Abstract
Background
Idiopathic Parkinson's disease (IPD) is a neurodegenerative disorder, with the severity of the disability usually increasing with disease duration. IPD affects patients' health‐related quality of life, disability, and impairment. Current rehabilitation approaches have limited effectiveness in improving outcomes in patients with IPD, but a possible adjunct to rehabilitation might be non‐invasive brain stimulation by transcranial direct current stimulation (tDCS) to modulate cortical excitability, and hence to improve these outcomes in IPD.
Objectives
To assess the effectiveness of tDCS in improving motor and non‐motor symptoms in people with IPD.
Search methods
We searched the following databases (until February 2016): the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library ; 2016 , Issue 2), MEDLINE, EMBASE, CINAHL, AMED, Science Citation Index, the Physiotherapy Evidence Database (PEDro), Rehabdata, and Inspec. In an effort to identify further published, unpublished, and ongoing trials, we searched trial registers and reference lists, handsearched conference proceedings, and contacted authors and equipment manufacturers.
Selection criteria
We included only randomised controlled trials (RCTs) and randomised controlled cross‐over trials that compared tDCS versus control in patients with IPD for improving health‐related quality of life , disability, and impairment.
Data collection and analysis
Two review authors independently assessed trial quality (JM and MP) and extracted data (BE and JM). If necessary, we contacted study authors to ask for additional information. We collected information on dropouts and adverse events from the trial reports.
Main results
We included six trials with a total of 137 participants. We found two studies with 45 participants examining the effects of tDCS compared to control (sham tDCS) on our primary outcome measure, impairment, as measured by the Unified Parkinson's Disease Rating Scale (UPDRS). There was very low quality evidence for no effect of tDCS on change in global UPDRS score ( mean difference (MD) ‐7.10 %, 95% confidence interval (CI ‐19.18 to 4.97; P = 0.25, I² = 21%, random‐effects model). However, there was evidence of an effect on UPDRS part III motor subsection score at the end of the intervention phase (MD ‐14.43%, 95% CI ‐24.68 to ‐4.18; P = 0.006, I² = 2%, random‐effects model; very low quality evidence). One study with 25 participants measured the reduction in off and on time with dyskinesia, but there was no evidence of an effect (MD 0.10 hours, 95% CI ‐0.14 to 0.34; P = 0.41, I² = 0%, random‐effects model; and MD 0.00 hours, 95% CI ‐0.12 to 0.12; P = 1, I² = 0%, random‐ effects model, respectively; very low quality evidence).
Two trials with a total of 41 participants measured gait speed using measures of timed gait at the end of the intervention phase, revealing no evidence of an effect ( standardised mean difference (SMD) 0.50, 95% CI ‐0.17 to 1.18; P = 0.14, I² = 11%, random‐effects model; very low quality evidence). Another secondary outcome was health‐related quality of life and we found one study with 25 participants reporting on the physical health and mental health aspects of health‐related quality of life (MD 1.00 SF‐12 score, 95% CI ‐5.20 to 7.20; I² = 0%, inverse variance method with random‐effects model; very low quality evidence; and MD 1.60 SF‐12 score, 95% CI ‐5.08 to 8.28; I² = 0%, inverse variance method with random‐effects model; very low quality evidence, respectively). We found no study examining the effects of tDCS for improving activities of daily living. In two of six studies, dropouts , adverse events, or deaths occurring during the intervention phase were reported. There was insufficient evidence that dropouts , adverse effects, or deaths were higher with intervention (risk difference (RD) 0.04, 95% CI ‐0.05 to 0.12; P = 0.40, I² = 0%, random‐effects model; very low quality evidence).
We found one trial with a total of 16 participants examining the effects of tDCS plus movement therapy compared to control (sham tDCS) plus movement therapy on our secondary outcome, gait speed at the end of the intervention phase, revealing no evidence of an effect (MD 0.05 m/s, 95% CI ‐0.15 to 0.25; inverse variance method with random‐effects model; very low quality evidence). We found no evidence of an effect regarding differences in dropouts and adverse effects between intervention and control groups (RD 0.00, 95% CI ‐0.21 to 0.21; Mantel‐Haenszel method with random‐effects model; very low quality evidence).
Authors' conclusions
There is insufficient evidence to determine the effects of tDCS for reducing off time ( when the symptoms are not controlled by the medication) and on time with dyskinesia ( time that symptoms are controlled but the person still experiences involuntary muscle movements ) , and for improving health‐ related quality of life, disability, and impairment in patients with IPD. Evidence of very low quality indicates no difference in dropouts and adverse events between tDCS and control groups.
Keywords: Adult, Humans, Transcranial Direct Current Stimulation, Dyskinesias, Dyskinesias/physiopathology, Dyskinesias/therapy, Parkinson Disease, Parkinson Disease/physiopathology, Parkinson Disease/therapy, Quality of Life, Randomized Controlled Trials as Topic
Plain language summary
Non‐invasive electrical brain stimulation for improving rehabilitation outcomes in patients with idiopathic Parkinson's disease (IPD)
Review question
To assess the effectiveness of electrical brain stimulation in improving motor and non‐motor symptoms in people with idiopathic Parkinson's disease (IPD) .
Background
IPD is a neurodegenerative disorder, with the severity of the disability usually increasing with disease duration. IPD affects patients' health‐related quality of life, disability, and impairment. Current rehabilitation strategies have limited effectiveness in improving these outcomes. One possibility for enhancing the effects of rehabilitation might be the addition of non‐invasive electrical brain stimulation through a technique known as transcranial direct current stimulation (tDCS). This technique can alter how the brain works and may improve health‐related quality of life, disability, and impairment in function in patients with IPD. However, the effectiveness of this intervention for improving rehabilitation outcomes is still unknown.
Search date
The latest search was performed on 17 February 2016.
Study characteristics
We included six trials involving 137 participants. The duration of treatment in the included trials ranged from a single session to five consecutive sessions of tDCS.
Key results
From the six trials involving 137 participants, we found there was insufficient evidence to determine how much of an effect there is from tDCS in enhancing rehabilitation outcomes regarding reduction in off time (when the symptoms are not controlled by the medication) and on time with dyskinesia (time that symptoms are controlled but the person still experiences involuntary muscle movements) , and for improving health‐ related quality of life, disability, and impairment in patients with IPD. However, tDCS may improve impairment regarding motor symptoms in patients with IPD. We found no study examining the effects of tDCS for improving activities of daily living. Proportions of adverse events and people discontinuing the study were comparable between groups.
Quality of evidence
All findings are based on evidence of very low quality. That means that we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Summary of findings
Summary of findings for the main comparison. Transcranial direct current stimulation (tDCS) versus sham tDCS for patients with idiopathic Parkinson's disease.
| Transcranial direct current stimulation (tDCS) versus sham tDCS for patients with odiopathic Parkinson's disease | ||||||
|
Patient or population: Patients with
idiopathic Parkinson's disease
Settings: inpatient and outpatient settings in high‐income countries
Intervention: transcranial direct current stimulation (tDCS) Comparison: sham tDCS | ||||||
| Outcomes | Illustrative comparative risks/scores* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk/scores | Corresponding risk/scores | |||||
| Control (sham tDCS) | Transcranial Direct Current Stimulation (tDCS) | |||||
| Impairment/ disability Unified Parkinson's Disease Rating Scale (UPDRS; change in global UPDRS score). Scale from: 0 to 199 (lower score means less impairment/disability) | The mean impairment/disability in the control groups was ‐9.5%1 | The mean impairment/disability in the intervention groups was 7.1% lower (19.18% lower to 4.97% higher) | 45 (2 studies) | ⊕⊝⊝⊝ very low2,3 | ||
| Off time Self assessment (logfile) Scale from: 0 to 72 (lower score means less impairment/disability) | The mean off time in the control groups was 2.4 hours4 | The mean off time in the intervention groups was 0.10 hours higher (0.14 lower to 0.34 higher) | 25 (1 study) | ⊕⊝⊝⊝ very low2,3 | ||
| On time with dyskinesia Self assessment (logfile) Scale from: 0 to 72 (lower score means less impairment/disability) | The mean on time with dyskinesia in the control groups was 3.2 hours4 | The mean on time with dyskinesia in the intervention groups was 0.00 hours higher (0.12 lower to 0.12 higher) | 25 (1 study) | ⊕⊝⊝⊝ very low2,3 | ||
| Gait speed at the end of the intervention phase Measures of timed gait. Scale from: 0 (lower score means less impairment/disability) | The mean gait speed at the end of the intervention phase in the control groups was 0 NA6 | The mean gait speed at the end of the intervention phase in the intervention groups was 0.50 standard deviations higher (0.17 lower to 1.18 higher) | 41 (2 studies) | ⊕⊝⊝⊝ very low2,3 | SMD 0.50 (‐0.17 to 1.18; a standard deviation of 0.50 represents a moderate effect (Cohen 1988)) | |
|
Health‐related quality of life ‐
physical health
SF‐ 12v2. Scale from: 0 to 100 (scores less than 50 indicate health above the mean and scores less than 50 indicate health below the mean) |
The mean health‐related quality of life ‐ physical health in the control groups was 41 SF‐12v2 composite score4 | The mean health‐related quality of life ‐ physical health in the intervention groups was 1.00 higher (5.2 lower to 7.2 higher) | 25 (1 study) | ⊕⊝⊝⊝ very low2,3 | ||
| Health‐related quality of life ‐ mental health SF‐12v2. Scale from: 0 to 100 (scores less than 50 indicate health above the mean and scores less than 50 indicate health below the mean) | The mean health‐related quality of life ‐ mental health in the control groups was 52.5 SF‐12 composite score4 | The mean health‐related quality of life ‐ mental health in the intervention groups was 1.60 higher (5.08 lower to 8.28 higher) | 25 (1 study) | ⊕⊝⊝⊝ very low2,3 | ||
| Safety/ acceptability Dropouts and adverse events (lower rate means better safety/acceptability) | Study population | See comment | 101 (4 studies) | ⊕⊝⊝⊝ very low3,5 | Risks were calculated from pooled RDs RD 0.04, 95% CI ‐0.05 to 0.12; I² = 0% |
|
| 0 per 1000 | 4 per 1000 (0 to 12) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RD : risk difference; RR: risk ratio; S MD : standard ised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded one level due to serious risk of bias (allocation sequence generation, concealment, and blinding of outcome assessors unclear). 2 Downgraded two levels due to very serious imprecision (total sample size being less than 400; 95% CI includes appreciable benefit and harm). 3 Down graded one level due to serious risk of bias (allocation concealment and blinding of participants and personnel unclear). 4 No calculation possible, since different studies used various outcome measurements for the same outcome.
Background
Description of the condition
Idiopathic Parkinson' s disease (IPD) is a degenerative disorder that is characterised clinically by tremor, rigidity, bradykinesia (slowness of movement) , and postural instability.
Parkinson' s disease is estimated to have a crude incidence rate of 4.5 to 19 per 100,000 population per year (Baker 2006). Age‐adjusted prevalence estimations range between 72 and 258.8 per 100,000 population, and age‐adjusted incidence rates range from 9.7 to 13.8 per 100,000 population per year (Baker 2006). The male‐to‐female ratio is estimated to be 1.9 (Baker 2006). A recent study from Scotland found no association between incidence and socioeconomic status (Caslake 2013).
People suffering from Parkinson' s disease utilise health services significantly more often than healthy individuals, and this is associated with costs that are approximately double that the of a control population (Baker 2006). Almost every second US Dollar (USD) of associated annual direct and indirect costs of the disease is generated by productivity loss, and every fifth USD of associated costs is generated by inpatient and uncompensated care (Huse 2005).
At all stages of the disease, disability occurs, and the severity of disability usually increases with disease duration. Pain, motor impairment, depression, and insomnia affect the health‐related quality of life of people with IPD (Shearer 2012), particularly in the early stages of the disease. During disease progression, other factors become more important, particularly dementia (Schrag 2000a), and the prevalence of psychosis increases (Aarsland 1999). Patients usually have gait impairment, difficulty linking movements together smoothly, and episodes of freezing. These problems, together with balance disturbances, lead to an increased incidence of falls with concurrent risk of fractures. Nearly one‐third of patients with IPD have had a hip fracture within 10 years of their diagnosis (Johnell 1992).
Current management of IPD focuses on pharmacological therapy; at present, levodopa is regarded as the most effective treatment, and is prescribed most often (Crosby 2003). However, many patients suffer from side effects of levodopa, including abnormal involuntary movements known as dyskinesias (Jankovic 2000). Drugs other than levodopa, such as dopamine agonists are used increasingly as first‐line treatment to reduce motor complications, but with the tradeoff of generating other relevant side effects, and leading to poorer symptom control (Stowe 2008).
A large proportion of patients taking medication suffer from significant motor complications, such as motor fluctuation or dyskinesias (Nutt 1990). These complications cause functional disability, affect the person's quality of life, and are difficult to manage with available drug strategies (Motto 2003). These patients could be considered candidates for deep brain stimulation of the basal ganglia (Motto 2003). However, debate continues concerning risks and benefits of this surgical approach; whereas motor symptoms can be improved by deep brain stimulation , evidence is insufficient to show improvement in non‐motor symptoms (Fasano 2012).
