Abstract
Background
This Cochrane review was first published in 2005 and updated in 2007, 2012 and now 2015. Acute bronchiolitis is the leading cause of medical emergencies during winter in children younger than two years of age. Chest physiotherapy is sometimes used to assist infants in the clearance of secretions in order to decrease ventilatory effort.
Objectives
To determine the efficacy of chest physiotherapy in infants aged less than 24 months old with acute bronchiolitis. A secondary objective was to determine the efficacy of different techniques of chest physiotherapy (for example, vibration and percussion and passive forced exhalation).
Search methods
We searched CENTRAL (2015, Issue 9) (accessed 8 July 2015), MEDLINE (1966 to July 2015), MEDLINE in‐process and other non‐indexed citations (July 2015), EMBASE (1990 to July 2015), CINAHL (1982 to July 2015), LILACS (1985 to July 2015), Web of Science (1985 to July 2015) and Pedro (1929 to July 2015).
Selection criteria
Randomised controlled trials (RCTs) in which chest physiotherapy was compared against no intervention or against another type of physiotherapy in bronchiolitis patients younger than 24 months of age.
Data collection and analysis
Two review authors independently extracted data. Primary outcomes were change in the severity status of bronchiolitis and time to recovery. Secondary outcomes were respiratory parameters, duration of oxygen supplementation, length of hospital stay, use of bronchodilators and steroids, adverse events and parents' impression of physiotherapy benefit. No pooling of data was possible.
Main results
We included 12 RCTs (1249 participants), three more than the previous Cochrane review, comparing physiotherapy with no intervention. Five trials (246 participants) evaluated conventional techniques (vibration and percussion plus postural drainage), and seven trials (1003 participants) evaluated passive flow‐oriented expiratory techniques: slow passive expiratory techniques in four trials, and forced passive expiratory techniques in three trials.
Conventional techniques failed to show a benefit in the primary outcome of change in severity status of bronchiolitis measured by means of clinical scores (five trials, 241 participants analysed). Safety of conventional techniques has been studied only anecdotally, with one case of atelectasis, the collapse or closure of the lung resulting in reduced or absent gas exchange, reported in the control arm of one trial.
Slow passive expiratory techniques failed to show a benefit in the primary outcomes of severity status of bronchiolitis and in time to recovery (low quality of evidence). Three trials analysing 286 participants measured severity of bronchiolitis through clinical scores, with no significant differences between groups in any of these trials, conducted in patients with moderate and severe disease. Only one trial observed a transient significant small improvement in the Wang clinical score immediately after the intervention in patients with moderate severity of disease. There is very low quality evidence that slow passive expiratory techniques seem to be safe, as two studies (256 participants) reported that no adverse effects were observed.
Forced passive expiratory techniques failed to show an effect on severity of bronchiolitis in terms of time to recovery (two trials, 509 participants) and time to clinical stability (one trial, 99 participants analysed). This evidence is of high quality and corresponds to patients with severe bronchiolitis. Furthermore, there is also high quality evidence that these techniques are related to an increased risk of transient respiratory destabilisation (risk ratio (RR) 5.4, 95% confidence interval (CI) 1.6 to 18.4, one trial) and vomiting during the procedure (RR 10.2, 95% CI 1.3 to 78.8, one trial). Results are inconclusive for bradycardia with desaturation (RR 1.0, 95% CI 0.2 to 5.0, one trial) and bradycardia without desaturation (RR 3.6, 95% CI 0.7 to 16.9, one trial), due to the limited precision of estimators. However, in mild to moderate bronchiolitis patients, forced expiration combined with conventional techniques produced an immediate relief of disease severity (one trial, 13 participants).
Authors' conclusions
None of the chest physiotherapy techniques analysed in this review (conventional, slow passive expiratory techniques or forced expiratory techniques) have demonstrated a reduction in the severity of disease. For these reasons, these techniques cannot be used as standard clinical practice for hospitalised patients with severe bronchiolitis. There is high quality evidence that forced expiratory techniques in severe patients do not improve their health status and can lead to severe adverse events. Slow passive expiratory techniques provide an immediate and transient relief in moderate patients without impact on duration. Future studies should test the potential effect of slow passive expiratory techniques in mild to moderate non‐hospitalised patients and patients who are respiratory syncytial virus (RSV) positive. Also, they could explore the combination of chest physiotherapy with salbutamol or hypertonic saline.
Plain language summary
Chest physiotherapy for acute bronchiolitis in children younger than two years of age
Review question
We reviewed the evidence about the effect of chest physiotherapy in infants younger than two years of age with acute bronchiolitis.
Background
Acute bronchiolitis is a frequent viral respiratory infection in children younger than two years of age. Most children have a mild disease and do not require hospitalisation. Those who do need to be hospitalised sometimes have difficulty clearing phlegm (thick mucous respiratory secretions caused by the infection). It has been proposed that chest physiotherapy may assist in the clearance of respiratory secretions and improve breathing. There are three different types of chest physiotherapy available: vibration and percussion, forced expiratory techniques and slow flow techniques that avoid blockage of the airway.
Study characteristics
The evidence is current to July 2015. This review has included 12 trials with a total of 1249 participants. By type of chest physiotherapy, five trials tested vibration and percussion techniques in 246 participants, three trials tested forced expiratory techniques in 624 participants, and four trials tested slow flow techniques in 375 participants.
Key results
Vibration and percussion techniques produce a thorax (chest) oscillation by fast compression or percussion with the physiotherapist's hands. Neither manoeuvre was shown to improve the clinical scores of patients with acute bronchiolitis in the trials. These techniques did not show improvements in respiratory measurements, time on oxygen therapy or length of hospital stay. There were no data on time to recovery from acute bronchiolitis, use of bronchodilators or steroids, or parents' assessment of physiotherapy benefit. The trials included in this review did not present data on adverse effects related to the intervention, but the literature cites cases of relevant adverse effects such as rib fractures related to these techniques.
Forced expiratory techniques consist of suddenly increasing the expiratory flow by compressing the thorax or the abdomen. In participants with severe bronchiolitis, such techniques failed to reduce time to recovery or time to clinical stability when compared to no physiotherapy. They also failed to improve clinical scores, oxygen saturation or respiratory rates except in mild to moderate bronchiolitis patients. There were no data on secondary outcomes such as duration of oxygen supplementation, length of hospital stay, or use of bronchodilators and steroids. Two studies reported no significant differences in parents' impression of the benefit of physiotherapy compared to controls. One of the trials reported a higher number of transient episodes of vomiting and respiratory instability after forced expiratory physiotherapy. This trial found no differences for bradycardias (decreases in heart rate), with and without desaturation (reduced oxygen levels in blood).
Slow flow techniques consist of compressing the rib cage and the abdominal cavity gradually and gently from the mid‐expiratory phase up to the end of exhalation. Slow flow techniques showed an overall lack of benefit on clinical scores of severity of the disease. However, in two trials they provided either a short‐lived relief in terms of clinical scores or a decrease in the need for oxygen support in children with moderate bronchiolitis. There were no changes in length of hospital stay, use of bronchodilators or steroids. There were no data on changes in time to recovery, change in respiratory measurements, or parents' impression of physiotherapy benefit. No severe adverse events were reported in the trials.
Quality of the evidence
Vibration and percussion techniques are not recommended in routine practice in hospital settings due to a lack of benefit and risk of potential adverse events. There is high quality evidence that forced expiratory techniques in severe bronchiolitis present no clinical benefit, while being related to adverse effects such as vomiting, bradycardia with desaturation, or transient respiratory destabilisation. There is low quality evidence that suggests that slow flow techniques do not provide a clear overall benefit, but could provide some transient benefits in some children with bronchiolitis. Except for one trial, related to forced expiration, the included trials are at unclear or high risk of bias. The risk of bias of the trials and the imprecision of the estimates led to the low quality of evidence for the effect of slow flow techniques on clinical scores. Further trials are needed before reaching firm conclusions.
Summary of findings
Background
Description of the condition
Acute bronchiolitis is the leading cause of Emergency Department visits during winter in children younger than two years of age. It results in high utilisation of healthcare resources and is an increasing burden on outpatient practices, Emergency Departments and hospitals (Carroll 2008). It also results in significant morbidity for infants. Infant mortality rates vary depending upon the population. In high‐income countries, incidence of bronchiolitis‐associated deaths is low, and due mainly to patients with severe comorbidities (e.g. congenital heart disease, etc.). For example, it was reported to be 2 per 10,000 live births in the USA in the 1990s (Holman 2003) and 1.82 per 100,000 in the UK in 2000 (Panickar 2005). Furthermore, there is strong evidence of irreversible airway damage and reduced lung function in adults who were hospitalised for bronchiolitis in infancy (Sigurs 2010). Children who have had respiratory syncytial virus (RSV) disease in early life have been shown to have a higher incidence of asthma/wheezing in later life (odds ratio 3.84: Régnier 2013).
Some years ago, the American Academy of Pediatrics published a statement on the diagnosis and treatment of bronchiolitis (AAPs 2006). However, criteria for diagnosing acute bronchiolitis vary greatly. Most doctors agree that the case definition for an episode of acute bronchiolitis should include children aged 24 months or younger who have a first episode of acute wheezing accompanied by physical findings of viral infection (for example, coryza, cough and fever) (González 2001; Videla 1998; Wainwright 2003). The most prevalent virus identified with the disease is RSV.
Most cases of acute bronchiolitis are mild and can be treated on an outpatient basis; 1% to 3% (depending on the severity of the disease) will require hospitalisation (Ralston 2014). Risk factors associated with the need for hospitalisation are young age, premature birth, chronic lung disease, congenital heart disease and a deficient immune system (AAPs 2006). In low‐income countries the most frequent risk factors associated with hospitalisation and severe disease include living in a low‐income family, malnourishment, low birthweight, age of the mother, mother's education level, being bottle‐fed and premature birth (Smyth 2006; Spencer 1996).
Description of the intervention
The standard treatment of acute bronchiolitis is to ensure adequate oxygenation, fluid intake and feeding of the infant (AAP 2006; SIGN 2006). Pharmacological strategies considered in acute bronchiolitis include bronchodilators, antibiotics and steroids but their effectiveness remains quite uncertain and current guidelines do not recommend their use (AAPs 2006; SIGN 2006). There is no evidence to support the use of glucocorticoids or antibiotics (Farley 2014; Fernandes 2013), and although there is some evidence that bronchodilators, nebulised hypertonic saline, epinephrine and heliox therapy may have some benefit in terms of improving clinical scores (Gadomski 2014; Hartling 2011; Liet 2010; Umoren 2011; Zhang 2011), this benefit must be weighed against the lack of benefit in reducing the duration or severity of illness, costs and adverse effects.
Chest physiotherapy has been proposed to assist in the clearance of tracheo‐bronchial secretions. The main goal is to clear the airway obstruction, reduce airway resistance, enhance gas exchange and reduce the work of breathing. Different techniques are used in paediatric patients: 1) the conventional chest physical therapy (cCPT) such as chest percussion and vibration in combination with postural drainage positions, chest shaking and directed coughing and 2) the flow‐based techniques: slow or forced passive expiration may help to mobilise secretions towards the trachea and trigger coughing that helps to remove secretions. Specific measures are recommended to prevent spreading of the disease during the procedure, such as cohort segregation, hand washing and wearing gowns, masks, gloves and goggles (Hall 1981). However, conventional chest physiotherapy techniques may have drawbacks: it has been claimed that they might cause distress to the infant and concerns have arisen about the safety of the procedure, especially in relation to rib fractures in patients at risk (Beeby 1998; Chalumeau 2002; Chanelière 2006).
Why it is important to do this review
At the time of the first publication of this review, there was uncertainty about the efficacy of conventional physiotherapy techniques (vibration and percussion). The review challenged their application in daily practice, prompting the recommendation that chest physiotherapy based on vibration and percussion not be applied routinely in hospital settings (AAP 2006; BGT 2005; SIGN 2006). However, chest physiotherapy is still being applied in outpatient and inpatient settings (Barben 2008; González 2010a). Parents' expectation and demand for chest physiotherapy in clinical daily practice may explain its widespread use (Sanchez 2007).
New and gentler passive expiratory physiotherapy techniques have become mainstream in several countries. In France, passive forced exhalation techniques are recommended by a consensus panel both for inpatient and outpatient cases (Beauvois 2001; Consensus 2001), with extremely high implementation in outpatient settings (David 2010; Halna 2005; Touzet 2007). However, lately there seems to be contrary practice to the routine use of respiratory physiotherapy in bronchiolitis. Other countries such as Chile also report using chest physiotherapy in outpatient and inpatient settings, although it is not clear which techniques are applied (Girardi 2001). These changes motivated a shift in the focus of the review, in order to assess the efficacy and safety of passive expiratory techniques, and to explore the differential effect of chest physiotherapy depending on the technique used, severity of the patients and setting of implementation.
Objectives
To determine the efficacy of chest physiotherapy in infants aged less than 24 months old with acute bronchiolitis. A secondary objective was to determine the efficacy of different techniques of chest physiotherapy (for example, vibration and percussion and passive forced exhalation).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) evaluating chest physiotherapy in acute bronchiolitis.
Types of participants
Infants younger than 24 months of age with acute bronchiolitis as defined by the trial authors, in all settings.
Types of interventions
We included trials that compared any type of chest physiotherapy (postural drainage, chest percussion, vibration, chest shaking, directed coughing, slow or forced expiration techniques) versus standard care or other physiotherapy, drainage or breathing techniques.
The interventions are classified into two main categories: vibration and percussion, and passive expiratory techniques. Passive expiratory techniques are further subdivided into slow passive expiratory techniques and forced passive expiratory techniques.
Types of outcome measures
Primary outcomes
Change in the severity status of bronchiolitis.
Time to recovery.
Secondary outcomes
Respiratory parameters (oxygen saturation levels, transcutaneous carbon dioxide partial pressure (PaCO2)).
Duration of oxygen supplementation.
Length of hospital stay.
Use of bronchodilators and steroids.
Parents' impression of physiotherapy benefit.
Adverse events. We defined adverse events as any undesired outcome due to the intervention. For example, rib fractures, bradycardia, respiratory instability, vomiting or long‐term neurological disabilities. We took all outcomes into consideration. We described the method used to measure any adverse events.
Search methods for identification of studies
Electronic searches
In this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 9) (accessed 8 July 2015), the Cochrane Acute Respiratory Infections Group's Specialised Register (October 2011 to July 2015), MEDLINE and MEDLINE in‐process and other non‐indexed citations (October 2011 to July 2015), EMBASE (October 2011 to July 2015), CINAHL (October 2011 to July 2015), LILACS (October 2011 to July 2015), Web of Science (October 2011 to July 2015) and Pedro (October 2011 to July 2015). See Appendix 1 for details of previous searches.
We used the search strategy described in Appendix 2 to search CENTRAL and MEDLINE. We did not combine the search strategy with a filter for identifying randomised trials as there were too few results. We adapted the search strategy to search MEDLINE in‐process (Appendix 3); EMBASE (Appendix 4); CINAHL (Appendix 5); LILACS (Appendix 6) and Web of Science (Appendix 7).
Searching other resources
In the first publication of this review, we examined the reference lists of general paediatric, infectious diseases, pneumatology and physiotherapy textbooks. We reviewed reference lists of all selected articles and recent review articles and also examined published abstracts from the Pediatric Academic Societies' Annual Meetings (US) (1999 to 2003). We handsearched the French journalsJournal Pédiatrie Puériculture (1999 to May 2004) and Archives de Pédiatrie (1994 to 1997; 2000 to May 2004). We also searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and www.ClinicalTrials.gov trials registers with the search terms bronchiolitis AND "chest physiotherapy" for completed and ongoing studies (latest search 8 July 2015).
Data collection and analysis
Selection of studies
Three review authors (CG, MG, MR) independently screened the results from the initial search of all the databases and reference lists to identify citations that seemed relevant to this review. We obtained the full‐text articles once pertinent abstracts or titles were identified. Four review authors (CG, MG, MR, JV) independently decided on which trials to include using a standard form. There were no disagreements in relation to the included trials. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (see Figure 1) (Moher 2009) and Characteristics of excluded studies table.
1.
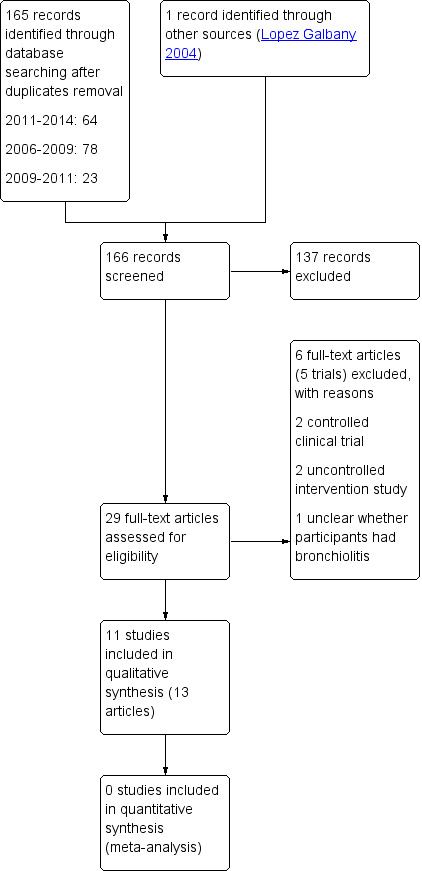
Study flow diagram
Data extraction and management
Two review authors (MR, MG) independently extracted the data. We used a standard form to extract the following data.
