Abstract
Background
Oral leukoplakia is a relatively common oral lesion that, in a small proportion of people, precedes the development of oral cancer. Most leukoplakias are asymptomatic; therefore, the primary objective of treatment should be to prevent onset of cancer. This review updates our previous review, published in 2006.
Objectives
To assess the effectiveness, safety and acceptability of treatments for leukoplakia in preventing oral cancer.
Search methods
We searched the following electronic databases: Cochrane Oral Health's Trials Register (to 16 May 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 4), MEDLINE Ovid (1946 to 16 May 2016), Embase Ovid (1980 to 16 May 2016) and CancerLit via PubMed (1950 to 16 May 2016). We searched the metaRegister of Controlled Trials (to 10 February 2015), ClinicalTrials.gov (to 16 May 2016) and the World Health Organization (WHO) International Clinical Trials Registry Platform for ongoing trials (to 16 May 2016). We placed no restrictions on the language or date of publication when searching electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) that enrolled people with a diagnosis of oral leukoplakia and compared any treatment versus placebo or no treatment.
Data collection and analysis
We collected data using a data extraction form. Oral cancer development, demonstrated by histopathological examination, was our primary outcome. Secondary outcomes were clinical resolution of the lesion, improvement of histological features and adverse events. We contacted trial authors for further details when information was unclear. When valid and relevant data were available, we conducted a meta‐analysis of the data using a fixed‐effect model when we identified fewer than four studies with no heterogeneity. For dichotomous outcomes, we calculated risk ratios (RRs) and 95% confidence intervals (CIs). We assessed risk of bias in studies by using the Cochrane tool. We assessed the overall quality of the evidence by using standardised criteria (Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE)).
Main results
We included 14 studies (909 participants) in this review. Surgical interventions, including laser therapy and cryotherapy, have never been studied by means of an RCT that included a no treatment or placebo arm. The included trials tested a range of medical and complementary treatments, in particular, vitamin A and retinoids (four studies); beta carotene or carotenoids (three studies); non‐steroidal anti‐inflammatory drugs (NSAIDs), specifically ketorolac and celecoxib (two studies); herbal extracts (four studies), including tea components, a Chinese herbal mixture and freeze‐dried black raspberry gel; bleomycin (one study); and Bowman‐Birk inhibitor (one study).
We judged one study to be at low risk of bias, seven at unclear risk and six at high risk. In general, we judged the overall quality of the evidence to be low or very low, so findings are uncertain and further research is needed.
Five studies recorded cancer incidence, only three of which provided useable data. None of the studies provided evidence that active treatment reduced the risk of oral cancer more than placebo: systemic vitamin A (RR 0.11, 95% CI 0.01 to 2.05; 85 participants, one study); systemic beta carotene (RR 0.71, 95% CI 0.24 to 2.09; 132 participants, two studies); and topical bleomycin (RR 3.00, 95% CI 0.32 to 27.83; 20 participants, one study). Follow‐up ranged between two and seven years.
Some individual studies suggested effectiveness of some proposed treatments, namely, systemic vitamin A, beta carotene and lycopene, for achieving clinical resolution of lesions more often than placebo. Similarly, single studies found that systemic retinoic acid and lycopene may provide some benefit in terms of improvement in histological features. Some studies also reported a high rate of relapse.
Side effects of varying severity were often described; however, it seems likely that interventions were well accepted by participants because drop‐out rates were similar between treatment and control groups.
Authors' conclusions
Surgical treatment for oral leukoplakia has not been assessed in an RCT that included a no treatment or placebo comparison. Nor has cessation of risk factors such as smoking been assessed. The available evidence on medical and complementary interventions for treating people with leukoplakia is very limited. We do not currently have evidence of a treatment that is effective for preventing the development of oral cancer. Treatments such as vitamin A and beta carotene may be effective in healing oral lesions, but relapses and adverse effects are common. Larger trials of longer duration are required to properly evaluate the effects of leukoplakia treatments on the risk of developing oral cancer. High‐quality research is particularly needed to assess surgical treatment and to assess the effects of risk factor cessation in people with leukoplakia.
Keywords: Humans; Leukoplakia, Oral; Leukoplakia, Oral/therapy; Mouth Neoplasms; Mouth Neoplasms/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Interventions for treating oral leukoplakia to prevent oral cancer
Review question
People with oral leukoplakia are at higher risk of developing oral cancer than those with normal oral mucosa. This review, produced through Cochrane Oral Health, seeks to evaluate whether people affected by leukoplakia can benefit from surgical, medical or complementary treatments, either local or systemic. In particular, we conducted this review to find out which, if any, treatment is able to prevent people with leukoplakia of the mouth from getting oral cancer. This review updates our previous review published in 2006.
Background
Oral leukoplakia is a white patch formed in the mouth lining that cannot be rubbed off. It often does not hurt and may go unnoticed for years. People with leukoplakia develop oral cancer more often than people without it. Preventing this is critical because rates of oral cancer survival longer than five years after diagnosis are low. Drugs, surgery and other therapies have been tried for treatment of oral leukoplakia.
Objectives
This review aimed to evaluate whether treatments for oral leukoplakia are effective in preventing oral cancer, and safe and acceptable to patients.
Study characteristics
The evidence on which this review is based is up‐to‐date as of May 2016. We found 14 randomised controlled trials (RCTs) of medical and complementary treatments, which involved 909 participants in total. Treatments included herbal extracts, anti‐inflammatory drugs, vitamin A, beta carotene supplements and others. Surgical treatment has not been compared with placebo or no treatment in an RCT.
Key results
Cancer development was measured in studies of three treatments: systemic vitamin A, systemic beta carotene and topical bleomycin. None of these treatments showed effectiveness in preventing cancer development, as measured up to two years for vitamin A and beta carotene, and seven years for bleomycin.
Some individual studies of vitamin A and beta carotene suggested that these treatments may be effective for improving or healing oral lesions. However, some studies observed a high rate of relapse in participants whose lesions were initially resolved by treatment.
Most treatments caused side effects of differing severity in a high proportion of participants.
It seems likely that interventions were well accepted by participants because drop‐out rates were similar between treatment and control groups.
Quality of the evidence
The available evidence is very limited. Most interventions were assessed by only one small study. Most studies had problems in the way they were conducted, making their results unreliable. We judged the quality of evidence for the outcome of cancer development to be very low.
Author conclusions
Larger, better studies of longer duration are required. As well as further studies of drug treatment and alternative treatments like vitamins, studies are needed to evaluate the effectiveness and safety of surgery, and of stopping risk factor habits such as smoking.
Summary of findings
Summary of findings for the main comparison. Vitamin A or retinoids versus placebo for treating oral leukoplakia.
| Systemic or topical vitamin A vs placebo for treating leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: vitamin A or retinoids Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with vitamin A or retinoids | |||||
| Cancer development at 24 months from start of treatment (treatment lasted 12 months) | 93 per 1000 | 10 per 1000 (1 to 191) | RR 0.11 (0.01 to 2.05) | 85 (1 RCT) | ⊕⊝⊝⊝ very lowa | This study evaluated systemic treatment |
| Clinical resolution (not completely resolved) at 4 to 12 months | Studies could not be combined in meta‐analysis One study evaluated topical treatment and did not find evidence of benefit 3 studies of systemic vitamin A ‐ 2 showed benefit in terms of clinical resolution, and 1 did not |
|||||
| Histological changes (not improved) at 3 months | 889 per 1000 | 382 per 1000 (213 to 676) | RR 0.43 (0.24 to 0.76) | 41 (1 RCT) |
⊕⊕⊕⊝ moderateb | This study evaluated systemic treatment |
| Safety of the intervention at 4 to 12 months | 3 studies (1 each evaluating topical acitretin, topical 13‐cis‐retinoic acid, 200,000 IU per week of vitamin A) found no adverse effects. Systemic 13‐cis‐retinoic acid (1 to 2 mg/kg/d) caused adverse effects of varying severity in 79% of participants | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; vs = versus; d = day | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to the estimate of the effect Moderate quality: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect but may be substantially different Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
*From event rate in control group aDowngraded 3 levels as single small study at unclear risk of bias with very imprecise result bDowngraded as single small study
Summary of findings 2. Systemic beta carotene or carotenoids vs placebo for treating oral leukoplakia.
| Systemic beta carotene or carotenoids vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: systemic beta carotene Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with beta carotene or carotenoids | |||||
| Cancer development at 24 months from start of treatment (treatment lasted 12 months) |
108 per 1000 | 79 per 1000 (26 to 238) | RR 0.73 (0.24 to 2.20) | 132 (2 RCTs) | ⊕⊕⊝⊝ very low1 | |
| Clinical resolution (not completely resolved) at 5 to 12 months |
The 3 studies could not be combined in meta‐analysis. 2 found benefit for systemic beta carotene, and 1 did not | |||||
| Histological changes (not improved) at 5 months (treatment lasted 3 months) |
833 per 1000 | 200 per 1000 (100 to 383) |
RR 0.24 (0.12 to 0.46) | 58 (1 RCT) |
⊕⊕⊝⊝ low2 | |
| Safety of the intervention at 5 to 12 months | Systemic beta carotene did not cause any adverse effects in 1 study supplementing 10 mg/d, and caused adverse effects of varying severity in 9% of participants in another study supplementing 360 mg/wk No adverse effects were reported by participants treated with systemic lycopene in one study |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; vs = versus; d = day; wk = week | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* From event rate in control group aDowngraded 3 levels for unclear or high risk of bias and serious imprecision bDowngraded 2 levels as single small study at unclear risk of bias
Summary of findings 3. Non‐steroidal anti‐inflammatory drugs (NSAIDs) vs placebo for treating oral leukoplakia.
| NSAIDs vs placebo for treating oral leukoplakia | ||||||
|
Patient or population: people with oral leukoplakia Intervention: NSAIDs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with NSAIDs | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 3 months | 1 study evaluated systemic treatment and 1 evaluated topical treatment. Neither found benefit for NSAIDs | |||||
| Histological changes (not improved) | Not measured | |||||
| Safety of the intervention over 3 months |
Systemic celecoxib (1 study) ‐ 32 intervention participants reported 56 adverse effects and 20 placebo participants in the placebo group reported 20 adverse effects. Minor adverse events included dizziness, diarrheoa and abdominal pain. 4 participants (2 from the placebo group and 2 from an intervention group) had grade 3 adverse events. 2 participants permanently discontinued treatment owing to an adverse event (grade 2 vision abnormality and hypertension in a participant receiving 400 mg twice daily of celecoxib and a grade 3 ischaemic cerebrovascular accident in a participant receiving 200 mg twice daily of celecoxib) Ketorolac oral rinse (1 study) caused adverse effects of varying severity in 29% of participants |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; vs = versus | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
Summary of findings 4. Herbal extracts vs placebo for treating oral leukoplakia.
| Herbal extracts vs placebo for treating oral leukoplakia | ||||||
|
Patient or population: people with oral leukoplakia Intervention: herbal extracts ‐ tea components; a Chinese herbal mixture; curcumin chewing gum; freeze‐dried black raspberry gel Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with herbal extracts | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 3 to 6 months | 3 studies (1 of freeze‐dried black raspberry gel, 1 of green tea extract capsules and 1 of mixed tea treatment) did not find evidence of benefit for the intervention | |||||
| Histological changes (not improved) at 3 months | 2 studies (1 of green tea extract capsules and 1 of freeze‐dried raspberry gel) did not find evidence of benefit from these interventions | |||||
| Safety of the intervention up to 3 months | 3 studies measured adverse effects: green tea extract capsules caused high frequency (93%) of adverse effects of varying severity in 1 study; the mixed tea treatment study did not mention adverse effects; freeze‐dried black raspberry gel did not cause any adverse effects | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
Summary of findings 5. Topical bleomycin vs placebo for treating oral leukoplakia.
| Topical bleomycin vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: topical bleomycin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with topical bleomycin | |||||
| Cancer development up to 7 years | 83 per 1000 | 250 per 1000 (27 to 1000) |
RR 3.00 (0.32 to 27.83) | 20 (1 RCT) | ⊕⊝⊝⊝ very lowa | |
| Clinical resolution (not completely resolved) at 3 months |
917 per 1000 | 504 per 1000 (266 to 954) | RR 0.55 (0.29 to 1.04) | 22 (1 RCT) |
⊕⊝⊝⊝ very lowa | |
| Histological changes (not improved) at 3 months | 818 per 1000 | 401 per 1000 (180 to 900) | RR 0.49 (0.22 to 1.10) | 21 (1 RCT) |
⊕⊝⊝⊝ very lowa | |
| Safety of the intervention up to 3 months | "All patients in the bleomycin group developed erythema with erosion by the end of the applications, whereas erythema developed in the placebo group. Discomfort was reported by 60% of the bleomycin group, but analgesics were not required. Taste of the topical application as well‐tolerated. There was no observed systemic toxicity in the patient groups" | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; vs = versus | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but may be substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
* From event rate in control group aDowngraded 3 levels as a single small study at unclear risk of bias with imprecise result
Summary of findings 6. Bowman‐Birk inhibitor versus placebo for oral leukoplakia.
| Bowman‐Birk inhibitor vs placebo for treating oral leukoplakia | ||||||
|
Patient or population: people with oral leukoplakia Intervention: Bowman‐Birk inhibitor Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Bowman‐Birk inhibitor | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 6 months | 957 per 1000 | 957 per 1000 (871 to 1000) |
RR 1.00 (0.91 to 1.09) | 21 (1 RCT) |
⊕⊕⊝⊝ lowa | |
| Histological changes (not improved) at 6 months | Not measured | |||||
| Safety of the intervention up to 6 months | Trial authors reported that there were no significant adverse effects. 33 participants in the intervention group reported 75 adverse effects. 25 participants in the placebo group reported 63 adverse effects | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI = confidence interval; RR = risk ratio; vs = versus | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
* From event rate in control group aDowngraded 2 levels as a single small study at high risk of bias
Background
Description of the condition
"The term leukoplakia should be used to recognize white plaques of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer" (Warnakulasuriya 2007). Such a definition is the result of the efforts of an international working group comprising specialists in the fields of epidemiology, oral medicine, pathology and molecular biology with a special interest in cancer and precancer, who met in London in 2005. This meeting was co‐ordinated by the World Health Organization (WHO) Collaborating Centre for Oral Cancer and Precancer in the UK, to review definitions, classifications, natural history and management of potentially malignant disorders on the basis of previously published work (Axell 1984; Axell 1996; Kramer 1978) and new scientific acquisitions. Thus, 'leukoplakia' is a clinical term that is used when the clinician has excluded any other condition of the oral mucosa that can present as a white lesion (e.g. frictional keratosis, lichen planus, white sponge nevus, hairy leukoplakia). Such lesions warrant biopsy and histopathological examination to assess the possible presence of epithelial dysplasia or carcinoma. Leukoplakia is often associated with tobacco smoking or chewing, although idiopathic forms are not rare (Axell 1987). The role of alcohol, viruses and systemic conditions needs further investigation (Dietrich 2004; Syrjänen 2011).
Clinical variants of leukoplakia are often classified into two groups: (1) homogeneous leukoplakia, a lesion of uniform flat appearance that may exhibit superficial irregularities, but with consistent texture throughout; and (2) non‐homogeneous leukoplakia, a predominantly white or white and red lesion (erythroleukoplakia) with an irregular texture that may include ulceration and may be characterised by a speckled, nodular or verrucous topography. Histological features of both forms of leukoplakia are variable and may include ortho‐keratosis or para‐keratosis of various degrees, acanthosis or atrophy of the squamous epithelium, mild inflammation in the corium, dysplastic changes of various grades (i.e. mild, moderate or severe), carcinoma in situ or carcinoma. Some cases of predominantly white lesions that are difficult to diagnose, in spite of the availability of a biopsy.
Leukoplakia is not uncommon, and although it is highly variable among geographical areas and demographic groups, the prevalence of leukoplakia in the general population varies from less than 1% to more than 5% (Axell 1984; Axell 1987; Bouquot 1986; Ikeda 1991; Reichart 2000). In a systematic review that included studies with more than 1000 individuals, the pooled prevalence was estimated to be between 1.49% and 4.27% (Petti 2003). Incidence data are very scarce. A study from Japan reported an age‐adjusted incidence rate per 100,000 person‐years of 409.2 among males and 70 among females (Nagao 2005), and an Indian study, conducted in a population with distinctive risk factors for oral cancer, reported lower figures: 240 among males and 3 among females (Gupta 1980).
Leukoplakia is one of a group of conditions defined as potentially malignant disorders (i.e. "morphological alterations amongst which some may have an increased potential for malignant transformation, [they] are also indicators of risk of likely future malignancies elsewhere in (clinically normal appearing) oral mucosa and not only site specific predictors") (Warnakulasuriya 2007). The rate of malignant transformation into squamous cell carcinoma varies from almost 0% to 36.4% (Arduino 2013), and a study investigating the natural limit of malignant transformation on the basis of European epidemiological data concluded that the upper limit of the annual transformation rate of oral leukoplakia is unlikely to exceed 1% (Scheifele 2003).
Non‐homogeneous leukoplakias carry a higher degree of risk of transformation when compared with homogeneous variants. Among patients with a histopathological diagnosis of dysplasia, about 1/10 of the total may be at higher risk. Other reported risk factors of statistical significance for cancer development in people with leukoplakia include female gender, long duration of leukoplakia, non‐smoking status, location on the lateral tongue and/or floor of the mouth, size > 200 mm2 (Holmstrup 2006) and the presence of Candida albicans (Van der Waal 2009). Studies investigating biomarkers and histological features have suggested methods that can be used to identify which patients with leukoplakia will develop oral cancer, and which will not (Pitiyage 2009; Smith 2009); however, a definitive, evidence‐based and clinically useful predictor of malignant transformation for dysplastic and non‐dysplastic leukoplakias is not available at the moment. Aneuploid lesions (i.e. with abnormal DNA content) are more likely to transform to cancer compared with diploid lesions (i.e. with normal DNA content) (Sperandio 2013; Torres‐Rendon 2009).
Description of the intervention
Most leukoplakias are asymptomatic; therefore, the need for treatment is based primarily on the precancerous nature of the lesion, and the primary aim of management should be to avoid development of cancer. This is particularly important in view of the poor prognosis associated with oral squamous cell carcinoma, in which only about 50% of patients are still alive five years after diagnosis (Scully 2009), and of the morbidity associated with oral cancer and complications of oral cancer therapy (Epstein 2012).
Many approaches to the treatment of leukoplakia have been proposed in an attempt to prevent cancer development and to evaluate clinical/histological resolution of oral leukoplakias (Lodi 2008). These approaches include surgical excision with different techniques (scalpel, cryosurgery, photodynamic therapy, laser surgery and vaporisation), medical treatment (topical or systemic), cessation of risk activities (smoking and alcohol) and no intervention but strict surveillance.
How the intervention might work
The rationale of surgical excision is that removing the clinically altered tissue could prevent the onset of oral cancer. For medical treatments, the rationale depends on the mechanism of action of the agent employed: retinoids, vitamin A and carotenoids might influence epithelial turnover; non‐steroidal anti‐inflammatory drugs (NSAIDs) block cyclo‐oxygenase activity, thereby modulating specific prostaglandins possibly involved in carcinogenesis; and chemotherapeutic agents act directly on early neoplastic cells. As many of these treatments have potentially serious adverse effects, the “wait and see” approach, based on strict clinical and histological surveillance, is generally employed to identify early cancer onset and to initiate cancer treatment to render the best possible prognosis. Although surgery and medical treatments aim to remove or reduce the lesion, it must be stressed that no evidence has shown a relationship between changes in size or resolution and decreased risk of oral cancer.