Despite optimal medical and surgical therapies for IPD, patients develop progressive disability (Deane 2001). Hence, novel treatment approaches, aimed at reducing movement disorders in IPD are needed (Mehrholz 2010).
Description of the intervention
Non‐invasive brain stimulation by transcranial direct current stimulation (tDCS) might offer such a treatment approach. tDCS modulates cortical excitability by applying a direct current to the skull (Bindman 1964; Nowak 2009; Purpura 1965). Stimulation of the central nervous system by tDCS is relatively inexpensive and easy to administer when compared with other techniques for brain stimulation, such as repetitive transcranial magnetic stimulation (rTMS) and epidural stimulation (Hesse 2011). tDCS usually is delivered via saline‐soaked surface sponge electrodes, which are connected to a direct current stimulator that produces low intensities of a current (Lang 2005). Different techniques might be applied: the anodal electrode might be placed over the presumed area of interest of the brain and the cathodal electrode placed above the contralateral orbit (anodal stimulation), or vice versa (cathodal stimulation) (Hesse 2011), or both at the same time (bi‐hemispheric or dual stimulation) (Lindenberg 2010).
How the intervention might work
Depending on the type of stimulation (anodal or cathodal), tDCS might lead to increased or decreased cortical excitability, respectively (Bindman 1964; Purpura 1965). This might be due to a shift in the resting potential of the brain's nerves (Purpura 1965). Stimulation lasting for longer than five minutes might induce significant after‐effects, which could last up to several hours (Nitsche 2001; Nitsche 2003). Anodal stimulation might lead to depolarisation of the neuronal membranes, and therefore may result in greater cortical excitability, and vice versa (Bindman 1964). This effect might be used to facilitate motor learning in healthy people (Boggio 2006; Jeffery 2007; Reis 2009), and seems therefore to be a promising option in neurorehabilitation of individuals with movement disorders because it could be delivered with standard rehabilitation simultaneously, and is inexpensive.
Why it is important to do this review
Recent studies have suggested that tDCS might be beneficial for improving movement disorders in people with Parkinson's disease (Benninger 2010; Fregni 2006; Gruner 2010; Wu 2008). However, no systematic review has examined the available literature on the effectiveness and acceptability of this treatment option.
Objectives
To assess the effectiveness of transcranial direct current stimulation (tDCS) in improving motor and non‐motor symptoms in people with idiopathic Parkinson's disease (IPD) in comparison to any active or passive comparator.
Methods
Criteria for considering studies for this review
Types of studies
We included genuine randomised controlled trials (RCTs) with parallel‐group or cross‐over designs. We did not include quasi‐RCTs.
Types of participants
We included adult participants (18+ years of age) with idiopathic Parkinson's disease (IPD) who have been diagnosed by the UK Parkinson' s Disease Brain Bank criteria (Hughes 1992), or by a clinical definition, regardless of medication, duration of illness, presence of motor fluctuations, duration of treatment, or level of initial impairment.
Types of interventions
We compared any kind of truly active transcranial direct current stimulation (tDCS) for improving movement disorders versus any kind of placebo or control intervention (i.e. sham tDCS or no intervention). We defined active tDCS as the longer‐lasting (longer than one minute) application of a direct current to the brain to stimulate the affected hemisphere or to inhibit the healthy hemisphere, or to do both simultaneously. We defined sham tDCS as a short‐term direct current stimulation (less than one minute; this is approximately the time it usually takes to fade in and fade out the current in sham‐controlled tDCS trials without producing perceivable sensations on the skin (Gandiga 2006)), or the placement of electrodes with no application of direct current.
Types of outcome measures
Primary outcomes
As primary outcomes, we considered the following.
Impairment/disability, measured by the Unified Parkinson's Disease Ranking Scale (UPDRS; Fahn 1987).
Reduction in off time (when the symptoms are not controlled by the medication)
Reduction in on time with dyskinesia (time that symptoms are controlled but the person still experiences involuntary muscle movements) .
We classified the clinically important difference in UPDRS change according to Shulman 2010 (i.e. 2.5 points for a minimal, 5.2 points for a moderate, and 10.8 points for a large clinically important difference ).
We reported primary outcome measures at the end of the intervention phase, and if sufficient data were available, at least three months after the end of the intervention phase.
Secondary outcomes
As secondary outcomes, we considered the following.
Specific measures of impairment.
Timed tests of gait.
Stride length.
Cadence.
Bradykinesia in the upper extremity.
Health‐related quality of life; possible outcome measures include the following.
PDQ‐39 (Marinus 2002).
PDQL (Marinus 2002).
EQ‐5D (Schrag 2000b).
Activities of daily living; a possible outcome measure is:
the Schwab and England A ctivities of D aily L iving scale (Schwab 1969).
-
Safety/acceptability .
Dropouts .
A dverse events (including death from al l causes) .
Search methods for identification of studies
Electronic searches
We used a modified search strategy developed by the Cochrane Stroke Group to search the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library ; 2016 , Issue 2 ), MEDLINE (from 1948 to 16 February 2016), EMBASE (from 1980 to 16 February 2016), CINAHL (from 1982 to 16 February 2016), AMED (from 1985 to 16 February 2016), Web of Science Core Collection (from 1900 to 16 February 2016), the Physiotherapy Evidence Database (PEDro; www.pedro.org.auto 16 February 2016), Rehabdata (from 1956 to 16 February 2016), and the engineering database Inspec (from 1969 to 16 February 2016 ).
The search strategy can be found in Appendix 1.
We identified and searched the following ongoing trial and research registers.
Current Controlled Trials (http://www.controlled‐trials.com/).
ClinicalTrials.gov (http://clinicaltrials.gov/ct2/home).
European Union (EU) Clinical Trials Register (https://www.clinicaltrialsregister.eu/).
World Health Organization (WHO) International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Searching other resources
To identify further published, unpublished, and ongoing trials not available in the aforementioned databases, we:
identified and handsearched relevant conference proceedings;
screened reference lists of relevant articles, reviews, and textbooks;
contacted authors of identified trials and other researchers in the field;
searched the Science Citation Index cited references;
-
contacted the following equipment manufacturers:
DJO global http://djoglobal.com/contact‐us;
Grindhouse http://www.grindhousewetware.com;
Trans Cranial Technologies http://www.trans‐cranial.com;
Soterix Medical http://soterixmedical.com/;
Activatek http://activatekinc.com;
Zhinheng Electronics http://cszhineng.diytrade.com;
Magstim www.magstim.com;
Neuroelectrics www.neuroelectrics.com;
Neuroconn www.neuroconn.de;
Newronika www.newronika.it;
and searched Google Scholar (http://scholar.google.com/).
We searched for relevant trials in all languages and arranged translation of trial reports published in languages other than English.
Data collection and analysis
Selection of studies
Two review authors (BE and JM) read the titles and abstracts of the records identified through the electronic searches and eliminated irrelevant references that clearly did not match our inclusion criteria. We retrieved the full‐ text of the remaining studies, and two review authors (JK and MP) independently checked relevant studies for inclusion according to our inclusion criteria (types of studies, participants, and aims of interventions). We resolved disagreements by discussion with all review authors. If we needed further information to resolve disagreements concerning including or excluding a study, we contacted the trial authors to request the required information. We listed in the 'Characteristics of excluded studies' table all studies that appeared to match our inclusion criteria regarding type of study, participants, or types of interventions, but did not actually match them.
Data extraction and management
Two review authors (BE and JM) independently extracted trial and outcome data from the selected trials. If one of the review authors was involved in an included trial, another review author extracted trial and outcome data from such a trial.
We used checklists to independently extract data regarding the following aspects and provided them in the 'Characteristics of included studies' table.
Methods of random sequence generation.
Methods of allocation concealment.
Blinding of assessors.
Use of an intention‐to‐treat (ITT) analysis.
Adverse effects and dropouts.
Important differences in prognostic factors.
Participants (country, number of participants, age, gender, stage of Parkinson's disease as assessed by Hoehn and Yahr at entry to the study ( Hoehn 1967 ), 'on' / 'off' state of dopaminergic medication, inclusion and exclusion criteria).
Comparison (details of interventions in treatment and control groups, duration of treatment, and details of co‐interventions in study groups).
Outcomes.
Time point of measurement.
Further, we extracted data of initial functional ability and of initial level of function, and we presented the content of each included study in detail in a table.
All review authors checked the extracted data for agreement. If necessary, we contacted trialists to request more information.
Assessment of risk of bias in included studies
Two review authors (JK and MP) assessed the risk of bias in the included trials according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We categorise d the risk of bias as ‘low’, ‘high’ or ‘unclear’ for the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Selective outcome reporting.
Incomplete outcome data.
Other bias.
We described the agreement between review authors for assessment of methodological quality, and we resolved disagreements in methodological assessment by reaching consensus through discussion. We contacted trialists for clarification and to request missing information.
Measures of treatment effect
For outcomes measured with continuous data, we used means and standard deviations (SDs) to generate effect estimates. We calculated a summary estimate of the mean difference (MD) with 95% confidence intervals (CIs). If studies assessing the same outcome used different scales, we calculated standardised mean differences (SMDs) instead of MDs. For all binary outcomes, we calculated risk ratios (RRs) with 95% CIs. If only few events occurred, we calculated risk differences (RDs) with 95% CIs instead.
For all statistical comparisons, we used the current version of Review Manager 5 (RevMan 2014).
Unit of analysis issues
We analysed both periods of randomised cross‐over trials. If sham or active control groups from the same study investigated the same content, we combined these into one group for each (e.g. if two sham control groups were used, we combined these into a single group for comparison with the intervention group).
Dealing with missing data
In case of missing information, we contacted the authors of the respective studies to ask for further clarification. If we were not able to receive the missing data of a study from the authors, we classified the study as 'awaiting classification'.
Assessment of heterogeneity
We used the I² statistic to assess heterogeneity. In cases in which I² was greater than 50%, we assumed substantial heterogeneity.
Assessment of reporting biases
We examined the presence of a reporting bias by visual inspection of funnel plots using all studies that met basic entry criteria, if appropriate (Sterne 2011).
Data synthesis
We used a random‐effects model, regardless of the level of heterogeneity. Thus, in the case of heterogeneity, we did not violate the preconditions of a fixed‐effect model approach.
'Summary of findings' table and assessing the quality of evidence
We assessed the quality of evidence using the grading system developed by the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) collaboration (Guyatt 2008). Following the methods developed by the GRADE Working Group, two review authors independently judged the quality of the body of evidence using a transparent structure that involved appraising factors such as research design, implementation, imprecision, inconsistency, indirectness, and reporting bias. We used the software, GRADEprofiler, to assist in the process of a GRADE assessment ( GRADEproGDT 2015). We implemented a 'S ummary of findings' table to describe the quality of the evidence for the main comparison 'transcranial direct current stimulation (tDCS) versus sham tDCS' . If data on more than seven outcomes had to be reported, we presented the outcomes with the highest prioritisation in the 'Summary of findings' table , regardless of the availability of data.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we conducted an analysis of the following subgroups for our primary outcome, impairment/disability, measured by the UPDRS .
On/off state of the illness.
Type of stimulation: cathodal versus anodal.
Type of control intervention (sham tDCS or nothing).
All stratified (subgroup) analyses were accompanied by appropriate tests for interaction (statistical tests for subgroup differences as described in the Cochrane Handbook for Systematic Review of Interventions (Deeks 2011), and as implemented in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We intended to undertake a sensitivity analysis regarding the risk of bias of included studies to assess the robustness of our results. We planned to analyse the influence of studies that did not clearly state or did not utilise proper methods for: (1) generating the randomisation schedule; (2) allocation concealment; and (3) did not use an intention‐to‐treat analysis.
Results
Description of studies
Results of the search
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification , and Characteristics of ongoing studies. We identified a total of 4420 unique records through the searches. After screening titles and abstracts, we excluded 4167 records and obtained the full‐ text of the remaining 53 articles. After further assessment, we determined that six studies met the review inclusion criteria. The flow of references is shown in Figure 1.
1.
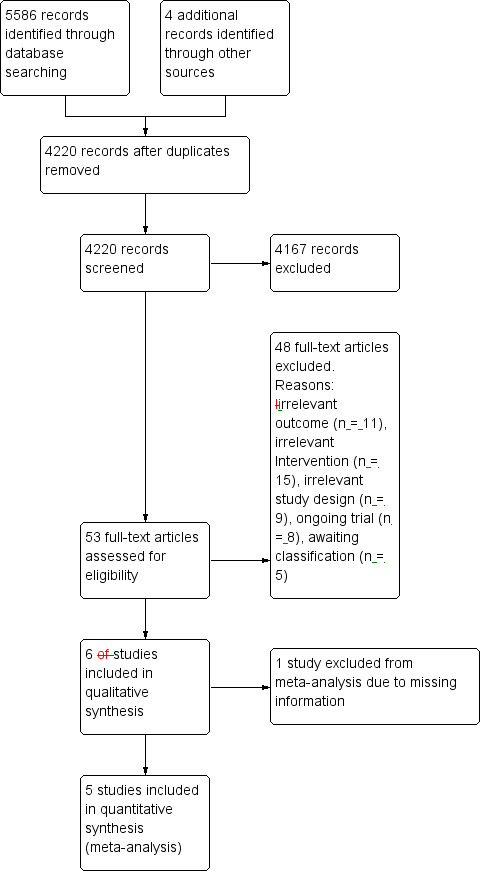
Study flow diagram. Please note, that the number of full‐texts may be unequal to the number of studies (i.e. the studies Kaski 2014a and Kaski 2014b have been reported in a single full‐text).
