Abstract
Background
Total hip arthroplasty (THA) is one of the most common orthopaedic operations performed worldwide. Painful osteoarthritis of the hip is the primary indication for THA. Following THA, people have conventionally been provided with equipment, such as raised toilet seats and chairs, and educated to avoid activities that could cause the hip joint to be in a position of flexion over 90 degrees, or adduction or rotation past the midline. These aspects of occupational therapy have been advocated to reduce the risks of prosthesis dislocation. However, the appropriateness of these recommendations has been questioned.
Objectives
To assess the effects of provision of assistive devices, education on hip precautions, environmental modifications and training in activities of daily living (ADL) and extended ADL (EADL) for people undergoing THA.
Search methods
We searched MEDLINE (1946 to April 2016), EMBASE (1947 to April 2016), the Cochrane Library including CENTRAL (Issue 4 of 12, 2016), Database of Reviews of Effects (DARE), Health Technology Assessment (HTA), Economic Evaluations Database (EED), CINAHL, PEDro and CIRRIE from inception to April 2016. In addition we checked Controlled Clinical Trials, Clinicaltrials.gov, the National Institutes of Health Trial Registry, the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and the OpenGrey database from inception to April 2016.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs that evaluated the effectiveness of the provision of assistive devices, education on hip precautions, environmental modifications, or training in ADL and EADL for people undergoing THA. The main outcomes of interest were pain, function, health‐related quality of life (HRQOL), global assessment of treatment success, reoperation rate, hip dislocation and adverse events.
Data collection and analysis
We used standard methodological procedures recognised by Cochrane. We conducted a systematic literature search using several databases and contacted corresponding authors, appraised the evidence using the Cochrane risk of bias tool, analysed the data using a narrative analysis approach (as it was not possible to conduct a meta‐analysis due to heterogeneity in interventions), and interpreted all outcomes using the GRADE approach.
Main results
We included three trials with a total of 492 participants who had received 530 THA. The evidence presented with a high risk of performance, detection and reporting bias.
One study (81 participants) compared outcomes for participants randomised to the provision of hip precautions, equipment and functional restrictions versus no provision of hip precautions, equipment or functional restrictions. Due to the quality of evidence being very low, we are uncertain if the provision of hip precautions, equipment and functional restrictions improved function measured using the Harris Hip Score at 12 month follow‐up, or health‐related quality of life (HRQOL) measured by the Short Form‐12 at four week follow‐up, compared to not providing this. There were no incidences of hip dislocation or adverse events in either group during the initial 12 postoperative months. The study did not measure pain score, global assessment of treatment success or total adverse events.
One study (265 participants; 303 THAs) evaluated the provision of hip precautions with versus without the prescription of postoperative equipment and restrictions to functional activities. Due to the quality of evidence being very low, we are uncertain if perceived satisfaction in the rate of recovery differed in people who were not prescribed postoperative equipment and restrictions (135/151 satisfied) compared to those prescribed equipment and restrictions (113/152) (risk ratio (RR) 0.83, 95% confidence interval (CI) 0.75 to 0.93; 265 participants, one trial; number needed to treat for an additional beneficial outcome (NNTB) = 7). Due to the low quality evidence, we are uncertain if the incidence of hip dislocation differed between participants provided with hip precautions with (1/152) compared to without providing equipment or restrictions post‐THA (0/151) (RR 2.98, 95% CI 0.12 to 72.59). The study did not measure pain, function, HRQOL, re‐operation rates or total adverse events.
One study (146 participants) investigated the provision of an enhanced postoperative education and rehabilitation service on hospital discharge to promote functional ADL versus a conventional rehabilitation intervention in the community. This study was of very low quality evidence. We were uncertain if the provision of enhanced postoperative education and rehabilitation improved function at six months follow‐up, when assessed using the Objective and Subjective Functional Capability Index (146 participants, one trial; P > 0.05; no numerical results provided) compared to conventional rehabilitation. The study did not measure pain score, HRQOL, global assessment of treatment success, hip dislocation, re‐operation rate or total adverse events.
Authors' conclusions
Very low quality evidence is available from single trials, thus we are uncertain if hip precautions with or without the addition of equipment and functional restrictions are effective in preventing dislocation and improving outcomes after THA. There is also insufficient evidence to support or refute the adoption of a postoperative community rehabilitation programme consisting of functional reintegration and education compared to conventional rehabilitation strategies based on functional outcomes.
Further high‐quality trials are warranted to assess the outcomes of different occupational therapy interventions both in the short and longer‐term for those who undergo THA. An assessment of the impact of such interventions on pain and restriction on personal ADL, EADL and instrumental ADL is needed, and also of functional integration‐type interventions rather than just hip precautions, equipment and restrictions.
Plain language summary
Occupational therapy after hip replacement
Background
Total hip arthroplasty (THA) is a common surgical procedure for the treatment of pain and disability cause by osteoarthritis. Following THA, people have usually been provided with equipment, such as raised toilet seats and chairs, and educated to avoid activities that could cause the hip joint to be in a position of bending, twisting or where people cross their legs. These interventions aim to reduce the chances of dislocating the new hip, which is a painful and disabling event. This advice and equipment provision is often led by occupational therapists after a THA. We wanted to find out whether these types of treatments improve a person's recovery following a THA.
Study characteristics
This Cochrane review is current to 29 April 2016. We searched the available evidence and included three studies, which had 492 people who had received a THA. Two of these studies investigated providing people with equipment, such as raised toilet seats and rails, and restricting their body movements (one of these studies also provided people with physiotherapy). One study investigated teaching participants about doing certain activities of daily living in a safe way to promote self‐care without the risk of dislocating the new hip. The interventions were different and thus we did not combine the results.
Key results
One study compared outcomes for participants randomised to the provision of hip precautions, equipment and functional restrictions versus no provision of hip precautions or equipment or functional restrictions. This is the main comparator in the review.
Health‐related quality of life (lower scores mean better quality of life)
We cannot tell from our results whether the intervention has an important effect on health‐related quality of life (no numerical results provided) because the sample size was small and the study design flawed.
Function
We cannot tell from our results whether the intervention has an important effect on functional outcomes (no numerical results provided) because the sample size was small and the study design flawed.
Complications and adverse events
There were no dislocations or adverse events.
Outcomes of interest not measured
Pain, treatment success and re‐operation rate were not measured.
Quality of the evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of the evidence. Due to issues relating to the small number of participants, size of studies and study conduct, including poorly blinding assessors to group allocation, we rated the quality of the evidence as 'very low'. Further research is highly likely to change the conclusions drawn from these results. We are uncertain whether the interventions improved outcomes.
Summary of findings
Background
Description of the condition
Total hip arthroplasty (THA) surgery involves replacement of the femoral head and acetabular components of the diseased hip joint with a new artificial joint that replicates the function of the hip. Usually, the prosthetic hip is constructed from either metal, plastic, ceramic materials or a combination. Although some THA surgery is performed following traumatic hip injuries, most THA surgery is for degenerative hip diseases and is planned in advance. This is termed ‘elective’ surgery.
THA is one of the most common orthopaedic operations performed worldwide. In 2013, the National Joint Registry for England and Wales recorded 89,945 THAs (National Joint Registry 2014). Of these, 80,194 were primary (first time) procedures and 9751 were revision (replacement of the prosthesis) surgeries. In 2012, the Swedish Joint Registry recorded that 18,261 THA procedures were performed, of which 15,978 were primary and 2283 were revisions (Swedish JRU 2013). Similarly, 44,308 (primary THA) and 2767 (revision THA) were performed in Canada from 2012 to 2013 (Canadian Joint Replacement Registry 2014) and over 193,000 THAs per annum in the USA (Graver 2010).
Osteoarthritis is the principal indication for THA, and accounts for between 83% (Swedish JRU 2013) to 93% (National Joint Registry 2014) of all primary THA procedures. With an ageing population, increasing rates of obesity and increasing quality of life expectations, the annual increase in operative rates is likely to continue (Birrell 1999; Kurtz 2007). Although THA is considered to be one of the most effective orthopaedic procedures performed for relieving pain and improving the quality of people’s lives (Hawker 2006; McMurray 2000; NICE 2000), its provision carries substantial associated costs. For example, in the USA, the cost in 2006 for THA was estimated as USD 5 billion, of which 70% of the costs related directly to hospital stay (Graver 2010). Although costs in other developed counties are lower, they are still substantial (Sigurdsson 2008). The high cost of the hospitalisation phase has resulted in a drive by healthcare providers to reduce the overall length of stay (Cookson 2011). As a result of this decreased length of stay, increased emphasis needs to be placed on pre‐admission education services, efficient discharge planning and immediate postoperative rehabilitation (Westby 2006).
Description of the intervention
Occupational therapists use purposeful activity or interventions designed to help people perform activities of daily living (ADL) at home or at work (AOTA 1994). For people undergoing THA, the interventions provided by occupational therapists generally aim to improve function and prevent dislocation following THA. These have been categorised as the following.
Provision of assistive devices designed to assist ADL (such as raised toilet seats, furniture raises, dressing aids, perching stools, long‐handled reaches and commodes).
Postoperative education in joint protection by advising on following 'hip precautions' that is, avoiding specific movements such as hip flexion beyond 90°, hip adduction beyond the midline, and internal and external rotation of the hip beyond 20° from neutral (Lucas 2008).
Environmental modifications (removal of trip hazards, layout of furniture to improve access around the home, installation of handrails or grab rails).
Training to improve basic ADL, such as washing, dressing, feeding and toileting.
Training to improve extended ADL (EADL) or instrumental ADL (IADL) (e.g. cooking, household activities, leisure pursuits and community engagement).
Provision of specific advice about coping strategies to manage pain.
Provision of specific advice on how to access other services for support following THA (e.g. access to other professional services for mental well‐being).
All these interventions may be provided preoperatively or postoperatively, or both, and may be delivered in acute hospitals, or in community or primary care.
It has been recommended that postoperative rehabilitation following THA should be delivered by multidisciplinary teams (Tian 2010). This has become common practice within Western Europe, the USA and Australasia (De Jong 2009; Grotle 2010; Tian 2010). However, it remains unclear whether this occurs in less developed nations that do not have access to occupational therapy as a specific profession (Fudge 1992; Krefting 1992; Wilson‐Braun 1992). Consequently, physiotherapists or nurses may administer the provision of hip precaution equipment and functional training rather than only by occupational therapists. Therefore, we reflected this potential variability in the professional group who provides these interventions in the inclusion criteria of this Cochrane review.
How the intervention might work
Although the overall aims of occupational therapy interventions may vary and are patient‐centred, in this context their general aim is to: empower people and reduce anxiety through education, provide advice postoperatively, maximise independence through training in EADL and IADL skills with a graded approach dependent on peoples' capabilities during their recovery, and enhance participation with increased functional capability through advice, training and preparation for hospital discharge (Orpen 2010). A variety of interventions may be used to reduce the risk of prosthesis dislocation. These can include education on which specific movements should be avoided to reduce the risk of prosthesis dislocation, and the provision of equipment such as raised toilet seats, furniture raises, perching stools and long‐handled reaches to avoid hip flexion over 90° (Drummond 2012). The assessment and provision of environmental adaptations, such as removal of trip hazards, evaluation of the layout of furniture and installation of handrails or grab rails, may be useful to reduce the risk of falls and facilitate functional capability during the recovery period (Pighills 2011).
Why it is important to do this review
A recent survey of occupational therapists working in orthopaedic settings in the UK reported that, on average, people who have had THA comprise 40% of their caseload, despite a paucity of evidence on the clinical or cost‐effectiveness of occupational therapy interventions (Drummond 2012). Most reviews to date that investigate rehabilitation following THA have focused predominantly on physiotherapy, exercise, preoperative education or multidisciplinary rehabilitation programmes (Ackerman 2004; Coudeyre 2007; Dauty 2007; Di Monaco 2009; Kuster 2002). Previous Cochrane systematic reviews that have addressed preoperative education (McDonald 2014) and multidisciplinary rehabilitation programmes (Khan 2008) specifically excluded unidisciplinary interventions and included studies that contained both THA and knee arthroplasty populations. Furthermore, a protocol for a review of postacute physiotherapy for THA patients is awaiting publication (Westby 2006). However, no review of the postoperative occupational therapy interventions for people following THA has been undertaken. Steultjens 2005, who assessed the efficacy of occupational therapy for different conditions, reiterated this. Steultjens 2005 concluded that no reviews have been undertaken on occupational therapy rehabilitation for people following THA.
Therefore, despite endorsements in the UK by NICE (NICE 2003) and the British Orthopaedic Association (BOA) (British Orthopaedic Association 2006) for the provision of assistive devices as a key aspect of occupational therapy in THA rehabilitation, there has been no specific assessment of the evidence‐base to underpin these recommendations. As a result, existing protocols on occupational therapy management following THA have been based on clinical experience, surgeon preference or anecdotal reports (Westby 2006). The UK College of Occupational Therapists recognised the limitations in practice guidelines and subsequently recently released their first clinical guidelines on this topic (College of Occupational Therapists 2012). They recommend the application of the interventions mentioned above, but acknowledge the paucity of literature that evaluates the effectiveness of these interventions for people after THA.
Objectives
To assess the effects of provision of assistive devices, education on hip precautions, environmental modifications and training in activities of daily living (ADL) and extended ADL (EADL) for people undergoing THA.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) (individual and cluster) and quasi‐RCTs. Quasi‐RCTs are those where the generated sequence to allocate participants is not strictly random, for example by hospital number. We excluded non‐RCTs. We did not place any restrictions on the inclusion of studies based on the language that papers are published in or the publication status of studies.
Types of participants
We included participants who underwent primary THA surgery for osteoarthritis or revision THA. If we had excluded studies that included a few participants who received a THA for reasons other than osteoarthritis, this may have limited the information available for inclusion in this review. Therefore, we included studies if most participants (over 80%) who underwent THA surgery for osteoarthritis. We included trials that included various pathologies and various orthopaedic surgeries (that is total knee arthoplasty, hip resurfacing, hemi‐arthroplasty) if the study authors presented results for THA for osteoarthritis. We considered all types of prostheses, fixation methods and surgical approaches for inclusion.
Types of interventions
We included studies that examined one or more of the following interventions.
Provision of and education about using assistive devices for preventing dislocation. Such assistive devices included: raised toilet seats, furniture raises, dressing aids, perching stools, long‐handled grabbers and commodes.
Postoperative education about hip precautions and specifically on teaching joint positions associated with joint dislocation (hip flexion beyond 90°, adduction beyond the midline, and to avoid internal and external rotation beyond 20° from neutral (Lucas 2008)).
Environmental modifications such as: removal of trip hazards; amended layout of furniture to improve access around the home; amended layout of specific rooms such as bathrooms, the kitchen and bedroom; and installation of handrails or grab rails.
Assessment, facilitation, practice and re‐assessment of self‐care activities of daily living (ADL) tasks to foster independence and skills in these activities.
Training of extended ADL (EADL) or (also known as) instrumental ADL (IADL) as these skills are aimed at improving health‐related quality of life (HRQOL). This may have included specific training to facilitate activities beyond personal or self‐care ADL and may therefore have included activities such as gardening, shopping and social pursuits.
Provision of specific advice about coping strategies to manage pain and activity pacing.
Postoperative education sessions designed to inform participants of their expected pathway from the operative procedure to recovery at home to reduce anxiety and improve preparation for hospital discharge, and specific advice on how to access other services for support following THA (e.g. access to other professional services).
