Abstract
Objective.
Obesity has been strongly linked to endometrial cancer (EC) risk. A number of potential EC risk bio-markers have been proposed, including heightened pro-inflammatory cytokines and adipokines. To evaluate if bariatric surgery can serve as a means for altering levels of such EC risk biomarkers, we investigated changes in these biomarkers after weight loss.
Methods.
Blood samples were collected pre-operatively and 6 months post-operatively in107 female bariatric surgery patients aged 18–72 years. Wilcoxon signed-rank tests were used to compare biomarker levels (measured using xMAP immunoassays) pre- and post-surgery. Normative comparisons were implemented to contrast 6-month post-surgery biomarker levels to levels in a sample of 74 age-matched non-obese women. Linear regression was used to evaluate the relationship between biomarker expression at baseline and 6 months post-surgery and the relationship between race and biomarker levels.
Results.
On average, participants lost 30.15 kg (SD: 12.26) after the bariatric intervention. Levels of C-peptide, insulin, CRP, leptin, IL-1Rα, and IL-6 significantly decreased, while levels of SHBG, IGFBP1, and adiponectin significantly increased with weight loss. Normative comparisons showed the levels of SHBG, C-peptide, insulin, IGFBP1, adiponectin, CRP, and TNFα after bariatric intervention approached the level of markers in comparison group. Multiple regression analyses revealed significant relationships between changes in BMI and changes in biomarker levels. The changes in IL-1Rα were significantly associated with race.
Conclusions.
Our findings demonstrate that normalization of EC risk biomarkers can be achieved with bariatric surgery. Improved understanding of biological mechanisms associated with weight loss may inform preventive strategies for EC.
Keywords: Endometrial cancer, Obesity, Biomarkers, Bariatric surgery, Weight loss
1. Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy among American women, and has been gradually increasing in incidence in recent years, with approximately 61,380 new diagnoses and 10,920 deaths expected in 2017 [1]. A recent publication from our group estimates a 55% increase in the incidence of EC by 2030 [2]. Although multiple factors are involved, increasing rates of obesity are thought to be the primary driver of increasing EC incidence [3–5]. Prospective studies indicate that EC risk increases 1.6-fold with each additional 5 kg/m2 in body mass index (BMI), reaching 9.1-fold higher risk at 42 kg/m2 [6]. In a recent publication, we indicated that increasing BMI is also associated with a greater risk of endometrial pathology among women with severe obesity (>35 BMI) [7]. As of 2016, no systemic biomarker (or panel of markers) is available to identify women at high risk of precancerous changes, at a time when preventive interventions such as weight loss or hormone therapy may still be possible.
Accumulating evidence from preclinical research, as well as prospective studies exploring associations between biomarker levels in peripheral blood and the development of EC, strongly implicates three basic biological pathways: pro-inflammatory factors, insulin resistance/metabolic factors, and steroid hormones [8–13]. Obesity is associated with a physiological state of chronic, low-grade inflammation, characterized by elevated systemic levels of circulating inflammatory biomarkers mediating, at least in part, the association between obesity and risk of EC [12,14,15]. Increased adipose tissue mass may contribute to the development of cancer via increased secretion of pro-inflammatory cytokines and chemokines [16,17]. A recent study found CRP, an acute-phase reactant protein that can influence production of inflammatory cytokines, to be positively associated with EC risk [13]. CRP, IL-6, and IL-1Rα have been implicated in EC risk in several prospective investigations [11–13,16]. Circulating adipokines (small protein molecules produced and secreted by white adipose tissue), such as adiponectin, have systemic immunomodulating effects that also play a major role in the development of several cancers [18]. Insulin, IGFBP2, leptin, adiponectin, and C-peptide have been implicated in EC development in prospective studies [11,13,19,20]. While a very limited number of publications have explored the biomarkers associated with endometrial hyperplasia (EC precursor lesion [21 ]), it is likely that the development of hyperplasia and EC are associated with abnormal activity of similar inflammatory, hormonal, and metabolic pathways. One such hormone is leptin, which has been found to be elevated in patients with both endometrial cancer and hyperplasia in comparison with pathology free controls [22].
Emerging literature suggests that the risk of EC may be particularly responsive to weight loss [3,4,23]. In a large-scale study, Ward et al. recently demonstrated that bariatric surgery is associated with a 71% reduction in risk for uterine malignancy [24]. Similar evidence has been recently published by the Swedish Obese Subject Study, reporting that bariatric surgery lowered the incidence of EC in the bariatric surgery group [25].
The study described in this manuscript was based on the idea that biomarkers in EC risk pathways will be ameliorated by weight loss among women with severe obesity. Our group previously reported that behavioral intervention for weight loss is associated with changes in adiponectin[26]. In this manuscript, we report on the modification of EC risk biomarkers with surgically induced weight loss, an area poorly investigated in existing studies. This study aims to fill an important gap by analyzing biomarkers associated with EC risk in women undergoing weight loss through bariatric surgery and comparing the levels of markers post-bariatric surgery with levels of the same markers in non-obese women.
