Abstract
Background: As iodine is a requisite micronutrient for infant brain development, infants are at risk for iodine deficiency during the weaning period when their diet transitions from milk (breast-milk, infant formula, or follow-on formula) to solid food. Dietary iodine intake during this weaning period is likely minimal, as the iodine content of commercial baby food is not regulated, and the addition of salt to baby food is not recommended. This study reports the current status of iodine nutrition among weaning infants in the United States.
Methods: Subjects (n = 60; 50% Caucasian, 30% black) were infants <12 months of age who were fed any combination of formula and/or baby food. Samples of all formula and food consumed in the previous 24 hours and a spot urine sample from each infant were obtained for the measurement of iodine. The estimated quantities of ingested formula and baby food were summed from a food diary recorded by the infants' parents.
Results: The mean age of the infants was 6.3 ± 3.5 months. The median urinary iodine concentration (UIC) was 117 μg/L (range 26.9–1302.8 μg/L). Estimated daily iodine intake obtained from the measured iodine content in infant formula/foods was 89 μg (range 0–288 μg). There was a positive correlation between the infants' UIC and the iodine content in the consumed foods (r = 0.4, p < 0.001).
Conclusions: Although the median UIC of infants fed a combination of infant formula and baby food would meet the criteria for iodine sufficiency in a larger sample, those consuming the lowest quartile of iodine-containing nutritional sources had a median UIC <100 μg/L.
Keywords: iodine, infants, nutrition, brain development, iodine nutrition
Introduction
Iodine, required for thyroid hormone synthesis, is a critical micronutrient for brain development (1), and iodine deficiency is the most common cause of preventable mental impairment worldwide (2). A low iodine supply in newborns is associated with an increased frequency of transient thyroid dysfunction in this population (3). By body weight, nutritional iodine requirements in infants are higher than at any other time in the life-span (4,5). A median urinary iodine concentration (UIC) of >100 μg/L in a population of at least 125 individuals (6) indicates population-level adequate iodine nutrition (7). Although the United States is regarded overall as iodine sufficient (2), there has been a downtrend of median UICs, including among pregnant women (8,9), in recent decades.
Dietary iodine intake is the primary contributor to iodine status. The U.S. Institute of Medicine and the World Health Organization recommend iodine intake of 110 μg/day for infants 0–6 months old and 130 μg/day for infants 7–12 months old (2,10). In breast-feeding infants, the only source of nutritional iodine is breast milk, which is dependent on the mother's intake of foods naturally containing or fortified with iodine. In one study, direct iodine supplementation in infants was less effective in improving infant iodine status compared to iodine dosing of their mothers (11). For infants who are not breast-fed, the iodine content of commercial infant formulas is regulated by the U.S. Food and Drug Administration (12). However, similar regulations for commercial baby food in the United States are lacking. Thus, the risk of iodine deficiency increases as infants transition from milk (breast milk, infant formula, or follow-on formula) to baby food (homemade or commercially prepared table foods) (13). In one European study, approximately half of the complementary baby-food products (defined as those fed to infants as they transition from exclusive breast-feeding to table food) studied contain sufficient iodine, and partially breast-fed infants receiving table food achieved <50% of the recommended iodine intake goals (14). Additionally, salt is not customarily added to homemade weaning foods, and thus infants do not benefit from iodized salt.
The purpose of this study is to report the current status of and factors affecting iodine nutrition of weaning infants in the United States.
Methods
Study approval was obtained from the Boston University Medical Campus and UCLA Institutional Review Boards. Inclusion criteria were full-term infants (i) ≤14 months of age, (ii) with no known history of a thyroid disorder, (iii) and who were exclusively formula-fed or fed a combination of formula (either infant or follow-on) and baby food. Infants who were exclusively breast-fed were excluded. Enrollment was completed within the greater Boston and Los Angeles metropolitan areas from 2012 to 2016 through online and paper advertisements.