Included studies
We included six studies involving a total of 137 participants (Benninger 2010; Kaski 2014a ; Kaski 2014b; Monte‐Silva 2013; Valentino 2014; Verheyden 2013 ; see Characteristics of included studies). Kaski 2014a; and Kaski 2014b are two arms of the same study. Four studies investigated the effects of transcranial direct current stimulation (tDCS) versus sham tDCS (Benninger 2010; Kaski 2014b; Valentino 2014; Verheyden 2013), whereas two trials with 28 participants investigated the effects of tDCS plus movement therapy versus sham tDCS plus movement therapy (Kaski 2014a; Monte‐Silva 2013). Four trials with 92 participants were randomly assigned cross‐over trials (Kaski 2014a; Kaski 2014b; Valentino 2014; Verheyden 2013), whereas the remaining two, with 45 analysed participants, were randomised parallel‐group trials (RCTs) (Benninger 2010; Monte‐Silva 2013). All six studies included one intervention group and one control group, or the patients received the intervention and served as their own control, respectively. Benninger 2010, Monte‐Silva 2013 and Valentino 2014 were the only studies that examined the effects of tDCS on the Unified Parkinson's Disease Ranking Scale (UPDRS) or UPDRS motor subscore , and four studies examined the effects of tDCS on gait speed (Benninger 2010; Kaski 2014a; Kaski 2014b; Verheyden 2013). One of the included studies was conducted in Italy, one in the USA, one in Brazil, and one in the UK. In two studies, the country was not clearly stated. Widely used outcomes were measures of timed gait and the UPDRS. For a comprehensive summary of participant characteristics, please see Table 2; for a comprehensive summary of intervention characteristics, dropouts and adverse events, please see Table 3.
1. Patient characteristics.
| Study ID | EXP: age, mean (SD) | CTL: age, mean (SD) | Hoehn and Yahr stages | EXP: duration of disease | CTL: duration of disease | EXP: female/male | CON: female/male |
| Benninger 2010 | 64 (9) years | 64 (9) years | 2 to 3 | 11 (7) months | 9 (3) years | 5/7 | 4/9 |
| Kaski 2014a | No demographical data provided | ||||||
| Kaski 2014b | No demographical data provided | ||||||
| Monte‐Silva 2013 | No demographical data provided | ||||||
| Valentino 2014 | 72 (4) years | 2.5 to 4 | 11 (5) years | 5/5 | |||
| Verheyden 2013 | 71 (7) years | 1 to 4 | 9 (4) years | not reported | |||
CTL: control group EEG: electroencephalography EXP: experimental group NA: not applicable SD: standard deviation
2. Demographics of studies, including dropouts and adverse events.
| Study ID | Type of stimulation (polarity) | Electrode position and size | Treatment intensity | Base treatment | Dropouts | Adverse events | Reasons for dropouts and adverse events in the experimental group | Reasons for dropouts and adverse events in the control group | Source of information | |
| Benninger 2010 | Anodal tDCS | Anode and cathode were placed on the forehead | 2 mA for 20 minutes | Anodal or sham tDCS for 4 days once a day | None | None | 1 | Skin defect (similar to 1st degree burn) due to malpositioned electrodes | NA | Published |
| Sham tDCS | 1 mA for 1 to 2 minutes | None | ||||||||
| Kaski 2014a; | Anodal tDCS | Saline‐soaked 40 cm² sponge electrode was placed over the Cz area of international 10‐20 EEG electrode placement system (anode) and over the inion (cathode), respectively | 2 mA for 15 minutes | Anodal or sham tDCS once | 15 minutes of physical training, focusing on improvement on gait initiation, stride length, gait velocity, arm swing and balance | None | Not reported | NA | NA | Published |
| Sham tDCS | 2 mA for 30 seconds | |||||||||
| Kaski 2014b | Anodal tDCS | Saline‐soaked 40 cm² sponge electrode was placed over the Cz area of international 10‐20 EEG electrode placement system (anode) and over the inion (cathode), respectively | 2 mA for 15 minutes | Anodal or sham tDCS once | None | None | Not reported | NA | NA | Published |
| Sham tDCS | 2 mA for 30 seconds | |||||||||
| Monte‐Silva 2013 | Not reported | Unpublished | ||||||||
| Valentino 2014 | Anodal tDCS | Anode was positioned in anterioposterior orientation over M1 corresponding to the leg with which the patient usually started walking after a freezing of gait period and the cathode at the contralateral supraorbital region | 2 mA for 20 minutes | Anodal or sham tDCS for five consecutive days | None | None | None | NA | NA | Published |
| Sham tDCS | 2 mA for 1 minute | |||||||||
| Verheyden 2013 | Anodal tDCS | Anode was positioned over M1 of the dominant hemisphere with the cathode placed on the contralateral supraorbital region | 1 mA for 15 minutes | Anodal or sham tDCS once | None | 1 in EXP group | None | Feeling of heat underneath the active electrode | NA | Publisihed and unpublished |
| Sham tDCS | 1 mA for 10 seconds (plus ramps) | |||||||||
EEG: electroencephalography EXP: experimental M1: primary motor cortex NA: not applicable
Excluded studies
Altogether we excluded 48 full‐text articles (Figure 1), as they did not fulfil our inclusion criteria. We have listed ten of them in the Characteristics of excluded studies t ables as these studies did not obviously violate our inclusion criteria, but were not suitable for inclusion in this review (Baijens 2012; Biundo 2015; Boggio 2006; Fregni 2006; Gruner 2010; Manenti 2014; Pereira 2013; Schollmann 2015; von Papen 2014; Yu 2011).
Risk of bias in included studies
We provided information about the risk of bias in the Characteristics of included studies tables. To complete the rating of methodological quality, we contacted principal investigators of the included trials, and of trials awaiting classification, to request further information about methodological issues, if necessary. We made contact via letter and email, including email reminders once a month, if we received no response. Some trialists provided all requested information, and some did not answer our requests. We used the 'Risk of bias' tool, as implemented in Review Manager 5 ( RevMan 2014 ), to assess risk of bias according to the aspects listed under Methods. Two review authors (BE and JM) independently assessed risk of bias of the included trials, and two other review authors (JK and MP) checked the extracted data for agreement. Information on risk of bias at the study level is provided in Figure 2. All review authors discussed disagreements and, if necessary, sought arbitration by another review author. A detailed description of risk of bias can be found in the 'Risk of bias' tables in Characteristics of included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of the six included studies ( 66 %) described a low risk of bias for sequence generation (Benninger 2010; Kaski 2014a; Kaski 2014b; Verheyden 2013), whereas two studies (34%) described a low risk of bias for allocation concealment (Monte‐Silva 2013; Verheyden 2013).
Blinding
We rated five of the six included studies (83%) at low risk of performance bias for blinding of participants and personnel (objective outcome measures) (Benninger 2010; Kaski 2014a; Kaski 2014b; Valentino 2014; Verheyden 2013); we rated Monte‐Silva 2013 at unclear risk of bias. For the subjective measures of blinding of participants and personnel , we judged Kaski 2014a and Kaski 2014b at low risk of performance bias; we rated all other studies at unclear risk of performance bias. Four studies (67%) described low risk of bias for blinding of outcome assessment ( objective out come measures ) (Benninger 2010; Kaski 2014a; Kaski 2014b; Verheyden 2013), whereas we judged one study (17%) to have high risk of bias for subjective outcomes (Valentino 2014), and one study at unclear risk of bias for both objective and subjective outcomes (Monte‐Silva 2013).
Incomplete outcome data
Four of the six included studies (67%) were at low risk of bias for incomplete outcome data (Benninger 2010; Kaski 2014a; Kaski 2014b; Valentino 2014).
Selective reporting
One of the five included studies (17%) was at low risk of bias for selective outcome reporting (Benninger 2010), whereas we rated the remaining five (83%) at unclear risk of bias.
Effects of interventions
See: Table 1
A summary of this review's main findings can be found in Table 1.
Comparison 1. transcranial direct current stimulation (tDCS) versus sham tDCS
Outcome 1.1. Unified Parkinson's Disease Ranking Scale (UPDRS; change in global UPDRS score)
We found two studies with 45 participants examining the effects of tDCS on impairment, as measured by UPDRS (Benninger 2010; Valentino 2014). We used percentage change, rather than absolute UPDRS change because one trial only provided percentage change from baseline ( Valentino 2014 ). We found no evidence of effect regarding impairment ( mean difference (MD) ‐7.10%, 95% confidence interval (CI) ‐19.18 to 4.97; P = 0.25, I² = 21%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 1.1).
1.1. Analysis.
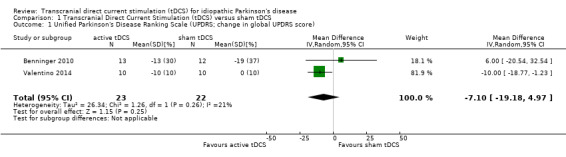
Comparison 1 Transcranial Direct Current Stimulation (tDCS) versus sham tDCS, Outcome 1 Unified Parkinson's Disease Ranking Scale (UPDRS; change in global UPDRS score).
Outcome 1.2. Unified Parkinson's Disease Ranking Scale (UPDRS; change in part III (motor section) score)
Two studies with 45 participants used UPDRS part III motor subsection to evaluate the effects of tDCS on (motor) impairment (Benninger 2010; Valentino 2014). We found evidence of an effect (MD ‐14.43%, 95% CI ‐24.68 to ‐4.18; P = 0.006, I² = 2%, inverse variance method with random‐effects model; very low quality evidence), but the confidence interval was wide (Analysis 1.2).
1.2. Analysis.
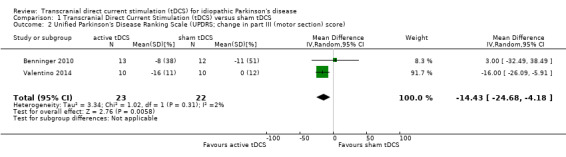
Comparison 1 Transcranial Direct Current Stimulation (tDCS) versus sham tDCS, Outcome 2 Unified Parkinson's Disease Ranking Scale (UPDRS; change in part III (motor section) score).
Outcome 1.3 Off time and on time with dyskinesia
1.3.1 Off time (when the symptoms are not controlled by the medication)
We found one study with 25 participants examining the reduction in off time (Benninger 2010). We found no evidence of an effect (MD 0.10 hours, 95% CI ‐0.14 to 0.34; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 1.3).
1.3. Analysis.
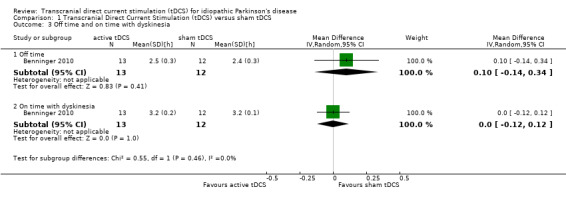
Comparison 1 Transcranial Direct Current Stimulation (tDCS) versus sham tDCS, Outcome 3 Off time and on time with dyskinesia.
1.3.2 On time with dyskinesia (time that symptoms are controlled but the person still experiences involuntary muscle movements)
We found one study with 25 participants examining the reduction in on time with dyskinesia (Benninger 2010). We found no evidence of an effect (MD 0.00 hours, 95% CI ‐0.12 to 0.12; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 1.3).
Outcome 1.4 Gait speed at the end of the intervention phase
We found two studies with 41 participants examining the effects of tDCS on gait speed, measured by timed tests of gait (Benninger 2010; Kaski 2014b). We found no evidence of effect regarding impairment ( standardised mean difference (SMD) 0.50, 95% CI ‐0.17 to 1.18; P = 0.14, I2 = 11%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 1.4).
1.4. Analysis.

Comparison 1 Transcranial Direct Current Stimulation (tDCS) versus sham tDCS, Outcome 4 Gait speed at the end of intervention phase.
Outcome 1.5 Health‐related quality of life
1.5.1 Physical health
We found one study with 25 participants examining the physical health aspect of health‐related quality of life (Benninger 2010). We found no evidence of an effect (MD 1.00 SF‐12 score, 95% CI ‐5.20 to 7.20; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 1.5).
1.5. Analysis.
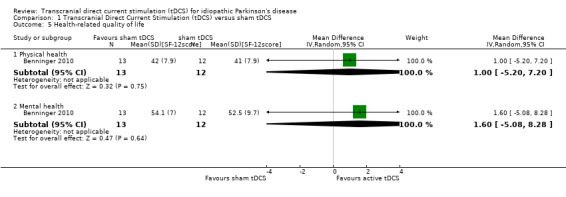
Comparison 1 Transcranial Direct Current Stimulation (tDCS) versus sham tDCS, Outcome 5 Health‐related quality of life.
