Abstract
Background
Psychological and educational interventions have been used as an adjunct to conventional therapy for children with atopic eczema to enhance the effectiveness of topical therapy. This is an update of the original Cochrane review.
Objectives
To assess the effect of psychological and educational interventions for atopic eczema in children.
Search methods
We updated our searches of the following databases to January 2013: the Cochrane Skin Group Specialised Register, CENTRAL in The Cochrane Library (2012, Issue 12), MEDLINE (from 1946), EMBASE (from 1974), OpenGrey, and PsycINFO (from 1806). We also searched six trials registers and checked the reference lists of included and excluded studies for further references to relevant randomised controlled trials (RCTs).
Selection criteria
Randomised controlled trials of psychological or educational interventions, or both, used to assist children and their carers in managing atopic eczema.
Data collection and analysis
Three authors independently applied eligibility criteria, assessed trial quality, and extracted data. A lack of comparable data prevented data synthesis, and we were unable to conduct meta‐analysis because there were insufficient data.
Main results
We included 10 RCTs, of which 5 were new to this update; all interventions were adjuncts to conventional therapy and were delivered in primary‐ and secondary‐care settings. There were 2003 participants in the 9 educational interventions and 44 participants in the 1 psychological study. Some included studies had methodological weaknesses; for example, we judged four studies to have high risk of detection bias, attrition bias, or other bias. Our primary outcomes were participant‐rated global assessment, reduction in disease severity (reported as objective SCORAD (SCORing Atopic Dermatitis)), and improvement in sleep and quality of life. No study reported participant‐rated global assessment or improvement of sleep.
The largest and most robust study (n = 992) demonstrated significant reduction in disease severity and improvement in quality of life, in both nurse‐ and dermatologist‐led intervention groups. It provided six standardised, age‐appropriate group education sessions. Statistically significant improvements in objective severity using the SCORAD clinical tool were recorded for all intervention groups when compared with controls. Improvements in objective severity (intervention minus no intervention) by age group were as follows: age 3 months to 7 years = 4.2, 95% confidence interval (CI) 1.7 to 6.8; age 8 to 12 years = 6.7, 95% CI 2.1 to 11.2; and age 13 to 18 years = 9.9, 95% CI 4.3 to 15.5. In three of five studies, which could not be combined because of their heterogeneity, the objective SCORAD measure was statistically significantly better in the intervention group compared with the usual care groups. However, in all of the above studies, the confidence interval limits do not exceed the minimum clinically important difference of 8.2 for objective SCORAD.
The largest study measured quality of life using the German 'Quality of life in parents of children with atopic dermatitis' questionnaire, a validated tool with five subscales. Parents of children under seven years had significantly better improvements in the intervention group on all five subscales. Parents of children aged 8 to 12 years experienced significantly better improvements in the intervention group on 3 of the 5 subscales.
Authors' conclusions
This update has incorporated five new RCTs using educational interventions as an adjunct to conventional treatment for children with atopic eczema. We did not identify any further studies using psychological interventions. The inclusion of new studies has not substantially altered the conclusions from the original review. The educational studies in both the original review and this update lack detail about intervention design and do not use a complex interventions framework. Few use an explicit theoretical base, and the components of each intervention are not sufficiently well described to allow replication. A relative lack of rigorously designed trials provides limited evidence of the effectiveness of educational and psychological interventions in helping to manage the condition of atopic eczema in children. However, there is some evidence from included paediatric studies using different educational intervention delivery models (multiprofessional eczema interventions and nurse‐led clinics) that these may lead to improvements in disease severity and quality of life. Educational and psychological interventions require further development using a complex interventions framework. Comparative evaluation is needed to examine their impact on eczema severity, quality of life, psychological distress, and cost‐effectiveness. There is also a need for comparison of educational interventions with stand‐alone psychosocial self‐help.
Keywords: Adolescent; Child; Humans; Infant; Biofeedback, Psychology; Caregivers; Caregivers/education; Dermatitis, Atopic; Dermatitis, Atopic/psychology; Dermatitis, Atopic/therapy; Family Health; Hypnosis; Outcome Assessment, Health Care; Parents; Parents/education; Patient Education as Topic; Patient Education as Topic/methods; Practice Patterns, Nurses'; Psychotherapy; Quality of Life; Randomized Controlled Trials as Topic; Steroids; Steroids/administration & dosage
Plain language summary
Psychological and educational interventions for atopic eczema in children
Atopic eczema is an itchy, inflammatory skin condition, which affects the quality of life of children with eczema and their parents or carers. It affects large and increasing numbers of children worldwide. Psychological and educational approaches have been used to complement medication in managing eczema, for example, by using simple psychological techniques to manage itching and scratching or sleep disturbance. Educational interventions, provided to individuals and groups by nurses or teams of specialists in hospital or community settings, have been used to help parents and children to understand the condition and their role in managing it successfully. However, the effect of these approaches has not been systematically measured.
We included 10 studies in this review: 5 were in the original review, and 5 were newly incorporated in this update.
Nine studies were educational and predominantly parent‐focused (total number of participants n = 2003), and the tenth was a child‐centred psychological intervention (n = 44).
The main finding of this review is that there is currently only limited research evidence about the effect of educational and psychological approaches when used alongside medicines for the treatment of childhood eczema. Included studies provided a range of interventions, from a single 15‐minute consultation to a comprehensive series of sessions delivered to groups of parents over a period of 12 hours. Details of the interventions used and the educational theory base are generally poorly described. Outcome measures varied between studies.
Although it is not possible to draw definitive conclusions from this review, several studies using educational interventions demonstrated improvements in eczema severity and quality of life for both children and families. In particular, two studies showed promise. One large study (n = 992) using a multi‐disciplinary group education intervention in a hospital setting showed modest improvements in disease severity and quality of life. The single study using psychological approaches indicated that relaxation methods reduced the severity of eczema when compared to discussion only.
There is a need for further research into this subject, and priority should be given to comparing the relative cost effectiveness of health professionals educating parents either in teams or by nurses alone. There is also a need for comparison with stand‐alone self‐help. The most appropriate timeframe for evaluating the effect of interventions should be considered.
Background
This is an updated version of an original Cochrane review (Ersser 2007).
Description of the condition
Definition, clinical features, and epidemiology
Atopic eczema (or atopic dermatitis) is an itchy, inflammatory skin disease, often involving skin creases (Williams 2005). The condition may be acute with redness, scaling, oozing, and vesicles, or it may be chronic with associated skin thickening, altered pigmentation, and exaggerated surface markings. Itching is a major symptom that can develop into a cycle of scratching, causing skin damage and in turn more itching (the itch‐scratch cycle). Atopic eczema is now the most common inflammatory skin disease of childhood, affecting large and increasing numbers of children worldwide (Asher 2006). Whilst the number of adults with atopic eczema is smaller (1% to 2%), their disease is frequently more chronic and severe (Herd 1996). Approximately 70% of cases start in children under the age of 5 (Hanifin 2007). Evidence suggests that the prevalence of atopic eczema has increased two‐ to threefold over the last 30 years (Schram 2010). The reasons for this are not entirely clear but are likely to be environmental, as significant differences in prevalence between populations of the same ethnic background have frequently been found, for instance between urban and rural areas (Schram 2010).
Causes
Nevertheless, genetic factors are important in the development of eczema, as has been repeatedly shown in association with carriage of filaggrin (FLG) loss‐of‐function mutations (Palmer 2006; Smith 2006); FLG is a gene that has a pivotal role in skin barrier function. Filaggrin forms part of the cornified cell envelope (the 'mortar' of the 'brick‐and mortar' structure of the epidermis). Reduced expression or complete lack of FLG therefore leads to an impaired skin barrier. Approximately 40% of children with moderate to severe eczema carry at least one FLG mutation, which predisposes to early onset eczema, disease severity, and chronicity. However, as a significant proportion of children with eczema do not carry a FLG mutation, other genetic factors are likely to play a role, too (Paternoster 2012).
The current hypothesis is that where people carry a skin barrier gene mutation, such as a loss‐of‐function mutation in the filaggrin gene, the skin barrier is impaired, leading to an increase in transepidermal water loss (water loss across the superficial skin) and therefore skin dryness (Flohr 2010). Probably in interaction with environmental factors, such as water hardness and frequent use of protease‐containing detergents and soaps, the integrity of the skin barrier is gradually broken down further, and this may lead to the typical immunological changes seen in eczematous skin (Cork 2006; McNally 1998; Sherriff 2002). Animal work suggests that environmental allergens, such as house dust mites, but also food protein, can make contact with the immune system via antigen‐presenting cells in the superficial epidermis, leading to sensitisation. This can make existing eczema worse and may also be an important precursor of food and respiratory allergies (Fallon 2009). This would explain why FLG mutations are only associated with asthma in the presence of eczema or allergic sensitisation (van den Oord 2009). However, prospective studies are required to examine the exact sequence of events.
Impact
Measurement of the impact of skin disease on quality of life and emotional well‐being is important for our understanding and management of skin conditions as psychosocial factors play an important role in the itch‐scratch cycle (Verhoeven 2008). Several studies suggest that atopic eczema has a greater detrimental impact on quality of life than other skin diseases, such as acne and psoriasis (Lewis‐Jones 1995); therefore, it is desirable to measure the impact on quality of life as a potential outcome of interventions (Lewis‐Jones 1995). It is notable that whilst detrimental impact on quality of life is common, non‐adherence to treatment regimens continues to be problematic, with parents reporting dissatisfaction with the 'trial and error' approach to treatment often experienced in primary care (Santer 2012). The relationship between the severity of atopic eczema in children and adolescents and quality of life has been established (Ben‐Gashir 2004). Problematic symptoms, such as itching, can adversely affect quality of life. Itch leads to scratching, which may have a significant detrimental impact on a child's sleep, quality of life (Lewis‐Jones 2001; Williams 1997), and family life (Elliott 1997; Johnson 1991). Because of the various impacts of atopic eczema, it is necessary to measure changes in disease severity as a key outcome measure. Also, since caregivers, especially parents, are often required to assist with treatments, their ability and confidence are relevant outcomes to measure. Given that children and adolescents with atopic eczema require special clothing, bedding, frequent applications of greasy ointments, and may need to avoid activities such as swimming (Reid 1995), treatment adherence becomes a relevant outcome to measure. There is also a substantial economic cost to the family (Kemp 2003) and the health service (Verboom 2002).
Description of the intervention
Educational and psychological interventions, where available, are invariably provided in conjunction with conventional therapy. Such interventions may be directed towards the parent or child, with parents tending to be the primary focus of the educational approaches and children the main target of psychological interventions. A child's ability to participate effectively in an educational or psychological intervention will depend on the suitability of the activity for their age and developmental stage. Educational interventions are often used in supporting people with long‐term conditions to optimise care. A recent example of this in the dermatology field is the Eczema Education Programme. This is one of the largest parental eczema education programmes in Europe and has been subject to extensive evaluation in a non‐controlled trial (Ersser 2013; Jackson 2013). An example of a psychological (primarily behavioural) intervention is habit reversal, identified as a method of eliminating nervous habits and tics, whereby an alternative or competing behaviour is adopted in place of the undesirable behaviour (Miltenberger 1998). Other types of psychological intervention might include cognitive behavioural therapy, counselling, and arousal reduction techniques, such as relaxation and mindfulness.
a) Psychological interventions
The main types of psychological intervention available are summarised briefly below. All of these approaches can be delivered either in an individual or group format, although more in‐depth psychological therapy tends to be provided on a one‐to‐one basis.
1. Psychological techniques using arousal reduction techniques
These are essentially all very similar relaxation techniques, which may include the following.
Progressive relaxation: a technique that relies on tensing different muscles in the body and then releasing that tension. This enables an individual to recognise areas of tension and to consciously learn to release that tension.
Autogenic training: a systematic form of relaxation involving increasing awareness of the body.
Guided imagery or 'visualisation': learning to use imagery associated with relaxation or calmness and attempting to induce the related feeling in one's own body.
Biofeedback: here a person learns how to recognise and manage physiological responses through feedback usually facilitated by the use of instrumentation.
Hypnotherapy: involves creating a state whereby an individual is suggestible. It is often used to create a feeling of relaxation and is consequently included here.
2. Behavioural interventions
Behaviour therapy involves the application of behavioural theory to modify behaviours.
Habit reversal is a form of behavioural intervention used to modify unhelpful scratching.
Other forms of behavioural intervention may include caregiver training programmes whereby carers are trained in the use of contingency management systems (for example, through the systematic use of charts to record and reward progress).
3. Psychological therapies focused on internal processes
There are a number of therapies that might broadly be referred to as 'talking therapies', and these are generally associated with raising insight and may or may not involve the use of specific techniques to change internal psychological processes, external behaviour, and coping styles.
Cognitive behavioural therapy takes a biopsychosocial perspective that involves the promotion of an empiricist approach, i.e. assisting an individual in understanding the links between their thoughts, thinking processes, and behaviours. As well as drawing on cognitive theories, it also draws on behavioural theories and techniques. It is problem‐focused and may use a range of techniques to raise awareness of ‐ and so to change specific thoughts ‐ cognitive processes, feelings, or behaviour and to enhance coping strategies.
Counselling: usually non‐directive, non‐judgemental, empathetic, and supportive approaches, which enable an individual to cope more effectively with their problems or inner states.
Family therapy: views the family, rather than the individual member, as the unit requiring assistance. Types of family therapy all involve encouraging family members to talk to one another, examining inflexibilities, family rules and beliefs, focusing on relationships within the family and those between the family and external agencies, e.g. health, education, occupation, and social services.
Psychodynamic approaches place emphasis on unconscious motives and drives. The aim of the therapy is the recognition of unhelpful defences and the linkage of these to underlying conflicts. This may include focusing on the past and making use of the relationship between the patient and the therapist to understand the origin and maintenance of distress.
b) Educational Interventions
Wolf 2002 defines educational interventions as, 'any intervention targeted at children (or their caregivers) designed to teach one or more management strategies related to prevention, management, or the use of social skills'. We included these interventions, which can use 'any instructional strategy or combination of strategies (problem solving, role‐playing, videotapes, computer‐assisted instruction, booklets, etc) and be presented either individually or in group sessions' (Wolf 2002), in this review.
Dermatological educational and psychological behaviour‐change techniques may be combined to support secondary prevention (Gieler 2000). Educational interventions are focused on the process of acquiring new knowledge or skills through teaching and learning activities. An approach where information‐giving and formal teaching leads the recipients to become more accurately informed about their condition means they are better prepared to understand the need for medical interventions and effective disease management. The content of educational interventions may include information on the disease, treatment instructions, management, and prevention strategies, and may be delivered in hospital or community settings. There is growing awareness that education, in the form of imparting knowledge alone, will not lead to improved outcome. In recent years, there has been increased use of self‐efficacy‐based interventions that build knowledge, skills, and confidence (Bandura 1997) to enable people to self‐manage long‐term conditions as effectively as possible (Ersser 2011). Motivation and intention to change are important factors in educational interventions, and it is well recognised that intention to change does not necessarily lead to health behaviour change (Webb 2006). Therefore, it is important to plan some follow up from such interventions.
How the intervention might work
Educational interventions have been used in the management of long‐term conditions in adults with positive outcomes. However, it is evident that interventions based solely on education are unlikely to bring about health behaviour change. Whilst the 'active ingredients' of successful interventions remain unclear (Coster 2009), they are likely to include the participant's motivation; shared decision‐making; development of problem‐solving skills; goal setting and agreeing action plans (Coulter 2006; Health Foundation 2011); and ensuring that people have sufficient knowledge, skills, and confidence to self‐manage as effectively as possible (Bandura 1997). A range of theories can be applied to the development of educational interventions in health care. Of particular importance is the relationship between intention and actual behaviour change. The theory of planned behaviour, the theory of reasoned action, and self‐regulation theory have been successfully integrated into interventions (Webb 2006).
Many existing educational programmes for long‐term conditions are based on Social Learning Theory (SLT) (Bandura 1997). A core concept of SLT is self‐efficacy, that is, the belief that a person has that they are able to successfully initiate and complete actions needed to achieve a specific outcome (Ersser 2011). To be able to self‐manage as effectively as possible, people need to have sufficient knowledge, skills, and confidence; attributes that can be developed through the application of self‐efficacy theory‐based educational interventions. This approach has been applied with promising results in adult psoriasis (Ersser 2011). In childhood eczema, any such intervention would primarily be aimed at the parent or carer.
Techniques such as habit reversal work on the premise that scratching has become unconscious and generalised beyond the experience of itch. It therefore aims to bring into conscious awareness the repetitive scratching by use of a recording technique, which may in itself reduce the urge to scratch. In addition, habit reversal teaches how to use alternative less damaging behaviours where the itch persists. Some of the other simple psychological techniques, such as relaxation, may simply work by reducing arousal and stress that may heighten the perception of itch. More complex psychological interventions may be necessary where there are secondary gains or unhelpful coping responses contributing to the presentation.
