Abstract
Background
This is an updated version of the original Cochrane review published in Issue 8, 2011, on 'Drug therapy for treating post‐dural puncture headache'.
Post‐dural puncture headache (PDPH) is the most common complication of lumbar puncture, an invasive procedure frequently performed in the emergency room. Numerous pharmaceutical drugs have been proposed to treat PDPH but there are still some uncertainties about their clinical effectiveness.
Objectives
To assess the effectiveness and safety of drugs for treating PDPH in adults and children.
Search methods
The searches included the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 6), MEDLINE and MEDLINE in Process (from 1950 to 29 July 2014), EMBASE (from 1980 to 29 July 2014) and CINAHL (from 1982 to July 2014). There were no language restrictions.
Selection criteria
We considered randomised controlled trials (RCTs) assessing the effectiveness of any pharmacological drug used for treating PDPH. Outcome measures considered for this review were: PDPH persistence of any severity at follow‐up (primary outcome), daily activity limited by headache, conservative supplementary therapeutic option offered, epidural blood patch performed, change in pain severity scores, improvements in pain severity scores, number of days participants stay in hospital, any possible adverse events and missing data.
Data collection and analysis
Review authors independently selected studies, assessed risk of bias and extracted data. We estimated risk ratios (RR) for dichotomous data and mean differences (MD) for continuous outcomes. We calculated a 95% confidence interval (CI) for each RR and MD. We did not undertake meta‐analysis because the included studies assessed different sorts of drugs or different outcomes. We performed an intention‐to‐treat (ITT) analysis.
Main results
We included 13 small RCTs (479 participants) in this review (at least 274 participants were women, with 118 parturients after a lumbar puncture for regional anaesthesia). In the original version of this Cochrane review, only seven small RCTs (200 participants) were included. Pharmacological drugs assessed were oral and intravenous caffeine, subcutaneous sumatriptan, oral gabapentin, oral pregabalin, oral theophylline, intravenous hydrocortisone, intravenous cosyntropin and intramuscular adrenocorticotropic hormone (ACTH).
Two RCTs reported data for PDPH persistence of any severity at follow‐up (primary outcome). Caffeine reduced the number of participants with PDPH at one to two hours when compared to placebo. Treatment with caffeine also decreased the need for a conservative supplementary therapeutic option.
Treatment with gabapentin resulted in better visual analogue scale (VAS) scores after one, two, three and four days when compared with placebo and also when compared with ergotamine plus caffeine at two, three and four days. Treatment with hydrocortisone plus conventional treatment showed better VAS scores at six, 24 and 48 hours when compared with conventional treatment alone and also when compared with placebo. Treatment with theophylline showed better VAS scores compared with acetaminophen at two, six and 12 hours and also compared with conservative treatment at eight, 16 and 24 hours. Theophylline also showed a lower mean "sum of pain" when compared with placebo. Sumatriptan and ACTH did not show any relevant effect for this outcome.
Theophylline resulted in a higher proportion of participants reporting an improvement in pain scores when compared with conservative treatment.
There were no clinically significant drug adverse events.
The rest of the outcomes were not reported by the included RCTs or did not show any relevant effect.
Authors' conclusions
None of the new included studies have provided additional information to change the conclusions of the last published version of the original Cochrane review. Caffeine has shown effectiveness for treating PDPH, decreasing the proportion of participants with PDPH persistence and those requiring supplementary interventions, when compared with placebo. Gabapentin, hydrocortisone and theophylline have been shown to decrease pain severity scores. Theophylline has also been shown to increase the proportion of participants that report an improvement in pain scores when compared with conventional treatment.
There is a lack of conclusive evidence for the other drugs assessed (sumatriptan, adrenocorticotropic hormone, pregabalin and cosyntropin).
These conclusions should be interpreted with caution, due to the lack of information to allow correct appraisal of risk of bias, the small sample sizes of the studies and also their limited generalisability, as nearly half of the participants were postpartum women in their 30s.
Plain language summary
Drugs for treating headache after a lumbar puncture
Lumbar puncture involves getting a sample of spinal fluid though a needle inserted into the lower back. Post‐dural puncture headache (PDPH) is the most common side effect of a lumbar puncture. The symptom of PDPH is a constant headache that gets worse when upright and improves when lying down. Lots of drugs are used to treat PDPH, so the aim of this review was to assess the effectiveness of these drugs.
This is an updated review, and we searched for new trials in July 2014. We included 13 small randomised clinical trials (RCTs), with a total of 479 participants. The trials assessed eight drugs: caffeine, sumatriptan, gabapentin, hydrocortisone, theophylline, adrenocorticotropic hormone, pregabalin and cosyntropin. Caffeine proved to be effective in decreasing the number of people with PDPH and those requiring extra drugs (2 or 3 in 10 with caffeine compared to 9 in 10 with placebo). Gabapentin, theophylline and hydrocortisone also proved to be effective, relieving pain better than placebo or conventional treatment alone. More people had better pain relief with theophylline (9 in 10 with theophylline compared to 4 in 10 with conventional treatment). No important side effects of these drugs were reported.
The quality of the studies was difficult to assess due to the lack of information available. Conclusions should be interpreted with caution.
Background
Description of the condition
This review is an update of a review previously published in the Cochrane Database of Systematic Reviews (Issue 8, 2011) on 'Drug therapy for treating post‐dural puncture headache'.
Post‐dural (post‐lumbar or post‐spinal) puncture headache (PDPH) is one of the most common complications of diagnostic, therapeutic or inadvertent lumbar punctures (Bezov 2010; Davignon 2002). PDPH is defined as any headache after a lumbar puncture that worsens within 15 minutes of sitting or standing and is relieved within 15 minutes of lying down (International Headache Society 2004). Ninety per cent of PDPHs occur within three days of the procedure and 66% start in the first 48 hours (Turnbull 2003).
The pathophysiology of PDPH has not been fully described. It is well known that the puncture in the dura allows cerebrospinal fluid (CSF) to leak from the subarachnoid space, resulting in a decrease of CSF volume and pressure (Grande 2005). This CSF volume loss may cause a downward pull on pain‐sensitive structures resulting in a headache (Ahmed 2006; Baumgarten 1987; Davignon 2002; Denny 1987; Harrington 2004). Alternatively, the loss of CSF may cause an increase in blood flow, resulting in arterial and venous vasodilatation and PDPH. A third explanation for PDPH involves the role of P substance and the regulation of neurokinin‐1 receptors (NK1R) (Clark 1996).
Occurrence of PDPH varies from 1% to 40%, according to the needle gauge, needle orientation, operator skill level and presence of risk factors such as age group or history of PDPH (Turnbull 2003). This frequency is related to the type of lumbar puncture. During anaesthetic procedures, such as epidural anaesthesia, PDPH is most commonly caused by an unintentional dural puncture (Thew 2008; Turnbull 2003). In contrast to the aforementioned, in diagnostic or therapeutic lumbar punctures, the need for adequate CSF flow requires an intentional lesion that may generate the PDPH phenomenon (Kuczkowski 2006). Estimated frequencies vary from less than 10% following spinal anaesthesia (Hafer 1997; Vallejo 2000), to 36% for diagnostic lumbar punctures (Lavi 2006; Vallejo 2000), and up to 81% in obstetric patients with inadvertent dural puncture during active labour (Banks 2001). Reported risk of inadvertent dural puncture placement during epidural anaesthesia in an obstetric population ranges from 0.04% to 6% (Berger 1998; Choi 2003). Therefore, obstetric analgesia is probably the main source of PDPH patients.
The features of PDPH are often variable. PDPH may be accompanied by neck stiffness, tinnitus, hearing loss, photophobia or nausea; other features, such as the location and duration, are also unpredictable (Grande 2005). Although PDPH is not a life‐threatening condition, physical activity is often restricted. Likewise patients are usually required to stay in bed the whole day and length of stay as well as medical attendance increases (Angle 2005).
The variability of symptoms makes PDPH a diagnosis of exclusion. Other alternative diagnoses should be ruled out (e.g. viral meningitis, sinus headache or intracranial haemorrhage) (Turnbull 2003). Once PDPH is diagnosed, the initial treatment involves conservative measures such as bed rest and analgesics. If PDPH continues for more than 72 hours, a more specific treatment is indicated (Ahmed 2006). Severe PDPH may respond to some therapeutic drugs and administration of an epidural blood patch (Lavi 2006).
How the intervention might work
Due to the fact that no clear pathophysiology has been asserted for PDPH, many therapeutic options are used to relieve headache in clinical practice and also essayed in clinical trials: epidural blood patch (EBP) mechanically blocks the leakage of CSF; postures such as a prone position reduce pressure in the subarachnoid space and allow a seal to form over the dura; hydration increases CSF production (Ahmed 2006); methylxanthines, sumatriptan and caffeine increase vasoconstriction of cerebral blood vessels and adrenocorticotropic hormone (ACTH) increases intravascular volume (Kuczkowski 2006).
Treatment drugs should help to decrease the duration of headache, reduce headache severity as much as possible, avoid the need for any other therapeutic option (e.g. EBP), improve daily activity, reduce the length of hospital stay and decrease the occurrence of adverse events overall.
Why it is important to do this review
Three Cochrane systematic reviews about the prevention of PDPH have been published (Arévalo‐Rodríguez 2013; Basurto 2013; Boonmak 2010), and one is in process (Newman 2010). The treatment and management of PDPH is also focused on in Boonmak 2010.
Numerous therapeutic drugs have been proposed, based on limited randomised controlled trials (RCTs) and case series, including: analgesics, caffeine, theophylline, sumatriptan, epidural route administration of adrenocorticotropic hormones, morphine, 0.9% sodium chloride or dextran (Choi 1996; Turnbull 2003). The sample sizes of most of these trials are small and there is inconsistency among them, therefore there is weak evidence to support the drug treatment of PDPH.
Current uncertainties about the clinical effectiveness of treatment drugs require a systematic review to clarify their potential benefits and inspire future guidelines on the topic.
Objectives
To assess the effectiveness and safety of drugs for treating PDPH in adults and children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) (parallel, cross‐over or factorial) in any setting. We excluded studies using alternation, date of birth, hospital record number or other quasi‐random methods of allocation of treatment.
Types of participants
Participants undergoing lumbar punctures for any of the reasons outlined: CSF sampling or pressure measurement, or both, spinal anaesthesia, myelography, intrathecal drug administration, or accidental puncture of the dura during epidural anaesthesia. We included individuals of all ages and either sex.
The use of a standardised diagnostic criteria for PDPH was not required, but we expected that it was at least described as orthostatic headache, which worsened on standing and was improved by lying down. We described the specific diagnostic criteria used in each included study.
Types of interventions
We considered any pharmacological drug used for treating PDPH. Acceptable control groups included: placebo, no intervention, any other drug treatments, behavioural and physical therapies. We considered interventions at any dose, formulation or route of administration given after lumbar puncture.
Types of outcome measures
Primary outcomes
PDPH persistence of any severity at follow‐up. We considered the rate of persistent PDPH at short (< 12 hours), medium (< 24 hours) or long‐term (≥ 24 hours) follow‐up.
Secondary outcomes
Daily activity limited by headache.
Conservative supplementary therapeutic option offered when trial drug intervention fails to relieve headache and following trial protocol (e.g. bed rest, fluid consumption, analgesics).
Epidural blood patch performed, administered when intervention drug and conservative option fail to relieve headache and following trial protocol.
Change in pain severity scores as defined by the trialist.
Improvements in pain severity scores as defined by the trialist.
Number of days participants stay in hospital.
Any possible adverse events from pharmacological drugs taken to treat PDPH.
Missing data (withdrawals, drop‐outs and participants lost to follow‐up).
Search methods for identification of studies
We designed the search in the context of an extensive review about the prevention and treatment drugs used for PDPH.
The Cochrane Central Register of Controlled Trials (CENTRAL) was our primary source for identifying studies.
Our search terms were a combination of thesaurus‐based and free‐text terms covering both the procedure of interest (dural puncture performed for diagnosis, anaesthesia or myelography) and headache. For MEDLINE, EMBASE and CINAHL we used a modified version of the strategy used to search CENTRAL.
We considered articles written in any language.
In addition, we searched the reference lists of all studies and review articles identified by electronic searching. We requested information about any potentially relevant studies when we contacted trialists from every included study.
Electronic searches
We searched:
the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 6);
MEDLINE and MEDLINE in Process (from 1950 to 29 July 2014);
EMBASE (from 1980 to 29 July 2014); and
CINAHL (from 1982 to July 2014).
The search strategies for CENTRAL, MEDLINE, EMBASE and CINAHL can be found in Appendix 1, Appendix 2, Appendix 3 and Appendix 4 respectively.
Searching other resources
For future updates, we will also search reference lists of reviews and retrieved articles for additional studies and perform citation searches on key articles. For future updates, we will also contact experts in the field for unpublished and ongoing trials.
Data collection and analysis
Selection of studies
Two independent review authors (XB, DO) screened titles and abstracts of studies identified by the literature search for eligibility. We resolved disagreements through discussion. We retrieved eligible studies in full to confirm whether or not they fulfilled the inclusion criteria. Review authors were not blinded to the authors' names and institutions, journal of publication or study results at this or any stage of the review.
