Abstract
Background
Tracheostomy formation is one of the most commonly performed surgical procedures in critically ill intensive care participants requiring long‐term mechanical ventilation. Both surgical tracheostomies (STs) and percutaneous tracheostomies (PTs) are used in current surgical practice; but until now, the optimal method of performing tracheostomies in critically ill participants remains unclear.
Objectives
We evaluated the effectiveness and safety of percutaneous techniques compared to surgical techniques commonly used for elective tracheostomy in critically ill participants (adults and children) to assess whether there was a difference in complication rates between the procedures. We also assessed whether the effect varied between different groups of participants or settings (intensive care unit (ICU), operating room), different levels of operator experience, different percutaneous techniques, or whether the percutaneous techniques were carried out with or without bronchoscopic guidance.
Search methods
We searched the following electronic databases: CENTRAL, MEDLINE, EMBASE, and CINAHL to 28 May 2015. We also searched reference lists of articles, 'grey literature', and dissertations. We handsearched intensive care and anaesthesia journals, abstracts, and proceedings of scientific meetings. We attempted to identify unpublished or ongoing studies by contacting manufacturers and experts in the field, and searching in trial registers.
Selection criteria
We included randomized and quasi‐randomized controlled trials (quasi‐RCTs) comparing percutaneous techniques (experimental intervention) with surgical techniques (control intervention) used for elective tracheostomy in critically ill participants (adults and children).
Data collection and analysis
Three authors independently checked eligibility and extracted data on methodological quality, participant characteristics, intervention details, settings, and outcomes of interest using a standardized form. We then entered data into Review Manager 5, with a double‐entry procedure.
Main results
Of 785 identified citations, 20 trials from 1990 to 2011 enrolling 1652 participants fulfilled the inclusion criteria. We judged most of the trials to be at low or unclear risk of bias across the six domains, and we judged four studies to have elements of high risk of bias; we did not classify any studies at overall low risk of bias. The quality of evidence was low for five of the seven outcomes (very low N = 1, moderate N = 1) and there was heterogeneity among the studies. There was a variety of adult participants and the procedures were performed by a wide range of differently experienced operators in different situations.
There was no evidence of a difference in the rate of the primary outcomes: mortality directly related to the procedure (Peto odds ratio (POR) 0.52, 95% confidence interval (CI) 0.10 to 2.60, I² = 44%, P = 0.42, 4 studies, 257 participants, low quality evidence); and serious, life‐threatening adverse events ‐ intraoperatively: risk ratio (RR) 0.93, 95% CI 0.57 to 1.53, I² = 27%, P = 0.78, 12 studies, 1211 participants, low quality evidence,and direct postoperatively: RR 0.72, 95% CI 0.41 to 1.25, I² = 24%, P = 0.24, 10 studies, 984 participants, low quality evidence.
PTs significantly reduce the rate of the secondary outcome, wound infection/stomatitis by 76% (RR 0.24, 95% CI 0.15 to 0.37, I² = 0%, P < 0.00001, 12 studies, 936 participants, moderate quality evidence) and the rate of unfavourable scarring by 75% (RR 0.25, 95% CI 0.07 to 0.91, I² = 86%, P = 0.04, 6 studies, 789 participants, low quality evidence). There was no evidence of a difference in the rate of the secondary outcomes, major bleeding (RR 0.70, 95% CI 0.45 to 1.09, I² = 47%, P = 0.12, 10 studies, 984 participants, very low quality evidence) and tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change (RR 1.36, 95% CI 0.65 to 2.82, I² = 22%, P = 0.42, 6 studies, 538 participants, low quality evidence).
Authors' conclusions
When compared to STs, PTs significantly reduce the rate of wound infection/stomatitis (moderate quality evidence) and the rate of unfavourable scarring (low quality evidence due to imprecision and heterogeneity). In terms of mortality and the rate of serious adverse events, there was low quality evidence that non‐significant positive effects exist for PTs. In terms of the rate of major bleeding, there was very low quality evidence that non‐significant positive effects exist for PTs.
However, because several groups of participants were excluded from the included studies, the number of participants in the included studies was limited, long‐term outcomes were not evaluated, and data on participant‐relevant outcomes were either sparse or not available for each study, the results of this meta‐analysis are limited and cannot be applied to all critically ill adults.
Plain language summary
Comparison of different techniques for planned opening of the trachea
Review question
We compared different techniques used for planned opening of the trachea in adult participants hospitalized in an intensive care unit (ICU).
Background
The term 'tracheotomy' refers to the surgical opening of the trachea (windpipe) through the front of the neck. The resulting opening between the trachea and the outer air space (stoma, tracheostomy) allows the person to breathe when the usual route for breathing is somehow obstructed or impaired. Tracheostomy is also necessary for persons in an ICU who are being ventilated by a machine for a long time (i.e. weeks). It is one of the most commonly performed surgical procedures in intensive care medicine. Both surgical techniques (surgical opening of the trachea) and percutaneous techniques (opening of the trachea with plastic dilators) are widely used in current practice. Compared to surgical tracheostomies, percutaneous tracheostomies seem to have a number of potential advantages.
Study characteristics
The evidence is current to May 2015. We included 20 studies from 1990 to 2011, enrolling 1652 adult participants hospitalized in the ICU, who were scheduled for planned tracheotomy. None of the studies were funded.
Key results
The application of percutaneous techniques, does not reduce the rate of death, of serious, life‐threatening complications (e.g. injuries to the windpipe or the oesophagus), major bleeding or problems with the tracheostomy tube (blockage, accidental loss, difficult tube change). There was some evidence that using percutaneous techniques results in fewer cases of wound infections (‐ 76%) and unfavourable scarring (‐ 75%).
Quality of the evidence The quality of the evidence varied by outcome from moderate (wound infection) to low (death, serious complications, unfavourable scarring, problems with the tracheostomy tube) and to very low (major bleeding). Reasons for the limitations are: great differences among the studies, results not similar across the studies, and not enough data.
Conclusions
Based on the available data, we conclude that percutaneous tracheostomies offer benefits for some of the outcomes when compared with surgical tracheostomies. However, because several groups of participants were excluded from the included studies (i.e. people with unfavourable neck structure, bleeding disorders or emergency situations), the number of participants in the included studies was limited, long‐term outcomes were not evaluated, and data on participant‐relevant outcomes were either sparse or not available for each study, the results of this meta‐analysis are limited and cannot be applied to all critically ill adults.
Summary of findings
Summary of findings for the main comparison. Percutaneous techniques compared to surgical techniques for tracheostomy.
| Percutaneous techniques compared to surgical techniques for tracheostomy | ||||||
| Patient or population: patients with tracheostomy Settings: hospital Intervention: percutaneous techniques Comparison: surgical techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Surgical techniques | Percutaneous techniques | |||||
| Mortality directly related to the procedure (Total mortality) Follow‐up: up to 21 days | Study population | POR 0.52 (0.10 to 2.60) | 257 (4 studies) | ⊕⊕⊝⊝ low1 | ||
| 31 per 1000 | 16 per 1000 (3 to 81) | |||||
| Intraoperative serious, life‐threatening adverse events | Study population | RR 0.93 (0.57 to 1.53) | 1211 (12 studies) | ⊕⊕⊝⊝ low1 | ||
| 43 per 1000 | 40 per 1000 (25 to 66) | |||||
| Direct postoperative serious, life‐threatening adverse events Follow‐up: up to 24 hours | Study population | RR 0.72 (0.41 to 1.25) | 984 (10 studies) | ⊕⊕⊝⊝ low1 | ||
| 55 per 1000 | 40 per 1000 (23 to 69) | |||||
| Wound infection/stomatitis Follow‐up: up to 2 years2 | Study population | RR 0.24 (0.15 to 0.37) | 936 (12 studies) | ⊕⊕⊕⊝ moderate3 | 2 Length of follow‐up ranges from not stated up to two years. | |
| 178 per 1000 | 43 per 1000 (27 to 66) | |||||
| Unfavourable scarring Follow‐up: up to 20 months4 | Study population | RR 0.25 (0.07 to 0.91) | 789 (6 studies) | ⊕⊕⊝⊝ low5 | 4 Length of follow‐up ranges from not stated up to 20 months. | |
| 296 per 1000 | 74 per 1000 (21 to 270) | |||||
| Major bleeding Follow‐up: up to 24 hours | Study population | RR 0.70 (0.45 to 1.09) | 984 (10 studies) | ⊕⊝⊝⊝ very low6 | ||
| 80 per 1000 | 56 per 1000 (36 to 87) | |||||
| Tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change Follow‐up: up to 6 months | Study population | RR 1.36 (0.65 to 2.82) | 538 (6 studies) | ⊕⊕⊝⊝ low1 | ||
| 40 per 1000 | 55 per 1000 (26 to 114) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; POR: Peto odds ratio; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded two levels due to serious concerns about study limitations and imprecision.
3 Downgraded one level due to serious concerns about study limitations.
5 Downgraded two levels due to serious concerns about inconsistency and imprecision.
6 Downgraded three levels due to serious concerns about study limitations, inconsistency, imprecision and strongly suspected publication bias.
Background
Description of the condition
Tracheostomy formation is one of the most commonly performed surgical procedures in the critically ill intensive care patient who requires long‐term mechanical ventilation (Cools‐Lartigue 2013; Durbin 2010; Kollef 1999; Wood 1996). Translaryngeal intubation is the preferred artificial airway for initial use in the mechanically‐ventilated patient but known benefits from tracheostomy are less need for deeper sedation, shorter weaning time, and shorter intensive care unit (ICU) and hospital stay. With a few exceptions, there is no difference in mortality with a tracheostomy or continued prolonged translaryngeal intubation (Caruso 1997; Durbin 2010; Kane 1997; Wood 1996) (see Table 2). While prolonged respiratory failure is probably the most common reason for performing tracheostomy, other indications such as decreased level of consciousness, poor airway protective reflexes, and severe alterations in physiology associated with trauma and medical illness are also indications for tracheostomy. Airway accidents may be more frequent and more severe in patients with tracheostomy tubes, and safety should modulate the decision to move a patient from an ICU (Durbin 2010).
1. Benefits from tracheostomy.
| Benefit | Type and quality of literature support showing benefit |
| Improved patient comfort | Uncontrolled reports, clinical opinion |
| Less need for sedation | Several RCTs |
| Lower work of breathing | Theoretical analysis; one small study |
| Improved patient safety | Clinical belief but minimal data, some contradictory |
| Improved oral hygiene | Clinical observation |
| Better long‐term laryngeal function | Large uncontrolled reports |
| Faster weaning from mechanical ventilation | One RCT |
| Lower risk of ventilator‐associated pneumonia | Controversial; data support for both sides |
| Lower mortality | RCT supports, many do not, but a large RCT supports mortality not higher with tracheostomy |
| Shorter intensive care unit and hospital stay | Several meta‐analyses |
RCT = randomised controlled trial
Traditionally, tracheostomy has been performed by surgeons or otolaryngologists in the operating room using standard surgical principles. Open tracheostomy has a number of possible complications including the loss of the airway, injuries to nearby structures, bleeding, pneumothorax, tracheoinnominate fistula, infection, and tracheal stenosis (Chew 1972; Durbin 2010; Hazard 1988; Heffner 1986b; Heffner 1988; Mulder 1969; Paul 1989; Stauffer 1981). Previous reports have documented complication rates associated with tracheostomy that vary from 6% to 66% and mortality rates ranging from 0% to 5% (Chew 1972; Friedman 1996b; Heffner 1986b; Skaggs 1969; Stauffer 1981; Stock 1986). There have been many attempts to reduce the number of complications associated with tracheostomy. These attempts have involved the development of ever new kinds of surgical and percutaneous puncture techniques and materials, as well as through the performance of percutaneous dilatational tracheostomy (PDT) under bronchoscopic control (Oberwalder 2004). Accordingly, the use of fibreoptic bronchoscopy to facilitate PDT has become the standard of care in most institutions (Barba 1995; Kornblith 2011). Recently, some authors suggested using ultrasonography of the neck to identify the underlying anatomy with more precision than palpation (Alansari 2015). Durbin stated that "Tracheal rings are usually easily appreciated and an overlying large vessel or thyroid gland can be seen and avoided during the procedure" (Durbin 2010; Sustic 2007). Rudus concluded that because of the paucity of randomized controlled trials (RCTs) addressing the safety and efficacy of preprocedural or real‐time intraprocedural ultrasound guidance or both, during PDT compared with the current standard of care, no recommendation for its use can be made (Rudas 2012) However, Alsansari stated, that "the best available evidence highly recommends the use of ultrasound scanning prior to, during, and after PDT to improve the safety of the procedure" (Alansari 2015)
Description of the intervention
In 1955, Sheldon et al first attempted percutaneous tracheostomy (PT) (Sheldon 1955). Since that time, many different techniques of PT have been reported (Cools‐Lartigue 2013): Pertrach® (Toy 1969); PDT (Ciaglia 1985); Rapitrach® (Schachner 1989); the guide wire dilating forceps (GWDF) method (Griggs 1990); translaryngeal tracheostomy (Fantoni 1997); and PercuTwist® (rotational dilation technique) (Frova 2002).
The PDT method proposed in Ciaglia 1985 has gained widespread acceptance as an alternative method to conventional surgical tracheostomy (ST) for airway access in patients requiring prolonged mechanical ventilation (Silvester 2006). Ciaglia originally introduced hydrophilic‐coated plastic dilators of increasing sizes over a guide wire (multiple dilator technique) inset in the trachea until the chosen tracheostomy tube could be placed. In the year 2000, changes in this technique consisted of creating a single dilator of appropriate size with a long taper to create the stoma with a single pass (single dilator technique, frequently cited by its brand names, Ciaglia Blue Rhino® or Ultraperc®). Another modification of the PDT method was the use of a high‐pressure angiographic balloon to create the stoma (balloon dilation technique, Ciaglia Blue Dolphin®) (Byhahn 2000; Gromann 2009a; Gromann 2009b; Zgoda 2005).
The various other percutaneous methods have not gained in popularity. This is mainly because of their difficulty of use, the lack of investigations documenting their safety and efficacy, and their relatively high perioperative complication rates (Brambrink 2004; Cabrini 2014; Cools‐Lartigue 2013; Powell 1998). On the other hand, several clinical trials have compared the various methods of performing PTs without any method being shown to be conclusively superior (Ambesh 2002; Byhahn 2001; Cools‐Lartigue 2013; Kaiser 2006; Nates 2000; Westphal 1999). Cabrini et al performed a systematic review and meta‐analysis of 13 randomized studies comparing at least two PT techniques in critically ill adult patients, to investigate if one of the six techniques found is superior to the others with regard to major intraprocedural complications, the early need to convert to other PT or surgical techniques, and mild complications. The main result was that the GWDF technique, the multiple dilator technique and the single dilator technique are largely equivalent for safety and rate of success, with the multiple dilator technique and the single dilator technique superior to the GWDF for mild complications. The other three methods (balloon dilation technique, translaryngeal tracheostomy, rotational dilation technique) appeared less safe and effective (Cabrini 2012). Cools‐Lartigue performed a review of 15 randomized trials and found that the single dilator technique is still the benchmark, with the best safety, success, and complication profile (Cools‐Lartigue 2013).
The proportion of patients receiving either PT or ST and the predominant tracheostomy technique varies greatly from country to country, hospital to hospital, and in different practice settings (Añón 2004; Blot 2005; Cooper 1998; Fikkers 2003; Fischler 2000; Kluge 2008; Krishnan 2005), however, PDT is increasingly the technique of choice for critically ill patients in ICUs throughout the world (Blondonnet 2014; Cabrini 2014; Delaney 2006; Dennis 2013; Groves 2007; Putensen 2014). PT was initially believed to be contraindicated in children, emergency situations, when patients were markedly obese or had anatomic abnormalities such as thyromegaly or neck cancer, or had uncorrectable coagulopathy. With growing experience, the indications for PT have been expanded and the patient exceptions which mandate a surgical tracheostomy have decreased. PT has been applied in various subgroups (Deppe 2013; Guzman 1995; Heffner 1986a; McCague 2012; Pandian 2010; Rosseland 2011; Takahashi 2014; Toursarkissian 1994a; Toursarkissian1994b). Toursarkissian 1994a stated that PT is the preferred method of tracheostomy placement in patients who have difficult anatomy, although large goitre is still a contraindication. For all of these reasons many authors believe that PT is the procedure of choice for most patients in the ICU who need a tracheostomy (Dennis 2013; Friedman 1996a; Griggs 1991; Groves 2007; Lebiedz 2010; Putensen 2014). However, Cabrini 2012 stated, that no recommendation for specific subgroups (obese patients, trauma patients, cardiac surgical patients,etc.) can be made, because of the paucity of RCTs addressing these high risk subgroups.
How the intervention might work
Compared to STs, PTs seem to have a number of potential advantages. For example, it is relatively simple to learn and perform (Barba 1995; Lukas 2007), thus even individuals who lack extensive surgical training may quickly become adept at this procedure (Petros 1997; Pothman 1997). PTs may be associated with fewer peri‐ and postoperative complications (5.5% to 40%) (Cheng 2000; Gysin 1999; Hill 1996), including bleeding (Guyatt 2008), and infection rates ( 5% versus 30%) (Delaney 2006; Freeman 2000; Friedman 1996a; Higgins 2007; Stauffer 1981). As a reason for the reduced incidence of wound infection Delaney 2006 suggested minimization of local tissue damage with the dilatational technique, and was in agreement with Iwanaka 1997, who wrote ‘...the relative preservation of immune functions when minimally invasive techniques are used when compared to an open technique’. The reasons for less perioperative bleeding is the use of smaller incisions and blunt dissection instead of cutting and transecting vessels, and that the tracheostomy tube fits exactly in the stoma, allowing compression of the surrounding tissues. PTs may be performed at the patient's bedside with a limited number of personnel. This eliminates the potential risks associated with transporting a critically ill patient (such as accidental disconnection of the breathing circuit or extubation, reduced monitoring during transfer) (Delaney 2006; Dulgerov 1999; Silvester 2006) as well as the inconvenience and expense of scheduling and utilizing operating room (OR) facilities (Barba 1995; Bowen 2001; Dulgerov 1999, Melker 1992). It is also a more rapid procedure, which is beneficial to unstable, critically ill patients. The time taken from the decision to perform a tracheostomy to the procedure being performed is significantly shorter when tracheostomies are performed using the PT method. `This may have additional implications for critically ill patients including decreased duration of sedation, earlier weaning from mechanical ventilation, and shorter overall length of stay in the ICU` (Arabi 2004; Griffiths 2005; Rumbak 2004; Shirawi 2005). ICU utilization can be improved as the earlier tracheostomy insertion may allow more aggressive and potentially more rapid weaning, or may allow earlier transfer of a patient with a more secure airway (Friedman 1996b; Wu 2003). Because of these and other advantages, the popularity of this technique has grown dramatically. On the other hand, complications that are unusual with conventional surgical methods (ST), including paratracheal insertion (Bodenham 1992; Hazard 1988; Leinhardt 1992; Marelli 1990), pneumothorax, tracheal laceration, tracheoesophageal fistula, haemorrhage, and loss of the airway, have been reported in association with PT (Alexander 1997; Douglas 1999; Kaloud 1997; Leinhardt 1992; Malthaner 1998; Pothman 1997). Further, it is unknown if the frequency of major late complications of tracheostomy, such as tracheoinnominate artery fistula and symptomatic subglottic stenosis, differ substantially when these two techniques are compared.
Why it is important to do this review
Avoiding complications which are associated with elective tracheostomies may have beneficial effects in terms of reduced morbidity and mortality in critically ill patients. A variety of publications and six previous meta‐analyses have compared the effectiveness and safety of percutaneous techniques to surgical techniques for tracheostomy, in order to evaluate the superiority of one technique over the other (Cheng 2000; Delaney 2006; Dulgerov 1999; Freeman 2000; Higgins 2007; Putensen 2014). However, these reviews do not include some recent studies. In the meta‐analysis from Higgins, Ahn 1998, Lukas 2007 and Silvester 2006 are missing. In the review from Delaney, Lukas 2007 is missing. In addition, all of these meta‐analyses included only multiple dilator tracheostomy, GWDF and translaryngeal tracheostomy (TLT) in the PT group. Since newer PT techniques, such as single‐step dilation tracheostomy, rotational dilation tracheostomy, or balloon dilation tracheostomy are used for PT, previous meta‐analyses may not reflect current clinical practice; this was the reason for the Putensen 2014 meta‐analysis. But even Putensen 2014, did not consider Ahn 1998, Lukas 2007, Massick 2001, Raine 1999, Xu 2007, or Youssef 2011. Despite the multitude of work published on this topic, the debate about the possible advantages from PT techniques over conventional ST techniques, and whether one PT technique is superior to another PT technique, continues. Therefore, we systematically reviewed the literature to assess both efficacy and safety outcomes of the use of percutaneous techniques and surgical techniques for tracheostomy to see if either of the two makes the procedure safer, faster, freer of complications and more often successful.
Objectives
We evaluated the effectiveness and safety of percutaneous techniques compared to surgical techniques commonly used for elective tracheostomy in critically ill participants (adults and children) to assess whether there was a difference in complication rates between the procedures. We also assessed whether the effect varied between different groups of participants or settings (intensive care unit (ICU), operating room), different levels of operator experience, different percutaneous techniques, or whether the percutaneous techniques were carried out with or without bronchoscopic guidance.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomized controlled trials (RCTs) and quasi‐RCTs comparing PTs with STs irrespective of publication status, date of publication, and blinding status in all languages eligible for inclusion in the review. We defined a RCT as a study in which participants were allocated to treatment groups on the basis of a random method (e.g. using a computer‐generated number table). We defined a quasi‐RCT as a study in which participants were allocated to treatment groups on the basis of a quasi‐random method (e.g. using hospital number, date of birth). We excluded studies containing cointerventions and non‐randomized trials. For trials which had cross‐over designs, we only considered results from the first randomized treatment period.
Types of participants
We included intubated and mechanically‐ventilated critically ill participants (children and adults) who required an elective tracheostomy. We excluded studies of tracheostomy in emergency situations, in non‐critically ill or homecare participants. We made no restrictions with respect to specific population characteristics (such as age, gender, race, or the presence of a particular condition or risk factors), settings (ICU, operating room,and participants being awake, sedated or anaesthetized), or the practitioner's experience.
Types of interventions
We included all studies in which a percutaneous technique for tracheotomy (experimental intervention) was compared with a surgical technique for elective tracheotomy (control intervention). We included all studies, irrespective of whether the percutaneous tracheostomy (PT) procedure was performed under bronchoscopic control or not.
Types of outcome measures
The outcome measures did not constitute criteria for including studies.
Primary outcomes
-
Mortality directly related to the procedure
Intraoperative mortality (measured as the proportion of participants who died intraoperatively)
Postoperative mortality (measured as the proportion of participants who died during the first 24 hours after the intervention)
-
Serious, life‐threatening adverse events
Intraoperative serious, life‐threatening adverse events (major vascular injury or excessive bleeding (determined by need for blood transfusion or an additional surgical procedure), tracheal or oesophageal injury (detected by intraoperative bronchoscopy), loss of the airway (loss of the tube or tracheostoma tube > 20 sec) or a misplaced airway (paratracheal insertion of the tube or the tracheostoma tube), a severe hypoxic episode, or cardiac arrest)
Direct postoperative serious, life‐threatening adverse events (major vascular injury or excessive bleeding (determined by need for blood transfusion or an additional surgical procedure), a severe hypoxic episode, or saturation < 90%.
Secondary outcomes
-
Non‐life threatening events
Intraoperative non‐life threatening events: minimal or moderate bleeding (where bleeding could be stopped by conservative measures), subcutaneous emphysema (detected during the first 24 hours by chest x‐ray), cuff puncture, transient hypotension, pneumothorax or pneumomediastinum (both detected by postoperative chest x‐ray), cannula misplacement or difficult tube placement.
Direct postoperative non‐life threatening events: pneumonia, atelectasis (detected by postoperative chest x‐ray), difficult tube change, tracheostomy tube occlusion/obstruction, accidental decannulation.
Late non‐life threatening events: tracheal stenosis, tracheal malacia, delayed wound healing, cosmetic deformity, tracheocutaneous or oesophageal fistula.
Total number of peri‐ and postoperative complications/adverse events
Duration of the procedure
Wound infection/stomatitis
Unfavourable scarring
Major bleeding
Tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change
Patient or caregiver satisfaction
All outcomes defined as stated by the study authors. We differentiated between intraoperative, postoperative and long‐term complications. We included studies irrespective of whether all of this information was available.
Search methods for identification of studies
We employed the standard methods of the Cochrane Anaesthesia, Critical and Emergency Care Group (ACE).
Three review authors (PB, JL, AL) independently assessed the titles and abstracts (when available) of all reports identified by electronic searching, manual searching, snowballing and contacts with experts and industry.
We retrieved and evaluated potentially relevant studies, chosen by at least one author, in full‐text versions. We masked all selected studies by obscuring authors' names and institutions, location of study, reference lists, journal of publication and any other potential identifiers.
Electronic searches
Three review authors (PB, BK, KH) searched the following databases for relevant trials.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5, see Appendix 1).
OVID MEDLINE (1966 to 28 May 2015, see Appendix 2).
OVID EMBASE (1980 to 28 May 2015, see Appendix 3)
CINAHL via EBSCOhost (1982 to 28 May 2015, see Appendix 4).
The same review authors searched medical databases: Current Contents Medicine (CC MED) and Medikat, Health Care Literature Information Network (Heclinet); and publisher databases: Springer, Kluwer, Karger and Thieme; Somed; NHS Economic Evaluation (NHSEED and INAHTA); Global Health Database; registers of clinical trials (from the International Register of Clinical Trials; registers compiled by Current Science). For this research we used 'grips', one of the DIMDI (German Institute for Medical Documentation and Information) platforms. We developed a specific strategy for the database (please see Appendix 5 for the grips web search).
We did not limit the search by language or publication status.
We used Cochrane's optimally sensitive strategies to identify RCTs for MEDLINE and EMBASE searches (Dickersin 1994; Lefebvre 2001; Robinson 2002). We combined the MEDLINE search strategy with Cochrane's Highly Sensitive Search Strategy as contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We adapted our MEDLINE search strategy for searching the other databases.
We attempted to identify unpublished or ongoing studies by searching the following two trial registries (searched on 28 May 2015) for all years available in all possible fields using the basic search function (using separately the following keyword terms: "tracheotomy", "tracheostomy"):
Current Controlled Trials: www.controlled‐trials.com
ClinicalTrials.gov:www.clinicaltrials.gov
Searching other resources
We performed an additional handsearch focused on intensive care and anaesthesia journals (e.g. Anästhesiologie; Intensivmedizin; Notfallmedizin; Schmerztherapie Thieme Verlag; Der Anaesthesist Springer Verlag; Intensivmedizin und Notfallmedizin ‐ German Interdisciplinary Journal of Intensive Care Medicine Springer Verlag), abstracts and proceedings of scientific meetings (for example, proceedings of the Annual Congress of the European Society of Intensive Care Medicine (ESICM), the Annual Congress of the German Society of Anaesthesia (DAK) and the Annual Congress of the European Society of Anaesthesia (ESA)) (2003 to 2014; last searched 31 January 2014); references lists, 'grey literature' (System for Information on Grey Literature in Europe (SIGLE and ZETOC); the Index to Scientific and Technical Proceedings (from the Institute for Scientific Information) and dissertations. We attempted to identify additional, unpublished or ongoing studies by contacting the companies, Cook, Smith and Portex.
