Anthropogenic influences alter tree mycorrhizal associations inducing nutrient acceleration in the U.S. forests.
Abstract
Plant-fungal symbioses play critical roles in vegetation dynamics and nutrient cycling, modulating the impacts of global changes on ecosystem functioning. Here, we used forest inventory data consisting of more than 3 million trees to develop a spatially resolved “mycorrhizal tree map” of the contiguous United States. We show that abundances of the two dominant mycorrhizal tree groups—arbuscular mycorrhizal (AM) and ectomycorrhizal trees—are associated primarily with climate. Further, we show that anthropogenic influences, primarily nitrogen (N) deposition and fire suppression, in concert with climate change, have increased AM tree dominance during the past three decades in the eastern United States. Given that most AM-dominated forests in this region are underlain by soils with high N availability, our results suggest that the increasing abundance of AM trees has the potential to induce nutrient acceleration, with critical consequences for forest productivity, ecosystem carbon and nutrient retention, and feedbacks to climate change.
INTRODUCTION
The forests of North America are experiencing unprecedented change owing to the combined effects of climate change, nitrogen (N) deposition, changes in disturbance regime, habitat fragmentation, and invasions of exotic species (1–5). While anthropogenic-induced shifts in the distribution and abundance of tree communities are well described (1, 6), far less is known about the direct and indirect impacts of global anthropogenic changes on plant-fungal associations (7, 8). More than 90% of vascular plants associate with mycorrhizal fungi (9, 10), and there is an emerging consensus that these plant-fungal associations have profound impacts on nutrient cycling and vegetation dynamics in ecosystems, particularly temperate forests (11–15). However, critical gaps remain in our understanding of biogeographic patterns of mycorrhizal associations, and our limited knowledge of the anthropogenic factors responsible for shifting plant-mycorrhizal distributions has hindered efforts to predict ecosystem feedbacks to climate change (16).
The two dominant types of fungi that associate with trees—arbuscular mycorrhizal (AM) and ectomycorrhizal (EM)—differ greatly in their forms and functions. Consequently, tree mycorrhizal associations have been hypothesized to represent trait-integrating phenotypes that give rise to “biogeochemical syndromes” in forests (13), although the relative contribution of the plants versus fungi to these syndromes is poorly quantified. EM-dominated forests often cycle carbon (C) and nutrients conservatively owing to the lower chemical quality of EM plant litter relative to that of AM-dominated forests, which have more “open” C and nutrient cycles (17, 18). These effects not only alter the degree to which these ecosystems store C and nutrients (19–22) but also likely affect their sensitivity to human-induced global changes (23–26). Here, using forest inventory data collected by the U.S. Department of Agriculture (USDA) Forest Service, Forest Inventory and Analysis (FIA) program across the contiguous United States, we (i) map the tree mycorrhizal association patterns and identify underlying drivers of these observed associations; (ii) quantify the impacts of human-induced global changes, primarily climate change, N deposition, and disturbance regimes, on tree mycorrhizal associations; and (iii) assess the potential feedback of mycorrhizal association shifts on soil C and nutrient dynamics.
RESULTS AND DISCUSSION
Distribution and drivers of tree mycorrhizal association patterns
Using FIA vegetation data and tree mycorrhizal type information, we mapped the relative abundance of more than 3 million AM and EM trees across the conterminous United States (Fig. 1A). While patterns of mycorrhizal associations and associated drivers have been reported previously, these studies were based on species occurrence data (27–29), and they covered lesser spatial extent (30) and examined fewer vegetation survey plots than our study (31). Our results indicate that AM trees are more dominant in dry and warm ecoregions, while EM trees are more dominant in humid and cold ecoregions (Fig. 1, A and B). In the western United States, AM trees are dominant in the subtropical desert and steppe regions, while EM trees are dominant in the northwestern and intermountain west regions (Fig. 1A and fig. S1). AM and EM trees are well mixed in the eastern United States, with more AM trees in the hot continental region and more EM trees in the western portion of the warm continental region (Fig. 1 and fig. S1). Using a mixed-effects model that accounts for the spatial heterogeneity between plots in different subecoregions, we found that climate is an important driver of tree mycorrhizal association patterns at the continental scale (Fig. 1C). Overall, AM tree dominance was negatively associated with mean annual precipitation (MAP) and positively associated with mean annual temperature (MAT) (Fig. 1C), although the magnitude and direction of effect sizes differed in some ecoregions. Forest tree basal area, which was used as an indicator of forest successional stage, had smaller effects on AM tree dominance compared with climatic factors, implying that climate is a more important driver of the continental tree mycorrhizal association patterns than successional stage (Fig. 1C).
Fig. 1. Distribution of forest tree mycorrhizal types and their associated factors in forests of the contiguous United States.
