Abstract
Background
Administration of oral sucrose with and without non‐nutritive sucking is the most frequently studied non‐pharmacological intervention for procedural pain relief in neonates.
Objectives
To determine the efficacy, effect of dose, method of administration and safety of sucrose for relieving procedural pain in neonates as assessed by validated composite pain scores, physiological pain indicators (heart rate, respiratory rate, saturation of peripheral oxygen in the blood, transcutaneous oxygen and carbon dioxide (gas exchange measured across the skin ‐ TcpO2, TcpCO2), near infrared spectroscopy (NIRS), electroencephalogram (EEG), or behavioural pain indicators (cry duration, proportion of time crying, proportion of time facial actions (e.g. grimace) are present), or a combination of these and long‐term neurodevelopmental outcomes.
Search methods
We used the standard methods of the Cochrane Neonatal. We performed electronic and manual literature searches in February 2016 for published randomised controlled trials (RCTs) in the Cochrane Central Register of Controlled Trials (CENTRAL; The Cochrane Library, Issue 1, 2016), MEDLINE (1950 to 2016), EMBASE (1980 to 2016), and CINAHL (1982 to 2016). We did not impose language restrictions.
Selection criteria
RCTs in which term or preterm neonates (postnatal age maximum of 28 days after reaching 40 weeks' postmenstrual age), or both, received sucrose for procedural pain. Control interventions included no treatment, water, glucose, breast milk, breastfeeding, local anaesthetic, pacifier, positioning/containing or acupuncture.
Data collection and analysis
Our main outcome measures were composite pain scores (including a combination of behavioural, physiological and contextual indicators). Secondary outcomes included separate physiological and behavioural pain indicators. We reported a mean difference (MD) or weighted MD (WMD) with 95% confidence intervals (CI) using the fixed‐effect model for continuous outcome measures. For categorical data we used risk ratio (RR) and risk difference. We assessed heterogeneity by the I2 test. We assessed the risk of bias of included trials using the Cochrane 'Risk of bias' tool, and assessed the quality of the evidence using the GRADE system.
Main results
Seventy‐four studies enrolling 7049 infants were included. Results from only a few studies could be combined in meta‐analyses and for most analyses the GRADE assessments indicated low‐ or moderate‐quality evidence. There was high‐quality evidence for the beneficial effect of sucrose (24%) with non‐nutritive sucking (pacifier dipped in sucrose) or 0.5 mL of sucrose orally in preterm and term infants: Premature Infant Pain Profile (PIPP) 30 s after heel lance WMD ‐1.70 (95% CI ‐2.13 to ‐1.26; I2 = 0% (no heterogeneity); 3 studies, n = 278); PIPP 60 s after heel lance WMD ‐2.14 (95% CI ‐3.34 to ‐0.94; I2 = 0% (no heterogeneity; 2 studies, n = 164). There was high‐quality evidence for the use of 2 mL 24% sucrose prior to venipuncture: PIPP during venipuncture WMD ‐2.79 (95% CI ‐3.76 to ‐1.83; I2 = 0% (no heterogeneity; 2 groups in 1 study, n = 213); and intramuscular injections: PIPP during intramuscular injection WMD ‐1.05 (95% CI ‐1.98 to ‐0.12; I2 = 0% (2 groups in 1 study, n = 232). Evidence from studies that could not be included in RevMan‐analyses supported these findings. Reported adverse effects were minor and similar in the sucrose and control groups. Sucrose is not effective in reducing pain from circumcision. The effectiveness of sucrose for reducing pain/stress from other interventions such as arterial puncture, subcutaneous injection, insertion of nasogastric or orogastric tubes, bladder catherization, eye examinations and echocardiography examinations are inconclusive. Most trials indicated some benefit of sucrose use but that the evidence for other painful procedures is of lower quality as it is based on few studies of small sample sizes. The effects of sucrose on long‐term neurodevelopmental outcomes are unknown.
Authors' conclusions
Sucrose is effective for reducing procedural pain from single events such as heel lance, venipuncture and intramuscular injection in both preterm and term infants. No serious side effects or harms have been documented with this intervention. We could not identify an optimal dose due to inconsistency in effective sucrose dosage among studies. Further investigation of repeated administration of sucrose in neonates is needed. There is some moderate‐quality evidence that sucrose in combination with other non‐pharmacological interventions such as non‐nutritive sucking is more effective than sucrose alone, but more research of this and sucrose in combination with pharmacological interventions is needed. Sucrose use in extremely preterm, unstable, ventilated (or a combination of these) neonates needs to be addressed. Additional research is needed to determine the minimally effective dose of sucrose during a single painful procedure and the effect of repeated sucrose administration on immediate (pain intensity) and long‐term (neurodevelopmental) outcomes.
Plain language summary
Sucrose for analgesia (pain relief) in newborn infants undergoing painful procedures
Review question
Cochrane reviewers investigated how well sucrose (table sugar) works as a reliever of pain in newborn babies who are having painful procedures (e.g. an injection, or heel lance, or insertion of a needle to obtain a blood sample (venipuncture), or eye examinations). The babies' pain responses (e.g. crying, grimacing) were assessed by scoring systems for pain used by health care professionals to measure the pain that babies are experiencing. In addition, the reviewers wanted to investigate whether the level of pain relief is related to the dose of sucrose, or the method of delivery (e.g. as a solution squirted into the mouth, or on a pacifier (also called a soother or dummy), and whether there are any safety concerns about using sucrose to relieve pain.
Background
Although there are ways to manage the pain of surgery, medical illness and major procedures, ways of preventing or reducing pain from minor medical procedures (e.g. heel lance and venipuncture) have, until relatively recently, been lacking. Sucrose has been examined for its calming effects in crying newborns and its pain‐relieving effects for invasive procedures in full‐term and premature newborns.
Study characteristics
We searched the medical literature widely up to February 2016 for studies that investigated the pain‐relieving effect of sucrose for minor medical procedures in newborn full‐term and premature babies. We included randomised controlled trials only, as these provide the most reliable medical evidence. We identified 74 studies that reported on a total of more than 7000 infants in this Cochrane Review.
Thirty‐eight studies included full‐term babies only, 31 included premature babies only, and five included both full‐term and premature babies. Heel lance was the painful procedure in 38 studies, and venipuncture in nine; the remaining studies investigated a wide variety of other minor painful procedures.
The studies used a variety of delivery methods for the sucrose solution (oral syringe, dropper or sucrose‐dipped pacifier), as well as a range of concentrations and volumes of dose. Sucrose treatment was compared with giving the babies a similar volume of water, a pacifier, routine care, breastfeeding, 'facilitated tucking' (holding the infant in a flexed position with arms close to the body and hands placed to promote sucking), laser acupuncture, swaddling, warmth, anaesthetic cream for the skin (EMLA), or a combination of these. The studies used a range of pain assessment scales to measure their results.
Study funding sources
We did not identify any studies that received funding from the industry.
Key results
There was high‐quality evidence that sucrose reduces different measures of newborn pain during heel lance, venipuncture and intramuscular injection. However, sucrose does not provide effective pain relief during circumcision. There is conflicting evidence for whether sucrose reduces pain for other minor painful procedures and further research is needed to investigate these more thoroughly.
Twenty‐nine studies reported on adverse events (harms of the sucrose and other treatments) and found that the number of minor adverse events (e.g. choking or gagging) was very low, and was similar in the different groups (so not attributable to the sucrose treatment). No major adverse events were reported.
Quality of evidence
Although sucrose has been widely studied as a pain reliever for newborn babies, most studies have included few babies and have used many different measures of pain to assess its effectiveness. We identified high‐quality evidence that sucrose reduces pain for heel lance, venipuncture and intramuscular injection. The quality of evidence was low or moderate in favour for the use of sucrose for other painful procedures.
Summary of findings
for the main comparison.
| Sucrose (20% to 33%) compared with water for pain associated with heel lance | |||||
|
Patient or population: neonates with heel lance‐associated pain Settings: hospital Intervention: sucrose (20% to 33%) Comparison: water | |||||
| Outcomes | Illustrative comparative risks (mean and range) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water | Sucrose (20% to 33%) | ||||
|
PIPP at 30 s after heel lance Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA. A lower score = less pain (Stevens 1996) |
The mean for PIPP ranged across control groups from 8.5 to 9.62 | The WMD for PIPP in the intervention groups was lower: ‐1.42 (95% CI ‐2.86 to 0.01) | 105 (2) | ⊕⊕⊝⊝ low | Bias: there were some concerns about bias in both studies (see RoB tables) Consistency: there was moderate inconsistency between the study point estimates (12 = 51 %) Precision: there was low precision in the point estimate with wide 95% CIs) Indirectness: all trials were conducted in the target population (no concern about indirectness) |
|
PIPP at 60 s after heel lance Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants >3 6 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean for PIPP in the control group was 8.59 | The mean for PIPP in the intervention groups was lower: ‐1.80 (95% CI ‐3.81 to 0.21) | 31 (1) |
⊕⊕⊝⊝ low | Bias: there were concerns about allocation concealment and performance bias in this single study Consistency: N/A as there was only one study Precision: there was lack of precision due to small sample size Indirectness: the study was conducted in the target population |
|
PIPP score during heel lance (1st heel lance) Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean for PIPP in the control group was 7.3 | The mean for PIPP in the intervention group was the same as in the control group: 0.00 (95% CI ‐1.52 to 1.52) | 107 (1) | ⊕⊕⊕⊝ moderate | Bias: there were no concerns about bias in this study Consistency: as there was only one study in this analysis concerns about consistency were N/A Precision: this was a relatively large single study (no lack of precision) Indirectness: the study was conducted in the target population |
|
DAN score at 30 s after heel lance Range of scale 0‐10 A lower score = less pain (Carbajal 1997) |
The mean DAN score in the control group was 9.5 | The mean DAN score in the intervention group was lower: ‐1.90 (95% CI ‐8.58 to 4.78) | 32 (1) | ⊕⊕⊝⊝ low | Bias: concerns about blinding of performance and detection bias Consistency: as there was only one study in this analysis concerns about consistency were not N/A Precision: small sample size Indirectness: the study was conducted in the target population |
|
NIPS during heel lance Range of scale 0‐7 A lower score = less pain Lawrence 1993 |
The mean NIPS score in the control group was 3 | The mean NIPS score in the intervention group was lower: ‐2.00 (95% CI ‐2.42 to ‐1.58) | 56 (1) |
⊕⊕⊕⊝ moderate | Bias: no concerns about bias Consistency: as there was only one study in this analysis concerns about consistency were N/A Precision: small sample size Indirectness: the study was conducted in the target population (no concern about indirectness) |
| *The basis for the assumed risk was 'The mean PIPP, DAN and NIPS scores across control groups according to the values reported in the Assumed risk column. The corresponding risk was the mean in the intervention groups for the PIPP, DAN and NIPS scores with their 95% CI'. CI: confidence interval; DAN: Douleur Aiguë du Nouveau‐né Scale; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A: not applicable; NIPS: Neonatal Infant Pain Scale; WMD: weighted mean difference | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
2.
| Sucrose (24%) compared with breastfeeding for heel lance‐associated pain | |||||
|
Patient or population: neonates with heel lance‐associated pain Settings: hospital Intervention: sucrose 24% Comparison: breastfeeding | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Breastfeeding | Sucrose (24%) | ||||
| PIPP ‐ Preterm infants | The mean PIPP score in the breast feeding group was 7 Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score in the sucrose group was lower: ‐1.75 (95% CI ‐2.22 to ‐1.28) | 47 (1) |
⊕⊕⊝⊝ low | Bias: there were concerns about performance and detection bias Consistency: as there was only one study in this analysis concerns about consistency were N/A Precision: small sample size Indirectness: the study was conducted in the target population (no concern about indirectness) |
| *The basis for the assumed risk was 'The mean PIPP score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the PIPP score with its 95% CI'. CI: confidence interval; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A: not applicable | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
3.
| Sucrose (24%) + NNS compared with water + NNS or pacifier dipped in sucrose compared with pacifier dipped in water for heel lance‐associated pain | |||||
|
Patient or population: newborns with heel lance‐associated pain Settings: hospital Intervention: sucrose (24%) + NNS Comparison: water + NNS | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water + NNS | Sucrose + NNS | ||||
|
NFCS Range of scale 0‐10 in term infants 0‐9 in preterm infants A lower score = less pain Grunau 1987 |
The mean NFCS score in the water + NNS groups was 2.1 | The mean NFCS score in the sucrose + NNS group was lower than in the water + NNS group: ‐0.60 (95% CI ‐1.47 to 0.47) | 100 (1) |
⊕⊕⊝⊝ low | Bias: many items scored 'unclear' on the RoB assessment Consistency: as there was only one study in this analysis concerns about consistency were N/A Precision: relatively large sample size (no lack of precision) Indirectness: the study was conducted in the target population (no concern about indirectness) |
|
PIPP 30 s after heel lance (term and preterm infants) Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score ranged across control groups from 6.3 to 10.19 | The WM PIPP score in the sucrose + NNS group was lower than in the control group: ‐ 1.70 (95% CI ‐2.13 to ‐1.26) | 278 (3) | ⊕⊕⊕⊕ high | Bias: there was low risk of bias in all three studies. Consistency: the findings of the three studies were consistent, I2 = 0% (no heterogeneity). Precision: large sample size (no lack of precision). Indirectness: the studies were conducted in the target population (no concern about indirectness). |
|
PIPP 60 s after heel lance Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score ranged across control groups from 10.54 to 11.2 |
The WM PIPP score in the sucrose + NNS group was lower than in the control group: ‐ 2.14 (95% CI ‐3.34 to ‐0.94) | 164 (2) |
⊕⊕⊕⊕ high | Bias: there was low risk of bias in both studies Consistency: the findings of the two studies were consistent, I2 = 0% (no heterogeneity) Precision: large sample size (no lack of precision) Indirectness: the studies were conducted in the target population (no concern about indirectness) |
| *The basis for the assumed risk was 'The mean NFCS and PIPP scores across control groups according to the values reported in the Assumed risk column. The corresponding risk was the mean in the intervention groups for the NFCS and PIPP scores with their 95% CI'. CI: confidence interval; RoB: risk of bias; N/A: not applicable;NFCS: Neonatal Facial Coding System;NNS: non‐nutritive sucking; WM: weighted mean | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
4.
| Sucrose (24%) + NNS + NIDCAP compared with breast milk (by breastfeeding) for heel lance‐associated pain | |||||
|
Patient or population: neonates with heel lance‐associated pain Settings: hospital Intervention: sucrose (24%) + NNS + NIDCAP Comparison: breast milk (by breastfeeding) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Breast milk (by breastfeeding) | Sucrose (24%) + NNS + NIDCAP | ||||
|
PIPP score Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score in the breast milk (by breast feeding) group was 7 | The mean PIPP score in the sucrose (24%) + NNS + NIDCAP group was lower than in the control group: ‐ 1.75 (95% CI ‐4.03 to 0.53) | 47 (1) |
⊕⊕⊝⊝ low | Bias: there was a high risk of performance and detection bias as the interventions could not be blinded Consistency: as there was only one study in this analysis concern about consistency was N/A Precision: small sample size (lack of precision) Indirectness: the study was conducted in the target population (no concern about indirectness) |
| *The basis for the assumed risk was 'The mean PIPP score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the PIPP score with its 95% CI'. CI: confidence interval; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A not applicable; NIDCAP: Newborn Individualized Developmental Care and Assessment Program;NNS: non‐nutritive sucking | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
5.
| Sucrose (24%) + NNS + NIDCAP support compared with breast milk (by syringe) for heel lance‐associated pain | |||||
|
Patient or population: neonates with heel lance‐associated pain Settings: hospital Intervention: sucrose (24%) + NNS + NIDCAP support Comparison: breast milk (by syringe) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Breast milk (by syringe) | Sucrose (24%) + NNS + NIDCAP support | ||||
|
PIPP score Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score in the breast milk (by syringe) group was 5.38 | The mean PIPP score in the sucrose (24%) + NNS + NIDCAP support group was lower than in the control group: ‐0.13 (95% CI ‐2.41 to 2.15) | 47 (1) |
⊕⊕⊝⊝ low | Bias: there was a high risk of performance and detection bias as the interventions could not be blinded Consistency: as there was only one study in this analysis concern about consistency was N/A Precision: small sample size (lack of precision) Indirectness: the study was conducted in the target population (no concern about indirectness) |
| *The basis for the assumed risk was 'The mean PIPP score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the PIPP score with its 95% CI'. CI: confidence interval; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A not applicable; NIDCAP: Newborn Individualized Developmental Care and Assessment Program;NNS: non‐nutritive sucking | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
6.
| Sucrose (24%) compared with laser acupuncture for pain associated with heel lance | |||||
|
Patient or population: neonates with pain associated with heel lance Settings: hospital Intervention: sucrose (24%) Comparison: laser acupuncture | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Laser acupuncture | Sucrose (24%) | ||||
|
NIPS score Range of scale 0‐7 A lower score = less pain Lawrence 1993 |
The mean NIPS score was 4.52 in the laser acupuncture group | The mean NIPS score in the sucrose group was lower than in the laser group: ‐0.86 (95% CI ‐1.43 to ‐0.29) | 42 (1) |
⊕⊕⊝⊝ low | Bias: There was high risk of selection bias and performance bias in this study Outcome assessments were made from video tapes (low risk of bias) Consistency: As there was only one study in this analysis concern about consistency was N/A Precision: small sample size (lack of precision) Indirectness: the study was conducted in the target population (no concern about indirectness) |
| *The basis for the assumed risk was 'The mean NIPS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NIPS score with its 95% CI'. CI: confidence interval; N/A: not applicable; NIPS: Neonatal Infant Pain Scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
7.
| Sucrose (24%) compared with sucrose (24%) + NNS for pain associated with heel lance | |||||
|
Patient or population: neonates with pain associated with heel lance Settings: hospital Intervention: sucrose (24%) Comparison: sucrose (24%) + NNS | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Sucrose (24%) + NNS | Sucrose (24%) | ||||
|
Revised NFCS Range of scale 0‐10 in term infants 0‐9 in preterm infants A lower score = less pain Grunau 1987 |
The mean NFCS score in the sucrose (24%) + NNS group was 0.02 | The mean NFCS score in the sucrose (24%) only group was higher than in the sucrose (24%) + NNS group: 0.43 (95% CI 0.23 to 0.63) | 343 (1) |
⊕⊕⊕⊝ moderate | Bias: there was a risk of performance and detection bias in this study as the coder could have distinguished the different groups Consistency: as this was a single study a rating of consistency was N/A Precision: this study had a very large sample size with a narrow 95% CI (no concerns about precision) Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean NFCS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NFCS score with its 95% CI'. CI: confidence interval; N/A: not applicable; NFCS: Neonatal Facial Coding System; NNS: non‐nutritive sucking. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
8.
| Sucrose (24%) compared with sucrose (24%) + swaddling for pain associated with heel lance | |||||
|
Patient or population: neonates with pain associated with heel lance Settings: hospital Intervention: sucrose (24%) Comparison: sucrose (24%) + swaddling | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Sucrose (24%) + swaddling | Sucrose (24%) | ||||
|
Revised NFCS Range of scale 0‐10 in term infants 0‐9 in preterm infants A lower score = less pain Grunau 1987 |
The mean NFCS score in the sucrose (24%) + swaddling group was 0.05 | The mean NFCS score in the sucrose (24%) group was higher than in the sucrose (24%) + swaddling group: 0.40 (95% CI 0.19 to 0.61) | 343 (1) |
⊕⊕⊕⊝ moderate | Bias: there was a risk of performance and detection bias in this study as the coder could have distinguished the different groups Consistency: as this was a single study a rating of consistency was N/A Precision: this study had a very large sample size with a narrow 95% CI (no concerns about precision) Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean NFCS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NFCS score with its 95% CI'. CI: confidence interval; N/A: not applicable; NFCS: Neonatal Facial Coding System | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
9.
| Sucrose (24%) compared with sucrose (24%) + NNS + swaddling for pain associated with heel lance | |||||
|
Patient or population: neonates with pain associated with heel lance Settings: hospital Intervention: sucrose (24%) Comparison: sucrose (24%) + NNS + swaddling | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Sucrose (24%) + NNS + swaddling | Sucrose (24%) | ||||
|
Revised NFCS Range of scale 0‐10 in term infants 0‐9 in preterm infants A lower score = less pain Grunau 1987 |
The mean NFCS score in the sucrose (24%) + NNS + swaddling group was 0.02 | The mean NFCS score in the sucrose (24%) group was higher than in the sucrose (24%) + NNS + swaddling group: 0.43 (95% CI 0.23 to 0.63) | 337 (1) |
⊕⊕⊕⊝ moderate | Bias: there was a risk of performance and detection bias in this study as the coder could have distinguished the different groups Consistency: as this was a single study a rating of consistency was N/A Precision: this study had a very large sample size with a narrow 95% CI (no concerns about precision) Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean NFCS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NFCS score with its 95% CI'. CI: confidence interval; N/A: not applicable; NFCS: Neonatal Facial Coding System; NNS: non‐nutritive sucking | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
10.
| Sucrose (20%) compared with facilitated tucking for pain associated with repeated heel lances | |||||
|
Patient or population: neonates with pain associated with repeated heel lances Settings: hospital Intervention: sucrose (20%) Comparison: facilitated tucking | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Facilitated tucking | Sucrose (20%) | ||||
|
Total Bernese Pain Scale for Neonates (BPSN) during heel lance Range: The BPSN contains 9 items; 3 physiologic (HR, RR, oxygen saturation) and 6 behavioural (grimacing, body movements, crying, skin colour, sleeping patterns, consolation) items. Each item is scored on a 3 point scale (0‐3) points. Higher scores for the behavioural items and greater changes in the physiological items indicate increased pain, whereas a total score of < 11 is considered nonpainful. Cignacco 2004 |
The mean Total Bernese Pain Scale in the control group was 9.75 | The mean Total Bernese Pain Scale was lower in the in the intervention group: MD ‐2.27 (95% CI ‐4.66 to 0.12) | 48 (1) |
⊕⊕⊕⊝ moderate | Bias: there was some risk of bias in this study as the intervention was not blinded Consistency: as this was a single study consistency was N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
Total Bernese Pain Scale for Neonates during recovery from heel lance Range: See comments above. Cignacco 2004 |
The mean Total Bernese Pain Scalein the control group was 5.18 | The mean Total Bernese Pain Scale was lower in the intervention group: MD ‐0.31 (95% CI ‐1.72 to 1.10) | 48 (1) | ⊕⊕⊕⊝ moderate | Bias: There was some risk of bias in this study as the intervention was not blinded. Consistency: As this was a single study consistency was N/A. Precision: This was a small study and the CI was wide around the point estimate. Directness: The study was conducted in the target population ‐ no concerns about indirectness. |
| *The basis for the assumed risk was 'The mean Total Bernese Pain Scale for Neonates score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the mean Total Bernese Pain Scale for Neonates score with its 95% CI'. CI: confidence interval; MD mean difference;N/A not applicable | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
11.
| Sucrose (20%) compared with facilitated tucking and sucrose (20%) for pain associated with repeated heel lances | |||||
|
Patient or population: neonates with pain associated with repeated heel lances Settings: hospital Intervention: sucrose (20%) Comparison: facilitated tucking and sucrose (20%) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Facilitated tucking and sucrose (20%) | Sucrose (20%) | ||||
|
Total Bernese Pain Scale for Neonates during heel lance (preterm infants) Range: The BPSN contains 9 items; 3 physiologic (HR, RR, oxygen saturation) and 6 behavioural (grimacing, body movements, crying, skin colour, sleeping patterns, consolation) items. Each item is scored on a 3 point scale (0‐3) points. Higher scores for the behavioural items and greater changes in the physiological items indicate increased pain, whereas a total score of < 11 is considered nonpainful. A lower score = less pain Cignacco 2004 |
The mean Total Bernese Pain Scale for Neonates in the control group was 7.53 | The mean Total Bernese Pain Scale for Neonates in the intervention group was lower than in the control group: MD ‐0.05 (95% CI ‐2.16 to 2.06) | 47 (1) | ⊕⊕⊕⊝ moderate | Bias: there was some risk of bias in this study as the intervention was not blinded Consistency: as this was a single study consistency was N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
Total Bernese Pain Scale for Neonates during recovery (preterm infants) Range: See information above. A lower score = less pain Cignacco 2004 |
The mean Total Bernese Pain Scale for Neonates in the control group was 4.23 | The mean Total Bernese Pain Scale for Neonates in the intervention groups was higher than in the control group: MD 0.64 (95% CI ‐0.73 to 2.01) | 47 (1) | ⊕⊕⊕⊝ moderate | Bias: there was some risk of bias in this study as the intervention was not blinded Consistency: as this was a single study consistency was N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean Total Bernese Pain Scale for Neonates score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the mean Total Bernese Pain Scale for Neonates score with its 95% CI'. CI: confidence interval; MD mean difference;N/A not applicable | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
12.
| Sucrose (12%) compared with water for pain associated with venipuncture | |||||
|
Patient or population: neonates with pain associated with venipuncture Settings: hospital Intervention: sucrose (12%) Comparison: water | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water | Sucrose (12%) | ||||
|
NIPS score in term and preterm infants Range of scale 0‐7 A lower score = less pain Lawrence 1993 |
The mean NIPS score was 3.8 in the water group | The mean NIPS score in the sucrose group was lower than in the water group: 0.90 (95% CI ‐1.81 to 0.01) | 111 (1) |
⊕⊕⊕⊝ moderate | Bias: it is uncertain if outcome assessors were blinded Consistency: as this was a single study a rating of consistency was N/A Precision: this study had a relatively large sample size with a narrow 95% CI (no concerns about precision) Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean NIPS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NIPS score with its 95% CI'. CI: confidence interval; N/A: not applicable; NIPS: Neonatal Infant Pain Scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
13.
| Sucrose (24% to 30%) compared with control (sterile water or no treatment) for pain associated with venipuncture | |||||
|
Patient or population: neonates with pain associated with venipuncture Settings: hospital Intervention: sucrose (24% to 30%) Comparison: sterile water or no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Sterile water or no treatment | Sucrose (24% to 30%) | ||||
|
PIPP score during venipuncture Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score ranged across control groups from 8.9 to 9.2 | The WM PIPP score in the intervention group was lower than in the control group: 2.79 (95% CI‐3.76 to ‐1.83) | 213 (2 groups in 1 study) |
⊕⊕⊕⊕ high | Bias: low risk of bias Consistency: this study reported on two groups of infants; one group was born to non‐diabetic mothers and the other group to diabetic mothers. There was no heterogeneity for the results of the two groups I2 = 0% Precision: this study (with the two groups combined) had a large sample size with a narrow 95% CI (no concerns about precision) Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean PIPP score in the control groups according to the values reported in the Assumed risk column. The corresponding risk was the mean in the intervention groups for the PIPP score with its 95% CI'. CI: confidence interval; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A: not applicable; WM weighted mean | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
14.
| Sucrose (24% to 30%) compared with sucrose (24% to 30%) + EMLA/liposomal lidocaine cream on the skin for pain associated with venipuncture | |||||
|
Patient or population: neonates with pain associated with venipuncture Settings: hospital Intervention: sucrose (24% to 30%) Comparison: sucrose (24% to 30%) + EMLA/liposomal lidocaine cream on the skin | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Sucrose (24% to 30%) + EMLA/liposomal lidocaine cream on the skin | Sucrose (24% to 30%) | ||||
|
PIPP score Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score was 7.2 in the control group | The mean PIPP score in the intervention group was higher than in the control group: 1.30 (95% CI ‐0.12 to 2.72) | 76 (1) |
⊕⊕⊕⊝ moderate | Bias: low risk of bias Consistency: as this was the only study consistency was N/A Precision: this was a relatively small study and the CIs were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
PIPP score during post‐injection period Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score was 7.1 in the control group | The mean PIPP in the intervention group was higher than in the control group: 0.60 (95% CI ‐0.73 to 1.93) | 76 (1) |
⊕⊕⊕⊝ moderate | Bias: low risk of bias Consistency: as this was the only study consistency was N/A Precision: this was a relatively small study and the CIs were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
DAN score during venipuncture (preterm infants) Range of scale 0‐10 A lower score = less pain (Carbajal 1997) |
The mean DAN score was 6.4 in the control group | The mean DAN score in the intervention group was higher than in the control group: 1.30 (95% CI 0.26 to 2.34) | 76 (1) |
⊕⊕⊕⊝ moderate | Bias: low risk of bias Consistency: as this was the only study consistency was N/A Precision: this was a relatively small study and the CIs were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
DAN score during the post‐injection period Range of scale 0‐10 A lower score = less pain (Carbajal 1997) |
The mean DAN score in the control group was 5.7 | The mean DAN score in the intervention groups was higher than in the control group: 1.40 (95% CI 0.03 to 2.77) | 76 (1) |
⊕⊕⊕⊝ moderate | Bias: low risk of bias Consistency: as this was the only study consistency was N/A Precision: this was a relatively small study and the CIs were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean PIPP scores and the DAN scores in the control groups according to the values reported in the Assumed risk column. The corresponding risk was the means in the intervention groups for the PIPP scores and the DAN scores with their 95% CI'. CI: confidence interval; DAN: Douleur Aiguë du Nouveau‐né Scale; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A: not applicable | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
15.
| Sucrose (20% to 25%) compared with water or no intervention for pain associated with intramuscular injection | |||||
|
Patient or population: neonates with pain associated with intramuscular injection Settings: hospital Intervention: sucrose (20% to 25%) Comparison: water or no intervention | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water or no intervention | Sucrose (20% to 25%) | ||||
|
NIPS during 1‐2 minutes after IM injection Range of scale 0‐7 A lower score = less pain Lawrence 1993 |
The mean NIPS score in the control group was 5.2 | The mean NIPS score in the intervention group was lower than in the control group: ‐2.30 (95% CI ‐2.93 to ‐1.67) | 60 (1) |
⊕⊕⊝⊝ low | Bias: concerns about bias for random sequence generation, allocation concealment and lack of blinding for performance and detection Consistency: as this was the only study consistency was N/A Precision: this was a relatively small study and the CIs were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
PIPP during IM injection (term infants) ‐ Infants of non‐diabetic and diabetic mothers Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score ranged across control groups from 7.2 to 8.5 | The WM PIPP score in the intervention groups was lower than in the control group: ‐1.05 (95% CI ‐1.98 to ‐0.12) | 232 (2 groups in 1 study) |
⊕⊕⊕⊕ high | Bias: no concerns about bias Consistency: there was high consistency between the two groups in this study. I2 = 0% Precision: this was a large study and the CIs were narrow around the point estimates Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean NIPP score and the PIPP scores in the control groups according to the values reported in the Assumed risk column. The corresponding risk was the mean in the intervention groups for the NIPP score and the PIPP scores with their 95% CI'. CI: confidence interval; IM: intramuscular; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; N/A: not applicable; WM: weighted mean | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
16.
| Sucrose (25%) compared with glucose (25%) for pain associated with intramuscular injection | |||||
|
Patient or population: neonates with pain associated with intramuscular injection Settings: hospital Intervention:sucrose (25%) Comparison: glucose (25%) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Glucose (25%) | Sucrose (25%) | ||||
|
NIPS during 1‐2 minutes after immunization Range of scale 0‐7 A lower score = less pain Lawrence 1993 |
The mean NIPS score was 3 in the control group | The mean NIPS score in the intervention group was lower than the mean score in the control group: ‐ 0.10 (95% CI ‐0.89 to 0.69) | 60 (1) |
⊕⊕⊝⊝ low | Bias: concerns about bias for random sequence generation, allocation concealment and lack of blinding for performance and detection Consistency: no concerns as this was the only study Precision: this was a relatively small study and the CIs were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean NIPS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NIPS score with its 95% CI'. CI: confidence interval; N/A: not applicable; NIPS: Neonatal Infant Pain Scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
17.
| Sucrose (24%) compared with sterile water for pain associated with bladder catheterization | |||||
|
Patient or population: neonates with pain associated with bladder catheterization Settings: hospital Intervention: sucrose (24%) Comparison: sterile water | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Sterile water | Sucrose (24%) | ||||
|
Change in DAN score Range of scale 0‐10 A lower score = less pain (Carbajal 1997) |
The mean change in DAN score in the control group was 5.29 | The mean change in DAN score was lower in the intervention group than in the control group: ‐ 2.43 (95% CI ‐4.50 to ‐0.36) | 33 (1) |
⊕⊕⊕⊝ moderate | Bias: There was a low risk of bias in this study Consistency: as this was a single study concerns about consistency were N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean Change in DAN score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the NIPS score with its 95% CI'. CI: confidence interval;DAN: Douleur Aiguë du Nouveau‐né Scale; N/A: not applicable | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
18.
| Sucrose (24%) compared with distilled water for pain associated with orogastric tube insertion | |||||
|
Patient or population: neonates with pain associated with orogastric tube insertion Settings: hospital Intervention: sucrose (24%) Comparison: distilled water | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Distilled water | Sucrose (24%) | ||||
|
PIPP score intra procedure Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score was 7.9 in the control group | The mean PIPP score in the intervention group was lower than in the control group: ‐0.30 (95% CI ‐1.33 to 0.73) | 105 (1) |
⊕⊕⊕⊕ high | Bias: there was a low risk of bias in this study. The protocol for the study was available to us and there were no deviations Consistency: as this was a single study concerns about consistency were N/A Precision: this was a moderately sized study and the CI was narrow around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
PIPP score 30 seconds post procedure Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score was 5.6 in the control group | The mean PIPP score in the intervention group was lower than in the control group ‐1.30 (95% CI ‐2.31 to ‐0.29) | 105 (1) |
⊕⊕⊕⊕ high | Bias: there was a low risk of bias in this study. The protocol for the study was available to us and there were no deviations Consistency: as this was a single study concerns about consistency were N/A Precision: this was a moderately sized study and the CI was narrow around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
PIPP score 1 min post procedure Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score was 4.6 in the control group | The mean PIPP score in the intervention group was lower than in the control group: ‐0.50 (95% CI ‐1.40 to 0.40) | 105 (1) |
⊕⊕⊕⊕ high | Bias: there was a low risk of bias in this study. The protocol for the study was available to us and there were no deviations Consistency: as this was a single study concerns about consistency were N/A Precision: this was a moderately sized study and the CI was narrow around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean PIPP scores in the control group according to the values reported in the Assumed risk column. The corresponding risk was the mean in the intervention groups for the PIPP scores with their 95% CI'. CI: confidence interval; N/A: not applicable; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
19.
| Sucrose (24%) by syringe + swaddled +pacifier compared with water by syringe + swaddled + pacifier for pain/distress associated with retinopathy of prematurity (ROP) examination | |||||
|
Patient or population: neonates with pain/distress associated with ROP examination Settings: hospital Intervention: sucrose (24%) by syringe + swaddled + pacifier Comparison: water by syringe + swaddled + soother | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water by syringe + swaddled + pacifier | Sucrose (24%) by syringe + swaddled +pacifier | ||||
|
PIPP during exam Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score was 14 in the control group. | The mean PIPP score in the intervention group was neither lower nor higher than in the control group: 0.00 (95% CI ‐2.08 to 2.08 | 32 (1) | ⊕⊕⊝⊝ low | Bias: the authors did not describe how the random sequence was generated, nor did they describe how allocation concealment was achieved Consistency: as this was the only study concerns about consistency were N/A Precision: this was a small study and the CI were wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean PIPP score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the PIPP scores with its 95% CI'. CI: confidence interval; N/A not applicable; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; ROP: retinopathy of prematurity examination | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
20.
| Sucrose (24% to 33%) (sucrose or sucrose + NNS) compared with water (or water + NNS) for pain/distress associated with retinopathy of prematurity (ROP) examination | |||||
|
Patient or population: neonates Settings: hospital Intervention: sucrose (24% to 33%) (sucrose or sucrose + NNS) Comparison: water (or water + NNS) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water (or water + NNS) | Sucrose (24% to 33%) (or sucrose + NNS) | ||||
|
PIPP score during eye examination Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score ranged across control groups from 11.4 to 16.4 | The WM PIPP score in the intervention groups was lower than in the control group: ‐2.15 (95% CI ‐2.86 to ‐1.43) | 134 (3) |
⊕⊕⊕⊝ moderate | Bias: there were some concerns about risk of bias in these studies for random sequence generation and allocation concealment Consistency: the findings were consistent with each other; I2 = 46% (low heterogeneity) Precision: this was a moderately sized meta‐analysis and the CI was narrow around the typical point estimate Directness: the studies were conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean PIPP score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the PIPP scores with its 95% CI'. CI: confidence interval; N/A: not applicable; NNS: non‐nutritive sucking; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age; ROP: retinopathy of prematurity examination; WM: weighted mean | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
21.
| Sucrose (24%) compared with EMLA for pain associated with circumcision | |||||
|
Patient or population: neonates undergoing circumcision Settings: hospital Intervention: sucrose (24%) Comparison: EMLA | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| EMLA | Sucrose (24%) | ||||
|
N‐PASS score during circumcision Range of scale 0‐13 A lower score = less pain (Hummel 2010) |
The mean N‐PASS score in the control group was 5.8 | The mean N‐PASS score in the intervention group was higher: MD 2.40 (95% CI 1.85 to 2.95) | 60 (1) | ⊕⊕⊝⊝ low | Bias: there were concerns about risk of bias in this study for random sequence generation (unclear risk) and high risk of performance and detection bias Consistency: as this was the only study concerns about consistency were N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
N‐PASS score after 5 min Range of scale 0‐13 A lower score = less pain (Hummel 2010) |
The mean N‐PASS score in the control group was 3.1 | The mean N‐PASS score in the intervention group was higher: MD 1.40 (95% CI 0.74 to 2.06) | 60 (1) | ⊕⊕⊝⊝ low | Bias: there were concerns about risk of bias in this study for random sequence generation (unclear risk) and high risk of performance and detection bias Consistency: as this was the only study concerns about consistency were N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean N‐PASS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the N‐PASS score with its 95% CI'. CI: confidence interval; EMLA: eutectic mixture of local anaesthetic; MD mean difference; N/A not applicable; N‐PASS: Neonatal Pain Agitation and Sedation Scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
22.
| Sucrose (24%) compared with EMLA + sucrose (24%) for pain associated with circumcision | |||||
|
Patient or population: neonates undergoing circumcision Settings: hospital Intervention: sucrose (24%) Comparison: EMLA + sucrose (24%) | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| EMLA + sucrose (24%) | Sucrose (24%) | ||||
|
N‐PASS score during circumcision Range 0‐13 A lower score = less pain (Hummel 2010) |
The mean N‐PASS score in the control group was 5.2 | The mean N‐PASS score in the intervention group was higher: MD 3.00 (95% CI 2.42 to 3.58) | 60 (1) | ⊕⊕⊝⊝ low | Bias: there were concerns about risk of bias in this study for random sequence generation (unclear risk) and high risk of performance and detection bias Consistency: as this was the only study concerns about consistency were N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
N‐PASS score after 5 min Range 0‐13 A lower score = less pain (Hummel 2010) |
The mean N‐PASS score in the control group was 3.3 | The mean N‐PASS score in the intervention group was higher: MD 1.20 (95% CI 0.49 to 1.91) | 60 (1) | ⊕⊕⊝⊝ low | Bias: there were concerns about risk of bias in this study for random sequence generation (unclear risk) and high risk of performance and detection bias Consistency: as this was the only study concerns about consistency were N/A Precision: this was a small study and the CI was wide around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean N‐PASS score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the N‐PASS score with its 95% CI'. CI: confidence interval; EMLA: eutectic mixture of local anaesthetic; MD mean difference; N/A not applicable; N‐PASS: Neonatal Pain Agitation and Sedation Scale | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
23.
| Sucrose (24%) compared with for water stress associated with echocardiography | |||||
|
Patient or population: neonates undergoing echocardiography Settings: hospital Intervention: sucrose (24%) Comparison: water | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| No intervention | Sucrose (24%) | ||||
| PIPP | The mean PIPP score was 7.4 in the control group Range of scale 0‐21 for infants < 28 weeks PMA and 0‐18 for infants > 36 weeks PMA A lower score = less pain (Stevens 1996; Stevens 2014a) |
The mean PIPP score in the intervention group was lower than in the control group: ‐ 2.15 (95% CI ‐3.30 to ‐1.00) | 104 (1) |
⊕⊕⊝⊝ low | Bias: there were concerns about allocation concealment bias and performance blinding in this study Consistency: as this was a single study concerns about consistency were N/A Precision: this was a moderately sized study and the CI was narrow around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean PIPP score in the control group according to the value reported in the Assumed risk column. The corresponding risk was the mean in the intervention group for the PIPP scores with its 95% CI'. CI: confidence interval; N/A: not applicable; PIPP: Premature Infant Pain Profile; PMA: postmenstrual age | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
24.
| Sucrose (24%) compared with water for potentially painful procedures for a period of seven days | |||||
|
Patient or population: neonates with pain associated with potentially painful procedures for 7 days Settings: hospital Intervention: sucrose (24%) Comparison: water | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Water | Sucrose (24%) | ||||
|
Motor development and vigor (MDV) domain of the NAPI tool Normative mean and SD at 36 weeks PMA 63 (14.5) Higher scores are associated with more mature behaviour (Snider 2005) |
The mean MDV score at 40 weeks PMA was 76.48 in the control group | The mean MDV score at 40 weeks PMA in the intervention group was lower than in the control group: ‐1.83 (95% CI ‐8.59 to 4.93) | 93 (1) |
⊕⊕⊕⊕ high | Bias: there were no concerns about bias in this study Consistency: as this was a single study concerns about consistency were N/A Precision: this was a moderately sized study and the CI was narrow around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
|
Alertness and orientation (AO) domain of the NAPI tool Normative mean and SD at 36 weeks PMA 54 (19.4) Higher scores are associated with more mature behaviour (Snider 2005) |
The mean AO score at 40 weeks PMA was 67.77 in the control group | The mean AO score at 40 weeks PMA in the intervention group was higher than in the control group: 3.09 (95% CI ‐6.49 to 12.67) | 93 (1) |
⊕⊕⊕⊕ high | Bias: there were no concerns about bias in this study Consistency: as this was a single study concerns about consistency was N/A Precision: this was a moderately sized study and the CI was narrow around the point estimate Directness: the study was conducted in the target population ‐ no concerns about indirectness |
| *The basis for the assumed risk was 'The mean MDV and the mean AO scores in the control group according to the values reported in the Assumed risk column. The corresponding risk was the means in the intervention groups for the MDV and AO scores with their 95% CI'. AO: 'alertness and orientation' CI: confidence interval; MDV: 'motor development and vigor'; N/A: not applicable; PMA post menstrual age; SD: standard deviation | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Background
Description of the condition
Infants hospitalized in the Neonatal Intensive Care Unit (NICU) undergo frequent painful tissue‐breaking procedures for diagnostic and therapeutic purposes. Epidemiological research from audits in NICUs in high‐income countries estimates that neonates undergo an average of four to 16 painful exposures per day (Carbajal 2008; Johnston 2011; Lago 2013; Roofthooft 2014; Stevens 2011). Similarly, estimates of procedural pain in infants in low‐ and middle‐income countries ‐ including those in South America (Linhares 2011), Asia (Chen 2012; Jeong 2014), and Africa (Kyololo 2014) ‐ are equally high.
Treatment for prevention or relief of procedural pain for neonates in the NICU varies widely in practice and is generally less than optimal (Carbajal 2008; Johnston 2011). An audit of 3508 tissue‐damaging procedures performed on 582 infants across a one week period in 14 Canadian NICUs indicated that only about 50% were accompanied by any form of pharmacologic, behavioural or physical pain‐relieving intervention (Johnston 2011). Although clinical practice guidelines outlining strategies to relieve pain from surgery, medical illness, and major procedures exist (Lee 2014), their quality is inconsistent.
Untreated pain in neonates, and particularly preterm infants, during a critical time in brain development, has the potential to result in significant immediate and long‐term consequences (Grunau 2013; Walker 2013). These consequences include: (a) changes in somatosensory processing and altered sensitivity to future painful stimuli; (b) impaired neuro‐anatomical development; and, (c) behavioural, emotional and learning disabilities (Bartocci 2006; Brummelte 2012; Grunau 2013; Holsti 2005; Slater 2006; Tu 2007; Vinall 2014a; Vinall 2014bWalker 2013; Zwicker 2013).
Description of the intervention
Administration of oral sucrose with or without non‐nutritive sucking (NNS) (e.g. pacifiers) and other sweet solutions (e.g. glucose) prior to and during painful procedures have been the most frequently studied interventions for relief of procedural pain in neonates (Bueno 2013; Stevens 2013). Analgesic effects persist up to approximately one year of age, although the robustness of the effect may decline in older infants compared to neonates (Harrison 2013).
How the intervention might work
Analgesic, calming and stress reducing effects of sucrose were first reported in infant rats (Blass 1989; Ren 1997; Shide 1989). These effects occurred rapidly, persisted for several minutes and were blocked by systemic opioid receptor antagonists. They were age dependent and had differential maturational effects consistent with changes in the endogenous analgesic mechanisms and the development of the gustatory and pain pathways (Anseloni 2002). Researchers contend that sucrose may act at, or be mediated at, the level of the brainstem (Anseloni 2002; Anseloni 2005; Fitzgerald 2015), which has been shown to be a primary relay in the ascending gustatory pathway in animals (Anseloni 2005).
In human infants, the analgesic and calming effects of sweet‐tasting solutions are speculated to influence endogenous opioid pathways activated by the sweet taste (Blass 1994). However, the underlying mechanisms for calming and pain may differ. These mechanisms may be additive or synergistic, but most likely depend on normal functioning of central mechanisms. Further research has demonstrated that the effects are associated with the potency of sweet taste (sweeter, more concentrated solutions), rather than volume of solution administered; with sucrose being more analgesic than glucose and fructose, and lactose not demonstrating any analgesic effects (Blass 1994). In a systematic review/meta‐analysis of the efficacy of sucrose for procedural pain management in 13 trials and 982 neonates, Stevens 1997a found that the proportion of time crying decreased with 0.24 g to 0.48 g sucrose (i.e. 2 mL of a 12% to 24% solution) administered orally two minutes prior to a painful procedure (e.g. heel lance or venipuncture).
Despite advances through research on the potential mechanisms of sucrose analgesia, further research is required to enhance our understanding of the opioid pathways involved in the developing infant, the effectiveness of sucrose when administered with concomitant opioids and/or other pain‐relieving interventions, and with repeated use for extended periods of time (Harrison 2012).
Why it is important to do this review
This systematic review is a substantive update of the original 1998 Cochrane Review (Stevens 1998), and the updates completed in 2001, 2004, 2010 and 2013 (Stevens 2001; Stevens 2004; Stevens 2010; Stevens 2013). The most recently updated version in 2013 included 57 studies ‐ with 4730 term and preterm neonates ‐ demonstrated that single doses of sucrose were effective and safe for reducing pain associated with several single painful procedures performed in stable full‐term and preterm neonates (Stevens 2013). Tissue‐damaging procedures included heel lance, venipuncture, eye examinations, circumcision, subcutaneous injections, bladder catheterization, and nasogastric tube insertion. A meta‐analysis of four studies indicated that a range of sucrose doses (from a few drops to 0.5 mL of 24% solution) significantly reduced composite infant pain scores.
Repeated use, or use in extremely preterm and sick infants has rarely been addressed (Harrison 2009). Johnston reported that preterm infants of less than 31 weeks gestational age who were exposed to more than 10 repeated doses of sucrose a day were more prone to poorer attention and motor development in early life (Johnston 2002; Johnston 2007). Other studies have not reported differences between sucrose and non‐sucrose groups (Banga 2015; Gaspardo 2008; Stevens 2005b; Taddio 2009a). However, comprehensive studies evaluating repeated dosing of sucrose for all painful procedures during hospitalizations of the neonate have not been undertaken. Concerns regarding the repeated use of sucrose and cumulative volumes administered during painful procedures in the developing brains of preterm infants have been raised (Ranger 2014).
Although 24% or 25% sucrose solutions are the most widely used concentrations for treatment of procedural pain in clinical practice, there is considerable variation in the volumes of sucrose reported to be effective in diminishing pain, and a more than 20‐fold variation in doses used in clinical practice (Taddio 2009a). There is currently no clear recommendation regarding the optimal volume of sucrose for analgesia in infants, or for the use of sucrose in the presence of opioid analgesia, despite frequent administration of opioids to infants during their NICU stay (Harrison 2009; Johnston 2011). Ideally the amount of sucrose (mg/kg body weight) administered should be reported. A critical evaluation of guidelines for incorporating sucrose as an intervention for preventing or ameliorating procedural pain in infants indicates that recommendations for practitioners are not clear (Lee 2014). These knowledge gaps and lack of consistent strategies for effective application of knowledge most likely contribute to the current suboptimal or non‐standardized utilization of sucrose in NICUs.
Objectives
To determine the efficacy, effect of dose, method of administration and safety of sucrose for relieving procedural pain in neonates as assessed by validated composite pain scores, physiological pain indicators (heart rate, respiratory rate, saturation of peripheral oxygen in the blood, transcutaneous oxygen and carbon dioxide (gas exchange measured across the skin ‐ TcpO2, TcpCO2), near infrared spectroscopy (NIRS), electroencephalogram (EEG), or behavioural pain indicators (cry duration, proportion of time crying, proportion of time facial actions (e.g. grimace) are present), or a combination of these and long‐term neurodevelopmental outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) that evaluated the effect of sucrose analgesia in newborn infants undergoing painful procedures. We did not include quasi‐randomised trials. We included only published studies and no abstracts were included as we have identified discrepancies in numbers of infants enrolled between abstracts and final publications (Walia 1999) (see Selection of studies). We did not impose language restrictions.
For the 2013 update, we broadened our inclusion criteria to include RCTs in which the efficacy of sucrose was assessed during any minor painful procedure (i.e. other than heel lance and venipuncture), as well as after repeated doses of sucrose. The same inclusion criteria were used for this 2016 update.
Types of participants
We included studies that assessed term, preterm, or both term and preterm neonates, with maximum postnatal age of 28 days after reaching 40 weeks' postmenstrual age (PMA).
Types of interventions
Interventions included administration of sucrose via oral syringe, dropper or in addition to a pacifier for treatment of procedural pain. For the 2013 update of the review we extended inclusion criteria to all studies that used sucrose as an intervention for any acute painful procedure including heel lance, venipuncture, subcutaneous injection, intramuscular injection, arterial puncture, circumcision, bladder catheterization, insertion of orogastric or nasogastric tube, and eye examination for retinopathy of prematurity (ROP). Control group interventions included breastfeeding, breast milk or milk formula, water (sterile, tap, distilled, spring), local anaesthetics, pacifier, positioning/containing, facilitated tucking, warmth no treatment, and various concentrations of glucose. For this 2016 update we added echocardiography as a stressful intervention and laser acupuncture as a control group intervention.
Types of outcome measures
For this 2016 update we reorganized the outcomes as follows:
Primary outcomes
Composite pain score: A composite pain score includes indicators of pain from multiple dimensions (e.g. behavioural and physical ‐ e.g.PIPP‐R). Multidimensional behavioural pain score: A multidimensional behavioural pain score includes multiple indicators of pain but from one dimension (e.g. NFCS). Validated pain scores in this review included the Premature Infant Pain Profile (PIPP; Stevens 1996), Revised PIPP (PIPP‐R; Stevens 2014a), Douleur Aiguë du Nouveau‐né Scale (DAN; Carbajal 1997), Neonatal Infant Pain Scale (NIPS; Lawrence 1993), Neonatal Facial Coding System (NFCS; Grunau 1987), Neurobehavioural Assessment of Preterm Infants (NAPI; Snider 2005), Neonatal Pain Agitation and Sedation Scale (N‐PASS; Hummel 2010) and the Bernese Pain Scale for Neonates (BPSN; Cignacco 2004). We did not include the results for the COMFORTneo Scale in the 'Summary of findings' tables (van Dijk 2009), as it has not been fully validated. We do report on the results from studies that used that pain scale in the Results section.
Long‐term neurodevelopmental outcomes (assessed by a standardized and validated assessment tool, a child developmental specialist, or both) at 18 to 24 months, or at any later age in childhood.
Secondary outcomes
Individual behavioural pain indicators:
cry duration;
proportion of time crying;
proportion of time facial actions were present;
facial actions.
Individual physiological pain indicators:
heart rate;
respiratory rate;
oxygen saturation of the blood;
TcpO2;
TcpCO2;
cortisol level;
NIRS;
EEG.
Any adverse effects reported.
Search methods for identification of studies
Electronic searches
We used the standard methods of Cochrane Neonatal. We carried out electronic searches for relevant RCTs in MEDLINE (PubMed; 1950 to February 2016), EMBASE (1980 to February 2016), CINAHL (1982 to February 2016) and the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 1, 2016). Search terms used in the PubMed search are shown in Appendix 1. Similar search terms were used in the other databases. One author (AO) selected the trials for inclusion or exclusion and one other author (JY) confirmed the selections.
Searching other resources
We searched bibliographies and personal files (BS, JY AO). We did not include unpublished studies as they have not been peer‐reviewed. We obtained additional information from published studies if needed. We have listed identified abstracts under excluded studies. We did not apply any language restrictions. We searched the ISRCTN database (www.isrctn.com), the National Institute of Health Clinical Trials database (clinicaltrials.gov), and the International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp) in February 2016. One author (AO) selected the ongoing trials for inclusion and exclusion and another author (JY) confirmed the selections.
Data collection and analysis
Selection of studies
We did not include abstracts as we have identified discrepancies in numbers of infants enrolled between abstracts and final publications (Walia 1999). In the 2013 update and the current 2016 update the types of participants were more clearly defined to include maximum postnatal age of 28 days after reaching 40 weeks' PMA. As sucrose has become more widely evaluated as an analgesic for a variety of different acute painful procedures, we no longer limited our search to those studies evaluating pain due to heel lance and venipuncture.
Data extraction and management
For this 2016 update three reviewers (AO, SH, AS) extracted data separately using pre designed forms. We compared the data and resolved differences. We had additional data provided by investigators for four studies we had previously included (Allen 1996; Harrison 2003; Johnston 1999; Stevens 1999).
Assessment of risk of bias in included studies
Three reviewers (AO, SH, AS), who were not blinded to trial authors or institutions, assessed the methodological quality of each study independently. Three authors (BS, JY, AO) have published trials that were included in this update of the review. For these trials two authors (SH, AS) did the data abstraction and RoB assessments.
For this update the following issues were evaluated and entered into the 'Risk of bias' tables.
-
Random sequence generation (selection bias): for each included study, we categorized the risk of selection bias as:
low risk ‐ adequate (any truly random process, e.g. random number table; computer random number generator);
high risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk ‐ no, or unclear, information provided.
-
Allocation concealment (selection bias): for each included study, we categorized the risk of bias regarding allocation concealment as:
low risk ‐ adequate (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk ‐ no, or unclear, information provided.
-
Blinding (performance bias): for each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received. As our study population consisted of neonates they would all be blinded to the study intervention:
low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group);
high risk ‐ inadequate, personnel aware of group assignment;
unclear risk ‐ no, or unclear, information provided.
-
Blinding (detection bias): for each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. (As our study population consisted of neonates they would all be blinded to the study intervention.) Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods used with regard to detection bias as:
low risk ‐ adequate follow‐up was performed with assessors blinded to group assignment;
high risk ‐ inadequate, assessors were aware of group assignment at follow‐up ;
unclear risk ‐ no, or unclear, information provided.
-
Incomplete outcome data (attrition bias): for each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re included missing data in the analyses. We categorized the methods with respect to the risk of attrition bias as:
low risk ‐ adequate (< 10% missing data);
high risk ‐ inadequate (> 10% missing data);
unclear risk ‐ no, or unclear, information provided.
-
Selective reporting (reporting bias): for each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk ‐ adequate (where it was clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review had been reported);
high risk ‐ inadequate (where not all the study's prespecified outcomes had been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; or the study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk ‐ no, or unclear, information provided (the study protocol was not available).
-
Other bias: for each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design, or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk ‐ no concerns of other bias raised;
high risk ‐ e.g. investigators were aware of results before the end of the study; differences exist between abstracts and final publications of papers concerning the number of participants enrolled;
unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the authors.
If necessary, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Quality of evidence
For this update the quality of the evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach in order to assess the quality of the body of evidence relating to the following primary outcomes for each of the comparisons listed under Objectives (Guyatt 2011a; Schünemann 2009):
composite pain score
long‐term neurodevelopmental outcomes (assessed by a standardized and validated assessment tool, a child developmental specialist, or both) at 18 to 24 months, or at any later age in childhood. No study reported on long‐term neurodevelopmental outcomes, and therefore this outcome could not be included in the GRADE assessment.
We used the RevMan table editor (RevMan 2014), which is based on GRADEprofiler (GRADEpro 2014), to create ’Summary of findings’ tables. We produced a summary of the intervention effect and a measure of quality for each of the main comparisons and outcomes listed above (if reported) using the GRADE approach (Guyatt 2011a). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. The GRADE approach considers evidence from RCTs as being of high quality, but this quality rating may be downgraded on the basis of any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias (Guyatt 2011a). The GRADE approach results in an assessment of the quality of a body of evidence as one of four grades explained below.
High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Further research is very likely to have and important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. We are very uncertain about the estimate (Schünemann 2013).
The review authors independently assessed the quality of the evidence found for outcomes identified as critical or important for clinical decision making, namely: composite pain score. No study reported on long‐term neurodevelopmental outcomes.
When we considered the risk of bias, the presence of inadequate concealment of allocation or randomised assignment, incomplete follow‐up or unblinded outcome assessment reduced our confidence in the effect estimates, and we downgraded the quality of evidence accordingly (Guyatt 2011b). We evaluated consistency by comparing the similarity of point estimates, extent of overlap of confidence intervals and statistical criteria, including measurement of heterogeneity (I2). We downgraded the quality of evidence when inconsistency across study results was present, large and unexplained (i.e. some studies suggested important benefit and others no effect or harm without a clinical explanation) (Guyatt 2011c). We assessed precision according to the 95% confidence interval around the pooled estimation (Guyatt 2011d). If trials had been conducted in populations other than the target population, we would have downgraded the quality of evidence because of indirectness (Guyatt 2011e). Publication bias was not applicable, as only three or fewer studies were included in each analysis. We entered data (i.e. pooled estimates of the effects and corresponding 95% confidence intervals) and made explicit judgments for each of the above aspects assessed in the 'Summary of findings' tables. Because of the large number of comparisons (n = 37) and outcomes under each comparison we elected to include only those comparisons that included outcomes for validated pain scores (BPSN, DAN, NAPI, NFCS, NIPS, N‐PASS, PIPP, PIPP‐R). All judgements involving the assessment of the study characteristics described above are explained in foot notes or comments in the 'Summary of findings' tables.
Measures of treatment effect
We performed statistical analyses using RevMan 5.3 (RevMan 2014). We analyzed categorical data using risk ratio (RR), risk difference (RD) and the number needed to treat for an additional beneficial outcome (NNTB) or additional harmful outcome (NNTH) if the RD was statistically significant. We analyzed continuous data using mean difference (MD). We reported the 95% confidence interval (CI) on all estimates. Three authors (AO, SH and AS) used the formulas reported in the Cochrane Handbook for Systematic Reviews of Interventions (Section 7.7.3.2) to convert 95% confidence intervals to standard deviations (SD) (Higgins 2011; RevMan 2014). Following advice from Dr Michael Bracken (statistician of the Cochrane Neonatal Review Group) we decided not to convert medians and interquartile or full ranges to means and SDs.
Unit of analysis issues
We did not identify any cluster randomised trials and so did not encounter any unit of analysis issues.
Dealing with missing data
Many authors did not report means and SDs for the outcomes. We transformed 95% CIs to SDs using the techniques described under Measures of treatment effect. Many studies reported the results in graph form only, and we could not incorporate the findings in RevMan‐analyses.
Assessment of heterogeneity
We report the I2 statistic for all analyses in which more than one trial was included. We categorized the heterogeneity using the following labels and cut‐offs for the results of the I2 test; less than 25%, no heterogeneity present; 25% to 49%, low heterogeneity; 50% to 74%, moderate heterogeneity; and 75% or more, high heterogeneity. If we detected statistical heterogeneity, we explored the possible causes (e.g. differences in study quality, participants (term or preterm infants), intervention regimens or outcome assessments) using post hoc subgroup analyses.
Assessment of reporting biases
We planned to construct forest plots if there were at least 10 studies included in one meta‐analysis that assessed the same outcome in comparable trials of the same intervention in the same population, however, no single meta‐analysis fulfilled this criterion.
Data synthesis
We used the statistical package RevMan 5.3 provided by Cochrane (RevMan 2014). For meta‐analyses, we reported a weighted mean difference (WMD) with 95% CI using a fixed‐effect model for continuous outcome measures if at least two studies were included in the analysis, otherwise we reported the mean difference (MD). For categorical outcomes we reported the typical (if at least two trials were included) risk ratio (RR), risk difference (RD), and, if the RD was statistically significant, we planned to report the NNTB or NNTH. All values were reported with their 95% CIs.
Subgroup analysis and investigation of heterogeneity
We did not make separate subgroup comparisons for different painful/stressful procedures (heel lance, venipuncture, arterial puncture, subcutaneous injections, pain associated with IM injections (hepatitis B immunization and injection of vitamin K), bladder catheterization, insertion of nasogastric or orogastric tubes, ROP eye examination, circumcision, and echocardiography exam) or for multiple procedures.
Sensitivity analysis
Under each comparison for the outcomes we report the results in the term and the preterm populations separately and combined these whenever data were available.
Results
Description of studies
Two authors (AO, JY) identified an additional 20 studies for inclusion in this current 2016 update through the searches of the literature (Abbasoglu 2015; Al Qahtani 2014; Asmerom 2013; Banga 2015; Cignacco 2012; Dilli 2014; Gray 2012; Gray 2015; Leng 2013; Leng 2015; Liaw 2011; Liaw 2013; Marin Gabriel 2013; Milazzo 2011; Pandey 2013; Potana 2015; Simonse 2012; Suhrabi 2014; Thakkar 2016; Tutag Lehr 2015). The flow chart for the literature searches is shown in Figure 1. In this current update the authors excluded the study by Scaramuzzo 2013 as it was a quasi‐randomised controlled trial. In addition two studies were classified as awaiting classification due to a need for translation (Moradi 2012b; Moradi 2012a). One newly identified study was excluded after translation as the infants were older (mean 8.5 months) than the accepted age for inclusion in this review (28 days) (Aziznejad 2013). We excluded the study by Fernandez 2003, which was added at the 2013 update, as it assessed the effects of heel stroke.
1.