We also contacted experts in the field to identify missed, unpublished or ongoing studies, and studies presented in abstract form at major international meetings.
We (PB, AL, JL) handsearched the reference lists of all identified studies and reviews to locate additional studies.
We repeated this approach until no further studies could be identified.
Data collection and analysis
Selection of studies
Three review authors (AL, JL, PB) independently scanned the titles and abstracts of reports identified by electronic searching, manual searching, snowballing and contacts with experts and industry for relevance. We performed this process without blinding of authors, institution, journal of publication or results. We only excluded citations which were clearly irrelevant at this stage. We obtained full copies of all potentially relevant papers.
Three authors (PB, AL or JL) independently screened the full papers, identified relevant studies and assessed eligibility of studies for inclusion. We selected trials that met the inclusion criteria, using a checklist designed in advance for that purpose (see Appendix 6). We resolved disagreements on the eligibility of studies through discussion. Where resolution was not possible, we consulted a third review author (JL or AL).
We assessed all studies meeting the inclusion criteria for quality and extracted data from them. We excluded all irrelevant records and recorded details of the studies and the reasons for exclusion in the Characteristics of excluded studies table.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2015).
Data extraction and management
Two review authors (PB, JL) independently extracted the data from all reports using specially designed data extraction forms (see Appendix 6).
We resolved disagreements by discussion; where necessary we consulted a third review author (AL). Once we had resolved disagreements, we recorded the extracted data on the final data extraction form.
We contacted study authors for clarification or missing information. If further clarification was not available, we were unable to obtain the missing information, or we were unable to reach an agreement, we placed these studies under the heading 'Studies awaiting classification' so that there is an opportunity to use the data in the future.
One review author (JL) transcribed the data into Review Manager 5 (RevMan 5), and the other review author (PB) checked the data entered, for any discrepancies (double data entry).
In addition to details relating to the risk of bias of the included studies, we extracted two sets of data.
Study characteristics: place of publication; date of publication; population characteristics; setting; detailed nature of intervention; detailed nature of comparator; and detailed nature of outcomes. A key purpose of this data was to define unexpected clinical heterogeneity in included studies independently from the analysis of the results.
Results of included studies with respect to each of the main outcomes indicated in the review question. We carefully recorded reasons why an included study did not contribute data on a particular outcome and considered the possibility of selective reporting of results on particular outcomes.
We recorded for each trial the following data.
Authors
Year of publication
Study design
Population
Inclusion procedure: (‐) equals non‐consecutive/unknown, (+) equals consecutive
Setting: university/other/unknown
Patient characteristics (age, gender, height, weight, body mass index (BMI)) recorded as stated in the study
Number of participants/procedures
Acute Physiology And Chronic Health Evaluation (APACHE) II Score
Simplified Acute Physiology Score (SAPS)
Period of intubation up to tracheotomy (days)
Number and experience of the practitioner(s)
Procedure setting (location PT and ST performed)
Intervention: puncture methods: Ciaglia, Fantoni, Griggs (with or without bronchoscopic guidance), standardized or not standardized, surgical techniques
Study design: P: prospective R: randomized C: controlled Cr.‐ o.: cross‐over; information on the randomization method; exclusion of participants after randomization: +: yes, ‐: no; intention‐to‐treat evaluation plan: +: yes, ‐: no
Monitoring: pulse oximetry, bronchoscopy
General anaesthesia, local anaesthesia, epinephrine
Details of the outcome (all studies included irrespective of whether they contained complete information on the overall success rate, the total number of attempts needed until success, the number of punctures which were successful at the first, second, third etc. attempt, the overall complication rate or the number of individual complications, and the time required until success, or whether some of this information was lacking
Conclusion of the authors
Assessment of risk of bias in included studies
Two review authors (PB, JL), independently and in duplicate, assessed the methodological quality of each included study using a simple form and following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following six domains as having either a low, unclear, or high risk of bias.
Selection bias (random sequence generation, allocation concealment).
Performance bias (blinding of participants and personnel).
Detection bias (blinding of outcome assessment).
Attrition bias (incomplete outcome data).
Reporting bias (selective reporting).
Other bias not covered elsewhere.
We reviewed the assessments and discussed any inconsistencies in the interpretation of inclusion criteria and their significance to the selected studies. We resolved any disagreements through discussion with a third review author.
We did not automatically exclude any study as a result of a rating of 'unclear' risk of bias or 'high' risk of bias. We presented the evaluation of the Risk of bias in included studies in tabular form in the Results section of the review. We predicted that, given the nature of the intervention, blinding of the practitioner would not be possible. We noted measures of clinical performance. For instance, where given, we recorded the experience and number of practitioners performing the procedures in a trial.
Within each study we described what was reported for each domain and contacted the authors for additional information, where necessary.
Yes: criteria appropriately applied and described in the report or ascertained in communication with the primary author of the study. Unclear: criteria not described and impossible to acquire from or clarify with the author. No: criteria inappropriately applied.
We classified included studies into one of the following categories.
Low risk of bias: all criteria met.
High risk of bias: one or more criteria not applied or met.
Unclear risk of bias: one or more criteria unclear.
At each stage we compared results. We discussed the impact of methodological quality on the results. We resolved any disagreements by discussion.
We reviewed the assessments and discussed any inconsistencies between the review authors in the interpretation of inclusion criteria and their significance to the selected studies. We resolved any disagreements through discussion with a third review author.
Measures of treatment effect
We analysed extracted data using Review Manager 5 (RevMan 5).
Dichotomous data
For dichotomous data, we described results both as a relative measure (risk ratio (RR)) with 95% confidence intervals (CIs) and an absolute measure (i.e. the number needed to treat for an additional beneficial outcome (NNTB)). Relative measures can be used to combine studies, but absolute measures can be more informative than relative measures because they reflect the baseline risk as well as the change in risk with the intervention. For the test for an overall pooled effect we used the Z statistic, taking a P value of less than 0.05 to be significant.
Continuous data
For continuous data, we used the mean difference (MD) and standard deviations (SDs) to summarize the data for each group. This has the advantage of summarizing results in natural units that are easily understood. We performed a meta‐analysis where there were studies making similar comparisons and reporting the same outcome measures.
Unit of analysis issues
We include cross‐over studies in this review but we did not analyse the endpoint success rate after cross‐over. We only used data from the first randomized treatment period of the cross‐over studies.
The unit of analysis was the individual participant. However, multiple complications may occur in a single participant and manuscripts are often unspecific regarding the number of participants with at least one complication. Thus, in order to include as many studies as possible for meta‐analysis, data on frequent complications were summarized by rate ratios (assuming multiple 'independent' complications and equal observation time per participant) and data on rare complications were summarized by risk ratios (assuming a single complication per participant).
Dealing with missing data
No simple solution exists for the problem of missing data. We handled this problem by contacting the investigators, whenever possible, to clarify some methodological issues and to request additional data. In addition, the assumptions of whatever method was used to cope with missing data was made explicit. We tried to check for selective outcome reporting by comparing publications with their protocols or official trial registrations, when available. We included studies irrespective of whether all of the outcome information were available. However, to date, we have not received any additional data to that presented in the primary reports. If we subsequently receive additional information, we plan to incorporate these data in the next update of this review.
Assessment of heterogeneity
We assessed heterogeneity between trials by visual inspection of forest plots and we quantified statistical heterogeneity by calculating the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2003). We regarded heterogeneity as low if the I2 statistic was less than 25%, as moderate if the I2 statistic was between 25% and 50%, and substantial if the I2 statistic was greater than 50%. If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
The predetermined significance level for the test of heterogeneity was 0.10. We interpreted both the total effect size and the effect size relative to specific study characteristics cautiously if there was significant heterogeneity.
Assessment of reporting biases
We made a great effort to identify unpublished studies and minimize the impact of possible publication bias by using a comprehensive research strategy .
Publication bias occurs when published studies are not representative of all studies that have been done, usually because positive results tend to be submitted and published more often than negative results. Because detecting publication bias is difficult, we tried to minimize it by comprehensive literature searching, the use of study registries and contacting the manufacturer of tracheostomy devices (Glasziou 2001).
We assessed reporting bias also by trying to identify whether the study was included in a trial registry, whether a protocol is available, and whether the methods section provided a list of outcomes. We compared the list of outcomes from those sources to the outcomes reported in the published paper.
We used a graphical display (funnel plot) of the size of the treatment effect against the precision of the trial (1/standard error) to investigate publication bias by examining for signs of asymmetry. Publication bias is associated with asymmetry (Light 1984). In the absence of publication bias, a plot of study sample size (or study weight) versus outcome (that is, log relative risk) should have a bell or inverted funnel shape with the apex near the summary effect estimate (a funnel plot). If there is asymmetry, reasons other than publication bias will also be sought, for example, poor methodological quality of smaller studies, true heterogeneity, artefact or chance (Egger 1997).
As suggested by the Cochrane Handbook for Systematic Reviews of Interventions we did not use funnel plots to assess publication bias when we found less than 10 trials for an endpoint, since asymmetry is difficult to detect with a small number of studies. We used the tests for funnel plot asymmetry only when there were at least 10 studies included in the meta‐analysis, and results were interpreted cautiously, with visual inspection of the funnel plots (Higgins 2011).
Data synthesis
We reviewed the data from included studies qualitatively and then, if appropriate, combined the data quantitatively by population, intervention and outcome, using Cochrane's statistical software, Review Manager 5 (RevMan 5).
We performed a meta‐analysis, where there were studies of similar comparisons reporting the same outcome measures. We used models with random‐effects, i.e. the Mantel‐Haenszel method for dichotomous data (using risk ratio (RR) as the effect measure) and the inverse variance method for continuous data (using SMD as the effect measure), due to apparent between‐study heterogeneity as assessed by Q and I2 statistics. We calculated 95% confidence intervals and considered corresponding P values equal or less than 5% (two‐sided alpha) as statistically significant.
For rare events, i.e. death directly related to the procedure, we used Peto's method (assuming a fixed‐effect) to pool odds ratios. Multiple complications may occur in a single participant. However, manuscripts are often unspecific regarding the number of participants with at least one complication. Thus, in order to include as many studies as possible for meta‐analysis, we summarized data on frequent complications using rate ratios (assuming multiple 'independent' complications and equal observation time per participant) and summarized data on rare complications using risk ratios (assuming a single complication per participant). We combined rate ratios using the inverse variance method.
We assessed the overall quality of evidence for each outcome that included pooled data from RCTs using the GRADE approach (Atkins 2004). We downgraded the evidence from high quality by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. GRADEproGDT 2015 allowed us to import data from Review Manager 5 to create 'Summary of findings' tables (RevMan 5). These tables provide outcome‐specific information concerning the overall quality of evidence from studies included in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes we considered.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses to determine whether the results differed by:
technique, with PDT according to the Ciaglia technique or guide wire dilating forceps (GWDF) method according to the Griggs' technique, Pertrach® according to Toy's technique, Rapitrach® according to Schachner's technique, translaryngeal tracheostomy according to Fantoni's technique, PercuTwist® according to Frova's technique versus conventional surgical procedures commonly used for tracheostomy (Ciaglia versus Griggs was executed; the other comparisons were not executed because we did not find sufficient studies);
experience of the practitioner (experienced versus not experienced);
Location where the tracheostomy was performed (ICU versus operating room);
PT with or without bronchoscopy;
age (adults versus children; not executed because we did not find any studies); and
urgency (elective versus an emergency; not executed because we did not find any studies).
Sensitivity analysis
A priori, we planned sensitivity analyses to test how sensitive the results are to reasonable changes in the assumptions that are made during the review process and in the protocol for combining the data (Lau 1998).
We planned to performed sensitivity analysis regarding 'randomized versus quasi‐randomized' and eventually 'good quality studies versus poor quality studies'. We defined a good quality study as one which has all of the following domains: adequate allocation concealment, blinding of outcome assessment, and data analysis performed according to the intention‐to‐treat principle. A poor quality study for the purposes of the proposed sensitivity analysis was defined as one which lacks one or more of these key domains.
We did not perform a sensitivity analysis since almost all the included studies had a low or unclear risk of bias. For example, in no study was the outcome assessor blinded; in only 11 studies an adequate sequence generation or an adequate allocation concealment was reported, and the control groups were adequately described at entry in only six of the studies.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies
Results of the search
The May 2015 search strategy and our previous searching identified a total of 687 citations from searching electronic databases.
The January 2014 search in other sources retrieved a total of 98 citations: zero from an additional handsearch focused on intensive care and anaesthesia journals, abstracts and proceedings of scientific meetings, 58 from reference lists and a further 40 from the companies we contacted for references.
Altogether, we identified 785 citations, including 276 duplicates. After we screened the title and abstracts of the 509 unique citations, 477 of those citations could be excluded. We screened a total of 32 full‐texts, of which we excluded 12 reports. The reasons for exclusion are as follows: six were not randomized trials (Beck 2007; Bowen 2001; Goldenberg 2003; Karvandian 2009; Pauliny 2012; Sulaiman 2006); five compared different PT techniques or early versus late tracheotomy (Birbicer 2008; Cianchi 2010; Montcriol 2011; Remacle 2008Yurtseven 2007); and one study was published twice (Melloni 2002; Muttini 1999). We identified no ongoing studies and no studies are awaiting classification.
Altogether, we included 20 studies in the quantitative synthesis (Figure 1).
1.
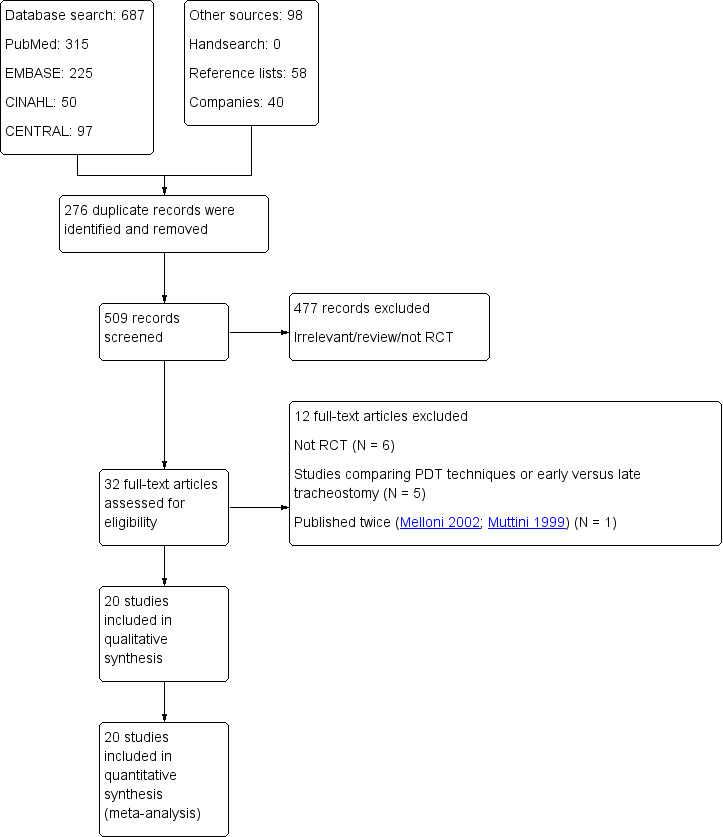
Study flow diagram.
Included studies
See:Characteristics of included studies
We included 20 studies from 1990 (Gysin 1990) to 2011 (Youssef 2011), with 1652 participants (percutaneous tracheostomy (PT) 854, surgical tracheostomy (ST) 798), described in the Characteristics of included studies tables. The individual studies involved sample sizes of 16 (Sustic 2002) to 205 participants (Lukas 2007). The studies took place in different hospital settings all over the world. Of the 20 studies, 13 were RCTs (Ahn 1998; Antonelli 2005; Freeman 2001; Gysin 1990; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Wu 2003; Xu 2007; Youssef 2011), four were quasi‐RCTs (Crofts 1995; Friedman 1996; Heikkinen 2000; Tabaee 2005) and in three studies it is unclear whether they are RCTs or controlled clinical trials (CCTs) (Hazard 1991; Melloni 2002; Sustic 2002).
One study was published twice (Melloni 2002; Muttini 1999).
Intention‐to‐treat (ITT) analyses were made in 19 studies and not made in one study (Wu 2003).
The inclusion and exclusion criteria were clearly defined in 19 studies (not clearly defined in Youssef 2011) and the treatment and the control groups were adequately described at entry only in six studies (Ahn 1998; Antonelli 2005; Friedman 1996; Hazard 1991; Massick 2001; Melloni 2002).
In 14 studies the Ciaglia technique with multiple dilatator was used (Ahn 1998; Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Holdgaard 1998; Massick 2001; Melloni 2002; Porter 1999; Silvester 2006; Tabaee 2005; Wu 2003; Xu 2007), in five studies the Griggs technique was used (Heikkinen 2000; Lukas 2007; Raine 1999; Sustic 2002; Youssef 2011), and in one the Fantoni technique was used (Antonelli 2005).
Participants were adults in all of the 20 studies and were from general intensive care units (ICUs), medical, surgical or neurosurgical ICUs.
Both procedures (percutaneous and surgical) were performed in the ICU in nine studies (Ahn 1998; Heikkinen 2000; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Tabaee 2005; Xu 2007; Youssef 2011), both in the operating room in one study (Holdgaard 1998), the PT was performed in the ICU and the ST was performed in the operating room in five studies (Crofts 1995; Freeman 2001; Friedman 1996; Sustic 2002; Wu 2003), both in the ICU or in the operating room in one study (Gysin 1990), in three studies the PT was performed in the ICU and the ST in the ICU or in the operating room (Antonelli 2005; Hazard 1991; Melloni 2002), and in one study no details were given (Lukas 2007).
Eight of the 18 studies, provided details on the number of operators who carried out the procedure (Antonelli 2005; Crofts 1995; Friedman 1996; Heikkinen 2000; Massick 2001; Melloni 2002; Tabaee 2005; Wu 2003).
In one study, details on the experience of the operators who carried out the procedure was not provided (Freeman 2001).
In three of the studies, the experience of the operators who carried out the procedure was different between the groups (Friedman 1996; Hazard 1991; Holdgaard 1998).
In five studies, the experience of the operators who carried out the procedure (trainees), the location (ICU) and the technique (Ciaglia technique with multiple dilatator) were the same in the two groups (Ahn 1998; Massick 2001; Porter 1999; Silvester 2006; Tabaee 2005).
In five studies (in which the experience of the operators who carried out the procedure were the same in each study), the location where the procedures were performed were different (Antonelli 2005; Crofts 1995; Gysin 1990; Melloni 2002; Sustic 2002).
In three of the studies, the experience of the operators who carried out the procedure and the location where the procedures were performed were different (Friedman 1996; Hazard 1991; Wu 2003).
In one study the experience of the operators who carried out the procedure was not stated and the location where the procedures were performed were different (Freeman 2001).
In two studies, the experience of the operators who carried out the procedure (staff), the location (ICU) and the technique (forceps, bronchoscopy, no details) were the same in the two groups (Heikkinen 2000; Raine 1999).
In one study, the experience of the operators who carried out the procedure and the technique (forceps, bronchoscopy, no details) were the same in the two groups but no details were stated about the location (Lukas 2007).
Excluded studies
We excluded 12 studies from the review for the following reasons. six studies were not randomized trials (Beck 2007; Bowen 2001; Goldenberg 2003; Karvandian 2009; Pauliny 2012 ; Sulaiman 2006), five studies compared different PT techniques or early versus late tracheostomy (Birbicer 2008; Cianchi 2010; Montcriol 2011; Remacle 2008; Yurtseven 2007), and one study was published twice (Melloni 2002; Muttini 1999). See the Characteristics of excluded studies tables.
Ongoing studies
There are no ongoing studies.
Studies awaiting classification
There are no studies awaiting classification.
Risk of bias in included studies
We used Cochrane's domain‐based evaluation table provided in RevMan 5 to assess the validity and quality of the included trials. We have detailed the methods of randomization, outcome assessment, and exclusion criteria in the Characteristics of included studies table. A summary of our assessment of methodological quality of included studies is presented in the 'Risk of bias' graph (Figure 2), and in the 'Risk of bias' summary (Figure 3). Most of the trials had low risk or unclear risk of bias across the six domains. We did not classify any trials overall at low risk of bias.
2.
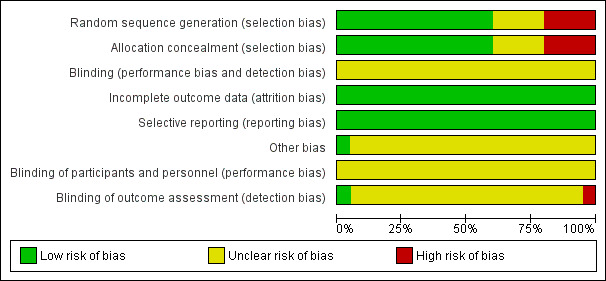
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
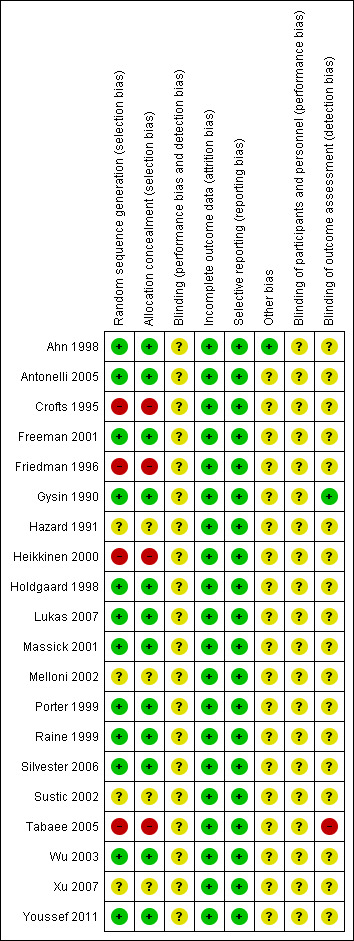
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In 12 trials, the allocation sequence generation and the allocation concealment were at low risk of bias (Ahn 1998; Antonelli 2005; Freeman 2001; Gysin 1990; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Wu 2003; Xu 2007), high risk of bias in four studies (random number tables (Friedman 1996), lots (Heikkinen 2000), odd/even number (Tabaee 2005), randomization per weeks (Crofts 1995)), and unclear in four of the studies (Hazard 1991; Melloni 2002; Sustic 2002; Xu 2007). We are aware that these studies are a potential risk of bias and have taken this into account when assessing their results.
Blinding
We felt that the inability to blind the practitioner performing the puncture, especially when the same person was performing all the punctures, was a potential source of performance bias. One further source of potential bias is that in only one of the included studies was the outcome assessor for the postoperative evaluation blinded (Gysin 1990). The outcome assessors for the follow‐up evaluation were blinded in four studies (Antonelli 2005; Gysin 1990; Raine 1999; Silvester 2006). For this reason, all the included trials should be considered as having at least a low risk of bias. We are aware that these studies are at potential risk of bias and have taken this into account when assessing their results.
Incomplete outcome data
In none of the studies were the data of the main outcomes reported completely. However, we think that the potential for attrition bias is nevertheless low in these studies.
Four studies evaluated the primary outcome: mortality (Freeman 2001; Friedman 1996; Massick 2001; Porter 1999); 16 did not (Ahn 1998; Antonelli 2005; Crofts 1995; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Melloni 2002; Raine 1999; Silvester 2006; Sustic 2002; Tabaee 2005; Wu 2003; Xu 2007; Youssef 2011). Fifteen studies evaluated the other primary outcome: intraoperative serious, life‐threatening adverse events, e.g. major vascular injury or excessive bleeding (determined by the need for blood transfusion or an additional surgical procedure), tracheal or oesophageal injury (detected by intraoperative bronchoscopy), loss of the airway (loss of the tube or tracheostoma tube > 20 sec), or a misplaced airway (paratracheal insertion of the tube or the tracheostoma tube), a severe hypoxic episode, or cardiac arrest (Ahn 1998; Antonelli 2005; Freeman 2001; Friedman 1996; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Tabaee 2005; Wu 2003; Xu 2007). None of the authors give an indication why the missing endpoints were not recorded.
A comparison of the outcomes mentioned in the publication with the endpoints planned in the study protocol was not possible in any of the studies because not a single protocol was published.
We did not find excessive drop‐outs in any of the studies.
Selective reporting
In no study can selective reporting (selective availability of data, selective reporting of outcomes, time points, subgroups or analyses) be excluded. This is because we were unable to find protocol or trial registration material for all of the studies to compare with the published material. However, in all studies with a methods section, all outcomes specified therein were reported in the results section.
Other potential sources of bias
Only the study by Ahn 1998 was free from other potential sources of bias.
Baseline imbalance
The inclusion and exclusion criteria were clearly defined in all 20 studies, and the treatment and the control groups were adequately described at entry only in six studies (Ahn 1998; Antonelli 2005; Friedman 1996; Hazard 1991; Massick 2001; Melloni 2002). The stated exclusion criteria were nearly similar in all included trials; we feel that the potential for exclusion bias is therefore low.
In all 20 studies, the participants included in the studies were selected. Unfavourable anatomy was identified as a restriction to the percutaneous technique in most studies, which reflects current practice, and the importance of determining anatomic landmarks for this procedure. In most of the studies, the lack of palpable midline structures (thyroid cartilage, cricoid cartilage, sternal notch) was a contraindication to perform a PT. Several further groups of participants (emergency tracheostomy, difficult anatomy, prior airway problems, coagulopathies and previous tracheostomy) were excluded from the included studies and therefore from this meta‐analysis, thus limiting the generalizability of the results of this meta‐analysis to all critically ill adult patients requiring tracheostomy.
The experience of the practitioners and their experience in both PT techniques and ST techniques, as well as the number of practitioners involved, varied across the trials. In eight of the studies, details on the number and/or the experience of the operators who carried out the procedure were either not provided (Freeman 2001), or incompletely provided (Antonelli 2005; Gysin 1990; Hazard 1991; Porter 1999; Raine 1999; Silvester 2006; Wu 2003).
Effects of interventions
See: Table 1
All the results of this systematic review need to be interpreted with caution considering the characteristics and the risk of bias profile of each included study (Characteristics of included studies, Table 1).
Primary outcomes
1. Mortality directly related to the procedure
This outcome was studied in 14 trials (Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Massick 2001; Melloni 2002; Porter 1999; Wu 2003; Silvester 2006; Tabaee 2005; Wu 2003; Xu 2007; Youssef 2011). However, mortality was only reported in four trials (257 participants) (Freeman 2001; Friedman 1996; Massick 2001; Porter 1999). There were four deaths in the ST group (Freeman 2001; Friedman 1996), and two in the PT group (Massick 2001; Porter 1999). The pooled result for mortality, using the fixed‐effect model, demonstrated that there was no evidence of a reduction in mortality with the use of a percutaneous technique (Peto odds ratio (POR) 0.52, 95% confidence interval (CI) 0.10 to 2.60, I² = 44%, P = 0.42) (Figure 4). The quality of evidence was low for this outcome (Table 1). We downgraded the quality of evidence from high to low because of serious risk of bias, serious imprecision, and because the total number of events is less than 300.
