Abstract
Background
There is considerable uncertainty regarding the optimal haemoglobin threshold for the use of red blood cell (RBC) transfusions in anaemic patients. Blood is a scarce resource, and in some countries, transfusions are less safe than others because of a lack of testing for viral pathogens. Therefore, reducing the number and volume of transfusions would benefit patients.
Objectives
The aim of this review was to compare 30‐day mortality and other clinical outcomes in participants randomized to restrictive versus liberal red blood cell (RBC) transfusion thresholds (triggers) for all conditions. The restrictive transfusion threshold uses a lower haemoglobin level to trigger transfusion (most commonly 7 g/dL or 8 g/dL), and the liberal transfusion threshold uses a higher haemoglobin level to trigger transfusion (most commonly 9 g/dL to 10 g/dL).
Search methods
We identified trials by searching CENTRAL (2016, Issue 4), MEDLINE (1946 to May 2016), Embase (1974 to May 2016), the Transfusion Evidence Library (1950 to May 2016), the Web of Science Conference Proceedings Citation Index (1990 to May 2016), and ongoing trial registries (27 May 2016). We also checked reference lists of other published reviews and relevant papers to identify any additional trials.
Selection criteria
We included randomized trials where intervention groups were assigned on the basis of a clear transfusion 'trigger', described as a haemoglobin (Hb) or haematocrit (Hct) level below which a red blood cell (RBC) transfusion was to be administered.
Data collection and analysis
We pooled risk ratios of clinical outcomes across trials using a random‐effects model. Two people extracted the data and assessed the risk of bias. We conducted predefined analyses by clinical subgroups. We defined participants randomly allocated to the lower transfusion threshold as 'restrictive transfusion' and to the higher transfusion threshold as 'liberal transfusion'.
Main results
A total of 31 trials, involving 12,587 participants, across a range of clinical specialities (e.g. surgery, critical care) met the eligibility criteria. The trial interventions were split fairly equally with regard to the haemoglobin concentration used to define the restrictive transfusion group. About half of them used a 7 g/dL threshold, and the other half used a restrictive transfusion threshold of 8 g/dL to 9 g/dL. The trials were generally at low risk of bias .Some items of methodological quality were unclear, including definitions and blinding for secondary outcomes.
Restrictive transfusion strategies reduced the risk of receiving a RBC transfusion by 43% across a broad range of clinical specialties (risk ratio (RR) 0.57, 95% confidence interval (CI) 0.49 to 0.65; 12,587 participants, 31 trials; high‐quality evidence), with a large amount of heterogeneity between trials (I² = 97%). Overall, restrictive transfusion strategies did not increase or decrease the risk of 30‐day mortality compared with liberal transfusion strategies (RR 0.97, 95% CI 0.81 to 1.16, I² = 37%; N = 10,537; 23 trials; moderate‐quality evidence) or any of the other outcomes assessed (i.e. cardiac events (low‐quality evidence), myocardial infarction, stroke, thromboembolism (high‐quality evidence)). Liberal transfusion did not affect the risk of infection (pneumonia, wound, or bacteraemia).
Authors' conclusions
Transfusing at a restrictive haemoglobin concentration of between 7 g/dL to 8 g/dL decreased the proportion of participants exposed to RBC transfusion by 43% across a broad range of clinical specialities. There was no evidence that a restrictive transfusion strategy impacts 30‐day mortality or morbidity (i.e. mortality at other points, cardiac events, myocardial infarction, stroke, pneumonia, thromboembolism, infection) compared with a liberal transfusion strategy. There were insufficient data to inform the safety of transfusion policies in certain clinical subgroups, including acute coronary syndrome, myocardial infarction, neurological injury/traumatic brain injury, acute neurological disorders, stroke, thrombocytopenia, cancer, haematological malignancies, and bone marrow failure. The findings provide good evidence that transfusions with allogeneic RBCs can be avoided in most patients with haemoglobin thresholds above 7 g/dL to 8 g/dL.
Plain language summary
Is it safe to use lower blood counts as a trigger for blood transfusion in order to give fewer blood transfusions?
Background
Doctors and healthcare professionals often give blood transfusions to people after loss of blood from surgery, bleeding, or medical illnesses. Blood is a limited resource, so for this reason, and because some low‐income countries do not test the blood used in transfusions for the presence of dangerous viruses such as HIV or hepatitis, it is helpful to give blood transfusions only when they are really necessary.
A normal blood count is above 12. This review summarised all randomized controlled trials (RCTs) that investigated whether it is safe to give blood transfusions when the blood count drops to between seven and eight (thereby reducing the number of transfusions), rather than giving transfusions at higher blood counts of nine to 10.
Study characteristics
We examined the results of RCTs that randomly allocated participants to one of two groups. In one group, trial participants received blood at lower blood counts. In the other group, trial participants received blood at higher blood counts. The data are current up to May 2016.
Key results
We identified a total of 31 relevant trials, which involved 12,587 participants. All of the studies compared different policies for blood transfusions. We found that participants who were assigned to receive blood at lower blood counts were 43% less likely to receive a blood transfusion than those who were given blood at higher blood counts. The risk of dying within 30 days of the transfusion was the same whether the participants received transfusion at lower or higher blood counts. We also evaluated harmful events that occurred after participants received, or did not receive, blood transfusions, including infection (pneumonia, wound infection, and blood poisoning), heart attacks, strokes, and problems with blood clots, and found that there was no clear difference in the instance of these events between the group that received transfusions at lower blood counts and the group that received transfusions at higher blood counts.
Quality of evidence
We found that most of the RCTs provided a high quality of evidence, in that they were adequately conducted and used appropriate methods that minimised any possible biases that could make the validity of the results uncertain.
Authors conclusions
We concluded that it was not harmful to the participants' health status to give blood at lower or higher blood counts. If a policy of giving blood only at lower blood counts were followed routinely in clinical practice, it would reduce the amount of blood patients receive substantially and reduce the risk of patients receiving blood transfusions unnecessarily, as transfusions can have harmful effects. Additional studies are needed to establish the blood count at which a blood transfusion is needed in patients who have suffered a heart attack, brain injury, or have cancer.
Summary of findings
for the main comparison.
| Restrictive compared with liberal transfusion protocols for guiding allogeneic red blood cell transfusion | ||||||
|
Patient or population: Adults and children (haemodynamically stable) with potential need for RBC transfusion Settings: Inpatient Intervention: Restrictive transfusion protocol Comparison: Liberal transfusion protocol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Liberal transfusion (Hb 9 g/dL to 10 g/dL) | Restrictive transfusion (Hb 7 g/dL to 8 g/dL) | |||||
| People receiving blood transfusions | 841 per 1000 | 479 per 1000 | RR 0.57 (0.49 to 0.65) | 12,587 (31) | ⊕⊕⊕⊕ High | ‐ |
| 30‐day mortality | 93 per 1000 | 90 per 1000 | RR 0.97 (0.81 to 1.16) | 10,537 (23) | ⊕⊕⊕⊝ Moderatea | ‐ |
| Myocardial infarction | 17 per 1000 | 19 per 1000 | RR 1.08 (0.74 to 1.60) | 8303 (16) | ⊕⊕⊕⊕ High | ‐ |
| Congestive heart failure | 36 per 1000 | 28 per 1000 | RR 0.78 (0.45 to 1.35) | 6257 (12) | ⊕⊕⊝⊝ Lowb,c | ‐ |
| Cerebrovascular accident (CVA) ‐ stroke | 17 per 1000 | 13 per 1000 | RR 0.78 (0.53 to 1.14) | 7343 (13) | ⊕⊕⊕⊕ High | ‐ |
| Rebleeding | 163 per 1000 | 144 per 1000 | RR 0.75 (0.51 to 1.10) | 3108 (6) | ⊕⊕⊝⊝ Lowd, e | ‐ |
| Pneumonia | 82 per 1000 | 76 per 1000 | RR 0.94 (0.80 to 1.11) | 6277 (14) | ⊕⊕⊕⊕ High | ‐ |
| Thromboembolism | 10 per 1000 | 8 per 1000 | RR 0.77 (0.41 to 1.45) | 4019 (10) | ⊕⊕⊕⊕ High | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RBC: red blood cell;RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
a. Downgraded for imprecision: could be up to 17 per 1000 more deaths in restrictive transfusion. b. Downgraded for inconsistency: moderately wide confidence intervals. c. Downgraded for risk of bias: blinding of participants and personnel impossible and blinding of outcome assessment inconsistent between trials. d. Downgraded for inconsistency: I² = 58%, P = 0.04. e. Downgraded for imprecision: could be up to 16 per 1000 more rebleeds in restrictive transfusion.
Background
Description of the condition
Patients who are ill in hospital are frequently anaemic, with low haemoglobin concentrations. The causes of anaemia are diverse, including loss of blood from surgery, bleeding, excessive blood sampling for laboratory tests, or as a consequence of illness. Patients with cancer may develop anaemia because of the underlying disease or chemotherapy affecting production of red cells in their bone marrow. Anaemia both decreases the oxygen content of the blood supplied to the tissues, including the myocardium, and increases myocardial oxygen demand by requiring a higher cardiac output to maintain adequate systemic oxygen delivery (Sabatine 2005).
Anaemia has been associated with worse outcomes in patients who are anaemic prior to surgery or who have cardiovascular disease (Carson 1996; Shander 2014). However, it does not necessarily follow that the correction of anaemia by red blood cell (RBC) transfusion will improve outcomes. Anaemia is generally well tolerated by many people, and therefore, the benefits of potentially corrective treatments such as red cell transfusions need to be weighed against their risks.
Description of the intervention
The main treatment option for raising the haemoglobin concentration rapidly in patients with anaemia remains RBC transfusion. Red cells for transfusion are collected from whole blood donations that are centrifuged to concentrate them, prior to the addition of anticoagulant and storage solutions. However, there are recognised risks of blood transfusion, as with any medical intervention. These risks and the general availability of RBC transfusion vary throughout the world. In countries with well‐regulated blood supplies, the safety of allogeneic red cell transfusion has improved significantly over the past 30 years, and the overall risks are very low. For example, in the USA, the estimated risk per unit for HIV is 1:1,467,000 (Zou 2010); for the hepatitis C virus (HCV), 1:1,149,000 (Zou 2010); and for the hepatitis B virus (HBV), 1:282,000 to 1:357,000 (Zou 2009). This has been primarily due to improvements in donor blood screening policies and the implementation of more stringent quality control measures (Klein 2007).
In resource‐poor countries, the supply of blood is inadequate and may not be safe because it is not often tested for viral pathogens. Blood donations are not routinely tested in 39 countries for transfusion‐transmissible infections that include HIV, hepatitis B, hepatitis C, and syphilis (WHO 2011). In 40 countries, less than 25% of the blood supply is collected from voluntary unpaid blood donors, with most coming from family or paid blood donors (WHO 2011). The prevalence of HIV in low‐income countries is 2.3% of blood donations compared with 0.001% in high‐income countries (WHO 2011).
Other general risks of transfusion have been described (although perhaps under‐reported) and include acute transfusion reactions, volume overload, bacterial contamination, infection with new ‐ but currently unknown ‐ blood‐borne pathogens, and transfusion‐related acute lung injury (Delaney 2016; Toy 2012). Additional possible adverse effects include loss of red cell nitric oxide production, which is thought to induce local vasodilatation; prothrombotic effects from factors in the supernatant; and variable immunomodulatory or proinflammatory effects from different cellular products in the red cell component. Overall, these harmful effects of red cell transfusions may be manifested as increased risks of infections in hospitals or cardiovascular events, including myocardial infarction or stroke.
There were concerns that the effects of storage on red cells may also render them less effective and potentially harmful. However, recent trials have not demonstrated clinical harm to blood stored for approximately 21 to 28 days compared with less than seven to 10 days (Dhabangi 2015; Fergusson 2013; Lacroix 2015; Steiner 2015).
Blood transfusion is expensive. The direct costs of each collected bag of red cells fail to capture the many associated costs related to hospital blood‐banking practice and safe patient administration. In 2008, the mean payment for one unit of leukoreduced RBCs in the USA was USD 223 (Whitaker 2011). However, if the costs of administration as well as the acquisition expenses of RBC transfusion are considered, the estimated cost derived from four USA and European hospitals rises to USD 761 per unit (standard deviation +/‐ USD 294) (Shander 2010).
Treatment options for anaemia other than red cell transfusions may include erythropoietin, and oral or intravenous iron therapy. These have been used in chronic anaemia, particularly in renal failure, for many years, but are the subjects of other systematic reviews, so we did not consider them further within this review.
How the intervention might work
The rationale for transfusing RBCs in anaemic patients is to improve oxygen delivery to the tissues and to the myocardium itself, to reduce the compensatory work done by the heart to increase cardiac output. Red blood cell transfusion is one of the few treatments that may restore tissue oxygenation adequately when oxygen demand exceeds supply (Klein 2007; Wang 2010).
Many randomized controlled trials that compared outcomes in participants allocated to different policies or schedules of using red cell transfusions have now been completed and reported. These studies presented results after randomising participants to either 'restrictive' triggers (typically, participants are transfused only when their haemoglobin concentration falls to around 7 g/dL to 8 g/dL) or 'liberal' triggers (participants are transfused at a higher haemoglobin concentration of around 9 g/dL to 10 g/dL). Historically, the widely accepted clinical standard was to transfuse patients when the haemoglobin level dropped below 10 g/dL or the haematocrit fell below 30%. Adams and Lundy first proposed this '10/30 rule' in 1942, and it served as a RBC transfusion trigger for decades (Madjdpour 2005; Wang 2010). However, the 1988 National Institutes of Health Consensus Conference in the USA reported that the evidence did not support a single criterion for transfusion (NIH 1988). Since then, most published guidelines have advised against a single threshold for RBC transfusion, recommending that a range of haemoglobin values between 6 g/dL and 10 g/dL can be used, depending on the presence of serious comorbidity (AAGBI 2008; ASA 2006; BCTMAG 2003; Carson 2012a; Napolitano 2009; NBUGI 2001). The American Association of Blood Banks (AABB) guidelines advise using a restrictive transfusion threshold of 7 g/dL to 8 g/dL in most clinical settings (Carson 2012a).
Why it is important to do this review
Much of the earlier evidence comparing restrictive and liberal thresholds comes from trials based in critical care. In 1999, the landmark TRICC trial (transfusion requirements in critical care) found a similar mortality in participants transfused at a restrictive trigger of less than 7 g/dL compared with a liberal trigger of less than 10 g/dL (Hébert 1999). Since the last review (Carson 2012b), the number of participants enrolled in trials has doubled from 6264 to 12,587. Therefore, there is a need to update this systematic review to ensure that new guidelines continue to be based on the most recent literature reporting on the effectiveness and safety of RBC transfusion.
The purpose of the review was to identify, appraise, and summarize the data from all randomized controlled trials (RCTs) that studied the clinical impact of varying thresholds for transfusion with RBCs. We were particularly interested in whether the results of RCTs support the trend for increasingly restrictive RBC transfusion practices across all patient groups and if RBC transfusions can be withheld in some circumstances without harming patients.
Objectives
The aim of this review was to compare 30‐day mortality and other clinical outcomes in participants randomized to restrictive versus liberal red blood cell (RBC) transfusion thresholds (triggers) for all conditions. The restrictive transfusion threshold uses a lower haemoglobin level to trigger transfusion (most commonly 7 g/dL or 8 g/dL), and the liberal transfusion threshold uses a higher haemoglobin level to trigger transfusion (most commonly 9 g/dL to 10 g/dL).
Methods
Criteria for considering studies for this review
Types of studies
To examine the evidence for the effect of transfusion thresholds on the use of red blood cell (RBC) transfusions and the evidence for any change in clinical outcomes, we included randomized controlled trials if the comparison groups were assigned on the basis of a transfusion 'threshold' (also known as a 'trigger'), defined as a haemoglobin or haematocrit level (with or without a specified level of haemodynamic instability) that had to be reached before a RBC transfusion was administered. We required that control group participants had to have been either transfused with allogeneic or autologous red blood cells, or both, at higher haemoglobin or haematocrit levels (transfusion threshold) than the intervention group, or transfused in accordance with current transfusion practices, which may not have included a well‐defined transfusion threshold, but involved liberal rather than restrictive transfusion practices. We excluded trials that were not designed to include any clinical outcomes relevant to this review.
Types of participants
We included trials of surgical or medical participants, involving adults or children, or both. We excluded studies enrolling neonates, given the distinct pathophysiology and clinical features of anaemia.
Types of interventions
The intervention considered was the use of transfusion thresholds ('triggers') as a means of guiding allogeneic or autologous RBC transfusion, or both. A liberal transfusion threshold most often refers to administration of blood transfusion when the haemoglobin level falls below 9 g/dL to 10 g/dL. A restrictive transfusion threshold most often refers to administration of blood transfusion when the haemoglobin level falls below 7 g/dL to 8 g/dL.
Types of outcome measures
Primary outcomes
In contrast to prior versions of this systematic review, the primary outcome for this analysis was 30‐day mortality. The primary outcome was changed because mortality is a more clinically relevant outcome and the number of participants enrolled in trials provided sufficient power to examine this outcome. Sample size calculations assuming baseline 30‐day mortality of 9% for restrictive transfusion, 90% power, alpha level of 0.05, indicate that to detect a 15%, 20%, or 25% relative decrease in mortality with the use of liberal transfusion, a study needs to enrol 17,500, or 9600, or 6000 participants, respectively.
Secondary outcomes
Other time periods we examined for mortality included: during hospital admission, at 90 days, and long term (median follow‐up of 3.1 years). We compared RBC transfusion use between the groups (listed below for the morbidity outcome) by proportion of participants exposed to transfusion, participants exposed to allogeneic or autologous transfusion, units of blood transfused, and units of blood transfused in those receiving any transfusion. We evaluated morbidity that occurred during hospitalisation, including cardiac events (composite of myocardial infarction, cardiac arrhythmias, cardiac arrest, pulmonary oedema, and angina), non‐fatal and fatal myocardial infarction, congestive heart failure, cerebral vascular accident (stroke), rebleeding, infection, thromboembolism, renal failure, mental confusion, function, and fatigue. Infection was defined in three ways: sepsis or bacteraemia, pneumonia alone, or pneumonia plus wound infection. We defined all morbidity outcomes according to their definitions in the individual trials.
As this review is an update, we have continued to include some of the secondary outcomes for historical reasons. As stronger evidence is accrued, we feel that in future updates of this review, some of these outcomes may need to be modified or omitted.
Search methods for identification of studies
Electronic searches
We searched the following databases and ongoing trial registries:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library;
MEDLINE via OvidSP (from 1946 to 27 May 2016);
Embase via OvidSP (from 1974 to 27 May 2016);
PubMed (for Epublications ahead of print only, on 27 May 2016);
Transfusion Evidence Library (www.transfusionevidencelibrary.com, 1950 to 27 May 2016);
Web of Science Conference Proceedings Citations Index (CPCI‐S, 1990 to 27 May 2016);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched to 27 May 2016); and
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched to 27 May 2016);
ISRCTN Registry (www.isrctn.com; searched to 27 May 2016).
We combined searches in MEDLINE and Embase with adaptations of the Cochrane randomized controlled trial (RCT) search filter as detailed in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). We did not restrict our search by date, language, or publication status. We present the search strategies for this update and the ongoing trial registries in Appendix 1 and Appendix 2 respectively. We present search strategies for the 2012 update in Appendix 3.
Searching other resources
We checked the references of all identified trials, relevant review articles, and current treatment guidelines for further literature. We limited these searches to the 'first‐generation' reference lists, i.e. reference lists of papers retrieved directly by the database searches.
We contacted experts in the field to identify information relevant to the review. Where possible and when necessary, we contacted authors of published studies for clarification of trial methodology and data. We emailed all authors of trials that did not report our primary outcome of 30‐day mortality, but this was not possible for older trials where contact information was not available. We searched the reference lists of relevant reviews and transfusion trials.
Data collection and analysis
Selection of studies
Two authors (JLC and SS) independently screened the titles or abstracts of the search results, or both, and selected trials that met the inclusion criteria. We resolved disagreements by discussion until we reached consensus. We identified trials in which participants were randomized to a restrictive transfusion strategy (transfusion threshold or protocol, or both) or to a control group that was randomized to a liberal transfusion strategy.
Data extraction and management
Previously, JLC and Paul Carless (prior author) extracted all the data for the earlier versions of this review. For this 2016 update, using a data extraction form, JLC and SS independently extracted study characteristics and outcomes of new trials since the last review. Information recorded on the extraction form included study type, methodology descriptions, the presence of a transfusion threshold, transfusion protocol, the type of surgery involved, clinical setting, treatment outcomes, and general comments. JLC then entered data into Review Manager 5 (Review Manager 5a); NR checked data. We contacted authors of trials to request missing data.
We used a data extraction form to record data on the following outcomes: the number of participants exposed to allogeneic blood, the amount of allogeneic blood transfused, the number of participants receiving any transfusion (allogeneic blood, autologous blood, or both). For trials involving surgical participants, we recorded the following outcomes: postoperative complications (infection, haemorrhage, non‐fatal myocardial infarction, cardiac events, renal failure, stroke, thromboembolism, pulmonary oedema, mental confusion), mortality, and length of hospital stay (not reported in the review). We recorded data for blood loss and haemoglobin and hematocrit levels (on admission, pre‐ and post‐transfusion, and at discharge). We recorded information regarding demographics (age, sex), type of surgery, and medical condition on the data extraction form. We extracted data for allogeneic blood transfusion if it was expressed as packed RBCs. We documented information regarding the use of fresh frozen plasma or platelets, or both.
Assessment of risk of bias in included studies
We used the Cochrane tool for assessing risk of bias as described in section 8.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
JLC and SS assessed the following domains for each study:
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective outcome reporting; and
other potential sources of bias.
We completed a 'Risk of bias' table for each study, incorporating a description of the study's performance against each of the above domains and our overall judgement of the risk of bias for each entry as follows: 'low', 'unclear' (indicating unclear or unknown risk of bias), or 'high' risk of bias.
