Abstract
Background
Respiratory syncytial virus infection causes acute lung infection in infants and young children worldwide, resulting in considerable morbidity and mortality. Children with cystic fibrosis are prone to recurrent lung inflammation, bacterial colonisation and subsequent chronic airway disease, putting them at risk for severe respiratory syncytial virus infections requiring intensive care and respiratory support. No treatment currently exists, hence prevention is important. Palivizumab is effective in reducing respiratory syncytial virus hospitalisation rates and is recommended for prophylaxis in high‐risk children with other conditions. It is unclear if palivizumab can prevent respiratory syncytial virus hospitalisations and intensive care unit admissions in children with cystic fibrosis. This is an update of a previously published review.
Objectives
To determine the efficacy and safety of palivizumab (Synagis®) compared with placebo, no prophylaxis or other prophylaxis, in preventing hospitalisation and mortality from respiratory syncytial virus infection in children with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register and scanned references of the eligible study and related reviews.
Date of last search: 05 May 2016.
Selection criteria
Randomised and quasi‐randomised studies.
Data collection and analysis
The authors independently extracted data and assessed risk of bias.
Main results
One study (186 infants up to two years old) comparing five monthly doses of palivizumab (N = 92) to placebo (N = 94) over one respiratory syncytial virus season was identified and met our inclusion criteria. We judged there to be a low risk of bias with respect to the concealment of the randomization schedule (although it was not clear how this was generated) and to blinding of participants and study personnel. There is also a low risk of bias with regards to incomplete outcome data. However, we judged there to be a high risk of bias from selective reporting (summary statements presented but no data) and the fact that this industry‐supported study has not been published as a full report in a peer‐reviewed journal.
At six months follow‐up, one participant in each group was hospitalised due to respiratory syncytial virus; there were no deaths in either group. In the palivizumab and placebo groups, 86 and 90 children experienced any adverse event, while five and four children had related adverse events respectively. Nineteeen children receiving palivizumab and 16 receiving placebo suffered serious adverse events; one participant receiving palivizumab discontinued due to this. At 12 months follow‐up, there were no significant differences between groups in number of Pseudomonas bacterial colonisations or change in weight‐to‐height ratio.
Authors' conclusions
We identified one randomised controlled trial comparing five monthly doses of palivizumab to placebo in infants up to two years old with cystic fibrosis. While the overall incidence of adverse events was similar in both groups, it is not possible to draw firm conclusions on the safety and tolerability of respiratory syncytial virus prophylaxis with palivizumab in infants with cystic fibrosis. Six months after treatment, the authors reported no clinically meaningful differences in outcomes. Additional randomised studies are needed to establish the safety and efficacy of palivizumab in children with cystic fibrosis.
Keywords: Humans, Infant, Antiviral Agents, Antiviral Agents/adverse effects, Antiviral Agents/therapeutic use, Cystic Fibrosis, Cystic Fibrosis/complications, Drug Administration Schedule, Palivizumab, Palivizumab/adverse effects, Palivizumab/therapeutic use, Pseudomonas aeruginosa, Pseudomonas Infections, Pseudomonas Infections/epidemiology, Randomized Controlled Trials as Topic, Respiratory Syncytial Virus Infections, Respiratory Syncytial Virus Infections/drug therapy
Plain language summary
Palivizumab vaccine for prevention of respiratory syncytial virus infection in children with cystic fibrosis
Review question
We reviewed the evidence about the effects of vaccinating children with cystic fibrosis with palivizumab to prevent respiratory syncytial virus.
Background
Respiratory syncytial virus commonly causes lung infections in infants and children. Although cases are not severe in most children, children with cystic fibrosis may be at higher risk for severe lung infections from the virus. During a respiratory virus season, children with cystic fibrosis are more likely to need admitting to hospital and to experience a deterioration in lung function, compared with children who don't have cystic fibrosis. Palivizumab (Synagis®) is a vaccine which has been shown to reduce hospitalisation rates due to respiratory syncytial virus in some high risk populations. Palivizumab is administered once a month for five months, beginning before the respiratory syncytial virus season each year. It is not yet known how effective and safe this vaccine is in children with cystic fibrosis. We looked for randomised controlled trials (trials where children are put into different treatment groups at random and then compared to each other) where palivizumab vaccinations were compared to either another preventive therapy or no preventive therapy in children with cystic fibrosis.
Search date
The evidence is current to: 05 May 2016.
Study characteristics
We found one study with 186 participants (infants with cystic fibrosis up to two years of age) which was run across 40 centres in the USA.
Key results
One infant (out of 92) who received palivizumab and one infant (out of 94) who received placebo were admitted to hospital due to infection from respiratory syncytial virus. No infants died. Overall, the number of adverse events in the palivizumab group was similar to that in the placebo group. No serious adverse events were reported to be related to the vaccine. Over the longer term (12 months), weight gain and the number of infections with Pseudomonas aeruginosa (a common bacterial infection in cystic fibrosis) were similar between groups.
The limitation of all these findings is that we only identified one study. More research is needed on the use of the palivizumab vaccination in children with cystic fibrosis.
Quality of the evidence
We thought there was a low risk that it would be know which treatment group the next participant would be put into, although it was not clear how this order was generated. We also thought that participants and study personnel were sufficiently blinded to the treatment to avoid bias and that any missing data were unlikely to bias the study results. However, we did have concerns about bias from selective reporting (summary statements were presented but without any data) and the fact that this industry‐supported study has not been published as a full report in a peer‐reviewed journal.
Background
Description of the condition
Respiratory syncytial virus (RSV) infection is a leading viral cause of acute lower respiratory tract infection (LRTI) in infants and young children worldwide, resulting in considerable morbidity and mortality. Seasonal peaks in rates of infection occur during winter in temperate climates and during the rainy season in tropical climates (Cane 2001). These peaks are often called 'RSV seasons'. By two years of age, almost all children in the United States of America (USA) have had their first infection and about half have been infected twice (Glezen 1986). In the USA, RSV bronchiolitis was the primary cause of hospitalisation for any reason among infants younger than one year between 1997 and 1999 (Leader 2002); while annual RSV hospitalisation rate in Spain was found to be 37 per 1000 among infants less than six months old, with a mean length of hospital stay of 5.9 days (Vicente 2003). In addition, RSV has been shown to be the most important viral cause of death in children under five years of age, especially in those younger than one year (Thompson 2003).
