Abstract
Background
Pseudomonas aeruginosa is the most common bacterial pathogen causing lung infections in people with cystic fibrosis and appropriate antibiotic therapy is vital. Antibiotics for pulmonary exacerbations are usually given intravenously, and for long‐term treatment, via a nebuliser. Oral anti‐pseudomonal antibiotics with the same efficacy and safety as intravenous or nebulised antibiotics would benefit people with cystic fibrosis due to ease of treatment and avoidance of hospitalisation. This is an update of a previous review.
Objectives
To determine the benefit or harm of oral anti‐pseudomonal antibiotic therapy for people with cystic fibrosis, colonised with Pseudomonas aeruginosa, in the: 1. treatment of a pulmonary exacerbation; and 2. long‐term treatment of chronic infection.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings.
We contacted pharmaceutical companies and checked reference lists of identified trials.
Date of last search: 08 July 2016.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing any dose of oral anti‐pseudomonal antibiotics, to other combinations of inhaled, oral or intravenous antibiotics, or to placebo or usual treatment for pulmonary exacerbations and long‐term treatment.
Data collection and analysis
Two authors independently selected the trials, extracted data and assessed quality. We contacted trial authors to obtain missing information.
Main results
We included three trials examining pulmonary exacerbations (171 participants) and two trials examining long‐term therapy (85 participants). We regarded the most important outcomes as quality of life and lung function. The analysis did not identify any statistically significant difference between oral anti‐pseudomonal antibiotics and other treatments for these outcome measures for either pulmonary exacerbations or long‐term treatment. One of the included trials reported significantly better lung function when treating a pulmonary exacerbation with ciprofloxacin when compared with intravenous treatment; however, our analysis did not confirm this finding. We found no evidence of difference between oral anti‐pseudomonal antibiotics and other treatments regarding adverse events or development of antibiotic resistance, but trials were not adequately powered to detect this. None of the studies had a low risk of bias from blinding which may have an impact particularly on subjective outcomes such as quality of life. The risk of bias for other criteria could not be clearly stated across the studies.
Authors' conclusions
We found no conclusive evidence that an oral anti‐pseudomonal antibiotic regimen is more or less effective than an alternative treatment for either pulmonary exacerbations or long‐term treatment of chronic infection with P. aeruginosa. Until results of adequately‐powered future trials are available, treatment needs to be selected on a pragmatic basis, based upon any available non‐randomised evidence, the clinical circumstances of the individual, the known effectiveness of drugs against local strains and upon individual preference.
Keywords: Adult; Child; Humans; Pseudomonas aeruginosa; Administration, Oral; Anti‐Bacterial Agents; Anti‐Bacterial Agents/adverse effects; Anti‐Bacterial Agents/therapeutic use; Chronic Disease; Cystic Fibrosis; Cystic Fibrosis/complications; Cystic Fibrosis/microbiology; Pseudomonas Infections; Pseudomonas Infections/drug therapy; Randomized Controlled Trials as Topic; Respiratory Tract Infections; Respiratory Tract Infections/drug therapy; Respiratory Tract Infections/microbiology; Treatment Outcome
Plain language summary
Oral antibiotics for treating infection with Pseudomonas aeruginosa in people with cystic fibrosis
Review question
We looked for evidence that antibiotics that are swallowed can treat Pseudomonas aeruginosa infections in people with cystic fibrosis.
Background
Treatment of Pseudomonas aeruginosa lung infection is very important in managing cystic fibrosis lung disease. If oral (taken by mouth) antibiotics are as effective and safe for treating infection with Pseudomonas aeruginosa as intravenous (given into a vein) or nebulised (breathed in as a mist) antibiotics the quality of life of people with cystic fibrosis would improve as it would be easier to administer the drugs administration and would avoid being admitted to hospital.
We looked for trials in which people had equal chances of being treated with oral antibiotics or an alternative treatment for Pseudomonas aeruginosa infections. We thought the most important outcomes to find results for were quality of life and lung function. This is an updated version of a previous review.
Search date
The evidence is current to: 08 July 2016.
Study characteristics
We included five trials with 256 participants. Three trials included people experiencing a flare up of disease (171 participants) and two trials looked at long‐term therapy (85 participants).
Key results
We found no conclusive evidence to show that oral antibiotics were more or less effective than an alternative treatment for either flare ups of disease or long‐term treatment of chronic infection with Pseudomonas aeruginosa. One of the trials with volunteers being treated for a flare up of disease reported significantly better lung function when using ciprofloxacin compared with intravenous treatment; but we did not agree with this finding when we analysed the same data. We did not find any evidence of differences between oral antibiotics and other treatments in terms of adverse events or the development of antibiotic resistance, but we do note that the trials were not designed to detect such differences.
Until the results of large trials are available, people should choose their treatment on a practical basis, basing decisions on any available evidence, their clinical circumstances, the known effectiveness of drugs against local strains of the bug and individual preference.
Quality of the evidence
The evidence we found was limited. The trials were very different in terms of design, drugs used, length of treatment and follow up and the outcomes measured. We judged the trials to be at different risks of bias, but we did not think any of them had a low risk of bias from blinding, which might affect the results of subjective outcomes like quality of life.
Background
Description of the condition
A consequence of the genetic abnormality in people with cystic fibrosis (CF) is an increased susceptibility to chronic lung infections, resulting in lung damage (FitzSimmons 1996). This lung damage is progressive, ultimately leading to respiratory failure; the principal cause of CF‐related mortality and morbidity (FitzSimmons 1993). By the end of the first decade of life, Pseudomonas aeruginosa (P. aeruginosa) is the predominant bacterial pathogen causing infection in the lungs of people with CF (Wang 2001). The CFF data registry reports that approximately 65% to 70% of 18 to 24 year olds have P. aeruginosa infection (CFF 2008). By 18 years of age, 80% of individuals are colonised with P. aeruginosa (Rajan 2002). In 2009 Millar reported that colonisation rates had remained stable between 1985 and 2005 at 77% to 82% (Millar 2009). Infection with P. aeruginosa seems to precede chronic infection (also known as colonisation) by 6 to 12 months (West 2002), and once chronic infection is established, there is evidence that mucoid strains of the isolates prevail and in progressively higher density (Burns 2001; Nixon 2001; Rosenfeld 2001). Additionally, it has been suggested that there is a relationship between the onset of chronic infection and increased morbidity (Kosorok 2001; Parad 1999).
Description of the intervention
Appropriate antibiotic therapy against the bacteriological pathogens in the respiratory tract is a vital component in managing CF lung disease (Ratjen 2006). Anti‐pseudomonal antibiotics are used in three clinical settings (Gibson 2003): to attempt eradication of P. aeruginosa at first evidence of infection so as to delay chronic infection that leads to progressive lung damage; as long‐term treatment in chronic infection to slow the decline in respiratory function and reduce frequency and morbidity of pulmonary exacerbations; as antibiotic treatment in pulmonary exacerbations to relieve symptoms and restore respiratory function to baseline values (Gibson 2003).
For each indication, there is a choice of antibiotics and method of administration (i.e. intravenous (IV), oral, nebulised). For long‐term therapy current evidence recommends the use of nebulised antibiotics (Döring 2000). For treating moderate to severe pulmonary exacerbations, IV administration of two different classes of antibiotics is suggested to be most effective (Döring 2000). This requires IV access and hospitalisation or home care which are costly and a major inconvenience for the individual with CF.
Oral anti‐pseudomonal antibiotics with the same efficacy and safety as the afore‐mentioned methods would improve quality of life of people with CF due to ease of drug administration. Both nebulised and IV treatments require significantly more time compared to oral treatments. Often the administration of IV antibiotics requires hospitalisation rather than home treatment, the subject of another Cochrane Review (Balaguer 2012). Furthermore, the administration of IV antibiotics may cause discomfort and is potentially a source of infection. In 1985, ciprofloxacin, now the most commonly used fluoroquinolone antibiotic for CF, was introduced as an effective oral treatment against P. aeruginosa (Döring 2000).
How the intervention might work
Ciprofloxacin has been shown to have excellent activity against a variety of micro‐organisms found in bronchial sputum of children and adults with CF (Richard 1997; Schaad 1997).
There is concern that the wide use of oral anti‐pseudomonal antibiotics has led to the emergence of resistant micro‐organisms (Ball 1990), but it is not clear how great the risk is of resistant P. aeruginosa developing after treatment with these antibiotics or the real effect of it on the disease process. The adverse effects of these drugs have been well described, for example central nervous system effects, phototoxicity, gastrointestinal effects and joint toxicity (Ball 1986; Patterson 1991). Generally pregnancy is a contra‐indication to use of these drugs, although it is difficult to know the level of risk (Schaefer 1996). In most clinical settings there are safe alternatives. Furthermore, while the risk of arthropathy in children is probably not sufficient to avoid use when there is benefit, fluoroquinolone‐induced resistance remains a concern (Schaad 2007).
Why it is important to do this review
The use of oral anti‐pseudomonal antibiotics to delay the onset of chronic infection with P. aeruginosa is covered in another Cochrane Review (Langton Hewer 2014). Evidence of the effect of macrolide antibiotics in people with CF and chronic infection with P. aeruginosa is discussed in another Cochrane Review (Southern 2012). We aim to assess oral anti‐pseudomonal antibiotics for people with CF chronically infected with P. aeruginosa, both as a treatment for pulmonary exacerbations and as a long‐term treatment in chronic infection.
This is an update of previous reviews (Remmington 2007; Remmington 2013).
Objectives
To determine the benefits or harms, or both, of oral anti‐pseudomonal antibiotic therapy for people with CF who are colonised with P. aeruginosa in two clinical settings:
treatment of a pulmonary exacerbation: and
long‐term treatment of chronic respiratory tract infection.
Methods
Criteria for considering studies for this review
Types of studies
Randomised (RCTs) and quasi‐randomised trials.
Types of participants
Adults and children (with all levels of disease severity) diagnosed with CF clinically and confirmed with sweat test or genetic testing or both.
Participants to have chronic infection with P. aeruginosa. We arbitrarily selected the UK Cystic Fibrosis Trust's definition of chronic infection, i.e. the culture of P. aeruginosa on two or more occasions over a six‐month period prior to the start of the trial (CF Trust 2004). A post hoc change to the review was made and we included trials in which participants were described as chronically infected, even if no further details were given.
Types of interventions
Oral anti‐pseudomonal antibiotics, given in any dose, compared with other combinations of inhaled, oral or IV antibiotics, or with placebo or with usual treatment (e.g. for long‐term treatment of chronic infection, no antibiotic treatment), for:
treatment of a pulmonary exacerbation, one course of oral anti‐pseudomonal antibiotics for less than one month;
long‐term treatment in chronic infection, course(s) of oral anti‐pseudomonal antibiotics of one month more.
A pulmonary exacerbation was regarded as an increase in symptoms requiring additional antibiotic treatment. Long‐term treatment was defined as any antibiotic regimen outside the treatment of a pulmonary exacerbation with the aim of preventing exacerbation of P. aeruginosa infection.
Trials which evaluated oral anti‐pseudomonal antibiotics for eradication of P. aeruginosa are the subject of another Cochrane Review (Langton Hewer 2014) and were not eligible for inclusion.
Types of outcome measures
Treatment of a pulmonary exacerbation
Primary outcomes
Quality of life (measured by a validated tool such as Cystic Fibrosis Questionnaire‐Revised version (CFQ‐R (Quittner 2009)) and Cystic Fibrosis Quality of Life Questionnaire (CFQoL (Gee 2000)))
-
Lung function
forced expiratory volume in one second (FEV1)
forced vital capacity (FVC)
Secondary outcomes
Weight
Time to next pulmonary exacerbation
Adverse effects of antibiotics used, e.g. abnormal liver function, diarrhoea, vomiting, renal and auditory impairment, sensitivity reactions (e.g. skin rash), bronchospasm, candidiasis
Frequency of need for additional antibiotic use and number of days receiving additional antibiotics
Isolation of antibiotic‐resistant strains of P. aeruginosa or other micro‐organisms with or without antibiotic resistance
Long‐term treatment for chronic infection of P. aeruginosa
Primary outcomes
Quality of life (measured by a validated tool such as Cystic Fibrosis Questionnaire‐Revised version (CFQ‐R (Quittner 2009)) and Cystic Fibrosis Quality of Life Questionnaire (CFQoL (Gee 2000)))
-
Lung function
FEV1
FVC
Mortality
Secondary outcomes
Time to next pulmonary exacerbation
Weight, growth velocity
Adverse effects of antibiotics used, e.g. abnormal liver function, diarrhoea, vomiting, renal and auditory impairment, sensitivity reactions (e.g. skin rash), bronchospasm, candidiasis
Number of admissions to hospital and number of days spent as an inpatient
Frequency of need for additional courses of antibiotics and number of days receiving additional antibiotics
Isolation of antibiotic‐resistant strains of P. aeruginosa or other micro‐organisms with or without antibiotic resistance
Search methods for identification of studies
Electronic searches
Relevant trials were identified from the Group's Cystic Fibrosis Trials Register using the terms: antibiotics AND (pseudomonas OR mixed) AND (oral OR *stated).
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of The Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group Module.
Date of the most recent search of the Group's Cystic Fibrosis Trials Register: 08 July 2016.
Searching other resources
We contacted pharmaceutical companies that manufacture oral anti‐pseudomonal antibiotics for any information on any relevant trials. We also checked the reference lists of all trials to identify further relevant trials.
Data collection and analysis
We included an arbitrary definition of chronic infection in the 'Types of participants' section. However, several trials, whilst stating that participants were chronically infected (as described below in the 'Description of studies' section) did not completely fulfil our definition. However, we did not feel that it was appropriate to exclude these trials on these grounds. Therefore, we subsequently made a post hoc change to the protocol to enable us to include these trials, i.e. we included trials in which participants were described as chronically infected, even if no further details were given.
Furthermore, we have regarded the development of antibiotic resistance as a result of therapy (a resistant strain that emerges soon after and in relation to antibiotic treatment) separately to a strain which is there at baseline and which does not respond to antibiotic treatment, i.e. persists.
Selection of studies
Two authors (TR, NJ) independently applied the inclusion criteria to all potential trials. We performed this without blinding. There was no discrepancy between authors in trial selection.
Data extraction and management
Two authors (TR, NJ) independently extracted the data using a customised data extraction form. Where information was lacking, we contacted primary authors for clarification.
