Abstract
Background
Depression is a recurrent illness with high rates of chronicity, treatment‐resistance and significant economic impact. There is evidence in the literature that S‐adenosyl methionine (SAMe), a naturally occurring compound in the human body, has antidepressant efficacy. This product may be an important addition to the armamentarium of antidepressant agents.
Objectives
To assess the effects of SAMe in comparison with placebo or antidepressants for the treatment of depression in adults.
Search methods
We searched the Cochrane Common Mental Disorders Group's Specialised Register (CCMDCTR Studies and Reference Register), MEDLINE, EMBASE, PsycINFO, international trial registers ClinicalTrials.gov and the World Health Organization trials portal (ICTRP). We checked reference lists, performed handsearching and contacted experts in the field. The CCMDCTR literature search was last updated on 5 February 2016.
Selection criteria
Randomised controlled trials comparing SAMe with placebo or antidepressants in adults with a diagnosis of major depression.
Data collection and analysis
Two authors independently performed extraction of data and assessment of risk of bias. We contacted trialists of included studies for additional information.
Main results
This systematic review included eight trials comparing SAMe with either placebo, imipramine, desipramine or escitalopram. We accepted trials that used SAMe as monotherapy or as add‐on therapy to selective serotonin reuptake inhibitors (SSRIs), and we accepted both oral and parenteral administration. The review involved 934 adults, of both sexes, from inpatient and outpatient settings.
The trials were at low risk of reporting bias. We judged the risk of selection, performance, detection and attrition bias as unclear or low, and one study was at high risk of attrition bias.
There was no strong evidence of a difference in terms of change in depressive symptoms from baseline to end of treatment between SAMe and placebo as monotherapy (standardised mean difference (SMD) ‐0.54, 95% confidence interval (CI) ‐1.54 to 0.46; P = 0.29; 142 participants; 2 studies; very low quality evidence). There was also no strong evidence of a difference in terms of drop‐out rates due to any reason between SAMe and placebo, when used as monotherapy (risk ratio (RR) 0.88, 95% CI 0.61 to 1.29; P = 0.52; 142 participants; 2 studies; low quality evidence).
Low quality evidence showed that the change in depressive symptoms from baseline to end of treatment was similar between SAMe and imipramine, both as monotherapy (SMD ‐0.04, 95% CI ‐0.34 to 0.27; P = 0.82; 619 participants; 4 studies). There was also no strong evidence of a difference between SAMe and a tricyclic antidepressant in terms of drop‐outs due to any reason (RR 0.61, 95% CI 0.28 to 1.31; P = 0.2; 78 participants; 3 studies; very low quality evidence).
There was little evidence of a difference in terms of change in depressive symptoms from baseline to end of treatment between SAMe and escitalopram, both as monotherapy (MD 0.12, 95% CI ‐2.75 to 2.99; P = 0.93; 129 participants; 1 study; low quality evidence). There was no strong evidence of a difference between SAMe and escitalopram in terms of drop‐outs due to any reason (RR 0.81, 95% CI 0.57 to 1.16; P = 0.26; 129 participants; 1 study; low quality evidence).
There was low quality evidence that SAMe is superior to placebo as add‐on to SSRIs in terms of change in depressive symptoms from baseline to end of treatment (MD ‐3.90, 95% CI ‐6.93 to ‐0.87; P = 0.01; 73 participants; 1 study). There was no strong evidence of a difference between SAMe and placebo as adjunctive therapy to an SSRI in terms of drop‐outs due to any reason (RR 0.70, 95% CI 0.31 to 1.56; P = 0.38; 73 participants; 1 study; very low quality evidence).
For all comparisons, secondary outcome measures of response and remission rates were consistent with these primary outcome measures.
With regard to all extractable measures of the acceptability of SAMe, the quality of the evidence was low to very low. SAMe was not different from placebo and established antidepressants. The exception was that compared to imipramine, fewer participants experienced troublesome adverse effects when treated with parenteral SAMe.
The specific adverse effects were not detailed in most of the included studies. There were two reports of mania/hypomania recorded for 441 participants in the SAMe arm.
Authors' conclusions
Given the absence of high quality evidence and the inability to draw firm conclusions based on that evidence, the use of SAMe for the treatment of depression in adults should be investigated further. Future trials should be in the form of large randomised controlled clinical trials of high methodological quality, with particular attention given to randomisation, allocation concealment, blinding and the handling of missing data. Comparator antidepressants from all classes should be used. Adverse events should be detailed for each participant, bearing in mind that induction of mania is of particular interest.
Plain language summary
S‐adenosyl methionine for depression in adults
Description of the illness
Depression is a common, recurrent mood disorder. Usually, affected people experience symptoms such as low mood and a loss of interest or pleasure. People with depression also often experience some of the following symptoms: weight loss or gain; a decrease or increase in appetite, insomnia or hypersomnia; restlessness or fatigue as well as excessive guilt; feelings of worthlessness, poor concentration and indecisiveness; recurrent thoughts of death and suicidal thoughts. The medicines most often used in the treatment of depression are antidepressants.
Description of the medicine
S‐adenosyl methionine (often referred to as SAMe) is naturally present in the human body and there is evidence that it is effective as an antidepressant. SAMe has been marketed in some European countries since the mid‐1980s for the treatment of depression and for other medical conditions such as osteoarthritis (joint disease that causes joint pain and stiffness), fibromyalgia (widespread pain and stiffness), liver disease and migraine headaches. However, SAMe is not formally approved in the UK for the treatment of depression, and in the USA it is classified only as a dietary supplement.
Aim of the review
Given the extent of the burden of depression, the high rates of chronicity and the high number of people who do not respond to the conventional treatments, there is an urgent need to examine alternative medications. In this review, we investigated the effectiveness of SAMe in the treatment of depression.
Results
We searched scientific databases for all randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) in adults with a diagnosis of major depression, where SAMe was compared to either placebo (a pretend treatment) or other antidepressant medicines (e.g. imipramine and escitalopram) carried out before February 2016.
We included eight studies involving 934 people in this review. There was no strong evidence of a difference in effectiveness between SAMe and imipramine or escitalopram when used alone. It was superior to placebo when used in combination with selective serotonin reuptake inhibitor antidepressants, but this evidence was of low quality. There was no significant difference in terms of effectiveness between SAMe and placebo alone, but again this evidence was of very low quality. The acceptability of SAMe did not differ from that of antidepressants or placebo. The exception was that fewer participants experienced side effects when treated with SAMe compared with imipramine. Though, the quality of the evidence for acceptability of SAMe was of low quality.
Limitations of this review were that not all the relevant data could be obtained despite efforts to contact the authors and some of the included studies were of low quality.
What should happen next
It is not possible to draw any firm conclusions from this review and the evidence included is of limited quality. There is a need to investigate the efficacy and acceptability of SAMe for the treatment of depression in adults further in larger and better planned trials.
Summary of findings
Summary of findings for the main comparison. S‐adenosyl methionine as monotherapy compared to placebo as monotherapy for depression in adults.
| S‐adenosyl methionine as monotherapy compared to placebo as monotherapy for depression in adults | ||||||
|
Patient or population: adults with depression Settings: inpatient and outpatient Intervention: SAMe as monotherapy Comparison: placebo as monotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo as monotherapy | SAMe as monotherapy | |||||
| Efficacy. Change in scores from baseline to end of treatment on the depression rating scale A larger negative SMD indicates greater improvement in the SAMe group Follow‐up: 3 to 12 weeks | ‐ | The mean change in scores from baseline to end of treatment on the depression rating scale in the SAMe groups was 0.54 standard deviations greater (1.54 lower to 0.46 higher) indicating more improvement. However, this was not statistically significant | ‐ | 142 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD ‐0.54 (95% CI ‐1.54 to 0.46) |
| Acceptability Participants dropping out of treatment during the study period for any reason Follow‐up: 3 to 12 weeks | Moderate |
RR 0.88
(0.61 to 1.29) not statistically significant |
142 (2 studies) | ⊕⊕⊝⊝ low4,5 | ‐ | |
| 37 per 100 | 32 per 100 (22 to 47) | |||||
|
Proportions of participants responding to treatment
≥ 50% reduction in depression score from baseline to end of treatment A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 3 to 12 weeks |
Moderate |
RR 1.77
(0.51 to 6.13) not statistically significant |
142 (2 studies) | ⊕⊕⊝⊝ low4,5,6 | ‐ | |
| 21 per 100 | 38 per 100 (11 to 100) | |||||
|
Proportions of participants achieving remission
Depression rating scale score within the normal range at the end of the study A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 12 weeks |
Moderate |
RR 1.69
(0.85 to 3.36) not statistically significant |
124 (1 study) | ⊕⊕⊝⊝ low4,5 | ‐ | |
| 17 per 100 | 28 per 100 (14 to 56) | |||||
| Acceptability. Participants experiencing troublesome adverse effects of any nature | No data | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SAMe: S‐adenosyl methionine; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 downgraded one point because of inconsistency caused by high level of heterogeneity (I2 = 72%; P = 0.06), related to the different duration of the trials and sample size (3 weeks, 18 participants for Kagan 1990; 12 weeks, 124 participants for Mischoulon 2014). 2 downgraded one point because of imprecision caused by small sample size, fewer than 400. 3 downgraded one point because of imprecision caused by a 95% confidence interval that included no effect and the upper and lower confidence limit crosses an effect size of 0.5 in either direction. 4 downgraded one point because of imprecision caused by a total number of events that was fewer than 300. 5 downgraded one point because of imprecision caused by a 95% confidence interval that includes both no effect and appreciable benefit and appreciable harm (the threshold for 'appreciable benefit' or 'appreciable harm' was a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%). 6 The two studies showed a non‐significant heterogeneity (I2 = 49%; P = 0.16). This was due to the Kagan 1990 study where the number of responder was bigger in the SAMe arm than the placebo arm, but it was not statistically significant (RR 4.80, 95% CI 0.72 to 32.15; P = 0.11).
Summary of findings 2. S‐adenosyl methionine as monotherapy compared to tricyclic antidepressant agent as monotherapy for depression in adults.
| S‐adenosyl methionine compared to tricyclic antidepressant agent as monotherapy for depression in adults | ||||||
|
Patient or population: adults with depression Settings: inpatient and outpatient Intervention: SAMe as monotherapy Comparison: TCA as monotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SAMe vs. TCA as monotherapy | |||||
| Efficacy. Change in scores from baseline to end of treatment on the depression rating scale A larger negative SMD indicates greater improvement in the SAMe group Follow‐up: 2 to 6 weeks | ‐ | The mean change in scores from baseline to end of treatment on the depression rating scale in the SAMe group was 0.04 standard deviations greater (0.34 lower to 0.27 higher), indicating more improvement. However, this was not statistically significant | ‐ | 619 (4 studies) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.04 (95% CI ‐0.34 to 0.27) |
| Acceptability Participants dropping out of treatment during the study period for any reason Follow‐up: 2 to 6 weeks | Study population |
RR 0.61
(0.28 to 1.31) not statistically significant |
78 (3 studies) | ⊕⊝⊝⊝ very low3,4,5 | ‐ | |
| 33 per 100 | 20 per 100 (9 to 44) | |||||
| Moderate | ||||||
| 27 per 100 | 16 per 100 (7 to 35) | |||||
|
Proportions of participants responding to treatment
≥ 50% reduction in depression score from baseline to end of treatment A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 2 to 6 weeks |
Study population |
RR 1.14
(0.83 to 1.56) not statistically significant |
622 (4 studies) | ⊕⊝⊝⊝
very low5,6,7 |
‐ | |
| 50 per 100 | 57 per 100 (42 to 79) | |||||
| Moderate | ||||||
| 34 per 100 | 39 per 100 (28 to 54) | |||||
| Proportions of participants achieving remission Depression rating scale score within the normal range at the end of the study | No data | ‐ | ‐ | ‐ | ‐ | ‐ |
| Acceptability. Participants experiencing troublesome adverse effects of any nature Follow‐up: 4 to 6 weeks | Study population |
RR 0.68
(0.52 to 0.88) statistically significant |
604 (3 studies) | ⊕⊕⊝⊝ low4,8 | ‐ | |
| 49 per 100 | 33 per 100 (25 to 43) | |||||
| Moderate | ||||||
| 47 per 100 | 32 per 100 (24 to 41) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; SAMe: S‐adenosyl methionine; SMD: standardised mean difference; TCA: tricyclic antidepressant. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 downgraded one point because of risk of bias: the Bell 1988 study was at unclear risk of selection, performance and detection bias; three studies were at unclear risk of selection, performance, detection and attrition bias (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). 2 downgraded one point because of inconsistency caused by significant heterogeneity between the studies (I2 = 57%; P = 0.07). It was due to one study that favoured SAMe over the active antidepressant (imipramine) (Bell 1988). The different result of the Bell 1988 study could be due to its shorter duration (two weeks) and to the fact that the therapeutic actions of imipramine usually are not immediate, but often delayed by two to four weeks. This could suggest a more rapid onset of action of the SAMe. Also, the doses and routes of administration of SAMe among the studies were heterogeneous. However, when subgroup analysis was conducted separating data regarding oral and parenteral administration of SAMe, the outcome was not affected. 3 downgraded one point because of risk of bias: the Bell 1988 study was at unclear risk of selection, performance and detection bias; the Bell 1994 study was at high risk of attrition bias and at unclear risk of selection bias; the De Vanna 1992 study was at unclear risk of selection, performance, detection and attrition bias. 4 downgraded one point because of imprecision caused by a total number of events that was fewer than 300. 5 downgraded one point because of imprecision caused by a 95% confidence interval that includes both no effect and appreciable benefit and appreciable harm (the threshold for 'appreciable benefit' or 'appreciable harm' was a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%). 6 downgraded one point because of risk of bias: the Bell 1988 study was at unclear risk of selection, performance and detection bias; the Bell 1994 study was at high risk of attrition bias and at unclear risk of selection bias; two studies were at unclear risk of selection, performance, detection and attrition bias (Delle Chiaie 2000a; Delle Chiaie 2000b). 7 downgraded one point because of inconsistency caused by significant heterogeneity among the studies (I2 = 58%, P = 0.07). It could be caused by the different durations of the treatment. Then, the studies differed in terms of antidepressants compared to the intervention, but it did not explain the heterogeneity. Also, the doses and routes of administration of SAMe among the studies were heterogeneous. However, when we conducted subgroup analysis separating data regarding oral and parenteral administration of SAMe, the outcome was not affected. 8 downgraded one point because of risk of bias: the three studies were at unclear risk of selection, performance, detection and attrition bias (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). 9 downgraded one point because of risk of bias: the Bell 1988 study was at unclear risk of selection, performance and detection bias; the De Vanna 1992 study was at unclear risk of selection, performance, detection and attrition bias.
Summary of findings 3. S‐adenosyl methionine as monotherapy compared to SSRI antidepressant agent as monotherapy for depression in adults.
| S‐adenosyl methionine compared to SSRI antidepressant agent as monotherapy for depression in adults | ||||||
|
Patient or population: adults with depression
Settings: outpatients
Intervention: SAMe as monotherapy Comparison: SSRI antidepressant agent as monotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | SAMe vs. SSRI antidepressant agent as monotherapy | |||||
| Efficacy. Change in scores from baseline to end of treatment on the depression rating scale A larger negative MD indicates greater improvement in the SAMe group Follow‐up: 12 weeks | ‐ | The mean change in scores from baseline to end of treatment on the depression rating scale in the SAMe group was 0.12 standard deviations lower (2.75 lower to 2.99 higher), indicating less improvement. However, this was not statistically significant | ‐ | 129 (1 study) | ⊕⊕⊝⊝ low1,2 | MD 0.12 (95% CI ‐2.75 to 2.99) |
| Acceptability Participants dropping out of treatment during the study period for any reason Follow‐up: 12 weeks | Study population |
RR 0.81
(0.57 to 1.16) not statistically significant |
129 (1 study) | ⊕⊕⊝⊝ low3,4 | ‐ | |
| 54 per 100 | 44 per 100 (31 to 62) | |||||
| Moderate | ||||||
| 54 per 100 | 44 per 100 (31 to 63) | |||||
|
Proportions of participants responding to treatment
≥ 50% reduction in depression score from baseline to end of treatment A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 12 weeks |
Study population |
RR 1.06
(0.66 to 1.7) not statistically significant |
129 (1 study) | ⊕⊕⊝⊝ low3,4 | ‐ | |
| 34 per 100 | 36 per 100 (22 to 58) | |||||
| Moderate | ||||||
| 34 per 100 | 36 per 100 (22 to 58) | |||||
|
Proportions of participants achieving remission
depression rating scale score within the normal range at the end of the study A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 12 weeks |
Study population |
RR 1.02
(0.58 to 1.77) not statistically significant |
129 (1 study) | ⊕⊕⊝⊝ low3,4 | ‐ | |
| 28 per 100 | 28 per 100 (16 to 49) | |||||
| Moderate | ||||||
| 28 per 100 | 28 per 100 (16 to 49) | |||||
| Acceptability. Participants experiencing troublesome adverse effects of any nature | No data | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SAMe: S‐adenosyl methionine; SSRI: selective serotonin reuptake inhibitor. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 downgraded one point because of imprecision caused by small sample size, fewer than 400. 2 downgraded one point because of imprecision caused by a 95% confidence interval that included no effect and the upper and lower confidence limit crossed an effect size of 0.5 in either direction. 3 downgraded one point because of imprecision caused by a total number of events that was fewer than 300. 4 downgraded one point because of imprecision caused by a 95% confidence interval that includes both no effect and appreciable benefit and appreciable harm (the threshold for 'appreciable benefit' or 'appreciable harm' was a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%).
Summary of findings 4. S‐adenosyl methionine as adjunctive treatment compared to placebo as add‐on to SSRI for depression in adults.
| S‐adenosyl methionine as adjunctive treatment compared to placebo as add‐on for depression in adults | ||||||
| Patient or population: adults with depression Settings: outpatients Intervention: SAMe as adjunctive treatment to SSRI Comparison: placebo as adjunctive treatment to SSRI | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo as add‐on | SAMe as adjunctive treatment | |||||
| Efficacy. Change in scores from baseline to end of treatment on the depression rating scale A larger negative MD indicates greater improvement in the SAMe group Follow‐up: 6 weeks | ‐ | The mean change in scores from baseline to end of treatment on the depression rating scale in the SAMe group was 3.9 greater (6.93 to 0.87 lower), indicating more improvement. This was statistically significant | ‐ | 73 (1 study) | ⊕⊕⊝⊝ low1,2 | MD ‐3.90 (‐6.93 to ‐0.87) |
| Acceptability Participants dropping out of treatment during the study period for any reason Follow‐up: 6 weeks | Moderate |
RR 0.7
(0.31 to 1.56) not statistically significant |
73 (1 study) | ⊕⊝⊝⊝ very low2,3,4 | ‐ | |
| 29 per 100 | 21 per 100 (9 to 46) | |||||
|
Proportions of participants responding to treatment
≥ 50% reduction in depression score from baseline to end of treatment. A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 6 weeks |
Moderate |
RR 2.62
(1.17 to 5.83) statistically significant |
73 (1 study) | ⊕⊕⊝⊝ low2,4 | ‐ | |
| 18 per 100 | 46 per 100 (21 to 100) | |||||
|
Proportions of participants achieving remission
depression rating scale score within the normal range at the end of the study. A larger RR indicates greater response to treatment in the SAMe group Follow‐up: 6 weeks |
Moderate |
RR 3.05
(1.11 to 8.39) statistically significant |
73 (1 study) | ⊕⊕⊝⊝ low2,4 | ‐ | |
| 12 per 100 | 36 per 100 (13 to 99) | |||||
| Acceptability. Participants experiencing troublesome adverse effects of any nature | No data | ‐ | ‐ | ‐ | ‐ | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio; SAMe: S‐adenosyl methionine; SSRI: selective serotonin reuptake inhibitor. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 downgraded one point because of imprecision caused by small sample size, fewer than 400. 2 downgraded one point because of indirectness caused by a population restricted to SSRI non‐responders 3 downgraded one point because of imprecision caused by a 95% confidence interval that included both no effect and appreciable benefit and appreciable harm (the threshold for 'appreciable benefit' or 'appreciable harm' was a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%. 4 downgraded one point because of imprecision caused by a total number of events that was fewer than 300.
Background
Description of the condition
Depression is a common recurrent illness with high rates of chronicity. It ranks first among mental illnesses in the causes of worldwide disability (Murray 1997). The main symptoms of depression are low mood and a loss of interest or pleasure. Physical symptoms include weight loss or gain, a decrease or increase in appetite, insomnia or hypersomnia, psychomotor agitation or retardation and fatigue. Psychological symptoms such as excessive guilt, feelings of worthlessness, poor concentration and indecisiveness occur. Recurrent thoughts of death and suicidal thoughts and actions may also feature (APA 1994). Anxiety is common in depression (Fawcett 1983), and its presence detrimentally affects the treatment outcome (Goldberg 2012). In cases of severe depression, mood‐congruent psychotic symptoms such as hallucinations and delusions may develop.
Atypical depression may be a distinct subtype of depression. Its atypical symptoms include reactivity of mood, increased sleep and increased appetite. This type of depression may preferentially respond to one particular class of antidepressants, monoamine oxidase inhibitors (MAOIs) (Henkel 2006).
Treatment‐resistant depression is a significant problem, with a 12‐month prevalence of 2% to 3% (Nemeroff 2007). Treatment resistance has been defined as occurring when "at least two trials with antidepressants from different pharmacologic classes (adequate in terms of dosage, duration, and compliance) fail to produce a significant clinical improvement" (Berlim 2007).
Depression has significant economic impact. It is associated with significant occupational underperformance and low earnings. It is also associated with an increased risk of chronic physical illnesses, early mortality and suicide (Kessler 2012). In the USA, the economic cost of depression was USD 83 billion in 2000; USD 26 billion were direct medical costs, USD 5.4 billion were suicide‐related mortality costs and USD 51.5 billion were workplace costs (Greenberg 2003). In Europe, the total annual cost of depression in 2004 was EUR 118 billion, approximately 1% of the gross domestic product. Direct costs corresponded to EUR 22 billion for outpatient care, EUR 10 billion for hospitalisation, while indirect costs due to morbidity and mortality were EUR 76 billion (Sobocki 2006). The total cost of services for depression in England in 2007 was estimated to be GBP 1.7 billion; lost employment brought the total cost to GBP 7.5 billion. The projection is that in 2026 the costs will increase to GBP 3 billion for total cost of services and GBP 12.2 billion for lost employment (King's Fund 2008).
Description of the intervention
Currently the various major US and European guidelines for the treatment of depression provide similar basic principles of treatment, which include individualising the treatment plan, preparing the person for potential long‐term treatment, providing measurement‐based care and treating to remission. With regard to mild depression, some, but not all, guidelines suggest that it may resolve with exercise or watchful waiting, but psychotherapy or antidepressants could be used if initial efforts fail. First‐line treatment recommendations for moderate major depressive disorder include antidepressant monotherapy, psychotherapy and the combination of both (Davidson 2010). In contrast, a combination of depression‐focused psychotherapy and pharmacotherapy is considered a useful treatment choice for people with severe or chronic forms of depression (APA 2010). With regard to drugs, normally a selective serotonin reuptake inhibitor (SSRI) is chosen because of its favourable risk‐benefit ratio. In the case of people with depression who have not responded to a first SSRI antidepressant after six to eight weeks of adequate treatment, switching to an alternative antidepressant may be considered. Initially, this may be a different SSRI or a better‐tolerated newer‐generation antidepressant. Subsequently, an antidepressant of a different pharmacological class may be chosen, although this may be less well tolerated, for example venlafaxine, a tricyclic antidepressant (TCA) or an MAOI (NICE 2009).
S‐Adenosyl methionine (SAMe) was originally discovered in Italy in 1952 (Cantoni 1952), where it is commonly used in clinical practice. SAMe has been marketed in some European countries since the mid‐1980s for the treatment of depression and for other medical conditions such as osteoarthritis, fibromyalgia, liver disease and migraine headaches (Chavez 2000; Di Rocco 2000; Papakostas 2003; Shippy 2004). However, SAMe is not formally approved in the UK for the treatment of depression. In the USA, it has not been classified as a drug but is available as a non‐prescription (over‐the‐counter) dietary supplement under the Dietary Health and Supplement Act of 1999 (Papakostas 2003).
SAMe occurs naturally in the human body. It may be synthesised from adenosine triphosphate and the alpha‐amino acid methionine. Cantoni discovered it to be an active cofactor in biological methylation reactions (Kresge 2005). As a physiological donor of methyl groups, it is involved in many cellular functions including the synthesis and metabolism of neurotransmitters (Gören 2004), and its potential epigenetic effects have been highlighted (Sugden 2006).
Recommended daily doses of SAMe range from 200 mg to 1600 mg taken in divided doses, depending upon the condition for which it is being taken and its severity, and upon the route of administration (Chavez 2000; Delle Chiaie 2002; Morelli 2000). Exogenous, orally administered SAMe has a short half‐life, undergoing first‐pass effects and rapid metabolism. However, oral doses of SAMe at 1600 mg/day are significantly bioavailable and non‐toxic (Gören 2004). Because SAMe is best absorbed on an empty stomach, it should be administered 30 to 60 minutes before meals or two hours after meals; people should be instructed to adhere strictly to these directions. It may also be administered parenterally, using intramuscular or intravenous routes (Williams 2005).
With regard to possible adverse effects, SAMe is reported to induce mania in some cases (Carney 1989; Lipinski 1984). In one open study, nine of 11 people with bipolar disorder experienced a switch to an 'elevated mood state' (hypomania, mania or euphoria) (Carney 1989). Reports of induced mania and hypomania were found even in cases with no prior suggestion of bipolar disorder (Kagan 1990). A transient mixed manic episode with suicidal ideation was reported in a person with no previous psychiatric history on SAMe; recovery followed discontinuation (Gören 2004). These findings must be interpreted with caution as bipolar II disorder (diagnosed by the presence of a hypomanic episode) is sometimes misdiagnosed as major depressive disorder when hypomanic episodes are overlooked.
There is a theoretical possibility of hyperhomocysteinaemia, a condition associated with cardiac and renal complications in the long term. However, in their four‐week study of SAMe treatment of healthy participants, Gören 2004 found no elevation in homocysteine levels. Mild gastrointestinal disturbance and headache have been reported (Gören 2004; Lipinski 1984). One Cochrane review on SAMe in the treatment of alcoholic liver disease found no significant increase in adverse or serious adverse effects (Rambaldi 2006).
The cost of SAMe seems comparable in different countries. In the USA, one local national chain sells 36 SAMe 400 mg tablets for USD 42.99 (Craig Nelson 2010). In Italy, the price of 20 SAMe 400 mg tablets amounts to EUR 25.63, whereas in the UK, one local national chain sells 30 SAMe 400 mg tablets for GBP 26.71. The mean cost per tablet in these three countries is EUR 1.08. In some countries such as Italy, Germany and Russia, pharmaceutical grade SAMe is available on physician prescription only. As of 2016, the cheapest antidepressant drugs available in the UK are fluoxetine and citalopram with an approximately comparable price, the net price of a 30‐capsules pack of fluoxetine 20 mg is GBP 1.11, the net price of a 28‐tablet pack of citalopram 20 mg is GBP 1.02 (BNF 2016). Although SAMe seems more expensive, considering its adverse‐effect profile and its rapidity of onset of the antidepressant effect, it may have a specific impact on the use of resources in terms of drug acquisition, treatment duration and dosage, inpatient and outpatient care, treatment of adverse events, management of people who discontinue therapy and time off work.
