Abstract
Background
Cancer pain is an important and distressing symptom that tends to increase in frequency and intensity as the cancer advances. For people with advanced cancer, the prevalence of pain can be as high as 90%. It has been estimated that 30% to 50% of people with cancer categorise their pain as moderate to severe, with between 75% and 90% of people with cancer experiencing pain that they describe as having a major impact on their daily life. Epidemiological studies suggest that approximately 15% of people with cancer pain fail to experience acceptable pain relief with conventional management. Uncontrolled pain can lead to physical and psychological distress and can, consequently, have a drastic effect on people's quality of life.
Objectives
To determine the analgesic efficacy of hydromorphone in relieving cancer pain, as well as the incidence and severity of any adverse events.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase and clinical trials registers up to April 2016. There were no language, document type or publication status limitations applied in the search.
Selection criteria
We included randomised controlled trials (RCTs) that compared hydromorphone with placebo or other active pain medication for cancer pain in both adults and children. The four main outcomes selected have previously been identified as important to people with cancer; pain no worse than mild pain, and the impact of the treatment on consciousness, appetite and thirst. We did not consider physician‐, nurse‐ or carer‐reported measures of pain.
Data collection and analysis
Two review authors independently extracted data. For binary outcomes, we calculated risk ratio (RR) and its 95% confidence interval (CI), on an intention‐to‐treat basis. For continuous data, we estimated the mean difference (MD) between groups and its 95% CI. We used a random‐effects model and assessed the risk of bias for all included studies. A meta‐analysis was not completed on any of the primary outcomes in this review due to the lack of data. We assessed the evidence using GRADE and created two 'Summary of findings' tables.
Main results
We included four studies (604 adult participants), which compared hydromorphone to oxycodone (two studies) or morphine (two studies). Overall, the included studies were at low or unclear risk of bias, rated unclear due to unknown status of blinding of outcome assessment; we rated three studies at high risk of bias for potential conflict of interest. Data for 504 participants were available for analysis. We collected data on endpoint participant‐reported pain intensity measured with a visual analogue scale (VAS) (mean ± standard deviation (SD): hydromorphone 28.86 ± 17.08, n = 19; oxycodone 30.30 ± 25.33, n = 12; scale from 0 to 100 with higher score indicating worse pain), and Brief Pain Inventory (BPI) 24 hours worst pain subscale (mean ± SD: hydromorphone 3.5 ± 2.9, n = 99; morphine 4.3 ± 3.0, n = 101, scale from 0 to 10 with higher score indicating worse pain). The data demonstrated a similar effect between groups with both comparisons. The pain intensity data showed that participants in all four trials achieved no worse than mild pain. There were several adverse events: some were the expected opioid adverse effects such as nausea, constipation and vomiting; others were not typical opioid adverse effects (for example, decreased appetite, dizziness and pyrexia, as shown in Table 1 in the main review), but generally showed no difference between groups. There were three deaths in the morphine group during the trial period, considered to be due to disease progression and unrelated to the drug. Three trials had over 10% dropout, but the reason and proportion of dropout was balanced between groups. The overall quality of evidence was very low mainly due to high risk of bias, imprecision of effect estimates and publication bias. There were no data available for children or for several participant‐important outcomes, including participant‐reported pain relief and treatment impact on consciousness, appetite or thirst.
Authors' conclusions
This review indicated little difference between hydromorphone and other opioids in terms of analgesic efficacy. Data gathered in this review showed that hydromorphone had a similar effect on participant‐reported pain intensity as reported for oxycodone and morphine. Participants generally achieved no worse than mild pain after taking hydromorphone, which is comparable with the other drugs. It produced a consistent analgesic effect through the night and could be considered for use in people with cancer pain experiencing sleep disturbance. However, the overall quality of evidence was very low mainly due to risk of bias, imprecision of effect estimates and publication bias. This review only included four studies with limited sample size and a range of study designs. Data for some important outcomes, such as impact of the treatment on consciousness, appetite or thirst, were not available. Therefore, we were unable to demonstrate superiority or inferiority of hydromorphone in comparison with other analgesics for these outcomes. We recommend that further research with larger sample sizes and more comprehensive outcome data collection is required.
Keywords: Adult; Female; Humans; Male; Analgesics, Opioid; Analgesics, Opioid/adverse effects; Analgesics, Opioid/therapeutic use; Hydromorphone; Hydromorphone/adverse effects; Hydromorphone/therapeutic use; Morphine; Morphine/adverse effects; Morphine/therapeutic use; Neoplasms; Neoplasms/complications; Oxycodone; Oxycodone/adverse effects; Oxycodone/therapeutic use; Pain; Pain/drug therapy; Pain/etiology; Pain Measurement; Randomized Controlled Trials as Topic
Hydromorphone for the treatment of cancer pain
Background
Over 75% of people with cancer experience pain. Around 30% to 50% of these people have moderate to severe pain, which can have a negative impact on daily life. Cancer pain is a distressing symptom that tends to worsen as the disease progresses. Hydromorphone may help relieve these symptoms.
Cancer‐related pain is usually treated with medicines such as morphine, oxycodone, fentanyl or hydromorphone. This review looked at how effective hydromorphone was in relieving symptoms of moderate to severe cancer pain.
Results
In April 2016, we searched for clinical trials looking at hydromorphone in people with moderate to severe cancer pain. We found four small, but well conducted, studies with 604 adults (none of the studies included children) comparing hydromorphone with oxycodone or morphine.
Based on very low quality evidence, we found no differences between the treatment groups relating to pain intensity and most people had good pain relief. Hydromorphone seemed to work as well as morphine and oxycodone. There were some side effects, such as confusion, constipation and diarrhoea, but generally there was no difference between people taking hydromorphone and people taking morphine or oxycodone.
Conclusions
The studies did not provide enough high quality evidence to draw conclusions from; however, based on very low quality evidence, hydromorphone seemed to work as well as morphine and oxycodone and had similar side effects. Hydromorphone provided consistent pain relief through the night and could be considered for use in people with cancer who find it difficult to sleep due to the pain.
Summary of findings
Summary of findings for the main comparison.
Hydromorphone compared to oxycodone for cancer pain
| Hydromorphone compared to oxycodone for cancer pain | ||||||
|
Patient or population: people with cancer pain Setting: unclear if these are inpatients or community patients Intervention: hydromorphone Comparison: oxycodone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oxycodone | Hydromorphone | |||||
| Participant‐reported pain intensity |
Pain intensity scores: 1 study (n = 31) using VAS (0 to 100, higher = worse outcome): mean (± SD) endpoint score for hydromorphone 28.86 ± 17.08 (n = 19); oxycodone 30.30 ± 25.33 (n = 12). SD of the oxycodone group was much more widespread than for the hydromorphone group. 1 study (n = 81) using BPI (0 to 10; higher = worse outcome): mean change score of 'pain at its worst in the past 24 hours' for hydromorphone ‐1.8 ± 3.29 (n = 40); oxycodone ‐1.7 ± 3.91 (n = 41). No worse than mild pain: 1 study (n = 31) using 5‐point categorical pain intensity scale (0 to 4; higher = worse outcome): mean (± SD) score across all days for hydromorphone 1.5 ± 0.1; oxycodone 1.4 ± 0.1. Both groups achieved no worse than mild pain. 1 study (n = 81) using BPI (0 to 10; higher = worse outcome): 'average pain in the past 24 hours' for hydromorphone 2.9; oxycodone 3.3, SD not reported. Both groups achieved no worse than mild pain (defined as score of ≤ 4). |
⊕⊝⊝⊝ Very low1,2,3 | For pain intensity, the results were similar in hydromorphone and oxycodone groups, although data were skewed. Both oxycodone and hydromorphone groups had mean pain levels of 'no worse than mild pain'. |
|||
| Adverse events ‐ nausea Follow‐up: 28 days | See comment | See comment | Not estimable | 254 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Adverse events ‐ constipation Follow‐up: 28 days | See comment | See comment | Not estimable | 254 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Adverse events ‐ vomiting Follow‐up: 28 days | See comment | See comment | Not estimable | 254 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Leaving the study early Follow‐up: 14‐28 days | Study population | RR 0.61 (0.2 to 1.87) | 304 (2 studies) | ⊕⊝⊝⊝ Very low1,2,4 | — | |
| 480 per 1000 | 293 per 1000 (96 to 898) | |||||
| Moderate | ||||||
| 470 per 1000 | 287 per 1000 (94 to 879) | |||||
| Death Follow‐up: 28 days | See comment | See comment | Not estimable | 260 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | We are uncertain if there is any difference between interventions. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPI: Brief Pain Inventory; CI: confidence interval; RR: risk ratio; SD: standard deviation; VAS: visual analogue scale | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded once: risk of bias: sample size of < 200 per treatment arm. 2 Downgraded once: imprecision: sample size was smaller than optimal information size; CI around estimate of effect was wide and included no effect and appreciable benefit/harm. 3 Downgraded once: publication bias: only 1 small trial was identified for this comparison, thus publication bias was highly suspected. 4 Downgraded once: inconsistency: unexplained heterogeneity was high between included studies.
Summary of findings 2.
Hydromorphone compared to morphine for cancer pain
| Hydromorphone compared to morphine for cancer pain | ||||||
|
Patient or population: people with cancer pain Setting: inpatients, outpatients and day patients Intervention: hydromorphone Comparison: morphine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Morphine | Hydromorphone | |||||
| Participant‐reported pain intensity |
Pain intensity scores: 1 study (n = 200) using subscale data derived from BPI scale (0 to 10; higher = worse outcome): mean (± SD) endpoint score for 'worst pain' hydromorphone 3.5 ± 2.9 (n = 99); morphine 4.3 ± 3.0 (n = 101) Mean scores on 'least pain' and 'average pain' were almost identical. No worse than mild pain: 1 study (n = 200) using subscale data derived from BPI scale (0 to 10; higher = worse outcome): the 'average pain' subscale data showed that both groups achieved no worse than mild pain. |
⊕⊝⊝⊝ Very low1,2,3 | Higher mean endpoint scores for 'worst pain' in morphine group. Similar scores for 'average' and 'least' pain in hydromorphone and morphine groups. Both morphine and hydromorphone groups had mean pain levels of 'no worse than mild pain'. |
|||
| Adverse events ‐ confusion Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Adverse events ‐ headache Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Adverse events ‐ insomnia Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Adverse events ‐ nausea Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Adverse events ‐ pyrexia Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Death Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| Leaving the study early ‐ overall Follow‐up: 24 days | See comment | See comment | Not estimable | 200 (1 study) | ⊕⊝⊝⊝ Very low1,2,3 | We are uncertain if there is any difference between interventions. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPI: Brief Pain Inventory; CI: confidence interval; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded once: risk of bias: sample size < 200 per treatment arm. 2 Downgraded once: imprecision: sample size was smaller than optimal information size; CI around estimate of effect was wide and included no effect and appreciable benefit/harm. 3 Downgraded once: publication bias: only 1 small trial was identified for this comparison, thus publication bias was highly suspected.
Background
This review updates and replaces the previously published 'Hydromorphone for acute and chronic pain' review which was withdrawn as the original author team were unavailable to update the review (Quigley 2013). The scope of the current review is limited to cancer pain.
Description of the condition
Pain is defined as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage" (IASP 2011).
Cancer pain is an important and distressing symptom of the disease, which tends to increase in frequency and intensity as the cancer advances. For people with cancer, pain can arise from the progression of the cancer itself as well as from treatments designed to alleviate the condition such as radiotherapy, chemotherapy and surgery. Cancer‐related pain can be classified as acute or chronic, though it is sometimes thought to be an ongoing acute pain. Acute pain is defined as having "a temporal pattern of onset... generally associated with subjective and objective physical signs" (Meier 2010), whereas chronic pain is more continuous, lasting six months or longer (Meier 2010).