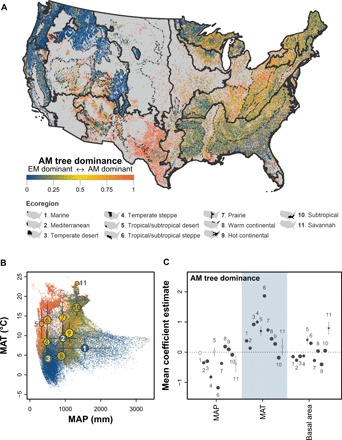
(A) Geographical distribution of AM tree dominance. (B) Distribution of AM tree dominance in climatic space. MAP, mean annual precipitation; MAT, mean annual temperature. (C) Relative effects of MAP, MAT, and tree basal area on AM tree dominance. Each dot in (A) and (B) represents a plot and is colored on the basis of the associated AM-EM tree dominance. Boundaries of ecoregions (solid line) and nested subecoregions (dashed lines) in (A) are based on Cleland et al. (58). Circles in (B) indicate ecoregion-level mean MAT and MAP values with the associated SDs. The circle is colored on the basis of the mean AM tree dominance, and the size is proportional to the number of plots (log scale). Effects of MAP, MAT, and basal area on AM tree dominance across ecoregions in the contiguous United States (C) were tested using generalized mixed-effects models with subecoregions included as a random effect in each model. Significant coefficient estimates are plotted in (C) as solid circles, and nonsignificant ones are plotted as open circles. Circle size is proportional to the number of plots (log scale). The number beside each dot in (B) and (C) represents the associated ecoregion in (A). Error bars in (C) are SEs.
Shifts in tree mycorrhizal associations and associated drivers
To understand the impacts of human-induced global changes on tree mycorrhizal associations, we used repeated measures of forest inventories from the FIA program during the past three decades in the eastern United States where rapid climate change has been observed (fig. S2) (1). AM tree dominance has significantly increased in all parts of the eastern United States during the past three decades (based on a paired Wilcoxon signed-rank test, P < 0.05; Fig. 2A), especially in the central regions (17% increase in prairie and 15% increase in hot continental regions), because of both an increase in AM tree abundance (i.e., basal area; fig. S3A) and a decrease in EM tree abundance (fig. S3B). AM tree dominance in southern and northern ecoregions also increased (5% increase in warm continental and 5% increase in subtropical; Fig. 2A), although these regions had statistically significant increases in both AM and EM tree abundance (fig. S3, A and B). In particular, the western portion of the warm continental region and northern portion of the subtropical region had similar increases in AM tree abundance, while the eastern portion of the warm continental region and southern portion of the subtropical region had the opposite trend (Fig. 2A).
Fig. 2. Changes in forest AM tree dominance during the past three decades and the relative impacts of environmental changes on the mycorrhizal association changes in forests of the eastern United States.
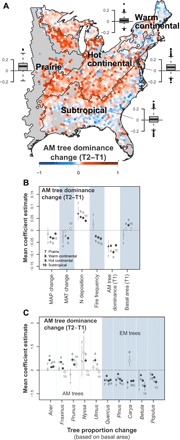
(A) Changes in AM tree dominance over the two inventories (T2-T1). All ecoregions had a significant increase in AM tree dominance during the period based on a paired Wilcoxon signed-rank test (P < 0.05; inset figures are boxplots of hexagon-level changes by ecoregions). (B) Relative effects of climate and basal area change, AM tree dominance at the first inventory (T1), N deposition, and fire frequency on AM tree dominance change. (C) Effects of tree abundance change of the top 10 most abundant tree genera (genera on the left without shaded background are AM trees, and genera on the right are EM trees) on AM tree dominance change. Mean coefficients in (B) and (C) were estimated at the ecoregion level based on generalized mixed-effects models with subecoregions included as a random effect. Significant coefficient estimates are plotted as solid circles, and nonsignificant ones are plotted as open circles with the size being proportional to the number of hexagons (log scale). Error bars in (B) and (C) are SEs.
Our analysis indicates that three factors—N deposition, fire frequency, and climate change—likely contributed to the increases in AM tree dominance. First, we found a strong positive correlation between N deposition and shifts in AM tree dominance, consistent with earlier studies based on smaller spatial extent and shorter temporal scale (31). In temperate forests, most AM tree species have nutrient acquisitive traits (e.g., rapid root growth into nutrient hot spots and narrower C:N in leaf and root tissues) (17, 32–34) and often dominate stands characterized by open (i.e., fast) N cycles (13, 30). Thus, the positive relationship between N deposition and AM dominance may result from AM trees being competitively superior at acquiring excess N—the nutrient that generally limits plant growth in these forests. Second, we found strong negative associations between fire frequency and AM tree dominance (Fig. 2B). While it is well established that fire suppression following European settlement has led to oak (Quercus) regeneration failure and forest “mesophication” in the eastern United States (35), our results indicate that this trend is not merely a result of EM-associating oaks being replaced by AM-associating maples (Acer). The change in AM tree dominance was not driven by a specific phylogenetic group of tree species, as the most common AM and EM tree genera had relatively similar effect sizes on the change (Fig. 2C). Changes of abundance in all five most common AM genera, with a few statistically nonsignificant exceptions, were positively associated with AM tree dominance change, while the changes of abundance in EM genera were nearly all negatively associated with the AM tree dominance change (Fig. 2C). Possible explanations for the observed nonsignificant outliers (e.g., Prunus in prairie and Carya in warm continental region) could be due to small sample sizes or potential preferential harvesting in these regions. The third factor contributing to increases in AM tree dominance is climate change. In general, increases in MAP were negatively associated with increases in AM tree dominance, while the associations with MAT were weak and variable (Fig. 2B).