4.
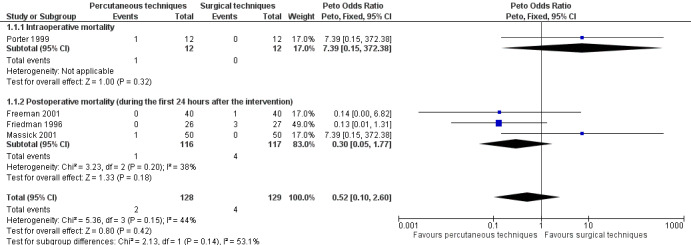
Forest plot of comparison: 1 Percutaneous technique versus surgical techniques for tracheostomy, outcome: 1.1 Mortality directly related to the procedure.
1.a. Intraoperative mortality (measured as the proportion of participants who died intraoperatively)
This outcome was studied in 11 trials (Freeman 2001; Gysin 1990; Hazard 1991; Massick 2001; Melloni 2002; Porter 1999; Silvester 2006; Tabaee 2005; Wu 2003; Xu 2007; Youssef 2011), but reported in only one trial (Porter 1999) (24 participants). There was one death in the PT group. The result for intraoperative mortality, using the fixed‐effect model, demonstrated that there was no evidence of a difference in this outcome (POR 7.39, 95% CI 0.15 to 372.38, P = 0.32) (Figure 4). The quality of evidence was very low for this outcome. We downgraded the quality of evidence from high to very low because of serious risk of bias, very serious imprecision, and because the total number of events is less than 300.
1.b. Postoperative mortality (measured as the proportion of participants who died during the first 24 hours after the intervention)
This outcome was measured in nine trials (Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Massick 2001; Porter 1999; Silvester 2006; Tabaee 2005; Wu 2003), but reported in only three trials (233 participants). There were four deaths in the ST group (Freeman 2001; Friedman 1996), and one in the PT group (Massick 2001). The result for postoperative mortality, using the fixed‐effect model demonstrated that there was no evidence of a difference in this outcome (POR 0.30, 95% CI 0.05 to 1.77, I² = 38%, P = 0.18) (Figure 4). The quality of evidence was low for this outcome. We downgraded the quality of evidence from high to low because of serious risk of bias, serious imprecision, and because the total number of events is less than 300.
2. Serious, life‐threatening adverse events
This outcome was studied in 19 of the 20 trials (Ahn 1998; Antonelli 2005; Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Sustic 2002; Tabaee 2005; Wu 2003; Xu 2007; Youssef 2011). No adverse events were reported in five of the 19 trials (Crofts 1995; Gysin 1990; Melloni 2002; Sustic 2002; Youssef 2011). Since some studies are listed in several (Xu 2007; Youssef 2011), or all three subgroups (Silvester 2006; Wu 2003), only the subtotal results, and not the total results are shown (Figure 5). We generated a funnel plot and found no publication bias for this endpoint (Figure 6).
5.
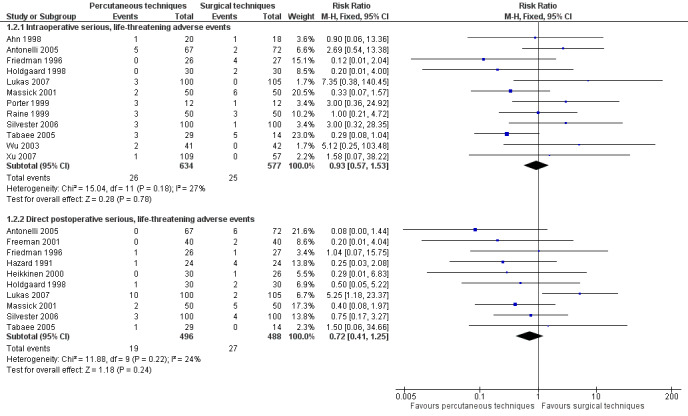
Forest plot of comparison: 1 Percutaneous technique versus surgical techniques for tracheostomy, outcome: 1.2 Serious, life‐threatening adverse events.
6.
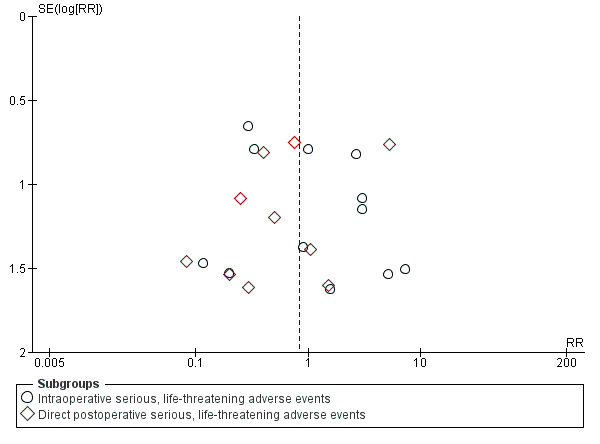
Funnel plot of comparison: 1 Percutaneous technique versus surgical techniques for tracheostomy, outcome: 1.2 Serious, life‐threatening adverse events.
2.a. Intraoperative serious, life‐threatening adverse events (major vascular injury or excessive bleeding (determined by the need for blood transfusion or an additional surgical procedure), tracheal or oesophageal injury (detected by intraoperative bronchoscopy), loss of the airway (loss of the tube or tracheostoma tube > 20 sec) or a misplaced airway (paratracheal insertion of the tube or the tracheostoma tube), a severe hypoxic episode, or cardiac arrest)
This outcome was measured in 17 trials (Ahn 1998; Antonelli 2005; Crofts 1995 ; Friedman 1996; Gysin 1990; Hazard 1991; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Sustic 2002; Tabaee 2005; Wu 2003; Xu 2007; Youssef 2011). No adverse events were reported in five of those 17 studies (Crofts 1995; Gysin 1990; Hazard 1991; Sustic 2002; Youssef 2011).
The result for intraoperative serious, life‐threatening adverse events, using the fixed‐effect model, demonstrated that there was no evidence of a difference in this outcome (risk ratio (RR) 0.93, 95% CI 0.57 to 1.53, I² = 27%, P = 0.78) (Figure 5) (12 studies, 1211 participants). The quality of evidence was low for this outcome (Table 1). We downgraded the quality of evidence from high to low because of serious risk of bias and serious imprecision.
2.b. Direct postoperative serious, life‐threatening adverse events (major vascular injury or excessive bleeding (determined by the need for blood transfusion or an additional surgical procedure), a severe hypoxic episode, or saturation < 90 %)
This outcome was measured in 13 trials (Antonelli 2005; Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Silvester 2006; Tabaee 2005). Three of the 13 trials reported no events (Crofts 1995; Gysin 1990; Porter 1999). So 10 studies (984 participants) were included in our analysis.
The result for the total number of direct postoperative serious, life‐threatening adverse events, demonstrated that for the use of PTs there was no evidence of a difference in this outcome (RR 0.72, 95% CI 0.41 to 1.25, I² = 24%, P = 0.24) (Figure 5). The quality of evidence was low for this outcome (Table 1). We downgraded the quality of evidence from high to low because of serious risk of bias and serious imprecision.
Secondary outcomes
1. Non‐life threatening events
This outcome was reported in all 20 trials (Ahn 1998; Antonelli 2005; Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Melloni 2002; Porter 1999; Raine 1999; Silvester 2006; Sustic 2002; Tabaee 2005; Wu 2003; Youssef 2011; Xu 2007). In Tabaee 2005, the authors divided the estimated blood loss into groups: 0 ml to 10 ml or 10 ml to 20 ml. For our analysis we took into account only the estimated blood loss of 10 ml to 20 ml. In Holdgaard 1998 there were 24 minimal or moderate bleeding events during the procedure in the ST group, and nine minimal or moderate bleeding events during the first 24 hours in the ST group. So 33 events occurred in 30 participants. We have chosen the conservative random‐effects model for all subgroups, because the heterogeneity is great in subgroup 1.3.3 (I² = 65%). We generated a funnel plot and found no publication bias for this endpoint.
1.a. Intraoperative non‐life threatening events (minimal or moderate bleeding (where bleeding could be stopped by conservative measures), subcutaneous emphysema (detected during the first 24 hours by chest x‐ray), cuff puncture, transient hypotension, pneumothorax or pneumomediastinum (both detected by postoperative chest x‐ray), cannula misplacement or difficult tube placement
This outcome was reported in 19 studies. In Tabaee 2005, the authors divided the estimated blood loss into groups. For our analysis we took into account only the estimated blood loss of 10 ml to 20 ml.
The result demonstrated that using the random‐effects model there was no evidence of a difference for the total number of intraoperative non‐life threatening events when using PTs (rate ratio 1.02, 95% CI 0.79 to 1.32, I² = 0%, P = 0.86) (Analysis 1.3).
1.3. Analysis.
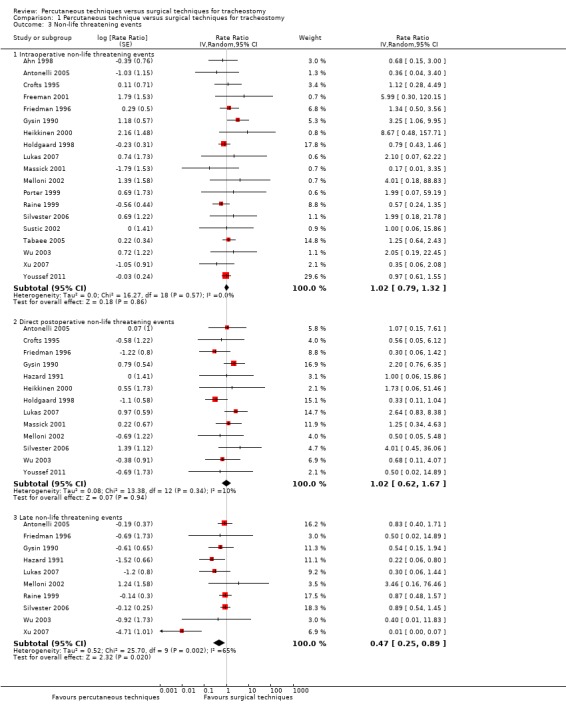
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 3 Non‐life threatening events.
1.b. Direct postoperative non‐life threatening events (pneumonia, atelectasis (detected by postoperative chest x‐ray), difficult tube change, tracheostomy tube occlusion/obstruction, accidental decannulation)
This outcome, was measured in 15 trials (Antonelli 2005; Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Melloni 2002; Porter 1999; Silvester 2006; Wu 2003; Youssef 2011). In two of the 15 trials no event was seen (Freeman 2001; Porter 1999). So 13 studies were included in our analysis.
The result demonstrated that using the random‐effects model there was no evidence of a difference for the total number of direct postoperative non‐life threatening events when using PTs (rate ratio 1.02, 95% CI 0.62 to 1.67, I² = 10%, P = 0.94) (Analysis 1.3).
1.c. Late non‐life threatening events (tracheal stenosis, tracheal malacia, delayed wound healing, cosmetic deformity, tracheocutaneous or oesophageal fistula)
This outcome was reported in 10 trials (Antonelli 2005; Friedman 1996; Gysin 1990; Hazard 1991; Lukas 2007; Melloni 2002; Raine 1999; Silvester 2006; Wu 2003; Xu 2007).
The result demonstrated that using the random‐effects model because of substantial (P = 0.002) heterogeneity (I² = 65%), PTs significantly reduced the total number of late non‐life threatening events (rate ratio 0.47, 95% CI 0.25 to 0.89, I² = 65%, P = 0.02) (Analysis 1.3).
2. Total number of peri‐ and postoperative complications/adverse events
The result demonstrated that using the random‐effects model because of substantial (P < 0.00001) heterogeneity (I² = 69%), PTs significantly reduced the total number of peri‐ and postoperative complications (20 studies, 1652 participants), by 29% (rate ratio 0.71, 95% CI 0.53 to 0.94, I² = 69%, P = 0.002) (Analysis 1.4). The quality of evidence was very low for this outcome. We downgraded the quality of evidence from high to low because of serious risk of bias, and unexplained substantial heterogeneity.
1.4. Analysis.
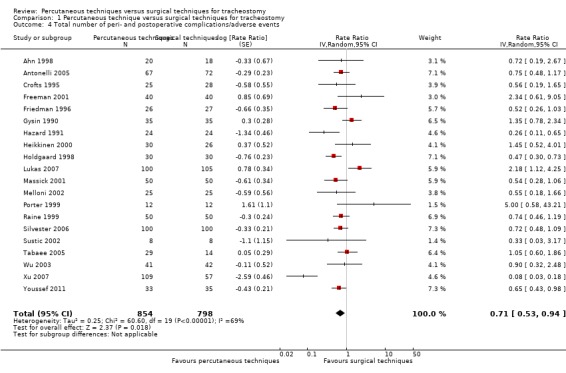
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 4 Total number of peri‐ and postoperative complications/adverse events.
3. Duration of the procedure
This outcome was reported in 17 trials (Ahn 1998; Antonelli 2005; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Melloni 2002; Porter 1999; Raine 1999; Silvester 2006; Sustic 2002; Tabaee 2005; Wu 2003). Due to the high heterogeneity (I² = 98%), we did not attempt a meta‐analysis and, thus, do not show totals for this outcome (Analysis 1.5).
1.5. Analysis.
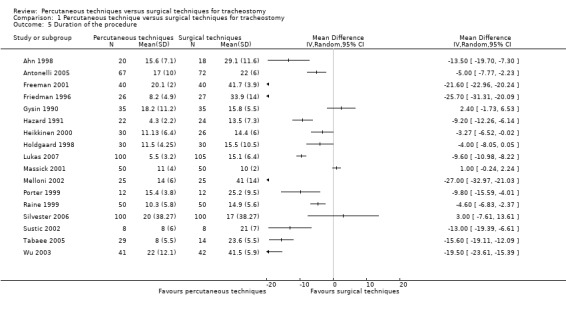
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 5 Duration of the procedure.
4. Wound infection/stomatitis
The number of wound infections and/or stomatitis (local inflammation, cellulitis or pus, necrosis or wound breakdown with or without antibiotic therapy) was measured in 15 studies (Antonelli 2005; Crofts 1995; Friedman 1996; Gysin 1990; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Massick 2001; Melloni 2002; Porter 1999; Silvester 2006; Sustic 2002; Wu 2003; Xu 2007; Youssef 2011). Antonelli 2005 measured infections and inflammation; we included only the infections. We do not include Xu 2007 because they looked only for inflammations. No event was reported by two of the 15 studies (Heikkinen 2000; Porter 1999), so 12 studies (936 participants) were included in our analysis. The pooled result demonstrated that using the fixed‐effect model, PTs significantly reduced the total number of wound infections and/or stomatitis (RR 0.24, 95% CI 0.15 to 0.37, I² = 0%, P = < 0.00001) (Analysis 1.6). The quality of evidence was moderate for this outcome (Table 1). We downgraded the quality of evidence from high to moderate because of serious risk of bias.
1.6. Analysis.
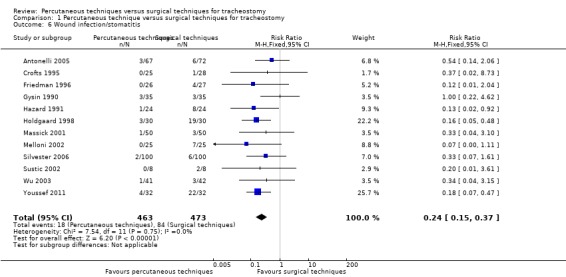
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 6 Wound infection/stomatitis.
5. Unfavourable scarring
The number of unfavourable scarring events was reported in six trials (Gysin 1990; Hazard 1991; Lukas 2007; Raine 1999; Silvester 2006; Xu 2007) (789 participants). The pooled result demonstrated that using the random‐effects model because of substantial (P < 0.00001) heterogeneity (I² = 86%), PTs significantly reduced the number of unfavourable scarring cases by 75% (RR 0.25, 95% CI 0.07 to 0.91, I² = 86%, P = 0.04) (Analysis 1.7). The quality of evidence was low for this outcome (Table 1). We downgraded the quality of evidence from high to low because of serious imprecision, and unexplained substantial heterogeneity.
1.7. Analysis.
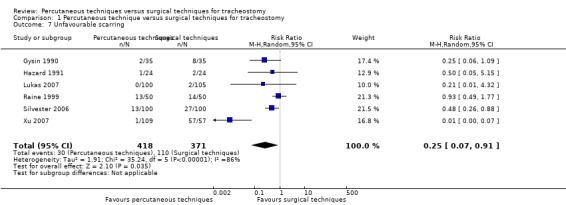
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 7 Unfavourable scarring.
6. Major bleeding
If one considers only the total number of major bleeding cases that was reported in 10 trials (Antonelli 2005; Freeman 2001; Friedman 1996; Hazard 1991; Heikkinen 2000; Holdgaard 1998; Lukas 2007; Massick 2001; Silvester 2006; Tabaee 2005) (984 participants), one can see, that using the fixed‐effect model there was no evidence of a difference in this outcome (RR 0.70, 95% CI 0.45 to 1.09, I² = 47%, P = 0.12) (Analysis 1.8). The quality of evidence was very low for this outcome (Table 1). We downgraded the quality of evidence from high to very low because of serious risk of bias, serious imprecision, unexplained moderate heterogeneity and strongly suspected publication bias.
1.8. Analysis.
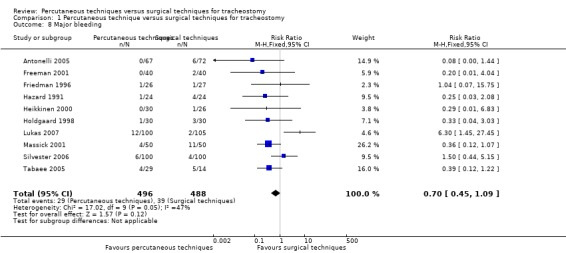
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 8 Major bleeding.
7. Tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change
The total number of tracheostomy tube occlusion/obstruction, accidental decannulation and difficult tube change was measured in nine trials (Friedman 1996; Gysin 1990; Holdgaard 1998; Lukas 2007; Massick 2001; Melloni 2002; Porter 1999; Raine 1999; Tabaee 2005). Three of the nine studies reported no events (Porter 1999; Raine 1999; Tabaee 2005), so, six studies (538 participants) were included in our analysis.
The pooled result for the total number of tracheostomy tube occlusion/obstruction, accidental decannulation and difficult tube changes in those six studies, demonstrated that using the fixed‐effect model there was no evidence of a difference in this outcome (RR 1.36, 95% CI 0.65 to 2.82, I² = 22%, P = 0.42) (Analysis 1.9). The quality of evidence was low for this outcome (Table 1). We downgraded the quality of evidence from high to low because of serious risk of bias and serious imprecision.
1.9. Analysis.
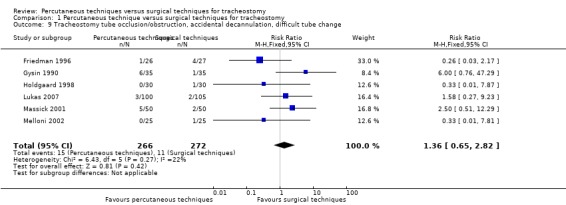
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 9 Tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change.
8. Patient or caregiver satisfaction
None of the studies assessed discomfort during the procedure. Only one study (Antonelli 2005) assessed patient satisfaction after a few months.
More than half of the interviewed survivors of both groups rated their physical health as moderately or severely compromised, and emotional health ratings were even lower. The physical and emotional subscores on the Short Form 12 Health Survey (SF‐12; Ware 1995) from the 14 participants whose tracheostomies were still open at the one year follow‐up (seven participants per group) were significantly lower than those of the 17 participants (11 in the translaryngeal tracheostomy (TLT) group) whose tracheostomies were closed. There were no significant differences between the TLT and the ST group. The study authors further noticed, that the number of participants examined for this outcome was too low to allow any hypotheses regarding causative factors as to why the tracheostomy technique used had no significant effect.
Subgroup analysis
We planned to perform a subgroup analysis to determine whether the results differed by age (adults versus children), urgency (elective versus emergency), PT technique (Ciaglia, Griggs, Fantoni), experience of practitioner (experienced versus not experienced), location where tracheostomy was performed (ICU versus operating theatre) or use of a bronchoscope. We did not perform subgroup analyses to determine whether the results differed by age or urgency because no studies were found for these comparisons. Due to the differences between the studies we were able to perform only the following subgroup analyses (see Characteristics of included studies).
Technique (Ciaglia versus Griggs)
We conducted a subgroup analysis to determine whether the results for the total number of peri‐ and postoperative complications/adverse events differed by the technique used for PT. In other words, we compared PTs versus STs according to the technique (Ciaglia or Griggs) used.
The Ciaglia technique was used in 14 trials (Ahn 1998; Crofts 1995; Freeman 2001; Friedman 1996; Gysin 1990; Hazard 1991; Holdgaard 1998; Massick 2001; Melloni 2002; Porter 1999; Silvester 2006; Tabaee 2005; Wu 2003; Xu 2007). When we compared PDT (using the Ciaglia technique) to ST, we found (using the random‐effects model because of substantial heterogeneity; I² = 72%), that Ciaglia PDTs significantly reduced the total number of peri‐ and postoperative complications by 38% (rate ratio 0.62, 95% CI 0.42 to 0.92, P = 0.02) (Analysis 2.1).
2.1. Analysis.
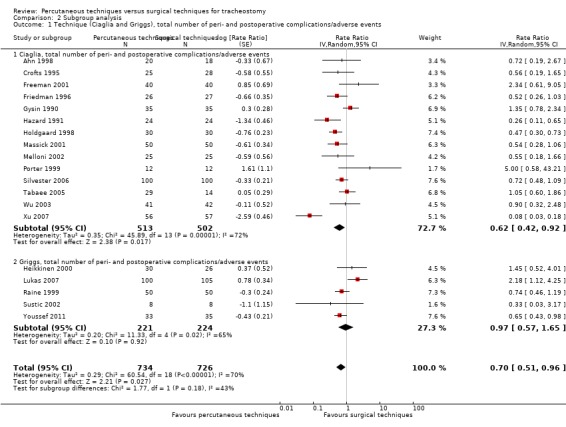
Comparison 2 Subgroup analysis, Outcome 1 Technique (Ciaglia and Griggs), total number of peri‐ and postoperative complications/adverse events.
The Griggs technique was used without bronchoscopy in five trials (445 participants) (Heikkinen 2000; Lukas 2007; Raine 1999; Sustic 2002; Youssef 2011). When we compared PT (using theGriggs technique) to ST, we found (using the random‐effects model because of heterogeneity; I² = 65%), that there was no evidence of a difference in this outcome (RR 0.97, 95% CI 0.57 to 1.65, P = 0.92) (Analysis 2.1).
For the other endpoints, results in both subgroups did not differ from the results of the whole analysis.
Our analysis shows that the Ciaglia technique is superior to the Griggs technique with respect to the total number of peri‐ and postoperative complications. However, this conclusion rests on the similarity of the studies included, especially regarding the performance of STs.
Experience of the practitioner (experienced versus not experienced)
We conducted a subgroup analysis to determine whether the results for the total number of peri‐ and postoperative complications differed according to the experience of the practitioner (experienced versus inexperienced). In other words, we compared PDTs (performed using the Ciaglia technique with multiple dilatator) versus STs according to experience of the practitioner (trainees (Ahn 1998; Crofts 1995; Friedman 1996; Hazard 1991; Massick 2001; Porter 1999; Silvester 2006; Xu 2007) or ICU staff (Melloni 2002; Wu 2003)).
When we compared PDTs carried out by trainees against STs, we found for the overall number of complications (using the random‐effects model because of substantial heterogeneity; I² = 74%), that PDTs performed with the Ciaglia technique with multiple dilatator by trainees in the ICU significantly reduced the total number of peri‐ and postoperative complications by 56% (rate ratio 0.46, 95% CI 0.26 to 0.83, P = 0.009) (Analysis 2.2).
2.2. Analysis.
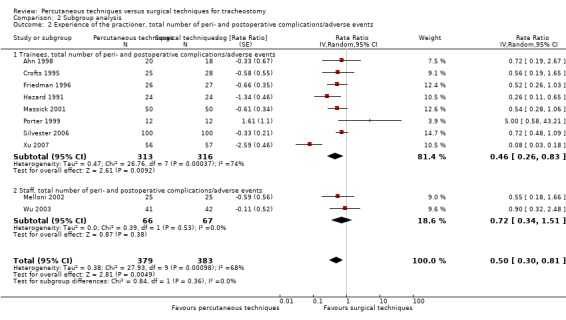
Comparison 2 Subgroup analysis, Outcome 2 Experience of the practioner, total number of peri‐ and postoperative complications/adverse events.
When we compared PDTs carried out by ICU staff against STs, we found for the overall number of complications (using the random‐effects model), that there was no evidence of a difference in this outcome (rate ratio 0.72, 95% CI 0.34 to 1.51, I² = 0%, P = 0.38) (Analysis 2.2).
Our analysis shows that PDT is more beneficial for trainees in terms of the total number of peri‐ and postoperative complications than for ICU staff. However, this conclusion rests on the similarity of the studies included, especially regarding the performance of STs.
We had planned to compare studies where both techniques are only performed by trained staff (Antonelli 2005; Gysin 1990; Lukas 2007; Melloni 2002; Raine 1999; Sustic 2002). This comparison was not possible because of the great level of heterogeneity between the studies (Antonelli 2005 used the Fantoni technique; Gysin 1990 used the Ciaglia/Björk technique; Lukas 2007 used the Griggs Forceps technique; Melloni 2002 used the Ciaglia/Fenster technique; Raine 1999 used the Griggs Forceps technique; and Sustic 2002 used the Griggs Forceps technique).
Location where the tracheostomy was performed (ICU versus operating theatre)
We conducted a subgroup analysis to determine whether the results for the overall number of complications differed according to the location where the tracheostomy was performed (ICU versus operating theatre). In other words, we compared PDTs (performed with the Ciaglia technique with multiple dilatator) against STs according to the location (ICU or operating theatre, both by staff).
When we compared PDTs performed in the ICU by staff against STs (Melloni 2002; Wu 2003), we found for the overall number of complications (using the fixed‐effect model because of no heterogeneity; I² = 0%), that there was no evidence of a difference in this outcome (rate ratio 0.72, 95% CI 0.34 to 1.51, P = 0.38) (Analysis 2.3).
2.3. Analysis.
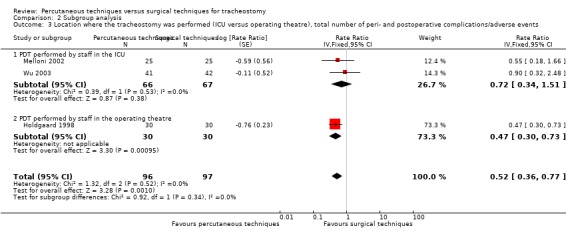
Comparison 2 Subgroup analysis, Outcome 3 Location where the tracheostomy was performed (ICU versus operating theatre), total number of peri‐ and postoperative complications/adverse events.
