Abstract
Background
Umbilical cord infection caused many neonatal deaths before aseptic techniques were used.
Objectives
To assess the effects of topical cord care in preventing cord infection, illness and death.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group trials register (September 2003) and the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 2, 2003). We also contacted experts in the field.
Selection criteria
Randomized and quasi‐randomized trials of topical cord care compared with no topical care, and comparisons between different forms of care.
Data collection and analysis
Two reviewers assessed trial quality and extracted data.
Main results
Twenty‐one studies (8959 participants) were included, the majority of which were from high‐income countries. No systemic infections or deaths were observed in any of the studies reviewed. No difference was demonstrated between cords treated with antiseptics compared with dry cord care or placebo. There was a trend to reduced colonization with antibiotics compared to topical antiseptics and no treatment. Antiseptics prolonged the time to cord separation. Use of antiseptics was associated with a reduction in maternal concern about the cord.
Authors' conclusions
Good trials in low‐income settings are warranted. In high‐income settings, there is limited research which has not shown an advantage of antibiotics or antiseptics over simply keeping the cord clean. Quality of evidence is low.
Plain language summary
Topical umbilical cord care at birth
No evidence that applying sprays, creams or powders are any better than keeping the baby's cord clean and dry at birth.
The umbilical cord connects the baby to its food and oxygen supply in the womb, and is clamped and cut at birth. The cord stump dries, shrivels and becomes black before falling off the baby's belly button, five to 15 days after birth. Without proper care, the baby may become infected through the stump. Usually the cord is kept clean and dry by loosely covering it with clean clothes. Hand washing is critical. The review found that not enough trials had been done to show if antiseptics or antibiotics were any better at keeping infection away. More research is needed.
Background
The umbilical cord which connects the baby and placenta in utero (the womb) is made of blood vessels and connective tissue. It is covered by a membrane which is bathed in amniotic fluid. After birth, cutting the cord physically and symbolically separates the mother and her baby. The cord stump dries, falls off and the wound heals.
As the umbilical stump dries, it shrivels turning black in colour. An area of separation forms between the drying cord and the abdominal wall in which polymorphonuclear leucocytes, a form of white blood cells, are present (OudesluysMurphy 1990). During the normal separation process, material may collect at this junction which sometimes looks like pus and is often wrongly identified as an infection. The cord usually separates between five and 15 days after birth. Before the separation, the remaining stump can be considered to be a healing wound and thus a possible route for infection through the vessels into the baby's blood stream.
Soon after a normal delivery, the skin of the newborn baby including the umbilical stump is colonized mainly by non‐pathogenic (non‐infection causing) bacteria such as coagulase‐negative Staphylococci and Diphtheroid bacilli. Pathogenic bacteria such as Coliforms and Streptococci may also be present on the skin (Sarkany 1967) and can track up the umbilical stump causing infection. It is therefore essential to keep the cord clean.
An umbilical cord infection may be clinically obvious, but is also sometimes hidden. In frank infections, the cord may be swollen, the surrounding skin inflamed, or the cord may be 'smelly' if infected with anaerobic bacteria. Tracking of bacteria along the umbilical vessels is not obvious to the eye, but can cause septicaemia (blood poisoning), or result in other focal infections as a result of blood‐borne spread such as septic arthritis (Cullen 1916; Forshall 1957). In such cases, affected babies may also present with fever, lethargy or poor feeding.
Cord cutting and care of the umbilical stump varies according to accepted practice and culture (Elhassani 1984). In many parts of the world the cord is cut with unsterile tools such as used razors or scissors after which various substances are applied including charcoal, grease, cow dung or dried banana to speed up cord separation. These practices are important sources of bacterial infection and neonatal tetanus (Bennett 1999; Meegan 2001).
While there is a general agreement about the 'clean' technique for cutting the cord using a sterile cutting instrument (blade or scissors) and clean hands to avoid infection, there is less agreement on what is the best care of the cord stump. Most frequent modern practice is applying antiseptic agents to the cord (usually alcohol, silver sulphadiazine, iodine, chlorhexidine; and dyes such as triple dye, gentian violet, acriflavine and eozine). Some authorities recommend routine topical application of antibiotics, including bacitracin, neomycin, nitrofurazone, or tetracycline, or moisture absorbing powders. These may be used as solutions in water, alcohol, detergent or ointments.
A practice often forgotten is to do nothing other than keep the cord clean and dry without applying anything(Dore 1998; Mugford 1986).
Bathing the baby soon after birth with an antimicrobial such as hexachlorophene may reduce skin contamination. However, hexachlorophene is no longer recommended in new born babies as it is absorbed through the skin and is neurotoxic (WHO 1998a). Cord separation may be delayed by topical antimicrobials, premature delivery, caesarean section or low birthweight (Novack 1988; OudesluysMurphy 1987) which can potentially increase the risk of bacterial entry. Delays in cord separation increase the midwives' workload in countries where there is a policy of continued home visits until cord separation (Mugford 1986).
Other practices can significantly contribute to preventing infection in the early neonatal period. Rooming‐in (nursing babies in rooms with their mothers) has been shown to be protective to babies who become colonized with their mothers' non‐pathogenic bacteria as opposed to other harmful micro‐organisms. This is now widely practiced in high‐income countries (Enkin 2000).
Despite the advent of asepsis (Cullen 1916), umbilical cord infections continue to cause many deaths in neonates in low and middle‐income countries (WHO 1998a). Contamination of the cord remains a common cause of neonatal tetanus in deprived populations (Thayaparan 1998; Woodruff 1984). Around 200,000 neonatal deaths (5%) that occur every year are the result of neonatal tetanus (WHO 1998b, CHRPSR 1999).
The World Health Organization and others emphasize good hygiene at delivery, and promote good cord care practice. However, recommendations for cord care are often based on traditional assessments of published literature and opinion. The aim of this review is to provide data useful for identifying good practice in both high and low‐income countries. The findings of the original review were incorporated in a review summarizing available consensus on best and appropriate practice (WHO 1998a).
Objectives
To assess the effectiveness of topical cord care compared with no routine care, and comparisons between different forms of care, in preventing cord infection, illness and death.
In particular, to answer the following questions: Is any intervention better than no routine cord care?
If so, which care is preferable:
Antiseptic or no antiseptic?
Antibiotic or no antibiotic?
Antibiotic or antiseptic?
For any intervention, what is the optimal frequency, formulation and duration of application?
Hypotheses to be explored:
Cord antiseptics or antibiotics are effective in reducing neonatal infection and death when babies are nursed together in a nursery but are less likely to have an impact on health when babies are roomed‐in or nursed at home.
When people are living in poverty such that basic hygiene at home delivery and postnatally is constrained, cord antiseptics or antibiotics are more likely to have an impact on serious illness or death in the neonate.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized trials.
Types of participants
Newborn infants of any gestation.
Types of interventions
Antiseptic versus no antiseptic or placebo;
antibiotic versus no antibiotic or placebo;
antibiotic versus antiseptic;
antiseptic versus antiseptic;
antibiotic versus antibiotic;
single versus multiple applications;
washing the cord versus dry care.
All interventions must be topical preparations; to be excluded if the intervention is a combination of an antiseptic and antibiotic.
Antiseptics to include alcohol, triple dye, silver sulphadiazine, acriflavine, iodine, chlorhexidine, gentian violet.
Antibiotics to include bacitracin, nitrofurazone, or tetracycline.
Types of outcome measures
Primary outcomes
Clinical evidence of local cord infection: redness, swelling, smell;
clinical evidence of disseminated bacterial infection: fever, meningitis, septic foci;
death.
Secondary outcomes
Time to cord separation;
bacterial colonization;
mother unhappy with treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group trials register (September 2003).
The Cochrane Pregnancy and Childbirth Group's trials register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
monthly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness search of a further 37 journals.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the 'Search strategies for identification of studies' section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are given a code (or codes) depending on the topic. The codes are linked to review topics. The Trials Search Co‐ordinator searches the register for each review using these codes rather than keywords.
In addition, we searched the Central Register of Controlled Trials (The Cochrane Library, Issue 2, 2003) using the search term umbilical cord*.
We contacted the World Health Organization and experts and individual researchers working in the field.
Data collection and analysis
Two reviewers scrutinized all eligible papers and applied the inclusion criteria independently. If there had been disagreement, consultation with a third person (an editor in the Pregnancy and Childbirth Group) would have been sought.
Concealment of allocation was graded as: A ‐ adequate measures used such as opaque envelopes, independent number generation; B ‐ uncertainty, whether or not allocation was adequately concealed; C ‐ allocation not adequately concealed; and D ‐ score not assigned.
We recorded the number of participants experiencing the event in each group of the trial for binary outcomes. For continuous outcomes (such as time to cord separation), we recorded the arithmetic means and standard deviations and we combined means using the weighted mean difference. The results were analyzed using relative risks and a fixed effect model. Heterogeneity was checked first visually and then by the I²statistic and the decision to use a random effects model was taken on a case by case basis.
Potential causes of heterogeneity for exploration using subgroup analysis:
Frequency of intervention (single at birth; every nappy change);
duration (until discharged; until cord separation);
formulation (aqueous based; alcohol based; powder; spray dressing);
maturity (full‐term versus premature).
The time when the trials were conducted could also be a factor since the patterns of newborn care (feeding, nursing, rooming‐in) have changed significantly over the past 25 years.
Results
Description of studies
Twenty‐one studies met the inclusion criteria (see 'Characteristics of included studies'). These were conducted in Canada (4), Israel (1), Italy (1), Norway (2), Spain (1), Taiwan (1), Thailand (1), UK (2) and USA (8). One study was published in Spanish (Perapoch 1993). The 23 excluded studies are listed in 'Characteristics of excluded studies'.
There were two large studies with over 1200 infants (Meberg 1990; Pezzati 2002) and the largest trial (Pezzati 2002) compared eight treatments in 1470 infants. Most of the studies were small with comparator groups of less than 300.
Nineteen studies were on full‐term infants and excluded those who were small for gestational age or had other neonatal conditions. Two studies were on preterm infants (Bain 1994; Rosenfeld 1990). Babies were nursed in hospital initially and most were followed up at home. Rooming‐in was practised in six studies (Barrett 1979; Dore 1998; Meberg 1985; Meberg 1990; Rush 1986; Wald 1977) but this was partial rooming‐in as most of the babies were also cared for in the nursery.
