Abstract
Background
Reablement, also known as restorative care, is one possible approach to home‐care services for older adults at risk of functional decline. Unlike traditional home‐care services, reablement is frequently time‐limited (usually six to 12 weeks) and aims to maximise independence by offering an intensive multidisciplinary, person‐centred and goal‐directed intervention.
Objectives
To assess the effects of time‐limited home‐care reablement services (up to 12 weeks) for maintaining and improving the functional independence of older adults (aged 65 years or more) when compared to usual home‐care or wait‐list control group.
Search methods
We searched the following databases with no language restrictions during April to June 2015: the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (OvidSP); Embase (OvidSP); PsycINFO (OvidSP); ERIC; Sociological Abstracts; ProQuest Dissertations and Theses; CINAHL (EBSCOhost); SIGLE (OpenGrey); AgeLine and Social Care Online. We also searched the reference lists of relevant studies and reviews as well as contacting authors in the field.
Selection criteria
We included randomised controlled trials (RCTs), cluster randomised or quasi‐randomised trials of time‐limited reablement services for older adults (aged 65 years or more) delivered in their home; and incorporated a usual home‐care or wait‐list control group.
Data collection and analysis
Two authors independently assessed studies for inclusion, extracted data, assessed the risk of bias of individual studies and considered quality of the evidence using GRADE. We contacted study authors for additional information where needed.
Main results
Two studies, comparing reablement with usual home‐care services with 811 participants, met our eligibility criteria for inclusion; we also identified three potentially eligible studies, but findings were not yet available. One included study was conducted in Western Australia with 750 participants (mean age 82.29 years). The second study was conducted in Norway (61 participants; mean age 79 years).
We are very uncertain as to the effects of reablement compared with usual care as the evidence was of very low quality for all of the outcomes reported. The main findings were as follows.
Functional status: very low quality evidence suggested that reablement may be slightly more effective than usual care in improving function at nine to 12 months (lower scores reflect greater independence; standardised mean difference (SMD) ‐0.30; 95% confidence interval (CI) ‐0.53 to ‐0.06; 2 studies with 249 participants).
Adverse events: reablement may make little or no difference to mortality at 12 months' follow‐up (RR 0.97; 95% CI 0.74 to 1.29; 2 studies with 811 participants) or rates of unplanned hospital admission at 24 months (RR 0.94; 95% CI 0.85 to 1.03; 1 study with 750 participants).
The very low quality evidence also means we are uncertain whether reablement may influence quality of life (SMD ‐0.23; 95% CI ‐0.48 to 0.02; 2 trials with 249 participants) or living arrangements (RR 0.92, 95% CI 0.62 to 1.34; 1 study with 750 participants) at time points up to 12 months. People receiving reablement may be slightly less likely to have been approved for a higher level of personal care than people receiving usual care over the 24 months' follow‐up (RR 0.87; 95% CI 0.77 to 0.98; 1 trial, 750 participants). Similarly, although there may be a small reduction in total aggregated home and healthcare costs over the 24‐month follow‐up (reablement: AUD 19,888; usual care: AUD 22,757; 1 trial with 750 participants), we are uncertain about the size and importance of these effects as the results were based on very low quality evidence.
Neither study reported user satisfaction with the service.
Authors' conclusions
There is considerable uncertainty regarding the effects of reablement as the evidence was of very low quality according to our GRADE ratings. Therefore, the effectiveness of reablement services cannot be supported or refuted until more robust evidence becomes available. There is an urgent need for high quality trials across different health and social care systems due to the increasingly high profile of reablement services in policy and practice in several countries.
Plain language summary
Time‐limited home‐care reablement services (up to 12 weeks) for supporting older adults to live independently
Review question
We aimed to assess the effectiveness of time‐limited reablement for older people (aged 65 years or more) in helping them to maintain or improve their independence. We included two studies in the review.
Background
Services that help older people to remain living in their own home have obvious appeal for service‐users, family members, care‐providers and policy makers alike, especially if those services help to reduce pressure on hospitals or the need for long‐term care, or both. Reablement (or restorative care) is one potentially useful service that helps an older person to continue living at home. The service is typically provided by a team of health/social care professionals and care‐workers who work with an older person to restore their independence. The service is time‐limited (usually six to 12 weeks) and normally involves multiple visits to a person's home. It sets out to achieve goals set by the older person, and help them to regain ability to complete everyday tasks and activities.
Study characteristics
The evidence is current to April 2015. The review included two studies, one each from Australia (750 participants) and Norway (61 participants). In both studies, half of the participants received a reablement‐based home‐care package and half usual home‐care provision.
Key results
The very low quality evidence for all of the results means that we are uncertain about the effects of reablement when compared with usual care.
Reablement may help some older adults to improve their abilities to engage in everyday activities (functional status) to a small degree, but may make little or no difference to death rates or admissions to hospital. The findings mean we are also uncertain whether reablement affects quality of life or living arrangements. Reablement may lead to a small decrease in numbers of people needing higher levels of personal care, and may decrease care costs to a small degree, but neither study reported satisfaction of those using the reablement service.
Quality of the evidence
While there may be some small positive effects of reablement, the evidence was very low quality, meaning that we are very uncertain about how large or important these effects may be. There is a need for more studies to be conducted in a range of countries and situations before the effectiveness and safety of reablement can be determined with certainty.
Summary of findings
Summary of findings for the main comparison. Reablement compared with usual home‐care for maintaining independence.
| Reablement compared with usual home‐care for maintaining independence | ||||||
|
Patient or population: adults aged ≥ 65 years Settings: clients' own home Intervention: reablement services Comparison: usual home‐care service | ||||||
| Outcomes | Illustrative comparative risks (95% CI) |
Effect estimate (95% CI) |
No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk* | Corresponding risk | |||||
| Usual care | reablement | |||||
|
Functional status** ADL and IADL (Lewin 2013) COPM (Tuntland 2015) Lower scores indicate greater independence Follow‐up: 9 to 12 months |
‐ | The mean score in the intervention group was 0.3 SD lower (0.53 to 0.06 lower) |
SMD ‐0.30 (‐0.53 to ‐0.06) |
249 (2 studies) |
⊕ooo very low a,b |
‐ |
|
Mortality*** Follow‐up: 9 to 12 months |
198 per 1000 |
6 fewer per 1000 (51 fewer to 57 more) |
RR 0.97 (0.74 to 1.29) |
811 (2 studies) |
⊕ooo very lowa,c |
‐ |
|
Unplanned hospital admission** Follow‐up: 24 months |
707 per 1000 |
42 fewer per 1000 (106 fewer to 21 more) |
RR 0.94 (0.85 to 1.03) | 750 (1 study) |
⊕ooo very lowa,c,d |
‐ |
|
Quality of life** AAQ (Lewin 2013) COOP/Wonka (overall health; Tuntland 2015) Lower scores indicate improvement Follow‐up: 9 to 12 months |
‐ | The mean score in the intervention group was 0.2 SD lower (0.48 lower to 0.02 higher) | SMD ‐0.23 (‐0.48 to 0.02) | 249 (2 studies) |
⊕ooo very lowa,b,c |
‐ |
|
Level of emerging personal care needs (approved for higher level of care)*** Follow‐up: 24 months |
643 per 1000 | 84 fewer per 1000 (148 fewer to 13 fewer) |
RR 0.87 (0.77 to 0.98) |
750 (1 study) |
⊕ooo very lowa,d |
‐ |
|
Living arrangements (transferred to residential care) Follow‐up: 12 months |
128 per 1000 |
10 fewer per 1000 (49 fewer to 45 more) |
RR 0.92 (0.62 to 1.34) | 750 (1 study) |
⊕ooo very lowa,c,d |
‐ |
|
Cost effectiveness*** Total aggregated costs for home‐ and healthcare (emergency department and unplanned hospital admissions) Follow‐up: 24 months |
The mean costs were AUD 2869 lower for the reablement group compared with usual care (AUD 19,888 with intervention versus AUD 22,757 with control) | ‐ | 750 (1 study) |
⊕ooo very lowa,d |
‐ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AAQ: Assessment of Quality of Life; ADL: activities of daily living; CI: confidence interval; COPM: Canadian Occupational Therapy Performance; IADL: instrumental activities of daily living; RR: risk ratio; SMD: standardised mean difference; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
* median control group risk calculated by GRADEpro.
** unpublished data.
*** Data from Lewin 2014.
a Downgraded twice (‐2) for risk of bias concerns (Lewin 2013 was at high risk of bias on all domains).
b Downgraded once (‐1) for imprecision (fewer than 400 participants).
c Downgraded once for imprecision (‐1) as confidence interval was likely to include minimally important difference and no difference).
d Downgraded once for imprecision (‐1) as data were from single study.
Background
Description of the condition
As the population ages and people live longer, the proportion of dependent older people is likely to increase (Brodsky 2003; Wittenberg 2004). As a result, the cost of long‐term care for people aged over 65 years living in OECD (Organisation for Economic Co‐operation and Development) countries is expected to double or even triple by 2050 (Martins 2006). Therefore, many high‐income countries have actively promoted a shift from residential to home‐based care as a potentially more effective and financially sustainable approach to meeting the health and social care needs of older adults (Rostgaard 2011). Importantly, most older people prefer to 'age in place' (Wiles 2012), and therefore, to remain in their own homes for as long as possible, provided they have appropriate levels of support to meet their changing needs (Cutchin 2009).
Government policies in various high‐income countries reflect the need to reconfigure health and social service provision in order to meet the current and future requirements of an ageing population. In England, for example, the Department of Health has articulated a vision for the integration of health and social care services, with a greater focus on individualised preventative services to delay the need for more costly forms of care (Xie 2012). Similar key policy goals of early intervention, person‐centred care and restoration of function have been identified and developed in Australia (Cartwright 2009), Sweden (Löfqvist 2012), and New Zealand (King 2012), mainly with a view to reducing pressure on health and social care systems. However, despite these changes, little is known about the effectiveness and cost‐effectiveness of models of care provision across different geographical and socioeconomic contexts.
Description of the intervention
In recent years, there has been increasing interest in high‐income countries in reablement (also known as restorative care in Australia and the USA) ‐ an innovative approach to improving home‐care services for older adults in need of care and support or at risk of functional decline (Francis 2011). There is a lack of clarity regarding the boundaries between reablement and other related interventions in health and social care (including intermediate care, occupational therapy and traditional domiciliary care) (Wood 2012). While reablement shares features with other interventions, it is distinguished by a re‐orientation of home care away from treating disease and creating dependency to maximising independence; it achieves this by offering intensive (i.e. multiple visits), time‐limited (typically six to 12 weeks' duration), multidisciplinary, person‐centred and goal‐directed home‐care services (Ryburn 2009). It is important to note that reablement is not designed to resolve specific healthcare issues (e.g. Crotty 2010), but may help an older person to regain confidence and functional abilities after recovering from an illness or a period of hospitalisation. Therefore, a reablement programme typically includes a range of targeted components designed to optimise functioning in the performance of activities of daily living (ADL). These may include exercise and training to support behavioural change, education about self management and healthy ageing, environmental adjustments, provision of equipment and use of local resources (Kent 2000; Lewin 2010; Tinetti 2002). So, for example, rather than providing a meals‐on‐wheels service, a reablement approach would enable an older person to develop the confidence and skills to prepare lunch through task analysis/redesign, the use of assistive technology and physical exercises (Glendinning 2010).
Thus, reablement differs from usual home care/domiciliary care, which tends to focus on doing things for older people rather than enabling/reabling them to do things for themselves. Indeed, traditional models of home care have been shown to increase dependency, with an associated loss of function (Parsons 2013). Furthermore, the assumption underpinning usual home‐care services is that they will continue indefinitely (Montgomery 2008), whereas reablement is specifically time‐limited and aims to reduce the need for home care into the future (King 2012; Ryburn 2009). Reablement, therefore, is particularly valued for its potential to decrease demand on home‐care services and to reduce the attendant costs of ongoing care (Jones 2009). Nevertheless, this form of care provision may have considerable resource implications in terms of retraining staff and effecting organisational change (Francis 2011).
The reablement approach has become increasingly popular and has been implemented widely in the UK (Department of Health 2010), as well as in a number of other countries (e.g. New Zealand (King 2012; Parsons 2013), Australia (Ryburn 2009), USA (Tinetti 2002)). The provision of reablement reflects a wider change agenda that promotes person‐centred care through individually tailored services that permit greater choice and control for consumers (Xie 2012). Additionally, the growth in this type of approach is in line with the increasing demands of people as they age; older consumers are becoming increasingly likely to demand greater choice, more personalised services and better quality home‐care support in the future (Rostgaard 2011).
How the intervention might work
The reablement approach emphasises the active participation of an older person in working towards agreed goals that are designed to maximise independence and confidence. For example, these goals might include regaining confidence in self care management and improving mobility. The content of the intervention may encompass graduated practice in completing tasks, environmental adjustments and adaptive equipment, or enabling an older person to build up social networks (Ryburn 2009). Improved outcomes across similar domains, including self care, mobility and quality of life (QoL), have been reported (Kent 2000; Tinetti 2002). Furthermore, the ability to function effectively in the home may reduce the need for unscheduled hospital admission, while postponing or preventing admission to residential care (Tinetti 2002). A reduction in the care hours required following the intervention is frequently used as a measure of success (Kent 2000; Lewin 2010), although this may not always be a desired or possible outcome for some older people, particularly people who are socially isolated or in failing health (Francis 2011). Arguably therefore, a decrease in hours of care with regard to older people with high dependency needs may not be an appropriate outcome measure. Importantly, additional outcomes that are valued by older people themselves as indicators of effective services should be measured (Clark 2001).
There may be different routes and thresholds for entry into a reablement‐based service. For instance, some hospital discharge support schemes select only older people who are most likely to benefit from the approach (i.e. people with relatively low levels of ongoing need), whereas a reablement service that accepts referrals directly from the community may adopt a more flexible approach and screen out only those people who are terminally ill or who have advanced dementia (Glendinning 2010). Nevertheless, it seems likely that outcomes will vary depending on the route of entry and also on the functional abilities of the older person on entry to the service. For example, people with a high level of need may not benefit as much as people with lower support requirements (Francis 2011). Indeed, reablement represents only one end of the continuum of care and may not be suitable for people with chronic or relatively intractable problems such as dementia that may require a different type of longer‐term service model (CSED 2007).
Why it is important to do this review
There has been strong international interest in developing effective and cost‐effective interventions to support older people living in their own homes and, in turn, to reduce the demand on acute hospital services and residential care provision. Arguably, a lack of, or poorly developed, rehabilitation services has contributed to increasing pressure on acute hospital beds, delayed discharge, more frequent re‐admissions to hospital, and increased use of costly residential and nursing home care (Audit Commission 2000). One approach to freeing up hospital beds is to support early discharge by providing acute care at home. For example, one Cochrane review of 'hospital at home' services found that older people with a mix of conditions were less likely to need residential care at follow‐up after receiving these services, although only a small proportion of older people were deemed to be eligible or were willing to take part (Shepperd 2011).
There is currently limited evidence as to which setting or model(s) of care may be most effective for the rehabilitation and maintenance of the independence of older adults (Huss 2008; Ward 2009). This appears to be due, in large part, to the challenges involved in comparing different, often multi‐component interventions across a range of settings. For example, Beswick 2008 (89 participants) and Huss 2008 (21 participants) reviewed a range of heterogeneous studies such as community‐based nursing care following discharge from hospital, falls prevention, group education and annual health assessments. These reviews concluded that, while multidimensional home‐based programmes had the potential to reduce the burden of disability among older adults, it was not possible, on the basis of the available evidence, to identify which one of the various models/types of care provision was the most effective. There is a need to undertake a more focused systematic review in order to assess the comparative effectiveness and disentangle the effects of each type of intervention and their potentially active ingredients or components.
While a number of previous Cochrane and non‐Cochrane reviews have examined a range of rehabilitation and home‐visiting programmes, as yet there has not been a systematic review that has focused specifically on the effects of reablement‐based interventions. Important questions about the effectiveness and cost‐effectiveness of these types of interventions remain unanswered. For example, does reablement reduce health service utilisation (such as hospital re‐admissions)? Do specific subgroups benefit more than others (e.g. younger populations), and people with lower levels of need? Is there evidence to support personalisation of the service? We undertook this review to try to address these important gaps in our knowledge provided that a sufficient number of randomised controlled trials (RCTs) were eligible for inclusion.
Objectives
To assess the effects of time‐limited home‐care reablement services (up to 12 weeks) for maintaining and improving the functional independence of older adults (aged 65 years or more) when compared to usual home‐care or wait‐list control group.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT), cluster randomised trials and quasi‐RCTs of reablement compared to usual care (i.e. home‐care support, which included unpaid informal care) or wait list.
We deemed the inclusion of cluster randomised and quasi‐RCTs necessary to consider trials where individual random assignment may have been impractical due to the nature of the intervention (e.g. where only the reablement intervention was available in one geographical area or there may have been restrictions in terms of the availability of care staff to deliver either reablement or usual care).
We also planned to include studies that examined the costs or cost‐effectiveness of the intervention versus usual care, which had been conducted alongside, or subsequent to, trials that met the eligibility criteria (Shemilt 2011).
Types of participants
Older adults aged 65 years or more living in their own home who required assistance to perform tasks of daily living and to participate in normal activities due to poor physical or mental health. We excluded trials involving older adults living outside their own homes (e.g. in nursing homes). We anticipated that we could encounter trials with mixed populations because reablement is offered to younger people in some settings. We included trials with 80% or more older adults (aged 65 years or more) in the overall sample, and contacted study authors to determine the age profile in situations where younger people had been recruited into the trial.
Types of interventions
Reablement interventions compared with groups receiving usual home‐care services or with a wait list control group. Studies were required to meet the following criteria:
participants must have had an identified need for formal care and support or be at risk of functional decline (Francis 2011);
the intervention must have been time‐limited (up to 12 weeks) and intensive (e.g. multiple home visits) (Ryburn 2009);
the intervention must have been delivered in the older person’s own home, and provided by an interdisciplinary team (Glendinning 2010);
the intervention must have been focused on maximising independence; and
the intervention must have been person‐centred and goal‐directed (Parsons 2013).
We excluded trials that focused on the provision of acute care (e.g. nursing care in the home), or those describing interventions outside of existing home‐care services.
The control group was in receipt of, or awaiting, usual home‐care services, which may have been defined as ongoing assistance with completion of household activities or personal care (or both) by an outside agency (i.e. paid support) or informal (unpaid) care (or both), with or without professional input (e.g. nurses, occupational therapists). The control group could also have included people waiting for the intervention (wait list).