Measures of treatment effect
We calculated the risk ratio (RR) for allogeneic blood transfusion in the intervention group compared with the control group and the corresponding 95% confidence intervals (CI) for each trial using a random‐effects model (Der Simonian 1986). We adopted a similar approach for other outcomes of transfusion. We also entered the mean number of units of RBCs transfused to each group and the corresponding standard deviations. We used the mean difference (MD) and 95% CI to express the average mean reduction in the number of units of RBC administered to the intervention group compared with the control group. When the event rate was low, we considered using the Peto odds ratio when criteria for this method were fulfilled.
Unit of analysis issues
The unit of analysis was the participant. In all of the trials except one (Jairath 2015), randomisation was at the individual participant level. In one trial in people with gastrointestinal bleeding (Jairath 2015), the randomisation was at the level of the hospital (cluster), but the analysis occurred at the level of the individual participant. The intraclass correlation coefficient (ICC) was very low (0.0001) for the outcome of mortality, and we therefore included the data considering the participant as the unit of randomisation and ignoring the clustering, but performed a sensitivity analysis excluding this trial, to see what effect, if any, it had on the analysis. We did not evaluate any outcomes with repeated measures.
Dealing with missing data
We performed all analyses on an intention‐to‐treat basis. We imputed no missing data. We received information on 30‐day mortality from three authors (DeZern 2016; Villanueva 2013; Webert 2008). The levels of missing data were acceptable.
Assessment of heterogeneity
We examined statistical heterogeneity using both the I² statistic and Chi² test. The I² statistic describes the percentage of total variation across studies due to heterogeneity rather than chance. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity; moderate or substantial heterogeneity is considered to exist when the I² exceeds 50% or 85% respectively (Higgins 2011). For the Chi² test, we used a P value of < 0.10 to indicate the presence of statistically significant heterogeneity. Because of the anticipated significant clinical heterogeneity of the trials, we analyzed data using a random‐effects model. We also anticipated a high level of heterogeneity related to transfusion rates because practice in the different specialties of the trials would vary considerably according to speciality‐specific protocols. Therefore, as described later, we chose to provide a summary statistic for the outcomes of transfusion even when I² was very high, because of the clinically relevant information it provides.
Assessment of reporting biases
When there were more than 10 studies available, we examined funnel plots for the primary outcome of 30‐day mortality and the proportion of participants transfused for evidence of publication bias. We used the proportion of participants transfused because all trials reported this outcome, and it may reflect overall risk of publication bias better than 30‐day mortality, which was not reported in all of the trials.
Data synthesis
We performed all analyses using Review Manager software (Review Manager 5a). We entered data for the numbers of participants exposed to allogeneic blood and the numbers of participants in each treatment group into Review Manager. When studies presented transfusion volume as millilitres (mL), we converted these amounts to units by dividing by 300. We converted studies reporting haematocrit to haemoglobin concentration by dividing by three. We pooled the data for all outcomes and presented data stratified by subgroups for the primary outcome of 30‐day mortality and proportion of participants transfused. We used Peto odds ratios for the outcomes with event rates less than 1%. For continuous variables, we estimated the pooled mean difference and 95% CI using the generic inverse variance method.
Subgroup analysis and investigation of heterogeneity
The prespecified subgroups evaluated were clinical specialties (acute blood loss/trauma, cancer, cardiac surgery, critical care, orthopaedic surgery, myocardial infarction, vascular surgery, and haematological malignancies). We examined 30‐day mortality and the proportion of participants exposed to transfusion stratified by the transfusion threshold (difference between the liberal and restrictive transfusion thresholds: > 2 g/dL and < 2 g/dL) and restrictive transfusion threshold of less than 7 g/dL versus one of 8 g/dL to 9 g/dL. We also examined a post hoc subgroup of participants enrolled with myocardial infarction compared with all other clinical specialties, and we combined cardiac surgery with myocardial infarction because of emerging evidence that participants with acute myocardial infarction might differ from other anaemic participants (Carson 2013).
Where appropriate, as part of the exploration for clinical heterogeneity, we distinguished between adult and paediatric trials, for example, in analysis of the quantity of blood transfused (as this would not be directly comparable between adults and children), or in clinical settings where the widely used paediatric transfusion protocols differ.
For the primary outcome, we also compared findings between registered and unregistered trials.
Sensitivity analysis
We performed a sensitivity analysis to assess the effects of studies with a high risk of bias for allocation concealment and blinding of outcome assessment for the primary outcome only, as in earlier versions of the review, sensitivity analyses for secondary outcomes were not informative.
'Summary of findings' tables
We have presented the judgements about the quality of the evidence in a 'Summary of findings' table (according to guidelines developed by the GRADE Working Group) (Schünemann 2011). This table includes the following outcomes: number of people receiving blood transfusions, 30‐day mortality, myocardial infarction, congestive heart failure, cerebrovascular accident (stroke), rebleeding, pneumonia, and thromboembolism.
Results
Description of studies
Results of the search
In the first published version of this review (Hill 2002), we ran searches to 1999 and identified 10 included studies and one excluded study. We conducted the first update search in November 2004 but did not identify any new studies at the time. We conducted additional searches in 2009 and 2011 and identified nine new included studies. In the last published version (Carson 2012b), we included a total of 19 studies, excluded one study, listed one as awaiting classification, and identified two ongoing studies.
We conducted the most recent searches in April 2015, December 2015 and May 2016, which together retrieved 5727 records. After deduplication and screening, we identified 16 new, potentially relevant studies (Figure 1). On closer inspection two of these were new reports of studies already included in the 2012 review (So‐Osman 2013 and Villanueva 2013), one was previously awaiting classification (Cooper 2011), and two were previously listed as ongoing (Carson 2013/NCT01167582; Murphy 2015/ISRCTN70923932). For this latest update we considered a total of 35 studies for inclusion; 14 new studies from searches run to May 2016 and 17 studies already included in the review. We excluded a total of four studies (two of these, Fortune 1987 and Zygun 2009, were previously included in the 2012 version of the review). We also performed a search of the international trial registers in May 2016 and identified a further 11 studies of interest; nine are ongoing and two are completed but unpublished, so we have listed these as awaiting classification. Further updates of this review will incorporate the results of these studies (as appropriate).
1.
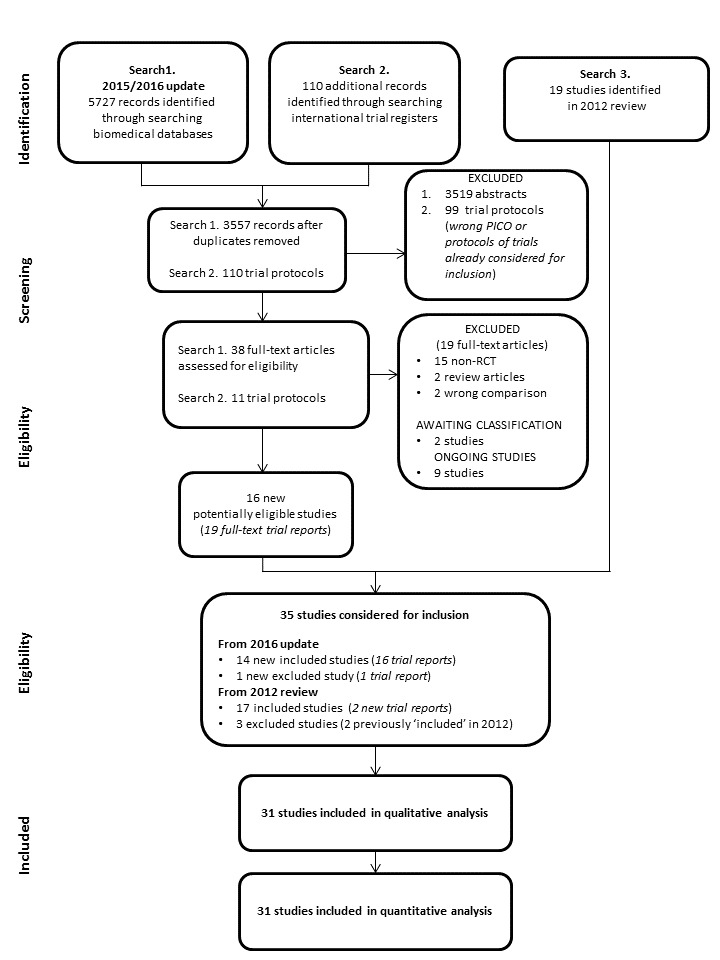
PRISMA Flow Diagram
Included studies
Participants
Thirty‐one studies were eligible for inclusion in this review. The clinical settings were varied: 10 studies were in orthopaedic surgery (Carson 1998; Carson 2011; Fan 2014; Foss 2009; Gregersen 2015; Grover 2005; Lotke 1999; Nielsen 2014; Parker 2013; So‐Osman 2013); six were in critical care (de Almeida 2015; Hébert 1995; Hébert 1999; Holst 2014; Lacroix 2007; Walsh 2013); five were in acute blood loss or trauma (Blair 1986; Fisher 1956; Jairath 2015; Prick 2014; Villanueva 2013); five were in cardiac surgery (Bracey 1999; Hajjar 2010; Johnson 1992; Murphy 2015; Shehata 2012); two were in acute coronary syndrome (Carson 2013; Cooper 2011); two were in leukaemia and haematological malignancies (DeZern 2016; Webert 2008); and one was in vascular surgery (Bush 1997). One trial dealt with paediatric participants (Lacroix 2007).
Interventions
There was considerable variation in the definition of restrictive transfusion strategies specified in the protocols. These varied from 7.0 g/dL to 9.7 g/dL, with two further trials specifying haematocrit values of 25% or 30% (equivalent to haemoglobin levels of around 8 g/dL and 10 g/dL respectively). One trial administered blood for symptoms of anaemia (Parker 2013); and in another trial in postpartum haemorrhage (included in the acute blood loss or trauma grouping), no transfusion was administered in the restrictive group (Prick 2014), although red blood cell (RBC) transfusion was permitted for severe symptoms of anaemia or if physicians believed it was indicated. The liberal transfusion triggers varied: 100% of 'normal red cell volume' (Fisher 1956); two units of blood irrespective of clinical state (immediately in one trial (Blair 1986), postoperatively in another (Lotke 1999)); transfusion sufficient to maintain haemoglobin levels at or above 12 g/dL (Webert 2008), 11.3 g/dL (Gregersen 2015), 10 g/dL (Bush 1997; Carson 1998; Carson 2011; Carson 2013; Foss 2009; Grover 2005; Hébert 1995; Hébert 1999; Hajjar 2010; Jairath 2015; Parker 2013), 9.5 g/dL (Lacroix 2007; Shehata 2012), 9 g/dL (Bracey 1999; de Almeida 2015; Holst 2014; Murphy 2015; Villanueva 2013; Walsh 2013), 8.9 g/dL (Prick 2014), and 8 g/dL DeZern 2016. Three trials specified the liberal triggers as haematocrit levels of 32%, Johnson 1992, and 33%, Cooper 2011.
Trial design
In 34 out of 35 trials, the participant was the unit of randomisation and analysis. One trial used cluster randomisation by hospital (Jairath 2015). Ten trials included more than 100 participants. Four trials included over 900 participants (Carson 2011; Holst 2014; Jairath 2015; Murphy 2015). This systematic review included a total of 12,587 trial participants.
Excluded studies
From the earlier searches, we excluded one trial confined to participants with sickle cell disease, because the trigger was based on the level of sickle haemoglobin, not the haemoglobin or haematocrit level (Vichinsky 1995).
We excluded two trials included in earlier versions of this review (Fortune 1987; Zygun 2009), because they were not designed to evaluate clinical outcomes relevant to this review.
From the most recent searches, we excluded one trial because participants received concomitant erythropoietin (Robertson 2014), and hence, it did not fulfil the inclusion criteria.
Studies awaiting classification
Brief details of two completed but unpublished studies are shown in the Studies awaiting classification section.
Ongoing studies
Brief details of nine ongoing studies identified by searching the international trial registers to May 2016 are shown in the Ongoing studies section.
Risk of bias in included studies
The 'Risk of bias' tables detail the performance of the studies for each domain and are summarised in Figure 2 and Figure 3.
2.
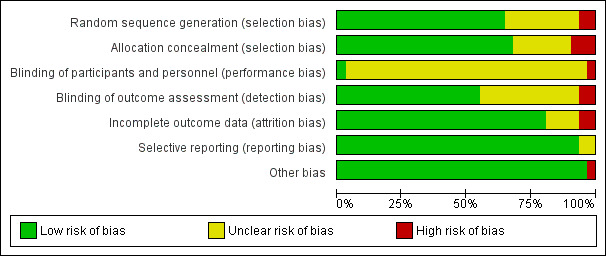
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies. Thirty studies are included in this review.
3.
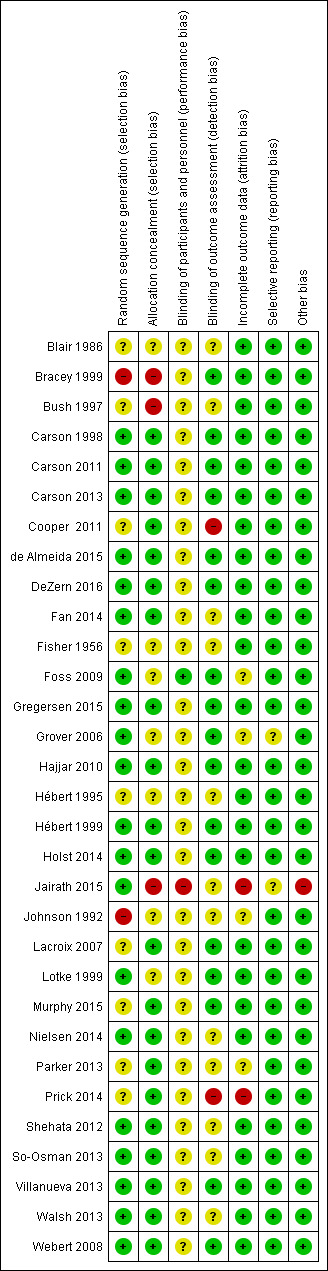
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation
We found low risk of bias for 20 trials, eight of which used computer randomisation and one of which used a table of random numbers to generate the allocation sequence for the participants. Another 11 trials used a variety of low‐risk methods. Two trials based the randomisation sequence on hospital record number, and we judged them to be at high risk of bias, while the remaining nine trials presented insufficient information to assess the adequacy of sequence generation, so we rated them as unclear.
Allocation concealment
We judged the risk of bias for this item to be low for 21 trials that used central allocation or sealed envelopes if appropriate safeguards (e.g. sequentially numbered envelopes) were used. We judged three trials to be at a high risk of bias; one of these trials used a cluster design, so everyone in hospital knew to which group all participants had been assigned (Jairath 2015). We rated seven studies as unclear because the publications did not provide any information about how allocation was concealed.
Blinding
Performance bias
The nature of the intervention means that blinding of clinicians involved in the care and administration of blood transfusions would not have been feasible. The extent to which this could have biased the results is unclear, but we rated 28 trials as being at unclear risk of bias for this domain. One trial reported that participants were blinded to treatment (Foss 2009). Thus, we have rated this study as being at low risk of bias for this domain. We rated one study as being at high risk because in a cluster design everyone knows the assigned group or cluster (Jairath 2015).
Detection bias
Outcomes are optimally assessed when assessors are blinded to assignment. It is possible to blind the assessment of many outcomes by using, for example, an adjudication committee. In contrast, for some outcomes such as death, blinded assessment is less useful. Since most trials evaluated multiple outcomes, it is possible that the potential bias for the assessment of one outcome would differ to that for another outcome. We classified risk of bias on the basis of the primary outcome of the trial. We judged the risk of bias to be low for 15 studies, high for three, and uncertain for 13 trials.
Incomplete outcome data
We rated 24 trials as being at low risk of bias for this domain as they either had no missing data or performed intention‐to‐treat analyses. Five trials reported a small number of exclusions, although the extent to which this may have introduced bias is uncertain; thus, we rated these trials as unclear. In two trials, the risk was high.
Selective reporting
We could not find any evidence of reporting bias. Although we did not have access to the trial protocols for the majority of trials, the results for the primary and secondary outcomes, as described in the methods sections of each trial, appeared clearly and concisely reported. Trial protocols were available for the trials with which the authors of this review had some degree of involvement (Carson 1998; Carson 2011; Hébert 1995; Hébert 1999; Lacroix 2007). We did communicate with one author who provided 30‐day mortality data (Villanueva 2013).
Other potential sources of bias
We identified no other sources of bias.
Effects of interventions
See: Table 1
There was substantial variation in the completeness of all review outcomes across the included trials. All of the trials contributed to the analysis comparing the proportion of participants transfused in the liberal and restrictive transfusion groups. Despite the heterogeneity in the methods and transfusion triggers reported in these randomized trials, it was possible to pool data, to varying degrees, for each of the review outcomes. See Table 1.
Primary outcome
Thirty‐day mortality
Thirty‐day mortality is the primary outcome, and 23 trials reported data (N = 10,537 participants). There was no difference in the 30‐day mortality between restrictive and liberal transfusion strategies (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.81 to 1.16; Analysis 1.1). Heterogeneity between these trials was not significant (Chi² = 29.75, df = 21 (P = 0.10); I² = 29%). The funnel plot demonstrates that the risk ratio for 30‐day mortality is symmetrically distributed, which indicates that there is not likely to be publication bias for this outcome (Figure 4).
1.1. Analysis.
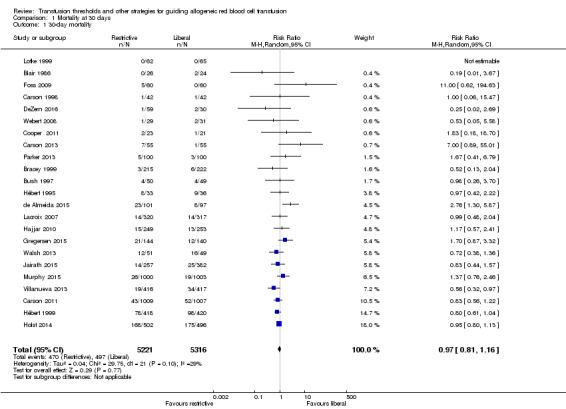
Comparison 1 Mortality at 30 days, Outcome 1 30‐day mortality.
4.
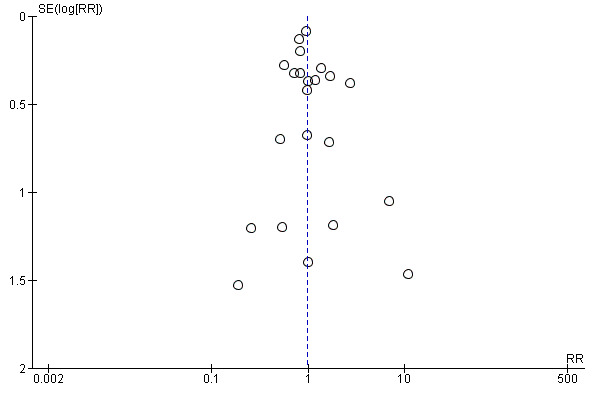
Funnel plot of comparison: 1 Mortality, outcome: 1.1 30‐day mortality.
Subgroup analysis of 30‐day mortality: restrictive threshold of 8 g/dL to 9 g/dL versus 7 g/dL
We examined 30‐day mortality and stratified it by the restrictive transfusion threshold used in the trials. Fourteen trials, with 4772 participants, used a restrictive threshold of 8 g/dL to 9 g/dL. The risk ratio for 30‐day mortality was 1.05 (95% CI 0.78 to 1.40). Nine trials, with 5765 participants, used a 7 g/dL restrictive threshold (Analysis 1.2). The risk ratio for 30‐day mortality was 0.94 (95% CI 0.74 to 1.19). The test for subgroup differences was not significant (Chi² = 0.34, df = 1 (P = 0.56); I² = 0%), indicating that there was no difference in the mortality risk between the two thresholds.
1.2. Analysis.
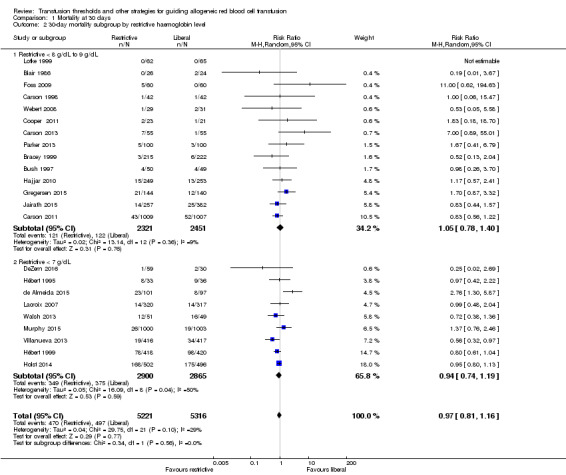
Comparison 1 Mortality at 30 days, Outcome 2 30‐day mortality subgroup by restrictive haemoglobin level.
Subgroup analyses of 30‐day mortality: clinical specialty
We examined 30‐day mortality and stratified it by the clinical speciality used in the trials: cardiac surgery, orthopaedic surgery, vascular surgery, acute blood loss or trauma (analyses for this grouping for 30‐day mortality included gastrointestinal (GI) bleeding only), critical care, acute myocardial infarction, or haematological malignancies. The overall risk ratio for 30‐day mortality stratified by clinical specialty was 0.97 (95% CI 0.81 to 1.16; Analysis 1.3). The test for differences in 30‐day mortality between the subgroups was not significant (Chi² = 9.78, df = 6 (P = 0.13); I² = 38.6%).