Interventions
Twelve studies had more than two arms and numerous comparisons between antiseptics and antibiotics were used (Table 1). Eight studies compared various antiseptics with no specific care (Bain 1994; Barrett 1979; Dore 1998; Meberg 1985; Medves 1997; Pezzati 2002; Speck 1980; Wald 1977). Ten studies compared antiseptics with other antiseptics (Arad 1981; Barrett 1979; Gladstone 1988; Panyavudhikrai 2002a; Panyavudhikrai 2002b; Perapoch 1993; Pezzati 2002; Rosenfeld 1990; Schuman 1985; Speck 1980). One study compared antibiotics with triple dye (Arad 1981). One study each compared antiseptic powder with astringent powder (Mugford 1986); and antiseptic with hydrophobic gauze dressing (Meberg 1990). One study compared daily bathing versus one initial bath and no additional cord care (Rush 1986).
1. Different combinations of antiseptics and antibiotics used in included trial.
| Antimicrobial | Placebo | Triple dye | Silver | Povidone‐iodine | Alcohol | Bacitracin | Neomycin | Chlorhexidene |
| Triple dye | X | X | X | X | X | X | X | |
| Silver sulfadiazine | X | X | X | X | X | |||
| Povidone‐iodine | X | X | X | X | ||||
| Alcohol | X | X | X | X | ||||
| Bacitracin | X | X | X | |||||
| Neomycin | X | |||||||
| Chlorhexidine | X | X | ||||||
| Mercurochrome | X | X | ||||||
| Salicylic sugar powder | X | X | X | |||||
| Zinc powder | X | X | X | |||||
| Green clay powder | X | X | X | |||||
| Katoxin powder | X | X | X | |||||
| Fuschine | X | X | X | |||||
| Hydrophobic gauze | X |
There were various other cleaning co‐interventions which were applied to both groups such as an initial bath (with soap, chlorhexidine or hexachlorophane) or daily bathing. In several studies alcohol was used to clean the umbilical stump following discharge home. Generally, treatment to the umbilical stump continued for a few days after or was stopped at cord separation.
Outcomes
(Table 2) Twelve studies reported cord infections; seven studies reported disseminated infections; one study reported deaths; twelve reported time of cord separation; eleven studies reported bacterial colonisation; and three studies reported parental satisfaction.
2. Outcomes.
| Study | Cord infection | Disseminated infect. | Death | Cord separation time | Bact. colonization | Mat. satisfaction |
| Arad 1981 | Y | Y | N | Y | N | N |
| Bain 1994 | Y | N | N | Y | N | N |
| Barrett 1979 | N | N | N | N | Y | N |
| Dore 1998 | Y | N | N | Y | N | Y |
| Gladstone 1988 | Y | N | N | Y | Y | Y |
| Golombek 2002 | Y | N | N | Y | N | N |
| Hsu 1999 | Y | N | N | Y | N | N |
| Janssen 2002 | Y | Y | N | N | Y | N |
| Meberg 1985 | Y | Y | N | N | Y | N |
| Meberg 1990 | Y | Y | N | Y | N | N |
| Medves 1997 | N | N | N | Y | Y | N |
| Mugford 1986 | N | N | N | Y | N | N |
| Panyavudhikrai 2002 | Y | N | N | N | N | N |
| Perapoch 1993 | Y | N | N | Y | Y | Y |
| Pezatti 2002 | Y | Y | Y | Y | Y | N |
| Rosenfeld 1990 | N | Y | N | N | Y | N |
| Rush 1986 | N | N | N | N | Y | N |
| Schuman 1985 | Y | N | N | Y | N | N |
| Speck 1977 | Y | Y | N | N | N | N |
| Speck 1980 | Y | N | N | N | Y | N |
| Wald 1977 | N | N | N | N | Y | N |
| Bact ‐ Bacterial; Mat ‐ Maternal |
Risk of bias in included studies
All studies were described as randomized. Generation of allocation was by computer (Barrett 1979; Schuman 1985); and random tables (Dore 1998; Gladstone 1988). Six trials were quasi‐randomized using: alternate allocation (Golombek 2002; Meberg 1990; Rosenfeld 1990; Wald 1977); the time of admission (Pezzati 2002); and the cot ID number (Rush 1986). Three trials with adequate allocation concealment used envelopes (Dore 1998; Janssen 2003; Mugford 1986).
As follow‐up times were generally up to separation of the cord, losses to follow up appeared minimal. Three studies continued follow up to six weeks (Meberg 1985; Meberg 1990; Wald 1977).
Effects of interventions
Twenty‐one studies with 8959 participants met the inclusion criteria.
Antiseptic versus dry cord care/placebo
Ten studies had treatment arms with this comparison. These compared dry cord care/placebo with: alcohol (Bain 1994; Dore 1998; Medves 1997; Pezzati 2002); triple dye (Barrett 1979; Speck 1980; Wald 1977); silver sulfadiazene (Barrett 1979; Speck 1980); and in one study each, zinc powder (Mugford 1986); chlorhexidine (Meberg 1985) and salicylic sugar powder; green clay powder; katoxin powder; and fuschine (all Pezzati 2002).
No deaths were reported in the single study reporting this outcome (Pezzati 2002). No severe bacterial systemic infections occurred in the two trials reporting this outcome (Meberg 1985; Pezzati 2002).
Two trials using alcohol as the comparator found no difference in cord infections (two trials; relative risk (RR) 0.63, 95% confidence interval (CI) 0.19 to 2.06; Bain 1994; Pezzati 2002). There was no significant difference in the incidence of cord infection with triple dye; chlorhexidine; salicylic sugar powder; green clay powder; katoxin powder; and fuschine compared with dry cord care/placebo. One study reported no cord infection in either group (Dore 1998). There was no significant difference in cord infection whether topical antiseptic was used or not (RR 0.53, 95% CI 0.25 to 1.13).
Meta‐analysis of four studies with alcohol as the comparator showed a trend towards cord separation being significantly later in the alcohol group but there was considerable heterogeneity (random effects, weighted mean difference (WMD) 3.51, 95% CI ‐0.41 to 7.43, test for heterogeneity p < 0.00001). Sensitivity analysis excluding the study with premature babies (Bain 1994) did not affect this result (three trials, random effects, WMD 4.54, 95% CI ‐0.49 to 9.57, test for heterogeneity p < 0.00001). Cord separation time was longer with triple dye (WMD 4.10, 95% CI 3.07 to 5.13) and fuchsine (WMD 2.80, 95% CI 2.01 to 3.59). Time to cord separation was generally shorter with powder applications: zinc (WMD ‐1.82, 95% CI ‐2.23 to ‐1.41; Mugford 1986); salicylic sugar (WMD ‐1.90, 95% CI ‐2.47 to ‐1.33); and green clay (WMD ‐0.80, 95% CI ‐1.36 to ‐0.24).
Compared with dry cord care/placebo, bacterial colonization by Staphylococcus aureus was significantly reduced by alcohol (RR 0.30, 95% CI 0.16 to 0.55); triple dye (three trials, RR 0.14, 95% CI 0.10 to 0.20); silver sulfadiazene (two trials: RR 0.72, 95% CI 0.59 to 0.87); chlorhexidene (RR 0.65, 95% CI 0.55 to 0.77); salicylic sugar powder (RR 0.32, 95% CI 0.17 to 0.58); green clay powder (RR 0.51, 95% CI 0.31 to 0.82); and fuschine (RR 0.52, 95% CI 0.32 to 0.84). Bacterial colonization by Streptococci was significantly reduced by alcohol (RR 0.20, 95% CI 0.04 to 0.89); triple dye (four trials: RR 0.57, 95% CI 0.44 to 0.73); silver sulfadiazene (two trials, RR 0.60, 95% CI 0.42 to 0.85) and fuschine (RR 0.19, 95% CI 0.04 to 0.85). Escherichia coli colonization was significantly reduced by triple dye (two trials: RR 0.76, 95% CI 0.63 to 0.91), silver sulfadiazene (RR 0.70, 95% CI 0.53 to 0.93) and chlorhexidene (RR 0.48, 95% CI 0.27 to 0.85).
More babies treated with green clay powder (RR 4.62, 95% CI 2.41 to 8.84) and katoxin powder (RR 5.87, 95%CI 3.12 to 11.05) were colonized by Streptococci. More infants treated with fuschine were colonized by Escherichia coli (RR 2.04, 95% CI 1.33 to 3.13).
Two studies using alcohol as the comparator reported parental satisfaction (Dore 1998; Pezzati 2002). There was no difference in maternal satisfaction in Dore 1998 mean score 1.49 (standard deviation (SD) 0.7) alcohol group versus 1.56 (SD 0.7) in the no alcohol group; t = ‐2.13, p‐value not significant, authors' calculation.) In Pezzati 2002, parents in the natural drying group were more satisfied with the treatment (RR 0.55, 95% confidence interval 0.45 to 0.66).
Antiseptic versus antibiotic
Two studies had treatment arms comparing: triple dye with neomycin (Arad 1981); triple dye with bacitracin (Gladstone 1988); silver sulfadiazene with neomycin (Arad 1981); silver sulfadiazene with bacitracin (Gladstone 1988); and povidone‐iodine with bacitracin (Gladstone 1988).
No clinical infections were reported in either study.
There was a trend to reduced colonization with Staphylococcus aureus with antibiotics compared with antiseptics (Gladstone 1988).
There was a trend towards time for cord separation being shorter with antiseptics compared with antibiotics. Time to cord separation was significantly shorter with triple dye compared to bacitracin (WMD ‐5.60, 95% CI ‐9.36 to ‐1.84; Gladstone 1988) and neomycin (WMD ‐4.30, 95% CI ‐6.27 to ‐2.33; Arad 1981) and for povidone‐iodine compared with bacitracin (WMD ‐2.00, 95% CI ‐3.67 to ‐0.33). Cord separation time was significantly longer with silver sulfadiazene compared with bacitracin (WMD 2.00, 95% CI 0.20 to 3.80).
Antiseptic versus antiseptic
Triple dye versus other antiseptic
Seven studies had treatment arms that compared triple dye with: silver sulphadiazine (Barrett 1979; Gladstone 1988); alcohol (Golombek 2002; Panyavudhikrai 2002a; Panyavudhikrai 2002b; Rosenfeld 1990; Schuman 1985); and povidone‐iodine (Gladstone 1988; Panyavudhikrai 2002a). Pezzati 2002 compared triple dye with six other antiseptics.
Fewer cord infections were reported with triple dye when compared with alcohol (four trials, RR 0.30, 95% CI 0.19 to 0.49) and povidone‐iodine (RR 0.15, 95% CI 0.07 to 0.32; Panyavudhikrai 2002a).
Fewer babies treated with triple dye were colonized with Staphylococcus aureus compared with alcohol (two trials RR 0.45, 95% CI 0.25 to 0.81: Pezzati 2002; Rosenfeld 1990) or silver sulfadiazine (two trials RR 0.28, 95% CI 0.17 to 0.46). More babies treated with triple dye were colonized with Escherchia coli compared with other antiseptics.