One previous systematic review indicated the prevalence of pain to be more than 50% in all cancer types (Van den Beuken‐van Everdingen 2007). For people with advanced cancer, the prevalence of pain can be as high as 90% (Laird 2008). It has been estimated that 30% to 50% of people with cancer categorise their pain as moderate to severe and that between 75% and 90% of people with cancer experience pain which has a major impact on their daily life (Portenoy 1999). Epidemiological studies suggest that approximately 15% of people with cancer who experience pain fail to experience acceptable pain relief with conventional management (Running 2011; Yakovlev 2008). Uncontrolled pain can lead to physical and psychological distress and can have a drastic effect on people's quality of life.
Description of the intervention
The options available for managing cancer‐related pain include pharmacological treatments (e.g. opioid analgesics), psychological therapy (e.g. cognitive behavioural therapy) and alternative treatments (e.g. acupuncture or massage). Opioid pharmacotherapy (such as morphine, oxycodone, fentanyl, hydromorphone and methadone) are the most effective of these therapies (Portenoy 2011).
The World Health Organization (WHO) has recommended oral morphine as the first choice for the management of moderate to severe cancer‐related pain (WHO 1986). This recommendation is largely due to its cost and availability rather than proven superiority (Caraceni 2012), with a previous review suggesting that a significant proportion of people do not achieve sufficient pain relief by taking morphine due to unmanageable adverse events, including nausea, delirium or myoclonus (muscle spasm) (Murray 2005). However, evidence from one Cochrane Review on oral morphine for cancer pain suggested that only around 5% of participants stopped taking morphine due to lack of pain relief or unacceptable adverse events (Wiffen 2013). Morphine has also been associated with toxicity in people with renal impairment (King 2011a).
Hydromorphone (also known as dihydromorphinone) is a semi‐synthetic derivative of morphine, and is marketed in various countries under a range of brand names. Since its clinical introduction in 1926, it has been used as an alternative opioid analgesic to morphine, as it has a similar chemical structure but is more lipid soluble (Urquhart 1988) and potent (Twycross 1994). Hydromorphone hydrochloride has high aqueous solubility and is beneficial for people who require higher doses (Portenoy 2011), and OROS (osmotic‐controlled release oral delivery system) hydromorphone extended‐release (ER) is five times as potent as morphine, and has 8.5 times the equianalgesic effect when administered intravenously (Binsfeld 2010; Sarhill 2001). This also allows a smaller dose of hydromorphone to be used for an equianalgesic effect. Hydromorphone is administered through several routes (e.g. oral, intravenous, subcutaneous, epidural and intrathecal) (Murray 2005).
Hydromorphone is represented in several international guidelines for the treatment of pain. For the management of chronic cancer pain, including break‐through pain, the WHO uses a model of a three‐step ladder, in which step‐one therapy consists of non‐opioid analgesics with or without adjuvant therapy. For persistent or increasing pain, an opioid for mild to moderate pain (e.g. tramadol, codeine) might be added. If this combination fails to relieve the pain or if the pain increases, an opioid for moderate to severe pain (e.g. morphine, methadone, hydromorphone, oxycodone or fentanyl) should be substituted (Ambrosio 2003). Recommendations issued by the European Association for Palliative Care in 2012 agree with the three‐step process and additionally suggest that hydromorphone be included as a step‐two opioid when used at low doses (e.g. 4 mg/day) (Caraceni 2012). Consensus‐based guidelines for the intrathecal treatment of cancer pain propose using intrathecal morphine as first‐, second‐ or third‐line therapy for people with moderate to severe intractable cancer pain (Deer 2011). For chronic non‐cancer pain, the American Society of Interventional Pain Physicians includes hydromorphone in their guidelines for the use of opioids (Trescot 2006).
How the intervention might work
Like morphine, hydromorphone is primarily an agonist at μ‐opioid receptors, displaying weak affinity for κ‐opioid receptors. μ‐Opioid receptors mediate pain‐relieving properties but they can also result in adverse events such as nausea, constipation and respiratory depression (Murray 2005). One systematic review showed that hydromorphone has similar analgesic and adverse effects to morphine (Miller 1999), while a more recent review concluded that no study has yet clearly demonstrated whether hydromorphone is better than oral morphine (Pigni 2011).
Hydromorphone, in common with other opioid analgesics, has the potential to produce adverse events that include respiratory depression, nausea, vomiting, constipation and itching. Tolerance may develop during chronic opioid therapy such that larger doses may be required to sustain the analgesic effect. In addition, people can be at risk of physiological dependence and experience opioid withdrawal syndrome upon sudden cessation of the opioid or administration of an antagonist. When used for the relief of pain in malignant disease, the actions of relieving anxiety, producing drowsiness and allowing sleep may be welcome (Grahame‐Smith 2002).
Why it is important to do this review
This is one of a suite of reviews investigating analgesics for cancer pain. Although WHO recommends oral morphine as a first‐line analgesia for cancer‐related pain, the use of hydromorphone remains a consideration in some circumstances (Wiffen 2013). Previous systematic reviews have compared the efficacy and adverse effects of hydromorphone with other medications, but the inconsistency of their conclusions and the limited (low to moderate) methodological quality of the studies that were included suggested that further research is needed (Pigni 2011). This review updates the evidence by evaluating the effectiveness of hydromorphone for cancer‐related pain and examines the incidence and severity of its adverse effects.
Objectives
To determine the analgesic efficacy of hydromorphone in relieving cancer pain, as well as the incidence and severity of any adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that focused on hydromorphone for the treatment of cancer pain and assessed pain as an outcome measure in this review. The RCTs included parallel or cross‐over studies of any duration. We excluded studies which did not state that participants were allocated at random.
Types of participants
We intended to include studies of both adults and children with moderate to severe cancer pain (as defined in each study) who were clinically assessed as requiring treatment with opioid analgesia.
Types of interventions
We included studies in which hydromorphone (any dose and route of administration) was the active intervention. Comparison treatments included placebo, an alternative opioid or another active control.
Types of outcome measures
We assessed participant‐reported pain intensity and pain relief through the use of validated pain scales, including visual analogue scales (VAS) and categorical scales. Where possible, we collected data on the four main outcomes previously highlighted as important to people with cancer (Moore 2013); pain no worse than mild, and the impact of the treatment on consciousness, appetite and thirst (Wiffen 2014).
Primary outcomes
Participant‐reported pain intensity levels as measured using a validated VAS or categorical pain scale. We were particularly interested in, but not limited to, numbers of participants who achieve 'no worse than mild pain' (Moore 2013). "No or mild pain" has been previously considered as: 3/10 on a numerical rating scale, or 30/100 mm on a VAS (Wiffen 2013). We did not consider physician, nurse or carer‐reported measures of pain.
Participant‐reported pain relief measured using a validated scale.
Secondary outcomes
Adverse events, for example, drowsiness/sedation, nausea and constipation, impact of the treatment on consciousness, appetite and thirst (incidence and severity, as defined and measured in each study).
Improvement in participants' quality of life measured using the EuroQol EQ‐5D, the World Health Organization Quality of Life Assessment or a similar validated quality of life instrument.
Leaving the study early or discontinuation of treatment for any reason.
Death.
Search methods for identification of studies
Electronic searches
To identify potentially relevant studies to be assessed for inclusion in this review, we searched the databases listed below. See Appendix 1 for search strategies.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 3) in the Cochrane Library.
MEDLINE (Ovid) 1946 to April 2016.
Embase (Ovid) 1974 to April 2016.
Searching other resources
In an attempt to identify any relevant published or unpublished reports not found in the electronic searches, we manually checked the references of each included paper. We contacted the authors of each included paper and of publications which were only available in abstract format. Where possible, we contacted representatives from the pharmaceutical companies marketing hydromorphone to ask for any relevant published or unpublished studies, or missing data.
There was no limitation on publication date or on language. Had there been any non‐English papers, we would have translated as necessary. We also searched for ongoing trials in ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/). We intended to search metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), however, the website was unavailable.
Data collection and analysis
Selection of studies
Two review authors (YJB and BJH) assessed the titles and abstracts of all studies identified by the searches and independently considered the full records of all potentially relevant studies for inclusion by applying the selection criteria outlined in the Criteria for considering studies for this review section. We resolved disagreements by discussion. We did not restrict the inclusion criteria by date or language. To promote transparency of the search and systematic review process, we produced a PRISMA flow diagram (Figure 1), as per the PRISMA statement (Moher 2009).
Figure 1.
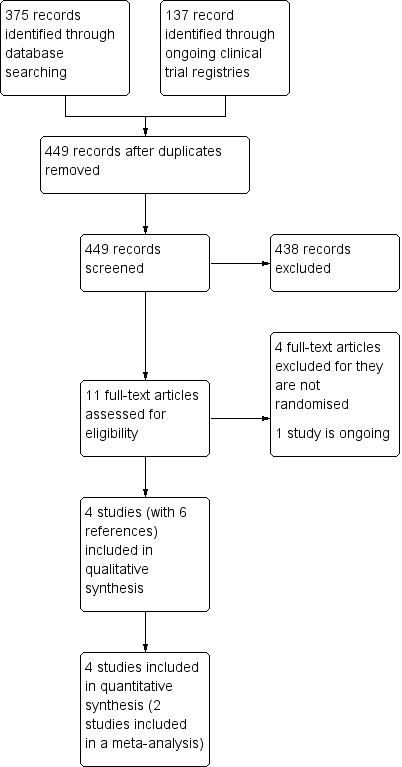
Study flow diagram.
Data extraction and management
We extracted data using the Cochrane Pain, Palliative and Supportive Care Group's recommended data extraction form and recorded baseline data on participants, details of interventions, outcomes and results relevant to our review. Had we identified any study that included a subset of participants who received hydromorphone, we would have extracted data from this group. We resolved any disputes by discussion.
Assessment of risk of bias in included studies
Two review authors (YJB and BJH) independently assessed the methodological quality of each included study using the 'Risk of bias' assessment method outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included the following risk of bias domains: allocation and randomisation methods; blinding and methods of maintaining blinding; selective reporting of outcome measures; incomplete outcome data and 'other' sources of bias (e.g. sources of funding). We rated the domains as 'low risk', 'high risk' or 'unclear risk' of bias. We completed a 'Risk of bias' table for each included study. We resolved any disagreements between the assessors by discussion. Small studies have been shown to overestimate treatment effects, probably due to methodological weaknesses (Moore 2012; Nüesch 2010); therefore, we considered studies to be at low risk of bias if they had 200 or more participants per treatment arm, at unclear risk if they had between 50 and 200 participants per treatment arm, and at high risk if they had fewer than 50 participants per treatment arm (Wiffen 2013).
Measures of treatment effect
For dichotomous outcomes, we calculated the risk ratio (RR) and the corresponding 95% confidence interval (CI) and P value. For continuous outcomes, we calculated the mean difference (MD) and its corresponding 95% CI when means and standard deviations (SD) were available. If such information was unavailable, we would have use the methods described in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions to calculate standardised mean differences (SMD) from, for example, F ratios, t values, Chi2 values and correlation coefficients (Higgins 2011). In cases where continuous measures were used to assess the same outcomes using different scales, we would have pooled these data using Hedges' g to estimate the SMD. When effect sizes could not be pooled, we would have reported study‐level effects narratively. We would also have calculated numbers needed to treat for an additional outcome (NNTB) and additional harmful outcomes (NNTH).
Unit of analysis issues
We only included studies that randomised the individual participant.
