Abstract
Background
Candida bloodstream infections most often affect those already suffering serious, potentially life‐threatening conditions and often cause significant morbidity and mortality. Most affected persons have a central venous catheter (CVC) in place. The best CVC management in these cases has been widely debated in recent years, while the incidence of candidaemia has markedly increased.
Objectives
The main purpose of this review is to examine the impact of removing versus retaining a CVC on mortality in adults and children with candidaemia who have a CVC in place.
Search methods
We searched the following databases from inception to 3 December 2015: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid SP), EMBASE (Ovid SP), the Commonwealth Agricultural Bureau (CAB), Web of Science and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). We searched for missed, unreported and ongoing trials in trial registries and in reference lists of excluded articles.
Selection criteria
We searched for randomized controlled trials (RCTs) and quasi‐RCTs involving adults and children with candidaemia and in which participants were randomized for removal of a CVC (the intervention under study), irrespective of publication status, date of publication, blinding status, outcomes published or language.
However, two major factors make the conduct of RCTs in this population a difficult task: the large sample size required to document the impact of catheter removal in terms of overall mortality; and lack of economic interest from the industry in conducting such a trial.
Data collection and analysis
Our primary outcome measure was mortality. Several secondary outcome measures such as required time for clearance of blood cultures for Candida species, frequency of persistent candidaemia, complications, duration of mechanical ventilation and length of stay in the intensive care unit (ICU) and in the hospital were planned, as were various subgroup and sensitivity analyses, according to our protocol. We assessed papers and abstracts for eligibility and resolved disagreements by discussion. However, we were not able to include any RCTs or quasi‐RCTS in this review and, as a result, have carried out no meta‐analyses. However, we have chosen to provide a brief overview of excluded observational studies.
Main results
We found no RCT and thus no available data for evaluation of the primary outcome (mortality) nor secondary outcomes or adverse effects. Therefore, we conducted no statistical analysis.
A total of 73 observational studies reported on various clinically relevant outcomes following catheter removal or catheter retention. Most of these excluded, observational studies reported a beneficial effect of catheter removal in patients with candidaemia. None of the observational studies reported results in favour of retaining a catheter. However, the observational studies were very heterogeneous with regards to population, pathogens and interventions. Furthermore, they suffered from confounding by indication and an overall high risk of bias. As a consequence, we are not able to provide recommendations or to draw firm conclusions because of the difficulties involved in interpreting the results of these observational studies (very low quality of evidence, GRADE ‐ Grades of Recommendation, Assessment, Development and Evaluation Working Group).
Authors' conclusions
Despite indications from observational studies in favour of early catheter removal, we found no eligible RCTs or quasi‐RCTs to support these practices and therefore could draw no firm conclusions. At this stage, RCTs have provided no evidence to support the benefit of early or late catheter removal for survival or other important outcomes among patients with candidaemia; no evidence with regards to assessment of harm or benefit with prompt central venous catheter removal and subsequent re‐insertion of new catheters to continue treatment; and no evidence on optimal timing of insertion of a new central venous catheter.
Plain language summary
Central venous catheter removal for adults and children suffering from bloodstream infections caused by Candida species
Review question
The main purpose of this review was to examine the impact of prompt removal of a central venous catheter (CVC) on the survival of patients with Candida species in the bloodstream (candidaemia) compared with keeping the CVC in place when treating with antifungal agents.
Background
A CVC is placed into a large vein to administer medications or fluids that cannot be taken by mouth or would harm a smaller peripheral vein. Catheters can be placed in veins in the neck, chest or groin, or through veins in the arms (peripherally inserted central catheters, also known as PICC lines). Candida (a genus of yeast) can be found in blood samples taken from the catheter and may cause acute, critical illness and even death in people already suffering from other diseases. Infections caused by Candida have markedly increased in numbers over past decades. Candida is now the fourth most common bloodstream infection contracted by people already in hospital. This type of infection considerably increases hospital costs.
Prompt catheter removal is recommended by international specialist societies. However, the catheter often provides important access for medical or fluid therapy for treating other illnesses. If a catheter is removed, then a new one is often required, and this can cause distress for the patient. Any time gap between removal of one catheter and insertion of a new catheter may interfere with treatment, leading to worsening of the situation. Additionally, inserting a new catheter is associated with risk of complications arising from accidental damage to large blood vessels, potentially causing severe bleeding or accidental puncture of a lung, causing the lung to collapse. Although rare, these complications may ultimately lead to death.
Search date
The evidence was up to date as of 3 December 2015.
Study characteristics
We found no clinical trials with a randomized controlled design that evaluated this topic and measured the number of deaths or any of our secondary outcomes.
We identified 73 observational studies that delivered descriptive data on catheter management and survival in people with bloodstream infections caused by Candida.
Key results
We identified no randomized controlled trials for statistical analyses and assessments. Therefore, we can present no results on the effect of catheter removal on survival when Candida is found in the bloodstream.
A total of 73 observational studies reported relevant outcomes after the catheter was removed or was kept in place. In all, 40 studies reported a beneficial effect of catheter removal in patients with candidaemia, and 34 presented results showing no clear differences between groups. No studies reported results in favour of retaining the catheter.
We found no reports on the harmful effects of removing a catheter and re‐inserting a new catheter.
Quality of evidence
No randomized controlled trials met the inclusion criteria. Consequently, we cannot assess the quality of evidence.
Background
Invasive Candida infections have markedly increased in frequency during the 1990s to become the fourth most common cause of nosocomial bloodstream infection (Colombo 2006; Edmond 1999). The estimated additional cost of an episode of candidaemia in adults is approximately USD 40,000 (Fridkin 2005; Morgan 2005). Most persons with candidaemia have a central venous catheter (CVC) in place, and the best CVC management in these patients has been highly debated (Nucci 2010; Pasqualotto 2008; Raad 2004).
Previous studies have shown that retention of vascular catheters colonized with Candida species is associated with prolonged fungaemia (Girmenia 1996; Rex 1995), increased risk of metastatic complications (Girmenia 1996; Lecciones 1992; Rex 1995) and death in adults with candidaemia (Asmundsdottir 2005; Lecciones 1992; Raad 2004). Other investigations have failed to confirm the benefit of early CVC removal (Nucci 2010; Rodriguez 2007). Removal of vascular catheters has been advocated as an adjunctive strategy for treating persons with candidaemia, particularly among non‐neutropenic adults (Mermel 2009; Pappas 2003; Pappas 2009).
However, other variables may impact the outcome, particularly severity of illness and persistence of neutropenia (Nucci 2002). A policy of systematic CVC removal in persons with candidaemia may result in mechanical complications associated with insertion of a new catheter, including bleeding, pneumothorax and eventually death. Although most international societies and experts recommend catheter removal in this scenario (Pappas 2009), no clinical trial has ever documented a survival benefit resulting from this intervention.
Description of the condition
Candidaemia describes the presence of any fungus of the species Candida in the bloodstream. It is a potentially devastating infection that predominantly affects severely ill, hospitalized people. Most studies report a relatively low prevalence (Blumberg 2001; Marchetti 2004; Petri 1997; Tortorano 2006), but an incidence as high as 9.8/1000 intensive care unit (ICU) admissions has been reported (Rangel‐Frausto 1999). Severity is inarguable, with reported crude mortality rates ranging from 30% to 60% and reported attributable mortality rates ranging from 25% to 40% (Blot 2002; Gudlaugsson 2003; Voss 1997; Wey 1988; Wisplinghoff 2004; Zaoutis 2005). Major risk factors include recent abdominal surgery, gastrointestinal perforation, compromised immune function, treatment with broad‐spectrum antibacterial agents, presence of CVC, major organ dysfunction, malignancy and extremes of age (Glockner 2013).
Candidaemia requires systemic antifungal treatment aimed at eradication of free‐floating Candida species as well as any primary focus or secondary manifestation. As mentioned, most people presenting with candidaemia have a CVC in place (Nucci 2010; Raad 2004); this evokes the controversial and much debated issue of whether it should be removed.
Description of the intervention
Removal of an indwelling CVC is a common and widely advocated strategy when candidaemia is suspected or diagnosed (Mermel 2009; Pappas 2009). A new CVC may be inserted immediately as a replacement if required for treatment. CVC removal may be performed as a sole intervention or may be done as part of a strategy in which all indwelling catheters are removed and possibly replaced.
Despite conflicting evidence, one might argue that the a priori possibility that this intervention will be effective in CVC‐related infection is considerable. However, it is not possible to formally categorize candidaemia as CVC‐related without removing the CVC in question, as this requires detection of a significant quantum of Candida species on the catheter tip.
Removal may be done early or late following the diagnosis of candidaemia. For the purposes of this review, we will consider removal on day zero or day one following the diagnosis of candidaemia as early, and removal from day two to day seven as late.
For comparison, a CVC may be retained in candidaemia while relevant treatment is initiated.
How the intervention might work
Similar to many other micro‐organisms, Candida species may produce and embed themselves within a protective biofilm. Biofilm acts both as a mechanical barrier and as an environment for genetic exchange, thereby contributing to protection from elimination by the innate host immune defence and to emerging antibiotic resistance (Raad 1993).
Most vascular devices develop biofilm within 24 hours after insertion (Raad 1993), and the occurrence of catheter‐related bloodstream infection is proportionate to the presence of micro‐organisms on the catheter tip. In case of catheter‐related candidaemia, removal of a catheter will eliminate the primary focus of infection and will prevent micro‐organisms embedded in the biofilm from further detachment of planktonic pathogens, embolization, establishment of metastatic infection and maintenance of systemic infection (Leonidou 2010; Schachter 2003). In cases of candidaemia not primarily related to an indwelling device, removal of such a device may prevent Candida species from embedding themselves in pre‐existing or new biofilm on this device.
Also, an indwelling device presents risk of complicating superinfections through extraluminal or intraluminal contamination (Miller 2012), which may negatively affect outcomes in those already struggling with candidaemia.
On the other hand, candidaemia always requires systemic antifungal treatment, which involves continuous intravenous access. Persons with candidaemia may require inotropics, haemodynamic monitoring or infusion of fluids or parenteral nutrition during illness, prompting insertion of a new CVC if one has been removed. This procedure involves risks of mechanical (bleeding, arterial puncture, pneumothorax, haemothorax) and infectious complications, which may negatively affect outcomes.
Why it is important to do this review
The issue of whether catheters should be removed from adults and children with candidaemia remains controversial.
Objectives
The main purpose of this review is to examine the impact of removing versus retaining a central venous catheter (CVC) on mortality in adults and children with candidaemia who have a CVC in place.
Methods
Criteria for considering studies for this review
Types of studies
We searched for randomized controlled trials (RCTs), irrespective of publication status, date of publication, blinding status or language. We planned to contact investigators and study authors to retrieve relevant data. We aimed to include unpublished trials only if trial data and methodological descriptions were provided in written form or could be retrieved from the study authors. We planned to include quasi‐randomized trials because of the expected low number of trials that could be included in the review, but we had no intention of including cross‐over trials or observational studies.
However, two major factors make the conduct of RCTs in this population a difficult task: the large sample size required to document the impact of catheter removal in terms of overall mortality; and lack of economic interest from the industry in conducting such a trial.
We had no knowledge of any existing RCTs exploring this subject and anticipated that we would find none. We did not plan to include non‐randomized studies but planned to provide a description of these studies and their results in the additional tables.
Types of participants
We planned to include participants of all ages with candidaemia who had a CVC in place. We excluded data from participants who did not have candidaemia (e.g. other forms of invasive Candida infection such as Candida peritonitis) and from individuals with no CVC in place. We considered only individuals for whom information about CVC management was available. We included participants irrespective of their underlying disease.
We searched papers considered eligible for assessment for the following data for each participant.
Demographic information.
Main underlying diseases.
Data on neutropenia.
Severity of illness.
CVC data and management.
Data on candidaemia.
Antifungal treatment.
Outcomes.
Demographic information included age and sex. Main underlying diseases included solid organ transplantation, haematopoietic stem cell transplantation, AIDS, diabetes mellitus, solid cancer and haematological neoplasm. We recorded abdominal surgeries performed during the two weeks preceding diagnosis of candidaemia, as well as receipt of steroids.
We defined the presence and duration of neutropenia by using an absolute neutrophil count ≤ 500 cells/µL in the last 30 days. We considered neutropenia to have persisted if the neutrophil count did not recover (i.e. with increases above 500 cells/µL) during the week following diagnosis of candidaemia.
We planned to obtain the following variables to determine the severity of candidaemia: Acute Physiology And Chronic Health Evaluation (APACHE II) score, stay in the ICU, shock requiring inotropic support, respiratory failure requiring invasive mechanical ventilation, renal failure (serum creatinine ≥ 2 mg/dL), renal failure requiring dialysis and liver insufficiency (aminotransferases or bilirubins above five or 10 times the upper limit of detection, respectively). We will collect these variables when we obtain the index blood culture.
CVC data include short versus long permanence of CVC and time taken for CVC removal. Both would be considered in relation to the date the blood culture was obtained and the date antifungal therapy was initiated. The diagnosis of candidaemia will be established when Candida species are recovered from a blood culture taken from an individual with sepsis. We will calculate duration of candidaemia and time taken for CVC removal from the day the first positive blood culture for Candida was obtained. We planned to stratify participants as having candidaemia lasting for: (1) three or fewer days; (2) four to seven days; and (3) longer than seven days. We planned to record the Candida species causing candidaemia.
We considered candidaemia to be CVC‐related if significant growth of Candida species was documented from the catheter tip. This could be determined by semi quantitative (> 15 colony‐forming units (CFUs)/catheter segment) or quantitative (> 103 CFUs/catheter segment) cultures. We did not consider differential time to positivity between blood taken from central lines and blood taken from peripheral veins for the diagnosis of CVC‐related candidaemia because this strategy has been validated only for use with bacterial infection (Mermel 2001; Mermel 2009).
We planned to stratify participants according to the antifungal drug they received because some drug classes (e.g. echinocandins, polyenes) are known to have antibiofilm activity. We aimed to collect data on the appropriateness of antibacterial therapy for candidaemic participants with a concomitant bacterial bloodstream infection. We considered therapy as appropriate if the prescribed antibacterial drug was shown to be active against bacteria isolated in the blood culture.
We aimed to record time to death and time to hospital discharge in the case of survivors. For the purpose of survival analysis, we planned to censor participants at week six after the diagnosis of candidaemia.
Types of interventions
For the purpose of this review, we considered CVC removal as removal or replacement of all central venous lines within seven days of the diagnosis of candidaemia (date on which the positive blood culture for Candida was drawn). This criterion would not apply when CVCs were exchanged over a guidewire; we planned to analyse these cases separately.
We considered a CVC not removed within seven days after the diagnosis of candidaemia to be a comparison.
Types of outcome measures
Primary outcomes
Overall mortality. We planned to use the longest follow‐up data from each trial, regardless of the duration of follow‐up*.
Secondary outcomes
Time required for clearance of blood culture for Candida species*.
Frequency of persistent candidaemia (defined as any blood culture that remained positive for Candida species after three days of effective antifungal therapy)*.
Complications probably related to candidaemia (metastatic foci of infection including endocarditis, endophthalmitis and hepatosplenic candidosis)*.
Complications probably related to the intervention: local suppurative and mechanical complications (e.g. pneumothorax, arterial puncture or bleeding requiring blood transfusion).*
Complications during in‐patient stay not specific to the trial intervention (e.g. pneumonia, congestive cardiac failure, respiratory failure, renal failure).
Duration of mechanical ventilation*.
Length of stay in the ICU*.
Length of stay in the hospital*.
Species‐related mortality.
* Indicates key outcomes that we planned to include in a 'Summary of findings' table for the review.