Two trials (Pezzati 2002; Schuman 1985) reporting cord separation with triple dye in comparison with alcohol gave opposite results and should be analysed separately because of considerable heterogeneity (p < 0.00001, random effects). Schuman 1985 was considerably smaller with 71 babies compared with 373 babies in Pezzati 2002. Similarly, two other trials comparing triple dye with silver sulfadiazene (Arad 1981; Gladstone 1988) also reported opposite results and were not combined because of significant heterogeneity (p < 0.002). Both the studies were small. Authors in Golombek 2002 comparing triple dye with alcohol reported median cord separation times as 13 days (n = 326; range 2 to 37) with triple dye compared to 10 days (n = 273, range 2 to 34) with alcohol (p < 0.0001, authors' calculation). Cord separation time was significantly longer with triple dye compared to povidone‐iodine (WMD 7.60, 95% CI 3.96 to 11.24; Gladstone 1988).
Povidone‐iodine versus other antiseptic
Two trials had treatment arms comparing povidone‐iodine with: silver sulfadiazine (Gladstone 1988); alcohol (Panyavudhikrai 2002a); and triple dye (Panyavudhikrai 2002a).
There was no difference in cord infection between povidone‐iodine and alcohol (RR 1.18, 95% CI 0.87 to 1.62; Panyavudhikrai 2002a). More cord infections were seen with povidone‐iodine compared to triple dye (RR 0.15, 95% CI 0.07 to 0.32; Panyavudhikrai 2002a).
Cord separation time with povidone‐iodine was significantly shorter compared with silver sulfadiazine (WMD ‐4.00, 95% CI ‐5.53 to ‐2.47) and triple dye (WMD ‐7.6, 95% CI ‐3.96 to ‐11.24).
Chlorhexidine versus other antiseptic
Two studies had treatment arms comparing chlorhexidine with: hydrophobic gauze (Meberg 1990); alcohol (Perapoch 1993); and mercurochrome (Perapoch 1993).
Perapoch 1993 reported cord infections and none occurred in any group. More cord infections were seen with chlorhexidine compared to hydrophobic gauze (RR 1.36, 95% CI 0.55 to3.36; Meberg 1990).
Cord separation time was significantly shorter with chlorhexidene compared with hydrophobic gauze (WMD ‐0.4, 95% CI ‐0.57 to ‐0.23). Cord separation time was significantly longer with chlorhexidene in comparison with alcohol (WMD ‐5.70, 95% CI ‐6.82 to ‐4.58); and mercurochrome (WMD 6.40, 95% CI 5.25 to 7.55).
Single versus multiple applications
Three studies compared single and multiple applications with: triple dye (Gladstone 1988; Hsu 1999) and dusting powders (Mugford 1986). Triple dye was applied once at birth or daily. Alcohol was also applied daily in addition to the triple dye in Hsu 1999.
Both trials using triple dye reported no cord or skin infections.
Cord separation was significantly prolonged with multiple applications of triple dye (two trials, WMD ‐4.27, 95% CI 5.48 to ‐3.05). There was no difference in cord separation time with the dusting powders (WMD ‐0.02, 95% CI ‐0.31 to 0.27).
One trial reported no difference in colonization by Staphylococcus aureus (Gladstone 1988).
Mothers were equally satisfied with both treatments in Gladstone 1988.
Impact on various specific outcomes
Cord separation (in days)
Summary analysis was explored with three comparisons but there was significant heterogeneity:
Alcohol versus dry cord care/placebo (Bain 1994; Dore 1998; Medves 1997; Pezzati 2002): The trend was towards cord separation being prolonged in the alcohol group but there was no significant difference in cord separation and considerable heterogeneity (four trials, WMD 3.51, 95% CI ‐0.41 to 7.43, test for heterogeneity p < 0.00001, random effects). Sensitivity analysis excluding the study with premature babies (Bain 1994) did not change this result (three trials, WMD 4.54, 95% CI ‐0.49 to 9.57, test for heterogeneity p < 0.00001, random effects).
Triple dye versus alcohol (Pezzati 2002; Schuman 1985): There was no significant difference in cord separation (two trials, WMD ‐0.16, 95% CI ‐10.25 to 9.94, test for heterogeneity p < 0.00001, random effects).
Triple dye versus silver sulfadiazene (Arad 1981; Gladstone 1988): There was no significant difference in cord separation (two trials, WMD 0.15, 95% CI ‐6.2 to 6.5, test for heterogeneity p < 0.002, random effects).
Studies which applied nothing to the cord had mean separation times of about nine days (four studies: Bain 1994; Dore 1998; Medves 1997; Pezzati 2002); with powders it was about seven (four studies: Arad 1981; Bain 1994; Mugford 1986; Pezzati 2002); alcohol it was about 11 days (six studies: Bain 1994; Dore 1998; Medves 1997; Perapoch 1993; Pezzati 2002; Schuman 1985); antibiotics, about 12 days (two studies: Arad 1981; Gladstone 1988); triple dye, about 14 days (six studies: Arad 1981; Gladstone 1988; Hsu 1999; Panyavudhikrai 2002b; Pezzati 2002; Schuman 1985); and silver sulphadiazine, about 12 days (two studies: Arad 1981; Gladstone 1988) (See Table 3).
3. Mean cord separation times.
| Cord care | Study | N | Mean (days) | SD |
| None | Bain 1994 | 25 | 8.61 | 2.88 |
| Dore 1998 | 909 | 8.16 | 3.1 | |
| Medves 1997 | 65 | 10.5 | 3.7 | |
| Pezzati 2002 | 177 | 7.5 | 3.1 | |
| COMBINED | 1176 | 8.7 | ||
| Powder | Arad 1981 | 34 | 6.4 | 1.75 |
| Bain 1994 | 24 | 7.3 | 2.09 | |
| Mugford 1986 | 199 | 6.29 | 1.73 | |
| Mugford 1986 | 202 | 6.93 | 1.95 | |
| Mugford 1986 | 197 | 7.19 | 1.75 | |
| Pezzati 2002 | 167 | 5.6 | 2.3 | |
| COMBINED | 789 | 6.63 | ||
| Alcohol | Panyavudhikrai 2002b | 214 | 11.5 | |
| Bain 1994 | 24 | 8.96 | 3.51 | |
| Dore 1998 | 907 | 9.8 | 4.6 | |
| Perapoch 1993 | 75 | 8.4 | 2.6 | |
| Medves 1997 | 71 | 13.1 | 5.7 | |
| Pezzati 2002 | 178 | 16.9 | 7.5 | |
| Schuman 1985 | 36 | 10.7 | 3.3 | |
| COMBINED | 1505 | 11.3 | ||
| Antibiotics | Arad 1981 | 26 | 12.0 | 4.1 |
| Gladstone 1988 | 48 | 11.8 | 4.8 | |
| COMBINED | 74 | 11.9 | ||
| Triple dye | Arad 1981 | 36 | 7.7 | 3.6 |
| Gladstone 1988 | 14 | 17.4 | 6.7 | |
| Hsu 1999 | 76 | 16.9 | 4.4 | |
| Pezzati 2002 | 195 | 11.6 | 6.6 | |
| Schuman 1985 | 35 | 15.7 | 3.6 | |
| Panyavudhikrai 2002b | 213 | 13.6 | ||
| COMBINED | 569 | 13.8 | ||
| Silver sulfadiazene | Arad 1981 | 25 | 10.6 | 4.1 |
| Gladstone 1988 | 42 | 13.8 | 3.9 | |
| COMBINED | 67 | 12.2 |
Examining the impact of interventions on these times suggests that alcohol or powder when compared with nothing have a minimal impact on separation times except in one study (Pezzati 2002), where daily alcohol applications more than doubled the time before separation compared to natural drying or antimicrobial powders. In one large study (Dore 1998), separation time was significantly shorter with alcohol but the time difference was only 1.8 days.
Discussion
There were few trials, considering the millions of newborns whose umbilical cords are treated with topical applications. All but two of the trials were conducted in high‐income countries, despite the fact that most neonatal deaths occur in low‐ and middle‐income countries where a significant proportion are due to tetanus associated with sub‐optimal cord care.
Most of the trials did not report our outcomes of interest as well as factors affecting methodological quality. We were, therefore, not able to assess trial quality. Follow up was generally to cord separation and outcomes subsequent to this (such as bacterial infection, whether local or generalized) would not have been detected. One of the studies that routinely followed all infants up to six weeks (Meberg 1985) showed relatively high levels of skin infections of various kinds, but there were no differences between the intervention and treatment group.
Over 40 different comparisons were seen with few trials using the same antiseptics or antibiotics. Meta‐analysis was therefore limited to few outcomes. Antiseptics such as triple dye; alcohol; silver sulfadiazene; and povidone‐iodine were used more frequently than antibiotics. This may be because their role is seen as more preventative than curative as antiseptics inhibit micro‐organisms and don't necessarily kill them whereas antibiotics both inhibit and kill micro‐organisms. Another important factor could be that antiseptics are cheaper than antibiotics.
Most trials reported on umbilical cord infections. They were rare. Information on disseminated infections as well as other topical infections (such as skin and eye infections) was limited. Death as an outcome was only reported in one study, which is probably because most of the trials were conducted in high‐income countries were neonatal mortality rates are low.
Eleven studies examined colonization of the skin. Measurement and reporting of these outcomes varied between studies, and was sometimes difficult to interpret. Colonization was reduced with antibiotic and antiseptic use, but the clinical significance of skin colonization is not known.
There were trends towards shorter cord separation times with no topical care compared to antiseptics, and shorter separation times with powder preparations compared to no intervention. Multiple applications compared to single applications of triple dye prolonged cord separation time in one trial. The clinical impact of delays of cord separation is unknown, but it has social and cost implications: delay makes mothers anxious, and increases the number of domiciliary midwife visits to the home (Mugford 1986).
Two studies were on well preterm infants. Such infants are at higher risk of infection because of their prematurity and are also have a higher risk of nosocomial infections. Prematurity is associated with longer cord separation times but there was no difference when alcohol was compared to placebo (Bain 1994). One trial specifically looked at bacterial colonization by methicillin‐resistant Staphylococcus aureus (MRSA) which was significantly reduced by triple dye compared with alcohol. None of the two trials reported on the potential risk of the topical antiseptic/antibiotic being absorbed systemically (percutaneous absorption) (Aggett 1981), which is associated with prematurity. As the trials were small, it is difficult to know how the findings relate to this particular subgroup of infants. Larger studies should be conducted in future.