Types of outcome measures
We recognised the possibility that specific outcomes may have been measured using different tools across trials. Where we found studies with more than one relevant outcome per outcome category we selected the primary outcome identified by the publication authors. If no primary outcome was identified, we planned to select the one specified in the sample size calculations; if the sample size calculation was not stated, we would have ranked the effect estimates and selected the median effect estimate.
We only included studies that assessed functional outcomes (e.g. ADL).
Primary outcomes
Functional status including measures of the skills and abilities to complete ADL.
Adverse events including mortality, hospital (re)admission.
Secondary outcomes
Quality of life (QoL). We evaluated studies that assessed health‐related quality of life (HRQoL) or social care‐related quality of life (SCRQoL) (or both) using validated uni‐ or multi‐dimensional questionnaires. Examples of generic HRQoL questionnaires include the 36‐item Short Form (SF‐36) and EuroQol five dimensions questionnaire (EQ‐5D); SCRQoL measures include ASCOT (Adult Social Care Outcome Toolkit; Netten 2011).
User satisfaction.
Service outcomes, including level of ongoing home‐care service (e.g. care hours) or use of external health services (e.g. visits to emergency department).
Living arrangements (i.e. in own home or other setting).
Cost‐effectiveness (as measured by comparing the costs of the intervention versus usual care; and health service utilisation). Full economic evaluations of reablement interventions may be relatively rare (e.g. Pilkington 2011), and as such, we also included cost analyses, provided that these were conducted alongside, or subsequent to, trials that otherwise met the eligibility criteria.
Timing of outcome assessment
All outcomes measured at baseline and on discharge from the reablement service (typically six to 12 weeks). We analysed follow‐up at nine and 12 months (and longer) as available.
Main outcomes for 'Summary of findings' table
We prepared Table 1 based on the methods described in theCochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), for the following main outcomes:
functional status;
adverse events (mortality; unplanned hospital admissions);
QoL;
service outcomes (level of ongoing/emergent personal care needs;
living arrangements (i.e. living in own home or elsewhere); and
cost‐effectiveness.
Search methods for identification of studies
We identified studies through: key word and text word searches of relevant electronic databases and government and non‐government agencies; searches of grey literature including conference papers, unpublished theses and reference lists of other rehabilitation reviews; and personal communications with experts in the field.
Electronic searches
We searched the following electronic databases with no language restrictions:
Cochrane Central Register of Controlled Trials (CENTRAL) (2015 Issue 5) (Appendix 1);
MEDLINE (OvidSP) (1945 to 21 April 2015) (Appendix 2);
Embase (OvidSP) (1988 to 4 April 2015) (Appendix 3);
PsycINFO (OvidSP) (1967 to 14 May 2015) (Appendix 4);
ProQuest (ERIC; Dissertations and Theses; Sociological Abstracts; earliest to 14 May 2015) (Appendix 5);
CINAHL complete (EBSCOhost) (1982 to 25 May 2015) (Appendix 6);
SIGLE (System for Information on Grey Literature in Europe) (1980 to 17 June 2015) (Appendix 7);
AgeLine (EBSCOhost) (1978 to 27 May 2014) (Appendix 6); and
Social Care Online (earliest to 20 May 2015) (Appendix 8).
Searches were up to date as of April 2015 (AgeLine was only available to us in May 2014); detailed search strategies are presented in the Appendices.
Searching other resources
We contacted key experts in the field and first authors of included studies for advice as to other relevant published, unpublished and ongoing studies (e.g. conference papers, unpublished dissertations, working papers or government reports) that might be eligible for inclusion. We searched reference lists of included studies and relevant reviews to identify further relevant studies.
We also searched online trial registers (Clinical Trials Register; ClinicalTrials.gov; WHO International Clinical Trials Registry) for ongoing and recently completed studies. Appendix 9 shows the search terms for these registers.
Data collection and analysis
Selection of studies
The lead author (AC) screened all titles and abstracts identified from searches to determine which met the inclusion criteria. We retrieved the full text of any papers identified as potentially relevant. Two authors (AC, MF) independently screened full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion and by consulting a third author (SMcG), where necessary. We collated duplicate publications and considered these by individual study. All potentially relevant papers excluded from the review at this stage are listed as excluded studies, with reasons provided in the Characteristics of excluded studies table. We presented the available information about three ongoing studies in the Characteristics of ongoing studies table. The screening and selection process is outlined in a PRISMA flow chart (Figure 1; Liberati 2009).
1.
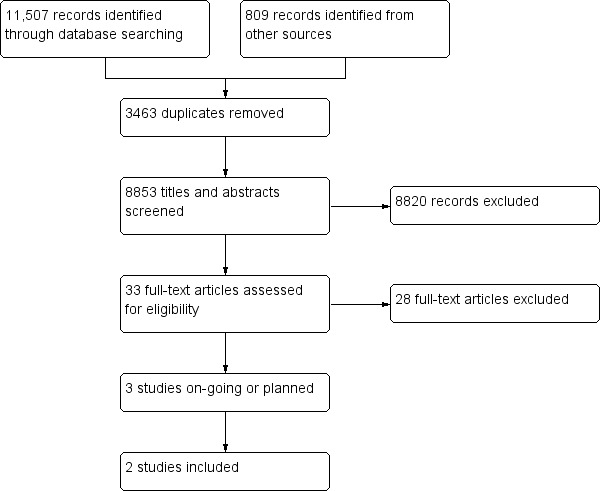
Study flow diagram.
Data extraction and management
Two authors (AC, MF) independently extracted data from the included studies. We resolved any discrepancies by discussion until we reached consensus, or through consultation with a third author (SMcG), where necessary. We used an adapted data extraction form based on the Cochrane Consumers and Communication Group Data Extraction Template (available at: cccrg.cochrane.org author‐resources). We piloted the adapted form using three trials (two of which we subsequently excluded) before finalising the design. Data extracted included: aim of intervention, study design, sample size and attrition, description of the comparison group, all outcomes and funding sources. See Characteristics of included studies for full details. One author (AC) entered extracted data into Review Manager 5 including outcome data and results (RevMan 2014), and one author (MF) independently checked for accuracy against the data extraction sheets.
Assessment of risk of bias in included studies
Two authors (AC, MF) independently assessed and reported on the methodological risk of bias of the included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and the Cochrane Consumers and Communication Group guidelines (Ryan 2011). Both recommended the explicit reporting of the following elements for RCTs: random sequence generation, allocation sequence concealment, blinding (participants and personnel), blinding (outcome assessment), completeness of outcome data (including data on attrition) and selective outcome reporting. We also considered other sources of bias: contamination, baseline comparability of groups and fidelity/delivery of interventions including any co‐interventions. We considered blinding separately for different outcomes. We judged each item as being at high, low or unclear risk of bias as outlined in the criteria provided by Higgins 2011, and we provided a quote from the study report as well as a justification for our judgement for each domain in 'Risk of bias' tables. If we had included any cluster‐RCTS we would have assessed and reported the risk of bias associated with selective recruitment of cluster participants and potential contamination between intervention and control groups. We would have assessed any quasi‐RCTs as high risk of bias on the sequence generation item of the 'Risk of bias' tool.
Two authors (AC, MF) independently assessed the risk of bias of the included studies, with any disagreements resolved by discussion to reach consensus. We contacted the study authors for additional information and for clarification of the study methods. We incorporated the results of the risk of bias assessment into the review by means of a standard table, and systematic narrative description/commentary about each of the elements, thereby providing an overall assessment of the risk of bias of the included studies.
With regard to the cost‐effectiveness analysis, we used the Drummond checklist to appraise the methodological quality of the included costs study critically (Shemilt 2011).
Measures of treatment effect
Dichotomous data
For dichotomous outcomes (e.g. living at home versus other location), we analysed data based on the number of events and the number of people assessed in the intervention and usual care group. We used these to calculate the risk ratio (RR) and 95% confidence intervals (CI) using a random‐effects model to analyse such data where pooled.
Continuous data
We planned to analyse continuous data (e.g. ADL) based on the mean and standard deviation (SD), and number of people assessed for both the intervention and usual care groups to calculate mean difference (MD) and 95% CI. However, the studies reported all continuous data using different scales for the same outcome (e.g. QoL). Therefore, we estimated SMDs and 95% CI and used the inverse‐variance method to analyse data in Review Manager 5 (RevMan 2014).
In cases where the mean and SD were not available in the published report, we obtained data from the study authors. If these had not been available, we planned to calculate effect sizes (e.g. from t tests, F tests or exact P values).
Time‐to‐event data
If we had encountered time‐to‐event (e.g. transfer to nursing home) data, we would have extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports. If these were not available, we would have estimated the log(HR) using published methods (Parmar 1998; Tierney 2007). We would have pooled HRs using the generic inverse‐variance method of Review Manager 5 (Deeks 2011).
Economic data
We had planned to tabulate the characteristics of any health economic studies included by subgroups (i.e. full economic evaluations, partial economic evaluations and analyses reporting more limited information). We would only present a pooled estimate if there was evidence of little variation in resource or cost use between studies. As we were unable to pool data, due to the lack of eligible data, we presented a narrative summary for the single study regarding the design and analytical viewpoints adopted, the primary outcome measures used for the evaluation and resource‐use cost data (Drummond 1996; Table 2).
1. Lewin 2014 economic evaluation ‐ risk of bias based on Drummond checklist.
| Study design | Issue addressed | Explanation |
| 1. The research question is stated | Yes | Compared health and aged care service use and costs of a restorative home care service versus a conventional service |
| 2. The economic importance of the research question is stated | Yes | Investigated whether an effective intervention was also cost‐effective |
| 3. The viewpoint(s) of the analysis are clearly stated and justified | Yes | Multi‐agency perspective including providers of health and social (aged) care |
| 4. The rationale for choosing alternative programmes or interventions compared is stated | Yes | Intervention and usual care services funded by the Government and provided by a not‐for‐profit organisation |
| 5. The alternatives being compared are clearly described | Yes | Time‐limited individualised restorative service delivered in the home compared to usual personal care service |
| 6. The form of economic evaluation used is stated | No | Not explicitly stated, information provided on the use and cost of home‐care and healthcare services |
| 7. The choice of form of economic evaluation is justified in relation to the questions asked | Yes | A partial evaluation that provided costs data on service utilisation in both the intervention and control conditions. An ICER was not conducted |
| Data collection | ||
| 8. The source(s) of effectiveness estimates are stated | Yes | RCT (Lewin 2013): 750 participants (aged ≥ 65 years) eligible for government‐funded home care and allocated to a time‐limited (12 weeks maximum) reablement intervention or to usual home‐care services |
| 9. Details of the design and results of effectiveness study are given (if based on a single study) | Yes | See Lewin 2013 |
| 10. Details of the methods of synthesis of estimates are given (if based on a synthesis of a number of effectiveness studies) | N/A | ‐ |
| 11. The primary outcome measure(s) for the economic evaluation are clearly stated | Yes | Comparison of intervention and control groups on:
|
| 12. Methods to value benefits are stated | N/A | Not stated |
| 13. Details of the participants from whom valuations were obtained were given | Yes | ‐ |
| 14. Productivity changes (if included) are reported separately | N/A | Productivity costs not included |
| 15. The relevance of productivity changes to the study question is discussed | N/A | Productivity costs not discussed |
| 16. Quantities of resource use are reported separately | No | The mean hours of aged‐care services provided, but only mean cost per client for first year and total 2‐year period reported rather than cost per hour Number of emergency department visits provided, but only mean total costs per client for 2‐year period rather than cost per visit reported Number of hospital visits and mean length of stay (episodic and cumulative) provided, but only mean total cost per client for all hospital admissions reported |
| 17. Methods for the estimation of quantities and unit costs are described | Yes |
Quantities data were obtained via the Western Australian Data Linkage System: the Emergency Department Data Collection; Hospital Morbidity Data System; the Mortality Register; the HACC database and the Aged Care Assessment Program database Unit costs: home care costs provided by the Western Australian DoH; emergency department costs provided by the National Hospital Cost Data Collection Cost Report (2007‐8); inpatient data were provided by the Public Sector Estimated Round 12 (2007‐8) AR‐DRG 5.1 Cost Report for Western Australia (DoHA 2008) |
| 18. Currency and price data are recorded | Australian dollars | |
| 19. Details of currency of price adjustments for inflation or currency conversion are given | No | ‐ |
| 20. Details of any model used are given | Yes | General linear model used to analyse aggregated health and social care costs over time (2‐year period) and adjusted for living arrangements, carer status, gender and dependency level |
| 21. The choice of model used and the key parameters on which it is based are justified | Yes | Aggregated health and social care costs presented |
| Analysis and interpretation of results | ||
| 22. Time horizon of costs and benefits is stated | Yes | Data collected for 3‐year period commencing 1 year prior to the date when the participant assigned to either intervention or control groups |
| 23. The discount rate(s) is stated | No | Not discussed |
| 24. The choice of discount rate(s) is justified | N/A | ‐ |
| 25. An explanation is given if costs and benefits are not discounted | No | None provided |
| 26. Details of statistical tests and confidence intervals are given for stochastic data | N/A | ‐ |
| 27. The approach to sensitivity analysis is given | N/A | Not conducted |
| 28. The choice of variables for sensitivity analysis is justified | N/A | Not conducted |
| 29. The ranges over which the variables are varied are justified | N/A | Sensitivity analyses not conducted |
| 30. Relevant alternatives are compared | No | ‐ |
| 31. Incremental analysis is reported | No | ‐ |
| 32. Major outcomes are presented in a disaggregated as well as aggregated form | Yes, in some cases only | Home‐care services, presentations to emergency departments and unplanned admissions were disaggregated. However, costs of the intervention itself were not disaggregated. The study authors reported that they were unable to include costs of residential/hospice care as the data they used related to approval for the service and there was no certainty that this translated into an actual admission |
| 33. The answer to the study question is given | Yes | Clients who received intervention were less costly to the aged and healthcare services over time than those who received standard home care, p. 334 |
| 34. Conclusions follow from the data reported | Yes | Statistical significance achieved more often in as‐treated analysis, suggesting that the success of the intervention depended heavily on compliance with the HIP [intervention] protocol. p. 334 |
| 35. Conclusions are accompanied by the appropriate caveats | Yes | Limitations and possible effects on the findings noted on p. 335. Specifically, authors were unable to match the dates of home‐care referral exactly with the financial year date of assessment or utilisation. Therefore, there may have been some over‐ or under‐estimation of the number of hours of service(s) the client used in each year. This measurement bias was likely to have affected the intervention and control groups equally |
AR‐DRG: Australian Refined Diagnosis Related Group; DoH: Department of Health; DoHA: Department of Health and Ageing; HACC: home and community care; HIP: Home Independence Program; ICER: incremental cost‐effectiveness ratio; RCT: randomised controlled trial.
Unit of analysis issues
We presented the relevant outcomes assessed at the end of the intervention (three months) and at follow‐up (nine, 12 and 24 months) separately.
If we had identified cluster RCTs for inclusion, we planned to check for unit‐of‐analysis errors. If we had found such errors and sufficient information was available, we planned to re‐analyse the data using the appropriate unit of analysis by taking account of the intracluster coefficient (ICC). We planned to contact study authors to obtain ICC estimates if these had not been clearly available from the trial reports, or to impute them using estimates from external sources (i.e. from a study of a similar population). If ICCs from other sources were used, we planned to undertake sensitivity analyses to investigate the effect of variation in the ICC. If it had not been possible to obtain sufficient information to re‐analyse the data, we planned to report the effect estimate and annotate unit‐of‐analysis error.
Dealing with missing data
We contacted the study authors to obtain data not included in the original article (i.e. means and SD of outcomes). We reported on the levels of loss to follow‐up and assessed this as a source of potential bias. We used intention‐to‐treat (ITT) data when available for our analyses. The ITT analysis in one study did not include all participants as randomised, and we contacted the study authors to determine if and how values for the missing data were imputed. The authors reported that they excluded cases if there was missing data and did not use any methods to impute these values (Lewin 2013).
We planned to conduct sensitivity analysis excluding studies with 20% or more of data missing for one of the primary outcomes to assess potential bias, but this was not possible due to the small number of included studies.
Assessment of heterogeneity
Where studies were sufficiently similar (e.g. based on considerations of population, intervention duration and intensity) to allow for pooling of data, we assessed the degree of heterogeneity by the visual inspection of the forest plots and by examining the Chi² test for heterogeneity. We quantified heterogeneity using the I² statistic (Higgins 2011). We considered an I² value of 50% or more to represent substantial heterogeneity, and interpreted it in view of size and direction of effects and the strength of the heterogeneity based on the P value from the Chi² test. If there was evidence of heterogeneity, we planned to discuss any possible reasons, and if there had been sufficient trials, we would have conducted subgroup analyses accordingly; the issue of sample size and power in each study would be considered in the interpretation and reporting of results.
If there had been substantial clinical, methodological or statistical heterogeneity across the included studies we would not have pooled results, but instead used a narrative approach to data synthesis. In addition, we would have explored possible clinical or methodological reasons for any variation to examine differences in intervention effects. Since the review included only two studies it was not possible to perform this analysis.
Assessment of reporting biases
We included insufficient studies to test for possible publication bias.
We had planned to assess reporting bias qualitatively based on the characteristics of the included studies (e.g. if only small studies that indicate positive findings are identified for inclusion), and if information that we had obtained from contacting experts and authors of studies suggested that there were relevant unpublished studies. If we had identified sufficient studies (at least 10) for inclusion, we planned to construct funnel plots to investigate any relationship between effect size and standard error. Such a relationship could be due to publication or related biases, or due to systematic differences between small and large studies. Where there was such a relationship, the methodological diversity of the studies was to be further examined as a possible explanation (Egger 1997). Findings were to be incorporated into 'Risk of bias' tables in the domain 'Other sources of bias'.
Data synthesis
We decided whether to meta‐analyse data based on if the included trials were sufficiently similar in terms of participants, interventions, comparisons and outcome measures to ensure meaningful conclusions from statistically pooled results. Due to the variability in the interventions and participants, we used a random‐effects model for meta‐analysis, with data analysis conducted in Review Manager 5 (RevMan 2014).
Lewin 2013 employed two different self report measures of functional status, ADL and IADL (instrumental activities of daily living), reported by the study authors as secondary outcomes. Therefore, to derive a summary effect estimate for this outcome from Lewin 2013, we calculated a mean effect size and standard error across both measures. We also calculated a mean effect size and standard error from the means, SDs and numbers of participants reported in Tuntland 2015 for their primary outcome (function measured using the Canadian Occupational Therapy Performance; COPM). Both summary estimates could then be pooled within the generic inverse‐variance analytic method, using the effect measure of SMD.
We calculated and reported the appropriate effect estimate (RR and 95% CI) where data were based on a single study.
Table 1 shows the findings for those outcomes most important to decision makers.
Subgroup analysis and investigation of heterogeneity
We were unable to conduct subgroup analyses due to an insufficient number of included studies. We had planned analyses based on the following subgroup parameters that emerged from the literature.
Context of recruitment to intervention. We anticipated that participants who had been recently discharged from hospital may have higher level of need or be at greater risk of re‐admission (or both) than participants recruited from the community, and thus some differences in outcome may emerge (Francis 2011).