All the educational and psychological interventions reviewed have been used as adjuncts to conventional eczema treatments, including topical and systemic therapies.
Why it is important to do this review
Since atopic eczema affects significant numbers of children and can be disabling for whole families, psychological support and education of the parent or carer are essential components of disease management. Little is known however of the measurable effects of such interventions. In the original version of this review, Ersser 2007 found only limited evidence to support psychological or educational interventions. However, management strategies to reduce scratching behaviours that exacerbate eczema are incorporating psychological interventions, and treatment guidelines are beginning to recommend them (Giannini 1997; Ring 2012). Despite the fact that parents are the primary carers for children with atopic eczema, very limited attention has been given to the psychological support of parents (by educational or psychological intervention). As such, the caregiver's ability to manage their child's eczema is an important outcome; therefore, the educational or psychological support given to parents is relevant to this review. However, it is recognised that psychological support to both caregiver and child are important. The general case for psychosocial interventions to improve clinical outcomes in organic disease is established (Williams 2002) and also in related areas, such as asthma (Guevara 2003).
The literature refers to a range of psychological interventions that have been used in atopic eczema, such as behavioural management (Bridgett 1995; Bridgett 2000; Norén 1989), relaxation therapy (de L Horne 1999), and cognitive behavioural therapy (Ehlers 1995). Clinical observations suggest that behavioural techniques can be a useful adjunct to topical therapy, and breaking the itch‐scratch cycle is argued to be a primary clinical aim (Hägermark 1995). However, evaluative research has been limited (Bridgett 2000; Ersser 2007; Simpson‐Dent 1999), especially with children.
Educational interventions have also been used to bring about behavioural change through patient education or patient teaching for those with eczema (Niebel 2000). These educational interventions are important since chronic disease management requires a degree of self management (or caregiver or parental support) and therefore educational and behavioural change (Holman 2000). A limited number of evaluative studies have examined the impact of parental education on the management of atopic eczema in children.
Objectives
To assess the effect of psychological and educational interventions for atopic eczema in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Children, adolescents, or infants with atopic eczema and their caregivers (including parents).
Types of interventions
We anticipated that most studies would be of conventional treatment alone versus conventional plus psychological or educational interventions and that we would be unlikely to find trials examining purely psychological or educational approaches. Some interventions that are psychologically‐ or educationally‐based, focused on the parent, the child, or both, and depended upon the developmental stage of the child.
Whilst some RCTs of therapies had an educational or psychological component, in this review, we only included studies where the educational or psychological intervention was the primary intervention to which the experimental group was exposed.
Types of outcome measures
Outcome measures for eczema interventions have recently been addressed by the HOME (Harmonising Outcome Measures for Eczema) initiative, following a Delphi exercise. The core outcomes that all eczema‐related RCTs should report on are as follows: clinical signs, symptoms, long‐term control of flares, and quality of life (Schmitt 2012).
The following outcomes, influenced by the HOME work, were of interest to us as measured by participant, carer, clinician, or other trial outcome observer, or any combination. Specifically, we were concerned with a clinically significant response in the following outcomes.
Primary outcomes
The participant‐rated global assessment was the primary outcome measure if available. We refer here to the evaluation of the participant deeming the intervention to be effective or helpful or ineffective or unhelpful as an outcome measure. If this was not available, we used the medical practitioner global rating (percentage with good or excellent improvement).
Reduction in disease severity as measured by a trained assessor (Minimal Clinically Important Difference (MCID) is 8.7 points for the SCORAD, 8.2 for the objective SCORAD, 6.6 for the EASI (Eczema Area and Severity Index), and 3.4 for the POEM (Patient Orientated Eczema Measure)) (Schram 2012).
Improvement in sleep.
Improvement in quality of life or reduction in distress of the child and parent (caregiver).
Secondary outcomes
Reduction in harmful scratching behaviour (using, for example, digital accelerometers or video recordings of patients (Benjamin 2004)).
Improvement in treatment adherence.
Reduction of medication usage (particularly anti‐inflammatory or immunosuppressant treatments)*.
Enhancement of caregivers' actual and perceived ability to manage atopic eczema in their child (e.g. self‐efficacy (self‐confidence), locus of control (distinguishing those who attribute events to either their own control or to external circumstances) and coping measures)**.
We took into account, in addition to the measures above, adverse effects such as inconvenience and cost. We accepted outcome measures however they were designed and implemented, although this was accompanied by a critical evaluation of the rigour of the measures used (attention to reliability and validity issues). The conventional treatment used in a trial will be an important characteristic that may influence the effectiveness of the psychological or educational intervention, and we considered this as a possible source of heterogeneity.
*It is recognised that medication usage may go up because of improved adherence, or it may go down because the eczema has improved as a result of psychological/educational intervention. **This outcome allows for the fact that the benefits of psychological support or education may not be primarily 'medical'.
Search methods for identification of studies
We aimed to identify all RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised and updated our search strategies, and searched the following databases up to 17 January 2013:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 12) using the strategy in Appendix 2;
MEDLINE via OVID (from 1946) using the strategy in Appendix 3;
EMBASE via OVID (from 1974) using the strategy in Appendix 4;
PsycINFO via OVID (from 1806) using the search strategy Appendix 5; and
CINAHL Plus with Full Text (1937 to 2013) using the search strategy in Appendix 6 (searched up to 22 November 2013).
A final prepublication search of the above databases was undertaken on 19 November 2013. Although it has not been possible to incorporate RCTs identified through this search within this review, relevant references are listed under Studies awaiting classification. They will be incorporated into the next update of the review.
Trials registers
For this update, we searched the following trials registers up to 22 November 2013:
Current Controlled Trials ISRCTN (www.controlled‐trials.com/isrctn/), using the following search phrase: (eczema OR dermat*) AND (child* OR infant*).
The UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk/default.aspx), searching for the conditions Eczema or “atopic dermatitis”.
The US National Institutes of Health Ongoing Trials Register (www.clinicaltrials.gov), using the terms (child OR children ORchildhood ORinfant ORinfants ORinfancy ORinfantile) AND (eczema OR dermatitis OR dermatology ) AND (psychology OR psychological OR education OR educational OR educating).
The Australian New Zealand Clinical Trials Registry (www.anzctr.org.au), using the terms eczema OR “atopic dermatitis”.
The World Health Organization International Clinical Trials Registry platform (www.who.int/trialsearch) using the terms eczema OR “atopic dermatitis” as a condition, then searching the subset of Clinical trials in children.
The EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/), using the terms eczema OR atopic dermatitis, limited to age range: children and infant and toddler.
Searching other resources
References from published studies
We checked the bibliographies of included and excluded studies for further references to relevant trials.
Unpublished literature
We searched for grey literature using the OpenGrey database (www.opengrey.eu/) up to 22 November 2013, using the following search terms: (eczema OR dermat*) AND (child* OR infant*) AND (psych* OR educ*).
Conference proceedings
We did not search Zetoc Alerts for additional conference proceeding that were not expected to be covered by the Cochrane Skin Group Specialised Register for this update.
Adverse effects
We did not perform a separate search for adverse effects. However, we did examine data on adverse effects from the included studies we identified.
Data collection and analysis
Selection of studies
We only considered randomised controlled trials (RCTs). Two authors (FC and SE) checked titles and abstracts identified from the searches. We excluded studies that did not refer to an RCT on atopic eczema. Three authors (FC, SE, and SML) obtained the full texts of studies for independent assessment to decide which trials fulfilled the inclusion criteria. They resolved any disagreement by discussion between all the authors.
Data extraction and management
Three authors (FC, SML, and EG) independently performed data extraction and management, entering data onto a data extraction form. They discussed all discrepancies and achieved a consensus for each paper. The authors entered all study information and the included RCTs results into Review Manager (RevMan) for data management. They were not blinded to the names of authors, journals, or institutions.
Assessment of risk of bias in included studies
We addressed the following four areas since there is reported evidence that these are associated with biased estimates of treatment effect (Juni 2001): a) randomisation (method of generation and concealment of allocation); b) blinding of observers (blinding of participants was not possible because of the nature of the intervention); c) loss to follow up (presence of dropouts and withdrawals and the analysis of these); and d) other bias.
The quality assessment included an evaluation of the following components for each included study. Each component was categorised as low risk, unclear risk, or high risk on the data extraction form as advised by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Criteria for judgement of adequacy are as follows.
Randomisation: Adequate studies used a randomised sequence from a computer‐generated procedure or shuffled envelopes. Unclear studies provided insufficient information, and we excluded those employing alternations.
Concealment of allocation sequences: In adequate studies, the assignment could not be foreseen (allocation concealment). Low risk included techniques such as the use of a third party or use of opaque sealed envelopes. High‐risk techniques included those such as having an open list or in accordance with days of the week.
Blinding: In adequate studies, this took place after allocation assignment and ensured the outcome assessor, participants, and clinicians were unaware of any allocation sequence. In our case, determining adequacy did not relate to all three areas of blinding as this was not practical for our included studies. We addressed this issue in the methodological quality assessment section.
Loss to follow up: when more than 80% of participants were followed up and then were analysed in the groups to which they were originally randomised (intention‐to‐treat). We also included as low risk those studies in which intention‐to‐treat (ITT) analysis was undertaken but with minimal missing outcome data. We specified inadequate loss to follow up when there was no ITT analysis or substantial missing data, as well as less than 80% follow up.
Selective outcome reporting bias: We checked whether findings for all outcomes listed in the Methods sections were reported.
In addition, we assessed the following as required: e) degree of certainty that participants have atopic eczema; f) baseline comparison for severity of disease; and g) comparability at baseline for all primary outcome variables.
Measures of treatment effect
If data synthesis were possible, we planned to calculate a weighted treatment effect across trials using a random‐effects model. For dichotomous outcomes, our planned treatment effect measure was the odds ratio, and for continuous data, our planned treatment effect measure was the weighted mean difference. We planned to used standardised mean differences if different studies used different scales for a continuous outcome.
Unit of analysis issues
We planned to analyse any cross‐over trials included in the review separately from the parallel group trials before pooling the results.
Dealing with missing data
If practical, we planned to carry out a sensitivity analysis to examine the impact on the overall treatment effect if some studies had substantial missing data. If feasible, we planned to do this by carrying out a meta‐analysis twice, firstly with all studies included and then secondly by excluding the studies with substantial missing data and also studies with higher levels of other potential biases.
Assessment of heterogeneity
We planned to test for heterogeneity of the intervention effect using the I² statistic. If this statistic suggested significant heterogeneity, we then planned to check if this was due to a single 'outlier' study. If so, we planned to perform and report meta‐analyses both with and without this study. On the other hand, if there were no clear outlying studies, we planned to try to establish the causes of heterogeneity and decide whether meta‐analysis was feasible.
Assessment of reporting biases
We planned to assess reporting bias using funnel plots if we included at least 10 studies in the review and a meta‐analysis was feasible.
Data synthesis
We planned to assess whether each of our outcomes of interest were measured in a large enough subset of studies for a meta‐analysis to be viable (i.e. the clinical diversity was not too great). We also planned to assess whether the intervention and control groups in each study and the study designs were sufficiently consistent for us to synthesise a global intervention effect. If the number of included studies in the review was very small or they were too diverse, we planned to present a narrative analysis that included details of individual study results instead of a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If sufficient study information was available, we planned to perform subgroup analysis using age or developmental stage as the grouping factor. As mentioned above, we planned to use the I² statistic to investigate heterogeneity.
Sensitivity analysis
If possible, we planned to do sensitivity analyses to examine the change in overall intervention effect estimates from a meta‐analysis by excluding studies with lower methodological quality.
Results
Description of studies
Results of the search
The search for this update identified 1844 studies. We assessed each title and abstract and rejected 1839 studies as they did not meet the inclusion criteria. The majority of studies identified were in English, but other languages encountered included German, Spanish, Italian, and French. We conducted translations as required.
The original review included five RCTs (Chinn 2002; Niebel 2000; Sokel 1993; Staab 2002; Staab 2006), and we added a further five in the update process (Grillo 2006; Moore 2009; Schuttelaar 2010; Shaw 2008; Weber 2008). The study by Kupfer was based on data from Staab 2006 that had already been included in the original review, so we added it as a subsidiary reference to Staab 2006. It is important to mention that all studies used conventional topical treatments in combination with either educational or psychological interventions. It was unlikely that we would find a study whereby psychological or educational interventions were assessed in isolation from conventional therapy; this was evident throughout the review.
Included studies
We included 10 RCTs in the review, with a total of 2003 participants in studies employing educational interventions, and 44 participants in the single psychological intervention study. We give details in the 'Characteristics of included studies' tables.
Design
All 10 studies employed a parallel group design.
Sample sizes
The number of participants randomised were as follows: Sokel 1993 (n = 44); Niebel 2000 (n = 47); Chinn 2002 (n = 240); Staab 2002 (n = 204); Staab 2006 (n = 992); Grillo 2006 (n = 61); Shaw 2008 (n = 151); Moore 2009 (n = 112); Weber 2008 (n = 36); and Schuttelaar 2010 (n = 160). Total number of participants = 2047.
Setting
Only one study was primary‐care‐based (Chinn 2002), and five were hospital‐based: Niebel 2000; Schuttelaar 2010; Shaw 2008; Staab 2002; Staab 2006. Two implied they were hospital‐based (Grillo 2006; Moore 2009), and the settings for the studies by Sokel 1993 and Weber 2008 remain unclear.
Three studies were conducted in Germany (Niebel 2000; Staab 2002; Staab 2006), two in the UK (Chinn 2002; Sokel 1993), two in Australia (Grillo 2006; Moore 2009), one in Brazil (Weber 2008), one in the USA (Shaw 2008), and one in the Netherlands (Schuttelaar 2010).
Participants
In all the educational studies, the participants were the child‐parent dyad; by this, we refer to the unit of both the parent and the child. In the Sokel 1993 study, the participant was the child only. The age of the children ranged from infants (age not specified) to 16 years old.
Interventions
Of the 10 RCTs included, 9 focused on educating parents to self‐manage their child's atopic eczema (Chinn 2002; Grillo 2006; Moore 2009; Niebel 2000; Schuttelaar 2010; Shaw 2008; Staab 2002; Staab 2006; Weber 2008), with one including a child component (Weber 2008). The other examined psychological or complementary intervention techniques (hypnotherapy and biofeedback) to improve the quality of life of children with atopic eczema (Sokel 1993). The nine RCTs focusing on parental education used a variety of intervention formats. Parents of children with atopic eczema were given multiple training sessions in five of the studies (Niebel 2000; Schuttelaar 2010; Staab 2002; Staab 2006; Weber 2008), but only one training session in the other four (Chinn 2002; Grillo 2006; Moore 2009; Shaw 2008). In relation to the health professionals administering the parental education programmes, four studies were nurse‐led (Chinn 2002; Moore 2009; Niebel 2000; Schuttelaar 2010), two were multi‐disciplinary (Staab 2002; Staab 2006), one was medically led (Weber 2008), one was led by a senior medical student (Shaw 2008), and the leadership of the Grillo 2006 study remains unclear. Niebel 2000; Moore 2009; Staab 2002; Staab 2006; and Weber 2008 delivered group interventions. Schuttelaar 2010 delivered a combination of individual and group input. Chinn 2002 and Shaw 2008 used one‐to‐one interventions. The delivery of the educational interventions varied in relation to their timing and duration of the various elements of delivery.
Outcomes
The main outcome data from the included studies used across more than one study was that of severity, for which different measures were used. SCORAD was used in the studies by Grillo 2006; Moore 2009; Niebel 2000; Schuttelaar 2010; Shaw 2008; Staab 2002; and Staab 2006. Despite this, the difference in intervention delivery (whether nurse‐led or multi‐disciplinary‐led) and the form in which the data were available for each study meant the scope for synthesis was limited. It was thought that little additional information would be gained by drawing together the data from the Staab 2002 and Staab 2006 studies. Two within‐study comparisons were theoretically possible for two of the included studies having two or more intervention groups. One compared different methods of relaxation‐biofeedback and hypnotherapy (Sokel 1993), and the other compared different types of educational delivery: direct and video‐mediated (Niebel 2000). The Sokel 1993 study used a newly developed, but unvalidated, severity measure in the comparison of the different intervention groups for three parameters of disease severity; this preceded the availability of SCORAD.
Several studies employed quality of life measures, predominately the Children's Dermatology Life Quality Index (CDLQI) +/‐ the Infant Dermatitis Quality of Life Index (IDQOL) (Chinn 2002; Grillo 2006; Schuttelaar 2010; Shaw 2008; Weber 2008), and Staab 2002 and Staab 2006 used a generic quality of life instrument. Again, differences in intervention delivery and the form in which the data were available for each study meant the scope for synthesis was limited.
None of the included studies addressed the following of our prespecified outcomes: participant global assessment; improvement of sleep as a separate measure, although there is an item within the SCORAD severity measure embracing sleep impact; reduction of medication usage; and enhancement of caregiver ability to manage atopic eczema in the child.