Overall care fashions have changed over the period of the studies reviewed, and this is likely to impact on the comparative effectiveness of various interventions. For example, there has been a shift from nursery care to rooming‐in high‐income countries, thus reducing the risk of infection.
Much of the concern from mothers and health workers relates to uncertainty about the normal process of drying and separation, including appearance and odour of decomposing tissue. Interventions in the West mainly relate to modifying this process in some way. Oudesluys‐Murphy et al described the histological findings during normal drying and separation of the umbilical stump and the perinatal factors modifying the process (OudesluysMurphy 1987; OudesluysMurphy 1990). Good research documenting the range of clinical variations of this normal process of cord separation is required.
Authors' conclusions
Implications for practice.
Based on the studies meeting the inclusion criteria for this review, we are unable to be sure what is best practice for cord care in institutions and at home in high‐income countries. Studies to date have insufficient evidence to know whether antiseptics or antibiotics have any additional advantage over keeping the cord clean and dry. Cord separation time with no topical care was shorter. Since home visits for cord care in developed countries may need to be frequent, earlier separation of the cord stump could decrease the need for the visits and thus reduce cost of postnatal care (Mugford 1986).
Some infants are at high risk of infection in hospital (such as premature babies or babies nursed on intensive care units). We identified two trials with premature babies. Given the higher risk of bacterial sepsis in these infants, use of antiseptics is unlikely to be harmful, and has the potential for reducing nosocomial infection by reducing umbilical cord and skin colonization.
This review provides no evidence on best cord care practice for settings where babies are at a higher risk of bacterial contamination of the cord such as those delivered in sub‐optimal hygienic conditions either at home or in institutions in poor‐resource countries or where harmful cord care practices prevail. Where the risk of bacterial infection appears high it might be prudent to use topical antiseptics. However, quality of evidence is not adequate to recommend the best antiseptic and the regimen for cord care. It would seem sensible, in situations where packages of care around improving umbilical cord sepsis are introduced, to conduct randomized comparisons to identify the best agents and regimens.
Implications for research.
In high‐income countries, where mortality is very low, important outcomes must include infections in the first month of life, maternal satisfaction, and time to cord separation. There is a good argument to conduct a study of existing inexpensive interventions with no specific topical cord care.
In low‐ and middle‐ income countries, neonates have a much higher risk of infection resulting in serious illness or death. The cord probably remains an important portal for bacteria, as demonstrated by neonatal tetanus. However, we still do not know what is the best cord care: what are the most appropriate agents (alcohol, antiseptics or antibiotics; powders, solutions or ointments) for routine use, and how often they should be applied. Any agent should be easily available, inexpensive and easy to apply. We also do not know what the best method is for cleaning the cord area. Trials should also address the best ways for replacing harmful cord practices.
Trials in low‐ and middle‐ income countries should be part of a package of care promoting good hygiene at delivery and until the cord separation in deprived areas where hygiene remains a problem. Within such an intervention programme, groups of women could be randomized to receive different preparations for the cord. The first set of trials could compare water and soap with an antiseptic (such as chlorhexidine, iodine or powders). Harmful effects of antimicrobials such as effect of iodine on thyroide gland function and possible interference with neonatal screening for congenital hypothyroidism, should be taken into account.
One potential intervention for cord care is colostrum which has bacteriostatic properties and could be applied to the cord stump. No research has been done and future trials should consider using this as one intervention arm.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2016 | Amended | The Published notes section has been updated to clarify that this review will not be updated because it has been replaced by a new review (prepared by a new review team) on 'Umbilical cord antiseptics for preventing sepsis and death among newborns' (Imdad 2013). |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 30 January 2013 | Amended | Contact details updated. |
| 2 December 2011 | Amended | Added information about the updating of this review to Published notes. |
| 20 September 2008 | Amended | Converted to new review format. |
| 6 May 2004 | New citation required and conclusions have changed | Substantive amendment |
| 1 September 2003 | New search has been performed | Eleven new studies with 3773 participants were included, two from less developed countries (Taiwan and Thailand). All but two were published in 1990 or later. Two studies included preterm infants and one study reported deaths. |
Notes
This review will not be updated because it has been replaced by a new review (prepared by a new review team) on 'Umbilical cord antiseptics for preventing sepsis and death among newborns' (Imdad 2013).
Acknowledgements
To Dr Iain Chalmers who compiled the first review and to Professor Justus Hofmeyr and Dr Metin Gulmezoglu for assistance.
As part of the pre‐publication editorial process, the first edition of this review was commented on by three peers (an editor and one referee external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Antiseptic vs dry cord care/placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cord infection | 4 | 2831 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.13] |
| 1.1 Alcohol | 2 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.19, 2.06] |
| 1.2 Triple dye | 2 | 968 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.13, 3.49] |
| 1.3 Chlorhexidine | 1 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Salicylic sugar powder | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.38] |
| 1.5 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.26] |
| 1.6 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.65] |
| 1.7 Fuschine | 1 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.04, 5.17] |
| 2 Bacterial colonization ‐ Staphylococcus aureus | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Alcohol | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.16, 0.55] |
| 2.2 Triple dye | 4 | 1319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.10, 0.20] |
| 2.3 Silver sulfadiazene | 2 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.59, 0.87] |
| 2.4 Chlorhexidene | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.55, 0.77] |
| 2.5 Benzine | 1 | 221 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.09] |
| 2.6 Salicylic sugar powder | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.17, 0.58] |
| 2.7 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.31, 0.82] |
| 2.8 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.02, 2.00] |
| 2.9 Fuschine | 1 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.84] |
| 3 Bacterial colonization ‐ Streptococci | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Alcohol | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.89] |
| 3.2 Triple dye | 4 | 1555 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.44, 0.73] |
| 3.3 Silver sulfadiazene | 2 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.42, 0.85] |
| 3.4 Chlorhexidene | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.27, 1.04] |
| 3.5 Salicylic sugar powder | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.29, 1.90] |
| 3.6 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [2.41, 8.84] |
| 3.7 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.87 [3.12, 11.05] |
| 3.8 Fuschine | 1 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.85] |
| 4 Bacterial colonization ‐ E.coli | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Alcohol | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.38, 1.21] |
| 4.2 Triple dye | 2 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.63, 0.91] |
| 4.3 Silver sulfadiazene | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.7 [0.53, 0.93] |
| 4.4 Chlorhexidene | 1 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.90] |
| 4.5 Salicylic sugar powder | 1 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.10] |
| 4.6 Green clay powder | 1 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.79, 2.05] |
| 4.7 Katoxin powder | 1 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.70, 1.81] |
| 4.8 Fuschine | 1 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.33, 3.13] |
| 5 Parental satisfaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Alcohol | 1 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.45, 0.66] |
1.1. Analysis.

Comparison 1 Antiseptic vs dry cord care/placebo, Outcome 1 Cord infection.
1.2. Analysis.

Comparison 1 Antiseptic vs dry cord care/placebo, Outcome 2 Bacterial colonization ‐ Staphylococcus aureus.
1.3. Analysis.

Comparison 1 Antiseptic vs dry cord care/placebo, Outcome 3 Bacterial colonization ‐ Streptococci.
1.4. Analysis.
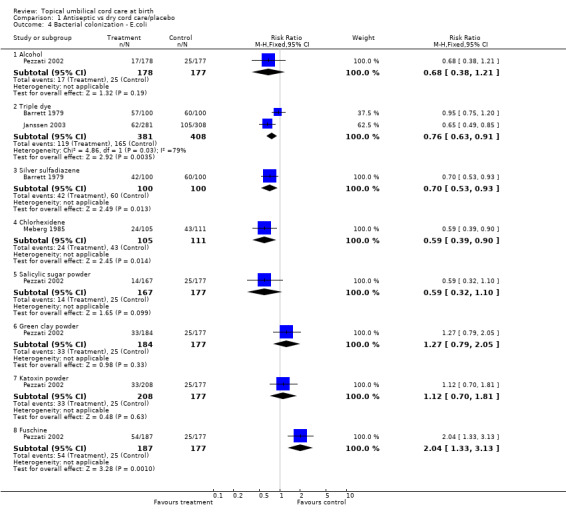
Comparison 1 Antiseptic vs dry cord care/placebo, Outcome 4 Bacterial colonization ‐ E.coli.
1.5. Analysis.

Comparison 1 Antiseptic vs dry cord care/placebo, Outcome 5 Parental satisfaction.
Comparison 2. Antiseptic vs dry cord care/placebo [continuous data].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to cord separation | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Alcohol | 4 | 2354 | Mean Difference (IV, Random, 95% CI) | 3.51 [‐0.41, 7.43] |
| 1.2 Zinc powder | 1 | 401 | Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.23, ‐1.41] |
| 1.3 Triple dye | 1 | 372 | Mean Difference (IV, Random, 95% CI) | 4.1 [3.07, 5.13] |
| 1.4 Salicylic sugar powder | 1 | 344 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐2.47, ‐1.33] |
| 1.5 Green clay powder | 1 | 361 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.36, ‐0.24] |
| 1.6 Katoxin powder | 1 | 385 | Mean Difference (IV, Random, 95% CI) | 0.80 [0.18, 1.42] |
| 1.7 Basic fuschine | 1 | 364 | Mean Difference (IV, Random, 95% CI) | 2.80 [2.01, 3.59] |
2.1. Analysis.

Comparison 2 Antiseptic vs dry cord care/placebo [continuous data], Outcome 1 Time to cord separation.
Comparison 3. Antiseptic vs antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bacterial colonization ‐ Staphylococcus aureus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Triple dye vs bacitracin | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.03, 12.87] |
| 1.2 Silver sulfadiazene vs bacitracin | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.44, 11.86] |
| 1.3 Povidone‐iodine vs bacitracin | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.42, 11.33] |
3.1. Analysis.

Comparison 3 Antiseptic vs antibiotic, Outcome 1 Bacterial colonization ‐ Staphylococcus aureus.