Data extraction and management
For included studies, we used specially designed, pre‐tested data forms to extract information from the original studies on participants, methods of randomisation and blinding, the comparison(s) of interest, the number of participants originally randomised in each arm of the study, any losses to follow‐up and the occurrence in each arm of the outcomes of interest. If information on any of these was incomplete, we attempted to obtain it by writing to the study author concerned.
One review author (XB) extracted the data from studies and a second review author (DO) checked data for accuracy, resolving any disagreement by discussion. We entered data into Review Manager 5.3.
When efficacy outcomes were reported in dichotomous form (primary outcome and all secondary outcomes except change in pain severity (outcome number 4) and number of days participants stay in hospital (outcome number 6)), we recorded the number of participants assigned to each treatment arm and the number with each outcome.
For outcomes reported on a continuous scale (change in pain severity (outcome number 4) and number of days participants stay in hospital (outcome number 6)), we recorded data on the variance associated with their means.
In future updates of this review, when a study reports pre and post‐treatment group means, without reporting data on the variance associated with these means, we will attempt to calculate or estimate variances based on primary data or test statistics, if these are reported. When a study uses pre and post‐treatment scores to calculate a change score for each participant, and then uses these within‐patient change scores to calculate a group mean change score, we will record and analyse these group mean change scores. When only post‐treatment data are available, we will use these, relying on allocation to achieve between‐group balance. If these calculations are needed, we will perform a sensitivity analyses excluding the studies involved, to assess the impact of the calculations.
We recorded the proportion of participants reporting adverse events for each treatment arm wherever possible. We recorded the identity and rates of specific adverse events.
Assessment of risk of bias in included studies
We used The Cochrane Collaboration's tool for assessing risk of bias in the studies included in this review, which addresses six specific domains (Higgins 2009), summarised in a specific table. For this review we assessed five of the domains (sequence generation, allocation concealment, blinding, incomplete outcome data and selective outcome reporting). Each domain has a description of what was reported. One review author (XB) completed the risk of bias judgements for each study and a second review author (DO) checked these for accuracy, resolving any disagreement by discussion.
Assessment of heterogeneity
This review did not include a meta‐analysis.
In future updates of this review, if needed, we will assess heterogeneity of effect sizes by means of the Q (Chi2 test) using the methods of Peto and Mantel‐Haenszel. If statistical evidence exists for homogeneity of effect sizes, the planned analysis will use a fixed‐effect model.
When significant heterogeneity is present (Chi2 test with P value < 0.1 or I2 statistic value greater than 50%), we will make an attempt to explain the differences based on the clinical characteristics of the included studies. We will not statistically combine studies that are dissimilar in terms of interventions and participants. However, when a group of studies with heterogeneous results appears to be similar, we will combine the study estimates using a random‐effects model (Higgins 2002; Higgins 2003).
Data synthesis
The differences between the studies included in this review, in terms of interventions assessed and outcomes measured, only permitted a narrative summary.
We analysed the results for different drugs separately using Review Manager 5.3. We performed analysis on an intention‐to‐treat (ITT) basis, i.e. all participants remained in their original trial arm, whether or not they actually received the intervention allocated.
We used dichotomous data to calculate risk ratios (RR) with 95% confidence intervals (CI). In future updates of this review, we hope to calculate the numbers needed to treat for an additional beneficial outcome (NNTB) with 95% CI, as the reciprocal of the risk difference (RD) (McQuay 1998). We will use data on the proportion of participants reporting adverse events to calculate the RD and numbers needed to treat for an additional harmful outcome (NNTH) with 95% CI for significant differences.
For continuous outcomes reported using the same scale, we calculated mean differences (MD) with 95% CI. In future updates of this review, we hope to calculate standardised mean differences (SMD) for pooling results of continuous outcomes measured with different scales.
Subgroup analysis and investigation of heterogeneity
In future updates of this review, when sufficient data are available, we plan to carry out the following subgroup analyses:
Follow‐up time subgroup analyses
When possible, we will assess the impact of the assessed interventions at short (< 12 hours), medium (12 to 24 hours) or long‐term time periods (≥ 24 hours) for the treatment drugs.
Population subgroup analyses
Where data allow in the future, we plan to conduct separate outcome analyses to test the following null hypotheses:
there is no difference between obstetric participants and all other participants;
there is no difference between men and non‐obstetric women participants;
there is no difference between young participants (18 to 35 years old) and all other adult participants.
Sensitivity analysis
In future updates of this review, we will conduct a sensitivity analyses formulated a priori:
We will examine the effect on the primary outcome of excluding any study judged to be at a high risk of bias by two of the domains, sequence generation and allocation concealment.
If applicable we will also perform a sensitivity analysis excluding those studies with a cross‐over design.
Results
Description of studies
See the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
We identified 1975 references in primary electronic databases on July 2014 from our extended search strategy for prevention and treatment drugs for PDPH. We excluded 1941 references after a detailed reading of the title and abstract. We obtained the full‐text report for the rest of the studies (33 papers) to check if they strictly fulfilled all the inclusion criteria. We finally excluded 18 studies after a complete full‐text review and we contacted the study authors by email in some cases when more information was needed to decide eligibility (Figure 1). Thirteen studies in 15 articles published completely fulfilled the inclusion criteria for this review (Alam 2012; Camann 1990; Connelly 2000; Dogan 2006; Erol 2011; Feuerstein 1986; Huseyinoglu 2011; Mahoori 2013; Noyan 2007; Rucklidge 2004; Sechzer 1978; Sen 2014; Zeger 2012).
1.
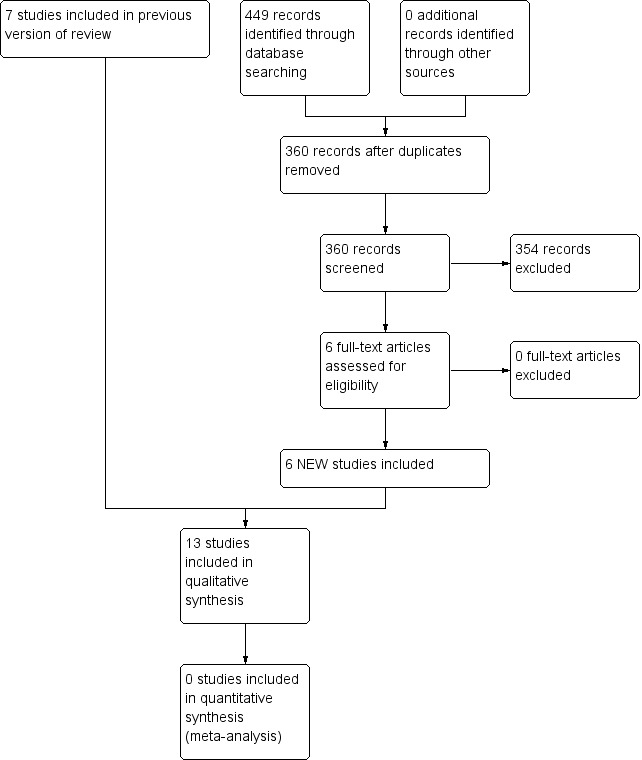
Study flow diagram.
Included studies
In the original version of this Cochrane review, only seven studies were included. In this update we added six new RCTs. All 13 included studies are detailed in the 'Characteristics of included studies' table.
Study design
All 13 included studies (involving a total of 479 participants) were RCTs with a parallel design. Most of them were placebo‐controlled, except five, which used a treatment control group (Erol 2011; Mahoori 2013; Noyan 2007; Sen 2014; Zeger 2012).
Setting
Only Rucklidge 2004 was a multicentric study with five hospitals involved.
Four studies were conducted in the USA (Camann 1990; Connelly 2000; Sechzer 1978; Zeger 2012), three in Turkey (Dogan 2006; Erol 2011; Huseyinoglu 2011), two in Iran (Noyan 2007; Mahoori 2013), one in the UK (Rucklidge 2004), one in Germany (Feuerstein 1986), one in Bangladesh (Alam 2012), and one in India (Sen 2014).
All the studies recruited the participants from hospital settings and the intervention took place while they were admitted.
Sample size
The studies included a total of 479 participants suffering from PDPH. The smallest study had 10 participants (Connelly 2000) and the largest studies had 60 (Alam 2012; Mahoori 2013; Noyan 2007).
Only three RCTs described how the sample size was calculated (Mahoori 2013; Rucklidge 2004; Zeger 2012).
Participants
Slightly more than half of the participants were women (at least 274/434), with 118 parturients after a lumbar puncture for a regional anaesthesia. All obstetric participants came from three RCTs that only included women (Camann 1990; Noyan 2007; Rucklidge 2004). Sechzer 1978 did not report statistics about gender. The median age among participants ranged from 24 to 47 years old.
Intervention
Eight of the 13 studies compared placebo with different drugs: oral theophylline (Feuerstein 1986), oral (Camann 1990) or intravenous (Sechzer 1978) caffeine, subcutaneous sumatriptan (Connelly 2000), oral gabapentin (Dogan 2006), intramuscular adrenocorticotropic hormone (ACTH) (Rucklidge 2004), oral pregabalin (Huseyinoglu 2011), or intravenous hydrocortisone (Alam 2012).
Intravenous hydrocortisone was compared with conventional care (bed rest, hydration, acetaminophen and pethidine) in Noyan 2007, oral gabapentin was compared with oral ergotamine plus caffeine in Erol 2011 and intravenous cosyntropin was compared with intravenous caffeine in Zeger 2012. Theophylline was compared with acetaminophen in Mahoori 2013 and with conservative treatment (bed rest, caffeinated beverages, opioid and/or non‐steroidal anti‐inflammatory drug (NSAID)) in Sen 2014.
Caffeine was assessed in five RCTs: compared with placebo in Camann 1990 and Sechzer 1978 with different routes of administration but at equipotent doses, and compared with cosyntropin in Zeger 2012. Also gabapentin was compared with caffeine plus ergotamine in Erol 2011 and also caffeine was used as a conservative treatment (bed rest, caffeinated beverages opioids and/or NSAID) compared with theophylline in Sen 2014.
Oral theophylline was assessed in three RCTs, compared with placebo in Feuerstein 1986, compared with acetaminophen in Mahoori 2013, and compared with conventional treatment in Sen 2014.
Six included studies used an epidural blood patch (EBP) as a supplementary analgesic in case the intervention drug failed to resolve the headache (Alam 2012; Camann 1990; Connelly 2000; Erol 2011; Noyan 2007; Rucklidge 2004).
Follow‐up differed between studies but the most common length of follow‐up was 48 hours in four studies (Connelly 2000; Huseyinoglu 2011; Noyan 2007; Rucklidge 2004). The shortest one was Zeger 2012, in which patients were discharged from the emergency room at 120 minutes if the pain was down and the longest was Huseyinoglu 2011, with five days. Two studies did not report length of follow‐up (Feuerstein 1986; Sechzer 1978).
Outcomes of interest
Sechzer 1978 and Zeger 2012 reported data on the primary outcome, proportion of participants with PDPH persistence of any level of severity at follow‐up.
The most reported secondary outcome, described by all included RCTs except Sechzer 1978, was the change in pain severity scores. The outcome was reported directly or could be calculated with the results for pain severity scores documented during the follow‐up. The second most reported secondary outcome was the number of any possible adverse events from the pharmacological drug, described by all included studies except by Dogan 2006 and Sechzer 1978.
Six RCTs reported data regarding the proportion of participants with EBP performed (Alam 2012; Camann 1990; Connelly 2000; Erol 2011; Noyan 2007; Rucklidge 2004).
Five RCTs reported the amount of missing data (withdrawals, drop‐outs and participants lost to follow‐up) (Erol 2011; Feuerstein 1986; Huseyinoglu 2011; Mahoori 2013; Zeger 2012).
The proportion of participants with a conservative supplementary therapeutic option offered when the trial drug intervention failed was reported in four RCTs (Feuerstein 1986; Huseyinoglu 2011; Sechzer 1978; Zeger 2012).
The proportion of participants showing improvements in pain severity scores was detailed in three RCTs (Camann 1990; Connelly 2000; Sen 2014).
There were two secondary outcomes not reported in the included RCTs: proportion of participants with daily activity limited by existence of headache and the number of days participants stayed in hospital.
Conflict of interest
Only one study stated that it had been funded by a grant from Glaxo (Connelly 2000). Only one study stated having no conflict of interest (Mahoori 2013).
Excluded studies
In this updated version of the review we did not exclude any of the six new eligible trials identified. In the original Cochrane review, 18 studies did not fulfil the inclusion criteria and we excluded them. The two most frequent reasons for exclusion were not being a RCT in five studies (Aguilera 1988; De las Heras 1997; Eldor 1990; Hakim 2005; Hodgson 1997), and not assessing an individual pharmacological drug intervention in five studies (Bart 1978; Naja 2009; Oedit 2005; Sandesc 2005; van Kooten 2008). In four studies the reason for exclusion was because the intervention was not aiming to treat PDPH (Basso 1985; Flaatten 1987; Widerlöv 1979; Zenglein 1978). In three RCTs the reason was not describing the orthostatic component of headache (Lang 1993; Schwalbe 1991; Torres 1986). Finally, in one case the reason was that the study used quasi‐randomisation (Ergün 2008). For a summary of the reasons for exclusion please see the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Risk of bias in the included studies is summarised in Figure 2 and Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Allocation sequence was adequately generated in five RCTs (Alam 2012; Connelly 2000; Mahoori 2013; Rucklidge 2004; Sechzer 1978). Connelly 2000 did not report the method used to generate the sequence but after contacting the study author, a computer random series was confirmed. Alam 2012, Mahoori 2013 and Rucklidge 2004 explicitly reported a computer‐generated random numbers sequence and Sechzer 1978 used a table of random numbers.