How the intervention might work
The mechanism of any antidepressant effect of SAMe is unclear. It may enhance the activity of the monoamine systems strongly associated with the aetiology and treatment of depression. Animal studies demonstrated an association between SAMe treatment and increased brain concentrations of noradrenaline (norepinephrine) and serotonin (5‐HT) (Algeri 1979; Curcio 1978; Otero‐Losado 1989a; Otero‐Losado 1989b). In humans, treatment is reported to increase cerebrospinal fluid concentrations of 5‐hydroxyindole acetic acid (the main metabolite of serotonin) (Agnoli 1976). In addition, through stimulation of phospholipid methylation, SAMe may increase the fluidity of cell membranes that is linked to an increase in β‐adrenoreceptor and muscarinic (M1) receptor density (Bottiglieri 2002). Further, SAMe may influence the expression of key genes in the brain affecting behaviour, memory, learning and cognition (Sugden 2006).
Why it is important to do this review
Given the extent of the burden of depression, the prevalence of treatment resistance described above, and the substantial economic cost associated with ineffective depression management compared with successfully treated depression (Byford 2011), there is an urgent need to examine less well recognised approaches to its pharmacological management. SAMe may be an important addition to the armamentarium of antidepressant agents. There is evidence that SAMe has antidepressant efficacy. Existing meta‐analyses of randomised controlled trials (RCT) of SAMe in depression have shown superior efficacy to placebo and efficacy equivalent to TCAs (Bressa 1994; Williams 2005), a long‐established category of antidepressant (Arroll 2009). In addition, SAMe is well established and widely used in some countries, such as Italy. Despite the clear need for new treatments for depression and the apparent evidence for its efficacy, SAMe is not formally approved or widely used as an antidepressant treatment in many countries. It is imperative that the potential role of this agent in depression should be rigorously examined. Further, it is important to consider whether use of SAMe is advantageous in the management of depression given the incremental costs (resource use) and benefits (effects) that may be associated with the intervention.
Objectives
To assess the effects of SAMe in comparison with placebo or antidepressants for the treatment of depression in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs and considered published and unpublished trials.
We included cross‐over trials in the review; however, as SAMe treatment may have a lasting effect on depressive symptoms, we only included data from the first phase of cross‐over studies.
We planned to include cluster RCTs, with assessment of their potential for unit of analysis errors (Higgins 2011a). However, we found no studies of this design.
We planned to include full economic evaluations, cost analyses and comparative resource utilisation studies conducted in the context of an RCT. However, we identified no trials providing economic analyses.
Types of participants
Participant characteristics
Men and women aged from 18 to 80 years.
Diagnosis
Participants with a diagnosis of major depression, with or without psychotic symptoms, according to Diagnostic and Statistical Manual of Mental Disorders (DSM)‐III/II‐R (APA 1980), DSM‐IV/IV‐TR (APA 1980; APA 2000), or International Classification of Diseases (ICD)‐9, ICD‐10 (WHO 1978; WHO 1992).
Subset data
We included trials examining a particular subgroup of participants with major depression in the meta‐analysis, such as people with psychotic features, anxiety symptoms, treatment resistance or atypical depression. We also analysed these subgroups separately.
Comorbidities
We excluded participants with bipolar depression or schizoaffective disorder. Where studies used heterogeneous groups of participants, we excluded these data unless data from those participants with 'unipolar' depression could be extracted separately. If there was any doubt regarding the diagnosis of participants, we approached the authors to obtain clarification. We excluded cyclothymia and dysthymia.
We excluded people with DSM‐IV Axis I and II and physical comorbidities.
Types of interventions
Experimental intervention
S‐adenosyl methionine (SAMe) as monotherapy or as an adjunct.
Comparator intervention
Placebo.
Alternative pharmacological treatment, limited to antidepressants.
We organised antidepressants into classes for the purposes of this review, as follows.
Tricyclic antidepressants (TCAs): amitriptyline, imipramine, trimipramine, doxepin, desipramine, protriptyline, nortriptyline, clomipramine, dothiepin, lofepramine.
Heterocyclic antidepressants: mianserin, trazodone, amoxapine, maprotiline.
Selective serotonin reuptake inhibitors (SSRIs): fluvoxamine, fluoxetine, paroxetine, sertraline, citalopram, escitalopram.
Monoamine oxidase inhibitors (MAOIs), irreversible: phenelzine, tranylcypromine, isocarboxazid; reversible: brofaramine, moclobemide, tyrima.
Other antidepressants, noradrenaline reuptake inhibitors (NARIs): reboxetine, atomoxetine; noradrenaline‐dopamine reuptake inhibitors (NDRIs): amineptine, bupropion; serotonin‐noradrenaline reuptake inhibitors (SNRIs): venlafaxine, milnacipram, duloxetine; noradrenergic and specific serotonergic antidepressants (NASSAs): mirtazapine; serotonin antagonist and reuptake inhibitor (SARIs): trazodone; unclassified: agomelatine, vilazodone.
Acute treatment was treatment instituted specifically to alleviate symptoms of an existing episode of depression. We considered trials in which SAMe was used as an adjunctive treatment separately.
When trials combined acute treatment and maintenance phases, we analysed acute treatment data separately. When this was not possible, we excluded the study from the review. We excluded studies with treatment durations of less than one week. We excluded discontinuation trials in which participants received SAMe prior to randomisation.
Types of outcome measures
Primary outcomes
Efficacy
1. *Change in mean scores from baseline to end of treatment on the depression rating scale used, such as the Hamilton Depression Rating Scale (HAM‐D; Hamilton 1960) and the Montgomery‐Åsberg Depression Rating Scale (MADRS; Montgomery 1979).
Acceptability
2. *Participants dropping out of treatment during study period for any reason.
3. Participants dropping out of the treatment during study period because of adverse effects.
Secondary outcomes
Efficacy
4. *Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment.
5. *Remission, defined as a depression rating scale score within normal range at end of the study.
We used data according to the definitions of the trialists (documented in the 'Outcomes' section of the Description of studies). The definitions were overall homogeneous.
Acceptability
6. *Participants experiencing troublesome adverse effects of any nature.
7. Specific adverse effects: mania or hypomania, headache, diarrhoea, flatulence, nausea, hyperhomocysteinaemia, emergent suicidal ideation or behaviours, completed suicide and attempted suicide, mortality excluding suicide, and verdicts of undetermined death and mortality due to iatrogenic causes; the numbers of participants experiencing these adverse events were presented in tabular form.
8. Participants dropping out for any reasons other than adverse effects.
*Outcomes to be reported in the 'Summary of findings' tables.
Economic data
Mean total direct medical cost per participant, including medication costs, consultant fees and inpatient treatment costs.
Direct resources use associated with complications of treatment.
Time to onset of antidepressant effect measured as change in depression score (days).
Time to return to work (days).
Incremental cost per disability‐adjusted life year (DALY).
Timing of outcome assessment
Outcomes were categorised as short‐term (up to six months from the beginning of treatment), medium‐term (six to 12 months) or long‐term (longer than 12 months). We considered the short‐term as our primary time point.
Hierarchy of outcome measures
If data on more than one efficacy of treatment measure were provided for a trial, we extracted the data according to the following hierarchy.
HAM‐D.
MADRS.
Other outcome measure of efficacy with depression rating scales.
Search methods for identification of studies
We used a comprehensive search strategy to identify all relevant studies regardless of language or publication status.
The literature search was last updated in February 2016.
Electronic searches
1. The Cochrane Common Mental Disorders Group's Specialised Register (CCMDCTR)
The Cochrane Common Mental Disorders Group maintains a specialised register of randomized controlled trials, the CCMDCTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies based register with >50% of reference records tagged to c12,500 individually PICO coded study records. Reports of trials for inclusion in the register are collated from (weekly) generic searches of Medline (1950‐), Embase (1974‐) and PsycINFO (1967‐), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the hand‐searching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website with an example of the core Medline search displayed in Appendix 1.
We searched the CCMDCTR (Studies and Reference Registers) to 5 February 2016 using the following free‐text terms: (*adenosyl* or SAM‐e or Samyr)
2. Biomedical databases The original search of MEDLINE, EMBASE and PsycINFO was conducted in May 2012 (Appendix 2). As the CCMDCTR includes these databases, further searches to February 2016 were conducted on the CCMDCTR alone.
3. International trial registries were searched in February 2016 via ClinicalTrials.gov and the WHO trials portal (ICTRP) for additional unpublished or ongoing studies.
We did not apply any restrictions on date, language or publication status to the searches.
Searching other resources
Reference checking
We checked the reference lists of all identified RCTs, other relevant papers, and major English, German and Italian textbooks of affective disorders. We searched the reference lists of identified studies for additional RCTs and health economics studies.
Handsearching
We handsearched the annual conference proceedings of the American Psychiatric Association, the British Association of Psychopharmacology, the Congress of the International College of Neuropsychopharmacology, the European College of Neuropsychopharmacology and the National Congress of the Italian Psychiatric Association to June 2014.
Personal communications
We identified the authors of significant papers since 2011 from authorship lists. We contacted them and other experts in the field and asked if they had knowledge of other studies, published or unpublished, relevant to the review. We requested pharmaceutical companies marketing SAMe products to provide relevant published and unpublished data.
Data collection and analysis
Selection of studies
Two authors (IG and LO) screened the results of the search using an over‐inclusive approach to construct a list of all papers that were potentially relevant. The two authors independently screened the abstracts for inclusion. We obtained the full‐texts of papers whenever there was any doubt about the relevance of an article or where the abstract and title looked relevant. Two authors (IG and LO) independently reviewed all the full‐text papers. We applied the full inclusion criteria to generate a list of studies to be considered for inclusion.
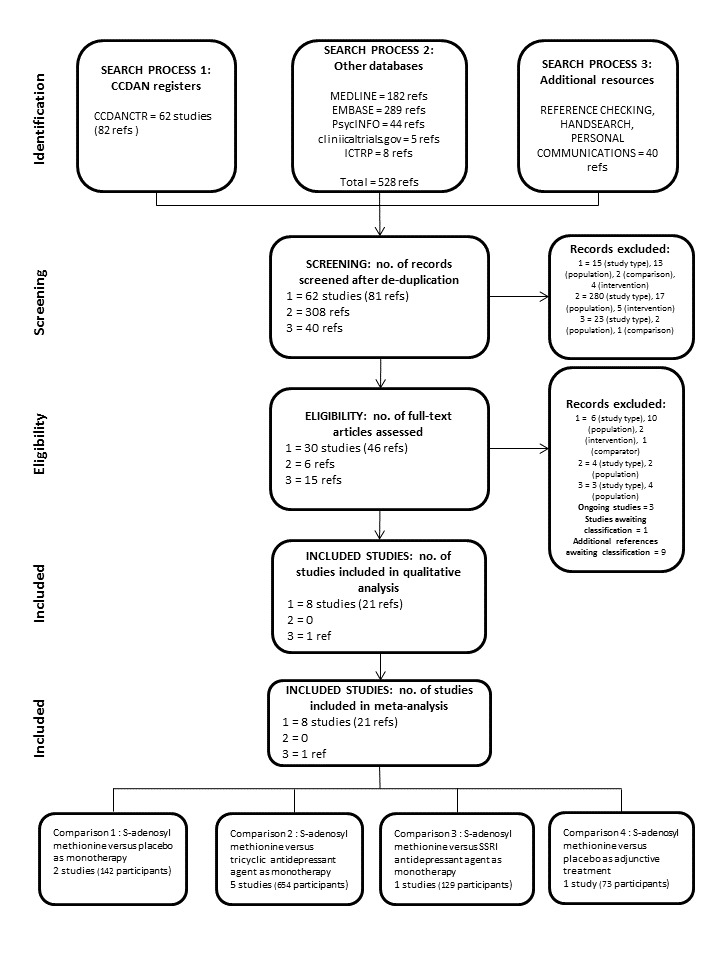
Two authors (IG and LO) independently reviewed the list of studies to see whether they met the previously defined inclusion criteria. We planned to resolve disagreements by consensus or discussions with a third member of the review team (AY) and report this in the final review. We did not calculate a kappa statistic for measuring the agreement between the two authors as the authors agreed. We documented the selection process was documented through the completion of a PRISMA flow chart (Figure 1). We described excluded studies in the Characteristics of excluded studies table. We listed multiple publications of the same study.
1.
Data extraction and management
For trials that met the inclusion criteria of the review, two review authors (IG and LO) independently extracted data concerning participant characteristics, intervention details and outcome measures using a previously piloted data collection form. We planned to solve any disagreements by consensus or discussions with a third member of the review team (AY). However, a kappa statistic for measuring the agreement between the two authors was not calculated as the authors agreed.
We extracted data on the following comparisons:
SAMe versus placebo as monotherapy;
SAMe versus a TCA as monotherapy;
SAMe versus SSRI as monotherapy;
SAMe versus placebo as adjunctive treatment.
We also planned to extract data on the comparison SAMe versus an active antidepressant agent as an adjunctive treatment; however, we found no studies.
We planned to develop a data collection form for use with health economic studies, based on the template used to produce UK National Health Service Economic Evaluation Database (NHS EED) structured abstracts (Craig 2007).
Assessment of risk of bias in included studies
Two review authors (KM and AY) independently assessed the risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). This tool gives special consideration to the generation of randomisation sequences, allocation concealment, blinding procedures, the completeness of final data sets and selective reporting. We planned to solve any disagreements by consensus or discussion with a third member of the review team (GM). A kappa statistic for measuring the agreement between the two authors was not calculated as the authors agreed.
Where inadequate details of randomisation and other characteristics of trials were provided, we contacted the trial authors for clarification. For studies considered to be at high risk of bias due to the method of sequence generation, the inadequate concealment of sequence allocation, the absence of double blinding or for any other reason, we identified the key mechanism of bias. Where this mechanism was likely to influence a particular outcome measure, we investigated the effect of including the study in the relevant meta‐analysis using a sensitivity analysis. Sensitivity analyses were also conducted on studies where the risk of bias remained unclear, despite contact with the study authors. We recorded the source of information for each risk of bias judgement, including judgements based on unpublished information.
For included health economic studies, we planned to assess the risk of bias and methodological quality using the Cochrane tool for assessing risk of bias (Higgins 2011b), and the BMJ Checklist (Drummond 1996).
We used the five GRADE considerations to assess the body of evidence for each outcome (Higgins 2011a). We justified and documented all such assessments.
Measures of treatment effect
Continuous data
For continuously distributed outcomes, we calculated the mean difference (MD) between the groups. Where measures were reported using different scales, we used the standardised mean difference (SMD), if this was clinically appropriate. We also reported 95% confidence intervals (CI).
Data would be checked for skew by calculation of the observed mean minus the lowest possible value minus (and by calculating the highest possible value minus the observed mean) and dividing this by the standard deviation. A ratio less than 2 suggests skew (Altman 1996a; Higgins 2011a). If the ratio is less than 1, there is strong evidence of a skewed distribution. When this was the case, we planned to exclude data from the analysis (Altman 1996a). In studies in which the ratio was between 1 and 2, suggesting less marked skew, we planned to subject data to a sensitivity analysis. Studies with more than 200 participants were exempt from these processes as skewed data were less problematic in large studies.
Dichotomous data
For dichotomous outcomes, we calculated the risk ratio (RR) of reported response, with 95% CI. We preferred the RR measure as the odds ratio is more difficult to interpret (Sackett 1996; Sinclair 1994).
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised studies are at risk of a 'unit of analysis' error (Divine 1992) and Type I errors (Bland 1997). Where clustering was suspected, we contacted the authors with a request that they supplied intra‐class correlation coefficients of their clustered data and adjust for this using methods described by Gulliford 1999. If clustering was incorporated, we planned to present the data in the form of a parallel‐group randomised study, with adjustments for clustering effects. If cluster studies were appropriately analysed, we planned to conduct synthesis with other studies using the generic inverse variance technique.
Cross‐over trials
For cross‐over studies, we considered only results from the first randomised portion. Data from the second phase of such studies are potentially subject to the confounding influences of discontinuation effects and persistent treatment effects.
Studies with multiple treatment groups
When a study had more than two intervention arms, we included only those intervention and control arms meeting the inclusion criteria of the review. We entered all relevant intervention groups of a multi‐intervention study in the Characteristics of included studies table and assessed these studies for any risk of bias. In particular, we sought reporting biases, such as the combining of groups on different doses of medication or the presentation of different outcomes in the comparison of different groups. Where appropriate, we combined data from all relevant experimental intervention groups of the study into a single group, and combined data from all relevant control intervention groups into a single control group (Higgins 2011a).
Dealing with missing data
We analysed all data on an intention‐to‐treat (ITT) basis. If it was not clear why data were missing, we contacted trialists to either provide the data or to explain why it was missing. However, we were unable to obtain any additional data. Careful consideration was given to the reason why data were missing, and whether the data were missing at random or their absence was in some way related to the outcome measure. We documented this where possible.
We considered the impact of missing data separately for different key outcomes. Where participants had withdrawn from the trial before reaching the end of the study period, we planned to assume that their condition would have remained unaltered had they continued to the end, that we would use the last observation carried forward (LOCF). However, it must be noted that ITT and LOCF methods have some limitations and can lead to bias as the means are likely to be distorted (Higgins 2011a). In the event, we were unable to use the LOCF method as, in all cases, individual raw participant data were not available. We addressed the missing data as follows:
For continuous efficacy outcomes, we imputed missing data using the conservative approach of assuming that these participants had no change in their mean score on the HAM‐D from baseline to the endpoint. As we did not have access to the raw participant baseline scores, we used the mean baseline score of all participants. To assess the robustness of the assumptions, we carried out sensitivity analyses where the participants were assumed to have had the same mean change as the other participants.
For dichotomous outcomes, we imputed missing data based on the consideration of a 'worst‐case' scenario. To assess the robustness of the assumption, we carried out sensitivity analyses based on a 'best‐case' scenario.
Variation in the degree of missing data was considered as a source of heterogeneity. We investigated the impact of these assumptions by undertaking a sensitivity analysis (Alderson 2004). Where standard error data were presented, we calculated standard deviations from the standard error (Altman 1996b). In the absence of any such data, we imputed standard deviations (Furukawa 2006), and undertook sensitivity analyses to assess the validity of this process.
For a detailed description of the procedures see Appendix 3 (Dealing with missing data).
Assessment of heterogeneity
We assessed heterogeneity using the I2 statistic. We used a P value of 0.10 as an indication of significant heterogeneity in meta‐analyses of small studies, as the Chi2 test may be underpowered to detect heterogeneity in these circumstances. According to the Cochrane Handbook for Systematic Reviews of Interventions, the bands of interpretation for I2 are as follows: 0% to 40%: may be unimportant; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity and 75% to 100%: may have considerable heterogeneity (Higgins 2011a). We took values above 30% to indicate moderate heterogeneity (Higgins 2011a), and sought sources of heterogeneity. We considered studies with heterogeneity greater than 75% too heterogeneous to combine in a meta‐analysis. Where we detected moderate or greater heterogeneity, we sought possible causes including the diagnosis, the demographic profile of the participants, the dose of agents used and the duration of treatment. We also considered variation in the degree of missing data as a source of heterogeneity.
Assessment of reporting biases
Where there were more than 10 studies contributing to an outcome, we planned to construct funnel plots to examine the data for small‐study effects (Higgins 2011a). In addition to publication bias, such effects may have been due to selective reporting, poor methodological quality leading to spuriously inflated effects in smaller studies, true heterogeneity of effect, artefact and chance (Higgins 2011a). We considered selective outcome reporting as part of the quality assessment procedure and reported any instances.
Data synthesis
Data from trials were combined in the meta‐analyses only if this was appropriate, that was the participants, interventions, comparisons and outcomes were sufficiently similar. The assessment of heterogeneity acted as a test of these judgements. We used a random‐effects model as it assumes that studies estimate different but related effects (DerSimonian 1986). We considered a random‐effects model appropriate because changes in the depression rating scales may measure similar but different effects. For instance, a change in total score may reflect improvements in physical symptoms of depression (e.g. sleep disturbance, appetite, lassitude), while in another study it may reflect a change in psychological symptoms such as feelings of guilt or hopelessness. Two authors (IG and LO) entered data into the Review Manager 5 (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
As discussed in the introduction, participants with certain subtypes of depression may respond differently to SAMe and to other treatments. We planned to undertake the following subgroup analyses, to examine the contribution of potential effect modifiers to heterogeneity.
Data from parenteral and oral administration of SAMe: different methods of administration may affect efficacy and the placebo response.
Depression with and without psychotic features: psychotic features are associated with more severe episodes of depression and they may respond differently to mild‐moderate depression or to non‐psychotic episodes of similar severity.
Treatment‐resistant depression: participants with this category of depression, by definition, will have failed to respond to at least two adequate trials of antidepressants and may respond differently to trial medications.
Atypical depression: participants with this category of depression characteristically respond optimally to MAOIs and may respond differently to trial medications.
Anxiety: anxiety detrimentally affects treatment outcome.
It is recognised that any findings from these analyses are hypothesis‐forming.
Sensitivity analysis
We planned to perform the following sensitivity analyses.
Studies where methodological factors may be sources of bias and likely to impact on the particular outcome under investigation. For instance, studies with inadequate blinding procedures may be liable to bias because of the effects of participants' and observers' expectations regarding their allocated treatment.
Studies with high levels of missing data (i.e. more than 30%). In studies with high drop‐out rates, the assumptions involved in the use of the LOCF approach may introduce considerable bias.
Studies using cluster randomisation. This method introduces the risk of bias in several ways. These include the possibility of recruitment bias, baseline imbalance and incorrect analysis (Higgins 2011a). There is also the question of how comparable these studies are with individually randomised trials. The influence of these potential sources of bias on the outcome measures identified in a review are difficult to predict. However, as there is a possibility of bias, we planned to investigate it routinely.
It is recognised that any findings from these analyses are hypothesis‐forming.
Economics issues
We planned to summarised characteristics and results of included economic evaluations using additional tables, supplemented by a narrative summary that would compare and evaluate methods used and principal results between studies.
In addition, we planned to tabulate unit cost data, when available.
'Summary of findings' tables
We constructed a 'Summary of findings' table according to the recommendations of theCochrane Handbook for Systematic Reviews of Interventions for each of the comparisons (Higgins 2011a). For each comparison, the table described the form of intervention, details of scales and time frames, the number of participants and studies for each outcome, a measure of the typical burden of non‐response to treatment (i.e. the assumed risks for non‐response, summary of the intervention effect: indices of absolute and relative magnitudes) and the quality of the body of evidence for each outcome.
Included outcomes were:
change in the mean score in the specified depression rating scores from baseline to end of treatment;
proportions of participants responding to treatment;
proportions of participants achieving remission;
participants dropping out of treatment during the study period for any reason;
participants experiencing troublesome adverse effects of any nature.
Notes on the 'Summary of findings' table
The 'Summary of findings' table presented the main group comparisons only.
For dichotomous outcomes, the table provided both a relative measure (the RR) of non‐response and the absolute risk reduction (ARR). For continuous data, the table presented the MD or SMD.
We presented typical assumed risks for non‐response in the control group and cited the sources of this information. Participants in the included studies were experiencing depression, and so the baseline assumed risk of non‐response for a median control group seemed the most helpful information to present. We cited the information on which this information was based. We calculated a corresponding intervention risk from the RR and the assumed control risk.
We used the GRADE approach to assessing the quality of the body of evidence. We adhered to the standard methods for the preparation and presentation of results outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Results
Description of studies
Results of the search
We identified 650 references: 82 through CCMDCTR, 528 through other electronic databases and 40 through additional resources. After we excluded 221 duplicates, we read the abstracts and excluded a further 362 references. We considered 67 references relevant for our review and tried to retrieve full‐text copies to assess their eligibility. Some studies that were possibly eligible reported heterogeneous groups of participants without the possibility to separate out data from those participants with 'unipolar' depression (Alvarez 1984; De Leo 1987; Delle Chiaie 1999; Janicak 1988; Kufferle 1982; Salmaggi 1991). We approached the authors in order to obtain original unpublished data but unsuccessfully and we excluded them. We also contacted Prof M. Fava to achieve clarification on randomisation in his study (Fava 1992); his answer did not permit us to include the study in our review. Finally, eight studies met inclusion criteria for our review and we included them in the qualitative and quantitative analysis. We categorised three studies as ongoing, nine references and one study awaiting classification and excluded the remaining studies for various reasons (see Figure 1 for PRISMA flow diagram).
We identified no RCTs providing economic analyses.
Included studies
This systematic review included eight studies with 934 participants. Although we contacted the authors of the included studies and received a response in some cases, attempts to obtain additional unpublished data and information regarding missing data were almost always unsuccessful.
See Characteristics of included studies table.
Study design
All the included studies were RCTs and were reported to be double blind. Two studies were multicentre (Delle Chiaie 2000a; Delle Chiaie 2000b). One study was three‐armed with SAMe, an alternative pharmacological treatment and placebo (Mischoulon 2014). Two studies were two‐armed with SAMe versus placebo as monotherapy (Kagan 1990) or as adjunctive therapy (Papakostas 2010a). The remaining five studies were two‐armed with SAMe versus an alternative pharmacological treatment (Bell 1988; Bell 1994; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). The Mischoulon 2014 study included a cross‐over phase in its design, though the report included in our review was focused on the data for the first phase of treatment.
There were no trials comparing SAMe with an active antidepressant as an adjunctive therapy.
Sample size
Overall, the review included 934 participants. Five studies recruited fewer than 100 participants (Bell 1988; Bell 1994; De Vanna 1992; Kagan 1990; Papakostas 2010a), and only three studies recruited more than 200 participants overall (Delle Chiaie 2000a; Delle Chiaie 2000b; Mischoulon 2014).
The mean sample size per arm was 55 participants (range 11 to 148).
Participants
Two studies enrolled only inpatients (Bell 1988; Kagan 1990), four studies only outpatients (Delle Chiaie 2000a; Delle Chiaie 2000b; Papakostas 2010a; Mischoulon 2014). One study enrolled both inpatients and outpatients (Bell 1994), and for the remaining trial the setting was unclear (De Vanna 1992). All studies enrolled people with a diagnosis of major depression, according to DSM‐III (Bell 1988; Kagan 1990), DSM‐III‐R (Bell 1994; De Vanna 1992), or DSM‐IV (Delle Chiaie 2000a; Delle Chiaie 2000b; Mischoulon 2014; Papakostas 2010a). All but one study (Kagan 1990, only men) recruited both women and men. Three studies provided participants over the age range of our review, including people aged 18 to 80 years (De Vanna 1992; Mischoulon 2014; Papakostas 2010a). Our protocol restricted the age range to 18 to 70 years (Galizia 2014). We decided to include these studies after consideration of the mean age of the participants.
Bell 1988 reported a past episode of mania in one participant in the comparison group. As per protocol, we should have excluded participants with bipolar depression. We decided to include this study because it was only one participant and nothing in the text showed that he reported different response to treatment or had a manic switch during the trial.
Only one study examined a particular subgroup of participants with major depression, namely SSRI non‐responders (Papakostas 2010a). We analysed this subgroup separately. Two studies excluded participants with history of resistance to TCA treatment (Bell 1988; Bell 1994).
Six studied excluded people who had psychotic symptoms (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992; Kagan 1990; Mischoulon 2014; Papakostas 2010a).
Intervention/comparisons
Studies used SAMe as monotherapy (Bell 1988; Bell 1994; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992; Kagan 1990; Mischoulon 2014) or as an adjunctive therapy (Papakostas 2010a). Specifically, SAMe was adjunct to SSRIs (Papakostas 2010a).
The mean duration of treatment was 5.3 weeks (range 2 to 12 weeks).