The extent to which other factors may contribute to future shifts in tree mycorrhizal associations is unknown. AM tree dominance tended to increase with basal area, an indicator of forest succession, as shade-tolerant AM trees increase their abundance with the progression of forest succession. However, the effects of basal area were relatively small compared with the other drivers, suggesting that anthropogenic drivers (i.e., climate change, N deposition, and fire suppression) had far greater impact on recent demographic shifts. Other factors such as land use change and forest management, which directly affect tree species dominance, could also affect shifts in tree mycorrhizal associations. In addition, to the extent that pollution control and reduction reduce N loading to U.S. forests, future shifts in mycorrhizal associations may be lessened in the coming decades.
A continuing shift to AM tree dominance is also predicted by our finding that saplings were more AM-dominated compared with adult trees in 7 of 11 ecoregions (Fig. 3). In the eastern United States, all ecoregions other than the warm continental region had greater AM tree dominance in saplings compared with adult trees (Fig. 3). The prairie, hot continental, and subtropical regions had more than 54% greater sapling AM tree dominance compared with adult trees. The differences in AM tree dominance between saplings and adult trees were mixed in the western United States (Fig. 3). Compared with adult trees, more AM saplings were observed in the marine, Mediterranean, and temperate steppe regions, but less AM saplings were observed in the temperate desert, tropical/subtropical desert, and tropical/subtropical steppe regions. In addition, the overall differences between adult and sapling AM tree dominance were smaller than those observed in the ecoregions in the eastern United States (Fig. 3).
Fig. 3. AM tree dominance differences between adult trees and saplings in forests across 11 ecoregions of the United States.
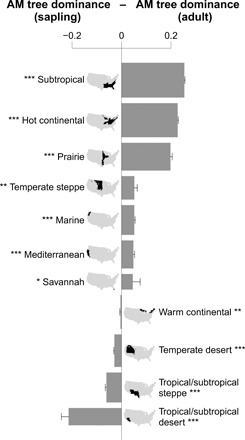
The difference in AM tree dominance between adults and saplings for each ecological region were tested on the basis of a paired Wilcoxon signed-rank test (*P < 0.05; **P < 0.01; ***P < 0.001). Error bars are SEs. The bar thickness is proportional to the number of plots (log scale). Only plots where both adult trees and saplings are present are used for the analysis (98,638 plots).
Relationships between tree mycorrhizal associations and soil C and N
To assess the potential consequences of mycorrhizal association shifts on C and N dynamics, we analyzed the relationships between AM tree dominance and soil C and N stocks (litter layer and 0- to 20-cm depth of the mineral soil) on plots where both soil attributes and associated vegetation were measured (2113 plots) using linear regression models. In general, the associations between AM tree dominance and soil C and N stocks were positive in mesic temperate ecoregions but negative in dry ecoregions; however, most ecoregions had a negative relationship between soil C:N ratio and AM tree dominance (Fig. 4, A to C). Across the study area, AM-dominated forests had 28% more soil N and 8% more soil C than EM-dominated forests (Fig. 4, A and B, and table S2). The higher increase in soil N stocks (relative to soil C stocks) in mesic ecoregions and the lower decrease in soil N stocks (relative to soil C stocks) in dry ecoregions resulted in a negative relationship between soil C:N ratio and AM tree dominance along the continental AM tree dominance gradient (Fig. 4, A to C).
Fig. 4. Associations between forest soil C and N with forest tree mycorrhizal type and environmental factors in forest ecosystems in the United States.
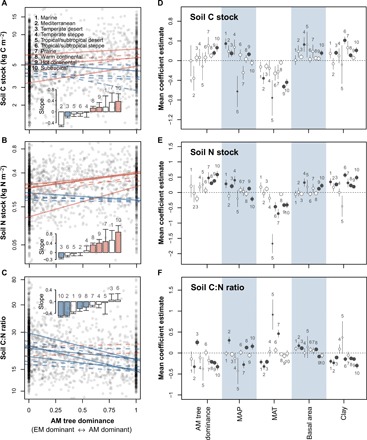
(A to C) Relationships between AM tree dominance and soil (A) C stock, (B) N stock, and (C) C:N ratio (based on 0- to 20-cm depth mineral soil and litter layer). Linear regression lines were fitted by ecoregion (solid line, P < 0.05; dotted line, P > 0.05; red, positive slope; blue, negative slope). Inset shows coefficient (slope) estimate of the fitted line for each ecoregion (colored bars indicate significant relationship at P < 0.05; red, positive; blue, negative). (D to F) Effects of AM tree dominance and environmental factors on soil (D) C stock, (E) N stock, and (F) C:N ratio across ecoregions of the United States. Coefficient estimates in (D) to (F) are based on mixed-effect models at ecoregion level with subecoregions as a random effect. Significant coefficient estimates are plotted as solid circles, and nonsignificant ones are plotted as open circles. Circle size is proportional to the number of plots (log scale). Error bars are SEs. The number beside each bar in (A) to (C) and each dot in (D) to (F) represents the associated ecoregion in (A). Soil data summary is available in table S2.
Using mixed-effects models, we further tested how forest mycorrhizal association and environmental factors are related to soil attributes for each ecoregion after accounting for the spatial heterogeneity between plots in different subecoregions by adding subecoregions as random intercepts in the models. We found that AM tree dominance, climate, and soil texture had significant associations with soil C and N stocks and C:N ratio, although with different effect sizes (Fig. 4, D to F). Overall, AM tree dominance was positively associated with soil C and N stocks and negatively with C:N ratio across the ecoregions, consistent with site-based patterns recently reported (22). Among the climatic factors, MAP tended to have positive associations with soil C and N stocks, while MAT have negative associations. Soil C and N stocks were similar between the top 20 cm of the mineral soil and the top 20 cm plus litter layer (fig. S4) owing to the much smaller size of the litter C and N pools.
