Abstract
Background
It has been suggested that the severity of autism spectrum disorder (ASD) symptoms is positively correlated with the level of circulating or stored toxic metals, and that excretion of these heavy metals, brought about by the use of pharmaceutical chelating agents, results in improved symptoms.
Objectives
To assess the potential benefits and adverse effects of pharmaceutical chelating agents (referred to as chelation therapy throughout this review) for autism spectrum disorder (ASD) symptoms.
Search methods
We searched the following databases on 6 November 2014: CENTRAL, Ovid MEDLINE, Ovid MEDLINE In‐Process, Embase, PsycINFO, Cumulative Index to Nursing and Allied Health Literature (CINAHL) and 15 other databases, including three trials registers. In addition we checked references lists and contacted experts.
Selection criteria
All randomised controlled trials of pharmaceutical chelating agents compared with placebo in individuals with ASD.
Data collection and analysis
Two review authors independently selected studies, assessed them for risk of bias and extracted relevant data. We did not conduct a meta‐analysis, as only one study was included.
Main results
We excluded nine studies because they were non‐randomised trials or were withdrawn before enrolment. We included one study, which was conducted in two phases. During the first phase of the study, 77 children with ASD were randomly assigned to receive seven days of glutathione lotion or placebo lotion, followed by three days of oral dimercaptosuccinic acid (DMSA). Forty‐nine children who were found to be high excreters of heavy metals during phase one continued on to phase two to receive three days of oral DMSA or placebo followed by 11 days off, with the cycle repeated up to six times. The second phase thus assessed the effectiveness of multiple doses of oral DMSA compared with placebo in children who were high excreters of heavy metals and who received a three‐day course of oral DMSA. Overall, no evidence suggests that multiple rounds of oral DMSA had an effect on ASD symptoms.
Authors' conclusions
This review included data from only one study, which had methodological limitations. As such, no clinical trial evidence was found to suggest that pharmaceutical chelation is an effective intervention for ASD. Given prior reports of serious adverse events, such as hypocalcaemia, renal impairment and reported death, the risks of using chelation for ASD currently outweigh proven benefits. Before further trials are conducted, evidence that supports a causal link between heavy metals and autism and methods that ensure the safety of participants are needed.
Plain language summary
Chelation for autism spectrum disorder (ASD)
Background
Autism spectrum disorders (ASD) are types of disorders characterised by difficulties in social interaction and communication, and restricted and repetitive behaviours. It has been suggested that increased levels of toxic metals result in more severe symptoms of ASD, and that excretion of these heavy metals brought about by use of pharmaceutical chelating agents (chemicals that are injected into the blood stream to bind to and remove toxic heavy metals from the body) may lead to improvement of symptoms.
Review question
The purpose of this review was to assess the evidence for the effects of pharmaceutical chelating agents for symptoms of ASD.
Study characteristics
We searched multiple databases to find studies that examined pharmaceutical chelating agents as treatment for ASD symptoms. We found only one randomised controlled trial that evaluated oral dimercaptosuccinic acid (DMSA) for ASD, but this trial did not use ideal methods for answering our question. The evidence is current to November 2014.
The trial that we found was conducted in two phases. During the first phase, 77 children with ASD were assigned randomly to receive seven days of glutathione lotion or placebo lotion, followed by three days of oral DMSA. Forty‐nine children who excreted high levels of heavy metals during phase one continued on to phase two to receive three days of oral DMSA or placebo followed by 11 days off, with the cycle repeated up to six times.
Key results
Results from the included study show that multiple rounds of oral DMSA did not have an effect on any of the ASD symptoms measured in children found to be high excreters who had already received three doses of a pharmaceutical chelating agent. Currently no clinical trial evidence suggests that pharmaceutical chelation is an effective intervention for ASD. Given prior reports of serious adverse events, such as changes to calcium levels in blood, kidney impairment and reported death, risks of using pharmaceutical chelating agents for ASD currently outweigh proven benefits.
Quality of the evidence
The quality of the evidence is poor, with only one study, which had methodological shortcomings, included in this review. These factors, when combined, preclude confidence in the findings. However, before further trials are conducted, more evidence is needed to show that heavy metals cause or worsen the severity of autism, and the safety of pharmaceutical chelating agents for participants must be established.
Background
Description of the condition
According to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), autism spectrum disorder (ASD) is defined as a set of pervasive neurodevelopmental conditions that are characterised by difficulties in social interaction and communication, and by the presence of restricted, repetitive behaviours (i.e. stereotypies) (APA 2013). Historically, Asperger’s disorder, autistic disorder, atypical autism and pervasive developmental disorder not otherwise specified (PDD NOS) were considered separate, diagnosable conditions that fall within the autism spectrum. However, the latest edition of the DSM (DSM‐5) (APA 2013) has replaced these diagnostic labels with one umbrella term: autism spectrum disorder (ASD).
The severity of ASD varies considerably from person to person, and great variability in symptoms and manifestations has been reported. Individuals with ASD have difficulty showing social‐emotional reciprocity (e.g. participating in reciprocal conversations, maintaining eye gaze), communicating verbally and non‐verbally, forming and maintaining relationships and understanding the social behaviour of others (Shattuck 2007; APA 2013). Individuals with ASD also exhibit preoccupations and restricted, repetitive patterns of interest and behaviours, which may include strong adherence to routines and stereotyped speech and motor movements (Lecavalier 2006; APA 2013). Some may present with behavioural symptoms (e.g. irritability, aggression, anxiety, self injury, hyperactivity); these features do not determine the diagnosis, rather they are co‐occurring symptoms. Specific causes of ASD are currently unknown. However, genetic factors and prenatal and perhaps postnatal environmental factors are believed to contribute to the onset of ASD, although the role of environmental triggers remains uncertain (Hallmayer 2011).
Many interventions are available for ASD, and although some, such as early intensive intervention, are effective in improving communication, social interaction and behaviours, none are capable of producing complete remission of all symptoms. Pharmacological interventions are often prescribed for individuals with ASD, primarily to target specific associated symptoms or co‐occurring features. Currently, however, no pharmacological interventions target the core symptoms of ASD. Individuals with ASD often use complementary and alternative medicine (CAM) too; approximately 75% of children with ASD use CAM (Hanson 2007). Examples of CAM used for ASD include exclusion diets, essential fatty acids, multi‐vitamins, acupuncture, auditory integration training and chelation therapy. To date, no consistent evidence indicates that CAM is an effective intervention for the core features and associated behaviours of ASD (Nye 2005; Millward 2008; Cheuk 2011; James 2011; Sinha 2011; Geretsegger 2014).
Description of the intervention
Chelation therapy involves administering a chelating substance that binds to heavy metals, such as lead and mercury, which then is excreted in urine. Pharmaceutical chelating substances (referred to as chelation therapy throughout this review) are approved for treating patients with heavy metal poisoning. However, they have also been used for unapproved reasons, including treatment of patients with Alzheimer’s disease, coronary heart disease and ASD (Ernst 2000; Dans 2002; Sinha 2006; Hedge 2009). See the section below for theories about the link between autism and heavy metals. Types of pharmaceutical chelating substances used to reduce heavy metal poisoning are outlined in Table 1. Chelation therapy is administered for approved uses in a highly controlled environment, which is different from the process followed by practitioners administering chelation for unapproved uses, such as for ASD.
1. Examples of chelating agents.
| Name | Target heavy metals | Route of administration | FDA‐approved indications | Pharmacokinetics | Pharmacodynamics | Common adverse effects |
| Dimercaptosuccinic acid (DMSA) | Lead; arsenic; mercury poisoning | Oral | Lead poisoning in adults and children > 12 months | Tmax 1 to 2 hours; elimination t1/2 2 hours to 2 days; primarily excreted renally |
Forms water‐soluble chelates with heavy metals, which are excreted renally | Rash; gastrointestinal upset |
| Edetate disodium (EDTA) | Calcium | Intravenous (IV) | Hypercalcaemia (emergency treatment); digitalis poisoning; ventricular arrhythmia in adults |
Elimination t1/2 1.4 to 3 hours |
Forms soluble chelate with calcium, resulting in a rapid decrease in plasma calcium concentrations | Fatigue; hypocalcaemia; thrombophlebitis |
| Sodium 2, 3 dimercaptopropanesulphonate (DMPS) | Arsenic; bismuth subcitrate; mercury; Wilson’s disease (copper) |
Intravenous (IV) and oral | Not approved for use in the United States of America | Elimination t1/2 1.8 hours | Forms water‐soluble chelates with many heavy metals, which are excreted renally High affinity for mercury |
Rash; nausea; dysgeusia |
| Edetate calcium disodium | Lead | Intravenous (IV) and intramuscular (IM) | Acute and chronic lead poisoning; lead encephalopathy in adults and children |
Elimination t1/2 20 to 60 minutes (IV), 1.4 to 3 hours (IM) |
An effective chelator of extracellular lead, resulting in increased urine excretion | Fatigue; nephrotoxicity |
From Osterloh 1986; Vamnes 2000; Drugs.com 2012.
FDA: Food and Drug Administration. Tmx: time until maximum concentration. t1/2: time until maximum concentration drops in half.
Patients with heavy metal poisoning, such as acute lead poisoning, require urgent hospitalisation and administration of chelating substances intravenously or by deep intramuscular injection for four hours. During treatment of patients with acute lead poisoning, blood and urine are monitored constantly, as significant shifts in the heavy metal can occur between the blood and the central nervous system with dire consequences. Moreover, because minerals and metal ions are essential elements that serve important functions in multiple biological processes, excessive removal can lead to deleterious results, for example, a child with ASD recently experienced fatal myocardial necrosis resulting from hypocalcaemia after receiving chelation therapy (Brown 2006).
Unapproved uses of chelation therapy, for example, when used as an intervention for ASD, may involve practitioners using various chelating substances and unlicensed routes of administration (such as through the rectum or the skin) to remove reported excess levels of mercury, other heavy metals or both (Semple 2011). Before treatment, individuals with ASD may undergo preliminary tests with the chelating substance to evoke a response, followed by timed urine collection to determine the levels of heavy metals in the body (Bradstreet 2003; Adams 2009). One of the more commonly used chelating substances, oral dimercaptosuccinic acid (DMSA; also called succimer), is given on a cyclical basis at doses of 10 mg/kg/d every eight hours for three days, followed by 11 days with no DMSA (Bradstreet 2003; Adams 2009). These two‐weekly cycles are repeated up to six times, totalling approximately three months of treatment (Adams 2009).
