Abstract
Background
A septic abortion refers to any abortion (spontaneous or induced) complicated by upper genital tract infection including endometritis or parametritis. The mainstay of treatment of septic abortion is antibiotic therapy alone or in combination with evacuation of retained products of conception. Regimens including broad‐spectrum antibiotics are routinely recommended for treatment. However, there is no consensus on the most effective antibiotics alone or in combination to treat septic abortion. This review aimed to bridge this gap in knowledge to inform policy and practice.
Objectives
To review the effectiveness of various individual antibiotics or antibiotic regimens in the treatment of septic abortion.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, and POPLINE using the following keywords: 'Abortion', 'septic abortion', 'Antibiotics', 'Infected abortion', 'postabortion infection'. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov for ongoing trials on 19 April, 2016.
Selection criteria
We considered for inclusion randomised controlled trials (RCTs) and non‐RCTs that compared antibiotic(s) to another antibiotic(s), irrespective of route of administration, dosage, and duration as well as studies comparing antibiotics alone with antibiotics in combination with other interventions such as dilation and curettage (D&C).
Data collection and analysis
Two review authors independently extracted data from included trials. We resolved disagreements through consultation with a third author. One review author entered extracted data into Review Manager 5.3, and a second review author cross‐checked the entry for accuracy.
Main results
We included 3 small RCTs involving 233 women that were conducted over 3 decades ago.
Clindamycin did not differ significantly from penicillin plus chloramphenicol in reducing fever in all women (mean difference (MD) ‐12.30, 95% confidence interval (CI) ‐25.12 to 0.52; women = 77; studies = 1). The evidence for this was of moderate quality. "Response to treatment was evaluated by the patient's 'fever index' expressed in degree‐hour and defined as the total quantity of fever under the daily temperature curve with 99°F (37.2°C) as the baseline".
There was no difference in duration of hospitalisation between clindamycin and penicillin plus chloramphenicol. The mean duration of hospital stay for women in each group was 5 days (MD 0.00, 95% CI ‐0.54 to 0.54; women = 77; studies = 1).
One study evaluated the effect of penicillin plus chloramphenicol versus cephalothin plus kanamycin before and after D&C. Response to therapy was evaluated by "the time from start of antibiotics until fever lysis and time from D&C until patients become afebrile". Low‐quality evidence suggested that the effect of penicillin plus chloramphenicol on fever did not differ from that of cephalothin plus kanamycin (MD ‐2.30, 95% CI ‐17.31 to 12.71; women = 56; studies = 1). There was no significant difference between penicillin plus chloramphenicol versus cephalothin plus kanamycin when D&C was performed during antibiotic therapy (MD ‐1.00, 95% CI ‐13.84 to 11.84; women = 56; studies = 1). The quality of evidence was low.
A study with unclear risk of bias showed that the time for fever resolution (MD ‐5.03, 95% CI ‐5.77 to ‐4.29; women = 100; studies = 1) as well as time for resolution of leukocytosis (MD ‐4.88, 95% CI ‐5.98 to ‐3.78; women = 100; studies = 1) was significantly lower with tetracycline plus enzymes compared with intravenous penicillin G.
Treatment failure and adverse events occurred infrequently, and the difference between groups was not statistically significant.
Authors' conclusions
We found no strong evidence that intravenous clindamycin alone was better than penicillin plus chloramphenicol for treating women with septic abortion. Similarly, available evidence did not suggest that penicillin plus chloramphenicol was better than cephalothin plus kanamycin for the treatment of women with septic abortion. Tetracyline enzyme antibiotic appeared to be more effective than intravenous penicillin G in reducing the time to fever defervescence, but this evidence was provided by only one study at low risk of bias.
There is a need for high‐quality RCTs providing reliable evidence for treatments of septic abortion with antibiotics that are currently in use. The three included studies were carried out over 30 years ago. There is also a need to include institutions in low‐resource settings, such as sub‐Saharan Africa, Latin America and the Caribbean, and South Asia, with a high burden of abortion and health systems challenges.
Keywords: Adult; Female; Humans; Pregnancy; Abortion, Septic; Abortion, Septic/drug therapy; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Cephalothin; Cephalothin/therapeutic use; Chloramphenicol; Chloramphenicol/therapeutic use; Clindamycin; Clindamycin/therapeutic use; Drug Therapy, Combination; Kanamycin; Kanamycin/therapeutic use; Length of Stay; Penicillins; Penicillins/therapeutic use; Randomized Controlled Trials as Topic; Tetracycline; Tetracycline/therapeutic use
Plain language summary
Antibiotics for treating septic abortion
Background
A septic abortion is any abortion with infection after a miscarriage or intentional pregnancy termination. One of the signs of septic abortion is fever. Antibiotic treatment is very important for the treatment of septic abortion. The recommended treatments include antibiotics that have effects on different types of bacteria. However, there is no agreement on the most effective antibiotics to be used either alone or in combination to treat septic abortion.