Data regarding mothers' age, ethnicity, birthplace, marital status, education, smoking status, and exposure to second-hand smoking were ascertained from subject questionnaires. Smoking status was included, as thiocyanate is a metabolite of cigarettes and competitive inhibitor of the sodium/iodide symporter in lactating mammary tissue, thereby resulting in potentially decreased breast-milk iodine content among smokers (15). The quantity and frequency of all infant formula and food ingested by the infant within the previous 24 hours of the visit was recorded by each infant's parents in a food diary. Parents were asked to bring a tablespoon-sized aliquot of all recorded infant formula and food items consumed by the infant in the past 24 hours in separate sealed bags or containers. If the parent had reported that the infant had ingested formula, sampling was performed of only the prepared and ready-to-consume formulation submitted by the parent. A spot urine sample was obtained from each infant using a urine diaper bag at the time of the study visit.
Samples of urine, infant formula, and food were stored at −20°C until they were batched shipped to the Boston University Iodine Research Laboratory for the measurement of iodine content spectrophotometrically using a Technicon Autoanalyzer (Technicon Instrument, Inc., Tarrytown, NY) by a modification of the method of Benotti et al. (16). Food iodine content was measured by the iodide catalysis of the redox reaction (catalytic activity) between ceric (Ce4+) and arsenic (As3+). Food samples, controls, and iodate standards were first digested with chloric acid (HClO3) then measured colorimetrically at 420 μm; calculations are based on a standard calibration curve. Samples were measured in duplicate, and the average levels are reported. In cases where the initial two measurements were not within 15% of each other, a third or a fourth measurement was obtained, and the average of all measurements was reported. Due to high and variable levels of urinary creatinine concentration in this age group (17), a urine iodine-to-creatinine ratio was not used for standardization.
The daily iodine intake for each infant was calculated from the measured iodine content of ingested food and formula and the ingested quantities recorded in the diary. For data collection and statistical analyses, Microsoft Excel v16.0 (Microsoft, Redmond, WA) and SAS v9.4 (SAS Institute, Inc., Cary, NC) were used. Group differences for continuous variables were tested by using Wilcoxon or Mann–Whitney U-tests with Bonferroni correction when indicated. Spearman or Pearson correlations were applied in univariate analyses. Multiple linear regression analyses were done assessing infant UIC to include sex and current breast-milk and formula consumption as covariates. p-Values of <0.05 were considered significant.
Results
Sixty infant–mother pairs (infants: Mage ±SD = 6.3 ± 3.5 months; median = 6 months (range 1–14 months); mothers: Mage ±SD = 31 ± 6.6 years) were studied. Mothers were primarily American born (87%), had less than a college-degree education (58%), and were nonsmokers (80%). The subject demographics are described in Table 1.
Table 1.
Subject Demographics for the Recruited Infant–Mother Pairs (n = 60)
| Variable | Frequency (%) |
|---|---|
| Infant's ethnicity | |
| White | 30 (50%) |
| Black | 18 (30%) |
| Hispanic | 7 (11.7%) |
| Asian | 1 (1.7%) |
| Other | 4 (6.7%) |
| Infant's birthplace | |
| United States | 52 (86.7%) |
| Other | 8 (13.3%) |
| Mother's marital status | |
| Single | 28 (46.7%) |
| Married | 28 (46.7%) |
| Other | 4 (6.7%) |
| Mother's educational status | |
| Less than high school | 9 (15%) |
| High-school graduate | 18 (30%) |
| Some college | 8 (13.3%) |
| College degree | 12 (20%) |
| Postgraduate | 13 (21.7%) |
| Mother's smoking status | |
| Nonsmoker | 48 (80%) |
| Smoker | 12 (20%) |
| Secondhand smoke exposure | |
| No | 32 (53.3%) |
| Yes | 4 (6.7%) |
| Unknown | 24 (40%) |
Examples of the most commonly ingested foods were formula (e.g., Enfamil, Earth's Best), commercially jarred baby food, steamed vegetables, steamed beans, baked potatoes, chicken, fish, eggs, and fruits. Sixty percent of infants were exclusively formula fed. The estimated daily iodine intake obtained from the measured iodine content in infant formula/foods was 89 μg/day (interquartile range [IQR] = 65–118 μg/day; range 0–288 μg/day; Fig. 1). Infants' median UIC was 117 μg/L (IQR = 76–182 μg/L; range 26.9–1302.8 μg/L), with 43% of infants having a UIC <100 μg/L (Fig. 2). There was a significant positive correlation between infants' UIC and total daily iodine consumed (r = 0.4, p < 0.001). However, infants' UIC was not significantly correlated with the amount of formula they consumed (categorized in quartiles; range 8–55 ounces/day; p = 0.26). In a multivariate analysis, infant age, ethnicity, place of birth, mothers' educational level, and mothers' cigarette smoking status were not predictive of infants' UIC.