Between 6% and 11% of families of children with ASD in various English‐speaking countries, including the United States, Canada and Australia, have sought out and tried chelation therapy; most of these families perceived that chelation therapy improved symptoms (Green 2006; Goin‐Kochel 2009; Christon 2010).
How the intervention might work
The theoretical basis for mercury or other heavy metals as a cause of ASD draws on a wide variety of hypotheses, none yet confirmed. One hypothesis is that mercury or other heavy metals are present in greater quantities in children with ASD, compared with their peers, as a result of intrauterine exposure to maternal stores or intake, increased intake from immunisations (thimerosal), oral ingestion (fish or medication), inhalation (airborne pollution), increased absorption, altered metabolism or decreased excretion (Bernard 2001; Goldman 2001; Holmes 2003; Levy 2003; Counter 2004; Kern 2007).
The excess of stored or circulating total body mercury or other heavy metals is thought to interfere with developmental processes implicated in ASD, and it has been suggested that symptoms of mercury poisoning and ASD share some characteristics (Bernard 2001). Mercury, through its ability to cross the blood‐brain barrier and the placental barrier, can affect the nervous system and disrupt normal development of the foetus (Aschner 1990; Liu 2008). Prenatal mercury poisoning may result in neurological impairment, global developmental delay and intellectual disability, and postnatal exposure can result in memory loss, irritability, fatigue, intention tremor, skin discolouration and other organ involvement, including kidney dysfunction (e.g. nephrotic syndrome, tubular dysfunction, or both) (Bakir 1973; Amin‐Zaki 1974; Grandjean 1997; Goldman 2001; Counter 2004).
Research has produced contradictory findings with regard to levels of heavy metals in individuals with ASD. Three studies (Cohen 1976; Cohen 1982; Adams 2013) (N = 34, N = 93 and N = 99, respectively), all from the USA, found higher levels of lead in individuals with ASD compared with individuals without ASD, with overlapping levels observed between diagnostic groups. One of these studies also reported elevated levels of heavy metals (lead, thallium and tungsten) in urine (Adams 2013). Two studies ‐ one from the USA (N = 452) and one from Jamaica (N = 130) ‐ exploring differences in mercury level in blood reported no association between ASD and higher levels of mercury when analyses were adjusted for fish eating and other relevant factors (Hertz‐Picciotto 2010; Rahbar 2013). Another smaller study from Italy (N = 37) found no difference in mercury, or in other heavy metals, between individuals with ASD and a group recruited from a neuropsychiatric service who did not have ASD (Albizzati 2012).
An alternative hypothesis is that mercury or other heavy metals can cause ASD through altered cellular functioning, which does not require increased body stores or circulating mercury or other heavy metals. In this context, it is thought that individuals with ASD have an impaired capacity to excrete heavy metals, and that the severity of autism symptoms is inversely correlated with excretion ability (Holmes 2003; Kern 2007). This hypothesis is currently being explored (Deth 2008; Zecavati 2009; Garrecht 2011).
Why it is important to do this review
Novel therapies are used frequently by individuals with ASD (Hanson 2007). Despite their increasing use, most types of CAM for ASD lack a robust evidence base (Nye 2005; Millward 2008; Cheuk 2011; James 2011; Sinha 2011; Geretsegger 2014). Chelation therapy is one CAM that continues to be used and promoted as efficacious, despite reports of harm, withdrawal of a trial before recruitment because of safety concerns (Mitka 2008) and discouragement by physicians (Golnik 2009). Adverse effects commonly reported with the use of pharmaceutical chelating agents are listed in Table 1. Deaths have also been reported (Brown 2006). A systematic review that examines potential beneficial and harmful effects of chelation for symptoms of ASD is urgently needed. Results from this systematic review will help families with ASD make well‐informed decisions about the use of chelation therapy and will assist relevant services and guide other organisations in making decisions about best practice.
Objectives
To assess the potential benefits and adverse effects of pharmaceutical chelating agents (referred to as chelation therapy throughout this review) for autism spectrum disorder (ASD) symptoms.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Individuals of any age diagnosed with ASD using established diagnostic criteria (e.g. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV)) or standardised diagnostic instruments (e.g. the Childhood Autism Rating Scale (CARS), the Autism Diagnostic Observation Scale (ADOS), the Autism Diagnostic Interview ‐ Revised (ADI‐R)) were eligible for inclusion in this review.
Types of interventions
Interventions were eligible for inclusion if they involved chelating substances of any type and dose, regardless of administration frequency or method, compared with placebo. Trials were also eligible for inclusion if the chelating substances were provided alone or as adjunctive treatment compared with placebo (e.g. chelation in combination with a behavioural intervention vs placebo in combination with a behavioural intervention).
Types of outcome measures
Primary outcomes
-
Changes in the following core symptoms of ASD, using any measure (e.g. the Aberrant Behavior Checklist (ABC), CARS), as assessed separately.
Social interaction.
Communication.
Stereotypy.
Adverse events.
Secondary outcomes
-
Changes in the following non‐core behaviours, using any measure (e.g. ABC, CARS), as assessed separately.
Irritability.
Aggression.
Hyperactivity.
Insomnia.
Self injury.
Quality of life for individual or family.
Heavy metal levels in blood or non‐provoked urine. Provoked urine will be examined cautiously (as provocation testing elevates urine heavy metal levels).
Search methods for identification of studies
We first searched databases for this review in December 2013 and repeated the searches, beginning on 6 November 2014, to find new studies published in the intervening period.
Electronic searches
We searched the following databases with no language or date restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL) 2014, Issue 11, part of the Cochrane Library.
Ovid MEDLINE, 1946 to October Week 5 2014.
Ovid MEDLINE In‐Process and Other Non‐indexed Citations, 5 November 2014.
Embase (Ovid), 1980 to Week 44.
PsycINFO (Ovid), 1967 to November Week 1 2014.
Science Citation Index (Web of Science), 1970 to 5 November 2014.
Social Sciences Citation Index (Web of Science), 1970 to 5 November 2014.
Conference Proceedings Citation Index – Science (Web of Science), 1990 to 5 November 2014.
Conference Proceedings Citation Index – Social Science & Humanities (Web of Science), 1990 to 5 November 2014.
Database of Abstracts of Reviews of Effects (DARE) 2014, Issue 4, part of the Cochrane Library.
Cochrane Database of Systematic Reviews (CDSR) 2014, Issue 11, part of the Cochrane Library.
Heatlh Technology Assessment Database 2014, Issue 4, part of the Cochrane Library.
CINAHL Plus (Cumulative Index to Nursing and Allied Health Literature) (EBSCOhost), 1937 to current.
Autism Data (www.autism.org.uk/autismdata).
WorldCat (limited to theses and dissertations) (www.worldcat.org/)).
metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com/mrct/search.html).
ClinicalTrials.gov (clinicaltrials.gov/).
International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/).
TOXNET (toxnet.nlm.nih.gov/).
Latin American Caribbean Health Sciences Literature (LILACS) (search.bvsalud.org/portal/advanced/?lang=en).
Scientific Electronic Library Online (SciELO) (scielo.br/cgi‐bin/wxis.exe/iah/).
Google Scholar (scholar.google.com/).
We reported the searches in detail in Appendix 1.
Searching other resources
We searched reference lists from the retrieved articles for studies not already identified, and we contacted known experts in the field to enquire about other sources of information. We also searched relevant websites, including Autism Speaks (www.autismspeaks.org/), Research Autism (http://researchautism.net/) and the US Department of Health and Human Services (www.hhs.gov/).
Data collection and analysis
Selection of studies
Two review authors (SJ and SS) independently screened the titles and abstracts of citations identified by the search. SJ and SS then obtained and reviewed the full text of studies that met, or seemed likely to meet, the inclusion criteria. In the event of uncertainties and differences of opinion, resolution was reached through discussions with the third and fourth review authors (NS and KW).
Data extraction and management
Two review authors (SJ and SS) independently extracted the following information from the included study using a data extraction form designed and piloted for this review.
Study methods and setting, including study duration, design and location.
Participant details, including age, gender, sample size and diagnosis.
Intervention details.
Outcomes.
No disagreements arose.
Assessment of risk of bias in included studies
Two review authors (SJ and SS) independently assessed risk of bias of the included study using the tool described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011, section 8.5.a). For the included study, we judged the risk of bias to be low, high or unclear for each of the following domains.
-
Sequence generation.
Low risk: if a random component was used in the sequence generation process, such as coin‐tossing, computer‐generated random numbers or a table of random numbers.
High risk: if a non‐random component was used in the sequence generation process.
Unclear risk: if the sequence generation process was not described.
-
Allocation concealment.
Low risk: if participants and trial investigators had no foreknowledge (i.e. before eligibility, decisions made and informed consent obtained) of intervention assignment through the use of, for example, central allocation or sequentially numbered envelopes that were opaque and sealed.
High risk: if participants and trial investigators had foreknowledge of intervention assignment.
Unclear risk: if the method of allocation concealment was not described.
-
Blinding of participants and personnel.
Low risk: if no blinding or incomplete blinding was reported but review authors judged the outcome as unlikely to have been influenced by lack of blinding, or if blinding of study participants and personnel was ensured and it is unlikely that blinding could have been broken.
High risk: if no blinding or incomplete blinding was reported and the outcome was likely influenced by lack of blinding, or if blinding of study participants and personnel was attempted but it is likely that blinding could have been broken and the outcome influenced by lack of blinding.
Unclear risk: if lack of information prohibits judgement of low or high risk of bias, or if the study did not address this outcome.
-
Blinding of outcome assessment.
Low risk: if no blinding of outcome assessment was reported but review authors judged the outcome measurement as unlikely to have been influenced by lack of blinding, or if blinding of outcome assessment was ensured and it is unlikely that blinding could have been broken.
High risk: if no blinding of outcome assessment was reported and the outcome measurement was likely influenced by lack of blinding, or if blinding of outcome assessment was reported but it is likely that blinding could have been broken and the outcome measurement influenced by lack of blinding.
Unclear risk: if lack of information prohibits judgement of low or high risk of bias, or if the study did not address this outcome.
-
Incomplete outcome data.
Low risk: if no missing data were reported or if appropriate methods were used to impute missing data, or if the reason for missing data is unlikely to be related to the true outcome.
High risk: if missing data were reported and no appropriate methods were used to impute missing data, or if the reason for missing data is likely to be related to the true outcome.
