Abstract
Background
Gamma aminobutyric acid (GABA) receptor agonists have been shown to have a neuroprotectant effect in reducing infarct size and improving functional outcome in animal models of cerebrovascular disease. However, the sedative effects of GABA receptor agonists have limited their wider application in people with acute stroke, due to the potential risk of stupor. This is an update of a Cochrane review first published in 2013, and previously updated in 2014.
Objectives
To determine the efficacy and safety of GABA receptor agonists in the treatment of acute stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (accessed March 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 3, part of the Cochrane Library (accessed March 2016), MEDLINE (from 1949 to March 2016), Embase (from 1980 to March 2016), CINAHL (from 1982 to March 2016), AMED (from 1985 to March 2016), and 11 Chinese databases (accessed March 2016). In an effort to identify further published, unpublished, and ongoing trials we searched ongoing trials registers, reference lists, and relevant conference proceedings, and contacted authors and pharmaceutical companies.
Selection criteria
We included randomized controlled trials (RCTs) investigating GABA receptor agonists versus placebo for people with acute stroke (within 12 hours after stroke onset), with the primary outcomes of efficacy and safety.
Data collection and analysis
Two review authors independently screened the titles and abstracts of identified records, selected studies for inclusion, extracted eligible data, cross‐checked the data for accuracy, and assessed the risk of bias.
Main results
We included five trials with 3838 participants (3758 analyzed). The methodological quality of the included trials was generally good, with an unclear risk for selection bias only. Four trials (N = 2909) measured death and dependency at three months for chlormethiazole versus placebo; pooled results did not find a significant difference (risk ratio (RR) 1.03, 95% confidence interval (CI) 0.96 to 1.11). One trial (N = 849) measured this outcome for diazepam versus placebo (RR 0.94, 95% CI 0.82 to 1.07). The most frequent adverse events related to chlormethiazole were somnolence (RR 4.56, 95% CI 3.50 to 5.95; two trials; N = 2527) and rhinitis (RR 4.75, 95% CI 2.67 to 8.46; two trials; N = 2527).
Authors' conclusions
This review provides moderate‐quality evidence that fails to support the use of GABA receptor agonists (chlormethiazole or diazepam) for the treatment of people with acute stroke. More well‐designed RCTs with large samples of participants with total anterior circulation syndrome are required to determine if there are benefits for this subgroup. Somnolence and rhinitis are frequent adverse events related to chlormethiazole.
Keywords: Humans, Acute Disease, Chlormethiazole, Chlormethiazole/adverse effects, Chlormethiazole/therapeutic use, Diazepam, Diazepam/therapeutic use, Disorders of Excessive Somnolence, Disorders of Excessive Somnolence/chemically induced, GABA Agonists, GABA Agonists/adverse effects, GABA Agonists/therapeutic use, Neuroprotective Agents, Neuroprotective Agents/adverse effects, Neuroprotective Agents/therapeutic use, Randomized Controlled Trials as Topic, Rhinitis, Rhinitis/chemically induced, Stroke, Stroke/drug therapy, Stroke/mortality
Gamma aminobutyric acid (GABA) receptor agonists for acute stroke
Question: Are GABA receptor agonist drugs effective and safe in the treatment of acute stroke? Background: GABA receptor agonists are a type of drug that may help protect the brain in acute stroke. This class of drugs, which includes diazepam and chlormethiazole, have been used as traditional sedatives for several decades, and have been found to be beneficial in animal models of stroke. However, the sedative effect of GABA receptor agonists could be harmful for people with acute stroke. Study characteristics: We identified five studies to March 2016 that met our inclusion criteria; they randomized 3838 participants and analyzed 3758. The quality of all the studies was generally good, with a low risk of bias. One study evaluated the efficacy and safety of diazepam for acute stroke in 849 participants within 12 hours of stroke onset. Four studies evaluated the efficacy and safety of chlormethiazole in 2909 participants with acute stroke, within 12 hours of stroke onset; 95 participants had hemorrhagic stroke and were analyzed separately. Key results: All five trials reported death and dependency at three months. There was no significant difference between the chlormethiazole and placebo groups or between the diazepam and placebo groups. The most frequent side effects caused by chlormethiazole were drowsiness and nasal irritation. Quality of the evidence: In conclusion, moderate‐quality evidence did not support the use of GABA receptor agonists for the treatment of people with acute stroke. The most frequently reported side effects of chlormethiazole were drowsiness and nasal irritation.
Summary of findings
Summary of findings for the main comparison.
Chlormethiazole compared with placebo for acute stroke
| Chlormethiazole compared with placebo for acute stroke | ||||||
|
Patient or population: people with acute stroke Settings: inpatients Intervention: chlormethiazole Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Chlormethiazole | |||||
| Death or dependency | 475 per 1000 | 487 per 1000 | RR 1.03 (0.96 to 1.11) | 2909 (4) | ⊕⊕⊕⊝ Moderate1 | — |
| Adverse events | Somnolence 113 per 1000 Rhinitis 25 per 1000 |
Somnolence 517 per 1000 Rhinitis 130 per 1000 |
RR 4.56 (3.50 to 5.95) RR 4.75 (2.67 to 8.46) |
2527 (2) | ⊕⊕⊕⊝ Moderate1 | — |
| Functional independence | 525 per 1000 | 513 per 1000 | RR 0.98 (0.92 to 1.05) | 2909 (4) | ⊕⊕⊕⊝ Moderate1 | — |
| Other stroke scales | — | — | — | NIHSS 1367 (2) SSS 2727 (3) |
⊕⊕⊕⊝ Moderate1 | In Lyden 2000, the mean change of the NIHSS score was ‐4.5 in the chlormethiazole group (N = 96) and ‐4.0 in the placebo group (N = 102; P = 0.36). In Lyden 2002, the change of NIHSS score (median (quartiles)) was ‐5.5 (‐11, 17) in the chlormethiazole group (N = 586) and ‐6.0 (‐10, 16) in the placebo group (N = 583; P = 0.68). In Wahlgren 1999, no significant difference was found between the placebo and chlormethiazole groups for the change in score in the SSS 48‐point (P = 0.56) and SSS motor power score (P = 0.96). In Lyden 2000 and Lyden 2002, the change in score in the SSS was not significant in the two groups (P = 0.06 and P = 0.23, respectively). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence Interval; NIHSS: National Institutes of Health Stroke Scale; RR: Risk Ratio; SSS: Scandinavian Stroke Scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to unclear risk of selection bias
Functional independence, defined as a BI score higher than 60 or a mRS score less than 3
Summary of findings 2.
Diazepam compared with placebo for acute stroke
| Diazepam compared with placebo for acute stroke | ||||||
|
Patient or population: people with acute stroke Settings: inpatients Intervention: diazepam Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Diazepam | |||||
| Death or dependency | 513 per 1000 | 481 per 1000 | RR 0.94 (0.82 to 1.07) | 849 (1) | ⊕⊕⊕⊝ Moderate1 | — |
| Adverse events | 357 per 1000 | 355 per 1000 | RR 0.99 (0.75 to 1.31) | 865 (1) | ⊕⊕⊕⊝ Moderate1 | — |
| Functional independence | 487 per 1000 | 519 per 1000 | RR 1.07 (0.93 to 1.22) | 849 (1) | ⊕⊕⊕⊝ Moderate1 | — |
| Other stroke scales | Not reported | Not reported | — | — | — | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level: one study with small sample size
Functional independence, defined as a BI score higher than 60 or a mRS score less than 3
Background
Description of the condition
Acute stroke is defined as a clinical syndrome of sudden onset of focal or global disturbance of central nervous system function, due to an interruption of the cerebral circulation (Warlow 2001). Ischemic stroke (80% of all strokes) is the most frequent type, followed by intracerebral hemorrhage (15%) and subarachnoid hemorrhage (5%). The estimated annual incidence of stroke is 0.25%, and increases with age (Simon 2009; WHO 2011). Two‐thirds of all strokes occur in those older than 65 years (AHA 2002). The common risk factors include smoking, hypertension, diabetes, carotid stenosis, hypercholesterolemia, hyperhomocysteinemia, alcohol abuse, and a high‐fat diet (Cruz‐Flores 2011). The prognosis is poor, with one‐third of patients dying and one‐third left with permanent disability (WHO 2011). In addition, stroke is a costly condition that incurs treatment, ongoing care, and indirect costs (Saka 2009).