FIG. 1.
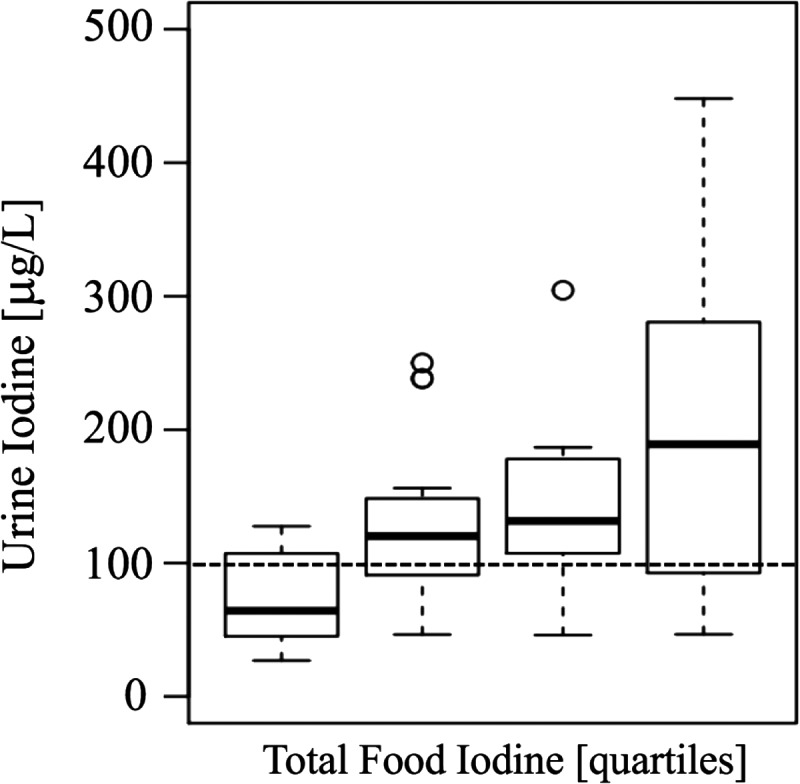
Urinary iodine concentration (UIC; μg/L) stratified by quartiles of total dietary iodine intake over the previous 24 hours. The error bars indicate the 25th/75th percentile. The dotted line indicates iodine sufficiency in a population of >125 individuals.
FIG. 2.