1.5.2 Mental health
We found one study with 25 participants examining the mental health aspect of health‐related quality of life (Benninger 2010). We found no evidence of an effect (MD 1.60 SF‐12 score, 95% CI ‐5.08 to 8.28; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 1.5).
Outcome 1.6 Activities of daily living
We found no study examining the effects of tDCS for improving activities of daily living.
Outcome 1.7 Dropouts and adverse events
We found four studies with 101 participants examining the effects of tDCS on dropouts and adverse events (Benninger 2010; Kaski 2014a; Valentino 2014; Verheyden 2013). We found no evidence of effect regarding dropouts and adverse events ( risk difference (RD) 0.04, 95% CI ‐0.05 to 0.12; P = 0.40, I2 = 0%, Mantel‐Haenszel method with random‐effects model; very low quality evidence) (Analysis 1.6).
1.6. Analysis.

Comparison 1 Transcranial Direct Current Stimulation (tDCS) versus sham tDCS, Outcome 6 Dropouts and adverse events.
Comparison 2. transcranial direct current stimulation (tDCS) plus movement therapy versus sham tDCS plus movement therapy
Outcome 2.1. Unified Parkinson's Disease Ranking Scale (UPDRS; change in global UPDRS score)
We found no study examining the effects of tDCS for improving UPDRS global score.
Outcome 2.2. Unified Parkinson's Disease Ranking Scale (UPDRS; change in part III (motor section) score)
We found no study examining the effects of tDCS for improving UPDRS part III (motor section) score.
Outcome 2.3 Reduction in off time and on time with dyskinesia
We found no study examining the effects of tDCS for improving off time (when the symptoms are not controlled by the medication) and on time with dyskinesia (when the symptoms are not controlled by the medication) (for people with more advanced disease).
Outcome 2.4 Gait speed at the end of the intervention phase
We found one randomised cross‐over study with eight participants examining the effects of tDCS on gait speed, measured by timed tests of gait (Kaski 2014a). We found no evidence of effect regarding impairment (MD 0.05 m/s, 95% CI ‐0.15 to 0.25; P = 0.62, I2 = 0%, inverse variance method with random‐effects model; very low quality evidence) (Analysis 2.1).
2.1. Analysis.
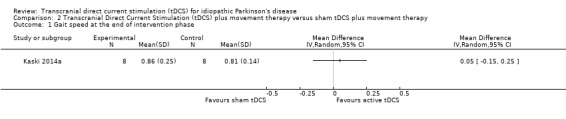
Comparison 2 Transcranial Direct Current Stimulation (tDCS) plus movement therapy versus sham tDCS plus movement therapy, Outcome 1 Gait speed at the end of intervention phase.
Outcome 2.5 Health‐related quality of life
We found no study examining the effects of tDCS for improving health‐related quality of life.
Outcome 2.6 Activities of daily living
We found no study examining the effects of tDCS for improving activities of daily living.
Outcome 2.7 Dropouts and adverse events
We found one randomised cross‐over study with eight participants examining the effects of tDCS on dropouts and adverse events (Kaski 2014a). We found no evidence of effect regarding dropouts and adverse events (RD 0.00, 95% CI ‐0.21 to 0.21; I2 = 0%, Mantel‐Haenszel method with random‐effects model; very low quality evidence) (Analysis 2.2).
2.2. Analysis.
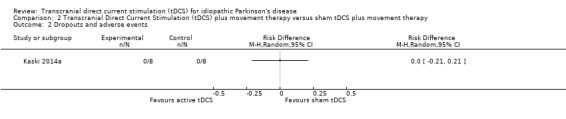
Comparison 2 Transcranial Direct Current Stimulation (tDCS) plus movement therapy versus sham tDCS plus movement therapy, Outcome 2 Dropouts and adverse events.
Sensitivity analyses
We discarded our planned sensitivity analysis, because there were too few included trials.
Discussion
Summary of main results
This review focused on evaluating the effectiveness of transcranial direct current stimulation (tDCS) (anodal, cathodal, or dual) versus control ( sham tDCS, any other approach, or no intervention) for improving health‐related quality of life, disability, and impairment in patients with idiopathic Parkinson's disease (IP D). We included six trials with a total of 137 participants.
tDCS versus sham tDCS
We found two studies with 45 participants examining the effects of tDCS on our primary outcome measure, impairment, as measured by the Unified Parkinson's Disease Rating Scale (UPDRS). We found no evidence of effect regarding impairment at the end of the intervention phase for global UPDRS score ( mean difference (MD) ‐7.10%, 95% confidence interval (CI) ‐19.18 to 4.97; inverse variance method with random‐effects model; very low quality evidence), whereas we found evidence of an effect of tDCS on UPDRS part III motor subsection score (MD ‐14.43%, 95% CI ‐24.68 to ‐4.18; inverse variance method with random‐effects model; very low quality evidence), but the CI was wide. We found one study with 25 participants examining the reduction in off time and in on time with dyskinesia. We found no evidence of an effect (MD 0.10 hours, 95% CI ‐0.14 to 0.34; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence; and MD 0.00 hours, 95% CI ‐0.12 to 0.12; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence, respectively).
One of our secondary outcome measures was gait speed: two trials with a total of 41 participants measured gait speed using measures of timed gait at the end of the intervention phase, revealing no evidence of an effect (standardised mean difference (SMD) 0.50, 95% CI ‐0.17 to 1.18; inverse variance method with random‐effects model; very low quality evidence). Another secondary outcome was health‐related quality of life and we found one study with 25 participants reporting on the physical health and mental health aspects of health‐related quality of life (MD 1.00 SF‐12 score, 95% CI ‐5.20 to 7.20; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence; and MD 1.60 SF‐12 score, 95% CI ‐5.08 to 8.28; I2 = 0%, inverse variance method with random‐effects model; very low quality evidence, respectively). We found no study examining the effects of tDCS for improving activities of daily living. In two of six studies (33%), dropouts and adverse events occurring during the intervention phase were reported. We found no evidence of an effect regarding differences in dropouts and adverse effects between intervention and control groups ( risk difference (RD) 0.04, 95% CI ‐0.05 to 0.12; Mantel‐Haenszel method with random‐effects model; very low quality evidence).
A summary of this comparison's main findings can be found in Table 1.
tDCS plus movement therapy versus sham tDCS plus movement therapy
One of our secondary outcome measures was gait speed: one trial with a total of 16 participants measured gait speed using measures of timed gait at the end of the intervention phase, revealing no evidence of an effect (MD 0.05 m/s, 95% CI ‐0.15 to 0.25; inverse variance method with random‐effects model; very low quality). We found no evidence of an effect regarding differences in dropouts and adverse effects between intervention and control groups (RD 0.00, 95% CI ‐0.21 to 0.21; Mantel‐Haenszel method with random‐effects model; very low quality evidence).
Overall completeness and applicability of evidence
Some factors suggest uncertainty in generalisations, for example, two studies had examined change in UPDRS scores ( Benninger 2010 ; Valentino 2014 ), which is appropriate for early patients with IPD. We only found one study that assessed the reduction in off time and on time with dyskinesia ( Benninger 2010), which are outcome measures suitable for people with more advanced disease. Completeness of evidence generally is lacking regarding studies on the non‐motor aspects of the disease and measures of health‐ related quality of life as well. The majority of patients have been analysed in the on‐state of the disease. Hence, the results may not be applicable to patients in the off‐state of the disease.
Quality of the evidence
Based on our assessments of the quality of evidence provided in Table 1, we downgraded quality of evidence due to unclear or high risk of bias (this would overestimate our findings) and the imprecision of effect estimates. We also found heterogeneity regarding trial design (parallel‐group or cross‐over design), therapy variables (active/passive comparator, location of stimulation, dosage of stimulation, base treatment), and participant characteristics (age, disease duration, severity of disease).
Potential biases in the review process
The methodological rigour of Cochrane reviews minimises bias during the process of conducting systematic reviews. However it is possible that publication bias could have affected our results. However, according to the Cochrane Handbook for Systematic Reviews of Interventions, methods for detecting publication bias, such as funnel plots and linear regression tests, as a rule of thumb, do not work properly in cases where there are less than ten included studies ( Sterne 2011) ; therefore we have not included such methods .
Another potential fact that could have introduced bias is the analysis of both periods of randomised cross‐over trials. On the one hand, the natural course of the disease as well as carry‐over effects are not regarded as a problem in this case. However, the analysis of both periods of randomised controlled trials (RCTs) as if they were a parallel‐ group trial may be too conservative, resulting in wide CIs, and giving too little weight to these studies.
Agreements and disagreements with other studies or reviews
Although there are systematic reviews dealing with the effects of repetitive transcranial magnetic stimulation (rTMS) in IPD (for example, Elahi 2009), we were not able to identify any systematic reviews dealing with the effects of tDCS on IPD. However, the results of our review seem to reflect the ambiguous results across clinical studies dealing with this topic. The apparent lack of benefit may be due to variations in the implementation of tDCS, since it is not known which are the optimal stimulation protocols for IPD (Chen 2010; Koch 2013). The results of Monte‐Silva 2013 corroborate the findings of Analysis 1.1.
There are two recent Cochrane reviews about the contemporary approach of tDCS for improving activities of daily living, function, and aphasia after stroke (Elsner 2013a; Elsner 2013b), which also revealed small or no effects of tDCS following stroke.
Authors' conclusions
Implications for practice.
At the moment, there is no clear evidence of effect of transcranial direct current stimulation (tDCS) versus control for reducing off time and on time with dyskinesia, and for improving health‐ related quality of life, disability, and impairment in patients with idiopathic Parkinson's disease (IPD), because there is some (albeit conflicting) evidence of an effect on the motor section of the Unified Parkinson's Disease Rating Scale (UPDRS). There is very low quality evidence that tDCS is not related to a higher risk of dropouts and adverse events than a control intervention.
Implications for research.
Further large‐scale randomised controlled trials (RCTs) with a parallel‐group design, broad inclusion criteria, and sample size estimation in this area are needed to strengthen the evidence base, particularly to develop systematic stimulation protocols. Methodological quality of future studies, particularly in relation to allocation concealment, blinding of personnel, and intention‐to‐treat analysis, needs to be improved, along with dropout and adverse event reporting.
Acknowledgements
We thank Ema Roque for giving us helpful support. We would like to thank all authors who answered our requests and/or provided additional data (in alphabetical order): David Benninger, Diego Kaski, Katia Monte‐Silva, and Geert Verheyden.
Appendices
Appendix 1. MEDLINE search strategy via Ovid SP (modified for the other databases)
Parkinson.tw.
Parkinson$.tw.
(PD or IPD).tw.
(Parkinson$ adj5 Diseas$).tw.
exp Parkinson Disease/
or/1‐5
Electric Stimulation Therapy/
Electric Stimulation/
Electrodes/
(transcranial adj5 direct current adj5 stimulation).tw.
(transcranial adj5 DC adj5 stimulation).tw.
(transcranial adj5 electric$ adj5 stimulation).tw.
(tDCS or A‐tDCS or C‐tDCS or S‐tDCS or electrode$ or anode or anodes or anodal or cathode or cathodes or cathodal).tw.
or/7‐13
Randomized Controlled Trials as Topic/
random allocation/
Controlled Clinical Trials as Topic/
control groups/
clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
double‐blind method/
single‐blind method/
Placebos/
placebo effect/
cross‐over studies/
Therapies, Investigational/
Research Design/
evaluation studies as topic/
randomized controlled trial.pt.
controlled clinical trial.pt.
(clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
(evaluation studies or comparative study).pt.
random$.tw.
(controlled adj5 (trial$ or stud$)).tw.
(clinical$ adj5 trial$).tw.
((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
(quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw.
((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
(coin adj5 (flip or flipped or toss$)).tw.
versus.tw.
(cross‐over or cross over or crossover).tw.
placebo$.tw.
sham.tw.
(assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw.
controls.tw.
or/15‐46
6 and 14 and 47
exp animals/ not humans.sh.
