Abstract
Background
Previous studies have shown potential benefits of rapamycin or rapalogs for treating people with tuberous sclerosis complex. Although everolimus (a rapalog) is currently approved by the FDA (U.S. Food and Drug Administration) and the EMA (European Medicines Agency) for tuberous sclerosis complex‐associated renal angiomyolipoma and subependymal giant cell astrocytoma, applications for other manifestations of tuberous sclerosis complex have not yet been established. A systematic review is necessary to establish the clinical value of rapamycin or rapalogs for various manifestations in tuberous sclerosis complex.
Objectives
To determine the effectiveness of rapamycin or rapalogs in people with tuberous sclerosis complex for decreasing tumour size and other manifestations and to assess the safety of rapamycin or rapalogs in relation to their adverse effects.
Search methods
Relevant studies were identified by authors from the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, and clinicaltrials.gov. Relevant resources were also searched by the authors, such as conference proceedings and abstract books of conferences, from e.g. the Tuberous Sclerosis Complex International Research Conferences, other tuberous sclerosis complex‐related conferences and the Human Genome Meeting. We did not restrict the searches by language as long as English translations were available for non‐English reports.
Date of the last searches: 14 March 2016.
Selection criteria
Randomized or quasi‐randomized studies of rapamycin or rapalogs in people with tuberous sclerosis complex.
Data collection and analysis
Data were independently extracted by two authors using standard acquisition forms. The data collection was verified by one author. The risk of bias of each study was independently assessed by two authors and verified by one author.
Main results
Three placebo‐controlled studies with a total of 263 participants (age range 0.8 to 61 years old, 122 males and 141 females, with variable lengths of study duration) were included in the review. We found high‐quality evidence except for response to skin lesions which was judged to be low quality due to the risk of attrition bias. Overall, there are 175 participants in the treatment arm (rapamycin or everolimus) and 88 in the placebo arm. Participants all had tuberous sclerosis complex as proven by consensus diagnostic criteria as a minimum. The quality in the description of the study methods was mixed, although we assessed most domains as having a low risk of bias. Blinding of treatment arms was successfully carried out in all of the studies. However, two studies did not report allocation concealment. Two of the included studies were funded by Novartis Pharmaceuticals.
Two studies (235 participants) used oral (systemic) administration of everolimus (rapalog). These studies reported response to tumour size in terms of the number of individuals with a reduction in the total volume of tumours to 50% or more relative to baseline. Significantly more participants in the treatment arm (two studies, 162 participants, high quality evidence) achieved a 50% reduction in renal angiomyolipoma size, risk ratio 24.69 (95% confidence interval 3.51 to 173.41) (P = 0.001). For the sub‐ependymal giant cell astrocytoma, our analysis of one study (117 participants, high quality evidence) showed significantly more participants in the treatment arm achieved a 50% reduction in tumour size, risk ratio 27.85 (95% confidence interval 1.74 to 444.82) (P = 0.02). The proportion of participants who showed a skin response from the two included studies analysed was significantly increased in the treatment arms, risk ratio 5.78 (95% confidence interval 2.30 to 14.52) (P = 0.0002) (two studies, 224 participants, high quality evidence). In one study (117 participants), the median change of seizure frequency was ‐2.9 in 24 hours (95% confidence interval ‐4.0 to ‐1.0) in the treatment group versus ‐4.1 in 24 hour (95% confidence interval ‐10.9 to 5.8) in the placebo group. In one study, one out of 79 participants in the treatment group versus three of 39 in placebo group had increased blood creatinine levels, while the median percentage change of forced expiratory volume at one second in the treatment arm was ‐1% compared to ‐4% in the placebo arm. In one study (117 participants, high quality evidence), we found that those participants who received treatment had a similar risk of experiencing adverse events compared to those who did not, risk ratio 1.07 (95% confidence interval 0.96 ‐ 1.20) (P = 0.24). However, as seen from two studies (235 participants, high quality evidence), the treatment itself led to significantly more adverse events resulting in withdrawal, interruption of treatment, or reduction in dose level, risk ratio 3.14 (95% confidence interval 1.82 to 5.42) (P < 0.0001).
One study (28 participants) used topical (skin) administration of rapamycin. This study reported response to skin lesions in terms of participants' perception towards their skin appearance following the treatment. There was a tendency of an improvement in the participants' perception of their skin appearance, although not significant, risk ratio 1.81 (95% confidence interval 0.80 to 4.06, low quality evidence) (P = 0.15). This study reported that there were no serious adverse events related to the study product and there was no detectable systemic absorption of the rapamycin during the study period.
Authors' conclusions
We found evidence that oral everolimus significantly increased the proportion of people who achieved a 50% reduction in the size of sub‐ependymal giant cell astrocytoma and renal angiomyolipoma. Although we were unable to ascertain the relationship between the reported adverse events and the treatment, participants who received treatment had a similar risk of experiencing adverse events as compared to those who did not receive treatment. Nevertheless, the treatment itself significantly increased the risk of having dose reduction, interruption or withdrawal. This supports ongoing clinical applications of oral everolimus for renal angiomyolipoma and subependymal giant cell astrocytoma. Although oral everolimus showed beneficial effect on skin lesions, topical rapamycin only showed a non‐significant tendency of improvement. Efficacy on skin lesions should be further established in future research. The beneficial effects of rapamycin or rapalogs on tuberous sclerosis complex should be further studied on other manifestations of the condition.
Plain language summary
Drugs that aim to relieve clinical symptoms of tuberous sclerosis complex
Review question
We reviewed the evidence about the effect of rapamycin or rapalogs for reducing the severity of clinical symptoms in people with tuberous sclerosis complex.
Background
Tuberous sclerosis or tuberous sclerosis complex is a genetic disease caused by mutations in tuberous sclerosis complex 1 or tuberous sclerosis complex 2 genes that affects several organs such as the brain, kidneys, heart, lungs and skin. The incidence has been reported to be one in approximately 6000. Previous studies have shown potential benefits of rapamycin or rapalogs for treating people with tuberous sclerosis complex. Although everolimus (a rapalog) is currently approved by the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) for tumours associated with tuberous sclerosis complex (renal angiomyolipoma and subependymal giant cell astrocytoma), the use of these drugs for treating other symptoms of the condition has yet to be established. This review aims to bring together clinical trials in this area to establish the clinical value of rapamycin and rapalogs for various symptoms of tuberous sclerosis complex.
Search date
The evidence is current to: 14 March 2016.
Study characteristics
The review included three studies with 263 people with tuberous sclerosis complex aged between 0.8 and 61 years of age. However, one study involved five people with sporadic lymphangioleiomyomatosis (without tuberous sclerosis complex) which we could not remove from the analysis. Studies compared rapamycin or rapalogs with placebo and people were selected for one treatment or the other randomly. The duration of the studies was variable. Two of the included studies were funded by Novartis Pharmaceuticals.
Key results
There is evidence that oral everolimus (rapalog) increased the number of people who achieved a 50% reduction in the size of subependymal giant cell astrocytoma and renal angiomyolipoma. Oral everolimus also showed benefit in terms of response to skin lesions, although applying rapamycin to the skin only showed a tendency for improvement. Those who received treatment had a similar risk of experiencing adverse events as compared to those who did not receive treatment. However, more people receiving the active treatment had severe adverse events causing them to withdraw from the trial, temporarily stop treatment or reduce their dose compared to the control group.
Quality of the evidence
Two of the included studies generally showed a low risk of bias in study design, except for one study where it was unclear whether people knew which group they would be put into. Another included study showed different degrees of risk of bias with regards to study design, for example, due to missing data and lack of clarity about how people where put into the different groups. The results from the studies were generally of high quality, except for response to skin lesions from topical rapamycin due to missing outcome data and seizure frequency due to the way participants were selected.
Summary of findings
Background
Description of the condition
Tuberous sclerosis or tuberous sclerosis complex (TSC) (OMIM#191100; OMIM#613254) is a genetic disease that affects several organs such as the brain, kidneys, heart, lungs and skin (NINDS 2013). The primary manifestation is as a consequence of growth of non‐malignant tumours in the various systems described. The incidence has been reported to be one in approximately 6000 (Osborne 1991). However, its true incidence is not known because of a number of undiagnosed cases consisting mostly of mildly affected or asymptomatic individuals (Osborne 1991).
Two disease‐causing genes have been identified by positional cloning, TSC1 (van Slegtenhorst 1997) and TSC2 (ECTSC 1993). The TSC1 gene is located on chromosome 9q34, and encodes the protein, hamartin (130 kDa, 1164 amino acids) (van Slegtenhorst 1997). The TSC2 gene is located on chromosome 16p13.3, and encodes another protein, tuberin (180 kDa, 1807 amino acids) (ECTSC 1993). However, 10% to 25% of people with TSC showed no TSC1/TSC2 mutation as identified by conventional genetic testing (Northrup 2013). A recent report of 53 people with TSC with no mutation identified, reported that mosaicism was observed in the majority (58%) and then followed by intronic mutations, which were seen in 40% of the study population. Two mutations were even identified in skin tumour biopsies only, and were not seen at appreciable frequency in blood or saliva DNA (Tyburczy 2015). These genetic abnormalities are generally inherited in an autosomal dominant manner. Nevertheless, in more than 60% of cases sporadic mutations are known to occur (van Slegtenhorst 1999).
The defective production of hamartin is caused by TSC1 mutations (NINDS 2013). Mutations on the TSC2 gene lead to the defective production of another protein, tuberin and are usually related to more severe manifestations (Dabora 2001). Both TSC1 and TSC2 are tumour suppressor genes, the defect of which will lead to an uncontrolled proliferation of benign tumours called tubers in various sites (Gao 2001). People with TSC present at different ages with a variety of clinical manifestations. Common presenting symptoms include skin lesions, seizures, learning disabilities or manifestations of tumours affecting organs such as the brain, heart, eyes, or the kidneys (Napolioni 2008). The disease can be diagnosed clinically by assessing individuals against major or minor criteria, depending on the signs and symptoms present (Northrup 2013).
Investigation of somatic mutations in a variety of TSC hamartomas supports classification of the TSC1 and TSC2 as tumour suppressor genes (Cheadle 2000). Interaction between hamartin and tuberin has a stoichiometry of 1:1 and is stable; a tight binding interaction between tuberin and hamartin forming a tumour suppressor heterodimer has been elucidated (Kwiatkowski 2003). This complex inhibits the mammalian target of rapamycin (mTOR) which is a key regulator in the signalling pathway of cell proliferation and organ size (Kwiatkowski 2003). It has been reported that hamartin‐tuberin complex regulates mTOR via hydrolysis of Rheb‐GTP into its inactive GDP bound state, Rheb‐GDP (Rosner 2004; Tee 2003).
Diagnostic criteria for TSC was recently updated (Northrup 2013) from its 1998 consensus (Roach 1998). The disease can now be diagnosed either genetically or clinically (Northrup 2013). Genetic diagnostic criteria include the identification of either a TSC1 or TSC2 pathogenic mutation in DNA from normal tissue. A pathogenic mutation is defined as a mutation that clearly inactivates the function of the TSC1 or TSC2 proteins (e.g., out‐of‐frame indel or nonsense mutation), prevents protein synthesis (e.g., large genomic deletion), or is a missense mutation whose effect on protein function has been established by functional assessment (Hoogeveen‐Westerveld 2012; Hoogeveen‐Westerveld 2013; LOVD TSC1; LOVD TSC2). Clinical diagnostic criteria remain with the major and minor clinical features that enable clinicians to clinically diagnose TSC (Northrup 2013). However, as compared to the older criteria (Roach 1998), less minor criteria were noted and 'cortical tubers' in major criteria was replaced by a broader feature 'cortical dysplasia' which includes tubers and cerebral white matter radial migration lines (Northrup 2013).
The new consensus for TSC clinical diagnostic criteria defines only possible and definitive diagnosis (Northrup 2013) without a probable diagnosis as defined in the old consensus (Roach 1998). A definitive diagnosis can be made when there are either two major features or one major feature with two or more minor features. A possible diagnosis can be made when there are either one major feature or two or more minor features (Northrup 2013). Major features in the new consensus include hypomelanotic macules (three or more, at least five mm diameter), angiofibromas (three or more) or fibrous cephalic plaque, ungual fibromas (two or more), shagreen patch, multiple retinal hamartomas, cortical dysplasias, subependymal nodules, subependymal giant cell astrocytoma, cardiac rhabdomyoma, lymphangioleiomyomatosis (LAM) and angiomyolipomas (two or more). It is of note, however, finding on the combination of LAM and angiomyolipomas still require another feature for a definitive diagnosis (Northrup 2013). Minor features include 'confetti' skin lesions, dental enamel pits (more than three), intraoral fibromas (two or more), retinal achromic patch, multiple renal cysts and nonrenal hamartomas (Northrup 2013).
Tumours of major clinical attentions include those involving the heart, brain, kidney and lung. Generally, tumour manifestations of TSC occur later in life, except for cardiac rhabdomyomas. Studies estimated that up to 70% to 90% of children with rhabdomyomas have TSC, and at least 50% of children with TSC have rhabdomyomas. Nearly 100% of foetuses with multiple rhabdomyomas have TSC, underscoring the practical importance of identifying additional tumours at the time of fetal assessment for diagnosis and prognosis (Hinton 2014). A recent report of a rhabdomyomas case series showed 31 out of 33 rhabdomyoma cases were diagnosed with TSC (Sciacca 2014). The case series reported 205 masses, mostly localized in interventricular septum, right ventricle and left ventricle. During follow up, a reduction of rhabdomyomas in terms of both the number and size was observed in all but one child (Sciacca 2014).
There are three types of brain lesions in TSC ‐ cortical dysplasia (cortical tubers and cerebral white matter radial migration lines), subependymal nodules (SEN; formed in the walls of the ventricles) and subependymal giant cell astrocytoma (SEGA; develops from SEN and grow such that they may block the flow of fluid within the brain, causing a buildup of fluid and pressure and leading to headaches and blurred vision) (NINDS 2013). Cortical tubers and cerebral white matter radial migration lines are commonly associated with intractable epilepsy and learning difficulties in TSC (Northrup 2013). The majority of people with TSC have SEN that remain static throughout their lifetime. However, approximately 20% of people with TSC demonstrate progressive growth of SEN to become SEGA, which may produce neurologic complications, including obstructive hydrocephalus (Crino 2010; Franz 2010).
Renal problems in TSC, including angiomyolipomas (which occur in 80% of people with TSC) and multiple renal cysts, comprise the second leading cause of premature death after severe intellectual disability (Shepherd 1991). Angiomyolipomas are benign tumours composed of vascular, smooth muscle, and adipose tissue. In the kidney, angiomyolipomas can cause serious issues with bleeding because of their vascular nature and can lead to the need for dialysis and even renal transplantation (Kozlowska 2008). Multiple renal cysts can be seen in people with TSC who have a TSC1 or TSC2 mutation or as part of a contiguous gene deletion syndrome involving the TSC2 and PKD1 genes (Bissler 2010).
In people with TSC, LAM is associated with interstitial expansion of the lung with benign‐appearing smooth muscle cells that infiltrate all lung structures (Johnson 2010; McCormack 2010). Matrix metalloproteinases (MMPs) activity was believed to facilitate tumour cell invasion by disrupting basement membranes (Kleiner 1999). Levels of urinary MMPs were elevated in people with LAM, and MMPs were found in LAM lung nodules (Glasgow 2010). Studies suggest a role for metalloproteinases in the cystic lung destruction of LAM (Glasgow 2010). Individuals typically present with progressive dyspnoea on exertion and recurrent pneumothoraces in the third to fourth decade of life. Cystic pulmonary parenchymal changes consistent with LAM are observed in 30% to 40% of females with TSC, but recent studies suggest that lung involvement may increase with age such that up to 80% of TSC females are affected by the age of 40 years (Cudzilo 2013). Cystic changes consistent with LAM are also observed in about 10% to 12% of males with TSC, but symptomatic LAM in males is very rare (Adriaensen 2011; Muzykewicz 2012).
