Abstract
Background
Group A streptococcus (GAS) accounts for 20% to 40% of cases of pharyngitis in children; the remaining cases are caused by viruses. Compared with throat culture, rapid antigen detection tests (RADTs) offer diagnosis at the point of care (within five to 10 minutes).
Objectives
To determine the diagnostic accuracy of RADTs for diagnosing GAS in children with pharyngitis. To assess the relative diagnostic accuracy of the two major types of RADTs (enzyme immunoassays (EIA) and optical immunoassays (OIA)) by indirect and direct comparison.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, Web of Science, CDSR, DARE, MEDION and TRIP (January 1980 to July 2015). We also conducted related citations tracking via PubMed, handsearched reference lists of included studies and relevant review articles, and screened all articles citing included studies via Google Scholar.
Selection criteria
We included studies that compared RADT for GAS pharyngitis with throat culture on a blood agar plate in a microbiology laboratory in children seen in ambulatory care.
Data collection and analysis
Two review authors independently screened titles and abstracts for relevance, assessed full texts for inclusion, and carried out data extraction and quality assessment using the QUADAS‐2 tool. We used bivariate meta‐analysis to estimate summary sensitivity and specificity, and to investigate heterogeneity across studies. We compared the accuracy of EIA and OIA tests using indirect and direct evidence.
Main results
We included 98 unique studies in the review (116 test evaluations; 101,121 participants). The overall methodological quality of included studies was poor, mainly because many studies were at high risk of bias regarding patient selection and the reference standard used (in 73% and 43% of test evaluations, respectively). In studies in which all participants underwent both RADT and throat culture (105 test evaluations; 58,244 participants; median prevalence of participants with GAS was 29.5%), RADT had a summary sensitivity of 85.6%; 95% confidence interval (CI) 83.3 to 87.6 and a summary specificity of 95.4%; 95% CI 94.5 to 96.2. There was substantial heterogeneity in sensitivity across studies; specificity was more stable. There was no evidence of a trade‐off between sensitivity and specificity. Heterogeneity in accuracy was not explained by study‐level characteristics such as whether an enrichment broth was used before plating, mean age and clinical severity of participants, and GAS prevalence. The sensitivity of EIA and OIA tests was comparable (summary sensitivity 85.4% versus 86.2%). Sensitivity analyses showed that summary estimates of sensitivity and specificity were stable in low risk of bias studies.
Authors' conclusions
In a population of 1000 children with a GAS prevalence of 30%, 43 patients with GAS will be missed. Whether or not RADT can be used as a stand‐alone test to rule out GAS will depend mainly on the epidemiological context. The sensitivity of EIA and OIA tests seems comparable. RADT specificity is sufficiently high to ensure against unnecessary use of antibiotics. Based on these results, we would expect that amongst 100 children with strep throat, 86 would be correctly detected with the rapid test while 14 would be missed and not receive antibiotic treatment.
Plain language summary
What is the performance of rapid tests for the diagnosis of strep throat in children?
Background and aims
Sore throat is very common in children. It can be caused by viruses or bacteria. The bacterium most frequently identified during sore throat in children is group A streptococcus ('strep throat'). Amongst children with sore throat, antibiotic treatment is only useful in those with strep throat.
Simple, rapid tests for the diagnosis of strep throat have been available since the 1980s. Physicians can do a rapid test at the point of care by swabbing the throat. Based on the result of the rapid test, they can then decide if antibiotics are needed.
We reviewed the evidence about the performance of rapid tests for correctly detecting strep throat in children seen in Outpatient departments with a main complaint of sore throat.
Study characteristics
We searched for studies published in any language from January 1980 to July 2015. We found 98 unique studies, for a total of 116 test evaluations, involving 101,121 children. The number of participants ranged from 42 to 11,644 across test evaluations. The proportion of children with strep throat ranged from 9.5% to 66.6% across test evaluations.
Quality of the evidence
Important study design features were frequently not reported. The overall methodological quality of included studies was poor. For most studies, we had concerns about the ways in which participants were selected.
Key results
On average, rapid tests for strep throat had a sensitivity (ability to correctly detect people with the disease) of 86% and a specificity (ability to correctly identify people who do not have the disease) of 95%. There was substantial variability in rapid test performance across studies, which was not explained by study characteristics, including methodological quality. The two types of rapid tests under evaluation seemed to have comparable sensitivity (85.4% versus 86.2% for enzyme immunoassays and optical immunoassays, respectively). Based on these results, we would expect that amongst 100 children with strep throat, 86 would be correctly detected with the rapid test while 14 would be missed and not receive antibiotic treatment. Of 100 children with non‐streptococcal sore throat, 95 would be correctly classified as such with the rapid test while 5 would be misdiagnosed as having strep throat and receive unnecessary antibiotics.
Summary of findings
Summary of findings'. 'Summary of findings table.
| Review questions | What is the diagnostic accuracy of rapid antigen detection tests (RADT) for detecting group A streptococcus (GAS)? What is the relative diagnostic accuracy of the two major types of RADTs (enzyme immunoassays (EIA) and optical immunoassays (OIA))? | ||||||
| Patients/population | Children with acute pharyngitis | ||||||
| Prior testing | Physical examination establishing the diagnosis of pharyngitis, with or without evaluating the likelihood of a streptococcal origin | ||||||
| Settings | Ambulatory care settings: mainly private offices, emergency departments and walk‐in clinics | ||||||
| Index tests | EIA and OIA test for GAS | ||||||
| Reference standard | Throat culture on a blood agar plate | ||||||
| Importance | Compared with culture, RADTs offer diagnosis at the point of care. Whether negative RADTs should be backed up by throat culture depends mainly on the reported sensitivity of the test | ||||||
| Studies | Cross‐sectional studies | ||||||
| Quality concerns | Methodological quality was generally poor, but quality appraisal was impeded by suboptimal reporting. Patient selection and reference standard methods were common risk of bias concerns (in 73% and 43% of test evaluations, respectively) | ||||||
| Heterogeneity | There was substantial heterogeneity in the results of the individual studies, especially for sensitivity, which could not be explained by the investigations | ||||||
| Quantity of evidence | Average diagnostic accuracy | Consequences in a cohort of 1000 patients… | |||||
| Studies (n) | Participants (n) | Sensitivity (95% CI) |
Specificity (95% CI) |
…given 20% prevalence of GAS cases? | …given 30% prevalence of GAS cases? | …given 40% prevalence of GAS cases? | |
| RADT for the diagnosis of GAS pharyngitis in children (EIA and OIA tests) | 105 | 58,244 | 85.6% (83.3 to 87.6) | 95.4% (94.5 to 96.2) | 200 children will have a positive culture for GAS. Of these, 171 will be identified (TP); 29 will be missed (FN). Of the 800 children without GAS, 763 will not be treated (TN); 37 may receive unnecessary antibiotics (FP) | 300 children will have a positive culture for GAS. Of these, 257 will be identified (TP); 43 will be missed (FN). Of the 700 children without GAS, 668 will not be treated (TN); 32 may receive unnecessary antibiotics (FP) | 400 children will have a positive culture for GAS. Of these, 342 will be identified (TP); 58 will be missed (FN). Of the 600 children without GAS, 572 will not be treated (TN); 28 may receive unnecessary antibiotics (FP) |
| Comparison of EIA versus OIA tests | |||||||
| EIA tests | 86 | 48,808 | 85.4% (82.7 to 87.8) |
95.8% (94.8 to 96.6) | Interpretation: EIA and OIA tests seem to have comparable accuracy (P value = 0.23) | ||
| OIA tests | 19 | 9436 | 86.2% (82.7 to 89.2) | 93.7% (91.5 to 95.4) | |||
CI: confidence interval EIA: enzyme immunoassay FN: false negative FP: false positive GAS: group A streptococcus OIA: optical immunoassay RADT: rapid antigen detection test TN: true negative TP: true positive
Background
Target condition being diagnosed
Pharyngitis is defined as an acute inflammation of the pharynx, tonsils or both. A sore throat is the most common symptom of pharyngitis. The terms 'pharyngitis', 'tonsillitis' and 'sore throat' are often used interchangeably. In this review, the more general term 'pharyngitis' is used. Viruses are the most common cause of pharyngitis but the bacterium most frequently identified during acute pharyngitis is Streptococcus pyogenes (S. pyogenes), also known as group A β‐haemolytic streptococcus (GAS). GAS is estimated to account for 20% to 40% of cases of pharyngitis in children and 5% to 15% in adults (Shaikh 2010; Wessels 2011). The estimated number of cases of GAS pharyngitis in children is 450 million/year worldwide (Carapetis 2005a). Most cases are benign and self limiting within a week but suppurative complications (cervical lymphadenitis, retropharyngeal abscess, peritonsillar cellulitis or abscess (quinsy), sinusitis, acute otitis media and mastoiditis) or non‐suppurative post‐streptococcal diseases (acute rheumatic fever and rheumatic heart disease, acute glomerulonephritis, Sydenham’s chorea, scarlet fever, streptococcal toxic shock syndrome and paediatric autoimmune neuropsychiatric disorder associated with group A streptococci) can occur (Gerber 2005; Shulman 2009).
Acute rheumatic fever is an autoimmune disorder resulting from infection with group A streptococcus, in which heart valves may be severely damaged (rheumatic heart disease). In low‐income countries, rheumatic heart disease remains the most commonly acquired heart disease in children, adolescents and young adults: a recent estimate of the number of deaths from rheumatic heart disease is 233,000 per year worldwide (Carapetis 2005a). In high‐income countries, acute rheumatic fever and rheumatic heart disease are rare (e.g., ≤ 10 cases/year/100,000 children for acute rheumatic fever) (Carapetis 2005b; Seckeler 2011), because of improvements in living conditions, hygiene, increased antibiotic usage, increased access to primary care providers and changes in GAS epidemiology (Carapetis 2007). In the US, about 50% to 70% of the visits by children with pharyngitis result in antibiotic agents being prescribed (Linder 2005). As a result, the public health goal is shifting from preventing rare GAS complications to minimising inappropriate use of antibiotics.
Index test(s)
Simple rapid antigen detection tests (RADTs) were developed in the 1980s to provide an immediate indication for the clinician about the presence or absence of GAS in children with pharyngitis. RADTs do not require any special equipment and can be performed at the point of care with a throat swab (Gerber 2004). They can provide immediate results and are calibrated to produce binary results (positive or negative).
All available RADTs involve the detection of the Lancefield group A carbohydrate, a GAS‐specific cell‐wall antigen. Different immunologic techniques are available for carbohydrate detection (Gerber 2004); from older to most recent:
Latex agglutination (LA) assay: the sample is placed in the presence of latex beads coupled with GAS‐specific antibodies; the result is determined by observing the agglutination of the beads if they are related to the specific antigen in the sample. These first‐generation tests are no longer used in clinical practice and were not considered in this review.
Enzyme immunoassay (EIA): the sample is placed at the end of a nitrocellulose strip and then migrates to an area where it forms an antigen‐antibody complex. These second‐generation tests are also known as immunochromatographic, sandwich or lateral‐flow assays. They are the most widespread and most used RADTs in clinical practice.
Optical immunoassay (OIA): the sample is placed on a silicon membrane in the presence of the reagent. The result is based on the change in optical properties of the inert membrane in the presence of an antigen‐antibody complex. These third‐generation tests seem to be more sensitive than EIAs but their use is limited because of their high cost.
Clinical pathway
Many experts recommend the prescription of antibiotics for children with GAS‐suspected or GAS‐proven pharyngitis (Matthys 2007). The goal of antibiotic treatment is to reduce the individual risk of suppurative or non‐suppurative complications, the duration of symptoms and the spread of the condition (Spinks 2013). Correct identification of GAS ensures against missing GAS‐positive cases that can lead to complications. The correct exclusion of GAS ensures against unnecessary use of antibiotics (thus reducing the incidence of adverse drug reactions, antibiotic resistance and associated costs).
There is a lack of consensus on the most suitable diagnostic method for GAS in children with pharyngitis and the 'standard' diagnostic practice varies greatly amongst countries. The signs and symptoms of GAS and viral pharyngitis overlap broadly (Shaikh 2011), therefore most guidelines that recommend antibiotic treatment of GAS also recommend confirmation of the presence of GAS on the basis of a throat swab (Matthys 2007). However, throat swabs are explicitly not recommended in some countries (e.g., the United Kingdom, Belgium and the Netherlands) (Matthys 2007). International discrepancies might be explained by academic reasons and 'clinical traditions', different targets of sensitivity and specificity because of local epidemiological differences (i.e., rheumatic fever and rheumatic heart disease prevalence), international differences in health systems and policies, and the sparseness of recent data on the incidence of GAS complications and the efficacy of antibiotic treatment for their prevention.
The standard criterion for the diagnosis of GAS in children with pharyngitis is a throat culture on a blood agar plate in a microbiology laboratory (AAP 2012). The major advantage of laboratory throat culture is its detection of GAS from swabs with a very low number of bacteria, but the major limitation is the 48‐hour delay in obtaining results. In addition, throat cultures cannot distinguish true GAS infection from GAS carriage with intercurrent viral pharyngitis. Asymptomatic pharyngeal GAS carriage is usually defined as positive throat culture results for GAS without a GAS‐specific immune response (anti‐streptolysin O and anti‐DNase B antibodies) (Tanz 2007). Asymptomatic GAS carriage occurs in 10% to 15% of healthy children (Shaikh 2010), and does not require antibiotic treatment (Tanz 2007). Agreement is lacking on the most suitable culture technique for diagnosing GAS in children with pharyngitis. Several parameters are likely to affect the sensitivity of the test (culture medium, atmosphere of incubation, duration of incubation, group A identification technique and the number of plates inoculated) (Kellogg 1990; Tanz 1997). These variables affect the diagnostic accuracy of the throat culture and thus the diagnostic accuracy of RADTs as compared to throat culture.
RADTs are widely used for diagnosing GAS pharyngitis at the point of care. In children, the reported sensitivity of RADTs is about 85% (Gerber 2004), but varies greatly amongst studies (from 66% (Van Limbergen 2006) to 99% (Harbeck 1993)), and the specificity is high and stable, about 95% (Gerber 2004). Due to this high specificity, most experts agree on prescribing antibiotics with positive RADT results, even if RADTs cannot differentiate GAS true infection from GAS carriage. However, the consequences of a negative RADT result depend on national guidelines. North American guidelines recommend backing up negative RADT results with throat culture to avoid not treating RADT false‐negative cases (Gerber 2009; Shulman 2012), but most recent European guidelines recommend relying on negative RADT results without culture confirmation (Pelucchi 2012). In low‐income countries, the clinical consequences of RADT results might be the same as in high‐income countries (treat RADT‐positive cases only) but resources for testing might be limited and practices may vary from generalised empiric antibiotic treatment to selective antibiotic treatment or selective rapid testing based on clinical scoring systems (Joachim 2010; Steinhoff 2005; WHO 1995).
Alternative test(s)
Office culture
Another test for the diagnosis of GAS in children with pharyngitis is a throat culture performed in the physician's office (office culture). Office culture has the same disadvantage as a laboratory culture (a 48‐hour delay in obtaining results), with the major limitation being insufficient sensitivity (from 50% to 85%) (Battle 1971; Mondzac 1967; Rosenstein 1970; Tanz 2009; Wegner 1992). Office culture is almost completely abandoned and was not considered in this review.
Streptococcal antibody tests
Assessment of GAS‐specific antibodies is the traditional reference test to differentiate true GAS infection and GAS carriage. The most commonly used GAS‐specific antibody assays tests are for anti‐streptolysin O and anti‐DNase B antibodies. Increased antibody titre assessment diagnoses true GAS infection better than a single absolute titre assessment (Gerber 1986b; Johnson 2010). Streptococcal antibody tests are not used for the diagnosis of GAS in children with pharyngitis because of the need for repeat blood samples. Moreover, the information about the kinetics of the immune response to GAS in children with pharyngitis is very limited and the most recent data show that the interpretation of streptococcal antibody test results is not straightforward (Johnson 2010). Therefore, their use is usually limited to documenting recent GAS infection in patients suspected of having GAS non‐suppurative complications or to epidemiologic studies (Gerber 1986b; Johnson 2010).
Clinical scoring systems
Clinical scoring systems have been developed to diagnose GAS on clinical grounds. The most popular of these scores are the Centor score (Centor 1981) and the McIsaac score (McIsaac 1998). The scores are based on assessing simple clinical criteria (history of fever, cough, tonsillar swelling or exudate, tender cervical adenopathy and age). Their use is recommended in adults but might be inappropriate in children; several authors have reported a lack of diagnostic accuracy in this population (Cohen 2012; Cohen 2015; Fischer Walker 2006; Shaikh 2011). Clinical scoring systems were not considered in this review.
Rapid molecular biology assays
Rapid molecular biology assays for GAS in children with pharyngitis have been recently developed (Group A Streptococcus Direct Test; GenProbe Inc., San Diego, CA; and LightCycler Strep‐A assay; Roche Applied Science, Indianapolis, IN) (Chapin 2002; Heelan 1996; Pokorski 1994; Uhl 2003). These techniques, based on DNA‐rRNA hybridisation or polymerase chain reaction (PCR), are highly sensitive but are not currently used widely because of their cost, the need for highly specialised equipment and personnel, and the two‐hour delay in results (Gerber 2004). Molecular assays are not antigen‐detection tests and were not considered in this review.
Rationale
Childhood pharyngitis is a significant public health problem with, on the one hand, suppurative and non‐suppurative complications of GAS pharyngitis (especially acute rheumatic fever and rheumatic heart disease) and, on the other, costly diagnostic tests and unnecessary antibiotics. RADTs for GAS are now widely available and their use in children with pharyngitis might increase accurate diagnosis and reduce antibiotic consumption.
According to local clinical guidelines, RADTs may be used as stand‐alone diagnostic tests in replacement of throat culture (e.g., in contexts where throat culture is unavailable or not used), or as triage tests, with negative results being supported by a throat culture. These international discrepancies might be explained in part by persistent gaps in knowledge regarding the diagnostic accuracy of RADTs:
What is the accuracy of RADTs for GAS in children with pharyngitis compared to the most consensual reference test (throat culture on a blood agar plate)?
Are there significant differences in diagnostic accuracy between EIAs and OIAs?
Which study‐level factors could explain variations in diagnostic accuracy across clinical studies?
We did not address in this review the questions of whether RADTs should be performed in all patients presenting with signs and symptoms of pharyngitis or only in selected patients on the basis of a clinical score (selective testing strategies), and whether clinical protocols that incorporate RADTs are sufficient to reduce antibiotic prescription. We aimed to provide information to help clinicians and public health decision makers better define the precise role of RADTs in the diagnosis of GAS in children with pharyngitis on the basis of unbiased evidence.
Objectives
To determine the diagnostic accuracy of RADTs for diagnosing GAS in children with pharyngitis. To assess the relative diagnostic accuracy of the two major types of RADTs (enzyme immunoassays (EIA) and optical immunoassays (OIA)) by indirect and direct comparison.
Secondary objectives
To assess the relative diagnostic accuracy of EIA and OIA tests by indirect and direct comparison.
Methods
Criteria for considering studies for this review
Types of studies
We included reports of cross‐sectional studies reporting the diagnostic accuracy of one or more RADTs for the diagnosis of GAS in children with pharyngitis, with laboratory throat culture as the reference standard. Reports of randomised controlled trials (RCTs) were also eligible if we could extract 2 x 2 tables for children. Reports of studies in which throat culture was selectively performed in participants with a positive or negative RADT result were included in the review but excluded from the meta‐analysis of sensitivity and specificity estimates.
Participants
We included reports of studies of children (age ≤ 21 years, according to the upper limit used by the American Academy of Pediatrics) seeking ambulatory medical care because of a sore throat or with a diagnosis of pharyngitis, who provided a throat swab for a RADT and laboratory throat culture. In this review, ambulatory care settings included private physicians' offices (general practitioners and paediatricians), walk‐in clinics, hospital outpatient clinics, emergency departments and family medicine centres; we excluded studies performed by specialised physicians (e.g., ear, nose and throat specialists).
We also included reports of studies with only a subgroup of participants eligible for inclusion in the review, provided that we could extract relevant data specific to that subgroup. Reports of studies were not excluded on the basis of whether studies were performed in high‐income or low‐income countries because no data exist to support variations in the accuracy of RADTs according to this criterion.
Index tests
We included only studies of EIA or OIA RADTs for GAS in children with pharyngitis, including those no longer marketed.
Target conditions
GAS in children with pharyngitis (dichotomous).
Reference standards
Studies were required to diagnose GAS with throat culture on a blood agar plate in a microbiology laboratory used as the reference test. Several parameters may affect the accuracy of throat culture. For studies involving more than one throat culture technique (different medium, duration or atmosphere of incubation), we a priori chose to extract data related to the culture technique recommended by a panel of North American content experts, i.e., simple blood agar plate (versus selective or enriched media), incubation 48 hours total (versus 18 to 24 hours only), aerobic atmosphere (versus other) (Shulman 2000), in order to avoid data‐driven approaches.
Search methods for identification of studies
Electronic searches
We searched MEDLINE via Ovid (1980 to May week 5, 2013) using the search strategy described in Appendix 1. The search strategy was developed in consultation with a medical librarian and the Trials Search Co‐ordinator for the Acute Respiratory Infections Group and was adapted to search EMBASE via Elsevier (1980 to June 2013) (Appendix 2) and Web of Science (1980 to June 2013) (Appendix 3). We did not use any filter related to age because many RADT studies enrol adults and children and could provide extractable data for children. We did not use methodological filters to identify diagnostic studies because such filters may result in omission of relevant studies (Leeflang 2006; Whiting 2011b). The searches were run from 1980 onwards because RADTs were not available prior to this date. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) for relevant studies.
We searched the following databases to identify potentially relevant studies referenced in reviews and guidelines:
the Cochrane Database of Systematic Reviews (2013, Issue 5);
DARE (Database of Abstracts of Reviews of Effects) (2013, Issue 2 of 4);
the MEDION database (for Systematic Reviews of Diagnostic Studies) (23 May 2013); and
TRIP (Turning Research Into Practice) (23 May 2013).
We also searched Conference Proceedings Citation Index (CPCI) and SCI‐Expanded for conference proceedings and abstracts. The literature search was updated by the Trials Search Co‐ordinator for the Acute Respiratory Infections Group on 7 July 2015.
Searching other resources
We handsearched reference lists of included articles and relevant review articles identified through the search and the ‘related articles’ function in PubMed (20 first related articles of each included article) for eligible articles. We used Google Scholar to search for reports that cited included articles. We contacted manufacturers of the most common RADTs to seek additional or unpublished studies. Manufacturers included Abbott, Beckman Coulter, Becton Dickinson, Genzyme, Inverness Medical, Polymedco and Quidel.
Data collection and analysis
Selection of studies
We considered studies published in any language. Two review authors (JFC, NB) independently excluded studies that were not related to pharyngitis or RADT on the basis of the titles and abstracts identified by the search strategy. Two review authors (JFC, NB) retrieved the full text of relevant articles and independently evaluated them for inclusion by using a pro forma as a guide. One review author (MC) acted as arbiter in case of discrepancies between two review authors (JFC, NB) who discussed the inclusion of the studies.
We selected the most recent or most complete report in cases of multiple reports for a given study or when we could not exclude the possibility of overlapping populations. We produced a flowchart to report the search process. We reported reasons for excluding studies but we did not report their references.
Data extraction and management
We extracted the number of true positives, true negatives, false positives and false negatives for each index test evaluated in each study to construct 2 x 2 tables. If such data were not provided by the trial authors, we calculated the number of true positives, true negatives, false positives and false negatives from the summary estimates of sensitivity and specificity of the index test, if available. For studies for which only a subgroup of patients were included in the review, we extracted, analysed and presented data for this subgroup only. If some data were unclear or missing, we attempted to contact study authors to obtain additional data.
Two authors (JFC, NB) independently extracted the data used for study quality assessment and statistical analysis (data from 2 x 2 tables and covariates used for investigations of heterogeneity) and resolved discrepancies by discussion until a consensus was reached; other descriptive data were extracted by one review author (JFC). See Table 2 for a description of which data were extracted for each study. Non‐English language reports were not translated: for reports in French, Italian, Spanish and German, members of our team extracted data; for other languages, the Cochrane Acute Respiratory Infections Group identified collaborators who kindly agreed to extract the data.
1. Data extracted from each study.
| Study ID | First author, year of publication |
| Type of study | Journal article or conference abstract |
| Clinical features and settings | Presenting signs and symptoms |
| Clinical selection of patients (none, clinical score, explicit criteria but not a score, implicit criteria) | |
| Exclusion if antibiotics use before inclusion (yes/no) | |
| Clinical setting (office‐based, emergency department, walk‐in clinic, mixed, other) | |
| Single‐ or multi‐centre study | |
| Age range for inclusion | |
| Participants | Sample size (n) |
| Age (distribution) | |
| GAS prevalence according to culture (with 95% confidence interval) | |
| Country of study | |
| Sex (% of girls) | |
| Clinical severity assessment (Centor score, McIsaac score, other, none) | |
| Study design | Cross‐sectional study or RCT |
| Retrospective or prospective design | |
| Sample (consecutive, random or unclear) | |
| Direct comparison of different RADTs (yes/no) | |
| Direct comparison of several throat culture techniques (yes/no) | |
| Throat swab (1 single, 1 double, 2 different) | |
| Person performing the throat sample (physician, nurse, laboratory personnel, other) | |
| Reference standard(s) | Throat culture medium (standard, enrichment, inhibitory) |
| Atmosphere of incubation (aerobic, aerobic with CO2 enrichment, anaerobic) | |
| Duration of incubation (≤ 24, 24 to 48, ≥ 48 hours) | |
| GAS confirmation (bacitracin disk, latex test, other, none) | |
| Number of plates inoculated (n) | |
| Assessment of GAS antibody response (yes/no) | |
| Relevant details | |
| Index tests | Commercial name of the RADT |
| Type of RADT (EIA, OIA) | |
| Data | Number of true positives, false positives, true negatives, false negatives and undetermined/uninterpretable results |
| Notes | Source of funding (whether any of the authors is affiliated with the manufacturer of the RADT, the study was directly funded by the manufacturer, authors reported conflicts of interests related to the manufacturer or other funding sources) |
| Anything else of relevance |
RADT: rapid antigen detection test EIA: enzyme immunoassay OIA: optical immunoassay CO2: carbon dioxide
Assessment of methodological quality
Methodological quality assessment involved use of a four‐domain tool adapted from QUADAS‐2 (Whiting 2011a). Two review authors (JFC, NB) independently collected the information needed to assess the methodological quality of each study using signalling questions (yes/no/unclear). We resolved disagreements on the signalling questions by discussion with a third author (MC) until a consensus was reached. One author (JFC) used this information to judge the risk of bias and concerns about applicability using pre‐defined rules. We tailored the quality assessment tool to our review question. We developed review‐specific guidance on how to assess each signalling question and how to use this information to judge the risk of bias and applicability. We refined the tool until satisfactory inter‐rater agreement on signalling questions was achieved. We summarised the methodological quality assessment in tables. See Table 3.
2. Methodological quality assessment table for each study.
| Domain 1: Patient selection | |
| Was a consecutive or random sample of patients enrolled? | Yes, No or Unclear |
| Was it a cross‐sectional study or a RCT? | Yes, No or Unclear |
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes, No or Unclear |
| Were patients seen in an ambulatory care setting? | Yes, No or Unclear |
| Was clinical selection of patients avoided? | Yes, No or Unclear |
| Could the selection of patients have introduced bias? | Risk: Low, High or Unclear |
| Is there concern that the included patients do not match the review question? | Concern: Low, High or Unclear |
| Domain 2: RADT (index test) | |
| Were RADTs conducted during consultation time? | Yes, No or Unclear |
| Were the RADT results interpreted with blinding of the results of culture? | Yes, No or Unclear |
| Was the type of the RADT mentioned (EIA or OIA)? | Yes, No or Unclear |
| Could the conduct or interpretation of the RADT have introduced bias? | Risk: Low, High or Unclear |
| Is there concern that the RADT, its conduct or interpretation differ from the review question? | Concern: Low, High or Unclear |
| Domain 3: Throat culture (reference standard) | |
| Were culture results interpreted with blinding of the results of the RADT? | Yes, No or Unclear |
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during ≥ 48 hr)? | Yes, No or Unclear |
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes, No or Unclear |
| Could the throat culture, its conduct or its interpretation have introduced bias? | Risk: Low, High or Unclear |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | Concern: Low, High or Unclear |
| Domain 4: Flow and timing | |
| Was the delay between the performance of the RADT and throat culture plating ≤ 48 hours? | Yes, No or Unclear |
| Did all patients receive a throat culture? | Yes, No or Unclear |
| Did patients receive the same throat culture method? | Yes, No or Unclear |
| Were undetermined/uninterpretable results reported? | Yes, No or Unclear |
| Were withdrawals from the study explained? | Yes, No or Unclear |
| Could the patient flow have introduced bias? | Risk: Low, High or Unclear |
Statistical analysis and data synthesis
We entered data for the 2 x 2 tables into RevMan 2014 and plotted estimates of sensitivity and specificity on forest plots and in the receiver‐operating characteristic (ROC) space to represent the variability in diagnostic test accuracy within and between studies.
We fitted the hierarchical bivariate model described by Reitsma 2005 by use of Stata/SE version 13 (using the user written program 'metandi'), which allowed for calculating summary estimates of sensitivity and specificity and the associated 95% confidence intervals (CIs). We also reported the estimate of correlation between sensitivity and specificity (rho). We put the results from the bivariate model into RevMan 2014 to provide plots of the estimated summary points and confidence regions, superimposed on the study‐specific estimates of sensitivity and specificity in the ROC space.
We included the same study in the same meta‐analysis more than once if needed, i.e., if one study reported different index tests. We presented results in groups according to commercial test name.
Investigations of heterogeneity
We initially visually inspected the forest plots and ROC space to check for heterogeneity between study results. To investigate sources of heterogeneity, we incorporated covariates in the bivariate model, i.e., meta‐regression (using the built in program 'xtmelogit' and routines available at http://methods.cochrane.org/sdt/software‐meta‐analysis‐dta‐studies). We assessed the significance of the difference in covariate by likelihood ratio test comparing the bivariate model with and without the covariate. We used a P value of less than 0.05 to denote statistical significance. With a significant test result, we assessed effects of covariates on sensitivity and specificity separately by testing the significance of the change in ‐2 log‐likelihood of the model (i.e., change in model deviance) with or without corresponding terms. We addressed the five following sources of heterogeneity by adding variables to the meta‐analysis model:
a. Effect of test type
Some authors have suggested that OIA may be more sensitive than EIA tests (Gerber 2004). Therefore, we tried to indirectly compare the RADT tests by using test type as a categorical covariate in the models (EIA versus OIA); in indirect comparisons, data originate from different studies in which participants underwent either the EIA or the OIA test. We also tried to perform direct comparisons of EIA versus OIA by restricting the analysis to studies in which all patients underwent both EIA and OIA tests.
b. Effect of the reference standard
In this review, the reference standard was throat culture on a blood agar plate. However, several parameters may affect the accuracy of throat culture on blood agar, including whether an enrichment broth was used before plating. We added this variable as a categorical covariate (yes/no) in the model.
c. Effect of age
The sensitivity of RADTs is known to be higher in younger children than in older ones (Cohen 2012; Edmonson 2005). This might be explained by higher GAS prevalence in school‐age children with pharyngitis than in older children. Therefore, we explored age as a potential source of heterogeneity by using the mean age of patients in the study as a categorised covariate in the model (i.e., below or above median of mean age across studies).