48 not 49
Data and analyses
Comparison 1. Transcranial Direct Current Stimulation (tDCS) versus sham tDCS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Unified Parkinson's Disease Ranking Scale (UPDRS; change in global UPDRS score) | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐7.10 [‐19.18, 4.97] |
| 2 Unified Parkinson's Disease Ranking Scale (UPDRS; change in part III (motor section) score) | 2 | 45 | Mean Difference (IV, Random, 95% CI) | ‐14.43 [‐24.68, ‐4.18] |
| 3 Off time and on time with dyskinesia | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Off time | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.14, 0.34] |
| 3.2 On time with dyskinesia | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.12, 0.12] |
| 4 Gait speed at the end of intervention phase | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Physical health | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐5.20, 7.20] |
| 5.2 Mental health | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐5.08, 8.28] |
| 6 Dropouts and adverse events | 3 | 85 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.05, 0.13] |
Comparison 2. Transcranial Direct Current Stimulation (tDCS) plus movement therapy versus sham tDCS plus movement therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gait speed at the end of intervention phase | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Dropouts and adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benninger 2010.
| Methods | Randomised controlled trial Method of randomisation: computer‐ generated randomisation list Blinding of outcome assessors: yes Adverse events: 1 in experimental group (skin defect) Deaths: none Dropouts : none ITT: yes | |
| Participants | Country: USA 25 patients (13 in treatment group, 12 in control group) Mean age: 64 years (control and treatment group) Inclusion criteria: age between 40 and 80 years, diagnosis of PD according to UK PD Brain Bank criteria, Hoehn and Yahr stage 2 to 4 while “off” medication, time to complete 10‐metre walk test > 6 s, optimal medication regimen with total levodopa equivalent dose > 300 mg Exclusion criteria: significant medical or psychiatric conditions, metal objects or stimulators in the head | |
| Interventions | 2 arms: (1) control group received sham tDCS, 3 times a week for 2.5 weeks (3‐6 minutes a week) at 1 mA with the electrodes attached to the forehead (2) experimental group used active tDCS, 3 times a week for 2.5 weeks (60 minutes a week) at 2 mA, alternating over premotor and motor or prefrontal areas | |
| Outcomes | Outcomes were recorded at baseline, at the end of intervention phase and at 1‐month and at 3‐month follow‐up: Gait speed (10‐metre walk test) Upper extremity bradykinesia (measured by the time taken to perform hand opening and closing, elbow flexion and elbow extension) UPDRS sum sco re UPDRS motor subsection Reaction time (serial reaction time task) Depression (Beck Depression Inventory) Health‐related quality of life (SF‐12) Sleep duration (in hours, self assessment) Duration of dyskinesia (in hours, self assessment) Time in 'on' and 'off' state (in hours, self assessment) Time off tremor (in hours, self assessment) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We randomly assigned patients to a real or sham group according to a computer generated number with equal probability” |
| Allocation concealment (selection bias) | Unclear risk | Not described by the authors |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants were blinded, whereas blinding of personnel was not clearly stated. Quote: "We set up the stimulating apparatus out of sight of the patients and blinded investigators" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Participants were blinded, whereas personnel were not Benninger 2014 [pers comm]. Quote: "We set up the stimulating apparatus out of sight of the patients and blinded investigators" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessors were blinded for primary outcomes (Quote): ”[...] motor tests and the UPDRS were assessed in the ‘best on’ and ‘practically defined off state’ by the same blinded raters for the entire study on the same day” |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcome assessors were blinded for primary outcomes (Quote): ”[...] motor tests and the UPDRS were assessed in the ‘best on’ and ‘practically defined off state’ by the same blinded raters for the entire study on the same day” |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No dropouts occurred |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in the protocol or in the "methods" section have been reported |
Kaski 2014a.
| Methods | Randomised cross‐over trial nested in a randomised controlled trial Method of randomisation: online random number generator Blinding of outcome assessors: no Adverse events: not reported Deaths: none Dropouts : none ITT: yes | |
| Participants | Country: not reported 8 patients (4 in treatment group, 4 in control group) Mean age: not reported Inclusion criteria: diagnosis of PD according to UK PD Brain Bank criteria, written informed consent Exclusion criteria: severe freezing, MMSE < 24 out of 30, other conditions affecting balance and gait, alternative central nervous diseases affecting gait confirmed by MRI | |
| Interventions | Each participant underwent the following conditions (with a washout period of one week): (A) a single session of physical training plus sham tDCS, (2 mA for 30s) over Cz area of international 10‐20 EEG electrode placement (Kaski 2012) (B) a single session of physical training plus anodal tDCS, 2 mA for 15 minutes, over Cz area of international 10‐20 EEG electrode placement | |
| Outcomes | Outcomes were recorded at baseline, and after each of the two treatment sessions: Gait velocity (calculated by the middle four minutes of the 6‐minute walk test) Gait endurance (6‐minute walk test) Stride length (video analysis) Timed Up and Go Test Postural Instability (Pull test) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by an online randomisation tool |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was performed by the unblinded researcher by entering the anonymised patient details into the software prior to the patient’s arrival. This generated a code relating to the intervention arm (physical training vs. no training), and stimulation type (real vs. sham)” |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants and personnel were blinded (Kaski 2014 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants and personnel were blinded (Kaski 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor was blinded to both the grouping (sham and real) and delivery of the stimulation (Kaski 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Outcome assessor was blinded to both the grouping (sham and real) and delivery of the stimulation (Kaski 2014 [pers comm]) |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No dropouts occurred |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the "methods" section have been reported; no protocol could be identified |
Kaski 2014b.
| Methods | Randomised cross‐over trial nested in a randomised controlled trial Method of randomisation: online random number generator Blinding of outcome assessors: no Adverse events: not reported Deaths: none Dropouts : none ITT: yes | |
| Participants | Country: not reported 8 patients (4 in treatment group, 4 in control group) Mean age: not reported Inclusion criteria: diagnosis of PD according to UK PD Brain Bank criteria, written informed consent Exclusion criteria: severe freezing, MMSE < 24 out of 30, other conditions affecting balance and gait, alternative central nervous diseases affecting gait confirmed by MRI | |
| Interventions | Each participant underwent the following conditions (with a washout period of one week): (A) a single session of no physical training plus sham tDCS, (2 mA for 30s) over Cz area of international 10‐20 EEG electrode placement (Kaski 2012) (B) a single session of no physical training plus anodal tDCS, 2 mA for 15 minutes, over Cz area of international 10‐20 EEG electrode placement | |
| Outcomes | Outcomes were recorded at baseline, and after each of the two treatment sessions: Gait velocity (calculated by the middle four minutes of the 6‐minute walk test) Gait endurance (6‐minute walk test) Stride length (video analysis) Timed Up and Go Test Postural Instability (Pull test) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by an online randomisation tool |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Randomization was performed by the unblinded researcher by entering the anonymised patient details into the software prior to the patient’s arrival. This generated a code relating to the intervention arm (physical training vs. no training), and stimulation type (real vs. sham)” |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants and personnel were blinded (Kaski 2014 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Participants and personnel were blinded (Kaski 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor was blinded to both the grouping (sham and real) and delivery of the stimulation (Kaski 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Outcome assessor was blinded to both the grouping (sham and real) and delivery of the stimulation (Kaski 2014 [pers comm]) |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No dropouts occurred |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the "methods" section have been reported; no protocol could be identified |
Monte‐Silva 2013.
| Methods | Randomised controlled trial with parallel assignment | |
| Participants | Participants: 10 people with PD (5 in experimental and 5 in control group) Inclusion criteria: diagnosis verified by a neurologist, regular treatment with dopamine or other anti‐Parkinsonian drugs Exclusion criteria: history of metal implants in the head, previous surgery for PD, change in medication during study, contamination with other physical therapy during study, pregnancy |
|
| Interventions | 2 arms: (1) Physiotherapy plus anodal tDCS with 1 mA over M1 (hemisphere not stated) twice for 13 minutes with 20 minutes interruption, 3 times a week for 10 sessions (2) Physiotherapy plus sham tDCS with 1 mA over M1 (hemisphere not stated) twice for 0.5 minutes with 20 minutes interruption, 3 times a week for 10 sessions |
|
| Outcomes | Outcome measures were collected at baseline, after the end of the intervention period and at 1‐ month follow‐up Primary outcome measures: UPDRS Secondary outcome measures: Jebsen Taylor Test Parkinson's Disease Quality of Life questionnaire cortical excitability (repetitive transcranial magnetic stimulation ) |
|
| Notes | The study was completed in September 2012 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was done by an independent person who selected one of the sealed, sequentially numbered opaque envelopes minutes before the intervention began" (Monte‐Silva 2014 [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
Valentino 2014.
| Methods | Randomised cross‐over trial Method of randomisation: not described Blinding of outcome assessors: not described Adverse events: none Deaths: none Dropouts : none ITT: yes | |
| Participants | Country: Italy 10 patients Mean age: 72 years Inclusion criteria: diagnosis of PD according to UK PD Brain Bank criteria, Hoehn and Yahr stage 2 to 4 while “off” medication, Freezing of Gait Questionnaire (FOG‐Q) > 3 and freezing of gait (FOG) in 'on' and 'off' state, written informed consent Exclusion criteria: contraindications to tDCS | |
| Interventions | Each participant underwent the following conditions (with a washout period of three months): (A) five consecutive sessions of sham tDCS, (2 mA for 20 minutes) over M1 of the leg with which the patient usually started walking after a FOG episode (B) five consecutive sessions of anodal tDCS, 2 mA for 1 minute, over M1 of the leg with which the patient usually started walking after a FOG episode | |
| Outcomes | The following outcomes were recorded at baseline, and after the 1st and 5th treatment session of the two treatment blocks, 2 days and 2 and 4 weeks after the last tDCS session: Italian validated Movement Disorders Society revision of the Unified Parkinson’s Disease Rating Scale (MDS‐UPDRS) (impairment) Stand Walk Sit (SWS) test (freezing of gait) The following outcomes were recorded at baseline, 2 days and 2 and 4 weeks after the last tDCS session FOG‐Q and Gait and falls questionnaire (gait, falls, freezing of gait episodes) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants apparently were blinded, but blinding of personnel not stated |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Participants apparently were blinded, but blinding of personnel not stated |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | No blinding of outcome assessors stated |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding of outcome assessors stated |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No dropouts occurred |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the "methods" section have been reported; no protocol could be identified |
Verheyden 2013.
| Methods | Randomised controlled cross‐over trial Method of randomisation: Computer‐generated random list Blinding of outcome assessors: yes Adverse events: none Deaths: none Dropouts : 1 (in the experimental group) ITT: unclear | |
| Participants | Country: UK 20 patients Mean age: 71 years Inclusion criteria: Confirmed diagnosis of IPD, independent ambulation, living in the community Exclusion criteria: Other neurological conditions, DBS, impaired cognitive function, metal implants, pacemaker, history of epilepsy and medication altering cortical excitability | |
| Interventions | Each participant underwent two different conditions: (A) sham tDCS (1 mA) once for 15 seconds and (B) anodal tDCS (1 mA) once for 15 minutes over M1 of the dominant hemisphere | |
| Outcomes | Outcomes were recorded at baseline and at the end of intervention phase Balance performance (Berg Balance Scale) Gait performance (GAITRite) at maximal walking speed Corticomotor activity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence (Verheyden 2014 [pers comm]) |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a lab technician for each eligible participant immediately after signing the informed consent. The lab technician was not involved in recruiting or selecting participants (Verheyden 2014 [pers comm]) |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Participants were blinded, whereas personnel were not (Verheyden 2014 [pers comm]) |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Participants were blinded, whereas personnel were not (Verheyden 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Outcome assessor was blinded (Verheyden 2014 [pers comm]) |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Outcome assessor was blinded (Verheyden 2014 [pers comm]) |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Not described by the author |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the "methods" section have been reported; no protocol could be identified |
DBS: d eep brain stimulation EEG : e lectroencephalography ITT: i ntention‐to‐treat analysis M1: p rimary motor cortex MMSE : Mini Mental State Examination MR I: m agnetic r esonance i maging PD: Parkinson's disease tDCS: transcranial d irect c urrent s timulation UPDRS : Unified Parkinson’s Disease Rating Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baijens 2012 | Irrelevant outcome for review: dysphagia |
| Biundo 2015 | Irrelevant outcome for review: attention/executive skills |
| Boggio 2006 | Irrelevant outcome for review: working memory |
| Fregni 2006 | No genuine randomised trial due to pseudo randomisation |
| Gruner 2010 | Effects of tDCS were contaminated with rTMS |
| Manenti 2014 | No genuine randomised trial due to pseudo randomisation |
| Pereira 2013 | Irrelevant outcome for review: functional connectivity |
| Schollmann 2015 | Irrelevant outcome measure for review: parieto‐occipital alpha activity |
| von Papen 2014 | Effects of tDCS were contaminated with rTMS |
| Yu 2011 | Both groups received tDCS. The intervention was a dual task during walking |
rTMS: repetitive transcranial magnetic stimulation tDCS: transcranial direct current stimulation
Characteristics of studies awaiting assessment [ordered by study ID]
Borgheresi 2013.
| Methods | Randomised controlled trial with randomisation before each of the five treatment sessions Adverse events: not reported Deaths: not reported Dropouts: not reported ITT: unclear |
| Participants | Country: Italy 9 patients Mean age: not reported Inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions | 2 arms: (1) A‐tDCS (2 mA for 20 minutes) over the leg motor cortex plus 5 consecutive daily sessions of physical therapy for improving freezing of gait (2) A‐tDCS (no details provided) plus 5 consecutive daily sessions of physical therapy for improving freezing of gait |
| Outcomes | Outcomes were recorded at baseline and 1 and 6 weeks after the end of intervention: Kinematic variables |
| Notes | Conference abstract |
Capecci 2014.