Dealing with missing data
We assessed missing data in the included studies. Where possible, we investigated and reported the reasons and numbers of those dropping out of each included study. Where studies were identified as having missing data, we initially attempted to contact the study authors to obtain this information. For dichotomous outcomes, we performed an intention‐to‐treat (ITT) analysis. If there was missing participant information, we recorded this and commented in the individual study's 'Risk of bias' table. We assigned participants with missing data to a 'zero improvement' category, and we performed a sensitivity analysis comparing the resulting effect sizes with those obtained using completer‐only data. For continuous outcomes, we intended to use baseline observation carried forward (BOCF), where rating scales were employed. However, this was not done as data of the few continuous outcomes were skewed. Where data are missing for substantial numbers of participants (greater than 10%), we rated the study as a high risk of bias.
Assessment of heterogeneity
We intended to assess for heterogeneity among primary outcome studies using the I2 statistic along with its corresponding P and Chi2 values (Higgins 2011), and discuss any observed heterogeneity and its magnitude. Had we identified heterogeneity, we would have investigated possible sources using subgroup analyses and sensitivity analyses.
Assessment of reporting biases
We looked for the original trial protocols of the included studies and compared the results to these when they were found. When no protocol was available, we compared the reported outcomes against the methods section of the paper to look for selective reporting of outcomes.
Data synthesis
We entered all extracted data into Review Manager 5 software for analysis (RevMan 2014). In order to take into account differences between studies, we synthesised data using a random‐effects model. We used a fixed‐effect model in a sensitivity analysis in order to investigate any differences in the estimate of effect. We meta‐analysed the data where possible. Where this was not feasible, we summarised data narratively in the results and discussion sections and the relevant tables.
Grading of evidence
This section is taken from the Cochrane Drugs and Alcohol Group recommended text. We assessed the overall quality of the evidence for each outcome using the GRADE system (Guyatt 2011), and presented it in the 'Summary of findings' tables, to present the main findings of a review in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grade of evidence:
high = further research is very unlikely to change our confidence in the estimate of effect;
moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low = any estimate of effect is very uncertain.
We decreased grade if there was:
serious (‐1) or very serious (‐2) limitation to study quality;
important inconsistency (‐1);
some (‐1) or major (‐2) uncertainty about directness;
imprecise or sparse data (‐1);
high probability of reporting bias (‐1).
'Summary of findings' table
We included two 'Summary of findings' tables to present the main findings in a transparent and simple tabular format. In particular, we include key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the outcomes participant‐reported pain intensity, adverse events, leaving the study early and death.
Subgroup analysis and investigation of heterogeneity
Had there been data available, we would have carried out the following subgroup analyses:
method of administration (long‐acting versus short‐acting);
single dose versus multiple dose;
type of cancer;
age (adults versus children).
Sensitivity analysis
Had there been sufficient data available, we would have examined the robustness of meta‐analyses by conducting a sensitivity analysis. In future updates of this review, we plan to exclude studies are at 'high risk of bias' across any one of the risk of bias domains in order to assess any differences in the estimate of treatment effect. We further plan to conduct a sensitivity analysis for high levels of attrition (greater than 10%) in individual studies, comparing completer‐only data with our assumptions of ITT and to assess any differences when synthesising data using a fixed‐effect rather than a random‐effects model.
Results
Description of studies
Results of the search
Details of the search results are illustrated in the PRISMA table (Figure 1).
In the original search, we found 512 (375 through databases and 137 through international ongoing clinical trial registries) records that were potentially relevant. After removing duplicate records, we screened 449 abstracts, of which we were able to exclude 438 records that were clearly irrelevant. We eventually identified 11 full‐text articles as potentially eligible for inclusion. Upon close inspection of these papers, we were able to include four studies (with six references) in this review. There was one ongoing study and there are no studies awaiting assessment.
Included studies
We found four RCTs in adults (604 participants) that satisfied the inclusion criteria of this review; see Characteristics of included studies for a full description. The search found no studies including children.
Design and setting
Three of the included studies were conducted in high‐income countries. Hagen 1997 was conducted in Canada; Hanna 2008 was a multicentre trial involving 37 centres in Belgium, Canada, France, Germany, the Netherlands, Spain, Sweden and the UK. This study reported that it included inpatients, outpatients and day patients. Moriarty 1999 was conducted in the UK. The remaining study was conducted in China (Yu 2014).
Two studies had a cross‐over study design (Hagen 1997; Moriarty 1999), and two had a two‐stage, parallel design that included an initial titration stage followed by a slow release (SR) or maintenance phase (Hanna 2008; Yu 2014).
Sample sizes
Hagen 1997 was the smaller trial of the four with 44 participants randomised, but only 31 people completed the trial. Hanna 2008 had a sample size of 200. Moriarty 1999 randomised 100 participants, but only 89 completed the trial. Yu 2014 randomised 260 people, but only 137 completed the trial through to the end of maintenance phase.
Participants
All four studies included adults with chronic cancer pain. The mean age in Hagen 1997, Hanna 2008, and Yu 2014 was about 56 to 59 years with evenly distributed gender; the age of Moriarty 1999 was over 18 years but no age range was given. We found no studies including children. The proportion of men in the study ranged from 42% (Hagen 1997) to 66% (Yu 2014).
The severity of cancer pain was unclear in Hagen 1997, but participants required stable analgesics. Participants in Hanna 2008 had moderate to severe pain and required 60 mg to 540 mg of oral morphine every 24 hours at baseline. Moriarty 1999 and Yu 2014 also involved people with moderate to severe cancer pain. The locations of the primary tumour were mainly breast, colorectal, lung, prostate, gastrointestinal and central nervous system. A smaller proportion of participants had cancer in the oral cavity, lymphoma, leukaemia and bone cancer.
Interventions
Interventions included hydromorphone versus oxycodone (Hagen 1997; Yu 2014), and hydromorphone versus morphine (Hanna 2008; Moriarty 1999).
Hagen 1997 compared controlled release (CR) hydromorphone versus CR oxycodone given every 12 hours for seven days. The mean (± SD) daily doses were given as 24 ± 4 mg for hydromorphone and 120 ± 22 mg for oxycodone. Cross‐over was completed without a washout period.
In the two‐stage Yu 2014 trial, the eight‐day titration phase was followed by a 28‐day maintenance phase. Both phases used CR formulations; OROS hydromorphone or oxycodone CR and the maximum daily doses were 32 mg for OROS hydromorphone and 80 mg for oxycodone CR.
The titration stage for Hanna 2008 used instant release (IR) formulations of either hydromorphone or morphine given every four hours (six times daily) for two to nine days. The titrated dosage of hydromorphone during this phase was 12 mg/day to 108 mg/day and for morphine was 62 mg/day to 540 mg/day. This was followed by a 10‐ to 15‐day SR stage, when the same drugs were given but in a CR formulation; OROS hydromorphone once daily or morphine CR twice daily. The starting dose was the same level as dose‐stable pain achieved in IR phase, adjusted as required every two days at most.
Moriarty 1999 used tablet formulation of hydromorphone CR 4 mg and morphine CR 30 mg.
Outcomes
We were able to collect data on pain intensity, but the data were skewed. Other outcomes reported by the studies included adverse events, death and leaving the study early.
Excluded studies
We excluded four studies. Although all of them had relevant participants and interventions, they were not RCTs. See Characteristics of excluded studies for further details (Han 2014; Lee 2012; Wirz 2008; Wirz 2009).
Studies awaiting assessment
There are no studies awaiting assessment.
Ongoing studies
The search found one ongoing RCT eligible for inclusion, which compared hydromorphone with oxycodone and fentanyl patch in adults with moderate to severe cancer pain (NCT02084355). The total sample size of this study was unclear. Expected completion date was January 2016.
Risk of bias in included studies
The general risk of bias in respect of study design and conduct was low. However, two trials were industry funded (Hagen 1997; Hanna 2008), which raised some concern over potential conflicts of interest. Furthermore, Hagen 1997 had a high dropout rate and those dropping out were excluded from their final analysis. See Figure 2 and Figure 3 for graphic representation of the risk of bias assessment.
Figure 2.
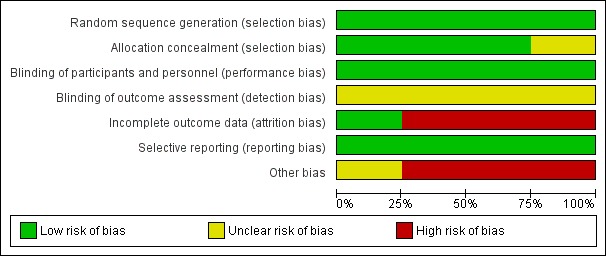
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
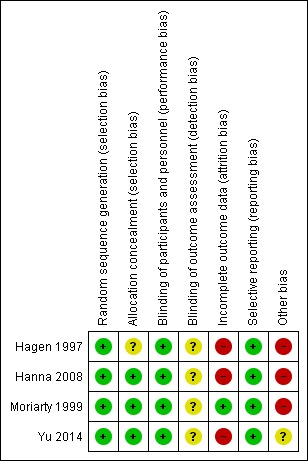
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We assigned all four included studies low risk of bias for random sequence generation. Hanna 2008 randomised participants on a 1:1 ratio via a central computer‐generated randomisation list. Similarly, Yu 2014 also used central randomisation (1:1) using an online dynamic minimisation allocation program. Hagen 1997 did not describe randomisation procedure in detail, but it was a double‐blind trial, thus it was likely to have had adequate randomisation. Moriarty 1999 employed a third‐party randomisation method.
Allocation concealment
None of the studies provided explicit detail on allocation concealment. We considered Hanna 2008 was more likely to have used concealment since the randomisation was done via a central list, and so we judged this study at low risk of bias. We also judged Moriarty 1999 and Yu 2014 at low risk of bias because they used third‐party randomisation, which typically conceals allocation. We judged Hagen 1997 at unclear risk of bias because there was no detail reported in the paper, thus review authors were unable to make any conclusive judgement.
Blinding
Blinding of participants and personnel
Three of the four studies were described as double‐blind (Hagen 1997; Hanna 2008; Moriarty 1999). Hanna 2008 was reported as double blind, but without further description of the blinding method; in this case, we accepted the author's reporting as true and accurate, thus rated it at low risk of bias. Hagen 1997 and Moriarty 1999 used double‐blind and double‐dummy to protect the blinding. Yu 2014 did not offer an explicit description on blinding; however, review authors felt that double‐blinding was likely to have been used, as the study employed over‐encapsulated tablet and placebo to mask blinding, hence we rated it at low risk of bias.
Blinding of outcome assessment
It was unclear in any of the studies if the outcome assessment was blinded, because none of the included studies provided an explicit description of this risk domain. Therefore, we rated this risk domain at unclear risk for all of the four studies.
Incomplete outcome data
Dropout was common and the proportion of dropout exceeded 10% in all four studies, thus the general risk of bias in this domain was high. Hanna 2008 had applied ITT analysis and the reasons and proportion for dropout was similar between groups, however, the dropout rate was greater than 10%, thus it was rated as high risk. Hagen 1997 was rated as high risk as it had over 10% dropout and these were excluded from final analysis, which further compromised the already weakened evidence. Moriarty 1999 had 11 (11%) participants drop out with reasons given and were included in the final analysis. The dropout rate was over 10%, but only marginally so. We felt the drop‐out was unlikely to have caused significant bias, as reasons and proportion of dropout were comparable between groups. We therefore judged this study to be at a low risk of bias for this domain. Sixty (46%) people dropped out of the hydromorphone group and 63 (48%) people dropped out of the oxycodone group in Yu 2014, but the proportion and reasons were balanced between groups. Nevertheless, we judged it as high risk because the dropout rate was greater than 10%.