Characteristics of the study (design, method of randomisation, withdrawals, drop‐outs).
Participants (age, gender, low birth weight or normal weight, ambulatory or hospital patients, disease severity, nutritional status).
Intervention (type of chest physiotherapy, administration, co‐interventions) and its comparator.
Outcomes (types of outcome measures, timing of outcomes, adverse effects).
Results.
Assessment of risk of bias in included studies
Two review authors (MG, MR) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
1. Sequence generation (selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
2. Allocation concealment (selection bias)
We described for each included study the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
3. Blinding of outcome assessment (detection bias)
Blinding of study participants and personnel was not possible due to the characteristics of the interventions studied. We described for each included study all the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We also provided information on whether the intended blinding was effective. Where blinding was not possible, we assessed whether the lack of blinding was likely to have introduced bias. We assessed the methods as:
adequate;
high risk of bias; or
unclear risk of bias.
4. Incomplete outcome data (attrition bias through withdrawals, drop‐outs, protocol deviations)
We described for each included study and for each outcome or class of outcomes the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported and whether missing data were balanced across groups or were related to outcomes. We assessed whether each study was at risk of attrition bias:
low risk of bias;
high risk of bias; or
unclear risk of bias.
5. Selective reporting bias
We described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all of the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
6. Other sources of bias
We described for each included study any important concerns we have about other possible sources of bias, in particular about contamination. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of bias;
high risk of bias; or
unclear risk of bias.
Measures of treatment effect
We estimated the effect of treatment by mean differences (MDs) in continuous outcomes and risks ratios (RRs) in dichotomous outcomes, with their corresponding confidence intervals (CIs).
Unit of analysis issues
We would have assessed their data analysis in search of possible unit of analysis errors if any cluster‐randomised trials had been included in the review. We would have combined them with individually randomised trials if no errors were observed. We did not expect to identify any cross‐over randomised trial on this topic given the short course of bronchiolitis.
Dealing with missing data
We assessed the impact of missing data on the results from the 'Risk of bias' assessment, considering for each trial the magnitude of missing data and how it was dealt with. We tried to assess how many patients were excluded from the trials analysis, which treatment group they belonged to, what were the causes for excluding them and whether their exclusion was biased the trials results. If a quantitative analyses had been performed, the main analysis would be based on available data and a secondary intention‐to‐treat (ITT) sensitivity analysis would have been performed for dichotomous outcomes. The ITT sub‐analysis would have used imputation, assuming that all missing data corresponded to a negative outcome.
Assessment of heterogeneity
We would have assessed statistical heterogeneity with the I² statistic, considering values I² ≥ 50% as a sign of moderate to high heterogeneity if the trials included had been similar enough to perform a quantitative analysis (Higgins 2003).
Assessment of reporting biases
We did not explore publication bias and other reporting biases statistically or graphically due to the lack of statistical data in the included studies.
Data synthesis
We did not perform a meta‐analysis due to clinical heterogeneity and statistical considerations. We described the individual results with the effect measures described in the original trials. If the included trials had been similar enough to combine them, a statistical pooling of effect measures would have been performed with a random‐effects model, applying the inverse‐variance method. We wrote the review using Review Manager 5.3 (RevMan 2014).
GRADE and 'Summary of findings' table
We added 'Summary of findings' tables to this 2014 update, comparing slow passive expiration techniques with no physiotherapy and forced expiration techniques with no physiotherapy. The outcomes included: time to recovery/clinical stability, clinical score and adverse effects. Since we did not perform a meta‐analysis in the review, we did not present illustrative comparative risks in the tables. We assessed the quality of evidence using the GRADE system. We used the guidelines of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group to assess the quality of the evidence related to selected outcomes (Guyatt 2008). The GRADE system assesses the quality of evidence based on the extent to which users can be confident that an association reflects the item being evaluated (Guyatt 2008). Assessment of the quality of evidence included risk of bias, heterogeneity, directness of the evidence, risk of publication bias and precision of effect estimates, among others (Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g). We developed 'Summaries of findings' tables with the GRADE profiler software (GRADEpro GDT 2015).
Subgroup analysis and investigation of heterogeneity
In the 2014 update, we proposed two subgroup analyses based on the hypothesis that performance of slow flow chest physiotherapy techniques could depend on the patient's severity and, consequently, on setting (inpatient versus outpatient). We introduced a subgroup analysis by patient severity, classifying trials into severe/moderate/unknown categories depending on the inclusion criteria of the trial, or on the characteristics of the included participants.
We proposed a subgroup analysis by setting, classifying trials into inpatient/outpatient categories, under the hypothesis that patients with more severe bronchiolitis would be seen in inpatient settings, while outpatient settings would attend a variable pool of patients, but mostly with moderate or low levels of bronchiolitis severity.
Sensitivity analysis
If a quantitative analyses had been performed, we would have carried out an ITT sensitivity analysis for dichotomous outcomes, imputing all missing data as a negative outcome.
Results
Description of studies
Results of the search
In the search update up to July 2015, we retrieved 129 unique records from the databases searched, and we included three new trials (Gomes 2012; Remondini 2014; Sanchez Bayle 2012). The Gomes paper corresponds to an ongoing trial included in previous review versions (Clinicaltrials.gov identifier NCT00884429). We identified one ongoing trial (Bella Lisbôa 2008).
Included studies
See Characteristics of included studies table.
We included 12 RCTs in this review totaling 1249 participants (Aviram 1992; Bohe 2004; De Córdoba 2008; Gajdos 2010; Gomes 2012; Lopez Galbany 2004; Nicholas 1999; Postiaux 2011; Remondini 2014; Rochat 2010; Sanchez Bayle 2012; Webb 1985).
A description of included trials by type of intervention is shown in Table 3. Five trials assessed percussion and vibration techniques in 246 randomised participants (Aviram 1992; Bohe 2004; De Córdoba 2008; Nicholas 1999; Webb 1985), while six trials assessed different passive flow‐oriented expiratory techniques in 974 randomised participants. Three of these trials assessed forced expiration techniques (Gajdos 2010; Remondini 2014; Rochat 2010), and four trials assessed slow flow techniques (Gomes 2012; Lopez Galbany 2004; Postiaux 2011; Sanchez Bayle 2012). The Gomes 2012 trial assessed the effect of slow flow passive expiratory techniques (slow flow) against vibration and percussion techniques. All 12 trials evaluated the efficacy of chest physiotherapy in hospitalised infants with a clinical diagnosis of acute bronchiolitis.
1. Chest physiotherapy effects grouped by the applied technique.
| Chest physiotherapy technique | Patients' severity | Comparison | Results | |||
| De Córdoba 2008 | Conventional vibration + PD (G1) |
Mild | Percus + PD (G2) Suctioning (G3) | SaO2 P15 = 0.05 (G1, G2) HR P15 = 0.05 (G1, G2) RR P15 = ns Aspired mucus = 0.02 (G3) Score discomfort P15 = 0.05 (G1, G2) |
||
| Bohe 2004 | Conventional | Moderate | No intervention + suctioning | LoS = ns Score = ns |
||
| Nicholas 1999 | Conventional | Severe | No intervention | Severity score = ns SaO2 = ns |
||
| Aviram 1992 | Conventional | na | No intervention | LoS = ns Socre = ns SaO2 = ns |
||
| Webb 1985 | Conventional | Moderate | No intervention | Score = ns LoS = ns |
||
| Sanchez Bayle 2012 | Slow expiration | Severe | Postural changes (Sham) | LoS = ns O2 supply = ns; if RSV+ = 0.04 Respiratory complications = ns Drug administration = ns |
||
| Gomes 2012 | Slow expiration + nasal drainage (G1) | Moderate | Conventional (G2) Suctioning (G3) |
Score pre‐post = 0.05 (G1, G2) Score 48 h = 0.05 (G1, G2) Score 72 h = 0.05 (G1) |
||
| Postiaux 2011 | Slow expiration + induced cough + albuterol 3 mL + 3% NaCl | Moderate | Albuterol 3 mL + 3% NaCl | Score T30 =0.02 Score T150 = ns Score day 1 to discharge = 0.002 LoS = ns |
||
| Lopez Galbany 2004 | Slow expiration | Moderate | No intervention | Score = ns LoS = ns O2 supply = ns |
||
| Remondini 2014 | Conventional physiotherapy (postural drainage associated with tapping or percussion and tracheal aspiration) + expiratory acceleration flow | Mild‐moderate | Conventional | SaO2 = ns Score 10 to 60 min = 0.001 (G1, G2) Time to recovery = ns |
||
| Rochat 2010 | Slow + forced expiration + induced cough | Severe | No intervention | Time to stability = ns Clinical score = ns Respiratory score = 0.04 |
||
| Gajdos 2010 | Forced expiration + assisted cough | Severe | Nasal suctioning | Time to recovery = ns Adverse side effects = 0.005 Parents perception = ns |
||
NaCl: hypertonic saline solution AE: adverse side effects AVB: acute viruses of bronchiolitis CG: control group Conventional: conventional chest physical therapy (CPT = postural drainage, percussion, vibration and suctioning) HR P15: heart rate post 15 minutes of intervention IG: intervention group LoS: length of stay NA: not applicable No intervention: usual medical care (bronchodilators, corticoids, oxygen therapy if needed and suctioning) ns: non‐significant O2 supply: time of oxygen delivered during treatment
PCO2: Carbon dioxide arterial pressure RR P15: respiratory rate post 15 minutes of intervention RSV: respiratory syncytial virus SaO2 P15: pulse blood oxygen saturation post 15 minutes of intervention
SpO2: pulse blood oxygen saturation Score T150: score evolution 150 minutes post treatment Score T30: score evolution 30 minutes post treatment Score: clinical score used to determine disease severity
The trials were classified by the clinical severity of the included infants, as reported in the papers or as estimated by the review authors. Clinical severity of participants was mild in one trial (De Córdoba 2008 1.9 mean Silverman‐Anderson score at baseline, out of 10 maximum score), moderate in six trials (Bohe 2004 5.7 mean Wang score at baseline; Gomes 2012 75% of participants with a four to eight points in Wang score; Postiaux 2011 5.75 mean Wang score at baseline; Webb 1985 11 mean clinical score at admission over 30 maximum score; Lopez Galbany 2004 5.6 mean Wang score at baseline; Remondini 2014 5.8 mean respiratory distress assessment instrument (RDAI) score at baseline) and severe in four trials (Gajdos 2010; Nicholas 1999; Rochat 2010; Sanchez Bayle 2012). Also, in these studies with severe bronchiolitis patients, they included infants who required nasogastric feeding or intravenous fluid. The severity of bronchiolitis in one trial was unknown (Aviram 1992).
The studies were carried out in the UK (Nicholas 1999; Webb 1985), Spain (Lopez Galbany 2004; Sanchez Bayle 2012), Brazil (De Córdoba 2008; Gomes 2012; Remondini 2014), France (Gajdos 2010), Belgium (Postiaux 2011), Israel (Aviram 1992), Argentina (Bohe 2004), and Switzerland (Rochat 2010).
Two of the included trials are unpublished and we contacted the trial authors for further clarification and data gathering (Aviram 1992; Lopez Galbany 2004). We contacted the authors of several trials asking for clarification and additional information, with positive responses (Aviram 1992; Gomes 2012; Lopez Galbany 2004; Postiaux 2011; Rochat 2010; Sanchez Bayle 2012).
Finally, only two studies reported specific funding from governmental organisations (Gajdos 2010; Rochat 2010). Two declared no conflicts of interest (Postiaux 2011; Sanchez Bayle 2012), and the other studies did not specify any conflicts of interest.
Published trials
A recent trial was conducted in Brazil included 29 infants younger than one year admitted to hospital with a diagnosis of acute bronchiolitis (Remondini 2014). Patients that presented with congenital heart diease, neuropathy, underlying lung disease, indication for ventilatory support, RDAI score ≤ four associated to SpO2 ≥ 92% were excluded. Patients were randomly allocated in two intervention groups. One (n = 16) underwent postural drainage associated to percussion and tracheal aspiration and the other group (n = 13), underwent postural drainage associated with forced passive expiratory technique and tracheal aspiration. Patients were assessed three times a day (before, 10 and 60 minutes after the physiotherapy intervention) by the same therapist. The endpoint was to compare the efficacy of both techniques in improving RDAI and SpO2. Trial authors considered discharging patients from the study when the RDAI score was ≤ four, which was associated with adequate oxygenation (SpO2 ≥ 92%). The total number of sessions was 83; 48 in conventional group and 35 in force expiratory group. The physiotherapist in charge of the infant determined the number of sessions according to the disease severity. The session numbers ranged from one to four a day.
A trial conducted in Spain and recruited 293 infants less than seven months old admitted to hospital with a diagnosis of first episode of acute bronchiolitis by the McConnochie 1993 criteria and at least one of the following signs: toxic aspect; history of apnoea or cyanosis; respiratory rate > 60; or pulse oxymetry < 94%. Inclusion criteria and signed informed consents were conducted after randomisation, leading to the exclusion of 40 randomised participants not meeting the criteria, and 16 participants whose parents refused consent because of the blinded design of the study that prevented knowing the intervention received (Sanchez Bayle 2012). Participants were allocated to receive either prolonged slow expiratory technique with manual vibration and assisted cough (n = 136) or postural changes plus oxygen therapy until pulse oximetry oxygen saturation (SpO2) >= 94% (n = 100). All interventions were administered twice a day and only the physiotherapists were aware of the allocation group of the infants. Parents, doctors and nurses were unaware of the treatment allocation during the study. The two groups were similar with regard to age, sex, duration of symptoms prior to hospital admission, fever, respiratory distress, clinical and respiratory severity score on admission, respiratory syncytial virus (RSV) positive, oxygen saturation and biochemical results. Two‐thirds of the participants were RSV‐positive. The primary outcomes were duration of oxygen supplementation and length of hospital stay. Secondary outcomes were salbutamol use, ipratropium bromide use, antibiotics use, adrenaline use and incidence of pneumonia.
Another recent trial was conducted in Brazil and included 30 infants up to two years of age, previously healthy, with a clinical diagnosis of acute viral bronchiolitis and positive outcome of RSV in nasopharyngeal aspirate detected by immunofluorescence technique (Gomes 2012). Participants were allocated to receive either prolonged slow expiration (slow passive and progressive expiration from the functional residual capacity into the expiratory reserve volume) and rhinopharyngeal retrograde clearance (forced inspiratory manoeuvre through the nose) (n = 10) or vibrations, expiratory compression, modified postural drainage only in the lateral decubitus position and clapping (n = 10) or suction of the upper airways (n = 10). The third group was only assessed at admission, and afterwards followed the standard chest physiotherapy regimen in the hospital; this group was not considered in this review. The two groups were similar with regard to age, sex, weight and clinical score. The primary outcomes were Wang's clinical score. Secondary outcomes were retractions and SpO2. Assessors were blinded to the treatment groups.
A trial conducted in Belgium recruited 20 infants with acute RSV bronchiolitis, with a mean age of 4.19 months (Postiaux 2011). Infants were randomised to inhalation of a 3% hypertonic saline solution and salbutamol (n = 8) or to a physiotherapy protocol combining prolonged slow expiration technique and coughing provoked after the same inhalation of saline solution and salbutamol (n = 12). The two groups were similar with regards to age, sex and Wang clinical severity score on admission (Wang 1992). The trial main outcome is Wang's clinical score, which assigns a value between zero and three to each of the four variables: respiratory rate, wheezing, retractions and general condition. The maximum Wang score is 12 and a higher Wang score indicates a worse condition. Secondary outcomes were SpO2 and heart rate (HR). All outcomes were assessed before the session, at the end of the session and two hours afterwards. Both of the paediatric evaluators were blinded to the applied treatment and goals. Physiotherapists in charge of administering the treatments were instructed to ignore the results of each evaluation until the end of the study. The participants' parents were unaware of the group in which their child was included. In both groups the periods of time spent in the room were identical, so outside observers were blinded to the applied treatment.
The largest trial was conducted in France, randomising 496 hospitalised infants with a first acute bronchiolitis episode between the ages of 15 days and 24 months (mean age two months, range 1.3 to 3.9 months) (Gajdos 2010). Infants had to present with at least one of the following on admission: toxic aspect; history of apnoea or cyanosis; respiratory rate > 60/minute, pulse oxymetry < 95%, alimentary intake < two‐thirds of the daily food requirements. The control group presented with a higher proportion of RSV‐positive patients than the intervention group (76.4% versus 73.3%), as well as the proportion of cases of lung atelectasis diagnosis on chest X‐ray (12.9% versus 7.6%). Patients were allocated to receive either the passive forced exhalation technique with assisted cough (n = 246) or nasal suction (n = 250). All interventions were administered three times a day, with the physiotherapist staying alone with the infant in a room with a covered window pane. The primary outcome was time to recovery, defined as eight hours without oxygen supplementation associated with minimal or no chest recession and ingesting more than two‐thirds of the daily food requirements. Survival analyses of time to recovery were adjusted for prognostic baseline covariates (personal eczema or history of atopy, age in months, hypoxaemia at randomisation, need for intravenous (IV) fluids at randomisation, atelectasis at randomisation, duration of symptoms, use of mucolytic before randomisation or RSV infection). The therapists were not involved in the evaluation of time to recovery. Secondary outcomes were intensive care unit admissions, artificial ventilation, antibiotic treatment, description of side effects during procedures and parental perception of comfort.