2. Methods
2.1. Participants and settings
This subanalysis included 107 women aged 18 to 72 (mean age 43.88 years (SD: 11.66 years)) who were participating in the “Effect of weight loss on biomarkers of immunity and inflammation” or Bariatric Marker (BAM) Study at Magee-Womens Hospital which had the goal of determining the extent to which women undergoing bariatric surgery, and its subsequent weight loss, had an overall improvement in inflammatory and endocrine biomarker status. Inclusion criteria for this study included: female, age 18+ years, BMI ≥ 35, approved and scheduled for bariatric surgery (Roux-en-Y gastric bypass, laparoscopic adjustable gastric banding, or sleeve gastrectomy), and life expectancy >3 years. Exclusion criteria included: refusal to sign informed consent, unable to attend study visits, plans to become pregnant within one year after surgery, presence of severe inflammatory disease, previous history of cancer (including gynecologic cancer), recent injury or surgery, and plans to move residence within one year.
Seventy-four female non-obese (BMI < 30) comparison participants aged 21 to 75 (mean age 42.04 years (SD: 13.53 years)) were recruited through a general recruitment campaign at the University of Pittsburgh campus. Comparison participants were matched to bariatric surgery patients on the basis of age. The University of Pittsburgh Human Research Protection Office approved this study. All participants signed informed consent documents. Exclusion criteria for comparisons were inability to sign informed consent, obesity, presence of severe inflammatory disease, previous history of cancer (other than non-melanoma skin cancer), and recent injury or surgery.
At each visit (pre- and 6 months post-operatively), participants in the bariatric surgery group completed a set of validated general health questionnaires, and anthropometric measurements and blood samples were obtained. The same procedure was performed at a single time point with participants in the comparison group. Registered research nurses and staff conducted study procedures in the Clinical and Translational Research Center (CTRC) at Magee-Womens Hospital of UPMC.
2.2. Measures
Anthropometric measurements for participants in both groups were obtained in the CTRC by research staff. Height was measured in centimeters using a wall-mounted stadiometer. Waist and hip circumference was measured in centimeters using a tape measure. Weight (kilograms) and BMI (kg/m2) were obtained from the Tanita body composition analyzer (Model TBF-310, Tanita Corporation of America) with participants wearing light clothing and no footwear.
Reproductive history, menstrual history, and history of hormone use (hormone therapy, birth control, fertility drugs) were obtained from the Reproductive Health Baseline (RHB) and the Screening Questionnaire for General Health History (SQHH). The RHB was used in the Longitudinal Assessment of Bariatric Surgery-2 Study (LABS) to collect information on the status of the reproductive health of women undergoing bariatric surgery [27]. The SQHH has been used in the Paving the road to everlasting food and exercise routines (PREFER) and Self-monitoring and recording using technology (SMART) studies, which tested several methods of behavioral weight management [28,29].
2.3. Statistical analysis
Basic descriptive statistics were used to summarize the characteristics of the bariatric surgery population at baseline and the 6-month post-operative visit. Continuous variables are reported as mean (standard deviation) and categorical variables are reported as N (%). Age, BMI, weight, waist circumference (WC), and waist-to-hip ratio (WHR) were analyzed as continuous variables. Race (dichotomized as European American (EA) or African American (AA)) was analyzed as a categorical variable. The time points were categorized as baseline and 6 months post-surgery.
Wilcoxon signed-rank tests were used to compare the mean levels of biomarkers of the participants in the bariatric surgery group at the baseline and 6-month post-operative visits. The Wilcoxon rank-sum test was used to compare mean biomarker levels of the participants in the bariatric surgery group at each time point and in the comparison group to determine if there were significant differences in mean levels between the groups. Fisher’s exact test was used to compare categorical variables of participants in the bariatric surgery group at baseline and in the comparison group to determine if there were significant differences between the groups.
Biomarker levels were log transformed to reduce skewness. Normative comparisons are a set of procedures that determine the clinical significance of an intervention used to treat participants compared to the untreated normal participants [30]. If the difference between means of the treated participants and normal participants falls in a specified range of closeness that centers on zero, the two groups are considered clinically equivalent. This range is bounded by a lower limit, negative delta ( − δ), and an upper limit, positive delta ( + δ). For our paper, we chose a range of closeness of one standard deviation of the normative mean for each biomarker level, which is consistent with the previously published article by Kendall et al. [30]. Thus, if the difference is within this range, we conclude that the two groups are clinically equivalent. Next, two one-sided t-tests were done to test the null that the difference of the means is less than the lower limit and greater than the upper limit, respectively. A statistically significant result for both tests indicated that the difference between the means was within the range of closeness and the post-treatment group and the normal group are clinically equivalent. Then, the means of the post-treatment group and the normal group were compared using a traditional two-sided hypothesis test [30] (e.g. two-sided t-test), where the null hypothesis was that the two means are equal. In the final step, the results from the traditional hypothesis test and the clinical equivalency test were compared via a 2 × 2 table adapted from Kendall et al. [30]. The overall goal of this approach was to demonstrate if weight loss resulted in normalization of the biomarker levels of the bariatric surgery participants.