Selective reporting
Two of the four trials had protocols (Hanna 2008; Yu 2014), and we did not identify any differences between the planned outcome measures in the protocol and the reported outcome measures in the full report. Two trials had no available protocols (Hagen 1997; Moriarty 1999). We compared the reported outcomes with the paper's methodology section and did not find any evidence of selective reporting. Therefore, we judged all four included studies as being at low risk of reporting bias.
Other potential sources of bias
We judged three included studies to be at a high risk of bias for this domain (Hagen 1997; Hanna 2008; Moriarty 1999). There were two major concerns of other bias regarding sample size and sponsorship. Two studies were funded by pharmaceutical companies (Hagen 1997; Hanna 2008). One of the authors of Moriarty 1999 was an employee of a pharmaceutical company, which raised concern over conflict of interest. Three of the four studies had a small sample size (fewer than 200 participants per treatment arm), which raised potential risk of bias (Hagen 1997; Hanna 2008; Moriarty 1999). Yu 2014 had between 50 and 199 participants per treatment arm, and so we judged this study at unclear risk of bias for this domain.
Effects of interventions
We were able to extract numerical data from three of the four included studies.
Comparison 1: hydromorphone versus oxycodone
This particular comparison had very low quality evidence from Hagen 1997 (n = 44) and Yu 2014 (n = 260) (Table 1), downgraded three times due to risk of bias, imprecision, publication bias or inconsistency.
1.1 Participant‐reported pain intensity
Data were presented in separate data tables because they were skewed. Hagen 1997 reported VAS score (0 to 100 with higher score indicating worse outcome). The mean VAS endpoint pain intensity scores at seven days were similar between groups (mean (± SD): hydromorphone 28.86 ± 17.08, n = 19; oxycodone 30.30 ± 25.33, n = 12), although the SD of the oxycodone group was larger than for the hydromorphone group indicating a wider spread of the data. Both groups achieved no worse than mild pain on the categorical pain intensity as measured on a four‐point VAS (higher = worse outcome) (hydromorphone 1.5 ± 0.1 points; oxycodone 1.4 ± 0.1 points).
Yu 2014 reported Brief Pain Inventory (BPI) score (0 to 10 with higher score indicating worse outcome). The BPI change score of 'pain at its worst in the past 24 hours' from baseline was similar between groups at 28 days (hydromorphone ‐1.8 ± 3.29, n = 40; oxycodone ‐1.7 ± 3.91, n = 41). BPI score for 'mean pain in the past 24 hours' of the same study showed that both groups achieved no worse than mild pain (hydromorphone 2.9; oxycodone 3.3, SDs not reported, n = 81) (Analysis 1.1).
Analysis 1.1.
Comparison 1 Hydromorphone versus oxycodone, Outcome 1 Participant‐reported pain intensity (skewed data).
Participant‐reported pain intensity (skewed data)
| Study | Interventions | Least square mean | Standard deviation | n |
|---|---|---|---|---|
| Visual analogue scale (VAS) endpoint pain intensity score (high score = poor outcome) | ||||
| Hagen 1997 | Hydromorphone | 28.86 | 17.08 | 19 |
| Hagen 1997 | Oxycodone | 30.30 | 25.33 | 12 |
| Brief Pain Inventory worst pain in past 24 hours (change score) | ||||
| Yu 2014 | Hydromorphone | ‐1.8 | 3.29 | 40 |
| Yu 2014 | Oxycodone | ‐1.7 | 3.91 | 41 |
Neither study reported 'no worse than mild pain'.
1.2 Participant‐reported pain relief
Neither study reported participant‐reported pain relief.
1.3 Adverse events
Hagen 1997 presented data measured using VAS at seven days in separate data tables because the continuous data for this outcome were skewed. The mean endpoint nausea scores were comparable between groups (hydromorphone 16.05 ± 17.51, n = 19; oxycodone 16.68 ± 21.53, n = 12). However, the oxycodone group had a higher mean endpoint sedation score than the hydromorphone group (hydromorphone 19.92 ± 20.62, n = 19; oxycodone 24.81 ± 25.73, n = 12), although we were not certain if the difference was statistically significant.
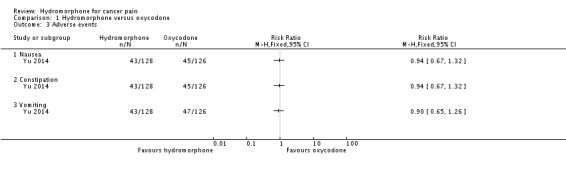
The above findings were consistent with Yu 2014, which indicated no significant differences between groups at 28 days on the following adverse events: nausea (RR 0.94, 95% CI 0.67 to 1.32); constipation (RR 0.94, 95% CI 0.67 to 1.32) and vomiting (RR 0.90, 95% CI 0.65 to 1.26). Other adverse events identified in this study showed no significant differences between groups. See Table 5 for further details.
Table 1.
Comparison 1: hydromorphone versus oxycodone (adverse events)
| Adverse event | Hydromorphone | Morphine | ||
| No of reports | Total (n) | No of reports | Total (n) | |
| Abdominal discomfort | 4 | 128 | 7 | 126 |
| Abdominal distension | 7 | 128 | 7 | 126 |
| Anaemia | 14 | 128 | 14 | 126 |
| Asthenia | 11 | 128 | 9 | 126 |
| Bone marrow failure | 9 | 128 | 9 | 126 |
| Chest discomfort | 9 | 128 | 6 | 126 |
| Decreased appetite | 20 | 128 | 21 | 126 |
| Diarrhoea | 12 | 128 | 9 | 126 |
| Dizziness | 21 | 128 | 22 | 126 |
| Hyperhidrosis | 3 | 128 | 8 | 126 |
| Hypoproteinaemia | 9 | 128 | 5 | 126 |
| Neutrophil count decreased | 7 | 128 | 5 | 126 |
| Oedema peripheral | 11 | 128 | 6 | 126 |
| Platelet count decreased | 8 | 128 | 7 | 126 |
| Pyrexia | 24 | 128 | 27 | 126 |
| Rash | 7 | 128 | 4 | 126 |
| Urinary tract infection | 4 | 128 | 7 | 126 |
| White blood cell count decreased | 13 | 128 | 17 | 126 |
n: number of participants
Neither study reported impact on consciousness, appetite or thirst.
1.4 Quality of life
Neither study reported quality of life.
1.5 Leaving the study early
Two studies involving 304 participants reported on participants leaving the study early (Hagen 1997; Yu 2014). We found no evidence of difference between hydromorphone and oxycodone (RR 0.61, 95% CI 0.20 to 1.87) (Analysis 1.4).
Analysis 1.4.
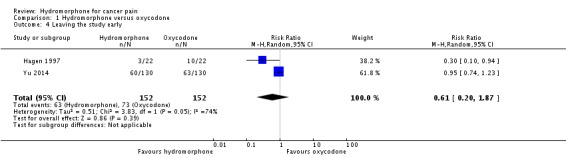
Comparison 1 Hydromorphone versus oxycodone, Outcome 4 Leaving the study early.
1.6 Death
One study reported death (n = 260) (Yu 2014). This was claimed to be a consequence of disease progression, and there was no statistically significant difference between groups (RR 0.50, 95% CI 0.22 to 1.13) (Analysis 1.5).
Analysis 1.5.
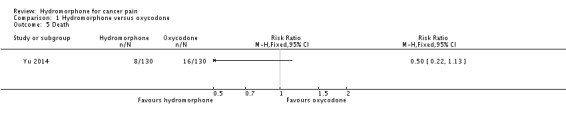
Comparison 1 Hydromorphone versus oxycodone, Outcome 5 Death.
Comparison 2: hydromorphone versus morphine
In this comparison, there was very low quality evidence from a single study (Hanna 2008, n = 200). The other study which investigated these interventions did not report any usable numerical data for analysis (Moriarty 1999) (Table 2, downgraded three times due to risk of bias, imprecision and publication bias).
2.1 Participant‐reported pain intensity: BPI endpoint (SR phase) subscale score (high = more pain; data skewed)
The continuous data from one RCT were too skewed to report in a graph. Therefore, we have presented them in a data table (Analysis 2.1). Subscale data derived from the BPI scale showed that the morphine group appeared to have a higher endpoint mean score on 'worst pain' (mean ± SD: hydromorphone 3.5 ± 2.9, n = 99; morphine 4.3 ± 3.0, n = 101), nevertheless, mean scores on 'least pain' and 'mean pain' were almost identical. The 'mean pain' subscale data showed that both groups achieved no worse than mild pain (see Analysis 2.1).
Analysis 2.1.
Comparison 2 Hydromorphone versus morphine, Outcome 1 Participant‐reported pain intensity: Brief Pain Inventory endpoint (slow‐release phase) subscale score (high = more pain; data skewed).
Participant‐reported pain intensity: Brief Pain Inventory endpoint (slow‐release phase) subscale score (high = more pain; data skewed)
| Study | Interventions | Least square mean | SD | n |
|---|---|---|---|---|
| Worst pain subscale score | ||||
| Hanna 2008 | Hydromorphone | 3.5 | 2.9 | 99 |
| Hanna 2008 | Morphine | 4.3 | 3.0 | 101 |
| Least pain subscale score | ||||
| Hanna 2008 | Hydromorphone | 1.8 | 2.0 | 99 |
| Hanna 2008 | Morphine | 1.8 | 2.0 | 101 |
| Mean pain | ||||
| Hanna 2008 | Hydromorphone | 3.4 | 3.0 | 99 |
| Hanna 2008 | Morphine | 3.2 | 3.0 | 101 |
Although it was not possible to extract and analyse data by groups from Moriarty 1999, the study gave a clear indication that participants in both groups achieved no worse than mild pain as measured by VAS. The mean score of both groups was below 20 on the 0‐ to 100‐mm VAS.
We found no studies reporting the number of participants who achieved 'no worse than mild pain'
2.2 Participant‐reported pain relief
Neither study reported participant‐reported pain relief.
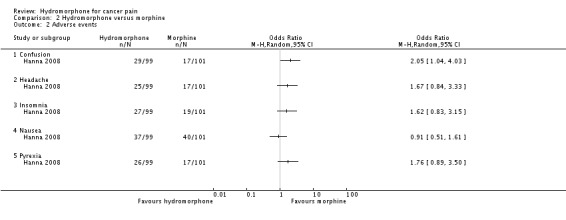
2.3 Adverse events
One study reported adverse events (Hanna 2008). There was no difference between groups for the following adverse events:
anaemia (hydromorphone 25/77; morphine 21/86; RR 1.21, 95% CI 0.73 to 2.02);
anorexia (hydromorphone 24/77; morphine 20/86; RR 1.22, 95% CI 0.72 to 2.07);
anxiety (hydromorphone 27/77; morphine 16/86; RR 1.72, 95% CI 0.99 to 2.99);
asthenia (hydromorphone 28/77; morphine 19/86; RR 1.50, 95% CI 0.9 to 2.51);
dizziness (hydromorphone 26/77; morphine 23/86; RR 1.15, 95% CI 0.71 to 1.88);
fatigue (hydromorphone 26/77; morphine 21/86; RR 1.26, 95% CI 0.76 to 2.09);
headache (hydromorphone 25/77; morphine 17/86; RR 1.50, 95% CI 0.87 to 2.60);
insomnia (hydromorphone 27/77; morphine 19/86; RR 1.45, 95% CI 0.86 to 2.43);
nausea (hydromorphone 37/77; morphine 40/86; RR 0.94, 95% CI 0.66 to 1.34);
peripheral oedema (hydromorphone 23/77; morphine 23/86; RR 1.02, 95% CI 0.61 to 1.69);
pruritus (hydromorphone 25/77; morphine 20/86; RR 1.28, 95% CI 0.76 to 2.14);
pyrexia (hydromorphone 26/77; morphine 17/86; RR 1.56, 95% CI 0.9 to 2.69);
somnolence (hydromorphone 30/77; morphine 27/86; RR 1.13, 95% CI 0.73 to 1.76);
vomiting (hydromorphone 29/77; morphine 34/86; RR 0.87, 95% CI 0.58 to 1.31).