| Methods | Randomised sham‐controlled cross‐over trial Adverse events: not reported Deaths: not reported Dropouts : not reported ITT: unclear |
| Participants | Country: Italy 7 patients (3 male, 4 female; mean age (SD) 61 (9) years; disease duration (SD) 17 (4) years) Inclusion criteria: not described Exclusion criteria: cognitive impairment (MMSE < 24) |
| Interventions | Each participant underwent one of the following conditions: (A) Single session of dual‐tDCS (2 mA, 20 minutes) over the prefrontal cortex (electrode montage not stated), at least 30 days resting period and a single session S‐tDCS (20 minutes) (B) Single session S‐tDCS (20 minutes), at least 30 days resting period and a single session of dual‐tDCS (2 mA, 20 minutes) over the prefrontal cortex (electrode montage not stated) |
| Outcomes | Time points of outcome measurement not stated: UPDRS motor subsection Timed Up and Go Test Timed Up and Go Test with motor dual task Timed Up and Go Test with cognitive dual task Digit span forward and backward Phonetic verbal fluency Semantic verbal fluency Stroop test |
| Notes | Conference abstract |
Falconer 2015.
| Methods | Randomised controlled trial Adverse events: not reported Deaths: not reported Dropouts : not reported ITT: unclear |
| Participants | Country: USA 7 patients, no demographic data provided Inclusion criteria: not stated Exclusion criteria: not stated |
| Interventions | 2 arms: (1) Dual‐tDCS over the frontal cortex (2 mA, 30 minutes) (2) S‐tDCS (15 seconds) |
| Outcomes | Outcomes were recorded at baseline and at the end of intervention phase 10 days after baseline: UPDRS Becks Depression Screen Montreal Cognitive Assessment Speech assessments |
| Notes | Conference abstract |
Heldman 2015.
| Methods | Randomised sham‐controlled cross‐over trial Adverse events: 1 (mild burning sensation) Deaths: not described Dropouts : not described ITT: unclear |
| Participants | Country: USA 6 patients (3 male, 3 female; aged 52 to 70 years; baseline MDS‐UPDRS‐III score (SD) 30 (9)) Inclusion criteria: idiopathic Parkinson's disease; treated with levodopa; morning akinesia Exclusion criteria: not described |
| Interventions | Each participant underwent one of the following conditions: (1) Single session of A‐tDCS during sleep (three 20‐ minute sessions, beginning 1 hour after sleep onset and separated by 1 hour each; electric current not stated) with the anode positioned over C3 or C4 on the 10‐20 system contralateral to the more affected limb and the four cathodal electrodes were placed in a ring fashion (exact location not stated), following 1 to 4 weeks resting period and a single session of S‐tDCS (no details provided) (2) Single session of S‐tDCS (no details provided), following 1 to 4 weeks resting period and a single session of A‐tDCS during sleep (three 20‐minute sessions, beginning 1 hour after sleep onset and separated by 1 hour each; electric current not stated) with the anode positioned over C3 or C4 on the 10‐20 system contralateral to the more affected limb and the four cathodal electrodes were placed in a ring fashion (exact location not stated) |
| Outcomes | Time point of outcome measurement not stated: MDS‐UPDRS part III (motor subsection) score |
| Notes |
Yotnuengnit 2013.
| Methods | Randomised controlled trial Adverse events: not reported Deaths: not reported Dropouts : not reported ITT: unclear |
| Participants | Country: Thailand 32 patients (age range 40 to 80 years; modified Hoehn and Yahr stages 2 and 3) Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | 3 arms:
(1) A‐tDCS (2 mA, duration and electrode montage not stated) over the lower extremity motor cortex) over 6 sessions in 2 weeks
(2) A‐tDCS (2 mA, duration and electrode montage not stated) over the lower extremity motor cortex) plus physical therapy over 6 sessions in 2 weeks (3) S‐tDCS (no details provided) plus physical therapy over 6 sessions in 2 weeks |
| Outcomes | Outcomes were recorded at baseline and at the 2nd, 4th and 8th week after the end of intervention phase: Gait speed Stride length Cadence |
| Notes | Conference abstract |
A‐tDCS : anodal transcranial direct current stimulation ITT: i ntention‐to‐treat analysis MDS‐UPDRS : Movement Disorders Society revision of the Unified Parkinson’s Disease Rating scale MMSE: Mini Mental State Examination SD: standard deviation S‐tDCS : sham transcranial direct current stimulatio n UPDRS : Unified Parkinson’s Disease Rating Scale
Characteristics of ongoing studies [ordered by study ID]
NCT01113086.
| Trial name or title | Selective modulation of cognitive, affective, and motor function by transcranial direct current stimulation as co‐adjuvant therapy in Parkinson's disease |
| Methods | Randomised controlled cross‐over study |
| Participants | Estimated enrolment: 66; aged 40 to 90 years Inclusion criteria: diagnosis of probable PD defined by > 2 out of 3 cardinal motor features and stable medications for at least 30 days Exclusion criteria: Other forms of parkinsonism syndromes, history of DBS or ablation surgery or mass brain lesions, history of psychiatric/psychological conditions, contraindications to tDCS, unstable medical conditions, pregnancy |
| Interventions | Each participant will undergo the following conditions: (A) A ‐tDCS over the left DLPFC (2 mA for 20 minutes in 10 sessions; 100 min per week) (B) A ‐tDCS over the right DLPFC (2 mA for 20 minutes in 10 sessions; 100 min per week) (C) sham tDCS as in (A) or (B) (2 mA for 30 seconds in 10 sessions; 2.5 minutes per week) For subjects who received S ‐tDCS there is an open label arm for receiving active stimulation free of charge: (D) application of active tDCS as in (A) or (B) |
| Outcomes | Outcomes will be recorded at baseline and at 1 and 2 months follow‐ up Primary outcome measures: general motor functioning (measured by UPDRS, Simple Reaction Time, 4‐Choice Reaction Time, Purdue, Pegboard Test, finger tapping, walking time, buttoning‐up, supination‐pronation) Secondary outcome measures: executive functioning, reasoning, visuo‐spatial ability, working memory (measured by the Stroop Test, Hooper Visual Organization Test, Digit Span Test, Trail Making Test B) |
| Starting date | December 2009 |
| Contact information | Felipe Fregni, MD, PhD Spaulding Rehabilitation Hospital Boston, Massachusetts United States 02114 |
| Notes |
NCT01602276.
| Trial name or title | The effect of transcranial direct current stimulation on subcortical brain functioning |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 150 Inclusion criteria: aged 18 to 79 years; fluent in English language; history of subcortical brain damage; no known neurological or cognitive impairment Exclusion criteria: appreciate deficits in hearing; schizophrenia; bipolar disorder; major depression; any neurological disorder associated with cognitive impairment or neuroanatomic abnormality; language‐based learning disorder; dementia or MMSE < 24 for normal control participants; any implanted metal devices (e.g. cardiac pacemaker) |
| Interventions | Each participant will undergo the following conditions: (A) A‐tDCS or C‐tDCS over the area of interest (no details provided) followed by S‐tDCS (no details provided) (B) S‐tDCS (no details provided) followed by A‐tDCS or C‐tDCS over the area of interest (no details provided) |
| Outcomes | Outcomes will be recorded at baseline and at 1‐ month follow‐ up Primary outcome measures: Improvement in motor functioning Improvement in level of consciousness and alertness Improvement in cognitive functioning |
| Starting date | February 2012 |
| Contact information | Barry Gordon, MD, PhD Johns Hopkins University |
| Notes |
NCT02205216.
| Trial name or title | Can transcranial direct stimulation enhance the efficacy of a rehabilitative intervention for the treatment of freezing of gait in Parkinson's disease? A double blind randomized controlled study |
| Methods | Randomised controlled trial |
| Participants | Estimated enrolment: 40 Inclusion criteria: aged 30 to 80 years; with DOPA‐responsive PD Hoehn and Yahr (HY) grade of 2 to 4 while off; on a regimen including levodopa (total dose of levodopa and dopamine agonists using dopamine equivalents > 300 mg/d; MDS‐UPDRS I score > 2 in Freezing of Gait; optimal conventional PD medication for > 1 month prior to screening; scheduled for rehabilitation for the treatment of freezing of gait Exclusion criteria: significant concurrent medical or psychiatric disease; history of seizures and epilepsy; dementia or other neurodegenerative disease besides PD; pallidotomy, implanted electrodes and generator for deep brain stimulation; pregnancy; surgically or traumatically implanted foreign bodies; undue risk or stress (for reasons such as tendency to fall, excessive fatigue, general frailty, or excessive apprehensiveness); significant postural instability with daily falls, inability to walk the parcours or inability to walk 10 metres ; presence of significant cognitive dysfunction as determined by Montreal Cognitive Assessment (MoCA) < 20 or mentally impaired patients having no capacity to provide their own consent; presence of other co‐morbid conditions that can contribute to gait dysfunction; clinically significant hallucinations; participation in any rehabilitation therapy for FOG within the last six months prior to screening |
| Interventions | 2 arms: (1) A‐tDCS (2 mA for 20 minutes) over the motor and premotor cortex with cathodes placed over both mastoids during a 45‐minute rehabilitation session; 2 times a week for 4 weeks (2) S‐tDCS (1 mA for 1‐2 minutes) over the motor and premotor cortex with cathodes placed over both mastoids during a 45‐minute rehabilitation session; 2 times a week for 4 weeks |
| Outcomes | Outcomes will be recorded at baseline and at 1‐ month follow‐ up Primary outcome measures: Walking parcours Secondary outcome measures: New Freezing of Gait Questionnaire (N‐FOGQ) MDS‐UPDRS 39‐Item Parkinson's Disease Questionnaire (PDQ‐39) Beck Depression Inventory 10‐metre Walk Test Timed Up and GO |
| Starting date | September 2014 |
| Contact information | David Benninger, MD Centre Hospitalier Universitaire Vaudois Lausanne, Vaud, Switzerland, 1011 |
| Notes |
NCT02250690.
| Trial name or title | Transcranial direct current stimulation (tDCS) associated with gait training in improving motor function in Parkinson's disease |
| Methods | Randomised controlled trial |
| Participants | Estimated enrolment: 24 Inclusion criteria: aged between 40 and 80 years; with IPD; Hoehn and Yahr stage 1 to 3; mild to severe gait disturbances; stable medication usage; absence of cognitive impairment and disorders interfering with participation in cueing therapy Exclusion criteria: presence of chronic disabling pathologies of lower limb; presence of pacemaker or severe cardiovascular conditions; history of tumour; prior neurosurgery on the brain; cardiac pacemaker; history of epilepsy or major psychiatric disorders; surgical implants; significant postural tremor dyskinesia; severe freezing or dementia |
| Interventions | 2 arms: (1) Active tDCS (13 minutes, no details provided) over SMA immediately followed by gait training with visual cues; 3 times a week for 4 weeks (2) Sham tDCS (13 minutes) over SMA immediately followed by gait training with visual cues; 3 times a week for 4 weeks |
| Outcomes | Outcomes will be recorded at baseline and before and after each session, before the 10th session and at 1 and 2 months after the 10th session Primary outcome measures: Change in bradykinesia Secondary outcome measures: Change in cortical excitability Other outcome measure: Change in balance |
| Starting date | September 2013 |
| Contact information | Adriana Carla Costa Ribeiro Clementino Universidade Federal de Pernambuco, Brazil |
| Notes |
NCT02266004.
| Trial name or title | Transcranial direct current stimulation for freezing of gait in patients with Parkinson's disease |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 40 Inclusion criteria: aged 18 to 50 years; Hoehn and Yahr stage 2 to 3; presence of freezing of gait; stable medication regimen Exclusion criteria: medical condition that would interfere with walking training for 30 minutes; unable to perform Timed Up and Go Test in the off condition; history of seizures; implanted deep brain stimulator, pacemaker or any other electronic device; dementia; adults unable to consent; pregnancy; prisoners |
| Interventions | Each participant will undergo the following conditions: (A) A‐tDCS (2 mA for 20 minutes; stimulation location not stated) plus locomotor training (treatment regimen not stated) followed by at least 1 week of resting period and S‐tDCS (stimulation variables not stated) plus locomotor training (B) S‐tDCS (stimulation variables not stated) plus locomotor training followed by at least 1 week of resting period and A‐tDCS (2 mA for 20 minutes; stimulation location not stated) plus locomotor training (treatment regimen not stated) |
| Outcomes | Outcomes will be recorded at baseline and at the end of intervention Primary outcome measures: Change in walking speed Secondary outcome measures: Change in UPDRS Freezing of gait questionnaire |
| Starting date | October 2014 |
| Contact information | Corneliu Luca, MD, PhD University of Miami Miami, Florida, United States, 33136 |
| Notes |
NCT02287207.