Search methods for identification of studies
We conducted searches to identify all published and unpublished studies evaluating CVC removal in participants with candidaemia. We applied no language restrictions; when necessary, we translated papers written in languages other than English. We planned to contact study authors and drug companies to obtain additional data from the selected trials but found no additional studies of relevance.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12) (Appendix 1); MEDLINE (interface PubMed) (1966 to 3 December.2015) (Appendix 2); EMBASE (1966 to 3 December.2015) (Appendix 3); Latin American Caribbean Health Sciences Literature (LILACS) (1982) (Appendix 4); Institute for Scientific Information (ISI) Web of Knowledge (1945 to 3 December.2015 ) (Appendix 5); and SCOPUS (1960 to 3 December.2015) (Appendix 6). We combined the strategies described in Section 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to search for RCTs in MEDLINE and EMBASE. We checked the reference lists of all retrieved studies of interest for additional relevant studies. Additionally, we checked all references of relevant reviews, society guidelines and commentaries identified in both PubMed and EMBASE.
Searching other resources
We handsearched the reference lists of reviews, randomized and non‐randomized studies and editorials to locate additional studies. We were not able to retrieve additional information from pharmaceutical companies nor from experts in the field. We searched for ongoing clinical trials and unpublished studies at the following Internet sites.
Current Controlled Trials (http://www.controlled‐trials.com).
ClinicalTrials.gov (http://clinicaltrials.gov).
CenterWatch (http://www.centerwatch.com).
Data collection and analysis
We used the standard methods of the Cochrane Anaesthesia, Critical and Emergency Care Group (ACE) to identify studies and to assess the methodological quality of eligible trials. We used the Review Manager statistical package (RevMan 2014) provided by The Cochrane Collaboration to analyse the data. We considered the frequency of autopsy and the frequency of daily blood culture in the five days following candidaemia and the percentage of participants excluded after screening for the purpose of quality evaluation.
Selection of studies
We searched for RCTs and quasi‐RCTs involving adult participants with candidaemia, and in which participants were randomized for CVC removal (the intervention under study). As already mentioned, we planned to select trials irrespective of their original language.
We independently read all abstracts in the records retrieved by our electronic search to identify eligible publications. We selected studies to be reviewed according to pre‐specified inclusion criteria. We completed this process without blinding of study authors, institution, journal of publication or results. We resolved disagreements by discussion, and if no agreement could be found, we planned to consult a third independent person from The Cochrane Collaboration.
We provide in the review a detailed description of this search and assessment in the form of a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow chart (Figure 1).
1.
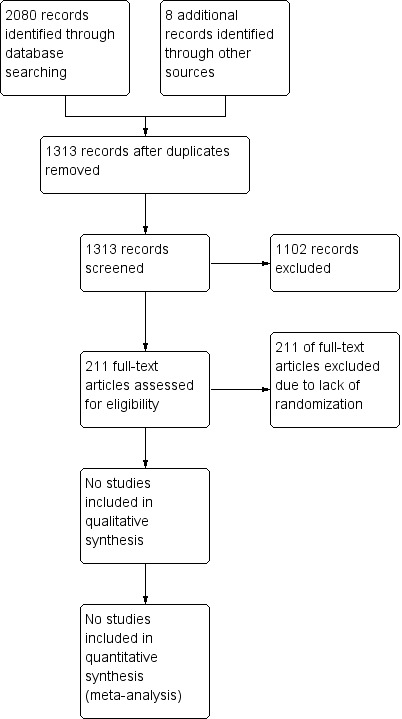
Study flow diagram.
Data extraction and management
We assessed the quality of eligible trials using criteria described by the Cochrane Effective Practice and Organisation of Care Group (EPOC) (Reeves 2008). We planned to independently extract data using a data extraction sheet developed for the purposes of this review (Appendix 7). We aimed to conduct an individual patient data (IPD) meta‐analysis for a subgroup of trials evaluating specific outcomes in the more homogeneous populations described below.
For each of these trials, we planned to record the following data.
Year of publication, country of origin and source of study funding.
Details of participants including demographic characteristics and criteria for inclusion.
Details of types of interventions.
Details of outcomes reported, including method of assessment and time intervals.
Individual patient data (IPD)
We aimed to contact the investigators of selected trials by email or by telephone to invite them to contribute individual patient data (IPD). We hoped to include in the review data from studies that did not provide IPD. In such cases, we planned to obtain aggregate data and to combine these with IPD.
Assessment of risk of bias in included studies
We independently assessed the risk of bias without blinding using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion, and when we could not reach agreement, we planned to consult a third person from The Cochrane Collaboration. We planned to assess each domain systematically, as described in Appendix 8.
Measures of treatment effect
We planned to calculate risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous data (binary outcome).
We planned to calculate mean differences (MDs) with 95% CIs for continuous data if they were measured the same way. To combine trials that measured the same outcome but in different ways, we planned to measure standardized mean differences (SMDs).
Unit of analysis issues
Cluster‐randomization
We planned to exclude cluster‐randomized trials, as factors related to clustering may contribute to outcomes.
Multiple intervention groups
In studies with multiple intervention groups, we planned to combine groups to create a single pair‐wise comparison (Higgins 2011).
We considered unit of analysis issues related to cross‐over trials, recurring events, repeated observations, multiple treatment attempts and interventions on multiple body parts to be not relevant to this intervention and outcome.
When meta‐analysis is used in combining results from several studies with binary outcomes (i.e. event or no event), adverse effects may be rare but serious, and hence may be important (Sutton 2002). Most meta‐analytical software does not include trials with 'zero event' in both arms (intervention vs control) when RR is calculated. Exempting these trials from calculation of RR and 95% CI may lead to overestimation of the treatment effect. The Cochrane Collaboration recommends applying the Peto odds ratio (OR) as the best method of estimating OR when many trials with no events in one or both arms are included (Higgins 2011). However, the Peto method is generally less useful when trials are small, or when treatment effects are large. We planned to conduct a sensitivity analysis by applying the Peto OR if this sensitivity analysis was seen as a valid option.
In a single trial, interim analysis increases the risk of type 1 errors. To avoid type 1 errors, group sequential monitoring boundaries (Lan 1983) are applied to reveal whether a trial could be terminated early because of a sufficiently small P value, that is, the cumulative z‐curve crosses monitoring boundaries. Sequential monitoring boundaries, called trial sequential monitoring boundaries, can be applied to meta‐analysis as well.
In trial sequential analysis (TSA), the addition of each trial to a cumulative meta‐analysis is regarded as an interim meta‐analysis and helps the investigator to decide whether additional trials are needed. The idea behind TSA is that if the cumulative z‐curve crosses the boundary, a sufficient level of evidence is reached, and no further trials are needed. If the z‐curve does not cross the boundary, evidence is insufficient to allow investigators to reach a conclusion. To construct trial sequential monitoring boundaries, the information size is required and is calculated as the smallest number of participants needed in a well‐powered single trial (Brok 2008; Pogue 1997; Pogue 1998; Wetterslev 2008; Wetterslev 2009).
We planned to apply TSA (TSA 2010) because this would prevent an increase in the risk of type 1 errors (< 5%) as the result of potential multiple updating and sparse data in a cumulative meta‐analysis, and would provide important information needed to estimate the level of evidence for the experimental intervention. Additionally, TSA provides important information regarding the need for additional trials and the required information size. We wanted to perform TSA in anticipation of an intervention effect, as indicated by the trials included in the traditional meta‐analysis, or even the intervention effect suggested by the upper confidence limit from the intervention effect estimate found in the traditional meta‐analysis, to cover any uncertainty displayed by the present data.
We aimed to calculate the diversity‐adjusted required information size by using the pooled variance from the traditional meta‐analysis (Turner 2013; Wetterslev 2009), as well as the control event proportion from the meta‐analysis of included trials.
Dealing with missing data
We planned to contact the corresponding authors of all studies with missing data in an attempt to retrieve the relevant data. For all included studies, we planned to note the number of exclusions and whether they were accounted for, and to assess the risk of attrition bias. In cases of missing data, we would choose a 'complete case analysis' for our primary outcome, which simply excludes from the analysis all participants with missing outcomes.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by examining types of participants, interventions and outcomes in each study. As a preliminary assessment of heterogeneity, we planned to examine statistical heterogeneity between the summary statistics of different studies by checking the usual statistical test in which P values were obtained by comparing the distribution of the Chi2 statistic. We aimed to take care in interpreting the Chi2 statistical test, as this has limited power in the (common) situation in which trials have a small sample size or are few in number. We would also assess statistical heterogeneity with the I2 statistic, thereby estimating the percentage of total variance across studies that is due to heterogeneity rather than to chance (Higgins 2002). We considered a value greater than 40% as definitely considerable if it is also significant. In combined analysis of IPD and abstracted data, as well as sensitivity analysis with IPD data only, we planned to use co‐variates and random study effects to attempt to explain between‐study heterogeneity.
Assessment of reporting biases
Selective outcome reporting occurs when non‐significant results are selectively withheld from publication (Chan 2004). It is defined as the selection, on the basis of results, of a subset of original variables recorded for inclusion in publication of trials (Hutton 2000). In future updates, we will check publications against their protocols or official registrations of trials when available, in an attempt to detect possible selective outcome reporting.
Publication bias arises when dissemination of research findings is influenced by the nature and direction of results (Higgins 2011).
In future updates, we will evaluate the level of publication bias related to the included trials by providing a funnel plot. For studies with binary outcomes, we will apply the test proposed in Rucker 2008. This test has the advantage of including trials with no events.
In future updates, if the number of included trials does not exceed 10, we will not carry out these tests, as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Funding bias is related to possible delay or discouragement to publish undesired results in trials sponsored by the industry (Higgins 2011). To explore the role of funding, in future updates we will conduct a sensitivity analysis based on our primary endpoint.
Data synthesis
We planned to perform the analysis by using Review Manager software (RevMan 2014) and other software if needed. As a general rule, we planned to use a random‐effects model because we did not expect an identical treatment effect across studies, and we intended to draw conclusions for the general population rather than only for participants in the included studies. We intended to compare outcomes across trials and treatment regimens to assess clinical heterogeneity and to compare patient populations. We planned that comparisons between health outcomes would be restricted by the different measurement tools and methods of reporting used in the included trials.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for subgroups of participants and for subgroups of the intervention by including the variables as listed before (see Types of participants).
Sensitivity analysis
We planned to perform sensitivity analyses of trials with low risk of bias versus high risk of bias. If evidence of small‐study effects was observed, we would also perform sensitivity analyses. We planned to test the robustness of results by repeating the analysis using different measures of effect size (e.g. RD (Risk Difference), OR (Odds Ratio)) and different statistical models (fixed‐effect and random‐effects models).
Summary of findings
We planned to use the principles of the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) system (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (overall mortality, duration of candidaemia, frequency of persistent candidaemia, incidence of metastatic infection, local suppurative/mechanical complications, length of hospital/ICU stay and species‐related mortality) in our review, and we planned to construct a 'Summary of findings' table using GRADE software. The GRADE approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence takes into consideration within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
By conducting electronic searches and reading the references of potentially relevant articles, we identified 1313 publications. We found no eligible studies. We reviewed a total of 211 publications in full text; 73 of these reported a relevant outcome following removal or retention of a catheter. We have provided a narrative or descriptive overview of these 73 papers in the Characteristics of excluded studies table and in additional tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Figure 1).
1. Table of excluded studies ‐ Prospective studies of adult populations.
| Prospective studies – adult populations | ||||||||||
| Author |
Population (study period) |
Sample size total (CVCs) | Primary intervention | Outcome | Catheter management intervention after positive blood culture | Analysis and results |
Overall mortality |
Results in favour of |
STROBE compatibility |
Comments |
| Chakrabarti 2015 | Adults with intensive care unit (ICU)‐acquired candidaemia (2011 to 2012) |
913 (676) | Systematic epidemiological study on (ICU)‐acquired candidaemia across India | 30‐Day mortality | Catheter removal AND antifungal treatment | Multi‐variate: odds ratio (OR) 0.39, 95% CI 0.18 to 0.84 P value = 0.016 |
0.45 | Removal | Yes | Analysis solely of participants subjected to both catheter removal and antifungal treatment No participants were excluded from analysis |
| Nucci 2010 | Adults with candidaemia and central venous catheter already included in 1 of 2 clinical trials (2003 to 2006) |
842 (842) | 2‐Arm study: micafungin at 100 mg/d or liposomal amphotericin B at 3 mg/kg/d and 3‐arm study: micafungin (100 mg daily) vs micafungin (150 mg daily) vs caspofungin (70 mg followed by 50 mg daily) | 28‐ and 42‐day survival | Removal within 48 hours | Multi‐variate: 28 days: OR 1.23, 95% CI 0.85 to 1.75, P value = 0.27 42 days: OR 1.25, 95% CI 0.88 to 1.75, P value = 0.20 |
0.32 | Not significant | Yes. Limited discussion of bias |
2 phase 3, multi‐centre, double‐blind, randomized, controlled trials. Participants included based on positive blood culture + ≥ 1 dose of study drug Results of 2‐arm study: finds that micafungin is non‐inferior to liposomal amphotericin B (Kuse 2007) Results of 3‐arm‐study: finds that micafungin in both doses is non‐inferior to caspofungin (Pappas 2007) |
| Garnacho‐Montero 2013 | Adults with candidaemia (2004 to 2009) |
188 (188) | Cohort | Mortality | Removal within 48 hours | Multi‐variate: hazard ratio (HR) 0.34, 95% CI 0.16 to 0.70, P value = 0.030 |
0.36 | Removal | Yes. Limited discussion of bias |
Participants were excluded from analysis if they died before day 2 after candidaemia diagnosis |
| Erard 2010 | Candidaemic participants in 27 Swiss hospitals (2004 to 2006) |
567 (567) |
Cohort | Crude mortality and attributable mortality (AM) | Catheter removal within 3 days | Multi‐variate: Increased AM OR 4.07, 95%CI 1.5 to 10.6, P value not provided |
0.41 | Removal | No. Eligibility criteria not defined | Participants were excluded from analysis if they did not receive antifungal treatment |
| Patel 2005 | Adults with candidaemia (2002 to 2003) |
119 (105) | Cohort | 6‐Week mortality | Removal within 24 hours | Multi‐variate: data not provided. Cited as non‐significant |
0.32 | Not significant | Yes | Participants were excluded from analysis if they died before blood cultures became positive for Candida or before antifungal treatment was initiated |
| Kuse 2007 | Adults with candidaemia (2003 to 2004) |
392 (277) | 2‐Arm study: micafungin 100 mg/d or liposomal amphotericin B 3 mg/kg/d | Investigator’s assessment of overall treatment success | Catheter removal | Uni‐variate: data not provided. Cited as non‐significant |
N/A | Not significant | No. RCT reporting subsidiary outcome not specified in methods | Double‐blind, randomized non‐inferiority study Removal of catheters was recommended and was to be done before the first dose of study drug was administered (constitute part of study byNucci 2010) Conclusion: finds that micafungin is non‐inferior to liposomal amphotericin B |
| Marriott 2009 | Non‐neutropenic adults with ICU‐acquired candidaemia (2001 to 2004) |
183 (199) | Cohort | 30‐Day mortality | Catheter removal | Uni‐variate: OR 0.41, 95% CI 0.26 to 0.67, P value < 0.001 |
0.56 | Removal | Yes | |
| Puig‐Asensio 2014a | Adults with candidaemia in ICU (CANDI‐POP) (2010 to 2011) |
168 (159) | Cohort | 7‐ and 30‐day mortality | Catheter removal within 48 hours | Uni‐variate: OR 0.41, 95% CI 0.17 to 1.01, P value = 0.054 |
0.47 | Not significant | Yes | Population‐based surveillance programme. No participants were excluded from analysis |
| CANDI‐POP: “Estudio Poblacional prospectivo sobre candidaemia en España”; CI: confindence interval; HR: hazard ratio; ICU: intensive care unit; mg: milligrams; N/A: not available; OR: odds ratio; STROBE: STrengthening the Reporting of OBservational studies in Epidemiology, strobe‐statement.org; vs: versus | ||||||||||