There was a statistically significant difference favouring the morphine group on the following outcomes. However, the stability of these analysis results is compromised by missing data, and two of the outcomes (confusion and diarrhoea) were no longer statistically significant once we had taken into account the missing data in a sensitivity analysis. See 'Comparison 2: sensitivity analysis for hydromorphone versus morphine' for further details.
Confusion (hydromorphone 29/77; morphine 17/86; RR 1.74, 95% CI 1.02 to 2.96).
Constipation (hydromorphone 52/77; morphine 34/86; RR 1.56, 95% CI 1.12 to 2.17).
Diarrhoea (hydromorphone 29/77; morphine 17/86; RR 1.74, 95% CI 1.02 to 2.96).
The Hanna 2008 study did not report impact on consciousness, appetite or thirst.
Moriarty 1999 observed similar adverse events, but did not report the number of participants, thus preventing pooling of the results. The type and number of adverse events appeared to be balanced between groups without any obvious differences. See Table 6 for a detailed account.
Table 2.
Comparison 2: hydromorphone versus morphine (adverse events)
| Adverse event | Hydromorphone | Morphine | ||
| No of reports | Treatment related | No of reports | Treatment related | |
| Abdominal discomfort/pain | 2 | 1 | 5 | 1 |
| Confusion | 1 | 1 | 1 | 0 |
| Constipation | 1 | 1 | 2 | 1 |
| Dizziness | 2 | 2 | 2 | 2 |
| Drowsiness | 0 | 0 | 2 | 2 |
| Fatigue | 0 | 0 | 1 | 1 |
| Nausea | 3 | 2 | 2 | 0 |
| Vomiting | 3 | 1 | 2 | 0 |
| Others | 22 | 0 | 38 | 0 |
| TOTAL | 33 | 8 | 55 | 7 |
2.4 Quality of life
We found no studies reporting quality of life.
2.5 Leaving the study early
2.5.1 Overall
One study (with 200 participants) reported leaving study early (Hanna 2008) (Analysis 2.3). For this subgroup, we found no evidence of a clear difference between OROS hydromorphone and morphine sulphate (RR 1.42, 95% CI 0.95 to 2.12).
Analysis 2.3.
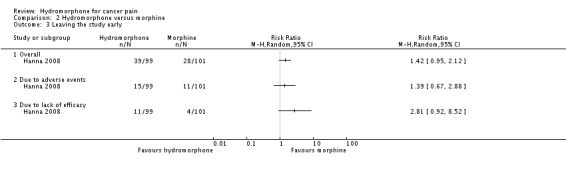
Comparison 2 Hydromorphone versus morphine, Outcome 3 Leaving the study early.
2.5.2 Due to adverse events
One study (with 200 people) reported leaving the study early due to adverse events (Hanna 2008) (Analysis 2.3). For this subgroup, we found no evidence of a clear difference between the two treatments (RR 1.39, 95% CI 0.67 to 2.88).
2.5.3 Due to lack of efficacy
One study (with 200 people) reported leaving the study early due to lack of efficacy (Hanna 2008) (Analysis 2.3). However, we found no evidence of a clear difference between the two treatments (RR 2.81, 95% CI 0.92 to 8.52).
2.6 Death
One trial (with 200 participants) reported death (Hanna 2008) (Analysis 2.4). There was no clear difference between hydromorphone and morphine (RR 0.15, 95% CI 0.01 to 2.78).
Analysis 2.4.
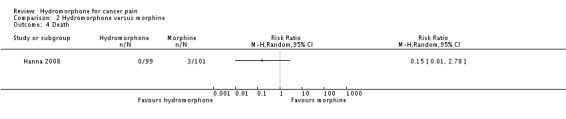
Comparison 2 Hydromorphone versus morphine, Outcome 4 Death.
Comparison 2: sensitivity analysis for hydromorphone versus morphine
In accordance with our protocol, we performed a sensitivity analysis for the adverse events data reported by Hanna 2008 comparing the effect size with and without drop‐outs. When we included dropouts in the analysis, we found a statistically significant difference favouring the morphine group for the following outcomes: confusion (RR 1.74, 95% CI 1.02 to 2.96), constipation (RR 1.56, 95% CI 1.12 to 2.17) and diarrhoea (RR 1.74, 95% CI 1.02 to 2.96). However, when we analysed completers data, only constipation remained statistically different (RR 1.76, 95% CI 1.09, 2.87).
Discussion
Summary of main results
We were only able to include four studies in this review, with a total sample size of 604 participants (data for 504 participants available for analysis). Two studies compared hydromorphone to oxycodone and two studies compared hydromorphone to morphine. Overall, there was no evident difference in treatment efficacy between groups, and participants achieved no worse than mild pain in all included studies (Hagen 1997; Hanna 2008; Moriarty 1999; Yu 2014). Data on pain intensity demonstrated a similar effect between groups. There were several adverse events, but most showed no difference between groups except for confusion, constipation and diarrhoea, which favoured the morphine group. However, the clinical significance of the observed differences are questionable due to the instability of analysis caused by missing data from the trial. Hanna 2008 reported three deaths in the morphine group during the trial period, but trialists claimed that they were not related to the drug but were the consequences of cancer. Yu 2014 also reported death (8 in the hydromorphone group and 16 in the oxycodone group), but the most common reason was disease progression. The two studies that contributed the most data in this review had over 10% dropout rates, but the reasons and proportion of dropouts were balanced between groups (Hagen 1997; Hanna 2008). We hoped to observe effects on consciousness, thirst and appetite, but the included studies did not report these data.
Prevalence of sleep disturbance in people with cancer ranges from 24% to 95% (Graci 2005; Mercadante 2004), and is more common among females with cancer, older people, and people with depression or anxiety (Akechi 2007; Graci 2005). Therefore, we suggest that more attention is given to pain control for increasing quality of sleep (Graci 2005; Kvale 2006). Opioids are useful for the initial restoration of night‐time sleep (Graci 2005; Kvale 2006); however, long‐term opioid use can cause sedation and daytime sleeping, as well as disturbed sleep patterns and circadian rhythms (Graci 2005; Hearson 2008). Unlike opioids and short‐acting hydromorphone, OROS hydromorphone is gradually absorbed over 24 hours without causing fluctuations in blood concentration (Nalamachu 2012). Hanna 2008 demonstrated that pain levels in the evening were significantly lower after OROS hydromorphone compared with CR morphine. Therefore, OROS hydromorphone could be considered for people with cancer pain with sleep disturbance.
Opioids are the mainstay of pain treatment. Knowledge regarding the use of opioids can improve the care provided to people with cancer. Opioid rotation is a common practice for the improvement of pain control or drug tolerability, or both (Quigley 2004). When these appropriate interventions have been exhausted or when adverse effects are rapid and severe (or both), rotation to an alternative opioid may help, but there is a lack of evidence to support rotation.
Overall completeness and applicability of evidence
There is a lack of data on children and younger adults. The mean age of participants included in this review was approximately 56 years. We were able to collect data on the primary outcome of no worse than mild pain and most of the secondary outcomes that we intended to measure, with the exception of quality of life. There was a lack of data on other participant‐important outcomes, such as the impact of the treatments on consciousness, appetite and thirst. Applicability of the evidence is further limited by the fact that only oxycodone and morphine were compared to hydromorphone in the included studies. Included studies were conducted in high‐income countries, which may have limited generalisability in some lower‐income countries.
There was a heterogeneity within the included trials with respects to their study design, formulations used and duration of follow‐up. Two studies were cross‐over design and two used a parallel design using two phases. All of the studies used CR or ER opioids, yet one study included an initial phase which used an IR formulation (Hanna 2008). The duration of follow‐up between the trials ranged from 7 to 28 days. These factors increased the difficulty of drawing specific conclusions from the studies.
It is worth noting that two of the trials in this review used the OROS formulation of hydromorphone which has some unique properties that differ from other formulations of hydromorphone (Hanna 2008, n = 200; Yu 2014, n = 260). It is a unique long‐acting opioid formulation that utilises Push‐Pull active osmotic technology and maintains consistent hydromorphone plasma concentrations throughout the 24‐hour dosing interval, providing long‐lasting analgesia (Angst 2001; Drover 2002; Palangio 2002). The dosage form controls the drug release into the body, almost independently from factors such as the surrounding pH or gastric motility (Bass 2002; Verma 2002). There is a minimal effect of food on the rate and extent of absorption of hydromorphone from OROS hydromorphone (Sathyan 2007). It has been reported that the pharmacokinetics of OROS hydromorphone are also minimally affected by alcohol. These unique features of OROS may further limit the generalisability of evidence. Similarly, we are aware of other formulations of hydromorphone (and other opioids) which may have potential benefits for specific groups of people with cancer but we found no evidence for these as part of this review.
Quality of the evidence
The four included studies (n = 604) were of low or unclear risk of bias overall as they adopted appropriate study design, adequate randomisation, and blinded participants and investigators. The two most prominent risks of bias concerned sample size and sponsorship. One of the studies only had 44 participants (Hagen 1997), and three studies were either funded or conducted by industry (Hagen 1997; Hanna 2008; Moriarty 1999). The quality of the body of evidence was very low, mainly due to risk of bias caused by small sample size and sponsorship with potential conflict of interest, as well as imprecision around effect estimates and publication bias. See Table 1; Table 2 for detailed assessment results of each individual outcome. The current body of evidence identified does not allow a robust conclusion, as the majority of the data for the outcomes were either skewed or had wide CIs around the estimated effect size.
Potential biases in the review process
Although we searched mainstream biomedical databases and clinical trials registries, searches beyond these resources to include other non‐English literature may improve the comprehensiveness of the search results. Two review authors independently performed screening and data extraction, but we were unable to extract any data from Moriarty 1999, which may have had some influence on the results.
Agreements and disagreements with other studies or reviews
The current review indicated little difference between hydromorphone and two other opioids, oxycodone and morphine, in terms of analgesic efficacy. This finding is consistent with the 2012 European Association for Palliative Care (EAPC) guidelines (Caraceni 2012), which included a series of systematic reviews reviewing the evidence for opioids in people with moderate to severe cancer pain (Caraceni 2011; King 2011a; King 2011b; Pigni 2011). The reviews concluded that there is a lack of evidence to demonstrate superiority or inferiority of hydromorphone in comparison with other analgesics and EAPC makes a weak recommendation that hydromorphone, morphine or oxycodone could be used as the first choice for step three of the WHO analgesic ladder. The results of this review do not differ from the results of the previous Cochrane Review (Quigley 2003).
Authors' conclusions
For people with cancer pain
Based on data gathered from the four included trials, it appears that hydromorphone has a similar effect on participant‐reported pain intensity as oxycodone and morphine for adults with moderate to severe cancer pain. There was no evident comparative difference in analgesic effect between hydromorphone and other opioids investigated in this review and, on average, participants achieved no worse than mild pain on all investigated treatments. There were several adverse events, but generally there was no difference between groups. In summary, the evidence suggests that hydromorphone has a similar therapeutic effect and adverse effects to oxycodone and morphine in adults with moderate to severe chronic cancer pain. However, this finding should be applied with caution for it only included four studies with different designs and limited sample size. There were no data available for children or for some important outcomes such as impact of the treatment on consciousness, appetite and thirst.