| Trial name or title | Effects of transcranial direct current stimulation on fine motor skills in Parkinson's disease: a pilot study |
| Methods | Randomised cross‐over trial |
| Participants | Estimated enrolment: 20 Inclusion criteria: diagnosed with IPD according to the UK Brain Bank criteria; Hoehn and Yahr stage 2 in the on‐phase of medication cycle; right dominant PD Exclusion criteria: cognitive impairment (MMSE < 23); visuo‐spatial deficits (measured by the Rey‐Osterrieth Complex Figure (ROCF) test); left‐handedness (evaluated by Edinburgh Handedness scale); depression; pregnancy; alcohol abuse; aneurysm clips; pacemaker; neurostimulator; implemented defibrillator; magnetically activated implant or device; implemented pump; spinal cord stimulator; implemented hearing aid; artificial or prosthetic limb; metal parts in the body; any external or internal metal; artificial heart valve; other implants; history of brain surgery; migraine; family history of epilepsy; other contra‐indications for tDCS and transcranial magnetic stimulation; other neurological, psychiatric or interfering upper limb problems |
| Interventions | Each participant will undergo the following conditions: (A) A‐tDCS (1 mA; 20 minutes; treatment regimen not described) over M1 (length of resting period not stated) followed by S‐tDCS (20 minutes; treatment regimen not described) (B) S‐tDCS (20 minutes; treatment regimen not described) (length of resting period not stated) followed by A‐tDCS (1 mA; 20 minutes; treatment regimen not described) over M1 |
| Outcomes | Outcomes will be recorded at baseline, at the end of intervention phase and at 1‐week follow‐up Primary outcome measures: Change in writing amplitude Change in writing velocity Secondary outcome measures: Change in speed on the Purdue Pegboard Test Change in cortical excitability Change in Short‐Latency Intracortical Inhibition Change in cortical silent period |
| Starting date | October 2015 |
| Contact information | Alice Nieuwboer, Professor Sanne Broeder, Msc CatholicULeuven Leuven, Belgium, 3000 |
| Notes |
NCT02503930.
| Trial name or title | The effect of transcranial direct cortical stimulation (tDCS) on a motor‐cognitive dual‐task performance of Parkinson's patients |
| Methods | Randomised controlled trial |
| Participants | Estimated enrolment: 60 Inclusion criteria: aged between 20 and 90 years; diagnosed with IPD according to the UK Brain Bank criteria; Hoehn and Yahr stage 1.5 to 3; on a treatment regimen with anti‐parkinsonian medications Exclusion criteria: MMSE score < 24; prior brain surgery; major depression (DSM‐IV criteria); cerebral infarction with residual deficits diagnosis; other neurological conditions; orthopaedic or cardiovascular conditions that may affect walking and cognitive abilities |
| Interventions | 2 arms: (1) A‐tDCS (20 minutes; electric current and electrode position not described) over the PFC (2) S‐tDCS (20 minutes; electric current and electrode position not described) over the PFC |
| Outcomes | Outcomes will be recorded at 1‐week post‐intervention Primary outcome measures: Changes in frequency and severity of the freezing of gait phenomenon (Freezing of Gait Questionnaire) Secondary outcome measures: Changes in grey matter volume (fMRI scan) Frontal lobe activation (fNIRS) Changes in cognitive performance (NeuroTrax software) Immediate change in gait speed (motion analysis system) Immediate change in gait variability (motion analysis system) |
| Starting date | July 2015 |
| Contact information | Anat Mirelman, PhD Jeffery M Hausdorff, PhD Tel Aviv Sourasky Medical Center Tel Aviv, Israel |
| Notes |
NCT02656316.
| Trial name or title | The effects of multi‐focal transcranial direct current stimulation (tDCS) on motor‐cognitive dysfunctions and freezing of gait in patients with Parkinson's disease: a randomized controlled trial |
| Methods | Randomised controlled trial |
| Participants | Estimated enrolment: 85 Inclusion criteria: aged between 25 and 90 years; diagnosed with IPD according to the UK Brain Bank criteria; Hoehn and Yahr stage 1.5 to 3; suffering from freezing of gait (measured by the new FOG questionnaire NFOG‐Q); no prior or anticipated change of medications within 1 month of the study; ability to walk independently Exclusion criteria: no signs of FOG; any diagnosed psychiatric or other neurologic disorder; unbalanced and high blood pressure; pregnancy; participation in any clinical trial in the last 3 months; unwillingness to be randomised; DBS, pacemakers intracranial electrodes; implanted defibrillators or any other prosthesis; perceived inability to complete the study; personal or family history of epilepsy; neuroactive medication; risk of metal fragments in the eyes or head |
| Interventions | 2 arms: (1) A‐tDCS (20 minutes; electric current and electrode position not described) (2) S‐tDCS (20 minutes; electric current and electrode position not described) |
| Outcomes | Outcomes will be recorded at the end of intervention phase and at 2 weeks follow‐up Primary outcome measures: Changes in frequency and severity of the freezing of gait phenomenon (NFOG‐Q) Secondary outcome measures: Changes in cognitive performance (NeuroTrax software) Immediate change in gait speed under usual and dual task conditions (motion analysis system) Immediate change in gait variability under usual and dual task conditions (motion analysis system) |
| Starting date | January 2016 |
| Contact information | Jeffery M Hausdorff, PhD Anat Mirelman, PhD Tel Aviv Sourasky medical Center Tel Aviv, Israel |
| Notes |
A‐tDCS: anodal transcranial direct current stimulation C‐tDCS: cathodal transcranial direct current stimulation DBS: deep brain stimulation DLPFC: dorsolateral prefrontal cortex DOPA ‐responsive : symptoms tipically improve with sustain ed use of L‐DOPA DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders fNIRS: functional near‐infrared spectroscopy FOG: freezing of gait IPD: idiopathic Parkinson's disease M1: primary motor cortex MDS‐UPDRS: Movement Disorder Society Unified Parkinson’s Disease Rating scale MMSE: Mini Mental‐State Examination PD: Parkinson's disease PFC: prefrontal cortex SMA: supplemental motor area S‐tDCS: sham transcranial direct current stimulation UPDRS: Unified Parkinson's Disease Rating scale
Differences between protocol and review
We were not able to search the engineering database COMPENDEX, because the subscription of our institution expired. To increase statistical power we decided to include both periods of randomised cross‐over trials, but we did not include quasi‐RCTs. Due to a lack of sufficient studies, we omitted our planned subgroup analyses regarding on/ off state, type of intervention (anodal, cathodal, and sham), and type of control intervention (rehabilitation versus no control intervention). Due to the low number of events in the analysis of our secondary outcomes dropouts and adverse events, we used risk differences (RDs) instead of risk ratios (RRs). We discarded our planned sensitivity analysis regarding trial methodology, because there were too few included trials.
Contributions of authors
All review authors contributed to the conception and design of the protocol; all review authors approved the protocol.
All review authors were involved in all stages of the review. BE and JM were involved in screening titles and abstracts of publications identified by the searches, extracted trial and outcome data from the selected trials, and analysed outcome data. JK and MP were involved in assessing the methodological quality of the studies. All review authors were involved in interpreting the results.
Sources of support
Internal sources
Gesundheitswissenschaften/Public Health, Medizinische Fakultät Carl Gustav Carus der TU Dresden, Fetscherstr. 74, 01307 Dresden, Germany.
Wissenschaftliches Institut, Private Europäische Medizinische Akademie der Klinik Bavaria in Kreischa GmbH, An der Wolfsschlucht 1‐2, 01731 Kreischa, Germany.
SRH Fachhochschule für Gesundheit Gera gGmbH, Neue Str. 28‐30, 07548 Gera, Germany.
External sources
No sources of support supplied
Declarations of interest
BE: none known.
JM: none known.
JK: none known.
MP: none known.
New
References
References to studies included in this review
Benninger 2010 {published data only}
- Benninger D, Lomarev M, Lopez G, Wassermann E, Considine E, Hallett M. Transcranial direct current stimulation for the treatment of Parkinson's disease: a randomized, double‐ blind, sham‐ controlled study. Neurology 2009; Vol. 72, issue 11:A413. [0028‐3878] [DOI] [PMC free article] [PubMed]
- Benninger D, Lomarev M, Lopez G, Wassermann E, Li X, Considine E, et al. Transcranial direct current stimulation for the treatment of Parkinson's disease: A randomized, double‐blind, sham‐controlled study. Movement Disorders 2009; Vol. 24:S256. [0885‐3185]
- Benninger DH, Lomarev M, Lopez G, Wassermann EM, Li X, Considine E, et al. Transcranial direct current stimulation for the treatment of Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 2010; Vol. 81, issue 10:1105‐11. [0022‐3050] [DOI] [PMC free article] [PubMed]
- NCT00082342. Transcranial direct current stimulation to treat symptoms of Parkinson's disease. www.ClinicalTrials.gov/show/NCT00082342 (accessed 25 February 2014).
Kaski 2014a {published data only}
- Kaski D, Dominguez R, Allum J, Islam A, Bronstein A. Combining physical training with transcranial direct current stimulation to improve gait in Parkinson's disease: a pilot randomized controlled study. Clinical Rehabilitation 2014 May 21 [Epub ahead of print] . [1477‐0873: (Electronic)] [DOI] [PubMed]
Kaski 2014b {published data only}
Monte‐Silva 2013 {unpublished data only}
- NCT01877148. Effects of transcranial direct current stimulation associated with physical therapy on motor rehabilitation in Parkinson's disease patients. www.ClinicalTrials.gov/show/NCT01877148 (accessed 25 February 2014).
Valentino 2014 {published data only}
Verheyden 2013 {published data only}
- Verheyden G, Purdey J, Burnett M, Cole J, Ashburn A. Immediate effect of transcranial direct current stimulation on postural stability and functional mobility in Parkinson's disease. Movement Disorders 2013;28(14):2040‐1. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baijens 2012 {published data only}
- Baijens LW, Speyer R, Passos VL, Pilz W, Roodenburg N, Clavé P. The effect of surface electrical stimulation on swallowing in dysphagic Parkinson patients. Dysphagia 2012;27(4):528‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biundo 2015 {published data only}
Boggio 2006 {published data only}
- Boggio PS, Ferrucci R, Rigonatti SP, Covre P, Nitsche M, Pascual‐Leone A, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson's disease. Journal of the Neurological Sciences 2006;249(1):31‐8. [0022‐510X] [DOI] [PubMed] [Google Scholar]
Fregni 2006 {published data only}
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, et al. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson's disease. Movement Disorders 2006;21(10):1693‐702. [0885‐3185] [DOI] [PubMed] [Google Scholar]
Gruner 2010 {published data only}
- Eggers C, Gruner U, Ameli M, Sarfeld AS, Nowak DA. 1Hz rTMS preconditioned by tDCS over the primary motor cortex in Parkinson's disease: Absence of effect on arm lift and hand grip force control. Motor Control 2012;16(2):284‐92. [1087‐1640] [DOI] [PubMed] [Google Scholar]
- Eggers C, Gruner U, Fink G, Nowak D. T‐DCS‐preconditioned low‐frequency rTMS of the hand area of M1 to improve bradykinesia in Parkinson's disease. Journal of Neurology 2009;256:S142. [0340‐5354] [Google Scholar]
- Gruner U, Eggers C, Ameli M, Sarfeld AS, Fink GR, Nowak DA. 1 Hz rTMS preconditioned by tDCS over the primary motor cortex in Parkinson's disease: effects on bradykinesia of arm and hand. Journal of Neural Transmission 2010; Vol. 117, issue 2:207‐16. [0300‐9564] [DOI] [PubMed]
Manenti 2014 {published data only}
- Manenti R, Brambilla M, Rosini S, Orizio I, Ferrari C, Borroni B, et al. Time up and go task performance improves after transcranial direct current stimulation in patient affected by Parkinson's disease. Neuroscience Letters 2014;580:74‐7. [0304‐3940] [DOI] [PubMed] [Google Scholar]
Pereira 2013 {published data only}
- Pereira JB, Junque C, Bartres‐Faz D, Marti MJ, Sala‐Llonch R, Compta Y, et al. Modulation of verbal fluency networks by transcranial direct current stimulation (tDCS) in Parkinson's disease. Brain Stimulation 2013;6(1):16‐24. [1935‐861X] [DOI] [PubMed] [Google Scholar]
Schollmann 2015 {published data only}
- Schollmann A, Weis D, Wasserka B, Kruger R, Plewnia C, Scholten M. Anodal tDCS to the left sensorimotor area induces a short‐lasting increase of parieto‐occipital alpha activity in Parkinson's disease. Clinical Neurophysiology 2015;8:e136‐7. [Google Scholar]
von Papen 2014 {published data only}
- Papen M, Fisse M, Sarfeld A‐S, Fink GR, Nowak DA. The effects of 1 Hz rTMS preconditioned by tDCS on gait kinematics in Parkinson's disease. Journal of Neural Transmission 2014;121(7):743‐54. [1435‐1463] [DOI] [PubMed] [Google Scholar]
Yu 2011 {published data only}
- Mak M, Yu L. Effects of transcranial direct current stimulation on dual‐task gait performance in patients with Parkinson's disease. Clinical Neurophysiology 2014; 125 ( Suppl 1 ):S127. [Google Scholar]
- Yu LL, Chan MY, Mak MKY. Effect of transcranial direct current stimulation (tDCS) on gait performance under concurrent cognitive task condition in patients with Parkinson's disease. Hong Kong Physiotherapy Journal 2011;29(2):97. [1013‐7025] [Google Scholar]
References to studies awaiting assessment
Borgheresi 2013 {published data only}
- Borgheresi A, Giovannelli F, Cozzi S, Antoniella L, Vanni P, Piccini C, et al. Effects of a short physical therapy program combined with transcranial direct current stimulation (tDCS) on freezing of gait in Parkinson's disease: Preliminary data from a randomized, sham‐controlled study. Clinical Neurophysiology 2013; 124 (11):e200. [Google Scholar]
Capecci 2014 {published data only}
- Capecci M, Andrenelli E, Orni C, Ceravolo MG. Bilateral prefrontal transcranial direct current stimulation (tDCS) in Parkinson's disease: A placebo controlled trial. Proceedings of the MDS 18th International Congress of Parkinson's Disease and Movement Disorders ; 2014 June 8‐12 ; Stockholm . Abstract Supplement . 2014; Vol. 29 :S229‐S230.