The other eight studies did not report the method used for sequence generation (Camann 1990; Dogan 2006; Erol 2011; Feuerstein 1986; Huseyinoglu 2011; Noyan 2007; Sen 2014; Zeger 2012).
Allocation concealment
Four studies had adequately concealed randomisation sequences: Connelly 2000 by sealed containers (confirmed by e‐mail), Rucklidge 2004 via an independent office (confirmed by e‐mail) and Sechzer 1978 and Zeger 2012 by pharmacy‐controlled randomisation.
The other nine included studies did not provide information regarding allocation concealment (Alam 2012; Camann 1990; Dogan 2006; Erol 2011; Feuerstein 1986; Huseyinoglu 2011; Mahoori 2013; Noyan 2007; Sen 2014)
Blinding
The blinding method was adequate in seven of the included studies (Camann 1990; Connelly 2000; Feuerstein 1986; Noyan 2007; Rucklidge 2004; Sechzer 1978; Zeger 2012). Six RCTs did not report detailed data to allow assessment of blinding (Alam 2012; Dogan 2006; Erol 2011; Huseyinoglu 2011; Mahoori 2013; Sen 2014).
Incomplete outcome data
All RCTs included in this review, except Feuerstein 1986 and Zeger 2012, had a low risk of attrition bias. The studies detailed data for all the participants that were randomised at the beginning of the trials. We judged Feuerstein 1986 and Zeger 2012 as at high risk of attrition bias.
Selective reporting
None of the studies (Alam 2012; Camann 1990; Connelly 2000; Dogan 2006; Erol 2011; Feuerstein 1986; Huseyinoglu 2011; Mahoori 2013; Noyan 2007; Rucklidge 2004; Sechzer 1978; Sen 2014 ), except Zeger 2012, reported results for key outcomes (PDPH persistence of any severity at follow‐up and number of any possible adverse events) that would be expected to have been reported for such a study.
Other potential sources of bias
For this update, we measured the size of study to check for possible biases confounded by small size. We assessed studies as being at low risk of bias (200 or more participants per treatment arm), unclear risk of bias (50 to 199 participants per treatment arm) and high risk of bias (fewer than 50 participants per treatment arm).
We considered all included trials as high risk of bias due to small size, because all had fewer than 50 participants per treatment arm.
Effects of interventions
We present in this section a narrative synthesis of the results for the different outcomes of interest.
Post‐dural puncture headache (PDPH) persistence of any severity at follow‐up
Two studies included data for the primary outcome of the review. Sechzer 1978 showed a statistically significant risk ratio when comparing intravenous caffeine sodium benzoate with placebo. This trial reported that 5 out of 20 participants in the caffeine group and 18 out of 21 participants in the placebo group had persistence of PDPH at follow‐up, with a risk ratio (RR) of 0.29, 95% confidence interval (CI) 0.13 to 0.64. Zeger 2012 showed a non‐significant risk ratio when comparing intravenous cosyntropin with intravenous caffeine, in which 8 out of 18 participants in the cosyntropin group and 3 out of 15 participants in the caffeine group had persistence of PDPH at follow‐up with a RR of 2.22, 95% CI 0.71 to 6.92.
Conservative supplementary therapeutic option offered when trial drug intervention fails to relieve headache
Four studies reported this outcome. Sechzer 1978 showed a statistically significant risk ratio for conservative supplementary therapeutic option when comparing intravenous caffeine sodium benzoate with placebo. This trial reported that 5 out of 20 participants in the caffeine group and 18 out of 21 participants in the placebo group needed conservative supplementary therapeutic options, with a RR of 0.29, 95% CI 0.13 to 0.64.
Feuerstein 1986 showed a non‐significant risk ratio when comparing theophylline with placebo, in which 2 out of 6 participants in the theophylline group and 4 out of 5 participants in the placebo group needed conservative supplementary therapeutic options, with a RR of 0.42, 95% CI 0.12 to 1.40.
Huseyinoglu 2011 showed a non‐significant risk ratio when comparing oral pregabalin with placebo, in which 6 out of 20 participants in the pregabalin group and 8 out of 20 participants in the placebo group needed conservative supplementary therapeutic options, with a RR of 0.50, 95% CI 0.05 to 5.08. Most of the events, 11 out of 14, were recorded at 24 hours of follow‐up.
Zeger 2012 showed a non‐significant risk ratio when comparing intravenous cosyntropin with intravenous caffeine, in which 8 out of 18 participants in the cosyntropin group and 3 out of 15 participants in the caffeine group needed conservative supplementary therapeutic options, with a RR of 2.22, 95% CI 0.71 to 6.92.
Epidural blood patch (EBP) performed
The studies that reported this outcome did not show significant differences (Alam 2012; Camann 1990; Connelly 2000; Erol 2011; Noyan 2007; Rucklidge 2004 ).
Camann 1990 showed that the risk ratio for EBP performed was statistically non‐significant when comparing caffeine with placebo. This trial reported that 7 out of 20 participants in the caffeine group and 11 out of 20 participants in the placebo group needed an EBP, with a RR of 0.64, 95% CI 0.31 to 1.30.
Connelly 2000 showed a non‐significant risk ratio when comparing sumatriptan with placebo. This trial reported that 4 out of 5 participants in the sumatriptan group and all 5 participants in the placebo group needed an EBP, with a RR of 0.82, 95% CI 0.49 to 1.38.
Rucklidge 2004 showed a non‐significant risk ratio when comparing adrenocorticotropic hormone (ACTH) with placebo, in which 6 out of 9 participants in the ACTH group and 7 out of 9 in the placebo group needed an EBP, with a RR of 0.86, 95% CI 0.48 to 1.53.
Noyan 2007 showed a non‐significant risk ratio when comparing hydrocortisone with control group where only one participant in the control group needed an EBP, with a RR of 0.33, 95% CI 0.01 to 7.87.
Erol 2011 reported that no patient received an EBP.
Alam 2012 showed a non‐significant risk ratio when comparing hydrocortisone with placebo, in which 1 out of 30 participants in each group needed an EBP, with a RR of 1.00, 95% CI 0.07 to 15.26.
Change in pain severity scores as defined by the trialist
All studies included in the review, except Sechzer 1978, reported this outcome. Pain severity was measured by means of visual analogue scale (VAS) scores (Alam 2012; Camann 1990; Connelly 2000; Dogan 2006; Erol 2011; Huseyinoglu 2011; Mahoori 2013; Rucklidge 2004; Noyan 2007; Sen 2014; Zeger 2012) or by mean sum of pain (Feuerstein 1986).
In Feuerstein 1986 a mean sum of pain among the participants during the treatment period was used to compare both groups. Treatment with theophylline showed a significant lower mean sum of pain when compared with placebo (11 participants; theophylline 16 (standard deviation (SD) 3.91), placebo 28 (SD 4.73), mean difference (MD) ‐12.00, 95% CI ‐17.19 to ‐6.81).
Camann 1990 reported statistically similar baseline VAS scores for the caffeine group and for the placebo group (40 participants; MD 9.00, 95% CI ‐0.80 to 18.80). At four hours, pain scores decreased in both groups, but did not show a significant difference (40 participants; MD ‐16.00, 95% CI ‐34.07 to 2.07). This result was also shown at 24 hours post‐treatment (40 participants; MD 7.00, 95% CI ‐18.10 to 32.10).
Connelly 2000 showed statistically similar VAS scores at baseline (10 participants; MD ‐26.00, 95% CI ‐55.14 to 3.14) and when comparing sumatriptan with placebo after one hour (10 participants; MD ‐18, 95% CI ‐55.73 to 19.73).
Rucklidge 2004 (18 participants) comparing a synthetic analogue of ACTH versus intramuscular saline 0.9% reported no significant differences for this outcome, but all the results were reported in a figure.
Dogan 2006 also reported a statistically similar baseline VAS score (20 participants; MD 0.20, 95% CI ‐0.17 to 0.57). Gabapentin showed a significant decrease in VAS scores when compared with placebo. The study showed a progressive reduction in VAS scores for participants receiving gabapentin after one, two and three days of follow‐up (20 participants; one day: gabapentin 4.1 (SD 0.31), placebo 5.7 (SD 0.42), MD ‐1.60, 95% CI ‐1.92 to ‐1.28; two days: gabapentin 1.8 (SD 0.29), placebo 4.4 (SD 0.33), MD ‐2.60, 95% CI ‐2.87 to ‐2.33; three days: gabapentin 0.3 (SD 0.15), placebo 3.2 (SD 0.29), MD ‐2.90, 95% CI ‐3.10 to ‐2.70). The effect was reduced after four days of follow‐up (20 participants; gabapentin 0.1 (SD 0.1), placebo 1.7 (SD 0.21), MD ‐1.60, 95% CI ‐1.74 to ‐1.46).
Noyan 2007 reported a statistically similar baseline VAS score (60 participants; MD 0.13, 95% CI ‐0.22 to 0.48). Hydrocortisone showed a significant decrease in VAS scores when compared with conventional care. The studies showed a progressive reduction in pain scores for the participant receiving hydrocortisone at six hours and 24 hours of follow‐up (60 participants; six hours: hydrocortisone 2.77 (SD 1.07), conventional treatment 6.63 (SD 1.35), MD ‐3.86, 95% CI ‐4.48 to ‐3.24; 24 hours: hydrocortisone 0.73 (SD 0.74), conventional treatment 3.87 (SD 1.63), MD ‐3.14, 95% CI ‐3.78 to ‐2.50). The effect was reduced at 48 hours of follow‐up (60 participants; hydrocortisone 0.63 (SD 0.61), conventional treatment 1.87 (SD 0.93), MD ‐1.24, 95% CI ‐1.64 to ‐0.84).
Erol 2011 reported a statistically similar baseline VAS score (42 participants; MD 0.20, 95% CI ‐0.07 to 0.47). Gabapentin showed a significant decrease in VAS scores when compared with control. After one day, VAS scores were statistically similar (42 participants; MD ‐0.15, 95% CI ‐0.73 to 0.43), but the studies showed a progressive reduction in pain scores for the participants receiving gabapentin at two, three and four days of follow‐up (42 participants; two days: gabapentin 1.23 (SD 1.17), conventional treatment 3.09 (SD 1.17), MD ‐1.86, 95% CI ‐2.57 to ‐1.15; three days: gabapentin 0.04 (SD 0.92), conventional treatment 2.42 (SD 0.92), MD ‐2.38, 95% CI ‐2.94 to ‐1.82). At four days (gabapentin 0 (SD 0), conventional treatment 1.09 (SD 0.04)) a mean difference could not be estimated.
Huseyinoglu 2011 reported change in VAS score between pregabalin and placebo at baseline and at one, two, three, four and five days only on a graph basis. The study did not report the numerical mean and standard deviation for pain VAS score during follow‐up. Interpreting the VAS score graph, pregabalin showed a significant decrease in VAS scores when compared with placebo during all follow‐up.
Alam 2012 reported a statistically similar baseline VAS score (60 participants; MD 0.15, 95% CI ‐0.52 to 0.82). Hydrocortisone showed a significant decrease in VAS scores when compared with placebo. The studies showed a reduction in pain scores for the participant receiving hydrocortisone at 6, 24 and 48 hours of follow‐up (60 participants; six hours: hydrocortisone 2.06 (SD 1.98), placebo 6.02 (SD 2.46), MD ‐3.96, 95% CI ‐5.09 to ‐2.83; 24 hours: hydrocortisone 0.94 (SD 2.67), placebo 3.77 (SD 1.85), MD ‐2.83, 95% CI ‐3.99 to ‐1.67; 48 hours: hydrocortisone 0.69 (SD 1.64), placebo 1.95 (SD 1.12), MD ‐1.26, 95% CI ‐1.97 to ‐0.55).
Zeger 2012 reported a statistically similar baseline VAS score (33 participants; MD ‐4.00, 95% CI ‐14.58 to 6.58). The mean difference between cosyntropin and caffeine at 60 and 120 minutes could not be estimated because the trial only reported means; the standard deviations were only reported in graphic. Analysing the VAS graph, there are no statistically significant differences between drugs at 60 and 120 minutes.
Mahoori 2013 reported a statistically similar baseline VAS score (60 participants; MD ‐0.5, 95% CI ‐1.14 to 0.14). Theophylline showed a significant decrease in VAS scores when compared with acetaminophen. The studies showed a consistent reduction in pain scores for the participant receiving theophylline at two, six and 12 hours of follow‐up (60 participants; two hours: theophylline 5 (SD 1.57), acetaminophen 5.97 (SD 1.27), MD ‐0.97, 95% CI ‐1.69 to ‐0.25; six hours: theophylline 3.43 (SD 1.73), acetaminophen 4.33 (SD 1.49), MD ‐0.90, 95% CI ‐1.72 to ‐0.08; 12 hours: theophylline 2.67 (SD 2.35), acetaminophen 4.24 (SD 1.97), MD ‐1.57, 95% CI ‐2.67 to ‐0.47).