For clinicians
Based on four included trials with different designs and limited number of participants, we found a lack of evidence to support a preference for hydromorphone over other opioid analgesics such as morphine and oxycodone. The treatment effect of hydromorphone appeared to be similar to that of the comparator drugs for adults with moderate to severe cancer pain. There were minor adverse events in all treatment groups and generally no significant difference between groups. However, most of the outcome data were based on single randomised controlled trials with small sample size, thus the findings of the current review should be interpreted and applied with caution. We found no data for children. The insufficient evidence requires clinicians to balance potential benefits against potential adverse events on the merit of each individual case when recommending treatment in clinical practice.
For policy makers
This review identified little evidence to support hydromorphone as the first‐, second‐ or third‐line treatment for cancer pain. However, evidence collated in the current review suggests hydromorphone has a similar analgesic effect as morphine and oxycodone, it can be considered as an alternative when other opioids result in excessive adverse events such as sedation and respiratory depression, and when people with cancer pain experience renal failure. We found no data for children. Included studies were conducted in high‐income countries, which may compromise the external validity of the review as some of the drugs investigated may have limited accessibility in some lower‐income countries. Finally, it is worth noting that findings from the current review are mainly based on small trials with different designs and limited sample size and some risk of bias, therefore, should be applied with caution.
This review reveals a general lack of research in this subject area. Future trials with significant numbers of participants (e.g. over 200 per treatment arm) are needed to evaluate the safety and effectiveness of hydromorphone for the management of moderate to severe cancer pain in adults. Future research is encouraged to involve children and young adults to provide direct evidence in this population. Further adequately powered randomised controlled trials should use standardised tools or scales to measure pain as a primary outcome. More data on other secondary outcomes, as well as the comparative effect of a more comprehensive range of medications, would also be useful to enable the review to draw a more reliable and conclusive effect. Longer‐term toxicity data should be collected if possible.
Acknowledgements
We would like to thank the staff of the Cochrane Pain, Palliative and Supportive Care (PaPaS) Group for their professional support on developing the search strategy and methodological advice of the protocol and review. We would like to thank Miss Katie Jones for her input on the development of the protocol and data extraction.
Cochrane Review Group funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: the views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (The Cochrane Library)
#1MeSH descriptor: [Hydromorphone] this term only
#2Hydromorphon*:ti,ab,kw (Word variations have been searched)
#3Dihydromorphinone:ti,ab,kw (Word variations have been searched)
#4Hydromorphon:ti,ab,kw (Word variations have been searched)
#5Palladone:ti,ab,kw (Word variations have been searched)
#6Laudacon:ti,ab,kw (Word variations have been searched)
#7Dilaudid:ti,ab,kw (Word variations have been searched)
#8#1 or #2 or #3 or #4 or #5 or #6 or #7
#9MeSH descriptor: [Neoplasms] this term only
#10neoplasm*:ti,ab,kw (Word variations have been searched)
#11malignan*:ti,ab,kw (Word variations have been searched)
#12tumour* or tumor*:ti,ab,kw (Word variations have been searched)
#13cancer*:ti,ab,kw (Word variations have been searched)
#14carcinoma*:ti,ab,kw (Word variations have been searched)
#15#9 or #10 or #11 or #12 or #13 or #14
#16MeSH descriptor: [Pain] explode all trees
#17MeSH descriptor: [Pain Measurement] this term only
#18MeSH descriptor: [Pain Threshold] this term only
#19Pain* or nocicept* or nocicept* or neuropath*:ti,ab,kw (Word variations have been searched)
#20#16 or #17 or #18 or #19
#21#8 and #15 and #20
MEDLINE (Ovid)
1. Hydromorphone/
2. Hydromorphon*.it,ab.
3. Dihydromorphinone.ti,ab.
4. Hydromorphon.ti,ab.
5. Palladone.ti,ab.
6. Laudacon.ti,ab.
7. Dilaudid.ti,ab.
8. or/1‐7
9. NEOPLASMS*:ME
10. neoplasm*
11. malignan*
12. tumour* OR tumor*
13. cancer*
14. carcinoma*
15. or/9‐14
16. exp Pain/
17. Pain Measurement/
18. Pain Threshold/
19. Pain* or nocicept* or nocicept* or neuropath*.ti.ab.
20. or/16‐19
21. randomized controlled trial.pt.
22. controlled clinical trial.pt.
23. randomized.ti,ab. or randomised.ti,ab.
24. placebo.ti,ab.
25. drug therapy.fs.
26. randomly.ab.
27. trial.ab.
28. groups.ab.
29. or/21‐28
30. (animals not (humans and animals)).sh.
31. 29 not 30
32. 8 and 15 and 20 and 31
Embase (Ovid)
1 Hydromorphone/
2 Hydromorphon*.ti,ab.
3 Dihydromorphinone.ti,ab.
4 Hydromorphon.ti,ab.
5 Palladone.ti,ab.
6 Laudacon.ti,ab.
7 Dilaudid.ti,ab.
8 or/1‐7
9 NEOPLASMS/
10 neoplasm*.tw.
11 malignan*.tw.
12 (tumour* or tumor*).tw.
13 cancer*.tw.
14 carcinoma*.tw.
15 or/9‐14
16 exp Pain/
17 Pain Measurement/
18 Pain Threshold/
19 (Pain* or nocicept* or nocicept* or neuropath*).tw.
20 or/16‐19
21 random$.tw.
22 factorial$.tw.
23 crossover$.tw.
24 cross over$.tw.
25 cross‐over$.tw.
26 placebo$.tw.
27 (doubl$ adj blind$).tw.
28 (singl$ adj blind$).tw.
29 assign$.tw.
30 allocat$.tw.
31 volunteer$.tw.
32 Crossover Procedure/
33 double‐blind procedure.tw.
34 Randomized Controlled Trial/
35 Single Blind Procedure/
36 or/21‐35
37 (animal/ or nonhuman/) not human/
38 36 not 37
39 8 and 15 and 20 and 38
Data and analyses
Comparison 1.
Hydromorphone versus oxycodone
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported pain intensity (skewed data) | Other data | No numeric data | ||
| 1.1 Visual analogue scale (VAS) endpoint pain intensity score (high score = poor outcome) | Other data | No numeric data | ||
| 1.2 Brief Pain Inventory worst pain in past 24 hours (change score) | Other data | No numeric data | ||
| 2 Adverse event: measured with VAS (high score = poor outcome, skewed data) | Other data | No numeric data | ||
| 2.1 Nausea | Other data | No numeric data | ||
| 2.2 Sedation | Other data | No numeric data | ||
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Constipation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Leaving the study early | 2 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.20, 1.87] |
| 5 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Analysis 1.2.
Comparison 1 Hydromorphone versus oxycodone, Outcome 2 Adverse event: measured with VAS (high score = poor outcome, skewed data).
Adverse event: measured with VAS (high score = poor outcome, skewed data)
| Study | Intervention | Least square mean | Standard deviation | n |
|---|---|---|---|---|
| Nausea | ||||
| Hagen 1997 | Hydromorphone | 16.05 | 17.51 | 19 |
| Hagen 1997 | Oxycodone | 16.68 | 21.53 | 12 |
| Sedation | ||||
| Hagen 1997 | Hydromorphone | 19.92 | 20.62 | 19 |
| Hagen 1997 | Oxycodone | 24.81 | 25.73 | 12 |
Analysis 1.3.
Comparison 1 Hydromorphone versus oxycodone, Outcome 3 Adverse events.
Comparison 2.
Hydromorphone versus morphine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participant‐reported pain intensity: Brief Pain Inventory endpoint (slow‐release phase) subscale score (high = more pain; data skewed) | Other data | No numeric data | ||
| 1.1 Worst pain subscale score | Other data | No numeric data | ||
| 1.2 Least pain subscale score | Other data | No numeric data | ||
| 1.3 Mean pain | Other data | No numeric data | ||
| 2 Adverse events | 1 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Confusion | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Headache | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Insomnia | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Nausea | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Pyrexia | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Leaving the study early | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Overall | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Due to lack of efficacy | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Analysis 2.2.
Comparison 2 Hydromorphone versus morphine, Outcome 2 Adverse events.
Differences between protocol and review
In accordance with the new Cochrane guidelines, we have used GRADE to assess the quality of evidence and added the method for using GRADE to the review. We expanded the 'Description of the intervention' section.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hagen 1997
| Methods | Allocation: randomised Blindness: double‐blind, double‐dummy Duration: pre‐cross‐over phase: 7 days in each phase. Total study duration 14 days Funding: 'Purdue Frederick', since renamed 'Purdue Pharma' Setting: Canada. Unclear if these were inpatients or community patients. Design: cross‐over |
|
| Participants | Diagnosis: people with chronic cancer pain and stable analgesic requirements n = 44 (31 analysed) Age (mean ± SD): 56 ± 3 years Sex: men 13; women 18 History: of the 31 participants who completed the study, location of primary tumour was breast (7), colorectal (5), lung (1), urological/prostate (5), central nervous system (4), unknown primary site (2) and other (7). Included: people with chronic cancer pain and stable analgesic requirements Exclusion: known hypersensitivity to opioid analgesics; intolerance of oxycodone or hydromorphone; presence of a medical or surgical condition likely to interfere with drug absorption in the gastrointestinal tract; concurrent use of other opioid analgesics during the study period; presence of intractable nausea or vomiting; people who had undergone or were expected to undergo therapeutic procedures likely to influence their pain during study period. Consent: "The study protocol and informed consent form received scientific and ethical approval, and patients gave written informed consent before participating in the study" (p. 1429). |
|
| Interventions |
Each intervention was administered every 12 hours for 7 days pre‐cross‐over. No other opioids permitted. Non‐opioid analgesics that had been part of the person's treatment before the study were permitted. Incident and non‐incident breakthrough pain was treated with IR oxycodone and hydromorphone matching the active opioid analgesic at a dosage of approximately 10% of the daily scheduled opioid dose. |
|
| Outcomes | Pain intensity VAS* Pain intensity ordinal* Sedation VAS* Nausea VAS* Dropouts* Unable to use Frequency of rescue analgesic use was not reported as there was no pre‐cross‐over data. |
|
| Notes | * Unpublished data obtained from Purdue Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data only analysed for 31/44 participants. Dropout rate > 10% |
| Selective reporting (reporting bias) | Low risk | None obvious |
| Other bias | High risk | Drug company‐funded. Sample size small, < 50 people per treatment arm |
Hanna 2008
| Methods | Allocation: randomised Blindness: double‐blind Duration: up to 24 days Funding: Johnson & Johnson (previously ALZA Corporation) Setting: multicentre (37 centres in Belgium, Canada, France, Germany, the Netherlands, Spain, Sweden and the UK); inpatients, outpatients and day patients Design: parallel |
|
| Participants | Diagnosis: people with moderate to severe chronic cancer pain requiring 60 mg to 540 mg of oral morphine (or equivalent) every 24 hours n = 200 Age (mean): 59.8 years Sex: 98 men (49%); 102 women (51%) History: cancer type: breast (56), lung (39), genitourinary (30), gastrointestinal (32), oral cavity (6), lymphoma (3), leukaemia (3), bone (2) and other (29) Included: inpatients, outpatients and day patients ≥ 18 years of age; moderate to severe chronic cancer pain; currently receiving strong oral or transdermal opioid analgesics (60 mg to 540 mg of oral morphine or equivalent every 24 hours); appropriate candidate for strong oral or transdermal analgesics (anticipated requirement, 60 mg to 540 mg of oral morphine or equivalent every 24 hours); pain suitable for treatment with a once‐daily formulation. Exclusion: pain not considered potentially responsive to opioids; pain present only upon movement; need for other opioid analgesics (except study medication and breakthrough pain medication) after randomisation; current or recent (within 6 months) history of drug or alcohol abuse (or both); pregnant or lactating, seeking pregnancy or failing to take adequate contraceptive precautions; intolerance of, or hypersensitivity to, hydromorphone or other opioids; presence of gastrointestinal disease of sufficient severity to likely interfere with oral analgesia (e.g. dysphagia, vomiting, no bowel movement or bowel obstruction due to impaction within 5 days of study entry, severe gut narrowing that may affect analgesic absorption or transit); use of monoamine oxidase inhibitors within 2 weeks prior to study entry; investigational drug use within 4 weeks of study entry; presence of conditions for which risks of opioid use outweigh potential benefits (e.g. raised intracranial pressure, hypotension, hypothyroidism, asthma, reduced respiratory reserve, prostatic hypertrophy, hepatic impairment, renal impairment, older and debilitated people, convulsive disorders, Addison's disease). Consent: "All patients who entered the trial were informed of the nature of the study, and provided written informed consent for participation. The study was conducted in accordance with the principles of the Declaration of Helsinki." |