1.3. Analysis.
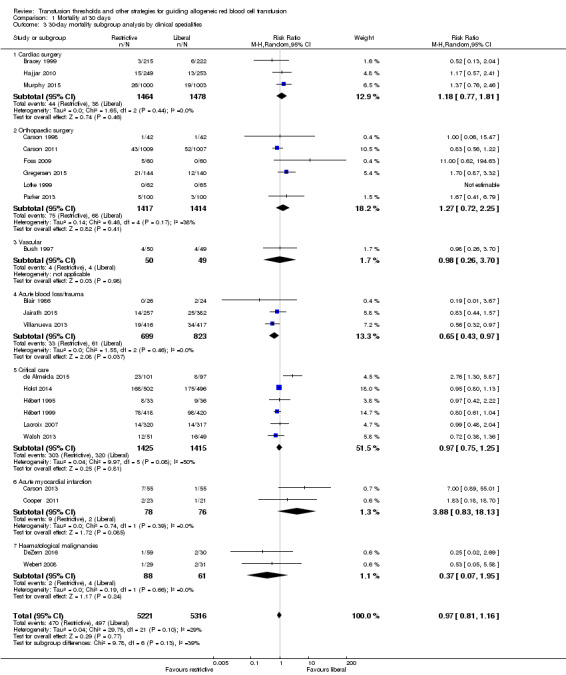
Comparison 1 Mortality at 30 days, Outcome 3 30‐day mortality subgroup analysis by clinical specialities.
In two trials that reported mortality at 30 days in 2221 participants with acute blood loss or trauma (GI bleeding), the mortality was significantly lower using the restrictive strategy compared with the liberal strategy (RR 0.65, 95% CI 0.43 to 0.97; Analysis 1.3).
Two trials recruited 154 participants who had acute myocardial infarction and evaluated mortality after random allocation; for this subgroup, the mortality risk was higher in the restrictive strategy group than in the liberal strategy group (RR 3.88, 95% CI 0.83 to 18.13). We carried out a post hoc subgroup analysis that compared the 30‐day mortality in the two trials that included 154 acute myocardial infarction participants versus all other participants, but found no differences. The P value for subgroup differences was 0.08 (Chi² = 3.09, df = 1; I² = 67.6%; Analysis 1.4). Although we observed a very high risk ratio for the myocardial infarction participants (RR 3.88), the two included studies were very small, and hence, the pooled estimate is not robust.
1.4. Analysis.
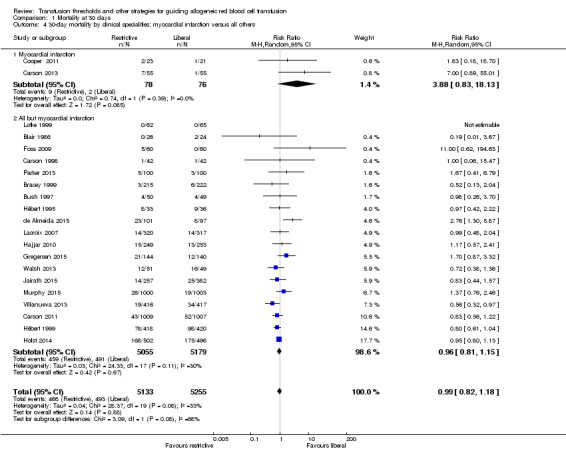
Comparison 1 Mortality at 30 days, Outcome 4 30‐day mortality by clinical specialities: myocardial infarction versus all others.
In a separate subgroup, we combined five trials, with 3096 participants, those with myocardial infarction and those undergoing cardiac surgery; the risk ratio for 30‐day mortality was 1.28 (95% CI 0.80 to 2.06; Analysis 1.5), and there was very little heterogeneity (I² = 12%).
1.5. Analysis.
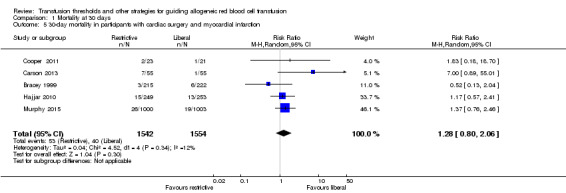
Comparison 1 Mortality at 30 days, Outcome 5 30‐day mortality in participants with cardiac surgery and myocardial infarction.
Subgroup analysis of 30‐day mortality: registered versus unregistered trials
Six of the included trials were not registered and were all conducted before 2000. This was expected, since more recent initiatives for research transparency dictate that all trials should be registered. There were no differences in the 30‐day mortality between the registered and unregistered trials (Analysis 2.1).
2.1. Analysis.
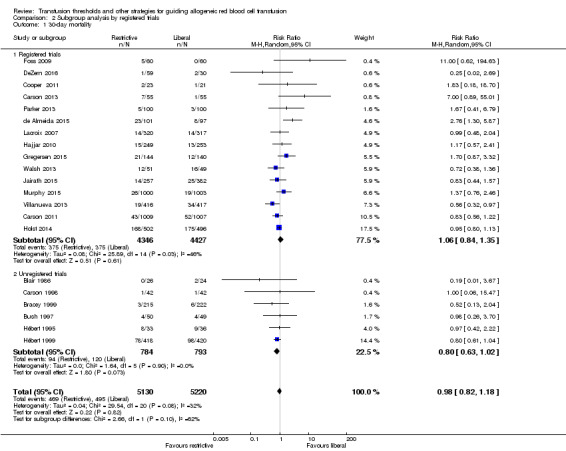
Comparison 2 Subgroup analysis by registered trials, Outcome 1 30‐day mortality.
Sensitivity analysis
There were no differences in 30‐day mortality between trials with low versus unclear or high risk of bias in two bias domains, i.e. allocation concealment and blinding of outcome assessment (Analysis 3.1 and Analysis 4.1 respectively).
3.1. Analysis.
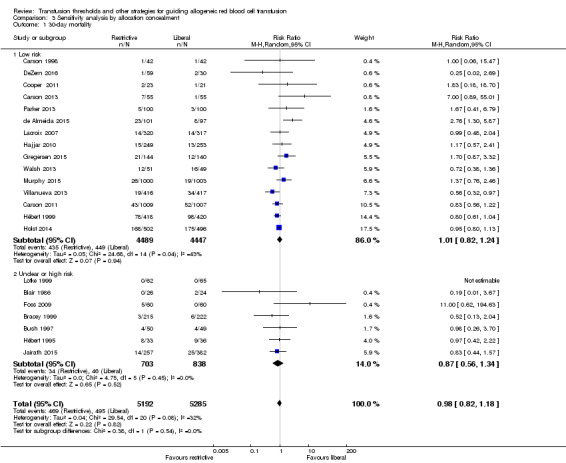
Comparison 3 Sensitivity analysis by allocation concealment, Outcome 1 30‐day mortality.
4.1. Analysis.
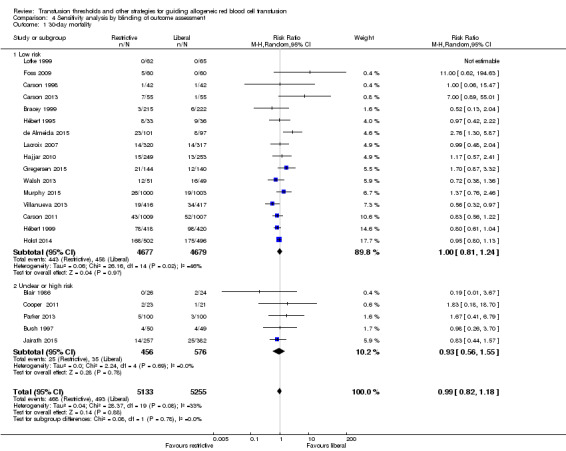
Comparison 4 Sensitivity analysis by blinding of outcome assessment, Outcome 1 30‐day mortality.
Secondary outcomes
Mortality at other time intervals
We analyzed mortality at hospital discharge (N = 5107, 10 trials; Analysis 5.1), 90 days (N = 3485, four trials; Analysis 5.2), and long term (N = 2016, one trial). There were no differences in mortality between transfusion strategies at each of the time points (mortality at hospital discharge: RR 0.86, 95% CI 0.73 to 1.01, Chi² = 8.67, df = 8 (P = 0.37); I² = 8%; 90‐day mortality: RR 1.15, 95% CI 0.95 to 1.40, Chi² = 3.76, df = 3 (P = 0.29); I² = 20%). In the one trial reporting long‐term mortality (Carson 2013), the hazard ratio was 1.09 (95% CI 0.95 to 1.25; P = 0.21). The results of mortality analyses at hospital discharge, 90 days, and long term are consistent with the results for mortality at 30 days.
5.1. Analysis.
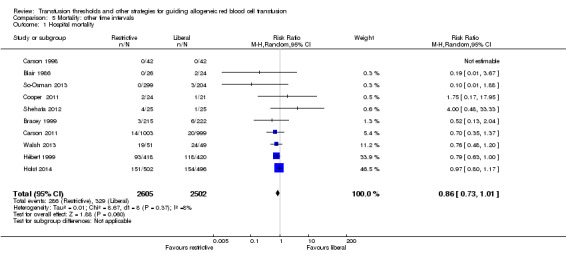
Comparison 5 Mortality: other time intervals, Outcome 1 Hospital mortality.
5.2. Analysis.
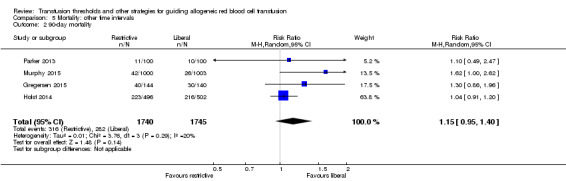
Comparison 5 Mortality: other time intervals, Outcome 2 90‐day mortality.
Blood transfusions
Historically, exploration of differences in the proportion of participants transfused has been the analysis of preference in earlier versions of this Cochrane Review. As indicated in the Methods, we anticipated high levels of heterogeneity in the analysis of transfusion outcomes, for several reasons. In particular, standard 'control' rates of transfusion practice are very variable across the clinical specialties in which trials were identified for this update. These differing rates of transfusion policy reflect practice defined in speciality guidelines and recommendations. It is usually recommended that pooled estimates are not presented when there is such high heterogeneity. However, we have chosen to present the pooled results here, and our further justification for presenting these results for transfusion outcomes is presented in the Discussion.
Proportion of participants transfused
This analysis demonstrates the difference in the proportion of participants transfused in the liberal and restrictive arms of the trials. Data on the proportion of transfused participants were available from 31 trials (12,547 participants). The implementation of a restrictive transfusion trigger across all trials reduced the relative risk of receiving a RBC transfusion by 43% (RR 0.57, 95% CI 0.49 to 0.65; Analysis 6.1). Heterogeneity between these trials was large and significant (Chi² = 948.58, df = 30 (P < 0.00001); I² = 97%).
6.1. Analysis.
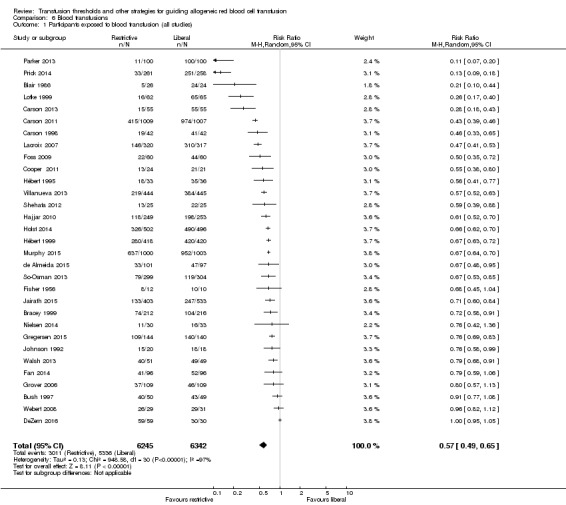
Comparison 6 Blood transfusions, Outcome 1 Participants exposed to blood transfusion (all studies).
The proportion of participants transfused in the liberal and restrictive arms of the trials were very different across the different clinical specialities, with the largest difference evident in the subgroup of participants with acute blood loss (Analysis 6.2). The test for subgroup differences was significant: Chi² = 175.97, df = 6 (P < 0.00001); I² = 96.6%. The acute blood loss/trauma subgroup included diverse underlying illnesses for haemorrhage, including comorbidities. For example, Prick 2014 recruited young (otherwise healthy) women with postpartum haemorrhage, while Jairath 2015 enrolled older participants with gastrointestinal bleeding, characterised by many comorbidities. Prick 2014 contributed to a large extent to the high heterogeneity in this subgroup, and temporarily removing it from the analysis reduced heterogeneity to 77%. By contrast, participants enrolled in the subgroup of cardiac surgery trials demonstrated less variability in risk of transfusion across trials, and in this subgroup, we observed no heterogeneity. In the subgroup of critical care trials, the high heterogeneity (I² = 88%) was substantially reduced to 35% by temporarily removing the paediatric trial (Lacroix 2007). This sensitivity analysis, although post hoc, highlights how transfusion policies in this setting differed from adult protocols in a critical care setting.
6.2. Analysis.
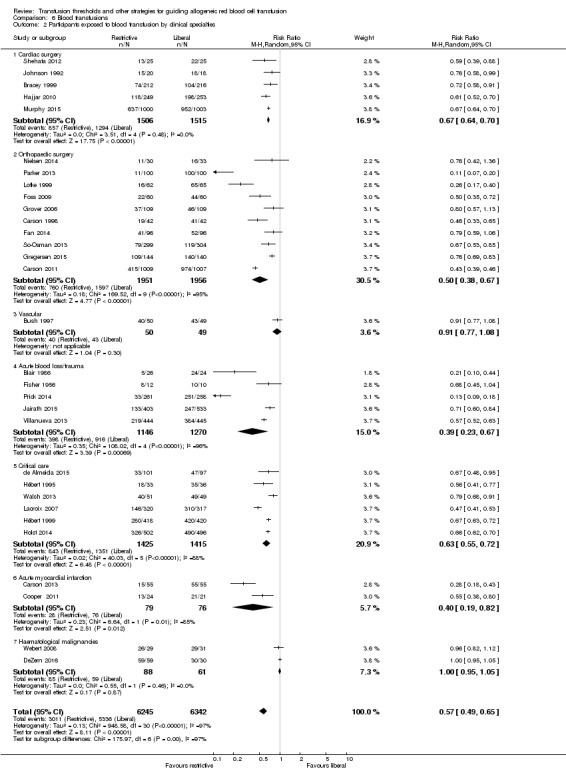
Comparison 6 Blood transfusions, Outcome 2 Participants exposed to blood transfusion by clinical specialties.
The relative risk of transfusion was higher when the differences in haemoglobin transfusion thresholds between the restrictive and liberal transfusion arms were 2 g/dL or more, compared with those in which the difference was less than 2 g/dL (test for overall effect: Z = 2.17 (P = 0.03); Analysis 6.3).
6.3. Analysis.
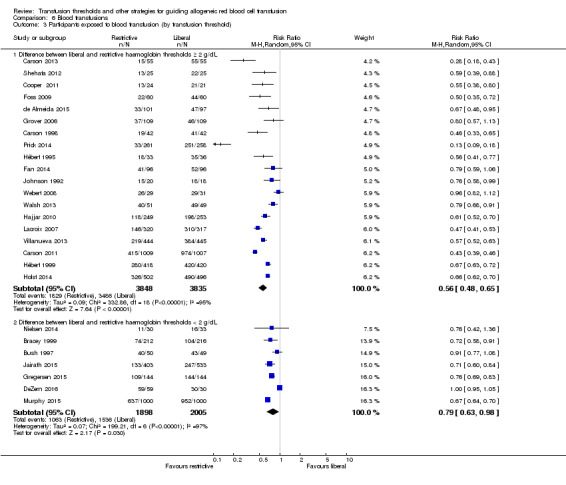
Comparison 6 Blood transfusions, Outcome 3 Participants exposed to blood transfusion (by transfusion threshold).
There was no difference in the proportion of participants transfused between trials that used a restrictive transfusion threshold of 8 g/dL to 9 g/dL versus less than 7 g/dL (test for subgroup differences: Chi² = 0.13, df = 1 (P = 0.72); I² = 0%; Analysis 6.4).
6.4. Analysis.
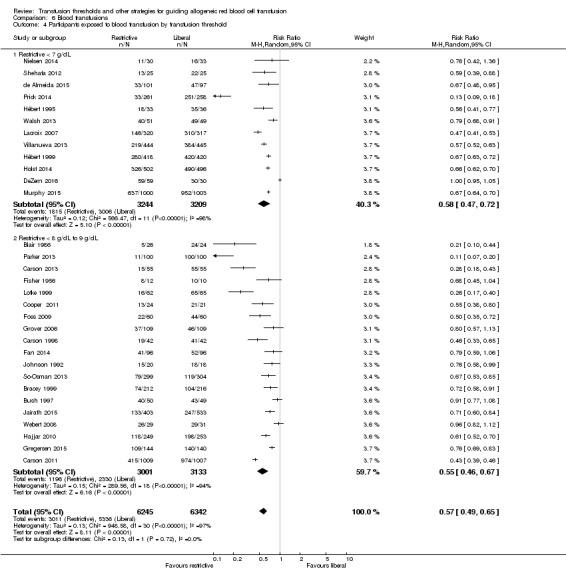
Comparison 6 Blood transfusions, Outcome 4 Participants exposed to blood transfusion by transfusion threshold.
The funnel plot for the proportion of participants transfused displays a grouping of trials with a risk ratio around 0.5 for receiving a transfusion in the restrictive transfusion arm (Figure 5), which is consistent with the overall observation that participants in the restrictive arm were transfused approximately half as often as those in the liberal arm. As expected, there were no studies in which participants in the restrictive arm were transfused more than the liberal arm.
5.
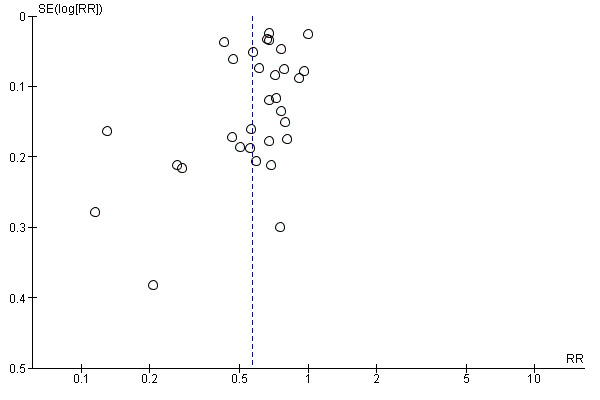
Funnel plot of comparison: 2 Blood transfusions, outcome: 2.1 Participants exposed to blood transfusion (all studies).
Quantity of RBCs transfused
Twelve trials reported the quantities of blood transfused. The use of a restrictive transfusion trigger resulted in an average saving of 1.30 units of RBCs per transfused participant (mean difference (MD) ‐1.30, 95% CI ‐1.85 to ‐0.75; Analysis 6.5). Heterogeneity between these trials was, again, large and significant (Chi² = 139.91, df = 11 (P < 0.00001); I² = 92%).
6.5. Analysis.
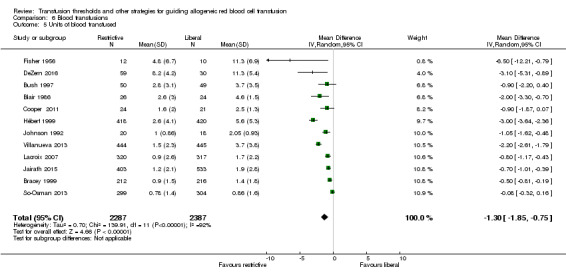
Comparison 6 Blood transfusions, Outcome 5 Units of blood transfused.
Haemoglobin or hematocrit concentration
Sixteen trials reported the difference in haemoglobin or haematocrit levels between the liberal and restrictive transfusion arms. The timing of measurement varied. When we pooled data (without regard to timing, which was consistent within studies), participants assigned to a restrictive strategy had a haemoglobin concentration on average 1.32 g/dL lower than participants assigned to a liberal transfusion strategy (MD ‐1.32, 95% CI ‐1.64 to ‐0.99; Analysis 7.1). Heterogeneity between these trials was large and significant (Chi² = 867.08, df = 15 (P < 0.00001); I² = 98%).
7.1. Analysis.
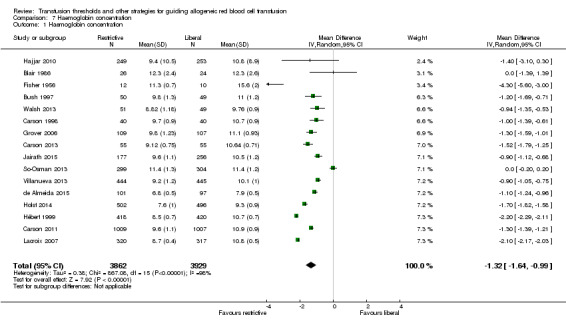
Comparison 7 Haemoglobin concentration, Outcome 1 Haemoglobin concentration.
Clinical outcomes
Cardiac events
Nine trials reported data on postenrolment cardiac events in 4849 participants. The risks of cardiac events (myocardial infarction, cardiac arrhythmias, cardiac arrest, pulmonary oedema, and angina) were not increased by the use of restrictive transfusion strategies (RR 1.04, 95% CI 0.79 to 1.39; Analysis 8.1). Heterogeneity between these trials was moderate and significant (Chi² = 21.25, df = 8 (P = 0.0007); I² = 62%). It is possible that participants were counted in more than one category of this composite outcome because these disorders are clinically inter‐related (for example, a participant could have angina that might lead to pulmonary oedema).
8.1. Analysis.
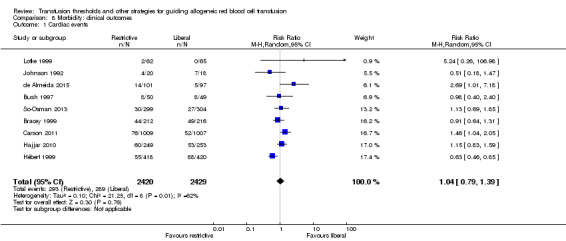
Comparison 8 Morbidity: clinical outcomes, Outcome 1 Cardiac events.
Myocardial infarction
Sixteen trials reported the outcome data on myocardial infarction (fatal and non‐fatal) in 8303 participants after random allocation to the liberal or restrictive transfusion arms. There was no difference between the restrictive and liberal transfusion strategies (RR 1.08, 95% CI 0.74 to 1.60; Analysis 8.2). There was no evidence of heterogeneity between trials (Chi² = 16.63, df = 15 (P = 0.34); I² = 10%).