Unclear risk: if lack of information prohibits judgement of low or high risk of bias, or if the study did not address this outcome.
-
Selective reporting.
Low risk: if a study has a protocol and all prespecified outcomes were reported in the prespecified manner, or if a study has no protocol but all expected outcomes have been reported.
High risk: if a study has a protocol and one or more prespecified outcomes were not reported or were reported in a manner that was not prespecified, or if a study has no protocol and all expected outcomes have not been reported.
Unclear risk: if lack of information prohibits judgement of low or high risk of bias.
-
Other bias.
Low risk: if other sources of bias (e.g. contamination, recruitment bias) do not appear to exist.
High risk: if other sources of bias exist.
Unclear risk: if lack of information permits judgement of whether other sources of bias exist.
No differences of opinions arose.
Measures of treatment effect
We did not conduct a meta‐analysis, given that only one study was included in this review. Analysis methods that we will use in updates of this review can be found in Table 2 and in our protocol (James 2013).
2. Analysis methods planned in our protocol that will be used in updates of this review.
| Measures of treatment effect | We will analyse dichotomous outcomes by calculating the risk ratio (RR) and the corresponding 95% confidence interval (CI). For continuous data, we will calculate mean difference (MD) and corresponding 95% CI if studies used the same rating scales. As recommended by Higgins 2011, we will focus on final values unless change scores are used in some of the studies. We will combine in the same meta‐analysis studies that reported final values with studies that reported only change scores, provided the studies used the same rating scale (Higgins 2011). A potential problem associated with including change scores is that the standard deviation of changes may not be reported in the original study (Higgins 2011). We will contact trial authors and will attempt to estimate the standard deviation of changes if not reported. We will calculate the standardised mean difference (SMD) with 95% CIs if studies used different scales to measure the same outcomes |
| Multiple outcomes | If studies provided multiple, interchangeable measures of the same construct at the same point in time, we will calculate the average SMD across outcomes and the average estimated variances (Higgins 2011) |
| Unit of analysis issues | When possible, we will obtain mean treatment differences and standard errors for cross‐over trials, and will enter these into RevMan under the generic inverse variance outcome type (Higgins 2011). We will create a single pair‐wise comparison for each identified multi‐arm study by combining all relevant experimental groups into a single group, and by combining all relevant control groups into a single group (Higgins 2011) |
| Dealing with missing data | We will attempt to contact trial investigators to request missing data. If missing data are provided by the trialists, we will conduct meta‐analysis according to intention‐to‐treat principles using all data and keeping participants in the treatment group to which they were originally randomly assigned, regardless of the treatment that they actually received (Higgins 2011). If missing data are not provided, we will analyse only available data, and we will not impute missing data given that symptoms of autism spectrum disorder (ASD) vary greatly. We will document missing data and attrition in the ’Risk of bias’ table, and we will discuss how missing data may affect interpretation of the results |
| Assessment of heterogeneity | We will assess clinical heterogeneity by comparing the between‐trials distribution of participant characteristics (e.g. children vs adults), trial characteristics (e.g. cross‐over vs parallel design) and intervention characteristics (e.g. treatment type, dose). We will evaluate statistical heterogeneity using the I² statistic and the Chi² test of heterogeneity, with statistical significance set at P value < 0.10. We will consider I² values as follows.
|
| Assessment of reporting biases | If 10 or more studies are found, we will use funnel plots to investigate the relationship between intervention effect and study size. Asymmetry of a funnel plot may indicate, among other things, publication bias or poor methodological quality (Egger 1997). We will explore possible reasons for any asymmetry found |
| Data synthesis | We will synthesise results in a meta‐analysis using a fixed‐effect model when studies are similar enough with regard to the intervention, population and methods to assume that the same treatment effect is estimated. We will synthesise results in a meta‐analysis using a random‐effects model when statistical heterogeneity is found, or when studies differ enough with regard to the intervention, population and methods to assume that different yet related treatment effects are estimated, and when it is deemed to be clinically relevant (Higgins 2011) |
| Subgroup analysis and investigation of heterogeneity | We will conduct the following subgroup analyses
|
| Sensitivity analysis | We will conduct sensitivity analyses to investigate the effect on overall results of excluding trials when
|
Dealing with missing data
We assessed and reported missing data and dropouts for the included study (see Characteristics of included studies). Based on our judgement that missing data and dropouts in the included study were unlikely related to the outcomes (and missing data appeared balanced across intervention groups, with similar reasons for missing data across groups), we did not contact the trial investigators for further information.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our search strategy identified 846 citations, of which 12 were deemed to be potentially eligible on the basis of title or abstract (see Figure 1). We obtained full‐text copies of these 12 reports and inspected them. After all full‐text copies had been reviewed, we included three reports of one study (Adams 2009) and excluded nine reports (Eppright 1996; Lonsdale 2002; Geier 2006a; Geier 2006b; Nataf 2006; Geier 2007; Patel 2007; Blaucok‐Busch 2012; NCT00376194).
1.
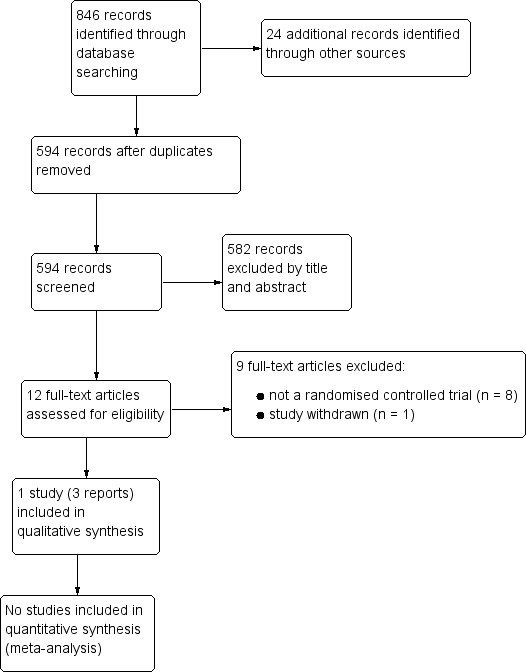
Study flow diagram.
Included studies
The study included in this review was reported to be randomised and double‐blind (Adams 2009). Although it fulfilled criteria for inclusion, the methods, which we have outlined here, are not appropriate to allow testing of the hypothesis of interest in this review. Instead, the included study was conducted in two phases: During the first phase, participants were randomly assigned to receive seven days of glutathione lotion (experimental group) or placebo lotion (control group) followed by three days of oral dimercaptosuccinic acid (DMSA) (for all children). This phase tested whether topical glutathione was absorbed and whether topical glutathione was effective for increasing the effectiveness of oral DMSA. During the second phase, a subset of participants ‐ high heavy metal excreters ‐ from phase one were randomly assigned to receive three days of oral DMSA (experimental group) or placebo (control group) followed by 11 days off, with this cycle repeated up to six times. This phase assessed the effectiveness of multiple doses of oral DMSA compared with placebo in children who were high excreters of heavy metals and who had received a three‐day course of oral DMSA.
We discussed at length whether the study should be included, as all children entering phase two had already received a chelating agent. Ultimately, however, we believed that we would not be deviating from protocol by including the study, because the second phase of the study was a randomised placebo‐controlled trial.
Participants
A total of 82 children, aged three to eight years, enrolled in the included study, of whom 77 completed the required initial blood collection (to assess baseline liver and kidney function, red blood cell (RBC) glutathione, and complete blood count (CBC)). Although the total number of children randomly assigned (and the number randomly assigned to each group) is not explicitly stated, it appears as though randomisation occurred after the initial blood collection (i.e. for the 77 children). All children (69 male and eight female; mean age 6.3 years) were Arizona residents who had been diagnosed with autism spectrum disorder (autism 95%, pervasive developmental disorder not otherwise specified (PDD NOS) 3%, Asperger's 3%) by a psychiatrist, a psychologist or a developmental paediatrician. Only 65 participants completed phase one, and 49 heavy metal excreting participants were included in phase two.
Interventions
For the first phase of the included study, children in the experimental group initially received glutathione lotion, which was administered once per day for seven days. Each daily dosage contained ˜ 180 mg of “reduced 1‐glutathione in a lotion of isopropyl myristate, mineral oil, caprylic/capric triglyceride, and vitamin E acetate” (Adams 2009, p 5). Children in the control group received a placebo lotion that was identical in packaging and contained a similar formulation (with the exception of glutathione). After receiving the glutathione or placebo lotion for seven days, all children received one round of oral DMSA, administered in a 10 mg/kg dose, three times per day for three days. All children were given one round of oral DMSA for screening purposes to ensure that only high heavy metal excreters continued on to the second phase.
For the second phase of the included study, all children had received a three‐day course of oral DMSA before randomisation. The experimental group received up to six more rounds of oral DMSA. Each round consisted of a 10 mg/kg dose, administered three times per day for three days, followed by 11 days of no DMSA. Children in the control group received up to six rounds of placebo (methyl cellulose), which was similar to DMSA in appearance and was packaged in identical bottles. Each round consisted of three days on placebo, followed by 11 days off.
Outcomes
Blood was collected to assess CBC and RBC glutathione. Blood was collected at the beginning of phase one, at the beginning of phase two, after the third round of oral DMSA/placebo in phase two and after the sixth round of oral DMSA/placebo in phase two. Urine was collected to assess excretion of toxic metals and essential minerals. Urine was collected at the beginning of phase one, after the first dose of oral DMSA in phase one, after the ninth dose in phase one and at the end of the second round in phase two. Severity of autism symptoms was assessed using the Pervasive Developmental Disorder Behavior Inventory (PDD‐BI), the Autism Treatment Evaluation Checklist (ATEC), the Severity of Autism Scale (SAS) and the Autism Diagnostic Observation Schedule (ADOS). The ATEC was administered at the beginning of phase one and at the end of phase two. The PDD‐BI, SAS and ADOS were administered at the beginning of phase two and at the end of phase two. Changes in autism symptoms were assessed at the end of phase two using the Parental Global Impressions (PGI) questionnaire.
As such, the included trial was able to answer only the following question.
Do repeated doses of oral DMSA decrease core features of ASD in children who have previously received a three‐day course of oral DMSA and are known to be high excreters?
Only outcomes that were available at the beginning of phase one and at the completion of phase two, or were available at the beginning and end of phase two, could assist with answering this question.
See also the Characteristics of included studies table.