Comparison 4. Antiseptic vs antibiotic [continuous data].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to cord separation | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Triple dye vs bacitracin | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐5.60 [‐9.36, ‐1.84] |
| 1.2 Triple dye vs neomycin | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐4.3 [‐6.27, ‐2.33] |
| 1.3 Silver sulfadiazine vs neomycin | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.65, 0.85] |
| 1.4 Silver sulfadiazene vs bacitracin | 1 | 90 | Mean Difference (IV, Random, 95% CI) | 2.0 [0.20, 3.80] |
| 1.5 Povidone‐iodine vs bacitracin | 1 | 92 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐3.67, ‐0.33] |
4.1. Analysis.
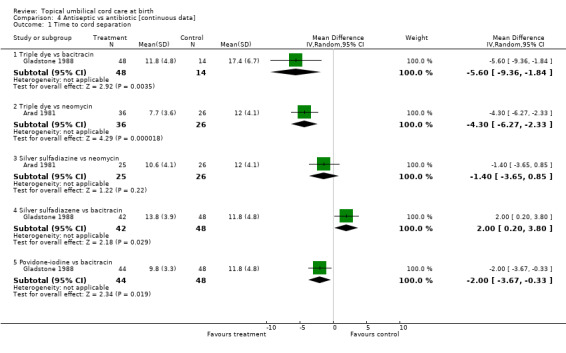
Comparison 4 Antiseptic vs antibiotic [continuous data], Outcome 1 Time to cord separation.
Comparison 5. Antiseptic vs antiseptic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cord infection | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Triple dye vs alcohol | 4 | 1560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.19, 0.49] |
| 1.2 Triple dye vs povidone‐iodine | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.07, 0.32] |
| 1.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [0.21, 88.65] |
| 1.4 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.17, 20.64] |
| 1.5 Triple dye vs katoxin | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.19, 23.34] |
| 1.6 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.18, 20.97] |
| 1.7 Povidone‐iodine vs alcohol | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.87, 1.62] |
| 1.8 Alcohol vs salicylic sugar powder | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.23, 97.03] |
| 1.9 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.19, 22.60] |
| 1.10 Alcohol vs katoxin | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.21, 25.56] |
| 1.11 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.10 [0.19, 22.97] |
| 1.12 Chlorhexidene vs hydrophobic gauze | 1 | 2441 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.55, 3.36] |
| 2 Bacterial colonization ‐ Staphylococcus aureus | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Triple dye vs alcohol | 2 | 487 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.25, 0.81] |
| 2.2 Triple dye vs silver sulfadiazene | 2 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.17, 0.46] |
| 2.3 Triple dye vs povidone‐iodine | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.03, 12.87] |
| 2.4 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.16, 1.12] |
| 2.5 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.11, 0.65] |
| 2.6 Triple dye vs katoxin powder | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.04, 0.22] |
| 2.7 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.63] |
| 2.8 Alcohol vs salicylic sugar powder | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.43, 2.03] |
| 2.9 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.30, 1.16] |
| 2.10 Alcohol vs katoxin powder | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.12, 0.37] |
| 2.11 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.12] |
| 2.12 Chlorhexidene vs hydrophobic gauze | 1 | 2441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.70, 1.15] |
| 2.13 Chlorhexidene vs alcohol | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.16] |
| 2.14 Chlorhexidene vs mercurochrome | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.04] |
| 3 Bacterial colonization ‐ Streptococci | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Triple dye vs sulfadiazine | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.99, 2.21] |
| 3.2 Triple dye vs alcohol | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.99] |
| 3.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.98] |
| 3.4 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.14] |
| 3.5 Triple dye vs katoxin powder | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.11] |
| 3.6 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.24] |
| 3.7 Alcohol vs salicylic sugar powder | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.06, 1.27] |
| 3.8 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.17] |
| 3.9 Alcohol vs katoxin powder | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.01, 0.14] |
| 3.10 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.15, 7.38] |
| 4 Bacterial colonization ‐ E.coli | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Triple dye vs sulfadiazine | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.02, 1.81] |
| 4.2 Triple dye vs alcohol | 1 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [2.10, 5.64] |
| 4.3 Triple dye vs salicylic sugar powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.92 [2.28, 6.72] |
| 4.4 Triple dye vs green clay powder | 1 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.27, 2.65] |
| 4.5 Triple dye vs katoxin powder | 1 | 403 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [1.43, 3.00] |
| 4.6 Triple dye vs fuchsine | 1 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.84, 1.54] |
| 4.7 Alcohol vs salicylic sugar powder | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.58, 2.24] |
| 4.8 Alcohol vs green clay powder | 1 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.31, 0.92] |
| 4.9 Alcohol vs katoxin powder | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.35, 1.04] |
| 4.10 Alcohol vs fuchsine | 1 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.20, 0.55] |
| 4.11 Chlorhexidene vs hydrophobic gauze | 1 | 2441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 2.00] |
| 4.12 Chlorhexidene vs alcohol | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.01, 3.27] |
| 4.13 Chlorhexidene vs mercurochrome | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
5.1. Analysis.
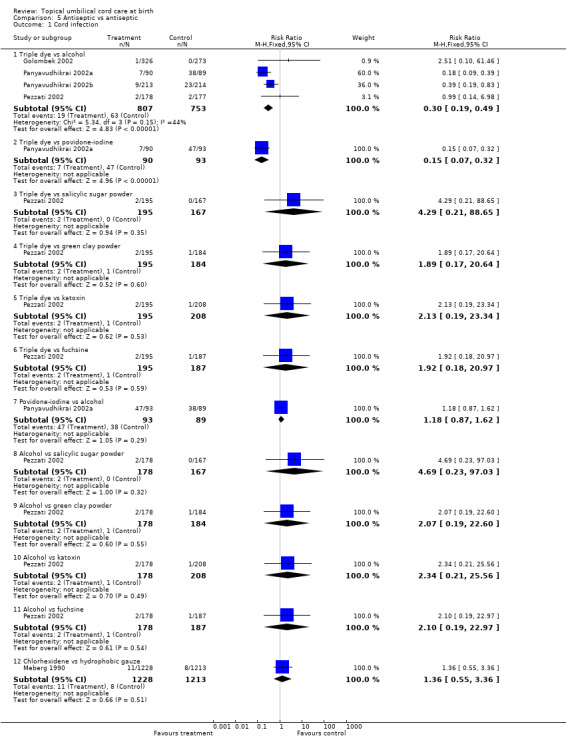
Comparison 5 Antiseptic vs antiseptic, Outcome 1 Cord infection.
5.2. Analysis.

Comparison 5 Antiseptic vs antiseptic, Outcome 2 Bacterial colonization ‐ Staphylococcus aureus.
5.3. Analysis.

Comparison 5 Antiseptic vs antiseptic, Outcome 3 Bacterial colonization ‐ Streptococci.
5.4. Analysis.

Comparison 5 Antiseptic vs antiseptic, Outcome 4 Bacterial colonization ‐ E.coli.
Comparison 6. Antiseptic vs antiseptic [continuous data].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to cord separation | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Triple dye vs alcohol | 2 | 444 | Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐10.25, 9.94] |
| 1.2 Triple dye vs silver sulfadiazine | 2 | 117 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐6.20, 6.51] |
| 1.3 Triple dye vs povidone‐iodine | 1 | 58 | Mean Difference (IV, Random, 95% CI) | 7.60 [3.96, 11.24] |
| 1.4 Triple dye vs salicylic sugar powder | 1 | 362 | Mean Difference (IV, Random, 95% CI) | 6.0 [5.01, 6.99] |
| 1.5 Triple dye vs green clay powder | 1 | 379 | Mean Difference (IV, Random, 95% CI) | 4.90 [3.92, 5.88] |
| 1.6 Triple dye vs katoxin powder | 1 | 403 | Mean Difference (IV, Random, 95% CI) | 3.30 [2.28, 4.32] |
| 1.7 Triple dye vs fuschine | 1 | 382 | Mean Difference (IV, Random, 95% CI) | 1.30 [0.17, 2.43] |
| 1.8 Alcohol versus chlorhexidine | 1 | 149 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐6.82, ‐4.58] |
| 1.9 Alcohol vs salicylic sugar powder | 1 | 345 | Mean Difference (IV, Random, 95% CI) | 11.30 [10.14, 12.46] |
| 1.10 Alcohol vs green clay powder | 1 | 362 | Mean Difference (IV, Random, 95% CI) | 10.2 [9.05, 11.35] |
| 1.11 Alcohol vs fuschine | 1 | 365 | Mean Difference (IV, Random, 95% CI) | 6.60 [5.32, 7.88] |
| 1.12 Alcohol vs katoxin powder | 1 | 386 | Mean Difference (IV, Random, 95% CI) | 8.60 [7.42, 9.78] |
| 1.13 Povidone‐iodine vs silver sulfadiazene | 1 | 86 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐5.53, ‐2.47] |
| 1.14 Chlorhexidene vs hydrophobic gauze | 1 | 2441 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.57, ‐0.23] |
| 1.15 Chlorhexidene vs mercurochrome | 1 | 152 | Mean Difference (IV, Random, 95% CI) | 6.40 [5.25, 7.55] |
6.1. Analysis.

Comparison 6 Antiseptic vs antiseptic [continuous data], Outcome 1 Time to cord separation.
Comparison 7. Single vs multiple applications [continuous data].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to cord separation | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Triple dye | 2 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐4.27 [‐5.48, ‐3.05] |
| 1.2 Zinc, sterzac or codocel powder | 1 | 800 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.31, 0.27] |
7.1. Analysis.

Comparison 7 Single vs multiple applications [continuous data], Outcome 1 Time to cord separation.
Comparison 8. Washing cord vs dry cord care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Bacterial colonization ‐ Staphylococcus aureus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
8.1. Analysis.
Comparison 8 Washing cord vs dry cord care, Outcome 1 Bacterial colonization ‐ Staphylococcus aureus.
Comparison 9. Antiseptic‐aqueous based vs powder [Subgroup analysis].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cord separation | 1 | 1144 | Mean Difference (IV, Fixed, 95% CI) | ‐4.76 [‐5.34, ‐4.19] |
| 1.1 Triple dye vs salicylic sugar | 1 | 362 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐6.99, ‐5.01] |
| 1.2 Triple dye vs green clay powder | 1 | 379 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐5.88, ‐3.92] |
| 1.3 Triple dye vs katoxin powder | 1 | 403 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐4.32, ‐2.28] |
9.1. Analysis.

Comparison 9 Antiseptic‐aqueous based vs powder [Subgroup analysis], Outcome 1 Cord separation.
Comparison 10. Antiseptic‐alcohol based vs powder [Subgroup analysis].