Mean age of participants. There was some indication that younger participants (aged under 75 years) may gain greater benefit from reablement (Glendinning 2010). Therefore, we had planned to examine two groups, people aged 65 to 75 years and people aged over 75 years, to explore this effect.
Living circumstances (i.e. alone or with others). Isolated older people may experience the service differently from people with a higher level of support (Francis 2011).
Duration of intervention. Defined as standard (six weeks); long (seven to 12 weeks) as some trials may offer an extended period of reablement to meet individual needs (Jones 2009).
Sensitivity analysis
We were unable to conduct the planned sensitivity analysis due to a small number of included studies. We had planned to evaluate the robustness of any pooled effect sizes across various components of methodological quality to examine the robustness of the various effect estimates. We would have analysed the effects of excluding trials that were judged at high risk of bias across one or more of the domains of sequence generation, allocation concealment, attrition (rates larger than 20%) and outcome reporting (greater than 20% of data missing) for meta‐analysis of the primary outcomes. We planned to include the data if the exclusion of trials at high risk of bias did not substantially alter the direction of effect or the precision of the effect estimates. We would also have undertaken a sensitivity analysis to assess the effects of including trials where we used imputed values (e.g. ICC values from external sources for cluster‐RCT trials).
'Summary of findings' table
We presented results for each of the major outcomes as outlined in Types of outcome measures (Schünemann 2011). We provided sources and rationales for the assumed risk cited in the table. two authors (AC, MF) independently assessed the quality of the evidence using the GRADE criteria for each of the following parameters: risk of bias, inconsistency, imprecision, indirectness and publication bias using the GRADEprofiler (GRADEpro) software (Schünemann 2011). We downgraded a rating of high quality evidence by one level for serious concerns, and by two levels for very serious concerns. The findings for the outcomes where meta‐analysis was not possible are presented in Table 1 using a narrative format (Chan 2011).
Consumer participation
Consumer participation and content expertise were considered important because reablement reflects a partnership between the older person and the service providers. Therefore, feedback on the protocol was received from a consumer referee (a Cochrane volunteer), while feedback from referees with content expertise was sought at both protocol and review stages.
Results
Description of studies
Results of the search
Searches of electronic databases carried out between April and June 2015 yielded 11,507 abstracts. Handsearching of the reference lists within included studies and previous reviews yielded 809 references. After removal of 3463 duplicates, we screened titles and abstracts and identified 33 as being potentially relevant. Following assessment of the full text of these papers, and in some cases contact with study authors, we identified two studies (one with an associated cost evaluation) that met our eligibility criteria (Lewin 2013; Tuntland 2015). We identified three potentially eligible studies but these were ongoing or in the planning stages and findings not yet available (Grimmer 2013; Langeland 2015; Whitehead 2014); see Characteristics of ongoing studies table for further details. See Figure 1 for the PRISMA study flow diagram. We excluded the remaining 28 studies; see Characteristics of excluded studies table.
Included studies
The Characteristics of included studies table describes the main features of the two included RCTs. One study was conducted in Perth, Western Australia (Lewin 2013), and the other in a rural municipality of Norway (Tuntland 2015).
Participants
Lewin 2013 included 750 older adults (aged 65 years or more), eligible for home‐care services defined as needing assistance with one or more activity of living. Recruitment took place between June 2005 and August 2007. The mean age of the participants was 82.28 years (SD 7.45), and the sample was predominantly female (67.33%; 505/750). At baseline, over half of the intervention (57.8%) and usual care (67.7%) groups had a carer available, and more of the intervention (51.2%) group lived alone relative to the usual care group (42.4%).
Tuntland 2015 recruited 61 people between May 2012 and February 2014. The participants reported activity limitations and had been referred to home‐based services available for people aged 18 years and over. We contacted the study authors to determine the age profile of participants; 8.2% (5/61) of the full sample were aged 64 years or younger. The trial therefore met our inclusion criterion because more than 80% of included participants were aged 65 years or over. The mean age of participants was 79 years (SD 10.1), and most were female (67.2%; 41/61). The study authors did not report information related to living situation and carer availability.
Reablement and usual care
The interventions were similar in the two studies and in both cases there was an emphasis on encouraging participants to achieve individualised goals and to perform daily activities themselves rather than letting others do it for them. In addition, the intervention included exercises to improve mobility, adaptations to tasks and equipment, and strategies to promote social connectedness. Both interventions involved interdisciplinary teams including occupational therapists and physiotherapists, who conducted the initial assessments and developed the rehabilitation plan tailored to the aims and needs of each participant. Tuntland 2015 reported that the reablement service lasted 10 weeks on average, with a mean number of seven home visits per person per week lasting on average 2.1 hours (based on a 12‐week period). The reablement group received more home visits from therapists than the usual care group reflecting the enhanced emphasis on rehabilitation (Tuntland 2015). The participants in Lewin 2013 continued with reablement until they achieved their goals or for up to 12 weeks, whichever occurred first; additional information regarding the mean number of visits or duration of these visits was not provided. Neither study provided data on the percentage of participants who received the full three months of sessions, nor how many achieved their goals earlier.
There were no changes to the usual home‐care services for the control groups in either study; Lewin 2013 described "standard" home care as typically involving three visits a week to help with personal care (bathing/showering) and house cleaning. Tuntland 2015 described usual care as the "compensating help they applied for" and for most participants this consisted of personal or practical assistance, meals on wheels or assistive technology; the participants received, on average, six visits per week lasting 1.7 hours. The limited information provided suggested that usual home care appears to be broadly comparable across the two studies. However, participants in the Norwegian study appeared to have received twice as many visits per week relative to Lewin 2013. Additionally, the participants in the usual care group in Tuntland 2015 accessed a significantly higher amount of co‐interventions in terms of outpatient physiotherapy during the first three months compared to the reablement group. Lewin 2013 did not report on co‐interventions, so we were unable to make any direct comparisons between the studies on this issue.
Outcomes
The two studies used different tools to measure functional status, the primary outcome for our review. Lewin 2013 used standardised measures of function (ADLs and IADLs; lower scores indicated greater functional independence) for a subgroup of participants recruited by a research assistant from the full sample (150 in the intervention group and 150 in the usual care group), at three and 12 months. Tuntland 2015 employed the COPM to measure self perceived activity performance and satisfaction with performance on individualised activity goals at baseline, and at three and nine months. The COPM was used to enable participants to identify and prioritise problems with their self care or other activities (or both) at baseline; each participant rated their five most important activities on a 10‐point scale (higher scores indicated better function; scores were transformed by inserting a minus sign in the analyses to be consistent with data from Lewin 2013 ); the COPM informed the individualised components and targets of the intervention as well as providing the outcome measure. Therefore, for the outcome of functional status a decrease represented an improvement.
Tuntland 2015 also measured separate domains of HRQoL (COOP/Wonka; Weel 1993; rated 1 to 5, lower scores indicating better QoL) whereas Lewin 2013 used a single measure (Assessment of Quality of Life Scale; Hawthorne 1999; rated 0 to 1, where higher scores indicated better QoL; scores were transformed by inserting a minus sign to be consistent with Tuntland 2015). Therefore, a decrease represented an improvement in QoL.
The study authors provided us with additional data (means and SDs) on request that had not been reported in the published studies.
Both studies reported on mortality rates at nine to 12 months' follow‐up. A paper linked to the Australian study, Lewin 2014, provided additional data related to mortality and service use at the one‐ and two‐year follow‐up. Additionally, Lewin 2014 examined the costs for the intervention and usual care groups for home and healthcare service utilisation at one‐ and two‐year follow‐up, and calculated mean costs per participant across three outcomes:
use of aged care services;
visits to emergency departments and
unplanned hospital admissions
The studies did not report the costs associated with the implementation of the intervention itself when compared to usual care, or the costs associated with residential or hospice care.
Excluded studies
We excluded 28 studies; see Characteristics of excluded studies table. Reasons for exclusion included design issues, that is not randomised at either individual or cluster level (Glendinning 2010; Heebøll 2012; Kent 2000; Le Mesurier 1999; Lewin 2010; McLeod 2009; Newbronner 2007; Tinetti 2002; Tinetti 2012; Winkel 2015); intervention did not meet criteria for duration or intensity (or both) (e.g. over six months or a limited number of visits to the home (or both); Gitlin 2006a; Gitlin 2006b; King 2012; Parsons 2012; Parsons 2013; Sheffield 2013; Szanton 2011); intervention was not exclusively delivered at home (Crotty 2008; Cunliffe 2004; Nikolaus 1999; Senior 2014); intervention focused on medical rather than social care (Avlund 2002; Friedman 2014; Li 2013; Martin 1994; Melis 2008); study did not measure the primary outcome (Crawford Shearer 2010); or the control group received an alternative intervention (i.e. not usual care; Gill 2002). We also excluded economic studies that included costs data based on ineligible studies.
Risk of bias in included studies
We conducted a risk of bias assessment in line with the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We sought further information from study authors to inform our assessment when necessary. See Characteristics of included studies table and Figure 2 for a summary. We used the Drummond checklist (Table 2) to assess any risk of bias of the costs paper (Lewin 2014); this information is presented in Other potential sources of bias, Economic evaluation).
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Overall, we rated Lewin 2013 at high risk of bias on all domains. Tuntland 2015 was deemed to be largely adequate, with issues around performance and detection bias and other sources of bias (use of co‐interventions and treatment fidelity) only.
Allocation
Lewin 2013 used a computerised system to randomise participants; however, the system was open to manipulation, and a 'handful' of operators had, in some cases, purposefully influenced the randomisation, based on the assumption that some participants (e.g. those living alone) might benefit more from allocation to either the intervention of usual care group; we judged this at high risk of bias. The recruitment of participants to subgroups in Lewin 2013 occurred after the initial group assignment; but there was insufficient information for us to be able to judge how these participants were selected from the full sample.
Tuntland 2015 reported appropriate randomisation and allocation concealment procedures, and so was rated at low risk of bias.
Blinding
It was not possible to blind participants or care providers (performance bias) to the allocated condition in either study due to the nature of the intervention, therefore both studies were rated as high risk of bias on this item.
Service outcomes
In Lewin 2013, the provider of home‐care services who collected data on service outcomes (e.g. ongoing personal care needs, hospital admissions) routinely were likely to be blind to group assignment. They used official records maintained by the Western Australian Data Linkage System to collect data relating to home and community care services as well as healthcare utilisation (Lewin 2014).
Functional status and quality of life
The research assistants involved in collecting outcome data from the subgroups in Lewin 2013 were initially blinded, but as the older people would often talk about the type of care they were receiving during the assessments, it was possible for research assistants to deduce which group participants had been allocated. Similarly, participants in Tuntland 2015 were urged not to reveal their group allocation to researchers, but the study authors reported this was only partly successful. Therefore, we rated both studies to be at a high risk of bias on this domain.
Incomplete outcome data
See the Characteristics of included studies table for details on incomplete outcome data.
Before the start of the Lewin 2013 trial, it had been agreed that any client randomised to receive the intervention who was not fully participating in the programme after two weeks would be reassigned to usual care; 20 participants (5.3%; 20/375) were transferred to the usual care group for this reason. A further 45 participants (6.0%; 45/750) were excluded from the as‐treated analysis because they did not receive sufficient levels of service (defined as three visits for the intervention group or three hours of personal care for the usual care group). Figure 1 in Lewin 2013 (page 74) suggested that all 45 participants were originally randomised to receive the intervention.
Dealing with missing data
Missing data due to mortality and participants' illness are commonly reported in research conducted with older populations. Imputing values for the deceased is inappropriate (Little 2002), but a number of different imputation approaches for ITT analysis have been proposed to address other sources of attrition by, for example, imputing missing values of the second follow‐up using information from both the baseline and first follow‐up (Ning 2013). There was no evidence from Lewin 2013 to suggest they used any specific statistical method to deal with missing data in their ITT analysis for personal care at three and 12 months or for the data collected for the functional status and QoL outcomes (provided by study authors), in which case this may be more correctly considered an 'available case analysis' (Higgins 2011). In our judgement, the missing outcome data were not managed appropriately for the purposes of an ITT analysis, so we rated this at high risk of bias.
Lewin 2013 did include all 750 participants in the descriptive data for mortality and service outcomes and Lewin 2014 included all 750 participants when reporting on the 12‐month and the full 24‐month period, and thus can be considered ITT analyses. Tuntland 2015 also included all participants in their ITT analyses.
The incomplete outcome data for the two studies are summarised below.
Mortality and service outcomes
We contacted the study authors to seek clarification on some minor differences in mortality rates reported in Lewin 2013 versus the costs paper Lewin 2014. The study authors indicated that the mortality data in the earlier paper were sourced from a database (maintained by the service provider) that was prone to occasional delays in updating, and that may therefore, have been less accurate due to some degree of under‐reporting. By contrast, the data used in the costs paper may be considered to be more reliable because these figures were obtained using a Mortality Register via the Western Australia Data Linkage System (Lewin 2014). This may explain the higher death rates reported in the costs study that was completed some time after the RCT.
The analysis for ongoing personal care included only 78.9% (592/750) of participants at three months and 63.1% (473/750) of participants at one year (Lewin 2013). People were excluded from the analysis if they: had died, moved either into residential care or out of the area, were terminally ill, declined follow‐up or had missing data for any included variable. The Characteristics of included studies table provides details on attrition. The incomplete data for service outcomes appear to be relatively comparable across the two groups, apart from 30 from the intervention group who declined follow‐up at three months (intervention: 8.0% (30/375); usual care: 2.4% (9/375)).
The ITT analysis conducted by Lewin 2014 included all participants, with the exception of people who had died in the first year and were removed from the second year analysis (intervention: 19.7% (74/375); usual care: 20.5% (77/375)). The ITT analyses for the full 24‐month follow‐up period included all 750 participants (Lewin 2014).
Functional and quality of life outcomes
Attrition was low for both groups in Tuntland 2015; we considered that missing data in the intervention group were unlikely to be due to adverse effects of the intervention, and thus rated the study low risk of bias for this domain.
Functional and QoL outcomes were examined for a subgroup of 40% (300/750) of the originally randomised sample (Lewin 2013). Furthermore, the analysis included only two‐thirds (66.7%) of the intervention subgroup (100/150) and a similar proportion (65.3% 98/150) of the usual care group. The proportions of missing data for functional (ADL and IADL) and QoL outcomes were similar for the intervention and usual care subgroups and at both time points (three and 12 months), with attrition rates ranging from 3.3% to 18.7%. These were related to participants who had declined follow‐up, whose health had deteriorated or who were no longer contactable. As these outcomes were assessed for a subgroup only, we rated this domain at high risk of bias.
See the Characteristics of included studies table for details on attrition,
Selective reporting
All outcomes proposed in the Tuntland 2014 protocol were reported and there was no evidence of selective reporting; we rated this domain at low risk of bias.
There was some indication of selective reporting in Lewin 2013; the outcomes for the subgroup (relating to both functional status and QoL) were not fully reported. However, we did receive unpublished data from the study authors. Some analyses reported in the study appeared to have been conducted on a post‐hoc basis (e.g. individual items of the IADL and ADL rather than full scales) thereby contributing to a high risk of reporting bias.
Other potential sources of bias
We noted several methodological issues that may have affected the magnitude of effect estimates. Lewin 2013 indicated that some participants may have already improved in self care abilities before the baseline assessments were conducted. Contamination of the group receiving usual care may have occurred in both studies. Tuntland 2015 did not monitor therapist and participant adherence to the intervention protocol, so it was not clear whether the reablement intervention led to the changes; the benefits achieved in the usual care group may have been due to the extra co‐interventions they received. We rated both studies to be at high risk of bias on this domain.
Economic evaluation
The reliability of any economic evaluation depends, at least in part, on its use of reliable clinical data (Shemilt 2011). As indicated, we judged the associated trial to be at high risk of bias in terms of randomisation and other domains (Lewin 2013), and this should therefore, be borne in mind when considering the costs reported in Lewin 2014. According to the Drummond checklist guidelines (Table 2), Lewin 2014 conducted a partial evaluation and used a regression‐based analysis to compare the costs associated with services used by the intervention and usual care groups over a two‐year period. The total costs per person were calculated for home‐care, visits to emergency departments and unplanned inpatient admissions for the first and second year, and for the 24 months combined. The cost of the 12‐week intervention when compared to 12 weeks of usual care were not reported separately; neither were the costs of residential or hospice care.
There were some additional limitations to the economic evaluation as indicated by the Drummond guidelines (Table 2). Specifically, an incremental cost‐effectiveness ratio linking the benefits to costs for the intervention and control conditions, was not calculated. In addition, the authors did not take into account discount rates to control for inflation and there was no mention of productivity costs. They did not conduct sensitivity analyses. The authors acknowledged they were unable to exactly match the date of home‐care referral with the financial year of assessment or with actual service use, so it is possible this may have led to some over‐ or under‐estimation of the hours of service(s) that participants had received in each year under investigation; however, this measurement bias was likely to have affected the intervention and usual care groups equally.
Effects of interventions
See: Table 1
We were able to pool data for functional status, mortality and QoL (at three‐month and nine‐ to 12‐month follow‐ups; Lewin 2013; Tuntland 2015). The remaining outcomes: hospital admission, emergency department presentation, level of personal care and living arrangements, came from a single study (Lewin 2013); and where indicated, from the associated costs paper (Lewin 2014). According to our GRADE assessment, the evidence was very low quality for all outcomes. Table 1 presents a summary of the main results.
We reported ITT analyses as described by the study authors in line with recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), although as indicated, we did not consider all analyses conducted by Lewin 2013 to meet the criteria for ITT.
Reablement versus usual care
Primary outcomes
Function
Self report measures
We pooled the self report function measures from Lewin 2013 (ADLs and IADLs) and Tuntland 2015 (COPM) using the generic inverse‐variance method. There was very low quality evidence that time‐limited reablement may slightly improve functional status at three months (SMD ‐0.40; 95% CI ‐0.81 to ‐0.00; Analysis 1.1; 2 studies; 252 participants), and at the nine‐ to 12‐month follow‐up period (SMD ‐0.30; 95% CI ‐0.53 to ‐0.06; Analysis 1.2; 2 studies; 249 participants).
1.1. Analysis.