Excluded studies
We excluded nine studies ‐ eight studies because they were non‐randomised (Eppright 1996; Lonsdale 2002; Geier 2006a; Geier 2006b; Nataf 2006; Geier 2007; Patel 2007; Blaucok‐Busch 2012) and one study because it was withdrawn before enrolment (NCT00376194). See the Characteristics of excluded studies table for more information.
Risk of bias in included studies
An overview of risk of bias is illustrated in Figure 2. See below for more detailed information on risk of bias.
2.
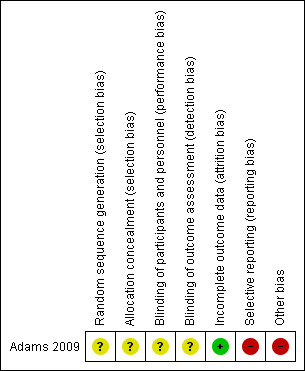
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Participants in the included study (Adams 2009) were reported to be randomly assigned, but no details were available on sequence generation or allocation concealment. As such, we rated risk of bias in these domains as unclear.
Blinding
Participants, caregivers and investigators in the included study were reported to be blinded to the treatment condition; however, it is unclear whether all lab technicians and ADOS evaluators were blinded. It is also uncertain whether parents knew that all children would receive at least one round of oral DMSA during the first phase, and that only high excreters would continue on to the second phase. Knowledge that all children received at least one round of chelation or awareness of who was in the high excreter group would likely yield high risk of bias in the second phase. Therefore, we rated risk of performance bias and risk of detection bias as unclear.
Incomplete outcome data
In phase two, two children (one in each treatment group) withdrew because of perceived lack of benefit, and four children dropped out as the result of adverse effects (two children in the control group for worsening of behaviours, and two children in the experimental group ‐ one for sleep problems and one for worsening of behaviours and skills). Additionally in phase two, one child dropped out as a result of elevated liver enzymes (reported to be due to psychiatric medication) and one child withdrew because of the death of a family member. Trialists did not report if these two children were included in the experimental group or in the control group. We rated risk of attrition bias as low, given that the reason for missing data was deemed unlikely to be related to the true outcome.
Selective reporting
The level of urinary excretion of toxic metals was not reported for participants in the control group during or after phase two, and no results were reported with regard to potential differences between the experimental group and the control group in excretion levels of toxic metals during or after phase two. As such, we rated risk of reporting bias as high.
Other potential sources of bias
Given that both the experimental group and the control group received an initial round of oral DMSA in phase one, carry‐over effects may have occurred. Trialists acknowledged that the effect of the phase one oral DMSA carried over into phase two: "...the single screening round of DMSA [in phase one] had an unexpectedly dramatic effect on improving abnormal glutathione and platelet levels, and the effect lasted until the end of phase two, so that phase two was not a placebo‐control‐led investigation" (Adams 2009, p 3).
The included study also appears to have biases related to the validity of the statistical conclusions. It is perplexing that the trialists would use an unconventional and atheoretical regression approach without the critical step of including a cross‐validation sample, and given the relatively small sample size and the apparently large set of variables (the number of tested variables was not revealed). Furthermore, use of adjusted R² instead of R² appears to be a less‐than‐satisfactory approach to the handling of sample uniqueness, which likely artificially inflated model effect size.
Finally, trialists downplayed the variability of heavy metal levels and the severity of ASD, stating, “Since the toxic metal excretions exhibit considerable correlation amongst themselves, one should refrain from reading too much into the relationships between specific metals and severity of autism" (p 6). This attempt to persuade readers to disregard differences across heavy metals, however, is undermined by the presented regression analyses, which show inconsistencies in the direction of the effects that metal excretion has on various change indices. For example, the unstandardised b‐weight signs suggest that increased excretion of As9 is associated with differential change patterns in PDD‐BI, ADOS and ATEC scores (although it is unclear how trialists created the change scores). Similar inconsistencies are evident upon examination of Pb9 with PDD‐BI and SAS. Incidentally, trialists did not report the b‐weight for SAS regressed on T19, although they reported on its statistical significance.
Given these factors, we rated other bias as high.
Effects of interventions
For the current version of this review, meta‐analysis was not possible, as only one study was included. In the included study, 77 children completed the required blood collection at the beginning of the trial. Of these, 65 children completed phase one, 49 high heavy metal excreters continued to phase two and 41 completed phase two.
Primary outcome: social interaction
ATEC sociability subscale
For children in the experimental group who completed phase two of the trial, the mean (standard deviation (SD)) score on the ATEC sociability subscale decreased from 16.6 (8.5) at the beginning of phase one, to 12.1 (6.5) at the end of phase two. For children in the control group who completed phase two of the trial, the mean (SD) score on the ATEC sociability subscale decreased from 14.9 (6.8) at the beginning of phase one, to 11.2 (6.5) at the end of phase two. The between‐group difference was reported to be non‐significant.
PDD‐BI social pragmatic problems subscale
For children in the experimental group who completed phase two of the trial, the mean (SD) score on the PDD‐BI social pragmatic problems subscale decreased from 16.9 (9.2) at the beginning of phase two, to 14.5 (9.2) at the end of phase two. For children in the control group who completed phase two of the trial, the mean (SD) score on the PDD‐BI social pragmatic problems subscale decreased from 13.9 (7.5) at the beginning of phase two, to 9.9 (7.5) at the end of phase two. The significance of the between‐group difference was not reported.
PDD‐BI social approach behaviours subscale
For children in the experimental group who completed phase two of the trial, the mean (SD) score on the PDD‐BI social approach behaviours subscale increased from 63.8 (20.6) at the beginning of phase two, to 70.8 (23.6) at the end of phase two. For children in the control group who completed phase two of the trial, the mean (SD) score on the PDD‐BI social approach behaviours subscale increased from 68.2 (25.4) at the beginning of phase two, to 72.6 (20.2) at the end of phase two. The significance of the between‐group difference was not reported.
ADOS sociability subscale
For children in the experimental group who completed phase two of the trial, the mean score on the ADOS sociability subscale decreased from 9.3 at the beginning of phase two, to 8.3 at the end of phase two. For children in the control group who completed phase two of the trial, the mean score on the ADOS sociability subscale decreased from 8.1 at the beginning of phase two, to 7.9 at the end of phase two. The between‐group difference was reported to be non‐significant.
Primary outcome: communication
ATEC speech/language communication subscale
For children in the experimental group who completed phase two of the trial, the mean (SD) score on the ATEC speech/language communication subscale decreased from 13.4 (7.7) at the beginning of phase one, to 10.6 (7.0) at the end of phase two. For children in the control group who completed phase two of the trial, the mean (SD) score on the ATEC speech/language communication subscale decreased from 12.0 (8.4) at the beginning of phase one, to 10.5 (8.9) at the end of phase two. The between‐group difference was reported to be non‐significant.
ADOS communication subscale
For children in the experimental group who completed phase two of the trial, the mean score on the ADOS communication subscale decreased from 7.8 at the beginning of phase two, to 7.1 at the end of phase two. For children in the control group who completed phase two of the trial, the mean score on the ADOS communication subscale decreased from 6.7 at the beginning of phase two, to 5.9 at the end of phase two. The between‐group difference was reported to be non‐significant.
Primary outcome: stereotypy
PDD‐BI ritualism/resistance to change subscale
For children in the experimental group who completed phase two of the trial, the mean (SD) score on the PDD‐BI ritualism/resistance to change subscale decreased from 13.9 (10.5) at the beginning of phase two, to 10.0 (7.8) at the end of phase two. For children in the control group who completed phase two of the trial, the mean (SD) score on the PDD‐BI ritualism/resistance to change subscale decreased from 15.0 (8.5) at the beginning of phase two, to 11.5 (8.2) at the end of phase two. The significance of the between‐group difference was not reported.
ADOS stereotyped behaviours and restricted interests subscale
For children in the experimental group who completed phase two of the trial, the mean score on the ADOS stereotyped behaviours and restricted interests subscale decreased from 3.9 at the beginning of phase two, to 3.5 at the end of phase two. For children in the control group who completed phase two of the trial, the mean score on the ADOS stereotyped behaviours and restricted interests subscale was 3.5 at the beginning of phase two and 3.5 at the end of phase two. The between‐group difference was reported to be non‐significant.
Primary outcome: adverse events
During phase one, one child experienced an adverse event (lethargy and diminished appetite), causing the child to withdraw from the study (treatment group not specified). During phase two, four children dropped out after experiencing adverse events, including sleep problems (n = 1; experimental group), worsening of behaviours and skills (n = 1; experimental group), increased self stimulatory behaviour (n = 1; control group) and regression of behaviour (n = 1; control group). Two additional children in the experimental group withdrew from the study as the result of low excretion of metals and worsening of symptoms, including sleep problems (n = 1) and sleep problems plus increased tantrums (n = 1).
Secondary outcome: aggression
PDD‐BI aggressiveness subscale
For children in the experimental group who completed phase two of the trial, the mean (SD) score on the PDD‐BI aggressiveness subscale decreased from 13.4 (9.8) at the beginning of phase two, to 9.8 (6.7) at the end of phase two. For children in the control group who completed phase two of the trial, the mean (SD) score on the PDD‐BI aggressiveness subscale decreased from 11.4 (8.1) at the beginning of phase two, to 8.4 (7.2) at the end of phase two. The significance of the between‐group difference was not reported.
Secondary outcome: heavy metal levels
No results were reported with regard to potential differences between the experimental group and the control group in excretion levels of toxic metals during or after phase two.
Discussion
Summary of main results
Few studies have examined the effectiveness of chelation for ASD, and no true randomised placebo‐controlled trials were identified for this review. This review included only one study, which compared children who received one round of oral dimercaptosuccinic acid (DMSA) versus children who received multiple rounds of oral DMSA, and found no significant differences with regard to autism spectrum disorder (ASD) symptoms.
Overall completeness and applicability of evidence
One trial, which had methodological issues and a relatively small sample size, is insufficient to provide robust evidence on chelation for ASD. The included study compared one round of oral DMSA versus multiple rounds of oral DMSA. Although this study sheds light on the impact of using different quantities of DMSA rounds, it does not address completely the question of whether DMSA is effective in the first place. Moreover, external validity is limited, given that the included study involved children from only one state (Arizona) in the United States of America, and given that only one chelating agent (DMSA) was assessed. Finally, most of the secondary outcomes presented in the protocol of this review (James 2013) were not investigated in the included study.