Study flow diagram: review update
The authors excluded one study that had been included at the previous 2013 update as they reclassified it as a quasi‐randomised trial (Ozdogan 2010), and excluded another that had previously been awaiting classification, as the word 'random' did not appear anywhere in the text (Akman 2002). The authors identified several papers as secondary publications to studies, and these are now listed under their primary study references, namely: the 2004 paper published by Boyer and co‐workers is now listed under Johnston 2002; the Angeles 2015 paper is now listed under Asmerom 2013; and the Yin 2015 paper is now listed under Liaw 2013. The study by Singh 2001 is still awaiting classification as we have not been able to obtain a reprint.
The authors identified a total of 18 ongoing studies for this 2016 update of the review, details are about these are provided in Characteristics of ongoing studies (Campbell‐Yeo 2013; ISRCTN59514984; ISRCTN73259137; NCT02344368; Montanholi 2012; NCT01742520; NCT01190995; NCT01438008; NCT01552993; NCT01800318; NCT01894659; NCT01931020; NCT02133716; Passariello 2014; Philip 2012; Roue 2013; Shah 2015; Stevens 2014).
For the 2013 and 2016 updates of this review, the inclusion criteria were expanded to include any painful/stressful procedure (rather than heel lance and venipuncture only), and included studies that assessed the efficacy of repeated doses of sucrose.
No studies assessing long‐term neurodevelopmental outcomes were identified in the 2013 update nor in this current 2016 update.
Included studies
A total of 74 studies (enrolling 7049 infants) are included in this systematic review. Thirty‐eight of these studies focused on term infants (Abbasoglu 2015; Allen 1996; Al Qahtani 2014; Altun‐Köroğlu 2010; Basnet 2010; Blass 1997; Blass 1999; Carbajal 1999; Codipietro 2008; Gormally 2001; Gray 2012; Gray 2015; Greenberg 2002; Guala 2001; Haouari 1995; Herschel 1998; Isik 2000a; Kaufman 2002; Leng 2013; Leng 2015; Liaw 2011; Marin Gabriel 2013; Mathai 2006; Ogawa 2005; Örs 1999; Overgaard 1999; Ramenghi 1996b; Rogers 2006; Rushforth 1993; Slater 2010; Stang 1997; Suhrabi 2014; Taddio 2008; Taddio 2011; Thakkar 2016; Tutag Lehr 2015; Unceta‐Barranechea 2008; Yilmaz 2010), while 31 included preterm infants only (Abad 1996; Acharya 2004; Asmerom 2013; Biran 2011; Boyle 2006; Bucher 1995; Cignacco 2012; Dilli 2014; Elserafy 2009; Gal 2005; Gaspardo 2008; Grabska 2005; Harrison 2003; Johnston 1997; Johnston 1999; Johnston 2002; Kristoffersen 2011; McCullough 2008; Milazzo 2011; Mitchell 2004; Mucignat 2004; O'Sullivan 2010; Okan 2007; Pandey 2013; Ramenghi 1996a; Ramenghi 1999; Rush 2005; Simonse 2012; Stevens 1999; Stevens 2005a; Storm 2002), and five included both preterm and term infants (Banga 2015; Gibbins 2002; Liaw 2013; Montoya 2009; Potana 2015). Details of each study are outlined in the Characteristics of included studies table.
The studies were conducted in 22 different countries; USA (17), Canada (9), UK (9), Turkey (7), India (5), France (3), Spain (3). In China, Italy, Norway, Saudi Arabia, Switzerland, and Taiwan two studies were conducted in each country and one study was conducted in each of Australia, Brasil, Colombia, Denmark, Iran, Ireland, Japan, Nepal, and the Netherlands.
Each included study is described in the Additional tables, which are organized according to the type of painful procedure to which the infants were exposed. As stated in the methods section for this update, three authors (AO, SH, AS) transcribed 95% CIs to SDs whenever possible. If authors reported data in such a way that we could not include the results in RevMan‐analyses, we reported the findings according to the authors in the Additional tables. The results of 35 studies could not be included in RevMan‐analyses (Abad 1996; Acharya 2004; Allen 1996; Altun‐Köroğlu 2010; Blass 1997; Blass 1999; Bucher 1995; Carbajal 1999; Codipietro 2008; Elserafy 2009; Gal 2005; Gaspardo 2008; Gormally 2001; Gray 2012; Johnston 1997; Johnston 2002; Kaufman 2002; Kristoffersen 2011; Leng 2013; Liaw 2013; Marin Gabriel 2013; McCullough 2008; Mucignat 2004; O'Sullivan 2010; Okan 2007; Örs 1999; Overgaard 1999; Ramenghi 1996a; Ramenghi 1996b; Ramenghi 1999; Rushforth 1993; Stevens 2005a; Storm 2002; Thakkar 2016; Yilmaz 2010).The results of those studies are summarized after the analyses for each comparison.
Painful procedures
Heel lance was the most predominant painful procedure, and was studied in 38 trials (Abbasoglu 2015; Altun‐Köroğlu 2010; Asmerom 2013; Blass 1997; Blass 1999; Bucher 1995; Cignacco 2012; Codipietro 2008; Gibbins 2002; Gormally 2001; Greenberg 2002; Guala 2001; Haouari 1995; Harrison 2003; Isik 2000a; Johnston 1997; Johnston 1999; Leng 2013; Leng 2015; Liaw 2013; Marin Gabriel 2013; Mathai 2006; Okan 2007; Overgaard 1999; Ramenghi 1996a; Ramenghi 1996b; Ramenghi 1999; Rushforth 1993; Simonse 2012; Slater 2010; Stevens 1999; Stevens 2005a; Storm 2002; Thakkar 2016; Tutag Lehr 2015; Unceta‐Barranechea 2008; Yilmaz 2010; Örs 1999) (Table 25). In nine studies, infants were observed during venipuncture (Abad 1996; Acharya 2004; Basnet 2010; Biran 2011; Carbajal 1999; Elserafy 2009; Gaspardo 2008; Montoya 2009; Taddio 2011) (Table 26). One study assessed both heel lance and venipuncture (Ogawa 2005) (Table 27), while another assessed arterial puncture (Milazzo 2011) (Table 28). In two studies, infants were assessed during subcutaneous injections (Allen 1996; Mucignat 2004) (Table 29), and in four studies during intramuscular injection (immunization for hepatitis B) (Gray 2012; Gray 2015; Liaw 2011; Suhrabi 2014) (Table 30). One study assessed infants during bladder catheterization (Rogers 2006) (Table 31), and three studies assessed infants during nasogastric or orogastric tube insertion (Kristoffersen 2011; McCullough 2008; Pandey 2013) (Table 32). Seven studies assessed infants undergoing an examination for ROP (Boyle 2006; Dilli 2014; Gal 2005; Grabska 2005; Mitchell 2004; O'Sullivan 2010; Rush 2005) (Table 33), while four studies involved circumcision (Al Qahtani 2014; Herschel 1998; Kaufman 2002; Stang 1997) (Table 34). Three studies assessed multiple painful procedures (Banga 2015; Johnston 2002; Taddio 2008) (Table 35). One trial assessed stress during echocardiography (Potana 2015) (Table 36).
1. Trials assessing pain during heel lances.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Abbasoglu 2015 | 42 term newborns, undergoing heel lancing between postnatal days 3 to 8 as part of neonatal screening | Heel lance |
|
NIPS Crying time |
Mean and SD | The NIPS score was significantly lower in the sucrose group 3.66 ± 1.01 vs 4.52 ± 0.87 in the laser group (P = 0.006) The crying time was significantly lower in the sucrose group 46.66 s ± 37.82 vs 97.95 s ± 34.23 in the laser group (P = 0.000) Data included in RevMan‐analyses |
|
Altun‐Köroğlu 2010 |
75 full‐term infants | Heel lance |
The volume of 3mL was provided in 3 doses (1 mL prior to, 1 mL immediately before, and 1mL after the procedure) |
NFCS, crying time, duration of crying, HR | Median and IQR |
Median crying time, duration of first cry and tachycardia, and time needed to return to baseline = longest in the distilled water group. Significantly shorter in the hind milk group when compared to distilled water group (P = 0.022, P = 0.008, P 0.009 and P = 0.038, respectively) No statistically significant differences observed between the hind milk and sucrose group for all measures Maximum HR in hind milk group was significantly lower than distilled water group (184 beats/min vs. 196 beats/min, P = 0.031) Significant reduction in average NFCS score. 1st min NFCS score and 5th min NFCS score in the hind milk group compared to the distilled water group (P = 0.006, P = 0.017 and P = 0.021, respectively) No data included in RevMan‐analyses |
| Asmerom 2013 | 131 preterm infants ≤ 36.5 weeks’ gestation who:
|
Heel lance |
|
PIPP after 2 min, plasma hypoxanthine, uric acid, xanthine, allantoin HR, oxygen saturation |
Mean and lowest and highest values | Oral sucrose given before a single heel lance significantly decreased behavioural markers of pain Sucrose increased markers of ATP use, as evidenced by significant increases over time in plasma hypoxanthine and uric acid concentrations We received the PIPP scores as means and SD from Dr D Angeles and the data were included in RevMan‐analyses |
| Blass 1997 | 72 term infants, 22 h to 40 h old | Heel lance | 2 mL of one of the following solutions:
n = 8 for all groups |
Crying time (%) during blood collection and 1, 2 and 3 min after heel lance Mean % of crying time per min at 1, 2 and 3 min after heel lance (recovery period) |
Mean proportions Graphically reported |
Significantly less crying time during blood collection in the sucrose group (47%) compared to the water group (92%, P = 0.015) No data could be used in RevMan‐analyses |
| Blass 1999 | 40 term newborn infants, 34 h to 55 h old | Heel lance | Prior to heel lance:
|
% time crying 3 min after heel lance Mean change in HR % time grimacing |
Mean percentage Mean change (beats/min) Graphically reported |
2 mL 12% (0.24 g) sucrose alone diminished cry duration from heel lance compared to water (8% vs. 50%, P = 0.003) and water with pacifier (8% vs. 35%, P = 0.002). Pacifier with 12% sucrose more effective in reducing cry duration compared to water with pacifier (5% vs. 35%, P = 0.001) or water alone (50%, P = 0.002) Mean HR increased significantly from treatment to heel lance in infants receiving water alone (mean increase of 17 beats/min, P = 0.002) and water with pacifier (mean increase of 20 beats/min, P = 0.005). Mean increase in HR also increased for the 12% sucrose and pacifier group (mean difference of 7.4 beats/min, P = 0.05) but not for infants receiving 12% sucrose alone (mean difference of 5.9 beats/min, P = 0.142) 12% sucrose reduced grimacing compared to water (P = 0.0003). 12% sucrose with pacifier reduced grimacing compared to water (P = 0.001) and pacifier alone (P = 0.04) No data could be used in RevMan‐analyses |
| Bucher 1995 | 16 preterm infants, 27 to 34 weeks' GA, PNA approximately 42 days | Heel lance |
|
% time crying Recovery time until crying stopped Increase in HR Recovery time for HR TcPO2 (max increase ‐ kPa); TcPO2 (max decrease ‐ kPa); TcPO2 (difference between baseline and 10 min after end of intervention ‐ kPa); TcPCO2 (max decrease ‐ kPa); TcPCO2 (difference between baseline and 10 min after the end of intervention), recovery time for respirations Cerebral near‐infrared spectroscopy (cerebral oxyhaemoglobin, deoxyhaemoglobin, total blood volume) |
Median, IQR | Cry duration (% of total duration of intervention) significantly reduced in 2 mL 50% (1.0 g) sucrose group (71.5%) compared to control group (93.5%, P = 0.002) Median increase in HR (beats/min) after heel lance was significantly reduced in the 2 mL 50% (1.0 g) sucrose group (35 beats/min) compared to water (51 beats/min), P = 0.005 No significant differences between groups with respect to measures for TcPO2 (P = 0.05) and TcPCO2 (P = 0.21) Decrease in cerebral blood volume was not significant between sucrose and placebo groups Data were not presented for effects of sucrose or water prior to cross over. No data could be used in RevMan‐analyses |
| Cignacco 2012 | 71 preterm infants between 24 weeks 0/7 and 32 weeks 0/7 PMA | Repeated heel lances |
|
Bernese Pain Scale for Neonates. Data collection occurred during:
The BPSN contains 9 items: 3 physiologic (HR, respiratory rate, and oxygen saturation) and 6 behavioral (grimacing, body movements, crying, skin colour, sleeping patterns, consolation) items. 3 phases (baseline, heel stick, recovery) of 5 heel stick procedures were videotaped for each infant |
Mean, SD | Sucrose with and without FT had pain‐relieving effects even in preterm infants of GA ≤ 32 weeks having repeated pain exposures. These interventions remained effective during repeated heel sticks across time. FT was not as effective Data included in RevMan‐analyses |
| Codipietro 2008 | 101 term infants Mean PMA:
|
Heel lance |
|
Duration of first cry (s), % crying time in first 2 min, and % crying time during blood sampling HR (beats/min) increase from baselines at 30 s following commencement of procedure Oxygen saturation decrease PIPP during blood sampling, 2 min after heel lance |
Median, range | Median duration (s) of first cry: breastfeeding group; 3 (range 0 to 120) compared to sucrose group; 21 (range 0 to 120), P = 0.004 % crying during first 2 min: breastfeeding group 4 (range 0 to 100) compared to sucrose; 45 (range 0 to 100) (P < 0.0001) % crying during sampling: breastfeeding group; 8 (range 0 to 100) compared to sucrose 56.5 (range 0 to 100) (P = 0.0003) Median increase in HR (beats/min) from baseline to 30 s after start of heel lance was significantly lower in breastfeeding group; 13 (range ‐12 to 54) compared to sucrose group; 22 (range ‐32 to 65) (P = 0.005) Median decrease in oxygen saturation (%) from baseline to 30 s after start of heel lance was significantly greater in sucrose group; ‐3 (range ‐30 to 1) compared to breastfeeding group; ‐1 (range ‐14 to 2)) (P = 0.001) Median PIPP scores significantly lower in breastfeeding group (3.0) compared to sucrose group (8.5) (P < 0.0001 Data could not be used in RevMan analyses |
| Gibbins 2002 | 190 preterm and term infants, mean GA 33.7 weeks, < 7 days PNA | Heel lance | 2 min prior to heel lance:
|
PIPP scores at 30 and 60 s after heel lance Incidence of adverse events |
Reported means, SD | Statistically significant difference in mean PIPP scores at both 30 s (P < 0.001) and 60 s (P < 0.001) after heel lance in favour of sucrose group and sucrose + NNS group. Post hoc Tukey tests showed infants who received sucrose + pacifier had significantly lower PIPP scores after heel lance at 30 s (mean 8.16, SD 3.24) compared to infants receiving sucrose alone (mean 9.77, SD 3.04, P = 0.007), or water + NNS (mean 10.19, SD 2.67, P < 0.001). At 60 s after heel lance, PIPP scores were significantly lower for sucrose + NNS group (mean 8.78, SD 4.03) compared to the sucrose alone group (mean 11.20, SD 3.25, P = 0.005) and water + NNS group (mean 11.20, SD 3.47, P = 0.007). No significant differences in PIPP scores found between sucrose alone group or water + NNS group at both follow‐up times 3 neonates in the sucrose alone group desaturated during the study period. No adverse events occurred with neonates randomized to the sucrose + NNS group Data used in RevMan‐analyses |
| Gormally 2001 | 94 term newborns, mean GA 39.4 weeks, 2nd or 3rd day of life | Heel lance |
All solutions given 3 times at 30‐s intervals |
% time crying 1, 2, 3 min after heel lance Mean HR before intervention, 1, 2, 3 min after heel lance, mean vagal tone index before intervention, 1, 2, 3 min after heel lance Pain concatenation scores for facial activity before intervention, 1, 2, 3 min after heel lance |
Not reported | Crying decreased over time (P < 0.001) but no significant interaction noted for time with holding, taste or holding and taste. Effect of taste on crying was significant (P < 0.05) in favour of sucrose group. Effect of holding not statistically significant (P = 0.09)). No statistically significant interaction between taste and holding to reduce crying (P = 0.37). Effect of combined interventions was additive Although no significant differences in mean HR due to holding or sucrose as main effects, there was significant interaction between holding and taste (P < 0.004), indicating synergistic effect that was also dependent on pre‐intervention HR (P < 0.004). No significant main effects noted for vagal tone; as with HR, effect of vagal tone was dependent on pre‐intervention vagal tone for both holding and taste interventions (P < 0.03). Pre‐intervention levels interacted to decrease HR and vagal tone in infants who had higher rates before interventions Pain concatenation scores measuring facial expressions of pain decreased over time (P < 0.001). Only the effect of holding reduced pain scores (P <0.02). No difference as to whether infant received sucrose (taste main effect P = 0.68) No data could be used in RevMan‐analyses |
| Greenberg 2002 | 84 term newborns, approximately 17 to 19 h old | Heel lance |
|
Duration of cry from procedure phase to 3 min postprocedure Vagal tone and vagal tone index Salivary cortisol levels |
Mean and SE reported for time crying (s) | Significant decrease in duration of cry for the sugar‐coated pacifier group compared to the control group (P = 0.001) and the water‐moistened pacifier group (P = 0.006). Lower vagal tone during heel lance in the sugar‐coated pacifier group compared to the control group (P = 0.008) and oral sucrose group (P = 0.018). Lower vagal tone index in the sugar‐coated pacifier group compared to control group at heel lance (P = 0.019), and 6 to 10 min after (P = 0.007) and 11 to 15 min (P = 0.049) after heel lance No significant differences were found in salivary cortisol levels across groups (no P value reported) Mean duration of cry used in RevMan‐analyses |
| Guala 2001 | 140 term, 38 to 41 weeks' GA | Heel lance |
|
HR before, during and 3 min after heel lance | Mean, SD | No significant differences were found between groups for differences in HR at each of the 3 phases of the heel lance (P value reported for 3 min after heel lance, P = 0.087; the difference between 3 min after heel lance and during heel lance, P = 0.068) Data used in RevMan‐analyses |
| Haouari 1995 | 60 term infants, 37 to 42 weeks' gestation, 1 to 6 days of age | Heel lance | 2 min prior to heel lance:
All solutions were given by syringe on the tongue over < 1 min |
Total time crying over 3 min. Time of first cry after lance % change in HR at 1, 3, 5 min after heel lance |
Median, IQR Reported means and SEM |
After heel lance, significant decreases in total crying time and duration of first cry in 50% sucrose group compared with water (P = 0.02). Significant reduction in median time crying at end of first min (P < 0.02) in 50% sucrose group (35 s; range 14 to 60) compared with water (60 s; range 50 to 60). In second min, duration of cry was significantly less in 50% sucrose group (0 s; range 0 to 25) and in 25% sucrose group (18 s; range 0 to 55) compared to water (60 s; range 40 to 60), P = 0.003 and P = 0.02, respectively A significant trend towards a reduction in crying time with greater concentrations of sucrose (P = 0.007). There was a similar trend in the reduction of the duration of first cry with increasing concentrations of sucrose (P = 0.004) Significant decrease in % change in HR 3 min after heel lancing (P = 0.02) in the 50% sucrose group (mean 0.1%, SEM 3.3) compared to water group (mean 17.5%, SEM 6.0) We transcribed SEMs to SDs and included data in RevMan‐analyses |
| Harrison 2003 | 99 sick hospitalised infants Mean GA (SD):
(author provided data on a subset of infants from a larger study (n = 128) that fulfilled our inclusion criteria) |
Heel lance |
For infants weighing ≤ 1500 g the dose was reduced to 0.5 mL |
Duration of cry until 5‐s pause, incidence and duration of crying time during the heel lance and squeeze and during the 3‐min recovery period HR at baseline, heel lance, during heel lance and 1, 2, 3 min post heel lance Oxygen saturation (%) at baseline, heel lance, during heel lance and 1, 2, 3 min post heel lance 4‐point subset of the NFCS (brow bulge, eye squeeze, nasolabial furrow, stretch mouth) at heel lance, during heel lance and 1, 2, 3 min post heel lance |
Mean, SD (collected from authors) | Mean (SD) length of first cry (s) was higher in the water group (70.5 (83.6)) compared to the sucrose group (46.8 (63.1)). The sucrose group cried 57.1% of the procedure time compared to 58.8% in the water group. The mean (SD) total duration of cry during the heel lance was 84.7 s (68.8) in the sucrose group and 87.4 s (87.1) in the water group. The mean (SD) HR upon heel lance was 163.0 (17.9) beats/min in the sucrose group and 159.5 (19.2) beats/min in the water group. HR at 30 s from the beginning of the procedure was 175.4 (22.2) and 172.8 (23.6) beats/min in the sucrose and water groups, respectively. The HR in both groups decreased after the procedure to 152.1 (22.5) beats/min in the sucrose group and 154.2 (29.1) beats/min in the water group 2 min post heel lance Results of oxygen saturation (%) were similar between the 2 groups Mean (SD) facial scores were significantly reduced at heel lance (2.74 (1.8)) in the sucrose group compared to the water group (2.94 (1.6)) (P = 0.02) and at 1 min (P = 0.04) and 2 min (P = 0.046) post heel lance. No significant differences occurred at 3 min post heel lance Data included in RevMan‐analyses |
| Isik 2000a | 113 healthy term newborns GA 37 to 42 weeks, median PNA 2 days, range 2 to 5 days | Heel lance | Solution syringed into the anterior third of the tongue for 1 min prior to heel lance:
|
Mean cry time during 3 min after lance Mean maximum HR 3 min from heel lance Mean recovery time for HR % change in HR at 1, 2, 3 min after heel lance |
Reported means and SEM | Infants who received 30% sucrose (mean crying time of 61 s) cried significantly less than those who received 30% glucose (mean crying time of 95 s), 10% glucose (mean crying time of 103 s) or sterile water (mean crying time of 105 s) (P = 0.02) No significant difference between groups with respect to maximum HR after heel lance (P = 0.71), or mean recovery time (P = 0.09). No significant difference found in % change in HR at 1 or 3 min after heel lance (P = 0.14, P = 0.53, respectively). At 2 min after heel lance, % change in HR favoured group receiving sucrose (P = 0.05) compared to other groups Data used in RevMan‐analyses |
| Johnston 1997 | 85 preterm infants, 25 to 34 weeks' GA, 2 to 10 days of age | Heel lance | Solutions via syringe into the mouth just prior to heel lance:
|
HR at baseline and 3 x 30‐s blocks Behavioural facial actions (NFCS) at baseline and 3 x 30‐s blocks |
Means and SD not reported | Although HR increased across all phases of procedure (P < 0.04), there were no significant differences noted between groups (P = 0.566) Decrease in % facial action in 24% sucrose alone group and combined 24% sucrose and rocking group compared to water group (P < 0.02) No data could be used in RevMan‐analyses |
| Johnston 1999 | 48 preterm neonates mean GA 31 weeks, range 25 to 34 weeks, within 10 days of birth | Heel lance |
|
PIPP scores in 5 x 30 s blocks | Reported means, SD in graph form | Statistically significant difference between groups (P < 0.0001) for mean PIPP scores. Post hoc analysis found significantly lower PIPP scores with repeated doses of 24% sucrose compared to placebo groups across all blocks of time (P < 0.05). PIPP scores for repeated doses of 24% sucrose were significantly lower compared to single doses of 24% sucrose (8.25 vs. 6.25) only at last block of time (P < 0.05). PIPP scores for single doses of 4% sucrose compared to placebo showed trend towards statistical significance in favour of 24% sucrose (P = 0.07) Data obtained from the author on 31 infants that could be used in RevMan‐analyses |
| Leng 2013 | 560 term neonates: GA: 37 to 42 weeks; birthweight: 2500 g‐4000 g; age 3 to 28 days |
Heel lance |
|
Increase in HR (beats/min) Decrease in oxygen saturation (%) Pain levels (test not specified) |
Reported means and full ranges, not IQRs | The average HR increase 3, 5 and 10 min after procedure in the 25% and 50% glucose groups, 12% and 24% and 30% sucrose groups was significantly lower than those in the placebo group (P < 0.01 or 0.05). The average HR increase 3 min after procedure in the sucrose groups was lower than that in the glucose groups (P < 0.01). At 3 min after heel lance neonates who received 30% sucrose had a significantly lower average HR increase than those who received 12% and 24% sucrose (both P < 0.05). The average oxygen saturation decrease 3, 5, 10 min after procedure was significantly lower than those in the placebo group (P < 0.01). The average oxygen saturation decrease 3 min after procedure in the sucrose groups was significantly lower than that in the glucose groups (P < 0.01). The average pain score 3, 5, 10 min after procedure was significantly lower than those in the placebo group (P < 0.01). The average pain score 3 min after procedure in the sucrose groups was significantly lower than that in the glucose groups (P < 0.01). We could not include in RevMan‐analyses data for 30% sucrose vs. sterile water for increase in HR and decrease in oxygen saturation at 3 min after heel lance as data were reported as means and full ranges In addition results were reported late at 3, 5 and 10 min after heel lance |
| Leng 2015 | 671 term neonates: GA 37 to 42 weeks at birth; PNA 3 to 28 days; birthweight 2500 g to 4000 g; Apgar score ≥ 8 at 5 min after birth; resting HR 120 to 140 beats/min; resting O2 saturation ≥ 95%; required neonatal congenital metabolism disease screening or blood glucose test |
Shallow (blood glucose test) and deep (congenital metabolism disease screening) heel lance |
|
Revised NFCS, increase in HR (%), decrease in oxygen saturation (%) | Factor analysis mean and SD |
A significant synergistic analgesic effect was observed between the sucrose + NNS and sucrose + swaddling groups in both the shallow (P = 0.015) and deep heel lance (P = 0.007) procedure. The sucrose + NNS + swaddling group exhibited the lowest pain score. For the deep heel lance procedure, the sucrose + NNS group had a significantly lower increase in HR % and decrease in oxygen saturation (%) than the sucrose group (P = 0.000, P = 0.001), while this difference was not observed in the shallow heel lance procedure. No difference was found between the sucrose and sucrose + swaddling groups, in terms of different physiological parameters NNS and swaddling had synergistic effects on pain relief when used with oral sucrose. For the deep heel lance procedure, oral sucrose combined with NNS and swaddling provided the best pain relief effect For the shallow heel lance procedure, addition of NNS and swaddling did not improve the effects Data included in RevMan‐analyses |
| Liaw 2013 | 110 infants (PMA 26.4 to 37 weeks) | Heel lance |
The 3 interventions outlined above were used in different combinations: NNS + FT (n = 22) FT + sucrose (n = 21) NNS + sucrose (n = 21) NNS + sucrose + FT (n = 23) Control: routine care (n = 23) |
Infants’ behavioural states (quiet sleep, active sleep, transition state, active awake, quiet awake, fussing or crying) | Graph form, CI, rate ratio and SE | Infants receiving NNS + sucrose + FT or NNS + sucrose experienced
52.8% (P = 0.023) and 42.6% (P = 0.063) more quiet‐sleep occurrences than those receiving routine care after adjusting for phase, baseline states, non‐treatment sucking during baseline and recovery, positioning, and infants’ characteristics. Infants receiving FT + sucrose, NNS + sucrose, NNS + sucrose + FT and NNS + FT experienced 77.3% (P < 0.001), 72.1% (P = 0.008), 51.5% (P = 0.017), and 33.0% (P = 0.105) fewer occurrences of fussing or crying, respectively, than those receiving routine care after adjusting for related factors No data could be used in RevMan‐analyses |
| Marin Gabriel 2013 | Healthy, term neonates, GA 37 to 41 weeks | Heel lance |
These interventions were used in different combinations (BF + SSC; Sucrose + SSC; SSC; Sucrose) |
NIPS; HR; crying time (s); crying in blood sampling; number of heel lances (%) | Median and IQR | The breastfeeding + SSC group achieved a significant lower median NIPS score (value = 1) compared with other groups (value = 2, 4 and 4, respectively). The percentage of neonates with moderate to severe pain was lower in the breastfeeding + SSC group. Both the breastfeeding + SSC group and
the sucrose + SSC group experienced a significantly lower percentage of crying compared with the SSC group No data used in RevMan‐analyses |
| Mathai 2006 | 104 term neonates, PNA > 24 h. Mean PNA: sucrose group: 48 h; distilled water group: 44 h |
Heel lance |
|
Time of first cry (s), total cry (s) HR before heel lance, 2 min after heel lance and 4 min after heel lance oxygen saturation (%) before heel lance, 2 min after heel lance and 4 min after heel lance DAN scale before the heel lance and 30 s, 1 min, 2 min, 4 min after heel lance |
Mean, SD | No significant difference between sucrose group and any other group for time of first cry NNS or rocking significantly reduced total duration of cry, P < 0.05 No significant difference in HR between the groups at any time point No significant difference in oxygen sasturation (%) between the groups at any time point Significantly reduced DAN scores at 30 s after the heel lance for the sucrose group (mean 7.6, SD 14, P < 0.05); however, this was not sustained at 1, 2 and 4 min NNS or rocking significantly decreased the DAN scores at 2 and 4 min post heel lance, P < 0.05 Reported data that could be used in RevMan‐analyses |
| Okan 2007 | 31 healthy, preterm newborns, mean GA 30.5 weeks, mean PMA 32.3 weeks | Heel lance |
The solutions were administered via syringe onto the anterior portion of the tongue Cross over study ‐ Infants received all 3 interventions at different times Data from the first treatment in each infant could not be derived |
Duration of first cry and total crying time HR, oxygen saturation (%), RR (breaths/min) and NFCS scores at baseline, during heel lance, and 1, 2, 3, 4 and 5 min post heel lance |
Mean, SD | Significantly increased duration of first cry and total crying time in the water group compared to the sucrose and glucose groups (P = 0.005 and P = 0.007, respectively). No significant differences in cry characteristics were observed between the sucrose and glucose groups Significantly higher HR in the water group (mean 175, SD 20.8) compared to the sucrose (mean 166, SD 17.6) and glucose groups (mean 165, SD 17.5) at 1 min following heel lance (P = 0.007). No significant differences between the sucrose and glucose groups The study found no significant results for respiratory rate or oxygen saturation between groups. Significantly higher NFCS score in the placebo group in the 4th min following heel lance (mean 1.3, SD 2.0) and 5th min following heel lance (mean 1.0, SD 1.0) compared to the sucrose (mean 0.5, SD 1.7; mean 0.3, SD 1.3, respectively) and glucose groups (mean 0.2, SD 0.5; mean 0.1, SD 0.3, respectively) (P = 0.009 at 4th min and P = 0.046 at 5th min). There were no significant differences between the sucrose and glucose groups Data could not be used in RevMan‐analyses. |
| Örs 1999 | 102 healthy, term infants, GA 37 to 42 weeks, median PNA 1.6 days, range 1 to 15 days | Heel lance |
All solutions syringed onto anterior part of tongue for 1 min Heel prick performed 2 min after intervention |
Median cry time during 3 min after lance % change HR 1, 2 and 3 min after heel lance |
Median, IQR | Significant decrease in crying times for 25% sucrose group (median 36, IQR 18 to 43) compared to human milk (median 62, IQR 29 to 107) and sterile water (median 52, IQR 32 to 158) (P = 0.0009). Recovery time for crying was significantly reduced in 25% sucrose group (median 72, IQR 48 to 116) compared to human milk (median 112, IQR 72 to 180) and sterile water (median 124, IQR 82 to 180) (P = 0.004) % change in HR after heel lance was significantly lower in the group receiving 25% sucrose compared to groups receiving human milk and sterile water at 1, 2 and 3 min (P = 0.008, P = 0.01, P = 0.002, respectively) No data were used in RevMan‐analyses |
| Overgaard 1999 | 100 newborn term infants, mean PNA 6 days, range 4 to 9 days | Heel lance |
In addition to the intervention to which the infants were assigned, parents were instructed to comfort the infant as much as possible |
Median crying time during heel lance, fraction of crying during sampling, crying time during first min after end of sampling, total crying time Change in HR at 0 and 1 min Oxygen saturation (%) at 0 and 1 min NIPS scores 1 min after heel lance and 1 min after blood sampling |
Median, 5th and 95th percentiles | Median duration of first cry in group receiving 50% sucrose was significantly lower (18 s (2 to 75)) compared to placebo group (22 s (11 to 143)) (P = 0.03). Median crying time during heel lance in the sucrose group was lower (26 s (2 to 183)) compared to placebo group (40 s (12 to 157)) (P = 0.07). Median fraction of crying during sampling in 50% sucrose group was significantly lower (43% (4 to 100)) compared to placebo group (83% (20 to 100)) (P = 0.004). Median crying time during first min after end of sampling in 50% sucrose group was significantly lower (3 s (0 ‐ 58)) compared to placebo group (16 s (0 to 59)) (P = 0.004). Median total time crying in 50% sucrose group was significantly lower (30 s (2 to 217)) compared to placebo group (71 s (13 to 176)) (P = 0.007) No significant HR differences between groups (P = 0.6) No significant differences between groups with respect to changes in oxygen saturation (%) (P = 0.9) Median NIPS scores 1 min after heel lance were lower in 50% sucrose group compared to placebo group (3 (0 to 7) and 6 (0 to 7), respectively; P = 0.04). Median NIPS scores 1 min after end of blood sampling were lower in 50% sucrose group (0 (0 to 7)) compared to placebo group (2 (0 to 7)) (P = 0.05) As means were not reported we could not include the results in RevMan‐analyses |
| Ramenghi 1996a | 15 preterm infants, GA 32 to 34 weeks, > 24 h of age | Heel lance |
The solutions were administered via syringe into the baby’s mouth for 1 min Cross‐over study |
Duration of first cry and % time crying 5 min after lance HR (at ‐2, 0, 1, 3 and 5 min from heel lance) Behavioural scores (4 facial expressions and the presence of crying) ‐2, ‐1 , 0, 1, 2, 3 and 5 min Quality/intensity of sucking |
||
| Ramenghi 1996b | 60 term infants, GA 37 to 42 weeks, 2 to 5 days old | Heel lance |
|
Duration of first cry after lance, % time crying over 3 min after heel lance % change in HR over 5 min (at ‐2, 0, 1, 3 and 5 min from heel lance) Behavioural scores (4 facial expressions and the presence of crying) ‐2, ‐1, 0, 1, 2, 3 and 5 min |
Median, IQR | Significant decrease in duration of first cry and % crying during 3 min after heel lance in the 25% sucrose, 50% sucrose and Calpol groups (P = 0.02) (data in graph form only) Significant increase in HR for 3 min after heel lance in water group compared with 50% sucrose group and Calpol group (P = 0.009) Pain score (0 to 5) was significantly higher in water group (score = 2, range 1 to 5) than in other 3 groups: 50% sucrose group (score = 0, range 0 to 3); 25% sucrose group (score = 0, range 0 to 2); Calpol group (score = 0, range 0 to 1) (P = 0.05) No data were used in RevMan‐analyses |
| Ramenghi 1999 | 30 preterm infants, GA 32 to 36 weeks, PNA > 24 h | Heel lance |
The cross‐over concerned mode of delivery of the solution ‐ intraorally or via NG tube. Infants stayed in their original group assigned to receive either sucrose or sterile water |
% cry over 5 min after sampling Behavioural scores (4 facial expressions and the presence of cry) at 1, 3 and 5 min after the lance for a total behavioural score |
Median, IQR | Median % cry in intraoral water group was 22% (IQR 10.6 to 40) and 27% (IQR 11.6 to 47) for infants in NG tube water group. Median % cry in intraoral 25% sucrose group was 6% (IQR 0.6 to 15) and 18.3% (IQR 11.6 to 41.6) for NG tube 25% sucrose group. Significant reduction in crying time (P = 0.006) noted in the 25% sucrose group compared with water group when infants received 25% sucrose intraorally, not via NG‐tube route. For infants in 25% sucrose group, significant reduction in crying time noted (P = 0.008) when solution given intraorally compared to via NG tube route Behavioural scores for the intraoral water group was 9 (IQR 6 to 12) and 10 (IQR 6 to 14) for NG tube water group. Behavioural scores for intraoral 25% sucrose group was 5 (IQR 3 to 6) and 9 (IQR 8 to 10) for NG tube sucrose group. Significant reduction in behavioural scores noted in 25% sucrose group (P = 0.002) compared with water group when infants received 25% sucrose intraorally but not via NG route. For infants in 25% sucrose group, there was significant reduction in behavioural score (P = 0.001) when solution was given intraorally compared to via NG tube Cross‐over design: data from the first assignment prior to cross‐over were presented. These data were not used in RevMan‐analyses |
| Rushforth 1993 | 52 term infants, GA 37 to 42 weeks, 2 to 7 days of age | Heel lance |
|
% crying during sampling and 3 min after sampling | Medians only | No significant differences in median % time crying between group receiving 7.5% sucrose (74.3%) compared to group receiving water (73.2%). No significant differences between groups in duration of cry after 1 min (P = 0.65), 2 min (P = 0.52) and 3 min (P = 0.72). No difference in time to cessation of crying (P = 0.16) No data could be used in RevMan‐analyses |
| Simonse 2012 | 71 preterm infants GA 32 to 36 + 6/7 weeks at birth undergoing heel lance with an automated piercing device. | Heel lance |
All 46 infants (in groups 2 and 3) were analyzed |
PIPP score; COMFORTneo Score, HR and oxygen saturation (%) | Mean and 95% CI | There was no significant difference in mean PIPP score between neonates receiving breast milk (6.1) and those receiving sucrose (5.5), with a mean difference of 0.6 (95% confidence interval ‐1.6 to 2.8; P = .58). No data were provided for HR and oxygen saturation 95% CI were transformed to SD and used in RevMan‐analyses |
| Slater 2010 | 44 term infants, GA 37 to 43 weeks, < 8 days old | Heel lance |
|
HR change, PIPP score, nociceptive‐specific brain activity, latency to change in facial expression (s), facial non‐responders, nociceptive reflex withdrawal activity | Mean, 95% CI, mean weight | Only mean baseline HR given: 24% sucrose 132. 6 beats/min (124.3 to 140.9); sterile water 131.8 (122.2 to 141.5) (P = 0.90) Only mean baseline oxygen saturation (%) given: 24% sucrose 99.4 (98.8 to 100.1); sterile water 97.4 (95.0 to 99.8) (P = 0.13) PIPP score during insertion: baseline PIPP score: 24% sucrose 1.3 (0.8 to 1.7), sterile water 1.3 (0.8 to 1.8) (P = 0.91); PIPP during procedure: 24% sucrose 5.8 (3.7 to 7.8), sterile water 8.5 (7.3 to 9.8) (P = 0.02) No significant differences in nociceptive‐specific brain activity (P = 0.46) latency to change in facial expression (P = 0.86), mean nociceptive reflex withdrawal activity (P = 0.49) or mean latency to nociceptive reflex withdrawal activity (P = 0.56); significant difference in facial non‐responders for 24% sucrose 35%, for sterile water 0% (P < 0.0001) Results presented as mean and 95% CIs Data used in RevMan‐analyses |
| Stevens 1999 | 122 preterm neonates, GA 27 to 31 weeks, ≤ 28 days of age | Heel lance |
NB: all infants were contained in SnuggleUp device for all interventions Each infant received all 4 interventions in random order (serving as his/her own control); there was 1 control group and 3 intervention groups |
PIPP scores at 30 s and 60 s | Reported means, SD | Main effect of treatment for mean PIPP scores (P < 0.0001). Post hoc analysis revealed significant reduction in PIPP scores 30 s after heel lance in sucrose group (pacifier dipped in 24% sucrose ‐ estimated at 0.02 g), (mean 7.87, SD 3.35), compared to control group (mean 9.80, SD 3.55) (P < 0.0001). Statistically significant reduction in PIPP scores in pacifier and water group (mean 8.44, SD 3.55) compared to control group (mean 9.80, SD 3.55) (P = 0.003). Trend towards lower PIPP scores with sucrose and pacifier group compared to water and pacifier group (P < 0.05) The first author provided data for 61 infants for PIPP at 30 s after heel lance and for 45 infants at 60 s after heel lance for infants before cross‐over |
| Stevens 2005a | 66 preterm infants, GA 26 to 30 weeks, PNA < 72 h | Heel lance |
These interventions were given every time there was a painful procedure during the first 28 days of life |
PIPP at days 7, 14, 21 and 28 at routine heel lance Adverse effects Neurobiological risk status |
Not reported | Significant main effect of group (there was a significant difference in a particular group) (P = 0.03) with differences occurring between the sucrose + pacifier group and the standard care group (at 60 s P = 0.01). Mean PIPP scores were generally higher in the standard care group Adverse effects: no group differences for adverse events, clinical outcomes or neurobiological risk status No data used in RevMan‐analyses |
| Storm 2002 | 48 preterm infants, median GA of 32 weeks, median PNA of 14 days | Heel lance |
All infants were given water prior to a second heel lance |
Differences in crying time for pre‐heel lance to heel lance procedure Changes in HR from pre‐heel lance to heel lance procedure Difference in skin conductance from pre‐heel lance to heel lance procedure |
Not reported | Significantly less crying in infants receiving 25% sucrose (P < 0.05) and food (milk) + 25% sucrose (P < 0.05) No significant differences between groups in changes in HR from pre heel lance to heel lance procedure (P value not reported) No statistically significant smaller increase in skin conductance variables compared to their water control session (P value not reported) No data could be included in RevMan‐analyses |
| Thakkar 2016 | 180 full‐term neonates, birthweight > 2000 g and age > 24 h | Heel lance |
|
PIPP Total crying time Adverse events |
Median, IQR | Median (IQR) PIPP score was 3 (2 to 4) in the sucrose + NNS group compared with 7 (6.5 to 8) in the sucrose only group, 9 (7 to 11) in the NNS group and 13 (10.5 to 15) in the no intervention group. The sucrose + NNS group had a significant decrease in the median PIPP score compared with other groups (P = 0.000). Median PIPP score decreased significantly with any intervention compared with no intervention (P = 0.000) A total of 5 episodes of adverse events were reported No data were used in RevMan‐analyses |
| Tutag Lehr 2015 | 56 term infants ≤ 7 days old: appropriate for gestational age |
Heel lance |
|
Primary outcome – mean skin blood flow (SBF); NIPS Skin blood flow (SBF), perfusion units (PU) measured by Laser Doppler Imager (LDI) during heel lance HR, RR (breaths per minute), oxygen saturation (%) |
Mean, SE, median, IQR | Mean SBF and median NIPS scores immediately post heel lance were lower in sucrose‐treated infants (167.9 PU ± 15.5 vs. 205.4 PU ± 16.0, P = 0.09; NIPS 1 (IQR 0‐4) vs. NIPS 3 (IQR 0‐6), P = 0.02) although no significant difference in mean SBF. During heel lance NIPS score was predictive of SBF. An increase of 1 in NIPS score was associated with 11 PU increase in SBF (R = 0.21; P = 0.09) for sucrose and 16 PU increase for placebo‐treated infants (R = 0.20; P = 0.014). Increased SBF assessed by LDI is a pain response among term neonates following routine heel lance that was not completely attenuated by oral sucrose administration. Increased SBF is associated with NIPS scores. Sucrose analgesic efficacy evidenced by decreased NIPS scores for the sucrose group. Association of SBF with NIPS scores suggests LDI is potentially useful for assessing newborn procedural pain SEs were transformed to SDs and were used in RevMan‐analyses for skin blood flow For heart rate, respiratory rate and oxygen saturation results were presented as means and SDs and were used in RevMan‐analyses |
| Unceta‐Barranechea 2008 | 150 term infants | Heel lance |
|
Mean crying time between groups Modified NFCS |
Mean, SD | Statistically significant differences in crying time between facilitated tucking and 2 intervention groups (P < 0.001). No significant difference between NNS + water and NNS + sucrose groups (P = 0.735) Statistically significant differences in pain score between control group and 2 intervention groups (P < 0.001). No significant difference between sucking with placebo and sucking with sucrose groups (P = 0.105) Data used in RevMan‐analyses |
| Yilmaz 2010 | 120 infants, GA 37 to 42 weeks Mean GA (SD):
|
Heel lance |
|
NIPS score, HR, respiratory rate, crying time | Mean, SD | No differences in HR and O2 saturation between groups After the procedure, the mean crying time of the sucrose group was shorter than those of the other groups. Comparing the crying times of the control and experimental groups according to the procedure time showed no statistically significant differences between the values for before and during the procedure (F = 1.50, P > 0.05); (F = 2.43, P > 0.05) Before the procedure, the lowest NIPS mean was in the sucrose group and the highest NIPS mean was in the pacifier group. During the procedure, no statistically significant differences were found between the groups for NIPS means (P > 0.05). After the procedure, the sucrose group showed the lowest response to pain, while the mother's milk group had the highest response. Comparing the NIPS means of the control and experimental groups according to the procedure times, statistically significant differences were found between the groups for values obtained before and after the procedure (F = 3.49, P < 0.05); (F = 6.71, P < 0.05) Outcome data were presented according to different procedure times and no data could be included in RevMan‐analyses |
Abbreviations
CI = confidence interval
BF = Breastfeeeding BPSN = Bernese Pain Scale for Neonates COMFORTneo = COOMFORT neo scale DAN = Douleur Aiguë du Nouveau‐né Scale GA = gestational age HR = heart rate IQR = interquartile range(s) min = minute(s) NFCS = Neonatal Facial Coding System NG = nasogastric NIPS = Neonatal Infant Pain Scale NNS = non‐nutritive sucking PIPP = Premature Infant Pain Profile PMA = postmenstrual age PNA = postnatal age RR = respiratory rate RSF = Ross Special Formula
SSC = skin‐to‐skin contact SD = standard deviation SE = standard error SEM = standard error of the mean SpO2 = oxygen saturation TcPO2 = transcutaneous oxygen pressure SSC = skin to skin contact
2. Trials assessing pain during venipunctures.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results | |
| Abad 1996 | 28 preterm, 29 to 36 weeks' PMA infants, PNA 1 to 26 days | Venipuncture | 2 min prior to venipuncture:
|
Time crying for 3 min after venipuncture HR: pre solution, post solution, 5 min after venipuncture Mean SpO2 and respiratory rate pre solution, post solution, 5 min after venipuncture |
Median, IQR Mean, SEM Mean, SD |
Significant group effect noted, (F(2, 25) = 4.26; P = 0.0256) for cry duration 3 min after venipuncture. Cry duration was significantly reduced in 24% sucrose group (19.1 s) compared to 12% sucrose (63.1 s) and water (72.9 s) groups (P < 0.05) Significant group effect for HR, F(2, 25) = 6.37, P = 0.006. Overall time effect, F(2, 50) = 14.15, P < 0.001. No significant interaction between treatment group and time. Post hoc Tukey test showed that group receiving 12% sucrose had lower HR compared to the 24% sucrose group or water group at all 3 time points (pre solution, P = 0.048; post solution, P = 0.010; 5 min after, P = 0.007) No significant differences noted between groups over time for SpO2 and respiratory rates (no P values reported) For time crying no SDs were reported by the authors Differences in oxygen saturation, respiratory rate and HR between 24% sucrose and water were reported only at 5 min after venipuncture and therefore the findings were not included in RevMan‐analyses |
|
| Acharya 2004 | 39 preterm neonates, mean 30.5 weeks' PMA, mean PNA 27.2 days | Venipuncture | 4 min prior to venipuncture:
Cross‐over trial |
Duration of first cry (beginning to end of first cry); total duration of crying (onset of first cry to cessation of all crying) Mean change in HR from pre procedure, procedure and postprocedure phase of venipuncture Mean SpO2 (%) at pre procedure, procedure and postprocedure NFCS changes across 3 phases of venipuncture |
Mean (SD) Mean (95% CI) |
Mean duration of first cry lower in infants who received sucrose (18.6 s (24.4)) compared to infants who received water (52.3 s (56.2)) (estimated treatment effect = 33.7, P < 0.001). Mean total duration of crying was significantly lower in infants who received sucrose (31.9 s (41.9)) compared to infants who received water (72.5 s (66.7)) (estimated treatment effect = 40.6, P < 0.001) Mean change in HR from pre procedure to procedure was lower in the infants receiving sucrose compared to water (estimated treatment effect = 7.5, P = 0.003). Mean change in HR from pre procedure to postprocedure was lower in the infants who received sucrose compared to water (estimated treatment effect = 4.16, P = 0.036) No significant differences between groups with respect to changes in SpO2 from pre‐procedure to procedure phase (P = 0.17) Changes in mean NFCS scores were significantly lower in the sucrose group compared to water group from pre procedure to procedure phase (estimated treatment effect = 1.08, P = 0.013) and between the pre procedure and postprocedure phase (estimated treatment effect = 2.39, P < 0.001) Data prior to cross‐over for the two groups were not presented so we could not include the data in RevMan‐analyses |
|
| Basnet 2010 | 50 term infants between 12 h to 8 days old
|
Venipuncture |
Method of administration (i.e. pacifier/syringe) was not reported |
Duration of cry, DAN scale, number of infants crying. HR and SpO2 were measured before, during and after the procedure | Mean and SD for duration of cry, IQR for DAN scale | 13 (52%) infants in sucrose group did not cry compared to 4 (16%) in no treatment group, P = 0.001, mean duration of cry was not significantly different between groups (P = 0.65) HR increased during procedure (P = 0.008) followed by decrease postprocedure (P = 0.001) in no treatment group; no significant changes in sucrose group (P = 0.39). Decrease in SpO2 in the no treatment group (P = 0.001) during the procedure; no significant changes in sucrose group (P = 0.3) Significantly lower DAN scores in the 30% sucrose group (score of 3 (1.5 to 5.5) compared to the no treatment group (score of 7 (5 to 9.5) (P = 0.0001) Duration of cry could be used in RevMan‐analyses |
|
| Biran 2011 | 76 preterm infants, mean (SD) PMA:
|
Venipuncture |
The sucrose solution was given 2 min before the procedure via syringe. |
DAN scale, PIPP, crying time Adverse effects |
Mean and SD | Mean (SD) DAN pain scores for the sucrose group and the sucrose + EMLA group were 7.7 (2.1) and 6.4 (2.5), respectively, during venipuncture and 7.1 (2.8) and 5.7 (3.3) during the recovery period. Significant time effect (P = 0.047) and treatment effect (P = 0.018) effect in favour of sucrose + EMLA group; no significant differences using PIPP No adverse effects after sucrose administration were observed Data used for meta‐analysis |
|
| Carbajal 1999 | 150 term newborn infants, 3 or 4 days old | Venipuncture |
Sucrose was administered over 30 s 2 min prior to the procedure |
DAN scale | Median, IQR | Median pain scores with IQRs during venipuncture were: no treatment 7 (5 to 10); sterile water group 7 (6 to 10); 30% glucose group 5 (3 to 7); 30% sucrose (0.6 g) group 5 (2 to 8); pacifier alone group 2 (1 to 4); 30% sucrose with pacifier group 1 (1 to 2). All groups had significantly lower pain scores compared to sterile water group: 30% glucose (P = 0.005), 30% sucrose (P = 0.01), pacifier (P < 0.0001), 30% sucrose with pacifier (P < 0.0001). The pacifier alone group had significantly lower pain scores than infants receiving 30% glucose (P = 0.0001) or 30% sucrose (P = 0.001). Trend towards lower pain scores for infants receiving 30% sucrose with pacifier compared to pacifier alone (P < 0.06) No data were used in RevMan‐analyses |
|
| Elserafy 2009 | 36 preterm infants, median (range): PMA 32 (27 to 36), mean (SD) PMA 32.4 (2.9) Note: two different mean PMAs reported in the article |
Venipuncture | Infants randomly allocated to 6 different regimens:
during a stay in intensive care of up to 15 days For the sucrose and water solutions, the tip of a 1 mL syringe without the needle was placed in the infant’s mouth and the solution was instilled with gentle movements to stimulate sucking for 30 s. Each treatment was given 2 min prior to the procedure. Every infant participating in the study received each of 6 different regimens during a maximum stay of 15 days from admission or the end of the NICU stay |
HR, SpO2, PIPP, respiratory rate, blood pressure, glucose check Crying time was assessed at 0, 1, 3, 5, 10 min |
Range, mean | PIPP score: significantly different between treatment groups P = 0.0005, over time P < 0.0005 24% sucrose + pacifier resulted in lowest pain scores (P < 0.05) No difference in respiratory rate (P = 0.193), no difference in blood pressure (P = 0.246); no difference in glucose check (P = 0.227) The sucrose groups had significantly shorter crying times compared to the other groups (P = 0.001) This was a cross‐over study and each infant got all the 6 different interventions No data that we could use in RevMan‐analyses |
|
| Montoya 2009 | 111 preterm and term neonates (55 in treatment group and 56 in control group) | Venipuncture | 5 min before venipuncture:
|
Overall NIPS score | Means and SDs | NIPS scores significantly lower for infants who received sucrose (2.9 (SD 2.3)) versus water (3.8 (SD 2.6)) (t = ‐2.063, P = 0.041) Data used in RevMan‐analyses (Article written in Spanish) |
|
| Taddio 2011 | 330 infants, mean PMA (SD) 39.5 (1.2):
|
Venipuncture |
Placebos were used for liposomal lidocaine and sucrose (i.e., double‐dummy design), so that all infants received a topically administered cream (liposomal lidocaine or placebo cream) and oral solution (sucrose or placebo water) Non‐randomised group of healthy neonates undergoing venipuncture were administered water |
Facial grimacing, cry duration (s), Observer‐rated pain using a VAS (0 to 10 cm), HR (beats/min), oxygen saturation (%) Safety/adverse events |
Means and 95% CI | The mean facial grimacing score differed among the randomised groups (P < 0.001). Post hoc analyses demonstrated a significantly lower score for the sucrose group compared with liposomal lidocaine group (MD 27; 95% CI ‐36 to ‐19; P < 0.001) and for the sucrose plus liposomal lidocaine group compared with the liposomal lidocaine group (MD 23; 95% CI ‐31 to ‐14; P < 0.001). No evidence of difference between the sucrose and sucrose + liposomal lidocaine groups (P = 0.3) Cry duration differed among groups (P < 0.001). Infants in the sucrose and sucrose + liposomal lidocaine groups cried less than infants in the liposomal lidocaine group (MD ‐38 s; 95% CI ‐52 to ‐25; P < 0.001; and MD 39 s; 95% CI ‐ 52 to ‐ 25; P < 0.001, respectively). There was no evidence of a difference in cry duration between the sucrose and sucrose + liposomal lidocaine group (MD 0 s; 95% CI ‐13 to 14; P = 0.95) No difference in VAS, HR or oxygen saturation (%) When compared with the non‐randomised placebo‐control group, the liposomal lidocaine group had significantly lower facial grimacing (mean difference ‐17; 95% CI ‐27 to ‐7; P < 0.001) and VAS scores (‐1.7 cm; 95% CI ‐2.5 to ‐0.9; P < 0.001). HR, oxygen saturation (%) and procedure duration were significantly higher in the liposomal lidocaine group compared to the control group. Cry duration and procedure success rate did not differ beyond chance No significant adverse events reported We transcribed 95% CI to SDs and included results in RevMan‐analyses |
Abbreviations
CI = confidence interval DAN = Douleur Aigue du Nouveau‐ne EMLA = eutectic mixture of local anaesthetics HR = heart rate IQR = interquartile range MD = mean difference NFCS = Neonatal Facial Coding System NIPS = Neonatal Infant Pain Scale PIPP = Premature Infant Pain Profile PMA = postmenstrual age PNA = postnatal age SD = standard deviation SEM = standard error of the mean VAS = visual analogue scale
3. Trials assessing pain during heel lances and venipunctures.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Ogawa 2005 | 100 healthy full‐term infants:
|
Heel lance or venipuncture |
2 min before procedure:
|
Duration of first cry (s), first crying time/total procedure time (%) and the ratio of crying: no crying NFCS score:
|
Reported medians, range and mean, SD Reported in graph form, median and IQR |
Significant reduction in duration of first cry in heel lance group given sucrose compared to heel lance alone (P < 0.001) Significantly reduced NFCS scores in sucrose group during heel lance (median 47, IQR 31 to 60) and during compression to stop bleeding (median 32, IQR 8 to 54) compared to the water group (median 58, IQR 54 to 65, median 52, IQR 41 to 61, respectively) (P < 0.001) Sucrose did not significantly reduce NFCS scores during or after venipuncture We included data for sucrose vs water for heel lance and for venipuncture for duration of first cry |
Abbreviations
GA = gestational age IQR = interquartile range NFCS = Neonatal Facial Coding System SD = standard deviation
4. Trials assessing pain during arterial puncture.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Milazzo 2011 | 47 neonates, GA 30 to 36 weeks, 48 h old | Arterial puncture | Sucrose group: 0.5 mL oral sucrose solution (Sweet‐Ease, preservative‐free, 24% sucrose solution 99044, Children’s Medical Ventures, Norwell, Massachusetts) in a 1‐mL syringe by the nurse caring for the infant (n = 24) Control group: no oral solution (n = 23) |
NIPS, HR, Oxygen saturation (%) | Mean, SD NIPS scores presented in graph form only |
Sucrose group had significantly less crying than the control group, both during, and immediately after arterial puncture (P .006 and .022, respectively). No significant changes in other pain subscales, HR, or oxygen saturation were found during or after the arterial puncture (P = ‐0.05) Data for HR and oxygen saturation could be used in RevMan‐analyses |
Abbreviations
GA = gestational age HR = heart rate NIPS = Neonatal Infant Pain Scale SD = standard deviation SpO2 = oxygen saturation
5. Trials assessing pain during subcutaneous injections.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Allen 1996 | 285 term infants Various age groups based on required immunisations. Age groups were:
Only data for neonates at 2 weeks of age are included in this review |
Subcutaneous injection |
The solutions were given 2 min prior to the procedure |
Cry duration (during and after procedure) | % time crying |
The overall P value for % time crying was significant (F = 5.92, P < 0.005) for the 2‐week‐old age group. Pairwise comparisons of the % time spent crying of sucrose and water groups versus the no treatment group showed significant differences (P < 0.01 for both comparisons) This was the only age group (< 2 weeks) in which significant differences were observed between sucrose, water and no treatment groups Means and SDs were not reported. No data could be used in RevMan‐analyses |
| Mucignat 2004 | 33 preterm neonates, mean (SD) GA at birth 30 weeks (6 days), GA at injection 32 weeks (6 days) | Subcutaneous injections |
Each infant was its own control |
Duration of cry from needle introduction until to 2 min after its removal HR before injection, during injection and after injection SpO2 before injection, during injections and after injection DAN and NFCS scores during injection |
Mean, SD | Crying time was significantly lower in the sucrose + EMLA + NNS group (P = 0.0002). The mean (SD) crying time in each group was as follows: 3.93 s (2.97) in the NNS group, 2.81 s (4.81) in the EMLA + NNS group, 2.32 s (7.51) in the sucrose + NNS group and 0.89 s (2.66) in the sucrose + EMLA + NNS group There were no significant differences in HR between the 4 groups The only significant difference in SpO2 between groups occurred during injection, which was lower in the NNS group (P = 0.02) Significant reduction in DAN and NFCS scores in EMLA + NNS, sucrose + NNS, and sucrose + EMLA + pacifier groups compared to NNS alone No data could be used in RevMan‐analyses |
Abbreviations
DAN = Douleur Aiguë du Nouveau‐né Scale EMLA = eutectic mixture of local anaesthetics GA = gestational age HR = heart rate NFCS = Neonatal Facial Coding System NNS = non‐nutritive sucking SD = standard deviation SpO2 = oxygen saturation
6. Trials assessing pain during intramuscular injection (immunization for hepatitis B or vitamin K injection).
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Gray 2012 | 47 healthy full‐term infants | Immunization for hepatitis B |
3 infants were subsequently excluded from data analysis (1 in the sucrose group and 2 in the warmth) |
Cumulative crying time (s) Mean HR Mean respiratory sinus arrhythmia Cumulative distribution of grimace time |
Graphs only | Infants in the warmth group cried significantly less than those in the sucrose or NNS groups after vaccination. HR patterns reflected this analgesia. Core temperature did not differ between study groups. "Providing natural warmth to newborn infants during a painful procedure decreases the crying and grimacing on par with the 'gold' standard treatments of sucrose or pacifier" (Gray 2012). No data could be used in RevMan‐analyses |
| Gray 2015 | 29 healthy, full‐term newborns | Immunization for hepatitis B |
|
Duration (s)of grimace and cry | Means and SDs | The sucrose + warmth group cried significantly less 12.8 s (SD 2.2) vs. 28.0 s (SD 6.9) and grimaced less 14.9 s (SD 2.4) vs 31.1 s (SD 7.2) than the sucrose only group Data included in RevMan‐analyses |
| Liaw 2011 | 165 healthy newborn infants PMA ≥ 36 weeks and birthweight ≥ 2200 g |
Immunization for hepatitis B |
|
NFCS Cry duration HR RR |
Cry duration (s) was reported as mean (SD) Other outcomes reported in graph form or in multiple regression models |
Pain was significantly lower among infants in the NNS (P < 0.001) and sucrose (P < 0.001) groups than that in the routine care group after adjusting for time effects, infant sleep/wake state, number of prior painful experiences, and baseline pain scores. Infants in the NNS and sucrose groups had significantly lower mean HR and RR than the controls. Cry duration of infants receiving sucrose was significantly shorter than those in the NNS (P < 0.001) and routine care groups (P < 0.001) Data for cry duration included in RevMan‐analyses |
| Suhrabi 2014 | 90 full‐term neonates | Immunization for hepatitis B |
Solutions applied 2 min before hepatitis B vaccination |
NIPS during 1‐2 min after vaccination | Mean, SD | Comparison of pain severity, mean and SD of pain, showed greater intensity of pain in the glucose group than the sucrose group (3 ± 1.66 vs. 2.90 ± 1.44), but this difference was not significant statistically (P = 0.78). Pain intensity was higher in the control group than in the intervention groups (P < 0.001) NIPS during 1‐2 min after vaccine injection were used in RevMan‐analyses |
Abbreviations
HR = heart rate NFCS = Neonatal Facial Coding System NIPS = Neonatal Infant Pain Scale NNS = non‐nutritive sucking PMA = postmenstrual age RR = respiratory rate SD = standard deviation
7. Trials assessing pain during bladder catheterization.
| Study | Participants | Procedures | Intervention | Outcomes | Metrics used | Results |
| Rogers 2006 | 83 infants ≤ 90 days of age requiring bladder catheterization. 3 infants were withdrawn after randomisation as a result of inappropriate enrolment or withdrawal of consent Subgroup analysis performed: infants 1 to 30, 31 to 60 and 61 to 90 days of age |
Bladder catheterization | 2 min before procedure:
|
% of subjects crying at maximal insertion Change in DAN scores |
Change in mean (SD) for DAN score | Subgroup analysis of youngest infants (1 to 30 days) in sucrose group showed smaller changes in DAN score compared to water group (2.86 vs. 5.29; P = 0.035) Subgroup analysis of infants (1 to 30 days) showed infants in sucrose group were significantly less likely to cry during maximal catheter insertion compared to water group (28.6% vs. 78.6%; P = 0.008) Change in DAN score and number of infants crying at maximal catheter insertion used in meta‐analysis |
Abbreviations
DAN = Douleur Aiguë du Nouveau‐né Scale SD = standard deviation
8. Trials assessing pain during naso‐ (NG) or orogastric (OG) intubations.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Kristoffersen 2011 | 24 preterm infants. PMA 28 to 32 weeks | NG tube insertion | 6 interventions:
The solutions were administered via syringe on the tip of the tongue immediately before tube insertion Cross‐over design: each infant acted as his or her own control over a 3‐week period during which the tube was changed 6 times |
PIPP scores | Median, range | Median PIPP score during the procedure was 9 and decreased gradually towards 4 after 5 min. The NNS + 30% sucrose intervention provided most effective pain reduction (P < 0.001 vs. no treatment). Highest pain score recorded in sterile water group Becasue of the cross‐over design of the study no data could be used in RevMan‐analyses |
| McCullough 2008 | 20 infants, mean (SD) PMA 30.7 weeks (2.3) |
NG tube insertion | 2 min prior to procedure:
Volume of solution was adjusted for current body weight:
The infants were randomised several times to either sterile water or 24% sucrose. This was not done in a cross‐over fashion 51 NG insertions (26 were in the sucrose group and 25 were in the water group) were performed in the 20 infants enrolled in the study |
Incidence of cry Baseline HR and change in HR from baseline during NG tube insertion Baseline SpO2 and change in SpO2 from baseline during NG tube insertion NFCS during NG tube insertion and after insertion |
% Mean, SD Median |
There was a non‐significant trend (P = 0.069) for fewer sucrose‐treated infants to cry during NG tube insertion (8/26), compared with the water group (14/25) Infants in the sucrose group had higher mean pre‐treatment baseline HR than water group but showed no change in HR during NG tube insertion (mean change ‐0.7 beats/min). The HR of the placebo group increased during NG tube insertion (mean change + 11). This difference approached statistical significance (P = 0.055) No significant changes in mean SpO2 occurred in either group Sucrose group had a significant lower median NFCS score during NG tube insertion compared with the water group (1 (range 0 to 4) vs. 3 (range 0 to 4), median difference 1 (95% CI 0 to 2); P = 0.004) After NG tube insertion, the NFCS scores fell to a median of 0 in both groups To see if NFCS is specific for pain, authors analyzed the 4 components on their own. Nasolabial folds showed a significant inhibition in the sucrose group (present in 4/26 (15%) compared with 12/25 (48%) in the water group; P = 0.012) Data could not be used for RevMan‐analyses as infants were randomised to the two different groups several times |
| Pandey 2013 | 120 clinically stable preterms (PMA < 37 weeks) within the first 7 postnatal days | OG tube insertion | 2 min before OGT insertion:
|
The primary outcome was painful response assessed by PIPP, while the secondary outcomes were heart rate and SpO2 changes. The pain response to the procedure according to the PIPP scale was evaluated at pre‐procedure, intra procedure, post 30 s, post 1 min and post 2 min. The secondary outcomes were the maximum heart rate and the minimum oxygen saturation recorded during the procedure. |
Mean, SD | The mean intraprocedure PIPP scores were significantly higher than the mean pre procedure PIPP scores, in the PMA groups of > 34 weeks, and 32 to 33 6/7 weeks, in both the water (7.25 vs. 3, and 8.14 vs. 3.14, respectively) and sucrose arm (8.06 vs.3.21, and 7.18 vs. 4.18, respectively). The mean PIPP scores assessed at 30 s postprocedure in the sucrose group were significantly lower than the water group (4.32 vs. 5.6, P = 00.014). No significant adverse events were seen. Data could be used in RevMan‐analyses for PIPP scores but not for heart rate and SpO2 changes. No significant difference was observed between the baseline and maximum HR, and between baseline and lowest SpO2, across the two study groups. However, there was a significant increase in mean HR from baseline in both the study groups during the procedure to 2 min postprocedure (19.44 beats/min in placebo group vs.22.5 beats/min in sucrose group) Data used in RevMan‐analyses |
Abbreviations
PMA = post menstrual age HR = heart rate NFCS = Neonatal Facial Coding Score NG = nasogastric NNS = non‐nutritive sucking OG = orogastric PIPP = Premature Infant Pain Profile SD = standard deviation SpO2 = oxygen saturation
9. Trials assessing pain during retinopathy of prematurity (ROP) examinations.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Boyle 2006 | 40 preterm infants, median PMA 29 weeks (24 to 34 weeks):
|
Eye examination for ROP | 2 min before start of eye examination:
|
PIPP during examination of eye | Mean, SD, 95% CI | Mean (SD) PIPP scores were: 15.3 (1.9), 14.3 (1.6), 12.3 (2.9), and 12.1 (3.4) for groups the sterile water group, sucrose group, water + NNS group, and sucrose + NNS group, respectively Significant differences in PIPP scores between the groups, P = 0.023 Infants in pacifier groups scored significantly lower than groups without pacifiers, P = 0.003 (95% CI ‐4.23 to ‐0.96) No significant differences between groups receiving sucrose vs. groups receiving water (P = 0.321) Data used in RevMan‐analyses |
| Dilli 2014 | 64 infants undergoing ROP eye examination. The groups had similar GA (28.5 ± 2.8 weeks), mean birthweight (1304 g ± 466 g) or corrected GA (35.4 ± 3.7 weeks) at examination | Eye examination for ROP | Topical anaesthetic (proxymetacaine; Alcaine(®) drop 0.5%: ALCON CANADA Inc., Mississauga, Canada) was applied 30 s before the eye examination in all infants
|
Mean PIPP score during examination; secondary outcome measurements were frequency of tachycardia (> 180 beats/min), bradycardia (< 100 beats/min), desaturations (< 85% for > 10 s) and crying time. |
Mean, SD | The intervention group had a significantly lower mean PIPP score during examination of the first eye, following insertion of the speculum (sucrose + NNS group: 13.7 ± 2.1 vs. water + NNS group: 16.4 ± 1.8, P = 0.001). Data used in RevMan‐analyses |
| Gal 2005 | 23 neonates, PMA mean (SD) 26.4 weeks (1.5) PNA 28 to 93 days | Eye examination for ROP |
Cross‐over design Mydriatic eye drops (phenylephrine HCl 1%, cyclopentolate HCl 0.2%) and local anaesthetic eye drops (proxymetacaine HCl 0.5%; 2 drops) given to both groups prior to examination |
SpO2 desaturation by ≥ 10% pre‐examination, at eye speculum insertion and postexamination PIPP scores at 5 and 1 min pre‐examination, eye speculum insertion, and 1 and 5 min postexamination |
Percentage of population Means, SD reported |
No significant difference in SpO2 desaturation by ≥ 10% pre‐examination, at eye speculum insertion between water group and sucrose group PIPP score at the eye examination significantly lower in the sucrose group (mean 8.3, SD 4.5) compared to the water group (mean 10.5, SD 4.0), P = 0.01); however, this effect was not sustained at 1 and 5 min post examination Results for the two groups prior to cross‐over were not reported. No data could be used in RevMan‐analyses |
| Grabska 2005 | 32 preterm infants, mean PMA 28 weeks, mean PNA 50.8 days |
Eye examination for ROP |
Doses were adjusted by weight:
All infants were swaddled and offered a pacifier All infants received tropicamide 0.5% and phenylephrine 2.5% eye drops approximately 30 min before examination. Topical tetracaine was instilled into the eyes just prior to the examination |
% of the eye examination the infant spent crying Mean HR, at baseline, post eye drop instillation, post study drug, during eye examination and post‐eye examination* RR and SpO2 at baseline, post‐eye drop instillation, post study drug, during eye examination and post‐eye examination* PIPP at baseline, during eye examination, post‐eye examination* *measures were taken at 1‐min intervals and were means for each study period ‐ study period times (in min) were not defined |
Mean, SD | No significant difference in crying time between the sucrose and water groups. Significant increases in HR, in both groups from baseline (P < 0.01) No differences between the sucrose and water groups in HR at any time point Significant reduction in SpO2 in sucrose group after the study drug (mean 95%, SD 4%) compared to the water group (mean 97%, SD 3%) Significant reduction in SpO2 in sucrose group during the eye examination (mean 93%, SD 5%; P < 0.05) compared to the water group (mean 96%, SD 3%; P < 0.05) No significant difference in RR and SpO2 at 2 min post examination No significant differences in PIPP scores between the sucrose and placebo groups before, during and after eye examinations Data for PIPP during examination used in RevMan‐analyses |
| Mitchell 2004 | 30 preterm infants:
|
Eye examination for ROP |
1st dose given 1.5 min before local anaesthetic eye drops, 2nd dose right at placement of the eye speculum, 3rd dose 120 s after 2nd dose All infants received proxymetacaine hydrochloride 0.5% eye drops and were swaddled before the eye examination |
PIPP at baseline, at eye drop instillation, at examination of left eye and at 30, 60, 90 and 120 s after the examination | Mean, SEM | Statistically significant differences in mean PIPP scores were found between sucrose group (mean 8.8, SEM 0.7) and the water group (mean 11.4, SEM 0.6) during the eye examination (P = 0.0077). However, this was not sustained after the eye examination Data presented that could be used in RevMan‐analyses |
| O'Sullivan 2010 | 40 preterm infants, corrected mean age:
|
Eye examination for ROP |
Infants were given mydriatic eye drops (cyclopentolate 0.2% and phenylephrine 1%) 60 min and 30 min prior to examination. Every neonate received local anaesthetic eye drops (tetracaine hydrochloride 1%) 30 s prior to examination. The interventions were given 2 min prior to the start of the eye examinations. The infants were swaddled in both groups |
N‐PASS, HR and SpO2 at baseline, number of episodes of bradycardia and desaturation, adverse events | Median, range | Significantly lower N‐PASS score at speculum insertion in sucrose compared to water group (6.5 vs. 5.0; P = 0.002); during procedure (9.5 vs. 7.5; P = 0.003). No significant differences between sucrose group and water group for episodes of desaturation, bradycardia, and adverse outcomes Data were not used in RevMan‐analyses |
| Rush 2005 | 30 preterm infants < 32 weeks PMA:
|
Eye examination for ROP | Prior to examination: instillation of 0.5% proxymetacaine and 1% tropicamide, then 15 min later eye drop instillation of 0.5% tropicamide, 2.5% phenylephrine and 0.5% tropicamide
|
Total crying time out of 5 min starting at the onset of the ROP examination HR 30 min before eye drop instillation and 5 min before ROP examination, during examination, and 5 min after the ROP examination SpO2 and RR at 30 min before eye drop instillation, 5 min before the ROP exam, 3 measurements during the ROP exam, and 5 min after the ROP exam |
Reported means and SEM Not reported SpO2 means and SEM reported RR not reported |
No significant differences in crying time between sucrose and water groups (P = 0.127) There was no significant difference in HR between groups No significant differences between treatment group and the control group for SpO2 and respiratory rate at any point Transcribed SEM to SD |
CI = confidence interval GA = gestational age HR = heart rate NNS = non‐nutritive sucking N‐PASS = Neonatal Pain Agitation and Sedation Scale PIPP = Premature Infant Pain Profile PMA = postmenstrual age PNA = postnatal age ROP = retinopathy of prematurity RR = respiratory rate SD = standard deviation SEM = standard error of the mean SpO2 = oxygen saturation
10. Trials assessing pain during circumcision.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Al Qahtani 2014 | 90 full‐term newborn males who underwent circumcision. PMA 38 weeks or more, 5‐min Apgar score of 8 or higher, PNA of 12 h or more, birthweight > 2500 g and free from jaundice, anomalies of the penis and analgesia or sedation in the previous 48 h |
Circumcision |
|
N‐PASS used to assess the severity of pain and neonatal response to pain, 5 min before, during and 5 min after the circumcision for all newborns. The scale measures both physiologic (HR, RR, blood pressure and oxygen saturation) and behavioural (crying irritability, behaviour state, facial expression and extremities tone) responses to pain | Mean, SD (Abstract says median) (Tables state mean and SD) |
N‐PASS scores were significantly lower in
EMLA + sucrose group (median EMLA + sucrose group = 5.2, EMLA group = 5.8, sucrose group = 8.5; P < 0.001). The endogenous response to pain
in terms of escalation of heart rate and reduction in O2 saturation were minimal among EMLA + sucrose group (P < 0.0001)
Duration of crying was comparable among all the groups Data used in RevMan‐analyses |
| Herschel 1998 | 120 healthy male newborns, ≥ 38 weeks PMA | Circumcision |
|
HR at baseline, restraint, skin preparation for procedure, lateral clamping, lysis of adhesions, dorsal clamping, dorsal cut, retraction, application of Gomco bell and clamp, tightening of clamp, excision of foreskin, removal of clamp, removal of bell, placement of dressing and overall change in HR from baseline SpO2 at baseline and throughout procedure; change from baseline during the circumcision procedure |
Mean, SD, mean differences and 95% CI | Mean change in HR from baseline through all follow‐up times were significantly different between groups (P < 0.001) Mean (95% CIs) HR differences:
Sucrose had a statistically significant effect compared to the no treatment controls (P < 0.001) Significant differences between groups in changes in SpO2 from baseline to circumcision (P < 0.001) Mean (95% CI) SpO2 differences between the 3 groups from baseline:
Differences between both the DPNB and sucrose groups compared to control were significant (P < 0.05) Control vs. sucrose: ‐3.3 (‐5.0 to ‐1.6) was statistically significant (P < 0.001) Data used in RevMan‐analyses |
| Kaufman 2002 | 57 term infants, mean age at time of procedure 30 h to 43 h | Circumcision |
All infants had EMLA cream applied 1 to 3 h before procedure |
Time spent crying during procedure Time spent grimacing Procedure stages:
|
Median and means, graphically Not reported |
Cumulative mean time crying for forceps to unrestraint interval in the Gomco + sucrose group was 56 s (median = 53 s) compared to 86 s (median = 78 s) in the Gomco + water group (P = 0.0001) Crying time in Mogen + sucrose and Mogen + water groups were not significantly different Overall, mean crying time significantly decreased in infants treated with sucrose compared to infants treated with water (P = 0.0001) Significantly less time spent grimacing in the Gomco + sucrose group compared to the Gomco + water group (P = 0.0001) No significant differences between Mogen + sucrose and the Mogen + water groups Overall, mean time grimacing was significantly reduced in infants treated with sucrose compared to infants treated with water (P = 0.0001) No data were presented that could be used in RevMan‐analyses |
| Stang 1997 | 80 healthy, term, newborn male infants, mean PMA 39.5 weeks | Circumcision |
|
Plasma cortisol level 30 min after beginning circumcision Behavioural arousal and behavioural distress scores were recorded at five scoring periods:
|
Mean, SD | Plasma cortisol levels not significantly different between groups Data used in RevMan‐analyses |
Abbreviations
CI = confidence interval DPNB = dorsal penile nerve block EMLA = eutectic mixture of local anaesthetics HR = heart rate N‐PASS = Neonatal Pain Agitation and Sedation Scale
PMA = Post menstrual age PNA = postnatal age RR = respiratory rate SD = standard deviation SpO2 = oxygen saturation
11. Trials assessing pain during multiple procedures.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Banga 2015 | 106 infants between 32 and 37 weeks PMA Sucrose group: mean (SD) age (h) at enrolment 3.78 (2.92), mean (SD) birthweight (g) 1555.58 (242.79) Water group: mean (SD) age (h) at enrolment 3.16 (2.18), mean (SD) birthweight 1575.24 (241.89) |
Repeated potentially painful procedures during the first 7 days after enrolment Mean (SD) number of procedures:
The authors did not describe which different potentially painful procedures were included (we have written to the corresponding author (drbhanu04@gmail.com) to get a description of these procedures) |
|
Primary outcome was score of motor development and vigor (MDV) and alertness and orientation (AO) domains of NAPI scale performed at 40 weeks PMA In addition, the highest HR and lowest SpO2 obtained during the procedure were recorded till 30 s after the prick, for newborns in both the groups (not reported) |
Means, SDs, 95% CIs | A total of 93 newborns were analyzed. The baseline characteristics of the groups were comparable. No statistically significant difference was observed in the assessment at 40 weeks PMA, among the groups. Use of sucrose analgesia, for repeated painful procedures on newborns, does not lead to any significant difference in the short‐term neurobehavioral outcome Data used in RevMan‐analyses |
| Gaspardo 2008 | 33 preterm infants, median PMA 30 weeks | Venipuncture, arterial puncture, heel lance, intravenous cannulation, endotracheal tube introduction, endotracheal tube suctioning, gavage insertion for feeding, removal of electrode leads and tape | On day 1, no treatment was given to any neonate in order to collect baseline data. After that, on days 2 to 4 before every minor painful procedure infants received either:
|
Pain was assessed over 4 days during morning blood collection (heel lance) Incidence of cry (% neonates crying), HR (% neonates with HR ≥ 160 beats per min), NFCS (% neonates with score ≥ 3), Activated Behavioural State (% neonates with score ≥ 4). The assessment was divided into five phases: Baseline Antisepsis, Puncture, Dressing, and Recovery. The neonates’ facial activity (NFCS), behavioural state, and HR were evaluated |
No means or standard deviations reported NFCS results reported in graph form only |
The data analysis used cut‐off scores for painful and distressful
responses. There were significantly fewer sucrose group neonates with facial actions signalling pain than water group neonates in puncture phase and in antisepsis phase. There were significantly fewer sucrose group neonates crying during antisepsis phase, puncture phase, and dressing phase. There was no statistical difference between groups for physiological response. The efficacy of sucrose was maintained for pain relief in preterm neonates with no side effects Data could not be used in RevMan‐analyses No side effects of using sucrose were detected |
| Johnston 2002 | 103 infants:
|
Every time the infant was to undergo an invasive (e.g. heel lance, intravenous cannulation, arterial puncture, injection) or non‐invasive but presumably uncomfortable procedure (e.g. endotracheal tube suctioning, tape or lead removal, gavage insertion for feeding) the infant received sucrose or water |
The solution was in a syringe and administered into the infant’s mouth at the beginning of the procedure, 2 min into the procedure, and another 2 min into the procedure. If the procedure was > 15 min, up to another 3 0.1 mL doses were to be given 2 min apart |
Neurobehavioural development assessed by the sub scales of alertness and orientation and motor development and vigour of the NAPI, SNAP and NBRS SNAP was measured for each 24‐hour period during the study week and NBRS was measured at 2 weeks’ PNA and at discharge |
Beta, CI (multiple regression) | On the basis of analysis of covariance with PMA at birth and the number of invasive procedures as covariates, there were no group differences (between sucrose and water) for any of the secondary outcomes of Neuro‐Biological Risk Scores (NBRS) at two weeks; postnatal age (P = 0.426) or at discharge (P = 0.965). In the sucrose group only, higher number of doses of sucrose predicted lower scores on motor development and vigor, and alertness and orientation at 36 weeks’, lower motor development and vigor at 40 weeks’, and higher NBRS at 2 weeks’ postnatal age. Higher number of invasive procedures was predictive of higher NBRS both times in the water group. No significant differences found between the sucrose and water groups for Neurobehavioral Assessment of the Preterm Infant (NAPI). Data could not be used in RevMan‐analyses |
| Taddio 2008 | 240 newborn infants born to non‐diabetic and diabetic mothers, PMA ≥ 36 weeks | 3 heel lances, venipuncture and intramuscular vitamin K injection Multiple painful stimuli |
|
PIPP scores overall, during IM injection, during venipuncture and all 3 heel lances Safety |
Mean, SD, 95% CI | Overall PIPP scores were significantly lower among newborns given sucrose (mean 6.8, SD 2.9) compared to water (mean 8.1, SD 2.5) (MD ‐1.3, 95% CI ‐2.0 to ‐0.6; P < 0.001) PIPP scores during IM injection did not differ between the sucrose and water group for non‐diabetic (P = 0.10) or diabetic mothers (P = 0.15) PIPP scores during venipuncture were significantly lower among infants of non‐diabetic mothers who received sucrose compared to water (mean score 5.7, 95% CI 4.7 to 6.7 vs. mean score 8.9, 95% CI 7.9 to 9.9; P < 0.001). Similar results were found among infants of diabetic mothers (sucrose: mean score 6.8, 95% CI 5.7 to 7.9 vs. water: mean score 9.2, 95% CI 8.4 to 10.1; P < 0.001) During first 3 heel lances, newborns from diabetic mothers receiving sucrose or water did not have significantly different PIPP scores Results for different painful stimuli reported separately Data could be used in RevMan‐analyses |
Abbreviations
CI = confidence interval PMA = gestational age HR = heart rate IM = intramuscular MD = mean difference NAPI = Neurobehavioral Assessment of the Preterm Infant NBRS = Neuro‐Biological Risk Score NFCS = Neonatal Facial Coding System PCA = postconceptional age PIPP = Premature Infant Pain Profile PMA = postmenstrual age PNA = postnatal age SD = standard deviation SNAP = Score for Neonatal Acute Physiology SpO2 = oxygen saturation
12. Trials assessing stress during echocardiography.
| Study | Participants | Procedure | Interventions | Outcomes | Metrics used | Results |
| Potana 2015 | Neonates with established enteral feeding, not on any respiratory support and with PMA 32 to 42 weeks requiring echocardiography |
Echocardiography |
|
PIPP | Mean, SD | The mean (SD) PIPP score was significantly lower in the sucrose group 5.25 (1.92) vs 7.40 (3.78) in the control group 4 (7.6%) neonates spat up the sucrose solution after administration. No episodes of hyperglycaemia, necrotizing enterocolitis, or feed intolerance were reported after sucrose administration Data could be used in RevMan‐analyses |
Abbreviations
PIPP = Premature Infant Pain Profile PMA = postmenstrual age SD = standard deviation
Twenty‐nine studies evaluated adverse effects.
Risk of bias in included studies
The studies we included enrolled between 15 and 671 infants. Forty‐eight studies (65%) enrolled < 100 infants. The largest study enrolled 671 term infants, who were randomised to four groups (Leng 2015); sucrose, sucrose + non‐nutritive sucking (NNS), sucrose + swaddling, and sucrose + NNS + swaddling. The second largest study enrolled 560 infants (Leng 2013), but we could not include data for sucrose versus sterile water for increase in heart rate and decrease in oxygen saturation at three minutes after heel lance in RevMan‐analyses, as they were reported as means and full ranges.
Few researchers provided a definition of pain or how it was conceptualised in relation to the outcomes. There were differences in study methods. Heel lance was studied as the pain stimulus in the majority of studies, however, little detail about this procedure (e.g. manual versus automated lance) was provided. Therefore, it is impossible to know if the painful stimuli were comparable in intensity, duration or frequency across studies. The length of infant observation following the heel lance was infrequently reported, and may have implications for the incidence of reported adverse effects.
The delivery method of sucrose differed between studies (syringe, dropper or sucrose‐dipped pacifier), as did the concentrations of sucrose and the volume used. Outcomes were reported inconsistently; as means with SDs, standard errors (SE) or 95% CIs, or medians with ranges and often in graphic form without reporting of numerical data.
The risk of bias is reported in the 'Risk of bias' graph (Figure 2) and the 'Risk of bias' summary (Figure 3).
2.
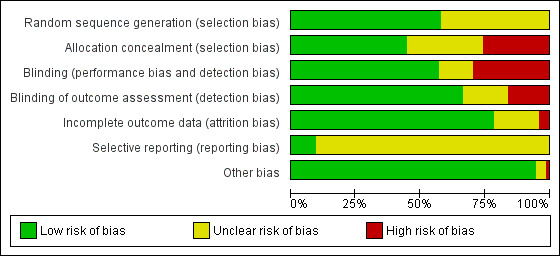
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
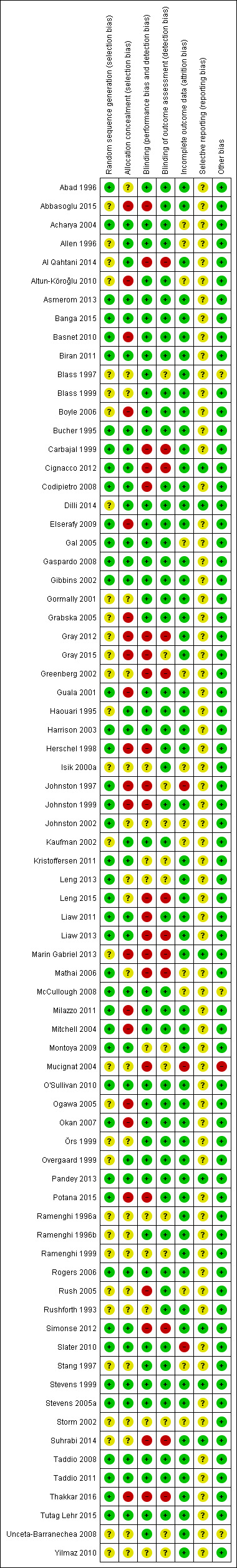
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Forty‐three studies (58%) reported an adequately generated allocation sequence, however, in the remaining 31 studies (42%) it was unclear how the random sequence was generated. In 33 studies (45%) the allocation was adequately concealed. There was a high risk of bias for allocation concealment in 19 studies (26%), and an unclear risk of bias in 22 studies (30%).
Blinding
There was a low risk of performance bias in 42 studies (57%) and a high risk in 22 studies (30%). There was an unclear risk of bias in the remaining 10 studies (14%).
There was a low risk of detection bias in 49 studies (66%), a high risk in 12 studies (16%), and an unclear risk in the remaining 13 studies (18%).
Incomplete outcome data
There was a low risk of attrition bias in 58 studies (78%), a high risk in three studies (4%), and an unclear risk in the remaining 13 studies (18%).
Selective reporting
The protocols for eight studies (11%) were available to us, these had a low risk of reporting bias. The risk of reporting bias was unclear for the remaining 66 studies (89%).
Other potential sources of bias
Other potential bias was detected in one study (1%) in which there was unequal distribution of allocated and received treatments amongst injected infants (Mucignat 2004): NNS = 41, eutectic mixture of local anaesthetic (EMLA; a topical mixture of lidocaine (2.5%) and prilocaine (2.5%) cream) = 71, sucrose = 86, EMLA + sucrose = 67. The risk of bias was unclear in three studies (4%), and we did not detect other bias in the remaining 70 studies (95%).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20; Table 21; Table 22; Table 23; Table 24
Tests for heterogeneity were not applicable when data from only one study were included in an analysis. For all included pain measures a lower score indicates lower level of pain.
Effectiveness of sucrose for heel lance
Sucrose (12% to 12.5 %) versus water/routine care (Comparison 1)
Secondary outcomes
Total crying time (s) (Outcome 1.1)
One study reported on 42 term infants (Greenberg 2002). There was a significantly shorter duration of total crying time (s) in the sucrose group compared with the water group; MD ‐48.09 (95% CI ‐ 93.04 to ‐3.14; Analysis 1.1).
1.1. Analysis.