We included studies where these interventions were applied postoperatively, either in a healthcare setting or in any community setting. Also we included trials that looked at complex packages of care delivered by multidisciplinary teams if we could independently evaluate the effect of the occupational therapy interventions. We included studies if therapy assistants provided interventions under the supervision of qualified occupational therapy staff. We accepted interventions which were provided by healthcare staff other than designated occupational therapists, ensuring that they were commensurate with accepted occupational therapy practice. One review author (AD) assessed any studies of this nature to ensure the intervention met accepted occupational therapy practice.
We included occupational therapy interventions provided as part of a multidisciplinary package if the study authors adequately described the nature of the occupational therapy intervention and we could independently assess the outcome or, if it could not be isolated, the occupational therapy aspects of the study constituted more than 75% of the time allocated to the whole multidisciplinary intervention package. If we could not isolate the nature of the occupational therapy intervention, or it formed less than 75% of the overall intervention package, we excluded the study. We did not include trials that investigated education interventions provided preoperatively since another Cochrane review has investigated this (McDonald 2014).
Comparison interventions included the following.
Rehabilitation therapy excluding the interventions of interest (assistive devices, hip precautions, environmental modifications).
No rehabilitation therapy provided.
One intervention of interest versus another.
Types of outcome measures
Major outcomes
Pain as measured with tools such as a visual analogue or rating scale, or formal tools such as the McGill Pain Questionnaire (Melzack 1971).
Function, as measured by WOMAC function (Bellamy 1988); Oxford Hip Score (Dawson 1996); Harris Hip Score (Harris 1969); Short Form (SF)‐36 Physical Component Score (Stewart 1988); SF‐12 (Ware 1996); Health Assessment Questionnaire (Fries 1980); Objective Functional Capability Index (OFCI) and Subjective Functional Capability Index (SFCI).
HRQOL (e.g. SF‐36 (Stewart 1988), SF‐12 (Ware 1996), Frenchay Activities Index (Schuling 1993), EuroQoL, Nottingham Health Profile (NHP) (Hunt 1980)).
Global assessment of treatment success.
Hip dislocation, as reported (e.g. the number of participants requiring a manipulation under anaesthetic to reduce a dislocated hip prosthesis, or the requirement of a revision procedure due to recurrent hip dislocation).
Reoperation rate.
Total adverse events (e.g. infection, thrombosis, falls).
We reported the major outcomes using a 'Summary of findings' table.
Minor outcomes
Limitations in personal ADL during the initial six weeks, which are defined as the basic activities that everyone undertakes to maintain a personal level of care (e.g. feeding, toileting, washing, bathing, transfer in and out of bed or on/off a chair, mobilising). Personal ADL may be assessed using instruments such as the Barthel Score (Collin 1988) or Iowa Level of Assistance Score (Shields 1995).
Restrictions in performance in extended ADL (EADL) or instrumental ADL (IADL), which are defined as the skills required to live independently and manage a dwelling (e.g. preparing own meals, doing housework, managing own money, shopping). This may be assessed using instruments such as the Oxford Hip Score (Dawson 1996) or the Nottingham extended ADL scale (Nouri 1987).
Societal reintegration or discretionary activities. These are the higher function activities such as driving, using local services, using public transport, socialising with friends, attending social or cultural events. This outcome measure differs from HRQOL measures since this outcome specifically relates to social interaction and participation activities rather than more generic ADL, which are captured through the HRQOL outcomes.
Length of hospital stay following THA.
Cost‐analysis. This includes specific occupational therapy costs, overall rehabilitation costs, or overall hospital costs.
Minor outcomes are reported in 'Additional tables'.
There is wide variation in outcome measures that assess ADL, EADL and IADL, quality of life (QOL) and pain. We analysed all validated outcome measures. The review team decided by consensus to analyse or reject non‐validated measures. We decided to reject or accept non‐validated measures before we examined the trial results.
Follow‐up time points
It is common in rehabilitation trials for outcome data to be collected at multiple follow‐up time points. If included trials measured outcomes at more than one time point, we categorised the follow‐up time points as follows.
Short term (less than six weeks following THA surgery).
Intermediate term (six weeks to six months following THA surgery).
Long term (greater than six months following THA surgery).
In the case of multiple time points within a category (e.g. four‐week and five‐week measurements in the short term category), we extracted the last time point (that is five weeks). For the 'Summary of findings' table, we chose the final time‐point reported for each comparison for each primary outcome measure.
Search methods for identification of studies
Electronic searches
We designed a sensitive search strategy to retrieve observational studies from electronic bibliographic databases. We identified items from the following databases on 29 April 2016.
MEDLINE via OVID (1946 to 29 April 2016).
EMBASE via OVID (1947 to 29 April 2016).
Cochrane Library via Wiley (Issue 4 of 12, 2016) including the CENTRAL, Database of Reviews of Effects (DARE), Health Technology Assessment (HTA), Economic Evaluations Database (EED).
CINAHL via EbscoHost (Cumulative Index to Nursing and Allied Health Literature).
PEDro (Physiotherapy Evidence database) via http://www.pedro.org.au/.
CIRRIE (Centre for International Rehabilitation Research Information and Exchange) via http://cirrie.buffalo.edu/database/.
We have presented the electronic search strategy for each search strategy in Appendix 1.
We searched the reference lists of included articles to ascertain if any relevant trials had not been identified by the electronic searches. We searched for ongoing trials through the following trials registers and their respective websites on 29 April 2016: Controlled Clinical Trials (www.controlled‐trials.com), the National Institutes of Health Trial Registry (http://clinicaltrials.gov) and the World Health Organiziation International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch/). Also, we used the OpenGrey database to identify relevant grey literature (http://www.opengrey.eu/).
Searching other resources
We searched conference abstracts from the European League Against Rheumatism (EULAR) and the Society of Research in Rehabilitation (SRR) to identify other unpublished studies from the earliest abstract archive (2005 and 2001 respectively) to the present. We checked the citations of key articles using the Web of Science citation search facility. We contacted national and international experts in occupational therapy orthopaedic research for any information regarding ongoing studies, published data unavailable electronically or unpublished work.
Data collection and analysis
Selection of studies
Two review authors (TS and GS) independently screened all titles and abstracts identified from the search against the selection criteria. They independently selected studies as possibly relevant (those that met the criteria and those where insufficient information was provided to definitively exclude studies based on title and abstract) and excluded those that clearly did not meet the inclusion criteria. We obtained the full‐text papers for all studies deemed possibly relevant. Two review authors (TS and GS) independently assessed whether they met the selection criteria. If necessary, they contacted the study authors for further information to determine if the study met the inclusion criteria. We consulted a researcher and registered occupational therapist (AD) about any uncertainty on occupational therapy involvement in the study. If they could not reach agreement about suitability of a study for inclusion, a third review author (AD) resolved this. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (TS and GS) independently extracted data from the full‐text paper of each included study. They recorded this on pre‐prepared data extraction forms. They extracted data on: setting (geographical location of study: acute hospital, rehabilitation hospital, community or domiciliary), population characteristics (age, gender, co‐morbidities), nature of the intervention and control (pre‐ or postoperative, or both; multidisciplinary or occupational therapy only), number and duration of participant contacts, nature of occupational therapy intervention, sample size, outcome measures used and timing of follow‐up assessments. We based the extracted 'Risk of bias' data on the domains itemised in the Cochrane 'Risk of bias' tool (Higgins 2011) detailed below. Two review authors (TS and GS) appraised each included study. They resolve any disagreements by consensus decision. If disagreement persisted, they consulted one of the three expert review authors (CS, ED or AB). We discussed any disagreement that specifically surrounded occupational therapy practice with the occupational therapy expert (AD) first before arbitration by the expert review authors. We attempted to contact the study authors and ask them to provide additional data and to clarify methods if insufficient detail was in the published report. We contacted all corresponding study authors by email to request verification on data extracted and missing measurements of variance (such as standard deviation (SD) values). However, none responded.
We established a priori decision rules to assist in the selection of which data to extract in the event of multiple outcome reporting.
Where trial authors reported outcomes for more than one pain score, we extracted data on the scale highest on the following list: (i) visual analogue or rating scale; (ii) formal tools such as the McGill Pain Questionnaire; (iii) any other pain score.
Where trial authors reported outcomes for more than one function scale, we extracted data on the scale that was highest on the following list: (i) WOMAC function; (ii) Oxford Hip Score; (iii) Harris Hip Score; (iv) SF‐36 Physical Component Score; (v) Health Assessment Questionnaire; (vi) any other function scale.
Where trial authors reported outcomes for more than one limitation in personal ADL score, we extracted data on the scale highest on the following list: (i) Iowa Level of Assistance Score; (ii) Barthel Score; (iii) any other personal ADL score.
Where trial authors reported outcomes for more than one HRQOL scale, we extracted data on the scale highest on the following list: (i) SF‐36; (ii) SF‐12; (iii) Frenchay Activities Index; (iv) EuroQoL; (v) Nottingham Health Profile; (vi) any other HRQOL scale.
Where trial authors reported outcomes for more than one limitation to extended ADL score, we extracted data on the scale highest on the following list: (i) Oxford Hip Score; (ii) the Nottingham extended ADL scale; (iii) any other extended ADL score.
If the study authors reported both final values and change from baseline values for the same continuous outcome, we used final scores rather than change from baseline scores.
If the study authors reported both unadjusted and adjusted values for the same outcome, we reported the unadjusted values but also extracted adjusted values for sensitivity analyses.
If data were analysed based on an intention‐to‐treat (ITT) sample and another sample (e.g. per protocol, as treated), we reported the ITT sample but also extracted the per protocol or as treated sample and analysed the results as a sensitivity analysis.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool (Higgins 2011) to assess the quality of the included studies. We assessed the following domains.
Random sequence generation.
Allocation concealment.
Blinding of outcome assessment.
Incomplete outcome data.
Selective reporting.
Other potential sources of bias such as: whether a potential source of bias was related to the specific study design; whether a trial stopped early due to some data‐dependent process; whether there were extreme baseline imbalances; and whether the trial has been claimed to be fraudulent (Higgins 2011).
In rehabilitation trials it is not usually possible for the participants or the study personnel to remain blinded to the intervention. However, we evaluated the ‘blinding of participants and personnel’ domain as the study may still be subject to performance bias even if it is not possible to blind the participants. Blinding of the outcome assessors is practicable and is considered highly important when using subjective outcomes (Boutron 2006). Furthermore, we separately assessed blinding of self‐reported subjective outcomes (such as pain, function, HRQOL) and blinding of independent outcome assessors of objective outcomes (such as re‐operation rate, adverse events).
Two review authors (TS and GS) independently assessed the risk of bias of the study for each domain and rated this as either at low, high or unclear risk of bias. If they were unable to agree, they consulted a third review author (CS).
Measures of treatment effect
We based our analyses on the ITT data from the included studies. We planned to express dichotomous outcome data (such as frequency of prosthesis dislocation, adverse events) as risk ratios (RR) with 95% confidence intervals (CIs) and continuous outcomes (such as the visual analogue pain score, Oxford Hip Score, McGill Pain Questionnaire) as mean differences (MDs) with 95% CIs for continuous outcomes if study authors used the same scale to measure the same outcome across studies. Where study authors used different scales to measure the same outcome, we planned to use the standardised mean difference (SMD) with 95% CIs. To enhance interpretability of results, we planned to back‐transform pooled SMDs to a representative original scale, highest on the prior hierarchy of outcomes reported, by multiplying the SMD and 95% CI values by a representative SD at baseline from one included trial.
Unit of analysis issues
We determined the unit of analysis as the participant, and a single measurement for each outcome from each participant was analysed. Therefore, we analysed participants who had bilateral THA as a single measurement. In the event of a study not presenting data by the individual participant, we contacted specific corresponding study authors to obtain these data at a participant rather than a THA unit level. In the event of trials with more than two treatment arms, we only extracted data from those interventions that related to the interventions of interest in this Cochrane review.
Dealing with missing data
We attempted to contact all authors of studies to obtain any missing data, and to gather all data to perform an ITT analysis. Due to difficulties in obtaining data and particularly ITT data, this was not possible. For dichotomous outcomes, we used the number of participants allocated to each group as the denominator for all analyses. For missing data, we assumed that all participants had the worst possible outcome. For continuous outcomes with no SDs reported, we planned to calculate these from standard errors, CIs or P values if reported. If it was not possible to calculate SDs, we first planned to use baseline SDs; if this was not possible, we planned to impute SDs from other included THA studies.
Assessment of heterogeneity
First we assessed all included trials for clinical homogeneity in terms of participants, interventions and comparators by a consensus decision. As stated, all included studies were heterogenous for the interventions under investigation. We planned to assess all studies we judged to be homogeneous for the potential statistical variability of the treatment effects due to heterogeneity via calculation of the I² statistic. This measure describes the percentage total variation across studies that results from heterogeneity rather than chance. We used the following guidelines for interpretation (Deeks 2011): 0% to 40% may be unimportant; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% considerable heterogeneity. We analysed the content of the occupational therapy interventions in the included studies and matched them to one or more of the categories listed in the 'Types of interventions' section.
We planned to combine studies for analysis in the following way.
Studies that contained the same intervention only with a common comparator.
Studies that combined training for basic ADL with training for EADL or IADL.
Complex occupational therapy interventions that contained intervention components which aimed to address specific treatment needs, e.g. increasing ADL, social reintegration and sleep hygiene.
Assessment of reporting biases
We searched the WHO ICTRP (http://apps.who.int/trialsearch/) to evaluate if selected reporting of outcomes was present (outcome reporting bias) by comparing outcomes specified in trial protocols with the outcomes reported in the corresponding trial publications. We decided a priori that if we had included 10 or more studies in the meta‐analyses, we would examine the data for reporting bias via visual inspection of a funnel plot. We planned to assess the presence of small study bias in the overall meta‐analysis by checking if the random‐effects model estimate was more beneficial than the fixed‐effect model estimate (Sterne 2011). Since we could not perform any meta‐analysis, we did not report this.
Data synthesis
We analysed data using Review Manager (RevMan) (RevMan 2014). We planned to combine data from individual trials for meta‐analyses if the interventions, participant groups and outcomes were sufficiently similar. We determined this by a consensus decision amongst the review authors. We did not report the results of any meta‐analysis we undertook if the I² statistic was greater than 75%. We planned to use a random‐effects model as the default analytical methodology.
Due to the heterogeneity of the interventions identified, it was inappropriate to undertake a meta‐analysis. We therefore adopted a narrative approach to data synthesis. We presented the results of the review separately by intervention to assess the effectiveness of each intervention.
We identified the following comparisons.
Provision of hip precautions, equipment, functional restrictions and outpatient physiotherapy versus outpatient physiotherapy without provision of hip precautions, equipment or functional restrictions.
Provision of hip precautions with versus without postoperative equipment and functional restriction.
Provision of an enhanced postoperative education and rehabilitation service with conventional hospital discharge to promote functional ADL versus a conventional traditional discharge and rehabilitation intervention in the community.
'Summary of findings' table
We presented the primary outcomes in a 'Summary of findings' table, which provides key information concerning the quality of the evidence, the magnitude of effect of the interventions examined, and the sum of available data measuring changes in all outcomes, as recommended by Cochrane (Schünemann 2011a). The outcomes were: (i) pain; (ii) function; (iii) HRQOL; (iv) global assessment of treatment success; (v) reoperation rate; (vi) hip dislocation; and (vii) adverse events (including infection, thrombosis, falls). The 'Summary of findings' table included an overall assessment of the quality of the evidence related to each primary outcome using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which assesses study limitations, consistency of effect, imprecision, indirectness and publication bias (Schünemann 2011b). For all outcomes, we included data for the latest time point available.
