Abstract
Background
Recurrent urinary tract infections (RUTI) are common in women who are pregnant and may cause serious adverse pregnancy outcomes for both mother and child including preterm birth and small‐for‐gestational‐age babies. Interventions used to prevent RUTI in women who are pregnant can be pharmacological (antibiotics) or non‐pharmacological (cranberry products, acupuncture, probiotics and behavioural modifications). So far little is known about the best way to prevent RUTI in pregnant women.
Objectives
To assess the effects of interventions for preventing RUTI in pregnant women.
The primary maternal outcomes were RUTI before birth (variously defined) and preterm birth (before 37 weeks). The primary infant outcomes were small‐for‐gestational age and total mortality.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (20 May 2015) and reference lists of retrieved articles.
Selection criteria
Published, unpublished and ongoing randomised controlled trials (RCTs), quasi‐RCTs, clustered‐randomised trials and abstracts of any intervention (pharmacological and non‐pharmacological) for preventing RUTI during pregnancy (compared with another intervention, placebo or with usual care).
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
The review included one trial involving 200 women and was at moderate to high risk of bias.The trial compared a daily dose of nitrofurantoin and close surveillance (regular clinic visit, urine cultures and antibiotics when a positive culture was found) with close surveillance only. No significant differences were found for the primary outcomes: recurrent pyelonephritis (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.31 to 2.53; one study, 167 women), RUTI before birth (RR 0.30, 95% CI 0.06 to 1.38; one study, 167 women), and preterm birth (before 37 weeks) (RR 1.18, 95% CI 0.42 to 3.35; one study, 147 women). The overall quality of evidence for these outcomes as assessed using GRADE was very low. There were no significant differences between the two comparison groups for any of the following secondary outcomes, birthweight less than 2500 (g) (RR 2.03, 95% CI 0.53 to 7.80; one study, 147 infants), birthweight (mean difference (MD) ‐113.00, 95% CI ‐327.20 to 101.20; one study, 147 infants), five‐minute Apgar score less than seven (RR 2.03, 95% CI 0.19 to 21.87; one study, 147 infants) and miscarriages (RR 3.11, 95% CI 0.33 to 29.29; one study, 167 women). The evidence for these secondary outcomes was also of very low quality. The incidence of asymptomatic bacteriuria (ASB) (at least 103 colonies per mL) (secondary outcome), only reported in women with a clinic attendance rate of more than 90% (RR 0.55, 95% CI 0.34 to 0.89; one study, 102 women), was significantly reduced in women who received nitrofurantoin and close surveillance. Data on total mortality and small‐for‐gestational‐age babies were not reported.
Authors' conclusions
A daily dose of nitrofurantoin and close surveillance has not been shown to prevent RUTI compared with close surveillance alone. A significant reduction of ASB was found in women with a high clinic attendance rate and who received nitrofurantoin and close surveillance. There was limited reporting of both primary and secondary outcomes for both women and infants. No conclusions can be drawn regarding the optimal intervention to prevent RUTI in women who are pregnant. Randomised controlled trials comparing different pharmacological and non‐pharmacological interventions are necessary to investigate potentially effective interventions to prevent RUTI in women who are pregnant.
Plain language summary
Interventions for preventing recurrent urinary tract infections during pregnancy
Recurrent urinary tract infections (RUTI) are common in women generally, and particularly in pregnant women. A urinary tract infection (UTI) is an infection of the urinary tract (bladder, kidneys) due to the presence of bacteria in the urine (bacteriuria). During pregnancy, UTI may be a serious complication that is associated with adverse pregnancy outcomes for both mother and child including preterm birth and small‐for‐gestational‐age babies. Therefore, it is important to define the optimal intervention for preventing RUTI during pregnancy to improve pregnancy outcomes. Interventions used to prevent RUTI in pregnant women can be pharmacological (antibiotics) or non‐pharmacological (cranberry products, acupuncture, probiotics and behavioural modifications). So far, little is known about the best way to prevent RUTI in pregnant women.
This review identified one study involving 200 pregnant women who received nitrofurantoin (antibiotics) and close surveillance (regular clinic visit, urine cultures and antibiotics when a positive culture was found) or close surveillance alone. Suppressive therapy with daily dose of nitrofurantoin and close surveillance was not shown to prevent RUTI compared with close surveillance alone but the evidence was of very low quality. A significant reduction of asymptomatic bacteriuria (presence of bacteria in the urine without the symptoms of a UTI) was found in women with a high clinic attendance rate who received nitrofurantoin and close surveillance. Due to lack of evidence no conclusions can be drawn. Future randomised controlled trials comparing different pharmacological and non‐pharmacological interventions are necessary to assess the optimal intervention to prevent RUTI in women who are pregnant. Such trials should report on a comprehensive range of outcomes for both women and infants.
Summary of findings
Summary of findings for the main comparison. Nitrofurantoin and close surveillance compared with close surveillance alone for preventing recurrent urinary tract infection during pregnancy.