Sen 2014 reported a statistically similar baseline VAS score (40 participants; MD ‐1.10, 95% CI ‐4.33 to 2.13). Theophylline showed a significant decrease in VAS scores when compared with conservative treatment. The studies showed a reduction in pain scores for the participants receiving theophylline at eight, 16 and 24 hours of follow‐up (40 participants; eight hours: theophylline 2.7 (SD 1.9), conservative treatment 46 (SD 40.3), MD ‐43.30, 95% CI ‐60.98 to ‐25.62; 16 hours: theophylline 13.4 (SD 28.3), conservative treatment 57.7 (SD 41.9), MD ‐44.30, 95% CI ‐66.46 to ‐22.14; 24 hours: theophylline 11.8 (SD 30), conservative treatment 66.5 (SD 39.1), MD ‐54.70, 95% CI ‐76.3 to ‐33.10).
Improvements in pain severity scores as defined by the trialist
Camann 1990 showed a marginally significant difference in the rate of participants with an improvement when receiving caffeine compared to placebo. This trial reported that 18 out of 20 participants in the caffeine group and 12 out of 20 participants in the placebo group improved in pain severity scores, with a RR of 1.50, 95% CI 1.02 to 2.21.
Connelly 2000 reported an improvement for two participants, one in each group (two events in 10 participants; RR 1.00, 95% CI 0.08 to 11.93). While the effect of sumatriptan was maintained until the end of follow‐up (48 hours), the participant in the placebo group worsened after 13 hours from the injection.
Sen 2014 showed a statistically significant risk ratio for improvements in pain severity scores when comparing theophylline with conservative treatment after eight, 16 and 24 of follow‐up. At eight hours all participants in the theophylline group and 10 out of 20 participants in the conservative group improved in pain severity, with a RR of 1.95, 95% CI 1.27 to 3.01. At 16 hours 18 out of 20 participants in the theophylline group and 8 out of 20 participants in the conservative group improved in pain severity, with a RR of 2.25, 95% CI 1.29 to 3.92. At 24 hours 18 out of 20 participants in the theophylline group and 6 out of 20 participants in the conservative group improved in pain severity, with a RR of 3.00, 95% CI 1.51 to 5.95.
Any possible adverse events from pharmacological drug taken to treat PDPH
Feuerstein 1986 reported one participant in each group with gastric pain. Camann 1990 reported one participant in each group with transient flushing and anxiety.
Huseyinoglu 2011 and Sen 2014 reported adverse events without severe clinical significance, but details on these adverse events or the group affected were not reported. Huseyinoglu 2011 reported "Dizziness and sleepiness were reported by some patients in the pregabalin group, but these symptoms were neither serious nor persistent and did not result in withdrawal from the study" (page 1366) and Sen 2014 reported that "we found least side‐effects" and "minimal side effects" (page 117).
Alam 2012, Dogan 2006, Erol 2011, Mahoori 2013, Noyan 2007, Rucklidge 2004 and Zeger 2012 reported no adverse events.
Missing data (withdrawals, drop‐outs and participants lost to follow‐up)
Feuerstein 1986 and Zeger 2012 did not report sufficient information about participants randomised who dropped out: 5/16 and 5/37 participants respectively.
Daily activity limited by headache
This outcome was not reported by the included RCTs.
Number of days participants stayed in hospital
This outcome was not reported by the included RCTs.
Discussion
Summary of main results
This updated systematic review identified three randomised controlled trials (RCTs) assessing caffeine for treating post‐dural puncture headache (PDPH) (Camann 1990; Sechzer 1978; Zeger 2012), three RCTs assessing theophylline (Feuerstein 1986; Mahoori 2013; Sen 2014), two RCTs assessing hydrocortisone (Alam 2012; Noyan 2007), two assessing gabapentin (Dogan 2006; Erol 2011), and four RCTs assessing other different drugs for treating PDPH: sumatriptan (Connelly 2000), adrenocorticotropic hormone (ACTH) (Rucklidge 2004), pregabalin (Huseyinoglu 2011), and cosyntropin (Zeger 2012). Some data were available for PDPH persistence of any severity at follow‐up (Sechzer 1978; Zeger 2012), and for changes in pain severity scores derived from visual analogue scale (VAS) measures.
For PDPH persistence (primary outcome), intravenous caffeine sodium benzoate showed a significant decrease in the proportion of participants with PDPH persistence when compared with placebo in Sechzer 1978.
For the changes in pain severity scores outcome, gabapentin showed a significant decrease in pain scores when compared to placebo in Dogan 2006, with differences at one, two and three days and decreased after four days of the intervention. Gabapentin also showed in Erol 2011 a significant decrease in pain scores when compared to ergotamine plus caffeine at two, three and four days. Hydrocortisone showed a significant decrease in pain scores when compared with conventional care in Noyan 2007, with differences that were sustained at six and 24 hours and decreased after 48 hours of the intervention. Hydrocortisone also showed in Alam 2012 a significant decrease in pain scores when compared with placebo at six, 24 and 48 hours of follow‐up. Theophylline showed a significant decrease in pain scores when compared with acetaminophen at two, six and 12 hours in Mahoori 2013 and also in Sen 2014 when theophylline was compared with conservative treatment at eight, 16 and 24 hours. Theophylline showed a significant lower mean sum of pain when compared with placebo in Feuerstein 1986.
The minimum clinically significant difference in acute pain VAS score has been poorly investigated, although some published studies have estimated it to be around 9 to 17 on a 0 to 100 VAS score (Gallagher 2002; Kelly 1998; Kelly 2001; Mark 2009; Todd 1996). RCTs included in this review with statistically significant mean differences in VAS scores reported numbers around 2 to 4 on a 0 to 10 VAS score, giving these values a clinically significant difference.
For the conservative supplementary therapeutic option, intravenous caffeine sodium benzoate showed a significant decrease in the proportion of participants needing supplementary interventions when compared with placebo in Sechzer 1978.
For the number of participants with improvements in pain severity scores, oral theophylline showed in Sen 2014 a significant and progressive better result in the proportion of participants that reported an improvement in pain scores when compared with conservative treatment (bed rest, caffeine beverages, injectable opioids and/or non‐steroidal anti‐inflammatory drug (NSAID)). Two studies showed a marginal effect favouring caffeine (Camann 1990) and sumatriptan (Connelly 2000) over placebo in the proportion of participants that reported an improvement in pain score.
The drugs assessed in the included studies did not show any relevant effect for the rest of outcomes of interest for this review. The proportion of participants that required an epidural blood patch (EBP) was similar between the interventions and their controls in six studies (Alam 2012; Camann 1990; Connelly 2000; Erol 2011; Noyan 2007; Rucklidge 2004).
The studies did not report any clinically significant adverse event derived from any of the assessed drugs (Alam 2012; Camann 1990; Dogan 2006; Erol 2011; Feuerstein 1986; Huseyinoglu 2011; Mahoori 2013; Noyan 2007; Rucklidge 2004; Sen 2014; Zeger 2012).
We found four different drugs (caffeine, theophylline, gabapentin and hydrocortisone) that were evaluated in several RCTs but we did not undertake meta‐analysis. Two RCTs used equipotent doses of caffeine but we did not undertake meta‐analysis because they reported different outcomes (Camann 1990; Sechzer 1978). Caffeine was used in three other RCTs and we could not undertake meta‐analysis because comparators were different, cosyntropin in Zeger 2012, or because caffeine was used in conjunction with other drugs, caffeine plus ergotamine in Erol 2011 and bed rest, caffeinated beverages, opioids and/or NSAID in Sen 2014. Oral theophylline was assessed in three RCTs and we did not undertake meta‐analysis because they were compared with different drugs; with placebo in Feuerstein 1986, with acetaminophen in Mahoori 2013, and with conservative treatment in Sen 2014. Gabapentin was assessed in two RCTs with different comparators: with placebo in Dogan 2006 and ergotamine plus caffeine in Erol 2011. Finally, intravenous hydrocortisone was evaluated in two RCTs: compared with placebo (Alam 2012) and compared with conventional care (bed rest, hydration, acetaminophen and pethidine) in Noyan 2007.
Overall completeness and applicability of evidence
All participants included in this review were recruited from acute care hospitals and their characteristics seemed to be similar to patients seen in usual clinical practice. The lumbar punctures were performed during a hospital stay, which is the most common setting for this technique. Most of the participants in the included studies underwent lumbar puncture to administer regional anaesthesia (spinal and epidural anaesthesia), which is the most common reason for lumbar puncture. No lumbar puncture in the included studies was done for diagnostic purposes. Sumatriptan, gabapentin, pregabalin, theophylline and hydrocortisone, used in the included studies, are widely marketed and frequently used. Caffeine, ACTH and cosyntropin are also commercialised but for more specific indications and therefore they are less widely available. Outcomes reported from the included studies were patient‐relevant but only two RCTs, Sechzer 1978 and Zeger 2012, reported data on the primary outcome, proportion of participants with PDPH persistence of any level of severity at follow‐up. The most reported secondary outcome, described by all included RCTs except Sechzer 1978, was the change in pain severity scores.
Quality of the evidence
The outlined results should be interpreted with caution due to the quality of the evidence. The limited number of studies identified, the diversity of drugs assessed and outcomes measured, the small sample sizes of the studies included and the bias presented limits the quality of the evidence identified in the review. We judged the size of the study as a risk of bias as high in all the trials included. We also judged the reporting bias risk as high in all except one of the included RCTs and there is also an important lack of data reported to allow correct appraisal of the risk of other sources of bias. The short duration of the included studies does not allow us to know the effect of the drugs that showed some effects in the mid‐term. This lack of applicability of the results is similar to that observed in another Cochrane review assessing EBP for treating PDPH (Boonmak 2010).
Larger studies (reporting how sample size was determined) with an extended duration, similar to the follow‐up in the study involving gabapentin (at least four days) (Dogan 2006), and the use of more pragmatic outcomes such as the persistence of pain at follow‐up and possible adverse events from pharmacological drugs, should provide more information on the impact of the assessed drugs in this setting and situation.
Potential biases in the review process
We conducted this review in accordance with the previously published protocol (Basurto 2009). We are unaware of any biases in the review process. To minimise bias, the selection, assessment for inclusion eligibility, risk of bias and data extraction were done independently by more than one review author. We also contacted study authors for clarification of study data. None of the review authors have been involved in any of the included studies and none have any commercial or other conflict of interest.
Agreements and disagreements with other studies or reviews
We have found no other systematic review specifically assessing the efficacy of drugs for treating PDPH. All the systematic reviews found refer to techniques and drugs for preventing PDPH; techniques such as needle bevel orientation, needle design and size, orientation and positioning following the procedure, prophylactic EBP, intrathecal catheters, combined spinal‐epidurals, loss of resistance medium, ultrasound‐guided insertion catheters, and drugs such as epidural morphine, dexamethasone, cosyntropin, epidural or intrathecal saline, or intravenous fluids (Arévalo‐Rodríguez 2013; Bradbury 2013; Heesen 2013; Rusch 2014; Shaikh 2013).
Authors' conclusions
Implications for practice.
For people with post‐dural puncture headache
We assessed eight different drugs: caffeine, sumatriptan, gabapentin, pregabalin, theophylline, hydrocortisone, cosyntropin and adrenocorticotropic hormone (ACTH). In this review update we have evaluated two new interventions, cosyntropin and pregabalin, without a significant benefit shown. New trials on theophylline, hydrocortisone and gabapentin are included, which add significant evidence in line with the previous review.
For clinicians
From the studies available caffeine shows a significant decrease in the proportion of participants with post‐dural puncture headache persistence and in those needing supplementary interventions, when compared with placebo. Gabapentin compared with placebo or with ergotamine plus placebo has shown a decrease in pain severity scores. Hydrocortisone compared with placebo or with conventional care has shown a decrease in pain severity scores. Theophylline has also shown a decrease in pain severity scores when compared with acetaminophen, with conservative treatment or with placebo. Theophylline has also shown better results in terms of the proportion of participants that reported an improvement in pain scores when compared with conservative treatment.
The other drugs assessed (sumatriptan, adrenocorticotropic hormone, pregabalin cosyntropin) have not shown a significant effect.
For policy‐makers
This conclusion should be interpreted with caution due to the quality of the evidence found: the limited number of studies, the diversity of drugs assessed and outcomes measured, the small sample sizes (13 studies involving a total of 479 participants) and the bias presented.
Implications for research.
General
The reporting of future trials in this field should be improved (i.e. using the CONSORT statement (Schulz 2010)) to allow medical literature users to appraise the results of these studies accurately.
Design
Future research in this field should focus on the design of trials with larger samples (reporting how sample size was determined) and with extended follow‐up periods (at least four days).
Measurement (endpoints)
The measurement of relevant outcomes for decision‐making should also be improved in future trials, such as the persistence of pain at follow‐up and possible adverse events from pharmacological drugs.