Long‐term morbidity that requires life‐long institutional care is also of concern in TSC. Best estimates from epidemiological populations suggest that about 45% of individuals with TSC have intellectual disability (Joinson 2003). Rates of self‐injury and aggression in children with TSC were 27% and 50%, respectively. These are high but not significantly different from rates in children with Down syndrome or other syndrome groups (Eden 2014). In addition, 30 out of 45 women who were diagnosed with TSC as adults, actually met the clinical criteria for TSC in childhood. Although these women had minimal morbidity during childhood, they were at risk of life‐threatening pulmonary and renal manifestations (Seibert 2011).
Once the diagnosis of TSC is established and initial diagnostic evaluations completed, continued surveillance is necessary to monitor the progression of known problems or lesions and the emergence of new ones. Although some manifestations that appear during childhood do not cause significant problems in adulthood and vice versa, some others may be present throughout the entire life‐span of the individual, such as epilepsy and TSC‐associated neuropsychiatric disorders (TAND) (Krueger 2013).
Description of the intervention
Rapamycin (sirolimus) and rapalogs (analogs of rapamycin) are first generation inhibitors of mTOR (mammalian target of rapamycin). This is a protein kinase that controls cell growth, proliferation, and survival. Often mTOR signaling is up‐regulated in cancer and there is a great interest in developing drugs that target this enzyme. Until recently, rapamycin sensitivity was the major criterion used to identify mTOR‐controlled effects (Ballou 2008).
During the 1980s, rapamycin (sirolimus) was discovered to show an anti‐cancer activity (Faivre 2006). However, due to its unfavourable pharmacokinetic properties, the development of rapamycin for the treatment of cancer was not successful at that time (Yuan 2009). Later on, analogs of rapamycin (rapalogs) with more favourable pharmakokinetic properties and reduced immunosuppressive effects were discovered (Faivre 2006). These include temsirolimus (CCI‐779), everolimus (RAD001), biolimus A9 and zotarolimus (ABT‐578) (Falotico 2005, Brachmann 2009). Both biolimus and zotarolimus are drug‐eluting coronary stents for preventing coronary artery restenosis.
Rapamycin (C51H79NO13) is a macrolide compound that was isolated in 1975 from Streptomyces hygroscopicus found in a soil sample on Easter Island. It prevents activation of T cells and B cells by inhibiting their response to interleukin‐2 (IL‐2). It is an FDA‐approved drug for immunosuppression after organ transplantation. Rapamycin also possesses both antifungal and antineoplastic properties. Rapamycin is administered orally once daily, with or without food as a tablet or a solution with a maximum daily dose of 40 mg (RxList 2015a). The most common adverse reactions (at least 30%) observed with rapamycin in clinical studies are: peripheral oedema; hypertriglyceridaemia; hypertension; hypercholesterolaemia; creatinine increase; constipation; abdominal pain; diarrhoea; headache; fever; urinary tract infection; anaemia; nausea; arthralgia; pain; and thrombocytopaenia (RxList 2015a).
Topical rapamycin has been used for facial angiofibromas in people with TSC (Haemel 2010; Mutizwa 2011). There are various preparations for topical rapamycin that have been reported with concentrations ranging from 0.1% to 1% (Madke 2013). An irritation and burning sensation is the most common side effect seen after topical administration of rapamycin. Individuals should be prescribed topical hydrocortisone 0.1% cream or desonide 0.05% lotion along with liberal emollients to counteract any irritation and ensure compliance. It is practical to use commercially available oral solution of rapamycin (1 mg/ml) as a topical formulation since compounding pharmacies are not always readily accessible and the stability and efficacy of compounded preparation cannot be ensured (Madke 2013).
Temsirolimus (C56H87NO16) has a molecular weight of 1030.30 and is non‐hygroscopic. It is insoluble in water and soluble in alcohol. It has no ionizable functional groups, and its solubility is independent of pH. In in vitro studies using renal cell carcinoma cell lines, temsirolimus inhibited the activity of mTOR and resulted in reduced levels of the hypoxia‐inducible factors HIF‐1 and HIF‐2 alpha, and the vascular endothelial growth factor. It has been indicated for the treatment of advanced renal cell carcinoma with a recommended dose of 25 mg infusion over a 30 to 60 minute period once a week. The most common adverse reactions (at least 30%) are rash, asthenia, mucositis, nausea, oedema, and anorexia. The most common laboratory abnormalities (at least 30%) are anaemia, hyperglycaemia, hyperlipaemia, hypertriglyceridaemia, lymphopenia, elevated alkaline phosphatase, elevated serum creatinine, hypophosphataemia, thrombocytopenia, elevated aspartate aminotransferase (AST), and leukopenia. Temsirolimus is contraindicated in individuals with bilirubin more than 1.5 times of upper limit of normal range. (RxList 2015b).
Everolimus (C53H83NO14) has a molecular weight of 958.2. It has been indicated for the treatment of post‐menopausal women with advanced hormone receptor‐positive, HER2‐negative breast cancer, adults with progressive neuroendocrine tumours of pancreatic origin (PNET), adults with advanced renal cell carcinoma (RCC), adults with renal angiomyolipoma, SEGA and TSC. However, further follow up of people with TSC is still required to determine long‐term outcomes. The recommended dose of everolimus tablets is 10 mg, to be taken once daily at the same time every day. The most common adverse reactions (incidence at least 30%) were stomatitis, infections, rash, fatigue, diarrhoea, and decreased appetite. The most common laboratory abnormalities (incidence at least 50%) were hypercholesterolaemia, hyperglycaemia, increased AST, anaemia, leukopenia, thrombocytopenia, lymphopenia, increased alanine transaminase (ALT), and hypertriglyceridaemia (RxList 2016).
While non‐randomised trials showed that rapamycin or rapalogs reduce the size of TSC‐associated tumours in humans, such as angiomyolipoma, LAM and SEGA, tumour regression does not occur in all cases and tumour regrowth is generally observed with the cessation of treatment (Bissler 2008; Cardamone 2014; Davies 2008; Franz 2006). Furthermore, evidence on the effect of rapamycin (Canpolat 2014, Cardamone 2014) or rapalogs (Muncy 2009, Krueger 2013) in improving epilepsy is not without opposition (Overwater 2014; Wiemer‐Kruel 2014). Rapamycin or rapalogs have also been tested on other manifestations of TSC with mostly promising results, albeit with a variable degree of evidence. Reports described improvement in facial angiofibroma (Foster 2012; Truchuelo 2012; Wataya‐Kaneda 2012; Wheless 2013), cardiac rhabdomyoma (Tiberio 2011), renal cell carcinoma (Pressey 2010) but not the optic nerve tumour (Sparagana 2010) among people with TSC. An open study of 10 people with TSC‐associated facial angiofibroma reported sustained improvement in erythema and in the size and extension of the lesions. Rapamycin plasma levels remained below detection limits (0.3 ng/mL) in all cases. The formula was well‐tolerated with no local or systemic adverse effects (Salido 2012). Although the response results in early human trials are encouraging, conflicting evidence is present and it is possible that a longer term use of rapamycin may be more effective. Identification of other active drugs is also of interest to improve the response rate or durability of response, or both (Lee 2009).
Rapamycin or rapalogs are extensively metabolized by O‐demethylation or hydroxylation (or both) in the intestinal wall and liver and undergo counter‐transport from enterocytes of the small intestine into the gut lumen. Seven major metabolites, including hydroxy, demethyl, and hydroxydemethyl, are identifiable in whole blood. Some of these metabolites are also detectable in plasma, faecal, and urine samples (RxList 2015a; RxList 2015b; RxList 2016).
In the human and rat liver, rapamycin or rapalogs is metabolized primarily by cytochrome P‐450 3A4 (Sattler 1992). Rapamycin or rapalogs are substrates for both CYP3A4 and P‐gp (P‐glycoprotein 1). Inducers of CYP3A4 and P‐gp may decrease rapamycin or rapalogs concentrations whereas inhibitors of CYP3A4 and P‐gp may increase their concentrations. Drugs and agents that could increase the concentration of rapamycin or rapalogs in the blood include cyclosporine, bromocriptine, cimetidine, cisapride, clotrimazole, danazol, diltiazem, fluconazole, protease inhibitors (e.g., for HIV and hepatitis C that include drugs such as ritonavir, indinavir, boceprevir, and telaprevir), metoclopramide, nicardipine, troleandomycin, verapamil, grapefruit (RxList 2015a), Seville oranges, limes and pomelos (Tanzi 2013). Drugs and agents that could decrease rapamycin or rapalogs concentrations include carbamazepine, phenobarbital, phenytoin, rifapentine, St. John's Wort (Hypericum perforatum).
In addition, verapamil concentration could increase when given with rapamycin or rapalogs. Immunosuppressants may affect response to vaccination. Therefore, during treatment with rapamycin or rapalogs, vaccination may be less effective. The use of live vaccines should be avoided, including, but not limited to, measles, mumps, rubella, oral polio, bacillus Calmette–Guérin (BCG), yellow fever, varicella, and TY21a typhoid (RxList 2015a).
How the intervention might work
As uncontrolled proliferation of benign tumours in various organs is part of the pathophysiology, due to the loss of tumour suppressor genes, inhibitors of such processes is a logical step in tumour suppression. Cell growth and proliferation function is largely regulated in its final pathway, by a set of processes involving a common regulatory mTOR protein. This protein regulates vital cell growth processes, receives external signals from growth factors, hormones, and proteins. It then gives the 'on' or 'off' signals for the cell to grow and divide. In normal circumstances, mTOR combines with several other cellular components to form two distinct complexes, termed mTORC1 and mTORC2 (Bhaskar 2007).
Inhibiting mTOR kinase was thought to be a useful approach to systemic therapy for TSC or LAM (or both) because rapamycin has been shown to normalize dysregulated mTOR signalling in cells that lack normal hamartin or tuberin (Gao 2001; Goncharova 2002; Inoki 2002; Kwiatkowski 2002; Manning 2002; Potter 2002).
A detailed look into the mode of action of rapamycin discovered that it binds to the cytosolic protein FK‐binding protein 12 (FKBP12). The sirolimus‐FKBP12 complex inhibits the mTOR from forming the mTORC1, thus inhibiting cell proliferation. A previous study utilising cohorts of Tsc2+/‐ mice and mouse model of Tsc2‐null tumours showed that treatment with an mTOR (mammalian target of rapamycin) kinase inhibitor (CCI‐779, a rapamycin analogue) reduced the severity of TSC‐related disease without significant toxicity (Lee 2005).
The mechanisms by which rapamycin or rapalogs inhibits mTOR are not fully understood, but rapamycin associates with FKBP12 to bind to the FRB (FKBP12–rapamycin‐binding) domain of mTOR. Binding of the rapamycin–FKBP12 complex to mTOR can destabilize the mTORC1 complex and interfere with the activation of mTOR by phosphatidic acid. Several new compounds are available to inhibit mTOR, either by interfering with complex formation (FKBP12‐dependent or FKBP12‐independent) or by directly inhibiting the catalytic domain of mTOR (Ehninger 2011).
Why it is important to do this review
There is currently no established therapy for TSC, despite the life‐threatening morbidities of the disorder. Previous studies have shown potential clinical applications of rapamycin for TSC. Although everolimus (a rapalog) is currently FDA‐ and EMA‐approved for TSC‐associated renal angiomyolipoma and SEGA, applications for other manifestations of TSC are yet to be established. This review aims to bring together clinical trials in this area to establish the clinical value of rapamycin or rapalogs for various manifestations in TSC.
Objectives
To determine the effectiveness of rapamycin or rapalogs administration in people with TSC for decreasing the size of TSC‐related tumours and other manifestations and to assess the safety of rapamycin or rapalogs relating to their adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised studies. Studies in which quasi‐randomised methods, such as alternation, are used were included when there is sufficient evidence that the treatment and control groups are similar at baseline.
Types of participants
People with known TSC as proven by the clinical features designated in the revised consensus on TSC diagnostic criteria (genetic or clinical (or both) manifestations) (Northrup 2013). Individuals diagnosed using older diagnostic criteria (Hyman 2000; Roach 1998; Roach 1999) would be included if we could not ascertain compliance with the latest diagnostic criteria through communications with trial authors.
Types of interventions
Rapamycin or rapalogs designed to reduce any TSC‐associated symptoms in people with TSC compared to placebo or any standard treatments, applied systemically or topically.
Types of outcome measures
Primary outcomes
Tumour size (any unit of analysis found)
Secondary outcomes
Skin lesion response
Aneurysm size for angiomyolipomas (any unit of analysis found)
Frequency of seizure (times)*
Forced expiratory volume at one second (FEV1) / forced vital capacity (FVC) ratio
Creatinine level (mg/dL)
Any reported adverse effect or toxicity
* Please refer to 'Differences between protocol and review' for information about this post hoc addition to outcomes.
Search methods for identification of studies
We did not restrict the searches by language as long as an English translation was available for non‐English reports.
Electronic searches
All electronic searches were carried out on 14 March 2016. Relevant studies were identified by authors from the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2012 to Issue 2, 2016) via the Central register of Studies Online (crso.cochrane.org), Ovid MEDLINE (published 1946 to 14 March 2016), and clinicaltrials.gov (www.clinicaltrials.gov). The search strategies used and filtering options were as outlined in the appendices (Appendix 1; Appendix 2; Appendix 3). The Ovid MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011).
Searching other resources
The authors also searched relevant resources, such as conference proceedings and books of conference abstracts (where accessible). This included the TSC International Research Conferences (2009 to 2015), 2012 International TSC Congress, 2014 World TSC Conference and the Human Genome Meeting (2010 to 2016). The authors will continue to search resources from similar conferences for future updates of this review. Investigators will be contacted whenever we identify eligible studies from these sources and if more detailed information is needed.
Data collection and analysis
Selection of studies
In order to select studies for inclusion in the review, the inclusion criteria were applied by two authors independently. The authors reached consensus by discussion between all four authors for any disagreements which arose on the suitability of a study for inclusion in the review.
Data extraction and management
Data were extracted by NFDI using the standard acquisition forms and then verified by one author (THS). The authors reached consensus by discussion upon any disagreements arise on the data extraction. For missing information as specified in other parts of this manuscript, the authors will contact study investigators for future update of this review. The eligible time period for endpoint analysis is at least one month after rapamycin administration. We excluded studies from analysis when the only time period reported was less than one month. While we planned to analyse data in three blocks of time (one to three months; over three months to six months; and longer than six months), none of the included studies reported enough information for such analyses. We aim, if possible, to undertake this for an update of this review, pending communication with study authors.
Assessment of risk of bias in included studies
The risk of bias of each study was assessed by NFDI and then verified by one author (ZAMHZH). The authors generated a risk of bias table for each study as described in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). In particular, details of the following components were examined:
sequence generation (e.g. whether randomization was adequate);
allocation concealment (e.g. whether the allocation was adequately concealed);
blinding of participants, personnel and outcome assessors (e.g. whether the the participants, personnel and outcome assessors were blinded);
incomplete outcome data (e.g. whether attrition and exclusion were reported);
selective outcome reporting (e.g. whether the study was free from selective outcome reporting);
other sources of bias (will be specified during the assessment).
All components were assessed by authors using the methods as described in the Cochrane Handbook for Systematic Reviews of Interventions. For each item, the table provided a description of what was reported in the study and the subjective judgement regarding possible bias (low, high or unclear risk of bias) (Higgins 2011).