| Nitrofurantoin and close surveillance compared with close surveillance alone for preventing recurrent urinary tract infection during pregnancy | ||||||
| Patient or population: pregnant women with a history of one or more UTI before or during pregnancy Settings: Los Angeles, USA. Intervention: nitrofurantoin and close surveillance Comparison: close surveillance alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Close surveillance alone | Nitrofurantoin and close surveillance | |||||
| Recurrent pyelonephritis | Study population | RR 0.89 (0.31 to 2.53) | 167 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 82 per 1000 | 73 per 1000 (26 to 208) | |||||
| Moderate | ||||||
| 82 per 1000 | 73 per 1000 (26 to 208) | |||||
| Recurrent UTI (cystitis) | Study population | RR 0.30 (0.06 to 1.38) | 167 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 82 per 1000 | 25 per 1000 (5 to 114) | |||||
| Moderate | ||||||
| 82 per 1000 | 25 per 1000 (5 to 114) | |||||
| Preterm birth (< 37 weeks) | Study population | RR 1.18 (0.42 to 3.35) | 147 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 81 per 1000 | 96 per 1000 (34 to 272) | |||||
| Moderate | ||||||
| 81 per 1000 | 96 per 1000 (34 to 272) | |||||
| Birthweight < 2500 (g) | Study population | RR 2.03 (0.53 to 7.80) | 147 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,2 | ||
| 41 per 1000 | 82 per 1000 (21 to 316) | |||||
| Moderate | ||||||
| 41 per 1000 | 82 per 1000 (21 to 316) | |||||
| Birthweight (g) | The mean birthweight (g) in the control group was 0 | The mean birthweight (g) in the intervention group was 113 lower (327.2 lower to 101.2 higher) | ‐ | 147 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,3 | |
| Five‐minute Apgar score < seven | Study population | RR 2.03 (0.19 to 21.87) | 147 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,4 | ||
| 14 per 1000 | 27 per 1000 (3 to 296) | |||||
| Moderate | ||||||
| 14 per 1000 | 27 per 1000 (3 to 295) | |||||
| Miscarriages | Study population | RR 3.11 (0.33 to 29.29) | 167 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1,4 | ||
| 12 per 1000 | 37 per 1000 (4 to 345) | |||||
| Moderate | ||||||
| 12 per 1000 | 37 per 1000 (4 to 346) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1One study with design limitations (‐1)
2One study of small sample size and few events. Wide CI crossing the line of no effect (‐2)
3One study of small sample size. Wide CI (‐1)
4One study of small sample size. Wide CI crossing the line of no effect (‐2)
Background
Description of the condition
Recurrent urinary tract infections (RUTI) are a common healthcare problem in women generally and particularly in pregnant women. Up to 30% of women who are not pregnant experience at least one recurrence within a year after the initial infection (Foxman 1990; Hooton 2001; Mabeck 1972; Scholes 2000). A urinary tract infection (UTI) is an infection of the urinary tract which can be divided in lower and upper UTI based on the location of the infection. A lower UTI is an infection of the bladder and results in a combination of significant bacteriuria and symptoms such as dysuria (painful urination) and frequency. In practice the diagnosis of UTI is often based on clinical symptoms alone. An upper UTI or pyelonephritis is an infection of the kidney accompanied by symptoms such as fever and renal tenderness. Asymptomatic bacteriuria (ASB) is significant bacteriuria without symptoms of a UTI (Schnarr 2008; Sobel 2014).
A UTI during pregnancy may be a serious complication as it is associated with adverse pregnancy outcomes for both mother and child (Anderson 2007; Bánhidy 2007; Dimetry 2007; Savage 1967; Schieve 1994; Sheiner 2009; Vazquez 2011). Important complications include preterm birth and small‐for‐gestational‐age babies (Dimetry 2007; Lang 1996; Mazor‐Dray 2009), although an association between UTI and preterm birth and small‐for‐gestational‐age babies has not been clearly established (Bánhidy 2007; Chen 2010; Dimetry 2007; Mann 2009; Mazor‐Dray 2009). Associations seen between UTI and adverse pregnancy outcomes in older studies (before the 1970s) may no longer be as evident with the advent of more antibiotic prescriptions (Bánhidy 2007; Savage 1967). While causal mechanisms remain unknown, there is evidence supporting the important role that prostaglandins, stimulated by bacterial and host signals following an infection, play in inducing preterm labour (Olson 2003; Romero 1988).
The exact incidence of symptomatic UTI in women who are pregnant is unknown (Gilstrap 2001). Two studies report an incidence between 1% to 2.3% during pregnancy for their particular population (Harris 1981; Mazor‐Dray 2009). Pyelonephritis (infection of the kidney) occurs in 2% of pregnancies, with a recurrence rate up to 23% within the same pregnancy or soon after the birth (Gilstrap 1981; McCormick 2008).
Some international guidelines recommend screening and treating ASB in women who are pregnant to prevent UTI and possible adverse pregnancy outcomes (U.S. Preventive Services Task Force 2008). This policy is followed by many countries and might have had an impact on the recently described incidences of both UTI and RUTI during pregnancy.
Varying definitions of RUTI exist, especially in pregnant women. In non‐pregnant women RUTI is frequently defined as three episodes of UTI in the previous 12 months, or two episodes in the last six months (Epp 2010; Foster 2008; Gopal 2007). For this review we used the following criteria for RUTI: pregnant women with a history of one or more UTI before or during pregnancy. We decided to include women with only one UTI as well because one UTI during pregnancy can be a reason to start prophylaxis during pregnancy both in practice and for research trials. Most UTI recurrences occur in the first three months following the initial infection (Foxman 1990). In studies on RUTI in pregnant women, one episode of UTI during pregnancy is often an indication to start prophylaxis to prevent RUTI (Harris 1974; Pfau 1992).
Uropathogens, generally originating in the rectal flora, may cause a UTI when they ascend to the bladder after they colonise the urethra and the periurethral area. The pathogenesis of a UTI in women who are suffering from RUTI is considered comparable with a single infection in women without a history of RUTI (Hooton 2010; Kodner 2010). In RUTI, uropathogens possibly recolonise the bladder after treatment because they are not eliminated from the rectal flora (Hooton 2001). E. coli is the most common UTI uropathogen (Kodner 2010; Sobel 2014). Particularly in the presence of structural abnormalities of the urinary tract, the following organisms are associated with RUTI: Proteus, Pseudomonas, Klebsiella and by Enterobacter spp. and enterococci and staphylococci (Sobel 2014).
There are four patterns of response of bacteriuria to therapy: cure, bacteriologic persistence, bacteriologic relapse or reinfection. Bacteriologic persistence is persistence of bacteriuria with the same microorganism after 48 hours of treatment (Sobel 2014). Relapse is an infection with the same microorganism that caused initial infection and usually occurs within one to two weeks after the cessation of treatment. A relapse indicates that the infecting organism has persisted in the urinary tract. Reinfection is an infection after sterilisation of the urine. Most of the time there is a change in bacterial species. Reinfection can be defined as a 'true' recurrence. Both persistence and relapse may be related to inadequate treatment (Hooton 2010; Sobel 2014). Although relapse and reinfection are two distinct outcomes, they both can be grouped under the wider outcome of recurrence.
During pregnancy, up to 90% of the women develop dilatation of the collecting system (ureters and renal pelvis) and decreased peristalsis of the ureters and bladder, which may facilitate bacterial colonisation and ascending infection due to urinary stasis (Brown 1991; Grenier 2000; McCormick 2008).
The main risk factors for RUTI in premenopausal women are: the age at first UTI (less than 15 years of age indicates a greater risk of RUTI), a family history of UTI in their mother, frequency of sexual intercourse, the use of spermicides and new sexual partners (Hooton 1996; Hooton 2001; Perotta 2008; Scholes 2000). In women who are pregnant, a high parity is a risk factor for UTI (Dwyer 2002; Haider 2010).
Description of the intervention
Interventions used to prevent RUTI in pregnant women can be pharmacological or non‐pharmacological. Pharmacological interventions consist of antibiotics that may be prescribed in different ways to prevent RUTI, continuous prophylaxis, post‐coital prophylaxis and patient‐initiated therapy based on symptoms of a UTI. The non‐pharmacological interventions include cranberries (juice or tablets), probiotics, acupuncture and behavioural modifications such as frequent and complete voiding, voiding after sexual intercourse, liberal fluid intake, and wiping techniques. Other potential interventions, such as vaccines and bacterial interference where one bacterial strain prevents colonisation with another strain and topical application of carbohydrates, are still under development (Epp 2010).