For treatment of a pulmonary exacerbation, we measured outcomes at less than a week, one to two weeks, more than two weeks to three weeks, more than three weeks to four weeks. We also considered additional follow‐up data recorded at other time periods.
For long‐term treatment for chronic infection of P. aeruginosa, we measured outcomes at one month, up to three months, up to six months, up to twelve months and then annually thereafter. For future updates, if outcome data are recorded at other time periods, we will consider examining these as well.
Assessment of risk of bias in included studies
Two authors (TR, NJ) assessed each trial using a simple form and followed the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We assessed the following domains as having either a low, unclear or high risk of bias.
Randomisation ('Low risk' ‐ random number table, computer‐generated lists or similar methods; 'Unclear risk' ‐ described as randomised, but no details given; 'High risk' ‐ e.g. alternation, the use of case record numbers, and dates of birth or day of the week).
Concealment of allocation ('Low risk' ‐ e.g. list from a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed opaque envelopes; 'Unclear risk' ‐ not described; 'High risk' ‐ if allocation sequence was known to, or could be deciphered by the investigators who assigned participants or if the trial was quasi‐randomised).
Blinding (of participants, personnel and outcome assessors) ('Low risk' ‐ e.g. there was no blinding, but we judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding, or at least outcome assessors were blinded; 'Unclear risk' ‐ not described; 'High risk' ‐ e.g. no or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding, or blinding was attempted, but likely to have been broken).
Incomplete outcome data (Whether investigators used an intention‐to‐treat analysis) ('Low risk' ‐ e.g. no missing data, or missing data have been imputed using appropriate methods; 'Unclear risk' ‐ e.g. insufficient reporting of attrition/exclusions; 'High risk' ‐ e.g. reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups).
Selective outcome reporting ('Low risk' ‐ e.g. the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; 'Unclear risk' ‐ e.g. insufficient information to permit judgement; 'High risk' ‐ e.g. not all of the study’s pre‐specified primary outcomes have been reported).
Other potential sources of bias ('Low risk' ‐ the study appears to be free of other sources of bias; 'Unclear risk' ‐ e.g. insufficient information to assess whether an important risk of bias exists; 'High risk' ‐ e.g. had a potential source of bias related to the specific study design used, or had extreme baseline imbalance).
We also reported on whether the investigators had performed a sample‐size calculation.
We compared assessments and resolved any inconsistencies by discussion.
Measures of treatment effect
For binary outcome measures, in order to allow an intention‐to‐treat analysis, we sought data on the number of participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the individual was later thought to be ineligible or otherwise excluded from treatment or follow up. We calculated a pooled estimate of the treatment effect for each outcome across trials using relative risk where appropriate.
For continuous outcomes, we recorded either mean relative change from baseline for each group or mean post‐treatment or post‐intervention values and standard deviation. If standard errors had been reported (and if it were possible) we planned to convert these to standard deviations. We calculated a pooled estimate of treatment effect by calculating the weighted mean difference. We are aware that some lung function data are skewed and therefore cannot be entered and analysed within RevMan (Review Manager (RevMan) 2011). Where this is the case we have reported the results narratively.
We have also reported count data narratively.
Unit of analysis issues
One of the trials included in the review was cross‐over in design, but the abstract did not contain any data for analysis (Wang 1988). Ideally when conducting a meta‐analysis combining results from cross‐over trials we would have liked to use the inverse variance methods that are recommended by Elbourne (Elbourne 2002). However, due to restrictions on the data that were available from the included trial, the only method that we have been able to use was to treat the cross‐over trial as if it was a parallel trial (assuming a correlation of zero as the most conservative estimate). Elbourne says that this approach produces conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant appears in both the treatment and control group, so the two groups are not independent.
Dealing with missing data
We assessed whether the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals. We contacted trial authors of five trials for clarification on any missing information, and following correspondence, these have all been excluded from the review (Black 1991; Kurz 1987; Postnikov 2001b; Romano 1991; Rubio 1987).
Assessment of heterogeneity
When sufficient trials are included in the review, we plan to perform a sensitivity analysis based on the methodological quality of the trials, including and excluding quasi‐randomized trials. We plan to assess the degree of heterogeneity between trials using the I2 statistic (Higgins 2003). This measure describes the percentage of total variation across studies that are due to heterogeneity rather than by chance (Higgins 2003). The values of I2 lie between 0% and 100%, and a simplified categorization of heterogeneity that we plan to use is of low (I2 value of 25%), moderate (I2 value of 50%), and high (I2 value of 75%) (Higgins 2003). If we find significant heterogeneity (over 50%), we will investigate the possible causes further by performing subgroup analyses based on the methodological quality of the included trials and the condition of the individuals (i.e. severity of disease, duration and type of treatment e.g. single or combined treatment). If no significant heterogeneity is identified, we will compute pooled estimates of the treatment effect for each outcome under a fixed effect model.
Assessment of reporting biases
In the 'Characteristics of included studies' table we have reported when measurements were taken by the primary investigators during the trial, what measurements were reported within the published paper and what data we reported in the review.
Within the review we have not reported baseline data. We have reported end of treatment data in line with the time‐frames which we pre‐specified in the protocol and additionally have included follow‐up data from one of the short‐term trials (Hodson 1987). Since this is longitudinal data we accept that we have treated these data as independent, although in reality they are not.
When a sufficient number of trials are included, we will attempt to assess whether our review is subject to publication bias by using a funnel plot. If asymmetry is detected, causes other than publication bias will be explored.
Data synthesis
We analysed the two clinical settings (pulmonary exacerbations and long‐term treatment of chronic infection) separately. We were only able to analyse data from four out of five included trials. Most of the outcome measures included in the meta‐analysis consisted of data from only one or two trials (Data and analyses).
Subgroup analysis and investigation of heterogeneity
If we had been able to include sufficient number of trials, we planned to split the trials by whether or not they fulfilled our definition of chronic infection or not. There were insufficient trials to do this, but this planned subgroup analysis will be carried out if sufficient trials are included in a future update of this review. When sufficient data are available, different antibiotic regimens will be analysed and compared to each other.
Results
Description of studies
Results of the search
Two authors initially assessed the trials identified in the searches for eligibility on the grounds of trial design, i.e. whether the trials were RCTs or quasi‐randomised controlled trials, and also according to whether participants were stated to be chronically infected with P. aeruginosa. The authors then further assessed the trials remaining after this initial evaluation according the the criteria stated above. Five trials (including 256 participants) are included in the review (Hodson 1987; Richard 1997; Schaad 1997; Sheldon 1993; Wang 1988). Of these five trials, three considered the treatment of a pulmonary exacerbation (171 participants) (Hodson 1987; Richard 1997; Wang 1988) and two trials examined long‐term treatment for chronic infection (85 participants) (Schaad 1997; Sheldon 1993).
A total of 41 trials are listed as excluded. One trial is listed as 'Awaiting assessment' until further details are available to allow the authors to judge eligibility (Xu 2012).
Included studies
Treatment of a pulmonary exacerbation
Three trials reported on this comparison (Hodson 1987; Richard 1997; Wang 1988).
Participants
Two trials included adult participants only (Hodson 1987; Wang 1988) and one trial included children only (Richard 1997).
It was difficult to establish that all participants in these trials had chronic infection with P. aeruginosa. On first reading only one trial clearly fulfilled our definition of 'chronic infection', as described in the 'Types of Participants' section (Hodson 1987). We then contacted trial authors and were able to confirm that one more trial fulfilled our definition (Wang 1988). The remaining trial described participants as being chronically infected (Richard 1997). After contact with the trial authors it was confirmed that the participants enrolled were "chronically colonised with P. aeruginosa, suffering from an acute bronchopulmonary exacerbation caused by P. aeruginosa as confirmed by sputum culture". The trial authors confirmed that the all participants did have more than just one positive sputum culture of P. aeruginosa (Richard 1997).
Interventions
Three trials compared oral with IV interventions (Hodson 1987; Richard 1997; Wang 1988). Of these trials, one 10‐day trial compared oral ciprofloxacin (500 mg three times daily) versus IV azlocillin (5 g three times daily) plus gentamicin (80 mg three times daily) (Hodson 1987); one 14‐day trial compared oral ciprofloxacin (15 mg/kg twice daily) with IV ceftazidine (50 mg/kg three times daily) plus tobramycin (3 mg/kg three times daily) (Richard 1997); one three‐arm trial ,with treatment periods of 14 days, compared oral ciprofloxacin (750 mg twice daily) with IV tobramycin plus ticarcillin versus IV tobramycin plus azlocillin (Wang 1988).
Outcomes
Please refer to the 'Review‐specified outcomes reported in included trials' table in 'Additional tables' for the outcomes reported in each trial (Table 1).
1. Review‐specified outcomes reported in included trials.
| Trial | Quality of life | FEV1 & FVC | Mortality | Time to next pulmonary exacerbation | Weight | Adverse effects | Additional ABs | AB‐resistant strains | Hospital admissions |
| Hodson 1987 | FEV1 & FVC at day 1, day 10 & 6 weeks. | Within 3 months post‐treatment. | Measured, but only at follow up. | Gastro‐intestinal, nervous system and other. | Further IV treatment reported between day 10 and 6 weeks. | P. aerugnosa‐resistant strains measured at day 1, day 10 and 6 weeks. | |||
| Richard 1997 | FEV1 and FVC at baseline, 5‐7 days, 14 days and at follow up at 20‐30 days | Measured and presented combined data over 9 to 30 days. | Gastro‐intestinal, musculoskeletal and other. | Additional antibiotics for new acute pulmonary exacerbations. | Bacteriologic outcome 48 hours before treatment and at the end of treatment (day 14) and at follow up (day 20 ‐ 30). | ||||
| Schaad 1997 | FVC at baseline, 6 weeks (although not presented) and 3 months | Measured, no data presented. | Gastro‐intestinal, nervous system, others. | P. aerugnosa‐resistant strains measured at the end of treatment (3 months). | |||||
| Sheldon 1993 | FEV1 and FVC assessed at baseline and every 3 months. Data reported at baseline and 12 months. | Within 12 months. | Measured at baseline and every 3 months. Data reported from baseline and 12 months. | Gastro‐intestinal. | Further IV treatment reported up to 12 months. | P. aerugnosa‐resistant strains measured at the end of treatment (12 months). | Reported mean number of days in hospital at end of 12 months. | ||
| Wang 1988 |
FEV1: forced expiratory volume at one second
FVC: forced vital capacity
IV: intravenous
P. aeruginosa: Pseudomonas aeruginosa
One trial reported on quality of life (Hodson 1987). All trials reported on lung function and adverse events (Hodson 1987; Richard 1997; Wang 1988). Two trials reported on time to next respiratory tract infection (Hodson 1987; Richard 1997). All three trials reported on isolation of antibiotic‐resistant strains of P. aeruginosa or other micro‐organisms with or without antibiotic resistance (Hodson 1987; Richard 1997; Wang 1988). No trials reported on weight; the frequency of need for additional courses of antibiotics and number of days receiving additional antibiotics.
Design
Two of the included trials were parallel in design (Hodson 1987; Richard 1997) and one of the included trials was cross‐over; having three arms (Wang 1988).
Setting
Two trials were single centre (Hodson 1987; Wang 1988) and one was multicentre carried out in 15 centres in 9 countries (France, Germany, Greece, Hungary, Israel, Italy, Portugal, South Africa and Switzerland) (Richard 1997).
Long‐term treatment for chronic infection of P. aeruginosa
Two trials reported on this comparison (Schaad 1997; Sheldon 1993).
Participants
One trial included adult participants only (Sheldon 1993) and one trial included both adults and children (Schaad 1997).
Again, it was difficult to establish whether all participants in these trials had chronic infection withP. aeruginosa. One trial described participants as being chronically infected (Schaad 1997). After contact with the authors of the Schaad paper, it was confirmed that the participants enrolled were "chronically colonised with P. aeruginosa, suffering from an acute bronchopulmonary exacerbation caused by P. aeruginosa as confirmed by sputum culture". The investigators confirmed that the all participants did have more than just one positive sputum culture of P. aeruginosa (Schaad 1997). The Sheldon paper reported that "Chronic infection implied isolation of P.aeruginosa from at least four sputum samples over the previous two years" (Sheldon 1993). Additionally, it was reported in the Sheldon paper that there was a difference in lung function between the groups at baseline; participants in the ciprofloxacin group started with worse lung function than those in the placebo group (Sheldon 1993).
Interventions
Two trials were included that examined long‐term treatment of chronic infection. One three‐month trial compared oral ciprofloxacin (30 mg/kg/day) versus oral ciprofloxacin (30 mg/kg/day) plus amikacin inhalation therapy (500 mg/day). Ciprofloxacin was given in two doses to a maximum of 1.5 g/day (Schaad 1997). It should be noted, however, that there was a 14‐day intensive hospital therapy with IV antibiotics and inhalation therapy prior to the randomisation for the oral antibiotic trial. A further 12‐month trial compared oral ciprofloxacin (500 mg three times daily) to identical placebo for 10 days every three months for four courses of treatment (Sheldon 1993).
Outcomes
Please refer to the 'Review‐specified outcomes reported in included trials' table in 'Additional tables' for a clear representation of the relevant outcomes reported in each trial (Table 1). One trial reported on quality of life (Sheldon 1993). Both trials reported on: lung function; adverse events; weight, growth velocity; and isolation of antibiotic‐resistant strains or P. aeruginosa or other micro‐organisms with or without antibiotic resistance (Schaad 1997; Sheldon 1993). Neither trial reported on time to next pulmonary exacerbation determined clinically or radiologically or both that cannot be attributed to concurrent isolates of other organisms. One trial reported on the number of admissions to hospital and number of days spent as an inpatient and on the frequency of need for additional courses of antibiotics and number of days receiving additional antibiotics (Sheldon 1993).
Design
Both of the included trials were parallel in design (Schaad 1997; Sheldon 1993).
Setting
Both of the included trials were single centre (Schaad 1997; Sheldon 1993).