Description of the intervention
Neuroprotective agents have attracted a lot of attention for the treatment of acute stroke, and are expected to be helpful in protecting vulnerable neurons and salvaging the ischemic penumbra (ischemic but still viable tissue). The most common neuroprotectants include excitatory amino acid antagonists, gangliosides, calcium channel antagonists, lubeluzole, methylxanthine derivatives, and tirilazad − each with different modes of action. It is disappointing that none of these treatments has been confirmed to be effective in the acute phase of stroke (Bath 2001; Bath 2004; Candelise 2001; Gandolfo 2002; Horn 2000; Muir 2003). Therefore, it is necessary to examine other potential neuroprotectants for stroke. Gamma aminobutyric acid (GABA) receptor agonists (e.g. diazepam and chlormethiazole) are traditional sedatives that have been used for several decades. They have also been found to be effective in reducing infarct size in histology, and improving functional outcome in animal models of cerebral ischemia (Gasior 2004; Marshall 2003; Sydserff 2002).
How the intervention might work
GABA is the main inhibitory neurotransmitter in the central nervous system and acts by reducing the depolarization‐induced and ischemia‐induced glutamate release (Nelson 2000; Vaishnav 2002). First, GABA can trigger hyperpolarization of neurons through anion channels (GABAA) and presynaptic G‐protein coupled receptors (GABAB) (Wilby 2004). This hyperpolarization counteracts the depolarization, which is the initiating event in the biochemical ischemic cascade (Tuttolomondo 2009). Secondly, there is no shortage of GABA in ischemic conditions, but the affinity of GABA receptors is decreased (Alicke 1995). Activation of GABA reduces respiratory rate, preserving glucose and reducing acidosis, which facilitates local cerebral blood flow (Chi 2011; Zubcevic 2010). Finally, GABA receptor agonists can induce hypothermia, which is also regarded as a neuroprotective condition for acute stroke (Klassman 2011; Visser 2005). In certain conditions, such as total anterior circulation syndrome (TACS), there is particular interest in neuroprotectants, because the large volume of infarction may suggest a large penumbra as a target for therapy.
Why it is important to do this review
There are existing clinical trials on the effects of GABA receptor agonists in acute stroke based on the results of preclinical in vivo studies. However, conflicting results limit their wider application in sedation caused by GABA receptor agonists in acute stroke with edema (Hanna 1996). This is an update of a Cochrane review first published in 2013 (Liu 2013), and previously updated in 2014 (Liu 2014), to evaluate the efficacy and safety of GABA receptor agonists through high‐quality randomized controlled trials (RCTs).
Objectives
To determine the efficacy and safety of GABA receptor agonists in the treatment of acute stroke.
Methods
Criteria for considering studies for this review
Types of studies
All randomized controlled trials (RCTs) investigating GABA receptor agonists for people with acute stroke were eligible. We excluded uncontrolled, non‐randomized or quasi‐randomized trials.
Types of participants
We included people who had suffered an acute stroke within the previous 12 hours. There were no limitations in gender, age, or subtype of stroke.
Types of interventions
Intervention: GABA receptor agonists administered orally or intravenously, regardless of length of treatment or dosage of treatment.
Comparator: placebo.
We considered interventions with concomitant therapies when they were administered in both treatment arms.
Types of outcome measures
We assessed the following outcomes measured at three‐month follow‐up.
Primary outcomes
Efficacy
Death or dependency at the end of follow‐up (at least three months). We defined dependency as a Barthel Index (BI) score of 60 or less, or the modified Rankin Scale (mRS) Grades 3 to 5 (Sulter 1999), or we used the definition provided by the researchers.
Safety
The number of people with adverse events (serious adverse events and frequent adverse events, e.g. somnolence and rhinitis).
Secondary outcomes
Functional independence
Defined as a BI score greater than 60, or a mRS less than 3.
Neurological function
Measured by other stroke scales, e.g. National Institutes of Health Stroke Scale (NIHSS) or Scandinavian Stroke Scale (SSS).
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers published in languages other than English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (March 2016) and the following electronic bibliographic databases:
Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 3, part of the Cochrane Library. www.cochranelibrary.com (accessed March 2016); Appendix 1;
MEDLINE Ovid (from 1949 to March 2016); Appendix 2;
Embase Ovid (from 1980 to March 2016); Appendix 3;
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; from 1982 to March 2016); Appendix 4;
AMED Ovid (Allied and Complementary Medicine Database; from 1985 to March 2016); Appendix 5;
Chinese Stroke Trials Register (accessed March 2016);
CBM‐disc (China Biological Medicine Databases; from 1979 to March 2016);
CNKI (China National Knowledge Infrastructure; from 1979 to March 2016);
Chinese MD and DD Dissertations in CNKI (accessed March 2016);
CACP (Chinese Academic Conference Papers Database; from 1998 to March 2016);
CDDB (Chinese Dissertations Database; from 1977 to March 2016);
Chinese Evidence‐Based Medicine Database (accessed March 2016);
CMAC (China Medical Academic Conferences; from 1994 to March 2016);
CMCC (Chinese Medical Current Contents; from 1994 to March 2016);
Chinese Science and Technique Journals Database (VIP; from 1989 to March 2016);
Wanfang Data. www.wanfangdata.com/ (from 1984 to March 2016).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and adapted it for the other databases.
To identify further published, unpublished, and ongoing trials, we searched the following trials registers in March 2016:
ClinicalTrials.gov (www.clinicaltrials.gov/);
EU Clinical Trials Register (www.clinicaltrialsregister.eu);
Stroke Trials Registry (www.strokecenter.org/trials/);
Current Controlled Trials (www.controlled‐trials.com);
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Searching other resources
We also:
used Science Citation Index Cited Reference Search for forward tracking of important articles;
searched reference lists of reviews and retrieved articles;
searched conference proceedings, including the 22nd, 23rd, and 24th European Stroke Conference (2013, 2014, and 2015) and the 7th, 8th, and 9th World Stroke Congress (2010, 2012, and 2014);
contacted authors for missing information, where necessary;
contacted the manufacturer (AstraZeneca pharmaceutical company) for updated information.
Data collection and analysis
Selection of studies
Two review authors (JL, LW) independently screened titles and abstracts of the citations produced by the database searches and excluded obviously irrelevant studies. We obtained the full‐text articles of all remaining citations and the same two authors independently selected studies that met the inclusion criteria. Both authors independently evaluated eligibility and assessed the risk of bias (methodological quality) of these studies. We resolved any disagreements by discussion, or referred them to an independent party (XM) if necessary.
Data extraction and management
Two review authors (JL, LW) independently extracted eligible data from the published reports onto pre‐standardized forms, and cross‐checked them for accuracy. We used checklists to independently record relevant details including methods of generating randomization schedule, method of concealment of allocation, blinding of assessors, intention‐to‐treat analysis, adverse events and dropouts for all reasons, important imbalance in prognostic factors, participants (socio‐demographic and related clinical information), interventions (medications and non‐pharmacological interventions), and outcomes. We resolved disagreements by consensus.
Assessment of risk of bias in included studies
Two review authors (JL, LW) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion among review authors. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We assessed the risk of bias for each domain as high, low, or unclear risk, and provided information from the study report with the description of the sources of bias. We judged a study to be at low risk of bias if all key domains were rated at low risk of bias. If one or more of the domains was rated at unclear risk of bias, we judged the study to be at unclear risk of bias. We judged the study to be at high risk of bias if one or more of the domains was rated at high risk of bias.
Measures of treatment effect
We expressed results for dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI). We entered and analyzed data in RevMan 5 (Review Manager 2014).
Unit of analysis issues
We dealt with any unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Dealing with missing data
We attempted to contact the authors of the studies for further details if any data were missing, and to establish the characteristics of unpublished trials, through correspondence with trial co‐ordinators or principal investigators.