Trial characteristics
This review included 3 small studies of 233 women with septic abortion. One study compared clindamycin alone to penicillin plus chloramphenicol; the second study compared penicillin plus chloramphenicol to cephalothin plus kanamycin; and the third study compared tetracycline enzyme‐based antibiotic with intravenous penicillin G.
Results
We found no strong evidence that clindamycin alone is better than penicillin plus chloramphenicol for treating women with septic abortion. Similarly, the available evidence did not suggest that penicillin plus chloramphenicol is better than cephalothin plus kanamycin for the treatment of women with septic abortion. Furthermore, performing D&C before starting antibiotic treatment was not better than performing D&C after antibiotic treatment has begun. The use of tetracycline‐enzyme antibiotic brought women's fever down faster than intravenous penicillin G.
Conclusion
The available evidence from three small trials, which involved some antibiotics not currently in use, is insufficient to advocate for a change in existing treatment recommendations for septic abortion. In spite of this, combinations of antibiotics may be administered to women with septic abortion because they are more likely to reduce fever faster, including in women with bacteria in the blood, than single antibiotic treatment. Only one study reported harm experienced by women: two women given clindamycin had treatment failure, and one woman had an adverse drug reaction. In addition, two women in the clindamycin group had pelvic abscess compared to one in the penicillin plus chloramphenicol group, although the difference was not significant. The limitation of this review is the inclusion of three small studies conducted over 30 years ago.
Summary of findings
Summary of findings for the main comparison. Clindamycin versus penicillin plus chloramphenicol compared to for.
| Clindamycin versus penicillin plus chloramphenicol compared to for treating septic abortion | ||||||
| Patient or population: patients with septic abortion Settings:In‐hospital Intervention: Clindamycin versus penicillin plus chloramphenicol Comparison: | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Clindamycin versus penicillin plus chloramphenicol | ||||||
| Fever index (mean degree‐hour) ‐ all women | ‐ | The mean fever index (mean degree‐hour) ‐ all women in the intervention groups was 12.3 lower (25.12 lower to 0.52 higher) | ‐ | 77 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ‐ |
| Fever index (mean degree‐hour) ‐ bacteraemic patients | ‐ | The mean fever index (mean degree‐hour) ‐ bacteraemic patients in the intervention groups was 11.7 lower (55.44 lower to 32.04 higher) | ‐ | 29 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ‐ |
| Duration of hospitalisation | ‐ | The mean duration of hospitalisation in the intervention groups was 0 higher (0.54 lower to 0.54 higher) | ‐ | 77 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ‐ |
| Complications | 25 per 1000 | 189 per 1000 (25 to 1000) | RR 7.57 (0.98 to 58.61) | 77 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Study reported patients were randomly assigned however there is a chance of selection bias 2 There is uncertainty about the magnitude of effect and wide confidence interval
Summary of findings 2. Penicillin plus chloramphenicol versus cephalothin plus kanamycin for treating septic abortion.
| Penicillin plus chloramphenicol versus cephalothin plus kanamycin for treating septic abortion | ||||||
| Patient or population: patients with treating septic abortion Settings:In‐hospital Intervention: Penicillin plus chloramphenicol versus cephalothin plus kanamycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Penicillin plus chloramphenicol versus cephalothin plus kanamycin | |||||
| Time to fever resolution (mean time) | ‐ | The mean time to fever resolution (mean time) in the intervention groups was 2.3 lower (17.31 lower to 12.71 higher) | ‐ | 56 (1 study) | ⊕⊕⊝⊝ low1,2 | ‐ |
| Absence of fever during D&C (mean time) | ‐ | The mean absence of fever during D&C (mean time) in the intervention groups was 1 lower (13.84 lower to 11.84 higher) | ‐ | 56 (1 study) | ⊕⊕⊝⊝ low1,2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment may have been ineffective and study did not report blinding among participants,study personnel or outcome assessors. 2 There is uncertainty about the magnitude of effect and wide confidence interval
Summary of findings 3. Antibiotics after D&C versus D&C during antibiotics for treating septic abortion.
| Antibiotics after D&C versus D&C during antibiotics for treating septic abortion | ||||||
| Patient or population: patients with treating septic abortion Settings:In‐hospital Intervention: Antibiotics after D&C versus D&C during antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics after D&C versus D&C during antibiotics | |||||
| Absence of fever (degree‐hour) ‐ Clindamycin after D&C versus D&C during clindamycin | ‐ | The mean absence of fever (degree‐hour) ‐ clindamycin after D&C versus D&C during clindamycin in the intervention groups was 6 lower (20.74 lower to 8.74 higher) | ‐ | 37 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ‐ |
| Absence of fever (degree‐hour) ‐ Penicillin + chloramphenicol after D&C versus D&C during penicillin + chloramphenicol therapy) | ‐ | The mean absence of fever (degree‐hour) ‐ penicillin + chloramphenicol after D&C versus D&C during penicillin + chloramphenicol therapy) in the intervention groups was 1.3 higher (24.84 lower to 27.44 higher) | ‐ | 39 (1 study) | ⊕⊕⊕⊝ moderate1,2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Study reported patients were randomly assigned however there is a chance of selection bias 2 There is uncertainty about the magnitude of effect and wide confidence interval
Summary of findings 4. Tetracycline versus penicillin G for treating septic abortion.