8.2. Analysis.
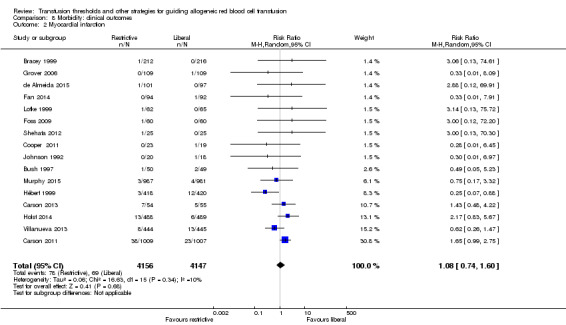
Comparison 8 Morbidity: clinical outcomes, Outcome 2 Myocardial infarction.
Congestive heart failure
Twelve trials reported data for congestive heart failure in 6257 participants. There was no significant difference between the restrictive and liberal transfusion strategies (RR 0.78, 95% CI 0.45 to 1.35; Analysis 8.3). Heterogeneity between the trials was moderate and significant (Chi² = 20.98, df = 10 (P = 0.02); I² = 52%).
8.3. Analysis.
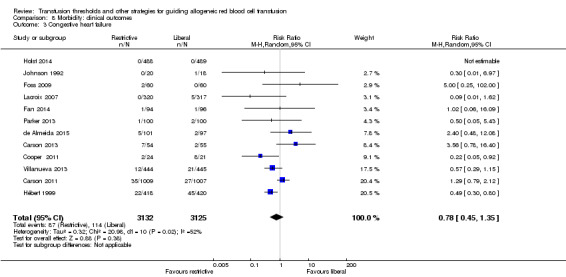
Comparison 8 Morbidity: clinical outcomes, Outcome 3 Congestive heart failure.
Cerebrovascular accident: stroke
Thirteen trials reported data for stroke in 7343 participants. There was no difference between transfusion strategies (RR 0.78, 95% CI 0.53 to 1.14; Analysis 8.4). Heterogeneity between the trials was not significant (Chi² = 9.28, df = 12 (P = 0.67); I² = 0%).
8.4. Analysis.
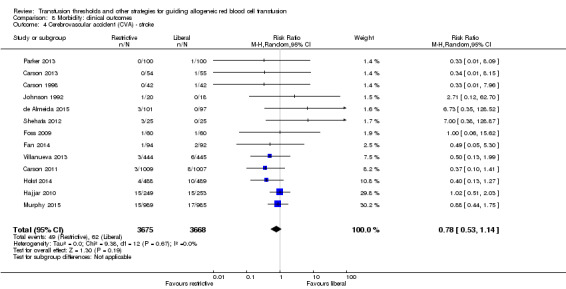
Comparison 8 Morbidity: clinical outcomes, Outcome 4 Cerebrovascular accident (CVA) ‐ stroke.
Rebleeding
Six trials reported data for rebleeding in 3108 participants. There was no difference between the restrictive and liberal transfusion strategies (RR 0.75, 95% CI 0.51 to 1.10; Analysis 8.5). Heterogeneity between these trials was statistically significant (Chi² = 11.95, df = 5 (P = 0.04); I² = 58%). In participants with acute blood loss or trauma, the risk of developing recurrent bleeding associated with restrictive transfusion was about half that of liberal transfusion (RR 0.75, 95% CI 0.51 to 1.10).
8.5. Analysis.
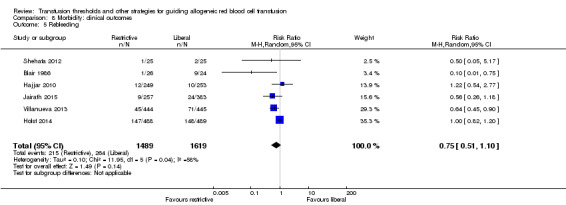
Comparison 8 Morbidity: clinical outcomes, Outcome 5 Rebleeding.
Sepsis/bacteraemia
Seven trials reported data for sepsis/bacteraemia in 3963 participants. There was no difference between the restrictive and liberal transfusion strategies (RR 1.03, 95% CI 0.79 to 1.35; Analysis 8.6). Heterogeneity between these trials was not statistically significant (Chi² = 6.28, df = 5 (P = 0.28); I² = 20%).
8.6. Analysis.
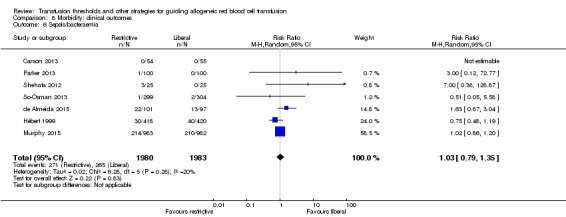
Comparison 8 Morbidity: clinical outcomes, Outcome 6 Sepsis/bacteraemia.
Pneumonia
Fourteen trials reported data for pneumonia in 6277 participants. There was no difference between the restrictive and liberal transfusion strategies (RR 0.94, 95% CI 0.80 to 1.11; Analysis 8.7). Heterogeneity between these trials was not statistically significant (Chi² = 9.83, df = 13 (P = 0.71); I² = 0.0%).
8.7. Analysis.
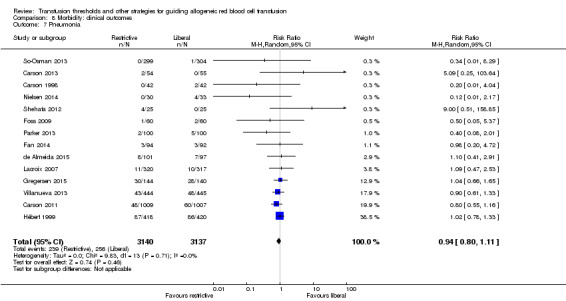
Comparison 8 Morbidity: clinical outcomes, Outcome 7 Pneumonia.
Pneumonia or wound infection
Fourteen trials reported data for infections in 9574 participants. The definition of infection was pneumonia or wound infection. There was no difference between the restrictive and liberal transfusion strategies (RR 0.96, 95% CI 0.86 to 1.07; Analysis 8.8). Heterogeneity between these trials was not statistically significant (Chi² = 19.00, df = 13 (P = 0.12); I² = 32%). Combining pneumonia with wound infections in the same analysis group has been the norm historically, but this was primarily due to a lack of sufficient data to separate these analyses. With the current number of trials included in the review and the large amount of participants, we had the ability to examine pneumonia separately rather than as a composite with wound infection ‐ and demonstrated that the results are similar.
8.8. Analysis.
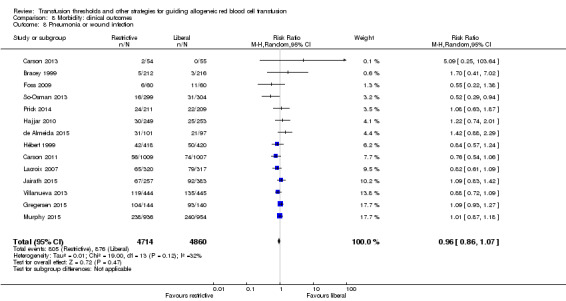
Comparison 8 Morbidity: clinical outcomes, Outcome 8 Pneumonia or wound infection.
Thromboembolism
Ten trials reported data for thromboembolism in 4019 participants. We calculated the risk ratio using the Peto method because the risk of thromboembolism was less than 1%. There was no difference between the restrictive and liberal transfusion strategies (RR 0.76, 95% CI 0.40 to 1.45; Analysis 8.9). Heterogeneity between these trials was not statistically significant (Chi² = 5.78, df = 9 (P = 0.76); I² = 0%).
8.9. Analysis.
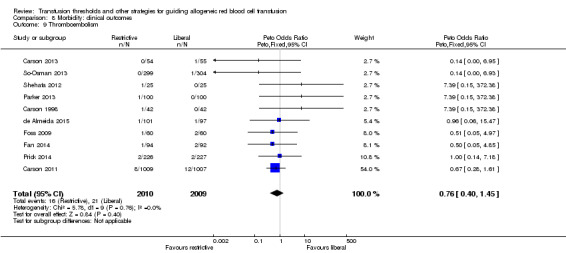
Comparison 8 Morbidity: clinical outcomes, Outcome 9 Thromboembolism.
Renal failure
Ten trials reported data for renal failure in 5929 participants. There was no difference between the restrictive and liberal transfusion strategies (RR 1.04, 95% CI 0.92 to 1.18; Analysis 8.10). Heterogeneity between these trials was not statistically significant (Chi² = 9.13, df = 9 (P = 0.43); I² = 1%).
8.10. Analysis.
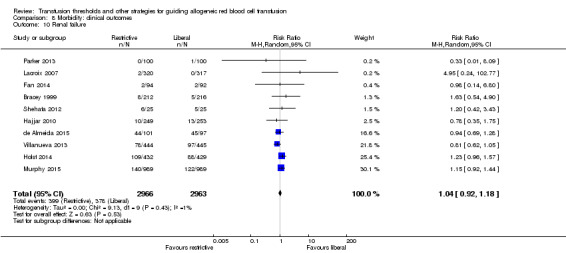
Comparison 8 Morbidity: clinical outcomes, Outcome 10 Renal failure.
Mental confusion
Six trials reported data for mental confusion in 1344 participants. There was no difference between the restrictive and liberal transfusion strategies (RR 0.92, 95% CI 0.65 to 1.30; Analysis 8.11). Heterogeneity between these trials was not statistically significant (Chi² = 5.53, df = 5 (P = 0.36); I² = 10%).
8.11. Analysis.
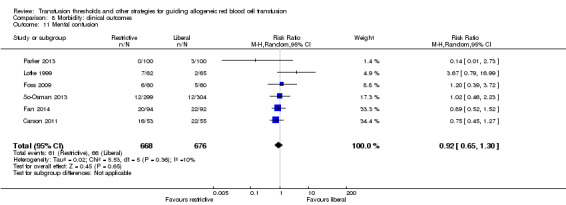
Comparison 8 Morbidity: clinical outcomes, Outcome 11 Mental confusion.
Functional recovery
Four trials reported functional outcomes in orthopaedic surgery participants. The functional measures were different in the trials, and hence, we could not pool them into a meta‐analysis, and only the numerical values from each trial are presented in graphs. Death or inability to walk at 30 days (RR 1.04, 95% CI 0.95 to 1.14) or 60 days (RR 0.99, 95% CI 0.87 to 1.11) was not significant between transfusion strategies (Analysis 9.1 ‐ pooled results not shown). No other measures of function were reported as significant between transfusion strategies in the single studies for each of the categories (Analysis 9.2 ‐ pooled results not shown).
9.1. Analysis.
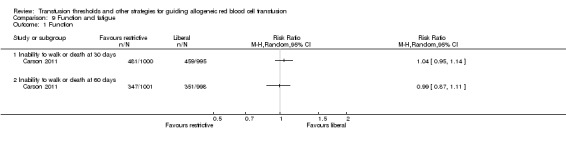
Comparison 9 Function and fatigue, Outcome 1 Function.
9.2. Analysis.
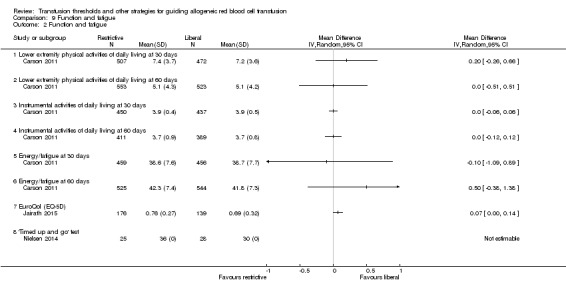
Comparison 9 Function and fatigue, Outcome 2 Function and fatigue.
Discussion
Summary of main results
We identified 31 randomized controlled trials that compared outcomes in participants allocated to receive transfusions of red blood cells (RBCs) at different haemoglobin concentration thresholds. These trials were undertaken between 1956 and 2016 and enrolled 12,587 participants across diverse patient populations. In the past four years, the number of participants in published trials evaluating transfusion thresholds has doubled.
The results of the meta‐analyses indicated that restrictive transfusion strategies led to a reduction of more than one‐third (43%) in the number of participants receiving at least one unit of blood, a red blood cell (RBC) transfusion requirement that was approximately 1.3 units lower, and a haemoglobin concentration that was around 1.32 g/dL lower than in the liberal transfusion groups. Most importantly, the meta‐analyses provided no evidence that restrictive transfusion policies harmed the participants or that they benefited from the use of liberal transfusion policies, within the parameters defined in the trials. Put another way, there was no evidence of an impact on clinically important outcomes when following a restrictive RBC transfusion policy compared with a liberal RBC transfusion policy. These findings may encourage the wider adoption of restrictive transfusion strategies, which would contribute to preserving blood supply.
Given the significant increase (a doubling) in the number of participants enrolled in transfusion threshold trials since this Cochrane Review was last published, this review has sufficient power to allow the primary outcome to be changed to 30‐day mortality. This increase also provided the opportunity to explore subgroups of participants with different underlying diseases, to ascertain whether the effects of RBC transfusion by haemoglobin thresholds are consistent across clinical specialities. This is important because there are pathophysiological reasons to postulate why transfusion might impact clinical outcomes differently in different patient populations, due to factors such as duration of anaemia (short term in critical illness versus long‐term transfusion dependence in bone marrow failure) or presence or not of an underlying restriction in cardiac function. Overall, across nearly all subgroups, the results indicated that risk of death and other adverse events were not impacted by either liberal or restrictive transfusion thresholds. Transfusion strategy did not influence the risk of cardiovascular events, including myocardial infarction, congestive heart failure, or stroke, although heterogeneity was observed in the trials that evaluated congestive heart failure (P = 0.01; I² = 57%).
In two subgroups of participant – those with acute blood loss and those with acute myocardial infarction, mortality may be influenced by a liberal or restrictive transfusion strategy, though the test for differences in 30‐day mortality between the subgroups was not significant (P = 0.13; I² = 41.2%). To be specific, in three trials (N = 1522) in participants with gastrointestinal bleeding (included in the acute blood loss or trauma grouping), a restrictive transfusion strategy was associated with a 35% lower risk of 30‐day mortality than a liberal transfusion strategy. The mechanism responsible for this significantly reduced risk of death may be due to a lower risk of rebleeding under restrictive transfusion regimens (risk ratio (RR) 0.65, 95% confidence interval (CI) 0.43 to 0.97; Analysis 1.3.4). The reason for this effect is not known, but may reflect higher vascular pressures following transfusion in the liberal transfusion group compared with the restrictive transfusion group. Participants enrolled with acute myocardial infarction constituted the other subgroup with apparently different results for 30‐day mortality. Two small trials included these participants (N = 154) for which the 30‐day mortality was 3.88 times higher in the restrictive transfusion group than in the liberal transfusion group (95% CI 0.83 to 18.13). These findings only provide a signal, since the results were not statistically significant and were based on a small number of participants.
Studies that used a restrictive threshold of 7 g/dL enrolled approximately half of the trial participants, and the other half used 8 g/dL as the restrictive threshold for transfusion. Most participants in the 7 g/dL restrictive transfusion threshold trials were based in critical care settings. The specialties were more varied in trials that tested an 8 g/dL restrictive transfusion threshold and included orthopaedic and cardiac surgery, gastrointestinal bleeding, and acute myocardial infarction. However, there was no apparent difference in the risk of death at 30 days between the two strata.
We compared 30‐day mortality in trials where the difference between the liberal and restrictive transfusion thresholds was at least 2 g/dL with those trials where the difference was less than 2 g/dL. Again, there was no evidence of dose‐effect of RBC transfusion by different trigger levels of haemoglobin concentration on clinical outcomes.
In this review, we compared the risk of infection in three ways but did not find evidence of a reduced risk of infection associated with restrictive transfusion. We combined pneumonia with wound infection (because they were the most common infections) and also examined sepsis or bacteraemia, and pneumonia (alone); the comparative risks of infection between the two transfusion strategies were nearly identical for all of these analyses. These results varied significantly from our prior analyses that had reported an elevated risk of infection in the liberal transfusion group (Rohde 2014). The change may be due to the incorporation of information from a recently reported cardiac surgery trial that had a large number of infections distributed equally between the liberal and restrictive transfusion strategies (Murphy 2015). Future trials may contribute to the power needed to examine subgroups.
Four trials assessed functional recovery, which used different measures, so meta‐analysis was not appropriate.
Overall completeness and applicability of evidence
With the expanding number of trials, the completeness of the evidence is increasing, and clinical trials have now evaluated many of the most common clinical specialities in which RBCs are transfused. Thus, the findings from this review are widely applicable to most clinical subgroups. However, we lack knowledge about the safety of different transfusion thresholds in groups of patients who frequently receive transfusion, but trials have not included. These understudied groups include those people with acute coronary syndrome, myocardial infarction, neurological injury/traumatic brain injury, acute neurological disorders, stroke, thrombocytopenia, cancer, haematological malignancies, and bone marrow failure.
Quality of the evidence
Overall, the quality of the evidence across the trials is good and shows improvement over time. The number of trials and participants enrolled has increased substantially, and the precision of the effect of transfusion has improved since the 2012 update of this review. We found relatively little heterogeneity for each clinical outcome across all analyses. However, we observed a significant amount of heterogeneity in the analyses evaluating the proportion of participants transfused, quantity of RBCs transfused, and differences in haemoglobin/haematocrit concentrations. It is conventional practice not to pool studies when there is such a large amount of heterogeneity; however, we chose to present the pooled results for these transfusion outcomes for several reasons. Firstly, the impact of restrictive transfusion on the proportion of participants transfused only varied by the magnitude of the reduction in transfusion, not the direction. In all of the trials, participants in the restrictive transfusion group received fewer transfusions, although the amount varied because the transfusion protocols were different and the clinical specialties required different frequencies of transfusion. Secondly, we expected the heterogeneity because of the variety of specialities for the clinical trials, including age; degree of comorbidities; and importantly, the policies for standard transfusion practice, which in turn reflect speciality‐specific guidelines and recommendations. At one extreme, nearly all participants, if not all, with leukaemia and cancer were transfused (DeZern 2016). Transfusion risk in participants in critical care, Hébert 1995; Hébert 1999; Lacroix 2007, or with acute blood loss, Villanueva 2013, was about 50% at the time of the studies.
In summary, we have chosen to present the pooled results for outcomes of transfusion because we are evaluating the effect of restrictive transfusion practice, and all study estimates for changes in transfusion are consistently in the same direction, and the substantial heterogeneity reflects the diversity in the strength of the estimates, rather than the efficacy of the policy. The reasons for the diversity in the strength of the trial estimates lies in the known and expected clinical specialities and the different practice guidelines used by different specialties. The subgroup explorations for transfusion outcomes reported earlier demonstrated these differences. The recognised differences in transfusion risk and amount by clinical specialities are a further justification for the more detailed analysis of all outcomes by clinical subgroups in this review, which we prespecified.
Potential biases in the review process
We performed extensive searches in an attempt to identify all eligible trials irrespective of publication status. Inspection of the funnel plots did not identify a major risk of publication bias (Figure 4; Figure 5).
'Risk of bias' evaluations revealed varying methodological issues between trials. We applied Cochrane methodology for defining high or low risk of bias to all trials, but acknowledged a number of challenges, including how to assign a single level of bias for multiple outcomes, for example, incomplete data or blinding (masking). Blinding the use of transfusion at the bedside is difficult to achieve unless study personnel are assigned to each participant, which would be an expensive procedure. The importance of blinding will differ according to the choice of primary trial outcome. For example, mortality is a hard endpoint and less open to bias than other functional outcomes.
Outcome assessment by observers who are blind to the treatment group is probably the most rigorous practical approach. Fourteen of the trials reviewed here reported this approach. Maintaining the integrity of the randomisation process becomes important if the trial is not to over‐estimate the benefit of the intervention (Schulz 1995). Some studies in this review did not report the methods used to conceal the allocation sequence from the treating clinicians. Ten trials used centralised allocation, and eight others used randomisation codes in sealed envelopes.
It is important to acknowledge limitations that apply to the findings of this review. The randomized trials may not have adequately evaluated some clinical outcomes specifically relevant to the use of RBC transfusions. Different grades of severity of cardiovascular events, such as myocardial infarction, congestive heart failure or stroke, or risk of overall infection, will occur in participants and may present in ways that are not always clinically overt and so are more subjective in interpretation. This is important because RBC transfusions may have both harmful and beneficial effects on the risk of these outcomes, for example, balancing prothrombotic tendencies against protective mechanisms to limit restrictions in myocardial oxygen delivery. Future studies need to establish robust definitions of all outcomes. The identified trials only evaluated the effect of transfusion in participants in hospital and not in outpatients in whom function and fatigue may be more important endpoints. Despite the large number of participants included in these trials, there remains inadequate power for many outcomes. Finally, a core rationale for RBC transfusion is to improve tissue and cellular oxygenation, but technologies for monitoring this directly are not available routinely; therefore, haemoglobin concentration is applied as a surrogate marker of need for transfusion, but it may not be a reliable biomarker.
Agreements and disagreements with other studies or reviews
The results of this review are consistent with other recent published systematic reviews. Holst 2015 recently identified 31 trials, with a total of 9813 randomized participants. We included 31 trials in our review, including a large, recently published trial in cardiac surgery (Murphy 2015), and a small pilot study in participants with haematological malignancies (DeZern 2016), but unlike Holst, we excluded studies in neonates (in whom the physiological responses to anaemia are considered very different from children and adults). The authors of Holst 2015 reported that restrictive transfusion strategies were associated with a reduction in the number of RBC units transfused and number of participants being transfused, with no effect on outcomes, including mortality, overall morbidity, and myocardial infarction. Restrictive compared with liberal transfusion strategies were not associated with risk of death (RR 0.86, 95% CI 0.74 to 1.01; 5707 participants), overall morbidity (RR 0.98, 95% CI 0.85 to 1.12; 4517 participants), or fatal or non‐fatal myocardial infarction (RR 1.28, 95% CI 0.66 to 2.49; 4730 participants). The inclusion of trials with unclear or high risk of bias did not affect results.