In a secondary analysis, we looked at the relationships between race and biomarker levels at baseline and 6 months post-bariatric surgery. Linear regression was used to evaluate the relationship between biomarker expression from baseline to 6 months and the biomarker levels during the same time period, controlling for baseline BMI, hormone use, diabetes status, and race. An interaction between BMI and race was examined to assess the relationship between biomarker expression, the change in BMI, and race. All statistical analyses were done with SAS version 9.4 (SAS Institute Inc., Cary, NC). α level was set at 0.01 to adjust for multiple comparisons and was two-sided.
3. Results
Table 1 lists the basic characteristics of the bariatric surgery group and the comparison group at baseline. Mean age of the bariatric surgery group participants was 43.88 years (SD: 11.66), mean weight was 123.88 kg (SD: 19.71), and mean BMI was 45.52 kg/m2 (SD: 6.19). Bariatric surgery group and control group were similar in terms of racial and age distribution.
Table 1.
Demographic and personal characteristics of bariatric surgery candidates at pre-operative visit and comparison participants.
| Bariatric surgery n = 107 |
Comparisons n = 74 |
p-Value | |
|---|---|---|---|
| Age (years), mean (SD) | 43.88 (11.66) | 42.04(13.53) | 0.2102* |
| Weight (kg), mean (SD) | 123.88 (19.71) | 60.01 (11.36) | <0.0001* |
| BMI (kg/m2), mean (SD) | 45.52 (6.19) | 22.61 (2.87) | <0.0001* |
| Race, N (%) | 0.8385** | ||
| European American | 82 (76.64) | 60 (81.08) | |
| African American | 25 (23.36) | 14 (18.92) | |
| Hormone Use (N = 105) | |||
| Yes | 17 (16.19%) | ||
| No | 88 (83.81%) | ||
| Diabetes (N = 100) | |||
| Yes | 15 (15.0%) | ||
| No | 85 (85.0%) | ||
p-Value from Wilcoxon rank sum test.
p-Value from Fisher’s exact test.
Bariatric surgery group participants lost an average of 30.15 kg (SD: 12.26) from baseline to the 6-month post-operative visit. BMI decreased an average of 11.03 kg/m2 (SD: 4.67), WC decreased by 21.10 cm (SD: 11.37), and the WHR decreased from a mean of 0.88 (SD: 0.07) to a mean of 0.86 (SD: 0.08) in the 6-month post-operative period. The decreases in weight, BMI, WC, and WHR from baseline to 6 months were statistically significant (all p < 0.01).
Fig. 1 shows the mean levels of the biomarkers for the 6-month visit for the bariatric surgery group and the comparison group. SHBG, adiponectin, IGFBP1, and IGFBP2 significantly increased (p-values < 0.01) from the baseline to the 6-month visit, and C-peptide, insulin, CRP, leptin, IL-1Rα, and IL-6 significantly decreased from the baseline to the 6-month visit (p-values < 0.01). When compared to biomarker levels in the comparison group, SHBG, IGFBP1, and adiponectin were significantly lower than comparison participants at both the baseline and 6-month visits, and C-peptide, insulin, CRP, resistin, leptin, IL-1Rα, IL-6, and TNFα were significantly higher in the bariatric surgery group than the levels of the comparison group at both visits. IGFBP2 was lower at baseline in the bariatric surgery group, but the levels increased significantly between baseline and the 6-month postoperative visit to levels significantly higher than was observed among comparison group participants.
Fig. 1.
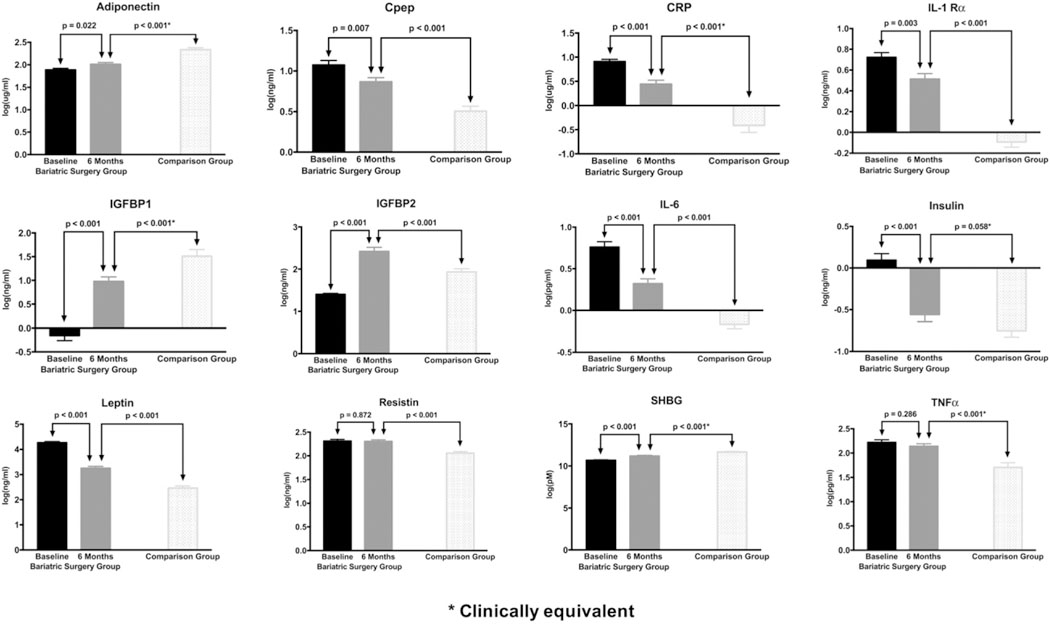
Biomarker levels over time in bariatric surgery patients and in non-obese comparison participants (log transformed values).