Rochat 2010 analysed 99 infants admitted to a Swiss hospital with bronchiolitis during two consecutive RSV seasons (2005 to 2006 and 2006 to 2007). Participants had a mean age of 3.9 months. All infants received standard care including oxygen therapy and rhinopharyngeal suctioning. Infants were either randomised to additionally receive a physiotherapy protocol combining prolonged slow expiratory technique, slow accelerated expiratory technique and coughing provoked (n = 51), or randomised to no physiotherapy (n = 53). The two groups were similar with regard to age, sex, clinical and respiratory severity score on admission, proportion who were RSV Enzyme‐Linked ImmunoSorbent Assay (ELISA) positive (overall proportion 75%) and history of eczema (overall proportion 7%). The trial assessed time to clinical stability, clinical and respiratory scores, respiratory rate, pulse oximetry oxygen saturation (SpO2) and complications such as transfer to the intensive care unit.
De Córdoba 2008 randomised 24 hospitalised infants below two years of age, in Brazil. Nineteen of those infants were analysed, of whom five were allocated to vibration and postural drainage, eight to percussion and postural drainage and six to the control group (bronchial aspiration). Infants had to present clinical and laboratory signs of acute viral bronchiolitis and bronchial hypersecretion (pulmonary auscultation). There was no information on percentage of RSV patients or patients with lung collapse/consolidation at baseline or during the trial. The three groups were similar with regard to age, sex, oxygen saturation and cardiac and respiratory frequency on admission. Mean age was 93 days, 131 days and 125 days in each intervention group. The main outcomes were: saturation of oxygen pulse, cardiac frequency, respiratory frequency, Silverman‐Anderson Score of respiratory discomfort (Silverman 1956), and amount of inhaled secretions. Outcomes were assessed immediately after treatment and 15 minutes later. Results were expressed as means and standard deviations (SDs).
In the Bohe 2004 study conducted in Argentina, 16 infants were randomly allocated to the physiotherapy group and 16 to the control group. Patients were included if they had a clinical diagnosis of acute bronchiolitis defined by an acute upper respiratory infection plus fever, tachypnoea or increase of respiratory effort. The mean age of the participants was 2.8 months and 78.1% of participants were positive for RSV. There was no information on the percentage of patients with atelectasis/consolidation at baseline or during the trial. The intervention was percussion, postural drainage, vibration and nasopharyngeal aspiration twice a day. The control group received only nasopharyngeal aspiration. The endpoints were length of hospital stay and a severity score constructed out of five clinical variables: respiratory rate, heart rate, lung auscultation and accessory muscle use.
A trial conducted in the UK randomly allocated 50 infants to control (n = 24) or treatment (n = 26) groups; their mean age was 2.8 months (range 0.4 to 7.6 months). Infants had to present clinical diagnoses of acute bronchiolitis and severe respiratory distress requiring nasogastric tube feeding or intravenous fluids (Nicholas 1999). The intervention and control groups presented similar proportions of RSV‐positive patients (79% versus 85%). There was no information on atelectasis/consolidation at study entry or afterwards. The physiotherapy protocol established manual techniques of percussion and vibrations performed in postural drainage positions with possible modifications as required in relation to infant tolerance. The main outcomes were clinical status and length of hospital stay. Secondary endpoints were oxygen requirements and change in oxygen saturation levels after physiotherapy; these outcomes were measured only in the intervention arm. Results were expressed using means but standard deviations (SDs) were not reported. The trial author could not provide clarification as she was no longer in possession of the complete database.
The oldest trial was conducted in the UK and analysed 90 infants with a mean age of 4.6 months (range 0 to 15 months) presenting a clinical diagnosis of acute viral bronchiolitis (Webb 1985). Forty‐four infants were allocated to physiotherapy and 46 infants to the control group. The two groups were similar with regards to age, sex, severity score on admission, proportion who were RSV‐positive (overall proportion 69%), proportion with a first‐degree family history of atopy (overall proportion 36%), those participants with smokers in their household (overall proportion 66%) and participants with some degree of atelectasis/consolidation on chest X‐rays (overall proportion 24.5%). The intervention tested consisted of "chest percussion with a cupped hand for three minutes in each of five postural drainage positions followed by assisted coughing" or "gentle oropharyngeal suction performed twice each day while in the hospital". Three medical doctors made clinical assessments of the severity of the illness at a fixed time every day. A score of zero to three was allocated for each of 10 clinical signs: heart rate, respiratory rate, hyperinflation, use of accessory muscles, recession, rhinitis, wheeze, cough, crepitations and rhonchi, to give a total severity clinical score of a maximum of 30 points. At hospital discharge, parents were asked to maintain a symptom record diary and children were reviewed in outpatient clinics after two weeks. The main outcomes were: clinical score on admission, every day and after five days, length of hospital stay and total length of illness. Results were expressed as medians and ranges. The trial author was unable to provide the mean and SD of each parameter because the raw data were no longer available.
Unpublished trials
In the Lopez Galbany 2004 pilot study conducted in Spain, 30 infants with RSV‐positive bronchiolitis were randomly allocated to receive physiotherapy with slow expiratory technique (n = 15) or no intervention (n = 15). Outcomes assessed were the Bierman Pierson modified severity clinical score and length of hospital stay.
The Aviram 1992 study was a randomised controlled intervention study conducted in Israel, which included 50 infants aged one to five months, paired by age and clinical severity score. Participants were allocated to receive chest physiotherapy or not, in addition to salbutamol inhalations every six hours. Although there is no information on the physiotherapy technique applied, it is assumed to be based on vibration and percussion. Outcomes assessed were length of stay in hospital, improvement in clinical score and changes in SaO2. Clinical scoring was performed in a blinded manner.
Excluded studies
See Characteristics of excluded studies table.
We excluded six studies. One study was a single‐blind randomised clinical trial including infants under two years of age with moderate acute wheezing episodes attending an outpatient clinic (Castro 2014). The study randomised 48 participants to receive salbutamol with or without chest physiotherapy using slow and long expiratory flow and assisted cough techniques. After inclusion of the participant by a family physician, those infants in the chest physiotherapy group received physiotherapy for one hour. Afterwards the patient was assessed by the including family physician, blinded to intervention status, for re‐evaluation of his or her clinical status, clinical score and SpO2 level. If the patient met the criteria of improvement, he or she was discharged. Otherwise, the participant received a second hour of treatment, according to his or her original randomised group. After the second hour, the participant was assessed again by the original family physician and referred to the hospital for admission if the criteria of improvement based on the clinical score was still not achieved. The study endpoints were clinical score, SpO2, number of hospital admissions and parents satisfaction.
Three other excluded studies were uncontrolled intervention studies (Bernard‐Narbone 2003; Postiaux 2004; Quitell 1988), and the last two were non‐randomised comparative trials (Belcastro 1984; Pupin 2009).
The two comparative trials' details are as follows:
Belcastro 1984 was a pilot study with 12 patients that compared:
osteopathic manipulative treatment to postural drainage in a non‐randomised fashion (first three patients received osteopathy and the rest postural drainage); and
bronchodilators to placebo in a randomised, double‐blind fashion.
The endpoints were number of hospital days and mean daily respiratory rates.
Pupin 2009 was a comparative controlled intervention study which included 81 infants with clinically and radiologically diagnosed acute viral bronchiolitis. Participants were non‐randomly allocated to receive expiratory flow increase technique (EFIT), vibration plus postural drainage or a control procedure (no respiratory therapy, only manual contact of the physical therapist on the thorax). Each procedure consisted of a single therapeutic session performed in the morning for 10 minutes. Heart rate, respiratory rate and SpO2 were assessed before the procedure and at 10, 30 and 60 minutes after it. The authors conclude that "In terms of overall improvement of cardiorespiratory parameters, neither the EFIT nor vibration/PD provided any benefit to infants with acute viral bronchiolitis. However, over time, respiratory physical therapy seems to contribute to decreasing the respiratory rate in these patients".
Risk of bias in included studies
The overall risk of bias for the comparison of vibration and percussion techniques is moderate to high, because of the uncertainties and limitations associated with the assessment of risk of bias in the five trials in this comparison (Figure 2; Figure 3).
2.
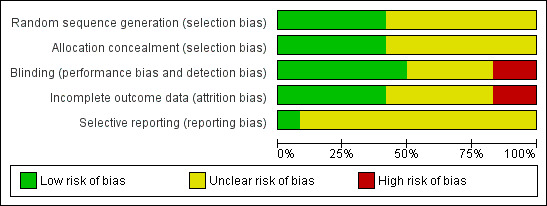
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
3.
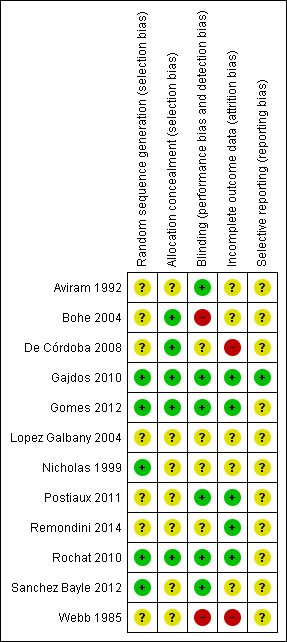
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study
The overall risk of bias for the comparison of passive expiratory techniques is uncertain. However, the two trials comparing forced expiration techniques are at low risk of bias (Gajdos 2010; Rochat 2010). The comparison of slow flow techniques has one low risk of bias trial (Gomes 2012), and four trials of uncertain risk of bias (Lopez Galbany 2004; Postiaux 2011; Remondini 2014; Sanchez Bayle 2012).
Allocation
Scant information was provided regarding randomisation methods and allocation concealment. Five trials described adequate sequence generation procedures (Gajdos 2010; Gomes 2012; Nicholas 1999; Rochat 2010; Sanchez Bayle 2012). Five trials either described procedures to conceal allocation (De Córdoba 2008; Gajdos 2010; Gomes 2012; Rochat 2010), or claimed to have concealed allocation (Bohe 2004).
Blinding
Masking of outcome assessment was most likely absent in all but two of the included trials. Five trials implemented rigorous procedures to mask outcome assessments (Gajdos 2010; Gomes 2012; Postiaux 2011; Rochat 2010; Sanchez Bayle 2012), but the other trials were admittedly open (Bohe 2004; Rochat 2010; Webb 1985), or most likely so (Aviram 1992; De Córdoba 2008; Lopez Galbany 2004; Nicholas 1999). Even though some outcomes were objective and not subject to bias (oxygen saturation, heart rate), other outcomes depended on observation and could be more vulnerable (clinical scores and respiratory discomfort questionnaire).
Incomplete outcome data
A single trial had a large sample size and had an adequate description of attrition of participants, as well as a description of how they were handled (ITT analysis) (Gajdos 2010). Another trial had a large sample and an adequate description of attrition of participants (Rochat 2010). The rest of the included trials were small and the attrition of participants was either null (Gomes 2012; Postiaux 2011), or low and unclearly dealt with (Bohe 2004; De Córdoba 2008; Nicholas 1999; Sanchez Bayle 2012; Webb 1985).
Selective reporting
A single trial had a low risk of selective reporting bias, as shown by comparing the trial protocol with the published paper (Gajdos 2010). Assessment of selective reporting bias is not possible for the rest of the trials due to the scarcity of available data.
Effects of interventions
Summary of findings for the main comparison. Forced expiration compared with standard care for acute bronchiolitis.
| Forced expiration compared with no physiotherapy for acute bronchiolitis | ||||
|
Patient or population: paediatric patients between 0 and 24 months old with acute bronchiolitis Settings: hospital Intervention: forced expiration Comparison: standard care | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Time to recovery/time to clinical stability (follow‐up until hospital discharge) |
Studies reported that no differences in time to recovery/clinical stability were observed | 624 (3 trials) | ⊕⊕⊕⊕ high | Participants with severe bronchiolitis (Gajdos 2010; Rochat 2010) Participants with mild‐moderate bronchiolitis (Remondini 2014) |
|
Adverse events (follow‐up until hospital discharge) |
Bradycardia with desaturation (RR 1.0, 95% CI 0.2 to 5.0) Bradycardia without desaturation (RR 3.6, 95% CI 0.7 to 16.9) Transient respiratory destabilisation (RR 5.4, 95% CI 1.6 to 18.4) Vomiting during procedure (RR 10.2, 95% CI 1.3 to 78.8) |
496 (2 trials) | ⊕⊕⊕⊕ high | Participants with severe bronchiolitis (Gajdos 2010; Rochat 2010) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
Summary of findings 2. Slow passive expiration compared with standard care for acute bronchiolitis.
| Slow passive expiration compared with no physiotherapy for acute bronchiolitis | ||||
|
Patient or population: paediatric patients between 0 and 24 months old with acute bronchiolitis Settings: hospital Intervention: slow passive expiration Comparison: standard care | ||||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
Change in the severity status of bronchiolitis Wang score and Bierman Pearson score (follow‐up ranging from 2.5 hours to discharge) |
2 studies did not find changes. 1 study found a transient small effect | 286 (3 trials) | ⊕⊕⊝⊝ low1 | Participants with moderate bronchiolitis (Gomes 2012; Lopez Galbany 2004; Postiaux 2011) |
|
Adverse events (follow‐up) |
Studies reported that no adverse events were observed | 256 (2 trials) | ⊕⊝⊝⊝ very low2 | Participants with moderate and severe bronchiolitis (Postiaux 2011; Sanchez Bayle 2012) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Downgraded quality due to uncertain risk of bias and imprecision of estimates. 2Downgraded quality due to uncertain risk of bias, imprecision of estimates and indirectness of assessments because the trials were unclear on the adverse effects assessment.
Although the included trials provided some data on change in the severity status of bronchiolitis (using clinical scores) and length of hospital stay, due to clinical and statistical considerations we were unable to pool the data. First of all, the clinical scores assessed in the included trials were heterogeneous:
the studies used different scores, although admittedly based on similar recordings;
the timing of the assessments was quite variable (15 minutes after the intervention (De Córdoba 2008), two hours after the intervention (Postiaux 2011), at hospital discharge (Bohe 2004), on the fifth day (Lopez Galbany 2004); and
not all trials provided data for this outcome, in particular the largest, most valid trial (Gajdos 2010).
It seems unreliable to present a statistical analysis that only partially incorporates the available evidence, lacking the most influential trial with a sample size that doubles that of the rest of the trials. Finally, length of hospital stay is quite an asymmetric variable, often presented as medians, and the usual meta‐analysis methods, based on symmetry, are not the right tools to analyse it.
Postural drainage plus percussion and vibration techniques
Primary outcomes
1. Change in the severity status of bronchiolitis
Five trials (241 analysed participants) in this comparison assessed the severity of bronchiolitis by means of clinical scores and none of them showed statistical differences between groups at day five (Aviram 1992; Bohe 2004; De Córdoba 2008; Nicholas 1999; Webb 1985).
Nicholas 1999 and Webb 1985 assessed this outcome using a common clinical score. In the Webb 1985 study there were no statistically significant differences between groups in relation to the clinical score or to the proportion who remained in hospital at day five. The clinical score was similar in both groups at baseline and on each of the first five days of assessment at the hospital. In the control group the median score on admission was 12 (range 4 to 24) in 46 participants and in the physiotherapy group the median score was 10 (range 4 to 22) in 44 participants. On the fifth day, 18 participants who remained in hospital had a median score of five (range 1 to 11) in the control group; 11 participants in the physiotherapy group had a median score of six (range not presented in the original article). The study also assessed the length of illness, which was not significantly different between the groups (Mann‐Whitney test (Mann 1947)). In the control group the median length of illness was 14 (range 4 to 27) and in the physiotherapy group the median was 13 (range 7 to 26). Nicholas 1999 expressed clinical scores using means but did not report standard deviations (SDs). There were no differences in the admission mean clinical scores (intervention group 9.1 versus control group 10.9) between groups. The trial authors reported that clinical scores did not show any statistically significant differences between groups during the five day trial. Data were provided on a graph but could not be extracted. Bohe 2004 used a different clinical severity score to the one used in the other two trials. The score at day five or the day of discharge was 3.25 (SD 1.27) in the physiotherapy group and 3.12 (SD 1.15) in the control group (mean difference (MD) 0.13, 95% confidence interval (CI) ‐0.71 to 0.97). The unpublished trial did not describe the clinical score used and it also failed to show differences between treatment groups (Aviram 1992).
2. Time to recovery
No trial presented data on time to recovery.
Secondary outcomes
1. Respiratory parameters
Data for respiratory parameters are available in only one of the included trials, assessed immediately after treatment and at 15 minutes (De Córdoba 2008). No significant differences were observed in oxygen saturation levels nor in respiratory frequency between the treatment groups in their 15‐minute results (Kruskal Wallis test (Kruskal 1952)). The amount of aspired secretions was significantly smaller in the control group than in the intervention groups (P = 0.02, Kruskal Wallis test). Respiratory discomfort was assessed by means of the Silverman‐Andersen Questionnaire (Silverman 1956), which significantly improved (P < 0.05, Friedman analysis of variance) post 15 minutes with respect to baseline in the two treatment groups but not in the control group. It is not clear from the paper whether differences across the groups were tested but it can be assumed that the lack of data means that there were not significant differences across the groups.