A number of systematic reviews have specifically examined participant subgroups. A review by Fominskiy aimed to assess the effect of liberal and restrictive RBC transfusion strategies on mortality in perioperative and critically ill adult participants through a meta‐analysis of relevant trials (Fominskiy 2015). Seventeen studies enrolled 7552 participants in perioperative settings, while 10 trials enrolled 3469 participants in critically ill settings. Participants in the perioperative period in the liberal transfusion strategy group had lower all‐cause mortality when compared with those allocated to the restrictive transfusion strategy group (odds ratio (OR) 0.81, 95% CI 0.66 to 1.00; P = 0.05; I² = 25%; number needed to treat for additional beneficial outcome = 97). There was no difference in mortality among critically ill participants receiving a liberal transfusion strategy when compared with the restrictive transfusion strategy (OR 1.10, 95% CI 0.99 to 1.23; P = 0.07; I² = 34%). The interpretation of systematic reviews that report only subgroups of the wider trial literature should be approached with some caution, to ensure that the patterns of findings are not inappropriately selective.
The results of these trials need to be viewed against large observational studies that compared clinical outcomes at varying haemoglobin levels in transfused and non‐transfused participants, finding conflicting results. In a study of 2202 participants undergoing coronary bypass surgery, the liberal transfusion group had a higher incidence of myocardial infarction than the conservative transfusion group (Spiess 1998). In a study of 8787 hip fracture participants, there was no difference in short‐ or long‐term mortality between participants transfused and not transfused (Carson 1998). In a study of 4470 intensive care unit participants, mortality was reduced in those receiving transfusion of up to six units of blood (Hébert 1997). A retrospective study of 78,974 Medicare beneficiaries found that blood transfusion was associated with a lower short‐term mortality rate among elderly patients with acute myocardial infarction if the haematocrit on admission was 30% or lower, and that blood transfusion may be effective with a haematocrit level as high as 33% on admission (Wu 2001). A study of 310,311 participants 65 years of age or older who underwent major non‐cardiac surgery found a 1.6% increase in 30‐day postoperative mortality for each 1% decrease in preoperative haematocrit (Wu 2007). Another study of 239,286 participants 65 years of age or older who underwent major non‐cardiac surgery found intraoperative blood transfusion was associated with a reduction in mortality in those with preoperative haematocrit levels of less than 24%, or in those with blood loss exceeding 500 mL (Wu 2010). The main limitation of these observational studies is that there may be residual confounding by indication, despite the extensive statistical adjustment of the results. It is possible that differences in participant characteristics between those that were transfused and non‐transfused may not have been identified or adequately adjusted for. This point is emphasised by the fact that a randomized controlled trial and an observational study (Hébert 1999 and Hébert 1997 respectively) in intensive care participants, performed by the same group of investigators, came to opposite conclusions. Even more convincing is how different ‐ and potentially biased ‐ the results of the meta‐analyses of observational data are; Chatterjee 2013 and Marik 2008 found a large increase in risk of death associated with transfusion. In contrast, the results of the meta‐analysis of clinical trials performed in this review showed no increased risk of death with liberal transfusion thresholds compared with restrictive transfusion thresholds. Despite recent assertions to the contrary (Benson 2000; Concato 2000), we believe that adequately powered, rigorously performed, randomized clinical trials are the only way of overcoming these limitations.
The transfusion policies reviewed here represent fairly small modifications to routine clinical practice. They are consistent with the recommendations of published clinical practice guidelines (AAGBI 2008; ASA 2006; BCTMAG 2003; Carson 2012a; Napolitano 2009; NBUGI 2001; Retter 2013; STSBCGTF 2011). The transfusion triggers (in terms of haemoglobin levels) were most often in the range of 7 g/dL to 10 g/dL. In fact, the 'restrictive' transfusion triggers in some trials were equivalent to the 'liberal' triggers used in other trials. Nevertheless, the trials documented significant reductions in the risk of RBC transfusion and worthwhile blood conservation. These effects are similar to what has been documented in meta‐analyses of trials of blood‐sparing techniques, such as cell salvage, Carless 2010a, and antifibrinolytic drugs, Henry 2011. Adoption of a conservative transfusion threshold appears to be as effective as these technologies in avoiding the need for transfusion and is likely to cost less.
Some guidelines have recommended RBC transfusion for symptoms or haemodynamic instability, rather than for a specific trigger haemoglobin level (AAGBI 2008; ASA 2006; Napolitano 2009; NBUGI 2001). A pilot study involving 84 participants tested this approach to transfusion (Carson 1998), as well as a trial involving 2016 participants (Carson 2011), and a 110‐participant trial in acute myocardial infarction (Carson 2013), in which participants could be transfused if they exhibited symptoms or had a haemoglobin concentration less than 8 g/dL. These studies found no difference in functional recovery, mortality, or morbidity in participants in the restrictive (symptomatic) transfusion group in the orthopaedic surgery trials (Carson 1998; Carson 2011); though in the trial involving participants with acute myocardial infarction (Carson 2013), there was a tendency toward worse outcomes in the restrictive transfusion group.
Authors' conclusions
Implications for practice.
Analysis of the published evidence shows that transfusing at a restrictive strategy of 7 g/dL to 8 g/dL, compared with a liberal haemoglobin threshold of 9 g/dL to 10 g/dL, across a broad range of hospitalised patients does not adversely affect clinical outcomes, including 30‐day mortality, cardiac morbidity, and infection. Evidence of a possible exception came from two small trials in participants with acute myocardial infarction, which suggested the possibility of increased 30‐day mortality with a restrictive transfusion threshold of 8 g/dL. Given there is no evidence of additional benefit of red blood cell (RBC) transfusion at higher haemoglobin concentration thresholds (9 g/dL to 10 g/dL), and that blood for transfusion is a costly and a scarce biological resource with finite risks, a restrictive transfusion trigger policy (7 g/dL to 8 g/dL) could be widely adopted. A restrictive transfusion policy is not associated with increased adverse events and reduces the risk of exposure to RBC transfusion and the total number of units transfused. There is insufficient evidence to evaluate the effect of the different strategies on functional recovery.
There was mixed evidence for adverse events associated with restrictive transfusion policies in two subsets of participants only. Firstly, as mentioned above, for two small trials that recruited participants with acute myocardial infarction, there was a raised risk for 30‐day mortality with restrictive transfusion, although in participants undergoing cardiac surgery, the risk of 30‐day mortality was not elevated. Secondly, in participants with acute blood loss from gastrointestinal bleeding, there may be a lower risk of 30‐day mortality with restrictive transfusion, but this requires confirmation. There were very limited data for participants with acute coronary syndrome, myocardial infarction, neurological injury/traumatic brain injury, acute neurological disorders, stroke, thrombocytopenia, cancer, haematological malignancies, and bone marrow failure. Many people in the latter categories will be transfusion‐dependent for prolonged periods of time and if concomitant bone marrow failure occurs will have a very different pathophysiology to other patient groups.
The trial interventions were split equally on the haemoglobin concentration used to define the restrictive transfusion group. About half of the trials used a 7 g/dL threshold, and the other half used a restrictive transfusion threshold of 8 g/dL to 9 g/dL. Therefore, it is unclear whether a 7 g/dL threshold could be used in most adult patients or only in critical care patients where most of those trials were conducted. Our clinical impression is that most adult patients would probably tolerate the lower 7 g/dL threshold, but specific trial data do not exist for some patient populations, and it is possible that an 8 g/dL threshold might improve function or reduce cardiovascular events. Thus, it is reasonable for clinicians to use an 8 g/dL threshold in settings where trial data for a 7 g/dL threshold are not available, such as orthopaedic surgery and in patients with cardiovascular disease.
In countries where there are concerns about the microbiological screening and safety of donated blood, the existing data constitute a stronger basis for avoiding liberal RBC transfusion in many clinical settings. The benefits of minimising allogeneic RBC transfusion are likely to be greatest where there is doubt about the safety of the blood supply.
Implications for research.
Further randomized trials should not be aimed at addressing the safety of RBC transfusion policies within the range of haemoglobin thresholds tested in the trials identified in this review or in unselected groups of patients across broad clinical settings. Rather, additional trials should be targeted to address specific research questions, where the strength of evidence‐based recommendations has significant uncertainty, as highlighted in this review. Subsets of patients where there is currently no adequately powered randomized controlled trial data to inform optimal RBC transfusion treatment include those with acute cardiovascular disease, neurological disorders including (traumatic) brain injury, and haematological and other malignancies. Outcomes of importance in trials would be mortality, but also functional and bleeding endpoints, specifically in transfusion‐dependent participants with cancer and haemological malignancies. We believe that in these clinical groups, the clinical goals and pathophysiology preclude generalisation from the completed studies included in this review. Trials are also needed to evaluate lower haemoglobin concentrations such as 6.0 g/dL, especially in countries with suboptimal blood safety and inadequate blood supply. Further research is needed to identify methods to measure oxygen delivery to vital organs directly. All trials should be large enough to measure the impact of lower thresholds on clinical outcomes and should apply consistent definitions for all clinical outcomes, such as myocardial infarction and ischaemic heart disease.
What's new
| Date | Event | Description |
|---|---|---|
| 27 May 2016 | New search has been performed | We made the following changes: 1) added 16 new trials; 2) used 30‐day mortality as the primary outcome because mortality is a more clinically relevant outcome and the number of participants enrolled in the trials provided sufficient power to examine this outcome; 3) added sensitivity analyses to evaluate heterogeneity for transfusion outcomes between trials; 4) changed authors. |
| 27 May 2016 | New citation required and conclusions have changed | The conclusions of the review have changed, and the search date is now 27 May 2016. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 20 December 2011 | New citation required and conclusions have changed | The searches were updated to February 2011. Data from two new trials are included and the results have been amended accordingly. One trial was identified through the updated search; the other had previously been included as an ongoing trial, and the results recently became available. The Background section of the review has been updated. The overall conclusions of the review remain similar, but the clinical specialties for which the results can be generalised have been extended. As part of this update, the assessment of methodological quality used in earlier versions of this review has been replaced with an assessment of the risk of bias. This amendment is in accordance with a change in Cochrane's methodological guidance. The authors of the review have changed. |
| 1 February 2011 | New search has been performed | The search for studies was updated to February 2011. |
| 9 September 2008 | Amended | Converted to new review format. |
| 17 November 2004 | New search has been performed | An updated search for new trials was conducted in November 2004. No new trials for inclusion were identified. |
Acknowledgements
We acknowledge the contribution of Suzanne Hill (World Health Organization), the first author of the original version, Hill 2000, and the 2005 update of the review, and Paul Carless, who was first author on the update published in 2010 (Carless 2010b). We also acknowledge the contribution of Kim Henderson in the original review first published in 2000. We acknowledge David Henry (Institute for Clinical Evaluative Sciences) and Brian McClelland who cowrote reviews up to 2010; Katharine Ker (London School of Hygiene & Tropical Medicine) undertook the following tasks for the 2010 update: screened search output, obtained articles, applied inclusion/exclusion criteria to retrieved papers, assessed risk of bias, extracted data, performed data analysis, and revised the text of the review. We thank Karen Blackhall (Injuries Group Trials Search Co‐ordinator), who ran electronic database searches in 2009 and 2011.
For this 2016 update, we thank Deirdre Beecher (Injuries Group Information Specialist), who ran the searches in 2015, and Sarah Dawson (Injuries Group Information Specialist), who updated the searching section in 2016. We are especially appreciative to Marialena Trivella, Emma Sydenham, Elizabeth Royle, and the other editors at Cochrane Injuries for the their extensive assistance in the analysis and presentation of this review.
This project was supported by the UK National Institute for Health Research (NIHR), through Cochrane Infrastructure funding to the Cochrane Injuries Group. The views and opinions expressed are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Search strategies May 2016
CENTRAL in the Cochrane Library #1 MeSH descriptor: [Blood Transfusion] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST, Trends ‐ TD] #2 MeSH descriptor: [Erythrocyte Transfusion] this term only and with qualifier(s): [Methods ‐ MT, Standards ‐ ST] #3 ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) near/5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)) #4 ((h?emoglobin or h?ematocrit or HB or HCT) near/5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)) #5 (blood near/3 (management or program*)) #6 ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)):ti #7 #1 or #2 or #3 or #4 or #5 or #6
MEDLINE (OvidSP) 1. *Blood Transfusion/ad, mt, st, td or *Erythrocyte Transfusion/mt, st, td 2. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) adj5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)).tw. 3. ((h?emoglobin or h?ematocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)).tw. 4. (blood adj3 (management or program*)).mp. 5. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)).ti. 6. or/1‐5 7. randomized controlled trial.pt. 8. controlled clinical trial.pt. 9. randomi*.tw. 10. placebo.ab. 11. clinical trials as topic.sh. 12. randomly.ab. 13. groups.ab. 14. trial.tw. 15. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 16. exp animals/ not humans/ 17. 15 not 16 18. 6 and 17
Embase (OvidSP) 1. *Blood Transfusion/ or Erythocyte Transfusion/ 2. ((red blood cell* or red cell* or RBC* or PRBC*) adj5 (therap* or transfus*)).mp. 3. 1 or 2 4. Standard/ or Gold Standard/ 5. 3 and 4 6. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) adj5 (trigger* or thresh?old* or target* or restrict* or liberal* or aggressive* or conservative* or prophylactic* or limit* or protocol* or policy or policies or practic* or indicat* or strateg* or regimen* or criteri* or standard* or management or program*)).tw. 7. ((h?emoglobin or h?ematocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or maintain* or indicator* or strateg* or criteri* or standard*)).tw. 8. (blood adj3 (management or program*)).mp. 9. ((transfus* or red cell* or red blood cell* or RBC* or PRBC*) and (critical* or intensive* or h?emorrhag* or bleed*)).ti. 10. or/5‐9 11. Randomized Controlled Trial/ 12. Randomization/ 13. Single Blind Procedure/ 14. Double Blind Procedure/ 15. Crossover Procedure/ 16. Placebo/ 17. exp Clinical Trial/ 18. Prospective Study/ 19. (randomi* or double‐blind* or single‐blind* or RCT*).tw. 20. (random* adj2 (allocat* or assign* or divid* or receiv*)).tw. 21. (crossover* or cross over* or cross‐over* or placebo*).tw. 22. ((treble or triple) adj blind*).tw. 23. or/11‐22 24. Case Study/ 25. case report*.tw. 26. (note or editorial).pt. 27. or/24‐26 28. 23 not 27 29. limit 28 to embase 30. 10 and 29
PubMed (for Epublications ahead of print only) #1 ((transfus*[TI] OR red cell*[TI] OR red blood cell*[TI] OR RBC*[TI] OR PRBC*) AND (trigger*[TI] OR threshold*[TI] OR target*[TI] OR restrict*[TI] OR liberal*[TI] OR aggressive*[TI] OR conservative*[TI] OR prophylactic*[TI] OR limit*[TI] OR protocol*[TI] OR policy[TI] OR policies[TI] OR practic*[TI] OR indicat*[TI] OR strateg*[TI] OR regimen*[TI] OR criteri*[TI] OR standard*[TI] OR management[TI] OR program*[TI])) #2 ((hemoglobin[TI] OR haemoglobin[TI] OR hematocrit[TI] OR haematocrit[TI] OR HB[TI] OR HCT[TI]) AND (polic*[TI] OR practic*[TI] OR protocol*[TI] OR trigger*[TI] OR threshold*[TI] OR maintain*[TI] OR indicator*[TI] OR strateg*[TI] OR criteri*[TI] OR standard*[TI])) #3 (blood[TI] AND (management[TI] OR program*[TI])) #4 ((transfus*[TI] OR red cell*[TI] OR red blood cell*[TI] OR RBC*[TI] OR PRBC*[TI]) and (critical*[TI] OR intensive*[TI] OR hemorrhag*[TI] OR haemorrhage*[TI] OR bleed*[TI])) #5 #1 OR #2 OR #3 OR #4 #6 (random* OR blind* OR "control group" OR placebo* OR controlled OR groups OR trial* OR "systematic review" OR "meta‐analysis" OR metaanalysis OR "literature search" OR medline OR cochrane OR embase) AND (publisher[sb] OR inprocess[sb] OR pubmednotmedline[sb]) #7 #5 AND #6
Transfusion Evidence Library Subject Area: Red Cells AND (trigger OR threshold OR target OR restrict OR restrictive OR liberal OR aggressive OR aggressively OR conservative OR prophylactic OR limit OR limits OR protocol OR policy OR policies OR practice OR indicator OR strategy OR strategies OR regimen OR criteria OR standard OR management OR program OR programme) OR Subject Area: Red Cells AND title:(critical OR critically OR intensive OR intensively OR hemorrhage OR haemorrhage OR hemorrhaging OR haemorrhaging OR bleed OR bleeding)
Web of Science Conference Proceedings Citation Index ‐ Science (CPCI‐S) ((TOPIC: ((transfus* OR "red cell*" OR "red blood cell*" OR RBC* OR PRBC*) NEAR/5 (trigger* OR threshold* OR target* OR restrict* OR liberal* OR aggressive* OR conservative* OR prophylactic* OR limit* OR protocol* OR policy OR policies OR practic* OR indicat* OR strateg* OR regimen* OR criteri* OR standard* OR management OR program*)) OR (TOPIC: ((hemoglobin OR haemoglobin OR hematocrit OR haematocrit OR HB OR HCT) NEAR/5 (polic* OR practic* OR protocol* OR trigger* OR threshold* OR maintain* OR indicator* OR strateg* OR criteri* OR standard*))) OR (TOPIC: (blood NEAR/3 (management OR program*))) AND (TOPIC: (random* OR blind* OR "control group" OR placebo* OR "controlled trial" OR "controlled study" OR "controlled clinical trial" OR groups OR trials OR systematic review OR meta‐analysis OR metaanalysis OR "literature search" OR medline OR cochrane OR embase))
Appendix 2. Search strategies ongoing trial registries May 2016
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (ICTRP)
Title/Intervention=(transfusion and (liberal or restrictive or threshold or Hb or haemoglobin or hemoglobin or haemaglobin or hemaglobin))
We also conducted an earlier search on the international trial registries in December 2015.
