Abstract
Polyploidy (the more than doubling of a cell’s genome) frequently arises during organogenesis, tissue repair, and age-associated diseases. Despite its prevalence, major gaps exist in how polyploid cells emerge and affect tissue function. Studies have begun to elucidate the signals required for polyploid cell growth as well as the advantages and disadvantages of polyploidy in health and disease. This review highlights the recent advances on the role and regulation of polyploidy in Drosophila and vertebrate models. The newly discovered versatility of polyploid cells has the potential to provide alternative strategies to promote tissue growth and repair, while limiting disease and dysfunction.
Keywords: Polyploidy, Endoreplication, Aneuploidy, Tissue Repair, Hippo, Mechanotransduction
A polyploid cell is defined as a cell that contains more than the diploid copy of its chromosomes. The total DNA content is represented by the amount of ‘chromatin’ in the cell, known as the C-value. Haploid cells (i.e. sperm) are 1C, diploid cells are 2C, and polyploid cells therefore are at least 3C or greater. Cells are either developmentally programmed to become polyploid or opt to adopt the polyploid fate as a stress resistance response. This review focuses on the recent observations of polyploidy in Drosophila and vertebrates (mice and zebrafish) that has given new insights into the functional significance and regulation of polyploidy in health and disease.
Generating a polyploid cell
Polyploid cells are generated by cell cycle dependent or independent mechanisms (Figure 1). The mitotic cell cycle depends on four sequential phases: G1-S-G2-M which is driven by cyclin-dependent kinases (CDKs). CDK-cyclin activation allows cells to progress through the canonical phases of the cell cycle. For example, CDK2-cyclin E complex drives S phase (DNA replication) and CDK1-cyclin B complex drives M phase, in which chromosomes are segregated and the cell undergoes cytokinesis resulting in two daughter cells (Figure 1A).
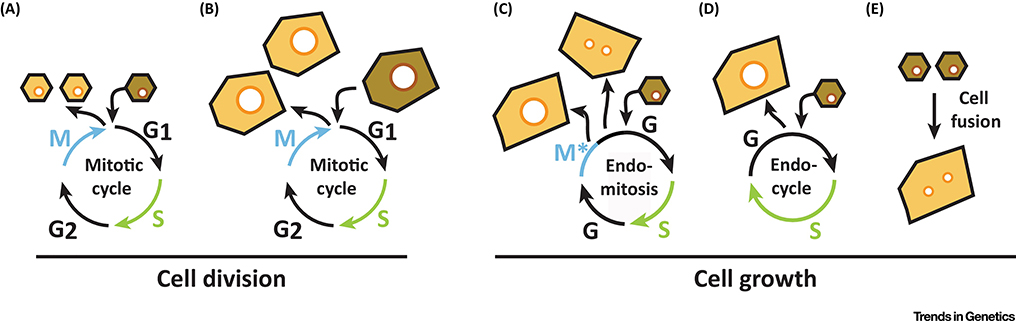
Figure 1. Generation of diploid and polyploid cells through division and growth.
The mitotic cell cycle generates either two diploid daughter cells (A) or two polyploid daughter cells (B) depending on whether the initiating cell is diploid (A) or polyploid (B). Incomplete cell cycles promote polyploid cell growth by either endomitosis (C), which results in a mono- or binucleated, polyploid cell or via the endocycle (D) resulting in a mononucleated polyploid cell. (E) Cell fusion also generates bi- and multinucleated polyploid cells independent of the cell cycle. Cell cycle markers do not discern polyploid vs diploid cell generation. A cell’s total ploidy (DNA content) has to be measured to rigorously distinguish between diploid (A) and polyploid (B-E) cell outcomes.
Endoreplication encompasses two types of incomplete cell cycles: endocycle and endomitosis [1]. Endomitosis refers to a cell cycle that initiates but does not complete M-phase (Figure 1C). This is either due to a block in cytokinesis resulting in a binucleated, polyploid cell or truncates M phase, before telophase, resulting in a mononucleated, polyploid cell (Figure 1C). In the endocycle, a cell replicates its genome in S phase, but completely bypasses M phase with alternating G and S phases (Figure 1D). Inhibition of cell’s mitotic machinery enables M phase to be skipped over. This often occurs by targeting mitotic cyclins for proteolytic degradation via the anaphase- promoting complex/ cyclosome (APC/C) E3 ligase or inhibition of CDK-cyclin complex activity by CDK inhibitors (CDI). The oscillation of CDK2-cyclin E activity from low to high in G to S phase allows cells to initiate sequential endocycles [2]. Each endocycle will double the cell’s genome allowing a cell to reach C-values as high as 200,000C [3]. Alternatively, bi- and multinucleated, polyploid cells can also form by cell fusion, which is not dependent on the cell cycle (Figure 1E).