Assessment of heterogeneity
We tested heterogeneity using the I² statistic and made a judgement as to whether significant heterogeneity was present (Higgins 2011). We took I² values over 50% as suggestive of substantial heterogeneity. However, the direction and magnitude of effects were taken into account.
Assessment of reporting biases
We had planned to use the funnel plot method if there were sufficient numbers of trials to allow for a meaningful presentation (Egger 1997).
Data synthesis
If a sufficient number of comparable studies with a low risk of bias were available, we had planned to carry out meta‐analyses. We calculated the overall effects using a random‐effects model, regardless of the level of heterogeneity. If substantial heterogeneity between the studies prevented us from combining outcome data, we gave a descriptive summary of the results.
Summary of findings and quality of the evidence (GRADE)
In a post‐hoc change, we have presented two summary of findings tables, one for each comparison (Table 1; Table 2). We reported all outcomes in the tables.
We determined the quality of the evidence using the GRADE approach, and downgraded evidence in the presence of risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, or a high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Subgroup analysis and investigation of heterogeneity
We analyzed subgroups of studies categorized by ischemic or hemorrhagic stroke, subtype of stroke (such as total anterior circulation infarcts, partial anterior circulation infarcts, posterior circulation infarcts, and lacunar infarcts), and time from stroke onset to treatment administration.
Sensitivity analysis
We undertook sensitivity analyses to assess the robustness of results in fixed‐effect versus random‐effects models, and studies at high risk versus low risk of bias. We also used these sensitivity analyses to examine potential sources of methodological heterogeneity.
Results
Description of studies
Results of the search
From our original review, we included five studies. When we re‐ran the searches in March 2016, we identified 545 papers after removing duplications (Figure 1). We acquired and screened the full text of only one article (Zhang 2014); this study did not meet the inclusion criteria because of their participants. Agreement between the review authors on exclusion was 100%. We found no ongoing RCTs.
Figure 1.
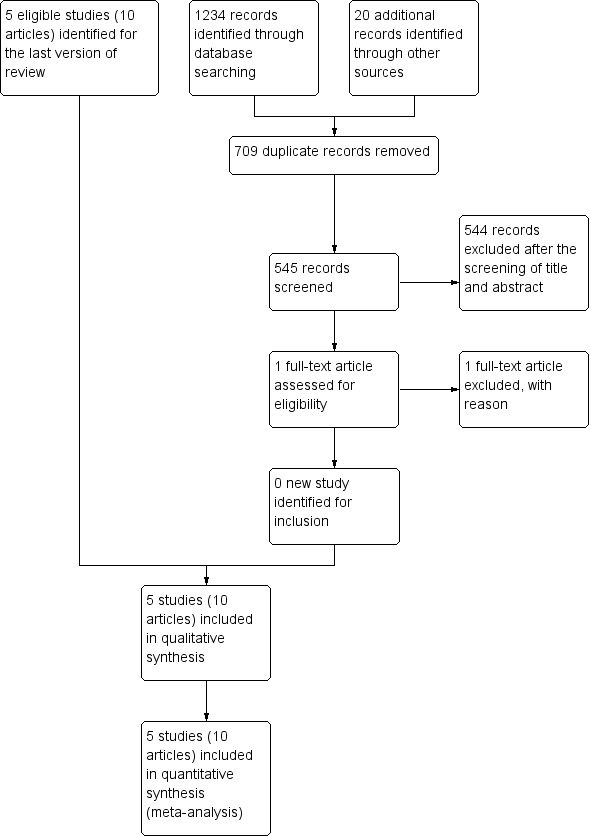
Study flow diagram.
Included studies
We included five studies, with 3838 participants. One study evaluated the efficacy and safety of diazepam for acute stroke in 879 participants, included within 12 hours of stroke onset (Lodder 2006). The Chlormethiazole Acute Stroke Study focused mainly on the efficacy and safety of chlormethiazole in 1360 participants with acute stroke, included within 12 hours of stroke onset (Wahlgren 1999): 95 participants had hemorrhagic stroke and were analyzed separately. We undertook a subgroup analysis of TACS in 545 participants. After the completion of Wahlgren 1999, another chlormethiazole acute stroke study in ischemic, hemorrhagic and t‐PA treated stroke (CLASS‐IHT) was designed. All participants were included within 12 hours after stroke onset. There were 1198 participants randomized in Lyden 2002, 201 in Lyden 2000, and 200 in Lyden 2001. We have provided relevant information about the included trials in the Characteristics of included studies table.
Excluded studies
We excluded nine studies after full‐text evaluation. We have provided the reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
Information regarding risk of bias is provided in Figure 2 and Figure 3.
Figure 2.
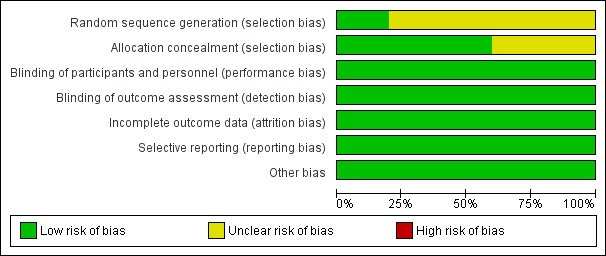
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
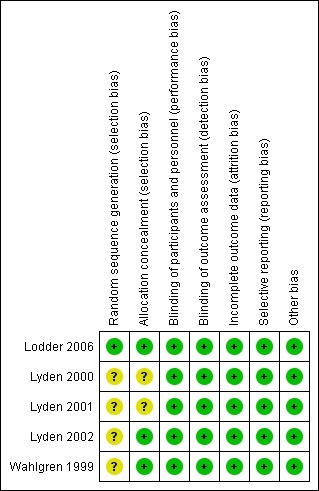
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the included trials stated that participants were randomized into intervention and placebo groups, but only one trial described the actual method of randomization and allocation concealment. Therefore, we judged the random sequence generation and allocation concealment of the trials as unclear risks of bias.
Blinding
All of the participants and investigators were blinded to the trial medication. We assessed blinding as low risk of bias.
Incomplete outcome data
All five studies reported the reason and number of participants who had discontinued treatment by the endpoint. Only one trial stated they had performed an intention‐to‐treat analysis (Lodder 2006). In general, 80/3838 (2%) of the randomized participants were not included in the efficacy analysis. Therefore, we assessed this as low risk of bias.
Selective reporting
All of the pre‐specified outcomes were reported. Therefore, we assessed this as low risk of bias.
Other potential sources of bias
We did not find any potential publication bias. Insufficient numbers of trials were available for a funnel plot analysis.
Effects of interventions
Primary outcome measures
Efficacy (death or dependency)
Four trials with 2909 participants reported death and dependency at three months after chlormethiazole or placebo administration; 710/1457 (49%) and 689/1452 (47%) deaths or dependencies occurred in the chlormethiazole and placebo groups, respectively (RR 1.03, 95% CI 0.96 to 1.11; Lyden 2000; Lyden 2001; Lyden 2002; Wahlgren 1999). One trial with 849 participantsthat compared diazepam and placebo, reported 206/428 (48%) and 216/421 (51%) deaths or dependencies in the diazepam and placebo groups, respectively (RR 0.94, 95% CI 0.82 to 1.07; Lodder 2006).
In total, 916/1885 (49%) participants in the gamma aminobutyric acid (GABA) receptor agonists group and 905/1873 (48%) participants in the placebo group experienced death or dependency at three months (RR 1.01, 95% CI 0.95 to 1.08; Analysis 1.1).
Analysis 1.1.
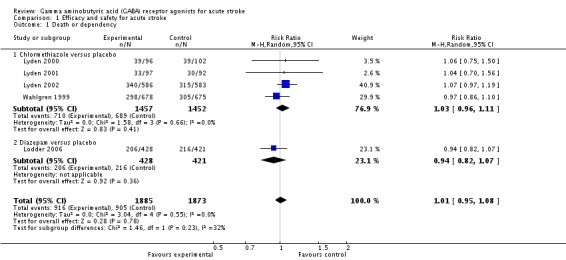
Comparison 1 Efficacy and safety for acute stroke, Outcome 1 Death or dependency.