From the normative comparisons, SHBG, C-peptide, IGFBP1, adiponectin, CRP, and TNFα levels in the bariatric surgery group were statistically different, but clinically equivalent to the comparison group at the 6-month post-operative visit (data not shown). IGFBP2, resistin, leptin, IL-1Rα, and IL-6 were clinically different from the comparison group at the 6-month post-operative visit. Insulin was clinically equivalent to the comparison group at the 6-month post-operative visit.
In the analysis by race for the surgery group, at baseline (data not shown), AA women had lower levels of potential EC risk biomarkers than EA women; however, only IGFBP1 and adiponectin were significantly lower in AA women (p < 0.01). At 6 months (data not shown), IGFBP1,IGFBP2, and IL-1 Rα were significantly lower in AA women (p < 0.01). From the linear regression models, the change in IL-1Rα was significantly associated with race (p-value = 0.0009). Hormone use and diabetes status were not significantly associated with any of the biomarkers. Table 2 details the results of the linear regression.
Table 2.
The linear regression equation: Δbiomarker level = age + race + baseline biomarker + baseline BMI + ΔBMI.
| Change (Δ)in biomarker levels | Age | Race | Baseline biomarker level | Baseline BMI | ΔBMI |
|---|---|---|---|---|---|
| ΔSHBG | –0.006 | – 0.297 | 0.374 | 0.017 | −0.041 |
| (0.26)* | (0.06) | (0.002) | (0.16) | (0.01) | |
| ΔC-peptide | –0.002 | 0.114 | 0.788 | – 0.006 | 0.025 |
| (0.57) | (0.30) | (<0.0001) | (0.49) | (0.03) | |
| ΔInsulin | 0.001 | – 0.043 | 0.794 | –0.010 | 0.041 |
| (0.85) | (0.81) | (<0.0001) | (0.43) | (0.02) | |
| ΔIGFBP1 | –0.010 | 0.047 | 0.620 | 0.036 | − 0.078 |
| (0.14) | (0.79) | (<0.0001) | (0.006) | (<0.0001) | |
| ΔIGFBP2 | –0.014 | 0.228 | – 0.401 | 0.044 | − 0.132 |
| (0.03) | (0.19) | (0.22) | (0.001) | (<0.0001) | |
| ΔAdiponectin | – 0.004 | – 0.039 | 0.227 | 0.014 | − 0.035 |
| (0.16) | (0.59) | (0.01) | (0.008) | (<0.0001) | |
| ΔCRP | – 0.005 | 0.446 | 0.226 | – 0.056 | 0.061 |
| (0.48) | (0.02) | (0.18) | (0.0001) | (0.002) | |
| ΔResistin | 0.0004 | 0.023 | 0.294 | –0.013 | 0.0003 |
| (0.84) | (0.65) | (<0.0001) | (0.0006) | (0.95) | |
| ΔLeptin | 0.001 | –0.121 | 0.518 | – 0.059 | 0.124 |
| (0.73) | (0.17) | (<0.0001) | (<0.0001 ) | (< 0.0001) | |
| ΔIL-1Rα | 0.004 | 0.360 | 0.458 | –0.016 | 0.023 |
| (0.27) | (0.0009) | (< 0.0001) | (0.04) | (0.03) | |
| ΔIL-6 | –0.0003 | – 0.093 | 0.342 | – 0.007 | 0.020 |
| (0.94) | (0.34) | (<0.0001) | (0.36) | (0.04) | |
| ΔTNFα | – 0.0005 | 0.141 | 0.315 | – 0.006 | 0.002 |
| (0.88) | (0.11) | (<0.0001) | (0.39) | (0.86) | |
p-Values are in parentheses.