Excluded studies
We excluded a total of 41 trials for the following reasons: seven trials were not RCTs or quasi‐RCTs (Denning 1977; Kapranov 1995; Ordonez 2001a; Pirzada 1999; Postnikov 2001a; Scully 1987; Strandvik 1989); in 15 trials not all participants were colonised or infected with P.aeruginosa (Beringer 2012; Bosso 1987; Bosso 1989; Equi 2002; Harrison 1985; Knight 1979; Connett 2015; Loening‐Baucke 1979; Nolan 1982; Owen 1991; Postnikov 2001b; Shapera 1981; Stutman 1987; Weaver 1994; Wolter 2002); three trials reported on macrolides, which we did not consider to be anti‐pseudomonal antibiotics (Anstead 2001; Saiman 2003; Sriram 2003); one trial assessed combined oral and inhaled therapy (no oral therapy alone) (Treggiari 2011); eight trials presented pharmacokinetic results (Cipolli 2001; Davies 1987; Goldfarb 1986; Johansen 1999; Mack 1991; Pai 2006; Smith 1997; Vitti 1975); one trial reported on a Cox‐2 inhibitor (Pukhalsky 2001); one trial reporting on chronic infection did not meet the criteria for treatment duration (Jensen 1987); and one trial compared a combination of oral and inhaled antibiotics for three weeks or three months (Frederiksen 2003). We were not able to clarify the eligibility of four trials after two attempted contacts with the authors (Black 1991; Kurz 1987; Romano 1991; Rubio 1987).
Studies awaiting assessment
One trial comparing 100 mg doxycycline to placebo for treating an exacerbation in people with CF has been listed as 'Awaiting assessment' (Xu 2012). We are not certain whether the participants were chronically infected with P. aeruginosa and therefore have list this trial as 'Awaiting assessment' until we have further information.
Risk of bias in included studies
For detailed information on the risk of bias of each included trial, please refer to the risk of bias tables attached to the 'Characteristics of included studies' section of this review. A summary is also presented in the figures (Figure 1). There are three trials reporting on the treatment of a pulmonary exacerbation (Hodson 1987; Richard 1997; Wang 1988). There are two trials reporting on long‐term treatment (Schaad 1997; Sheldon 1993).
1.
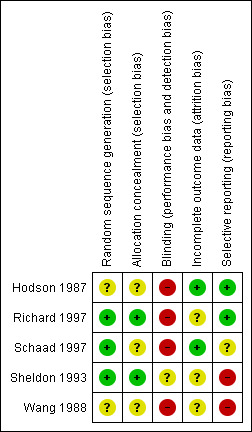
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Treatment of a pulmonary exacerbation
Generation of the allocation sequence
One trial provided information on the randomisation of participants and we assessed this trial as having a low risk of bias (Richard 1997). We judged the remaining two trials as 'unclear' as they failed to provide sufficient information (Hodson 1987; Wang 1988).
Allocation concealment
One trial provided information on the allocation concealment and was judged to have a low risk of bias (Richard 1997). The remaining two trials did not describe any method of allocation concealment and were assessed as 'unclear' (Hodson 1987; Wang 1988).
Long‐term treatment for chronic infection of P. aeruginosa
Generation of the allocation sequence
Both trials provided information on the randomisation of participants and they were judged to have a low risk of bias (Schaad 1997; Sheldon 1993).
Allocation concealment
One trial provided information on the allocation concealment and was thought to have a low risk of bias (Sheldon 1993). The remaining trial did not describe any method of allocation concealment and we assessed this as 'unclear' (Schaad 1997).
Blinding
Treatment of a pulmonary exacerbation
Clinicians or Persons delivering treatment
In three trials it was not possible to blind participants or clinicians given the interventions being compared, which means there is a potential risk of bias (Hodson 1987; Richard 1997; Wang 1988).
Participants
In all three trials it was not possible to blind participants given the interventions being compared (Hodson 1987; Richard 1997; Wang 1988).
Outcome assessor
Two trials reported that outcome assessors were blinded with regards to specific outcomes (see the 'Risk of bias' tables in Characteristics of included studies for further details) (Hodson 1987; Richard 1997). The remaining trial did not provide any information on the blinding of outcome assessors (Wang 1988).
Long‐term treatment for chronic infection of P. aeruginosa
Clinicians or Persons delivering treatment
In one trial it was reported that the person delivering the treatment was blinded to the treatment group (Sheldon 1993). In the remaining trial it was not possible to blind given the interventions being compared (Schaad 1997).
Participants
In one trial which compared oral treatments the participants were blinded to the treatment group (Sheldon 1993). In the remaining trial it was not possible to blind given the interventions being compared (Schaad 1997).
Outcome assessor
One trial was described as "double‐blind" although it was not specifically discussed whether the outcome assessors were blinded (Sheldon 1993). The remaining trial did not provide any information on the blinding of outcome assessors (Schaad 1997).
Incomplete outcome data
Treatment of a pulmonary exacerbation
All three trials in this setting described withdrawals from treatment, further details can be found in the 'Risk of bias' tables in Characteristics of included studies (Hodson 1987; Richard 1997; Wang 1988). None of the included trials specifically stated the use of an intention‐to‐treat analysis when presenting data.
Long‐term treatment for chronic infection of P. aeruginosa
Both trials described withdrawals from treatment, further details can be found in the 'Risk of bias' tables in Characteristics of included studies (Schaad 1997; Sheldon 1993). Neither of the included trials specifically stated the use of an intention‐to‐treat analysis when presenting data.
Selective reporting
Please refer to an additional table for information regarding the measurement and reporting of outcome data (Table 1).
Treatment of a pulmonary exacerbation
In summary, only one trial reported all time‐points that were measured within the trial and at follow up (Hodson 1987). A further trial did not report on one of the time‐points that was stated as having been measured, this was between baseline and end of treatment (Richard 1997). The remaining trial is in abstract form and only reported results narratively (Wang 1988).
Long‐term treatment for chronic infection of P. aeruginosa
In summary, neither trial reported on one of the time‐points that was stated as having been measured and in both cases these were between baseline and end of treatment (Schaad 1997; Sheldon 1993).
For the Schaad trial, we note that the investigators, while stating that lung function was measured at clinic visits, did not report on FEV1 (generally regarded as the standard lung function measurement and reported within most published trials in this area) (Schaad 1997).
Effects of interventions
Where the results generated by our analysis conflict with the results reported within the paper, we have reported both sets of results.
Treatment of a pulmonary exacerbation
All three trials (n = 171) included in this setting compared an oral intervention to an IV intervention (Hodson 1987; Richard 1997; Wang 1988).
Primary outcomes
1. Quality of life
This outcome was not reported on by any of the included trials (Hodson 1987; Richard 1997; Wang 1988).
2. Lung function
a. FEV1
All three trials reported this outcome. Only one reported data which we were able to enter into Data and analyses (Hodson 1987). This paper reported that at end of treatment (day 10), FEV1 improved significantly more in the ciprofloxacin group compared to the azlocillin plus gentamicin group (P < 0.05). However, we note that this result conflicts with the graph which we produced, which shows a non‐significant difference; we are unable to explain this difference from the information provided (Analysis 1.1). Furthermore, the trial authors reported that although FEV1 had decreased at follow up (six weeks) it was still significantly better in the oral ciprofloxacin group (P < 0.001). These data are skewed and therefore have not been entered into the data tables.
1.1. Analysis.
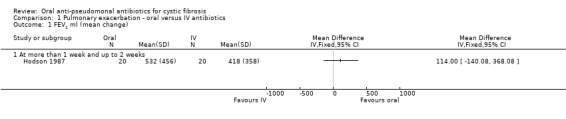
Comparison 1 Pulmonary exacerbation ‐ oral versus IV antibiotics, Outcome 1 FEV1 ml (mean change).
In the remaining two trials, Richard stated that the mean changes in FEV1 at the end of treatment were ciprofloxacin (7.4%) and ceftazidine plus tobramycin (7.5%) (P = 0.97) (Richard 1997). Wang briefly mentioned this outcome in the abstract, but did not present any specific results (Wang 1988).
b. FVC
All three trials reported on this outcome. Again, only one reported data which we were able to enter into the analysis (Hodson 1987). There was no significant difference between FVC at end of treatment (day 10) (Analysis 1.2). The investigators stated that at six weeks FVC had decreased, but was still significantly improved in the oral ciprofloxacin group (P < 0.005); these data are skewed and therefore have not been entered into the data tables. The Richard trial reported mean changes in FVC at the end of treatment for oral therapy (9.3%) and IV therapy (7.7%) (P = 0.60) (Richard 1997). In the abstract, Wang briefly mentioned this outcome, but did not present any specific results (Wang 1988).
1.2. Analysis.
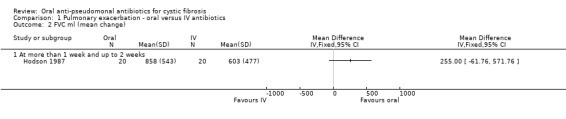
Comparison 1 Pulmonary exacerbation ‐ oral versus IV antibiotics, Outcome 2 FVC ml (mean change).
Secondary outcomes
1. Weight
This outcome was not reported on by any of the included trials (Hodson 1987; Richard 1997; Wang 1988).
2. Time to next pulmonary exacerbation
Two out of the three trials reported this outcome, but not in a form which allowed us to enter data into the data tables (Hodson 1987; Richard 1997). Hodson stated that there was no significant difference between the groups in the number of participants who received further treatment between day 10 and 6 weeks (Hodson 1987). Richard stated that 9 (out of 55) participants who had received ciprofloxacin and 5 (out of 53) who had received parenteral therapy suffered a further acute exacerbation between 9 and 30 days after the end of therapy (Richard 1997).
3. Adverse effects of antibiotics used
In the data tables, we have grouped adverse effects into gastro‐intestinal, central nervous system, musculoskeletal, sensitivity reactions and others.
All three trials reported on this outcome (Hodson 1987; Richard 1997; Wang 1988). Hodson and Richard reported details of the adverse events occurring in their respective trials and when pooled in a meta‐analysis none of these events reached statistical significance (Analysis 1.3). Wang stated within the abstract that "no toxic effects were observed in any of the patients" (Wang 1988).
1.3. Analysis.
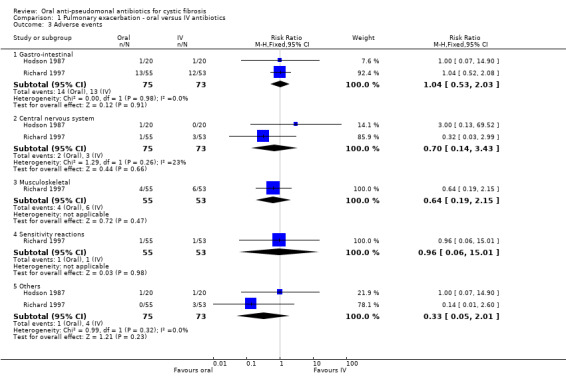
Comparison 1 Pulmonary exacerbation ‐ oral versus IV antibiotics, Outcome 3 Adverse events.
4. Frequency of need for additional antibiotic use and number of days receiving additional antibiotics
Two trials reported on this outcome (Hodson 1987; Richard 1997), but only one reported data which we were able to enter into the data tables (Hodson 1987). This showed no significant difference in the number of participants who received further treatment between day 10 and 6 weeks (Analysis 1.4). The remaining trial reported data "between 9 and 30 days after the end of therapy", due to the structured time periods defined in the protocol, we were not able to enter these data into a meta‐analysis (Richard 1997). During this time period it was reported that six participants in the ciprofloxacin group and three in the ofloxacin group were given additional antibiotics for new acute exacerbations.
1.4. Analysis.
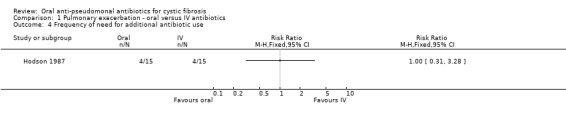
Comparison 1 Pulmonary exacerbation ‐ oral versus IV antibiotics, Outcome 4 Frequency of need for additional antibiotic use.
5. Isolation of antibiotic‐resistant strains of P. aeruginosa or other micro‐organisms with or without antibiotic resistance
Three trials reported on this outcome (Hodson 1987; Richard 1997; Wang 1988). Hodson reported no significant difference in the isolation of antibiotic‐resistant P. aeruginosa (Analysis 1.5) or S. aureus (Analysis 1.6) between the two groups at day 10 and at 6 weeks (Hodson 1987). Richard reported that, at day 14, resistant strains ("persistence") were found in 72% of the oral ciprofloxacin group and 33% of the IV group (Analysis 1.5). However, in the long term the IV group encountered a higher rate of recurrent infection with P. aeruginosa. Neither antibiotic therapy affected the resistant strains (Richard 1997). In the Wang trial weekly sputum cultures did not reveal any emergence or resistance to ciprofloxacin (Wang 1988).
1.5. Analysis.
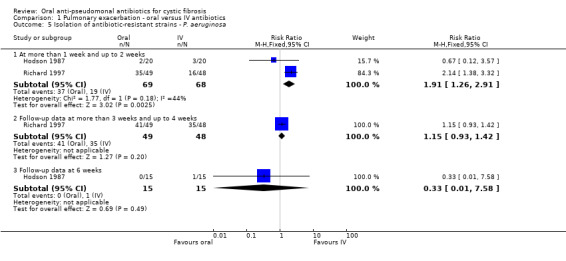
Comparison 1 Pulmonary exacerbation ‐ oral versus IV antibiotics, Outcome 5 Isolation of antibiotic‐resistant strains ‐ P. aeruginosa.
1.6. Analysis.
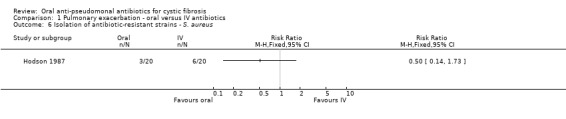
Comparison 1 Pulmonary exacerbation ‐ oral versus IV antibiotics, Outcome 6 Isolation of antibiotic‐resistant strains ‐ S. aureus.
Long‐term treatment for chronic infection of P. aeruginosa
Of the two included trials (n = 85), one compared an oral intervention to placebo (Sheldon 1993) and the other compared an oral intervention to oral plus inhaled therapy (Schaad 1997).
Primary outcomes
1. Quality of life
This outcome was not reported on by either of the included trials (Schaad 1997; Sheldon 1993).