d. Effect of disease severity
Spectrum effect has been demonstrated for RADTs, with increasing sensitivity with increasing disease severity, usually assessed by the McIsaac score (Cohen 2012; Edmonson 2005; Hall 2004; Tanz 2009). Therefore, disease severity might be a relevant source of heterogeneity to explore. We used the proportion of patients with a McIsaac score greater than two as a categorical covariate in the model; we compared studies with less than 70% of patients with a McIsaac score greater than two to studies with more than 70% of patients with a McIsaac score greater than two (arbitrary).
e. Effect of GAS prevalence
Diagnostic accuracy may vary with disease prevalence (Leeflang 2009; Leeflang 2013), usually with better performances in a population with higher disease prevalence. We considered GAS prevalence as a dichotomised covariate to define low‐risk versus high‐risk study populations (i.e., below or above median of GAS prevalence across studies).
Sensitivity analyses
We carried out the following sensitivity analyses to explore the robustness of the results:
include only studies judged at low risk of bias in each QUADAS‐2 domain;
include only studies judged at low risk of bias in at least 3 of 4 QUADAS‐2 domains (arbitrary);
include only studies judged to have low concerns about applicability in each QUADAS‐2 domain.
Additional analyses
We performed univariate logitnormal random‐effects meta‐analysis of the negative predictive value of RADTs (using the user written command ‘metan’) combining studies with complete verification and studies in which RADT results were selectively verified by throat culture only in RADT‐negative participants.
Assessment of reporting bias
We did not try to assess reporting bias (Macaskill 2010).
Results
Results of the search
The electronic search was performed on 7 July 2015. The search identified 5576 titles, of which we identified 82 as duplicates. We further excluded a total of 5166 titles on the basis of their title, abstract or both (Figure 1). After assessment of the full text of 328 articles, we excluded 235. Using the 'PubMed related articles' function and Google Scholar, and checking the references of included studies or reviews on the same topic (Gerber 2004; Lean 2014; Ruiz‐Aragon 2010; Stewart 2014), allowed us to include five additional studies (Nitsch‐Osuch 2010; Pauchard 2012; Sedki 2010; Tellechea 2012; Wong 1989). When possible, we contacted by email and postal mail authors of studies that included children and adults or in which the age of participants was unclear; eight trial authors shared or clarified paediatric data (Arribas Blanco 1988; Drulak 1991; Llor 2008; Mezghani Maleej 2010; Mlejnek 2014; Pauchard 2012; Pauchard 2013; Schwabe 1987; Schwabe 1991; Toepfner 2013). All included studies were cross‐sectional. Manufacturers of RADTs did not respond. Thus, this review includes a total of 98 unique study reports.
1.
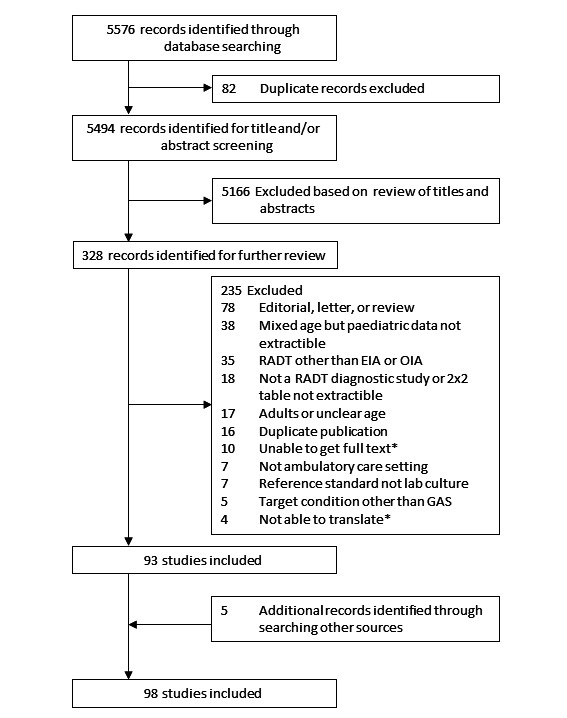
Flow diagram of studies in the review. *Studies awaiting classification (n = 14)
Included studies
Some studies were subdivided for the purpose of the review. One multi‐centre study conducted in four different countries was subdivided into four study cohorts (Rimoin 2010a). Some studies were also subdivided because they evaluated more than one RADT: nine studies compared two tests (Donatelli 1992a; Egger 1990a; Gieseker 2002a; Kaufhold 1991a; Mayes 2001a; Mirza 2007a; Roe 1995a; Schwartz 1997a; Wright 2007a), one compared three tests (Rogo 2010a), and one compared five tests (Chiadmi 2004a). Thus, this review includes a total of 116 test evaluations reporting a total of 101,121 test results. We performed descriptive analysis, methodological quality assessment and meta‐analysis at the test evaluation level.
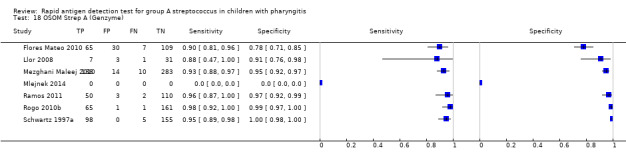
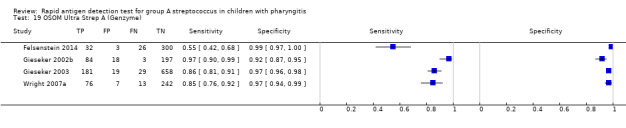
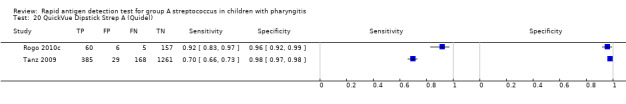
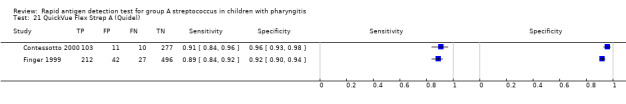
Included studies came from a variety of countries (n = 25); 53 (46%) test evaluations were conducted in the US. Forty‐two different commercial RADT kits were evaluated, and three studies mentioned evaluating an EIA test without providing any commercial name (further referred to as “EIA (no name)”). Six commercial kits were evaluated in at least five paediatric cohorts: OSOM Strep A, QuickVue InLine Strep A, Strep A OIA, Strep A OIA Max, TestPack Strep A and TestPack Plus.
Excluded studies
Amongst 328 full‐text articles assessed, we excluded 235 trials. Thirty‐five assessed RADTs relying on other technologies than EIA or OIA. We excluded 38 studies because they included children and adults but did not report specific data for children, and we could not obtain additional data by contacting the trial authors. The status of 10 studies is uncertain because we were unable to obtain articles in full text. The status of four articles is uncertain as they have not yet been translated (two articles in Turkish, one in Polish and one in Czech).
Methodological quality of included studies
The overall methodological quality of included study cohorts is summarised in Figure 2. The quality assessment results for the individual studies is shown in Figure 3. The median sample size per study cohort was 297 participants (interquartile range (IQR) 196 to 539). The median mean age of participants was 6.6 (IQR 5.8 to 7.7) years, as reported in 32 studies. The majority of study cohorts (82 of 116, 71%) did not clearly report whether participants formed a consecutive, random or convenience series. Fifty‐eight study cohorts (50%) avoided clinical selection of participants and therefore included a representative spectrum of patients.
2.
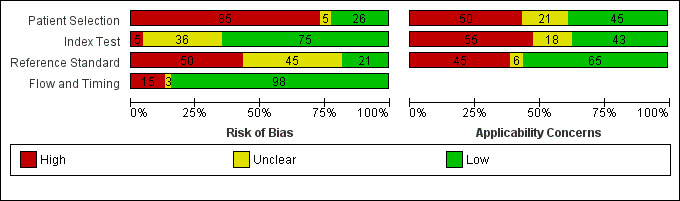
Risk of bias and applicability concerns graph: review authors' judgements about each domain across all included study cohorts (n = 116).
3.
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study cohort (n = 116).
Interpretation of the results of the RADT was done with blinding of the result of throat culture in 84 of 116 cases (72%). An appropriate reference standard (i.e., laboratory throat culture on a blood agar plate during 48 hours) was used in 72 study cohorts (62%). Interpretation of the results of the reference standard was done with blinding of the result of the RADT in 23 of 116 cases (20%).
Partial verification was avoided in a majority (105 of 116, 91%) of cases. In 10 study cohorts (42,319 participants), RADT results were verified by throat culture only in RADT negative participants (Ayanruoh 2009; Cohen 2004; Edmonson 2005; Hall 2004; Mayes 2001a; Mirza 2007a; Mlejnek 2014; Van Limbergen 2006); in one study (558 participants) RADT results were verified only in RADT positive participants (Cohen 1998).
Findings
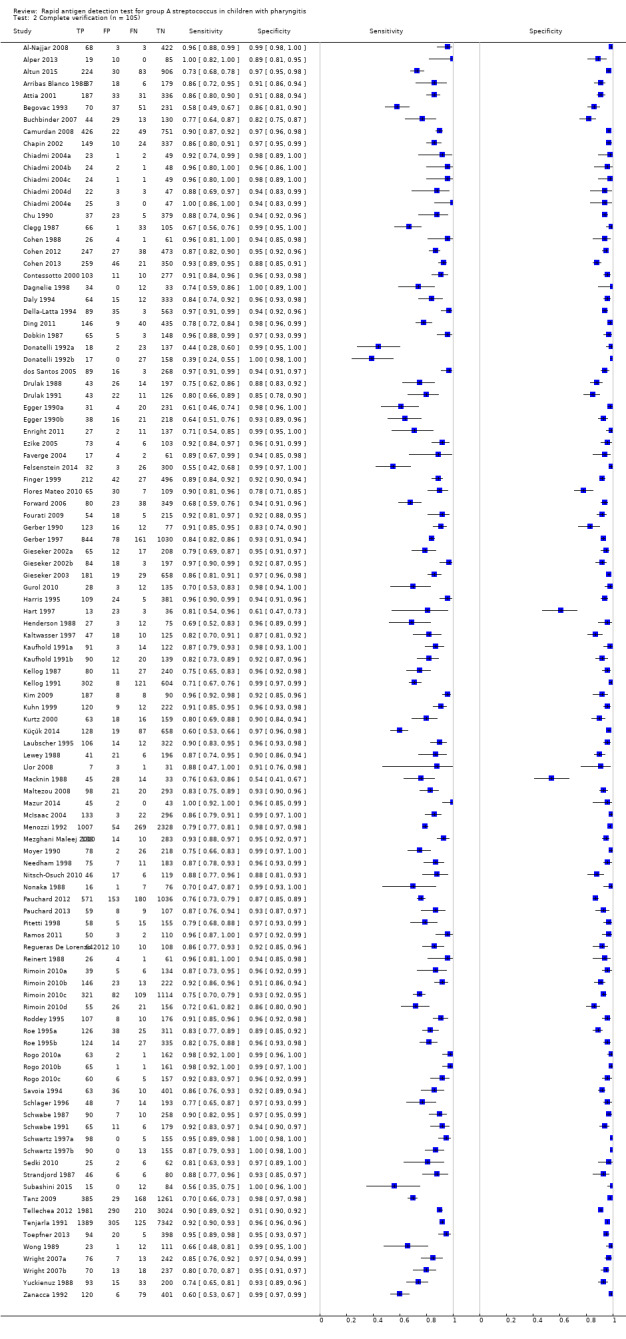
Across the 116 study cohorts included in the review, the sensitivity of rapid antigen detection tests (RADTs) ranged from 38.6% to 100% and the specificity from 54.1% to 100% (Figure 4). We excluded 11 study cohorts from the meta‐analysis of sensitivity and specificity estimates for a final dataset containing 105 pairs of sensitivity and specificity (58,244 participants), where partial verification was not avoided.
4.
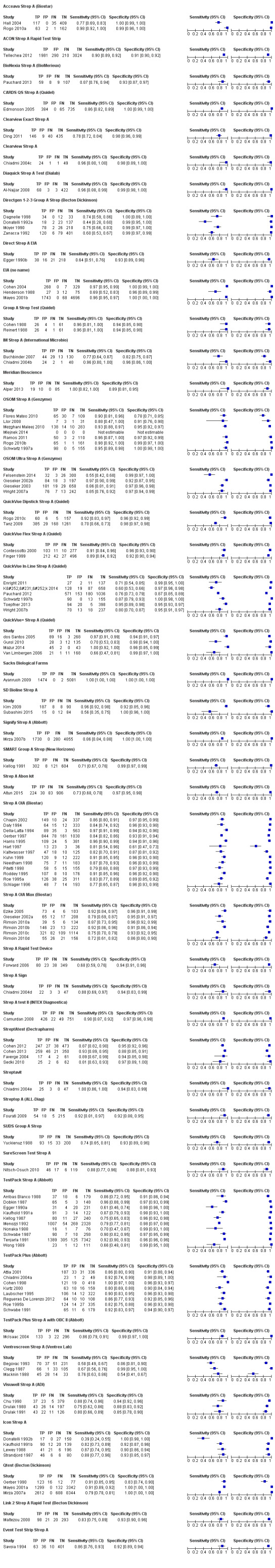
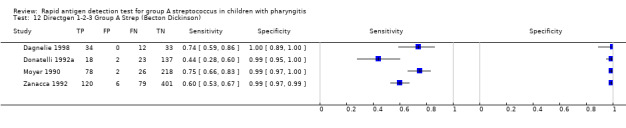
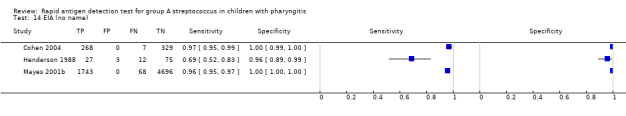
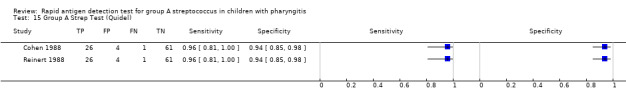
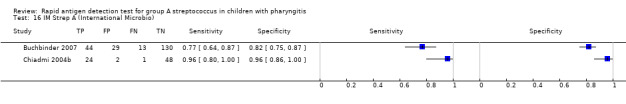
Forest plots of RADT sensitivity and specificity for GAS detection, ordered by commercial kit. TP = True Positive; FP = False Positive; FN = False Negative; TN = True Negative.
Summary estimates of sensitivity and specificity
Amongst 105 test evaluations included in the meta‐analysis (58,244 participants), the summary estimates of sensitivity and specificity were 85.6%; 95% confidence interval (CI) 83.3 to 87.6; and 95.4%; 95% CI 94.5 to 96.2, respectively (Figure 5). There was no statistical evidence of a correlation between sensitivity and specificity (correlation coefficient ‐0.17; 95% CI ‐0.39 to 0.07).
5.
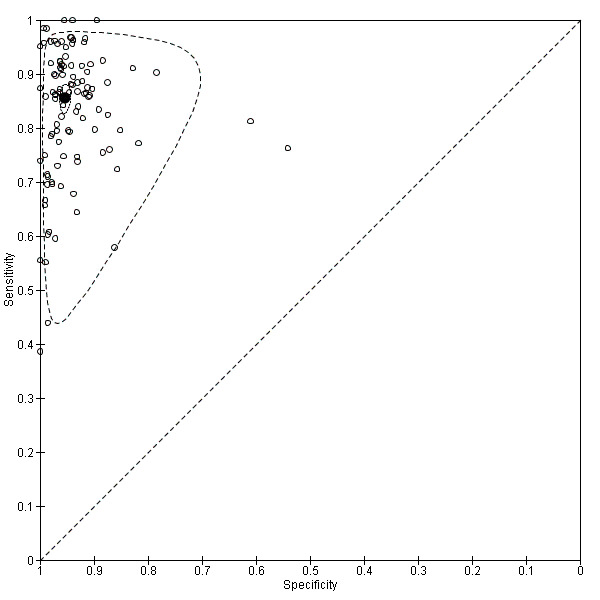
Summary ROC plot of RADT sensitivity and specificity for GAS detection (n = 105). Each individual study cohort is represented by an empty circle. The filled circle is the pooled summary estimate for sensitivity and specificity. The solid curve represents the 95% confidence region around the summary estimate; the dashed curve represents the 95% prediction region.
Enzyme immunoassay (EIA) tests
We included 86 evaluations of EIA RADTs (48,808 participants). The median sample size was 263 (IQR 178 to 454) and the median prevalence of group A streptococcus (GAS) on throat culture was 29.5% (IQR 23.8% to 34.9%). Sensitivity of EIA RADTs ranged from 38.6% to 100%, and specificity from 54.1% to 100%. The summary estimates of sensitivity and specificity for EIA tests were 85.4% (82.7 to 87.8) and 95.8% (94.8 to 96.6), respectively (Figure 6).
6.
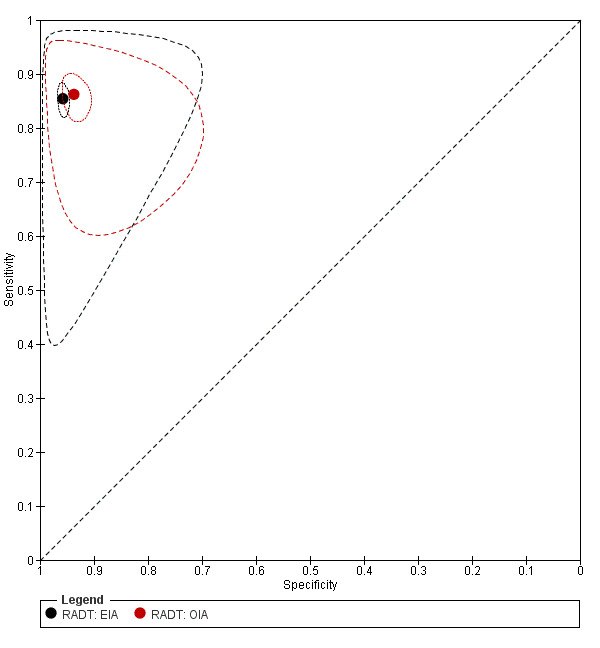
Summary ROC plot of RADT sensitivity and specificity for GAS detection: EIA (n = 86) versus OIA (n = 19). The filled black circle is the pooled summary estimate for sensitivity and specificity of EIA tests; the filled red circle is the pooled summary estimate for sensitivity and specificity of OIA tests The solid curves represent the 95% confidence region around the summary estimate; the dashed curves represent the 95% prediction region.
Optical immunoassay (OIA) tests
We included 19 evaluations of OIA RADTs (9436 participants). The median sample size was 302 (IQR 233 to 519), and the median prevalence of GAS on throat culture was 29.5% (IQR 23.7% to 36.4%). Sensitivity of OIA RADTs ranged from 72.4% to 96.7%, specificity from 61.0% to 97.1%. The summary estimates of sensitivity and specificity for OIA tests were 86.2% (82.7 to 89.2) and 93.7% (91.5 to 95.4), respectively (Figure 6).
Investigations of heterogeneity
Visual inspection of the forests plots and ROC space suggested substantial heterogeneity in accuracy estimates, especially amongst estimates of sensitivity, as reflected by the wide prediction areas around summary estimates. The results of investigations of heterogeneity are summarised in Table 4.
3. Results of investigations of heterogeneity.
| Study‐level covariate | Studies (n) | Sensitivity (95% CI) | Specificity (95% CI) | Interpretation | |
| Test typea | |||||
| Enzyme immuno‐assay | 86 | 85.4 (82.7 to 87.8) | 95.8 (94.8 to 96.6) | Accuracy does not seem influenced by test type (P value = 0.23) | |
| Optical immuno‐assay | 19 | 86.2 (82.7 to 89.2) | 93.7 (91.5 to 95.4) | ||
| Throat culture | |||||
| Without enrichment broth | 88 | 85.5 (82.8 to 87.8) | 95.6 (94.8 to 96.3) | Accuracy does not seem influenced by whether an enrichment broth was used (P value = 0.15) | |
| With enrichment broth | 10 | 86.3 (83.3 to 88.7) | 92.7 (87.9 to 95.7) | ||
| Mean age of participantsb | |||||
| Below the median | 16 | 87.1 (81.7 to 91.1) | 93.2 (90.5 to 95.2) | No evidence of association with age (P value = 0.39) | |
| Above the median | 13 | 83.7 (78.5 to 87.9) | 95.0 (92.7 to 96.6) | ||
| % of patients with McIsaac score > 2 | |||||
| ≤ 70% | 4 | 81.3 (69.8 to 89.1) | 94.9 (91.1 to 97.2) | No evidence of association with clinical severity (P value = 0.35) | |
| > 70% | 8 | 88.8 (82.9 to 92.9) | 94.2 (89.4 to 96.9) | ||
| Prevalence of group A streptococcusc | |||||
| Below the median | 54 | 84.9 (81.1 to 88.1) | 95.5 (94.2 to 96.4) | Accuracy does not seem influenced by the prevalence of group A streptococcus (P value = 0.70) | |
| Above the median | 51 | 86.2 (83.5 to 88.5) | 95.4 (94.0 to 96.5) | ||
aResults based on indirect comparisons; bthe median of mean age was 6.6 years; cthe median of group A streptococcus prevalence using throat culture as the reference standard was 29.5%.
CI: confidence interval
a. Effect of test type
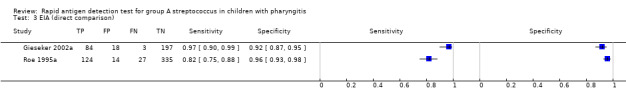
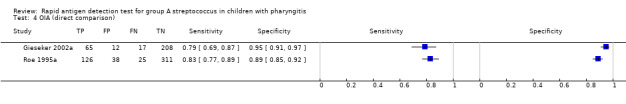
There were 86 evaluations of EIA tests (48,808 participants) and 19 evaluations of OIA tests (9436 participants). Based on analysis of all available data, there was no statistical evidence that sensitivity and/or specificity differed between EIA and OIA tests (sensitivity 85.4% versus 86.2%, respectively; specificity 95.8% versus 93.7%, respectively; change in model deviance = 2.90; P value = 0.23) (Figure 6).
Two studies directly compared EIA to OIA tests by applying both tests to each individual (802 participants; Figure 7) (Gieseker 2002a; Roe 1995a); data were too limited to perform additional statistical analysis. In Gieseker 2002a, EIA and OIA tests had comparable specificity (92% (87 to 95) versus 95% (91 to 97), respectively), and the EIA test had the highest sensitivity (97% (90 to 99) versus 79% (69 to 87), respectively). Contrarily, Roe 1995a found that EIA and OIA tests had comparable sensitivity (82% (75 to 88) versus 83% (77 to 89), respectively), with the specificity of EIA being higher than that of the OIA test under evaluation (96% (93 to 98) versus 89% (85 to 92)).
7.
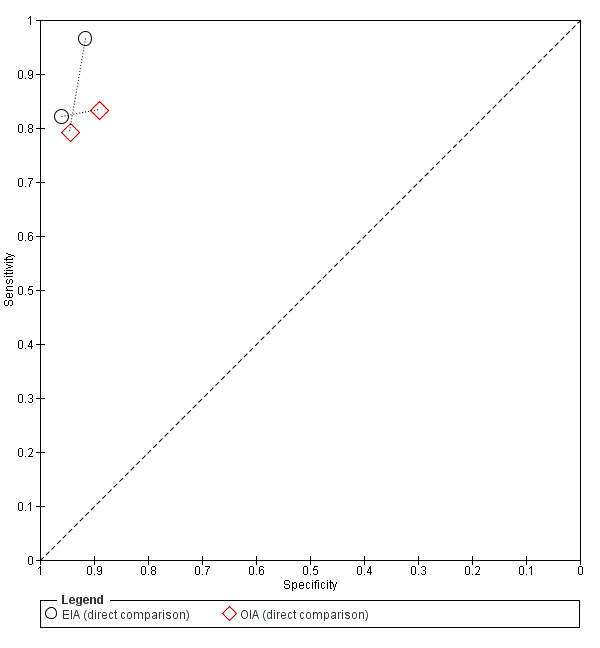
Summary ROC plot of RADT sensitivity and specificity for GAS detection: direct comparison of EIA versus OIA (n = 2). Each individual study cohort is represented by an empty black circle (EIA) and an empty red diamond (OIA), connected by a dotted line.
b. Effect of the reference standard
An enrichment broth was used before plating in 10 test evaluations; this was not done in 88 study cohorts, and the information was unclear for seven. Using an enrichment broth before plating was not associated with significantly different estimates of sensitivity and/or specificity (sensitivity 86.3% versus 85.5%, respectively; specificity 92.7% versus 95.6%, respectively; change in model deviance = 3.79; P value = 0.15).
c. Effect of age
Twenty‐nine studies reported the mean age of participants. The median of the mean age of participants was 6.6 years (IQR 5.8 to 7.4). Mean age was not associated with significantly different estimates of sensitivity and/or specificity (sensitivity 87.1% versus 83.7%, respectively; specificity 93.2% versus 95.0%, respectively; change in model deviance = 1.87; P value = 0.39).
d. Effect of disease severity
Twelve studies assessed clinical severity using the McIsaac score. The median proportion of severe patients (patients with a McIsaac score greater than two) was 85% (IQR 63% to 91%). The proportion of severe patients was below 70% in four study cohorts. Meta‐regression did not show evidence of significant associations between clinical severity and sensitivity and/or specificity (change in model deviance = 2.10; P value = 0.35).
e. Effect of GAS prevalence
Based on the proportion of throat culture results positive for GAS, the median prevalence of participants with streptococcal pharyngitis was 29.5% (IQR 23.8% to 34.9%). There was no significant effect of GAS prevalence on sensitivity and/or specificity when GAS prevalence was tested as a covariate in the bivariate model (change in model deviance = 0.71; P value = 0.70).
Sensitivity analysis
Compared with the overall results (summary sensitivity 85.6%), sensitivity was lower in the 20 studies at low risk of bias for the reference standard (81.0%), higher in the 33 studies with low concerns about applicability in the index test domain (89.1%), but stable in the 20 studies at low risk of bias in at least three QUADAS‐2 domains (84.0%) (Table 5). Summary estimates of specificity were robust across subgroups, at around 95%.
4. Results of sensitivity analyses.
| Concerns | Domain | Studies at low risk (n) | Sensitivity (95% CI) | Specificity (95% CI) |
| Risk of bias | Patient selection | 25 | 85.7 (82.1 to 88.6) | 93.0 (91.1 to 94.5) |
| Index test | 65 | 86.6 (84.0 to 88.8) | 95.2 (94.1 to 96.1) | |
| Reference standard | 20 | 81.0 (74.1 to 86.5) | 95.5 (93.4 to 96.9) | |
| Flow and timing | 98 | 85.4 (83.0 to 87.5) | 95.3 (94.4 to 96.1) | |
| ≥ 3 domains with low risk of bias | 20 | 84.0 (79.4 to 87.8) | 95.0 (93.1 to 96.4) | |
| Applicability | ||||
| Patient selection | 41 | 83.1 (79.7 to 86.0) | 94.9 (93.4 to 96.0) | |
| Index test | 33 | 89.1 (85.7 to 91.8) | 95.0 (93.2 to 96.4) | |
| Reference standard | 60 | 84.9 (81.6 to 87.6) | 94.7 (93.5 to 95.7) |
CI: confidence interval
Additional analysis
We excluded 10 studies from the main meta‐analysis of sensitivity and specificity estimates because RADT results were selectively verified by throat culture only in RADT negative participants (partial verification); four were very large studies (more than 3000 participants) (Ayanruoh 2009; Mayes 2001a; Mirza 2007a; Mlejnek 2014). We performed a meta‐analysis of the negative predictive value of RADTs, including those 10 additional studies. Across 115 test evaluations, the median prevalence of participants with streptococcal pharyngitis was 29.4%. Negative predictive value ranged from 70.2% to 100%; the summary estimate of negative predictive value was 93.9% (93.1 to 94.6).
Discussion
Summary of main results
In this systematic review, we included 116 cohorts (98 unique studies; 101,121 participants) that evaluated rapid antigen detection tests (RADTs) for the detection of group A streptococcus (GAS) in children with pharyngitis. The overall methodological quality of included studies was poor. Across 105 study cohorts (58,244 participants) in which all participants underwent both RADT and throat culture, the summary estimates of sensitivity and specificity were 85.6% (83.3 to 87.6) and 95.4% (94.5 to 96.2), respectively. There were substantial variations in sensitivity across studies, but specificity was more stable; there was no statistical evidence of a trade‐off between sensitivity and specificity. Heterogeneity in accuracy was not explained by study‐level characteristics such as test type (enzyme immunoassay (EIA) versus optical immunoassay (OIA)), use of an enrichment broth before plating, mean age and clinical severity of participants, and GAS prevalence. Summary estimates of sensitivity and specificity were stable in low risk of bias studies (84.0% and 95.0%, respectively). Across 115 test evaluations in which all negative RADT results were verified by throat culture, the negative predictive value of RADT was 93.9% (93.1 to 94.6).
Summary of findings
The Table 1 summarises the findings of the review by applying the results to a hypothetical cohort of 1000 children with pharyngitis, considering three scenarios where GAS prevalence varies from 20% to 40%. The consequence of a false negative result is that the patient may not receive antibiotic treatment, and thus may experience symptoms for a longer period and be at higher risk of developing non‐suppurative and suppurative complications of GAS infection (Spinks 2013). The consequence of a false positive result is that the patient may receive unnecessary antibiotics, which could result in adverse reactions and unwilling exposure to antibiotic‐resistant bacteria.
Comparison with previous findings
Our findings are in line with those from three published systematic reviews about the accuracy of RADTs for the diagnosis of streptococcal pharyngitis (Table 6) (Lean 2014; Ruiz‐Aragon 2010; Stewart 2014). Summary estimates of sensitivity and specificity were comparable across reviews, at around 85% and 95%, respectively.
5. Comparison between previous systematic reviews on the diagnostic accuracy of RADTs for streptococcal pharyngitis and the present one.
| Ruiz‐Aragon 2010a | Lean 2014 | Stewart 2014 | Present review | |
| Study participants | Adults and children | Adults and children | Adults and children | Children |
| Timeframe for searches | 2000 to 2009 | 1996 to 2013 | 2000 to 2012 | 1980 to 2015 |
| Number of studies included | 24 | 60b | 58c | 105b |
| Number of participants included | 14,936 | 29,934 | 55,766 | 58,244 |
| Summary estimate of sensitivity (95% CI) | 85% (84 to 87) | 86% (83 to 88) | 84% (83 to 85)d | 86% (83 to 88) |
| Summary estimate of specificity (95% CI) | 96% (96 to 97) | 96% (94 to 97) | 95% (94 to 95)d | 95% (95 to 96) |
| Investigations of heterogeneity | None performed | No evidence of significant variation in accuracy by test type (EIA versus OIA), and by age (children versus adults) | Did not identify sources of variabilityd | Did not identify sources of variability |
aIn Spanish; bpairs of sensitivity and specificity; c59 study cohorts; damongst high‐quality studies.
CI: confidence interval
Strengths and weaknesses of the review
We believe this dataset constitutes a fair representation of diagnostic accuracy studies evaluating RADTs in children with pharyngitis. However, it is known that studies of diagnostic test accuracy tend to be poorly indexed in electronic databases and we may therefore have missed some eligible studies. Moreover, we used an extensive literature search but we did not look systematically in conference abstracts, whereas it has been estimated that at least one‐fourth of abstracts of diagnostic accuracy studies presented at conferences are not published (Brazzelli 2009). Thirty‐eight studies did not differentiate between adults and children and so whilst they were identified, eligible subsets of data could not be included in the review.
The overall methodological quality of studies included in the review was poor, with less than one‐fifth (17%) of studies being judged at low risk of bias for at least three of four QUADAS‐2 domains, and half (50%) of estimates of diagnostic accuracy obtained from unselected groups of children presenting with signs and symptoms of pharyngitis. Poor quality mainly arose from high risk of selection bias and high risk of bias in the reference standard used (in 73% and 43% of test evaluations, respectively). Poor study reporting frequently impeded quality appraisal. Whether or not participants formed a consecutive or random series was reported in only 29% of cases, inclusion criteria in 46%, and whether readers of the reference standard were blinded to the result of the rapid test in 28%. We used QUADAS‐2 to assess the quality of included studies but did not use GRADE to rate the overall quality of the body of evidence; we will undertake GRADE assessment in future updates of this review.
We included sufficient numbers of studies and participants to obtain precise summary estimates. However, we were not able to identify sources of heterogeneity in accuracy through meta‐regression. It is known that sensitivity of RADTs is likely to vary across patient subgroups within a study; several studies, for example, found evidence of increasing sensitivity with increasing Centor or McIsaac scores (Cohen 2012; Edmonson 2005; Hall 2004; Tanz 2009). Due to aggregation bias, relationships across studies may not reflect relationships within studies; the relationship between accuracy and patient characteristics such as age and disease severity may be adequately estimated only using individual patient data; we strongly recommend such a future work. We dichotomised variables such as age and clinical severity when investigating heterogeneity, mostly because we lack routines for bivariate meta‐regression with continuous variables in Stata, but this may be at the cost of loss of information and statistical power. Study setting could also be a relevant source of heterogeneity to explore in future trials.
Other well described sources of variability in RADT sensitivity could not be explored in this review. For example, several studies reported increasing sensitivity with increasing amount of GAS found on culture (Cohen 2012; Kuhn 1999; Kurtz 2000), but we could not evaluate and compare such effects across studies because of the absence of any standard method to measure bacterial inoculum size. Also the level of expertise of the person performing the throat sample seems to affect the sensitivity of RADTs; several studies have shown improvement in sensitivity following dedicated training sessions (Fox 2006 ; Toepfner 2013).
The analysis was carried out at the test evaluation level, therefore some studies were included more than once in the meta‐analysis. This means that the summary estimates are partially based on duplicate use of individuals. This is likely to have introduced bias. However, we anticipate that the implications are rather marginal because such studies represent only a minority when compared to the total number of included studies (11 out of 98).
Applicability of findings to the review question
Included studies came from a variety of countries (n = 25) and ambulatory care settings (private offices, walk‐in clinics, emergency departments). However, only half of studies avoided clinical selection of participants; investigators often used clinical criteria, such as McIsaac’s, as inclusion criteria. Thus, the included studies may provide a distorted reflection of the diagnostic performance of RADT in unselected children with pharyngitis seen in ambulatory care. From the 41 studies judged at low risk of applicability concerns for patient selection, the summary estimate of sensitivity was slightly lower than the overall estimate (83.1% versus 85.6%, respectively).
We evaluated 42 different commercial kits in this review. All of them are binary tests giving either a positive or negative result, but the different commercial kits may not share a common positivity threshold (Charlier‐Bret 2004; Lasseter 2009). The absence of evidence for a significant correlation between sensitivity and specificity suggests that threshold effects may be negligible when evaluating the accuracy of RADTs. Recently, molecular rapid tests relying on DNA probes, polymerase chain reaction (PCR) and fluorescence in situ methods have been commercialised (Chapin 2002; Ding 2011; Slinger 2011). Their accuracy seems promising but they have rarely been evaluated in children and require specialised equipment and personnel.
Amongst 105 test evaluations included in the meta‐analysis of sensitivity and specificity estimates, we judged about one‐third (31%) to be of low concern regarding applicability in the index test domain because the RADT was processed and interpreted during consultation time. In this subgroup of studies, the summary estimate of sensitivity was higher than that from the overall analysis (89.1% versus 85.6%, respectively).
An appropriate reference standard (laboratory throat culture on a blood agar plate during 48 hours) was used in about two‐thirds (62%) of test evaluations. An enrichment broth was used to improve recovery of GAS on culture in 10% of test evaluations; this did not have any effect on RADT sensitivity on meta‐regression.
Authors' conclusions
Implications for practice.
The high specificity of rapid antigen detection tests (RADTs) implies that positive results may not require throat culture confirmation and could be used as a basis to prescribe antibiotics in children with pharyngitis. On average, RADT sensitivity and negative predictive value were 85.6% and 93.9%, respectively. Whether such performances are sufficient to use RADTs without backup culture of RADT negative results depends mainly on the epidemiological context. This includes the prevalence of group A streptococcus (GAS) pharyngitis, the rate of asymptomatic GAS carriage and the incidence of GAS complications such as acute rheumatic fever and quinsy. Clinicians and guideline developers should also take into account other elements that were beyond the scope of this review, such as effectiveness of antibiotics to prevent complications of GAS infection, accessibility of diagnostic tests, cost‐effectiveness and patient preferences. Our findings challenge the common view that optical immunoassay (OIA) tests may perform better than enzyme immunoassay (EIA) tests (AAP 2012; Gerber 2004).
Implications for research.
Further research should aim to define the minimal sensitivity that RADTs should achieve before such diagnostic tests would be accepted as stand‐alone tests in replacement of throat culture. This could be done by inviting a panel of experts or through simulation of patient outcomes. We also need to obtain consensus on which is the most appropriate reference standard to diagnose GAS pharyngitis in children. It remains controversial whether or not throat cultures yielding low GAS colony counts (less than 10 per plate) reflect true GAS infection or GAS carriage. Similarly, weakly positive results on molecular tests such as polymerase chain reaction (PCR) assays may reflect GAS carriage rather than true GAS infection.
Future accuracy studies should include more direct comparisons between different kits and types of RADTs. The best study design might be to randomise participants rather than to compare the accuracy of different tests in the same participants. Indeed, if a unique swab is used to perform two rapid tests, it is likely that the bacterial inoculum available for the second test will be insufficient to give a positive result. Thus, the first rapid test will look more sensitive than the second. Future diagnostic accuracy studies of RADTs should be reported according to the STARD reporting guideline to enhance data extraction and critical appraisal (Bossuyt 2003; Bossuyt 2015).
Beyond accuracy, further research is required to assess the impact of implementing RADTs on antibiotic prescribing and patient outcomes (Llor 2011). We need more test‐and‐treat randomised trials to evaluate whether rapid testing and/or antibiotics are beneficial to patients. Accuracy is only a proxy for more important outcomes such as pharyngitis‐related morbidity (e.g., quinsy, acute rheumatic fever, rheumatic heart disease) and mortality.
Acknowledgements
We thank Philippe Ravaud and Ludovic Trinquart (French Cochrane Centre, Université Paris Descartes, Paris, France) and the members of the Cochrane ARI Group and the Cochrane DTA Group for their comments and support. We thank the following people for commenting on the draft protocol: Noorin Bhimani, Samileh Noorbakhsh, Saleh Altamimi, Conor Teljeur and Jenny Doust. We thank Patrick Bossuyt (Department of Clinical Epidemiology, Biostatistics and Bioinformatics, University of Amsterdam, the Netherlands) for helpful discussions about methodological aspects of the review. We thank Drs. JM Arribas‐Blanco, M Drulak, C Llor, A Hammami, JR Mlejnek, JY Pauchard, LD Schwabe and N Toepfner for clarifying published data or sharing additional paediatric data. We also thank the following people for commenting on the draft review: Julie Gildie, Jennifer Larosa, Samileh Noorbakhsh, Helena Liira, Conor Teljeur and Rafael Perera. Finally, we thank Dr. Alexander Leis and Prof. Ryuki Kassai for translating articles in German and Japanese, respectively.
Appendices
Appendix 1. MEDLINE (Ovid) search strategy
1 Pharyngitis/ (6583) 2 pharyngitis.tw. (3961) 3 Tonsillitis/ (6246) 4 tonsillitis.tw. (3954) 5 (tonsillopharyngitis or pharyngotonsillitis).tw. (515) 6 sore throat*.tw. (3152) 7 ((throat* or pharyn* or tonsil*) adj5 (infect* or inflam*)).tw. (3411) 8 Pharynx/mi [Microbiology] (3411) 9 Streptococcal Infections/ (27643) 10 (strep* adj5 (throat* or pharyn* or tonsil*)).tw. (2750) 11 ("group a" adj5 streptococc*).tw. (7943) 12 gabhs.tw. (333) 13 (beta‐hemoly* or beta‐haemoly*).tw. (4341) 14 lancefield group a.tw. (110) 15 Streptococcus pyogenes/ (11396) 16 (streptococcus pyogenes or "s. pyogenes" or "s.pyogenes").tw. (6096) 17 or/1‐16 (54426) 18 Immunoassay/ (22034) 19 exp Immunoenzyme Techniques/ (183964) 20 (enzyme adj2 (immunoassay* or immuno‐assay* or immunosorbent)).tw. (80381) 21 Immunochromatography/ (203) 22 immunochromatograph*.tw. (1623) 23 Immunosorbent Techniques/ (6331) 24 exp Enzyme‐Linked Immunosorbent Assay/ (123418) 25 (elisa or elisas or eia or eias).tw. (112952) 26 (sandwich* adj2 assay*).tw. (1053) 27 (lateral flow adj2 assay).tw. (126) 28 (optical adj2 (immunoassay* or immuno‐assay*)).tw. (93) 29 (oia or oias).tw. (127) 30 Antigens, Bacterial/ (39874) 31 Reagent Kits, Diagnostic/ (14838) 32 Point‐of‐Care Systems/ (6609) 33 ((rapid or "point of care" or "near patient" or poc or poct or bedside) adj5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*)).tw. (57892) 34 (radt or radts or rdt or rdts).tw. (803) 35 (antigen* adj3 detect*).tw. (22067) 36 test pack strep a.tw. (5) 37 icon strep a.tw. (4) 38 link 2 strep a rapid test.tw. (1) 39 acceava strep a.tw. (2) 40 osom strep a.tw. (3) 41 poly stat strep a.tw. (0) 42 quickvue strep a.tw. (5) 43 or/18‐42 (395969) 44 17 and 43 (4273) 45 limit 44 to yr="1980 ‐Current" (3809) 46 exp animals/ not humans/ (3903550) 47 45 not 46 (3194)
Appendix 2. Embase (Elsevier) search strategy
#39 #35 NOT #382310 #38 #37 NOT #365030292 #37 [animals]/lim5557209 #36 'human'/exp AND [embase]/lim9108195 #35 #34 AND [embase]/lim AND [1‐1‐1980]/sd NOT [23‐5‐2013]/sd2579 #34 #17 AND #332757 #33 #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32321374 #32 'test pack strep a':ab,ti OR 'icon strep a':ab,ti OR 'link 2 strep a rapid test':ab,ti OR 'acceava strep a':ab,ti OR 'poly stat strep a':ab,ti OR 'quickvue strep a':ab,ti OR 'osom strep a':ab,ti AND [embase]/lim11 #31 (antigen* NEAR/3 detect*):ab,ti AND [embase]/lim19264 #30 radt:ab,ti OR radts:ab,ti OR rdt:ab,ti OR rdts:ab,ti AND [embase]/lim972 #29 ((rapid OR 'point of care' OR 'near patient' OR poc OR poct OR bedside) NEAR/5 (test OR tests OR testing OR detect* OR diagnos* OR screen* OR kit OR kits OR assay*)):ab,ti AND [embase]/lim56547 #28 'point of care testing'/de AND [embase]/lim3626 #27 'streptococcus group a rapid test'/de OR 'rapid test'/de OR 'elisa kit'/de AND [embase]/lim446 #26 'bacterial antigen'/de OR 'streptococcus antigen'/de AND [embase]/lim12962 #25 oia:ab,ti OR oias:ab,ti AND [embase]/lim112 #24 (optical NEAR/2 (immunoassay OR 'immuno‐assay')):ab,ti AND [embase]/lim76 #23 ((sandwich* OR 'lateral flow') NEAR/2 assay*):ab,ti AND [embase]/lim1180 #22 elisa:ab,ti OR elisas:ab,ti OR eia:ab,ti OR eias:ab,ti AND [embase]/lim124297 #21 immunochromatograph*:ab,ti AND [embase]/lim1576 #20 'immunoaffinity chromatography'/de AND [embase]/lim2788 #19 (enzyme NEAR/2 (immunoassay OR 'immuno‐assay' OR immunosorben*)):ab,ti AND [embase]/lim70295 #18 'immunoassay'/de OR 'enzyme linked immunosorbent assay'/de OR 'enzyme immunoassay'/de AND [embase]/lim212276 #17 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #1655997 #16 'streptococcus pyogenes':ab,ti OR 's. pyogenes':ab,ti OR 's.pyogenes':ab,ti AND [embase]/lim5883 #15 'streptococcus pyogenes'/de AND [embase]/lim8434 #14 'streptococcal pharyngitis'/de AND [embase]/lim442 #13 'lancefield group a':ab,ti AND [embase]/lim100 #12 (beta NEXT/1 hemoly*):ab,ti OR (beta NEXT/1 haemoly*):ab,ti AND [embase]/lim1830 #11 gabhs:ab,ti AND [embase]/lim372 #10 ('group a' NEAR/5 streptococc*):ab,ti AND [embase]/lim6931 #9 (strep* NEAR/5 (throat* OR pharyn* OR tonsil*)):ab,ti AND [embase]/lim2573 #8 'streptococcus infection'/de OR 'group a streptococcal infection'/exp AND [embase]/lim16432 #7 ((throat* OR pharyn* OR tonsil*) NEAR/5 (infect* OR inflam*)):ab,ti AND [embase]/lim3363 #6 'sore throat'/de AND [embase]/lim8235 #5 tonsillopharyngitis:ab,ti OR pharyngotonsillitis:ab,ti AND [embase]/lim633 #4 tonsillit*:ab,ti AND [embase]/lim3362 #3 'tonsillitis'/exp AND [embase]/lim7295 #2 pharyngit*:ab,ti AND [embase]/lim4095 #1 'pharyngitis'/exp AND [embase]/lim16213
Appendix 3. Web of Science (Thomson ISI) search strategy
| # 3 | 1,235 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All years |
|
| # 2 | 253,763 | Topic=(immunoassay or (enzyme NEAR/2 ("immuno‐assay" or immunosorben*)) or immunochromatograph* or elisa or elisas or eia or eias or (sandwich* NEAR/2 assay) or ("lateral flow" NEAR/2 assay) or (optical NEAR/2 (immunoassay or immuno‐assay)) or oia or oias) OR Topic=(((rapid or "point of care" or "near patient" or poc or poct or bedside) NEAR/5 (test or tests or testing or detect* or diagnos* or screen* or kit or kits or assay*))) OR Publication Name=(radt or radts or rdt or rdts) OR Topic=((antigen* NEAR/3 detect*) or "test pack strep a" or "icon strep a" or "link 2 strep a rapid test" or "acceava strep a" or "osom strep a" or "poly stat strep a" or "quickvue strep a") Databases=SCI‐EXPANDED, CPCI‐S Timespan=All years |
|
| # 1 | 26,550 | Topic=(pharyngitis or tonsillitis or tonsillopharyngitis or pharyngotonsillitis or "sore throat" or "sore throats" or ((throat* or pharyn* or tonsil*) NEAR/5 (infect* or inflam*)) or (strep* NEAR/5 (throat* or pharyn* or tonsil*)) or ("group a" NEAR/5 streptococc*) or gabhs or (streptococc* NEAR/3 infect*) or "beta‐hemolytic" or "beta‐haemolytic" or "lancefield group a" or "streptococcus pyogenes" or "s. pyogenes") Databases=SCI‐EXPANDED, CPCI‐S Timespan=All years |
Appendix 4. Trip database search strategy
(gabhs or group a streptococ* or strep throat) and (rapid test or immunoassay or radt or rapid antigen)
Appendix 5. Medion search strategy
Each term searched individually in the abstract field.
(pharyngitis, sore throat, gabhs, beta‐haemolytic, beta‐hemolytic, lancefield, streptococcal, streptococcus)
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
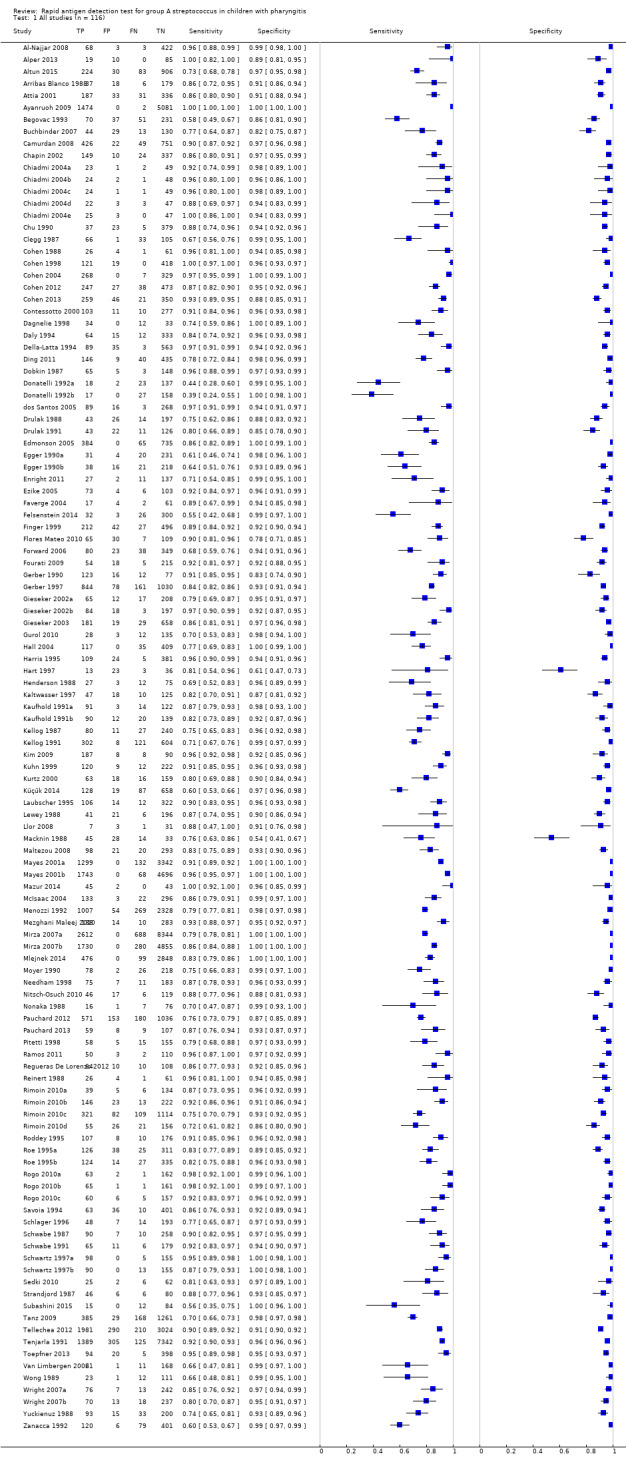
All studies (n = 116).
2. Test.
Complete verification (n = 105).
3. Test.
EIA (direct comparison).
4. Test.
OIA (direct comparison).
5. Test.
Acceava Strep A (Biostar).
6. Test.