How the intervention might work
Various antibiotic regimens, used as a continuous or as post‐coital prophylaxis, reduce the number of RUTI in women who are not pregnant (Albert 2008; Hooton 2010; Pfau 1992). The effect of post‐coital prophylaxis is related to frequency of sexual intercourse and mostly results in less antibiotic use in comparison with daily prophylaxis (Hooton 2001; Hooton 2010). Antibiotics may cause adverse effects such gastrointestinal symptoms and vaginal and oral candidiasis (Albert 2008; Epp 2010). Furthermore, not all antibiotics used as prophylaxis for RUTI in non‐pregnant women may be safe during pregnancy. Because of this, women who are pregnant often prefer not to use antibiotics during their pregnancy. In addition, the number of drug‐resistant bacteria is increasing, which may influence the potential prophylactic effect of different antibiotics in the future. Different antibiotics such as nitrofurantoin, amoxicillin and fosfomycin have been used to treat primary UTI in women who are pregnant (Vazquez 2011). Antibiotic effect depends on the concentration of the antimicrobial agent achieved in the urine in conjunction with the sensitivity of the organism(s) to that antibiotic (Sobel 2014).
Cranberry products (mainly juice) have been used as an intervention to prevent RUTI for decades. It has been shown in vitro that cranberries prevent bacteria adhering to the uro‐epithelial cells in the bladder (Jepson 2012; Zafiri 1989). Without adhesion the bacteria are unable to cause a UTI (Jepson 2012; Zafiri 1989). In some of the published studies on cranberries in pregnant and non‐pregnant women, there have been significant withdrawals or losses to follow‐up (Jepson 2012). Nausea and vomiting due to physiologic changes in pregnancy can further decrease adherence (Wing 2008). A trial in non‐pregnant premenopausal women showed that antibiotics (trimethoprim‐sulfamethoxazole) once daily is more effective in preventing RUTI than cranberry capsules twice daily, at the expense of emerging antibiotic resistance (Beerepoot 2011). Finally a recent cochrane review on 'Cranberries for preventing urinary tract infections' that included two studies in pregnant women concluded that more studies to assess the effectiveness of cranberry juice need ‘strong justification’ since the benefit is likely to be small especially in combination with poor adherence. Only in women with RUTI more studies of other cranberry products such as tablets and capsules may be useful (Jepson 2012).
Two small randomised controlled trials (with unclear risk of selection bias) have compared acupuncture with no treatment to prevent RUTI in women who are not pregnant. Both showed significant results in preventing RUTI (Alreak 2002; Aune 1998).
It is suggested that some Lactobacillus species prevent uropathogen colonisation of the vagina, a necessary step in ascending infection of the bladder. Studies show that certain Lactobacillus species can be given orally or vaginally and reduce RUTI through colonisation of the vagina and reducing vaginal coliform counts (Czaja 2007; Reid 2003). In postmenopausal women the use of lactobacilli capsules twice daily seems nearly as effective in preventing RUTI as the use of antibiotics once daily, without increase of antibiotic resistance (Beerepoot 2012).
Although behavioural modifications are unlikely to be harmful in women who are not pregnant, little information is available that these interventions actually work. Sexually active women who use spermicide while suffering from RUTI are recommended to use an alternative form of contraception (Epp 2010). Spermicide use increases the risk of colonisation of the vaginal and periurethral area with uropathogens and increases the adherence of E. coli to vaginal epithelial cells (Sobel 2014). Behavioural modifications may often be combined with other interventions (Epp 2010).
Why it is important to do this review
There are two Cochrane reviews on prevention of UTI, both in women. (Albert 2008; Jepson 2012). One of these reviews included two studies in pregnant women (Jepson 2012). The results described in the Cochrane review 'Antibiotics for preventing recurrent urinary tract infections in non‐pregnant women' show that continuous antibiotic prophylaxis for six to 12 months reduced the rate of UTI during prophylaxis when compared with placebo. However, women who used antibiotic prophylaxis had more adverse effects (Albert 2008). The results described in the updated Cochrane review 'Cranberries for preventing urinary tract infections' demonstrate that cranberry juice was not as effective as previously indicated and did not decrease the number of symptomatic UTIs over a 12‐month period. Besides, the authors conclude that cranberry juice may not be acceptable over long periods of time because there were large numbers of dropouts (Jepson 2012). A Cochrane protocol on 'Probiotics for preventing urinary tract infections in adults and children' will include studies in women who are pregnant (Schwenger 2010).
Preterm birth, one of the possible serious complications of a UTI during pregnancy, is the main cause of neonatal mortality and morbidity worldwide. The costs of preterm birth are enormous. These costs are mainly associated with intensive care for the neonates (Armstrong 2007; Clements 2007; Gilbert 2003). Prevention of RUTI and UTI will improve maternal and infant health and reduce the risk of preterm birth.
Different approaches have been proposed for prevention of RUTI in women who are not pregnant and include the use of low‐dose antibiotic prophylaxis daily or post‐coitally in sexually active women and non‐pharmacological therapies such as voiding after sexual intercourse or ingestion of cranberry juice (Albert 2008). Little is known about the best way to prevent RUTI in pregnant women, especially as not all approaches used in non‐pregnant women are applicable. Therefore, it is important to define the optimal interventions for preventing RUTI during pregnancy to improve pregnancy outcomes.
Objectives
To assess the effects of interventions for preventing recurrent urinary tract infections in pregnant women.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include all published, unpublished and ongoing randomised controlled trials (RCTs), quasi‐RCTs and clustered‐randomised trials of interventions aimed at preventing recurrent urinary tract infections (RUTI) during pregnancy. In future updates we will also include abstracts where sufficient information is available. Abstracts containing limited information will be classified as 'awaiting assessment' until further information can be obtained.
Types of participants
Pregnant women with a history of one or more urinary tract infections (UTI) before or during pregnancy.
Types of interventions
Any intervention (pharmacological and non‐pharmacological) for preventing recurrent urinary tract infection (RUTI) during pregnancy (compared with another intervention, placebo or with usual care).