2. Duration of oxygen supplementation
Nicholas 1999 found that the mean number of hours with supplemental oxygen in the control group was 63 (range 2.3 hours to 128 hours) compared with 86 (range 36 hours to 148 hours) in the physiotherapy group. Differences were reported as not significant using a non‐parametric test.
3. Length of hospital stay
In Bohe 2004, mean length of hospital stay was four days (SD 2) in the treatment group and 3.9 days (SD 1.3) in the control group. There were no statistically significant differences between them (MD 0.13, 95% CI ‐1 to 1.26). In the Nicholas 1999 study, mean length of hospital stay was 6.6 days (range 2.3 days to 11.5 days) in the control group and 6.7 days (range 3 days to 9.5 days) in the physiotherapy arm. Webb 1985 showed a median length of hospital stay of four days (range one day to 15 days) in the control group and a median of four days (range two days to 11 days) in the physiotherapy group.
4. Use of bronchodilators and steroids
No trial presented data on use of bronchodilators and steroids.
5. Parents' impression of physiotherapy benefit
No trial presented data on parents' impression of the benefit of physiotherapy in this comparison.
6. Adverse events
In the Bohe 2004 study one case of atelectasis was reported in the control arm. The participant was withdrawn from the trial and assigned to receive chest physiotherapy.
Passive expiratory techniques ‐ forced passive expiratory techniques
Primary outcomes
A summary of results is presented in Table 1.
1. Change in the severity status of bronchiolitis
One trial (103 participants) assessed severity of bronchiolitis through a clinical score assessing feeding, vomiting and sleep (Rochat 2010). No differences were observed in changes in the clinical score (mixed linear models P = 0.37).
One trial (29 participants) compared the addition of forced passive expiratory techniques to postural drainage. The trial assessed severity of bronchiolitis using respiratory distress assessment instrument (RDAI) (Remondini 2014). They observed significant differences immediately after forced passive expiratory physiotherapy + postural drainage (10 and 60 minutes post intervention; P < 0.001). However, when compared to conventional physiotherapy (postural drainage + manual percussion or tapping), no differences were found.
2. Time to recovery
Three trials (628 participants) in this comparison assessed resolution of bronchiolitis in terms of time to recovery (Gajdos 2010; Remondini 2014), and time to clinical stability (Rochat 2010). Overall, there were no significant differences between groups in any of these trials.
In Gajdos 2010, the physiotherapy intervention (forced expiratory technique with assisted cough) had no significant effect on time to recovery as assessed by the logrank test and a Cox regression. The median time to recovery was 2.31 days (95% CI 1.97 to 2.73) for the control group and 2.02 days (95% CI 1.96 to 2.34) for the physiotherapy group (hazard ratio (HR) 1.09, 95% CI 0.91 to 1.31, P = 0.33). In Rochat 2010, time to clinical stability, assessed as a primary outcome, was similar for increased exhalation technique (IET) and placebo (2.9 ± 2.1 versus 3.2 ± 2.8 days, logrank test P = 0.45).
For both primary outcomes, the quality of the evidence using GRADE was high.
Secondary outcomes
One trial comparing the addition of forced passive expiratory physiotherapy to postural drainage, Remondini 2014, did not observed differences in SpO2 during and after the intervention. There were no data on secondary outcomes such as duration of oxygen supplementation, length of hospital stay and use of bronchodilators and steroids.
1. Respiratory parameters
In Rochat 2010, the rate of improvement of a respiratory score, defined as secondary outcome, only showed a slightly faster improvement of the respiratory score in the prolonged slow expiration (PSE) technique group when including stethacoustic properties (mixed linear model P = 0.044). No differences were observed in oxygen saturation (SpO2) (mixed linear models P = 0.85) or respiratory rates (mixed linear models P = 0.24).
2. Duration of oxygen supplementation
No trial presented data on duration of oxygen supplementation.
3. Length of hospital stay
No trial presented data on length of hospital stay.
4. Use of bronchodilators and steroids
No trial presented data on use of bronchodilators and steroids.
5. Parents' impression of physiotherapy benefit
Two trials provided data on the parents' impression on the benefit of chest physiotherapy.
Remondini 2014 presented data on the parents' impression on the benefit of physiotherapy compared to conventional physiotherapy. Parents in both groups reported satisfaction related to improvements of breathing, feeding and nasal congestion, but no difference was observed between the intervention groups. Gajdos 2010 reported they did not observe any significant difference in the way the parents rated the influence of physiotherapy on respiratory status (risk ratio (RR) 0.99, 95% CI 0.90 to 1.08, P = 0.89) or comfort (RR 0.99, 95% CI 0.94 to 1.05, P = 0.84).
6. Adverse events
In the only trial in the review that specifically monitored adverse events, there were no significant differences between groups in the proportion of children who experienced one episode of bradycardia with desaturation (risk ratio (RR) 1.0, 95% CI 0.2 to 5.0, P = 1.00) or without desaturation (RR 3.6, 95% CI 0.7 to 16.9, P = 0.10) (Gajdos 2010). Conversely, in the IET physiotherapy group there were a higher proportion of children who had transient respiratory destabilisation (RR 5.4, 95% CI 1.6 to 18.4, P = 0.002) or vomited during the procedure (RR 10.2, 95% CI 1.3 to 78.8, P = 0.005).
Regarding the physiotherapy technique, in Rochat's study, complications were defined as concomitant bacterial infection or transfer to the intensive care unit due to respiratory fatigue (Rochat 2010). The trial authors state that complications related to bronchiolitis severity were rare and occurred more frequently in the control group (n = 19; 12 in the control group, seven in the intervention group), albeit not significantly (P = 0.21). Also, they state that no direct complications of physiotherapy, such as respiratory deterioration, occurred.
Remondini 2014 did not report any adverse events.
For adverse events, the quality of the evidence using GRADE was high.
Passive expiratory techniques ‐ slow passive expiratory techniques
Primary outcomes
A summary or results is presented in the Table 2.
1. Change in the severity status of bronchiolitis
Three trials analysing 286 participants assessed severity of bronchiolitis through clinical scores (Gomes 2012; Lopez Galbany 2004; Postiaux 2011). Overall, there were no significant differences between groups in any of these trials. Furthermore, the quality of the evidence for this outcome using GRADE was low.
In Lopez Galbany 2004 no significant differences were observed between groups in change from baseline values (P = 0.175). Mean values for a modified version of the Bierman Pierson score (Bierman 1974; Tal 1983) at five days were 2.46 for the physiotherapy group and 2.79 for the control group.
In Postiaux 2011, a significant small improvement in the Wang clinical score was observed immediately after the intervention in the group receiving slow flow physiotherapy and salbutamol (3.6 versus 5.1, ANOVA P = 0.02), which disappeared two hours later (4.6 versus 3.7, ANOVA P = 0.21). The authors report a "day‐to‐day baseline improvement in Wang score significantly better [in the CPT group] than that in the control group" but this conclusion is based on within‐group tests on a diminishing sample due to discharge of patients ("After 5 days, 6 of the 8 control group patients had been discharged, whereas all 12 of the new‐method‐CPT group had been discharged").
One trial (30 participants) compared severity of clinical scores between both physiotherapy techniques (Gomes 2012). The authors only applied statistical tests to within‐groups comparisons pre versus post. They found significant within‐group differences in clinical score values and retractions assessed at 48 hours for both physiotherapy regimens, and significant differences in clinical score and oxygen saturation assessed at 72 hours for the slow flow physiotherapy. Although not statistically tested, endpoint values at 48 and 72 hours for the clinical score and all its sub‐scales appear to be equal between both physiotherapy groups.
2. Time to recovery
No trial presented data on time to recovery.
Secondary outcomes
1. Respiratory parameters
No data were presented for this outcome.
2. Duration of oxygen supplementation
One trial (236 participants) compared the average hours with oxygen supplementation in the physiotherapy and control groups, which showed no statistically significant differences (Sanchez Bayle 2012). Mean hours of oxygen therapy needed were 49.98 ± 37.10 in the physiotherapy group and 53.53 ± 38.87 in the control group.
3. Length of hospital stay
This outcome was assessed in three trials (286 participants), and none of them detected statistically significant differences between the length of hospital stay of the physiotherapy and control groups. Mean length of stay in Sanchez Bayle 2012 was 4.56 ± 2.07 days in the physiotherapy group and 4.54 ± 1.72 days in the control group. Mean length of stay in Lopez Galbany 2004 was 6.18 days in the physiotherapy group and 5.88 in the control group. Average hospital stay in Postiaux 2011 was 5.3 ± 1.8 days in the physiotherapy group and 6.3 ± 2 days in the control group (Mann‐Whitney U test P = 0.25).
4. Use of bronchodilators and steroids
One trial including 236 participants recorded the percentages of participants that received salbutamol, ipratropium bromide or antibiotics, which showed no statistical differences between the intervention and control groups (Sanchez Bayle 2012).
5. Parents' impression of physiotherapy benefit
No trial presented data on parents' impression of physiotherapy benefit.
6. Adverse events
Two studies explicitly stated that no adverse events were observed but there is no definition on the events considered (Postiaux 2011; Sanchez Bayle 2012).
The quality of the evidence for adverse events using GRADE was very low.
Subgroup analyses
The subgroup analysis by participant severity was confused by interaction with techniques. Four trials included participants with severe bronchiolitis, corresponding to the comparison of vibration and percussion (Nicholas 1999), slow passive expiration (Sanchez Bayle 2012), and forced expiration (Gajdos 2010; Rochat 2010). Five trials included moderate cases of bronchiolitis, corresponding to the comparison of slow passive expiration (Gomes 2012; Lopez Galbany 2004; Postiaux 2011), and vibration and percussion (Bohe 2004; Webb 1985). One trial of vibration and percussion techniques included mild cases of bronchiolitis (De Córdoba 2008). While no formal meta‐analysis or test of subgroups could be conducted due to lack of data, it became clear that the evidence for the slow flow chest physiotherapy techniques was unevenly distributed, with slow flow techniques studied in less severe participants than forced expiratory techniques.
It was not possible to conduct the subgroup analysis by setting, since all the trials included hospitalised participants.
Subgroup analysis performed on the included trials
Sanchez Bayle 2012 conducted subgroup analyses of the effect of physiotherapy on length of hospital stay and duration of oxygen supplementation by subgroups of respiratory syncytial virus (RSV) status. They found statistical differences in the number of hours with oxygen supplementation in the subgroup of RSV‐positive participants that received physiotherapy compared to those RSV‐positive participants in the control group (mean hours 48.80 ± 37.70 versus 58.68 ± 36.78; P = 0.042, Mann‐Whitney test). There were no other statistical differences.
Gajdos 2010 performed subgroup analyses by personal eczema or history of atopy, RSV‐positive infection and hypoxaemia at randomisation. There was no statistically significant quantitative interaction on time to recovery between any of these subgroups.
Nicholas 1999 performed a subgroup analysis between participants who had more than 10 points on the baseline clinical score and those with a baseline clinical score below 9.5. There were no differences between the physiotherapy and control groups in this subgroup analysis.
Webb 1985 reports that there were no differences between treatments in daily scores or length of illness in the subset of participants with some degree of collapse/consolidation on chest X‐rays.
Discussion
Summary of main results
This review included 12 trials and 1249 participants exploring the efficacy of three physiotherapy modalities (vibration and percussion, slow passive expiratory techniques and forced passive expiratory techniques), compared to no intervention in hospitalised infants with acute bronchiolitis not on mechanical ventilation. None of the included trials showed a significant benefit of either chest physiotherapy techniques in change of disease severity, respiratory parameters, length of hospital stay or oxygen requirements in this population. One trial found transient immediate respiratory score improvements in moderate bronchiolitis patients that received slow expiratory techniques. The included trials did not report severe adverse events. In Gajdos 2010, a significant risk of vomiting (risk ratio (RR) > 10) and respiratory instability (RR > 5) was reported in children receiving physiotherapy with passive increased exhalation technique and assisted cough, while no complications related to physiotherapy and few complications related to bronchiolitis severity were observed in trials applying prolonged slow expiration techniques (Postiaux 2011; Rochat 2010).
Quality of the evidence
The quality of the evidence in the review is variable depending on the comparisons considered. While there is high quality evidence for forced expiration techniques, the quality of evidence is low for the techniques based on slow passive expiration and very low for vibration and percussion. The assessments of quality of evidence have relied heavily on the risk of bias of the trials and the imprecision of their results, mainly due to small sample sizes. For adverse events, there were concerns regarding indirectness of assessments for trials that were not clear enough on the adverse events assessment procedure.
The high quality evidence for forced expiration techniques in severe patients stems from the overall low risk of bias of the trials considered, the large number of patients considered and the consistency of the trials' results. Although the three trials assessed recovery with two different measures (time to recovery and time to clinical stability), the results were homogeneous and led to similar conclusions of no effect of the physiotherapy techniques. One of the trials had a very large sample size and good methodological quality, and was designed to detect a 20% decrease in time to recovery, assessed eight‐hourly (Gajdos 2010). Since this adequately powered trial was negative, our confidence in the lack of effect observed with this physiotherapy techniques is high. Also, the negative results are consistent in all the assessed outcomes, including respiratory parameters, which are more sensitive to the treatment and nevertheless do not show a statistical benefit. There are also negative results in length of hospital stay, a less relevant outcome since it is a crude measure of length of illness and it is sensitive to unrelated factors (i.e. hospital discharge practices, day of the week, parental wishes, etc).
The low quality of evidence for the slow flow techniques in moderate/severe patients stems from their uncertain risk of bias, moderate sample sizes and methodological limitations in adverse effects assessment. The included trials used different measures of clinical severity and some of them presented incomplete data. Although most data on clinical efficacy were negative overall, a transient effect was observed in one trial, leading to concerns of potential inconsistency in results and potential lack of power. The largest trial in the comparison and second largest trial in the review did not perform an a priori sample size estimation and thus we cannot assess the power of the trial or the potential lack of power of the conclusions (Sanchez Bayle 2012). The quality of evidence on the safety of the slow passive expiration techniques stems from the doubts regarding how was safety assessed in the trials. The safety issues observed in the forced expiratory techniques are related to the intrinsic characteristics of forcing expiration and it could be argued that these issues would be minor or non‐existent in the slow passive expiration procedures due to their gentler nature.
The very low quality of evidence for the vibration and percussion techniques stems from their high risk of bias and small sample sizes. However, the consistency between trials in showing a lack of effect and the external reports on safety of the procedures, give strength to a negative conclusion (Beeby 1998; Chalumeau 2002; Harding 1998; Knight 2001).
A methodological issue in the trials was the implementation of a valid placebo. Since all but one of the trials had a non‐intervention group, the researchers would have been expected to establish an outcome assessment procedure that prevented bias. Again, this was effectively and imaginatively established in the Gajdos 2010, Postiaux 2011 and Sanchez Bayle 2012 trials. Gajdos and Sanchez Bayle compared chest physiotherapy with nasal suctioning or postural changes, respectively. Postiaux administered in both groups an aerosol composed of albuterol (3 mL) and hypertonic saline (3% NaCl) and added to the intervention group the slow passive expiration techniques. However, none of these alternatives were shown to have an impact on the overall trial results as this lack of placebo alternative will usually over‐estimate the results, favouring the intervention.
Finally, it is important to consider that a limitation of the majority of the studies was that they did not analyse the effectiveness of the techniques in terms of duration of oxygen supplementation, time to recovery or other treatments used, such as bronchodilators and corticosteroids. Due to their importance in terms of disease improvement, it would be important to take these variables into account in future research,
Potential biases in the review process
To avoid biases in the review process, we have applied robust methods for searching, study selection, data collection and 'Risk of bias' assessment. To guarantee the comprehensiveness of the search, we sought both published and unpublished trials and contacted trial authors when possible to gather additional information about unpublished trials. Although pooling of data was not possible, we have considered its potential impact and performed a careful assessment of individual trials. In addition, we have performed a rigorous 'Risk of bias' assessment for the included trials.
Agreements and disagreements with other studies or reviews
The first publication of this review in 2005, Perrotta 2005, prompted the recommendation that chest physiotherapy based on vibration and percussion not be applied routinely in hospital settings (AAP 2006; BGT 2005; SIGN 2006). During recent years, a few systematic reviews have been published on this topic based on the same evidence and reaching similar conclusions to ours (Bourke 2010; González 2010b; Schechter 2007; Wainwright 2010). Also, in France, due to Cochrane evidence, two studies analysed the use of forced expiratory technique (AFE in French). They observed a decrease in chest physiotherapy prescription (Branchereau 2013), and a recommendation to not systematically prescribe chest physiotherapy for ambulatory patients (Verstraete 2014). As a consequence, this updated review includes the most recent randomised controlled trials (RCTs) and remains the main source of evidence on chest physiotherapy for acute bronchiolitis.
Authors' conclusions
Implications for practice.
Conventional chest physical therapy (postural drainage plus percussion and vibration techniques) has not been shown to improve the severity of bronchiolitis and has been associated with adverse events. For these reasons, conventional techniques cannot be not used in clinical practice for patients with bronchiolitis.