2. Table of excluded studies ‐ Prospective studies of mixed populations including both adult and paediatric cases.
| Prospective studies – populations of all ages | |||||||||
| Author |
Population (study period) |
Sample size (CVCs) | Outcome | Catheter management intervention | Analysis and results | Overall mortality | Results in favour of | STROBE compatibility | Comments |
| Puig‐Asensio 2014b | All participants with candidaemia + consent in the Barcelona area (2010 to 2011) |
729 (575) |
Early mortality (7 days + late mortality (8 to ‐30 days)) | Catheter removal within 48 hours | Multi‐variate: early mortality (0 to 7 days): OR 0.43, 95% CI 0.21 to 0.87, P value = 0.019; late mortality (8 to 30 days): OR 0.72, 95% CI 0.43 to 1.22, P value = 0.222 |
0.31 | Removal (outcome: early mortality) Not significant (outcome: late mortality) |
Yes | For early mortality, it was decided a priori that antifungal treatment and CVC removal would remain in the final multi‐variate analysis. No participants were excluded from analysis |
| Almirante 2006 | All cases in 14 hospitals in Barcelona (2002 to 2003) |
341 (302) |
Mortality on days 3 to ‐7 | Catheter removal | Multi‐variate: OR 0.3, 95% CI 0.1 to 0.9, P value = 0.04 |
N/A | Removal | Yes | Participants were excluded from analysis if they died on day 1 or 2 after diagnosis |
| Weinberger 2005 | All cases of candidaemia in 3 Israeli hospitals (1995 to 2000) |
272 (188) |
30‐Day mortality | Catheter removal | Multi‐variate: OR 0.38, 95% CI 0.14 to 1.04, P value = 0.06 |
0.36 | Not significant | No. Design not clear from title or abstract | No participants were excluded from analysis. Timing of catheter removal not documented Participants in neonatal ICU excluded owing to outbreak |
| Talarmin 2009 | All participants with candidaemia in 17 hospitals in France (2004) |
186 (135) |
30‐Day mortality | Catheter removal | Multi‐variate: OR 0.24, 95% CI 0.10 to 0.57, P value = 0.001 |
0.49 | Removal | Yes. Limited discussion of bias | No participants were excluded from analysis |
| Nucci 1998 | All participants with candidaemia in 6 tertiary hospitals in Brazil (22 months, not specified) |
145 (117) |
Mortality | Catheter retention | Multi‐variate: OR 4.81, 95% CI not provided, P value < 0.0001 |
N/A | Removal | No. Time period and follow‐up not specified | No participants were excluded from analysis |
| Bigni 2007 | Hospitalized participants with haematological malignancies in 1 hospital in Brazil (2001 to 2005) |
77 (N/A) |
Mortality | Catheter retention | Multi‐variate: adults: OR 6.41, 95% CI 1.04 to 39.55 |
0.47 | Removal | No. Design not clear from title | No participants were excluded from analysis Analysis of 47 adult cases. Results were non‐significant for paediatric cases |
| Kabbani 2014 | Laboratory surveillance programme, Georgia/Maryland, USA (2008 to 2013) |
3782 (84.6%) |
30‐Day mortality | 7 days | Univariate: adults: OR 0.25, 95% CI 0.21 to 0.3, P value not provided |
0.25 | Removal | No. Design not clear from title. Eligibility criteria not defined. Statistical methods vaguely described | No participants were excluded from analysis In paediatric cases, catheter removal was associated with lower odds of death (data not provided) |
| Nguyen 1995 | Observational study of participants receiving amphotericin B or fluconazole in 4 university hospitals, USA (1990 to 1994) |
427 (360) |
Mortality rate | Vascular catheter retention | Multi‐variate: mortality: retention 41%, removal 21%, 95% CI not provided, P value < 0.001 Microbiological failure: data not provided P value = 0,05 |
0.42 | Removal | No. Limited discussion of bias, interpretation and generalizability Conflicts of interest not stated |
No participants were excluded from analysis This study finds non‐inferiority between treatment regimens |
| Kibbler 2003 | All participants with candidaemia in 6 large hospitals, UK. Surveillance programme (1997 to 1999) |
136 (76.1%) |
30‐Day mortality | Catheter removal | No analysis: removal: mortality 15.7%, retention: mortality 48.8% |
0.26 | Not significant | Yes | No participants were excluded from analysis |
| Fernández‐Ruiz 2014 | Positive blood culture for C. parapsilosis (CANDI‐POP) (2010 to 2011) |
194 (163) | 30‐Day mortality | Catheter removal within 48 hours | Multi‐variate: OR 0.43, 95% CI 0.19 to 0.,96, P value = 0.04 |
0.24 | Removal | Yes | Participants who died < 72 hours were excluded from analysis Participants with recurrent infections were registered successively |
| Fernández‐Ruiz 2015 | Positive blood culture for C. tropicalis (CANDI‐POP) (2010 to 2011) |
59 (36) |
30‐Day mortality | Catheter removal within 48 hours | Uni‐variate: OR 0.07, 95% CI 0.01 to 0.62, P value = 0.006 |
0.18 | Removal | Yes | |
| CANDI‐POP: “Estudio Poblacional prospectivo sobre candidaemia en España”; CI: confidence interval; CVC: central venous catheter; ICU: intensive care unit; N/A: not available; OR: odds ratio; STROBE: STrengthening the Reporting of OBservational studies in Epidemiology, strobe‐statement.org | |||||||||
3. Table of excluded studies ‐ Retrospective cohort studies of adult populations.
| Retrospective studies – adult populations | |||||||||
| Author |
Population (study period) |
Sample size (CVCs) | Outcome | Intervention | Analysis and results | Overall mortality | Results in favour of | STROBE | Comments |
| Patino 2012 | Adult candidaemia cases with a central venous catheter (2008 to 2010) |
1027 (1027) |
Mortality | Catheter removal within 7 days | Multi‐variate: HR 0.64, 95% CI 0.45 to 0.92, P value not provided |
N/A | Removal | Yes. | Propensity score model showed no significant association between catheter removal and improved survival (HR 0.77, 95% CI 0.50 to 1.20) |
| Takesue 2015 | Non‐neutropenic participants > 17 years old treated with antifungals for candidaemia. (2011 to 2012) |
608 (510) | 28‐Day survival | Catheter removal within 24 hours of diagnosis | Multi‐variate: OR 2.97, 95% CI 1.51 to 5.85, P value not provided |
0.27 | Removal | No. Design not clear from title or abstract. Demographics not described | Participants who did not receive antifungal treatment were excluded from analysis |
| Anaissie 1998 | Adults with malignant disease and candidaemia (1988 to 1992) |
476 (364) |
90‐Day mortality or non‐cure | Catheter retention for longer than 0, 2 or 4 days | Multi‐variate: full exchange of catheter: OR 2.0, 95% CI 1.4 to 2.9, P value = 0.061. Full exchange or exchange over guidewire: OR 2.2, 95% CI 1.6 to 3.2, P value = 0.02 |
0.52 | Not significant | Yes | Study provides detailed data on timing of removal and complete exchange vs exchange over guidewire |
| De Rosa 2015 | All participants hospitalized with candidaemia in internal medical wards (2004 to 2012) |
274 (195) | 28‐Day mortality | Catheter removal < 48 hours | Multi‐variate: OR 0.14, 95% CI 0.07 to 0,30, P value not provided |
0.39 | Removal | Yes. Limited discussion of bias and generalizability | Subgroup analysis of treated participants renders insignificant result: OR 0.129, 95% CI 0.061 to 0.274, P value not provided |
| Chen 2015 | Adult participants with candidaemia caused by C. parapsilosis sensu lato (2000 to 2012) |
323 (299) | 30‐Day survival | Catheter removal | Multi‐variate: OR 0.35, 95% CI 0.19 to 0.62, P value = 0.02 |
0.25 | Removal | No. Limitations not discussed | No participants were excluded from analysis Includes untreated participants Data retrieved from database |
| Gürcüoğlu 2010 | Adult participants with candidaemia (1996 to 2007) |
256 (230) |
30‐Day survival | Catheter removal within 0 to > 3 days (stratified) | Multi‐variate: OR 1.98 95% CI 1.22 to 3.20. P value = 0.006 |
0.5 | Removal | No. Limitations not discussed | No significant difference in day of removal (day 0 to day 3) |
| Tang 2014 | Admitted cancer participant with candidaemia (2009 to 2012) |
242 (182) |
In‐hospital mortality | Catheter removal | Uni‐variate: OR 0.68, 95% CI 0.38 to 1.21, P value = 0.19 |
0.51 | Not significant | Yes | |
| Bassetti 2015 | All participants with candidaemia (2009 to 2014) |
204 (172) |
30‐Day survival | Catheter removal within 24 hours | Multi‐variate: OR 3.77, 95% CI 1.3 to 11.76, P value = 0.014 |
0.47 | Removal | No. Limitations not discussed | No participants were excluded from analysis Only 168 participants received empirical treatment |
| Luzzati 2000 | Adults. Participants 12 years and older with candidaemia (1992 to 1997) |
189 (122) |
30‐Day mortality | Catheter removal | Multi‐variate: OR 0.62 95% CI 0.38 to 0.99, P value = 0.0477 |
0.45 | Not significant | No. Limitations not discussed | |
| Tang 2015 | Adults 65 years and older with candidaemia (2009 to 2012) |
175 (NA) |
Mortality | N/A | Uni‐variate: data not provided. Cited as not significant with P value = 0.059 |
0.50 | Not significant | No. Limitations not discussed | |
| Rodriguez 2007 | Adult candidaemia cases with a central venous catheter (2002 to 2004) |
172 (172) |
30‐Day mortality | Catheter removal within 2 days | Multi‐variate: risk ratio (RR): 1.0, 95% CI 0.8 to 1.3, P value not provided |
0.35 | Not significant | Yes | Participants were excluded from analysis if they died or were discharged before day 2 post candidaemia onset |
| Arnold 2010 | Hospitalized adults with candidaemia (2004 to 2006) |
167 (144) |
Hospital costs + Length of stay | Catheter removal within 24 hours | No analysis: data not provided. Cited as not significant |
0.26 | Not significant | Yes | Hospital costs equal between intervention and control groups (P value = 0.97) |
| Meltem 2015 | Adults with blood culture positive for Candida spp (2012 to 2014) |
140 (N/A) | Mortality | Catheter removal | N/A: mortality significantly reduced with catheter removal, P value = 0.046 |
0.53 | Removal | No. Statistical methods not described. Results insufficiently described | |
| Lai 2012 | Adult cancer participants with a Port‐A‐Cath and candidaemia (2003 to 2009) |
98 (98) |
30‐Day mortality | Catheter retention (median 7 days, range 2 to ‐19 days) | Multi‐variate: OR 9.05, 95% CI 3.08 to 26.62, P value < 0.001 |
0.57 | Removal | Yes | Participants were excluded from analysis if they died within 72 hours of onset of candidaemia. Participants who retained catheters had higher APACHE II score and more |
| Pasqualotto 2007 | Cases of candidaemia among adult participants (1995 to 2003) |
93 (93) |
30‐Day mortality | Catheter removal | Multi‐variate: data not provided. Cited as not significant |
0.62 | Not significant | No. Data not provided | No participants were excluded from analysis |
| Liu 2009 | Adults with malignant disease and single, non‐tunnelled CVC in place for 24 hours before candidaemia diagnosis (2004 to 2007) |
92 (92) |
30‐Day mortality | Catheter retention > 72 hours | Multi‐variate: HR 7.15, 95% CI 3.51 to 14.53, P value ≤ 0.001 |
0.60 | Removal | No. Results of uni‐variate analysis not provided | Participants were excluded from analysis if they died within 72 hours post candidaemia onset |
| Choi 2009 | Adults with candidaemia caused by C. glabrata/krusei vs C. albicans (1997 to 2006) |
81 (73) |
30‐Day mortality | Catheter maintenance | Multi‐variate: OR 9.14, 95% CI 1.69 to 49.53, P value = 0.01 |
0.54 | Removal | Yes | Participants who received no antifungal therapy were excluded from the analysis |
| Wang 2014 | Elderly participants (> 65 years) with candidaemia (2008 to 2010) |
63 (32) | 30‐Day mortality | Catheter removal | Multi‐variate: data not provided. Cited as not significant |
0.20 | Not significant | No. Data not provided | No participants were excluded from analysis Untreated participants in study |
| Tsai 2011 | Non‐neutropenic adults on total parenteral nutrition with candidaemia (2003 to 2005) |
59 (N/A) |
30‐Day mortality | Catheter retention | Multi‐variate: HR 9.01, 95% CI 3.160 to 25.70, P value < 0.001 |
0.54 | Removal | Yes | No participants were excluded from analysis Untreated participants in study |
| Charles 2003 | Adults with candidaemia in ICU (1990 to 2000) |
51 (49) |
Survival in ICU | Catheter removal within 24 hours | Uni‐variate: HR 2.14, 95% CI 0.87 to 5.24, P value = 0.09 |
0.61 | Not significant | Yes | |
| Inoue 1995 | Adults with catheter‐related candidaemia (1985 to 1991) |
30 (29) |
30‐Day mortality | Catheter removal | No analysis: mortality: removal: 6/19, retention: 9/10 |
0.52 | Not significant | No. Statistical analysis not performed | |
| Launay 1998 | Human immunodeficiency virus (HIV)‐infected adults with nosocomial candidaemia (1990 to 1995) |
13 (9) |
26‐Day mortality | Catheter removal | No analysis: mortality: removal: 1/7, retention: 2/2 |
0.38 | Not significant | No. Statistical analysis not performed | |
| APACHE: Acute Physiology and Chronic Health Evaluation; Candida spp: Candida species; CI: confidence interval; HIV: human immunodeficiency virus; HR: hazard ratio; ICU: intensive care unit; N/A: not available; OR: odds ratio; RR: risk ratio; STROBE: STrengthening the Reporting of OBservational studies in Epidemiology, strobe‐statement.org; vs: versus | |||||||||
4. Table of excluded studies ‐ Other retrospective studies of adult populations.
| Other adult studies (case series and case‐control studies) | ||||||||
| Author |
Population (study period) |
Sample size (CVCs) | Outcome | Catheter management intervention | Analysis and results | Overall mortality | Results in favour of | STROBE compatibility |
| Clancy 2000 | Adults with late recurrent candidaemia by the same species occurring at least 1 month after complete resolution from the initial episode (N/A ‐ case series) |
5 (4) |
Mortality | N/A | No analysis: mortality: removal: 1/2 retention: 1/2 |
0.4 | Not significant | No |
| Gamaletsou 2014 | Hospitalized adult participants with haematological malignancies. Nested case‐control study of participants who developed candidaemia and contemporary controls who did not (2009 to 2012) |
40 (31) |
30‐Day mortality | Catheter removal within 3 days | No analysis: 11 catheters removed. Data not provided. Cited as not significant. P value = 0.073 |
0.45 | Not significant | No |
| Anaissie 1996 | Adult cancer participants with haematogenous candidiasis. Matched case‐control study of participants treated with fluconazole vs amphotericin B (1988 to 1992) |
90 (78) |
Clearance of Candida | N/A | No analysis: clearance of candidaemia: removal: 78% of 40 participants; retention: 71% of 38 participants. P value > 0.5 |
0.93 | Not significant | No |
| CI: confidence interval; CVC: central venous catheter; ICU: intensive care unit; N/A: not available; OR: odds ratio; STROBE: STrengthening the Reporting of OBservational studies in Epidemiology, strobe‐statement.org; vs: versus | ||||||||
5. Table of excluded studies ‐ Retrospective cohort studies of mixed populations including both adult and paediatric cases.
