Abstract
Background
The central venous catheter (CVC) is a device used for many functions, including monitoring haemodynamic indicators and administering intravenous medications, fluids, blood products and parenteral nutrition. However, as a foreign object, it is susceptible to colonisation by micro‐organisms, which may lead to catheter‐related blood stream infection (BSI) and in turn, increased mortality, morbidities and health care costs.
Objectives
To assess the effects of skin antisepsis as part of CVC care for reducing catheter‐related BSIs, catheter colonisation, and patient mortality and morbidities.
Search methods
In May 2016 we searched: The Cochrane Wounds Specialised Register; The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations and Epub Ahead of Print); Ovid EMBASE and EBSCO CINAHL Plus. We also searched clinical trial registries for ongoing and unpublished studies. There were no restrictions with respect to language, date of publication or study setting.
Selection criteria
We included randomised controlled trials (RCTs) that assessed any type of skin antiseptic agent used either alone or in combination, compared with one or more other skin antiseptic agent(s), placebo or no skin antisepsis in patients with a CVC in place.
Data collection and analysis
Two authors independently assessed the studies for their eligibility, extracted data and assessed risk of bias. We expressed our results in terms of risk ratio (RR), absolute risk reduction (ARR) and number need to treat for an additional beneficial outcome (NNTB) for dichotomous data, and mean difference (MD) for continuous data, with 95% confidence intervals (CIs).
Main results
Thirteen studies were eligible for inclusion, but only 12 studies contributed data, with a total of 3446 CVCs assessed. The total number of participants enrolled was unclear as some studies did not provide such information. The participants were mainly adults admitted to intensive care units, haematology oncology units or general wards. Most studies assessed skin antisepsis prior to insertion and regularly thereafter during the in‐dwelling period of the CVC, ranging from every 24 h to every 72 h. The methodological quality of the included studies was mixed due to wide variation in their risk of bias. Most trials did not adequately blind the participants or personnel, and four of the 12 studies had a high risk of bias for incomplete outcome data.
Three studies compared different antisepsis regimens with no antisepsis. There was no clear evidence of a difference in all outcomes examined, including catheter‐related BSI, septicaemia, catheter colonisation and number of patients who required systemic antibiotics for any of the three comparisons involving three different antisepsis regimens (aqueous povidone‐iodine, aqueous chlorhexidine and alcohol compared with no skin antisepsis). However, there were great uncertainties in all estimates due to underpowered analyses and the overall very low quality of evidence presented.There were multiple head‐to‐head comparisons between different skin antiseptic agents, with different combinations of active substance and base solutions. The most frequent comparison was chlorhexidine solution versus povidone‐iodine solution (any base). There was very low quality evidence (downgraded for risk of bias and imprecision) that chlorhexidine may reduce catheter‐related BSI compared with povidone‐iodine (RR of 0.64, 95% CI 0.41 to 0.99; ARR 2.30%, 95% CI 0.06 to 3.70%). This evidence came from four studies involving 1436 catheters. None of the individual subgroup comparisons of aqueous chlorhexidine versus aqueous povidone‐iodine, alcoholic chlorhexidine versus aqueous povidone‐iodine and alcoholic chlorhexidine versus alcoholic povidone‐iodine showed clear differences for catheter‐related BSI or mortality (and were generally underpowered). Mortality was only reported in a single study.
There was very low quality evidence that skin antisepsis with chlorhexidine may also reduce catheter colonisation relative to povidone‐iodine (RR of 0.68, 95% CI 0.56 to 0.84; ARR 8%, 95% CI 3% to 12%; ; five studies, 1533 catheters, downgraded for risk of bias, indirectness and inconsistency).
Evaluations of other skin antiseptic agents were generally in single, small studies, many of which did not report the primary outcome of catheter‐related BSI. Trials also poorly reported other outcomes, such as skin infections and adverse events.
Authors' conclusions
It is not clear whether cleaning the skin around CVC insertion sites with antiseptic reduces catheter related blood stream infection compared with no skin cleansing. Skin cleansing with chlorhexidine solution may reduce rates of CRBSI and catheter colonisation compared with cleaning with povidone iodine. These results are based on very low quality evidence, which means the true effects may be very different. Moreover these results may be influenced by the nature of the antiseptic solution (i.e. aqueous or alcohol‐based). Further RCTs are needed to assess the effectiveness and safety of different skin antisepsis regimens in CVC care; these should measure and report critical clinical outcomes such as sepsis, catheter‐related BSI and mortality.
Keywords: Adult; Humans; Anti-Infective Agents, Local; Anti-Infective Agents, Local/therapeutic use; Antisepsis; Antisepsis/methods; Catheter-Related Infections; Catheter-Related Infections/prevention & control; Central Venous Catheters; Central Venous Catheters/adverse effects; Central Venous Catheters/microbiology; Chlorhexidine; Chlorhexidine/therapeutic use; Ethanol; Ethanol/therapeutic use; Povidone-Iodine; Povidone-Iodine/therapeutic use; Randomized Controlled Trials as Topic; Skin; Skin/microbiology
Plain language summary
Skin antisepsis for reducing central venous catheter‐related infections
Review Question
We reviewed the evidence about whether using antiseptic treatments on people's skin helps reduce infections related to central venous catheters (CVCs).
Background
Central venous catheters (CVCs) are thin, flexible tubes that are inserted through the skin into a large vein, often in the arm or chest. The tube can then be used to give fluids, medicine and nutrition to chronically and critically ill patients. However, CVCs pose a significant risk of infection by providing a way for micro‐organisms (germs) to spread into the body at the point where the catheter is inserted. In order to try to reduce catheter‐related infections, healthcare staff frequently use antiseptic solutions to clean the skin around the catheter insertion site, both prior to insertion and whilst the catheter is in place. In this review, we summarise the evidence of the benefits and harms of using antiseptics on the skin, and the effects of different antiseptic solutions.
Search date
We searched multiple medical databases in May 2016.
Study characteristics
In May 2016 we searched medical databases to find randomised controlled trials looking at the use of skin antiseptics in people with CVCs. We included 13 studies in this review, although only 12 studies contributed data for a total of 3446 CVCs. The study participants were mainly adults in intensive care units or other specialist hospital units. We reported our findings in terms of the number of catheters, as some studies did not provide the number of patients assessed, and some patients had more than one CVC.One study was funded by a national research body, five studies were funded in whole or in part by at least a pharmaceutical company, and in the remaining seven studies funding sources were not stated.
Key results
Three studies examined the effect of cleansing versus no cleansing, and found no clear evidence of differences in blood infections, infections in the catheter and need for antibiotics between patients who received cleansing compared to those who did not. Chlorhexidine solution may reduce blood infections associated with the catheter compared with povidone‐iodine solution (reducing the infection rate from 64 cases per 1000 patients with a CVC with povidone iodine to 41 cases of infection per 1000 with chlorhexidine). This translates into the need to treat 44 people to avoid one additional bloodstream infection. Chlorhexidine solution may (compared with povidone iodine solution) also reduce the presence of infectious organisms within the catheter (reduced from 240 infected catheters per 1000 people to 189 infected catheters per 1000 people). It is unclear whether antiseptic skin cleansing influences mortality rates as only one study reported this and although similar death rates were observed with povidone iodine and chlorhexidine, small numbers mean a difference cannot be ruled out.
Quality of evidence
The overall quality of evidence was poor due to flaws in the way the studies were designed, small study sizes, inconsistency of the results between the included studies and the nature of the outcomes reported. These flaws have reduced our confidence in the results of the studies. This means we cannot be certain whether cleaning the skin around CVC insertion sites with antiseptic reduces catheter‐related blood stream infection and other harmful effects, such as overall blood infections and mortality compared with no skin cleansing. Cleansing with chlorhexidine solution may be more effective than povidone iodine but the quality of the evidence was very low.
Summary of findings
Summary of findings for the main comparison. Chlorhexidine compared to povidone‐iodine in reducing catheter related infections.
| Chlorhexidine compared to povidone‐iodine for patients with a central venous catheter | |||||
| Patient or population: patients with a central venous catheter Settings: hospital inpatients Intervention: chlorhexidine Comparison: povidone‐iodine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Povidone‐iodine | Chlorhexidine | ||||
| Catheter‐related BSI ‐ overall comparison between chlorhexidine and povidone‐iodine (during in‐patient stay) |
Study population | RR 0.64 (0.41 to 0.99) | 1436 (4 RCTs) | ⊕⊝⊝⊝ Very lowb,c | |
| 64 per 1000 | 41 per 1000 (26 to 63) | ||||
| Moderatea | |||||
| 46 per 1000 | 29 per 1000 (19 to 45) | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | Study population | RR 0.64 (0.32 to 1.28) | 452 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | |
| 86 per 1000 | 55 per 1000 (28 to 110) | ||||
| Moderate | |||||
| 84 per 1000 | 54 per 1000 (27 to 108) | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | Study population | RR 0.77 (0.39 to 1.53) | 503 (2 RCTs) | ⊕⊝⊝⊝ Very lowc,d | |
| 70 per 1000 | 54 per 1000 (27 to 108) | ||||
| Moderate | |||||
| 69 per 1000 | 53 per 1000 (27 to 106) | ||||
| Catheter‐related BSI ‐ subgroup: chlorhexidine in alcohol versus povidone‐iodine in alcohol | Study population | RR 0.4 (0.13 to 1.24) | 481 (1 RCT) | ⊕⊕⊕⊝ Moderatec | |
| 42 per 1000 | 17 per 1000 (5 to 52) | ||||
| Moderate | |||||
| 42 per 1000 | 17 per 1000 (5 to 52) | ||||
| Primary BSI or clinical sepsis | No studies under this comparison assessed this outcome. | ||||
| All‐cause mortality ‐ Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution Clinical assessment | Study population | RR 1.15 (0.72 to 1.83) | 213 (1 RCT) | ⊕⊕⊝⊝ lowc,e | |
| 236 per 1000 | 271 per 1000 (170 to 432) | ||||
| Moderate | |||||
| 236 per 1000 | 271 per 1000 (170 to 432) | ||||
| All‐cause mortality ‐ Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution Clinical assessment | Study population | RR 0.8 (0.48 to 1.34) | 222 (1 RCT) | ⊕⊕⊝⊝ lowc,e | |
| 236 per 1000 | 189 per 1000 (113 to 316) | ||||
| Moderate | |||||
| 236 per 1000 | 189 per 1000 (113 to 316) | ||||
| Mortality attributable the CVC‐related infections. | No studies under this comparison assessed this outcome. | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BSI: bloodstream infection; CI: Confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
a'Moderate risk' was calculated from the median control event rate for each outcome. bThree of the four included studies had unclear risks of bias in allocation concealment, and all had high risks of bias in blinding of participants and personnel. cThe 95% CI was wide. dThere was an overall very serious concern on risk of bias that resulted in downgrading of two levels: both studies had unclear risk of bias under allocation concealment and high risk of bias under blinding of participants and personnel, and one study had serious unit of analysis issue as the outcome was reported using catheters as the unit, and the number of catheters analysed exceeded the number of participants by over 50%, reflecting that fact that some patients received multiple catheters during the study, which could have seriously affected the effect estimate. eThe single study had unclear risk in allocation concealment, high risk in blinding of patients and personnel which might give rise to performance bias, which in turn might affect the risk of mortality, as well as high risk of attrition bias.
Background
Please refer to Appendix 1 for a glossary of terms (lay definitions in the context of this review only).
Description of the condition
The concept of central venous catheterisation was first introduced in the early part of the last century by Bleichroder, Forssmann, Duffy and Authaniac, after Bleichroder reportedly inserted the first central venous catheter (CVC) in a human in 1905 (Puri 2009). In the past four decades, the use of the CVC has become important in the management of many critically and chronically ill patients. Insertion of a CVC provides secure vascular access for the administration of intravenous medications, fluids, blood products and parenteral nutrition. It also serves as an essential conduit for blood sampling, haemodynamic monitoring, renal replacement therapy and plasmapheresis.
It is estimated that 5 million CVCs are inserted every year in the United States and 200,000 each year in the UK (Worthington 2005). One of the major problems associated with the use of CVCs is colonisation by micro‐organisms that could result in local or systemic infection. Research has shown that infectious complications associated with CVCs cause significant morbidity and mortality, with considerable costs to the healthcare system (CDC 2011; Cicalini 2004). In the USA, approximately 80,000 reported cases of CVC‐associated blood stream infections (BSIs) occur in intensive care units (ICUs) every year; this number more than triples when considering the entire hospital system (CDC 2011). Although the exact mortality attributable to these BSIs remains unclear, reports have cited figures up to 35% (CDC 2011). The associated cost incurred due to BSIs is considerable, including costs of additional medication, nursing time and increased length of hospital stay. The total annual cost of caring for patients with CVC‐associated BSIs in the USA alone is estimated to range anywhere from USD 296 million to USD 2.3 billion (CDC 2011).
Micro‐organisms colonise the CVCs and gain access to the blood stream of the patients via three main routes (CDC 2011; Cicalini 2004; Pagani 2008):
External surface of CVC through contaminated insertion site
Internal surface of CVC through contamination of catheter hubs, injection ports and lines; usually by the hands of healthcare workers or patients
Contaminated intravenous drugs, infusates and nutritional preparations.
For short‐term CVCs, investigators have proposed colonisation from the skin to the external surface of the CVCs as the major route of infection, while for long‐term CVCs, the internal surface route becomes increasingly important, as the micro‐organisms gain access to the internal surface as a result of contamination from repeated handling of the CVCs (Cicalini 2004).
Description of the intervention
A number of evidence‐based guidelines have been developed in recent years aimed at reducing CVC‐associated BSIs. Important measures recommended by two of the major guidelines include the following (CDC 2011; Pratt 2007):
Staff education
Quality assurance: systematically monitoring compliance to the established guidelines and evaluating issues relating to compliance
Hand hygiene
The use of aseptic technique during insertion and use of CVCs
Effective skin antisepsis at the insertion site
Maximum sterile barrier precautions (i.e. wearing sterile gloves, sterile gown, a cap and a mask and using a large sterile drape)
Use of subclavian vein as the preferred site of insertion rather than the internal jugular or femoral veins, as this has been shown to reduce infectious, mechanical and thrombotic complications (Hamilton 2007)
The use of antimicrobial or antiseptic impregnated CVCs.
Effective skin antisepsis throughout the in‐dwelling period of the catheter may prevent microbial contamination of the insertion site, thus delaying or reducing the risk of catheter colonisation and the subsequent development of infective complications. Given that insertion site contamination leads to colonisation on the external catheter surface and infection, one would expect skin antisepsis to have some impact on reducing BSIs, especially with short‐term CVCs.
Pioneering work by Pasteur, Semmelweis and Lister laid the foundation for the practice of antisepsis in medicine (Bankston 2005; Bynum 2008; Nuland 2003). Antisepsis is defined as the prevention of infection by inhibiting the growth of causative micro‐organisms, while antiseptics are antimicrobial substances capable of producing antisepsis (Taber 2016). An ideal antiseptic agent would need to be immediately and persistently effective when applied to living tissues, including when a small amount of blood is present, and to be effective against all pathogenic bacteria, viruses, fungi, protozoa, tubercle bacilli and bacterial spores (Taber 2016). At the same time it should be non‐toxic to living tissue, hypoallergenic and safe to use repetitively on all parts of the body (Edwards 2008; Hardin 1997). Human skin naturally has abundant microbiological flora which include resident (i.e. colonising) flora and transient (i.e. contaminating or non‐colonising) flora. Resident flora tend to inhabit deeper layers of the skin and therefore are not readily removed by the mechanical action of washing with soap and water. In contrast, transient flora are not consistently present in most people and can usually be removed by mechanical action (Larson 1995; Ryan 2004). Both resident and transient flora are implicated in the pathogenesis of CVC‐associated infections, thus effective skin antisepsis may require not only mechanical removal but also the chemical killing and inhibition of both the resident and transient flora of the human skin (Edwards 2008).
How the intervention might work
There is a large number of antiseptic agents available and three are considered particularly important in skin antisepsis: chlorhexidine, iodine and alcohol. All three agents have a broad spectrum of activity against gram positive, gram negative, aerobic and anaerobic bacteria, enveloped viruses such as human immunodeficiency virus (HIV), herpes simplex virus (HSV) and cytomegalovirus (CMV), as well as fungi, although they differ in their effects against tubercle bacilli and bacterial spores. We summarise their characteristics here:
Chlorhexidine, which is available mostly as chlorhexidine gluconate and less commonly as chlorhexidine acetate or hydrochloride (Martindale 2016), exercises its antimicrobial action chiefly by causing a disruption of microbial cell membranes. Its activity against tubercle bacilli and bacterial spores is limited (Larson 1995; Russell 1986). Chlorhexidine gluconate has an intermediate onset of effect, which is reported to be minimally affected by organic materials such as blood, pus or sputum. It also appears to cause relatively low level of skin irritation and has little allergenic potential. However, its activity is pH dependent, and its effect is known to be compromised by many substances, including those used in natural soaps (Larson 1995; Martindale 2016).
Iodine and iodophors exert their antimicrobial effects through chemical destruction of the microbial cell wall and cellular contents. They are effective against tubercle bacilli and bacterial spores. They kill bacteria within seconds to minutes but are rapidly inactivated in the presence of organic materials such as blood, pus or sputum. There have been reports of frequent skin irritation, allergic reactions and systemic toxicity in susceptible individuals (Edwards 2008; Hardin 1997; Larson 1995).
Alcohols are available as either ethyl (ethanol), normal‐propyl (n‐propyl) or isopropyl alcohol for use as antiseptic agents. Alcohols derive their antimicrobial activity from denaturation of cellular proteins. They are effective against tubercle bacilli but less so against bacterial spores. Alcohols have a rapid onset of action, but they lose their antimicrobial effects very quickly. Importantly for this review, they are often combined with other agents such as chlorhexidine gluconate or iodine to achieve optimal antisepsis. Alcohols are also poor cleaning agents, and their use is usually not recommended when significant amounts of blood or dirt are present. There have been reports of excessive skin drying and discomfort following application (Larson 1995; Martindale 2016).
Other antiseptic agents include the following (Larson 1995; Martindale 2016):
Triclosan
Hexachlorophene
Chloroxylenol
Quarternary ammonium compounds such as cetrimide and benzalkonium chloride
Octenidine dihydrochloride
Phenolic or carbolic acid compounds
Hydrogen peroxide.
Why it is important to do this review
A meta‐analysis showed that using chlorhexidine gluconate for catheter site care reduced the risk of catheter‐related BSIs by 49% when compared with povidone iodine (Chaiyakunapruk 2002). However, the meta‐analysis only evaluated chlorhexidine gluconate and povidone‐iodine as skin antiseptics, and some studies within it assessed a combination of arterial catheters as well as central and peripheral venous catheters. Some uncertainties remain regarding the best agent, or combination of agents, for use as skin antisepsis for CVCs alone; the optimal interval between application of antiseptics as well as the best method for applying these agents. Examination of the latest National Healthcare Safety Network report, which superseded the National Nosocomial Infections Surveillance (NNIS 2004), revealed that the CVC‐associated BSI rate in different ICUs in the USA ranges from 1.0 to 5.6 BSI per 1000 CVC‐days (Edwards 2008). These figures compare favourably with the previous NNIS figures of 2.7 to 7.4 BSI per 1000 CVC‐days (NNIS 2004). The observed improvement in CVC‐associated BSI rate is probably multifactorial in nature, but the recent educational and awareness campaigns about nosocomial infections and the implementation of infection control measures in many hospitals in the USA may have played a role. The impact of different skin antisepsis regimens in the presence of comprehensive infection control measures and lower baseline BSI rates remains unclear. Furthermore, the availability of new studies using different skin antiseptic preparations and the continuing emergence of drug resistant micro‐organisms necessitates a systematic review to aid clinical decision‐making and to highlight future research needs (O'Grady 2002; Parienti 2004; Pratt 2007).
Objectives
To assess the effects of skin antisepsis around central venous catheter sites, on rates of catheter‐related BSIs, catheter colonisation, and patient mortality and morbidities.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and cluster RCTs comparing one skin antiseptic regimen (a single agent or a combination of agents) with another regimen (a single agent or a combination of agents, placebo or no antisepsis). We excluded cross‐over studies due to the possible contaminating effect of one intervention over another. We also excluded studies assessing CVCs for haemodialysis, as this is covered by another Cochrane review (McCann 2010).
Types of participants
We included studies involving adults and children cared for in a hospital setting (in adult or paediatric wards or ICUs) with any underlying illness and a CVC inserted for any reason during the study period. Studies that enrolled a patient more than once were acceptable provided that the enrolment took place in separate hospital admissions. We excluded studies conducted in neonatal settings, for example in a neonatal intensive care unit (NICU), as the types of catheters used, the insertion site and techniques, the possible complications as well as the risk factors for sepsis are different compared with those in older children and adults (Trieschmann 2007).
Types of interventions
Intervention
The use of any skin antiseptic regimen (a single agent or a combination of agents) used for cleansing the skin around CVC insertion sites.
Comparisons
A different skin antisepsis regimen (a single agent or a combination of agents), placebo or no skin antisepsis for CVC insertion sites.
We required that the selection, insertion, use, maintenance and removal of CVCs in the intervention and comparison groups followed the standard protocol of the hospital setting in the study. The skin antisepsis regimen had to be the only systematic difference between comparison groups (i.e., not catheter material or concurrent CVC‐related antiseptic measures).
We accepted the duration of the studies as variously specified by the authors. We did not place any limit on the minimum and maximum duration of the follow‐up period for each study.
Types of outcome measures
Primary outcomes
Number of patients with CVC‐related blood stream infection (BSI)
Catheter‐related BSI confirmed by laboratory
Primary BSI or clinical sepsis.
We present the criteria for the diagnosis of CVC‐related BSI in Appendix 2 (Pagani 2008).
Mortality
All‐cause mortality
Mortality attributable to CVC‐related infections.
We included suitable studies using other definitions of CVC‐related and associated infections, provided the authors justified their definitions with valid sources.
Secondary outcomes
Number of patients with insertion site infection, either microbiologically documented (i.e. exudates at catheter insertion site yield a micro‐organism with or without concomitant BSI) or clinically documented (i.e. erythema or induration within 2 cm of the catheter insertion site in the absence of associated BSI and without accompanying purulence) (Pagani 2008)
Number of patients with catheter colonisation, as defined by the study authors using well‐accepted definitions such as a significant growth of micro‐organism (more than 15 colony‐forming units (CFU)) from the catheter tip, subcutaneous segment or catheter hub in the absence of clinical signs of infection (Pagani 2008)
Number of drug‐resistant organisms from cultures, including insertion site cultures, catheter cultures and blood cultures
Number of adverse events associated with the use of antiseptic agents, including skin irritation, contact dermatitis, systemic allergic reaction and anaphylaxis
Antibiotic usage during hospitalisation
Length of hospitalisation, either ICU stay or overall hospital stay
Cost of care, including cost of the antiseptic agent and the cost of treating any adverse effects
Quality of life, measured using validated tools.
Search methods for identification of studies
Electronic searches
We searched the following databases for relevant RCTs:
The Cochrane Wounds Specialised Register (searched 23 May 2016);
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library) (2016, Issue 4);
Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations and Epub Ahead of Print) (1946 to 23 May 2016);
Ovid EMBASE (1974 to 23 May 2016);
EBSCO CINAHL Plus (1937 to 23 May 2016).
We used the search strategy in Appendix 3 to search the Cochrane Central Register of Controlled Trials (CENTRAL). We adapted this strategy for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL Plus which can be found in Appendix 4, Appendix 5 and Appendix 6, respectively. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2011 revision) (Lefebvre 2011). We combined the EMBASE search with the Ovid EMBASE filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015).
We searched the following trial registries for details of ongoing clinical trials and unpublished studies.
ClinicalTrials.gov (http://www.clinicaltrials.gov/).
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/Default.aspx).
EU Clinical Trials Register (https://www.clinicaltrialsregister.eu/).
Searching other resources
We checked for further reports of eligible studies using the citation lists of papers identified by the above strategies. We also scanned references lists of relevant Cochrane reviews and guidelines and contacted experts in the field.
Data collection and analysis
Selection of studies
Two review authors (NML, EOR) independently assessed the first round of search results for potentially relevant studies. We retrieved in full those that appeared to meet the inclusion criteria, or where this could not be determined, for further assessment. Two review authors independently assessed the full papers retrieved, resolving any disagreement with input from a third review author (NC). We included the studies if they fulfilled the criteria for inclusion as outlined above and if the amount of information contained in the article enabled the extraction of outcome data for meta‐analysis.