| Tetracycline versus penicillin G for treating septic abortion | ||||||
| Patient or population: patients with septic abortion Settings:In‐hospital Intervention: Tetracycline versus penicillin G | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Tetracycline versus penicillin G | |||||
| Fever resolution time (days) | ‐ | The mean fever resolution time (days) in the intervention groups was 5.03 lower (5.77 to 4.29 lower) | ‐ | 100 (1 study) | ⊕⊕⊝⊝ low1 | ‐ |
| Leukocytosis resolution time (days) | ‐ | The mean leukocytosis resolution time (days) in the intervention groups was 4.88 lower (5.98 to 3.78 lower) | ‐ | 100 (1 study) | ⊕⊕⊝⊝ low1 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by two; method of randomisation not adequate as women were selected according to their history number. No attempts to blind participants, study personnel or outcome assessors.
Summary of findings 5. Adverse events.
| Adverse events | ||||||
| Patient or population:patients with septic abortion Settings:In‐hospital Intervention: Clindamycin versus penicillin plus chloramphenicol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Adverse events | |||||
| Pelvic abscess, salpingitis, urinary tract infection | 25 per 1000 | 108 per 1000 (13 to 924) | RR 4.32 (0.51 to 36.95) | 77 (1 study) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Treatment failure | 0 per 1000 | 0 per 1000 (0 to 0) | RR 5.39 (0.27 to 108.8) | 77 (1 study) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Drug reaction | 0 per 1000 | 0 per 1000 (0 to 0) | RR 3.24 (0.14 to 77.06) | 77 (1 study) | ⊕⊕⊕⊝ moderate1 | ‐ |
| Phlebitis | 25 per 1000 | 9 per 1000 (0 to 214) | RR 0.36 (0.02 to 8.56) | 77 (1 study) | ⊕⊕⊕⊝ moderate1 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There is uncertainty about the magnitude of effect and wide confidence interval
Background
Description of the condition
A septic abortion refers to any abortion (spontaneous or induced) complicated by upper genital tract infection including endometritis or parametritis (Stubblefield 1994). Septic abortions rarely complicate safe abortion (Raymond 2012; Henderson 2013). Several reasons may account for this including expertise of healthcare providers, adherence to standard sterile practices for infection prevention and control, and routine use of perioperative antibiotics during the procedure (Warriner 2006). Unsafe abortion is defined by the World Health Organization as "a procedure for terminating an unintended pregnancy either by individuals without the necessary skills or in an environment that does not conform to minimal medical standards or both" (WHO 2012). It is more prevalent in areas where access to safe abortion is restricted and where there is a dearth of trained providers and high incidence of untreated and unrecognised sexually transmitted infections (Achilles 2012; WHO 2012).
Unsafe abortion is a major public health issue and is associated with significant morbidity and mortality. Globally, 14% of pregnancy‐related deaths are due to induced and spontaneous abortions (Kassebaum 2014). Recent estimates suggest that 21.6 million unsafe abortions take place annually, with the majority (98%) occurring in low‐ and middle‐income countries (Grimes 2006; WHO 2011). Many unsafe abortions are reported to occur particularly in countries with restrictive abortion laws where access to treatment of abortion complications is also limited (Rasch 2011). Approximately 5 million women are admitted in low‐ and middle‐income countries with complications of unsafe abortion, with sepsis being the second most frequent complication and reportedly causing 10% of maternal deaths (Singh 2006; Adler 2012). The incidence of sepsis varies from 3% to 15% in primary and secondary health facilities to as high as 31% to 54% in tertiary centres (Nwogu‐Ikojo 2007; Adler 2012). Death from septic abortion is estimated at 10 to 100 deaths per 100,000 abortions in some low‐ and middle‐income countries (Ahman 2011). With regard to infectious complications, it has been estimated that approximately 20% to 30% of unsafe abortions cause reproductive tract infections, and that 20% to 40% of these lead to infection of the upper genital tract (WHO 2007). A recent systematic review of hospital‐based studies on morbidity from unsafe abortion reported severe abortion‐related infections in up to 52% of cases (Adler 2012). Spontaneous abortion, or miscarriage, is typically associated with a low likelihood of infection (Nielsen 1995; Trinder 2006). However, distinguishing women with spontaneous abortion from women seeking emergency treatment for unsafe abortion poses challenges in settings where access to abortion is restricted (Gerdts 2013). Across various settings, complications from septic abortion are the leading causes of abortion‐related deaths. (Toure 1992; Dragoman 2014).