The administration of SAMe was oral at a target dose of 1600 mg/day (Bell 1994; Delle Chiaie 2000a; De Vanna 1992; Kagan 1990; Papakostas 2010a) or parenteral at a dose of 200 mg/day to 400 mg/day (intravenous Bell 1988, intramuscularly Delle Chiaie 2000b). According to our protocol, we undertook a subgroup analysis to examine data from parenteral and oral administration of SAMe (Galizia 2014). In one study, for participants who complained of adverse effects the drug, dose could be reduced from the third week on, down to a minimal dose of imipramine of 100 mg/day and SAMe of 1200 mg/day; the study excluded participants who tolerated this dose poorly from the study (Delle Chiaie 2000a). The Papakostas 2010a trial withdrew participants who were unable to tolerate the study medications, per protocol. One study allowed a dose increase to 3200 mg/day for non‐responders (Mischoulon 2014); they allowed participants who experienced intolerable adverse effects at the higher dose to decrease the dose to the previous level. Four studies specified the exact formulation of the SAMe used in the trial: 1,4‐butanedisulphonate‐SAMe (Delle Chiaie 2000a; Delle Chiaie 2000b), and SAMe tosylate (Mischoulon 2014; Papakostas 2010a).
Two studies compared SAMe with placebo (Kagan 1990; Papakostas 2010a), four studies SAMe with imipramine (Bell 1988; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992), and one study SAMe with desipramine (Bell 1994). Three cases titrated imipramine up to 150 mg/day (Bell 1988; Delle Chiaie 2000a; Delle Chiaie 2000b), while in the De Vanna 1992 trial, participants received a dose of 140 mg/day; desipramine was titrated up to 250 mg/day. One study was three‐armed comparing SAMe with escitalopram 10‐20 mg/day and placebo (Mischoulon 2014).
Some studies allowed the use of benzodiazepine as a hypnotic (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). However, none of the studies analysed whether the use of benzodiazepines could have somehow affected the outcomes.
Outcomes
We categorised all outcomes in this review as short‐term, as the maximum endpoint of the included studies was 12 weeks.
Primary outcomes
All included studies evaluated the efficacy of treatment by administration of the Hamilton Depression Scale (HAM‐D). However, they used different versions of this rating scale: 31‐item HAM‐D in Bell 1988; and Bell 1994; 17‐item HAM‐D in Bell 1994; Mischoulon 2014; and Papakostas 2010a; 21‐item HAM‐D in Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992; and Kagan 1990. In our analysis, we applied the SMD in order to measure the treatment efficacy.
The efficacy assessments also included the Beck Depression Inventory in two studies (Bell 1988; Bell 1994), and the 14‐item Hamilton Rating Scale for Anxiety and 20‐item Zung's Self‐Rating Scale for Depression in one study (De Vanna 1992). Two studies evaluated the MADRS (De Vanna 1992; Delle Chiaie 2000a; Delle Chiaie 2000b). Other scales included the Clinical Global Impression scale (Delle Chiaie 2000a; Delle Chiaie 2000b), the Clinical Global Impression ‐ Severity (Mischoulon 2014; Papakostas 2010a), Clinical Global Impression ‐ Improvement versions (Mischoulon 2014; Papakostas 2010a). Further, secondary measures of efficacy included the Inventory of Depressive Symptomatology ‐ Clinician Rating and the Inventory of Depressive Symptomatology ‐ Self Report in the Mischoulon 2014 study, the Carroll Rating Scale for Depression in the Kagan 1990 study.
According to the hierarchy of outcome measures of this review, we prioritised the data from the HAM‐D.
In one study, the primary outcome was the correlation between plasma SAMe levels and the degree of clinical improvement; blood samples for the analysis of plasma SAMe levels were collected (Bell 1994).
In the De Vanna 1992 trial, we extrapolated data regarding the efficacy of the treatment from the figure reported in the paper, as no other information was available. To check the accuracy of our calculations, we verified if the extrapolated MDs matched with the per cent improvement in the mean scores shown in the paper. We conducted a sensitivity analysis to assess the robustness of this assumption.
We could extract data for the analysis of the outcome "Efficacy. Change in mean scores from baseline to end of treatment on the depression rating scale" in all but one study (Bell 1994).
The 'Risk of bias' table of the Characteristics of included studies table states the drop‐out rates of individual trials, the distribution of drop‐outs among trials arms and the reasons for drop‐out.
We could extract data for the analysis of the acceptability outcomes related to the drop‐outs in all but two studies (Delle Chiaie 2000a; Delle Chiaie 2000b). However, the Bell 1994 study did not provide data for the quantitative evaluation of the drop‐outs reasons.
Secondary outcomes
Response to treatment was defined as a reduction of more than 50% on HAM‐D (Bell 1988; Bell 1994; Mischoulon 2014) or at least 50% (Delle Chiaie 2000a; Delle Chiaie 2000b; Papakostas 2010a). Treatment‐responders were also defined those participants who had a Clinical Global Impression score of 2 or less at the end of the study (Delle Chiaie 2000a; Delle Chiaie 2000b) or a Clinical Global Impression ‐ Improvement score of less than 3 at endpoint (Papakostas 2010a).
All but one study (De Vanna 1992) provided data for the analysis of the outcome "Efficacy. Response to treatment".
Remission was determined as a final HAM‐D score of less than 7 (Mischoulon 2014) or 7 or less (Papakostas 2010a). In addition, Papakostas 2010a considered remission as a Clinical Global Impression ‐ Severity score of 1 at endpoint. Only data from these two studies could be extracted to evaluate the remission rates.
All studies evaluated the tolerability and safety of the treatment by reporting adverse effects. Some studies applied instruments, such as the Somatic Symptom Checklist (Bell 1988), Systematic Assessment for Treatment Emergent Events (Bell 1994), and the Systematic Assessment for Treatment of Emergent Events‐Specific Inquiry (Mischoulon 2014). Almost all studies performed laboratory tests, electrocardiogram (ECG) and assessment of vital signs.
Three studies provided the rates of adverse effects of any nature experienced by participants (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992).
Most studies did not provide enough detailed data regarding specific adverse effects to carry out complete quantitative analyses.
Excluded studies
Twenty‐eight studies initially considered for potential inclusion in the review and retrieved as full‐articles did not meet our inclusion criteria and were excluded for different reasons, as follows: inappropriate diagnosis, presence of comorbidity, inappropriate outcomes, inappropriate intervention, inappropriate comparator, unsuitable study design, methodological issues and heterogeneous group of participants.
See the Characteristics of excluded studies table for details of the respective reasons for excluding each study.
Studies awaiting classification
We identified nine additional references by screening reference lists; it is unclear as to whether these are reports of RCTs already included (e.g. Bell (personal communication)) or otherwise. As we could identify no abstract or full‐text reference to ascertain study characteristics, we have currently listed these as additional references (see Alvarez 1987; Bell (personal communication); Bell 1987; Di Padova 2000: Fazio 1974; Macher (in press); Macher 2000; Pancheri 1997; Pinzello 1972).
One additional study identified from the CCMDCTR search was a handsearch record submitted by the Iberoamerican Cochrane Centre in the 1980s (Quiros 1982, CENTRAL ID: CN‐00711163).
For further details of these studies see Characteristics of studies awaiting classification table.
Ongoing studies
We identified three ongoing studies, all described as double‐blind, randomised, placebo‐controlled trials involving adults with a diagnosis of major depressive disorder. Two studies were three‐armed with SAMe plus cofactors folinic acid and vitamin B12, enhanced SAMe combination nutraceutical formulation and placebo (ACTRN12613001299796; ACTRN12613001300763), while the other study compared adjunctive SAMe as adjunctive therapy versus adjunctive placebo (NCT01912196).
Risk of bias in included studies
Detailed assessment of risk of bias across all studies is presented in the Characteristics of included studies table and Figure 2 and Figure 3.
2.
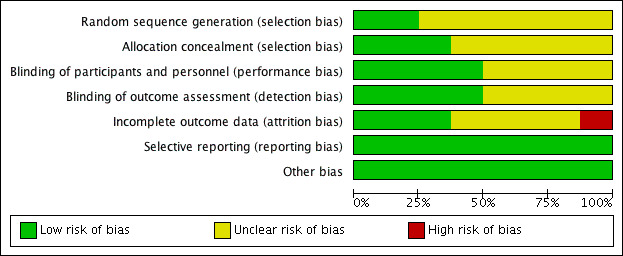
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
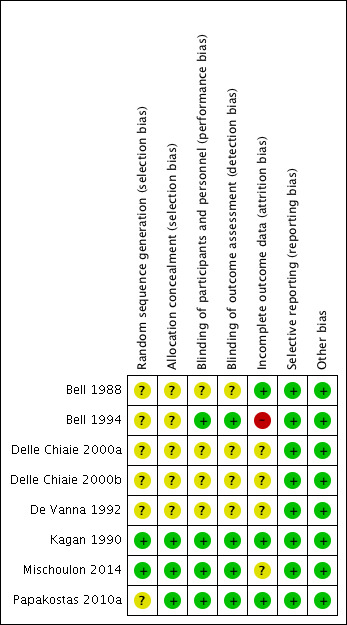
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Only two studies were at low risk of bias for random sequence generation, as the investigators sufficiently described the random component in the sequence generation process (Kagan 1990; Mischoulon 2014). The remaining studies were rated as having unclear risk of bias, since the methods of randomisation were not described in sufficient details.
Allocation concealment
Three studies were at low risk of bias for allocation concealment, as the investigators used a conceal allocation methods that did not allow participants and investigators enrolling participants to foresee assignment (Kagan 1990; Mischoulon 2014; Papakostas 2010a). The remaining studies were rated as unclear as the methods of concealment were not described in sufficient detail.
Blinding
Blinding of participants and personnel (performance bias)
With regard to the risk of performance bias, four studies were at low risk of bias as they ensured blinding of participants and personnel, and it was unlikely that the blinding could have been broken (Bell 1994; Kagan 1990; Mischoulon 2014; Papakostas 2010a).
The other studies were at unclear risk of performance bias, because the methods to secure the blinding were not fully described (Bell 1988; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). In addition, the allocation concealment was unclear.
Blinding of outcome assessment (detection bias)
With regard to the risk of detection bias, four studies were at low risk of bias as they ensured blinding of outcome assessment, and it was unlikely that the blinding could have been broken (Bell 1994; Kagan 1990; Mischoulon 2014; Papakostas 2010a).
The other studies were at unclear risk of detection bias, because the methods to secure the blinding were not fully described (Bell 1988; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). In addition, the allocation concealment was unclear.
Incomplete outcome data
Three studies were at low risk of attrition bias. In the Bell 1988 and Kagan 1990 trials, missing outcomes were too few to impact on the observed effect size. In both cases, the reason for drop‐outs were stated. In the Papakostas 2010a study, an ITT analysis was available and all non‐completers were mentioned with reasons for drop‐out.
One study was at high risk of attrition bias because they used only data on completers and did not state the reason for missing participants (Bell 1994).
The remaining four studies were at unclear risk of attrition bias. It was unclear whether De Vanna 1992 used an ITT analysis. Although Delle Chiaie 2000a and Delle Chiaie 2000b carried out an ITT analysis, they did not fully describe non‐completers. Mischoulon 2014 had a very large proportion of drop‐outs (almost 50%).
Selective reporting
Across all the studies, the risk of reporting bias was low. This was given careful consideration, as no study protocols were available. However, all expected outcomes, including those that were prespecified in the protocol section of the final reports, were included in the results.
Other potential sources of bias
The risk of other bias was low as we identified no other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We extracted data on the following comparisons:
SAMe versus placebo as monotherapy;
SAMe versus a TCA as monotherapy;
SAMe versus SSRI as monotherapy;
SAMe versus placebo as adjunctive treatment.
Where published data were not sufficient to evaluate our outcomes, we contacted the authors in order to obtain unpublished data; however, we received no responses.
Comparison 1: S‐adenosyl methionine versus placebo as monotherapy
Two studies were eligible for this comparison (Kagan 1990; Mischoulon 2014). See Table 1. The Kagan 1990 study was at low risk of selection, performance, detection, attrition, reporting and other bias. The Mischoulon 2014 study was at unclear risk of attrition bias and at low risk of bias in the other domains.
Primary outcomes
1.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
In this analysis, a reduction in depression rating scale score (indicated by a negative sign) represents an improvement.
Overall, there was no strong evidence of a difference between SAMe and placebo as monotherapy in terms of efficacy in the treatment of depression, measured as change in the mean scores on depression rating scale (SMD ‐0.54, 95% CI ‐1.54 to 0.46; P = 0.29; 142 participants; 2 studies) (Analysis 1.1). The evidence contributing to this outcome was very low quality.
1.1. Analysis.
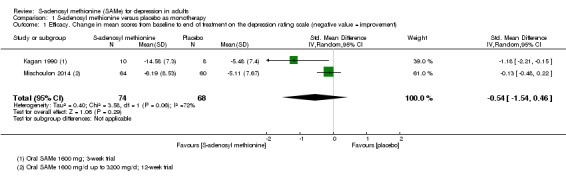
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 1 Efficacy. Change in mean scores from baseline to end of treatment on the depression rating scale (negative value = improvement).
The studies showed a high level of heterogeneity (I2 = 72%; P = 0.06), which has been investigated in the Discussion.
1.2 Acceptability. Participants dropping out of treatment during study period for any reason
There was no strong evidence of a difference between the two arms with regard to the level of drop‐outs (RR 0.88, 95% CI 0.61 to 1.29; P = 0.52; 142 participants; 2 studies; I2 = 0%) (Analysis 1.2). The evidence contributing to this outcome was low quality.
1.2. Analysis.
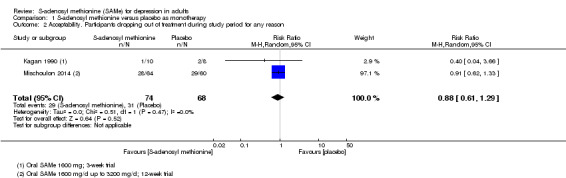
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 2 Acceptability. Participants dropping out of treatment during study period for any reason.
1.3 Acceptability. Participants dropping out of treatment during study period because of adverse effects
There was no strong evidence of a difference between SAMe and placebo as monotherapy in terms of drop‐outs due to adverse effects (RR 0.70, 95% CI 0.16 to 3.01; P = 0.64; 142 participants; 2 studies; I2 = 0%) (Analysis 1.3).
1.3. Analysis.
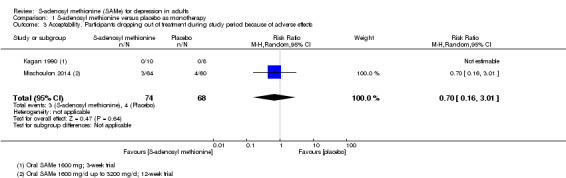
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 3 Acceptability. Participants dropping out of treatment during study period because of adverse effects.
Secondary outcomes
1.4 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
In this analysis, an increase of RR represents a positive outcome indicating response to treatment in the SAMe group.
There was no evidence that SAMe was superior to placebo as monotherapy in terms of response to treatment (RR 1.77, 95% CI 0.51 to 6.13; P = 0.37; 142 participants; 2 studies) (Analysis 1.4). The evidence contributing to this outcome was low quality.
1.4. Analysis.
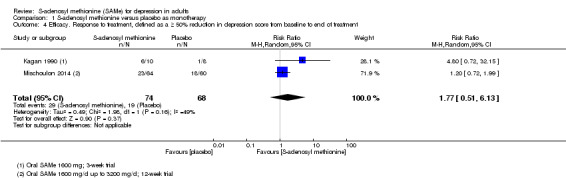
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 4 Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
The two studies included in this analysis showed a non‐significant heterogeneity (I2 = 49%; P = 0.16).
1.5 Efficacy. Remission, defined as a depression rating scale score within the normal range at end of study
In this analysis, an increase of RR represents a positive outcome indicating response to treatment in the SAMe group.
Only the Mischoulon 2014 study contributed to this outcome. There was no evidence of a difference between the two treatment arms with regard to remission (RR 1.69, 95% CI 0.85 to 3.36; P = 0.14; 124 participants; 1 study) (Analysis 1.5). The evidence contributing to this outcome was low quality.
1.5. Analysis.
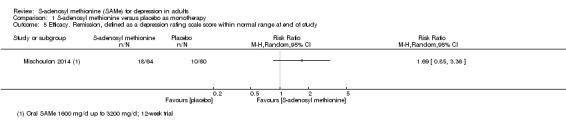
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 5 Efficacy. Remission, defined as a depression rating scale score within normal range at end of study.
1.6 Acceptability. Participants experiencing troublesome adverse effects of any nature
There were no available data to conduct this analysis.
1.7 Acceptability. Participants experiencing specific adverse effects identified in protocol
One study detailed the adverse effects (Kagan 1990). In the SAMe group, one participant experienced manic symptoms and headache (Analysis 1.6; Analysis 1.7); in the placebo group, two participants reported flatulence (Analysis 1.8). The conducted analyses showed no strong evidence of a difference between the two treatment arms (Analysis 1.6: RR 2.10, 95% CI 0.10 to 44.40; P = 0.63; 15 participants; 1 study; Analysis 1.7: RR 2.10, 95% CI 0.10 to 44.40; P = 0.63; 15 participants; 1 study; Analysis 1.8: RR 0.14, 95% CI 0.01 to 2.49; P = 0.18; 15 participants; 1 study).
1.6. Analysis.
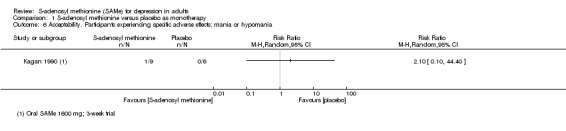
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 6 Acceptability. Participants experiencing specific adverse effects: mania or hypomania.
1.7. Analysis.
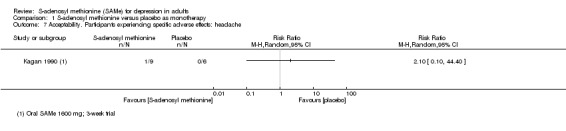
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 7 Acceptability. Participants experiencing specific adverse effects: headache.
1.8. Analysis.
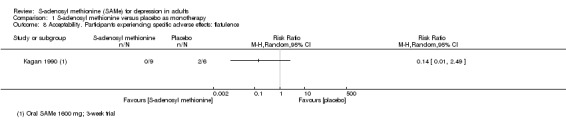
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 8 Acceptability. Participants experiencing specific adverse effects: flatulence.
The participant who experienced manic symptoms, on day 19 of treatment with SAMe, was noted to be energetic, talkative, irritable, grandiose and hyperkinetic; his clinical ratings had improved dramatically, but he was noting the return of insomnia and decreased appetite. This 65‐year‐old white man completed the three‐week long trial and subsequently developed a manic episode characterised by pressured speech, flight of ideas, poor judgement, extensive travel, insomnia, decreased appetite, weight loss and expenditure of large sums of money. His manic episode persisted even though SAMe treatment had been discontinued three months earlier. The participant's history revealed only one prior depressive episode (responsive to doxepin), no prior history of mania on hypomania and a family history of depression but not mania.
In both studies, there were no explicit reports of mortality during the treatment period (Kagan 1990; Mischoulon 2014). Overall, the trials did not systematically assess risk for suicidal ideation and behaviours. Neither study mentioned measurement of homocysteinaemia.
1.8 Acceptability. Participants dropping out for any reasons other than adverse effects
There was no evidence of a difference between SAMe and placebo as monotherapy in terms of drop‐outs for any reason other than adverse effects (RR 0.91, 95% CI 0.60 to 1.38; P = 0.66; 142 participants; 2 studies; I2 = 0%) (Analysis 1.9).
1.9. Analysis.
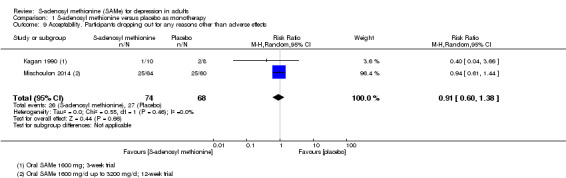
Comparison 1 S‐adenosyl methionine versus placebo as monotherapy, Outcome 9 Acceptability. Participants dropping out for any reasons other than adverse effects.
Comparison 2: S‐adenosyl methionine versus tricyclic antidepressant as monotherapy
Five studies were eligible for this comparison (Bell 1988; Bell 1994; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). See Table 2.
The Bell 1988 study was at unclear risk of selection, performance and detection bias and at low risk of attrition, reporting and other bias. The Bell 1994 study was at high risk of attrition bias, at unclear risk of selection bias and at low risk of bias in the remaining domains. Three studies were at unclear risk of selection, performance, detection and attrition bias, and at low risk of reporting and other bias (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992).
The antidepressant used as comparators in the included studies were imipramine and desipramine.
Primary outcomes
2.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
In this analysis, a reduction of depression rating scale score (indicated by a negative sign) represents an improvement.
The overall analysis showed that the efficacy of SAMe as monotherapy in the treatment of depression was not different from that of imipramine (SMD ‐0.04, 95% CI ‐0.34 to 0.27; P = 0.82; 619 participants; 4 studies; I2 = 57%) (Analysis 2.1). The evidence contributing to this outcome was low quality.
2.1. Analysis.
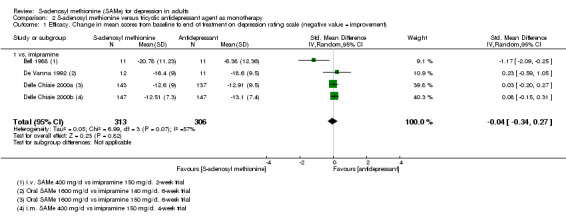
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
One study favoured SAMe over the active antidepressant (imipramine) and was likely responsible for the heterogeneity between the studies (I2 = 57%; P = 0.07) (Bell 1988).
2.2 Acceptability. Participants dropping out of treatment during study period for any reason
There was no evidence of a difference between SAMe and TCAs (imipramine and desipramine) with regard to drop‐outs during the study period for any reason (RR 0.61, 95% CI 0.28 to 1.31; P = 0.2; 78 participants; 3 studies; I2 = 0%) (Analysis 2.2). The evidence contributing to this outcome was very low quality.
2.2. Analysis.
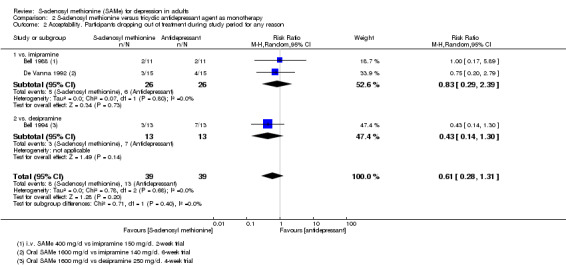
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 2 Acceptability. Participants dropping out of treatment during study period for any reason.
2.3 Acceptability. Participants dropping out of treatment during study period because of adverse effects
There was no evidence of a difference identified between drop‐outs because of adverse effects with SAMe as monotherapy versus imipramine (RR 0.75, 95% CI 0.20 to 2.79; P = 0.67; 52 participants; 2 studies; I2 = 0%) (Analysis 2.3).
2.3. Analysis.
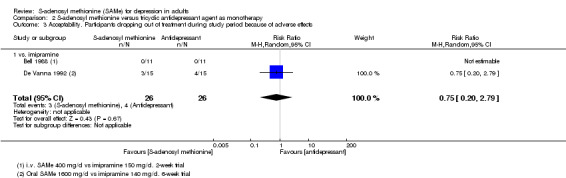
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 3 Acceptability. Participants dropping out of treatment during study period because of adverse effects.
Secondary outcomes
2.4 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
In this analysis, an increase of RR represents a response to treatment in the SAMe group.
There was no strong evidence of a difference in the response rate between SAMe and a TCA agent (imipramine and desipramine) as monotherapy (RR 1.14, 95% CI 0.83 to 1.56; P = 0.42; 622 participants; 4 studies; I2 = 58%) (Analysis 2.4). The evidence contributing to this outcome was very low quality.
2.4. Analysis.
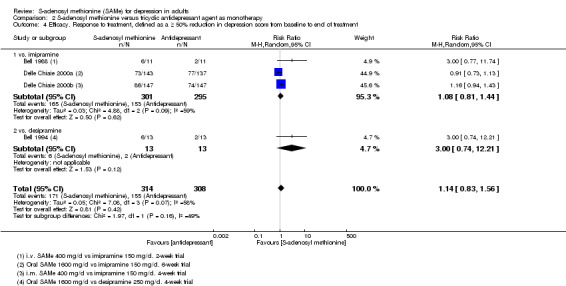
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 4 Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
We identified moderate heterogeneity among the studies (I2 = 58%; P = 0.07), which has been investigated in the Discussion.
2.5 Efficacy. Remission, defined as a depression rating scale score within the normal range at end of study
There were no available data.
One study provided details on remission for the four participants with psychotic features: the two participants in the experimental group were fully recovered by the end of the study, both having a HAM‐D score of less than 10; however, the two participants in the control group did not show remission (Bell 1988).
2.6 Acceptability. Participants experiencing troublesome adverse effects of any nature
There was evidence of low quality that, compared to imipramine, treatment with SAMe decreased the risk of experiencing troublesome adverse effects of any nature by 30% (RR 0.68, 95% CI 0.52 to 0.88; P = 0.004; 604 participants; 3 studies) (Analysis 2.5).
2.5. Analysis.
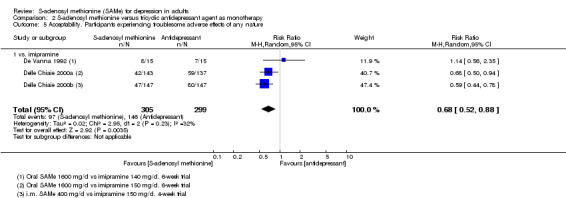
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 5 Acceptability. Participants experiencing troublesome adverse effects of any nature.
We identified moderate heterogeneity (I2 = 32%) between the studies that was not significant (P = 0.23) and was probably due to the De Vanna 1992 trial, which was the only one with the CI including 'no effect'. This study has a considerably smaller sample size than the other two; its removal from the analysis resulted in the elimination of the heterogeneity.
2.7 Acceptability. Participants experiencing specific adverse effects identified in protocol
Only one study detailed adverse effects (De Vanna 1992). In the Bell 1988 study, it was just reported that none of the participants became manic during the trial. The De Vanna 1992 study reported nausea and vomiting in six of the 15 participants in the SAMe group and in one of the 15 participants in the imipramine group, but we could not use these data in the analysis as these two symptoms were not reported separately. None of the participants reported headache, diarrhoea or flatulence. One participant exhibited hypomania in the SAMe arm (RR 3.00, 95% CI 0.13 to 68.26; P = 0.49; 48 participants; 2 studies) (Analysis 2.6).
2.6. Analysis.
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 6 Acceptability. Participants experiencing specific adverse effects: mania or hypomania.
In all studies, there were no explicit reports of mortality. Overall, the trials did not systematically assess and report the risk for suicidal ideation and behaviours. None of the studies mentioned measurement of homocysteinaemia.
2.8 Acceptability. Participants dropping out for any reasons other than adverse effects
There was no evidence that SAMe as monotherapy was more acceptable than a treatment with imipramine in terms of drop‐outs due to any reasons other than adverse effects (RR 1.00, 95% CI 0.17 to 5.89; P = 1; 52 participants; 2 studies; I2 = 0%) (Analysis 2.7).
2.7. Analysis.
Comparison 2 S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 7 Acceptability. Participants dropping out for any reasons other than adverse effects.
Comparison 3: S‐adenosyl methionine versus selective serotonin reuptake inhibitor as monotherapy
One study was eligible for this comparison (Mischoulon 2014). See Table 3.
The included study was at unclear risk of attrition bias and at low risk of bias in the other domains.
The antidepressant used as comparator was escitalopram.
Primary outcomes
3.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
In this analysis, lowering of a depression rating scale score (indicated by a negative sign) represents an improvement.
The analysis showed that there was no evidence of a difference in mean depression rating change scores between SAMe and escitalopram, both as monotherapy (MD 0.12, 95% CI ‐2.75 to 2.99; P = 0.93; 129 participants; 1 study) (Analysis 3.1). The evidence contributing to this outcome was low quality.