Distinguishing diploid vs polyploid growth and division
A major challenge in the tissue growth and regeneration field has been to faithfully distinguish between polyploidization and proliferative cell cycle events. The classical cell cycle markers, including S phase markers (PCNA, 3H-thymidine, EdU, or BrdU) do not distinguish endoreplication from a mitotic cell division, since in both cases cells will enter S phase (Figure 1A-1D). Likewise, the M phase marker, phospho-histone H3 (pH3), will not differentiate endomitosis and mitotic cell division, as chromosome condense during metaphase and are readily labeled with pH3 in both cell cycles [4]. The Ki67 marker labels all phases of cell cycle and only distinguishes cycling cells from post-mitotic cells [5]. Polyploid cells, in some circumstances, have the capacity to divide so cytokinesis markers are also not reliable (Figure 1B) [6, 7]. In fact, no cell cycle marker exists that can distinguish a cell division that will result in a diploid versus polyploid cell (Figure 1A and 1B).
More sophisticated strategies are now being employed to conclusively detect a complete mitotic cell cycle resulting in cytokinesis and generation of two diploid daughter cells versus endoreplication to generate polyploid cells. One strategy is to use single-cell analysis either by imaging fixed cells, fluorescence-activated cell sorting (FACS), or single cell DNA sequencing [8–10]. These methods allow a cell’s nuclear DNA content (ploidy) to be measured, a rigorous method to distinguish between diploid (2C) and polyploid (>3C) cells. Single cell DNA sequencing has additional advantage of being able to detect chromosome copy number variation thereby detecting aneuploidy [10]. Another strategy is to use live imaging to follow cell division and growth in vivo [11]. These methods in combination with fluorescent reporters that allow all phases of cell cycle including cytokinesis to be detected [12, 13]. This has led to a significant recognition of the prevalence of polyploidy in development, regeneration, and disease. Long-held views of how tissues grow and repair are being put into question using these emerging techniques.
Polyploidy: a common strategy for cellular differentiation and organogenesis
Polyploid cells are ubiquitous in plants and insects and have been well studied in these model organisms, Arabidopsis and Drosophila [14, 15]. In mammals, polyploidy was thought to be unique to only a few cell types, most notably the placenta giant trophoblasts (up to 512C), megakaryocytes (up to 128C), and liver hepatocytes (up to 16C) [14]. However, advances in imaging with fluorescent cellular markers has allowed intact mammalian tissues to be visualized enabling the detection of remarkable number of polyploid cells in nearly all mammalian organs. These new imaging tools enabled the identification of binucleated secretory alveolar cells in the lactating mammary gland [16]. The secretory alveolar cells become polyploid by endomitosis (also referred to as failed cytokinesis), which is required for milk production (Figure 2B) [16].
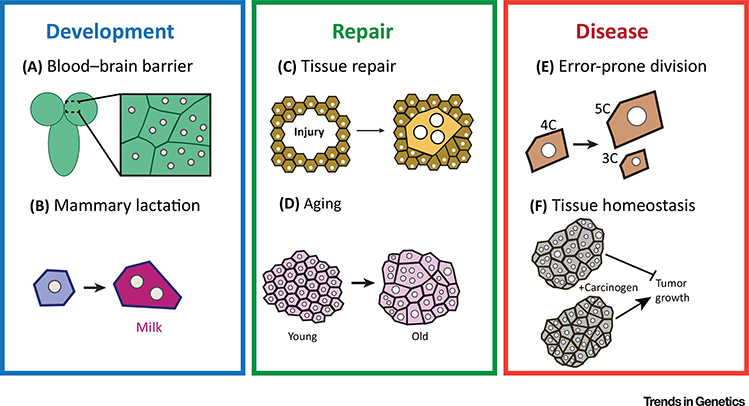
Figure 2. The functional significance of polyploidy in health and disease.
Illustrations of recent discoveries of polyploidy in health and disease. (A) Development and function of the blood brain barrier in Drosophila requires a balance of mono- and multinucleated polyploid cells generated by endocycle and endomitosis. (B) Mammary gland lactation requires polyploidization of secretory cells by endomitosis. (C) Wound-induced polyploidization occurs in Drosophila and vertebrate tissues to promote repair. (D) Polyploid cells arise with age and may help to compensate for cell loss. (E) Polyploid cells can retain the capacity to divide yet are susceptible to error-prone division resulting in aneuploidy. (F) Polypoid cells can also restrict tumor growth.
It is becoming appreciated that many mammalian tissues are composed of >50% polyploid cells as it is part of their developmentally programmed differentiation [17–21]. The switch to polyploidy often coincides with the onset of postnatal life. What still remains more poorly understood is functional significance of polyploidy. The atypical E2Fs (E2F7 and E2F8) regulate polyploidization by inhibition of mitotic gene expression, a requirement for the endocycle [18, 19]. However, conditional knockout of E2F7 and/ or E2F8 in liver hepatocytes and pancreas exocrine and endocrine cells has no apparent consequence to physiological organ function despite affected cell types being diploid, instead of polyploid [18, 19, 21]. In liver, two recent studies have now revealed that polyploid hepatocytes help to prevent tumor growth (Figure 2F) [22, 23]. While polyploidy may be dispensable for organ development in some cases, it’s appears to offer an adaptive advantage for disease resistance during adult mammalian life.