The included studies did not report adverse effects.
Excluded studies
In total, we excluded five studies from the review after the process of excluding by title and abstract. We give details in the 'Characteristics of excluded studies' tables.
Of the six excluded studies, three involved adults: Bae 2012 indicated in the abstract both child and adult involvement; however, the mean age was 23.5; the youngest participant was 12; and all data were presented collectively. Unpublished data obtained for the Greene 1997 study established that participants were adults. van Os‐Mendendorp 2012 included both children and adults in an RCT, and when contacted, the author confirmed that it was not possible to disaggregate the data.
Two RCTs designed to educate the parents of children with atopic eczema (Broberg 1990; Kardorff 2003) had originally been deemed suitable for inclusion. In one paper (Broberg 1990), missing data were derived from data figures and graphs, but after subsequent enquiry with the author, we excluded the paper because of inadequate randomisation. Translation of the Kardorff 2003 paper and further correspondence with the author also provided evidence to exclude this RCT since adequate randomisation of the participants had not occurred (Altman 1999). In each case, alternation was used; the participants were alternately allocated to the two study groups in order of their attendance at clinic: one into the control group, then experimental, then control, and so on. This is despite Broberg stating in the abstract that the participants were 'randomly assigned' and were 'divided into two random groups'; subsequent evidence demonstrated that this claim was inaccurate.
Studies awaiting classification
One study, Futamura 2013, is awaiting classification. For details, please see the 'Characteristics of studies awaiting classification' table.
Ongoing trials
Our searches of the trials registers retrieved 339 results, from which we identified four relevant trials.
ISRCTN98560867 (Supporting parents' and carers' management of childhood eczema).
N0060047013 (The project involved behavioural therapy (habit‐reversal) versus conventional medical management with children living with severe atopic eczema. The research question focused on whether a habit‐reversal programme might alter the natural history of atopic eczema and whether this is measurable in blood and skin samples. Correspondence with the trial authors revealed that the study had been suspended due to the loss of the principal investigator).
NCT01138761 (Health literacy for children with atopic dermatitis and their caregivers (active, not recruiting 2011)).
NCT01143012 (Group Eczema Education Visits: Impact on Patient and Family Quality of Life).
For details of these ongoing studies, please see the 'Characteristics of ongoing studies' tables.
Risk of bias in included studies
Please see Figure 1 for our judgements about each 'Risk of bias' item presented as percentages across all included studies, and please see Figure 2 for our judgements about each 'Risk of bias' item for each included study.
1.
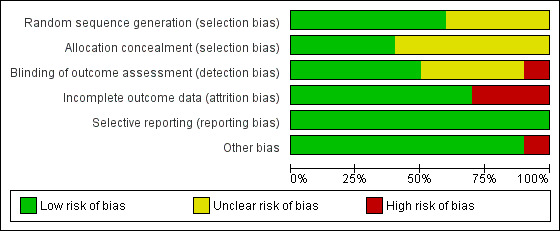
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
2.
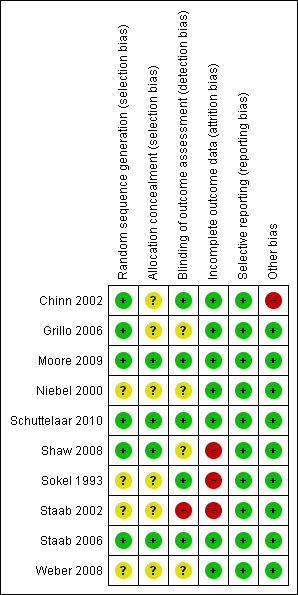
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Randomisation
According to the published papers, all 10 of the included studies randomly allocated the participants to either control or experimental groups. Six studies used computer software to generate random sequences (Chinn 2002; Grillo 2006; Moore 2009; Schuttelaar 2010; Shaw 2008; Staab 2006), so we judged these as at low risk of bias. The other four studies (Niebel 2000; Sokel 1993; Staab 2002; Weber 2008) claimed to have randomised the participants but did not state the precise method by which this was achieved.
Allocation concealment
We considered the concealment of participant allocation to groups as low risk in 4 of the 10 included studies (Moore 2009; Schuttelaar 2010; Shaw 2008; Staab 2006). We could not adequately assess the remaining six (Chinn 2002; Grillo 2006; Niebel 2000; Sokel 1993; Staab 2002; Weber 2008) because of a lack of information in the published reports. Correspondence with Dr Chinn indicated that a list of subject numbers were generated at the start of the study; participants were allocated according to this list in the order each participant returned their baseline questionnaire. This was conducted independently of their practice or their nurse; the nurse was then informed to which group each participant had been assigned.
Blinding
In all 10 included studies, it was impossible to blind the intervention, so participants were not blinded to their group allocation. Four studies blinded the outcome assessor (Chinn 2002; Schuttelaar 2010; Sokel 1993; Staab 2006), and 1 checked on 10 participants using an independent blinded assessor (Moore 2009); we judged these 5 studies as at low risk of bias. Four were unclear (Grillo 2006; Niebel 2000; Shaw 2008; Weber 2008), and one (Staab 2002) used parent‐documented outcome measures; we judged the latter as at high risk of bias for this domain.
Incomplete outcome data
Loss to follow up
Seven studies (Chinn 2002; Grillo 2006; Moore 2009; Niebel 2000; Schuttelaar 2010; Staab 2006; Weber 2008) presented data for > 80% of participants, and we judged them to be low risk for attrition bias. Staab 2002 was unclear in the description of loss to follow up; limited information suggests that follow‐up was 77% in 1 group and 66% in the other, so we assessed this as at high risk of bias. Follow‐up was more clearly presented in a different but later study (Staab 2006); no ITT analysis was undertaken, and twice as many participants were lost to follow up in the control arm than the intervention group. Shaw 2008 and Sokel 1993 had substantial missing data, and no ITT analysis was performed, so we assessed these as at high risk of bias.
Selective reporting
All 10 studies reported findings on all outcomes listed in the Methods section. Therefore, we judged selective reporting bias to be low for all 10 studies.
Other potential sources of bias
Topic‐specific considerations
All 10 included studies stated that their groups were comparable at baseline assessment. However, in the Chinn 2002 study, the distribution of baseline IDQOL and Family Dermatitis Index (FDI) scores differed significantly between those who returned all questionnaires and dropouts. The latter had worse quality of life (QoL) and FDI scores at baseline, so we judged this study as at high risk of bias for this domain.
Effects of interventions
Data synthesis and meta‐analysis were not possible for three reasons:
methodological weaknesses in the selected studies;
heterogeneity of the outcome measures; and
the heterogenous nature of the interventions.
Although data were available of a similar generic type (e.g. severity, quality of life data), there were insufficient comparative data on the specific measures used (e.g. severity data from the use of SCORAD). Consequently, we did not undertake the planned assessments of heterogeneity and the subgroup and sensitivity analyses. We presented a forest plot without a meta‐analysis of objective SCORAD for studies with available data and a narrative analysis of remaining studies for disease severity and for other outcome measures.
Primary outcome measures
(i) Participant‐rated global assessments
None of our included studies assessed participant‐rated global assessment or the medical practitioner global rating.
(ii) Reduction in disease severity
Follow‐up data on objective SCORAD were available from Grillo 2006; Moore 2009; Schuttelaar 2010; Shaw 2008; and Staab 2006, either directly from the paper or by contact with the authors, and are presented in Analysis 1.1; Figure 3. In the interpretation of the forest plot, it should be borne in mind that follow‐up time for the presented results varied from 1 month (Moore 2009) up to 12 months (Schuttelaar 2010; Staab 2006). The nature of the interventions also varied, as described below.
1.1. Analysis.
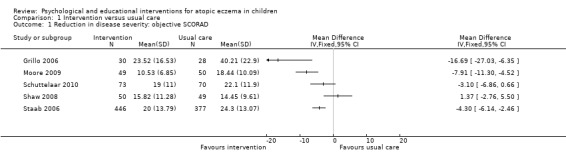
Comparison 1 Intervention versus usual care, Outcome 1 Reduction in disease severity: objective SCORAD.
3.
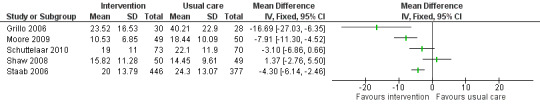
Forest plot of comparison: 1 Intervention versus usual care; outcome: 1.1 Reduction in disease severity: objective SCORAD
Grillo 2006 evaluated an intensive parental and child education programme, taking the form of a two‐hour workshop. As illustrated in Analysis 1.1, scores at follow‐up were significantly better in the intervention group, with a mean difference of ‐16.7. Additionally, the analyses presented in the paper that take into account baseline data demonstrate statistically significant improvements (P < 0.005) in the intervention group relative to the control group at both 1 month and 3 months. However, as a cautionary caveat, the lower 95% confidence limit for the group difference is 6.35, which is less than the minimum clinically important difference of 8.2 for objective SCORAD.
Moore 2009 evaluated the effect of a nurse‐led eczema workshop as their intervention in comparison to usual care at a dermatologist‐led clinic. Severity of atopic eczema, measured by the SCORAD, was the primary study outcome. Analysis 1.1 illustrates that the difference in objective SCORAD at follow‐up was statistically significant and in favour of the intervention. Once again, the 95% confidence interval does not exclude the minimum clinically important difference of 8.2.
Schuttelaar 2010 evaluated nurse practitioner care as their intervention in comparison to usual care with a dermatologist as the control. It was hypothesised that nurse practitioner care would be beneficial because the consultation time was greater and the care was more structured. Analysis 1.1 illustrates that the mean group difference of ‐3.1 on the objective SCORAD at 12‐month follow up was not statistically significant.
Shaw 2008 evaluated an intervention comprising a parental and child education programme involving an individual session at the initial study visit and further availability for advice throughout the study from a specialist atopic dermatitis educator. Analysis 1.1 shows that the mean group difference of 1.37 on objective SCORAD at follow‐up was not statistically significant.
Staab 2006 also evaluated an intervention comprising an educational programme, involving group training at six sessions once a week for two hours from a multiprofessional team. For younger children (3 months to 7 years), the intervention was directed at parents; for the intermediate age range (8 to 12 years), the intervention was directed at both parents and children; and for older children (13 to 18 years), the intervention was directed at the children themselves. Analysis 1.1 illustrates the effect of the intervention combined over all the age groups on objective SCORAD at 12‐month follow up. It can be seen that the mean difference of ‐4.30 is statistically significant in favour of the intervention. However, both the confidence interval limits of 2.46 and 6.14 are less than the minimum clinically important difference of 8.2 for objective SCORAD.
As the study was a large and robust study, we have also reported the effects for objective SCORAD broken down by age that were presented in the paper. We obtained these by comparing the 12‐month follow‐up data controlling for baseline measurements by an analysis of covariance (ANCOVA). Again, we found statistically significant group differences: There was greater improvement in the intervention group for all age groups, but none of the confidence intervals excludes the minimum clinically important difference.
Objective severity by age group (Staab 2006):
| Age group | Group difference in improvement over 12 months (intervention minus control) (95% CI) | P value |
| 3 to 7 months | 4.2 (1.7 to 6.8) | 0.0009 |
| 8 to 12 years | 6.7 (2.1 to 11.2) | 0.005 |
| 13 to 18 years | 9.9 (4.3 to 15.5) | < 0.0001 |
In the study by Sokel 1993, the dermatologist assessed severity with 'a scoring sheet showing the front and back of the body divided into 20 zones of approximately equal area'. A score of 0 to 3 was given for each zone in respect of erythema (redness), surface damage, and lichenification (thickening); the total maximum score being 60.
There are two sets of results:
percentage body coverage (area); and
mean severity score.
We summarised the latter in Table 1.
1. Mean severity scores: children completing 3 assessment sessions (Sokel 1993).
| Severity dimension |
Combined intervention groups ‐ control (Biofeedback and hypnotherapy) ‐ (discussion) (95% CI) |
P value |
| (A) Erythema | ||
| 8 weeks | ‐2.2 (‐9.58, 5.18) | 0.551 |
| 20 weeks | ‐8.2 (‐17.2, 0.78) | 0.072 |
| (B) Surface damage | ||
| 8 weeks | ‐1.2 (‐8.32, 5.92) | 0.735 |
| 20 weeks | ‐8.3 (‐16.2, ‐0.41) | 0.040 |
| (C) Lichenification | ||
| 8 weeks | ‐1.0 (‐7.60, 5.60) | 0.761 |
| 20 weeks | ‐8.8 (‐16.0, ‐1.55) | 0.019 |
CI = confidence interval
For body coverage, the paper states a key result as 'no significant difference in the percentage body area covered for either erythema, lichenification or surface damage'. Children in the combined hypnotherapy and biofeedback groups showed a statistically significant difference in the severity of surface damage and lichenification compared to the control group at visit 3 (20 weeks) (P = 0.04 and P = 0.02, respectively). We found no significant differences in erythema. Recalculated P values are marginally different to those reported in the paper; however, this is unlikely to be clinically significant (Table 1).
Niebel 2000 evaluated the effects of two interventions: direct parent education and video education of parents compared to dermatological standard treatment as the control. They used the Hanifin 1980, Rajka 1989, and SCORAD (summary scores only) methods to evaluate severity, measured pre‐intervention and at follow‐up after four months, and we summarised their results in Table 2. When controlling for pre‐intervention measures by analysis of covariance, there were statistically significant improvements in the direct parent education and video education groups relative to the control group on all severity criteria except pruritus measured by the Rajka 1989 method.
2. Childrens' skin condition (severity) after dermatology consultation (Niebel 2000).
| Severity score | DE (pre) | DE (post) | VE (pre) | VE (post) | Control (pre) | Control (post) | ANOVA/ANCOVA | P value |
| Rajka & Langeland criteria | ||||||||
| 1. General severity | 3.9 (SD 1.19) | 3.2 (SD 1.47) | 4.2 (SD 0.94) | 3 (SD 1.25) | 4 (SD 1.1) | 3.71 (SD 2.43) | Z:F (1/36) = 5.76 | P < 0.022 |
| 2. Surface area | 1.9 (SD 0.88) | 1.7 (SD 0.82) | 2 (SD 0.53) | 1.47 (SD 0.64) | 1.71 (SD 0.61) | 1.36 (SD 0.63) | Z:F (1/36) = 10.87 | P < 0.002 |
| 3. Pruritus | 2 (SD 0.67) | 1.5 (SD 0.71) | 2.2 (SD 0.56) | 1.53 (SD 0.64) | 2.29 (SD 0.61) | 2.36 (SD 2.34) | Z:F (1/36) = 2.09 | P < 0.15 |
| Hanifin criteria | ||||||||
| 1. Erythema | 1 (SD 0.65) | 0.58 (SD 0.61) | 2.4 (SD 0.66) | 1.53 (SD 1.06) | 1.71 (SD 0.8) | 1.36 (SD 1.15) | Z:F (1/39) = 11.34 | P < 0.002 |
| 2. Excoriation | 1.61 (SD 0.98) | 0.65 (SD 0.85) | 2.13 (SD 0.95) | 1.3 (SD 1.06) | 1.86 (SD 0.95) | 1.07 (SD 1.21) | Z:F (1/39) = 15.6 | P < 0.0001 |
| 3. Lichenification | 1.54 (SD 0.96) | 0.75 (SD 0.84) | 2.27 (SD 0.96) | 2 (SD 1.25) | 2.14 (SD 0.86) | 1.86 (SD 1.03) | Z:F (1/38) = 7.12 | P < 0.01 |
| 4. Flaking | 1.73 (SD 0.83) | 1.04 (SD 0.9) | 1.57 (SD 0.75) | 1.3 (SD 0.98) | 2.07 (SD 0.92) | 1.77 (SD 0.96) | Z:F (1/39) = 5.73 | P < 0.022 |
| 5. Induration | 0.83 (SD 0.94) | 0.42 (SD 0.7) | 1.7 (SD 0.78) | 0.93 (SD 1.16) | 1.11 (SD 1.08) | 0.68 (SD 0.72) | Z:F (1/38) = 14.48 | P < 0.0001 |
| 6. Inflammation | 1.13 (SD 0.86) | 0.5 (SD 0.56) | 1.53 (SD 1.13) | 0.67 (SD 1.13) | 0.93 (SD 0.99) | 0.29 (SD 0.61) | Z:F (1/38) = 13.48 | P < 0.001 |
| SCORAD (summary) | N/A | N/A | 55.91 (18.45) | 36.91 (25.95) | 48.66 (SD 15.43) | 32.33 (SD 17.75) | Z:F (1/27) = 22.42 | P < 0.0001 |
DE = Direkte ElternSchulung (direct parent education) VE = Video ElternSchulung (video education of parents) Control = dermatological standard treatment pre = prior to the intervention post = at follow‐up after 4 months SCORAD = Scoring Index of Atopic Dermatitis Analysis of variance (ANOVA) Analysis of covariance (ANCOVA)
For the Staab 2002 study, the difference between the severity score (SCORAD) for each study group was not significant (P = 0.43); limited statistical details are given, with only the mean decrease in score per group being specified other than the P value. Because of the weaknesses in results reporting, we have not tabulated the results.