Types of outcome measures
Primary outcomes
Maternal
RUTI before birth (variously defined e.g. recurrent pyelonephritis, recurrent cystitis)
Preterm birth (less than 37 weeks)
Infant
Small‐for‐gestational age
Total mortality (including stillbirth and babies born alive who die prior to primary hospital discharge)
Secondary outcomes
Recurrences
Proportion of pregnant women who experienced at least one UTI, identified using clinical criteria (dysuria)
Proportion of pregnant women who experienced at least one UTI, using microbiological criteria
Number of UTI per woman during index pregnancy, identified using clinical criteria (e.g. dysuria, fever)
Number of UTI per woman during index pregnancy, using microbiological criteria
Number of pregnant women who were admitted antenatally because of a UTI
Pregnancy and delivery (complications)
Maternal
Maternal death
Miscarriage
Antenatal pyrexia requiring the use of antibiotics
Asymptomatic bacteriuria (ASB) (variously defined)
Prelabour rupture of the membranes
Eclampsia/pre‐eclampsia (variously defined)
Induction of labour
Mode of birth (normal vaginal birth, operative vaginal birth, caesarean section)
Intrapartum fever requiring the use of antibiotics
Postpartum infection requiring the use of antibiotics
Postpartum haemorrhage
Chorioamnionitis (variously defined)
Postpartum fever requiring the use of antibiotics
Adverse effects of interventions (nausea, vomiting, diarrhoea)
Proportion of women who had severe adverse effects (defined as those requiring withdrawal of treatment)
Women's satisfaction with treatment
Infants
Stillbirths (variously defined)
Death of liveborn infants prior to hospital discharge
Gestational age at birth
Preterm birth less than 34 weeks’ gestation
Birthweight
Birthweight < 2500 (g) (not prespecified)
Birth centile (below 10th centile)
Small‐for‐gestational age
Five‐minute Apgar score less than seven
Chronic lung disease (variously defined)
Intraventricular haemorrhage (variously defined)
Periventricular leukomalacia
Necrotising enterocolitis (variously defined)
Respiratory distress syndrome (variously defined)
Hyperbilirubinaemia requiring treatment
Neonatal convulsions
Early neonatal infection requiring antibiotics (less than 48 hours)
Hypoxic ischaemic encephalopathy
Neonatal encephalopathy
Composite of severe neonatal morbidity (variously defined)
Use of resources, e.g. and/or costs utilisation
Antenatal admission of the mother
Days of antenatal admission of the mother
Admission to a neonatal intensive care unit
Days of admission to a neonatal intensive care unit
Admission to nursery care
Costs of interventions
Additional visits to clinicians
Costs to women and families for extra care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (20 May 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
In addition, we searched the reference lists of retrieved articles We did not apply any language or date restrictions to the search and in future updates, we will attempt to obtain translations of papers when necessary.
Data collection and analysis
For methods used in the previous version of this review, seeSchneeberger 2012.
Assessment of the quality of the evidence
For this update, no new reports were identified for assessment but we assessed the quality of evidence of the existing study using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the comparison 'nitrofurantoin and close surveillance versus close surveillance alone'.
Recurrent pyelonephritis
Recurrent UTI (cystitis)
Preterm birth (≤ 37 weeks)
Birthweight < 2500 (g)
Birthweight (g)
Five‐minute Apgar score less than seven
Miscarriages
We used GRADEprofiler (GRADEpro 2014) to import data from Review Manager 5.3 (RevMan 2014) in order to create a 'Summary of findings' table. A summary of the intervention effect and a measure of quality for each of the above outcomes has been produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
In future updates, if new reports are identified, we will use the methods described in Appendix 1.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved two reports relating to one trial eligible for consideration. This trial (involving 200 women) was included in the review (Lenke 1983).
Included studies
Only one trial, performed in Los Angeles, USA with 200 pregnant women was identified that met the inclusion criteria (Lenke 1983). In this study, nitrofurantoin 50 mg three times daily and close surveillance (regular clinic visit, urine cultures and antibiotics when a positive culture was found) was compared with close surveillance only to prevent recurrent urinary tract infections (RUTI) in women who were pregnant and were admitted for pyelonephritis earlier during the index pregnancy. Close surveillance consisted of a visit every two weeks to a special clinic and after 36 weeks, a weekly visit until birth. At each visit a clean‐catch, mid voided urine was obtained for a routine culture and nitrite testing. When necessary, treatment was provided.
Excluded studies
There are no excluded studies.
Risk of bias in included studies
See Figure 1 and Figure 2 for a summary of the 'Risk of bias' assessment.
1.
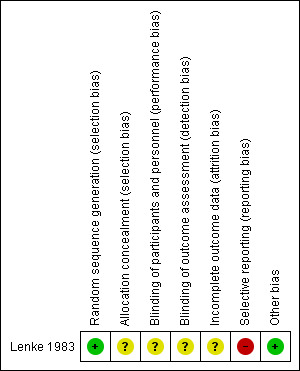
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
2.
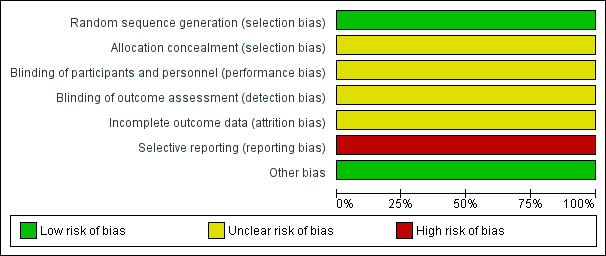
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In the one included study (Lenke 1983), a random number table was used to generate the sequence, which we considered a low risk of bias. The method of treatment allocation was unclear.
Blinding
No placebos were used and the care providers and the participants were not blinded. The 'Risk of bias' assessment was considered low for the culture results and delivery outcomes. Overall, we considered the risk of bias for performance and detection bias to be unclear.
Incomplete outcome data
There were 18 post‐randomisation losses to follow‐up in the nitrofurantoin and close surveillance group and 15 in close surveillance only group. No data about post randomisation data exclusions were reported. The outcome birthweight of infants was not available for 11 (13.4%) women in the nitrofurantoin group and close surveillance and for nine (10.6%) in the close surveillance only group. The outcomes of birthweight < 2500 (g), preterm birth (before 37 weeks) and five‐minute Apgar score less than seven were not available in nine (11.0%) of the women who received nitrofurantoin and close surveillance and 11 (12.9%) of the women who received close surveillance only.
Selective reporting
No data were reported on the following primary outcomes: total infant mortality and small‐for‐gestational‐age babies. Furthermore, only a small number of secondary outcomes were reported. Overall, we considered this domain to have a high risk of bias.
Other potential sources of bias
No obvious risk of other potential sources of bias for the included studies was apparent.
Effects of interventions
See: Table 1
This review included one trial (Lenke 1983) involving 200 women.