Chest physiotherapy using passive flow‐oriented expiratory techniques (which includes both forced expiratory techniques and slow flow techniques) has not been shown to improve the severity of bronchiolitis by means of clinical scores, nor to reduce time to recovery or length of stay in hospitalised patients. There is high quality evidence that forced expiratory techniques in severe patients do not improve their health status and can lead to severe adverse events. For these reasons, there are no argument in favour of routine use of these techniques as standard clinical practice for hospitalised patients with severe bronchiolitis.
However, there is a gap in the knowledge regarding the effects of slow passive expiratory techniques in patients with moderate bronchiolitis or respiratory syncytial virus (RSV)‐positive disease. There is low quality evidence from individual trials that slow passive expiratory techniques could have a short‐lived effect in reducing respiratory scores in patients presenting with moderate bronchiolitis and in reducing the need for oxygen supplementation in RSV‐positive patients with severe bronchiolitis. The findings of the review are that there is low quality evidence that slow flow techniques could induce temporary relief in some children, and for this reason we conclude that, under clinician judgement, these techniques could be considered in specific situations, to improve respiratory performance.
Implications for research.
Based on the review results, it seems clear that conventional and forced expiratory techniques will not change the course of the disease in hospitalised patients with severe disease. Therefore, further studies using these techniques in this population should not be a research priority.
However, there is uncertainty about the role of slow passive expiratory physiotherapy during a bronchiolitis episode, and the clinical relevance of transient short‐term relief for patients who are RSV‐positive should be discussed and studied. Other areas for further research are the effect of slow flow physiotherapy techniques combined or not with salbutamol or hypertonic saline, as well as the effect of chest physiotherapy in moderate bronchiolitis. Any research conducted on this topic should include a specific assessment of adverse events.
Finally, we recommend exploring the effects of slow passive expiratory techniques in mild to moderate non‐hospitalised patients. Until now, all reviewed studies were conducted in a hospital setting and the generalisation of these results to non‐hospitalised patients may not be straightforward due to differences in the health conditions and severity of disease between these two populations.
Feedback
Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old, 5 March 2012
Summary
We have read with much interest the last Cochrane review devoted to Chest Physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months. (1) M. Roqué and her co‐authors have reported the most recent publications in this field.
We would like to present some remarks:
The study of Postiaux et al. has been performed in Belgium, and not in France as mentioned in the Cochrane publication. (2) Even if this aspect is not scientifically relevant, it implies different methodological PT approaches.
M. Roqué et al. have merged two different PT approaches in a same appellation “forced expiration techniques”, adding to the confusion concerning the PT techniques. Indeed, their different functional features are essential. The first one is the Increased Exhalation Technique ‐ IET (augmentation/accélération du flux expiratoire) mainly used in France (see the Gajdos and Sanchez studies (3, 4)), which is a passive forced (i.e. rapid, robust) expiration technique ‐ FET, and the second one is the Prolonged Slow (i.e. progressive) Expiration technique – PSE (prlonge slow expiration technique) proposed by our group in 1992 to avoid the mechanical drawbacks of the IET ‐(Increased Exhalation Technique) such as the tracheal collapse. (5) PSE is more attuned to the infant’s specific ventilatory mechanics. (6)
It is important to stress that the therapeutic regimens are different. In the Postiaux’ study, PT is preceded by a hypertonic saline solution nebulization NaCL3% – HS3%, while it is not in the other studies. HS3% dilutes the bronchial secretions and helps the mucociliary transport. (7) Both, HS3% and PSE act in synergy.
The Cochrane Review states that in the Postiaux’ study, the effect of the treatment “disappeared two hours later”. However the study has shown that the effect of the treatment lasted at least two hours and that a significant day‐to‐day cumulative effect had been observed. These results envision a long term effect of such a treatment.
Explaining the apparent controversial results are also the different levels of severity of the patients samples. The Gajdos’, Sanchez’ and Rochat’ (8) studies were dealing with severe bronchiolitis while the Postiaux’ study dealt with moderate bronchiolitis. Severe bronchiolitis are known to be poorly tolerating any handling procedure, probably explaining the lack ok positive outcome of IET in this group.
We think that those elements are likely to clarify the PT methods and better define the indications/contraindications of PT in RSVB.
Roqué I Figuls M, Giné‐garriga M, Granados Rugeles C, Perrotta C. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane database of Systematic Review 2012 Issue 2.Art No.:CD004873, DOI:10.1002/14651858.CD004873.pub4.
Postiaux G, Louis J, Labasse HC, Patte C, Gerroldt J, Kotik AC, Lemuhot A. Effects of an alternative chest physiotherapy regimen protocol in infants with RSB bronchiolitis. Resp Care 2011;56,7:989‐94.
Gajdos V, Katsahian S, Beydon N, et al. Effectiveness of Chest Physiotherapy in Infants Hospitalized with Acute Bronchiolitis : A Multicenter, randomized, Controlled Trial. PLoS Med 2010;7(9) : e1000345.doi :10.1371/journal.pmed.1000345.
Sánchez Bayle M, et al. Estudio de la eficacia y utilidad de la fisioterapia respiratoria en la bronquiolitis aguda del lactante hospitalizado. Ensayo clínico aleatorizado y doble ciego. An Pediatr (Barc). 2012. doi:10.1016/j.anpedi.2011.11.026 5. Postiaux G., Lens E. De ladite Accélération du Flux Expiratoire…où forced is fast
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Dear Dr Postiaux, thank you for your comments that allow us to improve our work. In response to your feedback, we would like to formulate the following remarks:
We apologise for the confusion regarding countries, and we have amended the review accordingly.
Throughout the text we have tried to clarify the differences between these techniques, grouped now as passive expiratory techniques instead of forced expiratory techniques. Efficacy and safety results for both techniques have been clearly labelled in the results and discussion sections.
We have clarified this point in the discussion and conclusions sections.
We have added a quote in the results section mentioning the day‐to‐day cumulative effect. Nevertheless, we've considered that this result is inconclusive and doesn't change the overall results and conclusions of the review. The reasons are that this apparent cumulative effect is based on 1) within group comparisons and not between group comparisons, and 2) assessment of a reduced number of patients due to discharges during follow‐up.
We have added specific mentions to the severity of patients.
After careful consideration of this feedback we have introduced several changes in the review with the aim to clarify the differences between the diverse passive expiratory techniques, and to highlight their respective efficacy and safety results. This greater detail has lead to amend the implications for research section, given that the prolonged slow expiration technique appears to be safe and that it may be related to (at least) a transient effect. Nevertheless, the overall conclusion of the review and its implications for practice have not changed.
Contributors
Guy Postiaux. Occupation: An author cited in the Review Jacques Louis.
Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old, 29 April 2016
Summary
I noticed the values of relative risk etc related to ‘vomiting during procedure’ or ‘respiratory destabilisation’ published in the Cochrane review (from the Gajdos 2010 paper) have been incorrectly reversed – this is important in terms of readers understanding the actual consequences of treatment... The mistake is consistent throughout the text of the Cochrane review.
Reply
Thanks for pointing out this transcription error. The text and tables have been modified to show the correct risk values for respiratory destabilisation (RR 5.4, 95% CI 1.6 to 18.4, P = 0.002) and vomiting during the procedure (RR 10.2, 95% CI 1.3 to 78.8, P = 0.005). These values had been interchanged during transcription.
Contributors
Professor Eleanor Main FCSP (BSc, BA, MSc, PhD) Programme Director: UCL MSc, Diploma & Certificate in Physiotherapy
Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old, 4 May 2017
Summary
In the Cochrane review “Chest physiotherapy for acute bronchiolitis in pediatric patients between 0 and 24 months old (Review)”, published on Cochrane Database of Systematic Reviews 2016, Issue 2. Art. No.: CD004873. DOI: 10.1002/14651858.CD004873.pub5., you analyse, among other studies, one study from our authorship (Remondini R, Santos AZ, Castro G, Prado C, Silva Filho LV. Comparative analysis of the effects of two chest physical therapy interventions in patients with bronchiolitis during hospitalization period. Einstein. 2014;12(4):452‐8).
After a thoughtful review of your review, we identified some conclusions reached by you that do not fit with our study.
Under “Summary of findings for the main comparison”: In our study, we didn't compare expiratory acceleration flow with no‐physiotherapy for acute bronchiolitis, but the comparison was made between expiratory acceleration flow and conventional physiotherapy (manual percussion or tapping).
Under “Results – Included studies”: It classifies the study as forced expiration techniques, but the study compares expiratory acceleration flow and conventional techniques (manual percussion or tapping).
Under “Passive expiratory techniques ‐ forced passive expiratory techniques ‐ Primary outcomes ‐ Change in the severity status of bronchiolitis”: It mentions that “They observed significant differences immediately after forced passive expiratory physiotherapy + postural drainage (10 and 60 minutes post intervention; P <0.001). However, when compared to conventional physiotherapy (postural drainage), no differences were found”, nevertheless the conventional physiotherapy is defined as postural drainage + manual percussion or tapping, not postural drainage only.
Under "Results – Postural drainage percussion and vibration techniques – Primary outcomes 1 – Change in the severity status of bronchiolitis": Our study should be cited as “One trial (29 participants) compared the addition tapping to postural drainage. The trial assessed severity of bronchiolitis using respiratory distress assessment instrument (RDAI) (Remondini 2014). They observed significant differences immediately after conventional physiotherapy (tapping + postural drainage (10 and 60 minutes post intervention; P<0.001), the same result was observed after forced passive expiratory physiotherapy + postural drainage)”.
Under "Parents’ impression of physiotherapy benefit": it was mentioned “No trial presented data on parents’ impression of physiotherapy benefit except Gajdos”, however our study presented that parents answered positively about the effects of therapy in the majority of items in the questionnaire about the treatment applied, both for the expiratory acceleration flow technique and for tapping.
In summary, in our study we compare the effects of two chest physiotherapy interventions in patients hospitalised due to acute bronchiolitis, with randomised patients and two groups (Group 1, submitted to postural drainage, tapping and tracheal aspiration; and Group 2, submitted to postural drainage, expiratory acceleration flow and tracheal aspiration). We never compared Forced Passive Expiratory Physiotherapy with just postural drainage.
A relevant improvement was observed on the Respiratory Distress Assessment Instrument score with physical therapy, with reduction of the score 10 minutes after interventions, and the same score 60 minutes later, with no differences between techniques applied. No differences were observed between groups regarding the items assessed (time required to discharge from study, pulse oximetry in room air and disease severity according to the Respiratory Distress Assessment Instrument score).
We are available for any clarification. Best Regards, Renata Remondini PT (on behalf of the authors)
Reply
Under “Summary of findings for the main comparison” we changed the term “no‐physiotherapy” to “standard care” and deleted "(excluding chest physiotherapy)".
Under “Results – Included studies”: Remondini et al used the conventional terminology used by Gajdos 2010 for describing a type of technique commonly used in France. The “expiratory acceleration flow” AFE in France, is related to a manual chest compression during the expiratory phase that produces a high increase of flow in order to help mucus expectoration. This type of manoeuvre is globally called, force expiration technique. We changed the name in the review as suggested by Remondini in order to better fit with their original work but kept it in the same classification group. These changes also are made to the Table Remondini 2014 ‐ Interventions.
Under “Passive expiratory techniques ‐ forced passive expiratory techniques ‐ Primary outcomes ‐ Change in the severity status of bronchiolitis”: we agree with the feedback and followed their suggestion.
Under Results – Postural drainage percussion and vibration techniques – Primary outcomes 1 – Change in the severity status of bronchiolitis: again we agreed with the feedback
Under "Parents’ impression of physiotherapy benefit": we agree with the feedback. Firstly, we changed in “Postural drainage plus percussion and vibration techniques ‐ Secondary Outcomes”, outcome 5. Parents' impression of physiotherapy benefit "No trial presented data on parents' impression of physiotherapy benefit in this comparison. except Gajdos (Gajdos 2010). In it, they did not observe any significant difference in the way the parents rated the influence of physiotherapy on respiratory status (risk ratio (RR) 0.99, 95% CI 0.90 to 1.08, P = 0.89) or comfort (RR 0.99, 95% CI 0.94 to 1.05, P = 0.84). Secondly, we changed in “Passive expiratory techniques – forced passive expiratory techniques ‐ Secondary outcomes”, Outcome 5. Parents' impression of physiotherapy benefitTwo trials provided data on the parents' impression on the benefit of chest physiotherapy.
Remondini 2014 presented data on the parents' impression on the benefit of physiotherapy compared to conventional physiotherapy postural drainage alone. Parents' in both groups reported satisfaction related to improvements of breathing, feeding and nasal congestion, but, no difference was observed between the intervention groups. Gajdos 2010 reported they did not observe any significant difference in the way the parents rated the influence of physiotherapy on respiratory status (risk ratio (RR) 0.99, 95% CI 0.90 to 1.08, P = 0.89) or comfort (RR 0.99, 95% CI 0.94 to 1.05, P = 0.84).
We did not compare forced passive expiratory physiotherapy with just postural drainage.
Contributors
Jordi Vilaró and Marta Roqué i Figuls
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Feedback has been incorporated | Feedback comment and response from authors added to the review. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 10 October 2016 | Amended | Acknowledgement statement edited. |
| 19 May 2016 | Feedback has been incorporated | Data transcription error reported and corrected |
| 19 May 2016 | Amended | Data transcription error corrected in Abstract |
| 4 May 2016 | Feedback has been incorporated | Reader feedback and authors' responses and corrections incorporated |
| 8 July 2015 | New search has been performed | Searches updated. We included three new trials (Gomes 2012; Remondini 2014; Sanchez Bayle 2012) and excluded one new trial (Castro 2014). We identified one ongoing trial (Bella Lisbôa 2008). |
| 8 July 2015 | New citation required and conclusions have changed | Review amended to add a finer classification of interventions and to introduce the analysis of severity of disease. Dr Jordi Vilaró joined the review team to update this review. New evidence is presented for slow passive expiratory techniques. The role of respiratory syncytial virus (RSV) and severity of disease are discussed as potential modifiers of the effect of chest physiotherapy. |
| 9 November 2012 | Feedback has been incorporated | Reply to feedback comment added to the review. |
| 3 July 2012 | Feedback has been incorporated | Feedback comment added to the review. |
| 13 December 2011 | New citation required and conclusions have changed | New evidence shows no benefit of forced expiratory techniques. A new review author joined the original author team to update this review. |
| 13 December 2011 | New search has been performed | Searches conducted. Six new trials were included in this update (Aviram 1992; De Córdoba 2008; Gajdos 2010; Lopez Galbany 2004; Postiaux 2011; Rochat 2010) and one trial was excluded (Pupin 2009). |
| 14 May 2008 | Amended | Converted to new review format. |
| 19 July 2006 | New search has been performed | Updated review Issue 1, 2007. |
| 9 June 2004 | New search has been performed | First published Issue 2, 2005. |
Acknowledgements
We thank Dr Gadjós, Dr Asher Tal, Ms Núria Lopez, Dr Postiaux, Dr Gomes, Dr Rochat and Dr Sanchez Bayle for their help in providing information regarding their studies. We thank the following people for commenting on this 2014 update: Eman Sobh, Martin Chalumeau, Mark Jones and Hans van der Wouden. Marta Roqué i Fíguls is a PhD candidate at the Department of Paediatrics, Obstetrics and Gynecology and Preventive Medicine, Universitat Autònoma de Barcelona, Spain.
Appendices
Appendix 1. Details of searches
In the first version of this review we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2004, Issue 2), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (January 1966 to June 2004); EMBASE (1990 to June 2004); PASCAL, SCISEARCH, LILACS and Cumulative Index to the Nursing & Allied Health Literature (CINAHL) (1982 to May 2004).
In June 2006 we updated the searches of CENTRAL (The Cochrane Library 2006, Issue 2); MEDLINE (2004 to May Week 4 2006); EMBASE (July 2004 to December 2005) and CINAHL (1982 to May Week 4 2006).
In 2011 we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 4, part of The Cochrane Librarywww.thecochranelibrary.com (accessed 13 December 2011), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (May 2006 to November week 3, 2011), MEDLINE in‐process and other non‐indexed citations (8 December 2011), EMBASE.com (December 2005 to December 2011), CINAHL (2006 to December 2011), LILACS (2006 to December 2011) and Web of Science (2006 to December 2011).
In 2015 we conducted a top‐up search. We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 6) (accessed 8 July 2015), the Cochrane Acute Respiratory Infections Group's Specialised Register (October 2011 to July 2015), MEDLINE and MEDLINE in‐process and other non‐indexed citations (October 2011 to July 2015), EMBASE (October 2011 to July 2015), CINAHL (October 2011 to July 2015), LILACS (October 2011 to July 2015), Web of Science (October 2011 to July 2015) and Pedro (October 2011 to July 2015).
We used the following search strategy to search MEDLINE and CENTRAL in June 2006. The highly sensitive search strategy filter (Dickersin 1994) was combined with the search strategy and run over MEDLINE. The MEDLINE search was modified slightly to search CINAHL. No language restrictions were applied.