Measures of treatment effect
We pooled the analysis of different types of the different drugs types and plan to perform subgroup analysis for rapamycin and rapalogs if in future versions of the review we identify more studies. We separated the analysis of different modes of applications into systemic or topical administration.
We could not obtain tumour size in the form of continuous data. Instead, authors obtained response of treatment to tumour size (Bissler 2013;Franz 2013) and skin lesions (Bissler 2013; Franz 2013; Koenig 2012) and we analysed data as dichotomous. The studies reported creatinine levels as the number of participants with increased creatinine levels in both treatment or placebo arms. We therefore analysed these data as dichotomous. Two studies reported lung functions (Bissler 2013) and frequency of seizures (Franz 2013) as only median change. None of the included studies reported aneurysm size of angiomyolipoma. All studies reported adverse effects (Bissler 2013; Franz 2013; Koenig 2012).
The authors reported dichotomous outcomes (number of participants having 50% reduction in tumour size and skin lesions, presence or absence of adverse effects and treatment response to creatinine level). We calculated the risk ratios (RR) and 95% confidence intervals (95% CIs) based on the ratio of an outcome among treatment‐allocated participants to that among controls. We calculated the pooled estimate of the treatment effect for each outcome across studies by determining the RR.
In future updates of this review, if continuous data of these outcomes become available, we will record them as either mean change from baseline for each group or mean post‐treatment values and standard deviation (SD) for each group. We will assess continuous outcomes by the calculation of the mean difference (MD) and 95% CIs. Where trials report multiple measures for the same outcome (e.g. absolute change FEV1 per cent predicted, percentage change in FEV1 per cent predicted, or per cent change of absolute FEV1 volumes) we will calculate the standardised MDs (SMD). We will consider absolute changes in FEV1 in the context of comparable data being available for each participant before and after the intervention so that a calculation of the effect size is possible.
Unit of analysis issues
We did not include any cross‐over studies in the review; in future updates, we will only include cross‐over studies if we consider there to be a sufficient washout period between the treatment arms. We will analyse any data from such studies using paired analyses as described by Elbourne (Elbourne 2002).
For cluster randomised studies, if we regard these as not being analysed correctly by study authors, we will calculate the effective sample size and monitor and analyse them based on the method described by Donner (Donner 2002). We plan to analyse any such studies separately. We aim to address the risk of unit of analysis error caused by repeated observations on participants and whether the individual or the tumours are randomised based on the information provided in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). If the individual is randomised, we will include all tumours observed in the studies.
Dealing with missing data
If any included studies were only reported as abstracts, presented at meetings, or reported to the review authors, we sought full reports from the study investigators. For missing information as specified in other parts of this manuscript, we plan to contact study investigators for future updates of this review. In order to allow an intention‐to‐treat (ITT) analysis, we grouped data by allocated treatment groups, irrespective of later exclusion (regardless of cause) or loss to follow up.
Assessment of heterogeneity
We tested for heterogeneity between studies using a standard Chi2 test and the I2 statistic (Higgins 2003) (Analysis 1.1; Analysis 1.2; Analysis 1.5).
1.1. Analysis.
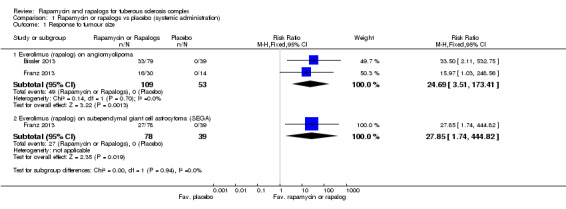
Comparison 1 Rapamycin or rapalogs vs placebo (systemic administration), Outcome 1 Response to tumour size.
1.2. Analysis.
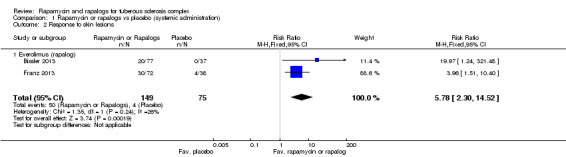
Comparison 1 Rapamycin or rapalogs vs placebo (systemic administration), Outcome 2 Response to skin lesions.
1.5. Analysis.
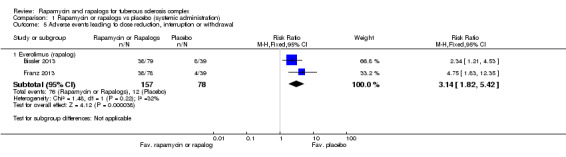
Comparison 1 Rapamycin or rapalogs vs placebo (systemic administration), Outcome 5 Adverse events leading to dose reduction, interruption or withdrawal.
The Chi2 test is a statistical test for heterogeneity, whereas I2 assesses the quantity of inconsistency across studies in the meta‐analysis. The authors used a cut‐off P value of 0.1 to determine significance. This is because of the generally anticipated low power of the reported studies due to the disease being rare. The authors used the following I2 ranges to interpret heterogeneity: 0% to 40%: might not be important; 30% to 60%: may represent moderate heterogeneity; 50% to 90%: may represent substantial heterogeneity; 75% to 100%: considerable heterogeneity.
The authors visually assessed the forest plot to see if CIs overlapped.
Assessment of reporting biases
For all studies, we compared the 'Methods' section of the full published paper with the 'Results' section to ensure that all measured outcomes were reported.
The review included fewer than 10 studies, so we did not construct any funnel plots for assessment, either visually or statistically (small‐study effects analyses). However, in future updates, provided that we include at least 10 studies, we will carry out a visual assessment of funnel plots and statistical tests for funnel plot asymmetry (small‐study effects analyses). If we identify any evidence of small‐study effects, we will attempt to understand the source, including the possibility of reporting bias.
Data synthesis
We employed a fixed‐effect analysis in this review. In future updates, if there is evidence of heterogeneity (I2 statistic more than 40%), we plan to use a random‐effects analysis.
We compared rapamycin or rapalogs versus placebo or other standard treatment. We determined the exact time‐points that we reported after analysing the included studies, but they were at least six months apart.
Subgroup analysis and investigation of heterogeneity
In future versions of this review, if we identify heterogeneity between the studies (I2 statistic more than 40%), we will examin subgroups, such as: age of participants (0 years to 10 years, over 10 years to 20 years, over 20 years) and the gene affected (TSC1 or TSC2).
In addition to this, where appropriate, we plan to perform subgroup analyses of different medications (rapamycin or rapalogs), different locations and types of tumour, and if rapamycin or rapalogs were administered in conjunction with other agent(s).
Sensitivity analysis
Authors plan to test the robustness of the results with the following sensitivity analyses:
studies where quasi‐randomisation methods are used;
studies where there are variations among one or more inclusion criteria;
studies of different designs (e.g. cross‐over studies).
In addition, we plan to undertake a sensitivity analysis to investigate the effects of combining endpoint analysis across all time‐periods.
Summary of findings table
Summary of findings tables were created with the following outcomes, depending on data available in the included studies:
50% reduction of tumour size;
response to skin lesions;
frequency of seizures (times);
creatinine level (mg/dL);
FEV1/FVC ratio; and
any reported adverse effect or toxicity.
Results
Description of studies
Results of the search
Please refer to the study flow diagram (Figure 1).
1.
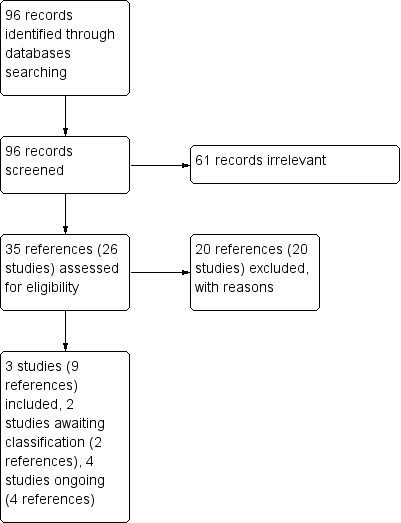
Study flow diagram.
The search identified 29 relevant studies, we excluded 20 studies; 14 of these are case series or case reports (Birca 2010; Foster 2012; Franz 2006; Herry 2007; Hofbauer 2008; Koenig 2008; Lam 2010; Pressey 2010; Salido 2012; Sparagana 2010; Staehler 2012; Wataya‐Kaneda 2011; Wienecke 2006; Yalon 2011); six are phase 2 clinical trials with no control arm or randomization (Cabrera 2011; Dabora 2011; Davies 2011; Krueger 2010; Tanaka 2013; Wataya‐Kaneda 2015). Two studies were awaiting classification in a future update of this review (NCT01289912; NCT01526356). Four studies are ongoing clinical trials (NCT01713946; NCT01730209; NCT01954693; NCT02635789). Three studies were eligible for inclusion in the current review (Bissler 2013; Franz 2013; Koenig 2012).
Included studies
Refer to Characteristics of included studies.
Trial characteristics
All three studies were described as double‐blind, randomized, placebo‐controlled studies (Bissler 2013; Franz 2013; Koenig 2012). Additionally, the Bissler and the Franz studies were described as phase 3 studies (Bissler 2013; Franz 2013).
The Bissler study was multicentre (24 centres in 11 countries) as was the Franz study (24 centres in 10 countries) (Franz 2013). The Koenig study was carried out in a single centre in Texas, USA (Koenig 2012).
Participants
The Bissler study randomised 118 participants having at least one angiomyolipoma (3 cm or larger in diameter), and a definite diagnosis of TSC per consensus criteria. There were 79 participants in the treatment group and 39 in the placebo group. This study included adults (18 years or older). People were excluded if their angiomyolipoma required surgery at randomisation, or if they had angiomyolipoma‐related bleeding or embolisation during the six months before randomisation. Those with lymphangioleiomyomatosis were excluded if their carbon monoxide diffusion capacity (DLco) was 35% or less, oxygen saturation was below normal at rest, or oxygen saturation was 88% or less on a six‐minute walking test with up to 6 L/min of oxygen (Bissler 2013).
The Franz study randomised 117 participants having at least one angiomyolipoma (3 cm or larger in diameter), and a definite diagnosis of TSC per consensus criteria. There were 78 participants in the treatment group and 39 in the placebo group. This study included participants aged up to 65 years old. Those individuals who had previously used antiproliferative agents were excluded from the study (Franz 2013).
The Koenig study randomised 28 (13 females and 15 males) participants with a diagnosis of TSC. There were 18 participants in the treatment group and 10 in the placebo group. This study included people over 13 years of age. Participants were excluded if they were currently pregnant, were using oral rapamycin, or had any form of immune dysfunction (Koenig 2012).
Interventions
The Bissler study compared everolimus and placebo; details of the placebo were not provided. Everolimus was given for six months at a dose of 10 mg per day, with dose modifications allowed on the basis of safety findings (systemic administration). Concomitant use of strong inhibitors or inducers of cytochrome P450 3A4 or p‐glycoprotein (PgP) was to be avoided during the study and participants were prohibited from using antiproliferative agents other than the study drug (Bissler 2013).
The Franz study compared everolimus and placebo; details of the placebo were not provided. Everolimus was administered orally at a starting dose of 4.5 mg/m² body surface area per day and subsequently adjusted to attain blood trough concentrations of 5 to 15 ng/mL with dose modifications allowed for the purpose of treatment‐related toxic effects (systemic administration). The starting dose for children with malignancies was chosen to be just less than the maximum‐tolerated dose (5 mg/m² per day). Participants were prohibited from using strong and moderate inhibitors of cytochrome P450 3A4 (CYP3A4) and P‐glycoprotein (except antiepileptic drugs), strong inducers of CYP3A4 (except antiepileptic drugs), and concomitant use of antiproliferative drugs. The participants were treated with everolimus for six months with an extension phase (upto four years after the last participant was randomly assigned to treatment) if the results of the core phase favoured everolimus (Franz 2013).
The Koenig study compared rapamycin and placebo; details of the placebo were not provided. Over a period of six months, rapamycin was given at a dose of either 1 mg per 30 cc (0.003%) or 5 mg per 30 cc (0.015%). A thin coating of rapamycin was applied on the skin directly over the treatment area every morning (topical administration). The product was allowed to air dry for 60 minutes following application and was removed by gentle washing in the morning (Koenig 2012).
Outcomes
The Bissler study reported the effect of everolimus on response rate of tumour (angiomyolipoma and skin lesion), tumour volume and time for angiomyolipoma progression. Adverse events were also reported (Bissler 2013).
The Franz study reported the effect of everolimus on tumour volume (subependymal giant cell astrocytomas), rate of tumour response, seizure frequency, time for tumour progression, skin lesions response and angiomyolipoma response. Adverse events were also reported (Franz 2013). se studies reported tumour response to the intervention in terms of the number of participants having a 50% or more reduction of volume of tumours relative to baseline (Bissler 2013; Franz 2013).
The Koenig study reported the effect of rapamycin on skin lesion appearance. Rapamycin concentrations and complete blood counts (including haemoglobin level and platelet count) were measured. Adverse events were also reported (Koenig 2012). Effects of intervention were measured as the participants' perception on their skin appearance. The participants were asked if they "got better on the treatment", "got worse on the treatment", or if "the treatment made no difference".
Excluded studies
Please refer to the Characteristics of excluded studies table.
A total of 20 studies were excluded from this review. Of these, six studies were non‐randomized without any control group and recruited participants from multiple centres based on TSC diagnostic criteria (Cabrera 2011; Dabora 2011,Davies 2011; Tanaka 2013; Wataya‐Kaneda 2015). A further 14 studies were case series and case reports (Birca 2010; Foster 2012; Franz 2006; Herry 2007; Hofbauer 2008; Koenig 2008; Lam 2010; Pressey 2010; Salido 2012; Sparagana 2010; Staehler 2012; Wataya‐Kaneda 2011; Wienecke 2006; Yalon 2011).
Risk of bias in included studies
Please refer to Figure 2 and Figure 3.
2.
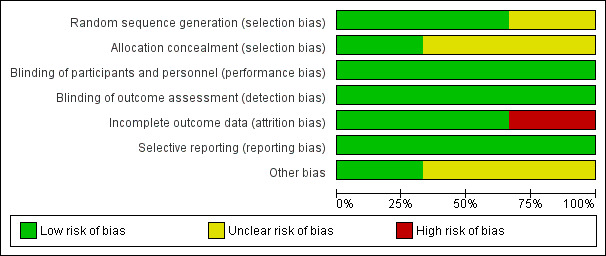
Risk of bias graph illustrating percentages of each risk of bias item for all three included studies (Bissler 2013, Koenig 2008 and Franz 2006).
3.
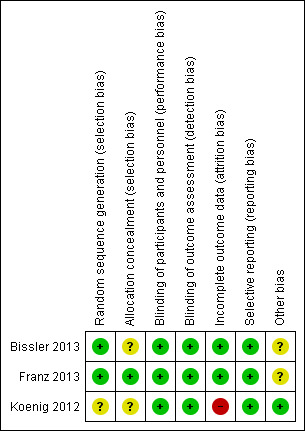
Risk of bias graph summarizing review authors' judgements about each risk of bias item for each included studies (Bissler 2013, Franz 2013, Koenig 2012).
Allocation
Sequence generation
Two studies described the use of a computer‐generated random sequence and were judged to have a low risk of bias (Franz 2013, Bissler 2013). The Koenig study was judged to have an risk of bias for allocation sequence as no process of generating allocation sequence was described (Koenig 2012).
Allocation concealment
The Franz study described the use of interactive Internet‐response system for allocation concealment and therefore, it was judged as having low risk for allocation concealment (Franz 2013). Allocation concealment was not described in the remaining two studies and was therefore graded as unclear (Bissler 2013; Koenig 2012).
Blinding
Participants and all study personnel (caregiver, investigator and outcomes assessor) were blinded in all three studies and so were assessed as having a low risk of bias (Bissler 2013; Franz 2013; Koenig 2012).