Nitrofurantoin and close surveillance versus close surveillance alone
Primary outcomes
Lenke 1983 found no differences in women who developed recurrent pyelonephritis (upper UTI) (risk ratio (RR) 0.89, 95% confidence interval (CI) 0.31 to 2.53; one study, 167 women) (Analysis 1.1), or RUTI before birth (RR 0.30, 95% CI 0.06 to 1.38; one study, 167 women) (Analysis 1.2), and preterm birth (before 37 weeks) (RR 1.18, 95% CI 0.42 to 3.35; one study, 147 women) (Analysis 1.3) between nitrofurantoin and close surveillance and close surveillance only. Data on total mortality and small‐for‐gestational‐age babies were not reported.
1.1. Analysis.
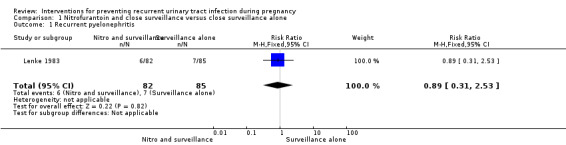
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 1 Recurrent pyelonephritis.
1.2. Analysis.
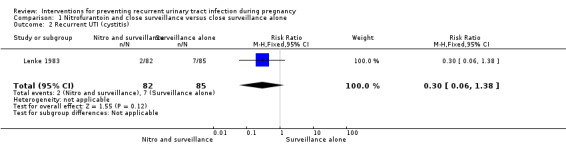
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 2 Recurrent UTI (cystitis).
1.3. Analysis.
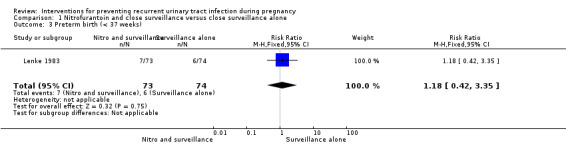
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 3 Preterm birth (< 37 weeks).
Secondary outcomes
The only secondary outcomes reported were birthweight less than 2500 (g) (RR 2.03, 95% CI 0.53 to 7.80; one study, 147 infants) (Analysis 1.4), birthweight (mean difference (MD) ‐113.00, 95% CI ‐327.20 to 101.20; one study, 147 infants) (Analysis 1.5), five‐minute Apgar score less than seven (RR 2.03, 95% CI 0.19 to 21.87; one study, 147 infants) (Analysis 1.6), and miscarriages (RR 3.11, 95% CI 0.33 to 29.29; one study, 167 women) (Analysis 1.7). There were no significant differences between the two comparison groups for any of these outcomes.
1.4. Analysis.
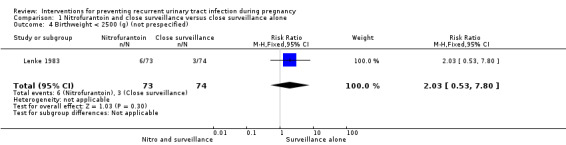
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 4 Birthweight < 2500 (g) (not prespecified).
1.5. Analysis.
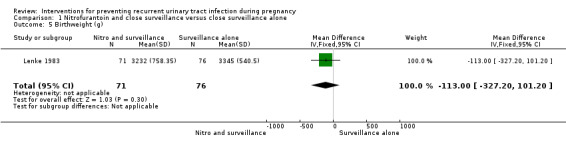
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 5 Birthweight (g).
1.6. Analysis.
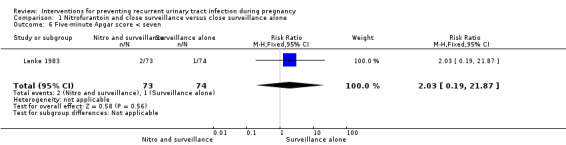
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 6 Five‐minute Apgar score < seven.
1.7. Analysis.
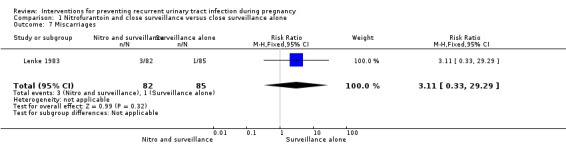
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 7 Miscarriages.
In women who received nitrofurantoin and close surveillance, the incidence of ASB defined as positive cultures with at least 103 colonies per mL is only reported in women with more than 90% clinic attendance rate (RR 0.55, 95% CI 0.34 to 0.89; one study, 102 women) (Analysis 1.8) and showed a significant reduction of asymptomatic positive cultures for women in the nitrofurantoin and close surveillance versus close surveillance alone. No symptomatic recurrences were seen in women with more than 90% clinic attendance rate.
1.8. Analysis.
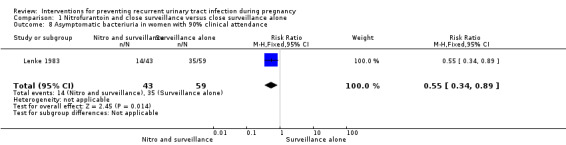
Comparison 1 Nitrofurantoin and close surveillance versus close surveillance alone, Outcome 8 Asymptomatic bacteriuria in women with 90% clinical attendance.
Several secondary outcomes including stillbirth and maternal deaths were not reported.
Discussion
Summary of main results
In this review we found no significant differences between a combination of suppressive therapy with a daily dose of nitrofurantoin and close surveillance and close surveillance alone in preventing recurrent urinary tract infections (RUTI). Only sub‐analyses in women with more than 90% follow‐up show a decreased incidence of asymptomatic bacteriuria (ASB) in women who received nitrofurantoin and close surveillance compared with close surveillance only. Since only one study was included no other interventions to prevent RUTI in pregnant women were assessed.
Overall completeness and applicability of evidence
The evidence for interventions preventing RUTI in pregnant women is incomplete. This review included only one relatively old (1983) trial involving 200 pregnant women with limited reporting of primary and secondary outcomes for both women and infants. Due to lack of randomised controlled trials (RCTs), no conclusions can be drawn regarding the optimal intervention to prevent RUTI in women who are pregnant.
Quality of the evidence
The included trial had moderate to high risk of bias. GradePro software was used to assess the quality of evidence for the main comparison 'nitrofurantoin and close surveillance versus close surveillance alone' for the outcomes listed above.The evidence was of very low quality for all outcomes "recurrent pyelonephritis, RUTI (cystitis), preterm birth (≤ 37 weeks), birthweight < 2500 (g), birthweight (g), five‐minute Apgar score less than seven, and miscarriages". Downgrading of evidence was based on including one small study with design limitations and imprecise results 'wide confidence interval (CI) crossing the line of no effect'.