A significant cause of infant morbidity is RSV infection. Longer‐term respiratory problems including increased rates of wheezing and allergy in affected infants have been demonstrated (Henderson 2005; Sigurs 1995; Sigurs 2000; Sigurs 2005; Stein 1999). Children born prematurely and those with pre‐existing chronic lung or congenital heart diseases have the highest risk of developing severe infection requiring intensive care and respiratory support (Purcell 2004). Mortality from severe RSV infection is also increased by the presence of pre‐existing diseases. In a hospital‐based cohort study in the United Kingdom (UK), RSV mortality was 8.6% with a standardised mortality ratio of 0.76 and all the RSV deaths were associated with pre‐existing conditions especially cardiac abnormalities and multiple co‐morbidities (Thorburn 2009). Pohl also showed RSV to be an important pathogen in patients after solid organ transplantation (Pohl 1992).
Besides inducing cytotoxic and chemokine‐mediated lung damage, RSV also directly impairs the mucus clearance mechanisms in the lungs (Tarran 2005). The resultant mucus stasis facilitates superimposition of bacteria, progressive lung disease and consequently respiratory failure. In addition, RSV has extra‐pulmonary manifestations e.g. pancreatic dysfunction, hepatitis, encephalopathy, and myocarditis.
Cystic fibrosis (CF) is a multi‐system genetic disorder characterised by mutation of the CF trans‐membrane conductance regulator gene. The lungs of affected children produce viscous secretions and mucus plugging develops in the airways. Airway inflammation may be present as early as four weeks of age with an associated increase in susceptibility to respiratory infections in the presence of structurally normal lungs (Khan 1995; Kirchner 1996).
Children with CF are prone to recurrent lung inflammation, bacterial colonisation and subsequent chronic airway disease. This underlying lung disease puts them at high risk for severe RSV infections. In a prospective study of 80 children with CF, 31 (39%) were hospitalised for severe or persistent respiratory disease in the first year of life and respiratory viruses were isolated in 52% of the cases. The leading viral pathogen detected among them was RSV (Armstrong 1998). Furthermore, RSV infection has been shown to be a frequent cause of hospitalisation for acute respiratory illness, prolonged hospitalisation and mechanical ventilation as well as an important contributor to the initiation and progression of respiratory symptoms and early lung injury among infants with CF (Abman 1988).
It has been reported that children with CF are more likely to develop acute lower respiratory tract infections (LRTI), require hospitalisation and develop a deterioration in lung function during a respiratory virus season, compared with children without CF. More importantly, this decline in lung function may persist for several months after the respiratory illness has cleared, further worsening the clinical course of lung disease (Hiatt 1999).
Description of the intervention
Currently, there is no cure for RSV infection, treatment mainly being supportive. Prevention is therefore very important. The development of active immunisation against RSV has been unsuccessful; however, passive immunisation with antibodies against RSV has been demonstrated to be effective in reducing RSV hospitalisation rates and serious complications among high risk children (Feltes 2003; Fenton 2004; IMpact‐RSV 1998; Pedraz 2003). In addition, the use of antibodies against RSV has been shown to diminish the risk of developing long‐term pulmonary complications (Simoes 2007).
Palivizumab (Synagis®) is a humanised monoclonal antibody directed against the RSV F glycoprotein. It is given intramuscularly at a dose of 15 mg/kg/monthly for five months and the first dose is given before the commencement of the RSV season (e.g. usually November in most states in the USA). The immunity elicited is only partial, hence the need for monthly injections throughout the duration of the RSV season.
In animal studies, palivizumab has been shown to reduce RSV‐induced airway inflammation and obstruction with a resultant improvement in acute disease severity and long‐term pulmonary function. It appears to decrease both the direct cell damage and immune inflammatory response caused by the virus (Mejias 2004) as well as prevent the persistent susceptibility to airway inflammation after the virus has been cleared (Piedimonte 2004). This is especially important because the airway inflammatory response is exaggerated in the CF mouse model (Colasurdo 2006).
In the large randomised controlled multi‐centre IMpact‐RSV trial, palivizumab prophylaxis was found to reduce the risk of hospitalisation for RSV by 55% among children with prematurity or bronchopulmonary dysplasia (BPD). Among those with prematurity alone this risk reduction was increased to 78%, while among those with BPD it was 39%. There was no significant difference in the number of reported adverse events between the treatment and control groups (IMpact‐RSV 1998).
Two non‐randomised studies on the use of palivizumab for RSV prophylaxis among children with CF have been published (Giebels 2008; Speer 2008). Using data from the Palivizumab Outcomes Registry between 2000 and 2004, Speer reported that none of the 91 infants with CF who had received palivizumab prophylaxis required hospitalisation for RSV LRTI during this period (Speer 2008). Giebels carried out a retrospective study among 75 children diagnosed with CF between 1997 and 2005 in Canada (Giebels 2008). Three out of 35 children who received palivizumab prophylaxis and seven out of 40 who did not, were admitted for acute respiratory illness (ARI) with none in the palivizumab group and three non‐treated children having laboratory‐confirmed RSV infection. Children who received palivizumab also had fewer mean ARI hospitalisation days per patient (0.8 versus 1.73) and a 54% reduction in the number of hospital days. However, these differences did not reach statistical significance (Giebels 2008).
Palivizumab is very expensive, and needs to be administered monthly for five months each RSV season. The wholesale price of a 100 mg vial was US$1922.65 in the USA in 2009 (Red Book 2009). This amounts to a cost of US$9613.25 per RSV season. In Germany, the cost per RSV season was €3637 in 2003 (Roeckl‐Wiedmann 2003). Also, because it is available only as a single‐dose vial, wastages occur. In addition, limited data on the long‐term effects of palivizumab use are available.
Presently, the American Academy of Paediatrics (AAP) recommends palivizumab prophylaxis in infants and children younger than two years with congenital heart disease (CHD), chronic lung disease of prematurity (CLD [formerly bronchopulmonary dysplasia]), and birth before 32 weeks 0 days of gestation (AAP 2009). It is recommended that these infants receive a maximal number of five doses. Among those with gestational age between 32 weeks 0 days and 34 weeks 6 days, the AAP recommends palivizumab prophylaxis if at least one of the following two risk factors are present: child care attendance; or one or more siblings or other children younger than five years live permanently in the child's household. These infants should receive palivizumab prophylaxis only until they reach 90 days of age or a maximum of three doses (whichever comes first). Although children with CF have morbidity rates comparable to those of children with CLD (Arnold 1999), a recommendation for routine palivizumab prophylaxis cannot be made for patients with CF due to insufficient evidence for the effectiveness of palivizumab among this population (AAP 2009).