3.1. Analysis.
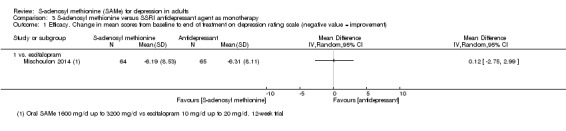
Comparison 3 S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
3.2 Acceptability. Participants dropping out of treatment during study period for any reason
There was no evidence of a difference between SAMe and escitalopram with regard to drop‐outs during the study period for any reason (RR 0.81, 95% CI 0.57 to 1.16; P = 0.26; 129 participants; 1 study) (Analysis 3.2). The evidence contributing to this outcome was low quality.
3.2. Analysis.
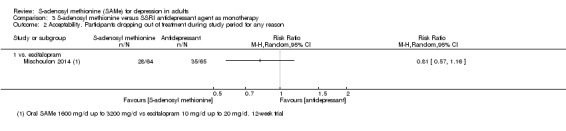
Comparison 3 S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 2 Acceptability. Participants dropping out of treatment during study period for any reason.
3.3 Acceptability. Participants dropping out of treatment during study period because of adverse effects
There was no evidence of a difference identified between drop‐outs because of adverse effects with SAMe as monotherapy versus escitalopram (RR 0.38, 95% CI 0.11 to 1.37; P = 0.14; 129 participants; 1 study) (Analysis 3.3).
3.3. Analysis.
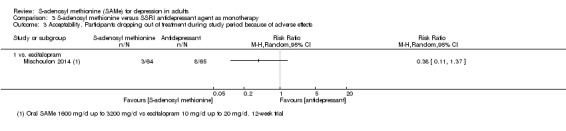
Comparison 3 S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 3 Acceptability. Participants dropping out of treatment during study period because of adverse effects.
Secondary outcomes
3.4 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
In this analysis, an increase of RR represents a positive outcome indicating response to treatment in the SAMe group.
There was no evidence of a difference in the response rate between SAMe and escitalopram as monotherapy (RR 1.06, 95% CI 0.66 to 1.70; P = 0.8; 129 participants; 1 study) (Analysis 3.4). The evidence contributing to this outcome was low quality.
3.4. Analysis.
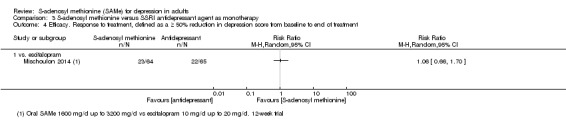
Comparison 3 S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 4 Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
3.5 Efficacy. Remission, defined as a depression rating scale score within the normal range at end of study
In this analysis, an increase of RR represents a positive outcome indicating response to treatment in the SAMe group.
There was no evidence that SAMe was more efficacious than an active antidepressant agent in reaching the remission from depression (RR 1.02, 95% CI 0.58 to 1.77; P = 0.96; 129 participants; 1 studies) (Analysis 3.5). The evidence contributing to this outcome was low quality.
3.5. Analysis.
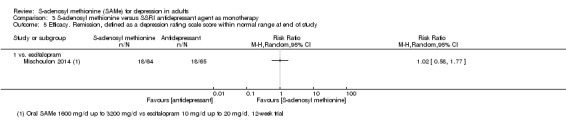
Comparison 3 S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 5 Efficacy. Remission, defined as a depression rating scale score within normal range at end of study.
3.6 Acceptability. Participants experiencing troublesome adverse effects of any nature
There were no available data for this outcome.
3.7 Acceptability. Participants experiencing specific adverse effects identified in protocol
There were no available data for this outcome. There were no explicit reports of mortality reported. The study did not systematically assess and report the risk for suicidal ideation and behaviours. The study did not mention measurement of homocysteinaemia.
3.8 Acceptability. Participants dropping out for any reasons other than adverse effects
There was no evidence that SAMe as monotherapy was more acceptable than a treatment with a SSRI antidepressant (escitalopram), in terms of drop‐outs due to any reasons other than adverse effects (RR 0.94, 95% CI 0.62 to 1.43; P = 0.77; 129 participants; 1 study) (Analysis 3.6).
3.6. Analysis.
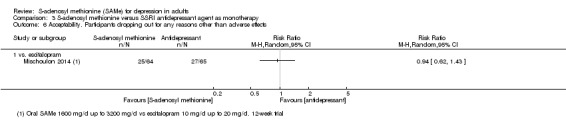
Comparison 3 S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 6 Acceptability. Participants dropping out for any reasons other than adverse effects.
Comparison 4: S‐adenosyl methionine versus placebo as adjunctive treatment
One study compared SAMe with placebo as an adjunctive treatment to their existing SSRI treatment (Papakostas 2010a). See Table 4. The study was at unclear risk of bias for random sequence generation and at low risk of bias in the other categories.
This study examined a particular subgroup of participants with major depression, namely SSRI non‐responders. We planned to analyse this subgroup separately. However, as it was the only study in this comparison, this was not necessary.
Primary outcomes
4.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
In this analysis, lowering of a depression rating scale score (indicated by a negative sign) represents an improvement.
There was low quality evidence that SAMe was superior to placebo as add‐on to SSRIs in terms of change in depressive symptoms from baseline to end of treatment (MD ‐3.90, 95% CI ‐6.93 to ‐0.87; P = 0.01; 73 participants; 1 study) (Analysis 4.1).
4.1. Analysis.
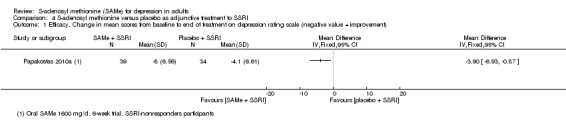
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
4.2 Acceptability. Participants dropping out of treatment during study period for any reason
There was no evidence of a difference between SAMe and placebo as adjunctive therapy with regard to drop‐outs during the study period for any reason (RR 0.70, 95% CI 0.31 to 1.56; P = 0.38; 73 participants; 1 study) (Analysis 4.2). The evidence contributing to this outcome was very low quality.
4.2. Analysis.
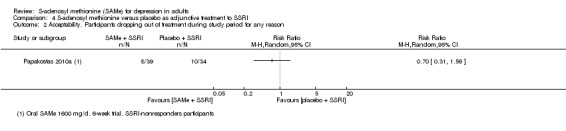
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 2 Acceptability. Participants dropping out of treatment during study period for any reason.
4.3 Acceptability. Participants dropping out of treatment during study period because of adverse effects
There was no evidence of a difference between SAMe and placebo as adjunctive therapy with regard to drop‐outs during the study period because of adverse effects (RR 0.58, 95% CI 0.10 to 3.28; P = 0.54; 73 participants; 1 study) (Analysis 4.3).
4.3. Analysis.
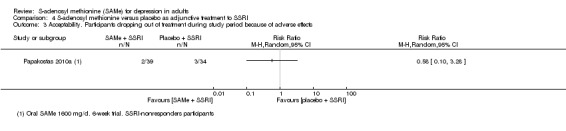
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 3 Acceptability. Participants dropping out of treatment during study period because of adverse effects.
Secondary outcomes
4.4 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
In this analysis, positive RR represents a positive outcome indicating response to treatment in the SAMe group.
We found evidence that the number of participants in the SAMe arm who obtained a reduction of 50% or greater in depression score was significantly higher than in the placebo arm (RR 2.62, 95% CI 1.17 to 5.83; P = 0.02; 73 participants; 1 study) (Analysis 4.4). The evidence contributing to this outcome was low quality.
4.4. Analysis.
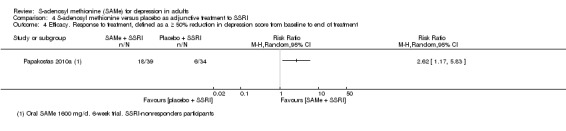
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 4 Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
4.5 Efficacy. Remission, defined as a depression rating scale score within the normal range at end of study
In this analysis, an increase of RR represents a positive outcome indicating response to treatment in the SAMe group.
For every person in the placebo group that achieved remission, three people in the SSRI/SAMe achieved remission although obviously alongside the caveat that this is based on only study that was low quality due to indirectness and imprecision (RR 3.05, 95% CI 1.11 to 8.39; P = 0.03; 73 participants; 1 study) (Analysis 4.5).
4.5. Analysis.
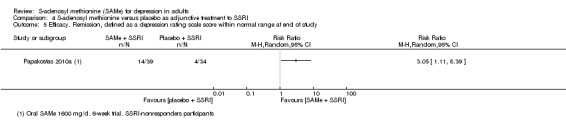
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 5 Efficacy. Remission, defined as a depression rating scale score within normal range at end of study.
4.6 Acceptability. Participants experiencing troublesome adverse effects of any nature
There was no available data for this outcome.
4.7 Acceptability. Participants experiencing specific adverse effects identified in protocol
The authors of the study reported only those adverse effects experienced by at least two participants, and so we could not include the study in the quantitative analysis of the all specific adverse effects. The study reported headache and diarrhoea; neither were significantly different compared with the placebo group (headache: RR 1.74, 95% CI 0.34 to 8.93; P = 0.50; 73 participants; 1 study; Analysis 4.6; diarrhoea: RR 1.22, 95% CI 0.43 to 3.49; P = 0.71; 73 participants; 1 study; Analysis 4.7).
4.6. Analysis.
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 6 Acceptability. Participants experiencing specific adverse effects: headache.
4.7. Analysis.
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 7 Acceptability. Participants experiencing specific adverse effects: diarrhoea.
There were no explicit reports of mortality during the study period. Overall, the trials did not systematically assess and report risk for suicidal ideation and behaviours. The study did not mention measurement of homocysteinaemia.
4.8 Acceptability. Participants dropping out for any reasons other than adverse effects
The number of drop‐outs for any reasons other than adverse effects was not significantly different between the two arms (RR 0.75, 95% CI 0.28 to 2.01; P = 0.56; 73 participants; 1 study) (Analysis 4.8).
4.8. Analysis.
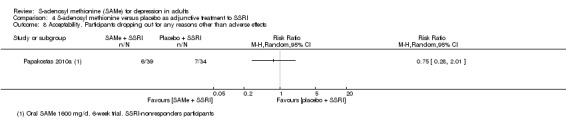
Comparison 4 S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI, Outcome 8 Acceptability. Participants dropping out for any reasons other than adverse effects.
Subgroup analyses
We conducted subgroup analyses only on data from parenteral and oral administration of SAMe.
We planned to perform other subgroup analyses but it was not possible, as explained below.
One study described psychotic symptoms in four participants (two in each treatment group) (Bell 1988); however, because it was impossible to separate data from these participants, a subgroup analysis was not undertaken. The authors commented that the two participants in the SAMe group who experienced major depression with psychotic features (somatic/nihilistic delusions) were fully recovered by the end of the study and each had a HAM‐D score of less than 10; both of the participants treated with imipramine with psychotic features did not respond to imipramine only. Six studies excluded participants who had psychotic symptoms (Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992; Kagan 1990; Mischoulon 2014; Papakostas 2010a).
No studies provided data regarding a specific group of participants with atypical depression or with anxiety symptoms. However, one study used the augmented 31‐item HAM‐D (Bell 1988). This scale added items that identified atypical depressive symptoms such as hyperphagia; hypersomnia; psychomotor retardation; and feelings of helplessness, hopelessness and worthlessness; in addition, the individual item anxiety on the same depression scale was pointed out. The authors commented that between‐group comparison of scores for individual items on the HAM‐D at the endpoint demonstrated a significantly greater improvement for participants treated with SAMe than participants treated with imipramine on items concerning psychic anxiety, helplessness, worthlessness and hypersomnia. One study evaluated participants using the 14‐item Hamilton Rating Scale for Anxiety (De Vanna 1992); the authors commented that at the endpoint, there was a significant difference versus baseline values on this scale in both treatment groups.
One study examined a particular subgroup of participants with major depression, namely SSRI non‐responders (Papakostas 2010a). We planned to analyse this subgroup separately. However, as it was the only study under the appropriate comparison, we did not perform a subgroup analysis.
Comparison: S‐adenosyl methionine versus tricyclic antidepressant as monotherapy
Primary outcomes
5.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
The subgroup analyses on data from oral and parenteral administration of SAMe yielded similar results (oral: SMD 0.06, 95% CI ‐0.17 to 0.28; P = 0.62; 303 participants; 2 studies; I2 = 0%; Analysis 6.1; parenteral: SMD ‐0.46, 95% CI ‐1.68 to 0.75; P = 0.45; 316 participants; 2 studies; I2 = 85%; Analysis 6.2).
6.1. Analysis.
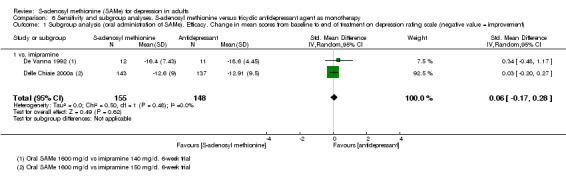
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 1 Subgroup analysis (oral administration of SAMe). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
6.2. Analysis.
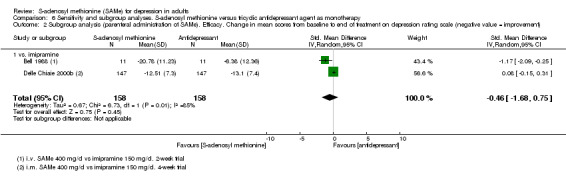
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 2 Subgroup analysis (parenteral administration of SAMe). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
5.2 Acceptability. Participants dropping out of treatment during study period for any reason
Considering data from oral and parenteral administration of SAMe separately, the lack of significant difference between SAMe and active antidepressant persisted (oral: RR 0.54, 95% CI 0.23 to 1.27; P = 0.16; 56 participants; 2 studies; I2 = 0%; Analysis 6.3; parenteral: RR 1.00, 95% CI 0.17 to 5.89; P = 1; 22 participants; 1 study; I2 = 0%; Analysis 6.4). We noted that in Bell 1988, of the 11 participants treated with intravenous SAMe, two refused to continue the study because of the discomfort of the intravenous procedure.
6.3. Analysis.
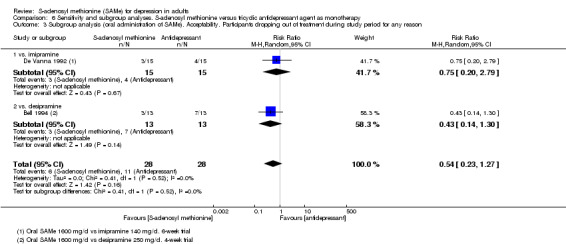
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 3 Subgroup analysis (oral administration of SAMe). Acceptability. Participants dropping out of treatment during study period for any reason.
6.4. Analysis.
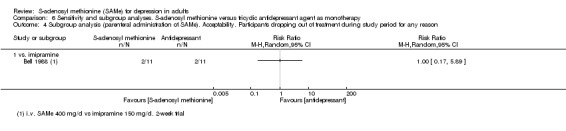
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 4 Subgroup analysis (parenteral administration of SAMe). Acceptability. Participants dropping out of treatment during study period for any reason.
5.3 Acceptability. Participants dropping out of treatment during study period because of adverse effects
The subgroup analyses on data from oral and parenteral administration of SAMe did not change the results and neither of the methods of administration differed from the two combined (oral: RR 0.75, 95% CI 0.20 to 2.79; P = 0.67; 30 participants; 1 study; I2 = 0%; Analysis 6.5; parenteral: no events; Analysis 6.6).
6.5. Analysis.
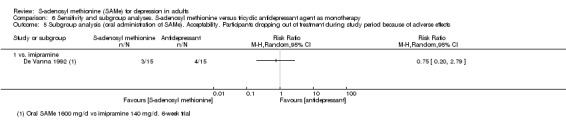
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 5 Subgroup analysis (oral administration of SAMe). Acceptability. Participants dropping out of treatment during study period because of adverse effects.
6.6. Analysis.
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 6 Subgroup analysis (parenteral administration of SAMe). Acceptability. Participants dropping out of treatment during study period because of adverse effects.
Secondary outcomes
5.4 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
When we conducted the subgroup analysis separating data from oral and parenteral administration, the outcome was not affected (oral: RR 1.35, 95% CI 0.44 to 4.09; P = 0.6; 306 participants; 2 studies; I2 = 64%; Analysis 6.7; parenteral: RR 1.46, 95% CI 0.66 to 3.26; P = 0.35; 316 participants; 2 studies; I2 = 46%; Analysis 6.8), and neither of the methods of administration differed from the two combined.
6.7. Analysis.
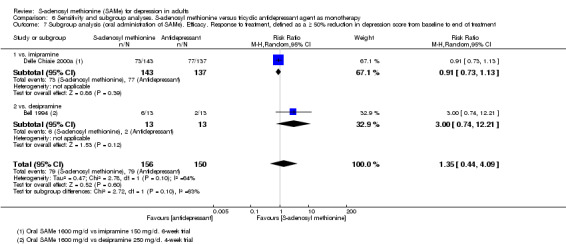
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 7 Subgroup analysis (oral administration of SAMe). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
6.8. Analysis.
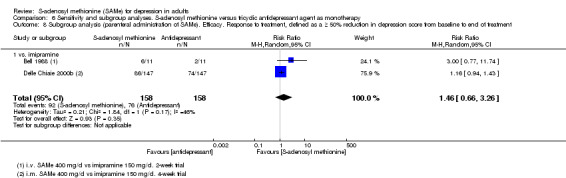
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 8 Subgroup analysis (parenteral administration of SAMe). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
5.5 Acceptability. Participants experiencing troublesome adverse effects of any nature
When we considered separately data from oral and parenteral administration of SAMe, we found evidence for better acceptability was found only for the parenteral administration (RR 0.59, 95% CI 0.44 to 0.78; P = 0.0002; 294 participants; 1 study; I2 = 0%; Analysis 6.9). The analysis of the data regarding the oral administration of SAMe showed no significant difference between the two groups (RR 0.80, 95% CI 0.50 to 1.27; P = 0.34; 310 participants; 2 studies; I2 = 40%; Analysis 6.10). In this last case, one out of the two studies contributing to the outcome, only one favoured SAMe in terms of acceptability. The two studies were heterogeneous in terms of sample size (180 participants Delle Chiaie 2000a; 30 participants De Vanna 1992). Further, in the Delle Chiaie 2000a study, in participants who complained of adverse effects, the drug dose could be reduced from the third week onward, to a minimum dose of imipramine 100 mg/day and SAMe 1200 mg/day. This could mean that a dose reduction of SAMe may minimise the risk of experiencing adverse effects more than a reduction of imipramine.
6.9. Analysis.
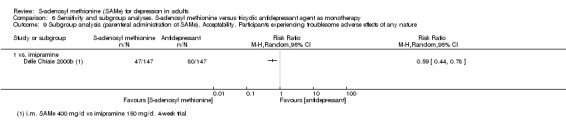
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 9 Subgroup analysis (parenteral administration of SAMe). Acceptability. Participants experiencing troublesome adverse effects of any nature.
6.10. Analysis.
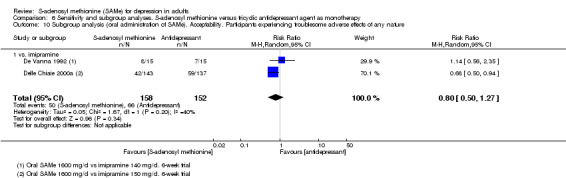
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 10 Subgroup analysis (oral administration of SAMe). Acceptability. Participants experiencing troublesome adverse effects of any nature.
5.6 Acceptability. Participants dropping out for any reasons other than adverse effects
When we considered data from oral and parenteral administration of SAMe separately, the outcome was not affected and neither of the methods of administration differed from the two combined (oral: no events; Analysis 6.11; parenteral: RR 1.00, 95% CI 0.17 to 5.89; P = 1; 22 participants; 1 study; I2 = 0%; Analysis 6.12).
6.11. Analysis.
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 11 Subgroup analysis (oral administration of SAMe). Acceptability. Participants dropping out for any reasons other than adverse effects.
6.12. Analysis.
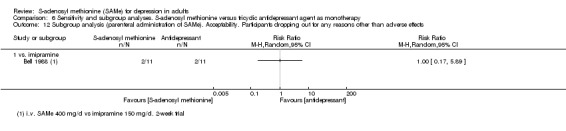
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 12 Subgroup analysis (parenteral administration of SAMe). Acceptability. Participants dropping out for any reasons other than adverse effects.
Sensitivity analyses
In the De Vanna 1992 study, we extrapolated the data regarding the efficacy of treatment from the figure reported in the paper, as no other information was available. To check the correctness of our calculations, we verified if the extrapolated MD matched with the per cent improvement in the mean scores shown in the paper. In addition, the authors did not specify how they dealt with missing data. We attempted to contact them in order to obtain clarification, but were unsuccessful. As they did not indicate whether they had or not conducted an ITT analysis, we decided to use only the reported data without any imputation, in order to be the most conservative possible. To assess the robustness of these assumptions, we conducted a sensitivity analysis.
We identified two studies with more than 30% of missing data and carried out a sensitivity analysis (Bell 1994; Mischoulon 2014). Further, as two studies provided no drop‐outs rates, we decided to exclude these studies in this sensitivity analysis (Delle Chiaie 2000a; Delle Chiaie 2000b).
The Bell 1994 study was at high risk of attrition bias; we performed a sensitivity analysis excluding this study.
Further, we conducted a sensitivity analysis where we had imputed missing data and standard deviations.
Comparison 1: S‐adenosyl methionine versus placebo as monotherapy
Primary outcomes
6.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
When we performed a sensitivity analysis excluding the Mischoulon 2014 trial (more than 30% missing data), the results with the remaining data from Kagan 1990 were significantly different (Analysis 5.4). However, it is important to remember that the Kagan 1990 study had a very small sample size (18 participants).
5.4. Analysis.

Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 4 Sensitivity analysis (excluding studies with high levels of missing data). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
Otherwise, the other conducted sensitivity analyses did not affect the results (sensitivity analysis for the imputation of continuous efficacy data with the assumption that missing participants had the same mean change as the other participants, Analysis 5.1; sensitivity analysis for the imputation of standard deviations, using correlation coefficient of 0.4, Analysis 5.3).
5.1. Analysis.
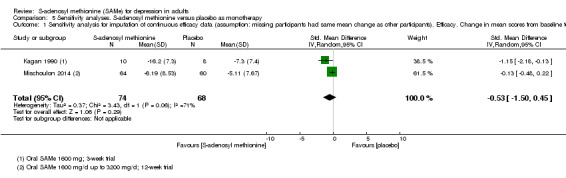
Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 1 Sensitivity analysis for imputation of continuous efficacy data (assumption: missing participants had same mean change as other participants). Efficacy. Change in mean scores from baseline to end of treatment on the depression rating scale (negative value = improvement).
5.3. Analysis.
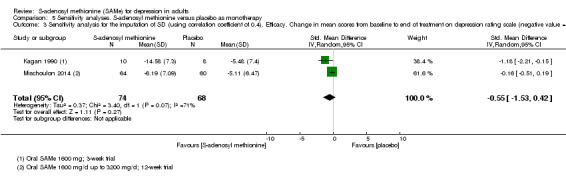
Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 3 Sensitivity analysis for the imputation of SD (using correlation coefficient of 0.4). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
6.2 Acceptability. Participants dropping out of treatment during study period for any reason
The sensitivity analysis excluding the study with high levels of missing data (Mischoulon 2014) did not change the result, although only one study remained (Kagan 1990) (Analysis 5.5).
5.5. Analysis.
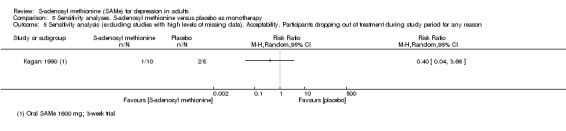
Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 5 Sensitivity analysis (excluding studies with high levels of missing data). Acceptability. Participants dropping out of treatment during study period for any reason.
Secondary outcomes
6.3 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
We performed two sensitivity analyses: sensitivity analysis for the imputation of dichotomous data with the assumption of 'best‐case' scenario (Analysis 5.2), and sensitivity analysis excluding study with high levels of missing data (Analysis 5.6). In both cases, the results were not affected.
5.2. Analysis.
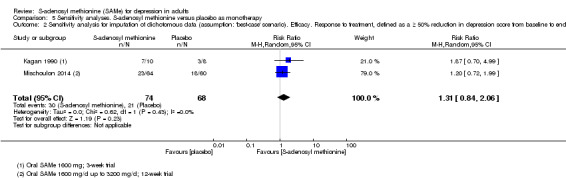
Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 2 Sensitivity analysis for imputation of dichotomous data (assumption: 'best‐case' scenario). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
5.6. Analysis.
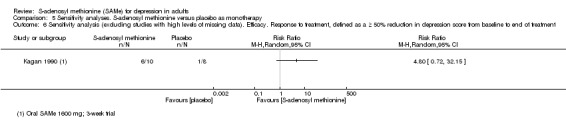
Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 6 Sensitivity analysis (excluding studies with high levels of missing data). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
6.4 Acceptability. Participants dropping out for any reasons other than adverse effects
The sensitivity analysis excluding Mischoulon 2014 (high level of missing data) had only one study and did not affect the results (Analysis 5.7).
5.7. Analysis.
Comparison 5 Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy, Outcome 7 Sensitivity analysis (excluding studies with high levels of missing data). Acceptability. Participants dropping out for any reasons other than adverse effects.
Comparison 2: S‐adenosyl methionine versus tricyclic antidepressant as monotherapy
Primary outcomes
7.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
We conducted the following sensitivity analyses, which did not affect the results: sensitivity analysis for the imputation of continuous efficacy data with the assumption that missing participants had the same mean change as the other participants (Analysis 6.13); sensitivity analysis for the imputation of standard deviations using correlation coefficient of 0.4 (Analysis 6.15); sensitivity analysis excluding studies with high levels of missing data (Delle Chiaie 2000a; Delle Chiaie 2000b) (Analysis 6.16); sensitivity analysis excluding the De Vanna 1992 study (Analysis 6.20).
6.13. Analysis.
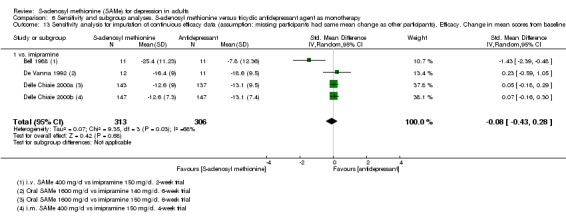
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 13 Sensitivity analysis for imputation of continuous efficacy data (assumption: missing participants had same mean change as other participants). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
6.15. Analysis.
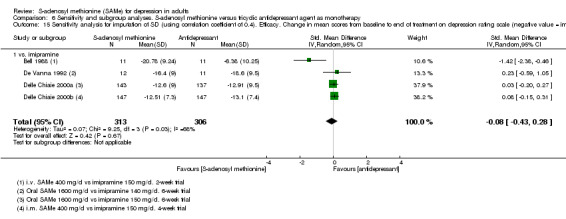
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 15 Sensitivity analysis for imputation of SD (using correlation coefficient of 0.4). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
6.16. Analysis.
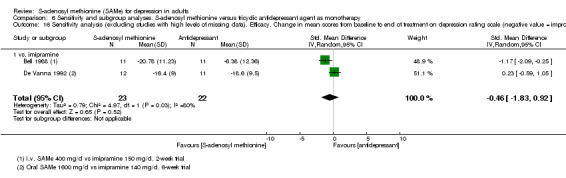
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 16 Sensitivity analysis (excluding studies with high levels of missing data). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
6.20. Analysis.
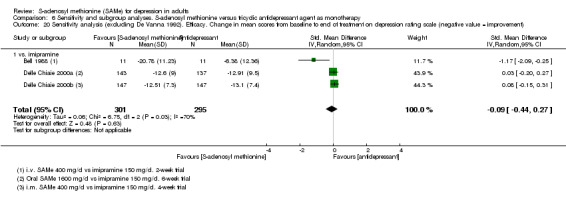
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 20 Sensitivity analysis (excluding De Vanna 1992). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
7.2 Acceptability. Participants dropping out of treatment during study period for any reason
The sensitivity analysis excluding studies with high levels of missing data (Bell 1994) (Analysis 6.17) and the sensitivity analysis excluding the Bell 1994 study that was at high risk of attrition bias (Analysis 6.21) did not change the results.