Falconer 2015 {published data only}
- Falconer RA, Rogers SL, Torres‐Yaghi Y, Turkeltaub P, Pagan F. Impact of tDCS in Parkinson's disease on mood, cognition, and motor deficits: A randomized, double‐blinded, placebo‐controlled trial. Proceedings of the MDS 19th International Congress of Parkinson's Disease and Movement Disorders ; 2015 June 14‐18 ; San Diego; California. Abstract Supplement . 2015; Vol. 30 :S81.
Heldman 2015 {published data only}
- Heldman DA, Pulliam CL, Blassucci LM, Giuffrida JP, Comella CL. Pilot study to evaluate transcranial direct current stimulation (tDCS) during sleep for the treatment of Parkinson's disease. Proceedings of the MDS 19th International Congress of Parkinson's Disease and Movement Disorders ; 2015 June 14‐18; San Diego; C alifornia. Abstract Supplement . 2015; Vol. 30 :S425‐S426.
- NCT02125383. ParkinStim: Transcranial direct current stimulation for Parkinson's disease. www.ClinicalTrials.gov/show/NCT02125383 (accessed 22 February 2016).
Yotnuengnit 2013 {published data only}
- Yotnuengnit P, Piravej K. Effects of transcranial direct current stimulation (TDCS) plus physical therapy on gait in Parkinson. Archives of Physical Medicine and Rehabilitation 2013; 9 4 (10):e12. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01113086 {published data only}
- NCT01113086. Use of noninvasive brain stimulation in Parkinson's disease. www.ClinicalTrials.gov/show/NCT01113086 (accessed 25 February 2014).
NCT01602276 {published data only}
- NCT01602276. The effect of tDCS on subcortical brain functioning. www.clinicaltrials.gov/show/NCT01602276 (accessed 22 February 2016).
NCT02205216 {published data only}
- NCT02205216. Can tDCS enhance efficacy of rehabilitative intervention for freezing of gait in Parkinson's disease?. www.clinicaltrials.gov/show/NCT02205216 (accessed 22 February 2016).
NCT02250690 {published data only}
- NCT02250690. Neuromodulation on motor function in Parkinson's disease. www.clinicaltrials.gov/show/NCT02250690 (accessed 22 February 2016).
NCT02266004 {published data only}
- NCT02266004. Transcranial direct current stimulation for freezing of gait. www.clinicaltrials.gov/show/NCT02266004 (accessed 23 February 2016).
NCT02287207 {published data only}
- NCT02287207. Effects of transcranial direct current stimulation on fine motor skills in Parkinson's disease: a pilot study. wwwclinicaltrials.gov/show/NCT02287207 (accessed 23 February 2016).
NCT02503930 {published data only}
- NCT02503930. The effect of tDCS on a motor‐cognitive dual‐task performance of Parkinson's patients. www.clinicaltrials.gov/show/NCT02503930 (accessed 23 February 2016).
NCT02656316 {published data only}
- NCT02656316. The effects of multi‐focal tDCS on motor‐cognitive dysfunctions in Parkinson's disease. www.clinicaltrials.gov/show/NCT02656316 (accessed 23 February 2016).
Additional references
Aarsland 1999
- Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community‐based study. Archives of Neurology 1999;56(5):595‐601. [0003‐9942: (Print)] [DOI] [PubMed] [Google Scholar]
Baker 2006
- Baker M, Gershanik OS. Parkinson's disease (3.8). Neurological Disorders. Public Health Challenges. Geneva, Switzerland: World Health Organization, 2006:140‐50. [92‐4‐156336‐2] [Google Scholar]
Benninger 2014 [pers comm]
- Benninger D. RE: Cochrane review about tDCS in Parkinson's disease: Request for further information [personal communication] . Email to: B Elsner 9 October 2014.
Bindman 1964
- Bindman LJ, Lippold OCJ, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long‐lasting after‐effects. Journal of Physiology 1964;172(3):369‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caslake 2013
- Caslake R, Taylor K, Scott N, Gordon J, Harris C, Wilde K, et al. Age‐, gender‐, and socioeconomic status‐specific incidence of Parkinson's disease and parkinsonism in northeast Scotland: the PINE study. Parkinsonism & Related Disorders 2013;19(5):515‐21. [1353‐8020] [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen R. Transcranial direct current stimulation as a treatment for Parkinson's disease ‐ interesting, but not ready for prime time. Journal of Neurology, Neurosurgery and Psychiatry 2010;81(10):1061. [1468‐330X] [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition. Mahwah, New Jersey: Lawrence Earlbaum Associates, 1988. [Google Scholar]
Crosby 2003
- Crosby NJ, Deane K, Clarke CE. Amantadine in Parkinson's disease. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003468] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deane 2001
- Deane K, Jones DE, Ellis‐Hill C, Clarke CE, Playford ED, Ben‐Shlomo Y. Physiotherapy for Parkinson's disease: a comparison of techniques. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002815] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Elahi 2009
- Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson motor function ‐ systematic review of controlled clinical trials. Movement Disorders 2009;24(3):357‐63. [1531‐8257: (Electronic)] [DOI] [PubMed] [Google Scholar]
Elsner 2013a
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving aphasia in patients after stroke. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009760.pub2] [DOI] [PubMed] [Google Scholar]
Elsner 2013b
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane Database of Systematic Reviews 2013, Issue 11 . [DOI: 10.1002/14651858.CD009645.pub2] [DOI] [PubMed] [Google Scholar]
Fahn 1987
- Martinez‐Martin P, Rodriguez‐Blazquez C, João Forjaz M, Chaudhuri KR. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden C, Goldstein M, Calne D editor(s). Recent Developments in Parkinson’s Disease. Vol. 2, Florham Park, NJ : Macmillan Healthcare Information , 1987:153‐63. [Google Scholar]
Fasano 2012
- Fasano A, Daniele A, Albanese A. Treatment of motor and non‐motor features of Parkinson's disease with deep brain stimulation. Lancet Neurology 2012;11(5):429‐42. [1474‐4465: (Electronic)] [DOI] [PubMed] [Google Scholar]
Gandiga 2006
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double‐blind sham‐controlled clinical studies in brain stimulation. Clinical Neurophysiology 2006;117:845‐50. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, et al. Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hesse 2011
- Hesse S, Waldner A, Mehrholz J, Tomelleri C, Pohl M, Werner C. Combined transcranial direct current stimulation and robot‐assisted arm training in subacute stroke patients: an exploratory, randomized multicenter trial. Neurorehabilitation and Neural Repair 2011;25(9):838‐46. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoehn 1967
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. . Neurology 1967 ; 17 ( 5 ):427‐42. [DOI] [PubMed] [Google Scholar]
Hughes 1992
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. Journal of Neurology, Neurosurgery and Psychiatry 1992;55(3):181‐4. [PUBMED: 1564476] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huse 2005
- Huse DM, Schulman K, Orsini L, Castelli‐Haley J, Kennedy S, Lenhart G. Burden of illness in Parkinson's disease. Movement Disorders 2005;20(11):1449‐54. [0885‐3185: (Print)] [DOI] [PubMed] [Google Scholar]
Jankovic 2000
- Jankovic J. Complications and limitations of drug therapy for Parkinson's disease. Neurology 2000;55(12 Suppl 6):S2‐6. [PUBMED: 11188970] [PubMed] [Google Scholar]
Jeffery 2007
- Jeffery DT, Norton JA, Roy FD, Gorassini MA. Effects of transcranial direct current stimulation on the excitability of the leg motor cortex. Experimental Brain Research 2007;182(2):281‐7. [DOI] [PubMed] [Google Scholar]
Johnell 1992
- Johnell O, Melton LJ 3rd, Atkinson EJ, O'Fallon WM, Kurland LT. Fracture risk in patients with parkinsonism: a population‐based study in Olmsted County, Minnesota. Age and Ageing 1992;21(1):32‐8. [PUBMED: 1553857] [DOI] [PubMed] [Google Scholar]
Kaski 2012
- Kaski D, Quadir S, Patel M, Yousif N, Bronstein AM. Enhanced locomotor adaptation aftereffect in the "broken escalator" phenomenon using anodal tDCS. Journal of Neurophysiology 2012;107(9):2493‐505. [0022‐3077] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaski 2014 [pers comm]
- Kaski D. RE: Cochrane review about tDCS in Parkinson's disease: Request for further information [personal communication] . Email to: B Elsner 18 August 2014.
Koch 2013
- Koch G. Do studies on cortical plasticity provide a rationale for using non‐invasive brain stimulation as a treatment for Parkinson's disease patients?. Frontiers in Neurology 2013;4:180. [1664‐2295: (Electronic)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 2005
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain?. European Journal of Neuroscience 2005;22(2):495‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindenberg 2010
- Lindenberg R, Nair D, Zhu LL, Renga V, Schlaug G. Non‐invasive motor cortex stimulation after stroke: the effect of number of sessions on outcome. Stroke 2011;42(3):e72. [Google Scholar]
Marinus 2002
- Marinus J, Ramaker C, Hilten JJ, Stiggelbout AM. Health related quality of life in Parkinson's disease: a systematic review of disease specific instruments. Journal of Neurology, Neurosurgery and Psychiatry 2002;72(2):241‐8. [0022‐3050: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehrholz 2010
- Mehrholz J, Friis R, Kugler J, Twork S, Storch A, Pohl M. Treadmill training for patients with Parkinson's disease. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007830.pub2] [DOI] [PubMed] [Google Scholar]
Monte‐Silva 2014 [pers comm]
- Monte‐Silva K. Re: Your trial NCT01877148 meets inclusion criteria of our Cochrane review about tDCS in Parkinson's disease ‐ Request of further information [personal communication] . Email to: B Elsner 25 August 2014.
Motto 2003
- Motto Cristina CM, Tamma F, Candelise L. Deep brain stimulation of subthalamic nucleus for Parkinson's disease. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD004491] [DOI] [Google Scholar]
Nitsche 2001
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001;57:1899‐901. [DOI] [PubMed] [Google Scholar]
Nitsche 2003
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Journal of Physiology 2003;114:600‐4. [DOI] [PubMed] [Google Scholar]
Nowak 2009
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemsipsheric competition after stroke: brain stimulation to enhance recovery of function of the affected hand. Neurorehabilitation and Neural Repair 2009;23(7):641‐56. [DOI] [PubMed] [Google Scholar]
Nutt 1990
- Nutt JG. Levodopa‐induced dyskinesia: review, observations, and speculations. Neurology 1990;40(2):340‐5. [PUBMED: 2405297] [DOI] [PubMed] [Google Scholar]
Purpura 1965
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology 1965;28(1):166‐85. [DOI] [PubMed] [Google Scholar]
Reis 2009
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, et al. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proceedings of the National Academy of Sciences of the United States of America 2009;106(5):1590‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schrag 2000a
- Schrag A, Jahanshahi M, Quinn N. What contributes to quality of life in patients with Parkinson's disease?. Journal of Neurology, Neurosurgery and Psychiatry 2000;69(3):308‐12. [0022‐3050: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schrag 2000b
- Schrag A, Selai C, Jahanshahi M, Quinn NP. The EQ‐5D—a generic quality of life measure—is a useful instrument to measure quality of life in patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 2000;69(1):67‐73. [0022‐3050: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwab 1969
- Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson IML editor(s). Third Symposium on Parkinson’s disease. Edinburgh: Livingstone, 1969:152–7. [Google Scholar]
Shearer 2012
- Shearer J, Green C, Counsell C, Zajicek J. The impact of motor and non motor symptoms on health state values in newly diagnosed idiopathic Parkinson’s disease. Journal of Neurology 2012;259(3):462‐8. [0340‐5354] [DOI] [PubMed] [Google Scholar]
Shulman 2010
- Shulman LM, Gruber‐Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the Unified Parkinson's Disease Rating Scale. Archives of Neurology 2010;67(1):64‐70. [0003‐9942] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stowe 2008
- Stowe R, Ives N, Clarke CE, Van H, Ferreira J, Hawker RJ, et al. Dopamine agonist therapy in early Parkinson's disease. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD006564.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verheyden 2014 [pers comm]
- Verheyden G. RE: Cochrane review on tDCS for improving movement disorders in Parkinson's disease [personal communication] . Email to: B Elsner 27 May 2014.
Wu 2008
- Wu AD, Fregni F, Simon DK, Deblieck C, Pascual‐Leone A. Noninvasive brain stimulation for Parkinson's disease and dystonia. Neurotherapeutics 2008;5(2):345‐61. [1933‐7213: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]