Comparison 1 Heel lance (term infants): sucrose (12% to 12.5%) versus water/routine care, Outcome 1 Total crying time (s).
Percentage (%) change in heart rate 1 minute after heel lance (Outcome 1.2)
One study reported on 30 term infants (Haouari 1995). There was no significant difference in percentage change (%) in heart rate one minute after heel lance between the sucrose group and the water group; MD 6.40 (95% CI ‐13.69 to 26.49; Analysis 1.2).
1.2. Analysis.
Comparison 1 Heel lance (term infants): sucrose (12% to 12.5%) versus water/routine care, Outcome 2 Percentage change in heart rate 1 min after heel lance.
Sucrose (20% to 33%) versus water (Comparison 2)
Primary outcomes
PIPP at 30 s after heel lance (Outcome 2.1)
One study reported on 44 term infants (Slater 2010), and another study reported on 61 preterm infants (Johnston 1999). For the combined group of term and preterm infants there was no significant difference in the PIPP scores between the sucrose and the water groups; MD ‐1.42 (95% CI ‐2.86 to 0.01); I2 = 51 % (moderate: Analysis 2.1). For term infants the PIPP score was significantly lower in the sucrose group; MD ‐2.70 (95% CI ‐4.96 to ‐0.44), but in the preterm infants group there was no significant difference in the PIPP scores between the sucrose and the water groups; MD ‐0.56 (95% CI ‐2.42 to 1.30).
2.1. Analysis.
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 1 PIPP at 30 s after heel lance.
PIPP at 60 s after heel lance (Outcome 2.2)
One study reported on 31 preterm infants (Johnston 1999). There was no significant difference in the PIPP scores in the sucrose group compared with the water group; MD ‐1.80 (95% CI ‐3.81 to 0.21; Analysis 2.2).
2.2. Analysis.
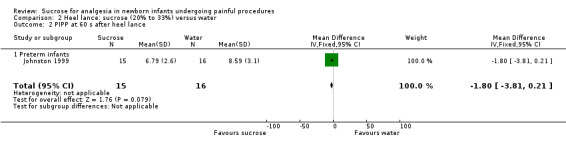
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 2 PIPP at 60 s after heel lance.
PIPP during (first) heel lance (Outcome 2.3)
One study reported on 107 newborns of diabetic mothers (Taddio 2008). There was no significant difference in the PIPP scores in the sucrose group compared with the water group; MD 0.00 (95% CI ‐1.52 to 1.52; Analysis 2.3).
2.3. Analysis.

Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 3 PIPP score during heel lance (1st heel lance).
DAN score at 30 s after heel lance (Outcome 2.4)
One study reported on 32 term infants (Mathai 2006). There was no significant difference in the DAN score 30 s after heel lance in the sucrose group compared with the water group; MD ‐1.90 (95% CI ‐8.58 to 4.78; Analysis 2.4).
2.4. Analysis.
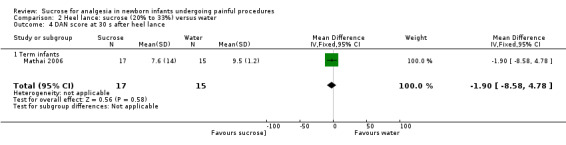
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 4 DAN score at 30 s after heel lance.
NIPS during heel lance (Outcome 2.5)
One study reported on 56 term infants (Tutag Lehr 2015). The NIPS score during heel lance was significantly lower in the sucrose group compared with the water group; MD ‐2.00 (95% CI ‐2.42 to ‐1.58; Analysis 2.5).
2.5. Analysis.
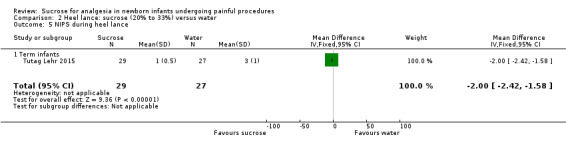
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 5 NIPS during heel lance.
Secondary outcomes
Duration of first cry (s) (Outcome 2.6)
One study reported on 32 term infants (Mathai 2006), and another reported on 110 preterm infants (Harrison 2003). When the two groups of infants (term and preterm) were combined (n = 142) there was no significant difference in duration of first cry (s) in the sucrose group compared with the water group; MD ‐8.63 (‐19.88 to 2.61); I2 = 46.0% (low heterogeneity; Analysis 2.6). In the study performed in term infants (n = 32) there was no significant difference in the crying time (s) between the sucrose group compared with the water group; MD ‐5.00 (95% CI ‐17.40 to 7.40). In preterm infants (n =110) there was no significant difference between the duration of first cry (s) between the sucrose and the water groups; MD ‐25.41 (95% CI ‐52.06 to 1.24).
2.6. Analysis.

Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 6 Duration of first cry (s).
Total crying time (s) (Outcome 2.7)
Two studies reported on a total of 88 term infants (Isik 2000a; Mathai 2006). There was a significantly shorter total crying time (s) in the sucrose group than the water group; MD ‐22.11 (95% CI ‐32.52 to ‐11.70; Analysis 2.7). There was moderate heterogeneity for this outcome; I2 = 59%.
2.7. Analysis.
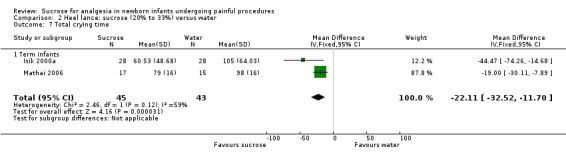
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 7 Total crying time.
Heart rate (beats/minute) during heel lance (Outcome 2.8)
Two studies reported on 96 term infants (Guala 2001; Tutag Lehr 2015). There was no significant difference in the heart rate (beats/minute) during heel lance; MD ‐0.81 (95% CI ‐8.57 to 6.94; Analysis 2.8). There was no heterogeneity for this outcome; I2 = 0%.
2.8. Analysis.
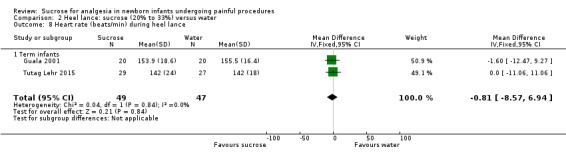
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 8 Heart rate (beats/min) during heel lance.
Percentage change in heart rate 1 minute after heel lance (Outcome 2.9)
Two studies reported on 86 term infants (Haouari 1995; Isik 2000a). There was no significant difference in percentage change in heart rate one minute after heel lance in the sucrose group compared with the water group; WMD 0.90 (95% CI ‐5.81 to 7.61; Analysis 2.9). There was high heterogeneity for this outcome; I2 = 86%. The high heterogeneity could be explained by the very different results for the two studies; the Haouari 1995 study showed a significant increase in percentage change in heart rate; MD 9.90 (95% CI 0.41 to 19.39), whereas the Isik 2000a study showed a non‐significant decrease; MD ‐8.10 (95% CI ‐17.59 to 1.39).
2.9. Analysis.
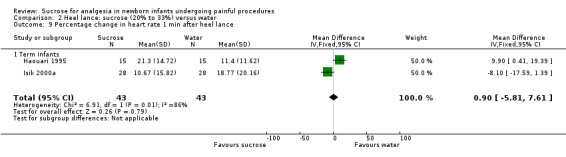
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 9 Percentage change in heart rate 1 min after heel lance.
Respiratory rate (breaths/minute) during heel lance (Outcome 2.10)
One study reported on 56 term infants (Tutag Lehr 2015). There was no significant difference in the respiratory rate (breaths/minute) between the sucrose and the water groups during heel lance; MD ‐1.00 (95% CI ‐7.64 to 5.64; Analysis 2.10).
2.10. Analysis.
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 10 Respiratory rate (breaths/min) during heel lance.
Oxygen saturation (%) during heel lance (Outcome 2.11)
One study reported on 56 term infants (Tutag Lehr 2015). There was no significant difference in the oxygen saturation (%) between the sucrose and the water groups during heel lance; MD ‐5.00 (95% CI ‐12.79 to 2.79; Analysis 2.11).
2.11. Analysis.
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 11 Oxygen saturation (%) during heel lance.
Skin blood flow during heel lance ‐ Perfusion Units (PU) (Outcome 2.12)
One study reported on 56 term infants (Tutag Lehr 2015). There was no significant difference in skin blood flow (PU) during heel lance between the sucrose and the water groups; MD ‐32.00 (95% CI ‐68.87 to 4.87; Analysis 2.12).
2.12. Analysis.
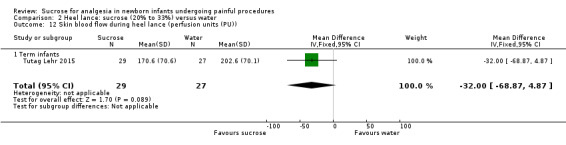
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 12 Skin blood flow during heel lance (perfusion units (PU)).
Nociceptive‐specific brain activity (mean weight) (Outcome 2.13)
One study reported on 44 term infants (Slater 2010). There was no significant difference in the nociceptive‐specific brain activity (mean weight by EEG) between the sucrose and the water groups; MD 0.02 (95% CI ‐0.05 to 0.09; Analysis 2.13).
2.13. Analysis.
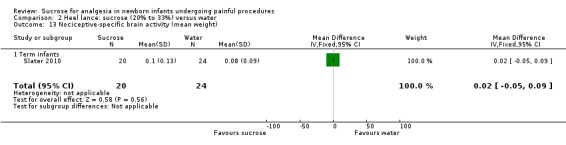
Comparison 2 Heel lance: sucrose (20% to 33%) versus water, Outcome 13 Nociceptive‐specific brain activity (mean weight).
Sucrose (50%) versus water (Comparison 3)
Secondary outcomes
Duration of first cry (s) (Outcome 3.1)
Two studies reported on 80 term infants (Haouari 1995; Ogawa 2005). There was a significantly shorter duration of first cry (s) in the sucrose group compared with the water group; MD ‐63.20 (95% CI ‐79.20 to ‐47.19; Analysis 3.1); I2 = 0% (none).
3.1. Analysis.
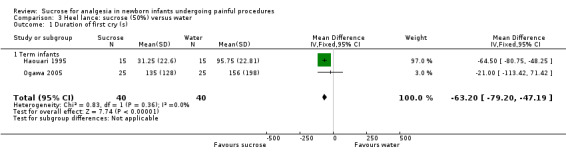
Comparison 3 Heel lance: sucrose (50%) versus water, Outcome 1 Duration of first cry (s).
Percent change in heart rate 1 minute after heel lance (Outcome 3.2)
One study reported on 30 term infants (Haouari 1995). There was no significant difference in percent change in heart rate one minute after heel lance in the sucrose group compared with the water group; MD 2.60 (95% CI ‐11.43 to 16.63; Analysis 3.2).
3.2. Analysis.

Comparison 3 Heel lance: sucrose (50%) versus water, Outcome 2 Percentage change in heart rate 1 min after heel lance.
Sucrose (24% to 25%) versus breastfeeding (Comparison 4)
Primary outcomes
PIPP (Outcome 4.1)
One study reported on 47 preterm infants (Simonse 2012). There was a significantly lower PIPP score in the sucrose group than in the breastfeeding group; MD ‐1.75 (95% CI ‐2.22 to ‐1.28; Analysis 4.1).
4.1. Analysis.
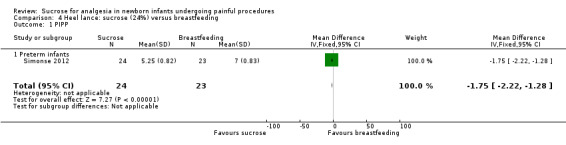
Comparison 4 Heel lance: sucrose (24%) versus breastfeeding, Outcome 1 PIPP.
Comfort score (Outcome 4.2)
One study reported on 47 preterm infants (Simonse 2012). The Comfort score was significantly lower in the sucrose group than in the breastfeeding group; MD ‐2.60 (95% CI ‐3.06 to ‐2.14; Analysis 4.2).
4.2. Analysis.

Comparison 4 Heel lance: sucrose (24%) versus breastfeeding, Outcome 2 Comfort score.
Sucrose (24%) + NNS versus water + NNS, or pacifier dipped in sucrose versus pacifier dipped in water (Comparison 5)
Primary outcomes
NFCS (Outcome 5.1)
One study reported on 100 term infants (Unceta‐Barranechea 2008). There was no significant difference in the NFCS score in the sucrose + NNS group compared to the water + NNS group; MD ‐0.60 (95% CI ‐1.47 to 0.27; Analysis 5.1).
5.1. Analysis.
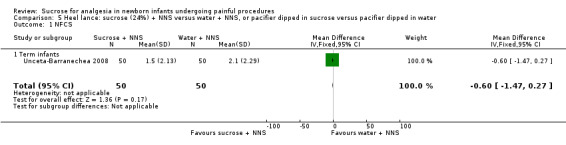
Comparison 5 Heel lance: sucrose (24%) + NNS versus water + NNS, or pacifier dipped in sucrose versus pacifier dipped in water, Outcome 1 NFCS.
PIPP 30 s after heel lance (term and preterm infants) (Outcome 5.2)
One study reported on 128 term and preterm infants (mean PMA 33.7 weeks for the entire group) (Gibbins 2002). There was a significantly reduced PIPP in the sucrose + NNS group compared to the water + NNS group; MD ‐2.03 (95% CI ‐3.06 to ‐1.00). Another study reported on 61 preterm infants, who received either a pacifier dipped in sucrose or a pacifier dipped in water (Stevens 1999). There were no significant differences in the PIPP score between the sucrose and water groups; MD ‐0.56 (95% CI ‐2.42 to 1.30). A third study, Asmerom 2013, reported on 89 preterm infants who received sucrose (24%) + NNS or sterile water + NNS. There was a significantly reduced PIPP in the sucrose + NNS group compared to the water + NNS group; MD ‐1.70 (95% CI ‐2.20 to ‐1.20). When the three studies were combined (n = 278) there was a significantly lower PIPP score at 30 s after heel lance in the sucrose group than the water group; WMD ‐1.70 (95% CI ‐2.13 to ‐1.26; Analysis 5.2; Figure 4); I2 = 0 % (no heterogeneity).
5.2. Analysis.
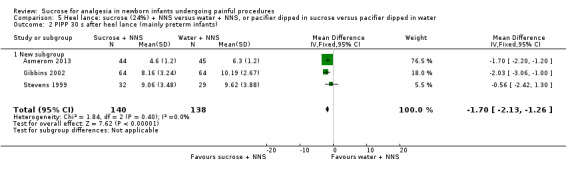
Comparison 5 Heel lance: sucrose (24%) + NNS versus water + NNS, or pacifier dipped in sucrose versus pacifier dipped in water, Outcome 2 PIPP 30 s after heel lance (mainly preterm infants).
4.
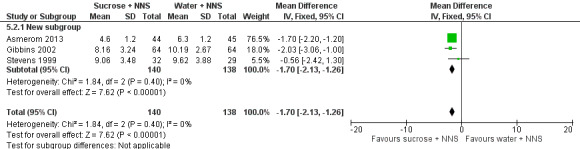
Forest plot of comparison: 6 Heel lance: Sucrose (24%) + NNS vs. water + NNS, outcome: 6.2 PIPP 30 s after heel lance (term and preterm infants).
PIPP 60 s after heel lance (term and preterm infants) (Outcome 5.3)
One study reported on 119 term and preterm infants (Gibbins 2002). There was a significantly reduced PIPP score in the sucrose + NNS group compared to the water + NNS group; MD ‐2.42 (95% CI ‐3.77 to ‐1.07). Another study reported on 45 preterm infants, who received either pacifier dipped in sucrose or pacifier dipped in water (Stevens 1999). There were no significant differences in the PIPP score between the sucrose and water groups; MD ‐1.06 (95% CI ‐3.70 to 1.58). When the two studies were combined there was a significantly lower PIPP score in the sucrose group compared with the water group; WMD ‐2.14 (95% CI ‐3.34 to ‐0.94; Analysis 5.3; Figure 5); I2 = 0% (no heterogeneity).
5.3. Analysis.
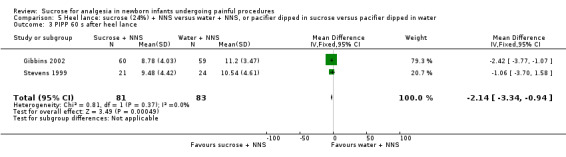
Comparison 5 Heel lance: sucrose (24%) + NNS versus water + NNS, or pacifier dipped in sucrose versus pacifier dipped in water, Outcome 3 PIPP 60 s after heel lance.
5.
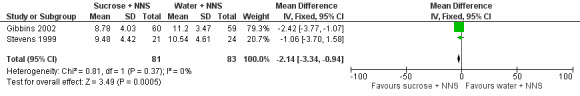
Forest plot of comparison: 6 Heel lance: Sucrose (24%) + NNS vs. water + NNS, outcome: 6.3 PIPP 60 s after heel lance.
Secondary outcomes
Crying time (s) (Outcome 5.4)
One study reported on 100 term infants and compared sucrose + NNS with water + NNS (Unceta‐Barranechea 2008). Another study reported on 42 term infants and compared a sucrose‐coated pacifier with a water‐moistened pacifier (Greenberg 2002). When the two studies were combined there was no significant difference in the crying time (s) in the sucrose + NNS group compared to the water + NNS group; MD ‐1.41 s (95% CI ‐9.87 to 7.04; Analysis 5.4); I2 = 83% (high).
5.4. Analysis.
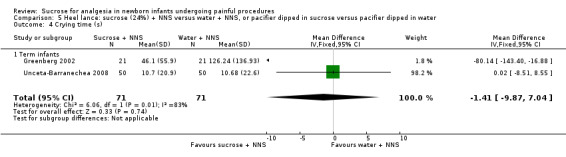
Comparison 5 Heel lance: sucrose (24%) + NNS versus water + NNS, or pacifier dipped in sucrose versus pacifier dipped in water, Outcome 4 Crying time (s).
Sucrose (20%) versus human milk (Comparison 6)
Secondary outcomes
Crying time (s) (Outcome 6.1)
One study reported on 35 term infants (Mathai 2006). There was no significant difference in crying time in the sucrose group compared with the human milk group; MD ‐8.00 s (95% CI ‐21.07 to 5.07; Analysis 6.1).
6.1. Analysis.
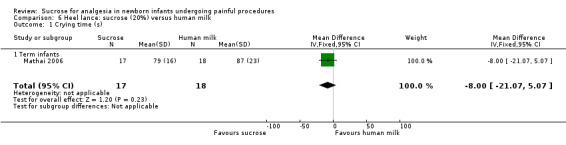
Comparison 6 Heel lance: sucrose (20%) versus human milk, Outcome 1 Crying time (s).
Sucrose (24%) + NNS + Newborn Individualized Developmental Care and Assessment Program (NIDCAP) support versus breast milk via breastfeeding (Comparison 7)
Primary outcomes
PIPP score (Outcome 7.1)
One study reported on 47 preterm infants (Simonse 2012). There was no significant difference in the PIPP score in the sucrose + NNS + NIDCAP support group compared with the breast milk by breastfeeding group; MD ‐1.75 (95% CI ‐4.03 to 0.53; Analysis 7.1).
7.1. Analysis.
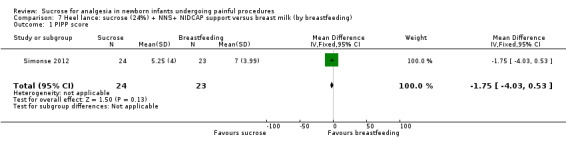
Comparison 7 Heel lance: sucrose (24%) + NNS+ NIDCAP support versus breast milk (by breastfeeding), Outcome 1 PIPP score.
COMFORTneo score (Outcome 7.2)
One study reported on 47 preterm infants (Simonse 2012). The COMFORTneo score was significantly lower in the sucrose + NNS + NIDCAP support group than in the breast milk via breastfeeding group; MD ‐2.60 (95% CI ‐4.84 to ‐0.36; Analysis 7.2).
7.2. Analysis.
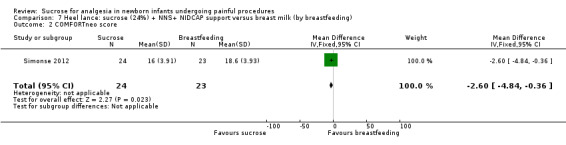
Comparison 7 Heel lance: sucrose (24%) + NNS+ NIDCAP support versus breast milk (by breastfeeding), Outcome 2 COMFORTneo score.
Sucrose (24%) + NNS + Newborn Individualized Developmental Care and Assessment Program (NIDCAP) support versus breast milk via syringe (Comparison 8)
PIPP score (Outcome 8.1)
One study reported on 47 preterm infants (Simonse 2012). There was no significant difference in the PIPP score in the sucrose + NNS + NIDCAP support group compared with the breast milk via syringe group; MD ‐0.13 (95% CI ‐2.41 to 2.15; Analysis 8.1).
8.1. Analysis.
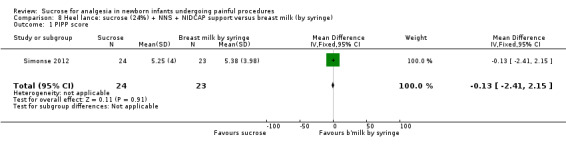
Comparison 8 Heel lance: sucrose (24%) + NNS + NIDCAP support versus breast milk (by syringe), Outcome 1 PIPP score.
COMFORTneo score (Outcome 8.2)
One study reported on 47 preterm infants (Simonse 2012). The COMFORTneo score was not significantly different in the sucrose + NNS + NIDCAP support group compared with the breast milk by syringe group; MD ‐0.50 (95% CI ‐2.74 to 1.74; Analysis 8.2).
8.2. Analysis.
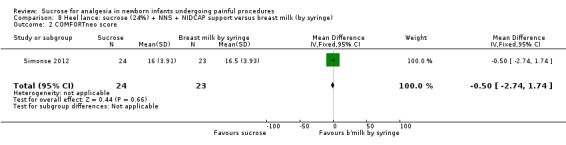
Comparison 8 Heel lance: sucrose (24%) + NNS + NIDCAP support versus breast milk (by syringe), Outcome 2 COMFORTneo score.
Repeated heel lances: sucrose (20%) versus facilitated tucking (Comparison 9)
Primary outcomes
Total Bernese Pain Scale for Neonates during heel lance (Outcome 9.1)
One study reported on 48 infants (Cignacco 2012). There was no significant difference in the total Bernese Pain Scale for Neonates during heel lancing between the sucrose and the facilitated tucking groups; MD ‐2.27 (95% CI ‐4.66 to 0.12; Analysis 9.1).
9.1. Analysis.