2. Lung function
a. FEV1
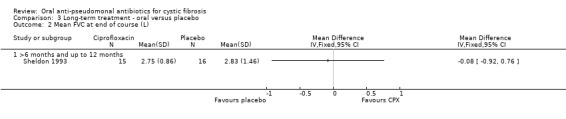
Only the trial comparing oral ciprofloxacin with placebo reported on this outcome (Sheldon 1993). There was no significant difference between treatment groups at the end of therapy (Analysis 3.1). However, after examining the graph produced, there are indications that data are skewed and so results should be interpreted with caution. Furthermore, it should be noted that participants in the ciprofloxacin group started with lower lung function than those in the placebo group.
3.1. Analysis.
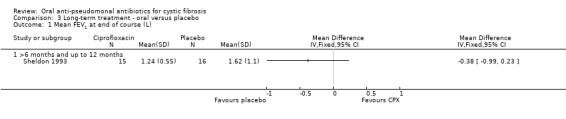
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 1 Mean FEV1 at end of course (L).
b. FVC
Both trials reported on this outcome. The trial comparing oral ciprofloxacin with placebo reported no significant difference between treatment groups at the end of therapy (Analysis 3.2) (Sheldon 1993). However, after examining the graph produced, there are indications that data are skewed and so results should be interpreted with caution. The trial comparing oral ciprofloxacin to oral ciprofloxacin plus inhaled amikacin reported geometric means and ranges which cannot be entered into a meta‐analysis (Schaad 1997). The paper reported that the mean change (range) in FVC % predicted at three months in the ciprofloxacin group was 67% (35% to 123%) compared to 55% (28% to 119%) in the combined group. The authors also stated that the improvements in FVC attained during IV therapy (the pre‐therapy before the start of the oral antibiotic trial, as discussed in the Description of studies section) gradually deteriorated during oral therapy (Schaad 1997).
3.2. Analysis.
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 2 Mean FVC at end of course (L).
3. Mortality
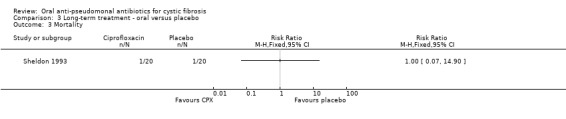
Only Sheldon reported on this outcome; there was one death (n = 20) in the treatment group and one death in the placebo group (n = 20) (Analysis 3.3) (Sheldon 1993). However, the trial was not adequately powered to detect a difference between groups in this outcome.
3.3. Analysis.
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 3 Mortality.
Secondary outcomes
1. Time to next pulmonary exacerbation
Neither trial reported on this outcome (Schaad 1997; Sheldon 1993).
2. Weight, growth velocity
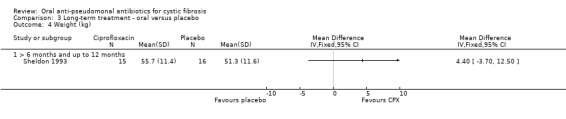
Both trials reported on this outcome. The trial comparing oral ciprofloxacin with placebo found no significant differences in weight between the groups (Sheldon 1993) (Analysis 3.4). The oral ciprofloxacin versus oral ciprofloxacin plus inhaled amikacin trial reported geometric means and ranges which cannot be entered into a meta‐analysis (Schaad 1997). The paper reported the mean change (range) in height at three months as 146.7 cm (103 cm to 187 cm) in the ciprofloxacin alone group, compared to 147.2 cm (103 cm to 180 cm) in the combined group. The authors further stated that growth curves revealed unchanged increase of the height measured by stadiometer along the individual percentile (Schaad 1997).
3.4. Analysis.
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 4 Weight (kg).
3. Adverse effects of antibiotics used
In the data tables, we have grouped adverse effects into gastro‐intestinal, central nervous system, musculoskeletal, sensitivity reactions and others.
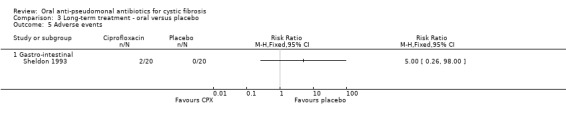
Both trials reported on this outcome. The trial comparing oral ciprofloxacin with placebo found no significant differences between the groups for the gastro‐intestinal events (Analysis 3.5) (Sheldon 1993). The oral ciprofloxacin versus oral ciprofloxacin plus inhaled amikacin trial reported that five participants in each group experienced adverse events, but only reported details on the number of events, not on the number of participants experiencing these events, therefore these data cannot be entered into a meta‐analysis (Schaad 1997). The trial authors reported that there were a total of five events for five participants in the ciprofloxacin group and 10 events for five participants in the combined group. These were split as follows: one gastro‐intestinal event in the ciprofloxacin group and four in the combined group; one central nervous system event in the ciprofloxacin group and four in the combined group; one musculoskeletal event in each of the treatment groups; and two 'other' events in the ciprofloxacin group and one in the combined group (Schaad 1997). We note that neither trial was of sufficient duration to detect any skeletal adverse events.
3.5. Analysis.
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 5 Adverse events.
4. Number of admissions to hospital and number of days spent as an inpatient
One trial reported on this outcome (Sheldon 1993). Participants in the ciprofloxacin group experienced a mean (SD) 7.50 (10.32) number of days in hospital compared to participants in the placebo group who experienced a mean (SD) 6.75 (14.29) number of days in hospital. These count data cannot be entered into the data tables.
5. Frequency of need for additional courses of antibiotics and number of days receiving additional antibiotics
One trial reported on this outcome (Sheldon 1993); it found no significant difference between the groups (Analysis 3.6).
3.6. Analysis.
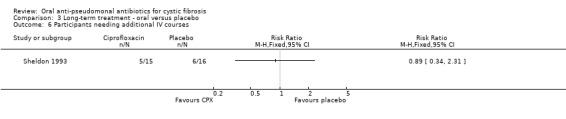
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 6 Participants needing additional IV courses.
6. Isolation of antibiotic‐resistant strains of P. aeruginosa or other micro‐organisms with or without antibiotic resistance
Both trials reported on this outcome. At the end of three months, Schaad reported a total of seven incidences of antibiotic‐resistant strains of P. aeruginosa; however, there was no significant difference between the oral ciprofloxacin versus oral ciprofloxacin plus inhaled amikacin treatment groups (Analysis 2.1) (Schaad 1997). The trial authors reported that at routine follow up, 10 to 15 weeks later, all seven isolates reversed to ciprofloxacin‐susceptible strains of P. aeruginosa (Schaad 1997). The Sheldon trial reported a total of 15 incidences of antibiotic‐resistant strains of P. aeruginosa (Sheldon 1993); however, there was no significant difference between the treatment groups at 12 months (Analysis 3.7). Sheldon also reported transient resistance to ciprofloxacin in four participants in the treatment group and two participants in the placebo group (Sheldon 1993). Furthermore, Sheldon also reported that S. aureus was persistently isolated from four participants in the treatment group and six participants in the placebo group during the trial (Analysis 3.8) (Sheldon 1993).
2.1. Analysis.
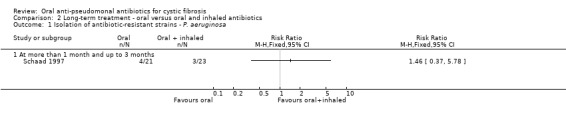
Comparison 2 Long‐term treatment ‐ oral versus oral and inhaled antibiotics, Outcome 1 Isolation of antibiotic‐resistant strains ‐ P. aeruginosa.
3.7. Analysis.
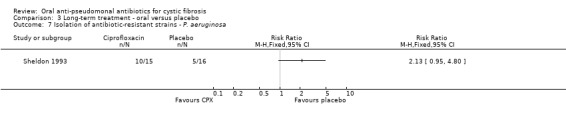
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 7 Isolation of antibiotic‐resistant strains ‐ P. aeruginosa.
3.8. Analysis.
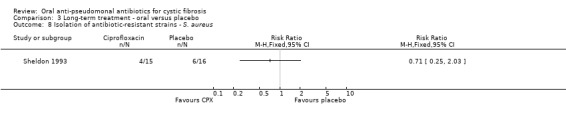
Comparison 3 Long‐term treatment ‐ oral versus placebo, Outcome 8 Isolation of antibiotic‐resistant strains ‐ S. aureus.
Discussion
Current standard treatment for a pulmonary exacerbation of cystic fibrosis (CF) lung disease is intravenous administration of two different classes of antibiotics (Döring 2000). Standard long‐term treatment is to use nebulised antibiotics (Döring 2000). There may be significant advantages of oral treatment for people with CF compared to an intravenous (IV) or inhaled drug regimen. We identified five trials which were eligible for inclusion in the review; three trials of treatment of pulmonary exacerbations and two trials of long‐term treatment for chronic infection of the respiratory tract.
Summary of main results
In summary, we were unable to find sufficient evidence of benefits and harms to provide guidance on the use of an oral anti‐pseudomonal antibiotic regimen (alone or in combination with another therapy) in treating a pulmonary exacerbation. Likewise, we were unable to present any conclusive evidence to show that an oral antibiotic regimen (alone or in combination with another therapy) is more or less effective than any other drug regimen for long‐term treatment of chronic infection with Pseudomonas aeruginosa (P. aeruginosa).
No sufficient data were identified to validate concerns surrounding the emergence of antibiotic resistance as a result of widespread and long‐term use of oral anti‐pseudomonal antibiotics. Similarly, no differences in frequency or severity of adverse effects of antibiotics were found. However, the trials were not adequately powered to detect these differences and may not have been of sufficient duration to detect long‐term adverse events.
Quality of the evidence
All of the trials were published over 15 years ago and did not always report outcome measures which clinicians and consumers currently perceive to be important. The trials were very heterogeneous in terms of design, drugs used, outcomes measured and duration of treatment and follow up. We used an arbitrary definition of chronic infection (CF Trust 2004); however, several trials, whilst stating that participants were chronically infected did not define this term clearly and consistently. After correspondence with several authors to clarify definitions of chronic infection, we were able to make a post hoc change to the review and include trials which we initially thought would be excluded. Furthermore, inconsistencies in expression of results and statistical reporting (e.g. different measures of lung function reported in a variety of ways) made meta‐analysis impossible in most cases. We would need to collect individual patient data from the trial authors to clarify these issues; however, given the age of the trials it is unlikely that this would be possible. It was disappointing that only four trials presented data which we were able to analyse. Most of the outcome measures included in the data tables consisted of data from only one or two trials. An insufficient number of trials and lack of data presented within the included trials meant we were unable to use sensitivity and subgroup analyses to examine for effects of methodological quality of trials, the condition of the individuals (i.e. severity of disease), duration of treatment or type of treatment (e.g. single or combined treatment).
Agreements and disagreements with other studies or reviews
We regarded the most important outcomes as quality of life and clinical improvement. In our analysis, we unable to present any significant results for these outcome measures for any of the comparisons, either for exacerbations or maintenance treatment. However, in her primary paper Hodson reported significant results for lung function when treating a pulmonary exacerbation with oral ciprofloxacin compared to a combination of IV azlocillin and gentamicin (Hodson 1987). The consensus for standard treatment of a pulmonary exacerbation is the use of IV antibiotics, but we were unable to identify any evidence from randomised controlled trials (RCTs) for this. For long‐term maintenance treatment, standard care consists of nebulised antibiotics; we were unable to demonstrate any evidence from RCTs that this is more effective than oral treatment.
Authors' conclusions
Implications for practice.
We found no evidence from RCTs that oral anti‐pseudomonal antibiotics, alone or in combination with another therapy, are any more or less effective in treating acute pulmonary infectious exacerbations or for long‐term treatment of chronic infection in people with CF than other therapies. Until results of adequately powered future trials are available, treatment needs to be selected on a pragmatic basis, based upon any available non‐RCT evidence, the clinical circumstances of the individual, the known effectiveness of drugs against local strains and upon individual preference.
Implications for research.
The effectiveness of oral antibiotics for treatment of lung infection in people with CF who have chronic Pseudomonas aeruginosa infection is an important question for people with CF. The evidence from available RCTs is insufficient to answer the question. Unfortunately, as far as we are aware, there have been no RCTs of this intervention since 1998, and this highlights the need for further research. Future trials should be designed to adequately compare oral treatment with current standard therapies for both acute pulmonary exacerbations and long‐term treatment of chronic infection. This review gives limited evidence of the frequency and variance of outcome measures to plan sample size. In order to enable future pooling of data, trial authors should endeavour to use standard methods and definitions to report on outcomes which are important to people with CF and their carers, such as quality of life, pulmonary exacerbations, antibiotic resistance; all of which are reasonably widely accepted in CF literature.
What's new
| Date | Event | Description |
|---|---|---|
| 13 July 2016 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Group identified 12 new references that were potentially eligible for inclusion in the review. Of these, six were additional references to an already excluded trial (Treggiari 2011); two references to one trial have been listed as 'Awaiting classification' until we can ascertain whether the participants were chronically infected with Pseudomonas aeruginosa (Xu 2012); four references to two trials have been excluded (Beringer 2012; Connett 2015). |
| 13 July 2016 | New citation required but conclusions have not changed | We have not been able to include any new trials or data at this update and therefore our conclusions remain the same. |
History
Protocol first published: Issue 3, 2005 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 16 October 2013 | New citation required but conclusions have not changed | None of the new references identified were eligible for inclusion in this review, hence the conclusions of the review remain the same. |
| 16 October 2013 | New search has been performed | There are no new trials added to the 'Included studies' section of the review. However, three trials have been added to the 'Excluded studies' section (Frederiksen 2003; Rubio 1987; Treggiari 2011). |
| 6 October 2010 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 35 potentially eligible new references, of which one has been listed under Excluded studies (Pai 2006). One trial previously listed under Studies awaiting classification has been excluded following correspondence with the trial author (Postnikov 2001b). Three trials previously listed under Studies awaiting classification have been excluded following no response from two attempted contacts with each author team (Black 1991; Kurz 1987; Romano 1991). A previously included trial has now been excluded (Jensen 1987). While treatment duration was only 14 days, on closer examination of the paper, we noted that the participants did not have a pulmonary exacerbation, but also, given that they were not treated for more than one month they were not eligible for inclusion in the comparison for long‐term treatment for chronic disease. |
| 3 April 2008 | New search has been performed | Search of the Group's Cystic Fibrosis Trials Register identified five new trials, however, none were eligible for inclusion in this review. |
| 3 April 2008 | Amended | Converted to new review format. |
| 18 May 2007 | New citation required and conclusions have changed | Review first published. |
Acknowledgements
We would like to thank Dr Gerard Ryan (Sir Charles Gairdner Hospital, Nedlands, Australia) for his considerable assistance in the formulation of this review and the 2010 update.