There are currently no national guidelines for the use of palivizumab in the UK (McCormick 2007). However, the Scottish Intercollegiate Guidelines Network (SIGN) does not recommend the routine use of palivizumab (SIGN 2006). It is recommended that palivizumab be considered for use, on a case‐by‐case basis, in infants less than 12 months old with extreme prematurity, acyanotic congenital heart disease, congenital or acquired significant orphan lung diseases, or immune deficiency (SIGN 2006).
Why it is important to do this review
Prospective cohort studies (Abman 1988; Armstrong 1998; Efthimiou 1984; Wang 1984) and an in‐vitro study (Tarran 2005) have suggested that RSV infection may worsen CF lung disease. Infection with RSV predisposes individuals to bacterial colonisation and deterioration in lung function. Since there is currently no effective treatment against RSV infection, prevention remains important.
A previous Cochrane Systematic Review, which has been withdrawn, showed that prophylaxis with RSV immunoglobulin among high risk infants (prematurity, congenital heart disease and BPD) was effective in reducing RSV hospitalisations and admissions into the intensive care unit compared with placebo or no prophylaxis (Wang 2006). However, it is unclear whether the same effect holds among children with CF.
Currently, universal newborn screening is being performed in the USA (CFF 2009) and the UK (NHS 2009). Roll‐out programmes for CF newborn screening are underway across Europe (Southern 2007).
A survey conducted among CF centres in the UK reported that less than 10% of infants identified had received RSV prophylaxis (McCormick 2007). The paucity of evidence and funding were discussed as the main reasons for the low rate of RSV prophylaxis among infants with CF in the UK. While RSV may become an important pathogen again in later life, the current review will focus on children with CF because of the severity of its clinical implications in this age group.
This version is an update of a previously published review (Robinson 2010; Robinson 2012; Robinson 2013; Robinson 2014).
Objectives
To determine the efficacy and safety of palivizumab (Synagis®) compared with either placebo or no prophylaxis or another type of prophylaxis, in preventing hospitalisation and mortality due to respiratory syncytial virus infection in children with cystic fibrosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised studies comparing palivizumab against placebo or no prophylaxis or another type of prophylaxis in the prevention of respiratory syncytial virus infection.
Types of participants
Infants and children (up to 18 years) of both sexes with diagnosis of cystic fibrosis made by either sweat test or genetic testing or clinical criteria, and of any disease severity.
Types of interventions
Prophylaxis with palivizumab (Synagis®) compared with either placebo or no prophylaxis or another type of prophylaxis. No limits were placed on setting, regimen or dose.
Types of outcome measures
Primary outcomes
Need for hospitalisation with respiratory syncytial virus (RSV) infection based on clinical diagnosis or validated laboratory diagnosis or both
Mortality
Secondary outcomes
-
Hospitalisation for RSV infection
Length of stay in hospital
Need for intensive care
-
Oxygen therapy for RSV infection
Need for oxygen therapy
Duration of oxygen therapy
-
Pulmonary function
forced expiratory volume in one second (FEV1) (absolute or per cent predicted for age, sex and height)
forced vital capacity (FVC) (absolute or per cent predicted for age, sex and height)
forced expiratory volume in 0.5 seconds (FEV0.5) measured by the raised volume rapid thoracic compression technique or thoracic gas volume (TGV) measured by whole body plethysmography in infants and young children
Nutritional status (weight, weight‐for‐age, weight‐for‐age Z‐score, height, height‐for‐age, height‐for‐age Z‐score, weight‐for‐height, body mass index (BMI))
Number of adverse events or number of children having adverse events
Number of acute exacerbations
Number of infections with Pseudomonas aeruginosa
Number of antibiotic courses
We accepted data from all provided time points for outcomes.
Search methods for identification of studies
No restrictions were placed on language.
Electronic searches
We identified relevant studies from the Group's Cystic Fibrosis Trials Register using the terms: respiratory syncytial virus AND palivizumab. The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group Module.
Date of most recent search: 05 May 2016.
Searching other resources
We searched the reference list of the eligible trial and existing review articles on palivizumab to identify additional relevant studies and trial reports. We contacted the drug manufacturer (MedImmune, Inc.) and authors to obtain information on ongoing or unpublished studies.
Data collection and analysis
Selection of studies
Two review authors independently screened the article identified by the search methods, first using the title and abstract and subsequently using the full‐text. Disagreements regarding eligibility were resolved by consensus or by consulting with a third review author.
Data extraction and management
Two review authors independently extracted data and assessed the risk of bias in the eligible study using custom data abstraction forms designed for this review. We abstracted the information about study and participant characteristics, the intervention, and the outcomes directly into custom data abstraction forms. We corresponded with trial authors to address uncertainties in the identified study and to obtain additional information on missing data. We checked the data for accuracy and consistency, and resolved disagreements by consensus or by consulting a third reviewer. Finally, we entered the data into Review Manager software for analysis (RevMan 2011).
We accepted data from all provided time‐points for outcomes.
Assessment of risk of bias in included studies
We employed the method described in The Cochrane Handbook for Systematic Reviews of Interventions to evaluate the risk of bias (Higgins 2011). We looked for adequacy of the random sequence generation; methods of concealment of treatment allocation; blinding of participants, personnel, and outcome assessors; completeness of outcome data for each main outcome; and presence of selective outcome reporting.
Measures of treatment effect
We calculated risk ratios and their associated 95% confidence intervals (CI) for each treatment group for dichotomous outcomes. We calculated odds ratios for adverse events.
No continuous outcomes were reported in the included study. If in future updates we identify studies reporting continuous outcomes, we will report the mean relative change from baseline or the mean post‐intervention value as well as the difference in means between treatment groups and their associated 95% CI. We will also report the standard deviations; where standard errors are provided, we will convert these to standard deviations.
Unit of analysis issues
We have not included any cross‐over trials as this study design is not appropriate in evaluating vaccines.
Dealing with missing data
We contacted the authors of the included trial to obtain missing data and to clarify uncertainties in the available abstract and poster. We requested information regarding sequence generation, masking (blinding), intention‐to‐treat analysis, baseline characteristics (gender, nutritional status, pulmonary function), and outcome data (nutritional status, pulmonary function, airway colonisations with Pseudomonas aeruginosa, oxygen therapy for RSV infection, acute exacerbations, antibiotic courses, and any other outcomes). We also sought clarification on the criteria used for classifying adverse events as 'any', 'related', or 'serious'.
Assessment of heterogeneity
If in future sufficient studies are included in the review, we will assess heterogeneity between study estimates using the Chi2 test (obtained from Forest plots) and the I2 statistic (Higgins 2003). We will consider a Chi2 P value of less than 0.10 indicative of heterogeneity. The Chi2 test must, however, be interpreted with caution since the test may be negative in the presence of heterogeneity, in studies with small sample sizes or where a small number of studies have been pooled together in the meta‐analysis. In addition, where there are many studies, it has excessive power to detect clinically insignificant heterogeneity.