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cord separation | 1 | 1093 | Mean Difference (IV, Fixed, 95% CI) | ‐10.05 [‐10.72, ‐9.38] |
| 1.1 Alcohol vs salicylic sugar | 1 | 345 | Mean Difference (IV, Fixed, 95% CI) | ‐11.30 [‐12.46, ‐10.14] |
| 1.2 Alcohol vs green clay powder | 1 | 362 | Mean Difference (IV, Fixed, 95% CI) | ‐10.2 [‐11.35, ‐9.05] |
| 1.3 Alcohol vs katoxin powder | 1 | 386 | Mean Difference (IV, Fixed, 95% CI) | ‐8.60 [‐9.78, ‐7.42] |
10.1. Analysis.
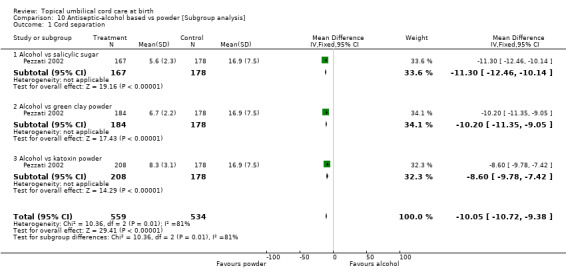
Comparison 10 Antiseptic‐alcohol based vs powder [Subgroup analysis], Outcome 1 Cord separation.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arad 1981.
| Methods | 'Randomly assigned'. | |
| Participants | Inborn, healthy term babies (36, 26, 25 and 34 in each experimental group). Hospital nursery. caesarean section births included. | |
| Interventions | Initial bath. Daily application during hospital stay. 1. Triple dye 2. Neomycin ointment 3. Sulphadiazine ointment 4. Bismuth powder. Daily alcohol applied at home. | |
| Outcomes | Separation time. Infection. | |
| Notes | Israel 1980. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Bain 1994.
| Methods | Randomization not described. | |
| Participants | Inborn, premature babies > 1000 g. Excluded if had umbilical line or abdominal surgery (26, 24, 24, 28 in each experimental group). | |
| Interventions | No information about initial bath 1. Alcohol wipe (Steret) +hexachlorophane and 3% zinc powder (Ster‐zac) 2. Ster‐zac 3. Sterets 4. Nothing. | |
| Outcomes | Separation time. Infection with negative swabs. Positive second bacterial swab. | |
| Notes | Scotland 1991‐2. Some babies received antibiotics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Barrett 1979.
| Methods | Computer generated random numbers. | |
| Participants | Inborn, hospital nursery (100 in each group). Some infants partial rooming‐in. | |
| Interventions | Initial bath. 1. Silver sulphadiazine, single application 2. Triple dye, single application 3. Dry cord care. | |
| Outcomes | Colonization of periumbilical area and anterior nares at 48 hours. | |
| Notes | USA 1976. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dore 1998.
| Methods | Table of random numbers. Opaque envelopes. | |
| Participants | Inborn, healthy term infants (902 experimental, 909 control). caesarean section births included. Rooming‐in. | |
| Interventions | Initial bath. 1. 70% isopropyl alcohol at least three times a day 2. Natural drying. | |
| Outcomes | Umbilical infection. Separation time. Maternal satisfaction. | |
| Notes | Canada 1995‐6. Two sites. Includes cost data. Loss to follow‐up: 65, equally divided between the two groups. 'High level of breast feeding'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gladstone 1988.
| Methods | Table of random numbers. | |
| Participants | Inborn healthy term infants > 2500 g (53, 48, 44, 42, and 48 in each group). Hospital nursery. | |
| Interventions | No information about initial bath 1. Triple dye once daily until separation 2. Triple dye once then alcohol until separation 3. Triple dye once only 4. Povidone iodine daily until separation 5. Silver sulphadiazine daily until separation 6. Bacitracin ointment until cord separation. | |
| Outcomes | Colonization at discharge from hospital. Separation time. Maternal satisfaction. Local or other infections. Nursing staff satisfaction. | |
| Notes | USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Golombek 2002.
| Methods | Randomized using alternate months. | |
| Participants | Inborn healthy term infants (273, 326). | |
| Interventions | No information about initial bath 1. Alcohol 2. Triple dye. | |
| Outcomes | Cord infection. Cord separation. Nursing staff satisfaction. | |
| Notes | USA 1998‐1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Hsu 1999.
| Methods | Randomization not described. | |
| Participants | Inborn healthy term infants (101 experimental, 79 control). Hospital nursery. | |
| Interventions | Daily whole body wash with soap. 1. Triple dye, single application 2. Triple dye, daily application. | |
| Outcomes | Separation time. Infection. | |
| Notes | Taiwan 1995‐6 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Janssen 2003.
| Methods | Randomization stratified according to clinical area in which infant resided. Adequate concealment. | |
| Participants | Inborn healthy infants (384, 382). Rooming in. | |
| Interventions | No information about initial bath 1. Triple dye ‐ 2 applications then alcohol thrice daily 2. Dry cord care Both groups had daily bath. | |
| Outcomes | Omphalitis. Staphylococcus aureus induced conjunctivitis or skin infection. Bacterial colonization. | |
| Notes | Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Meberg 1985.
| Methods | 'Consecutively and randomly selected'. | |
| Participants | Inborn healthy term infants (113 and 112 experimental, 108 control). Hospital ward. Day rooming‐in. Cesarean section births excluded. | |
| Interventions | Phase I: Daily whole body soap wash 1. Benzine daily 2. Chlorhexidine (0.05%) daily 3. No specific care. | |
| Outcomes | Colonization of stump at discharge. Infection (umbilical and severe) within six weeks. | |
| Notes | Norway. Phase 1: 1982; Phase 2: 1983. Roomed‐in with mothers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Meberg 1990.
| Methods | Consecutive birth numbers with alternate allocation. (even/odd). | |
| Participants | Hospital births (1213 experimental, 1228 control). Day time rooming‐in. | |
| Interventions | Daily whole body soap wash. 1. Hydrophobic gauze material bandage, applied daily 2. Chlorhexidine in alcohol, applied daily. | |
| Outcomes | Infections of skin, cord, eyes during stay and at six weeks. Separation time. | |
| Notes | Norway 1987‐1989. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Medves 1997.
| Methods | Block randomization. | |
| Participants | Inborn healthy term infants. (71 experimental, 65 control). | |
| Interventions | Initial bath. 1. Isopropyl alcohol 2. Sterile water | |
| Outcomes | Separation time. Colonization at birth and within 12 hrs of cord separation. | |
| Notes | Canada 1996. 'Intention to treat' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mugford 1986.
| Methods | Open randomized factorial design. Numbered sealed envelopes. | |
| Participants | Inborn babies and likely to receive normal postnatal care (199, 202, 197 experimental, 202 control). | |
| Interventions | Factor 1: Powder 1. Zinc/starch/talc 2. Sterzac (hexochlorophane, zinc and starch) 3. Cordocel 4. No powder. Factor 2: cleansing method: 1. Spirit 2. Water 3. No routine cleansing. Factor 3: frequency of treatment: 1. Daily 2. Once only. | |
| Outcomes | Use of additional cord treatments. Midwife visits after day 10. Separation time. Days of cord moisture and stickiness. | |
| Notes | United Kingdom 1984. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Panyavudhikrai 2002a.
| Methods | 'Simple randomization'. | |
| Participants | Inborn healthy term infants (93, 90, 89 in each group). | |
| Interventions | Phase 1 1. Povidone ‐iodine twice daily 2. Triple dye twice daily 3. Alcohol (70%) twice daily. | |
| Outcomes | Cord infection. | |
| Notes | Thailand. Phase 1: Nov ‐ Dec 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Panyavudhikrai 2002b.
| Methods | 'Simple randomization'. | |
| Participants | Inborn healthy term infants (213 and 214). | |
| Interventions | Phase 2 1. Triple dye twice daily 2. Alcohol (70%) twice daily. | |
| Outcomes | Cord infection. | |
| Notes | Thailand. Phase 2: Dec 1998 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Perapoch 1993.
| Methods | Randomization not described. | |
| Participants | Inborn healthy term infants (75, 84, 78, 74 in each group). | |
| Interventions | No information about initial bath 1. Alcohol (70%) 2. Alcohol + mercurochrome 3. Mercurochrome 4. Chlorhexidine (1%). | |
| Outcomes | Cord infection. Cord separation. Bacterial colonization. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Pezzati 2002.
| Methods | Randomized by month of admission. | |
| Participants | Inborn healthy term infants (167, 184, 177, 208, 174, 187, 195, 178). | |
| Interventions | Initial bath with soap. 1. Salicylic sugar powder 2. Green clay powder 3. Natural drying 4. Katoxin 5. Cicatrene 6. 1% basic fuschine 7. Triple dye 8. 70% alcohol | |
| Outcomes | Sepsis. Death. Cord infection. Cord separation. Cord bleeding. Compliance. Parental satisfaction. Bacterial colonization. | |
| Notes | Italy 1999. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Rosenfeld 1990.
| Methods | Alternate allocation. | |
| Participants | Premature babies < 2200 g (54 experimental, 60 control). Hospital nursery. | |
| Interventions | Initial bath with soap. 1. Triple dye, single application 2. Isopropyl alcohol each diaper change | |
| Outcomes | Colonization on day 4 and at discharge. | |
| Notes | USA 1988. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Rush 1986.
| Methods | Random allocation by 'cot ID'. | |
| Participants | Healthy term newborn infants (95 experimental, 86 control). Rooming‐in. | |
| Interventions | 1. Routine daily bath with water and soap 2. Initial bath only. | |
| Outcomes | Colonization on day 4 in the nose and umbilicus. | |
| Notes | Canada 1984. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Schuman 1985.
| Methods | Computer generated random numbers. | |
| Participants | Inborn healthy, term babies Hospital nursery (35 experimental, 36 control). caesarean section birth included. | |
| Interventions | Daily bath with Phisoderm 1. Triple dye 2. Isopropyl alcohol After discharge, isopropyl alcohol in both groups. | |
| Outcomes | Separation time. Cord infection. | |
| Notes | USA 1983. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Speck 1980.
| Methods | 'Randomly assigned'. | |
| Participants | Inborn healthy term babies (80 and 82 experimental, 78 control). Hospital nursery. Complicated labour and caesarean births excluded. | |
| Interventions | Initial bath.