The observational nature of our data precludes us from determining whether AM trees are causing elevated N levels in soil (as opposed to merely responding to them). It is possible that AM trees can elevate soil N (pool sizes and transformation rates) by releasing high chemical quality litter to soil. Several studies of temperate trees have shown that AM litters generally decay faster than EM litters in common garden studies (17) and meta-analyses (36). Given that higher chemical quality litter leads to both greater rates of N transformations (37) and the formation of more protected (i.e., stable) soil organic matter [sensu the MEMS (Microbial Efficiency-Matrix Stabilization) hypothesis] (38), increasing AM dominance may lead to elevated N levels in soil (22). Contemporary theory on soil organic matter stabilization and turnover predicts that changes in AM versus EM dominance can alter C and N cycling in 30 to 45 years (18), well within the time frame of changes detected in the FIA dataset. Thus, our contention that AM trees may be contributing, in part, to N accumulation is a testable hypothesis but requires further inquiry.
Last, separating cause and effect in this instance may be of limited importance given that tree species often modify soils in ways that tend to exacerbate or enhance the preexisting biogeochemical condition (39). Thus, while we cannot rule out that the species are merely responding to the high N soils, both processes likely contribute to the maintenance of the biogeochemical syndromes observed. More experiments and/or long-term repeated measures of soil stocks are needed to test the mechanisms of these associations. Nevertheless, the consistent large-scale patterns in forests across multiple ecoregions suggest that dominant tree mycorrhizal type could be an important driver of nutrient and C dynamics in forests through positive/negative feedbacks associated with AM/EM plant traits (39).
Given that AM-dominated forests tend to be underlain by soils with lower C:N than EM-dominated soils [(30, 33); Fig. 4C], the increasing dominance of AM trees could have consequences for forest ecosystem functions and services. Low or small ratios are often used as proxies for rates of microbial N transformations in soils such as nitrification and nitrate leaching losses (37), as well as for ecosystem sensitivity to N deposition (40). Thus, forests of the eastern United States may be experiencing an acceleration of N cycling—owing to the shifts in AM dominance. The consequences of nutrient acceleration would likely be profound for water quality if, for example, elevated nitrification rates enhance nitrate export to lakes and rivers.
The consequences of nutrient acceleration may be most profound for forest productivity, which can feed back to affect climate change. Most AM-dominated ecosystems cannot sustain a high level of productivity under elevated CO2 unless the availability of soil N is high (23). Thus, if increases in AM tree dominance lead to an acceleration of N cycling, then AM-dominated forests in the eastern United States may be strong sinks for atmospheric CO2. However, if the AM-induced acceleration of N cycling leads to substantial ecosystem N losses (25) or results in N stabilization in soil organic matter (22), then there may be little stimulation of forest productivity. In addition, shifting the balance of AM-EM vegetation could also be influenced by N-induced phosphorus (P) limitation and more severe drought periods (41). While N-induced P limitation has long been considered as something that would only occur in forests south of the Last Glacial Maximum (where mineral P levels are extremely low), recent evidence from northern ecosystems challenges that paradigm (42). Either way, our results indicate that changes in N cycling owing to increasing AM tree dominance could have profound consequences for C and N retention and loss in forests and hence, the degree to which forests feedback to climate change.
CONCLUSIONS
Our study provides the first comprehensive distribution map of tree mycorrhizal association in the contiguous United States. We provide empirical evidence based on national forest inventory data to suggest how global changes may have affected the shift of mycorrhizal associations at the continental scale. We found that AM tree dominance was positively associated with both soil C and N stocks, particularly for temperate forests, which challenges the prevailing idea that EM tree dominant ecosystems store more C than AM tree dominant ecosystems (15, 19–21). We note, however, that soil C:N ratio was negatively associated with AM tree dominance, supporting recent findings that showed a positive association between soil C:N ratio and EM tree dominance driven by low soil N instead of by high C stock in EM tree dominant ecosystems (22, 30). Our results suggest that increases in AM tree dominance in the eastern United States may increase soil N stocks, inducing a positive feedback of nutrient acceleration, at least in the upper surface soils. A better understanding of the role of dominant forest mycorrhizal association type in ecosystem processes at global scales and the mechanisms responsible for forest soil C storage is critical for improving ecosystem models to predict forest ecosystem processes and functions in global climate change.
MATERIALS AND METHODS
Tree data collection
Tree inventory data were obtained from forest plots across the United States by the FIA program (U.S. Forest Service; data are available at https://apps.fs.usda.gov/fia/datamart/). The FIA program monitors forest resources at the national level, using permanent plots, which have a sampling intensity of approximately one plot every 2428 ha. Each plot comprises four subplots (fixed radius, 7.3 m) spaced 37 m apart in a triangular arrangement with one subplot in the center. For each FIA plot, we extracted tree basal area by species with a diameter at breast height (dbh) of >12.7 cm as adult tree and a dbh of 5.1 to 12.7 cm as sapling (43).