ACON Strep A Rapid Test Strip.
7. Test.

BioNexia Strep A (BioMerieux).
8. Test.

CARDS QS Strep A (Quidel).
9. Test.

Clearview Exact Strep A.
10. Test.

Clearview Strep A.
11. Test.

Diaquick Strep A Test (Dialab).
12. Test.
Directgen 1‐2‐3 Group A Strep (Becton Dickinson).
13. Test.

Direct Strep A EIA.
14. Test.
EIA (no name).
15. Test.
Group A Strep Test (Quidel).
16. Test.
IM Strep A (International Microbio).
17. Test.

Meridian Bioscience.
18. Test.
OSOM Strep A (Genzyme).
19. Test.
OSOM Ultra Strep A (Genzyme).
20. Test.
QuickVue Dipstick Strep A (Quidel).
21. Test.
QuickVue Flex Strep A (Quidel).
22. Test.
QuickVue In‐Line Strep A (Quidel).
23. Test.
QuickVue+ Strep A (Quidel).
24. Test.

Sacks Biological Farms.
25. Test.
SD Bioline Strep A.
26. Test.

Signify Strep A (Abbott).
27. Test.

SMART Group A Strep (New Horizons).
28. Test.

Strep A Abon kit.
29. Test.
Strep A OIA (Biostar).
30. Test.
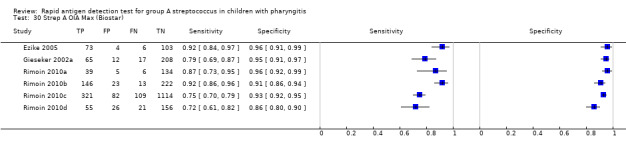
Strep A OIA Max (Biostar).
31. Test.

Strep A Rapid Test Device.
32. Test.

Strep A Sign.
33. Test.

Strep A test II (INTEX Diagnostica).
34. Test.
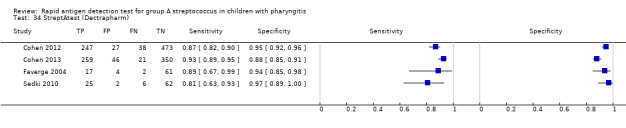
StreptAtest (Dectrapharm).
35. Test.

Streptavit.
36. Test.

Streptop A (ALL‐Diag).
37. Test.

SUDS Group A Strep.
38. Test.

SureScreen Test Strep A.
39. Test.
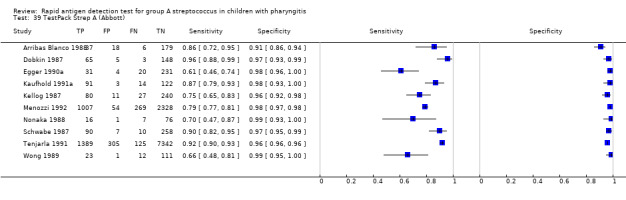
TestPack Strep A (Abbott).
40. Test.
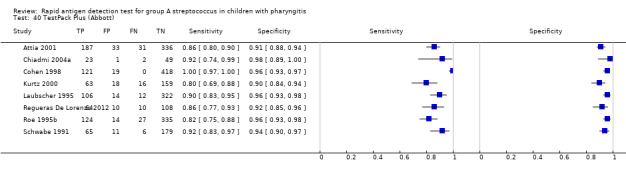
TestPack Plus (Abbott).
41. Test.

TestPack Plus Strep A with OBC II (Abbott).
42. Test.
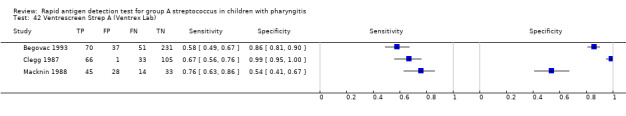
Ventrescreen Strep A (Ventrex Lab).
43. Test.
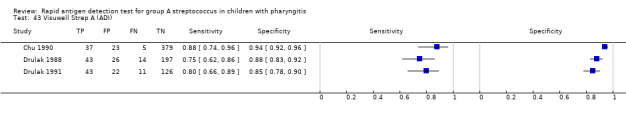
Visuwell Strep A (ADI).
44. Test.
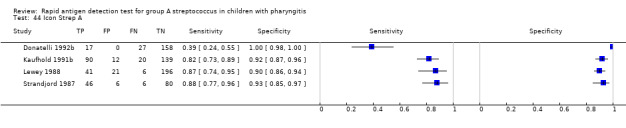
Icon Strep A.
45. Test.

Qtest (Becton Dickinson).
46. Test.

Link 2 Strep A Rapid Test (Becton Dickinson).
47. Test.