Symptoms and signs of abortion‐related infections include fever, pelvic/abdominal pain, prolonged vaginal bleeding, uterine tenderness, foul‐smelling uterine discharge, and elevated inflammatory markers, findings similar to those associated with pelvic inflammatory disease (Soper 2010; WHO 2012). Diagnostic imaging can aid in detecting retained products of conception, uterine perforation, and extension of the infection to the parametrium and peritoneum. Ultrasound scan enables visualisation of fluid‐filled dilated tubes, tubo‐ovarian abscess, and collections of exudate or pus in the peritoneal cavity. Microbiological studies of blood, urine, endocervical, and evacuated specimens enable the identification of involved bacteria (Stubblefield 1994). Typically, infections are polymicrobial, and isolated bacteria originate largely from vaginal flora and the bowel (ASRM 2013; ten Broek 2013). Gram‐positive and gram‐negative aerobes, as well as facultative or obligate anaerobes, have also been isolated (Hazra 2013). Isolated organisms include bacteria such as anaerobic Peptostreptococcus, antibiotic‐resistant and toxin‐producing Staphylococcus aureus, strains of Clostridia species such as Clostridium perfringens, Group A Streptococcus, and Escherichia coli (Eschenbach 2015). The presence of sexually transmitted infections at the time of abortion with organisms such as Chlamydia trachomatis or Neisseria gonorrhoeae is an established risk factor for septic abortion (Barbacci 1986; Blackwell 1993). Uterine infections involving Clostridium spp. such as Clostridium perfringens are infrequent but potentially fatal (Fischer 2005; Soper 2010). Clostridium sordellii,a rare cause of death from sepsis, has been reported among women who had medical abortions in Canada and the USA, with death thought to be related to a toxin this organism produced (Meites 2010; Zane 2011). Indeed, the majority of women infected with this organism are likely to present without a fever, making early diagnosis difficult (Fischer 2005; Aldape 2006; Meites 2010).
Severe acute medical consequences of septic abortion include septic shock, coagulopathy (bleeding disorder), multiple organ dysfunction, and death. Significant long‐term consequences can include persistent pelvic pain, chronic pelvic inflammatory disease, and infertility, which is commonly due to blocked fallopian tubes (Bhattacharya 2010; Butt 2012; ten Broek 2013).
Description of the intervention
The mainstay of treatment of septic abortion is antibiotic therapy in addition to removal of retained products of conception (WHO 2012). Regimens including broad‐spectrum antibiotics are routinely recommended for treatment. However, there is no consensus on the most effective antibiotics alone or in combination for the treatment of septic abortion (Porter 2008). Parenteral broad‐spectrum antibiotics are usually initiated because of the polymicrobial nature of the condition. The US National Guideline Clearinghouse suggests commencing intravenous broad‐spectrum antibiotic therapy within an hour of clinical diagnosis (NGCH 2012). This is then adjusted following a reassessment with microbiology and clinical data (Dellinger 2008; Porter 2008). However, in low‐ and middle‐income countries, data on the microbial aetiology of pregnancy‐related infections are scarce, perhaps because collecting samples and carrying out cultures is technically challenging (Gravett 2012). Furthermore, the emergence of strains of some organisms that are resistant to antibiotics such as ampicillin and fluoroquinolones has led to a change in the recommendations for their use (Abudu 1986; Cherpes 2002; CDC 2015).
While antibiotic therapy may offer benefits, women may also experience adverse effects dependent on the class and combinations of antibiotics. These range from relatively mild gastrointestinal disturbances such as nausea, vomiting, diarrhoea, and bloating to life‐threatening allergic reactions.
How the intervention might work
The mechanisms of action of the classes of antibiotics that may be used in the treatment of septic abortion vary and include inhibiting the microbial cell wall, protein, or nucleic acid synthesis, and altering microbial cell membranes (Kohanski 2010). Overall, effective antibiotics are expected to control the infection, ensuring a marked reduction in both short‐ and long‐term complications such as death and disabilities including infertility. In spite of this, the early identification and removal of infected retained products of conception in addition to administration of effective antibiotics appear to be the recommended key steps in the management of septic abortion (WHO 1994). Although most organisms that cause septic abortion are localised to the placenta, they can proliferate very rapidly in the dead tissue of the uterus so that antibiotic administration prior to uterine evacuation limits their spread and prevents the likelihood of septic shock (Eschenbach 2015).
Why it is important to do this review
A systematic review on the use of perioperative antibiotics to prevent upper genital infections following first‐trimester surgically induced abortions showed that antibiotics were effective in preventing genital tract infections. However, it did not provide evidence to recommend a policy of routine antibiotic use during these procedures (Low 2012). Another review did not provide evidence to recommend or abandon the use of prophylactic antibiotics in women with an incomplete abortion (May 2007). It would therefore seem plausible to assume that when abortion‐related infection ensues, effective antibiotic therapy may be an important life‐saving intervention. A recent scoping review of postabortion care interventions, as yet unpublished, found that no existing systematic review has evaluated the evidence regarding the use of antibiotics specifically for treating septic abortion (Nwagbara 2014 [pers comm]). Yet, healthcare providers will need guidance on what antibiotic regimen to recommend as either primary or adjunctive treatment following removal of retained and infected products of conception. This review aimed to bridge this gap in knowledge to inform policy and practice.