Comparison 1 Reablement versus usual care: functional status, Outcome 1 Functional status at 3 months.
1.2. Analysis.

Comparison 1 Reablement versus usual care: functional status, Outcome 2 Functional status at 9 to 12 months.
Mortality
There was very low quality evidence from the pooled data that reablement may lead to little or no difference in mortality at nine to 12 months (RR 0.97; 95% CI 0.74 to 1.29; Analysis 2.1; 2 studies; 811 participants). Only Lewin 2014 pooled mortality rates at 24 months of follow‐up; there is very low quality evidence that reablement may lead to little or no difference in mortality rates (RR 0.84; 95% CI 0.69 to 1.03; Analysis 2.2; 1 study; 750 participants).
2.1. Analysis.

Comparison 2 Reablement versus usual care: mortality, Outcome 1 Mortality at 9 to 12 months.
2.2. Analysis.

Comparison 2 Reablement versus usual care: mortality, Outcome 2 Mortality at 24 months.
Unplanned hospital admissions
Very low quality evidence from a single study with 750 participants suggested that the intervention may make little or no difference to unplanned hospital admissions at 12 months (RR 0.94; 95% CI 0.83 to 1.07; Analysis 3.1) or 24 months (RR 0.94; 95% CI 0.85 to 1.03; Analysis 3.2) (Lewin 2013; Lewin 2014).
3.1. Analysis.

Comparison 3 Reablement versus usual care: unplanned hospital admissions, Outcome 1 Hospital admissions at 12 months.
3.2. Analysis.

Comparison 3 Reablement versus usual care: unplanned hospital admissions, Outcome 2 Hospital admissions at 24 months.
Secondary outcomes
Quality of life
We combined QoL scores from Lewin 2013 (Assessment of Quality of Life Scale; three and 12 months) and the overall health rating from Tuntland 2015 (COOP/Wonka; three and nine months) so that lower scores indicated an improvement in QoL. The very low quality findings indicated that reablement may make little or no difference to QoL at three months (SMD ‐0.18; 95% CI ‐0.43 to 0.07; Analysis 4.1; 2 studies; 252 participants) or at the nine‐ to 12‐month follow‐up period (SMD ‐0.23; 95% CI ‐0.48 to 0.02; Analysis 4.2; 2 studies; 249 participants).
4.1. Analysis.

Comparison 4 Reablement versus usual care: health‐related quality of life, Outcome 1 Quality of life at 3 months.
4.2. Analysis.

Comparison 4 Reablement versus usual care: health‐related quality of life, Outcome 2 Quality of life at 9 to 12 months.
Service use ‐ level of personal care
The three‐ and 12‐month data are from Lewin 2013; there was very low quality evidence that the intervention may reduce the need for personal care at three months (RR 0.43; 95% CI 0.36 to 0.52; Analysis 5.1; 750 participants). At 12‐month follow‐up, Lewin 2014 reported on both people receiving ongoing care as well as new (emergent) clients. We combined these scores and found there was very low quality evidence that the reablement group may be less likely to need personal care services at 12 months (RR 0.45; 95% CI 0.36 to 0.56; Analysis 5.2; 750 participants). Lewin 2014 also reported on people assessed and approved for a higher level of care (residential care or equivalent home care) over the 24‐month follow‐up period. There was very low quality evidence that the reablement group may have been slightly less likely to have been approved for a higher level of care than the usual care group (RR 0.87; 95% CI 0.77 to 0.98; Analysis 5.3; 750 participants).
5.1. Analysis.

Comparison 5 Reablement versus usual care: level of personal care, Outcome 1 Personal care at 3 months.
5.2. Analysis.

Comparison 5 Reablement versus usual care: level of personal care, Outcome 2 Personal care at 12 months.
5.3. Analysis.

Comparison 5 Reablement versus usual care: level of personal care, Outcome 3 Approved for higher level of personal care at 24 months.
Service use ‐ visits to emergency departments
Only Lewin 2014 reported on presentations to emergency departments. The very low quality results suggest that the intervention may make little or no difference to the rates of emergency department visits at 12 months (RR 0.90; 95% CI 0.79 to 1.04; Analysis 6.1; 750 participants) or at 24‐month follow‐up (RR 0.93; 95% CI 0.84 to 1.03; Analysis 6.2; 750 participants).
6.1. Analysis.

Comparison 6 Reablement versus usual care: emergency department (ED) presentations, Outcome 1 ED presentation at 12 months.
6.2. Analysis.

Comparison 6 Reablement versus usual care: emergency department (ED) presentations, Outcome 2 ED presentation at 24 months.
Living arrangements
Lewin 2013 reported the number of people who were in residential care at three and 12 months. There was very low quality evidence that reablement may make little or no difference to the rates of transfer to a residential setting (RR 0.76; 95% CI 0.40 to 1.44; Analysis 7.1, three‐month data; RR 0.92 95% CI 0.62 to 1.34; Analysis 7.2, 12‐month data; 750 participants).
7.1. Analysis.

Comparison 7 Reablement versus usual care: living arrangements, Outcome 1 Residential care at 3 months.
7.2. Analysis.