Soil C and N stocks
FIA program collected soil samples on every 1/16th of the base intensity plot, distributed approximately every 38,848 ha (44). We compiled forest litter layer C and N concentrations and the associated litter layer thickness and bulk density, mineral soil C and N concentrations for 0- to 20-cm soil depth, and the associated soil bulk density and coarse fraction at subplot level where species-level vegetation inventory was available. Soil C and N measurements in FIA data were based on mineral soil (<2 mm), and coarse particles (>2 mm) were not included. Mineral soil C and N stocks (kg m−2) to a depth of 20 cm were calculated on the basis of soil C and N concentration (%) and soil bulk density (g cm3) after removing a proportion of coarse particle fraction (particle size, >2 mm) in the soil layer. Total soil C and N stocks were calculated by combining values for both mineral soil (0- to 20-cm depth) and litter layer. Since soil texture for mineral soil (0- to 20-cm depth) was categorized on the basis of field measure, we assigned mean clay proportion for each texture type (loamy, 45%; clayey, 60%; sandy or coarse sandy, 10%), following Zhu et al. (30). Soil data summary by ecoregion is available in table S2.
Climate, N deposition, and fire frequency data
At the plot level, MAT and MAP of current climate conditions were derived from the Global Climate Data–WorldClim Version 1.4 (1-km spatial resolution; available at www.worldclim.org) (45). At the hexagon level, MAT and MAP changes over the past three decades, calculated by subtracting mean values of the recent period (1981–2015) and the recent past period (1951–1980) from the PRISM (Parameter-elevation Regressions on Independent Slopes Model) Climate Group (4-km spatial resolution; available at http://prism.oregonstate.edu/) (46), were aggregated with mean. Annual mean of total N deposition (kg N ha−1 year−1) data over the past 15 years (2000–2015) were extracted from the National Atmospheric Deposition Program (available at http://nadp.slh.wisc.edu/) (47) and aggregated with mean at the hexagon level. Although wet N deposition data are available from 1985 to 2016, we used total N deposition data, which include wet and dry N deposition of both organic and inorganic forms, given that recent N deposition data reflect the historical N deposition patterns (31). Fire frequency data were compiled from spatial wildfire occurrence data in the United States over 24 years (1992–2015) (48). The point locations of fire occurrence during the period were converted to kernel density (per km2) raster (1-km spatial resolution) using ArcGIS (version 10.5, Esri Inc., USA) and then aggregated with mean at the hexagon level. Spatial patterns of these data are available in fig. S2.
Tree mycorrhizal type information
Mycorrhizal type was assigned for each tree species present in FIA plots based on peer-reviewed journal publications (49–52). If the species-level mycorrhizal type was not available, we assigned the most frequent mycorrhizal type within genus (or family). To avoid potential false conclusions due to misclassification of mycorrhizal association (53), we further revised the EM tree information based on Tedersoo and Brundrett (54). We then calculated the AM tree dominance (based on basal area) for each plot by dividing the total AM tree basal area by the sum of AM and EM mycorrhizal tree basal area. For some species categorized as both AM and EM, we tested whether assigning them as AM (or EM) changes the patterns of mycorrhizal associations and found no significant effects; therefore, we assigned them a half of the basal area each to AM and EM.
Changes in AM-EM tree dominance during the past three decades
To test changes in AM-EM tree dominance over the past three decades, we used repeated measures of forest plot inventory available in the eastern United States by U.S. Forest Service, the first inventory, collected between 1980 and 1995 (T1; mean inventory year: 1986; 83,866 plots), and the second inventory was the latest completed inventory, which was finished in 2015 for most states (T2; mean inventory year: 2015; 70,715 plots). We only included the eastern United States since repeated FIA measures are not widely available for the western United States. Because T2 inventory measures were not necessarily done in the same plot locations with T1 plots, we aggregated plot-level AM tree dominance and total tree basal area to the hexagon level (a spatial tessellation design used by FIA), following Fei et al. (1). The size of hexagon (1452 km2) was approximately the mean size of counties in the eastern United States. We only included hexagons with at least 10 plots each for both T1 and T2 inventories (mean plot number per each T1 hexagon, 47; mean plot number per each T2 hexagon, 40), resulted in 1785 hexagons for the final analysis.
Statistical analysis
We determined relative effects of climate and total tree basal area (as a surrogate for succession status) on AM tree dominance across the ecoregions (plot level data) using mixed-effects models with a beta distribution and logit link function using R package “glmmTMB” (55). Since AM tree dominance data in our inventory plots included many zeros (only EM trees present in the plot) and ones (only AM trees present), we transformed the data as , where y is the AM tree dominance and N is the sample size, following Averill et al. (31). We added subecoregions (nested units within ecoregion; Fig. 1A) as a random effect in the model to account for the spatial heterogeneity between plots in different subecoregions. We excluded plots with any missing variables and ended up with 132,956 plots for analyses. All predictor variables were standardized by subtracting mean and dividing by 2 SDs to make the regression coefficients for the predictors comparable. AM tree dominance differences between two inventories (T1 and T2) (hexagon-level data) and between saplings and adult trees (plot-level data) were tested using a paired Wilcoxon signed-rank test for each ecoregion. At the ecoregion level, effects of global change drivers (i.e., MAP and MAT changes, N deposition, and fire frequency), AM tree dominance at T1, and basal area on the AM tree dominance change during the past three decades (T2-T1) were tested using mixed-effects models with a Gaussian error distribution and subecoregions as a random effect (see fig. S2 for the spatial patterns of the predictor variables). We also tested the effects of genus-level tree dominance changes in 10 most abundant tree genera (56) in the studied plots on the AM tree dominance change using the same mixed-effects model structure described above. The AM tree genera included Acer, Fraxinus, Prunus, Nyssa, and Ulmus, and the EM tree genera included Quercus, Pinus, Carya, Betula, and Populus. Fire frequency was log-transformed to meet normality assumptions, and all predictor variables were standardized. Bivariate relationships between soil C and N stocks and C:N ratio and AM tree dominance were tested using a linear regression. We further determined relative effects of AM tree dominance and environmental factors on soil attributes using a mixed-effects modeling approach. At the ecoregion level, we modeled each soil attribute (soil C and N stocks and C:N ratio) with a Gaussian error distribution as a function of AM tree dominance, MAP, MAT, basal area, and clay proportion, nested within subecoregions. Soil attributes were log-transformed to meet normality assumptions, and all predictor variables were standardized. All statistical analyses were performed in the R statistical programming environment, version 3.3.2 (57).