We screened publications for duplicate reports of the same trial and contacted the trial authors for clarification when necessary. If we confirmed a duplicate publication, we identified a primary reference, but extracted unique data from all versions.
Data extraction and management
Two pairs of review authors (NAL and NML, PL and EOR) independently extracted and coded all data for each included study using a pro forma designed specifically for this review. Each pair was responsible for half of the total number of included studies. We extracted the following information on each study: study design, participants, setting, sample size, nature of intervention, comparison, outcomes, methods (unit of allocation and analysis) and results. We screened for duplicate entries of patients, where possible, by matching the initial number of patients recruited against the total number along each step in the conduct of the study.
We found a discrepancy between the number of catheter and the number of patients in most studies. This was due to multiple catheters being inserted in some patients who were enrolled after each insertion. We were unable to limit our analysis to one catheter per participant as none of the studies provided the data in this format.
We resolved any disagreement among the review authors by discussion and formulation of a consensus acceptable to all members of the review team.
Assessment of risk of bias in included studies
Two authors (NAL and NML) independently assessed each included study using the Cochrane tool for 'Risk of bias' assessment (Higgins 2011a). This tool addresses six specific domains.
Sequence generation
Allocation concealment
Blinding
Incomplete outcome data
Selective outcome reporting
Other issues (e.g. extreme baseline imbalance, design‐specific risks of bias such as recruitment in cluster for cluster‐RCT, block randomisation of unblinded trials or fraud).
We present detailed criteria on which we based our judgement in Appendix 7. We assessed blinding and completeness of outcome data for each outcome separately. We completed a 'Risk of bias' table for each eligible study. We resolved any disagreement among the review authors by discussion to achieve a consensus. We presented an overall assessment of the risk of bias using a 'Risk of bias summary figure', which presented all of the judgement in a cross‐tabulation of study by entry. This display of internal validity indicated the weight the reader may give to the results of each study.
In addition, we assessed whether trials followed a standard protocol for all groups under study with regard to the insertion, use, maintenance and removal of CVC, and regarding the concurrent use of other antiseptic measures such as antimicrobial impregnated CVCs, antiseptic‐soaked dressing and prophylactic antibiotics. We referred to the study protocol, where available, for further details if necessary. We made relevant remarks in the corresponding 'Risk of bias' table for each study if there were significant concerns in this aspect.
Measures of treatment effect
For dichotomous data, we used risk ratio (RR) to measure outcome estimates of the same scale. We estimated the number needed to treat for an additional beneficial outcome (NNTB) from the pooled risk difference (RD) using an online NNTB calculator (http://nntonline.net/visualrx/). For continuous data, we pooled measures at a similar time point using the mean difference (MD). Two studies reported the measure of variance as a standard error (SE) or 95% confidence intervals (CI) (Humar 2000; Dettenkofer 2010). We obtained standard deviations (SD) for the above‐mentioned studies from the SE using the formula SD = SE x square root of the number of participants, and from the 95% CI using the formula SD = square root of the number of participants x (upper limit or CI − lower limit of CI)/3.92.
Unit of analysis issues
One potential unit of analysis issue that we had anticipated was the issue that arose as a result of the studies using catheters, rather than patients, as the unit of analysis in catheter‐related outcomes such as catheter‐related BSI and catheter colonisation. Ideally, if the study performed randomisation and analysis based on the participants, and each participant had only one catheter evaluated, adjustment for clustering would not have been necessary. However, if a study included multiple catheters per patient and clearly stated so, we would have assessed whether the authors had undertaken statistical adjustment to account for the effects of clustering by using appropriate analysis models such as the 'generalised estimating equation' (GEE) model (Higgins 2011b). If investigators had made adjustments for clustering, we would have combined the study with other studies in the meta‐analysis. If they had not, or if it was unclear whether there were adjustments made, we would have assessed the number of catheters as well as participants in the study. If the studies had also reported the number of participants with events and the total number analysed, we would have only reported the outcomes using the participants, rather than catheters as the unit of analysis. However, if the study did not provide participant‐level data, we would not have been able to avoid the unit of analysis issues. We would have acknowledged this as a major limitation of the review in our discussion and undertaken sensitivity analysis to assess the pooled results after excluding studies with no adjustments for clustering.
However, in this review, none of the included studies provided participant‐level data for catheter‐specific outcomes. As a result, we could not adjust for the unit of analysis issue, nor could we perform sensitivity analysis to assess the results with and without studies with unadjusted unit of analysis issues. We have acknowledged this in our discussion, as planned.
Another possible unit of analysis issue that could have arisen was the effects of clustering that arose in cluster‐RCTs in which randomisation was performed at the unit, rather than the participant level. However, we did not include any cluster‐RCTs in this review.
Had we identified an eligible cluster‐RCT (e.g. trial in which the assignment to intervention or control group was made at the level of the unit or ward rather than the individual), we would have addressed the possible unit of analysis issues as follows.
First, we would have assessed whether the authors had made adjustments for the effects of clustering to account for non‐independence among the participants by using appropriate analysis models such as the 'generalised estimating equation' (GEE) model (Higgins 2011b).
If investigators did not make adjustments for the effects of clustering, we would have performed adjustment by multiplying the SEs of the final effect estimates by the square root of the 'design effect', represented by the formula '1 + (m − 1) x ICC', where m is the average cluster size (number of participants per cluster) and ICC is the intracluster correlation. We would have determined the average cluster size m by dividing the total number of participants by the total number of clusters. We would have used an assumed ICC of 0.10, which has been proposed to be a realistic general estimate based on previous similar studies (Campbell 2001). We would also have combined the adjusted final effect estimates from each trial with their SEs in our meta‐analysis using the generic inverse‐variance methods, as stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
If it were impossible to find out whether trialists made adjustments on the effect of clustering, we would still have included the studies concerned in our meta‐analysis using the effect estimates reported by the authors, and performed sensitivity analyses to assess how excluding those studies would affect the overall pooled estimates.
Dealing with missing data
We assessed whether there was a high attrition rate and whether an intention‐to‐treat analysis was performed. To assess whether the dropout rate was important, we inspected the absolute attrition rate and the attrition rate in relation to the event rates for the intervention and the comparison groups. If the absolute dropout rate was 20% or more, we judged the study to be at high risk of bias due to incomplete outcome data. If the dropout rate was lower than 20%, we used a 'worst‐case‐scenario' method for the primary outcomes (Guyatt 1993). For instance, for an unfavourable outcome such as catheter‐related BSI or mortality, if the results of a trial favoured the intervention group, we assumed all dropouts from the intervention group to have developed the outcome, and all dropouts from the comparison group to have not developed the outcome. We then analysed to see if such an assumption changed the direction of the results (e.g. from favouring the intervention group to favouring the comparison group). If so, we considered the dropout rate to be significant. We made the reverse assumption when a trial favoured the comparison group, or when the outcomes examined were favourable, such as survival or treatment success.
Assessment of heterogeneity
We assessed all the included studies in terms of their clinical and methodological characteristics.
Baseline characteristics of the participants
Clinical settings of the studies (e.g. intensive care units, oncology wards, renal units)
Co‐interventions
Methodological quality (as detailed in the 'Risk of bias' assessment, for example studies at high risk of bias are defined as studies with unclear or no allocation concealment, and studies where participants, caregivers or investigators are not blinded, or where blinding is unclear)
Nature of intervention (comparison between one skin antiseptic regimen and placebo as opposed to comparison of two active regimens)
Outcome assessment and unit of analysis.
We visually inspected the forest plots for any evidence of heterogeneity of treatment effects. We used the I2 statistic (Higgins 2003) to measure inconsistency in the results, with a value of 50% or greater indicating moderate to substantial statistical heterogeneity.
We found significant statistical heterogeneity in one analysis (Analysis 4.4) and provided a plausible explanation the possible reason for heterogeneity in the form of risk of attrition bias in some included studies. We decided to still provide the pooled estimate for this analysis and separated the studies based on the risk of attrition bias in our pre‐specified sensitivity analysis.
4.4. Analysis.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 4 Catheter colonisation.
Assessment of reporting biases
We planned to screen for publication bias in our review using a funnel plot if there were more than 10 studies included in the analysis. If publication bias was implied by a significant asymmetry of the funnel plot, we would have included a statement in our results with a corresponding note of caution in our discussion. We did not generate any funnel plot in this review as there were fewer than 10 studies included in the analysis across all the comparisons and outcomes.
Data synthesis
We used Review Manager software to perform meta‐analysis of the included studies (RevMan 2014). We used a fixed‐effect model for most of our analyses, as there was no substantial clinical and statistical heterogeneity. For the outcomes with substantial clinical and statistical heterogeneity that was not satisfactorily explained or reduced by subgroup analyses, we used a random‐effects model that took into account between‐study variability within the analysis and lessened the possibility of spurious inferences of significance compared to the fixed‐effect model. We used the Mantel‐Haenszel method to analyse all the dichotomous outcomes, as we anticipated relatively frequent events for most of our outcomes. For continuous outcomes, we employed the inverse variance methods using the effect measure of mean differences. In our assessment of the effects of missing data, we compared our adjusted analysis using the best‐ and worst‐case scenarios to the completer analysis as reported by the study authors.
When there were more than two arms evaluated in a study, for example, aqueous chlorhexidine versus alcoholic chlorhexidine versus aqueous povidone‐iodine, we set up separate pairwise comparisons as subgroups under the major comparison of chlorhexidine versus povidone‐iodine, as follows: aqueous chlorhexidine versus aqueous povidone‐iodine; and alcoholic chlorhexidine versus aqueous povidone‐iodine. In so doing, we halved the total number of participants and events in the povidone‐iodine group to avoid double‐counting.
Had we identified studies that assessed cost‐effectiveness, we planned to provide only a narrative review of their findings and not directly compare costs in studies using different units of measurement, due to the complexity of analysing cost‐effectiveness if different price‐years were used.
Subgroup analysis and investigation of heterogeneity
In this review, we created subgroups of comparisons based on the solution used, for example, a subgroup for chlorhexidine in aqueous solution versus povidone iodine in aqueous solution, and another subgroup for chlorhexidine in alcohol versus povidone‐iodine in aqueous solution.
Had data been available, we would have carried out the following subgroup analyses:
Short term CVCs (less than 10 days) versus longer term CVCs (10 days or more)
CVCs with antimicrobial modifications (antimicrobial impregnation, cuffs, hubs) versus CVCs with no antimicrobial modifications
Studies undertaken in paediatric patients versus adult patients
Studies undertaken in different patient populations with different levels of care (intensive care patients, oncology patients, renal patients and patients in general medical or surgical wards)
Studies undertaken with co‐interventions (e.g. sepsis prevention bundle) versus studies done without co‐interventions
Studies that used rigorous criteria (e.g. as outlined in Pagani 2008) for determining catheter‐related infections versus studies that used more liberal criteria.
Sensitivity analysis
We performed the following sensitivity analyses.
Best‐ and worst‐case scenarios to assess the impact of missing data, as described in the section 'Dealing with missing data'.
Including and excluding studies with unclear and high risks of selection bias, namely, studies with unclear or high risk for random sequence generation, allocation concealment or both.
Had sufficient data been available, we would have performed additional sensitivity analyses to include and exclude studies with methodological issues other than selection bias, such as a lack of blinding to the participants, caregivers or investigators, or where blinding was unclear.
'Summary of findings' table
We created a 'Summary of findings' table, which displayed seven major outcomes in our review, using the web‐based GRADEpro software (http://gdt.guidelinedevelopment.org) (Schünemann 2011a). We used the eight GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias, large effect, plausible confounding and dose response relationship) to assess the overall quality of the body of evidence (Schünemann 2011b). In generating the 'Summary of findings' table, we interpreted the median control group event rate for the outcome as 'moderate risk'.
Results
Description of studies
Results of the search
We identified 609 records from the initial search of the Cochrane Wounds Group Specialised Register, CENTRAL, MEDLINE, EMBASE and CINAHL. We performed additional searches from relevant published studies and identified two further studies that appeared to be relevant. After removing duplicates, there were 574 records. Of these, 107 articles appeared to be relevant after we inspected the titles. We evaluated the abstracts and if necessary, the full text of the articles, excluding 84 of the 107 records, including one duplicate publication of another excluded study. Of the remaining 23 articles, one was an ongoing study, and we could not fully assess six as we are still awaiting their full texts or further information from the authors. Ultimately, 16 articles describing 13 studies were available and met our inclusion criteria. Among these 16 articles, three were additional publications relating to three included studies. The flow diagram of the studies from the initial search to the meta‐analysis is shown in Figure 1. We describe all the included studies in the Characteristics of included studies table and note the reasons for excluding the others in the Characteristics of excluded studies table.
1.
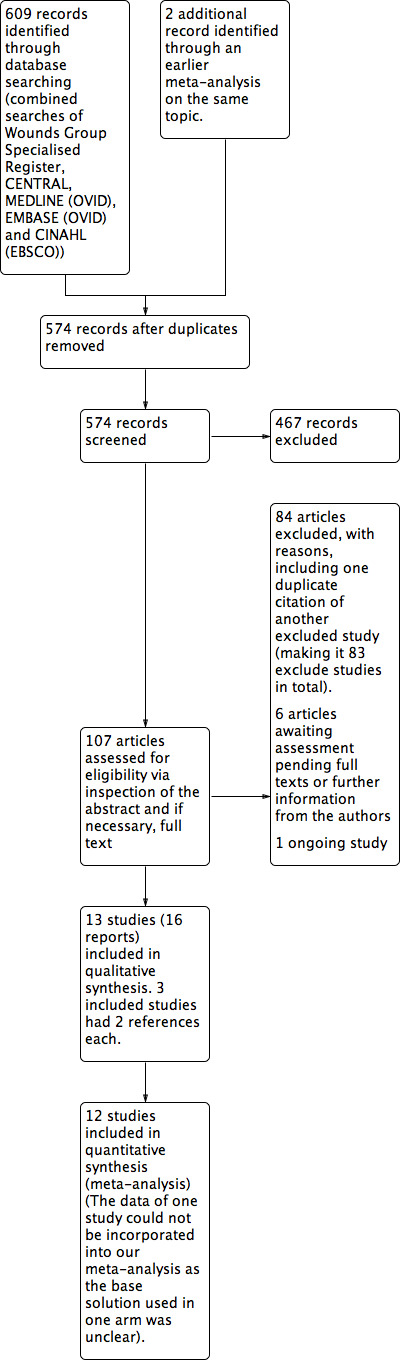
Study flow diagram.
Included studies
We included 13 RCTs, conducted in eight countries, including the USA (four studies), France (two studies), and Canada, Germany, Iran, Japan, Spain, Switzerland and Finland (1 study each). Ten trials were single centre RCTs and three were multicentre RCTs (Dettenkofer 2010; Humar 2000; Yasuda 2013) The number of patients recruited ranged from 50 (with 50 CVCs) in Sadowski 1988 to 420 (with 998 CVCs) in Vallés 2008. Mimoz 1996, Mimoz 2007 and Yasuda 2013 did not report the number of participants. Prager 1984 recruited children (n = 3) in addition to adults (in this case, n = 159), while Sadowski 1988 recruited children and adolescent from 10 weeks to 15 years of age. All studies included participants of both sexes.
Six studies recruited patients from the medical/surgical ICUs (Maki 1991; Mimoz 1996; Mimoz 2007; Vallés 2008; Tuominen 1981; Yasuda 2013), two studies recruited patients who were either pre‐ or post‐cardiac surgery (Levy 1988; Yousefshahi 2013), one study enrolled patients from a burns unit (Sadowski 1988), one from haematology and surgical units (Dettenkofer 2010) and the remaining three studies were conducted hospital‐wide, which included intensive‐care and non intensive‐care patients (Humar 2000; Langgartner 2004; Prager 1984). The average duration of catheterisation, where reported, varied from 2 to 21.1 days (range 1 to > 30 days).
There were ten basic comparisons between two or three arms in the included studies, with subgroups based on type of solution in two comparisons.
Comparison 1: povidone‐iodine (in aqueous solution) versus no skin antisepsis (Prager 1984).
Comparison 2: chlorhexidine (in aqueous solution) versus no skin antisepsis (Tuominen 1981).
Comparison 3: alcohol versus no skin antisepsis (Sadowski 1988).
Comparison 4: chlorhexidine versus povidone‐iodine (Humar 2000; Maki 1991; Mimoz 2007; Vallés 2008; Yasuda 2013). The specific subgroups for this comparison are listed below based on the different preparations of chlorhexidine and/or povidone‐iodine:
Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution (Maki 1991; Vallés 2008).
Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution (Humar 2000; Vallés 2008).
Chlorhexidine in alcohol versus povidone‐iodine in alcohol (Mimoz 2007).
Chlorhexidine in alcohol versus povidone‐iodine (base solution unknown) (Yasuda 2013).
Among the studies included in this comparison, two (Vallés 2008; Yasuda 2013) carried out three‐arm comparison. Vallés 2008 compared 2% chlorhexidine in aqueous solution (group 1), 0.5% chlorhexidine in alcohol (group 2) and 10% povidone‐iodine in aqueous solution (group 3), while Yasuda 2013 compared 1% chlorhexidine in alcohol (group 1), 0.5% chlorhexidine in alcohol (group 2) and 10% povidone‐iodine (base solution unknown). Because the authors of Yasuda 2013 did not specify the base solution for the povidone‐iodine group, we could not include this study in any subgroup in our meta‐analysis.
Comparison 5: chlorhexidine (aqueous) versus alcohol (Maki 1991).
-
Comparison 6: povidone‐iodine versus alcohol.
Comparison 7: alcohol versus octenidine in alcohol (Dettenkofer 2010).
Comparison 8: chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine in alcohol (Langgartner 2004).
Comparison 9: chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution) (Langgartner 2004).
Comparison 10: Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine 10% aqueous scrub (Yousefshahi 2013).
In terms of the timing of intervention, most studies assessed skin antisepsis prior to insertion and regularly thereafter during the in‐dwelling period of the catheters, ranging from every 24 h to every 72 h. Three studies evaluated the skin antisepsis intervention only prior to catheter insertion (Levy 1988; Yasuda 2013; Yousefshahi 2013), and one study examined skin antisepsis prior to removal of the catheters (Sadowski 1988). Maki 1991 and Mimoz 1996 evaluated central venous as well as arterial catheters, although only Maki 1991 provided a separate report of patients receiving CVCs for the outcomes of catheter‐related BSI and catheter colonisation, while only Mimoz 1996 provided CVC‐specific reports for both outcomes per 1000 catheter‐days.
The concentration of chlorhexidine‐based solution used in the studies ranged from 0.05% to 2%, with three studies using a combination of chlorhexidine plus alcohol. The concentration of povidone‐iodine was 10% in all studies except Mimoz 2007, which used 5% povidone‐iodine together with 70% ethanol. All of the studies that evaluated alcohol used 70% isopropyl alcohol except Dettenkofer 2010, which used a combination of 45% 2‐propanol or 74% ethanol with 10% 2‐propanol.
In terms of concomitant CVC‐related infection control measures, six studies clearly described the use of maximal sterile barrier precaution (Dettenkofer 2010; Humar 2000; Langgartner 2004; Mimoz 1996; Mimoz 2007; Vallés 2008), three studies described part of the maximal sterile precaution (such as the use of sterile gloves, gown or dressing) without explicitly mentioning maximal sterile precaution (Levy 1988; Maki 1991; Yousefshahi 2013), and four studies did not provide any clear description (Prager 1984; Sadowski 1988; Tuominen 1981; Yasuda 2013).
The included studies assessed almost exclusively two major outcomes, namely, catheter colonisation or equivalent (all 13 studies) and catheter‐related BSI or equivalent (8 studies). The other outcomes assessed were sepsis, skin colonisation, insertion site infection, number of patients who required antibiotics during the period of catheter use and adverse effects (only evaluated in one study). Only one study reported mortality (Vallés 2008), and no study reported cost of care or quality of life.
Control group risk of infection varied from 6.0% to 32.0% for catheter colonisation, and from 4.1% to 9.8% for catheter‐related BSI.
Of the eight studies that evaluated the primary outcome of catheter‐related BSI, all except Yasuda 2013 clearly defined this outcome in line with our definitions, detailed in Appendix 2. The exact wording varied among the studies, but the definitions involved a positive blood culture in the presence of catheter with clinical evidence of sepsis, improvement of the clinical signs following removal of the catheters or both. One study (Yousefshahi 2013) used the Centers for Disease Control and Prevention (CDC) definitions of catheter‐related BSI (CDC 2011), which were also consistent with the definitions adopted in this review. Most studies used previously validated laboratory methods to perform catheter and blood cultures, adopting microbiological definitions for colonisation and bloodstream infection that were consistent with published literature in the evaluation of catheter‐related infections, including the use of molecular subtyping. In Yasuda 2013, the published abstract did not contain the definition of catheter‐related BSI.
All studies reported catheter‐related outcomes such as catheter‐related BSI and catheter colonisation using the catheter as the unit of analysis. Ten of the 13 included studies provided the number of participants alongside the number of catheters, although none provided separate reports of the catheter‐related outcomes using participants as the unit of analysis. The number of catheters matched the number of participants in six studies (Dettenkofer 2010; Levy 1988;Humar 2000; Maki 1991; Sadowski 1988; Yousefshahi 2013); in three studies, the number of catheters exceeded the number of participants: by 10% in Prager 1984, 18% in Langgartner 2004 and 50% in Vallés 2008. In Tuominen 1981, there were fewer catheters analysed than participants enrolled, with no reason provided.
We did not incorporate the outcome data of Yasuda 2013 into our meta‐analysis, as it was published only as an abstract and did not state the base solution used (either aqueous or alcohol) for the povidone‐iodine group. We are awaiting further information from the authors.
In terms of funding source, one study (Dettenkofer 2010) received funding from a national research agency, five studies (Humar 2000; Maki 1991; Mimoz 1996; Mimoz 2007; Prager 1984) were funded in whole or in part by a pharmaceutical company, and in the remaining seven studies (Langgartner 2004; Levy 1988; Sadowski 1988; Tuominen 1981; Vallés 2008; Yasuda 2013; Yousefshahi 2013), the sources of funding were not stated.
Excluded studies
We excluded a total of 83 articles based on one or more of the following reasons.
Study design or article type (54 studies): the studies were either retrospective or prospective cohort studies, cross‐over study, before‐and‐after intervention studies, prospective non‐randomised intervention studies, meta‐analyses, economic analyses with no original trial data, in vitro experiments, studies with research questions or outcomes that did not match our review, commentaries or an abstract of an included study, excluded study or a study awaiting classification.
Population (17 studies): the participants in the studies were either neonates, people undergoing haemodialysis or all patients in ICU, not only those with CVCs in place.
Intervention (25 studies): the studies either assessed antimicrobial‐impregnated dressing or cerebral ventricular catheter.
Insufficient information (four studies): the studies either reported combined outcome data for arterial, venous or Swan Gantz catheters (or a combination of these), with no separate reporting for venous catheter and little possibility of contacting the authors for further information, or they reported outcome data that were unsuitable for meta‐analysis.
Among the excluded articles, three articles were merged with other articles as their secondary references on the basis of duplication of information as stated under reason number 1 above, including two included studies (Maki 1991; Mimoz 1996) and one excluded study (Garland 2009b).
A description of each study is available in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
There was a wide variation in the risk of bias of the included studies. Overall, there was approximately a one‐third split in the domains that were judged to be low risk, unclear risk and high risk. There was at least one high‐risk domain in each of the included studies. All studies were judged to be at high risk for blinding of participants, except Dettenkofer 2010 (low risk) and Yousefshahi 2013 (unclear risk). Yasuda 2013 had unclear risks of bias in all domains, as there was insufficient information in the published abstract. The proportions of included studies with low, high and unclear risks of bias in each domain is illustrated in Figure 2, and the risk of bias judgment of each included study in each domain is depicted in Figure 3. Additionally, we have provided a detailed description of the risk of bias of each study in the 'Characteristics of included studies' table. We summarise our risk of bias assessments for each domain below.
2.