We would also like to thank the peer reviewers who commented on the draft protocol or review or both: Chris Hyde, Ashley Jones, Heather McIntosh, Ken Olivier, Alan Smyth, Kevin Southern, Sarah Walters and Karen Welch.
Finally, we would like to thank all the trial authors who provided us with additional information in response to our queries: Clark Inderlied, Tim Jensen, Peter Ostrup Jensen, Claus Moser, Sandia Nousia‐Arvanitakis, S.S. Postnikov, Thomas Rubio, Urs Schaad and Birgitta Strandvik.
Data and analyses
Comparison 1. Pulmonary exacerbation ‐ oral versus IV antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 ml (mean change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 At more than 1 week and up to 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC ml (mean change) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At more than 1 week and up to 2 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Gastro‐intestinal | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.53, 2.03] |
| 3.2 Central nervous system | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.14, 3.43] |
| 3.3 Musculoskeletal | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.19, 2.15] |
| 3.4 Sensitivity reactions | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 15.01] |
| 3.5 Others | 2 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.01] |
| 4 Frequency of need for additional antibiotic use | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Isolation of antibiotic‐resistant strains ‐ P. aeruginosa | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At more than 1 week and up to 2 weeks | 2 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.26, 2.91] |
| 5.2 Follow‐up data at more than 3 weeks and up to 4 weeks | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.93, 1.42] |
| 5.3 Follow‐up data at 6 weeks | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 6 Isolation of antibiotic‐resistant strains ‐ S. aureus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Long‐term treatment ‐ oral versus oral and inhaled antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Isolation of antibiotic‐resistant strains ‐ P. aeruginosa | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At more than 1 month and up to 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Long‐term treatment ‐ oral versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean FEV1 at end of course (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 >6 months and up to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Mean FVC at end of course (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 >6 months and up to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Weight (kg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 > 6 months and up to 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Gastro‐intestinal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Participants needing additional IV courses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Isolation of antibiotic‐resistant strains ‐ P. aeruginosa | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Isolation of antibiotic‐resistant strains ‐ S. aureus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hodson 1987.
| Methods | RCT (generation of allocation sequence & allocation concealment both graded as 'unclear'). Parallel design. Single centre. | |
| Participants | 40 admitted with acute exacerbations of pulmonary symptoms associated with isolation of P. aeruginosa from sputum. 20 randomly allocated to each group. Aged 16 and over, diagnosed with CF and had grown P. aeruginosa consistently in their sputum for at least 6 months, were admitted to hospital with an exacerbation of pulmonary symptoms. All had chronic bronchopulmonary infection, malabsorption, and a sodium concentration in sweat of more than 70mmol/l. The P. aeruginosa isolated in sputum had to be sensitive to CPX, azlocillin and gentamicin. Excluded if had abnormal renal or hepatic function, previous adverse reactions to drugs in trial, were pregnant or taking theophyllines. Mean age: 23 years; range: 18 to 35 years. Sex: 11 males, 9 females in each group. Country: UK. |
|
| Interventions | Each treatment given 3 times a day for 10 days. Azlocillin (5 g) plus gentamicin (80 mg) both given intravenously or ciprofloxacin (500 mg) given orally. Time‐points when measurements were taken during the trial: day 1 (for all 40 participants), day 10 (for all 40 participants), 6 weeks (for 30 participants (15 in each group). On each of the 10 days of treatment temperature, max PEF and sputum weight were recorded. Time‐points reported in the trial: day 1, day 10, 6 weeks. |
|
| Outcomes | Sputum cultured and sensitivities for any isolates assessed by standard disc methods. Sputum weight PEF FEV1* FVC* Blood and liver function tests. Temperature Scores on diary cards: breathing; sputum colour and volume; whether chest felt wheezy or better/same/worse as day 1*. Any side‐effects noted (gastro‐intestinal, nervous system, others)*. CPX participants asked whether they preferred oral to IV treatment. Additional IV treatment required*. Isolation of antibiotic‐resistant strains*. Death within 3 months post‐treatment. Any treatment with IV anti‐pseudomonal drugs within 3 months post‐treatment*. | |
| Notes | Time‐points used in the review: day 10 and follow up at 6 weeks. Sample‐size calculation not discussed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "randomly allocated" but no further information on the methods used. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible to blind participants or clinicians given the interventions being compared. Reported that lung function was tested by an assessor not involved in the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis: not stated. Data recorded for all 40 participants on days 1 and 10, but only 15 in each group evaluated at 6 weeks. Some withdrawals described: 4 participants receiving azlocillin/gentamicin admitted and treated again with IV chemotherapy. 32 out of 40 participants completed diary cards. Sputum weight available for 38 out of 40 participants (19 in each group). 3 deaths by 3 months after start of treatment (1 azlocillin/gentamicin; 2 CPX), all had very severe lung disease at start of trial. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, however, all outcomes listed as being measured at clinic visits were described in full in the results section of the paper. |
Richard 1997.
| Methods | RCT (generation of allocation sequence & allocation concealment both graded as 'adequate'). Parallel design. Multi centre (15 centres in 9 countries). | |
| Participants | 108 people randomised (55 to oral CPX and 53 to parenteral combination therapy). Minimum age of 5 years and whose growth was not completed, hospitalised between May 1993 and April 1995, for treatment of an exacerbation of pulmonary infection. Treatment was confined to those who were infected with P. aeruginosa and microbiologically susceptible to CPX and at least 1 of the comparison drugs. Those people with advanced CF were excluded (Shwachman score < or = 40), so were those with previous or current joint abnormality, CF arthropathy, myasthenia or a history of allergy to quinolones, beta‐lactams or aminoglycosides. Mean age in CPX group: 10.2 years; in combination therapy group: 11.00 years. Age across groups ranged from 5 to 17 years. Sex: 59 males, 49 females in each group. CPX group (32 males, 23 females); combination therapy group (27 males, 26 females). Country: 9 countries. |
|
| Interventions | Each treatment given for 14 days. Oral CPX (15 mg/kg bd, maximum dose, 1500 mg/day) versus IV ceftazidine plus tobramycin (50 mg/kg tds, 3 mg/kg tds, respectively). The dosage was adjusted to achieve to achieve peak plasma concentrations between 6 & 12 mg/l and trough values <2 mg/l. Time‐points when measurements were taken during the trial: at baseline, at 5 to 7 days, at 14 days and at follow up (20 to 30 days). Time‐points reported in the trial: baseline, day 14 and follow up (day 20 to 30) data. |
|
| Outcomes | Shwachman score Chest radiographs (using Chrispin‐Norman score) Severity of acute exacerbations was assessed by a modified acute change clinical score system FEV1 (% of predicted value for height)* FVC (% of predicted value for height)* Baseline sputum samples were taken 48 hr before treatment. Bacteriologic outcome at the end of treatment and at follow up. Physical examination of the joints (knees, hips, shoulders) assessed four times* MRI evaluation Laboratory assessments (Days 5 to 7, chemistry) and at the end of therapy (all) and at follow‐up (haematology) Adverse events Additional antibiotics for new acute exacerbations* | |
| Notes | Time‐points used in the review: day 14 and at follow up (20 to 30 days). Authors confirmed colonisation according to our criteria. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors confirmed assignment was based on random code generated at the Institute of Biometry of Bayer AG, Wuppertal, Germany. |
| Allocation concealment (selection bias) | Low risk | Authors confirmed sealed envelope for each participant specifying individual drug schedule to be opened after enrolment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Clinician/person delivering treatment: not possible (oral vs IV). Participants: not possible (oral vs IV) Outcome assessor: yes (chest radiographs, ultrasound documents, MRI pictures, physical examination of joints undertaken by a specialist (usually a physiotherapist)). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis: no. Withdrawals described. 4 participants on CPX not evaluated ‐ 3 dropped out (1 withdrawal of consent, 1 recognition of previous joint disorder, 1 pneumothorax) and clinician reported indeterminate response in 1 participant. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, however, all outcomes listed as being measured at clinic visits were described in full in the results section of the paper. |
Schaad 1997.
| Methods | RCT (generation of allocation sequence graded as adequate & allocation concealment graded as 'unclear'). Parallel design. Single centre. | |
| Participants | 45 people randomised. 24 male and 21 female. 22 were randomly assigned to maintenance treatment with CPX alone and 23 received CPX with amikacin. 1 female excluded from CPX group after baseline culture showed no P. aeruginosa. Participants admitted because of deterioration in their condition were eligible for inclusion provided that P. aeruginosa was confirmed as the dominant pathogen by sputum culture. Those with advanced stage illness (bernese score, < or = 10) were excluded from entering the study, as were those with impaired cardiac, renal or liver function, hearing or balance disorders or any disease of the skeleton. Age range: 8 to 25 years. 28 participants < 15 years. CPX group mean age 13.4 years (range 4 to 25 years with 13 aged < 15 years). In CPX plus amikacin group mean age 14 years (range 5 to 26 years, with 15 aged < 15 years). Country: Switzerland |
|
| Interventions | Pre‐treatment began with an intensive, 2‐week hospital course of IV ceftazidime (300 mg/kg/day) and amikacin (36 mg/kg/day). Ceftazidime was given in 4 doses to a maximum of 12 g/day and amikacin was given in 3 doses to a maximum of 1.5 mg/kg/day. Each antibiotic was administered as a separate 5 minute IV injection. In all participants IV therapy was supplemented by twice daily inhalation of amikacin (500 mg in 2 ml) administered using a nebuliser. Patients who responded to hospital treatment were randomised to a 3‐month period of outpatient therapy with oral ciprofloxacin (30 mg/kg/day) either alone or in combination with supplemental amikacin inhalation therapy (500 mg/kg/day). CPX was given in 2 doses to a maximum dose of 1.5 g/day. Physiotherapy tds when participants were in hospital and bd thereafter. Inhaled salbutamol (2 ml in 0.9% saline) was administered by nebuliser immediately after each physiotherapy session, and nebulised amikacin was always administered after physiotherapy. Time‐points when measurements were taken during the trial: baseline; 6 weeks; and at 3 months (randomised section). Time‐points reported in the trial: baseline; and 3 months. |
|
| Outcomes | Participants were assessed at the start and end of hospital therapy (pre‐randomisation) and after 6 weeks and 3 months of outpatient treatment. At each assessment: sputum samples were taken for bacteriologic examination; lung function (respiratory rate, forced vital capacity*; residual volume; and airway resistance); weight and height*; adverse events were recorded as free text*. Blood and urine samples were taken for routine haematology, clinical chemistry and urinalysis tests; Strains of isolated P. aeruginosa were tested for antibiotic susceptibility*; Changes in clinical symptoms were scored at the end of maintenance therapy as "improved or "unchanged" compared with the end of hospital therapy, or as "clinical failure". |
|
| Notes | Time‐points used in the review: 3 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors confirmed that a computer‐generated randomisation list was used and strictly adhered to. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Clinician/person delivering treatment: not possible (oral vs oral plus inhaled). Participants: not possible (oral vs oral plus inhaled) Outcome assessor: no information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis: not clear. Withdrawals described. CPX group: 1 participant in the CPX group was excluded because the baseline culture had been negative for PA. CPX plus inhaled amikacin: 2 participants discontinued treatment; 1 (after 3 weeks of therapy) because of abdominal pain, tiredness and loss of concentration; and the other (at 4 weeks) because of subjective breathlessness after amikacin therapy inhalation. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available. The methods section of the paper states that lung function was measured at each assessment and the following were reported in a table for baseline, end of oral and end of IV therapy: respiratory rate, vital capacity, residual volume and airway resistance. One of the standard lung function measurements, FEV1, is not mentioned and it is not clear if this was not measured at each assessment or if the authors have selectively reported this outcome. |
Sheldon 1993.
| Methods | Double‐blind RCT (generation of allocation sequence & allocation concealment both graded as 'adequate'). Parallel design. Single centre. | |
| Participants | 40 randomised. 31 completed the trial. Eligible if over 18 years of age and chronically infected with P. aeruginosa. Participants were excluded from the trial if they had P. aeruginosa resistant to CPX in their sputum culture immediately prior to entering the trial, renal insufficiency, an intention to become pregnant, current treatment with theophyllines or a past history of poor compliance. Mean (SD) age of 15 participants in the active treatment group: 28.3 years (6.06 years) Mean (SD) age of 16 participants in the placebo group: 24.9 years (5.15 years) Sex: active treatment group: 13 males, 2 females; placebo group: 10 males, 6 females. Country: UK |
|
| Interventions | CPX (500 mg) tds or an identical placebo for 10 days every 3 months for 4 courses. Time‐points when measurements were taken during the trial: baseline; every 3 months up to 12 months. Time‐points reported in the trial: baseline; day 10 (every 3 months) for MIC only; final assessment. |
|
| Outcomes | At each visit the participants' clinical symptoms, signs, weight and drug history were recorded*. PEF FEV1* FVC* Oxygen saturation Diary card completed listing details of symptoms, sputum volume and PEF. Breathlessness was graded by the participants using a 3‐point scale*. Sputum volume was recorded using a 5‐point scale. Sputum samples were cultured at the start and finish (day 10) of each course of tablets. Sputum specimens were collected at each outpatient visit and at the end of each treatment period. Susceptibility of isolates of P. aeruginosa were stored for analysis of MIC at the end of the trial period. Mortality Adverse effects* | |
| Notes | Time‐points used in the review: 12 months. In this trial, participants in the ciprofloxacin group started with worse lung function than those in the placebo group. The trial had a power of 80% for detecting a real difference of 200 ml in the improvement of FEV1 between the groups significant at the 5% level. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On enrolment into the trial participants were given consecutive trial numbers, which corresponded to the treatment group randomised before the study. Randomisation of treatment courses was arranged prior to the start of the trial in blocks of 4: 2 each for treatment and placebo. |
| Allocation concealment (selection bias) | Low risk | Treatment courses were prepared by Bayer, none of the staff involved with the trial had knowledge of the treatment allocated to each participant. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Clinician/person delivering treatment: yes
Participants: yes
Outcome assessor: unclear (see below). Described as double‐blinded "None of the staff involved in the study had knowledge of the treatment allocated to each patient". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 withdrawals, all described. 5 participants receiving CPX were withdrawn for the following reasons: poor compliance (2), heart‐lung transplant (1), death (1), nausea & anorexia (1). 4 participants receiving placebo were withdrawn for the following reasons: poor compliance (2), death (1), desire to become pregnant (1). |
| Selective reporting (reporting bias) | High risk | Study protocol not available. All outcomes listed as being measured at clinic visits were described in full in the results section of the paper for baseline and 12 months. However, no data were presented for intermediate clinic visits. |
Wang 1988.