Supplementary Material
Acknowledgments
Funding: This research was partially supported by the NSF Macrosystems Biology Program (no. 1638702) and USDA Mcintire-Stennis program to S.F. and by the DOE Terrestrial Ecosystem Science program (no. DESC0016188) to R.P.P. Author contributions: I.J. and S.F. conceived the study. C.M.O. and G.M.D. provided the forest inventory data. I.J. performed the analyses and drafted the manuscript. All authors contributed substantially to revisions and confirmed the final version of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data used in the analyses are available at an open data repository (Purdue University Research Repository; DOI:10.4231/R76D5R7S). Vegetation and soil data are available at FIA DataMart–U.S. Forest Service (https://apps.fs.usda.gov/fia/datamart/). Climate data are available at the Global Climate Data–WorldClim (www.worldclim.org) and the PRISM Climate Group (http://prism.oregonstate.edu/). N deposition data are available at the National Atmospheric Deposition Program (http://nadp.slh.wisc.edu/). Fire frequency data are available at U.S. Forest Service Research Data Archive (DOI:10.2737/RDS-2013-0009.4).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/4/eaav6358/DC1
Fig. S1. Distribution of AM and EM forest trees in geographical and climatic space.
Fig. S2. Spatial patterns of climatic factors (MAP and MAT change), basal area change, total N deposition (2000–2015), and fire frequency (1992–2015) used in the model in Fig. 2B.
Fig. S3. Changes in AM and EM tree basal area in forests in eastern USA during the past three decades (T2-T1).
Fig. S4. Effects of AM tree dominance and environmental factors on the 0- to 20-cm depth mineral soil C and N stocks and C:N ratio in forest ecosystems.
Table S1. Vegetation and climate data summary used for tree mycorrhizal association distribution map in Fig. 1.
Table S2. Soil data summary.
Reference (58)
REFERENCES AND NOTES
- 1.Fei S., Desprez J. M., Potter K. M., Jo I., Knott J. A., Oswalt C. M., Divergence of species responses to climate change. Sci. Adv. 3, e1603055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas R., Canham C., Weathers K., Goodale C., Increased tree carbon storage in response to nitrogen deposition in the US. Nat. Geosci. 3, 13–17 (2009). [Google Scholar]
- 3.Dale V. H., Joyce L. A., McNulty S., Neilson R. P., Ayres M. P., Flannigan M. D., Hanson P. J., Irland L. C., Lugo A. E., Peterson C. J., Simberloff D., Swanson F. J., Stocks B. J., Wotton B. M., Climate change and forest disturbances: Climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduced species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 51, 723–734 (2001). [Google Scholar]
- 4.Riitters K. H., Wickham J. D., O’Neill R. V., Jones K. B., Smith E. R., Coulston J. W., Wade T. G., Smith J. H., Fragmentation of continental United States forests. Ecosystems 5, 815–822 (2002). [Google Scholar]
- 5.Ehrenfeld J. G., Kourtev P., Huang W., Changes in soil functions following invasions of exotic understory plants in deciduous forests. Ecol. Appl. 11, 1287–1300 (2001). [Google Scholar]
- 6.Parmesan C., Yohe G., A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003). [DOI] [PubMed] [Google Scholar]
- 7.van der Putten W. H., Bradford M. A., Brinkman E. P., van de Voorde T. F. J., Veen G. F., Where, when and how plant–soil feedback matters in a changing world. Func. Ecol. 30, 1109–1121 (2016). [Google Scholar]
- 8.Classen A. T., Sundqvist M. K., Henning J. A., Newman G. S., Moore J. A. M., Cregger M. A., Moorhead L. C., Patterson C. M., Direct and indirect effects of climate change on soil microbial and soil microbial-plant interactions: What lies ahead? Ecosphere 6, 1–21 (2015). [Google Scholar]
- 9.S. E. Smith, D. Read, Mycorrhizal Symbiosis (Academic Press, 2008). [Google Scholar]
- 10.van der Heijden M. G. A., Martin F. M., Selosse M.-A., Sanders I. R., Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 205, 1406–1423 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Bennett J. A., Maherali H., Reinhart K. O., Lekberg Y., Hart M. M., Klironomos J., Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355, 181–184 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Jo I., Potter K. M., Domke G. M., Fei S., Dominant forest tree mycorrhizal type mediates understory plant invasions. Ecol. Lett. 21, 217–224 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Phillips R. P., Brzostek E., Midgley M. G., The mycorrhizal-associated nutrient economy: A new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol. 199, 41–51 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Read D. J., Perez-Moreno J., Mycorrhizas and nutrient cycling in ecosystems – a journey towards relevance? New Phytol. 157, 475–492 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Taylor M. K., Lankau R. A., Wurzburger N., Mycorrhizal associations of trees have different indirect effects on organic matter decomposition. J. Ecol. 104, 1576–1584 (2016). [Google Scholar]
- 16.Shi M., Fisher J. B., Brzostek E. R., Phillips R. P., Carbon cost of plant nitrogen acquisition: Global carbon cycle impact from an improved plant nitrogen cycle in the Community Land Model. Glob. Chang. Biol. 22, 1299–1314 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen J. H. C., Aerts R., Cerabolini B., Werger M. J. A., van der Heijden M. G. A., Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129, 611–619 (2001). [DOI] [PubMed] [Google Scholar]