Safety (adverse events)
All of the trials reported serious adverse events (SAEs). Four trials compared chlormethiazole with placebo (Lyden 2000; Lyden 2001; Lyden 2002; Wahlgren 1999), and one trial compared diazepam with placebo (Lodder 2006). We found no significant differences in either the number of participants with SAEs or the number of SAEs in all trials.
The more frequent adverse events in the chlormethiazole group, reported in two trials, were somnolence and rhinitis (Lyden 2002; Wahlgren 1999). In the chlormethiazole and placebo groups, 654/1266 (52%) participants and 142/1261 (11%) participants, respectively experienced somnolence (RR 4.56, 95% CI 3.50 to 5.95; Analysis 1.2), while 165/1266 (13%) participants and 32/1261 (3%) participants, respectively, experienced rhinitis (RR 4.75, 95% CI 2.67 to 8.46; Analysis 1.3). There was significant heterogeneity (I² = 62% and I² = 53% respectively) for these two outcomes, therefore, we used a random‐effects model for the analyses.
Analysis 1.2.
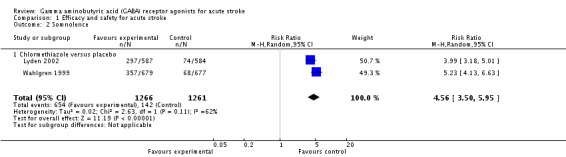
Comparison 1 Efficacy and safety for acute stroke, Outcome 2 Somnolence.
Analysis 1.3.
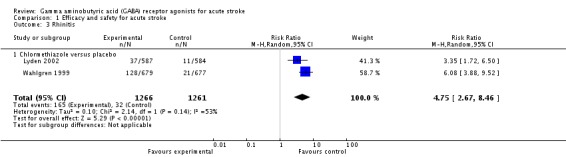
Comparison 1 Efficacy and safety for acute stroke, Outcome 3 Rhinitis.
Secondary outcomes measures
Functional independence
Functional independence, defined as a BI score higher than 60 or a mRS score less than 3, was measured in five trials. Four trials compared chlormethiazole with placebo, with 747/1457 (51%) participants and 763/1452 (53%) participants, respectively, scoring more than 60 on the BI score (RR 0.98, 95% CI 0.92 to 1.05; Lyden 2000; Lyden 2001; Lyden 2002; Wahlgren 1999). The Lodder 2006 trial, of diazepam versus placebo, reported that 222/428 (52%) participants and 205/421 (49%) participants, respectively, had a mRS score of less than three (RR 1.07, 95% CI 0.93 to 1.22; Analysis 1.4).
Analysis 1.4.
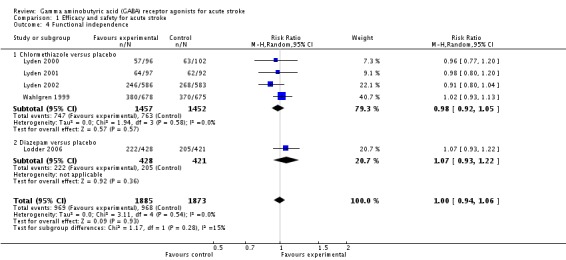
Comparison 1 Efficacy and safety for acute stroke, Outcome 4 Functional independence.
Neurological function
National Institutes of Health Stroke Scale (NIHSS) score
We had intended to calculate the mean change of NIHSS score, however, only two trials reported this and neither of them gave it as 'mean (SD)' (Lyden 2000; Lyden 2002). In Lyden 2000, the mean change in NIHSS score was ‐4.5 in the chlormethiazole group (N = 96) and ‐4.0 in the placebo group (N = 102; P = 0.36). In Lyden 2002, the change in NIHSS score (median (quartiles)) was ‐5.5 (‐11 to 17) in the chlormethiazole group (N = 586) and ‐6.0 (‐10 to 16) in the placebo group (N = 583, P = 0.68).
Scandinavian Stroke Scale (SSS) score
The results of the SSS score were given for three trials (Lyden 2000; Lyden 2002; Wahlgren 1999). In Wahlgren 1999, we found no significant difference between the placebo and chlormethiazole groups for the change in score in the 48‐point Scandinavian Stroke Scale (SSS‐48; P = 0.56) and the Scandinavian Stroke Scale motor power score (SSS‐MP; P = 0.96). In Lyden 2000 and Lyden 2002, the change in score in the SSS was not significant in the two groups (P = 0.06 and P = 0.23, respectively).
Subgroup analysis
Efficacy for acute ischemic stroke
Three trials reported death and dependency at three months in the chlormethiazole and placebo groups in 652/1327 (49%) and 628/1319 (48%) participants, respectively (RR 1.04, 95% CI 0.96 to 1.12; Analysis 2.1; Lyden 2001; Lyden 2002; Wahlgren 1999). The same three trials reported functional independence, in 662/1327 (50%) participants in the chlormethiazole group and 675/1319 (51%) participants in the placebo group (RR 0.98, 95% CI 0.91 to 1.05). One other trial reported functional independence in 203/380 (53%) participants in the diazepam group, and 178/368 (48%) participants in the placebo group (RR 1.10, 95% CI 0.96 to 1.27; Analysis 2.2; Lodder 2006).
Analysis 2.1.
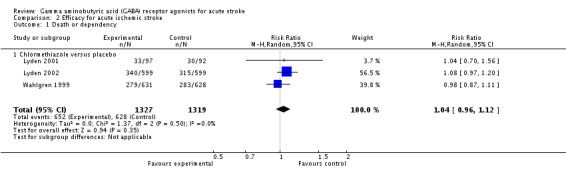
Comparison 2 Efficacy for acute ischemic stroke, Outcome 1 Death or dependency.
Analysis 2.2.
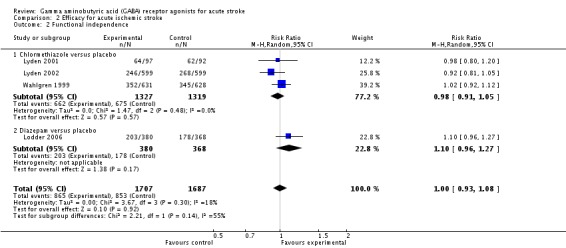
Comparison 2 Efficacy for acute ischemic stroke, Outcome 2 Functional independence.
Efficacy for acute hemorrhagic stroke
Two trials reported death and dependency at three months in the chlormethiazole and placebo groups in 58/143 (41%) and 61/149 (41%) participants, respectively (RR 0.99, 95% CI 0.75 to 1.30; Analysis 3.1; Lyden 2000; Wahlgren 1999). Functional independence was found in 85/143 (59%) in the chlormethiazole group and 88/149 (59%) in the placebo group (RR 1.00, 95% CI 0.83 to 1.21). In addition, Lodder 2006, which tested diazepam versus placebo, found that 18/46 (39%) and 24/49 (49%) participants, respectively, were functionally independent (RR 0.80, 95% CI 0.50 to 1.27; Analysis 3.2).
Analysis 3.1.
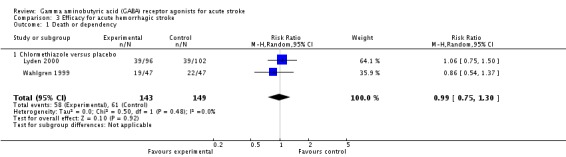
Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 1 Death or dependency.
Analysis 3.2.
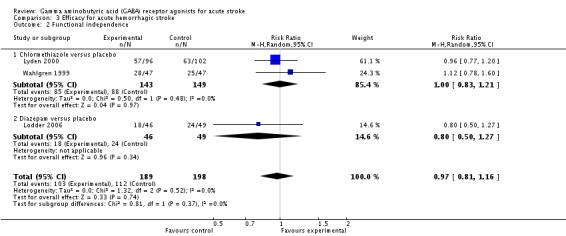
Comparison 3 Efficacy for acute hemorrhagic stroke, Outcome 2 Functional independence.
Efficacy for total anterior circulation syndrome (TACS)
For participants with a TACS, two trials measured functional independence at three months in the chlormethiazole and placebo groups (Wahlgren 1999; Lyden 2001). In total, 144/338 (43%) participants and 94/297 (32%) participants, respectively, were found to be functionally independent (RR 1.33, 95% CI 1.09 to 1.64; Analysis 4.1).