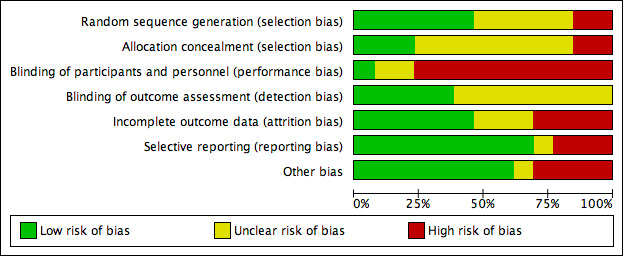
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
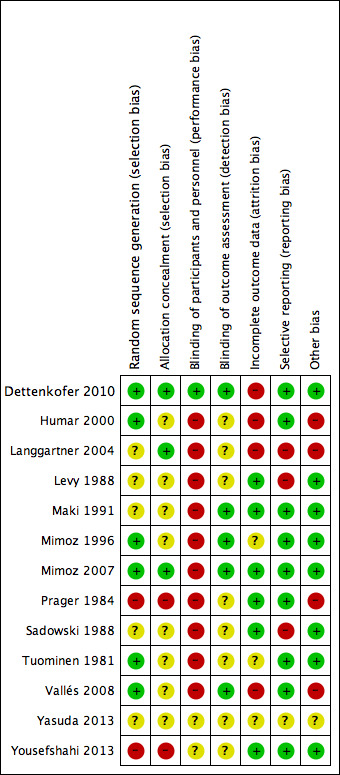
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For random sequence generation, we judged 6 of the 13 included studies to have low risk of bias (Dettenkofer 2010; Humar 2000; Mimoz 1996; Mimoz 2007; Tuominen 1981; Vallés 2008). For allocation concealment, three studies had low risk of bias (Dettenkofer 2010; Langgartner 2004; Mimoz 2007). In these studies, the authors clearly stated the method of sequence generation, which involved some form of random number scheme, mostly by computers. There were also clear statements in the 'Methods' that reassured the readers of the independence between sequence generation and allocation. Two studies were judged to be at high risk in sequence generation as well as allocation concealment, as they allocated participants either using an alternate sequence or based on their hospital registration numbers (Prager 1984; Yousefshahi 2013). There was an unclear risk of bias in one or both domains for 8 of the 13 included studies due to insufficient information provided in the articles.
Blinding
All of the studies except Dettenkofer 2010, Yasuda 2013 and Yousefshahi 2013 had a high risk of bias with regard to blinding of participants. Maki 1991, Mimoz 1996 and Mimoz 2007 clearly stated that they did not blind participants, while other studies did not specify. However, blinding was considered very unlikely in these studies because they compared either a skin antisepsis regimen against no regimen, one skin antisepsis solution against another with a different appearance, or a skin antisepsis regimen against a different and clearly distinguishable infection control measure with no documented attempt to mask the participants.
Eight studies did not report blinding of outcome assessors (Humar 2000; Langgartner 2004; Levy 1988; Prager 1984; Sadowski 1988; Tuominen 1981; Yasuda 2013; Yousefshahi 2013), while the other five did not make any clear statements one way or the other (Dettenkofer 2010; Maki 1991; Mimoz 1996; Mimoz 2007; Vallés 2008). Although investigators objectively measured the outcome of catheter colonisation, catheter‐related BSI required some degree of clinical judgment, which might have been affected by lack of blinding.
Incomplete outcome data
We judged studies to have a high risk of attrition bias for the following three reasons, alone or in combination:
High absolute attrition rates (≥ 20% attrition) or an attrition rate that was higher than the event rates in the control group
Vulnerability of the pooled estimates to best‐ and worst‐case scenarios using the dropouts in the assigned groups
Marked imbalance in the attrition rates between the assigned groups.
Four studies had high risk of bias in this domain either because they had more than 20% withdrawals (Dettenkofer 2010; Humar 2000; Langgartner 2004) or because their results changed significantly with best‐ and worst‐case scenarios (Vallés 2008). Six studies had low risk of bias (Levy 1988; Maki 1991; Mimoz 2007; Prager 1984; Sadowski 1988; Yousefshahi 2013), and the information on withdrawal was not sufficient in the remaining three studies (Mimoz 1996; Tuominen 1981; Yasuda 2013).
Selective reporting
Nine studies had low risk of reporting bias (Dettenkofer 2010; Humar 2000; Maki 1991; Mimoz 1996; Mimoz 2007; Prager 1984; Sadowski 1988; Tuominen 1981; Yousefshahi 2013), and three studies carried a high risk (Langgartner 2004; Levy 1988; Sadowski 1988). The three studies that were judged to have high risk of reporting bias did not report key outcomes that would be expected in such types of studies, such as catheter‐related BSI, clinical sepsis or mortality.
Other potential sources of bias
We screened for other potential sources of bias including extreme baseline imbalance, block randomisation of unblinded trials, unit of analysis issues and any evidence of fraud. As blinding was highly unlikely in most included studies, the use of block randomisation posed an additional risk of bias due to the possibility of disrupting the integrity of the random sequence with educated guess on the likely allocation of the future participants (Higgins 2011a). Two studies (Humar 2000; Vallés 2008) were judged to have high risk under 'other potential sources of bias' as they used block randomisation, and the authors did not state whether they used varying block sizes in either trial.
Unit of analysis issues were a particular concern in three studies (Langgartner 2004; Prager 1984; Vallés 2008), in which the number of catheters analysed exceeded the total number of participants. This meant that some participants had multiple catheters analysed in the study as the authors of the three studies did not limit one catheter per participants in the analyses. The results might have been affected as the outcomes data from multiple catheters from the same participants were most likely not independent from each other. A more detailed description of the risk of bias of the trials is provided in 'Assessment of risk of bias in included studies'.
Effects of interventions
See: Table 1
In this review, we assessed outcomes for a total of 3446 catheters in our meta‐analysis of 12 studies. The total number of participants was unclear as some studies did not report this detail. Overall, we carried out 10 comparisons, with variations related to the base solution in comparisons 4 and 6.
Comparison 1: povidone‐iodine (in aqueous solution) versus no skin antisepsis (Prager 1984).
Comparison 2: chlorhexidine (in aqueous solution) versus no skin antisepsis (Tuominen 1981).
Comparison 3: alcohol versus no skin antisepsis (Sadowski 1988).
-
Comparison 4: chlorhexidine versus povidone‐iodine.
Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution (Maki 1991; Vallés 2008).
Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution (Humar 2000; Vallés 2008).
Chlorhexidine in alcohol versus povidone‐iodine in alcohol (Mimoz 2007).
Comparison 5: chlorhexidine (in aqueous solution) versus alcohol (Maki 1991).
-
Comparison 6: povidone‐iodine versus alcohol.
Comparison 7: alcohol versus octenidine in alcohol (Dettenkofer 2010).
Comparison 8: chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine in alcohol (Langgartner 2004).
Comparison 9: chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution) (Langgartner 2004).
Comparison 10: Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine 10% aqueous scrub (Yousefshahi 2013).
Below, we report on our outcomes of interest in order of the comparisons that examined them.
Primary outcomes
Catheter‐related BSI
Comparison 1: aqueous povidone iodine versus no skin antisepsis (1 RCT, 179 catheters)
Prager 1984 was the only study that compared povidone iodine in aqueous solution versus with no skin antisepsis (dry dressing). There was no clear evidence of a difference in the rate of catheter‐related BSI (RR 0.99, 95% CI 0.37 to 2.61; 179 catheters; Analysis 1.1). The estimate is very uncertain as the comparison was underpowered to detect important differences in the outcome. The quality of evidence for this outcome was rated as very low due to very serious risk of bias issues (random sequence generation, allocation concealment, non‐blinding of participants and unit of analysis issue) as well as imprecision.
1.1. Analysis.
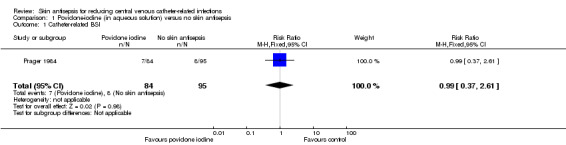
Comparison 1 Povidone‐iodine (in aqueous solution) versus no skin antisepsis, Outcome 1 Catheter‐related BSI.
Comparisons 2: aqueous chlorhexidine versus no skin antisepsis and comparison 3: alcohol versus no skin antisepsis
No study reported this outcome for these comparisons.
Comparison 4: chlorhexidine versus povidone‐iodine (4 RCTs, 1436 catheters)
Overall, chlorhexidine (any solution) was associated with a lower rate of catheter‐related BSI than povidone‐iodine (any solution) (absolute risk reduction (ARR) of 2.30%, 95% confidence interval (CI) 0.06% to 3.70%; risk ratio (RR) 0.64, 95% CI 0.41 to 0.99; NNTB 44, 95% CI 27 to 1563; four studies, 1436 catheters, I2 = 0%; Analysis 4.1; Figure 4). This evidence was very low quality, downgraded for imprecision (one level) and risks of bias (two levels) in allocation concealment, blinding of participants and unit of analysis issues under "other sources of bias". Analyses of subgroups according to the base solution used showed no clear differences between chlorhexidine and povidone‐iodine in the rates of catheter‐related BSI: chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution (RR 0.64, 95% CI 0.32 to 1.28, 2 studies, 452 catheters, I2 = 15%), chlorhexidine in alcohol versus povidone‐iodine in aqueous solution (RR 0.77, 95% CI 0.39 to 1.53; 2 studies, 503 catheters, I2 = 0%), chlorhexidine in alcohol versus povidone‐iodine in alcohol (RR 0.40, 95% CI 0.13 to 1.24; 1 study, 481 catheters). The small number of trials in each subgroup means that the comparisons were underpowered, and the results are uncertain. We considered the evidence from the data to be of very low overall quality (downgraded for imprecision (one level) and risks of bias (two levels) in allocation concealment, blinding of participants and unit of analysis issues. We have highlighted the results for these outcomes from the overall comparison of chlorhexidine versus povidone‐iodine as well as the three subgroup comparisons in our Table 1.
4.1. Analysis.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 1 Catheter‐related BSI.
4.
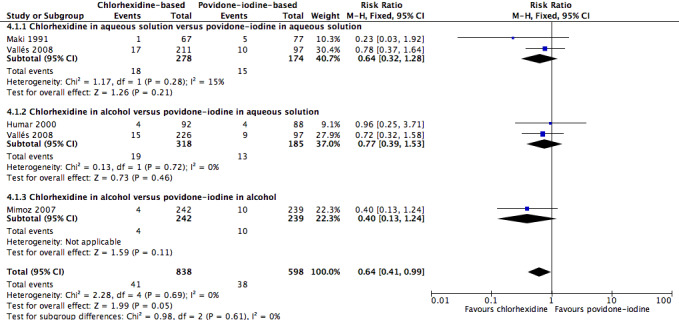
Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.1 Catheter‐related BSI.
For the outcome of catheter‐related BSI per 1000 catheter‐days, chlorhexidine was associated with an apparent lower BSI rate compared with povidone‐iodine (RR 0.53, 95% CI 0.30 to 0.94; 4 studies, 1450 catheters, I2 = 0%; Analysis 4.2). Analyses of subgroups according to the base solution used found evidence of a possible difference between chlorhexidine in alcohol versus povidone‐iodine in aqueous solution (RR 0.49, 95% CI 0.25 to 0.95; 3 studies, 661 catheters, I2 = 31%), but relative effects were unclear for the other base solutions in comparison (chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution (RR 0.82, 95% CI 0.23 to 2.93; 1 study, 308 catheters), and chlorhexidine in alcohol versus povidone‐iodine in alcohol (RR 0.41, 95% CI 0.06 to 2.92; 1 study, 481 catheters). All subgroup comparisons were underpowered and the overall quality of evidence for this outcome was very low due to very serious risk of bias issues (non‐blinding of participants, incomplete outcome data and unit of analysis issues).
4.2. Analysis.

Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 2 Catheter‐related BSI per 1000 catheter‐days.
Comparison 5: aqueous chlorhexidine versus alcohol (1 RCT, 99 catheters)
A single small study compared chlorhexidine in aqueous solution with alcohol (Maki 1991) and found no clear difference in the absolute rate of catheter‐related BSI between the alcohol‐based solution and the chlorhexidine‐based solution (RR 0.24, 95% CI 0.02 to 2.54; 99 catheters; Analysis 5.1). The comparison was underpowered and the quality of evidence for this outcome was low due to risk of bias of the study (non‐blinding) and imprecision.
5.1. Analysis.
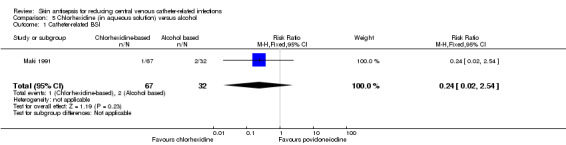
Comparison 5 Chlorhexidine (in aqueous solution) versus alcohol, Outcome 1 Catheter‐related BSI.
Comparison 6: aqueous povidone‐iodine versus alcohol (1 RCT, 109 catheters)
Maki 1991, the only study that compared povidone‐iodine in aqueous solution with alcohol did not find a clear difference in the rate of catheter‐related BSI between the two groups (RR 1.04, 95% CI 0.24 to 5.08; 109 catheters; Analysis 6.1). The comparison was underpowered and the quality of evidence for this outcome was low due to risk of bias issue (non‐blinding of the participants) and imprecision.
6.1. Analysis.
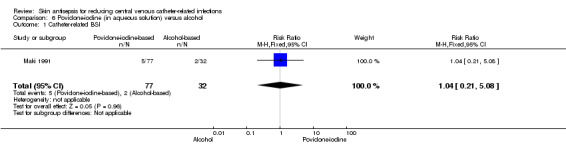
Comparison 6 Povidone‐iodine (in aqueous solution) versus alcohol, Outcome 1 Catheter‐related BSI.
Comparison 7: alcohol versus octenidine in alcohol (1 RCT, 387 catheters)
Dettenkofer 2010 was the only study to compare alcohol versus octenidine in alcohol, and found no clear difference between groups in the absolute rate of catheter‐related BSI (RR 2.01, 95% CI 0.88 to 4.59; 387 catheters; Analysis 7.1) or catheter‐related BSI per 1000 catheter‐days (RR 2.18, 95% CI 0.54 to 8.77; 387 catheters; Analysis 7.2). The comparison was underpowered and the quality of evidence for both outcomes was low due to risk of bias issue (incomplete outcome data) and imprecision.
7.1. Analysis.
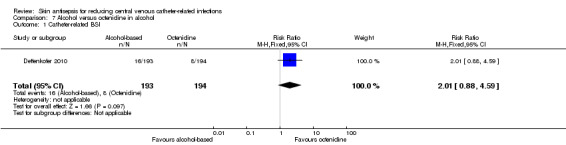
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 1 Catheter‐related BSI.
7.2. Analysis.
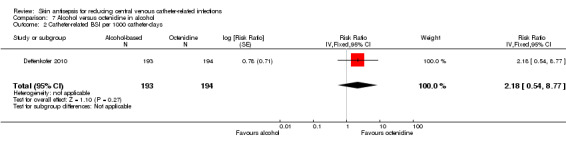
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 2 Catheter‐related BSI per 1000 catheter‐days.
Septicaemia (whether or not CVC‐related)
Comparison 2: chlorhexidine versus no skin antisepsis (1 RCT, 136 participants)
The only study that reported the outcome of septicaemia (irrespective of its relationship with CVC) was Tuominen 1981, which compared chlorhexidine with no skin antisepsis. This study of 136 participants compared the use of 0.05% chlorhexidine in aqueous solution with no skin antisepsis and found no clear difference in the rate of septicaemia between the two groups, but the result was inconclusive due to imprecision (RR 2.91, 95% CI 0.31 to 27.31; Analysis 2.1). The quality of evidence for this outcome was low due to risk of bias issue (non‐blinding of participants) and imprecision, as stated above.
2.1. Analysis.

Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 1 Septicaemia.
Mortality (all‐cause or CVC‐related)
Comparison 4: chlorhexidine versus povidone‐iodine (1 RCT, 329 participants analysed, 106 participants in povidone‐iodine group were included in both subgroup comparisons below)
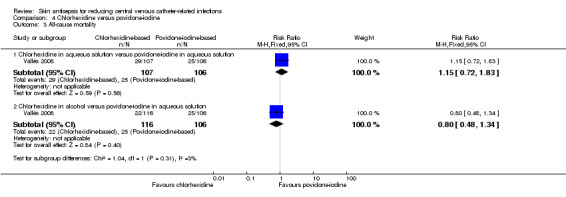
A single study (Vallés 2008) reported mortality. The study divided the participants into three groups: chlorhexidine in aqueous solution, chlorhexidine in alcohol and povidone‐iodine in aqueous solution. Analyses according to subgroups showed no clear differences in the rates of mortality between chlorhexidine in aqueous solution and povidone‐iodine in aqueous solution (RR 1.15, 95% CI 0.72 to 1.83; 213 participants) (Analysis 4.3), or between chlorhexidine in alcohol and povidone‐iodine in aqueous solution (RR 0.80, 95% CI 0.48 to 1.34; 222 participants) (Analysis 4.3)(Figure 5). However, the comparison was underpowered to detect important differences in the outcome, and the quality of evidence for both analyses was low due to a combination of risk of bias issues and imprecision in the outcome estimates (Table 1). Consequently true differences in the mortality associated with use of chlorhexidine or povidone iodine cannot be ruled out.
4.3. Analysis.
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 3 All‐cause mortality.
5.
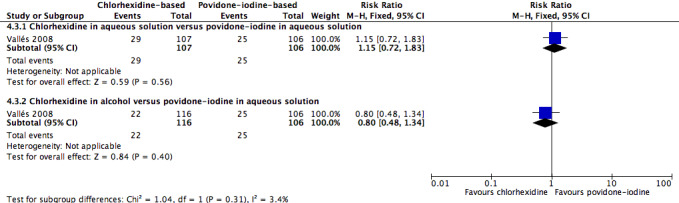
Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.3 All‐cause mortality.
Secondary outcomes
Catheter colonisation
Comparison 1: aqueous povidone‐iodine versus no skin antisepsis (1 RCT, 179 catheters)
Based on Prager 1984, the only study in this underpowered comparison, it is unclear whether there is any difference in the effect on catheter colonisation of aqueous povidone iodine and no skin antisepsis (RR 0.93, 95% CI 0.53 to 1.60; 179 catheters; Analysis 1.2). There was very low quality evidence due to serious risk of bias (random sequence generation, allocation concealment, non‐blinding of participants and unit of analysis issue) and indirectness of the outcome.
1.2. Analysis.

Comparison 1 Povidone‐iodine (in aqueous solution) versus no skin antisepsis, Outcome 2 Catheter colonisation.
Comparison 2: aqueous chlorhexidine versus no skin antisepsis (1 RCT, 124 catheters)
Based on Tuominen 1981, the only study to compare chlorhexidine in aqueous solution with no skin antisepsis, there was no clear difference in the rate of catheter colonisation and therefore uncertainty as to their relative effects remains (RR 1.26, 95% CI 0.61 to 2.59; 124 catheters; Analysis 2.2). The quality of evidence was very low due to risk of bias (non‐blinding of participants), indirectness of the outcome and imprecise estimate from an underpowered analysis.
2.2. Analysis.
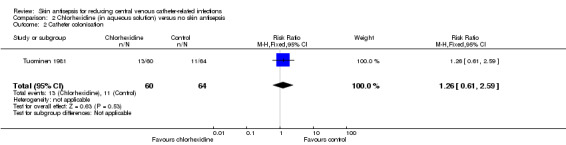
Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 2 Catheter colonisation.
Comparison 3: alcohol versus no skin antisepsis (1 RCT, 50 catheters)
Based on a single study in this underpowered analysis (Sadowski 1988), it remains unclear whether there is a difference between cleansing the skin with alcohol and no skin antisepsis prior to catheter removal (RR 0.75, 95% CI 0.30 to 1.85; 50 catheters; Analysis 3.1). The quality of evidence was very low due to risk of bias (non‐blinding of the participants), indirectness and imprecision.
3.1. Analysis.
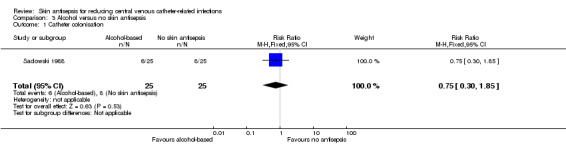
Comparison 3 Alcohol versus no skin antisepsis, Outcome 1 Catheter colonisation.
Comparison 4: chlorhexidine versus povidone‐iodine (5 RCTs, 1533 catheters)
Pooled analysis of five studies that compared chlorhexidine with povidone iodine showed an overall reduction in the risk of catheter colonisation with chlorhexidine (RR 0.68, 95% CI 0.56 to 0.84; ARR 8%, 95% CI 3 to 12%; NNTB 13, 95% CI 9 to 34; 5 studies, 1533 catheters, I2 = 55%; Analysis 4.4; Figure 6). Analysing subgroups according to the solution, there appeared to be reductions in rates of catheter colonisation favouring chlorhexidine in the following comparisons:
6.

Forest plot of comparison: 1 Chlorhexidine versus povidone‐iodine, outcome: 1.4 Catheter colonisation.
Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution (RR 0.60, 95% CI 0.40 to 0.91; 2 studies, 442 catheters, I2 = 56%).
Chlorhexidine in alcohol versus povidone‐iodine in alcohol (RR 0.52, 95% CI 0.34 to 0.80; 1 study, 481 catheters).
However, the rate of catheter colonisation between chlorhexidine in alcohol versus povidone‐iodine in aqueous solution appeared to be similar (RR 0.86, 95% CI 0.64 to 1.14; 3 studies, 600 catheters, I2 = 58%).
There was moderate heterogeneity present for the overall pooled analysis, as indicated by the I2 of 55%. The extent of heterogeneity remained even with the studies separated into subgroups according to the solution used, as shown above. We investigated other possible sources of heterogeneity by exploring factors that were present in the population, intervention, comparison, outcome definitions and risk of bias among the included studies. We noted that although there were some differences in the characteristics of the included studies in terms of population (surgical versus cardiac versus general ICUs) and intervention (different concentrations of chlorhexidine used, duration of catheterisation and the concurrent use of other antiseptic substances alongside chlorhexidine‐based solution), these differences did not plausibly explain the degree of heterogeneity, as separating the studies into subgroups according to these factors did not reduce the degree of heterogeneity.
However, we identified one plausible source of heterogeneity under the risk of bias criterion. We found that only two out of five included studies (Maki 1991; Mimoz 1996) had low risk of attrition bias, while the other three were at high risk of bias in this domain. The two studies with low risk of attrition bias showed significant benefits of chlorhexidine compared with povidone‐iodine, whilst the remaining studies showed no significant difference between the two groups. Grouping studies with low risk and high risk of attrition bias separately reduced the I2 statistic to 0% and 41%, respectively.
We undertook best‐ and worst‐case scenarios to determine the impact of missing data from these three studies and found that the overall pooled analysis was substantially altered, with the best‐case scenario moving the direction of the pooled estimate to significantly and substantially favour the chlorhexidine group, and the worst‐case scenario moving the pooled estimate to significantly favour the povidone‐iodine group (see 'Sensitivity analysis' for details).
Having identified a plausible explanation for the observed heterogeneity, we still decided to combine all five studies under three different subgroups according to the type of solution used (either aqueous or alcohol). Taking all considerations, the overall quality of evidence for this outcome was very low, as there were very serious concerns regarding risk of bias (non‐blinding of participants, incomplete outcome data and unit of analysis issue), indirectness of the outcome and inconsistency among the study results.
Comparison 5: aqueous chlorhexidine versus alcohol (1 RCT, 99 catheters)
According to a single study (Maki 1991), it remains unclear whether there is a difference in the rates of catheter colonisation between chlorhexidine in aqueous solution and alcohol (RR 0.38, 95% CI 0.11 to 1.33; 99 catheters; Analysis 5.2), but the comparison was underpowered. The quality of evidence for this outcome was very low due to risk of bias (non‐blinding of participants), indirectness and imprecision.
5.2. Analysis.
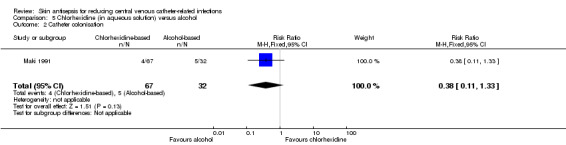
Comparison 5 Chlorhexidine (in aqueous solution) versus alcohol, Outcome 2 Catheter colonisation.