Incomplete outcome data
Two studies were judged to have a low risk of bias for incomplete outcome data (Bissler 2013; Franz 2013). In the Bissler study, efficacy analyses were undertaken on all randomised participants, and safety analyses were undertaken on all participants who received at least one dose of the study drug and had at least one post‐baseline assessment. Participants who were not able to be assessed (either by dropout or for other reasons) were considered as non‐responders (Bissler 2013). Therefore, the risk of bias for incomplete outcome data was low. In the Franz study, efficacy and safety analyses were carried out on all participants, therefore the study was assessed as having low risk of bias for incomplete outcome data (Franz 2013).
The Koenig study was assessed as having a high risk of bias for incomplete outcome data due to the fact that only 23 out of 28 participants were analysed upon completion of the intervention, no information pertaining to which arms the dropouts came from and outcomes were only reported as percentages of participants reporting improvements of their skin lesions on each arm (Koenig 2008).
Selective reporting
Overall, there is a low risk of bias for selective reporting for all studies (Bissler 2013; Franz 2013; Koenig 2012). For the Franz study, all outcomes recorded in the 'Methods' section were reported in 'Results' section (Franz 2013). The outcomes were response to tumour size, tumour progression, seizure frequency (median change from baseline to week 24), response of treatment to skin lesion and adverse effects (Franz 2013). For the Bissler study, all outcomes mentioned in the 'Methods' section were also reported in the 'Results' section (Bissler 2013). The outcomes included response of treatment to tumour size, tumour progression, response to skin lesion, FEV1, creatinine level, plasma VEGF‐D and collagen IV levels and adverse events (Bissler 2013). For the Koenig study, all outcomes mentioned in the 'Methods' section were also reported in the 'Results' section (Koenig 2012). They reported intervention effect on lesion appearance based on the participants' perception of their skin appearance, complete blood counts and adverse events (Koenig 2012).
Other potential sources of bias
For the Bissler and Franz studies it was noted that the authors who are employees, stock owners or consultants of the funder (Novartis) were involved in the study design, discussion, research, overseeing of data collection and data analysis and interpretation, we assessed this as having an unclear risk of bias.
Effects of interventions
Summary of findings for the main comparison. Summary of findings for systemic administration.
| Oral everolimus (rapalog) compared with placebo for people with TSC | |||||||
|
Patient or population: people with TSC Settings: outpatient Intervention: oral everolimus (rapalog) Comparison: placebo | |||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Assumed risk | Corresponding risk | ||||||
| Placebo | Oral Everolimus (Rapalog) | ||||||
| 50% reduction of tumour size1 | Renal angiomyolipoma | 0 out of 1000 (see comment) |
450 out of 1000 (see comment) |
RR: 24.7 (95% CI 3.5 ‐ 173.4) | 162 (2 studies) |
⊕⊕⊕⊕ high3 | P = 0.001 0 out of 53 participants in the placebo group and 49 out of 109 participants in the Rapalog group experienced 50% reduction in tumour size. Assumed and corresponding risk are calculated as the event rates in each group. |
| SEGA | 0 out of 1000 (see comment) |
346 out of 1000 (see comment) |
RR: 27.9 (95% CI 1.7 ‐ 444.8) |
117 (1 study) |
⊕⊕⊕⊕ high3 | P = 0.02 0 out of 39 participants in the placebo group and 27 out of 78 participants in the Rapalog group experienced 50% reduction in tumour size. Assumed and corresponding risk are calculated as the event rates in each group. |
|
| Response to skin lesions2 | 53 out of 1000 | 307 out of 1000 (122 to 769 out of 1000) | RR: 5.8 (95% CI 2.3 ‐ 14.5) | 224 (2 studies) |
⊕⊕⊕⊕ high3 | P = 0.0002 | |
| Frequency of seizure | Median change = ‐4.1/24 hr (95% CI ‐10.9 to 5.8) | Median change = ‐2.9/24 hr (95% CI ‐4.1 to ‐1.0) | ‐ | Not reported (1 study) |
⊕⊕⊕⊕ moderate4 | ‐ | |
| Creatinine level | Increased in 77 out of 1000 participants | Increased in 12 out of 1000 participants (2 to 118 participants) |
RR: 0.16 (95% CI 0.02 ‐ 1.53) | 118 (1 study) |
⊕⊕⊕⊕ high3 | ‐ | |
| Forced expiratory volume in 1 second (FEV1) | See comments | See comments | See comments | See comments | See comments | For the subset of participants with lymphangioleiomyomatosis and sporadic lymphangioleiomyomatosis the median percentage change of FEV1 in the treatment arm (in 24 out of 79 participants) was ‐1%, while in the placebo arm (10 out of 39 participants) this was ‐4%. | |
| Any adverse events | 897 out of 1000 | 960 out of 1000 (807 to 1000 out of 1000) |
RR: 1.07(0.9‐1.2) | 117 (1 study) |
⊕⊕⊕⊕ high3 | P = 0.24 | |
| Adverse Events leading to dose reduction, interruption or withdrawal | 154 out of 1000 | 484 out of 1000 (277 to 770 out of 1000) |
RR: 3.14(1.8‐5.4) | 235 (2 studies) |
⊕⊕⊕⊕ high3 | P < 0.0001 | |
| *The basis for the assumed risk was the event rate in the placebo group unless otherwise stated in the comments. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; TSC: tuberous sclerosis complex | |||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||||
This summary of finding table was generated based on analyses from two of the included studies (Bissler 2013, Franz 2013) which used oral (systemic) administration of everolimus (rapalog). Five of the participants from Bissler study were diagnosed with sporadic lymphangioleiomyomatosis (without TSC) which we could not separate from the current analysis (we will attempt to do this for a future update of the review) (Bissler 2013).
1. Response to tumour size is defined as reduction in at least 50% reduction from baseline in sum of volumes of target tumour in participant.
2. Definition of 'response' to skin lesions was not mentioned in the two study analysed (Bissler 2013; Franz 2013).
3. No downgrading of evidence: High quality of evidences were given even though there are large CIs. The number of participants is relatively large for a rare disease, thus judgement about the quality of evidence (particularly judgements about precision) may be based on the absolute effect. Here the quality rating was considered 'high' because the outcome was appropriately assessed.
4. Downgraded once due to lack of applicability: Most participants had no seizures at baseline or at follow up. Participants were selected for the trial on the basis of their need for intervention for progression of subependymal giant cell astrocytomas rather than presence of seizures (Franz 2013). Further randomised study designed specifically to select people with TSC with seizures may change the outcome.
Summary of findings 2. Summary of findings for topical administration.
| Topical rapamycin compared with placebo for people with TSC | ||||||
|
Patient or population: people with TSC Settings: outpatient Intervention: topical rapamycin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Topical Rapamycin | |||||
|
Response to skin lesions ‐ rapamycin (6 months) |
38% of participants1 | 73% of participants1 | RR: 1.81 (0.80 ‐ 4.06) | 28 (1) | ⊕⊕ low2 | P = 0.15 |
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. The study reported only the percentages. The absolute numbers of responder in each treatment arm were inferred from the percentages. While there are 5 dropouts, no reports on which treatment arm they came from. ITT principle is in effect for the analysis, assuming all participants completed the protocol.
2. We judged the quality of evidence as low due to incomplete outcome data reported from the study (Koenig 2012) and we needed to infer the results from percentages. The evidence is downgraded twice for this reason.
Rapamycin or rapalogs versus placebo
Three studies with a total of 263 participants were included (Bissler 2013; Franz 2013; Koenig 2012).
Primary outcome
1. Tumour size
The included studies reported the response of tumour size to oral administration of everolimus in terms of a reduction in the total volume of tumours to 50% or more relative to baseline (Bissler 2013; Franz 2013). The results were presented as dichotomous data as the number of participants experiencing this event in both the treatment and placebo groups.
1.1. Angimyolipoma
Bissler reported that 33 out of 79 participants showed at least a 50% reduction in the size of angiomyolipoma in the treatment group versus none out of 39 in the placebo group (Bissler 2013). Franz reported response to the size of renal angiomyolipomas; 16 out of 30 participants in treatment group versus none of 14 in placebo group showed at least a 50% reduction in angiomyolipoma size (Franz 2013). Our meta‐analysis showed that there was a significant increase in the proportion of participants who achieved a 50% reduction in angiomyolipoma size, RR 24.69 (95% CI 3.51 to 173.41) within the treatment arm (Analysis 1.1).
Given the ITT principle where we analysed all participants enrolled into both studies, it should be noted that five of 79 participants in the Bissler study had sporadic lymphangioleiomyomatosis (without TSC), which we could not separate from the analysis and which we will attempt to do so for a future update of this review (Bissler 2013).
1.2. Subependymal giant cell astrocytoma (SEGA)
1.2.1. Systemic administration
Franz reported a 50% reduction of SEGA volume in 27 out of 78 participants in the treatment group versus none out of 38 participants in the placebo group (Franz 2013). There was a significant increase within the treatment arm in the proportion of participants who achieved a 50% reduction in the volume of SEGA, RR 27.85 (95% CI 1.74 to 444.82) (Analysis 1.1).
Secondary outcomes
1. Response to skin lesions
1.1. Systemic administration
Two studies administered everolimus orally and reported the response to skin lesions; however, these studies did not define 'response' (Bissler 2013, Franz 2013).
Bissler reported 20 participants with a response to skin lesions out of 77 participants in treatment group as compared to no participants among 37 participants in the placebo group (Bissler 2013). Franz reported 30 participants with a response to skin lesions out of 72 participants in the treatment group as compared to four out of 38 participants in placebo group (Franz 2013). We analysed the data from these two studies and found that the proportion of participants who showed a skin response was significantly increased in the treatment arms, RR 5.78 (95% CI 2.30 to 14.52) (Analysis 1.2).
1.2. Topical administration
One study administered rapamycin topically (skin application), reported a defined response to skin lesions (seeCharacteristics of included studies) (Koenig 2012). The report of five withdrawals were not accompanied by an explanation from which arm the withdrawn participants came. Based on the ITT principle, we analysed this outcome by assuming that all participants completed the protocol.
The effects of the intervention in the Koenig study was measured based on the participants' perception of their skin condition (seeCharacteristics of included studies). While absolute numbers were not reported, it was stated that 73% of participants in the treatment group were said to have improved after treatment, versus 38% in the placebo group (Koenig 2012). We inferred the number of responders in each treatment arm from these percentages and analysed the data (Analysis 2.1). Although there is tendency for improvement in skin lesions in participants who received treatment, it was not significant, RR 1.81 (95% CI 0.80 to 4.06) (P = 0.15).
2.1. Analysis.
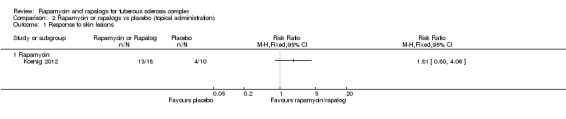
Comparison 2 Rapamycin or rapalogs vs placebo (topical administration), Outcome 1 Response to skin lesions.
For future updates of the review, we plan to contact the authors of two studies for clarification on the definition of skin response (Bissler 2013;Franz 2013) and type of skin lesions (Bissler 2013;Franz 2013). We also plan to contact the authors of a third study with regards to the absolute number of participants who responded and completed the protocol in each treatment arm (Koenig 2012).
2. Frequency of seizure (times)
One study reported seizure frequency in the form of median seizure frequency at baseline and at week 24 (Franz 2013). The effect of the intervention was reported as the median change of seizure frequency between these time points. However, a large proportion of participants did not experience seizures at baseline. The study reported sensitivity analyses on the subset of participants who had at least one seizure at baseline. The placebo group was reported to have higher median in the baseline of seizure frequency at baseline (11 in 24 hours) compared to treatment group (5.9 in 24 hours). The median change at 24 weeks was ‐2.9 in 24 hours (95% CI ‐4.0 to ‐1.0) in treatment group versus ‐4.1 in 24 hours (95% CI ‐10.9 to 5.8) in placebo group (Franz 2013).
3. Aneurysm size for angiomyolipomas (any unit of analysis found)
None of the included studies reported aneurysm size for angiomyolipomas (Bissler 2013; Franz 2013; Koenig 2012).
4. Creatinine level (mg/dL)
One study reported creatinine levels in terms of the number of participants with increased or decreased levels (dichotomous not continuous data) (Bissler 2013). One out of 79 participants in treatment group versus three out of 39 participants in placebo group had increased blood creatinine levels, our analysis showed a non‐significant difference between treatment groups, RR 0.16 (95% CI 0.02 to 1.53) (Analysis 1.3).
1.3. Analysis.
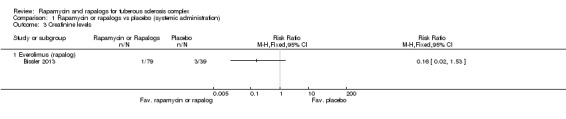
Comparison 1 Rapamycin or rapalogs vs placebo (systemic administration), Outcome 3 Creatinine levels.
5. Forced expiratory volume at one second (FEV1) / forced vital capacity (FVC) ratio
No study reported analysable data for FEV1 / FVC ratio were not reported. In the Bissler study, only median values at baseline and week 24 for FEV1 were reported. At baseline, the median values were 2.9 the in treatment group versus 2.7 in placebo group, while at week 24, the median values for both groups were 2.7. For the subset of participants with lymphangioleiomyomatosis and sporadic lymphangioleiomyomatosis the median percentage change of FEV1 in the treatment arm (in 24 out of 79 participants) was ‐1%, while in the placebo arm (10 out of 39 participants) this was ‐4% (Bissler 2013).
6. Any reported adverse effect or toxicity
All included studies reported adverse events during the course of each study, but none were definitely related to tretment (Bissler 2013; Franz 2013; Koenig 2012).
6.1. Systemic administration
Adverse events reported by the two studies which administered everolimus orally, were consistent with the known safety profile of the drug (Bissler 2013; Franz 2013). Adverse events were graded according to the 'Common Terminology Criteria for Adverse Events v3.0' (CTCAE v3.0); most were grade 1 or 2. The most common events in the Franz study were mouth ulceration, stomatitis, convulsion and pyrexia (Franz 2013), and in the Bissler study were nasopharyngitis, acne‐skin lesions, headache, cough and hypercholesterolaemia (Bissler 2013). Franz reported that 75 out of 78 participants within the treatment group and 35 out of 39 participants in the placebo group experienced an adverse events (Franz 2013). Bissler reported the proportion of specific adverse events in both arms (Bissler 2013). An additional table summarises adverse events from 157 participants in the treatment arm and 78 participants in the placebo arm from both studies (Table 3) (Bissler 2013; Franz 2013).
1. List of adverse events in oral (systemic) administration of everolimus (rapalog).
| Adverse events | Everolimus (n = 157) | Placebo (n = 78) |
| Stomatitis | 69 (44%) | 12 (15%) |
| Nasopahryngitis | 33 (21%) | 21 (27%) |
| Acne‐like skin lesions | 17 (11%) | 2 (3%) |
| Rash | 9 (6%) | 2 (3%) |
| Convulsion | 22 (14%) | 12 (15%) |
| Pyrexia | 22 (14%) | 6 (8%) |
| Headache | 17 (11%) | 8 (10%) |
| Cough | 26 (17%) | 9 (12%) |
| Hypercholesterolaemia | 16 (10%) | 1 (1%) |
| Aphthous stomatitis | 17 (11%) | 4 (5%) |
| Fatigue | 25 (16%) | 8 (10%) |
| Mouth ulceration | 41(26%) | 4 (5%) |
| Nausea | 13(8%) | 5 (6%) |
| Urinary tract infection | 12(8%) | 6 (8%) |
| Bronchitis | 11(7%) | 5 (6%) |
| Otitis media | 9 (6%) | 3 (4%) |
| Pharyngitis | 8 (5%) | 1 (1%) |
| Vomiting | 26 (17%) | 7 (9%) |
| Anaemia | 10 (6%) | 1 (1%) |
| Arthralgia | 10 (6%) | 2 (3%) |
| Diarrhoea | 20 (13%) | 4 (%) |
| Abdominal pain | 9 (6%) | 4 (5%) |
| Blood lactate dehydrogenase increased | 9 (6%) | 2 (3%) |
| Hypophosphataemia | 9 (6%) | 0 (0%) |
| Eczema | 8 (5%) | 3 (4%) |
| Leucopenia | 8 (5%) | 3 (4%) |
| Oropharyngeal pain | 8 (5%) | 4 (5%) |
| Upper respiratory tract infection | 21 (13%) | 9 (12%) |
Data were analysed from two studies (Bissler 2013; Franz 2013).