The Weber 2008 study measured body surface area with eczema at baseline but not at follow‐up. Pruritus intensity and its effect on the child's mood and feeding was measured using a McGill pain questionnaire adapted by Yosipovitch 2002. At follow‐up during the 24‐month period (precise time of follow‐up not reported), references to itch by participants from the intervention group reduced from daily to weekly (P = 0.023). The group differences for the effects of pruritus on mood and feeding were respectively statistically significant (P = 0.03) and of borderline significance (P = 0.052).
iii) Improvements in sleep
The included studies did not assess or record improvement in sleep. However, the impact on sleep is a component of some severity measures, such as SCORAD.
(iv) Quality of life of child and parent
In the assessment of a single nurse consultation on quality of life (Chinn 2002), the parent participants completed the CDLQI, the IDQOL, and the FDI. We summarised the results in Table 3. No significant differences between control and intervention groups were found between baseline and follow‐up at 4 and 12 weeks on the CDQOL and IDQOL measures (P > 0.05). However, the group difference for the change in FDI score at 4 weeks was of borderline significance (P = 0.06) in favour of the intervention group (Chinn 2002).
3. Change in quality of life scores across comparison groups (Chinn 2002).
| QoL measure |
Group difference (Intervention ‐ control) (95% CI) |
P value |
| (A) CDLQI | ||
| Baseline ‐ 4 weeks | ‐1.3 (‐3.2 to 0.6) | 0.17 |
| Baseline ‐ 12 weeks | 0.24 (‐1.5 to 2.0) | 0.7 |
| (B) IDQOL | ||
| Baseline ‐ 4 weeks | ‐0.05 (‐1.3 to 1.2) | 0.9 |
| Baseline ‐ 12 weeks | 1.2 (‐0.8 to 3.1) | 0.24 |
| (C) FDI | ||
| Baseline ‐ 4 weeks | ‐0.79 (‐1.62 to 0.04) | 0.06 |
| Baseline ‐ 12 weeks | 0.34 (‐0.8 to 1.5) | 0.5 |
QoL = quality of life CDLQI = Children's Dermatology Life Quality Index IDQOL = Infant Dermatitis Quality of life Index FDI = Family Dermatitis Index CI = confidence interval
Staab 2002 used the generic 'Daily life' measure to measure quality of life experienced by the mothers of children with eczema; it was stated that there was 'significant improvement in the psychic and somatic well‐being, daily life, joy of life and satisfaction with medical treatment sub‐scales', although no data are given, nor are the 'P' values reported. The validated disease‐specific quality of life questionnaire showed an improvement in the intervention group regarding a subsection of the questionnaire relating to confidence in the medical treatment group compared to the control group (P = 0.016).
The multicentred study by Staab 2006 also used a validated parental quality of life (of children aged less then 13 years) as a key outcome measure. They used a 26‐item German tool 'Quality of life of parents of children with atopic dermatitis' (Von Rueden 1999), which has five subscales:
psychosomatic well‐being;
effects on social life;
confidence in medical treatment;
emotional coping; and
disease acceptance.
Summary results for the primary outcomes reflect the analysis of covariance (ANCOVA) of parental quality of life at baseline and 12 months, comparing intervention minus no intervention, with adjustment for baseline scores. We gave details of the intervention minus no intervention estimates and 95% confidence intervals in Table 4. Parents of children with eczema aged under seven years had significantly better improvements in the intervention group on all five quality of life subscales. Parents of children aged 8 to 12 years experienced significantly better improvements in the intervention group on 3 of the 5 subscales; the changes in psychosomatic well‐being and effects on social life were not statistically significant.
4. Parental QoL by age group using ANCOVA (Staab 2006).
| Outcome by age group |
Group difference (Intervention ‐ control) (95% CI) |
P value |
| *3 months to 7 years* | ||
| Psychosomatic well‐being | ‐1.4 (‐2.5 to ‐0.2) | 0.0040 |
| Effects on social life | ‐0.8 (‐1.4 to ‐0.2) | < 0.0001 |
| Confidence on medical treatment | ‐2.1 (‐2.8 to ‐1.4) | < 0.0001 |
| Emotional coping | ‐1.9 (‐2.5 to ‐1.3) | < 0.0001 |
| Acceptance of disease | ‐0.6 (‐0.9 to ‐0.2) | < 0.0001 |
| *8 to 12 years* | ||
| Psychosomatic well‐being | ‐0.6 (‐2.4 to 1.2) | 0.360 |
| Effects on social life | ‐0.2 (‐1.2 to 0.8) | 0.940 |
| Confidence on medical treatment | ‐2.9 (‐4.1 to ‐1.7) | < 0.0001 |
| Emotional coping | ‐1.8 (‐2.8 to ‐0.9) | 0.002 |
| Acceptance of disease | ‐0.6 (‐1.2 to 0) | 0.031 |
CI = confidence interval
Grillo 2006 used three extensively validated measures of quality of life: IDQOL for children aged < 4 years, CDLQI for children aged 4 to 16 years, and the Dermatology Family Impact (DFI) questionnaire. We summarised the results in Table 5. There was no statistically significant difference between groups at either week 4 or week 12 for the IDQOL and the DFI. For the CDLQI, the group difference at week 4 was not statistically significant, but at week 12 it was statistically significant (P < 0.0001).
5. Difference in quality of life scores at weeks 4 and 12 (Grillo 2006).
| QoL measure |
Group difference (Intervention ‐ control) (95% CI) |
P value |
| (A) CLDQI | ||
| Week 4 | ‐1.79 (‐4.00, 0.42) | 0.110 |
| Week 12 | ‐5.33 (‐7.04 to ‐3.62) | < 0.0001 |
| (B) IDQOL | ||
| Week 4 | 2.10 (‐0.87 to 5.07) | 0.162 |
| Week 12 | 1.58 (‐0.612 to 3.77) | 0.154 |
| (C) DFI | ||
| Week 4 | 0.27 (‐3.38 to 3.92) | 0.883 |
| Week 12 | ‐0.42 (‐3.48 to 2.64) | 0.785 |
QoL = quality of life CDLQI = Children's Dermatology Life Quality Index IDQOL = Infant Dermatitis Quality of life Index DFI = Dermatitis Family Impact questionnaire CI = confidence interval
Weber 2008 used the CDLQI and DFI to evaluate the impact of attending a series of educational support groups. There was no significant difference in CDLQI between groups at baseline (P = 0.86). The intervention group showed a significant improvement relative to the control group at follow‐up (P < 0.01). Specifically, there was evidence of improvement in the quality of life, i.e. leisure (P = 0.04) and personal relationship (P = 0.02) domains in the intervention group relative to the control group. There were no group differences in the DFI scores following the intervention.
Secondary outcome measures
(i) Reduction of harmful scratching behaviour
No studies used this outcome.
(ii) Improvement in treatment adherence
In the Staab 2002 study after the education programme, inflammation of the skin was treated with significantly more steroids by the intervention group than the control group (P = 0.001), reflecting that adequate quantities were then being used.
Moore 2009 reported greater use of wet dressings in the nurse‐led group (76%) compared with the dermatologist‐led clinic (12%). Post‐intervention, 5/49 (10%) nurse‐led workshop attendees and 11/50 (22%) dermatologist clinic attendees were bathing twice daily. Of nurse‐led eczema workshop participants, 80% were applying emollients at least twice daily compared with 62% from the dermatologist‐led clinic. Both groups used comparable strength of steroid on the face. However, 5/50 (10%) children from the dermatologist‐led clinic were using a potent preparation on their face compared with 1/49 (2%) from the nurse‐led workshop (Moore 2009). More children were treated with antibiotics following initial consultation with the dermatologist (n = 10, 20%) compared with the nurse‐led (n = 3, 6%) clinics.
(iii) Reduction of medication usage
No studies used this outcome.
(iv) Enhancement of caregiver ability to manage atopic eczema in the child
No studies used this outcome.
(v) Cost and inconvenience
Staab 2002 assessed the direct treatment costs covered by public health insurance (medical consultations and prescriptions) by comparing six months prior to the study and one year after. Cost reduction was significantly greater in the intervention group than the control group (P = 0.043). There were no reports of inconvenience.
Discussion
Summary of main results
The data for this review were limited, comprising 10 studies. Nine studies focused on parental education interventions (Chinn 2002; Grillo 2006; Moore 2009; Niebel 2000; Schuttelaar 2010; Shaw 2008; Staab 2002; Staab 2006; Weber 2008), of which nurses delivered four interventions (Chinn 2002; Moore 2009; Schuttelaar 2010; Staab 2002), and three were multi‐disciplinary delivery (Staab 2002; Staab 2006; Weber 2008). The Shaw 2008 study used a senior medical student for intervention delivery, and it is unclear who delivered the intervention in the Grillo 2006 study. Only one study of psychological interventions met the inclusion criteria; this had two relaxation intervention groups: biofeedback and hypnotherapy (Sokel 1993). All interventions were provided as an adjunct to conventional topical therapy. Only a limited range of the psychological interventions available were employed. The included studies addressed two of our primary outcome measures: reduction in disease severity and quality of life, but they did not address the other two: participant‐rated global assessment and improvement in sleep. The included studies addressed only one of our secondary measures: improvement in treatment adherence. It was surprising not to find the use of sleep improvement as an outcome measure, given the reporting in the literature of sleep disruption as a significant consequence of childhood atopic eczema (Emerson 2000). We could not synthesise data from these studies because of the following factors: the heterogeneous nature of the outcome measures used, a lack of adequate data (both in quality and accessibility), and methodological weaknesses in study design. The evidence available to date is therefore derived from individual studies.
For parental educational interventions, four studies reported statistically significant improvements in clinical severity in the intervention groups compared to the control (Grillo 2006; Moore 2009; Niebel 2000; Staab 2006). The Schuttelaar 2010 study reported significant improvements in SCORAD, in both control (dermatologist) and intervention (nurse) groups. However, quality of reporting was variable, with Moore 2009 providing limited information, and Niebel 2000 omitting SCORAD data for the parental education group. The difference in SCORAD found between comparison groups was not significant in the Staab 2002 study. One multicentre study found significant impact on SCORAD (Staab 2006). However, we support Williams 2006 observation that it remains unclear whether the degree of the final differences observed between groups could be accounted for by the differential use of appropriate treatments (individual therapy remained the responsibility of the participants' doctors). The quality of reporting of SCORAD scores varied in the included studies.
The Staab 2006 study found statistically significant improvements in parental quality of life in all 5 subscales for their affected child within the '7 years and under' age group and in 3 of these subscales for the '8 to 12 years' age group.
We found no differences in quality of life outcomes at 4 and 12 weeks in the study by Chinn 2002. One multicentre study found significant impact on SCORAD (Staab 2006).
The single psychological study (Sokel 1993) identified significant differences in two of three elements of the multi‐dimensional clinical severity score (surface damage and lichenification) between the intervention groups (biofeedback and hypnotherapy) and the control group (discussion only) (Sokel 1993).
Overall completeness and applicability of evidence
We identified no studies that could not be subsequently located. A small number of studies met the inclusion criteria, employing a limited range of the potential psychological and educational interventions available. These included educational interventions: parental (and child), education (nurse‐ or multi‐disciplinary‐led), nurse‐led individually, or with groups of participants. They also included the use of technology to support education (video or not), relaxation‐based psychological interventions, or complementary interventions (hypnotherapy and biofeedback). Although we identified a number of relevant studies in terms of the type of intervention, design, and disease outcome measures used, because the population was made up of adults, we therefore recorded them as excluded studies, albeit ones of clinical and methodological relevance.
The main methodological weaknesses of our included studies were as follows:
unclear allocation concealment in several studies (Chinn 2002; Grillo 2006; Niebel 2000; Sokel 1993; Staab 2002; Weber 2008) due to lack of information from published papers and correspondence;
blinding of the outcome assessor was unclear in four studies (Grillo 2006; Niebel 2000; Shaw 2008; Weber 2008); in others, blinding was not possible (Staab 2002) as parents completed assessment;
loss to follow up was problematic in the Shaw 2008; Sokel 1993; and Staab 2002 studies, which had less than 80% follow up; and
finally, although all 10 studies said they used random allocation, the method by which this was achieved remains unclear in four studies (Niebel 2000; Sokel 1993; Staab 2002; Weber 2008).
Although the majority of these studies used validated outcome measures, exceptions included Sokel 1993, which used a non‐validated severity measure, and Staab 2002, which used an untitled disease‐specific parental quality of life measure and the Trier Scales of Coping (Staab 2002), which are used widely in German studies. Moore 2009 reported simply asking participants about changes in treatment adherence.
Furthermore, although most of the included studies focused on parental education, there were few parentally‐focused outcomes, other than the use of a parental or a family quality of life measure in five studies (Grillo 2006; Schuttelaar 2010; Staab 2002; Staab 2006; Weber 2008). It may also be speculated that the clinical outcomes used to measure the impact of the parentally directed interventions (that directly related to the child, e.g. clinical severity) may not have been a sufficiently sensitive measure of effectiveness. The issue of studies being underpowered is highlighted in the Chinn 2002 study; the estimation of sample size was unable to detect a significant change in primary care participants. There were reporting problems with some of the individual studies, with key quantitative results not being reported. For example, the Niebel 2000 study did not present SCORAD summary scores for the parental education group.
Educational interventions are by their nature complex and, as such, may interact in a complex way with the organisation of health services, which are influenced by socioeconomic and cultural factors. By way of illustration, variations may exist in the availability of specialist dermatology care and the staff to deliver these. Furthermore, the education and scope of practice of health professionals and the distribution and delivery of services across primary and secondary care vary within and between countries. In addition, educational and psychological interventions represent a highly heterogeneous grouping of interventions due to the wide range of methods employed and ways of utilising and delivering them. The range of psychological interventions that could be potentially employed is high, each with different theoretical underpinnings. This is reflected in the intervention summary earlier in the review. Interestingly, no included studies used 'theoretically based' interventions drawing on, for example, behavioural modification or self‐efficacy theory.
The capacity of an outcome measure to detect a clinically significant change in a person remains unclear for the primary outcome measures used in the included studies. The most renowned severity measure of atopic eczema is SCORAD. This measure has been validated several times on the basis of establishing good inter‐rater judgements and recognising the need for prior training (Kunz 1997; Pucci 2005), but it has yet to be assessed against global measures so that it can be correlated with a participant‐perceived measure of change. A systematic review of named outcome measures for atopic eczema found that SCORAD, POEM (Patient Orientated Eczema Measure), and EASI (Eczema Area and Severity Index) were the only adequately validated scores (Schmitt 2007).
Adult and child studies compared
A search of the literature on educational and psychological interventions for adults with eczema using the databases MEDLINE, CINAHL, and the Cochrane Database of Systematic Reviews from 2000 to 2013 revealed relatively few studies in this area. However, it is useful to briefly compare child studies to relevant adult studies because of methodological insights that may be gained from their discussion and comparison. This may help others when planning future robust studies in children. Nine of the included paediatric studies focused on educational interventions, involving either nurse‐led or multi‐disciplinary interventions, directed either at individual parents or groups and located in out‐patient or primary care practices as described (Chinn 2002; Grillo 2006; Moore 2009; Niebel 2000; Schuttelaar 2010; Shaw 2008; Staab 2002; Staab 2006; Weber 2008).
Four key adult educational studies (Armstrong 2011; Coenraads 2001; Gradwell 2002; Jaspers 2000) involved individual contact (Gradwell 2002), group education (Coenraads 2001; Jaspers 2000), and the use of online video‐based education versus the provision of an educational pamphlet (Armstrong 2011). Two studies highlighted improvements in clinical severity and improved self‐care ability (Coenraads 2001; Jaspers 2000). Armstrong 2011 demonstrated that knowledge of eczema and disease severity were both significantly improved in the video group when compared with the pamphlet group. There was also further evidence of a reduced need for consultations (Jaspers 2000). Standardised outcome measures used included the use of severity measures similar to those used in child studies, such as SCORAD (Coenraads 2001), and quality of life measures including DLQI (Gradwell 2002) and the Short Form (36) Health Survey (SF‐36) (Jaspers 2000). Armstrong 2011 used the Patient Oriented Eczema Measure (POEM).
The adult studies give a clearer indication of effectiveness than those for the child studies. This would appear to be due to the improved design and clarity about the stages of the research process and in reporting of results, not due to the nature of the interventions. The rigorously designed adult studies by Gradwell 2002 (educational intervention) and Ehlers 1995 (psychological intervention), in terms of the use of robust outcome measures and controls, may provide useful pointers toward effective study design for child intervention studies. These studies also highlight the need to give consideration to the scope for combining educational and psychological approaches (based on relaxation and habit reversal) in the management of atopic eczema in children.