Potential biases in the review process
Data extraction and assessment of risk of the included study was independently performed by two authors to minimise bias. A third review author was contacted when consensus was not reached. This review only includes one study therefore all conclusions need to be considered with caution. We are not aware of other potential biases in the review process.
Agreements and disagreements with other studies or reviews
Following the results of this review, suppressive therapy with a daily dose of nitrofurantoin and close surveillance has not been shown to prevent RUTI compared with close surveillance alone. These results are not consistent with a Cochrane review on antibiotics to prevent urinary tract infection (UTI) in women who are not pregnant, which showed that continuous antibiotic prophylaxis for six to 12 months reduced the rate of UTI during prophylaxis when compared with placebo (Albert 2008). In the latter review, the authors did not compare antibiotics with non‐pharmacological interventions such as close surveillance. Moreover, more adverse effects were seen in the antibiotic group including vaginal itching and nausea. These side effects are not desirable in pregnant women since both are already more frequent during pregnancy.
Little is known about the effect of close surveillance on preventing RUTI. Lenke 1983 reported that all of the symptomatic recurrences occurred in patients who either had poor clinic attendance and subsequent lack of follow‐up urine cultures or were not treated when gram‐negative organisms (mainly uropathogens) were found in their urine. These results explain that close surveillance itself already may have an effect on preventing RUTI in pregnant women.
Authors' conclusions
Implications for practice.
This review found that daily dose of nitrofurantoin and close surveillance was not more likely to prevent RUTI compared with close surveillance alone. However, a significant reduction in asymptomatic bacteriuria was found in women with a clinic attendance rate of more than 90% and who received nitrofurantoin and close surveillance. It is important to note that the results of this review were based on only one small trial with limited reporting of primary and secondary outcomes in both mother and child. Due to the lack of randomised controlled trials no conclusions can be drawn.
Implications for research.
It is important to have a standard definition for RUTI in women who are pregnant. Since pregnancy is a limited period during which a UTI may be associated with increased risks for both mother and baby, the definition for RUTI should be adapted for pregnant women. A possible definition of RUTI in pregnant women may be: at least one UTI during the current pregnancy or either three UTI in the 12 months or two in six months before onset of pregnancy.
Further large trials (with sufficient power) comparing different pharmacological and non‐pharmacological interventions are needed to assess the optimal intervention to prevent RUTI in women who are pregnant. Such trials should report on a broad range outcomes for both women and infants. Given the significant differences found in the greater than 90% follow‐up group, future trials should further asses the effects of close surveillance on preventing RUTI in pregnant women
What's new
| Date | Event | Description |
|---|---|---|
| 9 April 2015 | New citation required but conclusions have not changed | Review updated. |
| 9 April 2015 | New search has been performed | Search updated. No new reports identified. A 'Summary of findings' table has been incorporated. Background updated. |
Acknowledgements
Professor Ronald Stolk and Dr Jan Jaap Erwich for their continued advice and support.
The editorial team of the Cochrane Pregnancy and Childbirth Group in Liverpool, UK.
We would like to thank Nasreen Aflaifel for her support in the creation of the 'Summary of findings' table for the 2015 update. Nasreen Aflaifel's work was financially supported by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Methods to be used in future updates
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors will independently assess for inclusion all the potential studies identified as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third review author.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult the third review author. We will enter data into Review Manager software (RevMan 2014) and check for accuracy. When information regarding any of the above is unclear, we plan to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement will be resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We will describe for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported, or could have been supplied by the trial authors, we plan to re‐include missing data in the analyses which we undertake.
We will assess methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We will describe for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We will make explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we plan to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
We will use the mean difference if outcomes were measured in the same way between trials. We will use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust either their sample sizes or standard errors using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
It is unlikely that cross‐over designs will be a valid study design for Pregnancy and Childbirth reviews and so, if identified, we will exclude them.
Dealing with missing data
For included studies, we will note levels of attrition. If more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, that is, we will attempt to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We will regard heterogeneity as substantial if an I² is greater than 30% and either a Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identify substantial heterogeneity (above 30%), we plan to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar.
If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we used random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use random‐effects analysis to produce it.
We will carry out the following subgroup analyses.
Type of bacteriuria: asymptomatic bacteriuria (without symptoms) versus UTI (with symptoms) versus pyelonephritis (requiring hospitalisation). We wish to test whether results differ when UTI is variously defined, according to the severity of the condition and the presence of symptoms.
Definition of RUTI: history of RUTI before pregnancy versus no history of RUTI before pregnancy. We wish to test whether results differ when women already have a history of RUTI before their pregnancy.
Gestational age at which the intervention was started before 20 weeks versus equal to or greater than 20 weeks. We wish to test whether the effects of the interventions are different according to the stage of pregnancy in which they were started.
Types of interventions: pharmacological versus non‐pharmacological. We wish to test whether the results differ between pharmacological and non‐pharmacological interventions.
The following outcomes will be used in subgroup analyses.
Maternal
Recurrent urinary tract infections (RUTI) before birth (variously defined)
Preterm birth (less than 37 weeks)
Infant
Small‐for‐gestational age
Total mortality (including stillbirth and babies born alive who die prior to primary hospital discharge)
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We plan to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Data and analyses
Comparison 1. Nitrofurantoin and close surveillance versus close surveillance alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent pyelonephritis | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.31, 2.53] |
| 2 Recurrent UTI (cystitis) | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.06, 1.38] |
| 3 Preterm birth (< 37 weeks) | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.42, 3.35] |
| 4 Birthweight < 2500 (g) (not prespecified) | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.53, 7.80] |
| 5 Birthweight (g) | 1 | 147 | Mean Difference (IV, Fixed, 95% CI) | ‐113.0 [‐327.20, 101.20] |
| 6 Five‐minute Apgar score < seven | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [0.19, 21.87] |
| 7 Miscarriages | 1 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.33, 29.29] |
| 8 Asymptomatic bacteriuria in women with 90% clinical attendance | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.34, 0.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lenke 1983.