Polyploid cell generation in organogenesis
A variety of mechanisms are used to generate polyploid cells, but what drives polyploid cells to form by one strategy versus another is an open question. In the blood, megakaryocytes develop by endomitosis forming multinucleated polyploid cells required for platelet production [14]. Remarkably, megakaryocytes can be reprogrammed into an endocycle by genetically ablating the mitotic regulator, CDK1, with no inhibition of platelet formation [24]. This is an example where polyploidy generation is functionally interchangeable, but this is not always the case. The Drosophila blood brain barrier (BBB) was found to be composed of both mono- and multinucleated polyploid subperineurial glia (SPG), which are generated by the endocycle and endomitosis during larval development (Figure 2A) [25]. Notch signaling promotes the endocycle but inhibits endomitosis similar to its known role in mitotic to endocycle switch in the Drosophila intestinal and follicle epithelial cells [15, 25, 26]. Genetically manipulating Notch signaling or mitotic entry can shift the balance of mono- and multinucleated SPG cells but doing so impaired integrity of the BBB [26]. Therefore, in this tissue, mono- and multinucleated cells are not interchangeable and both endocycle and endomitosis are required for the development of a functional barrier. The advantages and constraints of making mono- versus multi-nucleated polyploid cells still remains to be elucidated. It may be a matter of cell size as the multinucleated SPG cells were found to attain a larger cell size despite having the same ploidy as their mononucleated counterparts [26].
Polyploid cell division in organogenesis
Polyploid cell division is unusual as most polyploid cells are terminally differentiated and unable to return to mitosis. This is in part because polyploidy poses a challenge to cell division since dividing polyploid cells readily accumulate mitotic errors, including chromosome aberrations and aneuploidy associated with cancer cells (Figure 2E) [27, 28]. Despite these constraints, mouse liver hepatocytes and Drosophila recital papillar cells retain the capacity to divide [6, 7, 29]. These models of polyploid cell division are providing new insights into how polyploid division is regulated with the risk of aneuploidy.
Drosophila’s rectal papillar first endocycle to become octoploid and then mitotically divide two times during development [6]. To retain mitotic competence, a unique endocycle program, including the retention of centrosomes and late replicating genomic regions, was discovered [30]. In addition, the polyploid papillar cell chromosomes are polytene, a state in which chromosome copies are bound within their chromatids. Frequent mitotic errors were found to be reduced by dissociating the polytene chromosomes into separated chromatid pairs prior to anaphase, named separation into recent sister chromatid pairs (SIRS) [31]. Inhibition of SIRS, by genetic loss of Mad2, delays SIRS resulting in significant increase in chromosome aberrations and aneuploidy after polyploid cell division [31].
In liver, polyploid hepatocytes also retain mitotic competence and were reported to generate aneuploid cells during development and homeostasis [7, 29]. However, the high percentage of aneuploid hepatocytes in the normal liver has been put into question using the single cell DNA sequencing method [10, 29]. Strikingly, it was found that the tissue environment dictates a polyploid cell’s susceptibility to aneuploidy during division. Dissociated polyploid hepatocytes grown in culture frequently become aneuploid, whereas polyploid division of hepatocytes within developing neonatal liver retained the capacity to accurately segregate chromosomes [10, 33]. In adult liver regeneration, the tissue architecture is also disrupted and aneuploidy arises in the dividing polyploid hepatocytes [7, 29]. Still it remains to be determined whether aneuploid hepatocytes persist or are eliminated after liver regeneration completes as aneuploidy can activate the immune response [33]. What would be the consequence if aneuploid cells persisted? A growth advantage by resulting in genetic diversity in liver or as an initiator of disease progression [28, 32, 34].
Polyploidy: the benefits and limits for tissue repair
Polyploidy as a driver of tissue repair
Many adult tissues lack a resident stem cell population and are solely composed of terminally differentiated cells that lack the capacity to proliferate. As a consequence, polyploidy serves as an alternative strategy to promote wound repair and compensate for cell loss when cell division is restricted. Recently, polyploid cell growth has been found to contribute to tissue repair and regeneration in several Drosophila and vertebrate tissues. In Drosophila, the adult abdominal epithelium heals puncture wounds by wound-induced polyploidization [8, 35, 36]. Enlarged multinucleated, polyploid cells form by cell fusion and endoreplication, which function simultaneously to re-establish the adult fly epithelial barrier (Figure 1D, 1E, and 2C) [35]. In Drosophila egg development, follicular epithelial cells switch from mitotic cycle to endocycle. Genetic ablation of the follicle cells or a reduction in follicle cell growth triggers an insulin-dependent compensatory cellular hypertrophy [37]. As result, the follicular epithelial cells compensate for cell loss by boosting the endocycle to generate epithelial cells with higher ploidy [37].
Many tissues utilize both polyploidization and cell proliferation to heal. The regenerative capacity of the mammalian liver relies on the ability of hepatocytes to polyploidize as well as divide as either diploid or polyploid cells [38]. Hepatocytes cell growth was found to precede hepatocyte division following surgical removal the liver mass [38]. The Drosophila gut relies on both stem cell proliferation and endoreplication of daughter cells to maintain homeostasis and regenerate [39]. Polyploid enterocytes, the major cell type of the fly intestine, differentially respond to physiological demands of the organ by boosting cell growth via the endocycle. InR, Pi3K, and Tor signaling activate the endocycle during homeostasis, whereas tissue damage activates EGFR, Ras, and MAPK signaling to induce polyploid growth of the enterocytes [39].