US National Institutes of Health Ongoing Trials Register Clinicaltrials.gov INFLECT EXACT "Interventional" [STUDY‐TYPES] AND ( "blood transfusion" OR "hemoglobin threshold" OR "haemoglobin threshold" OR "red blood cell transfusion" ) [TREATMENT] AND ( "01/02/2011" : "12/09/2015" ) [FIRST‐RECEIVED‐DATE]
World Health Organization International Clinical Trials Registry Platform (ICTRP) Intervention: "blood transfusion" OR "red blood cell transfusion" OR "hemoglobin threshold" OR "haemoglobin threshold" Recruitment status: ALL Date of registration: 01/02/2011 to 09/12/2015
ISRCTN Registry Intervention: "blood transfusion" Date applied: 01/02/2011 to 09/12/2015
Appendix 3. Search strategies 2011 (for 2012 update)
Cochrane Injuries Group's Specialised Register (searched 1 February 2011)
(Blood or "Red blood cell" or "Red blood cells" or RBC) and (therap* or transfus*) and (polic* or practice or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard* or restrict* or liberal* or management or program*)
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2011, Issue 1)
#1 MeSH descriptor Blood Transfusion, this term only with qualifiers: MT,ST #2 transfus* near5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*) #3 (Red blood cell* or RBC) near5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*) and (therap* or transfus*) #4 (H?emoglobin or h?emocrit or HB or HCT) near5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*) #5 transfus* near5 (restrict* or liberal*) #6 (blood transfus*) near3 (management or program*) #7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6)
MEDLINE (Ovid) 1948 to January Week 3 2011
1. *Blood Transfusion/ 2. ((Red blood cell* or RBC) adj3 (therap* or transfus*)).mp. 3. 1 or 2 4. exp Reference Standards/ 5. standards.fs. 6. methods.fs. 7. 4 or 5 or 6 8. 3 and 7 9. (transfus* adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 10. ((Red blood cell* or RBC) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 11. ((H?emoglobin or h?emocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 12. (transfus* adj5 (restrict* or liberal*)).mp. 13. ((blood or transfus*) adj3 (management or program*)).mp. 14. 8 or 9 or 10 or 11 or 12 or 13 15. randomi?ed.ab,ti. 16. randomized controlled trial.pt. 17. controlled clinical trial.pt. 18. placebo.ab. 19. clinical trials as topic.sh. 20. randomly.ab. 21. trial.ti. 22.15 or 16 or 17 or 18 or 19 or 20 or 21 23. (animals not (humans and animals)).sh. 24. 22 not 23 25. 24 and 14
Embase (Ovid) 1980 to 2011 Week 04
1. *Blood Transfusion/ 2. ((Red blood cell* or RBC) adj3 (therap* or transfus*)).mp. 3. 1 or 2 4. exp standard/ 5. 3 and 4 6. (transfus* adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 7. ((Red blood cell* or RBC) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 8. ((H?emoglobin or h?emocrit or HB or HCT) adj5 (polic* or practic* or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard*)).mp. 9. (transfus* adj5 (restrict* or liberal*)).mp. 10. ((blood or transfus*) adj3 (management or program*)).mp. 11. 5 or 6 or 7 or 8 or 9 or 10 12. exp Randomized Controlled Trial/ 13. exp controlled clinical trial/ 14. randomi?ed.ab,ti. 15. placebo.ab. 16. *Clinical Trial/ 17. randomly.ab. 18. trial.ti. 19. 12 or 13 or 14 or 15 or 16 or 17 or 18 20. exp animal/ not (exp human/ and exp animal/) 21. 19 not 20 22. 11 and 21
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to February 2011) and ISI Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990 to February 2011)
#1 TS=((Blood or "Red blood cell" or "Red blood cells" or RBC or Hemoglobin* or haemoglobin* or haemocrit or hemocrit or HB or HCT) SAME transfus*) #2 TS=(polic* or practice or protocol* or trigger* or threshold* or indicator* or strateg* or criteri* or standard* or restrict* or liberal* or management or program*) #3 #1 and #2 #4 TS=(randomised OR randomized OR randomly OR random order OR random sequence OR random allocation OR randomly allocated OR at random OR randomized controlled trial) OR Topic=(controlled clinical trial OR controlled trial OR clinical trial OR placebo) #5 TS=((singl* OR doubl* OR trebl* OR tripl*) SAME (blind* OR mask*)) #6 #2 or #3 #7 #3 and #6 #8 Topic=(human*) #9 #7 and #8
Data and analyses
Comparison 1. Mortality at 30 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day mortality | 23 | 10537 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2 30‐day mortality subgroup by restrictive haemoglobin level | 23 | 10537 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 2.1 Restrictive < 8 g/dL to 9 g/dL | 14 | 4772 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.78, 1.40] |
| 2.2 Restrictive < 7 g/dL | 9 | 5765 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.19] |
| 3 30‐day mortality subgroup analysis by clinical specialities | 23 | 10537 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.16] |
| 3.1 Cardiac surgery | 3 | 2942 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.77, 1.81] |
| 3.2 Orthopaedic surgery | 6 | 2831 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.72, 2.25] |
| 3.3 Vascular | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.26, 3.70] |
| 3.4 Acute blood loss/trauma | 3 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.43, 0.97] |
| 3.5 Critical care | 6 | 2840 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.75, 1.25] |
| 3.6 Acute myocardial infarction | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [0.83, 18.13] |
| 3.7 Haematological malignancies | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.07, 1.95] |
| 4 30‐day mortality by clinical specialities: myocardial infarction versus all others | 21 | 10388 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.18] |
| 4.1 Myocardial infarction | 2 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [0.83, 18.13] |
| 4.2 All but myocardial infarction | 19 | 10234 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.15] |
| 5 30‐day mortality in participants with cardiac surgery and myocardial infarction | 5 | 3096 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.80, 2.06] |
Comparison 2. Subgroup analysis by registered trials.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day mortality | 21 | 10350 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 1.1 Registered trials | 15 | 8773 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.84, 1.35] |
| 1.2 Unregistered trials | 6 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.02] |
Comparison 3. Sensitivity analysis by allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day mortality | 22 | 10477 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.18] |
| 1.1 Low risk | 15 | 8936 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.82, 1.24] |
| 1.2 Unclear or high risk | 7 | 1541 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.34] |
Comparison 4. Sensitivity analysis by blinding of outcome assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 30‐day mortality | 21 | 10388 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.82, 1.18] |
| 1.1 Low risk | 16 | 9356 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.81, 1.24] |
| 1.2 Unclear or high risk | 5 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.56, 1.55] |
Comparison 5. Mortality: other time intervals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital mortality | 10 | 5107 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.73, 1.01] |
| 2 90‐day mortality | 4 | 3485 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.40] |
Comparison 6. Blood transfusions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants exposed to blood transfusion (all studies) | 31 | 12587 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.49, 0.65] |
| 2 Participants exposed to blood transfusion by clinical specialties | 31 | 12587 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.49, 0.65] |
| 2.1 Cardiac surgery | 5 | 3021 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.64, 0.70] |
| 2.2 Orthopaedic surgery | 10 | 3907 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.38, 0.67] |
| 2.3 Vascular | 1 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.77, 1.08] |
| 2.4 Acute blood loss/trauma | 5 | 2416 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.23, 0.67] |
| 2.5 Critical care | 6 | 2840 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.55, 0.72] |
| 2.6 Acute myocardial infarction | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.19, 0.82] |
| 2.7 Haematological malignancies | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.95, 1.05] |
| 3 Participants exposed to blood transfusion (by transfusion threshold) | 26 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Difference between liberal and restrictive haemoglobin thresholds ≥ 2 g/dL | 19 | 7683 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.48, 0.65] |
| 3.2 Difference between liberal and restrictive haemoglobin thresholds < 2 g/dL | 7 | 3903 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 4 Participants exposed to blood transfusion by transfusion threshold | 31 | 12587 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.49, 0.65] |
| 4.1 Restrictive < 7 g/dL | 12 | 6453 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.47, 0.72] |
| 4.2 Restrictive < 8 g/dL to 9 g/dL | 19 | 6134 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.46, 0.67] |
| 5 Units of blood transfused | 12 | 4674 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐1.85, ‐0.75] |
Comparison 7. Haemoglobin concentration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Haemoglobin concentration | 16 | 7791 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐1.64, ‐0.99] |
Comparison 8. Morbidity: clinical outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cardiac events | 9 | 4849 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.79, 1.39] |
| 2 Myocardial infarction | 16 | 8303 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.74, 1.60] |
| 3 Congestive heart failure | 12 | 6257 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.45, 1.35] |
| 4 Cerebrovascular accident (CVA) ‐ stroke | 13 | 7343 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.53, 1.14] |
| 5 Rebleeding | 6 | 3108 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.51, 1.10] |
| 6 Sepsis/bacteraemia | 7 | 3963 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.35] |
| 7 Pneumonia | 14 | 6277 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.80, 1.11] |
| 8 Pneumonia or wound infection | 14 | 9574 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.07] |
| 9 Thromboembolism | 10 | 4019 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.40, 1.45] |
| 10 Renal failure | 10 | 5929 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.92, 1.18] |
| 11 Mental confusion | 6 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.65, 1.30] |
Comparison 9. Function and fatigue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Inability to walk or death at 30 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Inability to walk or death at 60 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Function and fatigue | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Lower extremity physical activities of daily living at 30 days | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Lower extremity physical activities of daily living at 60 days | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Instrumental activities of daily living at 30 days | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Instrumental activities of daily living at 60 days | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Energy/fatigue at 30 days | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 Energy/fatigue at 60 days | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 EuroQol (EQ‐5D) | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.8 'Timed up and go' test | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blair 1986.
| Methods | Randomised controlled trial | |
| Participants | 50 consecutive participants with severe upper gastrointestinal haemorrhage were randomized to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Blood usage (units), rebleeding, mortality, clotting times, Hct on admission/discharge, kaolin cephalin clotting time after 24 hours, impedance clotting time after 24 hours | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported no information regarding this domain. |
| Allocation concealment (selection bias) | Unclear risk | The trial reported no information regarding this domain. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial reported no information regarding this domain. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported no information regarding this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases identified. |
Bracey 1999.
| Methods | Randomised controlled trial | |
| Participants | 428 consecutive participants undergoing elective primary coronary artery bypass graft surgery were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, blood usage (units), blood loss, complications, infection rates, cardiac events | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly assigned on the basis of the last digit of their medical record number. |
| Allocation concealment (selection bias) | High risk | There was inadequate concealment (record number). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial reported no information regarding this domain. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome of mortality allows a judgement of low risk of bias. Morbidity information was collected from the hospital database. The trial provided no information regarding the survey questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial used intention‐to‐treat analysis and reported the exclusion of a small number of participants. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases identified. |
Bush 1997.
| Methods | Randomised controlled trial | |
| Participants | 99 participants undergoing elective aortic or infrainguinal arterial reconstruction were randomized to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Primary endpoints were myocardial ischaemia, myocardial infarction, and death. Secondary endpoints were length of intensive care unit stay, hospital stay, and graft patency. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported no information regarding this domain. |
| Allocation concealment (selection bias) | High risk | Sealed envelopes were chosen at random for participant assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both surgeons and anaesthesiologists were informed of the group of randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial reported no information regarding this domain. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The outcome data appeared to be complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases identified. |
Carson 1998.
| Methods | Randomised controlled trial | |
| Participants | 84 hip fracture participants undergoing surgical repair who had postoperative Hb levels < 10.0 g/dL were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, blood usage (units), complications, pneumonia, stroke, thromboembolism | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedules were stratified by clinical site and cardiovascular disease state. The randomisation was designed in blocks of 2 to 8 participants to avoid imbalance within a site. |
| Allocation concealment (selection bias) | Low risk | Study personnel at the clinical sites randomly assigned participants by contacting the data co‐ordinating centre's 24‐hour automated telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary outcome of mortality allowed a judgement of low risk of bias. Although function was assessed blinded, the morbidity outcomes were not assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were minimal missing data. |
| Selective reporting (reporting bias) | Low risk | The trial reported all outcomes. |
| Other bias | Low risk | No other biases identified. |
Carson 2011.
| Methods | Randomised, unblinded, parallel, 2‐group, multicentre trial | |
| Participants | Participants aged 50 years or older, who were undergoing surgical repair of a hip fracture, with Hb concentrations below 10.0 g/dL within 3 days after surgery and who had clinical evidence of cardiovascular disease or cardiovascular risk factors Sample size = 2016 |
|
| Interventions |
|
|
| Outcomes | The primary outcome was inability to walk 10 feet (or across a room) without human assistance or death prior to closure of the window for 60‐day mortality. Other outcomes were Hb concentration, acute coronary syndrome (ACS), in‐hospital myocardial infarction, unstable angina or death, disposition on discharge, survival, functional measures, fatigue/energy, readmission to hospital, pneumonia, wound infection, thromboembolism, stroke or transient ischaemic attack, cognition (Gruber‐Baldini), mortality at 30 days, and long‐term mortality (Carson 2014). | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data co‐ordinating centre staff prepared randomisation schedules for each site using randomly ordered block sizes of 2, 4, 6, or 8. |
| Allocation concealment (selection bias) | Low risk | The trial used an automated telephone randomisation system. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | After random allocation, clinical site staff, clinicians, and participants were not blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The primary and secondary outcomes were assessed blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was nearly complete reporting data for primary outcomes and most secondary outcomes. |
| Selective reporting (reporting bias) | Low risk | There was complete reporting. |
| Other bias | Low risk | No other biases identified. |
Carson 2013.
| Methods | Randomised clinical trial | |
| Participants | Participants with acute myocardial infarction or undergoing cardiac catheterisation with Hg less than 10 g/dL
|
|
| Interventions |
|
|
| Outcomes | Primary outcomes were death, myocardial infarction, and unscheduled revascularisation. Secondary outcomes were 30‐day and 6‐month mortality, long‐term mortality, myocardial infarction, congestive heart failure, stroke, thromboembolism, and pneumonia. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | The trial used central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and physicians were not blinded, but this was unlikely to impact the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All primary and most secondary outcomes were assessed blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 of 110 participants was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported. |
| Other bias | Low risk | No other biases identified. |
Cooper 2011.
| Methods | Randomised clinical trial | |
| Participants | 45 participants with acute myocardial infarction and haematocrit less than 30%
|
|
| Interventions | Liberal transfusion: transfusion occurred when haematocrit < 30% to maintain 30% to 33%. Conservative transfusion: transfusion occurred when haematocrit < 24% to maintain 24% to 27%. |
|
| Outcomes | The primary clinical safety measurements were in‐hospital death, recurrent myocardial infarction, or new or worsening congestive heart failure. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The paper did not describe random sequence generation. |
| Allocation concealment (selection bias) | Low risk | The trial used consecutively numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and investigators were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | A local investigator determined the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was complete in‐hospital follow‐up. 3 of 45 participants were lost to follow‐up at 30 days. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases identified. |
de Almeida 2015.
| Methods | Randomised clinical trial | |
| Participants | Adult participants who underwent a major surgical procedure for abdominal cancer and required postoperative care in the ICU
|
|
| Interventions | While in the ICU, the liberal transfusion group received transfusion when Hg < 9 g/dL, and the restrictive transfusion group received transfusion when Hg < 7 g/dL. | |
| Outcomes | The primary outcome was a composite of all‐cause mortality or severe clinical complications within 30 days. Severe clinical complications included major cardiovascular complications, septic shock, acute kidney injury requiring renal replacement therapy, ARDS, and reoperation. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The chief statistician ensured random sequence generation. |
| Allocation concealment (selection bias) | Low risk | The trial used opaque envelopes that were opened sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians or participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The participants and the study investigators who classified outcomes and those who conducted the follow‐up telephone assessments were blinded to the study‐group assignments and had no access to transfusion data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases identified. |
DeZern 2016.
| Methods | Randomised clinical trial | |
| Participants | Acute leukaemia participants (acute myeloid leukaemia, acute lymphoblastic leukaemia/lymphoma, acute promyelocytic leukaemia, treatment‐related myeloid neoplasm, high‐grade myelodysplastic syndrome) more than 18 years of age admitted to the inpatient leukaemia services with plans for inpatient myelosuppressive chemotherapy
|
|
| Interventions | 2 participants were assigned to the restrictive (LOW) Hb trigger (7 g/ dL) for every 1 participant assigned to the higher (HIGH) Hb trigger (8 g/dL) (90 (participants randomized 2:1). | |
| Outcomes | Primary outcomes: feasibility defined a priori as achieving the following 4 criteria: 1) more than 50% of the eligible participants consented, 2) more than 75% of the participants randomized to the 7 g/dL arm tolerated the transfusion trigger, 3) fewer than 15% of participants crossed over from the lower transfusion threshold arm to the higher transfusion threshold arm, and 4) no indications for the need to pause the study for safety concerns Secondary outcomes included fatigue, bleeding, response to therapy, vital status on day 60, length of hospital stay (days), and the number of units of RBCs and PLTs transfused per participant. The trial author provided the 30‐day mortality. |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer software generated the random number sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered envelopes were opened upon determination of inclusion for each participant in the trial. An investigator who did not enrol or consent participants for the trial performed the randomisation sequence and creation and numbering of the envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Risk varied by outcome. Detection bias was low risk for the outcome of mortality; other secondary outcomes had a high risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other bias was apparent. |
Fan 2014.
| Methods | Randomised clinical trial | |
| Participants | 186 participants 65 years of age or older undergoing elective unilateral total hip replacement
|
|
| Interventions |
|
|
| Outcomes | Delirium, cerebrovascular accident, cardiac failure, myocardial infarction, pulmonary embolism, pneumonia, superficial wound infection, urinary tract infection, acute renal failure | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a random number table. |
| Allocation concealment (selection bias) | Low risk | The trial used a sealed envelope technique. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The blinding of participants and personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was a low rate of missing data. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other bias was apparent. |
Fisher 1956.
| Methods | Randomised controlled trial | |
| Participants | 22 trauma participants were randomly allocated to 1 of 2 groups:
NB: no demographic data were reported. |
|
| Interventions |
|
|
| Outcomes | Blood usage (units), blood loss, wound healing, elevated temperature, number of participants transfused, Hb levels | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The use of random sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The trial used sealed envelopes. When the participant was considered eligible for the trial, they were placed in a severity grade and an envelope was opened to decide which transfusion schedule was to be used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not addressed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data set appeared to be complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Foss 2009.
| Methods | Randomised controlled trial | |
| Participants | 120 hip fracture participants were randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Ambulatory capacity, mortality, length of stay, cardiac complications, infectious complications | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a computer‐generated list. |
| Allocation concealment (selection bias) | Unclear risk | The trial used sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial reported that participants were blinded. Clinicians could not implement the protocol. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The physiotherapist who performed ambulation assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13 of 100 participants did not have ambulation assessment. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Gregersen 2015.
| Methods | Randomised clinical trial | |
| Participants | Participants aged 65 years or older undergoing hip fracture surgery and who had postoperative Hb levels between 9.7 g/dL and 11.3 g/dL during the first 6 postoperative days
|
|
| Interventions | The liberal transfusion group received transfusion when Hb was less than 11.3 g/dL, and the restrictive transfusion group received transfusion when Hg was less than 9.7 g/dL. | |
| Outcomes | The primary outcome was recovery from physical disabilities, with 3 tools being used to measure physical performance: Modified Barthel index, New Mobility score and cumulated ambulation score, total number of infections (pneumonia, urinary tract infection, other), cognition, depression, quality of life, modified Barthels index, and comprehensive frailty index. | |
| Notes | 3 publications reported results. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was not specifically stated, but it was likely since a clinical trial support system was used. |
| Allocation concealment (selection bias) | Low risk | A web‐based randomisation system was used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were blinded but not the clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The outcome data appeared to be complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Grover 2006.
| Methods | Randomised controlled trial | |
| Participants | 260 participants undergoing elective lower limb joint replacement surgery were randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Ischaemic load, blood load, Hb concentration, number of units transfused, length of hospital stay, adverse events, new infections requiring antibiotic therapy | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | The trial used sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The anaesthetists and surgical team responsible for treatment were aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of a recruited 260 participants, outcome data were presented for 218. The missing 42 participants did not have analysable tape recordings. |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Hajjar 2010.
| Methods | Randomised clinical trial | |
| Participants | 502 adult participants who underwent cardiac surgery with cardiopulmonary bypass
|
|
| Interventions |
|
|
| Outcomes | The primary outcome composite endpoint included 30‐day all‐cause mortality and severe morbidity (cardiogenic shock; ARDS or acute renal injury requiring dialysis or haemofiltration; respiratory, cardiac, neurologic, and infectious complications; inflammatory complications; bleeding; ICU and hospital lengths of stay, RBC transfusions). | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The chief statistician prepared a random number table to use. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were opened sequentially. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were blinded but not clinicians. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was undertaken. Follow‐up was complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Holst 2014.
| Methods | Randomised clinical trial | |
| Participants | Participants with septic shock and haemoglobin concentration less than 9 g/dL
|
|
| Interventions | The intervention was single units of cross‐matched, prestorage leukoreduced RBCs when the blood concentration of haemoglobin had decreased to the assigned transfusion threshold (≤ 7 g/dL (lower threshold) or ≤ 9 g/dL (higher threshold)). The intervention period was the entire ICU stay, to a maximum of 90 days after randomisation. | |
| Outcomes | The primary outcome was 90‐day mortality. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A centralised computer generated the assignment sequence. |
| Allocation concealment (selection bias) | Low risk | Use of a centralised computer ensured allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators assessing mortality (the DSMB) and the trial statistician were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was near‐complete follow‐up. |
| Selective reporting (reporting bias) | Low risk | Reporting was comprehensive. |
| Other bias | Low risk | There were no other biases. |
Hébert 1995.
| Methods | Randomised controlled trial | |
| Participants | 69 normovolaemic critically ill participants admitted to 1 of 5 tertiary level intensive care units with Hb values < 9.0 g/dL within 72 hours of admission were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, length of ICU stay, blood usage (units), complications, Hb levels | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were assigned to 1 of 2 groups by consecutive allocation from a random listing stratified by centre and disease severity. |
| Allocation concealment (selection bias) | Unclear risk | The trial reported no information regarding this domain. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of treatment allocation was not feasible, but it was unlikely to have been important. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not specified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Hébert 1999.
| Methods | Randomised controlled trial | |
| Participants | 838 critically ill participants with euvolaemia after initial treatment who had Hb concentrations < 9.0 g/dL within 72 hours after admission to the intensive care unit were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Mortality, length of hospital stay, length of ICU stay, blood usage (units), complications, infection rates, cardiac events, pulmonary oedema, pneumonia | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random order was computer generated. |
| Allocation concealment (selection bias) | Low risk | The data co‐ordinating centre prepared sealed opaque envelopes, which they distributed to each participating institution where they were opened up sequentially to determine the participants treatment assignment. The envelopes were returned periodically to the co‐ordinating centre for auditing. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "It was not feasible to mask the assigned transfusion strategy from health care providers." Participants were ICU patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mortality was the primary outcome. Most outcomes were based on laboratory measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Jairath 2015.
| Methods | Cluster‐randomised trial pilot study involving 6 hospitals | |
| Participants | Participants were 18 years of age or older and admitted to 1 of the participating hospitals with upper gastrointestinal bleeding. Participants with exsanguinating haemorrhage were excluded. Participants consented to permit data collection and follow‐up. |
|
| Interventions | The following transfusion strategy was encouraged at each participating hospital:
|
|
| Outcomes | Feasibility outcomes included recruitment rates, adherence to transfusion policy, difference in haemoglobin concentration, RBC exposure, and evidence for selection bias. Clinical outcomes included further bleeding, thromboembolic and ischaemic events, number of infections, mortality, serious adverse events, and health‐related quality of life. | |
| Notes | This was the only cluster trial in our review. It did not require participants to meet a haemoglobin threshold for enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospital was randomized, not the individual participant. |
| Allocation concealment (selection bias) | High risk | The hospital was randomized, so everyone knew which arm the participants were in. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Mortality allowed a judgement of low risk of bias. Assessment of other clinical outcomes was unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a high percentage of missing data. |
| Selective reporting (reporting bias) | Unclear risk | No reporting bias was apparent. |
| Other bias | High risk | There was differential enrolment by treatment arms. |
Johnson 1992.
| Methods | Randomised controlled trial | |
| Participants | 39 autologous blood donors undergoing elective myocardial revascularisation were randomized to 1 of 2 groups:
|
|
| Interventions |
NB: operative management included sequestration of 1 or more units of fresh autologous blood in participants with a Hct value greater than 35% who were haemodynamically stable after anaesthetic induction. Red cell conservation was practised through the salvage of oxygenator contents and reinfusion of postoperatively shed mediastinal blood. On the 5th postoperative day, all participants were asked to complete an exercise treadmill test. A second test was performed the following day. |
|
| Outcomes | Cardiac events, complications, postoperative blood loss, blood use (total units), allogeneic blood use (units), autologous blood use (units), all product blood use (units), number of participants receiving transfusions, mean cardiac index, mean systemic resistance, exercise capacity, Hct levels, length of ICU stay, length of hospital stay | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | A table of random numbers and an odd‐even designation randomized participants. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear if assignment was concealed prior to randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Surgeons and anaesthesiologists were blinded as to the group of randomisation until the participant reached the intensive care unit (ICU). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A small number of exclusions were reported. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Lacroix 2007.
| Methods | Randomised controlled trial | |
| Participants | 637 stable critically ill children with Hb concentrations below 9.5 g/dL within 7 days after admission to an ICU were randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | 28‐day mortality, sepsis, transfusion reactions, infections, length of stay | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial reported no information regarding this domain. |
| Allocation concealment (selection bias) | Low risk | Allocation was internet‐based and central. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinical staff and parents of the participants were aware of the assignments to study groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mortality was the primary outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were very few dropouts. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Lotke 1999.