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important reviews to maintain in The Cochrane Library (Worthington 2015). This review was identified as a priority title by the oral medicine expert panel (Cochrane OHG priority review portfolio). Treatment of leukoplakia continues to be based on expert opinion, and more research is needed. This review aims to provide evidence‐based support for clinicians and patients and a clinical research agenda for planning future studies.
Objectives
To assess the effectiveness, safety and acceptability of treatments for leukoplakia in preventing oral cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing effects of surgery, medical or complementary treatments (local or systemic) or risk factor cessation versus placebo.
Types of participants
Anyone with a diagnosis of oral leukoplakia (without histopathological evidence of carcinoma) as defined, at the time of the studies, by consensus conferences held in 1978, 1983, 1994 and 2005 (Axell 1984; Axell 1996; Kramer 1978, Warnakulasuriya 2007).
Types of interventions
Active
Surgical removal of the lesion, including surgical excision, laser surgery, cryotherapy
Systemic medical treatment
Topical medical treatment, including anti‐inflammatory agents, antimycotic agents, carotenoids and retinoids, cytotoxic agents, etc.
Removal of predisposing habits (e.g. tobacco, alcohol)
Other treatment (e.g. photodynamic therapy)
Combined treatment
Control
Placebo
No treatment
Types of outcome measures
In light of the pre‐cancerous nature of leukoplakia, the primary objective of treatment is to prevent cancer development.
Primary outcomes
Oral cancer development, demonstrated by histopathological examination
Secondary outcomes
Clinical resolution, in terms of the proportion of lesions that did not resolve (with relapse data when provided)
Improvement of histological features, in terms of the proportion of lesions that did not show improvement in histological features
Safety of the intervention, as measured by the incidence of adverse effects
Search methods for identification of studies
To identify studies included in or considered for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE Ovid but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised controlled trials (RCTs) in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). We have provided details of the MEDLINE search in Appendix 1.
Electronic searches
We searched the following databases.
Cochrane Oral Health's Trials Register (to 16 May 2016) (see Appendix 2);
The Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 4) (see Appendix 3);
MEDLINE Ovid (1946 to 16 May 2016) (see Appendix 1);
Embase Ovid (1980 to 16 May 2016) (see Appendix 4);
CancerLit via PubMed (1950 to 16 May 2016) (see Appendix 5).
We placed no restrictions on the language or date of publication when searching the electronic databases.
Searching other resources
We searched the following databases for ongoing trials (see Appendix 6 for details of the search strategy).
metaRegister of Controlled Trials (to 10 February 2015);
ClinicalTrials.gov (to 16 May 2016);
The WHO International Clinical Trials Registry Platform (to 16 May 2016).
We manually checked the reference lists of included studies and existing reviews. The metaRegister of Controlled Trials is no longer available and so was not searched in May 2016.
Data collection and analysis
Selection of studies
Two review authors (GL and RF) separately examined the title and abstract of each article identified by the different search strategies. When at least one review author considered the article relevant, it progressed in the review process and was included in a digital archive prepared by using dedicated software. We obtained full reports for all relevant studies.
Data extraction and management
All studies meeting the inclusion criteria underwent data extraction performed by at least two review authors, using a specially designed form. We present the characteristics of trial participants, interventions and outcomes for the included trials in the Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of included trials and resolved disagreements through discussion and consensus. We used the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011a). This approach addresses the following seven specific domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other bias
Each domain in the tool includes one or more specific entries in a ‘Risk of bias’ table. Within each entry, the first part of the tool describes what was reported to have happened in the study, in sufficient detail to support a judgement about risk of bias. The second part of the tool assigns a judgement related to the risk of bias for that entry ‐ ‘low risk’, ‘high risk’ or ‘unclear risk’. After taking into account the additional information provided by trial authors, we summarised the risk of bias in included studies as follows.
Low risk of bias: low risk of bias for all key domains
Unclear risk of bias: unclear risk of bias for one or more key domains
High risk of bias: high risk of bias for one or more key domains
We completed a 'Risk of bias' table for each included study (see Characteristics of included studies) and presented results graphically by study (Figure 1) and by domain over all studies (Figure 2).
1.
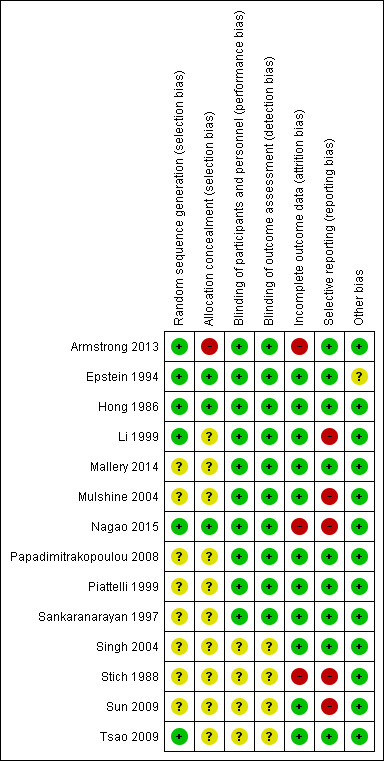
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
2.
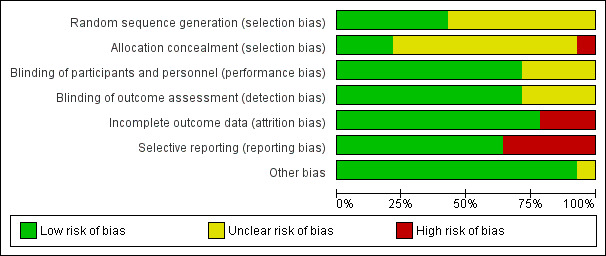
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Measures of treatment effect
The primary measure of intervention effect was onset of oral cancer. Dichotomous data were reported for this outcome measure: cancer development versus absence of cancer development.
Secondary outcomes, clinical resolution, histological changes and adverse effects were usually reported as ordinal measures. We dichotomised data: clinical resolution vs partial or no clinical response; decreased severity vs worsening of histology or no change in histological features.
For each intervention, we sought and summarised data on the number of participants from both intervention and control groups who experienced the event (outcome) and the total number of participants. We analysed dichotomous data by calculating risk ratios. As we anticipated pooling data from studies in which true treatment effects were likely to differ, we planned to use a random‐effects model in statistical analyses; however, we used a fixed‐effect model because of the very small number of studies combined.
Unit of analysis issues
The individual participant was the unit of analysis.
Dealing with missing data
Whenever possible, we obtained missing data from tables and graphs or through personal contact with study authors. When this was not possible, and we found no evidence that data were missing because of a specific bias, we analysed only available data (Higgins 2011). This represents a change from the previous version of the review, in which missing data were imputed with the assumption that all were poor outcomes.
Assessment of heterogeneity
We assessed the significance of discrepancies in the estimates of treatment effects provided by different trials by using Cochran’s test for heterogeneity and the I² statistic; the latter describes the percentage total variation across studies that is due to heterogeneity rather than to chance. Heterogeneity was considered statistically significant if the P value was less than 0.1. A rough guide to the interpretation of I² given in the Cochrane Handbook for Systematic Reviews of Interventions is as follows: 0 to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, 75% to 100% represents very substantial ('considerable') heterogeneity (Higgins 2011).
Assessment of reporting biases
We attempted to minimise reporting biases by conducting a thorough search of multiple sources including trial registries, and by making efforts to identify unpublished trials and non‐English language publications.
Data synthesis
When valid and relevant data were collected, we undertook a meta‐analysis of the data. We grouped and analysed studies on the basis of intervention category. We conducted meta‐analyses in Review Manager software, using the Mantel‐Haenszel method with a fixed‐effect model. We had planned to use a random‐effects model, but this would not have been appropriate because of the small number of studies included. We did not pool data when substantial heterogeneity was identified.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct subgroup analyses for smoking and non‐smoking participants, and for lesions with or without dysplasia. Unfortunately, as such data were not available, we did not perform subgroup analyses.
Sensitivity analysis
We had planned to undertake sensitivity analysis excluding studies at high risk and at unclear risk of bias.
Summarising findings and assessing the quality of the evidence
We constructed 'Summary of findings' tables for each comparison to present the results for our review outcomes. We assessed the quality of the evidence using GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) criteria.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
This review was originally published in 2001, and updates were published in 2004 and 2006. Since its first publication up until May 2016, we have identified a total of 3438 articles through the search strategy. We have examined titles and abstracts for eligibility and have eliminated those not matching the inclusion criteria. We identified 68 apparently eligible studies and rejected 30 because they were not pertinent. Nine of the studies are ongoing (see Characteristics of ongoing studies for details). After we obtained the full‐text version of the remaining 29 studies, we excluded 13 additional studies (Characteristics of excluded studies) ‐ one because it was quasi‐randomised, four because of inadequate allocation, four for problems in selection of participants and four for lack of an adequate control group. We categorised two studies as awaiting classification (Califano 2012; Chiba 2012; Characteristics of studies awaiting classification). Thus we included 14 studies in this review. See Figure 3.
3.

Study flow diagram
Included studies
Characteristics of trial setting and design
Location
Of the 15 studies included, six were conducted in the USA (Armstrong 2013; Hong 1986; Mallery 2014; Mulshine 2004; Papadimitrakopoulou 2008; Tsao 2009), three in India (Sankaranarayan 1997; Singh 2004; Stich 1988), one in Italy (Piattelli 1999), two in China (Li 1999; Sun 2009), one in Canada (Epstein 1994) and one in Japan (Nagao 2015). The setting for all studies was a university hospital.
Design
Ten trials had a two‐arm parallel design (Armstrong 2013; Epstein 1994; Hong 1986; Li 1999; Mallery 2014; Mulshine 2004; Nagao 2015; Piattelli 1999; Stich 1988; Sun 2009); three, a three‐arm parallel design (Papadimitrakopoulou 2008; Sankaranarayan 1997; Singh 2004); and one, a four‐arm parallel design (Tsao 2009). In three of the four studies with more than two arms, we pooled together data from the active arms: interventions differed in dosage in Papadimitrakopoulou 2008; Singh 2004; and Tsao 2009.
Duration
The trials varied in length. Three studies used an open follow‐up (Epstein 1994; Nagao 2015; Tsao 2009); one study lasted two years (Sankaranarayan 1997); and all other studies lasted less than one year.
Funding
Two trials did not specify any funding source (Epstein 1994; Sankaranarayan 1997). In two trials, some study authors worked for the company that supplied the study drug (Mulshine 2004; Tsao 2009); in another, the first study author had ownership interest in the patent of the drug tested (Mallery 2014). One study was supported by Central Soya Company and NIH (National Institutes of Health) (Armstrong 2013), one by Hoffmann‐La Roche and the National Cancer Institute (Hong 1986), one by the Chinese National Natural Science Foundation (Li 1999), one by NIH (Mallery 2014), one by the National Cancer Institute Specialized Programs of Reasearch Excellence (SPORE) Program (Mulshine 2004), one by the Butterfield Award of the Sasakawa Foundation GB and DSM Nutrition Japan (Nagao 2015), one by Pfizer (Papadimitrakopoulou 2008), one by the Italian National Research Council (CNR) and the Italian Ministry of University, Research, Science and Technology (MURST) (Piattelli 1999), one by Jagsonpal Pharmaceuticals Ltd., New Delhi, India (Singh 2004), one by the National Cancer Institute of Canada (Stich 1988), one by the Beijing Natural Science Foundation, the National Natural Science Foundation of China and the Tenth 5‐Year Plan of National Key Technologies R&D Program in China and NIH (Sun 2009) and one by Ito En Ltd. (Tsao 2009).
Characteristics of participants
The total number of participants randomised in the trials was 909, with a mean of 64.9 participants per study (ranged from 10 to 131 participants).
The reported proportion of smoking and drinking participants (the two main risk factors for oral cancer) varied from 8% (Papadimitrakopoulou 2008) to 86% (Mulshine 2004), and from 9% (Sun 2009) to 71% (Mulshine 2004), respectively. Use of tobacco products (Mallery 2014) and smoking (Nagao 2015) were exclusion criteria in two studies. None of the study authors reported significant changes in these habits during the course of the trial. In two studies, all participants recruited were chewers of tobacco‐containing betel quid (another well‐known risk factor for oral cancer) from the same Indian village (Trivandrum, Kerala) (Sankaranarayan 1997; Stich 1988). All participants enrolled in the studies underwent a confirmatory biopsy; however, only four studies reported the histological criteria employed (Epstein 1994; Papadimitrakopoulou 2008; Stich 1988; Sun 2009). Seven studies reported the percentage of dysplastic lesions, which ranged from 18.75% (Sun 2009) to 73.2% (Tsao 2009) (see Table 7). One study excluded lesions with severe dysplasia (Li 1999), and another study included cases with at least one of the following features: at least mild dysplasia, high‐risk location, significant extent of tissue involvement and presence of symptoms (Tsao 2009).
1. Participants with dysplastic leukoplakia.
| Study | Participants with dysplastic leukoplakia (any grade) |
| Armstrong 2013 | Not reported |
| Epstein 1994 | 22% |
| Hong 1986 | 27% |
| Li 1999 | 20% |
| Mallery 2014 | 72.5% |
| Mulshine 2004 | Not reported |
| Nagao 2015 | Not reported |
| Papadimitrakopoulou 2008 | Not reported |
| Piattelli 1999 | Not reported |
| Sankaranarayan 1997 | Not reported |
| Singh 2004 | 59% |
| Stich 1988 | Not reported |
| Sun 2009 | 18.75% |
| Tsao 2009 | 73.2% |
Characteristics of interventions
We did not identify any RCTs that compared surgical treatments with placebo or no treatment, nor did we identify any RCTs of risk factor cessation. All included trials compared medical or complementary treatment versus placebo, usually a preparation similar to the treatment, without the active ingredient; in one case, the placebo contained vitamin C, which we considered an inactive ingredient (Nagao 2015).
Four RCTs compared topical treatment versus placebo (129 participants) (Epstein 1994; Mallery 2014; Mulshine 2004; Piattelli 1999), and nine RCTs compared systemic treatment versus placebo (716 participants) (Armstrong 2013; Hong 1986; Nagao 2015; Papadimitrakopoulou 2008; Sankaranarayan 1997; Singh 2004; Stich 1988; Sun 2009; Tsao 2009). One RCT compared a combination of topical and systemic treatments versus placebo (64 participants) (Li 1999).
Four studies tested vitamin A or retinoids (Hong 1986; Piattelli 1999; Sankaranarayan 1997; Stich 1988); three studies tested beta carotene or carotenoids (Nagao 2015; Sankaranarayan 1997; Singh 2004); two studies tested NSAIDs: ketorolac (Mulshine 2004) and celecoxib (Papadimitrakopoulou 2008); and four studies tested herbal extracts, in particular, tea components (Li 1999 ‐ mixed; Tsao 2009 ‐ green tea extract capsules), a Chinese herbal mixture (Sun 2009) and gel containing freeze‐dried black raspberries (Mallery 2014). The other interventions tested were bleomycin (Epstein 1994) and Bowman‐Birk inhibitor (Armstrong 2013).
Characteristics of outcomes
Five studies reported data on oral cancer development (Epstein 1994; Nagao 2015; Papadimitrakopoulou 2008; Sankaranarayan 1997; Tsao 2009). In Epstein's trial, although seven out of 12 participants in the control group received the active treatment at the end of the study period, we conducted an intention‐to‐treat (ITT) analysis for this review.
All studies used lesion measurement as the clinical parameter to assess change; five studies also used pictures of the lesions for clinical evaluation (Armstrong 2013; Epstein 1994; Hong 1986; Mallery 2014; Piattelli 1999). In 11 RCTs, a complete response was defined as complete disappearance of the lesion (Armstrong 2013; Epstein 1994; Hong 1986; Li 1999; Mulshine 2004; Nagao 2015; Papadimitrakopoulou 2008; Piattelli 1999; Sankaranarayan 1997; Singh 2004; Tsao 2009), lasting at least four weeks in four of them (Hong 1986; Mulshine 2004; Sankaranarayan 1997; Singh 2004). For partial response, nine studies used the definition 'greater than 50% reduction', and one used a slightly different criterion ‐ 'greater than 30% reduction' (Li 1999). Three studies defined “stable disease” as a reduction of less than 50% of the lesion (Hong 1986; Mulshine 2004; Singh 2004); three studies adopted an otherwise non‐specified "unchanged clinical aspect" (Li 1999; Stich 1988; Sun 2009); and two studies defined "stable disease" as lesions not satisfying any other category (Papadimitrakopoulou 2008; Tsao 2009). Eight studies gave similar definitions of "disease progression" as an increase in the size of the lesion or the appearance of new lesions (Armstrong 2013; Hong 1986; Li 1999; Mulshine 2004; Papadimitrakopoulou 2008; Singh 2004; Sun 2009; Tsao 2009). One study included the "no response" category, indicating stable and progressive lesions (Sankaranarayan 1997). Three studies adopted different categories for clinical evaluation. Stich 1988 used the following: remission, no change, new leukoplakia. Sun 2009 used positive response (including complete and partial response), stable disease and progressive disease. One study reported the change in lesion measurement, expressed in mm2 (Mallery 2014). Clinical response was recorded immediately at the end of treatment in 10 studies (Armstrong 2013; Li 1999; Mallery 2014; Nagao 2015; Papadimitrakopoulou 2008; Piattelli 1999; Sankaranarayan 1997; Singh 2004; Stich 1988; Tsao 2009), two weeks after the end of treatment in Epstein 1994 and three months after the end of treatment in Sun 2009. In two studies, it was not clear when the reported clinical assessment was recorded (Hong 1986; Mulshine 2004).
Six studies reported assessment of histological changes (Armstrong 2013; Epstein 1994; Hong 1986; Papadimitrakopoulou 2008; Singh 2004; Tsao 2009). These studies did not use a unique histological classification, and the comparison between pre‐treatment and post‐treatment histological features was highly variable. One study defined histological response as an otherwise non‐specified "reversal" or "improvement" of dysplastic features (Papadimitrakopoulou 2008); another adopted a graphical method for evaluating histological changes (Armstrong 2013). Stich 1988 reported histological changes in the treatment group only. In one study, a control biopsy was taken only if development of cancer was suspected (Sankaranarayan 1997).
Biomarkers evaluated included bcl‐2 immunostaining (Piattelli 1999), AgNOR (silver‐stained nucleolar organizer region) and PCNA (proliferation cell nuclear antigen) labelling indexes (Li 1999; Sun 2009), biomarkers of DNA damage in exfoliated cells and peripheral blood lymphocytes (Li 1999), Neu protein of exfoliated cells and serum (Armstrong 2013), epidermal growth factor receptors (EGFRs) (Li 1999) and p53 and Ki67 (protein; cellular marker of neoplasia) (Nagao 2015).
Most trials monitored safety of the intervention. Only Li 1999 and Sun 2009 did not appear to measure adverse effects.