Comparison 9 Repeated heel lances: sucrose (20%) versus facilitated tucking, Outcome 1 Total Bernese Pain Scale for Neonates during heel lance.
Total Bernes Pain Scale for Neonates during recovery (Outcome 9.2)
One study reported on 48 infants (Cignacco 2012). There was no significant difference in the total Bernese Pain Scale for Neonates during recovery between the sucrose and the facilitated tucking groups; MD ‐0.31 (95% CI ‐1.72 to 1.10; Analysis 9.2).
9.2. Analysis.
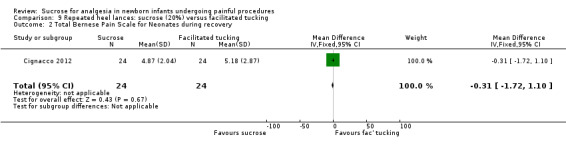
Comparison 9 Repeated heel lances: sucrose (20%) versus facilitated tucking, Outcome 2 Total Bernese Pain Scale for Neonates during recovery.
Repeated heel lances: sucrose (20%) versus facilitated tucking + sucrose (20%) (Comparison 10)
Primary outcomes
Total Bernese Pain Scale for Neonates during heel lance (Outcome 10.1)
One study reported on 47 infants (Cignacco 2012). There was no significant difference in the total Bernese Pain Scale for Neonates during heel lance between the sucrose and the facilitated tucking groups; MD ‐0.05 (95% CI ‐2.16 to 2.06; Analysis 10.1).
10.1. Analysis.
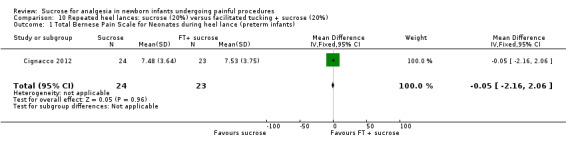
Comparison 10 Repeated heel lances: sucrose (20%) versus facilitated tucking + sucrose (20%), Outcome 1 Total Bernese Pain Scale for Neonates during heel lance (preterm infants).
Total Bernese Pain Scale for Neonates during recovery (Outcome 10.2)
One study reported on 47 infants (Cignacco 2012). There was no significant difference in the total Bernese Pain Scale for Neonates during recovery between the sucrose and the facilitated tucking groups; MD 0.64 (95% CI ‐0.73 to 2.01; Analysis 10.2).
10.2. Analysis.

Comparison 10 Repeated heel lances: sucrose (20%) versus facilitated tucking + sucrose (20%), Outcome 2 Total Bernese Pain Scale for Neonates during recovery (preterm infants).
Sucrose (30% to 33%) versus glucose (30% to 33%) (Comparison 11)
Secondary outcomes
Heart rate (beats/minute) during heel lance (Outcome 11.1)
One study reported on 40 term infants (Guala 2001). There was no significant difference n the heart rate (beats/minute) during heel lance in the sucrose group compared with the glucose group; MD 6.20 (95% CI ‐6.19 to 18.59; Analysis 11.1).
11.1. Analysis.
Comparison 11 Heel lance: sucrose (30% to 33%) versus glucose (30% to 33%), Outcome 1 Heart rate (beats/min) during heel lance.
Crying time (s) (Outcome 11.2)
One study reported on 56 term infants (Isik 2000a). There was a significantly shorter crying time (s) in the sucrose group compared with the glucose group; MD ‐34.89 (95% CI ‐61.67 to ‐8.11; Analysis 11.2).
11.2. Analysis.
Comparison 11 Heel lance: sucrose (30% to 33%) versus glucose (30% to 33%), Outcome 2 Crying time (s).
Percentage change in heart rate one minute after heel lance (Outcome 11.3)
One study reported on 56 term infants (Isik 2000a). there was no significant difference in the percentage change in heart rate (%) one minute after heel lance between the sucrose group and the glucose group; MD ‐6.58 (95% CI ‐14.85 to 1.69; Analysis 11.3).
11.3. Analysis.
Comparison 11 Heel lance: sucrose (30% to 33%) versus glucose (30% to 33%), Outcome 3 Percentage change in heart rate 1 min after heel lance.
Sucrose (50%) versus glucose (50%) (Comparison 12)
Secondary outcomes
Heart rate (beats/minute) during heel lance (Outcome 12.1)
One study reported on 40 infants (Guala 2001). The heart rate (beats/minute) during heel lance was significantly higher in the sucrose group compared with the glucose group; MD 16.30 (95% CI 1.93 to 30.67; Analysis 12.1).
12.1. Analysis.
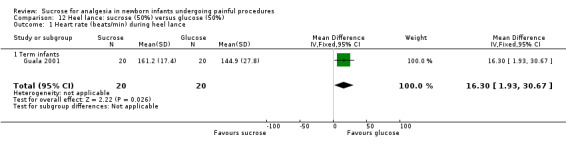
Comparison 12 Heel lance: sucrose (50%) versus glucose (50%), Outcome 1 Heart rate (beats/min) during heel lance.
Sucrose (24%) versus laser acupuncture (Comparison 13)
Primary outcomes
NIPS score (Outcome 13.1)
One study reported on 42 term infants (Abbasoglu 2015). The NIPS score was significantly lower in the sucrose group compared with the laser acupuncture group during heel lance; MD ‐0.86 (95% CI ‐1.43 to ‐0.29; Analysis 13.1).
13.1. Analysis.
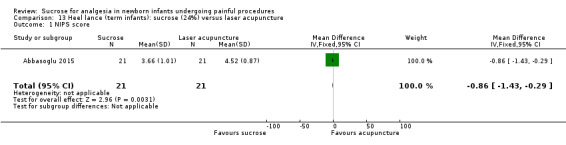
Comparison 13 Heel lance (term infants): sucrose (24%) versus laser acupuncture, Outcome 1 NIPS score.
Secondary outcomes
Crying time (s) (Outcome 13.2)
One study reported on 42 term infants (Abbasoglu 2015). The crying time (s) was significantly lower in the sucrose group than in the laser acupuncture group during heel lance; MD ‐51.29 (95% CI ‐73.11 to ‐29.47; Analysis 13.2).
13.2. Analysis.
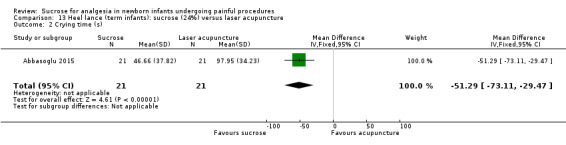
Comparison 13 Heel lance (term infants): sucrose (24%) versus laser acupuncture, Outcome 2 Crying time (s).
Sucrose (24%) versus sucrose (24%) + NNS (Comparison 14)
Primary outcomes
Revised NFCS (Outcome 14.1)
One study reported on 343 term infants (Leng 2015). The revised NFCS score was significantly higher in the sucrose group than in the sucrose + NNS group; MD 0.43 (95% CI 0.23 to 0.63; Analysis 14.1).
14.1. Analysis.
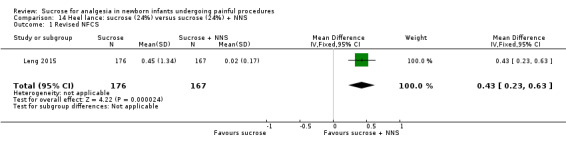
Comparison 14 Heel lance: sucrose (24%) versus sucrose (24%) + NNS, Outcome 1 Revised NFCS.
Secondary outcomes
Increase in heart rate (%) (Outcome 14.2)
One study reported on 343 term infants (Leng 2015). There was a significantly higher increase (%) in the heart rate in the sucrose group compared with the sucrose + NNS group; MD 2.29 (95% CI 0.44 to 4.14; Analysis 14.2).
14.2. Analysis.
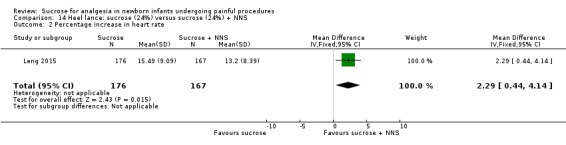
Comparison 14 Heel lance: sucrose (24%) versus sucrose (24%) + NNS, Outcome 2 Percentage increase in heart rate.
Decrease in oxygen saturation of blood (%) (Outcome 14.3)
One study reported on 343 term infants (Leng 2015). There was a significantly larger decrease (%) in the oxygen saturation of blood in the sucrose group compared with the sucrose + NNS group; MD 0.48 (95% CI 0.10 to 0.86; Analysis 14.3).
14.3. Analysis.
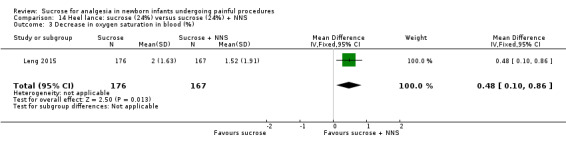
Comparison 14 Heel lance: sucrose (24%) versus sucrose (24%) + NNS, Outcome 3 Decrease in oxygen saturation in blood (%).
Sucrose (24%) versus sucrose (24%) + swaddling (Comparison 15)
Primary outcomes
Revised NFCS (Outcome 15.1)
One study reported on 343 term infants (Leng 2015). The revised NFCS score was significantly higher in the sucrose group compared with the sucrose + swaddling group; MD 0.40 (95% CI 0.19 to 0.61; Analysis 15.1).
15.1. Analysis.
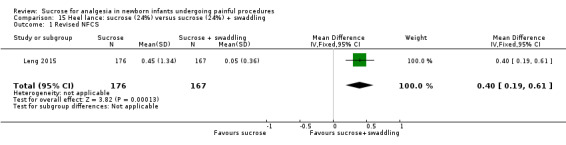
Comparison 15 Heel lance: sucrose (24%) versus sucrose (24%) + swaddling, Outcome 1 Revised NFCS.
Secondary outcomes
Increase in heart rate (%) (Outcome 15.2)
One study reported on 343 term infants (Leng 2015). There was no significant difference in the increase (%) in the heart rate in the sucrose group compared with the sucrose + swaddling group; MD 0.57 (95% CI ‐1.43 to 2.57; Analysis 15.2).
15.2. Analysis.
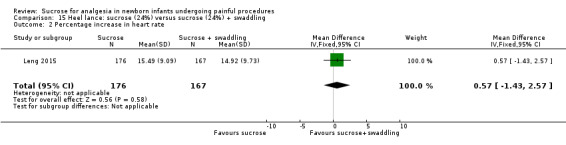
Comparison 15 Heel lance: sucrose (24%) versus sucrose (24%) + swaddling, Outcome 2 Percentage increase in heart rate.
Decrease in oxygen saturation of blood (%) (Outcome 15.3)
One study reported on 343 term infants (Leng 2015). There was no significant difference in the decrease (%) in the oxygen saturation of blood in the sucrose group compared with the sucrose + swaddling group; MD 0.30 (95% CI ‐0.07 to 0.67; Analysis 15.3).
15.3. Analysis.
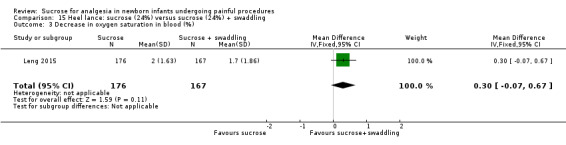
Comparison 15 Heel lance: sucrose (24%) versus sucrose (24%) + swaddling, Outcome 3 Decrease in oxygen saturation in blood (%).
Sucrose (24%) versus sucrose (24%) + NNS + swaddling (Comparison 16)
Primary outcomes
Revised NFCS (Outcome 16.1)
One study reported on 337 term infants (Leng 2015). The revised NFCS score was significantly higher in the sucrose group compared with the sucrose + NNS + swaddling group; MD 0.43 (95% CI 0.23 to 0.63; Analysis 16.1).
16.1. Analysis.

Comparison 16 Heel lance: sucrose (24%) versus sucrose (24%) + NNS + swaddling, Outcome 1 Revised NFCS.
Secondary outcomes
Increase in heart rate (%) (Outcome 16.2)
One study reported on 343 term infants (Leng 2015). There was a significantly larger increase (%) in the heart rate in the sucrose group compared with the sucrose + NNS + swaddling group; MD 3.25 (95% CI 1.43 to 5.07; Analysis 16.2).
16.2. Analysis.
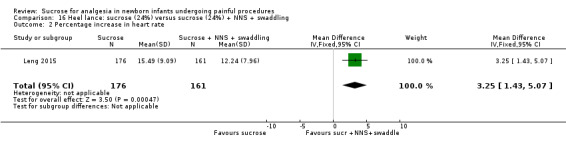
Comparison 16 Heel lance: sucrose (24%) versus sucrose (24%) + NNS + swaddling, Outcome 2 Percentage increase in heart rate.
Decrease in oxygen saturation (%) (Outcome 16.3)
One study reported on 343 term infants (Leng 2015). There was a significantly larger decrease (%) in the oxygen saturation of blood in the sucrose group compared with the sucrose + NNS + swaddling group; MD 0.79 (95% CI 0.44 to 1.44; Analysis 16.3).
16.3. Analysis.
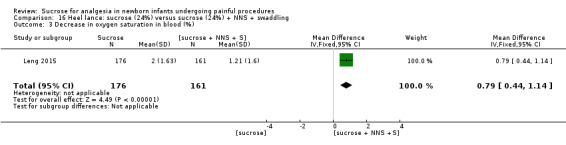
Comparison 16 Heel lance: sucrose (24%) versus sucrose (24%) + NNS + swaddling, Outcome 3 Decrease in oxygen saturation in blood (%).
Data from 21 studies could not be included in analyses in RevMan (Altun‐Köroğlu 2010; Blass 1997; Blass 1999; Bucher 1995; Codipietro 2008; Gormally 2001; Johnston 1997; Leng 2013; Liaw 2013; Marin Gabriel 2013 ; Okan 2007; Örs 1999; Overgaard 1999; Ramenghi 1996a; Ramenghi 1996b; Ramenghi 1999; Rushforth 1993; Stevens 2005a; Storm 2002; Thakkar 2016; Yilmaz 2010); for details see Table 25, though a brief summary is provided below.
In the Altun‐Köroğlu 2010 study there were no statistically significant differences observed between the hind milk group and the sucrose group for all measures. In Blass 1997 there was significantly less crying time during blood collection in the sucrose group than in the water group, and, in the later Blass 1999 study, sucrose diminished cry duration compared to water. In Bucher 1995 cry duration was significantly reduced by sucrose compared to water. In Codipietro 2008 the duration of first cry was shorter in the breastfeeding group than the sucrose group, as were the PIPP scores (two minutes after heel lance). Gormally 2001 reported a significant effect of holding on pain concatenation scores for facial activity, with no difference between infants who received sucrose and those who did not. Johnston 1997 found a significant decrease in percentage facial action in the sucrose alone group and the sucrose plus rocking group compared to the water group. In the large study, Leng 2013, the average pain score three minutes after the procedure was significantly lower in the sucrose groups compared to the glucose groups. Liaw 2013 found that infants receiving NNS + oral sucrose + tucking or NNS + oral sucrose experienced more quiet sleep occurrences than those receiving routine care. Marin Gabriel 2013 reported a significantly lower percentage of crying with both the breast fed + skin‐to‐skin contact group and also the sucrose + skin‐to‐skin contact group compared with the skin‐to‐skin contact group. In Okan 2007 the sucrose and glucose groups showed a shorter time for the duration of first cry and total crying time than the water group. In Overgaard 1999 the NIPS scores one minute after heel lance and one minute after blood sampling were statistically significantly lower in the sucrose group than the water group. Ramenghi 1996a noted lower mean pain scores in the group receiving sucrose at one and two minutes after heel lance compared to the water group. In Ramenghi 1996b the pain scores were significantly lower in the sucrose (25% and 50%) groups and the Calpol group compared to the water group. In the third study by the Ramenghi group (Ramenghi 1999), which was a cross‐over study (sucrose and water were given by nasogastric tube or intraorally, but the infant received either sucrose or water both times), significant reductions in behavioural scores were noted in the sucrose group compared with the water group. It is notable that infants in the 25% sucrose group displayed a significant reduction in behavioural score (P value 0.001) when sucrose was given intraorally compared to via a nasogastric tube. Rushforth 1993 used a low concentration of sucrose (7.5%) and found no difference in median percentage crying time between the sucrose group and the group that received water. In Stevens 2005a 66 infants were randomised to (1) standard of care (positioning + swaddling), (2) standard of care, sterile water via syringe and pacifier or (3) standard of care, 24% sucrose via syringe and pacifier two minutes prior to painful interventions during the first 28 days of life. PIPP scores were recorded at day 7, 14, 21, and 28 at routine heel lance. There was a significant main effect of group (P = 0.03) with differences occurring between the sucrose + pacifier group and standard care group at pain assessment at 60 s ( P value 0.01). Mean PIPP scores were generally higher in the standard care group. Storm 2002 reported significantly less crying in infants in the groups that received sucrose (25%) or sucrose (25%) + milk compared to the groups that received a lower concentration of sucrose (15%) or milk. Thakkar 2016 reported lower median PIPP scores in the sucrose + NNS group compared to the sucrose only, NNS only, or no intervention groups. Yilmaz 2010 reported that the mean crying time in the sucrose group was lower than in the groups where the infant was sitting on the mother's lap, received mother's milk by syringe, or was given a pacifier. Örs 1999 noted that there was a significant decrease in crying time for the sucrose group compared to the human milk or sterile water groups, and recovery time from crying was shorter in the sucrose group, as was the percentage change in heart rate after heel lance.
Effectiveness of sucrose for venipuncture
Sucrose (12%) versus water (Comparison 17)
Primary outcomes
NIPS scores in term and preterm infants (Outcome 17.1)
One study reported on 111 preterm and term infants (Montoya 2009). There was no significant difference in the NIPS score between the sucrose and the water groups; MD ‐0.90 (95% CI ‐1.81 to 0.01; Analysis 17.1).
17.1. Analysis.
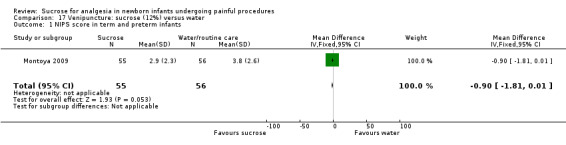
Comparison 17 Venipuncture: sucrose (12%) versus water, Outcome 1 NIPS score in term and preterm infants.
Sucrose (24% to 30%) versus control (sterile water or no treatment) (Comparison 18)
Primary outcomes
PIPP score during venipuncture (Outcome 18.1)
One study reported on 213 term infants (106 born to non‐diabetic mothers and 107 to diabetic mothers) (Taddio 2008). For the two groups of infants combined there was a significant reduction in the PIPP score in the sucrose group compared with the sterile water group; WMD ‐2.79 (95% CI ‐3.76 to ‐1.83; Analysis 18.1; Figure 6); I2 = 0 % (no heterogeneity). In the 106 infants born to non‐diabetic mothers there was a significant reduction in the PIPP score in the sucrose group compared with the water group; MD ‐3.20 (95% CI ‐4.58 to ‐1.82). In the 107 infants born to diabetic mothers there was a significant reduction in the PIPP score in the sucrose group compared with the water group; MD ‐2.40 (95% CI ‐3.76 to ‐1.04).
18.1. Analysis.
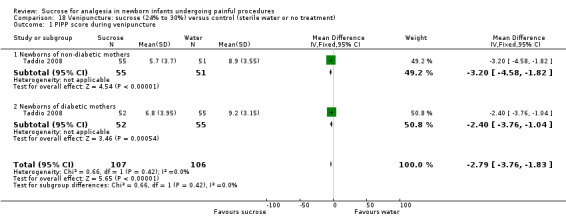
Comparison 18 Venipuncture: sucrose (24% to 30%) versus control (sterile water or no treatment), Outcome 1 PIPP score during venipuncture.
6.
Forest plot of comparison: 18 Venipuncture: sucrose (24% to 30%) versus control (sterile water or no treatment), outcome: 18.1 PIPP score during venipuncture.
Secondary outcomes
Duration of cry (s) in term infants (Outcome 18.2)
One study reported on 50 term infants (Basnet 2010). There was no significant difference between the sucrose and water groups in the duration of cry (s); MD ‐16.50 (95% CI ‐71.41 to 38.41; Analysis 18.2).
18.2. Analysis.
Comparison 18 Venipuncture: sucrose (24% to 30%) versus control (sterile water or no treatment), Outcome 2 Duration of cry (s).
Sucrose (50%) versus water (Comparison 19)
Secondary outcomes
Duration of first cry (s) in term infants (Outcome 19.1)
One study reported on 50 term infants (Ogawa 2005). There was no significant difference in the duration of first cry (s) between the sucrose and the water group; MD ‐14.00 (95% CI ‐51.79 to 23.79; Analysis 19.1).
19.1. Analysis.
Comparison 19 Venipuncture: sucrose (50%) versus water, Outcome 1 Duration of first cry (s).
Sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/liposomal lidocaine cream on the skin (Comparison 20)
Primary outcomes
PIPP score in preterm infants during venipuncture (Outcome 20.1)
One study reported on 76 preterm infants (Biran 2011). There was no significant difference in the PIPP score between the sucrose and the sucrose + EMLA/liposomal lidocaine group; MD 1.30 (95% CI ‐0.12 to 2.72; Analysis 20.1).
20.1. Analysis.
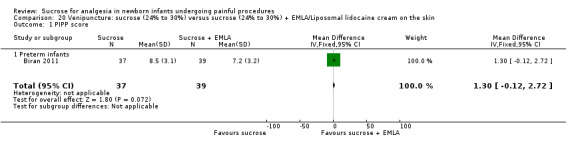
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 1 PIPP score.
PIPP score in preterm infants during recovery period (Outcome 20.2)
One study reported on 76 preterm infants (Biran 2011). There was no significant difference in the PIPP score during the recovery period between the sucrose and the sucrose + EMLA/liposomal lidocaine group; MD 0.60 (95% CI ‐0.73 to 1.93; Analysis 20.2).
20.2. Analysis.
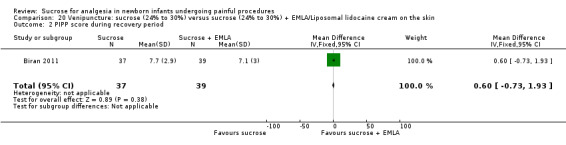
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 2 PIPP score during recovery period.
DAN score during venipuncture in preterm infants (Outcome 20.3)
One study reported on 76 preterm infants (Biran 2011). The DAN score during venipuncture was significantly higher in the sucrose group compared with the sucrose + EMLA/liposomal lidocaine group; MD 1.30 (95% CI 0.26 to 2.34; Analysis 20.3).
20.3. Analysis.
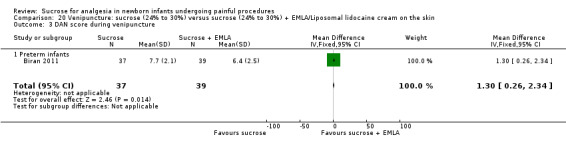
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 3 DAN score during venipuncture.
DAN score in preterm infants during recovery period (Outcome 20.4)
One study reported on 76 preterm infants (Biran 2011). The DAN score during the recovery period was significantly higher in the sucrose group compared with the sucrose + EMLA/liposomal lidocaine group; MD 1.40 (95% CI 0.03 to 2.77; Analysis 20.4).
20.4. Analysis.
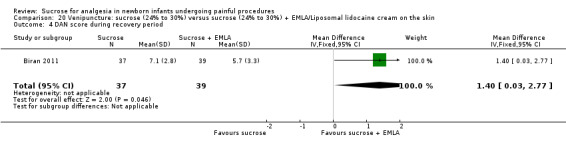
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 4 DAN score during recovery period.
Facial grimacing score in term infants (Outcome 20.5)
One study reported on 213 term infants (Taddio 2011). There was no significant difference between the sucrose group and the sucrose + EMLA/liposomal lidocaine group for the facial grimacing score; MD ‐5.00 (95% CI ‐13.48 to 3.48; Analysis 20.5).
20.5. Analysis.
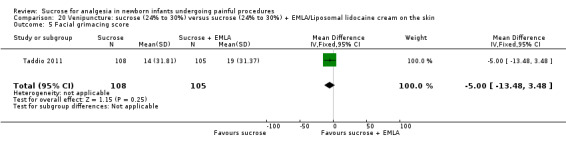
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 5 Facial grimacing score.
Observer‐rated pain visual analogue scale (VAS) (cm) (Outcome 20.6)
One study reported on 213 term infants (Taddio 2011). There was no significant difference between the sucrose group and the sucrose + EMLA/liposomal lidocaine group for observer rated pain (VAS) (cm); MD ‐0.70 (95% CI ‐1.55 to 0.15; Analysis 20.6).
20.6. Analysis.
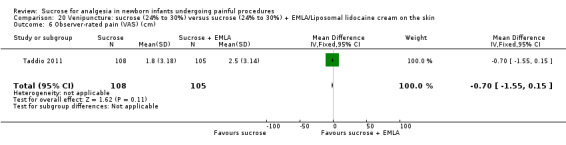
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 6 Observer‐rated pain (VAS) (cm).
Secondary outcomes
Mean crying time (s) during all procedures (Outcome 20.7)
One study reported on 213 term infants (Taddio 2011), and another study reported on 76 preterm infants (Biran 2011). For the two studies combined (n = 289) there was no significant difference between the sucrose group and the sucrose + EMLA/liposomal lidocaine group for mean crying time (s); MD 1.83 (95% CI ‐10.42 to 14.09; Analysis 20.7); I2 = 0% (no heterogeneity). In term infants (n = 213) there was no significant difference between the sucrose group and the sucrose + EMLA/liposomal lidocaine group for mean crying time (s); MD 0.00 (95% CI ‐13.79 to 13.79) (Taddio 2011). In preterm infants (n = 76) there was no significant difference between the sucrose group and the sucrose + EMLA/liposomal lidocaine group for mean crying time; 8.69 (95% CI ‐17.97 to 35.35) (Biran 2011).
20.7. Analysis.
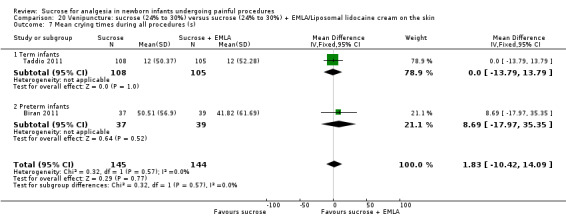
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 7 Mean crying times during all procedures (s).
Heart rate (beats/minute) in term infants (Outcome 20.8)
One study reported on 213 term infants (Taddio 2011). The heart rate (beats/minute) was significantly higher in the sucrose group than the sucrose + EMLA/liposomal lidocaine group; MD 5.00 (95% CI 0.39 to 9.61; Analysis 20.8).
20.8. Analysis.
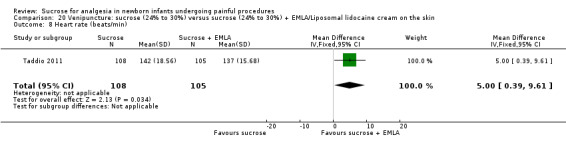
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 8 Heart rate (beats/min).
Oxygen saturation (%) in term infants (Outcome 20.9) Analysis 20.9
One study reported on 213 term infants (Taddio 2011). There was no significant difference between the sucrose group and the sucrose + EMLA/liposomal lidocaine group for oxygen saturation (%); MD 0.20 (95% CI ‐0.33 to 0.73; Analysis 20.9).
20.9. Analysis.
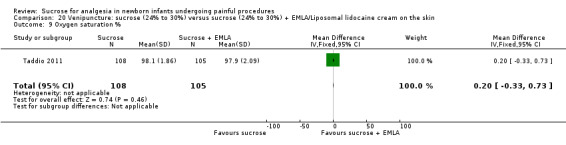
Comparison 20 Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin, Outcome 9 Oxygen saturation %.
Sucrose (24%) versus liposomal lidocaine (Comparison 21)
Primary outcomes
Facial grimacing score (Outcome 21.1)
One study reported on 216 term infants (Taddio 2011). There was a significantly lower facial grimacing score in the sucrose group compared to the liposomal lidocaine group; MD ‐28.00 (95% CI ‐36.48 to ‐19.52; Analysis 21.1).
21.1. Analysis.
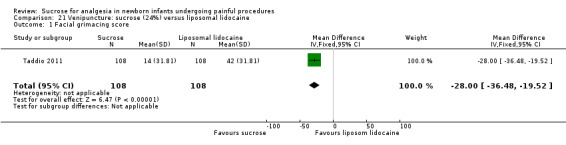
Comparison 21 Venipuncture: sucrose (24%) versus liposomal lidocaine, Outcome 1 Facial grimacing score.
Observer‐rated pain (VAS) (cm) (Outcome 21.2)
One study reported on 216 term infants (Taddio 2011). There was no significant difference in the observer‐rated pain (VAS) (cm) in the sucrose group compared to the liposomal lidocaine group; MD ‐0.40 (95% CI ‐1.25 to 0.45; Analysis 21.2).
21.2. Analysis.
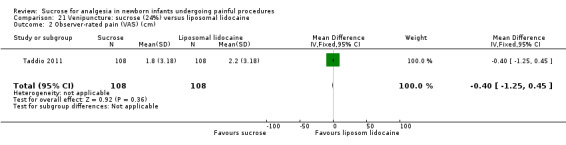
Comparison 21 Venipuncture: sucrose (24%) versus liposomal lidocaine, Outcome 2 Observer‐rated pain (VAS) (cm).
Cry duration (sec) (Outcome 21.3)
One study reported on 216 term infants (Taddio 2011). There was a significantly shorter cry duration (s) in the sucrose group compared to the liposomal lidocaine group; MD ‐39.00 (95% CI ‐52.43 to ‐25.57; Analysis 21.3).
21.3. Analysis.
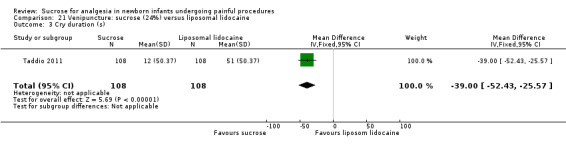
Comparison 21 Venipuncture: sucrose (24%) versus liposomal lidocaine, Outcome 3 Cry duration (s).
Heart rate (beats/minute) (Outcome 21.4)
One study reported on 216 term infants (Taddio 2011). There was no significant difference in the heart rate (beats/minute) in the sucrose group compared to the liposomal lidocaine group; MD 3.00 (95% CI ‐1.95 to 7.95; Analysis 21.4).
21.4. Analysis.
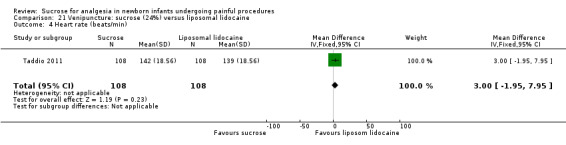
Comparison 21 Venipuncture: sucrose (24%) versus liposomal lidocaine, Outcome 4 Heart rate (beats/min).
Oxygen saturation (%) (Outcome 21.5) Analysis 21.5
One study reported on 216 term infants (Taddio 2011). There was no significant difference in the percentage oxygen (%) saturation in the sucrose group compared to the liposomal lidocaine group; MD 0.50 (95% CI ‐0.03 to 1.03; Analysis 21.5).
21.5. Analysis.
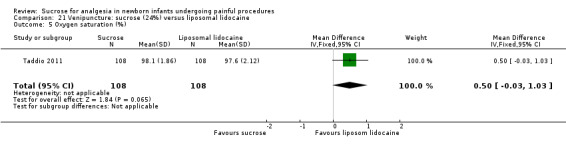
Comparison 21 Venipuncture: sucrose (24%) versus liposomal lidocaine, Outcome 5 Oxygen saturation (%).
Results from five studies could not be included in RevMan‐analyses, but are described in a narrative here. Abad 1996 reported shorter cry duration three minutes after venipuncture in the sucrose (24%) group compared to the sucrose (12%) group and the water group. In Acharya 2004 the duration of first cry was shorter in infants who received sucrose, as was total duration of crying. Mean change in heart rate from pre procedure to procedure and post procedure was lower in the infants who received sucrose. Changes in mean NFCS scores were significantly lower in the sucrose group than in the water group from pre procedure to the procedure phase and the post procedure phase. Carbajal 1999 reported lower DAN scores in the groups that received glucose, sucrose, pacifier, or sucrose + pacifier compared to water. There was a trend towards lower pain scores for infants receiving sucrose with a pacifier compared to a pacifier alone. In the Elserafy 2009 cross‐over study every infant received each of six different regimens during a maximum stay of 15 days. Sucrose + pacifier resulted in the lowest pain (PIPP) scores. The sucrose groups had significantly lower crying times compared to the other groups. Gaspardo 2008 (listed under additional table Table 35) studied different phases of venipuncture on different days. All significant results favoured the sucrose group. On day 2 the percentage of neonates crying showed a significant difference between the sucrose and control groups in the antisepsis phase and puncture phases. On day 3, there was a significant difference between groups in the percentage of neonates crying in the dressing phase. On day 4, significant differences existed between groups at the puncture phase. A significant difference in the NFCS ≥ 3 was seen between sucrose and control groups on day 2 at the puncture phase, and on day 3 a significant difference was observed at the antisepsis phase. There was a significant difference in the percentage of neonates with active behavioural state (ABS) score ≥ 4 between sucrose and control groups on day 2 at the puncture phase, and on day 4 at the antisepsis phase.
Effectiveness of sucrose for arterial puncture
Sucrose (24%) versus no intervention in preterm infants (Comparison 22)
Secondary outcomes
Heart rate (beats/minute) after needle insertion (Outcome 22.1)
One study reported on 47 preterm infants (Milazzo 2011). There was no significant difference in the heart rate (beats/minute) between the sucrose group and the no intervention group after needle insertion; MD ‐1.90 (95% CI ‐11.73 to 7.93; Analysis 22.1).
22.1. Analysis.
Comparison 22 Arterial puncture in preterm infants: sucrose (24%) versus no intervention, Outcome 1 Heart rate (beats/min) after needle insertion.
Heart rate (beats/minute) one minute after procedure completed (Outcome 22.2)
One study reported on 47 preterm infants (Milazzo 2011). There was no significant difference in the heart rate (beats/minute) between the sucrose group and the no intervention group one minute after procedure completion; MD ‐2.40 (95% CI ‐10.56 to 5.76; Analysis 22.2).
22.2. Analysis.
Comparison 22 Arterial puncture in preterm infants: sucrose (24%) versus no intervention, Outcome 2 Heart rate (beats/min) 1 min after procedure completed.
Oxygen saturation in blood (%) after needle insertion (Outcome 22.3)
One study reported on 47 preterm infants (Milazzo 2011). There was no significant difference in the oxygen saturation after needle insertion between the sucrose group and the no intervention group; MD ‐1.00 (95% CI ‐4.65 to 2.65; Analysis 22.3).
22.3. Analysis.
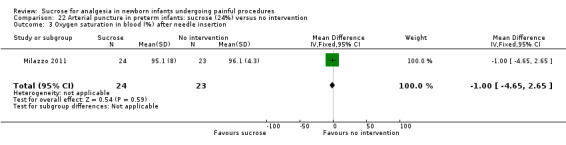
Comparison 22 Arterial puncture in preterm infants: sucrose (24%) versus no intervention, Outcome 3 Oxygen saturation in blood (%) after needle insertion.
Oxygen saturation in blood (%) one minute after procedure (Outcome 22.4)
One study reported on 47 preterm infants (Milazzo 2011). There was no significant difference in the oxygen saturation (%) one minute after the procedure between the sucrose group and the no intervention group; MD ‐2.90 (95% CI ‐5.95 to 0.15; Analysis 22.4).
22.4. Analysis.
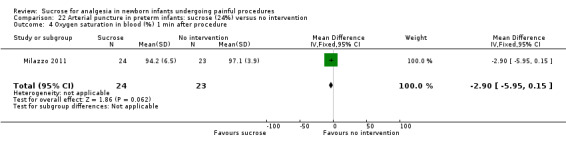
Comparison 22 Arterial puncture in preterm infants: sucrose (24%) versus no intervention, Outcome 4 Oxygen saturation in blood (%) 1 min after procedure.
Milazzo 2011 was the only study identified for arterial puncture.
Effectiveness of sucrose for subcutaneous injection
Two reports studied the effects of sucrose for subcutaneous injection (Allen 1996, Mucignat 2004). Neither of the studies reported the results in a way that permitted the use of data in RevMan‐analyses. For details see Table 29.
In the Allen 1996 study two‐week old infants who received either sterile water or sucrose solution cried significantly less than infants who received no intervention (P < 0.005). Mucignat 2004 found significant reductions in DAN and NFCS scores in the EMLA + NNS, sucrose + NNS, and sucrose + EMLA + pacifier groups.
Effectiveness of sucrose for pain associated with intramuscular injection (immunization or vitamin K) (term infants)
Sucrose (25%) versus water or no intervention (Comparison 23)
Primary outcomes
NIPS during one to two minutes after immunization (term infants) (Outcome 23.1)
One study reported on 60 term infants (Suhrabi 2014). There was a significant reduction in the NIPS score between one and two minutes after immunization in term infants in the sucrose group compared to the no intervention group; MD ‐2.30 (95% CI ‐2.93 to ‐1.67; Analysis 23.1).
23.1. Analysis.
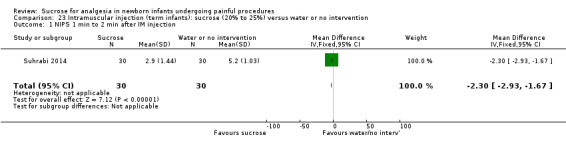
Comparison 23 Intramuscular injection (term infants): sucrose (20% to 25%) versus water or no intervention, Outcome 1 NIPS 1 min to 2 min after IM injection.
PIPP during intramuscular injection (term infants) (Outcome 23.2)
One study reported on 232 term infants (115 infants were born to non‐diabetic mothers and 117 were born to diabetic mothers) (Taddio 2008). The combined group (n = 232) showed a significantly lower PIPP score in the sucrose group compared with the water group; WMD ‐1.05 (95% CI ‐1.98 to ‐0.12; Analysis 23.2; Figure 7); I2 = 0% (no heterogeneity). Among the infants born to non‐diabetic mothers (n = 115) there was no significant difference between the group that received sucrose and the group that received water; MD ‐1.10 (95% CI ‐2.38 to 0.18). Among the infants born to diabetic mothers there was no significant difference between the group that received sucrose and the group that received water; MD ‐1.00 (95% CI ‐2.35 to 0.35).
23.2. Analysis.
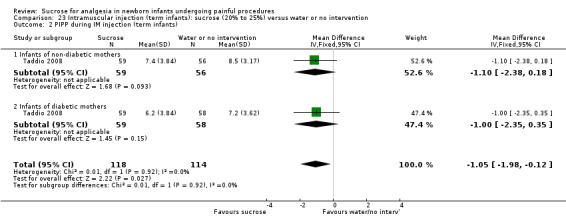
Comparison 23 Intramuscular injection (term infants): sucrose (20% to 25%) versus water or no intervention, Outcome 2 PIPP during IM injection (term infants).
7.
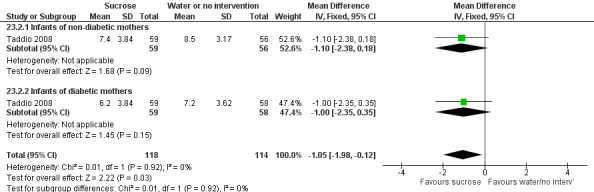
Forest plot of comparison: 23 Intramuscular injection (term infants): Sucrose (20‐25%) vs. water or no intervention, outcome: 23.2 PIPP during IM injection (term infants).
Secondary outcomes
Duration of cry (s) (Outcome 23.3)
One study reported on 110 infants (Liaw 2011). The duration of cry (s) was significantly shorter in the sucrose group than in the no intervention group; MD ‐163.83 (95% CI ‐192.58 to ‐135.08; Analysis 23.3).
23.3. Analysis.

Comparison 23 Intramuscular injection (term infants): sucrose (20% to 25%) versus water or no intervention, Outcome 3 Duration of cry (s).
Sucrose (25%) versus glucose (25%) (Comparison 24)
Primary outcomes
NIPS during one to two minutes after immunization (term infants) (Outcome 24.1)
One study reported on 60 infants (Suhrabi 2014). There was no significant difference in the NIPS score between one and two minutes after immunization in term infants in the sucrose group compared with the glucose group; MD ‐0.10 (95% CI ‐0.89 to 0.69; Analysis 24.1).
24.1. Analysis.
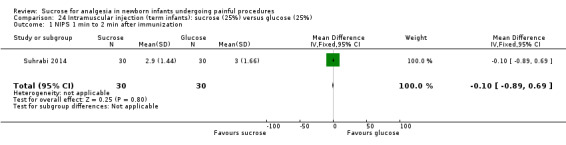
Comparison 24 Intramuscular injection (term infants): sucrose (25%) versus glucose (25%), Outcome 1 NIPS 1 min to 2 min after immunization.
Sucrose (25%) versus sucrose (25%) + warmth (Comparison 25)
Secondary outcomes
Crying time (s) (Outcome 25.1)
One study reported on 29 term infants (Gray 2015). There was a significantly longer crying time (s) in the sucrose group than the sucrose + warmth group; MD 15.20 (95% CI 11.52 to 18.88; Analysis 25.1).
25.1. Analysis.
Comparison 25 Intramuscular injection (term infants): sucrose (25%) versus sucrose (25%) + warmth, Outcome 1 Crying time (s).
Grimacing time (s) (Outcome 25.2)
One study reported on 29 term infants (Gray 2015). There was a significantly longer grimacing time (s) in the sucrose group compared with the sucrose + warmth group; MD 16.20 (95% CI 12.35 to 20.05; Analysis 25.2).
25.2. Analysis.
Comparison 25 Intramuscular injection (term infants): sucrose (25%) versus sucrose (25%) + warmth, Outcome 2 Grimacing time.
One study could not be included in RevMan‐analyses Gray 2012. For details see Table 30. This trial reported that warmer infants cried significantly less than infants exposed to sucrose taste or pacifier suckling after vaccination. Heart rate patterns reflected this analgesia. Core temperature did not differ between study groups.
Effectiveness of sucrose for bladder catheterization
Sucrose versus sterile water (Comparison 26)
Primary outcome
Change in DAN score (Outcome 26.1)
One study reported on 33 infants (Rogers 2006). There was significantly less change in the DAN score in the sucrose group than in the water group; MD ‐2.43 (95% CI ‐4.50 to ‐0.36; Analysis 26.1).
26.1. Analysis.
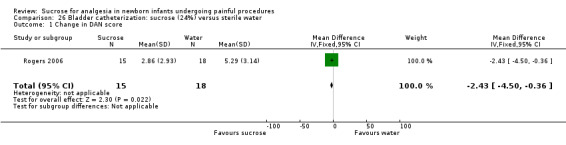
Comparison 26 Bladder catheterization: sucrose (24%) versus sterile water, Outcome 1 Change in DAN score.
Secondary outcomes
Infants crying at maximal catheter insertion (Outcome 26.2)
One study reported on 33 infants (Rogers 2006). The number of infants crying at maximal catheter insertion was significantly lower in the sucrose group than in the sterile water group; RR 0.34 (95% CI 0.14 to 0.82; Analysis 26.2); RD ‐0.51 (95% CI ‐0.81 to ‐0.22); NNTB = 2 (95% CI 1 to 5).
26.2. Analysis.
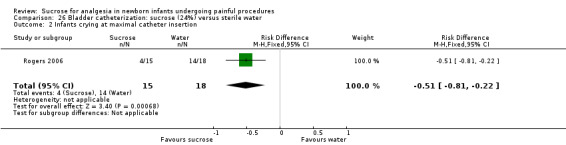
Comparison 26 Bladder catheterization: sucrose (24%) versus sterile water, Outcome 2 Infants crying at maximal catheter insertion.
Rogers 2006 was the only study identified for bladder catheterization.
Effectiveness of sucrose for orogastric or nasogastric tube insertion
Sucrose (25%) versus distilled water (Comparison 27)
Primary outcomes
PIPP score intraprocedure (Outcome 27.1)
One study reported on 105 infants subjected to orogastric tube insertion (Pandey 2013). There was no significant difference in the PIPP score during the procedure; MD ‐0.30 (95% CI ‐1.33 to 0.73; Analysis 27.1).
27.1. Analysis.
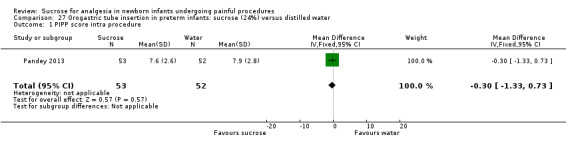
Comparison 27 Orogastric tube insertion in preterm infants: sucrose (24%) versus distilled water, Outcome 1 PIPP score intra procedure.
PIPP score at 30 s postprocedure (Outcome 27.2)
One study reported on 105 infants (Pandey 2013). There was a significantly lower PIPP score in the sucrose group than the water group at 30 s postprocedure; MD ‐1.30 (95% CI ‐2.31 to ‐0.29; Analysis 27.2).
27.2. Analysis.
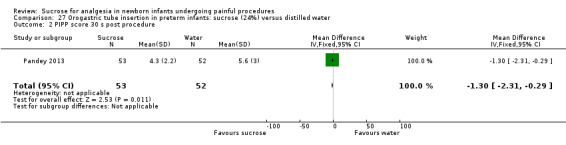
Comparison 27 Orogastric tube insertion in preterm infants: sucrose (24%) versus distilled water, Outcome 2 PIPP score 30 s post procedure.
PIPP score one minute postprocedure (Outcome 27.3)
One study reported on 105 infants (Pandey 2013). There was no significant difference between groups in the PIPP score one minute postprocedure; MD ‐0.50 (95% CI ‐1.40 to 0.40; Analysis 27.3).
27.3. Analysis.
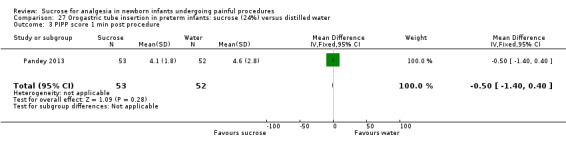
Comparison 27 Orogastric tube insertion in preterm infants: sucrose (24%) versus distilled water, Outcome 3 PIPP score 1 min post procedure.
Data from two studies that assessed the pain associated with nasogastric tube insertion could not be used in RevMan‐analyses (Kristoffersen 2011; McCullough 2008). For details see Table 32.
Kristoffersen 2011 found that a pacifier + 30% sucrose provided the most effective pain reduction (P value < 0.001 versus no treatment). The highest pain score was in sterile water group. McCullough 2008 reported that the sucrose group had a significant lower median NFCS score during NG tube insertion compared with the water group (1 (range 0 to 4) versus 3 (range 0 to 4), median difference 1 (95% CI 0 to 2); P = 0.004).
Effectiveness of sucrose for retinopathy of prematurity (ROP) examination
Sucrose (24%) by syringe + swaddle + pacifier versus water by syringe + swaddle + pacifier (Comparison 28)
Primary outcome
PIPP during the examination (Outcome 28.1)
One study reported on 32 infants (Grabska 2005). There was no significant difference in the PIPP score during the examination between the two groups; MD 0.00 (95% CI ‐2.08 to 2.08; Analysis 28.1).
28.1. Analysis.

Comparison 28 ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier, Outcome 1 PIPP during examination.
Secondary outcomes
Crying time (%) (Outcome 28.2)
One study reported on 32 infants (Grabska 2005). There was no significant reduction in the percentage of crying time (%) between the comparison groups; MD ‐10.00 s (95% CI ‐32.91 to 12.91; Analysis 28.2).
28.2. Analysis.
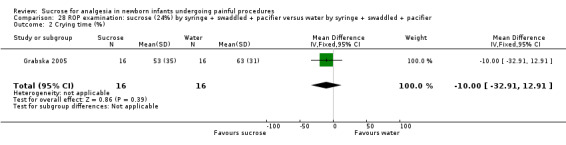
Comparison 28 ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier, Outcome 2 Crying time (%).
Heart rate (beats/minute) (Outcome 28.3)
One study reported on 32 infants (Grabska 2005). There was no significant reduction in the heart rate (beats/minute) between the comparison groups; MD ‐6.00 (95% CI ‐19.33 to 7.33; Analysis 28.3).
28.3. Analysis.
Comparison 28 ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier, Outcome 3 Heart rate (beats/min).
Mean blood pressure (mmHg) (Outcome 28.4)
One study reported on 32 infants. (Grabska 2005) There was no significant difference in the mean blood pressure (mmHg) between the comparison groups; MD ‐7.00 (95% CI ‐18.48 to 4.48; Analysis 28.4).
28.4. Analysis.
Comparison 28 ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier, Outcome 4 Mean blood pressure (mmHg).
Respiratory rate (breaths/minute) (Outcome 28.5)
One study reported on 32 infants (Grabska 2005). There was no significant reduction in the respiratory rate (breaths/minute) between the comparison groups; MD 2.00 (95% CI ‐5.07 to 9.07; Analysis 28.5).
28.5. Analysis.

Comparison 28 ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier, Outcome 5 Respiratory rate (breaths/min).
Oxygen saturation (%) (Outcome 28.6)
One study reported on 32 infants (Grabska 2005). There was a significant difference in the percentage oxygen saturation (%) between the comparison groups with a lower oxygen saturation in the sucrose group; MD ‐3.00 (95% CI ‐5.86 to ‐0.14; Analysis 28.6) favouring the water group.
28.6. Analysis.
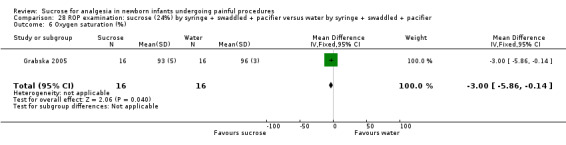
Comparison 28 ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier, Outcome 6 Oxygen saturation (%).
Sucrose (24%) + swaddled + held versus lying in the crib (Comparison 29)
Secondary outcomes
Total crying time (s) (Outcome 29.1)
One study reported on 30 infants (Rush 2005). There was no significant reduction in the total crying time (s) between the two groups; MD ‐33.90 (95% CI ‐76.22 to 8.42; Analysis 29.1).
29.1. Analysis.
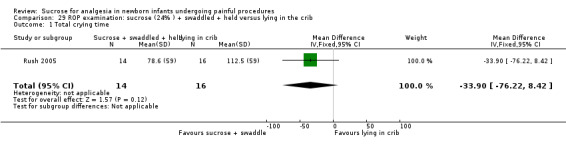
Comparison 29 ROP examination: sucrose (24% ) + swaddled + held versus lying in the crib, Outcome 1 Total crying time.
Oxygen saturation (%) during examination (Outcome 29.2)
One study reported on 30 infants (Rush 2005). There was no significant difference in the oxygen saturation (%) between the two groups; MD ‐1.71% (95% CI ‐5.85 to 2.43; Analysis 29.2).
29.2. Analysis.
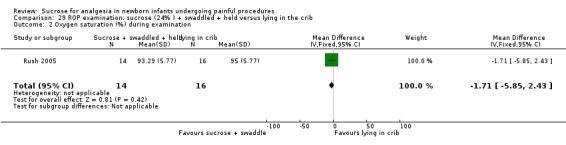
Comparison 29 ROP examination: sucrose (24% ) + swaddled + held versus lying in the crib, Outcome 2 Oxygen saturation (%) during examination.
Sucrose (24% to 33%) (sucrose or sucrose + NNS) versus control (water or water + NNS) (Comparison 30)
Primary outcomes
PIPP score during eye examination (Outcome 30.1)
Three studies reported on 134 infants for this outcome (Boyle 2006; Dilli 2014; Mitchell 2004). The Boyle 2006 study contributed to two analyses for this outcome. In the first analysis (n = 20), which compared sucrose 33% versus sterile water via syringe, there was no significant difference between the groups MD ‐1.00 (95% CI ‐2.54 to 0.54). There was a significant reduction in the PIPP score in the combined group of sucrose with or without NNS; WMD ‐2.15 (95% CI ‐2.86 to ‐1.43; Analysis 30.1; Figure 8). There was low heterogeneity for this analysis (I2 = 46%). Sucrose + pacifier was more effective than sterile water + pacifier; WMD ‐2.47 (95% CI ‐3.27 to ‐1.66; I2 = 29% (low heterogeneity); 3 studies, 114 infants; Analysis 30.1; Figure 8).
30.1. Analysis.
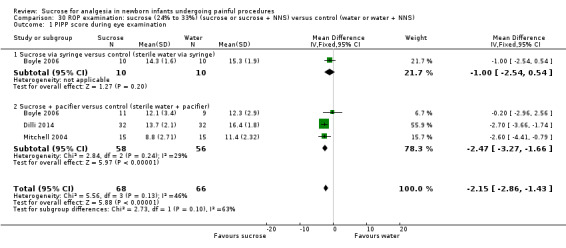
Comparison 30 ROP examination: sucrose (24% to 33%) (sucrose or sucrose + NNS) versus control (water or water + NNS), Outcome 1 PIPP score during eye examination.
8.
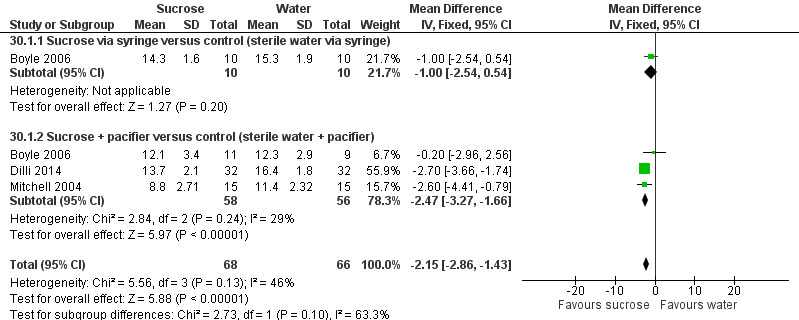
Forest plot of comparison: 30 ROP examination: sucrose (24% to 33%) (sucrose or sucrose + NNS) versus control (water or water + NNS), outcome: 30.1 PIPP score during eye examination.
Secondary outcomes
Crying time (s) during eye examination (Outcome 30.2)
One study reported on 64 infants (Dilli 2014). There was a significant reduction in the crying time (s) in the sucrose group + NNS compared to the NNS group; ‐21.10 (95% CI ‐33.10 to ‐9.10; Analysis 30.2).
30.2. Analysis.

Comparison 30 ROP examination: sucrose (24% to 33%) (sucrose or sucrose + NNS) versus control (water or water + NNS), Outcome 2 Crying time (s) during eye examination.
The results of two studies could not be used in RevMan‐analyses (Gal 2005; O'Sullivan 2010). For details see Table 33. Gal 2005 reported that the PIPP scores at the eye examination were significantly lower in the group given sucrose (mean 8.3, SD 4.5) compared to the placebo group (mean 10.5, SD 4.0), P value 0.01); however, this effect was not sustained at one and five minutes post examination. O'Sullivan 2010 found a significantly lower N‐PASS score in the sucrose group compared to the control group at speculum insertion (6.5 versus. 5.0; P value 0.002) and during the procedure (9.5 versus 7.5; P value 0.003). There were no significant differences between the sucrose group and the water group for episodes of desaturation, bradycardia, or adverse outcomes.
Effectiveness of sucrose for circumcision
Sucrose (50%) solution on a premature nipple, with a 2 x 2 cm sterile gauze pad inside the nipple moistened by the fluid versus no treatment (Comparison 31)
Secondary outcomes
Change from baseline in heart rate (beats/minute) (Outcome 31.1)
One study reported on 56 infants (Herschel 1998). There was no significant difference in the change from baseline in heart rate (beats/minute) between the sucrose and the no treatment groups; MD ‐9.70 (95% CI ‐19.82 to 0.42; Analysis 31.1).
31.1. Analysis.
Comparison 31 Circumcision: sucrose 50% solution on a premature nipple containing a 2 x 2 cm sterile gauze pad moistened by the fluid versus no treatment, Outcome 1 Change from baseline in heart rate (beats/min).
Sucrose (24%) versus eutectic mixture of local anaesthetic (EMLA) (Comparison 32)
Primary outcomes
N‐PASS score during circumcision (Outcome 32.1)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly higher N‐PASS score (indicating more pain) in the sucrose versus the EMLA group; MD 2.40 (95% CI 1.85 to 2.95; Analysis 32.1).
32.1. Analysis.
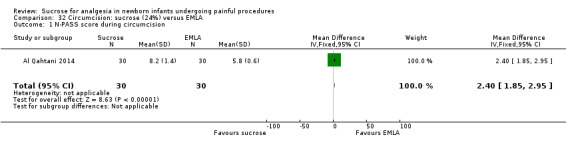
Comparison 32 Circumcision: sucrose (24%) versus EMLA, Outcome 1 N‐PASS score during circumcision.
N‐PASS score after 5 minutes (Outcome 32.2)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly higher N‐PASS score (indicating more pain) after 5 minutes in the sucrose versus the EMLA group; MD 1.40 (95% CI 0.74 to 2.06; Analysis 32.2).
32.2. Analysis.
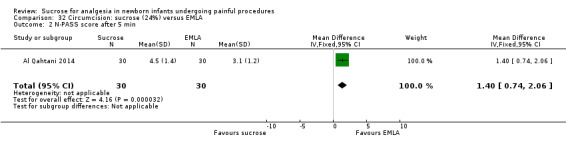
Comparison 32 Circumcision: sucrose (24%) versus EMLA, Outcome 2 N‐PASS score after 5 min.
Secondary outcomes:
Heart rate (beats/minute) during circumcision (Outcome 32.3)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly higher heart rate (beats/minute) in the sucrose group than in the EMLA group; MD 6.00 (95% CI 0.19 to11.81; Analysis 32.3).
32.3. Analysis.
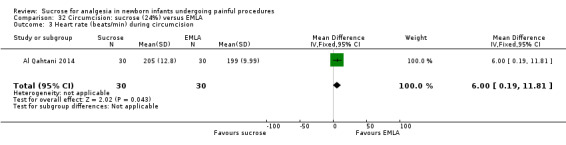
Comparison 32 Circumcision: sucrose (24%) versus EMLA, Outcome 3 Heart rate (beats/min) during circumcision.
Repiratory rate (breaths/minute) during circumcision (Outcome 32.4)
One study reported on 60 infants (Al Qahtani 2014). There was no significant difference in respiratory rate (breaths/minute) between the sucrose group and the EMLA group; MD ‐1.90 (95% CI ‐4.00 to 0.20; Analysis 32.4).
32.4. Analysis.
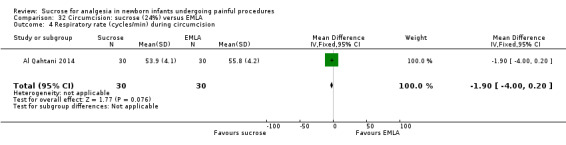
Comparison 32 Circumcision: sucrose (24%) versus EMLA, Outcome 4 Respiratory rate (cycles/min) during circumcision.
Oxygen saturation (%) during circumcision (Outcome 35.5)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly lower oxygen saturation (%) in the sucrose group compared to the EMLA group; MD ‐2.70 (95% CI ‐3.70 to ‐1.70; Analysis 32.5).
32.5. Analysis.
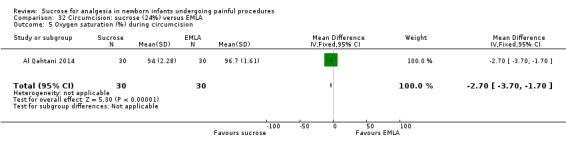
Comparison 32 Circumcision: sucrose (24%) versus EMLA, Outcome 5 Oxygen saturation (%) during circumcision.
Sucrose (24%) versus EMLA + sucrose (24%) (Comparison 33)
Primary outcomes
N‐PASS score during circumcision (outcome 33.1)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly higher N‐PASS score (indicating more pain) in the sucrose vs the EMLA group; MD 3.00 (95% CI 2.42 to 3.58; Analysis 33.1).
33.1. Analysis.
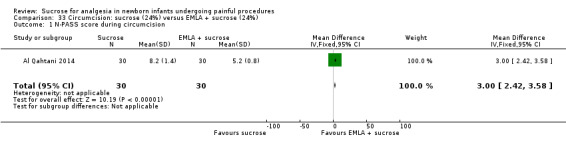
Comparison 33 Circumcision: sucrose (24%) versus EMLA + sucrose (24%), Outcome 1 N‐PASS score during circumcision.
N‐PASS score after five minutes (Outcome 33.2)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly higher N‐PASS score (indicating more pain) after five minutes in the sucrose group compared to the EMLA group; MD 1.20 (95% CI 0.49 to 1.91; Analysis 33.2).
33.2. Analysis.