We analysed total adverse event data from only one of the included studies (Franz 2013) and found the participants who received oral everolimus treatment had a similar risk of experiencing adverse events as compared to those who did not receive treatment, RR 1.07 (95% CI 0.96 to 1.20) (Analysis 1.4).
1.4. Analysis.
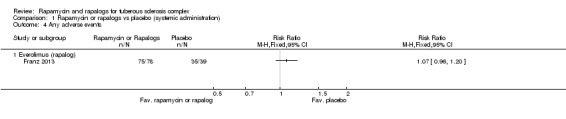
Comparison 1 Rapamycin or rapalogs vs placebo (systemic administration), Outcome 4 Any adverse events.
Oral everolimus treatment lead to significantly more adverse events resulting in withdrawal, interruption in treatment, or a reduction in dose level, RR 3.14 (with 95% confidence interval 1.82 to 5.42) (P < 0.0001) (Analysis 1.5) (Bissler 2013; Franz 2013).
6.2. Topical administration
The Koenig study, which administered rapamycin topically, reported that one participant from the treatment group experienced a serious adverse event, albeit unrelated to the study treatment (Koenig 2012). The participant aspirated during a seizure and developed pneumonia, which progressed to septic shock. The rapamycin concentrations were undetectable at the time of hospital admission, and the participant was immediately withdrawn from the study. This study did not list specific adverse events in the participants; however, the authors reported that there were no serious adverse events related to the study product and there was no detectable systemic absorption of the rapamycin during the study period (Koenig 2012).
Discussion
Summary of main results
Significantly more participants with angiomyolipoma who received oral everolimus (rapalog) had at least a 50% reduction in tumour size compared to those receiving placebo. Similarly, more participants with subependymal giant cell astrocytoma (SEGA) who received oral everolimus (rapalog) had at least a 50% reduction in tumour volume compared to those receiving placebo.
Two studies reported a significant increase in the chance of showing a response to skin lesions with oral everolimus (rapalog) (Bissler 2013, Franz 2013). However, the response of skin lesions to topical rapamycin showed a tendency to improvement, although this was not significant (Koenig 2012).
The effect of oral everolimus (rapalog) on seizure frequency was reported in one study, but the outcome was inconclusive (Franz 2013). These observations were based on the median values of seizure reduction, rather than the mean; probably because the data for this outcome was skewed (i.e. most participants might have had experienced a few seizures but a small number of participants may have experienced many) which will change the distribution of the data (the median would be a more appropriate statistic to use in this case). Moreover, participants were selected for the study on the basis of their need for intervention for progression of subependymal giant cell astrocytomas rather than presence of seizures. Further clarifications from the trial authors will be sought on this for a future update of the review.
One study reported an effect of oral everolimus (rapalog) on serum creatinine, were more participants in the placebo group were found to have elevated levels of serum creatinine compared to those in the intervention arm (Bissler 2013).
For a subset of participants with lymphangioleiomyomatosis and sporadic lymphangioleiomyomatosis, after 24 weeks, the median percentage reduction in FEV1 was greater in the placebo group than in the oral everolimus (rapalog) group (Bissler 2013).
Although our analysis on one of the included studies showed that participants who received oral everolimus (rapalog) had a similar risk of having adverse events as compared to participants who did not (Franz 2013), the treatment itself led to significantly more adverse events resulting in withdrawal, an interruption in treatment, or a reduction in dose level (Bissler 2013, Franz 2013). Nevertheless, these are adverse events of which correlation to the treatment per se is not certain. No serious adverse events related to the topical rapamycin administration were reported and there was no detectable systemic absorption during the study period (Koenig 2012)
The findings were summarized in the table (Table 1; Table 2).
Overall completeness and applicability of evidence
We identified three studies meeting our inclusion criteria and including outcomes of interest to this review. However, the objective of correlating intervention to adverse effects was not satisfactorily met, as most of the manifestations were observed as adverse events and not specifically treatment‐related adverse effects. It should be noted that small proportions of the participants (five of 162) analysed for renal angiomyolipoma have sporadic lymphangioleiomyomatosis (without tuberous sclerosis complex).
Quality of the evidence
Two of the included studies generally showed a low risk of bias (Bissler 2013, Franz 2013), except for an unclear risk in one study with no information on allocation concealment (Bissler 2013). Another included study showed a range of risk of biases across domains, where we judged this study to have high risk of attrition bias, unclear allocation concealment and unclear random sequence generation (Koenig 2012). The reporting outcomes across studies was generally of high quality, except for the response to skin lesions from topical rapamycin due to the attrition bias of the study and seizure frequency due to selection of participants. Evidence for an effect on skin lesions from topical rapamycin requires more data. Evidence for seizure frequency was inconclusive. The evidence was of high quality in relation to response to tumour size, even though there are large confidence intervals. The number of participants is relatively large for a rare disease, thus judgement about the quality of the evidence (particularly judgements about precision) may be based on the absolute effect. Here the quality rating was considered 'high' because the outcome was appropriately assessed.
Potential biases in the review process
The strengths of this review are based on the careful selection of randomised studies of a rare disease. We have noted that not all relevant data could be obtained, especially those data relating to adverse effects.
Agreements and disagreements with other studies or reviews
A systematic review on non‐randomised studies carried out by this author team showed similar findings, where absolute tumour size reduction was noted in people treated with rapamycin or rapalogs (Sasongko 2015).
Authors' conclusions
Implications for practice.
Convincing evidence of a reduction of tumour size after 24 weeks of treatment with oral everolimus (rapalogs) in both renal angiomyolipoma and SEGA would provide sufficient evidence for its use in clinical practice as the benefits outweigh the risks. With this in mind, and with the positive effects on the size reduction in renal angiomyolipoma and SEGA, this review concurs with the decision of the U.S. Food and Drug Administration (FDA) and European Medicine Agency (EMA) on the use of everolimus for both types of tumours. Rapamycin or rapalogs may also have a beneficial effect on skin lesions.
Implications for research.
More research is needed to further define the effects of rapamycin or rapalogs on other manifestations of tuberous sclerosis complex, such as seizures and neurocognitive problems. Beneficial effects noted in this review on skin lesions need to be further established. This research should take the form of a randomized study design, involving participants diagnosed according to the latest consensus tuberous sclerosis complex diagnostic criteria.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Amended | Contact details updated. |
Acknowledgements
The authors wish to thank Tracey Remmington and Jacqueline Ho for their helpful comments and guidance throughout the manuscript writing. The authors wish to thank Nik Mohd. Ariff Nik Abdul Malik for his great assistance during study searching and selection as well as data extraction and analyses.
Appendices
Appendix 1. Search Strategies for CENTRAL (through CRSO)
A. MeSH‐term‐based search strategy:
#1 MeSH descriptor Tuberous Sclerosis explode all trees
#2 MeSH descriptor Sirolimus explode all trees
#3 MeSH descriptor Everolimus explode all trees
#4 #2 OR #3
#5 #1 AND #4
B. Free‐text‐based search strategy:
(tuberous sclerosis OR Adenoma Sebaceum OR Bourneville* Disease OR Bourneville Phacomatosis OR Bourneville Phakomatosis OR Bourneville Pringle* Disease OR Bourneville Syndrome OR Bourneville Syndrome) AND ((Sirolimus OR Rapamycin OR Rapamune OR I‐2190A OR I2190A OR I 2190A OR AY 22‐989 OR AY 22989 OR AY 22 989) OR (Everolimus OR 001, RAD OR 40‐O‐(2‐hydroxyethyl)‐rapamycin OR Afinitor OR Certican OR RAD 001 OR RAD, SDZ OR RAD001 OR SDZ RAD OR SDZ‐RAD) OR (temsirolimus OR torisel OR CCI‐779 OR biolimus A9 OR zotarolimus OR ABT‐578 OR deforolimus OR AP23573 OR MK‐8669 OR ridaforolimus)):ti,ab,ky
C. Filtering options for CENTRAL:
We searched records owned by all groups. In MEDLINE, In EMBASE and In REVIEWS were ticked. We searched references in CRSO from 1 January 2006 to 14 March 2016, which were published in CENTRAL issue 1 2012 to issue 2 2016 with were published between 2000 to 2016.
Appendix 2. Search Strategy for MEDLINE
1 randomized controlled trial.pt.
2 controlled clinical trial.pt.
3 randomized.ab.
4 placebo.ab.
5 drug therapy.fs.
6 randomly.ab.
7 trial.ab.
8 groups.ab.
9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10 exp animals/ not humans.sh.
11 9 not 10
12 exp Tuberous Sclerosis Complex/
13 (tuberous sclerosis OR adenoma sebaceum OR bourneville* disease OR bourneville phacomatosis OR bourneville phakomatosis OR bourneville pringle* disease OR bourneville syndrome OR bourneville syndrome)
14 12 or 13
15 exp Sirolimus/
16 exp Everolimus/
17 (sirolimus OR rapamycin OR rapamune OR I‐2190A OR I2190A OR I 2190A OR AY 22‐989 OR AY 22989 OR AY 22 989)
18 (everolimus OR 001, RAD OR 40‐O‐(2‐hydroxyethyl)‐rapamycin OR Afinitor OR Certican OR RAD 001 OR RAD, SDZ OR RAD001 OR SDZ RAD OR SDZ‐RAD)
19 (temsirolimus OR torisel OR CCI‐779 OR biolimus A9 OR zotarolimus OR ABT‐578 OR deforolimus OR AP23573 OR MK‐8669 OR ridaforolimus)
20 15 or 16 or 17 or 18 or 19
21 11 and 14 and 20
22 remove duplicates from 20
Appendix 3. Search Strategy for clinicaltrials.gov
Search was done for "Conditions" using the following keywords: tuberous sclerosis OR adenoma sebaceum OR bourneville* disease OR bourneville phacomatosis OR bourneville phakomatosis OR bourneville pringle* disease OR bourneville syndrome OR bourneville syndrome. Only entries reporting phase 2 or phase 3 or phase 4 were selected.
Data and analyses
Comparison 1. Rapamycin or rapalogs vs placebo (systemic administration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to tumour size | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Everolimus (rapalog) on angiomyolipoma | 2 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 24.69 [3.51, 173.41] |
| 1.2 Everolimus (rapalog) on subependymal giant cell astrocytoma (SEGA) | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 27.85 [1.74, 444.82] |
| 2 Response to skin lesions | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.78 [2.30, 14.52] |
| 2.1 Everolimus (rapalog) | 2 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.78 [2.30, 14.52] |
| 3 Creatinine levels | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Everolimus (rapalog) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Any adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Everolimus (rapalog) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Adverse events leading to dose reduction, interruption or withdrawal | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Everolimus (rapalog) | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [1.82, 5.42] |
Comparison 2. Rapamycin or rapalogs vs placebo (topical administration).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to skin lesions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Rapamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bissler 2013.
| Methods | Double‐blind, randomised, placebo‐controlled, phase 3 study. Multicentre (24 centres in 11 countries). | |
| Participants | 118 participants (aged 18 ‐ 61 years old, 40 males and 78 females) having at least one angiomyolipoma 3 cm or larger in diameter, and a diagnosis of tuberous sclerosis per consensus criteria (Roach 1998, Hyman 2000) were recruited. 79 participants were in the treatment group and 39 participants were in the placebo group. Participants were 18 years or older. 23 participants in the placebo group withdrew, mainly due to disease progression. However, all participants were included in the analysis. There were 2/79 participants in intervention arm and 3/36 participants in the placebo arm with sporadic lymphangioleiomyomatosis (without TSC) which we could not separate from the analysis. For a future version of this review, we plan to contact study authors to obtain more detailed information so that we can analyse outcome from only people with TSC. We also plan to contact study authors to ascertain if the TSC participants meet the latest diagnostic criteria (Northrup 2013). |
|
| Interventions | Participants were randomized into either:
1. oral everolimus 10 mg/day, or
2. oral placebo. Median exposure of intervention was 38 weeks for the treatment group and 34 weeks for placebo group. Kidney CT or MRI was carried out at baseline, 12, 24, 48 weeks and annually after start of treatment. Although it was stated that the core phase of the trial lasted until the last randomised participant had been treated for 6 months, there was no clear statement on the exact study duration, especially in view of another statement about a subsequent open‐label phase. Study authors will be contacted for exact information about the timeline of randomisation. |
|
| Outcomes | Response rate of everolimus on angiomyolipomas (defined as the number of participants having 50% reduction in tumour size as compared to baseline), seizure frequency, response to skin lesions and adverse events. | |
| Notes | Authors who are employees, stock owners or consultants of the funder (Novartis) were involved in the study design, discussion, research, overseeing of data collection and data analysis and interpretation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 2:1 fashion to receive either everolimus or placebo, stratified by enzyme‐inducing antiepileptic drug use at randomisation and by the presence of sporadic lymphangio leiomyomatosis. Random sequence was generated using interactive web response system. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received blinded study treatment until angiomyolipoma progression, occurrence of unacceptable toxicity, or participant withdrawal for any other reason. Double blind (participant, caregiver, investigator, outcomes assessor). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Kidney CT or MRI (same modality used throughout the study for each participant) was assessed by a blinded central radiology review. Adverse effects were monitored via participant‐reported or caregiver‐reported responses as well as investigator assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy analyses were done on all randomised participants, and safety analyses were done on all participants who received at least one dose of the study drug and had at least one post‐baseline assessment. Participants not able to be assessed (either by dropout or other reasons) were considered as non responders. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were mentioned in 'Methods' were reported in 'Results' section of the study report. |
| Other bias | Unclear risk | It was noted that the authors who are employees, stock owners or consultants of the funder (Novartis) were involved in the study design, discussion, research, overseeing of data collection and data analysis and interpretation. |
Franz 2013.