Excluded studies
The primary reason for exclusion of each study is given in the Characteristics of excluded studies table. Many trials were ineligible for more than one reason; however, the more common reasons for exclusion were inappropriate selection of participants, lack of random allocation and absence of a proper control arm. In particular, although we found three randomised controlled trials evaluating surgical interventions (Chee 2013; López‐Jornet 2013; Schwarz 2005), we were unable to include them in the review as they did not include a no treatment or placebo group.
Risk of bias in included studies
On the basis of criteria used in the critical appraisal of studies, one study had an overall low risk of bias (Hong 1986). We judged seven studies as having unclear risk of bias (Epstein 1994; Mallery 2014; Papadimitrakopoulou 2008; Piattelli 1999; Sankaranarayan 1997; Singh 2004; Tsao 2009). We considered the remaining studies to be at high risk of bias (Armstrong 2013; Li 1999; Mulshine 2004; Nagao 2015; Stich 1988; Sun 2009). See Figure 1 and Figure 2.
Allocation
We assessed the generation of the randomisation sequence as having low risk of bias for six trials and unclear risk for eight trials.
We assessed the concealment of allocation as having low risk of bias for three trials, at unclear risk for 10 trials and at high risk for one trial (Armstrong 2013). In Armstrong 2013, the block size was two.
Blinding
We assessed blinding of participants and personnel, as well as blinding of outcome assessment, as low risk of bias for 11 trials and unclear risk for four studies in which not enough information was provided.
Incomplete outcome data
The reported drop‐out rate ranged from 0% (Epstein 1994; Mallery 2014; Singh 2004) to 32.5% (Armstrong 2013). We assessed 11 trials as having low risk of bias with regard to attrition bias because they reported there were no drop‐outs, or because drop‐out was not likely to influence findings. We assessed three studies with high drop‐out rates as having high risk of bias (Armstrong 2013; Nagao 2015; Stich 1988).
Selective reporting
Most trials reported important outcomes and were assessed as having low risk of bias. Five studies failed to report histological results (Li 1999; Mulshine 2004; Nagao 2015; Stich 1988; Sun 2009); we assessed these trials as having high risk of bias for this domain.
Other potential sources of bias
We assessed Epstein 1994, which had discrepant published and unpublished data, along with baseline imbalance, as having unclear risk of bias for this domain. See Figure 2.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Vitamin A and retinoids versus placebo
Four studies (one at high, two at unclear and one at low risk of bias) compared vitamin A and retinoids versus placebo. Three of these studies evaluated systemic treatment (Hong 1986; Sankaranarayan 1997; Stich 1988), and one evaluated topical treatment (Piattelli 1999).
Oral cancer development
One study reported effects of systemic vitamin A on cancer incidence (Sankaranarayan 1997). Investigators found no evidence of benefit compared with placebo (risk ratio (RR) 0.11, 95% confidence interval (CI) 0.01 to 2.05; 85 participants) (Analysis 1.1; Figure 4).
1.1. Analysis.
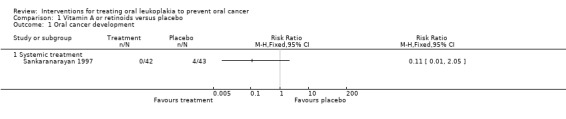
Comparison 1 Vitamin A or retinoids versus placebo, Outcome 1 Oral cancer development.
4.

Forest plot of comparison: 1 Vitamin A or retinoids vs placebo, outcome: 1.1 Cancer development
Clinical resolution
Five studies reported effects of vitamin A or retinoids on clinical features of leukoplakia, in particular, on its resolution (Analysis 1.2; Figure 5). In particular, three studies tested systemic treatment (Hong 1986; Sankaranarayan 1997; Stich 1988), but because heterogeneity was high (I2 = 94%), it was inappropriate to combine findings in a meta‐analysis. Two of the three studies at high or unclear risk of bias showed some benefit (Sankaranarayan 1997: RR 0.51, 95% 0.37 to 0.71; 85 participants; Stich 1988: RR 0.44, 95% CI 0.27 to 0.73; 54 participants), but Hong 1986, which was at low risk of bias, showed no clear evidence of benefit (RR 0.92, 95% CI 0.78 to 1.08; 40 participants).
1.2. Analysis.
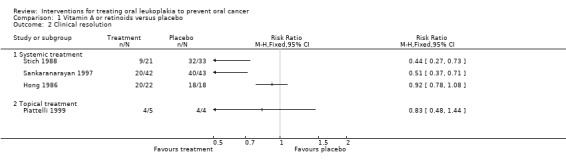
Comparison 1 Vitamin A or retinoids versus placebo, Outcome 2 Clinical resolution.
5.
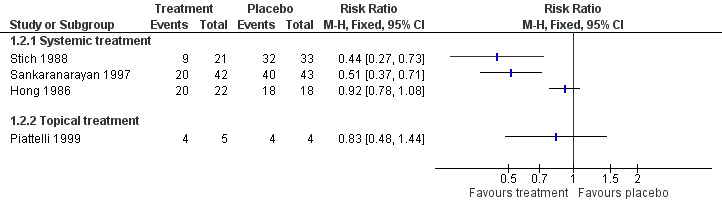
Forest plot of comparison: 1 Vitamin A or retinoids vs placebo, outcome: 1.2 Oral lesion not completely resolved
Hong 1986 provided relapse data: nine out of 16 (56%) participants who responded to treatment (partially or completely) subsequently relapsed (no information was available regarding the two partial responders from the placebo group). Sankaranarayan 1997 reported that 14 out of 22 (64%) complete responders developed recurrent lesions (no information was available regarding the three complete responders in the placebo group).
One study at unclear risk of bias tested topical treatment (nine participants) and found treatment was not more likely to completely resolve the lesion than placebo: RR 0.83, 95% CI 0.48 to 1.44 (Piattelli 1999).
In Piattelli 1999, one out of five (20%) participants responding completely or partially to the experimental treatment relapsed, and one out of four (25%) participants responding to placebo relapsed.
Improvement of histological features
A single study recorded histological improvement and showed that improvement in histological features of lesions was more likely in participants treated with systemic retinoic acid than in those treated with placebo (RR 0.43, 95% CI 0.24 to 0.76; 39 participants; Analysis 1.3) (Hong 1986).
1.3. Analysis.
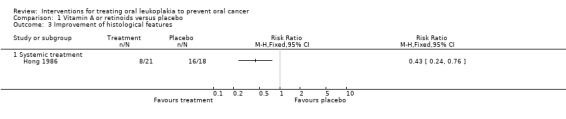
Comparison 1 Vitamin A or retinoids versus placebo, Outcome 3 Improvement of histological features.
Safety
Topical 13‐cis‐retinoic acid (Piattelli 1999) and 200,000 IU per week of vitamin A (Stich 1988) produced no adverse effects. Systemic 13‐cis‐retinoic acid (1 to 2 mg/kg/d) (Hong 1986) caused adverse effects of varying severity in 79% of participants (see Table 8). Two participants withdrew from Hong 1986 because of severe conjunctivitis and hypertriglyceridaemia.
2. Participants reporting adverse effects.
| Study | Arms | Active treatment | Placebo | Adverse effects |
| Armstrong 2013 | Bowman Birk inhibitor concentrate vs placebo | 75 reported from 33 of 67 participants | 63 reported from 25 of 65 participants | Minor adverse effects |
| Epstein 1994 | Topical bleomycin vs placebo | 10/10 | 0/12 | Bleomycin group ‐ erythema and erosion (100%), discomfort (60%) Placebo group ‐ erythema only |
| Hong 1986 | Systemic 13‐cis‐retinoic acid (from 1 to 2 mg/kg per day) vs placebo | 19/24 | 4/20 | Cheilitis, facial erythema, dryness and peeling of skin, conjunctivitis, hypertriglyceridaemia |
| Li 1999 | Systemic and topical tea vs placebo | Not measured or reported | ||
| Mallery 2014 | Freeze‐dried black raspberry gel vs placebo gel | 0/22 | 0/18 | "No participant experienced any treatment‐associated complications" |
| Mulshine 2004 | Ketorolac oral rinse vs placebo | 11/38 | 3/19 | Pain, toxicity grade 1 and 2 |
| Nagao 2015 | Beta carotene and vitamin C vs placebo | 0/23 | 0/23 | No untoward side effects were noted |
| Papadimitrakopoulou 2008 | Celecoxib vs placebo | 56 reported from 32 participants | 20 reported from 18 participants | 4 participants presented grade 3 adverse events: 2 in placebo arm and 2 in active treatment arm. 2 participants from intervention groups discontinued treatment owing to adverse effects (1 grade 2 and 1 grade 3). |
| Piattelli 1999 | Topical 13‐cis‐retinoic acid vs placebo | 0/5 | 0/5 | "No side effects from the use of the gel were ever observed" |
| Sankaranarayan 1997 | Vitamin A (300,000 IU per week) vs placebo | 13/50 | 1/55 | Headache, muscular pain, dry mouth |
| Sankaranarayan 1997 | Beta carotene (360 mg per week) vs placebo | 5/55 | 1/55 | Headache, muscular pain |
| Singh 2004 | Lycopene (8 mg or 4 mg) vs placebo | 0/40 | 0/18 | "No side effects, toxicity of any sort were encountered in the complete duration of the therapy" |
| Stich 1988 | Vitamin A (200,000 IU per week) vs placebo | 0/30 | 0/35 | "The administered doses of vitamin A did not produce any detectable adverse effects during the trial period" |
| Sun 2009 | Chinese herbal mixture vs placebo | Not measured or reported | ||
| Tsao 2009 | Green tea extract at different doses (500, 750 or 1000 mg/m2 daily) vs placebo | 28/30 | 8/11 | Grade 1 to 2 adverse events including insomnia, headache, nausea and nervousness |
vs = versus
Beta carotene or carotenoids versus placebo
Three studies compared systemic beta carotene or carotenoids versus placebo (Nagao 2015; Sankaranarayan 1997; Singh 2004).
Oral cancer development
Two studies reported the effects of systemic beta carotene on cancer incidence (Nagao 2015; Sankaranarayan 1997). Investigators found no evidence of benefit when compared with placebo (RR 0.71, 95% CI 0.24 to 2.09; 132 participants; I2 = 0%; Analysis 2.1; Figure 6).
2.1. Analysis.
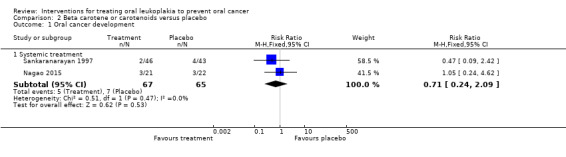
Comparison 2 Beta carotene or carotenoids versus placebo, Outcome 1 Oral cancer development.
6.
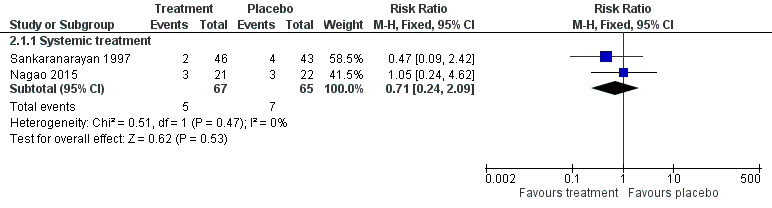
Forest plot of comparison: 2 Beta carotene or carotenoids vs placebo, outcome: 2.1 Cancer development
Clinical resolution
Three studies tested effects of systemic beta carotene and carotenoids on clinical resolution (Nagao 2015; Sankaranarayan 1997; Singh 2004) (Analysis 2.2; Figure 7). Owing to high heterogeneity (I2 = 87%), it was not appropriate to combine findings in a meta‐analysis. Two of the individual studies, which were at unclear risk of bias, found that systemic beta carotene was more effective than placebo for complete resolution of the lesion (Sankaranarayan 1997: RR 0.72, 95% CI 0.58 to 0.90; 89 participants; Singh 2004: RR 0.61, 95% CI 0.47 to 0.80; 58 participants). The other study, at high risk of bias, failed to show evidence of benefit (Nagao 2015: RR 0.95, 95% CI 0.84 to 1.08; 43 participants).
2.2. Analysis.
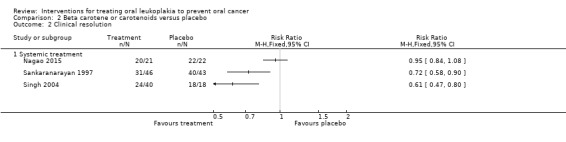
Comparison 2 Beta carotene or carotenoids versus placebo, Outcome 2 Clinical resolution.
7.
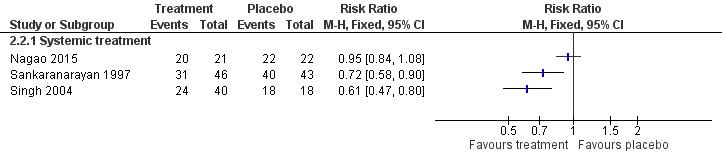
Forest plot of comparison: 2 Beta carotene or carotenoids vs placebo, outcome: 2.2 Oral lesion not completely resolved
Sankaranarayan 1997 reported that eight out of 15 (54%) complete responders developed recurrent lesions (no information was available regarding the three complete responders in the placebo group).
Improvement of histological features
Evidence of histological improvement was recorded when lycopene (a carotenoid) was compared with placebo (RR 0.24, 95% CI 0.12 to 0.46; one study; 58 participants; Analysis 2.3) (Singh 2004).
2.3. Analysis.
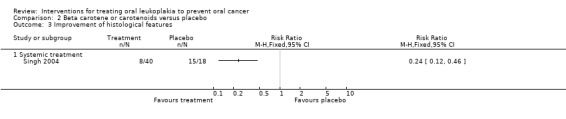
Comparison 2 Beta carotene or carotenoids versus placebo, Outcome 3 Improvement of histological features.
Safety
Systemic treatment with beta‐carotene produced no adverse effects in one study supplementing 10 mg/d (Nagao 2015). It caused adverse effects of varying severity in 9% of participants in another study supplementing 360 mg/wk (Sankaranarayan 1997). Researchers reported no adverse effects among participants treated with systemic lycopene (Singh 2004). See Table 8.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo
Two studies at unclear risk of bias compared NSAIDs ‐ ketorolac in Mulshine 2004 and celecoxib in Papadimitrakopoulou 2008 ‐ versus placebo.
Oral cancer development
Cancer development was among the outcomes reported in Papadimitrakopoulou 2008. This did not occur in either arm, probably because of the extremely short duration of the study (12 weeks).
Clinical resolution
Investigators found no evidence of benefit for systemic celecoxib (Papadimitrakopoulou 2008: RR 0.94, 95% CI 0.83 to 1.08; 46 participants) nor topical ketorolac (Mulshine 2004: RR 0.94, 95% CI 0.81 to 1.10; 56 participants) compared with placebo in terms of clinical resolution of lesions (Analysis 3.1).
3.1. Analysis.
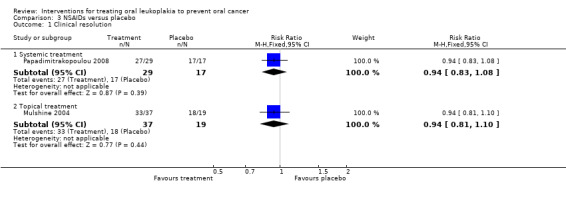
Comparison 3 NSAIDs versus placebo, Outcome 1 Clinical resolution.
Improvement of histological features
Histological changes were not among the outcomes in the studies testing NSAIDs.
Safety
In Papadimitrakopoulou 2008, which tested systemic celecoxib, trialists reported that the treatment was "safe and well tolerated". Thirty‐two intervention participants reported 56 adverse effects, and 20 placebo participants reported 20 adverse effects. Minor adverse events included dizziness, diarrheoa and abdominal pain. No participants had grade 4 adverse events. Four participants (two from the placebo group and two from an intervention group) had grade 3 adverse events. Two people discontinued treatment due to an adverse event (grade 2 vision abnormality and hypertension in a participant receiving 400 mgtwice daily of celecoxib and a grade 3 ischemic cerebrovascular accident in a participant receiving 200 mg twice daily of celecoxib.
Ketorolac oral rinse caused adverse effects of varying severity in 29% of participants (see Table 8). One person withdrew from the trial after the first dose because of mouth pain.
Herbal extracts versus placebo
Four studies compared herbal extracts, in particular, tea components (Li 1999; Tsao 2009), a Chinese herbal mixture (Sun 2009) and freeze‐dried black raspberry gel (Mallery 2014), versus placebo.
Oral cancer development
Cancer development was among the outcomes in Tsao 2009; however, it was not possible to analyse data because trial authors reported the cumulative number of cases, without specifying the allocation arm.
Clinical resolution
The four studies testing herbal extracts included clinical resolution among outcomes (Analysis 4.1).
4.1. Analysis.
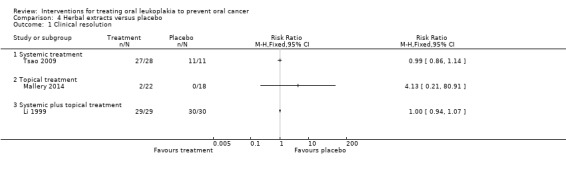
Comparison 4 Herbal extracts versus placebo, Outcome 1 Clinical resolution.
Systemic treatment with green tea extract showed no evidence of benefit in terms of clinical resolution of leukoplakia when compared with the control in one study at unclear risk of bias (Tsao 2009: RR 0.99, 95% CI 0.86 to 1.14; 39 participants). Li 1999, which was at high risk of bias, investigated a treatment integrating systemic (capsules containing 0.38 g of dried mixture of the whole water extract of green tea, green tea polyphenols and tea pigments) and topical preparations of mixed tea extract (mixed tea in glycerin at the concentration of 10%), but was not able to demonstrate benefit when compared with placebo in terms of clinical resolution (RR 1.00, 95% CI 0.94 to 1.07; 59 participants).
In one study investigating effects of a Chinese herbal mixture, it was not possible to extract data on clinical resolution (Sun 2009).
One topical herbal treatment (freeze‐dried black raspberries) showed no evidence of benefit when compared with placebo in a study that was at unclear risk of bias (Mallery 2014: RR 4.13, 95% CI 0.21 to 80.91; 40 participants). Among participants who did respond to such treatment, six of 22 (32%) in the treatment arm and seven of 17 (41%) in the placebo arm had visible evidence of lesion recurrence at former treatment sites at three months post trial follow‐up (Mallery 2014).
Improvement of histological features
In two studies reporting histological changes, neither active treatment (topical freeze‐dried black raspberries and systemic green tea extract) showed benefit when compared with placebo (Mallery 2014: RR 0.97, 95% CI 0.58 to 1.60; Tsao 2009: RR 0.86, 95% CI 0.66 to 1.13; Analysis 4.2).
4.2. Analysis.
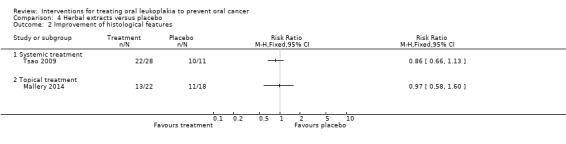
Comparison 4 Herbal extracts versus placebo, Outcome 2 Improvement of histological features.