Analysis 4.1.
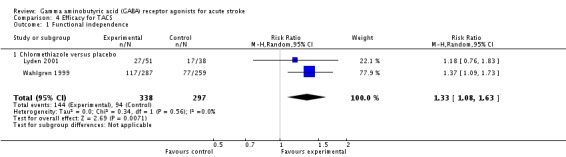
Comparison 4 Efficacy for TACS, Outcome 1 Functional independence.
Efficacy for early‐treated acute stroke
We extracted data for early‐treated acute stroke. Two trials reported functional independence at three months in participants treated within six hours of stroke onset, in 267/590 (45%) participants in the chlormethiazole group and 282/592 (48%) participants in the placebo group (RR 0.93, 95% CI 0.73 to 1.19; Lyden 2002; Wahlgren 1999). Lodder 2006 defined early treatment as within three hours of onset and measured functional independence at three months in the diazepam and placebo groups: 37/70 (53%) participants and 27/62 (44%) participants, respectively, were reported to be functionally independent (RR 1.21, 95% CI 0.85 to 1.74; Analysis 5.1).
Analysis 5.1.
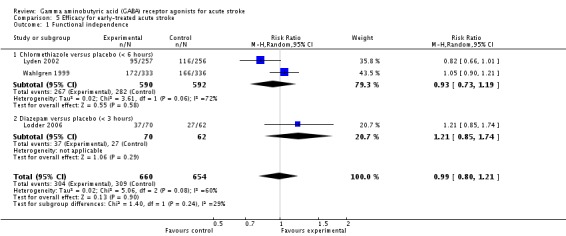
Comparison 5 Efficacy for early‐treated acute stroke, Outcome 1 Functional independence.
Sensitivity analysis
Fixed‐effect versus random‐effects models
We calculated the overall effects using a random‐effects model, regardless of the level of heterogeneity. We also assessed the robustness of results in fixed‐effect versus random‐effects models. We found no changes in the results.
Excluding studies with potential selection bias
Only one trial clearly described random sequence generation and allocation concealment, and it was the only trial of diazepam for acute stroke (Lodder 2006); the other trials tested chlormethiazole. Thus, the data were inadequate to conduct a sensitivity analysis.
Discussion
Summary of main results
We included five eligible trials with 3838 participants (3758 analyzed) in our review. The methodological quality of the included trials was generally good, with an unclear risk for selection bias only. There was no convincing evidence to support the use of gamma aminobutyric acid (GABA) receptor agonists (chlormethiazole or diazepam) for the treatment of people with acute ischemic or hemorrhagic stroke.
Our primary outcomes were efficacy and safety. Four trials reported death and dependency at three months in the chlormethiazole versus placebo groups; we found no significant difference (RR 1.03, 95% CI 0.96 to 1.11). One trial reported this outcome for diazepam versus placebo (RR 0.94, 95% CI 0.82 to 1.07).
The most frequent adverse events caused by chlormethiazole were somnolence (RR 4.56, 95% CI 3.50 to 5.95) and rhinitis (RR 4.75, 95% CI 2.67 to 8.46; two trials).
Overall completeness and applicability of evidence
None of the included studies demonstrated benefits from GABA receptor agonists (chlormethiazole or diazepam) for people with acute stroke when compared with placebo; that is, we found neither a decrease in death or dependency, nor an increase in functional independence. The subgroup analysis for those with total anterior circulation syndrome (TACS) illustrated a positive result, based on only two studies. Meanwhile, the subgroup analysis for acute hemorrhagic stroke did not find an increase in death or dependency, which meant that GABA receptor agonists did not appear to cause harm in hemorrhagic stroke when compared with placebo. Readers should note these conclusions need further confirmation by more RCTs with large samples. At present, no clear evidence supports the clinical administration of GABA receptor agonists in any type of acute stroke.
Quality of the evidence
This review provides moderate‐quality evidence. The search methods were rigorous and well performed. The methodological quality of the included trials was generally good, with an unclear risk for selection bias only. We also specified the reasons for excluding trials. However, the conclusions from subgroup analyses should be interpreted with caution, due to the limitations of the available data.
Potential biases in the review process
Some data were not provided for subgroup analyses. For instance, the Wahlgren 1999 study included 1360 participants with acute stroke, 7% of whom (95 participants) had a hemorrhagic stroke. However, they did not provide the independent data for ischemic stroke, and we used subtraction to calculate the ischemic stroke data for the outcomes. This may have led to potential bias.
Agreements and disagreements with other studies or reviews
We found only one review on this topic, which mainly focused on the pharmacokinetics of chlormethiazole in people with acute stroke (Zingmark 2003). Therefore, it is not comparable, because of the different objectives and outcomes.
Authors' conclusions
This review provides moderate‐quality evidence that fails to support the use of gamma aminobutyric acid (GABA) receptor agonists (chlormethiazole or diazepam) for the treatment of people with acute ischemic or hemorrhagic stroke. Somnolence and rhinitis seem to be the most frequent adverse events related to chlormethiazole, and further investigations are required.
Well‐designed, double‐blind RCTs would be required to test the efficacy of chlormethiazole in a large group of people with TACS.
Acknowledgements
The authors would like to acknowledge the help provided by the Cochrane Stroke Group.
Appendices
Appendix 1. Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [Basal Ganglia Cerebrovascular Disease]
#2 MeSH descriptor: [Brain Ischemia]
#3 MeSH descriptor: [Carotid Artery Diseases]
#4 MeSH descriptor: [Cerebrovascular Trauma]
#5 MeSH descriptor: [Intracranial Arterial Diseases]
#6 MeSH descriptor: [Intracranial Arteriovenous Malformations]
#7 MeSH descriptor: [Intracranial Embolism and Thrombosis]
#8 MeSH descriptor: [Intracranial Hemorrhages]
#9 MeSH descriptor: [Stroke] explode all trees
#10 MeSH descriptor: [Brain Infarction]
#11 MeSH descriptor: [Vasospasm, Intracranial] explode all trees
#12 MeSH descriptor: [Cerebrovascular Disorders] explode all trees
#13 (stroke or poststroke or "post‐stroke" or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab,kw
#14 ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*mi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw
#15 ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*))
#16 {or #1‐#15}
#17 MeSH descriptor: [GABA Agonists] explode all trees
#18 MeSH descriptor: [GABA‐A Receptor Antagonists]
#19 MeSH descriptor: [GABA‐B Receptor Agonists]
#20 MeSH descriptor: [GABA Modulators] explode all trees
#21 MeSH descriptor: [gamma‐Aminobutyric Acid]
#22 MeSH descriptor: [Receptors, GABA]
#23 (("gamma aminobutyric acid" or "gamma‐aminobutyric acid" or gaba or "gaba‐A" or "gaba‐B") near/5 (agonist* or modulator* or stimulat* or stimulant*)):ti,ab,kw
#24 ((gabaergic or "gaba‐ergic" or gabamimetic) near/5 (agent* or drug* or stimul*)):ti,ab,kw
#25 (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine* or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or "Hopantenate calcium" or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or "Oxybate sodium" or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or "Valproic acid" or Vigabatrin or Zolazepam or XP19986):ti,ab,kw
#26 {or #17‐#25}
#27 #16 and #26
Appendix 2. MEDLINE (Ovid) search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp cerebrovascular trauma/ or exp intracranial arterial diseases/ or exp intracranial arteriovenous malformations/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. ((transi$ adj3 isch?em$ adj3 attack$) or TIA$1).tw.
6. 1 or 2 or 3 or 4 or 5
7. gaba agonists/ or exp gaba‐a receptor agonists/ or exp gaba‐b receptor agonists/ or exp gaba modulators/
8. exp gamma‐Aminobutyric Acid/tu [Therapeutic Use]
9. exp Receptors, GABA/de [Drug Effects]
10. ((gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$)).tw.
11. ((gabaergic or gaba‐ergic or gabamimetic) adj5 (agent$ or drug$ or stimul$)).tw.
12. (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986).tw,nm.