Comparison 33 Circumcision: sucrose (24%) versus EMLA + sucrose (24%), Outcome 2 N‐PASS score after 5 min.
Secondary outcomes
Heart rate (beats/minute) during circumcision (Outcome 33.3)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly higher heart rate (beats/minute) in the sucrose group compared to the EMLA group; MD 12.00 (95% CI 06.62 to 17.38; Analysis 33.3).
33.3. Analysis.
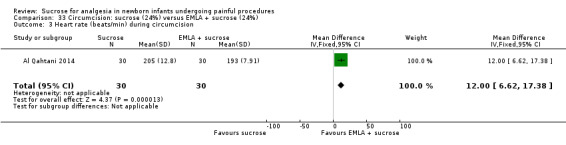
Comparison 33 Circumcision: sucrose (24%) versus EMLA + sucrose (24%), Outcome 3 Heart rate (beats/min) during circumcision.
Repiratory rate (breaths/minute) during circumcision (Outcome 33.4) Analysis 33.4
One study reported on 60 infants (Al Qahtani 2014). There was no significant difference in respiratory rate (cycles/minute) between the sucrose group and the EMLA group; MD 0.60 cycles/minute (95% CI ‐1.77 to 2.97; Analysis 33.4).
33.4. Analysis.
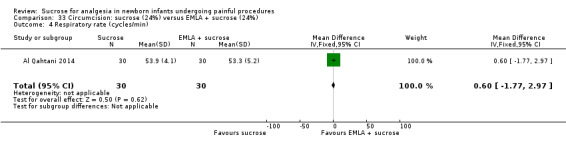
Comparison 33 Circumcision: sucrose (24%) versus EMLA + sucrose (24%), Outcome 4 Respiratory rate (cycles/min).
Oxygen saturation (%) during circumcision (Outcome 33.5)
One study reported on 60 infants (Al Qahtani 2014). There was a significantly lower percentage of oxygen saturation in the sucrose group compared to the EMLA group; MD ‐3.40 (95% CI ‐4.39 to ‐2.41; Analysis 33.5).
33.5. Analysis.
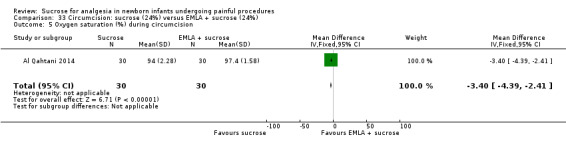
Comparison 33 Circumcision: sucrose (24%) versus EMLA + sucrose (24%), Outcome 5 Oxygen saturation (%) during circumcision.
Sucrose (50%) on a premature nipple, with a 2 x 2 cm sterile gauze pad inside the nipple moistened by the fluid versus dorsal penile nerve block (DPNB) (Comparison 34)
Secondary outcomes
Change in heart rate (beats/minute) from baseline (Outcome 34.1)
One study reported on 79 infants (Herschel 1998). There was a significantly greater change in heart (rate beats/minute) in the sucrose group than in the DPNB group (favours the DPNB group); MD 17.40 (95% CI 11.16 to 23.64; Analysis 34.1).
34.1. Analysis.
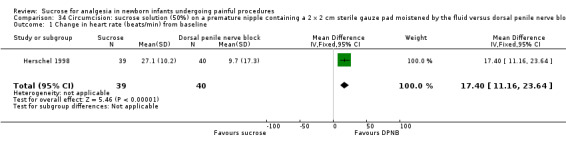
Comparison 34 Circumcision: sucrose solution (50%) on a premature nipple containing a 2 x 2 cm sterile gauze pad moistened by the fluid versus dorsal penile nerve block (DPNB), Outcome 1 Change in heart rate (beats/min) from baseline.
Pacifier dipped in 24% sucrose + DNPB versus pacifier dipped in water + DNPB (Comparison 35)
Primary outcomes
Mean Behavioural Distress Scale scores during circumcision (Outcome 35.1)
One study reported on 40 infants (Stang 1997). There was a significantly lower Behavioural Distress Scale score (indicating less pain) in the sucrose + DPNB group compared with the water + DPNB group; MD ‐0.67 (‐1.08 to ‐0.26; Analysis 35.1).
35.1. Analysis.
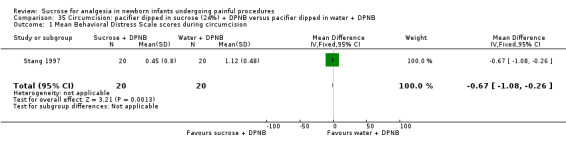
Comparison 35 Circumcision: pacifier dipped in sucrose (24%) + DPNB versus pacifier dipped in water + DPNB, Outcome 1 Mean Behavioral Distress Scale scores during circumcision.
Secondary outcomes
Mean plasma cortisol levels n mol/dL (Outcome 35.2)
One study reported on 40 infants (Stang 1997). There was no significant difference in the mean plasma cortisol levels (n mol/dL) in the sucrose + DPNB group compared with the water + DPNB group; MD 68.90 (‐53.93 to 191.73; Analysis 35.2).
35.2. Analysis.
Comparison 35 Circumcision: pacifier dipped in sucrose (24%) + DPNB versus pacifier dipped in water + DPNB, Outcome 2 Mean plasma cortisol levels n mol/dL.
Data from one study could not be used in RevMan‐analyses (Kaufman 2002); for details see Table 34. In this study the overall, mean crying time was significantly decreased in infants treated with sucrose compared to infants treated with water (P value 0.0001). Infants in the Gomco clamp + sucrose group spent significantly less time grimacing (P value 0.0001) compared to the Gomco clamp +water group.
Effectiveness of sucrose for stress during echocardiography
Sucrose (24%) versus no medication/placebo (Comparison 36)
Primary outcomes
PIPP score (Outcome 36.1)Analysis 36.1
36.1. Analysis.