Safety
People undergoing treatment with green tea reported very high frequency (93%) of adverse effects of varying severity in one study (Tsao 2009). Adverse effects were not mentioned in Li 1999. Freeze‐dried black raspberry gel caused no adverse effects (Mallery 2014). Sun 2009 stated, "drug toxicity was not monitored in the clinical trial". See Table 8.
Topical bleomycin versus placebo
Topical bleomycin was tested against placebo in a single small study at unclear risk of bias that included 22 participants (Epstein 1994). Following post‐treatment biopsy, seven participants in the placebo group were crossed over to receive the active intervention. An ITT analysis was conducted for outcomes measured after post‐treatment biopsy.
Mean follow‐up from the end of the study was 15 months for group A and 22 months for group B.
Oral cancer development
The trial found no evidence of benefit of topical bleomycin compared with placebo in reducing cancer development among participants affected by leukoplakia (RR 3.00, 95% CI 0.32 to 27.83; 20 participants; Analysis 5.1).
5.1. Analysis.
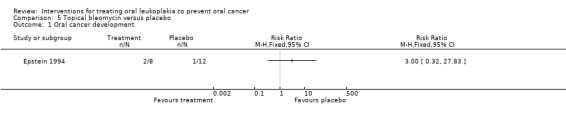
Comparison 5 Topical bleomycin versus placebo, Outcome 1 Oral cancer development.
Clinical resolution
Topical bleomycin showed no benefit for clinical resolution when compared with placebo: RR 0.55, 95% CI 0.29 to 1.04. In addition, among participants for whom follow‐up information was available, two out of four (50%) participants with a complete response relapsed and one out of two (50%) participants with a partial response relapsed (Analysis 5.2).
5.2. Analysis.
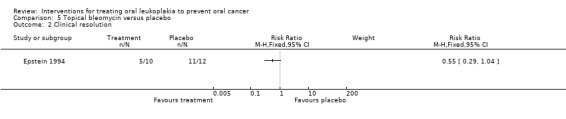
Comparison 5 Topical bleomycin versus placebo, Outcome 2 Clinical resolution.
Improvement of histological features
Epstein 1994 reported histological changes, showing no benefit of topical bleomycin when compared with placebo: RR 0.49, 95% CI 0.22 to 1.10 (Analysis 5.3).
5.3. Analysis.
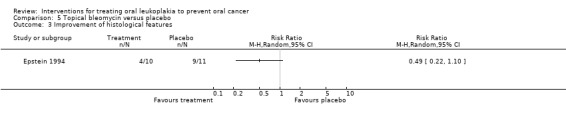
Comparison 5 Topical bleomycin versus placebo, Outcome 3 Improvement of histological features.
Safety
Topical bleomycin caused adverse effects of varying severity in 100% of participants (see Table 8). Participants in the bleomycin group developed erythema with erosion. Erythema developed in the placebo group; 60% of the bleomycin group reported discomfort but did not require analgesics. The trial found no evidence of systemic toxicity.
Bowman‐Birk Inhibitor versus placebo
A single study tested the Bowman‐Birk inhibitor against placebo (Armstrong 2013).
Oral cancer development
Cancer development was not among the outcomes of the study testing the Bowman‐Birk inhibitor.
Clinical resolution
The topical Bowman‐Birk inhibitor showed no benefit for clinical resolution when compared with placebo: RR 1.00, 95% CI 0.91 to 1.09 (Analysis 6.1).
6.1. Analysis.
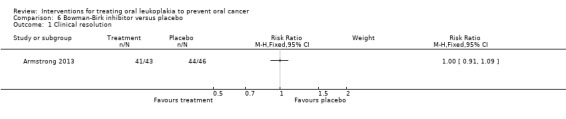
Comparison 6 Bowman‐Birk inhibitor versus placebo, Outcome 1 Clinical resolution.
Improvement of histological features
Data on histological changes from Armstrong 2013 were not available for analysis, but study authors reported no statistically significant differences in histological changes between study arms.
Safety
The Bowman‐Birk inhibitor caused adverse effects of varying severity in 49% of participants (see Table 8).
Sensitivity analysis
We did not undertake sensitivity analysis excluding studies at high or unclear risk of bias, as the only study at low risk of bias was not included in a meta‐analysis (Hong 1986).
Discussion
Leukoplakia is the most common potentially malignant oral disorder. Although rates of oral ccancer development may vary among studies, probably as the result of differences in diagnostic criteria for leukoplakia and follow‐up intervals, the morbidity and mortality associated with oral cancer suggest that leukoplakia is a relevant health issue for affected individuals. Yet, of the 14 studies included in the present review, none evaluated a surgical intervention nor the effect of habit cessation, and only three studies provided data on the effects of a medical or complementary treatment on cancer incidence.
Summary of main results
At present, there is no evidence that any of the medical or complementary treatments studied for people with leukoplakia can reduce the likelihood of oral cancer development. It should be noted that this conclusion is based on only three studies, namely, those testing systemic vitamin A, systemic beta carotene and topical bleomycin. These studies, which were at high or unclear risk of bias, included relatively few participants and had limited follow‐up. Overall, the quality of the evidence was very low.
Clinical change, in terms of variation in lesion size, was an outcome reported by all studies, although esearchers used different methods of measurement. Some single studies suggested effectiveness of some proposed treatments, namely, vitamin A, beta carotene and lycopene, in achieving complete clinical resolution of lesions more often than placebo (Sankaranarayan 1997; Singh 2004; Stich 1988). Similarly, single studies showed that vitamin A and lycopene provided some benefit in terms of improvement in histological features (Hong 1986; Singh 2004).
Leukoplakias generally are not associated with significant signs and symptoms, and the risk of developing cancer is relatively low (i.e. many patients with leukoplakia receive treatment that is not necessary). Therefore, proposed treatments should have minimal propensity for adverse effects. The proportion of participants reporting adverse effects varied between 0 and 100% in the active arms of the included trials, and between 0 and 90% in the placebo arms; however adverse effects were always more common in the study group than in the control group (see Table 8). It seems likely that interventions were well accepted by participants because drop‐out rates were similar between treatment and control groups (see Table 9); however, follow‐up may not have been long enough to permit this assessment. Adverse effects caused participants to withdraw in three studies: when systemic 13‐cis‐retinoic acid induced severe conjunctivitis and hypertriglyceridaemia (Hong 1986); when intolerable mouth pain followed the initial ketorolac mouthrinse (Mulshine 2004); and in two participants treated with celecoxib, because of vision abnormality and hypertension in one, and ischaemic cerebrovascular accident in another (Papadimitrakopoulou 2008).
3. Participants leaving the studies.
| Study | Arms | Active treatment | Placebo |
| Armstrong 2013 | Bowman Birk inhibitor concentrate vs placebo | 24/67 | 19/65 |
| Epstein 1994 | Topical bleomycin vs placebo | 0/10 | 1/12 |
| Hong 1986 | Systemic 13‐cis‐retinoic acid (from 1 to 2 mg/kg per day) vs placebo | 2/24 | 2/20 |
| Li 1999 | Systemic and topical tea vs placebo | 3/32 | 2/32 |
| Mallery 2014 | Freeze‐dried black raspberry gel vs placebo gel | 0/22 | 0/18 |
| Mulshine 2004 | Ketorolac oral rinse vs placebo | 1/38 | 0/19 |
| Nagao 2015 | Beta carotene and vitamin C vs placebo | 5/23 | 5/23 |
| Papadimitrakopoulou 2008 | Celecoxib vs placebo | 3/32 | 1/18 |
| Piattelli 1999 | Topical 13‐cis‐retinoic acid vs placebo | 0/5 | 1/5 |
| Sankaranarayan 1997 | Vitamin A (300,000 IU per week) vs placebo | 8/50 | 12/55 |
| Sankaranarayan 1997 | Beta carotene (360 mg per week) vs placebo | 9/55 | 12/55 |
| Singh 2004 | Lycopene (8 mg or 4 mg) vs placebo | 0/40 | 0/18 |
| Stich 1988 | Vitamin A (200,000 IU per week) vs placebo | 9/30 | 2/35 |
| Sun 2009 | Chinese herbal mixture vs placebo | 1/60 | 7/60 |
vs = versus
Overall completeness and applicability of evidence
Less than half (33% to 42%) of people with leukoplakia who develop oral cancer do so within two years of diagnosis (Lind 1987; Silverman 1984), and the incidence of oral cancer increases with the duration of follow‐up (Shiu 2000). Therefore, to properly test the effects of treatments on cancer incidence, it would be necessary to plan studies with large groups of participants and a long follow‐up period ‐ ideally, multi‐centre randomised controlled trials (RCTs) assessing outcomes at 10 years. As the duration of the studies included in this review was less than 12 months in all but four studies (Epstein 1994; Nagao 2015; Sankaranarayan 1997; Tsao 2009), cancer incidence rates are likely to be underestimated. Indeed, most of the studies did not include cancer incidence as an endpoint, but rather employed outcomes that assessed clinical or histological markers or both. Although easier to perform, studies using such outcomes pose a double problem: first, there is little evidence of the predictive value of many of those outcomes; second, they are difficult to compare. In addition, widespread outcomes, such as dysplasia grade, may be affected by high inter‐observer and even intra‐observer variation (Abbey 1995; Karabulut 1995).
It is noteworthy that, although surgery is the first choice in leukoplakia management for many clinicians, there is an absence of RCTs comparing the effects of surgical excision versus no treatment or placebo (Marley 1998). The only data available are from observational studies comparing rates of cancer incidence in people who did or did not undergo surgical treatment for oral leukoplakias. Such studies have differences in diagnostic and inclusion criteria, follow‐up interval, participant characteristics and surgical techniques employed (scalpel, laser, cryotherapy). They show highly variable results and sometimes are conflicting in their conclusions (Saito 2001; Schepman 1998). In addition, on the basis of animal and clinical studies, it has been speculated that surgery itself might act to promote carcinogenesis in pre‐malignant oral lesions (Holmstrup 2009). Trials evaluating interventions directed against risk factors (e.g. smoking) are also missing.
The applicability of results of two of the included studies (Sankaranarayan 1997; Stich 1988) should be considered in the context of their different risk factor profile as the participants were all betel quid chewers, a risk factor uncommon in individuals from geographical areas outside South Asia.
Leukoplakias with different histological or molecular characteristics may have different risks of transforming into cancer. However, the value of predictive factors proposed so far in the literature requires sound confirmatory data. The presence of epithelial dysplasia may be predictive of a transformation to oral cancer and the risk of cancer may increase with the severity of dysplastic changes (Lumerman 1995; Schepman 1998; Warnakulasuriya 2011), although this hypothesis has been recently challenged (Holmstrup 2006). Unfortunately, the available data did not allow us to perform a subgroup analysis of lesions with and without dysplasia, thus it is not possible to establish whether any particular treatment may be more indicated in the presence of dysplasia of different severity. Many different molecular biomarkers have been proposed, but no single marker or battery of markers seems predictive enough to be implemented during clinical care.
Quality of the evidence
Of the studies included in the present review, we judged one as having low risk of bias, six asunclear risk of bias and seven ashigh risk of bias. Although these studies were randomised trials, information about randomisation methods was missing or incomplete in most studies. In particular, methods used for random sequence generation were unclear in eight out of 15 studies, and details on allocation concealment were missing in 10 out of 15 studies. The body of evidence for cancer incidence comprises three studies investigating three different treatments: vitamin A, beta carotene and topical bleomycin. We assessed these studies as having very low quality according to GRADE (Grades of Recommendation, Assessment, Development and Evaluation) assessment criteria (see Table 1; Table 2; Table 5). Thus, the quality and the number of included trials, often with short follow‐up times, suggest cautious interpretation of results.
Authors' conclusions
Implications for practice.
No randomised controlled trials (RCTs) on surgical treatment for people who have oral leukoplakia have included placebo and active treatment arms. Nor have RCTs examined risk factor cessation (e.g. smoking). Therefore, the effectiveness of these interventions cannot be reliably assessed. None of the medical and complementary treatments studied (vitamin A, beta carotene, bleomycin) has been shown to be effective in preventing cancer onset in people with leukoplakia, and, despite the findings of some studies that vitamin A or beta carotene may be effective in reducing or even resolving oral leukoplakia in the short term, the risk of subsequent relapse seems high.
Implications for research.
Although surgery remains the treatment option favoured by most clinicians, the effectiveness of surgery compared with no treatment ("wait and see") has not been assessed in RCTs for prevention of cancer development in people with leukoplakia. Research is needed to assess surgical treatment of patients with leukoplakia and to evaluate effects of risk factor cessation in people with leukoplakia. Larger, better conducted trials of longer duration are required to properly evaluate the effects of any treatment on malignant transformation rates, which should be considered the primary outcome when effectiveness of treatments for leukoplakia is tested.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2016 | New citation required but conclusions have not changed | 6 new studies included. 1 previously included study has been excluded, as it was quasi‐randomised (Gaeta 2000) |
| 16 May 2016 | New search has been performed | Search updated |
History
Protocol first published: Issue 3, 1999 Review first published: Issue 4, 2001
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format |
| 4 July 2006 | New citation required but conclusions have not changed | Review updated. 2 new included studies (Mulshine 2004; Singh 2004), 3 new ongoing studies, 3 newly excluded studies. Conclusions remained essentially the same |
| 25 May 2004 | New citation required but conclusions have not changed | Review updated. 1 new study (Gaeta 2000) has been included, but summary estimates did not change significantly, and conclusions remained essentially the same |
Acknowledgements
The review authors wish to thank all researchers in cited studies who have provided some of the data used in the review. We also thank members of the Cochrane Oral Health editorial team (Anne Littlewood, Marco Esposito), who assisted with this update, and external referees Isaac van der Waal and Andrew Ness, who provided clinical feedback.
Appendices
Appendix 1. MEDLINE Ovid search strategy
1. exp Leukoplakia, Oral/ 2. (erythroplak$ or erythroleukoplak$).ti,ab. 3. (leukoplak$ adj (oral or mucosa$ or mouth$)).ti,ab. 4. (keratosis adj (oral or mucosa$ or mouth$)).ti,ab. 5. (leukokeratosis adj (oral or mucosa$ or mouth$)).ti,ab. 6. ((precancer$ or pre‐cancer$ or preneoplas$ or pre‐neoplas$) adj6 (oral or mouth$ or mucosa$)).ti,ab. 7. ((white adj (spot$ or lesion$ or patch$)) and (mouth$ or oral or mucosa$)).ti,ab. 8. "oral dysplasia".ti,ab. 9. or/1‐8
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomized trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 2. Cochrane Oral Health's Trials Register search strategy
(leukoplak* or erthroplak* or erythroleukoplak* or keratosis or leukokeratosis or precancer or pre‐cancer or preneoplas* or pre‐neoplas* or "white spot*" or "white lesion*" or "white patch*" or "oral dysplasia")
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Leukoplakia, Oral this term only #2 (erythroplak* in All Text or erythroleukoplak* in All Text) #3 (oral next leukoplak* in All Text or mucosa* next leukoplak* in All Text or mouth* next leukoplak* in All Text) #4 (oral next keratosis in All Text or muscoa* next keratosis in All Text or mouth* next keratosis in All Text) #5 (oral next leukokeratosis in All Text or muscoa* next leukokeratosis in All Text or mouth* next leukokeratosis in All Text) #6 ( (precancer* in All Text near/6 oral in All Text) or (pre‐cancer in All Text near/6 oral in All Text) or (preneoplas* in All Text near/6 oral in All Text) or (pre‐neoplas* in All Text near/6 oral in All Text) ) #7 ( (precancer* in All Text near/6 mouth* in All Text) or (pre‐cancer in All Text near/6 mouth* in All Text) or (preneoplas* in All Text near/6 mouth* in All Text) or (pre‐neoplas* in All Text near/6 mouth* in All Text) ) #8 ( (precancer* in All Text near/6 mucosa* in All Text) or (pre‐cancer in All Text near/6 mucosa* in All Text) or (preneoplas* in All Text near/6 mucosa* in All Text) or (pre‐neoplas* in All Text near/6 mucosa* in All Text) ) #9 ( ("white spot*" in All Text near/6 mouth* in All Text) or ("white lesion*" in All Text near/6 mouth* in All Text) or ("white patch*" in All Text near/6 mouth* in All Text) ) #10 ( ("white spot*" in All Text near/6 oral in All Text) or ("white lesion*" in All Text near/6 oral in All Text) or ("white patch*" in All Text near/6 oral in All Text) ) #11 ( ("white spot*" in All Text near/6 mucosa* in All Text) or ("white lesion*" in All Text near/6 mucosa* in All Text) or ("white patch*" in All Text near/6 mucosa* in All Text) ) #12 "oral dysplasia" in All Text #13 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12)
Appendix 4. Embase Ovid search strategy
1. Leukoplakia/ 2. (erythroplak$ or erythroleukoplak$).ti,ab. 3. (leukoplak$ adj (oral or mucosa$ or mouth$)).ti,ab. 4. (keratosis adj (oral or mucosa$ or mouth$)).ti,ab. 5. (leukokeratosis adj (oral or mucosa$ or mouth$)).ti,ab. 6. ((precancer$ or pre‐cancer$ or preneoplas$ or pre‐neoplas$) adj6 (oral or mouth$ or mucosa$)).ti,ab. 7. ((white adj (spot$ or lesion$ or patch$)) and (mouth$ or oral or mucosa$)).ti,ab. 8. "oral dysplasia".ti,ab. 9. or/1‐8
The above subject search was linked to the Cochrane Oral Health Group filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. CancerLit (PubMed) search strategy
#1 Oral leukoplakia [mh:exp] #2 oral AND leukoplak*[Title/Abstract] #3 mucosa* AND leukoplak*[Title/Abstract] #4 mouth* AND leukoplak*[Title/Abstract] #5 erthroplak* or erythroleukoplak*[Title/Abstract] #6 oral AND keratosis[Title/Abstract] #7 mouth* AND keratosis [tiab] #8 oral AND leukokeratosis [tiab] #9 mucosa* AND leukokeratosis [tiab] #10 mouth* AND leukokeratosis [tiab] #11 precancer* AND oral [tiab] #12 pre‐cancer* AND oral [tiab] #13 preneoplas* AND oral [tiab] #14 pre‐neoplas* AND oral [tiab] #15 precancer* AND mouth* #16 pre‐cancer* AND mouth* [tiab] #17 preneoplas* AND mouth* [tiab] #18 pre‐neoplas* AND mouth* [tiab] #19 precancer* AND mucosa* [tiab] #20 pre‐cancer* AND mucosa* [tiab] #21 preneoplas* AND mucosa* [tiab] #22 pre‐neoplas* AND mucosa* [tiab] #23 "white spot*" AND mouth* [tiab] #24 "white spot*" AND oral [tiab] #25 "white spot*" AND mucosa* [tiab] #26 "white lesion*" AND mouth* [tiab] #27 "white lesion*" AND oral [tiab] #28 "white lesion*" AND mucosa* [tiab] #29 "white patch*" AND mouth* [tiab] #30 "white patch*" AND oral [tiab] #31 "white patch*" AND mucosa* [tiab] #32 oral AND dysplasia [tiab] #33 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.a of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Higgins 2011).