In order to quantify inconsistency across studies, we will calculate the I2 statistic. This statistic describes the percentage of total variation across studies that are due to heterogeneity rather than by chance. The values of I2 lie between 0% and 100%, and a simplified categorisation of heterogeneity that we plan to use is: heterogeneity might not be important (I2 value of up to 40%), heterogeneity may be moderate (I2 value of 30% to 60%), heterogeneity may be substantial (I2 value of 50% to 90%), and considerable heterogeneity (I2 value of 75% or above) (Deeks 2011).
Assessment of reporting biases
If sufficient studies are included in the review in the future and if meta‐analysis is possible, we will assess reporting bias using funnel plots. Interpretation of funnel‐plots is primarily subjective and we will take this into account. To eliminate this subjectivity, the rank correlation test is commonly used (Lau 2006). But, this test relies on the presence of a large number of studies in the analysis (at least 30). Thus, small number of studies is a hindrance in the interpretation of funnel‐plots. In theory, the funnel‐plot evaluates the presence of differences in study results by size of study (precision of results). Thus, between‐study heterogeneity further limits the validity of conclusions made from funnel‐plots. We will interpret asymmetry of funnel‐plots with caution (Lau 2006).
The authors' choice of reported outcomes can be influenced by the results, potentially making published results misleading (Higgins 2011). We compared the 'Methods' section of the abstract and poster with the 'Results' section, and we also considered if an outcome commonly reported in related studies was not reported. We contacted the authors for additional data.
Data synthesis
We entered the extracted data into RevMan 5 for data analysis using a fixed‐effect model (RevMan 2011).
If sufficient studies are included in future updates, and if meta‐analyses are appropriate, we will pool those of the same design and assess effects of the interventions. We will consider performing analyses using both random‐effects and fixed‐effects models. We will use random‐effects models if there is indication of heterogeneity between trials and we can not explain the source of heterogeneity. We will use random‐effects models for analysis if the studies have small sample sizes or the number of trials is small, in which situation tests for heterogeneity may be underpowered (Higgins 2011). If there is no indication of heterogeneity after qualitative exploration of the included studies and the use of statistical tests (I2 statistic), we will conduct analyses using fixed‐effect models.
We analysed dichotomous outcomes using risk ratio (RR), but used the odds ratio (OR) for the analysis of adverse events. When available in future updates of the review, we will use the difference in means for continuous outcomes. Where different scales of measurement have been used, we will calculate a standardised difference in means (SMD).
When possible, we plan to conduct separate meta‐analyses for each type of control group i.e. placebo, no prophylaxis, and each individual other type of prophylaxis.
Subgroup analysis and investigation of heterogeneity
When appropriate during future review updates, we will perform subgroup analyses based on the following variables:
Gender (males and females);
Age group (0 to 2 years, 3 to 6 years, and 7 to 18 years);
Presence or absence of other risk factors for severe RSV infection (premature birth, CHD, chronic lung disease of prematurity);
Geographical setting (urban and rural);
Dose used (15 mg/kg and other doses);
Regimen used (five monthly regimen and other regimens);
Definition of outcomes (as provided in included articles);
Duration of follow‐up (up to one month, one month to six months, and beyond six months);
Delta F508 zygosity (homozygous and heterozygous).
Sensitivity analysis
When appropriate during future review updates, we will also conduct sensitivity analyses to assess the impact of including and excluding in the meta‐analysis:
studies with inadequate methods of random sequence generation;
studies with inadequate methods of allocation concealment
studies with inadequate methods of masking (blinding) of participants, personnel, or outcome assessors;
studies with incomplete outcome data;
studies with selective outcome reporting; and
unpublished studies.
Results
Description of studies
Results of the search
The search identified one study (Cohen 2005).
Included studies
The one study identified by the search strategy was eligible for inclusion in the review (Cohen 2005). This was a double‐blind, placebo‐controlled study conducted in 186 children (mean (range) age: 12.8 (0.4 to 24.4) months) with CF across 40 centres in the USA. Participants were randomised to receive either 15 mg/kg palivizumab or placebo injections monthly over five months of one RSV season. Outcomes such as the number of infants hospitalised for RSV infection, mortality and adverse events, were assessed at 30 days after the end of the intervention (150 days from start of study); and others such as nutritional status (weight to height ratio) and the number of infections with Pseudomonas aeruginosa, were assessed at 180 days after the end of the intervention (300 days from start of trial). Since the intervention lasted 120 days, we categorised those outcomes reported at 150 days from the start of the study as 'up to six months' and those reported at 300 days from the start of the study as 'up to 12 months'.
For this study, we abstracted data from the published abstract and from the poster presented by the authors at the 2005 American Thoracic Society International Conference (San Diego, CA, USA) (Cohen 2005). The poster was obtained from the drug manufacturer (Medimmune, Inc.). In addition, we contacted the authors and the drug manufacturer to obtain additional data relevant to the outcomes of interest in this review. We obtained some relevant information. This review (all versions published from 2013 onwards) discusses data abstracted from the available abstract and the poster as well as data obtained from the investigators (Cohen 2005).
Excluded studies
No studies were excluded.
Risk of bias in included studies
See the risk of bias table in the section 'Characteristics of included studies'.
Allocation
The method of random sequence generation was unclear. When contacted, the investigators clarified that the randomization schedule was controlled by a pharmacy (low risk).
Blinding
The included study was described as double‐blind and therefore this domain is graded as 'low risk of bias'. When contacted, the investigators clarified that participants, investigators, outcome assessors, and data analysts were all blinded.
Incomplete outcome data
The authors reported that five participants (2.7%) were lost to follow up: two (2.2%) in the palivizumab group and three (3.2%) in the placebo group, giving a low risk of bias.
Selective reporting
There is a high risk of bias for this domain as the authors mentioned that there were no clinically meaningful differences between treatment groups for all outcomes measured at 12 months follow up (weight gain (weight to height ratio), changes in use of pulmonary medications from baseline, incidence of Pseudomonas bacterial colonization, incidence of documented wheezing episodes, and duration of steroid usage), but no data were provided.
Other potential sources of bias
This industry‐supported study has not been published as a full report in a peer‐reviewed journal.