4. Discussion
In this longitudinal analysis of biomarker changes occurring with weight loss, we found that the mean levels of the following biomarkers in the bariatric surgery group after 6 months of weight loss approach normative levels in a comparison group: SHBG, C-peptide, insulin, IGFBP1, adiponectin, CRP, and TNFα. The increase in adiponectin reported with weight loss is consistent with findings reported by Arita [31]. Findings are also consistent with previously published literature on biomarker changes occurring with weight loss [26,32].
This is one of the first studies to evaluate changes in EC risk biomarkers in a group of women undergoing bariatric surgery and to demonstrate that these biomarkers trend toward normalization with weight loss. The idea of preventing EC by weight loss is an attractive idea, as weight loss would also improve cardiovascular fitness, reduce Type 2 diabetes, and possibly reduce the risk of other obesity-related cancers [3]. The possibility of preventing EC through weight loss is particularly important to investigate for AA women, who bear a disproportionate burden of EC mortality [33]. Our findings that race is associated with changes in several EC risk biomarkers is interesting in the context of our previous publication suggestion differential expression of EC-associated biomarkers in AA in comparison to EA women [34]. Bariatric surgery patients would be an ideal group in which to investigate EC prevention through weight loss because they exhibit a very substantial and rapid weight loss.
Although women can lose weight through other means, such as diet and exercise, the literature suggests that diet and exercise programs for individuals with severe obesity result in sustainable weight loss in <20% of program participants [35], while bariatric surgery results in sustainable weight loss in over 80% of patients [36]. In a prospective Swedish study of a cohort of bariatric surgery patients and matched comparisons, sustained weight loss was associated with a 38% reduction of cancer incidence in women [37]. In another prospective study, a 24% reduction in incident cancers was seen among 6596 women who underwent bariatric surgery, in comparison with controls, over a 12.5-year follow-up period [37]. The most impressive reduction in cancer risk was seen for EC with a seven-fold risk reduction [37]. Taken together, these studies strongly suggest that EC is preventable through bariatric surgery-induced weight loss [3]. Our results raise the possibility that these effects may be mediated by changes in EC risk biomarker levels.
Key limitations of this study include a relatively small sample size and a short follow-up period; however, the results of this initial study will help to form future research questions and research studies in the field of EC prevention. Additionally, future investigations will include more than one follow-up point and be expanded to multiple ethnic and racial groups. While this study focused on EC risk biomarkers, our future studies will also evaluate similar biomarkers in the context of confirmed cancer, as similar biomarkers have are associated with EC development, progression, and prognosis [38]. Furthermore, our future research will focus on exploring endometrial tissues of women at high risk, as our previous studies indicated that bariatric patients may have unrecognized endometrial pathologies that may resolve following bariatric intervention and may be associated with normalization of endometrial tissue biomarkers [7,39–41].
EC risk has been associated with a number of factors in addition to obesity, including diabetes, hormone use, and smoking history [42]. These variables, as well as depressive symptoms and quality of life, have been implicated in altered levels of several cytokines [43] and have been positively associated with inflammatory markers such as CRP and IL-6, with BMI implicated as a mediating/moderating factor [44]. Thus, additional contributions of these factors will need to be assessed in conjunction with further investigation of inflammatory cytokines, metabolic, and hormonal factors in future investigations. Results of this study will have important implications for weight loss-based EC prevention programs and alternative treatments for women with high EC risk due to obesity. This concept is consistent with EC prevention targets identified by Hursting [45], which include host factors such as inflammatory-related molecules, insulin, leptin, adiponectin, and other cytokines evaluated in conjunction with systems level look at EC prevention targets.
HIGHLIGHTS.
Endometrial cancer associated biomarker levels decreased after bariatric surgery.
The change in CRP and IL-1Rα, and SHBG were associated with race.
After surgery biomarker levels in surgery group were equivalent to healthy controls.
Acknowledgments
Funding
This research was funded by the American Cancer Society (MRSG-1—079–01-CPPB), Hillman Foundation, and the Scaife Foundation at the University of Pittsburgh School of Medicine. This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory that is supported in part by award P30CA047904. This publication was also partially supported by funds received from the NIH CTSA Grant Numbers UL1RR024153 and UL1TR000005.
Footnotes
Conflict of interest
None reported.
References
- [1].ACS, Available from: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html 2017.