Objectives
To review the effectiveness of various individual antibiotics or antibiotic regimens in the treatment of septic abortion.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and non‐RCTs that compared antibiotic(s) to another antibiotic(s), irrespective of route of administration, dosage, and duration as well as studies comparing antibiotics alone with antibiotics in combination with other interventions such as dilation and curettage (D&C).
Types of participants
We included women who had an abortion and any of the following symptoms and signs based on World Health Organization guidelines (WHO 1994): fever with chills or rigours, abdominal and/or pelvic pain, purulent or offensive vaginal or cervical discharge, pelvic inflammatory disease, uterine tenderness, prolonged vaginal bleeding or spotting, and/or an elevated white blood cell count. We also included studies that enrolled women with established septic abortion as defined by the authors.
Types of interventions
Studies that compared an antibiotic(s) alone or in combination with other interventions versus:
another antibiotic(s);
antibiotic(s) in combination with other interventions.
These other interventions may include intravenous fluids, D&C, manual or electric vacuum aspiration, misoprostol, hysterectomy, hysterotomy, analgesics, and blood transfusion.
Types of outcome measures
We considered trials for inclusion if they reported any of the following clinical or laboratory outcomes.
Primary outcomes
Clinical improvement: defined as absence of fever, vaginal discharge, or pelvic pain, or improvement in ultrasound findings such as a reduction in the size of a tubo‐ovarian abscess
Microbiological cure: defined as disappearance of the micro‐organism from appropriate samples that were previously positive
Death
Secondary outcomes
Duration of hospital admission: defined as time in days from enrolment in the trial until discharge
Fever resolution time: defined as time in days from the commencement of antibiotics until temperature becomes normal
Short‐term complications: e.g. multiple organ failure, bleeding disorder, tubo‐ovarian abscess
Reduction in levels of markers of inflammation, such as C‐reactive protein, erythrocyte sedimentation rate, total white cell count, and procalcitonin
Quality of life: score, chronic pelvic pain, chronic pelvic inflammatory disease, infertility, etc.
Adverse effects
Search methods for identification of studies
Electronic searches
Databases
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE via PubMed (1950 to 19 April, 2016), EMBASE (1974 to 19 April, 2016), LILACS (1982 to 19 April, 2016) and POPLINE (up till 19 April, 2016) using the following keywords: 'Abortion', 'septic abortion', 'Antibiotics', 'Infected abortion', 'postabortion infection' (Appendix 1). We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov for ongoing trials on 19 April, 2016 (Figure 1).
1.
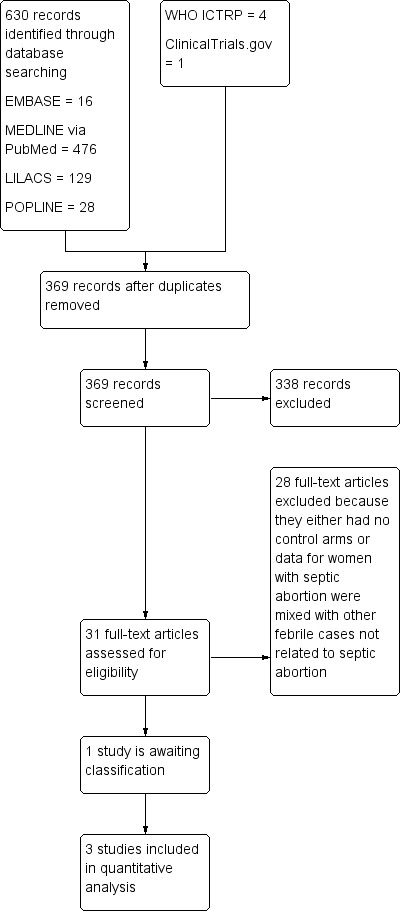
Study flow diagram.
Searching other resources
References lists
We handsearched the reference lists of all relevant articles obtained from our search and those from previously published systematic reviews to identify other possible articles. We also translated studies published in languages other than English.
Data collection and analysis
Selection of studies
Two review authors (AU and OO) independently screened search outputs for eligible studies based on a priori inclusion criteria for the review. A third (EEE) or fourth (BOO) review author resolved any disagreements regarding the inclusion of a study.
Data extraction and management
Two review authors (AU and OO) independently extracted data from the included trials using a pretested data extraction form. We resolved disagreements through consultation with a third review author (BOO). We attempted to contact the authors of included studies for relevant information not specified or unclear. We summarised details of the extracted data in a 'Characteristics of included studies' table (Characteristics of included studies)
For each outcome, we extracted the total number of participants randomised and the number that were analysed in each treatment arm for each trial. For dichotomous outcomes, we recorded the number of participants experiencing the event and the number that were analysed in each treatment arm. For continuous outcomes, we extracted arithmetic means and standard deviations, along with the number of participants that were analysed for each treatment arm. Where the standard deviation was not reported, we derived it using standard error of the mean and multiplied by square root of the sample size according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). One review author (OO) entered extracted data into Review Manager 5.3 (RevMan 2014), and a second review author (BOO) cross‐checked the entry for accuracy.