When we compared PDTs performed in the operating theatre by staff against STs (Holdgaard 1998), we found for the overall number of complications that PTs significantly reduced the total number of peri‐ and postoperative complications by 53% (rate ratio 0.47, 95% CI 0.30 to 0.73, P = 0.0010) (Analysis 2.3).
Our analysis shows that the reduction of the total number of peri‐ and postoperative complications was greater when the PDTs were carried out in the operating theatre. However, this conclusion rests on the similarity of the studies included, especially regarding the performance of STs.
Note that no study reported on PDTs performed in the operating theatre by trainees.
PDTs with or without bronchoscopy, massive and moderate bleeding
In eight studies the authors used bronchoscopic guidance for the PDT (Ahn 1998 (events two/procedures 20); Freeman 2001 (0/40); Gysin 1990 (0/35); Massick 2001 (2/50); Melloni 2002 (2/25); Porter 1999 (0/12); Silvester 2006 (4/100); Tabaee 2005 (28/29)). In one study bronchoscopic guidance was sometimes used (Wu 2003 (1/41)); in eight studies they did not use bronchoscopic guidance (Antonelli 2005 1/67; Crofts 1995 0/25; Friedman 1996 3/26; Hazard 1991 0/22; Heikkinen 2000 5/31; Holdgaard 1998 6/30; Raine 1999 5/50; Sustic 2002 1/8); and in one study, it is not stated (Lukas 2007 (2/100). With the use of bronchoscopic guidance for the PDT, 39 bleedings were seen in 352 procedures (11.1%); and without the use of bronchoscopic guidance for the PDT, 23 bleedings were seen in 359 procedures (6.4%).
Discussion
Summary of main results
The evidence comparing open/surgical tracheostomy (ST) versus percutaneous tracheostomy (PT) in adults with experienced or inexperienced operators is derived from the 20 included studies (16 randomised controlled trials (RCTs) and four quasi‐RCTs) from 1990 to 2011, enrolling 1652 participants. These studies have a variety of hospital settings, participants, interventions and outcome measures.
Most of the trials had low risk or unclear risk of bias across the six domains. We could not classify any trials at overall low risk of bias. None of the outcomes were of high quality evidence. We gave a GRADE rating of moderate quality for one outcome (wound infection/stomatitis), of low quality for five outcomes (mortality, postoperative mortality, intraoperative and direct postoperative serious life‐threatening adverse events, unfavourable scarring, and tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change) and of very low for two of the outcomes (intraoperative mortality, major bleeding). There was significant heterogeneity among the studies.
None of the studies addressed the impact of PTs on patient‐relevant outcomes (pain, discomfort, discomfort during the procedure, caregiver satisfaction). Only one study assessed patient satisfaction after a few months (Antonelli 2005). Only four of the studies addressed the impact of PTs on mortality (Freeman 2001; Friedman 1996; Massick 2001; Porter 1999), and none on the length of stay in the intensive care unit (ICU) or in hospital.
Our analyses of the available data suggested that the percutaneous technique improves some, but not all aspects concerned with the effectiveness and safety of tracheostomy.
Based on the available evidence, using the percutaneous technique, compared to usual practice (open/surgical technique) for elective tracheostomy, significantly reduces the rate of late non‐life threatening events (tracheal stenosis, tracheal malacia, delayed wound healing, cosmetic deformity, tracheocutaneous or oesophageal fistula) by 53% (rate ratio 0.47, 95% confidence interval (CI) 0.25 to 0.89, I² = 65%, P = 0.02, 10 studies, 643 participants), the total number of peri‐ and postoperative complications/adverse events by 29% (rate ratio 0.71, 95% CI 0.53 to 0.94, I² = 69%, P = 0.02, 20 studies, 1652 participants, low quality evidence), the rate of wound infection/stomatitis by 76% (risk ratio (RR) 0.24, 95% CI 0.15 to 0.37, I² = 0%, P < 0.00001, 12 studies, 936 participants, moderate quality evidence), and the rate of unfavourable scarring by 75% (RR 0.25, 95% CI 0.07 to 0.91, I² = 86%, P = 0.04, 6 studies, 789 participants, low quality evidence) in a variety of adult patients in different settings, performed by a wide range of variously experienced operators in different situations. This may be due to minimization of local tissue damage with a dilatational technique, the ease of performance, and of performing the procedure at the bedside in the ICU.
Non‐significant positive effects were seen with respect to postoperative mortality (‐70%, P = 0.18) and total mortality (‐48%) (Peto odds ratio (POR) 0.52, 95% CI 0.10 to 2.60, I² = 44%, P = 0.42, 4 studies, 257 participants, low quality evidence), the rate of serious life‐threatening adverse events ‐ intraoperative (‐7%) (RR 0.93, 95% CI 0.57 to 1.53, I² = 27%, P = 0.78, 12 studies, 1211 participants, low quality evidence), postoperative (‐28%) (RR 0.72, 95% CI 0.41 to 1.25, I² = 24%, P = 0.24, 10 studies, 984 participants, low quality evidence), and the rate of major bleeding (‐30%) (RR 0.70, 95% CI 0.45 to 1.09, I² = 47%, P = 0.12, 10 studies, 984 participants, very low quality evidence). In addition, the review shows a trend towards an (not significantly) increase of the rate of intraoperative mortality (+639%, P = 0.32), the rate of intraoperative (+2%, P = 0.86) and direct postoperative (+2%, P = 0.94) non‐life threatening events (intraoperatively: rate ratio 1.02, 95% CI 0.79 to 1.32, I² = 0%, P = 0.86, 19 studies, 1600 participants; direct postoperatively: rate ratio 1.02, 95% CI 0.62 to 1.67, I² = 10%, P = 0.94, 13 studies, 1133 participants), and the rate of tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change (+36%) (RR 1.36, 95% CI 0.65 to 2.82, I² = 22%, P = 0.42, 6 studies, 538 participants, low quality evidence).
Our subgroup analysis shows that the Ciaglia technique (‐38%, P = 0.02) is superior to the Griggs technique (‐3%, P = 0.92) with respect to the total number of peri‐ and postoperative complications, that PDTs are more beneficial for trainees (‐54%, P = 0.009) in terms of the total number of peri‐ and postoperative complications than for staff (‐28%, P = 0.38), and that the greatest reduction of the total number of peri‐ and postoperative complications is shown when the PDTs performed by staff were carried out in the operating theatre (‐53%, P = 0.0010) versus (‐28%, P = 0.38).
Also, data on patient‐relevant outcomes such as mortality or patient discomfort are either sparse (patient satisfaction) or not available for any study (caregiver satisfaction) to adequately evaluate the efficacy of using PT techniques.
However, because several groups of participants were excluded from the included studies (unfavourable anatomy, participants requiring emergency tracheostomy, evidence or suspicion of difficult anatomy, prior airway problems, coagulopathies and previous tracheostomy) or because outcomes were not evaluated (long‐term outcomes), the generalizability of the few results of this meta‐analysis to all critically ill adult populations is limited.
Overall completeness and applicability of evidence
Because of our comprehensive search strategy, the additional handsearch, and contact with different companies and experts in the field, we are confident that we have identified all randomized trials comparing surgical and percutaneous techniques for tracheostomy in adults with experienced or inexperienced operators.
The 20 included studies (16 RCTs and four quasi‐RCTs) recruited participants with a variety of underlying diseases, in a variety of settings, and a variety of operators (different disciplines and experience), which should increase the applicability of the results.
Quality of the evidence
We gave a GRADE rating of moderate quality for one outcome (wound infection/stomatitis), a rating of low quality for six outcomes (mortality, postoperative mortality, intraoperative and direct postoperative serious life‐threatening adverse events, unfavourable scarring, and tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change) and very low for two of the outcomes (intraoperative mortality, major bleeding). Most of the trials had a low or unclear risk of bias across the six domains, and there was significant heterogeneity among the studies. The main limiting factor (which was the reason for downgrading the quality of evidence in eleven outcomes), was the serious imprecision of the results. Other reasons for downgrading the quality of evidence in the outcomes were the strongly suspected publication bias in three outcomes and unexplained heterogeneity in three outcomes.
We originally planned to undertake an exploratory subgroup analysis to find out if contextual factors (type of operators, settings) or intervention factors (type of percutaneous tracheostomy (PT) or ST method) were the cause of the heterogeneity. However, due to the wide variety of procedures, operators and circumstances under which the procedure took place, we could not justify performing all of the planned analyses.
It is not easy to isolate the reasons for heterogeneity because a tracheostomy is a complex process. It is plausible that the discordance in results among studies may be due to contextual factors (differences in patient populations and practice) or intervention factors. In relation to intervention factors, there were many methodological differences among studies that may have contributed to heterogeneity. In relation to risk of bias within studies, methodological quality ranged from high to low. The intervention could not be blinded to personnel, which is understandable. It is plausible therefore, that the unblinded nature of the intervention may have prompted a change in behaviour and this may have affected results.
In 12 trials, the allocation sequence generation and the allocation concealment were at low risk of bias (Ahn 1998; Antonelli 2005; Freeman 2001; Gysin 1990; Holdgaard 1998; Lukas 2007; Massick 2001; Porter 1999; Raine 1999; Silvester 2006; Wu 2003; Xu 2007), high risk of bias in four studies (random number tables (Friedman 1996), lots (Heikkinen 2000), odd/even number (Tabaee 2005), randomization per weeks (Crofts 1995)), and unclear in four of the studies (Hazard 1991; Melloni 2002; Sustic 2002; Xu 2007). We are aware that these studies are a potential risk of bias and have taken this into account when assessing their results.
There were further potential sources of bias. In only one of the studies was the outcome assessor for the direct postoperative outcome blinded (Gysin 1990); in two of the studies the follow‐up examiners were blinded (Antonelli 2005; Raine 1999), and in all the remaining studies it remains unclear. There was also considerable clinical heterogeneity in terms of the surgical approaches used (different techniques, Jackson, Björk, Grillo), and the PT devices (Ciaglia/Cook, Griggs/Portex, Ciaglia/Portex) used. Furthermore, different studies used different methods (bedside, operating theatre; with or without bronchoscopic guidance), experienced and inexperienced (not blinded) operators who carried out the procedure, and time periods for the procedure. In none of the trials was it stated whether an attempt was made (apart from the general anaesthesia used during the intervention) to blind the patients to the technique being used for tracheostomy. Because subjective endpoints (for example, patient satisfaction) were examined in none of the studies, no source of detection bias exists in all.
The performance of tracheostomy is clearly dependent on the expertise of the operator for the surgical and the percutaneous technique used. Crucially, advances in medicine do not come simply from the availability of new technology, but depend on how the technology is actually applied (Guimares 2009). The experience of practitioners, and their faculties, in both groups, as well as the number of practitioners involved, varied across the included trials. In ten of the 20 studies, details of the number (Ahn 1998; Freeman 2001; Gysin 1990; Hazard 1991; Holdgaard 1998; Lukas 2007; Porter 1999; Raine 1999; Silvester 2006; Sustic 2002), and/or the experience of the operators who carried out the procedure (Freeman 2001), were either not provided or were incompletely provided.
Furthermore, whatever the experience of the operator, there are certain 'tacit' factors to do with performing practical procedures which are not (and indeed cannot be) recorded in the report of a clinical trial, but nevertheless influence the effectiveness and safety of the procedure (Pope 2003; Smith 2006). Some of these will be non‐technical skills and, although less obvious, they are an essential part of expert performance (Smith 2009; Smith 2010; Smith 2011).
We performed a subgroup analysis to determine whether the results for the overall number of complications differed by the experience of the practitioner (experienced versus inexperienced). We therefore compared PDTs performed with the Ciaglia technique with multiple dilatator in the ICU by trainees (Ahn 1998; Crofts 1995; Friedman 1996; Hazard 1991; Massick 2001; Porter 1999; Silvester 2006; Xu 2007) with those performed by staff (Melloni 2002; Wu 2003). The result for the overall number of complications in this first subgroup (Analysis 2.2), demonstrated that PDTs performed with the Ciaglia technique with multiple dilatator by trainees in the ICU significantly reduced the total number of peri‐ and postoperative complications by 54% (rate ratio 0.46, 95% CI 0.26 to 0.83, I² = 74%, P = 0.009) (Analysis 2.2). The result for the overall number of complications in this second subgroup, demonstrated that there was no evidence of a difference in this outcome (rate ratio 0.72, 95% CI 0.34 to 1.51, I² = 0%, P = 0.38) (Analysis 2.2). Our comparison shows that PDTs are more beneficial for trainees in terms of the total number of peri‐ and postoperative complications than for staff.
The most recent study included by us in this review is from 2011 (Youssef 2011). The 20 included studies cover a period of 21 years, during which time frame there has been a considerable change in the surgical procedures, the devices used for PT, and the routine use of PT in clinical practice.
Potential biases in the review process
Our systematic approach to searching, study selection and data extraction should have minimized the likelihood of missing relevant studies. We applied a comprehensive search strategy to identify all potential studies and their reports. However, although we included 20 studies in this review, information on several relevant outcome data pre‐specified in our protocol were not always (mortality, patient satisfaction after a few month) or never (patient discomfort during the procedure, caregiver satisfaction) reported. Several of these outcome measures are important in order to allow the operator to make an informed and balanced decision of which technique should be used in which situation. Some of these outcome measures were most likely not ascertained during the trial, however, others could well have been collected, but not reported. Unfortunately, we have not been able to obtain any additional data. We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), e.g. extracting data independently in duplicate to minimize errors and reduce bias in the process of doing this systematic review.
Agreements and disagreements with other studies or reviews
Five meta analyses compared the effectiveness of percutaneous tracheostomy (PT) with open/surgical tracheostomy (ST) (Cheng 2000; Delaney 2006; Freeman 2000; Higgins 2007; Putensen 2014).
In the meta‐analysis of prospective trials comparing PT and ST in critically ill participants, Freeman 2000, pooled data from five studies with 236 participants (Crofts 1995; Friedman 1996; Hazard 1991; Holdgaard 1998; Porter 1999). They concluded that only a limited number of small studies prospectively evaluated PT and ST. Their meta‐analysis suggests the potential advantages of PT relative to ST, including ease of performance, lower incidence of peristomal bleeding and postoperative infection, as well as postoperative complications in general. They speculate that the differences in rates of bleeding and infection can be explained by differences in the tracheostomy stoma following these two techniques. Specifically, following percutaneous placement, the stoma fits snugly around the tracheostomy tube. This lack of dead space conceivably serves to both tamponade bleeding vessels and to impede infection. In contrast, following ST, the stoma fits loosely around the tracheostomy tube. Thus, no tamponading effect or barrier to infection occurs. This tamponading effect may similarly explain why PT was associated with less operative bleeding. Because PT is a bedside procedure it avoids both the delays inherent in procedures performed in the operating room (PT can be carried out 10 minutes more quickly) and the potential risks and inconvenience associated with patient transport. Freeman 2000, also saw the need for more adequately powered prospective trials to support these findings.
In their meta‐analysis, Cheng 2000, included four RCTs that compared the complications of ST and PT (Crofts 1995; Friedman 1996; Hazard 1991; Holdgaard 1998). Although their meta‐analysis was published in September 2000, the Porter 1999 study from February 1999, is not included. Cheng 2000, found that ST had a five‐fold higher complication rate than PT, and that these complications were often more severe. They conclude that PT is a safer procedure for elective tracheostomy in patients with a normal sized neck.
The systematic review with meta‐analysis on percutaneous dilatational tracheostomy (PDT) versus ST in critically ill participants by Delaney 2006 has 17 included studies, and until now, was the most extensive work on this topic. The Ahn 1998 trial was included in Delaney et al following translation by Jong Joon. The Lukas 2007 study was not included in Delaney 2006; neither was the meta‐analysis from Higgins 2007.
A comparison between the data used by Delaney 2006 and those we used, show the following differences.
In the table "Characteristic of randomized trials ...", Delaney et al stated that in the Gysin 1999 study, STs and PTs were performed by staff. In the original publication, however, it was recorded that, "All tracheostomies were performed or supervised by one of the first two authors (CG, PD), both of whom have extensive experience with this procedure."
Further, they stated that in the Heikkinen 2000 study, ST and PT were performed by staff. In the original publication, however, it was recorded that "All tracheostomies were performed by one of four surgeons."
In the "Summary of long‐term complications", Delaney 2006 reported that 11 from 56 patients were available for long‐term follow‐up in each group, but altogether 11 from 57 patients were available.
Delaney 2006 wrote that in the Holdgaard 1998 study, ST was performed by trainees and PT was performed by staff. However, in the original publication it was stated "the (ST) procedure was carried out by an experienced specialist in surgery or by senior registrars in their final training." And, in the original publication, it was stated that "The PT procedures were performed by one of the authors."
In the original publication from Holdgaard 1998, eight authors are named. Table 3 in this publication, however, indicated the number of operators for PDT to be four. Whether the eight authors are experienced in terms of PDT remains open.
Delaney 2006, stated that in the Silvester 2006 study, ST and PDT were performed by trainees. In the original publication, however, it is stated that "Only intensivists or supervised senior trainees who had completed at least ten PTs performed the PTs, ... . The STs were carried out in the ICU by one of two thoracic surgeons (SK, SS) or their supervised senior trainees who had completed at least ten STs."
Furthermore, Delaney 2006 stated that the incidence of significant bleeding in the Silvester 2006 study was10/100 in the PDT group and 6/100 in the ST group. In the original publication (see table 4 from Silvester 2006), the authors stated that there was moderate and severe operative bleeding in four (PDT) patients and one (ST) patient, and moderate and severe postoperative bleeding in seven (PDT) and five (ST) patients.
Unlike the details in the Delaney 2006 meta‐analysis, we found sufficient randomization in the Gysin 1999 and Wu 2003 studies.
Delaney 2006, concluded that their systematic review and meta‐analysis demonstrated that the technique of PT has a number of important advantages over ST in critically ill patients who require an elective tracheostomy. Theyconcluded that PT was associated with a reduction in the incidence of clinically important wound infections compared with traditional ST;this is consistent with the results of our investigation. Secondly, Delaney 2006 found that there was no evidence overall that PT resulted in an increased incidence of clinically significant bleeding (consistent with our results), major periprocedural complications (overall complications decreased by 15% (RR 0.85, 95% CI 0.65 to 1.12, P = 0.25), intraoperative serious, life‐threatening adverse events increased by 31% (RR 1.31, 95% CI 0.62 to 2.79, P = 0.48), direct postoperative serious, life‐threatening adverse events decreased by 13% (RR 0.87, 95% CI 0.49 to 1.54, P = 0.63)) or long‐term complications (consistent with our results (RR 0.73, 95% CI 0.55 to 0.97, P = 0.03)).
As a reason for the reduced incidence of wound infection, Delaney 2006 suggested minimization of local tissue damage with a dilatational technique, or in agreement with Iwanaka 1997, who wrote "...the relative preservation of immune functions when minimally invasive techniques are used when compared to an open technique. Delaney 2006 stated further, that one considerable advantage of PT is the relative safety and convenience of performing the procedure at the bedside in the ICU, which obviates the need to transport a critically ill patient, and a shorter waiting period prior to performing the tracheotomy (these assumptions correspond to our estimates, but cannot be confirmed with the study results). The transport of critically ill patients is often logistically difficult and exposes the patient to increased likelihood for adverse events and risk to safety (Beckmann 2004; Lovell 2001; Warren 2004; Waydhas 1999). The results of the Delaney 2006 analysis supports the assertion that the elective transport of critically ill patients to the operating theatre for STs may pose undue and increased risk of complications and death.
In their meta‐analysis, Higgins 2007 include 15 RCTs, in the English language. This meta‐analysis included nearly 1000 patients comparing the complication rates, the cost‐effectiveness, and the procedure length of STs and PTs. Although the meta‐analysis was published in March 2007, it did not include the following three studies (Ahn 1998; Lukas 2007; Silvester 2006).
In reviewing the numbers underlying the calculations of the authors, we found discrepancies with the numbers mentioned in the original publications.
Figure 1: Comparison for decannulation/obstruction: in Higgins 2007: PDT 10/35, ST 2/35; the original Gysin 1990 study had no data for decannulation; obstruction in Gysin 1999:PDT 1/35, ST 0/35; in Higgins 2007: PDT 1/40, ST 0/40; the original publication (Freeman 2001) had no data; in Higgins 2007: PDT 2/41, ST 0/42; in the original publication (Wu 2003), premature extubation of translaryngeal tube: PDT 2, ST 0. So this evaluation was based on three incorrect data records.
Figure 2: Comparison for wound infection/stomatitis: in Higgins 2007: PT 0/30, ST 0/26; in the original publication, Heikkinen 2000: PT 0/31, ST 0/26; in Higgins 2007: PDT 1/26, ST 4/27; in the original publication, Friedman 1996: PDT 0/26, ST 4/27; in Higgins 2007: PDT 3/30, ST 8/30; in the original publication, Holdgaard 1998, minor infection: PDT 3, ST 11; major infection: PDT 0, ST 8. So this evaluation was also based on three incorrect data records.
Figure 3. Comparison for unfavourable scarring: in Higgins 2007: PT 1/67, ST 3/72; in the original publication, Antonelli 2005, no data were found; in Higgins 2007: PT 0/30, ST 1/26; in the original publication, Heikkinen 2000, eleven of 57 patients were alive 18 months postoperatively and answered a questionnaire; in Higgins 2007: PT 13/50, ST 18/50; in the original publication, Raine 1999: PT 13/50, ST 14/50. So this evaluation was also based on three incorrect data records.
Higgins 2007 found a decreased incidence of unfavourable scarring, wound infection/stomatitis, and decreased case length favouring PT. For complications relating to decannulation and airway obstruction, ST was favoured. There was no statistical difference between groups for false passage rates, minor or major haemorrhage, death, long‐term complications and rates of subglottic stenosis.
Higgins 2007 concluded that their meta‐analysis has shown that PTs trend toward fewer overall complications than open techniques (STs) (consistent with the results of our investigation) and appear to be more cost‐effective (not examined in our investigation) by releasing operating room resources, including time and personnel, provide greater feasibility in terms of bedside capability, and allow non‐surgeons to safely perform the procedure. Higgins 2007 concluded further that PT appears to be a safe alternative to traditional open ST, but that there is no body of literature favouring one over the other in terms of perioperative complication rates.
The conclusion that complications of tracheostomy, such as tube occlusion/obstruction, accidental decannulation and difficult tube change, were significantly more likely to occur in PTs and strongly favoured the open surgical technique, failed to confirm with our results. Higgins 2007 speculated that this may relate to the fact that the surgical techniques produce a well defined insertion tract that allows the insertion of a tracheotomy tube, with an inner and outer cannula, that facilitates nursing and allows an easier cannula changing. However, the percutaneous method was significantly better for wound infection/stomatitis and scarring. Higgins 2007 demanded that future directions should include a comparison between open bedside and percutaneous bedside tracheotomy, with detailed cost‐effectiveness analysis. Although Higgins 2007 evaluated 443 patients less, and there are differences between the characteristics and the results used in their meta‐analysis and our meta‐analysis, the conclusions derived from the results are very similar.
The meta‐analysis presented by Putensen 2014, was performed according to Cochrane guidelines and includes 14 RCTs with 973 patients. In our review, we included 20 trials from 1990 to 2011, enrolling 1646 patients; Putensen 2014 does not include the following studies: Ahn 1998; Lukas 2007; Massick 2001; Raine 1999; Xu 2007; Youssef 2011. They observed a reduced risk of major postprocedural bleeding with PT (odds ratio (OR) 0.39, 95% CI 0.15 to 0.97, P = 0.04, I2 = 0%). Like other meta‐analyses, we did not distinguish between intraoperative and postoperative bleeding, and also did not observe a reduction in bleeding following PT. They did not include four studies in their meta‐analysis: Hazard 1991 (PDT 1/24; ST 4/24); Massick 2001 (PDT 2/50; ST 5/50); Lukas 2007 (PT 10/100; ST 2/105); Tabaee 2005 (PDT 1/29; ST 0/14). They looked for the rate of stoma infection and stated that in the postprocedural period, PT techniques reduced odds for this outcome (OR 0.22, 95% CI 0.11 to 0.41, P < 0.00001, I2 = 0%). We have additionally taken into account the studies from Massick 2001 and Youssef 2011, but the result is still the same. In addition, Putensen 2014 observed that the pooled summary data indicated a faster procedure with PT than ST, but that this has to be viewed with caution because of the significant heterogeneity among the studies (I2 = 97%). Because of this high heterogeneity, we decided to dispense with a summary of the data. We did not look for the following outcomes: minor bleeding, tracheal stenosis; major intraprocedural bleeding, technical difficulties. A further comparison of results is therefore not possible. Putensen 2014 came to the conclusion that on the basis of available evidence from RCTs in critically ill adult patients, PT techniques can be performed faster and reduce stoma inflammation and infection, but are associated with increased technical difficulties when compared with ST. These results correspond (as far as we have been considering this outcome) to our own.
Authors' conclusions
Implications for practice.
There are several important implications for practice arising from our systematic review and meta‐analysis.
Our systematic review clearly shows that there are some benefits in terms of effectiveness and safety of the use of percutaneous techniques for tracheostomy. These regard rate of late non‐life threatening complications, total number of peri‐ and postoperative complications/adverse events, rate of wound infection/stomatitis and rate of unfavourable scarring in a variety of different adult patients in different settings, performed by a wide range of differently experienced operators in different situations.
Non‐significant positive effects based on low quality evidence were seen with respect to postoperative mortality and total mortality, rate of serious postoperative life‐threatening adverse events and the rate of major bleeding. In addition, the review shows a trend towards a (not significant) increase in the rate of intraoperative mortality, rate of intraoperative and direct postoperative non‐life threatening events, and rate of tracheostomy tube occlusion/obstruction, accidental decannulation or difficult tube change. Our subgroup analysis shows that the Ciaglia (multiple dilator) technique is superior to the Griggs technique with respect to the total number of peri‐ and postoperative complications, that percutaneous dilatational tracheostomies (PDTs) are more beneficial for trainees in terms of the total number of peri‐ and postoperative complications than for staff, and that the greatest reduction of the total number of peri‐ and postoperative complications is shown, when the PDTs performed by staff were carried out in the operating theatre. The statements made above apply exclusively to the multiple dilator technique but not to the single dilator technique (frequently cited by its brand names Ciaglia Blue Rhino® or Ultraperc®) nor the balloon dilation technique (Ciaglia Blue Dolphin®) because these techniques have not been evaluated in any of the studies included in our systematic review and meta‐analysis.
However, because several groups of patients were excluded from the included studies (unfavourable anatomy, patients requiring emergency tracheostomy, evidence or suspicion of difficult anatomy, prior airway problems, coagulopathies and previous tracheostomy), or patient‐relevant outcomes were not evaluated (long‐term outcomes, patient discomfort, patient satisfaction, caregiver satisfaction) or not completely evaluated (mortality), the generalizability of the few results of this meta‐analysis to all critically ill adults is limited.
Applying guidelines to real‐life clinical practice can be difficult because their effectiveness is dependent upon many factors, including clinician acceptance of the guidelines, workload, availability of the equipment, frequency of assessments, and continuation of assessment and feedback to ensure compliance with guidelines. Also, data on patient‐relevant outcomes such as mortality or patient discomfort are either sparse (low number of studies (N = 4) and events for mortality (N = 6); one study assessed patient satisfaction after a few months) or not available for any study (discomfort during the procedure, caregiver satisfaction) to adequately evaluate the efficacy of using percutaneous tracheostomy (PT) techniques.