6.17. Analysis.
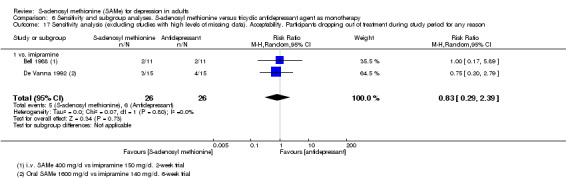
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 17 Sensitivity analysis (excluding studies with high levels of missing data). Acceptability. Participants dropping out of treatment during study period for any reason.
6.21. Analysis.
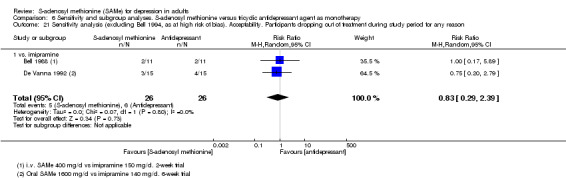
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 21 Sensitivity analysis (excluding Bell 1994, as at high risk of bias). Acceptability. Participants dropping out of treatment during study period for any reason.
Secondary outcomes
7.3 Efficacy. Response to treatment, defined as a 50% reduction or greater in depression score from baseline to end of treatment
We carried out the sensitivity analyses as per protocol: sensitivity analysis for the imputation of dichotomous data with the assumption of 'best‐case' scenario (Analysis 6.14); sensitivity analysis excluding studies with high levels of missing data (Bell 1994; Delle Chiaie 2000a; Delle Chiaie 2000b) (Analysis 6.18); sensitivity analysis excluding the Bell 1994 study that was at high risk of attrition bias (Analysis 6.22). This did not change the results.
6.14. Analysis.
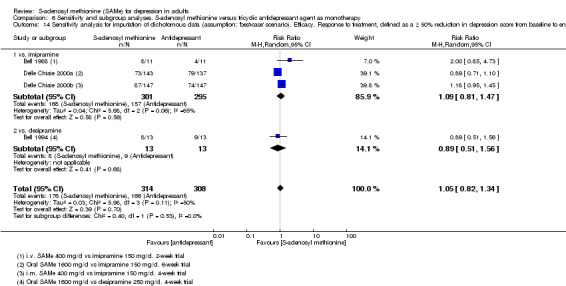
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 14 Sensitivity analysis for imputation of dichotomous data (assumption: 'best‐case' scenario). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
6.18. Analysis.
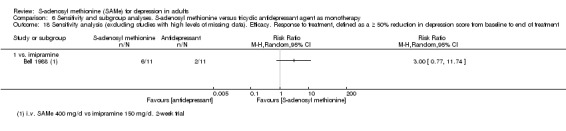
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 18 Sensitivity analysis (excluding studies with high levels of missing data). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
6.22. Analysis.
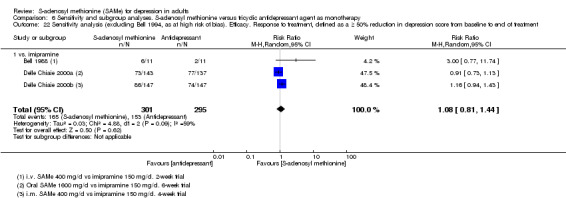
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 22 Sensitivity analysis (excluding Bell 1994, as at high risk of bias). Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment.
7.4 Acceptability. Participants experiencing troublesome adverse effects of any nature
When we performed a sensitivity analysis excluding studies with high levels of missing data (Delle Chiaie 2000a; Delle Chiaie 2000b), only the De Vanna 1992 study remained. The results changed and we found no evidence of SAMe superiority (Analysis 6.19).
6.19. Analysis.
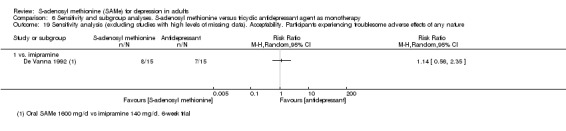
Comparison 6 Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy, Outcome 19 Sensitivity analysis (excluding studies with high levels of missing data). Acceptability. Participants experiencing troublesome adverse effects of any nature.
Comparison 3: S‐adenosyl methionine versus selective serotonin reuptake inhibitor as monotherapy
Primary outcomes
8.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
We conducted a sensitivity analysis for the imputation of standard deviations using correlation coefficient of 0.4, which did not affect the results (Analysis 7.1).
7.1. Analysis.
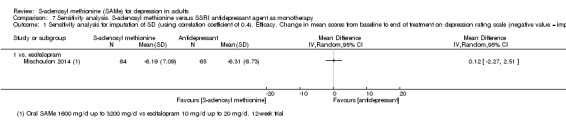
Comparison 7 Sensitivity analysis. S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy, Outcome 1 Sensitivity analysis for imputation of SD (using correlation coefficient of 0.4). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
Comparison 4: S‐adenosyl methionine versus placebo as adjunctive treatment
Primary outcomes
9.1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale
We performed a sensitivity analysis to assess the robustness of the imputation of standard deviations using a correlation coefficient of 0.4 (Analysis 8.1). The outcome was not affected.
8.1. Analysis.
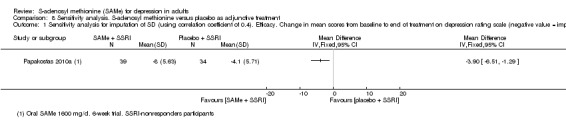
Comparison 8 Sensitivity analysis. S‐adenosyl methionine versus placebo as adjunctive treatment, Outcome 1 Sensitivity analysis for imputation of SD (using correlation coefficient of 0.4). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement).
Discussion
Summary of main results
This systematic review included eight trials and 934 participants. The quality of the evidence, reflected in the GRADE analysis, ranged from low to very low. This limits the applicability of the findings and the interpretation of the treatment effects should be made with caution.
We did not find that SAMe was more efficacious than placebo in monotherapy, reflected in mean change data in depression rating scale and in response and remission findings; there were two studies in the first two analyses and one study in the last analysis. These results should be interpreted with caution. The quality of the evidence contributing to these outcomes was low to very low. The result of these analyses were determined by data from a large three‐arm trial in which neither SAMe nor escitalopram were superior to placebo. Further, in Analysis 1.1 (change in mean scores from baseline to end of treatment on the depression rating scale) the studies showed a high level of heterogeneity (I2 = 72%; P = 0.06). The Kagan 1990 study favoured SAMe over placebo in terms of efficacy (SMD ‐1.18; CI ‐2.21, ‐0.15; P = 0.02), but the Mischoulon 2014 study reported no evidence that the active treatment was more efficacious than placebo (SMD ‐0.13; CI ‐0.48 to 0.22; P = 0.46). This heterogeneity could be related to the different duration of the trials (three weeks, Kagan 1990; 12 weeks, Mischoulon 2014). As the risk of spontaneous improvement is cumulative (Posternak 2000), it could be maximised in a 12‐week trial, increasing the placebo response. However, it is noted that in the Mischoulon 2014 study, there was significant separation between SAMe and placebo on the depression rating scale only at treatment at weeks eight and 10, not at week three and dose increase was allowed for non‐responders at week six. It is also noted that in Mischoulon 2014, a three‐armed study comparing placebo, SAMe and escitalopram, at the endpoint, all three treatments arms demonstrated a significant improvement in the depression rating scale but there was no significant difference between any of the treatments. Another potential source of heterogeneity could be that different formulations of SAMe may have been used: Mischoulon 2014 used SAMe tosylate, while Kagan 1990 did not specify the formulation. Another source of heterogeneity could be that participants in Kagan 1990 trial were all inpatients while in Mischoulon 2014 trial they were all outpatients; this could suggest a different severity of the disease. In addition, one study recruited only men (Kagan 1990), while Mischoulon 2014 recruited both men and women, raising the issue of a possible gender effect of SAMe (Sarris 2015a). In addition, it is relevant that the two studies were highly diverged in terms of sample size: Mischoulon 2014 had the largest number of participants (124), but also had a high level of missing data.
SAMe was more efficacious than placebo as an adjunctive treatment, using mean change data and in terms of response and remission. The quality of this evidence was low. Only one small study contributed to these outcomes, only SSRIs were tested as an add‐on therapy and the data regarded a specific subgroup of participants (SSRI non‐responders).
We did not find the efficacy of SAMe as monotherapy to be different from that of TCA and SSRI antidepressants in the treatment of depression, using mean change data and response/remission findings; four studies were in the comparison SAMe versus TCAs and one study in the comparison SAMe versus SSRI. There was a very small selection of antidepressants tested: the TCAs imipramine and desipramine, and the SSRI escitalopram. In addition, the quality of the evidence contributing to outcomes was low or very low. Under the comparison of SAMe versus a TCA for the mean change score outcome, the studies involved in the analysis were heterogeneous (I2 = 57%; P = 0.07). One study favoured SAMe over imipramine (Bell 1988). This could be due to its shorter duration (two weeks). In addition, the therapeutic action of imipramine is often delayed by two to four weeks, and these results may suggest a more rapid onset of action of the SAMe. Another potential source of heterogeneity could be that different formulations of SAMe could have been used: the Delle Chiaie 2000a and Delle Chiaie 2000b studies used butanedisulphonate‐SAMe, while the other trials did not specify formulations. In addition, the doses and routes of administration of SAMe among the studies varied. However, when we conducted subgroup analyses separating data regarding oral and parenteral administration of SAMe, neither of the methods of administration differed from the two combined and the outcome was not affected. Under the same comparison examining response, we identified moderate heterogeneity among the studies (I2 = 58%; P = 0.07). The reasons above could explain this heterogeneity as well as the different comparator antidepressants used (even if there was no pattern of efficacy to explain the heterogeneity).
Overall, the quality of the evidence related to acceptability outcomes was low or very low.
We did not find any evidence that SAMe was more acceptable than antidepressant agents in terms of drop‐outs. For the comparison SAMe versus TCA, there were three studies in the analysis 'Participants dropping out of treatment during the study period for any reason' and two studies in the analysis 'Participants dropping out of the treatment during the study period because of adverse effects'; for the comparison SAMe versus SSRI, there was only one study. Interestingly, there was no strong evidence of a difference in rates of drop‐outs between SAMe and placebo; there were two studies in the analyses where SAMe and placebo were used as monotherapy, and one study in the analyses where SAMe and placebo were used as an adjunctive treatment. With the exception of the De Vanna 1992 study where all drop‐outs were caused by adverse effects, withdrawals were generally due to other causes such as clinical worsening, non‐adherence and scheduling.
With regard to tolerability in terms of number of participants experiencing troublesome adverse effects of any nature, we found parenteral SAMe to be more tolerable than imipramine (three studies in this analysis). Data regarding tolerability of SAMe for the other comparison were not available; however, the studies reported in the text that SAMe was overall safe and well‐tolerated.
Most studies did not detail the specific adverse effects or they were often described with considerable heterogeneity, precluding a complete quantitative analysis of these data. Therefore, no definitive conclusions can be drawn on this issue. Using the available data, we found no evidence of a difference between SAMe and the comparisons with regard to the specific adverse effects of headache, diarrhoea and flatulence (only one study and a very small number of participants were included in each analyses). With regard to mania/hypomania, the analyses involved two studies: in one study, one participant exhibited hypomania and in another study, one participant with no prior history of mania experienced manic symptoms, both while taking SAMe. There were no explicit reports of mortality. Overall, the trials did not systematically assess and report risk for suicidal ideation and behaviours.
The parenteral and oral administration of SAMe seemed to be comparable. Trials used lower doses for parenteral administration than oral. This probably reflects the better bioavailability of SAMe when given parenterally (Stramentinoli 1979). However, the specific dose equivalence between the oral and parenteral routes of administration of SAMe is not established yet.
Overall completeness and applicability of evidence
This systematic review included only RCTs and they were similar in design. They differed in duration of treatment, routes of administration and sample size. The assessment of the quality of the trials was often hindered by the lack of detail regarding key methodological issues, such as randomisation, allocation concealment, blinding and missing data.
The number of included studies, the number of trials used in each comparison, and, in general, the number of participants in those studies were small. Although all the objectives of the review were addressed (see Objectives), not all studies provided appropriate data for all the outcomes we considered. In addition, for some outcomes the CIs were large. These factors limit the applicability of the findings and the interpretation of the treatment effects.
The use of SAMe in the included studies reflects its use in practice. Trials tested standard doses and both oral and parenteral routes. However, in the clinical practice lower doses of SAMe are also administered for the oral route (800 mg/day to 1200 mg/day as per leaflet of the commercialised drug).
Studies analysed both men and women with a diagnosis of major depression according to DSM‐III or DSM‐IV criteria, the population of interest for this review. However, with the exception of one study examining a particular subgroup of participants with major depression (SSRI non‐responders), we could not obtain data from other subgroup of participants such as with atypical depression or anxiety symptoms or psychotic symptoms or with treatment resistance. Therefore, we could not investigate if participants with certain subtypes of depression may respond differently to SAMe. We also excluded participants with bipolar depression, schizoaffective disorder, cyclothymia and dysthymia; therefore, the results cannot be generalised to depressive episodes occurring in these contexts. Overall, it is accepted that one of the main limitations of efficacy trials is to include participants far from "real world" (Rothwell 2005). In clinical practice, patients are usually very heterogeneous, even among groups of people with the same diagnosis. Similarly, in clinical practice a large proportion of people with depression have physical comorbidities. As we excluded studies of participants with physical comorbidities, this further limits the generalisability of the findings. This issue may be considered in a future version of this review.
We found SAMe to be more efficacious than placebo as an adjunctive treatment. However, the applicability of this evidence is limited. Only one trial contributed to the outcome, participants were only SSRI non‐responders and SAMe was added only to one category of antidepressant (the SSRI). Further, under the comparisons of SAMe versus an active treatment, overall studies included only three antidepressant agents (imipramine, escitalopram and desipramine), limiting the applicability of the evidence.
The economic case for the use of SAMe in clinical practice cannot be made as the trials did not provide information on the comparative costs of the treatments.
Quality of the evidence
We assessed the quality of evidence and constructed the 'Summary of findings' tables for each comparison. See Table 1; Table 2; Table 3; Table 4.
From the GRADE evaluation documented in the 'Summary of findings' tables, this systematic review found no evidence of high or moderate quality. The quality of the evidence for SAMe was generally low/very low.
It is concluded that higher quality evidence from further research would be required to increase our confidence in the estimate of the effect of this intervention. We rated the quality of the body of evidence for the outcomes accounting the following factors: risk of bias, inconsistency, indirectness, imprecision and publication bias.
All included studies were RCTs. However, often, we were unable to assess the quality of the trials because of the lack of information regarding randomisation, allocation concealment, blinding and missing data (Bell 1988; Delle Chiaie 2000a; Delle Chiaie 2000b; De Vanna 1992). On addition, one of the included studies had a high risk of attrition bias (Bell 1994). We downgraded, for risk of bias, the quality of evidence by one level for the outcomes determined by these studies.
We downgraded, for inconsistency, the evidence for the outcomes where the contributing studies showed a significant heterogeneity (P < 0.1).
We downgraded the evidence for the outcomes where the Papakostas 2010a study was a contributor, because of indirectness. This trial examined a particular subgroup of participants with major depression, namely SSRI non‐responders.
In most outcomes, we downgraded the quality of evidence for imprecision because the sample size was small, the number of relevant events was small and the CIs were wide and included 'no effect'.
In accordance with the protocol, we did not formally assess publication bias by funnel plot analyses, because the review included fewer than 10 studies (Galizia 2014).
Potential biases in the review process
Some limitations and biases could be noted in the review process.
We performed a broad and thorough literature search, exploring all sources detailed in the protocol (Galizia 2014). Therefore, it is likely that we identified all relevant studies. However, it is possible that we missed studies that are still unpublished or are currently being conducted and plan to include these in future updates of the review. Then, although we made exhaustive attempts to retrieve as much data as possible, by asking pharmaceutical companies and study authors to supply all available information, data from some trials are still lacking. Further, this review lists a number of references and one study classified as 'awaiting classification', apparently published but proved unretrievable.
All included studies were RCTs. However, the assessment of the trial quality was often complicated by the lack of information regarding randomisation, allocation concealment, blinding and missing data. Further, some of the older included studies did not reflect current methodological practice.
Overall, the included studies did not always report the data we needed to assess all our outcomes. For example, only a few studies reported detailed data on the specific adverse effects occurring during the trials as well as the rates of adverse effects of any nature experienced by participants.
The meta‐analyses often combined data from studies of different durations and routes of medication administration. Where identified, we investigated heterogeneity and conducted subgroup analyses if possible.
In the case of missing data, we analysed data on an ITT basis. Some of the studies included data from 'completer' data‐sets only. Although we contacted the trialists, attempts to obtain additional unpublished data and information regarding missing data were almost always unsuccessful. We were unable to use the LOCF approach for dealing with missing data, as individual raw participant data were not available. Therefore, where needed, we imputed the missing data as well as the standard deviations. Although the sensitivity analyses conducted to assess the robustness of the assumptions yielded similar results, the imputation may have led to biases.
Although all included studies evaluated the efficacy of treatment by administration of the HAM‐D, they used different versions of this rating scale. Therefore, we had to apply the SMD in order to measure the treatment efficacy and this could have led to some biases.
We evaluated both the rates of response to treatment and the remission. 'Treatment response' describes an improvement in the person's condition of sufficient quality to result in a reduction of at least 50% in depressive symptomatology. However, what is clinically relevant is achieving remission, which correlates with better longer‐term functional recovery and lower risk of relapse. Only two studies in this review reported remission rates.
Three studies provided participants over the age range of our review, including participants aged 18 to 80 years (De Vanna 1992; Mischoulon 2014; Papakostas 2010a). Our protocol restricted the age range to 18 to 70 years but did not stipulate methods for how to deal with this issue (Galizia 2014). We decided to include these studies after consideration of the mean age of the participants.
One study was not designed to test efficacy of SAMe as a primary outcome, but focused on the correlation between plasma SAMe levels and the degree of clinical improvement (Bell 1994). In this study, a subset of participants from a previously reported double‐blind study comparing oral SAMe and desipramine participated in the trial. Therefore, though the study included data for efficacy and tolerability, it may not have been sufficiently powered to detect differences in depressive symptoms.
Another potential bias in the review process could come from the method in which data were obtained from the De Vanna 1992 study. We extrapolated data regarding the efficacy of treatment from the figure reported in the paper, as no other information was available. We checked the correctness of our calculations verifying that the extrapolated MDs matched the per cent improvement in the mean scores cited in the paper. In addition, the authors of this study did not specify how they dealt with missing data. We attempted to contact authors to obtain clarification but were unsuccessful. As they did not indicate whether they had conducted an ITT analysis, we decided to use the reported data without any imputation. These factors could have led to bias, although the sensitivity analysis conducted yielded similar results.
Some studies allowed the use of benzodiazepines as a hypnotic, but in none of the studies did the authors analyse its potential confounding effects. This could have led to bias in the included studies and so in this review.
In the present review no RCTs reported economic outcomes. This represents a limit for the applicability of findings as comprehensive economic estimates of antidepressant treatment effect would better inform healthcare policy.
Agreements and disagreements with other studies or reviews
Meta‐analyses (Bressa 1994; Hardy 2002) and systematic reviews (Carpenter 2011; Mischoulon 2002; Papakostas 2003; Williams 2005) have consistently concluded that SAMe is effective for treating depression, with an efficacy superior to placebo and equivalent to the conventional antidepressants. Further, when used in combination with conventional antidepressants, it has been reported that SAMe seems to have the potential to enhance response or limit adverse effects, by allowing lower doses of the conventional antidepressant to be prescribed (Sarris 2010).
In our review, we found that the efficacy of SAMe for the treatment of depression was not different from TCA and SSRI antidepressants and higher than placebo as an adjunctive treatment, though the comparisons with SSRI antidepressants and placebo as an adjunctive treatment included only one study. In contrast to the findings of other reviews, we did not demonstrate superior efficacy of SAMe in comparison with placebo in monotherapy. In our review, this analysis was undermined by a high level of heterogeneity and included only two studies.
With regard to acceptability, SAMe has generally reported to be safe and well‐tolerated with a more favourable adverse effect profile than conventional antidepressants (Carpenter 2011; Hardy 2002; Rambaldi 2006). In our review, SAM‐e was not found to be different from either placebo or established antidepressants.
SAMe has been reported to be associated (in rare instances) with induction of mania (Carney 1989; Carpenter 2011; Gören 2004; Lipinski 1984) ‐ similarly to conventional antidepressants. Two studies in our review reported one participant each who presented with manic/hypomanic symptoms in the SAMe arm.
Where our findings differ from results of previous reviews, it could be considered that the quality and the methodological rigour of many of the individual studies included in the previous reviews is questionable. Most of these studies were older, had a heterogeneous population of participants with a diagnosis not necessarily restricted to major depression. Many of the studies exhibited methodological flaws, had a small sample size and a short duration of treatment. Often they include data from 'completer' data‐sets only and exclude participants dropping out for any reason from the efficacy analysis.
We strictly adhered to the inclusion criteria of our protocol, aiming to improve the quality of the body of the evidence (Galizia 2014). This was probably the reason we have included fewer studies in this review than other reviews. Nevertheless, we met the same limitations of the previous reviews. Some studies were methodologically dated, lacked information on methodological key issues, had an inadequate sample size, included data from completers participants only, did not report data in an extractable way or a combination of these.
Authors' conclusions
Implications for practice.
Given the absence of high quality evidence and the inconsistency of our findings, we are unable to draw firm conclusions about the use of S‐adenosyl methionine (SAMe) for the treatment of depression in adults. It should be investigated further by additional larger high quality randomised controlled trials.
There were two reports of mania/hypomania in a review of 441 participants in the SAMe arm. Although this adverse effect was not commonly found in our analysis, it is an important factor to be considered in clinical settings.
Implications for research.
Further research of high methodological quality is required to establish the efficacy and acceptability of SAMe confidently. Double‐blind randomised controlled trials should be conducted. Specific attention should be paid to the process of randomisation, allocation concealment, blinding and handling of missing data.
In addition, the following methodological issues should be considered.
The population studied. Future studies should include people with a diagnosis of major depression of both sexes; aged 18 years and over; of all ethnicities; and in inpatient, outpatient and primary care settings. Data from particular subgroups of participants with major depression, such as those with psychotic features, mixed features, anxiety symptoms, treatment resistance or atypical depression should be analysed separately.
The nature of the intervention. SAMe should be studied in monotherapy and as an adjunctive therapy to antidepressant medication. A dose of 800 mg/day to 1600 mg/day for oral administration and 200 mg/day to 400 mg/day for parenteral administration should be used. There is little available information about the comparability of oral and parenteral doses of SAMe. Clearly, it would be important to establish this in studies where oral and parenteral SAMe are directly compared. Because SAMe is best absorbed on an empty stomach, it should be administered 30 to 60 minutes before meals or two hours after meals; future studies should instruct the participants and monitor for compliance with these instructions. In addition, as the stability of different formulae and of the tablets varies, in future studies the formulation of SAMe should be reported in detail. Trials should also be of at least eight weeks' duration. Comparison interventions should include placebo and alternative antidepressants or adjunctive agents from all classes. The antidepressants tested should not be limited to TCAs and SSRI.
Relevant outcome measures. Detailed and complete information should be provided on depression rating scale scores, remission and response rates, drop‐outs with the reasons why and accounts of specific adverse events. It would be desirable if these were documented for each participant. Reports of mania and hypomania are important, particularly with regard to the safety of prescribing this treatment in people with depression at high risk of later developing a bipolar illness. Hyperhomocysteinaemia is also of interest. It would be worthwhile to investigate specific adverse effects that can limit the participants' compliance, such as sexual adverse effects and weight gain; SAMe could potentially have an advantage with regard to adverse effects that are commonly caused by recognised antidepressants. Outcome measures of relevance to participants and their carers should also be included. Further, there could be a gender effect with regard to response to SAMe; this has been reported previously (Sarris 2015a), and may have been a possible explanation for the heterogeneity between the Kagan 1990 and Mischoulon 2014 studies. Therefore, it would be interesting to conduct analyses by the subgroup gender in order to investigate a possible gender effect.
Data on the comparative costs of treatment should be included in future studies, to allow economic analyses.
Acknowledgements
CRG Funding Acknowledgement
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Core Ovid MEDLINE search ‐ CCMDCTR
Core Ovid MEDLINE search used to inform the Cochrane Common Mental Disorders Group's specialised register (CCMDCTR), a weekly search alert based on condition + RCT filter. 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID EMBASE and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. MEDLINE, EMBASE and PsycINFO search strategies
Ovid MEDLINE was searched using the following terms (30‐May‐2012):
1. S‐ADENOSYLMETHIONINE/
2. 29908‐03‐0.rn.
3. (s adenosyl$).tw.
4. (SAM‐e or Samyr or Ademetionine or Adomet or Adenosylmethionine or Adenoylmethionine or Adenosyl levo Methionine
or Adenosyl l Methionine or Active Methionine or Acylcarnitine or Methioninyladenylate or Gumbaral or fo 1561 or fo1561).tw.
5. or/1‐4
6. exp MOOD DISORDERS/
7. DEPRESSION/
8. (depress$ or dysthymi$ or affective disorder$ or adjustment disorder$ or cyclothym$).tw.
9. or/6‐8
10. randomised controlled trial.pt.
11. controlled clinical trial.pt.
12. randomi#ed.ti,ab.
13. placebo$.tw.
14. drug therapy.fs.
15. trial$.ti,ab.
16. groups.ab.
17. randomly.ab.
18. (clinic$ adj3 (trial$ or study or studies$)).ti,ab.
19. ((singl$ or doubl$ or tripl$) adj (blind$ or mask$ or dummy)).ti,ab.
20. (control$ or prospectiv$ or volunteer$).ti,ab.
21. or/10‐20
22. exp animals/ not humans.sh.
23. 21 not 22
24. 23 and 9 and 5
Ovid EMBASE was searched using the following terms (30‐May‐2012):
1. S ADENOSYLMETHIONINE/
2. (s adenosyl$).tw.
3. (SAM‐e or Samyr or Ademetionine or Adomet or Adenosylmethionine or Adenoylmethionine or Adenosyl levo Methionine
or Adenosyl l Methionine or Active Methionine or Acylcarnitine or Methioninyladenylate or Gumbaral or fo 1561 or fo1561).tw.
4. 29908‐03‐0.rn.
5. or/1‐4
6. exp MOOD DISORDER/
7. exp DEPRESSION/
8. exp BIPOLAR DISORDER/
9. ADJUSTMENT DISORDER/
10. (depress$ or dysthymi$ or affective disorder$ or adjustment disorder$ or cyclothym$).tw.
11. or/6‐10
12. clinical trial.de.
13. controlled clinical trial.de.
14. randomised controlled trial.de.
15. major clinical study.de.
16. double blind procedure.de.
17. single blind procedure.de.
18. randomization.de.
19. placebo.de.
20. prospective study.de.
21. comparative study.de.
22. follow up.de.
23. (randomi#ed or randomly).ti,ab.
24. ((singl$ or doubl$ or tripl$) adj (blind$ or mask$ or dummy)).ti,ab.
25. placebo$.tw.
26. (clinic$ adj3 (trial$ or study or studies$)).ti,ab.
27. comparative stud$.ti,ab.