Comparison 7 Reablement versus usual care: living arrangements, Outcome 2 Residential care at 12 months.
User satisfaction
Neither of the included trials reported user satisfaction.
Costs of services
The intervention group used fewer hours of personal care and other home‐care services and this translated into lower costs per client relative to people in the usual care group for the total two‐year period (Lewin 2014; intervention AUD 5833; usual care AUD 8374). The costs related to hospital admissions over the two‐year period were only slightly lower for the intervention group (intervention AUD 13,369; usual care AUD 13,675). The mean total aggregated costs for home‐care and healthcare (emergency department and unplanned hospital admissions) in the intervention group over 24 months was AUD 19,888 compared to AUD 22,757 for the usual care group (RR 0.89; 95% CI 0.78 to 1.02; P = 0.08 as reported in Lewin 2014).
Discussion
Summary of main results
The findings of this review were based on two studies (Table 1), and the evidence was uniformly very low quality for all outcomes. From the results of meta‐analyses of the two included studies, reablement may slightly improve functional status but may have little or no effect on QoL of older adults, or mortality rates at nine to 12 months. Other outcomes were measured by one study (Lewin 2013), and an associated costs paper (Lewin 2014). The very low quality evidence suggested there is uncertainty regarding the effects of reablement on living arrangements, unplanned hospital admissions or visits to an emergency department at both the 12‐month follow‐up and for the overall 24‐month period, or for mortality at 24 months (Lewin 2014).
There was very low quality evidence from one study to indicate that the reablement intervention may reduce need for either ongoing home‐care, or a new episode of personal care at 12‐month follow‐up (Lewin 2013), and may slightly reduce the likelihood of being assessed as needing a higher level of care (i.e. residential care or equivalent home care) at 24 months (Lewin 2014). Neither study measured user satisfaction, which is possibly an important factor in ensuring uptake and adherence related to such interventions. The accompanying cost data, again of very low quality, indicated that health and social‐care services cost per client were lowered to a small degree in the intervention group when compared to people receiving usual care at 24 months' follow‐up, suggesting that reduced need for personal care may translate into lower costs per client in the intervention group. However, these results were based on very low quality evidence and so we are uncertain about the size and importance of these effects.
Overall completeness and applicability of evidence
Despite a thorough search of the existing evidence for the effectiveness of reablement type services, we identified only two trials that were eligible for inclusion in this review. Our review focused on reablement services that were time‐limited (12 weeks or less) as this model has been widely adopted in practice (Mishra 2016; e.g. UK: Glendinning 2010, Denmark: Winkel 2015). We excluded trials lasting longer than 12 weeks; in one study with an extended time frame (Parsons 2012), a reablement‐type intervention continued until the clients were admitted to residential care or died. We concluded that interventions that continued indefinitely would not be directly comparable to time‐limited services for our outcomes of interest, and especially in terms of achieving functional independence and a reduced need for personal care services in the home. We also focused our search on those studies that included a measure of functional outcomes. These two factors resulted in excluding a number of studies, and we may consider broadening our inclusion criteria to enable a comparison of time‐limited versus extended reablement services in an update of this review. We identified no trials that compared reablement with a wait‐list control, a finding that may reflect ethical concerns related to withholding a service to a vulnerable group. Nevertheless, as usual home care itself could have some beneficial effects on the outcomes of interest, a wait‐list comparison may have provided an opportunity to determine the absolute effect of reablement.
While acknowledging these limitations, Lewin 2013 and Tuntland 2015 did yield some interesting information on reablement services developed and delivered in two different countries and health/social care settings. The characteristics of the participants were broadly similar to those in the studies that we excluded (e.g. not time‐limited or non‐randomised) in terms of age, level of dependency and proportion living alone, thereby suggesting they were generally representative of older adults requiring home care. The components and ethos of the two services are comparable to each other and to those offered elsewhere (e.g. Glendinning 2010), although there may be limits in the extent to which the findings can be generalised to other settings with differing levels of integration between health and social care systems. There are also likely to be differences in the content and delivery of usual care provided in different jurisdictions; for example, there were some differences in the frequency of visits for the usual care group between the included studies. Indeed, home‐care can be provided and resourced in a number of different ways (e.g. public or private sector funds/publicly or privately funded organisations; Rostgaard 2011), all of which may add to the complexity associated with delivering and evaluating this intervention elsewhere.
Many of our objectives could not be addressed in this review due to the lack of trials that met our inclusion criteria. Unfortunately, it was not possible to undertake analyses to determine whether reablement is more suitable for particular groups of older people, such as people living alone, or younger versus older cohorts. Future studies are required to address these gaps in our knowledge. Encouragingly, one large multicentre trial is currently underway in Norway using the same design as Tuntland 2015, and data should be available by 2017 (Langeland 2015).
Quality of the evidence
We are very uncertain of the effectiveness of reablement because the evidence was very low quality for all outcomes according to our GRADE assessment (Table 1). We downgraded for risk of bias and imprecision (primarily as some of the outcomes were from a single study only) and as a result, any interpretation of the findings and effects should be treated with considerable caution. To their credit, the authors of both trials highlighted methodological concerns that are probably not uncommon in RCTs of this type. The randomisation process was compromised in Lewin 2013, in that some personnel involved had manipulated the procedure in the well‐intentioned belief that particular clients might benefit more from assignment to either the intervention or usual care group. Neither study was able to blind participants/personnel adequately, something that is difficult to achieve in this type of intervention. Furthermore, blinding of outcome assessors was not completely successful in either trial. There were also some potential issues related to treatment fidelity and adherence. Incomplete data collection resulting from participant drop‐out was also evident in Lewin 2013; indeed, reaching, recruiting and retaining older participants whose health may deteriorate over the course of the study presents a methodological challenge for studies of this type. Similarly, issues such as contamination, baseline differences or differences in what control groups receive, and fidelity of such complex interventions are all challenging when undertaking research in this area.
Potential biases in the review process
It is possible that we may have missed some studies when searching, including those not published in English. Following advice from Cochrane Consumers and Communication, we excluded all non‐randomised trials. Arguably, these would have provided a more extensive analysis of the effectiveness of reablement, but it seems unlikely that such research designs would be rated as higher quality evidence than the identified RCTs, and therefore would not necessarily have added to the findings with any certainty. Our criteria did limit the number of trials for inclusion although we do not consider this to have introduced bias to the review.
Agreements and disagreements with other studies or reviews
A number of previous reviews were unable to identify which of the various models/types of care provision may be most effective for the rehabilitation and maintenance of the independence of older adults. One (non‐Cochrane) review examined interventions designed to reduce dependency in personal care in adults aged 18 years and older (Whitehead 2015). The review considered a range of single and multi‐component interventions and non‐randomised designs. Whitehead 2015 included 13 studies, five of which (including Lewin 2013) were classified as reablement/restorative services. We excluded four of these studies from the current review because they did not meet our inclusion criteria (i.e. non‐randomised designs; not time‐limited). Whitehead 2015 focused on changes in ADL scores as a measure of dependency and concluded that there was some limited evidence to support the effectiveness of the interventions. Consistent with our findings, these authors indicated that such interventions could reduce the use and costs associated with ongoing care services. However, the use of broad selection criteria led to considerable heterogeneity in the content of the interventions; this was something we were keen to avoid by using more focused inclusion criteria.
Another non‐Cochrane review aimed to determine whether home‐care reablement interventions reduced the need for support and assistance from social care home‐care services (Legg 2016). Consistent with our review, Legg 2016 focused on time‐limited services, but used six weeks as their cut‐off; Legg 2016 found no studies that met their inclusion criteria and interestingly did not list Lewin 2013 as one of their excluded studies. Legg 2016 concluded that there was no evidence to suggest reablement is effective at increasing independence or reducing the use of personal care services.
A further non‐Cochrane review included 10 studies, six of which were non‐randomised designs, and suggested that reablement had a positive impact on HRQoL and service utilisation; however, the inclusion of non‐randomised studies may have introduced some bias and uncertainty into their findings, and this may have inflated the effect estimates in this case (Tessier 2016). In addition, while a time‐limit to the service was part of the inclusion criteria, Tessier 2016 included some trials that could continue beyond the six to 12 weeks, which we excluded on that basis from our review.
Another non‐Cochrane review is currently underway that will examine the impact of a range of programmes for older adults in receipt of home‐care services (Sims‐Gould 2015). The review will consider services that aim to provide recipients with the capacity and ability to self manage some aspects of their care, principally ADLs; it will include trials lasting a few weeks to a few months, while excluding long‐term programmes (those lasting more than six months).
Authors' conclusions
Implications for practice.
Despite an exhaustive search of the literature, we identified only two eligible studies to include in our review, and the findings were of very low quality, thereby limiting our ability to provide definitive evidence with any certainty. While the effectiveness of reablement services cannot be supported or refuted until more robust evidence becomes available, there are some issues emerging from this review that may warrant attention. For example, Lewin 2013 anticipated that some clients may not fully engage with a reablement‐based service, and made provisions for participants who were not participating in the programme after two weeks to be reallocated to usual care. This may relate to the participants' expectations of more conventional home‐care support or a lack of understanding of the aims of the service (or both) (Glendinning 2010). It is important therefore, that practitioners promote a shared understanding of the ethos associated with any new service to ensure that expectations are compatible among clients, careers and service providers. This may be particularly vital when changing an established usual care paradigm. Furthermore, there is a need to consider the challenges and opportunities associated with working in an interdisciplinary team when developing, implementing and evaluating such interventions.
Implications for research.
Reablement services are becoming widely available, particularly in the UK, and interest is also growing in a number of high‐income countries (Mishra 2016). Despite this, there remains a lack of rigorous evidence about their overall effectiveness due to a dearth of eligible trials. Overall, the complexity associated with reablement makes it difficult to assess in a rigorous trial design, and there is little agreement about the most appropriate tools to measure relevant outcomes. A number of areas would benefit from further research including, in particular, a focus on delineating more clearly, population and intervention characteristics. First, there is a need to identify which groups of older people are most likely to benefit from a reablement approach, such as younger populations or people with lower levels of need. Second, more trials are needed to identify the critical components or processes of reablement that are most effective in promoting or maintaining (or both) independence in older adults. Third, there is a need to identify the most appropriate outcomes and assessment tools with which to measure meaningful changes in this population; for example, what are the benefits of using the goals set by the individual as an outcome measure, as in Tuntland 2015, versus a standardised tool as in Lewin 2013? Reablement is not a passive process and it is important to identify the reasons why some people do not wish to, or cannot engage, with these programmes. There is, therefore, a need for more process evaluations to assess participants' experiences, views and attitudes, and to identify, for example, the contexts and mechanisms associated with effective reablement services, including the role of the interdisciplinary team. While Lewin 2013 did assess the effects of reablement over 24 months, there remains a need to establish the ongoing or longer‐term effects of reablement, something that has not been covered in the available literature to date. More evidence is needed on the extent to which these services should, or should not, be time‐limited.
In conclusion, it is important to note that the marked lack of RCTs in this area appear to reflect some of the challenges inherent in conducting rigorous research on social care in real world community settings. For example, the recruitment of frail older people in the community can be problematic as many may be lost to follow‐up for reasons including deteriorating health, hospital admissions and transfers to residential settings. It can also be difficult to identify and recruit usual care groups when a service has already been established in a particular setting or when service providers believe the service to be effective (or both) and, therefore, it should be available to all who might need it. Last, RCTs are expensive (and time‐consuming) and the funding for such studies may be limited; nevertheless, there is a need for high‐quality RCTs in this area.
Notes
This review drew on standard text and guidance provided by Cochrane Consumers and Communication (CCCG 2013).
Acknowledgements
We would like to thank the editors and staff of the Cochrane Consumers and Communication Group who have provided us with valuable guidance and advice, and, in particular, Managing Editors Megan Prictor, Sue Cole and Ann Jones, Deputy Co‐ordinating editor Rebecca Ryan and John Kis‐Rigo the Information Specialist.
Appendices
Appendix 1. CENTRAL search strategy
1. (home near/5 (care or visit*)):ti,ab,kw
2. (homecare or domiciliary‐care):ti,ab,kw
3. (community near/3 (dwell* or setting)):ti,ab,kw
4. ("independent living" or "community health nursing" or "house calls"):kw
5. (own‐home* or home‐environment):ti,ab,kw
6. {or #1‐#5}
7. [mh rehabilitation]
8. daily‐life‐activit*:ti,ab,kw
9. (rehab* or (activit* near/2 daily‐living)):ti,ab,kw
10. (re‐abl* or reabl* or enablement or empower* or restor* or re‐learn* or relearn*):ti,ab,kw
11. ((recover* or optim* or maintain* or increas* or improv* or independen* or abilit* or outcome*) near/3 function*):ti,ab,kw
12. ((enabl* or recover* or maintain* or develop* or living) near/3 independen*):ti,ab,kw
13. (self next (care or manag*)):ti,ab,kw
14. {or #7‐#13}
15. #6 and #14
Appendix 2. MEDLINE (OvidSP) search strategy
1. home care services/
2. (home adj5 (care or visit*)).tw.
3. homecare.tw.
4. house calls/
5. domiciliary care.tw.
6. own home?.tw.
7. (community dwelling or community setting or living in the community or home based).tw.
8. community health nursing/
9. or/1‐8
10. exp rehabilitation/
11. (rehab* or (activit* adj2 daily living)).tw.
12. (re‐abl* or reabl* or enablement or empower* or restor* or re‐learn* or relearn*).tw.
13. "recovery of function"/
14. ((recover* or optim* or maintain* or increas* or improv* or independen* or ability or outcome*) adj3 function*).tw.
15. ((enabl* or recover* or maintain* or develop* or living) adj3 independen*).tw.
16. self care/
17. (self adj (care or manag*)).tw.
18. or/10‐17
19. 9 and 18
20. randomized controlled trial.pt.
21. controlled clinical trial.pt.
22. randomized.ab.
23. placebo.ab.
24. drug therapy.fs.
25. randomly.ab.
26. trial.ab.
27. groups.ab.
28. or/20‐27
29. exp animals/ not humans.sh.
30. 28 not 29
31. 19 and 30
Appendix 3. Embase (OvidSP) search strategy
1. exp home care/
2. (home adj5 (care or visit*)).ti,ab,kw.
3. (homecare or domiciliary care).ti,ab,kw.
4. independent living/
5. own home?.ti,ab,kw.
6. (community adj3 (dwell* or setting)).ti,ab,kw.
7. community health nursing/
8. or/1‐7
9. exp rehabilitation/
10. daily life activity/
11. (rehab* or (activit* adj2 daily living)).mp.
12. (re‐abl* or reabl* or enablement or empower* or restor* or re‐learn* or relearn*).ti,ab,kw.
13. ((recover* or optim* or maintain* or increas* or independen* or ability) adj3 function*).ti,ab,kw.