Polyploid cells can also be a source for the generation of new intestinal stem cells. Under conditions of severe stem cell loss, polyploid (4C) enteroblasts, the immediate stem cell daughter, are capable of undergoing a process of reductive cell division known as amitosis [40]. The reductive division of the polyploid enteroblasts into two diploid daughter cells enabled the fly intestinal stem cell pool to be restored [40]. Reductive polyploid divisions have also been observed during mouse liver regeneration, suggesting the amitosis may be conserved strategy to resupply the organ with a diploid cell pool that has more proliferative potential [7, 32, 38].
In vertebrates, wound-induced polyploidization has also been discovered to play a beneficial role in kidney and heart repair [11, 41]. The mouse kidney is capable of withstanding acute injury as tubular cells were thought to be replaced by de-differentiation and cell division [42]. However, a recent study has put this model into question by taking advantage of combination of cellular and genetic tools, including a tubular cell-lineage analysis, the FUCCI cell cycle reporter, and ploidy analysis [41]. As a result, tubular epithelial cells were found to endocycle in response to acute kidney injury and polyploid cell growth of tubular cells helped to restore renal function [41].
In the zebrafish heart epicardium, live cell imaging, unexpectedly, revealed that large, multinucleated polyploid epicardial cells form during heart regeneration (Figure 3) [11]. Both the endocycle and endomitosis contribute to generation of polyploid epicardial cells. However, after heart regeneration concluded the polyploid cells were cleared by apoptosis and replaced by dividing epicardial cells [11]. The upregulation of mechanical tension at the leading edge was found to be sufficient to drive endoreplication, suggesting that specific mechanical inputs could favor polyploid cell growth, instead of diploid cell division (Figure 3) [11]. Mechanical stimuli are known to be required for tissue morphogenesis during development by controlling cell proliferation, but the biophysical cues instructing polyploid cell growth in development and disease remain to be identified.
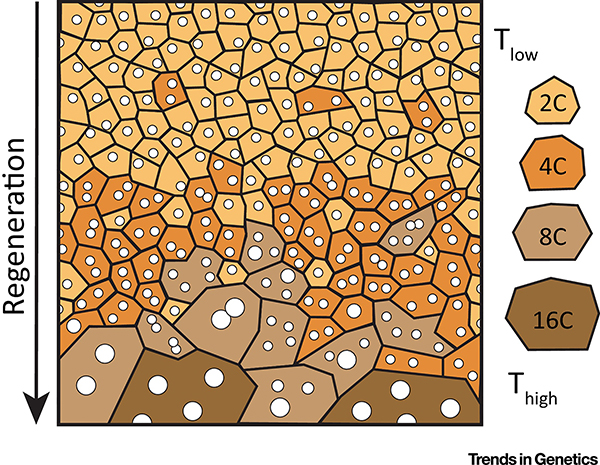
Figure 3. Mechanical tension is a driver for polyploid cell growth.
The zebrafish epicardium heals by cell division and polyploidization. The tissue is composed of mostly diploid cells (2C) prior to injury. During regeneration, higher tension (Thigh) occurs at the leading edge and is sufficient to induce polyploid growth by boosting the endocycle and endomitosis.
Polyploidy as a barrier to tissue repair
Damage to the adult mammalian heart results in permanent loss of myocardium and formation of fibrotic scar tissue that impairs heart function and increases the risk of heart failure [43]. The block in heart regeneration correlates with the onset of cardiomyocyte polyploidization at mouse postnatal day 7 [20, 44]. The cardiomyocytes are capable of limited cell cycle activity, where polyploidization predominates, yet is not sufficient to restore myocardial mass post injury [20, 44, 45]. In contrast, the adult zebrafish regenerates its myocardium through proliferation of preexisting diploid cardiomyocytes [46]. Genetically inducing cardiomyocyte polyploidization in zebrafish heart also impaired regeneration [47, 48]. Likewise, genetically selecting for inbred mouse strains with higher frequency of diploid cardiomyocytes was found to improve heart functional recovery after coronary artery ligation [48]. Taken together, cardiomyocyte polyploidization appears to be a barrier for heart repair. It is surprising that polyploidy acts as both a driver and repressor of tissue repair depending on the tissue/ organ context. For lost tissue mass to be regenerated, polyploid cells must retain the capacity to endoreplicate or divide (Figure 1B-1D). It remains to be determined if the impaired regenerative capacity of polyploid cardiomyocytes is due to the inhibition of polyploid growth and/ or division.