Event Test Strip Strep A.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Najjar 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (exclusion if antibiotics during the preceding week) Clinical selection of patients: explicit criteria but not a score Presenting signs and symptoms: fever, acute catarrh and acutely inflamed throat/tonsils with or without exudates Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 505 (but the contingency table includes 496 participants)
Age (distribution): 81% under 5 years of age (mean or median not reported) GAS prevalence according to culture (with 95% confidence interval): 14.1% (95% CI not reported) Country of study: United Arab Emirates Sex (% of girls): 45% Clinical severity assessment: none Clinical setting: walk‐in clinics Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab Commercial name of the RADT: Diaquick Strep A Test (Dialab) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | ‐ | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Unclear | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Unclear | |||
Alper 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (within 3 days before inclusion) Clinical selection of patients: none Presenting signs and symptoms: patients with a chief complaint of sore throat Age range for inclusion: 7 to 15 years |
||
| Patient characteristics and setting | Sample size: 114
Age (distribution): mean (SD) = 10.0 (0.24) years GAS prevalence according to culture (with 95% confidence interval): 16.7% (95% CI not reported) Country of study: Turkey Sex (% of girls): not reported Clinical severity assessment: Centor score Clinical setting: walk‐in clinic (family practice centre) Single‐centre study |
||
| Index tests | Throat swab: unclear Commercial name of the RADT: only the name of the manufacturer was reported (Meridian Bioscience) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: 48 hours GAS confirmation: bacitracin disk Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by academic funding (Uludag University Scientific Research Projects) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Altun 2015.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: non‐consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score Presenting signs and symptoms: clinical exudative tonsillopharyngitis Age range for inclusion: 0 to 18 years |
||
| Patient characteristics and setting | Sample size: 1243
Age (distribution): mean (SD) = 5.5 (3.1) years GAS prevalence according to culture (with 95% confidence interval): 24.7% (95% CI not reported) Country of study: Turkey Sex (% of girls): 48.5% Clinical severity assessment: none Clinical setting: paediatric outpatient clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: Strep A Abon kit Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: < 24 hours GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Arribas Blanco 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician (most of the time) or nurse (sometimes) Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: acute pharyngo‐tonsillitis Age range for inclusion: < 21 years |
||
| Patient characteristics and setting | Sample size: 240
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 18.9% (95% CI not reported) Country of study: Spain Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: TestPack Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in Spanish) | ||
| Notes | We thank Dr JM Arribas Blanco for sharing unpublished paediatric data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Attia 2001.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: yes (within 5 days of enrollment) Clinical selection of patients: none Presenting signs and symptoms: patients with signs and symptoms of acute pharyngitis Age range for inclusion: 1 to 18 years |
||
| Patient characteristics and setting | Sample size: 587
Age (distribution): mean (SD) = 6.7 (3.9) years GAS prevalence according to culture (with 95% confidence interval): 37.1% (95% CI not reported) Country of study: USA Sex (% of girls): 51% Clinical severity assessment: other (Attia score) Clinical setting: mixed (paediatric emergency department and 2 paediatric outpatient clinics) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for the RADT) Commercial name of the RADT: TestPack Plus (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Funded by a grant from the Nemours Research Programmes | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Ayanruoh 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: yes (within 14 days of presentation) Clinical selection of patients: none Presenting signs and symptoms: patients with clinical signs of pharyngitis Age range for inclusion: 3 to 18 years |
||
| Patient characteristics and setting | Sample size: 6557
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 22.5% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: not reported Commercial name of the RADT: only the name of the manufacturer was reported (Sacks Biological Farms) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Throat culture performed only for children with negative RADT results (partial verification) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| High | |||
Begovac 1993.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: symptoms and signs of pharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 389 (age < 15 years = 389, age > 15 years = 115)
Age (distribution): mean or median not reported GAS prevalence according to culture (with 95% confidence interval): 31.1% (95% CI not reported) Country of study: Croatia Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic (outpatient clinic of a University Hospital) Single‐centre study |
||
| Index tests | Throat swab: 1 double swab Commercial name of the RADT: Venterscreen Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard and inhibitory (2 plates)
Atmosphere of incubation: aerobic with CO2 enrichment
Duration of incubation: 48 hours
GAS confirmation: latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: ‐ |
||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Buchbinder 2007.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: random
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: yes (time frame not reported) Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: sore throat associated with pharyngeal erythema or exudate and fever Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 216
Age (distribution): mean (SD) = 4.8 (3.6) years GAS prevalence according to culture (with 95% confidence interval): 26.4% (95% CI not reported) Country of study: France Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for the RADT) Commercial name of the RADT: IM Strep A (International Microbio) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard (no details) Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated (n): not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Camurdan 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: signs and symptoms of acute upper respiratory tract infections Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 1248
Age (distribution): mean (SD) = 6.3 (3.6) years GAS prevalence according to culture (with 95% confidence interval): 38.1% (95% CI not reported) Country of study: Turkey Sex (% of girls): 48% Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab (1 single, 1 double, 2 different): unclear Commercial name of the RADT: Strep A Test II (Intex Diagnostica) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: latex test Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chapin 2002.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no (comparison of a RADT with a DNA probe test)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: symptoms of pharyngitis Age range for inclusion: not reported ("paediatric outpatient clinics") |
||
| Patient characteristics and setting | Sample size: 520
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 33.1% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic ("paediatric outpatient clinics") Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab was used for the RADT, 1 swab for the DNA probe technique, and the pledget was used for culture) Commercial name of the RADT: Strep A OIA (Thermo Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: enrichment
Atmosphere of incubation: anaerobic
Duration of incubation: 48 hours
GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ |
||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The RADT was compared to a DNA probe technique; such molecular tests are not in the scope of this review. Travel grant support provided by Gen‐Probe, manufacturer of the DNA probe assay under evaluation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chiadmi 2004a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (within 7 days before inclusion) Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: signs and symptoms of pharyngitis or pharyngo‐tonsillitis (fever, sore throat, inflammation of pharynx) Age range for inclusion: 8 to 14 years |
||
| Patient characteristics and setting | Sample size: 75
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 33.3% (95% CI not reported) Country of study: France Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: other ("paediatric consultation") Single‐centre study |
||
| Index tests | Throat swab (1 single, 1 double, 2 different): unclear Commercial name of the RADT: Test Pack Plus (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | A total of 7 RADTs were performed in the same sample of children (5 EIAs and 2 LAs). We only extracted data regarding the evaluation of EIA tests | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chiadmi 2004b.
| Study characteristics | |||
| Patient sampling | See Chiadmi 2004a | ||
| Patient characteristics and setting | See Chiadmi 2004a | ||
| Index tests | Throat swab (1 single, 1 double, 2 different): unclear Commercial name of the RADT: IM Strep A (International Microbio) Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Chiadmi 2004a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | A total of 7 RADTs were performed in the same sample of children (5 EIAs and 2 LAs). We only extracted data regarding the evaluation of EIA tests | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chiadmi 2004c.
| Study characteristics | |||
| Patient sampling | See Chiadmi 2004a | ||
| Patient characteristics and setting | See Chiadmi‐a | ||
| Index tests | Throat swab (1 single, 1 double, 2 different): unclear Commercial name of the RADT: Clearview Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Chiadmi 2004a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | A total of 7 RADTs were performed in the same sample of children (5 EIAs and 2 LAs). We only extracted data regarding the evaluation of EIA tests | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chiadmi 2004d.
| Study characteristics | |||
| Patient sampling | See Chiadmi 2004a | ||
| Patient characteristics and setting | See Chiadmi 2004a | ||
| Index tests | Throat swab (1 single, 1 double, 2 different): unclear Commercial name of the RADT: Strep A Sign Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Chiadmi 2004a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | A total of 7 RADTs were performed in the same sample of children (5 EIAs and 2 LAs). We only extracted data regarding the evaluation of EIA tests | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chiadmi 2004e.
| Study characteristics | |||
| Patient sampling | See Chiadmi 2004a | ||
| Patient characteristics and setting | See Chiadmi 2004a | ||
| Index tests | Throat swab (1 single, 1 double, 2 different): unclear Commercial name of the RADT: Streptavit Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Chiadmi 2004a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | A total of 7 RADTs were performed in the same sample of children (5 EIAs and 2 LAs). We only extracted data regarding the evaluation of EIA tests | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Chu 1990.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study Prospective design Sample: consecutive Direct comparison of different RADTs: no Direct comparison of several throat culture techniques: no Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: one or more of the following: sore throat, tonsil exudate, pharyngeal erythema, enlarged anterior cervical lymph node, fever or skin rash suggestive of scarlet fever Age range for inclusion: 3 to 18 years |
||
| Patient characteristics and setting | Sample size: 444
Age (distribution): mean = 9.8 years GAS prevalence according to culture (with 95% confidence interval): 9.5% (95% CI not reported) Country of study: Taiwan Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (hospital outpatient clinics, emergency department and a private office clinic) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: Visuwell Strep A (ADI) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard
Atmosphere of incubation: anaerobic
Duration of incubation: 24 hours
GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ |
||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Clegg 1987.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study Prospective design Sample: unclear Direct comparison of different RADTs: no Direct comparison of several throat culture techniques: no Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: acute pharyngitis Age range for inclusion: not reported ("paediatric patients") |
||
| Patient characteristics and setting | Sample size: 205
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 48.3% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Ventrescreen (Ventrex Laboratories) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: not reported GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Cohen 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: pharyngitis with fever Age range for inclusion: 2 to 14 years |
||
| Patient characteristics and setting | Sample size: 92
Age (distribution): mean age = 6.3 years GAS prevalence according to culture (with 95% confidence interval): 29.3% (95% CI not reported) Country of study: France Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (office‐based and hospital) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: Group A Strep Test (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: enrichment and inhibitory Atmosphere of incubation: aerobic Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | No | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| High | |||
Cohen 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes (within 7 days before inclusion) Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: acute pharyngitis or tonsillitis with dysphagia or fever Age range for inclusion: 4 to 15 years |
||
| Patient characteristics and setting | Sample size: 563
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 21.5% (95% CI not reported) Country of study: France Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 2 different (first one for the RADT, second one only if RADT+) Commercial name of the RADT: TestPack Plus Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | The study was supported by ASTRA laboratories | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Cohen 2004.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: signs and symptoms of pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 604
Age (distribution): median age = 5.5 years GAS prevalence according to culture (with 95% confidence interval): 45.5% (95% CI not reported) Country of study: France Sex (% of girls): not reported Clinical severity assessment: McIsaac score and Wald score Clinical setting: mixed (office‐based and emergency department) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: not reported ("EIA no name") Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Throat culture performed only for children with negative RADT results (partial verification) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | No | ||
| Were RADTs conducted during consultation time? | Yes | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| High | |||
Cohen 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes (within 7 days before inclusion) Clinical selection of patients: none Presenting signs and symptoms: pharyngitis (inflammation of the pharynx and/or tonsils) Age range for inclusion: 3 to 15 years |
||
| Patient characteristics and setting | Sample size: 785
Age (distribution): mean (SD) = 6.1 (2.5) years GAS prevalence according to culture (with 95% confidence interval): 36.3% (95% CI 32.9 to 39.8) Country of study: France Sex (% of girls): 44.7% Clinical severity assessment: McIsaac score Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: StreptAtest (Dectrapharm) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: latex agglutination (Prolex) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Funded by Dectrapharm (manufacturer of the RADT) and educational grants | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Cohen 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes (within 7 days before inclusion) Clinical selection of patients: none Presenting signs and symptoms: children with a diagnosis of pharyngitis Age range for inclusion: 3 to 14 years |
||
| Patient characteristics and setting | Sample size: 676
Age (distribution): mean (SD) = 6.1 (2.5) years GAS prevalence according to culture (with 95% confidence interval): 41.4% (95% CI 37.7 to 45.2) Country of study: France Sex (% of girls): 46.3% Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: StreptAtest (Dectrapharm) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory and enrichment Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 2 (the second plate was inoculated if the first one was negative after 48 hours of incubation) Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Funded by Dectrapharm (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Contessotto 2000.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (within 3 days before inclusion) Clinical selection of patients: none Presenting signs and symptoms: acute pharyngitis and/or tonsillitis Age range for inclusion: 6 months to 14 years |
||
| Patient characteristics and setting | Sample size: 401
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 28.2% (95% CI +/‐ 4.4%) Country of study: Spain Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (office‐based and emergency department) Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: QuickVue Flex Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation (aerobic, aerobic with CO2 enrichment, anaerobic): not reported Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (Spanish) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Dagnelie 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: sore throat for less than 15 days Age range for inclusion: 4 to 14 years |
||
| Patient characteristics and setting | Sample size: 79 (total of 558 patients but only 79 children)
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 58.2% (95% CI not reported) Country of study: the Netherlands Sex (% of girls): not reported Clinical severity assessment: Centor score Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: Directgen 1‐2‐3 (Becton Dickinson) Type of RADT: EIA (liposomal test) |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic and anaerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: unclear Assessment of GAS antibody response: yes Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study included children and adults; we extracted data only for children | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Daly 1994.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 424
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 17.9% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: child medical centre Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: enrichment in a Todd‐Hewitt broth followed by culture on a selective medium Atmosphere of incubation: 35°C, aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test (and PYR test) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by a grant from Biostar (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Unclear | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Della‐Latta 1994.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: acute pharyngitis Age range for inclusion: 2 to 19 years |
||
| Patient characteristics and setting | Sample size: 690
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 13.3% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (BioStar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex agglutination Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: pledgets were also incubated in a Todd‐Hewitt broth to improve GAS recovery (data not extracted) | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Ding 2011.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: signs and symptoms of acute upper respiratory infection Age range for inclusion: 6 months to 14 years |
||
| Patient characteristics and setting | Sample size: 630
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 29.5% (95% CI not reported) Country of study: China Sex (% of girls): 39.5% Clinical severity assessment: none Clinical setting: walk‐in clinic Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab was used for the RADT, 1 swab for a FISH technique, and the pledget for culture) Commercial name of the RADT: Clearview Exact Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The RADT was compared to a FISH technique; such techniques were out of the scope of this review | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Dobkin 1987.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients with acute pharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 221
Age (distribution): not reported ("Almost all swabs were obtained from children younger than 16 years of age") GAS prevalence according to culture (with 95% confidence interval): 30.8% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: unclear Single‐centre study |
||
| Index tests | Throat swab: not reported Commercial name of the RADT: Test Pack Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by a grant from Abbott (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Donatelli 1992a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: laboratory personnel (data from nurses not extracted) Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients presenting with and symptoms of acute pharyngitis Age range for inclusion: not reported (performed in a children's hospital) |
||
| Patient characteristics and setting | Sample size: 180
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 22.8% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (general paediatric clinic and emergency department) Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: Directgen 1‐2‐3 Type of RADT: EIA (liposome assay) |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: other (PYR test during first third of the study, then latex test) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study compared results obtained by nurses and by laboratory technologists; we extracted data only for laboratory technologists. This study was funded in part by Health and Welfare Canada | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Donatelli 1992b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: laboratory personnel (data from nurses not extracted) Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients presenting with and symptoms of acute pharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 203
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 21.7% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (general paediatric clinic and emergency department) Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: ICON Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: other (PYR test during first third of the study, then latex test) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study compared results obtained by nurses and by laboratory technologists; we extracted data only for laboratory technologists. This study was funded in part by Health and Welfare Canada | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
dos Santos 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: the "researcher" Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: children with a painful throat and evidence of inflammation of throat or tonsils and no sign of viral respiratory infection (rhinorrhoea, coryza, conjunctivitis, coughing and/or sneezing) Age range for inclusion: 2 to 13 years |
||
| Patient characteristics and setting | Sample size: 376
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 24.5% (95% CI not reported) Country of study: Brazil Sex (% of girls): 54% Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: QuickVue Plus Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk (and PYR test) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The first author received public funding (Coordination for the Improvement of Higher Education Personnel, Brazilian Ministry of Higher Education) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Drulak 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: other ("staff") Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngitis Age range for inclusion: < 18 years |
||
| Patient characteristics and setting | Sample size: 280
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 20.4% (95% CI not reported) Country of study: Canada Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: outpatient clinic ("outpatient department of a large paediatric institution") Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: Visuwell Strep A (ADI) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic during 24 hours then aerobic with CO2 enrichment during 24 hours Duration of incubation: 48 hours GAS confirmation: other (capillary tube precipitation) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study had 2 parts: 1 with adults and children (n = 585), the second with children only (n = 280). The data used for this systematic review were restricted to the second part because paediatric data were not extractable from the first part of the study Study conducted by the manufacturer of the RADT under investigation (Visuwell, ADI) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Drulak 1991.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngitis Age range for inclusion: < 18 years |
||
| Patient characteristics and setting | Sample size: 202
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 26.7% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: outpatient clinic Single‐centre study |
||
| Index tests | Throat swab: 1 swab (used for culture and then for the RADT) Commercial name of the RADT: Visuwell Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: bacitracin disk followed by latex test Number of plates inoculated: unclear Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | First and last author affiliated with the manufacturer. We thank Dr M Drulak for sharing unpublished paediatric data | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Edmonson 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no (exclusion only if current antimicrobial therapy) Clinical selection of patients: patients enrolled retrospectively if they had a diagnotic test to detect GAS Presenting signs and symptoms: n/a (see above) Age range for inclusion: < 24 years |
||
| Patient characteristics and setting | Sample size: 1184
Age (distribution): 63% between 5 and 15 years of age GAS prevalence according to culture (with 95% confidence interval): 38% (95% CI 35 to 41) Country of study: USA Sex (% of girls): 53% Clinical severity assessment: McIsaac score Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 1 single swab for culture and then for the RADT during first 11 months then 1 double swab (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: CARDS QS Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Throat culture performed only for children with negative RADT results (partial verification) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Egger 1990a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: clinical pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 579
Age (distribution): range 9 months to 14 years 1 month GAS prevalence according to culture (with 95% confidence interval): 19.0% (95% CI not reported) Country of study: Switzerland Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADTs: Test Pack Strep A Type of RADTs: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic during 24 hours and if negative reincubated during 24 hours in CO2 enriched atmosphere Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by grants from the manufacturers of the RADTs (Abbott and Hoffmann‐La Roche) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Egger 1990b.
| Study characteristics | |||
| Patient sampling | See Egger 1990a | ||
| Patient characteristics and setting | See Egger 1990a | ||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADTs: Direct Strep A Type of RADTs: EIA |
||
| Target condition and reference standard(s) | See Egger 1990a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by grants from the manufacturers of the RADTs (Abbott and Hoffmann‐La Roche) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Enright 2011.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: nurse Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: presentation consistent with symptomatic pharyngitis Age range for inclusion: 0 to 13 years |
||
| Patient characteristics and setting | Sample size: 177
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 21.5% (95% CI not reported) Country of study: Scotland Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: QuickVue In‐Line Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | No specific funding reported but the RADTs were made available by the manufacturer (Quidel) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Ezike 2005.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: convenience
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: acute pharyngitis without rhinorrhoea or conjunctivitis (considered suggestive of viral infection) Age range for inclusion: 5 to 18 years |
||
| Patient characteristics and setting | Sample size: 186 (group 2)
Age (distribution): mean (SD) = 9.9 (3.7) years GAS prevalence according to culture (with 95% confidence interval): 42.5% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: McIsaac score Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: Strep A OIA MAX Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: enrichment Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | This study was supported by the Sarnaik Endowment Resident and Fellow Research Fund, Children’s Hospital of Michigan, Detroit. Some rapid test kits were provided by Thermo Electron Corporation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Faverge 2004.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: not reported Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 84
Age (distribution): range 7 months to 14 years GAS prevalence according to culture (with 95% confidence interval): 22.6% (95% CI not reported) Country of study: France Sex (% of girls): 42% Clinical severity assessment: none Clinical setting: mixed (paediatric ward, outpatient clinic, emergency department from a general hospital) Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: StreptAtest Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Felsenstein 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: nurse Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngitis, fever of unknown origin, upper respiratory tract symptoms, or subjective complaints of throat pain or discomfort on swallowing Age range for inclusion: not reported ("paediatric patients") |
||
| Patient characteristics and setting | Sample size: 361
Age (distribution): mean (SD) = 7.4 (4.2) years GAS prevalence according to culture (with 95% confidence interval): 16.1% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: Centor and McIsaac scores (only in those with positive throat culture or RADT result Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: OSOM Ultra Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | No specific funding reported but the manufacturer of a rapid molecular test also evaluated in the study (illumigene, Meridian Biosciences) supplied assay kits, incubator and reader for the study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Finger 1999.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study Prospective design Sample: unclear Direct comparison of different RADTs: no Direct comparison of several throat culture techniques: no Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: complaint of sore throat with at least one sign of pharyngitis (redness of throat, purulent exudate in throat, or anterior cervical lymphadenopathy) Age range for inclusion: 3 to 16 years |
||
| Patient characteristics and setting | Sample size: 777
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 30.8% (95% CI not reported) Country of study: Vietnam Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (emergency department and outpatient clinics) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: QuickVue Flex Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: 48 hours GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: during the first half of the study, the laboratory investigators read cultures with knowledge of the result of the RADT | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | No specific funding reported but the manufacturer of the RADT (Quidel) provided the RADTs | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Flores Mateo 2010.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: yes (within 15 days before enrollment) Clinical selection of patients: none Presenting signs and symptoms: sore throat for less than 5 days Age range for inclusion: 1 to 14 years |
||
| Patient characteristics and setting | Sample size: 211
Age (distribution): mean (SD) = 6.6 (3.8) years GAS prevalence according to culture (with 95% confidence interval): 34.1% (95% CI not reported) Country of study: Spain Sex (% of girls): 55.8% Clinical severity assessment: McIsaac score Clinical setting: walk‐in clinic Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: OSOM Strep A (Gemzyme) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard
Atmosphere of incubation: aerobic with CO2 enrichment
Duration of incubation: 48 hours
GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ |
||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in Spanish) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Forward 2006.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study Prospective design Sample: unclear Direct comparison of different RADTs: no Direct comparison of several throat culture techniques: no Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: not reported ("pharyngeal swabs received from children") Age range for inclusion: < 16 years |
||
| Patient characteristics and setting | Sample size: 490
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 24.1% (95% CI not reported) Country of study: Canada Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: unclear (laboratory study) Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A Rapid test Device Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: PYR test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Fourati 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngotonsillitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 292
Age (distribution): mean (SD) = 6.7 (3.5) years GAS prevalence according to culture (with 95% confidence interval): 20.2% (95% CI not reported) Country of study: Tunisia Sex (% of girls): 39% Clinical severity assessment: none Clinical setting: mixed (emergency department and walk‐in clinics) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: Streptop A (ALL‐Diag) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: not reported Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Gerber 1990.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: "clinical findings suggestive of GA(BH)S pharyngitis" Age range for inclusion: not reported ("private pediatric practice") |
||
| Patient characteristics and setting | Sample size: 228
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 59.2% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for the RADT, 1 swab for culture) Commercial name of the RADT: QTest Strep (Becton Dickinson) Type of RADT: EIA (liposomal assay) |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Gerber 1997.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: acute pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 2113
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 47.6% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard culture and culture following incubation in a Todd‐Hewitt enrichment broth Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk +/‐ latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: composite reference standard (office standard culture + laboratory enriched culture). Office tests (culture and RADT) were reviewed in the laboratory. The same swab was used for multiple purposes (office culture, RADT and lab culture). | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by a grant from Biostar (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Gieseker 2002a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria ("children suspected of having S. pyogenes pharyngitis") Presenting signs and symptoms: not reported Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 302
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 28.8% (plate 1) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (child health clinic and emergency department) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADTs: Strep A OIA Max (Biostar) Type of RADTs: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 4 Assessment of GAS antibody response: no Relevant details: the study used a composite reference standard relying on 4 media plated for each culture but we only extracted the data corresponding to the "same swab single plate standard", i.e., single inhibitory plate using the same swab first for culture and then for performing the RADT. This standard may resemble what is used in practice in most settings. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by a grant from one of the manufacturers of the RADTs under evaluation (Genzyme) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Gieseker 2002b.
| Study characteristics | |||
| Patient sampling | See Gieseker 2002a | ||
| Patient characteristics and setting | See Gieseker 2002a | ||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADTs: OSOM Ultra Strep A (Genzyme) Type of RADTs: EIA |
||
| Target condition and reference standard(s) | See Gieseker 2002a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by a grant from one of the manufacturers of the RADTs under evaluation (Genzyme) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Gieseker 2003.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: patients suspected of having S. pyogenes pharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 887
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 23.7% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: OSOM Ultra Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: 2 swabs were taken for each participant. We extracted the data corresponding to Swab #2 because it was fully processed in the microbiology laboratory whereas Swab #1 was processed in the paediatrician's office. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded by Genzyme (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Gurol 2010.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: patients for whom RADT and culture were requested Presenting signs and symptoms: not reported Age range for inclusion: not reported ("all patients", i.e., adults and children; data extractable for children 0 to 9 years of age) |
||
| Patient characteristics and setting | Sample size: 178 (total sample 453, paediatric sample 0 to 9 years 178)
Age (distribution): not reported in this age group (0 to 9 years) GAS prevalence according to culture (with 95% confidence interval): 22.5% (95% CI not reported) Country of study: Turkey Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: outpatient clinic of a university hospital Single‐centre study |
||
| Index tests | Throat swab: unclear Commercial name of the RADT: QuickVue Plus Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The RADT is referred to as "QuickVue Strep A cassette test" from Quidel. Quidel manufactures 2 cassette 2: QuickVue In‐Line and QuickVue Plus. The accuracy mentioned by the authors as being reported in the package insert corresponds to those from the QuickVue Plus test. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Hall 2004.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: nurse or medical assistant Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria ("all children with suspected GAS pharyngitis") Presenting signs and symptoms: unclear (see above) Age range for inclusion: 2 to 17 years |
||
| Patient characteristics and setting | Sample size: 561
Age (distribution): median age = 9 years GAS prevalence according to culture (with 95% confidence interval): 27.1% (95% CI not reported) Country of study: USA Sex (% of girls): 53% Clinical severity assessment: Centor score (modified) Clinical setting: mixed ("departments of pediatrics, family medicine, urgent care, and emergency medicine and primary care satellite centers") Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for the RADT) Commercial name of the RADT: Acceava Strep A (Biostar) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard (plate 1) and inhibitory (plate 2) Atmosphere of incubation: aerobic (plate 1) and aerobic with CO2 enrichment (plate 2) Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Throat culture performed only for children with negative RADT results (partial verification). Funded by the US Centers for Disease Control and Prevention. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Harris 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: signs of pharyngitis Age range for inclusion: 2 to 18 years |
||
| Patient characteristics and setting | Sample size: 519
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 22.0% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: other (in‐house score) Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | RADT kits were provided by the manufacturer (Biostar) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Hart 1997.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: nurses Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients presenting with pharyngitis Age range for inclusion: not reported ("adults" and "children"; data extractable for patients ≤ 18 years) |
||
| Patient characteristics and setting | Sample size: total sample 263, paediatric sample 75
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 21% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic ("family practice clinic") Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (each swab was used first for culture and then for performing the RADT; paired swabs were collected to study swab‐to‐swab variability but only the result from one randomly selected swab was used for estimating diagnostic accuracy) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory and enrichment (using the pledget) Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk Number of plates inoculated: 2 plates per swab (1 selective plate and 1 selective plate following Todd‐Hewitt enrichment) Assessment of GAS antibody response: no Relevant details: 2 swabs were collected to study swab‐to‐swab variability but only the result from one randomly selected swab was used for estimating accuracy measurements | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Technical and partial financial assistance was provided by Biostar (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Henderson 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes (EIA versus LA)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: birth to 17 years |
||
| Patient characteristics and setting | Sample size: 117 (total sample 218; 117 were tested by EIA)
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 33.3% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: unclear Commercial name of the RADT: not reported ("EIA no name") Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: unclear Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract (published in the American Journal of Diseases in Children) | ||
| Notes | The study compared an EIA rapid test to a LA test and compared the accuracy of both tests performed in the emergency room or in the microbiology laboratory. We extracted data only for the EIA test performed in the emergency room. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kaltwasser 1997.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (and culture versus PCR)
Person performing the throat sample: other ("emergency department personnel") Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria (enrollment if "the medical staff evaluating the patient determined that detection of GAS was needed") Presenting signs and symptoms: pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 200