We had planned to perform subgroup analyses to determine whether the results differed by age, urgency, technique, experience of the practitioner or the location where the tracheostomy was performed. We only performed subgroup analyses to determine whether the results differed by experience of the practitioner (experienced versus not experienced) or the location where the tracheostomy was performed because the included studies were very heterogeneous (Characteristics of included studies).
No difference in the overall number of complications was seen if the PTs were performed in the intensive care unit (ICU) by trainees or staff.
There are a number of potential limitations of our review that warrant discussion.
First, unfavourable anatomy was identified as a restriction to the percutaneous technique in most studies, which reflects current practice, and the importance of determining anatomic landmarks for this procedure. In most of the studies, the lack of palpable midline structures (thyroid cartilage, cricoid cartilage, sternal notch) was a contraindication to perform a PT. So these patients were excluded from the included studies.
Second, several further groups of patients (patients requiring emergency tracheostomy, evidence or suspicion of difficult anatomy, prior airway problems, coagulopathies and previous tracheostomy) or outcomes (long‐term outcomes) were excluded from the included studies.
Third, several outcomes were not always (mortality, patient satisfaction after a few months) or never (patient discomfort during the procedure, caregiver satisfaction) reported or evaluated.
Fourth, there is a paucity of evidence demonstrating that one technique of PT is clearly superior to any other.
Fifth, there was great heterogeneity between definitions for outcomes/definitions of complications used in the studies (e.g. wound infection, major/minor bleeding, duration).
Because of these limitations, generalizability of the few results of this meta‐analysis to all critically ill adult patients requiring tracheostomy, is limited.
Thus, surgical tracheostomy (ST) may still be indicated for selected patients, despite the continuing broader indications for use of PT.
Implications for research.
In many studies, many important details were not described in sufficient detail. More well designed clinical trials with high methodological quality are needed to quantify differences of contextual factors (type of operators ‐ experienced, inexperienced; patient populations ‐ children, patients with unfavourable anatomy or coagulopathies and patients requiring emergency tracheostomy; settings ‐ ICU, operating room; and intervention factors ‐ type of percutaneous or surgical technique, ultrasound examination before tracheotomy) on the complication rate, the overall mortality, the procedure length and costs. Great emphasis must be placed on attempts to reduce bias and increase power to show differences in patient‐relevant clinical outcomes (i.e. mortality). With such studies it may be possible to developed an algorithm which enables clinicians to find the best tracheotomy proceedings for a particular patient in a particular condition.
Furthermore, such trials must fully evaluate the components of this complex intervention by focusing on mixed methods research. Future studies of the efficacy of PT should follow a framework that incorporates process evaluation (such as that advocated by MRC 2008) to understand how context influences outcome, and to provide insights to aid implementation in other settings. In addition, an economic evaluation taking into consideration the cost‐effectiveness of the method, not only from the payer's perspective, but also from that of service users and society as a whole, would be useful for decision‐makers.
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Notes
We would like to thank Mathew Zacharias, Martin Birchall and Bradley D Freeman for their help and editorial advice during the preparation of the protocol (Brass 2009).
Acknowledgements
We thank Dr Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia Review Group (CARG)) for his assistance in providing our different search strategies. Many special thanks to Jane Cracknell (Managing Editor, CARG) for her great patience, help and editorial advice during the entire process. We would like to acknowledge Professor Ulf Börner's contribution to the protocol. Professor Börner was listed as an author of the protocol for the systematic review, prior to his death in 2008 (Brass 2009).
We also thank A. Delaney for providing requested information, and Bettina Kullmer for her contribution to the protocol (Brass 2009). We would like to thank Lei Yang for extracting data from an article.
Finally we would like to thank Rodrigo Cavallazzi (content editor), Marialena Trivella and Jing Xie (statistical editors), Bradley Freeman and Bernard G Fikkers (peer reviewers) for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. Search strategy for CENTAL, The Cochrane Library
#1 surg* near tracheo?tomy #2 (tracheostomy or tracheotomy):ab,ti #3 guide wire dilation #4 forceps method* #5 PercuTwist #6 MeSH descriptor Tracheostomy, this term only #7 MeSH descriptor Tracheotomy, this term only #8 (dilational or percutaneous or translaryngeal) near tracheo?tomy #9 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
(surg* adj5 tracheo?tomy).mp. or (tracheostomy or tracheotomy).ti,ab. or ((dilational or percutaneous or translaryngeal) adj5 tracheo?tomy).mp. or guide wire dilation.mp. or forceps method*.mp. or PercuTwist.mp. or exp Tracheostomy/ or exp Tracheotomy/
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
1 and 2
Appendix 3. Search strategy for EMBASE (Ovid SP)
(surg* adj5 tracheo?tomy).mp. or (tracheostomy or tracheotomy).ti,ab. or ((dilational or percutaneous or translaryngeal) adj5 tracheo?tomy).mp. or guide wire dilation.mp. or forceps method*.mp. or PercuTwist.mp. or tracheostomy/ or tracheotomy/
(placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh.
1 and 2
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 (MM "Tracheostomy") or (TX surg* and TX tracheo?tomy) or (TI tracheostomy or tracheotomy) or (TX ( dilational or percutaneous or translaryngeal ) and tracheo?tomy) or (TX forceps and TX method*) S2 (MM "Random Assignment") OR "random" OR (MM "Prospective Studies") OR (MH "Clinical Trials") OR (MH "Clinical Trial Registry") OR (MH "Double‐Blind Studies") OR (MH "Single‐Blind Studies") OR (MH "Triple‐Blind Studies") OR (MM "Placebos") S3 S1 and S2
Appendix 5. Grips websearch
| Search terms |
| #1 AQS01 CC00 CDAR94 CDSR93 HN69 INAHTA KL97 KP05 KR03 MK77 SM78 SP97 SPPP TV01 CCTR93 ME60 NHSEED AZ72 EM74 TVPP #2 ct=tracheostomy #3 ct=tracheotomy #4 ct=tracheostomy #5 (percutan? AND surg?) #6 (perkutan? AND chirurg?) #7 5 OR 6 #8 3 OR 4 #9 7 AND 8 #10 check duplicates: unique in s=9 #11 (study; studie#) #12 10 AND 11 AQS01 DIQ‐Projekte 0 CC00 CCMed 0 CDAR94 NHS‐CRD‐DARE 2 CDSR93 Cochrane Library ‐ CDSR 0 HN69 HECLINET 0 INAHTA NHS‐CRD‐HTA 0 KL97 Kluwer‐Verlagsdatenbank 0 KP05 Krause & Pachernegg Verlagsdatenbank 0 KR03 Karger‐Verlagsdatenbank 0 MK77 MEDIKAT 0 SM78 SOMED 0 SP97 Springer‐Verlagsdatenbank 0 SPPP Springer‐Verlagsdatenbank PrePrint 0 TV01 Thieme‐Verlagsdatenbank 0 CCTR93 Cochrane Library ‐ Central 31 ME60 MEDLINE 197 NHSEED NHS‐EED 3 AZ72 GLOBAL Health 0 EM74 EMBASE 164 TVPP Thieme‐Verlagsdatenbank PrePrint 0 |
Appendix 6. Data extraction form
Study ID:
Authors:
Medline Journal ID:
Year of publication:
Language:
Type of study:
Type of Study: RCT_____ Q‐RCT_____ CCT_____ Non‐randomized_____
| Comments on Study Design: |
Quality of concealment of allocation points
| Allocation was not concealed (e.g. quasi‐randomization) D | |
| Allocation concealment was inadequate C | |
| Methods of concealment were unclear B | |
| Concealment was adequate (e.g. numbered, sealed opaque envelopes drawn consecutively) A | |
| Inclusion and exclusion criteria were not clearly defined in the text | |
| Outcomes of patients who withdrew or were excluded after allocation were NEITHER detailed separately NOR included in an intention to treat | |
| Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention to treat analysis OR the text stated there were no withdrawals | |
| Treatment and control groups were NOT adequately described at entry | |
| Treatment and control groups were adequately described at entry. A minimum of 4 admission details were described |
| Patient selection | Yes_____ | No_____ | Unclear_____ |
| Withdrawls | Yes_____ | No_____ | Unclear_____ |
| Post random exclusion |
Yes_____ | No_____ | Unclear_____ |
| Intension to treat analysis | Yes_____ | No_____ | Unclear_____ |
Participants
| Number of eligible participants | Number enrolled in study | |||
| Sex: males females | No information/ Unclear_____ | |||
| Weight | No information/ Unclear_____ | |||
| Height | No information/ Unclear_____ | |||
| BMI | No information/ Unclear_____ | |||
| APACHE II, SAPS | No information/ Unclear_____ | |||
| Age | No information/ Unclear_____ | |||
| Duration of translaryngeal intubation before TS | No information/ Unclear_____ | |||
| Number of days with TS before decannulation | No information/ Unclear_____ | |||
| Number of patients who were successfully decannulated | No information/ Unclear_____ | |||
| Admission details not described only “equal demographic data” | ||||
| Participants in narcosis | Participants awake | Part. in narcosis or awake | No information | |
Operators
| Number: |
| Experience: |
Methods
| Subject ‐ Blinded | Yes_____ | No_____ | Unclear_____ |
| Physician ‐ Blinded | Yes_____ | No_____ | Unclear_____ |
| Outcome Assessor ‐ Blinded | Yes_____ | No_____ | Unclear_____ |
Intervention
| N= | TS‐Technique: Sheldon (PT), Toye et al. (Pertrach®), Ciaglia et al. (PDT), Schachner et al. (Rapitrach®), Griggs et al. (GWDF), Fantoni et al. (Translaryngeal Tracheostomy), Frova et al. (PercuTwist®) |
Percutaneous tracheostomy with (B) or without bronchoscopic guidance (_) |
Technique standardized | Location where the procedure is carried out (ICU/OP) |
|
| PT | ____ | Yes_____ No_____ Unclear____ |
____ ____ |
||
| ST | ____ | Yes_____ No_____ Unclear____ |
____ ____ |
Comments on treatment
Outcomes
Outcome measures defined
| No_____ | |
| Yes_____ | _________ |
Outcomes
|
Overall success rate (N/N, %) |
Mortality(N/N, %) |
Serious, life threatening, adverse events (e.g. major vascular injury or excessive bleeding (blood transfusion or need for additional surgical procedure), tracheal or oesophageal injury (detected by intraoperative bronchoscopy), loss of airway (loss of the tube or the tracheostoma tube > 20 sec.) or misplaced airway (paratracheal insertion of the tube or the tracheostoma tube), severe hypoxic episode (SaO2 < 90%) or cardiac arrest) (N/N, %) |
Non‐life threatening events during the procedure (e.g. minimal or moderate bleeding (bleeding could be stopped by conservative measures) , subcutaneous emphysema (detected during the first 24 hr by chest x‐ray), pneumothorax, pneumomediastinum (both detected by postoperative chest x‐ray), or difficult tube placement) (N/N, %) |
Postoperative complications (e.g. pneumonia, atelectasis (detected by postoperative chest x‐ray), stomal infections (local inflammation, cellulitis or pus, necrosis or wound breakdown with or without antibiotic therapy), difficult tube change and hoarseness) (N/N, %) |
Late complications (e.g. tracheal stenosis (mild 10% to 30%, moderate 30% to 50%, severe > 50%), tracheal malacia, delayed wound healing (> 3 weeks), cosmetic deformity (colour changes at the scar, level difference with the surrounding skin, surgical revision needed), tracheocutaneous, ‐oesophageal fistula) (N/N, %) |
Duration of the procedure (minutes) |
Patient/ caregiver satisfaction (N/N, %) |
|
| PT | |
|
Overall success rate (N/N, %) |
Mortality(N/N, %) |
Serious, life threatening, adverse events (N/N, %) |
Non‐life threatening events during the procedure (N/N, %) |
Postoperative complications (N/N, %) |
Late complications (N/N, %) |
Duration of the procedure (minutes). |
Patient/ care giver satis‐faction (N/N, %) |
|
| ST | |
Cross over
| PT ‐ ST |
Changes in protocol
Contact with author
Other comments on this study
Data and analyses
Comparison 1. Percutaneous technique versus surgical techniques for tracheostomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality directly related to the procedure | 4 | 257 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.10, 2.60] |
| 1.1 Intraoperative mortality | 1 | 24 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 1.2 Postoperative mortality (during the first 24 hours after the intervention) | 3 | 233 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.05, 1.77] |
| 2 Serious, life‐threatening adverse events | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intraoperative serious, life‐threatening adverse events | 12 | 1211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.53] |
| 2.2 Direct postoperative serious, life‐threatening adverse events | 10 | 984 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.41, 1.25] |
| 3 Non‐life threatening events | 20 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 Intraoperative non‐life threatening events | 19 | Rate Ratio (Random, 95% CI) | 1.02 [0.79, 1.32] | |
| 3.2 Direct postoperative non‐life threatening events | 13 | Rate Ratio (Random, 95% CI) | 1.02 [0.62, 1.67] | |
| 3.3 Late non‐life threatening events | 10 | Rate Ratio (Random, 95% CI) | 0.47 [0.25, 0.89] | |
| 4 Total number of peri‐ and postoperative complications/adverse events | 20 | 1652 | Rate Ratio (Random, 95% CI) | 0.71 [0.53, 0.94] |
| 5 Duration of the procedure | 17 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Wound infection/stomatitis | 12 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.15, 0.37] |
| 7 Unfavourable scarring | 6 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.07, 0.91] |
| 8 Major bleeding | 10 | 984 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.09] |
| 9 Tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change | 6 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.65, 2.82] |
1.1. Analysis.
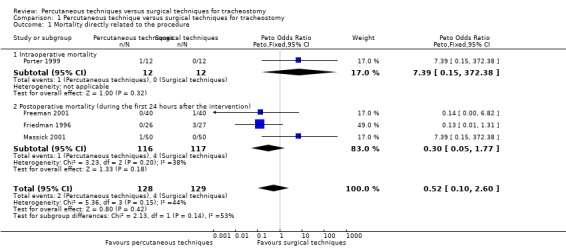
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 1 Mortality directly related to the procedure.
1.2. Analysis.
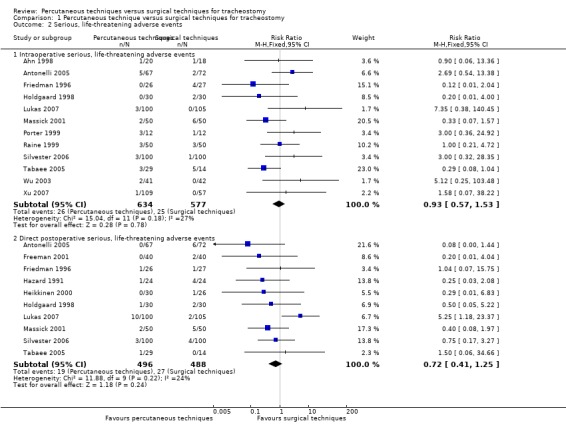
Comparison 1 Percutaneous technique versus surgical techniques for tracheostomy, Outcome 2 Serious, life‐threatening adverse events.
Comparison 2. Subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Technique (Ciaglia and Griggs), total number of peri‐ and postoperative complications/adverse events | 19 | 1460 | Rate Ratio (Random, 95% CI) | 0.70 [0.51, 0.96] |
| 1.1 Ciaglia, total number of peri‐ and postoperative complications/adverse events | 14 | 1015 | Rate Ratio (Random, 95% CI) | 0.62 [0.42, 0.92] |
| 1.2 Griggs, total number of peri‐ and postoperative complications/adverse events | 5 | 445 | Rate Ratio (Random, 95% CI) | 0.97 [0.57, 1.65] |
| 2 Experience of the practioner, total number of peri‐ and postoperative complications/adverse events | 10 | 762 | Rate Ratio (Random, 95% CI) | 0.50 [0.30, 0.81] |
| 2.1 Trainees, total number of peri‐ and postoperative complications/adverse events | 8 | 629 | Rate Ratio (Random, 95% CI) | 0.46 [0.26, 0.83] |
| 2.2 Staff, total number of peri‐ and postoperative complications/adverse events | 2 | 133 | Rate Ratio (Random, 95% CI) | 0.72 [0.34, 1.51] |
| 3 Location where the tracheostomy was performed (ICU versus operating theatre), total number of peri‐ and postoperative complications/adverse events | 3 | 193 | Rate Ratio (Fixed, 95% CI) | 0.52 [0.36, 0.77] |
| 3.1 PDT performed by staff in the ICU | 2 | 133 | Rate Ratio (Fixed, 95% CI) | 0.72 [0.34, 1.51] |
| 3.2 PDT performed by staff in the operating theatre | 1 | 60 | Rate Ratio (Fixed, 95% CI) | 0.47 [0.30, 0.73] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahn 1998.
| Methods | RCT Randomization method: computer‐generated random assignments were concealed in sealed envelopes Allocation concealment was adequate |
|
| Participants | Number of participants/procedures (PDT/ST): 20/18 Population: medical ICU Gender male/female: PDT: ST: "female to male ratio was higher in the ST group" Mean age (years): no details Population: medical ICU APACHE II Score: PDT: ST: no details SAPS Score: PDT: ST: no details Period intubation up to tracheotomy (days): PDT: ST: no details Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): third grade medical resident and pulmonologist/second year residents of the department of otolaryngology Procedure Setting (location PDT performed/location ST performed): bedside/bedside Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: patients who were under mechanical ventilation for more than 7 days or required airway protection Exclusion criteria:uncorrectable coagulopathy, previous tracheostomy or neck surgery, anatomic distortion of the tracheal region, or skin or soft‐tissue infection at the proposed tracheostomy Treatment and control groups description |
|
| Interventions | Technique/method: Ciaglia/Cook, multiple dilator ST: different Use of bronchoscopic guidance for PDT: yes |
|
| Outcomes | Operation time (min): PDT: 15.6 ± 7.1 ST: 29.1 ± 11.6 (S) Complications |
|
| Notes | Abstract in English language, original article in Korean language, translation required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignments |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes Allocation concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Low risk | Treatment and control groups were adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
Antonelli 2005.
| Methods | RCT Randomization method: computer‐generated random assignments were concealed in sealed envelopes Allocation concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PT/ST): 67/72 Gender male/female: PT: 41/26 (NS) ST: 42/30 (NS) Mean age (years): PT: 63 ± 1 (NS) ST: 64 ± 17 (NS) Population: medical/surgical ICU (general ICU) APACHE II Score: PT: no details ST: no details SAPS Score: PT: 43 ± 14 (NS) ST: 44 ± 11 (NS) Period intubation up to tracheotomy (days): PT: 10 ± 4 (NS) ST: 11 ± 5 (NS) Total number of operators (PT/ST): no details Experience of the operators (PT/ST): experienced/no details (ICU physicians, who completed 1 yr of TLT training and had already performed an average of 30 tracheostomies when the study began/team of ear, nose and throat surgeons (all staff doctors)) Procedure setting (location PT performed/location ST performed): bedside/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: all patients in the unit requiring tracheostomies (difficulties in weaning the patient from mechanical ventilation, need for long‐term ventilation (e.g. patients with severe traumatic or post‐anoxic brain damage, cerebral infarction, other neurologic disorders, such as spinal cord injury or Guillain‐Barre syndrome, and any other patient expected to require mechanical ventilation for 10 days)) Exclusion criteria: indications for emergency tracheostomy, age 18 yrs, severe coagulopathy, surgical wounds near the tracheostomy site, previous or pre‐existing tracheostomy, conditions that compromised adequate visualization of normal anatomic landmarks and generally accepted contraindications for PT (i.e. inability to extend the neck adequately, significant thyroid gland enlargement, palpable neck vessels that left insufficient space for percutaneous dilatational tracheostomy insertion) Treatment and control groups were adequately described at entry (sex, age, SAPS II score, comorbid conditions, kind of admissions, reason for ICU admission) |
|
| Interventions | Technique/method: PT: Fantoni (TLT) ST: different Monitoring: pulse oximetry Use of bronchoscopic guidance for PT: no (PT/ST): anaesthetized with propofol and sufentanil and paralysed with atracurium bromide and with local anaesthesia (lidocaine 2%) |
|
| Outcomes | Survival rate (%): no details Discharged from ICU: PT: 67 (NS) ST: 65 (NS) Discharged from hospital: PT: 50 (NS) ST: 46 (NS) Alive at 1 yr PT: 28 (NS) ST: 24 (NS) Days up to decannulation: PT: no details ST: no details Lowest PaO2 (%): PT: no details ST: no details Stay in the hospital (days): PT: no details ST: no details Operation time (min): PT: 17 ± 10 (NS) ST: 22 ± 6 (NS) Complications Length of follow‐up: 1 year Percentage lost to follow‐up (total, PT, open): 77.6, 73, 82 |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random assignments |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes Allocation concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were adequately described at entry Patient selection: No _X_ Withdrawls: Yes _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
Crofts 1995.
| Methods | Quasi‐RCT Randomization method: randomization per weeks Allocation was not concealed (e.g. quasi‐randomization) Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 25/28 Gender male/female: PDT: 12/13 (NS) ST:19/9 (NS) Mean age (years): PDT: 59.2 ± 16.4 (NS) ST: 59.4 ± 18.3 (NS) Population: medical/surgical ICU (consecutive, ventilated critically ill patients of internal, surgical and neurosurgical ICUs) APACHE II Score: PDT: 16.0 ± 6.2 (NS) ST: 17.5 ± 7.9 (NS) SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 12.5 ± 6.3 (NS) ST: 10.5 ± 5.0 (NS) Total number of operators (PDT/ST): multiple surgeons performed the conventional tracheostomies. The percutaneous tracheostomies were supervised by one ENT surgeon but technically carried out by several ENT residents Experience of the operators (PDT/ST): trainee/trainee (consulting surgical team/otolaryngology house staff, small group of experienced surgeons under the supervision of a staff otolaryngologist) Procedure Setting (location PDT performed/location ST performed): bedside/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: Consecutive patients requiring tracheostomy as an elective procedure in either the medical/surgical or neurosurgical intensive care units. Exclusion criteria: included children < 16 yr, enlarged thyroid gland, previous tracheostomy, cervical spine fracture, evidence of coagulopathy defined as platelet count < I00,000 ml ‐l or prothrombin time > 1.5 times control Treatment and control groups were not adequately described at entry (sex, age, APACHE II score) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: different Monitoring: none Use of bronchoscopic guidance for PDT: no PDT: general anaesthesia and local anaesthesia (2% lidocaine, 1:100,000 epinephrine) ST: general anaesthesia |
|
| Outcomes | Survival rate (%): PDT: 68 (3 months) (NS) ST: 50 (3 months) (NS) Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: no details ST: no details Complication rate: measured Length of follow‐up: 2 weeks Lost to follow‐up (total, PDT, open) (%): no details, 36, 50 |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization per weeks |
| Allocation concealment (selection bias) | High risk | Randomization per weeks |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Freeman 2001.
| Methods | RCT Randomization method: no details Allocation concealment was adequate sealed envelopes Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 40/40 Gender male/female: PDT: 18/22 (NS) ST: 19/21 (NS) Mean age (years): PDT: 65.44 ± 2.82 (NS) ST: 61.4 ± 2.89 (NS) Population: medical/surgical ICU (patients from the medical, surgical and coronary ICUs) APACHE II Score: PDT: 16.87 ± 0.84 (NS) ST: 17.88 ± 0.92 (NS) SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 12.7 ± 1.1 (NS) ST: 15.6 ± 1.9 (NS) Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): no details Procedure setting (location PDT performed/location ST performed): bedside/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: were as follows: a) age > 18 yrs; b) the necessity of mechanical ventilation for >= 1 week; c) haemodynamic stability (e.g. not requiring vasopressor support); d) ventilatory support of no greater than FIO2 of 0.40 and positive end‐expiratory pressure of 5 cm H2O; and e) no signs of active infection (i.e. afebrile [temperature 38.5°C], white blood cell count under 10,000/mm3 or decreasing by 2000/mm3 per day for the preceding 3 days) Exclusion criteria: included distorted neck anatomy that precluded the operating surgeon from identifying surface landmarks necessary for safely performing PDT, refractory coagulopathy, and patients considered to have a difficult airway for translaryngeal intubation in the event that airway control was inadvertently lost. Also, patients were excluded who were being transported to the operating room for another purpose (such as an orthopedic procedure, abdominal exploration, or gastrostomy tube placement). Children < 16 yr, enlarged thyroid gland, previous tracheostomy, cervical spine fracture, evidence of coagulopathy defined as platelet count < I00,000 ml ‐l or prothrombin time > 1.5 times control Treatment and control groups were not adequately described at entry (gender, age, severity of illness, principle diagnosis) |
|
| Interventions | Technique/method: PDT: Ciaglia/Sims, multiple dilator ST: Zollinger/Fenster Monitoring: bronchoscopy Use of bronchoscopic guidance for PDT: yes PDT: sedation and analgesia (e.g. intravenous benzodiazepine, narcotics, and propofol) as well as paralysis if necessary and local anaesthesia (1% lidocaine) ST: adequate anaesthesia |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: 46.7 ± 4.2 (NS) ST: 43.8 ± 3.5 (NS) Operation time (min): PDT: 20.1 ± 2,0 ST: 41.7 ± 3.9 Complication rate, costs Length of follow‐up: PDT: no details ST: no details Lost to follow‐up (total, PDT, open) (%): no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No details |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry. Patient selection: No _X_ Withdrawls: Unclear _X_ Post‐random exclusion: Yes _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Friedman 1996.
| Methods | Quasi‐RCT Randomization method: random number tables Allocation was not concealed (e.g. quasi‐randomization) Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 26/27 Gender male/female: PDT: 14/12 (NS) ST: 17/10 (NS) Mean age (years): PDT: 56 ± 20 (NS) ST: 53 ± 17 (NS) Population: medical/surgical ICU (patients of medical, surgical, trauma, or neurosurgical ICUs) APACHE II Score: PDT: 18.0 ± 7.8 (NS) ST: 14.8 ± 6.5 (NS) SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 17.2 ± 7.5 (NS) ST: 21.3 ± 26.2 (NS) Total number of operators (PDT/ST): 2/4 Experience of the operators: PDT: performed or supervised by one of the two intensivists ST: performed by one of the four surgeons Procedure Setting (location PDT performed/location ST performed): bedside/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: > 18 years of age and required a tracheostomy for long‐term ventilator support or airway protection Exclusion criteria: clinical instability, positive end‐expiratory pressure greater than 15 cm H2O, uncorrectable coagulopathy, previous tracheostomy or neck surgery, thyromegaly, anatomic distortion of the tracheal region, or skin or soft‐tissue infection at the proposed tracheostomy Treatment and control groups were adequately described at entry (gender, age, severity of illness, principle diagnosis, APACHE II score, coagulation status) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: Jackson Monitoring: none Use of bronchoscopic guidance for PDT: no PDT: sedation and local anaesthesia ST: general anaesthesia |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Lowest SaO2 (%): PDT: 97.6 ± 3.1 ST: 95.4 ± 3.9 Stay in the hospital (days): PDT: 24.5 ± 20.1 (NS) ST: 18.5 ± 11.8 (NS) Operation time (min): PDT: 8.2 ± 4.9 ST: 33.9 ± 14.0 Complication rate, effectiveness Length of follow‐up: PDT: no details ST: no details Lost to follow‐up (total, PDT, open) (%): no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number tables |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Gysin 1990.