|
| Interventions |
Initial IR phase and subsequent SR phase
* dose‐stable pain control: defined as participants who experience 2 consecutive days with ≤ 3 breakthrough pain episodes requiring rescue medication ‐ they could then begin SR phase of the study. Participants not achieving dose‐stable pain control by day 9 were withdrawn from the study. |
|
| Outcomes | Pain ('worst pain in the past 24 hours' item of the BPI) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised 1:1, with a central computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | There was no explicit description on allocation concealment, but we considered concealment is likely to have been used since the randomisation was done via a central list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind ‐ no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals from study were addressed Quote: "All efficacy variables were analyzed using the intent‐to‐treat (ITT) population, which included all patients who took at least one dose of study medication and had at least one assessment from each study phase" The proportion of dropout appeared to be higher in hydromorphone group; however, the reasons for dropout were comparable between groups, thus review authors consider the proportional difference between groups is unlikely to have had an effect on effect estimate. However, because the dropout rate was > 10%, we rated this domain as high risk |
| Selective reporting (reporting bias) | Low risk | Detailed data of the following 2 scales were not given: Mini‐Mental State Examination and Eastern Cooperative Oncology Group performance status was administered, but authors did comment that the assessment scores were similar between groups |
| Other bias | High risk | Drug company‐funded One of writers was employed by Johnson & Johnson (study sponsor) Study size small, < 200 participants per treatment arm |
Moriarty 1999
| Methods | Allocation: randomised Blindness: double‐blind, double‐dummy Duration: 6 days (with run‐in period of 1 to 3 days) Funding: not stated Setting: not stated. Unclear if these were inpatients or community patients. Design: cross‐over |
|
| Participants | Diagnosis: people with cancer pain n = 100 Age: not stated Sex: men 53; women 47 History: most common of primary malignancies presented by participants were lung, breast, gastrointestinal and genitourinary. Included: people aged ≥ 18 years, with cancer and achieving pain control with CR morphine sulphate Exclusion: people with significant respiratory depression; severe renal or hepatic impairment; people taking strong opioid analgesics other than 12‐hourly morphine sulphate, and people taking monoamine oxidase inhibitors currently or within the previous 2 weeks; pregnant (or not adequately protected from becoming pregnant) or lactating women. Consent: no details |
|
| Interventions |
|
|
| Outcomes | Pain VAS Adverse events Treatment preference Use of rescue medication |
|
| Notes | No pre‐cross‐over data available. No contact details available to request necessary information. We are also unclear about the intensity of people's cancer pain. This review only intended to include people with moderate to severe cancer pain. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...according to a randomisation schedule previously prepared by the clinical supplies department at Napp Laboratories Limited..." Comment: third‐party randomisation was used and is likely to be low risk of bias |
| Allocation concealment (selection bias) | Low risk | Third‐party randomisation was used, thus we considered allocation concealment is likely to have been done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Marched placebos were taken throughout to maintain the blinding of the study (double‐dummy technique)" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 people left the study early and were not included in the final analysis. Although the dropout rate was marginally > 10%, it is unlikely to have had a biased effect on the results, as reasons and proportion for dropout were given and comparable between groups |
| Selective reporting (reporting bias) | Low risk | None obvious |
| Other bias | High risk | 1 of the study authors is an employee of Napp Laboratories Ltd, a pharmaceutical company that produces analgesics Sample size small, 100 participants in total in the 2 arms |
Yu 2014
| Methods | Allocation: randomised Blindness: double‐blind Duration: 3‐phase study: screening period up to 14 days prior to randomisation; dose titration phase up to 8 days, and a 28‐day dose maintenance phase Funding: not stated Setting: China. Unclear if these are inpatients or community patients. Design: parallel |
|
| Participants | Diagnosis: Chinese people with moderate to severe cancer pain n = 260 Age: 18 to 70 years Sex: men and women History: most common of primary malignancies presented by people were lung, breast, gastrointestinal and genitourinary. Included: people who required or were expected to require between 40 mg and 184 mg of oral morphine or morphine equivalents every 24 hours for chronic management of cancer pain and people who were reasonably expected to achieve a stable dose of opioid study medication during the study. Exclusion: people with pure neuropathic pain or pain of unknown origin (where a mechanism or physical cause could not be identified), only had pain on movement or acute pain, required other opioid analgesics (apart from the morphine hydrochloride, in IR formulation, allowed as rescue medication for break through pain), had any significant central nervous system disorder, risk of treatment with study medication could outweigh the potential benefits, women of childbearing potential who were pregnant or lactating. Consent: written informed consent was obtained before entering the study. |
|
| Interventions |
Study completed a dose titration phase (up to 8 days), and a 28‐day dose maintenance phase. Dose titration phase: randomised participants were converted from their prior opioids to their morphine equivalents and titrated to adequate effect (as determined by the pain assessments and supplementary analgesic requirements), and dosage adjustments were made no more frequently than every 2 days. Upward and downward dose titrations were allowed, but the maximum total daily dose was not to exceed hydromorphone ER 32 mg or oxycodone CR 80 mg. Participants had to achieve a stable dose providing pain control at least in the last 2 days of the titration phase (2 to 8 days) to be eligible to enter the maintenance phase. Maintenance phase: the titrated dose was continued for 28 consecutive days. Upward and downward dose titrations were not to exceed a total daily dose of hydromorphone ER 32 mg or oxycodone CR 80 mg. |
|
| Outcomes | BPI pain intensity Participant assessment of pain at its worst in the past 24 hours (assessed with BPI) Pain at its least in the past 24 hours Pain relief in the past 24 hours Adverse events, assessed with treatment‐emergent adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central randomisation (1:1) by an online dynamic minimization allocation programme as the stratification factors was implemented" Comment: third‐party central randomisation was used, thus is likely to be low risk of bias |
| Allocation concealment (selection bias) | Low risk | Quote: "Interactive web based response system designated a unique patient number and treatment code, which dictated the treatment assignment for each patient" Comment: judging from the above description, allocation was concealed by the third party who conducted randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Hydromorphone ER, oxycodone CR and placebo were provided in the form of over‐encapsulated tablets. Dosing had to start in the morning and the study drug was administrated twice daily, with placebo tablet substitute for 1 dose of hydromorphone ER to maintain blinding. The blinding was broken only if specific emergency treatment dictated knowing the treatment status" Comment: placebo was employed to mask blinding where necessary, thus is likely to be low risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 137/260 participants completed the study, loss to follow‐up > 10% |
| Selective reporting (reporting bias) | Low risk | It appears all measured outcomes were reported |
| Other bias | Unclear risk | 50 to 199 participants per treatment arm |
BPI: Brief pain inventory; CR: controlled release; ER: extended release; IR: instant release; n: number of participants; OROS: osmotic‐controlled release oral delivery system; SD: standard deviation; SR: slow release; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Han 2014 | Not a randomised controlled study. |
| Lee 2012 | Not a randomised controlled study. |
| Wirz 2008 | Not a randomised controlled study. Participants who were already taking the experimental and control medication were randomly selected to be consented to take part in the study. |
| Wirz 2009 | Not a randomised controlled study. Participants who were already taking the experimental and control medication were randomly selected to be consented to take part in the study. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Study Efficacy and Safety of Opioid Rotation Compared with Opioid Dose Escalation in Patients with Moderate to Severe Cancer Pain ‐ Open Label, Randomized, Prospective Study |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open label Primary purpose: supportive care |
| Participants | People with cancer pain Inclusion criteria: aged > 18 years; being treated with 1 strong opioid including oral oxycodone, oral hydromorphone or fentanyl patch with range from 60 mg to 200 mg of oral morphine equivalent daily dose; moderate to severe cancer pain (numeric rating scale > 3) at screening; uncontrolled adverse effects associated with currently applied opioid. Exclusion criteria: previous opioid rotation; unable to take oral medication; life expectancy < 1 month; newly started chemotherapy or radiotherapy (or both) within past 2 weeks of screening; serum aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase > 2.5 times of upper normal limit; serum total bilirubin or creatinine > 1.5 times of upper normal limit. |
| Interventions | Oral oxycodone Oral hydromorphone Fentanyl patch |
| Outcomes | Not described in the registration record. |
| Starting date | April 2014 |
| Contact information | Se‐Il Go; Tel: +82 55 750 9454 ext 9454; Email: gose1@hanmail.net |
| Notes | Estimated completion date: January 2016 |
Contributions of authors
Protocol development: all authors contributed equally. Study screening: YJB, BJH. Data extraction: YJB, BJH. Data analysis: WH, XYK, LPY. Report writing: YJB, BJH, JX, RK. Future update: all authors in the existing team will be responsible for future updates.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Science and Technology Department, China.
The current work was partially supported by the National Special Issue Research Program of Science and Technology Department of China (no. 2010ZX09102‐216)
-
National Natural Science Foundation Project, China.
Project No. 81273718 and No. 81302961
Declarations of interest
YJB: none known; YJB is a specialist oncology physician and manages patients with cancer pain.
WH: none known; WH is a specialist oncology physician and manages patients with cancer pain.
XYK: none known; XYK is a pharmacologist.
LPY: none known; LPY is a specialist nephropathy physician and manages patients with nephropathy.
JX: none known; JX is a methodologist and does not have a clinical connection.
BJH: none known; BJH is a specialist oncology physician and manages patients with cancer pain.
RK: none known; RK is a pharmacist and manages patients with pain.
Notes
This review replaces the original review 'Hydromorphone for acute and chronic pain' as the original author team were unavailable to complete the update (Quigley 2013). The new review focuses on cancer pain only and adheres to current Cochrane standards.
New
References
References to studies included in this review
- Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled‐release oxycodone formulation and controlled‐release hydromorphone in the treatment of cancer pain. Cancer1997; Vol. 79, issue 7:1428‐37. [PubMed]
- Hanna M, Thipphawong J, 118 Study Group. A randomized, double‐blind comparison of OROS(R) hydromorphone and controlled‐release morphine for the control of chronic cancer pain. BMC palliative care 2008;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00410540. A study of OROS hydromorphone HCl vs morphine in cancer pain patients [A randomized, double‐blind, controlled trial of hydromorphone (immediate and sustained‐ release) vs morphine (immediate and sustained‐release) in cancer pain]. clinicaltrials.gov/ct2/show/NCT00410540 First received: 12 December 2006.
- Moriarty M, McDonald CJ, Miller AJ. A randomised crossover comparison of controlled release hydromorphone tablets with controlled release morphine tablets in patients with cancer pain. Journal of Clinical Research1999; Vol. 2:1‐8.