Assessment of risk of bias in included studies
Two review authors (OO and EEE) independently assessed the risk of bias in each included study using The Cochrane Collaboration's 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed whether adequate steps were taken to reduce the risk of bias across six domains: generation of the randomisation sequence, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias such as the trial being stopped earlier than planned. We categorised our judgements as 'yes' (low risk of bias), 'no' (high risk of bias), or 'unclear'. We compared our judgements and resolved any disagreements by discussion or by consulting a third review author (AU). We attempted to contact the authors of included studies for relevant information not specified or unclear. We entered the results of our assessment into a 'Risk of bias' table and presented them in a 'Risk of bias' graph (Figure 2) and a 'Risk of bias' summary (Figure 3).
2.
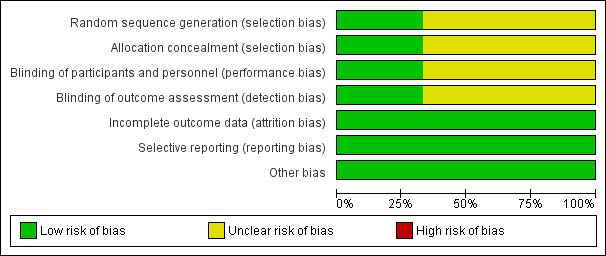
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
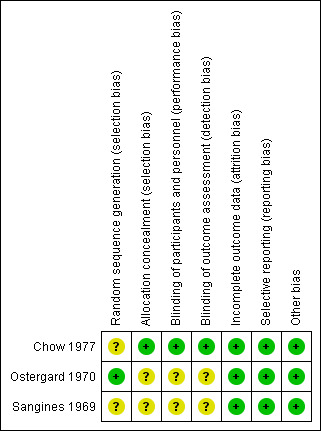
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We used the risk ratio to summarise dichotomous outcomes and reported the mean difference for continuous outcomes. Where these effect estimates were reported as adjusted values, we extracted these with their corresponding standard errors or confidence intervals. We presented all measures of effect with their corresponding 95% confidence intervals.
Unit of analysis issues
Not applicable.
Dealing with missing data
We performed an intention‐to‐treat analysis by including all randomised participants in the analysis according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where we could not obtain missing data, we conducted the analysis using only the available data as presented by the authors. In such a circumstance, we assumed the data to be missing at random.
Assessment of heterogeneity
We planned to estimate the I2 statistic, with values of 30% to 59%, 60% to 89%, and 90% to 100% representing moderate, substantial, and considerable levels of heterogeneity, respectively. However, investigation of heterogeneity was not feasible because we did not aggregate data of included studies into a meta‐analysis, as the study interventions were different across all three included studies.
Assessment of reporting biases
We could not explore the presence of publication bias by looking for funnel plot asymmetry because the number of included studies was too small.
Data synthesis
We could not aggregate the data of included studies into a meta‐analysis because the interventions in the studies differed. Where the study authors did not report standard deviation (SD), we used the standard error (SE) of the mean to obtain SD according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We extracted the mean differences (MD) and SE of the mean for some outcomes and calculated 95% confidence intervals of the MDs using generic inverse variance. We used RevMan 2014 to perform an inverse‐variance meta‐analysis using a fixed‐effect method for differences in mean between comparisons: clindamycin versus penicillin plus chloramphenicol, penicillin plus chloramphenicol versus cephalothin plus kanamycin, and tetracycline versus penicillin G. We used the Mantel‐Haenszel method to analyse the risk ratio for adverse events.
We graded the evidence using the GRADE (Grading of Recommendations, Assessment and Evaluation of Evidence) methodology for the assessment of overall quality of evidence (Guyatt 2011). We also used the GRADEpro software (GradeproGDT 2015) to produce a 'Summary of findings' table and evidence profiles for specific outcomes of significant interest to patients and caregivers.
Subgroup analysis and investigation of heterogeneity
Data were available for three small studies. As a result, investigation of heterogeneity was not feasible. For two studies (Ostergard 1970; Chow 1977), we also did a subgroup analysis of the different types of antibiotics plus D&C (Analysis 3.1).
3.1. Analysis.
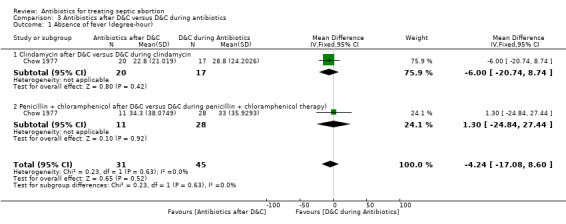
Comparison 3 Antibiotics after D&C versus D&C during antibiotics, Outcome 1 Absence of fever (degree‐hour).
Sensitivity analysis
We did not perform a sensitivity analysis because we included only three small trials.
Results
Description of studies
Results of the search
Our search yielded 630 studies. Only three studies met our inclusion criteria (Sangines 1969; Ostergard 1970; Chow 1977). All review authors agreed on the relevance of these trials. One study is ongoing (NCT02309346) (see Figure 1).