| Methods | Randomised controlled trial | |
| Participants | 152 participants undergoing primary total knee arthroplasty (TKA) were randomly assigned to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Complications, cardiac events, Hb levels, blood usage (units), mental confusion, lethargy, orthostatic hypotension, number of participants transfused | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a computer random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were made by a person blind to the group to which the participant was assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appear to have been complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Murphy 2015.
| Methods | Randomised clinical trial | |
| Participants | Participants older than 16 years of age who were undergoing nonemergency cardiac surgery with haemoglobin level below 9 g/dL
|
|
| Interventions | The liberal transfusion threshold group received transfusion when the haemoglobin level was < 9 g/dL, and the restrictive transfusion threshold group received transfusion when the haemoglobin level was less than 7.5 g/dL. | |
| Outcomes | The primary outcome was a composite of a serious infection (sepsis or wound infection) or an ischaemic event (permanent stroke, myocardial infarction, infarction of the gut, or acute kidney injury) within 3 months after randomisation. Secondary outcomes included units transfused, infection, ischaemic events, acute kidney injury, hospital stay and ICU stay, and cost. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The use of random sequence generation was not stated. |
| Allocation concealment (selection bias) | Low risk | The trial used an internet‐based system that concealed assignments and used cohort minimisation to balance assignments according to centre and type of surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Physicians and nurses were aware of the group assignments. "We intended participants to be unaware of the group assignments and tested our success in keeping the study groups blinded by asking the patients if they were aware of the group they were in." At discharge 15.1% of patients believed they knew treatment and 75.6% were correct. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were adjudicated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The was a low loss to follow up. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Nielsen 2014.
| Methods | Randomised clinical trial | |
| Participants | Participants were at least 18 years of age and scheduled for elective hip revision surgery.
|
|
| Interventions | The participants were randomized to a restrictive strategy receiving transfusion of RBC at a Hb of 7.3 g/dL (4.5 mmol/L) or a liberal strategy receiving transfusion of RBC at a Hb of 8.9 g/dL (5.5 mmol/L). The target level of haemoglobin in the restrictive group was 7.3 g/dL to 8.9 g/dL and above 8.9 g/dL in the liberal group. | |
| Outcomes | The primary outcome was the 'Timed up and go' test. Other outcomes were pneumonia, wound infection, gastrointestinal complications, dizziness, hypotension, fatigue, deep vein thrombosis, and fall. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A dedicated computer program (Idefix) was used after entering participants' baseline data. The allocation was written on a form, which was kept in the investigator's office, and the allocation could only be accessed by the investigator in charge of administrating red blood cells. |
| Allocation concealment (selection bias) | Low risk | Only 1 investigator had access to the programme. Investigators at the other hospital had to call this investigator to randomise. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The allocation and Hb during the testing period were concealed from the participants but the investigator, the staff in the operating room, and the staff at the ward could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The physiotherapist testing the participant was blinded, but it was not stated who reviewed medical records for other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other bias was apparent. |
Parker 2013.
| Methods | Randomised clinical trial | |
| Participants | Participants 60 years of age or older with hip fracture and whose postoperative haemoglobin level on postoperative days 1 or 2 was between 8.0 g/dL to 9.5 g/dL
|
|
| Interventions | Liberal transfusion maintained haemoglobin > 10.0 g/dL, or the symptomatic group received transfusion for symptoms of anaemia. These included recurrent vaso‐vagal episodes on mobilisation, chest pain of cardiac origin, congestive cardiac failure, unexplained tachycardia, hypotension or dyspnoea that was felt to be due to anaemia, decreased urine output that is unresponsive to fluid replacement, or symptoms felt appropriate by the medical staff. | |
| Outcomes | Mobility, mental agility, physical status using the American Society of Anesthesiologists grade | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The random sequence generation was not documented. |
| Allocation concealment (selection bias) | Low risk | The trial used opaque numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and personnel was not addressed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The mobility score was missing for 94 of 200 participants. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Prick 2014.
| Methods | Randomised controlled trial, not blinded | |
| Participants | Postpartum haemorrhage (blood loss of ≥1000 ml or a decrease in Hb concentration of ≥1.9 g/dL, or both) and had an Hb between 4.8 g/dL and 7.9 g/dL 12 to 24 hours after delivery
|
|
| Interventions | In the liberal group, participants received at least 1 unit of red blood cells; the trialists aimed to reach an Hb concentration of at least 8.9 g/dL. In the restrictive group, participants received no transfusion. | |
| Outcomes | Primary outcome was physical fatigue 3 days postpartum using the Multidimensional Fatigue Inventory scale | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The use of random sequence generation was not stated. |
| Allocation concealment (selection bias) | Low risk | The trial used a web‐based application with block randomisation of variable block size. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The primary outcome was based on a questionnaire. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% of data for the primary outcome was missing. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Shehata 2012.
| Methods | Randomised clinical trial | |
| Participants | Adults participants undergoing cardiac surgery with a CARE score (a score for cardiac surgery participants used to predict morbidity and mortality) of 3 or 4, or participants of advanced age defined as greater than or equal to 80 years
|
|
| Interventions | Those on the restrictive transfusion strategy received RBC transfusions if their Hb was 7.0 g/dL or less during cardiopulmonary bypass and 7.5 g/dL or less postoperatively after bypass. Those on the liberal transfusion strategy received RBC transfusions if their Hb concentration was 9.5 g/dL or less during and less than 10 g/dL after bypass. | |
| Outcomes | The primary outcome was enrolment rate and overall adherence to the transfusion strategies. Clinical outcomes were assessed. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician generated the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque sequential sealed envelopes were opened at the start of surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians and participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding for clinical outcomes was not addressed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data appeared complete. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
So‐Osman 2013.
| Methods | Randomised clinical trial | |
| Participants | Elective orthopedic surgery
|
|
| Interventions | Restrictive transfusion was compared with liberal transfusion regimens. | |
| Outcomes | The primary outcome variable was RBC use. Secondary outcomes included postoperative complications and quality of life. | |
| Notes | We re‐analysed the prior report (So‐Osman 2010) comparing restrictive versus liberal transfusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial provided a detailed description of statistical procedures. |
| Allocation concealment (selection bias) | Low risk | A research nurse opened sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians caring for the participants were aware of allocation status. There was no blinding information on participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The trial did not state who collected outcome data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition bias was apparent. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Villanueva 2013.
| Methods | Randomised clinical trial | |
| Participants | Participants older than 18 years of age who had haematemesis or melena, or both (due to upper GI bleeding)
|
|
| Interventions | The restrictive transfusion group was transfused for haemoglobin < 7 g/dL, and the liberal transfusion group was transfused when Hg was < 9 g/dL. In both groups, 1 unit of RBCs was transfused initially. | |
| Outcomes | Death at 45 days | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was computer generated. |
| Allocation concealment (selection bias) | Low risk | The trial used sealed consecutively numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians and participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mortality was the primary outcome. Assessors of other outcomes were not documented to be blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial had good follow up. |
| Selective reporting (reporting bias) | Low risk | Reporting was complete. |
| Other bias | Low risk | No other biases were apparent. |
Walsh 2013.
| Methods | Randomised clinical trial | |
| Participants | ICU participants aged ≥ 55 years, Hg < 9 g/dL, mechanical ventilation for ≥ 96 hours, and were expected to require ≥ 24 hours of further mechanical ventilation when assessed
|
|
| Interventions | The restrictive transfusion group received transfusion with haemoglobin≤ 7.0g/dL and a target Hb concentration of 7.1 g/dL to 9.0g/dL, and the liberal transfusion group received transfusions with haemoglobin ≤ 9.0 g/dL and a target of 9.1 g/dL to 11.0 g/dL during intervention. | |
| Outcomes | The primary feasibility outcome was the difference in mean Hb among groups. Clinical outcomes were assessed. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Minimisation by centre and the presence of IHD, including a random element, was used. |
| Allocation concealment (selection bias) | Low risk | The trial used telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Clinicians were not blinded. Most surviving participants stated that they were unaware of group allocation at 180 days (restrictive group: 67%; liberal group: 78%); 23% of participants in the restrictive group and 9% in the liberal group correctly stated their treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Researchers concealed from group allocation collected questionnaire‐based measures at 60 and 180 days postrandomisation. Assessment of clinical outcomes was not documented to have been done blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was good follow up. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Webert 2008.
| Methods | Randomised controlled trial | |
| Participants | 60 adult participants with acute leukaemia were randomly allocated to 1 of 2 groups:
|
|
| Interventions |
|
|
| Outcomes | Transfusions, bleeding risk, 30‐day mortality provided by authors | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Allocation was internet‐based and central. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and clinicians were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data. |
| Selective reporting (reporting bias) | Low risk | No reporting bias was apparent. |
| Other bias | Low risk | No other biases were apparent. |
Abbreviations
ACS = acute coronary syndrome ARDS = acute respiratory distress syndrome CARE = Cardiac Anesthesia Risk Evaluation DSMB = Data Safety Monitoring Board GI = gastrointestinal Hb = haemoglobin Hct = haematocrit ICU = intensive care unit IHD = ischaemic heart disease M/F = male/female PLTs = platelets RBC = red blood cells SD = standard deviation TKA = total knee arthroplasty
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fortune 1987 | This study measured oxygen utilisation. The trial planned no clinical outcomes of interest. |
| Robertson 2014 | The trial used a 2 x 2 factorial design in which participants received erythropoietin or transfusion at different thresholds. We were unable to isolate the effect of transfusion from erythropoietin. |
| Vichinsky 1995 | The transfusion trigger was based on the level of sickle haemoglobin (HbS), not the haemoglobin or haematocrit level. |
| Zygun 2009 | This trial measured oxygen utilisation and planned no clinical outcomes of interest. |
Abbreviation
HbS = sickle haemoglobin
Characteristics of studies awaiting assessment [ordered by study ID]
ISRCTN26088319.
| Methods | Randomised clinical trial |
| Participants | Participants with myelodysplastic syndrome, transfusion dependent, and life expectancy > 6 months |
| Interventions | Liberal: to maintain haemoglobin concentration Restrictive: to maintain a haemoglobin level between 8.5 g/dL and 10 g/dL |
| Outcomes | Percentage of compliance of pretransfusion haemoglobin levels and achievement of at least 2 g/dL difference between liberal and restrictive transfusion |
| Notes | ‐ |
NCT02203292.
| Methods | Randomised clinical trial |
| Participants | Moderate or severe traumatic brain injury and a haemoglobin level less than 9 g/dL |
| Interventions | Liberal transfusion: transfusion if haemoglobin level was less than 9 g/dL Restrictive transfusion: transfusion only if haemoglobin level was less than 7 g/dL |
| Outcomes | Primary outcome: haemoglobin difference at 14 days |
| Notes | ‐ |
Characteristics of ongoing studies [ordered by study ID]
NCT01079247.
| Trial name or title | A randomized clinical trial of restrictive vs. traditional blood transfusion practices in burn patients |
| Methods | Randomised clinical trial |
| Participants | > 20% TBSA burn with anticipated operation needed on admission |
| Interventions | Liberal transfusion: transfusion to maintain haemoglobin at 10g/dL to 11 g/dL Restrictive transfusion: transfusion to maintain haemoglobin level at 7g/dL to 8 g/dL |
| Outcomes | Blood stream infection |
| Starting date | February 2010 |
| Contact information | Tina L Palmieri, MD University of California, Davis |
| Notes | ‐ |
NCT02042898.
| Trial name or title | An international, multi‐center, randomized controlled trial to assess transfusion thresholds in patients undergoing cardiac surgery (TRICS‐III) |
| Methods | Randomised clinical trial |
| Participants | Cardiac surgery using cardiopulmonary bypass and preoperative European System for Cardiac Operation Risk Evaluation (euroSCORE) of 6 or more |
| Interventions | Liberal: transfusion if haemoglobin level is less than 9.5 g/dL Restrictive: transfusion if haemoglobin level is less than 7.5 g/dL |
| Outcomes | Composite of any of the following: all‐cause mortality, myocardial infarction, new renal failure requiring dialysis, new focal neurological deficit (primary outcomes) |
| Starting date | January 2014 |
| Contact information | David Mazer or Nadine Shehata |
| Notes | ‐ |
NCT02099669.
| Trial name or title | Red blood cell transfusion thresholds and QOL in MDS (EnhanceRBC): a pilot, feasibility Study |
| Methods | Randomised clinical trial |
| Participants | All participants with MDS ≥18 years of age, transfusion dependent: at least 1 transfusion per month in the last 8 weeks, haemoglobin < 10 g/dL |
| Interventions | Liberal transfusion strategy: to maintain Hb level between 11 g/dL and 12 g/dL Restrictive transfusion strategy: to maintain Hb level between 8.5 g/dL and 10 g/dL |
| Outcomes | Percentage compliance of q2weekly haemoglobin |
| Starting date | March 2014 |
| Contact information | Kristina Commisso kristina.commisso@sunnybrook.ca |
| Notes | ‐ |
NCT02465125.
| Trial name or title | The transfusion triggers in vascular surgery trial: two different transfusion triggers for postoperative haemoglobin separation and adherence to transfusion strategies in vascular surgery: a randomized clinical feasibility trial |
| Methods | Randomised clinical trial |
| Participants | Open repair of abdominal aorta or infrainguinal arterial bypass |
| Interventions | Restrictive transfusion: transfusion when haemoglobin level is less than 5 mmol/L (approximately 8 g/dL) Liberal transfusion: transfusion when haemoglobin level is less than 6 mmol/L (approximately 10 g/dL) |
| Outcomes | Postoperative haemoglobin measured at the time of arrival to recovery room or ICU (primary outcome) |
| Starting date | June 2015 |
| Contact information | Anders Moller dr.andersm@gmail.com |
| Notes | ‐ |
NCT02483351.
| Trial name or title | Aneurysmal subarachnoid hemorrhage: red blood cell transfusion and outcome ‐ a pilot randomized controlled trial (SaHARA pilot) |
| Methods | Randomised clinical trial |
| Participants | Subarachnoid haemorrhage |
| Interventions | Liberal transfusion (10 g/dL) versus restrictive transfusion |
| Outcomes | Randomised rate |
| Starting date | October 2015 |
| Contact information | Shane English, MD senglish@ohri.ca |
| Notes | ‐ |
NCT02619136.
| Trial name or title | Myocardial ischemia and transfusion: a pilot, multi‐centre, open label randomized clinical trial of two commonly used transfusion strategies in patients with myocardial infarction |
| Methods | Randomised clinical trial |
| Participants | Myocardial infarction |
| Interventions | Liberal transfusion: transfusion if haemoglobin level is less than 10 g/dL for up to 30 days postrandomisation Restrictive transfusion: transfusion is permitted if haemoglobin level is less than 8 g/dL and required below 7 g/dL |
| Outcomes | Enrolment rate (primary outcome) |
| Starting date | February 2016 |
| Contact information | Paul Hebert paul.hebert.chum@ssss.gouv.qc.ca |
| Notes | ‐ |
NCT02648113.
| Trial name or title | Cost‐effectiveness and cost‐utility of liberal vs restrictive red blood cell transfusion strategies in patients with acute myocardial infarction and anemia. The REALITY (restrictive and liberaltransfusion strategies in patients with acute myocardial infarction) randomized trial |
| Methods | Randomised clinical trial |
| Participants | Acute myocardial infarction |
| Interventions | Liberal transfusion: transfusion when haemoglobin ≤ 10 Restrictive transfusion: transfusion when haemoglobin ≤ 8 |
| Outcomes | Cost‐effectiveness ratio at 30 days |
| Starting date | March 2016 |
| Contact information | Phillippe‐Gabriel Steg gabriel.steg@aphp.fr |
| Notes | ‐ |
NTR3244.
| Trial name or title | Perioperative transfusion study (PETS): does a liberal transfusion protocol improve outcome in high‐risk cardiovascular patients undergoing non‐cardiac surgery? |
| Methods | Randomised clinical trial |
| Participants | Elective high‐risk cardiac surgery participants |
| Interventions | Liberal transfusion: 11 g/dL Restrictive transfusion: 9.7 g/dL |
| Outcomes | Troponin elevation above 99th percentile |
| Starting date | August 2015 |
| Contact information | Felix van Lier |
| Notes | ‐ |
Tay 2011.
| Trial name or title | Transfusion of red cells in hematopoietic stem cell transplantation: the TRIST study |
| Methods | Randomised clinical trial stratified by centre and type of transplant |
| Participants | Participants undergoing hematopoietic stem cell transplantation |
| Interventions | Restrictive (target haemoglobin of 7 g/dL to 9 g/dL) or liberal (target haemoglobin of 9 g/dL to 11 g/dL) RBC transfusion strategy, based on daily haemoglobin values up to 100 days post‐transplant |
| Outcomes | Feasibility and clinical outcomes including transfusion requirements, transplant‐related mortality, maximum grade of acute graft versus host disease, veno‐occlusive disease, serious infections, Bearman Toxicity Score, bleeding, quality of life, number of hospitalisations, and number of intensive care unit admissions. |
| Starting date | March 2011 |
| Contact information | Jason Tay, MD Department of Medicine University of Calgary |
| Notes | ‐ |
Abbreviations
Hb = haemoglobin ICU = intensive care unit MDS = myelodysplastic syndromes QOL = quality of life RBC = red blood cells TBSA = total body surface area
Differences between protocol and review
The following differences applied in this (2016) version of the review.
The primary outcome has changed in this version of the review from 'the proportion of patients 'at risk' who were transfused with red blood cells', to '30‐day mortality'. Previously, 30‐day mortality was a secondary outcome. Now the proportion of participants 'at risk' who were transfused with red blood cells is a secondary outcome. The primary outcome was changed because mortality is a more clinically relevant outcome and the number of participants enrolled in trials provided sufficient power to examine this outcome. Sample size calculations assuming baseline 30‐day mortality of 9% for restrictive transfusion, 90% power, alpha level of 0.05, indicate that to detect a 15%, 20%, or 25% relative decrease in mortality with the use of liberal transfusion, a study needs to enrol 17,500, or 9600, or 6000 participants, respectively.
We added one new exclusion criterion: we excluded trials that were not designed to include any clinical outcomes relevant to this review.
We added a new sensitivity analysis: registered studies versus unregistered studies.
We separated blinding of participants and personnel from blinding of outcome assessment.
The authors of the review have changed.
Contributions of authors
Please note that names are listed alphabetically.
For the 2012 update
Paul Carless (University of Newcastle) performed original database literature searches, screened abstracts and titles for relevant articles, obtained relevant papers, applied inclusion/exclusion criteria to retrieved papers, extracted data from the trials, quality‐assessed trials, entered data into Meta‐View 4.1, entered all study details into Review Manager 5.1 (Review Manager 5b), and co‐wrote the review. Jeffrey Carson (Rutgers Robert Wood Johnson Medical School) screened abstracts and titles for relevant articles, obtained relevant papers, applied inclusion/exclusion criteria to retrieved papers, extracted data from the trials, quality‐assessed trials, entered data and all study details into Review Manager 5.1, and co‐wrote the review. Paul Hebert (Ottawa General Hospital) reviewed the manuscript and provided expertise with analysis and content expert opinion.
For the 2016 review
Jeffrey Carson and Simon Stanworth screened the abstracts and titles identified in the searches, applied inclusion and exclusion criteria, and assessed the quality of the trials. Simon Stanworth led the quality review, and Jeffrey Carson entered the data into Review Manager 5.3 (Review Manager 5a), performed the initial analyses, and prepared the first draft of the manuscript. Nareg Roubinian checked the accuracy of the data and assisted with the manuscript. Dean Fergusson provided methodological and statistical expertise and assisted with the manuscript. Darrell Triulzi and Paul Hebert reviewed the manuscript and provided content expertise. Carolyn Doree performed additional literature searches.
Sources of support
Internal sources
No sources of support supplied
External sources
New South Wales Ministerial Advisory Committee on Quality in Health Care, Australia.
New South Wales Health Department, Australia.
Declarations of interest
Jeffrey Carson reports receiving grant support to his institution from the US National Institutes of Health. He is involved with guideline development and has received a grant from the US National Institutes of Health to evaluate transfusion thresholds in patients with acute myocardial infarction.
Carolyn Doree: nothing to declare.
Dean Ferugussion is a Co‐Prinical Investigator on the TRICSIII trial, and a member of the Steering Committee for the MINT trial.
Paul Hebert and Jeffrey Carson have received a grant from the Canadian Institutes of Health Research for a pilot trial of transfusion in patients with acute myocardial infarction.
Nareg Roubinian: nothing to declare.
Simon Stanworth has received funding for two RBC transfusion trials in patients with haematological malignancies.