| Retrospective studies – populations of all ages | |||||||||
| Author |
Population (study period) |
Sample size (CVCs) | Outcome | Catheter management intervention | Analysis and results | Overall mortality | Results in favour of | STROBE | Comments |
| Farmakiotis 2015 | Cancer participants with candidaemia caused by C. glabrata (2005 to 2013) |
146 (131) | 28‐Day mortality | Catheter removal < 48 hours | Multi‐variate: data not provided. Cited as non‐significant |
0.40 | Not significant | No. Design not clear from abstract or title | Significance in uni‐variate analysis. Exclusion of participants who died < 48 hours from outcome analysis did not change significance |
| Viudes 2002 | All cases of candidaemia, 1 hospital, Spain (1995 to 1997) |
145 (120) |
Mortality | Catheter not changed within 5 days | Multi‐variate: OR 3.54, 95% CI 1.16 to 10.77, P value = 0.03 |
0.44 | Removal | No. Data from uni‐variate analysis insufficiently reported and limitations not discussed | No participants were excluded from analysis. Differences between adults and children |
| Taur 2010 | All participants with candidaemia, tertiary care hospital, New York, USA (2005 to 2007) |
106 (93) |
Mortality | Catheter removal | Multi‐variate: OR 1.17, 95% CI 0.40 to 3.44, P value = 0.769 |
0.23 | Not significant | No. Design not clear from abstract or title. Outcome insufficiently defined | Participants were excluded from analysis if they died before the culture result became positive, if no antifungal treatment was given or if the participant was already receiving pre‐existing systemic antifungal therapy Primary endpoint was time (incubation, notification, initiation of therapy) |
| Murthy 2008 | All episodes of candidaemia in 1 institution (2004 to 2005) |
107 (105) |
90‐Day mortality | Complete removal vs partial exchange or retention | Multi‐variate: OR 0.10, 95% CI 0.02 to 0.66, P value = 0.017 |
0.20 | Removal | No. Design not clear from title | No participants were excluded from analysis Only 84 participants, but 107 episodes |
| Takakura 2004 | All participants with candidaemia – 156 Japanese institutions. Surveillance programme, laboratory (2001 to 2002) |
326 (208) |
30‐Day survival | Catheter removal or lack of CVC vs retention of catheter | Multi‐variate: OR 5.96, 95% CI 2.20 to 16.1, P value < 0.001 |
0.31 | Removal | No. Limitations not discussed | Participants without an indwelling central venous catheter at diagnosis were included in the “removal of CVC” group because the study authors' topic of interest was the potential risk associated with a retained CVC Primary outcome of study was factors associated with fluconazole resistance |
| Labelle 2008 | All participants with candidaemia Divided into hospital and ICU cohorts (2004 to 2006) |
245 (217) |
Hospital mortality | CVC retention for > 24 hours | Multi‐variate: OR 4.85, 95% CI 2.54 to 9.29, P value = 0.015 ICU: OR 6.21, 95% CI 3.02 to 12.77, P value = 0.011 |
0.29 | Removal | No. Results of uni‐variate analysis not provided | Participants who died before receiving antifungal therapy were excluded |
| Asmundsdottir 2005 | All participants with candidaemia in Iceland Laboratory data combined with national registry (1980 to 1999) |
165 (130) |
30‐Day mortality | Prompt removal of CVC (< 48 hours) | Multi‐variate: all participants: OR 0.22, 95% CI 0.08 to 0.61, P value = 0.004 Participants surviving > 72 hours after positive blood culture: OR 0.26, 95% CI 0.09 to 0.76, P value = 0.014 |
0.36 | Removal | Yes. Limited discussion of bias and generalizability | Improvement over time during almost 20 years of registration CVCs more frequently removed in adult cases vs paediatric cases (82% vs 49%) |
| Shorr 2011 | All participants in studies with C. glabrata or C. krusei (N/A) |
183 (N/A) |
28‐Day survival | N/A | Muti‐variate: removal: OR 3.72, 95% CI 1.52 to 9.09, P value not provided |
0.31 | Removal | No. Results of uni‐variate analysis not provided | Subgroups of Kuse 2007 and Pappas 2007 |
| Al‐Tawfiq 2007 | All participants with candidaemia at Saudi Aramco Hospital (1996 to 2004) |
98 (60) |
7‐ and 30‐day mortality | Removal of CVC | Uni‐variate: RR 1.11, 95% CI no provided, P value = 0.,86 |
0.43 | Not significant | No. Results of uni‐variate analysis not provided and limitations not discussed | No participants were excluded from analysis Consecutive episodes not included |
| Chalmers 2011 | All episodes of candidaemia at 5 centres in Scotland/Wales (2008) |
96 (49) |
30‐Day mortality | Removal of catheter within 48 hours | No analysis: mortality: removal: 36%, retention: 29% |
0.40 | Not significant | No. Statistical analysis not performed | No participants were excluded from analysis Consecutive episodes included if > 4 weeks after primary |
| Rodriguez 2005 | All cases of primary candidaemia in participants with CVCs (2002 to 2003) |
299 (299) |
30‐Day mortality | Catheter removal by day 3 | Uni‐variate analysis: mortality: removal: 9%, retainment: 27%, P value < 0.01 |
N/A | Removal | No. Design not clear from title | No participants were excluded from analysis |
| AM: attributable mortality; CI: confidence interval; CVC: central venous catheter; ICU: intensive care unit; N/A: not available; OR: odds ratio; RR: risk ratio; STROBE: STrengthening the Reporting of OBservational studies in Epidemiology, strobe‐statement.org; vs: versus | |||||||||
6. Table of excluded studies ‐ Studies of paediatric populations of various designs.
| All studies – paediatric populations | ||||||||||
| Author | Design |
Population (study period) |
Sample size (CVCs) | Outcome | Catheter management intervention | Analysis and results | Overall mortality | Results in favour of | STROBE | Comments |
| Benjamin 2006 | Prospective | Neonates born at < 1000 g and surviving > 3 days after birth, who developed candidaemia during the post‐natal period (1998 to 2001) |
320 (189) |
Mortality, neurodevelopmental impairment (NDI) | Early catheter removal (1 day after initiation of therapy) |
Multi‐variate: OR (NDI/death): 2.69, 95% CI 1.25 to 5.79, P value = 0.01. Death: 21% vs 37%, P value = 0.02, NDI 45% vs 63%, P value = 0.08 |
0.32 | Removal | Yes | Inclusion criteria positive blood culture (n = 307), cerebrospinal fluid (CSF) (n = 13) or both CSF and blood (n = 14) |
| Fisher 2015 | Retrospective | Cohort study of children < 19 years with candidaemia (2000 to 2012) |
285 (285) |
30‐Day mortality | Retention > 1 day after positive culture | Multi‐variate: OR 2.50, 95% CI 1.06 to 5.91, P value not provided |
0.11 | Removal | Yes. | |
| Karadag‐Oncel 2015 | Retrospective | Cohort study of children < 18 years with positive blood culture. Subdivided into participants < 3months and participants ≥ 3 months (2004 to 2012) |
248 (218) | 30‐Day mortality | CVC removal | Multi‐variate: < 3 months: OR 20.5, 95% CI 3.9 to 106, P value < 0,001 ≥ 3 months: OR 23, 95% CI 7.48 to 70.77, P value < 0.001 |
0.29 | Removal | No. Results of uni‐variate analysis not providedLimitations not discussed | |
| Zaoutis 2004 | Nested case‐control study | Cohort of hospitalized children with persistent candidaemia (≥ 3 days) (1998 to 2001) |
168 (44) |
Disseminated candidiasis at 3 months (chorioretinitis, endocarditis or solid organ) | CVC left in situ for > 3 days with persistent candidaemia | Multi‐variate: increased risk of dissemination: OR 3.0, 95% CI 1.2 to 7.8, P value = 0.02 |
0.26 | Removal | Yes. | Cases: participants with evidence of disseminated candidiasis Controls: participants with no evidence of disseminated candidiasis |
| Chan 2015 | Retrospective | Cohort of children < 21 years with candidaemia (2000 to 2009) |
106 (102) |
Mycological eradication < 5 days; 30‐day mortality |
Catheter removal | Multi‐variate: mycological eradication < 5 days: OR 1.28, 95% CI 0.13 to 12.1, P value = 0.83. 30‐Day mortality: OR 0.3, 95% CI 0.033 to 3.5, P value = 0.4 |
0.12 | Not significant | Yes | No participants were excluded from analysis Very few CVCs were not removed (7 out of 102) |
| Pasqualotto 2008 | Retrospective | All cases of candidaemia in paediatric participants (1995 to 2003) |
61 (61) |
Early mortality (7 days) Late mortality (8 to ‐30 days) |
Catheter removal | Multi‐variate: 7 days: OR 16.0, 95% CI 2.9 to 87.8, P value < 0.001 8 to 30 days: data not provided. Cited as not significant |
0.36 | Removal | No. 'Catheter removal' not clearly defined. Not clear which results are from uni‐variate and which are from multi‐variate analysis | No participants were excluded from analysis Median time to removal 5.0 days |
| Karlowicz 2000 | Retrospective (prospective reporting to database) | Infants with candidaemia
and CVC (1994 to 1998) |
104 (104) | Case fatality: Death < 3 days of pos blood culture Autopsy evidence of dissemination Death attributable to candidaemic complication (e.g. thrombus) |
Early catheter removal (< 3 days) | Uni‐variate: candidaemia case fatality 2% vs 19% (P value = 0.008) |
0.23 | Removal | No. Not clearly stated whether study is prospective or retrospective. No attempt to control for potential. bias. No discussion of potential bias related to design | Participants were excluded from analysis if they died within 2 days of onset of candidaemia |
| Stamos 1995 | Retrospective | Episodes of candidaemia in children (1988 to 1992) |
70 (66) | Mortality | Catheter removal within 3 days | Uni‐variate: mortality: removed: 0%, retained: 36%, P value < 0.0001 |
0.19 | Removal | No. Design not clearly stated. Definitions unclear. No attempt to control for potential. bias. No discussion of potential bias related to design | Participants were excluded from analysis if they died before diagnosis |
| San Miguel 2006 | Retrospective | Cases of candidaemia in children with congenital cardiopathy (1988 to 2000) |
52 (52) |
Mortality | Maintenance of catheter | Multi‐variate: OR: 6.0, 95% CI 1.0 to 37.2, P value = 0.05 |
0.385 | Removal | No. Design not clear from abstract. Limitations not discussed | No participants were excluded from analysis |
| Eppes 1989 | Retrospective | Hospitalized children with candidaemia and a central line treated with amphotericin B (1978 to 1987) |
21 (21) |
Persistent candidaemia Median duration Subsequent complications Mortality Combined adverse outcome |
Removal of catheter within 3 days | Uni‐variate: mortality: data not provided Cited as not significant P value = 0.13 |
0.10 | Not significant | No. Design not clear from abstract or title. No attempt to control for potential. bias. No discussion of potential bias related to design | Risk of persistent candidaemia and median duration of candidaemia reduced with early removal. Combined risk of adverse outcomes (mortality or morbidity) significantly increased with late removal |
| Benjamin 2000 | Retrospective | Children with candidaemia in neonatal intensive care unit (1995 to 1998) |
37 (33) |
Mortality (epidemiologic study of prognostic factors for and risk factors in candidaemia vs bacteraemia with CoNS in neonates) |
N/A | N/A Data not provided Cited as not significant |
0.38 | Not significant | No. Data not provided. 'Catheter removal' not clearly defined | Cites later catheter removal in candidaemia caused by C. parapsilosis |
| Dato 1990 | Retrospective | Children < 18 years old with CVC‐related candidaemia (1981 to 1986) |
31 (31) |
Case fatality | Catheter removal between 1 and 7 days (stratified) | Uni‐variate: cites later catheter removal in fatal cases |
0.16 | Not significant | No No attempt to control for potential. bias and no discussion of potential bias related to design Results not accurately provided |
Eleven participants were excluded from analysis owing to sickness and poor prognosis |
| Donowitz 1995 | Case series | Children < 17 years with candidaemia (laboratory data) (1983 to 1990) |
31 (28) |
Mortality | Catheter removal (single data on timing available |
N/A Cites increased risk with removal, but study is strongly confounded |
0.3 | Not significant | Not relevant. Case series | Distinction between therapeutic regimens (short‐course vs non‐short cause) All deaths among participants with persistent candidaemia |
| Vogiatzi 2013 | Retrospective | Children in ICU with candidaemia > 48 hours after admission (2005 to 2009) |
22 (22) |
30‐Day mortality | Catheter removal | No analysis States that outcome did not correlate with removal |
0.18 | Not significant | No. Definitions unclear Results not accurately provided | Time of CVC removal was not recorded (18 out of 22 CVCs removed) |
| Echave 2010 | Retrospective | Children in neonatal ICU with candidaemia (2003 to 2008) |
18 (14) |
Mortality | Timing of removal | Uni‐variate: survivors: catheter removed after 1.9 days; deceased: catheter removed after 5.8 days, P value = 0.02 |
0.28 | Removal | No. Design not clear from title | Comparison between survivors and deceased participants |
| Ridola 2004 | Retrospective | Children with a solid tumour and candidaemia (1988 to 2000) |
17 (17) |
Mortality | Removal of central line | No analysis: removal: 1/13 died; retention: 3/4 died |
0.24 | Not significant | No. No statistical analysis performed | No participants were excluded from analysis Three children with retained catheters died < 72 hours |
| Devrim 2014 | Retrospective | Children with a port receiving chemotherapy, who had positive blood culture for C. parapsilosis (2001 to 2012) |
12(12) | Time to clearance of C. parapsilosis | Removal of port | No analysis: all ports eventually removed; 2 fatalities |
0.17 | Not significant | No. Design not clear from title or abstract. No statistical analysis performed | Median recovery from fever after Port removal was 4 days |
| Chakrabarti 2003 | Case‐control (retrospective in terms of CVCs) | Infants < 6 months with complex congenital heart disease who underwent cardiac surgery and developed candidaemia < 2 months (1999 to 2001) |
6 (6) | Clearance of candidaemia and survival to discharge | Removal of catheter | No analysis: data not provided. Cited as not significant Removed between 4 and ‐20 days after candidaemia onset. All had Candida thrombophlebitis |
0.83 | Not significant | Not relevant Case series |
Five out of 6 catheters eventually removed Five out of 6 children do not survive to discharge Three out of 6 children clear candidaemia |
| CI: confidence interval; CoNS: coagulase‐negative staphylococci; CSF: cerebrospinal fluid; CVC: central venous catheter; g: grams; ICU: intensive care unit; n: number; N/A: not available; NDI: neurodevelopmental impairment; OR: odds ratio; pos: positive; STROBE: STrengthening the Reporting of OBservational studies in Epidemiology, strobe‐statement.org; vs: versus | ||||||||||
Included studies
We identified no eligible studies.
Excluded studies
We excluded no RCTs, but we excluded 73 observational studies because the design of the studies did not meet our inclusion criteria (Criteria for considering studies for this review). We referred to these 73 studies as 'excluded' to provide a narrative and descriptive overview of published literature on this topic.