For dichotomous outcomes, such as adverse events, we planned to calculate the number needed to treat from the control group event rate and the relative risk using the visual treatment number needed to treat for an additional beneficial outcome (NNTB) calculator (Cates 2008). We planned to calculate the NNTB for continuous measures using the Wells calculator (available at the Cochrane Musculoskeletal Group (CMSG) Editorial office, http://musculoskeletal.cochrane.org/). However, we were unable to calculate this due to the limited data available.
For dichotomous outcomes, we planned to calculate the absolute risk difference using the risk difference statistic in RevMan (RevMan 2014) and to express the result as a percentage. For continuous outcomes, we planned to calculate the absolute benefit as the improvement in the intervention group minus the improvement in the control group, in the original units.
We planned to determine the relative per cent change for dichotomous data as the risk ratio ‐ 1 and expressed as a percentage. For continuous outcomes, we planned to calculate the relative difference in the change from baseline as the absolute benefit divided by the baseline mean of the control group. However, we were unable to calculate this as none of the included papers presented baseline data.
Subgroup analysis and investigation of heterogeneity
We planned to conduct three subgroup analyses based on sufficient numbers of trials being available. Whilst we had planned the following subgroup analyses, there were insufficient data to perform the analyses.
Primary versus revision THA procedure.
Delivery of the intervention by occupational therapists or other health professionals.
Comparison of multiple interventions (e.g. assistive devices plus hip precautions plus environmental modifications) versus single interventions alone.
Sensitivity analysis
We planned a sensitivity analysis to assess the effect of adequate allocation concealment on the treatment effect for the main outcome measurements. Removal of trials identified in the 'Risk of bias' section as having inadequate or unclear allocation concealment from the meta‐analyses may change the overall treatment effect. We planned to perform a sensitivity analysis to analyse the effect of adequate blinding of self‐reported subjective outcomes (e.g. pain, function, HRQOL) on treatment effects. However, there were insufficient data to perform any of the planned sensitivity analyses.
Results
Description of studies
We have presented a summary of the included and excluded studies in the 'Characteristics of included studies' and 'Characteristics of excluded studies' tables.
Results of the search
We have presented the search strategy results in Figure 1. From the search strategy, we identified a total of 4736 citations after removal of duplicates. We found no studies after we searched conference abstracts from the European League Against Rheumatism (EULAR) and the Society of Research in Rehabilitation (SRR). We checked the citations of key articles, as determined by articles specifically providing clinical recommendations and national guidelines on hip precautions and equipment provision post‐total hip arthroplasty (post‐THA), including College of Occupational Therapists 2012 and Drummond 2012, using the Web of Science citation search facility.
1.
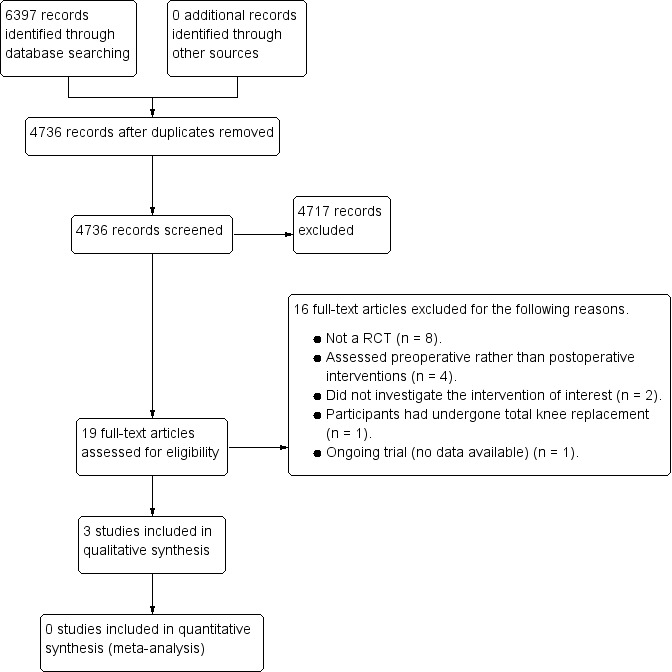
Study flow diagram.
Of the identified articles, we considered 19 citations potentially eligible after screening the titles/abstracts. After we reviewed the full‐text articles to re‐assess eligibility, 15 papers did not satisfy the eligibility criteria. Three papers did and we included them in the review. One study is currently ongoing (Peters 2015), and we have summarised it in the 'Characteristics of ongoing studies' table.
We contacted all three corresponding authors from each included study for additional information. None responded after repeated attempts.
Included studies
The three included trials randomised a total of 492 participants (530 THAs). This consisted of 287 participants who received an 'experimental' rehabilitation approach following THA and 242 participants who received a control or 'usual treatment' approach following THA. Two trials were conducted in the USA (Peak 2005; Ververeli 2009), whilst Wong 1990 was undertaken in Canada.
Participant characteristics
Surgery
All participants underwent THA. Peak 2005 and Ververeli 2009 described this as an uncemented procedure, whilst Wong 1990 did not document the method of prosthesis fixation. All participants in the Peak 2005 cohort underwent an anterolateral surgical approach, whilst a modified anterolateral approach was adopted in the Ververeli 2009 cohort. Wong 1990 did not document the surgical approach for their participants. Whilst Wong 1990 did not document whether this was a primary or revision procedure, all participants were primary THA in Peak 2005 and Ververeli 2009 cohorts. Ververeli 2009 used a 32, 36 to 40 mm femoral head in their cohort. The most common femoral head size used in the Peak 2005 cohort was a 28 mm (range 22 mm to 36 mm). Wong 1990 did not document this. The required position of acetabular component anteversion was 10° in Ververeli 2009 and between 10° and 15° in Peak 2005. Wong 1990 did not document this.
Gender
In total the three included studies randomised 243 males and 249 females.
Age
The mean age of the cohort groups ranged from 57.4 years (Ververeli 2009) to 71.1 years (Wong 1990).
Body Mass Index (BMI)
Two studies presented data on baseline BMI (Peak 2005; Ververeli 2009). Mean BMI ranged from 27.8 kg/m² in females in the control group of Ververeli 2009 to 29.8 kg/m² for males in the control group of Ververeli 2009. Mean BMI was 29.3 kg/m² in in the control group and 28.7 kg/m² in the experimental (unrestricted hip movements) in Peak 2005.
Indication for THA
Only Wong 1990 presented data on the indication for THA. In this cohort the most frequent reason for THA was osteoarthritis of the hip in 87% to 92% of cases dependent on group allocation. The remaining reasons were due to rheumatoid arthritis and fractured neck of femur in up to 8% of cases.
Co‐morbidities
Only Wong 1990 presented data on the frequency of co‐morbidities. Whilst the study did not indicate what co‐morbidities the cohort reported, Wong 1990 documented that, dependent on group allocation, between 30% to 32% of their groups presented with a concurrent medical condition.
Intervention
The three included studies investigated three interventions.
The provision of hip precautions, equipment and functional restriction versus no provision of hip precautions or equipment or functional restrictions (Ververeli 2009).
The provision of hip precautions with versus without postoperative equipment and functional restrictions (Peak 2005).
The provision of an enhanced postoperative education and rehabilitation service with early hospital discharge to promote functional activities of daily living (ADL) versus a conventional traditional discharge and rehabilitation intervention in the community (Wong 1990).
All people in the intervention group in Ververeli 2009 received instruction on hip precautions (avoid hip flexion greater than 90°, avoid crossing the legs at the thighs, avoid riding in a car, provision of an elevated toilet seat and elevated chair, instructed to sleep supine with a pillow between their legs) plus home physiotherapy. During the second and third postoperative month, participants were permitted to ride in a car and attend an outpatient physiotherapy programme, but were instructed to maintain their hip precautions with no hip flexion greater than 90° or adduction greater than 5°. All people in the comparator group received no specific hip precautions (except an instruction to avoid crossing legs at the thighs) or postoperative equipment, such as toilet raises, chair raises or abduction pillows, and received outpatient physiotherapy. The studies did not report participant deviation from group allocation or compliance to interventions allocated to either group. The studies did not report on the outpatient physiotherapy treatment received by either group.
In Peak 2005, all participants allocated to the intervention group received an abduction pillow, elevated toilet seats and elevated chairs, and instructions to avoid sleeping on their side, avoid driving or being a passenger in an automobile plus hip precautions, which consisted of limiting hip range of motion for the initial six weeks to less than 90° flexion, 45° external and internal rotation and to avoid hip adduction versus the comparator group who received hip precautions alone. Peak 2005 provided self‐reported data on participant compliance to equipment and functional restriction during the initial six postoperative weeks. Whilst they reported 100% compliance for the use of a postoperative abduction pillow and 96% compliance to range of movement restriction, compliance for the use of elevated toilet seats (78%) and elevated chairs (56%), and for avoiding being a passenger in an automobile (34%) was lower.
Whilst both Peak 2005 and Ververeli 2009 investigated the use of hip precautions and equipment, all participants in Ververeli 2009 also received outpatient physiotherapy whereas no participants in Peak 2005 received this co‐intervention. Accordingly, we deemed it inappropriate to pool the data from these two studies as this intervention may have had a significant impact on outcomes.
Wong 1990 randomised participants to one of three groups. For the purposes of this Cochrane review on hip precautions and equipment, we determined that the two groups who received a conventional inpatient discharge pathway were the groups of interest. From these two groups, the intervention group received a supportive discharge intervention consisted of patient information delivered by a pamphlet and videotape, and community nurse review. The pamphlet and videotape provided information on the safe performance of ADL to avoid hip dislocation, postdischarge exercises, early detection of complications, safe and correct use of aids for walking, bathing, dressing and toileting, expected stages of progress during the first six months, and the potential impact of the operation on the participant's perception and the availability of community resources. In addition participants received regular postdischarge home visits by a community health nurse. The objective of this visit was to procure walking and ADL aids as appropriate, counsel the participant on planning and implementing changes, provide supportive actions and reinforce teaching initiated in the hospital. These visits were during the fifth or sixth postoperative day in hospital, on the day before discharge, and then one week and three and six months postdischarge at the participant's home. The comparator group received a traditional rehabilitation programme after a conventional inpatient discharge pathway without this supportive discharge regime. No data were provided on participant deviation from group allocation or on compliance to interventions allocated to either group.
Outcome measures
The follow‐up periods ranged from six months (intermediate term: Peak 2005; Wong 1990) to 12 months (longer‐term; Ververeli 2009).
The primary outcomes reported were: function as assessed with the Harris Hip Score in Ververeli 2009 and Wong 1990 using the Objective Functional Capability Index (OFCI) and Subjective Functional Capability Index (SFCI); two studies assessed global assessment of treatment success using patient satisfaction (Peak 2005; Ververeli 2009); hip dislocation (Peak 2005); and Ververeli 2009 recorded the frequency of complications. This included the incidence of hip dislocation as well as more general complications, such as infection, thrombosis and falls. No studies assessed the primary outcomes: pain, health‐related quality of life (HRQOL) or reoperation rates.
The secondary outcomes reported were: presence of a limp on observation of gait pattern, the number of days until participants walked without a limp (Ververeli 2009), and the frequency of return to ADL at follow‐up interval (Peak 2005), which were considered limitations in personal ADL. Ververeli 2009 also assessed the number of days until participants walked with a stick, the number of days until participants walked without a stick, the number of days until participants drove and the number of days required before participants walked without a limp (Peak 2005) percentage of their usual ADL which participants could perform at the time of assessment (Peak 2005). The frequency to which participants had returned to work was considered as a measure of societal reintegration (Peak 2005). Peak 2005 also assessed societal reintegration or discretionary activities such as duration until participants returned to work, and travelled in a car. Finally, Peak 2005 explored hospital length of stay and costs associated with the interventions, but the other included studies did not examine this. No studies assessed the secondary outcomes of restrictions in performance in extended ADL (EADL).
Outcomes that we did not consider in this Cochrane review but that an included trial reported were: adherence to postoperative guidelines (Peak 2005), adherence to use of equipment (Peak 2005) and time‐point when participants stopped using equipment (Peak 2005).
Excluded studies
We excluded 16 studies after we assessed the full‐texts of these papers (Figure 1). We have presented the reasons for exclusion in the 'Characteristics of excluded studies' table and one study is still ongoing (Peters 2015; Characteristics of ongoing studies). We excluded eight studies as they were not randomised controlled trials (RCTs) (Gillen 2007; Gromov 2015; McMurray 2000; Mikkelsen 2014; Satoh 2007; Stewart 2011; Stinnett 1996; Thomas 2010). We excluded four trials as they evaluated interventions that were delivered preoperatively rather than postoperatively (Bitterli 2011; Jepson 2016; Lewis 2002; McGregor 2004). We excluded two studies as they did not investigate an intervention that was of relevance to this review (Akarcali 2003; Bai 2009). Bai 2009 did not satisfy the inclusion criteria as the intervention was solely exercise‐based. We excluded one study as it investigated an intervention for participants who had undergone a total knee arthroplasty (Jacofsky 2010).
Risk of bias in included studies
We have presented a summary of the 'Risk of bias' assessment for each included study in Figure 2 and Figure 3 and in the text below.
2.
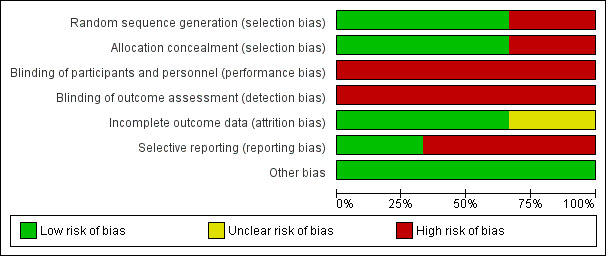
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
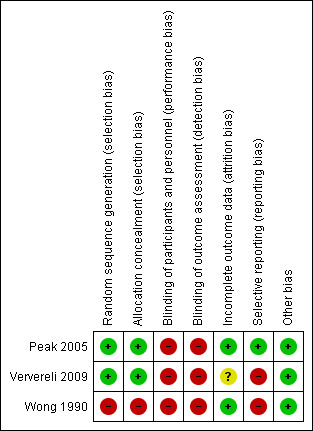
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Two studies were at low risk of selection bias, due to adequate random sequence generation and allocation concealment (Peak 2005; Ververeli 2009). Peak 2005 generated the allocation sequence using a computer number generator and blinded to group allocation, whereas Ververeli 2009 generated the sequence allocation using a random number table and the research co‐ordinator assigned it. Wong 1990 provided no information on sequence generation and we assigned this study as at high risk of bias for this 'Risk of bias' domain.
Peak 2005 and Ververeli 2009 assigned allocation through a sealed envelope method. Wong 1990 did not report allocation and therefore we judged this study as at high risk of bias for this 'Risk of bias' domain.
Blinding
We considered all three included studies at high risk of bias for performance bias. This was understandable given that it would have been logistically difficult to be able to blind participants or personnel to group allocation due to the nature of the equipment and interventions under investigation in these studies. However, assessors could have been blinded to group allocation during each of the data collection phases. Whilst Wong 1990 reported blinding their assessors to group allocation for the OFCI, since the SFCI was a subjective assessment, there was a high risk of bias as the participants were not (and could not be) blinded to group allocation. Two studies did not clearly document that their assessors were blinded to group allocation (Peak 2005; Ververeli 2009). Therefore, we judged these as at high risk of detection bias.