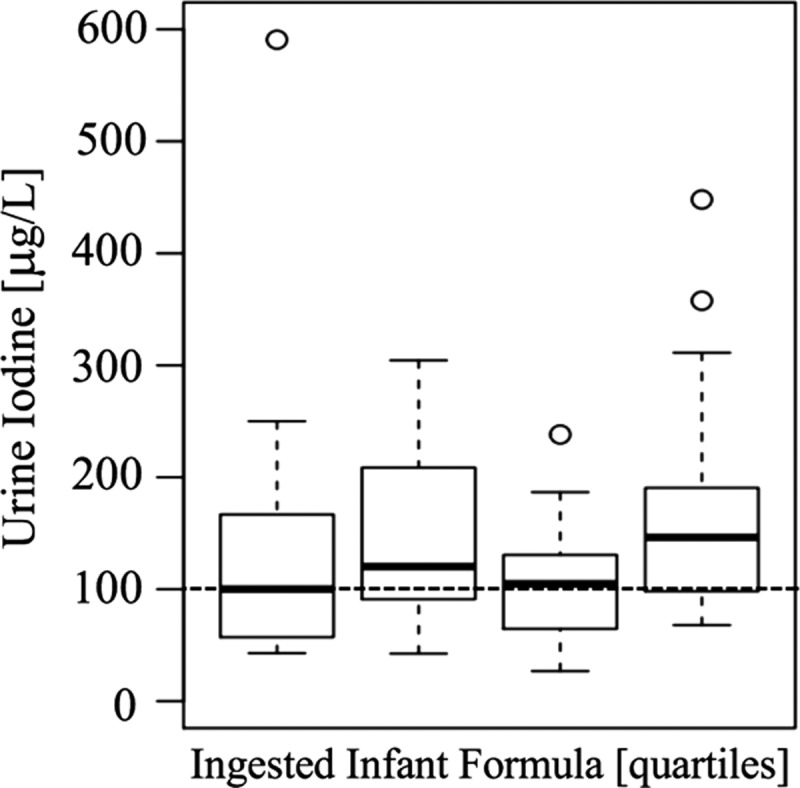
UIC (μg/L) stratified by quartiles of the quantities of ingested infant formula (in ounces) over the previous 24 hours. The error bars indicate the 25th/75th percentile. The dotted line indicates iodine sufficiency in a population of >125 individuals.
Discussion
Iodine is a crucial component of thyroid hormones, which are important for normal brain development and overall growth (18). Iodine deficiency during pregnancy is associated with adverse neurocognitive outcomes in children (19) and is the most common preventable cause of intellectual disabilities worldwide. Recommendations for micronutrient intake are based on the needs of most healthy individuals in specific age groups (10). The current study adds further understanding to the limited data regarding infant iodine nutrition in the United States.
Although the present study demonstrates that infants who were fed a combination of infant formula, follow-on formula, and baby food would be overall iodine sufficient in a larger cohort, the median UIC in those infants who consumed the lowest quartile amount of iodine-containing foods was <100 μg/L, suggesting that a subset of infants with low dietary iodine intake may be at risk for iodine insufficiency.
These findings support the concern that among countries regarded as generally iodine sufficient, such as the United States (2), weaning infants may be at risk for iodine deficiency during this transitional period (14). The American Academy of Pediatrics recommends exclusive breast-feeding until at least four to six months of life (20), although commercial infant formulas are alternatively used by caregivers if breast-feeding is not an option. Some studies have shown that exclusively breast-fed infants receive sufficient iodine in breast milk when their mothers ingest at least 250 μg/day of iodine from the diet (21,22). It has been suggested that breast-milk iodine concentrations of 150–180 μg/L would confer sufficient iodine to the lactating infant (23), although breast-milk iodine concentrations may fluctuate substantially relative to the timing of iodine ingestion by the mother (24). A recent study showed that iodine content (2.5th–97.5th percentiles) of breast milk in exclusively breast-feeding women living in iodine-sufficient regions ranges between 60 and 465 μg/kg (25). Another report showed that UICs among breast- and formula-fed infants in the greater Boston area are similar (26).
Strengths of this study include the measurement of the iodine content in ingested infant formula and baby foods, since iodine nutrition based on food package labeling may be misleading (27). Limitations of this pilot study include a relatively small sample size that is inadequate to determine overall iodine sufficiency in this population based on median UIC, as well as the relative different proportions of infant formulae and food consumed by infants at any given age within this sample. Additionally, infants' iodine intake resulted from a combination of infant formula (mandated to contain iodine) and baby food (unregulated for iodine content), therefore supporting the need for further studies examining the nutritional iodine contributions of each separately. It is also acknowledged that 24-hour UICs may be more accurate than those measured from spot urine samples (28).