28. (control$ or prospectiv$ or volunteer$).ti,ab.
29. or/12‐28
30. ((animal or nonhuman) not (human and (animal or nonhuman))).de.
31. 29 not 30
32. 31 and 11 and 5
Ovid PsycINFO was searched using the following terms (30‐May‐2012):
1. (s adenosyl$).tw.
2. (SAM‐e or Samyr or Ademetionine or Adomet or Adenosylmethionine or Adenoylmethionine or Adenosyl levo Methionine
or Adenosyl l Methionine or Active Methionine or Acylcarnitine or Methioninyladenylate or Gumbaral or fo 1561 or fo1561).tw.
3. or/1‐2
4. exp AFFECTIVE DISORDERS/
5. ADJUSTMENT DISORDERS/
6. (depress$ or dysthymi$ or affective disorder$ or adjustment disorder$ or cyclothym$).tw.
7. or/4‐6
8. treatment effectiveness evaluation.de.
9. clinical trials.de.
10. placebo.de.
11. treatment outcomes.de.
12. mental health program evaluation.de.
13. evaluation.de.
14. followup studies.de.
15. random$.ti,ab.
16. placebo$.tw.
17. comparative stud$.ti,ab.
18. (clinical adj3 trial$).ti,ab.
19. (research adj3 design).ti,ab.
20. (evaluat$ adj3 stud$).ti,ab.
21. (prospectiv$ adj3 stud$).ti,ab.
22. ((singl$ or doubl$ or tripl$) adj3 (blind$ or mask$ or dummy)).ti,ab.
23. or/8‐22
24. (animal NOT (animal and (human or inpatient or outpatient))).po.
25. 23 not 24
26. 25 and 7 and 3
Appendix 3. Dealing with missing data
We contacted trialists to request information and data on missing participants, but were unable to obtain any additional data.
We analysed data on an intention‐to‐treat (ITT) basis. We were unable to use the last observation carried forward (LOCF) approach as, in all cases, individual raw participant data were not available. We addressed the missing data as follows.
1. For continuous efficacy outcomes, we imputed missing data using the conservative approach of assuming that these participants had no change in their mean score on the Hamilton Depression Rating Scale (HAM‐D) from baseline to endpoint. As we did not have access to the raw participant data for their baseline score, we used the mean baseline score of all participants.
To assess the robustness of the assumptions, we carried out sensitivity analyses: we assumed the participants had the same mean change as the other participants.
Where present, we kept the same original change‐from‐baseline standard deviation (SD) (Delle Chiaie 2000a; Delle Chiaie 2000b); otherwise we imputed them. Where possible, we used the SD from another study in the review sufficiently homogeneous in terms of measurement scales and time period (Delle Chiaie 2000a to impute SD in De Vanna 1992; Delle Chiaie 2000b to impute SD in Kagan 1990). Otherwise, we calculated the correlation coefficient from the Delle Chiaie 2000a study (c = 0.1) and imputed the change‐from‐baseline SDs in the other studies, making use of this imputed correlation coefficient (Bell 1988; Mischoulon 2014; Papakostas 2010a). We then used the SDs thus calculated for the imputed means in each study.
We undertook a sensitivity analysis trying a different value of the correlation coefficient. We used a value of 0.4, as it was the highest correlation coefficient obtained from the studies reported in considerable detail to calculate it (correlation coefficient of 0.1 was the lowest value obtained).
In the De Vanna 1992 study, the authors did not specify how they dealt with missing data. We attempted to contact the authors in order to obtain clarification, but were unsuccessful. As they did not indicate whether they had or not conducted an ITT analysis, we decided to use the reported data without any imputation, in order to be the most conservative possible. We conducted a sensitivity analysis for the efficacy data.
2. For dichotomous outcomes, we imputed missing data based on the consideration of a 'worst‐case' scenario. To assess the robustness of the assumption, we carried out sensitivity analyses based on a 'best‐case' scenario.
With regard to the outcome 'Acceptability. Participants experiencing specific adverse effects', in handling missing data, we departed from the protocol and performed an available‐case analysis (Galizia 2014). Given the restricted number of trials and small number of events, we did not want to overestimate the specific adverse events, such as manic symptoms, etc., by imputing them.
Four studies provided an ITT analysis, using the LOCF approach (Delle Chiaie 2000a;Delle Chiaie 2000b;Papakostas 2010aMischoulon 2014). However, in the Mischoulon 2014 study, there were tolerability data available for 166 participants (59 participants in SAMe group, 55 in escitalopram group and 52 in placebo group) of the 189 randomised. Through contact with the authors, we established that for the tolerability analysis they focused on participants who had completed at least one postbaseline visit with adverse effects recorded.
In the Delle Chiaie 2000a trial, of the 281 randomised participants, they excluded three participants in the control group from the ITT efficacy analysis because one participant received no treatment and two participants received no postbaseline assessment. In the Delle Chiaie 2000b trial, of the 295 randomised participants, one in the control group received no treatment and one in the SAMe group received no postbaseline assessment; these two participants were excluded from the ITT efficacy analysis. In the Delle Chiaie 2000a trial, we imputed missing data for two of the three participants who received treatment. Similarly, in the Delle Chiaie 2000b trial, we imputed data for one of the two missed participants who received treatment. Instead, in both studies, all treated participants were included in the safety evaluation.
Overall, we imputed missing data as follows.
For the outcome 'Change in mean score from baseline to end of treatment on depression rating scale', under the comparison 'S‐adenosyl methionine versus placebo as monotherapy', we imputed missing data for Kagan 1990; under the comparison 'S‐adenosyl methionine versus an active antidepressant agent as monotherapy', we imputed missing data for Bell 1988;Delle Chiaie 2000a; Delle Chiaie 2000b; and De Vanna 1992.
For the outcome 'Efficacy. Response to treatment', under the comparison 'S‐adenosyl methionine versus placebo as monotherapy', we imputed missing data for Kagan 1990; under the comparison 'S‐adenosyl methionine versus an active antidepressant agent as monotherapy', we imputed missing data for Bell 1988; Bell 1994; Delle Chiaie 2000a; and Delle Chiaie 2000b.
For the outcome 'Acceptability. Participants experiencing troublesome adverse effects of any nature', under the comparison 'S‐adenosyl methionine versus placebo as monotherapy' we imputed missing data for Kagan 1990.
There was no need to impute missing data in the Papakostas 2010a and Mischoulon 2014 studies, for the analysed outcomes.
Data and analyses
Comparison 1. S‐adenosyl methionine versus placebo as monotherapy.
Comparison 2. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement) | 4 | 619 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.34, 0.27] |
| 1.1 vs. imipramine | 4 | 619 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.34, 0.27] |
| 2 Acceptability. Participants dropping out of treatment during study period for any reason | 3 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.31] |
| 2.1 vs. imipramine | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.29, 2.39] |
| 2.2 vs. desipramine | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.14, 1.30] |
| 3 Acceptability. Participants dropping out of treatment during study period because of adverse effects | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.79] |
| 3.1 vs. imipramine | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.20, 2.79] |
| 4 Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment | 4 | 622 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.83, 1.56] |
| 4.1 vs. imipramine | 3 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.81, 1.44] |
| 4.2 vs. desipramine | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.74, 12.21] |
| 5 Acceptability. Participants experiencing troublesome adverse effects of any nature | 3 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.52, 0.88] |
| 5.1 vs. imipramine | 3 | 604 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.52, 0.88] |
| 6 Acceptability. Participants experiencing specific adverse effects: mania or hypomania | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 6.1 vs. imipramine | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 3.00 [0.13, 68.26] |
| 7 Acceptability. Participants dropping out for any reasons other than adverse effects | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.17, 5.89] |
| 7.1 vs. imipramine | 2 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.17, 5.89] |
Comparison 3. S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 vs. escitalopram | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Acceptability. Participants dropping out of treatment during study period for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 vs. escitalopram | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Acceptability. Participants dropping out of treatment during study period because of adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 vs. escitalopram | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Efficacy. Response to treatment, defined as a ≥ 50% reduction in depression score from baseline to end of treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 vs. escitalopram | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Efficacy. Remission, defined as a depression rating scale score within normal range at end of study | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.1 vs. escitalopram | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Acceptability. Participants dropping out for any reasons other than adverse effects | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 vs. escitalopram | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. S‐adenosyl methionine versus placebo as adjunctive treatment to SSRI.
Comparison 5. Sensitivity analyses. S‐adenosyl methionine versus placebo as monotherapy.
Comparison 6. Sensitivity and subgroup analyses. S‐adenosyl methionine versus tricyclic antidepressant agent as monotherapy.
Comparison 7. Sensitivity analysis. S‐adenosyl methionine versus SSRI antidepressant agent as monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis for imputation of SD (using correlation coefficient of 0.4). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 vs. escitalopram | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Sensitivity analysis. S‐adenosyl methionine versus placebo as adjunctive treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis for imputation of SD (using correlation coefficient of 0.4). Efficacy. Change in mean scores from baseline to end of treatment on depression rating scale (negative value = improvement) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bell 1988.
| Methods | Double‐blind, randomised, controlled study | |
| Participants | 22 inpatients included and treated Age (years): range 20 to 65 (mean ± SD age of the 18 participants who completed the study 43 ± 13) Sex: 5 men, 13 women Diagnosis: DSM‐III diagnosis of major depression and a score at baseline > 23 on an augmented 31‐item version of the HAM‐D Exclusion criteria: histories of major medical illness, personality disorder or substance or alcohol abuse in the 6 months preceding the study; refusal to given written informed consent; history of failure to respond to the equivalent of 150 mg/day or more of imipramine given for 4 weeks |
|
| Interventions | SAMe: 11 participants; increasing doses of SAMe iv, from 200 mg/day to 400 mg/day iv, over 3 days and then were maintained at 400 mg/day throughout the rest of the study Imipramine: 11 participants; imipramine titrated up to 150 mg/day (capsules) in divided doses over 4 days and then received that dose through the rest of the trial. Also given iv saline infusions each day Duration of treatment: 14 days, preceded by 2‐day baseline evaluation and medication washout |
|
| Outcomes | Efficacy of treatment evaluated by 31‐item HAM‐D assessed daily and BDI completed by the participants at baseline and on the last day of study. A reduction of more than 50% on HAM‐D was considered a successful treatment response Tolerability of treatment evaluated by performing CBC, SMA‐18, urinalysis, thyroid function tests, ECG, and DST, at baseline and on the last day of the study; vital signs, including blood pressure, pulse, and respiration, recorded at baseline evaluation and on each study day 15, 30 and 60 minutes after the infusion was started Somatic Symptom Checklist assessed daily |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients randomly assigned". Method not described |
| Allocation concealment (selection bias) | Unclear risk | Identical tablets and saline infusions. No other measures described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Nursing staff, raters and participants blind to the type of infusions. Not specified about tablets. Allocation concealment was not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Raters blind to type of infusions. Concern since allocation concealment not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcomes not enough to have clinically relevant impact on observed effect size. Reason for drop‐outs stated 22 participants included and treated. 4 participants (2 in each drug treatment group) did not complete the study; 1 withdrawn after receiving a diagnosis of antisocial personality disorder, 2 refused to continue because of discomfort with the iv procedure, and 1 withdrawn because results of a test for syphilis were positive |
| Selective reporting (reporting bias) | Low risk | No formal a priori statement of primary outcome measure, but did report HAM‐D |
| Other bias | Low risk | |
Bell 1994.
| Methods | Double‐blind, randomised, controlled study | |
| Participants | 26 participants (21 inpatients and 5 outpatients) included and treated Age (years): range 20 to 70 (mean ± SD age of the 17 participants who completed at least 2 weeks of active treatment 39 ± 14; 43 ± 16 in SAMe group, 33 ± 8 in desipramine group) Sex: 4 men, 13 women (4 men, 7 women in SAMe group; 0 men, 6 women in desipramine group) Diagnosis: DSM‐III‐R diagnosis of major depression and a baseline score > 20 on 17‐item HAM‐D or > 23 on an augmented 31‐item HAM‐D Exclusion criteria: history of significant medical illness, personality disorder, substance or alcohol abuse in the 6 months prior to the study, refusal to give written informed consent, history of failed response to the equivalent of 150 mg/day or more of imipramine for 4 weeks, HAM‐D scores fallen to ≤ 80% of baseline scores after an initial 3‐day evaluation and medication washout period |
|
| Interventions | SAMe: 11 participants received 1600 mg/day (tablets) in divided dose every day for the 4 weeks Desipramine: 6 participants titrated to 250 mg/day (tablets) in divided dose over the first 5 days and continued, whenever possible, at 250 mg/day until the end of the trial Duration of treatment: 4 weeks, preceded by 3‐day evaluation and medication washout period |
|
| Outcomes | Relationship between plasma levels of SAMe and clinical response Efficacy of treatment evaluated by 17‐item and 31‐item HAM‐D, assessed at baseline and weekly thereafter, as well as by BDI, completed by participants at baseline and weekly throughout the protocol. A participant classified as a treatment responder if he or she showed a reduction of > 50% on total HAM‐D‐17 scores at week 4 from baseline. Blood samples for the analysis of plasma SAMe levels collected after the washout period and at the end of study, in order to evaluate the correlation between plasma SAMe levels and the degree of clinical improvement Tolerability of treatment was evaluated by Systematic Assessment for Treatment Emergent Events, assessed at baseline and weekly thereafter. In addition, thyroid function, CBC, SMA‐18, urinalysis and ECG measured at baseline and on the last day of the study Vital signs, including blood pressure, pulse and respiration, recorded at baseline and at each weekly visit |
|
| Notes | Quote: "A subset of patients from our previously reported double‐blind study comparing oral SAMe and desipramine in the treatment of major depression participated in this study". There was no mention in the literature of this previous study (Bell, American Journal of Psychiatry). In addition, though we contacted the authors, this did not solve the issue. Therefore, we have used just this subset This study was carried out before the US Food and Drug Administration requested that all US sites conducting clinical investigations on SAMe discontinue their studies until more information was available from the company making SAMe tablets |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly assigned". Method not stated |
| Allocation concealment (selection bias) | Unclear risk | Method not stated. Pharmacist not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical tablets. States that nursing staff, raters and participants were blind to type of medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated that raters were blind to type of medication |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26 participants randomised. 17 participants completed ≥ 2 weeks of the study (11 on SAMe and 6 on desipramine). Only used data on completers. Reason not stated |
| Selective reporting (reporting bias) | Low risk | HAM‐D was primary outcome measure (also BDI but not reported) |
| Other bias | Low risk | |
De Vanna 1992.
| Methods | Double‐blind, randomised, controlled study | |
| Participants | 30 participants included and treated Age (years): range 27 to 80 (mean age 48.5 overall; 48.4 in SAMe group; 48.5 in imipramine group) Sex: 9 men, 21 women (3 men, 12 women in SAMe group; 6 men, 9 women in imipramine group) Diagnosis: DSM‐III‐R diagnosis of major depression and a score at baseline ≥ 18 on HAM‐D Exclusion criteria: contraindication to tricyclic antidepressants; suicidal ideation; psychotic episodes; severe liver, renal, cardiovascular, endocrine, or neurological diseases; pregnant or nursing women; chronic alcohol abusers; drug abusers. Before starting the trial, participants received placebo during a 7‐day drug‐free, washout period. Excluded placebo responders from study |
|
| Interventions | SAMe: 15 participants received 1600 mg/day (tablets) Imipramine: 15 participants received 140 mg/day (tablets) Duration of treatment: 6 weeks No concomitant medications allowed. In some cases, a sleep‐inducing benzodiazepine (triazolam 0.25 mg) was permitted for not more 2 weekly administrations |
|
| Outcomes | Efficacy of treatment evaluated by 10‐item MADRS, 21‐item HAM‐D, 14‐item HAM‐A, 20‐item Zung's Self‐Rating Scale for Depression, assessed at baseline and days 10, 20, and 42 CBC, blood urea nitrogen, blood sugar, creatinine, transaminases, bilirubin and urinalysis performed at baseline and end of trial. Adverse effects reported | |
| Notes | Authors stated that 3 participants in SAMe group and 5 in control group dropped out. However, incongruity was evident in the paper, because the authors cited elsewhere that 4 participants dropped out in the imipramine group. We attempted to contact the authors to obtain clarification, but were unsuccessful. As the authors quoted also "Twenty‐three patients, 12 in the SAMe group and 11 in the imipramine group, completed the treatment", we concluded that 4 participants dropped out in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, more than "patients were randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Method not described. Called a double‐blind trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not fully described. Called a double‐blind trial and indistinguishable tablets used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. Called a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 enrolled participants. 15 to SAMe group; 15 to imipramine group. 3 drop‐outs on SAMe (nausea and vomiting), 4 drop‐outs on imipramine (1 for nausea and vomiting, 1 for excessive sweating, 2 for mouth dryness). Unclear whether LOCF/ITT used |
| Selective reporting (reporting bias) | Low risk | Did not state primary outcome measure. Did report on all measures they listed |
| Other bias | Low risk | |
Delle Chiaie 2000a.
| Methods | Multicentre, double‐blind, randomised, controlled study | |
| Participants | 281 outpatients included and treated Age (years): range 18 to 70 (mean ± SD: 45.3 ± 11.92 in SAMe group; 44.6 ± 13.2 in imipramine group) Sex: 82 men, 196 women (40 men, 103 women in SAMe group; 42 men, 93 women in imipramine group) Diagnosis: DSM‐IV diagnosis of major depressive episode with a unipolar (depressive) course and no psychotic symptoms; a score at baseline ≥ 18 on the 21‐item HAM‐D with the score on the first item of the scale (depressed mood) being ≥ 2, and a severity score ≥ 4 on the CGI rating scale. Baseline assessment performed after 1 week, during which no treatment was administered; at this point, participants who still satisfied the inclusion and exclusion criteria began the double‐blind treatment phase |
|
| Interventions | SAMe: 143 participants received 1,4‐butanedisulphonate‐SAMe 1600 mg (tablets) Imipramine: 138 participants received 150 mg/day (tablets) according to a gradual titration, and full doses of imipramine reached after 15 days In participants who complained of adverse effects, the drug dose could be reduced from the third week on, down to a minimal dose of imipramine 100 mg/day and SAMe 1200 mg/day. Participants who tolerated this dose poorly excluded from study Duration of treatment: 6 weeks During the study, only lorazepam (1 mg/day to 2.5 mg/day orally) was allowed to facilitate sleep induction if required |
|
| Outcomes | Main efficacy measures were HAM‐D total score at endpoint and percentage of treatment responders (i.e. those participants who had a CGI score ≤ 2 at end of study) Secondary efficacy measures were MADRS total score at endpoint and percentage of treatment responders (i.e. those participants who had a decrease in HAM‐D score from baseline of ≥ 50% at end of study) 21‐item version of HAM‐D assessed at baseline and at days 14, 28 and 42 to evaluate depressive symptoms CGI assessed at baseline and at days 14, 28 and 42 to evaluate severity of illness and degree of improvement after treatment MADRS assessed at baseline and at days 14, 28 and 42 to detect the rapid mood variations occurring during antidepressant therapy Tolerability and safety of treatment: incidence of adverse events assessed during treatment period, including changes in laboratory measures. Laboratory analyses, ECG and vital signs performed at baseline and at final visit |
|
| Notes | Authors stated, "As is usual in a multicenter clinical study, each center was provided with a portion of an overrepresented randomisation list, on the basis of the number of patients the center planned to enrolled. The slight difference in the number of patients in each group resulted because of a discrepancy between the planned number of patients and the number actually enrolled in some centers" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation. "Randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Use of placebo tablets indistinguishable in appearance from active compound |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and dummy tablets used but allocation concealment was unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind and dummy tablets used but allocation concealment was unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 281 participants met criteria. 143 participants in SAMe group, 138 participants in imipramine group. 3 in imipramine group randomised but not included in ITT efficacy analysis because 1 participant received no treatment and 2 participants received no postbaseline assessment. ITT analysis carried out, but no other non‐completers described |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure was HAM‐D total score at endpoint and percentage of responders on CGI. Reported on these |
| Other bias | Low risk | |
Delle Chiaie 2000b.
| Methods | Multicentre, double‐blind, randomised, controlled study | |
| Participants | 295 outpatients included and treated Age (years): range 18 to 70 (mean ± SD; 48.2 ± 12.2 in SAMe group; 48.8 ± 14.0 in imipramine group) Sex: 108 men, 185 women (44 men, 102 women in SAMe group; 64 men, 83 women in imipramine group) Diagnosis: DSM‐IV diagnosis of major depressive episode with a unipolar (depressive) course and no psychotic symptoms; a score at baseline ≥ 18 on the 21‐item HAM‐D, with the score on the first item of the scale (depressed mood) being ≥ 2, and a severity score ≥ 4 on the CGI rating scale. Baseline assessment performed after 1 week, during which no treatment was administered; at this point, participants who still satisfied the inclusion and exclusion criteria began the double‐blind treatment phase |
|
| Interventions | SAMe: 147 participants received 1,4‐butanedisulphonate‐SAMe 400 mg/day intramuscularly Imipramine: 148 participants received imipramine 150 mg/day (tablets) Duration of treatment: 4 weeks During the study, only lorazepam (1 mg/day to 2.5 mg/day orally) was allowed to facilitate sleep induction if required |
|
| Outcomes | Main efficacy measures were HAM‐D total score at endpoint and percentage of treatment responders (i.e. those participants who had a CGI score ≤ 2 at end of study) Secondary efficacy measures were MADRS total score at endpoint and percentage of treatment responders (i.e. those participants who had a decrease in HAM‐D score from baseline of ≥ 50% at end of study) 21‐item HAM‐D assessed at baseline and at days 14 and 28 to evaluate depressive symptoms CGI assessed at baseline and at days 14 and 28 to evaluate severity of illness and degree of improvement after treatment MADRS assessed at baseline and at days 14 and 28 to detect the rapid mood variations occurring during antidepressant therapy Tolerability and safety of treatment: incidence of adverse events assessed during treatment period, including changes in laboratory measures. Laboratory analyses, ECG and vital signs performed at baseline and at final visit |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation. "Randomisation list" |
| Allocation concealment (selection bias) | Unclear risk | Use of placebo tablets indistinguishable in appearance from active compound |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind and dummy vials used but allocation concealment was unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind and dummy vials used but allocation concealment was unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 295 participants met criteria. 147 participants in SAMe group, 148 participants in imipramine group. 1 participant in SAMe group received no postbaseline assessment and 1 in imipramine group received no treatment; these 2 participants were excluded from the ITT efficacy analysis. ITT analysis carried out but no other non‐completers described |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure was HAM‐D total score at endpoint and percentage of responders on CGI. Reported on these |
| Other bias | Low risk | |
Kagan 1990.
| Methods | Double‐blind, randomised, placebo‐controlled study | |
| Participants | 18 inpatients included and treated Age (years): 18 to 65 (mean ± SD age of 15 participants who completed study 42.2 ± 16.3) Sex: men Diagnosis: DSM‐III criteria for major depression, unipolar, without psychotic features and a scores > 20 on the 21‐item HAM‐D Exclusion criteria: actively suicidal, bipolar, substance abuse, significant medical problems, people who would experience undue loss (e.g. financial) from participation in the trial, major abnormalities in physical examinations, routine laboratory (SMA‐18, CBC, urinalysis, thyroid function tests), and ECGs. All participants underwent 7‐day drug‐free washouts on the ward and were rated again before entering the trial; participants who no longer met the criteria for the study were excluded |
|
| Interventions | SAMe: 9 participants received SAMe 1600 mg/day (tablets) Placebo: 6 participants received placebo tablets First 5 participants received gradually increasing doses; placebo was given to 2 and SAMe to 3. Their doses increased from 200 mg/day to 800 mg twice daily by day 7. The dose remained at 800 mg twice daily for days 8 to 21. Since the oral SAMe was extremely well tolerated by these first 5 participants, authors decided to eliminate the graduated‐dose phase of the study. The remaining participants received 800 mg twice daily for the entire trial Duration of treatment: 21 days |
|
| Outcomes | Efficacy of treatment evaluated by 21‐item HAM‐D and Carroll Rating Scale for Depression, assessed at baseline and at days 3, 7, 14 and 21 Vital signs recorded each day. Adverse effects reported | |
| Notes | Trial initially planned to include 30 participants, but the authors were forced to stop after 18 participants were enrolled because approval of the SAMe 200‐mg tablet was withdrawn by the US Food and Drug Administration. This withdrawal was not related to the clinical performance of SAMe but to technical issues regarding data on the dissolution of the tablets | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned by a computer‐generated random string of 0s and 1s |
| Allocation concealment (selection bias) | Low risk | No participant, families, ward staff or investigators aware of code and identical tablets used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No participant, families, ward staff or investigators aware of code and identical tablets used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No participant, families, ward staff or investigators aware of code and identical tablets used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 participants recruited. 15 participants completed. 2 in placebo group withdrawn (1 because of worsening depression, 1 had hypothyroidism); 1 in SAMe group was non‐compliant and was dropped from the study. Only reported completers. Missing outcomes not enough to have clinically relevant impact on observed effect size |
| Selective reporting (reporting bias) | Low risk | Use HAM‐D and Carroll Rating for Depression. Both reported |
| Other bias | Low risk | |
Mischoulon 2014.
| Methods | 3‐armed, double‐blind, randomised, controlled study | |
| Participants | 189 outpatients included and treated Age (years): range 18 to 80 years (mean ± SD 45 ± 15) Sex: 95 men, 94 women Diagnosis: DSM‐IV diagnosis of major depressive disorder and a score ≥ 25 on the IDS‐Clinician Rated Exclusion criteria: pregnancy or women of childbearing potential who were not using a medically accepted means of contraception; serious suicidality or homicidality; unstable medical illness including cardiovascular, hepatic, renal, respiratory, endocrine, neurological or haematological; organic mental disorders; substance‐ or alcohol‐use abusers, active within the preceding 6 months; schizophrenia and other psychotic disorders or psychotic features; bipolar disorder; acute bereavement; severe borderline or antisocial personality disorder; current primary diagnoses of panic disorder or obsessive‐compulsive disorder; seizure disorder; concurrent use of other psychotropic drugs; hypothyroidism; ≥ 6‐week treatment with escitalopram ≥ 10 mg/day or SAMe ≥ 1200 mg/day during the current depressive episode; intolerance to SAMe or escitalopram; having taken an investigational psychotropic drug within the last year; failure to respond to ≥ 2 antidepressant trials at adequate doses (e.g. fluoxetine ≥ 40 mg/day) and duration (≥ 6 weeks) during the current depressive episode; any depression‐focused ongoing psychotherapy; history of bleeding diatheses, low platelet counts, gastrointestinal bleeding, or use of medications that alter bleeding risk; CGI‐Improvement scale score of 'much' or 'very much improved' between the screening and baseline visits or an IDS‐Clinician Rated score < 25 at either the screening or the baseline visit, or both of these |
|
| Interventions | SAMe: 64 participants received SAMe tosylate 1600 mg/day (tablets) during the first 6 weeks Escitalopram: 65 participants received escitalopram 10 mg/day (tablets) during the first 6 weeks Placebo: 60 participants received placebo tablets Dose increase was allowed for non‐responders (participants with a < 50% HAM‐D‐17 score reduction) at week 6; escitalopram could be increased to 20 mg/day and SAMe to 3200 mg/day for weeks 7 to 12. Participants who experienced intolerable adverse effects at the higher dose were allowed to decrease the dose to the previous level Duration of treatment: 12 weeks |
|
| Outcomes | Primary efficacy measure was change in 17‐item HAM‐D over 12 weeks. Response defined as > 50% decrease in the HAM‐D ‐17 items and remission as a final HAM‐D < 7 Secondary measures of efficacy included changes in scores on the IDS‐Clinician Rated, IDS‐Self Report, CGI‐Severity and CGI‐Improvement ratings over time Adverse events documented with the Systematic Assessment for Treatment of Emergent Events ‐ Specific Inquiry Outcomes were assessed at weeks 1, 2, 4, 6, 8, 10 and 12 |
|
| Notes | Study include a cross‐over phase in which non‐responders to either escitalopram or SAMe received the combination of the 2 drugs, though this report focused on the main outcome data for the first 12 weeks of double‐blind treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in a 1:1:1 manner. Randomisation numbers assigned by a biostatistician, in consecutive order, stratified by site |
| Allocation concealment (selection bias) | Low risk | Research pharmacists at both sites maintained codes/allocations. All participants, clinicians and research co‐ordinators blinded to intervention |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy design to maintain blinding, and allocation codes kept by pharmacists. No information regarding effectiveness of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded by allocation concealment. No information regarding effectiveness of blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis, including all participants allocated to the 3 treatment arms with LOCF analysis. 189 randomised: 64 in SAMe group; 65 in escitalopram group; 60 in placebo group. 92 participants dropped out. All drop‐outs reported in CONSORT diagram, and reasons for drop‐out given 28 participants dropped out in the SAMe group (3 because of adverse events, 3 for clinical worsening, 5 because of ineffectiveness, 1 for scheduling, 4 because of non‐adherence, 4 lost to follow‐up and 8 for unknown reasons) 35 participants dropped out in the escitalopram arm (8 because of adverse events, 4 for clinical worsening, 3 for scheduling, 6 because of non‐adherence, 7 lost to follow‐up and 7 for unknown/unspecified reasons) 29 participants discontinued placebo (4 because of adverse events, 2 for clinical worsening, 4 because of ineffectiveness, 3 because of non‐adherence, 2 lost to follow‐up and 14 for unknown reasons) A very large proportion of drop‐outs occurred |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure specified and reported. Some but not all secondary measures, e.g. not IDS‐C |
| Other bias | Low risk | |
Papakostas 2010a.