Comparison 6: aqueous povidone‐iodine versus alcohol (3 RCTs, 169 catheters)
It is unclear whether there is a difference in the rates of catheter colonisation between patients who received CVC cleansing with povidone‐iodine and those who receive cleansing with alcohol, either overall (RR 1.76, 95% CI 0.76 to 4.09; 2 studies, 169 catheters, I2 = 43%), or in subgroups comparing povidone‐iodine in aqueous solution versus alcohol (RR 1.25, 95% CI 0.49 to 3.14; 1 study, 109 catheters) or povidone‐iodine‐impregnated adherent film versus alcohol (RR 9.00, 95% CI 0.51 to 160.17; 1 study, 60 catheters; Analysis 6.2). The comparisons were underpowered, and the overall quality of evidence for this outcome was very low due to risk of bias (non‐blinding of participants), indirectness of the outcome and imprecision.
6.2. Analysis.
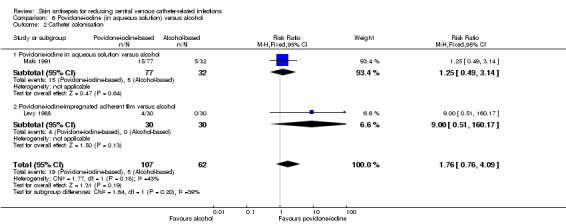
Comparison 6 Povidone‐iodine (in aqueous solution) versus alcohol, Outcome 2 Catheter colonisation.
Comparison 7: alcohol versus octenidine in alcohol (1 RCT, 322 catheters)
Dettenkofer 2010, the only study to compare alcohol versus octenidine in alcohol, showed that alcohol alone is probably associated with a higher rate of catheter colonisation compared to octenidine (RR 2.26, 95% CI 1.22 to 4.21; 322 catheters; Analysis 7.3). However, there appeared to be no clear difference between the two groups in terms of catheter colonisation per 1000 catheter‐days (RR 2.23, 95% CI 0.79 to 6.29; 322 catheters; Analysis 7.4). The quality of evidence for both outcomes was low, due to concerns in risk of bias (non‐blinding of participants) and indirectness of the outcomes.
7.3. Analysis.
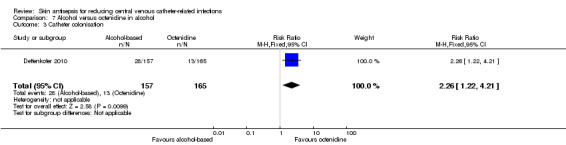
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 3 Catheter colonisation.
7.4. Analysis.
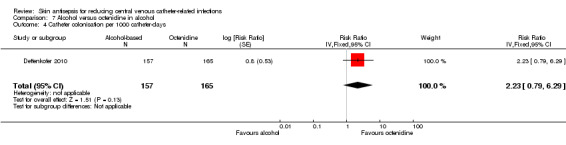
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 4 Catheter colonisation per 1000 catheter‐days.
Comparison 8: chlorhexidine in alcohol plus povidone‐iodine in aqueous solution versus chlorhexidine in alcohol (1 RCT, 88 catheters)
In an underpowered analysis from a single study (Langgartner 2004), a combination of chlorhexidine plus povidone‐iodine appeared to be associated with lower rate of catheter colonisation (RR 0.19, 95% CI 0.04 to 0.81; 88 catheters; Analysis 8.1) as well as catheter colonisation per 1000 catheter‐days (RR 0.19, 95% CI 0.06 to 0.59; 88 catheters; Analysis 8.2) compared with chlorhexidine alone, although the effects were uncertain due to the very low quality of evidence, which was reduced by risk of bias (non‐blinding of participants, incomplete outcome data, unit of analysis issue), indirectness and imprecision.
8.1. Analysis.
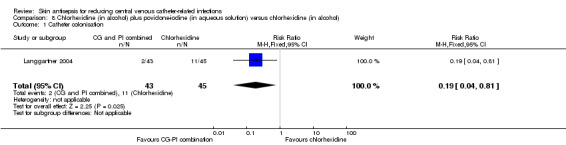
Comparison 8 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol), Outcome 1 Catheter colonisation.
8.2. Analysis.

Comparison 8 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol), Outcome 2 Catheter colonisation per 1000 catheter‐days.
Comparison 9: chlorhexidine in alcohol plus povidone‐iodine in aqueous solution versus povidone‐iodine in aqueous solution (1 RCT, 95 catheters)
In another single‐study, underpowered analysis based on Langgartner 2004, there appeared to be lower rate of catheter colonisation (RR 0.15, 95% CI 0.04 to 0.62; 95 catheters; Analysis 9.1) as well as catheter colonisation per 1000 catheter‐days (RR 0.17, 95% CI 0.05 to 0.52; 95 catheters; Analysis 9.2) using a combination of chlorhexidine and povidone‐iodine compared with using povidone‐iodine alone, but the effects were very uncertain due to the very low quality of evidence, which was reduced by risk of bias (non‐blinding of participants, incomplete outcome data, unit of analysis issue), indirectness and imprecision.
9.1. Analysis.
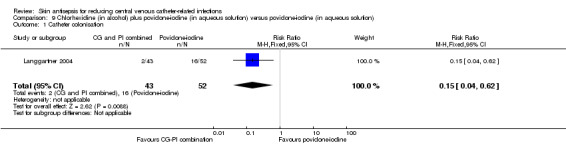
Comparison 9 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution), Outcome 1 Catheter colonisation.
9.2. Analysis.

Comparison 9 Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution), Outcome 2 Catheter colonisation per 1000 catheter‐days.
Comparison 10: Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine 10% aqueous scrub (1 RCT, 249 catheters)
From the single study in this underpowered comparison (Yousefshahi 2013), it is uncertain whether there is any clear difference between the two groups in the rate of catheter colonisation (RR 1.08, 95% CI 0.68 to 1.72; 249 catheters; Analysis 10.1) due to the very low quality of evidence, which was reduced by risk of bias (random sequence generation, allocation concealment), indirectness and imprecision.
10.1. Analysis.

Comparison 10 Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine aqueous 10% scrub, Outcome 1 Catheter colonisation.
Insertion site infection
Comparison 4: Chlorhexidine versus povidone‐iodine (1 RCT, 242 catheters)
Based on the result of a single study (Humar 2000) in an underpowered analysis, it is uncertain whether there is any clear difference between chlorhexidine (in alcohol) and povidone‐iodine (in aqueous solution) with regard to insertion site infection, as the quality of evidence was very low due to risk of bias (non‐blinding of the participants, incomplete outcome data), indirectness and imprecision. The authors reported this outcome as the mean CFU count (MD − 2.80, 95% CI − 9.10 to 3.50; 242 catheters; Analysis 4.6).
4.6. Analysis.
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 6 Insertion site infection.
Skin colonisation
Comparison 7: Alcohol versus octenidine in alcohol (1 RCT, 365 catheters)
Based on the results of Dettenkofer 2010, using alcohol alone probably resulted in higher mean CFU compared with octenidine in alcohol (MD 79.00 CFUs, 95% CI 32.76 to 125.24; 365 catheters; Analysis 7.5). The quality of evidence was moderate as it was reduced by imprecision of the effect estimates from an underpowered analysis.
7.5. Analysis.
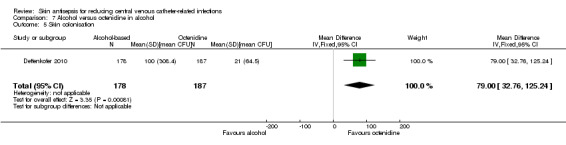
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 5 Skin colonisation.
Adverse effects
Comparison 7: Alcohol versus octenidine in alcohol (1 RCT, 398 participants)
A single study, Dettenkofer 2010, reported the rates of various adverse effects on the skin, the definitions of which appeared to overlap. For example, the authors reported "skin irritation", "burning", "skin irritation and burning", "itching", "skin lesions", "burning and skin lesions", "itching and skin irritation" as the outcomes under adverse effects. To avoid duplication, we included only the most commonly reported adverse effect, namely, skin irritation. For this outcome, there was moderate quality evidence showing no clear difference between in adverse effect rates between patients whose CVC sites were cleansed with alcohol and those who were cleansed with octenidine in alcohol (RR 0.85, 95% CI 0.60 to 1.20; 398 participants; Analysis 7.6). The quality of evidence was reduced by imprecision of the effect estimates from an underpowered analysis.
7.6. Analysis.
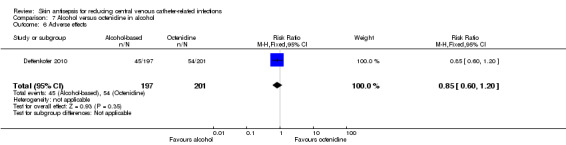
Comparison 7 Alcohol versus octenidine in alcohol, Outcome 6 Adverse effects.
Number of patients who were on antibiotics during the period of catheter use
Comparison 2: Chlorhexidine in aqueous solution versus no skin antisepsis ( 1 RCT, 136 participants)
The only study that evaluated this outcome, Tuominen 1981 found no clear difference between the two groups with regard to the number of patients who required antibiotics during the period of catheter use (RR 0.84, 95% CI 0.55 to 1.27; 136 participants; Analysis 2.3). The quality of evidence was low due to risk of bias (non‐blinding of participants) and imprecision from an underpowered analysis.
2.3. Analysis.
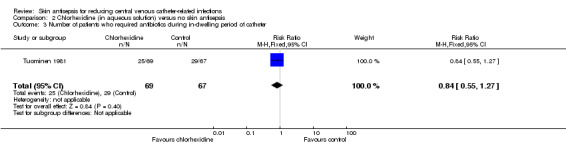
Comparison 2 Chlorhexidine (in aqueous solution) versus no skin antisepsis, Outcome 3 Number of patients who required antibiotics during in‐dwelling period of catheter.
Number of drug‐resistant organisms from culture, length of hospitalisation, cost of care and quality of life
No studies in any comparison assessed these outcomes.
Subgroup analyses
Other than separating the subgroups according to the type of solution used in comparisons 4 and 6, we did not perform any additional subgroup analyses as specified in our 'Methods' because the data in each study were not presented separately for various potential subgroups (for short term versus longer term CVCs, for paediatric versus adult patients and for patients in ICU versus those in other wards). Likewise, there was only a single study included in many comparisons, and all studies in the meta‐analysis used diagnostic criteria for catheter‐related infections that were in line with our pre‐specified criteria (see 'Included studies').
Sensitivity analysis
We performed sensitivity analyses on the most commonly assessed outcomes, namely, catheter‐related BSI (primary outcome) and catheter colonisation (secondary outcome) to evaluate the impact of excluding some studies based our predefined criteria (unclear or no allocation concealment (selection bias) and significant dropout rates (attrition bias)). We assessed the impact of missing data in studies with high dropout rates using the best‐ and worst‐case scenarios. We did not carry out a sensitivity analysis according to the criterion of blinding because we considered that all but one study were at high risk in this domain.
Catheter‐related BSI
Comparison 4: Chlorhexidine versus povidone‐iodine
Selection bias: None of the four studies included were at low risk of bias for both random sequence generation and allocation concealment. Maki 1991 was at unclear risk for both items while the other three studies were at low risk for at least one of the items. We decided to perform the sensitivity analysis by excluding Maki 1991. While the point estimate changed only slightly, the confidence interval expanded to cross the line of no effect, shifting the result to become non‐significant (before exclusion: RR 0.64, 95% CI 0.41 to 0.99; after exclusion: RR 0.68, 95% CI 0.43 to 1.08).
Attrition bias: Two studies had a high risk of attrition bias (Humar 2000, Vallés 2008). We conducted best‐ and worst‐case scenarios by assuming the outcome for the patients with missing data as described in the Dealing with missing data section. The direction of the pooled estimate differed markedly between the best‐ and worst‐case scenarios as well as from the actual results reported, namely the 'completer analysis' (best‐case scenario: RR 0.35, 95% CI 0.24 to 0.50, I2: 0%; worst‐case scenario: RR 1.47, 95% CI 1.01 to 2.14, I2: 64%; actual results reported: RR 0.64, 95% CI 0.41 to 0.99, I2: 0%).
There was only a single study included for all the other comparisons.
Catheter colonisation
Comparison 4: Chlorhexidine versus povidone‐iodine
Selection bias: One study (Maki 1991) had unclear risk of bias in both random sequence generation and allocation concealment, whilst the other studies were at low risk of bias for at least one item. The exclusion of Maki 1991 did not result in a substantial change in the pooled estimates (before exclusion: RR 0.68, 95% CI 0.56 to 0.84; after exclusion: RR 0.72, 95% CI 0.58 to 0.88).
Attrition bias: Three of the five studies (Humar 2000; Langgartner 2004; Vallés 2008) included in this comparison had high or unclear risk of attrition bias. We conducted best‐ and worst‐case scenarios. With the best‐case scenario, the pooled estimate showed substantial reduction in the risk of catheter colonisation favouring the chlorhexidine group (RR 0.56, 95% CI 0.47 to 0.68, I2: 73%), and with the worst‐case scenario, there was no significant difference between the two groups (RR 0.90, 95% CI 0.74 to 1.09, I2: 72%). Results from both the best‐ and worst‐case scenarios differed markedly with the actual results reported, namely the 'completer analysis' (RR 0.68, 95% CI 0.56 to 0.84, I2: 55%).
There were insufficient studies in all the other comparisons to enable a meaningful sensitivity analysis.
Discussion
Summary of main results
This review identified a wide variety of skin antisepsis regimens that comprised different combinations of an active substance (such as chlorhexidine) and base solution (such as aqueous or alcoholic solution). However, a limited number of studies (and sometimes just one) examined each regimen. Based on very limited evidence, there were no clear differences between various skin antisepsis regimens for our primary outcome of catheter‐related BSI, although for the overall comparison between chlorhexidine and povidone‐iodine, there appeared to be a reduction in catheter‐related BSI associated with chlorhexidine. Notably, two studies conducted in the 1980s, one comparing povidone‐iodine in aqueous solution with no skin antisepsis and the other comparing chlorhexidine in aqueous solution with no skin antisepsis, found no difference in the rates of BSI between the intervention group and the control group (Prager 1984; Tuominen 1981). However, these were small studies with some methodological issues, and the evidence they provide is very inconclusive.
Based on a single study (Vallés 2008), there were similar rates of mortality between chlorhexidine‐based solution and povidone‐iodine based solution. However, the analyses were underpowered for any clear conclusion to be drawn with regards to this outcome.
In the outcome of catheter colonisation, some differences existed between different skin antisepsis regimens, with regimens containing chlorhexidine appearing to be more effective than regimens containing povidone‐iodine in reducing risk.
One trial showed that octenidine in alcohol appeared to be more effective than alcohol alone in reducing catheter colonisation. Three separate studies that compared chlorhexidine, povidone‐iodine and alcohol‐based solution, respectively, with no skin antisepsis did not find any clear difference in the rates of catheter colonisation between the intervention group and the control group, although the amount of evidence based on these studies is insufficient to draw any clear conclusion. Analysis based on very small number of studies and catheters suggested that a combination of chlorhexidine and povidone‐iodine appeared to be more effective than either agent alone in reducing catheter colonisation. Single‐study analyses showed that there were no clear differences in the rates of insertion site infection, skin colonisation or adverse events between different skin antisepsis regimens examined. Overall, the results of this meta‐analysis need to be interpreted with caution, as the majority of the included studies were not sufficiently powered to detect a clear difference in the outcomes, and some significant results came from small, methodologically flawed studies, as mentioned above.
Overall completeness and applicability of evidence
We identified 13 studies that matched our selection criteria in terms of population, intervention, comparison and outcomes, and data were unavailable for analysis in 1 out of 13 studies. A total of 3446 catheters were assessed. The studies took place in Europe, the USA and Asia, from 1981 to 2013, in settings where CVCs are commonly used, such as the ICUs and haematology and oncology units. However, there are certain limitations in the completeness of this review. For example, among the participants, children were grossly underrepresented, and most of the included studies did not adequately asses some of the key prespecified outcomes of this review, including primary BSIs, mortality, adverse effects and costs. Furthermore, we were unable to undertake most of the subgroup analyses because there were insufficient data.
Quality of the evidence
Overall, the quality the evidence for the majority of outcomes assessed was very low to moderate due to the small number of studies included in each comparison and variable risk of bias of the included studies. The strongest evidence comes from the overall comparison between antiseptic solutions containing chlorhexidine against antiseptic solutions containing povidone‐iodine, for which there were five studies. However, all comparisons in this review suffered from a lack of power in the analysis, as evidenced by the small number of trials and catheters in each comparison. The lack of power in the analysis has seriously affected our confidence in interpreting the results in general, as we were unable to determine whether non‐statistically significant results were indicative of true (null) effects or of insufficient data for detecting differences. Also, in the case of a statistically significant difference, an analysis with a small number of trials and catheters lessens the reliability of the results due to concerns about the effects of small studies in exacerbating the impact of biases (Sterne 2011).
A second major limitation in the quality of the evidence gathered was the risk of attrition bias, as four studies had high risks and three had unclear risks. In studies with high risk of attrition bias, the pooled results varied substantially between the best‐ and worst‐case scenarios and from the actual results reported, and this precluded us from drawing a firm conclusion on the results of the outcomes concerned. Besides, a lack of blinding of the participants in most studies, as well as the unit of analysis issues in some studies in which multiple catheters in the same participants were analysed as separate units has further affected the overall methodological rigour of the included studies, and in turn the quality of evidence. Overall, the body of evidence gathered in this review did not allow us to reach a robust conclusion regarding the effectiveness and safety of various skin antisepsis regimes in reducing CVC‐related infections (see Table 1 for the outcome data under the major comparisons in this review).
Potential biases in the review process
We performed a comprehensive search of multiple databases with independent screening, selection and assessment of eligible studies. However, we were unable to obtain all relevant data; five studies are awaiting assessment, as there were difficulties obtaining full texts, and another one is an ongoing study. Many of the excluded studies assessed a combination of arterial and venous catheters but did not report outcome data separately for CVC, which prevented us from including a larger body of potentially relevant information. We are currently waiting for authors of the studies concerned to provide us relevant data for our future updates.
There are some unresolved unit of analysis issues in this review: for catheter‐specific outcomes such as catheter‐related BSI and catheter colonisation, we reported the results in the same way as the original studies, using catheters rather than participants as the unit of analysis. As a result, the review included multiple catheters in the same participants. Our failure to adjust for this unit of analysis issue might have affected the results.
Agreements and disagreements with other studies or reviews
The findings of this review are broadly in line with two other reviews on this topic (Adams 2007; Chaiyakunapruk 2002), which concluded that antiseptic solutions containing chlorhexidine are more effective than those containing povidone‐iodine in reducing catheter colonisation and catheter‐related BSI. Of the two reviews, Adams 2007 was a narrative review and Chaiyakunapruk 2002 was a systematic review that evaluated chlorhexidine against povidone‐iodine in all vascular catheters, including arterial and central and peripheral venous catheters. Chaiyakunapruk 2002 showed that antiseptic solutions containing chlorhexidine reduced catheter‐related BSI on average by 49% compared with povidone‐iodine, although there was a great degree of uncertainty on the magnitude of its benefit, as reflected by a wide confidence interval (RR 0.51, CI 0.27 to 0.97; 8 RCTs including three that evaluated only CVCs, 4143 arterial and venous catheters combined including 1493 CVCs). Our review, which is focused only on central venous catheters, included two more trials but a slightly smaller number of CVCs, and we showed that a solution containing chlorhexidine reduced catheter‐related BSI by an average of 36% (relative effect) compared with povidone‐iodine (RR 0.64, CI 0.41 to 0.99; 5 studies, 1436 CVCs).
Authors' conclusions
Implications for practice.
Very low quality evidence suggests that antiseptic solutions containing chlorhexidine may reduce catheter colonisation and catheter‐related BSI compared with antiseptic solutions containing povidone‐iodine. It is unclear whether skin cleansing for CVCs with any solution is beneficial compared with no skin antisepsis. It is still unclear whether skin antisepsis as part of CVC care reduces overall sepsis and mortality. While the evidence gathered in this review does not change the current recommendations that favour the use of chlorhexidine‐containing solution for skin antisepsis in CVC care, uncertainties remain on its value in improving patient mortality and morbidity.
Implications for research.
Further trials in skin antisepsis in patients with a CVC are warranted. This review highlights the paucity of high‐quality research answering questions on whether skin antisepsis in patients with CVC reduces overall rates of sepsis and mortality. Furthermore, the evolving patterns of hospital‐associated infections, accompanying progress in infection control measures and microbiological diagnostic techniques have resulted in changing effectiveness of various interventions employed. Future trials should include the two key outcomes, overall rate of sepsis and mortality, alongside catheter‐specific outcomes such as catheter‐related BSI and catheter colonisation, with a clear description of the settings, participants and concurrent infection control measures to enable an evaluation of the results in relation to these factors. If possible, investigators should blind participants and personnel, or at the very least outcome assessors, with measures in place (such as training of care personnel on handling the catheters for the purpose of research and the implementation of a standard protocol with regards to the handling of study catheters during and after office hours) to reduce loss of data.
Acknowledgements
We are grateful for the contribution of peer reviewers Joan Webster, Susan O’Meara, Judith Tanner, Ankur Barua, Clifford Richardson, Mark Rodgers, Jane Nadel, Marian Brady, Gill Worthy, Victoria Steelman and Dayanithee Chetty for kindly spending time to comment on our draft protocol and review and suggesting improvements. We thank Meggan Harris for copyediting the review.
We also acknowledge the contribution by Dr Rachel Wel Lynn Ooi in assisting Nai Ming Lai in screening through the search results from CENTRAL to identify potentially relevant articles.
Appendices
Appendix 1. Glossary of terms (lay definitions in the context of this review only)
Colonisation: occupation by bacteria or other micro‐organisms in a specific body part or a device in the body without causing infection Erythema: redness Induration: a term usually used to describe the hardening of a small area of the skin Infusates: liquid that is being infused through a device, such as a line, from the source (such as the fluid bag) to the patient Nosocomial infection: also known as a hospital‐acquired infection or HAI, an infection whose development is favoured by a hospital environment, such as one acquired by a patient during a hospital visit or one developed among hospital staff. Such infections include fungal and bacterial infections and are aggravated by the reduced resistance of individual patients. Pathogenesis: the chain of events leading to the appearance of a disease or a medical problem, described scientifically in detail Placebo: a simulated or 'sham' treatment that is designed to be indistinguishable from the actual treatment in all aspects except for the active component tested Plasmapheresis: a medical procedure in which a person's blood is channeled out of his body to a special 'filtering machine' and then returned to the body after the removal of the unwanted substance. It is used to treat a variety of medical problems in which unwanted substances, usually in the form of harmful antibodies, are produced Purulence: the state where pus appears at or around a lesion such as a wound Regimen: a systematic plan of single or multiple measures designed to improve the health of a patient Single agent: the use of only one antiseptic agent A combination of agents: the use of more than one antiseptic agent together Transient flora: bacteria that occupy a specific place in the body or a device for a short‐term period Subclavian vein: large blood vessels on each the side of the neck; commonly used as a site for inserting a central venous catheter.