#1 randomized controlled trial [pt] #2 controlled clinical trial [pt] #3 randomized [tiab] #4 placebo [tiab] #5 drug therapy [sh] #6 randomly [tiab] #7 trial [tiab] #8 groups [tiab] #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 animals [mh] not humans [mh] #11 #9 NOT #10
[limits: Cancer]
Appendix 6. Trials registries search strategies
metaRegister of Controlled Trials search strategy
leukoplakia
ClinicalTrials.gov search strategy
"oral leukoplakia"
WHO International Clinical Trials Registry Platform search strategy
"oral leukoplakia"
Data and analyses
Comparison 1. Vitamin A or retinoids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral cancer development | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical resolution | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Systemic treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improvement of histological features | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Beta carotene or carotenoids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral cancer development | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Systemic treatment | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.09] |
| 2 Clinical resolution | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Systemic treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improvement of histological features | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. NSAIDs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Systemic treatment | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.08] |
| 1.2 Topical treatment | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.10] |
Comparison 4. Herbal extracts versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Systemic plus topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement of histological features | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Topical bleomycin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral cancer development | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical resolution | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Improvement of histological features | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Bowman‐Birk inhibitor versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical resolution | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armstrong 2013.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in USA Number of centres: 8 Recruitment period: May 1999 to September 2009 Funding source: Central Soya Company, NIH Study duration: 6 months |
|
| Participants |
Inclusion criteria At least 18 years old, with histologically proven oral leukoplakia and/or erythroplakia, capable of being measured bi‐dimensionally; pre‐menopausal and perimenopausal women were required to agree to use adequate birth control methods and to have a negative pregnancy test. At completion of the 4‐week run‐in phase of the study, during which participants self administered the placebo compound, 75% compliance with administration of drug measured by counting unused drug packets was required for continuation in the study If recent (within 3 months) histological analysis had not been documented with review of biopsy, 3‐mm punch biopsy of representative lesions was conducted after measurement and photodocumentation of the lesion Exclusion criteria Use of systemic or topical oral steroids within 3 months, currently pregnant or lactating, presence of head and neck cancer (including in situ disease), history of such within 2 years, retinoid or beta carotene therapy for any reason within 2 years or beta carotene capsules of any size within 6 months (participants were allowed up to 2 multi‐vitamins per day), participation in another randomised clinical trial within 6 months Histological criteria for leukoplakia Not reported 132 people randomised: 48/132 (36%) reported use of beer, wine or liquor, whereas 56/132 (42%) did not answer questions on alcohol consumption; 28/132 (21%) reported using tobacco (viz., cigarettes, cigars, pipe, oral use), whereas 66/132 (50%) did not answer questions on tobacco use. Percentage of dysplastic lesions was not reported 89 people completed the study: 32/89 females, mean age 60.7 (range 29 to 82), ethnic group 69/89 white, tobacco users 20/89 (but 40/89 unknown), alcohol users 35/89 (but 34/89 unknown) Group A: randomised 67; 43 completed the study. 41 available for histological analysis Group B: randomised 65; 46 completed the study. 46 available for histological analysis |
|
| Interventions |
Group A: 3 grams Bowman‐Birk Inhibitor concentrate twice a day for 6 months Group B: 3 grams placebo (corn tortilla mix) twice a day for 6 months Compliance control: not reported |
|
| Outcomes |
Clinical response The primary end point was relative per cent change in total lesion area after 6 months in the study, and the percentage of participants showing a clinical response on that measure. A complete response was declared if the relative per cent change in total lesion area was minus 100%. A partial response was a relative per cent decrease in total lesion area of 50% or more, without a complete response. Disease progression was a relative per cent increase in total lesion area of at least 50%. Remaining cases were declared to be stable disease A secondary clinical response measure was change in clinical impression from photographs of lesions based on blinded, comparative judgements of pairs of photographs of the same lesion at baseline and at 6 months on the study using a 7‐point scale Histological response A single, experienced pathologist compared pre‐treatment and post‐treatment pairs of tissue specimens. The pathologist was blinded to study arm assignment (drug or placebo), but not to time point of the specimen. For each specimen, the pathologist marked a continuum to indicate the degree of tissue abnormality. The continuum was 140 mm long, and was anchored by the word ‘normal’ on the left and ‘malignant’ on the right. The distance from the left edge of the continuum to the pathologist’s mark, in millimetres, was determined. For analyses, a score was determined by subtracting the pre‐treatment value from the 6‐month value. The central pathologist also made a direct comparison of pre‐treatment and post‐treatment specimens, marking a 170‐mm continuum anchored on the left by "posttreatment shows no dysplasia in comparison with pretreatment," and on the right by "posttreatment shows greater dysplasia than pretreatment". The centre of the continuum was labelled, "pretreatment and posttreatment show no difference." The reviewer’s mark was coded as the distance from the left edge, in millimetres. On this measure, low scores denote improvement over time, a score of 85 denotes no change and a value greater than 85 indicates histological worsening Other outcomes included changes in buccal cell and serum Neu protein, buccal cell protease activity, adverse events |
|
| Notes | Initially it was required that the total lesion area estimated at baseline be at least 100 mm2, but later this requirement was relaxed to facilitate accrual Early in the study, participants on the drug arm who showed a partial or complete response at 6 months were allowed to continue treatment for an additional 12 months (total 18 months) with final follow‐up at 21 months. However, because of the limited supply of drugs, the protocol was soon modified to limit treatment to 6 months Bowman‐Birk inhibitor concentrate was initially produced and supplied by Central Soya Company, but was later supplied by the NIH/National Cancer Institute/Division of Cancer Prevention pharmacy Both treatment and placebo groups showed a statistically significant decrease in total lesion area |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Independent randomisation schedules were created for each performance centre (so study‐arm assignment would not be confounded with geography). For those centres expected to accrue at a faster rate, the randomisation schedule incorporated a block size of 4. A block size of 2 was used for centres with lighter accrual goals" |
| Allocation concealment (selection bias) | High risk | Comment: block size 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high number of lost participants: 43/132 (32.5%) |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Epstein 1994.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in Canada Number of centres: 1 Recruitment period: unspecified Funding source: unspecified Study duration: 2 weeks for treatment plus open follow‐up |
|
| Participants |
Inclusion criteria Patients older than 18 years with clinically visible leukoplakia and pathological diagnosis of the lesion Exclusion criteria Pregnant women, women of childbearing age in whom contraception was not confirmed, cases of carcinoma in situ, invasive SCC and lesions identified as inflammatory in nature Histological criteria for leukoplakia Histological diagnosis of hyperkeratosis or acanthosis with or without dysplasia. 22% of the lesions were dysplastic 22 participants randomised: 12/22 females, mean age 56.6 (range 25 to 79), ethnic group not reported, 14 (63%) tobacco users, 10 (45%) alcohol users. Leukoplakia was significantly larger in the placebo group (320 mm2) compared with the test group (76 mm2) at randomisation Group A: randomised 10; 10 completed the study Group B: randomised 12; 12 completed the study (12 analysed clinically, 11 analysed histologically) |
|
| Interventions |
Group A: 1 daily topical application of 1% w/v bleomycin in dimethylsulphoxide for 14 days Group B: 1 daily topical application of placebo (dimethylsulphoxide only) for 14 days Compliance control: yes |
|
| Outcomes |
Cancer incidence Clinical response Measurement of the lesion before the start of treatment and weekly during treatment: (1) complete response was defined as no clinical and histological evidence of leukoplakia, (2) partial response was defined as a greater than 50% reduction in the size of the lesion or elimination of dysplasia. Data from the first follow‐up visit (2 weeks from the end of treatment) are included in the meta‐analysis Histological response Histological grading before the start of treatment and 4 weeks after treatment Other outcomes: assessment of oral burning and pain during application, between applications and with eating. Adverse effects |
|
| Notes | After the post‐treatment biopsy (4 weeks after treatment), 7 participants in the placebo group were crossed over to receive 1% w/v bleomycin in dimethylsulphoxide. We received unpublished data on malignant transformation and based our ITT analysis on those data Mean follow‐up from the end of the study: 15 months (group A) and 22 months (group B) Based our analyses on raw data supplied by trial authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “patients were randomised to the drug or placebo arm by the department of Pharmacy, with the use of a table of random numbers” |
| Allocation concealment (selection bias) | Low risk | Quote: “patients were randomised to the drug or placebo arm by the department of Pharmacy” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data with the exception of 1/12 (8.3%) histological outcome in the control group |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Unclear risk | Some areas of concern: discrepancies between published and raw data; baseline imbalance in lesion size; cross‐over to intervention of more than half of participants in the placebo group |
Hong 1986.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in USA Number of centres: unspecified Recruitment period: unspecified Funding source: supported by a grant from Hoffmann‐La Roche and in part by a grant from the National Cancer Institute Study duration: 9 months (3 months of treatment plus 6 months of follow‐up) |
|
| Participants |
Inclusion criteria Histologically confirmed oral leukoplakia Exclusion criteria Women with reproductive capacity, persons taking megadoses of vitamin A (> 25,000 USP units/d), patients who had an oral cancer within the 2 years preceding the study Histological criteria for leukoplakia Not reported 44 participants randomised:
Group A: randomised 24; 22 completed the study (analysed 22 clinically, 21 histologically) Group B: randomised 20; 18 completed the study |
|
| Interventions |
Group A: capsules of 13‐cis‐retinoic acid (1 to 2 mg/kg/d) for 3 months Group B: capsules of placebo for 3 months Compliance control: yes, pill counts at each visit |
|
| Outcomes |
Clinical response Measurement of the lesion, colour photography performed before the start of treatment and every 2 to 3 weeks during treatment: (1) complete response was defined as no clinical evidence of leukoplakia for at least 4 weeks; (2) partial response was defined as a greater than 50% reduction in the product of the longest diameters of the lesion; (3) a response was classified as stable when the decrease in lesion size was less than 50%; (4) disease progression was defined as an unequivocal increase in the size of any lesion during treatment or as the appearance of a new lesion Histological response Histological grading was done before the start of treatment and upon its completion. Histological grading included (1) atypical hyperplasia; (2) mild dysplasia; (3) moderate dysplasia; (4) severe dysplasia or carcinoma in situ Other outcomes included laboratory analysis, including fasting serum triglycerides and liver function testing |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “the randomisation code was computer‐generated” |
| Allocation concealment (selection bias) | Low risk | Quote: “randomisation was performed by the pharmacy” and “The code for treatment assignment was broken after the patients had completed the full nine months of study” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “neither the patient nor the physician was aware of which treatment was assigned” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “neither the patient nor the physician was aware of which treatment was assigned” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likely to have a clinically relevant impact on the intervention effect estimate: 4/44 (9%) |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Li 1999.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in China Number of centres: 1 Recruitment period: unspecified Funding source: supported by a grant from the Chinese National Natural Science Foundation Study duration: 6 months |
|
| Participants |
Inclusion criteria Not specified Exclusion criteria Patients with severe dysplasia Histological criteria for leukoplakia Not reported 64 participants randomised: 24 females, mean age 54.5 (range 23 to 78), ethnic group not reported, 46 (71.9%) tobacco users. 20% of lesions were dysplastic Group A: randomised 32, 29 completed the study Group B: randomised 32, 30 completed the study |
|
| Interventions |
Group A: systemic (capsules) and topical (paint) mixed tea (3 grams/d and 3 paintings/d) for 6 months Group B: systemic (capsules) and topical (paint) placebo for 6 months Compliance control: not reported |
|
| Outcomes |
Clinical response Size and number of lesions of each participant were recorded at baseline and at the end of the trial: (1) complete regression was defined as complete disappearance of the lesion; (2) partial regression was defined as a 30% or greater reduction in the size of a single lesion or in the sum of sizes of multiple lesions; (3) lesions with no change in size were recorded as no change; (4) deterioration referred to the occurrence of new lesions Histological response Oral biopsies were conducted at the beginning and at the end of the trial. Besides routine histopathological examination, lesional tissue investigations included also silver‐stained nucleolar organizer regions (AgNOR), proliferation cell nuclear antigen (PCNA) and epidermal growth factor receptor (EGFR) analysis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Personal communication: random sequence based on random number table. Randomisation stratified for presence of dysplasia |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likely to have a clinically relevant impact on the intervention effect estimate: 5/64 (7.8%) |
| Selective reporting (reporting bias) | High risk | Comment: missing histological assessment of lesions at the end of the study |
| Other bias | Low risk | Comment: no other sources of bias identified |
Mallery 2014.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in US Number of centres: 3 Recruitment period: unspecified Funding source: supported by NIH NCI RC2 CA148099. S.R.Mallery has ownership interest (including patents) in BRB gel patent Study duration: 6 months (12 weeks plus 3 months of follow‐up) |
|
| Participants |
Inclusion criteria Microscopically confirmed pre‐malignant oral epithelial lesions, no use of tobacco products for 6 weeks before and during the 3‐month study, no previous history of cancer (except for basal cell carcinoma of the skin). Participants screened before entrance into and during (10 to 12 days recall intervals) the trial for no tobacco use compliance via unannounced saliva testing for nicotine (NicAlert, JANT Pharmacal Corporation) Exclusion criteria Previous or current history of non‐basal cell cancer, use of tobacco products, and either a microscopic diagnosis of no pre‐malignant change or OSCC in the pre‐trial biopsy Histological criteria for leukoplakia Not reported 40 participants randomised: 22 females, mean age 60.15 (range 32 to 78), ethnic group not reported, 24 (60%) former smokers. 72.5% of lesions were dysplastic: 63% (14/22) in Group A and 83% (15/18) in Group B Group A: randomised 22, 22 completed the study. 21 available for post‐trial examination Group B: randomised 18, 18 completed the study. 17 available for post‐trial examination |
|
| Interventions |
Group A: topical application of a bioadhesive gel that contained 10% w/w freeze‐dried black raspberries (0.5 g, q.i.d. for 12 weeks) Group B: topical application of a bioadhesive placebo gel that contained 10% w/w sucrose and food colorants (0.5 g, q.i.d. for 12 weeks) Compliance control: yes |
|
| Outcomes |
Clinical response Clinical photographs of lesions were taken with a calibrated measuring device (Puritan) placed parallel to the long axis of the lesion. Acquired clinical images were analysed using ImagePro 6.2 software (Media Cybernetics). Lesional sizes were normalised to square millimetres (mm2) according to the following formula: lesional size mm2 = pixels of lesional area × 100/(pixels of 1 centimetre unit on the calibration device in the same image). The remaining lesional area after the initial biopsy and before gel treatment was the pre‐treatment size. Post‐treatment lesional size was the residual lesional area after 3 months of gel treatment and just before the final biopsy Histological response Histological grading was done before the start of treatment and upon its completion. A 0 to 8 grade scale (0 = normal with or without hyperkeratosis, 1 = atypia with crisply defined clinical margins, 2 = mild dysplasia, 3 = mild‐moderate dysplasia, 4 = moderate dysplasia, 5 = moderate‐severe dysplasia, 6 = severe dysplasia, 7 = carcinoma in situ, 8 = invasive SCC) was used to rank light microscopic diagnoses |
|
| Notes | 30 participants had OIN lesions (16 in BRB ‐ 72.7% and 14 in placebo ‐ 77.7%) that were recalcitrant to surgery and had recurred multiple times (2 to 8) at the same site before trial participation. 12 Group A and 3 Group B participants had a history of multiple lesions dispersed throughout the mouth, consistent with a diagnosis of proliferative verrucous leukoplakia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All participating oral pathologists, surgeons, and patients were blinded to the patients’ gel assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All participating oral pathologists, surgeons, and patients were blinded to the patients’ gel assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. All randomised participants included in analysis of results |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Mulshine 2004.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in USA Number of centres: 3 Recruitment period: unspecified Funding source: supported by a grant from the National Cancer Institute Specialized Programs of Reasearch Excellence (SPORE) Program Study duration: 4 months (90 days of treatment plus 1 month of follow‐up) |
|
| Participants |
Inclusion criteria Patients with bi‐dimensionally measurable leukoplakia of the oral cavity or of the oropharynx. In cases of previous oral cancer diagnosis, individuals had to be free from disease for at least 3 months, excellent performance status, general good health Exclusion criteria Hypersensitivity to aspirin, lidocaine, NSAIDs, retinoids. Use of antibiotics, steroids, NSAIDs, aspirin, probenecid, antihistamines for > 10 consecutive days, or any immunosuppressants, anticoagulants, dilantin, lithium, methotrexate, phenothiazines or drugs that could compromise test product safety during the 30 days immediately preceding the first treatment visit, debilitating oral conditions requiring extensive dental procedures or conditions interfering with compliance. Respiratory or cardiovascular problems Histological criteria for leukoplakia Not reported 57 participants randomised: 19 females; age not reported; ethnic group: non‐white participants: 6/57, white participants: 51/57; 48/56 (86%) smokers, 40/56 (71%) alcohol users. Percentage of dysplastic lesions not reported Group A: randomised 38, 37 completed the study Group B: randomised 19, 19 completed the study |
|
| Interventions |
Group A: mouthwash with ketorolac 0.1%, twice a day, for 90 days Group B: mouthwash with placebo, twice a day, for 90 days Compliance control: yes |
|
| Outcomes |
Clinical response Measurement of the lesion. (1) Complete response was defined as no clinical evidence of leukoplakia for at least 30 days from inception of treatment. (2) Partial response was defined as a greater than 50% reduction in the product of the longest diameters of a single lesion (in the sum of these figures for all lesions, in the setting of multiple lesions) for at least 30 days. (3) A response was classified as stable when the decrease in lesion size was less than 50%. (4) Disease progression was defined as an unequivocal increase in size greater than 10%, or as the appearance of a new lesion. Clinical evaluations were made at study entry, monthly during the intervention and 1 month after cessation of study drugs Histological response Histological grading was done before the start of treatment and upon its completion |
|
| Notes | Nine of the 56 participants who completed the study had an oropharyngeal leukoplakia (6 in Group A and 3 in Group B) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data are balanced in numbers across intervention groups and are not likely to have a clinically relevant impact on the intervention effect estimate 1/57 (1.7%) |
| Selective reporting (reporting bias) | High risk | Comment: data on histological modifications reported only partially |
| Other bias | Low risk | Comment: no other sources of bias identified |
Nagao 2015.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in Japan Number of centres: 3 Recruitment period: not reported Funding source: supported by Butterfield Award of the Sasakawa Foundation GB and DSM Nutrition Japan Study duration: 1 year plus open follow‐up |
|
| Participants |
Inclusion criteria Patients with leukoplakia who had never smoked or were ex‐smokers Exclusion criteria Current smokers or ex‐smokers within 3 months of cessation Histological criteria for leukoplakia Not reported 46 participants randomised: 21 females; median age 65 (range 38 to 80 years); ethnic group: all Japanese; never smoked or ex‐smokers; alcohol users not reported Percentage of dysplastic lesions not reported Group A: randomised 23, 16 completed the study Group B: randomised 23, 17 completed the study |
|
| Interventions |
Group A: 10 mg/d of beta carotene and 500 mg/d of vitamin C for 1 year Group B: 50 mg/d of vitamin C for 1 year Compliance control: not reported |
|
| Outcomes |
Cancer incidence Study authors provided unpublished cancer incidence after a median follow‐up of 86 months. Trial authors intended to exclude from the analysis any cases that progressed to oral cancer within 6 months of the start of the study, but there were no cancer cases within 6 months Clinical response Complete remission, partial remission, no change, disease progression Histological response Severity degree of epithelial dysplasia p53 and Ki67 expression Serum levels of antioxidant micronutrients |
|
| Notes |
Follow‐up since the end of treatment: 60 months for clinical response, 86 months for cancer incidence 2 participants in the experimental arm and 1 in the control arm did not receive allocated interventions. During intervention, 5 participants in the experimental arm and 5 in the control arm dropped out. Among these, 3 in the experimental arm and 4 in the control arm returned for follow‐up at the close of the study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation was performed [...] with stratification by blocking randomisation according to presence or absence of dysplasia" |
| Allocation concealment (selection bias) | Low risk | Quote: "a trial coordinator not involved in the routine care of patients generated the allocation sequence and enrolled participants. The central randomisation by numbered containers was used for allocation concealment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High number of drop‐outs (stopping treatment) 13/46 (28.2%), although not lost to follow‐up and included in ITT analysis |
| Selective reporting (reporting bias) | High risk | Comment: data on histological outcomes not reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Papadimitrakopoulou 2008.