Included studies
We included 3 small RCTs involving 233 women in the review (Sangines 1969; Ostergard 1970; Chow 1977). In Chow 1977, 77 women were randomised in a double‐blind trial. The study included all febrile women with fever equal to or greater than 100.4°F (38°C) with a clinical diagnosis of septic abortion and no history of allergy to penicillin. The mean age was 24.1 years (SD ± 1.01) in the clindamycin group and 24.7 years (SD ± 0.69) in the penicillin plus chloramphenicol group. Gestational age was also similar in the two groups: 11.6 ± 0.61 and 13.4 ± 0.61 in the clindamycin and penicillin plus chloramphenicol groups, respectively. More than half of women (54%) were white. Baseline characteristics between the two comparison groups were similar. The second study involved 56 women admitted with clinical diagnosis of septic abortion (Ostergard 1970). Neither the participants nor the assessors were blinded. This study compared penicillin plus chloramphenicol versus cephalothin plus kanamycin. The study provided no information on baseline characteristics of women. In Sangines 1969, 100 women with septic abortion were randomised to receive either tetracycline with streptokinase and streptodornase or intravenous 10,000,000 units of penicillin G sodium. In addition, most women (39 versus 47 respectively) had dilation and curettage (D&C).
Excluded studies
We excluded Sivasamboo 1968, Horta 1971, Dahm 1973, Abudu 1986, and Savaris 2011. Abudu 1986 did not have a control arm. Dahm 1973 treated the control arm with penicillin alone or in combination with streptomycin or chloramphenicol, and the study arm received only ampicillin. The effect of each treatment was not separated, and it was therefore impossible to know which treatment contributed to improvement or to harm. Horta 1971 did not define how treatment was administered to the control arm, while Sivasamboo 1968 included cases of threatened abortion and septic abortion. Data for septic abortion were not disaggregated. Savaris 2011 randomised women to treatments arms 48 hours after they were discharged from the hospital after having been treated with intravenous antibiotics.
Risk of bias in included studies
We have presented a summary of the 'Risk of bias' assessment in Figure 3. Other details are provided in Characteristics of included studies.
Allocation
The generation of the randomisation sequence in Chow 1977 was unclear, although assignment to a treatment regimen was said to be determined by sequentially drawing a sealed envelope containing the antibiotic protocol. In Ostergard 1970, treatment protocol was placed in a plain envelope. It is unclear if the envelopes were opaque or transparent. Sangines 1969 reported random assignment to treatment arm, but did not state the exact method of sequence generation.
Blinding
The baseline characteristics did not differ significantly between treatment groups in Chow 1977. The study personnel and participants were blinded to the intervention arms. All medications were mixed by the hospital pharmacy in unmarked infusion bottles. Doses of 5 x 106 units of penicillin and 1 g of chloramphenicol were administered by separate intravenous infusions every 6 hours. Clindamycin (300 mg) was administered intravenously every 6 hours, and a bottle of 0.85% NaCl was also administered intravenously to keep the procedure double blind (Figure 2). Ostergard 1970 did not blind participants and investigators, while it was unclear if participants or personnel were blinded in Sangines 1969 (Figure 3).
Incomplete outcome data
Two included studies accounted for all participants by including them in the final analysis (Ostergard 1970; Chow 1977). For Sangines 1969, data for one outcome (fever resolution time) was incomplete.
Selective reporting
We could not ascertain whether there was selective reporting in the three included studies, especially since we had no access to the study protocols of the trials. In particular, Ostergard 1970 and Sangines 1969 did not report any adverse events.
Other potential sources of bias
Ostergard 1970 was funded in part by a clinical research grant from a pharmaceutical company and so has a potential for bias (Higgins 2011).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Primary outcomes
1. Clinical improvement
Reports of assessment of clinical improvement varied. In Chow 1977, response to therapy was assessed as fever index, a quantitative measure of the overall amount of fever reported in degree hours (Ledger 1975). Ostergard 1970 and Sangines 1969 measured the time to fever defervescence, in hours and days, respectively.
In Chow 1977, clindamycin did not differ significantly from penicillin plus chloramphenicol in terms of the fever index (mean difference (MD) ‐12.30, 95% confidence interval (CI) ‐25.12 to 0.52; women = 77; studies = 1) (Analysis 1.1). The evidence for this was of moderate quality (Table 1).
1.1. Analysis.
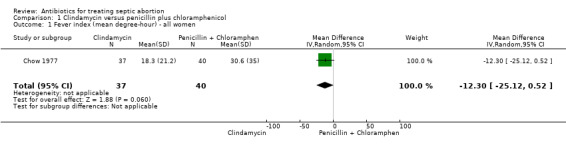
Comparison 1 Clindamycin versus penicillin plus chloramphenicol, Outcome 1 Fever index (mean degree‐hour) ‐ all women.