Quality of the evidence
The strength and consistency of evidence available is limited because of the lack of robust studies with data and design of a similar nature sufficient to allow data synthesis. The data from individual studies remain inconclusive in terms of the effectiveness of the interventions studied given that there is a combination of some clinically significant results in some outcome measures and no differences in others, together with methodological weaknesses in all studies.
Potential biases in the review process
There were no known biases operating in the review process.
Agreements and disagreements with other studies or reviews
This is an update of the first Cochrane systematic review to focus on evaluating the impact of psychological and educational interventions on children with atopic eczema (Ersser 2007). A Health Technology Assessment generic review of interventions for atopic eczema (Hoare 2000) embraced such strategies.
More recent and wide‐ranging systematic reviews and meta‐analyses of educational and psychological interventions for adults include patient education in chronic skin disease (de Bes 2011) and the effectiveness of psychological interventions for adults with skin conditions (Lavda 2012). de Bes 2011 concluded that patient education for adults appears to be effective in improving quality of life and in reducing perceived severity of skin disease. It is suggested by Lavda 2012 that whilst psychological interventions are beneficial for people with skin disease, there is a need for more robust randomised controlled studies and development of a wider range of interventions developed with a wider range of skin conditions.
Authors' conclusions
Implications for practice.
This review draws on evidence from 10 trials. It is interesting that the studies focused on interventions directed at the parent rather than the child, particularly when 'atopic schools' offering multi‐disciplinary therapeutic patient education, involving both parents and children, are becoming more common (Barbarot 2013). Based on the Chinn 2002; Moore 2009; Niebel 2000; and Schuttelaar 2010 studies, there is limited evidence that parental education delivered by nurses who are caring for children with atopic eczema may improve the clinical severity of the atopic eczema when used as an adjunct to conventional treatment. Details of the precise nature of educational activity within nurse‐led clinics are limited; consideration needs to be given to this issue and its reporting. Evidence from the robust GADIS (German Atopic Dermatitis Intervention Study) multicentre study (Staab 2006) of multi‐disciplinary intervention using an eczema school curriculum indicates that children and their parental carers may benefit from structured education, albeit using a complex intervention. There appear, in consequence, to be two main service delivery models ‐ nurse‐led and multi‐disciplinary ‐ in operation; however, we have no comparative evaluation of their relative effectiveness, either clinically or in terms of cost. Furthermore, reliable conclusions cannot be drawn on the effectiveness of psychological and complementary approaches, namely biofeedback and hypnotherapy, from one satisfactory but small study.
Since the management of atopic eczema requires an adaptation in health and illness behaviour and effective actions by the carer, it is logical to develop and evaluate both psychological and educational strategies as an adjunct to conventional therapy. It is surprising that despite the wide range of psychological interventions available, few have been subject to application, and there has been little robust evaluation. Educational interventions directed towards parents also appear to be worthy of development and robust testing, with attention given to finding both effective and resource‐efficient models. Current case‐based indications of good practice in prominent dermatology departments reveal recognition of the potential of such approaches (e.g. Lawton 2005). Educational interventions require careful consideration of both the content of learning and of the most effective process, including who is best placed to teach affected people, at what frequency and duration, and whether or not educational technology should be employed. Nine of the 10 RCTs focused on parental education and used a variety of intervention formats.
An important issue for consideration is the scope and limits of the application and effectiveness of psychological interventions that have been used with adults could be used with children and their parents. The adult studies provide some additional, useful and relevant information on both interventions and their evaluation, which was not found within the child studies under review. For example, although based on small studies, there are indications that the habit reversal technique used in conjunction with conventional treatment may improve atopic eczema outcomes (Melin 1986; Norén 1989). However, its application to children will depend on the child's developmental stage. Similarly, Ehlers 1995 showed that although a combined approach (patient education and cognitive‐behavioural treatment) led to a significantly larger improvement in atopic dermatitis than intensive patient education or conventional dermatological treatment, such treatment will be limited to some older children of the appropriate developmental stage. In contrast, those educational studies that have sought to improve effective health behaviour through adult education have direct applicability to parental carers of children with atopic eczema. For example, Gradwell 2002 showed that a single 20‐minute appointment with a nurse to demonstrate the use of therapies (as well as the standard consultant appointment and follow‐up) was useful in improving the participants' understanding of the treatments. Therefore, there may be some limited scope to explore psychological interventions as an additional therapy for children of the appropriate developmental stage. There may also be opportunities to apply the interventions used to teach adults with atopic eczema to the parents of children with atopic eczema.
This review suggests that there is scope for both multi‐disciplinary teams and suitably qualified individual clinicians, such as nurses, as well as psychologists, to deliver educational interventions in conjunction with conventional therapy. In some countries, such as the UK, nurse‐led clinics provide an opportunity for focused intervention. In countries such as Germany, the eczema school multi‐disciplinary model is more established. There is scope to debate the relative merits of these different service delivery models that employ suitably qualified professionals to deliver both psychological and educational interventions. There is also scope to study how educational activity can most effectively be integrated with the resource efficient provision of conventional dermatological therapy.
Implications for research.
A relatively small number of studies fulfilled our inclusion criteria, and of these, we assessed only three as at low risk of bias. As such, there are significant opportunities to improve research design to evaluate psychological and educational interventions for children with atopic eczema and the reporting standards of such studies.
It is important that in the development of future trials, those people who will actually use the intervention are involved at an early stage (Medical Research Council 2008). Interventions should have a robust and explicit theoretical base (NICE 2013), and consideration should be given to duration and frequency.
The Harmonising Outcome Measures for Eczema (HOME) initiative concluded that the core outcomes that all eczema‐related RCTs should report on are clinical signs, symptoms, long‐term control of flares, and quality of life (Schmitt 2012). At the HOME III meeting (HOME 2013), it was agreed that EASI should be the instrument for measuring signs of eczema. It is advised that self‐efficacy measures are incorporated particularly as this is likely to be a key mediator in changing the health behaviour of parents caring for children with eczema (Ersser 2013). Quality of life, for both the child and their family, and sleep are also important measures (HOME 2013). Consideration needs to be given to clinically meaningful time frames for applying selected outcome measures to assess sustained change.
Useful information to inform the design of more robust trials may be obtained from this review of existing studies examining the delivery of psychological and educational interventions to the parents of children with atopic eczema and those adult studies discussed above. These include ensuring the following:
the use of (and reporting of) adequate methods of random allocation and allocation concealment;
the use of validated outcome measures (for validity and reliability, for use with the appropriate populations under study); and
the pursuit of loss to follow up is addressed within the study design.
In addition, given the nature of the interventions and outcomes examined in this review, there is scope to consider a wider range of research designs other than RCTs within any subsequent reviews, since these may help us to better understand the behavioural nature and effects of educational and psychological interventions.
In conclusion, there is significant scope to undertake intervention development and then design robust trials to evaluate theoretically based psychological and educational interventions, which may enhance the management of atopic eczema in children.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2017 | Amended | Contact author information (affiliation) updated. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 12 October 2016 | Amended | Author information (affiliation) updated. |
| 10 November 2015 | Amended | Author information (affiliation) updated. |
| 20 December 2013 | New search has been performed | Five new trials have been added, making a total of 10 randomised controlled trials (RCTs) |
| 20 December 2013 | New citation required but conclusions have not changed | There has been no significant alteration to the conclusions of the original review |
| 2 October 2008 | Amended | Converted to new review format. |
| 23 May 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
2013 update
Professor Peter Thomas (Bournemouth University) and Dr Matthew Ridd (Univerity of Bristol) for their contributions to the early part of this review.
The constructive comments and guidance of Dr Finola Delamere and the support of Miss Laura Prescott from the Cochrane Skin Group were much appreciated.
The Cochrane Skin Group editorial base wishes to thank Sue Jessop who was the Cochrane Dermatology Editor for this review; Matthew Grainge and Phillipa Middleton who were the Statistical and Methods Editors, respectively; the clinical referees, Miriam Santer and Sebastien Barbarot; and the consumer referee, Rosemary Humphreys.
2007 review
Ms Katja Schmidt (formerly of University of Exeter), Dr Katja Doerholt and Dr Maja Mockenhaupt (translation assistance, University of Freiberg), Dr Mike Weaver (translation, University of Southampton), Dr Phil Wiffen (RevMan assistance, UK Cochrane Centre), Dr Rafael Perera and Dr Peter Nichols (statistical assistance, Universities of Oxford and Southampton, respectively), and Ms Anne Eisinga (searching strategy audit, UK Cochrane Centre).
Assistance at protocol development phase: Ms Heidi Surridge and Ms Pauline Buchanan.
Assistance with study searching from Mr Philip Satherley, Cardiff University.
The constructive comments and guidance of Dr Tina Leonard, Dr Jo Leonardi‐Bee, Phillipa Middleton, and Dr Finola Delamere from the Cochrane Skin Group were much appreciated.
The authors are grateful for the comments provided by their lead editor, Dr Sam Gibbs.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register search strategy
(eczema or dermatitis or neurodermatitis) and (psychotherap* or “behavio* management” or autogenic or counsel* or hypnosis or hypnotherapy or relaxation or “psychotherapeutic technique*” or “self help” or mindfulness or imagery or biofeedback or “health promotion” or education or “patient teaching” or “patient training” or psychology or psychiatry or ((psychodynamic or cognitive or famil* or behavio*) and therap*))
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor Eczema explode all trees #2 MeSH descriptor Dermatitis, Atopic explode all trees #3 MeSH descriptor Neurodermatitis explode all trees #4 MeSH descriptor Dermatitis explode all trees #5 (eczema or dermatitis or neurodermatitis):ti,ab,kw #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Psychotherapy explode all trees #8 MeSH descriptor Patient Education as Topic explode all trees #9 MeSH descriptor Education explode all trees #10 MeSH descriptor Health Education explode all trees #11 MeSH descriptor Psychology explode all trees #12 MeSH descriptor Behavior Therapy explode all trees #13 MeSH descriptor Cognitive Therapy explode all trees #14 MeSH descriptor Autogenic Training explode all trees #15 MeSH descriptor Suggestion explode all trees #16 MeSH descriptor Hypnosis explode all trees #17 MeSH descriptor Counseling explode all trees #18 MeSH descriptor Relaxation explode all trees #19 MeSH descriptor Relaxation Therapy explode all trees #20 MeSH descriptor Imagery (Psychotherapy) explode all trees #21 MeSH descriptor Biofeedback, Psychology explode all trees #22 MeSH descriptor Family Therapy explode all trees #23 MeSH descriptor Health Promotion explode all trees #24 MeSH descriptor Parent‐Child Relations explode all trees #25 MeSH descriptor Skin Care explode all trees #26 MeSH descriptor Self‐Help Groups explode all trees #27 MeSH descriptor Psychiatry explode all trees #28 (psychodynamic therap*) or (behavio* management) or (behavio* therapy) or (autogenic training) or (counsel*) #29 (psychotherapy) or (suggestion):ti,ab,kw or (hypnosis or hypnotherapy) or (cognitive therap*) or (relaxation) #30 (psychotherapeutic technique*) or (self help) or (support):ti,ab,kw or (mindfulness or imagery or biofeedback ) or (family therap*) #31 (health promotion) or (health education) or (patient education) or (patient teaching) or (patient training) #32 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31) #33 (#6 AND #32)
Appendix 3. MEDLINE (OVID) search strategy
1. exp Eczema/ or eczema.mp. 2. exp Dermatitis, Atopic/ 3. neurodermatitis.mp. or exp Neurodermatitis/ 4. exp Dermatitis/ or dermatitis.mp. 5. or/1‐4 6. exp Psychotherapy/ 7. exp Patient Education Handout/ 8. exp Education/ 9. exp Health Education/ 10. exp Psychology/ 11. exp Behavior Therapy/ 12. exp Cognitive Therapy/ 13. exp Autogenic Training/ 14. exp Suggestion/ 15. exp Hypnosis/ 16. exp Counseling/ 17. exp Relaxation/ 18. exp Relaxation Therapy/ 19. exp "Imagery (Psychotherapy)"/ 20. exp Biofeedback, Psychology/ 21. exp Family Therapy/ 22. exp Health Promotion/ 23. exp Patient Education as Topic/ 24. exp Parent‐Child Relations/ 25. exp Skin Care/ 26. exp Self‐Help Groups/ 27. exp Psychiatry/ 28. psychodynamic therap$.ti,ab. 29. behavio$ management.ti,ab. 30. behavio$ therapy.ti,ab. 31. autogenic training.ti,ab. 32. counsel$.ti,ab. 33. psychotherapy.ti,ab. 34. suggestion.ti,ab. 35. (hypnosis or hypnotherapy).ti,ab. 36. cognitive therap$.ti,ab. 37. relaxation.ti,ab. 38. psychotherapeutic technique$.ti,ab. 39. self help.ti,ab. 40. support.ti,ab. 41. mindfulness.ti,ab. 42. imagery.ti,ab. 43. biofeedback.ti,ab. 44. family therap$.ti,ab. 45. health promotion.ti,ab. 46. health education.ti,ab. 47. patient education.ti,ab. 48. patient teaching.ti,ab. 49. patient training.ti,ab. 50. or/6‐49 51. exp Eczema/px [Psychology] 52. exp Dermatitis, Atopic/px [Psychology] 53. exp Neurodermatitis/px [Psychology] 54. exp Dermatitis/px [Psychology] 55. 51 or 52 or 53 or 54 56. randomized controlled trial.pt. 57. controlled clinical trial.pt. 58. randomized.ab. 59. placebo.ab. 60. clinical trials as topic.sh. 61. randomly.ab. 62. trial.ti. 63. 56 or 57 or 58 or 59 or 60 or 61 or 62 64. (animals not (humans and animals)).sh. 65. 63 not 64 66. 5 and 50 and 65 67. 55 and 65 68. 66 or 67
Appendix 4. Embase (OVID) search strategy
1. psychodynamic therap$.ti,ab. 2. behavio$ management.ti,ab. 3. behavio$ therapy.ti,ab. 4. autogenic training.ti,ab. 5. counsel$.ti,ab. 6. psychotherapy.ti,ab. 7. suggestion.ti,ab. 8. (hypnosis or hypnotherapy).ti,ab. 9. cognitive therap$.ti,ab. 10. relaxation.ti,ab. 11. psychotherapeutic technique$.ti,ab. 12. self help.ti,ab. 13. support.ti,ab. 14. mindfulness.ti,ab. 15. imagery.ti,ab. 16. biofeedback.ti,ab. 17. family therap$.ti,ab. 18. health promotion.ti,ab. 19. health education.ti,ab. 20. patient education.ti,ab. 21. patient teaching.ti,ab. 22. patient training.ti,ab. 23. exp psychotherapy/ 24. exp education/ 25. exp patient education/ 26. exp health education/ 27. exp psychodynamics/ 28. exp behavior therapy/ 29. exp autogenic training/ 30. exp suggestion/ 31. exp hypnosis/ 32. exp cognitive therapy/ 33. exp counseling/ 34. exp relaxation training/ 35. exp imagery/ 36. exp feedback system/ 37. exp family therapy/ 38. exp health promotion/ 39. exp child parent relation/ 40. exp skin care/ 41. exp psychology/ 42. exp self help/ 43. exp psychiatry/ 44. or/1‐43 45. eczema.mp. or exp ECZEMA/ 46. exp DERMATITIS/ or dermatitis.mp. 47. exp atopic dermatitis/ 48. neurodermatitis.mp. or exp NEURODERMATITIS/ 49. or/45‐48 50. random$.mp. 51. factorial$.mp. 52. (crossover$ or cross‐over$).mp. 53. placebo$.mp. or PLACEBO/ 54. (doubl$ adj blind$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 55. (singl$ adj blind$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 56. (assign$ or allocat$).mp. 57. volunteer$.mp. or VOLUNTEER/ 58. Crossover Procedure/ 59. Double Blind Procedure/ 60. Randomized Controlled Trial/ 61. Single Blind Procedure/ 62. 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 63. 44 and 49 and 62
Appendix 5. PsycINFO (OVID) search strategy
1. eczema.ti,ab. or exp Eczema/ 2. dermatitis.ti,ab. or exp Dermatitis/ 3. neurodermatitis.ti,ab. or exp Neurodermatitis/ 4. 1 or 2 or 3 5. double‐blind.tw. 6. random$ assigned.tw. 7. control.tw. 8. 5 or 6 or 7 9. 4 and 8
Appendix 6. CINAHL (OVID) search strategy
S1. (MH "Eczema")
S2. eczema
S3. (MH "Dermatitis, Atopic")
S4. neurodermatitis
S5. (MH "Dermatitis+")
S6. dermatitis
S7. S1 or S2 or S3 or S4 or S5 or S6
S8. (MH "Psychotherapy+")
S9. (MH "Patient Education+")
S10. (MH "Education+")
S11. (MH "Health Education+")
S12. (MH "Psychology+")
S13. (MH "Behavior Therapy+")
S14. (MH "Cognitive Therapy")
S15. (MH "Autogenic Training (Iowa NIC)")
S16. (MH "Hypnosis")
S17. (MH "Counseling+")
S18. (MH "Relaxation")
S19. (MH "Biofeedback")
S20. (MH "Family Therapy")
S21. (MH "Health Promotion+")
S22. (MH "Parent‐Child Relations+")
S23. (MH "Skin Care+")
S24. (MH "Support Groups+")
S25. (MH "Psychiatry+")
S25. TI psychodynamic therap* OR AB psychodynamic therap*
S27. TI behavio* management OR AB behavio* management
S28. TI behavio* therapy OR AB behavio* therapy
S29. TI autogenic training OR AB autogenic training
S30. TI counsel* OR AB counsel*
S31. TI psychotherapy OR AB psychotherapy
S32. TI suggestion OR AB suggestion
S33. TI ( hypnosis or hypnotherapy ) OR AB ( hypnosis or hypnotherapy )
S34. TI cognitive therap* OR AB cognitive therap*
S35. TI relaxation OR AB relaxation
S36. TI psychotherapeutic technique* OR AB psychotherapeutic technique*
S37. TI ( "self‐help" or "self help" ) OR AB ( "self‐help" or "self help" )
S38. TI support OR AB support
S39. TI mindfulness OR AB mindfulness
S40. TI imagery OR AB imagery
S41. TI biofeedback OR AB biofeedback
S42. TI family therap* OR AB family therap*
S43. TI health promotion OR AB health promotion
S44. TI health education OR AB health education
S45. TI patient education OR AB patient education
S46. TI patient teaching OR AB patient teaching
S47. TI patient training OR AB patient training
S48. S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or
S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33
or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or
S46 or S47
S49. (MH "Clinical Trials+")
S50. PT clinical trial
S51. TX clinic* n1 trial*
S52. (MH "Random Assignment")
S53. TX random* alloc*
S54. TX placebo*
S55. (MH "Placebos")
S56. (MH "Quantitative Studies")
S57. TX allocat* random*
S58. "randomi#ed control* trial*"
S59. TX singl* n1 blind* OR TX singl* n1 mask* OR TX doubl* n1 blind* OR TX doubl* n1 mask* OR
TX tripl* n1 blind OR TX tripl* n1 mask* OR TX trebl* n1 blind* OR TX trebl* n1 mask*
S60. S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59
S61. S7 and S48 and S60
Data and analyses
Comparison 1. Intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in disease severity: objective SCORAD | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chinn 2002.