Incomplete outcome data
We judged two papers as at low risk of attrition bias (Peak 2005; Wong 1990). There was no clear loss to study follow‐up in Peak 2005 and Wong 1990 papers. However, in Ververeli 2009 it was unclear whether or not participant attrition had occurred and was accounted for in the analyses. Therefore, we considered this study as at unclear risk of attrition bias.
Selective reporting
One study, Peak 2005, was at low risk of reporting bias. Peak 2005 reported all outcomes acknowledged within the paper. However, there was no study protocol provided or a reference number to a study protocol. We judged that both Ververeli 2009 and Wong 1990 papers were at high risk of reporting bias. Ververeli 2009 did not provide any data regarding participant satisfaction and provided only limited data for Harris Hip Score or Short Form‐12 (SF‐12). Finally, due to the presentation of Wong 1990 data, it was difficult to interpret the descriptive statistical results of their outcomes, which limited the reporting of the data.
Other potential sources of bias
We did not detect any other forms of bias in the included studies (Peak 2005; Ververeli 2009; Wong 1990).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. 'Summary of findings' table 1.
| Provision of hip precautions or equipment or functional restrictions compared with no provision of hip precautions, equipment and functional restrictions for people following primary total hip arthroplasty (THA) | ||||||
|
Patient or population: people following primary uncemented THA Settings: hospital and home settings Intervention: provision of hip precautions, equipment and functional restrictions and outpatient physiotherapy Comparison: no provision of hip precautions or equipment or functional restrictions and outpatient physiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Outpatient physiotherapy without hip precautions, equipment and functional restrictions | Hip precautions, equipment and functional restrictions and outpatient physiotherapy | |||||
| Pain | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Function Harris Hip Score Follow‐up: 3 months |
Mean score not reported. | Mean score not reported. | Not estimable | 81 (1) | ⊕⊝⊝⊝ very low3,4,5 | Mean scores not reported, thus we could not calculate the relative effect2 The trial authors reported MD 0.41 (no 95% CIs reported) |
| Health‐related quality of life Short Form‐12 Follow‐up: 12 months |
Mean score not reported. | Mean score not reported. | Not estimable | 81 (1) | ⊕⊝⊝⊝ very low3,4,5 | Mean scores not reported, thus relative effect could not be calculated2 Trialists report MD 0.38 (no 95% CIs) at 4 weeks. |
| Global assessment of treatment success | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Hip dislocation Incidence of events Follow‐up: 12 months |
No hip dislocations | No hip dislocation | Not estimable | 81 (1) | ⊕⊝⊝⊝ very low3,4,6 | There were no hip dislocations in either group |
| Reoperation rate | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Total adverse events Incidence of events Follow‐up: 12 months |
No adverse events | No adverse events | Not estimable | 81 (1) | ⊕⊝⊝⊝ very low3,4,6 | There were no reported adverse events in either group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; MD: mean difference; RR: risk ratio; SD: standard deviation; THA: total hip arthroplasty. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Outcome not measured. 2Mean value not provided to calculate the effect and 95% CI. 3No blinding of assessors to group allocation therefore risk of detection bias. 4No blinding of participants or personnel therefore risk of performance bias. 5Limited data provided on Harris Hip Score or Short Form‐12 assessments and therefore downgrade for reporting bias and imprecision. 6This outcome was based on a small number of events.
Summary of findings 2. 'Summary of findings' table 2.
| Provision of hip precautions with compared to without the provision of postoperative equipment and functional restriction following primary total hip arthroplasty (THA) | ||||||
|
Patient or population: people following primary uncemented THA Settings: hospital and home settings Intervention: provision of hip precautions with the provision of postoperative equipment and functional restriction Comparison: provision of hip precautions without the provision of postoperative equipment and functional restriction | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hip precautions, but no equipment or functional restrictions | Hip precautions, with equipment and with functional restrictions | |||||
| Pain | Not assessed | Not assessed | Not estimable | Not assessed | Not assessable | No studies measured this outcome1 |
| Function | Not assessed | See comment | Not estimable | Not assessed | Not assessable | No studies measured this outcome1 |
| Health‐related quality of life | Not assessed | Not assessed | Not estimable | Not assessed | Not assessable | No studies measured this outcome1 |
| Global assessment of treatment success Satisfactory pace of recovery Follow‐up: 6 months |
894 per 1000 | 742 per 1000 (180 to 704) | RR: 0.83 (0.75 to 0.93) | 303 (1) | ⊕⊝⊝⊝ very low2,3,4 | Global assessment of treatment success, as assessed with satisfaction with pace of recovery was favoured in the unrestricted group. Patients in the unrestricted group could perform 106.4% (range 25% to 350%) of their preoperative daily activities compared with 96.5% (range 25% to 200%) in the restricted group (P = 0.015). NNTB: 7 (95% CI: 4 to 16). |
| Hip dislocation Incidence of event Follow‐up: 6 months |
0 per 1000 | 7 per 1000 (1 to 480) | RR 2.98 (0.12 to 72.59) | 303 (1) | ⊕⊝⊝⊝ very low2,3,4,5 | No dislocations occurred in the unrestricted group. One dislocation occurred in the restricted group as a consequence of a component of the restrictions (abduction pillow) and was managed successfully with closed reduction. NNTB: not analysed as no statistically significant difference between the groups. |
| Reoperation rate1 | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Total adverse events Incidence of event Follow‐up: 6 months |
0 per 1000 | 0 per 1000 (0 to 0) | RR 0 (0 to 0) | 303 (1) | ⊕⊝⊝⊝ very low2,3,4,5 | There was no statistically significant difference between the groups. NNTB: Not analysed as no statistically significant difference between the groups. Thus, based on an assumed risk of 0 out of 1000 people receiving hip precautions, equipment and functional restrictions having an adverse event 6 months after hip arthroplasty, no hip precautions, equipment and functional restrictions resulted in 0 fewer (CI 0 fewer to 0 more) people per 1000 having an adverse event during this time. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio; THA: total hip arthroplasty. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1This outcome was not measured. 2There was no blinding of assessors to group allocation. Therefore there was risk of detection bias. 3There was no blinding of participants or personnel. Therefore there was risk of performance bias. 4The period of time that hip precautions, equipment and functional restrictions were applied was not stipulated to participants a priori. 5This outcome was based on a small number of events.
Summary of findings 3. 'Summary of findings' table 3.
| Provision of an enhanced postoperative education and rehabilitation service with early hospital discharge to promote functional activities of daily living (ADL) compared with an conventional discharge and rehabilitation intervention in the community for people following primary total hip arthroplasty (THA) | ||||||
|
Patient or population: people following primary uncemented THA Settings: hospital, rehabilitation centre and home settings Intervention: provision of an enhanced postoperative education and rehabilitation intervention Comparison: conventional rehabilitation intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional rehabilitation intervention | Enhanced postoperative education and rehabilitation intervention | |||||
| Pain | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Function Objective Functional Capacity Index and Subjective Functional Capacity Index Follow‐up: 6 months |
Insufficient data provided to assess this outcome. | Insufficient data provided to assess this outcome | Not estimable | 146 (1) | ⊕⊝⊝⊝ very low2,3,4,5 | There was no statistical difference (P > 0.05) between the groups. |
| Health‐related quality of life1 | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Global assessment of treatment success1 | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Hip dislocation1 | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Reoperation rate1 | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| Total adverse events1 | Not assessed | Not assessed | Not estimable | Not assessed | Not assessed | No studies measured this outcome1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; GRADE: Grading of Recommendations Assessment, Development and Evaluation; THA: total hip arthroplasty. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1This outcome was not measured. 2Sequence generation and allocation procedure were not clearly reported. Therefore there was high risk of bias for selection bias. 3There was no blinding of assessors to group allocation. Therefore there was risk of detection bias. 4There was no blinding of participants or personnel. Therefore there was risk of performance bias. 5There was limited reporting of descriptive statistical data for outcomes. Therefore there was high risk of reporting bias.
The three included studies investigated three interventions. Due to the heterogeneity in intervention, we did not pool the data from these studies but have presented them individually by intervention.
1. Provision of hip precautions, equipment, functional restrictions and outpatient physiotherapy versus outpatient physiotherapy without provision of hip precautions, equipment or functional restrictions
A single trial (81 participants), Ververeli 2009, compared rehabilitation with hip precautions, equipment, functional restrictions and outpatient physiotherapy versus outpatient physiotherapy with no specific hip precautions, equipment or functional restrictions. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we downgraded the quality of the evidence for Ververeli 2009 for all outcomes except the frequency of complications by two levels (nominally two levels for limitations in design and implementation that related to potential risk of bias) to low, whilst we downgraded the frequency of complications by three levels for limitations in design and implementation related to the risk of bias, and one for imprecision due to the small number of complication events (see 'Summary of findings' table 1: Table 1). Accordingly, we are very uncertain about the estimated effects for this trial’s outcomes.
Major outcomes
The trial authors reported no statistically significant difference in functional assessment by Harris Hip Score between the no precautions group and the hip precaution, equipment and restriction group at three months (intermediate term) (mean difference (MD) 0.41, P = 0.07). Similarly the trial authors reported no statistically significant difference between the groups for SF‐12 result across the follow‐up periods, except for a MD of 0.38 between groups at four weeks (no 95% CIs reported), in favour of the rehabilitation group. As the trial did not report groups means and measures of variance, we could not substantiate their results. No participants in either group (0/38 in the no precautions group versus 0/43 in the precautions group) reported hip joint dislocation during the 12 month follow‐up period, or postoperative complication at 12 months follow‐up.
The trial did not report the following outcomes: pain, global assessment of treatment rates or total adverse events.
Minor outcomes
Participants in the group with provision of hip precautions reported a significantly slower recovery in respect to functional outcomes compared to those who were not advised to follow and use hip precautions, equipment and restrictions. People who received precautions mobilised slower with only a stick, compared to those who were not provided with hip precautions, equipment and functional restrictions. Those in the precautions group walked with a stick at a mean of 16.4 days, compared to 12.6 days in the no provision of precautions, equipment and functional restriction group (MD 3.80 days, 95% CI 0.47 to 7.13; Table 4). Similarly, the precautions group reported a longer time until they could walk without a stick (mean: 39 days), compared to the no precautions, equipment and functional restrictions group (mean: 27 days) (MD 12.40, 95% CI 6.48 to 18.32; Table 4). The precautions group reported a longer period of time until they recommenced driving (mean: 30 days precautions group versus 23 days non‐precautions group) (MD 7.20, 95% CI 2.78 to 11.62; Table 4), whilst those allocated to the precautions group also reported a longer period of time until they could walk without a limp (mean: 67 days), when compared to those who did not receive precautions, equipment and functional restrictions (mean: 50 days) (MD 17.30, 95% CI 6.90 to 27.90; Table 4).
1. Secondary outcomes results: no provision of hip precautions or equipment or functional restrictions compared with provision (Ververeli 2009).
| Functional Outcome | Restricted rehabilitation (mean/SD) | Unrestricted rehabilitation (mean/SD) | Mean difference (95% CI) |
| Days until walked with a stick only | 16.4 (9.5) | 12.6 (5.5) | 3.80 (0.47 to 7.13) |
| Days until walked without a stick | 39 (15.4) | 26.6 (11.7) | 12.40 (6.48 to 18.32) |
| Days until drove a car | 30.1 (8.0) | 22.9 (11.7) | 7.20 (2.78 to 11.62) |
| Days until walked without a limp | 67.2 (27.2) | 49.9 (20.4) | 17.30 (6.90 to 27.90) |
Abbreviations: SD: standard deviation; CI: confidence interval.
2. Provision of hip precautions with versus without postoperative equipment and functional restriction
A single trial (265 participants; 303 THAs) compared the provision of hip precautions with versus without postoperative equipment and functional restriction. Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we downgraded the quality of the evidence for Peak 2005 outcomes by three levels (nominally two levels for limitations in design and implementation that related to potential risk of bias and one for imprecision) to very low, meaning that we are very uncertain about the estimated effect (see 'Summary of findings' table 2: Table 2).
Major outcomes
Peak 2005 measured global assessment of treatment success through patient satisfaction questionnaires in the intermediate period. There was a significant difference between the groups with those allocated to the restricted group significantly less satisfied with the pace of their recovery than the unrestricted group (RR 0.83, 95% CI 0.75 to 0.93; NNTB: 7 (4 to 16); Analysis 1.1).
1.1. Analysis.

Comparison 1 Provision of hip precautions with versus without postoperative equipment and functional restriction, Outcome 1 Global assessment of treatment success: participant reported questionnaire of perceived pace of recovery at 6 month follow‐up.
There was no statistically significant difference in the incidence of hip joint dislocation between the groups with hip precautions (1/152 participants) with versus without postoperative equipment and functional restriction (0/151 participants) (RR 2.98, 95% CI 0.12 to 72.59; Analysis 1.2). However, as there was only one event, this estimate is very uncertain.
1.2. Analysis.

Comparison 1 Provision of hip precautions with versus without postoperative equipment and functional restriction, Outcome 2 Hip dislocation: incidence of event at 6 month follow‐up.
One participant in the postoperative equipment and functional restriction group dislocated during transfer from the operating table to a bed postoperatively. No subsequent dislocation or instability occurred after this was managed with closed reduction in either the no equipment and functional restriction group (0/151 participants) nor the equipment and functional restriction group (0/152 participants). Similarly, no participants in either the no equipment and functional restriction group (0/151 participants) nor the equipment and functional restriction group (0/152 participants) experienced an adverse event.
The trial did not assess the following primary outcomes of interest in this Cochrane review: pain, function, HRQOL, reoperation rate or total adverse events.
Minor outcomes
In respect to limitation of ADL, at six months participants in the restricted group reported statistically significantly less patient satisfaction regarding return to preoperative levels of ADL compared to the group who did not received postoperative equipment and functional restriction (P = 0.02). The study author did not provide any numerical results for this outcome in the paper or after we contacted the study authors. Accordingly, we reported these results directly from the trial. There was a difference between the groups for time to return to sleeping on their side; those in the restricted group took a longer period of time (mean: 5.8 weeks) compared to the unrestricted group (mean: 3.2 weeks; P < 0.001). There were no standard deviation (SD) values for this outcome and therefore we reported the data directly from the trial paper for this outcome. There was no statistically significant difference between the two groups for the prevalence of a limp at six months (RR 1.06, 95% CI 0.59 to 1.90; Table 5).
2. Secondary outcomes results: provision of hip precautions with versus without postoperative equipment and functional restriction (Peak 2005).
| Outcome | Restricted rehabilitation (events/total) | Unrestricted rehabilitation (events/total) | Relative risk (95% CI) |
| Functional outcomes: presence of a limp at 6 month follow‐up | 19/152 | 20/151 | 1.06 (0.59 to 1.90) |
| Number of people who returned to work in less than 6 weeks post‐THA | 16/85 | 49/98 | 2.66 (1.64 to 4.31) |
| Number of people who required a rehabilitation stay post‐THA | 125/152 | 100/151 | 0.81 (0.70 to 0.92) |
Abbreviations: CI: confidence interval; THA: total hip arthroplasty.