Appendix 2. Definitions of infections linked to vascular access
Table 1. Definitions of infections linked to vascular access (Pagani 2008)
| Type of infection | Criteria |
| Catheter colonisation | A significant growth of a micro‐organism (> 15 CFU) from the catheter tip, subcutaneous segment or catheter hub in the absence of clinical signs of infection |
| Exit‐site/insertion site infection | Microbiologically documented: exudates at catheter exit site yield a micro‐organism with or without concomitant bloodstream infection. Clinically documented: erythema or induration within 2 cm of the catheter insertion site in the absence of associated bloodstream infection and without concomitant purulence |
| Positive blood culture | Micro‐organism, potentially pathogenic, cultured from one or more blood culture |
| Bloodstream infection | Positive blood culture with a clinical sepsis (see below) |
| Primary bloodstream infection | Laboratory‐confirmed bloodstream infection or clinical sepsis occurring without documented infection |
| Secondary bloodstream infection | Laboratory‐confirmed bloodstream infection secondary to another documented infection |
| Clinical sepsis | Requires one of the following with no other recognised cause: fever (> 38° C), hypotension (SBP < 90 mmHg), oliguria (< 20 ml/h); and all of the following: blood culture not performed or no organism detected in blood, no apparent infection at another body site and clinical response to therapy following catheter removal or change |
| Catheter‐associated bloodstream infection | Primary bloodstream infection or clinical sepsis in the presence of an intravascular device |
| Catheter‐related bloodstream infection | Laboratory‐confirmed bloodstream infection in the presence of an intravascular access: at least 1 positive blood culture obtained from a peripheral vein, clinical manifestation of infection and no apparent source of the bloodstream infection except the vascular access, and with 1 of the microbiological methods: a positive result of semi‐quantitative (> 15 CFUs per catheter segment) or quantitative culture (> 103 CFU/catheter segment) with the same organism, paired quantitative blood cultures with a > 5:1 ratio device versus peripheral, differential time to positivity (blood culture obtained from a CVC is positive at least 2 h earlier than a peripheral blood culture) |
| CFU: colony‐forming units; CVC: central venous catheter; S BP: systolic blood pressure. | |
Appendix 3. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 MeSH descriptor: [Catheterization, Central Venous] explode all trees #2 central next venous next catheter*:ti,ab,kw #3 central next venous next line*:ti,ab,kw #4 {or #1‐#3} #5 MeSH descriptor: [Antisepsis] explode all trees #6 antisepsis:ti,ab,kw #7 MeSH descriptor: [Hand Hygiene] explode all trees #8 (handwash* or hand wash* or "hand hygiene"):ti,ab,kw #9 aseptic next technique*:ti,ab,kw #10 barrier next precaution*:ti,ab,kw #11 MeSH descriptor: [Anti‐Infective Agents, Local] explode all trees #12 MeSH descriptor: [Chlorhexidine] explode all trees #13 MeSH descriptor: [Iodine] explode all trees #14 MeSH descriptor: [Povidone] explode all trees #15 MeSH descriptor: [Triclosan] explode all trees #16 MeSH descriptor: [Hexachlorophene] explode all trees #17 MeSH descriptor: [Cetrimonium Compounds] explode all trees #18 MeSH descriptor: [Phenol] explode all trees #19 MeSH descriptor: [Hydrogen Peroxide] explode all trees #20 MeSH descriptor: [Alcohols] explode all trees #21 MeSH descriptor: [Soaps] explode all trees #22 (iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or "hydrogen peroxide" or alcohol or alcohols or antiseptic* or soap*):ti,ab,kw #23 skin near/3 disinfect*:ti,ab,kw #24 {or #5‐#23} #25 {and #4, #24} in Trials
Appendix 4. Ovid MEDLINE search strategy
1 exp Catheterization, Central Venous/ 2 central venous catheter*.tw. 3 central venous line*.tw. 4 or/1‐3 5 exp Antisepsis/ 6 antisepsis.tw. 7 exp Hand Hygiene/ 8 (handwash* or hand wash* or hand hygiene).tw. 9 aseptic technique*.tw. 10 barrier precaution*.tw. 11 exp Anti‐Infective Agents, Local/ 12 exp Chlorhexidine/ 13 exp Iodine/ 14 exp Povidone/ 15 exp Triclosan/ 16 exp Hexachlorophene/ 17 exp Cetrimonium Compounds/ 18 exp Phenol/ 19 exp Hydrogen Peroxide/ 20 exp Alcohols/ 21 exp Soaps/ 22 (iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap*).tw. 23 (skin adj3 disinfect*).tw. 24 or/5‐23 25 4 and 24 26 randomized controlled trial.pt. 27 controlled clinical trial.pt. 28 randomi?ed.ab. 29 placebo.ab. 30 clinical trials as topic.sh. 31 randomly.ab. 32 trial.ti. 33 or/26‐32 34 exp animals/ not humans.sh. 35 33 not 34 36 and/25,35
Appendix 5. Ovid EMBASE search strategy
1 exp central venous catheter/ 2 central venous catheter*.tw. 3 central venous line*.tw. 4 or/1‐3 5 exp antisepsis/ 6 antisepsis.tw. 7 exp hand washing/ 8 (handwash* or hand wash* or hand hygiene).tw. 9 aseptic technique*.tw. 10 barrier precaution*.tw. 11 exp topical antiinfective agent/ 12 exp chlorhexidine/ 13 exp iodine/ 14 exp povidone/ 15 exp povidone iodine/ 16 exp triclosan/ 17 exp hexachlorophene/ 18 exp cetrimide/ 19 exp benzalkonium/ 20 exp octenidine/ 21 exp phenol/ 22 exp hydrogen peroxide/ 23 exp alcohol/ 24 exp soap/ 25 (iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap*).tw. 26 (skin adj3 disinfect*).tw. 27 or/5‐26 28 4 and 27 29 Randomized controlled trials/ 30 Single‐Blind Method/ 31 Double‐Blind Method/ 32 Crossover Procedure/ 33 (random$ or factorial$ or crossover$ or cross over$ or cross‐over$ or placebo$ or assign$ or allocat$ or volunteer$).ti,ab. 34 (doubl$ adj blind$).ti,ab. 35 (singl$ adj blind$).ti,ab. 36 or/29‐35 37 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 38 human/ or human cell/ 39 and/37‐38 40 37 not 39 41 36 not 40 42 28 and 41
Appendix 6. EBSCO CINAHL Plus search strategy
S1 (MH "Central Venous Catheters+") S2 (MH "Catheterization, Central Venous+") S3 TI central venous catheter* or AB central venous catheter* S4 TI central venous line* or AB central venous line* S5 S1 or S2 or S3 or S4 S6 TI antisepsis or AB antisepsis S7 (MH "Handwashing+") S8 TI ( handwash* or hand wash* or hand hygiene ) or AB ( handwash* or hand wash* or hand hygiene ) S9 TI aseptic technique* or AB aseptic technique* S10 TI barrier precaution* or AB barrier precaution* S11 (MH "Antiinfective Agents, Local+") S12 (MH "Chlorhexidine") S13 (MH "Iodine") S14 (MH "Povidone‐Iodine") S15 (MH "Hexachlorophene") S16 (MH "Benzalkonium Compounds") S17 (MH "Phenols") S18 (MH "Hydrogen Peroxide") S19 (MH "Alcohols+") S20 (MH "Soaps") S21 TI iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap* S22 AB iodine* or povidone* or chlorhexidine or betadine or triclosan or hexachlorophene or chloroxylenol or cetrimide or benzalkonium or benzylkonium or octenidine or phenol* or carbolic or hydrogen peroxide or alcohol or alcohols or antiseptic* or soap* S23 TI skin N3 disinfect* or AB skin N3 disinfect* S24 S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 S25 S5 and S24 S26 MH "Clinical Trials+" S27 PT Clinical trial S28 TI clinic* N1 trial* or AB clinic* N1 trial* S29 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S30 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S31 TI randomi?ed control* trial* or AB randomi?ed control* trial* S32 MH "Random Assignment" S33 TI random* allocat* or AB random* allocat* S34 MH "Placebos" S35 TI placebo* or AB placebo* S36 MH "Quantitative Studies" S37 S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 S38 S25 and S37
Appendix 7. Risk of bias criteria
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes used without appropriate safeguards (e.g. envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following:
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Either of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following:
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following:
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following:
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used;
had extreme baseline imbalance;
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Povidone‐iodine (in aqueous solution) versus no skin antisepsis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related BSI | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.37, 2.61] |
| 2 Catheter colonisation | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.53, 1.60] |
Comparison 2. Chlorhexidine (in aqueous solution) versus no skin antisepsis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Septicaemia | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.91 [0.31, 27.31] |
| 2 Catheter colonisation | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.61, 2.59] |
| 3 Number of patients who required antibiotics during in‐dwelling period of catheter | 1 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.27] |
Comparison 3. Alcohol versus no skin antisepsis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter colonisation | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.30, 1.85] |
Comparison 4. Chlorhexidine versus povidone‐iodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related BSI | 4 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 0.99] |
| 1.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 2 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.28] |
| 1.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 2 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.39, 1.53] |
| 1.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.13, 1.24] |
| 2 Catheter‐related BSI per 1000 catheter‐days | 4 | 1450 | Risk Ratio (Fixed, 95% CI) | 0.53 [0.30, 0.94] |
| 2.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 308 | Risk Ratio (Fixed, 95% CI) | 0.82 [0.23, 2.93] |
| 2.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 3 | 661 | Risk Ratio (Fixed, 95% CI) | 0.49 [0.25, 0.95] |
| 2.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (Fixed, 95% CI) | 0.41 [0.06, 2.92] |
| 3 All‐cause mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.72, 1.83] |
| 3.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 1 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.48, 1.34] |
| 4 Catheter colonisation | 5 | 1533 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.08 [‐0.12, ‐0.03] |
| 4.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 2 | 452 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.09 [‐0.17, ‐0.02] |
| 4.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 3 | 600 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 4.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.11 [‐0.17, ‐0.04] |
| 5 Catheter colonisation per 1000 catheter‐days | 5 | 1547 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.50, 0.81] |
| 5.1 Chlorhexidine in aqueous solution versus povidone‐iodine in aqueous solution | 1 | 308 | Risk Ratio (Fixed, 95% CI) | 0.69 [0.40, 1.20] |
| 5.2 Chlorhexidine in alcohol versus povidone‐iodine in aqueous solution | 4 | 758 | Risk Ratio (Fixed, 95% CI) | 0.64 [0.48, 0.85] |
| 5.3 Chlorhexidine in alcohol versus povidone‐iodine in alcohol | 1 | 481 | Risk Ratio (Fixed, 95% CI) | 0.53 [0.24, 1.17] |
| 6 Insertion site infection | 1 | 242 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐9.10, 3.50] |
4.5. Analysis.
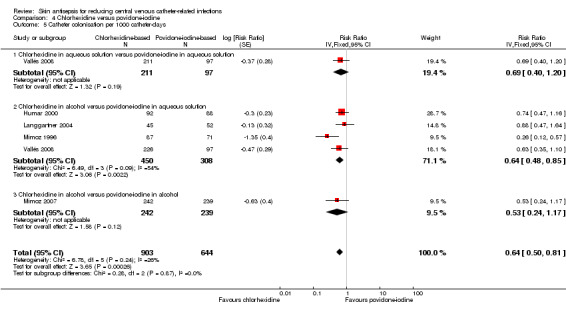
Comparison 4 Chlorhexidine versus povidone‐iodine, Outcome 5 Catheter colonisation per 1000 catheter‐days.
Comparison 5. Chlorhexidine (in aqueous solution) versus alcohol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related BSI | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.02, 2.54] |
| 2 Catheter colonisation | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.33] |
Comparison 6. Povidone‐iodine (in aqueous solution) versus alcohol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related BSI | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.21, 5.08] |
| 2 Catheter colonisation | 2 | 169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.76, 4.09] |
| 2.1 Povidone‐iodine in aqueous solution versus alcohol | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.49, 3.14] |
| 2.2 Povidone‐iodine‐impregnated adherent film versus alcohol | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.51, 160.17] |
Comparison 7. Alcohol versus octenidine in alcohol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related BSI | 1 | 387 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.88, 4.59] |
| 2 Catheter‐related BSI per 1000 catheter‐days | 1 | 387 | Risk Ratio (Fixed, 95% CI) | 2.18 [0.54, 8.77] |
| 3 Catheter colonisation | 1 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.22, 4.21] |
| 4 Catheter colonisation per 1000 catheter‐days | 1 | 322 | Risk Ratio (Fixed, 95% CI) | 2.23 [0.79, 6.29] |
| 5 Skin colonisation | 1 | 365 | Mean Difference (IV, Fixed, 95% CI) | 79.00 [32.76, 125.24] |
| 6 Adverse effects | 1 | 398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.20] |
Comparison 8. Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus chlorhexidine (in alcohol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter colonisation | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.04, 0.81] |
| 2 Catheter colonisation per 1000 catheter‐days | 1 | 88 | Risk Ratio (Fixed, 95% CI) | 0.19 [0.06, 0.59] |
Comparison 9. Chlorhexidine (in alcohol) plus povidone‐iodine (in aqueous solution) versus povidone‐iodine (in aqueous solution).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter colonisation | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.62] |
| 2 Catheter colonisation per 1000 catheter‐days | 1 | 95 | Risk Ratio (Fixed, 95% CI) | 0.17 [0.05, 0.52] |
Comparison 10. Sanosil (hydrogen peroxide and silver) versus water as adjunct to chlorhexidine 2% aqueous bath plus povidone‐iodine aqueous 10% scrub.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter colonisation | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dettenkofer 2010.
| Methods | Multicentre RCT (Switzerland) Study period: May 2002 to June 2005 Setting: 2 haematology units and 1 surgical unit in 2 university hospitals |
|
| Participants | Adult patients who required a CVC. Number of participants: 400 Number of catheters; 400 Age: median age of 59 years (25% quartile of 48 to 70 years) Sex: 66% male overall |
|
| Interventions | 2‐arm comparison of skin antisepsis prior to catheter insertion.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | The unit of analysis was patient, and it appeared that 1 catheter per patient was analysed although this was not stated explicitly. Funding source: the study was funded partly by the Swiss National Science Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Methods', 'Randomisation and interventions': "The randomisation code was produced by the independent Centre for Clinical Studies using computerised random number generator… used a stratification factor and block randomisation with randomly varying block length" |
| Allocation concealment (selection bias) | Low risk | 'Methods', 'Randomisation and interventions': As above, and "The randomisation was realised using closed envelopes, ensuring that the sequence was concealed before patients entered the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Methods', 'Randomisation and interventions': "The patients, staff administering the intervention, the microbiology lab were all blinded to the assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Methods', 'Randomisation and interventions': "The patients, staff administering the intervention, the microbiology lab were all blinded to the assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Discussion, paragraph 2: "20% of the catheters were not cultured, however they were equally distributed". The absolute rate of post randomisation exclusion was high for the outcome of catheter colonisation. However, the authors appeared to follow the intention‐to‐treat principle as they analysed the patients for whom the data was available in the originally assigned group. |
| Selective reporting (reporting bias) | Low risk | Authors reported all 4 major outcomes as stated in the 'Methods', namely, catheter colonisation, skin colonisation, catheter‐related BSI and adverse effects in sufficient detail in the 'Results'. |
| Other bias | Low risk | None identified |
Humar 2000.
| Methods | Multicentre RCT (Canada) Study period: Period of study not specified but authors stated that study conducted over 1 year (paragraph 1, results) Setting: hospital‐wide |
|
| Participants | 'Patients and methods', 'Patients': "All patients > 18 years of age who had CVCs inserted for any purpose were eligible for inclusion in the study, provided the treating physician felt the inserted catheter would be present for a minimum of 72 hours." Number of participants: 242 Number of catheters; 374 Age: mean of 58.3 years +/‐ range of 16.8 years (chlorhexidine group ) and 62.2 years +/‐ range of 16.0 years (povidone‐iodine group) Sex: 78% male in chlorhexidine group and 72% male in povidone‐iodine group. |
|
| Interventions | Comparison of 2 active agents for initial and subsequent cutaneous antisepsis for catheter care.
Outcomes assessed at various points during in‐patient stay. |
|
| Outcomes |
|
|
| Notes | Funding source: the study was funded by Physicians Services Incorporated (North York, Ontario, Canada) and Medi‐Flex (Overland Park, KS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Methods', 'Study design': Randomisation was achieved "by the use of blinded block randomisation schedule". |
| Allocation concealment (selection bias) | Unclear risk | Although the authors stated that the block randomisation schedule was "blinded", there was no further information provided on how treatment assignment was allocated using the random sequence generated at the time of enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors did not report whether blinding was achieved; blinding for clinical outcome assessment was highly unlikely because the antiseptic solutions used differed in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding for microbiological outcome assessment was unclear as this was not stated in the paper. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the outcomes of catheter‐related BSI and catheter colonisation, trialists only analysed 180 out of 242 patients that were initially enrolled (74%). The authors stated that 62 catheters were not analysed because the catheter tips were not available for culture, the underlying reasons of which were not provided. For the outcome of insertion site ("exit site") infection which was not dependent on catheter culture, trialists included all 242 patients in the analysis. The authors appeared to follow the intention‐to‐treat principle as they analysed the patients for whom the data was available in the originally assigned group. |
| Selective reporting (reporting bias) | Low risk | Authors reported all the outcomes stated in the 'Methods' with sufficient detail in the 'Results'. |
| Other bias | High risk | The study employed a block randomisation schedule with high likelihood that blinding of participants and personnel could not be achieved. This posed a risk to the integrity of the random sequence which would be vulnerable to disruption following educated guesses by those involved in the study on the likely assigned group of the future participants. |
Langgartner 2004.
| Methods | Single‐centre RCT (Germany) Study period: May 1999 to August 2002. Setting: Inpatient hospital wards and ICUs |
|
| Participants | 'Materials and methods': "Adult inpatients scheduled for elective CVC placement during normal working hours were eligible for participation in the study. Patients from normal wards as well as from the intensive care units were included. Patients known to be allergic to iodine or chlorhexidine were excluded as were all patients who needed a CVC placed under emergency conditions. No underlying disease was defined as an exclusion criteria." Number of participants: 119 Number of catheters: 200 (140 analysed) Age: mean age ranged from 50.5 to 56.6 years (SD ranged from 14.8 to 17.2 years)(reported separately according to three groups). Sex: overall 60.7% male. |
|
| Interventions | Skin disinfection prior to catheter insertion and daily during the change of dressings with 1 of the 3 regimens.
Outcomes assessed at various points during in‐patient stay. |
|
| Outcomes | Catheter colonisation | |
| Notes | Funding source: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | 'Materials and methods': "Sealed and numbered envelopes contained the randomisation code together with the instructions for skin disinfection and forms for the documentation of the procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of patients and carers not reported, although blinding appeared very unlikely because the number of antiseptic solution used for each group and their appearances were different. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether the personnel taking the swabs and the interpreter of the microbiological tests were blinded to the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 'Materials and methods': "In addition to the 140 catheters evaluated, 60 more catheters had been included but had to be excluded from analysis: in 5 cases, patients had died with the catheter in place, in 38 cases microbiological analysis of the catheter tip had not been performed and 17 catheters were lost during follow‐up (e.g. the patient was taken to a different clinic with the CVC in place).” In total, 200 catheters were recruited but only 140 were evaluated, which represented an overall dropout rate of 30%. It was unclear why trialists did not perform microbiological analyses in the 38 catheters as mentioned. However, the authors appeared to follow the intention‐to‐treat principle as they analysed the patients for whom the data was available in the originally assigned group. |
| Selective reporting (reporting bias) | High risk | The only outcome stated in the 'Methods' and reported was catheter colonisation. Some important outcomes such as catheter‐related blood stream infection, clinical sepsis and mortality were not reported. |
| Other bias | High risk | There was a unit of analysis issue in which the number of catheters analysed exceeded the number of participants by nearly 18%, and the outcome was reported using catheters as the units. |
Levy 1988.
| Methods | Single‐centre RCT (USA) Study period: not reported Setting: no clear description of the study setting except that the study was conducted on "patients undergoing coronary artery surgery". |
|
| Participants | 'Patients and methods': "60 patients scheduled for coronary artery surgery were studied during right internal jugular vein cannulation for PA catheter insertion." Number of participants: 60 Number of catheters;60 Age: not reported Sex: not reported |
|
| Interventions | Comparison of 2 skin preparation regimes before insertion of CVC.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Funding source: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients and methods': "Patients were assigned randomly assigned to one of two groups." There was no further information, including on random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was no information in the paper to enable an assessment on whether random sequence generation was independent from allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the authors did not explicitly say, blinding of the patient and personnel was highly unlikely because the 2 skin antisepsis regimes differed in the way of administration (1 using a liquid solution and an additional adherent film and the other using a swab without an adherent film). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding for microbiological outcome assessment not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors analysed all 60 participants initially enrolled and seemed to follow the intention‐to‐treat principle. |
| Selective reporting (reporting bias) | High risk | Authors reported both major outcomes named in the 'Methods', catheter colonisation and positive glove culture, in sufficient detail in the 'Results'. However, they did not include major patient‐related outcomes such as catheter‐related BSI, sepsis or mortality. |
| Other bias | Low risk | None identified |
Maki 1991.
| Methods | Single‐centre RCT (USA) Study period: 1986‐1987. Setting: surgical ICU |
|
| Participants | All adult patients over 18 years old Number of participants:176 Number of catheters;176 Age: mean age ranged from 51 to 53 years (SD of 19 in all three groups) Sex: not reported. |
|
| Interventions | Skin antisepsis prior to CVC insertion and every 48 h thereafter using 1 of 3 antiseptic solutions.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Although not clearly stated, it appeared that each patient had only 1 catheter included in the study, as Table 1 in the article suggested. Authors studied both venous and arterial catheters and reported outcome data separately. Funding source: partly funded by Stuart Corporation (ICI, Ltd) of Wilmington, Delaware. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Materials and methods', 'Procedures for insertion and care of catheters': "At the time of insertion, each catheter was randomised to one of three antiseptic solutions . . ." There was no description of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Materials and methods', 'Source of clinical data': "Although it was not possible for the users or the research nurses to be blinded to the antiseptic agent used . . ." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Materials and methods', 'Source of clinical data': "[T]he research microbiologist who processed all cultures had no knowledge of the antiseptic group to which the catheter had been assigned" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appeared that there were no withdrawals, as the number of catheters analysed matched the number of catheters enrolled initially. The authors appeared to follow the intention‐to‐treat principle by analysing the catheters in the originally assigned groups. |
| Selective reporting (reporting bias) | Low risk | Authors reported both major outcomes of catheter colonisation and catheter‐related BSI as stated in the 'Methods' in sufficient detail in the 'Results'. An additional outcome of adverse event was reported, although this was reported as an overall percentage without separating venous from arterial catheters. |
| Other bias | Low risk | None identified |
Mimoz 1996.
| Methods | Single‐centre RCT (France) Study period: 1 July 1992 to 31 October 1993 Setting: surgical‐trauma ICU |
|
| Participants | Consecutive patients aged 18 years and above who were scheduled to receive a non‐tunnelled central venous catheter, an arterial catheter or both Number of participants: not reported Number of catheters; 158 Age: mean age from 51 to 54 years (SD 18 to 19)(reported separately in two groups) Sex: not reported |
|
| Interventions | Comparison of the following 2 skin antiseptic regimens prior to catheter insertion and every 48 h post insertion.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Trialists studied both arterial catheters and CVCs. They did not report data separately for CVC and arterial catheters except for the outcomes of catheter colonisation per 1000 catheter‐days and catheter‐related sepsis per 1000 catheter‐days. Funding source: funded in part by Les Laboratoires Nicholas, Gaillard, France. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Materials and methods', 'Randomisation procedure': "Each patient requiring at least one catheter was randomly allocated to one of two groups by drawing envelopes from an urn." |
| Allocation concealment (selection bias) | Unclear risk | 'Materials and methods', 'Randomisation procedure': ""Each patient requiring at least one catheter was randomly allocated to one of two groups by drawing envelopes from an urn." It was unclear who drew the envelopes and when. It was also unclear whether the envelops were sealed and opaque. If the envelop was drawn by the investigator involved in the enrolment, there was a high risk of violating allocation concealment, for example, by redrawing. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Materials and methods', 'Blood cultures': "Although it was not possible for the research team to be blinded to the antiseptic agents used, the research microbiologist who processed all cultures had no knowledge of the antiseptic group to which the catheter had been assigned." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Materials and methods', 'Blood cultures': "Although it was not possible for the research team to be blinded to the antiseptic agents used, the research microbiologist who processed all cultures had no knowledge of the antiseptic group to which the catheter had been assigned." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no information on post randomisation withdrawals, nor any description on the use of intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Authors reported the major outcomes stated in the 'Methods', namely catheter colonisation and catheter related sepsis, in sufficient details in the 'Results'. The authors provided separate data for CVCs and arterial catheters for the outcomes of catheter colonisation per 1000 catheter‐days and catheter‐related sepsis per 1000 catheter‐days. |
| Other bias | Low risk | None identified |
Mimoz 2007.