| Methods | Double‐blind, randomised, placebo‐controlled study | |
| Participants | 73 participants included and treated Age (years): range 18 to 80 Sex: 29 men, 44 women (18 men, 21 women in SAMe group, 11 men, 23 women in placebo group) Diagnosis: SSRI non‐responders with DSM‐IV current major depressive disorder and a score of ≥ 16 on HAM‐D; treatment with an SSRI at adequate doses (a minimally adequate dose was defined as fluoxetine, citalopram or paroxetine at ≥ 20 mg/day; escitalopram ≥ 10 mg/day; sertraline ≥ 50 mg/day; duloxetine ≥ 60 mg/day and venlafaxine ≥ 150 mg/day; this was defined historically); treatment with SSRIs for an adequate duration (defined as treatment at an adequate dose for at least 6 weeks). At baseline visit, participants must have been taking a stable dose of an SSRI for the past 4 weeks Exclusion criteria: breastfeeding or pregnant women, or women of childbearing potential who were not using a medically accepted means of contraception; a decrease in depressive symptoms as reflected by the HAM‐D total score between the screen and baseline visits > 15%; serious suicide or homicide risk, unstable medical illness; active alcohol‐ or drug‐use disorder within the last 6 months; history of mania, hypomania (including antidepressant‐induced), psychotic symptoms, or seizure disorder; clinical evidence of untreated hypothyroidism; failure to experience sufficient symptom improvement following more than 4 antidepressant trials during the current major depressive episode; prior course of SAMe or intolerance to SAMe at any dose |
|
| Interventions | Adjunctive SAMe: 39 participants received 2 SAMe tosylate 400 mg tablets daily Adjunctive placebo: 34 participants received placebo tablets All participants had their number of tablets doubled upon completion of 2 weeks of treatment (target dose of SAMe was 800 mg twice daily) Participants continued to receive their SSRI treatment at a stable dose throughout the 6‐week trial. Participants who were unable to tolerate the study medications, per protocol, were withdrawn Duration of treatment: 6‐weeks |
|
| Outcomes | Primary outcome measure was defined as difference in response rates, according to 17‐item HAM‐D, between the 2 treatment groups. Response according to HAM‐D ratings was defined as a ≥ 50% reduction in scores during treatment (or a final score of ≤ 7) Secondary outcome measures included continuous change in HAM‐D scores and CGI‐Severity ratings during treatment; proportion of participants meeting remission status according to HAM‐D scores (final score of ≤ 7) or CGI‐Severity ratings (score of 1 at endpoint) and response status according to CGI‐Improvement ratings (score of < 3 at endpoint) Adverse effects were reported Postbaseline study visits occurred weekly |
|
| Notes | The authors inadvertently made a calculation error in the results. The quoted percentage is 36.1% for SAMe + antidepressant treatment among responders, whereas the actual number of responders indicated on page 945 was 18/39, or 46.1%. Likewise, the quoted percentage for SAMe + antidepressant treatment among participants who remitted was 25.8%, whereas the actual number of these participants cited on page 945 was 14/39, or 35.1%. So, 36.1% was given instead of 46.1% and 25.8% instead of 35.8% (Fleisch 2010; Papakostas 2010b) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation process not described ‐ "randomly assigned" |
| Allocation concealment (selection bias) | Low risk | Dummy pills used for placebo |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind trial, but methods not detailed, except above |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Described as double‐blind trial, but methods not detailed, except above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73 participants assigned to treatment. 55 completed (31 in SAMe group; 24 in placebo group). All non‐completers were mentioned, with reasons for drop‐out. 4 participants in the control group and 2 in SAMe group dropped out because of inefficacy; 3 in placebo and 2 in SAMe discontinued because of intolerance; 2 in placebo and 3 in SAMe discontinued for other reasons; and 1 in each group was lost to follow‐up LOCF used |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcome measures not stated, but HAM‐D, CGI‐Severity and CGI‐Improvement used and reported |
| Other bias | Low risk | |
BDI: Beck Depression Inventory; CBC: complete blood count; CGI: Clinical Global Impression; DSM: Diagnostic and Statistical Manual of Mental Disorders; DST: dexamethasone suppression test; ECG: electrocardiogram; HAM‐D: Hamilton Depression Rating Scale; HAM‐D: Hamilton Depression Rating Scale; IDS‐C: Inventory of Depressive‐Symptomatology ‐ Clinician‐Rated; ITT: intention‐to‐treat; iv: intravenous; LOCF: last observation carried forward; MADRS: Montgomery‐Åsberg Depression Rating Scale; SAMe: S‐adenosyl methionine; SD: standard deviation; SMA‐18: 18 panel blood test; SSRI: selective serotonin reuptake inhibitor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agnoli 1975 | Inappropriate diagnosis |
| Agnoli 1976 | Inappropriate diagnosis |
| Agnoli 1978 | Inappropriate diagnosis |
| Alvarez 1984 | Heterogeneous group of participants (depression with both unipolar and bipolar course). Although we approached the authors to request original data, we obtained no further information and data from those participants with 'unipolar' depression could not be separated out |
| Bambling 2015 | Inappropriate comparator |
| Barberi 1978 | Inappropriate diagnosis |
| Berlanga 1992 | The adjunctive treatment (imipramine) was increased during the first week of the trial and the period with a stable dose of imipramine was limited at only 1 week |
| Blasi Ras 1985 | Inappropriate diagnosis |
| Bottiglieri 1986 | Inappropriate outcomes |
| Bottiglieri 1990 | Inappropriate outcomes |
| Calandra 1979 | Inappropriate diagnosis |
| Carney 1986 | Inappropriate diagnosis |
| De Leo 1987 | Heterogeneous group of participants (also diagnosis of dysthymia). Although we approached the author to request original data, we obtained no further information and data from those participants with major depression could not be separated out |
| Del Vecchio 1978 | Inappropriate diagnosis |
| Delle Chiaie 1999 | Heterogeneous group of participants (depression with both unipolar and bipolar course). Although we approached the author to request original data, we obtained no further information and data from those participants with 'unipolar' depression could not be separated out |
| Di Pierro 2015 | Inappropriate intervention |
| Fava 1992 | Initial randomisation of the treated participants not stated |
| Janicak 1988 | Heterogeneous group of participants (depression with both unipolar and bipolar course). Although we approach the author to request original data, we obtained no further information and data from those participants with 'unipolar' depression could not be separated out |
| Kufferle 1982 | Heterogeneous group of participants (depression with both unipolar and bipolar course). Although we contacted the author to request original data, we obtained no further information and data from those participants with 'unipolar' depression could not be separated out |
| Lanaia 1977 | Inappropriate diagnosis |
| Mantero 1976 | People with comorbidity of skin disease |
| Muscettola 1982 | Inappropriate diagnosis |
| Rabassini 1979 | Inappropriate diagnosis |
| Salmaggi 1991 | Heterogeneous group of participants (also diagnosis of dysthymia). Although we approached the author to request original data, we obtained no further information and data from those participants with major depression could not be separated out |
| Sarris 2015b | Inappropriate intervention |
| Scarzella 1978 | Inappropriate diagnosis |
| Schifano 1993 | Inappropriate diagnosis |
| Thomas 1987 | Inappropriate diagnosis |
Characteristics of studies awaiting assessment [ordered by study ID]
Quiros 1982.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Study not retrievable |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613001299796.
| Trial name or title | Nutraceuticals as Monotherapy Treatments in Major Depressive Disorder: a Double‐Blind, Randomised, Placebo‐Controlled Trial |
| Methods | 8 weeks, 3‐arm, double‐blind, randomised, placebo‐controlled trial |
| Participants | Inclusion criteria: aged 18 to 70 years; both men and women; fluent in written and spoken English; has the capacity to consent to the study and follow its procedures; fulfils the DSM‐IV‐TR and DSM‐V diagnostic criteria for major depressive disorder on structured interview (MINI‐Plus); presents with mild‐to‐moderate depression (MADRS 14‐25) at time of study entry; meets SAFER 2.0 criteria for a stable episode of depression Exclusion criteria: currently taking any antidepressant medication (SSRIs, SNRIs, TCAs, MAOIs, mood stabilisers, etc.); current use of any nutraceutical including a multivitamin, omega‐3, or psychotropic herbal medicine e.g. St John's wort (a 2‐week washout can occur before inclusion); presents with suicidal ideation (> 1 on MADRS suicidal thoughts domain) at time of study entry; ≥ 3 failed trials of pharmacotherapy or somatic therapy (e.g. ECT, TMS) for the current major depressive episode; recently commenced psychotherapy (> 4 weeks of stable treatment acceptable); taking warfarin or phenytoin; diagnosis of bipolar disorder I/II or schizophrenia on structured interview (MINI‐Plus); a primary clinical diagnosis of a substance‐/alcohol‐use disorder within the last 12 months on structured interview (MINI‐Plus); known or suspected clinically unstable systemic medical disorder (including cancer, organ failure or serious cardio/cerebrovascular disease); pregnancy or breastfeeding; not using medically approved contraception (including abstinence) if women and of childbearing age; allergy to seafood |
| Interventions | Group A: SAMe 800 mg/day + cofactors folinic acid 500 μg/day and vitamin B12 200 μg/day Group B: enhanced SAMe combination nutraceutical formulation consisting of SAMe 800 mg/day, omega‐3 concentrate (EPA esters 1000 mg/day, DHA esters 656 mg/day), 5‐HTP 200 mg/day, zinc picolinate 30 mg/day + cofactors folinic acid 500 μg/day, vitamin B12 200 μg/day, vitamin B6 200 mg/day, vitamin E 40 IU/day, vitamin C 60 mg/day and magnesium amino acid chelate 40 mg/day) Group C: placebo tablets and capsules, identical in appearance to the active treatments, made of microcrystalline cellulose (an inert plant product) and containing no active ingredients |
| Outcomes | Severity of depressive symptoms measured with MADRS Anxiety measured with HAM‐A Health‐related quality of life measured with SF‐12 Self reported quality of sleep measured with LSEQ Severity of self reported depressive symptoms measured with the BDI‐II Symptom severity and global improvement measured with CGI‐Severity and CGI‐Improvement scales The CORE Assessment of Psychomotor Change |
| Starting date | 21 November 2013 |
| Contact information | Dr Jerome Sarris, jerome.sarris@unimelb.edu.au Ms Jenifer Murphy, NAT‐Dstudy@unimelb.edu.au |
| Notes |
ACTRN12613001300763.
| Trial name or title | The Efficacy of S‐Adenosyl Methionine (SAMe) and a Combination Nutraceutical as Adjunctive Treatments in Depression: a Double‐Blind, Randomised, Placebo‐Controlled Trial |
| Methods | 3‐arm, double‐blind, randomised, placebo‐controlled trial |
| Participants | Men and women aged 18 to 70 years DSM‐IV‐TR and DSM‐V diagnosis of major depressive disorder with a score ≥ 18 on MADRS; meet SAFER 2.0 criteria for a stable episode of depression; not currently suicidal (< 4 on the MADRS suicidal thoughts domain) Exclusion criteria: diagnosis of bipolar disorder I/II or schizophrenia; a primary clinical diagnosis of a substance/alcohol‐use disorder within the last 12 months; currently taking MAOIs or TCAs; current use of any nutraceutical or psychotropic herbal medicine including a multivitamin, omega‐3, or St John's wort (a 2‐week washout can occur before inclusion); ≥ 3 failed trials of pharmacotherapy or somatic therapy (e.g. ECT) for the current major depressive episode; recently commenced psychotherapy (> 4 weeks of stable treatment acceptable); taking warfarin or phenytoin; known or suspected clinically unstable systemic medical disorder (including cancer, organ failure or serious cardio/cerebrovascular disease); pregnancy or breastfeeding; not using medically approved contraception (including abstinence) if female and of childbearing age; allergy to seafood; unable to read or understand (or both) English |
| Interventions | Group A: SAMe 800 mg/day + cofactors folinic acid 500 μg/day and vitamin B12 200 μg/day Group B: enhanced SAMe combination nutraceutical formulation consisting of SAMe 800 mg/day, omega‐3 concentrate (EPA‐esters 1000 mg/day, DHA‐esters 656 mg/day, 5‐HTP 200 mg/day, folinic acid 500 μg/day, zinc 30 mg/day + cofactors vitamin B6 200 mg/day, vitamin B12 200 μg/day, vitamin E 40 IU/day, magnesium 40 mg/day and vitamin C 60 mg/day) Group C: placebo 8‐week |
| Outcomes | MADRS, BDI‐II, SF‐12, CGI‐Severity, CGI‐Improvement, Anxiety measured with the HAM‐A, self reported quality of sleep measured with the LSEQ: measured at baseline and weeks 2, 4, 6 and 8 CORE Assessment of Psychomotor Change: measured at baseline and week 8 |
| Starting date | October 2013 |
| Contact information | Dr Jerome Sarris, jsarris@unimelb.edu.au |
| Notes |
NCT01912196.
| Trial name or title | Add‐on Study of MSI‐195 (S‐Adenosyl‐L‐Methionine, SAMe) for Patients with Major Depressive Disorder (MDD) |
| Methods | Double‐blind, randomised, placebo‐controlled trial |
| Participants | Men and women aged 21 to 70 years DSM‐IV‐TR diagnosis of major depressive disorder with a total score ≥ 16 on the HAM‐D17 at screening and baseline visits, with a score of ≥ 2 on mood item 1, have experienced 1 to 4 prior major depressive episodes, have failed 1 to 3 treatment regimens in the current depressive episode, have received an adequate dose and duration of antidepressant therapy (on antidepressant therapy for at least 6 weeks with a stable dose for at least 3 weeks) Exclusion criteria: failed ≥ 4 adequate treatment regimens in current episode of depression, significant risk for suicidal behaviour, intolerance to SAMe, prior use of MSI‐195; history of any of the following psychiatric disorders: eating disorder within 6 months, obsessive compulsive disorder, psychotic disorder, bipolar disorder, mental retardation, dementia or other forms of cognitive impairment at any time or alcohol‐ or substance‐use abuse; > 3 x ULN alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase; > 1.5 x ULN total bilirubin; pregnant or lactating women; any history of seizures, excluding febrile seizures; known positivity for human immunodeficiency virus |
| Interventions | Adjunctive MSI‐195: 2 tablets (800 mg) of MSI‐195 plus ongoing antidepressant therapy Adjunctive placebo: 2 tablets placebo plus ongoing antidepressant therapy 8 weeks |
| Outcomes | HAM‐D17: assessed from baseline to week 8 MADRS: assessed at baseline, weeks 2, 4, 6, 7 and 8 CGI‐Severity: assessed at baseline, weeks 2, 4, 7 and 8 IDS‐Self Rated 30: assessed at baseline, weeks 2, 4, 6 and 8 Adverse events: assessed at baseline, weeks 1, 2, 3, 4, 6, 8 and 9 (follow‐up) Columbia Suicide Severity Rating Scale: assessed at baseline, weeks 2, 4, 6 and 8 |
| Starting date | October 2013 |
| Contact information | Clayton Janik, clayton.janik@ppdi.com; Scott Smith, scott.smith@ppdi.com |
| Notes |
5‐HTP: 5‐hydroxytryptophan; BDI: Beck Depression Inventory; CGI: Clinical Global Impression; DHA: docosahexaenoic acid; DSM: Diagnostic and Statistical Manual of Mental Disorders; ECT: electroconvulsive therapy; EPA: eicosapentaenoic acid; HAM‐A: Hamilton Rating Scale for Anxiety; IDS: Inventory of Depressive Symptomatology; IU: international unit; LSEQ: Leeds Sleep Evaluation Questionnaire; MADRS: Montgomery‐Åsberg Depression Rating Scale; MAOI: monoamine oxidase inhibitor; MINI‐Plus: Mini‐International Neuropsychiatric Interview; SAFER: Massachusetts General Hospital SAFER interview; SAMe: S‐adenosyl methionine; SF‐12: 12‐item Short Form; SNRI: serotonin‐noradrenaline reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; TCA: tricyclic antidepressant; TMS: transcranial magnetic stimulation; ULN: upper limit of normal.
Differences between protocol and review
Our protocol restricted the age range of participants to 18 to 70 years (Galizia 2014). Three studies provided participants over the range 18 to 80 years (De Vanna 1992; Mischoulon 2014; Papakostas 2010a). We decided to include these studies after consideration of the participants mean age.
In the Bell 1988 study, one participant in the comparison group reported a past episode of mania. As per protocol, we should have excluded participants with bipolar depression. We decided to include this study because this condition was limited to only one participant and nothing in the text showed that he experienced a different response to treatment or had a manic switch during the trial.
As per protocol, we analysed data on an ITT basis. However, we were unable to use the LOCF approach and imputed the missing data differently (for a full explanation of imputation strategy see Appendix 3). We did not perform any imputation for the De Vanna 1992 study: because the authors did not specify how they dealt with missing data (by ITT or not) and we decided to use only the reported data, in order to be as conservative as possible. Further, we departed from the protocol in dealing with missing data for the outcome 'Participants experiencing specific adverse effects'. Given the restricted number of trials and events, we performed an available‐case analysis, in order to avoid overestimating the specific adverse events, such as manic symptoms, etc., through the imputation of data.
In the protocol, we defined the outcome 'Response to treatment' as a 50% reduction or greater in depression score from baseline to end of treatment where this was clinically meaningful. However, it was difficult to define clinical meaningfulness so we used 50% reduction only.
Lastly, in the 'Summary of findings' table, we added the outcome 'Participants experiencing troublesome adverse effects of any nature' as we found evidence that we considered to be of interest to the reader. In addition, as we exceeded the maximum number of outcomes for 'Summary of findings' tables, which is seven, we removed the outcome 'Reported adverse events'; most studies did not detail the specific adverse effects or were often described with considerable heterogeneity, precluding a complete quantitative analysis of these data.
Contributions of authors
IG and LO co‐wrote the protocol. They performed the literature search, data extraction, data analysis and selection of trials.
IG drafted the final review.
KM co‐wrote the protocol and supervised the selection of trials, performed the assessment of the risk of bias, data analysis, and contributed to write the final review.
EA contributed to the writing of the protocol and to a preliminary literature search.
JM commented on the protocol and helped perform a preliminary literature search.
DD, TNJ, LY and RWL commented on the protocol and on the final draft.
AHY commented on the protocol and on the final draft, performed assessment of risk of bias, and was a third author to resolve any disputes on study inclusion, study quality and data extraction.
Declarations of interest
Dr Karine Macritchie has no conflicts of interest relevant to this review, but has attended educational symposia and conferences sponsored by several pharmaceutical companies. She has received a non‐promotional educational grant from Sanofi‐Synthélabo to support research. She has been employed at the University of Edinburgh on an award from the Translational Medicine Research Collaboration ‐ a consortium made up by the Universities of Aberdeen, Dundee, Edinburgh and Glasgow, the four associated National Health Service (NHS) Health Boards, Scottish Enterprise and Pfizer (formerly Wyeth).
Dr Ilaria Galizia, Dr Lucio Oldani, Ms Erica Amari, Dr Jacopo Massei, Dr Dominic Dougall and Dr Tessa Jones have no conflicts of interest to declare.
Dr Lam has been a speaker for, sat on advisory boards for, or has received research funds from: Advanced Neuromodulation Systems Inc, AstraZeneca, BrainCells Inc, Biovail, Canadian Institutes of Health Research, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Research Foundation, Eli Lilly, Janssen, Litebook Company Ltd, Lundbeck, Lundbeck Institute, Mathematics of Information Technology and Advanced Computing Systems, Michael Smith Foundation for Health Research, Servier, Takeda, UBC Institute of Mental Health/Coast Capital Savings and Wyeth.
Dr Lakshmi Yatham has received grants from and has been a speaker or member of advisory boards for AstraZeneca, Lilly, Janssen, Bristol Myers Squibb, Pfizer, Novartis, GlaxoSmithKline (GSK), Sanofi, Servier and Scherring Plough.
Professor Allan Young has no conflicts of interest relevant to this review, but he has received honoraria for lectures from Eli Lilly, AstraZeneca, Sanofi, GSK, Sanofi‐Aventis, Pfizer, Janssen, Servier, BMS, Biovail and Wyeth. He has been a member of advisory boards for Eli Lilly, AstraZeneca, Sanofi, GSK, Sanofi‐Aventis, Pfizer, Janssen, Servier, BMS and Wyeth. He has received research support from SMRI, MRC (UK), CIHR, NIH, Wellcome Trust (UK) and the following foundations: the University of British Columbia‐Vancouver General Hospital Foundations, BC Credit Unions, CoastCapital Savings and the Kelty Patrick Dennehy Foundation.
New
References
References to studies included in this review
Bell 1988 {published data only}
- Bell KM, Plon L, Bunney WE Jr, Potkin SG. S‐adenosylmethionine treatment of depression: a controlled clinical trial. American Journal of Psychiatry 1988;145(9):1110‐14. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Bell K, Plon L, Bunney WE Jr. Rapid antidepressant response with SAMe. A double‐study. Alabama Journal of Medical Sciences 1988;25(3):313‐6. [PubMed] [Google Scholar]
Bell 1994 {published data only}
- Bell KM, Carreon D, Plon L, Bunney We Jr, Potkin SG. Oral S‐adenosylmethionine in the treatment of depression: a double‐blind comparison with desipramine. Study report. BioResearch File 1990:53‐4.
- Bell KM, Potkin SG, Carreon D, Plon L. S‐adenosylmethionine blood levels in major depression: changes with drug treatment. Acta Neurologica Scandinavica 1994;154(Suppl 1):15‐8. [DOI] [PubMed] [Google Scholar]
Delle Chiaie 2000a {published data only}
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular s‐adenosyl‐l‐methionine 1,4‐butanedisulfonate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicentrum studies. American Journal of Clinical Nutrition 2002;76(5):1172‐6. [DOI] [PubMed] [Google Scholar]
- Delle Chiaie R, Pancheri P, Scapicchio P. Mc3: multicentre, controlled efficacy and safety trial of oral S‐adenosyl‐methionine (SAMe) vs oral imipramine in the treatment of depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S230. [Google Scholar]
- Delle Chiaie R, Pancheri P, Scapicchio P. Multicentre, controlled efficacy and safety trial of S‐adenosyl‐methionine (SAMe) vs oral imipramine in the treatment of depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):230. [Google Scholar]
- Delle Chiaie R, Pancheri P, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA. 2002.
- Delle Chiaie R, Pancheri R, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10, New Orleans. 2000:NR499.
Delle Chiaie 2000b {published data only}
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular s‐adenosyl‐l‐methionine 1,4‐butanedisulfunate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicenter studies. American Journal of Clinical Nutrition 2002;76(5):1172‐6. [DOI] [PubMed] [Google Scholar]
- Delle Chiaie R, Pancheri P, Scapicchio P. Mc4: multicentre, controlled efficacy and safety trial of intramuscular S‐adenosylmethionine (SAMe) versus oral imipramine in the treatment of depression. International Journal of Neuropsychopharmacology 2000;3(Suppl 1):S230. [Google Scholar]
- Delle Chiaie R, Pancheri P, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10, New Orleans. 2001:NR499.
- Delle Chiaie R, Pancheri P, Scapicchio P. S‐adenosyl‐methionine (SAMe) in major depression: two multicenter trials versus imipramine. 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA. 2002.