| Methods | RCT (generation of allocation sequence & allocation concealment both graded as 'unclear'). 3‐way cross‐over design. Single centre. | |
| Participants | 23 people randomised. CF adults over 18 years of age with an exacerbation of pulmonary infection. Moderately severe disease, treated during exacerbation of pulmonary infection. Age: "young CF adults over 18 years of age". Country: USA. |
|
| Interventions | Each treatment given for 2 weeks. CPX 750 mg bd versus IV tobramycin plus ticarcillin versus IV tobramycin plus azlocillin. Time‐points when measurements were taken during the trial: before, at week 1 and after completion of treatment. Time‐points reported in the trial: no data reported. Narrative "...after completion…" |
|
| Outcomes | Laboratory tests (before and after completion of treatments) Chest X‐rays Pulmonary function tests* Toxic effects* Sputum cultures (weekly) | |
| Notes | Time‐points used in the review: no specific data reported. Pilot (abstract only). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as "were placed at random on 1 of 3 regimens". |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not possible for clinicians or participants as the comparison was oral versus IV antibiotics. Outcome assessor: not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals: Described as "16 patients received 17 courses of CPX (with 1 receiving CPX twice). All but 1 then went on to receive IV tobramycin plus ticarcillin. Similarly, 4 out of 16 also went on to receive IV tobramycin and azlocillin. The remaining 7 in regimen 3 received tobramycin plus ticarcillin on other admissions". Intention‐to‐treat analysis: unclear. |
| Selective reporting (reporting bias) | High risk | Abstract states data measured: baseline, at week 1 and after completion of treatment. However, paper reports narratively with no data. |
bd: twice daily CF: cystic fibrosis CPX: ciprofloxacin ESR: erythrocyte sedimentation rate FEV1: forced expiratory volume in 1 second FVC: forced vital capacity IV: intravenous MIC: minimum inhibitory concentration MRI: magnetic resonance imaging PEF: peak expiratory flow P. aeruginosa: Pseudomonas aeruginosa RCT: randomised controlled trial SD: standard deviation tds: three times a day *: indicates an outcome used in the review
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anstead 2001 | Macrolide (azithromycin) is not a conventional anti‐pseudomonal drug. |
| Beringer 2012 | Participants received single dose of intravenous doxycycline before 4 weeks of differing doses of oral doxycycline; not clear if participants were chronically infected with P. aeruginosa. |
| Black 1991 | Eligibility for inclusion not clear. Contacted author team on two occasions for clarification of eligibility. No response received. |
| Bosso 1987 | Not all participants colonised or infected with P. aeruginosa. |
| Bosso 1989 | Participants not colonised with P. aeruginosa. |
| Cipolli 2001 | Pharmacokinetic study, no relevant outcomes. |
| Connett 2015 | Particpants not chronically infected with P. aeruginosa. |
| Davies 1987 | Pharmacokinetic study, no relevant outcomes. Colonisation unclear. |
| Denning 1977 | Not an RCT or quasi‐randomised. |
| Equi 2002 | Not all participants colonised with P. aeruginosa. |
| Frederiksen 2003 | A study of a combination of oral and inhaled antibiotic for three weeks compared to three months ‐ no arm where participants received only oral antibiotics. |
| Goldfarb 1986 | A pharmacokinetic trial administering 3 single variable doses. |
| Harrison 1985 | Not all participants were colonised with P. aeruginosa. |
| Jensen 1987 | The participants did not have a pulmonary exacerbation, nor were they treated for more than one month (the eligibility criteria for inclusion in long‐term treatment for chronic disease comparison). |
| Johansen 1999 | Those participants colonised with P. aeruginosa were only part of a pharmacokinetic study ‐ not part of the clinical trial. |
| Kapranov 1995 | Not an RCT or quasi‐randomised. |
| Knight 1979 | Participants not colonised with P. aeruginosa. |
| Kurz 1987 | Eligibility for inclusion not clear. Contacted author team on two occasions for clarification of eligibility. No response received. |
| Loening‐Baucke 1979 | Broad spectrum of disease severity. Not all colonised with P. aeruginosa. |
| Mack 1991 | Pharmacokinetic study, no relevant outcomes. |
| Nolan 1982 | Not all participants colonised. |
| Ordonez 2001a | Not an RCT or quasi‐randomised. |
| Owen 1991 | Not colonised ‐ recruiting newborns. |
| Pai 2006 | Pharmacokinetic study regarding interaction of levofloxacin with calcium carbonate. |
| Pirzada 1999 | Not randomised. Case‐control study. |
| Postnikov 2001a | Not an RCT or quasi‐randomised. |
| Postnikov 2001b | Not all participants colonised with P. aeruginosa. |
| Pukhalsky 2001 | Cox‐2 inhibitor. Not looking at an oral anti‐pseudomonal. |
| Romano 1991 | Eligibility for inclusion not clear. Contacted author team on two occasions for clarification of eligibility. No response received. |
| Rubio 1987 | Unclear if colonisation was a prerequisite for trial entry. Unable to clarify with trial authors. |
| Saiman 2003 | Macrolide (azithromycin) is not a conventional anti‐pseudomonal drug. |
| Scully 1987 | Not an RCT or quasi‐randomised. |
| Shapera 1981 | Not all participants colonised. |
| Smith 1997 | Pharmacokinetic study. |
| Sriram 2003 | Macrolide (azithromycin) is not a conventional anti‐pseudomonal drug. |
| Strandvik 1989 | Trialists 'intended' to treat participants in a random fashion, but actual process not clear. 20 participants were recruited of which 8 received each treatment. Data presented for courses of treatment, rather than for each participant. |
| Stutman 1987 | Not all participants infected or colonised with P. aeruginosa. |
| Treggiari 2011 | Trial looks at combined oral and inhaled therapy (no oral therapy alone). |
| Vitti 1975 | Pharmacological study looking at Interaction of enzyme supplement with antibiotics. |
| Weaver 1994 | Not colonised ‐ recruiting newborns. |
| Wolter 2002 | Participants not all infected with P. aeruginosa and treatment drug was a macrolide. |
P.aeruginosa: Pseudomonas aeruginosa RCT: Randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Xu 2012.
| Methods | Randomised double‐blind placebo‐controlled trial. Single centre. |
| Participants | Inclusion criteria included participants undergoing uncomplicated inpatient CF exacerbation who were colonized with Pseudomonas aeruginosa. |
| Interventions | Oral doxycycline (100 mg) twice a day versus placebo. |
| Outcomes | Primary outcomes: safety and tolerability; change in MMP‐9 activity in sputum and blood samples. Secondary outcomes: measures of inflammatory biomarkers in sputum and blood; change in lung function; sputum and blood doxycycline levels. Sputum and blood samples were collected at the beginning of hospitalization and prior to doxycycline; sputum and blood were also collected at end of hospitalization. |
| Notes | Randomisation was conducted by UAB Research Pharmacy. Participants were followed up 1 month after trial completion with phone call. |
CF: cystic fibrosis
Differences between protocol and review
November 2006: The term colonisation has been replaced with more suitable term 'chronic infection'.
September 2010: Following editorial advice, the previously listed fourth primary outcome for the comparison 'Long‐term treatment for chronic infection of P. aeruginosa' has been amended from 'Frequency of infective respiratory tract exacerbation (time to next exacerbation) determined clinically or radiologically or both that cannot be attributed to concurrent isolates of other organisms' to 'Time to next pulmonary exacerbation'. This outcome has also been moved from 'Primary outcomes' to 'Secondary outcomes' in line with current CFGD Group policy regarding the maximum number of primary outcomes which should be listed within a review.
Contributions of authors
Protocol
Tracey Remmington took the lead on the write up of the protocol with significant input on all draft stages from Nikki Jahnke. Christian Harkensee commented on several draft versions.
Review & updates
Tracey Remmington and Nikki Jahnke independently selected trials and extracted data for inclusion in the review. Each then worked together in inputting the data and drafting the text and should be regarded as joint first authors on the review. Christian Harkensee advised on some clinical aspects of the review and commented on several draft versions.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hodson 1987 {published data only}
- Hodson ME, Roberts CM, Butland RJA, Batten JC. Ciprofloxacin compared with intravenous azlocillin and gentamicin in the treatment of Pseudomonas aeruginosa infection in cystic fibrosis (CF) [abstract]. Pediatric Pulmonology 1987;3(2):126. [Google Scholar]
- Hodson ME, Roberts CM, Butland RJA, Batten JC. Ciprofloxacin compared with intravenous azlocillin and gentamicin in the treatment of Pseudomonas aeruginosa infection in cystic fibrosis (CF) [abstract]. Proceedings of the 14th Annual Meeting of the European Working Group for Cystic Fibrosis; 1‐2 September 1986; Budapest. 1986:20.
- Hodson ME, Roberts CM, Butland RJA, Smith MJ. Oral ciprofloxacin compared with conventional intravenous treatment for Pseudomonas aeruginosa infection in adults with cystic fibrosis. Lancet 1987;1(8527):235‐7. [DOI] [PubMed] [Google Scholar]
Richard 1997 {published and unpublished data}
- Richard DA, Nousia‐Arvanitakis S, Sollich V, Hampel BJ, Sommerauer B, Schaad UB. Oral ciprofloxacin vs. intravenous ceftazidime plus tobramycin in pediatric cystic fibrosis patients: comparison of antipseudomonas efficacy and assessment of safety with ultrasonography and magnetic resonance imaging. Pediatric Infectious Disease Journal 1997;16(6):572‐8. [DOI] [PubMed] [Google Scholar]
Schaad 1997 {published and unpublished data}
- Schaad UB, Wedgwood J, Ruedeberg A, Kraemer R, Hampel B. Ciprofloxacin as antipseudomonal treatment in patients with cystic fibrosis. Pediatric Infectious Disease Journal 1997;16(6):106‐11. [DOI] [PubMed] [Google Scholar]
Sheldon 1993 {published data only}
- Assoufi BK, Sheldon CD, Hodson ME. Double blind comparison of regular 3 monthly treatment with ciprofloxacin or placebo in cystic fibrosis patients who are chronically colonised with Pseudmonas aeruginosa [abstract]. Pediatric Pulmonology 1990;Suppl 5:204. [Google Scholar]
- Assoufi BK, Sheldon CD, Hodson ME. Double blind comparison of regular three monthly treatment with ciprofloxacin or placebo in cystic fibrosis (CF) patients who are chronically colonised with Pseudmonas aeruginosa [abstract]. 16th Annual Meeting of the European Working Group for Cystic Fibrosis; 1989; Prague, Czechoslovakia. 1989:32.
- Sheldon CD, Assoufi BK, Hodson ME. Regular three monthly oral ciprofloxacin in adult cystic fibrosis patients infected with Pseudomonas aeruginosa. Respiratory Medicine 1993;87:587‐93. [DOI] [PubMed] [Google Scholar]
Wang 1988 {published data only}
- Wang CI, Inderlied CB, Armer C, Roldan MA, Osher AB. Comparison of the efficacy and safety of oral ciprofloxacin with that of i.v. tobramycin plus azlocillin and/or tobramycin plus ticarcillin in patients with cystic fibrosis [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:R(c)19. [Google Scholar]
References to studies excluded from this review
Anstead 2001 {published data only}
- Anstead M, Kuhn RJ, Halsey S, Doherty DE, D'Souza N, Kanga JF. Effect of azithromycin on lung function, sputum bacteriology, and sputum inflammatory markers in cystic fibrosis [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A565. [Google Scholar]
Beringer 2012 {published data only}
- Beringer P, Owens H, Nguyen A, Benitez D, Boyd‐King A, Rao AP. Safety, pharmacokinetics and preliminary evaluation of the antiinflammatory effect of doxycycline in CF [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:370, Abstract no: 422. [CENTRAL: 867114; CFGD Register: PI256a; CRS: 5500100000011256] [Google Scholar]
- Beringer PM, Owens H, Nguyen A, Benitez D, Rao A, D'Argenio DZ. Pharmacokinetics of doxycycline in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2012;56(1):70‐4. [CFGD Register: PI256b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Black 1991 {published data only}
- Black A, Redmond A, Scott E. Randomised study comparing courses or oral ciprofloxacin alone with intermittent courses of intravenous azlocillin plus tobramycin or oral ciprofloxacin in the treatment of acute exacerbations of respiratory infection in cystic fibrosis [abstract]. Proceedings of the 16th Annual Meeting of the European Working Group for Cystic Fibrosis; 1989; Prague, Czechoslovakia. 1989:31.
- Black A, Redmond AOB, Steen HJ, Oborska IT. Tolerance and safety of ciprofloxacin in paediatric patients. Journal of Antimicrobial Chemotherapy 1990;26(Suppl F):25‐9. [DOI] [PubMed] [Google Scholar]
- Black A, Stevenson M, Scott E, Redmond A. Randomised study comparing oral ciprofloxacin alone with ciprofloxacin alternating with intravenous therapy in cystic fibrosis [abstract]. Proceedings of the 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:80.
Bosso 1987 {published data only}
- Bosso JA, Black PG, Matsen JM. Ciprofloxacin versus tobramycin plus azlocillin in pulmonary exacerbations in adult patients with cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4a):180‐4. [PubMed] [Google Scholar]
Bosso 1989 {published data only}
- Bosso JA. Use of ciprofloxacin in cystic fibrosis patients. American Journal of Medicine 1989;87(Suppl 5a):123s‐7s. [DOI] [PubMed] [Google Scholar]
Cipolli 2001 {published data only}
- Cipolli M, Cazzola G, Novelli A, Cassetta MI, Fallani S, Mazzeri T. Azithromycin concentrations in serum bronchial secretions of patients with cystic fibrosis. Clinical Drug Investigation 2001;21(5):353‐60. [Google Scholar]
Connett 2015 {published data only}
- Connett GJ, Pike KC, Legg JP, Cathie K, Dewar A, Foote K, et al. Ciprofloxacin during upper respiratory tract infections to reduce Pseudomonas aeruginosa infection in paediatric cystic fibrosis: a pilot study. Therapeutic Advances in Respiratory Disease 2015;9(6):272‐80. [CFGD Register: PI282b; CRS: 5500135000001465; PUBMED: 26341118] [DOI] [PubMed] [Google Scholar]
- Legg J, Pike K, Cathie K, Dewar A, Foote K, Harris A, et al. A randomized double‐blind, placebo‐controlled trial of ciprofloxacin in the treatment of upper respiratory tract infections in children with cystic fibrosis [ abstract]. Pediatric Pulmonology 2014;49 Suppl 38:333, Abstract no: 325. [CENTRAL: 1012529; CFGD Register: PI282a; CRS: 5500131000000185] [Google Scholar]
Davies 1987 {published data only}
- Davies RL, Koup JR, Williams‐Warren J, Weber A, Heggen L, Stempel D, et al. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrobial Agents and Chemotherapy 1987;31(6):915‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Denning 1977 {published data only}
- Denning CR, Park S, Grece CA, Mellin GW. Continuous vs intermittent oral antibiotics in the management of patients with cystic fibrosis [abstract]. Proceedings of the 18th Annual Meeting Cystic Fibrosis Club Abstracts; 1977. 1977:23.