- 18.Sulman B. N., Brzostek E. R., Medici C., Shevliakova E., Menge D. N. L., Phillips R. P., Feedbacks between plant N demand and rhizosphere priming depend on type of mycorrhizal association. Ecol. Lett. 20, 1043–1053 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Averill C., Turner B. L., Finzi A. C., Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543–545 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Averill C., Hawkes C. V., Ectomycorrhizal fungi slow soil carbon cycling. Ecol. Lett. 19, 937–947 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Soudzilovskaia N. A., van der Heijden M. G. A., Cornelissen J. H. C., Makarov M. I., Onipchenko V. G., Maslov M. N., Akhmetzhanova A. A., van Bodegom P. M., Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytol. 208, 280–293 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Craig M. E., Turner B. L., Liang C., Clay K., Johnson D. J., Phillips R. P., Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob. Chang. Biol. 24, 3317–3330 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Terrer C., Vicca S., Hungate B. A., Phillips R. P., Prentice I. C., Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 353, 72–74 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Terrer C., Vicca S., Stocker B. D., Hungate B. A., Phillips R. P., Reich P. B., Finzi A. C., Prentice I. C., Ecosystem responses to elevated CO2 governed by plant–soil interactions and the cost of nitrogen acquisition. New Phytol. 217, 507–522 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Midgley M. G., Phillips R. P., Mycorrhizal associations of dominant trees influence nitrate leaching responses to N deposition. Biogeochemistry 117, 241–253 (2014). [Google Scholar]
- 26.Gehring C. A., Sthultz C. M., Flores-Rentería L., Whipple A. V., Whitham T. G., Tree genetics defines fungal partner communities that may confer drought tolerance. Proc. Natl. Acad. Sci. U.S.A. 114, 11169–11174 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.N. A. Soudzilovskaia, S. Vaessen, M. van’t Zelfde, N. Raes, Global patterns of mycorrhizal distribution and their environmental drivers, in Biogeography of Mycorrhizal Symbiosis, L. Tedersoo, Ed. (Springer International Publishing, 2017), vol. 230, pp. 223–235. [Google Scholar]
- 28.Swaty R., Michael H. M., Deckert R., Gehring C. A., Mapping the potential mycorrhizal associations of the conterminous United States of America. Fungal Ecol. 24, 139–147 (2016). [Google Scholar]
- 29.Read D. J., Mycorrhizas in ecosystems. Experientia 47, 376–391 (1991). [Google Scholar]
- 30.Zhu K., McCormack M. L., Lankau R. A., Egan J. F., Wurzburger N., Association of ectomycorrhizal trees with high carbon-to-nitrogen ratio soils across temperate forests is driven by smaller nitrogen not larger carbon stocks. J. Ecol. 106, 524–535 (2018). [Google Scholar]
- 31.Averill C., Dietze M. C., Bhatnagar J. M., Continental-scale nitrogen pollution is shifting forest mycorrhizal associations and soil carbon stocks. Glob. Chang. Biol. 24, 4544–4553 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Chen W., Koide R. T., Adams T. S., DeForest J. L., Cheng L., Eissenstat D. M., Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. U.S.A. 113, 8741–8746 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin G., McCormack M. L., Ma C., Guo D., Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol. 213, 1440–1451 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Zhang H.-Y., Lü X.-T., Hartmann H., Keller A., Han X.-G., Trumbore S., Phillips R. P., Foliar nutrient resorption differs between arbuscular mycorrhizal and ectomycorrhizal trees at local and global scales. Glob. Ecol. Biogeogr. 27, 875–885 (2018). [Google Scholar]
- 35.Nowacki G. J., Abrams M. D., The demise of fire and “mesophication” of forests in the eastern United States. BioScience 58, 123–138 (2008). [Google Scholar]
- 36.Keller A. B., Phillips R. P., Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytol. 222, 556–564 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Lovett G. M., Weathers K. C., Arthur M. A., Control of nitrogen loss from forested watersheds by soil carbon: Nitrogen ratio and tree species composition. Ecosystems 5, 712–718 (2002). [Google Scholar]
- 38.Cotrufo M. F., Wallenstein M. D., Boot C. M., Denef K., Paul E., The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 19, 988–995 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Hobbie S. E., Plant species effects on nutrient cycling: Revisiting litter feedbacks. Trends Ecol. Evol. 30, 357–363 (2015). [DOI] [PubMed] [Google Scholar]
- 40.Midgley M. G., Phillips R. P., Resource stoichiometry and the biogeochemical consequences of nitrogen deposition in a mixed deciduous forest. Ecology 97, 3369–3378 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Treseder K. K., Allen E. B., Egerton-Warburton L. M., Hart M. M., Klironomos J. N., Maherali H., Tedersoo L., Arbuscular mycorrhizal fungi as mediators of ecosystem responses to nitrogen deposition: A trait-based predictive framework. J. Ecol. 106, 480–489 (2018). [Google Scholar]
- 42.Goswami S., Fisk M. C., Vadeboncoeur M. A., Garrison-Johnston M., Yanai R. D., Fahey T. J., Phosphorus limitation of aboveground production in northern hardwood forests. Ecology 99, 438–449 (2018). [DOI] [PubMed] [Google Scholar]
- 43.U.S. Department of Agriculture, Forest Service (USDA Forest Service), “The forest inventory and analysis database: Database description and user guide for Phase 2 (version 7.2)” (USDA Forest Service, 2018); www.fia.fs.fed.us/library/database-documentation/current/ver72/FIADB%20User%20Guide%20P2_7-2_final.pdf.