- [2].Sheikh MA, Althouse AD, Freese KE, Soisson S, Edwards RP, Welburn S, Sukumvanich P, Comerci J, Kelley J, LaPorte RE, Linkov F, USA endometrial cancer projections to 2030: should we be concerned? Future Oncol. 10 (16) (2014) 2561–2568. [DOI] [PubMed] [Google Scholar]
- [3].Mackintosh ML, Crosbie EJ, Obesity-driven endometrial cancer: is weight loss the answer? BJOG 120 (7) (2013) 791–794. [DOI] [PubMed] [Google Scholar]
- [4].Schmandt RE, Iglesias DA, Co NN, Lu KH, Understanding obesity and endometrial cancer risk: opportunities for prevention, Am. J. Obstet. Gynecol 205 (6) (2011) 518–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shaw E, Farris M, McNeil J, Friedenreich C, Obesity and endometrial cancer, Recent Results Cancer Res. 208 (2016) 107–136. [DOI] [PubMed] [Google Scholar]
- [6].Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M, Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies, Lancet 371 (9612) (2008) 569–578. [DOI] [PubMed] [Google Scholar]
- [7].Kaiyrlykyzy A, Freese KE, Elishaev E, Bovbjerg DH, Ramanathan R, Hamad GG, McCloskey C, Althouse AD, Huang M, Edwards RP, Linkov F, Endometrial histology in severely obese bariatric surgery candidates: an exploratory analysis, Surg. Obes. Relat. Dis 11 (3) (2015) 653–658. [DOI] [PubMed] [Google Scholar]
- [8].Cust AE, Allen NE, Rinaldi S, Dossus L, Friedenreich C, Olsen A, Tjonneland A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Chang-Claude J, Boeing H, Schulz M, Benetou V, Trichopoulou A, Trichopoulos D, Palli D, Berrino F, Tumino R, Mattiello A, Vineis P, R Quiros J, Agudo A, Sanchez MJ, Larranaga N, Navarro C, Ardanaz E, Bueno-de-Mesquita HB, Peeters PH, van Gils CH, Bingham S, Khaw KT, Key T, Slimani N, Riboli E, Kaaks R, Serum levels of C- peptide, IGFBP-1 and IGFBP-2 and endometrial cancer risk; results from the European prospective investigation into cancer and nutrition, Int. J. Cancer 120 (12) (2007) 2656–2664. [DOI] [PubMed] [Google Scholar]
- [9].Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, Rinaldi S, Dossus L, Slimani N, Lundin E, Tjonneland A, Olsen A, Overvad K, Clavel- Chapelon F, Mesrine S, Joulin V, Linseisen J, Rohrmann S, Pischon T, Boeing H, Trichopoulos D, Trichopoulou A, Benetou V, Palli D, Berrino F, Tumino R, Sacerdote C, Mattiello A, Quiros JR, Mendez MA, Sanchez MJ, Larranaga N, Tormo MJ, Ardanaz E, Bueno-de-Mesquita HB, Peeters PH, van Gils CH, Khaw KT, Bingham S, Allen N, Key T, Jenab M, Riboli E, Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women, J. Clin. Endocrinol. Metab 92 (1) (2007)255–263. [DOI] [PubMed] [Google Scholar]
- [10].Dossus L, Becker S, Rinaldi S, Lukanova A, Tjonneland A, Olsen A, Overvad K, Chabbert-Buffet N, Boutron-Ruault MC, Clavel-Chapelon F, Teucher B, Chang-Claude J, Pischon T, Boeing H, Trichopoulou A, Benetou V, Valanou E, Palli D, Sieri S, Tumino R, Sacerdote C, Galasso R, Redondo ML, Bonet CB, Molina- Montes E, Altzibar JM, Chirlaque MD, Ardanaz E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Onland-Moret NC, Lundin E, Idahl A, Khaw KT, Wareham N, Allen N, Romieu I, Fedirko V, Hainaut P, Romaguera D, Norat T, Riboli E, Kaaks R, Tumor necrosis factor (TNF)-alpha, soluble TNF receptors and endometrial cancer risk: the EPIC study, Int. J. Cancer 129 (8) (2011) 2032–2037. [DOI] [PubMed] [Google Scholar]
- [11].Dossus L, Lukanova A, Rinaldi S, Allen N, Cust AE, Becker S, Tjonneland A, Hansen L, Overvad K, Chabbert-Buffet N, Mesrine S, Clavel-Chapelon F, Teucher B, Chang-Claude J, Boeing H, Drogan D, Trichopoulou A, Benetou V, Bamia C, Palli D, Agnoli C, Galasso R, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Onland-Moret NC, Redondo ML, Travier N, Sanchez MJ, Altzibar JM, Chirlaque MD, Barricarte A, Lundin E, Khaw KT, Wareham N, Fedirko V, Romieu I, Romaguera D, Norat T, Riboli E, Kaaks R, Hormonal, metabolic, and inflammatory profiles and endometrial cancer risk within the EPIC cohort—a factor analysis, Am. J. Epidemiol. 177 (8) (2013) 787–799. [DOI] [PubMed] [Google Scholar]
- [12].Dossus L, Rinaldi S, Becker S, Lukanova A, Tjonneland A, Olsen A, Stegger J, Overvad K, Chabbert-Buffet N, Jimenez-Corona A, Clavel-Chapelon F, Rohrmann S, Teucher B, Boeing H, Schutze M, Trichopoulou A, Benetou V, Lagiou P, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Redondo ML, Travier N, Sanchez MJ, Altzibar JM, Chirlaque MD, Ardanaz E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Onland-Moret NC, Peeters PH, Hallmans G, Lundin E, Khaw KT, Wareham N, Allen N, Key TJ, Slimani N, Hainaut P, Romaguera D, Norat T, Riboli E, Kaaks R, Obesity, inflammatory markers, and endometrial cancer risk: a prospective case-control study, Endocr. Relat. Cancer 17 (4) (2010) 1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang T, Rohan TE, Gunter MJ, Xue X, Wactawski-Wende J, Rajpathak SN, Cushman M, Strickler HD, Kaplan RC, Wassertheil-Smoller S, Scherer PE, Ho GY, A prospective study of inflammation markers and endometrial cancer risk in postmenopausal hormone nonusers, Cancer Epidemiol. Biomark. Prev 20 (5) (2011) 971–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balistreri CR, Caruso C, Candore G, The role of adipose tissue and adipokines in obesity-related inflammatory diseases, Mediat. Inflamm 2010 (2010) 802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Harvey AE, Lashinger LM, Hursting SD, The growing challenge of obesity and cancer: an inflammatory issue, Ann. N. Y. Acad. Sci. 1229 (2011) 45–52. [DOI] [PubMed] [Google Scholar]