Equi 2002 {published data only}
- Equi A, Balfour‐Lynn IM, Bush A, Rosenthal M. Long term azithromycin in children with cystic fibrosis: a randomised, placebo‐controlled crossover trial. Lancet 2002;360(9338):978‐84. [DOI] [PubMed] [Google Scholar]
- Equi A, Bush A, Alton EW, Balfour‐Lynn I, Rosenthsal M. A prospective double‐blind randomised placebo controlled crossover trial of long term azithromycin in children [abstract]. Pediatric Pulmonology 2001;Suppl 22:307. [Google Scholar]
- Equi A, Bush A, Balfour‐Lynn IM, Rosenthsal M. A prospective double‐blind randomised placebo controlled crossover trial of azithromycin in paediatric cystic fibrosis [abstract]. Thorax 2002;57(Suppl iii):38. [Google Scholar]
Frederiksen 2003 {published data only}
- Frederiksen B, Hansen A, Koch C, Hoiby N. Delay of recurrence of Pseudomonas aeruginosa in patients with cystic fibrosis with inhaled colistin and oral ciprofloxacin: a comparison between 3 weeks and 3 months of treatment [abstract]. Pediatric Pulmonology 1997;23 Suppl 14:288, Abstract no: 298. [CFGD Register: PI118a] [Google Scholar]
- Frederiksen B, Pressler T, Koch C, Hoiby N. Endpoints for evaluating early anti‐pseudomonal treatment: changes in pseudomonas prevalence and in pulmonary function [abstract]. Pediatric Pulmonology 2003;36 Suppl 25:334. [CFGD Register: PI118b; ] [Google Scholar]
Goldfarb 1986 {published data only}
- Goldfarb J, Wormser GP, Inchiosa MA, Guideri G, Diaz M, Gandhi R, et al. Single dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. Journal of Clinical Pharmacology 1986;26:222‐6. [DOI] [PubMed] [Google Scholar]
Harrison 1985 {published data only}
- Harrison CJ, Marks MI, Welch DF, Sharma BB, Baker D, Dice J, et al. A multicenter comparison of related pharmacologic features of cephalexin and dicloxacillin given for two months to young children with cystic fibrosis. Pediatric Pharmacology 1985;5(1):7‐16. [PubMed] [Google Scholar]
Jensen 1987 {published data only}
- Jensen T, Pedersen SS, Høiby N, Koch C. Efficacy of oral fluoroquinolones versus conventional intravenous antipseudomonal chemotherapy in treatment of cystic fibrosis. European Journal of Clinical Microbiology 1987;6(6):618‐22. [DOI] [PubMed] [Google Scholar]
- Jensen T, Pedersen SS, Nielsen CH, Hoiby N, Koch C. The efficacy and safety of ciprofloxacin and ofloxacin in chronic Pseudomonas aeruginosa infection in cystic fibrosis. Journal of Antimicrobial Chemotherapy 1987;20(4):585‐94. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Jensen T, Hvidberg EF. Comparative pharmacokinetics of ciprofloxacin and ofloxacin in cystic fibrosis patients. Journal of Antimicrobial Chemotherapy 1987;20(4):575‐83. [DOI] [PubMed] [Google Scholar]
Johansen 1999 {published data only}
- Johansen HK, Børch K, Espersen F, Koch C, Høiby N. Pivmecillinam + pivampicillin vs. pivampicillin in cystic fibrosis patients with Haemophilus influenzae infection [abstract]. Proceedings of 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:89.
- Johansen HK, Børch K, Espersen F, Koch C, Høiby N. Randomised trial of pivampicillin plus pivmecillinam vs. pivampicillin in children and youg adults with chronic obstructive pulmonary disease and infection with Haemophilus influenzae. Current Medical Research and Opinion 1999;15(4):300‐9. [DOI] [PubMed] [Google Scholar]
Kapranov 1995 {published data only}
- Kapranov NI, Belousov YB, Kashyrskaya NY, Smirnova EY. Quinoline therapy in children with cystic fibrosis [abstract]. Proceedings of 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:P19.
Knight 1979 {published data only}
- Knight RK, Batten JC, Mearns M. A double blind trial of cephalexin in cystic fibrosis patients with Pseudomonas in their sputum. Proceedings of 9th Meeting European Working Group for Cystic Fibrosis; 1979 June 12‐13; Noordwijkerhout, The Netherlands. 1979:52.
Kurz 1987 {published data only}
- Kurz CC, Marget W, Harms K, Bertele RM. A cross‐over study on the effectiveness of ofloxacin and ciprofloxacin administered orally [Kreuzstudie über die Wirksamkeit von Ofloxacin und Cirpofolxacin bei oraler Anwendung]. Infection 1986;14(Suppl 1):S82‐S86. [DOI] [PubMed] [Google Scholar]
- Kurz CC, Marget W, Harms K, Bertele RM. Comparison of ciprofloxacin and ofloxacin in cystic fibrosis: a cross‐over study [Vergleichende Untersuchung mit Ciprofloxacin und Ofloxacin im Kreuzversuch bei zystischer Fibrose]. Fortschritte der Antimikrobiellen u. Antineoplastischen Chemotherapie 1987;6:2107‐13. [Google Scholar]
Loening‐Baucke 1979 {published data only}
- Loening‐Baucke V, Mischler EH, Myers MG. A placebo‐controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. Journal of Pediatrics 1979;95(4):630‐7. [DOI] [PubMed] [Google Scholar]
- Loening‐Baucke V, Mischler EH, Myers MG. Cephalexin compared to placebo in the management of patients with cystic fibrosis [abstract]. Cystic Fibrosis Club Abstracts. 1978:69.
- Loening‐Baucke V, Mischler EH, Myers MG. Cephalexin in cystic fibrosis: a placebo‐controlled study [abstract]. Pediatric Research 1978;12(4 Pt 2):495. [Google Scholar]
Mack 1991 {published data only}
- Mack G, Cooper PJ, Buchannan N. Effects of enzyme supplementation on oral absorption of ciprofloxacin in patients with cystic fibrosis. Antimicrobial Agents and Chemotherapy 1991;35(7):1484‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolan 1982 {published data only}
- Nolan G, McIvor P, Levison H, Fleming PC, Corey M, Gold R. Antibiotic prophylaxis in cystic fibrosis: inhaled cephaloridine as an adjunct to oral cloxacillin. Journal of Pediatrics 1982;101(4):626‐30. [DOI] [PubMed] [Google Scholar]
Ordonez 2001a {published data only}
- Ordonex CL, Stulbarg M, Grundland H, Liu JT, Boushey HA. Effect of clarithromycin on airway obstruction and inflammatory markers in induced sputum in cystic fibrosis. Pediatric Pulmonology 2001;32(1):29‐37. [DOI] [PubMed] [Google Scholar]
Owen 1991 {published data only}
- Owen G, West J, Macguire S, Riley H, Goodchild M, Weller P. Continuous and intermittent antibiotic therapy in CF patients to age four years [abstract]. Proceedings of 17th European Cystic Fibrosis Conference; 1991 June 18‐21; Copenhagen, Denmark. 1991:95.
- West J, Smith AW, Brown MRW, Weller PH. The longitudinal relationship between clinical status lung infection and immune responses in young cystic fibrosis patients [abstract]. Pediatric Pulmonology 1990;Suppl 5:222. [Google Scholar]
- Williams J, Alfaham M, Riley HC, Goodchild MC, Weller PH, Dodge JA. Screening for cystic fibrosis in Wales and the West Midlands. 2: Clinical Evaluation [abstract]. Excerpta Medica, Asia Pacific Congress Series 1988;74:G(b)3. [Google Scholar]
Pai 2006 {published data only}
Pirzada 1999 {published data only}
- Pirzada OM, Taylor CJ. Long term macrolide antibiotics improve pulmonary function in cystic fibrosis [abstract]. Pedaitric Pulmonology 1999;28(Suppl 19):263. [Google Scholar]
Postnikov 2001a {published data only}
- Postnikov SS, Semykin SJ, Najimov VP. Safety of fluoroquinolones in children [abstract]. Proceedings of 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P213.
Postnikov 2001b {published data only}
- Postnikov SS, Yu S, Semykin V, Nazhimov P, Kapranov NI. Comparative growth rate of a year at the children with mucoviscidosis treated and not treated with Ciprofloxacin: clinicomorphological comparisons. Antibiotiki I Khimioterapiia 2001;46(10):11‐3. [PubMed] [Google Scholar]
Pukhalsky 2001 {published data only}
- Pukhalsky AL, Shmarina GV, Kapranov NI, Kashirskaja NJ, Kokarovtseva SN, Shabalova LA, et al. Increase of the sputum neutrophil elastase activity is a paradoxical effect of the successful lung disease treatment in cystic fibrosis [abstract]. Pediatric Pulmonology 2001;Suppl 22:274. [Google Scholar]
Romano 1991 {published data only}
- Romano L, Girosi D, Spallone E, Parisi F, Minicucci L, Romano C. The use of ofloxacin in cystic fibrosis patients [Uso dell'ofloxacin nei pazienti con fibrosi cistica]. Minerva Pediatrica 1992;44(3):79‐86. [PubMed] [Google Scholar]
- Romano L, Minicucci L, Spallone E, Girosi D, Campelli A, Fabbri A, et al. Role of home therapy with ofloxacin in patients with cystic fibrosis (CF) [Ruolo della terapia domiciliare conofloxacin in pazienti con fibrosi cistica]. Giornale italiano di chemioterapia 1991;38(1‐3):181‐3. [PubMed] [Google Scholar]
Rubio 1987 {published data only}
- Rubio TT. Ciprofloxacin: comparative data in cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4a):185‐8. [PubMed] [Google Scholar]
Saiman 2003 {published data only}
- Nguyen D, Mayor‐Hamblett N, Marshall BC, Saiman L, Burns JL. Phenotypic characterization of Pseudomonas aeruginosa as a potential predictor of clinical response to azithromycin in CF patients [abstract]. Pediatric Pulmonology 2004;38(Suppl 27):286. [Google Scholar]
- Saiman L. What have we learned from further analysis of the U.S. macrolide trial? Subgroup analysis of azithromycin trial. Pediatric Pulmonology 2003;Suppl 25:165‐7. [Google Scholar]
- Saiman L, Marshall BC, Mayer‐Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290(13):1749‐56. [DOI] [PubMed] [Google Scholar]
- Saiman L, Mayer‐Hamblett N, Campbell P, Marshall BC, Macrolide Study Group. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2005;172(8):1008‐12. [DOI] [PubMed] [Google Scholar]
Scully 1987 {published data only}
- Scully BE, Nakatomi M, Ores C, Davidson S, Neu HC. Ciprofloxacin therapy in cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4a):196‐201. [PubMed] [Google Scholar]
Shapera 1981 {published data only}
- Shapera RM, Warwick WJ, Matsen JM. Clindamycin therapy of staphylococcal pulmonary infections in patients with cystic fibrosis. Journal of Pediatrics 1981;99(4):647‐50. [DOI] [PubMed] [Google Scholar]
Smith 1997 {published data only}
- Smith A, Weber A, Pandher R, Williams‐Warren J, Cohen ML, Ramsey B. Utilization of salivary concentrations of ciprofloxacin in subjects with cystic fibrosis. Infection 1997;25(2):106‐8. [DOI] [PubMed] [Google Scholar]
Sriram 2003 {published data only}
- Sriram S, Young J, Waterhouse JC, Bucknall CE, Stack BHR. The antiinflammatory effect of clarithromycin in CF [abstract]. Journal of Cystic Fibrosis 2003;2(Suppl 1):S52. [Google Scholar]
Strandvik 1989 {published data only}
- Christensson BA, Nilsson‐Ehle I, Ljungberg B, Lindblad A, Malmborg AS, Hjelte L, et al. Increased oral bioavailability of ciprofloxacin in cystic fibrosis patients. Antimicrobial Agents and Chemotherapy 1992;36(11):2512‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandvik B, Hjelte L, Lindblad A, Ljungberg B, Malmborg AS, Nilsson‐Ehle I. Comparison of efficacy and tolerance of intravenously and orally administered ciprofloxacin in cystic fibrosis patients with acute exacerbations of lung infection. Scandinavian Journal of Infectious Diseases. Supplementum 1989;60:84‐8. [PubMed] [Google Scholar]
Stutman 1987 {published data only}
- Shalit I, Stutman HR, Marks MI, Chartrand SA, Hilman BC. Randomized study of two dosage regimens of ciprofloxacin for treating chronic bronchopulmonary infection in patients with cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4a):189‐95. [PubMed] [Google Scholar]
- Stutman HR, Shalit I, Marks MI, Greenwood R, Chartrand SA, Hilman BC. Pharmacokinetics of two doses regimens of ciprofloxacin during a two‐week therapeutic trial in patients with cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4a):142‐5. [PubMed] [Google Scholar]
Treggiari 2011 {published data only}
- Anstead M, Heltshe SL, Khan U, Barbieri JT, Langkamp M, Doring G, et al. Pseudomonas aeruginosa serology and risk for re‐isolation in the EPIC trial. Journal of Cystic Fibrosis 2013;12(2):147‐53. [CENTRAL: 1089772; CFGD Register: PI202m; CRS: 5500135000000084; PUBMED: 22944725] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstead M, Lymp J, Khan U, Barbieri J, Langkamp M, Doring G, et al. Pseudomonas aeruginosa serology predicts response to treatment and re‐infection in the EPIC clinical study [abstract]. Pediatric Pulmonology 2011;46(S34):303, Abstract no: 254. [CFGD Register: PI202g] [Google Scholar]
- Anstead M, Saiman L, Mayer‐Hamblett N, Lands LC, Kloster M, Goss CH, et al. Pulmonary exacerbations in CF patients with early lung disease. Journal of Cystic Fibrosis 2014;13(1):74‐9. [CFGD Register: PI202l] [DOI] [PubMed] [Google Scholar]
- Hamblett NM, Retsch‐Bogart GZ, Treggiari M, Kronmal RA, Khan U, Williams J, et al. Safety and efficacy of anti‐pseudomonal therapy for early eradication of Pseudomonas aeruginosa: the EPIC study [abstract]. Pediatric Pulmonology 2009;44(S32):183. [CFGD CF Register: PI202b; MEDLINE: ] [Google Scholar]