Darryl Triluizi is a member of the Steering Committee for the MINT trial and a member of the scientific advisory board for Fresenius‐Kabi.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Blair 1986 {published data only}
- Blair SD, Janvrin SB, McCollum CN, Greenhalgh RM. Effect of early blood transfusion on gastrointestinal haemorrhage. British Journal of Surgery 1986;73(10):783‐5. [DOI] [PubMed] [Google Scholar]
Bracey 1999 {published data only}
- Bracey AW, Radovancevic R, Riggs SA, Houston S, Cozart H, Vaughn WK, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion 1999;39(10):1070‐7. [DOI] [PubMed] [Google Scholar]
Bush 1997 {published data only}
- Bush RL, Pevec WC, Holcroft JW. A prospective, randomized trial limiting perioperative red blood cell transfusions in vascular patients. American Journal of Surgery 1997;174(2):143‐8. [DOI] [PubMed] [Google Scholar]
Carson 1998 {published data only}
- Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin‐level‐driven red blood cell transfusions following hip fracture. Transfusion 1998;38(6):522‐9. [DOI] [PubMed] [Google Scholar]
Carson 2011 {published data only}
- Carson JL, Sieber F, Cook DR, Hoover DR, Noveck H, Chaitman BR, et al. Liberal versus restrictive blood transfusion strategy: 3‐year survival and cause of death results from the FOCUS randomised controlled trial. Lancet 2015;385(9974):1183‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. New England Journal of Medicine 2011;365(26):2453‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber‐Baldini AL, Marcantonio E, Orwig D, Magaziner J, Terrin M, Barr E, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. Journal of the American Geriatrics Society 2013;61(8):1286‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carson 2013 {published data only}
- Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. American Heart Journal 2013;165(6):964‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2011 {published data only}
- Cooper HA, Rao SV, Greenberg MD, Rumsey MP, Mckenzie M, Alcorn KW, et al. Conservative versus liberal red cell transfusion in acute myocardial infarction (the CRIT randomized pilot study). American Journal of Cardiology 2011;108(8):1108‐11. [DOI] [PubMed] [Google Scholar]
de Almeida 2015 {published data only}
- Almeida JP, Vincent JL, Galas FR, Almeida EP, Fukushima JT, Osawa EA, et al. Transfusion requirements in surgical oncology patients: a prospective, randomized controlled trial. Anesthesiology 2015;122(1):29‐38. [DOI] [PubMed] [Google Scholar]
DeZern 2016 {published and unpublished data}
- DeZern AE, Williams K, Zahurak M, Hand W, Stephens RS, King KE, et al. Red blood cell transfusion triggers in acute leukemia: a randomized pilot study. Transfusion 2016;56(7):1750‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2014 {published data only}
- Fan YX, Liu FF, Jia M, Yang JJ, Shen JC, Zhu GM, et al. Comparison of restrictive and liberal transfusion strategy on postoperative delirium in aged patients following total hip replacement: a preliminary study. Archives of Gerontology and Geriatrics 2014;59(1):181‐5. [DOI] [PubMed] [Google Scholar]
Fisher 1956 {published data only}
- Fisher MR, Topley ET. The illness of trauma. British Journal of Clinical Practice 1956;10(11):770‐6. [PubMed] [Google Scholar]
Foss 2009 {published data only}
- Foss NB, Kristensen MT, Jensen PS, Palm H, Krasheninnikoff M, Kehlet H. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion 2009;49(2):227‐34. [DOI] [PubMed] [Google Scholar]
Gregersen 2015 {published data only}
- Gregersen M, Borris LC, Damsgaard EM. Blood transfusion and overall quality of life after hip fracture in frail elderly patients‐‐the transfusion requirements in frail elderly randomized controlled trial. Journal of the Americal Medical Directors Association 2015;16(9):762‐6. [DOI] [PubMed] [Google Scholar]
- Gregersen M, Borris LC, Damsgaard EM. Postoperative blood transfusion strategy in frail, anemic elderly patients with hip fracture: the TRIFE randomized controlled trial. Acta Orthopaedica 2015;86(3):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen M, Damsgaard EM, Borris LC. Blood transfusion and risk of infection in frail elderly after hip fracture surgery: the TRIFE randomized controlled trial. European Journal of Orthopaedic Surgery & Traumatology 2015;25(6):1031‐8. [DOI] [PubMed] [Google Scholar]
Grover 2006 {published data only}
- Grover M, Talwalkar S, Casbard A, Boralessa H, Contreras M, Boralessa H, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sanguinis 2006;90(2):105‐12. [DOI] [PubMed] [Google Scholar]
- Grover M, Talwalker SA, Contreras, Soni N. Blood transfusion threshold and silent myocardial ischemia after lower limb arthroplasty: a randomized controlled trial of transfusion strategy. Transfusion Alternatives in Transfusion Medicine 2005;7(1 (Suppl)):62‐3. [Google Scholar]
Hajjar 2010 {published data only}
- Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304(14):1559‐67. [DOI] [PubMed] [Google Scholar]
Hébert 1995 {published data only}
- Hébert PC, Wells G, Marshall J, Martin C, Tweeddale M, Pagliarello G, et al. Transfusion requirements in critical care. A pilot study. Canadian Critical Care Trials Group [published erratum appears in JAMA 1995 Sep 27;274(12):944]. JAMA 1995;273(18):1439‐44. [DOI] [PubMed] [Google Scholar]
Hébert 1999 {published data only}
- Carson JL, Terrin ML, Barton FB, Aaron R, Greenburg AG, Heck DA, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin‐level‐driven red blood cell transfusions following hip fracture. Transfusion 1998;38(6):522‐9. [DOI] [PubMed] [Google Scholar]
- Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. New England Journal of Medicine 1999;340(6):409‐17. [DOI] [PubMed] [Google Scholar]
- McIntyre L, Hebert PC, Wells G, Fergusson D, Marshall J, Yetisir E, et al. Canadian Critical Care Trials Group. Is a restrictive transfusion strategy safe for resuscitated and critically ill trauma patients?. The Journal of Trauma 2004;57(3):563‐8. [DOI] [PubMed] [Google Scholar]
Holst 2014 {published and unpublished data}
- Holst LB, Haase N, Wetterslev J, Wernerman J, Guttormsen AB, Karlsson S, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. New England Journal of Medicine 2014;371(15):1381‐91. [DOI] [PubMed] [Google Scholar]
Jairath 2015 {published data only}
- Jairath V, Kahan BC, Gray A, Doré CJ, Mora A, James MW, et al. Restrictive versus liberal blood transfusion for acute upper gastrointestinal bleeding (TRIGGER): a pragmatic, open‐label, cluster randomised feasibility trial. Lancet 2015;386(9989):137‐44. [DOI] [PubMed] [Google Scholar]
Johnson 1992 {published data only}
- Johnson RG, Thurer RL, Kruskall MS, Sirois C, Gervino EV, Critchlow J, et al. Comparison of two transfusion strategies after elective operations for myocardial revascularization. Journal of Thoracic and Cardiovascular Surgery 1992;104(2):307‐14. [PubMed] [Google Scholar]
Lacroix 2007 {published data only}
- Lacroix J, Hébert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. New England Journal of Medicine 2007;356(16):1609‐19. [DOI] [PubMed] [Google Scholar]
Lotke 1999 {published data only}
- Lotke PA, Barth P, Garino JP, Cook EF. Predonated autologous blood transfusions after total knee arthroplasty: immediate versus delayed administration. Journal of Arthroplasty 1999;14(6):647‐50. [DOI] [PubMed] [Google Scholar]
Murphy 2015 {published data only}
- Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes EA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. New England Journal of Medicine 2015;372:997‐1008. [DOI] [PubMed] [Google Scholar]
Nielsen 2014 {published data only}
- Nielsen K, Johansson PI, Dahl B, Wagner M, Frausing B, Børglum J, et al. Perioperative transfusion threshold and ambulation after hip revision surgery‐‐a randomized trial. BMC Anesthesiology 2014;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2013 {published data only}
- Parker MJ. Randomised trial of blood transfusion versus a restrictive transfusion policy after hip fracture surgery. Injury 2013;44(12):1916‐8. [DOI] [PubMed] [Google Scholar]
Prick 2014 {published data only}
- Prick BW, Jansen AJ, Steegers EA, Hop WC, Essink‐Bot ML, Uyl‐de Groot CA, et al. Transfusion policy after severe postpartum haemorrhage: a randomised non‐inferiority trial. BJOG 2014;121(8):1005‐14. [DOI] [PubMed] [Google Scholar]
Shehata 2012 {published data only}
- Shehata N, Burns LA, Nathan H, Hebert P, Hare GM, Fergusson D, et al. A randomized controlled pilot study of adherence to transfusion strategies in cardiac surgery. Transfusion 2012;52(1):91‐9. [DOI] [PubMed] [Google Scholar]
So‐Osman 2013 {published data only}
- So‐Osman C. A restrictive transfusion trigger is a method for blood saving in elective orthopaedic surgery. Vox Sanguinis 2004;87(Suppl 3):52. [Google Scholar]
- So‐Osman C, Nelissen R, Brand R, Faber F, Slaa RT, Stiggelbout A, et al. The impact of a restrictive transfusion trigger on post‐operative complication rate and well‐being following elective orthopaedic surgery: a post‐hoc analysis of a randomised study. Blood Transfusion (Trasfusione del sangue) 2013;11(2):289‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So‐Osman C, Nelissen R, Slaa R, Coene L, Brand R, Brand A. A randomized comparison of transfusion triggers in elective orthopaedic surgery using leucocyte‐depleted red blood cells. Vox Sanguinis 2010;98(1):56‐64. [DOI] [PubMed] [Google Scholar]
Villanueva 2013 {published and unpublished data}
- Colomo A, Hernandez‐Gea V, Muniz‐Diaz E, Madoz P, Aracil C, Alvarez‐Urturi C. Transfusion strategies in patients with cirrhosis and acute gastrointestinal bleeding. Hepatology 2008;48(4 (Suppl)):413A. [Google Scholar]
- Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez‐Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. New England Journal of Medicine 2013;368(1):11‐21. [DOI] [PubMed] [Google Scholar]
Walsh 2013 {published data only}
- Walsh TS, Boyd JA, Watson D, Hope D, Lewis S, Krishan A, et al. Restrictive versus liberal transfusion strategies for older mechanically ventilated critically ill patients: a randomized pilot trial. Critical Care Medicine 2013;41(10):2354‐63. [DOI] [PubMed] [Google Scholar]
Webert 2008 {published and unpublished data}
- Webert KE, Cook RJ, Couban S, Carruthers J, Lee KA, Blajchman MA, et al. A multicenter pilot‐randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion 2008;48(1):81‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fortune 1987 {published data only}
- Fortune JB, Feustel PJ, Saifi J, Stratton HH, Newell JC, Shah DM. Influence of hematocrit on cardiopulmonary function after acute hemorrhage. The Journal of Trauma 1987;27(3):243‐9. [DOI] [PubMed] [Google Scholar]
Robertson 2014 {published data only}
- Robertson CS, Hannay HJ, Yamal JM, Gopinath S, Goodman JC, Tilley BC, et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. JAMA 2014;312(1):36‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vichinsky 1995 {published data only}
- Vichinsky EP, Haberkern CM, Neumayr L, Earles AN, Black D, Koshy M, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. New England Journal of Medicine 1995;333(4):206‐13. [DOI] [PubMed] [Google Scholar]
Zygun 2009 {published data only}
- Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Critical Care Medicine 2009;37(3):1074‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ISRCTN26088319 {unpublished data only}
- ISRCTN26088319. Red cell transfusion and QoL in myelodysplastic syndromes [Red blood cell transfusion thresholds and quality of life in myelodysplastic syndromes: a pilot, feasibility study]. www.isrctn.com/ISRCTN26088319 (first received 13 November 2014).
NCT02203292 {unpublished data only}
- NCT02203292. Transfusion requirements after head trauma (TRAHT). www.clinicaltrials.gov/ct2/show/NCT02203292 (first received 26 July 2014).
References to ongoing studies
NCT01079247 {unpublished data only}
- NCT01079247. A trial of restrictive versus traditional blood transfusion practices in burn patients [A randomized clinical trial of restrictive vs. traditional blood transfusion practices in burn patients]. www.clinicaltrials.gov/ct2/show/NCT01079247 2010 (accessed 9/9/16).
NCT02042898 {unpublished data only}
- NCT02042898. Transfusion requirements in cardiac surgery III (TRICS‐III) [An international, multi‐centre, randomized controlled trial to assess transfusion thresholds in patients undergoing cardiac surgery]. www.clinicaltrials.gov/ct2/show/NCT02042898 2014 (accessed 9/9/16).
NCT02099669 {unpublished data only}
- NCT02099669. Red blood cell transfusion thresholds and QOL in MDS (EnhanceRBC) [Red blood cell transfusion thresholds and QOL in MDS (EnhanceRBC): a pilot, feasibility study]. www.clinicaltrials.gov/ct2/show/NCT02099669 2014 (accessed 9/9/16).
NCT02465125 {unpublished data only}
- NCT02465125. The transfusion triggers in vascular surgery trial (TV) [The transfusion triggers in vascular surgery trial: two different transfusion triggers for postoperative haemoglobin separation and adherence to transfusion strategies in vascular surgery: a randomised clinical feasibility trial]. www.clinicaltrials.gov/ct2/show/NCT02465125 2015 (accessed 9/9/16).
NCT02483351 {unpublished data only}
- NCT02483351. Aneurysmal subarachnoid hemorrhage: red blood cell transfusion and outcome (SAHaRA Pilot) [Aneurysmal subarachnoid hemorrhage: red blood cell transfusion and outcome ‐ a pilot randomized controlled trial]. www.clinicaltrials.gov/ct2/show/NCT02483351 2015 (accessed 9/9/16).
NCT02619136 {unpublished data only}
- NCT02619136. Myocardial ischemia and transfusion (MINT) [Myocardial ischemia and transfusion: a pilot, multi‐centre, open‐label randomized controlled trial of two commonly used transfusion strategies in patients with myocardial infarction]. www.clinicaltrials.gov/ct2/show/NCT02619136 2015 (accessed 9/9/16).
NCT02648113 {unpublished data only}
- NCT02648113. Cost‐effectiveness and cost‐utility of liberal vs restrictive red blood cell transfusion strategies in patients with acute myocardial infarction and anaemia (REALITY). www.clinicaltrials.gov/ct2/show/NCT02648113 2016 (accessed 9/9/16).
NTR3244 {unpublished data only}
- NTR3244. Blood transfusion study in patients at risk for cardiac complications after non‐cardiac surgery [Perioperative Transfusion Study (PETS): does a liberal transfusion protocol improve outcome in high‐risk cardiovascular patients undergoing non‐cardiac surgery?]. www.trialregister.nl/trialreg/admin/rctview.asp?TC=3244 2012 (accessed 9/9/16).
Tay 2011 {published data only}
- NCT01237639. Study of red blood cell transfusion triggers in patients undergoing hematopoietic stem cell transplantation (TRIST) [Transfusion of red cells in hematopoietic stem cell transplantation: the TRIST Study]. www.clinicaltrials.gov/ct2/show/NCT01237639 2010 (accessed 9/9/16).
- Tay J, Tinmouth A, Fergusson D, Allan D. Transfusion of red cells in hematopoietic stem cell transplantation (TRIST): study protocol for a randomized controlled trial. Trials 2011;12:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
AAGBI 2008
- Association of Anaesthetists of Great Britain and Ireland. Blood transfusion and the anaesthetist ‐ red cell transfusion. www.aagbi.org/publications/guidelines/docs/red_cell_08.pdf 2008 (accessed 11 October 2016).
ASA 2006
- American Society of Anesthesiologists (ASA) Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology 2006;105(1):198‐208. [DOI] [PubMed] [Google Scholar]
BCTMAG 2003
- British Columbia Transfusion Medicine Advisory Group (BCTMAG). Guidelines for red blood cell transfusion. British Columbia Transfusion Medicine Advisory Group 2003; Vol. November:1‐3.
Benson 2000
- Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. New England Journal of Medicine 2000;342(25):1878‐86. [DOI] [PubMed] [Google Scholar]
Carless 2010a
- Carless PA, Henry DA, Moxey AJ, O'Connell D, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD001888.pub4] [DOI] [PubMed] [Google Scholar]
Carson 1996
- Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996;348(9034):1055‐60. [DOI] [PubMed] [Google Scholar]
Carson 2012a
- Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, et al. Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Annals of Internal Medicine 2012;157(1):49‐58. [DOI] [PubMed] [Google Scholar]
Chatterjee 2013
- Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: a meta‐analysis and diversity‐adjusted study sequential analysis. JAMA Internal Medicine 2013;173(2):132‐9. [DOI] [PubMed] [Google Scholar]
Concato 2000
- Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. New England Journal of Medicine 2000;342(25):1887‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delaney 2016
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dhabangi 2015
- Dhabangi A, Ainomugisha B, Cserti‐Gazdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. JAMA 2015;314(23):2514‐23. [DOI] [PubMed] [Google Scholar]
Fergusson 2013
- Fergusson D, Tinmouth A. Storage age of red blood cells for transfusion of premature infants‐‐reply. JAMA 2013;309(6):545. [DOI] [PubMed] [Google Scholar]
Fominskiy 2015
- Fominskiy E, Putzu A, Monaco F, Scandroglio AM, Karaskov A, Galas FR, et al. Liberal transfusion strategy improves survival in perioperative but not in critically ill patients. A meta‐analysis of randomised trials. British Journal of Anaesthesia 2015;115(4):511‐9. [DOI] [PubMed] [Google Scholar]
Henry 2011
- Henry DA, Carless PA, Moxey AJ, O'Connell D, Stokes BJ, Fergusson DA, et al. Anti‐fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001886.pub4] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holst 2015
- Holst LB, Petersen MW, Haase N, Perner A, Wetterslev J. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta‐analysis and trial sequential analysis. BMJ 2015;350:h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hébert 1997
- Hébert PC, Wells G, Tweeddale M, Martin C, Marshall J, Pham B, et al. Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. American Journal of Respiratory and Critical Care Medicine 1997;155(5):1618‐23. [DOI] [PubMed] [Google Scholar]
Klein 2007
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet 2007;370(9585):415‐26. [DOI] [PubMed] [Google Scholar]
Lacroix 2015
- Lacroix J, Hébert PC, Fergusson DA, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. New England Journal of Medicine 2015;372(15):1410‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Madjdpour 2005
- Madjdpour C, Spahn DR. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. British Journal of Anaesthesia 2005;95(1):33‐42. [DOI] [PubMed] [Google Scholar]
Marik 2008
- Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Critical Care Medicine 2008;36(9):2667‐74. [DOI] [PubMed] [Google Scholar]
Napolitano 2009
- Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Critical Care Medicine 2009;37(12):3124‐57. [DOI] [PubMed] [Google Scholar]
NBUGI 2001
- National Blood Users Group Ireland (NBUGI). A guideline for transfusion of red blood cells in surgical patients. www.dohc.ie/publications/transfusion_of_red_blood_cells.html (accessed 11 October 2016).
NIH 1988
- National Institutes of Health (NIH). Perioperative red blood cell transfusion. JAMA 1988;260(18):2700‐3. [PubMed] [Google Scholar]
Retter 2013
- Retter A, Wyncoll D, Pearse R, Carson D, McKechnie S, Stanworth S, et al. Guidelines on the management of anaemia and red cell transfusion in adult critically ill patients. British Journal of Haematology 2013;160(4):445‐64. [DOI] [PubMed] [Google Scholar]
Review Manager 5a [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Review Manager 5b [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.1. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rohde 2014
- Rohde JM, Dimcheff DE, Blumberg N, Saint S, Langa KM, Kuhn L, et al. Health care‐associated infection after red blood cell transfusion: a systematic review and meta‐analysis. JAMA 2014;311(13):1317‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabatine 2005
- Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, et al. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 2005;111(16):2042‐9. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DJ. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled clinical trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Flasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Review of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shander 2010
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity‐based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50(4):753‐65. [DOI] [PubMed] [Google Scholar]
Shander 2014
- Shander A, Javidroozi M, Naqvi S, Aregbeyen O, Caylan M, Demir S, et al. An update on mortality and morbidity in patients with very low postoperative hemoglobin levels who decline blood transfusion (CME). Transfusion 2014;54(10 Pt 2):2688‐95. [DOI] [PubMed] [Google Scholar]
Spiess 1998
- Spiess BD, Ley C, Body SC, Siegel LC, Stover EP, Maddi R, et al. Hematocrit value on intensive care unit entry influences the frequency of Q‐wave myocardial infarction after coronary artery bypass grafting. The Institutions of the Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Journal of Thoracic & Cardiovascular Surgery 1998;116(3):460‐7. [DOI] [PubMed] [Google Scholar]
Steiner 2015
- Steiner ME, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Effects of red‐cell storage duration on patients undergoing cardiac surgery. New England Journal of Medicine 2015;372(15):1419‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
STSBCGTF 2011
- Society of Thoracic Surgeons Blood Conservation Guideline Task Force (STSBCGTF), Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. The Annals of Thoracic Surgery 2011;91(3):944‐82. [DOI] [PubMed] [Google Scholar]
Toy 2012
- Toy P, Gajic O, Bacchetti P, Looney MR, Gropper MA, Hubmayr R, et al. Transfusion‐related acute lung injury: incidence and risk factors. Blood 2012;119(7):1757‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2010
- Wang JK, Klein HG. Red blood cell transfusion in the treatment and management of anaemia: the search for the elusive transfusion trigger. Vox Sanguinis 2010;98(1):2‐11. [DOI] [PubMed] [Google Scholar]
Whitaker 2011
- U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health. Report of the US Department of Health and Human Services. The 2009 national blood collection and utilization survey report. www.hhs.gov/ophs/bloodsafety/2007nbcus_survey.pdf (accessed 11 October 2016).
WHO 2011
- WHO. Blood safety: key global facts and figures in 2011. www.who.int/worldblooddonorday/media/who_blood_safety_factsheet_2011.pdf (accessed 11 October 2016); Vol. Fact Sheet 279:1‐9.
Wu 2001
- Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. New England Journal of Medicine 2001;345(17):1230‐6. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu WC, Schifftner TL, Henderson WG, Eaton CB, Poses RM, Uttley G, et al. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA 2007;297(22):2481‐8. [DOI] [PubMed] [Google Scholar]
Wu 2010
- Wu WC, Smith TS, Henderson WG, Eaton CB, Poses RM, Uttley G, et al. Operative blood loss, blood transfusion, and 30‐day mortality in older patients after major noncardiac surgery. Annals of Surgery 2010;252(1):11‐7. [DOI] [PubMed] [Google Scholar]
Zou 2009
- Zou S, Stramer SL, Notari EP, Kuhns MC, Krysztof D, Musavi F, et al. Current incidence and residual risk of hepatitis B infection among blood donors in the United States. Transfusion 2009;49(8):1609‐20. [DOI] [PubMed] [Google Scholar]
Zou 2010
- Zou S, Dorsey KA, Notari EP, Foster GA, Krysztof DE, Musavi F, et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 2010;50(7):1495‐504. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Carless 2010b
- Carless PA, Henry DA, Carson JL, Hebert PPC, McClelland B, Ker K. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD002042.pub2] [DOI] [PubMed] [Google Scholar]
Carson 2012b
- Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD002042.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 2000
- Hill S, Carless PA, Henry DA, Carson JL, Hebert PPC, Henderson KM, et al. Transfusion thresholds and otherstrategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]
Hill 2002
- Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]
Hill 2005
- Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DBL, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002042] [DOI] [PubMed] [Google Scholar]