There was a difference between the groups in societal reintegration and discretionary activities. It took a longer period of time for participants allocated to the restricted group to return to driving a car (mean: 6.8 weeks) compared to the unrestricted group (mean: 4.9 weeks; P < 0.001). There was also a statistically significant difference between the groups where it was a longer period of time for those in the restricted group to be passengers in cars (mean: 1.9 weeks) compared to the unrestricted group (mean: 1.5 weeks; P = 0.26). Regarding return to work, participants in the restricted group returned to work significantly later (mean: 9.5 weeks) compared to the unrestricted group (mean: 6.5 weeks; P < 0.001). There were no SD values for these outcomes and therefore we reported the data directly from the trial paper for these outcomes. The proportion of participants who returned to work in less than six weeks was also lower in the restricted group compared to the participants who were not provided with postoperative equipment and functional restriction (RR 2.66, 95% CI 1.64 to 4.31; Table 5).
There was no significant difference between the groups for acute hospital length of stay where both the restricted and unrestricted group had a mean length of stay of 3.5 days (P = 0.88). There were no SD values presented for acute hospital length of stay. Therefore we reported the data directly from the trial paper for this outcome. However, there was a significant difference between the groups in requirement for rehabilitation, where there were a greater number of participants who required a rehabilitation stay if they received postoperative equipment and functional restriction compared to no equipment and restriction (RR 0.81, 95% CI 0.70 to 0.92; Table 5).
Peak 2005 calculated the costs associated with the additional equipment. Peak 2005 estimated that the cost associated with the provision of this equipment was an additional USD 655 per participant, where the abduction pillow cost USD 120, elevated toilet seat USD 65, and elevated chair USD 955 to purchase or USD 15 per day to rent. This estimation did not include cost of transport required for people in the restricted group, loss of wages related to delayed return to work or greater rehabilitation requirements in the restricted group.
This trial did not report data on restriction to extended ADL.
3. Provision of an enhanced postoperative education and rehabilitation service with conventional hospital discharge to promote functional ADL versus a conventional traditional discharge and rehabilitation intervention in the community
A single trial (146 participants), Wong 1990, compared these interventions. Using the GRADE approach, we downgraded the quality of the evidence for the hospital length of stay and functional assessments using the OFCI and SFCI by the maximum of three levels (nominally, two levels for limitations in design and implementation that related to potential risk of bias; and one level for inconsistency in results) to very low, meaning that we are very uncertain about the estimated effect. The recorded outcomes were hospital length of stay, OFCI and SFCI, evaluated at six months.
Major outcomes
Regarding function through the OFCI and SFCI, there was no statistically significant difference between those randomised to the enhanced postoperative education and rehabilitation programme at conventional discharge compared to the conventional discharge and traditional rehabilitation programme (P > 0.33). The study authors did not provide any numerical results in the paper nor after we contacted the study authors. Accordingly, we reported the results directly from the trial.
Primary outcomes that Wong 1990 did not report included: pain, HRQOL, global assessment of treatment success, hip dislocation, re‐operation rate and total adverse events.
Minor outcomes
Whilst not assessed through inferential statistical tests, those allocated to the conventional discharge and rehabilitation regime had a shorter hospital length of stay (mean: 12.75 days) compared to those allocated to the conventional hospital discharge and enhance postoperative education and rehabilitation programme (mean: 13.85 days). There were no SD values presented for hospital length of stay. Therefore, we reported the data directly from the trial paper for this outcome.
Secondary outcomes that this trial did not report included: limitations in personal ADL, restrictions in EADL, societal reintegration and cost‐analysis.
Discussion
Summary of main results
The three included studies, which were at moderate to high risk of bias, investigated three different interventions following total hip arthroplasty (THA). Evidence from a single trial suggests there may be some benefit for earlier recovery for postoperative functional capability in participants that receive no advice on hip precautions, but there was uncertainty regarding adverse events and complications including hip dislocation events between those prescribed hip precautions, functional restriction and equipment following primary THA. However, due to the small number of events and low quality of the evidence, we are uncertain whether receiving no advice on hip precautions and the provision (or not) of equipment has an important effect on dislocation rates or adverse events; the results were too imprecise to rule out a small or no effect, and the number of adverse events were rare. Overall, the quality of the evidence was very low, which mostly reflected the limitations in study design (all outcomes) and imprecision of point estimates and inconsistency in results (particularly Wong 1990). This means that we are uncertain about the estimates of effect (see the 'Summary of findings' tables: Table 1; Table 2; Table 3). Accordingly, there is insufficient high‐quality evidence to support or refute the adoption of a hip precautions, functional restriction, equipment or postoperative community rehabilitation programmes consisting of functional reintegration and education compared to conventional rehabilitation strategies.
Overall completeness and applicability of evidence
There is insufficient evidence to draw conclusions on the use of postoperative equipment and functional limitations, which appears to unnecessarily limit recovery following THA. However, this is only generalisable for those following primary THA and those with an anterolateral surgical approach. Due to the nature of surgical approaches, the anterior, anterolateral (i.e. the modified Hardinge approach (detachment of the anterior fibres of the abductor) or the Watson‐Jones approach where the abductor is preserved (more of an minimally invasive surgery approach), lateral and posterior approach each have a specific impact on the specific soft tissues affected through the THA procedure. Therefore, it may be suggested that the specific motions and activities which one approach has should reflect the risk for dislocation. This means that there is incomplete knowledge on whether the outcomes on postoperative equipment and functional activity requirement is different dependent on surgical approach adopted.
The other main surgical issue which can influence prosthesis dislocation is femoral head size. Whilst Peak 2005 and Ververeli 2009 reported femoral head size, all studies should consistently report this. Over the last decade, there has been a gradual increase in the size of femoral head due to the changes in bearing material. This may have an important role in reducing the rates of dislocation further when compared to older papers previously reported, and should be considered when reviewing future trials.
The included studies were based on primary THA procedures. It is unclear whether results would be similar in participants with revision procedures, which may have different risks of dislocation events and complications due to the greater risk of poorer soft tissue and suboptimal component orientation through repeated surgery. Generalisability of these findings to this different population is therefore difficult.
Only one study assessed the adoption of a specific enhanced recovery programme to improve ADL post‐THA (Wong 1990). We considered this study as at high risk of bias and it was underpowered in sample size. Further studies to address this with greater rigour, through improved randomisation procedures, reporting of results and reporting of attrition, are therefore important to improve the quality of this clinical message.
None of the included studies assessed pain as an outcome for any of the interventions investigated. There was also limited reporting of function and health‐related quality of life (HRQOL) and reoperation rates. The available studies were also too small to detect if there was a difference in incidence of hip dislocation or adverse events. There is also a paucity of evidence on disruption to activities of daily living (ADL) and levels of extended ADL (EADL) and instrumental ADL (IADL). Further research is need to determine the impact of postoperative interventions on these.
Finally, because of the lack of studies with similar intervention comparisons, we were unable to pool data in a meta‐analysis. Furthermore, due to the limited number of included papers, it was not possible to perform subgroup and sensitivity analyses. Considering the findings in particular subgroups or for particular interventions would assist in the dissemination of findings to more specific populations and would thus be more clinically valuable.
Quality of the evidence
We are uncertain if the provision of hip precautions, equipment and functional restrictions and outpatient physiotherapy provides any benefits following total hip replacement, due to very low quality evidence available from a single trial. Using the GRADE approach, we downgraded the quality of the evidence for function, quality of life, hip dislocation and adverse events by three levels to very low: twice for limitations in design and implementation (given that no study was blinded, introducing performance and detection bias), once for imprecision (given that the results for each outcome, were based on a small number of events, thereby being underpowered to detect a difference if one existed) ('Summary of findings' table 1: Table 1). This indicates that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Pain, treatment success and re‐operation rate were not measured.
Evidence was scant for the comparison, provision of postoperative equipment and functional restriction plus standard hip precautions versus standard hip precautions alone. Only very low evidence was available from a single trial for treatment success, hip dislocations and adverse events. Pain, function, quality of life and reoperation rate were not measured ('Summary of findings' table 2: Table 2).
Similarly, the evidence was scant for the comparison, provision of an enhanced postoperative education and rehabilitation service with conventional hospital discharge to promote functional ADL versus a conventional traditional discharge and rehabilitation intervention in the community. There was only very low evidence available from a single trial of 146 participants for function ('Summary of findings' table 3: Table 3). Pain, HRQOL, global assessment of treatment success, hip dislocation, reoperation rate and the frequency of total adverse events were not measured. Therefore for all comparisons, the effect of the intervention was uncertain due to imprecision and flawed design (very low quality evidence throughout).
Overall the evidence presented with moderate to high risk of bias. We judged two included studies as at moderate risk of bias (Peak 2005; Ververeli 2009), whilst one study was at high risk of bias (Wong 1990). Consistent limitations across the three studies were the high risk of bias for not blinding participants or personnel and for not blinding of outcome assessors in Peak 2005 or Ververeli 2009 studies. Whilst the latter assessment would have been feasible to minimise detection bias, it would not have been possible logistically to blind participants to whether or not they had been allocated to the restriction of movement, adoption of hip precautions and provision of equipment due to the nature of these participatory interventions. We considered Wong 1990 was as at high risk of bias. This was largely related to the poor reporting of their randomisation procedures and sequence generation for randomisation, as well as the ambiguity in reporting their outcomes with selective data reporting. Accordingly, the assessment of an enhanced postoperative education and rehabilitation service with early hospital discharge to promote functional ADL is limited and these results should be interpreted with caution.
Potential biases in the review process
We designed the review to minimise the risks of potential biases. Therefore we included strategies such as searching a variety of published and unpublished literature sources on health and social care to limit publication bias. Secondly, two review authors independently screened the studies, extracted data and assessed the risk of bias of included studies to maximise rigour in the conduct of the review.
Therefore we attribute potential biases in the review more to the limitations in the included studies, both in the risk of bias and reporting quality. Since the only three included studies presented three specific interventions, it was inappropriate to pool data. The quality of these studies was moderate to low, based on the risk of bias and imprecision though the sample sizes being small, and the number of events, such as hip dislocation and adverse events, being rare. Accordingly the results should be viewed with caution.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first Cochrane review to report the findings of randomised evaluations of postoperative assistive devices, hip precautions, environmental modifications and training to prevent dislocation and improve function for people with THA. Two recent Cochrane reviews have previously investigated the provision of hip precautions (Barnsley 2015) and lifestyle restrictions and precautions following THA (van der Weegen 2016).
Firstly Barnsley 2015 identified two studies that examined the prescription of hip movement precautions after primary THA undertaken through an anterolateral surgical approach. We included both of these studies in this review (Peak 2005; Ververeli 2009). We did not identify any studies that investigated the effect of using or not using hip precautions following THA when the surgical procedure was undertaken through a posterior approach.
Barnsley 2015 drew the same conclusions to our review, where the provision of hip precautions provided no additional benefit in preventing dislocation compared to not providing hip precautions.
Secondly van der Weegen 2016 identified six studies in their review of lifestyle restrictions and precautions following THA. Three studies were randomised controlled trials (RCTs), including two RCTs included in this review (Peak 2005; Ververeli 2009). It also included Barrett 2013, which we excluded from this review since it investigated clinical outcomes, including dislocation rates, between participants randomised to receive a THA through a direct anterior surgical (DAA) approach versus a posterior‐lateral surgical approach. Whilst there was a difference between the surgical procedures where those who underwent a DAA did not receive hip precautions, due to the design adopted, it was not possible to ascertain whether the difference in outcomes was related to the surgical procedure or the precautions (or not) adopted. The other three included studies included one retrospective matched‐cohort study, and one retrospective and one prospective cohort study. The authors made similar conclusions to our review, where participants randomised to unrestricted postoperative programmes resumed activities faster and were more satisfied with the pace of their recovery compared to those who were restrictive. However, there was no clear assessment of the risk of bias which this data may have exhibited. Therefore, the findings of van der Weegen 2016 should be viewed with caution particularly given that our review highlighted that the present evidence is very low quality.
Two reviews have previously investigated interventions around the management of people with THA. These have not been directly related to hip precautions, restrictions or equipment. McDonald 2014 identified 13 trials that assessed preoperative education and advice for people awaiting THA. They reported similar concerns regarding the quality of the evidence‐base, and concluded that, based on this low‐quality evidence, preoperative education did not appear to offer additional benefit to usual care (the consent process preoperatively) for outcomes including pain, function, postoperative anxiety, total adverse events and re‐operation rates. Khan 2008 identified five trials that assessed multidisciplinary rehabilitation programmes for people following THA and total knee arthroplasty. As with our Cochrane review and McDonald 2014, the quality of the evidence was of low and very low quality. They reported some support for improved outcomes of functional activity and participation for people provided with early multidisciplinary rehabilitation compared to usual care. However in agreement with our review and McDonald 2014, the quality of the evidence‐base limits the confidence to which these results can be implemented into clinical practice.
Authors' conclusions
Implications for practice.
It is uncertain if the prescription of postoperative equipment and placing functional limitations on patients following primary antero‐lateral THA is beneficial due to the very low quality evidence available from three single studies. It is uncertain whether the provision of functional limitations and postoperative equipment is beneficial for functional recovery and societal reintegration of patients following THA due to the very low quality evidence available.
There is insufficient evidence to provide any recommendations on whether hip precautions (limiting hip flexion, adduction or rotation) are required in the initial six postoperative weeks following THA. From the single study of very low quality evidence ( Peak 2005), it is uncertain whether there is a difference in complication rates such as hip dislocation, but modifying this advice has yet to be assessed in isolation, having only been assessed with the addition of equipment and functional restrictions.
Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, we judged the quality of the evidence as 'very low', and downgraded the quality of the evidence due to limitations in design and implementation and for imprecision. There is insufficient evidence to support or refute the adoption of an enhanced postoperative intervention and community rehabilitation consisting of functional reintegration and education compared to conventional rehabilitation strategies. The single study that investigated this was underpowered, poorly reported and we judged it as at high risk of bias.
Implications for research.
The current evidence‐base requires further development. Firstly, the findings of this Cochrane review are based on a primary anterolateral approach to THA. It is therefore unknown whether the evidence can be generalised to revision procedures dependent on their fixation and postoperative stability, to the other surgical approaches such as the anterior, lateral or posterior approach, or to differences in femoral head sizes. Further evaluations in people that receive THA with different surgical approaches or femoral head sizes, and in people that receive revision THA are warranted.
Whilst Peak 2005 provided some indication on the costs associated with providing their equipment component in their control intervention, further full economic analysis is required to assess the cost‐effectiveness of the provision of equipment and of provision of instructions and education relating to hip precautions and functional limitations. Whilst the current evidence suggests that social reintegration, most notably return to work, is faster in the unrestricted group, further assessment of the indirect costs attributed to the intervention is required. This could provide further data on whether the difference in capability to drive or be a passenger in a car, the perceived difference in recovery and difference in rehabilitation requiring more domiciliary rather than outpatient visits, has an impact on cost‐effectiveness.
The interventions under‐investigation have centred largely on the provision or non‐provision of equipment or functional restriction. Wong 1990 was the only included study that investigated the provision of specific teaching and enhanced education on ADL or extended ADL (EADL). The reporting of this study was limited and we judged it as at high risk of bias. Further research is therefore needed to explore the clinical and cost‐effectiveness of occupational therapy interventions to aid ADL and EADL at home or in rehabilitation settings.
A number of outcome measures which we identified as important a priori, were not reported in the literature. These included pain, function, the assessment of restrictions in performance in EADL or instrumental ADL (IADL), which are defined as the skills required to live independently and manage, and the capability in performing a variety of personal ADL. These should be considered when designing future trials in this area.