| Methods | RCT Randomization method: computer‐generated number table Allocation concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 35/35 Gender male/female: PDT: 27/8 (NS) ST: 27/8 (NS) Mean age (years): PDT: 55 ± 15.4 (NS) ST: 56 ± 13.8 (NS) Population: medical/surgical ICU APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 8.3 ± 12.4 (NS) ST: 4.9 ± 7.5 (NS) Total number of operators (PDT/ST): no details Experience of the operators: PDT: no details Procedure setting (location PDT performed/location ST performed): 8 bedside, 27 operating room/13 bedside, 22 operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: all patients older than 18 years, who underwent an elective tracheostomy Exclusion criteria: tracheostomy in the past or those with previous tracheal pathology Treatment and control groups were not adequately described at entry (sex ratio, age, anatomical distinctiveness) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: Björck Monitoring: tracheoscopy Use of bronchoscopic guidance for PDT: no, tracheoscopy PDT: general anaesthesia ST: general anaesthesia |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 18.2 ± 11.2 ST: 15.8 ± 5.5 Complication rate Length of follow‐up: 3 months Lost to follow‐up (total, PDT, open) (%): 57, no details, no details |
|
| Notes | 1 cross‐over PDT ‐> ST | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number table |
| Allocation concealment (selection bias) | Low risk | Computer‐generated number table |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Yes__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded: Yes__X__ |
Hazard 1991.
| Methods | RCT Randomization method: no details Methods of concealment were unclear Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 22/24 / 24/24 Gender male/female: PDT: 12/10 (NS) ST: 13/11 (NS) Mean age (years): PDT: 61 ± 19 (NS) ST: 65 ± 18 (NS) Population: medical/surgical ICU APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: 11.9 ± 4.6 (NS) ST: 11.8 ± 4.2 (NS) Period intubation up to tracheotomy (days): PDT: 7.7 ± 3.9 (NS) ST: 9.2 ± 3.2 (NS) Total number of operators (PDT/ST): three general surgeons, four cardiothoracic surgeons and one neurosurgeon Experience of the operators (PDT / ST): board‐certified and experienced in the performance of tracheostomy/investigators, or by house officers under their direct supervision Procedure Setting (location PDT performed/location ST performed): bedside/bedside and operating room (numbers no details) Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: adult patients who required an oral or nasal endotracheal tube for > 5 days Exclusion criteria: age < 15 yrs, high potential for extubation within the next 4 days, the presence of any haemostatic defect (platelet count < 40,000/mm3, or activated partial thromboplastin time or prothrombin time >1.5 times the control value) that could not be corrected by replacement of blood components, any gross anatomic distortion of the trachea, as by tumour, thyromegaly, or scarring from prior tracheostomy, any evidence of infection in the soft tissues of the neck Treatment and control groups were adequately described at entry (sex, age, SAPS score, principle diagnosis, coagulation status) |
|
| Interventions | Technique/method: PDT: Ciaglia, multiple dilator ST: different (the specific techniques used by these individuals were not under the control of the investigators) Monitoring: none Use of bronchoscopic guidance for PDT: no PDT: local anaesthetic combined with intravenous narcotic or benzodiazepine sedation ST: general or local anaesthesia |
|
| Outcomes | Survival rate (%): PDT: 54 ST: 33 Days up to decannulation: PDT: 16.9 ± 12.9 ST: 22.0 ± 10.0 Lowest PaO2 (%): PDT: 96 ± 5 ST: 92 ± 11 Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 4.3 ± 2.2 (NS) ST: 13.5 ± 7.3 (NS) Complication rate Length of follow‐up: 12 weeks Lost to follow‐up (total, PDT, open) (%): no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment were unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Heikkinen 2000.
| Methods | Quasi‐RCT Randomization method: lot Allocation concealment was inadequate (e.g. quasi‐randomization) Blinding unclear |
|
| Participants | Number of participants/procedures (PT/ST): 30/26 Gender male/female: PT: 24/7 (NS) ST: 16/10 (NS) Mean age (years): PT: 64.2 ± 11 (NS) ST: 65 ± 11 (NS) Population: medical/surgical ICU Indications for tracheostomy: prolonged mechanical ventilation, prolonged coma after severe head injury, severe neuromuscular disease or compromised airways in severe maxillofacial injury APACHE II Score: PT: no details ST: no details SAPS Score: PT: no details ST: no details Period intubation up to tracheotomy (days): PT: 11.5 ± 3.7 (NS) ST: 13 ± 4.5 (NS) Total number of operators (PT/ST): one of four surgeons Experience of the operators (PT/ST): trainee/trainee (the four surgeons who did the procedures had no previous experience with PDT) Procedure Setting (location PT performed/location ST performed): bedside/bedside Inclusion and exclusion criteria were clearly defined in the text Inc lusion criteria: All patients were receiving long‐term ventilatory support in the ICU. The indications for the tracheostomy were as follows: prolonged mechanical ventilation, prolonged coma after severe head injury, severe neuromuscular disease, or compromised airways in severe maxillofacial injury. Exclusion criteria: previous tracheostomy or neck surgery, thyromegaly, infection at the proposed tracheostomy site, or an extremely obese patient Treatment and control groups were not adequately described at entry (age, gender, principle diagnosis) |
|
| Interventions | Technique/method: PT: Griggs/Portex, forceps and nasal speculum ST: Björck Monitoring: none Use of bronchoscopic guidance for PT: no PT/ST: sedation (propofol, midazolam, or diazepam), analgesics and muscle relaxants were used as needed, local anaesthesia (1% lidocaine with epinephrine) |
|
| Outcomes | Survival rate (%): PT: no details ST: no details Days up to decannulation: PT: no details ST: no details Lowest PaO2 (%): PT: no details ST: no details Stay in the hospital (days): PT: no details ST: no details Operation time (min): PT: 11.13 ± 6,4 ST: 14.4 ± 6 Complication rate, costs, time expenditure Length of follow‐up: 18 months Lost to follow‐up (total, PT, open) (%): 80, no details, no details |
|
| Notes | 1 cross‐over PT ‐> ST | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization method: Lot |
| Allocation concealment (selection bias) | High risk | Allocation concealment was inadequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Holdgaard 1998.
| Methods | Randomized controlled trial (RCT) Randomization method: single‐blinded envelope Allocation concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 30/30 Gender male/female: PDT: 24/6 (NS) ST: 22/8 (NS) Mean age (years): PDT: 54.5 (18‐79) (NS) ST: 65.5 (18‐78) (NS) Population: medical/surgical ICU APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (hrs): PDT: 7 (156 (42‐340)) (NS) ST: 6.5 (168 (1‐264)) (NS) Total number of operators (PDT/ST): 4/18 Experience of the operators (PDT/ST): no details/experienced specialist in surgery or by senior registrars in their final training Procedure setting (location PDT performed/location ST performed): operating room/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: Consecutive patients selected for elective tracheostomy in either the medical or surgical intensive care units. Exclusion criteria: age < 18 yrs, previous tracheostomy, pathology of the neck or neck deformities and with unidentifiable anatomy of the neck, patients treated with radiotherapy, excessive clinical bleeding, platelet count less than 60X 109/1 Treatment and control groups were not adequately described at entry (age, gender, underlying disorders) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: different (the surgical procedure and the size of the tracheostomy tube (Portex Ltd., England) was at the surgeon’s discretion) Monitoring: none Use of bronchoscopic guidance for PDT: no PDT/ST: general anaesthesia, (lidocaine with 1% epinephrine) |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: 9 (NS) ST: 10.8 (NS) Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: 25 (8‐75) (NS) ST: 26 (11‐132) (NS) Operation time (min): PDT: 11.5 (7‐24) 4.25 (NS) ST: 15.5 (5‐47) 10.50 (NS) Complication rate, effectiveness Length of follow‐up: to stoma closure Lost to follow‐up (total, PDT, open) (%): no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Single‐blinded envelope |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Lukas 2007.
| Methods | RCT Randomization method: sealed envelope Allocation concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PT/ST): 100/105 Gender male/female: PT: 62/38 (NS) ST: 63/42 (NS) Mean age (years): PT: 65 (22‐88) (NS) ST: 64 (17‐96) (NS) Population: ICU APACHE II Score: PT: no details ST: no details SAPS Score: PT: no details ST: no details Period intubation up to tracheotomy (years??): PT: 7 (3+‐3.4) (NS) ST: 8 (0+‐3.2) (NS) Total number of operators (PT/ST): 4/4 Experience of the operators (PT/ST): one of three ICU physicians and one otorhinolaryngologist/one of four otorhinolaryngologists Procedure setting (location PT performed/location ST performed): no details/no details Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: the need for long‐term mechanical pulmonary ventilation, tracheal toilet, or ICU physician indication, and patients aged > 15 years Exclusion criteria: laryngotracheal intubation longer than 21 days, haemodynamic instability‐coagulopathy, thrombocytopenia < 50.109/l, or INR > 1.5, positive end‐expiratory pressure > 15 cm H2O, patients who had already undergone tracheostomy, or with oncological disease in the head and neck area, or with unsuitable anatomical conditions such as kyphoscoliosis of the cervical spine, enlarged thyroid, cervical spine injury, or skin infection in the neck area Treatment and control groups were not adequately described at entry (age, sex) |
|
| Interventions | Technique/method: PT: Griggs/Portex, multiple dilator ST: Björck, elective tracheostomies Monitoring: blood pressure, oxygen, and carbon dioxide levels, and electrocardiogram Use of bronchoscopic guidance for PT: no details PT/ST: intravenous analgo sedative and neuromuscular blockade agents, 1% trimecaine with adrenaline |
|
| Outcomes | Survival rate: PT: 60/100 ST: 58/105 Days up to decannulation: PT: 30.2 (NS) ST: 32.2 (NS) Lowest PaO2 (%): PT: no details ST: no details Stay in the hospital (days): PT: no details ST: no details Operation time (min) (initial skin incision ‐ introduction of the tracheostomal cannula: PT: 5.5 (+‐3.2, 2‐22) (S) ST: 15,1 (+‐ 6.4, 4.5‐60) (S) Complication rate Length of follow‐up: to stoma closure Lost to follow‐up (total, PDT, open) (%): no details |
|
| Notes | 1 PT cross‐over to ST | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization method: sealed envelope |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: Yes _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Massick 2001.
| Methods | RCT Randomization method: serial of sequentially sealed envelopes Allocation concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 50/50 (164 consecutive intubated patients selected for elective tracheostomy were enrolled. 100 patients met selection criteria for bedside tracheostomy and were randomly assigned to either open surgical tracheostomy (50) or endoscopically guided percutaneous dilatational tracheotomy (50). The remaining 64 patients received open surgical tracheostomies in the operating room) Gender male/female: 76/88 (NS) Mean age (years): 63 ± 16.9 (19‐92) (NS) Population: medical/surgical ICU (all intubated patients in the medical ICU selected for tracheostomy placement) APACHE II Score: 4,67 ± 6,7 (1‐43) (NS) SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): 9.7 ± 4.1 (NS) Total number of operators (PDT/ST): 3/4 Experience of the operators: (PDT/ST): one senior otolaryngology resident assisted by a junior otolaryngology resident with staff in attendance, a senior critical care physician and a respiratory therapist or an anaesthesiologist Procedure Setting (location PDT performed/location ST performed): bedside/bedside Inclusion criteria were clearly defined in the text Inclusion criteria: age greater than 18 years, elective tracheostomy with preexisting endotracheal tube in place, absence of a cervical infection, coagulopathy correctable before procedure (prothrombin time, 1.5; INR, 1.4; partial thromboplastin time, 40), palpable cricoid cartilage at least 3 cm above the sternal angle with appropriate head extension, history of uneventful/uncomplicated translaryngeal intubation, positive end‐expiratory pressure requirement of less than 10 cm H2O, informed consent before enrolment. Exclusion criteria: no details Treatment and control groups were adequately described at entry (age, sex, reason for admission, indication for tracheostomy, APACHE II score) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: Björck Monitoring: bronchoscopy Use of bronchoscopic guidance for PDT: yes PDT/ST: Intravenous sedation (propofol, midazolam, and morphine) and lidocaine 1% to epinephrine 1:100,000 |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 11 ± 4 (NS) ST: 10 ± 2 (NS) Complication rate, costs Length of follow‐up: 21 days Percentage Lost to follow‐up (total, PDT, open): 0, 0, 0 |
|
| Notes | 2 cross‐over PDT ‐> ST | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Serial of sequentially sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Melloni 2002.
| Methods | RCT Randomization method: no details Methods of concealment were unclear Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 25/25 Gender male/female: PDT: 14/11 (NS) ST: 17/8 (NS) Mean age (years): PDT: 61 ± 13 (NS) ST: 52 ± 18 (NS) Population: medical/surgical ICU (adult patients requiring elective tracheostomy in general or in neurosurgical ICUs) APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: 46 ± 13 (NS) ST: 49 ± 19 (NS) Period intubation up to tracheotomy (days): PDT: 6.9 ± 2.4 (NS) ST: 7.5 ± 3.1 (NS) Total number of operators (PDT/ST): 4/4 Experience of the operators (PDT/ST): experienced staff intensivists, thoracic surgeons Procedure Setting (Location PDT performed/location ST performed): bedside/15 bedside, 10 operating room Inclusion and exclusion criteria were clearly defined in the text I nclusion criteria: All adult patients requiring elective tracheostomy in general or in neurosurgical intensive care units were randomized to undergo either ST or PDT. Exclusion criteria: age < 15 years, oral‐ or nasal‐intubation for more than 10 days, enlarged thyroid gland, a history of laryngeal or tracheal disease, previous tracheostomy or major laryngeal, tracheal or neck surgery, acute burns, uncorrectable coagulopathy, evidence of subcutaneous infection or emphysema of the neck, spine fracture Treatment and control groups were adequately described at entry (sex, age, SAPS II score, underlying disorders) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: Grillo Monitoring: bronchoscopy Use of bronchoscopic guidance for PDT: yes PDT/ST: general anaesthesia |
|
| Outcomes | Survival rate (%): PDT and ST: 84 Days up to decannulation: PDT: 24.1 ± 12.6 (NS) ST: 33.1 ± 37.3 (NS) Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 14 ± 6 ST: 41 ± 14 Complication rate, long‐term follow‐up Length of follow‐up: 6 months Percentage lost to follow‐up (total, PDT, open): no details, 40, 48 |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment were unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Porter 1999.
| Methods | RCT Randomization method: sealed manila envelopes Concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 12/12 Gender male/female: PDT: 7/5 ST: 12/0 Mean age (years): PDT: 48 ± 18 ST: 41.9 ± 16 Population: patients from a surgical ICU APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 9.8 ± 4 ST: 12.4 ± 6.0 Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): residents and critical care fellows, under direct supervision by one of the two authors (performed well over 250 percutaneous tracheostomies) Procedure setting (location PDT performed/location ST performed): bedside/bedside Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: severe head injury leading to the inability to control the airway, inability to wean from the ventilator because of pneumonia, chronic obstructive pulmonary disease, adult respiratory distress syndrome, or multiorgan dysfunction syndrome and severe central nervous system abnormality, leading to the inability to control the airway or to wean from the ventilator Exclusion criteria: need for surgical airway in an emergency, coagulopathy Treatment and control groups were not adequately described at entry (sex, age, underlying disorders) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: Jackson Monitoring: bronchoscopy and pulse oximetry Use of bronchoscopic guidance for PDT: yes PDT/ST: local anaesthesia and intravenous sedation |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 14.5 ± 3.8 ST: 25.2 ± 9.5 Complication rate Length of follow‐up: no details Percentage lost to follow‐up (total, PDT, open): no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed manila envelopes |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Raine 1999.
| Methods | RCT Randomization method: sequentially numbered envelopes Allocation concealment was adequate |
|
| Participants | Number of participants/procedures (PT/ST): 50/50 Gender male/female: PT: 35/15 (NS) ST: 31/19 (NS) Mean age (years): PT: 42.7 ± 15.3 (NS) ST: 43.4 ± 15.9 (NS) Population: Respiratory and surgical ICU patients with respiratory failure, needing tracheostomy for anticipated prolonged intubation APACHE II Score: PT: no details ST: no details SAPS Score: PT: no details ST: no details Period intubation up to tracheotomy (days): PT: 7.5 ± 4.2 (NS) ST: 7.5 ± 4.3 (NS) Total number of operators (PT/ST): no details/no details Experience of the operators (PT/ST): operators were trained in this technique and had performed a minimum of ten procedures each, before performing study procedures Procedure setting (location PT performed/location ST performed): bedside ICU/bedside ICU Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: patients with respiratory failure needing who tracheostomy for anticipated prolonged intubation Entry criteria: separate consents obtained from patient or an adult relative Exclusion criteria: anatomical abnormalities of the neck, previous neck surgery, INR > 1.5 or platelet count < 60 x 10 mm Treatment and control groups were not adequately described at entry (sex, age, diagnosis). |
|
| Interventions | Technique/method: PT: Griggs/Portex, multiple dilator ST: no details Monitoring: pulse oximetry Use of bronchoscopic guidance for PT: no. All patients underwent fibre‐optic inspection of the airways via the endotracheal tube, with the tube withdrawn into the larynx, to evaluate the tracheal mucosa prior to performance of the procedure (PT/ST): intravenous sedation and local anaesthesia (lidocaine with ornipressin). Sedative and neuromuscular blocking drugs were used as needed |
|
| Outcomes | Difficult procedure: PT: 3 ST: 3 SaO2 < 90%: Survival rate (%): ICU no details, hospital no details Days up to decannulation: PT: no details ST: no details Lowest PaO2 (%): PT: no details ST: no details Stay in the hospital (days): PT: no details ST: no details Operation time (min): PT: 10.3 ± 5.8 (S) ST: 14.9 ± 5.6 (S) Complications bleeding, hypoxia, skin scarring and the airways were inspected fibre‐optically under local anaesthesia to assess laryngeal and tracheal injury Length of follow‐up: 60 days after decannulation Percentage lost to follow‐up (total, PT, open): 50% |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes Allocation concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: Yes _X_ Post‐random exclusion: Yes _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
Silvester 2006.
| Methods | RCT Randomization method: sealed envelopes Allocation concealment was adequate Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 100/100 Gender male/female: PDT: 69/31 (NS) ST: 68/32 (NS) Mean age (years): PDT: 67 (50‐77) (NS) ST: 61 (46‐73) (NS) Population: medical/surgical ICU (combined medical/surgical ICU, a case mix of postsurgical, trauma and sepsis, and multiple organ failure patients) APACHE II Score: PDT: 19 (15‐24) (NS) ST: 17(14‐22) (NS) SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 6 (4‐10) (NS) ST: 6 (3‐8) (NS) Total number of operators (PDT/ST): no details/3 Experience of the operators (PDT/ST): experienced/trainee (intensivists or supervised senior trainees who had completed at least ten PTs performed the PTs/2 thoracic surgeons or their supervised senior trainees who had completed at least ten STs) Procedure setting (location PDT performed/location ST performed): bedside/bedside Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: critically ill mechanically‐ventilated patients who required tracheostomy; age >= 16 yrs, separate consents obtained from patient or next of kin for the procedure and the study, and availability of procedural list to per form either PT or ST Exclusion criteria: coagulopathy (INR 2) or platelet count 40 109/L; anatomical abnormality in the anterior neck involving trachea, vessels, or thyroid; previous tracheostomy scar; or cervical spinal injury that had not been internally fixed Treatment and control groups were adequately described at entry (sex, age, APACHE II score, diagnosis, coagulation status) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: different Monitoring: pulse oximetry, bronchoscopy Use of bronchoscopic guidance for PDT: yes (PDT/ST): intravenous general anaesthetic (fentanyl and propofol) and local anaesthetic (lidocaine 1% with adrenaline 1:200,000) |
|
| Outcomes | Survival rate (%): ICU 91/94, hospital 74/77 Days up to decannulation: PDT: 19 (11‐28) ST: 21 (11‐27) Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 20 (15‐30) (NS) ST: 17 (15‐20) (NS) Complications Length of follow‐up: 1 year Percentage lost to follow‐up (total, PDT, open): 77.6, 73, 82 |
|
| Notes | 3 cross‐over PDT ‐> ST There were 12 protocol violations |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes Allocation concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: postop: Unclear __X__ 1 year follow‐up examiners: Yes__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: Yes _X_ Post‐random exclusion: Yes _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded postop: Unclear __X__ 1 year follow‐up examiners Yes:__X__ |
Sustic 2002.
| Methods | RCT Randomization method: no details Methods of concealment were unclear Blinding unclear |
|
| Participants | Number of participants/procedures (PT/ST): 8/8 Gender male/female: PT: 7/1 (NS) ST:6/2 (NS) Mean age (years): PT: 35 (24‐59) (NS) ST: 37 (18‐47) (NS) Population: surgical ICU APACHE II Score: PT: no details ST: no details SAPS Score: PT: no details ST: no details Period intubation up to tracheotomy (days): PT: 16 (12‐33) (NS) ST: 18 (11‐42) (NS) Total number of operators (PT/ST): 1/1 Experience of the operators (PT/ST): no details Procedure setting (location PT performed/location ST performed): bedside/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: patients aged 18 years or older who have had anterior cervical spine fixation (ACSF) (Smith‐Robinson interbody grafting) after acute cervical cord injury and in whom tracheostomy was indicated after 7 days of their stay in the ICU. The indication for tracheostomy in all cases was prolonged mechanical ventilation and/or difficulties in separating from respiratory support Exclusion criteria: purulent infection of the surgical scar before or on the day of tracheostomy or refractory coagulopathy Treatment and control groups were not adequately described at entry (sex, age, underlying disorders) |
|
| Interventions | Technique/method: PT: Griggs/Portex, multiple dilator ST: Björck Monitoring: ultrasound, bronchoscopy Use of bronchoscopic guidance for PT: no PT/ST: analgesic sedation (propofol or etomidate plus fentanyl or sufentanil) and relaxation (vecuronium) and Xylocaine with epinephrine, 1:200,000 ST: adequate anaesthesia |
|
| Outcomes | Survival rate (%): PT: no details ST: no details Days up to decannulation: PT: no details ST: no details Lowest PaO2 (%): PT: no details ST: no details Stay in the hospital (days): PT: no details ST: no details Operation time (min): PT: 8 ± 6 (4‐21) ST: 21 ± 7 (12‐47) Complication rate Length of follow‐up: no details Percentage lost to follow‐up (total, PDT, open): no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: no details |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment were unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry. Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Tabaee 2005.
| Methods | Quasi‐RCT Randomization method: patients with medical record numbers ending in an odd number were randomized to PDT and those with medical numbers ending in an even number were randomized to ST Allocation was not concealed (e.g. quasi‐randomization) Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 29/14 Gender male/female: PDT: 12/17 (NS) ST: 5/9 (NS) Mean age (years): PDT: 61.2 (18‐88) (NS) ST: 57.7 (20‐90) (NS) Population: medical ICU (medical, cardiac, and neurologic ICUs) APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: no details ST: no details Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): no details/no details (pulmonary fellow or an otolaryngology resident supervised by the senior author/one senior and one junior otolaryngology resident) Procedure Setting (location PDT performed/location ST performed): bedside/bedside Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: critically ill mechanically‐ventilated patients who required tracheostomy; age >= 16 yrs, separate consents obtained from patient or next of kin for the procedure and the study, and availability of procedural list to per form either PT or ST Exclusion criteria: tracheotomy site infection, tracheotomy site mass or goiter, unstable or immobile cervical spine, inability to palpate external tracheal landmarks, history of previous tracheotomy, palpable or sonographically detected excess vascularity, age < 18, pregnant female, inability to obtain informed institutional or study consent, minute ventilation greater than 15 L/min, positive end‐expiratory pressure greater than 10 cm H2O, fractional inspired oxygen of greater than 70%, known or suspected difficult endotracheal intubation, non‐intubated patient, emergency or non‐elective tracheotomy, prothrombin time or partial thromboplastin time 1.5 times control, platelet count less than 75,000/mm3 Treatment and control groups were not adequately described at entry (sex, age, indication for tracheotomy, anatomical distinctiveness) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, single dilator ST: see text Monitoring: pulse oximetry, bronchoscopy Use of bronchoscopic guidance for PDT: yes (PDT/ST): intravenous sedation and a short‐acting paralytic agent and 1% lidocaine with 1:100,000 epinephrine |
|
| Outcomes | Survival rate (%): PDT: no details ST: no details Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 8 (3‐25) (NS) ST: 23,6 (18‐40) (NS) Complications Length of follow‐up: PDT: no details ST: no details Lost to follow‐up (total, PDT, open) (%): no details |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization method: Patients with medical record numbers ending in an odd number were randomized to PDT and those with medical numbers ending in an even number were randomized to ST |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed (e.g. quasi‐randomization) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: No__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: Yes _X_ Post‐random exclusion: Yes _X_ Intension‐to‐treat analysis: Yes _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor blinded: No__X__ |
Wu 2003.
| Methods | RCT Randomization method: computer generated number table Concealment was adequate |
|
| Participants | Number of participants/procedures (PDT/ST): 41/42 Gender male/female: PDT: 31/10 (NS) ST: 33/9 (NS) Mean age (years): PDT: 72 ± 14.4 (NS) ST: 65.6 ± 14.8 (NS) Population: medical/surgical ICU APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): PDT: 21.5 ± 14.6 (NS) ST: 26.,7 ± 17.0 (NS) Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): PDT technique was new to PDT team/ICU attending staff, chief residents or residents of thoracic section being supervised Procedure setting (location PDT performed/location ST performed): bedside/operating room Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: all adult patients from the general ICU services requiring elective tracheostomy Exclusion criteria: previous neck operation, unable to identify the land marks, severe coagulopathy Treatment and control groups were not adequately described at entry (sex, age, underlying disorders) |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: Björck Monitoring: bronchoscopy (12 cases), pulse oximetry (all cases) Use of bronchoscopic guidance for PDT: yes (12 cases) PDT/ST: heavy sedation and local infiltrative anaesthesia/general anaesthesia |
|
| Outcomes | Survival rate (%): PDT: 34 ST: 40 Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT: 22 ± 12.10 ST: 41.5 ± 5,9 Complication rate, costs, time expenditure Length of follow‐up: 2‐4 years Percentage lost to follow‐up (total, PDT, open): 63, no details, no details |
|
| Notes | No cross‐over | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization method: computer generated number table |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: No _X_ Withdrawls: No _X_ Post‐random exclusion: No_X_ Intension‐to‐treat analysis: No _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Xu 2007.