Studies mentioned in Table 1; Table 2; Table 3; Table 4; Table 5; and Table 6 report a relevant outcome following removal or retention of a central venous catheter in participants with candidaemia. Studies including participants diagnosed with 'invasive candidiasis' (also referring to peritonitis, abscess, etc.) or fungaemia are not mentioned in this review, nor are studies referring to 'intravascular catheters' and not precisely central venous catheters, or studies with a 'source control' as the major intervention, as this may refer to a wider range of interventions (e.g. drainage of abscesses) and not specifically to central venous catheter management.
For the purpose of providing an overview, we added a column with the title 'Results in favour of'. If a study reports a significant result in favour of a specific catheter management strategy ‐ defined as a P value < 0.05 ‐ we marked this in the column as 'Removal' or 'Retaining'. If a study reports a P value > 0.05, or if no comparative analysis was conducted, we marked this as 'Not significant'.
We performed no systematic qualitative assessment of studies, but for each study we have provided in a separate column an assessment of reporting in accordance with STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) recommendations. Further information and checklists can be found at strobe‐statement.org (accessed 2 June 2016).
Studies awaiting classification
We identified no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We were not able to assess risk of bias as we included no eligible studies in this review.
Allocation
This cannot be assessed as we included no eligible studies in this review.
Blinding
This cannot be assessed as we included no eligible studies in this review.
Incomplete outcome data
This cannot be assessed as we included no eligible studies in this review.
Selective reporting
This cannot be assessed as we included no eligible studies in this review.
Other potential sources of bias
This cannot be assessed as we included no eligible studies in this review.
Effects of interventions
We included no eligible studies in this review. Therefore, no data were available for evaluation of the primary outcome (mortality) nor of secondary outcomes or adverse effects. We conducted no statistical analyses.
Discussion
Summary of main results
We were able to find no eligible studies and no ongoing trials that met our inclusion criteria. In this review, we provide only a narrative overview of the observational studies and thereby refrain from synthesizing the results of observational studies on the basis of a substantial degree of clinical heterogeneity or high risk of bias.
Overall completeness and applicability of evidence
We were not able to assess overall completeness and applicability of the evidence, as we included no randomized controlled trials (RCTs) in the review. However, candidaemia in adults and children with a central venous catheter in situ represents an important clinical problem with considerable attributable morbidity and mortality. The question of whether a central venous catheter should be removed upon identification of Candida species in the bloodstream has been the topic of some dispute (Lazzarini 2002; Luzzati 2008; Nucci 2002; Nucci 2005).
We have summarized the available evidence in the Characteristics of excluded studies table and in additional tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6); this summary represents an extremely heterogeneous mass of few prospective and more retrospective studies that differ greatly in methods of data collection, sample size, population, clinical setting and modus of intervention. The heterogeneity of the intervention in particular and the lack of studies in which the intervention is randomized make interpretation rather difficult. Most of the 73 observational studies described in this review favour removal of central venous catheters (40 studies present results in favour of removal, and 34 present results with no significant differences between groups). One study presents both significant and non‐significant results for two different outcomes: early and late mortality (Puig‐Asensio 2014b). No studies report results indicating a significant benefit from catheter retention. When we considered only the 28 studies that report results in accordance with the STROBE statement, we noted that 17 studies favoured removal, 10 studies presented non‐significant differences between groups and, finally, one study presented both significant and non‐significant results for two different outcomes, as mentioned above (Puig‐Asensio 2014b). Thus, a slightly higher proportion of observational studies have reported results in favour of removal, which may be considered to introduce lower risk of bias. However, this trend does not challenge the impression that ‐ as a general rule ‐ the quality of observational studies does not match their results. However, any further attempt at qualitative ranking of these studies would involve splitting hairs on the basis of assumptions, relations and the impact of a wide range of patient characteristics and treatment‐related factors on morbidity and mortality ‐ still not rendering a firm conclusion meaningful.
Studied populations range from premature infants (Benjamin 2000; Benjamin 2006; Karlowicz 2000) or children with haematological cancers or solid tumours (Ridola 2004) to selected subgroups of adults with advanced malignant (Anaissie 1998; Liu 2009) or haematological disease (Bigni 2007; Gamaletsou 2014). Data may be retrieved simply from laboratory records and cross‐linked to death records (Asmundsdottir 2005; Donowitz 1995; Kabbani 2014; Takakura 2004), thus including a broad spectrum of participants. Removal of a catheter may have different implications for different groups of people depending on their age, co‐morbidity or intercurrent acute illness, or the feasibility of managing treatment with only peripheral lines. Also, the most likely primary focus of a candidaemic episode may vary between these populations, which would likely affect the impact of removing versus retaining a central venous catheter. Especially interesting is the distinction between neutropenic and non‐neutropenic individuals, which is seldom described in detail.
Quality of the evidence
We found no studies or ongoing trials that met our inclusion criteria. All studies reporting any outcome related to catheter management are observational and are predominantly retrospective. Studies described as prospective are generated from data registered prospectively as part of randomized controlled trials evaluating other interventions (Kuse 2007; Nucci 2010) or as reports to a database or surveillance programme with evaluation of catheter management performed as a post hoc analysis (Fernández‐Ruiz 2014; Fernández‐Ruiz 2015; Kabbani 2014; Kibbler 2003; Puig‐Asensio 2014a; Puig‐Asensio 2014b).
In referral to the above, the decision to remove or retain a catheter in an observational setting would likely be based on the attending clinician's assessment of individual patient‐related risk factors, other treatment regimens and previous personal preferences in combination with institutional and international guidelines. Therefore, confounding by indication in observational studies ‐ prospective or retrospective ‐ is to be expected. In addition, several studies include the data of individuals in whom no active treatment was ever initiated and of those who succumb to their illness before therapy is initiated, possibly even before culture results become available (Asmundsdottir 2005; Dato 1990; Puig‐Asensio 2014a), introducing possible immortal time bias. Inclusion of these cases with the most grave prognosis, possibly during terminal phases of illness, in observational studies represents a bias enhancing the calculated impact of catheter removal, although an actual intention or option to treat may not have occurred..
Some studies analyse data on catheter removal extracted from randomized controlled trials (Kuse 2007; Nucci 2010; Pappas 2007), such as Nucci 2010, which conjoined and summarized selected data from the first two studies. Although inclusion in a randomized controlled trial eliminates the above problem of unrecognized and untreated individuals and secures some degree of homogeneity in non‐pharmacological treatments and populations, catheter removal still is performed at the discretion of the responsible clinician with possible bias as described above. Inclusion also very clearly exemplifies the problem of different treatment regimens given throughout observational studies. Additionally, the heterogeneous time frame of catheter removal seen throughout studies ‐ ranging from less than 24 hours to seven days, and with a large number of studies distinguishing merely between removing and retaining with no defined time frame – makes a summarizing interpretation meaningless, as a large number of participants would have been included in the opposite intervention group, if they had been included in a different study.
Observational studies on this topic have been conducted over decades, ranging from the late 1970s and early 1980s to the present. Over these years, distribution of Candida species has changed, diagnostic measures have developed with improved sensitivity and specificity reducing the time to diagnosis, newer antifungal drugs have emerged and both treatment paradigms and resistance patterns have shifted (Kullberg 2015). All of these factors may impact the effect of catheter removal and the clinician's decision to remove or retain a central venous catheter, and may represent a temporal bias.
Potential biases in the review process
As with all systematic reviews, our findings and interpretations are limited by the quality and quantity of available evidence; therefore, the major limitation of our review is reflected by the lack of published RCTs in this area.
We have adhered to methods of The Cochrane Collaboration to strengthen our conclusions.
Agreements and disagreements with other studies or reviews
We are not aware of any other published systematic review on this topic. Nevertheless, the complete absence of clinical trials represents a major limitation. Evidence concerning whether or not an indwelling central venous catheter should be removed after isolation of Candida species from the bloodstream consists only of observational studies with an obviously biased nature and extreme heterogeneity. Review authors have made no attempt to pool these studies, as this would increase only the quantity of observed participants ‐ not the quality of the evidence.
Another limitation of this review is the scarce mention of potential harms related to removal of catheters. These are potentially non‐trivial adverse events that may not be fatal and may not affect mortality, which is the most common outcome in these studies. Harms related to this intervention are not systematically assessed in any studies.
Guidelines published by the Infectious Diseases Society of America in 2009 state: "If feasible, initial nonmedical management should include removal of all existing central venous catheters (B‐II)" (Pappas 2009), with B‐II referring to moderate evidence derived from '1 well‐designed, non‐randomized trial', specifically referring to three trials ‐ Luzzati 2000; Nguyen 1995; Rex 1995 ‐ the latter of which was excluded from mention in Table 1, as intravascular catheters were not necessarily central venous catheters. These guidelines also clearly state that the question of removal is more controversial in neutropenic individuals, for whom the evidence is scarce, arguing that an abdominal focus is more likely in neutropenic individuals (Pappas 2009). This review evaluates and grades available evidence similarly.
Authors' conclusions
Implications for practice.
In conclusion, optimal central venous catheter management upon presentation with a positive blood culture remains a controversial issue. Observational studies come to diverging conclusions, but all are multiply biased in nature; no firm conclusions can be drawn from these, and pooling of these results makes no sense. This evidence ‐ along with a clinician's experience and individual patient assessment ‐ may provide a basis for qualified suggestions, rather than evidence‐based practices.
The question of removal or retention, however, is important to the individual and to the provider. Removal and re‐insertion may be associated with marked discomfort, severe risks and possible disruption or delay of other treatment for the individual, and represents some degree of logistical challenge and cost for the provider. Yet retaining the catheter and providing optimal medical treatment cannot be deemed safe from the current body of evidence, and no evidence has been found to document a reduction of possible harms when the catheter is retained. Likewise, no qualified and applicable evidence is available to document that the proposed benefits of catheter removal outweigh potential harms.
Implications for research.
Large‐scale, well‐designed randomized controlled trials are required to answer this pivotal question while providing clinicians with qualified evidence on catheter management that can be applied when they become aware of a positive blood culture for Candida species.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Nicola Petrucci (Content Editor), Djillali Annane (Peer Reviewer) and Janet Wale (Consumer Editor) for help and editorial advice provided during preparation of this systematic review
We are in debt to Alessandro C Pasqualotto, David Andes, Dimitrios P Kontoyiannis, Felipe Tuon, Elias Anaissie, Marcio Nucci, Issam Raad, Junfeng Sun and Andre C Kalil for their great work on preparations for this protocol (Janum 2014).
We are grateful to Abjørn Hrobjartsson and Anne Juul Wikkelsø for sharing their advice and experience during preparation of this protocol (Janum 2014).
We owe a very special thank you to Karen Hovshanniyan for his skilled work in refining the search strategy.
We would also like to thank the editors and peer reviewers of the initial protocol (Nicola Petrucci (Content Editor); Cathal Walsh (Statistical Editor); Hilmar Wisplinghoff, Mazen Bader, Peter Pappas (Peer Reviewers); and Karl Gallegos (Cochrane Consumer Network)) (Janum 2014).
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Candidiasis] explode all trees #2 MeSH descriptor: [Fungemia] explode all trees #3 (candidias* or candid?emia or fung?emia):ti,ab #4 #1 or #2 or #3 #5 MeSH descriptor: [Central Venous Catheters] explode all trees #6 MeSH descriptor: [Catheterization, Central Venous] explode all trees #7 (central venous line* or catheter* or CVC) #8 #5 or #6 or #7 #9 #4 and #8
Appendix 2. MEDLINE (Ovid SP) search strategy
1. (exp Candidiasis/ not (Candidiasis, Vulvovaginal/ or Candidiasis, Oral/ or Candidiasis, Cutaneous/ or Urinary Tract Infections/)) or candid?emia.ti,ab. or (exp Fungemia/ not (Cryptococcosis/ or Aspergillosis/ or Zygomycosis/ or Fusarium/ or Scedosporium/ or Histoplasmosis/ or Penicillium/ or phialemonium.mp. or Geotrichum/ or Rhodotorula/ or Paecilomyces/ or Trichosporon/))
2. exp Central Venous Catheters/ or Catheterization, Central Venous/ or "Severity of Illness Index"/ or CVC.ti,ab. or ((central venous line* or catheter* or CVC) adj5 (remov* or replace* or switch* or chang* or swap* or management or retention)).mp. or (((catheter* or CVC) adj5 (remov* or replace*)) and (impact* or effect* or influenc* or systematic or adjunctive strategy)).mp.
3. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
4. 1 and 2 and 3
Appendix 3. EMBASE (Ovid SP) search strategy
1. (candidiasis/ not (esophagus candidiasis/ or vagina candidiasis/ or genital candidiasis/ or oropharynx candidiasis/ or thrush/ or skin candidiasis/ or nose candidiasis/ or mucocutaneous candidiasis/ or pulmonary candidiasis/ or urinary tract infection.mp.)) or candid?emia.ti,ab. or (fungemia/ not (cryptococcosis/ or aspergillosis/ or zygomycosis/ or Fusarium/ or Scedosporium/ or histoplasmosis/ or Penicillium/ or phialemonium.mp. or Geotrichum/ or Rhodotorula/ or Paecilomyces/ or Trichosporon/))
2. central venous catheter/ or central venous catheterization/ or catheter removal/ or "severity of illness index"/ or CVC.ti,ab. or ((central venous line* or catheter* or CVC) adj3 (remov* or replace* or switch* or chang* or swap* or management or retention)).ti,ab. or (((catheter* or CVC) adj3 (remov* or replace*)) and (impact* or effect* or influenc* or systematic or adjunctive strategy)).ti,ab.
3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh.