14. ((enabl* or recover* or maintain* or living) adj3 independen*).ti,ab,kw.
15. self care/
16. (self adj (care or manag*)).ti,ab,kw.
17. or/9‐16
18. 8 and 17
19. randomized controlled trial/
20. controlled clinical trial/
21. single blind procedure/ or double blind procedure/
22. crossover procedure/
23. random*.tw.
24. placebo*.tw.
25. ((singl* or doubl*) adj (blind* or mask*)).tw.
26. (crossover or cross over or factorial* or latin square).tw.
27. (assign* or allocat* or volunteer*).tw.
28. or/19‐27
29. 18 and 28
Appendix 4. PsycINFO search strategy
1. home care/
2. home visiting programs/
3. (home adj5 (care or visit*)).ti,ab,hw,id.
4. (homecare or domiciliary care).ti,ab,hw,id.
5. home environment/
6. (own home? or home environment*).ti,ab,id.
7. (community adj3 (dwell* or setting)).ti,ab,hw,id.
8. or/1‐7
9. exp rehabilitation/
10. "activities of daily living"/
11. (rehab* or (activit* adj2 daily living)).ti,ab,id.
12. (re‐abl* or reabl* or enablement or empower* or restor* or re‐learn* or relearn*).ti,ab,hw,id.
13. independent living programs/
14. ((recover* or optim* or maintain* or increas* or independen* or ability) adj3 function*).ti,ab,hw,id.
15. ((enabl* or recover* or maintain* or living) adj3 independen*).ti,ab,hw,id.
16. (self adj (care or manag*)).ti,ab,hw,id.
17. or/9‐16
18. 8 and 17
19. random*.ti,ab,hw,id.
20. trial*.ti,ab,hw,id.
21. controlled stud*.ti,ab,hw,id.
22. placebo*.ti,ab,hw,id.
23. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
24. (cross over or crossover or factorial* or latin square).ti,ab,hw,id.
25. (assign* or allocat* or volunteer*).ti,ab,hw,id.
26. treatment effectiveness evaluation/
27. mental health program evaluation/
28. exp experimental design/
29. "2000".md.
30. or/19‐29
31. 18 and 30
Appendix 5. ProQuest search strategy
all((home near/5 (care or visit*)) or (community near/3 (dwell* or setting)) or homecare or "domiciliary care" or "independent living" or "community health nursing" or "house calls" or "own home*" or "home environment*") and all(rehab* or (activit* near/2 ("daily living" or "daily life")) or "re‐abl*" or reabl* or enablement or empower* or restor* or "re‐learn*" or relearn* or ((recover* or optim* or maintain* or increas* or improv* or independen* or abilit* or outcome*) near/3 function*) or ((enabl* or recover* or maintain* or develop* or living) near/3 independen*) or "self care" or selfcare or "self manag*") and all(random* or trial* or placebo* or assign* or allocat* or volunteer* or ((singl* or doubl* or tripl* or trebl*) and (blind* or mask*)) or crossover or "cross over" or factorial* or "latin square")
Appendix 6. CINAHL and AgeLine (EBSCO) search strategy
1. MH home health care+
2. home N5 (care or visit*)
3. homecare or "domiciliary care" or "independent living" or "community health nursing" or "house calls" or "own home*" or "home environment" or "home based"
4. community N3 (dwell* or setting or living)
5. MH community health nursing+
6. s1 or s2 or s3 or s4 or s5
7. MH rehabilitation+
8. rehab* or (activit* N2 ("daily living" or "daily life"))
9. "re‐abl*" or reabl* or enablement or empower* or restor* or "re‐learn*" or relearn*
10. (recover* or optim* or maintain* or increas* or improv* or independen* or abilit* or outcome*) N3 function*
11. (enabl* or recover* or maintain* or develop* or living) N3 independen*
12. MH self care+
13. self N1 (care or manag*)
14. s7 or s8 or s9 or s10 or s11 or s12 or s13
15. s6 and s14
16. "randomi?ed controlled trial" or PT randomized controlled trial
17. PT Clinical Trial
18. MH Clinical Trials+
19. MH Random Assignment
20. MH Placebos
21. MH Quantitative Studies
22. AB (random* or trial or placebo*) or TI (random* or trial or placebo*)
23. AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*)
24. TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*)
25. S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24
26. s15 and s25
27. s26
Appendix 7. SIGLE (now OpenGrey)
home care AND (reablement OR re‐ablement OR restorative);
Appendix 8. Social Care Online
Three searches using the following terms: "reablement" OR "re‐ablement", "restorative", home care OR "home‐care" and each search re‐run including AND "Randomised clinical trial"
Appendix 9. Searches of clinical trial registries
WHO Clinical Trial Search Portal (www.who.int/trialsearch)
Three searches using the following terms: "reablement" OR "re‐ablement", "restorative", home care OR "home‐care"
Clinical Trials.gov (www.clinicaltrials.gov)
Three searches using the following terms: "reablement" OR "re‐ablement", "restorative", home care OR "home‐care"
Clinical Trials Register (www.clinicaltrialsregister.eu)
Three searches using the following terms: "reablement" OR "re‐ablement", "restorative", home care OR "home‐care"
Data and analyses
Comparison 1. Reablement versus usual care: functional status.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status at 3 months | 2 | 252 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.81, 0.00] |
| 2 Functional status at 9 to 12 months | 2 | 249 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.53, ‐0.06] |
Comparison 2. Reablement versus usual care: mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality at 9 to 12 months | 2 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.29] |
| 2 Mortality at 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Comparison 3. Reablement versus usual care: unplanned hospital admissions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital admissions at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Hospital admissions at 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 4. Reablement versus usual care: health‐related quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life at 3 months | 2 | 252 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.43, 0.07] |
| 2 Quality of life at 9 to 12 months | 2 | 249 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.48, 0.02] |
Comparison 5. Reablement versus usual care: level of personal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Personal care at 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Personal care at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Approved for higher level of personal care at 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Reablement versus usual care: emergency department (ED) presentations.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ED presentation at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 ED presentation at 24 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Reablement versus usual care: living arrangements.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Residential care at 3 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Residential care at 12 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Lewin 2013.
| Methods | RCT | |
| Participants | Older people living in the suburbs of Perth (Western Australia):
RCT inclusion criteria: aged > 65 years; referred for personal care; not diagnosed with dementia or other progressive neurological disorder, or receiving palliative care; able to communicate in English Sample: 750 participants randomised to intervention or usual care group 67.3% women (n = 505); mean age 82.3 years. 48.8% born in Australia (48.8%); of the remainder, 18.4% born in England, 4.8% born in Italy and 28% classified as 'other' At baseline, 57.8% of the intervention group and 67.7% of the usual care group had a carer available, and more of the intervention (51.2%) group lived alone compared to the usual care group (42.4%) Subgroup (n = 300) was recruited from the original sample for the assessment of secondary outcomes Recruitment: June 2005 to August 2007 |
|
| Interventions | Intervention and usual care were delivered in client's own home Intervention: HIP "designed to target individuals when they are first referred for home care or existing home‐care clients who request an increase in service input" (p. 72). Service had multiple components and was goal‐directed. The overall aim was to promote "active engagement in a range of daily living activities using tasks analysis and redesign; work simplification and assistive technology; balance and endurance programmes for improving or maintaining mobility; chronic disease self‐management; falls prevention strategies; medication, continence and nutrition management; and improvement or maintenance of skin integrity" (p. 72). The service continued until the client achieved their goals or for up to a maximum of 12 weeks Usual care: standard home‐care service (HACC), care coordinator assessed individual needs, and completed a care plan; this most commonly included 3 personal care visits a week to help with bathing/showering and a fortnightly visit for housecleaning |
|
| Outcomes | Assessed on 3 occasions: baseline, 3 months and 12 months Primary outcome:
Secondary outcomes: collected for a subgroup of participants only, using a Primary Assessment Tool developed for use by community service providers:
The following outcomes were also reported at 3 and 12 months (Lewin 2013) and in a follow‐up paper for the first and second year and overall 24 months (Lewin 2014):
Emergency department visits were reported by Lewin 2014 only |
|
| Sample Size | 2108 participants referred to the service; 826 did not meet the inclusion criteria; 532 were not included as the service was not available or the target sample size had been reached Final sample of 750 randomised to either intervention or usual care (sample size calculation indicated that this number was sufficient to detect a difference of 12% in service outcomes with 90% power and 5% levels of significance. p. 71) A subgroup (n = 300) was recruited from the main RCT sample, comprising 150 participants in the intervention, 150 in the usual care group |
|
| Notes | The research was funded by an Australian Health Ministers' Advisory Council priority‐driven research programme grant, and supported by Western Australia's Home and Community Care programme and Silver Chain (service provider) Primary outcome data at 3 months were sourced from Lewin 2013; at 1‐year and 2‐year follow‐up Lewin 2014 ADLs, IADLs and QoL unpublished data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Email communication: "the system worked out the number of tenths of seconds in time when the customer completed the eligibility questionnaire and depending whether it was an odd or even 10th the person was allocated to one group rather than the other" Quote: "following achievement of approximately half the sample an imbalance in numbers between the two groups had not righted itself and it was thought that Operators [at the customer centre] may somehow have been purposefully influencing the randomisation process" p. 71 Comment: randomisation was at high risk as the operators at the customer centre were able to circumvent the process Quote: "Prior to commencement of the trial it was agreed that clients randomised to receive HIP [the intervention] who after 2 weeks were not participating in the program for any reason would be reassigned to receive usual home care" (p. 72) Comment:Figure 1 (p. 74, Lewin 2013) suggested that 20 people originally randomised to receive the intervention were reassigned to usual care. Additionally, a further 45 participants were reported as receiving neither the intervention nor usual care, Figure 1 suggests that all of these participants were originally assigned to the intervention. The ITT analysis for service outcomes included all 375 participants |
| Allocation concealment (selection bias) | High risk | Quote: "The operators could not therefore be blind to group allocation" (p. 71) Quote: "a handful of staff admitted they tried to circumvent the process in the belief that particular clients would benefit most from assignment to the usual care or intervention group" (p. 78) Comment: this manipulation suggests a high risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk |
Participants: quote "participants would often talk about the type of assistance they were receiving" (p. 73) suggesting that they knew they were either receiving usual care or the intervention; therefore, they could not be blinded Personnel: home‐care workers providing the service(s) could not be blind to group assignment. RAs were blind at first interview "however, participants would often talk about the type of assistance they were receiving. So it was impossible to prevent the RAs from deducing over the course of the RCT whether the participant was in the intervention or group" (p. 73) Comment: blinding was not achieved and therefore at high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | High risk |
Service outcomes (e.g. whether a participant was receiving ongoing personal care): "service data are collected at intervals throughout an individual's episode of care" (p. 72) and accessed from the service provider's database Comment: unclear risk of bias Other outcomes (QoL, ADL, IADL): collected for subgroup by RAs Comment: as the RAs were likely to have deduced whether the participant was in the intervention or usual care group (p. 73), this was at high risk of bias |
| Incomplete outcome data (attrition bias) | High risk |
Service outcomes Quote: "All 750 clients randomised to the study were included in the ITT analysis, whereas 45 were not included in the as‐treated analysis because they did not receive sufficient service (defined as three HIP [the intervention] visits for the intervention group or three hours of personal care for the control group)" (p. 74) Comment: the study did not report how many of the 45 were originally assigned to the intervention or usual care group. Figure 1 (Lewin 2013) suggested that all 45 were originally assigned to the intervention. As we have no additional information as to why participants did not receive sufficient service, we have judged this at unclear risk Quote: "Assessment at referral (baseline) and service data (including outcomes at 3 and 12 months) were available for all participants" (p. 74) Comment: in the analysis examining service outcomes (logistic regression p. 76) the ITT analysis was reduced to n = 592/750 at 3 months and n = 473/750 at 12 months Excluded: participants who died (3 months: intervention 4.5% (17/375), usual care 6.6% (25/375); 12 months: intervention 17.3% (65/375), usual care 19.2% (72/375); moved into residential care or out of the area (3 months: intervention 4.2% (16/375), usual care 6.4% (24/375); 12 months: intervention 12% (45/375), usual care 14.1% (53/375); receiving hospice care (3 months: intervention 2.4% (9/375), usual care 0% (0/375); 12 months: intervention 1.1% (4/375), control 0.3% (1/375); declined/terminated follow‐up (3 months: intervention 8% (30/375), usual care 2.4% (9/375); 12 months: intervention 1.6% (6/375), usual care 1.1% (4/375); and participants who had missing data for any variable (n not available) The as‐treated analysis reduced to n = 558/705 at 3 months and n = 444/705 at 12 months Excluded participants who died (3 months: intervention 4.2% (13/310), usual care 6.6% (26/395); 12 months: intervention 18.1% (56/375), usual care 18.7% (74/375); moved into residential care or out of the area (3 months: intervention 4.5% (14/310), usual care 6% (24/395); 12 months: intervention 11.6% (36/310), usual care 14.2% (56/395); receiving hospice care (3 months: intervention 2.9% (9/310), usual care 0% (0/395); 12 months: intervention 0.96% (3/310), usual care 0.5% (2/395); declined/terminated follow‐up (3 months: intervention 3.9% (12/310); usual care 2.3% (9/395); 12 months: intervention 1.3% (4/310), usual care 1% (4/395); and participants who had missing data for any variable (n not available) The incomplete data for service outcomes appeared to be comparable across the 2 groups, apart from the 30 from the intervention group who declined follow‐up at 3 months (8% (30/375) with intervention versus 2.4% (9/375) with usual care). There is also a lack of clarity with regard to the strategy used to manage missing data in the ITT analysis and we have therefore judged this at high risk Subgroup only Participants recruited to subgroups: n = 150 intervention and n = 150 control Quote: "Both intention to treat (ITT) and as‐treated analysis was performed" p. 73 "only those for whom data was available at initial collection, 3 and 12 months were included in the analysis" p. 76 Unpublished data received from trial authors showed that the ITT analysis included: IADL and ADL: intervention 66.67% (100/150) at 3 and 12 months; usual care 65.34% (98/150) at 3 and 12 months; and AQOL: intervention 66.67% (100/150) at 3 months and 12 months; usual care 65.34% (98/150) at 3 months and 64.67% (97/150) at 12 months Attrition rates and reasons given (p.73):
Quote from authors email communication: "The full subgroup for the ITT at the completion of 12 months was 198 (98 HACC, 100 HIP)... these are the numbers for most outcomes but there are a couple that are slightly lower due to missing data (e.g. because there was a few individuals that might not have completed all three time point measures so there is some missing data and 3 people did not complete the score at the 3 month time point as were in hospital or uncontactable. Those individuals did complete the other measures though and finished the study so were included in the final analysis" Comment: missing outcome data does seem relatively comparable across the 2 subgroups; but it remains unclear whether the ITT analysis made appropriate adjustments to account for missing data, therefore we judged this at high risk of bias As‐treated analysis reported: IADL and ADL: intervention 58.67% (88/150) at 3 and 12 months; usual care 69.34% (104/150) at 3 and 12 months; and AQOL: intervention 58.67% (88/150) at 3 months and 12 months; usual care 69.34% (104/150) at 3 months and 68.67% (103/150) at 12 months Attrition rates and reasons given (Figure 1, p. 73):
Comment: missing outcome data did seem relatively comparable across the 2 subgroups, but as the functional outcomes were only reported for 25.6% (192/750) in the as‐treated and 26.4% (198/750) in the ITT analysis of the original sample, we judged this at high risk of bias |
| Selective reporting (reporting bias) | High risk | Results were not fully reported for functional and QoL outcomes but it was stated that "Some improvement on all measures was shown by both groups during the first 3 months... maintained by both groups over the next 9 months" (p. 76) Comment: there seems to be some level of selective reporting, and this is therefore considered at high risk of bias |