Finally, we assessed the quality of the evidence as very low. Two notable ways in which the quality of the evidence could be improved through study design are around blinding of assessors and evaluating intervention fidelity. The 'Risk of bias' assessment highlighted that trials did not blind assessors to group allocation. Whilst it would be impossible to blind a participant to a movement restriction or use of equipment, it would be possible to blind assessors to group allocation. This should be considered to reduce the risk of subsequent assessor bias in future trials. Secondly, whilst no papers reported whether there were examples of participant cross‐over between intervention groups post‐randomisation, only Peak 2005 assessed adherence to allocated interventions. It is therefore unclear whether there were issues on fidelity of group interventions. Given that the use of equipment and functional restriction could significantly impact on an individual's lifestyle and behaviour, assessing compliance to the interventions allocated would be valuable in future studies to better understand how the findings work in the 'real world'.
Acknowledgements
This article outlines independent research commissioned by the National Institute for Health Research (NIHR) in England under its Programme Grants for Applied Research funding scheme (RP‐PG‐0407‐10070).
The review author (PJ) is also in receipt of a West Midlands Strategic Health Authority Nursing, Midwifery and Allied Health Professionals Pre‐PhD training award.
We thank Stephanie Smith (A&E/Acute Stroke/Urology, City Hospital, Birmingham, UK) for her initial assistance during the development phase of the Cochrane protocol.
Appendices
Appendix 1. Search strategies
1. MEDLINE search strategy (1946 to 29 April 2016)
1 exp Occupational Therapy/ (9972)
2 exp Self‐Help Devices/ (8208)
3 exp Splints/ (7291)
4 exp Protective Clothing/ (9759)
5 exp Protective Devices/ (31396)
6 exp Orthotic Devices/ (9237)
7 exp Health Education/ (133188)
8 exp Patient Education/ (68894)
9 exp ACTIVITIES OF DAILY LIVING/ (49787)
10 exp ACCIDENT PREVENTION/ (58144)
11 exp Discharge Planning/ (18361)
12 exp Counseling/ (32261)
13 exp Social Support/ (49755)
14 exp ADAPTATION, PSYCHOLOGICAL/ (99746)
15 exp Pain Management/ (16378)
16 exp Postoperative Care/ (51253)
17 (hip adj (protector$ or pad$ or cushion$)).tw. (371)
18 (activit$ adj2 (daily adj2 (life or liv$))).tw. (17933)
19 advice.tw. (31033)
20 (social adj1 (work$ or support)).tw. (31440)
21 (occupational adj1 therap$).ti,ab. (9000)
22 splint$.ti,ab. (10802)
23 ((assist$ or help$) adj5 (device$ or technolog$)).ti,ab. (19635)
24 ((sel$ or home$) adj5 (care$ or manage$)).ti,ab. (76765)
25 ((environment$ or home$ or domestic$ or house$) adj5 adapt$).ti,ab. (13123)
26 ((daily or domestic$ or house$ or home$) adj5 (activit$ or task$ or skill$ or chore$)).ti,ab. (42558)
27 (functional adj train$).tw. (235)
28 (patient adj2 (educat$ or coach$)).tw. (13385)
29 or/1‐28 (695693)
30 exp Arthroplasty, Replacement, Hip/ (15964)
31 exp Arthroplasty, Replacement, Knee/ (12037)
32 exp Knee Prosthesis/ (8382)
33 exp Hip Prosthesis/ (18097)
34 Joint Prosthesis/ (8681)
35 ((hip$ or knee$) adj10 (replace$ or arthroplast$ or prosthe$ or implant$)).ti,ab. (45622)
36 (tkr or thr or tka or tha).tw. (29001)
37 or/30‐36 (82122)
38 29 and 37 (4529)
39 randomized controlled trial.pt. (363614)
40 controlled clinical trial.pt. (87597)
41 randomized.ab. (283849)
42 placebo.ab. (150067)
43 drug therapy.fs. (1664960)
44 randomly.ab. (206377)
45 trial.ab. (292654)
46 groups.ab. (1319311)
47 or/39‐46 (3258478)
48 exp animals/ not humans.sh. (3882912)
49 47 not 48 (2792120)
50 38 and 49 (1054)
2. EMBASE (1947 to 29 April 2016)
1 exp Occupational Therapy/ (18817)
2 exp Self‐Help Devices/ (11609)
3 exp Splints/ (9317)
4 exp Protective Clothing/ (10300)
5 exp Protective Devices/ (38266)
6 exp Orthotic Devices/ (18357)
7 exp Health Education/ (240171)
8 exp Patient Education/ (88205)
9 exp ACTIVITIES OF DAILY LIVING/ (57221)
10 exp ACCIDENT PREVENTION/ (14137)
11 exp Discharge Planning/ (62928)
12 exp Counseling/ (108802)
13 exp Social Support/ (57872)
14 exp ADAPTATION, PSYCHOLOGICAL/ (48990)
15 exp Pain Management/ (108841)
16 exp Postoperative Care/ (70560)
17 (hip adj (protector$ or pad$ or cushion$)).tw. (470)
18 (activit$ adj2 (daily adj2 (life or liv$))).tw. (25194)
19 advice.tw. (46313)
20 (social adj1 (work$ or support)).tw. (42702)
21 (occupational adj1 therap$).ti,ab. (15145)
22 splint$.ti,ab. (15424)
23 ((assist$ or help$) adj5 (device$ or technolog$)).ti,ab. (28306)
24 ((sel$ or home$) adj5 (care$ or manage$)).ti,ab. (103242)
25 ((environment$ or home$ or domestic$ or house$) adj5 adapt$).ti,ab. (16015)
26 ((daily or domestic$ or house$ or home$) adj5 (activit$ or task$ or skill$ or chore$)).ti,ab. (59406)
27 (functional adj train$).tw. (478)
28 (patient adj2 (educat$ or coach$)).tw. (18560)
29 or/1‐28 (1018408)
30 exp total hip prosthesis/ (22523)
31 exp Arthroplasty, Replacement, Hip/ (14556)
32 exp total knee replacement/ (13895)
33 exp Arthroplasty, Replacement, Knee/ (24637)
34 exp Knee Prosthesis/ (6738)
35 exp Hip Prosthesis/ (32693)
36 Joint Prosthesis/ (10029)
37 ((hip$ or knee$) adj10 (replace$ or arthroplast$ or prosthe$ or implant$)).ti,ab. (60451)
38 (tkr or thr or tka or tha).tw. (34328)
39 or/30‐37 (83894)
40 29 and 39 (8766)
41 random$.tw. (913091)
42 factorial$.tw. (24026)
43 crossover$.tw. (51219)
44 cross over.tw. (23185)
45 cross‐over.tw. (23185)
46 placebo$.tw. (212764)
47 (doubl$ adj blind$).tw. (155189)
48 (singl$ adj blind$).tw. (14878)
49 assign$.tw. (248925)
50 allocat$.tw. (86120)
51 volunteer$.tw. (190565)
52 crossover procedure/ (40412)
53 double blind procedure/ (125534)
54 randomized controlled trial/ (371203)
55 single blind procedure/ (19144)
56 or/41‐55 (1488486)
57 40 and 56 (1563)
3. The Cochrane Library, 2016 Issue 4
#1 MeSH descriptor: [Occupational Therapy] explode all trees
#2 MeSH descriptor: [Self‐Help Devices] explode all trees
#3 MeSH descriptor: [Foot Orthoses] explode all trees
#4 MeSH descriptor: [Health Education] this term only
#5 MeSH descriptor: [Activities of Daily Living] explode all trees
#6 MeSH descriptor: [Accident Prevention] explode all trees
#7 MeSH descriptor: [Counseling] explode all trees
#8 MeSH descriptor: [Social Support] explode all trees
#9 MeSH descriptor: [Patient Discharge] explode all trees
#10 MeSH descriptor: [Adaptation, Psychological] explode all trees
#11 MeSH descriptor: [Postoperative Care] explode all trees
#12 hip protector
#13 activities of daily living
#14 advice or counseling
#15 occupation*
#16 social support
#17 environment*
#18 assist*
#19 functional training
#20 self care
#21 coping
#22 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21
#23 MeSH descriptor: [Arthroplasty, Replacement, Hip] explode all trees
#24 MeSH descriptor: [Arthroplasty, Replacement, Knee] explode all trees
#25 MeSH descriptor: [Knee Prosthesis] explode all trees
#26 MeSH descriptor: [Hip Prosthesis] explode all trees
#27 MeSH descriptor: [Joint Prosthesis] explode all trees
#28 (knee or hip) near/3 (replace* or arthroplast* or prosthe* or implant*)
#29 (tkr or thr or tka or tha)
#30 #23 or #24 or #25 or #26 or #27 or #28 or #29
#31 #22 and #30 Publication Year from 2014 to 2016, in Trials
4. CINAHL Ebscohost (1981 to 29 April 2016)
S1 (MM "Occupational Therapy")
S2 (MM "Assistive Technology Devices")
S3 (MM "Splints")
S4 (MH "Protective Devices")
S5 (MH "Hip Protectors")
S6 (MH "Hip Protectors")
S7 (MH "Patient Education") OR (MH "Patient Discharge Education") OR (MH "Preoperative Education")
S8 (MM "Counseling")
S9 (MH "Support, Psychosocial")
S10 (MH "Postoperative Pain")
S11 (MM "Postoperative Care")
S12 (MM "Postoperative Care")
S13 (MH "Home Environment")
S14 (MH "Functional Training")
S15 (MH "Balance Training, Physical")
S16 (MH "Activities of Daily Living") OR (MH "Instrumental Activities of Daily Living (Saba CCC)") OR (MH "Assisted Living")
S17 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16
S18 (MH "Arthroplasty, Replacement, Hip")
S19 (MH "Arthroplasty, Replacement, Knee")
S20 (MH "Arthroplasty, Replacement")
S21 "hip prosthesis"
S22 "knee prosthesis"
S23 "knee replacement"
S24 "hip replacement"
S25 S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24
S26 S17 AND S25
5. Centre for International Rehabilitation Research Information and Exchange (CIRRIE) via http://cirrie.buffalo.edu/database/
Replacement or arthroplasty or prosthesis or implant
6. Physiotherapy Evidence database (PEDro) via http://www.pedro.org.au/ (advanced search)
Replacement or arthroplasty or prosthesis or implant
7. Clinicaltrials.gov (advanced search)
Subject: Replacement or arthroplasty or prosthesis or implant
Intervention: occupat* or support or assist* or device or counseling or training or patient education
Data and analyses
Comparison 1. Provision of hip precautions with versus without postoperative equipment and functional restriction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global assessment of treatment success: participant reported questionnaire of perceived pace of recovery at 6 month follow‐up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Hip dislocation: incidence of event at 6 month follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Peak 2005.
| Methods | A single centre, prospective, randomised study, to evaluate the role of postoperative functional restrictions on the prevalence of dislocation following total hip arthroplasty (THA). | |
| Participants | Number of participants (N): 265 participants; 303 THA. Gender: 139 males; 126 females. Age: mean 58.3 years (range: 14 to 88 years). Body Mass Index (BMI): restricted group: mean 29.3 kg/m² (range: 15.9 to 50.2 kg/m²). Unrestricted group: 28.7 kg/m² (range: 17.6 to 45.7 kg/m²). Inclusion criteria: all participants undergoing primary uncemented THA through an anterolateral approach. Exclusion criteria: a history of surgery on the ipsilateral hip; hyperflexibility syndromes; neuromuscular compromise (e.g. Alzheimer's or Parkinson's disease). |
|
| Interventions | Restricted group (N = 152): hip precautions were advised: (1) to limit the range of motion of the hip for the first 6 weeks to < 90° of flexion and 45° of external and internal rotation; and (2) to avoid adduction (crossing the legs). Immediate weight‐bearing as tolerated permitted, and instructed to use a walking aid for as long as they required. All in the restricted group were provided with an abduction pillow in the operating room before bed transfer and instructed to use pillows to maintain abduction while in bed. These participants were provided and instructed to use an elevated toilet seat and elevated chairs from immediately postoperatively in hospital, rehabilitation centre (if transferred) and at home. These participants were instructed to avoid sleeping on their side, from driving and from being a passenger in an automobile. Unrestricted group (N = 151): hip precautions were advised: (1) to limit the range of motion of the hip for the first six weeks to < 90° of flexion and 45° of external and internal rotation and (2) to avoid adduction (crossing the legs). Immediate weight‐bearing as tolerated permitted, and instructed to use a walking aid for as long as they required. |
|
| Outcomes | Follow‐up period: 6 months postoperatively. Outcome measures: incidence of hip dislocation; adherence to postoperative guidelines; use of equipment; presence of a limp; time‐point they stopped using equipment; percentage of activities of daily living (ADL) they could perform; patient satisfaction; return to ADL; return to work; length of hospital stay; costs (outcomes we included in this review are highlighted in italics). |
|
| Notes | The study authors did not stipulate the period of time hip precautions, equipment or functional restriction should be adhered to. They presented a sample size calculation to provide an estimate of power for the incidence of hip dislocation. No data was presented in the primary outcomes: pain, functional outcomes; health‐related quality of life (HRQOL); global assessment of treatment success; reoperation rate; total adverse events; and restrictions in extended ADL (EADL). We requested the following information from the study authors: mean and standard deviation (SD) values and sample size in each group for data on: patient satisfaction scores; time to return to sleeping on their side; time to return to driving a car; time until was a passenger in a car; time until returned to work and length of hospital stay. The study authors did not respond to this request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study generated a random sequence using a “random‐number table”. Page 249. |
| Allocation concealment (selection bias) | Low risk | The study performed randomisation performed preoperatively but the study co‐ordinator only opened the sealed‐envelope at the end of surgery. Designation of the participant “double‐blinded until completion of wound closure to avoid patient‐selection bias or alteration in surgical technique”. Page 249. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It would be difficult to blind participants to group allocation due to the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor blinding was not assured which could affect assessment of subjective outcomes including: pain, function, quality of life, rather than of re‐operation or dislocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study followed up all participants who were randomised, with no clear attrition (Results, Page 249; Table 1 and Table 2). |
| Selective reporting (reporting bias) | Low risk | The study authors reported all outcomes acknowledged, as documented in the Methods and Results section. However, there was no study protocol provided or reference number to a study protocol. The study did not report any standard deviation (SD) values or other measures of variance. |
| Other bias | Low risk | There are no apparent other sources of bias. |
Ververeli 2009.