What's new
| Date | Event | Description |
|---|---|---|
| 17 September 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 8, 2011
| Date | Event | Description |
|---|---|---|
| 15 July 2015 | Review declared as stable | See Published notes. |
| 7 December 2014 | New citation required but conclusions have not changed | The results of the six new included studies have not changed the conclusions of the previous version of the review. |
| 7 December 2014 | New search has been performed | In this review update, we included six new randomised clinical trials after identifying 360 new references, increasing the number of participants from 200 to 479. This new included evidence has led to substantial amendment of the characteristics of the included participants, with a decrease in the proportion of women and obstetric participants from around 80% to 50% because none of the new trials include participants with post‐dural puncture headache after obstetric analgesia. We have evaluated two new interventions, cosyntropin (one new study) and pregabalin (one new study), which did not show any significant benefit. Two new trials on theophylline, one on hydrocortisone and one on gabapentin, are included in this update and add significant evidence that is concordant with the previous review. In this update, a new review author has been added, Dimelza Osorio, who worked on the review in all phases of the process. We have also added 'Risk of bias' summary tables. |
Notes
2015
Protocol title split from 'Drug therapy for preventing and treating post‐dural puncture headache' (Sudlow 2009) into two separate titles; one on prevention (Basurto 2013) and this one on treatment.
This Cochrane Review will be assessed for further updating in 2020. Further research is unlikely to change our confidence in the estimate of effect.
Assessed for updating in 2020
At June 2020, we identified one potentially relevant study (Peralta 2020) but the new information is unlikely to change the review findings. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be assessed for updating in five years. If appropriate we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Peralta 2020: Prophylactic intrathecal morphine and prevention of post‐dural puncture headache: a randomized double‐blind trial. Peralta FM, Wong CA, Higgins N, Toledo P, Jones MJ, McCarthy RJ.Anesthesiology. 2020 May;132(5):1045‐1052. DOI: 10.1097/ALN.0000000000003206
Acknowledgements
Xavier Basurto is a PhD student at the Pediatrics, Obstetrics and Gynecology, and Preventive Medicine Department, Universitat Autònoma de Barcelona, Barcelona, Spain.
We are grateful to Joanne Abbott (Trials Search Co‐ordinator of the Cochrane Pain, Palliative and Supportive Care Review Group) and Caroline Struthers (previously the Trials Search Co‐ordinator of the Review Group) for undertaking the searches; to Marta Roqué (Iberoamerican Cochrane Center) for her help in the statistical analysis; to Sera Tort for her help in editing the review; and to Cathie Sudlow and Charles Warlow for writing the first draft of the protocol.
We acknowledge the contribution of Laura Martínez and Ivan Solà who were involved in the original review, but not the update.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor Anesthesia, Epidural explode all trees
#2 MeSH descriptor Anesthesia, Spinal explode all trees
#3 MeSH descriptor Injections, Spinal explode all trees
#4 MeSH descriptor Myelography explode all trees
#5 MeSH descriptor Spinal Puncture explode all trees
#6 (spine or spinal or intraspinal or dura* or intradural or epidural or lumbar* or theca* or intrathecal or subarachnoid*) near/10 (puncture* or inject* or anesth* or anaesth* or needle*)
#7 myelogra*
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Headache Disorders explode all trees
#10 headach* or cephalgia or (head near/2 pain) or (cranial near/2 pain)
#11 (#9 OR #10)
#12 (#8 AND #11)
Appendix 2. MEDLINE Ovid search strategy
1 exp Anesthesia, Epidural/
2 exp Anesthesia, Spinal/
3 Injections, Spinal/
4 exp Myelography/
5 exp Spinal Puncture/
6 ((spine or spinal or intraspinal or dura* or intradural or epidural or lumbar* or theca* or intrathecal or subarachnoid*) adj10 (puncture* or inject* or anesth* or anaesth* or needle*)).mp.
7 myelogra*.mp.
8 1 or 2 or 3 or 4 or 5 or 6 or 7
9 exp Headache Disorders/
10 (headach* or cephalgia or (head adj2 pain) or (cranial adj2 pain)).mp.
11 9 or 10
12 8 and 11
13 randomised controlled trial.pt.
14 controlled clinical trial.pt.
15 randomized.ab.
16 placebo.ab.
17 drug therapy.fs.
18 randomly.ab.
19 trial.ab.
20 groups.ab.
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22 12 and 21
key:
p = title, original title, abstract, name of substance word, subject heading word, unique identifier
pt = publication type, ab = abstract, fs = floating subheading
Appendix 3. EMBASE Ovid search strategy
1 exp spinal anaesthesia/
2 exp lumbar puncture/
3 exp MYELOGRAPHY/
4 ((spine or spinal or intraspinal or dura* or intradural or epidural or lumbar* ot theca* or intrathecal or subarachnoid*) adj10 (puncture* or inject* or anesth* or anaesth* or needle*)).mp.
5 myelogra*.mp.
6 1 or 2 or 3 or 4 or 5
7 exp "headache and facial pain"/
8 (headach* or cephalgia or (head adj2 pain) or (cranial adj2 pain)).mp.
9 7 or 8
10 6 and 9
11 random*.mp.
12 factorial*.mp.
13 (crossover* or cross over* or cross‐over*).mp.
14 placebo*.mp.
15 (doubl* adj blind*).mp.
16 (singl* adj blind*).mp.
17 assign*.mp.
18 allocat*.mp.
19 volunteer*.mp.
20 crossover procedure/
21 double blind procedure/
22 randomised controlled trial/
23 single blind procedure/
24 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 10 and 24
key:
mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer
Appendix 4. CINAHL search strategy
1 anaesthesia, epidural/ or analgesia, epidural/ or "epidural analgesia administration (iowa nic)"/ or exp injections, epidural/
2 exp injections, intraspinal/
3 myelography/
4 spinal puncture/ or anaesthesia, spinal/
5 ((spine or spinal or intraspinal or dura* or intradural or epidural or lumbar* or theca* or intrathecal or subarachnoid*) and (puncture* or inject* or anesthe* or anaesthe* or needle*)).ti,ab
6 myelogra*.ti,ab
7 1 or 2 or 3 or 4 or 5 or 6
8 *headache/
9 (headach* or cephalgi* or cephalalgi*).ti,ab
10 8 or 9
11 7 and 10
12 exp clinical trials/
13 (clinical and trial*).ti
14 ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti
15 (randomi?ed and control* and trial*).ti
16 random assignment/
17 (random* and allocat*).ti
18 placebo*.ti
19 placebos/
20 quantitative studies/
21 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22 11 and 21
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alam 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study type: single‐centre study Location: Bangladesh (Chittagong) Study design: parallel Randomisation: computer random numbers series Allocation concealment: not described Blinding: double‐blind, patients and observer Follow‐up period: 48 hours | |
| Participants | Randomised: 60 (intervention group: 30; control group: 30)
Excluded (post‐randomisation): not described
Gender (women): 32 (53%)
Age (years); mean (standard deviation ‐ SD): intervention group 30.32 (5.83), control group 32.49 (4.69)
Baseline VAS score: mean (SD): intervention group 9.32 (0.83), control group 9.17 (1.69)
Inclusion criteria: Adult patients (ASA I and II) who developed PDPH after non‐obstetric surgery Exclusion criteria: History of cluster headache, convulsion, cerebrovascular accident, pre‐eclampsia, eclampsia, coagulopathy or previous neurological diseases |
|
| Interventions |
Intervention group: 100 mg hydrocortisone, diluted in 2 ml, intravenous 8‐hourly for 48 hours
Control group: 2 ml of normal saline intravenously (placebo) 8‐hourly for 48 hours
Co‐interventions:
|
|
| Outcomes | 1. Change in pain severity VAS score after 6, 24 and 48 hours 2. Number of any possible adverse events 3. Number of participants with EBP performed | |
| Notes | Post‐dural puncture headache (PDPH): Quote "The mean of headache intensity was measured in all 60 patients after 1 min in upright position." (page 191) Visual analogue scale (VAS) 10 cm: 0 to 1, no headache; 2 to 4, mild headache; 5 to 7, moderate headache; and 8 to 10, severe headache Sample size calculation: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigator reported the use of a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The patients and the single observer were blinded to this study." (Page 191) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 60 (intervention group: 30; control group: 30) |
Camann 1990.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study type: single‐centre study Location: USA (Massachusetts) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: blinding of participants and key study personnel. Investigational pharmacist was not blinded Follow‐up period: 24 hours |
|
| Participants | Randomised: 40 (intervention group: 20; control group: 20) Excluded (post‐randomisation): not described Gender (women): 40 (100%) Age (years); mean (standard deviation ‐ SD): intervention group 29.8 (6.26), control group 30.6 (5.36) Baseline VAS score mean (SD): intervention group 69 (13.42), control group 60 (17.89) Inclusion criteria: Postpartum women Exclusion criteria: Hypertension, pre‐eclampsia, seizure disorder, intolerance to caffeine or consumed beverages containing caffeine within the previous 4 hours |
|
| Interventions |
Intervention group: one oral capsule with 300 mg of caffeine Control group: one oral placebo capsule with lactose Co‐interventions:
|
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "Frontal and/or occipital discomfort worsened by upright posture and relieved by lying supine" (page 181) Visual analogue scale (VAS): 0 = no headache and 100 = worst headache imaginable Sample size calculation: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Reported as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Capsules, prepared by our investigational pharmacy, contained either anhydrous caffeine powder (USP 300 mg, Spectrum Chemical Mfg. Corp., Gardena, Calif.) or placebo (lactose powder) and appeared identical." (Pages 181 to 182) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 40 (intervention group: 20; control group: 20) |
Connelly 2000.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study type: single‐centre study Location: USA (Massachusetts) Study design: parallel Randomisation: computer random numbers series Allocation concealment: sealed container with a random code Blinding: blinding of participants and key study personnel Follow‐up period: 48 hours |
|
| Participants | Randomised: 10 (intervention group: 5; control group: 5) Excluded (post‐randomisation): not described Gender (women): 8 (80%); intervention group 3 (60%); control group 5 (100%) Age (years); mean (SD): intervention group 43 (12); control group 24 (8) Baseline VAS score: mean (SD): intervention group 61 (24), control group 87 (23) Inclusion criteria: Patients with severe PDPH Exclusion criteria: History of migraine, a contraindication to an EBP, or contraindication to sumatriptan (ischaemic heart disease, hypertension, pregnancy, pre‐eclampsia or being treated with ergot medications or MAO inhibitors) |
|
| Interventions |
Intervention group: once subcutaneous sumatriptan, 6 mg (0.5 ml) Control group: once subcutaneous saline (0.5 ml) Co‐interventions:
|
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "Headache which is characterized by relieved with recumbency". (Page 316) Visual analogue scale (VAS): 0 = no headache and 100 = worst headache imaginable Sample size calculation: not described Email contact with MD Neil Roy Connelly on January 2010 for clarification about randomisation, allocation concealment, blinding and statistical questions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigator reported the use of a computer random number generator |
| Allocation concealment (selection bias) | Low risk | The investigator reported the use of a sealed container with a random code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Patients received, in a randomised fashion, either subcutaneous sumatriptan, 6 mg (0.5 mL), or saline (0.5 mL) using the Glaxo injector". (Page 317) The investigator reported blinding the VAS recorder |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcomes (PDPH persistence of any severity at follow‐up and number of any possible adverse events) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 10 (intervention group: 5; control group: 5) |
Dogan 2006.
| Study characteristics | ||
| Methods | Randomised, placebo‐controlled trial Study type: single‐centre study Location: Turkey (Afyon) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: not described Follow‐up period: 4 days |
|
| Participants | Randomised: 20 (intervention group: 10; control group: 10) Excluded (post‐randomisation): not described Gender (women): 8 (40%); intervention group 4 (40%); control group 4 (40%) Age (years); mean (SD): intervention group 36.30 (9.54); control group 46.60 (17.10) Baseline VAS score: mean (SD): intervention group 7.5 (0.428); control group 7.3 (0.423) Inclusion criteria:
Exclusion criteria: Known allergy or contraindications (pancreatitis, galactosaemia) to gabapentin, migraine, asthma and hepatic or renal insufficiency |
|
| Interventions |
Intervention group: gabapentin 900 mg/day orally (300 mg every 8 hours) during 4 days Control group: placebo Co‐interventions:
|
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "PDPH was diagnosed by the postural component of the pain". (Page 170) Visual analogue scale (VAS): 0 = no pain and 10 = worst pain imaginable Sample size calculation: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 20 (intervention group: 10; control group: 10) |
Erol 2011.