| Methods | RCT Methods of concealment were unclear Blinding unclear |
|
| Participants | Number of participants/procedures (PDT/ST): 166/166 Gender male/female: 84/82 Mean age (years): PDT: 43.5 ± 14.2 (NS) ST: 49.2 ± 13.6 (NS) Population: no details APACHE II Score: PDT: no details ST: no details SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): no details Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): attending at least Procedure setting (location PDT performed/location ST performed): ICU/ICU Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: patients requiring tracheostomies Exclusion criteria: emergency tracheostomy, age <18 yrs, severe coagulopathy, surgical wounds near the tracheostomy site, previous or pre‐existing tracheostomy Treatment and control groups were not adequately described at entry (sex, age). |
|
| Interventions | Technique/method: PDT: Ciaglia/Cook, multiple dilator ST: no details Monitoring: bronchoscopy (53 cases), other no details Use of bronchoscopic guidance for PDT: yes (53 cases) PDT/ST: no details if heavy sedation, local infiltrative anaesthesia or general anaesthesia |
|
| Outcomes | Survival rate (%): PDT: 109/109 ST: 57/57 Days up to decannulation: PDT: no details ST: no details Lowest PaO2 (%): PDT: no details ST: no details Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT without BSK: 8.5 ± 1.1 PDT with BSK: 4:6 ± 1.2 ST: 28.7 ± 2.5 Complication rate, costs, time expenditure Length of follow‐up: no details Percentage lost to follow‐up (total, PDT, open): no details, no details |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method: unclear |
| Allocation concealment (selection bias) | Unclear risk | Methods of concealment were unclear |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: Unclear _X_ Withdrawls: No _X_ Post‐random exclusion: Unclear _X_ Intension‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Unclear__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
Youssef 2011.
| Methods | RCT Randomization method: computer generated number table Concealment was adequate |
|
| Participants | Number of participants/procedures (PDT/ST): 32/32 Gender male/female: PDT: 18/14 (NS) ST: 16/16 (NS) Mean age (years): PDT: 43.12 ± 15.3 (NS) ST: 41.58 ± 18.6 (NS) Population: intensive care patients, 22 patients were admitted to the ICU due to neurological disease, 20 patients had respiratory disease, 18 patients with cardiovascular disease and 4 patients due to head trauma APACHE II Score: PDT: 15 to 26 with mean 19.1 ST: 7 to 25 with mean 18.4 SAPS Score: PDT: no details ST: no details Period intubation up to tracheotomy (days): duration of endotracheal intubation ranged from 6‐21 days with mean 12.3 days Total number of operators (PDT/ST): no details Experience of the operators (PDT/ST): no details Procedure setting (location PDT performed/location ST performed): ICU/ICU Inclusion and exclusion criteria were clearly defined in the text Inclusion criteria: all adult patients from the ICU services requiring elective tracheostomy Exclusion criteria: previous neck operation, distorted anatomy , bleeding disorder, goiter, neck masses, unstable general condition, cervical spine trauma Treatment and control groups were not adequately described at entry (sex, age, APACHE) |
|
| Interventions | Technique/method: PT: Griggs/Portex, forceps and nasal speculum ST: Türkmen Monitoring: arterial blood gases, blood pressure, ECG, pulse oximetry Use of bronchoscopic guidance for PDT: no details PDT/ST: general anaesthesia |
|
| Outcomes | Survival rate (%): PDT: 32/32 ST: 32/32 Days up to decannulation: PDT ST: 14‐22 days, mean 16.3 Lowest PaO2 (%): PDT: 99.4 ± 0.6 ST: 99 ± 0.5 (NS) Stay in the hospital (days): PDT: no details ST: no details Operation time (min): PDT 20.1 ST: 19.3 Complication rate, time expenditure, length of scar Length of follow‐up: one year Percentage lost to follow‐up (total, PDT, open): no details, no details |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | RCT Randomization method: computer‐generated number table |
| Allocation concealment (selection bias) | Low risk | Concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Subject blinded: Yes__X__ Physician blinded: No__X__ Outcome assessor blinded: Unclear__X__ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes of patients who withdrew or were excluded after allocation were EITHER detailed separately OR included in an intention‐to‐treat analysis OR the text stated there were (no withdrawals) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective or incomplete reporting |
| Other bias | Unclear risk | Treatment and control groups were not adequately described at entry Patient selection: Unclear _X_ Withdrawls: No _X_ Post‐random exclusion: Unclear _X_ Intension‐to‐treat analysis: Unclear _X_ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Subject blinded: Yes__X__ Physician blinded: No__X__ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinded: Unclear__X__ |
APACHE = Acute Physiology And Chronic Health Evaluation Score ECG = electrocardiography ENT = ears, nose, throat surgeon GWDF = guide wire dilating forceps method ICU = intensive care unit INR = international normalized ratio (NS) = not significant PDT = percutaneous dilatational tracheostomy PT = percutaneous tracheostomy RCT = randomized controlled trial (S) = significant SAPS = Simplified Acute Physiology Score ST = surgical tracheostomy TLT = translaryngeal tracheostomy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beck 2007 | Not a study; comment on Silvester 2006 |
| Birbicer 2008 | Comparison of different percutaneous tracheostomy techniques |
| Bowen 2001 | Percutaneous versus surgical tracheotomy, but not RCT. A retrospective medical chart review was performed |
| Cianchi 2010 | Comparison of different percutaneous tracheostomy techniques |
| Goldenberg 2003 | Percutaneous versus surgical tracheotomy, but not RCT. A prospective study of 75 percutaneous dilatational tracheotomies and a retrospective study of 75 surgical tracheotomies were performed |
| Karvandian 2009 | Percutaneous versus surgical tracheotomy, but not RCT. Prospective clinical trial. No further details |
| Montcriol 2011 | Comparison of different percutaneous tracheostomy techniques |
| Muttini 1999 | Published twice; see Melloni 2002 |
| Pauliny 2012 | Observational study; not RCT |
| Remacle 2008 | Comparison of different percutaneous tracheostomy techniques |
| Sulaiman 2006 | Percutaneous versus surgical tracheotomy, but not RCT |
| Yurtseven 2007 | Comparison of different percutaneous tracheostomy techniques |
RCT = randomized controlled trial
Differences between protocol and review
The differences between the review and the published protocol (Brass 2009), are as follows.
We used Cochrane's new domain‐based evaluation to assess the validity and quality of the included studies. This was released after publication of the protocol.
We planned to performed sensitivity analysis regarding 'randomized versus quasi‐randomized' and possibly 'good quality studies versus poor quality studies' to test how sensitive the results are to reasonable changes in the assumptions that are made and in the protocol for combining the data. We have not undertaken the sensitivity analysis since almost all the included studies have a high risk of bias.
We planned to examine the outcomes 'patient satisfaction after a few months', 'discomfort during the procedure' or 'patient/caregiver satisfaction.' We have not undertaken these analyses since only one of the studies assessed patient satisfaction after a few months (Antonelli 2005), and none of the studies assessed discomfort during the procedure or patient/caregiver satisfaction.
In the methods, data synthesis section of the published protocol we stated that 'for dichotomous data, we will describe results both as a relative measure (relative risk (RR) with 95% confidence intervals (CIs)) and an absolute measure (number needed to treat and risk difference). Relative measures can be used to combine studies but absolute measures can be more informative than relative measures because they reflect the baseline risk as well as the change in risk with the intervention.' And 'we will analyse results using both fixed‐effect and random‐effects analysis because for each model there are situations where the result is counterintuitive.' In the review we used random‐effects models, i.e. the Mantel‐Haenszel method for dichotomous data (using risk ratio (RR) as effect measure) and the inverse variance method for continuous data (using SMD as effect measure), due to apparent between‐study heterogeneity, as assessed by Q and I2 statistics.
Bettina Kullmer is no longer an author of the review.
A new author (Anna Wrzosek) has joined the review team.
In the abstract and the objective section of the published protocol we wrote that 'we will evaluate the efficacy and safety of percutaneous techniques (PT) for tracheostomy as the primary objective.' In the sections of the review we write "... for elective tracheostomy in critically ill patients (adults and children)." And we added the following statement to the outcomes section "We excluded studies of tracheotomy in emergency situations, in not critically ill or homecare patients." In the protocol, we expressed ourselves imprecisely. We never intended to include emergency tracheotomies or tracheotomies in non‐critically ill patients.
We added the following statement to the outcomes section, "We differentiated between intraoperative, postoperative and long‐term complications. We included studies irrespective of whether all of this information was available."
In the protocol we planned to consider the following outcomes.
Primary outcomes: 1. mortality directly related to the procedure (measured as the proportion of patients who died intraoperatively or during the first 24 hours after the intervention) (*absolute numbers (N/N) and expressed as percentage (%) (*)); 2. serious, life‐threatening, adverse events e.g. major vascular injury or excessive bleeding (blood transfusion or need for additional surgical procedure), tracheal or oesophageal injury (detected by intraoperative bronchoscopy), loss of airway (loss of the tube or the tracheostoma tube > 20 sec.) or misplaced airway (paratracheal insertion of the tube or the tracheostoma tube), severe hypoxic episode (SaO2 < 90%) or cardiac arrest) (*).
Secondary outcomes: 1. non‐life threatening events during the procedure e.g. minimal or moderate bleeding (bleeding could be stopped by conservative measures), subcutaneous emphysema (detected during the first 24 hours by chest x‐ray), pneumothorax, pneumomediastinum (both detected by postoperative chest x‐ray), or difficult tube placement) (*); 2. postoperative complications e.g. pneumonia, atelectasis (detected by postoperative chest x‐ray), stomal infections (local inflammation, cellulitis or pus, necrosis or wound breakdown ‐ with or without antibiotic therapy), difficult tube change and hoarseness) (*); 3. late complications e.g. tracheal stenosis (mild 10% to 30%, moderate 30% to 50%, severe > 50%), tracheal malacia, delayed wound healing (> 3 weeks), cosmetic deformity (colour changes at the scar, level difference with the surrounding skin, surgical revision needed), tracheo‐cutaneous, ‐oesophageal fistula) (*); 4. patient/caregiver satisfaction (*); 5. duration of the procedure (minutes).
During our evaluation, we determined that it is more useful to look at the outcomes differentiated. For this reason, we decided to subdivide the outcomes and distinguish them as follows.
Primary outcomes: 1. Mortality directly related to the procedure: A. intraoperative mortality (measured as the proportion of patients who died intraoperatively); and B. postoperative mortality (measured as the proportion of patients who died during the first 24 hours after the intervention). 2. Serious, life‐threatening adverse events: A. Intraoperative serious, life‐threatening adverse events (major vascular injury or excessive bleeding (determined by need for blood transfusion or an additional surgical procedure)), tracheal or oesophageal injury (detected by intraoperative bronchoscopy), loss of the airway (loss of the tube or tracheostoma tube > 20 sec) or a misplaced airway (paratracheal insertion of the tube or the tracheostoma tube), a severe hypoxic episode, or cardiac arrest); and B. Direct postoperative serious, life‐threatening adverse events (major vascular injury or excessive bleeding (determined by need for blood transfusion or an additional surgical procedure), a severe hypoxic episode, or saturation < 90 %).
Secondary outcomes: 1. Non‐life threatening events: A. Intraoperative non‐life threatening events (minimal or moderate bleeding (where bleeding could be stopped by conservative measures)), subcutaneous emphysema (detected during the first 24 hours by chest x‐ray), cuff puncture, transient hypotension, pneumothorax or pneumomediastinum (both detected by postoperative chest x‐ray), cannula misplacement or difficult tube placement); B. Direct postoperative non‐life threatening events (pneumonia, atelectasis (detected by postoperative chest x‐ray), difficult tube change, tracheostomy tube occlusion/obstruction, accidental decannulation); and C. Late non‐life threatening events (tracheal stenosis, tracheal malacia, delayed wound healing, cosmetic deformity, tracheocutaneous or oesophageal fistula). 2. Total number of peri‐ and postoperative complications/adverse events. 3. Duration of the procedure. 4. Wound infection/stomatitis. 5. Unfavourable scarring. 6. Major bleeding. 7. Tracheostomy tube occlusion/obstruction, accidental decannulation, difficult tube change. 8. patient/caregiver satisfaction.
We think that this structure is clearer and easier to understand.
Contributions of authors
Patrick Brass (PB), Martin Hellmich (MH), Angelika Ladra (AL), Jürgen Ladra (JL), Anna Wrzosek (AW)
Conceiving the review: Professor Ulf Börner, JL, PB
Designing the review: JL, PB
Co‐ordinating the review: PB
Undertaking manual searches: JL, AL, PB
Undertaking electronic searches: BK
Screening search results: JL, AL, PB
Organizing retrieval of papers: JL, AL, PB
Screening retrieved papers against inclusion criteria: JL, AL, PB
Appraising quality of papers: PB, JL, AL
Abstracting data from papers: PB, JL
Writing to authors of papers for additional information: JL, AL
Providing additional data about papers: JL, AL
Obtaining and screening data on unpublished studies: PB, JL, AL
Data management for the review: MH
Entering data into Review Manager (RevMan 5): PB, JL
Analysis of data: PB, JL, MH, AW
Interpretation of data: PB, JL, MH, AW
Writing the review: PB, JL, AW
Performing previous work that was the foundation of the present study: JL, AL, PB
Guarantor for the review (one author): PB
Statistical analysis: PB, JL, MH, AW
Sources of support
Internal sources
Professor M Hellmich, Institute of Medical Statistics, Informatics and Epidemiology, University of Cologne, Germany.
Mrs Kullmer, public relations and mediated literature searching, local library services German Central Library for Medicine, University of Cologne, Germany.
External sources
Jane Cracknell, Managing Editor, Cochrane Anaesthesia Review Group, Denmark.
Karen Hovhannisyan, Trials Search Co‐ordinator, Cochrane Anaesthesia Review Group, Denmark.
Declarations of interest
Patrick Brass: None known.
Martin Hellmich: None known.
Angelika Ladra: None known.
Jürgen Ladra: None known.
Anna Wrzosek: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Ahn 1998 {published data only}
- Ahn JJ, Koh YS, Chin JY, Lee KM, Park W, Hong SB, et al. Comparison of clinical efficacy between percutaneous dilatational tracheostomy and surgical tracheostomy. Tuberculosis and Respiratory Diseases 1998;45(6):1277‐83. [Google Scholar]
Antonelli 2005 {published data only}
- Antonelli M, Michetti V, Palma A, Conti G, Pennisi MA, Arcangeli A, et al. Percutaneous translaryngeal versus surgical tracheostomy: A randomized trial with 1‐yr double blind follow‐up. Critical Care Medicine 2005;33(5):1015‐20. [DOI] [PubMed] [Google Scholar]
Crofts 1995 {published data only}
- Crofts SL, Alzeer A, McGuire GP, Wong DT, Charles D. A comparison of percutaneous and operative tracheostomy in intensive care patients. Canadian Journal of Anaesthesia = Journal Canadien d'anesthésie 1995;42(9):775‐9. [DOI] [PubMed] [Google Scholar]
Freeman 2001 {published data only}
- Freeman BD, Isabella K, Cobb JP, Boyle WA 3rd, Schmieg RE Jr, Kolleff MH, et al. A prospective, randomized study comparing percutaneous with surgical tracheostomy in critically ill patients. Critical Care Medicine 2001;29(5):926‐30. [DOI] [PubMed] [Google Scholar]
Friedman 1996 {published data only}
- Friedman Y, Fildes J, Mizock B, Samuel J, Patel S, Appavu S, et al. Comparison of percutaneous and surgical tracheostomies. Chest 1996;110(2):480‐5. [DOI] [PubMed] [Google Scholar]
Gysin 1990 {published data only}
- Gysin C, Dulguerov P, Guyot JP, Perneger TV, Abajo B, Chevrolet JC. Percutaneous versus surgical tracheostomy: a double‐blind randomized trial. Annals of Surgery 1999;230(5):708‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazard 1991 {published data only}
- Hazard P, Jones C, Benitone J. Comparative clinical trial of standard operative tracheostomy with percutaneous tracheostomy. Critical Care Medicine 1991;19(8):1018‐24. [DOI] [PubMed] [Google Scholar]
Heikkinen 2000 {published data only}
- Heikkinen M, Aarnio P, Hannukainen J. Percutaneous dilational tracheostomy or conventional surgical tracheostomy?. Critical Care Medicine 2000;28(5):1399‐402. [DOI] [PubMed] [Google Scholar]
Holdgaard 1998 {published data only}
- Holdgaard HO, Pedersen J, Jensen RH, Outzen KE, Midtgaard T, Johansen LV, et al. Percutaneous dilational tracheostomy versus conventional surgical tracheostomy. A clinical randomised study. Acta Anaesthesiologica Scandinavica 1998;42(5):545‐50. [DOI] [PubMed] [Google Scholar]
Lukas 2007 {published data only}
- Lukas J, Duskova J, Lukas D, Paska J, Stritesky M, Haas T. Standard surgical versus percutaneous dilatational tracheostomy in intensive care patients. Saudi Medical Journal 2007;28(10):1529‐33. [PUBMED: 17914514 ] [PubMed] [Google Scholar]
Massick 2001 {published data only}
- Massick DD, Yao S, Powell DM, Griesen D, Hobgood T, Allen JN, et al. Bedside tracheostomy in the intensive care unit: a prospective randomized trial comparing open surgical tracheostomy with endoscopically guided percutaneous dilational tracheotomy. The Laryngoscope 2001;111(3):494‐500. [DOI] [PubMed] [Google Scholar]
Melloni 2002 {published data only}
- Melloni G, Muttini S, Gallioli G, Carretta A, Cozzi S, Gemma M, et al. Surgical tracheostomy versus percutaneous dilational tracheostomy: A prospective randomized study with long‐term follow‐up. The Journal of Cardiovascular Surgery 2002;43(1):113‐21. [PubMed] [Google Scholar]
- Muttini S, Melloni G, Gemma M, Casati A, Carretta A, Giudici D, et al. Percutaneous or surgical tracheotomy. Prospective, randomized comparison of the incidence of early and late complications [Tracheotomia percutanea e chiurgica. Confronto prospettico, randomizzato dellincidenza di complicanze precoci e tardive]. Minerva Anestesiologica 1999;65(7‐8):521‐7. [PUBMED: 10479839] [PubMed] [Google Scholar]
Porter 1999 {published data only}
- Porter JM, Ivatury RR. Preferred route of tracheostomy ‐ percutaneous versus open at the bedside: a randomized, prospective study in the surgical intensive care unit. The American Surgeon 1999;65(2):142‐6. [PubMed] [Google Scholar]
Raine 1999 {published data only}
- Raine RI, Michell WL, Ruttman TG, Bloch MB, Thorp MA. Late outcome after guide‐wire forceps percutaneous tracheostomy ‐ a prospective randomised comparison with open surgical tracheostomy. BMJ 1999;82 (Suppl 1):168. [Google Scholar]
Silvester 2006 {published data only}
- Silvester W, Goldsmith D, Uchino S, Bellomo R, Knight S, Seevanayagam S, et al. Percutaneous versus surgical tracheostomy: A randomized controlled study with long‐term follow‐up. Critical Care Medicine 2006;34(8):2145‐52. [DOI] [PubMed] [Google Scholar]
Sustic 2002 {published data only}
- Sustić A, Krstulović B, Eskinja N, Zelić M, Ledić D, Turina D. Surgical tracheostomy versus percutaneous dilational tracheostomy in patients with anterior cervical spine fixation: preliminary report. Spine 2002;27(17):1942‐5. [DOI] [PubMed] [Google Scholar]
Tabaee 2005 {published data only}
- Tabaee A, Geng E, Lin J, Kakoullis S, McDonald B, Rodriguez H, et al. Impact of neck length on the safety of percutaneous and surgical tracheotomy: a prospective, randomized study. The Laryngoscope 2005;115(9):1685‐90. [DOI] [PubMed] [Google Scholar]
Wu 2003 {published data only}
- Wu JJ, Huang MS, Tang GJ, Shih SC, Yang CC, Kao WF, et al. Percutaneous dilatational tracheostomy versus open tracheostomy ‐ a prospective, randomized, controlled trial. Journal of the Chinese Medical Association 2003;66(8):467‐73. [PubMed] [Google Scholar]
Xu 2007 {published data only}
- Xu JG, Chen XG, Pan YJ. Comparison between percutaneous dilational tracheotomy and surgical tracheotomy [article in Chinese]. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese Journal of Otorhinolaryngology Head and Neck Surgery 2007;42(11):869‐70. [PUBMED: 18300458] [PubMed] [Google Scholar]
Youssef 2011 {published data only}
- Youssef TF, Ahmed MR, Saber A. Percutaneous dilatational versus conventional surgical tracheostomy in intensive care patients. North American Journal of Medical Sciences 2011;3(11):508‐12. [DOI: 10.4297/najms.2011.3508.; PUBMED: 22361497] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Beck 2007 {published data only}
- Beck A. Percutaneous versus surgical tracheotomy [Perkutane versus chirurgische tracheotomie]. Der Anaesthesist 2007;56(3):281‐2. [Google Scholar]
Birbicer 2008 {published data only}
- Birbicer H, Doruk N, Yapici D, Atici S, Altunkan AA, Epozdemir S, et al. Percutaneous tracheostomy: a comparison of PercuTwist and multi‐dilatators techniques. Annals of Cardiac Anaesthesia 2008;11(2):131. [DOI] [PubMed] [Google Scholar]
Bowen 2001 {published data only}
- Bowen CP, Whitney LR, Truwit JD, Durbin CG, Moore MM. Comparison of safety and cost of percutaneous versus surgical tracheostomy. The American Surgeon 2001;67(1):54‐60. [PubMed] [Google Scholar]
Cianchi 2010 {published data only}
- Cianchi G, Zagli G, Bonizzoli M, Batacchi S, Cammelli R, Biondi S, et al. Comparison between single‐step and balloon dilatational tracheostomy in intensive care unit: a single‐centre, randomized controlled study. British Journal of Anaesthesia 2010;104(6):728‐32. [DOI] [PubMed] [Google Scholar]
Goldenberg 2003 {published data only}
- Goldenberg D, Golz A, Huri A, Netzer A, Joachims HZ, Bar‐Lavie Y. Percutaneous dilation tracheotomy versus surgical tracheotomy: our experience. Otolaryngology and Head and Neck Surgery 2003;128(3):358‐63. [DOI] [PubMed] [Google Scholar]
Karvandian 2009 {published data only}
- Karvandian K, Mahmoodpoor A, Beigmohammadi M, Sanaie S. Complications and safety of percutaneous dilatational tracheostomy with griggs method versus surgical tracheostomy: a prospective trial with six months follow‐up. Pakistan Journal of Medical Sciences 2009;25(1):41‐5. [Google Scholar]
Montcriol 2011 {published data only}
- Montcriol A, Bordes J, Asencio Y, Prunet B, Lacroix G, Meaudre E. Bedside percutaneous tracheostomy: a prospective randomised comparison of PercuTwist versus Griggs' forceps dilational tracheostomy. Anaesthesia and Intensive Care 2011;39(2):209‐16. [DOI] [PubMed] [Google Scholar]
Muttini 1999 {published data only}
- Muttini S, Melloni G, Gemma M, Casati A, Carretta A, Giudici D, et al. Percutaneous or surgical tracheotomy. Prospective, randomized comparison of the incidence of early and late complications [Tracheotomia percutanea e chiurgica. Confronto prospettico, randomizzato dellincidenza di complicanze precoci e tardive]. Minerva Anestesiologica 1999;65(7‐8):521‐7. [PUBMED: 10479839] [PubMed] [Google Scholar]
Pauliny 2012 {published data only}
- Pauliny M, Christova E, Mackova J, Liska M. Percutaneous dilation tracheostomy versus surgical tracheostomy in critically ill patients. Bratislava Medical Journal 2012;113(7):409‐11. [PUBMED: 22794514] [DOI] [PubMed] [Google Scholar]
Remacle 2008 {published data only}
- Remacle M, Lawson G, Jamart J, Trussart C, Bulpa P. Comparison between the Percutwist and the Ciaglia percutaneous tracheotomy techniques. European Archives of Otorhinolaryngology 2008;265(12):1515‐9. [DOI] [PubMed] [Google Scholar]
Sulaiman 2006 {published data only}
- Sulaiman L, Tighe SQ, Nelson RA. Surgical vs wire‐guided cricothyroidotomy: a randomised crossover study of cuffed and uncuffed tracheal tube insertion. Anaesthesia 2006;61(6):565‐70. [DOI] [PubMed] [Google Scholar]
Yurtseven 2007 {published data only}
- Yurtseven N, Aydemir B, Karaca P, Aksoy T, Komurcu G, Kurt M, et al. PercuTwist: a new alternative to Griggs and Ciaglia's techniques. European Journal of Anaesthesiology 2007;24(6):492‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Alansari 2015
- Alansari M, Alotair H, Al Aseri Z, Elhoseny MA. Use of ultrasound guidance to improve the safety of percutaneous dilatational tracheostomy: a literature review. Critical Care 2015;18(19):229. [DOI: 10.1186/s13054-015-0942-5; PUBMED: 25981550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 1997