| Methods | Design: parallel group Unit of randomisation: the child Unit of analysis: the child‐parent dyad | |
| Participants | Setting: primary care (general practice) Diagnostic criteria: yes, BAD guidelines Disease severity: children aged 6 months to 5 years, parents rated severity on a 'five point scale' Inclusion criteria of the trial
Participants randomised: 240 in total = 120 (intervention) and 120 (control) Participants who took part: 235 in total = 115 (intervention) and 120 (control) Age: 6 months to 4 years (younger group) = 61 (intervention) and 54 (control); 4 years to 16 years = 58 (intervention) and 62 (control) Sex: not stated Duration of condition: new cases and parents requesting repeat prescriptions (intervention and control) Severity of condition: At baseline, parent completed a 'five‐point scale for severity'. The majority of cases were 'fairly good' (29%) or 'average' (43%). 25% of parents reported their child's eczema as 'severe' or 'extremely severe' Withdrawals Number of: 1 (intervention) and 4 (control) Reason for: 'Moved out of the area' or otherwise withdrawn (intervention and control) Loss to follow up: 14 (intervention) and 24 (control) ITT analysis: not stated |
|
| Interventions |
Intervention Nature: nurse‐led parental education consultation Format: face‐to‐face session with a trained dermatology nurse Theoretical basis: Duration: 30 minutes Frequency: one‐off session No extant theoretical base |
|
| Outcomes | 1. Quality of Life using the Children's Dermatology Life Quality Index (4 to 16 years) or Infant Dermatitis Quality of Life questionnaire (< 4 years) 2. Family Dermatitis Index |
|
| Notes | Group comparability at baseline: yes
Conventional topical treatment
Allocation concealment: "I generated a list of subject numbers (1‐240??) at the beginning of the study and those that volunteered were allocated according to this list in the order each patient returned their baseline questionnaire. I did this independent of the practice or their nurse. The nurse was informed which group each patient had been assigned and she then arranged the nurse interview for those in the intervention group" Funding source: Northern and Yorkshire R&D fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sample Size software generated a computer random numbers list in blocks of 20 |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of how the randomisation list was concealed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not possible to blind participants or healthcare providers, but the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was > 80% follow up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | High risk | Dropouts differed significantly: Yes, the distribution of baseline IDQOL and FDI scores differed significantly between those who returned all questionnaires and dropouts. The latter had worse QoL FDI scores at baseline |
Grillo 2006.
| Methods | Design: parallel group Unit of randomisation: the child Unit of analysis:
Only 3 dropouts, so statistical comparisons not useful |
|
| Participants | Setting: not clear where education took place or the follow‐up measures, although limitations section refers to data collected from 1 hospital site only Diagnostic criteria: 'diagnosed by physician' Disease severity: baseline mean SCORAD, intervention = 50.97 (SD 21.83), control = 47.73 (SD 22.61) Inclusion criteria of the trial
Participants randomised: 61 in total = 32 (intervention) and 29 (control) Participants who took part: 61 in total = 32 (intervention) and 29 (control) Age: 38 infants aged < 5 years, 23 children aged 5 + years (intervention/control numbers not stated) Sex: 35 boys, 26 girls (intervention/control numbers not stated) Duration of condition: not stated Withdrawals Number of: not stated Reason for: not stated Loss to follow up: total of 3 (change of address, not possible to contact them) ITT analysis: not stated |
|
| Interventions |
Intervention Nature: parental education workshop Format: face‐to‐face session Theoretical basis: not stated Duration: 2 hours Frequency: one‐off session |
|
| Outcomes | 1. Severity of eczema: Scoring Atopic Dermatitis (SCORAD) 2. Quality of life: Children's Dermatology Life Quality Index (CDLQI) or Infant Dermatitis Quality of Life Questionnaire (IDQOL) (< 4 years) 3. Family impact: Dermatitis Family Impact Questionnaire (DFI) |
|
| Notes | Funding source: The study was partially funded by a Flinders Medical Centre Volunteer Study Award | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator was used to place participants into either the intervention or control group |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details were provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was unclear for the outcome assessor. Participants were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was > 80% follow up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Moore 2009.
| Methods | Design: parallel group Unit of randomisation: the child Unit of analysis: the child |
|
| Participants | Setting: dermatology clinic (secondary care implied) Diagnostic criteria: SCORAD at new referral visit Disease severity: baseline mean SCORAD, intervention = 38 (SD 11), control = 42 (SD 15) Inclusion criteria of the trial
Participants randomised: 165 in total = 80 (intervention) and 85 (control) Participants who took part: 112 in total = 54 (intervention) and 58 (control) Mean age (months: SD): intervention 34 (33), control 45 (44) ‐ 0 to 24 months (n) intervention 27, control 21 ‐ 25 to 144 months (n) intervention 21, control 27 ‐ 145 to 192 months (n) intervention 1, control 2 Sex: intervention men = 30, control men = 24 Duration of condition: mean age of onset (months: SD): intervention = 5 (5) and control = 9 (16) Withdrawals Loss to follow up: 5 (intervention) and 8 (control) Final number of participants evaluable: intervention = 49, control = 50 ITT analysis: not stated |
|
| Interventions |
Intervention Nature: nurse‐led parental education workshop Format: face‐to‐face session Theoretical base: not stated Duration: 90 minutes contact time Frequency: one‐off session |
|
| Outcomes | 1. Severity of eczema: Scoring Atopic Dermatitis (SCORAD) 2. Comparison of treatments used 'at review' |
|
| Notes | Funding source: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Clinics were randomised in blocks of 10 using statistical software |
| Allocation concealment (selection bias) | Low risk | The clinical epidemiology and biostatistics unit at the participating hospital prepared sequentially numbered sealed opaque envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Complete blinding was not possible, but 10 blinded assessments showed good reliability with an independent assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was > 80% follow up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Niebel 2000.
| Methods | Design: parallel group Blinding: not explained Unit of randomisation: the parent Unit of analysis: the child‐parent dyad | |
| Participants | Setting: dermatology clinic (secondary)
Diagnostic criteria: yes (Hanifin 1980)
Disease severity: medium to severe level of AE
Inclusion criteria of the trial
Participants randomised: 47 in total = 14 (control), 18 (intervention 1), and 15 (intervention 2) Age ranges not stated in paper Mean age: children = 3 yrs (control), 4.7 yrs (intervention 1), and 4 yrs (intervention 2) Sex: 8 M, 6 F (control); 12 M, 6 F (intervention 1); and 8 M, 7 F (intervention 2) Mean duration of condition: 1.58 yrs (control), 1.6 yrs (intervention 1), and 1.25 yrs (intervention 2) Severity of condition: SCORAD baseline = 4 (control), 3.9 (intervention 1), and 4.2 (intervention 2) Withdrawals N/A Loss to follow up: no dropouts from study Dropouts differed significantly: N/A |
|
| Interventions |
Intervention 1
Nature: parental educational training program delivered in groups (details given of the topic content)
Format: nurse‐led sessions on theoretical and practical information
Theoretical basis:
Frequency: 10 X 2‐hr sessions
Duration: maximum of 16 weeks Intervention 2 Nature: parental educational training program Format: video film (100 minutes) and booklet with information on theoretical and practical information Theoretical basis: theory element and practical element, designed to promote more therapeutically effective self‐help Frequency duration: maximum 16 weeks Control group: conventional dermatology consultation with no other intervention |
|
| Outcomes | 1. Disease severity (SCORAD‐summary scores given only). Timing: pre‐ and post‐assessment 2. Psychological problems with mothers |
|
| Notes | Group comparability at baseline: The parents' (mothers') age and sociodemographic features were comparable (except for level of school education). Children, comparable age and severity distribution across groups
Conventional topical treatment: For both groups, when an exacerbation occurred, topical steroids were used for approximately 1 week. Wet lesions were treated with antiseptic compressions Funding source: Ministerium für Arbeit, Soziales, Jugend und Gesundheit des Landes Schleswig‐Holstein |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stated in the text, but the method was not explained |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not explained |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The process was not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was > 80% follow up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Schuttelaar 2010.
| Methods | Design: parallel group Unit of randomisation: the parent Unit of analysis: the child‐parent dyad |
|
| Participants | Setting: dermatology clinic (secondary)
Diagnostic criteria: yes
Disease severity: mean SCORAD at baseline, control = 31.6, intervention = 34.3
Inclusion criteria of the trial
Participants randomised: 160 in total = 81 (nurse practitioner (NP) group), 79 (dermatologist (D)) group Age ranges: 0 to 16 years Mean age: ≤ 4, NP = 1.5 years, D = 1.6 years; 4 to 16, NP = 9.1 years, D = 9.3 years Sex: ≤ 4, NP = 30 male, 10 female, D = 29 male, 11 female; 4 to 16, NP = 20 male, 21 female, D = 19 male, 20 female Mean duration of condition: not stated |
|
| Interventions |
Intervention 1
Nature: parental educational, individual +/‐ group
Format: nurse‐led sessions with theoretical and practical input including treatment of eczema, practical demonstrations, education, and support
Theoretical basis: social cognitive theory
Frequency: 1 individual session with follow‐up in person or by telephone +/‐ group session
Duration: individual session 1. 30 minutes, 2. 20 minutes in person or 10‐minute telephone consultation. Group session 2 hours with a maximum of 8 parents Control group: conventional consultation with dermatologist, no other intervention |
|
| Outcomes | 1. Child quality of life (IDQOL, CDLQI) at 4, 8, and 12 months 2. Family quality of life (DFI) at 4, 8, and 12 months 3. Severity of eczema SCORAD at 4, 8, and 12 months 4. Participant satisfaction (CSQ‐8) at 4, 8, and 12 months |
|
| Notes | Funding source: This study was supported by the Healthcare Efficiency Research Programme of the University Medical Center Groningen, Groningen, the Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation scheme was used |
| Allocation concealment (selection bias) | Low risk | Participants opened consecutive closed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was not possible to blind participants or healthcare providers, but the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 95% of participants were followed up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Shaw 2008.
| Methods | Design: parallel group Unit of randomisation: the parent Unit of analysis: the child‐parent dyad |
|
| Participants | Setting: dermatology clinic (secondary care)
Diagnostic criteria: referral to hospital clinic, no criteria used Disease severity: not stated Inclusion criteria of the trial
Participants randomised: 151 in total = 74 (control) and 77 (intervention) Age ranges: newborn to 18 years Mean age: children = 4.62 (control) and intervention (6.34) Sex: control men = 25, control women = 27, intervention men = 21, intervention women = 29 Mean duration of condition: not stated Severity of condition: SCORAD baseline, control mean = 32.02, intervention = 33.54 Withdrawals N/A |
|
| Interventions | Intervention Nature: parental education, 15‐minute individual session following outpatient appointment, given verbal and written information training programme delivered in groups (outline given of the topic content) Format: senior medical student‐led session giving theoretical and practical information Theoretical basis: not stated Frequency: 1 x 15‐minute session Duration: once only, but telephone and email support available post‐session | |
| Outcomes | 1. Child quality of life (IDQOL, CDLQI) 2. Disease severity |
|
| Notes | Funding source: The Doris Duke Clinical Research Fellowship Program | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator in Microsoft Excel was used |
| Allocation concealment (selection bias) | Low risk | The caregiver opened sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not possible to blind the parents or educator; it was not clear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 70% of complete data were analysed |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Sokel 1993.
| Methods | Design: parallel group Unit of randomisation: the child Unit of analysis: the child | |
| Participants | Setting: unclear
Diagnostic criteria: no, but 'all had AE that was inadequately controlled'
Disease severity: not for recruitment standardisation, only as an outcome measure
Inclusion criteria of the trial
Participants randomised: 44 in total = 16 (C), 18 (I1), and 10 (I2) Mean age (months): 117.25 (C), 111.38 (I1), and 108.8 (I2) Sex: 8 M, 8 F (C); 9 M, 9 F (I1); and 6 M, 4 F (I2) Duration of condition: not specified Severity of condition: not specified Withdrawals Number of: 12 in total (6 = C) Reason for: not stated ITT analysis: not stated |
|
| Interventions |
Intervention 1
Nature: relaxation technique: hypnotherapy
Format: focused specifically on reducing itching through guided imagery, face‐to‐face with a clinical psychologist
Theoretical basis: precise technique based on Karle & Boys (1987) and Olness & Gardner (1988)
Duration: 30‐minute sessions
Frequency: 4 sessions at 2, 3, 5, and 8 weeks after enrolment Intervention 2 Nature: relaxation technique: biofeedback Format: A relaxometer gave feedback to participants about their level of relaxation using skin conductance Theoretical basis: Biofeedback techniques can engage the participant to actively manage the stress‐response initiated by anxiety about their health problem Duration: 30‐minute sessions Frequency: 4 sessions at 2, 3, 5, and 8 weeks after enrolment Discussion‐only group (control): Children were encouraged to keep an eczema diary that would be discussed at the next session. Parents were encouraged to help the children complete this. No specific psychological therapy was given Duration: 30‐minute sessions Frequency: 4 sessions at 2, 3, 5, and 8 weeks after enrolment |
|
| Outcomes | 1. Mean per cent of body coverage for (i) erythema, (ii) surface damage, and (iii) lichenification 2. Mean severity score for (i) erythema, (ii) surface damage, and (iii) lichenification | |
| Notes | Group comparability at baseline: yes, no differences between the 3 groups for age or vocabulary test at enrolment
Conventional topical treatment: All participants were stabilised on conventional topical and oral treatments for 2 weeks before being randomly allocated to 1 of the groups Funding source: The Lowe‐Costello fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper stated a randomised trial, but the method was not explained |
| Allocation concealment (selection bias) | Unclear risk | The process was not explained |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and clinicians were not blinded, but the outcome assessor was |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow up: There were substantial missing data and no ITT analysis Dropouts differed significantly: There were 13 dropouts from the 44 initial participants, but no reasons were offered within the paper. No intention‐to‐treat analysis was performed, so it is not clear what affect the high number of dropouts had on the results |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Staab 2002.