1. Daily wash with castile soap
2. Triple dye
3. Silver sulphadiazine.
Routine daily sponge bath with tap water. After discharge, daily application of isopropanol. |
|
| Outcomes | Bacterial culture from the nose day 3, 14. Cord infection. Conjunctivitis. Impetigo. | |
| Notes | USA 1975‐76. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Wald 1977.
| Methods | Babies with same intervention admitted to one room. Room assignments rotated after every 100 infants admitted. | |
| Participants | Inborn healthy term babies (409, 197, 199). Hospital nursery with partial rooming‐in. | |
| Interventions | No initial bath. 1. Triple dye 2. Hexachlorophene 3. Control. | |
| Outcomes | Bacterial colonization with Group B streptococci. | |
| Notes | USA 1974. Treatment arm using hexachlorophene excluded from review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alder 1980 | Comparison between hexachlorophene and chlorhexidine. Hexachlorophene not recommended anymore because of central nervous toxicity. |
| Barclay 1994 | Not a randomized trial. 890 babies in comparison between chlorhexidine and no specific treatment to cord. |
| Bhakoo 1969 | Quasi‐randomized trial but wrong intervention. Comparison between bathing and not bathing the baby. In both groups the umbilical stump was cleaned daily with savlon solution. |
| Birenbaum 1990 | Randomized trial but wrong intervention. Comparison of gowning with no‐gowning of visitors and hospital personnel to investigate effect on nose and umbilical colonization. |
| Bourke 1990 | Not a randomized trial. All babies born in two designated wards were entered into study. Experimental group received no treatment and the control group was treated with alcohol (70%). |
| Bradshaw 1993 | Randomized trial but combined intervention of alcohol and hexacholorphane. Hexachlorophene not recommended anymore because of central nervous toxicity. |
| Branchi 1998 | Not a randomized trial. 346 babies in comparison between alcohol and salicylic sugar powder. |
| Coyer 1975 | Abstract refers to a trial with 271 babies randomized to neosporin cord care compared with triple dye and no specific cord care. No publication arising from this trial, and no results available. Numbers of babies in the various arms incompatible with randomization. |
| Gezon 1964 | Daily whole body bath with hexachlorophene during the hospital stay and three weeks at home was compared to daily bath with a detergent. Hexachlorophene not recommended because of central nervous toxicity. |
| Gluck 1963 | Comparison between entire body wash with hexachlorophene, no wash and dry skin care. Hexachlorophene not recommended because of central nervous toxicity. |
| Henningsson 1981 | Alternate allocation. Interventions were bathing or washing the baby. Outcomes were bacterial colonization, clinical infection, body temperature and crying. |
| Hnatko 1977 | Whole body wash with hexachlorophene compared to three other antiseptic agents. Hexachlorophene not recommended because of central nervous toxicity. Allocation to groups was according to predetermined schedule. |
| Jellard 1957 | Allocation of treatment group to one of three wards in a hospital. Triple dye versus nothing; all received surgical spirit. |
| Kwong 1973 | Daily bath with hexachlorophene compared to bath with tap water. Hexachlorophene not recommended because of central nervous toxicity. |
| Olowe 1980 | Randomized trial on 58 babies with glucose‐6‐phosphate dehydrogenase deficiency. The effect of different dressing powders versus no dressing powder on the severity of neonatal jaundice. |
| Oxford 1991 | The authors did not give details of cord care that could have influenced the separation time. Additional information is being sought. |
| Pildes 1973 | Not a randomized trial. |
| Pyati 1977 | Controlled trial in which multiple applications of povidone iodine were compared with a single application. None of the outcomes for inclusion into the review were reported. |
| Smales 1988 | Not a randomized trial. Two hospitals with different regimens reversed after two months. Chlorhexidine detergent solution compared with iodine in surgical spirit. Outcomes: bacterial colonization and time of cord separation. |
| Thomas 1979 | Not a randomized trial. Treatment and control groups allocated by ward. Interventions were chlorhexidine powder versus chlorhexidine zinc oxide powder and chlorhexidene bath. Outcomes were infection, cord separation, bacterial colonization, days to discharge, and number of times cord re‐clamped. |
| Verber 1993 | Not a randomized trial. Treatment and control groups allocated by ward. Cross‐over trial comparing chlorhexidine with dry cord care. |
| Watkinson 1992 | Not a randomized trial. 50 babies delivered by caesarean section. Comparison between alcohol and hexachlorophane with no antiseptic. |
| Wojciechowska 1989 | Study completed but not analysed. No data available. |
Contributions of authors
Jelka Zupan and Paul Garner extracted the data, analysed the data and wrote the first edition of the review. Aika Omari extracted and analysed the data for the second edition of the review which was checked by Jelka Zupan. Jelka Zupan and Aika Omari wrote the second edition of the review and Paul Garner advised on the final draft.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
WHO Division of Reproductive Health, Switzerland.
External sources
Department for International Development, UK.
European Commission (Directorate General XII), Belgium.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Arad 1981 {published data only}
- Arad I, Eyal F, Fainmesser P. Umbilical care and cord separation. Archives of Disease in Childhood 1981;56:887‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bain 1994 {published data only}
- Bain J. Umbilical cord care in pre‐term babies. Nursing Standard 1994;8(15):32‐6. [DOI] [PubMed] [Google Scholar]
Barrett 1979 {published data only}
- Barrett FF, Mason EO, Fleming D. The effect of three cord care regimens on the bacterial colonization of normal newborn infants. Journal of Pediatrics 1979;94:796‐800. [Google Scholar]
Dore 1998 {published data only}
- Dore S, Buchan D, Coulas S, Hamber L, Stewart M, Cowan D, et al. Alcohol versus natural drying for newborn cord care. Journal of Obstetric, Gynecologic and Neonatal Nursing 1998;27:621‐7. [DOI] [PubMed] [Google Scholar]
Gladstone 1988 {published data only}
- Gladstone IM, Clapper L, Thorp JW, Wright DI. Randomized study of six umbilical cord care regimens. Clinical Pediatrics 1988;27:127‐9. [DOI] [PubMed] [Google Scholar]
Golombek 2002 {published data only}
- Golombek SG, Brill PE, Salice AL. Randomized trial of alcohol versus triple dye for umbilical cord care. Clinical Pediatrics 2002;41(6):419‐23. [DOI] [PubMed] [Google Scholar]
Hsu 1999 {published data only}
- Hsu CF, Wang CC, Yuh YS, Chen YH, Chu ML. The effectiveness of single and multiple applications of triple dye on umbilical cord separation time. European Journal of Pediatrics 1999;158:144‐6. [DOI] [PubMed] [Google Scholar]
Janssen 2003 {published data only}
- Janssen PA, Selwood BL, Dobson SR, Peacock D, Thiessen PN. To dye or not to dye: a randomized clinical trial of a triple dye/alcohol regime versus dry cord care. Pediatrics 2003;111(1):15‐20. [DOI] [PubMed] [Google Scholar]
Meberg 1985 {published data only}
- Meberg A, Schoyen R. Bacterial colonization and neonatal infections. Acta Paediatrica Scandinavica 1985;74:366‐71. [DOI] [PubMed] [Google Scholar]
Meberg 1990 {published data only}
- Meberg A, Schoyen R. Hydrophobic material in routine umbilical cord care and prevention of infections in newborn infants. Scandinavian Journal of Infectious Diseases 1990;22:729‐33. [DOI] [PubMed] [Google Scholar]
Medves 1997 {published data only}
- Medves JM, O'Brien BAC. Cleaning solutions and bacterial colonization in promoting healing and early separation of the umbilical cord in healthy newborns. Canadian Journal of Public Health 1997;88(6):380‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mugford 1986 {published data only}
- Mugford M, Somchiwong M, Waterhouse I. Treatment of umbilical cords: a randomised trial to assess the effect of treatment methods on the work of midwives. Midwifery 1986;2:177‐86. [DOI] [PubMed] [Google Scholar]
Panyavudhikrai 2002a {published data only}
- Panyavudhikrai S, Danchaivijity S, Vantanasiri C, Trakulsomboon S, Kolatat T, Dhiraputra C, et al. Antiseptics for preventing omphalitis. Journal of Medical Association of Thailand 2002;85(2):229‐33. [PubMed] [Google Scholar]
Panyavudhikrai 2002b {published data only}
- Panyavudhikrai S, Danchaivijity S, Vantanasiri C, Trakulsomboon S, Kolatat T, Dhiraputra C, et al. Antiseptics for preventing omphalitis. Journal of Medical Association of Thailand 2002;85(2):229‐33. [PubMed] [Google Scholar]
Perapoch 1993 {published data only}
- Perapoch Lopez JP, Abizanda SS, Catala AG, Monforte GP, Caro MC, Perez CB, et al. Colonization of the umbilical cord in normal neonates: comparative assessment of four antiseptic methods applied to the umbilical stump [Colonizacion umbilical en recien nacidos normales. Estudio comparativo de cuatro metodos de antisepsia umbilical]. Anales Espanoles de Pediatria 1993;39(3):195‐8. [PubMed] [Google Scholar]
Pezzati 2002 {published data only}
- Pezzati M, Biagioli EC, Martelli E, Gambi B, Biagotti R, Rubaltelli FF. Umbilical cord care: the effect of eight different cord‐care regimens on cord separation time and other outcomes. Biology of the Neonate 2002;81:38‐44. [DOI] [PubMed] [Google Scholar]
Rosenfeld 1990 {published data only}
- Rosenfeld CR, Laptook AR, Jeffery J. Limited effectiveness of triple dye (TD) in prevention of colonization with methacillin‐resistant staphylococcus aureus (MRSA) in a special care nursery (SCN). Pediatric Research 1989;25:281A. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Laptook AR, Jeffery J. Limited effectiveness of triple dye in preventing colonization with methicillin‐resistant staphylococcus aureus in a special care nursery. Pediatric Infectious Disease Journal 1990;9(4):290‐1. [DOI] [PubMed] [Google Scholar]
Rush 1986 {published data only}
- Rush J. Does routine newborn bathing reduce staphylococcus aureus colonization rates? A randomized controlled trial. Birth 1986;13:176‐80. [DOI] [PubMed] [Google Scholar]
Schuman 1985 {published data only}
- Schuman AJ, Oksol BA. The effect of isopropyl alcohol and triple dye on umbilical cord separation time. Military Medicine 1985;150:49‐50. [PubMed] [Google Scholar]
Speck 1980 {published data only}
- Speck WT, Driscoll JM, O'Neil J, Rosenkranz HS. Effect of antiseptic cord care on bacterial colonization in the newborn infant. Chemotherapy 1980;26:372‐6. [DOI] [PubMed] [Google Scholar]
Wald 1977 {published data only}
- Wald ER, Snyder MJ, Gutberlet RL. Group B ß‐hemolytic streptococcal colonization. American Journal of Diseases of Children 1977;131(2):178‐80. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alder 1980 {published data only}
- Alder VG, Burnam D, Simpson RA, Fysh J, Gillespie WA. Comparison of hexachlorophene and chlorhexidine. Archives of Disease in Childhood 1980;55:277‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barclay 1994 {published data only}
- Barclay L, Harrington A, Conroy R, Royal R, LaForgia J. A comparative study of neonates’ umbilical cord management. Australian Journal of Advanced Nursing 1994;11:34‐40. [PubMed] [Google Scholar]
Bhakoo 1969 {published data only}
- Bhakoo ON, Lall JC, Agarwal KC. Prevention of hospital infections in neonates: an evaluation of no bath regimen. Indian Pediatrics 1969;6(11):697‐700. [PubMed] [Google Scholar]
Birenbaum 1990 {published data only}
- Birenbaum HJ, Glorioso L, Rosenberger C, Arshad C, Edwards K. Gowning on a postpartum ward fails to decrease colonization in the newborn infant. American Journal of Diseases of Children 1990;144:1031‐3. [DOI] [PubMed] [Google Scholar]
Bourke 1990 {published data only}
- Bourke E. Cord care: too much or too little. Australian Journal of Advanced Nursing 1990;7(2):19‐22. [PubMed] [Google Scholar]
Bradshaw 1993 {unpublished data only}
- Bradshaw C. An experimental study to compare treatment vs non treatment of the umbilical cord. National Conference on Research in Midwifery, Birmingham; 1993 Sept 14; Birmingham, UK, 1993.