4. 1 and 2 and 3
Appendix 4. LILACS (BIREME) search strategy
(candid$ or candidaemia or candidemia or fungemia or fungaemia or Infecção or infección$) and ((Cateter venoso central or CVC) or ((central venous line$ or catheter$ or CVC or catéter$ or línea venosa central) and (remoción or reemplazo or conmutación or cambiar or intercambio or gestión or administración or retención)))
Appendix 5. ISI Web of Science and BIOSIS Citation Index search strategy
#1 (TS=candidiasis not TS=(candidiasis, vulvovaginal or candidiasis, oral or candidiasis, cutaneous or urinary tract infections)) or TI=candid?emia or (TS=fungemia not TS=(cryptococcosis or aspergillosis or zygomycosis or fusarium or scedosporium or histoplasmosis or penicillium or phialemonium.mp. or geotrichum or rhodotorula or paecilomyces or trichosporon))
# 2 TS=(central venous SAME catheter*) or TI=CVC or TS=((central venous line* or catheter* or cvc) SAME (remov* or replace* or switch* or chang* or swap* or management or retention)) or TS=(((catheter* or cvc) SAME (remov* or replace*)) and (impact* or effect* or influenc* or systematic or adjunctive strategy))
#3 #1 and #2
Appendix 6. SCOPUS search strategy
(TITLE‐ABS‐KEY ( candid?emia OR fung?emia OR candidiasis)) AND (ABS ((central venous catheter* OR cvc OR central venous line* OR catheter* ) AND ( remov* OR replace* OR switch* OR chang* OR swap* OR management OR retention )))
Appendix 7. Cochrane Anaesthesia, Critical and Emergency Care Group ‐ Data extraction form
Study selection, quality assessment and data extraction form
| First author | Journal/Conference proceedings, etc | Year |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear | Yes/No*/Unclear |
* Issue relates to selective reporting – when study authors may have taken measurements for particular outcomes, but did not report these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded
| Do not proceed if any of the above answers are ‘No’. If study is to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’ |
Participants and trial characteristics
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers/%, etc) | |
| Disease status/type, etc (if applicable) | |
| Other | |
Methodological quality
| Adequate sequence generation | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| Adequate (random) (YES) | |
| Inadequate (e.g. alternate) (NO) | |
| Unclear | |
|
Allocation concealment Process used to prevent foreknowledge of group assignment in an RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate (YES) | |
| Inadequate (NO) | |
| Unclear | |
| Blinding | |
| Person responsible for participant's care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes/No/Not clear
Discuss if appropriate…………………………………………………………………………………………
…………………………………………………………………………………………………………
| Incomplete outcome data addressed? | |
| Completeness of outcome data including attritions and exclusions | Grade (circle) |
| Adequate (YES) | |
| Inadequate (NO) | |
| Unclear | |
| Free of selective reporting? | |
| Possibility of selective outcome reporting | Grade (circle) |
| Adequate (YES) | |
| Inadequate (NO) | |
| Unclear | |
|
Free of other bias? (bias not addressed in the other domains) | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate (YES) | |
| Inadequate (NO) | |
| Unclear | |
Data extraction
|
Outcomes relevant to your review Copy and paste from ‘Types of outcome measures’ | |
| Reported in paper (circle) | |
| Overall mortality | Yes/No |
| Overall 28‐day mortality (30 days M included) | Yes/No |
| Time required for clearance of blood culture for Candida species | Yes/No |
| Frequency of persistent candidaemia (positive culture after 3 days of effective antifungal therapy) |
Yes/No |
| Complications probably related to candidaemia (e.g. metastatic foci, endocarditis, endophthalmitis, hepatosplenic candidosis) | Yes/No |
| Complications probably related to the intervention (e.g. pneumothorax, arterial puncture, bleeding requiring blood transfusion, local suppurative complications) | Yes/No |
| Complications during in‐patient stay not specific to trial intervention (e.g. pneumonia, congestive heart failure, respiratory failure, renal failure) | Yes/No |
| Duration of mechanical ventilation | Yes/No |
| Ventilator‐free days | Yes/No |
| Mean length of stay in hospital | Yes/No |
| Mean length of stay in intensive care unit (ICU) | Yes/No |
| Species‐related mortality | Yes/No |
| For continuous data | |||||||
| Code of paper | Outcomes (rename) | Unit of measurement | Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| A, etc. | Time required for clearance of blood culture for Candida species | ||||||
| Duration of mechanical ventilation | |||||||
| Ventilator‐free days | |||||||
| Mean length of stay in hospital | |||||||
| For dichotomous data | |||
| Code of paper | Outcomes (rename) |
Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| A | Overall mortality | ||
| Overall mortality (28 days) | |||
| Persistent candidaemia (positive culture after 3 days of effective antifungal therapy) |
|||
| Complications probably related to candidaemia (e.g. metastatic foci, endocarditis, endophthalmitis, hepatosplenic candidosis) | |||
| Complications probably related to the intervention (e.g. pneumothorax, arterial puncture, bleeding requiring blood transfusion, local suppurative complications) | |||
| Complications during in‐patient stay not specific to trial intervention (e.g. pneumonia, congestive heart failure, respiratory failure, renal failure) | |||
|
Other information that you believe is relevant to the results Indicate if any data were obtained from the primary author; if results were estimated from graphs, etc. or were calculated by you using a formula (this should be stated and the formula given). In general, if results not reported in paper(s) are obtained, this should be made clear here to be cited in the review |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First study author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give contact names and details | ||
| Trial characteristics | |
| Further details | |
| Single‐centre/Multi‐centre | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Drug treatment(s) used | |
| Dose/Frequency of administration | |
| Duration of treatment (state weeks/months, etc.; if cross‐over trial, give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years, or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Appendix 8. Assessment of risk of bias in included studies
Random sequence generation
Assessment of randomization: sufficiency of the method in producing 2 comparable groups before intervention.
-
Grading.
'Low risk’ (a truly random process, e.g. random computer number generator, coin tossing, throwing dice).
'High risk’ (any non‐random process, e.g. date of birth, date of admission by hospital, clinic record number, availability of the intervention).
'Unclear risk'.
Allocation concealment
Allocation method prevented investigators or participants from foreseeing assignment.
-
Grading.
'Low risk’ (central allocation or sealed envelopes).
'High risk’ (using open allocation schedule or other unconcealed procedure).
'Unclear risk’.
Blinding of participants and personnel
Assessment of appropriate blinding of investigation team and participants: person responsible for participants’ care, participants and eventual others
-
Grading.
'Low risk': We consider blinding as adequate if participants and personnel are kept unaware of intervention allocations after inclusion of participants in the study, and if the method of blinding involves placebo or an intervention disguised in the same manner as a placebo, because mortality is an objective outcome.
'High risk’: not double‐blinded; categorized as an open‐label study or without use of placebo or an intervention disguised in the same manner as a placebo.
'Unclear': blinding not described.
Blinding of outcome assessor
Assessment of appropriate blinding of outcome assessor.
-
Grading.
'Low risk’: We consider blinding as adequate if outcome assessors are kept unaware of intervention allocations after inclusion of participants in the study, and if the method of blinding involves placebo or an intervention disguised in the same manner as a placebo, because mortality is an objective outcome.
'High risk’: not double‐blinded; categorized as an open‐label study or without use of placebo or an intervention disguised in the same manner as a placebo.
'Unclear risk’: blinding not described.
Incomplete outcome data
Completeness of outcome data including attrition and exclusions.
-
Grading.
'Low risk’: if numbers and reasons for dropouts and withdrawals in the intervention groups are described, or if it is specified that no dropouts or withdrawals occurred.
'High risk': if no description of dropouts and withdrawals is provided).
'Unclear risk’: if the report gives the impression that no dropouts or withdrawals occurred, but this is not specifically stated.
Selective reporting
Possibility of selective outcome reporting.
-
Grading.
'Low risk’: if reported outcomes are those pre‐specified in an available study protocol or official trial registration; if this is not available, published report includes all expected outcomes.
'High risk': if not all pre‐specified outcomes have been reported, or if they have been reported using non‐pre‐specified subscales or have been reported incompletely or if report fails to include a key outcome that would have been expected to have been reported for such a study).
'Unclear risk'.
Other bias
Assessment of any possible sources of bias not addressed in the first five domains.
-
Grading.
'Low risk’: if the report appears to be free of such bias.
'High risk’: if at least 1 important bias related to study design is present, or early stopping owing to some data‐dependent process, extreme baseline imbalance, claimed fraudulence or other problems).
'Unclear risk’: insufficient information or evidence that an identified problem will introduce bias.
With reference to the domains above, we planned to assess the likely magnitude and direction of bias and whether we could consider it likely that it would have an impact on our findings. We planned to assess the impact of bias in the sensitivity analyses
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Tawfiq 2007 | Excluded owing to study design ‐ retrospective. For details, see Table 5 |
| Almirante 2006 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Anaissie 1996 | Excluded owing to study design ‐ case‐control study. For details, see Table 4 |
| Anaissie 1998 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Arnold 2010 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Asmundsdottir 2005 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Bassetti 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Benjamin 2000 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Benjamin 2006 | Excluded owing to study design ‐ prospective cohort. For details, see Table 6 |
| Bigni 2007 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Chakrabarti 2003 | Excluded owing to study design ‐ case‐control study. For details, see Table 6 |
| Chakrabarti 2015 | Excluded owing to study design ‐ prospective cohort. For details, see Table 1 |
| Chalmers 2011 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Chan 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Charles 2003 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Chen 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Choi 2009 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Clancy 2000 | Excluded owing to study design ‐ retrospective case series. For details, Table 4 |
| Dato 1990 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| De Rosa 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Devrim 2014 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Donowitz 1995 | Excluded owing to study design ‐ case series. For details, see Table 6 |
| Echave 2010 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Eppes 1989 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Erard 2010 | Excluded owing to study design ‐ prospective cohort. For details, see Table 1 |
| Farmakiotis 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Fernández‐Ruiz 2014 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Fernández‐Ruiz 2015 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Fisher 2015 | Excluded owing to study design ‐ prospective cohort. For details, see Table 6 |
| Gamaletsou 2014 | Excluded owing to study design ‐ case‐control study. For details, see Table 4 |
| Garnacho‐Montero 2013 | Excluded owing to study design ‐ prospective cohort. For details, see Table 1 |
| Gürcüoğlu 2010 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Inoue 1995 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Kabbani 2014 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Karadag‐Oncel 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Karlowicz 2000 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Kibbler 2003 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Kuse 2007 | Excluded owing to study design ‐ randomized clinical trial with other intervention. Catheter management not randomized. For details, see Table 1 |
| Labelle 2008 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Lai 2012 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Launay 1998 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Liu 2009 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Luzzati 2000 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Marriott 2009 | Excluded owing to study design ‐ prospective cohort. For details, see Table 1 |
| Meltem 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Murthy 2008 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Nguyen 1995 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Nucci 1998 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Nucci 2010 | Excluded owing to study design ‐ randomized clinical trial with other intervention. Catheter management not randomized. For details, see Table 1 |
| Pasqualotto 2007 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Pasqualotto 2008 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Patel 2005 | Excluded owing to study design ‐ prospective cohort. For details, see Table 1 |
| Patino 2012 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Puig‐Asensio 2014a | Excluded owing to study design ‐ prospective cohort. For details, see Table 1 |
| Puig‐Asensio 2014b | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Ridola 2004 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Rodriguez 2005 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Rodriguez 2007 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| San Miguel 2006 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Shorr 2011 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Stamos 1995 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Takakura 2004 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Takesue 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Talarmin 2009 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Tang 2014 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Tang 2015 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Taur 2010 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Tsai 2011 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 3 |
| Viudes 2002 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Vogiatzi 2013 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
| Wang 2014 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 5 |
| Weinberger 2005 | Excluded owing to study design ‐ prospective cohort. For details, see Table 2 |
| Zaoutis 2004 | Excluded owing to study design ‐ retrospective cohort. For details, see Table 6 |
Differences between protocol and review
We made the following changes to the protocol (Janum 2014).
BIOSIS Citation Index was not searched.
Contributions of authors
Arash Afshari (AA), Susanne Janum (SJ).
Conceiving the review: Alessandro C Pasqualotto (ACP).
Co‐ordinating the review: AA.
Screening search results: SJ and AA.
Organizing retrieval of papers: SJ and AA.
Screening retrieved papers against inclusion criteria: SJ and AA.
Appraising quality of papers: SJ and AA.
Abstracting data from papers: SJ and AA.
Writing to authors of papers for additional information: SJ.
Providing additional data about papers: SJ and AA.
Obtaining and screening data on unpublished studies: SJ and AA.
Managing data for the review: SJ and AA.
Entering data into Review Manager (RevMan 2014): SJ.
Conducting RevMan statistical analysis: SJ and AA.
Conducting other statistical analysis not using RevMan: SJ and AA.
Performing double entry of data: data entered by person one: SJ; data entered by person two: AA.
Interpreting data: SJ and AA.
Making statistical inferences: SJ and AA.
Writing the review: SJ and AA.
Securing funding for the review: SJ.
Performing previous work that was the foundation of the present study: AA.
Serving as guarantor for the review (one author): AA.
Taking responsibility for reading and checking the review before submission: SJ and AA.
Sources of support
Internal sources
No source of support supplied, Other.
External sources
No sources of support supplied
Declarations of interest
The review authors (Arash Afshari and Susanne Janum) declare that they have no financial or non‐financial conflicts of interest.
In accordance with The Cochrane Collaboration sponsorship policy, this review was not funded by any companies whose products are mentioned in the review.
Edited (no change to conclusions)
References
References to studies excluded from this review
Almirante 2006 {published data only}
- Almirante B, Rodriguez D, Cuenca‐Estrella M, Almela M, Sanchez F, Ayats J, et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case‐control population‐based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. Journal of Clinical Microbiology 2006;44(5):1681‐5. [PUBMED: 16672393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Tawfiq 2007 {published data only}
- Al‐Tawfiq JA. Distribution and epidemiology of Candida species causing fungemia at a Saudi Arabian hospital, 1996‐2004. International Journal of Infectious Diseases 2007;11(3):239‐44. [PUBMED: 16859945] [DOI] [PubMed] [Google Scholar]
Anaissie 1996 {published data only}
- Anaissie EJ, Vartivarian SE, Abi‐Said D, Uzun O, Pinczowski H, Kontoyiannis DP, et al. Fluconazole versus amphotericin B in the treatment of hematogenous candidiasis: a matched cohort study. The American Journal of Medicine 1996;101(2):170‐6. [PUBMED: 8757357] [DOI] [PubMed] [Google Scholar]
Anaissie 1998 {published data only}
- Anaissie EJ, Rex JH, Uzun O, Vartivarian S. Predictors of adverse outcome in cancer patients with candidemia. The American Journal of Medicine 1998;104(3):238‐45. [PUBMED: 9552086] [DOI] [PubMed] [Google Scholar]
Arnold 2010 {published data only}
- Arnold HM, Micek ST, Shorr AF, Zilberberg MD, Labelle AJ, Kothari S, et al. Hospital resource utilization and costs of inappropriate treatment of candidemia. Pharmacotherapy 2010;30(4):361‐8. [PUBMED: 20334456] [DOI] [PubMed] [Google Scholar]
Asmundsdottir 2005 {published data only}
- Asmundsdottir LR, Erlendsdottir H, Gottfredsson M. Improving survival of patients with candidaemia: analysis of prognostic factors from a long‐term, nationwide study in Iceland. Scandinavian Journal of Infectious Diseases 2005;37(2):111‐20. [PUBMED: 15764202] [DOI] [PubMed] [Google Scholar]
Bassetti 2015 {published data only}
- Bassetti M, Merelli M, Ansaldi F, Florentiis D, Sartor A, Scarparo C, et al. Clinical and therapeutic aspects of candidemia: a five year single centre study. PloS One 2015;10(5):e0127534. [PUBMED: 26010361] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamin 2000 {published data only}
- Benjamin DK Jr, Ross K, McKinney RE Jr, Benjamin DK, Auten R, Fisher RG. When to suspect fungal infection in neonates: a clinical comparison of Candida albicans and Candida parapsilosis fungemia with coagulase‐negative staphylococcal bacteremia. Pediatrics 2000;106(4):712‐8. [PUBMED: 11015513] [DOI] [PubMed] [Google Scholar]
Benjamin 2006 {published data only}
- Benjamin DK Jr, Stoll BJ, Fanaroff AA, McDonald SA, Oh W, Higgins RD, et al. Neonatal candidiasis among extremely low birth weight infants: risk factors, mortality rates, and neurodevelopmental outcomes at 18 to 22 months. Pediatrics 2006;117(1):84‐92. [PUBMED: 16396864] [DOI] [PubMed] [Google Scholar]
Bigni 2007 {published data only}
- Bigni RS, Velasco ED, Dobbin JA. The prognostic role of clinical and microbiological factors on mortality related to Candida bloodstream infections in hospitalized patients with hematologic malignancies. Blood (ASH Annual Meeting Abstracts). 2007; Vol. 110:Abstract 3861.