| Methods | Double‐blind, randomised, placebo‐controlled, phase 3 trial. Multicentre (24 centres in 10 countries). | |
| Participants | 117 participants (aged 0.8 ‐ 26.6 years old, 67 males and 50 females) having at least one angiomyolipoma 3 cm or larger in diameter, and a definite diagnosis of tuberous sclerosis per consensus criteria (Hyman 2000; Roach 1998) were recruited. There are 78 participants in the treatment group and 39 participants in the placebo group. In the treatment group, 1 participant was lost to follow up and another participant withdrew consent. In the placebo group, 6 discontinued due to disease progression, 1 withdrew consent and another participant discontinued due to administrative problems. Data from all 117 recruited participants were analysed. For the future version of this review, we plan to contact study authors to ascertain if the people with TSC meet the latest diagnostic criteria (Northrup 2013). | |
| Interventions | Participants were were randomised into either:
1. oral everolimus, from 4.5 mg/m² body surface area per day and subsequently adjusted to attain blood trough concentrations of 5 ‐ 15 ng/mL with dose modifications allowed on for the purpose of treatment‐related toxic effects. Starting dose for children with malignancies was chosen to be just less than the maximum tolerated dose (5 mg/m² per day); or 2. oral placebo. Participants were treated for 6 months with an extension phase (upto 4 years after the last participant was randomly assigned to treatment) if the results of the core phase favoured everolimus. The median duration of study treatment was 41.9 weeks (range 24.0 ‐ 78.9) for individuals in the everolimus group and 36.1 weeks (13.9 ‐ 79.7) for those in the placebo group. |
|
| Outcomes | Response rate (defined the number of participants having 50% reduction in tumour size as compared to baseline) of everolimus on SEGA, angiomyolipomas, seizure frequency, response to skin lesions and adverse events. | |
| Notes | Authors who are employee or consultants of the funder (Novartis) were involved in the study design, discussion, research, overseeing of data collection and data analysis and interpretation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in a 2:1 ratio to everolimus or matching placebo, stratified according to the use of enzyme‐inducing antiepileptic drugs. It is assumed that the researchers used computer‐generated random sequence: "An interactive internet‐response system was used for random assignment of patients...". |
| Allocation concealment (selection bias) | Low risk | The researchers concealed allocation according to the following statement: "An interactive internet‐response system was used...for management of their treatment to maintain allocation concealment". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded according to the following statement: "Patients were given masked study treatment (identical everolimus and placebo) unless discontinued..." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All study personnel were masked to treatment assignment, including outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy and safety analyses included all participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were mentioned in 'Methods' were reported in 'Results' sections of the study report. |
| Other bias | Unclear risk | Authors who are employees or consultants of the funder (Novartis) were involved in the study design, discussion, research, overseeing of data collection and data analysis and interpretation. |
Koenig 2012.
| Methods | Double‐blind, randomised, placebo‐controlled study. Single centre in Texas, USA. | |
| Participants | 28 participants (mean age at 23 years old, ranging from 13 to > 30 years old, 15 males and 13 females) diagnosed as TSC based on the consensus criteria (Roach 1999) were recruited. 18 participants in the treatment group and 10 participants in the placebo group. Five participants were withdrawn from the study, but it is unclear if they come from the treatment arm or from placebo arm. For a future version of this review, we plan to contact study authors to ascertain if the people with TSC meet the latest diagnostic criteria (Northrup 2013). | |
| Interventions | Participants were were randomised into either: 1. topical (skin application) rapamycin 1 mg per 30 cc (0.003%); or 2. topical (skin application) rapamycin 5 mg per 30 cc (0.015%); or 3. topical (skin application) placebo. Participants were treated for 6 months. |
|
| Outcomes | Effect of rapamycin on participant' perception towards their skin appearance following the treatment. The participants were asked if they "got better on the treatment", "got worse on the treatment" or if "the treatment made no difference". Rapamycin concentrations and complete blood counts (including haemoglobin level and platelet count) were measured. Adverse events were reported. | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done by the manufacturer of the investigational product: "The company randomized the investigational product". However, there was no statement as to how the allocation sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and study personnel were blinded: "Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Out of 28 participants included into the study, 23 participants completed the intervention. Outcome data were reported only as percentages. While there are dropouts, no reports as to how many dropouts in each treatment arm. |
| Selective reporting (reporting bias) | Low risk | All outcomes that were mentioned in 'Methods' were reported in 'Results' sections of the study report. |
| Other bias | Low risk | ‐ |
CT: computed tomography MRI: magnetic resonance imaging SEGA: subependymal giant cell astrocytomas TSC: tuberous sclerosis complex
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Birca 2010 | Case report. |
| Cabrera 2011 | Non‐randomised, non‐controlled study. |
| Dabora 2011 | Non‐randomised, non‐controlled study. |
| Davies 2011 | Non‐randomised, non‐controlled study. |
| Foster 2012 | Case series. |
| Franz 2006 | Case series. |
| Herry 2007 | Case report. |
| Hofbauer 2008 | Case report. |
| Koenig 2008 | Case report. |
| Krueger 2010 | Non‐randomised, non‐controlled study. |
| Lam 2010 | Case series. |
| Pressey 2010 | Case report. |
| Salido 2012 | Case series. |
| Sparagana 2010 | Case report. |
| Staehler 2012 | Case series. |
| Tanaka 2013 | Non‐randomised; left‐right cheek comparison of topical rapamycin vs vehicle on facial angiofibroma on the same participants. |
| Wataya‐Kaneda 2011 | Case series. |
| Wataya‐Kaneda 2015 | Non‐randomised, non‐controlled study. |
| Wienecke 2006 | Case report. |
| Yalon 2011 | Case report. |
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01289912.
| Methods | Phase II randomised, double‐blinded, placebo‐controlled study. |
| Participants | 52 participants diagnosed with TSC between the ages of 6 and 21 years of age and had an IQ of greater than or equal to 60. |
| Interventions | Drug: RAD001 (other names: everolimus; afinitor). RAD001 is formulated as tablets of 5.0 mg strength, blister‐packed under aluminium foil in units of 10 tablets and dosed on a regular basis. Matching placebo will be provided as a matching tablet and will also be blister packed under aluminium foil in units of 10. RAD001 or matching placebo tablets should be opened only at the time of administration as drug is both hygroscopic and light‐sensitive. Participants will be instructed to take 4.5 mg/m2 of RAD001 or matching placebo orally with a glass of water at regular intervals at the same time (delete: each day) in the morning after a light, non‐fat breakfast. |
| Outcomes | Primary outcomes: neurocognition and safety. Secondary outcomes: frequency of epileptiform events; sleep disturbances; autism spectrum disorders features; academic skills; behavioral problems. |
| Notes | Sponsored by Mustafa Sahin (Children's Hospital, Boston) in collaboration with Tuberous Sclerosis Alliance, Autism Speaks, Novartis Pharmaceuticals and Seizure Tracker LLC. |
NCT01526356.
| Methods | Multicentre prospective, randomised, double‐blind, placebo‐controlled study. |
| Participants | 177 participants diagnosed with TSC and visible facial angiofibromas. |
| Interventions | Participants were randomised into either: 1. topical placebo cream; OR 2. topical 0.1% rapamycin cream; OR 3. topical 1% rapamycin cream. Study cream is applied nightly to the affected areas on the face. |
| Outcomes | Primary outcome: reduction in lesion size and appearance. Photographic, visual, and measurable reduction in the size and severity of the lesions. Secondary outcomes: systemic uptake of topically applied rapamycin; dermatologic sensitivity at the site of application. |
| Notes | Sponsored by The University of Texas Health Science Center, Houston. |
IQ: intelligence quotient TSC: tuberous sclerosis complex
Characteristics of ongoing studies [ordered by study ID]
NCT01713946.
| Trial name or title | A placebo‐controlled study of efficacy and safety of 2 trough‐ranges of everolimus as an adjunctive therapy in people with TSC and refractory partial‐onset seizures (EXIST‐3). |
| Methods | 3‐arm, randomised, double‐blind, placebo‐controlled study. |
| Participants | People with TSC who have refractory partial‐onset seizures. |
| Interventions | Participants are randomised into either: 1. placebo; OR 2. low trough everolimus (titrated to 3 to 7 ng/mL); OR 3. high trough everolimus (titrated to 9 to 15 ng/mL). |
| Outcomes | Primary outcomes: response rate (at least a 50% reduction from baseline in partial‐onset seizure frequency); percentage reduction in partial onset seizure frequency. Secondary outcomes: seizure‐free rate (100% reduction in partial onset‐seizure frequency); proportion of participants with at least a 25% reduction in partial onset seizure frequency; categorical variable of 6 levels of reduction from baseline in partial‐onset seizure frequency (≤ ‐25% (exacerbation); > ‐25% to < 25% (no change); ≥ 25% to < 50%; ≥ 50% to < 75%; ≥ 75% to < 100%; 100% (seizure‐freedom); frequency of seizure‐free days; treatment duration (time from randomisation until treatment discontinuation in the core phase); overall QoL global scores; sub‐test scores for neurocognitive, neurodevelopmental, and neurobehavioral tests (changes in the Vineland Adaptive Behavior Scales‐II and the Wechsler Non‐Verbal Scale of Ability); percentage reduction in seizure frequency/frequency of selected adverse events; pre‐dose concentrations of AEDs alone and post‐baseline (AEDs plus everolimus); 50% response rate from baseline by time interval over the extension phase; seizure‐free days in partial onset seizure by time interval over the extension phase; frequency of adverse events; frequency of abnormal laboratory values; frequency of Columbia Suicide Severity Rating Scale (C‐SSRS) outcomes; frequency of SAEs referring to a positive suicidal evaluation. |
| Starting date | April 2013. |
| Contact information | Not provided by the time of access. |
| Notes | Sponsored by Novartis Pharmaceuticals. |
NCT01730209.
| Trial name or title | Efficacy of RAD001/everolimus in autism and neuropsychological deficits in children with TSC (RAPIT) |
| Methods | Randomised double‐blind placebo controlled intervention study. |
| Participants | Children with TSC between age 4 and 15 years with an IQ estimated < 80 and/or special schooling and/or autism spectrum disorder and/or learning disability requiring remedial teaching. |
| Interventions | Participants are randomised into either: 1. placebo tablet OR 2. everolimus tablet (titrated to trough level 5 ‐ 10 ng/ml). |
| Outcomes | Primary outcomes: cognitive ability measured by IQ and Wechsler Intelligence Scale for Children (WISC‐III‐NL). Secondary outcomes: autistic features as assessed by Autism Diagnostic Observation Schedule (ADOS); social and communicational skills as assessed by social responsiveness scale (SRS) and Dutch Children's Communication Checklist (CCC‐2‐NL) questionnaires; working memory and attention, information processing as assessed by Cambridge Neuropsychological Test Automated Battery (CANTAB); visual‐motor integration as assessed by BEERY Visual‐Motor Integration (BEERY VMI), grooved pegboard; child behavior as assessed by Child Behavior Checklist (CBCL) and Teacher's Report Form (TRF) questionnaires; executive functioning as assessed by Behavior Rating Inventory of Executive Functioning (BRIEF) questionnaire Dutch version; sleeping problems as assessed by Sleep Disturbance Scale for Children (SDSC) questionnaire; child health as assessed by Child Health Questionnaire Parent Form (CHQ‐PF50) questionnaire; sensory related difficulties as assessed by Short Sensory Profile (SSP) questionnaire; epilepsy (comparison of epilepsy frequency during month previous to study start and last month of trial participation.EEG abnormalities). Other outcomes: school level as assessed by the school CITO (centraal instituut voor toetsontwikkeling) scores or reading and arithmetic scores; pharmacokinetics as assessed by measuring trough levels of everolimus; safety (levels of and abnormalities in blood control value). |
| Starting date | November 2012. |
| Contact information | M.C.Y. de Wit, MD. PhD. Tel: +31 10 703 6956. Email: tubereuzesclerose@erasmusmc.nl. |
| Notes | Sponsored by Erasmus Medical Center. |
NCT01954693.
| Trial name or title | A study of everolimus in the treatment of neurocognitive problems in tuberous sclerosis (TRON). |
| Methods | Single centre, 2‐arm, individually randomised, phase II, double‐blind, placebo‐controlled study. |
| Participants | People aged 16 to 60 years with TSC who have IQ > 60 and a significant deficit in one or more primary outcome measures. |
| Interventions | Participants are randomised into either: 1. placebo tablet OR 2. everolimus tablet (2 x 2.5mg daily). |
| Outcomes | Primary outcomes: list Learning test (from the BIRT Memory and Information Processing Battery); complex Figure test (from the BIRT Memory and Information Processing Battery); CANTAB ‐ Stockings of Cambridge (SOC); CANTAB ‐ Spatial Working Memory (SWM); telephone search dual task (from the Test of Everyday Attention). Secondary outcomes: CANTAB ‐ Rapid Visual Information Processing Battery (RVIP); CANTAB ‐ Spatial Span (SSP); CANTAB ‐ Attentional Set‐shifting (IDED); Verbal Fluency /Controlled Oral Word Association Test (COWAT); cancellation task; Symptom Checklist 90R (SCL‐90R); QoL in Epilepsy (QOLIE); Liverpool Seizure Severity Scale (LSSS); Vineland Adaptive Behavior Scales‐II (VABS‐II) (survey form); Social Responsiveness Scale ‐ Adult version (SRS‐A); Social communication questionnaire (SCQ); National Adult Reading Test (NART). Other outcomes: Wechsler Abbreviated Scale of Intelligence (WASI) (4 subtests) (eligibility visit screening measure); Edinburgh Handedness Test (eligibility visit screening measures). |
| Starting date | June 2012. |
| Contact information | Prof. Julia Sampson. Email: sampson@cardiff.ac.uk. |
| Notes | Sponsored by Cardiff University in collaboration with Novartis. |
NCT02635789.
| Trial name or title | Phase III study of topical formulation of sirolimus to skin lesions in people with TSC. |
| Methods | Double‐blind, randomised, placebo‐controlled phase III study. |
| Participants | People with TSC with angiofibroma, hypomelanotic macules, plaque. |
| Interventions | Participants are randomized into either: 1. placebo gel OR 2. NPC‐12G gel containing 0.2% sirolimus administered topically twice‐a‐day for 12 weeks. |
| Outcomes | Primary outcome: improvements in angiofibroma after 12 weeks (improvements comparing with baseline as assessed using photograph by the central photo‐judgement committee). Secondary outcomes: improvements in angiofibroma after 4, 8 and 16 weeks (improvements comparing with baseline is assessed using photograph by the central photo‐judgement committee); improvements in angiofibroma after 4, 8, 12 and 16 weeks (improvements comparing with baseline as assessed by the investigator); improvements in redness of angiofibroma (improvement comparing with baseline is assessed by the central photo‐judgement committee and the investigator); improvements in hypomelanotic macule and plaque of upper neck (improvement comparing with baseline is assessed by the central photo‐judgement committee and the investigator); the rate of patients who are evaluated as ''improvement'' or more (improvement rate) in primary outcome measure and in secondary outcome measures above outcome 1 to 5; change in total score from baseline for DLQI and CDLQI (DLQI for subjects 16 years old and greater, or CDLQI for children of less than 16 years old as assessed by participants); AEs during the study period; SAEs during the study period; laboratory findings during the study period; vital signs during the study period; sirolimus blood concentration as assessed by drug monitoring. |
| Starting date | December 2015. |
| Contact information | Mr Taihei Hio (Tel.: +81‐3‐5651‐1177, email: hio@nobelpharma.co.jp) and Mr. Kenji Shimizu (Tel.: +81‐3‐5651‐1177, email: shimizu@nobelpharma.co.jp). |
| Notes | Sponsored by Nobelpharma. |
AE: adverse event AEDs: anti‐epileptic drugs IQ: intelligence quotient QoL: quality of life SAEs: serious adverse events TSC: tuberous sclerosis complex
Differences between protocol and review
We included in this review an additional secondary outcome, skin lesion response. At the time of protocol writing, we did not consider the outcome of 'skin lesion response'; however, we now realise the clinical relevance of this outcome which is measured in all included studies (Bissler 2013; Franz 2013; Koenig 2012). We added the new outcome into the summary of findings tables, which was highlighted as a post hoc change. Whenever applicable, we separated the analyses of efficacy and adverse effects into systemic administration and topical administration, including the summary of findings tables. For sensitivity analyses, we decided to remove the requirement "if 10 or more studies are included in the review" in view of the fact that this disorder is rare. For an assessment of heterogeneity, we decided to remove the following sentence "When there are more than five studies included, the authors will test for heterogeneity between studies using a standard Chi2 test and I2 statistic". We added rapalogs to the search terms.
Contributions of authors
NFDI searched for studies and applied the inclusion criteria for including studies into this review as well as extracted the data from the included studies and entered the data into RevMan. THS and NFDI carried out the analyses. THS and ZAMH interpreted the analyses. THS and ZAMH drafted the final review. THS and ZAMHZH secured funding for the review. All authors approved the final version of the review.