Comparison 36 Echocardiography (term and preterm infants): sucrose (24%) versus no medication/placebo, Outcome 1 PIPP.
One study reported on 104 infants (Potana 2015). The PIPP score in the sucrose group was significantly lower compared with the group that got no intervention or placebo; MD ‐2.15 (95% CI ‐3.30 to ‐1.00; Analysis 36.1).
This was the only study we identified that used sucrose during echocardiography.
Effectiveness of sucrose for potentially painful procedures for seven days after enrolment
Sucrose (24%) versus water (Comparison 37)
Primary outcomes
Motor development and vigor (MDV) domain of the NAPI tool at 40 weeks PMA (Outcome 37.1)
One study reported on 93 infants who had been given sucrose or water for every potentially painful procedure for seven days after enrolment in the study (Banga 2015). There was no significant difference in the MDV domain of the NAPI tool between the sucrose and the water groups; MD ‐1.83 (95% CI ‐8.59 to 4.93; Analysis 37.1).
37.1. Analysis.
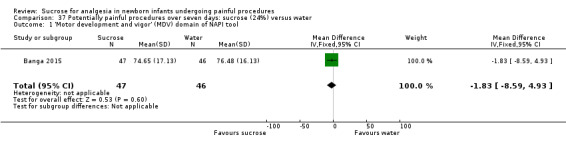
Comparison 37 Potentially painful procedures over seven days: sucrose (24%) versus water, Outcome 1 'Motor development and vigor' (MDV) domain of NAPI tool.
Alertness and orientation (AO) domain of the NAPI tool at 40 weeks PMA (Outcome 37.2)
One study reported on 93 infants who had been given sucrose or water for every potentially painful procedure for seven days after enrolment in the study (Banga 2015). There was no significant difference in the AO domain of the NAPI tool between the sucrose and the water groups; MD 3.09 (95% CI ‐6.49 to 12.67; Analysis 37.2).
37.2. Analysis.
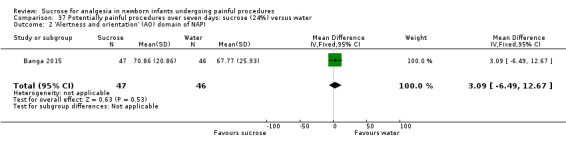
Comparison 37 Potentially painful procedures over seven days: sucrose (24%) versus water, Outcome 2 'Alertness and orientation' (AO) domain of NAPI.
The results from the Taddio 2008 study are included under the heel lance and intramuscular injection sections.
The results of the Gaspardo 2008 and Johnston 2002 studies could not be included in RevMan‐analyses. See Table 35 for details. In the Gaspardo 2008 study the neonates were evaluated during blood collection each morning. The assessment was divided into five phases: baseline, antisepsis, puncture, dressing, and recovery. The neonates’ facial activity (NFCS), behavioural state, and heart rate were evaluated. The data analysis used cut‐off scores for painful and distressed responses. Significantly fewer neonates in the sucrose group than the control group exhibited facial actions that signalled pain during the puncture phase and the antisepsis phase. Significantly fewer neonates in the sucrose group cried during the antisepsis phase, the puncture phase, and the dressing phase. There was no statistical difference between groups for physiological response. The efficacy of sucrose was maintained for pain relief in preterm neonates with no side effects.
Johnston 2002 reported that on the basis of analysis of covariance with PMA at birth and the number of invasive procedures as covariates, there were no group differences (between sucrose and water) for any of the secondary outcomes of Neuro‐Biological Risk Scores (NBRS) at two weeks; postnatal age (P = 0.426) or at discharge (P = 0.965). In the sucrose group only, higher number of doses of sucrose predicted lower scores on motor development and vigor, and alertness and orientation at 36 weeks’, lower motor development and vigor at 40 weeks’, and higher NBRS at 2 weeks’ postnatal age. Higher number of invasive procedures was predictive of higher NBRS both times in the water group.
No significant differences found between the sucrose and water groups for Neurobehavioral Assessment of the Preterm Infant (NAPI).
Effects of sucrose on long‐term neurodevelopmental outcomes
We identified no studies that reported on long‐term neurodevelopmental outcomes (assessed by a standardized and validated assessment tool, a child developmental specialist, or both) at 18 to 24 months or at any later age in childhood.
Adverse effects
In the previous update (Stevens 2013), 16 studies evaluated adverse effects of sucrose compared to placebo (Ramenghi 1996a; Carbajal 1999; Stevens 1999; Guala 2001; Gibbins 2002; Acharya 2004; Gal 2005; Grabska 2005; Stevens 2005a; Rogers 2006; Codipietro 2008; McCullough 2008; Taddio 2008; O'Sullivan 2010; Biran 2011; Taddio 2011). Six of these studies observed minor side effects in infants (Gibbins 2002; Grabska 2005; Stevens 2005a; McCullough 2008; Taddio 2008; O'Sullivan 2010). Gibbins 2002 described minor adverse effects in six infants, none of which occurred in the sucrose + pacifier group. One neonate who received water with pacifier choked when the water was administered but stabilized within 10 s. Three infants randomised to the sucrose group and two infants randomised to the water + pacifier groups experienced oxygen desaturation when the study intervention (sucrose or water) was administered. Each neonate recovered spontaneously with no medical intervention required. Grabska 2005 confirmed choking and oxygen desaturation as possible adverse effects of administering sucrose for pain. McCullough 2008 reported that there was no significant difference between the sucrose and control groups with regard to adverse effects; the investigators observed brief apnoea or self‐limiting bradycardia in some infants, but none required clinical intervention. Stevens 2005a reported that the adverse events related to repeated use of sucrose over the first 28 days of life were 'low' and all immediate adverse events resolved spontaneously. Taddio 2008 reported no significant differences between groups in blood glucose levels monitored during the study, as well as the incidence of spitting up the sucrose solution. Lastly, O'Sullivan 2010 reported that four neonates in the water group and one in the sucrose group experienced oxygen desaturation or bradycardia.
In this current 2016 update we included 20 newly identified studies and evaluated adverse effects in 13 of them. Dilli 2014 did not notice any choking episode or vomiting in any of the study infants. Gray 2012 observed no adverse events or side effects from the brief exposure to a heat source or change in the infants’ ambient temperature, and did not mention any adverse effects related to sucrose. The Tutag Lehr 2015 study assessed a number of adverse events including gagging, choking, and vomiting and noted that only one infant in the sucrose group experienced a mild episode of 'spitting up'. Thakkar 2016 reported on a total of five episodes of adverse events in all four groups. One neonate each in the sucrose group, the NNS group and the sucrose + NNS group desaturated, while two neonates desaturated in the no intervention group. Leng 2015; Marin Gabriel 2013, Milazzo 2011, Pandey 2013, and Simonse 2012 noted no adverse events in any infant. Potana 2015 reported no episode of hyperglycaemia, necrotizing enterocolitis, or feed intolerance after sucrose administration. Abbasoglu 2015 stated that no child developed any clinically visible changes on the skin, and that no side effects were observed in their comparison of laser acupuncture with oral sucrose. Banga 2015 reported that there was no significant difference in the frequency of adverse effects (fall in heart rate or oxygen saturation) across the two groups (sucrose and water).
Seven newly included studies did not report on adverse effects (Al Qahtani 2014; Asmerom 2013; Cignacco 2012; Leng 2013; Liaw 2013; Suhrabi 2014; Gray 2015). In the current review, it would appear that researchers are being much more vigilant in observing and reporting adverse events. The proportion of minor adverse events remains very low with no major adverse events reported.
Discussion
Summary of main results
Below we report for each painful procedure the results from the 'Summary of findings' tables. In those tables we included only results from the most validated pain assessment scales (DAN, NFCS, NIPS, N‐PASS, domains of NAPI, PIPP, PIPP‐R and Total Bernese Pain Scale for Neonates) and provide GRADE assessments for the quality of the evidence. In brief paragraphs we summarize the results from outcomes that could be included in analyses in RevMan and provide short comments on the results from studies that could not be included in the RevMan‐analyses.
As shown in Figure 1, results from only 39 of the 74 included studies could be included in RevMan‐analyses, and the remaining studies are included in narrative qualitative syntheses.
The largest study included in any analysis was Leng 2015, which reported on 342 infants, and the two meta analyses with the largest numbers of participants included 278 infants (3 studies) (Figure 4) and 232 infants (2 groups of infants from one study) (Figure 7).
Heel lance
Heel lance was the most common painful procedure and was included in 38 studies. There was moderate quality evidence that sucrose (20% to 30%) compared with water significantly reduced NIPS scores during heel lance (indicating less pain with sucrose). No significant differences were noted in PIPP scores at 30 s or 60 s after heel lance, or during heel lance. There was no difference in DAN scores 30 s after heel lance (Table 1).
Sucrose (24%) was a more effective analgesic than breastfeeding (low quality evidence) (Table 2).
For sucrose (24%) + NNS compared with water + NNS, or pacifier dipped in sucrose compared with pacifier dipped in water there was high quality evidence that PIPP scores at 30 s (Figure 4) and 60 s (Figure 5) were significantly reduced (indicating less pain) and that the NFCS score was non‐significantly reduced (Table 3).
No significant difference was found between sucrose (24%) + NNS + NIDCAP compared with breast milk (by breastfeeding or by syringe) (low quality evidence) (Table 4; Table 5).
There was low quality evidence that sucrose was more effective than laser acupuncture in reducing heel lance‐associated pain (Table 6).
There was moderate quality evidence that sucrose (24%) + NNS or sucrose (24%) + swaddling or sucrose (24%) + NNS + swaddling were more effective than sucrose alone to reduce pain associated with heel lance (Table 7; Table 8; Table 9).
Many studies that could not be included in RevMan‐analyses, or reported on outcomes other than validated pain assessment scores supported these findings (see summary in the results section).
Sucrose in concentrations of 20% to 30% reduces composite and multidimensional behavioural pain scores, as well as individual behavioural and physiological pain indicators associated with heel lance. Other pain reducing interventions such as NNS and swaddling in association with sucrose provides further pain relief.
Venipuncture
Venipuncture was the painful intervention in nine studies. In addition one study assessed both heel lance and venipuncture (Ogawa 2005), but results were reported separately for the two interventions and we present the results under the two different headings (heel lance and venipuncture).
There was moderate quality evidence that sucrose (12% to 12.5%) versus water had no significant effect on the NIPS score (Table 12).
There was high quality evidence that the PIPP score was significantly lower in the sucrose group (24% to 30%) than the sterile water group during venipuncture (Table 13).
Comparison of sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/liposomal lidocaine cream on the skin showed that the DAN scores during venipuncture and the recovery period were significantly higher (indicating more pain) in the sucrose group (moderate quality evidence). There were no significant differences in the PIPP scores at the same time points (Table 14).
The studies that could not be included in RevMan‐analyses or reported outcomes using other non‐validated pain assessment scores supported these findings (see summary in the results section) including lower DAN scores, NFCS scores and shorter durations of crying.
Sucrose (24% to 30%) reduces composite and multidimensional behavioural pain scores and cry variables associated with venipuncture.
Arterial puncture
Arterial puncture was the painful intervention in one trial that reported on 47 infants (Milazzo 2011). There was no significant difference between the groups in the physiological outcomes measured; heart rate (beats/minute) or oxygen saturation after needle insertion, or one minute after the procedure. This one small study might have lacked power to ascertain a true difference.
Currently there is lack of evidence for or against the use of sucrose for arterial puncture.
Effectiveness of sucrose for pain during subcutaneous injections
Subcutaneous injection was studied in two trials (Allen 1996; Mucignat 2004). The results could not be included in RevMan‐analyses. Allen 1996 found that sucrose decreased the percentage of time that two‐week‐old infants spent crying. Mucignat 2004 found that crying time was significantly lower in the sucrose + EMLA + pacifier group. There was a significant reduction in DAN and NFCS scores in the EMLA + NNS, sucrose + NNS, and sucrose + EMLA + pacifier groups compared to NNS alone.
There is not sufficient evidence to judge whether sucrose is beneficial or not for reducing pain associated with subcutaneous injections.
Effectiveness of sucrose for pain associated with intramuscular injection (immunization against hepatitis B or injection of vitamin K)
Intramuscular injection was studied in four trials (Gray 2012; Gray 2015; Liaw 2011; Suhrabi 2014).
There was low quality evidence that sucrose (20% to 25%) versus water or no intervention significantly lowers NIPS scores during the one to two minutes after immunization (Table 15).
There was high quality evidence that PIPP scores during intramuscular injections were significantly lower (indicating less pain) in the sucrose group than the water group for infants born to both non‐diabetic and diabetic mothers (Table 15).
For sucrose (25%) versus glucose (25%) there was no significant difference in the NIPS score one to two minutes after immunization (low quality evidence) (Table 16).
Data from one trial could not be entered in RevMan‐analyses. Gray 2012 showed that providing warmth during immunization for hepatitis B decreased crying and grimacing as much as sucrose or a pacifier did, and in a similar study, Gray 2015, showed that infants exposed to sucrose + warmth cried significantly less and grimaced less compared to the infants who received sucrose only.
There is some high quality evidence that sucrose reduces pain associated with intramuscular injections. Adding a body warmer to administration of sucrose may provide further pain relief. Further research is recommended for that co‐intervention.
Bladder catheterization
The painful intervention of bladder catheterization was reported in one study. There was a significantly smaller change in the DAN score in the sucrose group than the water group, and the number of infants crying at maximal catheter insertion was significantly lower in the sucrose group (moderate quality evidence) (Table 17).
Further research is required to assess the effectiveness of sucrose to reduce pain during bladder catheterization.
Orogastric or nasogastric tube insertion
Orogastric or nasogastric tube insertion was studied in three trials.
Although the sample size was small, there was high quality evidence from one trial of a significantly lower PIPP score 30 s after the procedure in the sucrose (24%) group than in the water group, but no difference in PIPP score during the procedure or one minute after the procedure (Table 18).
Two studies of nasogastric tube insertion could not be included in RevMan‐analyses (Kristoffersen 2011; McCullough 2008). Kristoffersen 2011 found that pacifier + 30% sucrose provided the most effective pain reduction (P value < 0.001 versus no treatment). The highest pain score was for infants in the sterile water group. McCullough 2008 reported that the sucrose group had a significant lower median NFCS score during NG tube insertion compared with the water group.
Further research is required to assess the effectiveness of sucrose to reduce pain during orogastric or nasogastric tube insertion.
ROP examination
ROP examinations were studied in seven trials.
There was no significant difference in the PIPP score between groups for sucrose (24%) by syringe + swaddled + pacifier compared with water by syringe + swaddled + pacifier (low quality evidence) (Table 19).
The pooling of three studies revealed a significant reduction in the PIPP score for the sucrose group when sucrose (24% to 33%; sucrose ± NNS) was compared with water (water ± NNS). There was a significant reduction in the crying time in the sucrose + NNS group (moderate quality evidence) (Table 20) (Figure 8).
There is limited evidence that sucrose may confer some pain relief when combined with other pain reducing interventions. Further research on sucrose in combination with other pain reducing interventions is required.
Circumcision
Circumcision was studied in four trials.
For sucrose (24%) versus EMLA there was a significantly higher N‐PASS score (indicating more pain for sucrose) during circumcision and 5 minutes after circumcision. The heart rate (beats/minute) was significantly higher and the oxygen saturation was significantly lower in the sucrose group during circumcision (low quality evidence) (Table 21).
For sucrose (24%) versus EMLA + sucrose (24%) there were significantly higher N‐PASS scores in the sucrose only group during circumcision and five minutes after circumcision (low quality evidence) (Table 22). During circumcision the heart rate (beats/minute) was significantly higher and the oxygen saturation significantly lower in the sucrose only group.
Secondary outcomes
When a pacifier dipped in 24% sucrose + DPNB was compared with a pacifier dipped in water + DNPB there was a significantly lower behavioural score in the group that received sucrose. There was no significant difference in the mean plasma cortisol levels between the groups.
When sucrose (50%) solution on a premature nipple, with a 2 x 2 cm gauze pad inside the nipple moistened by fluid was compared with DPNB there was an increase (indicating more pain) in heart rate (beats/minute) in the sucrose group.
Based on low quality of evidence the use of sucrose alone is insufficient for pain relief from circumcision.
Echocardiography
The stress/pain associated with echocardiography examination was reported in one low‐quality evidence study. The mean PIPP score was significantly lower in the sucrose group compared with the no intervention group (Table 23).
The use of sucrose during echocardiography deserves further study.
Potentially painful procedures for seven days after study entry
One high‐quality evidence study reported on two domains of the NAPI score; motor development and vigor (MDV) and alertness and orientation (AO). The potentially painful procedures included; venipuncture, heel lance, peripheral venous catheterization, orogastric or nasogastric tube insertion, intramuscular injection, suprapubic bladder tap, ROP examination and removal of adhesive tapes.
There were no significant differences in these two domains between the sucrose (24%) and water groups at 40 weeks PMA.
Long‐term neurodevelopmental outcomes
Long‐term neurodevelopmental outcomes were not assessed in any of the included studies.
Adverse effects
Adverse effects were evaluated in 29 studies and in most no side effects were reported. Minor, rare and untoward events included 'spitting up', oxygen desaturation, bradycardia, choking, and brief apnoea. Most of these occurred in both the sucrose and the control groups and were self‐resolved. One study that measured blood glucose levels did not find any significant difference between the sucrose and the water group (Taddio 2008).
Adverse effects following the short term use of sucrose are currently not a concern.
Overall completeness and applicability of evidence
Although sucrose has been studied in 74 trials that included 7049 infants, the large variation in painful procedures, dose of sucrose (concentration and volume), co‐interventions in the comparison groups, assessment tools used and differences in the population (term or preterm infants) resulted in a large number of RevMan‐analyses that include one or few trials. There were few infants included for each comparison and each outcome, which weakens our ability to draw firm conclusions. However, if the effectiveness of sucrose for a specific intervention were measured by a variety of instruments, and the results showed a similar reduction, this would strengthen the results and the conclusions.
To date, the best studied use of sucrose is for heel lance, venipuncture and intramuscular injections and for these interventions sucrose appears to offer pain relief.
Sucrose does not seem to relieve the pain associated circumcision adequately and there is no strong indication that further studies are indicated. For pain/stress associate with arterial puncture, subcutaneous injection, bladder catheterization, orogastric or nasogastric tube insertion, ROP examination and echocardiography examination further research is warranted. For these procedures, we would recommend that if trials are done, a rescue dose should be available for infants in obvious distress, where the sucrose alone does not seem to be effective in preventing moderate to severe pain.
Quality of the evidence
The quality of the evidence varied from low to high and for each of the painful interventions and comparisons the quality of the evidence is reported in separate 'Summary of findings' tables (Table 1 to Table 24). We excluded quasi‐randomised trials and trials reported in abstract form only. We could include only a few studies for most comparisons and outcomes. For most outcomes we included the results of a single study only, and so tests for heterogeneity were not applicable. The main reasons for our inability to include study results in RevMan‐analyses were that the results were not presented as means and SDs or were presented in graph form only.
By early February 2016, we had identified 74 RCTs that tested the effectiveness of sucrose in reducing stress or pain associated with common, potentially painful procedures in neonates. These studies reported on 7049 neonates. Most studies had a small sample size: the sample sizes ranged from 15 to 671 infants, and 48 studies reported on fewer than 100 infants.
Potential biases in the review process
We are not aware of any biases in the review process. Two authors (AO, JY) selected the trials from the literature searches and there was complete agreement. One author (AO) abstracted data and filled in pre designed forms for 'Risk of bias' assessments and data abstraction, transformed 95% CIs to SDs and two authors (SH, AS) checked the data abstraction and transformation of data. Any discrepancies were discussed and resolved by consensus. Three authors (BS, JY, AO) are coauthors of some included trials. For these trials two authors (SH, AS) did the data abstraction and RoB assessments. One author (BS) is the developer of the PIPP and PIPP‐R measure.
Agreements and disagreements with other studies or reviews
In our previous versions of this review (Stevens 2001; Stevens 2004; Stevens 2010; Stevens 2013), we reported inconsistency in effective sucrose dosage, although we identified studies in which the dose ranged from 0.012 g to 0.12 g. Johnston 1997 and Stevens 1999 identified that very small volumes of 24% sucrose (estimated at 0.01 g to 0.02 g) significantly reduced pain. However, the meta‐analyses in Stevens 1997a showed 0.18 g sucrose was ineffective in reducing crying and did not differ from the control solution (water). Doses of 0.24 g or more were more effective; there was some additional benefit of administering 0.48 g to 0.50 g sucrose, but effectiveness did not increase when sucrose doses greater than 0.50 g were administered. In this updated review, a significant reduction in PIPP scores was demonstrated with sucrose doses between 0.012 g to 0.12 g (0.05 mL to 0.5 mL of 24% sucrose solution) at 30 and 60 seconds after heel lance and 0.12 g (0.5 mL) prior to ROP examinations. In these studies, there was a one‐ to two‐point reduction in the PIPP score. Shah 2004 reported that clinicians and researchers consider a 20% reduction in pain to be the minimal clinically important difference, although other researchers suggest a 10% reduction may suffice (Powell 2001). Lemyre 2006 used a three‐point reduction on the PIPP scale as being clinically meaningful; however, a rationale for this decision was not reported. Determining the level of clinical improvement is challenging in infants, given their inability to self‐report their pain and the level of improvement that could make significant differences either in treatment strategy or the affective component of pain (i.e. how bad pain makes you feel).
The greatest analgesic effect occurs when sucrose is administered approximately two minutes before the painful stimulus. This interval is thought to coincide with the release of endogenous opioids (Blass 1994). Johnston 1999 reported increased analgesia when sucrose solution was repeatedly administered in small aliquots (i.e. 0.05 mL of 24% sucrose) at two‐minute intervals throughout the painful procedure. The peak effect appears to last about four minutes; therefore, the analgesic effect may wear off if procedures are prolonged. The infant's postnatal age may influence the effectiveness of sucrose (Taddio 2008).
Adverse effects following the short‐term use of sucrose are currently not a concern. We reported the possible adverse effects in the Effects of interventions. Adverse effects would be more likely to occur when multiple doses of are used. Stevens 2005a found no significant differences in incidence rates for necrotizing enterocolitis between infants who received repeated doses of sucrose over 28 days of life compared to control groups. Johnston 2002 studied 107 preterm infants of less than 31 weeks' postmenstrual age where 1 mL of 24% sucrose or sterile water was administered up to three times, two minutes apart, for all painful procedures over a seven‐day period. Johnston indicated that higher frequency of sucrose doses was predictive of lower awareness, orientation (AO), motor development and vigour (MDV) on the NAPI scale at 36 weeks, and lower (MDV) at 40 weeks. At two weeks' postnatal age, a higher number of doses of sucrose were predictive of higher Neuro‐Biological Risk Score (NBRS) scores. Proposed explanations were that: (a) low neurodevelopmental scores could be related to infants receiving sucrose during the one‐week study period only, and ongoing exposure to painful procedures might have resulted in heightened sensitivity to pain; or (b) the sample size was inadequate to identify other explanatory variables. Further analysis revealed that 10 or fewer doses of sucrose over a 24‐hour period were unlikely to be related to poorer neurodevelopmental scores (Johnston 2007). Banga 2015 reported that 93 infants randomised to either repeated doses of sucrose or water for painful procedures for seven consecutive days showed no significant differences in MDV or AO scales of the NAPI scale or adverse events. Stevens 2005a reported no statistically significant differences between sucrose + pacifier, water + pacifier, or the standard care group on neurobiological risk status outcomes
Generally, infants in this review were healthy term and preterm neonates and very few were under 27 weeks' PMA at birth. Although the preterm infant's pain response is generally consistent with that of the term infant, it is often more subtle, less sustained and affected by the infant's behavioural state and severity of illness (Gibbins 2008). There was no significant difference in this review between crying in term and preterm infants; however, the incidence of crying following painful stimuli is reported to be 50% less in preterm infants compared to term infants (Stevens 1994); therefore, crying alone may not be a reliable indicator of pain in the preterm infant population and is precluded as an indicator in many validated infant pain measures.
Few researchers provided a definition or conceptualisation of pain as an outcome. If the reported outcomes reflect the investigators' concept of pain, then we can assume that most investigators considered proportion, percentage or duration of time crying to be the most valid indicator of pain in neonates. Although research on infant crying has delineated certain crying characteristics, such as pitch, intensity, melody and harmonics, as being good indicators of pain, these were not assessed in the sucrose studies reviewed. Cry duration may give some indication of distress. However, cry duration does not necessarily confirm or deny that the infant is in pain. For unstable ventilated infants who often do not cry following painful procedures, any cry characteristic would be an inappropriate outcome. Attempts at cry or a silent cry in ventilated infants may be reasonable to consider. A multivariate approach looking at multiple indicators or a composite pain score may be a more comprehensive approach.
The majority of researchers studied heel lance as the painful procedure. However, they provided little detail about this procedure (e.g. type of lancet used, number of attempts, number of squeezes, duration of the procedure). Therefore, it was impossible to determine if the painful stimuli (or painful procedures) were comparable in intensity, duration or frequency between studies. Similarly, details about other procedures (e.g. subcutaneous injection, ROP examination, bladder catheterization and circumcision) and co‐interventions such as skin‐to‐skin (kangaroo care), breastfeeding or comforting strategies (e.g. containment, bundling, tucking or positioning) that may enhance the effect of sucrose would be desirable. The length of observation and return to baseline parameters (e.g. heart rate) of infants following procedures was not reported frequently.
The delivery of sucrose (by syringe, dropper or dipping pacifier) varied among studies. The pacifier promotes NNS and calming that may contribute to reducing pain‐elicited distress (Campos 1994). Blass 1994 suggests that sucking exerts a profound behavioural effect and induces feelings of calm. Other researchers have found that NNS reduces heart rate and metabolic rate, causes infants to self‐soothe and elevates the pain threshold. NNS has not been shown to affect cortisol response, vagal tone or oxygen saturation of blood (DiPietro 1994; Gunnar 1992). The calming effects are not sustained following cessation of the NNS alone. This is in contrast to NNS + sucrose administration, where the effects persist for several minutes beyond the cessation of contact. Results from this 2016 update indicate that the use of sucrose with NNS appears to have synergistic effect with both single and repeated doses of sucrose.
Codipietro 2008 concluded that breastfeeding was more effective than sucrose for reducing pain from heel lance in term neonates. Shah 2006 recommended that breastfeeding, when available, should be used to reduce procedural pain in neonates who are exposed to single painful procedures; breast milk alone in small volumes is shown to be as effective as water for the relief of procedural pain (much less so than sucrose), and its effectiveness for repeated painful procedures has not yet been established.
Harrison 2010 conducted a systematic review of 14 RCTs with 1674 injections to compare the efficacy of oral sweet solutions to water or no treatment in infants aged one to 12 months. Infants who received sucrose or glucose before immunisation had moderately reduced incidence and duration of crying. Pillai Riddell 2015 in a systematic review of non‐pharmacological interventions found that the most established evidence for managing procedural pain in preterm and term neonates and older infants was for NNS, swaddling, facilitated tucking, and rocking/holding. Bueno 2013 in a systematic review demonstrate that 20% to 30% glucose reduces pain scores and crying during single heel lances and venipunctures and can be recommended as an alternative to sucrose for procedural pain reduction in healthy term and preterm neonates.
Slater 2010 demonstrated no significant differences in the nociceptive brain activity measured with neonatal EEG or magnitude or latency of the spinal nociceptive reflex withdrawal measured with EMG between neonates given sucrose and those given sterile water; however, the PIPP scores were significantly lower in the infants given sucrose. The authors suggested that oral sucrose does not affect activity in the neonatal brain or spinal cord and many not be an effective analgesic. The small sample size, moderate attrition rates and methods used to measure and analyse EEG and EMG recordings limit the generalization of these findings.
This evidence has been integrated into evidence‐based sucrose consensus protocols and guidelines (Dunbar 2006; Lefrak 2006; Sharek 2006). However, in an evaluation of infant pain guidelines (Lee 2014), recommendations were not sufficient, clear or consistent to guide clinical practice. Furthermore, in a cross‐sectional survey of painful procedures in NICUs, procedural pain was managed using sweet solutions only 3.5% of the time (Carbajal 2008).
We still have significant gaps in our understanding of how pain is processed in the developing brain and optimal assessment and treatment approaches (Fitzgerald 2015). Methodological and pain treatment issues need to be addressed, and new evidence generated in light of the existing evidence. A comprehensive approach to validating various measures to evaluate pain in neonates and a standardized approach to measuring outcomes is critical, especially when evaluating pain‐relieving interventions that form the basis for clinical decision making.
There is a need to assess the long‐term effects of sucrose administration to neonates. Long‐term neurodevelopmental outcomes should be assessed by a standardized and validated assessment tool, a child developmental specialist, or both at 18 to 24 months or at any later age in childhood.
Authors' conclusions
Implications for practice.
We included 74 studies in this review, 38 of which examined pain associated with heel lancing. The results from these studies provide further evidence to support the efficacy of sucrose for reducing pain from single and, to a lesser extent, repeated heel lances. The included studies reported on the use of sucrose for venipuncture, intramuscular injection, arterial puncture, subcutaneous injections, nasogastric or orogastric tube insertion, bladder catheterization, retinopathy of prematurity (ROP) examinations, circumcision, and echocardiography exam in hospitalized neonates. However, except for venipuncture and intramuscular injection for which there was high‐quality evidence for the use of sucrose, further studies of these other painful procedures are required due to conflicting evidence on the effect of sucrose in reducing pain. Sucrose reduces procedural pain with minimal to no reported adverse effects. Small doses of 24% sucrose (0.01 g to 0.02 g) are efficacious in preterm infants, while larger doses (0.24 g to 0.50 g) reduce the proportion of time term infants spend crying.There is some moderate‐quality evidence that sucrose in combination with other non‐pharmacological interventions such as non‐nutritive sucking is more effective than sucrose alone.
Implications for research.
The optimal dose of sucrose for pain relief in term and preterm infants has not yet been established. Researchers should consider establishing more precise and tailored doses based on the infant (e.g. postmenstrual age, severity of illness) and context in which it is to be used. Optimal sucrose doses could be assessed further using sensitivity/meta‐regression techniques. More research is needed to address the analgesic and calming effects of sucrose and its interaction with other behavioural (e.g. facilitated tucking, kangaroo care), and pharmacological (e.g. morphine, fentanyl), interventions for more invasive procedures such as ROP exam and circumcision. Strategies need to be initiated to increase understanding of the underlying mechanisms of pain and sucrose for pain relief in infants. The use of repeated administrations of sucrose in neonates needs to be investigated further in terms of clinical, developmental and economic outcomes.
Investigators should be cautious when utilising existing evidence to answer questions on efficacy in other painful procedures that have been minimally addressed to date (e.g. lumbar punctures, peripherally inserted central catheter insertions, endotracheal intubation, chest tube insertions). Strengthened study design and methods with particular attention to the adequacy of sample size, acknowledgment of conceptualisation of pain, use of validated pain measures to determine outcomes, and context are required. Use of sucrose in neonates that are of extremely low birthweight, unstable, ventilated, or a combination of these factors needs to be addressed.
Replication of existing studies of high methodological quality, using a valid pain assessment measure and standard set of validated outcomes would allow for further combination of results in meta‐analyses. Researchers should report on means and standard deviations (SD) in addition to medians and ranges, if the data are not normally distributed, to allow for the use of meta‐analytic techniques. If researchers choose to present data in graph form, they should include the means and SDs in the text (or additional appendices). Preferably the amount of sucrose administered should be reported in g/kg body weight. Future research should focus on long‐term effects of repeated sucrose administration with other behavioural and pharmacological interventions in sick and very‐preterm neonates. Results should be presented for individual indicators and any composite pain scores constructed from these indicators. The relationship between pain measures and individual physiological and cognitive indicators of nociceptive brain activity should be explored further.
Additional research is urgently needed regarding the understanding of the neurophysiological basis of pain, as well as addressing the pressing clinical need by determining the minimally effective dose of sucrose during a single painful procedure, and the effect of repeated sucrose administration on immediate (pain intensity) and long‐term (neurodevelopmental) outcomes.
Effective knowledge translation strategies are required to translate research evidence on sucrose into practice effectively (Stevens 2014b). For healthcare providers these strategies could include the use of reminders, interactive education and educational outreach and regular audit and feedback sessions.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Added external source of support |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 31 March 2016 | New citation required but conclusions have not changed | Authors' conclusions are not changed with the inclusion of additional studies. |
| 15 March 2016 | New search has been performed | This review was updated in 2016. An additional 20 studies were accepted for inclusion for a total of 74 studies. The total number of infants included in the review is now 7049. We converted 95% confidence intervals to standard deviations. We performed the comparisons based on the different concentrations of sucrose used and on the different control interventions. This resulted in a large number of comparisons and RevMan‐analyses. Most comparisons and outcomes included a limited number of studies and infants. Although we included a total of 37 comparisons, we include 'Summary of findings' tables for primary outcomes (validated pain scales) and GRADE assessments based on the GRADE assessment tables that are available in RevMan. |
| 17 February 2012 | New citation required but conclusions have not changed | For the purpose of the current updated review, the inclusion criteria were expanded to include all minor painful procedures (rather than heel lance and venipuncture only). The updated review criteria included studies that assessed the efficacy of repeated doses of sucrose. |
| 17 February 2012 | New search has been performed | This updates the review "Sucrose for analgesia in newborn infants undergoing painful procedures" published in The Cochrane Library, Issue 3, 2010 (Stevens 2010). Thirteen new studies were added in the current update. |
| 3 February 2008 | Amended | Converted to new review format. |
| 20 April 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to acknowledge the assistance of:
Ms Moira Lynch for conducting an extensive updated search of MEDLINE, EMBASE and CENTRAL in April 2001 and Ms Tamsin Adams‐Webber for her assistance with our 2004 and 2009 updates;
Ms Yolanda Brosseau, who conducted the searches in February 2016;
Dr Celeste Johnston, Dr Aage Knudsen, Dr Sharyn Gibbins and Dr Denise Harrison for providing unpublished data;
Dr Denise Harrison, Jasmine Lamba, Alison Dickson and Sobia Khan for their assistance with quality assessment and data extraction of the studies for the 2013 review update; Ms. Grace Y Lee, who made major contributions to the 2013 update of the review;
Mr David Corpman for translating the paper by Leng 2013 from Chinese to English;
Dr Reza Assadi, Mashhad University of Medical Sciences, Mashhad, Iran, for translating one article from Persian to English (Aziznejad 2013);
Dr Danilyn Angeles for providing additional analyses for the Asmerom 2013 study;
Dr Shreshtha Banga provided us with additional information regarding the study Banga 2015.
Appendices
Appendix 1. Search terms used for searches of PubMed
Sucrose AND pain AND ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomised controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomised [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh])).
Data and analyses
Comparison 1. Heel lance (term infants): sucrose (12% to 12.5%) versus water/routine care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total crying time (s) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐48.09 [‐93.04, ‐3.14] |
| 1.1 Term infants | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐48.09 [‐93.04, ‐3.14] |
| 2 Percentage change in heart rate 1 min after heel lance | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 6.4 [‐13.69, 26.49] |
| 2.1 Term infants | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 6.4 [‐13.69, 26.49] |
Comparison 2. Heel lance: sucrose (20% to 33%) versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP at 30 s after heel lance | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐2.86, 0.01] |
| 1.1 Term infants | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐4.96, ‐0.44] |
| 1.2 Preterm infants | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐2.42, 1.30] |
| 2 PIPP at 60 s after heel lance | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.81, 0.21] |
| 2.1 Preterm infants | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.81, 0.21] |
| 3 PIPP score during heel lance (1st heel lance) | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.52, 1.52] |
| 3.1 Newborns of diabetic mothers | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.52, 1.52] |
| 4 DAN score at 30 s after heel lance | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐8.58, 4.78] |
| 4.1 Term infants | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐8.58, 4.78] |
| 5 NIPS during heel lance | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.42, ‐1.58] |
| 5.1 Term infants | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐2.42, ‐1.58] |
| 6 Duration of first cry (s) | 2 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐8.63 [‐19.88, 2.61] |
| 6.1 Term infants | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐17.40, 7.40] |
| 6.2 Preterm infants | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐25.41 [‐52.06, 1.24] |
| 7 Total crying time | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐22.11 [‐32.52, ‐11.70] |
| 7.1 Term infants | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐22.11 [‐32.52, ‐11.70] |
| 8 Heart rate (beats/min) during heel lance | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐8.57, 6.94] |
| 8.1 Term infants | 2 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐8.57, 6.94] |
| 9 Percentage change in heart rate 1 min after heel lance | 2 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.81, 7.61] |
| 9.1 Term infants | 2 | 86 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐5.81, 7.61] |
| 10 Respiratory rate (breaths/min) during heel lance | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.64, 5.64] |
| 10.1 Term infants | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐7.64, 5.64] |
| 11 Oxygen saturation (%) during heel lance | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐12.79, 2.79] |
| 11.1 Term infants | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐12.79, 2.79] |
| 12 Skin blood flow during heel lance (perfusion units (PU)) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐32.0 [‐68.87, 4.87] |
| 12.1 Term infants | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐32.0 [‐68.87, 4.87] |
| 13 Nociceptive‐specific brain activity (mean weight) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 13.1 Term infants | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
Comparison 3. Heel lance: sucrose (50%) versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first cry (s) | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐63.20 [‐79.20, ‐47.19] |
| 1.1 Term infants | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐63.20 [‐79.20, ‐47.19] |
| 2 Percentage change in heart rate 1 min after heel lance | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐11.43, 16.63] |
| 2.1 Term infants | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐11.43, 16.63] |
Comparison 4. Heel lance: sucrose (24%) versus breastfeeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐2.22, ‐1.28] |
| 1.1 Preterm infants | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐2.22, ‐1.28] |
| 2 Comfort score | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐3.06, ‐2.14] |
| 2.1 Preterm infants | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐3.06, ‐2.14] |
Comparison 5. Heel lance: sucrose (24%) + NNS versus water + NNS, or pacifier dipped in sucrose versus pacifier dipped in water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NFCS | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.47, 0.27] |
| 1.1 Term infants | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.47, 0.27] |
| 2 PIPP 30 s after heel lance (mainly preterm infants) | 3 | 278 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.13, ‐1.26] |
| 2.1 New subgroup | 3 | 278 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐2.13, ‐1.26] |
| 3 PIPP 60 s after heel lance | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐2.14 [‐3.34, ‐0.94] |
| 4 Crying time (s) | 2 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐9.87, 7.04] |
| 4.1 Term infants | 2 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐9.87, 7.04] |
Comparison 6. Heel lance: sucrose (20%) versus human milk.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Crying time (s) | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐21.07, 5.07] |
| 1.1 Term infants | 1 | 35 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐21.07, 5.07] |
Comparison 7. Heel lance: sucrose (24%) + NNS+ NIDCAP support versus breast milk (by breastfeeding).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐4.03, 0.53] |
| 2 COMFORTneo score | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐4.84, ‐0.36] |
Comparison 8. Heel lance: sucrose (24%) + NNS + NIDCAP support versus breast milk (by syringe).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐2.41, 2.15] |
| 2 COMFORTneo score | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐2.74, 1.74] |
Comparison 9. Repeated heel lances: sucrose (20%) versus facilitated tucking.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Bernese Pain Scale for Neonates during heel lance | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐4.66, 0.12] |
| 2 Total Bernese Pain Scale for Neonates during recovery | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.72, 1.10] |
Comparison 10. Repeated heel lances: sucrose (20%) versus facilitated tucking + sucrose (20%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Bernese Pain Scale for Neonates during heel lance (preterm infants) | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐2.16, 2.06] |
| 2 Total Bernese Pain Scale for Neonates during recovery (preterm infants) | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [‐0.73, 2.01] |
Comparison 11. Heel lance: sucrose (30% to 33%) versus glucose (30% to 33%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate (beats/min) during heel lance | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐6.19, 18.59] |
| 1.1 Term infants | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 6.20 [‐6.19, 18.59] |
| 2 Crying time (s) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐34.89 [‐61.67, ‐8.11] |
| 2.1 Term infants | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐34.89 [‐61.67, ‐8.11] |
| 3 Percentage change in heart rate 1 min after heel lance | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐6.58 [‐14.85, 1.69] |
| 3.1 Term infants | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐6.58 [‐14.85, 1.69] |
Comparison 12. Heel lance: sucrose (50%) versus glucose (50%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate (beats/min) during heel lance | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 16.30 [1.93, 30.67] |
| 1.1 Term infants | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 16.30 [1.93, 30.67] |
Comparison 13. Heel lance (term infants): sucrose (24%) versus laser acupuncture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NIPS score | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.43, ‐0.29] |
| 2 Crying time (s) | 1 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐51.29 [‐73.11, ‐29.47] |
Comparison 14. Heel lance: sucrose (24%) versus sucrose (24%) + NNS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Revised NFCS | 1 | 343 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.23, 0.63] |
| 2 Percentage increase in heart rate | 1 | 343 | Mean Difference (IV, Fixed, 95% CI) | 2.29 [0.44, 4.14] |
| 3 Decrease in oxygen saturation in blood (%) | 1 | 343 | Mean Difference (IV, Fixed, 95% CI) | 0.48 [0.10, 0.86] |
Comparison 15. Heel lance: sucrose (24%) versus sucrose (24%) + swaddling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Revised NFCS | 1 | 343 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [0.19, 0.61] |
| 2 Percentage increase in heart rate | 1 | 343 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [‐1.43, 2.57] |
| 3 Decrease in oxygen saturation in blood (%) | 1 | 343 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.07, 0.67] |
Comparison 16. Heel lance: sucrose (24%) versus sucrose (24%) + NNS + swaddling.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Revised NFCS | 1 | 337 | Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.23, 0.63] |
| 2 Percentage increase in heart rate | 1 | 337 | Mean Difference (IV, Fixed, 95% CI) | 3.25 [1.43, 5.07] |
| 3 Decrease in oxygen saturation in blood (%) | 1 | 337 | Mean Difference (IV, Fixed, 95% CI) | 0.79 [0.44, 1.14] |
Comparison 17. Venipuncture: sucrose (12%) versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NIPS score in term and preterm infants | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐0.9 [‐1.81, 0.01] |
Comparison 18. Venipuncture: sucrose (24% to 30%) versus control (sterile water or no treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score during venipuncture | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐2.79 [‐3.76, ‐1.83] |
| 1.1 Newborns of non‐diabetic mothers | 1 | 106 | Mean Difference (IV, Fixed, 95% CI) | ‐3.2 [‐4.58, ‐1.82] |
| 1.2 Newborns of diabetic mothers | 1 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐3.76, ‐1.04] |
| 2 Duration of cry (s) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐16.5 [‐71.41, 38.41] |
| 2.1 Term infants | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐16.5 [‐71.41, 38.41] |
Comparison 19. Venipuncture: sucrose (50%) versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of first cry (s) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐51.79, 23.79] |
| 1.1 Term infants | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐14.0 [‐51.79, 23.79] |
Comparison 20. Venipuncture: sucrose (24% to 30%) versus sucrose (24% to 30%) + EMLA/Liposomal lidocaine cream on the skin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.12, 2.72] |
| 1.1 Preterm infants | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐0.12, 2.72] |
| 2 PIPP score during recovery period | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.73, 1.93] |
| 3 DAN score during venipuncture | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.26, 2.34] |
| 3.1 Preterm infants | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [0.26, 2.34] |
| 4 DAN score during recovery period | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [0.03, 2.77] |
| 5 Facial grimacing score | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐13.48, 3.48] |
| 6 Observer‐rated pain (VAS) (cm) | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐1.55, 0.15] |
| 7 Mean crying times during all procedures (s) | 2 | 289 | Mean Difference (IV, Fixed, 95% CI) | 1.83 [‐10.42, 14.09] |
| 7.1 Term infants | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐13.79, 13.79] |
| 7.2 Preterm infants | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | 8.69 [‐17.97, 35.35] |
| 8 Heart rate (beats/min) | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [0.39, 9.61] |
| 9 Oxygen saturation % | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.33, 0.73] |
Comparison 21. Venipuncture: sucrose (24%) versus liposomal lidocaine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Facial grimacing score | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐28.0 [‐36.48, ‐19.52] |
| 2 Observer‐rated pain (VAS) (cm) | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.25, 0.45] |
| 3 Cry duration (s) | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐39.0 [‐52.43, ‐25.57] |
| 4 Heart rate (beats/min) | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐1.95, 7.95] |
| 5 Oxygen saturation (%) | 1 | 216 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.03, 1.03] |
Comparison 22. Arterial puncture in preterm infants: sucrose (24%) versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate (beats/min) after needle insertion | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐11.73, 7.93] |
| 2 Heart rate (beats/min) 1 min after procedure completed | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐10.56, 5.76] |
| 3 Oxygen saturation in blood (%) after needle insertion | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐4.65, 2.65] |
| 4 Oxygen saturation in blood (%) 1 min after procedure | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐2.90 [‐5.95, 0.15] |
Comparison 23. Intramuscular injection (term infants): sucrose (20% to 25%) versus water or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NIPS 1 min to 2 min after IM injection | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐2.93, ‐1.67] |
| 2 PIPP during IM injection (term infants) | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐1.98, ‐0.12] |
| 2.1 Infants of non‐diabetic mothers | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐2.38, 0.18] |
| 2.2 Infants of diabetic mothers | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.35, 0.35] |
| 3 Duration of cry (s) | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐163.83 [‐192.58, ‐135.08] |
Comparison 24. Intramuscular injection (term infants): sucrose (25%) versus glucose (25%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 NIPS 1 min to 2 min after immunization | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.89, 0.69] |
Comparison 25. Intramuscular injection (term infants): sucrose (25%) versus sucrose (25%) + warmth.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Crying time (s) | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 15.2 [11.52, 18.88] |
| 2 Grimacing time | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 16.20 [12.35, 20.05] |
Comparison 26. Bladder catheterization: sucrose (24%) versus sterile water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in DAN score | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐2.43 [‐4.50, ‐0.36] |
| 2 Infants crying at maximal catheter insertion | 1 | 33 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.51 [‐0.81, ‐0.22] |
Comparison 27. Orogastric tube insertion in preterm infants: sucrose (24%) versus distilled water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score intra procedure | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐1.33, 0.73] |
| 2 PIPP score 30 s post procedure | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐1.30 [‐2.31, ‐0.29] |
| 3 PIPP score 1 min post procedure | 1 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.40, 0.40] |
Comparison 28. ROP examination: sucrose (24%) by syringe + swaddled + pacifier versus water by syringe + swaddled + pacifier.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP during examination | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.08, 2.08] |
| 2 Crying time (%) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐10.0 [‐32.91, 12.91] |
| 3 Heart rate (beats/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐19.33, 7.33] |
| 4 Mean blood pressure (mmHg) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐5.00 [‐18.48, 4.48] |
| 5 Respiratory rate (breaths/min) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐5.07, 9.07] |
| 6 Oxygen saturation (%) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | ‐1.00 [‐5.86, ‐0.14] |
Comparison 29. ROP examination: sucrose (24% ) + swaddled + held versus lying in the crib.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total crying time | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐33.90 [‐76.22, 8.42] |
| 2 Oxygen saturation (%) during examination | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐5.85, 2.43] |
Comparison 30. ROP examination: sucrose (24% to 33%) (sucrose or sucrose + NNS) versus control (water or water + NNS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP score during eye examination | 3 | 134 | Mean Difference (IV, Fixed, 95% CI) | ‐2.15 [‐2.86, ‐1.43] |
| 1.1 Sucrose via syringe versus control (sterile water via syringe) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐2.54, 0.54] |
| 1.2 Sucrose + pacifier versus control (sterile water + pacifier) | 3 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐2.47 [‐3.27, ‐1.66] |
| 2 Crying time (s) during eye examination | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐21.10 [‐33.10, ‐9.10] |
Comparison 31. Circumcision: sucrose 50% solution on a premature nipple containing a 2 x 2 cm sterile gauze pad moistened by the fluid versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change from baseline in heart rate (beats/min) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐9.70 [‐19.82, 0.42] |
Comparison 32. Circumcision: sucrose (24%) versus EMLA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 N‐PASS score during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [1.85, 2.95] |
| 2 N‐PASS score after 5 min | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.4 [0.74, 2.06] |
| 3 Heart rate (beats/min) during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [0.19, 11.81] |
| 4 Respiratory rate (cycles/min) during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐4.00, 0.20] |
| 5 Oxygen saturation (%) during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐2.70 [‐3.70, ‐1.70] |
Comparison 33. Circumcision: sucrose (24%) versus EMLA + sucrose (24%).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 N‐PASS score during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 3.00 [2.42, 3.58] |
| 2 N‐PASS score after 5 min | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.49, 1.91] |
| 3 Heart rate (beats/min) during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 12.0 [6.62, 17.38] |
| 4 Respiratory rate (cycles/min) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐1.77, 2.97] |
| 5 Oxygen saturation (%) during circumcision | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐3.40 [‐4.39, ‐2.41] |
Comparison 34. Circumcision: sucrose solution (50%) on a premature nipple containing a 2 x 2 cm sterile gauze pad moistened by the fluid versus dorsal penile nerve block (DPNB).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in heart rate (beats/min) from baseline | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 17.40 [11.16, 23.64] |
Comparison 35. Circumcision: pacifier dipped in sucrose (24%) + DPNB versus pacifier dipped in water + DPNB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean Behavioral Distress Scale scores during circumcision | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.08, ‐0.26] |
| 2 Mean plasma cortisol levels n mol/dL | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 68.90 [‐53.93, 191.73] |
Comparison 36. Echocardiography (term and preterm infants): sucrose (24%) versus no medication/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PIPP | 1 | 104 | Mean Difference (IV, Fixed, 95% CI) | ‐2.15 [‐3.30, 1.00] |
Comparison 37. Potentially painful procedures over seven days: sucrose (24%) versus water.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 'Motor development and vigor' (MDV) domain of NAPI tool | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐8.59, 4.93] |
| 2 'Alertness and orientation' (AO) domain of NAPI | 1 | 93 | Mean Difference (IV, Fixed, 95% CI) | 3.09 [‐6.49, 12.67] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abad 1996.
| Methods | Double‐blind, RCT Painful intervention: venipuncture Study location: Department of Pediatrics, University Hospital, La Laguna, Tenerife, Spain Study period: not stated |
|
| Participants | 28 (29 to 36 weeks' GA) healthy infants, PNA age 1 to 26 days | |
| Interventions | 2 mL 12% sucrose via syringe (n = 8) 2 min prior to venipuncture 2 mL 24% sucrose via syringe (n = 8) 2 min prior to venipuncture 2 mL spring water via syringe (n = 12) 2 min prior to venipuncture | |
| Outcomes | Oxygen saturation, respiratory rate, HR (just before and just after administering the solution and 5 min after venipuncture), time spent in audible crying for 3 min following venipuncture | |
| Notes | 1‐way and 2‐way ANOVA used to evaluate outcomes
Data were reported as means and SDs for the 3 physiological outcomes and as medians and IQRs for cry duration (in graph form only). Data were collected at 3 time points; just before the administration of the solution, just after administration of the solution and 5 min after venipuncture
Adverse effects were not evaluated Data for oxygen saturation, respiratory rate and HR were reported at 5 min after venipuncture and were not included in meta‐analyses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed in advance using numbers taken from a randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interventions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed blinded to interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomised infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Abbasoglu 2015.
| Methods | RCT Painful intervention: heel lance Study location: Baskent University Hospital, Ankara, Turkey Study period: not stated |
|
| Participants | 42 term newborns undergoing heel lance between postnatal days 3 and 8 as part of routine neonatal inpatient screening for phenylketonuria and hypothyroidism | |
| Interventions | 0.5 mL 24% sucrose solution given orally via syringe 2 min before heel lancing Laser acupuncture – 0.3 J energy applied to the Yintang point using a Laser PREMIO‐30 unit for 30 s |
|
| Outcomes | NIPS, cry duration (s) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Blank envelope containing a card indicated 1 of 2 groups. Not stated whether the envelopes were opaque, sealed and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | The nurse was blinded to group allocation before the envelope was opened, but not afterwards |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were made from video tapes (NIPS and cry duration) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all infants enrolled |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Acharya 2004.
| Methods | Double‐blind, randomized, controlled, cross‐over trial Painful intervention: venipuncture Study location: NICU at Leicester Royal Infirmary, UK Study period: not stated. |
|
| Participants | 39 healthy preterm neonates (mean 30.5 (SD 2.3) weeks' GA), mean PNA 27.2 (SD 24.4) days | |
| Interventions | 2 mL 25% (0.5 g) sucrose (n = 39) via syringe over 2 min into infant's mouth before 2 routine venipunctures 2 mL water (n = 39) via syringe over 2 min into infant's mouth before 2 routine venipunctures | |
| Outcomes | Rise in HR, oxygen saturation, duration of first cry, total duration of crying, NFCS at the 3 phases of the venipuncture | |
| Notes | Data were reported using means, SDs over the 3 phases of the venipuncture. Data could not be abstracted for the 2 groups prior to cross‐over. Adverse effects were evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Selected from random number table by a hospital pharmacist |
| Allocation concealment (selection bias) | Low risk | Allocation controlled by a hospital pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Interventions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments were done blinded to intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inconsistent number of infants reported in Methods section (n = 39) versus discussion section (n = 28) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Al Qahtani 2014.
| Methods | RCT Painful intervention: circumcision Study location: Day Care Surgery Department of Maternity and Children Hospital, Dammam City, Kingdom of Saudi Arabia Study period: January 2011 and April 2011 |
|
| Participants | 90 full‐term newborn males who underwent circumcision GA of 38 weeks or beyond, 5 min Apgar score of 8 or higher, PNA of 12 h or older and birthweight > 2500 g, and to be free from jaundice, anomalies of the penis, and analgesia or sedation in the previous 48 h |
|
| Interventions | 2 mL oral sucrose (24% w/v) given through a dropper onto the tongue 2 min before the procedure (n = 30) EMLA cream: applied to the shaft of the penis with an occlusive dressing 1 h before the procedure (n = 30) Combination of EMLA cream + oral sucrose (n = 30): 1 g EMLA cream applied to the shaft of the penis with an occlusive dressing 1 h before the procedure + 2 mL oral sucrose (24% w/v) given through a dropper onto the tongue 2 min before the procedure |
|
| Outcomes | N‐PASS used to assess the severity of pain and neonatal response to pain, 5 min before, during and 5 min after the circumcision for all newborns. The scale measures both physiologic responses (HR, respiratory rate, blood pressure and oxygen saturation) and behavioural responses (crying irritability, behaviour state, facial expression and extremities tone) to pain | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | The sample was divided randomly into 3 groups. The envelope was opened to classify the neonate randomly to 1 of the groups in order to carry out the appropriate action |
| Blinding (performance bias and detection bias) All outcomes | High risk | Staff were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Video imaging of the neonate 5 min before, during and until 10 min after the procedure showed the newborn reaction to pain and recorded the duration of crying. The videotapes were reviewed by an individual who was unaware of the infant’s treatment group, however, since the sucrose was applied 2 min before the procedure, it was probably possible to tell from the tapes which babies received sucrose |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported for all 90 infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Allen 1996.
| Methods | RCT Painful intervention: immunization injection Study location: a university hospital ambulatory paediatric clinic, Omaha, USA Study period: not stated |
|
| Participants | 285 infants aged between 2 weeks and 18 months; 50 included in this review (only neonates at 2 weeks of age) | |
| Interventions | 2 mL 12% sucrose (n = 16) 2 mL sterile water (n = 15) No treatment (n = 19) |
|
| Outcomes | Mean cry duration and percentage time crying during and 3 min after subcutaneous injection | |
| Notes | Data for percentage time crying were presented in graphical form only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Solutions in coded syringes prepared by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Low risk for sucrose and water; high risk for no intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 285 infants recruited from a continuous sample. Unsure of the number included in the analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Altun‐Köroğlu 2010.
| Methods | Double‐blind placebo‐controlled study Painful intervention: heel lance Study location: Marmara University Hospital, Istanbul, Turkey Study period: not stated |
|
| Participants | 75 full‐term infants undergoing heel lance | |
| Interventions | 3 mL hind milk (n = 25) 3 mL 12.5% sucrose solution (n = 25) 3 mL distilled water (n = 25) |
|
| Outcomes | NFCS, crying time, duration of crying, HR. Results reported as medians and IQRs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were used, however, there was no information regarding whether envelopes were opaque and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The test solution was prepared in a covered syringe |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The researchers were blind to the groups and utilized only the video recordings for scoring |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample sizes were not provided in Tables 2 and 3. We assumed the numbers from Table 1. Demographic features of the study groups were correct with 25 infants in each group |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Asmerom 2013.
| Methods | Randomized, double‐blind, controlled trial Painful intervention: heel lance Study location: Loma Linda University Children’s Hospital NICU, Loma Linda, California, USA Study period: July 2009 to February 2012 |
|
| Participants | 131 preterm infants ≤ 36.5 weeks’ PMA who weighed ≥ 800 g, had a central catheter in place, and required a heel lance | |
| Interventions | Sucrose 24% with a pacifier (n = 44): 2 mL for neonates > 2 kg; 1.5 mL for neonates 1.5 kg‐2 kg; and 0.5 mL for neonates < 1.5 kg Placebo with pacifier (n = 45) 42 infants received no heel lance no sucrose or placebo |
|
| Outcomes | PIPP after 2 min, plasma hypoxanthine, uric acid, xanthine, allantoin HR, oxygen saturation. We received unpublished data for means and SDs from Dr Angeles |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by a research pharmacist, who used a permuted block randomization table generated by the study statistician |
| Allocation concealment (selection bias) | Low risk | The study drug was prepared immediately before the experimental procedure by the research pharmacist and labelled as 'study drug' to ensure blinding |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neonates randomized to the sucrose group received a single dose of 24% sucrose in the following volumes: 2 mL for neonates >2 kg, 1.5 mL for neonates 1.5‐2 kg, and 0.5 mL for neonates that were <1.5 kg. Neonates randomized to the placebo group received an equal volume of sterile water to the anterior portion of the tongue along with a pacifier |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The neonate’s face was videotaped by trained research staff to record facial action at 0 min, during the heel lance and up to 30 s post heel lance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data reported on all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Banga 2015.
| Methods | RCT Painful intervention: each potentially painful procedure for a period of 7 days after enrolment Study location: a tertiary–level teaching hospital in North India Study period: April 2010 to April 2011 |
|
| Participants | 106 newborns, between completed 32 weeks and 37 weeks PMA were randomized to 2 groups sucrose (n = 53) and water (n = 53). 93 infants were available for analysis (47 in the sucrose group and 46 in the water group) | |
| Interventions | Sterile solution 24% sucrose (0.5 mL in 1mL syringe) for every potentially painful procedure during the first 7 days after enrolment Double‐distilled water (0.5 mL in 1mL syringe) for every potentially painful procedure during the first 7 days after enrolment |
|
| Outcomes | Primary outcome: score of motor development and vigor (MDV) and alertness and orientation (AO) domains of NAPI scale performed at 40 weeks PMA In addition, the highest HR and lowest SpO2 obtained during the procedure were recorded until 30 s after the painful stimulus, for newborns in both groups (not reported) |
|
| Notes | We wrote to the authors and Dr Banga provided us with this information: The potentially painful procedures included: venipuncture, heel lance, peripheral venous catheterization, OG or NG tube insertion, intramuscular injection, suprapubic bladder tap, retinopathy of prematurity examination, removal of adhesive tapes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization using computer‐generated random sequences was used with a static block size of 6 each |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was generated and maintained confidentially by the co‐investigator from department of Pharmacology. At the time of enrolment, the group allocation was telephonically conveyed to the research candidate, to ensure allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical‐looking packets carrying sucrose and the double‐distilled water, prepared and serially labelled according to confidential randomization code by pharmacy, were available at neonatal units. The primary care team members were responsible for administrating the intervention/control to the enrolled newborn according to the allocated serially numbered packet, unaware of the randomization |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants, the research candidate, and the primary care team members assessing the painful response were blinded to the group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 infants lost to follow‐up in the sucrose group, intervention discontinued in 1 and 2 died. 5 infants lost to follow1up in the water group and 2 died |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Basnet 2010.
| Methods | RCT Painful intervention: venipuncture Study location: Neonatal ward of Tribhuvan University Teaching Hospital, Kathmandu, Nepal Study period: February to August 2006 |
|
| Participants | 50 term infants aged between 12 h to 8 days Mean post‐natal age: 59.92 h no treatment group; 68.76 h sucrose group |
|
| Interventions | No treatment group (n = 25) Sucrose group (n = 25): received 2 ml of 30% sucrose orally 2 minutes before venipuncture |
|
| Outcomes | DAN score, duration of cry, number of infants crying | |
| Notes | Data for DAN scores were reported as median and IQRs. Duration of cry was reported as mean and SD and was included in meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used random numbers from 1 to 50 developed from a random number table |
| Allocation concealment (selection bias) | High risk | Authors did not report whether the opaque, sealed envelopes used to allocate participants were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | One rating scale (DAN) listed in methods section and is reported in results table. The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Biran 2011.
| Methods | RCT Painful intervention: venipuncture Study location: NICUs at Hôpital Armand Trousseau, Paris, France and Centre Hospitalier de Meaux, Meaux, France Study period: July to September 2007 |
|
| Participants | 76 preterm infants, mean (SD) PMA: sucrose group (n = 37): 32.6 (2.33) weeks; sucrose + EMLA group: (n = 39): 32.3 (2.01) weeks | |
| Interventions | Sucrose group: 0.5 mL 30% sucrose solution orally and placebo cream Sucrose + EMLA group: 0.5 mL 30% sucrose solution orally and EMLA cream on the skin |
|
| Outcomes | DAN scale, PIPP score | |
| Notes | Discussed adverse effects observed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done in advance in blocks of 8 using a random number table |
| Allocation concealment (selection bias) | Low risk | Used opaque, sealed and sequentially numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes not reported for 4 infants because of problems with video recording |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Blass 1997.
| Methods | RCT Painful intervention: heel lance Study location: Tompkins Community Hospital, Ithaca, New York, USA Study period: not stated |
|
| Participants | 72 newborn infants (PNA 22 h to 40 h) | |
| Interventions | 2 mL 12% sucrose (n = 8)
2 mL protein solution (Provimin) (n = 8)
2 mL lactose (n = 8)
2 mL dilute fat (coconut and soy oil blend) (n = 8)
2 mL concentrated fat (n = 8)
2 mL fat and lactose solution (n = 8)
2 mL Ross Special Formula (RSF ‐ artificial milk) (n = 8)
2 mL Similac (artificial milk) (n = 8) Solutions were given via syringe over a 2‐min period |
|
| Outcomes | Crying time (percentage of procedure time spent crying, percentage of time spent crying during 3‐min recovery period, number of infants that cried 20% or more during each recovery minute) | |
| Notes | Sucrose vs. water, Similac vs. water and RSF vs. water were compared using Mann‐Whitney U test. Most results were presented in graph form and means were reported in the text and could not be combined in meta‐analyses Adverse events were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Similac group could not be concealed because appearance differed from other intervention solutions |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded However, Similac group was high risk as its appearance differed from sucrose or water |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Sucrose and water solutions blinded. However, Similac group was high risk as its appearance differed from sucrose or water |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Unclear risk | Several test solutions were gifts from Ross Laboratories |
Blass 1999.
| Methods | RCT Painful intervention: heel lance Study location: Boston Medical Center, Boston, MA, USA Study period: not stated. |
|
| Participants | 40 term newborn infants, 34 h to 55 h old | |
| Interventions | All interventions given for 2 min prior to heel lance: 2 mL 12% sucrose over 2 min via syringe (n = 10) 2 mL water via syringe over 2 min (n = 10) Pacifier dipped every 30 s in 12% sucrose solution for 2 min (n = 10) Pacifier dipped in water every 30 s for 2 min (n = 10) |
|
| Outcomes | Percentage of time spent crying during 3 min after heel lance, percentage of time spent grimacing, change in mean HR | |
| Notes | Data were reported in graph forms only Results of ANOVA reported as P values only (we have contacted the authors to request additional information, but have received none) Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water alone groups blinded; pacifier + water, and pacifier + sucrose groups were blinded (although assessors could see pacifiers, they did not know which solution was being tested) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sucrose and water alone groups blinded; pacifier + water, and pacifier + sucrose groups were blinded (although assessors could see pacifiers, they did not know which solution was being tested) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Boyle 2006.
| Methods | RCT Painful intervention: screening for ROP Study location: Neonatal Unit, Royal Infirmary of Edinburgh, Edinburgh, Scotland, UK and Neonatal Unit, Birmingham Heartlands Hospital, Birmingham, UK Study period: not stated |
|
| Participants | 40 preterm infants < 32 weeks' PMA Sterile water group: mean PMA 27 weeks; mean PNA 45 days Sucrose group: mean PMA 29 weeks; mean PNA 43 days Water + pacifier group: mean PMA 30 weeks; mean PNA 41 days Sucrose + pacifier group: mean PMA 29 weeks; mean PNA 42 days |
|
| Interventions | 2 min before start of eye examination: 1 mL sterile water (n = 10) 1 mL sucrose 33% (n = 10) 1 mL sterile water + pacifier (n = 9) 1 mL sucrose 33% + pacifier (n = 11) Water or sucrose was given by mouth using a syringe | |
| Outcomes | PIPP during eye examination | |
| Notes | Data were presented in graph form and reported as means and SDs
Results of ANOVA and independent t‐tests reported as P values Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | High risk | Sealed opaque envelopes. Did not state if the envelopes were sealed and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water alone groups blinded; pacifier + water, and pacifier + sucrose groups were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sucrose and water alone groups blinded; pacifier + water, and pacifier + sucrose groups were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Bucher 1995.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over trial Painful intervention: heel lance Study location: Neonatal Clinic, Department of Obstetrics and Gynaecology, University Hospital, Zurich, Switzerland Study period: not stated |
|
| Participants | 16 preterm infants (27 to 34 weeks' PMA), PNA approximately 42 days | |
| Interventions | 2 mL 50% sucrose via syringe 2 min before heel lance
2 mL distilled water via syringe 2 min before heel lance
(n = 16, cross‐over design) Each infant was assessed twice receiving2ml of sucrose 50%or 2 ml of distilled water in random order immediately before heel lance |
|
| Outcomes | Increase in HR (beats/min); recovery time for HR (s); recovery time for respirations (s); crying (percentage of total intervention); recovery time until crying stopped (s); oxygen saturation (maximum increase in kPa; maximum decrease in kPa; and difference between baseline and 10 min after end of intervention in kPa), and cerebral blood volume | |
| Notes | Results were presented in graph form without mean values and SDs, in tables with medians with IQRs, or both. Used Wilcoxon signed rank test. Data for sucrose and placebo groups prior to cross‐over were not presented Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated from random number table |
| Allocation concealment (selection bias) | Low risk | Vials containing solutions were coded and contents could not be identified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Carbajal 1999.
| Methods | RCT Painful intervention: venipuncture Study location: maternity ward, Poissy Hospital, Poissy, France Study period: April to end of June 1997 |
|
| Participants | 150 term newborn infants, 3 to 4 days old | |
| Interventions | 2 min prior to venipuncture the allocated solution was adminstered for 30 seconds by a sterile syringe into the infant's mouth No treatment (n = 25) 2 mL sterile water (n = 25) 2 mL 30% glucose (n = 25) 2 mL 30% sucrose (n = 25) Pacifier alone (n = 25) 2 mL 30% sucrose followed by pacifier (n = 25) |
|
| Outcomes | DAN scale during venipuncture, reported as median and IQR | |
| Notes | Mann‐Whitney U test used to evaluate pain scores Adverse effects were evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by random number table |
| Allocation concealment (selection bias) | Low risk | Allocated by sequentially numbered, opaque and sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Low risk for sucrose and water solutions High risk for pacifier groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Low risk for sucrose and water solutions High risk for pacifier groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Cignacco 2012.
| Methods | RCT Painful intervention: repeated heel lances Study location: 3 NICUs in Switzerland Study period: 12 January to 31 December 2009 |
|
| Participants | 71 preterm infants between 24 and 32 weeks PMA | |
| Interventions | Sucrose group (n = 24): sucrose 20% (0.2 mL/kg), administered orally ∼2 min before the heel lance. If the infant seemed to be in pain during the heel lance phase, up to 2 additional doses of sucrose were administered and noted in the study chart Sucrose + facilitated tucking (FT) (n = 23): combination of sucrose and facilitated tucking; the FT was started at the beginning of the baseline phase and sucrose was given 2 min before the heel lance FT (n = 24): FT was started at the beginning of the baseline phase, and the infant was 'tucked' through all 3 phases |