Early life represents an important foundational period critical for later health outcomes, thus representing a time-sensitive opportunity to ensure healthy growth and development (29). These results indicate that although U.S. infants fed a combination of baby formula and food would be overall iodine sufficient in a larger sample, those consuming sources containing low nutritional iodine content had median UICs <100 μg/L. Iodine-containing infant formula and baby foods are important sources of iodine nutrition during infancy, and further research is needed to determine the specific iodine contributions of each during this important developmental period, identify at-risk subgroups, and take preventive measures to achieve iodine sufficiency for all infants.
Acknowledgments
This study was supported by U.S. National Institutes of Health grant K23 HD068552 (A.M.L.).
Author Disclosure Statement
The authors have no conflicts of interest, commercial associations, or financial ties to disclose.
References
- 1. Zimmermann MB. 2012. The effects of iodine deficiency in pregnancy and infancy. Paediatr Perinat Epidemiol 26:108–117 [DOI] [PubMed] [Google Scholar]
- 2. Iodine Global Network. Available at: www.ign.org (last accessed May22, 2018)
- 3. Delange F, Heidemann P, Bourdoux P, Larsson A, Vigneri R, Klett M, Beckers C, Stubbe P. 1986. Regional variations of iodine nutrition and thyroid function during the neonatal period in Europe. Biol Neonate 49:322–330 [DOI] [PubMed] [Google Scholar]
- 4. Delange F. 1998. Screening for congenital hypothyroidism used as an indicator of the degree of iodine deficiency and of its control. Thyroid 8:1185–1192 [DOI] [PubMed] [Google Scholar]
- 5. Dold S, Zimmermann MB, Baumgartner J, Davaz T, Galetti V, Braegger C, Andersson M. 2016. A dose–response crossover iodine balance study to determine iodine requirements in early infancy. Am J Clin Nutr 104:620–628 [DOI] [PubMed] [Google Scholar]
- 6. Andersen S, Karmisholt J, Pedersen KM, Laurberg P. 2008. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br J Nutr 99:813–818 [DOI] [PubMed] [Google Scholar]
- 7. International Council for the Control of Iodine Deficiency Disorders 2007 Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination. Third edition. World Health Organization, Geneva, Switzerland [Google Scholar]
- 8. Pearce EN, Leung AM. 2013. The state of U.S. Iodine nutrition: how can we ensure adequate iodine for all? Thyroid 23:924–925 [DOI] [PubMed] [Google Scholar]
- 9. Hollowell JG, Staehling NW, Hannon WH, Flanders DW, Gunter EW, Maberly GF, Braverman LE, Pino S, Miller DT, Garbe PL, DeLozier DM, Jackson RJ. 1998. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971–1974 and 1988–1994). J Clin Endocrinol Metab 83:3401–3408 [DOI] [PubMed] [Google Scholar]
- 10. Institute of Medicine Food and Nutrition Board 2006 Dietary Reference Intakes. National Academies Press, Washington, DC [Google Scholar]
- 11. Bouhouch RR, Bouhouch S, Cherkaoui M, Aboussad A, Stinca S, Haldimann M, Andersson M, Zimmermann MB. 2014. Direct iodine supplementation of infants versus supplementation of their breastfeeding mothers: a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol 2:197–209 [DOI] [PubMed] [Google Scholar]
- 12. Trumbo PR. 2016. FDA regulations regarding iodine addition to foods and labeling of foods containing added iodine. Am J Clin Nutr 104:864S–867S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexy U, Drossard C, Kersting M, Remer T. 2009. Iodine intake in the youngest: impact of commercial complementary food. Eur J Clin Nutr 63:1368–1370 [DOI] [PubMed] [Google Scholar]