- [16].Friedenreich CM, Langley AR, Speidel TP, Lau DC, Courneya KS, Csizmadi I, Magliocco AM, Yasui Y, Cook LS, Case-control study of inflammatory markers and the risk of endometrial cancer, Eur. J. Cancer Prev. 22 (4) (2013) 374–379. [DOI] [PubMed] [Google Scholar]
- [17].Prieto-Hontoria PL, Perez-Matute P, Fernandez-Galilea M, Bustos M, Martinez JA Moreno-Aliaga MJ, Role of obesity-associated dysfunctional adipose tissue in cancer: a molecular nutrition approach, Biochim. Biophys. Acta 1807 (6) (2011) 664–678. [DOI] [PubMed] [Google Scholar]
- [18].Cancello R, Clement K, Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue, BJOG 113 (10) (2006) 1141–1147. [DOI] [PubMed] [Google Scholar]
- [19].Wang PP,He XY, Wang R, Wang Z, Wang YG, High leptin level is an independent risk factor of endometrial cancer: a meta-analysis, Cell. Physiol. Biochem. 34 (5) (2014) 1477–1484. [DOI] [PubMed] [Google Scholar]
- [20].Dallal CM, Brinton LA, Bauer DC, Buist DS, Cauley JA, Hue TF, Lacroix A, Tice JA, Chia VM, Falk R, Pfeiffer R, Pollak M, Veenstra TD, Xu X, Lacey JV Jr., Obesity-related hormones and endometrial cancer among postmenopausal women: a nested case-control study within the B007EFIT cohort, Endocr. Relat. Cancer 20 (1) (2013) 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shaarawy M, El-Sharkawy SA, Biomarkers of intrinsic angiogenic and anti-angiogenic activity in patients with endometrial hyperplasia and endometrial cancer, Acta Oncol. 40 (4) (2001) 513–518. [DOI] [PubMed] [Google Scholar]
- [22].Cymbaluk A, Chudecka-Glaz A, Rzepka-Gorska I, Leptin levels in serum depending on body mass index in patients with endometrial hyperplasia and cancer, Eur. J. Obstet. Gynecol. Reprod. Biol 136 (1) (2008) 74–77. [DOI] [PubMed] [Google Scholar]
- [23].Luo J, Chlebowski RT, Hendryx M, Rohan T, Wactawski-Wende J, Thomson CA, Felix AS, Chen C, Barrington W, Coday M, Stefanick M, LeBlanc E, Margolis KL, Intentional weight loss and endometrial cancer risk, J. Clin. Oncol 35 (11) (2017) 1189–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ward KK, Roncancio AM, Shah NR, Davis MA, Saenz CC, McHale MT, Plaxe SC, Bariatric surgery decreases the risk of uterine malignancy, Gynecol. Oncol. 133 (1) (2014) 63–66. [DOI] [PubMed] [Google Scholar]
- [25].Anveden A, Taube M, Peltonen M, Jacobson P, Andersson-Assarsson JC, Sjoholm K, Svensson PA, Carlsson LMS, Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study, Gynecol. Oncol. 145 (2) (2017) 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Linkov F, Burke LE, Komaroff M,Edwards RP, Lokshin A, Styn MA, Tseytlin E, Freese KE, Bovbjerg DH, An exploratory investigation of links between changes in adipokines and quality oflife in individuals undergoing weight loss interventions: possible implications for cancer research, Gynecol. Oncol. 133 (1) (2014) 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gosman GG, King WC, Schrope B, Steffen KJ, Strain GW, Courcoulas AP, Flum DR, Pender JR, Simhan HN, Reproductive health ofwomen electing bariatric surgery, Fertil. Steril. 94 (4) (2010) 1426–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Burke LE, Choo J, Music E, Warziski M, Styn MA, Kim Y, Sevick MA, PREFER study: a randomized clinical trial testing treatment preference and two dietary options in behavioral weight management — rationale, design and baseline characteristics, Contemp. Clin. Trials 27 (1) (2006) 34–38. [DOI] [PubMed] [Google Scholar]
- [29].Burke LE, Styn MA, Glanz K, Ewing LJ, Elci OU, Conroy MB, Sereika SM, Acharya SD, Music E, Keating AL, Sevick MA, SMART trial: a randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings, Contemp. Clin. Trials 30 (6) (2009) 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kendall PC, Marrs-Garcia A, Nath SR, Sheldrick RC, Normative comparisons for the evaluation of clinical significance, J. Consult. Clin. Psychol 67 (3) (1999) 285–299. [DOI] [PubMed] [Google Scholar]
- [31].Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y, Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. 1999, Biochem. Biophys. Res. Commun. 425 (3) (2012) 560–564. [DOI] [PubMed] [Google Scholar]
- [32].Linkov F, Maxwell GL, Felix AS, Lin Y, Lenzner D, Bovbjerg DH, Lokshin A, Hennon M, Jakicic JM, Goodpaster BH, DeLany JP, Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: implications for cancer risk reduction, Gynecol. Oncol. 125 (1) (2012) 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Key statistics for endometrial cancer, Available from: http://www.cancer.org/cancer/endometrialcancer/detailedguide/endometrial-uterine-cancer-key-statistics 2016.