|
| Outcomes | BPSN: data collection occurred: at baseline (before any manipulation); at heel lance (skin preparation, heel stick, and haemostasis after blood was drawn); and during recovery (3 min after the heel lance) The BPSN contains 9 items: 3 are physiological (HR, respiratory rate, and oxygen saturation) and 6 are behavioural (grimacing, body movements, crying, skin colour, sleeping patterns, consolation) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization by using SPSS, version 16 |
| Allocation concealment (selection bias) | Low risk | For each site, group assignments were sealed in opaque, consecutively numbered envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | When parents consented to participation, the envelope was opened by a study nurse |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The intervention was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all 71 randomized infants |
| Selective reporting (reporting bias) | Low risk | The trial was registered as: NCT00758511 In the registry it said that 25% sucrose would be used. In the paper it said that 20% sucrose was used. We did not see any other deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Codipietro 2008.
| Methods | RCT Painful intervention: heel lance Study location: Neonatal Unit of Agnelli Hospital, Pinerolo, Turin, Italy Study period: January to April 2007 |
|
| Participants | 51 term infants: mean PMA 39.3 weeks (SD 1.2) in breastfeeding group; 50 term infants: mean PMA 39.4 weeks (SD 1.1) in sucrose group | |
| Interventions | 1 mL 25% sucrose (n = 50) Breastfeeding (n = 51) |
|
| Outcomes | PIPP during blood sampling, 2 min after heel lance, HR increase from baseline at 30 s following commencement of procedure, oxygen saturation decrease, duration of first cry, percentage crying time in first 2 min and during blood sampling Data reported as median and full range |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence created by statistician and masked to investigators |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Breastfeeding could not be blinded. Nurses and parents not blinded to assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only assistants listening to voice recordings of cry for PIPP scoring were blind to intervention. High risk for PIPP‐R outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Dilli 2014.
| Methods | RCT Painful intervention: screening for ROP Study location: Dr Sami Ulus Maternity and Children Training and Research Hospital, Ankara, Turkey Study period: July 2011 to June 2012 |
|
| Participants | 64 infants undergoing eye examination for ROP. The groups had similar PMA (28.5 ± 2.8 weeks), mean birthweight (1304 ± 466 g) or corrected PMA (35.4 ± 3.7 weeks) at examination | |
| Interventions | All infants received topical anaesthetic (proxymetacaine, Alcaine) drop 0.5%: ALCON CANADA Inc, Mississauga, Canada) applied 30 s before the eye examination. In addition: Sucrose group (n= 32): received 0.5 mL/kg 24% sucrose with a pacifier Control group (n = 32): received 0.5 mL/kg sterile water with a pacifier |
|
| Outcomes | Mean PIPP score during examination Secondary outcome measurements were frequency of tachycardia (> 180 beats/min), bradycardia (< 100 beats/min), desaturations (< 85% for > 10 s) and crying time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Low risk | Syringes of either 24% sucrose (A) or sterile water (B) were provided by the pharmacy in sealed envelopes. Both of the solutions were colourless |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The parents, the nurse, the ophthalmologist and investigators were blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the infants were video‐recorded until completion of the eye examination. Primary outcome measurement was PIPP score which was performed by the same investigator who had web‐based training |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all 64 infants |
| Selective reporting (reporting bias) | Low risk | Clinical Trials.gov Identifier: NCT01811979. There did not seem to be any deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Elserafy 2009.
| Methods | Cross‐over RCT Painful intervention: venipuncture Study location: NICU at King Faisal Specialist Hospital, Jeddah, Saudi Arabia Study period: January 2005 to May 2007 |
|
| Participants | 36 infants: median (range): 32 weeks' PMA (27 to 46), mean (SD) GA: 32.4 (2.0) ‐ 2 different mean PMAs reported in the article | |
| Interventions | 0.5 mL sterile water with pacifier 0.5 mL sterile water without pacifier 0.5 mL 24% sucrose with pacifier 0.5 mL 24% sucrose without pacifier Pacifier alone Control group (the authors do not state what this grooup received ‐ we assume no intervention) |
|
| Outcomes | Duration of cry, PIPP, HR, respiratory rate, glucose check | |
| Notes | All infants received all of the 6 interventions and so we could not use the results in meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A paper was randomly picked so that assignments were random and double‐blinded for the sucrose and water solutions |
| Allocation concealment (selection bias) | High risk | Consecutively numbered envelopes, but report did not specify whether they were opaque or sealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Personnel were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Gal 2005.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study Painful intervention: screening for ROP Study location: Department of Neonatology, Women’s Hospital, Greensboro, North Carolina, USA Study period: January 2003 to June 2004 |
|
| Participants | 23 preterm infants mean PMA 26.4 weeks (range 24 to 29), PNA 28 to 93 days | |
| Interventions | Mydriatic eye drops (phenylephrine HCl 1%, cyclopentolate HCl 0.2%) and local anaesthetic eye drops (proxymetacaine HCl 0.5%: 2 drops) were given to both groups prior to examination. In addition infants received: Sucrose group: 2 mL 24% sucrose via syringe (n = 23) Water group: 2 mL sterile water via syringe (n = 23) |
|
| Outcomes | PIPP score at 5 min and 1 min pre‐examination, PIPP score at eye speculum insertion, PIPP score 1 min and 5 min post examination | |
| Notes | Results were reported as means and SDs after cross‐over. Results for the 2 groups prior to cross‐over were not available
Results of paired t‐tests were reported as P values Adverse events reported, but no adverse events experienced |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was made in groups of 6 based on the results from a dice roll |
| Allocation concealment (selection bias) | Low risk | Allocation centrally controlled by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported to have 23 neonates in study but only 22 neonates included in demographic information and PIPP Scores in Table 1 of the paper |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Stopped at 23 neonates due to change in ophthalmologist in order to maintain consistency in examinations; however, statistical power calculated determined that 24 neonates were needed for the study. This does not seem to have affected the results |
Gaspardo 2008.
| Methods | Randomized, double‐blind, controlled trial Painful intervention: venipuncture, arterial puncture, heel lance, intravenous cannulation, endotracheal tube introduction, endotracheal tube suctioning, gavage insertion for feeding, removal of electrode leads and tape Study location: NICU of the Hospital of Clinics, School of Medicine, University of São Paulo at Ribeirão Preto, Preto, Brasil Study period: April 2003 and September 2005 |
|
| Participants | 33 preterm infants, median PMA 30 weeks | |
| Interventions | On day 1, no treatment was given to any neonate in order to collect baseline data. On days 2 to 4 solutions (sucrose or water) were administered to neonates before every painful procedure (listed above): 0.5 mL/kg 25% sucrose before every minor painful procedure listed above (n = 17) 0.5 mL/kg sterile water before every minor painful procedure listed above (n = 16) |
|
| Outcomes | Incidence of cry (percentage of neonates crying), HR (percentage of neonates with HR ≥ 160 beats/min), NFCS (percentage of neonates with score ≥ 3), Activated Behavioural State (percentage of neonates with score ≥ 4) | |
| Notes | Pain was assessed over 4 days during morning blood collection (heel lance) The Mann‐Whitney U test was used to calculate the difference between sucrose and water groups for continuous variables. The Chi2 test was used to calculate the difference between sucrose and water groups for categorical variables No means or standard deviations were reported. NFCS results were reported in graph form only Adverse events were assessed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Solutions prepared by pharmacist labelled 'A' or 'B' to keep identity from investigators. Co‐ordinator kept identities of solutions in sealed and opaque envelopes until after analysis |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11/ 44 enrolled infants were discharged from the NICU while the data collection was in progress and 33 infants completed the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Gibbins 2002.
| Methods | RCT Painful intervention: heel lance Study location: a University‐affiliated metropolitan Level III NICU, Toronto, Ontario, Canada Study period: 16‐month period during 1998‐1999 |
|
| Participants | 190 preterm and term infants, mean PMA 33.7 weeks, under 7 days' PNA | |
| Interventions | 2 min prior to heel lance: Sucrose + NNS group (N = 64): 0.5 mL 24% sucrose via syringe to the anterior surface of the tongue followed by pacifier Sucrose group (N = 62): 0.5 mL 24% sucrose without pacifier Water + NNS group (N = 64): 0.5 mL sterile water with pacifier |
|
| Outcomes | PIPP at 30 s and 60 s after heel lance | |
| Notes | 1‐way ANOVA to evaluate mean pain scores Results were reported as means and SDs Adverse effects were evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated using a centralized randomization table |
| Allocation concealment (selection bias) | Low risk | Centrally allocated by pharmacist. Pharmacist labelled all solutions as 'study drug' and delivered it to neonate's bedside |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Facial coders were not informed about the purpose of the study, phases of the heel lance, or group allocation for the 2 pacifier groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 neonates were lost to follow‐up due to equipment failure |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other biases |
Gormally 2001.
| Methods | RCT, factorial design Painful intervention: heel lance Study location: Lakeshore General Hospital, Montreal, Quebec, Canada Study period: not stated |
|
| Participants | 94 normally developing newborns, mean PMA 39.4 weeks on 2nd or 3rd day of life 9 infants did not complete the study for the following reasons: early discharge, nurse or testing room unavailability to obtain heel lance, infant removed from study prior to start date, technical difficulties | |
| Interventions | No holding + sterile water given by pipette (n = 21) No holding + 0.25 mL 24% sucrose solution (0.06 g) given by pipette (n = 22) Holding + sterile water given by pipette (n= 20) Holding + 0.25 mL 24% sucrose solution (0.06 g) given by pipette (n = 22) All solutions given 3 times at 30‐s intervals | |
| Outcomes | Percentage of time crying, pain concatenation scores for facial activity, mean HR, mean vagal tone index, measurements prior to intervention and at 1, 2, and 3 min after heel lance | |
| Notes | Factorial ANOVA to assess effects on behavioural and physiological measures No means or standard deviations reported in numbers, only in graph form Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Facial coders were blind to solution assignment only but not to holding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all infants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Grabska 2005.
| Methods | Prospective, randomized, blinded, placebo‐controlled study Painful intervention: screening for ROP Study location: NICUs at Conneticut Children's Medical Center and John Dempsey Hospital, USA Study period: not stated |
|
| Participants | 32 preterm infants with birthweight < 1.5 kg or PMA < 28 weeks PMA (mean ± standard deviation) 28 ± 1.6 weeks, PNA (mean ± standard deviation) 50.8 ± 20.3 days | |
| Interventions | Sterile water (n = 16) 24% sucrose (n = 16): dose was adjusted according to weight: 0.5 mL (0.12 g) for infants < 1 kg; 1.0 mL (0.24 g) for infants 1 kg‐1.5 kg; 1.5 mL (0.36 g) for infants > 1.5 kg‐2 kg; 2.0 mL (0.48 g) for infants > 2 kg | |
| Outcomes | HR, respiratory rate and oxygen saturation at baseline, post mydriatic, post study drug, during eye examination, post eye examination PIPP at baseline, during eye examination, post eye examination Crying time during eye examination Blood pressure at baseline, post mydriatic, during eye examination and post eye examination |
|
| Notes | Results were reported as means and standard deviations
Results of t‐tests and ANOVAs were reported as P values where a value of < 0.05 was considered significant Adverse events were evaluated and included choking, and transient oxygen desaturation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | High risk | Pharmacy provided solutions in sealed envelopes after randomization, but did not specify whether envelopes were sequentially numbered and opaque |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although not explicitly stated, it can be inferred that nurses administering solutions and those assessing videotapes were blinded to assigned solution |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Gray 2012.
| Methods | RCT Painful intervention: vaccination (hepatitis B) Study location: University of Chicago Medicalm Center, Chicago, Illinois, USA Study period: June to July 2007 |
|
| Participants | 47 healthy full‐term infants undergoing vaccination | |
| Interventions | Sucrose group (n = 15): 1.0 mL 25% sucrose solution administered via syringe Warmth group (n = 14): 100% radiant warmth from Ohmeda warmer on the manual setting Pacifier group (n = 15): hospital‐issued pacifier held lightly to their mouths 3 infants were subsequently excluded from data analysis (1 in the sucrose group and 2 in the warmth group) |
|
| Outcomes | Cumulative crying time, mean HR, mean respiratory sinus arrhythmia, cumulative distribution of grimace time | |
| Notes | All outcomes were provided in graph form only and could not be used in meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Infants were randomly assigned using a sealed envelope system into 1 of 3 groups: warmth (n = 14), sucrose (n = 15), pacifier (n = 15). Did not state whether the envelopes were sequentially numbered or not |
| Blinding (performance bias and detection bias) All outcomes | High risk | Infants in the warmth group had their clothing removed except for the diaper and were placed under an Ohmeda‐Ohio 3000 Infant Warmer System. Infants in the other 2 study groups (sucrose and pacifier) remained in their bassinets (cots) clothed in a shirt, diaper, and hat |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The infant’s face was videotaped for offline coding of grimace and cry. The research assistants could probably tell to which group the infant belonged |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 47 enrolled infants, 3 infants were subsequently excluded from data analysis due to technical problems with HR recording (1 in the sucrose group and 2 in the warmth group) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Gray 2015.
| Methods | RCT Painful intervention: vaccination (hepatitis B) Study location: University of Chicago Hospital, Chicago, Illinois, USA Study period: July to August 2008 |
|
| Participants | 29 healthy, full‐term newborns undergoing vaccination Exclusion criteria included preterm birth (< 37 weeks’ completed PMA), birthweight < 2 kg, any Apgar score < 6, congenital abnormalities, medical complications, or drug exposure. Infants with previous oxygen administration, ventilatory support, or NICU admission were excluded |
|
| Interventions | Sucrose group (n = 15): 1.0 mL 24 % sucrose 2 min before vaccination Sucrose + warmth group (n = 14): 1.0 mL 24% sucrose 2 min before vaccination + radiant warmth from an infant warmer before the vaccination Infants in the sucrose + warmth group were placed under an Ohmeda Ohio Infant Warmer (Model No. 3000; GE Healthcare, Fairfield, CT), and their clothing was removed, except for a diaper (nappy) |
|
| Outcomes | Duration of cry and grimace (s), HR variability and HR | |
| Notes | Duration of cry and grimace were provided as means and SDs and in graph form. Respiratory sinus arrhythmia and HR reported in graph form Both groups received sucrose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We randomly assigned each infant in the study to sucrose alone or sucrose plus warmer groups by using a sealed envelope randomisation system" |
| Allocation concealment (selection bias) | High risk | No information about whether the envelopes were opaque and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | The study could not be blinded to warmth vs. no warmth |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Video tapes were analyzed by assessors blinded to group assignment – probably, but could the warmer be seen? |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it. We could not find a Trils registration number |
| Other bias | Low risk | Appears free of other bias |
Greenberg 2002.
| Methods | RCT Painful intervention: heel lance Study location: a moderate sized hospital in Southern California, USA Study period: not stated |
|
| Participants | 84 term newborns, approximately 17 h to 19 h old | |
| Interventions | Sugar‐coated pacifier held in infant's mouth before procedure to 3 min after procedure (n = 21) Water‐moistened pacifier (n = 21) 2 mL 12% sucrose via syringe into side of infant's mouth (n = 21) Routine care (n = 21) | |
| Outcomes | Salivary cortisol levels, duration of cry, vagal tone | |
| Notes | Analysis using MANOVA to evaluate outcomes by groups Results were presented in graph forms without mean values and SDs. Means and SEs were provided for "time crying by group in seconds" Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Use of pacifier precluded blinding. No blinding between pacifier groups either, as one was moistened with water and one dipped in sugar packet |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome measurement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement indicating how many infants were recruited and how many dropped out |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Guala 2001.
| Methods | RCT Painful intervention: heel lance Study location: Hospital of Vigevano, Italy Study period: not stated |
|
| Participants | 140 term (38 to 41 weeks' PMA) | |
| Interventions | Nothing (n = 20) Water (n = 20) 5% glucose (n = 20) 33% glucose (n = 20) 50% glucose (n = 20) 33% sucrose (n = 20) 50% sucrose (n = 20) Administered via syringe into infant's mouth over 30 s | |
| Outcomes | HR before, during and 3 min after heel lance | |
| Notes | ANOVA to evaluate HR across groups at each phase of the heel lance. Means and SDs provided Adverse effects were evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by random number table |
| Allocation concealment (selection bias) | High risk | Allocated by sealed opaque envelopes. Did not state if the envelopes were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 infants were allocated to each group and results for all infants were presented |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Haouari 1995.
| Methods | Randomized, double‐blind, placebo‐controlled trial Painful intervention: heel lance Study location: Leeds General Infirmary, Leeds, UK Study period: 6 months (dates not provided) |
|
| Participants | 60 term (37 to 42 weeks' PMA) infants, 1 to 6 days of age | |
| Interventions | 2 mL 12.5% sucrose 2 min prior to heel lance (n = 15) 2 mL 25% sucrose 2 min prior to heel lance (n = 15) 2 mL 50% sucrose 2 min prior to heel lance (n = 15) 2 mL sterile water 2 min prior to heel lance (n = 15) All solutions were given by syringe on the tongue over < 1 min | |
| Outcomes | Total time (s) crying over 3 min following heel lance, time of first cry (s) following heel lance, percentage change in HR after heel lance (at 1, 3 and 5 min) | |
| Notes | Analysis of non‐parametric data was by the Mann‐Whitney U test or a trend test. Total time crying in the first 3 min after heel lance was reported as medians and IQRs. Changes in HR were expressed in means and SDs as a percentage of resting HR Adverse effects were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Preprepared solutions in coded bottles |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions administered blinded to staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Harrison 2003.
| Methods | Randomized, blinded, controlled trial Painful intervention: heel lance Study location: Royal Children's Hospital, University of Melboourne, Victoria, Australia Study period: May 2000 to July 2001 |
|
| Participants | Our sample was a subset of a larger study (n = 128) that included older infants Authors provided us with data for a subset of infants that fulfilled our inclusion criteria The subset included 99 hospitalized infants Mean (SD) PMA of placebo group: 36.7 weeks (3.3) Mean (SD) PMA of treatment group: 36.8 weeks (3.7) |
|
| Interventions | 1 mL water 2 min prior to heel lance (n = 46)
1 mL 25% sucrose 2 min prior to heel lance (n = 53) For infants weighing ≤ 1500 g the dose was reduced to 0.5 mL |
|
| Outcomes | NFCS at baseline, upon heel lance, during heel squeeze and completion of heel squeeze at 1, 2 and 3 min of recovery Duration of cry until 5‐s pause, percentage of crying time during heel lance and squeeze, percentage of crying time during 3 min recovery period HR and oxygen saturation (SpO2) |
|
| Notes | Results were presented in graphs
Results of Student's t‐test, Pearson's Chi2 test, Fisher's exact test and Mann‐Whitney U test were reported as P values Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Pharmacy‐prepared solutions in consecutively numbered syringes. Contents of syringes obscured |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | NNS with pacifier was provided as comfort measure if part of regular infant care. This was addressed by the authors and adjusted analyses were performed to assess the effect of pacifier across groups |
Herschel 1998.
| Methods | RCT Painful intervention: circumcision Study location: General Care Nursery of the University of Chicago Hospitals, Chicago, Ill, USA Study period: not stated |
|
| Participants | 119 full‐term male neonates undergoing circumcision, PMA ≥ 38 weeks, PNA ≥ 12 h | |
| Interventions | No treatment (n = 40) DPNB (0.8 mL 1% lidocaine) (n = 40) Pacifier dipped in and packed with gauze soaked in 50% sucrose (n = 39) | |
| Outcomes | HR and oxygen saturation (change from baseline and means for each interval of circumcision) | |
| Notes | Results of change in HR and oxygen saturation for each group were reported as mean and SD. Mean HRs for each interval of circumcision were presented in graph form Mean HR and oxygen saturation were compared between groups using ANOVA. Characteristics of infants in the 3 groups were compared using Chi2 test, Fisher exact test or ANOVA Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffled opaque unmarked envelopes to generate sequence |
| Allocation concealment (selection bias) | High risk | Group assignments contained in opaque unmarked envelopes. Did not state if the envelopes were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | Intervention was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment was blinded. Outcome not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 1 exclusion: an infant randomized to sucrose was not circumcised. After the operator visualized the location of the meatus, she thought the surgery was contraindicated |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Isik 2000a.
| Methods | RCT Painful intervention: heel lance Study location: Marmara University Hospital, Istanbul, Turkey Study period: August 1997 to May 1998 |
|
| Participants | 113 healthy newborns PMA: 37 to 42 weeks, median PNA: 2 days (range 2 to 5 days) | |
| Interventions | 2 mL 30% sucrose (n = 28) 2 mL 10% glucose (n = 29) 2 mL 30% glucose (n = 28) 2 mL distilled water (n = 28) Syringed into the anterior third of the tongue for 1 min 2 min prior to heel lance | |
| Outcomes | Mean cry time during 3 min after heel lance; mean maximum HR 3 min after heel lance; mean recovery time for HR; percentage change in HR at 1, 2, 3 min after heel lance | |
| Notes | 1‐way ANOVA was used to evaluate mean cry time, recovery time and percentage change in HR Results reported as means and standard errors of the mean Adverse effects were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Could not tell if intervention was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Could not tell if HR assessment was blinded; however, it was stated that assessment of crying was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No clear statement given. Indicated that any baby that cried prior to the heel lance was excluded, but number in methods is same as number in results, so unsure if there were more recruited but dropped out/excluded for results |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Johnston 1997.
| Methods | RCT Painful intervention: heel lance Study location: University‐affiliated level III NICU, Canada Study period: not stated |
|
| Participants | 85 preterm infants (25 to 34 weeks' PMA), 2 to 10 days of age | |
| Interventions | 0.05 mL 24% sucrose via syringe into the mouth just prior to heel lance (n = 27) 0.05 mL 24% sucrose via syringe into the mouth just prior to heel lance and simulated rocking 15 min prior to heel lance (n = 14) 0.05 mL sterile water via syringe into the mouth just prior to heel lance and simulated rocking 15 min prior to heel lance (n= 24) 0.05 mL sterile water via syringe into the mouth just prior to heel lance (n = 20) | |
| Outcomes | HR, oxygen saturation, behavioural facial actions, behavioural state; NFCS baseline and at 3 x 30‐s blocks | |
| Notes | Data were analyzed using MANOVA (facial action). For HR repeated measures ANOVA was used with mean values but no SDs presented in graph form For state repeated measures ANOVA was performed and no univariate means and SDs were presented Oxygen saturation (SpO2) was dropped from analysis Adverse effects were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence |
| Allocation concealment (selection bias) | High risk | Sequentially numbered envelopes, but did not specify whether envelopes were opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The research nurse who actually conducted the heel stick procedure was not naive as to the interventions". "Not only was it obvious whether or not the infant was on the rocking bed, the nurse participated in preparing the infants for the conditions" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Similarily, in instances where the pulse oximeter signal was lost and heart rate was recorded by hand, the researcher collecting the data knew to which group the infant belonged". "The research assistant who coded the behavioral data in the laboratory did not know the purpose of the study, the nature of the interventions, nor the infants' group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The original design called for 28 infants/group based on anticipated effect size; however Table 1 shows that sample size of each group varied from 14 to 27; not equal groups |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Johnston 1999.
| Methods | RCT Painful intervention: heel lance Study location: Level III NICU, Canada Study period: not stated |
|
| Participants | 48 preterm neonates, mean PMA of 31 weeks (range 25 to 34 weeks) within 10 days of birth | |
| Interventions | Interventions given by syringe to anterior surface of the tongue at: 2 min prior to heel lance, just prior to lancing, and 2 min after lancing 0.05 mL 24% sucrose as a single dose, followed by 2 doses of sterile water (n = 15) 3 doses 0.05 mL 24% sucrose (n = 17) 3 doses 0.05 mL sterile water (n = 16) |
|
| Outcomes | PIPP, measured over 5 x 30‐s blocks of time | |
| Notes | Repeated measures ANOVA was used to evaluate the effect of single vs. repeated doses of sucrose Means and SDs for pain scores were obtained from the author Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignment |
| Allocation concealment (selection bias) | High risk | Once parental consent was obtained, the research assistant opened the next sealed study envelope that contained the computer‐generated random assignment to 1 of 3 treatment groups: single sucrose, repeated sucrose, and sterile water. Trial report did not state whether or not the envelopes were opaque |
| Blinding (performance bias and detection bias) All outcomes | High risk | Sucrose and water solutions blinded for research nurses, but not the research assistants as they prepared the syringes with the solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The video tapes were later coded according to the NFCS in the university laboratory by research assistants who were blind to the purpose of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Johnston 2002.
| Methods | RCT Painful intervention: multiple invasive procedures Study location: 3 level III university‐affiliated NICUs in Canada Study period: 27 months (dates not stated) |
|
| Participants | 103 preterm infants completed the study (107 infants entered the study; 2 infants died and 2 were withdrawn) Sucrose group: mean (SD) PMA: 28.18 weeks (1.72) Water (control) group: mean (SD) PMA 28.05 weeks (2.06) |
|
| Interventions | Sucrose or water was administered orally up to 3 times, 2 min apart, for every invasive procedure during a 7‐day period: 0.1 mL 24% sucrose (n = 51) 0.1 mL water (n = 52) |
|
| Outcomes | Neurobehavioural development assessed by the sub scales of alertness and orientation and motor development and vigour of the NAPI, Score for Neonatal Acute Physiology (SNAP) and Neuro‐Biological Risk Score (NBRS) | |
| Notes | Data could not be used for meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random computer‐generated program |
| Allocation concealment (selection bias) | Unclear risk | Did not specify how allocation was done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Research assistants not blinded to group, but blinded to purpose of study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistants not blinded to group, but blinded to purpose of study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 infants were withdrawn from the study during the week of intervention and another 2 infants died |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Kaufman 2002.
| Methods | RCT Painful intervention: circumcision Study location: normal newborn Nursery of the Boston Univeristy Medical Centre, Boston, MA, USA Study period: March 1999 to August 2000 |
|
| Participants | 57 male infants undergoing circumcision | |
| Interventions | Mogen method and water (n = 15)
Mogen method and 24% sucrose (n = 14)
Gomco method and 24% sucrose (n = 14)
Gomco method and water (n = 14) Solutions were given via a dipped pacifier |
|
| Outcomes | Cry and grimacing during real time 10‐s intervals | |
| Notes | Results were reported graphically. A 2‐factor analysis of variance evaluated raw and percentage duration of crying and grimacing. The Kolmogorov‐Smirnov test for the equivalence of empiric distribution functions was used to evaluate differences in the distribution of cumulative crying and grimacing Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not adequately described |
| Allocation concealment (selection bias) | Low risk | Solutions prepared and coded by pharmacy and stored in dark vials to make them indistinguishable |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions were prepared and coded by pharmacy department and stored in individual darkened vials, each containing 60 mL aliquots. Investigators, research assistants, and hospital staff who participated in the circumcision did not know the contents of a given vial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators who were unaware of the experimental condition scored the audio‐video tapes using software |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is unclear how many infants were included in the analyses |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Kristoffersen 2011.
| Methods | RCT, cross‐over design Painful intervention: NG intubation Study location: NICU at St Olav's University Hospital, Trondheim, Norway Study period: January 2005 to June 2008 |
|
| Participants | 24 preterm infants, 28 to 32 weeks' PMA | |
| Interventions | Each infant acted as his or her own control over a 3‐week period 6 times. On these occasions, 6 different treatment combinations were given in randomized order: Pacifier or no pacifier, combined with no fluid, sterile water, or 30% sucrose | |
| Outcomes | PIPP scores | |
| Notes | Infants acted as their own controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random list generated by computer |
| Allocation concealment (selection bias) | Low risk | Used a unique sequence from list ‐ only the study leader had access to the list |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Nurses doing PIPP scores were asked to "turn away" before solution was given but authors did not mention how to control for that |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nurses doing PIPP scores were asked to "turn away" before solution was given but authors did not mention how to control for that |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 infants were transferred to another hospital and did not complete the study. 24 infants completed the study and they had complete observations. The 6 treatment combinations resulted in 144 discreet events being observed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Leng 2013.
| Methods | RCT Painful intervention: heel lance Study location: Children's Hospital of Chongqing Medical University, Chongqing 400014, China Study period: not stated |
|
| Participants | 560 full‐term neonates (male 295, female 265) PMA 37 to 42 weeks; weight at birth 2500 g to 4000 g; Apgar scores at 1 min and 5 min after birth averaged Inclusion criteria: ≥ 8 points; age 3 to 28 days; had not undergone surgery; baseline HR 120‐140 beats/min; oxygen saturation ≥ 0.90; planned screening for congenital metabolic disease Exclusion criteria: neonates presenting with asphyxia, congenital heart disease, and neuromuscular disease during birth; oxygen inhalation; hyperglycaemia; fasting; received sedative injection within last 48 h; fructose intolerance; maternal methadone dependence; vertebral injury |
|
| Interventions | The infants were randomized to 7 groups: Placebo group (plain boiled water) 10% glucose 25% glucose 50% glucose 12% sucrose 24% sucrose 30% sucrose The solutions were administered through a syringe dripping into the neonate's mouth 2 min before heel lance |
|
| Outcomes | The heel lance procedure was recorded by video. HR, oxygen saturation and pain scores were assessed at 1 min before heel lance and 3, 5 and 10 min after heel lance. Results were reported as means and full ranges. | |
| Notes | The article was translated for us by Mr David Corpman | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used |
| Allocation concealment (selection bias) | Unclear risk | A lottery method was used to assign the 7 groups to a boiled water placebo control group (placebo group), glucose groups (10%, 25%, and 50% concentration (mass concentration)), and sucrose groups (12%, 24%, and 30% concentration) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There was no statement that staff and assessors were blinded to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The heel lance procedure was recorded by video. HR, oxygen saturation and pain scores were assessed at 1 min before heel lance and 3, 5 and 10 min after the heel lance. It is not stated if the assessors were blinded to intervention groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for 80 infants in each group |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Leng 2015.
| Methods | RCT Painful intervention: heel lance Study location: Children’s Hospital of Chongqing Medical University, Chongqing, and Hunan Children’s Hospital, Hunan, Shenzhen Children’s Hospital, Shenzhen, and Chengdu Women’s & Children’s Central Hospital, Chengdu, China Study period: 25 June 2012 to 25 February 2013 |
|
| Participants | New born infants (n = 671) with PMA between 37 and 42 weeks at birth; PNA between 3 and 28 days; birthweight 2500 g to 4000 g; Apgar score ≥ 8 at 5 min after birth; resting HR 120‐140 beats/min and resting oxygen saturation ≥ 95%; and requiring neonatal congenital metabolism disease screening or blood glucose test | |
| Interventions | The interventions in the 4 groups were as follows: Sucrose + routine care group: 2 mL 24% sucrose administered to the infant’s mouth by syringe 2 min before the heel lance procedure Sucrose + NNS: 2 mL 24% sucrose administered to the infant’s mouth by syringe 2 min before the heel lance procedure, and then a standard silicone newborn pacifier was placed into the infant’s mouth until the end of the process Sucrose + swaddling: infants were swaddled with a cotton blanket, upper but not lower limb movements were restricted by the blanket, and then 2 mL 24% sucrose administered to the infant’s mouth by syringe 2 min before the heel lance procedure. The lower limbs were swaddled right after the heel lance procedure until the end of the process Sucrose + NNS + swaddling): infants were swaddled with a cotton blanket, upper but not lower limb movements were restricted by the blanket, then 2 mL 24% sucrose administered into the infant’s mouth by syringe before the heel lance procedure, then a standard silicone newborn pacifier was placed into the infant’s mouth, the lower limbs were swaddled right after the heel lance procedure until the end of the process |
|
| Outcomes | Revised NFCS, increase in HR (%), decrease in oxygen saturation (%). There was no significant difference in the frequency of adverse effects (fall in HR or oxygen saturation) across groups. No adverse events were observed during the procedure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each infant was assigned a random digit by using a random number table generated with SPSS19.0 |
| Allocation concealment (selection bias) | Unclear risk | A simple calculation utilizing the random digit was used to determine a remainder (remainder = random digit/4), which was then used to decide which group the infant belonged to. For example, if the remainder was 1, then the infant was assigned to the sucrose group. If the remainder was 2, then the infant was assigned to the sucrose + NNS group. If the remainder was 3 then the infant was assigned to the sucrose + swaddling group. If the remainder was 0 then the group assigned was the sucrose + NNS + swaddling group. Randomization codes were kept in a secure location that could not be accessed by study personnel |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "Although we made efforts to limit bias from the coder, there is a possibility that the coder could still have distinguished the different groups by assessing with or without NNS". Nurses performing heel sticks were blinded to study and infant’s clinical information |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Although we made efforts to limit bias from the coder, there is a possibility that the coder could still have distinguished the different groups by assessing with or without NNS" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appears that outcome data were reported on all infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Liaw 2011.
| Methods | RCT Painful intervention: IM injection Study location: a neonatal nursery at a medical centre in Taipei, Taiwan Study period: not stated |
|
| Participants | 165 newborns, ≥ 36 weeks PMA receiving IM injections. Birthweight ≥ 2200 g, Apgar score ≥ 7 at 1 and 5 min after birth | |
| Interventions | 20% sucrose orally NNS Routine care |
|
| Outcomes | NFCS, cry duration, HR and respiratory rate | |
| Notes | We reported on cry duration in the sucrose and the routine care groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Each infant enrolled in the study was randomly assigned to 1 of 3 pain relief methods by a statistician blind to the study purpose and using random allocation software |
| Blinding (performance bias and detection bias) All outcomes | High risk | The senior research nurse was trained to follow the nursery's standard procedures for IM injections of hepatitis vaccine. The IM injection procedures were controlled to be administered within 1 min in all newborns of the 3 groups. The other research nurse was trained to offer NNS or oral sucrose adeptly to infants in the experimental groups before the IM injection procedures |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The other research assistant, blinded to the study purpose and the infants' clinical information and intervention groups, was trained to code facial actions, to score pain using the NFCS and to measure cry duration. Facial actions and cry duration were recorded using a real‐time colour video recorder. Video signals were directly transmitted to a computer, and a time code was recorded and entered into videotapes by software The NNS group was probably known to the research assistant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other biases |
Liaw 2013.
| Methods | RCT Painful intervention: heel lance Study location: Level III NICU and a neonatal special care unit at a medical centre in Taipei, Taiwan Study period: not stated |
|
| Participants | 110 infants (PMA 26.4 to 37 weeks) needing heel lances | |
| Interventions | 3 interventions were used in different combinations: NNS + FT (n = 22); FT + sucrose (n = 21); NNS + sucrose (n = 21); NNS + sucrose + FT (n = 23) Sucrose intervention: infants were fed 0.2 mL–2.0 mL 20% sucrose through a syringe 2 min before the heel lance procedures. Volume depended on the infant’s PMA (PMA 26 to 28 weeks: 0.2 mL; PMA 28.1 to 30 weeks: 0.5 mL; PMA 30.1 to 32 weeks: 1 mL; PMA 32.1 to 37 weeks: 1.5 mL; PMA > 37 weeks: 2.0 mL) NNS intervention: infants were given a standard silicone newborn pacifier to stimulate sucking 1 min before touching the foot to initiate heel lance procedures FT intervention: infants were placed in a flexed posture and gently held by the intervener’s warm hands without strongly restraining the infant’s head and body, one hand on the infant’s head, and the other on the trunk Control group: routine care (n = 23) |
|
| Outcomes | Infants’ behavioural states (quiet sleep, active sleep, transition state, active awake, quiet awake, fussing or crying) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated Infants meeting the study criteria were randomly assigned to control and treatment interventions by a statistician blind to the study purpose using Clinstat block randomization |
| Allocation concealment (selection bias) | Low risk | See random sequence generation |
| Blinding (performance bias and detection bias) All outcomes | High risk | We could not see how staff and assessors could be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | We could not see how staff and assessors could be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Marin Gabriel 2013.
| Methods | RCT Painful intervention: heel lance Study location: Hospital Puerta de Hierro‐Majadahonda, Madrid, Spain Study period: not stated |
|
| Participants | 136 healthy term neonates (PMA 37 to 41 weeks) | |
| Interventions | Sucrose group (N = 32; analyzed): 2 mL 24% sucrose given into mouth with a sterile syringe 2 min before heel lance to neonates laid supine on a cot; procedure was done in presence of mother Sucrose + skin‐to‐skin contact (SSC) group (n = 35; analyzed): neonates held prone between their mother's breasts at least 5 min before sampling and 2 mL 24% sucrose was given into mouth with a sterile syringe 2 min before heel lance SSC group (n = 31? Written to authors, could be 32): neonates held prone between their mother's breasts at least 5 min before sampling Breastfeeding (BF) + SSC group (n = 29; analyzed): neonates were held prone with skin‐to‐skin contact with mother; BF started at last 5 min before heel lance and was maintained during sampling Mothers were allowed to speak and touch their babies in all groups |
|
| Outcomes | NIPS; HR; crying time (s); crying in blood sampling; number of heel lances (%) Data presented as medians and IQRs, HR as means with SDs |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | High risk | Randomization was by closed envelopes and 268 opaque (but not sequentially numbered) envelopes with the group assignment were prepared at the beginning of the study and mixed. Parents selected 1 envelope |
| Blinding (performance bias and detection bias) All outcomes | High risk | Nurses and parents were not blinded to the treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Nurses and parents were not blinded to the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the sucrose group 1 infant was not analyzed because of technical problem. In the BF + SSC group 3 infants were excluded from the analysis because of non‐effective BF, 2 because of incorrect SSC and 1 because of technical problem. All participants from the sucrose + SSC group were included. In the SSC group there were technical problem in 1 infant but Figure 1 in the trial report indicates that 31/33 infants were analyzed, and we do not know what happened to this 1 infant |
| Selective reporting (reporting bias) | Low risk | Trial registration number (ClinicalTrials.gov): NCT01576432. There did not seem to be any deviations from the protocol in the full report |
| Other bias | Low risk | Appears free of other bias |
Mathai 2006.
| Methods | Randomized study (blinded for cry but not for DAN) Painful intervention: heel lance Study location: transitional care unit and postnatal ward of a large, teaching hospital, Mumbai, India Study period: not stated |
|
| Participants | 104 term neonates > 24 h old Sucrose group mean PNA = 48 h Distilled water group mean PNA = 44 h | |
| Interventions | 2 mL 20% sucrose (n = 17) 2 mL distilled water (n = 15) 2 mL expressed breast milk (n = 18) NNS (n = 20) Rocking (n = 17) Massage (n = 17) |
|
| Outcomes | DAN before heel prick and 30 s and 1, 2 and 4 min after heel prick; time of first cry (s) (i.e. until baby took first inspiration after beginning of cry), total cry (s); HR and oxygen saturation (SpO2) before heel prick and 2 and 4 min after heel prick | |
| Notes | Results were graphed and reported as means and SDs (2 SD)
ANOVA, Fischer's exact 't' test, multivariate analysis, Pearson's correlation test ‐ some P values were reported Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by random number table |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Interventions could not be blinded. 1 observer left the room during the intervention to be able to assess the DAN score blindly. A second observer was in the room during the interventions and was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 1 observer left the room during the intervention to be able to assess the DAN score blindly. A second observer was in the room during the interventions and was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Physiological parameters (HR, oxygen saturation) not reported, but were listed as outcomes variables in the Methods sections |
| Selective reporting (reporting bias) | Unclear risk | Physiological parameters (HR, oxygen saturation) not reported, but were listed as outcomes variables in the Methods sections |
| Other bias | Low risk | Appears free of other bias |
McCullough 2008.
| Methods | Randomized, double‐blind, controlled trial Painful intervention: NG tube insertion Study location: Department of Child Health, Rotherham General Hospital, Rotherham, South Yorkshire, UK Study period: not stated |
|
| Participants | 20 preterm infants, mean PMA 30 weeks Sucrose group: mean PNA 23 days Water group: mean PNA 27 days |
|
| Interventions | Total of 51 NG tube insertions. Each infant was randomised to either the water or sucrose group prior to each insertion. This was not a cross‐over study. In each instance where NG tube insertion was required, the infant was randomised separately and independently of any previous allocation to receive either placebo (sterile water) or 24% sucrose solution 0.5 mL to 2 mL 24% sucrose (n = 26) 0.5 mL to 2 mL sterile water (n = 25) |
|
| Outcomes | Incidence of crying, HR, oxygen saturation, NFCS (median) | |
| Notes | Incidence of crying reported as percentage of neonates who cried. HR and oxygen saturation measured as change (in beats/min and percentage saturation, respectively) from baseline Adverse events were evaluated; brief apnoea and self‐limiting bradycardia reported in a few neonates, but no clinical intervention needed. No statistically significant differences between sucrose and water groups regarding incidence of adverse events |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list |
| Allocation concealment (selection bias) | Low risk | Used sealed opaque envelopes to allocate to groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions administered in a blinded manner |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about why enrolled infants did not participate in the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Unclear risk | Infants were randomised several times ‐ could they be "remembering" their previous experience, which may affect the results? |
Milazzo 2011.
| Methods | Double‐blind, RCT Painful intervention: arterial puncture Study location: a 40‐bed, Level III, NICU of a 564‐bed community‐based hospital in midwest USA Study period: 12‐month period (Dates not reported) |
|
| Participants | 47 neonates, 30 to 36 weeks’ PMA, 48 h old, nil by mouth status, and medical requirement for an arterial puncture | |
| Interventions | Sucrose group (n = 24): 0.5 mL solution oral sucrose (Sweet‐Ease, preservative‐free, 24% sucrose solution 99044, Children’s Medical Ventures, Norwell, Massachusetts) given in a 1 mL syringe 1‐3 min before arterial puncture. A pacifier was then held in place lightly and infant's extremities swaddled by nurses assigned to infant’s care. Arterial puncture was performed by another nurse Control group (n = 23): a pacifier was held in place lightly and infant's extremities swaddled by nurses assigned to infant’s care. Arterial puncture was performed by another nurse |
|
| Outcomes | NIPS, HR, oxygen saturation (%) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to groups was done using a computer randomization scheme |
| Allocation concealment (selection bias) | High risk | Envelopes were sealed, but not sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Immediately prior to the therapeutically required arterial puncture, the study investigator left the infant’s room while the nurse caring for the infant opened the sealed envelope containing the randomization assignment to treatment group. Infants assigned to the sucrose solution treatment group were then given a 0.5 mL solution of oral sucrose in a 1‐mL syringe by the nurse caring for the infant. Infants assigned to the placebo group did not receive any oral solution The investigator was then called back to the infant’s bedside, remaining blinded to treatment group assignment, and obtained baseline study data (NIPS; HR; oxygen saturation) immediately after the arterial puncture needle was inserted and again 1 min after the arterial puncture procedure was completed For infants assigned to the sucrose treatment group, the time from sucrose administration to arterial puncture was at least 1 min but not more than 3 min. Group assignment codes were not revealed to study investigators until study enrolment was completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See blinding of participants and personnel. Group assignment codes were not revealed to study investigators until study enrolment was completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were presented for 47/49 infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Mitchell 2004.
| Methods | Randomized, double‐blind, placebo‐controlled trial Painful intervention: screening for ROP Study location: level‐3 university‐affiliated NICU, Monroe, Louisiana, USA Study period: February 2002 to August 2002 |
|
| Participants | 30 preterm infants Sucrose group: mean PMA 26.5 weeks, mean PNA 8.5 weeks Water group: mean PMA 27.3 weeks, mean PNA 8.2 weeks |
|
| Interventions | Sucrose group (n = 15): pacifier and 3 doses of 0.1 mL 24% sucrose drops Water group (n = 15): pacifier and 3 doses of 0.1 mL sterile water drops Both groups received proxymetacaine hydrochloride 0.5% and were swaddled before the eye examination |
|
| Outcomes | PIPP at baseline; at eye drops instillation; during examination of left eye and at 30 s, 60 s, 90 s and 120 s after completion of the eye examination | |
| Notes | Results were in graph form and reported as means and standard errors of the means. A series of t‐tests were conducted and their P values reported Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | High risk | Allocated using sealed envelopes, but did not specify whether envelopes were opaque or sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions administered blinded to staff Nurse administering interventions was aware of group allocation. All other personnel and investigators were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Montoya 2009.
| Methods | Randomized, double‐blind, controlled trial Painful intervention: venipuncture Study location: Unidad de Cuidado Intensivo Neonatal, Clinica Universitaria Bolivariana, Medellin, Colombia Study period: January to June 2008 |
|
| Participants | 111 preterm and term infants: 55 in sucrose group, 56 in water group | |
| Interventions | 1 mL 12% sucrose 1 mL water |
|
| Outcomes | NIPS score | |
| Notes | Trial report was written in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Low risk | Coded solutions |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Staff were blinded to the solutions used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Trial report did not mention blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data reported on all 111 randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Mucignat 2004.
| Methods | Randomized, prospective, cross‐over study Painful intervention: SC injection Study location: Service de Néonatologie, Hôpital Armand‐Trousseau, Paris, France Study period: 1 April to 31 August 2002 |
|
| Participants | 33 preterm neonates, < 33 weeks' PMA Mean ± SD PMA at birth: 30 ± 6 weeks Mean ± SD PMA at injection: 32 ± 6 weeks |
|
| Interventions | NNS group: non‐nutritive pacifier Sucrose + NNS group: 0.2 mL to 0.5 mL 30% sucrose with pacifier EMLA + NNS group: local application of EMLA cream with pacifier Sucrose + EMLA + NNS group: 0.2 mL to 0.5 mL 30% sucrose with EMLA and pacifier |
|
| Outcomes | DAN and NFCS scores; HR, respiratory rate and oxygen saturation (%) measured before, during and after injection | |
| Notes | Fisher test, ANOVA of fixed‐effect and the Tukey method were used to compare groups Results were reported as means and SDs Each infant acted as its own control. For each consecutive EPO injection, patients were randomised between four groups of intervention. The numbers of injections reported for the different interventions in Table 1 of the trial report varied from 41 to 86. NNSgroup; n = 41; Sucrose + NNS group; n = 86; EMLA + NNS group; n = 71; and Sucrose + EMLA + NNS group; n = 67. Data could not be used in RevMan‐analyses Adverse effects were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Interventions could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not state that outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Each infant acted as its own control. The numbers reported for the different interventions in Table 1 vary from 41 to 86 |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | High risk | Unequal distribution of allocated and received treatments amongst injections: NNS; n = 41, EMLA + NNS; n = 71, sucrose + NNS; n = 86, sucrose + EMLA + NNS; n = 67 |
O'Sullivan 2010.
| Methods | Randomized, prospective, placebo‐controlled study Painful intervention: screening for ROP Study location: Dublin, Ireland Study period: not stated |
|
| Participants | 40 preterm infants Water group: mean PMA ± SD = 29.5 ± 2.3 weeks, mean corrected age at first eye examination ± SD = 33.1 ± 1.2 weeks Sucrose group: mean PMA ± SD = 29.8 ± 2.4 weeks, mean corrected age at first eye examination ± SD = 33.0 ± 1.1 weeks |
|
| Interventions | Sucrose group: 0.2 mL 24% sucrose given by mouth using a syringe and a pacifier (N = 20) Water group: 0.2 mL sterile water given by mouth using a syringe and a pacifier (N = 20) Both groups were swaddled |
|
| Outcomes | N‐PASS; HR and oxygen saturation at baseline; number of episodes of bradycardia, desaturation | |
| Notes | Recorded number of adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomization process used |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque and sealed envelopes used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Only pharmacist was aware of the identify of solutions, all other personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Ogawa 2005.
| Methods | Randomized, double‐blind, placebo‐controlled trial Painful intervention: heel lance and venipuncture Study location: NICU of Osaka Medical College, Osaka, Japan Study period: November 1999 to March 2000 |
|
| Participants | 100 healthy, full‐term infants ≥ 37 weeks' PMA | |
| Interventions | 1 mL sterile water 2 min before heel lance (n = 25) 1 mL 50% sucrose 2 min before heel lance (n = 25) 1 mL sterile water 2 min before venipuncture (n = 25) 1 mL 50% sucrose 2 min before venipuncture (n = 25) |
|
| Outcomes | NFCS score after skin puncture, during blood sampling and during compression to stop bleeding; duration of first cry, ratio of crying to no crying, total procedure time | |
| Notes | Intergroup comparisons were performed by the Kruskal‐Wallis test Mann Whitney U test for continuous variables or by the Chi2 test for categorical data Results were reported as medians and ranges and means and SDs. P values were reported Adverse effects were evaluated for the procedure itself, not sucrose |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | High risk | 100 sealed envelopes. Did not state if they were opaque and sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions were administered blindly |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data provided for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appeears free of other bias |
Okan 2007.
| Methods | Randomized, blinded, cross‐over trial Painful intervention: heel lance Study location: NICU, Istanbul, Turkey Study period: not stated |
|
| Participants | 31 preterm infants: mean (SD) PMA 30.5 weeks (2.7); PNA 20 days (16) | |
| Interventions | 2 mL sterile water
2 mL 20% sucrose
2 mL 20% glucose All solutions were given 2 min before heel lance. The infants were tested 3 times in a cross‐over manner |
|
| Outcomes | HR, respiratory rate, oxygen saturation and NFCS score at baseline, heel lance and at 1, 2, 3 and 4 min post heel lance; duration of first cry and total crying time | |
| Notes | The differences in duration of crying time and blood collection were analyzed using the Friedman test Results were reported as means and SDs Adverse effects were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes drawn randomly to determine sequence |
| Allocation concealment (selection bias) | High risk | Allocated by sealed envelopes. Solutions contained in identical bottles coded by nurse who was not part of the study. Did not mention whether envelopes were opaque or sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions were provided blinded to staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Videotape records were later analyzed by two observers who were not aware of which solution was used. Each observer assessed the data independently and could not communicate their findings to the other.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Overgaard 1999.
| Methods | Double‐blind RCT Painful intervention: heel lance Study location: Department of Obstetrics and Gynaecology, Aalborg University Hospital, Aalborg, Denmark Study period: 3 months (dates not provided) |
|
| Participants | 100 newborn term infants, mean age 6 days (range 4 to 9) | |
| Interventions | 2 mL 50% sucrose solution via syringe into the mouth over 30 s, 2 min prior to heel lance (n = 50) 2 mL sterile water via syringe into the mouth over 30 s, 2 min prior to heel lance (n = 50) | |
| Outcomes | NIPS score, crying time (duration of first cry, crying time during heel lance, fraction of crying during sampling, crying time during first minute after end of sampling, total crying time), NIPS 1 min after heel lance and 1 min after blood sampling, change in HR at 0 min and 1 min, change in oxygen saturation at 0 min and 1 min | |
| Notes | Results were reported as medians and 5% and 95% percentiles ‐ we did not attempt to convert to means and SDs. Four infants were excluded after randomisation due to failure of the videotaping leaving 96 newborns for analysis; sucrose n = 49; placebo n = 47 Statistical testing used Mann Whitney U and Fisher's exact test Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | 100 syringes manufactured at random to contain sucrose or water. Numbered and administered consecutively. Contents were unknown to investigators and parents |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions were administered blinded to investigators and parents |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 infants were excluded due to failure of videotaping, leaving 96 newborns for analysis |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Pandey 2013.
| Methods | RCT Painful intervention: OG tube insertion Study location: NICUs of Kalawati Saran Children's Hospital, Lady Hardinge Medical College, New Delhi, India Study period: not stated |
|
| Participants | 120 clinically stable preterm infants (< 37 weeks PMA) were enrolled within the first 7 postnatal days, they had not received any painful stimulus 30 min prior to intervention, and required routine OG tube insertion. 105 infants were analyzed | |
| Interventions | 1 mL 24% sucrose administered to the tongue 2 min before OG tube insertion. Total number randomized: n = 60; final analysis n = 53 1 mL distilled water administered to the tongue 2 min before OG tube insertion. Total number randomized: n = 60; final analysis n = 52 |
|
| Outcomes | The primary outcome was the response to the pain, assessed by the PIPP scale, prior to the procedure, during the procedure, and at 30 s, 1 min and 2 min postprocedure Secondary outcomes were the maximum HR and minimum oxygen saturation recorded during the procedure |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used block randomization with computer generated random sequences and a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was done by the hospital pharmacy which packed 2 mL of the sucrose and the double distilled water into syringes and provided opaque sealed envelopes sequentially labelled according to randomization code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 2 min prior to the procedure, 1 mL of the solution marked with infant's serial number was administered orally to the infant by a healthcare provider (blinded to the contents of the solution) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A consultant of the unit, who was unrelated to the study and was blinded to the study methodology, evaluated the video‐recordings and assigned the PIPP scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 infants were excluded from the sucrose group as the monitor malfunctioned, and 1 infant in the sucrose group went into sudden cardiorespiratory arrest and had to be excluded. In the water group the OGT was displaced before the 2 min post procedure PIPP scores could be assigned for 3 infants, and the monitor malfunctioned for 5 infants. 53 infants were analyzed in the sucrose group and 52 in the water group (88% of enrolled infants) |
| Selective reporting (reporting bias) | Low risk | The protocol for the study was available to us and there did not seem to be any deviation from the protocol. ClinicalTrials.gov (Registration number: NCT 00949104) |
| Other bias | Low risk | Appears free of other bias |
Potana 2015.
| Methods | RCT Stressful intervention: echocardiography Study location: a level III NICU in Gujarat, India Study period: August to November 2013 |
|
| Participants | 104 neonates with established enteral feeding, not on any respiratory support and with PMA between 32 and 42 weeks requiring echocardiography Exclusion criteria: neonates who were nil by mouth, had poor neurological status, and those who were paralysed or sedated with pharmacological agents |
|
| Interventions | Sucrose group: Arbineo 24% w/v oral solution, dose: 1 mL for infants 32 to 40 weeks PMA, 2 mL for infants > 40 weeks PMA, administered 2 min prior to echocardiography by a dropper Control group: no medication or placebo |
|
| Outcomes | PIPP, adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated using GraphPad software |
| Allocation concealment (selection bias) | High risk | Sealed opaque envelopes but not stated if they were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | Could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators performing the video analysis were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled infants were analyzed |
| Selective reporting (reporting bias) | Unclear risk | The study was not entered into a trials registry and the protocol for the study was not available to us. We could not judge if there was a deviation from the protocol or not |
| Other bias | Low risk | Appears free of other bias |
Ramenghi 1996a.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study Study intervention: heel lance Study location: Leeds General Infirmary, Leeds, UK Study period: not stated |
|
| Participants | 15 infants (32 to 34 weeks' PMA) > 24 h of age | |
| Interventions | 1 mL 25% sucrose via syringe into mouth 2 min prior to heel lance 1 mL sterile water via syringe into mouth via syringe 2 min before heel lance Cross‐over design | |
| Outcomes | Duration of first cry (s) following heel lance, percentage of time crying 5 min after heel lance, HR (at ‐2, 0, 1, 3, 5 min from heel lance), behavioural scores (4 facial expressions and the presence of cry at ‐2, 0, 1, 3, 5 min from heel lance) | |
| Notes | Medians and ranges were reported for duration of first cry, percentage cry over 5 min and HR. For composite behavioural outcome scores data were presented in graph form only with no indication if data represented medians or means. Wilcoxon matched pairs signed rank test used to evaluate outcomes Adverse effects were evaluated. Cross‐over study and results from the first assignment to sucrose or water were not reported. No data available to use in meta‐analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Unsure if staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unsure of whether outcome assessments were performed blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Ramenghi 1996b.
| Methods | Randomized, single‐blind, placebo‐controlled trial Painful intervention: heel lance Study location: Leeds General Infirmary, Leeds, UK Study period: not stated |
|
| Participants | 60 infants (37 to 42 weeks' PMA) 2 to 5 days old | |
| Interventions | 2 mL 25% sucrose via syringe into mouth 2 min prior to heel lance (n = 15) 2 mL 50% sucrose via syringe into mouth 2 min prior to heel lance (n =15) 2 mL commercial sweet‐tasting solution (Calpol) via syringe into mouth 2 min prior to heel lance (N = 15) 2 mL sterile water via syringe into mouth 2 min prior to heel lance (n = 15) | |
| Outcomes | Duration of first cry (s) following heel lance, percentage time crying over 3 min following heel lance, percentage change in HR over 7 min (‐2, 0, 1, 3, 5 min from heel lance), behavioural scores (4 facial expressions and the presence of cry (‐2, 0, 1, 3, 5 min after heel lance) | |
| Notes | Results were presented as medians and IQRs for the pain score. For cry duration and percentage crying over 3 min the data were presented as medians and IQRs. Percentage change in HR was reported in graph form without indicating whether the data represented means or medians with SDs or errors. Mann‐Whitney U test used to evaluate outcomes Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators were blind to the nature of the sucrose and water solutions, but the Calpol could not be disguised because of its pink colour |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments were performed blinded to groups for sucrose and water but not for Calpol |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Ramenghi 1999.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over trial for the mode of delivery of sucrose or water by mouth or intragastrically Painful intervention: heel lance Study location: Leeds General Infirmary, Leeds, UK Study period: Dates not provided |
|
| Participants | 30 preterm infants (PMA 32 to 36 weeks, PNA < 24 h) | |
| Interventions | Each infant received either sucrose or water and was not crossed over for the solution received, only for the method of delivery Sucrose group (n = 15): 25% sucrose solution (volume not reported) given via syringe into the mouth or via NG tube 2 min prior to first heel lance, and via the alternate route for the second heel lance within 48 h Water group (n = 15) : sterile water via syringe into the mouth or via NG tube 2 min prior to first heel lance and via the alternate route for the second heel lance within 48 h Cross‐over design |
|
| Outcomes | Percentage crying over 5 min after sampling, behavioural scores (4 facial expressions and the presence of cry) at 1, 3 and 5 min after the lance for a total behavioural score | |
| Notes | Mann Whitney‐U and Wilcoxon matched pairs signed ranked test used to evaluate outcomes Results reported as median and IQR and total range Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not describe measures taken to ensure blinding of intervention and outcome assessment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not describe measures taken to ensure blinding of intervention and outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Rogers 2006.
| Methods | Randomized, double‐blind trial Painful intervention: bladder catheterization Study location: a single tertiary‐care dedicated paediatric emergency department, USA Study period: June 2003 to November 2004 |
|
| Participants | 83 infants ≤ 90 days old, born at least 34 weeks' PMA were enrolled and randomized, 3 infants were withdrawn after randomization Separated into 3 age groups:
|
|
| Interventions | 2 mL 24% sucrose via syringe 2 min before bladder catheterization (n = 40) 2 mL sterile water via syringe 2 min before bladder catheterization (n = 40) |
|
| Outcomes | DAN scale, percentage cry, time to return to behavioural baseline | |
| Notes | Post hoc subgroup analyses, t‐tests, Chi2 tests, Mann‐Whitney test, ANOVA and Breslow‐Day (BD) test for homogeneity used to evaluate outcomes Results were reported as means and SDs. P values were reported Adverse events were evaluated; no adverse effects experienced |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generated by random number table |
| Allocation concealment (selection bias) | Low risk | Syringes were coded by pharmacy and solutions were indistinguishable |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurses were blinded to the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 infants were enrolled and randomized, but 3 were withdrawn after randomizations as a result of inappropriate enrolment or withdrawal of consent |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Rush 2005.
| Methods | Prospective RCT Painful intervention: screening for ROP Study location: NICU at a university‐affiliated hospital, Amarillo, Texas, USA Study period: not stated |
|
| Participants | 30 infants < 32 weeks' GA or weighing < 1500 g Sucrose group mean GA 29.57 weeks (range 26 to 32) Control group mean GA 28.8 weeks (range 25 to 31) |
|
| Interventions | Sucrose group: swaddled in a warm blanket, pacifier packed with gauze soaked in 24% sucrose and held by a nurse until 15 min after the eye examination Control: no swaddling, no sucrose and not held by nurse All infants received eye drop instillation of 0.5% proxymetacaine and 1% tropicamide, then 15 min later eye drop instillation of 0.5% tropicamide and 2.5% phenylephrine All eye drops were instilled into both infant's eyes before the ROP examination |
|
| Outcomes | Pulse rate, respiratory rate and oxygen saturation at baseline (30 min before instillation of proxymetacaine), 5 min before eye examination, 3 different times during eye examination and 5 min after the completion of the examination; total crying time; time required to return to baseline value | |
| Notes | ANOVA and Wilcoxon signed rank test and the Pearson test were used to evaluate outcomes Results were reported as medians, means and standard errors of the means (SEM). P values were also reported Adverse events were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding to interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Pulse rate, respiratory rate data not reported |
| Selective reporting (reporting bias) | Unclear risk | Pulse rate, respiratory rate data not reported |
| Other bias | Low risk | Appears free of other bias |
Rushforth 1993.
| Methods | Randomized, double‐blind, placebo‐controlled study Painful intervention: heel lance Study setting: Leeds General Infirmary, Leeds, UK Study period: not stated |
|
| Participants | 52 infants, 37 to 42 weeks' PMA, 2 to 7 days old | |
| Interventions | 2 mL 7.5% sucrose administered by a dropper into the mouth over a 1‐min period prior to heel lance (n = 26) 2 mL sterile water administered by dropper into the mouth over a 1‐min period prior to heel lance (n = 26) | |
| Outcomes | Percentage time crying during sampling and 3 min following the completion of the heel lance recorded on a standard audio tape recorder and analyzed blindly at a later date | |
| Notes | Results presented as medians only with no ranges Mann Whitney U test to evaluate duration of cry Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not clear whether staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Simonse 2012.
| Methods | RCT Painful intervention: heel lance Study location: neonatal ward of Amphia Hospital, a secondary health care centre in Breda, the Netherlands Study period: January 2010 to May 2011 |
|
| Participants | 71 preterm infants, PMA 32 to 36 6/7 weeks at birth undergoing heel lance with an automated piercing device | |
| Interventions | Sucrose group (n= 25; 24 analyzed): neonates lay in their cots and received 1 mL to 2 mL 24% sucrose solution 2 min before the heel lance, combined with NNS and Newborn Individualized Developmental Care and Assessment Breastfed group (n = 23; all analyzed): infants were breastfed while held in mothers' arms, heel lance was performed after continuous sucking was observed (i.e. during feeding) Bottle‐fed group (n = 23; all analyzed): infants were bottle‐fed breast milk. The non‐breast feeding group infants were held in arms of an experienced nurse and were given supplemental breast milk by a sterile syringe and heel lance was performed after continuous sucking was observed |
|
| Outcomes | PIPP score; COMFORTneo Score, HR and SpO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was created by using a fixed block size of 8 for a maximum of 75 neonates with a 1:1:1 allocation |
| Allocation concealment (selection bias) | Low risk | Neonates were allocated to 1 of the 3 groups according to the method of sequentially numbered and opaque sealed envelopes created by an independent employee and masked for the investigator |
| Blinding (performance bias and detection bias) All outcomes | High risk | It was not possible to blind investigators for the allocated intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It was not possible to blind investigators for the allocated intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 infant was excluded from the sucrose group |
| Selective reporting (reporting bias) | Low risk | This trial was registered at www.clinicaltrials.gov (identifier NCT01276366). There did not seem to be any deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Slater 2010.
| Methods | Randomized, prospective study Painful intervention: heel lance Study location: Elizabeth Garret Anderson Wing, University College Hospital, London, UK Study period: 25 February 2009 to 25 March 2010 |
|
| Participants | 59 infants; 29 assigned to sucrose group and 30 to water group 44 term infants 37 to 43 weeks' PMA, < 8 days old were included in the analysis of the primary outcome (pain‐specific brain activity recorded with electroencephalography and identified by principal component analysis) |
|
| Interventions | Sucrose group (n = 20): 0.5 mL 24% sucrose given via syringe Water group (n = 24): 0.5 mL sterile water |
|
| Outcomes | HR change, PIPP score, nociceptive‐specific brain activity, latency to change in facial expression(s), facial non‐responders, nociceptive reflex withdrawal activity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomized code |
| Allocation concealment (selection bias) | Low risk | Only the hospital pharmacy had access to the randomization codes that could be used to identify the solution. A sealed copy of the randomization chart was also stored in the neonatal unit in case an adverse event was reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, personnel and outcome assessors blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 59 infants were enrolled, but the primary outcome was ascertained in 44 infants (75%) |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Stang 1997.
| Methods | Prospective, randomized, double‐blind, placebo‐controlled trial Painful intervention: circumcision Study location: Fairview Riverside Medical Center, Minneapolis, Minnesota, USA Study period: 1993 to 1994 |
|
| Participants | 80 healthy term male newborns, mean PMA 39.5 weeks, mean PNA 31.5 h | |
| Interventions | DPNB with non‐buffered lidocaine (0.8 mL lidocaine, 0.2 mL saline), new padded restraint chair and pacifier dipped in water (n = 20) DPNB with buffered lidocaine (0.8 mL lidocaine, 0.2 mL sodium bicarbonate), rigid plastic restraint chair and pacifier dipped in water (n = 20) DPNB with non‐buffered lidocaine (0.8 mL lidocaine, 0.2 mL saline), rigid plastic restraint chair and pacifier dipped in 24% sucrose (n = 20) DPNB with non‐buffered lidocaine (0.8 mL lidocaine, 0.2 mL saline), rigid plastic restraint chair and pacifier dipped in water (n = 20) |
|
| Outcomes | Behavioural Distress Scale (scores prior to injection, at injection for DPNB, 2 min post injection, 4 min post injection and at circumcision); plasma cortisol level (30 min after start of circumcision); percentage of sleep during circumcision | |
| Notes | Results were reported as mean and SDs
ANOVA with repeated measures were used to compare distress scores. 1‐way ANOVAs were used to examine plasma cortisol and sleep data Adverse effects were not evaluated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data results did not specify number of infants with data |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Stevens 1999.
| Methods | Randomized, cross‐over, controlled trial Painful intervention: heel lance Study location: NICUs of 3 metropolitan university‐affiliated teaching hospitals and 1 children's hospital in Canada and the USA Study period: a 15‐month period (dates not given) |
|
| Participants | 122 preterm neonates, 27 to 31 weeks' PMA, < 28 days old | |
| Interventions | Each infant received all 4 interventions in a random order (serving as his/her own control); there was 1 control intervention and 3 treatment interventions
|
|
| Outcomes | PIPP | |
| Notes | Repeated measures ANOVA and ANCOVA used to evaluate efficacy of treatment interventions
Means and SDs provided for pain scores prior to cross‐over for sucrose + pacifier and water + pacifier
Adverse effects were evaluated The first author provided us with unpublished information regarding the study methods. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was enclosed in sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study solutions were prepared in each of the study units by the Pharmacist and labelled as Study Solution 1 and 2. The research nurses (who performed the heel lance and administered each intervention) drew up the solution specified in the intervention sequence from a dark coloured bottle, so were blind to which solution (e.g. water or 24% sucrose) was used. Nurses were not blind to the prone positioning or control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study nurse collected all of the data (e.g. videotaped facial expressions, recorded cry) and was blind to study solutions; the data coders were also blinded to which study solution was used ‐ but NOT to the prone positioning or control, as they could visualize this on the videotapes. The data analysts were blinded to all of the interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Low risk | The primary author assured us that there were no deviations from the protocol |
| Other bias | Low risk | Appears free of other bias |
Stevens 2005a.
| Methods | Prospective RCT Painful intervention: heel lance and other procedures Study location: 1 tertiary‐level NICU in Canada Study period: not stated |
|
| Participants | 66 preterm infants, 26 to 30 weeks' PMA, < 72 h PNA | |
| Interventions | No intervention (N = 22)
0.1 mL 24% sucrose via syringe and pacifier (n = 23) 0.1 mL sterile water via syringe and pacifier (n = 21) Solutions were given 2 min before every procedure during the first 28 days of life |
|
| Outcomes | PIPP, neonatal clinical outcomes and neurobiological risk scores | |
| Notes | Actual PIPP scores (mean, standard deviation) were not reported. PIPP scores were analyzed by RMANOVA. Chi2 analyses were used to compare the incidence of immediate and long‐term adverse events Adverse events were evaluated. Adverse events were reported as 'low' and all immediate adverse events resolved spontaneously |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Delivered to baby by pharmacist. Solutions carried in dark glass bottles. Water and sucrose solutions appeared to be the same |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 infants dropped out of the study prior to any data collection |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Storm 2002.
| Methods | RCT Painful intervention: heel lance Study location: Section of Neonatology, Department of Paediatrics, the National Hospital, Oslo, Norway Study period: not reported |
|
| Participants | 48 preterm infants, median PMA 32 weeks, median PNA 14 days | |
| Interventions | 2 mL 15% sucrose (n = 12) 1 mL 25% sucrose, n = 12) Milk via NG tube, (n = 12) Milk via NG tube + 25% sucrose, (n = 12) All infants were given water prior to a second heel lance Oral solutions were administered via syringe into infant's mouth 2 min prior to heel lance NG tube solutions (milk) given during the last hour prior to heel lance | |
| Outcomes | Changes from before heel lance to during heel lance for: crying time, changes in behavioural state, skin conductance, HR | |
| Notes | Paired non‐parametric tests (Wilcoxon test) used to compare the infant's intervention and control session No median or IQR reported for each outcome Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. Randomly divided into 4 groups |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 2 groups fasting; 2 groups fed (milk) during the last hour prior to heel lanceup via NG tube |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not provide any information about blinding of assessor |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on number of participants included in the Methods or Results sections. Results in figures and P values only. Presented no data that could be meta‐analysed |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us, so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Suhrabi 2014.