| Methods |
Study design: RCT, parallel‐group, 3 arms Conducted in USA Number of centres: 8 Recruitment period: November 2000 to January 2004 Funding source: Pfizer (grant support) Study duration: 6 months |
|
| Participants |
Inclusion criteria Histologically confirmed early or advanced oral pre‐malignant lesion; age ≥ 18 years; Zubrod performance status 0 to 1; haemoglobin level above lower limit of normal, WBC count > 3000/mm3, platelet count > 125,000/mm3, total bilirubin, aspartate aminotransferase and alanine aminotransferase levels V1.5 upper limit of normal, serum creatinine V1.5 upper limit of normal; no anticipated need for treatment with oral or i.v. corticosteroids for more than 2 consecutive weeks over any 6‐month period during the study; willingness to limit aspirin use to V100 mg/d and to abstain from chronic use of all other non‐steroidal anti‐inflammatory drugs and COX‐2 inhibitors for the duration of the study Exclusion criteria Diagnosis of or treatment for oesophageal, gastric, pyloric channel or duodenal ulceration within 30 days before randomisation; history of head and neck cancer in the past 18 months or of another cancer in the past 3 years (patients with a history of non‐melanoma skin cancer, cervical carcinoma in situ or chronic lymphocytic leukaemia stage 0 were not excluded); chronic or acute renal or hepatic disorder or significant bleeding disorder; history of or active inflammatory bowel syndrome or pancreatic disease; current use of fluconazole or lithium Histological criteria for leukoplakia Early oral pre‐malignant lesion: atypical hyperplasia, atypical hyperkeratosis or mild dysplasia. Advanced oral pre‐malignant lesion: moderate to severe dysplasia 50 participants randomised: 26 females; age range 34 to 84; ethnic group: 2/50 non‐white participants, 48/50 white participants; 4/50 current smokers (8%), 27/50 current drinkers (54%), prior oral cancer history 10/50. Early/advanced oral pre‐malignant lesion: Group A 15/2, Group B 14/1, Group C 16/2. Ten out of 32 (31%) participants in combined active arms vs 0 of 18 in the placebo arm had a history of squamous cell cancer of the oropharynx (9) or larynx (1). Percentage of participants with any degree of dysplasia not reported Group A: randomised 17, 16 completed the study Group B: randomised 15, 13 completed the study Group C (placebo): randomised 18, 17 completed the study |
|
| Interventions |
Group A: oral celecoxib 100 mg twice for 12 weeks Group B: oral celecoxib 200 mg twice for 12 weeks Group C: oral placebo twice daily for 12 weeks Groups A and B were considered together in the present review Compliance control: Compliance was measured by telephone queries and remaining capsule counts at weeks 8 and 12 |
|
| Outcomes |
Cancer incidence Cancer development at 12 weeks Clinical response Clinical changes at 12 weeks: complete response (disappearance of all evidence of lesions), partial response (50% or greater decrease in the sum of products of diameters of all measured lesions); stable disease (any response that did not meet the criteria for the other categories), progressive disease (increase ≥ 25% in size of lesions or appearance of new lesions or progression to invasive cancer) Histological response Histological changes at 12 weeks: (1) reversal of dysplasia, (2) improvement in degree of dysplasia Adverse events classified according to the National Cancer Institute Common Toxicity Criteria version 2.0 |
|
| Notes | Follow‐up from the end of treatment: 14 weeks Quote: “After a protocol amendment in March 2003, only patients with early OPLs continued to be randomised as just described, whereas all patients with advanced OPLs received open‐label oral celecoxib at 400 mg twice daily, based again on data of the familial adenomatous polyposis study showing significant efficacy of celecoxib only at 400 mg twice daily. Because this decision was made while the study was already accruing, the 400 mg twice daily open‐label arm was included in an exploratory intent as an attempt to preserve the feasibility of the study while offering a possibly superior intervention for patients at higher cancer risk (i.e., advanced OPL). [….] Shortly after opening the 400 mg twice daily arm for patients with developed OPLs, the first reports potentially linking use of selective COX‐2 inhibitors with serious adverse cardiovascular events emerged, leading to early closure of this arm” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likely to have a clinically relevant impact on the intervention effect estimate 4/50 (8%) |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Piattelli 1999.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in Italy Number of centres: 1 Recruitment period: unspecified Funding source: partially supported by the National Research Council (CNR) and by the Ministry of University, Research, Science and Technology (MURST), Rome, Italy Study duration: 4 months |
|
| Participants |
Inclusion criteria Histologically confirmed oral leukoplakia Exclusion criteria Women of childbearing age Histological criteria for leukoplakia Not reported 10 participants randomised: 4 females, mean age 61 (range 40 to 71), ethnic group: Caucasian, 4 (40%) tobacco users. Mean duration of lesions: 5.8 years (range 0.5 to 20 years). Percentage of dysplastic lesions not reported Group A: 5 randomised, 5 completed the study Group B: 5 randomised, 4 completed the study |
|
| Interventions |
Group A: 3 times daily topical application of 0.1% isotretinoin (13‐cis‐retinoic acid ‐ Roaccutane Roche) for 4 months Group B: 3 times daily topical application of placebo (gel only), for 4 months Compliance control: not reported |
|
| Outcomes |
Clinical response Measurement of the lesion: Photography was performed before the start of treatment and every month during treatment: Complete response was defined as complete disappearance of the lesion as assessed by visual inspection; partial response was defined as a 50% or greater reduction in the size of lesions Histological response Evaluation of bcl‐2 immunostaining. Laboratory studies (including serum cholesterol, triglycerides, aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase) were performed before the start of treatment and every month during treatment |
|
| Notes | At the end of the study period (4 months), participants who received placebo started 4‐month treatment with active medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likey to have a clinically relevant impact on the intervention effect estimate 1/10 (10%) |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Sankaranarayan 1997.
| Methods |
Study design: RCT, parallel‐group, 3 arms Conducted in India Number of centres: 1 Recruitment period: unspecified Funding source: unspecified Study duration: 2 years (1 year of treatment + 1 year of follow‐up) |
|
| Participants |
Inclusion criteria Not reported Exclusion criteria Not reported Histological criteria for leukoplakia Not reported Participant details were available only for those who completed the trial (131 participants: 47 female; mean age 50.7; 127 (97%) chewers, 41 (31%) smokers, 72 (55%) drinkers. Percentage of dysplastic lesions not reported Group A : randomised 50, 42 completed the study Group B : randomised 55, 46 completed the study Group C: randomised 55, 43 completed the study |
|
| Interventions |
Group A: capsules of vitamin A (300,000 IU/wk) for 1 year Group B: capsules of beta carotene (360 mg/wk) for 1 year Group C: capsules of placebo for 1 year Compliance control: yes |
|
| Outcomes |
Cancer incidence Malignant transformation: Biopsies were taken at baseline and during the study, whenever a malignant transformation was suspected. Malignant transformation was scored if malignancy was histologically established in the lesions during follow‐up Clinical response Number, type and dimension of lesion(s) were recorded at baseline and at each review. (1) Complete response was defined as no clinical evidence of leukoplakia. (2) Partial response was defined as a greater than 50% reduction in the size of the single lesion or in the sum of sizes of multiple lesions. (3) Stable and progressive lesions were scored as no response |
|
| Notes | 160 participants randomised, all with tobacco chewing habits and leukoplakia, belonging to the fisherman community of Trivandrum City, Kerala, India ‐ a population with high incidence of leukoplakia and oral cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “double blind” and “subjects were examined every 2 months during visits by dentists and physician, both of whom were blinded to the treatment group” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “double blind” and “subjects were examined every 2 months during visits by dentists and physician, both of whom were blinded to the treatment group” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likely to have a clinically relevant impact on the intervention effect estimate 29/160 (16.9%) |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Singh 2004.
| Methods |
Study design: RCT, parallel‐group, 3 arms Conducted in India Number of centres: unspecified Recruitment period: unspecified Funding source: supported by Jagsonpal Pharmaceuticals Ltd., New Delhi, India Study duration: 3 months plus 2 months of follow‐up |
|
| Participants |
Inclusion criteria Not reported Exclusion criteria Not reported Histological criteria for leukoplakia Not reported Characteristics of 58 participants who completed the study: 14 females; age: 12 participants were between 10 and 30 years, 42 were between 31 and 60 years, 4 were between 61 and 80 years; ethnic group: not reported; smoking status: not reported; alcohol status: not reported. Percentage of dysplastic lesions: 59% Group A: randomised 20, 20 completed the study Group B: randomised 20, 20 completed the study Group C: randomised 18, 18 completed the study |
|
| Interventions |
Group A: capsules of lycopene at high dose (8 mg/d) divided into 2 daily doses for 3 months Group B: capsules of lycopene at low dose (4 mg/d) divided into 2 daily doses for 3 months Group C: capsules of placebo in 2 daily doses for 3 months Groups A and B were considered together in the present review Compliance control: not reported |
|
| Outcomes |
Clinical response Clinical assessment was made at the third month of the study. (1) Complete response was defined as no clinical evidence of leukoplakia for at least 4 weeks. (2) Partial response was defined as a greater than 50% reduction in the product of the longest diameters of the lesion. (3) Stable response was defined to occur when the decrease in lesion size was < 50%. (4) Disease progression was defined as an unequivocal increase in the size of any lesion during treatment, or as the appearance of a new lesion Histological response Histological grading was done before the start of treatment and upon its completion. For histological evaluation, 5 stages were taken as normal, atypical hyperplasia, mild dysplasia, moderate dysplasia and severe dysplasia. They were ranked as 0, 1, 2, 3 and 4, respectively, so that change could be quantified in terms of these ranks. For example, a case from stage moderate dysplasia comes to stage mild dysplasia post treatment; improvement was considered as 32¼ for 1 unit |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. All randomised participants included in analysis of results |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
Stich 1988.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in India Number of centres: unspecified Recruitment period: unspecified Funding source: supported by a grant from National Cancer Institute of Canada Study duration: 6 months |
|
| Participants |
Inclusion criteria Betel quid chewers Exclusion criteria Not reported Histological criteria for leukoplakia WHO 1978 The 65 participants had tobacco chewing habits and leukoplakia, belonging to the fisherman community of Trivandrum City, Kerala, India ‐ a population with high incidence of leukoplakia and oral cancer. 2% tobacco users, 37% alcohol users, 28% tobacco + alcohol users. Percentage of dysplastic lesions not reported Group A: randomised 30, 21 completed the study Group B: randomised 35, 33 completed the study |
|
| Interventions |
Group A: systemic capsules of vitamin A (200,000 IU/wk) for 6 months Group B: systemic capsules of placebo for 6 months Compliance control: yes, capsules were administered twice weekly under strict supervision of a local nurse |
|
| Outcomes |
Clinical response Leukoplakias were evaluated before the start of treatment and at the end of the study (6 months): (1) remission of leukoplakia, (2) no change, (3) development of new leukoplakia Histological response Biopsies were taken before the start of treatment and at the end of the study (6 months). Histological markers evaluated were (1) loss of polarity of basal cells, (2) lymphocytic infiltration, (3) nuclei with condensed chromatin |
|
| Notes | Questionnaires completed during the trial demonstrated that habits such as chewing, smoking and drinking did not change during the course of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of lost participants (11/65) with imbalance across intervention groups (30% vs 5.7%) |
| Selective reporting (reporting bias) | High risk | Histological data available for only 18 participants in the study group and for none in the control group |
| Other bias | Low risk | Comment: no other sources of bias identified |
Sun 2009.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in China Number of centres: 1 Recruitment period: 1998 to 2001 Funding source: Beijing Natural Science Foundation, National Natural Science Foundation of China, Tenth 5‐Year Plan of National Key Technologies R&D Program in China (No. 2004BA720A28), NIH grants Study duration: 8 to 12 months plus 3 months of follow‐up |
|
| Participants |
Inclusion criteria Oral leukoplakia was defined as a white patch or plaque that cannot be characterised clinically or pathologically as any other disease. All participating patients had general good health without other uncontrolled medical conditions. Patients underwent a baseline biopsy to confirm the absence of invasive cancer Exclusion criteria Those with a previous diagnosis of head and neck or oral cancer; those currently treated by other drugs or having drug hypersensitivity; those requiring extensive dental procedures; those with a history of social or psychiatric situations interfering with study compliance Histological criteria for leukoplakia Not reported Characteristics of the 112 participants who completed the study: 43 females; age: Group A 52.9 ± 10.4, Group B 44.4 ±11.8; ethnic group: not reported; smokers: 53/112 (47%); alcohol drinkers: 10/112 (9%). Percentage of participants with any dysplasia: 18.75% (11.61% mild dysplasia, 3.57% moderate dysplasia, 3.57% severe dysplasia) Group A: randomised 60, 59 completed the study Group B: randomised 60, 53 completed the study |
|
| Interventions |
Group A: ZengShengPing ‐ a mixture of 6 medical herbs (0.3 g per tablet) ‐ 4 tablets each time, 3 times a day, for 8 to 12 months (not better specified: length of the study) Group B: placebo 4 tablets each time, 3 times a day, for 8 to 12 months (not better specified: length of the study) Compliance control: self report during monthly visit |
|
| Outcomes |
Clinical response Measurement of the lesion: (1) positive response defined as disappearance or reduction in size by more than 50% at final checkup (3 months after cessation of treatment), (2) stable disease defined as insignificant change in the size of the lesion, (3) progressive disease defined as increase in size of the lesion by > 50%, or development of new lesions Histological evaluation AgNOR and PCNA labelling index in tissues were evaluated in tissue samples before and after treatment. Histological assessment was available for pre‐treatment samples only |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “randomly assigned to two groups” |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likely to have a clinically relevant impact on the intervention effect estimate 8/120 (6.6%) |
| Selective reporting (reporting bias) | High risk | Comment: information about histological features of lesions not available |
| Other bias | Low risk | Comment: no other sources of bias identified |
Tsao 2009.
| Methods |
Study design: RCT, parallel‐group, 4 arms Conducted in Texas (USA) Number of centres: 1 Recruitment period: August 2002 to March 2008 Funding source: supported by Ito En Ltd. Study duration: 12 weeks and open follow‐up (median 27.5 months) |
|
| Participants |
Inclusion criteria Presence of 1 or more histologically confirmed, bi‐dimensionally measurable OPLs that could be sampled by biopsy and had at least 1 of the following high‐risk features of malignant transformation: harbouring at least mild dysplasia, located in a high‐risk area (i.e. floor of mouth, ventrolateral tongue, and soft palate), significant extent of OPL tissue involvement, and presence of symptoms (pain or substantial discomfort). Additional inclusion criteria included age between ≥ 18 and ≤ 75 years; Zubrod performance status < 2; adequate haematological, liver and renal function; adequate cardiac function (defined as no clinically significant electrocardiogram abnormality, unstable atrial or ventricular arrhythmias requiring medical control or ischaemic event experienced within the prior 6 months); negative pregnancy test in females of childbearing potential within 7 days before first dose of study medication; use of effective contraceptive method while in the trial; written informed consent for participation Exclusion criteria Known hypersensitivity to oral green tea extract (GTE) or its analogous, use of prior investigational agents within 30 days, any serious intercurrent illness, history of prior malignancy with less than a 1‐year disease‐free interval before study entry, lactating females, patients who were not able to abstain from consumption of methylxanthine‐containing products (including coffee, tea, chocolate, caffeinated soft drinks and theophylline) and decaffeinated tea Histological criteria for leukoplakia Not reported 41 participants randomised: 22 females; mean age 57 (range 33 to 76); ethnic group: Caucasian 37/41, Hispanic 2/41, Asian 2/41; smoking status: never smoked 15/41, former smoker 22/41, current smoker 4/41; alcohol status: never 8/41, former 9/41, current 24/41. Mean duration of lesions: 5.8 years (range 0.5 to 20 years). Percentage of participants with any dysplasia: 73.2% (56.1% mild dysplasia, 17.1% moderate/carcinoma in situ). 34.1 % of participants had prior HNSCC, 12.2 % had prior radiotherapy (not specified whether head and neck radiotherapy), 90.2% had prior surgery (not specified which type of surgical therapy) Group A: randomised 11, 11 completed the study Group B: randomised 9, 8 completed the study Group C: randomised 10, 9 completed the study Group D: randomised 11, 11 completed the study Groups A, B and C were considered together in the present review |
|
| Interventions |
Group A: GTE capsules (500 mg/m2 daily), given orally thrice a day after meals for 12 weeks Group B: GTE capsules (750 mg/m2 daily) given orally thrice a day after meals for 12 weeks Group C: GTE capsules (1000 mg/m2 daily) given orally thrice a day after meals for 12 weeks Group D: placebo capsules given orally thrice a day after meals for 12 weeks Each capsule contained 350 mg of GTE. GTE dosage calculations were based on participant's body surface area according to the following formula: body surface area (m2) = weight (kg) 0.425 × height (cm) 0.725 × 0.007184. Calculated dose was adjusted downward to the closest dose that could be administered by using 1 or more 350‐mg capsules Groups A, B and C were considered together in the present review Compliance control: yes |
|
| Outcomes |
Cancer incidence Cancer incidence was recorded during an open follow‐up period Clinical response Bi‐dimensional measurement of the lesion: (1) Disappearance of all lesions was considered a complete response. (2) 50% or greater decrease in the sum of products of diameters of all measured lesions was considered a partial response. (3) Increase of 25% or greater in size of lesions or appearance of new lesions or progression to invasive cancer was considered progressive disease. (4) Any response that did not meet criteria for CR, PR or PD was considered stable disease Histological response (1) Complete response was defined as a complete reversal of pre‐malignancy to normal epithelium with no new lesions. (2) Partial response was defined as improvement in degree of maturation of epithelium with no new lesions and no progression of any lesion. (3) Stable disease was defined as no change in histology and no appearance of new lesions or progression of any lesion. (4) Progression of disease was defined as progression from hyperplasia and/or hyperkeratosis to dysplasia, or from a lower to a higher degree of dysplasia or invasive carcinoma, or appearance of any new lesions |
|
| Notes | Quote: "With a median follow‐up time of 27.5 months, 15 patients subsequently developed oral cancer with a median time to oral cancer development of 46.4 months." It is not specified how they were distributed in the 3 arms, but (quote): "There was no difference in oral cancer–free survival between the GTE and placebo arms" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation was done with the Pocock‐Simon dynamic allocation scheme to balance three prognostic factors in each of the four arms. The prognostic factors were tobacco use (current, stopped, never), alcohol use (current, stopped, never), and tea use (zero to four 8‐oz cups daily or five or more 8‐oz cups daily) |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups and not likely to have a clinically relevant impact on the intervention effect estimate 2/41 (5%) |
| Selective reporting (reporting bias) | Low risk | Comment: important outcomes and adverse effects reported |
| Other bias | Low risk | Comment: no other sources of bias identified |
AgNOR = silver‐stained nucleolar organizer region CNR = National Research Council COX = cyclo‐oxygenase CR = complete response EGFR = epidermal growth factor receptor FU = follow‐up HNSCC = head and neck squamous cell carcinoma ITT = intention‐to‐treat MURST = Ministry of University, Research, Science and Technology NIH = National Institutes of Health OIN = oral intraepithelial neoplasia OPL = oral pre‐malignant lesion OSCC = oral squamous cell carcinoma PCNA = proliferation cell nuclear antigen PD = progressive disease PR = partial response RCT = randomised controlled trial SCC = squamous cell carcinoma WHO = World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bocharova 2004 | The study was not randomised, and both arms of the study employed active treatments, i.e. no placebo (or no treatment) group was included in the study |
| Boisnic 1994 | The study included participants with traumatic lesions |
| Chee 2013 | Both arms of the study employed active treatments, i.e. no placebo (or no treatment) group was included in the study |
| Chiesa 2005 | All participants underwent active treatment. The aim of the treatment tested was to prevent recurrence of leukoplakia; all participants were randomised after surgical removal (active treatment) of the oral lesion |
| Femiano 2001 | The study was not randomised, and participants were allocated to study arms by researchers |
| Gaeta 2000 | Quasi‐randomised trial |
| Garewal 1999 | Participants randomised were a selected group who responded to the drug tested in the randomised phase (beta carotene) |
| Krishnaswamy 1995 | The study used an inadequate method of allocation, and only 66% of participants had lesions, none with a histological diagnosis |
| Lippman 1993 | Participants randomised were a selected group who responded to 1 of the 2 drugs tested during the randomised phase (isotretinoin) |
| López‐Jornet 2013 | Both arms of the study employed active treatments, i.e. no placebo (or no treatment) group was included in the study |
| Mathew 1995 | The study was not randomised, as controls were taken from another study control group |
| Schwarz 2005 | Both arms of the study employed active treatments, i.e. no placebo (or no treatment) group was included in the study |
| Zaridze 1993 | The oral lesions diagnosed as leukoplakia were not biopsied for histological examination. Data, as presented in the paper, do not allow analysis |
Characteristics of studies awaiting assessment [ordered by study ID]
Califano 2012.