Effects of interventions
Primary outcomes
1. Need for hospitalisation with respiratory syncytial virus (RSV) infection based on clinical diagnosis or validated laboratory diagnosis or both
Although a total of 13 (14.1%) participants in the palivizumab group and 14 (14.9%) participants in the placebo group were hospitalised within the first six months, only one participant (1.1%) in each group was identified as hospitalised due to RSV infection (as identified by a positive RSV antigen test) (Cohen 2005). We calculated the risk ratio (RR) for RSV hospitalisations comparing palivizumab and placebo and there was no significant difference between groups, RR 1.02 (95% CI 0.06 to 16.09) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 1: Need for hospitalisation for RSV infection
2. Mortality
There were no deaths among either group of participants during the first six months follow up (Cohen 2005). This outcome was not reported at 12 months follow up.
Secondary outcomes
1. Hospitalisation for RSV infection
a. Length of stay in hospital
This outcome was not assessed in the included study.
b. Need for intensive care
This outcome was not assessed in the included study.
2. Oxygen therapy for RSV infection
a. Need for oxygen therapy
When contacted, the investigators of the included study reported that one participant in the palivizumab treatment group and none in the placebo treatment group needed oxygen therapy during the trial (Cohen 2005). We calculated the RR as 3.06 (95% CI 0.13 to 74.27) (Analysis 1.3).
1.3. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 3: Need for oxygen therapy
b. Duration of oxygen therapy
This outcome was not assessed in the included study.
3. Pulmonary function
a. forced expiratory volume in one second (FEV1)
This outcome was not assessed in the included study.
b. forced vital capacity (FVC)
This outcome was not assessed in the included study.
c. forced expiratory volume in 0.5 seconds (FEV0.5)
This outcome was not assessed in the included study.
4. Nutritional status
a. weight
i. weight
When contacted, the investigators reported that both treatment groups experienced a mean weight gain of 2.7 (standard error (SE) = 0.1) kg in the palivizumab group, weight gain ranged from 1.1 kg to 6.3 kg while in the placebo group, it ranged from 0.3 kg to 6.9 kg (Cohen 2005).
ii. weight‐for‐age
This outcome was not assessed in the included study.
iii. weight‐for‐age Z‐score
This outcome was not assessed in the included study.
iv. weight‐for‐height
This outcome was not reported in the included trial. But, the authors reported no clinically significant differences between treatment groups for change in weight to height ratio at 12 months follow up (Cohen 2005). Data were not provided.
iv. body mass index (BMI)
This outcome was not assessed in the included study.
b. height
i. height
This outcome was not assessed in the included study.
ii. height‐for‐age
This outcome was not assessed in the included study.
iii. height‐for‐age Z‐score
This outcome was not assessed in the included study.
5. Adverse events
a. number of adverse events
This outcome was not assessed in the included study.
b. number of children having adverse events
The authors of the included study reported that the number of children experiencing adverse events was similar comparing palivizumab and placebo groups at six months follow up (Cohen 2005). We analysed adverse event data that were provided and calculated ORs and associated 95% CIs. The investigators defined an adverse event as "any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporarily associated with the use of a medicinal product, whether or not considered related to the medicinal product" (Cohen 2005). The number of children with any adverse event were 89 (96.7%) and 90 (95.7%) in the palivizumab and placebo groups respectively, OR 1.32 (95% CI 0.29 to 6.06) (Analysis 1.4), while 5 (5.4%) and 4 (4.3%) children respectively had adverse events classified as related, OR 1.29 (95% CI 0.34 to 4.98) (Analysis 1.5). The investigators classified serious adverse events as those that resulted in any of the following outcomes: death; life‐threatening; inpatient hospitalisation or prolongation of existing hospitalisation; persistent or significant disability or incapacity; congential anomaly or birth defect (in the offspring of a participant); or an important medical event that may not result in death, threaten life or require hospitalisation but, when based upon appropriate medical judgement, may jeopardise the patient and may require medical or surgical intervention to prevent one of the outcomes listed above. In the palivizumab group 19 (20.7%) children suffered adverse events classified as serious and the placebo group this number was 16 children (17.0%), OR 1.27 (95% CI 0.61 to 2.65) (Analysis 1.6). To assess whether a serious adverse event was related to a study drug, the investigators classified the relationship as unlikely (none or remote relationship) or likely relationship (possible, probable, or definite relationship). No children in the palivizumab group and two children (2.1%) in the placebo group suffered related serious adverse events, OR 0.20 (95% CI 0.01 to 4.22) (Analysis 1.7).
Permanent discontinuation due to a serious adverse event occurred in one participant (1.1%) in the palivizumab group (Cohen 2005).
6. Number of acute exacerbations
This outcome was not assessed in the included study.
7. Number of infections with Pseudomonas aeruginosa
When contacted, the authors of the included study reported similar number of participants with Pseudomonas aeruginosa infections in the palivizumab and the placebo treatment groups (Cohen 2005); there were 14 participants (15.2%) and 12 participants (12.8%) respectively at 12 months follow up, RR 1.19 (95% CI 0.58 to 2.44) (Analysis 1.8).
8. Number of antibiotic courses
This outcome was not assessed in the included study.
Discussion
Summary of main results
Palivizumab has been shown to be effective in reducing respiratory syncytial virus (RSV) hospitalisation rates and has been recommended for infants at high risk with other conditions. This systematic review identified only one randomised study assessing the use of palivizumab in infants with cystic fibrosis (CF). The overall incidence of adverse events was similar in palivizumab and placebo groups. Thus, it is not possible to comment on the safety and tolerability of RSV prophylaxis with palivizumab in infants with CF from these data. The authors reported that there were no clinically meaningful differences in outcomes at 12‐months follow up.
Overall completeness and applicability of evidence
We identified only one randomised trial addressing the use of palivizumab in children with CF. The study included CF infants up to two years of age.
Quality of the evidence
Although the authors mentioned that the study was double‐blinded, there was no mention about which two parties were blinded. Similarly, no information was provided about the method of random sequence generation or whether or not allocation was concealed. They reported that there were no clinically meaningful differences in weight gain, change in pulmonary medications, incidence of Pseudomonas aeruginosa colonisation, incidence of documented wheezing episodes, and duration of steroid usage between palivizumab and placebo recipients at 12 months follow up. However no data were provided for these outcomes.
Potential biases in the review process
Given our comprehensive search strategy and contact with the authors and the drug manufacturer, it is unlikely that we missed any relevant studies. We included the only randomised study that was identified by our search. We were successful in obtaining additional data from the investigators of this study.
Agreements and disagreements with other studies or reviews
The report of only one participant (1.1%) in each group being hospitalised due to RSV infection is in keeping with non‐randomised studies in the literature; Speer reported that none of the 91 infants with CF from the Palivizumab Outcomes Registry who had received palivizumab prophylaxis required hospitalisation for RSV between 2000 and 2004 (Speer 2008). Giebels reported that 3 out of 35 children with CF who received palivizumab prophylaxis and 7 out of 40 who did not, were admitted for acute respiratory illness with none in the palivizumab group and three non‐recipients having confirmed RSV infection (Giebels 2008).