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 28.5% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab used first for culture and then for the RADT, 1 swab used for broth‐enhanced culture and PCR) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory and enrichment Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: bacitracin disk +/‐ latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: only data for the simple selective plate were extracted (no enrichment) | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study compared the RADT to 2 types of culture and to PCR. We extracted data regarding OIA versus simple agar plating. Study supported in part by an unrestricted grant from Biostar (manufacturer of the RADT). |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kaufhold 1991a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective or prospective design: unclear
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: data not extracted Clinical selection of patients: none Presenting signs and symptoms: suspicion of streptococcal pharyngitis Age range for inclusion: 0 to 16 years |
||
| Patient characteristics and setting | Sample size: 230
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 45.6% (95% CI not reported) Country of study: Germany Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (paediatric hospital and private offices) Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab Commercial name of the RADT: TestPack Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 24 to 48 hours GAS confirmation: latex test Number of plates inoculated (n): data not extracted Assessment of GAS antibody response: data not extracted Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in German) | ||
| Notes | The manufacturers provided the rapid test kits. We thank Dr A Leis for translating this study report. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kaufhold 1991b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective or prospective design: unclear
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: data not extracted Clinical selection of patients: none Presenting signs and symptoms: suspicion of streptococcal pharyngitis Age range for inclusion: 0 to 16 years |
||
| Patient characteristics and setting | Sample size: 261
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 42.1% (95% CI not reported) Country of study: Germany Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (paediatric hospital and private offices) Multi‐centre study |
||
| Index tests | Throat swab: 1 double swab Commercial name of the RADT: Tandem Icon Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 24 to 48 hours GAS confirmation: latex test Number of plates inoculated (n): data not extracted Assessment of GAS antibody response: data not extracted Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in German) | ||
| Notes | The manufacturers provided the rapid test kits. We thank Dr A Leis for translating this study report. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kellog 1987.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (office versus laboratory culture)
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients with symptoms of pharyngitis Age range for inclusion: not reported (only age range of included patients) |
||
| Patient characteristics and setting | Sample size: 358
Age (distribution): mean 7.2 years (range 7 months to 19 years) GAS prevalence according to culture (with 95% confidence interval): 29.9% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: TestPack Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: latex test or direct fluorescent antibody procedure Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: 2 swabs were taken from each patient. Swab #1 was used in the office for culture (office culture) and then for performing the RADT. Swab #2 was sent to the laboratory for culture and then for performing the RADT. We only extracted data related to analyses performed in the microbiology laboratory. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | 2 swabs were taken from each patient. Swab #1 was used in the office for culture (office culture) and then for performing the RADT. Swab #2 was sent to the laboratory for culture and then for performing the RADT. We only extracted data related to analyses performed in the microbiology laboratory. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kellog 1991.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (office culture versus laboratory culture)
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: symptoms of pharyngitis Age range for inclusion: not reported ("pediatric offices") |
||
| Patient characteristics and setting | Sample size: 1035
Age (distribution): mean = 8.0 years (1030 children and 5 parents included) GAS prevalence according to culture (with 95% confidence interval): 40.9% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 1 duplicate swab (swab #1 used first for culture and then for performing the RADT in the office; swab #2 used first for culture and then for performing the RADT in the microbiology laboratory) Commercial name of the RADT: SMART Group A test (New Horizons) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: when the SMART result was positive but the culture was negative, the primary inoculum zone was subcultured to both an aerobically incubated standard blood agar plate and aerobically (with CO2 enrichment) selective blood agar plate. | ||
| Flow and timing | No follow‐up. | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Swab #1 was used first for culture and then for performing the RADT in the office and swab #2 was used first for culture and then for performing the RADT in the microbiology laboratory. In the laboratory, RADTs were read after 5 minutes of incubation and tests with negative results were reincubated overnight and reread. We extracted data only for the RADT performed in the laboratory and read after 5 minutes of incubation. RADT kits were provided by the manufacturer (New Horizons). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | No | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Kim 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: patients with "suspected bacterial pharyngitis on the basis of the symptoms or signs" Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 293
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 66.6% (95% CI not reported) Country of study: Korea Sex (% of girls): 44.7% Clinical severity assessment: none Clinical setting: mixed (office‐based and walk‐in clinics) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (unclear how they were used) Commercial name of the RADT: SD Bioline Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 24 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The manufacturer of the RADT (SD) provided the kits for this study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kuhn 1999.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (within the previous 72 hours) Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: sore throat and one of the following signs: pharyngeal injection or exudate, fever > 38.4°C, or cervical lymphadenopathy Age range for inclusion: 2 to 18 years |
||
| Patient characteristics and setting | Sample size: 363 throat swabs from 248 children (multiple visits allowed)
Age (distribution): median 6.6 years (range 2.2 to 15.9 years) GAS prevalence according to culture (with 95% confidence interval): 36.4% (95% CI not reported) Country of study: Canada Sex (% of girls): 49.2% Clinical severity assessment: none Clinical setting: mixed (emergency department and office‐based) Multi‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 standard (+1 after broth enrichment, data not extracted) Assessment of GAS antibody response: no Relevant details: the throat swab was used for standard culture and the pledget was used for a broth‐enriched culture. We only extracted data relevant to the standard agar culture technique. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The throat swab was used for standard culture and the pledget was used for a broth‐enriched culture. We only extracted data relevant to the standard agar culture technique. The first author was supported by the Canadian Infectious Diseases Society Glaxo Wellcome Research Fellowship Award. The study was supported by a grant from the Alberta Children’s Hospital Foundation. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Kurtz 2000.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no (but comparison of single‐swab versus double‐swab antigen extraction)
Direct comparison of several throat culture techniques: yes (standard blood agar versus selective medium)
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (previous 7 days) Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: children with clinical signs of S. pyogenes pharyngitis ("fever, sore throat, and/or cervical adenitis and the absence of cough, rhinorrhea, lower respiratory infection, and otitis media") Age range for inclusion: 4 to 15 years |
||
| Patient characteristics and setting | Sample size: 256
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 30.9% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (each used first for culture and then for performing the RADT; we randomly chose to extract data for swab B) Commercial name of the RADT: Test Pack Plus (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard and inhibitory (composite 2‐plate reference standard) Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: 2 swabs were taken (A and B). Each swab was first inoculated onto a culture plate (standard or selective) and then used for performing the RADT. Culture positivity was defined as growth from either of the 2 plates. For the results of the RADT, we only extracted data for 1 swab, which was randomly chosen to be swab B. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | 2 swabs were taken (A and B). Each swab was inoculated onto a culture plate (standard or selective) and then used for antigen detection. Culture positivity was defined as growth from either of the 2 plates. For the results of the RADT, we only extracted data for 1 swab, which was randomly chosen to be swab B. Funded in part by Abbott (manufacturer of the RADT). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Küçük 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (without precision) Clinical selection of patients: explicit criteria but not a score Presenting signs and symptoms: acute sore throat, fever and acutely inflamed throat/tonsils with or without exudates Age range for inclusion: 0 to 17 years |
||
| Patient characteristics and setting | Sample size: 892
Age (distribution): mean = 5.3 years GAS prevalence according to culture (with 95% confidence interval): 24.1% (95% CI not reported) Country of study: Turkey Sex (% of girls): 42% Clinical severity assessment: none Clinical setting: mixed (paediatric emergency department and outpatient clinics) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: QuickVue In‐Line Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The authors reported using the "QuickVue Strep A (Quidel) cassette". Quidel manufactures several RADTs that use a cassette; we assumed the study evaluated the most simple one, QuickVue In‐Line Strep A kit. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Laubscher 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (within the last 5 days) Clinical selection of patients: none Presenting signs and symptoms: all patients with a clinical diagnosis of pharyngitis Age range for inclusion: not reported ("pediatric patients") |
||
| Patient characteristics and setting | Sample size: 454
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 26.0% (95% CI not reported) Country of study: Switzerland Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: Test Pack Strep A Plus (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Supported by the manufacturer of the RADT (Abbott), which provided the kits | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Lewey 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: sore throat and fever Age range for inclusion: 1 to 21 years |
||
| Patient characteristics and setting | Sample size: 264
Age (distribution): mean = 10.4 years GAS prevalence according to culture (with 95% confidence interval): 17.8% (95% CI not reported) Country of study: USA Sex (% of girls): 59% Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (2 double swabs were taken, for a total of 4 swabs, but we extracted data only for swab #1) Commercial name of the RADT: Icon Strep A (Hybritech) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: 2 double swabs (swab #1 and #2) were taken for each patient, for a total of 4 swabs per participant. For each double swab, swab A was used for the RADT and swab B was used for culture. We randomly chose 1 double swab for which we extracted data (swab #1). | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | 2 double swabs (swab #1 and #2) were taken for each patient, for a total of 4 swabs per participant. For each double swab, swab A was used for the RADT and swab B was used for culture. We randomly chose 1 double swab for which we extracted data (swab #1). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Llor 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes Clinical selection of patients: clinical score (Centor score) Presenting signs and symptoms: clinical symptoms of odynophagia and 2 or more of Centor criteria Age range for inclusion: 14 to 21 years |
||
| Patient characteristics and setting | Sample size: 42
Age (distribution): not reported, in patients 14 to 21 years GAS prevalence according to culture (with 95% confidence interval): 19.0% (95% CI not reported) Country of study: Spain Sex (% of girls): not reported, in patients 14 to 21 years Clinical severity assessment: Centor score Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: OSOM Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The manufacturer provided the rapid test kits. We thank Dr C Llor for sharing unpublished paediatric data. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Macknin 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes (EIA versus LA, data extracted only for EIA)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: patients with fever and sore throat Age range for inclusion: 2 to 18 years |
||
| Patient characteristics and setting | Sample size: 120
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 49.2% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 3 different swabs (1 for each RADT and 1 for culture) Commercial name of the RADT: Ventrescreen Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: anaerobic Duration of incubation: not reported GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Maltezou 2008.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (within the previous week) Clinical selection of patients: clinical score (Centor) Presenting signs and symptoms: clinical evidence of pharyngitis including one of the 4 Centor criteria (fever, tonsillar exudate, tender enlarged anterior cervical lymph nodes and absence of cough) Age range for inclusion: 2 to 14 years |
||
| Patient characteristics and setting | Sample size: 432
Age (distribution): mean = 6.8 years (calculated from data in table 1) GAS prevalence according to culture (with 95% confidence interval): 27.3% (95% CI not reported) Country of study: Greece Sex (% of girls): 53.9% Clinical severity assessment: Centor score Clinical setting: mixed (office‐based and hospital outpatient clinic) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: Link 2 Strep A Rapid Test (Becton Dickinson) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Funded by the Hellenic Center for Disease Control and Prevention | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Mayes 2001a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria (RADTs were not used for all patients presenting with pharyngitis; different physicians used varying individual criteria to determine whether or not to use the RADT or throat culture as the primary diagnostic test) Presenting signs and symptoms: unclear Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: total 4847
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 28.8% (assuming all RADT positive results are true positives; 95CI not reported) Country of study: USA Sex (% of girls): 45% Clinical severity assessment: none Clinical setting: office‐based (laboratory records of the Elmwood Pediatric Group) Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for performing the RADT, 1 swab for culture) Commercial name of the RADT: Qtest (Becton Dickinson) Type of RADT: EIA (liposomal test) |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | We sub‐divided the study into 2 time periods (Mayes 2001a and Mayes 2001b) to take into account the fact that different criteria were used to determine whether or not a RADT should be performed, and because different RADTs were used during those 2 time periods. Funded in part by an academic grant (Strong Children's Research Center, Summer Student Scholar Program, University of Rochester). Throat culture performed only for children with negative RADT results (partial verification). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Unclear | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Mayes 2001b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: unclear
Direct comparison of different RADTs: no (different RADTs used but not compared)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: total 6580
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 27.8% (assuming all RADT positive results are true positives: 95% CI not reported) Country of study: USA Sex (% of girls): 45% Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for performing the RADT, 1 swab for culture) Commercial name of the RADT: Signify (Abbott) and Acceava (Biostar), data aggregated and test further referred to as "EIA (no name)" Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Mayes 2001a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | See Mayes 2001a | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Unclear | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Mazur 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes (within the previous 2 weeks) Clinical selection of patients: clinical score (McIsaac) Presenting signs and symptoms: clinical and epidemiological signs of acute pharyngitis suggesting GAS aetiology and McIsaac score ≥ 2 Age range for inclusion: 2 to 15 years |
||
| Patient characteristics and setting | Sample size: 90
Age (distribution): mean (SD) = 6.6 (3.4) years GAS prevalence according to culture (with 95% confidence interval): 50.0% (95% CI not reported) Country of study: Poland Sex (% of girls): 42.2% Clinical severity assessment: McIsaac score Clinical setting: paediatric outpatient clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: QuickVue+ Strep A Test (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Academic funding (Medical University of Lublin, Poland) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
McIsaac 2004.
| Study characteristics | |||
| Patient sampling | RCT (comparing 2 different antibacterial therapies for pharyngitis)
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria ("a throat swab was collected when the physician believed it was warranted") Presenting signs and symptoms: patients with acute sore throat Age range for inclusion: 3 to 17 years (adults also included in the study but data extracted only for children) |
||
| Patient characteristics and setting | Sample size: total 787; children 454
Age (distribution): not reported among children GAS prevalence according to culture (with 95% confidence interval): 34.1% (95% CI not reported) Country of study: Canada Sex (% of girls): not reported Clinical severity assessment: McIsaac score Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: TestPack Plus Strep A with OBC II (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: not reported GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded by Abbott (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Menozzi 1992.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians and nurses Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients with symptoms of pharyngitis Age range for inclusion: unclear |
||
| Patient characteristics and setting | Sample size: 3658
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 34.9% (95% CI not reported) Country of study: Italy Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: paediatric outpatient clinic Single‐ or multi‐centre study: unclear |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: TestPack Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Mezghani Maleej 2010.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: acute pharyngitis, excluding those with signs suggesting viral aetiology Age range for inclusion: 2 to 10 years |
||
| Patient characteristics and setting | Sample size: 504 (445 participants in the contingency table)
Age (distribution): mean = 5.7 years (range 2 years and 2 months to 10 years) GAS prevalence according to culture (with 95% confidence interval): 32.9% (95% CI not reported) Country of study: Tunisia Sex (% of girls): 46% Clinical severity assessment: McIsaac score Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: OSOM Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard and inhibitory (2 plates) Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in French) | ||
| Notes | We thank Prof. A Hammami for providing data from the contingency table (not extractable in the original publication) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Mirza 2007a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: nurses and medical assistants Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: < 18 years |
||
| Patient characteristics and setting | Sample size: total 11,644 (only 9032 included in the meta‐analysis, i.e., those with RADT negative results also cultured)
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 28.3% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: QTest (Becton Dickinson) Type of RADT: EIA (liposomal test) |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was sub‐divided into 2 study cohorts (Mirza 2007a and Mirza 2007b). In Mirza 2007a, the data came from 3 paediatric practices and the RADT used was the QTest (Abbott). In Mirza 2007b, the data came from a children's hospital and the RADT used was the Signify (Abbott). Throat culture performed only for children with negative RADT results (partial verification). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Mirza 2007b.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: nurses Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: < 18 years |
||
| Patient characteristics and setting | Sample size: total 6865 (only 5135 included in the meta‐analysis, i.e., those with RADT negative results also cultured)
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 29.3% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: unclear ("children's hospital") Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for performing the RADT, 1 swab for culture) Commercial name of the RADT: Signify Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | See Mirza 2007a | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Mlejnek 2014.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: not reported ("all patients who had rapid strep screens") Age range for inclusion: < 21 years |
||
| Patient characteristics and setting | Sample size: 3423
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 16.8% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab (1 single, 1 double, 2 different): not reported Commercial name of the RADT: OSOM Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: not reported Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract (Annual Meeting of the Society for Academic Emergency Medicine, Dallas, Texas, USA, May 2014) | ||
| Notes | We thank Dr. JR Mlejnek for sharing additional information that was not part of the original conference abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Moyer 1990.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (24 versus 48 hour reading)
Person performing the throat sample: physician or nurse Exclusion if recent antibiotics use before inclusion: yes (within 2 weeks prior to the onset of pharyngitis) Clinical selection of patients: not reported Presenting signs and symptoms: not reported Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: total 649, children 324
Age (distribution): range 7 months to 16 years GAS prevalence according to culture (with 95% confidence interval): 32.1% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Directgen 1‐2‐3 Group A Strep Test Type of RADT: EIA (liposomal test) |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: plates were examined at 24 and 48 hours and variations in the accuracy of the RADT by incubation time were evaluated. We only extracted data related to the 48 hour reference standard. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study included children and adults. We extracted data for paediatric participants. RADT kits were provided by the manufacturer (BBL). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Needham 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (standard versus enriched)
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 276
Age (distribution): mean = 6.4 years GAS prevalence according to culture (with 95% confidence interval): 31.2% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study (regarding paediatric participants) |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard and enrichment
Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: swabs were inoculated on a standard blood agar plate and the pledget from the transport tube was used for culture following incubation in a Todd‐Hewitt enrichment broth. Enriched culture did not identify additional positive specimens as compared to standard culture. The results of the 2 culture techniques were considered equivalent. |
||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded in part by Biostar (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Nitsch‐Osuch 2010.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: fever > 38°C and sore throat, no cough and sneezing Age range for inclusion: 2 to 15 years |
||
| Patient characteristics and setting | Sample size: 188
Age (distribution): mean (SD) = 5.5 (2.6) years GAS prevalence according to culture (with 95% confidence interval): 33.5% (95% CI not reported) Country of study: Poland Sex (% of girls): 48% Clinical severity assessment: none Clinical setting: unclear Single‐ or multi‐centre study: unclear |
||
| Index tests | Throat swab (1 single, 1 double, 2 different): not reported Commercial name of the RADT: Test Strep A (SureScreen) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Nonaka 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective or prospective design: unclear
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: unclear Clinical selection of patients: unclear Presenting signs and symptoms: pharyngitis or tonsillitis Age range for inclusion: 0 to 16 years |
||
| Patient characteristics and setting | Sample size: 100
Age (distribution): unclear GAS prevalence according to culture (with 95% confidence interval): 23% (95% CI not reported) Country of study: Japan Sex (% of girls): 42% Clinical severity assessment: none Clinical setting: hospital paediatric outpatient clinic Single‐centre study |
||
| Index tests | Throat swab: unclear Commercial name of the RADT: TestPack Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: unclear Atmosphere of incubation: unclear Duration of incubation: unclear GAS confirmation: bacitracin disk Number of plates inoculated: not extracted Assessment of GAS antibody response: not extracted Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in Japanese) | ||
| Notes | The study was funded by Tokyo Kosei‐Nenkin Hospital. We thank Prof. Ryuki Kassai for translating this article. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Unclear | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Unclear | ||
| Low | |||
Pauchard 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (within the previous week) Clinical selection of patients: none Presenting signs and symptoms: sore throat Age range for inclusion: 3 to 18 years |
||
| Patient characteristics and setting | Sample size: 1940
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 38.7% (95% CI not reported for this group) Country of study: Switzerland Sex (% of girls): not reported Clinical severity assessment: McIsaac score Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: QuickVue In‐Line Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract (Annual Meeting of the Swiss Society of Paediatrics, Lucerne, Switzerland, June 2012) | ||
| Notes | We thank Dr. JY Pauchard for sharing additional information that was not part of the original conference abstract | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Pauchard 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (within the previous week) Clinical selection of patients: none Presenting signs and symptoms: sore throat Age range for inclusion: 3 to 18 years |
||
| Patient characteristics and setting | Sample size: 183
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 37.2% (95% CI not reported for this group) Country of study: Switzerland Sex (% of girls): not reported Clinical severity assessment: McIsaac Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: BioNexia Strep A (BioMerieux) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract (Annual Meeting of the Swiss Society of Paediatrics, Geneva, Switzerland, June 2012). | ||
| Notes | We thank Dr. JY Pauchard for sharing additional information that was not part of the original conference abstract. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Pitetti 1998.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: yes (within one week before presentation) Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: patients with a sore throat with erythematous posterior pharynx, tonsillar exudate or scarlatiniform rash; or patients without a complaint of sore throat but with either an erythematous posterior pharynx, with or without exudate, or a scarlatiniform rash Age range for inclusion: 1 to 18 years |
||
| Patient characteristics and setting | Sample size: 233
Age (distribution): mean = 8.6 years (range 1.5 to 18.9 years) GAS prevalence according to culture (with 95% confidence interval): 31.3% (95% CI not reported) Country of study: USA Sex (% of girls): 44.6% Clinical severity assessment: none Clinical setting: mixed (emergency department, walk‐in clinic and acute concern clinic of a children hospital) Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Funded in part by a grant from Biostar (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Ramos 2011.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: not reported ("pediatric services") |
||
| Patient characteristics and setting | Sample size: 165
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 31.5% (95% CI not reported) Country of study: Spain Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: other ("pediatric services") Multi‐centre study |
||
| Index tests | Throat swab: unclear Commercial name of the RADT: OSOM Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract | ||
| Notes | Funding not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Regueras De Lorenzo 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design Sample: consecutive Direct comparison of different RADTs: no Direct comparison of several throat culture techniques: no Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: yes (within a week before enrollment) Clinical selection of patients: none Presenting signs and symptoms: acute tonsillitis and/or pharyngitis Age range for inclusion: 2 to 14 years |
||
| Patient characteristics and setting | Sample size: 192
Age (distribution): mean (SD) = 7.2 (2.8) years GAS prevalence according to culture (with 95% confidence interval): 38.5% (95% CI not reported) Country of study: Spain Sex (% of girls): 48.4% Clinical severity assessment: Centor score Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 for culture, 1 for performing the RADT) Commercial name of the RADT: TestPack Plus (Inverness) Type of RAD: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in Spanish) | ||
| Notes | Supported by a public research grant (Institute of Health Carlos III) and EU funding (FEDER) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Reinert 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: febrile sore throat Age range for inclusion: 2 to 14 years |
||
| Patient characteristics and setting | Sample size: 92
Age (distribution): mean age = 6 years and 4 months GAS prevalence according to culture (with 95% confidence interval): 29.4% (95% CI not reported) Country of study: France Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for performing the RADT, 1 swab for culture) Commercial name of the RADT: Group A Strep Test (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: enrichment and inhibitory Atmosphere of incubation: aerobic Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Unclear | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Unclear | |||
Rimoin 2010a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (oral use in the 3 days prior to screening or parenteral use in the 28 days before screening) Clinical selection of patients: none Presenting signs and symptoms: sore throat Age range for inclusion: 2 to 12 years |
||
| Patient characteristics and setting | Sample size: 184
Age (distribution): mean (SD) = 5.8 (0.21) years GAS prevalence according to culture (with 95% confidence interval): 24.5% (95% CI not reported) Country of study: Brazil Sex (% of girls): 43.3% Clinical severity assessment: Centor score Clinical setting: walk‐in clinic Multi‐centre study (see Rimoin 2010b‐d) |
||
| Index tests | Throat swab: 2 different swabs (1 swab for performing the RADT, 1 swab for culture) Commercial name of the RADT: Strep A OIA Max (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Multi‐centre study conducted in Brazil, Croatia, Egypt and Latvia (see Rimoin 2010b‐d). This study was supported by USAID and WHO. The rapid test kits were provided by Biostar (manufacturer of the RADT). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Rimoin 2010b.
| Study characteristics | |||
| Patient sampling | See Rimoin 2010a | ||
| Patient characteristics and setting | Sample size: 404
Age (distribution): mean (SD) = 5.8 (0.14) years GAS prevalence according to culture (with 95% confidence interval): 39.4% (95% CI not reported) Country of study: Croatia Sex (% of girls): 51.6% Clinical severity assessment: Centor score Clinical setting: walk‐in clinic Multi‐centre study (see Rimoin 2010a) |
||
| Index tests | See Rimoin 2010a | ||
| Target condition and reference standard(s) | See Rimoin 2010a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | See Rimoin 2010a | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Rimoin 2010c.
| Study characteristics | |||
| Patient sampling | See Rimoin 2010a | ||
| Patient characteristics and setting | Sample size: 1626
Age (distribution): mean (SD) = 4.8 (0.06) years GAS prevalence according to culture (with 95% confidence interval): 26.4% (95% CI not reported) Country of study: Egypt Sex (% of girls): 42.3% Clinical severity assessment: Centor score Clinical setting: walk‐in clinic Multi‐centre study (see Rimoin 2010a) |
||
| Index tests | See Rimoin 2010a | ||
| Target condition and reference standard(s) | See Rimoin 2010a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | See Rimoin 2010a | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Rimoin 2010d.
| Study characteristics | |||
| Patient sampling | See Rimoin 2010a | ||
| Patient characteristics and setting | Sample size: 258
Age (distribution): mean (SD) = 6.6 (1.9) years GAS prevalence according to culture (with 95% confidence interval): 29.5% (95% CI not reported) Country of study: Latvia Sex (% of girls): 46.1% Clinical severity assessment: Centor score Clinical setting: walk‐in clinic Multi‐centre study (see Rimoin 2010a) |
||
| Index tests | See Rimoin 2010a | ||
| Target condition and reference standard(s) | See Rimoin 2010a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | See Rimoin 2010a | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Roddey 1995.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (standard versus enriched culture)
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (during the precedent week) Clinical selection of patients: none Presenting signs and symptoms: acute pharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 301
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 38.9% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: paediatric office Single‐centre study |
||
| Index tests | Throat swab: 2 throat swabs were taken for each patient. Swab #1 was used for standard culture and then for performing the RADT. Swab #2 was incubated in a Todd‐Hewitt enrichment broth and subsequently inoculated on a blood agar plate. We extracted data only for swab #1. Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard and enrichment (data extracted only for standard culture) Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: bacitracin disk Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded by a research grant from the American Academy of Pediatrics and the manufacturer of the RADT (Biostar) provided the test kits | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Roe 1995a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: yes (1 plate versus 2 plates versus enrichment broth)
Person performing the throat sample: other ("clinical personnel") Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: symptomatic pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 500
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 30.2% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (children's hospital clinic and emergency department) Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs (each swab used for culture and then for the RADT) Commercial name of the RADT: Strep A OIA (Biostar) Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory and enrichment Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 2 or 3 Assessment of GAS antibody response: no Relevant details: 2 swabs were taken for each patient. Each swab was used for culture on a selective medium and then for antigen detection by one or the other RADTs. If both selective plates were negative for GAS, the pledgets were incubated in a Todd‐Hewitt enrichment broth with subsequent culture. The reference standard was the isolation of GAS by any one (or more than one) of the plates. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | A co‐author was affiliated with the manufacturer of one of the RADTs under evaluation (Abbott) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | No | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Roe 1995b.
| Study characteristics | |||
| Patient sampling | See Roe 1995a | ||
| Patient characteristics and setting | See Roe 1995a | ||
| Index tests | Throat swab: 2 different swabs (each swab used for culture and then for the RADT) Commercial name of the RADT: Test Pack Plus Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Roe 1995a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | See Roe 1995a | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | No | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Rogo 2010a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: signs and symptoms of pharyngitis Age range for inclusion: not reported ("pediatric office setting") |
||
| Patient characteristics and setting | Sample size: 228
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 28.1% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 3 different swabs (each swab used for culture and then for the RADT) Commercial name of the RADT: Acceava Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: 24 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded by the manufacturer of one of the 3 RADTs under evaluation (Acceava) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Rogo 2010b.
| Study characteristics | |||
| Patient sampling | See Rogo 2010a | ||
| Patient characteristics and setting | See Rogo 2010a | ||
| Index tests | Throat swab: 3 different swabs (each swab used for culture and then for the RADT) Commercial name of the RADT: OSOM Strep A (Genzyme) Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Rogo 2010a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded by the manufacturer of one of the 3 RADTs under evaluation (Acceava) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Rogo 2010c.
| Study characteristics | |||
| Patient sampling | See Rogo 2010a | ||
| Patient characteristics and setting | See Rogo 2010a | ||
| Index tests | Throat swab: 3 different swabs (each swab used for culture and then for the RADT) Commercial name of the RADT: QuickVue Dipstick (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Rogo 2010a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The study was funded by the manufacturer of one of the 3 RADTs under evaluation (Acceava) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Savoia 1994.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: patients with pharyngotonsillitis Age range for inclusion: 1 to 14 years |
||
| Patient characteristics and setting | Sample size: 510
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 14.3% (95% CI not reported) Country of study: Italy Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: not reported Single‐ or multi‐centre study: not reported |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: Event test strip Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 2 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Schlager 1996.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: unclear Presenting signs and symptoms: pharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 262
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 24% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (paediatric and family practice clinics in a primary care centre) Single‐centre study |
||
| Index tests | Throat swab: 1 double Commercial name of the RADT: Strep A OIA Type of RADT: OIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: the study compared the accuracy of different throat culture techniques. We extracted data used by the authors to calculate accuracy estimates for the rapid test ("standard culture"). | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Schwabe 1987.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: yes (2 weeks before throat swab collection) Clinical selection of patients: none Presenting signs and symptoms: current respiratory tract infection Age range for inclusion: unclear (but Dr. LD Schwabe confirmed that study specimens were primarily from children < 21 years seen in paediatric offices) |
||
| Patient characteristics and setting | Sample size: 365
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 27.4% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: paediatric offices Single‐centre study |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: TestPack Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic for the first 24 hours and then aerobic with CO2 enrichment for the second 24 hours Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | We thank Dr. LD Schwabe for confirming that study specimens were primarily from children < 21 years seen in paediatric offices | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Schwabe 1991.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes
Person performing the throat sample: mixed (physicians, nurses, technologists, other) Exclusion if recent antibiotics use before inclusion: yes (2 weeks prior to throat swab collection) Clinical selection of patients: unclear Presenting signs and symptoms: unclear Age range for inclusion: unclear from the study report (but Dr. LD Schwabe confirmed 98.6% of participants were paediatric patients) |
||
| Patient characteristics and setting | Sample size: 261
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 27.1% (95 CI% not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (paediatric offices, a university student health centre and a general community hospital outpatient laboratory) Multi‐centre study |
||
| Index tests | Throat swab: 1 single swab (culture then RADT) Commercial name of the RADT: Test Pack Plus Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic for the first 24 hours and aerobic with CO2 enrichment for the second 24 hours Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: only data for culture on the nonselective medium were extracted | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | We thank Dr. LD Schwabe for confirming that numbers in the published contingency table are from paediatric patients (99%) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Schwartz 1997a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes (2 different EIAs)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: not reported Presenting signs and symptoms: unclear Age range for inclusion: unclear |