Quality of the evidence
The quality of the evidence is poor. Only one trial was included in this review, and we judged it to have high or uncertain risk of bias and methodological problems that limited the interpretation of outcomes presented. Of particular concern are the trialists’ questionable data analytical approach and interpretation of findings. It is interesting that trialists found differential directions of heavy metal excretion and change in ASD indices, yet they attempted to convince the reader not to read too much into these differences. Given the deleterious effects of chelation, misinterpretation and misuse of the study of Adams et al to justify the use of chelation for ASD is unethical and potentially places children unnecessarily in harm’s way. Moreover, if these findings are in fact valid, they actually undermine the heavy metal toxicity theory and the rationale for chelation treatment, suggesting that it should not be used in the first place.
The inclusion of only one study, which had a relatively small sample size and a high likelihood of carry‐over effects and other biases, precludes confidence in the findings. Further well‐designed studies from multiple locations and using larger numbers of participants are needed to better ascertain the effects of chelation for ASD.
Potential biases in the review process
We attempted to minimise bias by having two review authors independently screen studies for inclusion, extract and manage data and assess risk of bias in included studies. Although we attempted to minimise publication bias by using a comprehensive search strategy and by searching multiple sources, we may have failed to identify relevant trials.
Agreements and disagreements with other studies or reviews
A systematic review evaluating the effectiveness of chelation for ASD (Davis 2013) was published after the title for this review was registered. The Davis 2013 review did not limit inclusion criteria to randomised controlled trials; whereas we included one trial, Davis 2013 included five trials. Nonetheless, all of the trials included in the Davis 2013 review were reported to have substantial methodological limitations, and the results of our review support the findings reported by Davis 2013; we agree that no available evidence supports the use of chelation to treat individuals with ASD symptoms.
Authors' conclusions
Implications for practice.
This review found no high‐quality evidence to suggest that chelation is an effective treatment for improving ASD symptoms. Cochrane reviews typically avoid making recommendations for practice; however, given that harm resulting from the use of chelation therapy has been reported, and that no proven benefits have been found, it seems reasonable to conclude that use of chelation for the treatment of individuals with ASD symptoms should not be recommended.
Implications for research.
At the present time, the theory that heavy metals may cause autism or might worsen symptoms has not been established. This underlying theory needs to be tested and confirmed before future trials that assess chelation for ASD symptoms are implemented. However, the numerous side effects of chelation therapy, including hypocalcaemia, renal impairment, musculoskeletal and gastrointestinal symptoms and even death (Morgan 2002; Brown 2006; Kosnett 2010), have led to the withdrawal of at least one planned study. It is therefore unlikely that institutional review boards will approve future trials for chelation for ASD unless safety in children can be assured through the current approach to research.
If evidence emerges that supports a causal link between heavy metals and autism, further trials with methods suitable to ensure safety and to demonstrate that chelation removes heavy metals, improves social communication and reduces restricted repetitive behaviours seen in autism will be needed.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2016 | Amended | Slight amendments have been made to the 'Background' section of the Plain Language Summary, to clarify details of both intervention and condition. |
Acknowledgements
We would like to thank Laura MacDonald, Geraldine Macdonald and Joanne Wilson for feedback and guidance, and Margaret Anderson for assistance with the development of our search strategy. We would also like to thank Dr. Yashwant Sinha for pharmacological advice about topical glutathione and dimercaptosuccinic acid (DMSA). Finally, we are extremely grateful for invaluable feedback provided by external reviewers and statisticians.
Appendices
Appendix 1. Search strategy
Cochrane Central Register of Controlled Trials (CENTRAL), part of theCochrane Library
2013 (Issue 11), searched 5 December 2013 (2 records) 2014 (Issue 11), searched 6 November 2014. Limited to year=2013 to 2014 (no records)
ID Search #1 [mh ^"child development disorders, pervasive"] #2 [mh "Developmental Disabilities"] #3 pervasive next development* next disorder* #4 (pervasive near/3 child*) #5 (PDD or PDDs or PDD next NOS or ASD or ASDs) #6 autis* #7 asperger* #8 kanner* #9 childhood next schizophreni* #10 Rett* #11 {or #1‐#10} #12 [mh "Chelation Therapy"] #13 MeSH descriptor: [Chelating Agents] explode all trees #14 [mh "Iron Chelating Agents"] #15 [mh siderophores] #16 [mh ferrozine] or [mh "pentetic acid"] or [mh deferoxamine] or [mh enterobactin] or [mh Fursultiamin] #17 (Fursultiamin* or TTFD or "thiamine tetrahydrofurfuryl disulfide") #18 complexon* #19 (chelation or chelating or chelator*) #20 (metal near/3 antagonist*) #21 ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or "caloxetic acid" or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or "choline tetrathiomolybdate" or "citric acid" or clathrin* or (crown next (compound or ether)) or cyclodextrin or cyclophane or cuprizone) #22 (dimercaprol or "dimercapto succinic acid" or "dimercaptosuccinic acid" or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb) #23 (edetate or Edetic Acid or Egtazic Acid or "Fura‐2" or "Humic Substances" or "Nitrilotriacetic Acid" or Penicillamine or "Pentetic Acid" or "phytic acid" or Phytochelatins or Razoxane or "salicylhydroxamic acid" or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine* or tropantiol or Unithiol or versetamide) #24 (siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin) #25 {or #12‐#24} #26 #11 and #25
Ovid MEDLINE
1946 to November Week 3 2013, searched 5 December 2014 (130 records) 1946 to October Week 5 2014, searced 6 November 2014. Limited to ed=20131101‐20141106 (6 records)
1 exp child development disorders, pervasive/ 2 Developmental Disabilities/ 3 pervasive development$ disorder$.tw. 4 (pervasive adj3 child$).tw. 5 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 6 autis$.tw. 7 asperger$.tw. 8 kanner$.tw. 9 childhood schizophrenia.tw. 10 Rett$.tw. 11 or/1‐10 12 Chelation Therapy/ 13 Chelating Agents/ 14 2,2'‐dipyridyl/ or caseins/ or chitosan/ or citric acid/ or cuprizone/ or dimercaprol/ or dithizone/ or ditiocarb/ or edetic acid/ or egtazic acid/ or fura‐2/ or humic substances/ or nitrilotriacetic acid/ or penicillamine/ or pentetic acid/ or phytochelatins/ or razoxane/ or succimer/ or technetium tc 99m pentetate/ or thenoyltrifluoroacetone/ or trientine/ or unithiol/ 15 Iron Chelating Agents/ 16 siderophores/ 17 ferrozine/ or pentetic acid/ or deferoxamine/ or enterobactin/ 18 Fursultiamin/ or (Fursultiamin$ or TTFD or thiamine tetrahydrofurfuryl disulfide).tw. 19 complexon$.tw. 20 (chelation or chelating or chelator$).tw. 21 (metal adj3 antagonist$).tw. 22 ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or caloxetic acid or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or choline tetrathiomolybdate or citric acid or clathrin$ or (crown adj (compound or ether)) or cyclodextrin or cyclophane or cuprizone).mp. 23 (dimercaprol or dimercapto succinic acid or dimercaptosuccinic acid or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb).mp. 24 (edetate or Edetic Acid or Egtazic Acid or Fura‐2 or Humic Substances or Nitrilotriacetic Acid or Penicillamine or Pentetic Acid or phytic acid or Phytochelatins or Razoxane or salicylhydroxamic acid or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine$ or tropantiol or Unithiol or versetamide).mp. (77430) 25 (siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin).mp. 26 or/12‐25 27 11 and 26 28 exp animals/ not humans.sh. 29 27 not 28 30 remove duplicates from 29
Ovid MEDLINE In‐Process & other Non‐indexed Citations
Database content last updated 4 December 2014. Searched 6 December 2013 (9 records) Database content last updated 5 November 2014. Searched 6 November 2014 (12 records)
1 pervasive development$ disorder$.tw. 2 (pervasive adj3 child$).tw. 3 (PDD or PDDs or PDD‐NOS or ASD or ASDs).tw. 4 autis$.tw. 5 asperger$.tw. 6 kanner$.tw. 7 childhood schizophrenia.tw. 8 Rett$.tw. 9 complexon$.tw. 10 (chelation or chelating or chelator$).tw. 11 (metal adj3 antagonist$).tw. 12 ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or caloxetic acid or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or choline tetrathiomolybdate or citric acid or clathrin$ or (crown adj (compound or ether)) or cyclodextrin or cyclophane or cuprizone).mp. 13 (dimercaprol or dimercaptosuccinic acid or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb).mp. ) 14 (edetate or Edetic Acid or Egtazic Acid or Fura‐2 or Humic Substances or Nitrilotriacetic Acid or Penicillamine or Pentetic Acid or phytic acid or Phytochelatins or Razoxane or salicylhydroxamic acid or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine$ or tropantiol or Unithiol or versetamide).mp. 15 (siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin).mp. 16 or/1‐8 17 or/9‐15 18 16 and 17
Embase (Ovid)
1980 to Week 48, searched 5 December 2013 (252 records) 1980 to Week 44, searched 6 November 2014. Limited to em=201349‐201444 (35 records)
1 exp autism/ 2 pervasive development$ disorder$.tw. 3 (PDD or PDDs or ASD or ASDs).tw. 4 autis$.tw. 5 asperger$.tw. 6 kanner$.tw. 7 childhood schizophreni$.tw. 8 Rett$.tw. 9 (pervasive adj3 child$).tw. 10 or/1‐9 11 exp chelation/ 12 chelation therapy/ 13 exp chelating agent/ 14 enterochelin/ 15 (Fursultiamin$ or TTFD or thiamine tetrahydrofurfuryl disulfide).tw. 16 complexon$.tw. 17 (chelation or chelating or chelator$).tw. 18 (metal adj3 antagonist$).tw. 19 ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or caloxetic acid or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or choline tetrathiomolybdate or citric acid or clathrin$ or (crown adj (compound or ether)) or cyclodextrin or cyclophane or cuprizone).mp. 20 (dimercaprol or dimercaptosuccinic acid or dimercapto succinic acid or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb).mp. 21 (edetate or Edetic Acid or Egtazic Acid or Fura‐2 or Humic Substances or Nitrilotriacetic Acid or Penicillamine or Pentetic Acid or phytic acid or Phytochelatins or Razoxane or salicylhydroxamic acid or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine$ or tropantiol or Unithiol or versetamide).mp. 22 (siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin).mp. (21516) 23 or/11‐22 24 10 and 23 25 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 26 human/ or normal human/ or human cell/ 27 25 and 26 28 25 not 27 29 24 not 28