Restoring organ size via polyploid cell growth
The regulation of organ size (or mass) is essential to generating a functional and correctly proportioned tissue for both tissue growth and regeneration. A master regulator of organ size in both flies and mammals is the Hippo signal transduction pathway that allows tissues to grow during development and regrow during repair to defined sizes [49, 50]. Hippo pathway was originally discovered to control tissue growth by regulating cell division through Yki/ Yap (Drosophila/ mammalian) dependent transcription of cell survival and cell cycle genes [51]. It is now appreciated that Yki/ Yap also regulates endoreplication, thereby controlling organ size by regulating cell size.
In Drosophila development and wound repair, entry into the endocycle is dependent on Yki [8, 52]. The Yki-dependent induction of S phase genes induces entry into cell cycle, but expression of E3 ligase Fizzy-related (Fzr) bypasses M phase resulting in endocycle instead of mitosis (Figure 1D) [52, 53, 54]. In mice, Yap promotes polyploid cell growth by regulating Skp2, an E3 ligase that targets the CDI p27 for proteolytic degradation [55]. The accumulation of p27 causes hepatocytes to default into an endocycle and boost ploidy [56]. The regulation of polyploid cell growth by Hippo signaling is a crucial, but a more poorly understood factor in organ size control. It remains to be determined whether mechanical or other signals regulate the Hippo pathway to instruct the extent of polyploid growth required to develop and regrow tissues after injury.
These studies have revealed that a key detriment of whether polyploidy is used for tissue growth and repair is decided by the cell’s mitotic competence. Therefore, how feasible is it to switch between polyploid cell growth and diploid cell division? Some cell types have plasticity and can be switched by genetic or pharmacological regulation of the cell cycle [11, 54, 56, 57]. Remarkably, polyploid cell growth is sufficient to restore tissue mass without relying on cell division (Figure 4) [8, 11, 54, 56, 57]. However, switching mechanisms of tissue repair may not be feasible for all cell types. Genetically forcing adult Drosophila epithelial cells to divide impaired wound healing demonstrating that polyploid cell growth, in some tissues, may be the only tolerated mechanism to heal [53]. There may also be long-term detrimental consequences to switching from polyploidization to proliferative growth as mouse liver and Drosophila hindgut were found to be more sensitive to tumor growth upon oncogenic activation [22, 23, 54].
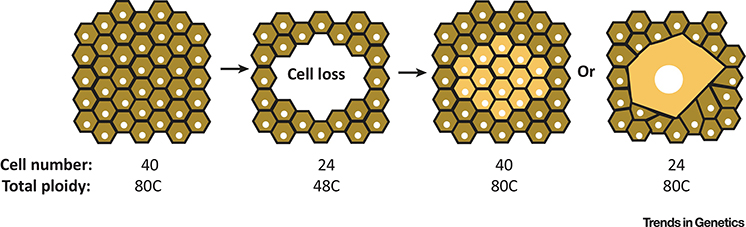
Figure 4. Organ size (mass) is determined by cell number or cell size.
Model illustrates how organ mass is restored by either cell proliferation (cell number) or polyploidization (cell size) following cell loss caused by various tissue insults, including injury or cell turnover as result of aging and disease. Polyploidization allows organ mass to be restored with fewer total cells as total ploidy of tissue remains unchanged.
Role of polyploidy in age-associated diseases
Polyploidization is known in many mammalian organs to increase with age, including in the liver, heart, brain, and eye [43, 58–60]. Polyploidy may accumulate with age due to the decline in cell proliferation and resident tissue stem cell loss. The increase in polyploidization with age could function as either a beneficial tissue repair strategy or a driver of disease. In the mammalian eye, cornea endothelial cells are frequently lost with age and disease including, Fuchs endothelial corneal dystrophy (FECD) [60, 61]. The surviving endothelial cells enlarge in size and often become multi-nucleated (Figure 2D). In a mouse model of FECD, polyploidization was found to accompany the enlargement of cornea endothelial cells [8]. Like in Drosophila and vertebrate models, polyploid endothelial cells precisely compensated for the endothelial cell loss, as total tissue ploidy did not change despite reduced endothelial cell number (Figure 2D and Figure 4) [8, 11, 54, 56, 57]. Therefore, corneal endothelial polyploidization serves as an example where age-associated polyploidy promotes repair by compensating for cell loss.
Polyploidy is more often than not associated with many age-associated diseases, including cancer. The discovery that tetraploidy predisposes cells to aneuploidy and tumor formation upon cell division, along with reports that most cancer cells are in a polyploid state has created renewed interest in polyploidy’s role in tumorigenesis [27, 28, 62]. The hallmarks of cancer cells, including DNA damage resistance and aneuploidy, are known traits that polyploid cells have acquired for physiological function.
Polyploid cells alter their genome copy number as regions of the genome have been found to be either amplified or underreplicated during the endocycle in both Drosophila cells and mouse giant trophoblast cells [63–67]. The re-replication of genome causes DNA damage due to stalled replication forks, yet p53-dependent DNA damage response is epigenetically silenced during polyploid growth [68–70]. The division competent rectal papillar cells are also able to divide in the presence of DNA damage as alignment and segregation of acentric chromosome fragments occurs via the Fanconi anemia protein, FANCD2 [71]. By becoming polyploid, cancer cells may exploit the survival and growth mechanisms that polyploid cells have adapted thereby driving tumorigenesis and disease.