| Study characteristics | ||
| Methods | Randomised, treatment‐controlled trial Study type: single‐centre study Location: Turkey (Afyonkarahisar) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: not described Follow‐up period: 4 days | |
| Participants | Randomised: 42 (intervention group: 21; control group: 21)
Excluded (post‐randomisation): not described
Gender (women): 17 (40%)
Age (years); mean: intervention group 45, control group 47 (SD not described)
Baseline VAS score: mean(SD): intervention group 7.5 (0.48), control group 7.3 (0.42)
Inclusion criteria: Adult PDPH patients after spinal or epidural anaesthesia Exclusion criteria: Known allergy to, or contraindications (pancreatitis, galactosaemia) to the use of gabapentin or Cafergot, other medication use (postoperative analgesics, phenytoin, carbamazepine, valproic acid, phenobarbital, naproxen, hydrocodone, morphine, cimetidine, oral contraceptive, antacid, probenecid), migraine, asthma, coronary artery disease, and hepatic or renal insufficiency |
|
| Interventions |
Intervention group: gabapentin 900 mg/day orally (300 mg every 8 hours) during 4 days Control group: ergotamine 1 mg and caffeine 100 mg orally, every 8 hours, during for 4 days Co‐interventions:
|
|
| Outcomes | 1. Change in pain severity VAS score after 1, 2, 3 and 4 days 2. Number of any possible adverse events 3. Number of participants with EBP performed 4. Missing data (withdrawals) | |
| Notes | Post‐dural puncture headache (PDPH): Quote "For diagnosis we used the criteria suggested by the International Headache Society (IHS)." (page 26) Visual analogue scale (VAS): 0 (denoting no pain) to 10 (denoting worst possible imaginable pain) Sample size calculation: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. Quote: "No patients withdrew" (Page 26) |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 42 (intervention group: 21; control group: 21) |
Feuerstein 1986.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study type: single‐centre study Location: Germany (Freiburg) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: blinding of participants and evaluation personnel Follow‐up period: until healing of the headache |
|
| Participants | Randomised: 16 (not described the number of participants initially allocated to each group) Excluded (post‐randomisation): 5 (not described how many from each group). Analysed: 11 (intervention group: 6; control group: 5) Gender (women): 6 (54%) Age (years); n (%): 4 (36.4%) 10 to 30 years old (intervention group 3; control group 1); 4 (36.4%) 31 to 50 years old (intervention group 1; control group 3); 3 (27.3%) > 50 years old (intervention group 2; control group 1) Baseline VAS score: not evaluated Inclusion criteria: patients with a diagnostic lumbar puncture performed, with no headache during the last week before the lumbar puncture and a severe headache Exclusion criteria: not described |
|
| Interventions |
Intervention group: oral theophylline 281.7 mg tablets (verum Euphyllin retard tablets) 3 times a day Control group: oral placebo tablets 3 times a day Co‐interventions: analgesics and hypotonic saline solution as needed |
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "Subjective severe headache occurring only after arising and ceasing within a few minutes after lying down.". (Page 217) Sum of pain: 1 = slight headache; 2 = intermediate headache and 3 = severe headache. 3 values per day Sample size calculation: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Reported as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo tablets, indistinguishable from the verum Euphyllin retard tablets (281.7 mg theophylline), were kindly provided by Byk Gulden Pharmazeutika, Konstanz, FRG." (Page 217) Quote: "Verum and placebo tablets were randomised so that neither the patient nor the examining medical staff knew the real content of the administered tablets." (Page 217) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 of 16 participants randomised dropped out, with insufficient information provided Quote: "Five patients of 16 dropped out (transferal to other clinical departments, dismissal before the end of the study or insufficient compliance)." (Page 217) |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 11 (intervention group: 6; control group: 5) |
Huseyinoglu 2011.
| Study characteristics | ||
| Methods | Randomised, single‐blind, placebo‐controlled trial Study type: single‐centre study Location: Turkey (Kars) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: single‐blind but not described who was blinded Follow‐up period: 5 days | |
| Participants | Randomised: 40 (intervention group: 20; control group: 20)
Excluded (post‐randomisation): not described
Gender (women): 28 (70%)
Age (years); mean (standard deviation ‐ SD): intervention group 39.95 (12.62), control group 34.85 (10.91)
Baseline VAS score: mean(SD): text reported that there was no significant difference (P value = 0.947). No explicit numbers reported, only data from a graphic
Inclusion criteria: ASA I‐II patients under spinal anaesthesia for various surgical procedures or diagnostic or therapeutic lumbar puncture with PDPH according to IHS criteria Exclusion criteria: < 18 years of age, weight < 40 kg, renal or liver dysfunction, severe cardiovascular disease, a history of peptic ulcer and gastrointestinal system bleeding, a known allergy to any component of the treatment drugs, or glucose/lactose intolerance. Pregnancy or breast‐feeding |
|
| Interventions |
Intervention group: 75 mg pregabalin orally, every 12 hours for 3 days and 150 mg pregabalin orally, every 12 hours for 2 more days Control group: placebo orally, every 12 hours, for 5 days Co‐interventions:
|
|
| Outcomes | 1. Change in pain severity VAS score after 1, 2, 3, 4 and 5 days 2. Number of participants with a conservative supplementary therapeutic option offered 3. Missing data (withdrawals, drop‐outs and participants lost to follow‐up) 4. Number of any possible adverse events | |
| Notes | Post‐dural puncture headache (PDPH): Quote "diagnosed with PDPH according to the International Headache Society criteria" (page 1366) Visual analogue scale (VAS): 0 = no headache and 10 = worst, unbearable headache Sample size calculation: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "randomized, single‐blinded, placebo‐controlled study" (Page 1366). No information about who was blinded and how |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study. Not clear how many patients had side effects |
| Size of study | High risk | Total 40 (intervention group: 20; control group: 20) |
Mahoori 2013.
| Study characteristics | ||
| Methods | Randomised, single‐blind, treatment‐controlled trial Study type: single‐centre study Location: Iran (Urmia) Study design: parallel Randomisation: computer random numbers series Allocation concealment: not described Blinding: blinding of participants Follow‐up period: 12 hours | |
| Participants | Randomised: 60 (intervention group: 30; control group: 30)
Excluded (post‐randomisation): not described
Gender (women): 19 (31.6%)
Age (years); mean (standard deviation ‐ SD): intervention group 40.06 (5.95), control group 40 (6.43)
Baseline VAS score: mean(SD): intervention group 5.46 (1.33), control group 5.96 (1.20)
Inclusion criteria: ASA I patients under spinal anaesthesia for various surgical procedures with PDPH according to ICHD‐II definition Exclusion criteria: Central nervous disorders, hypertension, ischaemic heart disease, cardiac arrhythmias, hyperthyroidism, age higher than 60 years old and past history of migraine headaches |
|
| Interventions | Intervention group: 250 mg theophylline orally, every 8 hours Control group: 500 mg acetaminophen orally, every 8 hours Co‐interventions: not clearly described. Quote "other therapies (including fluids and drugs) were similar in patient of both groups" (Page 291) | |
| Outcomes | 1. Change in pain severity VAS score after 2, 6 and 12 hours 2. Number of any possible adverse events 3. Missing data (withdrawals, drop‐outs and participants lost to follow‐up) | |
| Notes | Post‐dural puncture headache (PDPH): Quote "subjects have experienced PDPH according to the definition of International classification of headache disorders (ICHD‐II)" (page 290) Visual analogue scale (VAS): 0 (denoting no pain) to 10 (denoting worst possible imaginable pain) Sample size calculation: 54 patients needed according to the calculations described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The investigator reported the use of a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "TDS administration to ascertain the blindness of subjects in both study groups" (Page 290) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 60 (intervention group: 30; control group: 30) |
Noyan 2007.
| Study characteristics | ||
| Methods | Randomised, double‐blind, controlled trial Study type: single‐centre study Location: Iran (Tehran) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: blinding of participants and key study personnel Follow‐up period: 48 hours |
|
| Participants | Randomised: 60 (intervention group: 30; control group: 30) Excluded (post‐randomisation): not described Gender (women): 60 (100%) Age (years); mean (SD): 27.1 (3.45) Baseline VAS score: mean (SD): intervention group 9.20 (0.71); control group 9.07 (0.69) Inclusion criteria:
Exclusion criteria: Cluster headache, convulsion, cerebrovascular accident, pre‐eclampsia, eclampsia, high intracranial pressure, coagulopathy or previous neurologic disease |
|
| Interventions |
Intervention group: 200 mg hydrocortisone intravenously as a bolus and 100 mg hydrocortisone every 8 hours for 48 hours Co‐interventions:
|
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "Headache after spinal anaesthesia". (Page 416) Visual analogue scale (VAS): 0 to 1 no headache; 2 to 4 mild headache, 5 to 7 moderate headache; 8 to 10 severe headache Sample size calculation: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Parturient women and observer did not know which patient had received hydrocortisone". (Page 418) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 60 (intervention group: 30; control group: 30) |
Rucklidge 2004.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study type: multicentre trial Location: UK Study design: parallel Randomisation: computer‐generated random numbers Allocation concealment: central randomisation Blinding: blinding of participants and key study personnel Follow‐up period: 48 hours |
|
| Participants | Randomised: 18 (intervention group: 9; control group: 9) Excluded (post‐randomisation): not described Gender (women): 18 (100%) Age (years); mean (SD): intervention group 24.1 (3.8); control group 29.3 (4.7) Baseline VAS score: (graphical data) Inclusion criteria: Women with PDPH following deliberate or accidental dural puncture associated with obstetric regional anaesthesia or analgesia Exclusion criteria: Asthma, severe allergy or diabetes |
|
| Interventions |
Intervention group: intramuscular synthetic analogue of ACTH (Synacthen Depot®) 1 mg (1 ml) Control group: intramuscular saline 0.9% (1 ml) Co‐interventions:
|
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "severe headache occurring within 48 hours of dural puncture; exacerbation on sitting or standing with relief on lying or on abdominal compression; and the absence of focal neurological signs". (Page 138) Visual analogue scale (VAS): 0 = no pain and 10 = worst pain imaginable The study reported data on neck stiffness and nausea as 2 side effects derived from the progression of the condition. These 2 side effects were not considered as adverse events related to the assessed intervention Email contact with Dr. Steve Yentis in March 2010 for clarification about VAS numerical results Sample size calculation: estimate VAS reduction = 30%, β = 80%; α = 0.05 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Parturients were randomly allocated to receive Synacthen Depot 1 mg (1 ml) or 0.9% saline (1 ml) according to computer‐generated random numbers held at the Chelsea and Westminster Hospital, London". (Page 138) |
| Allocation concealment (selection bias) | Low risk | Quote: "Parturients were randomly allocated to receive Synacthen Depot 1 mg (1 ml) or 0.9% saline (1 ml) according to computer‐generated random numbers held at the Chelsea and Westminster Hospital, London". (Page 138) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The study agent was dispatched from the relevant pharmacy in a prefilled syringe and the contents were blinded to the parturient, investigator and midwife administering the drug". (Page 138) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (PDPH persistence of any severity at follow‐up) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 18 (intervention group: 9; control group: 9) |
Sechzer 1978.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled trial Study type: single‐centre study Location: USA (New York) Study design: parallel Randomisation: table of random numbers Allocation concealment: central randomisation Blinding: blinding of participants and key study personnel Follow‐up period: not described |
|
| Participants | Randomised: 41 (intervention group: 20; control group: 21) Excluded (post‐randomisation): not described Gender (women): not reported Age (years): not reported Baseline VAS score: not evaluated Inclusion criteria: patients with PDPH after a spinal anaesthesia, with usual symptomatic treatment unsatisfactory and headache through a 2‐ to 4‐day course Exclusion criteria: not described |
|
| Interventions |
Intervention group: intravenous caffeine sodium benzoate (CSB) (0.5 g/2 ml) Control group: intravenous physiologic saline solution (2 ml) Co‐interventions: supplementary caffeine (0.5 g/2 ml) was administered if headache was not relieved after 1 to 2 hours |
|
| Outcomes |
|
|
| Notes | Post‐dural puncture headache (PDPH): Quote "Aggravated by sitting or standing". (Page 308) Sample size calculation: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After informed consent was obtained, the patients were treated in random fashion with initial intravenous injection A which was either: physiologic saline solution (2 ml) or CSB (0.5 gm / 2 ml)." (Page 308) Quote: "The solutions were prepared in the hospital pharmacy according to a list derived from a table of random numbers." (Page 308) |
| Allocation concealment (selection bias) | Low risk | Quote: "The solutions were prepared in the hospital pharmacy according to a list derived from a table of random numbers." (Page 308) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The syringes were coded so that the observers were not aware of the contents." (Page 308) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (number of any possible adverse events) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 41 (intervention group: 20; control group: 21) |
Sen 2014.