PsycINFO (Ovid)
1967 to November Week 4 2013, searched 5 December 2013 (143 records) 1967 to November Week 1 2014, searched 6 November 2014. Limited to up=20131125‐20141103 (23 records)
1 exp pervasive developmental disorders/ 2 Developmental disabilities/ 3 pervasive development$ disorder$.tw. 4 (pervasive adj3 child$).tw. 5 autis$.tw. 6 asperger$.tw. 7 (ASD or ASDs or PDD or PDDs).tw. 8 Rett$.tw. 9 Kanner$.tw. 10 or/1‐9 11 ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or caloxetic acid or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or choline tetrathiomolybdate or citric acid or clathrin$ or (crown adj (compound or ether)) or cyclodextrin or cyclophane or cuprizone).af. 12 (dimercaprol or dimercapto succinic acid or dimercaptosuccinic acid or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb).af. 13 (edetate or Edetic Acid or Egtazic Acid or Fura‐2 or Humic Substances or Nitrilotriacetic Acid or Penicillamine or Pentetic Acid or phytic acid or Phytochelatins or Razoxane or salicylhydroxamic acid or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine$ or tropantiol or Unithiol or versetamide).af. 14 (siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin).af. 15 (chelation or chelating or chelator$).af. 16 (metal adj3 antagonist$).af. 17 complexon$.af. 18 alternative medicine/ 19 or/11‐18 20 10 and 19 21 remove duplicates from 20
Web of Science databases
Science Citation Index ‐ expanded: 1970 to 4 December 2013, searched 5 December 2013 (18 records) Science Citation Index ‐ expanded: 1970 to 5 November 2014, searched 6 November 2014. Limited to year= 2013 to 2014 (2 records) Social Sciences Citation Index: 1970 to 4 December 2013, searched 5 December 2013 (18 records) Social Sciences Citation Index: 1970 to 5 November 2014, searched 6 November 2014. Limited to year= 2013 to 2014 (2 records) Conference Proceedings Citation Index ‐ Science and Conference Proceedings Citation Index ‐ Social Science & Humanities, 1990 to 4 December 2013, searched 5 December 2013 (3 records) Conference Proceedings Citation Index ‐ Science and Conference Proceedings Citation Index ‐ Social Science & Humanities, 1990 to 5 November 2014, searched 6 November 2014. Limited to year= 2013 to 2014 (no records)
#13 #4 AND #3 DocType=All document types; Language=All languages; #12 #11 AND #3 DocType=All document types; Language=All languages; #11 #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 DocType=All document types; Language=All languages; #10 Ts=(siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin) DocType=All document types; Language=All languages; #9 TS= (edetate or Edetic Acid or Egtazic Acid or "Fura‐2" or "Humic Substances" or "Nitrilotriacetic Acid" or Penicillamine or "Pentetic Acid" or "phytic acid" or Phytochelatins or Razoxane or "salicylhydroxamic acid" or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine* or tropantiol or Unithiol or versetamide) DocType=All document types; Language=All languages; #8 TS=(dimercaprol or "dimercapto succinic acid" or "dimercaptosuccinic acid" or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb) DocType=All document types; Language=All languages; #7 TS= ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or "caloxetic acid" or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or "choline tetrathiomolybdate" or "citric acid" or clathrin* or (crown NEAR/1(compound or ether)) or cyclodextrin or cyclophane or cuprizone) DocType=All document types; Language=All languages; #6 TS =(metal near/3 antagonist*) DocType=All document types; Language=All languages; #5 TS= (complexon* or Fursultiamin* or TTFD or "thiamine tetrahydrofurfuryl disulfide") DocType=All document types; Language=All languages; #4 TS=(chelation or chelating or chelator*) DocType=All document types; Language=All languages; #3 #2 OR #1 DocType=All document types; Language=All languages; #2 Ts=("childhood schizophren*") DocType=All document types; Language=All languages; #1 TS=(autis* or asperger* or ASD or ASDs or PDD or PDDs or Pdd‐NOS or ("pervasiv* development* disorder*" ) or kanner* or Rett*) DocType=All document types; Language=All languages;
CINAHL Plus (EBSCOhost)
1937 to current, searched 5 December 2013 (57 records) 1937 to current, searched 6 November 2014. Limited to EM>=2013120 (1 record)
# Query Limiters/Expanders Last Run Via Results Action S20 S9 AND S19 S19 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 S18 (siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin) S17 (edetate or Edetic Acid or Egtazic Acid or Fura‐2 or Humic Substances or Nitrilotriacetic Acid or Penicillamine or Pentetic Acid or phytic acid or Phytochelatins or Razoxane or salicylhydroxamic acid or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine* or tropantiol or Unithiol or versetamide) S16 (dimercaprol or "dimercapto succinic acid" or "dimercaptosuccinic acid" or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb) S15 ("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or "caloxetic acid" or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or "choline tetrathiomolybdate" or "citric acid" or clathrin* or (crown N1 (compoundor ether)) or cyclodextrin or cyclophane or cuprizone) S14 (chelation or chelating or chelator*) S13 complexon* S12 (MH "Chelating Agents+") S11 (MH "Detoxification, Alternative Therapy") S10 (MH "Chelation Therapy") S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S8 Rett* S7 childhood schizophren* S6 kanner* S5 (PDD or PDDs or PDD‐NOS or ASD or ASDs) S4 (pervasive N3 child*) S3 pervasive development* disorder* S2 autis* or asperger* S1 (MH "Child Development Disorders, Pervasive+")
Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Reviews of Effects (DARE) and Health Technology Assessment (HTA) databases (all part of theCochrane Library)
CDSR 2013, Issue 12, searched 5 December 2013 (no records) CDSR 2014, Issue 11, searched 6 november 2014. Limited to 2013 to 2014 (no records) DARE 2013, Issue 4 , searched 5 December 2013 (no records) DARE 2014, Issue 4, searched 6 November 2014. Limited to 2013 to 2014 (no records) HTA 2013, Issue 4, searched 5 December 2013 (no records) HTA 2014, Issue 4, searched 6 November 2014. Limited to 2013 to 2014 (no records)
#1[mh ^"child development disorders, pervasive"] #2[mh "Developmental Disabilities"] #3(pervasive next development* next disorder*):ti,ab #4(pervasive near/3 child*):ti,ab #5(PDD or PDDs or PDD next NOS or ASD or ASDs):ti,ab #6autis*:ti,ab #7asperger*:ti,ab #8kanner*:ti,ab #9childhood next schizophreni*:ti,ab #10Rett*:ti,ab #11{or #1‐#10} #12[mh "Chelation Therapy"] #13MeSH descriptor: [Chelating Agents] explode all trees #14[mh "Iron Chelating Agents"] #15[mh siderophores] #16[mh ferrozine] or [mh "pentetic acid"] or [mh deferoxamine] or [mh enterobactin] or [mh Fursultiamin] #17(Fursultiamin* or TTFD or "thiamine tetrahydrofurfuryl disulfide"):ti,ab #18complexon*:ti,ab #19(chelation or chelating or chelator*):ti,ab #20(metal next/3 antagonist*):ti,ab #21("2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or "caloxetic acid" or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or "choline tetrathiomolybdate" or "citric acid" or clathrin* or (crown next (compound or ether)) or cyclodextrin or cyclophane or cuprizone):ti,ab #22(dimercaprol or "dimercapto succinic acid" or "dimercaptosuccinic acid" or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb):ti,ab #23(edetate or Edetic Acid or Egtazic Acid or "Fura‐2" or "Humic Substances" or "Nitrilotriacetic Acid" or Penicillamine or "Pentetic Acid" or "phytic acid" or Phytochelatins or Razoxane or "salicylhydroxamic acid" or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine* or tropantiol or Unithiol or versetamide):ti,ab #24(siderophores or ferrozine or pentetic acid or deferoxamine or enterobactin):ti,ab #25{or #12‐#24} #26#11 and #25
LILACS
(search.bvsalud.org/portal/advanced/?lang=en) Searched 9 December 2013 (no records) Searched 7 November 2014. Limited to year=2013 to 2014 (7 records)
(tw:(autis* OR asperger* OR "pdd‐nos" OR asd OR asds) OR mh:("Autistic Disorder" OR "Asperger Syndrome" OR "Child Development Disorders, Pervasive")) AND (mh:("Chelating Agents" OR "Chelation Therapy" OR "Iron Chelating Agents" OR "Siderophores") OR tw:(chelating OR chelate* OR chelator* OR complexon* or DMSA))
TOXNET
(toxnet.nlm.nih.gov/) Searched 20 November 2014
Autism and chelation
Google Scholar
(scholar.google.com/) Searched 20 November 2014
Autism and chelation
SciELO
(scielo.br/cgi‐bin/wxis.exe/iah/) Searched 9 December 2013 (no records) Searched 7 November 2014 (no records)
Database : article Search on : autism OR autistic OR asperger OR ASD OR pervasive [All indexes] and chelation OR chelating OR DMSA [All indexes]
Worldcat (limited to theses/dissertations)
(www.worldcat.org) Searched 9 December 2013 (2 records) Searched 7 November 2014. Limited to 2013 to 2014 (no records)
(kw:autis* OR kw:asperger* OR kw:ASD OR kw:ASDs) AND (kw:chelation OR kw:chelator OR kw:DMSA)
metaRegister of Controlled Trials (mRCT)
(www.controlled‐trials.com/mrct/search.html) Searched 10 December 2013 (9 records) Searched 7 November 2014 (no new records)
Autis* AND (chelat* OR DMSA)
ClinicalTrials.gov (CT.gov)
clinicaltrials.gov
Seached 9 December 2013 ( 5 records)
Searched 7 November 2014 (1 record)
Advanced search: autism OR aspergers OR ASD OR pervasive | chelation OR chelating OR chelator OR DMSA
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
(www.who.int/ictrp/en/) Searched 9 December 2013 (12 records) Searched 7 November 2014. Limited to Date of registration, between: 01/12/2013 and 7/11/2014 (no new records)
Search 1
autism AND chleation
Search 2
Condition autis% or asperger% or PDD% or ASD% or pervasive AND Intervention "2,2'‐Dipyridyl" or alcaligin or antipyrylazo or arsenazo or bixalomer or cabiotraxetan or calcobutrol or caldiamide or calixarene or calteridol or "caloxetic acid" or "carboxymethyl beta cyclodextrincaseins" or catenane or chitson or "choline tetrathiomolybdate" or "citric acid" or clathrin*
Search 3
Condition autis% or asperger% or PDD% or ASD% or pervasive AND Intervention dimercaprol or “dimercapto succinic acid” or “dimercaptosuccinic acid” or DMSA or deferasirox or deferiprone or deferitrin or deferoxamine or deferriferrithiocin or diglycine or dimercaprol or dimethyldithiocarbamate or dithizone or ditiocarb
Search 4
Condition autis% or asperger% or PDD% or ASD% or pervasive AND Intervention edetate or Edetic Acid or Egtazic Acid or "Fura‐2" or "Humic Substances" or "Nitrilotriacetic Acid" or Penicillamine or "Pentetic Acid" or "phytic acid" or Phytochelatins or Razoxane or "salicylhydroxamic acid" or sugammadex or Succimer or "Technetium Tc 99m Pentetate " or Thenoyltrifluoroacetone or Trientine or triethylenetetramine* or tropantiol or Unithiol or versetamide
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adams 2009.