Branchi 1998 {published data only}
- Branchi M, Bernardini E, Bordont G, Siani A, Bonora G. Bacterial colonisation and time of detachment of umbilical cord: comparative study between alcohol and salicylic sugar [Colonizzazione batterica e tempo di caduta del moncone ombelicale: confronto fra trattamento con alcool e con zucchero salicilico]. Rivista Italiana di Pediatria 1998;24:994‐1004. [Google Scholar]
Coyer 1975 {published data only}
- Coyer WF. Neonatal skin care and the prevention of staphylococcal aureus (staph) colonization. Pediatric Research 1975;9:339. [Google Scholar]
Gezon 1964 {published data only}
- Gezon HM, Thompson DJ, Rogers KD, Hatch TF, Taylor PM. Hexachlorophene bathing in early infancy. New England Journal of Medicine 1964;270:379‐86. [DOI] [PubMed] [Google Scholar]
Gluck 1963 {published data only}
- Gluck L, Wood HF. Staphylococcal colonisation in newborn infants with and without antiseptic skin care. New England Journal of Medicine 1963;268:1265‐8. [DOI] [PubMed] [Google Scholar]
Henningsson 1981 {published data only}
- Henningsson A, Nystrom B, Tunnell R. Bathing or washing babies after birth?. Lancet 1981;2:1401‐3. [DOI] [PubMed] [Google Scholar]
Hnatko 1977 {published data only}
- Hnatko SI. Alternatives to hexachlorophene bathing of newborn infants. Canadian Medical Association Journal 1977;117:223‐6. [PMC free article] [PubMed] [Google Scholar]
Jellard 1957 {published data only}
- Jellard J. Umbilical cord as reservoir of infection in a maternity hospital. BMJ 1957;1:925‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwong 1973 {published data only}
- Kwong MS, Loew AD, Anthony FA, Oh W. The effect of hexachlorophene on staphylococcal colonization rates in the newborn infant: a controlled study using a single‐bath method. Journal of Pediatrics 1973;82:982‐6. [DOI] [PubMed] [Google Scholar]
Olowe 1980 {published data only}
- Olowe SA, Ransome‐Kuti O. The risk of jaundice in glucose‐6‐phosphat dehydrogenase deficient babies exposed to menthol. Acta Paediatrica Scandinavica 1980;69:341‐5. [DOI] [PubMed] [Google Scholar]
Oxford 1991 {published data only}
- Oxford Midwives Research Group. A study of the relationship between the delivery to cord clamping interval and the time of cord separation. Midwifery 1991;7:167‐76. [DOI] [PubMed] [Google Scholar]
Pildes 1973 {published data only}
- Pildes RS, Ramamurth RS, Vidyasagar D. Effect of triple dye on staphylococcal colonisation in the newborn infant. Journal of Pediatrics 1973;82:987‐90. [DOI] [PubMed] [Google Scholar]
Pyati 1977 {published data only}
- Pyati SP, Ramamurthy RS, Krauss MT, Pildes RS. Absorption of iodine in the neonate following topical use of povidone iodine. Journal of Pediatrics 1977;91(5):825‐8. [DOI] [PubMed] [Google Scholar]
Smales 1988 {published data only}
- Smales O. A comparison of umbilical cord treatment in the control of superficial infection. New Zealand Medical Journal 1988;101:453‐5. [PubMed] [Google Scholar]
Thomas 1979 {published data only}
- Thomas WR. The prevention of superficial infection in neonates. Journal of Antimicrobial Chemotherapy 1979;5(2):235‐6. [DOI] [PubMed] [Google Scholar]
Verber 1993 {published data only}
- Verber IG, Pagan FS. What cord care ‐ if any?. Archives of Disease in Childhood 1993;68:594‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watkinson 1992 {published data only}
- Watkinson M, Dyas A. Staphylococcus aureus still colonizes the untreated neonatal umbilicus. Journal of Hospital Infection 1992;24:131‐6. [DOI] [PubMed] [Google Scholar]
Wojciechowska 1989 {published data only}
- Wojciechowska L. Trial to assess the effects of different treatments of the umbilical cord in newborn infants. Personal Communication 1989.
References to studies awaiting assessment
Bhalla 1975 {published data only}
- Bhalla JN, Nafis N, Robargi P, Singh J. Some observations on separation of the umbilical stump in the newborn. Indian Journal of Paediatrics 1975;42:329‐34. [DOI] [PubMed] [Google Scholar]
Guinsburg 1991 {unpublished data only}
- Guinsburg R, Ikezawa MK, Foglilano RRF, Reichert MCF, Carvalho ES, Cardo DM, et al. Umbilical bacterial colonization (UBC) of normal newborn (NB) infants: effect of four antiseptic regimens. Pediatric Research 1991;29:282A. [Google Scholar]
Huang 2001 {published data only}
- Huang C, Yeh L, Chuang M, Yuh Y. Umbilical separation time delayed by alcohol application. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 2):648. [Google Scholar]
Pezzati 2003 {published data only}
- Pezzati M, Rossi S, Tronchin M, Dani C, Filippi L, Rubaltelli FF. Umbilical cord care in premature Infants: the effect of two different cord‐care regimens (salicylic sugar powder vs chlorhexidine) on cord separation time and other outcomes. Pediatrics 2003;112(4):e275. [DOI] [PubMed] [Google Scholar]
Ronchera‐Oms 1994 {published data only}
- Ronchera‐Oms C, Hernandez C, Jimemez NV. Antiseptic cord care reduces bacterial colonisation but delays cord detachment. Archives of Disease in Childhood Fetal and Neonatal Edition 1994;70:F70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speck 1977 {published data only}
- Speck WT, Driscoll JM, Polin RA, O'Neill J, Rosenkranz HS. Staphylococcal and streptococcal colonization of the newborn infant. American Journal of Diseases in Childhood 1977;131:1005‐8. [DOI] [PubMed] [Google Scholar]
- Speck WT, Driscoll JM, Polin RA, Rosenkranz HS. Bacterial colonization in the newborn ‐ effect of cord care. Pediatric Research 1976;10:335. [Google Scholar]
Additional references
Aggett 1981
- Aggett PJ, Cooper LV, Ellis SH, McAinsh J. Percutaneous absorption of chlorhexidine in neonatal cord care. Archives of Disease in Childhood 1981;56(11):878‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 1999
- Bennett J, Ma C, Traverso H, Agha SB, Boring J. Neonatal tetanus associated with topical umbilical ghee: covert role of cow dung. International Journal of Epidemiology 1999;28:1172‐5. [DOI] [PubMed] [Google Scholar]
CHRPSR 1999
- Anonymous. Reducing perinatal and neonatal mortality. Child Health Research Project Special Report; 1999 May 10 ‐12; Baltimore, Maryland USA. Baltimore: Johns Hopkins University, 1999.
Cullen 1916
- Cullen TS. Embryology, anatomy and diseases of the umbilicus. Philadelphia: WB Saunders, 1916. [Google Scholar]
Elhassani 1984
- Elhassani SB. The umbilical cord: care, anomalies, and diseases. Southern Medical Journal 1984;77(6):730‐6. [DOI] [PubMed] [Google Scholar]
Enkin 2000
- Enkin M, Keirse MJNC, Neilson J, Crowther C, Duley L, Hodnett E, et al. Mother and baby. A guide to effective care in pregnancy and childbirth. 3rd Edition. Oxford: Oxford University Press, 2000:429‐38. [Google Scholar]
Forshall 1957
- Forshall. Septic umbilical arteritis. Archives of Disease in Childhood 1957;32:25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meegan 2001
- Meegan ME, Conroy RM, Ole Lengeny S, Renhault K, Nyangole J. Effect on neonatal tetanus mortality after a culturally‐based health promotion programme. Lancet 2001;358:640‐1. [DOI] [PubMed] [Google Scholar]
Novack 1988
- Novack AH, Mueller B, Ochs H. Umbilical cord separation in the normal newborn. American Journal of Diseases in Childhood 1988;142:220‐3. [DOI] [PubMed] [Google Scholar]
OudesluysMurphy 1987
- Oudesluys‐Murphy AM. The time of separation of the umbilical cord. European Journal of Pediatrics 1987;146(4):387‐9. [DOI] [PubMed] [Google Scholar]
OudesluysMurphy 1990
- Oudeluys‐Murphy AM, Hollander JC. Separation of the umbilical cord ‐ histological findings. Biology of the Neonate 1990;58:54‐6. [DOI] [PubMed] [Google Scholar]
Sarkany 1967
- Sarkany I, Gaylarde CC. Skin flora of the newborn. Lancet 1967;1(7490):589‐90. [DOI] [PubMed] [Google Scholar]
Thayaparan 1998
- Thayaparan B, Nicoll A. Prevention and control of tetanus in childhood. Current Opinion in Pediatrics 1998;10(1):4‐8. [DOI] [PubMed] [Google Scholar]
WHO 1998a
- World Health Organization. Care of the umbilical cord: a review of the evidence. World Health Organization WHO/RHT/MSM/98.4 1998.
WHO 1998b
- World Health Organization. Epi information systems: Global summary, September 1998. Geneva: WHO/EPI/GEN/98.10, 1998. [Google Scholar]
Woodruff 1984
- Woodruff AW, Grant J, Bashir EA, Baya EI, Yugusuk AZ, Sumi A. Neonatal tetanus: mode of infection, prevalence, and prevention in southern Sudan. Lancet 1984;1(8373):378‐379. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
CDSR 2004
- Zupan J, Garner P. Topical umbilical cord care at birth (Cochrane Review). The Cochrane Library 2004, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2013
- Imdad A, Bautista RMM, Senen KAA, Uy MEV, Mantaring III JB, Bhutta ZA. Umbilical cord antiseptics for preventing sepsis and death among newborns. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD008635.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