||
| Patient characteristics and setting | Sample size: 258
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 40.0% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based (paediatric clinic) Single‐centre study |
||
| Index tests | Throat swab: not reported Commercial name of the RADT: OSOM Strep A (Wyntek) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 24 hours GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Schwartz 1997b.
| Study characteristics | |||
| Patient sampling | See Schwartz 1997a | ||
| Patient characteristics and setting | See Schwartz 1997a | ||
| Index tests | Throat swab: not reported Commercial name of the RADT: QuickVue In‐Line Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Schwartz 1997a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Sedki 2010.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: yes Clinical selection of patients: clinical score (Centor) Presenting signs and symptoms: pharyngitis with at least 2 Centor criteria Age range for inclusion: 3 to 15 years |
||
| Patient characteristics and setting | Sample size: 95
Age (distribution): median = 8.98 years (range 3.3 to 13.8) GAS prevalence according to culture (with 95% confidence interval): 32.6% (95% CI not reported) Country of study: Egypt Sex (% of girls): 58% Clinical severity assessment: none Clinical setting: mixed (outpatient clinic of health centre or the school dispensary room) Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: StreptAtest Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: other (penicillin susceptibility and gram stain microscopy) Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The rapid test kits were supplied by the manufacturer | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Unclear | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Strandjord 1987.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes (LA versus EIA; data extracted only for EIA)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: "patients who were suspect of having GAS pharyngitis" Age range for inclusion: 2 to 18 years |
||
| Patient characteristics and setting | Sample size: 138
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 37.7% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: mixed (emergency department and acute care clinic) Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: Icon Strep A (Hybritech) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 24 hours GAS confirmation: fluorescent antibody technique Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Subashini 2015.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective or prospective design: unclear
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: not reported Presenting signs and symptoms: pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 111
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 24.3% (95% CI not reported) Country of study: India Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: outpatient clinic Single‐centre study |
||
| Index tests | Throat swab (1 single, 1 double, 2 different): not reported Commercial name of the RADT: SD Bioline Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: not reported GAS confirmation: latex test Number of plates inoculated (n): not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Tanz 2009.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (office culture versus laboratory culture)
Person performing the throat sample: physician Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngitis Age range for inclusion: 3 to 18 years |
||
| Patient characteristics and setting | Sample size: 1848
Age (distribution): 13% under 5 years of age (mean or median not reported) GAS prevalence according to culture (with 95% confidence interval): 30% (95% CI not reported) Country of study: USA Sex (% of girls): 53% Clinical severity assessment: McIsaac score Clinical setting: office‐based Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (swab A used first for office culture and then for performing the RADT; swab B used for laboratory culture) Commercial name of the RADT: QuickVue dipstick (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: latex agglutination Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: swab A was streaked on a blood agar plate for office culture and then used for the RADT; data for office culture not extracted | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Last author (Dr Shulman) is on the medical advisory board of Quidel (manufacturer of the RADT) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | No | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
Tellechea 2012.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Retrospective design
Sample: non‐consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: laboratory personnel Exclusion if recent antibiotics use before inclusion: yes (within the previous week) Clinical selection of patients: implicit criteria (see below) Presenting signs and symptoms: symptoms compatible with GAS Age range for inclusion: 3 to 15 years |
||
| Patient characteristics and setting | Sample size: 5505
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 39.8% (95% CI not reported) Country of study: Argentina Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: paediatric emergency department Single‐centre study |
||
| Index tests | Throat swab: not reported Commercial name of the RADT: ACON Strep A Rapid Test Strip (ACON Lab) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article (in Spanish) | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | No | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Unclear | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Unclear | |||
Tenjarla 1991.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: physicians and office staff Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: clinical tonsillopharyngitis Age range for inclusion: "pediatric patients" |
||
| Patient characteristics and setting | Sample size: 9161 children (among a total of 11,088)
Age (distribution): 3 months to 18 years ("pediatric population") GAS prevalence according to culture (with 95% confidence interval): 16.5% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐ or multi‐centre study: unclear |
||
| Index tests | Throat swab: 1 double swab (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: TestPack Strep A (Abbott) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: anaerobic Duration of incubation: 16 to 42 hours GAS confirmation: TestPack Strep A used on beta‐haemolytic colonies Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: in this study the RADT was also used as a confirmation technique to identify beta‐haemolytic colonies as S. pyogenes | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | Included adults and children; data extracted only for children | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | No | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Toepfner 2013.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: tonsillopharyngitis Age range for inclusion: not reported |
||
| Patient characteristics and setting | Sample size: 517 (324 in 2009 and 193 in 2010)
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 17.6% (95% CI not reported) Country of study: Germany Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: unclear Single‐ or multi‐centre study: unclear |
||
| Index tests | Throat swab: 1 single swab (used for culture and then for the RADT) Commercial name of the RADT: QuickVue In‐Line Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | In this study, the accuracy of the rapid test was compared between physicians and laboratory technicians. Our review focused on the accuracy of RADT with laboratory culture as the reference standard, therefore we extracted data only for laboratory technicians. The study also comprised 2 phases: before (2009) and after (2010) training of physicians by laboratory technicians. We extracted data only for laboratory technicians, therefore we pooled the data from 2009 and 2010. We thank Dr. M Hufnagel for confirming that numbers in the published contingency table are from paediatric patients. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Van Limbergen 2006.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: consecutive
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: nursing staff Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: clinical diagnosis of pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 201
Age (distribution): mean (SD) = 3.85 (3.15) years GAS prevalence according to culture (with 95% confidence interval): 15.9% (95% CI not reported) Country of study: Scotland Sex (% of girls): 48.4% Clinical severity assessment: none Clinical setting: emergency department Single‐centre study |
||
| Index tests | Throat swab: unclear Commercial name of the RADT: QuickVue Plus Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: not reported Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: throat culture technique not described | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The test kits were provided by Quidel (manufacturer of the RADT) Throat culture performed only for children with negative RADT results (partial verification) |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | No | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | Yes | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Wong 1989.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: convenience
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: unclear Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: symptoms of viral or streptococcal pharyngitis Age range for inclusion: < 18 years (data for adults not extracted) |
||
| Patient characteristics and setting | Sample size: 147 children (data for 151 adults not extracted)
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 23.8% (95% CI not reported) Country of study: Canada Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic Single‐centre study |
||
| Index tests | Throat swab: 2 different swabs Commercial name of the RADT: TestPack Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic with CO2 enrichment Duration of incubation: 48 hours GAS confirmation: latex test Number of plates inoculated: 1 Assessment of GAS antibody response: no Relevant details: ‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | No | ||
| Low | High | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Wright 2007a.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: yes (2 EIAs)
Direct comparison of several throat culture techniques: no
Person performing the throat sample: other ("medical technician") Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: explicit criteria but not a score (see below) Presenting signs and symptoms: "Criteria for throat swab included sore throat, erythematous tonsils or pharynx, cervical lymphadenopathy, and exudates" Age range for inclusion: 0 to 18 years |
||
| Patient characteristics and setting | Sample size: 338
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 26.0% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: military air force base Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (each swab used for antigen detection and culture) Commercial name of the RADT: OSOM Ultra Strep A Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic Duration of incubation: 48 hours GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details: unclear if the reference standard was a single‐plate culture or a composite of both plates. | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Wright 2007b.
| Study characteristics | |||
| Patient sampling | See Wright 2007a | ||
| Patient characteristics and setting | See Wright 2007a | ||
| Index tests | Throat swab: 1 double swab Commercial name of the RADT: QuickVue In‐Line Strep A (Quidel) Type of RADT: EIA |
||
| Target condition and reference standard(s) | See Wright 2007a | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | Yes | ||
| Was clinical selection of patients avoided? | Unclear | ||
| Were patients seen in an ambulatory care setting? | No | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Yes | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| Low | |||
Yuckienuz 1988.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: yes (office culture versus laboratory culture)
Person performing the throat sample: physicians Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: pharyngitis Age range for inclusion: not reported ("children") |
||
| Patient characteristics and setting | Sample size: 341
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 37.0% (95% CI not reported) Country of study: USA Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: office‐based Single‐centre study |
||
| Index tests | Throat swab: 1 double swab (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: SUDS Group A Strep (Murex) Type of RADT: EIA |
||
| Target condition and reference standard(s) | Throat culture medium: standard Atmosphere of incubation: aerobic during 24 hours (office culture) and then anaerobic during 24 hours (laboratory) Duration of incubation: 48 hours GAS confirmation: bacitracin disk and latex test Number of plates inoculated: 1 plate initially inoculated in the office but several subcultures performed in the laboratory Assessment of GAS antibody response: no Relevant details: 1 swab was used for office culture (aerobic 24‐hour incubation) and the plates were then transferred to the laboratory for further exploration (anaerobic 24‐hour reincubation +/‐ subcultures of suspect colonies) | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Journal article | ||
| Notes | The manufacturer of the RADT (Murex) financially supported the study and provided the test kits | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | No | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Yes | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | Yes | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Yes | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | No | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | Yes | ||
| High | |||
Zanacca 1992.
| Study characteristics | |||
| Patient sampling | Cross‐sectional study
Prospective design
Sample: unclear
Direct comparison of different RADTs: no
Direct comparison of several throat culture techniques: no
Person performing the throat sample: not reported Exclusion if recent antibiotics use before inclusion: no Clinical selection of patients: none Presenting signs and symptoms: symptoms of pharyngitis Age range for inclusion: not reported ("patients from the pediatric outpatients departments") |
||
| Patient characteristics and setting | Sample size: 606
Age (distribution): not reported GAS prevalence according to culture (with 95% confidence interval): 32.8% (95% CI not reported) Country of study: Italy Sex (% of girls): not reported Clinical severity assessment: none Clinical setting: walk‐in clinic Multi‐centre study |
||
| Index tests | Throat swab: 2 different swabs (1 swab for culture, 1 swab for performing the RADT) Commercial name of the RADT: Directgen 1‐2‐3 Group A Strep (Becton Dickinson) Type of RADT: EIA (liposomal test) |
||
| Target condition and reference standard(s) | Throat culture medium: inhibitory Atmosphere of incubation: not reported Duration of incubation: not reported GAS confirmation: not reported Number of plates inoculated: not reported Assessment of GAS antibody response: no Relevant details:‐ | ||
| Flow and timing | No follow‐up | ||
| Comparative | |||
| Type of study | Conference abstract | ||
| Notes | — | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was it a cross‐sectional study or a RCT? | Yes | ||
| Were selection criteria clearly described (at least presenting signs and symptoms and age limits for inclusion)? | No | ||
| Was clinical selection of patients avoided? | Yes | ||
| Were patients seen in an ambulatory care setting? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the RADT results interpreted with blinding of the results of culture? | Yes | ||
| Was the type of the RADT mentioned (EIA or OIA)? | Yes | ||
| Were RADTs conducted during consultation time? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Were culture results interpreted with blinding of the results of the RADT? | Unclear | ||
| Is the throat culture method likely to correctly identify GAS (laboratory culture on a blood agar plate during 48 hr)? | Unclear | ||
| Were the culture medium, atmosphere, duration of incubation and GAS‐confirmation technique described? | No | ||
| High | High | ||
| DOMAIN 4: Flow and Timing | |||
| Was the delay between the performance of the RADT and throat culture plating less than 48 hours? | Unclear | ||
| Did all patients receive a throat culture? | Yes | ||
| Did patients receive the same throat culture method? | Yes | ||
| Were undetermined/uninterpretable results reported? | No | ||
| Were withdrawals from the study explained? | No | ||
| Low | |||
CI: confidence interval EIA: enzyme immunoassay FISH: fluorescence in situ hybridisation GAS: group A streptococcus LA: latex agglutination n/a: not applicable OIA: optical immunoassay PCR: polymerase chain reaction PYR: pyrrolidonyl peptidase RADT: rapid antigen detection test SD: standard deviation USAID: United States Agency for International Development WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Sabaah 2006 | Not ambulatory care setting |
| Andersen 1994 | Mixed age but no paediatric data |
| Andersen 2003a | Duplicate publication |
| Andersen 2003b | Not ambulatory care setting |
| Anhalt 1992 | Mixed age but no paediatric data |
| Anonymous 1985a | Editorial, letter or review |
| Anonymous 1985b | Duplicate publication |
| Anonymous 1985c | Duplicate publication |
| Anonymous 1986 | Editorial, letter or review |
| Anonymous 1991 | Editorial, letter or review |
| Anonymous 1992 | Editorial, letter or review |
| Araj 1986 | RADT other than EIA or OIA |
| Araujo 2005 | Adults or unclear age |
| Armengol 2004a | Reference standard not laboratory culture |
| Armengol 2004b | Reference standard not laboratory culture |
| Arya 1993 | Editorial, letter or review |
| Atlas 2005 | Adults or unclear age |
| Ausina 1987 | Editorial, letter or review |
| Ba‐Saddik 2014 | RADT other than EIA or OIA |
| Badgett 1996 | Editorial, letter or review |
| Baker 1995 | Mixed age but no paediatric data |
| Baselski 1988 | Adults or unclear age |
| Berger‐Jekic 1987 | RADT other than EIA or OIA |
| Berke 1989 | Editorial, letter or review |
| Betriu 1988 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Betriu 1989 | Adults or unclear age |
| Bischoff 2007 | Editorial, letter or review |
| Bjerrum 2013 | Editorial, letter or review |
| Blade 1991 | Mixed age but no paediatric data |
| Blanco 1988 | Duplicate publication |
| Boccazzi 2011 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Bodino 1987 | RADT other than EIA or OIA |
| Boss 1992 | Editorial, letter or review |
| Bourbeau 1993 | Mixed age but no paediatric data |
| Brahmadathan 1986 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Burke 1988 | Mixed age but no paediatric data |
| Calvino 2015 | Adults or unclear age |
| Cardoso 2013 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Carey 1991 | Mixed age but no paediatric data |
| Centor 1984 | RADT other than EIA or OIA |
| Centor 1985 | RADT other than EIA or OIA |
| Chen 2000 | Editorial, letter or review |
| Chessman 1998 | Editorial, letter or review |
| Choi 1995 | Adults or unclear age |
| Coban 2013 | Not ambulatory care setting |
| Cohen 1993 | Editorial, letter or review |
| Cohen 2000 | Editorial, letter or review |
| Cohen 2012a | Duplicate publication |
| Cohen 2013a | Duplicate publication |
| Corneli 2001 | Editorial, letter or review |
| Dale 1994 | Adults or unclear age |
| Dale 1997 | Editorial, letter or review |
| De Lorenzo 2012 | Duplicate publication |
| Demeyere 1992 | Mixed age but no paediatric data |
| Diaz‐Berenguer 1992 | Mixed age but no paediatric data |
| Dimatteo 2001 | Adults or unclear age |
| Dingle 2014 | Mixed age but paediatric data not extractable |
| DiNicola 1986 | RADT other than EIA or OIA |
| DuBois 1986 | RADT other than EIA or OIA |
| DuBose 1996 | Editorial, letter or review |
| Eaton 1987 | Editorial, letter or review |
| Edmonson 2003 | Duplicate publication |
| Edouard 2014 | Editorial, letter or review |
| Ehrlich 1993 | Reference standard not laboratory culture |
| Enright 2009 | Duplicate publication |
| Esteban 2004 | Editorial, letter or review |
| Fellah 1988 | RADT other than EIA or OIA |
| Figura 1981 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Fischer 1992 | Editorial, letter or review |
| Foong 1992 | Mixed age but no paediatric data |
| Fox 2006a | Reference standard not laboratory culture |
| Fox 2006b | Reference standard not laboratory culture |
| Frei 1991 | Editorial, letter or review |
| Fries 1995 | Reference standard not laboratory culture |
| Gaustad 1991 | Editorial, letter or review |
| Gerber 1986a | RADT other than EIA or OIA |
| Gerber 1989 | Editorial, letter or review |
| Gerber 1990a | RADT other than EIA or OIA |
| Gerber 1997a | Editorial, letter or review |
| Gerber 1997b | Editorial, letter or review |
| Gerber 1998 | Editorial, letter or review |
| Ghanassia 1996 | Editorial, letter or review |
| Gnehm 1987 | RADT other than EIA or OIA |
| Gonsu 2015 | Not ambulatory care setting |
| Greiver 1999 | Editorial, letter or review |
| Gupta 1992 | Target condition other than GAS |
| Gupta 1997 | Adults or unclear age |
| Gutman 1996 | Editorial, letter or review |
| Hadfield 1987 | RADT other than EIA or OIA |
| Hallander 1988 | Editorial, letter or review |
| Handrick 2006 | Editorial, letter or review |
| Hansen 1992a | RADT other than EIA or OIA |
| Hansen 1992b | Editorial, letter or review |
| Harbeck 1993 | Mixed age but no paediatric data |
| Harbeck 1995 | Editorial, letter or review |
| Hasin 1989 | Mixed age but no paediatric data |
| Haym 1986 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Hedges 1991 | Adults or unclear age |
| Heiter 1993 | Mixed age but no paediatric data |
| Heiter 1995 | Mixed age but no paediatric data |
| Hinfey 2010 | Mixed age but no paediatric data |
| Hodgins 1988 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Hoffmann 1987 | Editorial, letter or review |
| Hoffmann 1990 | Mixed age but no paediatric data |
| Holbrook 1998 | Editorial, letter or review |
| Hufnagel 2010 | Duplicate publication |
| Humair 2006 | Editorial, letter or review |
| Issa 2014 | Editorial, letter or review |
| Johansson 2003 | Mixed age but no paediatric data |
| Johnson 1995 | Editorial, letter or review |
| Joslyn 1995 | Mixed age but no paediatric data |
| Joubaud 2003 | Mixed age but no paediatric data |
| Kawakami 2003 | Mixed age but no paediatric data |
| Kayaba 1996 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Keahey 2002 | RADT other than EIA or OIA |
| Kechrid 1988 | RADT other than EIA or OIA |
| Kellogg 1986a | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Kellogg 1986b | Editorial, letter or review |
| Kellogg 1987 | Mixed age but no paediatric data |
| Kellogg 1988 | Mixed age but no paediatric data |
| Kellogg 1990 | Editorial, letter or review |
| Klein 1986 | Adults or unclear age |
| Kljakovic 2009 | Editorial, letter or review |
| Kojima 2002 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Kramer 1980 | Editorial, letter or review |
| Kurtz 1999 | Duplicate publication |
| Larkin 2001 | Editorial, letter or review |
| Laubscher 1994 | Editorial, letter or review |
| Lind 1988 | Editorial, letter or review |
| Lindbaek 2004 | Mixed age but no paediatric data |
| Lindsay 1985 | Editorial, letter or review |
| Llor 2009a | Editorial, letter or review |
| Llor 2009b | Mixed age but no paediatric data |
| Llor 2010 | Editorial, letter or review |
| Luebbert 1989 | Editorial, letter or review |
| Lutticken 1991 | Editorial, letter or review |
| Manasse 1989 | Adults or unclear age |
| Mateo 2010 | Duplicate publication |
| Mathur 1992 | Editorial, letter or review |
| Matthys 2006 | Editorial, letter or review |
| Mayefsky 1985 | Editorial, letter or review |
| McCusker 1984 | RADT other than EIA or OIA |
| Meier 1990 | RADT other than EIA or OIA |
| Messina 2010 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Morandi 2003 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Morandi 2010 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Morlan 1988 | RADT other than EIA or OIA |
| Nahata 1986 | Editorial, letter or review |
| Nerbrand 2002 | Mixed age but no paediatric data |
| Nissinen 2009 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Noorbakhsh 2011 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Norris 1993 | Editorial, letter or review |
| Omurzakova 2008 | Target condition other than GAS |
| Omurzakova 2009 | Target condition other than GAS |
| Omurzakova 2010 | Target condition other than GAS |
| Patel 1987 | Mixed age but no paediatric data |
| Penalba Citores 2007 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Petts 1985 | RADT other than EIA or OIA |
| Petts 1988 | RADT other than EIA or OIA |
| Pichichero 1992 | Editorial, letter or review |
| Portier 2003 | Editorial, letter or review |
| Prakash 1985 | Editorial, letter or review |
| Preston 1987 | Editorial, letter or review |
| Putto 1987 | RADT other than EIA or OIA |
| Radetsky 1985 | Editorial, letter or review |
| Radetsky 1987 | Editorial, letter or review |
| Raich 1990 | Mixed age but no paediatric data |
| Rasaiah 1986 | Editorial, letter or review |
| Raz 1987 | Editorial, letter or review |
| Razongles 1993 | RADT other than EIA or OIA |
| Redd 1988 | RADT other than EIA or OIA |
| Reed 1990 | Mixed age but no paediatric data |
| Reichardt 2009 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Rimoin 2004 | Duplicate publication |
| Roosevelt 2001 | Reference standard not laboratory culture |
| Santos 2003 | Not ambulatory care setting |
| Sarikaya 2010 | Adults or unclear age |
| Savoia 1992 | Not a RADT diagnostic study or 2 x 2 table not extractable |
| Schafer 1995 | Editorial, letter or review |
| Schmuziger 1996 | Mixed age but no paediatric data |
| Schmuziger 2003 | Mixed age but no paediatric data |
| Schwartz 1985 | RADT other than EIA or OIA |
| Seaberg 1997 | Mixed age but no paediatric data |
| Seecamp 1993 | Adults or unclear age |
| Seguido 1987 | RADT other than EIA or OIA |
| Seki 1986 | RADT other than EIA or OIA |
| Serra 1989 | Not ambulatory care setting |
| Shaughnessy 2015 | Editorial, letter or review |
| Sheeler 2002 | Adults or unclear age |
| Shekelle 1992 | Editorial, letter or review |
| Shriner 1985 | Editorial, letter or review |
| Shulman 1994 | Editorial, letter or review |
| Shulman 1995 | Editorial, letter or review |
| Skellern 1993 | Adults or unclear age |
| Smith 1989 | RADT other than EIA or OIA |
| Smith 1995 | Mixed age but no paediatric data |
| Solé 2009 | Editorial, letter or review |
| Stillstrom 1991 | Mixed age but no paediatric data |
| Stingu 2009 | Not ambulatory care setting |
| Supon 1998 | Mixed age but no paediatric data |
| Syriopoulou 2011 | RADT other than EIA or OIA |
| Taeron 2006 | Editorial, letter or review |
| Tagami 1997 | Target condition other than GAS |
| Tenjarla 1990 | Duplicate publication |
| Tocks 1992 | Editorial, letter or review |
| Todd 1987 | Editorial, letter or review |
| True 1986 | RADT other than EIA or OIA |
| Uhl 2003 | Mixed age but no paediatric data |
| Vakkila 2015 | RADT other than EIA or OIA |
| Waagepetersen 2009 | Editorial, letter or review |
| Wagener 1985 | RADT other than EIA or OIA |
| Warner 1985 | Editorial, letter or review |
| Waseem 2009 | Duplicate publication |
| Wegner 1992 | Mixed age but no paediatric data |
| Wegner 1996 | Editorial, letter or review |
| White 1986 | RADT other than EIA or OIA |
| Wolinsky 1986 | RADT other than EIA or OIA |
| Wong 2002 | Mixed age but no paediatric data |
| Woodburn 2007 | Mixed age but no paediatric data |
| Wright 1987 | RADT other than EIA or OIA |
| Yu 1988 | Adults or unclear age |
EIA: enzyme immunoassay GAS: group A streptococcus OIA: optical immunoassay RADT: rapid antigen detection test
Characteristics of studies awaiting classification [ordered by study ID]
Briko 1997.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Gajos 1997.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | In Polish | ||
Gnehm 1986.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Grevnina 1992.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Herranz 2007.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Mirjat 2012a.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Mirjat 2012b.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Nestorovic 2004.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Sanz Moreno 2010.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Shikhman 1988.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Soyletir 1988.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | In Turkish | ||
Sramek 1992.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | In Czech | ||
Vylegzhanina 1994.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | Unable to obtain full text | ||
Yilmaz 2008.
| Study characteristics | |||
| Patient sampling | — | ||
| Patient characteristics and setting | — | ||
| Index tests | — | ||
| Target condition and reference standard(s) | — | ||
| Flow and timing | — | ||
| Comparative | — | ||
| Notes | In Turkish | ||
Differences between protocol and review
Authors: One author (NB) contributed to the review but not to the protocol.
Search methods for identification of studies: We intended to search the Cochrane Register of Diagnostic Test Accuracy Studies but did not do so. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) but this was not initially planned. In the protocol, we stated that we would search Science Citation Index for reports that cited included articles, and OpenSIGLE and OAISTER databases for grey literature; due to the number of citations returned by our search (more than 5000) and the number of included studies (n = 98), we judged that these searches were not required.
Data collection and analysis: Two review authors independently excluded studies that were not related to pharyngitis or RADT on the basis of the titles and abstracts, instead of one. We did not use ReSyWeb, an online tool, for study selection. We initially planned to extract all study‐level data in duplicate; due to the number of included studies (n = 98), independent double data extraction was restricted to signalling questions used for study quality assessment and data used for statistical analysis (data from 2 x 2 tables and covariates used for investigating heterogeneity); other descriptive data were extracted by one review author (JFC). In the protocol we stated that we would not present results in groups according to commercial test name but we finally did so because we found this grouping informative for readers.
Investigation of heterogeneity and sensitivity analyses: We intended to assess the effect of the following characteristics of the reference standard: culture medium, atmosphere of incubation, duration of incubation, use of an enrichment broth before plating, group A identification technique and number of plates inoculated; to contain the risk of false positive findings we finally decided to assess the effect of only one of such parameters (i.e. whether an enrichment broth was used before plating); we took this decision before analysing the data. We intended to investigate the effect of age of participants as a 4‐class categorical covariate; in almost all studies in which mean age was reported, mean age was in one of our pre‐specified age categories; we finally used a median split. We intended to investigate the effect of disease severity by using the proportion of participants with a McIsaac score greater than two as a continuous covariate; because we lack routines to investigate the effect of continuous covariates in the bivariate model in Stata, we dichotomised this variable using an arbitrary cut‐off of 70%.
Sensitivity analyses: In the protocol, we intended to carry out sensitivity analyses on the following groups: studies for which patient selection was avoided, studies for which patients were excluded on the basis of antibiotics use within seven days before inclusion, studies for which GAS antibody response was used as the reference test, and studies of high quality according to QUADAS‐2; we finally decided to explore only groups based on QUADAS‐2, as such criteria are explicitly meant to identify studies at low risk of bias and concerns about applicability; this decision was taken before analysing the data. We had the intention to study the effect of partial verification in a sensitivity analysis, but after discussion within the review team, we decided to exclude studies with partial verification from the meta‐analysis of sensitivity and specificity estimates but to include them in a separate additional meta‐analysis of the negative predictive value of RADTs.
Contributions of authors
MC and JFC had the original idea for the review and wrote the first draft of the protocol. RC edited the protocol. JFC and NB selected studies and extracted data. JFC performed the statistical analysis. JFC and MC interpreted the results and drafted the manuscript. All authors provided critical revisions to the manuscript. The study was supervised by MC.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Laboratoires Guigoz ‐ Société Française de Pédiatrie ‐ Groupe de Pédiatrie Générale – Groupe de Recherches Epidémiologiques en Pédiatrie, France.
Educational Grant to JFC (2010)
-
Agence Régionale de Santé d'Ile‐de‐France, France.
Educational Grant to JFC (2011)
-
French Ministry of Health, France.
Research grant PHRC régional AOR 12089 (2012)
-
Association Française de Pédiatrie Ambulatoire, France.
Research grant to JFC (2014)
Declarations of interest
Jérémie F Cohen: None known. Robert Cohen: My relevant financial activities are only in the field of vaccines. Martin Chalumeau: No financial competing interest. Potential academic competing interest (as any expert in the field). Nathalie Bertille: I am supported by educational grants from Laboratoires Guigoz ‐ Société Française de Pédiatrie ‐ Groupe de Pédiatrie Générale ‐ Groupe de Recherches Epidémiologiques en Pédiatrie and Ecole Doctorale 393 (Sorbonne Universités, UPMC Univ Paris 06) and I have no patents, products in development or marketed products to declare. JFC, RC and MC have been involved in studies that were included in the review.
New
References
References to studies included in this review
Al‐Najjar 2008 {published data only}
- Al‐Najjar FY, Uduman SA. Clinical utility of a new rapid test for the detection of group A Streptococcus and discriminate use of antibiotics for bacterial pharyngitis in an outpatient setting. International Journal of Infectious Diseases 2008;12(3):308‐11. [DOI] [PubMed] [Google Scholar]
Alper 2013 {published data only}
- Alper Z, Uncu Y, Akalin H, Ercan I, Sinirtas M, Bilgel NG. Diagnosis of acute tonsillopharyngitis in primary care: a new approach for low‐resource settings. Journal of Chemotherapy 2013;25(3):148‐55. [DOI] [PubMed] [Google Scholar]
Altun 2015 {published data only}
- Altun HU, Meral T, Aribas ET. The specificity and sensitivity results of the rapid antigen test used in the diagnosis of group A beta‐hemolytic streptococcal tonsillopharyngitis. Acta Medica Mediterranea 2015;31:287‐90. [Google Scholar]
Arribas Blanco 1988 {published and unpublished data}
- Arribas Blanco JM, Frieyro Segui JE, Baos Vicente V, Casarrubios Sagastibelza E, Gonzalez Martinez LA, Morera Montes J, et al. Rapid diagnosis of pharyngotonsillitis using an ELISA method (Test Pack Strep A): comparison with culture and clinical data. Analysis of 306 cases. Medicina Clinica 1988;91(15):561‐4. [PubMed] [Google Scholar]
Attia 2001 {published data only}
- Attia MW, Zaoutis T, Klein JD, Meier FA. Performance of a predictive model for streptococcal pharyngitis in children. Archives of Pediatrics & Adolescent Medicine 2001;155(6):687‐91. [DOI] [PubMed] [Google Scholar]
Ayanruoh 2009 {published data only}
- Ayanruoh S, Waseem M, Quee F, Humphrey A, Reynolds T. Impact of rapid streptococcal test on antibiotic use in a pediatric emergency department. Pediatric Emergency Care 2009;25(11):748‐50. [DOI] [PubMed] [Google Scholar]
Begovac 1993 {published data only}
- Begovac J, Bejuk D, Tesovic G, Bobinac E. The Venterscreen Strep A antigen test for rapid diagnosis of streptococcal pharyngitis. Periodicum Biologorum 1993;95(2):255‐7. [Google Scholar]
Buchbinder 2007 {published data only}
- Buchbinder N, Benzdira A, Belgaid A, Dufour D, Paon JC, Morel A, et al. Streptococcal pharyngitis in pediatric emergency unit: value and impact of rapid antigen detection test [Angine streptococcique aux urgences pédiatriques : performances et impact d’un test de diagnostic rapide]. Archives de Pédiatrie 2007;14(9):1057‐61. [DOI] [PubMed] [Google Scholar]
Camurdan 2008 {published data only}
- Camurdan AD, Camurdan OM, Ok I, Sahin F, Ilhan MN, Beyazova U. Diagnostic value of rapid antigen detection test for streptococcal pharyngitis in a pediatric population. International Journal of Pediatric Otorhinolaryngology 2008;72(8):1203‐6. [DOI] [PubMed] [Google Scholar]
Chapin 2002 {published data only}
- Chapin KC, Blake P, Wilson CD. Performance characteristics and utilization of rapid antigen test, DNA probe, and culture for detection of group A streptococci in an acute care clinic. Journal of Clinical Microbiology 2002;40(11):4207‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiadmi 2004a {published data only}
- Chiadmi F, Schlatter J, Mounkassa B, Ovetchkine P, Vermerie N. Fast diagnostic tests in the management of group A beta‐haemolytic streptococcal pharyngitis [Tests de diagnostic rapide dans la prise en charge des angines à streptocoques béta‐ hémolytiques du groupe A]. Annales de Biologie Clinique 2004;62(5):573‐7. [PubMed] [Google Scholar]
Chiadmi 2004b {published data only}
- Chiadmi F, Schlatter J, Mounkassa B, Ovetchkine P, Vermerie N. Fast diagnostic tests in the management of group A beta‐haemolytic streptococcal pharyngitis [Tests de diagnostic rapide dans la prise en charge des angines à streptocoques béta‐ hémolytiques du groupe A]. Annales de Biologie Clinique 2004;62(5):573‐7. [PubMed] [Google Scholar]
Chiadmi 2004c {published data only}
- Chiadmi F, Schlatter J, Mounkassa B, Ovetchkine P, Vermerie N. Fast diagnostic tests in the management of group A beta‐haemolytic streptococcal pharyngitis [Tests de diagnostic rapide dans la prise en charge des angines à streptocoques béta‐ hémolytiques du groupe A]. Annales de Biologie Clinique 2004;62(5):573‐7. [PubMed] [Google Scholar]
Chiadmi 2004d {published data only}
- Chiadmi F, Schlatter J, Mounkassa B, Ovetchkine P, Vermerie N. Fast diagnostic tests in the management of group A beta‐haemolytic streptococcal pharyngitis [Tests de diagnostic rapide dans la prise en charge des angines à streptocoques béta‐ hémolytiques du groupe A]. Annales de Biologie Clinique 2004;62(5):573‐7. [PubMed] [Google Scholar]
Chiadmi 2004e {published data only}
- Chiadmi F, Schlatter J, Mounkassa B, Ovetchkine P, Vermerie N. Fast diagnostic tests in the management of group A beta‐haemolytic streptococcal pharyngitis [Tests de diagnostic rapide dans la prise en charge des angines à streptocoques béta‐ hémolytiques du groupe A]. Annales de Biologie Clinique 2004;62(5):573‐7. [PubMed] [Google Scholar]
Chu 1990 {published data only}
- Chu JM, Chen JM, Wu MH, Hong JY, Wang YM, Hsu HH, et al. Rapid diagnosis of streptococcal pharyngitis with enzyme immunoassay. Acta Paediatrica Sinica 1990;31(3):151‐7. [PubMed] [Google Scholar]
Clegg 1987 {published data only}
- Clegg HW, Roddey OF, Mauney CU, Swetenburg RL, Martin ES. Rapid diagnosis of streptococcal pharyngitis using enzyme immunoassay. Pediatric Infectious Disease Journal 1987;6(7):696‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1988 {published data only}
- Cohen R, Bouhanna A, Geslin P, Reinert P. Interest of a rapid diagnosis test for streptococcus A in management of pharyngitis (Quidel Group A Strep Test) [Intérêt d'un test de diagnostic rapide du streptocoque du groupe A (Quidel Group A Strep Test) pour le traitement des angines]. Médecine et Maladies Infectieuses 1988;18(10b):518‐20. [Google Scholar]
Cohen 1998 {published data only}
- Cohen R, Gouvello A, Levy C, Rocque F, Boucherat M, Portier H. Utilization of rapid diagnostic tests for group A streptococcus and bacteriologic and clinical correlations with acute angina in general medicine. Presse Médicale 1998;27(23):1131‐4. [PubMed] [Google Scholar]
Cohen 2004 {published data only}
- Cohen R, Levy C, Ovetchkine P, Boucherat M, Weil‐Olivier C, Gaudelus J, et al. Evaluation of streptococcal clinical scores, rapid antigen detection tests and cultures for childhood pharyngitis. European Journal of Pediatrics 2004;163(4‐5):281‐2. [DOI] [PubMed] [Google Scholar]
Cohen 2012 {published data only}
- Cohen JF, Chalumeau M, Levy C, Bidet P, Thollot F, Wollner A, et al. Spectrum and inoculum size effect of a rapid antigen detection test for group A streptococcus in children with pharyngitis. PLoS One 2012;7(6):e39085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 2013 {published data only}
- Cohen JF, Cohen R, Bidet P, Levy C, Deberdt P, d'Humières C, et al. Rapid‐antigen detection tests for group A streptococcal pharyngitis: revisiting false‐positive results using polymerase chain reaction testing. Journal of Pediatrics 2013;162(6):1282‐4. [DOI] [PubMed] [Google Scholar]
Contessotto 2000 {published data only}
- Contessotto Spadetto C, Camara Simon M, Aviles Ingles MJ, Ojeda Escuriet JM, Cascales Barcelo I, Rodriguez Sanchez F. Rational use of antibiotics in pediatrics: impact of a rapid test for detection of beta‐haemolytic group A streptococci in acute pharyngotonsillitis [Empleo racional de los antibioticos en pediatria: impacto de la aplicacion de un test rapido de deteccion de estreptococo beta‐hemolitico del grupo A en la faringoamigdalitis aguda]. Anales Espanoles de Pediatria 2000;52(3):212‐9. [PubMed] [Google Scholar]
Dagnelie 1998 {published data only}
- Dagnelie CF, Bartelink ML, Graaf Y, Goessens W, Melker RA. Towards a better diagnosis of throat infections (with group A beta‐haemolytic streptococcus) in general practice. British Journal of General Practice 1998;48(427):959‐62. [PMC free article] [PubMed] [Google Scholar]
Daly 1994 {published data only}
- Daly JA, Korgenski EK, Munson AC, Llausas‐Magana E. Optical immunoassay for streptococcal pharyngitis: evaluation of accuracy with routine and mucoid strains associated with acute rheumatic fever outbreak in the intermountain area of the United States. Journal of Clinical Microbiology 1994;32(2):531‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Della‐Latta 1994 {published data only}
- Della‐Latta P, Whittier S, Hosmer M, Agre F. Rapid detection of group A streptococcal pharyngitis in a pediatric population with optical immunoassay. Pediatric Infectious Disease Journal 1994;13(8):742‐3. [DOI] [PubMed] [Google Scholar]
Ding 2011 {published data only}
- Ding JY, Wang P. Methods for the rapid screening of group A streptococci: fluorescent in situ hybridization versus immunochromatography. Medical Principles and Practice 2011;20(6):504‐8. [DOI] [PubMed] [Google Scholar]
Dobkin 1987 {published data only}
- Dobkin D, Shulman ST. Evaluation of an ELISA for group A streptococcal antigen for diagnosis of pharyngitis. Journal of Pediatrics 1987;110(4):566‐9. [DOI] [PubMed] [Google Scholar]
Donatelli 1992a {published data only}
- Donatelli J, Macone A, Goldmann DA, Poon R, Hinberg I, Nanji A, et al. Rapid detection of group A streptococci: comparative performance by nurses and laboratory technologists in pediatric satellite laboratories using three test kits. Journal of Clinical Microbiology 1992;30(1):138‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donatelli 1992b {published data only}
- Donatelli J, Macone A, Goldmann DA, Poon R, Hinberg I, Nanji A, et al. Rapid detection of group A streptococci: comparative performance by nurses and laboratory technologists in pediatric satellite laboratories using three test kits. Journal of Clinical Microbiology 1992;30(1):138‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
dos Santos 2005 {published data only}
- dos Santos AGP, Berezin EN. Comparative analysis of clinical and laboratory methods for diagnosing streptococcal sore throat. Jornal de Pediatria 2005;81(1):23‐8. [PubMed] [Google Scholar]
Drulak 1988 {published data only}
- Drulak M, Raybould TJ, Yong J, Hsiung D, Smith H, Winston S. Comparison of Visuwell enzyme immunoassay to culture for detection of group A Streptococcus in throat swab specimens. Diagnostic Microbiology & Infectious Disease 1988;11(4):181‐7. [DOI] [PubMed] [Google Scholar]
Drulak 1991 {published and unpublished data}
- Drulak M, Bartholomew W, LaScolea L, Amsterdam D, Gunnersen N, Yong J, et al. Evaluation of the modified Visuwell Strep‐A enzyme immunoassay for detection of group‐A Streptococcus from throat swabs. Diagnostic Microbiology & Infectious Disease 1991;14(4):281‐5. [DOI] [PubMed] [Google Scholar]