Sources of support
Internal sources
-
Grants, Malaysia.
This work is supported by grants from the Universiti Sains Malaysia (1001/PPSP/812137 for Teguh Haryo Sasongko and 304/CNEURO/652205/K134 for Zabidi Azhar Mohd. Hussin).
External sources
No sources of support supplied
Declarations of interest
Teguh H Sasongko: none known. Nur Farrah Dila Ismail: none known. ZAMH Zabidi‐Hussin: attended as a consultant for Affinitor® at two meetings held by Novartis.
Edited (no change to conclusions)
References
References to studies included in this review
Bissler 2013 {published data only}
- Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST‐2): a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet 2013;381(9869):817‐24. [PUBMED: 23312829] [DOI] [PubMed] [Google Scholar]
- NCT00790400. Efficacy and safety of RAD001 in patients aged 18 and over with Angiomyolipoma associated with either tuberous sclerosis complex (TSC) or sporadic lymphangioleiomyomatosis (LAM) (EXIST‐2). https://www.clinicaltrials.gov/ct2/show/study/NCT00790400?term=NCT00790400&rank=1 (accessed 14 March 2016).
Franz 2013 {published data only}
- Franz D, Lam D, Cauwel H, Jozwiak S. Effect of everolimus on subependymal nodules (SENs) and tubers in patients with subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC): Results from the exist‐1 trial [abstract]. Proceedings of the 65th American Academy of Neurology Annual Meeting San Diego, CA United States. Lippincott Williams and Wilkins, 2013.
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST‐1): a multicentre, randomised, placebo‐controlled phase 3 trial. Lancet 2013;381(9861):125‐32. [PUBMED: 23158522] [DOI] [PubMed] [Google Scholar]
- Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, et al. Everolimus for subependymal giant cell astrocytoma in patients with tuberous sclerosis complex: 2‐year open‐label extension of the randomised EXIST‐1 study. The Lancet. Oncology 2014;15(13):1513‐20. [PUBMED: 25456370] [DOI] [PubMed] [Google Scholar]
- Kingswood JC, Jozwiak S, Belousova ED, Frost MD, Kuperman RA, Bebin EM, et al. The effect of everolimus on renal angiomyolipoma in patients with tuberous sclerosis complex being treated for subependymal giant cell astrocytoma: subgroup results from the randomized, placebo‐controlled, Phase 3 trial EXIST‐1. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association ‐ European Renal Association 2014;29(6):1203‐10. [PUBMED: 24729041] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00789828. Efficacy and safety of Everolimus (RAD001) in patients of all ages with subependymal giant cell astrocytoma associated with tuberous sclerosis complex (TSC)(EXIST‐1). https://www.clinicaltrials.gov/ct2/show/NCT00789828?term=NCT00789828&rank=1 (accessed 14 March 2016).
Koenig 2012 {published data only}
- Koenig MK, Hebert AA, Roberson J, Samuels J, Slopis J, Woerner A, et al. Topical rapamycin therapy to alleviate the cutaneous manifestations of tuberous sclerosis complex: a double‐blind, randomized, controlled trial to evaluate the safety and efficacy of topically applied rapamycin. Drugs in R&D 2012;12(3):121‐6. [PUBMED: 22934754] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01031901. Topical rapamycin therapy to alleviate cutaneous manifestations of tuberous sclerosis complex (TSC) and neurofibromatosis I (NF1). https://www.clinicaltrials.gov/ct2/show/NCT01031901?term=NCT01031901&rank=1 (accessed 14 March 2016).
References to studies excluded from this review
Birca 2010 {published data only}
- Birca A, Mercier C, Major P. Rapamycin as an alternative to surgical treatment of subependymal giant cell astrocytomas in a patient with tuberous sclerosis complex. Journal of Neurosurgery. Pediatrics 2010;6(4):381‐4. [PUBMED: 20887114] [DOI] [PubMed] [Google Scholar]
Cabrera 2011 {published data only}
- Cabrera Lopez C, Marti T, Catala V, Torres F, Mateu S, Ballarin Castan J, et al. Effects of rapamycin on angiomyolipomas in patients with tuberous sclerosis. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia 2011;31(3):292‐8. [PUBMED: 21629335] [DOI] [PubMed] [Google Scholar]
Dabora 2011 {published data only}
- Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ Jr, Miles D, et al. Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF‐ D levels decrease. PloS one 2011;6(9):e23379. [PUBMED: 21915260] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2011 {published data only}
- Davies DM, Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, et al. Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17(12):4071‐81. [PUBMED: 21525172] [DOI] [PubMed] [Google Scholar]
Foster 2012 {published data only}
- Foster RS, Bint LJ, Halbert AR. Topical 0.1% rapamycin for angiofibromas in paediatric patients with tuberous sclerosis: a pilot study of four patients. The Australasian Journal of Dermatology 2012;53(1):52‐6. [PUBMED: 22309333] [DOI] [PubMed] [Google Scholar]
Franz 2006 {published data only}
- Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, et al. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Annals of Neurology 2006;59(3):490‐8. [PUBMED: 16453317] [DOI] [PubMed] [Google Scholar]
Herry 2007 {published data only}
- Herry I, Neukirch C, Debray MP, Mignon F, Crestani B. Dramatic effect of sirolimus on renal angiomyolipomas in a patient with tuberous sclerosis complex. European Journal of Internal Medicine 2007;18(1):76‐7. [PUBMED: 17223050] [DOI] [PubMed] [Google Scholar]
Hofbauer 2008 {published data only}
- Hofbauer GF, Marcollo‐Pini A, Corsenca A, Kistler AD, French LE, Wuthrich RP, et al. The mTOR inhibitor rapamycin significantly improves facial angiofibroma lesions in a patient with tuberous sclerosis. The British Journal of Dermatology 2008;159(2):473‐5. [PUBMED: 18547304] [DOI] [PubMed] [Google Scholar]
Koenig 2008 {published data only}
- Koenig MK, Butler IJ, Northrup H. Regression of subependymal giant cell astrocytoma with rapamycin in tuberous sclerosis complex. Journal of Child Neurology 2008;23(10):1238‐9. [PUBMED: 18952591] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krueger 2010 {published data only}
- Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant‐cell astrocytomas in tuberous sclerosis. The New England Journal of Medicine 2010;363(19):1801‐11. [PUBMED: 21047224] [DOI] [PubMed] [Google Scholar]
Lam 2010 {published data only}
- Lam C, Bouffet E, Tabori U, Mabbott D, Taylor M, Bartels U. Rapamycin (sirolimus) in tuberous sclerosis associated pediatric central nervous system tumors. Pediatric Blood & Cancer 2010;54(3):476‐9. [PUBMED: 19856393] [DOI] [PubMed] [Google Scholar]
Pressey 2010 {published data only}
- Pressey JG, Wright JM, Geller JI, Joseph DB, Pressey CS, Kelly DR. Sirolimus therapy for fibromatosis and multifocal renal cell carcinoma in a child with tuberous sclerosis complex. Pediatric Blood & Cancer 2010;54(7):1035‐7. [PUBMED: 20108343] [DOI] [PubMed] [Google Scholar]
Salido 2012 {published data only}
- Salido R, Garnacho‐Saucedo G, Cuevas‐Asencio I, Ruano J, Galan‐Gutierrez M, Velez A, et al. Sustained clinical effectiveness and favorable safety profile of topical sirolimus for tuberous sclerosis ‐ associated facial angiofibroma. Journal of the European Academy of Dermatology and Venereology : JEADV 2012;26(10):1315‐8. [PUBMED: 21834948] [DOI] [PubMed] [Google Scholar]
Sparagana 2010 {published data only}
- Sparagana SP, Wilkes DC, Thompson CE, Bowers DC. Optic nerve tumor in tuberous sclerosis complex is not responsive to sirolimus. Pediatric Neurology 2010;42(6):443‐6. [PUBMED: 20472200] [DOI] [PubMed] [Google Scholar]
Staehler 2012 {published data only}
- Staehler M, Sauter M, Helck A, Linsenmaier U, Weber L, Mayer K, et al. Nephron‐sparing resection of angiomyolipoma after sirolimus pretreatment in patients with tuberous sclerosis. International Urology and Nephrology 2012;44(6):1657‐61. [PUBMED: 23054313] [DOI] [PubMed] [Google Scholar]
Tanaka 2013 {published data only}
- Tanaka M, Wataya‐Kaneda M, Nakamura A, Matsumoto S, Katayama I. First left‐right comparative study of topical rapamycin vs. vehicle for facial angiofibromas in patients with tuberous sclerosis complex. The British journal of dermatology 2013;169(6):1314‐8. [PUBMED: 23909960] [DOI] [PubMed] [Google Scholar]
Wataya‐Kaneda 2011 {published data only}
- Wataya‐Kaneda M, Tanaka M, Nakamura A, Matsumoto S, Katayama I. A topical combination of rapamycin and tacrolimus for the treatment of angiofibroma due to tuberous sclerosis complex (TSC): a pilot study of nine Japanese patients with TSC of different disease severity. The British Journal of Dermatology 2011;165(4):912‐6. [PUBMED: 21692771] [DOI] [PubMed] [Google Scholar]
Wataya‐Kaneda 2015 {published data only}
- Wataya‐Kaneda M, Tanaka M, Yang L, Yang F, Tsuruta D, Nakamura A, et al. Clinical and Histologic Analysis of the Efficacy of Topical Rapamycin Therapy Against Hypomelanotic Macules in Tuberous Sclerosis Complex. JAMA Dermatology 2015;151(7):722‐30. [PUBMED: 25692384] [DOI] [PubMed] [Google Scholar]
Wienecke 2006 {published data only}
- Wienecke R, Fackler I, Linsenmaier U, Mayer K, Licht T, Kretzler M. Antitumoral activity of rapamycin in renal angiomyolipoma associated with tuberous sclerosis complex. American Journal of Kidney Diseases : the official journal of the National Kidney Foundation 2006;48(3):e27‐9. [PUBMED: 16931204] [DOI] [PubMed] [Google Scholar]
Yalon 2011 {published data only}
- Yalon M, Ben‐Sira L, Constantini S, Toren A. Regression of subependymal giant cell astrocytomas with RAD001 (Everolimus) in tuberous sclerosis complex. Child's Nervous System : ChNS : official journal of the International Society for Pediatric Neurosurgery 2011;27(1):179‐81. [PUBMED: 20703486] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01289912 {published data only}
- NCT01289912. Trial of RAD001 and Neurocognition in Tuberous Sclerosis Complex (TSC). https://clinicaltrials.gov/ct2/show/NCT01289912?term=NCT01289912&rank=1 (accessed 14 March 2016).
NCT01526356 {published data only}
- NCT01526356. Topical Rapamycin to Erase Angiofibromas in TSC (Treatment). https://clinicaltrials.gov/ct2/show/NCT01526356?term=NCT01526356&rank=1 (accessed 14 March 2016).
References to ongoing studies
NCT01713946 {published data only}
- NCT01713946. A Placebo‐controlled Study of Efficacy & Safety of 2 Trough‐ranges of Everolimus as Adjunctive Therapy in Patients With Tuberous Sclerosis Complex (TSC) & Refractory Partial‐onset Seizures (EXIST‐3). https://clinicaltrials.gov/ct2/show/NCT01713946?term=NCT01713946&rank=1 (accessed 14 March 2016).
NCT01730209 {published data only}
- NCT01730209. Efficacy of RAD001/Everolimus in Autism and NeuroPsychological Deficits in Children With TuberousSclerosis Complex (RAPIT). https://clinicaltrials.gov/ct2/show/NCT01730209?term=NCT01730209&rank=1 (accessed 14 March 2016).
NCT01954693 {published data only}
- NCT01954693. A Study of Everolimus in the Treatment of Neurocognitive Problems in Tuberous Sclerosis (TRON). https://clinicaltrials.gov/ct2/show/NCT01954693?term=NCT01954693&rank=1 (accessed 14 March 2016).
NCT02635789 {published data only}
- NCT02635789. Phase III Trial of Topical Formulation of Sirolimus to Skin Lesions in Patients With Tuberous SclerosisComplex (TSC). https://clinicaltrials.gov/ct2/show/NCT02635789?term=NCT02635789&rank=1 (accessed 14 March 2016).
Additional references
Adriaensen 2011
- Adriaensen ME, Schaefer‐Prokop CM, Duyndam DA, Zonnenberg BA, Prokop M. Radiological evidence of lymphangioleiomyomatosis in female and male patients with tuberous sclerosis complex. Clinical Radiology 2011;66(7):625‐8. [DOI] [PubMed] [Google Scholar]
Ballou 2008
- Ballou LM, Lin RZ. Rapamycin and mTOR kinase inhibitors. Journal of Chemical Biology 2008;1(1‐4):27‐36. [PUBMED: 19568796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhaskar 2007
- Bhaskar PT, Hay N. The two TORCs and Akt. Developmental Cell 2007;12(4):487‐502. [PUBMED: 17419990] [DOI] [PubMed] [Google Scholar]
Bissler 2008
- Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. The New England Journal of Medicine 2008;358(2):140‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bissler 2010
- Bissler J, Henske E. Renal manifestations of tuberous sclerosis complex. In: Kwiatkowsi D, Whittemore V, Thiele E editor(s). Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim:Wiley‐Blackwell, 2010. [Google Scholar]
Brachmann 2009
- Brachmann S, Fritsch C, Maira SM, Garcia‐Echeverria C. PI3K and mTOR inhibitors: a new generation of targeted anticancer agents. Current opinion in cell biology 2009;21(2):194‐8. [PUBMED: 19201591] [DOI] [PubMed] [Google Scholar]
Canpolat 2014
- Canpolat M, Per H, Gumus H, Yikilmaz A, Unal E, Patiroglu T, et al. Rapamycin has a beneficial effect on controlling epilepsy in children with tuberous sclerosis complex: results of 7 children from a cohort of 86. Child's Nervous System : ChNS : official journal of the International Society for Pediatric Neurosurgery 2014;30(2):227‐40. [PUBMED: 23743820] [DOI] [PubMed] [Google Scholar]
Cardamone 2014
- Cardamone M, Flanagan D, Mowat D, Kennedy SE, Chopra M, Lawson JA. Mammalian target of rapamycin inhibitors for intractable epilepsy and subependymal giant cell astrocytomas in tuberous sclerosis complex. The Journal of Pediatrics 2014;164(5):1195‐200. [PUBMED: 24518170] [DOI] [PubMed] [Google Scholar]
Cheadle 2000
- Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Human Genetics 2000;107(2):97‐114. [PUBMED: 11030407] [DOI] [PubMed] [Google Scholar]
Crino 2010
- Crino P, Mehta R, Vinters H. Pathogenesis of TSC in the brain. In: Kwiatkowsi D, Whittemore V, Thiele E editor(s). Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim: Wiley‐Blackwell, 2010:159‐185. [Google Scholar]
CTCAE v3.0 [Computer program]
- National Cancer Institute. Common terminology criteria for adverse events, (CTCAE), version 3.0.. Bethesda, MD: Cancer Therapy Evaluation Program, Aug 9, 2006..