MEDLINE (OVID) 1 exp BRONCHIOLITIS 2 exp Bronchiolitis, Viral/ 3 bronchiolitis.mp. 4 exp Respiratory Syncytial Viruses/ 5 exp Respiratory Syncytial Virus Infections/ 6 respiratory syncytial virus$.mp. 7 exp Physical Therapy Techniques/ 8 chest physiotherapy.mp. 9 exp Drainage, Postural/ 10 postural drainage.mp. 11 chest percussion.mp. 12 exp VIBRATION/ 13 vibration.mp. 14 chest shaking.mp. 15 directed coughing.mp. 16 forced exhalation.mp. 17 exp Breathing Exercises/ 18 breathing exercise$.mp. 19 or/1‐6 20 or/7‐18 21 19 and 20
EMBASE (WebSpirs) #1 explode 'bronchiolitis‐' / all subheadings in DEM,DER,DRM,DRR #2 (bronchiolitis in ti) or (bronchiolitis in ab) #3 explode 'Respiratory‐syncytial‐pneumovirus' / all subheadings in DEM,DER,DRM,DRR #4 (respiratory syncytial virus* or RSV) in ti #5 #1 or #2 or #3 or #4 #6 explode 'physiotherapy‐' / all subheadings in DEM,DER,DRM,DRR #7 (physiotherapy in ti) or (physiotherapy in ab) #8 explode 'postural‐drainage' / all subheadings in DEM,DER,DRM,DRR #9 (postural drainage in ti) or (postural drainage in ab) #10 (chest percussion in ti) or (chest percussion in ab) #11 explode 'vibration‐' / all subheadings in DEM,DER,DRM,DRR #12 (vibration in ti) or (vibration in ab) #13 (chest shaking in ti) or (chest shaking in ab) #14 (directed coughing in ti) or (directed coughing in ab) #15 (forced exhalation in ti) or (forced exhalation in ab) #16 explode 'breathing‐exercise' / all subheadings in DEM,DER,DRM,DRR #17 (breathing exercise* in ti) or (breathing exercise* in ab) #18 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 #5 and #18
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Bronchiolitis/ 2 bronchiolit*.tw. 3 exp Respiratory Syncytial Viruses/ 4 Respiratory Syncytial Virus Infections/ 5 (repiratory syncytial virus* or rsv).tw. 6 or/1‐5 7 exp Physical Therapy Modalities/ 8 (chest adj2 (physiotherap* or physical therap*)).tw. 9 Drainage, Postural/ 10 (postural adj2 drainage*).tw. 11 Percussion/ 12 (chest* adj3 percuss*).tw. 13 Vibration/ 14 vibrat*.tw. 15 (chest* adj3 shak*).tw. 16 directed cough*.tw. 17 forced exhalation.tw. 18 forced expiration.tw. 19 Breathing Exercises/ 20 breathing exercise*.tw. 21 or/7‐20 22 6 and 21
Appendix 3. MEDLINE (Ovid) in‐process and other non‐indexed citations
1 bronchiolit*.tw. 2 (repiratory syncytial virus* or rsv).tw. 3 (chest adj2 (physiotherap* or physical therap*)).tw. 4 (postural adj2 drainage*).tw. 5 (chest* adj3 percuss*).tw. 6 vibrat*.tw. 7 (chest* adj3 shak*).tw. 8 directed cough*.tw. 9 forced exhalation.tw. 10 forced expiration.tw. 11 breathing exercise*.tw. 12 (physiotherap* or physical therap*).tw. 13 1 or 2 14 or/3‐12 15 13 and 14
Appendix 4. EMBASE (Elsevier) search strategy
21. #6 AND #20 20. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 19. (breathing NEAR/2 exercise*):ab,ti 18. 'breathing exercise'/de 17. 'forced exhalation':ab,ti OR 'forced expiration':ab,ti 16. 'directed coughing':ab,ti 15. (chest* NEAR/3 shak*):ab,ti 14. vibrat*:ab,ti 13. 'vibration'/de 12. (chest* NEAR/3 percuss*):ab,ti 11. 'percussion'/de 10. 'postural drainage':ab,ti 9. 'postural drainage'/de 8. (physiotherapy NEAR/4 chest):ab,ti 7. 'physiotherapy'/exp 6. #1 OR #2 OR #3 OR #4 OR #5 5. 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti 4. 'respiratory syncytial virus infection'/de 3. 'respiratory syncytial pneumovirus'/de 2. bronchiolit*:ab,ti 1. 'bronchiolitis'/exp
Appendix 5. CINAHL (EBSCO) search strategy
S24 S6 and S23 S23 S21 or S22 S22 S7 or S8 or S9 or S10 or S11 or S12 or S13 S21 S14 or S15 or S16 or S17 or S18 or S19 or S20 S20 TI breathing exercise* or AB breathing exercise* S19 (MH "Breathing Exercises+") S18 TI ( "forced exhalation" or "forced expiration" ) or AB ( "forced exhalation" or "forced expiration" ) S17 TI directed N3 cough* or AB directed N3 cough* S16 TI chest N3 shak* or AB chest N3 shak* S15 TI vibrat* or AB vibrat* S14 (MH "Vibration") S13 TI chest N3 percuss* or AB chest N3 percuss* S12 (MH "Percussion") S11 TI "postural drainage" or AB "postural drainage" S10 TI chest N3 "physical therapy" or AB chest N3 "physical therapy" S9 TI chest N3 physiotherap* or AB chest N3 physiotherap* S8 (MH "Chest Physical Therapy+") S7 (MH "Physical Therapy") S6 S1 or S2 or S3 or S4 or S5 S5 TI ( respiratory syncytial virus* or rsv ) or AB ( respiratory syncytial virus* or rsv ) S4 (MH "Respiratory Syncytial Virus Infections") S3 (MH "Respiratory Syncytial Viruses") S2 TI bronchiolit* or AB bronchiolit* S1 (MH "Bronchiolitis+")
Appendix 6. LILACS (BIREME) search strategy
(MH:bronchiolitis OR MH:C08.127.446.135$ OR MH:C08.381.495.146.135$ OR MH:C08.730.099.135$ OR bronchiolit$ OR Bronquiolitis OR Bronquiolite OR MH:"Respiratory syncytial viruses" OR "respiratory syncytial virus" OR "respiratory syncytial viruses" OR "Virus Sincitiales Respiratorios" OR MH:"respiratory syncytial virus infections" OR "Infecciones por Virus Sincitial Respiratorio" OR rsv OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial") AND (MH:"physical therapy modalities" OR MH:E02.779$ OR "physical therapy" OR "physical therapies" OR "Modalidades de Terapia Física" OR "Modalidades de Fisioterapia" OR physiotherap$ OR Fisioterap$ OR Fisioterápicas OR "Terapia Física" OR MH:"Drainage, Postural" OR "postural drainage" OR "Drenaje Postural" OR "Drenagem Postural" OR MH:Percussion OR Percusión OR Percussão OR percus$ OR MH:vibration OR vibrat$ OR Vibración OR Vibração OR shak$ OR "directed coughing" OR "directed cough" OR "forced exhalation" OR "forced expiration" OR expiración OR Expiração OR MH:"Breathing exercises" OR "breathing exercise" OR "breathing exercises" OR "Ejercicios Respiratorios" OR "Exercícios Respiratórios")
Appendix 7. Web of Science (Thomson Reuters) search strategy
Topic=(bronchiolit* or rsv or respiratory syncytial virus*) AND Topic=(chest physical therap* or chest physiotherap* or postural drainage or chest percussion or chest vibration or chest shaking or directed coughing or forced exhalation or breathing exercises) Timespan=2006‐2009. Databases=SCI‐EXPANDED, CPCI‐S.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aviram 1992.
| Methods | Randomised, single‐blinded controlled trial | |
| Participants | 50 young infants with acute bronchiolitis, paired by age and severity of disease. Diagnostic criteria not described | |
| Interventions | Group 1: chest physiotherapy (N = 25) Group 2: no intervention (N = 25) All participants were treated with fluids, oxygen (when SaO2 in room < 92%) and received inhaled salbutamol every 6 hours |
|
| Outcomes | ‐ Length of stay in hospital ‐ Improvement in clinical score (12 hours) (Tal 1983) ‐ Changes in SaO2 |
|
| Notes | No information on funding. Authors confirmed trial unpublished (July 2010) and provided additional information Personal communication: the decision to discharge was based on improvement of the infant to a score of < 5 and no need for oxygen. There was no difference whatsoever between the 2 groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Clinical scoring was done by a physician who was blinded to the [chest physiotherapy] therapy" Personal communication: "Patient's condition was monitored using our clinical score by one of two physicians, twice a day, blinded to the yes or no chest physiotherapy done by a third person, who was blinded to the scores." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 50 infants were randomised and analysed |
| Selective reporting (reporting bias) | Unclear risk | No information available |
Bohe 2004.
| Methods | Randomised, open controlled trial | |
| Participants | Infants admitted to the hospital with a clinical diagnosis of acute bronchiolitis defined as acute respiratory tract infection, preceded or simultaneous to fever and/or rhinitis, plus tachypnoea, wheezing and increased respiratory effort N = 32 patients randomised and patients analysed: 16 allocated to the control group and 16 to the intervention arm |
|
| Interventions | Group 1: drainage, percussion, vibration and nasopharyngeal aspiration twice a day (N = 16) Group 2: nasopharyngeal aspiration (N = 16) All participants received nebulised B2 adrenergic, and inhaled and intravenous corticoids |
|
| Outcomes | Primary outcome: clinical score (Wood 1972) with range 0 to 12, scoring 0 to 3 to heart rate, respiratory rate, auscultation, use of accessory muscles. Assessment at discharge Secondary outcome: length of stay (days) |
|
| Notes | 1 patient in the control group developed atelectasis at day 4, and was withdrawn and received chest respiratory physiotherapy Children were assessed every evening up to discharge or day 5 No information on funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patient allocation was random, by means of concealed allocation according to admission number, independently assigned by the hospital admission centre |
| Allocation concealment (selection bias) | Low risk | Allocation was described as concealed |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study described as open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 32 patients were randomised and analysed. A child included in Group 2 presented right basal atelectasis by 4th day of hospitalisation; he received respiratory physiotherapy and was excluded from the trial. It is not clear how the data were treated |
| Selective reporting (reporting bias) | Unclear risk | Not described |
De Córdoba 2008.
| Methods | Randomised, open controlled trial Participants were allocated by opaque, sealed envelopes |
|
| Participants | Children below 2 years admitted to the hospital and emergency department, with clinical and radiological diagnosis of acute viral bronchiolitis, presenting with bronchial hypersecretion (pulmonary auscultation) N = 24 patients randomised, 19 patients analysed: 5 in Group 1, 8 in Group 2 and 6 in Group 3. Exclusions due to haemodynamic instability (2), heart disease (1), non‐invasive mechanical ventilation (1), prematurity (1) Mean age: 93 days in Group 1, 131.1 days in Group 2, 125.0 days in Group 3 |
|
| Interventions | Group 1: vibration + postural drainage + bronchial aspiration in dorsal decubitus (N = 5) Group 2: percussion + postural drainage + bronchial aspiration in dorsal decubitus (N = 8) Group 3: bronchial aspiration in dorsal decubitus (N = 6) Postural drainage for 5 minutes in each decubitus (right and left lateral randomly chosen) + bronchial aspiration in dorsal decubitus. All participants received nasotracheal aspiration with saline solution |
|
| Outcomes | The primary outcome is not clear. Outcomes assessed were: saturation of oxygen pulse, cardiac frequency, respiratory frequency, Silverman‐Anderson score of respiratory discomfort, amount of inhaled secretion | |
| Notes | Treatment was delivered once. Outcomes were assessed immediately after treatment and after 15 minutes No information on funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Patients were randomised by means of opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24 randomised patients and 5 exclusions described with reasons but not the group they belonged to. 2 due to haemodynamic instability, 1 by heart disease, 1 in non‐invasive mechanical ventilation and 1 preterm baby. Results are presented for 19 patients |
| Selective reporting (reporting bias) | Unclear risk | Not described |
Gajdos 2010.
| Methods | Randomised, double‐blind controlled trial | |
| Participants | Child aged 15 days to 24 months with first acute bronchiolitis and indication of hospitalisation, and one or more of these criteria at admission: toxic aspect; apnoea or cyanosis; respiratory rate > 60/minute; pulse oxymetry < 95%; alimentary intake < 2/3 of the needs. Bronchiolitis was diagnosed on the basis of a history of upper respiratory tract infection and clinical findings consistent with bronchiolitis, including wheezing or wheezing with crackles and respiratory distress N = 496 patients randomised and analysed: 246 allocated to the control group and 250 to the intervention arm |
|
| Interventions | Group 1: chest physiotherapy with increased exhalation technique plus with assisted cough plus nasopharyngeal aspiration (N = 246)
Group 2: nasopharyngeal aspiration (N = 250) Increased exhalation technique involved the generation of synchronised thoracic‐abdominal movement by the hands of the physiotherapist at the beginning of expiration with one hand on the thorax, meanwhile with the other on the abdomen, centred on the umbilicus, the physiotherapist applied an abdominal counter‐weight. The manoeuvre began at the end of the inspiratory plateau and was pursued until the end of expiration, according to the infant's thoraco‐pulmonary compliance and up to his or her chest wall and lung resistance limits. The procedure was repeated until meeting auscultation‐efficacy criteria (decrease or disappearance of wheezing and/or increase of rhonchi), but did not last longer than 10 to 15 minutes. The procedure was stopped in the case of respiratory status aggravation. If no spontaneous coughing occurred, coughing could be triggered by pressure on the suprasternal notch |
|
| Outcomes | Primary outcome: time to recovery defined in the study protocol as verifying, for at least 8 hours in a row, the following requirements: pulse oxymetry >= 95% AND normal feeding AND specific respiratory distress score lower than one as described in the protocol AND normal respiratory rate Secondary outcomes: safety of the forced expiratory technique; comparison of pulse oxymetry before/after chest physiotherapy; quality of life scale |
|
| Notes | ClinicalTrials.gov identifier: NCT00125450 Study received funding from governmental organisations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random allocation computer generated with SAS software packages in advance by the biostatistician", "permutation blocks with a block size of four" |
| Allocation concealment (selection bias) | Low risk | "physiotherapist opening a sealed sequentially numbered envelope" "block size of four that was not mentioned to the physicians involved in the patient recruitment" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "all paediatric department staff, parents and guardians were blind to treatment assignment." "Those involved in the evaluation of primary outcome or in the decision of the co interventions were blinded to group assignment." "The treatment was performed by the physiotherapist staying alone with the infant, in a room with a covered window pane" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analysis was performed on an intent‐to‐treat basis and all patients included in the study were analysed, including the two lost to follow‐up (one in each group)" |
| Selective reporting (reporting bias) | Low risk | Protocol available and consistent with report |
Gomes 2012.
| Methods | Randomised, single‐blinded controlled trial | |
| Participants | Infants aged from 28 days to 24 months, previously healthy, with a clinical diagnosis of AVB and positive outcome of RSV in nasopharyngeal aspirate detected by immunofluorescence technique 30 infants N = 30 patients randomised, 30 patients analysed at baseline, 20 analysed at 48 hours, 17 analysed at 72 hours. 10 allocated to the control group only assessed at baseline, 10 to the conventional physiotherapy arm and 10 to the new physiotherapy arm Mean age 125 days |
|
| Interventions | Group 1: new physiotherapy group (N = 10) received prolonged slow expiration (slow passive and progressive expiration from the functional residual capacity into the expiratory reserve volume) and clearance rhinopharyngeal retrograde (forced inspiratory manoeuvre) Group 2: conventional physiotherapy group (N = 10) received vibrations, expiratory compression, modified postural drainage only in the lateral decubitus position and clapping Group 3: control group, only assessed at baseline (n = 10), received suction of the upper airways | |
| Outcomes | Primary outcome: Wang's clinical score Secondary outcomes: transcutaneous PCO2 |
|
| Notes | Assessments performed at 2 hours, 48 hours and 72 hours after admission and again one hour prior to discharge ClinicalTrials.gov identifier: NCT00884429 No information on funding Authors contacted and provided information (21 March 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Infants were randomised by using sealed opaque envelopes containing the instructions to be followed in each of three groups" |
| Allocation concealment (selection bias) | Low risk | "Infants were randomised by using sealed opaque envelopes containing the instructions to be followed in each of three groups" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Assessors were blinded to the treatment groups. These raters were trained specifically for this assessment. The time spent caring for children was similar in all groups and parents were unaware of their child's group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost at 72 hours (1 in new physiotherapy and 2 in conventional physiotherapy) due to hospital discharge |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
Lopez Galbany 2004.
| Methods | Randomised, single‐blind controlled trial | |
| Participants | Pilot study enrolled 30 participants
N = 32 patients randomised, 32 patients analysed: 16 allocated to the control group and 16 to the intervention arm |
|
| Interventions | Group 1: forced expiratory technique for 10 minutes, single daily session during the first 5 days of hospitalisation Group 2: no intervention |
|
| Outcomes | ‐ Severity clinical score (Bierman Pierson modified score) (Bierman 1974; Tal 1983) ‐ Length of stay |
|
| Notes | No information on funding. Authors confirmed trial unpublished (July 2010) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
Nicholas 1999.