| Methods | Single‐centre RCT (France) Study period: 14 May 2004 to 29 June 2006 Setting: surgical ICU |
|
| Participants | Adult inpatients Number of participants: not reported Number of catheters; 538 Age: mean age 57‐58 years (SD 18‐19) (reported separately in two groups) Sex: 67.4% men in chlorhexidine group and 75.7% men in povidone‐iodine group. |
|
| Interventions | Skin antisepsis using the following 2 regimens prior to CVC insertion and thereafter every 72 h.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Funding source: this study was supported by Centre Hospitalier et Universitaire de Poitiers and unrestricted grants from Bayer HealthCare and Viatris Pharmaceuticals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Methods', 'Randomisation': "The randomisation sequences were generated by computer and conveyed to the investigators by means of sealed envelopes, 1 for each catheter, with instructions to select envelopes in numerical order." |
| Allocation concealment (selection bias) | Low risk | 'Methods', 'Randomisation': "The randomisation sequences were generated by computer and conveyed to the investigators by means of sealed envelopes, 1 for each catheter, with instructions to select envelopes in numerical order." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | 'Methods', 'Randomisation': "Although it was not possible for the nurses and attending physicians to be blinded to the antiseptic agent used because of different colours of the 2 solutions (brown for the povidone‐iodine and colourless for the chlorhexidine‐based solution), the microbiologists who processed all of the cultures and the research team who reviewed the outcomes were unaware of the type of antiseptic solution used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Methods', 'Randomisation': "Although it was not possible for the nurses and attending physicians to be blinded to the antiseptic agent used because of different colours of the 2 solutions (brown for the povidone‐iodine and colourless for the chlorhexidine‐based solution), the microbiologists who processed all of the cultures and the research team who reviewed the outcomes were unaware of the type of antiseptic solution used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was 11% withdrawal, with a similar number of catheters excluded from analysis from the 2 groups. The authors have clearly stated the reasons for withdrawal and appeared to follow the intention‐to‐treat principle by analysing the available patient data in the originally assigned groups. |
| Selective reporting (reporting bias) | Low risk | Authors reported the 2 major outcomes stated in the 'Methods', namely, catheter colonisation and catheter‐related BSI, in sufficient details in the 'Results'. |
| Other bias | Low risk | None identified |
Prager 1984.
| Methods | Single‐centre RCT (USA) Study period: not reported Setting: hospital departments of General Surgery (123), Medicine (20), Thoracic Surgery (19), Neurosurgery (8), Obstretrics and Gynaecology (3), Paediatrics (3) and others (3) |
|
| Participants | All hospital inpatients who required a CVC Number of participants: 159 adults, 3 children Number of catheters; 179 Age: not reported Sex: not reported |
|
| Interventions | Skin antisepsis applied daily after CVC insertion.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Funding source: supported in part by the Purdue Frederick Company, Wilmington, Delaware. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The exact method of sequence generated was not described. However, in the 'Methods', the authors stated that patients were randomised according to hospital registration number, suggesting that they used alternation, instead of true randomisation. |
| Allocation concealment (selection bias) | High risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although the authors did not explicitly say, it was unlikely that the participants and the care providers were blinded, as the study assessed skin antisepsis versus no skin antisepsis. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of microbiological outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although the authors did not describe any withdrawals, it appeared that all catheters that were initially enrolled were analysed in the originally assigned groups. |
| Selective reporting (reporting bias) | Low risk | Authors reported the major outcomes of catheter colonisation and catheter‐related BSI as stated in the 'Methods' in sufficient detail in the 'Results'. The authors also reported an additional outcome of skin erythema. However, this was reported as an overall percentage of patients with colonised catheters, not according to the allocated groups, and so it did not allow data extraction for meta‐analysis. Nevertheless, this did not affect our judgment on the overall risk of reporting bias in any major way. |
| Other bias | High risk | There was a unit of analysis issue in which the number of catheters analysed exceeded the number of participants by nearly 10%, and the outcomes were reported using catheters as the units. |
Sadowski 1988.
| Methods | Single‐centre RCT (USA) Study period: November 1982 to December 1985 Setting: surgical ICU |
|
| Participants | Adult burn patients with a CVC in place Number of participants: 50 Number of catheters; 50 Age: mean age of 5.4 years (10 weeks to 15 years) Sex: 68% male |
|
| Interventions | Skin antisepsis prior to catheter removal:
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Funding source: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Materials and methods': Patients were "randomly assigned to one of two groups". Method of random sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not stated in the article, blinding appeared highly unlikely because the intervention involved an additional measure in catheter site care. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of microbiological outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although not clearly stated, it appeared that all 50 patients were analysed in their originally assigned groups as the tabulated results suggest. |
| Selective reporting (reporting bias) | High risk | There were 2 major outcomes reported, namely, catheter colonisation (positive catheter tip culture) and positive blood culture. However, the data from positive blood culture was unsuitable to be included in the meta‐analysis as it was reported only as an overall figure and not according to the allocated groups. |
| Other bias | Low risk | None identified |
Tuominen 1981.
| Methods | Single‐centre RCT (Finland) Study period:not reported. Setting: ICU |
|
| Participants | Adult inpatients admitted to ICU who required a CVC. No exclusion criteria stated Number of participants:136 Number of catheters; 136 (124 analysed) Age: not reported Sex: not reported |
|
| Interventions | Skin antisepsis applied prior to CVC insertion and regularly thereafter.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | No adverse effects were recorded in either group, so we do not include the data in our analysis. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients and methods': The patients were "randomly allocated to one of two groups". |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated in the paper, but blinding appears unlikely as the trial involved a comparison between the application of chlorhexidine‐soaked gauze versus a dry sterile gauze. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of microbiological outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not provide information on the initial number of patients and catheters recruited or the eventual number analysed. |
| Selective reporting (reporting bias) | Low risk | The outcomes were not defined in the 'Methods'. However, authors reported all major outcomes, including septicaemia, catheter colonisation and adverse effects, in sufficient detail. |
| Other bias | Low risk | None identified |
Vallés 2008.
| Methods | Single‐centre RCT (Spain) Study period: 1 Jan 2005 to 3 June 2006 Setting: adult medical‐surgical ICU in a university hospital |
|
| Participants | Patients requiring a CVC Number of participants: 420 Number of catheters; 998 (631 analysed) Age: mean age from 60 to 61 years (SD 16‐17) (reported separately in three groups) Sex: not reported. |
|
| Interventions | 3‐arm comparison of the following skin antiseptic regimens applied prior to CVC insertion and every 72 h thereafter.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | Funding source: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Methods', 'Study design': The random sequence was generated by "[b]y use of a blinded block randomisation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not adequately reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Although not stated by the authors, blinding to patients and caregivers appeared highly unlikely, as the antiseptic solutions used differed in appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Methods', 'Bacteriologic methods': "The microbiologists who performed the catheter‐tip cultures had no knowledge of the antiseptic group to which the catheter had been assigned." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors excluded from analysis 367/998 (36.7%) of the catheters initially randomised for various reasons (Figure 1 of the paper). They excluded 279 catheters post enrolment because they did not meet the inclusion criteria. However, among these excluded catheters, the reason given for 179 of them was that they were "not cultured". It was unclear what the underlying reasons were for failure to obtain culture in these catheters, and whether the excluded data here were missing at random. Trialists excluded 88 further catheters because they were inserted beyond 72 h after discharge from ICU. These 88 catheters were evenly distributed among the 3 assigned groups (61 between the 2 chlorhexidine groups and 27 in the povidone‐iodine group). However, following the construction of the best‐ and worst‐case scenarios using the dropouts, the direction of the effect estimates swung from significantly favouring the chlorhexidine group (best‐case scenario for chlorhexidine group) to significantly favouring the povidone‐iodine group (worst‐case scenario for chlorhexidine group). It was unclear whether the authors followed the intention‐to‐treat principle by analysing all available data according to the originally assigned groups, as there was no mention of participants who crossed over to the other group. We accorded the study high risk in this domain due to the high absolute dropout rate including the 179 catheters that were not adequately accounted for, as mentioned above, and the vulnerability of the result estimates to best‐ and worst‐case scenarios. |
| Selective reporting (reporting bias) | Low risk | Authors reported all 3 outcomes stated in the 'Methods', namely, catheter colonisation, catheter‐related BSI ("catheter‐related sepsis") and catheter‐related bacteraemia in sufficient detail in the 'Results'. In addition, they also reported the important outcome of mortality in the "Patient characteristics" table. although this was not a pre‐specified outcome in the methods.. |
| Other bias | High risk | The study employed a block randomisation schedule with high likelihood that blinding of participants and personnel were not achieved. This posed a risk to the integrity of the random sequence, which would be vulnerable to disruption following educated guesses by those involved in the study on the likely assigned group of the future participants. There was a serious unit of analysis issue in which the number of catheters analysed exceeded the number of participants by over 50%, and the major outcomes were reported using catheters as the units. |
Yasuda 2013.
| Methods | Multicentre RCT (Japan) Study period: March 2014 (not further details provided) Setting: 23 Japanese ICUs |
|
| Participants | 'Participants': "Patients over 18 years of age undergoing CVC and AC placement for more than 72 hours" Number of participants:not reported Number of catheters; 137 Age: not reported Sex: not reported |
|
| Interventions | 3‐arm comparison for skin antisepsis prior to catheter insertion.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | For this review, we combined the data for 1% CHG and 0.5% CHG as there was no significant difference in the results between the 2 groups. This was an interim analysis of the full study and was published in abstract form. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the published abstract |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the published abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the published abstract |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the published abstract |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned in the published abstract |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned in the published abstract |
| Other bias | Unclear risk | Insufficient information in the published abstract to assess the risks of bias |
Yousefshahi 2013.
| Methods | Single‐centre RCT (Iran) Study period: not reported. Setting: cardiac‐surgical ICU |
|
| Participants | Adult patients admitted to ICU after cardiac surgery Number of participants: 249 Number of catheters; 249 Age: mean age of 57 and 60 years (range 51 to 68) (reported separately in two groups) Sex: 76.1% and 76.5% male (reported separately in two groups) |
|
| Interventions | Skin antisepsis prior to CVC insertion.
|
|
| Outcomes |
Outcomes assessed at various points during in‐patient stay. |
|
| Notes | The number of CVCs evaluated matched the number of participants. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | From the authors' description, it appeared that an alternate sequence was used following an initial coin toss to determine the daily order of the grouping. 'Methods': "[A]ll the patients were separated into the intervention and control groups based on simple randomisation and entry sequence to the pre‐operation room. Each day, a simple coin randomisation technique was used to determine the group for the first patient and the spraying of pure water or Sanosil 2% on the catheter location (from the upper chest to the mandible). Subsequently, odd and even numbers were used to determine the group of the other patients." |
| Allocation concealment (selection bias) | High risk | From the authors' description, it appeared that an alternate sequence was used following an initial coin toss to determine the daily order of the grouping. 'Methods': "[A]ll the patients were separated into the intervention and control groups based on simple randomisation and entry sequence to the pre‐operation room. Each day, a simple coin randomisation technique was used to determine the group for the first patient and the spraying of pure water or Sanosil 2% on the catheter location (from the upper chest to the mandible). Subsequently, odd and even numbers were used to determine the group of the other patients." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | From the authors' description, it appeared that the patients and the person who removed the catheters to send for culture were blinded (see below). However, the authors did not state whether the nurse who sprayed the study substance was blinded to the study materials. 'Methods': "Both spray bottles were similar in shape and cover. Sanosil does not have any colour or smell and is similar to water, and the patients were blinded to the study." 'Methods': "Each day, two trained ICU nurses, blinded to the group type of the patients, collected the tips of five removed catheters aseptically..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It appeared that all patients recruited initially had their CVCs analysed. |
| Selective reporting (reporting bias) | Low risk | Authors reported the 2 key outcomes specified in the 'Methods', namely, catheter colonisation and sepsis, in the 'Results'. As no patient in either group developed sepsis, we did not include this outcome in our meta‐analysis. |
| Other bias | Low risk | None identified |
AC: arterial catheter; BSI: bloodstream infection; CHG: chlorhexidine‐gluconate; CVC: central venous catheter; ICU: intensive care unit; PA: pulmonary artery; RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almeida 2009 | Before‐and‐after study. Basis of exclusion: design |
| Apisarnthanarak 2010 | Quasi‐experimental before‐and‐after study. Basis of exclusion: design |
| Assadian 2004 | A commentary to Parienti 2004. Basis of exclusion: article type |
| Astle 2005 | An RCT that assessed ExSept versus chlorhexidine for patients with haemodialysis catheters. Basis of exclusion: population |
| Balamongkhon 2007 | Non‐randomised trial that assessed CVC site care using 2% chlorhexidine gluconate versus povidone‐iodine. Basis of exclusion: design |
| Bilir 2009 | This is a conference abstract of an study awaiting classification (BIlir 2013) |
| Borghesi 2011 | A review article on infection control strategies for the newborn. Basis of exclusion: article type and population |
| Bowling 2010 | A before‐and‐after study that assessed a multifaceted programme in decreasing blood culture contamination. Basis of exclusion: study design |
| Camins 2010 | Cross‐over study that assessed chlorhexidine‐impregnated foam dressing for prevention of catheter‐related BSI in patients undergoing haemodialysis. Basis of exclusion: design, population and intervention |
| Carrer 2005 | RCT that compared maximal sterile barrier (consisting of mask, cap, sterile gloves, gown, large drape) versus control precautions (mask, cap, sterile gloves, small drape) and transparent polyurethane film versus gauze dressing for reduction of CVC‐related infections. Basis of exclusion: intervention |
| Casey 2003 | A single‐centre RCT (UK) that compared the PosiFlow needleless connector against the standard luer cap attached to the CVCs for adult patients admitted for cardiac surgery. The authors used a factorial design which enabled the concurrent 3‐arm comparison of 3 different skin antiseptic solutions (0.5% chlorhexidine/alcohol, 70% isopropyl alcohol and 10% povidone–iodine) applied prior to the insertion of the catheters. However, the major outcome assessed was "stopcock entry port microbial contamination" rather than catheter colonisation, and this is not part of the prespecified outcomes in our review. Basis of exclusion: study design (design of the outcome) |
| Casey 2007 | RCT that compared a needless connector set (Clearlink Y‐type extension set) against standard 3‐way stopcocks with caps for reducing CVC related infections. Basis of exclusion: intervention |
| Cepkova 2006 | A review article on reducing catheter‐related infections in the ICU. Basis of exclusion: article type |
| Chaiyakunapruk 2003 | Cost‐effectiveness analysis on chlorhexidine gluconate versus povidone‐iodine for catheter site care. Basis of exclusion: article type |
| Crawford 2004 | Cost‐benefit analysis of chlorhexidine gluconate dressing in reducing catheter‐related infections. Basis of exclusion: article type |
| Daghistani 1996 | RCT that assessed antibiotic flush for CVCs in children with cancer. Basis of exclusion: intervention |
| Darouiche 2007 | A review article on strategies to prevent catheter‐related infections. Basis of exclusion: article type |
| Darouiche 2008 | A review article on strategies to prevent catheter‐related infections. Basis of exclusion: article type |
| Dean 2011 | A cross‐over study that compared the use of chlorhexidine solution against chlorhexidine‐impregnated cloth for CVC care. Basis of exclusion: study design |
| Dettenkofer 2002 | A quasi‐randomised trial in which patients were assigned on an alternate basis to either octenidine‐based skin antiseptic solution versus propanol‐based solution. Additionally, the results were presented in 25th centile, median and 75th centile of quantitative skin culture (in CFU/24 cm2) which does not allow extraction for meta‐analysis. Basis of exclusion: study design and data reporting |
| Eggimann 2010 | A prospective non‐randomised study that assessed catheter‐related infections following the introduction of various infection control strategies. Basis of exclusion: study design |
| Eyberg 2008 | RCT that assessed chlorhexidine gluconate gel dressing versus chlorhexidine gluconate disk in reducing CVC‐related infections. Basis of exclusion: intervention |
| Freiberger 1992 | A quasi‐experimental study comparing 2 skin antisepsis regimens (chlorhexidine and povidone‐iodine) and 2 types of dressing (Tegaderm and standard gauze) in a 4‐arm comparison of different combinations. The authors only reported the results in F or X2 values along with the P values, without reporting the raw data, which precluded data extraction for meta‐analysis. Basis of exclusion: study design and data reporting |
| Fukunaga 2004 | A non‐randomised study with historical cohort that assessed povidone‐iodine ointment in addition to dressing in reducing CVC‐related infections. Basis of exclusion: study design |
| Garcia 2010 | A non‐randomised study that assessed the effect of chlorhexidine scrub of the CVC hub during each access in reducing CVC‐related infections. Basis of exclusion: study design |
| Garcia‐Teresa 2007 | A multicentre observational study that evaluated CVC‐related infections in children. Basis of exclusion: study design |
| Garcia‐Vazquez 2011 | A before‐and‐after study that evaluated the effect of a hand hygiene promotion programme in reducing infections in an ICU. Basis of exclusion: study design |
| Garland 1996 | An RCT that assessed the local reaction to a chlorhexidine gluconate‐impregnated antimicrobial dressing in very low birth weight infants. Basis of exclusion: population and intervention |
| Garland 2001 | An RCT that compared chlorhexidine gluconate‐impregnated dressing with povidone‐iodine skin scrub for prevention of CVC‐related infections in neonates. Basis of exclusion: population |
| Garland 2009a | An RCT that compared chlorhexidine gluconate with povidone‐iodine as skin antisepsis prior to CVC placement in neonates. Basis of exclusion: population |
| Garland 2009b | An RCT that assessed the safety of chlorhexidine gluconate in neonates with percutaneously inserted central venous catheters. Basis of exclusion: population |
| Gilad 2006 | A review article on prevention of catheter‐related BSI in the neonatal intensive care setting. Basis of exclusion: article type |
| Girard 2012 | A longitudinal cohort study that compared two CVC cleaning protocols (containing alcohol‐based povidone‐iodine solution (Betadine alcolique) and chlorhexidine‐based antiseptic (Biseptine), respectively) administered in different periods. Basis of exclusion: study design |
| Gnass 2004 | A prospective, non‐randomised study that evaluated the effect of multiple infection control strategies in reducing catheter‐related infections. Basis of exclusion: study design |
| Gunst 2011 | A non‐randomised trial that compared antiseptic‐impregnated CVC with peripherally‐inserted central line in reducing catheter‐related infections. Basis of exclusion: study design and intervention |
| Habibzadeh 2013 | A commentary on an included study (Yousefshahi 2013) |
| Hachem 2002 | A review article on prevention of catheter‐related infection in long‐term catheters. Basis of exclusion: article type |
| Halpin 1991 | An RCT that evaluated the effect of povidone‐iodine connection shield that is incorporated in the catheter hub in reducing CVC‐related infections. Basis of exclusion: intervention |
| Hanazaki 1999 | An RCT that assessed the effect of chlorhexidine dressing in reducing catheter colonisation. Basis of exclusion: intervention |
| Hill 1990 | An RCT that assessed the effect of mupirocin ointment on colonisation rate of internal jugular vein catheters. Basis of exclusion: intervention |
| Huang 2006 | A retrospective study that assessed the effect of multiple infection control measures on the rates of methicillin‐resistant Staphylococcus aureus infection in an adult ICU. Basis of exclusion: study design |
| Hutchinson 1990 | An RCT that assessed occlusive versus non‐occlusive right atrial catheter dressing change procedures in children with cancer. Basis of exclusion: intervention |
| Ishikawa 2010 | An RCT comparing maximal sterile barrier precaution versus standard sterile barrier precaution measures during CVC insertion in reducing CVC‐related infections. Basis of exclusion: intervention |
| Ishizuka 2009 | A non‐randomised trial that compared the use of chlorhexidine versus povidone‐iodine for CVC site skin disinfection in 2 separate cohorts of patients. Basis of exclusion: study design |
| Johnson 2005 | An RCT that compared honey versus mupirocin applied at the catheter exit site for preventing catheter‐related infections in patients undergoing haemodialysis. Basis of exclusion: population and intervention |
| Khattak 2010 | An RCT that evaluated the absorption of silver in very low birthweight infants who received silver alginate‐impregnated central venous catheter. Basis of exclusion: population and intervention |
| Khouli 2009 | A conference abstract that reports the impact of simulation training on residents' performance in adhering to maximum sterile barrier precaution during CVC insertion. Basis of exclusion: research question and design |
| Krein 2007 | A national survey on measures to reduce catheter‐related BSI. Basis of exclusion: study design |
| Kruse 1999 | This is a commentary on an included study (Mimoz 1996). Basis of exclusion: article type |
| Kulkarni 2013 | An RCT that compared the use of 10% povidone‐iodine versus 2% chlorhexidine for skin disinfection prior to insertion of epidural or central venous catheters. The study combined both epidural and CVCs is the outcome reporting with no separate data for CVC, and more importantly, the outcome of skin colonisation was assessed based on a skin swab that was taken immediately after the application of the skin antiseptic agent, which did not fit in with our question of whether the application of skin antiseptic agent reduces catheter‐related infection during the period of catheter use. Excluded on th basis of research question and design |
| Lange 1997 | A non‐randomised trial that assessed a multifaceted strategy in CVC management in reducing catheter‐related infection in children with chronic illness. Basis of exclusion: study design |
| Le Corre 2003 | An RCT comparing transparent dressing versus a dry gauze applied at the exit site of the catheter on haemodialysis patients. Basis of exclusion: population and intervention |
| Legras 1997 | An RCT comparing alcohol‐chlorhexidine against povidone‐iodine for skin antisepsis for intravascular catheters. The study evaluated a mixture of venous, arterial and Swan Gantz catheters with no separate outcome reporting for venous catheters. There were no contact details provided in the paper to request for separate data for venous catheters. Basis of exclusion: insufficient information |
| Levy 2005 | An RCT that assessed the effectiveness of chlorhexidine gluconate‐impregnated dressing in reducing catheter‐related infections in children. Basis of exclusion: intervention |
| Madeo 1998 | An RCT comparing 2 different dressings for arterial and venous catheters in reducing catheter‐related infections. Basis of exclusion: intervention |
| Mahieu 2001 | A prospective cohort study that evaluated the effect of catheter manipulation on catheter‐related BSI in neonates. Basis of exclusion: study design, population and intervention |
| Maki 1981 | A commentary on disinfectant for vascular catheters. Basis of exclusion: article type |
| Maki 1992 | An RCT comparing different antibiotic ointments for preventing catheter‐related infection. Basis of exclusion: intervention |
| McCann 2016 | A pilot RCT involving in 3 Irish outpatient hemodialysis units compared 2% chlorhexidine gluconate (CHG) in 70% isopropyl alcohol with CHG solutions for central venous catheter exit site antisepsis. Basis of exclusion: population. |
| Montecalvo 2012 | A prospective cohort study that evaluated the rates of catheter‐related BSI over 3 study periods: pre‐intervention (phase 1), in which all patients were bathed with soap and water or non‐medicated washcloths; active intervention (phase 2), in which patients were bathed with 2% chlorhexidine gluconate cloths with the number of baths administered and skin tolerability assessed; and post‐intervention (phase 3), in which chlorhexidine bathing continued but without oversight by research personnel. Basis of exclusion: study design |
| Munoz‐Price 2009 | A non‐randomised study that evaluated a step‐wise infection control approach in reducing catheter‐related infection. Basis of exclusion: study design, intervention |
| Munoz‐Price 2012 | A non‐randomised study that evaluated the use of daily chlorhexidine bath in reducing catheter‐related infection. Basis of exclusion: study design |
| Nikoletti 1999 | An RCT comparing transparent polyurethane and hydrocolloid dressings for CVC in reducing catheter‐related infection. Basis of exclusion: intervention |
| Noto 2014 | A cluster‐RCT that assessed the effects of daily chlorhexidine bathing on the rates of healthcare associated infection in general for all ICU patients, not specific to patients with CVC in place. Basis of exclusion: population |
| Parienti 2004 | A cluster‐randomised cross‐over study that assessed the effectiveness of alcoholic povidone‐iodine in preventing catheter‐related infection. Basis of exclusion: study design |
| Peterson 2011 | An evidence‐based summary on the effectiveness of chlorhexidine versus 70% alcohol for CVC injection cap disinfection. Basis of exclusion: article type |
| Raad 1994 | An RCT that assessed the effectiveness of maximal sterile precaution during CVC insertion in reducing catheter‐related infection. Basis of exclusion: intervention |
| Render 2006 | A cluster‐randomised trial that assessed the effectiveness of 2 multifaceted infection control projects in reducing central line infections. Basis of exclusion: study design |
| Rezaei 2009 | An RCT that assessed the effectiveness of mupirocin ointment in reducing catheter‐related infection. Basis of exclusion: intervention |
| Richardson 2006 | A commentary on Parienti 2004. Basis of exclusion: article type |
| Rickard 2004 | An RCT that assessed the effectiveness of changing intravenous administration set for reducing catheter‐related infection. Basis of exclusion: intervention |
| Rijnders 2003 | An RCT that assessed the use of full sterile barrier precaution in reducing catheter‐related infection. Basis of exclusion: intervention |
| Rubinson 2004 | A review article on measures to reduce catheter‐related infection during insertion of CVC. Basis of exclusion: article type |
| Rupp 2008 | A non‐randomised, comparative, cross‐over trial that evaluated the effectiveness of alcohol‐based hand gel in reducing hospital‐acquired infections. Basis of exclusion: research question, study design |
| Ruschulte 2009 | An RCT that assessed the effectiveness of chlorhexidine‐impregnated wound dressing in reducing CVC‐related infection in patients undergoing chemotherapy. Basis of exclusion: intervention |
| Schwebel 2012 | An economic analysis on chlorhexidine‐impregnated sponges for reducing catheter‐related infection. Basis of exclusion: article type |
| Sheehan 1993 | An article identified through a related review paper in the form of a conference abstract. The text of the conference abstract could not be traced after contacting the author of the review article. We were unable to locate the contact details of the authors of this conference paper to request for further information. The conference abstract did not appear to be published subsequently in full. Basis of exclusion: insufficient information |
| Spiegler 2010 | A review article comparing central venous line and arterial line infections. Basis of exclusion: article type |
| Swan 2014 | A cluster‐RCT that compared chlorhexidine bathing versus soap and water bathing in decreasing the rates of healthcare associated infection for all patients in ICUs, and not only patients with a CVC in place. Basis of exclusion: population |
| Tietz 2005 | A prospective observational study that assessed the effectiveness of octenidine hydrochloride for CVC site care in patients receiving bone marrow transplant. Basis of exclusion: study design |
| Van Esch 2002 | An evidence‐based summary that examined the role of chlorhexidine versus povidone‐iodine antisepsis for reducing catheter‐related infection in neonates. Basis of exclusion: article type |
| Zingg 2008 | An overview on catheter‐related BSI. Basis of exclusion: article type |
| Zingg 2009 | A before‐and‐after study that assessed the effectiveness of an educational programme on promoting hand hygiene measures in reducing catheter‐related BSI. Basis of exclusion: study design |
BSI: bloodstream infection; CFU: colony‐forming units; CVC: central venous catheter; RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Atahan 2012.