Concluding Remarks and Future Perspectives
Polyploidization is a conserved part of our tissues’ ability to develop, grow, and heal. In the last five years, the mystery of polyploid function in health and disease has begun to be unraveled. Polyploid cells provide many advantages over their diploid counterparts. Polyploidy allows cell to grow by orders of magnitude, which is critical for tissue development and maintenance of tissue integrity. Final organ size can also be reached by polyploid cell growth and division. As a result, animal tissues can grow and regrow with fewer, yet larger cells. Conserved signal transduction pathways regulate endoreplication, but how a cell senses how big to grow remains unknown (see Outstanding Questions). Mechanical cues from the tissue environment instruct cell proliferation and may also direct polyploidization through inactivation of the Hippo signal transduction pathway. In addition, polyploid cells possess unique traits (DNA damage resistance and genome copy number variation), which may be exploited to drive cancer and other diseases. Continued research on the role and regulation of polyploidy in health and disease will uncover the versatility of polyploid cells to support life. In the future, the goal will be to exploit the advantages of polyploidy to improve our ability to heal and prevent disease, while identifying the mechanisms that restrict polyploidy when disease and tissue dysfunction arise.
Highlights:
Advances in imaging and fluorescent cellular markers has revealed that polyploidization is a common strategy for tissue growth and repair in many organisms and organs.
Polyploid cells grow in size via endomitosis, the endocycle, and/ or cell fusion, but can also retain the capacity to divide.
Hippo signaling and mechanical forces regulate polyploid cell growth to maintain and restore organ size.
Depending on the context, polyploidy either promotes repair and maintains homeostasis or impairs regeneration and drives disease.
Outstanding Questions:
How do cells decide to fuse or endoreplicate to generate a polyploid cell? Are there advantages and limitations of mono- and multinucleated polyploid cells for tissue function?
What determines whether polyploidy is a barrier or driver of tissue repair? Does cell cycle competence dictate the regenerative capacity of polyploid cells?
Does mechanical stimuli signal through Hippo pathway to regulate polyploid cell growth and control organ size? Do specific mechanical inputs favor polyploid cell growth, instead of diploid cell division?
How does polyploidy alter long-term tissue function, when polyploid cells arise in response to injury?
When polyploid cells arise with age do they serve to promote tissue repair or as a source for disease initiation?
Acknowledgments
K.J.G., R.B., and V.P.L are supported by NIH R35GM124691 and MDI Biological Laboratory.
Glossary Box:
- Aneuploidy
The cell’s total chromosome number is either increased or reduced compared to the biological number of chromosomes in the organism
- Amitosis
A cell division that occurs without spindle formation by cleavage of nucleus and division of the cytoplasm
- C-value
The total DNA content of a cell as measured by the total chromatin content. Haploid cells (i.e. sperm and egg) are 1C and diploid cells are 2C
- Endocycle
The process by which a cell replicates its genome through alternating G and S phases, but completely bypasses M phase of the cell cycle. Each endocycle doubles the C-value
- Endomitosis
A cell cycle that initiates but does not complete mitosis either due to a block in cytokinesis or earlier truncation of M phase
- Endoreplication
An incomplete cell cycle that replicates DNA during the S phase without the completion of mitosis or cytokinesis
- Polyploidy
A cell that has more than diploid copy of its genome. Polyploid cells arise by cell cycle dependent and independent mechanisms thereby generating both mono- or multinucleated cells
- Polytene
Large chromosomes composed of many copies of the DNA strands bound together by repeated rounds of DNA replication in the endocycle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ovrebo JI and Edgar BA, Polyploidy in tissue homeostasis and regeneration. Development, 2018. 145(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zielke N, et al. , Control of Drosophila endocycles by E2F and CRL4(CDT2). Nature, 2011. 480(7375): p. 123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lasek RJ and Dower WJ, Aplysia californica: analysis of nuclear DNA in individual nuclei of giant neurons. Science, 1971. 172(3980): p. 278–80. [DOI] [PubMed] [Google Scholar]
- 4.Hans F and Dimitrov S, Histone H3 phosphorylation and cell division. Oncogene, 2001. 20(24): p. 3021–7. [DOI] [PubMed] [Google Scholar]
- 5.Scholzen T and Gerdes J, The Ki-67 protein: from the known and the unknown. J Cell Physiol, 2000. 182(3): p. 311–22. [DOI] [PubMed] [Google Scholar]
- 6.Fox DT, Gall JG, and Spradling AC, Error-prone polyploid mitosis during normal Drosophila development. Genes Dev, 2010. 24(20): p. 2294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan AW, et al. , The ploidy conveyor of mature hepatocytes as a source of genetic variation. Nature, 2010. 467(7316): p. 707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losick VP, Jun AS, and Spradling AC, Wound-Induced Polyploidization: Regulation by Hippo and JNK Signaling and Conservation in Mammals. PLoS One, 2016. 11(3): p. e0151251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson GD, Simultaneous Assessment of Cardiomyocyte DNA Synthesis and Ploidy: A Method to Assist Quantification of Cardiomyocyte Regeneration and Turnover. J Vis Exp, 2016(111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knouse KA, et al. , Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc Natl Acad Sci U S A, 2014. 111(37): p. 13409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao J, et al. , Tension Creates an Endoreplication Wavefront that Leads Regeneration of Epicardial Tissue. Dev Cell, 2017. 42(6): p. 600–615 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielke N and Edgar BA, FUCCI sensors: powerful new tools for analysis of cell proliferation. Wiley Interdiscip Rev Dev Biol, 2015. 4(5): p. 469–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielke N, et al. , Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell reports, 2014. 7(2): p. 588–98. [DOI] [PubMed] [Google Scholar]