| Methods | Randomised, open controlled trial Participants were randomly allocated to control and treatment groups using a random sequence number |
|
| Participants | Infants admitted to the hospital with a clinical diagnosis of acute bronchiolitis and with respiratory distress severe enough that required nasogastric tube feeding or intravenous fluids N = 50 patients randomised and analysed: 24 were allocated to control group and 26 to treatment Mean age of control group: 3.2 (range 0.4 to 8.3); intervention group 2.4 (range 0.4 to 6.9). RSV‐positive: control 79%, intervention 85% |
|
| Interventions | Group 1: vibration and postural drainage techniques twice a day (N = 26)
Group 2: no intervention (N = 24) In the physiotherapy arm, the participant was treated on the physiotherapist's knee, percussion and vibration lying on right side, lying on left side and sitting; suction performed after on each side, if necessary, until clear; no oxygen required during treatment. Modifications were allowed if participant did not tolerate the procedure. Oxygen was allowed depending on infant tolerability |
|
| Outcomes | Primary outcome: validated clinical score (Dick 1991) with values 0 to 20, assigning scores 0 to 2 to heart rate, respiratory rate, blood gases, rhinitis, hyperinflation, use of accessory muscles, recession, cough, wheeze, crackles Secondary outcomes: length of stay (days); provision of inspired oxygen; requirement for nasogastric feeding; oxygen saturation |
|
| Notes | The study ended at 5 days or if the patient was transferred to the intensive care unit
Authors did not report the standard deviation No information on funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random sequence number generated by the Medical Statistics Unit of the University of Edinburgh" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 50 patients were randomised and assessed, although 1 child was excluded from the trial after being admitted to the intensive care unit. It is not clear how data were treated. Saturation of oxygen pulse assessments comprised those of 2 excluded children which were not assessed for clinical outcomes |
| Selective reporting (reporting bias) | Unclear risk | Not described |
Postiaux 2011.
| Methods | Randomised, single‐blinded controlled trial | |
| Participants | Hospitalised infants less than 1 year of age presenting with acute RSV bronchiolitis and a clinical Wang score >= 3
N = 20 infants randomised and analysed: 8 allocated to the control group and 12 to the intervention arm Mean age: 4.19 months |
|
| Interventions | Group 1: 3% hypertonic saline solution and salbutamol (HS therapy) (n = 8 totaling 27 sessions) Group 2: HS therapy followed by one session of 10 to 15 minutes of prolonged slow expiration technique and coughing provoked (n = 12, totaling 31 sessions) Sessions lasted 30 minutes |
|
| Outcomes | Primary outcome: Wang's clinical score (respiratory rate, wheezing, retraction, general appearance) Secondary outcomes: SpO2; heart rate |
|
| Notes | Outcomes were evaluated at t0, t30 and t150 Authors report no conflict of interest/funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Both of our paediatrician evaluators were blinded to the applied treatment and goals"; "Physiotherapists in charge of administering the treatments were instructed to ignore the results of each evaluation until the end of the study. The patient' parents were unaware of the group in which their child was included. In both groups the periods of time spent in the room were identical, so outside observers were blinded to the applied treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 patients were randomised and assessed |
| Selective reporting (reporting bias) | Unclear risk | No information available |
Remondini 2014.
| Methods | Randomised controlled trial | |
| Participants | Hospitalised infants younger than 1 year with clinical diagnosis of bronchiolitis N = 29 infants randomised in 2 groups. G1 = 16 infants, 48 sessions, and G2 = 13 infants, 35 sessions. The trial authors considered the patient ready to be discharged from the study when the patient presented a lower disease severity score (RDAI score ≤ 4) associated with adequate oxygenation on RA (SpO2 ≥ 92%) |
|
| Interventions | Group 1: underwent postural drainage associated with tapping or percussion and tracheal aspiration Group 2: underwent postural drainage associated with expiratory acceleration flow (EAF) and tracheal aspiration |
|
| Outcomes | Primary outcome: Respiratory Distress Assessment Instrument (RDAI) score system and oxygen saturation (SpO2) Secondary outcomes: time required to discharge, and parents treatment perception |
|
| Notes | Patients were assessed before, 10 minutes after, and 60 minutes after the physical therapy intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients were excluded because refusal of parents for no acceptance of AEF manoeuvre Patients were assessed before, 10 minutes after, and 60 minutes after the physical therapy intervention |
| Selective reporting (reporting bias) | Unclear risk | No information available |
Rochat 2010.
| Methods | Randomised open clinical trial | |
| Participants | Infants <= 1 year admitted with diagnosis of RSV‐positive bronchiolitis during 2 consecutive RSV seasons N = 103 children randomised, 99 analysed. 51 allocated to physiotherapy and 52 to control. Mean age was 109 days. RSV test positive: 74% intervention, 75.5% control |
|
| Interventions | Group 1: physiotherapy group (n = 51) received 2 daily physiotherapy sessions at least 2 hours after feeds (prolonged slow expiratory technique obtained by slow manual pressure over the abdomen, exerted at the start of the expiratory phase down to the residual volume and maintained for 2 to 3 respiratory cycles; manual vibration exerted at the start of the expiratory phase; induced cough) plus same treatment as control group Group 2: control group (n = 52) received rhinopharyngeal suctioning after instillation of normal saline solution if needed; minimal handling; oxygen to achieve SpO2 ≥ 92% and fractionated meals. Topical bronchodilators and steroids were not routinely used. Nasal drops such as xylometazoline were often employed to decrease nasal congestion. Antibiotics were administered when concomitant bacterial infection was suspected (prolonged fever, otitis media and increased white cell count) |
|
| Outcomes | Primary outcome: time to clinical stability, defined by feeding more than 50% of the required amount, the absence of vomiting, undisrupted sleep and SpO2 ≥ 92% for more than 10 hours Secondary outcome: change in clinical state, measured by a general score made of 3 well‐being items (feeding, vomiting and quality of sleep); change in respiratory state, measured by a respiratory score made of 7 items (respiratory rate, SpO2, presence and severity of retractions, adventitious respiratory sounds, presence of vesicular murmur, thoracic distension); occurrence of complications | |
| Notes | Study received funding from governmental organisations Outcomes assessed daily at a fixed time point, prior physiotherapy sessions Authors contacted and provided information (March 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation list in blocks of random length (8, 10 or 12) by the study epidemiologist, not involved in the clinical phase of the study." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done by the attribution of a number contained in a sealed opaque envelope opened following the inclusion consent. Envelopes were prepared according to a randomisation list in blocks of random length (8, 10 or 12) by the study epidemiologist, not involved in the clinical phase of the study (TP)." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Open trial. Nevertheless, "All children underwent daily clinical evaluations at a fixed time point prior to the physiotherapy sessions when allocated to the group with CP. Evaluations were performed by a study physiotherapist who was different from the physiotherapist administering the treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 103 randomised infants, 4 of whom were later excluded (1 in physiotherapy, 3 in control) for the following reasons: parental withdrawal of content, erroneous initial diagnosis and direct admission to intensive care, or age > 12 months. Results presented for the 99 remaining eligible infants |
| Selective reporting (reporting bias) | Unclear risk | An abstract presented to a scientific meeting in 2010 focuses its conclusions on the daily improvement of a severity score, while the published paper states that the primary outcome is the time to clinical stability. Nevertheless, we believe this change does not introduce bias into the results since both outcomes are related and non‐significant |
Sanchez Bayle 2012.
| Methods | Randomised, single‐blinded, controlled trial Participants were randomised before checking of inclusion criteria and signing of informed consent, leading to the exclusion of 40 randomised participants not meeting the criteria, and 16 participants that refused consent because of blinding of intervention received |
|
| Participants | Infants < 7 months with a first episode of acute bronchiolitis diagnosed by McConnochie criteria, admitted in a paediatric hospital during 2 consecutive winter seasons 293 children where randomised (149 to physiotherapy and 144 to control) and 236 analysed. Mean age was 2.77 months. RSV test positive: 66% intervention, 67% control | |
| Interventions | Group 1: physiotherapy group (n = 136) received 2 daily physiotherapy sessions of 10 minutes (prolonged slow expiratory technique obtained by slow manual pressure over the abdomen, exerted at the start of the expiratory phase down to the residual volume and maintained for 2 to 3 respiratory cycles; manual vibration exerted at the start of the expiratory phase; induced cough) plus oxygen therapy until SpO2 >= 94% Group 2: control group (n = 100) received postural changes plus oxygen therapy until SpO2 >= 94% |
|
| Outcomes | Primary outcome: duration of oxygen supplementation, length of stay in hospital Secondary outcomes: salbutamol use, ipratropium bromide use, antibiotics use, adrenaline use, pneumonia |
|
| Notes | Outcomes were assessed at discharge. Authors reported no conflicts of interest/funding. Authors contacted and provided information (March 2014) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a random number table |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Only the physiotherapists were aware of the allocation group of the infants", "The placebo group received postural changes, so parents, doctors and nurses couldn't guess the allocation group" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 236 analysed participants of 293 initially recruited. 40 initially recruited participants (10 in treatment and 30 in control) did not meet inclusion criteria. The unequal distribution may be related to selection bias |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
Webb 1985.
| Methods | Randomised, open, controlled trial | |
| Participants | Infants admitted with a clinical diagnosis of acute bronchiolitis. Unreported diagnostic criteria N = 90 patients randomised and analysed: 46 allocated to the control group and 44 to the intervention arm Mean age 46 months (range 0.5 to 15) 69% had respiratory syncytial virus, 36% had a first‐degree family history of atopy, 66% had smokers in the household |
|
| Interventions | Group 1: chest physiotherapy comprising standard techniques applied by a trained paediatric physiotherapist Group 2: no intervention They performed chest percussion with a cupped hand for 3 minutes in each of 5 postural drainage positions followed by assisted coughing or gentle oropharyngeal suction twice a day |
|
| Outcomes | Primary outcome: clinical score, with values 0 to ‐30, assigning scores 0 to 3 to heart rate, respiratory rate, hyperinflation, use of accessory muscles, recession, rhinitis, wheeze, cough, crepitations and rhonchi Secondary outcome: length of stay (days) |
|
| Notes | Clinical assessment of severity illness made at a fixed time each day for 5 days. There was a follow‐up after 2 weeks at the outpatient clinic Authors did not report mean and standard deviation of the mean. Results were expressed as median values and range No information on funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Strictly speaking, [assessments] could not be ‘blind' with respect to treatment status though in practice that status was not obvious at each assessment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 90 analysed patients but it is not clear how many were randomised and if there was any attrition of patients |
| Selective reporting (reporting bias) | Unclear risk | Not described |
ELISA: enzyme‐linked immunosorbent assay RSV: respiratory syncytial virus SaO₂: oxygen saturation t: time point
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belcastro 1984 | Controlled clinical trial |
| Bernard‐Narbone 2003 | Uncontrolled intervention study |
| Castro 2014 | To be included, participants had to present an acute wheezing episode, which it not necessarily correlated to bronchiolitis |
| Postiaux 2004 | Uncontrolled intervention study |
| Pupin 2009 | Controlled clinical trial |
| Quitell 1988 | Uncontrolled intervention study |
Characteristics of ongoing studies [ordered by study ID]
Bella Lisbôa 2008.
| Trial name or title | Comparison of effectiveness between Anglo‐Saxon chest physiotherapy techniques and European chest physiotherapy techniques in infants diagnosed with acute bronchiolitis |
| Methods | Blinded randomised clinical trial |
| Participants | Infants aged between 0 and 24 months, with a recent acute bronchiolitis diagnostic attested by a physician and a posteroanterior (PA) Thorax X‐Ray incidence |
| Interventions | Group 1: Anglo‐Saxon chest physiotherapy techniques: inhalotherapy, vibration, postural drainage, percussion and induced cough Group 2: European chest physiotherapy techniques: inhalotherapy, ELPr (French: expiration length prolonged‐passive, slow expiration) induced cough |
| Outcomes | Wang severity clinical score, hospitalisation period, pulse oxymetry, heart rate |
| Starting date | 1 July 2008 (start of enrolment) |
| Contact information | Alice Bella Lisbôa. Rua Abolição, 1827, Swift, Campinas‐SP, Brazil. Phone: +55 19 32373878 +55 19 92475175. E‐mail: bella.lisboa@gmail.com |
| Notes | Conducted in Brazil |
Differences between protocol and review
In this 2015 update, we classified the trials by type of physiotherapy technique into vibration and percussion techniques and passive expiratory techniques. We further subdivided this subgroup into slow passive expiratory techniques and forced passive expiratory techniques. We no longer considered respiratory parameters to be primary outcomes; they are now secondary outcomes.
We added subgroup analyses by severity of participants and setting to this update, after the feedback received on previous versions made it clear that the review included trials of participants with wide‐ranging severity, and there was a plausible hypothesis that the efficacy of the interventions varied with severity and setting (a covariate highly correlated with severity of participants).
Finally, in this 2015 update, we added 'Summary of findings' tables for the comparisons of forced expiration versus standard care for acute bronchitis and slow passive expiration versus standard care for acute bronchitis.
To better reflect the secondary objective to determine the efficacy of different techniques of chest physiotherapy (for example, vibration and percussion and passive forced exhalation), we modified the type of intervention section to explicitly allow inclusion of studies with active comparators.
Contributions of authors
Contributions of authors have changed with the review versions. The following list corresponds to the current update:
Marta Roqué was responsible for updating the review. Marta Roqué, Maria Giné and Jordi Vilaró performed 'Risk of bias' assessment and data extraction, interpretation of results and drafting of the updated review text. Carla Perrota and Claudia Granados conducted reference screening. All authors commented on the interpretation of results and text of the review, and contributed to the final version of the review.
Sources of support
Internal sources
Iberoamerican Cochrane Center, Barcelona, Spain.
UCD School of Public Health and Population Sciences, Ireland.
External sources
Instituto de Salud Carlos III Subdireccion General de Investigacion Sanitaria (01/A060), Spain.
Declarations of interest
Marta Roqué i Figuls: none known. Maria Giné‐Garriga: none known. Claudia Granados Rugeles: none known. Carla Perrotta: none known. Jordi Vilaró: I received fees from Smiths Medical for giving a scientific conference.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Aviram 1992 {published and unpublished data}
- Aviram M, Damri A, Yekutielli C, Bearman J, Tal A. Chest physiotherapy in acute bronchiolitis [Abstract]. European Respiratory Journal 1992;5(Suppl 15):229‐30. [CENTRAL: CN‐00492981] [Google Scholar]
Bohe 2004 {published data only}
- Bohe L, Ferrero ME, Cuestas E, Polliotto L, Genoff M. Indications of conventional chest physiotherapy in acute bronchiolitis. Medicina de Buenos Aires 2004;64(3):198‐200. [PubMed] [Google Scholar]
De Córdoba 2008 {published data only}
- Córdoba F, Rodrigues M, Luque A, Cadrobbi C, Faria R, Solé D. Fisioterapia respiratória em lactentes com bronquiolite: realizar ou não?. Mundo Saúde 2008;32(2):183‐8. [Google Scholar]
Gajdos 2010 {published data only}
- Gajdos V, Katsahian S, Beydon N, Abadie V, Pontual L, Larrar S, et al. Effectiveness of chest physiotherapy in infants hospitalized with acute bronchiolitis: a multicenter, randomized, controlled trial. PLoS Medicine 2010;7(9):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gomes 2012 {published and unpublished data}
- Gomes ELFD, Postiaux G, Medeiros DRL, Monteiro KKDS, Sampaio LMM, Costa D. Chest physical therapy is effective in reducing the clinical score in bronchiolitis: randomized controlled trial [A fisioterapia respiratória é eficaz na redução de escore clínico na bronquiolite:ensaio controlado randomizado]. Revista Brasileira de Fisioterapia 2012;16:241‐7. [DOI] [PubMed] [Google Scholar]
Lopez Galbany 2004 {unpublished data only}
- Lopez Galbany N. Oral presentation in local meeting. Presentation slides on file 2004 (accessed 1 January 2011).