Edmonson 2005 {published data only}
- Edmonson MB, Farwell KR. Relationship between the clinical likelihood of group A streptococcal pharyngitis and the sensitivity of a rapid antigen‐detection test in a pediatric practice. Pediatrics 2005;115(2):280‐5. [DOI] [PubMed] [Google Scholar]
Egger 1990a {published data only}
- Egger P, Siegrist CA, Strautmann G, Belli D, Auckenthaler R. Evaluation of two ELISA tests for the rapid detection of group A streptococci. European Journal of Pediatrics 1990;149(4):256‐8. [DOI] [PubMed] [Google Scholar]
Egger 1990b {published data only}
- Egger P, Siegrist CA, Strautmann G, Belli D, Auckenthaler R. Evaluation of two ELISA tests for the rapid detection of group A streptococci. European Journal of Pediatrics 1990;149(4):256‐8. [DOI] [PubMed] [Google Scholar]
Enright 2011 {published data only}
- Enright K, Kalima P, Taheri S. Should a near‐patient test be part of the management of pharyngitis in the pediatric emergency department?. Pediatric Emergency Care 2011;27(12):1148‐50. [DOI] [PubMed] [Google Scholar]
Ezike 2005 {published data only}
- Ezike EN, Rongkavilit C, Fairfax MR, Thomas RL, Asmar BI. Effect of using 2 throat swabs vs 1 throat swab on detection of group A streptococcus by a rapid antigen detection test. Archives of Pediatrics & Adolescent Medicine 2005;159(5):486‐90. [DOI] [PubMed] [Google Scholar]
Faverge 2004 {published data only}
- Faverge B, Marie‐Cosenza S, Bietrix M, Attou D, Bensekhria S, Dookna P. Use in hospital of a rapid diagnosis test of group A streptococcal pharyngotonsillitis in children [Utilisation à l’hôpital d’un test de diagnostic rapide des angines à streptocoque du groupe A de l’enfant]. Archives de Pédiatrie 2004;11(7):862‐3. [DOI] [PubMed] [Google Scholar]
Felsenstein 2014 {published data only}
- Felsenstein S, Faddoul D, Sposto R, Batoon K, Polanco CM, Dien Bard J. Molecular and clinical diagnosis of group A streptococcal pharyngitis in children. Journal of Clinical Microbiology 2014;52(11):3884‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finger 1999 {published data only}
- Finger R, Ho SH, Ngo TT, Ritchie CD, Nguyen TN. Rapid streptococcal testing in Vietnamese children with pharyngitis. Asia‐Pacific Journal of Public Health 1999;11(1):26‐9. [DOI] [PubMed] [Google Scholar]
Flores Mateo 2010 {published data only}
- Flores Mateo G, Conejero J, Grenzner Martinel E, Baba Z, Dicono S, Echasabal M, et al. Early diagnosis of streptococcal pharyngitis in paediatric practice: validity of a rapid antigen detection test [Diagnostico precoz de faringitis estreptococica en pediatria: validacion deuna tecnica antigenica rapida]. Atencion Primaria 2010;42(7):356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forward 2006 {published data only}
- Forward KR, Haldane D, Webster D, Mills C, Brine C, Aylward D. A comparison between the Strep A Rapid Test Device and conventional culture for the diagnosis of streptococcal pharyngitis. Canadian Journal of Infectious Diseases and Medical Microbiology 2006;17(4):221‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fourati 2009 {published data only}
- Fourati S, Smaoui H, Jegiurim H, Berriche I, Taghorti R, Ben Bader M, et al. Use of the rapid antigen detection test in group A streptococci pharyngitis diagnosis in Tunis, Tunisia [Utilisation d'un test de diagnostic rapide des angines à streptocoque béta‐hémolytique du groupe A, auprès d'un échantillon d'enfants à Tunis, Tunisie]. Bulletin de la Société de Pathologie Exotique 2009;102(3):175‐6. [PubMed] [Google Scholar]
Gerber 1990 {published data only}
- Gerber MA, Randolph MF, DeMeo KK. Liposome immunoassay for rapid identification of group A streptococci directly from throat swabs. Journal of Clinical Microbiology 1990;28(6):1463‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerber 1997 {published data only}
- Gerber MA, Tanz RR, Kabat W, Dennis E, Bell GL, Kaplan EL, et al. Optical immunoassay test for group A beta‐hemolytic streptococcal pharyngitis. An office‐based, multicenter investigation. JAMA 1997;277(11):899‐903. [PubMed] [Google Scholar]
Gieseker 2002a {published data only}
- Gieseker KE, Mackenzie T, Roe MH, Todd JK. Comparison of two rapid Streptococcus pyogenes diagnostic tests with a rigorous culture standard. Pediatric Infectious Disease Journal 2002;21(10):922‐7. [DOI] [PubMed] [Google Scholar]
Gieseker 2002b {published data only}
- Gieseker KE, Mackenzie T, Roe MH, Todd JK. Comparison of two rapid Streptococcus pyogenes diagnostic tests with a rigorous culture standard. Pediatric Infectious Disease Journal 2002;21(10):922‐7. [DOI] [PubMed] [Google Scholar]
Gieseker 2003 {published data only}
- Gieseker KE, Roe MH, MacKenzie T, Todd JK. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics 2003;111(6):e666‐70. [DOI] [PubMed] [Google Scholar]
Gurol 2010 {published data only}
- Gurol Y, Akan H, Izbirak G, Tekkanat ZT, Gunduz TS, Hayran O, et al. The sensitivity and the specifity of rapid antigen test in streptococcal upper respiratory tract infections. International Journal of Pediatric Otorhinolaryngology 2010;74(6):591‐3. [DOI] [PubMed] [Google Scholar]
Hall 2004 {published data only}
- Hall MC, Kieke B, Gonzales R, Belongia EA. Spectrum bias of a rapid antigen detection test for group A beta‐hemolytic streptococcal pharyngitis in a pediatric population. Pediatrics 2004;114(1):182‐6. [DOI] [PubMed] [Google Scholar]
Harris 1995 {published data only}
- Harris R, Paine D, Wittler R, Bruhn F. Impact on empiric treatment of group A streptococcal pharyngitis using an optical immunoassay. Clinical Pediatrics 1995;34(3):122‐7. [DOI] [PubMed] [Google Scholar]
Hart 1997 {published data only}
- Hart AP, Buck LL, Morgan S, Saverio S, McLaughlin JC. A comparison of the BioStar Strep A OIA rapid antigen assay, group A Selective Strep Agar (ssA), and Todd‐Hewitt broth cultures for the detection of group A Streptococcus in an outpatient family practice setting. Diagnostic Microbiology & Infectious Disease 1997;29(3):139‐45. [DOI] [PubMed] [Google Scholar]
Henderson 1988 {published data only}
- Henderson EL, Meier FA, Fortner CA, Dalton HP, Zanga JR. Physician bias and the interpretation of rapid tests for group‐A streptococcal pharyngitis. American Journal of Diseases of Children 1988;142(4):405‐6. [Google Scholar]
Kaltwasser 1997 {published data only}
- Kaltwasser G, Diego J, Welby‐Sellenriek PL, Ferrett R, Caparon M, Storch GA. Polymerase chain reaction for Streptococcus pyogenes used to evaluate an optical immunoassay for the detection of group A streptococci in children with pharyngitis. Pediatric Infectious Disease Journal 1997;16(8):748‐53. [DOI] [PubMed] [Google Scholar]
Kaufhold 1991a {published data only}
- Kaufhold A, Krug E, Lutticken R, Knoop U, Blaker F. Evaluation of a rapid test for direct detection of group A streptococcal antigen in throat swabs. Study of 4 commercial test systems. Monatsschrift Kinderheilkunde 1991;139(4):208‐13. [PubMed] [Google Scholar]
Kaufhold 1991b {published data only}
- Kaufhold A, Krug E, Lutticken R, Knoop U, Blaker F. Evaluation of a rapid test for direct detection of group A streptococcal antigen in throat swabs. Study of 4 commercial test systems. Monatsschrift Kinderheilkunde 1991;139(4):208‐13. [PubMed] [Google Scholar]
Kellog 1987 {published data only}
- Kellogg JA, Landis RC, Nussbaum AS, Bankert DA. Performance of an enzyme immunoassay test and anaerobic culture for detection of group A streptococci in a pediatric practice versus a hospital laboratory. Journal of Pediatrics 1987;111(1):18‐21. [DOI] [PubMed] [Google Scholar]
Kellog 1991 {published data only}
- Kellogg JA, Bankert DA, Schonauer TD, Landis RC, Nussbaum AS, Levisky JS. Detection of group A streptococci by aerobic culture and a new simplified immunoassay in three pediatric practices and a hospital laboratory. Journal of Clinical Laboratory Analysis 1991;5(5):367‐71. [DOI] [PubMed] [Google Scholar]
Kim 2009 {published data only}
- Kim S. The evaluation of SD Bioline Strep A rapid antigen test in acute pharyngitis in pediatric clinics. Korean Journal Of Laboratory Medicine 2009;29(4):320‐3. [DOI] [PubMed] [Google Scholar]
Küçük 2014 {published data only}
- Küçük O, Biçer S, Giray T, Cöl D, Erdag GC, Gürol Y, et al. Validity of rapid antigen detection testing in group A beta‐hemolytic streptococcal tonsillopharyngitis. Indian Journal of Pediatrics 2014;81(2):138‐42. [DOI] [PubMed] [Google Scholar]
Kuhn 1999 {published data only}
- Kuhn S, Davies HD, Katzko G, Jadavji T, Church DL. Evaluation of the Strep A OIA assay versus culture methods: ability to detect different quantities of group A Streptococcus. Diagnostic Microbiology & Infectious Disease 1999;34(4):275‐80. [DOI] [PubMed] [Google Scholar]
Kurtz 2000 {published data only}
- Kurtz B, Kurtz M, Roe M, Todd J. Importance of inoculum size and sampling effect in rapid antigen detection for diagnosis of Streptococcus pyogenes pharyngitis. Journal of Clinical Microbiology 2000;38(1):279‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laubscher 1995 {published data only}
- Laubscher B, Melle G, Dreyfuss N, Crousaz H. Evaluation of a new immunologic test kit for rapid detection of group A streptococci, the Abbott Testpack Strep A plus. Journal of Clinical Microbiology 1995;33(1):260‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewey 1988 {published data only}
- Lewey S, White CB, Lieberman MM, Morales E. Evaluation of the throat culture as a follow‐up for an initially negative enzyme immunosorbent assay rapid streptococcal antigen detection test. Pediatric Infectious Disease Journal 1988;7(11):765‐9. [DOI] [PubMed] [Google Scholar]
Llor 2008 {published data only}
- Llor C, Hernandez Anadon S, Gomez Bertomeu FF, Santamaria Puig JM, Calvino Dominguez O, Fernandez Pages Y. Validation of a rapid antigenic test in the diagnosis of pharyngitis caused by group A beta‐haemolytic Streptococcus. Atencion Primaria 2008;40(10):489‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macknin 1988 {published data only}
- Macknin ML, Indich N, Easley KA, Imrie R, Shapiro DJ. Comparison of two rapid diagnostic tests for group A streptococcus. Pediatric Infectious Disease Journal 1988;7(10):735‐6. [DOI] [PubMed] [Google Scholar]
Maltezou 2008 {published data only}
- Maltezou HC, Tsagris V, Antoniadou A, Galani L, Douros C, Katsarolis I, et al. Evaluation of a rapid antigen detection test in the diagnosis of streptococcal pharyngitis in children and its impact on antibiotic prescription. Journal of Antimicrobial Chemotherapy 2008;62(6):1407‐12. [DOI] [PubMed] [Google Scholar]
Mayes 2001a {published data only}
- Mayes T, Pichichero ME. Are follow‐up throat cultures necessary when rapid antigen detection tests are negative for group A streptococci?. Clinical Pediatrics 2001;40(4):191‐5. [DOI] [PubMed] [Google Scholar]
Mayes 2001b {published data only}
- Mayes T, Pichichero ME. Are follow‐up throat cultures necessary when rapid antigen detection tests are negative for group A streptococci?. Clinical Pediatrics 2001;40(4):191‐5. [DOI] [PubMed] [Google Scholar]
Mazur 2014 {published data only}
- Mazur E, Bochynska E, Juda M, Koziol‐Montewka M. Empirical validation of Polish guidelines for the management of acute streptococcal pharyngitis in children. International Journal of Pediatric Otorhinolaryngology 2014;78(1):102‐6. [DOI] [PubMed] [Google Scholar]
McIsaac 2004 {published data only}
- McIsaac WJ, Kellner JD, Aufricht P, Vanjaka A, Low DE. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA 2004;291(13):1587‐95. [DOI] [PubMed] [Google Scholar]
Menozzi 1992 {published data only}
- Menozzi MC, Zanacca C, Boschi G, Carmeli G, Capatti C, Morini C. Evaluation of a kit for rapid detection of S. pyogenes in throat swabs. New perspectives on streptococci and streptococcal infections: proceedings of the XI Lancefield International Symposium on Streptococci and Streptococcal Diseases (Orefici G, ed). 1992:106‐7.
Mezghani Maleej 2010 {published data only}
- Mezghani Maalej S, Rekik M, Boudaouara M, Jardak N, Turki S, Arous R, et al. Childhood pharyngitis in Sfax (Tunisia): epidemiology and utility of a rapid streptococcal test. Médecine et Maladies Infectieuses 2010;40(4):226‐31. [DOI] [PubMed] [Google Scholar]
Mirza 2007a {published data only}
- Mirza A, Wludyka P, Chiu TT, Rathore MH. Throat culture is necessary after negative rapid antigen detection tests. Clinical Pediatrics 2007;46(3):241‐6. [DOI] [PubMed] [Google Scholar]
Mirza 2007b {published data only}
- Mirza A, Wludyka P, Chiu TT, Rathore MH. Throat culture is necessary after negative rapid antigen detection tests. Clinical Pediatrics 2007;46(3):241‐6. [DOI] [PubMed] [Google Scholar]
Mlejnek 2014 {published and unpublished data}
- Mlejnek JR, Almulhem K, Spadafore S. Utility and cost effectiveness of throat culture in the treatment of patients with negative rapid strep screens. Academic Emergency Medicine 2014;21(Suppl 1):51. [Google Scholar]
Moyer 1990 {published data only}
- Moyer NP, Quinn PJ, Showalter CA. Evaluation of the Directigen 1,2,3 Group A Strep Test for diagnosis of streptococcal pharyngitis. Journal of Clinical Microbiology 1990;28(7):1661‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Needham 1998 {published data only}
- Needham CA, McPherson KA, Webb KH. Streptococcal pharyngitis: impact of a high‐sensitivity antigen test on physician outcome. Journal of Clinical Microbiology 1998;36(12):3468‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nitsch‐Osuch 2010 {published data only}
- Nitsch‐Osuch A, Dyk S, Gyrczuk E, Wardyn K, Zycinska K. Validity of a rapid streptococcal test (Test Strep A) in children with acute pharyngitis treated in an outpatient setting. Abstract book of the Annual meeting of the European Society for Paediatric Infectious Diseases. 2010:207.
Nonaka 1988 {published data only}
- Nonaka C, Togasaki K, Ushijima H, Kon Y, Kawaoi T. Rapid diagnosis of the group A streptococcal infection‐‐comparison with culture method, latex agglutination and enzyme immunoassay. Kansenshogaku Zasshi 1988;62(1):32‐8. [DOI] [PubMed] [Google Scholar]
Pauchard 2012 {published and unpublished data}
- Pauchard JY, Verga ME, Bersier J, Prod'Hom G, Gehri M, Vaudaux B. Performance of rapid antigen diagnostic test for group A β‐haemolytic streptococcal pharyngitis in a tertiary paediatric emergency department. Swiss Medical Weekly 2012;142(Suppl 192):35S. [Google Scholar]
Pauchard 2013 {published and unpublished data}
- Pauchard JY, Verga ME, Bersier J, Durusell C, Gehri M, Vaudaux B. Performance of rapid antigen detection test in group A β‐haemolytic streptococcal pharyngitis in comparison with three clinical decision rule in a tertiary paediatric emergency department. Swiss Medical Weekly 2013;143(Suppl 197):6S. [Google Scholar]
Pitetti 1998 {published data only}
- Pitetti RD, Drenning SD, Wald ER. Evaluation of a new rapid antigen detection kit for group A beta‐hemolytic streptococci. Pediatric Emergency Care 1998;14(6):396‐8. [DOI] [PubMed] [Google Scholar]
Ramos 2011 {published data only}
- Ramos JL, Fraile MT, Chanza M, Tormo N, Lurbe A, Gimeno C. Rapid detection of Streptococcus pyogenes in peripheral medical centres. A pilot custody assay. Clinical Microbiology and Infection 2011;17:S250. [Google Scholar]
Regueras De Lorenzo 2012 {published data only}
- Regueras De Lorenzo G, Santos Rodriguez PM, Villa Bajo L, Perez Guirado A, Arbesu Fernandez E, Barreiro Hurle L, et al. Use of the rapid antigen technique in the diagnosis of Streptococcus pyogenes pharyngotonsillitis [Utilidad de una técnica antigénica rápida en el diagnósticode faringoamigdalitis por Streptococcus pyogenes]. Anales de Pediatria 2012;77(3):193‐9. [DOI] [PubMed] [Google Scholar]
Reinert 1988 {published data only}
- Reinert P, Cohen R, Rocque F, Bouhanna A, Geslin P. Value of rapid tests for the detection of streptococcal infections. Rhinology 1988;5(Suppl):75‐9. [PubMed] [Google Scholar]
Rimoin 2010a {published data only}
- Rimoin AW, Walker CLF, Hamza HS, Elminawi N, Ghafar HA, Vince A, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low‐resource settings. International Journal of Infectious Diseases 2010;14:e1048‐53. [DOI] [PubMed] [Google Scholar]
Rimoin 2010b {published data only}
- Rimoin AW, Walker CLF, Hamza HS, Elminawi N, Ghafar HA, Vince A, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low‐resource settings. International Journal of Infectious Diseases 2010;14:e1048‐53. [DOI] [PubMed] [Google Scholar]
Rimoin 2010c {published data only}
- Rimoin AW, Walker CLF, Hamza HS, Elminawi N, Ghafar HA, Vince A, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low‐resource settings. International Journal of Infectious Diseases 2010;14:e1048‐53. [DOI] [PubMed] [Google Scholar]
Rimoin 2010d {published data only}
- Rimoin AW, Walker CLF, Hamza HS, Elminawi N, Ghafar HA, Vince A, et al. The utility of rapid antigen detection testing for the diagnosis of streptococcal pharyngitis in low‐resource settings. International Journal of Infectious Diseases 2010;14:e1048‐53. [DOI] [PubMed] [Google Scholar]
Roddey 1995 {published data only}
- Roddey OF, Clegg HW, Martin ES, Swetenburg RL, Koonce EW. Comparison of an optical immunoassay technique with two culture methods for the detection of group A streptococci in a pediatric office. Journal of Pediatrics 1995;126(6):931‐3. [DOI] [PubMed] [Google Scholar]
Roe 1995a {published data only}
- Roe M, Kishiyama C, Davidson K, Schaefer L, Todd J. Comparison of BioStar Strep A OIA optical immune assay, Abbott TestPack Plus Strep A, and culture with selective media for diagnosis of group A streptococcal pharyngitis. Journal of Clinical Microbiology 1995;33(6):1551‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roe 1995b {published data only}
- Roe M, Kishiyama C, Davidson K, Schaefer L, Todd J. Comparison of BioStar Strep A OIA optical immune assay, Abbott TestPack Plus Strep A, and culture with selective media for diagnosis of group A streptococcal pharyngitis. Journal of Clinical Microbiology 1995;33(6):1551‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogo 2010a {published data only}
- Rogo T, Schwartz RH, Ascher DP. Comparison of the Inverness Medical Acceava Strep A Test with the Genzyme OSOM and Quidel QuickVue Strep A Tests. Clinical Pediatrics 2010;49(11):1050‐2. [DOI] [PubMed] [Google Scholar]
Rogo 2010b {published data only}
- Rogo T, Schwartz RH, Ascher DP. Comparison of the Inverness Medical Acceava Strep A Test with the Genzyme OSOM and Quidel QuickVue Strep A Tests. Clinical Pediatrics 2010;49(11):1050‐2. [DOI] [PubMed] [Google Scholar]
Rogo 2010c {published data only}
- Rogo T, Schwartz RH, Ascher DP. Comparison of the Inverness Medical Acceava Strep A Test with the Genzyme OSOM and Quidel QuickVue Strep A Tests. Clinical Pediatrics 2010;49(11):1050‐2. [DOI] [PubMed] [Google Scholar]
Savoia 1994 {published data only}
- Savoia D, Francesetti C, Millesima M, Dotti G, Gatti G, Rurali C. Evaluation of the diagnostic accuracy of a kit for the rapid detection of group A streptococci. Microbios 1994;77(313):253‐9. [PubMed] [Google Scholar]
Schlager 1996 {published data only}
- Schlager TA, Hayden GA, Woods WA, Dudley SM, Hendley JO. Optical immunoassay for rapid detection of group A beta‐hemolytic streptococci. Should culture be replaced?. Archives of Pediatrics and Adolescent Medicine 1996;150(3):245‐8. [DOI] [PubMed] [Google Scholar]
Schwabe 1987 {published data only}
- Schwabe LD, Small MT, Randall EL. Comparison of TestPack Strep A test kit with culture technique for detection of group A streptococci. Journal of Clinical Microbiology 1987;25(2):309‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwabe 1991 {published data only}
- Schwabe LD, Gobbo AF, Gottschall RL, Randall EL. Comparison of TestPack Plus Strep A with selective and nonselective culture media for detection of group‐A streptococci. Diagnostic Microbiology and Infectious Disease 1991;14(5):367‐72. [DOI] [PubMed] [Google Scholar]
Schwartz 1997a {published data only}
- Schwartz RH. Evaluation of rapid streptococcal detection tests. Pediatric Infectious Disease Journal 1997;16(11):1099‐100. [DOI] [PubMed] [Google Scholar]
Schwartz 1997b {published data only}
- Schwartz RH. Evaluation of rapid streptococcal detection tests. Pediatric Infectious Disease Journal 1997;16(11):1099‐100. [DOI] [PubMed] [Google Scholar]
Sedki 2010 {published data only}
- Sedki M, Salama H, Salama E, Abdalla N, Ezz H. Rapid diagnostic test for streptococcal throat infection in Egyptian children. Medical Journal of Cairo University 2010;78(2):177‐82. [Google Scholar]
Strandjord 1987 {published data only}
- Strandjord TP, Rich EJ, Quan L. Comparison of two antigen detection techniques for group A streptococcal pharyngitis in a pediatric emergency department. Pediatric Infectious Disease Journal 1987;6(11):1071‐2. [PubMed] [Google Scholar]
Subashini 2015 {published data only}
- Subashini B, Anandan S, Balaji V. Evaluation of a rapid antigen detection test for the diagnosis of group‐A beta‐hemolytic Streptococcus in pharyngotonsillitis. Journal of Global Infectious Diseases 2015;7(2):91‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanz 2009 {published data only}
- Tanz RR, Gerber MA, Kabat W, Rippe J, Seshadri R, Shulman ST. Performance of a rapid antigen‐detection test and throat culture in community pediatric offices: implications for management of pharyngitis. Pediatrics 2009;123(2):437‐44. [DOI] [PubMed] [Google Scholar]
Tellechea 2012 {published data only}
- Tellechea AL, Salvo MG, Mendez JH, Cavagnari BM. Group A beta‐hemolytic Streptococcus frequency in the throat of symptomatic patients younger than 15 years, by age group. Archivos Argentinos de Pediatria 2012;110(6):516‐9. [DOI] [PubMed] [Google Scholar]
Tenjarla 1991 {published data only}
- Tenjarla G, Kumar A, Dyke JW. TestPack Strep A kit for the rapid detection of group A streptococci on 11,088 throat swabs in a clinical pathology laboratory. American Journal of Clinical Pathology 1991;96(6):759‐61. [DOI] [PubMed] [Google Scholar]
Toepfner 2013 {published data only}
- Toepfner N, Henneke P, Berner R, Hufnagel M. Impact of technical training on rapid antigen detection tests (RADT) in group A streptococcal tonsillopharyngitis. European Journal of Clinical Microbiology and Infectious Diseases 2013;32(5):609‐11. [DOI] [PubMed] [Google Scholar]
Van Limbergen 2006 {published data only}
- Limbergen J, Kalima P, Taheri S, Beattie TF. Streptococcus A in paediatric accident and emergency: are rapid streptococcal tests and clinical examination of any help?. Emergency Medicine Journal 2006;23(1):32‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 1989 {published data only}
- Wong T, Tiessen E. Evaluation of TestPack Strep A for rapid identification of group A streptococci. Canadian Family Physician 1989;35(Sep):1767‐70. [PMC free article] [PubMed] [Google Scholar]
Wright 2007a {published data only}
- Wright M, Williams G, Ludeman L. Comparison of two rapid tests for detecting group A streptococcal pharyngitis in the pediatric population at Wright‐Patterson air force base. Military Medicine 2007;172(6):644‐6. [DOI] [PubMed] [Google Scholar]
Wright 2007b {published data only}
- Wright M, Williams G, Ludeman L. Comparison of two rapid tests for detecting group A streptococcal pharyngitis in the pediatric population at Wright‐Patterson air force base. Military Medicine 2007;172(6):644‐6. [DOI] [PubMed] [Google Scholar]
Yuckienuz 1988 {published data only}
- Yuckienuz SA, Thorne GM, Macone AB, Goldmann DA, Pierre J, Marcus EP. Performance of a solid phase enzyme immunoassay for detection of group A streptococci in a pediatric office laboratory as refereed by a hospital laboratory. Pediatric Infectious Disease Journal 1988;7(6):393‐8. [DOI] [PubMed] [Google Scholar]
Zanacca 1992 {published data only}
- Zanacca C, Boschi G, Capatti C, Carmeli G, Corsini M, Morini C. Liposome technology for a rapid diagnosis of streptococcal pharyngitis. New perspectives on streptococci and streptococcal infections: proceedings of the XI Lancefield International Symposium on Streptococci and Streptococcal Diseases (Orefici G, ed). 1992:104‐5.
References to studies excluded from this review
Abu‐Sabaah 2006 {published data only}
- Abu‐Sabaah AH, Ghazi HO. Better diagnosis and treatment of throat infections caused by group A beta‐haemolytic streptococci. British Journal of Biomedical Science 2006;63:155‐8. [DOI] [PubMed] [Google Scholar]
Andersen 1994 {published data only}
- Andersen JS, Borrild NJ, Hoffmann S. Diagnosis of sore throat. A multipractice study of 3 different ways of antigenic determination for detection of group A streptococci in throat swabs. Ugeskrift for Laeger 1994;156:6869‐73. [PubMed] [Google Scholar]
Andersen 2003a {published data only}
- Andersen JB, Dahm TL, Nielsen CT, Frimodt‐Moller N. Diagnosis of streptococcal tonsillitis in the pediatric department with the help of antigen detection test. Ugeskrift for Laeger 2003;165:2291‐5. [PubMed] [Google Scholar]
Andersen 2003b {published data only}
- Andersen JB, Dahm TL, Nielsen CT, Frimodt‐Moller N. Antigen detection test for the diagnosis of streptococcal tonsillitis in the paediatric hospital department. Ugeskrift for Laeger 2003;165:2291‐5. [PubMed] [Google Scholar]
Anhalt 1992 {published data only}
- Anhalt JP, Heiter BJ, Naumovitz DW, Bourbeau PP. Comparison of three methods for detection of group A streptococci in throat swabs. Journal of Clinical Microbiology 1992;30:2135‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 1985a {published data only}
- Anonymous. Rapid office diagnostic tests for streptococcal pharyngitis. Medical Letter on Drugs & Therapeutics 1985;27:49‐51. [PubMed] [Google Scholar]
Anonymous 1985b {published data only}
- Anonymous. Rapid diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1985;107:154‐6. [DOI] [PubMed] [Google Scholar]
Anonymous 1985c {published data only}
- Anonymous. Rapid diagnostic assay for strep throat. Analytical Chemistry 1985;57:1372A‐4A. [DOI] [PubMed] [Google Scholar]
Anonymous 1986 {published data only}
- Anonymous. Rapid detection of beta haemolytic streptococci. Lancet 1986;1:247‐8. [PubMed] [Google Scholar]
Anonymous 1991 {published data only}
- Anonymous. Rapid diagnostic tests for group A streptococcal pharyngitis. Medical Letter on Drugs & Therapeutics 1991;33:40‐1. [PubMed] [Google Scholar]
Anonymous 1992 {published data only}
- Anonymous. Rapid antigen test vs. culture in streptococcal pharyngitis. American Family Physician 1992;46:552. [Google Scholar]
Araj 1986 {published data only}
- Araj GF, Majeed HA. Evaluation of a two‐minute strep A direct swab test (SADST) on patients with pharyngitis at a primary care clinic. Journal of Hygiene 1986;97:133‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Araujo 2005 {published data only}
- Araujo Filho BC, Imamura R, Sennes LU, Sakae FA. Role of rapid antigen detection test for the diagnosis of group A beta‐hemolytic streptococcus in patients with pharyngotonsillitis. Revista Brasileira de Otorrinolaringologia 2005;71:168‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Armengol 2004a {published data only}
- Armengol CE, Hendley JO, Schlager TA. Could repetition of the rapid antigen detection test for group a streptococci on a second swab replace the backup throat culture?. Pediatric Research 2004;55:341A. [DOI] [PubMed] [Google Scholar]
Armengol 2004b {published data only}
- Armengol CE, Schlager TA, Hendley JO. Sensitivity of a rapid antigen detection test for group A streptococci in a private pediatric office setting: answering the Red Book's request for validation. Pediatrics 2004;113:924‐6. [DOI] [PubMed] [Google Scholar]
Arya 1993 {published data only}
- Arya SC. Treatment of streptococcal sore throat. Immunoassays for rapid diagnosis. BMJ 1993;306:1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atlas 2005 {published data only}
- Atlas SJ, McDermott SM, Mannone C, Barry MJ. The role of point of care testing for patients with acute pharyngitis. Journal of General Internal Medicine 2005;20:759‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ausina 1987 {published data only}
- Ausina V, Coll P. Rapid technics for antigen detection in acute streptococcal pharyngitis. Medicina Clinica 1987;89:413‐4. [PubMed] [Google Scholar]
Badgett 1996 {published data only}
- Badgett JT, Hesterberg LK. Management of group A streptococcus pharyngitis with a second‐generation rapid strep screen: Strep A OIA. Microbial Drug Resistance 1996;2:371‐6. [DOI] [PubMed] [Google Scholar]
Baker 1995 {published data only}
- Baker DM, Cooper RM, Rhodes C, Weymouth LA, Dalton HP. Superiority of conventional culture technique over rapid detection of group A Streptococcus by optical immunoassay. Diagnostic Microbiology & Infectious Disease 1995;21:61‐4. [DOI] [PubMed] [Google Scholar]
Ba‐Saddik 2014 {published data only}
- Ba‐Saddik IA, Munibari AA, Alhilali AM, Ismail SM, Murshed FM, Coulter JB. Prevalence of Group A beta‐haemolytic Streptococcus isolated from children with acute pharyngotonsillitis in Aden, Yemen. Tropical Medicine & International Health 2014;19:431‐9. [DOI] [PubMed] [Google Scholar]
Baselski 1988 {published data only}
- Baselski VS, Hansen VR, Ikner SB, Osborne PT. Concurrent evaluation of 4 rapid group A streptococcus tests. American Journal of Clinical Pathology 1988;89:447. [Google Scholar]
Berger‐Jekic 1987 {published data only}
- Berger‐Jekic O. A direct, rapid method for the identification of Streptococcus pyogenes from throat smears. Srpski Arhiv Za Celokupno Lekarstvo 1987;115:927‐34. [PubMed] [Google Scholar]
Berke 1989 {published data only}
- Berke CM. Development of rapid strep test technology. Pediatric Infectious Disease Journal 1989;8:825‐8. [DOI] [PubMed] [Google Scholar]
Betriu 1988 {published data only}
- Betriu C, Torre F, Munoz P, Fernandez A, Picazo JJ. Evaluation of four methods for the detection of streptococcal group A antigen directly from throat swabs. Microbiologia 1988;4:177‐9. [PubMed] [Google Scholar]
Betriu 1989 {published data only}
- Betriu Cabeceran C, Igea Benito A, Picazo de la Garza JJ, Valor Perea R. Comparative study of 2 rapid antigenic technics for diagnosing streptococcal pharyngitis. Revista Clinica Espanola 1989;184:389. [PubMed] [Google Scholar]
Bischoff 2007 {published data only}
- Bischoff A. Diagnosis of streptococcal tonsillitis. Rapid test prevents treatment error. MMW Fortschritte der Medizin 2007;149:17. [PubMed] [Google Scholar]
Bjerrum 2013 {published data only}
- Bjerrum L, Cordoba Currea GC, Llor C, Lindbaek M. Lower threshold for rapid antigen detection testing in patients with sore throats would reduce antibiotic use. BMJ 2013;347:f7055. [DOI] [PubMed] [Google Scholar]
Blade 1991 {published data only}
- Blade J, Alaman E, Cartana A, Guinea I, Liberal A, Herreros M. Evaluation of clinical data and a technique of rapid detection (TestPack Strep A) in the diagnosis of acute streptococcal pharyngo‐tonsillitis. Atencion Primaria 1991;8:92, 94, 96‐8. [PubMed] [Google Scholar]
Blanco 1988 {published data only}
- Blanco JMA, Segui JEF, Vicente VB, Sagastibelza EC, Martinez LAG, Montes JM. Rapid diagnosis of pharyngoamigdalitis by ELISA (Test Pack Strep‐A) ‐ comparison with culture and clinical‐data ‐ analysis of 306 cases. Medicina Clinica 1988;91:561‐4. [PubMed] [Google Scholar]
Boccazzi 2011 {published data only}
- Boccazzi A, Garotta M, Pontari S, Agostoni CV. Streptococcal tonsillopharyngitis: clinical vs. microbiological diagnosis. Infezioni in Medicina 2011;19:100‐5. [PubMed] [Google Scholar]
Bodino 1987 {published data only}
- Bodino JA, Lopez EL, Rubeglio E, Giavedoni CG. Evaluation of a rapid test for group A Streptococcus at a physician's office and hospital laboratory in Buenos Aires, Argentina. Pediatric Infectious Disease Journal 1987;6:762‐4. [PubMed] [Google Scholar]
Boss 1992 {published data only}
- Boss DJ. Culture and antigen detection tests for streptococcal tonsillopharyngitis. American Family Physician 1992;46:1658; author reply 1658‐9, 1662. [PubMed] [Google Scholar]
Bourbeau 1993 {published data only}
- Bourbeau PP, Heiter BJ, Anhalt JP, Naumovitz DW. Comparison of direct specimen testing utilizing TestPack strep A with testing of specimens following a two‐hour broth enrichment. Diagnostic Microbiology & Infectious Disease 1993;17:93‐6. [DOI] [PubMed] [Google Scholar]
Brahmadathan 1986 {published data only}
- Brahmadathan KN, Pandian R, Joseph A, Koshi G. Use of plastic kits for rapid recovery of streptococci in epidemiological studies. Indian Journal of Medical Research 1986;84:331‐3. [PubMed] [Google Scholar]
Burke 1988 {published data only}
- Burke P, Bain J, Lowes A, Athersuch R. Rational decisions in managing sore throat: evaluation of a rapid test. BMJ 1988;296:1646‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvino 2015 {published data only}
- Calvino DO, Hernandez AS, Teresa MBM, Hernandez AM. Validation Analyz‐Strep A Rapid Test in the diagnosis of acute pharyngitis. Atencion Primaria 2015;47:69‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardoso 2013 {published data only}
- Cardoso DM, Gilio AE, Hsin SH, Machado BM, Paulis M, Lotufo JPB. Impact of the rapid antigen detection test in diagnosis and treatment of acute pharyngotonsillitis in a pediatric emergency room. Revista Paulista de Pediatria 2013;31:4‐9. [DOI] [PubMed] [Google Scholar]
Carey 1991 {published data only}
- Carey RD, Tilyard MW, Morris RW. Evaluation of a rapid diagnostic test for group A beta‐haemolytic streptococcus in general practice. New Zealand Medical Journal 1991;104:401‐3. [PubMed] [Google Scholar]
Centor 1984 {published data only}
- Centor RM, Dalton HP, Campbell MS, Lynch MR. Rapid diagnosis of group A beta streptococcal throat infection. Clinical Research 1984;32:A841. [Google Scholar]
Centor 1985 {published data only}
- Centor RM, Garner BK, Campbell MS, Lynch MR, Dalton HP. Rapid diagnosis of group‐A beta‐streptococcal throat infection ‐ could the gold standard be fools gold. Clinical Research 1985;33:A245. [Google Scholar]
Chen 2000 {published data only}
- Chen FM. Culture confirmation of negative rapid strep test results. Journal of Family Practice 2000;49:371‐2. [PubMed] [Google Scholar]
Chessman 1998 {published data only}
- Chessman A. A streptococcal antigen detection test had low sensitivity and high specificity for detecting group A (beta)‐haemolytic streptococcus. Evidence‐Based Medicine 1998;3:190. [Google Scholar]
Choi 1995 {published data only}
- Choi YH, Lee C, Jung JA, Kang J. A rapid immunochromatographic assay for detection of group‐A streptococci. Clinical Chemistry 1995;41(Suppl):72‐3. [Google Scholar]
Coban 2013 {published data only}
- Coban B, Kaplan H, Topal B, Ulku N. The sensitivity and the specifity of rapid antigen test in group A streptococcal tonsillopharyngitis. Cocuk Enfeksiyon Dergisi 2013;7:143‐6. [Google Scholar]
Cohen 1993 {published data only}
- Cohen R, Geslin P. Rapid diagnostic tests of Streptococcus group A in pharyngitis. Value and limitations. Revue du Praticien 1993;43:2233‐5. [PubMed] [Google Scholar]
Cohen 2000 {published data only}
- Cohen R. Rapid diagnostic tests of community‐acquired infection. Archives de Pédiatrie 2000;7(Suppl 2):325‐7. [DOI] [PubMed] [Google Scholar]
Cohen 2012a {published data only}
- Cohen JF, Levy C, Bidet P, Benani M, Thollot F, Koskas M. Sensitivity of rapid diagnostic test for group A streptococcus in healthy carriers and children with pharyngitis. Archives de Pédiatrie 2012;19:H143‐4. [Google Scholar]
Cohen 2013a {published data only}
- Cohen JF, Chalumeau M, Levy C, Bidet P, Benani M, Koskas M. Effect of clinical spectrum, inoculum size and physician characteristics on sensitivity of a rapid antigen detection test for group A streptococcal pharyngitis. European Journal of Clinical Microbiology and Infectious Diseases 2013;32:787‐93. [DOI] [PubMed] [Google Scholar]
Corneli 2001 {published data only}
- Corneli HM. Rapid strep tests in the emergency department: an evidence‐based approach. Pediatric Emergency Care 2001;17:272‐8. [DOI] [PubMed] [Google Scholar]
Dale 1994 {published data only}
- Dale JC, Vetter EA, Contezac JM, Iverson LK, Wollan PC, Cockerill FR 3rd. Evaluation of two rapid antigen assays, BioStar Strep A OIA and Pacific Biotech CARDS O.S., and culture for detection of group A streptococci in throat swabs. Journal of Clinical Microbiology 1994;32:2698‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 1997 {published data only}
- Dale JC, Wollan P, Cockerill FR 3rd. Use of optical immunoassay to diagnose streptococcal pharyngitis. JAMA 1997;278:23‐4. [PubMed] [Google Scholar]
De Lorenzo 2012 {published data only}
- Lorenzo GR, Rodriguez PMS, Bajo LV, Guirado AP, Fernandez EA, Hurle LB. Use of the rapid antigen technique in the diagnosis of Streptococcus pyogenes pharyngotonsillitis. Anales De Pediatria 2012;77:193‐9. [DOI] [PubMed] [Google Scholar]
Demeyere 1992 {published data only}
- Demeyere M, Blondeel L, Bellon J, Verschraegen G. Directigen 1‐2‐3 group‐A Streptest (DGAST) ‐ new liposome immunoassay for rapid identification of group‐A streptococci directly from throat swabs. In: Orefici G editor(s). New Perspectives on Streptococci and Streptococcal Infections. 22. Stuttgart: Gustav Fischer, 1992:101‐3. [Google Scholar]
Diaz‐Berenguer 1992 {published data only}
- Diaz‐Berenguer JA, Ibrahim F. Evaluation of a rapid technique for detecting the type A Streptococcus antigen (Test Pack Strep A). Atencion Primaria 1992;9:245‐9. [PubMed] [Google Scholar]
Dimatteo 2001 {published data only}
- Dimatteo LA, Lowenstein SR, Brimhall B, Reiquam W, Gonzales R. The relationship between the clinical features of pharyngitis and the sensitivity of a rapid antigen test: evidence of spectrum bias. Annals of Emergency Medicine 2001;38:648‐52. [DOI] [PubMed] [Google Scholar]
Dingle 2014 {published data only}
- Dingle TC, Abbott AN, Fang FC. Reflexive culture in adolescents and adults with group A streptococcal pharyngitis. Clinical Infectious Diseases 2014;59:643‐50. [DOI] [PubMed] [Google Scholar]
DiNicola 1986 {published data only}
- DiNicola AF. Putting rapid group A strep throat screening tests into perspective. American Journal of Diseases of Children 1986;140:852. [DOI] [PubMed] [Google Scholar]
DuBois 1986 {published data only}
- DuBois D, Ray VG, Nelson B, Peacock JB. Rapid diagnosis of group A strep pharyngitis in the emergency department. Annals of Emergency Medicine 1986;15:157‐9. [DOI] [PubMed] [Google Scholar]
DuBose 1996 {published data only}
- DuBose Ravenel S, Ellis GC, Michal WN. Rapid streptococcal tests. Pediatrics 1996;97:288. [PubMed] [Google Scholar]
Eaton 1987 {published data only}
- Eaton CB. Rapid diagnostic test and throat cultures for streptococcal pharyngitis. Journal of Family Practice 1987;24:342‐3. [PubMed] [Google Scholar]
Edmonson 2003 {published data only}
- Edmonson MB, Weix KR. Relationship of pre‐test likelihood of group A streptococcal (GAS) pharyngitis and sensitivity of a rapid antigen detection test (RADT) in pediatric practice. Pediatric Research 2003;53:180A. [DOI] [PubMed] [Google Scholar]
Edouard 2014 {published data only}
- Edouard S, Michel‐Lepage A, Raoult D. Does it make sense to detect Streptococcus pyogenes during tonsillitis in Europe to prevent acute rheumatic fever?. Clinical Microbiology and Infection 2014;20(12):O981‐2. [DOI] [PubMed] [Google Scholar]
Ehrlich 1993 {published data only}
- Ehrlich TP, Schwartz RH, Wientzen R, Thorne MM. Comparison of an immunochromatographic method for rapid identification of group A streptococcal antigen with culture method. Archives of Family Medicine 1993;2:866‐9. [DOI] [PubMed] [Google Scholar]
Enright 2009 {published data only}
- Enright K, Taheri S, Beattie T. Emergency department testing for Streptococcus in children with sore throats. Emergency Medicine Journal 2009;26:310. [DOI] [PubMed] [Google Scholar]
Esteban 2004 {published data only}
- Esteban M, Pertierra A, Garcia‐Tornel S, Gaspa J. Rapid detection tests for pharyngotonsillitis. Pediatria Catalana 2004;64:141‐2. [Google Scholar]
Fellah 1988 {published data only}
- Fellah H, Benslimane A, Jai J, Veysseyre C, Carraz M. Evaluation of a fast test for direct research on streptococcal group A from pharyngeal samples. Pathologie Biologie 1988;36:885‐7. [PubMed] [Google Scholar]
Figura 1981 {published data only}
- Figura N, Partini N, Rossolini A. New methods for the group identification of beta‐hemolytic Streptococci. Quaderni Sclavo di Diagnostica Clinica e di Laboratorio 1981;17:438‐49. [PubMed] [Google Scholar]
Fischer 1992 {published data only}
- Fischer PM. Culture and antigen detection tests for streptococcal tonsillopharyngitis. American Family Physician 1992;46:1658; author reply 1658‐9, 1662. [PubMed] [Google Scholar]
Foong 1992 {published data only}
- Foong HB, Yassim M, Chia YC, Kang BH. Streptococcal pharyngitis in a primary care clinic. Singapore Medical Journal 1992;33:597‐9. [PubMed] [Google Scholar]
Fox 2006a {published data only}
- Fox JW, Cohen DM, Marcon MJ, Cotton WiH, Bonsu BK. Performance of rapid streptococcal antigen testing varies by personnel. Journal of Clinical Microbiology 2006;44:3918‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fox 2006b {published data only}
- Fox JW, Marcon MJ, Bonsu BK. Diagnosis of streptococcal pharyngitis by detection of Streptococcus pyogenes in posterior pharyngeal versus oral cavity specimens. Journal of Clinical Microbiology 2006;44:2593‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frei 1991 {published data only}
- Frei R. Diagnosis of streptococcal pharyngitis. Schweizerische Rundschau fur Medizin Praxis 1991;80:1471‐3. [PubMed] [Google Scholar]
Fries 1995 {published data only}
- Fries SM. Diagnosis of group A streptococcal pharyngitis in a private clinic: comparative evaluation of an optical immunoassay method and culture. Journal of Pediatrics 1995;126:933‐6. [DOI] [PubMed] [Google Scholar]
Gaustad 1991 {published data only}
- Gaustad P, Hjortdahl P. Use of streptococcal antigen tests in acute tonsillitis. Tidsskrift for Den Norske Laegeforening 1991;111:1130‐1. [PubMed] [Google Scholar]
Gerber 1986a {published data only}
- Gerber MA, Randolph MF, Tilton RC. Enzyme fluorescence procedure for rapid diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1986;108:421‐3. [DOI] [PubMed] [Google Scholar]
Gerber 1989 {published data only}
- Gerber MA. Comparison of throat cultures and rapid strep tests for diagnosis of streptococcal pharyngitis. Pediatric Infectious Disease Journal 1989;8:820‐4. [DOI] [PubMed] [Google Scholar]
Gerber 1990a {published data only}
- Gerber MA, Facklam RR, Randolph MF, DeMeo KK. New colorimetric test for rapid diagnosis of streptococcal pharyngitis: a warning. Pediatrics 1990;86:457‐9. [PubMed] [Google Scholar]
Gerber 1997a {published data only}
- Gerber MA. Use of antigen detection tests in the diagnosis and management of patients with group A streptococcal pharyngitis. Pediatric Infectious Disease Journal 1997;16:1187. [DOI] [PubMed] [Google Scholar]
Gerber 1997b {published data only}
- Gerber MA, Tanz RR, Kaplan EL. Use of optical immunoassay to diagnose streptococcal pharyngitis ‐ reply. JAMA 1997;278:23‐4. [PubMed] [Google Scholar]
Gerber 1998 {published data only}
- Gerber MA. Diagnosis of group A streptococcal pharyngitis. Pediatric Annals 1998;27:269‐73. [DOI] [PubMed] [Google Scholar]
Ghanassia 1996 {published data only}
- Ghanassia JP, Reinert P, Berche P, Bianchi M, Bingen E, Cohen R. How to diagnose streptococcal pharyngitis?. Archives de Pédiatrie 1996;3:749‐51. [DOI] [PubMed] [Google Scholar]
Gnehm 1987 {published data only}
- Gnehm HE, Salfinger M. Rapid antigen tests for the diagnosis of group A streptococci in practice. Schweizerische Rundschau fur Medizin Praxis 1987;76:188‐91. [PubMed] [Google Scholar]
Gonsu 2015 {published data only}
- Gonsu HK, Bomki CM, Djomou F, Toukam M, Ndze VN, Lyonga EE. A comparative study of the diagnostic methods for group a streptococcal sore throat in two reference hospitals in Yaounde, Cameroon. Pan African Medical Journal 2015;20:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greiver 1999 {published data only}
- Greiver M. Practice tips. Incorporating a rapid group A streptococcus assay with the sore throat score. Canadian Family Physician 1999;45:1181‐2. [PMC free article] [PubMed] [Google Scholar]
Gupta 1992 {published data only}
- Gupta R, Talwar GP, Gupta SK. Rapid antibody capture assay for detection of group‐A streptococci using monoclonal antibody and colloidal gold‐monospecific polyvalent antibody conjugate. Journal of Immunoassay 1992;13:441‐55. [DOI] [PubMed] [Google Scholar]
Gupta 1997 {published data only}
- Gupta R, Kalia A, Rattan A, Kumar R, Gupta SK. Comparative evaluation of two indigenously developed tests for rapid detection of group‐A streptococci directly from throat swabs. Indian Journal of Medical Research 1997;105:200‐5. [PubMed] [Google Scholar]
Gutman 1996 {published data only}
- Gutman S. Rapid streptococcal tests. Pediatrics 1996;97:783‐4. [PubMed] [Google Scholar]
Hadfield 1987 {published data only}
- Hadfield SG, Petts DN, Kennedy P, Lane A, McIllmurray MB. Novel color test for rapid detection of group A streptococci. Journal of Clinical Microbiology 1987;25:1151‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallander 1988 {published data only}
- Hallander HO. Rapid diagnosis of group A streptococci in the throat cannot yet substitute for conventional culture technics. Lakartidningen 1988;85:2316‐8. [PubMed] [Google Scholar]