| Other bias | High risk | Baseline differences: the intervention group was less likely to have a carer and more likely to live alone. There were also differences (judged by the study authors to be clinically insignificant) in IADL and ADL (pp. 74‐5) 2 methodological issues may have introduced bias and affected the size of any effects:
|
Tuntland 2015.
| Methods | Randomised controlled superiority trial | |
| Participants | Home‐dwelling people aged ≥ 18 years living in a rural municipality in Norway:
RCT inclusion criteria: ≥ 18 years; functional decline in ≥ daily activities; understand Norwegian Excluded if: "in need of institution‐based rehabilitation or a nursing home placement, terminally ill, or were cognitively reduced" Sample: 61 participants randomised to intervention or usual care group. The sample comprised 67.2% females (n = 41); mean age 79 (SD 10.1) years Information related to living situation and carer availability was not reported in the article Recruited: May 2012 to February 2014 |
|
| Interventions | Intervention and usual care delivered in client's own home Intervention: time‐limited (maximum 3 months). Intervention tailored to the needs and goals of the individual participant, and consisted of both general and individual features. An occupational therapist and physiotherapist used a standardised measure (CMOP‐E) to identify activity limitations that were perceived as important for participant. This information used to develop a rehabilitation plan. The therapists supervised the home‐care personnel; the focus was on encouraging the participants to perform daily activities themselves. The intervention included "training in daily activities, adaptations to the environment or the activity, and exercise programs" Mean intervention duration 10 weeks, with a mean of 7 home visits per week (mean 2.1 hours, based on a 12‐week period) Usual care: standard care/treatment offered to homebound participants: personal or practical assistance, meals on wheels, safety alarm and assistive technology; with or without occupational therapist/physiotherapist support. Not time‐limited and could continue after 3 months if needed Mean 6 visits per week lasting 1.7 hours on average |
|
| Outcomes | Assessed on 3 occasions: baseline, 3 months and 9 months Primary outcome:
Secondary outcomes:
Mortality was also reported for the 3‐ and 9‐month follow‐up periods |
|
| Sample Size | 81 participants assessed for eligibility; 6 did not meet the inclusion criteria and 2 died before consenting; 73 were invited to take part and 12 declined Final sample of 61 were randomised to either intervention or usual care. A sample size calculation was based on an earlier study that indicated that 42 participants were needed to detect a statistically significant change of 2 points for COPM (with a 2‐sided 5% level and power of 80%). The authors anticipated a high drop‐out rate due to the frailty of the participants and decided to recruit 60 participants (30 in each group) |
|
| Notes | Proportion of participants aged ≤ 64 years was 8.2% (email correspondence) The participants in the usual care group accessed a significantly higher amount of co‐interventions in terms of outpatient physiotherapy during the first 3 months compared to the reablement group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes: "We performed a parallel‐group randomised controlled superiority trial in which all participants were assessed at baseline, and after 3 and 9 months" (p. 3) "The randomisation with an allocation ratio of 1:1 using a computer‐generated permuted block randomisation sequence, with randomly selected block sizes of lengths 2 and 4, was performed by a biostatistician not involved in the assignment of participants to groups" (p. 3) Comment: randomisation appeared to have been conducted in an appropriate manner |
| Allocation concealment (selection bias) | Low risk | Quote: "We concealed the allocation sequence in sequentially numbered, opaque, sealed envelopes. The allocation list was stored in a safe deposit box in a central office in the municipality. Neither health‐care providers enrolling participants nor research assistants had influence on group allocation" (p. 3) Comment: low risk |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: it was not possible to blind therapists or participants to allocated condition |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quotes: "The participants were urged not to reveal their group allocation to the research assistants during follow‐up assessments. The success of the research assistants' blinding was recorded. Researchers conducting data entry and data analysis were blinded to group allocation" (p. 3) "The blinding of research assistants at follow‐ups was not completely successful" (p. 11) "Blinding of research assistants had a success rate of 63% at the 3‐month and 64% at the 9‐month follow‐up" (p. 6) Comment: despite best efforts, blinding was not successful in 36‐37% of cases. Insufficient blinding may be less serious for self report measures but more serious for the observer‐reported measures, e.g. the TUG test and the Jamar Dynamometer hydraulic instrument. Therefore, blinding was at high risk |
| Incomplete outcome data (attrition bias) | Low risk | Quotes: "Sixty‐one participants were randomised to reablement (n = 31) or to usual care (n = 30). Due to continuous monitoring of missing data during the trial period, there were few missing outcomes data. The dropout rate was 11% and 16% at the 3‐month and 9‐months follow‐ups respectively, and was mainly due to deaths among participants" (p. 5) "All participants were analysed according to initial group allocation (intention‐to‐treat)" (p. 5) Missing data at 3‐month follow‐up: 9.7% (3/31) in intervention group and 13.3% (4/30) in usual care group. Reasons for missing data included death, withdrawal and loss to follow‐up (p. 6 flowchart) and were balanced across groups Missing data at 9‐month follow‐up: 19.4% (6/31) in intervention group and 13.3% (4/30) in usual care group. Reasons for extra missing data in intervention group included death and loss to follow‐up (p. 6 flowchart). % attrition balanced across groups Comment: attrition low in both groups at 3‐ and 9‐month follow‐ups and was due to death and loss to follow‐up. Reasons for missing data in intervention group were unlikely to be due to adverse effects of the intervention. The study authors did not impute values for the missing data but since attrition was low, it is unlikely that missing data would unduly affect the effect size |
| Selective reporting (reporting bias) | Low risk | Comment: there does not appear to be any evidence of selective reporting. Outcomes specified in the study protocol were reported on |
| Other bias | High risk | Quotes: "Further, all co‐interventions were not equally distributed between the groups. Treatment fidelity, i.e. if the treatment was delivered as intended, was not adequately monitored. Consequently, we do not know whether assistants delivered the intervention as intended. Moreover, the compliance to the interventions was not systematically recorded, and there was a possibility of contamination from one arm of the study to the other" (p. 11) "The improvements in the control group may also have been caused by contamination from the intervention arm of the study to the control arm. Due to problems with recruitment in a sparsely inhabited municipality, the intervention was implemented in all home‐care districts in the municipality. Thus, it was not possible to avoid the situation where the same health‐care personnel provided both the experimental and control interventions, however to different participants" (p. 7) "Also, the significantly higher amount of co‐interventions in terms of outpatient physiotherapy received by participants in the control group during the first 3 months might have had an impact" (p. 7) Comment: there were a number of areas of possible bias in the study. First, given unequal co‐interventions within the groups, it was possible that the benefits achieved in the usual care group were due to the extra co‐interventions they received. Second, therapist and participant adherence to the intervention protocol was not monitored so it was not clear whether the reablement intervention led to the changes. Third, there was a high risk of contamination between the groups due to the same healthcare personnel providing the intervention and control treatments; therefore, there was a risk that the usual care group may also have received elements of the reablement intervention. We rated this as high risk |
ADL: activities of daily living; AQoL: Assessment of Quality of Life Scale; CMOP‐E: Canadian Model of Occupational Performance and Engagement; HACC: home and community care; HIP: Home Independence Program; IADL: instrumental activities of daily living; ITT: intention to treat; n: number of participants; QoL: quality of life; RA: research assistant; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avlund 2002 | Intervention was visits at home by a member of the geriatric team and focused on medical care; both intervention and control groups received the same home service |
| Crawford Shearer 2010 | Did not measure the primary outcome for this review ‐ functional status |
| Crotty 2008 | Home‐based intervention compared to rehabilitation in day hospital rather than usual home care |
| Cunliffe 2004 | Intervention designed to promote early hospital discharge and offered intensive support for up to 4 weeks only; control group received usual hospital care until fit for home and rehabilitation in outpatients or day hospital |
| Friedman 2014 | Intervention focused on disease management and health promotion rather than reablement; did not meet criteria for duration (long‐term) or intensity (once per month) |
| Gill 2002 | Did not meet the criteria for duration ‐ intervention lasted 6 months; control group underwent an educational programme rather than usual home care |
| Gitlin 2006a | Did not meet the criteria for duration ‐ Intervention lasted 6 months, and not intensive (5 home visits and 1 telephone call) |
| Gitlin 2006b | Reports on same trial as Gitlin 2006a; Intervention lasted 6 months, and not intensive (5 home visits and 1 telephone call) |
| Glendinning 2010 | Not randomised; before‐and‐after study; comparison group control from sites that did not offer reablement service |
| Heebøll 2012 | Emailed 6 November 2014: before‐and‐after studies only |
| Kent 2000 | Not randomised; measured secondary outcomes only |
| King 2012 | Intervention not time‐limited and lasted > 12 weeks |
| Le Mesurier 1999 | Not randomised |
| Lewin 2010 | Non‐randomised cluster study |
| Li 2013 | Same study as Friedman 2014; intervention focused on disease management and health promotion rather than reablement; did not meet criteria for duration (long‐term) or intensity (once per month) |
| Martin 1994 | Intervention facilitated discharge from hospital, and supported recovery at home. "Tasks performed [as the intervention] included personal care and domestic assistance" (p. 229), did not appear to be reablement, rather more intensive "conventional community services" |
| McLeod 2009 | Not randomised, "matched control group in another area receiving the traditional service" |
| Melis 2008 | Intervention nurse led and focused on medical problems. Control group 'usual' care from primary care physician |
| Newbronner 2007 | No control group |
| Nikolaus 1999 | Part of the intervention took place while still in hospital; mean duration of postdischarge follow‐up treatment was 7.6 days only |
| Parsons 2012 | Intervention (site A) was not time‐limited email from author 9 December 2014: "services were continuous until death or entry into residential care" |
| Parsons 2013 | Intervention not time‐limited and lasted > 12 weeks |
| Senior 2014 | Intervention included some time spent in residential setting |
| Sheffield 2013 | Occupational therapist intervention only; did not meet criteria for intensity with mean of 4 visits only (author's dissertation p. 145 mean 3.6 (± 1.32); some participants received intervention plus usual care |
| Szanton 2011 | Did not meet criteria for duration or intensity (10 in‐home sessions over 6 months); control group received an attention and education intervention (reminiscence and sedentary activities) rather than usual home‐care service |
| Tinetti 2002 | Not randomised at either cluster or individual level; matched pairs |
| Tinetti 2012 | Not randomised at either cluster or individual level; matched pairs |
| Winkel 2015 | Not randomised at either cluster or individual level |
Characteristics of ongoing studies [ordered by study ID]
Grimmer 2013.
| Trial name or title | TRialing Individualised Interventions to Prevent Functional DecLine in At Risk Older Adults (TRIIFL) |
| Methods | RCT |
| Participants | Older adults aged ≥ 65 years presenting to emergency department with non‐catastrophic health conditions that do not results in admission to hospital for further care Exclusions:
|
| Interventions |
Intervention: aims to slow functional decline, individualised person‐centred community based and goal directed. Provided during a 3‐ to 14‐week period depending on need Control: usual care equating to no intervention |
| Outcomes | Measured at 1, 4, 7 and 13 months after recruitment:
|
| Starting date | Awaiting funding |
| Contact information | julie.luker@unisa.edu.au |
| Notes | Contacted via email 14 August 2014. Quote: "Sadly we have not been able to start on this TRIIFL project as we were unsuccessful in securing a grant to fund it this year. We plan to reapply for funding in future grant rounds" |
Langeland 2015.
| Trial name or title | Multicenter investigation of reablement in Norway |
| Methods | Clinical controlled trial |
| Participants |
Sample size: proposed 107 control group and 400 intervention group from primary care centres in 44 municipalities Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: time‐limited (3 to 10 weeks) intensive, multidisciplinary intervention, including occupational therapists, physiotherapists, nurses and social educators. Rehabilitation plan developed following interview using the COPM to identify and target individualised goals for improvement. Intervention will include daily training, including performance of individual tasks, to build confidence and re‐learn skills Control: standard treatment that usually includes personal and practical assistance, meals on wheels, safety alarm or assistive technology. May also involve rehabilitation by health professionals such as nurses, occupational therapists and physiotherapists. Not time‐limited and may continue after 3 months if needed |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 2014 |
| Contact information | eva.langeland@hib.no |
| Notes | Proportion of participants aged ≤ 64 years is approximately 9.56% (email correspondence) |
Whitehead 2014.
| Trial name or title | Occupational Therapy in HomEcare Re‐ablement Services (OTHERS) |
| Methods | Feasibility RCT |
| Participants |
Sample size: 50 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: usual 6‐week reablement home care plus enhanced programme delivered by an occupational therapist targeting ADLs Control: usual 6‐week reablement home care |
| Outcomes | Feasibility of conducting a larger trial Participant outcomes:
Cost evaluation |
| Starting date | April 2014 |
| Contact information | philip.j.whitehead@nottingham.ac.uk |
| Notes | Recruitment closed December 2014; results expected to be published September 2016 |
ADL: activities of daily living; COPM: Canadian Occupational Performance Measure; GP: general practitioner; IADL: instrumental activities of daily living; RCT: randomised controlled trial.
Differences between protocol and review
Reablement is now a commonly used term in services and services research and we have therefore changed the term 're‐ablement' as written in the protocol to 'reablement' for the review. The focus on time‐limited reablement services is also now reflected in the title for purposes of clarity; and we have specified the cut‐off at 12 weeks under Types of interventions. We also included in our criteria that the service should be delivered by an interdisciplinary team as this is an important component of the reablement model.
We did not search SCOPUS or the Campbell Collaboration's Social, Psychological, Educational and Criminological Trials Register as planned due to difficulties in access.
Contributions of authors
AC developed the search strategy for this review in conjunction with John Kis‐Rigo, the Information Specialist with Cochrane Consumers and Communication.
AC and MF independently assessed study eligibility and risk of bias, and conducted data extraction.
AC entered data into Review Manager 5 and conducted analyses, which were checked for accuracy by MF.
AC and MF wrote the initial draft of the review and members of the review team contributed to subsequent revisions.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland.
A 2‐year Cochrane Training Fellowship to Andy Cochrane, from November 2012 to November 2014
Declarations of interest
AC: this review was conducted with the support of a Cochrane Training Fellowship (Health Research Board, Ireland) to the first author.
MF: worked as a consultant researcher with the Social Research and Evaluation Services (SocRES) in 2012 and 2013. SocRES has been commissioned by Atlantic Philanthropies to evaluate their Ageing Programme in the Republic of Ireland. It is not expected that her involvement with SocRES should adversely bias the completion of the review.
SMcG: none known.
DWM: none known.
MS: other than 140 shares in AstraZeneca, none known.
MD: none known.
New
References
References to studies included in this review
Lewin 2013 {published and unpublished data}
- Lewin G, San Miguel K, Knulman M, Alan J, Boldy D, Hendrie D, et al. A randomised controlled trial of the Home Independence Program, an Australian restorative home‐care programme for older adults. Health & Social Care in the Community 2013;21(1):69‐78. [DOI] [PubMed] [Google Scholar]
Tuntland 2015 {published and unpublished data}
- Tuntland H, Aaslund MK, Espehaug B, Førland O, Kjeken I. Reablement in community‐dwelling older adults: a randomised controlled trial. BMC Geriatrics 2015;15:145. [DOI: 10.1186/s12877-015-0142-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Avlund 2002 {published data only}
- Avlund K, Jepsen E, Vass M, Lundemark H. Effects of comprehensive follow‐up home visits after hospitalisation on functional ability and readmissions among older patients. A randomised controlled study. Scandinavian Journal of Occupational Therapy 2002;9(1):17‐22. [Google Scholar]