Nicholas 1999 {published data only}
- Nicholas KJ, Dhouieb MO, Marshal TG, Edmunds AT, Grant MB. An evaluation of chest physiotherapy in the management of acute bronchiolitis. Changing clinical practice. Physiotherapy 1999;85(12):669‐74. [Google Scholar]
Postiaux 2011 {published data only}
- Postiaux G, Louis J, Gerroldt J, Kotik A‐C, Lemuhot A, Patte C. Effects of a new chest physiotherapy protocol in infant RSV bronchiolitis, a RCT. European Respiratory Society Annual Congress, Berlin, Germany, October 4‐8. 2008:E1772. [CENTRAL: CN‐00679586]
- Postiaux G, Louis J, Labasse HC, Gerroldt J, Kotik AC, Lemuhot A, et al. Effects of an alternative chest physiotherapy regimen protocol in infants with RSV bronchiolitis. Respiratory Care 2011;56(7):989‐94. [DOI: 10.4187/respcare.00721; PUBMED: 21352671 ] [DOI] [PubMed] [Google Scholar]
Remondini 2014 {published data only}
- Remondini R, Zamprônio dos Santos A, Castro G, do Prado C, Ribeiro Ferreira da Silva Filho LV. Comparative analysis of the effects of twochest physical therapy interventions in patients withbronchiolitis during hospitalization period [Análise comparativa dos efeitos de duas intervenções de fisioterapia respiratória empacientes com bronquiolite durante o período de internação hospitalar]. Einstein (Sao Paulo) 2014;12(4):452‐8. [[Empty name]">[DOI: 10.1590/S1679‐45082014AO3230; PubMed: 25628196]] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rochat 2010 {published data only}
- Rochat I, Leis P, Bouchardy M, Oberli C, Sourial H, Friedli‐Burri M, et al. Chest physiotherapy in bronchiolitis: a randomised trial assessing passive expiratory manoeuvres. Paediatric Respiratory Reviews 2010;11(Suppl 1526):85‐6. [Google Scholar]
- Rochat I, Leis P, Bouchardy M, Oberli C, Sourial H, Friedli‐Burri M, et al. Chest physiotherapy using passive expiratory techniques does not reduce bronchiolitis severity: a randomised controlled trial. European Journal of Pediatrics 2011 Sep 17 [Epub ahead of print]. [DOI] [PubMed]
Sanchez Bayle 2012 {published and unpublished data}
- Sanchez Bayle M, Martin Martin R, Cano Fernandez J, Martínez Sánchez G, Gómez Martín J, Yep Chullen G, et al. Chest physiotherapy and bronchiolitis in the hospitalised infant. Double‐blind clinical trial [Estudio de la eficacia y utilidad de la fisioterapia respiratoria en labronquiolitis aguda del lactante hospitalizado. Ensayo clínico aleatorizado y doble ciego]. Anales de Pediatria 2012;77:5‐11. [DOI] [PubMed] [Google Scholar]
Webb 1985 {published data only}
- Webb MS, Martin JA, Cartlidge PH, Ng YK, Wright NA. Chest physiotherapy in acute bronchiolitis. Archives of Disease in Childhood 1985;60:1078‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Belcastro 1984 {published data only}
- Belcastro M, Backes C, Chila A. Bronchiolitis: a pilot study of osteopathic manipulative treatment, bronchodilators and other therapy. Journal of the American Osteopathic Association 1984;83(9):672‐5. [PubMed] [Google Scholar]
Bernard‐Narbone 2003 {published data only}
- Bernard‐Narbonne F, Daoud P, Castaing H, Rousset A. Effectiveness of chest physiotherapy in ventilated children with acute bronchiolitis [Efficacité de la kinésithérapie respiratorire chez des enfants intubés ventilés atteinets de bronchiolite aiguë]. Archives de Pédiatrie 2003;10:1043‐7. [DOI] [PubMed] [Google Scholar]
Castro 2014 {published data only}
- Castro‐Rodriguez JA, Sanchez I. Chest physiotherapy for acute wheezing episodes: an inappropriate interpretation of the first trial in outpatient infants. Acta Paediatrica, International Journal of Paediatrics 2014;103(17):e326‐e7. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Castro‐Rodriguez JA, Silva R, Tapia P, Salinas P, Tellez A, Leisewitz T, et al. Chest physiotherapy is not clinically indicated for infants receiving outpatient care for acute wheezing episodes. Acta Pediatrica 2014;103(23):518‐23. [DOI] [PubMed] [Google Scholar]
Postiaux 2004 {unpublished data only}
- Postiaux G, Dubois R, Marchand E, Jacquy J, Mangiaracina M. Chest physiotherapy in infant bronchiolitis: a new approach ‐ nCPT. Proceedings of the 6th International Meeting of Pediatric Neumonology, Lisboa, Portugal. 2004; Vol. Suppl:117‐25.
Pupin 2009 {published data only}
- Pupin M, Riccetto A, Ribeiro J, Baracat E. Comparison of the effects that two different respiratory physical therapy techniques have on cardiorespiratory parameters in infants with acute viral bronchiolitis. Jornal Brasileiro De Pneumologia: Publicacao Oficial Da Sociedade Brasileira De Pneumologia E Tisilogia 2009;35(9):860‐7. [DOI] [PubMed] [Google Scholar]
Quitell 1988 {published data only}
- Quitell LM, Wolfson MR, Schidlow DV. The effectiveness of chest physical therapy in infants with bronchiolitis. American Review of Respiratory Disease 1988;137:406A. [Google Scholar]
References to ongoing studies
Bella Lisbôa 2008 {published data only}
- Bella Lisbôa A. Chest physiotherapy effectiveness in infants with acute bronchiolitis. www.anzctr.org.au/ACTRN12608000601336.aspx 2008.
Additional references
AAP 2006
- American Academy of Pediatrics. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774–93. [DOI] [PubMed] [Google Scholar]
AAPs 2006
- Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774‐93. [DOI] [PubMed] [Google Scholar]
Barben 2008
- Barben J, Kuehni CE, Trachsel D, Hammer J, on behalf of the Swiss Paediatric Respiratory Research Group. Management of acute bronchiolitis: can evidence based guidelines alter clinical practice?. Thorax 2008;63:1103‐9. [DOI: 10.1136/thx.2007.094706] [DOI] [PubMed] [Google Scholar]
Beauvois 2001
- Beauvois E. Role of physiotherapy in the treatment of acute bronchiolitis in the infant [Place de la kinésithérapie dans le traitement des bronchiolites aiguës du norrisson. Conférence de consensus]. Archives de Pédiatrie 2001;8(Suppl 1):128‐31. [DOI] [PubMed] [Google Scholar]
Beeby 1998
- Beeby PJ, Henderson‐Smart DJ, Lacey JL, Rieger I. Short and long term neurological outcomes following neonatal chest physiotherapy. Journal of Pediatrics and Child Health 1998;34:60‐2. [DOI] [PubMed] [Google Scholar]
BGT 2005
- Bronchiolitis Guideline Team. Evidence based clinical practice guideline for medical management of bronchiolitis in infants year of age or less presenting with a first time episode. Cincinnati Children’s Hospital Medical Center 2005.
Bierman 1974
- Bierman CW, Pierson WE. The pharmacologic management of status asthmaticus in children. Pediatrics 1974;54:245‐7. [PubMed] [Google Scholar]
Bourke 2010
- Bourke T, Shields M. Bronchiolitis. Clinical Evidence 2011;4(308):1‐43. [PMC free article] [PubMed] [Google Scholar]
Branchereau 2013
- Branchereau E, Branger B, Launay E, Verstraete M, Vrignaud B, Levieux K, et al. Management of bronchiolitis in general practice and determinants of treatment being discordant with guidelines of the HAS [État des lieux des pratiques médicales en médecine générale en matière de bronchiolite et déterminants deprises en charge thérapeutiques discordantes par rapport aux recommandations de l’HAS]. Archives de Pédiatrie 2013;20:1369–75. [DOI] [PubMed] [Google Scholar]
Carroll 2008
- Carroll KN, Gebretsadik T, Griffin MR, Wu P, Dupont WD, Mitchel EF, et al. The increasing burden and risk factors for bronchiolitis‐related medical visits in infants enrolled in a state healthcare insurance plan. Pediatrics 2008;122(1):58‐64. [PUBMED: PMC2655142] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalumeau 2002
- Chalumeau M, Foix‐L'Helias L, Scheinmann P, Zuani P, Gendrel D, Ducou‐le‐Pointe. Rib fractures after chest physiotherapy for bronchiolitis or pneumonia in infants. Pediatric Radiology 2002;32(9):644‐7. [DOI] [PubMed] [Google Scholar]
Chanelière 2006
- Chanelière C, Moreux N, Pracros JP, Bellona G, Reixa P. Rib fractures after chest physiotherapy: a report of 2 cases [Fractures costales au cours des bronchiolites aiguës virales: à propos de 2 cas]. Archives de Pédiatrie 2006;13:1410–2. [DOI] [PubMed] [Google Scholar]
Consensus 2001
- Agence nationale d'accréditation d'évaluation en santé. Consensus conference on acute bronchiolitis in infants [Conférence de consensus sur la bronchiolite du norrisson]. Archives de Pédiatrie 2001;8(Suppl 1):11‐23. [Google Scholar]
David 2010
- David M, Luc‐Vanuxem C, Loundou A, Bosdure E, Auquier P, Dubus JC. Assessment of the French consensus conference for acute viral bronchiolitis on outpatient management: progress between 2003 and 2008. Archives of Pediatrics 2010;17(2):125‐31. [PUBMED: 19959347] [DOI] [PubMed] [Google Scholar]
Dick 1991
- Dick KJ. Investigation and evaluation of physiotherapy intervention in acute bronchiolitis of infancy. Masters thesis. Department of Physiotherapy, Queen Margaret College, Edinburgh 1991.
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farley 2014
- Farley R, Spurling GKP, Eriksson L, Mar CB. Antibiotics for bronchiolitis in children under two years of age. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD005189.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernandes 2013
- Fernandes RM, Bialy LM, Vandermeer B, Tjosvold L, Plint AC, Patel H, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD004878.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gadomski 2014
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001266.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Girardi 2001
- Girardi G, Astudillo P, Zuniga F. The IRA program in Chile: milestones and history [El programa IRA en Chile: hitos e historia]. Revista Chilena de Pediatria 2001;72(4):292‐300. [Google Scholar]
González 2001
- Gonzalez Caballero D, González Pérez‐Yarza E. Acute bronchiolitis: basics for rational guidelines [Bonquilitis aguda: bases para un protocolo racional]. Anales Españoles de Pediatria 2001;55:355‐64. [PubMed] [Google Scholar]
González 2010a
- González J, Ochoa C, Grupo Investigador del Proyecto aBREVIADo (BRonquiolitis‐Estudio de Variabilidad, Idoneidad y ADecuación). Study of variability in the management of acute bronchiolitis in Spain in relation to age of patients. National multicenter study (aBREVIADo project) [Estudio de variabilidad en el abordaje de la bronquiolitis aguda en España en relación con la edad de los pacientes]. Anales de Pediatria (Barcelona) 2010;72(1):4‐18. [DOI] [PubMed] [Google Scholar]
González 2010b
- González de Dios J, Ochoa Sangrador C. Consensus conference on acute bronchiolitis (IV): treatment of acute bronchiolitis. Review of scientific evidence [Conferencia de Consenso sobre bronquiolitis aguda (IV): tratamiento de la bronquiolitis aguda. Revisión de la evidencia científica]. Anales de Pediatria (Barcelona) 2010;72(4):285.e1‐285.e42. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Available from www.gradepro.org. McMaster University (developed by Evidence Prime, Inc.), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Hall 1981
- Hall CB, Douglas RG Jr. Modes of transmission of respiratory syncytial virus. Journal of Pediatrics 1981;99:100. [DOI] [PubMed] [Google Scholar]
Halna 2005
- Halna M, Leblond P, Aissi E, Dumonceaux A, Delepoulle F, Kohen R, et al. Impact of the consensus conference on outpatient treatment of infant bronchiolitis. Three‐year study in the Nord district of France. Presse Médicale 2005;34:277‐81. [DOI] [PubMed] [Google Scholar]
Harding 1998
- Harding J, Miles F, Becroft D, Allen B, Knight D. Chest physiotherapy may be associated with brain damage in extremely premature infants. Journal of Pediatrics 1998;132:440‐4. [DOI] [PubMed] [Google Scholar]
Hartling 2011
- Hartling L, Bialy LM, Vandermeer B, Tjosvold L, Johnson DW, Plint AC, et al. Epinephrine for bronchiolitis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003123.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holman 2003
- Holman RC, Shay DK, Curns AT, Lingahojr Anderson LJ. Risk factors for bronchiolitis associated deaths among infants in the US. Pediatric Infectious Diseases Journal 2003;22(6):483‐90. [DOI] [PubMed] [Google Scholar]
Knight 2001
- Knight DB, Bevan CJ, Harding JE, Teele RL, Kuschel CA, Battin MR, et al. Chest physiotherapy and porencephalic brain lesions in very preterm infants. Journal of Pediatrics and Child Health 2001;37(6):554‐8. [DOI] [PubMed] [Google Scholar]
Kruskal 1952
- Kruskal WH, Wallis WA. Use of ranks in one‐criterion variance analysis. Journal of the American Statistical Association 1952;47(260):583–621. [Google Scholar]
Liet 2010
- Liet JM, Ducruet T, Gupta V, Cambonie G. Heliox inhalation therapy for bronchiolitis in infants. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD006915.pub2] [DOI] [PubMed] [Google Scholar]
Mann 1947
- Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Annals of Mathematical Statistics 1947;18(1):50‐60. [DOI: 10.1214/aoms/1177730491] [DOI] [Google Scholar]
McConnochie 1993
- McConnochie KM. Bronchiolitis: what's in the name?. American Journal of Diseases of Children (1960) 1993;137:11‐3. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Panickar 2005
- Panickar JR, Dodd SR, Smyth RL, Couriel JM. Trends in deaths from respiratory illness in children in England and Wales from 1968 to 2000. Thorax 2005;60:1035‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ralston 2014
- Ralston S, Comick A, Nichols E, Parker D, Lanter P. Effectiveness of quality improvement in hospitalization for bronchiolitis: a systematic review. Pediatrics 2014;134(3):571‐81. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Régnier 2013
- Régnier SA, Huels J. Association between respiratory syncytial virus hospitalizations in infants and respiratory sequelae: systematic review and meta‐analysis. Pediatric Infectious Disease Journal 2013;32(8):820‐6. [DOI] [PubMed] [Google Scholar]
Sanchez 2007
- Sánchez Etxaniz J, Benito Fernández J, Mintegi Raso S. Bronquiolitis aguda: ¿por qué no se aplica lo que se publica? Barreras en la transmisión del conocimiento. Evidencias en Pediatria 2007;3:88. [Google Scholar]
Schechter 2007
- Schechter MS. Airway clearance applications in infants and children. Respiratory Care 2007;52(10):1382‐90; discussion 1390‐1. [PubMed] [Google Scholar]
SIGN 2006
- SIGN 91. Bronchiolitis in children. A national clinical guideline. http://www.sign.ac.uk (accessed 13 April 2011).
Sigurs 2010
- Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010;65:1045‐52. [DOI] [PubMed] [Google Scholar]
Silverman 1956
- Silverman W, Anderson D. A controlled clinical trial of effects of water mist on obstructive respiratory signs, death rate and necropsy findings among premature infants. Pediatrics 1956;17(1):1‐10. [PubMed] [Google Scholar]
Smyth 2006
- Smyth RL, Openshaw PJ. Bronchiolitis. Lancet 2006;368:312–22. [DOI] [PubMed] [Google Scholar]
Spencer 1996
- Spencer N, Logan S, Scholey S, Gentle S. Deprivation and bronchiolitis. Archives of Disease in Childhood 1996;74:50‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tal 1983
- Tal A, Bavilski C, Yohai D, Bearman JE, Gorodischer R, Moses SW. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71:13‐8. [PubMed] [Google Scholar]
Touzet 2007
- Touzet S, Réfabert L, Letrilliart L, Ortolan B, Colin C. Impact of consensus development conference guidelines on primary care of bronchiolitis: are national guidelines being followed?. Journal of Evaluation in Clinical Practice 2007;13(4):651‐6. [PUBMED: 176833310] [DOI] [PubMed] [Google Scholar]
Umoren 2011
- Umoren R, Odey F, Meremikwu MM. Steam inhalation or humidified oxygen for acute bronchiolitis in children up to three years of age. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006435.pub2] [DOI] [PubMed] [Google Scholar]
Verstraete 2014
- Verstraete M, Crosb P, Gouina M, Oillica H, Bihouea T, Denoualb H, et al. Update on the management of acute viral bronchiolitis: proposed guidelines of Grand Ouest University Hospitals [Prise en charge de la bronchiolite aiguë du nourrisson de moins de 1 an: actualisation et consensus médical au sein des hôpitaux universitaires du Grand Ouest (HUGO)]. Archives de Pédiatrie 2014;21:53‐62. [DOI] [PubMed] [Google Scholar]
Videla 1998
- Videla C, Carballal G, Misirlian A, Aguilar M. Acute lower respiratory infections due to respiratory syncytial virus and adenovirus among hospitalized children from Argentina. Clinical and Diagnostic Virology 1998;10:17‐23. [DOI] [PubMed] [Google Scholar]
Wainwright 2003
- Wainwright C, Altamirano L, Cheney M, Cheney J, Barber S, Price D, et al. A multicenter, randomized, double‐blind, controlled trial of nebulized epinephrine in infants with acute bronchiolitis. New England Journal of Medicine 2003;349:27‐35. [DOI] [PubMed] [Google Scholar]
Wainwright 2010
- Wainwright C. Acute viral bronchiolitis in children ‐ a very common condition with few therapeutic options. Paediatric Respiratory Reviews 2010;11(1):39‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1992
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oxymetry in infants hospitalized with lower respiratory infections. American Review of Respiratory Disease 1992;145(1):106‐9. [DOI] [PubMed] [Google Scholar]
Wood 1972
- Wood DW. A clinical score system for the diagnosis of respiratory failure. American Journal of Diseases of Children 1972;123:227‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2011
- Zhang L, Mendoza‐Sassi RA, Wainwright C, Klassen TP. Nebulised hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006458.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Perrotta 2004
- Perrotta C, Ortiz Z, Roque M, Gallo M. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004873] [DOI] [Google Scholar]
Perrotta 2005
- Perrotta C, Ortiz Z, Roque M. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004873.pub3] [DOI] [PubMed] [Google Scholar]
Perrotta 2007
- Perrotta C, Ortiz Z, Roque M. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004873.pub3] [DOI] [PubMed] [Google Scholar]
Roqué 2012
- Roqué i Figuls M, Giné‐Garriga M, Granados Rugeles C, Perrotta C. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004873.pub4] [DOI] [PubMed] [Google Scholar]