13. 7 or 8 or 9 or 10 or 11 or 12
14. Randomized Controlled Trials as Topic/
15. Random Allocation/
16. Controlled Clinical Trials as Topic/
17. control groups/
18. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
19. double‐blind method/
20. single‐blind method/
21. Placebos/
22. placebo effect/
23. Drug Evaluation/
24. Research Design/
25. randomized controlled trial.pt.
26. controlled clinical trial.pt.
27. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
28. (random$ or RCT or RCTs).tw.
29. (controlled adj5 (trial$ or stud$)).tw.
30. (clinical$ adj5 trial$).tw.
31. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
32. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseud or random$).tw.
33. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
34. placebo$.tw.
35. controls.tw.
36. exp animals/ not humans.sh.
37. 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35
38. 6 and 13 and 37
39. 38 not 36
40. limit 39 to yr="2013 ‐Current"
Appendix 3. Embase (Ovid) search strategy
1. cerebrovascular disease/ or exp basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or exp cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or vertebrobasilar insufficiency/ or stroke/ or stroke patient/ or stroke unit/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. 1 or 2 or 3 or 4
6. exp 4 aminobutyric acid receptor stimulating agent/
7. 4 aminobutyric acid/ct, ad, dt or exp 4 aminobutyric acid receptor/ct, dt
8. ((gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) adj5 (agonist$ or modulator$ or stimul$)).tw.
9. ((gabaergic or gaba‐ergic or gabamimetic) adj5 (agent$ or drug$ or stimul$)).tw.
10. (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986).tw.
11. 6 or 7 or 8 or 9 or 10
12. 5 and 11
13. Randomized Controlled Trial/ or Randomization/
14. Controlled Study/
15. control group/
16. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
17. Double Blind Procedure/
18. Single Blind Procedure/ or triple blind procedure/
19. placebo/
20. "types of study"/
21. trial.ti.
22. (random$ or RCT or RCTs).tw.
23. (controlled adj5 (trial$ or stud$)).tw.
24. (clinical$ adj5 trial$).tw.
25. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
26. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
27. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
28. placebo$.tw.
29. controls.tw.
30. or/13‐29
31. 12 and 30
32. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
33. 31 not 32
34. limit 33 to yr="2013 ‐Current"
Appendix 4. CINAHL (EBSCO) search strategy
S1. (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
S2. (MH "Stroke Patients") OR (MH "Stroke Units")
S3. TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
S4. TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral)
S5. TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
S6. S4 and S5
S7. TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
S8. TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
S9. S7 and S8
S10. S1 OR S2 OR S3 OR S6 OR S9
S11. (MH "GABA Agonists+") or (MH "GABA Modulators+")
S12. (MH "GABA/TU")
S13. TI (gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) or AB (gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B)
S14. TI (agonist* or modulator* or stimulat* or stimulant*) or AB (agonist* or modulator* or stimulat* or stimulant*)
S15. S13 and S14
S16. TI (gabaergic or gaba‐ergic or gabamimetic) or AB (gabaergic or gaba‐ergic or gabamimetic)
S17. TI (agent* or drug* or stimul*) or AB (agent* or drug* or stimul*)
S18. S16 and S17
S19. TI (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986) or AB (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986)
S20. S11 OR S12 or S15 or S18 or S19
S21. S10 AND S20
Appendix 5. AMED (Ovid) search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. 1 or 2 or 3 or 4
6. ((gamma aminobutyric acid or gamma‐aminobutyric acid or gaba or gaba‐A or gaba‐B) adj5 (agonist$ or modulator$ or stimulat$ or stimulant$)).tw.
7. ((gabaergic or gaba‐ergic or gabamimetic) adj5 (agent$ or drug$ or stimul$)).tw.
8. (Adipiplon or Alprazolam or Amobarbital or Arbaclofen or Atagabalin or AZD3355 or AZD9343 or Baclofen or Barbital or Benzodiazepine$ or Bromazepam or Chlordiazepoxide or Chlormethiazole or Clomethiazole or Clonazepam or Clorazepate or Dipotassium or Diazepam or Dihydromuscimol or Estazolam or Fengabine or Flumazenil or Flunitrazepam or Flurazepam or Gabapentin or Gaboxadol or Hexobarbital or Hopantenate calcium or Lesogaberan or Lorazepam or Medazepam or Mephobarbital or Midazolam or muscimol or Nitrazepam or Nordazepam or Oxazepam or Oxybate sodium or Pagoclone or Pentobarbital or Phenobarbital or Picamilon or Prazepam or Pregabalin or Progabide or Secobarbital or Temazepam or Thiamylal or Thiopental or THIP or TPA023 or Triazolam or Valproic acid or Vigabatrin or Zolazepam or XP19986).tw.
9. 6 or 7 or 8
10. 5 and 9
11. limit 10 to yr="2013 ‐Current"
Data and analyses
Comparison 1.
Efficacy and safety for acute stroke
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.95, 1.08] |
| 1.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.96, 1.11] |
| 1.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.07] |
| 2 Somnolence | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 2.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.56 [3.50, 5.95] |
| 3 Rhinitis | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 3.1 Chlormethiazole versus placebo | 2 | 2527 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [2.67, 8.46] |
| 4 Functional independence | 5 | 3758 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.94, 1.06] |
| 4.1 Chlormethiazole versus placebo | 4 | 2909 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.92, 1.05] |
| 4.2 Diazepam versus placebo | 1 | 849 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.93, 1.22] |
Comparison 2.
Efficacy for acute ischemic stroke
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 1.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.96, 1.12] |
| 2 Functional independence | 4 | 3394 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.93, 1.08] |
| 2.1 Chlormethiazole versus placebo | 3 | 2646 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 2.2 Diazepam versus placebo | 1 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.96, 1.27] |
Comparison 3.
Efficacy for acute hemorrhagic stroke
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 1.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 2 Functional independence | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2.1 Chlormethiazole versus placebo | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.21] |
| 2.2 Diazepam versus placebo | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.27] |
Comparison 4.
Efficacy for TACS
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional independence | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
| 1.1 Chlormethiazole versus placebo | 2 | 635 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.08, 1.63] |
Comparison 5.
Efficacy for early‐treated acute stroke
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional independence | 3 | 1314 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.80, 1.21] |
| 1.1 Chlormethiazole versus placebo (< 6 hours) | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 1.2 Diazepam versus placebo (< 3 hours) | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.85, 1.74] |
What's new
Last assessed as up‐to‐date: 31 March 2016.