| Methods | Randomised controlled trial. Phase 1 involved 7 days of glutathione lotion (experimental group) or placebo lotion (control group), followed by 3 days of oral DMSA. Phase 2 involved 3 days on oral DMSA (experimental group) or placebo (control group), followed by 11 days off, with the cycle repeated up to 6 times Duration: 4 months |
|
| Participants | Setting: Arizona residents Diagnosis of autism spectrum disorder made by a psychologist, a psychiatrist or a developmental paediatrician Of the 82 children enrolled in the study, 77 (69 male, 8 female) completed the initial blood draw and 65 completed phase 1. 1 child was excluded as the result of elevated liver enzymes, 4 children dropped out after their physical examination, 11 children dropped out after the initial blood draw and an additional child did not submit urine for analysis after taking DMSA. 49 of the 65 children who completed phase 1 continued into phase 2 ‐ 8 children did not meet phase 2 inclusion criteria (i.e. they did not have high urinary excretion of toxic metals), 1 child had exceedingly high levels of lead excretion (and this child was referred to a physician for potential acute lead exposure), 7 families discontinued for personal reasons and 1 family discontinued because of a mild adverse effect (lethargy, diminished appetite). Of the 49 children who began phase 2, 41 finished ‐ 1 child dropped out because of elevated liver enzymes resulting from psychiatric medication, 1 child discontinued because of the death of a family member, 2 children dropped out because of perceived lack of benefit (1 child in the experimental group and 1 child in the control group) and 4 children discontinued as the result of adverse effects (2 children in the experimental group (sleep problems, worsening of behaviours and skills) and 2 children in the control group (worsening of behaviours)) Age range: 3 to 8 years (M 6.3 years for the 77 children who completed the initial blood draw) |
|
| Interventions |
Experimental group: glutathione lotion, followed by 1 round of oral DMSA (phase 1), followed by up to 6 more rounds of oral DMSA (phase 2)
Control group: placebo lotion, followed by 1 round of oral DMSA (phase 1), followed by up to 6 rounds of placebo (phase 2)
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although each of the 3 reports stated randomised, no details were given |
| Allocation concealment (selection bias) | Unclear risk | No details were given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | ClinicalTrials.gov indicates that participants, caregivers and investigators were blinded, and efforts were made to make the placebo comparable with oral DMSA in appearance and smell: "It [the placebo] was packed in identical pills and bottles as the DMSA. Since DMSA has a strong smell, each bottle included a small slotted container that contained DMSA, so that the medication smell was present in the container" (p 5). However, it is uncertain whether parents knew that all children would receive at least 1 round of oral DMSA during the first phase, and that only high excreters would continue on to the second phase |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although ClinicalTrials.gov indicates that caregivers (who assessed certain behavioural outcomes) were blind, no details were given about whether all lab technicians and ADOS evaluators were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The reason for missing data is unlikely to be related to the true outcome |
| Selective reporting (reporting bias) | High risk | Level of urinary excretion of toxic metals was reported for all combined participants after phase 1, and for participants in the experimental group after phase 2, but not for participants in the control group after phase 2 |
| Other bias | High risk | Given that both groups received an initial round of oral DMSA in phase 1, it is likely that a carry‐over effect occurred |
ADOS: Autism Diagnostic Observation Schedule. ATEC: Autism Treatment Evaluation Checklist. CBC: complete blood count. DMSA: dimercaptosuccinic acid. PDD‐BI: Pervasive Developmental Disorder Behavior Inventory. PGI: Parental Global Impressions. RBC: red blood cell. SAS: Severity of Autism Scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blaucok‐Busch 2012 | Not a randomised controlled trial |
| Eppright 1996 | Not a randomised controlled trial |
| Geier 2006a | Not a randomised controlled trial |
| Geier 2006b | Not a randomised controlled trial |
| Geier 2007 | Not a randomised controlled trial |
| Lonsdale 2002 | Not a randomised controlled trial |
| Nataf 2006 | Not a randomised controlled trial |
| NCT00376194 | Study withdrawn before enrolment |
| Patel 2007 | Not a randomised controlled trial |
Contributions of authors
Stephen James: wrote the protocol and the review, screened records, reviewed the included study, assessed risk of bias and managed production of the review.
Shawn W Stevenson: contributed to the write‐up of the protocol and the review, screened records, reviewed the included study and assessed risk of bias.
Natalie Silove: contributed to the write‐up of the protocol and the review and reviewed the included study.
Katrina Williams: contributed to the write‐up of the protocol and the review, reviewed the included study and assessed risk of bias.
Sources of support
Internal sources
-
University of Melbourne, Australia.
Shawn Stevenson received a salary through support of the Williams Collie Trust, University of Melbourne, for autism research during the time of this protocol. Katrina Williams received a salary through support of the APEX Australia Chair of Developmental Medicine, University of Melbourne. The University provided IT, office and library facilities during the time of this protocol.
-
NSW Health Service, Australia.
Natalie Silove's salary is paid by NSW Health Service ‐ The Sydney Children's Hospital Network
External sources
No sources of support supplied
Declarations of interest
Stephen James is employed by Southwest Autism Research and Resource Center. His work is not related to chelation, and he receives no personal funds.
Shawn W Stevenson was employed by Autism Victoria at the time of this review. He was an unpaid Board Member of Support and Advocacy for Autism Spectrum Individuals and Families. Shawn is currently employed by the University of Melbourne and has received no personal funds.
Natalie Silove works at the Children's Hospital Westmead, which is enrolling up to eight participants in a phase two drug trial in adolescents with fragile X syndrome. It is not related to autism or to chelation. Natalie receives no personal funds.
Katrina Williams gave a talk about treatments for autism at a symposium organised by Janssen‐Cilag Pty Ltd. Janssen‐Cilag had no control over the contents of the talk, and the speaker's fee was paid to the University that employs her. She does not have an ongoing relationship with Janssen‐Cilag. She is not involved in any funded work relevant to chelation therapy.
Edited (no change to conclusions)
References
References to studies included in this review
Adams 2009 {published data only}
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part a ‐ medical results. BMC Pharmacology & Toxicology 2009;9(16):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part b ‐ behavioral results. BMC Pharmacology & Toxicology 2009;9(17):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00811083. Dimercaptosuccinic acid (DMSA) treatment of children with autism and heavy metal toxicity. http://clinicaltrials.gov/show/NCT00811083 (accessed 19 December 2013).
References to studies excluded from this review
Blaucok‐Busch 2012 {published data only}
- Blaucok‐Busch E, Amin OR, Dessoki HH, Rabah T. Efficacy of DMSA therapy in a sample of Arab children with autistic spectrum disorder. Maedica 2012;7(3):214‐21. [PMC free article] [PubMed] [Google Scholar]
Eppright 1996 {published data only}
- Eppright TD, Sanfacon JA, Horwitz EA. Attention deficit hyperactivity disorder, infantile autism, and elevated blood‐lead: a possible relationship. Missouri Medicine 1996;93(3):136‐8. [PubMed] [Google Scholar]
Geier 2006a {published data only}
- Geier DA, Geier MR. A clinical trial of combined anti‐androgen and anti‐heavy metal therapy in autistic disorders. Neuro Endocrinology Letters 2006;27(6):833‐8. [PubMed] [Google Scholar]
Geier 2006b {published data only}
- Geier DA, Geier MR. A prospective assessment of porphyrins in autistic disorders: a potential marker for heavy metal exposure. Neurotoxicity Research 2006;10(1):57‐64. [DOI] [PubMed] [Google Scholar]
Geier 2007 {published data only}
- Geier DA, Geier MR. A prospective study of mercury toxicity biomarkers in autistic spectrum disorders. Journal of Toxicology and Environmental Health, Part A 2007;70(20):1723‐30. [DOI] [PubMed] [Google Scholar]
Lonsdale 2002 {published data only}
- Lonsdale D, Shamberger RJ, Audhya T. Treatment of autism spectrum children with thiamine tetrahydrofurfuryl disulfide: a pilot study. Neuro Endocrinology Letters 2002;23(4):303‐8. [PubMed] [Google Scholar]
Nataf 2006 {published data only}
- Nataf R, Skorupka C, Amet L, Lam A, Springbett A, Lathe R. Porphyrinuria in childhood autistic disorder: implications for environmental toxicity. Toxicology and Applied Pharmacology 2006;214(2):99‐108. [DOI] [PubMed] [Google Scholar]
NCT00376194 {published data only}
- NCT00376194. Mercury chelation to treat autism. http://clinicaltrials.gov/show/NCT00376194 (accessed 19 December 2013).