- 14. Andersson M, Aeberli I, Wüst N, Piacenza AM, Bucher T, Henschen I, Haldimann M, Zimmermann MB. 2010. The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J Clin Endocrinol Metab 95:5217–5224 [DOI] [PubMed] [Google Scholar]
- 15. Laurberg P, Nøhr SB, Pedersen KM, Fuglsang E. 2004. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J Clin Endocrinol Metab 89:181–187 [DOI] [PubMed] [Google Scholar]
- 16. Benotti J, Benotti N, Pino S, Gardyna H. 1965. Determination of total iodine in urine, stool, diets, and tissue. Clin Chem 11:932–936 [PubMed] [Google Scholar]
- 17. Dorey CM, Zimmermann MB. 2008. Reference values for spot urinary iodine concentrations in iodine-sufficient newborns using a new pad collection method. Thyroid 18:347–352 [DOI] [PubMed] [Google Scholar]
- 18. Redman K, Ruffman T, Fitzgerald P, Skeaff S. 2016. Iodine deficiency and the brain: effects and mechanisms. Crit Rev Food Sci Nutr 56:2695–2713 [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann MB. 2009. Iodine deficiency in pregnancy and the effects of maternal iodine supplementation on the offspring: a review. Am J Clin Nutr 89:668S–672S [DOI] [PubMed] [Google Scholar]
- 20. Kramer MS, Kakuma R. 2012. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jorgensen A, O'Leary P, James I, Skeaff S, Sherriff J. 2016. Assessment of breast milk iodine concentrations in lactating women in Western Australia. Nutrients 8:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nazeri P, Kabir A, Dalili H, Mirmiran P, Azizi F. 2018. Breast-milk iodine concentrations and iodine levels of infants according to the iodine status of the country of residence: a systematic review and meta-analysis. Thyroid 28:124–138 [DOI] [PubMed] [Google Scholar]
- 23. Fisher W, Wang J, George NI, Gearhart JM, McLanahan ED. 2016. Dietary iodine sufficiency and moderate insufficiency in the lactating mother and nursing infant: a computational perspective. PLoS One 11:e0149300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leung AM, Braverman LE, He X, Heeren T, Pearce EN. 2012. Breastmilk iodine concentrations following acute dietary iodine intake. Thyroid 22:1176–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dold S, Zimmermann MB, Aboussad A, Cherkaoui M, Jia Q, Jukic T, Kusic Z, Quirino A, Sang Z, San Luis TO, Vandea E, Andersson M. 2017. Breast milk iodine concentration is a more accurate biomarker of iodine status than urinary iodine concentration in exclusively breastfeeding women. J Nutr 147:528–537 [DOI] [PubMed] [Google Scholar]
- 26. Gordon JH, Leung AM, Hale AR, Pearce EN, Braverman LE, He X, Belfort MB, Nelson SM, Brown RS. 2014. No difference in urinary iodine concentrations between Boston-area breastfed and formula-fed infants. Thyroid 24:1309–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pearce EN, Pino S, He X, Bazrafshan HR, Lee SL, Braverman LE. 2004. Sources of dietary iodine: bread, cows' milk, and infant formula in the Boston area. J Clin Endocrinol Metab 89:3421–3424 [DOI] [PubMed] [Google Scholar]
- 28. Vejbjerg P, Knudsen N, Perrild H, Laurberg P, Andersen S, Rasmussen LB, Ovesen L, Jørgensen T. 2009. Estimation of iodine intake from various urinary iodine measurements in population studies. Thyroid 19:1281–1286 [DOI] [PubMed] [Google Scholar]
- 29. Vaivada T, Gaffey MF, Bhutta ZA. 2017. Promoting early child development with interventions in health and nutrition: a systematic review. Pediatrics 140 [DOI] [PubMed] [Google Scholar]