| Methods | A single‐centre, randomised prospective study, to evaluating the need for hip restrictions following primary THA. | |
| Participants | N: 81 participants, 81 THA. Gender: experimental (unrestricted): 16 females, 22 males; control (restricted): 16 females, 27 males. Age: experimental (unrestricted): women mean age 60.8 years (range 39 to 76), males mean age 58.8 years (range 46 to 71). Control (restricted): women mean age 59.8 years (range 42 to 71), males mean age 57.4 years (range 40 to 75). BMI: experimental (unrestricted): females mean BMI 28.2 kg/m² (range: 21 to 40); males mean BMI 28.2 kg/m² (range: 24 to 34). Control (restricted): women mean BMI 27.8 kg/m² (range: 22 to 35), male mean BMI 29.8 kg/m² (range: 24 to 37) Inclusion criteria: all participants undergoing elective primary uncemented THA through a modified anterolateral approach. Exclusion criteria: a previous THA to the operative side; a hearing impairment despite the aid of a hearing device; previous diagnosis with dementia or Alzheimer’s; no family support at home; younger than 21 years; weighed > 275 pounds; unable to ambulate 30 feet without an assistive device preoperatively; or unable to attend postoperative outpatient physical therapy at a designated location |
|
| Interventions | Restricted (N = 43): participants in the standard rehabilitation group were instructed to refrain from bending the operative hip > 90°, crossing their legs at the thighs, and travelling in a car with the exception of travelling home from the hospital. Participants were to use an elevated toilet seat, sit only on an elevated chair and sleep flat on their back with a pillow between their legs. These hip restrictions were to be observed for the first postoperative month. Participants had home physical therapy 3 times a week for that month. During the second and third postoperative months, the participants were still limited to flexion < 90° and adduction < 5°, but were permitted to ride in a car and begin outpatient physical therapy. Unrestricted (N = 38): participants in the early rehabilitation group were instructed not to cross their legs at the thighs with no other restrictions. They could bend the hip where they were comfortable and travel in a car without any restrictions. They were to use a regular toilet seat and were permitted to sit on any standard chair. They were able to sleep in any comfortable position without a pillow between their legs. Participants began outpatient physical therapy on hospital discharge. |
|
| Outcomes | Follow‐up intervals: 1, 3 and 12 months. Outcome measures: Short Form‐12 (SF‐12); Harris Hip Score; number of days until they walked with a stick; number of days until they walked without a stick; number of days until they drove; number of days until they walked without a limp (as assessed as limitations in personal ADL); participant satisfaction; complications (we included all these outcomes in this review). Data on the Harris Hip Score was only presented at the 3 month follow‐up interval. |
|
| Notes | Limited information provided on SF‐12, Harris Hip Score or participant satisfaction. Outcomes of interest not presented included: pain; HRQOL; global assessment of treatment success; reoperation rate; total adverse events; restriction in EADL; societal reintegration; length of hospital stay and cost‐analysis. Power calculation for the estimation of incidence of dislocation reported. We requested the following data from the study authors: mean and SD values and sample size in each group, at each follow‐up intervals, for data on: Harris Hip Score, SF‐12 and total adverse events (if available). The study authors did not respond to this request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Group assignments were generated by the research coordinator using a random‐numbers table” (Page 2, Study Protocol). |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope system was “used on acquisition of informed consent during each patient’s preoperative assessment” (Page 2, Study Protocol). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no blinding of participants or personnel, although we acknowledged that it would have been difficult to blind participants due to the nature of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study authors did not blind assessors to group allocation, which could affect assessment of subjective outcomes including: pain, function, quality of life, rather than of re‐operation or dislocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was unclear whether or not there was attrition or loss to follow‐up at final follow‐up based on the Results section (Page 3). |
| Selective reporting (reporting bias) | High risk | The study authors did not report measures of variance such as SD for the Harris Hip Score, SF‐12 or participant satisfaction (Page 3, Results). |
| Other bias | Low risk | There were no apparent other sources of bias. |
Wong 1990.
| Methods | A multi‐centre (3 sites) randomised controlled trial evaluating the effects of an experimental program on post‐hospital adjustment of early discharged patients after THA. | |
| Participants | N: 146 participants, 146 THA. Gender: early discharge and enhanced recovery: males 24, females 26; conventional discharge and enhanced recovery males 12, females 35; conventional discharge and traditional recovery: males 19, females 29 Mean age: early discharge and enhanced recovery 63.3 years; conventional discharge and enhanced recovery: 71.1 years; conventional discharge and traditional recovery: 64.8 years. BMI: not documented Concurrent medical condition (yes): early discharge and enhanced recovery: 46 (92%); conventional discharge and enhanced recovery: 42 (87.5%); conventional discharge and traditional recovery: 42 (87.4%). Inclusion criteria: all participants listed for THA in the three participating hospitals; if they were English‐speaking; did not experience severe postoperative complications during hospitalisation; and met the following medical discharge criteria: satisfactory range of motion of the operated hip and satisfactory ambulation ability. Exclusion criteria: abnormality of mental state; people who suffered from apparent visual or auditory impairment, or both; and people with severe diseases such as peripheral vascular diseases of the lower extremities and advanced rheumatoid arthritis. |
|
| Interventions | Conventional discharge and enhanced recovery (N = 50): the study authors did not provide any information as to what constituted a ‘conventional’ discharge. The experimental enhanced recovery programme included the following: provision of a pamphlet and videotape and regular posthospital visits by a community health nurse on information: performance of selected ADL e.g. safe method of carrying out activities to promote participants’ self‐care without risk of prosthesis dislocation; required postdischarge exercises for muscle strength and movement; warning signs and symptoms of common complications post‐total hip replacement including deep infection; safe and proper use of different walking aids, bathing, dressing and toileting; expected stages of recovery during the first 6 months; potential impact of operation on participant’s psychological welfare to prevent unrealistic expectations postoperatively; list of all available community resources for people to use. The videotape was shown to these participants between the 5th and 6th postoperative day and the day before discharge. Posthospital visits were made at 1 week, 3 months and 6 months posthospital discharge to assess the ability to cope, planning and implementing the interventions and strategies such as procurement of aids, counselling and reinforcing teaching initiated at hospital. The study authors did not provide information as to what constituted an ‘early’ discharge. Conventional discharge and traditional rehabilitation programme control (N = 48): the study did not provide information as to what constituted a ‘conventional’ discharge. Participants received a “yoked attention‐placebo” visits from the research assistant at 1 week, 3 months and 6 months postdischarge, which consisted of advice on seeking medical and rehabilitation assistance if required regarding surgery‐related problems. Early discharge with the enhanced recovery programme (N = 48): participants allocated to this group received the same enhanced recovery programme as those in the early discharge and enhanced recovery programme. The study did not provide information as to what constituted an ‘early’ discharge. Given the lack of information regarding what constituted 'early' discharge, we included only the comparison between enhanced recovery and traditional rehabilitation as these were the interventions of interest. Therefore, we did not extract or include data from this specific treatment arm in the analysis. |
|
| Outcomes | Follow‐up intervals: 1 week, 3 months and 6 months. Outcome measures: the Objective Functional Capability Index (OFCI); the Subjective Functional Capability Index (SFCI); the Subjective Psychosocial Capability Index (SPSCI); the Knowledge Test Post Hip Arthroplasty Complications (KTPHAC); the Perceived Preparedness For Discharge Scale (PPFDS); the Patient Compliant Scale (PCS) consisted of two subsets: the Compliant Behavior Index (CBI) and the Exercise Compliance Scores (ECS) (outcomes included in this review were OFCI and SFCI). The study authors did not present data on outcomes of interest including: pain, HRQOL; global assessment of treatment success; hip dislocation; reoperation rate; total adverse events; limitations in personal ADL; restrictions in EADL; societal reintegration; length of hospital stay; and cost‐analysis. |
|
| Notes | The study authors did not present a power calculation to base sample size on. There was limited information on the study interventions, particularly on the discharge (early versus conventional) criteria. We requested data from the study authors, including mean and SD values and sample size in each group, at each follow‐up interval, for data on: the OFCI and SFCI. The study authors did not respond to this request. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The study authors did not provide information regarding sequence generation. |
| Allocation concealment (selection bias) | High risk | The study authors did not provide information on whether allocation was concealed or not. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This would have been logistically difficult to achieve for the participant and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study employed assessors blinded to group allocation (“impartial observers”) to collect data on all follow‐up points (Data Collection Procedure, Page 13). However as the participant is the assessor in this case, there was a high risk for the subjective outcome, the SFCI. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There appears to be sufficient evidence that the study authors accounted for all participants in the analyses (Results, Page 13). |
| Selective reporting (reporting bias) | High risk | The study authors only presented P values and no mean/median or SD/interquartile range data (Results, Page 13 to 15). |
| Other bias | Low risk | There were no apparent other sources of bias. |
Abbreviations: ADL ‐ activities of daily living; CBI ‐ Compliant Behavior Index; EADL ‐ extended activities of daily living; ECS ‐ Exercise Compliance Scores; HRQOL ‐ health‐related quality of life; kg/m2 ‐ kilograms per meter squared; KTPHA ‐ Knowledge Test Post Hip Arthroplasty Complications; BMI ‐ body mass index; N ‐ number of participants; OFCI ‐ Objective Functional Capability Index; PPFDS ‐ Perceived Preparedness For Discharge Scale; SD ‐ standard deviation; SF‐12 ‐ Short‐Form 12; SFCI ‐ Subjective Functional Capability Index; THA ‐ total hip arthroplasty
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akarcali 2003 | This study did not assess an intervention of interest to this Cochrane review. |
| Bai 2009 | This study did not assess an intervention of interest to this Cochrane review. |
| Bitterli 2011 | This study assessed a preoperative rather than a postoperative intervention. |
| Gillen 2007 | This was not a randomised controlled trial (RCT). |
| Gromov 2015 | This was not a RCT. |
| Jacofsky 2010 | This study assessed participants after a total knee replacement rather than a total hip replacement. |
| Jepson 2016 | This study assessed a preoperative rather than a postoperative intervention. |
| Lewis 2002 | This study assessed a preoperative rather than a postoperative intervention. |
| McGregor 2004 | This study assessed a preoperative rather than a postoperative intervention. |
| McMurray 2000 | This was not a RCT. |
| Mikkelsen 2014 | This was not a RCT. |
| Satoh 2007 | This was not a RCT. |
| Stewart 2011 | This was not a RCT. |
| Stinnett 1996 | This was not a RCT. |
| Thomas 2010 | This was not a RCT. |
Abbreviations: RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Peters 2015.
| Trial name or title | The need for supine position advise during sleep in the first eight week after a THA to prevent hip dislocation. |
| Methods | Stratified block randomised non‐inferiority controlled trial. The study aim is to test the non‐inferiority hypothesis of differences in early hip dislocation between a group of participants who will be restricted to sleep in supine position and a group without restricted sleeping position during the first 8 weeks after a THA following a posterolateral surgical approach. |
| Participants | Inclusion criteria: people who have/are planned to undergo a primary THA via the posterolateral approach by a high volume orthopaedic surgeon; patients with a ASA‐classification of I or II. Exclusion criteria: blindness; THA within 6 months of the contralateral hip; insufficient knowledge of the Dutch language; Collum fracture; infection of the THA; cognitive dysfunction; wheelchair dependability; hypermobility; alcohol abuse; neurological disorders such as Parkinson's disease and stroke. |
| Interventions | Experimental: sleep position: no restrictions. Participants do not have any restrictions in sleeping position during the first 8 weeks after a THA following a posterolateral surgical approach. Control: sleep position: supine. Participants will be instructed to sleep in a supine position during the first 8 weeks after a total hip replacement following a posterolateral surgical approach |
| Outcomes | Primary outcome measure: percentage of early dislocations within the first 8 weeks after THA. Secondary outcome measures: Hip Disability and Osteoarthritis Outcome Score; quality of sleep (VHS), EQ‐5D, visual analogue scale/numerical rating scale hip pain intensity; compliance to anti‐hip dislocation instructions assessed using a diary for patients to report their compliance with the set of anti‐dislocation instructions, among which is the (daily) reporting of their sleeping position in bed at night; sleeping position preferences. Follow‐up: 8 weeks and 6 months postoperative |
| Starting date | Start date: June 2014 Estimated study completion date: March 2020 Esimated primary competition date: March 2019 (final data collection date for primary outcome measure). |
| Contact information | Anil Peters (Orthopedisch Centrum Oost Nederland). Email: a.peters@ocon.nl |
| Notes | Sponsor: Orthopedisch Centrum Oost Nederland ClinicalTrials.gov identifier: NCT02107248. Protocol published: Peters 2015 |
Abbreviations: THA: total hip arthroplasty.
Differences between protocol and review
Due to the limited number of eligible papers we identified by the search strategy, we were unable to: construct a funnel plot to assess small sample size publication bias; perform a meta‐analysis to pool the data from the included studies; or undertake subgroup or sensitivity analyses for pooled data.
Contributions of authors
PJ, CS and AD developed the search strategy. TS and GS assessed the eligibility of all papers, extracted the data and undertook the 'Risk of bias' assessment for all included studies. TS and AB prepared the narrative synthesis.
TS wrote the first draft of the review.
TS, PJ, AB, GS, AD, ED and CS prepared, reviewed and agreed on the final review. CS is guarantor of this Cochrane review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
The funding for this review is from the NIHR programme grant 'improving patients' experience and outcome of total joint replacement' (RP‐PG‐0407‐10070)
-
West Midlands Strategic Health Authority (WMSHA), UK.
The main review author (PJ) is in receipt of a WMSHA Nursing, Midwifery and Allied health professionals Pre‐PhD training award.
Declarations of interest
Toby Smith has no known conflicts of interest.
Paul Jepson has no known conflicts of interest.
Andrew Beswick has no known conflicts of interest.
Gina Sands has no known conflicts of interest.
Avril Drummond has no known conflicts of interest.
Edward Davis has no known conflicts of interest.
Catherine Sackley has no known conflicts of interest.