Cudzilo 2013
- Cudzilo C, Szczesniak R, Brody A, Rattan MS, Krueger DA, Bissler JJ, et al. Lymphangioleiomyomatosis screening in women with tuberous sclerosis. Chest 2013;144(2):578‐85. [DOI] [PubMed] [Google Scholar]
Dabora 2001
- Dabora SL, Jozwiak S, Franz DN, Roberts PS, Nieto A, Chung J, et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. American Journal of Human Genetics 2001;68(1):64‐80. [PUBMED: 11112665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2008
- Davies DM, Johnson SR, Tattersfield AE, Kingswood JC, Cox JA, McCartney DL, et al. Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. The New England Journal of Medicine 2008;358(2):200‐3. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomised trials. Statistics in Medicine 2002;21(19):2971‐80. [DOI] [PubMed] [Google Scholar]
ECTSC 1993
- European Chromosome 16 Tuberous Sclerosis Consortium. Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 1993;75(7):1305‐15. [PUBMED: 8269512] [DOI] [PubMed] [Google Scholar]
Eden 2014
- Eden KE, Vries PJ, Moss J, Richards C, Oliver C. Self‐injury and aggression in tuberous sclerosis complex: cross syndrome comparison and associated risk markers. Journal of Neurodevelopmental Disorders 2014;6(1):10. [PUBMED: 24822087] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehninger 2011
- Ehninger D, Silva AJ. Rapamycin for treating tuberous sclerosis and autism spectrum disorders. Trends in Molecular Medicine 2011;17(2):78‐87. [PUBMED: 21115397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins PT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials:methodological issues. International Journal of Epidemiology 2002;30(1):140‐9. [DOI] [PubMed] [Google Scholar]
Faivre 2006
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nature reviews. Drug discovery 2006;5(8):671‐88. [PUBMED: 16883305] [DOI] [PubMed] [Google Scholar]
Falotico 2005
- Falotico R. Are all sirolimus analogs alike?. Conference presentation at Angioplasty Summit; TCT Asia Pacific; 2005 Apr 28; Seoul, Korea. 2005.
Franz 2010
- Franz DN, Krueger DA, Balko MG. Subependymal Giant Cell Astrocytoma. In: Kwiatkowsi D, Whittemore V, Thiele E editor(s). Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim: Wiley‐Blackwell, 2010:211‐228. [Google Scholar]
Gao 2001
- Gao X, Pan D. TSC1 and TSC2 tumor suppressors antagonize insulin signaling in cell growth. Genes & Development 2001;15(11):1383‐92. [PUBMED: 11390358] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2010
- Glasgow CG, Steagall WK, Taveira‐Dasilva A, Pacheco‐Rodriguez G, Cai X, El‐Chemaly S, et al. Lymphangioleiomyomatosis (LAM): molecular insights lead to targeted therapies. Respiratory Medicine 2010;104 Suppl 1:S45‐58. [PUBMED: 20630348] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goncharova 2002
- Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation. A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). The Journal of Biological Chemistry 2002;277(34):30958‐67. [PUBMED: 12045200] [DOI] [PubMed] [Google Scholar]
Haemel 2010
- Haemel AK, O'Brian AL, Teng JM. Topical rapamycin: a novel approach to facial angiofibromas in tuberous sclerosis. Archives of dermatology 2010;146(7):715‐8. [PUBMED: 20644030] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7417):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinton 2014
- Hinton RB, Prakash A, Romp RL, Krueger DA, Knilans TK. Cardiovascular manifestations of tuberous sclerosis complex and summary of the revised diagnostic criteria and surveillance and management recommendations from the International Tuberous Sclerosis Consensus Group. Journal of the American Heart Association 2014;3(6):e001493. [PUBMED: 25424575] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoogeveen‐Westerveld 2012
- Hoogeveen‐Westerveld M, Ekong R, Povey S, Karbassi I, Batish SD, Dunnen JT, et al. Functional assessment of TSC1 missense variants identified in individuals with tuberous sclerosis complex. Human Mutation 2012;33(3):476‐9. [PUBMED: 22161988] [DOI] [PubMed] [Google Scholar]
Hoogeveen‐Westerveld 2013
- Hoogeveen‐Westerveld M, Ekong R, Povey S, Mayer K, Lannoy N, Elmslie F, et al. Functional assessment of TSC2 variants identified in individuals with tuberous sclerosis complex. Human Mutation 2013;34(1):167‐75. [PUBMED: 22903760] [DOI] [PubMed] [Google Scholar]
Hyman 2000
- Hyman MH, Whittemore VH. National Institutes of Health consensus conference: tuberous sclerosis complex. Archives of neurology 2000;57(5):662‐5. [PUBMED: 10815131] [DOI] [PubMed] [Google Scholar]
Inoki 2002
- Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nature Cell Biology 2002;4(9):648‐57. [PUBMED: 12172553] [DOI] [PubMed] [Google Scholar]
Johnson 2010
- Johnson SR, Cordier JF, Lazor R, Cottin V, Costabel U, Harari S, et al. European Respiratory Society guidelines for the diagnosis and management of lymphangioleiomyomatosis. European Respiratory Journal 2010;35(1):14‐26. [DOI] [PubMed] [Google Scholar]
Joinson 2003
- Joinson C, O'Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychological Medicine 2003;33(2):335‐44. [PUBMED: 12622312] [DOI] [PubMed] [Google Scholar]
Kleiner 1999
- Kleiner DE, Stetler‐Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemotherapy and Pharmacology 1999;43 Suppl:S42‐51. [PUBMED: 10357558] [DOI] [PubMed] [Google Scholar]
Kozlowska 2008
- Kozlowska J, Okon K. Renal tumors in postmortem material. Polish Journal of Pathology 2008;59:21‐5. [PubMed] [Google Scholar]
Krueger 2013
- Krueger DA, Wilfong AA, Holland‐Bouley K, Anderson AE, Agricola K, Tudor C, et al. Everolimus treatment of refractory epilepsy in tuberous sclerosis complex. Annals of Neurology 2013;74(5):679‐87. [PUBMED: 23798472] [DOI] [PubMed] [Google Scholar]
Kwiatkowski 2002
- Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el‐Hashemite N, et al. A mouse model of TSC1 reveals sex‐dependent lethality from liver hemangiomas, and up‐regulation of p70S6 kinase activity in Tsc1 null cells. Human Molecular Genetics 2002;11(5):525‐34. [PUBMED: 11875047] [DOI] [PubMed] [Google Scholar]
Kwiatkowski 2003
- Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Annals of Human Genetics 2003;67(Pt 1):87‐96. [PUBMED: 12556239] [DOI] [PubMed] [Google Scholar]
Lee 2005
- Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, et al. Efficacy of a rapamycin analog (CCI‐779) and IFN‐gamma in tuberous sclerosis mouse models. Genes, Chromosomes & Cancer 2005;42(3):213‐27. [PUBMED: 15578690] [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacology 2009;9:8. [PUBMED: 19368729] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
LOVD TSC1
- Povey S, Ekong R. Tuberous sclerosis database: Tuberous sclerosis 1 (TSC1). http://chromium.liacs.nl/LOVD2/TSC/home.php?select_db=TSC1 (accessed 14 August 2014).
LOVD TSC2
- Povey S, Ekong R. Tuberous sclerosis database : Tuberous sclerosis 2 (TSC2). http://chromium.liacs.nl/LOVD2/TSC/home.php?select_db=TSC2 (accessed 14 August 2014).
Madke 2013
- Madke B. Topical rapamycin (sirolimus) for facial angiofibromas. Indian dermatology online journal 2013;4(1):54‐7. [PUBMED: 23439391] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manning 2002
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex‐2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3‐kinase/akt pathway. Molecular Cell 2002;10(1):151‐62. [PUBMED: 12150915] [DOI] [PubMed] [Google Scholar]
McCormack 2010
- McCormack F, Henske E. Lymphangioleiomyomatosis and pulmonary disease in TSC. In: Kwiatkowsi D, Whittemore VH, Thiele EA editor(s). Tuberous Sclerosis Complex: Genes, Clinical Features, and Therapeutics. Weinheim: Wiley‐Blackwell, 2010. [Google Scholar]
Muncy 2009
- Muncy J, Butler IJ, Koenig MK. Rapamycin reduces seizure frequency in tuberous sclerosis complex. Journal of Child Neurology 2009;24(4):477. [PUBMED: 19151365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutizwa 2011
Muzykewicz 2012
- Muzykewicz D, Black M, Muse V, Numis AL, Rajagopal J, Thiele EA, et al. Multifocal micronodular pneumocyte hyperplasia: computed tomographic appearance and follow‐up in tuberous sclerosis complex. Journal of Computer Assisted Tomography 2012;36(5):518‐22. [DOI] [PubMed] [Google Scholar]
Napolioni 2008
- Napolioni V, Curatolo P. Genetics and molecular biology of tuberous sclerosis complex. Current Genomics 2008;9(7):475‐87. [PUBMED: 19506736] [DOI] [PMC free article] [PubMed] [Google Scholar]
NINDS 2013
- National Institute of Neurological Disorders and Stroke (NINDS). Tuberous sclerosis fact sheet. www.ninds.nih.gov/disorders/tuberous_sclerosis/detail_tuberous_sclerosis.htm (accessed 1 August 2013).
Northrup 2013
- Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatric Neurology 2013;49(4):234‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
OMIM#191100 [Computer program]
- Online Mendelian Inheritance in Man® ‐ National Center for Biotechnology Information. TUBEROUS SCLEROSIS 1; TSC1. Online Mendelian Inheritance in Man® ‐ National Center for Biotechnology Information, 12 February 2015 (Last update).
OMIM#613254 [Computer program]
- Online Mendelian Inheritance in Man® ‐ National Center for Biotechnology Information. TUBEROUS SCLEROSIS 2; TSC2. Online Mendelian Inheritance in Man® ‐ National Center for Biotechnology Information, 12 February 2015 (Last Update).
Osborne 1991
- Osborne JP, Fryer A, Webb D. Epidemiology of tuberous sclerosis. Annals of the New York Academy of Sciences 1991;615:125‐7. [PUBMED: 2039137] [DOI] [PubMed] [Google Scholar]
Overwater 2014
Potter 2002
- Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nature Cell Biology 2002;4(9):658‐65. [PUBMED: 12172554] [DOI] [PubMed] [Google Scholar]
Roach 1998
- Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. Journal of Child Neurology 1998;13(12):624‐8. [PUBMED: 9881533] [DOI] [PubMed] [Google Scholar]
Roach 1999
- Roach ES, DiMario FJ, Kandt RS, Northrup H. Tuberous Sclerosis Consensus Conference: recommendations for diagnostic evaluation. National Tuberous Sclerosis Association. Journal of child neurology 1999;14(6):401‐7. [PUBMED: 10385849] [DOI] [PubMed] [Google Scholar]
Rosner 2004
- Rosner M, Freilinger A, Hengstschlager M. Proteins interacting with the tuberous sclerosis gene products. Amino Acids 2004;27(2):119‐28. [PUBMED: 15351877] [DOI] [PubMed] [Google Scholar]
RxList 2015a
- RxList: The Internet Drug Index. Rapamune (Sirolimus). www.rxlist.com/rapamune‐drug.htm 30 November 2015 (accessed 28 February 2016).
RxList 2015b
- RxList: The Internet Drug Index. Torisel (Temsirolimus). http://www.rxlist.com/torisel‐drug.htm 18 March 2015 (accessed 28 February 2016).
RxList 2016
- RxList: The Internet Drug Index. Afinitor (Everolimus). http://www.rxlist.com/afinitor‐drug.htm 16 February 2016 (accessed 28 February 2016).
Sasongko 2015
- Sasongko TH, Ismail NFD, Nik Mohd Ariff NAM, Zabidi‐Hussin ZAMH. Rapamycin and its analogues (rapalogs) for tuberous sclerosis complex‐associated tumors: a systematic review on non‐randomized studies using meta‐analysis. http://www.ojrd.com/content/10/1/95 (accessed 18 August 2015). [DOI] [PMC free article] [PubMed]
Sattler 1992
- Sattler M, Guengerich FP, Yun CH, Christians U, Sewing KF. Cytochrome P‐450 3A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metabolism & Disposition 1992;20(5):753‐61. [PubMed] [Google Scholar]
Sciacca 2014
- Sciacca P, Giacchi V, Mattia C, Greco F, Smilari P, Betta P, et al. Rhabdomyomas and tuberous sclerosis complex: our experience in 33 cases. BMC cardiovascular disorders 2014;14:66. [PUBMED: 24884933] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seibert 2011
- Seibert D, Hong CH, Takeuchi F, Olsen C, Hathaway O, Moss J, et al. Recognition of tuberous sclerosis in adult women: delayed presentation with life‐threatening consequences. Annals of Internal Medicine 2011;154(12):806‐13, W‐294. [PUBMED: 21690595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shepherd 1991
- Shepherd C, Gomez M, Lie J, Crowson C. Causes of death in patients with tuberous sclerosis. Mayo Clinic Proceedings 1991;66(8):792‐6. [DOI] [PubMed] [Google Scholar]
Tanzi 2013
- Tanzi MG. Juice interactions: What patients need to know. http://www.pharmacist.com/juice‐interactions‐what‐patients‐need‐know (accessed 01 August 2014).
Tee 2003
- Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, Tuberin and Hamartin, control mTOR signaling by acting as a GTPase‐activating protein complex toward Rheb. Current Biology 2003;13(15):1259‐68. [PUBMED: 12906785] [DOI] [PubMed] [Google Scholar]
Tiberio 2011
- Tiberio D, Franz DN, Phillips JR. Regression of a cardiac rhabdomyoma in a patient receiving everolimus. Pediatrics 2011;127(5):e1335‐7. [PUBMED: 21464184] [DOI] [PubMed] [Google Scholar]
Truchuelo 2012
- Truchuelo T, Diaz‐Ley B, Rios L, Alcantara J, Jaen P. Facial angiofibromas treated with topical rapamycin: an excellent choice with fast response. Dermatology Online Journal 2012;18(1):15. [PUBMED: 22301052] [PubMed] [Google Scholar]
Tyburczy 2015
- Tyburczy ME, Dies KA, Glass J, Camposano S, Chekaluk Y, Thorner AR, et al. Mosaic and Intronic Mutations in TSC1/TSC2 Explain the Majority of TSC Patients with No Mutation Identified by Conventional Testing. PLoS Genetics 2015;11(11):e1005637. [PUBMED: 26540169] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Slegtenhorst 1997
- Slegtenhorst M, Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, et al. Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science (New York, N.Y.) 1997;277(5327):805‐8. [PUBMED: 9242607] [DOI] [PubMed] [Google Scholar]
van Slegtenhorst 1999
- Slegtenhorst M, Verhoef S, Tempelaars A, Bakker L, Wang Q, Wessels M, et al. Mutational spectrum of the TSC1 gene in a cohort of 225 tuberous sclerosis complex patients: no evidence for genotype‐phenotype correlation. Journal of Medical Genetics 1999;36(4):285‐9. [PUBMED: 10227394] [PMC free article] [PubMed] [Google Scholar]
Wataya‐Kaneda 2012
- Wataya‐Kaneda M, Tanaka M, Nakamura A, Matsumoto S, Katayama I. A novel application of topical rapamycin formulation, an inhibitor of mTOR, for patients with hypomelanotic macules in tuberous sclerosis complex. Archives of Dermatology 2012;148(1):138‐9. [PUBMED: 22250258] [DOI] [PubMed] [Google Scholar]
Wheless 2013
- Wheless JW, Almoazen H. A novel topical rapamycin cream for the treatment of facial angiofibromas in tuberous sclerosis complex. Journal of Child Neurology 2013;28(7):933‐6. [PUBMED: 23680945] [DOI] [PubMed] [Google Scholar]
Wiemer‐Kruel 2014
- Wiemer‐Kruel A, Woerle H, Strobl K, Bast T. Everolimus for the treatment of subependymal giant cell astrocytoma probably causing seizure aggravation in a child with tuberous sclerosis complex: a case report. Neuropediatrics 2014;45(2):129‐31. [PUBMED: 24293099] [DOI] [PubMed] [Google Scholar]
Yuan 2009
- Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. Journal of Hematology & Oncology 2009;2:45. [PUBMED: 19860903] [DOI] [PMC free article] [PubMed] [Google Scholar]