Authors' conclusions
Implications for practice.
The strength of the current evidence (only one included randomised study, with limited data) is insufficient to allow conclusions about the efficacy and safety of palivizumab prophylaxis in children with CF to be made.
Implications for research.
Well‐designed adequately powered multi‐centre studies are required to provide evidence for safety and benefit of the use of palivizumab prophylaxis in children with CF.
No deaths were observed in the study included in this review (Cohen 2005) and mortality was not reported as an outcome in other studies of the use of palivizumab prophylaxis in children with CF (Giebels 2008; Speer 2008). Reductions in mortality rates associated with palivizumab prophylaxis in studies of other populations ranged from 21% to 78% (Feltes 2003; IMpact‐RSV 1998; Pedraz 2003). While a reduction in mortality rates is the ultimate aim of this intervention, it would appear that in children with CF these rates are very low and powering a study to detect changes in these rates is not feasible.
Existing studies of children with CF (Giebels 2008) and other populations (Feltes 2003; IMpact‐RSV 1998; Pedraz 2003; Singleton 2003) suggest an approximately 50% reduction in RSV hospitalisation rates associated with palivizumab prophylaxis. The study included in this review reported a 1.06% rate of hospitalisation due to RSV infection in the placebo group (Cohen 2005), while an observational study in infants with CF reported the control group rate to be higher (7.5%) (Giebels 2008). Depending on which rate is assumed, a randomised controlled study would need a per treatment group sample size of 4777 or 644 participants respectively to detect a 50% or larger difference in hospitalisation rates between palivizumab and placebo groups (assuming Type I error = 0.05, power = 80%, 1:1 allocation ratio, no dropouts, and using Fisher’s exact test) (Dupont 1990) (Figure 1). Alternatively, we could use the rate of hospitalisation due to any respiratory cause in the sample size calculations. The study included in this review reported this rate in the placebo group to be 14.9% (Cohen 2005), while the observational study in infants with CF reported it to be 17.5% in the control group (Giebels 2008). Depending on which is assumed, a randomised controlled study would need a per treatment group sample size of 306 or 255 participants respectively to detect a 50% or larger difference in hospitalisation rates between palivizumab and placebo groups (assuming Type I error = 0.05, power = 80%, 1:1 allocation ratio, no dropouts, and using Fisher’s exact test) (Dupont 1990) (Figure 2).
Whichever estimate of control group hospitalisation rate is used, the resulting sample size needed for a randomised clinical study would be prohibitively large for the CF community. Physicians and other health‐care workers need to consider longer‐term outcomes when prescribing this intervention for infants with CF. Such long‐term outcomes could include chronic airway infection, pulmonary function at school entry, etc.
1.
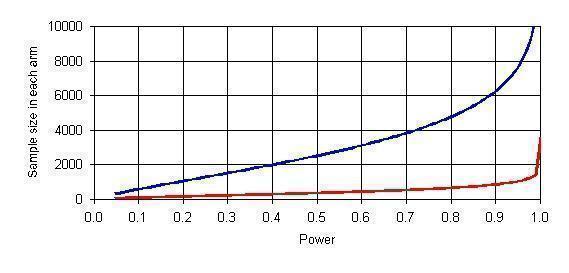
Sample size of needed randomised controlled trial as a function of power to detect a 50% reduction in rate of hospitalisation due to RSV infection (Type I error=0.05, 1:1 allocation ratio, no dropouts, and using Fisher’s exact test)
Upper Curve ‐ Assuming rate of hospitalisation in control group = 1.06% (Cohen 2005)
Lower Curve ‐ Assuming rate of hospitalisation in control group = 7.5% (Giebels 2008)
2.

Sample size of needed randomised controlled trial as a function of power to detect a 50% reduction in rate of hospitalisation due to any respiratory cause (Type I error=0.05, 1:1 allocation ratio, no dropouts, and using Fisher’s exact test)
Upper Curve ‐ Assuming rate of hospitalisation in control group = 14.9% (Cohen 2005)
Lower Curve ‐ Assuming rate of hospitalisation in control group = 17.5% (Giebels 2008)
What's new
| Date | Event | Description |
|---|---|---|
| 13 October 2023 | Amended | This review will no longer be updated as no new data likely to be forthcoming. Cystic fibrosis is no longer included in palivizumab guidance. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 2, 2010
| Date | Event | Description |
|---|---|---|
| 28 June 2016 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register did not identify any new references potentially eligible for inclusion in this review. The format of the plain language summary has been updated. |
| 28 June 2016 | New citation required but conclusions have not changed | Since no new data have been added to this review, our conclusions remain the same. |
| 12 May 2014 | New citation required but conclusions have not changed | No new information has been added to this review, hence our conclusions remain the same. One author (Naomi McKoy) has stepped down from the review team. |
| 12 May 2014 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Review Group's Trials Register did not identify any new references which were eligible for inclusion in this review. |
| 17 April 2013 | New citation required but conclusions have not changed | Despite the inclusion of additional data from the only included trial in this review, the authors' conclusions have not changed. |
| 17 April 2013 | New search has been performed | A search of the Group's Cystic Fibrosis Register did not identify any new references for this review. However, we obtained data from the sponsor of the only eligible trial and have included these data in the current update of this review. |
| 9 November 2010 | New search has been performed | A search of the Group's CF Trials Register did not identify any new references for inclusion in this review. We have revised the section 'Implications for research' with updated sample size calculations for a required randomized controlled trial based on rates of hospitalizations due to any respiratory cause and specifically due to RSV infection. We have also done separate calculations using estimates from the one included randomized control trial and an observational study. |
Notes
None.