| Study characteristics | ||
| Methods | Randomised, treatment‐controlled trial Study type: single‐centre study Location: India (Faridabad) Study design: parallel Randomisation: not described Allocation concealment: not described Blinding: not described Follow‐up period: 24 hours | |
| Participants | Randomised: 40 (intervention group: 20; control group: 20)
Excluded (post‐randomisation): not described
Gender (women): 18 (45%)
Age: text reported that there was no significant difference. Quote "Groups did not differ in age" (Page 115). Table 1 describes age in the intervention and control groups by type of surgery
Baseline VAS score: mean (SD): intervention group 93.5 (5.9), control group 94.6 (4.4)
Inclusion criteria: Patients of ASA I and II suffering from post‐dural puncture headache Exclusion criteria: not described |
|
| Interventions | Intervention group: 400 mg theophylline orally Control group: conservative treatment comprising bed rest in a supine position without a head pillow, caffeine‐containing beverages, injectable opioid and/or non‐steroid anti‐inflammatory drug (NSAID) Co‐interventions: not described | |
| Outcomes | 1. Change in pain severity VAS score after 8, 16 and 24 hours 2. Number of participants showing improvements in pain severity 3. Number of any possible adverse events | |
| Notes | Post‐dural puncture headache (PDPH): Quote "Patients under study were asked to mark a 0 to 100 mm VAS in sitting position to facilitate a maximal score at the onset of PDPH." (page 115) Visual analogue scale (VAS) 0 to 100 mm Sample size calculation: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | The study report fails to include results for a key outcome (number of any possible adverse events) that would be expected to have been reported for such a study |
| Size of study | High risk | Total 40 (intervention group: 20; control group: 20) |
Zeger 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blind, treatment‐controlled trial Study type: single‐centre study Location: USA (Washington) Study design: parallel Randomisation: not described Allocation concealment: central randomisation by the hospital pharmacy Blinding: blinding of participants and health care provider Follow‐up period: 120 minutes or discharged from the emergency department | |
| Participants | Randomised: 37 (intervention group: 17; control group: 16)
Excluded (post‐randomisation): 4 patients were excluded for analysis of the primary endpoint (33 available) and 1 more for secondary endpoint analysis (32 available). It is not described to which group excluded patients belonged
Gender (women): 20/33 (60.6%)
Age (years); mean (standard deviation ‐ SD): intervention group 25 (8), control group 33 (11)
Baseline VAS score: mean (SD): intervention group 78 (16), control group 82 (15)
Inclusion criteria: Presenting to the emergency department within 7 days of a lumbar puncture and meeting the diagnostic criteria for a PDPH Exclusion criteria: Life‐threatening aetiology, evidence of elevated intracranial pressure (e.g. papilloedema), pregnancy, history of congestive heart failure, severe hypertension, allergy to caffeine, ACTH or its analogues or if they elected a blood patch as an initial therapeutic intervention |
|
| Interventions | Intervention group: 0.75 mg cosyntropin in 1 litre of normal saline intravenously over 60 minutes followed by 1 litre of normal saline given over 60 minutes Control group: 500 mg caffeine in 1 litre of normal saline given intravenously over 60 minutes followed by a repeated dose of 500 mg caffeine in 1 litre of normal saline over 60 minutes Co‐interventions: • All patients received 975 mg of acetaminophen and 2 litres of normal saline over a 2‐hour period | |
| Outcomes | 1. PDPH persistence of any severity at 120 minutes 2. Change in pain severity VAS score after 60 and 120 minutes 3. Number of any possible adverse events 4. Number of participants with a conservative supplementary therapeutic option offered 5. Missing data (withdrawals, drop‐outs and participants lost to follow‐up) | |
| Notes | Post‐dural puncture headache (PDPH): Quote "headache clinically consistent with a PDPH, the presence of a postural component," (page 183) Visual analogue scale (VAS): 0 = no headache and 100 = worst headache imaginable Sample size calculation: 270 patients in each arm needed according to the calculations described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Described as randomised |
| Allocation concealment (selection bias) | Low risk | Central randomisation. Quote: "Each drug was premixed, coded, and randomised by the hospital pharmacy without the knowledge of the health care provider." (Page 183) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Each drug was premixed, coded, and randomized by the hospital pharmacy without the knowledge of the health care provider." (Page 183) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from 5 patients were not available because of protocol violation (3), withdrawal (1) and incomplete assessment (1). Is is not described to which group the excluded patients belonged |
| Selective reporting (reporting bias) | Low risk | Results presented according to objectives stated in the introductory section |
| Size of study | High risk | Total 37 (intervention group: 17; control group: 16) |
ASA: American Society of Anesthesiologists (ASA) Physical Status classification
CSB: caffeine sodium benzoate EBP: epidural blood patch h: hours ICHD: International Classification of Headache Disorders IHS: International Headache Society MAO: monoamine oxidase (inhibitor) PDPH: post‐dural puncture headache SD: standard deviation VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aguilera 1988 | Not a RCT (case series) |
| Bart 1978 | No individual pharmacological drug assessed |
| Basso 1985 | Intervention did not aim to treat PDPH |
| De las Heras 1997 | Not a RCT (case report) |
| Eldor 1990 | Not a RCT (case series) |
| Ergün 2008 | Randomisation by alternation (odd hospital record numbers assigned to intervention and even numbers to control; information provided by the trialists) |
| Flaatten 1987 | Intervention did not aim to treat PDPH |
| Hakim 2005 | Not a RCT (clinical trial without control group) |
| Hodgson 1997 | Not a RCT (case report) |
| Lang 1993 | The orthostatic component of headache was not described |
| Naja 2009 | No individual pharmacological drug assessed |
| Oedit 2005 | No individual pharmacological drug assessed |
| Sandesc 2005 | No individual pharmacological drug assessed |
| Schwalbe 1991 | The orthostatic component of headache was not described |
| Torres 1986 | The orthostatic component of headache was not described |
| van Kooten 2008 | No individual pharmacological drug assessed |
| Widerlöv 1979 | Intervention did not aim to treat PDPH |
| Zenglein 1978 | Intervention did not aim to treat PDPH |
PDPH: post‐dural puncture headache RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Gherghina 2013.
| Methods | Double‐blind, randomly assigned clinical trial |
| Participants | 50 participants with headache after spinal anaesthesia in Romania |
| Interventions | 25 patients with intravenous methylprednisolone (one dose of 500 mg intravenous) plus conventional therapy (complete bed rest, hydration, acetaminophen and pethidine) and 25 patients with conventional therapy |
| Outcomes | Mean (+/‐ SD) of headache intensity at 0, 6, 24 and 48 hours measured using visual analogue scale |
| Notes | 2013 conference abstract. Email sent asking for more information |
Haghbin 2012.
| Methods | Double‐blind, randomly assigned clinical trial |
| Participants | 60 patients (ASA I‐II) with PDPH after spinal anaesthesia for gynaecological or urological intervention in Iran |
| Interventions | Propranolol compared with acetaminophen |
| Outcomes | Pain severity measured by visual analogue scale |
| Notes | 2012 conference abstract. Email sent asking for more information |
Moghaddam 2011.
| Methods | Randomly assigned clinical trial |
| Participants | 60 patients (ASA I‐II) with PDPH after spinal anaesthesia were enrolled in Iran |
| Interventions | Gabapentin compared with pregabalin |
| Outcomes | Pain severity measured by numeric rating scale |
| Notes | 2011 conference abstract. Email sent asking for more information |
Saghaleini 2014.
| Methods | Single‐blind randomised clinical trial |
| Participants | 90 patients with PDPH who underwent elective surgery under spinal anaesthesia in Iran |
| Interventions | Gabapentin, pregabalin and acetaminophen |
| Outcomes | Pain severity measured by visual analogue scale at the onset of the headache, 24, 48 and 72 hours |
| Notes | Email sent asking for more information |
ASA: American Society of Anesthesiologists (ASA) Physical Status classification
PDPH: post‐dural puncture headache
SD: standard deviation
Differences between protocol and review
Types of participants: "The use of a standardised diagnostic criteria for PDPH will not be required, but it should at least be described as orthostatic headache which worsens on standing and is improved by lying down." The "it should at least be" has been added to emphasise the need to include only those RCTs that have used orthostatic headache criteria to include participants.
PaPaS Review Group Specialised Register electronic search eliminated.
CINAHL search strategy included.
Contributions of authors
Conceiving the review (guarantor): Xavier Basurto (XB).
Screening search results: XB, Dimelza Osorio (DO).
Screening retrieved papers against inclusion criteria: XB, DO, Xavier Bonfill Cosp (XBC).
Appraising quality of papers: XB, DO.
Extracting data from papers: XB, DO.
Data management for the review: XB, DO.
Entering data into Review Manager (RevMan 5.3): XB, DO.
Interpretation of data: XB, DO, XBC, DO.
Statistical analysis: XB, DO.
Writing the review: XB, XBC, DO.
Comment and editing of review drafts: XB, XBC, DO.
Responsible for reading and checking review before submission: XB, XBC, DO.
Responsible for initiating and running the update of this review: XB.
Sources of support
Internal sources
CIBER de Epidemiología y Salud Pública (CIBERESP), Spain
Iberoamerican Cochrane Centre, Spain
External sources
Agencia de Calidad para el Sistema Nacional de Salud, Ministerio de Sanidad, Política Social e Igualdad, Spain
Declarations of interest
Xavier Basurto, Dimelza Osorio and Xavier Bonfill Cosp have no conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Alam 2012 {published data only}
- Alam MR, Rahman MA, Ershad R. Role of very short-term intravenous hydrocortisone in reducing postdural puncture headache. Journal of Anaesthesiology, Clinical Pharmacology 2012;28(2):190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Camann 1990 {published data only}
- Camann WR, Murray RS, Mushlin PS, Lambert DH. Effects of oral caffeine on postdural puncture headache. A double-blind, placebo-controlled trial. Anesthesia and Analgesia 1990;70(2):181-4. [DOI] [PubMed] [Google Scholar]
Connelly 2000 {published and unpublished data}
- Connelly NR, Parker RK, Rahimi A, Gibson CS. Sumatriptan in patients with postdural puncture headache. Headache 2000;40(4):316-9. [DOI] [PubMed] [Google Scholar]
Dogan 2006 {published data only}
- Dogan D. The effect of oral gabapentin on postdural puncture headache. Acute Pain 2006;8:169-73. [Google Scholar]
Erol 2011 {published data only}
- Erol DD. The analgesic and antiemetic efficacy of gabapentin or ergotamine/caffeine for the treatment of postdural puncture headache. Advances in Medical Sciences 2011;56(1):25-9. [DOI] [PubMed] [Google Scholar]
Feuerstein 1986 {published data only}
- Feuerstein TJ, Zeides A. Theophylline relieves headache following lumbar puncture. Placebo-controlled, double-blind pilot study. Klinische Wochenschrift 1986;64(5):216-8. [DOI] [PubMed] [Google Scholar]
Huseyinoglu 2011 {published data only}
- Huseyinoglu U, Huseyinoglu N, Hamurtekin E, Aygun H, Sulu B. Effect of pregabalin on post-dural-puncture headache following spinal anesthesia and lumbar puncture. Journal of Clinical Neuroscience Journal 2011;18(10):1365–8. [DOI] [PubMed] [Google Scholar]
Mahoori 2013 {published data only}
- Mahoori A, Hassani E, Noroozinia H, Javaheri N, Hatami S. Theophylline versus acetaminophen in the treatment of post-dural puncture headache (PDPH). Middle East Journal of Anesthesiology 2013;22(3):289–92. [PubMed] [Google Scholar]
Noyan 2007 {published data only}
- Erratum. Middle East Journal of Anesthesiology 2007;19(3):706.