- 14.Edgar BA, Zielke N, and Gutierrez C, Endocycles: a recurrent evolutionary innovation for post-mitotic cell growth. Nat Rev Mol Cell Biol, 2014. 15(3): p. 197210. [DOI] [PubMed] [Google Scholar]
- 15.Orr-Weaver TL, When bigger is better: the role of polyploidy in organogenesis. Trends in genetics : TIG, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rios AC et al. Essential role for a novel population of binucleated mammary epithelial cells in lactation. Nat. Commun (2016) 7, 11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanet J, et al. , A mitosis block links active cell cycle with human epidermal differentiation and results in endoreplication. PLoS One, 2010. 5(12): p. e15701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HZ, et al. , Canonical and atypical E2Fs regulate the mammalian endocycle. Nat Cell Biol, 2012. 14(11): p. 1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandit SK, et al. , E2F8 is essential for polyploidization in mammalian cells. Nat Cell Biol, 2012. 14(11): p. 1181–91. [DOI] [PubMed] [Google Scholar]
- 20.Raulf A, et al. , Visualization of Cell Cycle Variations and Determination of Nucleation in Postnatal Cardiomyocytes. J Vis Exp, 2017(120). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matondo RB, et al. , Atypical E2f functions are critical for pancreas polyploidization. PLoS One, 2018. 13(1): p. e0190899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, et al. , The Polyploid State Plays a Tumor-Suppressive Role in the Liver. Dev Cell, 2018. 44(4): p. 447–459 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson PD, et al. , The polyploid state restricts hepatocyte proliferation and liver regeneration. Hepatology, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trakala M, et al. , Functional reprogramming of polyploidization in megakaryocytes. Dev Cell, 2015. 32(2): p. 155–67. [DOI] [PubMed] [Google Scholar]
- 25.Unhavaithaya Y and Orr-Weaver TL, Polyploidization of glia in neural development links tissue growth to blood-brain barrier integrity. Genes Dev, 2012. 26(1): p. 31–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Von Stetina JR, et al. , Variant cell cycles regulated by Notch signaling control cell size and ensure a functional blood-brain barrier. Development, 2018. 145(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coward J and Harding A, Size Does Matter: Why Polyploid Tumor Cells are Critical Drug Targets in the War on Cancer. Front Oncol, 2014. 4: p. 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu Z, Row S, and Deng WM, Endoreplication: The Good, the Bad, and the Ugly. Trends Cell Biol, 2018. 28(6): p. 465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knouse KA, et al. , Chromosome Segregation Fidelity in Epithelia Requires Tissue Architecture. Cell, 2018. 175(1): p. 200–211 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoenfelder KP, et al. , Indispensable pre-mitotic endocycles promote aneuploidy in the Drosophila rectum. Development, 2014. 141(18): p. 3551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stormo BM and Fox DT, Distinct responses to reduplicated chromosomes require distinct Mad2 responses. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duncan AW, et al. , Frequent aneuploidy among normal human hepatocytes. Gastroenterology, 2012. 142(1): p. 25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santaguida S et al. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell (2017) 41, 638–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenfelder KP and Fox DT, The expanding implications of polyploidy. J Cell Biol, 2015. 209(4): p. 485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losick VP, Fox DT, and Spradling AC, Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol, 2013. 23(22): p. 2224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Losick VP, Wound-Induced Polyploidy Is Required for Tissue Repair. Adv Wound Care (New Rochelle), 2016. 5(6): p. 271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamori Y and Deng WM, Tissue repair through cell competition and compensatory cellular hypertrophy in postmitotic epithelia. Developmental cell, 2013. 25(4): p. 350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyaoka Y, et al. , Hypertrophy and unconventional cell division of hepatocytes underlie liver regeneration. Current biology : CB, 2012. 22(13): p. 1166–75. [DOI] [PubMed] [Google Scholar]
- 39.Xiang J, et al. , EGFR-dependent TOR-independent endocycles support Drosophila gut epithelial regeneration. Nat Commun, 2017. 8: p. 15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lucchetta EM and Ohlstein B, Amitosis of Polyploid Cells Regenerates Functional Stem Cells in the Drosophila Intestine. Cell Stem Cell, 2017. 20(5): p. 609–620 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazzeri E, et al. , Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat Commun, 2018. 9(1): p. 1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomasova D and Anders HJ, Cell cycle control in the kidney. Nephrol Dial Transplant, 2015. 30(10): p. 1622–30. [DOI] [PubMed] [Google Scholar]