Acknowledgements
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed here are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Palivizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Need for hospitalisation for RSV infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1.1 Up to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2.1 Up to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Need for oxygen therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3.1 Up to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Adverse events ‐ any | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4.1 Up to 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Related adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5.1 Up to 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6 Any serious adverse event | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.6.1 Up to 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7 Related serious adverse event | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7.1 Up to 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Pseudomonas aeruginosa infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 Up to 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.2. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 2: Mortality
1.4. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 4: Adverse events ‐ any
1.5. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 5: Related adverse events
1.6. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 6: Any serious adverse event
1.7. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 7: Related serious adverse event
1.8. Analysis.

Comparison 1: Palivizumab versus placebo, Outcome 8: Pseudomonas aeruginosa infections
Characteristics of studies
Characteristics of included studies [author‐defined order]
Cohen 2005.
| Study characteristics | ||
| Methods | Multi‐centre double‐blind placebo‐controlled trial in the USA. Parallel design. 40 centres. | |
| Participants | 186 participants, all analysed. Mean (range) age = 12.8 (0.4 to 24.4) months. | |
| Interventions | Placebo versus palivizumab. | |
| Outcomes | At 6 months follow up ‐ number of patients hospitalised for RSV infection, mortality, adverse events. At 12 months follow up ‐ nutritional status (weight/height ratio), number of infections with Pseudomonas aeruginosa. |
|
| Intention‐to‐treat analysis | Yes. | |
| Notes | Of 186 participants, 5 were lost to follow‐up. All who received at least one dose were analysed (n = 186). Data abstracted from poster and from additional results provided by the investigators. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Clarification sought from authors, but not obtained. |
| Allocation concealment (selection bias) | Low risk | Assignment of participants to groups was done by a pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded, including participants, investigators, outcome assessors, data analysts, and sponsors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Five participants (2.7%) were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | Methods mention weight gain, change in pulmonary medications, incidence of Pseudomonas colonization, incidence of documented wheezing episodes, and duration of steroid usage assessed at 12 months follow‐up. Results state that there were no significant differences in outcomes at that time point, but no data provided. When we contacted the authors of the trial, we received information on weight gain and incidence of Pseudomonas colonization. |
| Other bias | Unclear risk | Clarification sought from authors, not obtained. |
RSV: respiratory syncytial virus USA: United States of America
Differences between protocol and review
None.
Contributions of authors
Link with editorial base: Karen Robinson Draft the protocol: all authors Develop the search strategy: editorial base and Karen Robinson Search for studies: all authors and editorial base Retrieve copies of the studies: Olaide Odelola and Naomi Mckoy Screening and abstraction of data: all authors Enter data into RevMan and carry out the analysis: Olaide Odelola Interpret analysis: all authors Draft final review: all authors Update the review: all authors
Sources of support
Internal sources
No sources of support provided
External sources
Partially funded by the Cystic Fibrosis Foundation, USA
-
National Institute for Health Research, UK
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Karen Robinson declares no potential conflict of interest.
Ian Saldanha declares no potential conflict of interest.
Olaide Odelola declares no potential conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Cohen 2005 {published and unpublished data}
- Cohen AH, Boron ML, Dingivan C. A phase IV study of the safety of Synagis® (Palivizumab) for prophylaxis of respiratory syncytial virus disease in children with cystic fibrosis [abstract]. In: Proceedings of the American Thoracic Society International Conference; 2005 May 20-25; San Diego, USA. 2005:A178.
- Cohen AH, Boron ML, Dingivan C. A phase IV study of the safety of Synagis® (Palivizumab) for prophylaxis of respiratory syncytial virus disease in children with cystic fibrosis. In: Poster session presented at the American Thoracic Society International Conference 2005 May 20-25; San Diego, USA. 2005.
Additional references
AAP 2009
- American Academy of Pediatrics Committee on Infectious Diseases. Policy statement modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics 2009;124(6):1694-701. [DOI: 10.1542/peds.2009-2345] [DOI] [PubMed] [Google Scholar]
Abman 1988
- Abman SH, Ogle JW, Butler-Simon N, Rumack CM, Accurso FJ. Role of respiratory syncytial virus in early hospitalization for respiratory distress of young infants with cystic fibrosis. Journal of Pediatrics 1988;113(5):826-30. [DOI] [PubMed] [Google Scholar]
Armstrong 1998
- Armstrong D, Grimwood K, Carlin JB, Carzino R, Hull J, Olinsky A, et al. Severe viral respiratory infections in infants with cystic fibrosis. Pediatric Pulmonology 1998;26(6):371-9. [DOI] [PubMed] [Google Scholar]
Arnold 1999
- Arnold SR, Wang EE, Law BJ, Boucher FD, Stephens D, Robinson JL, et al. Variable morbidity of respiratory syncytial virus infection in patients with underlying lung disease: a review of the PICNIC RSV Database. Pediatric Investigators Collaborative Network on Infections in Canada. The Pediatric Infectious Disease Journal 1999;18(10):866-9. [DOI] [PubMed] [Google Scholar]
Cane 2001
- Cane PA. Molecular epidemiology of Respiratory Syncytial Virus. Reviews in medical virology 2001;11(2):103-16. [DOI] [PubMed] [Google Scholar]
CFF 2009
- Cystic Fibrosis Foundation. Newborn screening for cystic fibrosis. http://www.cff.org/GetInvolved/Advocate/NewbornScreening (accessed December 03, 2009).
Colasurdo 2006
- Colasurdo GN, Fullmer JJ, Elidemir O, Atkins C, Khan AM, Stark JM. Respiratory syncytial virus infection in a murine model of cystic fibrosis. Journal of Medical Virology 2006;78(5):651-8. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta-analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Dupont 1990
- Dupont WD, Plummer WD. Power and Sample Size Calculations: A Review and Computer Program. Controlled Clinical Trials 1990;11:116-28. [DOI] [PubMed] [Google Scholar]
Efthimiou 1984
- Efthimiou J, Hodson ME, Taylor P, Taylor AG, Batten JC. Importance of viruses and Legionella pneumophila in respiratory exacerbations of young adults with cystic fibrosis. Thorax 1984 Feb;39(2):150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feltes 2003
- Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Journal of Pediatrics 2003;143(4):532-40. [DOI] [PubMed] [Google Scholar]
Fenton 2004
- Fenton C, Scott LI, Plosker GL. Palivizumab: A review of its use as prophylaxis for serious respiratory syncytial virus infection. Pediatric Drugs 2004;6(3):177-97. [DOI] [PubMed] [Google Scholar]
Giebels 2008
- Giebels K, Marcotte JE, Podoba J, Rousseau C, Denis MH, Fauvel V, et al. Prophylaxis against respiratory syncytial virus in young children with cystic fibrosis. Pediatric Pulmonology 2008;43(2):169-74. [DOI] [PubMed] [Google Scholar]
Glezen 1986
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. American Journal of Diseases in Children 1986;140(6):543-6. [DOI] [PubMed] [Google Scholar]
Henderson 2005
- Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM. Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatr Allergy Immunol 2005 Aug;16(5):386-92. [DOI] [PubMed] [Google Scholar]
Hiatt 1999
- Hiatt PW, Grace SC, Kozinetz CA, Raboudi SH, Treece DG, Taber LH, et al. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 1999;103(3):619-26. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistence in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
IMpact‐RSV 1998
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102(3):531-7. [PubMed] [Google Scholar]
Khan 1995
- Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1995;151(4):1075-82. [DOI] [PubMed] [Google Scholar]
Kirchner 1996
- Kirchner KK, Wagener JS, Khan TZ, Copenhaver SC, Accurso FJ. Increased DNA levels in bronchoalveolar lavage fluid obtained from infants with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1996;154(5):1426-9. [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ 2006;333(7568):597-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leader 2002
- Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatric Infectious Disease Journal 2002;21(7):629-32. [DOI] [PubMed] [Google Scholar]
McCormick 2007
- McCormick J, Southern KW. A survey of palivizumab for infants with cystic fibrosis in the UK. Archives of disease in childhood 2007;92(1):87-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mejias 2004
- Mejias A, Chavez-Bueno S, Rios AM, Saavedra-Lozano J, Aten MF, Hatfield J, et al. Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrobial Agents and Chemotherapy 2004;48(5):1811-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS 2009
- National Health Service. Newborn Blood Spot screening across the UK. http://www.screening.nhs.uk/bloodspot-compare (accessed December 03, 2009).