Chakrabarti 2003 {published data only}
- Chakrabarti C, Sood SK, Parnell V, Rubin LG. Prolonged candidemia in infants following surgery for congenital heart disease. Infection Control and Hospital Epidemiology 2003;24(10):753‐7. [PUBMED: 14587937] [DOI] [PubMed] [Google Scholar]
Chakrabarti 2015 {published data only}
- Chakrabarti A, Sood P, Rudramurthy SM, Chen S, Kaur H, Capoor M, et al. Incidence, characteristics and outcome of ICU‐acquired candidemia in India. Intensive Care Medicine 2015;41(2):285‐95. [PUBMED: 25510301] [DOI] [PubMed] [Google Scholar]
Chalmers 2011 {published data only}
- Chalmers C, Gaur S, Chew J, Wright T, Kumar A, Mathur S, et al. Epidemiology and management of candidaemia ‐ a retrospective, multicentre study in five hospitals in the UK. Mycoses 2011;54(6):e795‐800. [PUBMED: 21615542] [DOI] [PubMed] [Google Scholar]
Chan 2015 {published data only}
- Chan S, Baley ED, Hossain J, Pentima MC. Candida species bloodstream infections in hospitalised children: a 10‐year experience. Journal of Paediatrics and Child Health 2015;51(9):857‐61. [PUBMED: 25941056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Charles 2003 {published data only}
- Charles PE, Doise JM, Quenot JP, Aube H, Dalle F, Chavanet P, et al. Candidemia in critically ill patients: difference of outcome between medical and surgical patients. Intensive Care Medicine 2003;29(12):2162‐9. [PUBMED: 13680110] [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen CY, Sheng WH, Huang SY, Chou WC, Yao M, Tang JL, et al. Clinical characteristics and treatment outcomes of patients with candidaemia due to Candida parapsilosis sensu lato species at a medical centre in Taiwan, 2000‐12. The Journal of Antimicrobial Chemotherapy 2015;70(5):1531‐8. [PUBMED: 25558079] [DOI] [PubMed] [Google Scholar]
Choi 2009 {published data only}
- Choi HK, Jeong SJ, Lee HS, Chin BS, Choi SH, Han SH, et al. Blood stream infections by Candida glabrata and Candida krusei: a single‐center experience. The Korean Journal of Internal Medicine 2009;24(3):263‐9. [PUBMED: 19721864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clancy 2000 {published data only}
- Clancy CJ, Barchiesi F, Falconi DiFrancesco L, Morris AJ, Snydman DR, Yu VL, et al. Clinical manifestations and molecular epidemiology of late recurrent candidemia, and implications for management. European Journal of Clinical Microbiology & Infectious Diseases 2000;19(8):585‐92. [PUBMED: 11014620] [DOI] [PubMed] [Google Scholar]
Dato 1990 {published data only}
- Dato VM, Dajani AS. Candidemia in children with central venous catheters: role of catheter removal and amphotericin B therapy. The Pediatric Infectious Disease Journal 1990;9(5):309‐14. [PUBMED: 2352815] [DOI] [PubMed] [Google Scholar]
De Rosa 2015 {published data only}
- Rosa FG, Corcione S, Filippini C, Raviolo S, Fossati L, Montrucchio C, et al. The effect on mortality of fluconazole or echinocandin treatment in candidemia in internal medicine wards [corrected]. PloS One 2015;10(5):e0125149. [PUBMED: 25938486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Devrim 2014 {published data only}
- Devrim I, Yaman Y, Demirag B, Oymak Y, Carti O, Ozek G, et al. A single center's experience with Candida parapsilosis related long‐term central venous access device infections: the port removal decision and its outcomes. Pediatric Hematology and Oncology 2014;31(5):435‐41. [PUBMED: 24383767] [DOI] [PubMed] [Google Scholar]
- Donowitz LG, Hendley JO. Short‐course amphotericin B therapy for candidemia in pediatric patients. Pediatrics 1995;95(6):888‐91. [PUBMED: 7761216] [PubMed] [Google Scholar]
Donowitz 1995 {published data only}
- Donowitz LG, Hendley JO. Short‐course amphotericin B therapy for candidemia in pediatric patients. Pediatrics 1995;95(6):888‐91. [PubMed] [Google Scholar]
Echave 2010 {published data only}
- Echave CI, Praino ML, Vozza ML, Manso C, Russman R, Enfedaque C, et al. Risk factors for candidemia‐related mortality in a neonatal intensive care unit (NICU). Abstracts of the 14th International Congress on Infectious Diseases (ICID). Presentation number 30.019. 2010.
Eppes 1989 {published data only}
- Eppes SC, Troutman JL, Gutman LT. Outcome of treatment of candidemia in children whose central catheters were removed or retained. The Pediatric Infectious Disease Journal 1989;8(2):99‐104. [PUBMED: 2704608] [PubMed] [Google Scholar]
Erard 2010 {published data only}
- Erard V, Flückiger U, Zimmerli S, Garbino J, Imhof A, Boggian K, et al. FUNGINOS INVESTIGATORS. Impact of catheter removal and timely appropriate antifungal therapy on mortality attributable to candidemia: prospective study of the Fungal Infection Network of Switzerland (FUNGINOS). Abstracts of ICAAC: Interscience Conference of Antimicrobial Agents and Chemotherapy (ICAAC) Sep. 12‐15, 2010. Session 157 ‐ Clinical Mycology and Epidemiology. Presentation number: M‐1311. 2010.
Farmakiotis 2015 {published data only}
- Farmakiotis D, Kyvernitakis A, Tarrand JJ, Kontoyiannis DP. Early initiation of appropriate treatment is associated with increased survival in cancer patients with Candida glabrata fungaemia: a potential benefit from infectious disease consultation. Clinical Microbiology and Infection 2015;21(1):79‐86. [PUBMED: 25636931] [DOI] [PubMed] [Google Scholar]
Fernández‐Ruiz 2014 {published data only}
- Fernández‐Ruiz M, Aguado JM, Almirante B, et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: a propensity score analysis. Clinical Infectious Diseases 2014;58(10):1413‐21. [PUBMED: 24642553] [DOI] [PubMed] [Google Scholar]
Fernández‐Ruiz 2015 {published data only}
- Fernández‐Ruiz M, Puig‐Asensio M, Guinea J, et al. Candida tropicalis bloodstream infection: incidence, risk factors and outcome in a population‐based surveillance. Journal of Infection 2015;71:385‐94. [PUBMED: 26033696] [DOI] [PubMed] [Google Scholar]
Fisher 2015 {published data only}
- Fisher BT, Vendetti N, Bryan M, Prasad PA, Russell Localio A, Damianos A, et al. Central venous catheter retention and mortality in children with candidemia: a retrospective cohort analysis. Journal of the Pediatric Infectious Diseases Society 2015;pii:piv048. [PUBMED: 26407279] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamaletsou 2014 {published data only}
- Gamaletsou MN, Walsh TJ, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clinical Microbiology and Infection 2014;20(1):O50‐7. [PUBMED: 23889746] [DOI] [PubMed] [Google Scholar]
Garnacho‐Montero 2013 {published data only}
- Garnacho‐Montero J, Diaz‐Martin A, Garcia‐Cabrera E, Ruiz Perez de Pipaon M, Hernandez‐Caballero C, Lepe‐Jimenez JA. Impact on hospital mortality of catheter removal and adequate antifungal therapy in Candida spp. bloodstream infections. The Journal of Antimicrobial Chemotherapy 2013;68(1):206‐13. [PUBMED: 22945914] [DOI] [PubMed] [Google Scholar]
Gürcüoğlu 2010 {published data only}
- Gürcüoğlu E, Akalin H, Ener B, Ocakoğlu G, Sinirtas M, Akcağlar S, et al. Nosocomial candidemia in adults: risk and prognostic factors [Candidémie nosocomiale chez adultes: facteurs de risque et de prognostic]. Journal de Mycologie Médicale 2010;20:269‐78. [Google Scholar]
Inoue 1995 {published data only}
- Inoue Y, Kohno S, Fujii T, Otsubo T, Mori N, Ishino T, et al. Clinical evaluation of catheter‐related fungemia and bacteremia. Internal Medicine (Tokyo, Japan) 1995;34(6):485‐90. [PUBMED: 7549129] [DOI] [PubMed] [Google Scholar]
Kabbani 2014 {published data only}
- Kabbani S, Stein B, Hollick R, Harrison LH, Farley M. A population‐based investigation of outcomes of candidemia. Journal of Investigative Medicine (Southern Regional Meetings Abstracts, Infectious Diseases 1) 2014;62(2):531‐2. [Google Scholar]
Karadag‐Oncel 2015 {published data only}
- Karadag‐Oncel E, Kara A, Ozsurekci Y, Arikan‐Akdagli S, Cengiz AB, Ceyhan M, et al. Candidaemia in a paediatric centre and importance of central venous catheter removal. Mycoses 2015;58(3):140‐8. [PUBMED: 25678411] [DOI] [PubMed] [Google Scholar]
Karlowicz 2000 {published data only}
- Karlowicz MG, Hashimoto LN, Kelly RE Jr, Buescher ES. Should central venous catheters be removed as soon as candidemia is detected in neonates?. Pediatrics 2000;106(5):E63. [PUBMED: 11061800] [DOI] [PubMed] [Google Scholar]
Kibbler 2003 {published data only}
- Kibbler CC, Seaton S, Barnes RA, Gransden WR, Holliman RE, Johnson EM, et al. Management and outcome of bloodstream infections due to Candida species in England and Wales. The Journal of Hospital Infection 2003;54(1):18‐24. [PUBMED: 12767842] [DOI] [PubMed] [Google Scholar]
Kuse 2007 {published data only}
- Kuse ER, Chetchotisakd P, Cunha CA, Ruhnke M, Barrios C, Raghunadharao D, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double‐blind trial. Lancet 2007;369(9572):1519‐27. [PUBMED: 17482982] [DOI] [PubMed] [Google Scholar]
Labelle 2008 {published data only}
- Labelle AJ, Micek ST, Roubinian N, Kollef MH. Treatment‐related risk factors for hospital mortality in Candida bloodstream infections. Critical Care Medicine 2008;36(11):2967‐72. [PUBMED: 18824910] [DOI] [PubMed] [Google Scholar]
Lai 2012 {published data only}
- Lai YC, Huang LJ, Chen TL, Yang YW, Hsiao LT, Teng HW, et al. Impact of Port‐A‐Cath device management in cancer patients with candidaemia. The Journal of Hospital Infection 2012;82(4):281‐5. [PUBMED: 23084483] [DOI] [PubMed] [Google Scholar]
Launay 1998 {published data only}
- Launay O, Lortholary O, Bouges‐Michel C, Jarrousse B, Bentata M, Guillevin L. Candidemia: a nosocomial complication in adults with late‐stage AIDS. Clinical Infectious Diseases 1998;26(5):1134‐41. [PUBMED: 9597242] [DOI] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu CY, Huang LJ, Wang WS, Chen TL, Yen CC, Yang MH, et al. Candidemia in cancer patients: impact of early removal of non‐tunneled central venous catheters on outcome. The Journal of Infection 2009;58(2):154‐60. [PUBMED: 19162330] [DOI] [PubMed] [Google Scholar]
Luzzati 2000 {published data only}
- Luzzati R, Amalfitano G, Lazzarini L, Soldani F, Bellino S, Solbiati M, et al. Nosocomial candidemia in non‐neutropenic patients at an Italian tertiary care hospital. European Journal of Clinical Microbiology & Infectious Diseases 2000;19(8):602‐7. [PUBMED: 11014622] [DOI] [PubMed] [Google Scholar]
Marriott 2009 {published data only}
- Marriott DJE, Playford EG, Chen S, Slavin M, Nguyen Q, Ellis D, et al. Determinants of mortality in non‐neutropenic ICU patients with candidaemia. Critical Care 2009;13(4):R115. [PUBMED: 19594912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meltem 2015 {published data only}
- Meltem T, Kutsoylu O, Pullukcu H, et al. Effectiveness and safety of anidulafungin. A real‐life multicenter data study in Turkey. Conference: 7th Trends in Medical Mycoloy in Lisbon, Portugal. October 9‐12, 2015. Blackwell Publishing Ltd., 2015; Vol. 58:pp. 148‐9.
Murthy 2008 {published data only}
- Murthy MH, Vanschooneveld TC, Shafer LR, Kalil AC, Freifeld AG. Impact of intravascular catheter removal on Candida bloodstream infection mortality. Abstracts of IDSA: Infectious Diseases Society of America, 46th Annual Meeting. Poster session: Clinical Mycology 1. Abstract M‐2132.. 2008.
Nguyen 1995 {published data only}
- Nguyen MH, Peacock JE Jr, Tanner DC, Morris AJ, Nguyen ML, Snydman DR, et al. Therapeutic approaches in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Archives of Internal Medicine 1995;155(22):2429‐35. [PUBMED: 7503601] [PubMed] [Google Scholar]
Nucci 1998 {published data only}
- Nucci M, Colombo AL, Silveira F, Richtmann R, Salomao R, Branchini ML, et al. Risk factors for death in patients with candidemia. Evaluation in a multicenter, prospective, observational study. Infection Control and Hospital Epidemiology 1998;19(11):846‐50. [PUBMED: 9831941] [DOI] [PubMed] [Google Scholar]
Nucci 2010 {published data only}
- Nucci M, Anaissie E, Betts RF, Dupont BF, Wu C, Buell DN, et al. Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clinical Infectious Diseases 2010;51(3):295‐303. [PUBMED: 20578829] [DOI] [PubMed] [Google Scholar]
Pasqualotto 2007 {published data only}
- Pasqualotto AC, Moraes AB, Zanini RR, Severo LC. Analysis of independent risk factors for death among pediatric patients with candidemia and a central venous catheter in place. Infection Control and Hospital Epidemiology 2007;28(7):799‐804. [PUBMED: 17564981] [DOI] [PubMed] [Google Scholar]
Pasqualotto 2008 {published data only}
- Pasqualotto AC, Severo LC. The importance of central venous catheter removal in patients with candidaemia: time to rethink our practice?. Clinical Microbiology and Infection 2008;14(1):2‐4. [PUBMED: 18005175] [DOI] [PubMed] [Google Scholar]
Patel 2005 {published data only}
- Patel M, Kunz DF, Trivedi VM, Jones MG, Moser SA, Baddley JW. Initial management of candidemia at an academic medical center: evaluation of the IDSA guidelines. Diagnostic Microbiology and Infectious Disease 2005;52(1):29‐34. [PUBMED: 15878439] [DOI] [PubMed] [Google Scholar]
Patino 2012 {published data only}
- Patino AM, Roy M, Farley MM, Harrison LH, Stein B, Hollick R, et al. Adding fuel to the fire: central venous catheter removal and survival among patients with candidemia ‐ A propensity score analysis using results from population‐based surveillance, 2008‐2010. 18th Congress of the International Society for Human and Animal Mycology Berlin, Germany. 2012.
Puig‐Asensio 2014a {published data only}
- Puig‐Asensio M, Pemán J, Zaragoza R, Garnacho‐Montero J, Martín‐Mazuelos E, Cuenca‐Estrella M, et al. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Critical Care Medicine 2014;42(6):1423‐32. [PUBMED: 24557426] [DOI] [PubMed] [Google Scholar]
Puig‐Asensio 2014b {published data only}
- Puig‐Asensio M, Padilla B, Garnacho‐Montero J, Zaragoza O, Aguado JM, Zaragoza R, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population‐based surveillance in Spain. Clinical and Microbiological Infection 2014;20(4):245‐54. [PUBMED: 24125548] [DOI] [PubMed] [Google Scholar]
Ridola 2004 {published data only}
- Ridola V, Chachaty E, Raimondo G, Corradini N, Brugieres L, Valteau‐Couanet D, et al. Candida infections in children treated with conventional chemotherapy for solid tumors (transplant recipients excluded): The Institut Gustave Roussy Pediatrics Department experience. Pediatric Blood & Cancer 2004;42(4):332‐7. [PUBMED: 14966829] [DOI] [PubMed] [Google Scholar]
Rodriguez 2005 {published data only}
- Rodriguez D, Almirante B, Cuenca‐Estrella M, Park BJ, Planes AM, Mensa J, et al. Impact of venous catheter removal in patients with candidemia. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 2005. 2005;45:426, Presentation number: M‐980. [Google Scholar]
Rodriguez 2007 {published data only}
- Rodriguez D, Park BJ, Almirante B, Cuenca‐Estrella M, Planes AM, Mensa J, et al. Impact of early central venous catheter removal on outcome in patients with candidaemia. Clinical Microbiology and Infection 2007;13(8):788‐93. [PUBMED: 17610598] [DOI] [PubMed] [Google Scholar]
San Miguel 2006 {published data only}
- San Miguel LG, Cobo J, Martos I, et al. Candidemia in children with congenital cardiopathy. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy. 2002; Vol. 42:389.