Crawford Shearer 2010 {published data only}
- Crawford Shearer NB, Fleury JD, Belyea M. Randomised control trial of the Health Empowerment Intervention: feasibility and impact. Nursing Research 2010;59(3):203‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crotty 2008 {published data only}
- Crotty M, Giles LC, Halbert J, Harding J, Miller M. Home versus day rehabilitation: a randomised controlled trial. Age and Ageing 2008;37(6):628‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunliffe 2004 {published data only}
- Cunliffe AL, Gladman JRF, Husbands SL, Miller P, Dewey ME, Harwood RH. Sooner and healthier: a randomised controlled trial and interview study of an early discharge rehabilitation service for older people. Age and Ageing 2004;33(3):246‐52. [DOI] [PubMed] [Google Scholar]
Friedman 2014 {published data only}
- Friedman B, Li Y, Liebel DV, Poweres, BA. Effects of a home visiting nurse intervention versus care as usual on individual activities of daily living: a secondary analysis of a randomised controlled trial. BMC Geriatrics 2014;14:24. [DOI: 10.1186/1471-2318-14-24] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 2002 {published data only}
- Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail elderly persons who live at home. New England Journal of Medicine 2002;347(14):1068‐74. [DOI] [PubMed] [Google Scholar]
Gitlin 2006a {published data only}
- Gitlin LN, Winter L, Dennis MP, Corcoran M, Schinfeld S, Hauck WW. A randomised trial of a multicomponent home intervention to reduce functional difficulties in older adults. Journal of the American Geriatrics Society 2006;54(5):809‐16. [DOI] [PubMed] [Google Scholar]
Gitlin 2006b {published data only}
- Gitlin LN, Hauck WW, Winter L, Dennis MP, Schulz R. Effect of an in‐home occupational and physical therapy intervention on reducing mortality in functionally vulnerable older people: preliminary findings. Journal of the American Geriatric Society 2006;54(6):950‐5. [DOI] [PubMed] [Google Scholar]
Glendinning 2010 {published data only}
- Glendinning C, Jones K, Baxter K, Rabiee P, Curtis LA, Wilde A, et al. Home care re‐ablement services: investigating the longer‐term impacts (prospective longitudinal study). Social Policy Unit, University of York 2010.
Heebøll 2012 {published data only}
- Heebøll K, Hansen M, Dalgaard AM. Life long living ‐ maintaining independent living as long as possible, 2012. www.fredericia.dk/LMIEL/Sider/About‐the‐project.aspx (accessed 5 September 2016).
Kent 2000 {published data only}
- Kent J, Payne C, Stewart M, Unell J. Leicester County Council. External evaluation of the home care reablement pilot, 2000. www.scie‐socialcareonline.org.uk/leicestershire‐county‐council‐external‐evaluation‐of‐the‐home‐care‐reablement‐pilot‐project/r/a11G00000017zziIAA (accessed 5 September 2016).
King 2012 {published data only}
- King AI, Parsons M, Robinson E, Jörgensen D. Assessing the impact of a restorative home care service in New Zealand: a cluster randomised trial. Health & Social Care in the Community 2012;20(4):365‐74. [DOI] [PubMed] [Google Scholar]
Le Mesurier 1999 {published data only}
- Mesurier N, Cumella S. Enhancing independence: the effectiveness of re‐ablement provision in South Worcestershire. Managing Community Care 1999;7(4):27‐32. [Google Scholar]
Lewin 2010 {published data only}
- Lewin G, Vandermeulen S. A non‐randomised controlled trial of the Home Independence Program (HIP): an Australian restorative programme for older home‐care clients. Health & Social Care in the Community 2010;18(1):91‐9. [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li Y, Liebel DV, Friedman B. An investigation into which individual instrumental activities of daily living are affected by a home visiting nurse intervention. Age and Ageing 2013;42(1):27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1994 {published data only}
- Martin F, Oyewole A, Moloney A. A randomised controlled trial of a high support hospital discharge team for elderly people. Age and Ageing 1994;23(3):228‐34. [DOI] [PubMed] [Google Scholar]
McLeod 2009 {published data only}
- McLeod B, Mair M. Evaluation of City of Edinburgh Council Home Care Re‐ablement Service, 2009. www.gov.scot/Publications/2009/11/25100200/ (accessed 5 September 2016).
Melis 2008 {published data only}
- Melis RJF, Eijken MIJ, Teerenstra S, Achterberg T, Parker SG, Borm GF, et al. A randomised study of a multidisciplinary program to intervene on geriatric syndromes in vulnerable older people who live at home (Dutch EASYcare Study). Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2008;63(3):283‐90. [DOI] [PubMed] [Google Scholar]
Newbronner 2007 {published data only}
- Newbronner E, Baxter M, Chamberlain R, Maddison J, Arksey H, Glendinning C. Research into the longer term effects/impacts of reablement services, 2007. www.york.ac.uk/inst/spru/pubs/pdf/reab.pdf (accessed 5 September 2016).
Nikolaus 1999 {published data only}
- Nikolaus T, Specht‐Leible N, Bach M, Oster P, Schlierf G. A randomized trial of comprehensive geriatric assessment and home intervention in the care of hospitalized patients. Age and Ageing 1999;28(6):543‐50. [DOI] [PubMed] [Google Scholar]
Parsons 2012 {published and unpublished data}
- Parsons M, Senior HEJ, Kerse N, Chen M‐H, Jacobs S, Vanderhoorn S, et al. The assessment of service promoting independence and recovery in elders trial (ASPIRE): a pre‐planned meta‐analysis of three independent randomised controlled trial evaluations of ageing in place initiatives in New Zealand. Age and Ageing 2012;41:722‐8. [DOI] [PubMed] [Google Scholar]
Parsons 2013 {published data only (unpublished sought but not used)}
- Parsons JGM, Sheridan N, Rouse P, Robinson E, Connolly M. A randomized controlled trial to determine the effect of a model of restorative home care on physical function and social support among older people. Archives of Physical Medicine and Rehabilitation 2013;94(6):1015‐22. [DOI] [PubMed] [Google Scholar]
Senior 2014 {published data only (unpublished sought but not used)}
- Senior HEJ, Parsons M, Kerse N, Chen M‐H, Jacobs S, Vander Hoorn S, et al. Promoting independence in frail older people: a randomised controlled trial of a restorative care service in New Zealand. Age and Ageing 2014;43(3):418‐24. [DOI] [PubMed] [Google Scholar]
Sheffield 2013 {published data only}
- Sheffield C, Smith CA, Becker M. Evaluation of an agency‐based occupational therapy intervention to facilitate aging in place. Gerontologist 2013;53(6):907‐18. [DOI] [PubMed] [Google Scholar]
Szanton 2011 {published data only}
- Szanton SL, Thorpe RJ, Boyd C, Tanner EK, Leff B, Agree E, et al. Community aging in place, advancing better living for elders: a biobehavioural environmental intervention to improve function and health‐related quality of life in disabled older adults. Journal of the American Geriatrics Society 2011;59(12):2314‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinetti 2002 {published data only}
- Tinetti ME, Baker D, Gallo WT, Nanda A, Charpentier P, O'Leary J. Evaluation of restorative care vs usual care for older adults receiving an acute episode of home care. Journal of the American Medical Association 2002;287(16):2098‐105. [DOI] [PubMed] [Google Scholar]
Tinetti 2012 {published data only}
- Tinetti ME, Charpentier P, Gottschal M, Baker DI. Effect of a restorative model of posthospital home care on hospital readmissions. Journal of the American Geriatrics Society 2012;60(8):1521‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkel 2015 {published data only}
- Winkel A, Langberg H, Wǽhrens EE. Reablement in a community setting. Disability and Rehabilitation 2015;37(15):1347‐52. [DOI: 10.3109/09638288.2014.963707] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Grimmer 2013 {published data only}
- Grimmer K, Luker J, Beaton K, Kumar S, Crockett A, Price K. TRialing individualised interventions to prevent functional decline in at‐risk older adults (TRIIFL): study protocol for a randomised controlled trial nested in a longitudinal observational study. Trials 2013;14:266. [http://www.trialsjournal.com/content/14/1/266] [DOI] [PMC free article] [PubMed] [Google Scholar]
Langeland 2015 {published data only}
- Langeland E, Huntland H, Førland O, Aaa E, Folkestad FF, Kjeken I. Study protocol for a multicenter investigation of reablement in Norway. BMC Geriatrics 2015;15:111. [DOI: 10.1186/s12877-015-0108-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitehead 2014 {published data only}
- Whitehead PJ, Drummond AER, Walker MF, Parry RH, McGeorge ID, Latif Z. Occupational Therapy in HomEcare Re‐ablement Services (OTHERS): study protocol for a randomized controlled trial. Trials 2014;15:447. [DOI: 10.1186/1745-6215-15-447] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Audit Commission 2000
- Audit Commission (UK). The way to go home: rehabilitation and remedial services for older people, 2000. www.scie‐socialcareonline.org.uk/the‐way‐to‐go‐home‐rehabilitation‐and‐remedial‐services‐for‐older‐people/r/a11G000000180UwIAI (accessed 5 September 2016).
Beswick 2008
- Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman‐Hill R, Horwood J, et al. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta‐analysis. Lancet 2008;371(1):725‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodsky 2003
- Brodsky J, Habib J, Hirschfeld MJ (editors). Key policy issues on long‐term care. WHO report, 2003. www.who.int/chp/knowledge/publications/policy_issues_ltc/en/ (accessed 5 September 2016).
Cartwright 2009
- Cartwright C. Re‐ablement of older people in North Coast NSW: report to NSW Department of Ageing Disability and Home Care (DADHC) July 2009. trove.nla.gov.au/work/38091556?selectedversion=NBD46072276 2009.
CCCG 2013
- Cochrane Consumers and Communication Group. Standard review text and additional guidance for review authors, 2013. cccrg.cochrane.org (accessed 5 September 2016).
Chan 2011
- Chan RJ, Webster J, Marquart L. Information interventions for orienting patients and their carers to cancer care facilities. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008273.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2001
- Clark J. Preventive home visits to elderly people. Their effectiveness cannot be judged by randomised trials. BMJ 2001;323(7315):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crotty 2010
- Crotty M, Unroe K, Cameron ID. Rehabilitation interventions for improving physical and psychosocial functioning after hip fracture in older people. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007624.pub3] [DOI] [PubMed] [Google Scholar]
CSED 2007
- Care Services Efficiency Delivery (CSED) Programme. Homecare Re‐ablement Workstream (discussion document HRA 002). London: Department of Health, 2007. [Google Scholar]
Cutchin 2009
- Cutchin MP, Coppolan S, Talley V, Svihula J, Catelier D, Shank KH. Feasibility and effects of preventative home visits for at‐risk older people: design of a randomised controlled trial. BMC Geriatrics 2009;9:54. [DOI: 10.1186/1471-2318/9/54] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgin JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Department of Health 2010
- Department of Health (UK). A vision for adult social care: Capable communities and active citizens, 2010. www.cpa.org.uk/cpa_documents/vision_for_social_care2010.pdf (accessed 5 September 2016).
Drummond 1996
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ, The BMJ Economic Evaluation Working Party. BMJ 1996;313:275‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Francis 2011
- Francis J, Fisher M, Rutter D. SCIE briefing 36. Reablement: a cost‐effective route to better outcomes, 2011. www.scie.org.uk/publications/briefings/briefing36/ (accessed 5 September 2016).
Hawthorne 1999
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hills 1996
- Hill KD, Schwarz JA, Kalogeropoulos AJ, Gibson SJ. Fear of falling revisited. Archives of Physical Medicine and Rehabilitation 1996;77:1025‐9. [DOI] [PubMed] [Google Scholar]
Huss 2008
- Huss A, Stuck AE, Rubenstein LZ, Egger M, Clough‐Gorr KM. Multidimensional preventative home visit programs for community‐dwelling older adults: a systematic review and meta‐analysis of randomised controlled trials. Journal of Gerontology 2008;53A(3):298‐307. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones KC, Curtis LA, Arksey H, Forder JE, Glendinning C, Rabiee P. Investigating the longer term impact of home care re‐ablement services: the short‐term outcomes and costs of home care re‐ablement services, interim report, 2009. www.york.ac.uk/inst/spru/research/pdf/ReablementOutcomes.pdf (accessed 5 September 2016).
Kent 2000
- Kent J, Payne C, Stewart M, Unell J. Leicestershire County Council: External Evaluation of the Home Care Reablement Pilot Project, 2000. www.scie‐socialcareonline.org.uk/leicestershire‐county‐council‐external‐evaluation‐of‐the‐home‐care‐reablement‐pilot‐project/r/a11G00000017zziIAA (accessed 5 September 2016).
Legg 2016
Lewin 2014
- Lewin G, Allan J, Patterson C, Knulman M, Boldy D, Hendrie D. A comparison of the home‐care and healthcare service use and costs of older Australians randomised to receive a restorative or a conventional home‐care service. Health and Social Care in the Community 2014;22(3):328‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: exploration and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2002
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley & Sons, 2002. [Google Scholar]
Löfqvist 2012
- Löfqvist C, Eriksson S, Svensson T, Iwarsson S. First steps towards evidence‐based preventive home visits: experiences gathered in a Swedish municipality. Journal of Aging Research 2012;2012:352942. [DOI: 10.1155/2012/352942] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martins 2006
- Martins JO, Maisonneuve C. The drivers of public expenditure on health and long‐term care: an integrated approach, OECD Economic Studies no. 42, 2006. www.oecd.org/eco/public‐finance/40507566.pdf (accessed 5 September 2016).
Mishra 2016
- Mishra V, Barratt J. Reablement and older people. IFA Copenhagen 2016 Summit Final Report. Copenhagen: IFA and DaneAge, 2016.
Montgomery 2008
- Montgomery P, Mayo‐Wilson E, Dennis J. Personal assistance for older adults (65+) without dementia. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006855.pub2] [DOI] [PubMed] [Google Scholar]
Netten 2011
- Netten A, Beadle‐Brown J, Caiels J, Forder J, Malley J, Smith N, et al. ASCOT Adult Social Care Outcomes Toolkit: Main Guidance. Discussion Paper DP2716/3. Vol. v2.1, Canterbury: Personal Social Care Services Research Unit, University of Kent, 2011. [Google Scholar]
Ning 2013
- Ning Y, McAvay G, Chaudhry Sarwat I, Arnold AM, Allore, HG. Results differ by approaching distinctive multiple imputation approaches on the Longitudinal Cardiovascular Health Study data. Experimental Aging Research 2013;39(1):27‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pilkington 2011
- Pilkington G. The cost effectiveness of homecare re‐ablement: a discussion paper to explore the conclusions that can be drawn from the body of evidence, 2011. www.scie.org.uk/prevention‐library/getdetailedresultbyid?id=a11G000000181oxIAA (accessed 5 September 2016).
Podsiadlo 1991
- Podsiadlo D, Richardson S. The Timed "Up & Go": a test of basic functional mobility for frail elderly persons. Journal of the American Geriatric Society 1991;39(2):142‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rostgaard 2011
- Rostgaard T, Glendinning C, Gori C, Kröger T, Österle A, Szebehely M, et al. LIVINDHOME: Living independently at home. Reforms in home care in 9 European countries. Copenhagen: Danish National Centre for Social Research, 2011. [Google Scholar]
Ryan 2011
- Ryan R, Hill S, Prictor M, McKenzie J. Study Quality Guide, 2011. cccrg.cochrane.org/sites/cccrg.cochrane.org/files/uploads/StudyQualityGuide_May%202013.pdf (accessed 5 September 2016).
Ryburn 2009
- Ryburn B, Wells Y, Foreman P. Enabling independence: restorative approaches to home care provision for frail older adults. Health and Social Care in the Community 2009;17(3):225‐34. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shemilt 2011
- Shemilt I, Mugford M, Byford S, Drummond M, Eisenstein E, Knapp M, et al. Chapter 15: Incorporating economic evidence. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shepperd 2011
- Shepperd S, Doll H, Broad J, Gladman J, Iliffe S, Langhorne P, et al. Hospital at home early discharge. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD000356.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sims‐Gould 2015
Tessier 2016
- Tessier A, Beaulieu M‐D, McGinn CA. Effectiveness of reablement: a systematic review. Healthcare Policy 2016;11(4):49‐59. [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuntland 2014
- Tuntland H. Espehaug B, Forland O, Hole AD, Kjerstad E, Kjeken I. Reablement in community‐dwelling adults: study protocol for a randomised controlled trial. BMC Geriatrics 2014;14:139. [www.biomedcentral.com/1471‐2318/14/139] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2009
- Ward D, Drahota A, Gal D, Severs M, Dean TP. Care home versus hospital and own home environments for rehabilitation of older people. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD003164.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weel 1993
- Weel C. Functional status on primary care: COOP/Wonka charts. Disability Rehabilitation 1993;15(2):96‐101. [DOI] [PubMed] [Google Scholar]
Whitehead 2015
- Whitehead PJ, Worthington EJ, Parry RH, Walker MF, Drummond AER. Interventions to reduce dependency in personal activities of daily living in community dwelling adults who use homecare services: a systematic review. Clinical Rehabilitation 2015;29(11):1064‐76. [DOI: 10.1177/0269215514564894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiles 2012
- Wiles JL, Leibing A, Guberman MSW, Reeve J, Allen RES. The meaning of "ageing in place" to older adults. Gerontologist 2012;52(3):357‐66. [DOI] [PubMed] [Google Scholar]
Wittenberg 2004
- Wittenberg R, Comas‐Herrera A, Pickard L, Hancock R. Future demand for long‐term care in the UK, 2004. www.jrf.org.uk/file/35988/download?token=Nwf5e06h (accessed 5 September 2016).
Wood 2012
- Wood C, Salter J. The home cure 2012. www.demos.co.uk/files/Home_Cure_‐_web_1_.pdf?1340633545. London: Demos, (accessed 16th September 2016).
Xie 2012
- Xie C, Hughes J, Sutcliffe C, Chester H, Challis D. Promoting personalization in social care services for older people. Journal of Gerontological Social Work 2012;55(3):218‐32. [DOI] [PubMed] [Google Scholar]