- 43.Laflamme MA and Murry CE, Heart regeneration. Nature, 2011. 473(7347): p. 326–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porrello ER, et al. , Transient regenerative potential of the neonatal mouse heart. Science, 2011. 331(6020): p. 1078–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hesse M, et al. , Direct visualization of cell division using high-resolution imaging of M-phase of the cell cycle. Nat Commun, 2012. 3: p. 1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poss KD, Wilson LG, and Keating MT, Heart regeneration in zebrafish. Science, 2002. 298(5601): p. 2188–90. [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Rosa JM, et al. , Myocardial Polyploidization Creates a Barrier to Heart Regeneration in Zebrafish. Dev Cell, 2018. 44(4): p. 433–446 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson M, et al. , Frequency of mononuclear diploid cardiomyocytes underlies natural variation in heart regeneration. Nat Genet, 2017. 49(9): p. 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaspar P and Tapon N, Sensing the local environment: actin architecture and Hippo signalling. Curr Opin Cell Biol, 2014. 31: p. 74–83. [DOI] [PubMed] [Google Scholar]
- 50.Hariharan IK, Organ Size Control: Lessons from Drosophila. Dev Cell, 2015. 34(3): p. 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh H and Irvine KD, Yorkie: the final destination of Hippo signaling. Trends in cell biology, 2010. 20(7): p. 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Djabrayan NJ, et al. , Specification of differentiated adult progenitors via inhibition of endocycle entry in the Drosophila trachea. Cell Rep, 2014. 9(3): p. 859–65. [DOI] [PubMed] [Google Scholar]
- 53.Grendler J, Lowgren S, Mills M, and Losick VP, Wound-induced polyploidization is driven by Myc and supports tissue repair in the presence of DNA damage. bioRxiv, 2018. [DOI] [PMC free article] [PubMed]
- 54.Cohen E, et al. , Fizzy-Related dictates A cell cycle switch during organ repair and tissue growth responses in the Drosophila hindgut. Elife, 2018. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang S, et al. , Hippo Signaling Suppresses Cell Ploidy and Tumorigenesis through Skp2. Cancer Cell, 2017. 31(5): p. 669–684 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diril MK, et al. , Cyclin-dependent kinase 1 (Cdk1) is essential for cell division and suppression of DNA re-replication but not for liver regeneration. Proc Natl Acad Sci U S A, 2012. 109(10): p. 3826–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lazzerini Denchi E, Celli G, and de Lange T, Hepatocytes with extensive telomere deprotection and fusion remain viable and regenerate liver mass through endoreduplication. Genes & development, 2006. 20(19): p. 2648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang MJ, et al. , Hepatocyte polyploidization and its association with pathophysiological processes. Cell Death Dis, 2017. 8(5): p. e2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andriani GA, Vijg J, and Montagna C, Mechanisms and consequences of aneuploidy and chromosome instability in the aging brain. Mech Ageing Dev, 2017. 161(Pt A): p. 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ikebe H, et al. , Age-dependent changes in nuclear DNA content and cell size of presumably normal human corneal endothelium. Experimental eye research, 1986. 43(2): p. 251–8. [DOI] [PubMed] [Google Scholar]
- 61.Vedana G, Villarreal G Jr., and Jun AS, Fuchs endothelial corneal dystrophy: current perspectives. Clin Ophthalmol, 2016. 10: p. 321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujiwara T, et al. , Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature, 2005. 437(7061): p. 1043–7. [DOI] [PubMed] [Google Scholar]
- 63.Hannibal RL and Baker JC, Selective Amplification of the Genome Surrounding Key Placental Genes in Trophoblast Giant Cells. Curr Biol, 2016. 26(2): p. 230236. [DOI] [PubMed] [Google Scholar]
- 64.Hannibal RL, et al. , Copy number variation is a fundamental aspect of the placental genome. PLoS Genet, 2014. 10(5): p. e1004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nordman J, et al. , Developmental control of the DNA replication and transcription programs. Genome Res, 2011. 21(2): p. 175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nordman JT, et al. , DNA copy-number control through inhibition of replication fork progression. Cell Rep, 2014. 9(3): p. 841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yarosh W and Spradling AC, Incomplete replication generates somatic DNA alterations within Drosophila polytene salivary gland cells. Genes Dev, 2014. 28(16): p. 1840–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang B, et al. , Low levels of p53 protein and chromatin silencing of p53 target genes repress apoptosis in Drosophila endocycling cells. PLoS Genet, 2014. 10(9): p. e1004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassel C, et al. , Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development, 2014. 141(1): p. 112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qi S and Calvi BR, Different cell cycle modifications repress apoptosis at different steps independent of developmental signaling in Drosophila. Mol Biol Cell, 2016. 27(12): p. 1885–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bretscher HS and Fox DT, Proliferation of Double-Strand Break-Resistant Polyploid Cells Requires Drosophila FANCD2. Dev Cell, 2016. 37(5): p. 444–57. [DOI] [PMC free article] [PubMed] [Google Scholar]