| Methods | |
| Participants |
Inclusion criteria (1) Presence of 3p, 9p21 or 17p LOH, and/or (2) surgically unresectable high‐grade pre‐malignant lesions and/or (3) high‐grade pre‐malignancy after curative therapy for HNSCC |
| Interventions | Participants received cetuximab 400 mg/m2 week 1 followed by 250 mg/m2 weeks 2 to 8 or observation, with the option for cross‐over to cetuximab for participants originally randomised to the observation arm |
| Outcomes | Histological grade (1 = benign, 2 = mild dysplasia, 3 = moderate dysplasia, 4 = severe dysplasia/carcinoma in situ, 5 = invasive cancer) and change in grade of dysplasia were evaluated. Malignant transformation was evaluated |
| Notes |
Chiba 2012.
| Methods |
Study design: RCT, parallel‐group, 2 arms Conducted in Sri Lanka Number of centres: 15 Recruitment period: unspecified Funding source: unspecified Study duration: unspecified |
| Participants |
Inclusion criteria Betel quid chewers with pathological diagnosis of oral pre‐cancer Exclusion criteria Lesion not measurable Histological criteria for leukoplakia Not reported 72 participants randomised: mean age 53.9, 90.2% male, ethnic 98.6% Sinhala. Percentage of dysplastic lesions not reported Group A: unspecified Group B: unspecified |
| Interventions |
Group A: curcumin‐coated chewing gum Group B: placebo chewing gum Compliance control: not reported |
| Outcomes |
Clinical response Lesions were measured every 6 months. Participants were followed up every month by oral and maxillofacial surgeons at each hospital Histological response Not reported |
| Notes | The only available report of this study is an abstract |
HNSCC = head and neck squamous cell carcinoma LOH = loss of heterozygosity RCT = randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00004161.
| Trial name or title | Fenretinide in treating patients with leukoplakia of the mouth |
| Methods | Study design: RCT, parallel‐group |
| Participants | Inclusion criteria: histologically proven dysplastic leukoplakia > 1 cm in diameter, age 18 and older |
| Interventions |
Group A: oral fenretinide daily (except days 1 to 3 each month) for 6 months Group B: oral placebo daily (except days 1 to 3 each month) for 6 months, then oral fenretinide daily (except days 1 to 3 each month) for 6 months. Participants are followed up every 3 months |
| Outcomes | Regression of oral dysplastic leukoplakia Intermediate endpoint markers Surrogate endpoint biomarkers Recurrence rate of oral dysplastic leukoplakia after administration of fenretinide, both at the same site and at new sites |
| Starting date | February 2000 |
| Contact information | Samuel W. Beenken; University of Alabama at Birmingham |
| Notes | Status: completed (the study has ended normally, and participants are no longer being examined or treated) |
NCT00014404.
| Trial name or title | Celecoxib in treating patients with precancerous lesions of the mouth |
| Methods | Study design: RCT, parallel‐group |
| Participants | Inclusion criteria: histologically confirmed index oral pre‐malignant lesion |
| Interventions |
Group A: lower‐dose oral celecoxib twice daily Group B: higher‐dose oral celecoxib twice daily Group C: oral placebo twice daily Treatment continues in all 3 arms for 12 weeks in the absence of disease progression or unacceptable toxicity. Participants are followed at 18, 24 and 26 weeks |
| Outcomes | |
| Starting date | October 2000 |
| Contact information | Jay O Boyle, Study Chair; Memorial Sloan‐Kettering Cancer Center |
| Notes | This study is no longer recruiting participants |
NCT00101335.
| Trial name or title | Celecoxib in preventing head and neck cancer in patients with oral leukoplakia |
| Methods | Study design: cross‐over RCT |
| Participants | Inclusion criteria: diagnosis of oral leukoplakia with hyperplasia or dysplasia |
| Interventions |
Group A: oral celecoxib twice daily for 3 months Group B: oral placebo twice daily for 3 months All participants undergo biopsy. Participants then cross over to the opposite treatment arm for 3 months |
| Outcomes |
Primary: regression of oral leukoplakia lesions in participants with hyperplastic or dysplastic oral leukoplakia Secondary: multiple intermediate biomarkers (e.g. COX‐2, PPARγ, PPARδ) in normal and hyperplastic or dysplastic oral epithelia of participants; safety; cost‐effectiveness |
| Starting date | 2005 |
| Contact information | Paul F. Engstrom; Fox Chase Cancer Center |
| Notes | Status: completed (the study has ended normally, and participants are no longer being examined or treated) |
NCT00155337.
| Trial name or title | Photodynamic therapy for oral leukoplakia and erythroleukoplakia |
| Methods | Study design: RCT, parallel‐group |
| Participants | Inclusion criteria: patients with leukoplakia or erythroleukoplakia, age 20 to 80 years Exclusion criteria: oral cancers |
| Interventions |
Group A: photodynamic therapy Group B: unclear |
| Outcomes | Regression of lesions |
| Starting date | 2005 |
| Contact information | Chun‐Pin Chiang; National Taiwan University Hospital |
| Notes | Status: completed (the study has ended normally, and participants are no longer being examined or treated) |
NCT00176566.
| Trial name or title | A phase II trial to assess the effects of green tea in oral leukoplakia |
| Methods | Study design: RCT, parallel‐group |
| Participants |
Inclusion criteria: patients with oral leukoplakia without evidence of active infection Exclusion criteria: allergy to caffeine, GI ulcers, pregnancy, previous invasive mouth cancer |
| Interventions |
Group A: green tea lozenge Group B: placebo |
| Outcomes | Primary outcomes: prevalence, size, histological severity of oral leukoplakia |
| Starting date | 2005 |
| Contact information | Susan Goodin, PharmD; University of Medicine and Dentistry New Jersey |
| Notes | Status: terminated (the study has stopped recruiting or enrolling participants early and will not start again; participants are no longer being examined or treated) |
NCT00299195.
| Trial name or title | A randomized study of sulindac in oral premalignant lesions |
| Methods | Study design: RCT, parallel‐group |
| Participants | Inclusion criteria: oral pre‐malignant lesion (OPL) defined as a lesion that can include atypical hyperplasia, atypical hyperkeratosis, leukoplakia and erythroplakia/erythro‐leukoplakia |
| Interventions |
Drug: sulindac 150 mg p.o. b.i.d. × 24 weeks Drug: placebo b.i.d. × 24 weeks |
| Outcomes | To evaluate the efficacy of sulindac in participants with early or advanced oral pre‐malignant lesion (OPL) by both clinical response (reduction in size of all lesions) and histological response (change in histological grade) |
| Starting date | 2006 |
| Contact information | Jay O. Boyle; Memorial Sloan‐Kettering Cancer Center |
| Notes | Status: This study is ongoing but is not recruiting participants |
NCT00402779.
| Trial name or title | Erlotinib prevention of oral cancer (EPOC) |
| Methods | Study design: RCT, parallel‐group |
| Participants |
Inclusion criteria Male or female patients with 1 of the following: (1) loss of heterozygosity (LOH) at 3p14 and/or 9p21 in the oral Intraepithelial neoplasia (IEN) of patients with a history of curatively treated oral cancer, or (2) LOH at 3p14 and/or 9p21 plus at 1 other chromosomal region in the IEN of participants with no oral cancer history; participants must have confirmed diagnosis of oral IEN lesion with LOH; age ≥ 18 years |
| Interventions |
Group A: erlotinib 150 mg for 1 year Group B: placebo for 1 year |
| Outcomes |
Primary outcome: oral cancer‐free survival Secondary outcomes: Size, number and appearance of oral IEN will be assessed and correlated with cancer risk; a panel of molecular markers will be used for correlation with oral cancer development in our participants with oral IEN |
| Starting date | 2006 |
| Contact information | Vassiliki Papadimitrakopoulou; M.D. Anderson Cancer Center |
| Notes | Status: This study is ongoing but is not recruiting participants |
NCT00951379.
| Trial name or title | Pioglitazone for oral premalignant lesions |
| Methods | |
| Participants |
Inclusion criteria STAGE I: males or females with suspected or histologically confirmed oral premalignant lesion(s) |
| Interventions |
Group A: pioglitazone hydrochloride p.o. q.d. for 24 weeks Group B: placebo p.o. q.d. for 24 weeks |
| Outcomes | Primary outcome measures Clinical and histological response defined as 50% or greater reduction in the sum of measured products of perpendicular dimensions of target lesion(s), or improvement in the degree of dysplasia or hyperplasia Secondary outcome measures Tissue levels of PPAR gamma, cyclin D1 and p21 as indirect measures of pharmacological effect, TUNEL for apoptosis and Ki‐67 for proliferation, transglutaminase and involucrin as markers of squamous differentiation, 15‐PGDH and loss of heterozygosity. Level of C‐reactive protein in plasma. Tobacco and alcohol use. Adverse events and clinical laboratory toxicity assessed by National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 |
| Starting date | 2010 |
| Contact information | Jay Boyle; M.D. Anderson Cancer Center |
| Notes | Status: This study is currently recruiting participants |
NCT01497951.
| Trial name or title | Photodynamic therapy for oral precursor lesions (PDT) |
| Methods | Study design: RCT, parallel‐group |
| Participants |
Inclusion criteria Existing leukoplakia simplex SIN III (diagnostics by biopsy); leukoplakia verrucosa without indications of malignant changes (diagnostics by biopsy); oral lichen planus SIN III (diagnostics by biopsy) |
| Interventions |
Group A: aminolaevulinic acid Group B: placebo |
| Outcomes |
Primary outcome: changes in per cent (%) of initial area in mm2 Secondary outcomes: pain due to treatment, assessed by visual analogue scale (VAS) |
| Starting date | 2011 |
| Contact information | Georg Watzek; Medical University of Vienna |
| Notes | Status: This study is currently recruiting participants |
COX = cyclo‐oxygenase CTCAE = Common Terminology Criteria for Adverse Events EPOC = excess post‐exercise oxygen consumption IEN = intraepithelial neoplasia Ki‐67 = protein; cellular marker of neoplasia NCI = National Cancer Institute OPL = oral pre‐malignant lesion PDT = photodynamic therapy PGDH = 15‐hydroxyprostaglandin dehydrogenase PPAR = peroxisome proliferator‐activated receptor RCT = randomised controlled trial SIN = squamous intraepithelial neoplasia TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labeling VAS = visual analogue scale
Differences between protocol and review
We changed the title to specify that oral leukoplakia is treated to prevent oral cancer.
When it was not possible to obtain missing data from trial authors, and we found no evidence that data were missing because of a specific bias, we analysed only available data (Higgins 2011). This represents a change from the previous version of the review, wherein missing data were imputed with the assumption that all were poor outcomes.
We used fixed‐effect rather than random‐effects meta‐analysis because of the small number of included studies.
In accordance with the methodological recommendations of Cochrane Oral Health, we now include only randomised controlled trials to reduce risk of bias from quasi‐randomised studies. Therefore, we have excluded the small, quasi‐randomised study (Gaeta 2000) from this version of the review.
Contributions of authors
Giovanni Lodi: main review author, participation in all phases of review preparation Roberto Franchini: selection of articles, data extraction, text preparation Elena Varoni: data extraction, interpretation of studies Saman Warnakulasuriya: interpretation of results, text preparation Andrea Sardella: selection of articles, interpretation of results Alexander R Kerr: interpretation of results, text preparation Antonio Carrassi: group co‐ordinator, interpretation of results LCI MacDonald: updated text, data extraction and analysis, quality assessment Helen V Worthington: data extraction and analysis, quality assessment
Sources of support
Internal sources
Università degli Studi di Milano, Italy.
External sources
School of Dentistry, The University of Manchester, UK.
-
Cochrane Oral Health Group Global Alliance, Other.