Handrick 2006 {published data only}
- Handrick W. What is the value of rapid tests for streptococci?. Krankenhauspharmazie 2006;27:542‐3. [Google Scholar]
Hansen 1992a {published data only}
- Hansen FH. "When throats are visiting...." A profitable business for a rapid test?. Lakartidningen 1992;89:3155; discussion 3155‐6. [PubMed] [Google Scholar]
Hansen 1992b {published data only}
- Hansen FH. When "throats are visiting". Increased demands should be put on the rapid test. Lakartidningen 1992;89:3573. [PubMed] [Google Scholar]
Harbeck 1993 {published data only}
- Harbeck RJ, Teague J, Crossen GR, Maul DM, Childers PL. Novel, rapid optical immunoassay technique for detection of group A streptococci from pharyngeal specimens: comparison with standard culture methods. Journal of Clinical Microbiology 1993;31:839‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbeck 1995 {published data only}
- Harbeck RJ. Evaluation of two rapid antigen assays, BioStar Strep A OIA and Pacific Biotech CARDS O.S., and culture for detection of group A streptococci in throat swabs. Journal of Clinical Microbiology 1995;33:3365‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hasin 1989 {published data only}
- Hasin M, Furst A. Sore throat in family practice: comparison of blood agar throat culture with a rapid enzyme immunoassay test for diagnostic purposes. Journal of the Royal College of General Practitioners 1989;39:332‐4. [PMC free article] [PubMed] [Google Scholar]
Haym 1986 {published data only}
- Haym JL, DiTomasso RA, Colameco S. Use of selective vs standard sheep blood agar for the diagnosis of hemolytic streptococcus group A pharyngitis. Journal of Family Practice 1986;23:580‐1. [PubMed] [Google Scholar]
Hedges 1991 {published data only}
- Hedges JR, Singal BM, Estep JL. The impact of a rapid screen for streptococcal pharyngitis on clinical decision making in the emergency department. Medical Decision Making 1991;11:119‐24. [DOI] [PubMed] [Google Scholar]
Heiter 1993 {published data only}
- Heiter BJ, Bourbeau PP. Comparison of the Gen‐Probe Group A streptococcus Direct Test with culture and a rapid streptococcal antigen detection assay for diagnosis of streptococcal pharyngitis. Journal of Clinical Microbiology 1993;31:2070‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heiter 1995 {published data only}
- Heiter BJ, Bourbeau PP. Comparison of two rapid streptococcal antigen detection assays with culture for diagnosis of streptococcal pharyngitis. Journal of Clinical Microbiology 1995;33:1408‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinfey 2010 {published data only}
- Hinfey P, Nicholls BH, Garcia F, Ripper J, Cameron Y, Joshi S. Sensitivity of a rapid antigen detection test for the diagnosis of group A streptococcal pharyngitis in the emergency department. Annals of Emergency Medicine 2010;56(Suppl):132. [Google Scholar]
Hodgins 1988 {published data only}
- Hodgins GW, Raybould TJ. Comparison of the sensitivity and specificity of eight commercially‐available reagents for clinical detection of group A streptococcus to different extracts of streptococci. Medical Laboratory Sciences 1988;45:34‐9. [PubMed] [Google Scholar]
Hoffmann 1987 {published data only}
- Hoffmann S. Rapid office methods for the diagnosis of streptococcal tonsillitis‐‐reliability and impact on patient management. Scandinavian Journal of Primary Health Care 1987;5:129‐30. [DOI] [PubMed] [Google Scholar]
Hoffmann 1990 {published data only}
- Hoffmann S. Detection of group A streptococcal antigen from throat swabs with five diagnostic kits in general practice. Diagnostic Microbiology & Infectious Disease 1990;13:209‐15. [DOI] [PubMed] [Google Scholar]
Holbrook 1998 {published data only}
- Holbrook T. Rapid strep tests in the pediatric clinical setting. Journal of Pediatric Nursing 1998;13:131‐3. [DOI] [PubMed] [Google Scholar]
Hufnagel 2010 {published data only}
- Hufnagel M, Blessing K, Brell K, Fukala S, Schmidt U, Henneke P. Comparison of two Group A Streptococcal Rapid test (Latex agglutination vs. "Lateral‐Flow‐Immunoassay") consequences for clinical practice. Klinische Padiatrie 2010;222:S8. [Google Scholar]
Humair 2006 {published data only}
- Humair JP, Revaz SA, Bovier P, Stalder H. Acute pharyngitis: No reliability of rapid streptococcal tests and clinical findings ‐ in reply. Archives of Internal Medicine 2006;166:2285‐6. [DOI] [PubMed] [Google Scholar]
Issa 2014 {published data only}
- Issa N, Demant X, Bonnet G, Camou F. Negative rapid antigen detection test do not exclude streptococcal pharyngitis. Annales Françaises de Médecine d'Urgence 2014;5:54‐5. [Google Scholar]
Johansson 2003 {published data only}
- Johansson L, Mansson NO. Rapid test, throat culture and clinical assessment in the diagnosis of tonsillitis. Family Practice 2003;20:108‐11. [DOI] [PubMed] [Google Scholar]
Johnson 1995 {published data only}
- Johnson SL, Shulman ST. Value of new rapid tests for the diagnosis of group A streptococcal pharyngitis. Pediatric Infectious Disease Journal 1995;14:923‐4. [PubMed] [Google Scholar]
Joslyn 1995 {published data only}
- Joslyn SA, Hoekstra GL, Sutherland JE. Rapid antigen detection testing in diagnosing group A beta‐hemolytic streptococcal pharyngitis. Journal of the American Board of Family Practice 1995;8:177‐82. [PubMed] [Google Scholar]
Joubaud 2003 {published data only}
- Joubaud P. About a rapid technique for detection of group a streptococci from pharyngeal specimens. Immuno‐Analyse et Biologie Spécialisée 2003;18:302‐6. [Google Scholar]
Kawakami 2003 {published data only}
- Kawakami S, Ono Y, Yanagawa Y, Miyazawa Y. Basic and clinical evaluation of the new rapid diagnostic kit for detecting group A streptococci with the immunochromatographical method. Rinsho Biseibutsu Jinsoku Shindan Kenkyukai Shi 2003;14:9‐16. [PubMed] [Google Scholar]
Kayaba 1996 {published data only}
- Kayaba H, Tamura H, Fujiwara Y. Evaluation of the therapy for streptococcal pharyngitis using Abbott Test Pack strep A. Acta Paediatrica Japonica 1996;38:8‐11. [DOI] [PubMed] [Google Scholar]
Keahey 2002 {published data only}
- Keahey L, Bulloch B, Jacobson R, Tenenbein M, Kabani A. Diagnostic accuracy of a rapid antigen test for GABHS performed by nurses in a pediatric ED. American Journal of Emergency Medicine 2002;20:128‐30. [DOI] [PubMed] [Google Scholar]
Kechrid 1988 {published data only}
- Kechrid A, Ben M'rad N, Ben Salah N, Maherzi H, Boujnah A. Rapid diagnosis of Streptococcus A angina. Tunisie Médicale 1988;66:31‐5. [PubMed] [Google Scholar]
Kellogg 1986a {published data only}
- Kellogg JA, Bankert DA, Levisky JS. Suitability of a throat culture method for evaluation of group A streptococcal antigen detection kits. American Journal of Clinical Pathology 1986;86:624‐8. [DOI] [PubMed] [Google Scholar]
Kellogg 1986b {published data only}
- Kellogg JA, Manzella JP. Detection of group A streptococci in the laboratory or physician's office. Culture vs antibody methods. JAMA 1986;255:2638‐42. [PubMed] [Google Scholar]
Kellogg 1987 {published data only}
- Kellogg JA, Bankert DA, Levisky JS. Comparison of the TestPack Strep A enzyme immunoassay system with anaerobically incubated cultures for detection of group A streptococci from oropharyngeal swabs. American Journal of Clinical Pathology 1987;88:631‐4. [DOI] [PubMed] [Google Scholar]
Kellogg 1988 {published data only}
- Kellogg JA, Bankert DA, Levisky JS. Performance of the Tandem ICON Strep A enzyme immunoassay system for detection of group A streptococci from oropharyngeal swabs. Journal of Clinical Laboratory Analysis 1988;2:205‐8. [Google Scholar]
Kellogg 1990 {published data only}
- Kellogg JA. Suitability of throat culture procedures for detection of group A streptococci and as reference standards for evaluation of streptococcal antigen detection kits. Journal of Clinical Microbiology 1990;28:165‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klein 1986 {published data only}
- Klein G, Ziering R. Dipstick enzyme‐immunoassay (EIA) for rapid detection of group A streptococcal antigen (GAS). Annals of Allergy 1986;56:524. [Google Scholar]
Kljakovic 2009 {published data only}
- Kljakovic M. The office‐based blood agar plate culture was more sensitive than the rapid antigen detection test for detecting pharyngitis. Evidence‐Based Medicine 2009;14:183. [DOI] [PubMed] [Google Scholar]
Kojima 2002 {published data only}
- Kojima T, Arai M, Sadamoto S, Ikedo M, Yui I. Evaluation of the diagnostic reagents which detect group A Streptococcus with the immunochromatographical method. Rinsho Biseibutsu Jinsoku Shindan Kenkyukai Shi 2002;12:91‐5. [PubMed] [Google Scholar]
Kramer 1980 {published data only}
- Kramer II, Goldstein EO. Rapid diagnosis of streptococcal infection. Journal of Pediatrics 1980;97:1039‐40. [DOI] [PubMed] [Google Scholar]
Kurtz 1999 {published data only}
- Kurtz B, Kurtz M, Roe M, Todd J. Importance of inoculum size and sampling effect in rapid antigen detection of S‐pyogenes pharyngitis. Pediatric Research 1999;45:166A. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larkin 2001 {published data only}
- Larkin M. A single, rapid test suffices for pharyngitis diagnosis in high‐risk patients. Lancet 2001;358:1969. [Google Scholar]
Laubscher 1994 {published data only}
- Laubscher B. Streptococcal sore throat: the role of rapid diagnostic tests in the doctor's office. Revue Médicale de la Suisse Romande 1994;114:873‐7. [PubMed] [Google Scholar]
Lind 1988 {published data only}
- Lind L, Roos K. A rapid test for tonsillitis/pharyngitis as an aid in the diagnostic arsenal. Lakartidningen 1988;85:4209‐10. [PubMed] [Google Scholar]
Lindbaek 2004 {published data only}
- Lindbaek M, Hoiby EA, Lermark G, Steinsholt IM, Hjortdahl P. Which is the best method to trace group A streptococci in sore throat patients: culture or GAS antigen test?. Scandinavian Journal of Primary Health Care 2004;22:233‐8. [DOI] [PubMed] [Google Scholar]
Lindsay 1985 {published data only}
- Lindsay AN, Swensen PH. Rapid diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1985;107:154. [DOI] [PubMed] [Google Scholar]
Llor 2009a {published data only}
- Llor C, Madurell J. Rapid diagnostic test for streptococcal pharyngitis. Formacion Medica Continuada en Atencion Primaria 2009;16:219‐21. [Google Scholar]
Llor 2009b {published data only}
- Llor C, Calvino O, Hernandez S, Crispi S, Perez‐Bauer M, Fernandez Y. Repetition of the rapid antigen test in initially negative supposed streptococcal pharyngitis is not necessary in adults. International Journal of Clinical Practice 2009;63:1340‐4. [DOI] [PubMed] [Google Scholar]
Llor 2010 {published data only}
- Llor C. Does a pharyngeal culture have to be requested when using rapid antigen techniques?. Atencion Primaria 2010;42:362‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luebbert 1989 {published data only}
- Luebbert PP. Group A streptococci rapid testing. Laboratory Medicine 1989;20:863‐4. [Google Scholar]
Lutticken 1991 {published data only}
- Lutticken R. Rapid diagnosis of streptococci. Laboratoriums Medizin 1991;15:409‐19. [Google Scholar]
Manasse 1989 {published data only}
- Manasse RJ. Evaluation of the pacific biotech CARDS STREP A test for detecting group A streptococci from cases of pharyngitis. Journal of Clinical Microbiology 1989;27:1657‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mateo 2010 {published data only}
- Mateo GF, Conejero J, Martinel EG, Baba Z, Dicono S, Echasabal M. Early diagnosis of streptococcal pharyngitis in paediatric practice: validity of a rapid antigen detection test. Atencion Primaria 2010;42:356‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mathur 1992 {published data only}
- Mathur NB. Bacteriological examination of pharyngeal secretions. Indian Pediatrics 1992;29:1071‐5. [PubMed] [Google Scholar]
Matthys 2006 {published data only}
- Matthys J, Meyere M. Acute pharyngitis: no reliability of rapid streptococcal tests and clinical findings. Archives of Internal Medicine 2006;166:2285; author reply 2285‐6. [DOI] [PubMed] [Google Scholar]
Mayefsky 1985 {published data only}
- Mayefsky JH. Rapid diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1985;107:156. [DOI] [PubMed] [Google Scholar]
McCusker 1984 {published data only}
- McCusker JJ, McCoy EL, Young CL, Alamares R, Hirsch LS. Comparison of Directigen Group A Strep Test with a traditional culture technique for detection of group A beta‐hemolytic streptococci. Journal of Clinical Microbiology 1984;20:824‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meier 1990 {published data only}
- Meier FA, Howland J, Johnson J, Poisson R. Effects of a rapid antigen test for group A streptococcal pharyngitis on physician prescribing and antibiotic costs. Archives of Internal Medicine 1990;150:1696‐700. [PubMed] [Google Scholar]
Messina 2010 {published data only}
- Messina A, Bottaro G, Morselli I. Utility of rapid antigen detection test for group a streptococci in a family paediatrician office setting. Acta Medica Mediterranea 2010;26:101‐5. [Google Scholar]
Morandi 2003 {published data only}
- Morandi PA, Deom A, Mauris A, Rohner P. External quality control of direct antigen tests to detect group A streptococcal antigen. European Journal of Clinical Microbiology & Infectious Diseases 2003;22:670‐4. [DOI] [PubMed] [Google Scholar]
Morandi 2010 {published data only}
- Morandi PA, Kesseler D, Deom A. Performances of rapid antigen detection kits for group A Streptococcus. Revue Médicale Suisse 2010;6:358‐60. [PubMed] [Google Scholar]
Morlan 1988 {published data only}
- Morlan SA, Gonzalez SFJ, Pedreira CF, Rada MR, Castell YL. Rapid diagnosis of streptococcal pharyngo‐tonsillitis in a primary care unit. Anales Espanoles de Pediatria 1988;28:23‐6. [PubMed] [Google Scholar]
Nahata 1986 {published data only}
- Nahata MC. Rapid diagnostic tests for streptococcal pharyngitis. Clinical Pharmacy 1986;5:160‐1. [PubMed] [Google Scholar]
Nerbrand 2002 {published data only}
- Nerbrand C, Jasir A, Schalen C. Are current rapid detection tests for Group A Streptococci sensitive enough? Evaluation of 2 commercial kits. Scandinavian Journal of Infectious Diseases 2002;34:797‐9. [DOI] [PubMed] [Google Scholar]
Nissinen 2009 {published data only}
- Nissinen A, Stranden P, Myllys R, Takkinen J, Bjorkman Y, Leinikki P. Point‐of‐care testing of group A streptococcal antigen: performance evaluated by external quality assessment. European Journal of Clinical Microbiology & Infectious Diseases 2009;28:17‐20. [DOI] [PubMed] [Google Scholar]
Noorbakhsh 2011 {published data only}
- Noorbakhsh S, Tabatabaei A, Farhadi M, Ebrahimi T F. Immunoasssay chromatographic antigen test for rapid diagnosis of group a beta hemolytic streptococcus pharyngitis in children: a cross‐sectional study. Iranian Journal of Microbiology 2011;3:99‐103. [PMC free article] [PubMed] [Google Scholar]
Norris 1993 {published data only}
- Norris RJ. The diagnosis of streptococcal pharyngitis by optical immunoassay. American Clinical Laboratory 1993;12:24‐5. [PubMed] [Google Scholar]
Omurzakova 2008 {published data only}
- Omurzakova NA, Yamano Y, Sato T, Izumi T, Azakami K, Hasegawa D. Increased prevalence of group A (beta)‐hemolytic streptococcus among an ethnic population in Kyrgyzstan detected by the rapid antigen detection test. Molecular Medicine Reports 2008;1:869‐74. [DOI] [PubMed] [Google Scholar]
Omurzakova 2009 {published data only}
- Omurzakova NA, Yamano Y, Nishioka K, Nakajima T. Prevalence of group a Streptococcus among children with tonsillopharyngitis in Kyrgyzstan. Internal Medicine Journal 2009;39:A63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omurzakova 2010 {published data only}
- Omurzakova NA, Yoshihisa Y, Guli S, Toshihiro N, Mayramkan A. Prevalence of group A b‐hemolytic Streptococcus among children with tonsillopharyngitis in Kyrgyzstan: the difficulty of diagnostics and therapy. International Journal of Rheumatic Diseases 2010;13:212‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 1987 {published data only}
- Patel K, Chittom AL, Toshniwal R, Kocka FE. Rapid commercial test for direct detection of group A streptococci in throat swabs. European Journal of Clinical Microbiology 1987;6:193‐4. [DOI] [PubMed] [Google Scholar]
Penalba Citores 2007 {published data only}
- Penalba Citores AC, Riano Mendez B, Maranon Pardillo R, Miguez Navarro C, Vazquez Lopez P, Guerrero Soler Maria M. Incidence of streptococcal pharyngitis. Anales de Pediatria 2007;67:220‐4. [DOI] [PubMed] [Google Scholar]
Petts 1985 {published data only}
- Petts DN, Mantell GA. Rapid detection of group A streptococci on throat swabs. Medical Laboratory Sciences 1985;42:291‐2. [PubMed] [Google Scholar]
Petts 1988 {published data only}
- Petts DN, Lane A, Kennedy P, Hadfield SG, McIllmurray MB. Direct detection of groups A, C and G streptococci in clinical specimens by a trivalent colour test. European Journal of Clinical Microbiology & Infectious Diseases 1988;7:34‐9. [DOI] [PubMed] [Google Scholar]
Pichichero 1992 {published data only}
- Pichichero ME, Disney FA, Green JL, Francis AB, Marsocci SM, Lynd AM. Comparative reliability of clinical, culture, and antigen detection methods for the diagnosis of group A beta‐hemolytic streptococcal tonsillopharyngitis. Pediatric Annals 1992;21:798‐805. [DOI] [PubMed] [Google Scholar]
Portier 2003 {published data only}
- Portier H. Rapid diagnosis of streptococcal pharyngitis: what's new?. Revue de Médecine Interne 2003;24:347‐9. [DOI] [PubMed] [Google Scholar]
Prakash 1985 {published data only}
- Prakash K. Rapid diagnosis of streptococcal infections. Indian Journal of Pediatrics 1985;52:391‐3. [DOI] [PubMed] [Google Scholar]
Preston 1987 {published data only}
- Preston EN. Use of rapid group A strep throat screening tests. American Journal of Diseases of Children 1987;141:397. [DOI] [PubMed] [Google Scholar]
Putto 1987 {published data only}
- Putto A. Febrile exudative tonsillitis: viral or streptococcal?. Pediatrics 1987;80:6‐12. [PubMed] [Google Scholar]
Radetsky 1985 {published data only}
- Radetsky M, Wheeler RC, Roe MH, Todd JK. Comparative evaluation of kits for rapid diagnosis of group A streptococcal disease. Pediatric Infectious Disease 1985;4:274‐81. [DOI] [PubMed] [Google Scholar]
Radetsky 1987 {published data only}
- Radetsky M, Solomon JA, Todd JK. Identification of streptococcal pharyngitis in the office laboratory: reassessment of new technology. Pediatric Infectious Disease Journal 1987;6:556‐63. [DOI] [PubMed] [Google Scholar]
Raich 1990 {published data only}
- Raich T, Allerberger F, Sandholzer C, Kofler J, Arnold G, Moser G. Acute tonsillitis: clinical symptoms; bacteriologic culture and rapid test as deciding criteria for the use of antibiotics. Wiener Klinische Wochenschrift 1990;102:111‐4. [PubMed] [Google Scholar]
Rasaiah 1986 {published data only}
- Rasaiah B. Rapid diagnosis of streptococcal pharyngitis from throat swabs. CMAJ 1986;135:10‐1. [PMC free article] [PubMed] [Google Scholar]
Raz 1987 {published data only}
- Raz R, Bitnun S. Dilemmas of streptococcal pharyngitis. American Family Physician 1987;35:187‐92. [PubMed] [Google Scholar]
Razongles 1993 {published data only}
- Razongles P, Bastien P. Use of rapid beta‐hemolytic strept testing in the office‐based treatment of pharyngitis. Médecine et Maladies Infectieuses 1993;23:348‐53. [Google Scholar]
Redd 1988 {published data only}
- Redd SC, Facklam RR, Collin S, Cohen ML. Rapid group A streptococcal antigen detection kit: effect on antimicrobial therapy for acute pharyngitis. Pediatrics 1988;82:576‐81. [PubMed] [Google Scholar]
Reed 1990 {published data only}
- Reed BD, Huck W, French T. Diagnosis of group A beta‐hemolytic Streptococcus using clinical scoring criteria, Directigen 1‐2‐3 group A streptococcal test, and culture. Archives of Internal Medicine 1990;150:1727‐32. [PubMed] [Google Scholar]
Reichardt 2009 {published data only}
- Reichardt B, Pichlhofer O, Zehetmayer S, Maier M. Current diagnosis of acute pharyngitis. Wiener Medizinische Wochenschrift 2009;159:202‐6. [DOI] [PubMed] [Google Scholar]
Rimoin 2004 {published data only}
- Rimoin AW, Vince A, Hamza H, Cunha ALA, Chitale R, Oazi S. Evaluation of a rapid test for streptococcal pharyngitis in children in 3 countries. Pediatric Research 2004;55:279A. [Google Scholar]
Roosevelt 2001 {published data only}
- Roosevelt GE, Kulkarni MS, Shulman ST. Critical evaluation of a CLIA‐waived streptococcal antigen detection test in the emergency department. Annals of Emergency Medicine 2001;37:377‐81. [DOI] [PubMed] [Google Scholar]
Santos 2003 {published data only}
- Santos O, Weckx LLM, Pignatari ACC, Pignatari SSN. Detection of Group A beta‐hemolytic Streptococcus employing three different detection methods: culture, rapid antigen detecting test, and molecular assay. Brazilian Journal of Infectious Diseases 2003;7:297‐300. [DOI] [PubMed] [Google Scholar]
Sarikaya 2010 {published data only}
- Sarikaya S, Aktas C, Ay D, Cetin A, Celikmen F. Sensitivity and specificity of rapid antigen detection testing for diagnosing pharyngitis in the emergency department. Ear, Nose, & Throat Journal 2010;89:180‐2. [PubMed] [Google Scholar]
Savoia 1992 {published data only}
- Savoia D, Biglino S, Cestaro A, Scarsi G, Dotti G, Fiorucci GC. Group A streptococci ‐ evaluation of a rapid direct kit and T‐serotypes isolated in children. In: Orefici G editor(s). New Perspectives on Streptococci and Streptococcal Infections. 22. Cambridge: The Faculty Press, 1992:108‐9. [Google Scholar]
Schafer 1995 {published data only}
- Schafer S. Rapid antigen detection testing. Journal of the American Board of Family Practice 1995;8:428‐30. [PubMed] [Google Scholar]
Schmuziger 1996 {published data only}
- Schmuziger N, Frei R, Hauser R, Wengen D, Probst R. Reliability of the rapid Streptococcus A test. HNO 1996;44:365‐9. [PubMed] [Google Scholar]
Schmuziger 2003 {published data only}
- Schmuziger N, Schneider S, Frei R. Reliability and general practice value of 2 rapid Streptococcus A tests. HNO 2003;51:806‐12. [DOI] [PubMed] [Google Scholar]
Schwartz 1985 {published data only}
- Schwartz RH. Rapid diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1985;107:154‐5. [DOI] [PubMed] [Google Scholar]
Seaberg 1997 {published data only}
- Seaberg DC, Gettings G, Rosenthal B, Geiger T. Rapid, optical immunoassay for streptococcal pharyngitis. Academic Emergency Medicine 1997;4:81‐3. [DOI] [PubMed] [Google Scholar]
Seecamp 1993 {published data only}
- Seecamp CH. Rapid strep assessment. Minnesota Medicine 1993;76:8. [PubMed] [Google Scholar]
Seguido 1987 {published data only}
- Seguido P, Hidalgo MA, Lobos JM, Lahoz F, Garcia‐Perea A, Conthe P. Predictive value of rapid antigenic technics in the diagnosis of acute streptococcal pharyngitis. Medicina Clinica 1987;89:405‐6. [PubMed] [Google Scholar]
Seki 1986 {published data only}
- Seki I, Yabana T, Sakuta R, Ohkuni M. The rapid diagnosis of group A streptococcal infection. Japanese Circulation Journal 1986;50:1249‐50. [DOI] [PubMed] [Google Scholar]
Serra 1989 {published data only}
- Serra A, Grillo C, Saita V, Allegra E, Mantia I. Group A streptococcal tonsillitis: comparative evaluation of kits for rapid diagnosis. Journal of Chemotherapy 1989;1:20. [PubMed] [Google Scholar]
Shaughnessy 2015 {published data only}
- Shaughnessy AF. Back‐up culture not needed for negative rapid strep test results. American Family Physician 2015;91:643‐8. [Google Scholar]
Sheeler 2002 {published data only}
- Sheeler RD, Houston MS, Radke S, Dale JC, Adamson SC. Accuracy of rapid strep testing in patients who have had recent streptococcal pharyngitis. Journal of the American Board of Family Practice 2002;15:261‐5. [PubMed] [Google Scholar]
Shekelle 1992 {published data only}
- Shekelle PG. Rapid antigen and culture detection of streptococcal pharyngitis. Annals of Internal Medicine 1992;117:22. [Google Scholar]
Shriner 1985 {published data only}
- Shriner HC Jr, Spodaccini LJ, Wright LL, Deutsch L. A quick strep test. Pediatric Infectious Disease 1985;4:301. [DOI] [PubMed] [Google Scholar]
Shulman 1994 {published data only}
- Shulman ST. Streptococcal pharyngitis: diagnostic considerations. Pediatric Infectious Disease Journal 1994;13:567‐71. [DOI] [PubMed] [Google Scholar]
Shulman 1995 {published data only}
- Shulman ST. Value of new rapid tests for the diagnosis of group A streptococcal pharyngitis. Pediatric Infectious Disease Journal 1995;14:923‐4. [PubMed] [Google Scholar]
Skellern 1993 {published data only}
- Skellern P, Mitchell J, MacCulloch D, Lang S. Direct antigen test for group A streptococcal pharyngitis. New Zealand Medical Journal 1993;106:111. [PubMed] [Google Scholar]
Smith 1989 {published data only}
- Smith TD, Wilkinson V, Kaplan EL. Group A streptococcus‐associated upper respiratory tract infections in a day‐care center. Pediatrics 1989;83:380‐4. [PubMed] [Google Scholar]
Smith 1995 {published data only}
- Smith JM, Bauman MC, Fuchs PC. An optical immunoassay for the direct detection of group A strep antigen. Laboratory Medicine 1995;26:408‐10. [Google Scholar]
Solé 2009 {published data only}
- Solé JD, Rodríguez LH, Donaire MTB, Macias MD. Rapid antigen tests for beta‐haemolytic streptococcus in primary care. Atencion Primaria 2009;41:468; author reply 469‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stillstrom 1991 {published data only}
- Stillstrom J, Schwan A, Bjorklind A. Streptococcal throat infection: calculation of test standards and a comparison between an antigen detection test and culture. Scandinavian Journal of Primary Health Care 1991;9:149‐54. [DOI] [PubMed] [Google Scholar]
Stingu 2009 {published data only}
- Stingu CS, Turcu T, Dimitriu S. A comparison between a rapid antigen test and culture in diagnosis of group A streptococcal pharyngitis. Infectious Diseases in Clinical Practice 2009;17:354‐5. [Google Scholar]
Supon 1998 {published data only}
- Supon PA, Tunnell S, Greene M, Ostroff RM. Rapid detection of group A streptococcal antigen with a new optical immunoassay. Pediatric Infectious Disease Journal 1998;17:349‐51. [DOI] [PubMed] [Google Scholar]
Syriopoulou 2011 {published data only}
- Syriopoulou T, Konstantelos D, Papoula M, Karachanidi E, Maggana I, Straka K. Laboratory methods for diagnosing streptococcal pharyngitis: predictive value, usefulness. Clinical Biochemistry 2011;44:534‐5. [Google Scholar]
Taeron 2006 {published data only}
- Taeron C. The diagnostic rapid test for angina. Actualités Pharmaceutiques 2006;45:32‐3. [Google Scholar]
Tagami 1997 {published data only}
- Tagami H. Triggering factors. Clinics in Dermatology 1997;15:677‐85. [DOI] [PubMed] [Google Scholar]
Tenjarla 1990 {published data only}
- Tenjarla G, Kumar A, Dyke JW. Evaluation of TestPack Strep A kit for rapid detection of group‐A streptococci in a laboratory setting. Clinical Research 1990;38:A823. [DOI] [PubMed] [Google Scholar]
Tocks 1992 {published data only}
- Tocks JB. Culture and antigen detection tests for streptococcal tonsillopharyngitis. American Family Physician 1992;46:1657‐8; author reply 1658‐9, 1662. [PubMed] [Google Scholar]
Todd 1987 {published data only}
- Todd JK. Defining the limits of office (and home) laboratory testing: rapid streptococcal antigen tests. Bulletin of the New York Academy of Medicine 1987;63:467‐74. [PMC free article] [PubMed] [Google Scholar]
True 1986 {published data only}
- True BL, Carter BL, Driscoll CE, House JD. Effect of a rapid diagnostic method on prescribing patterns and ordering of throat cultures for streptococcal pharyngitis. Journal of Family Practice 1986;23:215‐9. [PubMed] [Google Scholar]
Uhl 2003 {published data only}
- Uhl JR, Adamson SC, Vetter EA, Schleck CD, Harmsen WS, Iverson LK. Comparison of LightCycler PCR, rapid antigen immunoassay, and culture for detection of group A streptococci from throat swabs. Journal of Clinical Microbiology 2003;41:242‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vakkila 2015 {published data only}
- Vakkila J, Koskinen JO, Brandt A, Muotiala A, Liukko V, Soittu S. Detection of group A Streptococcus from pharyngeal swab samples by bacterial culture is challenged by a Novel mariPOC point‐of‐care test. Journal of Clinical Microbiology 2015;53:2079‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waagepetersen 2009 {published data only}
- Waagepetersen R. Strep A test and ear irrigation. Ugeskrift for Laeger 2009;171:156; author reply 157. [PubMed] [Google Scholar]
Wagener 1985 {published data only}
- Wagener S, Remington JS. Rapid diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1985;107:155‐6. [DOI] [PubMed] [Google Scholar]
Warner 1985 {published data only}
- Warner MD. Rapid diagnostic assay for strep throat. Analytical Chemistry 1985;57:1372. [DOI] [PubMed] [Google Scholar]
Waseem 2009 {published data only}
- Waseem M, Ayanruoh S, Humphrey A, Reynolds T. Impact of rapid streptococcal test on antibiotic use in a pediatric emergency department. Annals of Emergency Medicine 2009;54:S41. [DOI] [PubMed] [Google Scholar]
Wegner 1992 {published data only}
- Wegner DL, Witte DL, Schrantz RD. Insensitivity of rapid antigen detection methods and single blood agar plate culture for diagnosing streptococcal pharyngitis. JAMA 1992;267:695‐7. [PubMed] [Google Scholar]
Wegner 1996 {published data only}
- Wegner DL. Proper evaluation of rapid antigen detection methods for diagnosing streptococcal pharyngitis. Archives of Pediatrics & Adolescent Medicine 1996;150:241, 245‐8. [DOI] [PubMed] [Google Scholar]
White 1986 {published data only}
- White CB, Bass JW. Rapid enzyme fluorescence test for diagnosis of streptococcal pharyngitis. Journal of Pediatrics 1986;109:564. [DOI] [PubMed] [Google Scholar]
Wolinsky 1986 {published data only}
- Wolinsky E, Adams W. Diagnosis of streptococcal pharyngitis by rapid antigen detection from the throat swab. Journal of Family Practice 1986;22:277‐8, 280. [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong MCK, Chung CH. Group A streptococcal infection in patients presenting with a sore throat at an accident and emergency department: prospective observational study. Hong Kong Medical Journal 2002;8:92‐8. [PubMed] [Google Scholar]
Woodburn 2007 {published data only}
- Woodburn JD, Smith KL, Nelson GD. Quality of care in the retail health care setting using national clinical guidelines for acute pharyngitis. American Journal of Medical Quality 2007;22:457‐62. [DOI] [PubMed] [Google Scholar]
Wright 1987 {published data only}
- Wright A, Crabtree B, O'Connor P. Evaluation of a rapid method for diagnosing streptococcal pharyngitis in an office laboratory. Journal of Family Practice 1987;25:505‐6, 508. [PubMed] [Google Scholar]
Yu 1988 {published data only}
- Yu PK, Germer JJ, Torgerson CA, Anhalt JP. Evaluation of TestPack Strep A for the detection of group A streptococci in throat swabs. Mayo Clinic Proceedings 1988;63:33‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Briko 1997 {published data only}
- Briko NI, Bovin NV, Shevelev BI, Dynga LO, Blinnikova EI, Kuksiuk PP. Immunoenzyme test system for detecting antibodies to group‐specific antigens of group A Streptococcus on the base of conjugated N‐acetylglucosamine and its use in medical practice. Klinicheskaia Laboratornaia Diagnostika 1997;9:43‐6. [PubMed] [Google Scholar]
Gajos 1997 {published data only}
- Gajos A, Janeczek T, Wilczynski K, Bogdan P, Winiewicz A, Szychowska Z, et al. Diagnostic value of rapid streptococcal antigen test provided by Abbott "test pack strep A": current report. Otolaryngologia Polska 1997;51:168‐70. [PubMed] [Google Scholar]
Gnehm 1986 {published data only}
- Gnehm HE. Rapid diagnosis for group‐A streptococci in practice. Helvetica Paediatrica Acta 1986;41:250. [Google Scholar]
Grevnina 1992 {published data only}
- Grevnina GS, Pavlova EB, Iontova IM, Bystriakova LV. The rapid diagnosis of acute streptococcal infection in children. Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii 1992;9‐10:31‐4. [PubMed] [Google Scholar]
Herranz 2007 {published data only}
- Herranz B, Rodriguez‐Salinas E, Orden B. From laboratory to clinic: usefulness of rapid diagnostic techniques for the diagnostic techniques of Streptococcus pyogenes. Anales de Pediatria Continuada 2007;5:92‐5. [Google Scholar]
Mirjat 2012a {published data only}
- Mirjat KA, Valiram P, Fatima I. Role of rapid antigen detection test (RADT) and throat culture in the diagnosis of streptococcal pharyngotonsillitis. Medical Forum Monthly 2012;23:60‐3. [Google Scholar]
Mirjat 2012b {published data only}
- Mirjat KA, Fatima I, Mustafa F. Prevalence of pharyngitis and tonsilitis among children. Medical Forum Monthly 2012;23:64‐7. [Google Scholar]
Nestorovic 2004 {published data only}
- Nestorovic B, Laban‐Nestorovic S, Paripovic V, Milosevic K. Value of a rapid test for identification of beta‐hemolytic streptococcus antigens in children with streptococcal pharyngitis. Srpski Arhiv Za Celokupno Lekarstvo 2004;132(Suppl 1):39‐41. [DOI] [PubMed] [Google Scholar]
Sanz Moreno 2010 {published data only}
- Sanz Moreno J. Differential diagnostic protocol of pharyngoamygdalitis. Medicine 2010;10:4015‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shikhman 1988 {published data only}
- Shikhman AR, Shmakova ZF, Briko NI. Enzyme‐immunoassay of Streptococcus Pyogenes group‐specific A polysaccharide ‐ relationship between the results and the bacterial‐cell biodegradation. Laboratornoe Delo 1988;4:18‐22. [PubMed] [Google Scholar]
Soyletir 1988 {published data only}
- Soyletir G, Ener B, Basaran M, Cakan N, Pamukcu A, Goral M. Direct antigen detection for group A streptococcal pharyngitis: comparison of throat cultures and the direct antigen test. Mikrobiyoloji Bulteni 1988;22:322‐6. [PubMed] [Google Scholar]
Sramek 1992 {published data only}
- Sramek J, Havlicek J, Benes O. The importance of a rapid, direct method of detection of group A streptococci in the treatment of pharyngitis. Ceskoslovenska Pediatrie 1992;47:733‐6. [PubMed] [Google Scholar]
Vylegzhanina 1994 {published data only}
- Vylegzhanina ES, Dmitrieva NF, Shevelev BI, Kondrakova OA. Use of enzyme immunoassay in the study of Group A Streptococcus adhesion. Klinicheskaia Laboratornaia Diagnostika 1994;4:40‐1. [PubMed] [Google Scholar]
Yilmaz 2008 {published data only}
- Yilmaz F, Karabay O, Ince NK, Ekerbicer H, Kocoglu E. Effectiveness of rapid antigen test with throat gargle in detecting group A beta‐hemolytic streptococci. Kulak Burun Bogaz Ihtisas Dergisi 2008;18:280‐3. [PubMed] [Google Scholar]
Additional references
AAP 2012
- American Academy of Pediatrics. Group A streptococcal infections. In: Pickering L, Baker C, Long S, McMillan J editor(s). Red Book: 2012 Report of the Committee on Infectious Disease. 29th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 2012. [Google Scholar]
Battle 1971
- Battle CU, Glasgow LA. Reliability of bacteriologic identification of beta‐hemolytic streptococci in private offices. American Journal of Diseases of Children 1971;122(2):134‐6. [DOI] [PubMed] [Google Scholar]
Bossuyt 2003
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clinical Chemistry 2003;49(1):1‐6. [DOI] [PubMed] [Google Scholar]
Bossuyt 2015
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brazzelli 2009
- Brazzelli M, Lewis SC, Deeks JJ, Sandercock PA. No evidence of bias in the process of publication of diagnostic accuracy studies in stroke submitted as abstracts. Journal of Clinical Epidemiology 2009;62(4):425‐30. [DOI] [PubMed] [Google Scholar]
Carapetis 2005a
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet 2005;5(11):685‐94. [DOI] [PubMed] [Google Scholar]
Carapetis 2005b
- Carapetis JR, McDonald M, Wilson NJ. Acute rheumatic fever. Lancet 2005;366(9480):155‐68. [DOI] [PubMed] [Google Scholar]
Carapetis 2007
- Carapetis JR. Rheumatic heart disease in developing countries. New England Journal of Medicine 2007;357(5):439‐41. [DOI] [PubMed] [Google Scholar]
Centor 1981
- Centor RM, Witherspoon JM, Dalton HP, Brody CE, Link K. The diagnosis of strep throat in adults in the emergency room. Medical Decision Making 1981;1(3):239‐46. [DOI] [PubMed] [Google Scholar]
Charlier‐Bret 2004
- Charlier‐Bret N, Boucher B, Poyart C, Quesne G, Bingen E, Doit C, et al. Rapid antigen detection tests for diagnosis of group A streptococcal pharyngitis: comparative evaluation of sensitivity and practicability of 16 in vitro diagnostics medical devices performed in July 2002 by the French health products safety agency (Afssaps) as part of its market control mission. Pathologie Biologie (Paris) 2004;52(8):438‐43. [DOI] [PubMed] [Google Scholar]
Cohen 2015
- Cohen JF, Cohen R, Levy C, Thollot F, Benani M, Bidet P, et al. Selective testing strategies for diagnosing group A streptococcal infection in children with pharyngitis: a systematic review and prospective multicentre external validation study. CMAJ 2015;187(1):23‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fischer Walker 2006
- Fischer Walker CL, Rimoin AW, Hamza HS, Steinhoff MC. Comparison of clinical prediction rules for management of pharyngitis in settings with limited resources. Journal of Pediatrics 2006;149(1):64‐71. [DOI] [PubMed] [Google Scholar]
Fox 2006
- Fox JW, Cohen DM, Marcon MJ, Cotton WiH, Bonsu BK. Performance of rapid streptococcal antigen testing varies by personnel. Journal of Clinical Microbiology 2006;44:3918‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerber 1986b
- Gerber MA, Randolph MF, Chanatry J, Wright LL, DeMeo KK, Anderson LR. Antigen detection test for streptococcal pharyngitis: evaluation of sensitivity with respect to true infections. Journal of Pediatrics 1986;108(5 Pt 1):654‐8. [DOI] [PubMed] [Google Scholar]
Gerber 2004
- Gerber MA, Shulman ST. Rapid diagnosis of pharyngitis caused by group A streptococci. Clinical Microbiology Reviews 2004;17(3):571‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerber 2005
- Gerber MA. Diagnosis and treatment of pharyngitis in children. Pediatric Clinics of North America 2005;52(3):729‐47, vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gerber 2009
- Gerber MA, Baltimore RS, Eaton CB, Gewitz M, Rowley AH, Shulman ST, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation 2009;119(11):1541‐51. [DOI] [PubMed] [Google Scholar]
Heelan 1996
- Heelan JS, Wilbur S, Depetris G, Letourneau C. Rapid antigen testing for group A Streptococcus by DNA probe. Diagnostic Microbiology and Infectious Disease 1996;24(2):65‐9. [DOI] [PubMed] [Google Scholar]
Joachim 2010
- Joachim L, Campos D Jr, Smeesters PR. Pragmatic scoring system for pharyngitis in low‐resource settings. Pediatrics 2010;126(3):e608‐14. [DOI] [PubMed] [Google Scholar]
Johnson 2010
- Johnson DR, Kurlan R, Leckman J, Kaplan EL. The human immune response to streptococcal extracellular antigens: clinical, diagnostic, and potential pathogenetic implications. Clinical Infectious Diseases 2010;50(4):481‐90. [DOI] [PubMed] [Google Scholar]
Lasseter 2009
- Lasseter GM, McNulty CA, Hobbs RFD, Mant D, Little P, PRISM investigators. In vitro evaluation of five rapid antigen detection tests for group A beta‐haemolytic streptococcal sore throat infections. Family Practice 2009;26(6):437‐44. [DOI] [PubMed] [Google Scholar]
Lean 2014
- Lean WL, Arnup S, Danchin M, Steer AC. Rapid diagnostic tests for group A streptococcal pharyngitis: a meta‐analysis. Pediatrics 2014;134(4):771‐81. [DOI] [PubMed] [Google Scholar]
Leeflang 2006
- Leeflang MM, Scholten RJ, Rutjes AW, Reitsma JB, Bossuyt PM. Use of methodological search filters to identify diagnostic accuracy studies can lead to the omission of relevant studies. Journal of Clinical Epidemiology 2006;59(3):234‐40. [DOI] [PubMed] [Google Scholar]
Leeflang 2009
- Leeflang MM, Bossuyt PM, Irwig L. Diagnostic test accuracy may vary with prevalence: implications for evidence‐based diagnosis. Journal of Clinical Epidemiology 2009;62(1):5‐12. [DOI] [PubMed] [Google Scholar]
Leeflang 2013
- Leeflang MM, Rutjes AW, Reitsma JB, Hooft L, Bossuyt PM. Variation of a test's sensitivity and specificity with disease prevalence. CMAJ 2013;185(11):E537‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Linder 2005
- Linder JA, Bates DW, Lee GM, Finkelstein JA. Antibiotic treatment of children with sore throat. JAMA 2005;294(18):2315‐22. [DOI] [PubMed] [Google Scholar]
Llor 2011
- Llor C, Madurell J, Balagué‐Corbella M, Gómez M, Cots JM. Impact on antibiotic prescription of rapid antigen detection testing in acute pharyngitis in adults: a randomised clinical trial. British Journal of General Practice 2011;61(586):e244‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. Chichester, UK: The Cochrane Collaboration, 2010. [Google Scholar]
Matthys 2007
- Matthys J, Meyere M, Driel ML, Sutter A. Differences among international pharyngitis guidelines: not just academic. Annals of Family Medicine 2007;5(5):436‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
McIsaac 1998
- McIsaac WJ, White D, Tannenbaum D, Low DE. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. Canadian Medical Association Journal 1998;158(1):75‐83. [PMC free article] [PubMed] [Google Scholar]
Mondzac 1967
- Mondzac AM. Throat culture processing in the office ‐ a warning. JAMA 1967;200(12):1132‐3. [PubMed] [Google Scholar]
Pelucchi 2012
- Pelucchi C, Grigoryan L, Galeone C, Esposito S, Huovinen P, Little P, et al. ESCMID Guideline for the management of acute sore throat. Clinical Microbiology and Infection 2012;18(Suppl 1):1‐28. [DOI] [PubMed] [Google Scholar]
Pokorski 1994
- Pokorski SJ, Vetter EA, Wollan PC, Cockerill FR, 3rd. Comparison of Gen‐Probe Group A streptococcus Direct Test with culture for diagnosing streptococcal pharyngitis. Journal of Clinical Microbiology 1994;32(6):1440‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenstein 1970
- Rosenstein BJ, Markowitz M, Gordis L. Accuracy of throat cultures processed in physicians' offices. Journal of Pediatrics 1970;76(4):606‐9. [DOI] [PubMed] [Google Scholar]
Ruiz‐Aragon 2010
- Ruiz‐Aragon J, Rodriguez Lopez R, Molina Linde JM. Evaluation of rapid methods for detecting Streptococcus pyogenes. systematic review and meta‐analysis. Anales de Pediatría (Barcelona, Spain) 2010;72(6):391‐402. [DOI] [PubMed] [Google Scholar]
Seckeler 2011
- Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clinical Epidemiology 2011;3:67‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaikh 2010
- Shaikh N, Leonard E, Martin JM. Prevalence of streptococcal pharyngitis and streptococcal carriage in children: a meta‐analysis. Pediatrics 2010;126(3):e557‐64. [DOI] [PubMed] [Google Scholar]
Shaikh 2011
Shulman 2000
- Shulman ST, Tanz RR, Gerber MA. Streptococcal pharyngitis. In: Stevens DL, Kaplan EL editor(s). Streptococcal Infections: Clinical Aspects, Microbiology, and Molecular Pathogenesis. New York: Oxford University Press, 2000:76‐101. [Google Scholar]
Shulman 2009
- Shuman ST. Pediatric autoimmune neuropsychiatric disorders associated with streptococci (PANDAS): update. Current Opinion in Pediatrics 2009;21(1):127‐30. [DOI] [PubMed] [Google Scholar]
Shulman 2012
- Shulman ST, Bisno AL, Clegg HW, Gerber MA, Kaplan EL, Lee G, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2012;55(10):1279‐82. [DOI] [PubMed] [Google Scholar]
Slinger 2011
- Slinger R, Goldfarb D, Rajakumar D, Moldovan I, Barrowman N, Tam R, et al. Rapid PCR detection of group A Streptococcus from flocked throat swabs: a retrospective clinical study. Annals of Clinical Microbiology and Antimicrobials 2011;10:33. [DOI: 10.1186/1476-0711-10-33] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spinks 2013
- Spinks A, Glasziou PP, Mar CB. Antibiotics for sore throat. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD000023.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinhoff 2005
- Steinhoff MC, Walker CF, Rimoin AW, Hamza HS. A clinical decision rule for management of streptococcal pharyngitis in low‐resource settings. Acta Paediatrica 2005;94(8):1038‐42. [DOI] [PubMed] [Google Scholar]
Stewart 2014
- Stewart EH, Davis B, Clemans‐Taylor BL, Littenberg B, Estrada CA, Centor RM. Rapid antigen group A streptococcus test to diagnose pharyngitis: a systematic review and meta‐analysis. PLoS One 2014;9(11):e111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanz 1997
- Tanz RR, Gerber MA, Shulman ST. What is a throat culture?. Advances in Experimental Medicine and Biology 1997;418:29‐33. [DOI] [PubMed] [Google Scholar]
Tanz 2007
- Tanz RR, Shulman ST. Chronic pharyngeal carriage of group A streptococci. Pediatric Infectious Disease Journal 2007;26(2):175‐6. [DOI] [PubMed] [Google Scholar]
Wessels 2011
- Wessels MR. Clinical practice. Streptococcal pharyngitis. New England Journal of Medicine 2011;364(7):648‐55. [DOI] [PubMed] [Google Scholar]
Whiting 2011a
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]
Whiting 2011b
- Whiting P, Westwood M, Beynon R, Burke M, Sterne JA, Glanville J. Inclusion of methodological filters in searches for diagnostic test accuracy studies misses relevant studies. Journal of Clinical Epidemiology 2011;64(6):602‐7. [DOI] [PubMed] [Google Scholar]
WHO 1995
- World Health Organization. The Management of Acute Respiratory Infectious in Children: Practical Guidelines for Outpatient Care. Geneva: World Health Organization, 1995. [Google Scholar]
References to other published versions of this review
Cohen 2013
- Cohen JF, Cohen R, Chalumeau M. Rapid antigen detection test for group A streptococcus in children with pharyngitis. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010502] [DOI] [PMC free article] [PubMed] [Google Scholar]