| Methods | Design: parallel group design Unit of randomisation: the child Unit of analysis: the child‐parent dyad | |
| Participants | Setting: secondary‐care evening sessions
Diagnostic criteria: yes (Hanifin 1980)
Age range: 5 months to 12 years
Disease severity: Participants had moderate to severe symptoms (SCORAD scale > 20 points)
Inclusion criteria of the trial
Participants randomised: 204 in total = 93 (intervention) and 111 (control) Mean age: child 2.7 yrs (treatment group) and child 3.4 yrs (control group) Sex: not stated Duration of condition: 2.1 yrs (intervention group) and 2.4 yrs (control group) Severity of condition: SCORAD 44 SD +/‐ 17 (intervention group) and 42 SD +/‐ 15 (control group) Withdrawals Number of: not stated Reason for: not stated Number lost to follow up: 21 (control) and 38 (intervention) ITT analysis: not stated |
|
| Interventions | Intervention Nature: parental educational training program Format: group sessions with presentations from various experts Theoretical basis: Frequency: once a week and for 2 hours in an evening session Duration: 6 weeks | |
| Outcomes | Primary outcomes: 1. Disease severity (SCORAD ‐ NB: does not distinguish between objective and subjective scores) 2. Disease‐specific (atopic eczema) parental QoL (untitled) 3. Generic parental QoL (Daily Life Questionnaire) 4. Coping strategies (Trier Scales of Coping) Secondary outcomes: 1. Questionnaire (unspecified), 2 key items: (1) treatment behaviour ‐ regularity of use of skin medication (topical steroids) and help seeking from unconventional treatments (indoor allergy reduction) (and initiated dietary restrictions); (2) direct cost of treatment ‐ calculated costs for previous 6 months and after 1 year |
|
| Notes | Group comparability at baseline: yes
Conventional topical treatment
Allocation concealment: The families in this study were randomly assigned to education or waiting control group. We did not stratify for age or severity. They were enrolled in the randomisation program in the computer by the time of their first evaluation visit. After this visit, they were told in what group they had been allocated Funding source: German Federal Ministry of Education, Science, Research and Technology (no. 01EG9523) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The process was not explained |
| Allocation concealment (selection bias) | Unclear risk | The process was not explained |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not possible as most outcome measures were obtained from questionnaires completed by the families who were aware of the group to which they were assigned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 77% of the intervention group and 66% of the control group were followed up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Staab 2006.
| Methods | Design: parallel group design Unit of randomisation: child Unit of analysis: child‐parent dyad |
|
| Participants | Setting: 7 centres (hospital out‐patients) = 3 from children's hospitals, 3 from hospitals specialising in dermatology, and 1 from the Department of Psychosomatic Medicine
Diagnostic criteria: yes (Hanifin 1980) Disease severity: eczema duration, minimum of 3 months, and severity of =/> 20 points on SCORAD Inclusion criteria of the trial
Participants randomised: 992, with 496 allocated to each group (645 in the < 7 band; 214 in the 8 to 12 band; and 151 in the 13 to 18 band) Mean age (SD): < 7 band = I group: 2.4 (1.8), C group: 2.4 (1.9); 8 to 12 band = I group: 9.5 (1.6), C group: 9.5 (1.5); 13 to 18 band = I group: 14.9 (1.7), C group: 14.8 (1.7) Sex (per cent male): < 7 band = both groups: 52; 8 to 12 band = I group: (40), C group: (48); 13 to 18 band = I group: (41), C group: (36) Condition (duration): not specified other than minimum of 3 months Withdrawls Loss to follow up: 169 (I = 50, C = 119) Reasons: tabulated, most gave 'no sufficient response' |
|
| Interventions | Intervention Nature: standardised (structured) educational programme delivered by a multiprofessional team (dermatologists, paediatricians, psychologists, dieticians) who had undergone 40 hours of training Format: The content and structure of the programme and teaching methods were agreed by an interdisciplinary consensus group. Parents of children aged 8 to 12 attended separate sessions. Adolescents aged 13 to 18 attended tailored sessions. A manual and handouts were used. NB: did not contain a therapy mandate, remained responsibility of patients' doctors Theoretical basis: not specified Duration: 6 once‐weekly sessions lasting 2 hours each Control conditions: no education | |
| Outcomes | 1. Severity of eczema: i) SCORAD ii) subjective severity score (the 'Skin detective' tool) iii) Itch questionnaires: used 2 standardised tools (abbreviations only given in paper): JUCKKI 15‐item tool for 8 to 12 age group and JUCKJU of 18 items for the 13 to 18 group 2. Quality of life of parents of children aged < 13 years: Tool (German): 'Quality of life in parents of children with AD'. 26‐item validated tool structured by factor analysis into 5 subscales (with abbreviations): psychosomatic well‐being (pw); well‐being (w); effects on social life (esl); confidence in medical treatment (cmt); emotional coping (ec); acceptance of disease (aod) |
|
| Notes | Also known as the GADIS trial Group comparability at baseline: Yes. In all age groups the severity of eczema or parental quality of life (of children aged < 13 years) did not differ significantly between the intervention and control groups at baseline Funding source: German Federal Ministry of Health and Social Services (grant No 01GL0010) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent study centre provided computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in closed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was not possible for participants who provided most of the outcome data from questionnaires used. The scoring of the AD severity scale involved staff not involved in intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No ITT analysis was undertaken, but overall follow‐up rates of 83% met the review threshold (a good rate for a long‐term study). The dropout rate was 17% (10% in the I group, 24% in the C group). There were twice as many losses to follow up in the control arm than the intervention group |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Weber 2008.
| Methods | Design: parallel group design Unit of randomisation: child‐parent dyad Unit of analysis:
|
|
| Participants | Setting: not stated Diagnostic criteria: yes (Hanifin 1980) Disease severity: AD defined by Hanifin and Rajka's 21 criteria as moderate or severe and that did not respond appropriately to conventional treatment Inclusion criteria of the trial
Participants randomised: 36 Participants who took part: 36 Age: average intervention = 79.31 +/‐ 49.82 months and control = 79.44 +/‐ 53.86 months Sex: intervention men = 11 and women = 5, control men = 7 and women = 9 Duration of condition: average intervention = 61.25 +/‐ 42.84 months and control = 56.25 +/‐ 51.59 months Withdrawals Number of: 32/36 completed the follow‐up over a 24‐month period Loss to follow up: 4 (reasons not stated) ITT analysis: not stated |
|
| Interventions |
Intervention
Nature: children's group meetings (co‐ordinated by child psychiatrist and volunteers, education and play) Parents' group meetings (co‐ordinated by dermatologists education and discussion) Format: face‐to‐face sessions Theoretical basis: not stated Duration: 90 minutes Frequency: fortnightly meetings for 6 months (minimum 75% audience) |
|
| Outcomes | 1. Quality of life: CDLQI 2. Family impact: Family Dermatitis Impact questionnaire 3. Pruritus: based on the McGill pain questionnaire, adapted from Yosipovitch 2002 |
|
| Notes | Funding source: not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information was provided |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information was provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was > 80% follow up |
| Selective reporting (reporting bias) | Low risk | The publication reported findings on all outcomes listed in the Methods section |
| Other bias | Low risk | Results were reported for all outcomes listed in the Methods section, so there was low risk of selective outcome reporting bias |
Legend: Gender: F = female, M = male; QoL = quality of life; Age: Yr = year; LFU = loss to follow up; ITT = intention‐to‐treat analysis; Study groups: C = control, I = intervention; CI = confidence interval; BAD = British Association of Dermatologists; Outcome measures: IDQOL = Infant Dermatitis Quality of life Index, CDLQI = Children's Dermatology Life Quality Index, FDI = Family Dermatitis Index, DFI = Dermatitis Family Impact questionnaire, SCORAD = Scoring Index of Atopic Dermatitis, CSQ‐8 Client Satisfaction Questionnnare‐8; N/A = not applicable; SD = standard deviation; R&D = research and development; AE = atopic eczema; AD = atopic dermatitis.
References: Hanifin & Rajka (1980), Kardoff & Schnelle‐Parker (2001), Karle & Boys (1987), Olness & Gardner (1988), Yosipovitch (2002).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bae 2012 | The trial included adult participants (RCT) with an age range of 12 to 40 and a mean age of 23.5 years, which was not stated in the abstract |
| Broberg 1990 | Inadequate randomisation was the reason for exclusion |
| Greene 1997 | On obtaining unpublished data, we discovered that the participants were adults |
| Kardorff 2003 | Inadequate randomisation was the reason for exclusion |
| van Os‐Mendendorp 2012 | Aggregated child and adult data were presented. We contacted the author who advised that it was not possible to provide child‐only data |
Characteristics of studies awaiting assessment [ordered by study ID]
Futamura 2013.
| Methods | RCT |
| Participants | Mothers of 59 children |
| Interventions | Parental education programme on childhood AD |
| Outcomes | 1. Participants in the intervention group had a significantly lower SCORAD and objective SCORAD score than the control group at 6 months 2. Sleeplessness symptom score and corticosteroid score in the intervention group were significantly better than those in the control group at 6 months There was no significant difference in medication use or quality of life |
| Notes | ‐ |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN98560867.
| Trial name or title | Supporting parents' and carers' management of childhood eczema (SPaCE) |
| Methods | RCT |
| Participants | Carers of children aged 5 years or less with a diagnosis of eczema on their GP record |
| Interventions | LifeGuide, internet‐based behavioural intervention |
| Outcomes | 1. Dermatitis Family Impact questionnaire 2. Infants' Dermatology Quality of Life index 3. Patient Oriented Outcome Measure |
| Starting date | March 2011 |
| Contact information | Dr Miriam Santer Primary Care Medical Aldermoor Health Centre Aldermoor Close Southampton, UK m.santer@soton.ac.uk |
| Notes | Recruitment completed |
N0060047013.
| Trial name or title | Atopic eczema and Habit Reversal |
| Methods | ‐ |
| Participants | Severe atopic patients |
| Interventions | Behavioural therapy versus conventional medical management |
| Outcomes | 1. Subjective and objective clinical improvement according to benchmarked disease severity indices |
| Starting date | After correspondence with the author, we found that the study is currently discontinued |
| Contact information | Dr Richard CD Staughton Dermatology Department Chelsea & Westminster Hospital 369 Fulham Road London SW10 9NH Telephone: 0181 746 8170 richard.staughton@chelwest.nhs.uk or sharon.singh@chelwest.nhs.uk |
| Notes | Does habit reversal programme alter natural history of atopic dermatitis? Is this measurable in blood and skin samples? |
NCT01138761.
| Trial name or title | Health literacy for children with atopic dermatitis and their caregivers |
| Methods | ‐ |
| Participants | 60 children |
| Interventions | Behavioural nurse instruction |
| Outcomes | ‐ |
| Starting date | June 2010 |
| Contact information | ‐ |
| Notes | Reported as active, not recruiting |
NCT01143012.
| Trial name or title | Group Eczema Education Visits: Impact on Patient and Family Quality of Life |
| Methods | RCT |
| Participants | Parents of 60 children aged 2 months to 6 years with diagnosis of atopic dermatitis |
| Interventions | Group eczema education session |
| Outcomes | 1. Difference between 2 groups in Childhood Atopic Dermatitis Impact Scale (CADIS) score. Average number of follow‐up telephone calls |
| Starting date | May 2010 |
| Contact information | Susan Tofte Oregon Health and Science University toftes@ohsu.edu |
| Notes | Register refreshed 17.10.12; trial still recruiting |
Differences between protocol and review
There were no differences between the protocol and review completed in 2007.
Differences between the 2007 review and this update are as follows.
The objective has, for clarity, been changed from 'To assess the effectiveness of psychological and educational interventions in changing outcomes for children with atopic eczema' to 'To assess the effect of psychological and educational interventions for atopic eczema in children'.
Secondary outcomes now include adverse effects in terms of cost and inconvenience.
Types of intervention: The description and organisation of psychological and educational interventions has been amended.
Analysis plan: In this update, it was planned, if feasible, to assess the impact of missing data with a sensitivity analysis, to use the I² statistic to assess heterogeneity, and to use funnel plots to assess reporting bias.
Search methods. Not all sources searched for the 2007 review have been searched for this update. We have incorporated some additional resources.
Contributions of authors
FC was the contact person with the editorial base. FC co‐ordinated contributions from the co‐authors and wrote the final draft of the review. FC, SE, AT, and FW developed the revised protocol and search terms. FC, SE, and SML screened papers against eligibility criteria. FC obtained data on ongoing and unpublished studies. FC, SML, and EG appraised the quality of papers. SML, EG, and FC extracted data for the review and sought additional information about papers. FC and EG entered data into RevMan. EG and SE analysed and interpreted data. SE, FC, HF, and KJ worked on the methods sections. AT, FC, SE, SML, and KF drafted the clinical sections of the background and responded to the clinical comments of the referees. FC, EG, HF, and SE responded to the methodology and statistics comments of the referees. AD was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. SE and FC are guarantors of the update.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health, UK.
Sources of support
Internal sources
None, Other.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Chinn 2002 {published data only}
- Chinn DJ, Poyner T, Sibley G. Randomized controlled trial of a single dermatology nurse consultation in primary care on the quality of life of children with atopic eczema. British Journal of Dermatology 2002;146(3):432‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grillo 2006 {published data only}
- Grillo M, Ng, M, Gassner L, Marshman G, Dunn S, Hudson P. Pediatric atopic eczema: the impact of an educational intervention. Pediatric Dermatology 2006;23(5):428‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moore 2009 {published data only}
- Moore EJ, Williams A, Manias E, Varigos G, Donath S. Eczema workshops reduce severity of childhood atopic eczema. Australasian Journal of Dermatology 2009;50(2):100‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niebel 2000 {published data only}
- Niebel G, Kallweit C, Lange I, Folster‐Holst R. Direct versus video‐aided parental education in atopic eczema in childhood as supplement to specialty physician treatment. A controlled pilot study [Direkte versus videovermittelte Elternschulung bei atopischem Ekzem im Kindesalter als Erganzung facharztlicher Behandlung. Eine Kontrollierte Pilotstudie]. Hautarzt 2000;51(6):401‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schuttelaar 2010 {published data only}
- Schuttelaar ML, Vermeulen KM, Drukker N, Coenraads PJ. A randomized controlled trial in children with eczema: nurse practitioner vs. dermatologist. British Journal of Dermatology 2010;162(1):162‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shaw 2008 {published data only}
- Shaw M, Morrell DS, Goldsmith LA. A study of targeted enhanced patient care for pediatric atopic dermatitis (STEP PAD). Pediatric Dermatology 2008;25(1):19‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sokel 1993 {published data only}
- Sokel B, Christie D, Kent A, Glover M, Lansdown R, Knibbs J, et al. A comparison of hypnotherapy and biofeedback in the treatment of childhood atopic eczema. Contemporary Hypnosis 1993;10(3):145,54. [Google Scholar]
Staab 2002 {published data only}
- Staab D, Rueden U, Kehrt R, Erhart M, Wenninger K, Kamtsiuris P, et al. Evaluation of a parental training program for the management of atopic dermatitis. Pediatric Allergy and Immunology 2002;13(2):84‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Staab 2006 {published data only}
- Kupfer J, Gieler U, Diepgen TL, Fartasch M, Lob‐Corzilius T, Ring J, et al. Structured education program improves the coping with atopic dermatitis in children and their parents ‐ a multicenter, randomized controlled trial. Journal of Psychosomatic Research 2010;68(4):353‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Staab D, Diepgen TL, Fartasch M, Kupfer J, Lob‐Corzilius T, Ring J, et al. Age related, structured educational programmes for the management of atopic dermatitis in children and adolescents: multicentre, randomised controlled trial. BMJ 2006;332(7547):933‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2008 {published data only}
- Weber MB, Fontes Neto Pde T, Prati C, Soirefman M, Mazzotti NG, Barzenski B, et al. Improvement of pruritus and quality of life of children with atopic dermatitis and their families after joining support groups. Journal of the European Academy of Dermatology and Venereology 2008;22(8):992‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bae 2012 {published data only}
- Bae BG, Oh SH, Park CO, Noh S, Noh JY, Kim KR, et al. Progressive muscle relaxation therapy for atopic dermatitis: objective assessment of efficacy. Acta Dermato‐Venereologica 2012;92(1):57‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Broberg 1990 {published data only}
- Broberg A, Kalimo K, Lindblad B, Swanbeck G. Parental education in the treatment of childhood atopic eczema. Acta Dermato‐Venereologica 1990;70(6):495‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Greene 1997 {published data only}
- Greene DH. The comparative effects of relaxation techniques in the treatment of atopic dermatitis. Dissertation Abstracts International: Section B; The Sciences and Engineering (California School of Professional Psychology ‐ San Diego, US) 1996; Vol. 57, issue 7726:ISSN: 0419–4217.