| Methods | RCT. | |
| Participants | Number of pregnant women randomised: 200. Inclusion criteria
Exclusion criteria
Setting: Los Angeles, USA. Period: October 1979 ‐ May 1981. Definitions
|
|
| Interventions |
Intervention group (n = 100): Nitrofurantoin 50 mg orally, 3 times daily, for the remainder of the pregnancy plus close surveillance. Control group (n = 100): close surveillance only. ALL WOMEN Follow‐up (close surveillance): all patients were followed in the special clinic every 2 weeks until the 36 weeks when they were seen weekly until delivery. At each visit a clean‐catch, mid voided urine was obtained for a routine culture and nitrite testing. When culture results were positive, attempts were made to reach patients to schedule a return appointment within 1 week. Treatment: irrespective of group, patients received a short course of antibiotics in clinic under 3 circumstances:
|
|
| Outcomes | Maternal
Infants
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random number tables." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant: no blinding. Clinician: no blinding. Describe: “the control group received no pills” “ the doctors responsible for patient care were aware of whether the patient was in the treated or control group”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low for culture results. Low for pregnancy outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Lost to follow‐up:
No reasons given. |
| Selective reporting (reporting bias) | High risk | Overall, very few pregnancy outcomes were measured. "observation period ended at the time of delivery, as logistics prevented longer follow‐up". |
| Other bias | Low risk | No major baseline differences. |
CVA: costovertebral angle mL: millilitre RCT: randomised controlled trials UTI: urinary tract infection
Differences between protocol and review
We did not seek unpublished trials by contacting experts in the field or scan the Internet and abstracts submitted to major international congresses as stated in our published protocol. We added birthweight < 2500 (g) to the list of outcomes since this is commonly used primary outcome especially in older studies.
A 'Summary of findings' table was added for this 2015 update.
Contributions of authors
The protocol was drafted jointly by Caroline Schneeberger, Suzanne Geerlings, Caroline A Crowther and Philippa Middleton. Caroline Schneeberger is guarantor for the review.
For the 2015 update, all authors were involved
Sources of support
Internal sources
ARCH, Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
University Medical Center Groningen (UMCG), Netherlands.
External sources
Australian Department of Health and Ageing, Australia.
UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Caroline Schneeberger, Caroline A Crowther and Philippa Middleton: none known.
Suzanne E Geerlings is project leader of the following study: Non‐antibiotic versus antibiotic prophylaxis for recurrent urinary tract infections (NAPRUTI)‐study. Trial Number: ISRCTN50717094. This study did not include pregnant women. For this study, placebo lactobacilli capsules were donated by Chr Hansen, Denmark. Cranberry capsules and placebo capsules for the study were provided by Springfield Nutraceuticals, Oud Beijerland, the Netherlands.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Lenke 1983 {published data only}
- Lenke RR, Dorsten JP, Schifrin BS. Pyelonephritis in pregnancy: a prospective randomized trial to prevent recurrent disease evaluating suppressive therapy with nitrofurantoin and close surveillance. American Journal of Obstetrics and Gynecology 1983;146:953‐7. [DOI] [PubMed] [Google Scholar]
- Dorsten JP, Lenke RR, Schifrin BS. Pyelonephritis in pregnancy. The role of in‐hospital management and nitrofurantoin suppression. Journal of Reproductive Medicine 1987;32:895‐900. [PubMed] [Google Scholar]
Additional references
Albert 2008
- Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non‐pregnant women. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD001209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alreak 2002
- Alraek T, Soedal LI, Fagerheim SU, Digranes A, Baerheim A. Acupuncture treatment in the prevention of uncomplicated recurrent lower urinary tract infections in adult women. American Journal of Public Health 2002;92(10):1609‐11. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2007
- Anderson BL, Simhan HN, Simons KM, Wiesenfeld HC. Untreated asymptomatic group B streptococcal bacteriuria early in pregnancy and chorioamnionitis at delivery. American Journal of Obstetrics and Gynecology 2007;196(6):524.e1‐524.e5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Armstrong 2007
- Armstrong J, Meis PJ. Clinical, family, and cost outcomes of preterm births: an overview of the problem and prevention opportunities. Journal of Clinical Outcomes Management 2007;14(10):547‐53. [Google Scholar]
Aune 1998
- Aune A, Alraek T, LiHua H, Baerheim A. Acupuncture in the prophylaxis of recurrent lower urinary tract infection in adult women. Scandinavian Journal of Primary Health Care 1998;16(1):37‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beerepoot 2011
- Beerepoot MA, ter Riet G, Nys S, Wal WM, Borgie CA, Reijke TM, et al. Cranberries vs antibiotics to prevent urinary tract infections: a randomized double‐blind noninferiority trial in premenopausal women. Archives of Internal Medicine 2011;171(14):1270‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Beerepoot 2012
- Beerepoot MA, ter Riet G, Nys S, Wal WM, Borgie CA, Reijke TM, et al. Lactobacilli vs antibiotics to prevent urinary tract Infections: a randomized, double‐blind, noninferiority trial in postmenopausal women. Archives of Internal Medicine 2012;172(9):704‐12. [DOI] [PubMed] [Google Scholar]
Brown 1991
- Brown MA. Urinary tract dilatation in pregnancy. American Journal of Obstetrics and Gynecology 1991;164(2):642‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bánhidy 2007
- Bánhidy F, Acs N, Puhó EH, Czeizel AE. Pregnancy complications and birth outcomes of pregnant women with urinary tract infections and related drug treatments. Scandinavian Journal of Infectious Diseases 2007;39(5):390‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2010
- Chen YK, Chen SF, Li HC, Lin HC. No increased risk of adverse pregnancy outcomes in women with urinary tract infections: a nationwide population‐based study. Acta Obstetricia et Gynecologica Scandinavica 2010;89(7):882‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Clements 2007
- Clements KM, Barfield WD, Ayadi MF, Wilber N. Preterm birth‐associated cost of early intervention services: an analysis by gestational age. Pediatrics 2007;119(4):e866‐e874. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Czaja 2007
- Czaja CA, Stapleton AE, Yarova‐Yarovaya Y, Stamm WE. Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infectious Diseases in Obstetrics and Gynecology 2007;2007:1‐8. [DOI: 10.1155/2007/35387; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dimetry 2007
- Dimetry SR, El‐Tokhy HM, Abdo NM, Ebrahim MA, Eissa M. Urinary tract infection and adverse outcome of pregnancy. Journal of the Egyptian Public Health Association 2007;82(3‐4):203‐18. [MEDLINE: ] [PubMed] [Google Scholar]
Dwyer 2002
- Dwyer PL, O'Reilly M. Recurrent urinary tract infection in the female. Current Opinion in Obstetrics & Gynecology 2002;14(5):537‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Epp 2010
- Epp A, Larochelle A, Lovatsis D, Walter JE, Easton W, Farrell SA, et al. Recurrent urinary tract infection. Journal of Obstetrics and Gynaecology Canada 2010;32(11):1082‐101. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foster 2008