| Date | Event | Description |
|---|---|---|
| 31 March 2016 | New citation required but conclusions have not changed | Conclusions not changed. |
| 31 March 2016 | New search has been performed | We updated all the searches for this review to March 2016 but did not identify any new information for inclusion. Therefore, the conclusions of the review remain unchanged. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lodder 2006
| Methods | A multicenter, randomized, stratified, double‐blind, placebo‐controlled clinical trial to examine the efficacy and safety of diazepam in acute stroke | |
| Participants | Adult males and females were included within 12 hours after stroke onset. CT or MRI within 7 days was mandatory. People with a clear indication for, or contraindication to benzodiazepines (at the discretion of the attending physician) were excluded, as were people with unresponsive coma. 879 eligible people from 35 hospitals in 5 European countries were randomized into the trial |
|
| Interventions | Diazepam 10 mg or placebo by rectiole, as soon as possible, followed by 10 mg tablets twice daily for 3 days versus placebo | |
| Outcomes | Independence (mRS < 3); complete recovery (BI ≧ 95 or mRS ≦ 1); adverse events; mortality | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized using a computer‐generated random listing of the 2 treatment assignments, blocked in groups of 4 and stratified for center |
| Allocation concealment (selection bias) | Low risk | Trial medication was packed and labeled by the hospital's pharmacist according to a medication code schedule generated before the trial, and sent to the participating centers in boxes of 20 treatment packs |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All the participants were blinded to trial medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the investigators, treating physicians and nurses were blinded to trial medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31 participants (3.5%) discontinued the study after randomization, with explicit reasons |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | All efficacy and safety outcomes were analyzed by intention‐to‐treat |
Lyden 2000
| Methods | The safety of chlormethiazole versus placebo in hemorrhagic stroke patients was evaluated in a randomized, double‐blind trial | |
| Participants | Conscious participants aged 18 to 90 years were included within 12 hours after stroke onset. 201 eligible participants were recruited and randomized into the trial |
|
| Interventions | Chlormethiazole (68 mg/kg) or placebo was given as an intravenous infusion over a 24‐hour period | |
| Outcomes | Adverse events; mortality; independence (BI ≧ 60 or mRS < 3); NIHSS; SSS‐48 | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Chlormethiazole and placebo were supplied in identical bottles to keep the treatment assignment blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the measurements were made by an assessor who was not involved during the administration of the study drug, to maintain blinding of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study drug was not administered to 1 participant in the chlormethiazole group and to 2 participants in the placebo group. Therefore, 3/201 (1%) participants were not included in the analysis of safety or efficacy |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
Lyden 2001
| Methods | A randomized, double‐blind, multicenter, placebo‐controlled study to explore the safety of t‐PA combined with chlormethiazole | |
| Participants | There were 101 participants randomized to the chlormethiazole group and 99 to the placebo group by 76 of the 142 hospitals involved in the study | |
| Interventions | All participants received 0.9 mg/kg t‐PA, beginning within 3 hours of stroke onset and then either 68 mg/kg chlormethiazole (N = 97) iv over 24 hours or placebo (N = 93) beginning within 12 hours of stroke onset | |
| Outcomes | Adverse events; mortality; independence (BI ≧ 60) | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The stratified randomization was implemented but the method of random sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Chlormethiazole and placebo were supplied in identical bottles to keep the treatment assignment blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the measurements were made by an assessor who was not involved during the administration of the study drug, to maintain blinding of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomization, 10/200 (5%) participants (4 in the chlormethiazole group and 6 in the placebo group) did not receive the study drug and thus were not included in the safety analysis. All 10 participants showed signs of clinical deterioration after randomization before the study drug could be initiated |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
Lyden 2002
| Methods | A randomized, double‐blind, multinational, placebo‐controlled investigation of the efficacy and safety of chlormethiazole for acute ischemic stroke | |
| Participants | Conscious participants aged 18 to 90 years were included within 12 hours after stroke onset. NIHSS score ≧ 3. 1198 eligible participants were recruited from 139 US and 14 Canadian centers and randomized into the trial |
|
| Interventions | Chlormethiazole (68 mg/kg) or placebo was given as an intravenous infusion over a 24‐hour period | |
| Outcomes | Independence (BI ≧ 60 or mRS < 3); NIHSS; SSS‐48; adverse events; mortality | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of random sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | The allocation was conducted by a central randomization scheme via telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Chlormethiazole and placebo were supplied in identical bottles to keep the treatment assignment blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All the measurements were made by an assessor who was not involved during the administration of the study drug, to maintain blinding of treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from 29/1198 (2%) participants were not available for the efficacy analysis. Treatment was never started in 27 participants: 12 in the chlormethiazole group and 15 in the placebo group. In addition, 2 participants (1 per group) provided no efficacy data but were included in the safety analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
Wahlgren 1999
| Methods | Randomized, double‐blind, multicenter, placebo‐controlled study to test the efficacy and safety of the neuroprotective drug chlormethiazole for acute stroke | |
| Participants | Participants aged 40 to 90 years with full consciousness before treatment were included. The symptoms should have lasted more than 1 hour and less than 12 hours. SSS‐48 of ≦ 40, with a sum of scores on arm, hand and leg motor items of ≦ 14. 1360 eligible participants from 85 clinical centers in 7 European countries and Canada were randomized; 546 participants had TACS and 95 participants had hemorrhagic stroke |
|
| Interventions | Chlormethiazole (75 mg/kg) or placebo were given as an intravenous infusion over a 24‐hour period | |
| Outcomes | Independence (BI ≧ 60); SSS‐48; SSS‐MP; adverse events; mortality | |
| Notes | Follow‐up: 3 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization was stratified by center, but the method of random sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | All validations were made with the treatment allocation blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the independent data monitoring committee had access to unblinded data during the course of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only the independent data monitoring committee had access to unblinded data during the course of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16/1360 (1%) randomized participants did not complete the study. 4 participants did not receive treatment (1 randomized to chlormethiazole, 3 to placebo). 4/1360 (0.3%) randomized participants were not available for the safety analysis. In subgroup analyses, data from 1/95 (1%) randomized hemorrhagic stroke participants and 6/546 (1%) randomized TACS participants were not available for analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | No other bias was found |
BI: Barthel Index score CT: computerized tomography iv: intravenous MRI: magnetic resonance imaging mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale SSS‐48: 48‐point Scandinavian Stroke Scale SSS‐MP: Scandinavian Stroke Scale motor power score t‐PA: tissue‐type plasminogen activator TACS: total anterior circulation syndrome
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cucchiara 2003 | Not an RCT |
| Cucchiara 2004 | Not an RCT |
| Lodder 2000 | Not an RCT |
| Lyden 1998 | Neurological outcome of patients was not addressed |
| Lyden 2004 | Not an RCT |
| Wester 1998 | Not an RCT |
| Zhang 2014 | The participants were not eligible |
RCT: randomized controlled trial
Contributions of authors
Jia Liu and Lu‐Ning Wang formulated the idea for the review and developed the basis for the review. Jia Liu took the primary role in searching, identifying and assessing studies; extracting and analyzing the data; and writing up the full review. Lu‐Ning Wang provided general advice on this review, as well as helping to identify trials, assess studies and extract data. Xin Ma served as the independent third author in selection of studies and assessment of risk of bias. Jia Liu supervised the quality of the methodology and statistics used. The manuscript was written by Jia Liu and Lu‐Ning Wang, and revised by Xunming Ji. Jia Liu will be responsible for updating the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Jia Liu: none known. Lu‐Ning Wang: none known. Xin Ma: none known. Xunming Ji: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Lodder J, Raak L, Hilton A, Hardy E, Kessels A. Diazepam to improve acute stroke outcome: results of the early GABA‐ergic activation study in stroke trial. A randomized double‐blind placebo controlled trial. Cerebrovascular Diseases 2006;21:120‐7. [DOI] [PubMed] [Google Scholar]; Raak L, Hilton A, Kessels F, Lodder J. Implementing the EGASIS trial, an international multicenter acute intervention trial in stroke. Controlled Clinical Trials 2002;23:74‐9. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Shuaib A, Ng K, Atkinson R, Ashwood T, Nordlund A, et al. The Clomethiazole Acute Stroke Study in Hemorrhagic Stroke (CLASS‐H): final results. Journal of Stroke and Cerebrovascular Diseases 2000;9:268‐75. [Google Scholar]