- Hoffman LR, Ramsey BW, Kulasekara HD, Retsch‐Bogart GZ, Wolter DJ, Pope CE, et al. Pseudomonas aeruginosa (PA) phenotypes associated with persistent early infection in CF patients in the EPIC Clinical Trial [abstract]. Pediatric Pulmonology 2012;47(S35):317, Abstract no: 266. [CFGD Register: PI202j] [Google Scholar]
- Jorth P, Hisert KB, Garudathri J, Wolter D, Hoffman L, Singh P. Studies on the effects of ciprofloxacin on Pseudomonas aeruginosa evolution in cystic fibrosis patients [abstract]. Pediatric Pulmonology 2014;49:349. [CENTRAL: 1057036; CFGD Register: PI202n; CRS: 5500050000000261; EMBASE: 71616423] [Google Scholar]
- Jorth P, Rezayat A, Hisert KB, Garudathri J, Khan U, Hamblett NM, et al. Early evolution of pseudomonas aeruginosa during cystic fibrosis infection [abstract]. Pediatric Pulmonology 2015;50 Suppl 41:304, Abstract no: 300. [CENTRAL: 1092204; CFGD Register: PI202p; CRS: 5500135000001393] [Google Scholar]
- Khan U, Mayer‐Hamblett N, Retsch‐Bogart G, Treggiari M, Ramsey B. Association between baseline pseudomonas aeruginosa positivity in EPIC clinical trial participants & prior antibiotic exposure [abstract]. Pediatric Pulmonology 2010;45(S33):335, Abstract no: 326. [CFGD Register: PI202f] [Google Scholar]
- Mayer‐Hamblett N, Kloster M, Rosenfeld M, Gibson RL, Retsch‐Bogart GZ, Emerson J, et al. Impact of Sustained Eradication of New Pseudomonas aeruginosa Infection on Long‐term Outcomes in Cystic Fibrosis. Clinical Infectious Diseases 2015;61(5):707‐15. [CENTRAL: 1099084; CFGD Register: PI202o; CRS: 5500135000001411; PUBMED: 25972024] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer‐Hamblett N, Rosenfeld M, Treggiari MM, Konstan MW, Retsch‐Bogart G, Morgan W, et al. Standard care versus protocol based therapy for new onset Pseudomonas aeruginosa in cystic fibrosis. Pediatric Pulmonology 2013;48(10):943‐53. [CFGD Register: PI202k] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer‐Hamblett N, Kronmal RA, Gibson RL, Rosenfeld M, Retsch‐Bogart G, Treggiari MM, et al. Initial Pseudomonas aeruginosa treatment failure is associated with exacerbations in cystic fibrosis. Pediatric Pulmonlogy 2012;47(2):125‐34. [CFGD Register: PI202i; DOI: 10.1002/ppul.21525] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey B. TOBI use in infants and children with early Pseudomonas Aeruginosa infection ‐ duration of effect and epic update [abstract]. Pediatric Pulmonology 2005;40(S28):146. [CFGD CF Register: PI202a] [Google Scholar]
- Treggiari M, Retsch‐Bogart G, Mayer‐Hamblett N, Khan U, Kronmal R, Ramsey B, et al. Comparative efficacy and safety of four randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S54, Abstract no: 209. [CFGD Register: PI202e] [Google Scholar]
- Treggiari M, Retsch‐Bogart GZ, Mayer‐Hamblett N, Kronmal R, Khan U, Williams J, et al. Early anti‐pseudomonal infection in children with CF: study population and conduct of the "EPIC" clinical trial [abstract]. Pediatric Pulmonology 2009;44(S32):316, Abstract no: 299. [CFGD CF Register: PI202c; MEDLINE: ] [Google Scholar]
- Treggiari MM, Retsch‐Bogart G, Mayer‐Hamblett N, Khan U, Kulich M, Kronmal R, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Archives of Pediatrics and Adolescent Medicine 2011;165(9):847‐56. [CFGD Register: PI202h] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treggiari MM, Rosenfeld M, Mayer‐Hamblett N, Retsch‐Bogart G, Gibson RL, Williams J, et al. Early anti‐pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contempory Clinical Trials 2009;30(3):256‐68. [CFGD CF Register: PI202d; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitti 1975 {published data only}
- Vitti TG, Berg TJ, Pagtakhan BS, Pagtakhan RD. The effect of pancreatic enzyme supplement on the intestinal absorption of ampicillin and cloxacillin in children with cystic fibrosis [abstract]. Proceedings of the 16th Annual Meeting Cystic Fibrosis Club Abstracts; 1975. 1975:56.
Weaver 1994 {published data only}
- Beardsmore CS, Thompson JR, Williams A, McArdle EK, Gregory GA, Weaver LT, et al. Pulmonary function in infants with cystic fibrosis: the effect of antibiotic treatment. Archives of Disease in Childhood 1994;71(2):133‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver LT, Green MR, Nicholson K, Heeley ME, Mills J, Kuzemko JA. Continuous prophylactic flucloxacillin improves outcome of infants with cystic fibrosis (CF) detected soon after birth [abstract]. Proceedings of 63rd Annual Meeting of the British Paediatric Association; Warwick, UK; 1991. 1991:24.
- Weaver LT, Green MR, Nicholson K, Mills J, Heeley ME, Kuzemko JA, et al. Prognosis in cystic fibrosis treated with continuous flucloxacillin from the neonatal period. Archives of Disease in Childhood 1994;70(2):84‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolter 2002 {published data only}
- Bell SC, Seeney S, Walmsley K, Wolter JM, Bowler SD, McCormack JG. Long term treatment with azithromycin results in reduced ex‐vivo inflammatory cytokine production in adults with cystic fibrosis [abstract]. Pediatric Pulmonology 2002;Suppl 24:289‐90. [Google Scholar]
- Bowler SD. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis. Japanese Journal of Antibiotics 2003;56(Suppl A):38. [PubMed] [Google Scholar]
- Bowler SD, Masel PJ, Bell SC, Seeney SL, Wolter JM, McCormack JG. A prospective randomised trial of long term azithromycin (AZM) versus placebo in cystic fibrosis; impact on clinical, laboratory and quality of life (QOL) outcomes [abstract]. Pediatric Pulmonology. 2000; Vol. Suppl 20:280.
- Bowler SD, Seeney S, Walmsley K, Wolter JM, Bell SC, McCormack JG. Long‐term treatment with azithromycin results in reduced in vitro inflammatory cytokine production in adults with cystic fibrosis [abstract]. Respirology 2002;7(Suppl):A9. [Google Scholar]
- Seeney SL, Bowler SD, Wolter JM, Bell SC, Masel PJ, McCormack JG. A prospective randomised trial of long term azithromycin (AZM) versus placebo in cystic fibrosis (CF): impact on clinical, laboratory and quality of life (QOL) outcomes [abstract]. Internal Medicine Journal 2001;31(Suppl):A12. [Google Scholar]
- Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002;57(3):212‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Xu 2012 {published data only}
- Xu X, Abdalla T, Sabbatini G Roberts T, Bratcher P, Jackson PL, Blalock JE, et al. A randomized double blinded placebo controlled trial of doxycycline as an adjunctive therapy for the treatment of inpatient cystic fibrosis exacerbation [abstract]. Pediatric Pulmonology 2014;49 Suppl 38:312, Abstract no: 270. [CENTRAL: 1012523; CFGD Register: PI270b; CRS: 5500131000000174] [Google Scholar]
- Xu X, Sabbatini GM, Hathorne H, Clancy JP, Gaggar A. A randomized double blinded placebo control trial of doxycycline as an adjunctive anti‐inflammatory agent during CF exacerbation (DOXY) [abstract]. Pediatric Pulmonology 2012;47(S35):296, Abstract no: 209. [CENTRAL: 962120; CFGD Register: PI270a; CRS: 5500125000000029] [Google Scholar]
Additional references
Balaguer 2012
- Balaguer A, González de Dios J. Home versus hospital intravenous antibiotic therapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD001917.pub3] [DOI] [PubMed] [Google Scholar]
Ball 1986
- Ball P. Ciprofloxacin: an overview of adverse experiences. Journal of Antimicrobial Chemotherapy 1986;18(Suppl D):187‐93. [DOI] [PubMed] [Google Scholar]
Ball 1990
- Ball P. Emergent resistance to ciprofloxacin amongst Pseudomonas aeruginosa and Staphylococcus aureus: clinical significance and therapeutic approaches. Journal of Antimicrobial Chemotherapy 1990;26(Suppl F):165‐79. [DOI] [PubMed] [Google Scholar]
Burns 2001
- Burns JL, Gibson R, McNamara S, Yim D, Emerson J, Rosenfeld M, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. Journal of Infectious Diseases 2001;183(3):444‐52. [DOI] [PubMed] [Google Scholar]
CF Trust 2004
- UK Cystic Fibrosis Trust Infection Control Group. Pseudomonas aeruginosa infection in people with cystic fibrosis: Suggestions for prevention and infection control. Report of the UK Cystic Fibrosis Infection Control Group. 2nd edition 2004.
CFF 2008
- Cystic Fibrosis Foundation. Patient Registry Report. www.cff.org/research/ClinicalResearch/PatientRegistryReport/ (accessed 26 August 2010).
Döring 2000
- Döring G, Conway SP, Heijerman HG, Hodson ME, Hoiby N, Smyth A, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. European Respiratory Journal 2000;16(4):749‐67. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
FitzSimmons 1993
- FitzSimmons SC. The changing epidemiology of cystic fibrosis. Journal of Pediatrics 1993;122(1):1‐9. [DOI] [PubMed] [Google Scholar]
FitzSimmons 1996
- FitzSimmons SC. The Cystic Fibrosis Foundation Patient Registry Report 1996. Pediatric Pulmonology 1996;21(Suppl):267‐75. [Google Scholar]
Gee 2000
- Gee L, Abbott J, Conway S, Etherington C, Webb A. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55(11):946‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibson 2003
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;168(8):918‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Kosorok 2001
- Kosorok MR, Zeng L, West SE, Rock MJ, Splaingard ML, Laxova A, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatric Pulmonology 2001;32(4):277‐87. [DOI] [PubMed] [Google Scholar]
Langton Hewer 2014
- Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD004197.pub4] [DOI] [PubMed] [Google Scholar]
Millar 2009
- Millar FA, Simmonds NJ, Hodson ME. Trends in pathogens colonising the respiratory tract of adult patients with cystic fibrosis, 1985‐2005. Journal of Cystic Fibrosis 2009;8(6):386‐91. [DOI] [PubMed] [Google Scholar]
Nixon 2001
- Nixon GM, Armstrong DS, Carzino R, Carlin JB, Olinsky A, Robertson CF, et al. Clinical outcome after early Pseudomonas aeruginosa infection in cystic fibrosis. Journal of Pediatrics 2001;138(5):699‐704. [DOI] [PubMed] [Google Scholar]
Parad 1999
- Parad RB, Gerard CJ, Zurakowski D, Nichols DP, Pier GB. Pulmonary outcome in cystic fibrosis is influenced primarily by mucoid Pseudomonas aeruginosa infection and immune status and only modestly by genotype. Infection & Immunity 1999;67(9):4744‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patterson 1991
- Patterson DR. Quinolone toxicity: methods of assessment. American Journal of Medicine 1991;91(6A):35S‐37S. [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajan 2002
- Rajan S, Saiman L. Pulmonary infections in patients with cystic fibrosis. Seminars in Respiratory Infections 2002;17(1):47‐56. [DOI] [PubMed] [Google Scholar]
Ratjen 2006
- Ratjen F. Diagnosing and managing infection in CF. Paediatric Respiratory Review 2006;7(Suppl 1):S151‐3. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenfeld 2001
- Rosenfeld M, Gibson RL, McNamara S, Emerson J, Burns JL, Castile R, et al. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatric Pulmonology 2001;32(5):356‐66. [DOI] [PubMed] [Google Scholar]
Schaad 2007
- Schaad UB. Will fluoroquinolones ever be recommended for common infections in children?. Pediatric Infectious Diseases Journal 2007;26(10):865‐7. [DOI] [PubMed] [Google Scholar]
Schaefer 1996
- Schaefer C, Amoura‐Elefant E, Vial T, Ornoy A, Garbis H, Robert E, et al. Pregnancy outcome after prenatal quinolone exposure. Evaluation of a case registry of the European Network of Teratology Information Services (ENTIS). European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69(2):83‐9. [DOI] [PubMed] [Google Scholar]
Southern 2012
- Southern KW, Barker PM, Solis‐Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002203.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2001
- Wang S, Fitzsimmons S, O'Leary L, Rock M, Gwinn M, Khoury M. Early diagnosis of cystic fibrosis in the newborn period and risk of Pseudomonas aeruginosa in the first 10 years of life: a registry‐based longitudinal study. Pediatrics 2001;107(2):274‐9. [DOI] [PubMed] [Google Scholar]
West 2002
- West SE, Zeng L, Lee BL, Kosorok MR, Laxova A, Rock MJ, et al. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA 2002;287(22):2958‐67. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Remmington 2007
- Remmington T, Jahnke N, Harkensee C. Oral anti‐pseudomonal antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005405.pub2] [DOI] [PubMed] [Google Scholar]
Remmington 2013
- Remmington T, Jahnke N, Harkensee C. Oral anti‐pseudomonal antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD005405.pub3] [DOI] [PubMed] [Google Scholar]