- 44.U.S. Department of Agriculture, Forest Service (USDA Forest Service), “The forest inventory and analysis database: Database description and user guide for Phase 3 (version 6.0.1)” (USDA Forest Service, 2014); www.fia.fs.fed.us/library/database-documentation/current/ver60/FIADB%20User%20Guide%20P3_6-0-1_final.pdf.
- 45.Hijmans R. J., Cameron S.E., Parra J.L., Jones P.G., Jarvis A., Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 (2005). [Google Scholar]
- 46.PRISM Climate Group, Oregon State University, “PRISM climate data” (2004); http://prism.oregonstate.edu.
- 47.NADP Program Office, “National atmospheric deposition program, (NRSP-3)” (WI, USA, 2018); http://nadp.slh.wisc.edu/.
- 48.K. C. Short, Spatial wildfire occurrence data for the United States, 1992–2015 [FPA FOD 20170508] (4th Edition). Forest Service Research Data Archive (Fort Collins, CO, USA, 2017); 10.2737/RDS-2013-0009.4. [DOI]
- 49.Brundrett M. C., Murase G., Kendrick B., Comparative anatomy of roots and mycorrhizae of common Ontario trees. Can. J. Bot. 68, 551–578 (1990). [Google Scholar]
- 50.Bueno C. G., Moora M., Gerz M., Davison J., Öpik M., Pärtel M., Helm A., Ronk A., Kühn I., Zobel M., Plant mycorrhizal status, but not type, shifts with latitude and elevation in Europe. Glob. Ecol. Biogeogr. 26, 690–699 (2017). [Google Scholar]
- 51.Wang B., Qiu Y. L., Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16, 299–363 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Akhmetzhanova A. A., Soudzilovskaia N. A., Onipchenko V. G., Cornwell W. K., Agafonov V. A., Selivanov I. A., Cornelissen J. H. C., A rediscovered treasure: Mycorrhizal intensity database for 3000 vascular plant species across the former Soviet Union: Ecological Archives E093-059. Ecology 93, 689–690 (2012). [Google Scholar]
- 53.Brundrett M., Tedersoo L., Misdiagnosis of mycorrhizas and inappropriate recycling of data can lead to false conclusions. New Phytol. 221, 18–24 (2019). [DOI] [PubMed] [Google Scholar]
- 54.L. Tedersoo, M. C. Brundrett, Evolution of ectomycorrhizal symbiosis in plants, in Biogeography of Mycorrhizal Symbiosis, L. Tedersoo, Ed. (Springer International Publishing, 2017), vol. 230, pp. 407–467. [Google Scholar]
- 55.A. Magnusson, H. Skaug, A. Nielsen, C. Berg, K. Kristensen, M. Maechler, K. van Bentham, B. Bolker, M. Brooks, Generalized linear mixed models using template model builder (2017); https://cran.r-project.org/web/packages/glmmTMB/glmmTMB.pdf.
- 56.Knott J. A., Desprez J. M., Oswalt C. M., Fei S., Shifts in forest composition in the eastern United States. Forest Ecol and Mgmt 433, 176–183 (2019). [Google Scholar]
- 57.R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2014).
- 58.D. T. Cleland, J. A. Freeouf, J. E. Keys, G. J. Nowacki, C. A. Carpenter, W. H. McNab, Ecological subregions: Sections and subsections for the conterminous United States (General Technical Report WO-76D, USDA Forest Service, 2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/4/eaav6358/DC1
Fig. S1. Distribution of AM and EM forest trees in geographical and climatic space.
Fig. S2. Spatial patterns of climatic factors (MAP and MAT change), basal area change, total N deposition (2000–2015), and fire frequency (1992–2015) used in the model in Fig. 2B.
Fig. S3. Changes in AM and EM tree basal area in forests in eastern USA during the past three decades (T2-T1).
Fig. S4. Effects of AM tree dominance and environmental factors on the 0- to 20-cm depth mineral soil C and N stocks and C:N ratio in forest ecosystems.
Table S1. Vegetation and climate data summary used for tree mycorrhizal association distribution map in Fig. 1.
Table S2. Soil data summary.
Reference (58)