- [34].Linkov F, Goughnour SL, Edwards RP, Lokshin A, Ramanathan RC, Hamad GG, McCloskey C, Bovbjerg DH, Endometrial cancer associated biomarkers in bariatric surgery candidates: exploration of racial differences, Surg. Obes. Relat. Dis 13 (5) (2017) 862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Patterson EJ, Urbach DR, Swanstrom LL, A comparison of diet and exercise therapy versus laparoscopic Roux-en-Y gastric bypass surgery for morbid obesity: a decision analysis model, J. Am. Coll. Surg 196 (3) (2003) 379–384. [DOI] [PubMed] [Google Scholar]
- [36].Schweitzer M, Lidor A, Magnuson T, Bariatric surgery, Adv. Psychosom. Med 27 (2006) 53–60. [DOI] [PubMed] [Google Scholar]
- [37].Sjostrom L, Gummesson A, Sjostrom CD, Narbro K, Peltonen M, Wedel H, Bengtsson C, Bouchard C, Carlsson B, Dahlgren S, Jacobson P, Karason K, Karlsson J, Larsson B, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Carlsson LM, Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial, Lancet Oncol. 10 (7) (2009) 653–662. [DOI] [PubMed] [Google Scholar]
- [38].Schmid M, Schneitter A, Hinterberger S, Seeber J, Reinthaller A, Hefler L, Association of elevated C-reactive protein levels with an impaired prognosis in patients with surgically treated endometrial cancer, Obstet. Gynecol. 110 (6) (2007) 1231–1236. [DOI] [PubMed] [Google Scholar]
- [39].Argenta PA, Kassing M, Truskinovsky AM, Svendsen CA, Bariatric surgery and endometrial pathology in asymptomatic morbidly obese women: a prospective, pilot study, BJOG 120 (7) (2013) 795–800. [DOI] [PubMed] [Google Scholar]
- [40].Argenta P, Svendsen C, Elishaev E, Gloyeske N, Geller MA, Edwards RP, Linkov F, Hormone receptor expression patterns in the endometrium of asymptomatic morbidly obese women before and after bariatric surgery, Gynecol. Oncol. 133 (1) (2014) 78–82. [DOI] [PubMed] [Google Scholar]
- [41].Linkov F, Elishaev E, Gloyeske N, Edwards R, Althouse AD, Geller MA, Svendsen C, Argenta PA, Bariatric surgery-induced weight loss changes immune markers in the endometrium of morbidly obese women, Surg. Obes. Relat. Dis. 10 (5) (2014) 921–926. [DOI] [PubMed] [Google Scholar]
- [42].Modugno F, Ness RB, Chen C, Weiss NS, Inflammation and endometrial cancer: a hypothesis, Cancer Epidemiol. Biomark. Prev. 14 (12) (2005) 2840–2847. [DOI] [PubMed] [Google Scholar]
- [43].Anisman H, Merali Z, Cytokines, stress, and depressive illness, Brain Behav. Immun. 16 (5) (2002)513–524. [DOI] [PubMed] [Google Scholar]
- [44].Soczynska JK, Kennedy SH, Woldeyohannes HO, Liauw SS, Alsuwaidan M, Yim CY, McIntyre RS, Mood disorders and obesity: understanding inflammation as a pathophysiological nexus, NeuroMolecular Med. 13 (2) (2011) 93–116. [DOI] [PubMed] [Google Scholar]
- [45].Hursting SD, Digiovanni J, Dannenberg AJ, Azrad M, Leroith D, Demark- Wahnefried W, Kakarala M, Brodie A, Berger NA, Obesity, energy balance, and cancer: new opportunities for prevention, Cancer Prev. Res. 5 (11) (2012) 1260–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]