Pedraz 2003
- Pedraz C, Carbonell-Estrany X, Figueras-Aloy J, Quero J. Effect of palivizumab prophylaxis in decreasing respiratory syncytial virus hospitalizations in premature infants. Pediatric Infectious Disease Journal 2003;22(9):823-7. [DOI] [PubMed] [Google Scholar]
Piedimonte 2004
- Piedimonte G, Hegele RG, Auais A. Persistent airway inflammation after resolution of respiratory syncytial virus infection in rats. Pediatric Research 2004;55(4):657-65. [DOI] [PubMed] [Google Scholar]
Pohl 1992
- Pohl C, Green M, Wald ER, Ledesma-Medina J. Respiratory syncytial virus infections in pediatric liver transplant recipients. Journal of Infectious Diseases 1992;165(1):166-9. [DOI] [PubMed] [Google Scholar]
Purcell 2004
- Purcell K, Fergie J. Driscoll Children's Hospital respiratory syncytial virus database: risk factors, treatment and hospital course in 3308 infants and young children, 1991 to 2002. Pediatric Infectious Disease Journal 2004;23(5):418-23. [DOI] [PubMed] [Google Scholar]
Red Book 2009
- Physician's Desk Reference. Red Book Drug Topics. Montvale, NJ: Thomson Reuters, 2009. [Google Scholar]
RevMan 2011 [Computer program]
- Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roeckl‐Wiedmann 2003
- Roeckl-Wiedmann I, Liese JG, Grill E, Fischer B, Carr D, Belohradsky BH. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. European Journal of Pediatrics 2003;162(4):237-44. [DOI] [PubMed] [Google Scholar]
SIGN 2006
- Scottish Intercollegiate Guidelines Network (SIGN). Bronchiolitis in Children: A National Clinical Guideline. www.sign.ac.uk/pdf/sign91.pdf (accessed 22 January 2009).
Sigurs 1995
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Björkstén B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics 1995;95(4):500-5. [PubMed] [Google Scholar]
Sigurs 2000
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1501-7. [DOI] [PubMed] [Google Scholar]
Sigurs 2005
- Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, Kjellman B. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. American Journal of Respiratory and Critical Care Medicine 2005;171(2):137-41. [DOI] [PubMed] [Google Scholar]
Simoes 2007
- Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. Journal of Pediatrics 2007;151(1):34-42, 42.e1. [DOI] [PubMed] [Google Scholar]
Singleton 2003
- Singleton R, Dooley L, Bruden D, Raelson S, Butler JC. Impact of palivizumab prophylaxis on respiratory syncytial virus hospitalizations in high risk Alaska Native infants. Pediatric Infectious Disease Journal 2003;22(6):540-5. [DOI] [PubMed] [Google Scholar]
Southern 2007
- Southern KW, Munck A, Pollitt R, Travert G, Zanolla L, Dankert-Roelse J, et al. A survey of newborn screening for cystic fibrosis in Europe. Journal of Cystic Fibrosis 2007;6(1):57-65. [DOI] [PubMed] [Google Scholar]
Speer 2008
- Speer ME, Fernandes CJ, Boron M, Groothuis JR. Use of palivizumab for prevention of hospitalization as a result of respiratory syncytial virus in infants with cystic fibrosis. The Pediatric Infectious Disease Journal 2008;27(6):559-61. [DOI] [PubMed] [Google Scholar]
Stein 1999
- Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354(9178):541-5. [DOI] [PubMed] [Google Scholar]
Tarran 2005
- Tarran R, Button B, Picher M, Paradiso AM, Ribeiro CM, Lazarowski ER, et al. Normal and cystic fibrosis airway surface liquid homeostasis. The effects of phasic shear stress and viral infections. Journal of Biological Chemistry 2005;280(42):35751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2003
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179-86. [DOI] [PubMed] [Google Scholar]
Thorburn 2009
- Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus (RSV) infection. Archives of Disease in Childhood 2009;94(2):99-103. [DOI: 10.1136/adc.2008.139188] [PMID: ] [DOI] [PubMed] [Google Scholar]
Vicente 2003
- Vicente D, Montes M, Cilla G, Perez-Yarza EG, Perez-Trallero E. Hospitalization for respiratory syncytial virus in the pediatric population in Spain. Epidemiology and Infection 2003;131(2):867-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1984
- Wang EE, Prober CG, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. New England Journal of Medicine 1984;311(26):1653-8. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang EEL, Tang NK. Immunoglobulin for preventing respiratory syncytial virus infection. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD001725. [DOI: 10.1002/14651858.CD001725.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Robinson 2010
- Robinson KA, Odelola OA, Saldanha IJ, Mckoy NA. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No: CD007743. [DOI: 10.1002/14651858.CD007743.pub2] [DOI] [Google Scholar]
Robinson 2012
- Robinson KA, Odelola OA, Saldanha IJ, Mckoy NA. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 2. Art. No: CD007743. [DOI: 10.1002/14651858.CD007743.pub3] [DOI] [PubMed] [Google Scholar]
Robinson 2013
- Robinson KA, Odelola OA, Saldanha IJ, Mckoy NA. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD007743. [DOI: 10.1002/14651858.CD007743.pub4] [DOI] [PubMed] [Google Scholar]
Robinson 2014
- Robinson KA, Odelola OA, Saldanha IJ. Palivizumab for prophylaxis against respiratory syncytial virus infection in children with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 5. Art. No: CD007743. [DOI: 10.1002/14651858.CD007743.pub5] [DOI] [PubMed] [Google Scholar]