| Methods | RCT (Czech Republic) |
| Participants | Adult surgical patients who required a CVC |
| Interventions | CVC insertion site disinfection with 10% povidone‐iodine (Poviiodeks) versus Savlosol (15% cetrimide, 1.5% chlorhexidine‐gluconate, ethanol) |
| Outcomes | Catheter colonisation and catheter‐related BSI |
| Notes | — |
BIlir 2013.
| Methods | RCT (Turkey) |
| Participants | Adult ICU patients who required a CVC |
| Interventions | 3‐arm comparison: skin antisepsis using 4% chlorhexidine gluconate (n = 19), 10% povidone iodine (n = 19) or octenidine hydrochlorodine (n = 19) |
| Outcomes | Catheter colonisation and catheter‐related BSI ("catheter‐related sepsis"), determined using "standard microbiological methods" ('Materials and methods') |
| Notes | The study evaluated a mixture of venous and arterial catheters with no separate analysis for venous catheters. This appears to be a conference abstract. We are awaiting further information from the authors. |
Giles 2002.
| Methods | RCT |
| Participants | Surgical patients who required a CVC |
| Interventions | Transparent occlusive dressing versus daily CVC site care with povidone‐iodine 10% solution |
| Outcomes | Catheter colonisation and catheter‐related sepsis |
| Notes | Awaiting full text |
Knasinski 2000.
| Methods | RCT |
| Participants | Unclear |
| Interventions | 1% chlorhexidine plus 75% alcohol versus 10% povidone iodine for cutaneous disinfection and follow‐up site care with central venous and arterial catheters |
| Outcomes | Catheter colonisation and catheter‐related BSI |
| Notes | This title was identified as a conference abstract from an earlier meta‐analysis on a similar topic. There is no further information at this stage other than the title. The author of the meta‐analysis paper with the title could not locate the abstract paper, and the study appeared not to be subsequently published in full. The study included both venous and arterial catheters, and it was unclear whether a separate outcome report for venous catheters would be available. We are awaiting the response of the study author for further information. |
Mimoz 2015.
| Methods | Open‐label multi‐centre RCT with a two‐by‐two factorial design |
| Participants | Adults (age >/=18 years) admitted to one of 11 French intensive‐care units and requiring at least one of central‐venous, haemodialysis, or arterial catheters |
| Interventions | All intravascular catheters prepared with 2% chlorhexidine‐70% isopropyl alcohol (chlorhexidine‐alcohol) or 5% povidone iodine‐69% ethanol (povidone iodine‐alcohol), with or without scrubbing of the skin with detergent before antiseptic application |
| Outcomes | "catheter‐related infections", catheter colonisation, adverse effects |
| Notes | Awaiting full‐text report for specific information on central venous catheters |
Yamamoto 2014.
| Methods | A comparative study (it is unclear from the abstract whether it is an RCT) |
| Participants | Haematology patients (age range unclear) |
| Interventions | 1% chlorhexidine‐gluconate ethanol versus 10% povidone‐iodine for skin antisepsis of CVC sites |
| Outcomes | Catheter‐related BSI, catheter colonisation |
| Notes | Awaiting full text from the authors |
Characteristics of ongoing studies [ordered by study ID]
Goudet 2013.
| Trial name or title | Comparison of four skin preparation strategies to prevent catheter‐related infection in intensive care unit (CLEAN trial): a study protocol for a randomized controlled trial |
| Methods | "A prospective multicenter, 2 × 2 factorial, randomized‐controlled, assessor‐blind trial" |
| Participants | Setting: 11 intensive care units in 6 French hospitals. Participants: All adult patients aged over 18 years requiring the insertion of 1 or more of the following: peripheral arterial catheter, non‐tunnelled central venous catheter, haemodialysis catheter and arterial pulmonary catheter |
| Interventions | Patients are allocated to 1 of the 4 skin preparation strategies: 2% chlorhexidine/70% isopropyl alcohol or 5% povidone iodine/69% ethanol, with and without prior skin scrubbing |
| Outcomes | Catheter‐related BSI, catheter colonisation, cutaneous tolerance, length of hospitalisation, mortality and cost. |
| Starting date | October 2012, lasting approximately 14 months |
| Contact information | Corresponding author: Olivier Mimoz o.mimoz@chu‐poitiers.fr |
| Notes | Clinicaltrials.gov number NCT01629550. Protocol published in Trials, 2013:14: 114 |
Differences between protocol and review
1. We have amended the title of the review by omitting the phrase "during catheter insertion". This was considered appropriate as all of our included studies examined skin antisepsis throughout the in‐dwelling period of the catheters with or without including the period of insertion, and keeping the phrase "during catheter insertion" would be misleading. We have revised the text of our review from Background through to the Methods where appropriate to reflect the change.
2. Under 'Why it is important to do this review', we changed the original statements "However, in some studies within the meta‐analysis, a combination of antiseptics were used; for example, chlorhexidine gluconate was sometimes evaluated in combination with alcohol. There remain some uncertainties regarding the best agent or combination of agents to be used for skin antisepsis" to the following: "However, the meta‐analysis only evaluated chlorhexidine gluconate and povidone‐iodine as skin antiseptics, and some studies within it assessed a combination of arterial catheters as well as central and peripheral venous catheters. Some uncertainties remain regarding the best agent, or combination of agents, for use as skin antisepsis for CVCs alone . . .". This was because in this review, the studies included also used a combination of agents, and there were no studies that assessed chlorhexidine gluconate separately, so the original statements did not justify the need for this review. Instead, the new statements more clearly reflect the differences between this review and the earlier review mentioned.
3. Under 'Types of studies', we added the following statements to further define the scope of our selection of studies: "We excluded cross‐over studies due to the possible contaminating effect of one intervention over another. We also excluded studies assessing CVCs for haemodialysis, as this is covered by another Cochrane review (McCann 2010)."
4. Under 'Selection of studies', we omitted the reference to unpublished studies because we did not find any unpublished study in our search of the trials registries.
5. Under Electronic searches, we updated the CENTRAL and MEDLINE search strategies in line with the updated indexing terms in each database.
6. Under 'Data extraction and management', we have re‐written paragraph 2 to the following to reflect what was actually done in the review.
"We found a discrepancy between the number of catheter and the number of patients in most studies, and this was due to multiple catheters being enrolled in some patients. However, we were unable to limit our analysis to one catheter per participant as none of the individual studies provided the adjusted results based on one catheter per participant."
7. We have added the section 'Unit of analysis issues' to describe how we would handle cluster‐RCTs.
8. Under 'Dealing with missing data', we revised our statement to include the absolute dropout rate in our consideration in assessing the risk of attrition bias, as a number of included studies had very high absolute dropout rates. Our revised statements are shown below:
"To assess whether the dropout rate was significant, we inspected the absolute dropout rate and the dropout rate in relation to the event rates for the intervention and the comparison groups. If the absolute dropout rate was 20% or more, we judged the study to be at high risk for incomplete outcome data. If the dropout rate was lower than 20%, we used a 'worst‐case‐scenario' method . . ."
9. Under 'Assessment of heterogeneity', we revised the statement to reflect what was actually done in the review, as follows:
"We found significant statistical heterogeneity in one analysis (Analysis 4.4) and provided a plausible explanation the possible reason for heterogeneity in the form of risk of attrition bias in some included studies. We decided to still provided the pooled estimate for this analysis and separated the studies based on the risk of attrition bias in our pre‐specified sensitivity analysis."
10. Under 'Sensitivity analysis', we re‐wrote the section as follows to reflect the information that we gathered in the review and removed any mention of intention‐to‐treat analysis:
"We performed the following sensitivity analyses.
Best‐ and worst‐case scenarios to assess the impact of missing data, as described in the section 'Dealing with missing data'.
Including and excluding studies with unclear and high risks of selection bias, namely, studies with unclear or high risk for random sequence generation, allocation concealment or both.
Had sufficient data been available, we would have performed additional sensitivity analyses to include and exclude studies with methodological issues other than selection bias, such as a lack of blinding to the participants, care givers or investigators, or where blinding was unclear."
11. Under 'Subgroup analysis and investigation of heterogeneity', we added the following statement to describe the separation of comparisons into subgroups based on the solution used, in response to the referees' comments in our draft review:
"In this review, we created subgroups of comparisons based on the solution used, for example, a subgroup for chlorhexidine in aqueous solution versus povidone iodine in aqueous solution, and another subgroup for chlorhexidine in alcohol versus povidone‐iodine in aqueous solution."
Contributions of authors
Nai Ming Lai conceived the review question, coordinated and developed the review, performed the CENTRAL search, screened and selected the studies, entered the data, performed the analyses, developed the 'Summary of findings' tables, drafted the results, discussion, conclusions and abstract, edited the review, made an intellectual contribution to the draft review writing, approved the final version prior to submission and is guarantor for the review. Nai An Lai made an intellectual contribution to the review writing and approved the final version prior to submission. Elizabeth O’Riordan made an intellectual contribution to the review writing and approved the final version prior to submission. Nathorn Chaiyakunapruk assisted in searching, provided some full‐text articles, made an intellectual contribution to the review writing and approved the final version prior to submission. Kenneth Tan participated in study selection, data entry and cross‐checking, made an intellectual contribution to the review writing and approved the final version prior to submission. Jacqueline Taylor participated in study selection, data entry and cross‐checking, edited the review draft and approved the final version prior to submission.
Contributions of editorial base:
Nicky Cullum (Editor): edited the protocol and the review; advised on methodology, interpretation and review content; approved the final review prior to submission. Sally Bell‐Syer and Gill Rizzello (Managing Editors): coordinated the editorial process. Advised on methodology, interpretation and content. Edited the review. Ruth Foxlee designed the search strategy, Amanda Briant and Reetu Child ran the searches and edited the search methods section. Denise Mitchell: assisted in searching and provided full‐text articles
Sources of support
Internal sources
No sources of support supplied
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
Nai Ming Lai: none known.
Nai An Lai: none known.
Elizabeth O’Riordan: none known.
Nathorn Chaiyakunapruk: none known.
Jacqueline Taylor: none known.
Kenneth Tan: none known.
New
References
References to studies included in this review
Dettenkofer 2010 {published data only}
Humar 2000 {published data only}
- Humar A, Ostromecki A, Direnfeld J, Marshall JC, Lazar N, Houston PC, et al. Prospective randomized trial of 10% povidone‐iodine versus 0.5% tincture of chlorhexidine as cutaneous antisepsis for prevention of central venous catheter infection. Clinical Infectious Diseases 2000; Vol. 31, issue 4:1001‐7. [DOI] [PubMed]
Langgartner 2004 {published data only}
Levy 1988 {published data only}
Maki 1991 {published data only}
- Maki DG, Ringer M, Alvarado CJ. Prospective randomised trial of povidone‐iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. The Lancet 1991; Vol. 338, issue 8763:339‐43. [DOI] [PubMed]
- Maki DG, Ringer M, Alvarado CJ. Prospective randomized trial of povidone‐iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. CINA: Official Journal of the Canadian Intravenous Nurses Association 1993;9(1):10‐15. [DOI] [PubMed] [Google Scholar]
Mimoz 1996 {published data only}
- Mimoz O, Pieroni L, Lawrence C, Edouard A, Costa Y, Samii K, et al. Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients. Critical Care Medicine 1996;24(11):1818‐23. [DOI] [PubMed] [Google Scholar]
- Mimoz O, Pieroni L, Lawrence C, Edouard A, Samii K. Prospective trial of povidone‐iodine (PI) and chlorhexidine (CH) for prevention of catheter‐related sepsis (CRS). Proceedings of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy; 1994 Oct 4‐7; Orlando (FL). 1994.
Mimoz 2007 {published data only}
Prager 1984 {published data only}
Sadowski 1988 {published data only}
- Sadowski DA, Harrell DA, Maley MP, Warden GD. The value of culturing central‐line catheter tips in burn patients. The Journal of Burn Care and Rehabilitation 1988;9(1):66‐8. [DOI] [PubMed] [Google Scholar]
Tuominen 1981 {published data only}
- Tuominen M, Valtonen VV, Nikki P. The effect of local antiseptic, chlorhexidine, in preventing infection from central venous catheterization: a clinical study. Annals of Clinical Research 1981; Vol. 13, issue 6:425‐8. [PubMed]
Vallés 2008 {published data only}
- Vallés J, Fernández I, Alcaraz D, Chacón E, Cazorla A, Canals M, et al. Prospective randomized trial of 3 antiseptic solutions for prevention of catheter colonization in an intensive care unit for adult patients. Infection Control and Hospital Epidemiology 2008; Vol. 29, issue 9:847‐53. [DOI] [PubMed]
Yasuda 2013 {published data only}
- Yasuda H, Sanui M, Fujitani S. Comparison of three cutaneous antiseptic solutions for the prevention of catheter colonization. Proceedings of the 43rd Critical Care Congress of the Society of Critical Care Medicine, SCCM 2014; 2014 Jan 9‐13; San Francisco. Critical Care Medicine 2013;41(12 Suppl 1):A195. [Google Scholar]
- Yasuda H, Sanui M, Komuro T, Hatakeyama J, Matsukubo S, Kawano S, et al. Comparison of three cutaneous antiseptic solutions for the prevention of catheter colonization in an ICU for adult patients: A multicenter prospective randomized controlled trial. Critical Care 2015;19(Suppl 1):73. [DOI: 10.1186/cc14153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yousefshahi 2013 {published data only}
- Yousefshahi F, Azimpour K, Boroumand MA, Najafi M, Barkhordari K, Vaezi M, et al. Can a new antiseptic agent reduce the bacterial colonization rate of central venous lines in post‐cardiac surgery patients?. Journal of Tehran University Heart Center 2013;8(2):70‐5. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Almeida 2009 {published data only}
- Almeida M, Ferreira A, Reis P, Alves V, Dias C, Granja C. Reducing catheter‐related bloodstream infections (CRBSI) in the ICU with an evidence‐based intervention. Intensive Care Medicine 2009;35(Suppl 1):S271. [Google Scholar]
Apisarnthanarak 2010 {published data only}
- Apisarnthanarak A, Thongphubeth K, Yuekyen C, Warren DK, Fraser VJ. Effectiveness of a catheter‐associated bloodstream infection bundle in a Thai tertiary care center: a 3‐year study. American Journal of Infection Control 2010;38(6):449‐55. [DOI] [PubMed] [Google Scholar]
Assadian 2004 {published data only}
- Assadian O. Skin antiseptic in reducing the risk of central venous catheter‐related infections. Critical Care Medicine 2004;32(3):887‐8. [DOI] [PubMed] [Google Scholar]
Astle 2005 {published data only}
- Astle CM, Jensen L. A trial of ExSept for hemodialysis central venous catheters. Nephrology Nursing Journal 2005;32(5):517‐25. [Google Scholar]
Balamongkhon 2007 {published data only}
- Balamongkhon B, Thamlikitkul V. Implementation of chlorhexidine gluconate for central venous catheter site care at Siriraj Hospital, Bangkok, Thailand. American Journal of Infection Control 2007;35(9):585‐8. [DOI] [PubMed] [Google Scholar]
Bilir 2009 {published data only}
- Bilir A, Yelken B, Erkan A. Chlorhexidine, octenidine or povidone iodine for catheter related infections: a randomised controlled trial. Critical Care 2009; Vol. 13, issue Suppl 1:S79. [PMC free article] [PubMed]
Borghesi 2011 {published data only}
- Borghesi A, Tzialla C, Decembrino L, Manzoni P, Stronati M. New possibilities of prevention of infection in the newborn. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(Suppl 2):28‐30. [DOI] [PubMed] [Google Scholar]
Bowling 2010 {published data only}
- Bowling G, Leykum L. Decreasing the rate of contaminated blood cultures. Proceedings of 2010 Annual Meeting of the Society of Hospital Medicine; 2010 Apr 8‐11; Washington, DC. Journal of Hospital Medicine 2010;5(Suppl 1):85. [Google Scholar]
Camins 2010 {published data only}
- Camins BC, Richmond AM, Dyer KL, Zimmerman HN, Coyne DW, Rothstein M, et al. A crossover intervention trial evaluating the efficacy of a chlorhexidine‐impregnated sponge in reducing catheter‐related bloodstream infections among patients undergoing hemodialysis. Infection Control and Hospital Epidemiology 2010;31(11):1118‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrer 2005 {published data only}
- Carrer S, Bocchi A, Bortolotti M, Braga N, Gilli G, Candini M, et al. Effect of different sterile barrier precautions and central venous catheter dressing on the skin colonization around the insertion site. Minerva Anestesiologica 2005;71(5):197‐206. [PubMed] [Google Scholar]
Casey 2003 {published data only}
Casey 2007 {published data only}
- Casey AL, Burnell S, Whinn H, Worthington T, Faroqui MH, Elliott TS. A prospective clinical trial to evaluate the microbial barrier of a needleless connector. The Journal of Hospital Infection 2007;65(3):212‐8. [DOI] [PubMed] [Google Scholar]
Cepkova 2006 {published data only}
- Cepkova M, Matthay MA. Reducing risk in the ICU: vascular catheter‐related infections. Infections in Medicine 2006;23(4):141‐52. [Google Scholar]
Chaiyakunapruk 2003 {published data only}
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Sullivan SD, Saint S. Vascular catheter site care: the clinical and economic benefits of chlorhexidine gluconate compared with povidone iodine. Clinical Infectious Diseases 2003;37(6):764‐71. [DOI] [PubMed] [Google Scholar]
Crawford 2004 {published data only}
- Crawford AG, Fuhr JP, Rao B. Cost‐benefit analysis of chlorhexidine gluconate dressing in the prevention of catheter‐related bloodstream infections. Infection Control and Hospital Epidemiology 2004;25(8):668‐74. [DOI] [PubMed] [Google Scholar]
Daghistani 1996 {published data only}
- Daghistani D, Horn M, Rodriguez Z, Schoenike S, Toledano S. Prevention of indwelling central venous catheter sepsis. Medical and Pediatric Oncology 1996;26(6):405‐8. [DOI] [PubMed] [Google Scholar]
Darouiche 2007 {published data only}
- Darouiche RO. Preventing catheter‐related infectious complications. Journal of Supportive Oncology 2007;5(2):70‐1. [PubMed] [Google Scholar]
Darouiche 2008 {published data only}
- Darouiche RO. Prevention of infections associated with vascular catheters. International Journal of Artificial Organs 2008;31(9):810‐9. [DOI] [PubMed] [Google Scholar]
Dean 2011 {published data only}
- Dean R, Dillworth J, Phillips M. Assessment of daily bathing protocols: a comparison of chlorhexidine solution and chlorhexidine impregnated cloths. Proceedings of Critical Care Congress; 2012 Feb 4‐8; Houston, TX. Critical Care Medicine 2011;39(Suppl 12):142. [Google Scholar]
Dettenkofer 2002 {published data only}
Eggimann 2010 {published data only}
- Eggimann P, Joseph C, Thevenin MJ, Voirol P, Bellini C, Pagani JL, et al. Impact of chlorhexidine‐impregnated sponges on catheter‐related infections rate. Intensive Care Medicine 2010;36(Suppl 2):S128. [Google Scholar]
Eyberg 2008 {published data only}
- Eyberg CI, Pyrek J. A controlled randomized prospective comparative pilot study to evaluate the ease of use of a transparent chlorhexidine gluconate gel dressing versus a chlorhexidine gluconate disk in healthy volunteers. The Journal of the Association for Vascular Access 2008;13(3):112‐7. [Google Scholar]
Freiberger 1992 {published data only}
Fukunaga 2004 {published data only}
Garcia 2010 {published data only}
- Garcia J, Island E, McLaughlin G, Langshaw A, Socarra M, Lalanne J, et al. Prevention of central venous catheter infections using chlorhexidine: Scrub the Hub campaign in transplant patients. American Journal of Transplantation 2010;10(2):422‐3. [Google Scholar]
Garcia‐Teresa 2007 {published data only}
- Garcia‐Teresa MA, Casado‐Flores J, Delgado‐Dominguez MA, Roqueta‐Mas J, Cambra‐Lasaosa F, Concha‐Torre A, et al. Infectious complications of percutaneous central venous catheterization in pediatric patients: a Spanish multicenter study. Intensive Care Medicine 2007;33(3):466‐76. [DOI] [PubMed] [Google Scholar]
Garcia‐Vazquez 2011 {published data only}
- Garcia‐Vazquez E, Murcia‐Paya J, Canteras M, Gomez J. Influence of a hygiene promotion programme on infection control in an intensive‐care unit. Clinical Microbiology and Infection 2011;17(6):894‐900. [DOI] [PubMed] [Google Scholar]
Garland 1996 {published data only}
- Garland JS, Alex CP, Mueller CD, Cisler‐Kahill LA. Local reactions to a chlorhexidine gluconate‐impregnated antimicrobial dressing in very low birth weight infants. The Pediatric Infectious Disease Journal 1996;15(10):912‐4. [DOI] [PubMed] [Google Scholar]
Garland 2001 {published data only}
- Garland JS, Alex CP, Mueller CD, Otten D, Shivpuri C, Harris MC, et al. A randomized trial comparing povidone‐iodine to a chlorhexidine gluconate‐impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 2001;107(6):1431‐6. [DOI] [PubMed] [Google Scholar]
Garland 2009a {published data only}
- Garland JS, Alex CP, Uhing MR, Peterside IE, Rentz A, Harris MC. Pilot trial to compare tolerance of chlorhexidine gluconate to povidone‐iodine antisepsis for central venous catheter placement in neonates. Journal of Perinatology 2009;29(12):808‐13. [DOI] [PubMed] [Google Scholar]
Garland 2009b {published data only}
- Garland JS, Harris MC, Uhing MR, Alex CP, Peterside I, Rentz A. Randomized pilot trial to assess safety of 2% chlorhexidine gluconate (CHG) in neonates with percutaneously placed central venous catheters (PICC). Proceedings of the Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, MD. 2009.