- Foster RT Sr. Uncomplicated urinary tract infections in women. Obstetrics & Gynecology Clinics of North America 2008;35(2):235‐48. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Foxman 1990
- Foxman B. Recurring urinary tract infection: incidence and risk factors. American Journal of Public Health 1990;80(3):331‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2003
- Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: quantification by gestational age and birth weight. Obstetrics & Gynecology 2003;102(3):488‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gilstrap 1981
- Gilstrap LC 3rd, Cunningham FG, Whalley PJ. Acute pyelonephritis in pregnancy: a retrospective study. Obstetrics & Gynecology 1981;57(4):409‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Gilstrap 2001
- Gilstrap LC 3rd, Ramin SM. Urinary tract infections during pregnancy. Obstetrics and Gynecology Clinics of North America 2001;28(3):581‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gopal 2007
- Gopal M, Northington G, Arya L. Clinical symptoms predictive of recurrent urinary tract infections. American Journal of Obstetrics and Gynecology 2007;197(1):74.e1‐74.e4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Grenier 2000
- Grenier N, Pariente JL, Trillaud H, Soussotte C, Douws C. Dilatation of the collecting system during pregnancy: physiologic vs obstructive dilatation. European Radiology 2000;10(2):271‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haider 2010
- Haider G, Zehra N, Munir AA, Haider A. Risk factors of urinary tract infection in pregnancy. Journal of Pakistan Medical Association 2010;60(3):213‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Harris 1974
- Harris RE, Gilstrap LC 3rd. Prevention of recurrent pyelonephritis during pregnancy. Obstetrics & Gynecology 1974;44(5):637‐41. [MEDLINE: ] [PubMed] [Google Scholar]
Harris 1981
- Harris RE, Gilstrap LC 3rd. Cystitis during pregnancy: a distinct clinical entity. Obstetrics & Gynecology 1981;57(5):578‐80. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hooton 1996
- Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New England Journal of Medicine 1996;335(7):468‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hooton 2001
- Hooton TM. Recurrent urinary tract infection in women. International Journal of Antimicrobial Agents 2001;17(4):259‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hooton 2010
- Hooton TM. Recurrent urinary tract infections in women. UpToDate (http://www.uptodate.com/home/about/index.html) (accessed 2011) 2010:1‐16.
Jepson 2012
- Jepson RG, Williams G, Craig J. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001321.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kodner 2010
- Kodner CM, Thomas Gupton EK. Recurrent urinary tract infections in women: diagnosis and management. American Family Physician 2010;82(6):638‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Lang 1996
- Lang JM, Lieberman E, Cohen A. A comparison of risk factors for preterm labor and term small‐for‐gestational‐age birth. Epidemiology 1996;7(4):369‐76. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mabeck 1972
- Mabeck CE. Treatment of uncomplicated urinary tract infection in non‐pregnant women. Postgraduate Medical Journal 1972;48(556):69‐75. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Mann 2009
- Mann JR, McDermott S, Gregg A, Gill TJ. Maternal genitourinary infection and small for gestational age. American Journal of Perinatology 2009;26(9):667‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mazor‐Dray 2009
- Mazor‐Dray E, Levy A, Schlaeffer F, Sheiner E. Maternal urinary tract infection: is it independently associated with adverse pregnancy outcome?. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(2):124‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McCormick 2008
- McCormick T, Ashe RG, Kearney PM. Urinary tract infection in pregnancy. Obstetrician & Gynaecologist 2008;10(3):156‐62. [Google Scholar]
Olson 2003
- Olson DM. The role of prostaglandins in the initiation of parturition. Best Practice & Research Clinical Obstetrics & Gynaecology 2003;17(5):717‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perotta 2008
- Perotta C, Aznar M, Meija R, Albert X, Ng CW. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD005131.pub2] [DOI] [PubMed] [Google Scholar]
Pfau 1992
- Pfau A, Sacks TG. Effective prophylaxis for recurrent urinary tract infections during pregnancy. Clinical Infectious Diseases 1992;14(4):810‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reid 2003
- Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR‐1 and L. fermentum RC‐14 significantly alters vaginal flora: randomized, placebo‐controlled trial in 64 healthy women. FEMS Immunology and Medical Microbiology 2003;35(2):131‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romero 1988
- Romero R, Mazor M. Infection and preterm labor. Clinical Obstetrics and Gynecology 1988;31(3):553‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Savage 1967
- Savage WE, Hajj SN, Kass EH. Demographic and prognostic characteristics of bacteriuria in pregnancy. Medicine 1967;46(5):385‐407. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schieve 1994
- Schieve LA, Handler A, Hershow R, Persky V, Davis F. Urinary tract infection during pregnancy: its association with maternal morbidity and perinatal outcome. American Journal of Public Health 1994;84(3):405‐10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnarr 2008
- Schnarr J, Smaill F. Asymptomatic bacteriuria and symptomatic urinary tract infections in pregnancy. European Journal of Clinical Investigation 2008;38 Suppl 2:50‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Scholes 2000
- Scholes D, Hooton TM, Roberts PL, Stapleton AE, Gupta K, Stamm WE. Risk factors for recurrent urinary tract infection in young women. Journal of Infectious Diseases 2000;182(4):1177‐82. [PUBMED: 10979915] [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Schwenger 2010
- Schwenger EM, Tejani AM, Loewen PS. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD008772] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheiner 2009
- Sheiner E, Mazor‐Drey E, Levy A. Asymptomatic bacteriuria during pregnancy. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(5):423‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sobel 2014
- Sobel JD, Kaye D. Urinary tract infections. In: Bennett JE, Dolin R, Blaser MJ editor(s). Principles and Practice of Infectious Disease. 8. Vol. 1, Philadephia: Churchill Livingstone Elsevier, 2014:886‐914. [Google Scholar]
U.S. Preventive Services Task Force 2008
- U.S. Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults: U.S. Preventive Services Task Force reaffirmation recommendation statement. Annals of Internal Medicine 2008;149(1):43‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vazquez 2011
- Vazquez JC, Abalos E. Treatments for symptomatic urinary tract infections during pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD002256.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 2008
- Wing DA, Rumney PJ, Preslicka CW, Chung JH. Daily cranberry juice for the prevention of asymptomatic bacteriuria in pregnancy: a randomized, controlled pilot study. Journal of Urology 2008;180(4):1367‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zafiri 1989
- Zafriri D, Ofek I, Adar R, Pocino M, Sharon N. Inhibitory activity of cranberry juice on adherence of type 1 and type P fimbriated Escherichia coli to eucaryotic cells. Antimicrobial Agents and Chemotherapy 1989;33(1):92‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Schneeberger 2012
- Schneeberger C, Geerlings SE, Middleton P, Crowther CA. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD009279.pub2] [DOI] [PubMed] [Google Scholar]