- NCT01205126. An efficacy and safety study of oral osmotic therapeutic system (OROS) hydromorphone hydrochloride (HCl) in participants with cancer related pain [A randomized, double‐blind, active controlled, multi‐center study to investigate the safety and efficacy of OROS hydromorphone HCl once‐daily compared with oxycodone HCL controlled‐release twice daily in subjects with cancer pain]. clinicaltrials.gov/ct2/show/NCT01205126 First received: 5 August 2010. ; Yu S, Shen W, Yu L, Hou Y, Han J, Richards HM. Safety and efficacy of once‐daily hydromorphone extended‐release versus twice‐daily oxycodone hydrochloride controlled‐release in Chinese patients with cancer pain: a phase 3, randomized, double‐blind, multicenter study. Journal of Pain : official journal of the American Pain Society 2014;15(8):835‐44. [PUBMED: 24846822] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Han HS, Lee KH, Lee KH, Ryu JS, Kim YC, Park SW, et al. A prospective, open‐label, multicenter study of the clinical efficacy of extended‐release hydromorphone in treating cancer pain inadequately controlled by other analgesics. Supportive Care in Cancer : official journal of the Multinational Association of Supportive Care in Cancer 2014;22(3):741‐50. [PUBMED: 24203087] [DOI] [PubMed] [Google Scholar]
- Lee KH, Kim MK, Hyun MS, Kim JY, Park KU, Song HS, et al. Clinical effectiveness and safety of OROS hydromorphone in breakthrough cancer pain treatment: a multicenter, prospective, open‐label study in Korean patients. Journal of Opioid Management2012; Vol. 8, issue 4:243‐52. [DOI] [PubMed]
- Wirz S, Wartenberg HC, Nadstawek J. Less nausea, emesis, and constipation comparing hydromorphone and morphine? A prospective open‐labeled investigation on cancer pain. Support Care Cancer2008; Vol. 16, issue 9:999‐1009. [DOI] [PubMed]
- Wirz S, Wittmann M, Schenk M, Schroeck A, Schaefer N, Mueller M, et al. Gastrointestinal symptoms under opioid therapy: a prospective comparison of oral sustained‐release hydromorphone, transdermal fentanyl, and transdermal buprenorphine. European Journal of Pain2009; Vol. 13, issue 7:737‐43. [DOI] [PubMed]
References to ongoing studies
- NCT02084355. Study of opioid rotation versus opioid escalation in patients with moderate to severe cancer pain [Efficacy and safety of opioid rotation compared with opioid dose escalation in patients with moderate to severe cancer pain ‐ open label, randomized, prospective study]. clinicaltrials.gov/ct2/show/NCT02084355 Date first received: 5 March 2014.
Additional references
- Akechi T, Okuyama T, Akizuki N, Shimizu K, Inagaki M, Fujimori M, et al. Associated and predictive factors of sleep disturbance in advanced cancer patients. Psycho‐oncology 2007;16(10):888‐94. [PUBMED: 17086580] [DOI] [PubMed] [Google Scholar]
- Ambrosio F, Paoletti F, Savoia G, Amantea B, Arcuri E, Avogaro F, et al. SIAARTI recommendations on the assessment and treatment of chronic cancer pain. Minerva Anestesiologica 2003;69(9):697‐729. [PUBMED: 14564240] [PubMed] [Google Scholar]
- Angst MS, Drover DR, Lotsch J, Ramaswamy B, Naidu S, Wada DR, et al. Pharmacodynamics of orally administered sustained‐release hydromorphone in humans. Anesthesiology 2001;94(1):63‐73. [PUBMED: 11135723] [DOI] [PubMed] [Google Scholar]
- Bass DM, Prevo M, Waxman DS. Gastrointestinal safety of an extended‐release, nondeformable, oral dosage form (OROS): a retrospective study. Drug Safety 2002;25(14):1021‐33. [PUBMED: 12408733] [DOI] [PubMed] [Google Scholar]
- Binsfeld H, Szczepanski L, Waechter S, Richarz U, Sabatowski R. A randomized study to demonstrate noninferiority of once‐daily OROS® hydromorphone with twice‐daily sustained‐release oxycodone for moderate to severe chronic noncancer pain. Pain Practice 2010;10(5):404‐15. [DOI] [PubMed] [Google Scholar]
- Caraceni A, Pigni A, Brunelli C. Is oral morphine still the first choice opioid for moderate to severe cancer pain? A systematic review within the European Palliative Care Research Collaborative guidelines project. Palliative Medicine 2011;25(5):402‐9. [DOI] [PubMed] [Google Scholar]
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence‐based recommendations from the EAPC. Lancet Oncology 2012;13(2):e58‐68. [DOI] [PubMed] [Google Scholar]
- Deer TR, Smith HS, Burton AW, Pope JE, Doleys DM, Levy RM, et al. Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician 2011;14(3):E283‐312. [PUBMED: 21587338] [PubMed] [Google Scholar]
- Drover DR, Angst MS, Valle M, Ramaswamy B, Naidu S, Stanski DR, et al. Input characteristics and bioavailability after administration of immediate and a new extended‐release formulation of hydromorphone in healthy volunteers. Anesthesiology 2002;97(4):827‐36. [PUBMED: 12357147] [DOI] [PubMed] [Google Scholar]
- Graci G. Pathogenesis and management of cancer‐related insomnia. Journal of Supportive Oncology 2005;3(5):349‐59. [PUBMED: 16218258] [PubMed] [Google Scholar]
- Grahame‐Smith DG, Aronson JK. Oxford Textbook of Clinical Pharmacology and Drug Therapy. 3rd Edition. Oxford: Oxford University Press, 2002. [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
- Hearson B, Sawatzky JA. Sleep disturbance in patients with advanced cancer. International Journal of Palliative Nursing 2008;14(1):30‐7. [PUBMED: 18414330] [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Merskey H, Bogduk N. Part III: pain terms, a current list with definitions and notes on usage. Classification of Chronic Pain. 2nd Edition. Seattle, WA: IASP Task Force on Taxonomy, IASP Press, 2011 [update from 1994]. [Google Scholar]
- King S, Forbes K, Hanks GW, Ferro CJ, Chambers EJ. A systematic review of the use of opioid medication for those with moderate to severe cancer pain and renal impairment: a European Palliative Care Research Collaborative opioid guidelines project. Palliative Medicine 2011;25(5):525‐52. [DOI] [PubMed] [Google Scholar]
- King SJ, Reid C, Forbes K, Hanks G. A systematic review of oxycodone in the management of cancer pain. Palliative Medicine 2011;25(5):454‐70. [PUBMED: 21708852] [DOI] [PubMed] [Google Scholar]
- Kvale EA, Shuster JL. Sleep disturbance in supportive care of cancer: a review. Journal of Palliative Medicine 2006;9(2):437‐50. [PUBMED: 16629573] [DOI] [PubMed] [Google Scholar]
- Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. European Journal of Cancer 2008;44:1078‐82. [DOI] [PubMed] [Google Scholar]
- Meier DE, Isaacs SL, Hughes R. Palliative Care: Transforming the Care of Serious Illness. 1st Edition. San Francisco, CA: Jossey‐Bass, 2010. [Google Scholar]
- Mercadante S, Girelli D, Casuccio A. Sleep disorders in advanced cancer patients: prevalence and factors associated. Supportive Care in Cancer : official journal of the Multinational Association of Supportive Care in Cancer 2004;12(5):355‐9. [PUBMED: 15064937] [DOI] [PubMed] [Google Scholar]
- Miller MG, McCarthy N, O'Boyle CA, Kearney M. Continuous subcutaneous infusion of morphine vs. hydromorphone: a controlled trial. Journal of Pain and Symptom Management 1999;18(1):9‐16. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI] [PubMed] [Google Scholar]
- Murray A, Hagen NA. Hydromorphone. Journal of Pain and Symptom Management 2005;29(5):57‐66. [DOI] [PubMed] [Google Scholar]
- Nalamachu SR, Kutch M, Hale ME. Safety and tolerability of once‐daily OROS(®) hydromorphone extended‐release in opioid‐tolerant adults with moderate‐to‐severe chronic cancer and noncancer pain: pooled analysis of 11 clinical studies. Journal of Pain and Symptom Management 2012;44(6):852‐65. [PUBMED: 22795050] [DOI] [PubMed] [Google Scholar]
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palangio M, Northfelt DW, Portenoy RK, Brookoff D, Doyle RT Jr, Dornseif BE, et al. Dose conversion and titration with a novel, once‐daily, OROS osmotic technology, extended‐release hydromorphone formulation in the treatment of chronic malignant or nonmalignant pain. Journal of Pain and Symptom management 2002;23(5):355‐68. [PUBMED: 12007754] [DOI] [PubMed] [Google Scholar]
- Pigni A, Brunelli C, Caraceni A. The role of hydromorphone in cancer pain treatment: a systematic review. Palliative Medicine 2011;25(5):471‐7. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Lesage P. Management of cancer pain. Lancet 1999;353:1695‐700. [DOI: 10.1016/S0140-6736(99)01310-0] [DOI] [PubMed] [Google Scholar]
- Portenoy RK. Treatment of cancer pain. Lancet 2011;377(9784):2236‐47. [DOI] [PubMed] [Google Scholar]
- Quigley C, Wiffen P. A systematic review of hydromorphone in acute and chronic pain. Journal of Pain and Symptom Management 2003;25(2):169‐78. [PUBMED: 12590032] [DOI] [PubMed] [Google Scholar]
- Quigley C. Opioid switching to improve pain relief and drug tolerability. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004847.pub2] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Running A, Turnbeaugh E. Oncology pain and complementary therapy. Clinical Journal of Oncology Nursing 2011;15(4):374‐9. [DOI] [PubMed] [Google Scholar]
- Sarhill N, Walsh D, Nelson KA. Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Cancer 2001;9(2):84‐96. [DOI] [PubMed] [Google Scholar]
- Sathyan G, Xu E, Thipphawong J, Gupta SK. Pharmacokinetic profile of a 24‐hour controlled‐release OROS formulation of hydromorphone in the presence and absence of food. BMC Clinical Pharmacology 2007;7:2. [PUBMED: 17270055] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trescot AM, Boswell MV, Atluri SL, Hansen HC, Deer TR, Abdi S, et al. Opioid guidelines in the management of chronic non‐cancer pain. Pain Physician 2006;9(1):1‐39. [PUBMED: 16700278] [PubMed] [Google Scholar]
- Twycross RG. Pain Relief in Advanced Cancer. Singapore: Churchill Livingstone, 1994:279. [Google Scholar]
- Urquhart ML, Klapp K, White PF. Patient controlled analgesia: a comparison of intravenous versus subcutaneous hydromorphone. Anesthesiology 1988;69:428‐32. [PubMed] [Google Scholar]
- Beuken‐van Everdingen MHJ, Rijke JM, Kessels AG, Schouten HC, Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Annals of Oncology 2007;18(9):1437‐49. [DOI] [PubMed] [Google Scholar]
- Verma RK, Krishna DM, Garg S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. Journal of Controlled Release : official journal of the Controlled Release Society 2002;79(1‐3):7‐27. [PUBMED: 11853915] [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cancer Pain Relief. 2nd Edition. Geneva: WHO, 1986. [Google Scholar]
- Wiffen PJ, Wee B, Moore RA. Oral morphine for cancer pain. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003868.pub3] [DOI] [PubMed] [Google Scholar]
- Wiffen PJ, Derry S, Moore RA. Impact of morphine, fentanyl, oxycodone or codeine on patient consciousness, appetite and thirst when used to treat cancer pain. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD011056.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovlev AE, Ellia Y. Spinal cord stimulation as a treatment option for intractable neuropathic cancer pain. Clinical Medicine & Research 2008;6(3‐4):103‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Quigley C. Hydromorphone for acute and chronic pain. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD003447.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