- Pancheri P, Scapicchio P, Delle Chiaie R. A double‐blind, randomized parallel‐group, efficacy and safety study of intramuscular S‐adenosyl‐L‐methionine 1,4‐butanedisulphonate (SAMe) versus imipramine in patients with major depressive disorder. International Journal of Neuropsychopharmacology 2002;5(4):287‐94. [DOI] [PubMed] [Google Scholar]
De Vanna 1992 {published data only}
- Vanna M, Rigamonti R. Oral S‐adenosyl‐l‐methionine in depression. Current Therapeutic Research, Clinical and Experimental 1992;52(3):478‐85. [Google Scholar]
Kagan 1990 {published data only}
- Kagan BL, Sultzer L, Rosenlicht N, Gerner RH. Oral S‐adenosylmethionine in depression: a randomized, double‐blind, placebo‐controlled trial. American Journal of Psychiatry 1990;147(5):591‐5. [DOI] [PubMed] [Google Scholar]
Mischoulon 2014 {published data only}
- Fava M. A double‐blind, placebo‐controlled study of the alternative therapy S‐adenosyl‐l‐methionine (SAMe) versus escitalopram in major depressive disorder. clinicaltrials.gov/show/NCT00101452 (accessed 3 August 2016). [http://clinicaltrials.gov/show/NCT00101452]
- Gerbarg PL, Muskin PR, Bottiglieri T, Brown RP. Failed studies should not be used to malign good treatments [NCT00101452]. Journal of Clinical Psychiatry 2014;75(11):e1328. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Fava M. Dr. Mischoulon and colleagues reply [NCT00101452]. Comment on failed studies should not be used to malign good treatments. Journal of Clinical Psychiatry 2014;75(11):e1328‐9. [DOI] [PubMed] [Google Scholar]
- Mischoulon D, Price LH, Carpenter LL, Tyrka AR, Papakostas GI, Fava M, et al. A double‐blind, randomized, placebo‐controlled clinical trial of S‐adenosyl‐l‐methionine (SAMe) versus escitalopram in major depressive disorder. Journal of Clinical Psychiatry 2014;75(4):370‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarris J, Papakostas GI, Vitolo O, Fava M, Mischoulon D. S‐adenosylmethionine (SAMe) versus escitalopram and placebo in major depression RCT: efficacy and effects of histamine and carnitine as moderators of response. Journal of Affective Disorders 2014;164:76‐81. [DOI] [PubMed] [Google Scholar]
- Sarris J, Price LH, Carpenter LL, Tyrka AR, Ng CH, Papakostas GI, et al. Is S‐adenosyl methionine (SAMe) for depression only effective in males? A re‐analysis of data from a randomized controlled trial. Pharmacopsychiatry 2015;48(4‐5):141‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papakostas 2010a {published data only}
- Mischoulon D, Alpert JE, Arning E, Bottiglieri T, Fava M, Papakostas GI. Bioavailability of S‐adenosyl methionine and impact on response in a randomized, double‐blind, placebo‐controlled trial in major depressive disorder [NCT00093847]. Journal of Clinical Psychiatry 2012;73(6):843‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakostas GI. S‐Adenosyl methionine (SAMe) augmentation of selective serotonin reuptake inhibitors (SSRIs) for treatment‐resistant depression (TRD). clinicaltrials.gov/show/NCT00093847 (accessed 3 August 2016). [http://clinicaltrials.gov/show/NCT00093847]
- Papakostas GI, Mischoulon D, Shyu I, Alpert JE, Fava M. S‐adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double‐blind, randomized clinical trial. American Journal of Psychiatry 2010;167(8):942‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agnoli 1975 {published data only}
- Agnoli A, Fazio C, Andreoli V. Neuropsychiatric disorders and transmethylations: therapeutic effects of S adenosyl L methionine [Disturbi neuropsichiatrici e transmetilazioni: effetti terapeutici della S adenosil l metionina]. Clinica Terapeutica 1975;75(6):567‐79. [PubMed] [Google Scholar]
Agnoli 1976 {published data only}
- Agnoli A, Andreoli V, Casacchia M, Cerbo R. Effect of S‐adenosyl‐l‐methionine (SAMe) upon depressive symptoms. Journal of Psychiatric Research 1976;13(1):43‐54. [DOI] [PubMed] [Google Scholar]
Agnoli 1978 {published data only}
- Agnoli A, Andreoli VM, Casacchia M, Maffei F, Fazio C. Results and possible developments of the clinical use of S‐adenosyl‐L‐methionine (SAMe) in psychiatry. Monographien aus dem Gesamtgebiete der Psychiatrie 1978;18:170‐82. [DOI] [PubMed] [Google Scholar]
Alvarez 1984 {published data only}
- Alvarez E, Udina C, Guillamat R. Double blind study on the antidepressant activity of sulfoadenosyl‐L‐methionine (SAMe): clinical and biological factors [Estudio doble ciego sobre la efectividad antidepresiva del SAMe; factores clínicos y biológicos]. Revista del Departamento de Psiquiatría de la Facultad de Medicina de Barcelona 1984;11(4):275‐88. [Google Scholar]
Bambling 2015 {published data only}
- Bambling M, Parham SC, Coulson S, Vitetta L. S‐adenosylmethionine (SAMe) and magnesium orotate as adjunctives to SSRIs in sub‐optimal treatment response of depression in adults: a pilot study. Advances in Integrative Medicine 2015;2(1):56‐62. [Google Scholar]
Barberi 1978 {published data only}
- Barberi A, Pusateri C. The clinical effects of S‐adenosyl‐L‐methionine (SAMe) in cases of depression [Sugli effetti clinici della S‐adenosil‐L‐metionina (SAMe) nelle sindromi depressive]. Minerva Psichiatrica 1978;19(4):235‐43. [Google Scholar]
Berlanga 1992 {published data only}
- Berlanga C, Ortega Soto HA, Ontiveros M, Senties H. Efficacy of S‐adenosyl‐L‐methionine in speeding the onset of action of imipramine. Psychiatry Research 1992;44(3):257‐62. [DOI] [PubMed] [Google Scholar]
Blasi Ras 1985 {published data only}
- Blasi Ras R. Comparative study of sulfoadenosyl‐l‐methionine and amoxapine in depression [Estudio comparativo sulfoadenosil‐l‐metionina con amoxapina en la patologia depresiva]. Psiquis, Revista de Psiquiatria, Psicologia y Psicosomatica 1985;6(4):35‐44. [Google Scholar]
Bottiglieri 1986 {published data only}
- Bottiglieri T, Carney MWP, Edeh J, Laundy M, Martin R, Reynolds EH, et al. A biochemical study of depressed patients receiving S‐adenosyl‐L‐methionine (SAM). Biological Methylation and Drug Design. Experimental Biology and Medicine 1986;12:327‐38. [Google Scholar]
Bottiglieri 1990 {published data only}
- Bottiglieri T, Godfrey P, Flynn T, Carney MWP, Toone BK, Reynolds EH. Cerebrospinal fluid S‐adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S‐adenosylmethionine. Journal of Neurology, Neurosurgery, and Psychiatry 1990;53(12):1096‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calandra 1979 {published data only}
- Calandra C, Roxas M, Rapisarda V. The antidepressant action of SAMe compared with that of chloripramine and an interpretation of its mechanism [Azione antidepressiva della SAMe a paragone della clorimipramina. Ipotesi interpretative del meccanismo d’azione]. Minerva Psichiatrica 1979;20(2):147‐52. [PubMed] [Google Scholar]
Carney 1986 {published data only}
- Carney MW, Edeh J, Bottiglieri T, Reynolds EM, Toone BK. Affective illness and S adenosylmethionine: a preliminary report. Clinical Neuropharmacology 1986;9(4):379‐85. [DOI] [PubMed] [Google Scholar]
De Leo 1987 {published data only}
- Leo D. S‐adenosylmethionine as an antidepressant: a double‐blind trial versus placebo. Current Therapeutic Research, Clinical and Experimental 1987;41(6):865‐70. [Google Scholar]
Delle Chiaie 1999 {published data only}
- Delle Chiaie R, Boissard G. Meta‐analysis of two European multicenter controlled trials with ademetionine (SAMe) in major depression. 6th World Congress of Biological Psychiatry; 1997 22‐27 Jun; Nice, France. 1997.
- Delle Chiaie R, Pancheri P. Combined analysis of two controlled, multicentric, double‐blind studies to assess efficacy and safety of sulfo‐adenosyl‐methionine (SAMe) vs. placebo (MC1) and SAMe vs. clomipramine (MC2) in the treatment of major depression [Analisi combinata di due studi controllati, multicentrici, in doppio cieco per la valutazione di efficacia e tollerabilita' di SAMe vs. placebo (MC1) e di SAMe vs. clomipramina (MC2) nel trattamento della depressione maggiore]. Giornale Italiano di Psicopatologia 1999;5:1‐16. [Google Scholar]
Del Vecchio 1978 {published data only}
- Vecchio M, Iorio G, Cocurullo M, Vacca L, Amati A. Has SAMe (Ado Met) and antidepressant effect: a preliminary trial versus chlorimipramine. Rivista Sperimentale di Freniatria 1978;102:344‐58. [Google Scholar]
Di Pierro 2015 {published data only}
- Pierro F, Settembre R. Preliminary results of a randomized controlled trial carried out with a fixed combination of S‐adenosyl‐L‐methionine and betaine versus amitriptyline in patients with mild depression. International Journal of General Medicine 2015;8:73‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fava 1992 {published data only}
- Fava M, Rosenbaum JF, Birnbaum R, Kelly K, Otto MW, MacLaughlin R. The thyrotropin response to thyrotropin‐releasing hormone as a predictor of response to treatment in depressed outpatients. Acta Psychiatrica Scandinavica 1992;86(1):42‐5. [DOI] [PubMed] [Google Scholar]
Janicak 1988 {published data only}
- Janicak PG, Lipinski J, Davis JM, Altman E, Sharma RP. Parenteral S‐adenosylmethionine (SAMe) in depression; literature review and preliminary data. Psychopharmacology Bulletin 1989;25(2):238‐42. [PubMed] [Google Scholar]
- Janicak PG, Lipinski J, Davis JM, Comaty JE, Waternaux C, Cohen B, et al. S‐adenosylmethionine in depression. A literature review and preliminary report. Alabama Journal of Medical Sciences 1988;25(3):306‐13. [PubMed] [Google Scholar]
Kufferle 1982 {published data only}
- Kufferle B, Grunberger J. Early clinical double‐blind study with S‐adenosyl‐L‐methionine: a new potential antidepressant. Advances in Biochemical Psychopharmacology 1982;32:175‐80. [PubMed] [Google Scholar]
Lanaia 1977 {published data only}
- Lanaia F, Savoca G, Zappala E, Magro E, Calandra A. SAMe in depressive syndromes [La SAMe nelle sindromi depressive]. Igiene Mentale 1977;2:397‐406. [Google Scholar]
Mantero 1976 {published data only}
- Mantero M, Pastorino P. Depressive syndromes, skin disease and transmethylations. Therapeutic effects of S adenosyl L methionine [Sindromi depressive, malattie cutanee e transmetilazioni. Effetti terapeutici della S adenosil L metionina]. Gazzetta Medica Italiana 1976;135(12):707‐16. [Google Scholar]
Muscettola 1982 {published data only}
- Muscettola G, Galzenati M, Balbi A. SAMe versus placebo: a double blind comparison in major depressive disorders. Advances in Biochemical Psychopharmacology 1982;32:151‐6. [PubMed] [Google Scholar]
Rabassini 1979 {published data only}
- Rabassini A, Russo G. Effects of the association of maprotiline + SAMe in depressive syndromes [Effetti dell’associazione maprotilina + SAMe nelle sindromi depressive]. Psichiatria Generale e Dell'eta' Evolutiva 1979;3:305‐23. [Google Scholar]
Salmaggi 1991 {published data only}
- Salmaggi P, Bressa GM, Nicchia G, Coniglio M, Greca P, Grazie C. Double‐blind, placebo‐controlled study of S‐adenosyl‐L‐methionine in depressed postmenopausal women. Psychotherapy and Psychosomatics 1993;59(1):34‐40. [DOI] [PubMed] [Google Scholar]
- Salmaggi P, Bressa GM, Niccia G, Coniglio M, Greca P, Grazie C. Oral S‐adenosylmethionine (SAMe) in the treatment of menopausal depressive disorder (MDD): a double‐blind placebo controlled trial. European Journal of Clinical Investigation 1991;21(2 pt 2):58. [Google Scholar]
Sarris 2015b {published data only}
- Sarris J, Stough C, Bousman C, Murphy J, Savage K, Smith DJ, et al. An adjunctive antidepressant nutraceutical combination in treating major depression: study protocol, and clinical considerations. Advances in Integrative Medicine 2015;2(1):49‐55. [Google Scholar]
Scarzella 1978 {published data only}
- Scarzella R, Appiotti A. A double‐blind clinical comparison of SAMe vs clomipramine in depressive disorders [Confronto clinico in doppio cieco della SAMe versus clorimipramina nelle sindromi depressive]. Rivista Sperimentale di Freniatria 1978;102:359‐65. [Google Scholar]
Schifano 1993 {published data only}
- Schifano F, Garofoli F. Dothiepin vs S‐adenosylmethionine (SAMe) for the treatment of major depression in weaned alcoholics: a pilot study. European Neuropsychopharmacology 1993;3(3):330. [Google Scholar]
Thomas 1987 {published data only}
- Thomas CS, Bottiglieri T, Edeh J, Carney MW, Reynolds EH, Toone BK. The influence of S‐adenosylmethionine (SAM) on prolactin in depressed patients. International Clinical Psychopharmacology 1987;2(2):97‐102. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Quiros 1982 {published data only}
- Quiros G. Assessment of a new drug for depression: S‐adenosyl‐L‐methionine [Valoracion de un nuevo farmaco en el tratamiento de las depresiones: la S‐Adenosyl‐L‐Metionina]. Psicopatologia 1982;2(3):265‐74. [Google Scholar]
References to ongoing studies
ACTRN12613001299796 {published data only}
- Sarris J. Nutraceuticals as monotherapy treatments in major depressive disorder: a double‐blind, randomised, placebo‐controlled trial, 2013. www.anzctr.org.au/ACTRN12613001299796.aspx (accessed 3 August 2016). [www.anzctr.org.au/ACTRN12613001299796.aspx]
- Sarris J, Murphy J. The efficacy of S‐adenosyl methionine (SAMe) and a combination nutraceutical as monotherapy treatments in major depressive disorder: a double‐blind, randomised, placebo‐controlled trial. www.anzctr.org.au/ACTRN12613001299796.aspx (accessed 3 August 2016).
ACTRN12613001300763 {published data only}
- Sarris J. The efficacy of S‐adenosylmethionine (SAMe) and a combination nutraceutical as adjunctive treatments in depression: a double‐blind, randomized, placebo‐controlled trial, 2013. www.anzctr.org.au/ACTRN12613001300763.aspx (accessed 3 August 2016). [www.anzctr.org.au/ACTRN12613001300763.aspx]
NCT01912196 {published data only}
- MSI Methylation Sciences, Inc. A double‐blind, placebo‐controlled, randomized add‐on study of MSI‐195 (Methylation Sciences Inc. S‐adenosyl‐L‐methionine, SAMe) for patients with major depressive disorder (MDD) who have had an inadequate response to current antidepressant therapy, 2013. clinicaltrials.gov/show/NCT01912196 (accessed 3 August 2016). [clinicaltrials.gov/show/NCT01912196]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins JPT. Cochrane Reviewers' Handbook 4.2.2 [updated December 2003]. 4.2.2. Chichester, UK: Wiley & Sons, Ltd, 2004. [Google Scholar]
Algeri 1979
- Algeri S, Catto E, Curcio M, Ponzi F, Stamentinoli G. Changes in rat brain noradrenaline and serotonin after administration of S‐adenosylmethionine. In: Zappia V, Usdin E, Salvatore F editor(s). Biochemical and Pharmacological Roles of Adenosylmethionine and the Central Nervous System. New York: Permagon Press, 1979:81‐7. [Google Scholar]
Altman 1996a
- Altman DG, Bland JM. Statistics notes: presentation of numerical data. BMJ 1996;312:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1996b
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez 1987
- Alvarez E, Udina C, Guillamat R. Shortening of latency period in depressed patients treated with SAMe and other antidepressant drugs. Cell Biology Reviews 1987;S1:103‐10. [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Third Edition (DSM III). Washington DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV). Washington DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2010
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder, 3rd edition. 2010. [PubMed]
Arroll 2009
- Arroll B, Elley CR, Fishman T, Goodyear‐Smith FA, Kenealy T, Blashki G, et al. Antidepressants versus placebo for depression in primary care. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell (personal communication)
- Bell KM, Potkin SG, Carreon L, Plot L, Bunney W. Oral S‐adenosylmethionine in the treatment of depression: a double‐blind, randomized comparison with desipramine. American Journal Psychiatry (submitted).
Bell 1987
- Bell K, Plon L, Nabel M. A controlled clinical trial of S‐adenosylmethionine treatment of depression. Annual Review of Cell and Developmental Biology 1987;S1:111‐20. [Google Scholar]
Berlim 2007
- Berlim MT, Turecki G. Definition, assessment, and staging of treatment‐resistant refractory major depression: a review of current concepts and methods. Canadian Journal of Psychiatry 2007;52:46‐54. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF 2016
- Royal Pharmaceutical Society of Great Britain, British Medical Association. British National Formulary. London: British Medical Association, September 2016. [Google Scholar]
Bottiglieri 2002
- Bottiglieri T. S‐adenosyl‐L‐methionine (SAMe): from the bench to the bedside ‐ molecular basis of a pleiotrophic molecule. American Journal of Clinical Nutrition 2002;78:1151S‐7S. [DOI] [PubMed] [Google Scholar]
Bressa 1994
- Bressa GM. S‐adenosyl‐l‐methionine (SAMe) as antidepressant: meta‐analysis of clinical studies. Acta Neurologica Scandinavica. Supplementum 1994;154:7‐14. [DOI] [PubMed] [Google Scholar]
Byford 2011
- Byford S, Barrett B, Despiégel N, Wade A. Impact of treatment success on health service use and cost in depression: longitudinal database analysis. Pharmacoeconomics 2011;29(2):157‐70. [DOI: 10.2165/11537360-000000000-00000] [DOI] [PubMed] [Google Scholar]
Cantoni 1952
- Cantoni GL. The nature of the active methyl donor formed enzymatically from L‐methionine and adenosine triphosphate. Journal of the American Chemical Society 1952;74(11):2942‐3. [Google Scholar]
Carney 1989
- Carney MW, Chary TK, Bottiglieri T, Reynolds EH. The switch mechanism and the bipolar/unipolar dichotomy. British Journal of Psychiatry 1989;154:48‐51. [DOI] [PubMed] [Google Scholar]
Carpenter 2011
- Carpenter DJ. St. John's wort and S‐adenosyl methionine as "natural" alternatives to conventional antidepressants in the era of the suicidality boxed warning: what is the evidence for clinically relevant benefit?. Alternative Medicine Review 2011;16(1):17‐39. [PubMed] [Google Scholar]
Chavez 2000
- Chavez M. SAMe: S‐Adenosylmethionine. American Journal of Health‐System Pharmacy 2000;57(2):119‐23. [DOI] [PubMed] [Google Scholar]
Craig 2007
- Craig D, Rice S. NHS Economic Evaluation Database Handbook. York, UK: Centre for Reviews and Dissemination, University of York, 2007. [Google Scholar]
Craig Nelson 2010
- Craig Nelson J. S‐Adenosyl Methionine (SAMe) augmentation in major depressive disorder. American Journal of Psychiatry 2010;167:889‐91. [10.1176/appi.ajp.2010.10040627] [DOI] [PubMed] [Google Scholar]
Curcio 1978
- Curcio M, Catto E, Stramentinoli G, Algeri S. Effect of S‐adenosyl‐L‐methionine on serotonin metabolism in rat brain. Progress in Neuro‐Psychopharmacology 1978;2(1):65‐71. [DOI] [PubMed] [Google Scholar]
Davidson 2010
- Davidson JR. Major depressive disorder treatment guidelines in America and Europe. Journal of Clinical Psychiatry 2010;71 Suppl E1:e04. [DOI: 10.4088/JCP.9058se1c.04gry] [DOI] [PubMed] [Google Scholar]
Delle Chiaie 2002
- Delle Chiaie R, Pancheri P, Scapicchio P. Efficacy and tolerability of oral and intramuscular S‐adenosyl‐L‐methionine 1,4‐butanedisulfonate (SAMe) in the treatment of major depression: comparison with imipramine in 2 multicenter studies. American Journal of Clinical Nutrition 2002;76(5) :1172S‐6S. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in Clinical Trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Di Padova 2000
- Padova C, Giudici A, Boissard G. Ademetionine and depression. IV Workshop on methionine metabolism: molecular mechanisms and clinical implications; 2000 Feb 20‐24; Granada, Spain. 2000:295‐9.
Di Rocco 2000
- Rocco A, Rogers JD, Brown R, Werner P, Bottiglieri T. S‐Adenosyl methionine improves depression in patients with Parkinson's disease in an open‐label clinical trial. Movement Disorders 2000;15 (6):1225‐9. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7:623‐9. [DOI] [PubMed] [Google Scholar]
Drummond 1996
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fawcett 1983
- Fawcett J, Kravitz HM. Anxiety syndromes and their relationship to depressive illness. Journal of Clinical Psychiatry 1983;44(8, Pt2):8‐11. [PubMed] [Google Scholar]
Fazio 1974
- Fazio C, Andreoli V, Agnoli A, Casacchia M, Cerbo R. Therapy of schizophrenic and depressive disorders with S‐adenosylmethionine. Clinical Pharmacology & Therapeutics 1974;2:1015. [Google Scholar]
Fleisch 2010
- Fleisch SB, Travis MJ, Ryan ND. Discrepancy in response and remission rates for SAMe‐treated patients in a double‐blind, randomized clinical trial [comment]. American Journal of Psychiatry 2010;167(12):1533. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambillac P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Goldberg 2012
- Goldberg D, Fawcett J. The importance of anxiety in both major depression and bipolar disorder. Depression and Anxiety 2012;29(6):471‐8. [DOI] [PubMed] [Google Scholar]
Greenberg 2003
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000?. Journal of Clinical Psychiatry 2003;64(12):1465‐75. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Gören 2004
- Gören JL, Stoll AL, Damico KE, Sarmiento IA, Cohen BM. Bioavailability and lack of toxicity of S‐adenosyl‐L‐methionine (SAMe) in humans. Pharmacotherapy 2004;24(11):1501‐7. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardy 2002
- Hardy M, Coulter I, Morton SC, Favreau J, Venuturupalli S, Chiappelli F, et al. S‐adenosyl‐L‐methionine for treatment of depression, osteoarthritis, and liver disease. Evidence Report/Technology Assessment Number 64. Rockville, MD: Agency for Healthcare Research and Quality October 2002. [PMC free article] [PubMed]
Henkel 2006
- Henkel V, Mergi R, Allgaier AK, Kohnen R, Möller HJ, Hegeri U. Treatment of depression with atypical features: a meta‐analytic approach. Psychiatry Research 2006;141:89‐101. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kessler 2012
- Kessler RC. The costs of depression. Psychiatric Clinics of North America 2012;35(1):1‐14. [DOI: 10.1016/j.psc.2011.11.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
King's Fund 2008
- McCrone P, Dhanasiri S, Pate A, Knapp M, Lawton‐Smith S. Paying the Price. The Cost of Mental Health Care in England to 2026. London: The King's Fund, 2008. [Google Scholar]
Kresge 2005
- Kresge N, Tabor H, Simoni RD, Hill RL. An escape from Italy, the discovery of S‐adenosylmethionine, and the biosynthesis of creatine by Giulio L. Cantoni. Journal of Biological Chemistry 2005;280:e35. [PubMed] [Google Scholar]
Lipinski 1984
- Lipinski JF, Cohen BM, Frankenburg F, Tohen M, Waternaux C, Altesman R, Jones B. Open trial of S‐adenosylmethionine for treatment of depression. American Journal of Psychiatry 1984;141:448‐50. [DOI] [PubMed] [Google Scholar]
Macher (in press)
- Macher JR. A double‐blind, placebo‐controlled trial of intravenous ademetionine (SAMe) in the treatment of major depression (DSM III‐R). International Clinical Psychopharmacology (in press). [Google Scholar]
Macher 2000
- Macher JP. A double blind placebo controlled trial of intravenous ademetionine (SAMe) in the treatment of major depression. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2000;24(2):207‐25. [DOI] [PubMed] [Google Scholar]
Mischoulon 2002
- Mischoulon D, Fava M. Role of S‐adenosyl L‐methionine in the treatment of depression: a review of the evidence. American Journal of Clinical Nutrition 2002;76:1158S‐61S. [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
Morelli 2000
- Morelli V, Zoorob RJ. Alternative therapies: Part I. Depression, diabetes, obesity. American Family Physician 2000;62(5):1051‐60. [PubMed] [Google Scholar]
Murray 1997
- Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349(9063):1436‐42. [DOI] [PubMed] [Google Scholar]
Nemeroff 2007
- Nemeroff CB. Prevalence and management of treatment‐resistant depression. Journal of Clinical Psychiatry 2007;68 Suppl 8:17‐25. [PubMed] [Google Scholar]
NICE 2009
- National Institute for Health and Care Excellence. Depression in adults. The treatment and management of depression in adults. NICE clinical guideline 90 October 2009.
Otero‐Losado 1989a
- Otero‐Losado ME, Rubio MC. Acute effects of S‐adenosylmethionine on catecholaminergic central function. European Journal of Pharmacology 1989;163:353‐6. [DOI] [PubMed] [Google Scholar]
Otero‐Losado 1989b
- Otero‐Losado ME, Rubio MC. Acute changes in 5HT metabolism after S‐adenosylmethionine administration. General Pharmacology 1989;20:403‐6. [DOI] [PubMed] [Google Scholar]
Pancheri 1997
- Pancheri P, Racagni G, Delle Chiaie RD. Recent experimental and clinical findings of the efficacy and safety of adementionine (SAMe) in the pharmacological treatment of depression. Giornale Italiano di Psicopatologia 1997;3:1‐23. [Google Scholar]
Papakostas 2003
- Papakostas GI, Alpert JE, Fava M. S‐adenosyl methionine in the treatment of depression: a comprehensive review of the literature. Current Psychiatry Reports 2003;5:460‐6. [DOI] [PubMed] [Google Scholar]
Papakostas 2010b
- Papakostas GI. 'Discrepancy in response and remission rates for SAMe‐treated patients in a double‐blind, randomized clinical trial': reply to Fleisch et al [letter]. American Journal of Psychiatry 2010;167(12):1533. [DOI] [PubMed] [Google Scholar]
Pinzello 1972
- Pinzello A, Andreoli V. SAMe‐dependent transmethylation in the depressive syndromes: evaluation of the therapeutic effect of S‐adenosylmethionine using Hamilton Rating Scale [Le transmetilazioni SAM‐dipendenti nelle sindromi depressive. Valutazione dell'effetto terapeutico della S‐adenosilmetionina con la scala di Hamilton]. Quaderno Terapeutico Sperimentale; Supplemento Biochimica e Biol Sperimentale 1972;10(2):3‐11. [Google Scholar]
Posternak 2000
- Posternak MA, Zimmermann M. Short‐term spontaneous improvement rates in depressed outpatients. Journal of Nervous and Mental Disease 2000;188(12):799‐804. [DOI] [PubMed] [Google Scholar]
Rambaldi 2006
- Rambaldi A, Gluud C. S‐adenosyl‐L‐methionine for alcoholic liver diseases. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD002235.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rothwell 2005
- Rothwell PM. External validity of randomised controlled trials: to whom do the results apply?. Lancet 2005;365:82‐93. [DOI] [PubMed] [Google Scholar]
Sackett 1996
- Sackett DL, Deeks JJ, Altman DG. Down with odds ratios!. Evidence Based Medicine 1996;1:164‐6. [Google Scholar]
Sarris 2010
- Sarris J, Kavanagh DJ, Byrne G. Adjuvant use of nutritional and herbal medicines with antidepressants, mood stabilizers and benzodiazepines. Journal of Psychiatric Research 2010;44:32‐41. [DOI] [PubMed] [Google Scholar]
Sarris 2015a
- Sarris J, Price LH, Carpenter LL, Tyrka AR, Ng CH, Papakostas GI, et al. Is S‐adenosyl methionine (SAMe) for depression only effective in males? A re‐analysis of data from a randomized controlled trial. Pharmacopsychiatry 2015;48(4‐5):141‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shippy 2004
- Shippy RA, Mendez D, Jones K, Cergnul I, Karpiak SE. S‐adenosylmethionine (SAM‐e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry 2004;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinclair 1994
- Sinclair JC, Bracken MB. Clinically useful measures of effect in binary analyses of randomized trials. Journal of Clinical Epidemiology 1994;47:881‐9. [DOI] [PubMed] [Google Scholar]
Sobocki 2006
- Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. Journal of Mental Health Policy and Economics 2006;9(2):87‐98. [PubMed] [Google Scholar]
Stramentinoli 1979
- Stramentinoli G, Gualano M, Galli‐Kienzle M. Intestinal absorption of S‐adenosyl‐L‐methionine. Journal of Pharmacology and Experimental Therapeutics 1979;209:323‐6. [PubMed] [Google Scholar]
Sugden 2006
- Sugden C. One‐carbon metabolism in psychiatric illness. Nutrition Research Reviews 2006;19:117‐36. [DOI] [PubMed] [Google Scholar]
WHO 1978
- World Health Organization. Mental disorders: glossary and guide to their classification in accordance with the ninth revision of the International Classification of Diseases. Geneva: WHO, 1978. [Google Scholar]
WHO 1992
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva: World Health Organization, 1992. [Google Scholar]
Williams 2005
- Williams AL, Girard C, Jui D, Sabina A, Katz Dl. S‐adenosylmethionine (SAMe) as treatment for depression: a systematic review. Clinical and Investigative Medicine 2005;28:132‐9. [PubMed] [Google Scholar]
References to other published versions of this review
Galizia 2014
- Galizia I, Oldani L, Macritchie K, Amari E, Dougall D, Jones TN, et al. S‐adenosyl methionine (SAM‐e) for depression in adults. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011286] [DOI] [PMC free article] [PubMed] [Google Scholar]