- Lyden P, Jacoby M, Schim J, Albers G, Mazzeo P, Ashwood T, et al. The clomethiazole acute stroke study in tissue‐type plasminogen activator‐treated stroke (CLASS‐T). Neurology 2001;57:1199‐205. [DOI] [PubMed] [Google Scholar]
- Lyden P, Shuaib A, Levin K, Atkinson RP, Rajput A, Wechsler L, et al. Clomethiazole acute stroke study in ischemic stroke (CLASS‐I). Stroke 2002;33:122‐9. [DOI] [PubMed] [Google Scholar]; Millis SR, Straube D, Iramaneerat C, Smith EV Jr, Lyden P. Measurement properties of the National Institutes of Health Stroke Scale for people with right‐ and left‐hemisphere lesions: further analysis of the CLomethiazole for Acute Stroke Study‐Ischemic (CLASS‐I) trial. Archives of Physical Medicine and Rehabilitation 2007;88:302‐8. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG, Bornhov S, Sharma A, Cederin B, Rosolacci T, Ashwood T, et al. The Clomethiazole Acute Stroke Study (CLASS): Efficacy results in 545 patients classified as Total Anterior Circulation Syndrome (TACS). Journal of Stroke and Cerebrovascular Diseases 1999;8:231‐9. [DOI] [PubMed] [Google Scholar]; Wahlgren NG, Diez‐Tejador E, Teitelbaum J, Arboix A, Leys D, Ashwood T, et al. Results in 95 hemorrhagic stroke patients included in CLASS, a controlled trial of clomethiazole versus placebo in acute stroke patients. Stroke 2000;31:82‐5. [DOI] [PubMed] [Google Scholar]; Wahlgren NG, Matias Guiu J, Lainez JM, Veloso F, Ranasinha K, Grossman E, et al. The Clomethiazole Acute Stroke Study (CLASS): safety results in 1,356 patients with acute hemispheric stroke. Journal of Stroke and Cerebrovascular Diseases 2000;9:158‐65. [DOI] [PubMed] [Google Scholar]; Wahlgren NG, Ranasinha KW, Rosolacci T, Franke CL, Erven PMM, Ashwood T, et al. Clomethiazole acute stroke study (CLASS). Results of a randomized, controlled trial of clomethiazole versus placebo in 1360 acute stroke patients. Stroke 1999;30:21‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Cucchiara B, Kasner SE, Wolk DA, Lyden PD, Knappertz VA, Ashwood T, et al. Lack of hemispheric dominance for consciousness in acute ischaemic stroke. Journal of Neurology, Neurosurgery and Psychiatry 2003;74:889‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchiara BL, Kasner SE, Wolk DA, Lyden PD, Knappertz VA, Ashwood T, et al. Early impairment in consciousness predicts mortality after hemispheric ischemic stroke. Critical Care Medicine 2004;32:241‐5. [DOI] [PubMed] [Google Scholar]
- Lodder J, Luijckx GJ, Raak L, Kessels F. Diazepam treatment to increase the cerebral GABAergic activity in acute stroke: a feasibility study in 104 patients. Cerebrovascular Diseases 2000;10:437‐40. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Ashwood T, Claesson L, Odergren T, Friday GH, Martin‐Munley S. The clomethiazole acute stroke study in ischemic, hemorrhagic, and t‐PA treated stroke: design of a phase III trial in the United States and Canada. Journal of Stroke and Cerebrovascular Diseases 1998;7:435‐41. [DOI] [PubMed] [Google Scholar]
- Lyden P, Claesson L, Havstad S, Ashwood T, Lu M. Factor analysis of the National Institutes of Health Stroke Scale in patients with large strokes. Archives of Neurology 2004;61:1677‐80. [DOI] [PubMed] [Google Scholar]
- Wester P, Strand T, Wahlgren NG, Ashwood T, Osswald G. An open study of clomethiazole in patients with acute cerebral infarction. Cerebrovascular Diseases 1998;8:188‐90. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zhang R, Zhang S, Xu M, Zhang S. Baclofen for stroke patients with persistent hiccups: a randomized, double‐blind, placebo‐controlled trial. Trials 2014;15:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- American Heart Association. 2002 Heart and Stroke Facts Statistical Update. Dallas: American Heart Association, 2002. [Google Scholar]
- Alicke B, Schwartz‐Bloom RD. Rapid down‐regulation of GABAA receptors in the gerbil hippocampus following transient cerebral ischemia. Journal of Neurochemistry 1995;65:2808‐11. [DOI] [PubMed] [Google Scholar]
- Bath PM, Iddenden R, Bath FJ, Orgogozo JM, Tirilazad International Steering Committee. Tirilazad for acute ischemic stroke. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD002087] [DOI] [PubMed] [Google Scholar]
- Bath PMW, Bath‐Hextall FJ. Pentoxifylline, propentofylline and pentifylline for acute ischemic stroke. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000162.pub2] [DOI] [PubMed] [Google Scholar]
- Candelise L, Ciccone A. Gangliosides for acute ischemic stroke. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000094] [DOI] [PubMed] [Google Scholar]
- Chi OZ, Hunter C, Liu X, Chi Y, Weiss HR. Effects of GABA(A) receptor blockade on regional cerebral blood flow and blood‐brain barrier disruption in focal cerebral ischemia. Journal of Neurological Sciences 2011;301:66‐70. [DOI] [PubMed] [Google Scholar]
- Cruz‐Flores S. Ischemic stroke in emergency medicine. Available from: emedicine.medscape.com/article/1916852‐overview (accessed 9 June 2011).
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo C, Sandercock P, Conti M. Lubeluzole for acute ischemic stroke. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD001924] [DOI] [PubMed] [Google Scholar]
- Gasior M, Witkin JM, Goldberg SR, Munzar P. Chlormethiazole potentiates the discriminative stimulus effects of methamphetamine in rats. European Journal of Pharmacology 2004;494:183‐9. [DOI] [PubMed] [Google Scholar]
- Hanna JP, Frank JI, Furlan AJ, Sila SA, Secic S. Prediction of worsening consciousness from edema after hemispheric infarction. Journal of Stroke and Cerebrovascular Diseases 1996;6:25‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2008. Available from: handbook.cochrane.org.
- Horn J, Limburg M. Calcium antagonists for acute ischemic stroke. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001928] [DOI] [PubMed] [Google Scholar]
- Klassman L. Therapeutic hypothermia in acute stroke. Journal of Neuroscience Nursing 2011;43:94‐103. [DOI] [PubMed] [Google Scholar]
- Marshall JW, Green AR, Ridley RM. Comparison of the neuroprotective effect of clomethiazole, AR‐R15896AR and NXY‐059 in a primate model of stroke using histological and behavioural measures. Brain Research 2003;972:119‐26. [DOI] [PubMed] [Google Scholar]
- Muir KW, Lees KR. Excitatory amino acid antagonists for acute stroke. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001244] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RM, Green AR, Lambert DG, Hainsworth AH. On the regulation of ischemia‐induced glutamate efflux from rat cortex by GABA: in vitro studies with GABA, clomethiazole and pentobarbitone. British Journal of Pharmacology 2000;130:1124‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age and Ageing 2009;38:27‐32. [DOI] [PubMed] [Google Scholar]
- Simon RP, Greenberg DA, Aminoff MJ. Stroke. Clinical Neurology. 7th Edition. New York: McGraw‐Hill, 2009:292‐327. [Google Scholar]
- Sulter G, Steen C, Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999;30:1538‐41. [DOI] [PubMed] [Google Scholar]
- Sydserff SG, Borelli AR, Green AR, Cross AJ. Effect of NXY‐059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat: studies on dose, plasma concentration and therapeutic time window. British Journal of Pharmacology 2002;135:103‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttolomondo A, Sciacca R, Raimondo D, Arnao V, Renda C, Pinto A, et al. Neuron protection as a therapeutic target in acute ischemic stroke. Current Topics in Medicinal Chemistry 2009;9:1317‐34. [DOI] [PubMed] [Google Scholar]
- Vaishnav A, Lutsep HL. GABA agonist: clomethiazole. Current Medical Research and Opinion 2002;18:S5‐8. [DOI] [PubMed] [Google Scholar]
- Visser SA, Pozarek S, Martinsson S, Forsberg T, Ross SB, Gabrielsson J. Rapid and long‐lasting tolerance to clomethiazole‐induced hypothermia in the rat. European Journal of Pharmacology 2005;512:139‐51. [DOI] [PubMed] [Google Scholar]
- Warlow CP, Dennis MS, Gijn J, Hankey GJ, Sandercock PAG, Bamford JM, et al. What caused this transient or persistent ischemic event?. Stroke: A Practical Guide to Management. 2nd Edition. Oxford: Wiley‐Blackwell, 2001. [Google Scholar]
- World Health Organization. The atlas of heart disease and stroke. Available from: who.int/cardiovascular_diseases/resources/atlas/en (accessed 9 June 2011).
- Wilby MJ, Hutchinson PJ. The pharmacology of chlormethiazole: a potential neuroprotective agent?. CNS Drug Reviews 2004;10:281‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingmark PH, Ekblom M, Odergren T, Ashwood T, Lyden P, Karlsson MO, et al. Population pharmacokinetics of clomethiazole and its effect on the natural course of sedation in acute stroke patients. British Journal of Clinical Pharmacology 2003;56:173‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubcevic J, Potts JT. Role of GABAergic neurons in the nucleus tractus solitarii in modulation of cardiovascular activity. Experimental Physiology 2010;95:909‐18. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Liu J, Wang LN. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009622.pub2] [DOI] [PubMed] [Google Scholar]
- Liu J, Wang LN. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD009622.pub3] [DOI] [PubMed] [Google Scholar]