Kardorff 2003 {published data only}
- Kardorff B, Schelle‐Parker G, Kardoff M, Wahlen M, d'Orville IH, Dorittke P. Successful reduction of the SCORAD score by a short‐time teaching method using a simplified skin model in children with atopic eczema in a 6‐week comparison. [Erfolgreiche Reduktion des SCORAD‐Scores bei Kindern mit atopischem Ekzem im 6‐Wochen‐Vergleich durch Kurzschulung mit einem vereinfachten Hautmodell.]. Journal der Deutschen Dermatologischen Gesellschaft 2003;1(6):451‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Os‐Mendendorp 2012 {published data only}
- Os‐Medendorp H, Koffijberg H, Eland‐de Kok PC, Zalm A, Bruin‐Weller MS, Pasmans SG, et al. E‐health in caring for patients with atopic dermatitis: a randomized controlled cost‐effectiveness study of internet‐guided monitoring and online self‐management training. British Journal of Dermatology 2012;166(5):1060‐8. [PUBMED: 22268960] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Futamura 2013 {published data only}
- Futamura M, Masuko I, Hayashi K, Ohya Y, Ito K. Effects of a short‐term parental education program on childhood atopic dermatitis: A randomized controlled trial. Pediatric Dermatology 2013;30(4):438‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN98560867 {published data only}
- ISRCTN98560867. Supporting Parents and Carer's management of Childhood Eczema (SPaCE). www.controlled‐trials.com/ISRCTN98560867 (accessed 12 February 2013).
N0060047013 {published data only (unpublished sought but not used)}
- N0060047013. Atopic eczema and habit reversal. www.nihr.ac.uk/Profile/Pages/NRRResults.aspx?publication_id=N0060047013 (accessed 12 February 2013).
NCT01138761 {published data only}
- NCT01138761. Health Literacy for Children With Atopic Dermatitis and Their Caregivers. clinicaltrials.gov/ct2/show/NCT01138761 (accessed 12 February 2013).
NCT01143012 {unpublished data only}
- NCT01143012. Group Eczema Education Visits: Impact on Patient and Family Quality of Life. clinicaltrials.gov/show/NCT01143012 (accessed 12 February 2013).
Additional references
Altman 1999
- Altman DG. Practical Statistics for Medical Research. CRC Press, 1999. [Google Scholar]
Armstrong 2011
- Armstrong AW, Kim RH, Idriss NZ, Larsen LN, Lio PA. Online video improves clinical outcomes in adults with atopic dermatitis: a randomized controlled trial. Journal of the American Academy of Dermatology 2011;64(3):502‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Asher 2006
- Asher MI, Montefort S, Björkstén, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006;368(9537):733‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bandura 1997
- Bandura, A. Self‐efficacy: the exercise of control. New York: WH Freeman, 1997. [Google Scholar]
Barbarot 2013
- Barbarot S, Bernier C, Deleuran M, Raeve L, Eichenfield L, Hachem M, et al. Therapeutic patient education in children with atopic dermatitis: position paper on objectives and recommendations. Pediatric Dermatology 2013;30(2):199‐206. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ben‐Gashir 2004
- Ben‐Gashir MA, Seed PT, Hay RJ. Quality of life and disease severity are correlated in children with atopic dermatitis. British Journal of Dermatology 2004;150(2):284–90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benjamin 2004
- Benjamin K, Waterston K, Russell M, Schofield O, Diffey B, Rees JL. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. Journal of the American Academy of Dermatology 2004;50(1):33‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bridgett 1995
- Bridgett CK, Roberts N. Cognitive therapy of itch and scratch in atopic dermatitis ‐ a review of 50 cases. Proceedings of the 6th International Congress of Dermatological Psychiatry. Amsterdam, 1995.
Bridgett 2000
- Bridgett C. Psychodermatology and atopic skin disease in London 1989‐1999 ‐ Helping patients to help themselves. Dermatology Psychosomatics 2000;1(4):183‐6. [DOI: 10.1159/000057975] [DOI] [Google Scholar]
Coenraads 2001
- Coenraads PJ, Span L, Jaspers JP, Fidler V. Intensive patient education and treatment program for young adults with atopic eczema. Hautarzt 2001;52(5):428‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cork 2006
- Cork MJ, Robinson DA, Vasilopoulos Y, Ferguson A, Moustafa M, MacGowan A, et al. New perspectives on epidermal barrier dysfunction in atopic dermatitis: gene‐environment interactions. Journal of Allergy and Clinical Immunology 2006;118(1):3‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coster 2009
- Coster S, Norman I. Cochrane reviews of education al and self‐management interventions to guide nursing practice: a review. International Journal of Nursing Studies 2009;46(4):508‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coulter 2006
- Coulter A, Ellins J. Patient‐focused interventions: a review of the evidence. www.health.org.uk/publications/patient‐focused‐interventions/ (accessed 19 November 2013):1‐277.
de Bes 2011
- Bes J, Legierse CM, Prinsen CA, Korte J. Patient education in chronic skin diseases: a systematic review. Acta Dermato‐Venereologica 2011;91(1):12‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de L Horne 1999
- L Horne DJ, Taylor M, Varigos G. The effects of relaxation with and without imagery in reducing anxiety and itchy skin in patients with eczema. Behavioural & Cognitive Psychotherapy 1999;27(2):143‐51. [EMBASE: 1999201504] [Google Scholar]
Ehlers 1995
- Ehlers A, Stangier U, Gieler U. Treatment of atopic dermatitis: a comparison of psychological and dermatological approaches to relapse prevention. Journal of Consulting and Clinical Psychology 1995;63(4):624‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elliott 1997
- Elliott BE, Luker K. The experiences of mothers caring for a child with severe atopic eczema. Journal of Clinical Nursing 1997;6(3):241‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Emerson 2000
- Emerson RM, Charman CR, Williams HC. The Nottingham Eczema Severity Score: a preliminary refinement of the Rajka and Langeland grading. British Journal of Dermatology 2000;142(2):288‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ersser 2011
- Ersser SJ, Cowdell FC, Nicholls PG, Latter SM, Healy E. A pilot randomised controlled trial to examine the feasibility and efficacy of an educational nursing intervention to improve self‐management practices in patients with mild‐moderate psoriasis. Journal of the European Academy of Dermatology and Venereology 2011;26(6):738‐45. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ersser 2013
- Ersser SJ, Farasat H, Jackson K, Dennis H, Sheppard ZA, More A. A service evaluation of the Eczema Education Programme: an analysis of child, parent and service impact outcomes. British Journal of Dermatology 2013;169(3):629‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fallon 2009
- Fallon PG, Sasaki T, Sandilands A, Campbell LE, Saunders SP, Mangan NE, et al. A homozygous frameshift mutation in the mouse FLG gene facilitates enhanced percutaneous allergen priming. Nature Genetics 2009;41(5):602‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flohr 2010
- Flohr C, England K, Radulovic S, McLean WH, Campbell LE, Barker J, et al. Filaggrin loss‐of‐function mutations are associated with early onset eczema, eczema severity and transepidermal water loss at 3 months of age. British Journal of Dermatology 2010;163(6):1333‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giannini 1997
- Giannini AV. Habit reversal technique and eczema. Journal of Allergy and Clinical Immunology 1997;100(4):580. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gieler 2000
- Gieler U, Kupfer J, Niemeier V, Brosig B, Standier U. Atopic eczema prevention programmes ‐ a new therapeutic concept for secondary prevention. Dermatology & Psychosomatics 2000;1(4):138‐47. [DOI: 10.1159/000057969] [DOI] [Google Scholar]
Gradwell 2002
- Gradwell C, Thomas KS, English JS, Williams HC. A randomized controlled trial of nurse follow‐up clinics: do they help patients and do they free up consultants’ time?. British Journal of Dermatology 2002;147(3):513‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guevara 2003
- Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of interventions for self management of asthma in children and adolescents: systematic review and meta analysis. BMJ 2003;326(7402):1308‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanifin 1980
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato‐Venereologica 1980;92:44‐7. [Google Scholar]
Hanifin 2007
- Hanifin JM, Reed ML, Eczema Prevalence and Impact Working Group. A population‐based survey of eczema prevalence in the USA. Dermatitis 2007;18(2):82‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Health Foundation 2011
- Health Foundation, Silva D. Helping people help themselves. A review of the evidence considering whether it is worthwhile to support self‐management. www.health.org.uk/publications/evidence‐helping‐people‐help‐themselves/ (accessed 19 November 2013).
Herd 1996
- Herd RM, Tidman MJ, Prescott RJ, Hunter JA. Prevalence of atopic eczema in the community: the Lothian atopic dermatitis study. British Journal of Dermatology 1996;135(1):18‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org., Available from www.cochrane‐handbook.org.
Hoare 2000
- Hoare C, Li Wan Po A, Williams H. Systematic review of treatments for atopic eczema. Health Technology Assessment 2000;4(37):1‐191. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Holman 2000
- Holman H, Long K. Patients as partners in managing chronic illness. Partnership is a prerequisite for effective and efficient health care. BMJ 2000;320(7234):526‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
HOME 2013
- Harmonising Outcome Measures for Eczema (HOME). HOME III Meeting. homeforeczema.org/ (accessed 19 November 2013).
Hägermark 1995
- Hägermark Ö, Wahlgren CF. Treatment of itch. Seminars in Dermatology 1995;14(4):320‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jackson 2013
Jaspers 2000
- Jaspers JPC, Span L, Molier L, Coenraads PJ. A multimodal education and treatment program for young adults with atopic dermatitis: a randomized controlled trial. Dermatology and Psychosomatics 2000;1(4):148‐53. [DOI: 10.1159/000057970] [DOI] [Google Scholar]
Johnson 1991
- Johnson CM. Infant and toddler sleep: a telephone survey of parents in one community. Journal of Developmental and Behavioral Pediatrics 1991;12(2):108‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemp 2003
- Kemp AS. Cost of illness of atopic dermatitis in children: a societal perspective. Pharmacoeconomics 2003;21(2):105‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kunz 1997
- Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997;195(1):10‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lavda 2012
- Lavda AC, Webb TL, Thompson AR. A meta‐analysis of the effectiveness of psychological interventions for adults with skin conditions. British Journal of Dermatology 2012;167(5):970‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lawton 2005
- Lawton S, Roberts A, Gibb C. Supporting the parents of children with atopic eczema. British Journal of Nursing 2005;14(13):693‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lewis‐Jones 1995
- Lewis‐Jones MS, Finlay AY. The children's dermatology life quality index (CDLQI): initial validation and practical use. British Journal of Dermatology 1995;132(6):942‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lewis‐Jones 2001
- Lewis‐Jones MS, Finlay AY, Dykes PJ. The infants' dermatitis quality of life index. British Journal of Dermatology 2001;144(1):104‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McNally 1998
- McNally NJ, Phillps DR, Williams HC. The problem of atopic eczema: aetiological clues from the environment and lifestyles. Social Science & Medicine 1998;46(6):729‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Medical Research Council 2008
- Medical Research Council. Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council, 2008. [Google Scholar]
Melin 1986
- Melin L, Frederiksen T, Noren P, Swebilius BG. Behavioural treatment of scratching in patients with atopic dermatitis. British Journal of Dermatology 1986;115(4):467‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Miltenberger 1998
- Miltenberger RG, Fuqua RW, Woods DW. Applying behavioral analysis to clinical problems: review and analysis of habit reversal. Journal of Applied Behavior Analysis 1998;31(3):447‐69. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health And Clinical Excellence. Behaviour change. www.nice.org.uk/guidance/index.jsp?action=download&o=64039 2013.
Norén 1989
- Norén P, Melin L. The effect of combined topical steroids and habit‐reversal treatment in patients with atopic dermatitis. British Journal of Dermatology 1989;121(3):359‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Palmer 2006
- Palmer CN, Irvine AD, Terron‐Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss‐of‐function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics 2006;38(4):441‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paternoster 2012
- Paternoster L, Standl M, Chen CM, Ramasamy A, Bønnelykke K, Duijts L, et al. Meta‐analysis of genome‐wide association studies identifies three new risk loci for atopic dermatitis. Nature Genetics 2012;44(2):187‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pucci 2005
- Pucci N, Novembre E, Cammarata MG, Bernardini R, Monaco MG, Calogero C, et al. Scoring atopic dermatitis in infants and young children: distinctive features of the SCORAD index. Allergy 2005;60(1):113‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rajka 1989
- Rajka G, Langeland T. Grading of the severity of atopic dermatitis. Acta Dermato‐Venereologica. Supplementum 1989;144:13‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reid 1995
- Reid P, Lewis‐Jones MS. Sleep difficulties and their management in pre‐schoolers with atopic eczema. Clinical and Experimental Dermatology 1995;20(1):38‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ring 2012
- Ring J, Alomar A, Bieber T, Deleuran M, Fink‐Wagner A, Gelmetti C, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. Journal of the European Academy of Dermatology and Venereology 2012;26(9):1176‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Santer 2012
- Santer M, Burgess H, Yardley L, Ersser S, Lewis‐Jones S, Muller I, et al. Experiences of carers managing childhood eczema and their views on its treatment: a qualitative study. British Journal of General Practice 2012;62(597):e261‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitt 2007
- Schmitt J, Langan S, Williams H, European Dermato‐Epidemiology Network. What are the best outcome measurements for atopic eczema? A systematic review. Journal of Allergy and Clinical Immunology 2007;120(6):1389‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmitt 2012
- Schmitt J, Spuls P, Boers M, Thomas K, Chalmers J, Roekevisch E, et al. Towards global consensus on outcome measures for atopic eczema research: results of the HOME II meeting. Allergy 2012;67(9):1111‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schram 2010
- Schram ME, Tedja AM, Spijker R, Bos JD, Williams HC, Spuls PI. Is there a rural/urban gradient in the prevalence of eczema? A systematic review. British Journal of Dermatology 2010;162(5):964‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schram 2012
- Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy 2012;67(1):99‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherriff 2002
- Sherriff A, Golding J, Alspac Study Team. Hygiene levels in a contemporary population cohort are associated with wheezing and atopic eczema in preschool infants. Archives of Disease in Childhood 2002;87(1):26‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson‐Dent 1999
- Simpson‐Dent SL, Staughton RCD, Bridgett CK, Farell A. The combined approach to chronic atopic eczema. A prospective comparison of behavioural modification with standard dermatological treatment against standard treatment alone. Proceedings of the International Congress of Dermatological Psychiatry. Paris, 1999.
Smith 2006
- Smith FJ, Irvine AD, Terron‐Kwiatkowski A, Sandilans A, Campbell LE, Zhao Y, et al. Loss‐of‐function mutations in the gene encoding filaggrin causes ichthyosis vulgaris. Nature Genetics 2006;38(3):337‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van den Oord 2009
- Oord RA, Sheikh A. Filaggrin gene defects and risk of developing allergic sensitization and allergic disorders: systematic review and meta‐analysis. BMJ 2009;339:b2433. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verboom 2002
- Verboom P, Hakkaart‐Van L, Sturkenboom M, Zeeuw R, Menke H, Rutten F. The cost of atopic dermatitis in the Netherlands: an international comparison. British Journal of Dermatology 2002;147(4):716‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Verhoeven 2008
- Verhoeven EW, Klerk S, Kraaimaat FW, Kerkhof PC, Jong EM, Evers AW. Biopsychosocial mechanisms of chronic itch in patients with skin disease: a review. Acta Dermato‐Veneroelogica 2008;88(3):211‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Von Rueden 1999
- Rueden U, Kerht R, Staab D, Wahn U. Development and validation of a disease specific questionnaire on quality of life of parents of children with atopic eczema. Zeitschrift für Gesundheitswissenschaften = Journal of public health 1996;4:335‐50. [Google Scholar]
Webb 2006
- Webb TL, Sheeran P. Does changing behavioral intentions engender behavior change? A meta‐analysis of the experimental evidence. Psychological Bulletin 2006;132(2):249‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1997
- Williams HC. Dermatology. Health Care Needs Assessment. Oxford: Radcliffe Medical Press, 1997. [Google Scholar]
Williams 2002
- Williams RB, Schneidermann, N. Psychosocial interventions can improve clinical outcomes in organic disease. Psychosomatic Medicine 2002;64(4):552‐7. [DOI: 10.1097/01.PSY.0000023410.02546.5D] [DOI] [PubMed] [Google Scholar]
Williams 2005
- Williams HC. Clinical practice. Atopic dermatitis. New England Journal of Medicine 2005;352(22):2314‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 2006
- Williams, HC. Educational programmes for young people with eczema. BMJ 2006;332(7547):923‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2002
- Wolf F, Guevara JP, Grum CM, Clark NM, Cates CJ. Educational interventions for asthma in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000326] [DOI] [PubMed] [Google Scholar]
Yosipovitch 2002
- Yosipovitch G, Goon AT, Wee J, Chan YH, Zucker I, Goh CL. Itch characteristics in Chinese patients with atopic dermatitis using a new questionnaire for the assessment of pruritus. International Journal of Dermatology 2002;41(4):212‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ersser 2007
- Ersser SJ, Latter S, Sibley A, Satherley PA, Welbourne S. Psychological and educational interventions for atopic eczema in children. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004054.pub2] [DOI] [PubMed] [Google Scholar]