Patel 2007 {published data only}
- Patel K, Curtis LT. A comprehensive approach to treating autism and attention‐deficit hyperactivity disorder: a prepilot study. Journal of Alternative and Complementary Medicine 2007;13(10):1091‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2013
- Adams JB, Audhya T, McDonough‐Means S, Rubin RA, Quig D, Geis E, et al. Toxicological status of children with autism vs. neurotypical children and the association with autism severity. Biological Trace Element Research 2013;151(2):171‐80. [DOI] [PubMed] [Google Scholar]
Albizzati 2012
- Albizzati A, More L, Candia D, Saccani M, Lenti C. Normal concentrations of heavy metals in autistic spectrum disorders. Minerva Pediatrica 2012;64(1):27‐31. [PubMed] [Google Scholar]
Amin‐Zaki 1974
- Amin‐Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood M. Intra‐uterine methylmercury poisoning in Iraq. Pediatrics 1974;54(5):587‐95. [PubMed] [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5). 5th Edition. Arlington, VA: American Psychiatric Publishing, 2013. [Google Scholar]
Aschner 1990
- Aschner M, Aschner JL. Mercury neurotoxicity: mechanisms of blood‐brain barrier transport. Neuroscience and Biobehavioral Reviews 1990;14(2):169‐76. [DOI] [PubMed] [Google Scholar]
Bakir 1973
- Bakir F, Damluji SF, Amin‐Zaki L, Murtadha M, Khalidi A, Al‐Rawi NY, et al. Methylmercury poisoning in Iraq. Science 1973;181(4096):230‐41. [DOI] [PubMed] [Google Scholar]
Bernard 2001
- Bernard S, Enayati A, Redwood L, Roger H, Binstock T. Autism: a novel form of mercury poisoning. Medical Hypotheses 2001;56(4):462‐71. [DOI] [PubMed] [Google Scholar]
Bradstreet 2003
- Bradstreet J, Geier DA, Kartzinel JJ, Adams JB, Geier MR. A case‐control study of mercury burden in children with autistic spectrum disorders. Journal of American Physicians and Surgeons 2003;8(3):76‐9. [Google Scholar]
Brown 2006
- Brown MJ, Willis T, Omalu B, Leiker R. Deaths resulting from hypocalcemia after administration of edetate disodium: 2003‐2005. Pediatrics 2006;118(2):e534‐6. [DOI] [PubMed] [Google Scholar]
Cheuk 2011
- Cheuk DKL, Wong V, Chen WX. Acupuncture for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD007849.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Christon 2010
- Christon LM, Mackintosh VH, Myers BJ. Use of complementary and alternative medicine (CAM) treatments by parents of children with autism spectrum disorders. Research in Autism Spectrum Disorders 2010;4(2):249‐59. [Google Scholar]
Cohen 1976
- Cohen DJ, Johnson WT, Caparulo BK. Pica and elevated blood lead level in autistic and atypical children. American Journal of Diseases of Children 1976;130(1):47‐8. [DOI] [PubMed] [Google Scholar]
Cohen 1982
- Cohen DJ, Paul R, Anderson GM, Harcherik DF. Blood lead in autistic children. Lancet 1982;320(8289):94‐5. [DOI] [PubMed] [Google Scholar]
Counter 2004
- Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicology and Applied Pharmacology 2004;198(2):209‐30. [DOI] [PubMed] [Google Scholar]
Dans 2002
- Dans AL, Tan FN, Villarruz‐Sulit EC. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002785] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2013
- Davis TN, O'Reilly M, Kang S, Lang R, Rispoli M, Sigafoos J, et al. Chelation treatment for autism spectrum disorders: a systematic review. Research in Autism Spectrum Disorders 2013;7(1):49‐55. [Google Scholar]
Deth 2008
- Deth R, Muratore C, Benzecry J, Power‐Charnitsky V, Waly M. How environmental and genetic factors combine to cause autism: a redox/methylation hypothesis. Neurotoxicology 2008;29(1):190‐201. [DOI] [PubMed] [Google Scholar]
Drugs.com 2012
- Drugs.com. Drug information online. www.drugs.com/drug‐class/chelating‐agents.html (accessed 10 January 2012).
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 2000
- Ernst E. Chelation therapy for coronary heart disease: an overview of all clinical investigations. American Heart Journal 2000;140(1):139‐41. [DOI] [PubMed] [Google Scholar]
Garrecht 2011
- Garrecht M, Austin DW. The plausibility of a role for mercury in the etiology of autism: a cellular perspective. Toxicological and Environmental Chemistry 2011;93(5‐6):1251‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geretsegger 2014
- Geretsegger M, Elefant C, Mössler KA, Gold C. Music therapy for people with autism spectrum disorder. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD004381.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goin‐Kochel 2009
- Goin‐Kochel RP, Mackintosh VH, Myers BJ. Parental reports on the efficacy of treatments and therapies for their children with autism spectrum disorders. Research in Autism Spectrum Disorders 2009;3(2):528‐37. [Google Scholar]
Goldman 2001
- Goldman LR, Shannon MW, American Academy of Pediatrics: Committee on Environmental Health. Technical report: mercury in the environment: implications for pediatricians. Pediatrics 2001;108(1):197‐205. [DOI] [PubMed] [Google Scholar]
Golnik 2009
- Golnik AE, Ireland M. Complementary alternative medicine for children with autism: a physician survey. Journal of Autism and Developmental Disorders 2009;39(7):996‐1005. [DOI] [PubMed] [Google Scholar]
Grandjean 1997
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, et al. Cognitive deficit in 7‐year‐old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology 1997;19(6):417‐28. [DOI] [PubMed] [Google Scholar]
Green 2006
- Green VA, Pituch KA, Itchon J, Choi A, O'Reilly M, Sigafoos J. Internet survey of treatments used by parents of children with autism. Research in Developmental Disabilities 2006;27(1):70‐84. [DOI] [PubMed] [Google Scholar]
Hallmayer 2011
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Archives of General Psychiatry 2011;68(11):1095‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanson 2007
- Hanson E, Kalish L, Bunce E, Curtis C, McDaniel S, Ware J, et al. Use of complementary and alternative medicine among children diagnosed with autism spectrum disorder. Journal of Autism and Developmental Disorders 2007;37(4):629‐36. [DOI] [PubMed] [Google Scholar]
Hedge 2009
- Hedge ML, Bharathi P, Suram A, Venugopal C, Jagannathan R, Poddar P, et al. Challenges associated with metal chelation therapy in Alzheimer's disease. Journal of Alzheimer's Disease 2009;17(3):457‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hertz‐Picciotto 2010
- Hertz‐Picciotto I, Green PG, Delwiche L, Hansen R, Walker C, Pessah LN. Blood mercury concentrations in CHARGE study children with and without autism. Environmental Health Perspectives 2010;118(1):161‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmes 2003
- Holmes AS, Blaxill MF, Haley BE. Reduced levels of mercury in first baby haircuts of autistic children. International Journal of Toxicology 2003;22(4):277‐85. [DOI] [PubMed] [Google Scholar]
James 2011
- James S, Montgomery P, Williams K. Omega‐3 fatty acids supplementation for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD007992.pub2] [DOI] [PubMed] [Google Scholar]
Kern 2007
- Kern JK, Grannemann BD, Trivedi MH, Adams JB. Sulfhydryl‐reactive metals in autism. Journal of Toxicology and Environmental Health, Part A 2007;70(8):715‐21. [DOI] [PubMed] [Google Scholar]
Kosnett 2010
- Kosnett MJ. Chelation for heavy metals (arsenic, lead, and mercury): protective or perilous?. Clinical Pharmacology & Therapeutics 2010;88(3):412‐5. [DOI] [PubMed] [Google Scholar]
Lecavalier 2006
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders 2006;36(8):1101‐14. [DOI] [PubMed] [Google Scholar]
Levy 2003
- Levy SE, Hyman SL. Use of complementary and alternative treatments for children with autistic spectrum disorders is increasing. Pediatric Annals 2003;32(10):685‐91. [DOI] [PubMed] [Google Scholar]
Liu 2008
- Liu J, Goyer RA, Waalkes MP. Toxic effects of metals. In: Klaassen CD editor(s). Casarett & Doull's Toxicology: The Basic Science of Poisons. 7th Edition. New York: McGraw‐Hill, 2008:931‐79. [Google Scholar]
Millward 2008
- Millward C, Ferriter M, Calver SJ, Connell‐Jones GG. Gluten‐ and casein‐free diets for autistic spectrum disorder. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003498.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mitka 2008
- Mitka M. Chelation therapy trials halted. JAMA 2008;300(19):2236. [DOI] [PubMed] [Google Scholar]
Morgan 2002
- Morgan BW, Kori S, Thomas JD. Adverse effects in 5 patients receiving EDTA at an outpatient chelation clinic. Veterinary and Human Toxicology 2002;44(5):274‐6. [PubMed] [Google Scholar]
Nye 2005
- Nye C, Brice A. Combined vitamin B6‐magnesium treatment in autism spectrum disorder. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003497.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osterloh 1986
- Osterloh J, Becker CE. Pharmacokinetics of CaNa2EDTA and chelation of lead in renal failure. Clinical Pharmacology and Therapeutics 1986;40(6):686‐93. [DOI] [PubMed] [Google Scholar]
Rahbar 2013
- Rahbar MH, Samms‐Vaughan M, Loveland KA, Ardjomand‐Hessabi M, Chen Z, Bressler J, et al. Seafood consumption and blood and blood mercury concentrations in Jamaican children with and without autism spectrum disorders. Neurotoxicity Research 2013;23(1):22‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Semple 2011
- Semple S, Hewton C, Paterson F, Angley M. Complementary medicine products used in autism ‐ evidence for efficacy and safety. In: Williams T editor(s). Autism Spectrum Disorders ‐ From Genes to Environment. Rijeka, Croatia: InTech, 2011:77‐100. [Google Scholar]
Shattuck 2007
- Shattuck PT, Seltzer MM, Greenberg JS, Orsmond GI, Bolt D, Kring S, et al. Change in autism symptoms and maladaptive behaviors in adolescents and adults with an autism spectrum disorder. Journal of Autism and Developmental Disorders 2007;37(9):1735‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2006
- Sinha Y, Silove N, Williams K. Letters. Chelation therapy and autism. BMJ 2006;333(7571):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2011
- Sinha Y, Silove N, Hayen A, Williams K. Auditory integration training and other sound therapies for autism spectrum disorders (ASD). Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003681.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vamnes 2000
- Vamnes JS, Eide R, Isrenn R, Hol PJ, Gjerdet NR. Diagnostic value of a chelating agent in patients with symptoms allegedly caused by amalgam fillings. Journal of Dental Research 2000;79(3):868‐74. [DOI] [PubMed] [Google Scholar]
Zecavati 2009
- Zecavati N, Spence SJ. Neurometabolic disorders and dysfunction in autism spectrum disorders. Current Neurology and Neuroscience Reports 2009;9(2):129‐36. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
James 2013
- James S, Williams K, Silove N, Stevenson SW. Chelation for autism spectrum disorder (ASD). Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010766] [DOI] [Google Scholar]