- Jeffery S, Garland JS, Harris MC, Uhing MR, Alex CP, Peterside I, Rentz A. Randomized pilot trial to assess safety of 2% chlorhexidine gluconate (CHG) in neonates with percutaneously placed central venous catheters (PICC). Proceedings of the Pediatric Academic Societies Annual Meeting; 2009 May 2‐5; Baltimore, MD. Baltimore: Pediatric Academic Societies, 2009.
Gilad 2006 {published data only}
- Gilad J, Borer A. Prevention of catheter‐related bloodstream infections in the neonatal intensive care setting. Expert Review of Anti‐Infective Therapy 2006;4(5):861‐73. [DOI] [PubMed] [Google Scholar]
Girard 2012 {published data only}
- Girard R, Comby C, Jacques D. Alcoholic povidone‐iodine or chlorhexidine‐based antiseptic for the prevention of central venous catheter‐related infections: in‐use comparison. Journal of Infection and Public Health 2012;5(1):35‐42. [DOI] [PubMed] [Google Scholar]
Gnass 2004 {published data only}
- Gnass SA, Barboza L, Bilicich D, Angeloro P, Treiyer W, Grenovero S, et al. Prevention of central venous catheter‐related bloodstream infections using non‐technologic strategies. Infection Control and Hospital Epidemiology 2004;25(8):675‐7. [DOI] [PubMed] [Google Scholar]
Gunst 2011 {published data only}
- Gunst M, Matsushima K, Vanek S, Gunst R, Shafi S, Frankel H. Peripherally inserted central catheters may lower the incidence of catheter‐related blood stream infections in patients in surgical intensive care units. Surgical Infections 2011;12(4):279‐82. [DOI] [PubMed] [Google Scholar]
Habibzadeh 2013 {published data only}
- Habibzadeh F, Yousefshahi F, Rouhipour N. Unethical conduct of underpowered clinical trials. Journal of Tehran University Heart Center 2013;8(4):213‐4. [PMC free article] [PubMed] [Google Scholar]
Hachem 2002 {published data only}
- Hachem R, Raad I. Prevention and management of long‐term catheter related infections in cancer patients. Cancer Investigation 2002;20(7‐8):1105‐13. [DOI] [PubMed] [Google Scholar]
Halpin 1991 {published data only}
- Halpin DP, O'Byrne P, McEntee G, Hennessy TP, Stephens RB. Effect of a betadine connection shield on central venous catheter sepsis. Nutrition 1991;7(1):33‐4. [PubMed] [Google Scholar]
Hanazaki 1999 {published data only}
- Hanazaki K, Shingu K, Adachi W, Miyazaki T, Amano J. Chlorhexidine dressing for reduction in microbial colonization of the skin with central venous catheters: a prospective randomized controlled trial. The Journal of Hospital Infection 1999;42(2):165‐8. [PubMed] [Google Scholar]
Hill 1990 {published data only}
- Hill RL, Fisher AP, Ware RJ, Wilson S, Casewell MW. Mupirocin for the reduction of colonization of internal jugular cannulae: a randomized controlled trial. The Journal of Hospital Infection 1990;15(4):311‐21. [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang SS, Yokoe DS, Hinrichsen VL, Spurchise LS, Datta R, Miroshnik I, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital‐wide methicillin‐resistant Staphylococcus aureus bacteremia. Clinical Infectious Diseases 2006;43(8):971‐8. [DOI] [PubMed] [Google Scholar]
Hutchinson 1990 {published data only}
- Hutchinson SK, Waskerwitz M, Martin K, Faubion W, Revesz S. Nonocclusive, clean permanent right atrial catheter dressing change procedures compared with occlusive, sterile permanent right atrial catheter dressing change procedures in children with cancer. Journal of Pediatric Oncology Nursing 1990;7(2):71. [DOI] [PubMed] [Google Scholar]
Ishikawa 2010 {published data only}
- Ishikawa Y, Kiyama T, Haga Y, Ishikawa M, Takeuchi H, Kimura O, et al. Maximal sterile barrier precautions do not reduce catheter‐related bloodstream infections in general surgery units: a multi‐institutional randomized controlled trial. Annals of Surgery 2010;251(4):620‐3. [DOI] [PubMed] [Google Scholar]
Ishizuka 2009 {published data only}
- Ishizuka M, Nagata H, Takagi K, Kubota K. Comparison of 0.05% chlorhexidine and 10% povidone‐iodine as cutaneous disinfectant for prevention of central venous catheter‐related bloodstream infection: a comparative study. European Surgical Research 2009;43(3):286‐90. [DOI] [PubMed] [Google Scholar]
Johnson 2005 {published data only}
- Johnson DW, Eps C, Mudge DW, Wiggins KJ, Armstrong K, Hawley CM, et al. Randomized, controlled trial of topical exit‐site application of honey (Medihoney) versus mupirocin for the prevention of catheter‐associated infections in hemodialysis patients. Journal of the American Society of Nephrology 2005;16(5):1456‐62. [DOI] [PubMed] [Google Scholar]
Khattak 2010 {published data only}
- Khattak AZ, Ross R, Ngo T, Shoemaker CT. A randomized controlled evaluation of absorption of silver with the use of silver alginate (Algidex) patches in very low birth weight (VLBW) infants with central lines. Journal of Perinatology 2010;30(5):337‐42. [DOI] [PubMed] [Google Scholar]
Khouli 2009 {published data only}
- Khouli HI, Jahnes K, Mathew J, Gohil A, Shapiro J, Rose K, et al. Medical residents' performance in maximum barrier precautions during central venous catheter placement: effect of simulation‐based training. Chest 2009;136(4):12S. [Google Scholar]
Krein 2007 {published data only}
- Krein SL, Hofer TP, Kowalski CP, Olmsted RN, Kauffman CA, Forman JH, et al. Use of central venous catheter‐related bloodstream infection prevention practices by US hospitals. Mayo Clinic Proceedings 2007;82(6):672‐8. [DOI] [PubMed] [Google Scholar]
Kruse 1999 {published data only}
- Kruse JA. Chlorhexidina gluconate solution prevented catheter colonization and infection [La solucion de gluconato de clorhexidina previene la colonizacion e infeccion del cateter]. Enfermedades Infecciosas y Microbiologia Clinica 1999;19(1):37‐8. [Google Scholar]
Kulkarni 2013 {published data only}
- Kulkarni AP, Awode M. A prospective randomised trial to compare the efficacy of povidone‐iodine 10% and chlorhexidine 2% for skin disinfection. Indian Journal of Anaesthesia 2013;57(3):270‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lange 1997 {published data only}
- Lange BJ, Weiman M, Feuer EJ, Jakobowski D, Bilodeau J, Stallings VA, et al. Impact of changes in catheter management of infectious complications among children with central venous catheters. Infection Control and Hospital Epidemiology 1997;18(5):326‐32. [DOI] [PubMed] [Google Scholar]
Le Corre 2003 {published data only}
- Corre I, Delorme M, Cournoyer S. A prospective, randomized trial comparing a transparent dressing and a dry gauze on the exit site of long term central venous catheters of hemodialysis patients. Journal of Vascular Access 2003;4(2):56‐61. [PubMed] [Google Scholar]
Legras 1997 {published data only}
- Legras A, Cattier B, Dequin PF, Boulain T, Perrotin D. Prospective randomised trial for prevention of vascular‐catheter infection: Alcohol chlorhexidine versus povidone‐iodine. [Etude prospective randomisée pour la prévention des infections liées aux cathéters: chlorhexidine alcoolique contre polyvidone iodée]. Réanimation Urgences 1997; Vol. 6, issue 1:5‐11.
Levy 2005 {published data only}
- Levy I, Katz J, Solter E, Samra Z, Vidne B, Birk E, et al. Chlorhexidine‐impregnated dressing for prevention of colonization of central venous catheters in infants and children: a randomized controlled study. The Pediatric Infectious Disease Journal 2005;24(8):676‐9. [DOI] [PubMed] [Google Scholar]
Madeo 1998 {published data only}
- Madeo M, Martin CR, Turner C, Kirkby V, Thompson DR. A randomized trial comparing Arglaes (a transparent dressing containing silver ions) to Tegaderm (a transparent polyurethane dressing) for dressing peripheral arterial catheters and central vascular catheters. Intensive and Critical Care Nursing 1998;14(4):187‐91. [DOI] [PubMed] [Google Scholar]
Mahieu 2001 {published data only}
- Mahieu LM, Dooy JJ, Lenaerts AE, Leven MM, Muynck AO. Catheter manipulations and the risk of catheter‐associated bloodstream infection in neonatal intensive care unit patients. Journal of Hospital Infection 2001;48(1):20‐6. [DOI] [PubMed] [Google Scholar]
Maki 1981 {published data only}
- Maki DG, Band JD. A comparative study of polyantibiotic and iodophor ointments in prevention of vascular catheter‐related infection. The American Journal of Medicine 1981;3:739‐44. [DOI] [PubMed] [Google Scholar]
Maki 1992 {published data only}
- Maki D. Choosing a disinfectant for central catheters. Nursing Times 1992;12:54‐5. [PubMed] [Google Scholar]
McCann 2016 {published data only}
- McCann M, Fitzpatrick F, Mellotte G, Clarke M. Is 2% chlorhexidine gluconate in 70% isopropyl alcohol more effective at preventing central venous catheter‐related infections than routinely used chlorhexidine gluconate solutions: a pilot multicenter randomized trial (ISRCTN2657745)?. American Journal of Infection Control 2016:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Montecalvo 2012 {published data only}
- Montecalvo MA, McKenna D, Yarrish R, Mack L, Maguire G, Haas J, et al. Chlorhexidine bathing to reduce central venous catheter‐associated bloodstream infection: impact and sustainability. American Journal of Medicine 2012;125(5):505‐11. [DOI] [PubMed] [Google Scholar]
Munoz‐Price 2009 {published data only}
- Munoz‐Price LS, Hota B, Stemer A, Weinstein RA. Prevention of bloodstream infections by use of daily chlorhexidine baths for patients at a long‐term acute care hospital. Infection Control and Hospital Epidemiology 2009;30(11):1031‐5. [DOI] [PubMed] [Google Scholar]
Munoz‐Price 2012 {published data only}
- Munoz‐Price LS, Dezfulian C, Wyckoff M, Lenchus JD, Rosalsky M, Birnbach DJ, et al. Effectiveness of stepwise interventions targeted to decrease central catheter‐associated bloodstream infections. Critical Care Medicine 2012;40(5):1464‐9. [DOI] [PubMed] [Google Scholar]
Nikoletti 1999 {published data only}
- Nikoletti S, Leslie G, Gandossi S, Coombs G, Wilson R. A prospective, randomized, controlled trial comparing transparent polyurethane and hydrocolloid dressings for central venous catheters. American Journal of Infection Control 1999;27(6):488‐96. [DOI] [PubMed] [Google Scholar]
Noto 2014 {published data only}
- Noto M, Domenico H, Talbot T, Byrne D, Wheeler A. Healthcare‐associated infections and chlorhexidine bathing: a pragmatic cluster‐randomized trial. Proceedings of the 2015 Critical Care Congress 2015; 2015 Jan 17‐21; Phoenix, AZ. Critical Care Medicine 2014;42(12 Suppl 1):A1478‐9. [Google Scholar]
Parienti 2004 {published data only}
- Parienti JJ, Du Cheyron D, Ramakers M, Malbruny B, Leclercq R, Coutour X, et al. Alcoholic povidone‐iodine to prevent central venous catheter colonization: A randomized unit‐crossover study. Critical Care Medicine 2004;32(3):708‐13. [DOI] [PubMed] [Google Scholar]
Peterson 2011 {published data only}
- Peterson K. Central venous catheter injection cap disinfection: chlorhexidine versus 70% alcohol. Journal of Pediatric Nursing 2011;26(2):e6. [Google Scholar]
Raad 1994 {published data only}
- Raad II, Hohn DC, Gilbreath BJ, Suleiman N, Hill LA, Bruso PA, et al. Prevention of central venous catheter‐related infections by using maximal sterile barrier precautions during insertion. Infection Control and Hospital Epidemiology 1994;15(4 Pt 1):231‐8. [PubMed] [Google Scholar]
Render 2006 {published data only}
- Render ML, Brungs S, Kotagal U, Nicholson M, Burns P, Ellis D, et al. Evidence‐based practice to reduce central line infections. Joint Commission Journal on Quality and Patient Safety 2006;32(5):253‐60. [DOI] [PubMed] [Google Scholar]
Rezaei 2009 {published data only}
- Rezaei J, Esfandiari Kh, Tavakoli H, Sadooghi M, Hasibi M, Behzadi M. Evaluation of mupirocin ointment in control of central venous catheter related infections: a randomized clinical trial. Tehran University Medical Journal 2009;67(6):428‐34. [Google Scholar]
Richardson 2006 {published data only}
- Richardson D. Literature review [Alcoholic povidone‐iodine to prevent central venous catheter colonization: a randomized unit‐crossover study]. Journal of the Association for Vascular Access 2006;11(2):76. [Google Scholar]
Rickard 2004 {published data only}
- Rickard CM, Lipman J, Courtney M, Siversen R, Daley P. Routine changing of intravenous administration sets does not reduce colonization or infection in central venous catheters. Infection Control and Hospital Epidemiology 2004;25(8):650‐5. [DOI] [PubMed] [Google Scholar]
Rijnders 2003 {published data only}
- Rijnders BJ, Wijngaerden E, Wilmer A, Peetermans WE. Use of full sterile barrier precautions during insertion of arterial catheters: a randomized trial. Clinical Infectious Diseases 2003;36(6):743‐8. [DOI] [PubMed] [Google Scholar]
Rubinson 2004 {published data only}
- Rubinson L, Diette GB. Best practices for insertion of central venous catheters in intensive‐care units to prevent catheter‐related bloodstream infections. Journal of Laboratory and Clinical Medicine 2004;143(1):5‐13. [DOI] [PubMed] [Google Scholar]
Rupp 2008 {published data only}
- Rupp ME, Fitzgerald T, Puumala S, Anderson JR, Craig R, Iwen PC, et al. Prospective, controlled, cross‐over trial of alcohol‐based hand gel in critical care units. Infection Control and Hospital Epidemiology 2008;29(1):8‐15. [DOI] [PubMed] [Google Scholar]
Ruschulte 2009 {published data only}
Schwebel 2012 {published data only}
- Schwebel C, Lucet JC, Vesin A, Arrault X, Calvino‐Gunther S, Bouadma L, et al. Economic evaluation of chlorhexidine‐impregnated sponges for preventing catheter‐related infections in critically ill adults in the Dressing Study. Critical Care Medicine 2012;40(1):11‐17. [DOI] [PubMed] [Google Scholar]
Sheehan 1993 {published data only}
- Sheehan G, Leicht K, O'Brien M, Taylor G, Rennie R. Chlorhexidine versus povidone‐iodine as cutaneous antisepsis for prevention of vascular‐catheter infection. Proceedings of the 33rd Interscience Conference on Antimicrobial Agents and Chemotherapy; 1993 Oct 17‐20; New Orleans, LA. 1993:a1616.
Spiegler 2010 {published data only}
- Spiegler P. Comparing central line and arterial line infections. Clinical Pulmonary Medicine 2010;17(5):248‐9. [Google Scholar]
Swan 2014 {published data only}
- Swan J, Bui L, Pham V, Shirkey B, Graviss E, Hai S, et al. RCT Of chlorhexidine vs. soap & water bathing for prevention of hospital‐acquired infections in SICU. Proceedings of the 2015 Critical Care Congress; 2015 Jan 17‐21; Phoenix, AZ. Critical Care Medicine 2014;42(12 Suppl 1):A1369‐70. [Google Scholar]
Tietz 2005 {published data only}
- Tietz A, Frei R, Dangel M, Bolliger D, Passweg JR, Gratwohl A, et al. Octenidine hydrochloride for the care of central venous catheter insertion sites in severely immunocompromised patients. Infection Control and Hospital Epidemiology 2005;26(8):703‐7. [DOI] [PubMed] [Google Scholar]
Van Esch 2002 {published data only}
- Esch J. Chlorhexidine reduced catheter tip colonisation more than 10% povidone‐iodine in critically ill neonates. Evidence‐Based Nursing 2002;5(3):73. [DOI] [PubMed] [Google Scholar]
Zingg 2008 {published data only}
- Zingg W, Cartier‐Fassler V, Walder B. Central venous catheter‐associated infections. Best Practice and Research: Clinical Anaesthesiology 2008;22(3):407‐21. [DOI] [PubMed] [Google Scholar]
Zingg 2009 {published data only}
- Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter‐related bloodstream infections. Critical Care Medicine 2009;37(7):2167‐73. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Atahan 2012 {published data only}
- Atahan K, Cokmez A, Bekoglu M, Durak E, Tavusbay C, Tarcan E. The effect of antiseptic solution in central venous catheter care. Bratislavske Lekarske Listy 2012;113(9):548‐51. [DOI] [PubMed] [Google Scholar]
BIlir 2013 {published data only}
- Bilir A, Yelken B, Erkan A. Chlorhexidine, octenidine or povidone iodine for catheter related infections: a randomised controlled trial. Journal of Research in Medical Sciences 2013;18(6):510‐2. [PMC free article] [PubMed] [Google Scholar]
Giles 2002 {published data only}
- Giles Y, Aksoy M, Tezelman S. What really affects the incidence of central venous catheter‐related infections for short‐term catheterization?. Acta Chirurgica Belgica 2002;102(4):256‐8. [DOI] [PubMed] [Google Scholar]
Knasinski 2000 {unpublished data only}
- Knasinski V, Maki DG. A prospective, randomized, controlled trial of 1% chlorhexidine 75% alcohol vs. 10% povidone iodine for cutaneous disinfection and follow‐up site care with central venous and arterial catheters. Proceedings of the National Association of Vascular Access Network Conference; 2000 September 6‐10; San Diego,CA. San Diego, 2000.
Mimoz 2015 {published data only}
- Mimoz O, Lucet JC, Kerforne T, Pascal J, Souweine B, Goudet V, et al. Skin antisepsis with chlorhexidine‐alcohol versus povidone iodine‐alcohol, with and without skin scrubbing, for prevention of intravascular‐catheter‐related infection (CLEAN): an open‐label, multicentre, randomised, controlled, two‐by‐two factorial trial. Lancet 2015;386(10008):2069‐2077. [DOI: 10.1016/S0140-6736] [DOI] [PubMed] [Google Scholar]
Yamamoto 2014 {published data only}
- Yamamoto N, Kimura H, Misao H, Matsumoto H, Imafuku Y, Watanabe A, et al. Efficacy of 1.0% chlorhexidine‐gluconate ethanol compared with 10% povidone‐iodine for long‐term central venous catheter care in hematology departments: a prospective study. American Journal of Infection Control 2014;42(5):574‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Goudet 2013 {published data only}
- Goudet V, Timsit JF, Lucet JC, Lepape A, Balayn D, Seguin S, et al. Comparison of four skin preparation strategies to prevent catheter‐related infection in intensive care unit (CLEAN trial): a study protocol for a randomized controlled trial. Trials 2013;14:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2007
- Adams D, Elliot TS. Skin antiseptics used prior to intravascular catheter insertion. British Journal of Nursing 2007;16(5):278‐80. [DOI] [PubMed] [Google Scholar]
Bankston 2005
- Bankston J. Joseph Lister and the History of Antiseptics. Hockessin: Mitchell Lane Publishers, 2005. [Google Scholar]
Bynum 2008
- Bynum W. The History of Medicine: A Very Short Introduction. New York: Oxford University Press, 2008. [Google Scholar]
Campbell 2001
- Campbell MK, Mollison J, Grimshaw JM. Cluster trials in implementation research: estimation of intracluster correlation coefficients and sample size. Statistics in Medicine 2001;20(3):391‐9. [DOI] [PubMed] [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention (CDC). Guidelines for the prevention of intravascular catheter‐related infections, 2011. http://www.cdc.gov/hicpac/BSI/BSI‐guidelines‐2011.html (accessed 4 June 2015).
Chaiyakunapruk 2002
- Chaiyakunapruk N, Veenstra DL, Lipsky BA, Saint S. Chlorhexidine compared with povidone‐iodine solution for vascular catheter‐site care: a meta‐analysis. Annals of Internal Medicine 2002;136(11):792‐801. [DOI] [PubMed] [Google Scholar]
Cicalini 2004
- Cicalini S, Palmieri F, Petrosillo N. Clinical review: new technologies for prevention of intravascular catheter‐related infections. Critical Care 2004;8(3):157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 2008
- Edwards JR, Peterson KD, Andrus ML, Dudeck MA, Pollock DA, Horan TC. National Healthcare Safety Network (NHSN) Report, data summary for 2006 through 2007, issued November 2008. American Journal of Infection Control 2008;36(9):609‐26. [DOI] [PubMed] [Google Scholar]
Guyatt 1993
- Guyatt GH, Sackett DL, Cook DJ, Evidence‐Based Medicine Working Group. Users' guides to the medical literature II. How to use an article about therapy or prevention A. Are the results of the study valid?. JAMA 1993;270(21):2598‐601. [DOI] [PubMed] [Google Scholar]
Hamilton 2007
- Hamilton HC, Foxcroft DR. Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long‐term intravenous therapy. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004084.pub2] [DOI] [PubMed] [Google Scholar]
Hardin 1997
- Hardin W, Nichols R. Handwashing and patient skin preparation. In: Malangoni MA editor(s). Critical Issues in Operating Room Management. Philadelphia: Lippincott‐Raven, 1997:133‐49. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks J, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Larson 1995
- Larson EL. APIC guideline for handwashing and hand antisepsis in health care settings. American Journal of Infection Control 1995;23(4):251‐69. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Martindale 2016
- Brayfield, A (editor). Martindale: the complete drug reference. www.medicinescomplete.com/mc/martindale/current/ (accessed 6 July 2016).
McCann 2010
- McCann M, Moore ZEH. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006894.pub2] [DOI] [PubMed] [Google Scholar]
NNIS 2004
- US Department of Health and Human Services. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. American Journal of Infection Control 2004;32(8):470‐85. [DOI] [PubMed] [Google Scholar]
Nuland 2003
- Nuland SB. The Doctors' Plague: Germs, Childbed Fever and the Strange Story of Ignaz Semmelweis. New York: WW Norton, 2003. [Google Scholar]
O'Grady 2002
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter‐related infections. Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report (MMWR) 2002; Vol. 51, issue RR‐10:1‐29. [PubMed]
Pagani 2008
- Pagani JL, Eggimann P. Management of catheter‐related infection. Expert Review of Anti‐infective Therapy 2008;6(1):31‐7. [DOI] [PubMed] [Google Scholar]
Pratt 2007
- Pratt RJ, Pellowe CM, Wilson JA, Loveday HP, Harper PJ, Jones SR, et al. epic2: National evidence‐based guidelines for preventing healthcare‐associated infections in NHS hospitals in England. Journal of Hospital Infection 2007;65(Suppl 1):S1‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puri 2009
- Puri N, Puri V, Dellinger RP. History of technology in the intensive care unit. Critical Care Clinics 2009;25(1):185‐200, ix. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 1986
- Russell AD. Chlorhexidine: antibacterial action and bacterial resistance. Infection 1986;14(5):212‐5. [DOI] [PubMed] [Google Scholar]
Ryan 2004
- Ryan KJ, Ray G (editors). Sherris Medical Microbiology. 4th Edition. New York: McGraw‐Hill, 2004. [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http:www.sign.ac.uk/methodology/filters.html#random (accessed 4 June 2015).
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Taber 2016
- Venes D (editor). Taber's Cyclopedic Medical Dictionary Online. http://www.tabers.com/tabersonline/view/Tabers‐Dictionary/738695/0/antiseptic?q=antiseptic&ti=0 (accessed 6 July 2016).
Trieschmann 2007
- Trieschmann U, Cate UT, Sreeram N. Central venous catheters in children and neonates ‐ what is important?. Images in Paediatric Cardiology 2007;9(4):1‐8. [PUBMED: 22368674] [PMC free article] [PubMed] [Google Scholar]
Worthington 2005
- Worthington T, Elliott TS. Diagnosis of central venous catheter related infection in adult patients. Journal of Infection 2005;51(4):267‐80. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lai 2012
- Lai NA, Lai NM, O'Riordan E, Chaiyakunapruk N, Taylor JE, Tan K. Skin antisepsis during catheter insertion for reducing central venous catheter related infections. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010140] [DOI] [PMC free article] [PubMed] [Google Scholar]