| Methods | RCT Painful intervention: vaccination pain (hepatitis B) Study location: Shahid Mostafa Khomini Hospital of Ilam, Islamic Republic of Iran Study period: May 2013 to June 2013 |
|
| Participants | 90 full‐term neonates, who were not receiving analgesics, not receiving nutrition during 30 min before vaccination and absence of diarrhoea and common cold | |
| Interventions | The hepatitis B vaccine was injected 2 min after administration of sucrose, glucose or no treatment and pain severity measured by the NIPS scale during 1‐2 min Sucrose group (n = 30): 2 mL 25% sucrose through a syringe in 30 s Glucose group (n = 30): 2 mL 25% glucose through a syringe in 30 s Control group (n = 30): no intervention |
|
| Outcomes | NIPS during 1‐2 min after vaccine injection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The cases were randomly (simple randomization method) divided into 3 groups of 30 neonates each |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | The cases were randomly (simple randomization method) divided into 3 groups of 30 neonates each |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The cases were randomly (simple randomization method) divided into 3 groups of 30 neonates each |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | NIPS reported on all 90 infants |
| Selective reporting (reporting bias) | Low risk | The registered protocol was available to us and there did not seem to have been any deviation from the protocol. IRCT 201304096790N3). Date registered: 22 May 2013. Registration timing: Registration while recruiting |
| Other bias | Low risk | Appears free of other bias |
Taddio 2008.
| Methods | Double‐blind, RCT Painful intervention: IM injections, venipunctures and heel lances Study location: Mount Sinai Hospital, Toronto, Ontario, Canada Study period: 15 September 2003 to 27 July 2004 |
|
| Participants | 240 newborns, mean PMA 38.7 to 39.9 weeks, mean PNA 0.5 h to 0.8 h | |
| Interventions | 2 mL 24% sucrose given to newborns of non‐diabetic mothers (n = 60) 2 mL sterile water given to newborns of non‐diabetic mothers (n = 60) 2 mL 24% sucrose given to newborns of diabetic mothers (n = 60) 2 mL sterile water given to newborns of diabetic mothers (n = 60) Solutions were given before all IM injections, venipunctures and heel lances during the first 2 days of life |
|
| Outcomes | PIPP score during procedure | |
| Notes | Student's t‐test used to compare average PIPP scores between groups. Post hoc analyses were performed after adjusting for baseline characteristics by use of a general linear model for IM injection and venipuncture and linear mixed‐model analysis for heel lances. Adverse events were analyzed using the Chi2 test or the Student t‐test Adverse effects were reported ‐ no significant differences between groups in the incidence of adverse events, which included spitting up and blood glucose levels |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table: allocation was done on a 1:1:1 basis |
| Allocation concealment (selection bias) | Low risk | Centralized at the hospital pharmacy. Solutions carried in identical bottles only labelled with patient identification |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessments blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Taddio 2011.
| Methods | RCT Painful intervention: venipuncture Study location: Mother and Infant Unit, Mount Sinai Hospital, Toronto, Ontario, Canada Study period: 20 August 2007 to 22 February 2009 |
|
| Participants | 330 infants mean PMA (SD) 39.5 weeks (1.2) Liposomal lidocaine group (n = 110) mean PMA (SD) 39.6 weeks (1) Sucrose group (n = 110) mean PMA (SD) 39.6 weeks (1.3) Sucrose liposomal lidocaine group (n = 110) mean PMA (SD) 39.6 weeks (1.3) |
|
| Interventions | Liposomal lidocaine group: 1 g liposomal lidocaine 4% cream to the dorsum of the hand, occluded by a dressing (Tegaderm) for 30‐40 min Sucrose group: 2 mL 24% sucrose solution, administered by mouth using a syringe over 1‐2 min Sucrose liposomal lidocaine group: both sucrose and liposomal lidocaine as described above Placebos were used for liposomal lidocaine and sucrose (i.e. double‐dummy design), so that all infants received a topically administered cream (liposomal lidocaine or placebo cream) and oral solution (sucrose or placebo water) |
|
| Outcomes | Facial grimacing, cry duration (s),observer‐rated pain using a visual analogue scale (VAS) (0 to 10 cm), HR (beats/min), oxygen saturation (%) | |
| Notes | Reported no significant adverse effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used |
| Allocation concealment (selection bias) | Low risk | Concealment of treatment allocation was achieved by carrying out randomization and dispensing functions offsite |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy outcomes were not reported in 9 infants and safety outcomes were not repotted in 2 infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Thakkar 2016.
| Methods | RCT Painful intervention: heel lance Study location: tertiary‐level NICU in Western India Study period; 1 year, dates not stated |
|
| Participants | Participants were full‐term infants (>37 weeks PMA), with birthweight > 2200 g, > 24 h old and were exclusively breastfed Exclusion criteria: neonates with history of birth asphyxia, sepsis, meningitis, respiratory distress, congenital malformations, receiving nil by mouth, being mechanically ventilated and who received any pharmacological agent for analgesia in the 72 h before enrolment |
|
| Interventions | Sucrose group (n = 45): 30% sucrose solution delivered by sterile syringe Sucrose + NNS (n = 45): 30% sucrose solution delivered by sterile syringe plus NNS NNS group (n = 45): sterile gauze was held gently in neonate’s mouth and the palate tickled to stimulate sucking No treatment group (n = 45): received no treatment |
|
| Outcomes | PIPP, total crying time. Results presented as medians and IQRs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐sequence numbers |
| Allocation concealment (selection bias) | High risk | Opaque sealed envelopes were used. Did not say if the envelopes were sequentially numbered |
| Blinding (performance bias and detection bias) All outcomes | High risk | Staff knew if the infants were given sucrose by syringe or if they were given NNS or no intervention. Quote: ” ... blinding was not possible because the interpreter was performing the procedure” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | ” ... blinding was not possible because the interpreter was performing the procedure” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Tutag Lehr 2015.
| Methods | RCT Painful intervention: heel lance Study location: Hutzel Harper University Hospital, Detroit, MI, USA Study period 2005 to 2007 |
|
| Participants | 56 term infants ≤ 7 days old: appropriate for PMA (weight between 5th to 95th percentile) scheduled to undergo routine heel lance for newborn metabolic screening | |
| Interventions | Sucrose group (n = 29): infants received 2.0 mL 24% sucrose orally Water group (n = 27): infants received sterile water orally Feedings were withheld 2.0 h prior to heel lance to minimize risk of aspiration. No infant received NNS (pacifiers) during the study |
|
| Outcomes | Primary outcomes: mean skin blood flow; NIPS Skin blood flow, perfusion units measured by Laser Doppler Imager during heel lance HR, RR, oxygen saturation in blood |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated block method |
| Allocation concealment (selection bias) | Low risk | Pharmacy Investigational Drug Service team at Hutzel Harper University Hospital maintained the double‐blinded randomization sequence |
| Blinding (performance bias and detection bias) All outcomes | Low risk | A computer‐generated block method assigned infants to receive either 2 mL 24% sucrose or 2 mL sterile water placebo prior to heel lance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Doses were dispensed in plastic oral syringes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the sucrose group (n = 30) 1 infant was excluded secondary to withdrawn consent. In the placebo group (n = 30) 3 infants were not included: 1 had a significant Laser Doppler Imager scan artefact, 1 received oral acetaminophen prior to heel lance, and 1 developed persistent tachypnoea prior to the study |
| Selective reporting (reporting bias) | Unclear risk | We could not determine whether this study was registered in a trials registry, so we could not tell if there were any deviations from the protocol |
| Other bias | Low risk | Appears free of other bias. |
Unceta‐Barranechea 2008.
| Methods | Prospective RCT Painful intervention: heel lance Study location: Maternity Unit Hospital de Basurto, Bilbao, Vizcaya, Spain Study period: 3 months in 2007 |
|
| Participants | 150 term infants | |
| Interventions | 2 mL 24% sucrose with NNS NNS with water Control: facilitated tucking |
|
| Outcomes | Modified NFCS, mean crying time | |
| Notes | Paper translated from Spanish to English | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Staff were blinded to sucrose and water solutions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was unclear if outcome assessments were performed staff blinded to interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Unclear risk | Appears free of other bias |
Yilmaz 2010.
| Methods | RCT Painful intervention: heel lance Study location: Trabzon Delivery and Children's Diseases Hospital, Erzurum, Turkey Study period: February 2007 to January 2008 |
|
| Participants | 120 infants GA 37 to 42 weeks Sucrose group (n = 30): mean PMA (SD) 39.10 weeks (0.71) Mother's milk group (n = 30): mean PMA (SD) 39.10 weeks (1.03) Pacifier group (n = 30): mean PMA (SD) 39.20 weeks (0.93) Control group (n = 30): mean PMA (SD) 39.67 weeks(0.80) |
|
| Interventions | Sucrose group: 2 mL 20% sucrose via syringe 2 min before the procedure (using a syringe with the needle removed and avoiding contact of the syringe with the mouth and lips) Mother's milk group: 2 mL mother's milk via syringe 2 min before the procedure (using a syringe with the needle removed and avoiding contact of the syringe with the mouth and lips) Pacifier group: given a pacifier Control group: newborns were in their mothers' lap; no interventions were made before the painful procedure |
|
| Outcomes | NIPS score, HR, respiratory rate, crying time. Data were presented according to different procedure times and could not be used in meta‐analyses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not specify method of randomization |
| Allocation concealment (selection bias) | Unclear risk | Did not specify method of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Did not report on blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Did not report on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no withdrawals |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Örs 1999.
| Methods | RCT Painful intervention: heel lance Study location: Marmara University Hosppital, Istanbul, Turkey Study period: September 1996 to January 1997 |
|
| Participants | 102 healthy term infants, PMA 37 to 42 weeks, median PNA 1.6 days (range 1 to 15 days) | |
| Interventions | 2 mL 25% sucrose (n = 35) 2 mL human milk (n = 33) 2 mL sterile water (n = 34) Solution syringed to anterior part of tongue for 1 min Heel prick done 2 min after intervention | |
| Outcomes | Median crying time 3 min after heel lance; percentage change in HR 1, 2, and 3 min after heel lance | |
| Notes | Kruskal‐Wallis 1‐way ANOVA used to assess differences between groups Medians and IQRs reported for outcomes Adverse effects were not evaluated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sucrose and water solutions were administered blinded to staff, but human milk was probably not blinded to staff |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data provided on all randomized infants |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available to us so we could not judge whether there were any deviations from it |
| Other bias | Low risk | Appears free of other bias |
Abbreviations
BF = breastfeeding BPSN = Bernese Pain Scale for Neonates DAN = Douleur Aiguë du Nouveau‐né Scale DPNB = dorsal penile nerve block EMLA = eutectic mixture of local anaesthetic (a topical mixture of lidocaine (2.5%) and prilocaine (2.5%) cream) HR = heart rate IM = intramuscular IQR = interquartile range(s) min = minute(s) NAPI = Neurobehavioural Assessment of Preterm Infants NFCS = Neonatal Facial Coding System NG = nasogastric NICU = neonatal intensive care unit NIPS = Neonatal Infant Pain Scale N‐PASS = Neonatal Pain Agitation and Sedation Scale NNS = non‐nutritive sucking PIPP = Premature Infant Pain Profile PMA = postmenstrual age PNA = postnatal age RCT = randomised controlled trial RR = respiratory rate ROP = retinopathy of prematurity SC = subcutaneous SD = standard deviation SpO2 = oxygen saturation SSC = skin to skin contact w/v = weight by volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abad 1993 | Available as an abstract only |
| Abad 2001 | Although this was an RCT, 4 newborns were included twice (i.e. there were 55 events recorded for 51 participants), therefore, it was not possible to separate data for 51 newborns |
| Ahuja 2000 | This was a non‐randomised study. A single cohort was studied. The intervention was a non‐sucrose sweetener |
| Akman 2002 | The word 'random' does not appear anywhere in the text. It is unlikely that this is an RCT |
| Aziznejad 2013 | The age of the infants was 8.5 months |
| Barbier 1994 | Study did not include the use of sucrose |
| Barr 1993 | Although an RCT, the authors did not provide information on the number of infants in each group. Results were presented in graph form without indicating whether means or medians were used. No standard deviations were presented |
| Barr 1995 | Excluded based on PNA (2 and 4 months PNA) |
| Bilgen 2001 | This manuscript was published previously in the European Journal of Pediatrics ("Comparison of sucrose and human milk on pain response in newborns" by Ors et al, Eur J Pediatr, 158:63‐66, 1999) and so, this article has been retracted by the Journal of Pain The editor of the Journal of Pain states that "Anyone citing this article must cite from the European Journal of Pediatrics and not from the Journal of Pain" |
| Blass 1991 | Although this was an RCT the number of neonates in each group was not stated |
| Blass 1995 | This was a controlled trial without randomisation. The number of patients in each group was not stated |
| Blass 2001 | Study not fully randomised |
| Bucher 2000 | This study used an artificial sweetener, glycine or breast milk as the intervention |
| Curtis 2007 | PNA 0 to 6 months |
| Dilli 2009 | PNA 0 to 48 months. A group < 1 month old could not be separated |
| Efe 2007 | Study not fully randomised |
| Fernandez 2003 | The noxious stimulus was heel stroke, which is non‐invasive |
| Gibbins 2000 | Available as an abstract only |
| Gormally 1996 | Available as an abstract only |
| Graillon 1997 | An RCT cross‐over study in which no painful stimulus was applied to the neonates |
| Harrison 2011 | Not an RCT |
| Isik 2000b | Available as an abstract only |
| Johnston 2000 | Available as an abstract only |
| Joung 2010 | Non‐randomised study |
| Lewindon 1998 | The infants in this study were too old for inclusion in this review (mean age 17.1 weeks) |
| Mandel 2012 | All infants received sucrose |
| Mellah 1999 | Randomised double blind cross‐over study. Data analyzed by paired t‐test. Results from the first exposure to sucrose or placebo could not be isolated |
| Mohan 1998 | Control group was not randomised |
| Ozdogan 2010 | This was a quasi‐randomised trial. "Healthy newborns (n = 142) were consecutively allocated to one of the six groups: ..." |
| Ramenghi 2002 | Immunisations performed at 2, 3 or 4 months, so infants were too old for this review |
| Razmus 2004 | Study not fully randomised |
| Reis 2003 | Mean PNA 9.5 weeks |
| Sahebihag 2011 | Infants were too old for this review |
| Scaramuzzo 2013 | Dr Scaramuzzo informed us that “our randomisation method was simply one yes‐one not”. This was therefore a quasi‐randomised trial which we did not include in the review |
| Skogsdal 1997 | This study used glucose and breast milk as the interventions |
| Stevens 1997b | Available as an abstract only |
| Stevens 2000 | Available as an abstract only |
| Taddio 2000 | Not an RCT and included older infants |
| Taddio 2003 | Infants did not receive a painful procedure |
| Taddio 2009b | Population subset of a larger study already included in the review |
| Vederhus 2006 | Sucrose was not used as an intervention |
| Yoon 2001 | Not fully randomised |
Abbreviations
PNA = postnatal age RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Moradi 2012a.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting classification ‐ written in Persian |
Moradi 2012b.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study awaiting classification – written in Persian |
Singh 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | We have not been able to locate a copy of the article at libraries in Canada or the US. |
Characteristics of ongoing studies [ordered by study ID]
Campbell‐Yeo 2013.
| Trial name or title | Trial of repeated analgesia with kangaroo care (TRAKC) |
| Methods | RCT |
| Participants | Infants < 36.0 weeks PMA |
| Interventions | Kangaroo mother care Sucrose Kangaroo mother care + sucrose |
| Outcomes | PIPP; NAPI |
| Starting date | June 2012 |
| Contact information | Marsha Campbell‐Yeo, RN, NNP, PhD; Marsha.CampbellYeo@iwk.nshealth.ca |
| Notes | ClinicalTrials.gov Identifier: NCT01561547 |
ISRCTN59514984.
| Trial name or title | RCT of sucrose analgesia for repeated capillary blood sampling |
| Methods | RCT Painful intervention: repeated capillary blood sampling |
| Participants | PMA or PNA not provided |
| Interventions | Sucrose Water |
| Outcomes | Outcome measures not provided |
| Starting date | September 2006 |
| Contact information | Dr Simon Mitchell, SMH Central Manchester & Manchester Children's University Hospitals St Mary's Hospital for Women & Children Oxford Road Manchester M13 0JH UK |
| Notes | ISRCTN59514984 |
ISRCTN73259137.
| Trial name or title | Kangaroo mother care combined with sucrose to reduce pain responses in preterm infants |
| Methods | RCT Painful intervention: venipuncture |
| Participants | 1. PMA between 28 and 36 weeks 2. PNA < 28 days |
| Interventions | Kangaroo mother care + sucrose Sucrose |
| Outcomes | Pain responses measured by:
Primary outcome measures were taken at baseline (30 s before venipuncture); during skin cleansing; needle stick and blood harvesting; compression after needle removal; and rest (up to 5 min after the end of the procedure) |
| Starting date | March 2007 |
| Contact information | Ms Ananda Fernandes, Praceta Falcão Resende 1 R/Ch Coimbra 3000‐164 Portugal +351 (0)917 500 541 Email: amfernandes@esenfc.pt |
| Notes | ISRCTN73259137 |
Montanholi 2012.
| Trial name or title | Effect of skin‐to‐skin compared to sucrose for pain relief in infants undergoing repeated painful procedures: randomised clinical trial |
| Methods | RCT |
| Participants | Newborns 4 to 12 h old with PMA ≥ 36 weeks |
| Interventions | The therapeutic intervention is skin‐to‐skin contact and the control interventions is 25% sucrose |
| Outcomes | NFCS |
| Starting date | June 2013 |
| Contact information | Liciane Langona Montanholi Av. Bandeirantes, 3900, City: Ribeirão Preto/Brazil Zip Code: 14040‐902 Telephone: +55 16 3602 3411 Email: licianelm@gmail.com Affiliation: Escola de Enfermagem de Ribeirão Preto‐ Universidade de São Paulo |
| Notes | RBR‐7nynr7 |
NCT01190995.
| Trial name or title | Role of repeated painful procedures in preterm neonates on short term neuro behavioural outcome |
| Methods | RCT |
| Participants | Infants up to 28 days of age |
| Interventions | 24% sucrose Placebo |
| Outcomes | Short‐term neurobehavioral status at enrolment in the study and at 40 weeks post‐conceptional age using the NAPI scale |
| Starting date | July 2010 |
| Contact information | Vikram Datta, MD., Lady Hardinge Medical College, New Delhi, Delhi1, India, 110001 Email: drvikramdatta@gmail.com |
| Notes | ClinicalTrials.gov Identifier: NCT01190995 |
NCT01438008.
| Trial name or title | Pilot study of sucrose to reduce pain in sick babies |
| Methods | RCT |
| Participants | Infants who are inpatients of the NICU:
|
| Interventions | 24% sucrose solution |
| Outcomes | PIPP; total crying time: skin conductance activity |
| Starting date | May 2012 |
| Contact information | Denise Harrison, Children's Hospital of Eastern Ontario |
| Notes | ClinicalTrials.gov Identifier NCT01438008 |
NCT01552993.
| Trial name or title | Registration and treatment of pain during eye examination of prematurity |
| Methods | RCT |
| Participants | Ages eligible for study: 31 to 37 weeks |
| Interventions | Paracetamol mixture 20 mg/kg + pacifier + glucose Pacifier + sucrose |
| Outcomes | Pain ‐ time frame 5 minutes using PIPP |
| Starting date | March 2012 |
| Contact information | Hakon Bergseng, PhD Email: hakon.bergseng@stolav.no |
| Notes | ClinicalTrials.gov Identifier: NCT01552993 |
NCT01742520.
| Trial name or title | Neurodevelopmental outcomes of preterm infants treated for pain management with repeated doses of sucrose 24% |
| Methods | RCT |
| Participants | Preterm infants born 27‐33 weeks PMA |
| Interventions | Breast milk or formula prior to every painful procedure Multiple doses of sucrose 1‐3 min prior to every invasive procedure |
| Outcomes | Primary outcome measures: neurodevelopmental outcomes at 6 month corrected age; Griffith mental developmental scale (gross and fine motor, language, performance, social) and general movements by Prechtel Secondary outcome measures: neurodevelopment at 15 weeks corrected age; Griffith mental developmental scaled (gross and fine motor, language, performance, social) and general movements by Prechtel |
| Starting date | January 2013 |
| Contact information | Dr Iris Morag, Sheba Medical Center Email: irismorag@gmail.com |
| Notes | ClinicalTrials.gov Identifier: NCT01742520 |
NCT01800318.
| Trial name or title | Does noninvasive electrical stimulation of acupuncture points (NESAP) reduce heel stick pain in infants? |
| Methods | RCT |
| Participants | Infants up to 3 days of age |
| Interventions | Sham NESAP with 24% oral sucrose NESAP with oral water NESAP with 24% oral sucrose Sham NESAP with oral water |
| Outcomes | Changes from baseline PIPP; change in salivary cortisol after heel stick; change in HR variability during heel stick; change in HR; change in oxygen saturation; duration of crying after TENS unit initiated; duration of crying during heel stick |
| Starting date | March 2013 |
| Contact information | Richard W Hall MD University of Arkansas |
| Notes | ClinicalTrials.gov Identifier: NCT01800318 |
NCT01894659.
| Trial name or title | Oral sucrose versus glucose for procedural pain in premature neonates |
| Methods | RCT |
| Participants | Preterm neonates ≤ 34 weeks PMA and ≤ 7 days of age postnatally |
| Interventions | Oral sucrose versus glucose for procedural pain in premature neonates |
| Outcomes | Urinary markers of ATP utilization, oxidative stress and cell injury |
| Starting date | February 2014 |
| Contact information | Danilyn Angeles, PhD, Loma Linda University, 909‐558‐7563; Email: dangeles@ll.edu |
| Notes | ClinicalTrials.gov Identifier: NCT01894659 |
NCT01931020.
| Trial name or title | Analgesic effect of oral 25% glucose versus oral 24% sucrose for pain relief during heel lance in preterm neonates |
| Methods | RCT |
| Participants | Infants at 34 to 37 weeks PMA |
| Interventions | Sucrose 1 mL of 24% sucrose administered prior to heel lance vs. 1 mL of 25% glucose administered prior to heel lance |
| Outcomes | Painful response:PIPP 30 s after heel lance Duration of crying Within 2 min following the procedure |
| Starting date | July 2013 |
| Contact information | Vikram Datta, MD., Lady Hardinge Medical College, New Delhi, Delhi1, India, 110001; Email: drvikramdatta@gmail.com |
| Notes | ClinicalTrials.gov Identifier: NCT01931020 |
NCT02133716.
| Trial name or title | Efficacy of breast milk expressed and sucrose in procedural pain in preterm (LACTEET) |
| Methods | RCT |
| Participants | Preterm infants (PMA 25 to 37 weeks and body weight < 2500 g) |
| Interventions | Expressed breast milk vs. oral 24% sucrose |
| Outcomes | PIPP; percentage of crying |
| Starting date | October 2013 |
| Contact information | Laura Collados Gómez, Hospital University Gregorio Marañon, Madrid, Spain, 28007 |
| Notes | ClinicalTrials.gov Identifier: NCT02133716 |
NCT02344368.
| Trial name or title | The effect of sucrose on pain relief during venous blood sampling in preterm infants |
| Methods | RCT |
| Participants | Preterm infants weighing > 1000 g |
| Interventions | The aim of this study is to find the minimal effective dose of 25% sucrose to reduce pain during a single venous blood sampling procedure: 0.2 mL or 0.5 mL |
| Outcomes | Pain assessed by PIPP‐R. Pain score related to the blood sampling will be performed twice: at skin puncture and immediately after the needle has been removed |
| Starting date | January 2015 |
| Contact information | Contact: Laila Kristoffersen (Email: laila.kristoffersen@ntnu.no); Contact: Håkon Bergseng, MD PhD (Email: hakon.bergseng@ntnu.no); St Olavs hospital, Trondheim, Norway |
| Notes | NCT02344368 |
Passariello 2014.
| Trial name or title | Efficacy of oral sucrose in newborns exposed to painful stimuli |
| Methods | RCT |
| Participants | All infants (< 1 month of age) admitted to the Unit of Neonatal Intensive Care of Monaldi Hospital, Naples, Italy. |
| Interventions | 24% sucrose oral solution administered during capillary and arterial blood sample taking, directly by a disposable plastic vial at dosage of 1.5 mL for newborns with a birthweight > 3 kg and 1 mL for newborns with a birthweight < 3 kg Sterile water administered directly by a disposable plastic vial during capillary and arterial blood sample taking |
| Outcomes | Skin conductance algesimeter was used to monitor pain. Skin conductance activity was measured for 3 min before, during, and for 3 min after the intervention |
| Starting date | June 2013 |
| Contact information | Dr Annalisa Passariello Address Via S. Pansini 5‐80100‐Naples. University of Naples “Federico II”. Italy Phone +39 3395077349 Email: annalisa_passariello@libero.it |
| Notes | ACTRN12614000164695 |
Philip 2012.
| Trial name or title | Effectiveness of human breast milk, table sugar vs sterile water for pain relief in premature infants undergoing a heel prick procedure in nursery of Christian Medical College and Hospital, Vellore |
| Methods | RCT |
| Participants | Haemodynamically stable preterm (28 to 36 weeks PMA) infants, aged 0 to 28 days, who tolerate feeds, and are admitted to the nursery and undergo heel‐prick procedure |
| Interventions | 24% sucrose (0.6 mL/kg body weight) to be given orally over 15 s (single dose) Expressed breast milk (0.6 mL/kg body weight) Placebo (sterile water 0.6 mL/kg body weight) |
| Outcomes | Preintervention PIPP score (infant at rest) and postintervention pain at 0, 2, and 5 min using PIPP scale |
| Starting date | June 2012 |
| Contact information | Ms Sophia Philip, College of Nursing, Christian Medical College, Vellore, Tamil Nadu 632004 India. Phone: 9894143708. Email: sopiantony@gmail.com |
| Notes | CTRI/2012/06/002730 |
Roue 2013.
| Trial name or title | Prevention of the procedural pain in the newborn (ACTISUCROSE) |
| Methods | RCT |
| Participants | Newborns with PMA of 37 to 42 weeks |
| Interventions | 20% saccharose Breastfeeding |
| Outcomes | Increase of the total concentration of haemoglobin (delta HbT) measured by the spectrometer during the intravenous injection |
| Starting date | June 2013 |
| Contact information | Jean‐Michel Roue, University Hospital, Brest, France |
| Notes | NCT02109263 |
Shah 2015.
| Trial name or title | Comparing efficacy of music therapy, sucrose and combination of the two in neonates for pain relief during heel prick procedure |
| Methods | RCT. This is a cross‐over study with each neonate getting all 3 interventions in random order. There will be a minimum of 40 minutes of 'wash‐out' period between interventions |
| Participants | Newborns ≥ 32 weeks, stable clinical condition with no need of CPAP/high flow/ventilation; age 0 h to 28 days |
| Interventions | Music therapy Oral 24% sucrose 0.5 mL with no music Oral 24% sucrose with music therapy |
| Outcomes | PIPP score, HR stability, saturation stability |
| Starting date | Anticipated date of first participant enrolment 1 June 2015 |
| Contact information | Dr Swapnil Shah, Department of Neonatology, Royal North Shore Hospital, Reserve Road, St Leonards NSW 2065, Australia. Phone: +61 2 9463 2141 Fax: +61 2 9463 2004 Email: swapnilshah12@yahoo.co.in |
| Notes |
Stevens 2014.
| Trial name or title | Sucrose practices for pain in neonates |
| Methods | RCT |
| Participants | Newborns up to 2 weeks of age. Infants 24 to 42 weeks PMA at birth, admitted to the NICU, and scheduled to receive a heel lance |
| Interventions | 0.1 mL 24% sucrose concurrent opioids 0.5 mL 24% sucrose concurrent opioids 1.0 mL 24% sucrose concurrent opioids 0.1 mL 24% sucrose no opioids 0.5 mL 24% sucrose no opioids 1.0 mL 24% sucrose no opioids |
| Outcomes | The primary outcome is pain intensity measured using the PIPP‐R to assess change from baseline to 30 s post painful procedure |
| Starting date | July 2013 |
| Contact information | Bonnie Stevens, RN, PhD Email: bonnie.stevens@sickkids.ca |
| Notes | ClinicalTrials.gov Identifier: NCT02134873 |
Abbreviations
ATP = adenosine triphosphate CPAP = continuous positive airway pressure PMA = post menstrual age HR = heart rate min = minute(s) NAPI = Neurobehavioural Assessment of Preterm Infants NESAP = Non−invasive Electrical Stimulation of Acupuncture Points NFCS = Neonatal Facial Coding System NICU = neonatal intensive care unit PIPP = Premature Infant Pain Profile PIPP‐R = Premature Infant Pain Profile‐Revised PMA = postmenstrual age PNA = postnatal age RCT = randomised controlled trial TENS = transcutaneous electrical nerve stimulation
Differences between protocol and review
For the update of the review in 2013, inclusion criteria were extended to all studies that used sucrose as an intervention for any acute painful procedure, including subcutaneous injections, circumcision, bladder catheterizations and eye examinations for retinopathy of prematurity, as well as repeated doses of sucrose. Long‐term neurodevelopmental outcomes were added for that update. For this update in 2016 echocardiographic exam was added as a potentially stressful intervention.
Contributions of authors
For this update of the review the authors made these contribution:
Bonnie Stevens:
writing the text of the background and discussion sections of the review;
editing the text of the review.
Janet Yamada:
literature search and identification of trials for inclusion;
editing the text of the review.
Arne Ohlsson:
literature search and identification of trials for inclusion;
evaluation of methodological quality of included trials;
abstraction of data;
entering and verifying and data inRevMan;
writing the text of the methods, results and reference sections of the review;
completing the tables; characteristics of studies and additional tables;
completing the summary of findings tables.
Sarah Haliburton:
literature search and identification of trials for inclusion;
evaluation of methodological quality of included trials;
abstraction of data;
verifying data and results in RevMan;
checking for data accuracy in the results section;
writing of text of review.
Allyson Shorkey:
literature search and identification of trials for inclusion;
evaluation of methodological quality of included trials;
abstraction of data;
verifying data and results in RevMan;
checking for data accuracy in the results section;
writing of text of review.
Sources of support
Internal sources
Faculty of Nursing, University of Toronto, Canada.
Mount Sinai Hospital, Toronto, Canada.
The Hospital for Sick Children, Toronto, Canada.
External sources
Canadian Institutes of Health Research (CIHR) Synthesis Grant: Knowledge Translation, Canada.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Bonnie Stevens ‐ is an author of the following included trials: Gibbins 2002; Johnston 1997; Mitchell 2004; Stevens 1999; Stevens 2005a. For these trials two authors (SH, AS) did the data abstraction and RoB assessments. No other conflict of interest to declare
Janet Yamada ‐ is an author of the following included trial: Stevens 2005a. For this trial two other authors (SH, AS) did the data abstraction and RoB assessments. No other conflict of interest to declare.
Arne Ohlsson ‐ ‐ is an author of the following included trial: Gibbins 2002. For this trial two other authors (SH, AS) did the data abstraction and RoB assessments. No other conflict of interest to declare.
Sarah Haliburton ‐ No conflict of interest to declare
Allyson Shorkey ‐ No conflict of interest to declare
Edited (no change to conclusions)
References
References to studies included in this review
Abad 1996 {published data only}
- Abad F, Diaz NM, Domenech E, Robayna M, Rico J. Oral sweet solution reduces pain‐related behavior in preterm infants. Acta Paediatrica 1996;85:854‐8. [DOI] [PubMed] [Google Scholar]
Abbasoglu 2015 {published data only}
- Abbasoglu A, Cabioglu MT, Tugcu AU, Yapakci E, Tekindal MA, Tarcan A. Laser acupuncture before heel lancing for pain management in healthy term newborns: a randomised controlled trial. Acupuncture in Medicine 2015;6:445‐50. [PUBMED: 26438556] [DOI] [PubMed] [Google Scholar]
Acharya 2004 {published data only}
- Acharya AB, Annamali S, Taub NA, Field D. Oral sucrose analgesia for preterm infant venepuncture. Archives of Disease in Childhood. Fetal and Neonatal Edition 2004;89:F17‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 1996 {published data only}
- Allen KD, White DD, Walburn JN. Sucrose as an analgesic agent for infants during immunization injections. Archives of Pediatrics & Adolescent Medicine 1996;150:270‐4. [DOI] [PubMed] [Google Scholar]
Al Qahtani 2014 {published data only}
- Al Qahtani R, Abu‐Salem LA, Pal K. Effect of lidocaine‐prilocaine eutectic mixture of local anaesthetic cream compared with oral sucrose or both in alleviating pain in neonatal circumcision procedure. African Journal of Paediatric Surgery 2014;11(1):56‐61. [PUBMED: 24647296] [DOI] [PubMed] [Google Scholar]
Altun‐Köroğlu 2010 {published data only}
- Altun‐Köroğlu O, Özek E, Bilgen H, Cebeci D. Hindmilk for procedural pain in term neonates. The Turkish Journal of Pediatrics 2010;52(6):623‐9. [PUBMED: 21428195] [PubMed] [Google Scholar]
Asmerom 2013 {published data only}
- Asmerom Y, Slater L, Boskovic DS, Bahjri K, Holden MS, Phillips R, et al. Oral sucrose for heel lance increases adenosine triphosphate use and oxidative stress in preterm neonates. The Journal of Pediatrics 2013;163(1):29‐35.e1. [PUBMED: 23415615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banga 2015 {published data only}
- Banga S, Datta V, Rehan HS, Bhakhri BK. Effects of sucrose analgesia, for repeated painful procedures, on short‐term neurobehavioral outcome of preterm neonates: a randomized controlled trial. Journal of Tropical Pediatrics 2015;Epub ahead of print(2):101‐6. [PUBMED: 26615181] [DOI] [PMC free article] [PubMed] [Google Scholar]
Basnet 2010 {published data only}
- Basnet S, Shrestha L, Shrestha PS. Sucrose as an analgesic in relieving procedural pain in neonates. Journal of Neonatal‐Perinatal Medicine 2010;3:325‐31. [Google Scholar]
Biran 2011 {published data only}
- Biran V, Gourrier E, Cimerman P, Walter‐Nicolet E, Mitanchez D, Carbajal R. Analgesic effects of EMLA cream and oral sucrose during venipuncture in preterm infants. Pediatrics 2011;128:e63‐70. [DOI] [PubMed] [Google Scholar]
Blass 1997 {published data only}
- Blass EM. Milk‐induced hypoalgesia in human newborns. Pediatrics 1997;99:825‐9. [DOI] [PubMed] [Google Scholar]
Blass 1999 {published data only}
- Blass EM, Watt LB. Suckling‐ and sucrose‐induced analgesia in human newborns. Pain 1999;83:611‐23. [DOI] [PubMed] [Google Scholar]
Boyle 2006 {published data only}
- Boyle EM, Freer Y, Khan‐Orakzai, Watkinson M, Wright E, Ainsworth JR, et al. Sucrose and non‐nutritive sucking for the relief of pain in screening for retinopathy of prematurity: a randomized controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2006;91:F166‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucher 1995 {published data only}
- Bucher H‐U, Moser T, Siebenthal K, Keel M, Wolf M, Duc G. Sucrose reduces pain reaction to heel lancing in preterm infants: a placebo‐controlled, randomized and masked study. Pediatric Research 1995;38:332‐5. [DOI] [PubMed] [Google Scholar]
Carbajal 1999 {published data only}
- Carbajal R, Chauvet X, Couderc SD, Olivier‐Martin M. Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. BMJ 1999;319:1393‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cignacco 2012 {published data only}
- Cignacco EL, Sellam G, Stoffel L, Gerull R, Nelle M, Anand KJ, et al. Oral sucrose and "facilitated tucking" for repeated pain relief in preterms: a randomized controlled trial. Pediatrics 2012;129(2):299‐308. [PUBMED: 22232305] [DOI] [PubMed] [Google Scholar]
- Gerull R, Cignacco E, Stoffel L, Sellam G, Nelle M. Physiological parameters after nonpharmacological analgesia in preterm infants: a randomized trial. Acta Pædiatrica 2013;102(8):e368–73. [PUBMED: 23651076] [DOI] [PubMed] [Google Scholar]
Codipietro 2008 {published data only}
- Codipietro L, Ceccarelli M, Ponzone A. Breastfeeding or oral sucrose solution in term neonates receiving heel lance: a randomized controlled trial. Pediatrics 2008;122:e716‐21. [DOI] [PubMed] [Google Scholar]
Dilli 2014 {published data only}
- Dilli D, Ilarslan NE, Kabatas EU, Zenciroglu A, Simsek Y, Okumus N. Oral sucrose and non‐nutritive sucking goes some way to reducing pain during retinopathy of prematurity eye examinations. Acta Paediatrica 2014;103(2):e76‐9. [PUBMED: 24730361] [DOI] [PubMed] [Google Scholar]
Elserafy 2009 {published data only}
- Elserafy FA, Alsaedi SA, Louwrens J, Bin Sadiq B, Mersal AY. Oral sucrose and a pacifier for pain relief during simple procedures in preterm infants: a randomized controlled trial. Annals of Saudi Medicine 2009;29(3):184‐8. [PUBMED: 19448377] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gal 2005 {published data only}
- Gal P, Kissling GE, Young WO, Dunaway KK, Marsh VA, Jones SM, et al. Efficacy of sucrose to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Annals of Pharmacotherapy 2005;39:1029‐33. [DOI] [PubMed] [Google Scholar]
Gaspardo 2008 {published data only}
- Gaspardo CM, Miyase CI, Chimello JT, Martinez FE, Martins Linhares MB. Is pain relief equally efficacious and free of side effects with repeated doses of oral sucrose in preterm neonates?. Pain 2008;137:16‐25. [DOI] [PubMed] [Google Scholar]
Gibbins 2002 {published and unpublished data}
- Gibbins S. Efficacy and Safety of Sucrose for Procedural Pain Relief in Preterm and Term Neonates [PhD Thesis]. Toronto: University of Toronto, 2001. [DOI] [PubMed] [Google Scholar]
- Gibbins S, Stevens B. The influence of gestational age on the efficacy and short‐term safety of sucrose for procedural pain relief. Advances in Neonatal Care 2003;3:241‐9. [PubMed] [Google Scholar]
- Gibbins S, Stevens B, Hodnett E, Pinelli J, Ohlsson A, Darlington G. Efficacy and safety of sucrose for procedural pain relief in preterm and term neonates. Nursing Research 2002;51:375‐82. [DOI] [PubMed] [Google Scholar]
Gormally 2001 {published data only}
- Gormally S, Barr RG, Wertheim L, Alkawaf R, Calinoiu N, Young SN. Contact and nutrient caregiving effects on newborn infant pain responses. Developmental Medicine and Child Neurology 2001;43:28‐38. [DOI] [PubMed] [Google Scholar]
Grabska 2005 {published data only}
- Grabska J, Walden P, Lerer T, Kelly C, Hussain N, Donovan T, et al. Can oral sucrose reduce the pain and distress associated with screening for retinopathy for prematurity?. Journal of Perinatology 2005;25:33‐5. [DOI] [PubMed] [Google Scholar]
Gray 2012 {published data only}
- Gray L, Lang CW, Porges SW. Warmth is analgesic in healthy newborns. Pain 2012;153(5):960‐6. [PUBMED: 22424877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2015 {published data only}
- Gray L, Garza E, Zageris D, Heilman KJ, Porges SW. Sucrose and warmth for analgesia in healthy newborns: an RCT. Pediatrics 2015;135(3):e607‐14. [PUBMED: 25687147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenberg 2002 {published data only}
- Greenberg CS. A sugar‐coated pacifier reduces procedural pain in newborns. Pediatric Nursing 2002;28:271‐7. [PubMed] [Google Scholar]
Guala 2001 {published data only}
- Guala A, Pastore G, Liverani ME, Giroletti G, Gulino F, Meriggi AI, et al. Glucose or sucrose as an analgesic for newborns: a randomized controlled blind trial. Minerva Pediatrica 2001;53:271‐4. [PubMed] [Google Scholar]
Haouari 1995 {published data only}
- Haouari N, Wood C, Griffiths G, Levene M. The analgesic effect of sucrose in full term infants: a randomised controlled trial. BMJ 1995;310:1498‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2003 {published data only}
- Harrison D, Johnston L, Loughnan P. Oral sucrose for procedural pain in sick hospitalized infants: a randomized‐controlled trial. Journal of Paediatric and Child Health 2003;39:591‐7. [DOI] [PubMed] [Google Scholar]
Herschel 1998 {published data only}
- Herschel M, Khoshnood B, Ellman C, Maydew N, Mittendorf R. Neonatal circumcision. Randomized trial of sucrose pacifier for pain control. Archives of Pediatrics and Adolescent Medicine 1998;152:279‐84. [DOI] [PubMed] [Google Scholar]
Isik 2000a {published data only}
- Isik U, Ozek E, Bilgen H, Cebeci D. Comparison of oral glucose and sucrose solutions on pain response in neonates. Journal of Pain 2000;1:275‐8. [DOI] [PubMed] [Google Scholar]
Johnston 1997 {published data only}
- Johnston CC, Stremler RL, Stevens BJ, Horton LJ. Effectiveness of oral sucrose and simulated rocking on pain response in preterm neonates. Pain 1997;72:193‐9. [DOI] [PubMed] [Google Scholar]
Johnston 1999 {published data only}
- Johnston CC, Stremler R, Horton L, Friedman A. Effect of repeated doses of sucrose during heel stick procedure in preterm neonates. Biology of the Neonate 1999;75:160‐6. [DOI] [PubMed] [Google Scholar]
Johnston 2002 {published data only}
- Boyer K, Johnston C, Walker CD, Filion F, Sherrard A. Does sucrose analgesia promote physiological stability in preterm neonates?. Biology of the Neonate 2004;85(1):26‐31. [PUBMED: 14631163] [DOI] [PubMed] [Google Scholar]
- Johnston CC, Filion F, Snider L, Majnemer A, Limperopolous C, Walker CD, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks' postconceptional age. Pediatrics 2002;110:523‐8. [DOI] [PubMed] [Google Scholar]
Kaufman 2002 {published data only}
- Kaufman GE, Cimo S, Miller LW, Blass EM. An evaluation of the effects of sucrose on neonatal pain with 2 commonly used circumcision methods. American Journal of Obstetrics and Gynecology 2002;186:564‐8. [DOI] [PubMed] [Google Scholar]
Kristoffersen 2011 {published data only}
- Kristoffersen L, Skogvoll E, Hafström M. Pain reduction on insertion of a feeding tube in preterm infants: a randomized controlled trial. Pediatrics 2011;127:e1449‐54. [DOI] [PubMed] [Google Scholar]
Leng 2013 {published data only}
- Leng HY, Zheng XL, Yan L, Zhang XH, He HY, Xiang M. [Effects of different types and concentration of oral sweet solution on reducing neonatal pain during heel lance procedures]. Zhonghua er ke za zhi. Chinese Journal of Pediatrics 2013;51(9):654‐8. [PUBMED: 24330983] [PubMed] [Google Scholar]
Leng 2015 {published data only}
- Leng HY, Zheng XL, Zhang XH, He HY, Tu GF, Fu Q. Combined non‐pharmacological interventions for newborn pain relief in two degrees of pain procedures: a randomized clinical trial. European Journal of Pain 2016;20(6):989‐97. [PUBMED: 26685099] [DOI] [PubMed] [Google Scholar]
Liaw 2011 {published data only}
- Liaw J‐J, Zeng W‐P, Yang L, Yuh Y‐S, Yin T, Yang M‐H. Nonnutritive sucking and oral sucrose relieve neonatal pain during intramuscular injection of hepatitis vaccine. Journal of Pain and Symptom Management 2011;42(6):918‐30. [PUBMED: 21620644] [DOI] [PubMed] [Google Scholar]
Liaw 2013 {published data only}
- Liaw JJ, Yang L, Lee CM, Fan HC, Chang YC, Cheng LP. Effects of combined use of non‐nutritive sucking, oral sucrose, and facilitated tucking on infant behavioural states across heel‐stick procedures: a prospective, randomised controlled trial. International Journal of Nursing Studies 2013;50(7):883‐94. [PUBMED: 23068310] [DOI] [PubMed] [Google Scholar]
- Yin T, Yang L, Lee T‐Y, Li C‐C, Hua Y‐M, Liaw J‐J. Development of atraumatic heel‐stick procedures by combined treatment with non‐nutritive sucking, oral sucrose, and facilitated tucking: a randomised, controlled trial. International Journal of Nursing Studies 2015;52(8):1288‐99. [PUBMED: 25939641] [DOI] [PubMed] [Google Scholar]
Marin Gabriel 2013 {published data only}
- Marin Gabriel MA, Rey Hurtado de Mendoza B, Jimenez Figueroa L, Medina V, Iglesias Fernandez B, Vazquez Rodriguez M, et al. Analgesia with breastfeeding in addition to skin‐to‐skin contact during heel prick. Archives of Disease in Childhood. Fetal and neonatal edition 2013;98(6):F499‐503. [PUBMED: 23839984] [DOI] [PubMed] [Google Scholar]
Mathai 2006 {published data only}
- Mathai S, Natrajan N, Rajalakshmi NR. A comparative study of non‐pharmacological methods to reduce pain in neonates. Indian Pediatrics 2006;43:1070‐5. [PubMed] [Google Scholar]
McCullough 2008 {published data only}
- McCullough S, Halton T, Mowbray D, Macfarlane PI. Lingual sucrose reduces the pain response to nasogastric tube insertion: a randomised clinical trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93:F100‐3. [DOI] [PubMed] [Google Scholar]
Milazzo 2011 {published data only}
- Milazzo W, Fielder J, Bittel A, Coil J, McClure M, Tobin P, et al. Oral sucrose to decrease pain associated with arterial puncture in infants 30 to 36 weeks’ gestation. A randomized clinical trial. Advances in Neonatal Care 2011;11(6):406‐11. [PUBMED: 22123473] [DOI] [PubMed] [Google Scholar]
Mitchell 2004 {published data only}
- Mitchell A, Stevens B, Mungan N, Johnson W, Lobert S, Boss B. Analgesic effects of oral sucrose and pacifier during eye examinations for retinopathy of prematurity. Pain Management Nursing 2004;5:160‐8. [DOI] [PubMed] [Google Scholar]
Montoya 2009 {published data only}
- Montoya IG, Gázquez MAR, Cadavid LAM, Jaramillo AQ. [The use of sucrose for the prevention of pain during venipuncture in neonates]. Enfermería Clínica 2009;19:267‐74. [DOI] [PubMed] [Google Scholar]
Mucignat 2004 {published data only}
- Mucignat V, Ducrocq S, Lebas F, Mochel F, Baudon JJ, Gold F. Analgesic effects of EMLA cream and saccharose solution for subcutaneous injections in preterm newborns: a prospective study of 265 injections [Effet analgésique de la crème EMLA®, du saccharose et de leur association pour les injections sous‐cutanées chez le nouveau‐né prématuré: étude prospective de 265 injections]. Archives de Pédiatrie 2004;11:921‐25. [DOI] [PubMed] [Google Scholar]
O'Sullivan 2010 {published data only}
- O'Sullivan A, O'Connor M, Brosnahan D, McCreery K, Dempsey EM. Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2010;95:F419–22. [DOI] [PubMed] [Google Scholar]
Ogawa 2005 {published data only}
- Ogawa S, Ogihara T, Fujiwara E, Ito K, Nakano M, Nakayama S, et al. Venepuncture is preferable to heel lance for blood sampling in term neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition 2005;90:F432‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okan 2007 {published data only}
- Okan F, Coban A, Ince Z, Yapici Z, Can G. Analgesia in preterm newborns: the comparative effects of sucrose and glucose. European Journal of Pediatrics 2007;166:1017‐24. [DOI] [PubMed] [Google Scholar]
- Ozdogan T, Akman I, Cebeci D, Bilgen H, Ozek E. Comparison of two doses of breast milk and sucrose during neonatal heel prick. Pediatrics International 2010;52:175‐9. [DOI] [PubMed] [Google Scholar]
Örs 1999 {published data only}
- Örs R, Özek E, Baysoy G, Cebeci D, Bilgen H, Türküner M, et al. Comparison of sucrose and human milk on pain response in newborns. European Journal of Pediatrics 1999;158:63‐6. [DOI] [PubMed] [Google Scholar]
Overgaard 1999 {published data only}
- Overgaard C, Knudesen A. Pain‐relieving effect of sucrose in newborns during heel prick. Biology of the Neonate 1999;75:279‐84. [DOI] [PubMed] [Google Scholar]
Pandey 2013 {published data only}
- Pandey M, Datta V, Rehan HS. Role of sucrose in reducing painful response to orogastric tube insertion in preterm neonates. Indian Journal of Pediatrics 2013;80(6):476‐82. [PUBMED: 23263970] [DOI] [PubMed] [Google Scholar]
Potana 2015 {published data only}
- Potana NT, Dongara AR, Nimbalkar SM, Patel DV, Nimbalkar AS, Phatak A. Oral sucrose for pain in neonates during echocardiography: a randomized controlled trial. Indian Pediatrics 2015;52(6):493‐7. [DOI] [PubMed] [Google Scholar]
Ramenghi 1996a {published data only}
- Ramenghi LA, Wood CM, Griffith GC, Levene MI. Reduction of pain response in premature infants using intraoral sucrose. Archives of Disease in Childhood. Fetal and Neonatal Edition 1996;74:F126‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramenghi 1996b {published data only}
- Ramenghi LA, Griffith GC, Wood CM, Levene MI. Effect of non‐sucrose sweet tasting solution on neonatal heel prick responses. Archives of Disease in Childhood. Fetal and Neonatal Edition 1996;74:F129‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramenghi 1999 {published data only}
- Ramenghi LA, Evans DJ, Levene MI. "Sucrose analgesia": absorptive mechanism or taste perception?. Archives of Disease in Childhood. Fetal and Neonatal Edition 1999;80:F146‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 2006 {published data only}
- Rogers AJ, Greenwald MH, DeGuzman MA, Kelly ME, Simon HK. A randomized, controlled trial of sucrose analgesia in infants younger than 90 days of age who require bladder catheterization in pediatric emergency department. Academic Emergency Medicine 2006;13:617‐22. [DOI] [PubMed] [Google Scholar]
Rush 2005 {published data only}
- Rush R, Rush S, Ighani F, Anderson B, Irwin M, Naqvi M. The effects of comfort care on the pain responses in preterm infants undergoing screening for retinopathy of prematurity. Retina 2005;25:59‐62. [DOI] [PubMed] [Google Scholar]
Rushforth 1993 {published data only}
- Rushforth JA, Levene MI. Effect of sucrose on crying in response to heel stab. Archives of Disease in Childhood 1993;69:388‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simonse 2012 {published data only}
- Simonse E, Mulder PG, Beek RH. Analgesic effect of breast milk versus sucrose for analgesia during heel lance in late preterm infants. Pediatrics 2012;129(4):657‐63. [PUBMED: 22392168] [DOI] [PubMed] [Google Scholar]
Slater 2010 {published and unpublished data}
- Slater R, Cornelissen L, Fabrizi L, Patten D, Yoxen J, Worley A, et al. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. The Lancet 2010;376:1225‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stang 1997 {published data only}
- Stang HJ, Snellman LW, Condon LM, Conroy MM, Liebo R, Brodersen L, et al. Beyond dorsal penile nerve block: a more humane circumcision. Pediatrics 1997;100:E3. [DOI] [PubMed] [Google Scholar]
Stevens 1999 {published data only}
- Johnston CC, Sherrard A, Stevens B, Franck L, Stremler R, Jack A. Do cry features reflect pain intensity in preterm neonates?. Biology of the Neonate 1999;76:120‐4. [DOI] [PubMed] [Google Scholar]
- Stevens B, Johnston C, Franck P, Petryshen P, Jack A, Foster G. The efficacy of developmentally sensitive interventions and sucrose for relieving pain in very low birth weight infants. Nursing Research 1999;48:35‐43. [DOI] [PubMed] [Google Scholar]
Stevens 2005a {published data only}
- Stevens B, Yamada J, Beyene J, Gibbins S, Petryshen P, Stinson J, et al. Consistent management of repeated procedural pain with sucrose in preterm neonates: is it effective and safe for repeated use over time?. The Clinical Journal of Pain 2005;21:543‐8. [DOI] [PubMed] [Google Scholar]
Storm 2002 {published data only}
- Storm H, Fremming A. Food intake and oral sucrose in preterms prior to heel prick. Acta Paediatrica 2002;91:555‐60. [DOI] [PubMed] [Google Scholar]
Suhrabi 2014 {published data only}
- Suhrabi Z, Taghinejad H, Valian K, Sayehmiri K, Taheri S. A comparative study on the efficacy of glucose and sucrose on the vaccination pain: a randomized controlled clinical trial. Journal of Clinical and Diagnostic Research 2014;8(10):PC01‐03. [PUBMED: 25478418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taddio 2008 {published data only}
- Taddio A, Shah V, Hancock R, Smith RW, Stephens D, Atenafu E, et al. Effectiveness of sucrose analgesia in newborns undergoing painful medical procedures. Canadian Medical Association Journal 2008;179:37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taddio 2011 {published data only}
- Taddio A, Shah V, Stephens D, Parvez E, Hogan ME, Kikuta A, et al. Effect of liposomal lidocaine and sucrose alone and in combination for venipuncture pain in newborns. Pediatrics 2011;127:e940‐7. [DOI] [PubMed] [Google Scholar]
Thakkar 2016 {published data only}
- Thakkar P, Arora K, Goyal K, Das RR, Javadekar B, Aiyer S, et al. To evaluate and compare the efficacy of combined sucrose and non‐nutritive sucking for analgesia in newborns undergoing minor painful procedure: a randomized controlled trial. Journal of Perinatology 2016;36(1):67‐70. [PUBMED: 26583940] [DOI] [PubMed] [Google Scholar]
Tutag Lehr 2015 {published data only}
- Tutag Lehr V, Cortez J, Grever W, Cepeda E, Thomas R, Aranda JV. Randomized placebo controlled trial of sucrose analgesia on neonatal skin blood flow and pain response during heel lance. The Clinical Journal of Pain 2015;31(5):451‐8. [PUBMED: 24918475] [DOI] [PubMed] [Google Scholar]
Unceta‐Barranechea 2008 {published data only}
- Unceta‐Barrenechea A, Saitua Iturriaga G, Sainz de Rozas Aparicio I, Riveira Fernández D. Analgesia when taking heel lance blood in the newborn [Analgesia en la toma sanguínea de talónen los recién nacidos]. Anales de Pediatria (Barcelona) 2008;69:544‐7. [DOI] [PubMed] [Google Scholar]
Yilmaz 2010 {published data only}
- Yilmaz F, Arikan D. The effects of various interventions to newborns on pain and duration of crying. Journal of Clinical Nursing 2010;20(7‐8):1008–17. [PUBMED: 21054600] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abad 1993 {published data only}
- Abad F, Diaz NM, Domenech E, Robayna M, Rico J, Arrecivita A, et al. Attenuation of pain related behavior in neonates given oral sweet solutions. 7th World Congress on Pain. Paris, 1993.
Abad 2001 {published data only}
- Abad F, Diaz‐Gomez NM, Domenech E, Gonzalez D, Robayna M, Feria M. Oral sucrose compares favourably with lidocaine‐prilocaine cream for pain relief during venepuncture in neonates. Acta Paediatrica 2001;90:160‐5. [PubMed] [Google Scholar]
Ahuja 2000 {published data only}
- Ahuja VK, Daga SR, Gosavi DV, Date AM. Non‐sucrose sweetener for pain relief in sick newborns. Indian Journal of Pediatrics 2000;67:487‐9. [DOI] [PubMed] [Google Scholar]
Akman 2002 {published data only}
- Akman I, Özek E, Bilgen H, Ozdogan T, Cebeci D. Sweet solutions and pacifiers for pain relief in newborn infants. Journal of Pain 2002;3(3):199‐202. [PUBMED: 14622773] [DOI] [PubMed] [Google Scholar]
Aziznejad 2013 {published data only}
- Aziznejad P, Zahed Pasha Y, Ahmadpour Kacho M, Haji Ahmadi M, Mohammadzadeh I, Arzani A, et al. Comparing the effect of oral sucrose, breast milk and EMLA cream on acute pain during venipuncture in full term neonates. Journal of Babol University of Medical Sciences 2013;15(3):16‐23. [Google Scholar]
Barbier 1994 {published data only}
- Barbier P, Lionnet C, Jonville AP, Hamon B, Autret E, Laugier J, et al. Does the placebo effect exist in newborn infants?. Therapie 1994;49:113‐6. [PubMed] [Google Scholar]
Barr 1993 {published data only}
- Barr RG, Oberlander T, Quek V, Brian J, Cassidy K‐L, Beauparlant J, et al. Dose‐response analgesic effect of intraoral sucrose in newborns [abstract]. Proceedings of the Society for Research in Child Development. 1993.
Barr 1995 {published data only}
- Barr RG, Young SN, Wright JH, Cassidy KL, Hendricks L, Bedad Y, et al. "Sucrose analgesia" and diphtheria‐tetanus‐pertussis immunizations at 2 and 4 months. Journal of Developmental Behavioral Pediatrics 1995;16:220‐5. [PubMed] [Google Scholar]
Bilgen 2001 {published data only}
- Bilgen H, Ozek E, Cebeci D, Ors R. Comparison of sucrose, expressed breast milk, and breast‐feeding on the neonatal response to heel prick. Journal of Pain 2001;2:301‐5. [DOI] [PubMed] [Google Scholar]
Blass 1991 {published data only}
- Blass EM, Hoffmeyer LB. Sucrose as an analgesic for newborn infants. Pediatrics 1991;87:215‐8. [PubMed] [Google Scholar]
Blass 1995 {published data only}
- Blass EM, Shah A. Pain‐reducing properties of sucrose in human newborns. Chemical Senses 1995;20:29‐35. [DOI] [PubMed] [Google Scholar]
Blass 2001 {published data only}
- Blass EM, Miller EW. Effects of colostrum in newborn humans. Dissociation between analgesic and cardiac effects. Journal of Developmental and Behavioral Pediatrics 2001;22:385‐90. [DOI] [PubMed] [Google Scholar]
Bucher 2000 {published data only}
- Bucher HU, Baumgartner R, Bucher N, Seiler M, Fauchere JC. Artificial sweetener reduces nociceptive reaction in newborn infants. Early Human Development 2000;59:56‐60. [DOI] [PubMed] [Google Scholar]
Curtis 2007 {published data only}
- Curtis SJ, Jou H, Ali S, Vandermeer B, Klassen T. A randomized controlled trial of sucrose and/or pacifier as analgesia for infants receiving venipuncture in a pediatric emergency department. BMC Pediatrics 2007;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dilli 2009 {published data only}
- Dilli D, Küçük IG, Dallar Y. Interventions to reduce pain during vaccination in infancy. Journal of Pediatrics 2009;154:385‐90. [DOI] [PubMed] [Google Scholar]
Efe 2007 {published data only}
- Efe E, Savaser S. The effect of two different methods used during peripheral venous blood collection on pain reduction in neonates. Journal of the Turkish Society of Algology 2007;19:49‐56. [PubMed] [Google Scholar]
Fernandez 2003 {published data only}
- Fernandez M, Blass EM, Hernandez‐Reif M, Field T, Diego M, Sanders C, et al. Sucrose attenuates a negative electroencephalographic response to an aversive stimulus for newborns. Journal of Developmental and Behavioral Pediatrics 2003;24:261‐6. [DOI] [PubMed] [Google Scholar]
Gibbins 2000 {published data only}
- Gibbins S, Stevens B, Ohlsson A, Hodnett E, Pinelli J. Safety and efficacy of sucrose for procedural pain in neonates. The 5th International Symposium on Paediatric Pain. London, 2000:P98.
Gormally 1996 {published data only}
- Gormally SM, Barr RG, Young SN, Alhawaf R, Wersheim L. Combined sucrose and carrying reduces newborn pain response more than sucrose or carrying alone. Archives of Pediatrics and Adolescent Medicine 1996;150:47. [Google Scholar]
Graillon 1997 {published data only}
- Graillon A, Barr RG, Young SN, Wright JH, Hendricks LA. Differential response to intraoral sucrose, quinine and corn oil in crying human newborns. Physiology and Behavior 1997;62:317‐25. [DOI] [PubMed] [Google Scholar]
Harrison 2011 {published data only}
- Harrison D, Loughnan P, Manias E, Smith K, Johnston L. Effect of concomitant opioid analgesics and oral sucrose during heel lancing. Early Human Development 2011;87:147–9. [DOI] [PubMed] [Google Scholar]
Isik 2000b {published data only}
- Isik U, Ozek E, Bilgen H, Ors R, Cebeci D, Basaran M. Comparison of oral dextrose and sucrose solutions on pain response in neonates. Pediatric Research 2000;47:403A. [DOI] [PubMed] [Google Scholar]
Johnston 2000 {published data only}
- Johnston C. The efficacy of sucrose analgesia for procedural pain in preterm infants < 32 weeks in the first week of life [abstract]. Pediatric Research 2000;47:405A. [Google Scholar]
Joung 2010 {published data only}
- Joung KH, Cho SC. The effect of sucrose on infants during a painful procedure. Korean Journal of Pediatrics 2010;53:790‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewindon 1998 {published data only}
- Lewindon PJ, Harkness L, Lewindon N. Randomized controlled trial of sucrose by mouth for the relief of infant crying after immunization. Archives of Disease in Childhood 1998;78:453‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandel 2012 {published data only}
- Mandel R, Ali N, Chen J, Galic IJ, Levesque L. Nitrous oxide analgesia during retinopathy screening: a randomised controlled trial. Archives of Disease in Childhood. Fetal Neonatal Edition 2012;97:F83‐87. [DOI] [PubMed] [Google Scholar]
Mellah 1999 {published data only}
- Mellah D, Gourrier E, Merbouche S, Mouchino G, Crumiere C, Leraillez J. Analgesia with saccharose during heel capillary prick. A randomized study in 37 newborns of over 33 weeks of amenorrhea [Analgesie au saccharose lors des prelevements capillaires au talon. Etude randomisee chez 37 nouveau‐nes de plus de 33 semaines d'amenorrhee]. Archives of Pediatrics 1999;6:610‐6. [DOI] [PubMed] [Google Scholar]
Mohan 1998 {published data only}
- Mohan CG, Risucci DA, Casimir M, Gulrajani‐LaCorte M. Comparison of analgesics in ameliorating the pain of circumcision. Journal of Perinatology 1998;18:13‐9. [PubMed] [Google Scholar]
Ozdogan 2010 {published data only}
- Ozdogan T, Akman I, Cebeci D, Bilgen H, Ozek E. Comparison of two doses of breast milk and sucrose during neonatal heel prick. Pediatrics International 2010;52:175‐9. [DOI] [PubMed] [Google Scholar]
Ramenghi 2002 {published data only}
- Ramenghi LA, Webb AV, Shevlin PM, Green M, Evans DJ, Levene MI. Intra‐oral administration of sweet‐tasting substances and infants' crying response to immunization: a randomized, placebo‐controlled trial. Biology of the Neonate 2002;81:163‐9. [DOI] [PubMed] [Google Scholar]
Razmus 2004 {published data only}
- Razmus IS, Dalton ME, Wilson D. Pain management for newborn circumcision. Pediatric Nursing 2004;30:414‐7. [PubMed] [Google Scholar]
Reis 2003 {published data only}
- Reis EC, Roth EK, Syphan JL, Tarbell SE, Holubkov R. Effective pain reduction for multiple immunization injections in young infants. Archives of Pediatrics and Adolescent Medicine 2003;157:1115‐20. [DOI] [PubMed] [Google Scholar]
Sahebihag 2011 {published data only}
- Sahebihag MH, Hosseinzadeh M, Mohammadpourasl A, Kosha A. The effect of breastfeeding, oral sucrose and combination of oral sucrose and breastfeeding in infant's pain relief during the vaccination. Iranian Journal of Nursing and Midwifery Research 2011;16:9–15. [Google Scholar]
Scaramuzzo 2013 {published data only}
- Scaramuzzo RT, Faraoni M, Polica E, Pagani V, Vagli E, Boldrini A. Skin conductance variations compared to ABC scale for pain evaluation in newborns. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(14):1399–403. [PUBMED: 23566033] [DOI] [PubMed] [Google Scholar]
Skogsdal 1997 {published data only}
- Skogsdal Y, Eriksson M, Schollin J. Analgesia in newborns given oral glucose. Acta Paediatrica 1997;86:217‐20. [DOI] [PubMed] [Google Scholar]
Stevens 1997b {published data only}
- Stevens B, Johnston C, Franck L, Petryshen P, Jack A, Narciso J, et al. Nonpharmacologic interventions for decreasing procedural pain in preterm neonates. Fourth International Symposium on Pediatric Pain. Helsinki, 1997:154.
Stevens 2000 {published data only}
- Stevens B, Petryshen P, Johnston C, Franck L, Jack A. The influence of consistent pain management on neonatal outcomes: preliminary findings. The 5th International Symposium on Paediatric Pain. London, UK, 2000:P96.
Taddio 2000 {published data only}
- Taddio A, Pollock N, Gilbert‐MacLeod C, Ohlsson K, Koren G. Combined analgesia and local anesthetic to minimize pain during circumcision. Archives of Pediatrics and Adolescent Medicine 2000;154:620‐3. [DOI] [PubMed] [Google Scholar]
Taddio 2003 {published data only}
- Taddio A, Shah V, Shah P, Katz J. Beta‐endorphin concentration after administration of sucrose in preterm infants. Archives of Pediatrics and Adolescent Medicine 2003;157:1071‐4. [DOI] [PubMed] [Google Scholar]
Taddio 2009b {published data only}
- Taddio A, Shah V, Katz J. Reduced infant response to a routine care procedure after sucrose analgesia. Pediatrics 2009;123:e425‐9. [DOI] [PubMed] [Google Scholar]
Vederhus 2006 {published data only}
- Vederhus BJ, Eide GE, Natvig G. Psychometric testing of a Norwegian version of the Premature Infant Pain Profile: an acute pain assessment tool. A clinical validation study. International Journal of Nursing Practice 2006;12:334‐44. [DOI] [PubMed] [Google Scholar]
Yoon 2001 {published data only}
- Yoon HB. Pain relieving effect of intraoral sucrose replacement in neonates. Korean Journal of Child Health 2001;7:35‐50. [Google Scholar]
References to studies awaiting assessment
Moradi 2012a {published data only}
- Moradi F, Imani A, Keyghobadi S, Nazari H, Ghorbani R, Keyghobadi T. Assessment of the effect of 20% oral sucrose on pain relief from Hepatitis B vaccine injection in full term infants. Journal of Zanjan University of Medical Sciences and Health Services 2012;20:79. [Google Scholar]
Moradi 2012b {published data only}
- Moradi F, Imani A, Keyghobadi S, Nazari H, Ghorbani R, Keyghobadi T, et al. Effects of intra‐oral intake of different concentrations of sucrose on biobehavioral pain response to immunizations in infants. Koomesh 2012;13(4):414‐9. [Google Scholar]
Singh 2001 {published data only}
- Singh M. The need for analgesia and sedation in newborn babies. Perinatology 2001;3:240‐2. [Google Scholar]
References to ongoing studies
Campbell‐Yeo 2013 {published data only}
- Campbell‐Yeo M, Johnston C, Benoit B, Latimer M, Vincer M, Walker CD, et al. Trial of repeated analgesia with Kangaroo Mother Care (TRAKC Trial). BMC Pediatrics 2013; Vol. 13:182. [PUBMED: 24284002] [DOI] [PMC free article] [PubMed]
ISRCTN59514984 {published data only}
- ISRCTN59514984. Randomised controlled trial of sucrose analgesia for repeated capillary blood sampling. Controlled‐trials.com. Identifier ISRCTN59514984, 2006.
ISRCTN73259137 {published data only}
- ISRCTN73259137. Kangaroo mother care combined with sucrose to reduce pain responses in preterm infants. Controlled‐trials.com Identifier:, 2010.
Montanholi 2012 {published data only}
- Montanholi LL. Effect of skin‐to‐skin compared to sucrose for pain relief in infants undergoing repeated painful procedures: randomized clinical trial. www.ensaiosclinicos.gov.br/rg/RBR‐7nynr7/. RBR‐7nynr7, 2012.
NCT01190995 {published data only}
- Datta V. Role of repeated painful procedures in preterm neonates on short term neurobehavioural outcome. ClinicalTrials.gov. Identifier: NCT01190995, 2010.
NCT01438008 {published data only}
- Harrison D. Pilot study of sucrose to reduce pain in sick babies. NCT01438008. ClinicalTrials.gov Identifier: NCT01438008. ClinicalTrials.gov, 2011.
NCT01552993 {published data only}
- Bergseng H. Registration and treatment of pain during eye examination of prematurity. ClinicalTrials.gov. Identifier: NCT01552993, 2012.
NCT01742520 {published data only}
- Morag I. Neurodevelopmental outcomes of preterm infants treated for pain management with repeated doses of sucrose 24%. ClinicalTrials.gov. Identifier: NCT01742520, 2012.
NCT01800318 {published data only}
- Hall RW. Does noninvasive electrical stimulation of acupuncture points (NESAP) reduce heel stick pain in infants?. ClinicalTrials.gov. Identifier: NCT01800318, 2011.
NCT01894659 {published data only}
- Angeles DM. Oral Sucrose Versus Glucose for Procedural Pain in Premature Neonates. ClinicalTrials.gov. NCT01894659 2013.
NCT01931020 {published data only}
- Datta V. Analgesic effect of oral 25% glucose versus oral 24% sucrose for pain relief during heel lance in preterm neonates. ClinicalTrials.gov. Identifier: NCT01931020, 2013.
NCT02133716 {published data only}
- Gómez LC. Efficacy of breast milk expressed and sucrose in procedural pain in preterm (LACTEET). ClinicalTrials.gov. Identifier: NCT02133716, 2013.
NCT02344368 {published data only}
- NCT02344368. The effect of sucrose on pain relief during venous blood sampling in preterm infants: a comparison of two different doses. ClinicalTrials.gov NCT02344368. ClinicalTrials.gov, 2015.
Passariello 2014 {published data only}
- Passariello A. Efficacy of oral sucrose in newborns exposed to painful stimuli. ANZCTR 12614000164695, 2014.
Philip 2012 {published data only}
- Philip S. Effectiveness of human breast milk, table sugar vs sterile water for pain relief in premature infants undergoing a heel prick procedure in nursery of Christian Medical College and Hospital, Vellor. http://ctri.nic.in/Clinicaltrials/login.php. CTRI/2012/06/002730, 2012.
Roue 2013 {published data only}
- Roue J‐M. Prevention of the procedural pain in the newborn (ACTISUCROSE). ClinicalTrials.gov. NCT02109263, 2013.
Shah 2015 {published data only}
- Shah S. Comparing efficacy of music therapy, sucrose and combination of the two in neonates for pain relief during heel prick procedure. [Computer program]. Version ACTRN12615000271505. ANZCTR (Australian New Zealand Trials Registry), 2015.
Stevens 2014 {published data only}
- Stevens B. Sucrose practices for pain in neonates. NCT02134873. ClinicalTrials.gov 2014.
Additional references
Anseloni 2002
- Anseloni VC, Weng HR, Terayama R, Letizia D, Davis BJ, Ren K, et al. Age‐dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain 2002;97:93‐103. [DOI] [PubMed] [Google Scholar]
Anseloni 2005
- Anseloni VCZ, Ren K, Dubner R, Ennis M. A brainstem substrate for analgesia elicited by intraoral sucrose. Neuroscience 2005;133(1):231‐43. [DOI] [PubMed] [Google Scholar]
Bartocci 2006
- Bartocci M, Bergqvist LL, Lagercrantz H, Anand KJ. Pain activates cortical areas in preterm newborn brain. Pain 2006;122(1‐2):109‐17. [DOI] [PubMed] [Google Scholar]
Blass 1989
- Blass EM, Shide DJ, Weller A. Stress‐reducing effects of ingesting milk, sugars, and fats. A developmental perspective. Annals of the New York Academy of Sciences 1989;575:292‐305. [DOI] [PubMed] [Google Scholar]
Blass 1994
- Blass E, Ciaramitaro V. A new look at some old mechanisms in human newborns: taste and tactile determinants of state, affect and action. Monographs of the Society for Research in Child Development 1994;59:1‐80. [PubMed] [Google Scholar]
Brummelte 2012
- Brummelte S, Grunau RE, Chau V, Poskitt KJ, Brant R, Vinall J, et al. Procedural pain and brain development in premature newborns. Annals of Neurology 2012;71(3):385‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bueno 2013
- Bueno M, Yamada J, Harrison D, Khan S, Ohlsson A, Adams‐Webber T, et al. A systematic review and meta‐analyses of non‐sucrose sweet solutions for pain relief in neonates. Pain Research & Management 2013;18(3):153‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campos 1994
- Campos RG. Rocking and pacifier; two comforting interventions for heel stick pain. Research in Nursing and Health 1994;17:321‐31. [DOI] [PubMed] [Google Scholar]
Carbajal 1997
- Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier Martin M. APN: evaluation behavioral scale of acute pain in newborn infants [DAN : une échelle comportementale d’évaluation de la douleur aiguë du nouveau‐né]. Archives Pediatrie 1997;4(7):623‐8. [DOI] [PubMed] [Google Scholar]
Carbajal 2008
- Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. Journal of the American Medical Association 2008;300:60‐70. [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen M, Shi X, Chen Y, Cao Z, Cheng R, Xu Y, et al. A prospective study of pain experience in a neonatal intensive care unit in China. Clinical Journal of Pain 2012;28(8):700‐4. [DOI] [PubMed] [Google Scholar]
Cignacco 2004
- Cignacco E, Mueller R, Hamers JPH, Gessler P. Pain assessment in the neonate using the Bernese Pain Scale for Neonates. Early Human Development 2004;78:125–31. [DOI] [PubMed] [Google Scholar]
DiPietro 1994
- DiPietro JA, Cusson RM, Caughy MO, Fox NA. Behavioral and physiologic effects of nonnutritive sucking during gavage feeding in preterm infants. Pediatric Research 1994;36:207‐14. [DOI] [PubMed] [Google Scholar]
Dunbar 2006
- Dunbar AE 3rd, Sharek PJ, Mickas NA, Coker KL, Duncan J, McLendon D, et al. Implementation and case‐study results of potentially better practices to improve pain management of neonates. Pediatrics 2006;118:S87‐94. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2015
- Fitzgerald M. What do we really know about newborn infant pain?. Experimental Physiology 2015;100(12):1451‐7. [DOI] [PubMed] [Google Scholar]
Gibbins 2008
- Gibbins S, Stevens B, McGrath PJ, Yamada J, Beyene J, Breau L, et al. Comparison of pain responses in infants of varying gestational ages. Neonatology 2008;93:10‐8. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version 20150131. Hamilton, ON: GRADE Working Group, McMaster University, 2014.
Grunau 1987
- Grunau RE, Craig KD. Pain expression in neonates: facial action and cry. Pain 1987;28(3):395‐410. [DOI] [PubMed] [Google Scholar]
Grunau 2013
- Grunau RE. Long‐term effects of pain in children. In: McGrath P, Stevens B, Walker S, Zempsky W editor(s). Oxford Textbook of Paediatric Pain. Oxford, UK: Oxford University Press, 2013:30‐8. [Google Scholar]
Gunnar 1992
- Gunnar MR. Reactivity of the hypothalamic‐pituitary‐adrenocortical system to stressors in normal infants and children. Pediatrics 1992;90:491‐7. [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt G, Oxman AD, Aklm EA, Kunz R, Vist G, Brozeka J. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407–15. [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294–302. [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐CoelloP, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283–93. [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303–10. [DOI] [PubMed] [Google Scholar]
Harrison 2009
- Harrison D, Loughnan P, Mania E, Gordon I, Johnston L. Repeated doses of sucrose in infants continue to reduce procedural pain during prolonged hospitalizations. Nursing Research 2009;58(6):427‐34. [DOI] [PubMed] [Google Scholar]
Harrison 2010
- Harrison D, Stevens B, Bueno M, Yamada J, Adams‐Webber T, Beyene J, et al. Efficacy of sweet solutions for analgesia in infants between 1 and 12 months of age: a systematic review. Archives of Disease in Childhood 2010;95:406‐13. [DOI] [PubMed] [Google Scholar]
Harrison 2012
- Harrison D, Beggs S, Stevens B. Sucrose for procedural pain management in infants. Pediatrics 2012;130(5):918‐25. [DOI] [PubMed] [Google Scholar]
Harrison 2013
- Harrison D, Anseloni VCZ, Yamada J, Bueno M. Sucrose and sweet taste. In: McGrath P, Stevens B, Walker S, Zempsky W editor(s). Oxford Textbook of Paediatric Pain. Oxford, UK: Oxford University Press, 2013:508‐16. [Google Scholar]
Higgins 2011 [Computer program]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version Version 5.1.0. The Cochrane Collaboration, 2011.
Holsti 2005
- Holsti L, Grunau RE, Oberlander TF, Whitfield MF. Prior pain induces heightened motor responses during clustered care in preterm infants in the NICU. Early Human Development 2005;81(3):293‐302. [DOI] [PubMed] [Google Scholar]
Hummel 2010
- Hummel P, Lawlor‐Klean P, Weiss MG. Validity and reliability of the N‐PASS assessment tool with acute pain. Journal of Perinatology 2010;30:474‐8. [DOI] [PubMed] [Google Scholar]
Jeong 2014
- Jeong IS, Park SM, Lee JM, Choi YJ, Lee J. The frequency of painful procedures in neonatal intensive care units in South Korea. International Journal of Nursing Practice 2014;20(4):398‐407. [DOI] [PubMed] [Google Scholar]
Johnston 2007
- Johnston CC, Filion F, Snider L, Limperopoulos C, Majnemer A, Pelausa E, et al. How much sucrose is too much sucrose?. Pediatrics 2007;119:226. [DOI] [PubMed] [Google Scholar]
Johnston 2011
- Johnston CC, Barrington KJ, Taddio A, Carbajal R, Filion F. Pain in Canadian NICUs: have we improved over the past 12 years?. Clinical Journal of Pain 2011;27(3):225‐32. [DOI] [PubMed] [Google Scholar]
Kyololo 2014
- Kyololo OM, Stevens B, Gastaldo D, Gisore P. Procedural pain in neonatal units in Kenya. Archives of Disease in Childhood. Fetal and Neonatal Edition 2014;99(6):F464‐7. [DOI] [PubMed] [Google Scholar]
Lago 2013
- Lago P, Boccuzzo G, Garetti E, Pirelli A, Pieragostini L, Merazzi D, et al. Pain management during invasive procedures at Italian NICUs: has anything changed in the last five years?. The Journal of Maternal‐Fetal and Neonatal Medicine 2013;26(3):303‐5. [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12:59‐66. [PubMed] [Google Scholar]
Lee 2014
- Lee GY, Yamada J, Kyololo O, Shorkey A, Stevens B. Pediatric pain clinical practice guidelines for acute procedural pain: a systematic review. Pediatrics 2014;133(3):500‐15. [DOI] [PubMed] [Google Scholar]
Lefrak 2006
- Lefrak L, Burch K, Caravantes R, Knoerlein K, DeNolf N, Duncan J, et al. Sucrose analgesia: identifying potentially better practices. Pediatrics 2006;118:S197‐202. [DOI] [PubMed] [Google Scholar]
Lemyre 2006
- Lemyre B, Sherlock R, Hogan D, Gaboury I, Blanchard C, Moher D. How effective is tetracaine 4% gel, before a peripherally inserted central catheter, in reducing procedural pain in infants: a randomized double‐blind placebo controlled trial. BMC Medicine 2006;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linhares 2011
- Linhares MB, Gaspardo CM, Martinez FE. Oral sucrose for procedural pain in infants: letter. Lancet 2011;377:27‐8. [DOI] [PubMed] [Google Scholar]
Pillai Riddell 2015
- Pillai Riddell RR, Racine NM, Gennis HG, Turcotte K, Uman LS, Horton RE, et al. Non‐pharmacological management of infant and young child procedural pain. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD006275.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Powell 2001
- Powell CV, Kelly AM, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Annals of Emergency Medicine 2001;37:28‐31. [DOI] [PubMed] [Google Scholar]
Ranger 2014
- Ranger M, Grunau RE. Early repetitive pain in preterm infants in relation to the developing brain. Pain Management 2014;4(1):57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ren 1997
- Ren K, Blass EM, Zhou Q, Dubner R. Suckling and sucrose ingestion suppress persistent hyperalgesia and spinal Fos expression after forepaw inflammation in infant rats. Proceedings of the National Academy of Sciences of the United States of America 1997;94(4):1471‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roofthooft 2014
- Roofthooft DW, Simmons SH, Anand KJ, Tibboel D, Dijk M. Eight years later, are we still hurting newborn infants?. Neonatology 2014;105(3):218‐26. [DOI] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Übertragbarkeit von Studienergebnissen]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendations. http://gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html 2013.
Shah 2004
- Shah V, Ipp M, Sam J, Einarson TR, Taddio A. Eliciting the minimally clinically important difference in the pain response from parents of newborn infants and nurses. Canadian Pediatric Society 81st Meeting. Montreal, QC: Pulsus Group, 2004.
Shah 2006
- Shah PS, Aliwalas LL, Shah V. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004950.pub2] [DOI] [PubMed] [Google Scholar]
Sharek 2006
- Sharek PJ, Powers R, Koehn A, Anand KJ. Evaluation and development of potentially better practices to improve pain management in neonates. Pediatrics 2006;118:S78‐86. [DOI] [PubMed] [Google Scholar]
Shide 1989
- Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10‐day‐old rats. Behavioral Neuroscience 1989;103(6):1168‐75. [DOI] [PubMed] [Google Scholar]
Slater 2006
- Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, et al. Cortical pain responses in human infants. Journal of Neuroscience 2006;26(14):3662‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Snider 2005
- Snider L, Tremblay S, Limperopoulos C, Majnemer A, Filion F, Johnston C. Construct validity of the Neurobehavioral Assessment of Preterm Infants. Physical & Occupational Therapy in Pediatrics 2005;25(3):81‐95. [PubMed] [Google Scholar]
Stevens 1994
- Stevens B, Johnston C, Horton L. Factors that influence the behavioral responses of premature infants. Pain 1994;59:101‐9. [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile; development and initial validation. Clinical Journal of Pain 1996;12(1):13‐22. [DOI] [PubMed] [Google Scholar]
Stevens 1997a
- Stevens B, Taddio A, Ohlsson A, Einarson T. The efficacy of sucrose for relieving procedural pain in neonates ‐ a systematic review and meta‐analysis. Acta Paediatrica 1997;86:837‐42. [DOI] [PubMed] [Google Scholar]
Stevens 2005b
- Stevens B, Yamada J, Beyene J, Gibbins S, Petryshen P, Stinson J, et al. Consistent management of repeated procedural pain with sucrose in preterm neonates: is it effective and safe for repeated use over time?. Clinical Journal of Pain 2005;12(6):543‐48. [DOI] [PubMed] [Google Scholar]
Stevens 2011
- Stevens B, Craig K, Johnston C, Harrison D, Ohlsson A. Oral sucrose for procedural pain in infants: letter. Lancet 2011;377:27‐8. [DOI] [PubMed] [Google Scholar]
Stevens 2014a
- Stevens B, Gibbins S, Yamada Y, Dionne K, Lee G, Taddio A. The Premature Infant Pain Profile ‐ revised: initial validation and feasibility. Clinical Journal of Pain 2014;30(3):238‐43. [DOI] [PubMed] [Google Scholar]
Stevens 2014b
- Stevens B, Yamada J, Promislow S, Stinson J, Harrison D, and the CIHR Team in Children's Pain. Implementation of multidimensional knowledge translation strategies to improve procedural pain in hospitalized children. Implementation Science 2014;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taddio 2009a
- Taddio A, Yiu A, Smith RW, Katz J, McNair C, Shah V. Variability in clinical practice guidelines for sweetening agents in newborn infants undergoing painful procedures. Clinical Journal of Pain 2009;25(2):153‐5. [DOI] [PubMed] [Google Scholar]
Tu 2007
- Tu MT, Grunau RE, Petrie‐Thomas J, Haley DW, Weinberg J, Whitfield MF. Maternal stress and behavior modulate relationships between neonatal stress, attention, and basal cortisol at 8 months in preterm infants. Developmental Psychobiology 2007;49(2):150‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dijk 2009
- Dijk M, Roofthooft DWE, Anand KJS, Guldemond F, Graaf J, Simmons S, et al. Taking up the challenge of measuring prolonged pain in neonates. The COMFORTneo Scale seems promising. Clinical Journal of Pain 2009;25(7):607‐16. [DOI] [PubMed] [Google Scholar]
Vinall 2014a
- Vinall J, Miller SP, Bjornson BH, Fitzpatrick KPV, Poskitt KJ, Brant R, et al. Invasive procedures in preterm children: brain and cognitive development at school age. Pediatrics 2014;133(3):412‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinall 2014b
- Vinall J, Grunau RE. Impact of repeated procedural pain‐related stress in infants born very preterm. Pediatric Research 2014;75(5):584‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walia 1999
- Walia R, Ohlsson A. Clinical trials in neonatal medicine. 7th Annual Cochrane Colloquium, Abstracts, October 1999. Rome, 1999.
Walker 2013
- Walker SM. Neuropathic pain in children. In: McGrath P, Stevens B, Walker S, Zempsky W editor(s). Oxford Textbook of Paediatric Pain. Oxford University Press, 2013:205‐14. [Google Scholar]
Zwicker 2013
- Zwicker JG, Grunau RE, Adams E, Chau V, Brant R, Poskitt KJ, et al. Score for neonatal acute physiology‐II and neonatal pain predict corticospinal tract development in premature newborns. Pediatric Neurology 2013;48(2):123‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Stevens 1998
- Stevens B, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD001069.pub2] [DOI] [PubMed] [Google Scholar]
Stevens 2001
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001069.pub2] [DOI] [PubMed] [Google Scholar]
Stevens 2004
- Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001069.pub2] [DOI] [PubMed] [Google Scholar]
Stevens 2010
- Stevens B, Yamada J, Ohlsson, A. Sucrose for analgesia in newborns infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD001069.pub3] [DOI] [PubMed] [Google Scholar]
Stevens 2013
- Stevens B, Yamada J, Lee GY, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD001069.pub4] [DOI] [PubMed] [Google Scholar]