New
References
References to studies included in this review
Peak 2005 {published data only}
- Peak EL, Parvizi J, Ciminiello M, Purtill JJ, Sharkey PF, Hozack WJ, et al. The role of patient restrictions in reducing the prevalence of early dislocation following total hip arthroplasty: a randomized, prospective study. Journal of Bone and Joint Surgery 2005;87(2):247‐53. [DOI] [PubMed] [Google Scholar]
Ververeli 2009 {published data only}
- Ververeli PA, Lebby EB, Tyler C, Fouad C. Evaluation of reducing postoperative hip precautions in total hip replacement: a randomized prospective study. Orthopedics 2009;32(12):889. [DOI] [PubMed] [Google Scholar]
Wong 1990 {published data only}
- Wong J, Wong S, Nolde T, Yabsley RH. Effects of an experimental program in post‐hospital adjustment of early discharged patients. International Journal of Nursing Studies 1990;27(1):7‐20. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akarcali 2003 {published data only}
- Akarcali I, Tugay N, Erden N, Kaya D, Atill B, Tokgözoğlu AM. Effect of preoperative education on early functional activities of patients undergoing total hip replacement. Fizyoterapi Rehabilitasyon 2002;13(1):19‐23. [Google Scholar]
Bai 2009 {published data only}
- Bai X. Clinical effects of comprehensive rehabilitation after minimally invasive total hip arthroplasty. Zhongguo Gu Shang [China Journal of Orthopaedics and Traumatology] 2009;22(6):417‐20. [PubMed] [Google Scholar]
Bitterli 2011 {published data only}
- Bitterli R, Sieben JM, Hartmann M, Bruin ED. Pre‐surgical sensorimotor training for patients undergoing total hip replacement: a randomised controlled trial. International Journal of Sports Medicine 2011;32(9):725‐32. [DOI] [PubMed] [Google Scholar]
Gillen 2007 {published data only}
- Gillen G, Berger SM, Lotia S, Morreale J, Siber MI, Trudo WJ. Improving community skills after lower extremity joint replacement. Physical & Occupational Therapy in Geriatrics 2007;25(4):41‐54. [Google Scholar]
Gromov 2015 {published data only}
- Gromov K, Troelsen A, Otte KS, Ørsnes T, Ladelund S, Husted H. Removal of restrictions following primary THA with posterolateral approach does not increase the risk of early dislocation. Acta Orthopaedica 2015;86(4):463‐8. [DOI: 10.3109/17453674.2015.1028009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacofsky 2010 {published data only}
- Jacofsky DJ, Kocisky S, Dixon D, Jacofsky MC. Secure tracks devices improves functional recovery and pain after total knee arthroplasty: a prospective, randomized, pilot study. Surgical Technology International 2010;20:357‐61. [PubMed] [Google Scholar]
Jepson 2016 {published data only}
- Jepson P, Sands G, Beswick AD, Davis ET, Blom AW, Sackley CM. A feasibility randomised controlled trial of pre‐operative occupational therapy to optimise recovery for patients undergoing primary total hip replacement for osteoarthritis (PROOF‐THR). Clinical Rehabilitation 2016;30(2):156‐66. [DOI: 10.1177/0269215515576811] [DOI] [PubMed] [Google Scholar]
Lewis 2002 {published data only}
- Lewis C, Gunta K, Wong D. Patient knowledge, behavior, and satisfaction with the use of a preoperative DVD. Orthopaedic Nursing 2002;21(6):41‐9. [Google Scholar]
McGregor 2004 {published data only}
- McGregor AH, Rylands H, Owen A, Doré CJ, Hughes SP. Does preoperative hip rehabilitation advice improve recovery and patient satisfaction. Journal of Arthroplasty 2004;19(4):464‐8. [DOI] [PubMed] [Google Scholar]
McMurray 2000 {published data only}
- McMurray R, Heaton J, Sloper P, Nettleton S. Variations in the provision of occupational therapy for patients undergoing primary elective total hip replacement in the United Kingdom. British Journal of Occupational Therapy 2000;63(9):451‐5. [Google Scholar]
Mikkelsen 2014 {published data only}
- Mikkelsen LR, Petersen MK, Søballe K, Mikkelsen S, Mechlenburg I. Does reduced movement restrictions and use of assistive devices affect rehabilitation outcome after total hip replacement? A non‐randomized, controlled study. European Journal of Physical and Rehabilitation Medicine 2014;50(4):383‐93. [PUBMED: 24476806] [PubMed] [Google Scholar]
Satoh 2007 {published data only}
- Satoh M, Kawaguchi T. Study on environmental transition of patients after total hip arthroplasty ‐‐ characteristics of dislocation aversion movements. Journal of Japan Academy of Nursing Sciences 2007;27(2):3‐14. [Google Scholar]
Stewart 2011 {published data only}
- Stewart LSP, McMillan IR. How necessary are hip restrictions for avoiding dislocation following hemiarthroplasty or total hip arthroplasty in older patients with a hip fracture?. British Journal of Occupational Therapy 2011;74(3):110‐8. [Google Scholar]
Stinnett 1996 {published data only}
- Stinnett KA. Occupational therapy intervention for the geriatric client receiving acute and subacute services following total hip replacement and femoral fracture repair. Topics in Geriatric Rehabilitation 1996;12(1):23‐31. [Google Scholar]
Thomas 2010 {published data only}
- Thomas WN, Pinkelman LA, Gardine CJ. The reasons for noncompliance with adaptive equipment in patients returning home after a total hip replacement. Physical & Occupational Therapy in Geriatrics 2010;28(2):170‐80. [Google Scholar]
References to ongoing studies
Peters 2015 {published data only}
- Peters A, Tijink M, Veldhuijzen A, Huis in 't Veld R. Reduced patient restrictions following total hip arthroplasty: study protocol for a randomized controlled trial. Trials 2015;16:360. [DOI: 10.1186/s13063-015-0901-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ackerman 2004
- Ackerman IN, Bennell KL. Does pre‐operative physiotherapy improve outcomes from lower limb joint replacement surgery? A systematic review. The Australian Journal of Physiotherapy 2004;50(1):25‐30. [DOI] [PubMed] [Google Scholar]
AOTA 1994
- American Occupational Therapy Association. Policy 5.3.1: Definition of occupational therapy practice for state regulation. American Journal of Occupational Therapy 1994;48(11):1072‐3. [Google Scholar]
Barnsley 2015
- Barnsley L, Barnsley L, Page R. Are hip precautions necessary post total hip arthroplasty? A systematic review. Geriatric Orthopaedic Surgery and Rehabilitation 2015;6(3):230‐5. [DOI: 10.1177/2151458515584640] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrett 2013
- Barrett WP, Turner SE, Leopold JP. Prospective randomized study of direct anterior vs postero‐lateral approach for total hip arthroplasty. Journal of Arthroplasty 2013;28(9):1634‐8. [PUBMED: 23523485] [DOI] [PubMed] [Google Scholar]
Bellamy 1988
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. The Journal of Rheumatology 1988;15(12):1833‐40. [PubMed] [Google Scholar]
Birrell 1999
- Birrell F, Johnell O, Silman A. Projecting the need for hip replacement over the next three decades: influence of changing demography and threshold for surgery. Annals of the Rheumatic Diseases 1999;58(9):569‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2006
- Boutron I, Estellat C, Guittet L, Dechartres A, Sackett DL, Hróbjartsson A, et al. Methods of blinding in reports of randomized controlled trials assessing pharmacologic treatments: a systematic review. PLoS Medicine 2006;3(10):e425. [DOI] [PMC free article] [PubMed] [Google Scholar]
British Orthopaedic Association 2006
- British Orthopaedic Association. Primary total hip replacement: a guide to good practice. 1st Edition. London: British Orthopaedic Association, 2006. [Google Scholar]
Canadian Joint Replacement Registry 2014
- Canadian Joint Replacement Registry. Canadian Joint Replacement Registry: 2012‐2013 Annual Report. 1st Edition. Ottawa, Ontario: Canadian Institute for Health Information, 2014. [Google Scholar]
Cates 2008 [Computer program]
- Cates C. Visual Rx 2.0 NNT Calculator. Dr Chris Cates EBM Website, www.nntonline.net, 2008.
College of Occupational Therapists 2012
- College of Occupational Therapists. Specialist section. Trauma and Orthopaedics. Occupational therapy for adults undergoing total hip replacement. Practice guideline. 1st Edition. London: British Association of Occupational Therapists, 2012. [Google Scholar]
Collin 1988
- Collin C, Wade DT, Davies S, Horne V. The Barthel ADL Index: a reliability study. International Disability Studies 1988;10(2):61‐3. [DOI] [PubMed] [Google Scholar]
Cookson 2011
- Cookson R, Laudicella M. Do the poor cost much more? The relationship between small area income deprivation and length of stay for elective hip replacement in the English NHS from 2001 to 2008. Social Science & Medicine 2011;72(2):173‐84. [DOI] [PubMed] [Google Scholar]
Coudeyre 2007
- Coudeyre E, Jardin C, Givron P, Ribinik P, Revel M, Rannou F. Could preoperative rehabilitation modify postoperative outcomes after total hip and knee arthroplasty? Elaboration of French clinical practice guidelines. Annales de Réadaptation et de Médecine Physique 2007;50(3):189‐97. [DOI] [PubMed] [Google Scholar]
Dauty 2007
- Dauty M, Genty M, Ribinik P. Physical training in rehabilitation programs before and after total hip and knee arthroplasty. Annales de Réadaptation et de Médecine Physique 2007;50(6):462‐8. [DOI] [PubMed] [Google Scholar]
Dawson 1996
- Dawson J, Fitzpatrick R, Carr A, Murray D. Questionnaire on the perceptions of patients about total hip replacement. The Journal of Bone and Joint Surgery. British volume 1996;78(2):185‐90. [PubMed] [Google Scholar]
De Jong 2009
- Jong G, Hsieh CH, Gassaway J, Horn SD, Smout RJ, Putman K, et al. Characterizing rehabilitation services for patients with knee and hip replacement in skilled nursing facilities and inpatient rehabilitation facilities. Archives of Physical Medicine and Rehabilitation 2009;90(8):1269‐83. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Di Monaco 2009
- Monaco M, Vallero F, Tappero R, Cavanna A. Rehabilitation after total hip arthroplasty: a systematic review of controlled trials on physical exercise programs. European Journal of Physical and Rehabilitation Medicine 2009;45(3):303‐17. [PubMed] [Google Scholar]
Drummond 2012
- Drummond A, Coole C, Brewin C, Sinclair E. Hip precautions following primary total hip replacement; a national survey of current occupational therapy practice. British Journal of Occupational Therapy 2012;75(4):164‐70. [Google Scholar]
Fries 1980
- Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis and Rheumatism 1980;23(2):137‐45. [DOI] [PubMed] [Google Scholar]
Fudge 1992
- Fudge S. A perspective on consulting in Guatemala. Occupational Therapy in Health Care 1992;8(1):15‐37. [DOI] [PubMed] [Google Scholar]
Graver 2010
- Graver RM, Deppo L, Harris EM, Feinglass SR. The total “economic cost” of primary total joint replacement surgery in the United States. Proceedings of the American Academy of Orthopaedic Surgeons Annual Meeting; 2010 Mar 9‐13; New Orleans. New Orleans, USA: American Academy of Orthopaedic Surgeons Annual Meeting, 2010.
Grotle 2010
- Grotle M, Garratt AM, Klokkerud M, Løchting I, Uhlig T, Hagen KB. What's in team rehabilitation care after arthroplasty for osteoarthritis? Results from a multicenter, longitudinal study assessing structure, process, and outcome. Physical Therapy 2010;90(1):121‐31. [DOI] [PubMed] [Google Scholar]
Harris 1969
- Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end‐result study using a new method of result evaluation. The Journal of Bone and Joint Surgery. American Edition 1969;51(4):737‐55. [PubMed] [Google Scholar]
Hawker 2006
- Hawker GA. Who, when, and why total joint replacement surgery? The patient’s perspective. Current Opinion in Rheumatology 2006;18(5):526‐30. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hunt 1980
- Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. Journal of Epidemiology and Community Health 1980;34(4):281‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2008
- Khan F, Ng L, Gonzalez S, Hale T, Turner‐Stokes L. Multidisciplinary rehabilitation programmes following joint replacement at the hip and knee in chronic arthropathy. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD004957.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krefting 1992
- Krefting L. Strategies for the development of occupational therapy in the Third World. American Journal of Occupational Therapy 1992;46(8):758‐61. [DOI] [PubMed] [Google Scholar]
Kurtz 2007
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. The Journal of Bone and Joint Surgery. American volume 2007;89(4):780‐5. [DOI] [PubMed] [Google Scholar]
Kuster 2002
- Kuster MS. Exercise recommendations after total joint replacement: a review of the current literature and proposal of scientifically based guidelines. Sports Medicine 2002;37(7):433‐45. [DOI] [PubMed] [Google Scholar]
Lucas 2008
- Lucas B. Total hip and total knee replacement: postoperative nursing management. British Journal of Nursing 2008;17(22):1410‐4. [DOI] [PubMed] [Google Scholar]
McDonald 2014
- McDonald S, Page MJ, Beringer K, Wasiak J, Sprowson A. Preoperative education for hip or knee replacement. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003526.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melzack 1971
- Melzack R, Torgerson WS. On the language of pain. Anesthesiology 1971;34(1):50‐9. [DOI] [PubMed] [Google Scholar]
National Joint Registry 2014
- The National Joint Registry for England, Wales, Northern Ireland. 11th Annual Report 2014. Available at: http://www.njrreports.org.uk/Portals/0/PDFdownloads/NJR%2011th%20Annual%20Report%202014.pdf (accessed 12 December 2014).
NICE 2000
- National Institute for Clinical Excellence. Guidance on the selection of prostheses for primary total hip replacement. Technology Appraisal Guidance ‐ No.2. 1st Edition. London: Department of Health, 2000. [Google Scholar]
NICE 2003
- National Institute for Clinical Excellence. Guidance on the selection of prostheses for primary total hip replacement. NICE Technology Appraisal Guidance No.2. 1st Edition. London: Department of Health, 2003. [Google Scholar]
Nouri 1987
- Nouri FM, Lincoln NB. An extended activities of daily living scale for stroke patients. Clinical Rehabilitation 1987;1(4):301‐5. [Google Scholar]
Orpen 2010
- Orpen N, Harris J. Patients’ perspectives of preoperative home‐based occupational therapy and/or physiotherapy interventions prior to hip replacement. British Journal of Occupational Therapy 2010;73(10):461‐9. [Google Scholar]
Pighills 2011
- Pighills AC, Torgerson TJ, Sheldon TA, Drummond AE, Bland JM. Environmental assessment and modification to prevent falls in older people. Journal of the American Geriatrics Society 2011;59(1):26‐33. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schuling 1993
- Schuling J, Haan R, Limburg M, Groenier KH. The Frenchay Activities Index. Assessment of functional status in stroke patients. Stroke 1993;24(8):1173‐7. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings’ tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shields 1995
- Shields RK, Enloe LJ, Evans RE, Smith KB, Steckel SD. Reliability, validity, and responsiveness of functional tests in patients with total joint replacement. Physical Therapy 1995;75(3):169‐76. [DOI] [PubMed] [Google Scholar]
Sigurdsson 2008
- Sigurdsson E, Siggeirsdottir K, Jonsson H Jr, Gudnason V, Matthiasson T, Jonsson B. Early discharge and home intervention reduces unit costs after total hip replacement: results of a cost analysis in a randomized study. International Journal of Health Care Finance and Economics 2008;8(3):181‐92. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Steultjens 2005
- Steultjens EMJ, Dekker JJ, Bouter LM, Leemrijse CJ, Ende CHM. Evidence of the efficacy of occupational therapy in different conditions: an overview of systematic reviews. Clinical Rehabilitation 2005;19(3):247‐54. [DOI] [PubMed] [Google Scholar]
Stewart 1988
- Stewart AL, Hays RD, Ware JE Jr. The MOS short‐form general health survey. Reliability and validity in a patient population. Medical Care 1988;26(7):724‐35. [DOI] [PubMed] [Google Scholar]
Swedish JRU 2013
- Swedish Hip Arthroplasty Register. [Svenska Höftprotesregistret Årsrapport 2013]. Swedish Hip Arthroplasty Annual Report 2013. 1st Edition. Göteborg: Svenska Höftprotesregistret Registercentrum VGR, 2013. [Google Scholar]
Tian 2010
- Tian W, DeJong G, Munin MC, Smout R. Patterns of rehabilitation after hip arthroplasty and the association with outcomes: an episode of care view. American Journal of Physical Medicine and Rehabilitation 2010;89(11):905‐18. [DOI] [PubMed] [Google Scholar]
van der Weegen 2016
- Weegen, Kornuijt A, Das D. Do lifestyle restrictions and precautions prevent dislocation after total hip arthroplasty? A systematic review and meta‐analysis of the literature. Clinical Rehabilitation 2016;30(4):329‐39. [DOI: 10.1177/0269215515579421] [DOI] [PubMed] [Google Scholar]
Ware 1996
- Ware J Jr, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care 1996;34(3):220‐33. [DOI] [PubMed] [Google Scholar]
Westby 2006
- Westby MD, Kennedy D, Carr S, Brander V, Bell M, Backman C. Post‐acute physiotherapy for primary total hip arthroplasty. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005957] [DOI] [Google Scholar]
Wilson‐Braun 1992
- Wilson‐Braun CE. An occupational therapy experience in Ecuador. Occupational Therapy in Health Care 1992;8(1):1‐14. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jepson 2013
- Jepson P, Beswick A, Smith TO, Sands G, Drummond A, Davis ET, et al. Assistive devices, hip precautions, environmental modifications and training to prevent dislocation and improve function after hip arthroplasty. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010815] [DOI] [PMC free article] [PubMed] [Google Scholar]