Through our Global Alliance (http://ohg.cochrane.org/partnerships‐alliances), the Cochrane Oral Health Group has received support from British Association for the Study of Community Dentistry, UK; British Association of Oral Surgeons, UK; British Orthodontic Society, UK; British Society of Paediatric Dentistry, UK; British Society of Periodontology, UK; Canadian Dental Hygienists Association, Canada; Mayo Clinic, USA; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland (NES); and Royal College of Surgeons of Edinburgh, UK
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Oral Health Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health
Declarations of interest
Giovanni Lodi: none known Roberto Franchini: none known Saman Warnakulasuriya: none known Elena Maria Varoni: none known Andrea Sardella: none known Alexander R Kerr: none known Antonio Carrassi: none known LCI MacDonald: none known. LM is a salaried member of staff with Cochrane Oral Health Helen V Worthington: none known. HW is a Co‐ordinating Editor with Cochrane Oral Health
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Armstrong 2013 {published data only}
- Armstrong WB, Taylor TH, Kennedy AR, Melrose RJ, Messadi DV, Gu M, et al. Bowman Birk inhibitor concentrate and oral leukoplakia: a randomized phase IIb trial. Cancer Prevention Research (Philadelphia, Pa.) 2013;6:410‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 1994 {published and unpublished data}
- Epstein JB, Wong FL, Millner A, Le ND. Topical bleomycin treatment of oral leukoplakia: a randomized double‐blind clinical trial. Head & Neck 1994;16(6):539‐44. [DOI] [PubMed] [Google Scholar]
Hong 1986 {published data only}
- Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, et al. 13‐cis‐retinoic acid in the treatment of oral leukoplakia. New England Journal of Medicine 1986;315(24):1501‐5. [DOI] [PubMed] [Google Scholar]
Li 1999 {published and unpublished data}
- Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proceedings of the Society for Experimental Biology and Medicine 1999;220(4):218‐24. [DOI] [PubMed] [Google Scholar]
Mallery 2014 {published data only}
- Mallery SR, Tong M, Shumway BS, Curran AE, Larsen PE, Ness GM, et al. Topical application of a mucoadhesive freeze‐dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo‐controlled clinical trial. Clinical Cancer Research 2014;20(7):1910‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulshine 2004 {published data only}
- Mulshine JL, Atkinson JC, Greer RO, Papadimitrakopoulou VA, Waes C, Rudy S, et al. Randomized, double‐blind, placebo‐controlled phase IIb trial of the cyclooxygenase inhibitor ketorolac as an oral rinse in oropharyngeal leukoplakia. Clinical Cancer Research 2004;10(5):1565‐73. [DOI] [PubMed] [Google Scholar]
Nagao 2015 {published and unpublished data}
- Nagao T, Warnakulasuriya S, Fukano H, Nakamura T, Kato S, Yamamoto K, et al. A randomized controlled trial for the treatment of oral leukoplakia with the aid of chemopreventive agents. Journal of Oral and Maxillofacial Surgery 2007;65(9 Suppl):41.e6. [Google Scholar]
- Nagao T, Warnakulasuriya S, Fukano H, Suzuki K, Shimozato K, Hashimoto S. A randomised trial for preventing malignant transformation of oral leukoplakia. Oral Surgery 2010;3:103–14. [Google Scholar]
- Nagao T, Warnakulasuriya S, Nakamura T, Kato S, Yamamoto K, Fukano H, et al. Treatment of oral leukoplakia with a low‐dose of beta‐carotene and vitamin C supplements: a randomized controlled trial. International Journal of Cancer 2015;136:1708‐17. [DOI] [PubMed] [Google Scholar]
- Nagao T, Warnakulasuriya S, Sakuma H, Suzuki K, Hashimoto S. Is response to chemoprevention in oral leukoplakia determined by p53 expression?. Oral Diseases 2010;16:518. [Google Scholar]
Papadimitrakopoulou 2008 {published data only}
- Papadimitrakopoulou VA, William WN Jr, Dannenberg AJ, Lippman SM, Lee JJ, Ondrey FG, et al. Pilot randomized phase II study of celecoxib in oral premalignant lesions. Clinical Cancer Research 2008;14:2095‐101. [DOI] [PubMed] [Google Scholar]
Piattelli 1999 {published and unpublished data}
- Piattelli A, Fioroni M, Santinelli A, Rubini C. bcl‐2 expression and apoptotic bodies in 13‐cis‐retinoic acid (isotretinoin)‐topically treated oral leukoplakia: a pilot study. Oral Oncology 1999;35(3):314‐20. [DOI] [PubMed] [Google Scholar]
Sankaranarayan 1997 {published data only}
- Sankaranarayanan R, Mathew B, Varghese C, Sudhakaran PR, Menon V, Jayadeep A, et al. Chemoprevention of oral leukoplakia with vitamin A and beta carotene: an assessment. Oral Oncology 1997;33(4):231‐6. [DOI] [PubMed] [Google Scholar]
Singh 2004 {published data only}
- Singh M, Krishanappa R, Bagewadi A, Keluskar V. Efficacy of oral lycopene in the treatment of oral leukoplakia. Oral Oncology 2004;40(6):591‐6. [DOI] [PubMed] [Google Scholar]
Stich 1988 {published data only}
- Stich HF, Hornby AP, Mathew B, Sankaranarayanan R, Nair MK. Response of oral leukoplakias to the administration of vitamin A. Cancer Letters 1988;40(1):93‐101. [DOI] [PubMed] [Google Scholar]
Sun 2009 {published data only}
- Sun Z, Guan X, Li N, Liu X, Chen X. Chemoprevention of oral cancer in animal models, and effect on leukoplakias in human patients with ZengShengPing, a mixture of medicinal herbs. Oral Oncology 2010;46:105‐10. [DOI] [PubMed] [Google Scholar]
Tsao 2009 {published data only}
- Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El‐Naggar AK, et al. Phase II randomized, placebo‐controlled trial of green tea extract in patients with high‐risk oral premalignant lesions. Cancer Prevention Research (Philadelphia, Pa.) 2009;2:931‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bocharova 2004 {published data only}
- Bocharova OA, Pozharitskaya MM, Chekalina TL, Lyzhenkova MA, Karpova RV, Mezentseva MV, et al. Leukoplakia of oral mucosa: pathogenesis and possible correction with phytoadaptogen. Bulletin of Experimental Biology and Medicine 2004;138(6):578‐83. [DOI] [PubMed] [Google Scholar]
Boisnic 1994 {published data only}
- Boisnic S, Branchet MC, Pascal F, Ben Slama L, Rostin M, Szpirglas H. Topical tretinoin in the treatment of lichen planus and leukoplakia of the mouth mucosa. A clinical evaluation [Trétinoine topique dans le traitement des lichens plans et des leucoplasies de la muqueuse buccale. Evaluation clinique]. [French]. Annals of Dermatology and Venereology 1994;121(6‐7):459‐63. [PubMed] [Google Scholar]
Chee 2013 {published data only}
- Chee M, Sasaki C. Carbon dioxide laser fiber for the excision of oral leukoplakia. Annals of Otology, Rhinology, and Laryngology 2013;122:547‐9. [DOI] [PubMed] [Google Scholar]
Chiesa 2005 {published data only}
- Chiesa F, Tradati N, Grigolato R, Boracchi P, Biganzoli E, Crose N, et al. Randomized trial of fenretinide (4‐HPR) to prevent recurrences, new localizations and carcinomas in patients operated on for oral leukoplakia: long‐term results. International Journal of Cancer 2005;115(4):625‐9. [DOI] [PubMed] [Google Scholar]
- Chiesa F, Tradati N, Marazza M, Rossi N, Boracchi P, Mariani L, et al. Fenretinide (4‐HPR) in chemoprevention of oral leukoplakia. Journal of Cellular Biochemistry Supplement 1993;17F:255‐61. [DOI] [PubMed] [Google Scholar]
- Chiesa F, Tradati N, Marazza M, Rossi N, Boracchi P, Mariani L, et al. Prevention of local relapses and new localisations of oral leukoplakias with the synthetic retinoid fenretinide (4‐HPR). Preliminary results. European Journal of Cancer. Part B, Oral Oncology 1992;28B(2):97‐102. [DOI] [PubMed] [Google Scholar]
- Costa A, Formelli F, Chiesa F, Decensi A, Palo G, Veronesi U. Prospects of chemoprevention of human cancers with the synthetic retinoid fenretinide. Cancer Research 1994;54(7 Suppl):2032s‐7s. [PubMed] [Google Scholar]
- Palo G, Veronesi U, Marubini E, Camerini T, Chiesa F, Nava M, et al. Controlled clinical trials with fenretinide in breast cancer, basal cell carcinoma and oral leukoplakia. Journal of Cellular Biochemistry Supplement 1995;22:11‐7. [DOI] [PubMed] [Google Scholar]
Femiano 2001 {published data only}
- Femiano F, Gombos F, Scully C, Battista C, Belnome G, Esposito V. Oral leukoplakia: open trial of topical therapy with calcipotriol compared with tretinoin. International Journal of Oral and Maxillofacial Surgery 2001;30(5):402‐6. [DOI] [PubMed] [Google Scholar]
Gaeta 2000 {published and unpublished data}
- Gaeta GM, Gombos F, Femiano F, Battista C, Minghetti P, Montanari L, et al. Acitretin and treatment of the oral leucoplakias. A model to have an active molecules release. Journal of the European Academy of Dermatology and Venereology 2000;14(6):473‐8. [DOI] [PubMed] [Google Scholar]
Garewal 1999 {published data only}
- Garewal H, Pitcock J, Friedman S, Alberts D, Meyskens F, Ramsey L, et al. Beta‐carotene in oral leukoplakia. Proceedings, Annual Meeting of the American Society of Clinical Oncology 1992;11:141. [Google Scholar]
- Garewal HS, Katz RV, Meyskens F, Pitcock J, Morse D, Friedman S, et al. Beta‐carotene produces sustained remissions in patients with oral leukoplakia: results of a multicenter prospective trial. Archives of Otolaryngology ‐ Head and Neck Surgery 1999;125(12):1305‐10. [DOI] [PubMed] [Google Scholar]
Krishnaswamy 1995 {published data only}
- Krishnaswamy K, Prasad MP, Krishna TP, Annapurna VV, Reddy GA. A case study of nutrient intervention of oral precancerous lesions in India. European Journal of Cancer. Part B: Oral Oncology 1995;31B(1):41‐8. [DOI] [PubMed] [Google Scholar]
Lippman 1993 {published data only}
- Lippman SM, Batsakis JG, Toth BB, Weber RS, Lee JJ, Martin JW, et al. Comparison of low‐dose isotretinoin with beta carotene to prevent oral carcinogenesis. New England Journal of Medicine 1993;328(1):15‐20. [DOI] [PubMed] [Google Scholar]
- Papadimitrakopoulou VA, Lippman SM, Lee JS, Toth BB, Martin JW, Lee JJ, et al. Long term follow‐up of low‐dose isotretinoin (13‐cRA) versus beta carotene to prevent oral carcinogenesis. Proceedings, Annual Meeting of the American Society of Clinical Oncology 1996;15:A340. [Google Scholar]
López‐Jornet 2013 {published data only}
- López‐Jornet P, Camacho‐Alonso F. Comparison of pain and swelling after removal of oral leukoplakia with CO₂ laser and cold knife: a randomized clinical trial. Medicina Oral, Patologia Oral y Cirugia Bucal 2013;18:38e‐44e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathew 1995 {published data only}
- Mathew B, Sankaranarayanan R, Nair PP, Varghese C, Somanathan T, Amma BP, et al. Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutrition and Cancer 1995;24(2):197‐202. [DOI] [PubMed] [Google Scholar]
Schwarz 2005 {published data only}
- Schwarz F, Maraki D, Yalcinkaya S, Bieling K, Bocking A, Becker J. Cytologic and DNA‐cytometric follow‐up of oral leukoplakia after CO2‐ and Er:YAG‐laser assisted ablation: a pilot study. Lasers in Surgery and Medicine 2005;37(1):29‐36. [DOI] [PubMed] [Google Scholar]
Zaridze 1993 {published data only}
- Zaridze D, Evstifeeva T, Boyle P. Chemoprevention of oral leukoplakia and chronic esophagitis in an area of high incidence of oral and esophageal cancer. Annals of Epidemiology 1993;3(3):225‐34. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Califano 2012 {published data only}
- Califano JA, Ferris RL, Epstein JB, Gillespie MB, Feldman LE, Gibson MK, et al. A phase II trial of cetuximab in high‐risk premalignant lesions of the upper aerodigestive tract. Journal of Clinical Oncology 2012;30(No 15_suppl (May 20 Suppl)):5528. [Google Scholar]
Chiba 2012 {published data only}
- Chiba I, Takeshima M, Abiko Y, Kobayashi H, Muthumala M, Sugiura C, et al. Curcumin is an effective chemopreventive substance for betel quid chewer's oral precancer in Sri Lanka. Cancer Prevention Research 2012;5(11 Suppl 1):PR‐04. [DOI: 10.1158/1940-6207.PREV-12-PR-04] [DOI] [Google Scholar]
References to ongoing studies
NCT00004161 {published data only}
- NCT00004161. Fenretinide in treating patients with leukoplakia of the mouth. https://clinicaltrials.gov/ct2/show/NCT00004161 (accessed 16 May 2016).
NCT00014404 {published data only}
- NCT00014404. Celecoxib in treating patients with precancerous lesions of the mouth. https://clinicaltrials.gov/ct2/show/NCT00014404 (accessed 16 May 2016).
NCT00101335 {published data only}
- NCT00101335. Celecoxib in preventing head and neck cancer in patients with oral leukoplakia. https://clinicaltrials.gov/ct2/show/NCT00101335 (accessed 16 May 2016).
NCT00155337 {published data only}
- NCT00155337. Photodynamic therapy for oral leukoplakia and erythroleukoplakia. https://clinicaltrials.gov/ct2/show/NCT00155337 (accessed 16 May 2016).
NCT00176566 {published data only}
- NCT00176566. A phase II trial to assess the effects of green tea in oral leukoplakia. https://clinicaltrials.gov/ct2/show/NCT00176566 (accessed 16 May 2016).
NCT00299195 {published data only}
- NCT00299195. A randomized study of sulindac in oral premalignant lesions. https://clinicaltrials.gov/ct2/show/NCT00299195 (accessed 16 May 2016).
NCT00402779 {published data only}
- NCT00402779. Erlotinib prevention of oral cancer (EPOC). https://clinicaltrials.gov/ct2/show/NCT00402779 (accessed 16 May 2016).
NCT00951379 {published data only}
- NCT00951379. Pioglitazone for oral premalignant lesions. https://clinicaltrials.gov/ct2/show/NCT00951379 (accessed 16 May 2016).
NCT01497951 {published data only}
- NCT01497951. Photodynamic therapy for oral precursor lesions (PDT). https://clinicaltrials.gov/ct2/show/NCT01497951 (accessed May 2016).
Additional references
Abbey 1995
- Abbey LM, Kaugars GE, Gunsolley JC, Burns JC, Page DG, Svirsky JA, et al. Intraexaminer and interexaminer reliability in the diagnosis of oral epithelial dysplasia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 1995;80(2):188‐91. [DOI] [PubMed] [Google Scholar]
Arduino 2013
- Arduino PG, Bagan J, El‐Naggar AK, Carrozzo M. Urban legends series: oral leukoplakia. Oral Diseases 2013;19:642‐59. [DOI: 10.1111/odi.12065] [DOI] [PubMed] [Google Scholar]
Axell 1984
- Axell T, Holmstrup P, Kramer IRH, Pindborg JJ. International seminar on oral leukoplakia and associated lesions related to tobacco habits. Community Dentistry and Oral Epidemiology 1984;12:145‐54. [Google Scholar]
Axell 1987
- Axell T. Occurrence of leukoplakia and some other oral white lesions among 20,333 adult Swedish people. Community Dentistry and Oral Epidemiology 1987;15(1):46‐51. [DOI] [PubMed] [Google Scholar]
Axell 1996
- Axell T, Pindborg JJ, Smith CJ, Waal I. Oral white lesions with special reference to precancerous and tobacco‐related lesions: conclusions of an international symposium held in Uppsala, Sweden, May 18‐21, 1994. International Collaborative Group on Oral White Lesions. Journal of Oral Pathology & Medicine 1996;25(2):49‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bouquot 1986
- Bouquot JE, Gorlin RJ. Leukoplakia, lichen planus, and other oral keratoses in 23,616 white Americans over the age of 35 years. Oral Surgery, Oral Medicine and Oral Pathology 1986;61(4):373‐81. [DOI] [PubMed] [Google Scholar]
Dietrich 2004
- Dietrich T, Reichart PA, Scheifele C. Clinical risk factors of oral leukoplakia in a representative sample of the US population. Oral Oncology 2004;40(2):158‐63. [DOI] [PubMed] [Google Scholar]
Epstein 2012
- Epstein JB, Thariat J, Bensadoun RJ, Barasch A, Murphy BA, Kolnick L, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA: A Cancer Journal for Clinicians 2012;62:400‐22. [DOI] [PubMed] [Google Scholar]
Gupta 1980
- Gupta PC, Mehta FS, Daftary DK, Pindborg JJ, Bhonsle RB, Jalnawalla PN, et al. Incidence rates of oral cancer and natural history of oral precancerous lesions in a 10‐year follow‐up study of Indian villagers. Community Dentistry and Oral Epidemiology 1980;8(6):283‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Chichester, UK: John Wiley & Sons, Ltd, 2011. [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Chichester, UK: John Wiley & Sons, Ltd, 2011. [Google Scholar]
Holmstrup 2006
- Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long‐term treatment outcome of oral premalignant lesions. Oral Oncology 2006;42(5):461‐74. [DOI] [PubMed] [Google Scholar]
Holmstrup 2009
- Holmstrup P. Can we prevent malignancy by treating premalignant lesions?. Oral Oncology 2009;45:549‐50. [DOI] [PubMed] [Google Scholar]
Ikeda 1991
- Ikeda N, Ishii T, Iida S, Kawai T. Epidemiological study of oral leukoplakia based on mass screening for oral mucosal diseases in a selected Japanese population. Community Dentistry and Oral Epidemiology 1991;19(3):160‐3. [DOI] [PubMed] [Google Scholar]
Karabulut 1995
- Karabulut A, Reibel J, Therkildsen MH, Praetorius F, Nielesen HW, Dabelsteen E. Observer variability in the histologic assessment of oral premalignant lesions. Journal of Oral Pathology Medicine 1995;24(5):198‐200. [DOI] [PubMed] [Google Scholar]
Kramer 1978
- Kramer IR, Lucas RB, Pindborg JJ, Sobin LH. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surgery, Oral Medicine and Oral Pathology 1978;46(4):518‐39. [MEDLINE: ] [PubMed] [Google Scholar]
Lind 1987
- Lind PO. Malignant transformation in oral leukoplakia. Scandinavian Journal of Dental Research 1987;95(6):449‐55. [DOI] [PubMed] [Google Scholar]
Lodi 2008
- Lodi G, Porter S. Management of potentially malignant disorders: evidence and critique. Journal of Oral Pathology & Medicine 2008;37:63‐9. [DOI] [PubMed] [Google Scholar]
Lumerman 1995
- Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 1995;79(3):321‐9. [DOI] [PubMed] [Google Scholar]
Marley 1998
- Marley JJ, Cowan CG, Lamey PJ, Linden GJ, Johnson NW, Warnakulasuriya KA. Management of potentially malignant oral mucosal lesions by consultant UK oral and maxillofacial surgeons. British Journal of Oral and Maxillofacial Surgery 1998;34(1):28‐36. [DOI] [PubMed] [Google Scholar]
Nagao 2005
- Nagao T, Ikeda N, Fukano H, Hashimoto S, Shimozato K, Warnakulasuriya S. Incidence rates for oral leukoplakia and lichen planus in a Japanese population. Journal of Oral Pathology and Medicine 2005;34(9):532‐9. [DOI] [PubMed] [Google Scholar]
Petti 2003
- Petti S. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral Oncology 2003;39:770‐80. [DOI] [PubMed] [Google Scholar]
Pitiyage 2009
- Pitiyage G, Tilakaratne WM, Tavassoli M, Warnakulasuriya S. Molecular markers in oral epithelial dysplasia: review. Journal of Oral Pathology & Medicine 2009;38:737‐52. [DOI] [PubMed] [Google Scholar]
Reichart 2000
- Reichart PA. Oral mucosal lesions in a representative cross‐sectional study of aging Germans. Community Dentistry and Oral Epidemiology 2000;28(5):390‐8. [DOI] [PubMed] [Google Scholar]
Saito 2001
- Saito T, Sugiura C, Hirai A, Notani K, Totsuka Y, Shindoh M, et al. Development of squamous cell carcinoma from pre‐existent oral leukoplakia: with respect to treatment modality. International Journal of Oral and Maxillofacial Surgery 2001;30(1):49‐53. [DOI] [PubMed] [Google Scholar]
Scheifele 2003
- Scheifele C, Reichart PA. Is there a natural limit of the transformation rate of oral leukoplakia?. Oral Oncology 2003;39(5):470‐5. [DOI] [PubMed] [Google Scholar]
Schepman 1998
- Schepman KP, Meij EH, Smeele LE, Waal I. Malignant transformation of oral leukoplakia: a follow‐up study of a hospital‐based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncology 1998;34(4):270‐5. [PubMed] [Google Scholar]
Scully 2009
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncology 2009;45:301‐8. [DOI] [PubMed] [Google Scholar]
Shiu 2000
- Shiu MN, Chen TH, Chang SH, Hahn LJ. Risk factors for leukoplakia and malignant transformation to oral carcinoma: a leukoplakia cohort in Taiwan. British Journal of Cancer 2000;82(11):1871‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 1984
- Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow‐up study of 257 patients. Cancer 1984;53(3):563‐8. [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith J, Rattay T, McConkey C, Helliwell T, Mehanna H. Biomarkers in dysplasia of the oral cavity: a systematic review. Oral Oncology 2009;45:647‐53. [DOI] [PubMed] [Google Scholar]
Sperandio 2013
- Sperandio M, Brown AL, Lock C, Morgan PR, Coupland VH, Madden PB, et al. Predictive value of dysplasia grading and DNA ploidy in malignant transformation of oral potentially malignant disorders. Cancer Prevention Research (Philadelphia, Pa.) 2013;6:822‐31. [DOI] [PubMed] [Google Scholar]
Syrjänen 2011
- Syrjänen S, Lodi G, Bültzingslöwen I, Aliko A, Arduino P, Campisi G, et al. Human papillomaviruses in oral carcinoma and oral potentially malignant disorders: a systematic review. Oral Diseases 2011;17 Suppl 1:58‐72. [DOI] [PubMed] [Google Scholar]
Torres‐Rendon 2009
- Torres‐Rendon A, Stewart R, Craig GT, Wells M, Speight PM. DNA ploidy analysis by image cytometry helps to identify oral epithelial dysplasias with a high risk of malignant progression. Oral Oncology 2009;45:468‐73. [DOI] [PubMed] [Google Scholar]
Van der Waal 2009
- Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncology 2009;45:317‐23. [DOI] [PubMed] [Google Scholar]
Warnakulasuriya 2007
- Warnakulasuriya S, Johnson NW, Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. Journal of Oral Pathology & Medicine 2007;36:575‐80. [DOI] [PubMed] [Google Scholar]
Warnakulasuriya 2011
- Warnakulasuriya S, Kovacevic T, Madden P, Coupland VH, Sperandio M, Odell E, et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10‐year period in South East England. Journal of Oral Pathology & Medicine 2011;40:677‐83. [DOI] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lodi 2002
- Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Systematic review of randomized trials for the treatment of oral leukoplakia. Journal of Dental Education 2002;66(8):896‐902. [PubMed] [Google Scholar]
Lodi 2004
- Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Interventions for treating oral leukoplakia. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001829.pub2] [DOI] [PubMed] [Google Scholar]
Lodi 2006
- Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Interventions for treating oral leukoplakia. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001829.pub3] [DOI] [PubMed] [Google Scholar]