- Alexander R, Pappachan J. Timing of surgical tracheostomy after failed percutaneous tracheostomy [letter]. Anaesthesia and Intensive Care 1997; Vol. 25, issue 1:91. [ISSN: 0310‐057X; PUBMED: 9075528] [PubMed]
Ambesh 2002
- Ambesh SP, Pandey CK, Srivastava S, Agarwal A, Singh DK. Percutaneous tracheostomy with single dilatation technique: a prospective, randomized comparison of Ciaglia blue rhino versus Griggs' guidewire dilating forceps. Anesthesia and Analgesia 2002;95(6):1739‐45. [PUBMED: 12456450] [DOI] [PubMed] [Google Scholar]
Arabi 2004
- Arabi Y, Haddad S, Shirawi N, Al Shimemeri A. Early tracheostomy in intensive care trauma patients improves resource utilization: a cohort study and literature review. Critical Care 2004;8(5):R347‐52. [PUBMED: 15469579] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Añón 2004
- Añón JM, Escuela MP, Gómez V, García de Lorenzo A, Montejo JC, López J. Use of percutaneous tracheostomy in intensive care units in Spain. Results of a national survey. Intensive Care Medicine 2004;30(6):1212‐5. [PUBMED: 15118816] [DOI] [PubMed] [Google Scholar]
Barba 1995
- Barba CA, Angood PB, Kauder DR, Latenser B, Martin K, McGonigal, et al. Bronchoscopic guidance makes percutaneous tracheostomy a safe, cost‐effective and easy‐to‐teach procedure. Surgery 1995;118(5):879‐83. [ISSN: 0039‐6060; PUBMED: 7482276] [DOI] [PubMed] [Google Scholar]
Beckmann 2004
- Beckmann U, Gillies DM, Berenholtz SM, Wu AW, Pronovost P. Incidents relating to the intra‐hospital transfer of critically ill patients. An analysis of the reports submitted to the Australian incident monitoring study in intensive care. Intensive Care Medicine 2004;30(8):1579‐85. [PUBMED: 14991102] [DOI] [PubMed] [Google Scholar]
Blondonnet 2014
- Blondonnet R, Chabanne R, Godet T, Pascal J, Pereira B, Kauffmann S, et al. Tracheostomy in French ICUs and patient outcome: national opinion survey [Trachéotomies en réanimation et devenir des patients: enquête déclarative nationale]. Annales Françaises d'Anesthèsie et de Rèanimation 2014;33(4):227‐31. [DOI: 10.1016/j.annfar.2014.01.020; PUBMED: 24636791] [DOI] [PubMed] [Google Scholar]
Blot 2005
- Blot F, Melot C. Indications, timing, and techniques of tracheostomy in 152 French ICUs. Chest 2005;127(4):1347‐52. [PUBMED: 15821214] [DOI] [PubMed] [Google Scholar]
Bodenham 1992
- Bodenham A, Diament R, Cohen A, Webster N. Percutaneous dilatational tracheostomy. A bedside procedure on the intensive care unit. Anaesthesia 1992;46(7):570‐2. [ISSN: 0003‐2409; PUBMED: 1862899] [DOI] [PubMed] [Google Scholar]
Brambrink 2004
- Bambrink A. Percutaneous dilatation tracheostomy: which technique is the best for critically ill patient, and how can we gather further scientific evidence?. Critical Care 2004;8(5):R299‐305. [PUBMED: 15469590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byhahn 2000
- Byhahn C, Lischke V, Halbig S, Scheifler G, Westphal K. Ciaglia blue rhino: a modified technique for percutaneous dilatation tracheostomy. Technique and early clinical results [Ciaglia Blue Rhino: Ein weiter entwickeltes Verfahren der perkutanen Dilatationstracheotomie]. Anaesthesist 2000;49(3):202‐6. [PUBMED: 10788989] [DOI] [PubMed] [Google Scholar]
Byhahn 2001
- Byhahn C, Wilke HJ, Lischke V, Rinne T, Westphal K. Bedside percutaneous tracheostomy: clinical comparison of Griggs and Fantoni techniques. World Journal of Surgery 2001;25(3):296‐301. [PUBMED: 11343179] [DOI] [PubMed] [Google Scholar]
Cabrini 2012
- Cabrini L, Monti G, Landoni G, Biondi‐Zoccai G, Boroli F, Mamo D, et al. Percutaneous tracheostomy, a systematic review. Acta Anaesthesiologica Scandinavica 2012;56(3):270‐81. [DOI: 10.1111/j.1399-6576.2011.02592.x.; PUBMED: 22188176] [DOI] [PubMed] [Google Scholar]
Cabrini 2014
- Cabrini L, Landoni G, Greco M, Costagliola R, Monti G, Colombo S, et al. Single dilator vs. guide wire dilating forceps tracheostomy: a meta‐analysis of randomised trials. Acta Anaesthesiologica Scandinavica 2014;58(2):135‐42. [DOI: 10.1111/aas.12213; PUBMED: 24410105] [DOI] [PubMed] [Google Scholar]
Caruso 1997
- Caruso DM, al‐Kasspooles MF, Mathews MR, Weiland DE, Schiller WR. Rationale for "early" percutaneous dilational tracheostomy in patients with burn injuries. The Journal of Burn Care and Rehabilitation 1997;18(5):424‐8. [ISSN: 0273‐8481; PUBMED: 9313124] [DOI] [PubMed] [Google Scholar]
Cheng 2000
- Cheng E, Fee WE Jr. Dilatational versus standard tracheostomy: a meta‐analysis. The Annals of Otology, Rhinology, and Laryngology 2000;109(9):803‐7. [PUBMED: 11007080] [DOI] [PubMed] [Google Scholar]
Chew 1972
- Chew JY, Cantrell RW. Tracheostomy. Complications and their management. Archives of Otolaryngology 1972;96(6):538‐45. [ISSN: 0003‐9977; PUBMED: 4621255] [DOI] [PubMed] [Google Scholar]
Ciaglia 1985
- Ciaglia P, Firsching R, Syniec C. Elective percutaneous dilatational tracheostomy. A new simple bedside procedure; preliminary report. Chest 1985;87(6):715‐9. [ISSN: 0012‐3692; PUBMED: 3996056] [DOI] [PubMed] [Google Scholar]
Cools‐Lartigue 2013
- Cools‐Lartigue J, Aboalsaud A, Gill H, Ferri L. Evolution of percutaneous dilatational tracheostomy‐ a review of current techniques and their pitfalls. World journal of surgery 2013;37(7):1633‐46. [DOI: 10.1007/s00268-013-2025-6; PUBMED: 23571862.] [DOI] [PubMed] [Google Scholar]
Cooper 1998
- Cooper RM. Use and safety of percutaneous tracheostomy in intensive care. Report of a postal survey of ICU practice. Anaesthesia 1998;53(12):1209‐12. [PUBMED: 10193228] [DOI] [PubMed] [Google Scholar]
Delaney 2006
- Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta‐analysis. Critical Care 2006;10(2):R55. [PUBMED: 16606435] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dennis 2013
- Dennis BM, Eckert MJ, Gunter OL, Morris JA Jr, May AK. Safety of bedside percutaneous tracheostomy in the critically ill: evaluation of more than 3,000 procedures. Journal of the American College of Surgeons 2013;216(4):858‐65. [10.1016/j.jamcollsurg.2012.12.017; PUBMED: 23403139] [DOI] [PubMed] [Google Scholar]
Deppe 2013
- Deppe AC, Kuhn E, Scherner M, Slottosch I, Liakopoulos O, Langebartels G, et al. Coagulation disorders do not increase the risk for bleeding during percutaneous dilatational tracheotomy. The Thoracic and Cardiovascular Surgeon 2013;61(3):234‐9. [DOI: 10.1055/s-0032-1322608; PUBMED: 23344764] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309(6964):1286‐91. [PUBMED: 7718048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Douglas 1999
- Douglas WE, Flabouris A. Surgical emphysema following percutaneous tracheostomy. Anaesthesia and Intensive Care 1999;27(1):69‐72. [ISSN: 0310‐057X; PUBMED: 10050229] [DOI] [PubMed] [Google Scholar]
Dulgerov 1999
- Dulguerov P, Gysin C, Perneger TV, Chevrolet JC. Percutaneous or surgical tracheostomy: a meta‐analysis. Critical Care Medicine 1999;27(8):1617‐25. [PUBMED: 10470774] [DOI] [PubMed] [Google Scholar]
Durbin 2010
- Durbin CG Jr. Tracheostomy: why, when, and how?. Respiratory Care 2010;55(8):1056‐68. [PUBMED: 20667153] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [PUBMED: 9432252] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fantoni 1997
- Fantoni A, Ripamonti D. A non‐derivative, non‐surgical tracheostomy: the translaryngeal method. Intensive Care Medicine 1997;23(4):386‐92. [ISSN: 0342‐4642; PUBMED: 9142576] [DOI] [PubMed] [Google Scholar]
Fikkers 2003
- Fikkers BG, Fransen GA, Hoeven JG, Briede IS, Hoogen FJ. Tracheostomy for long‐term ventilated patients: a postal survey of ICU practice in The Netherlands. Intensive Care Medicine 2003;29(8):1390‐3. [PUBMED: 12879247] [DOI] [PubMed] [Google Scholar]
Fischler 2000
- Fischler L, Erhart S, Kleger GR, Frutiger A. Prevalence of tracheostomy in ICU patients. A nation‐wide survey in Switzerland. Intensive Care Medicine 2000;26:1428‐33. [PUBMED: 11126252] [DOI] [PubMed] [Google Scholar]
Freeman 2000
- Freeman BD, Isabella K, Lin N, Buchman TG. A meta‐analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest 2000;118(5):1424‐8. [ISSN: 0012‐3692; PUBMED: 11083694] [DOI] [PubMed] [Google Scholar]
Friedman 1996a
- Friedman Y. Indications, timing, techniques, and complications of tracheostomy in the critically ill patients. Current Opinion in Critical Care 1996;2(1):47‐53. [Google Scholar]
Friedman 1996b
- Friedman Y, Fildes J, Mizock B, Samuel J, Patel S, Appavau S, et al. Comparison of percutaneous and surgical tracheostomy. Chest 1996;110(2):480‐5. [ISSN: 0012‐3692; PUBMED: 8697854] [DOI] [PubMed] [Google Scholar]
Frova 2002
- Frova G, Quintel M. A new simple method for percutaneous tracheostomy: controlled rotating dilatation. A preliminary report. Intensive Care Medicine 2002;28(3):299‐303. [ISSN: 0342‐4642] [DOI] [PubMed] [Google Scholar]
Glasziou 2001
- Glasziou P, Irwig L, Bain C, Colditz G. Systematic Reviews in Health Care: A Practical Guide. 1st Edition. Cambridge, UK: Cambridge University Press, 2001. [NLM ID: 101133142] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEproGDT: GRADEpro Guideline Development Tool [www.guidelinedevelopment.org]. Hamilton: McMaster University (developed by Evidence Prime, Inc.), 2015.
Griffiths 2005
- Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta‐analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ 2005;28(330):1243. [PUBMED: 15901643] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griggs 1990
- Griggs WM, Worthley LI, Gilligan JE, Thomas PD, Myburg JA. A simple percutaneous tracheostomy technique. Surgery 1990;170(6):543‐5. [ISSN: 0039‐6087; PUBMED: 2343371] [PubMed] [Google Scholar]
Griggs 1991
- Griggs WM, Myburgh JA, Worthley LI. A prospective comparison of a percutaneous tracheostomy technique with standard surgical tracheostomy. Intensive Care Medicine 1991;17(5):261‐3. [ISSN: 0342‐4642; PUBMED: 1939869] [DOI] [PubMed] [Google Scholar]
Gromann 2009a
- Gromann TW, Birkelbach O, Hetzer R. Balloon dilatational tracheostomy: initial experience with the Ciaglia Blue Dolphin method. Anesthesia and Analgesia 2009;108(6):1862‐6. [DOI: 10.1213/ane.0b013e3181a1a494; PUBMED: 19448213] [DOI] [PubMed] [Google Scholar]
Gromann 2009b
- Gromann TW, Birkelbach O, Hetzer R. Ballon dilatational tracheostomy. Technique and first clinical experience with the Ciaglia Blue Dolphin method [Tracheotomie mittels Ballondilatation]. Der Chirurg 2009;80(7):622‐7. [DOI: 10.1007/s00104-008-1651-2; PUBMED: 19050838] [DOI] [PubMed] [Google Scholar]
Groves 2007
- Groves DS, Durbin CG Jr. Tracheostomy in the critically ill: indications, timing and techniques. Current Opinion in Critical Care 2007;13(1):90‐7. [PUBMED: 17198055] [DOI] [PubMed] [Google Scholar]
Guimares 2009
- Guimaraes MM, Dib R, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006058.pub2] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐YtterY, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians. BMJ 2008;336:995–8. [PUBMED: 18456631]] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guzman 1995
- Guzman J, Bander J, Weinmann MD. Percutaneous dilational tracheostomy: a safe technique in patients at risk for bleeding. American Journal of Respiratory and Critical Care Medicine 1995;151:A489. [Google Scholar]
Gysin 1999
- Gysin C, Dulguerov P, Guyot JP, Perneger TV, Abajo B, Chevrolet JC. Percutaneous versus surgical tracheostomy. A double‐blind randomized trial. Annals of Surgery 1999;230(5):708‐14. [PUBMED: 10561096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazard 1988
- Hazard PB, Garrett H Jr, Adams JW, Robbins ET, Aguillard RN. Bedside percutaneos tracheostomy: experience with 55 elective procedures. The Annals of Thoracic Surgery 1988;46(1):63‐7. [PUBMED: 3382289] [DOI] [PubMed] [Google Scholar]
Heffner 1986a
- Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 1: Indications, technique, management. Chest 1986;90(2):269‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heffner 1986b
- Heffner JE, Miller KS, Sahn SA. Tracheostomy in the intensive care unit. Part 2: Complications. Chest 1986;90(3):430‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heffner 1988
- Heffner JE. Tracheal intubation in mechanically ventilated patients. Clinics in Chest Medicine 1988;9(1):23‐35. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2007
- Higgins KM, Punthakee X. Meta‐analysis comparison of open versus percutaneous tracheostomy. Laryngoscope 2007;117(3):447‐54. [PUBMED: 17334304] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 1996
- Hill BB, Zweng TN, Maley RH, Charash WE, Toursarkissian B, Kearney PA. Percutaneous dilational tracheostomy: report of 356 cases. Journal of Trauma 1996;41(2):238‐43. [PUBMED: 8760530] [DOI] [PubMed] [Google Scholar]
Iwanaka 1997
- Iwanaka T, Arkovitz MS, Arya G, Ziegler MM. Evaluation of operative stress and peritoneal macrophage function in minimally invasive operations. Journal of the American College of Surgeons 1997;184(4):357‐63. [PUBMED: 9100680] [PubMed] [Google Scholar]
Kaiser 2006
- Kaiser E, Cantais E, Goutorbe P, Salinier L, Palmier B. Prospective randomized comparison of progressive dilational vs forceps dilational percutaneous tracheostomy. Anaesthesia and Intensive Care 2006;34(1):51‐4. [PUBMED: 16494150] [DOI] [PubMed] [Google Scholar]
Kaloud 1997
- Kaloud H, Smolle‐Juettner F, Prause G, List WF. Iatrogenic rupture of the tracheobronchial tree. Chest 1997;112(3):774‐8. [PUBMED: 9315814] [DOI] [PubMed] [Google Scholar]
Kane 1997
- Kane TD, Rodriquez JL, Luchette FA. Early versus late tracheostomy in the trauma patient. Respiratory Care Clinics of North America 1997;3(1):1‐20. [PUBMED: 9390900] [PubMed] [Google Scholar]
Kluge 2008
- Kluge S, Baumann HJ, Maier C, Klose H, Myer A, Nierhaus A, et al. Tracheostomy in the intensive care unit: a nationwide survey. Anesthesia and Analgesia 2008;107(5):1639‐43. [DOI: 10.1213/ane.0b013e318188b818; PUBMED: 18931225] [DOI] [PubMed] [Google Scholar]
Kollef 1999
- Kollef MH, Ahrens TS, Shannon W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Critical Care Medicine 1999;27(9):1714‐20. [PUBMED: 10507588] [DOI] [PubMed] [Google Scholar]
Kornblith 2011
- Kornblith LZ, Burlew CC, Moore EE, Haenel JB, Kashuk JL, Biffl WL, et al. One thousand bedside percutaneous tracheostomies in the surgical intensive care unit: time to change the gold standard. Journal of the American College of Surgeons 2011;212(2):163‐70. [DOI: 10.1016/j.jamcollsurg.2010.09.024; PUBMED: 21193331] [DOI] [PubMed] [Google Scholar]
Krishnan 2005
- Krishnan K, Elliot SC, Mallick A. The current practice of tracheostomy in the United Kingdom: a postal survey. Anaesthesia 2005;60(4):360‐4. [PUBMED: 15766339] [DOI] [PubMed] [Google Scholar]
Lau 1998
- Lau J, Ioannidis JPA, Schmid CH. Quantitative synthesis in systematic reviews. In: Mulrow C, Cook D editor(s). Systematic Reviews: synthesis of best evidence for healthcare decisions. 1st Edition. Philadelphia: American College of Physicians, 1998:91‐101. [Google Scholar]
Lebiedz 2010
- Lebiedz P, Suca A, Gümüs E, Radke RM, Kaya E, Hilker E, et al. 7‐year survey after percutaneous dilatational tracheotomy on a medical intensive care unit. Journal of Investigative Medicine High Impact Case Reports 2010;58(8):977‐81. [DOI: 10.231/JIM.0b013e3181f65ca3; PUBMED: 20926957] [DOI] [PubMed] [Google Scholar]
Lefebvre 2001
- Lefebvre C, Clarke M. Identifying randomised trials. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London (UK): BMJ Publishing Group, 2001. [NLM ID: 101093083] [Google Scholar]
Leinhardt 1992
- Leinhardt DJ, Mughal M, Bowles B, Glew R, Kishen R, MacBeath J. Appraisal of percutaneous tracheostomy. The British Journal of Surgery 1992;79(3):255‐8. [PUBMED: 1555095] [DOI] [PubMed] [Google Scholar]
Light 1984
- Light RJ, Pillemer DB. Summing Up: The Science of Reviewing Research. 1st Edition. Cambridge, Massachusetts: Harvard University Press, 1984. [NLM ID: 8505340] [Google Scholar]
Lovell 2001
- Lovell MA, Mudaliar MY, Klineberg PL. Intrahospital transport of critically ill patients: complications and difficulties. Anaesthesia and Intensive Care 2001;29(4):400‐5. [PUBMED: 11512652] [DOI] [PubMed] [Google Scholar]
Malthaner 1998
- Malthaner RA, Telang H, Miller JD. Percutaneous tracheostomy: is it really better?. Chest 1998;144(6):1771‐2. [PUBMED: 9872217] [DOI] [PubMed] [Google Scholar]
Marelli 1990
- Marelli D, Paul A, Manolidis S, Walsh G, Odim JN, Burdon TA. Endoscopic guided percutaneous tracheostomy: early results of a consecutive trial. Journal of Trauma 1990;30(4):433‐5. [PUBMED: 2325175] [PubMed] [Google Scholar]
McCague 2012
- McCague A, Aljanabi H, Wong DT. Safety analysis of percutaneous dilational tracheostomies with bronchoscopy in the obese patient. Laryngoscope 2012;122(5):1031‐4. [DOI: 10.1002/lary.22505; PUBMED: 22294288] [DOI] [PubMed] [Google Scholar]
Melker 1992
- Melker RJ, Gallagher TJ. Transport of the critically ill/injured patient. In: Civetta JM, Taylor RW, Kirby RR editor(s). Critical Care. 2nd Edition. Philadelphia: J B Lippincott, 1992:1797‐808. [Google Scholar]
Moher 2015
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA, PRISMA‐P Group. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Systematic reviews 2015;Jan 1(4):1. [DOI: 10.1186/2046-4053-4-1; PUBMED: 25554246] [DOI] [PMC free article] [PubMed] [Google Scholar]
MRC 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: new guidance. www.mrc.ac.uk/complexinterventionsguidance (accessed September 2014).
Mulder 1969
- Mulder DS, Rubush JL. Complications of tracheostomy: relationship to long term ventilatory assistance. Journal of Trauma 1969;9(5):389‐402. [PUBMED: 5771756] [PubMed] [Google Scholar]
Nates 2000
- Nates JL, Cooper DJ, Myles PS, Scheinkestel CD, Tuxen DV. Percutaneous tracheostomy in critically ill patients: a prospective, randomized comparison of two techniques. Critical Care Medicine 2000;28(11):3734‐9. [PUBMED: 11098982] [DOI] [PubMed] [Google Scholar]
Oberwalder 2004
- Oberwalder M, Weis H, Nehoda H, Kafka‐Ritsch R, Bonatti H, Prommegger R, et al. Videobronchoscopicguidance makes percutaneous dilational tracheostomy safer. Surgical Endoscopy 2004;18(5):839‐42. [PUBMED: 15216870] [DOI] [PubMed] [Google Scholar]
Pandian 2010
- Pandian V, Vaswani RS, Mirski MA, Haut E, Gupta S, Bhatti NI. Safety of percutaneous dilational tracheostomy in coagulopathic patients. Ear, Nose, & Throat Journal 2010;89(8):387‐95. [PUBMED: 20737378] [PubMed] [Google Scholar]
Paul 1989
- Paul A, Marelli D, Chiu RC, Vestweber KH, Mulder DS. Percutaneous endoscopic tracheostomy. The Annals of Thoracic Surgery 1989;47(2):314‐5. [PUBMED: 2919922] [DOI] [PubMed] [Google Scholar]
Petros 1997
- Petros S, Engelmann L. Percutaneous dilatational tracheostomy in a medical ICU. Intensive Care Medicine 1997;23(6):630‐4. [PUBMED: 9255641] [DOI] [PubMed] [Google Scholar]
Pope 2003
- Pope C, Smith A, Goodwin D, Mort M. Passing on tacit knowledge in anaesthesia: a qualitative study. Medical Education 2003;37:650‐5. [PUBMED: 12925468] [DOI] [PubMed] [Google Scholar]
Pothman 1997
- Pothmann W, Tonner PH, Schulte am Esch J. Percutaneous dilatational tracheostomy: risks and benefits. Intensive Care Medicine 1997;23(6):610‐2. [PUBMED: 9255636] [DOI] [PubMed] [Google Scholar]
Powell 1998
- Powell DM, Price PD, Forrest LA. Review of percutaneous tracheostomy. Laryngoscope 1998;108(2):170‐7. [PUBMED: 947306] [DOI] [PubMed] [Google Scholar]
Putensen 2014
- Putensen C, Theuerkauf N, Guenther U, Vargas M, Pelosi P. Percutaneous and surgical tracheostomy in critically ill adult patients: a meta‐analysis. Critical Care 2014;18(6):544. [DOI: 10.1186/s13054-014-0544-7; PUBMED: 25526983] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 2002
- Robinson KA, Dickersin K. Development of a highly sensitive search strategy for the retrieval of controlled trials using PubMed. International Journal of Epidemiology 2002;31:150‐3. [PUBMED: 11914311] [DOI] [PubMed] [Google Scholar]
Rosseland 2011
- Rosseland LA, Laake JH, Stubhaug A. Percutaneous dilatational tracheotomy in intensive care unit patients with increased bleeding risk or obesity. A prospective analysis of 1000 procedures. Acta Anaesthesiologica Scandinavica 2011;55(7):835‐41. [DOI: 10.1111/j.1399-6576.2011.02458.x; PUBMED: 21615346] [DOI] [PubMed] [Google Scholar]
Rudas 2012
- Rudas M, Seppelt I. Safety and efficacy of ultrasonography before and during percutaneous dilatational tracheostomy in adult patients: a systematic review. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine 2012;14(4):297‐301. [PUBMED: 23230879] [PubMed] [Google Scholar]
Rumbak 2004
- Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients. Critical Care Medicine 2004;32(8):1689‐94. [PUBMED: 15286545] [DOI] [PubMed] [Google Scholar]
Schachner 1989
- Schachner A, Ovil Y, Sidi J, Rogev M, Heilbronn Y, Levy M. Percutaneous tracheostomy: a new method. Critical Care Medicine 1989;17(10):1052‐6. [PUBMED: 2791567] [DOI] [PubMed] [Google Scholar]
Sheldon 1955
- Sheldon CH, Pudenz RH, Freshwater DB, Crue BL. A new method for tracheostomy. Journal of Neurosurgery 1955;12(4):428‐31. [PUBMED: 14392499] [DOI] [PubMed] [Google Scholar]
Shirawi 2005
- Shirawi N, Arabi Y. Bench‐to‐bedside review: early tracheostomy in critically ill trauma patients. Critical Care 2006;10(1):201. [PUBMED: 16356202] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skaggs 1969
- Skaggs JA, Cogbill CL. Tracheostomy: management, mortality, complications. The American Surgeon 1969;35(6):393‐6. [PUBMED: 5770204] [PubMed] [Google Scholar]
Smith 2006
- Smith AF, Pope C, Goodwin D, Mort M. What defines expertise in regional anaesthesia? An observational analysis of practice. British Journal of Anaesthesia 2006;97:401‐7. [PUBMED: 16835256] [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith AF. In search of excellence in anesthesiology. Anesthesiology 2009;110:4‐5. [DOI: 10.1097/ALN.0b013e318190b263] [DOI] [PubMed] [Google Scholar]
Smith 2010
- Smith AF, Greaves JD. Beyond competence: defining and promoting excellence in anaesthesia. Anaesthesia 2010;65:184‐91. [PUBMED: 20003114] [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith AF, Glavin R, Greaves JD. Defining excellence in anaesthesia: the role of personal qualities and practice environment. British Journal of Anaesthesia 2011;106:38‐43. [PUBMED: 21118845] [DOI] [PubMed] [Google Scholar]
Stauffer 1981
- Stauffer JL, Olson DE, Petty TL. Complications and consequences of endotracheal intubation and tracheostomy. A prospective study of 150 critically ill adult patients. American Journal of Medicine 1981;70(1):65‐76. [PUBMED: 7457492] [DOI] [PubMed] [Google Scholar]
Stock 1986
- Stock MC, Woodward CG, Shapiro BA, Cane RD, Lewis V, Pecaro B. Perioperative complications of elective tracheostomy in critically ill patients. Critical Care Medicine 1986;14(10):861‐3. [PUBMED: 3757526] [DOI] [PubMed] [Google Scholar]
Sustic 2007
- Sustic A. Role of ultrasound in the airway management of critically ill patients. Critical Care Medicine 2007;35(5 Suppl):S173‐7. [PUBMED: 17446776] [DOI] [PubMed] [Google Scholar]
Takahashi 2014
- Takahashi M, Itagaki S, Laskaris J, Filsoufi F, Reddy RC. Percutaneous tracheostomy can be safely performed in patients with uncorrected coagulopathy after cardiothoracic surgery. Innovations 2014;9(1):22‐6. [DOI: 10.1097/IMI.0000000000000041; PUBMED: 24534764] [DOI] [PubMed] [Google Scholar]
Toursarkissian 1994a
- Toursarkissian B, Zweng TN, Kearney PA, Pofahl WE, Johnson SB, Barker DE. Percutaneous dilational tracheostomy: report of 141 cases. The Annals of Thoracic Surgery 1994;57(4):862‐7. [PUBMED: 8166532] [DOI] [PubMed] [Google Scholar]
Toursarkissian1994b
- Toursarkissian B, Fowler CL, Zweng TN, Kearney PA. Percutaneous dilational tracheostomy in children and teenagers. Journal of Pediatric Surgery 1994;29(11):1421‐4. [PUBMED: 7844712] [DOI] [PubMed] [Google Scholar]
Toy 1969
- Toy FJ, Weinstein JD. A percutaneous tracheostomy device. Surgery 1969;65(2):384‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Ware 1995
- Ware JE, Kosinski M, Keller SD. SF‐12: How to Score the SF‐12 Physical and Mental Health Summary Scales. Second Edition. Boston, MA: The Health Institute, New England Medical Center, 1995. [ISBN: 1891810022 9781891810022] [Google Scholar]
Warren 2004
- Warren J, Fromm RE Jr, Orr RA, Rotello LC, Horst HM. Guidelines for the inter‐ and intrahospital transport of critically ill patients. Critical Care Medicine 2004;32(1):256‐62. [PUBMED: 14707589] [DOI] [PubMed] [Google Scholar]
Waydhas 1999
- Waydhas C. Intrahospital transport of critically ill patients. Critical Care 1999;3(5):R 83‐9. [PMCID: PMC137237; PUBMED: 11094486] [DOI] [PMC free article] [PubMed] [Google Scholar]
Westphal 1999
- Westphal K, Byhahn C, Wilke HJ, Lischke V. Percutaneous tracheostomy: a clinical comparison of dilatational (Ciaglia) and translaryngeal (Fantoni) techniques. Anesthesia and Analgesia 1999;89(4):938‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wood 1996
- Wood DE. Tracheostomy. Chest Surgery Clinics of North America 1996;6(4):749‐64. [ISSN: 1052‐3359] [PubMed] [Google Scholar]
Zgoda 2005
- Zgoda MA, Berger R. Balloon‐facilitated percutaneous dilational tracheostomy tube placement: preliminary report of a novel technique. Chest 2005;128(5):3688‐90. [PUBMED: 16304334] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Brass 2009
- Brass P, Hellmich M, Kullmer B, Ladra A, Ladra J. Percutaneous technique versus surgical techniques for tracheostomy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD008045] [DOI] [PMC free article] [PubMed] [Google Scholar]