- San Miguel LG, Cobo J, Otheo E, Martos I, Muriel A, Fortun J, et al. Candidemia in pediatric patients with congenital heart disease. Diagnostic Microbiology and Infectious Disease 2006;55(3):203‐7. [PUBMED: 16545936] [DOI] [PubMed] [Google Scholar]
Shorr 2011 {published data only}
- Shorr AF, Wu C, Kothari S. Outcomes in patients with potentially fluconazole resistant Candida isolates treated with micafungin: insights from randomized trials. Society of Critical Care Medicine's 39th Critical Care Congress. 2009; Vol. 37, issue 12 (supplement):A25.
- Shorr AF, Wu C, Kothari S. Outcomes with micafungin in patients with candidaemia or invasive candidiasis due to Candida glabrata and Candida krusei. The Journal of Antimicrobial Chemotherapy 2011;66(2):375‐80. [PUBMED: 21147825] [DOI] [PubMed] [Google Scholar]
Stamos 1995 {published data only}
- Stamos JK, Rowley AH. Candidemia in a pediatric population. Clinical Infectious Diseases 1995;20(3):571‐5. [PUBMED: 7756477] [DOI] [PubMed] [Google Scholar]
Takakura 2004 {published data only}
- Takakura S, Fujihara N, Saito T, Kudo T, Iinuma Y, Ichiyama S. Clinical factors associated with fluconazole resistance and short‐term survival in patients with Candida bloodstream infection. European Journal of Clinical Microbiology & Infectious Diseases 2004;23(5):380‐8. [PUBMED: 15112070] [DOI] [PubMed] [Google Scholar]
Takesue 2015 {published data only}
- Takesue Y, Ueda T, Mikamo H, Oda S, Takakura S, Kitagawa Y, et al. Management bundles for candidaemia: the impact of compliance on clinical outcomes. The Journal of Antimicrobial Chemotherapy 2015;70(2):587‐93. [PUBMED: 25326087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Talarmin 2009 {published data only}
- Talarmin JP, Boutoille D, Tattevin P, Dargère S, Weinbreck P, Ansart S, et al. Epidemiology of candidemia: a one‐year prospective observational study in the west of France [Épidémiologie des candidémies: étude observationnelle prospectived’un an dans l’Ouest de la France]. Médecine et Maladies Infectieuses 2009;39(12):877‐85. [PUBMED: 19346088] [DOI] [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang HJ, Liu WL, Lin HL, Lai CC. Epidemiology and prognostic factors of candidemia in cancer patients. PloS One 2014;9(6):e99103. [PUBMED: 24901336] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang HJ, Liu WL, Lin HL, Lai CC. Epidemiology and prognostic factors of candidemia in elderly patients. Geriatrics & Gerontology International 2015;15(6):688‐93. [PUBMED: 25256556] [DOI] [PubMed] [Google Scholar]
Taur 2010 {published data only}
- Taur Y, Cohen N, Dubnow S, Paskovaty A, Seo SK. Effect of antifungal therapy timing on mortality in cancer patients with candidemia. Antimicrobial Agents and Chemotherapy 2010;54(1):184‐90. [PUBMED: 19884371] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2011 {published data only}
- Tsai CC, Lay CJ, Wang CL, Lin ML, Yang SP. Prognostic factors of candidemia among nonneutropenic adults with total parenteral nutrition. Journal of Microbiology, Immunology, and Infection 2011;44(6):461‐6. [PUBMED: 21576041] [DOI] [PubMed] [Google Scholar]
Viudes 2002 {published data only}
- Viudes A, Peman J, Canton E, Ubeda P, Lopez‐Ribot JL, Gobernado M. Candidemia at a tertiary‐care hospital: epidemiology, treatment, clinical outcome and risk factors for death. European Journal of Clinical Microbiology & Infectious Diseases 2002;21(11):767‐74. [PUBMED: 12461585] [DOI] [PubMed] [Google Scholar]
Vogiatzi 2013 {published data only}
- Vogiatzi L, Ilia S, Sideri G, Vagelakoudi E, Vassilopoulou M, Sdougka M, et al. Invasive candidiasis in pediatric intensive care in Greece: a nationwide study. Intensive Care Medicine 2013;39(12):2188‐95. [PUBMED: 23942859] [DOI] [PubMed] [Google Scholar]
Wang 2014 {published data only}
- Wang H, Liu N, Yin M, Han H, Yue J, Zhang F, et al. The epidemiology, antifungal use and risk factors of death in elderly patients with candidemia: a multicentre retrospective study. BMC Infectious Diseases 2014;14:609. [PUBMED: 25420435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2005 {published data only}
- Weinberger M, Leibovici L, Perez S, Samra Z, Ostfeld I, Levi I, et al. Characteristics of candidaemia with Candida albicans compared with non‐albicans Candida species and predictors of mortality. The Journal of Hospital Infection 2005;61(2):146‐54. [PUBMED: 16009456] [DOI] [PubMed] [Google Scholar]
Zaoutis 2004 {published data only}
- Zaoutis TE, Greves HM, Lautenbach E, Bilker WB, Coffin SE. Risk factors for disseminated candidiasis in children with candidemia. The Pediatric Infectious Disease Journal 2004;23(7):635‐41. [PUBMED: 15247602] [DOI] [PubMed] [Google Scholar]
Additional references
Blot 2002
- Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Effects of nosocomial candidemia on outcomes of critically ill patients. The American Journal of Medicine 2002;113(6):480‐5. [PUBMED: 12427497] [DOI] [PubMed] [Google Scholar]
Blumberg 2001
- Blumberg HM, Jarvis WR, Soucie JM, Edwards JE, Patterson JE, Pfaller MA, et al. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. The National Epidemiology of Mycosis Survey. Clinical Infectious Diseases 2001;33(2):177‐86. [PUBMED: 11418877] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Chan 2004
- Chan AW, Hrobjartsson A, Haahr MT, Gotzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291(20):2457‐65. [PUBMED: 15161896] [DOI] [PubMed] [Google Scholar]
Colombo 2006
- Colombo AL, Nucci M, Park BJ, Nouér SA, Arthington‐Skaggs B, Matta DA, et al. Epidemiology of candidemia in Brazil: a nationwide sentinel surveillance of candidemia in eleven medical centers. Journal of Clinical Microbiology 2006;44(8):2816‐23. [PUBMED: 16891497] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmond 1999
- Edmond MB, Wallace SE, McClish DK, Pfaller MA, Jones RN, Wenzel RP. Nosocomial bloodstream infections in United States hospitals: a three‐year analysis. Clinical Infectious Diseases 1999;29(2):239‐44. [PUBMED: 10476719] [DOI] [PubMed] [Google Scholar]
Fridkin 2005
- Fridkin SK. The changing face of fungal infections in health care settings. Clinical Infectious Diseases 2005;41(10):1455–60. [PUBMED: 16231257] [DOI] [PubMed] [Google Scholar]
Girmenia 1996
- Girmenia C, Martino P, Bernardis F, Gentile G, Boccanera M, Monaco M, et al. Rising incidence of Candida parapsilosis in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clinical Infectious Diseases 1996;23(3):506‐14. [PUBMED: 8879773] [DOI] [PubMed] [Google Scholar]
Glockner 2013
- Glockner A, Cornely OA. Practical considerations on current guidelines for the management of non‐neutropenic adult patients with candidaemia. Mycoses 2013;56(1):11‐20. [PUBMED: 22574925] [DOI] [PubMed] [Google Scholar]
Gudlaugsson 2003
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clinical Infectious Diseases 2003;37(9):1172‐7. [PUBMED: 14557960] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, GRADE WORKING GROUP. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thopson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919 ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hutton 2000
- Hutton JL, Williamson PR. Bias in meta‐analysis due to outcome variable selection within studies. Journal of the Royal Statistical Society Series C 2000;49:359–70. [Google Scholar]
Kullberg 2015
- Kullberg BJ, Arendrup MC. Invasive candidiasis. The New England Journal of Medicine 2015;373(15):1445‐56. [PUBMED: 26444731] [DOI] [PubMed] [Google Scholar]
Lan 1983
- Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983; Vol. 70, issue 3:659–63.
Lazzarini 2002
Lecciones 1992
- Lecciones JA, Lee JW, Navarro EE, Witebsky FG, Marshall D, Steinberg SM, et al. Vascular catheter‐associated fungemia in patients with cancer: analysis of 155 episodes. Clinical Infectious Diseases 1992;14(4):875‐83. [PUBMED: 1576282] [DOI] [PubMed] [Google Scholar]
Leonidou 2010
- Leonidou L, Gogos CA. Catheter‐related bloodstream infections: catheter management according to pathogen. International Journal of Antimicrobial Agents 2010;36 Suppl 2:S26‐32. [PUBMED: 21129929] [DOI] [PubMed] [Google Scholar]
Luzzati 2008
Marchetti 2004
- Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J, et al. Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991‐2000. Clinical Infectious Diseases 2004;38(3):311‐20. [PUBMED: 14727199] [DOI] [PubMed] [Google Scholar]
Mermel 2001
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Infectious Diseases Society of America, American College of Critical Care Medicine, Society for Healthcare Epidemiology of America. Guidelines for the management of intravascular catheter‐related infections. Clinical Infectious Diseases 2001;32(9):1249‐72. [PUBMED: 11303260] [DOI] [PubMed] [Google Scholar]
Mermel 2009
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter‐related infection: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;49(1):1‐45. [PUBMED: 19489710] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2012
- Miller SE, Maragakis LL. Central line‐associated bloodstream infection prevention. Current Opinion in Infectious Diseases 2012;25(4):412‐22. [PUBMED: 22766647] [DOI] [PubMed] [Google Scholar]
Morgan 2005
- Morgan J, Meltzer MI, Plikaytis BD, Sofair AN, Huie‐White S, Wilcox S, et al. Excess mortality, hospital stay, and cost due to candidemia: a case‐control study using data from population‐based candidemia surveillance. Infection Control and Hospital Epidemiology 2005;26(6):540‐7. [PUBMED: 16018429] [DOI] [PubMed] [Google Scholar]
Nucci 2002
- Nucci M, Anaissie E. Should vascular catheters be removed from all patients with candidemia? An evidence‐based review. Clinical Infectious Diseases 2002;34(5):591‐9. [PUBMED: 11810600] [DOI] [PubMed] [Google Scholar]
Nucci 2005
Pappas 2003
- Pappas PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. NIAID Mycoses Study Group. A prospective observational study of candidemia: epidemiology, therapy, and influences on mortality in hospitalized adult and pediatric patients. Clinical Infectious Diseases 2003;37(5):634‐43. [PUBMED: 19191635] [DOI] [PubMed] [Google Scholar]
Pappas 2007
- Pappas PG, Rotstein CM, Betts RF, Nucci M, Talwar D, Waele JJ, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clinical Infectious Diseases 2007;45(7):883‐93. [PUBMED: 17806055] [DOI] [PubMed] [Google Scholar]
Pappas 2009
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2009;48(5):503‐35. [PUBMED: 19191635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petri 1997
- Petri MG, Konig J, Moecke HP, Gramm HJ, Barkow H, Kujath P, et al. Epidemiology of invasive mycosis in ICU patients: a prospective multicenter study in 435 non‐neutropenic patients. Paul‐Ehrlich Society for Chemotherapy, Divisions of Mycology and Pneumonia Research. Intensive Care Medicine 1997;23(3):317‐25. [PUBMED: 9083235] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93; discussion 661‐6. [PUBMED: 9408720] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Raad 1993
- Raad I, Costerton W, Sabharwal U, Sacilowski M, Anaissie E, Bodey GP. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. The Journal of Infectious Diseases 1993;168(2):400‐7. [PUBMED: 8335977] [DOI] [PubMed] [Google Scholar]
Raad 2004
- Raad I, Hanna H, Boktour M, Girgawy E, Danawi H, Mardani M, et al. Management of central venous catheters in patients with cancer and candidemia. Clinical Infectious Diseases 2004;38(8):1119‐27. [PUBMED: 15095217] [DOI] [PubMed] [Google Scholar]
Rangel‐Frausto 1999
- Rangel‐Frausto MS, Wiblin T, Blumberg HM, Saiman L, Patterson J, Rinaldi M, et al. National epidemiology of mycoses survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clinical Infectious Diseases 1999;29(2):253‐8. [PUBMED: 10476721] [DOI] [PubMed] [Google Scholar]
Reeves 2008
- Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13: Including non‐randomised studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. Chichester: John Wiley & Sons, 2008. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rex 1995
- Rex JH, Bennett JE, Sugar AM, Pappas PG, Serody J, Edwards JE, et al. Intravascular catheter exchange and duration of candidemia. Clinical Infectious Diseases 1995;21(4):994‐6. [PUBMED: 8645855] [DOI] [PubMed] [Google Scholar]
Rucker 2008
- Rucker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27(5):746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Schachter 2003
- Schachter B. Slimy business ‐ the biotechnology of biofilms. Nature Biotechnology 2003;21(4):361‐5. [PUBMED: 12665817] [DOI] [PubMed] [Google Scholar]
Sutton 2002
- Sutton AJ, Cooper NJ, Lambert PC, Jones DR, Abrams KR, Sweeting MJ. Meta‐analysis of rare and adverse event data. Expert Review of Pharmacoeconomics and Outcomes Research 2002;2(4):367‐79. [PUBMED: 19807443] [DOI] [PubMed] [Google Scholar]
Tortorano 2006
- Tortorano AM, Kibbler C, Peman J, Bernhardt H, Klingspor L, Grillot R. Candidaemia in Europe: epidemiology and resistance. International Journal of Antimicrobial Agents 2006;27(5):359‐66. [PUBMED: 16647248] [DOI] [PubMed] [Google Scholar]
TSA 2010 [Computer program]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C, Copenhagen Trial Unit. Trial Sequential Analysis Software. Version 0.8. Copenhagen: Copenhagen Trial Unit, 2010.
Turner 2013
- Turner RM, Bird SM, Higgins JP. The impact of study size on meta‐analyses: examination of underpowered studies in Cochrane reviews. PloS One 2013;8(3):e59202. [PUBMED: 23544056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voss 1997
- Voss A, Noble JL, Verduyn Lunel FM, Foudraine NA, Meis JF. Candidemia in intensive care unit patients: risk factors for mortality. Infection 1997;25(1):8‐11. [PUBMED: 9039530] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wey 1988
- Wey SB, Mori M, Pfaller MA, Woolson RF, Wenzel RP. Hospital‐acquired candidemia. The attributable mortality and excess length of stay. Archives of Internal Medicine 1988;148(12):2642‐5. [PUBMED: 3196127] [DOI] [PubMed] [Google Scholar]
Wisplinghoff 2004
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clinical Infectious Diseases 2004;39(3):309‐17. [PUBMED: 15306996] [DOI] [PubMed] [Google Scholar]
Zaoutis 2005
- Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clinical Infectious Diseases 2005;41(9):1232‐9. [PUBMED: 16206095] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Janum 2014
- Janum S, Afshari A. Central venous catheter (CVC) removal for adult patients with candidaemia. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD011195] [DOI] [PMC free article] [PubMed] [Google Scholar]