- Noyan MA, Sadeghi A, Azarbakht Z, Salehi S, Hamediseresht E. Evaluation of intravenous hydrocortisone in reducing headache after spinal anesthesia: a double blind controlled clinical study [corrected]. Middle East Journal of Anesthesiology 2007;19(2):415-22. [PubMed] [Google Scholar]
Rucklidge 2004 {published and unpublished data}
- Rucklidge MW, Yentis SM, Paech MJ. Synacthen Depot® for the treatment of postdural puncture headache. Anaesthesia 2004;59(2):138-41. [DOI] [PubMed] [Google Scholar]
Sechzer 1978 {published data only}
- Sechzer PH, Abel L. Post-spinal anesthesia headache treated with caffeine. Evaluation with demand method. Part I. Current Therapeutic Research Clinical and Experimental 1978;24(3):307-12. [Google Scholar]
- Sechzer PH. Post-spinal anesthesia headache treated with caffeine. Part II: intracranial vascular distention, a key factor. Current Therapeutic Research Clinical and Experimental 1979;26(4):440-8. [Google Scholar]
Sen 2014 {published data only}
- Sen J, Sen B. Non invasive management of post dural puncture headache – a comparison. Bangladesh Journal of Medical Science 2014;13(02):114-8. [Google Scholar]
Zeger 2012 {published data only}
- Zeger W, Younggren B, Smith L. Comparison of cosyntropin versus caffeine for post-dural puncture headaches: A randomized double-blind trial. World Journal of Emergency Medicine 2012;3(3):182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aguilera 1988 {published data only}
- Aguilera L, Rodriguez-Sasiain JM, Castrillo J, Martinez-Garbizu I, Callejo A, Ortega LF et al. Intravenous caffeine in the treatment of dural post-puncture headache. Revista Española de Anestesiología y Reanimación 1988;35(3):170-1. [PubMed] [Google Scholar]
Bart 1978 {published data only}
- Bart AJ, Wheeler AS. Comparison of epidural saline placement and epidural blood placement in the treatment of post lumbar puncture headache. Anesthesiology 1978;48(3):221-3. [DOI] [PubMed] [Google Scholar]
Basso 1985 {published data only}
- Basso N, Marcelli M, Ginaldi A, De Marco M. Intrathecal dermorphine in postoperative analgesia. Peptides 1985;6(Suppl 3):177-9. [DOI] [PubMed] [Google Scholar]
De las Heras 1997 {published data only}
- las Heras-Rosas MA, Rodríguez-Pérez A, Ojeda-Betancor N, Boralla-Rivera G, Gallego-Alonso JI. Failure of sumatriptan in post-dural puncture headache. Revista Española de Anestesiología y Reanimación 1997;44(9):378-9. [PubMed] [Google Scholar]
Eldor 1990 {published data only}
- Eldor J, Gozal Y, Lavie A, Guedj P. Late postspinal headache treated with epidural morphine. Anaesthesia 1990;45(12):1099. [DOI] [PubMed] [Google Scholar]
Ergün 2008 {published and unpublished data}
- Ergün U, Say B, Ozer G, Tunc T, Sen M, Tüfekcioglu S et al. Intravenous theophylline decreases post-dural puncture headaches. Journal of Clinical Neuroscience 2008;15(10):1102-4. [DOI] [PubMed] [Google Scholar]
Flaatten 1987 {published data only}
- Flaatten H, Rodt S, Rosland J, Vamnes J. Postoperative headache in young patients after spinal anaesthesia. Anaesthesia 1987;42(2):202-5. [DOI] [PubMed] [Google Scholar]
Hakim 2005 {published data only}
- Hakim S, Khan RM, Maroof M, Usmani H, Huda W, Jafri F. Methylergonovine maleate (methergine) relieves postdural puncture headache in obstetric patients. Acta Obstetricia et Gynecologica Scandinavica 2005;84(1):100. [DOI] [PubMed] [Google Scholar]
Hodgson 1997 {published data only}
- Hodgson C, Roitberg-Henry A. The use of sumatriptan in the treatment of postdural puncture headache. Anaesthesia 1997;52(8):808. [PubMed] [Google Scholar]
Lang 1993 {published data only}
- Lang SA, Yip RW, Comfort VK. Intravenous caffeine as a treatment for postdural puncture headaches: will it replace the epidural blood patch? Anesthesia and Analgesia 1993;76(S1):S207. [Google Scholar]
Naja 2009 {published data only}
- Naja Z, Al-Tannir M, El-Rajab M, Ziade F, Baraka A. Nerve stimulator-guided occipital nerve blockade for postdural puncture headache. Pain Practice 2009;9(1):51-8. [DOI] [PubMed] [Google Scholar]
Oedit 2005 {published data only}
- Oedit R, Kooten F, Bakker SL, Dippel DW. Efficacy of the epidural blood patch for the treatment of post lumbar puncture headache BLOPP: a randomised, observer-blind, controlled clinical trial [ISRCTN 71598245]. BMC Neurology 2005;5(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandesc 2005 {published data only}
- Sandesc D, Lupei MI, Sirbu C, Plavat C, Bedreag O, Vernic C. Conventional treatment or epidural blood patch for the treatment of different etiologies of post dural puncture headache. Acta Anaesthesiologica Belgica 2005;56(3):265-9. [PubMed] [Google Scholar]
Schwalbe 1991 {published data only}
- Schwalbe SS, Schiffmiller MW, Marx GF. Theophylline for post-dural puncture headache. Anesthesiology 1991;75:A1082. [Google Scholar]
Torres 1986 {published data only}
- Torres LM, Llamas C, Martín ML, Carrasco MS. Epidural blood patch for the treatment of postlumbar puncture headache. Revista Española de Anestesiología y Reanimación 1986;33(3):167-9. [PubMed] [Google Scholar]
van Kooten 2008 {published data only}
- Kooten F, Oedit R, Bakker SL, Dippel DW. Epidural blood patch in post dural puncture headache: a randomised, observer-blind, controlled clinical trial. Journal of Neurology Neurosurgery and Psychiatry 2008;79(5):553-8. [DOI] [PubMed] [Google Scholar]
Widerlöv 1979 {published data only}
- Widerlöv E, Lindström L. D.D.A.V.P. and headache after lumbar puncture. Lancet 1979;1(8115):548. [DOI] [PubMed] [Google Scholar]
Zenglein 1978 {published data only}
- Zenglein JP, Baldauf E, Wasser PH. Effect of tiapride on the side effects of cerebrospinal fluid depletions in spinal puncture, pneumoencephalography and air myelography. Semaine des Hopitaux 1978;54(9-12):413-23. [PubMed] [Google Scholar]
References to studies awaiting assessment
Gherghina 2013 {unpublished data only}
- Gherghina VI, Nicolae G, Cîndea I, Balcan A, Popescu R, Costea D. Effect of intravenous methylprednisolone in the treatment of post‐dural puncture headache: a double blind controlled clinical study: 8AP3‐9. European Journal of Anaesthesiology 2013;30:124. [Google Scholar]
Haghbin 2012 {unpublished data only}
- Haghbin M. Therapeutic effect of propranolol on headache after spinal anesthesia. Pain 2012;150:63. [Google Scholar]
Moghaddam 2011 {unpublished data only}
- Moghaddam MJ, Mirkheshti A, Yahyavi P. Comparison between analgesic effect of gabapentin and pregabalin in controlling delayed onset post dural puncture headache in non-pregnant patients. European Journal of Anaesthesiology 2011;28(Suppl 48):2013. [Google Scholar]
Saghaleini 2014 {published data only}
- Saghaleini SH, Ghorbanian EA. Comparison of the effect of gabapentin, pregabalin and acetaminophen in post-dural puncture headache. Iranian Journal of Obstetrics, Gynecology and Infertility 2014;16(82):1-7. [Google Scholar]
Additional references
Ahmed 2006
- Ahmed SV, Jayawarna C, Jude E. Post lumbar puncture headache: diagnosis and management. Postgraduate Medicine 2006;82(973):713-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angle 2005
- Angle P, Tang SL, Thompson D, Szalai JP. Expectant management of postdural puncture headache increases hospital length of stay and emergency room visits. Canadian Journal of Anaesthesia 2005;52(4):397-402. [DOI] [PubMed] [Google Scholar]
Arévalo‐Rodríguez 2013
- Arévalo-Rodríguez I, Ciapponi A, Munoz L, Roqué i Figuls M, Bonfill Cosp X. Posture and fluids for preventing post-dural puncture headache. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD009199. [DOI: 10.1002/14651858.CD009199] [DOI] [PubMed] [Google Scholar]
Banks 2001
- Banks S, Paech M, Gurrin L. An audit of epidural blood patch after accidental dural puncture with a Tuohy needle in obstetric patients. International Journal of Obstetric Anesthesia 2001;10(3):172-6. [DOI] [PubMed] [Google Scholar]
Basurto 2013
- Basurto Ona X, Uriona Tuma SM, Martínez García L, Solà I, Bonfill Cosp X. Drug therapy for preventing post-dural puncture headache. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD001792. [DOI: 10.1002/14651858.CD001792] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumgarten 1987
- Baumgarten RK. Should caffeine become the first-line treatment for postdural puncture headache? Anesthesia and Analgesia 1987;66(9):913-4. [PubMed] [Google Scholar]
Berger 1998
- Berger CW, Crosby ET, Grodecki W. North American survey of the management of dural puncture occurring during labour epidural analgesia. Canadian Journal of Anesthesia 1998;45(2):110-4. [DOI] [PubMed] [Google Scholar]
Bezov 2010
- Bezov D, Lipton RB, Ashina S. Post-dural puncture headache: part I diagnosis, epidemiology, etiology, and pathophysiology. Headache 2010;50(7):1144-52. [DOI] [PubMed] [Google Scholar]
Boonmak 2010
- Boonmak P, Boonmak S. Epidural blood patching for preventing and treating post-dural puncture headache. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD001791. [DOI: 10.1002/14651858.CD001791.pub2] [DOI] [PubMed] [Google Scholar]
Bradbury 2013
- Bradbury CL, Singh SI, Badder SR, Wakely LJ, Jones PM. Prevention of post-dural puncture headache in parturients: a systematic review and meta-analysis. Acta Anaesthesiologica Scandinavica 2013;57(4):417-30. [DOI] [PubMed] [Google Scholar]
Choi 1996
- Choi A, Laurito CE, Cunningham FE. Pharmacologic management of postdural puncture headache. Annals of Pharmacotherapy 1996;30(7-8):831-9. [DOI] [PubMed] [Google Scholar]
Choi 2003
- Choi PT, Galinski SE, Takeuchi L, Lucas S, Tamayo C, Jadad AR. PDPH is a common complication of neuraxial blockade in parturients: a meta-analysis of obstetrical studies. Canadian Journal of Anesthesia 2003;50(5):460-9. [DOI] [PubMed] [Google Scholar]
Clark 1996
- Clark JW, Solomon GD, Senanayake PD, Gallagher C. Substance P concentration and history of headache in relation to postlumbar puncture headache: towards prevention. Journal of Neurology, Neurosurgery and Psychiatry 1996;60(6):681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davignon 2002
- Davignon KR, Dennehy K. Update on postdural puncture headache. International Anesthesiology Clinics 2002;40(4):89-102. [DOI] [PubMed] [Google Scholar]
Denny 1987
- Denny N, Masters R, Pearson D, Read J, Sihota M, Selander D. Postdural puncture headache after continuous spinal anesthesia. Anesthesia and Analgesia 1987;66(8):791-4. [PubMed] [Google Scholar]
Gallagher 2002
- Gallagher EJ, Bijur PE, Latimer C, Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. American Journal of Emergency Medicine 2002;20(4):287-90. [DOI] [PubMed] [Google Scholar]
Grande 2005
- Grande PO. Mechanisms behind postspinal headache and brain stem compression following lumbar dural puncture--a physiological approach. Acta Anaesthesiologica Scandinavica 2005;49(5):619-26. [DOI] [PubMed] [Google Scholar]
Hafer 1997
- Hafer J, Rupp D, Wollbrück M, Engel J, Hempelmann G. The effect of needle type and immobilization on postspinal headache. Anaesthetist 1997;46(10):860-6. [DOI] [PubMed] [Google Scholar]
Harrington 2004
- Harrington BE. Postdural puncture headache and the development of the epidural blood patch. Regional Anesthesia and Pain Medicine 2004;29(2):136-63. [DOI] [PubMed] [Google Scholar]
Heesen 2013
- Heesen M, Klöhr S, Rossaint R, Van De Velde M, Straube S. Can the incidence of accidental dural puncture in laboring women be reduced? A systematic review and meta-analysis. Minerva Anestesiologica 2013;79(10):1187-97. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21:1539-58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
International Headache Society 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(Suppl 1):9-160. [DOI] [PubMed] [Google Scholar]
Kelly 1998
- Kelly AM. Does the clinically significant difference in visual analog scale pain scores vary with gender, age, or cause of pain? Academic Emergency Medicine 1998;5:1086–90. [DOI] [PubMed] [Google Scholar]
Kelly 2001
- Kelly AM. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emergency Medicine Journal 2001;18(3):205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuczkowski 2006
- Kuczkowski KM. The treatment and prevention of post-dural puncture headache. Acta Anaesthesiologica Belgica 2006;57(1):55-6. [PubMed] [Google Scholar]
Lavi 2006
- Lavi R, Yarnitsky D, Rowe JM, Weissman A, Segal D, Avivi I. Standard vs atraumatic Whitacre needle for diagnostic lumbar puncture: a randomized trial. Neurology 2006;67(8):1492-4. [DOI] [PubMed] [Google Scholar]
Mark 2009
- Mark MSM, Au TTS, Choi YF, Wong TW. The minimum clinically significant difference in visual analogue scale pain score in a local emergency setting. Hong Kong Journal of Emergency Medicine 2009;16:233-6. [Google Scholar]
McQuay 1998
- McQuay HJ, Moore RA. An Evidence-based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [Google Scholar]
Newman 2010
- Newman MJ, Cyna AM, Middleton P. Epidural catheter replacement and intrathecal catheter techniques for preventing post-dural puncture headache following an inadvertent dural puncture in labour. Cochrane Database of Systematic Reviews 2010, Issue 1. Art. No: CD008266. [DOI: 10.1002/14651858.CD008266] [DOI] [Google Scholar]
Review Manager 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rusch 2014
- Rusch R, Schulta C, Hughes L, Withycombe JS. Evidence-based practice recommendations to prevent/manage post-lumbar puncture headaches in pediatric patients receiving intrathecal chemotherapy. Journal of Pediatric Oncology Nursing 2014;31(4):230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Annals of Internal Medicine 2010;152:726-32. [DOI] [PubMed] [Google Scholar]
Shaikh 2013
- Shaikh F, Brzezinski J, Alexander S, Arzola C, Carvalho JC, Beyene J et al. Ultrasound imaging for lumbar punctures and epidural catheterisations: systematic review and meta-analysis. BMJ (Clinical research ed.) 2013;346:f1720. [DOI] [PubMed] [Google Scholar]
Thew 2008
- Thew M, Paech MJ. Management of postdural puncture headache in the obstetric patient. Current Opinion in Anesthesiology 2008;21(3):288-92. [DOI] [PubMed] [Google Scholar]
Todd 1996
- Todd KH. Clinical versus statistical significance in the assessment of pain relief. Annals of Emergency Medicine 1996;27:439–41. [DOI] [PubMed] [Google Scholar]
Turnbull 2003
- Turnbull DK, Shepherd DB. Post-dural puncture headache: pathogenesis, prevention and treatment. British Journal of Anaesthesia 2003;91(5):718-29. [DOI] [PubMed] [Google Scholar]
Vallejo 2000
- Vallejo MC, Mandell GL, Sabo DP, Ramanathan S. Postdural puncture headache: a randomized comparison of five spinal needles in obstetric patients. Anesthesia and Analgesia 2000;91(4):916-20. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Basurto 2009
- Basurto Oña X, Solà I, Bonfill Cosp X. Drug therapy for treating post-dural puncture headache. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No: CD007887. [DOI: 10.1002/14651858.CD007887] [DOI] [PubMed] [Google Scholar]
Sudlow 2009
- Sudlow CLM, Warlow CP. Drug therapy for preventing and treating post-dural puncture headache. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD001792. [DOI: 10.1002/14651858.CD001792] [DOI] [Google Scholar]