When the effect of the two interventions on fever was compared in women with bacteraemia, there was no difference (MD ‐11.70, 95% CI ‐55.44. to 32.04; women = 29; studies = 1) (Analysis 1.2). The evidence for this was of moderate quality. However, one woman in the clindamycin group had bacteraemia two weeks after hospital discharge and was re‐admitted for acute salpingitis and endometritis (Analysis 5.1).
1.2. Analysis.
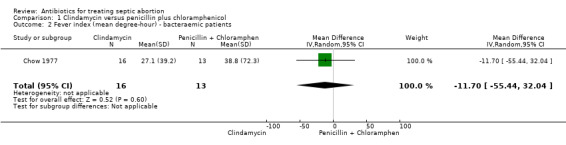
Comparison 1 Clindamycin versus penicillin plus chloramphenicol, Outcome 2 Fever index (mean degree‐hour) ‐ bacteraemic patients.
5.1. Analysis.
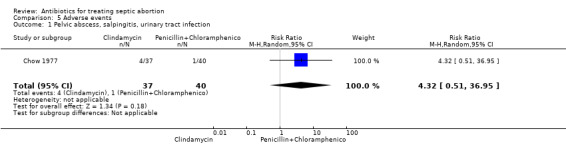
Comparison 5 Adverse events, Outcome 1 Pelvic abscess, salpingitis, urinary tract infection.
Ostergard 1970 evaluated the effect of penicillin plus chloramphenicol versus cephalothin plus kanamycin before and after D&C. Response to therapy was evaluated by "the time from start of antibiotics until fever lysis and time from D&C until patients become afebrile". The effect of penicillin plus chloramphenicol on fever resolution was not different from the effect of cephalothin plus kanamycin (MD ‐2.30, 95% CI ‐17.31 to 12.71; women = 56; studies = 1) (Analysis 2.1). The evidence for this was of low‐quality. Similarly, when D&C was performed during antibiotic therapy, no significant difference was detected in the duration of fever between penicillin plus chloramphenicol versus cephalothin plus kanamycin (MD ‐1.00, 95% CI ‐13.84 to 11.84; women = 56; studies = 1) (Analysis 2.2). As the study was at high risk of bias, we graded the quality of evidence as low (Table 2).
2.1. Analysis.
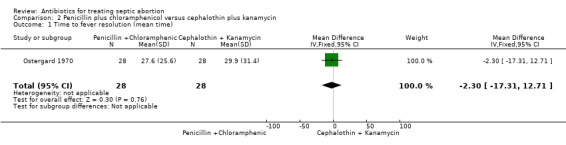
Comparison 2 Penicillin plus chloramphenicol versus cephalothin plus kanamycin, Outcome 1 Time to fever resolution (mean time).
2.2. Analysis.
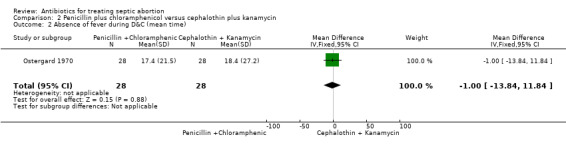
Comparison 2 Penicillin plus chloramphenicol versus cephalothin plus kanamycin, Outcome 2 Absence of fever during D&C (mean time).
Antibiotics after D&C and D&C during antibiotics
Performing D&C before commencing antibiotic therapy on women with septic abortion resulted in a lower fever index compared to D&C 12 to 24 hours after antibiotics. However, this difference was not statistically significant (MD ‐4.24, 95% CI ‐17.08 to 8.60; women = 76; studies = 1) (Analysis 3.1). The quality of evidence for this outcome was moderate (Table 3).
Moderate‐quality evidence showed that clindamycin after D&C was not significantly better than when D&C was performed during antibiotic therapy for the outcome fever index (MD ‐6.00, 95% CI ‐20.74 to 8.74; women = 37; studies = 1). Similarly, penicillin plus chloramphenicol after D&C was not better than D&C during penicillin plus chloramphenicol therapy (MD 1.30, 95% CI ‐24.84 to 27.44; women = 39; studies = 1) (Analysis 3.1).
2. Microbiological cure
None of the three included studies reported this outcome.
3. Death
No included study set out to study mortality as an outcome, hence death was not reported among women in the studies.
Secondary outcomes
1. Duration of hospitalisation
Only Chow 1977 evaluated the effect of treatment on duration of hospital stay. Moderate‐quality evidence showed that clindamycin did not differ from penicillin plus chloramphenicol (MD 0.00, 95% CI ‐0.54 to 0.54; women = 77; studies = 1). Average stay for each treatment arm was five days (Analysis 1.3).
1.3. Analysis.
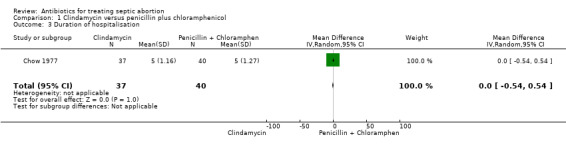
Comparison 1 Clindamycin versus penicillin plus chloramphenicol, Outcome 3 Duration of hospitalisation.
2. Fever resolution time
Sangines 1969 reported fever resolution time as a measure of effectiveness. Tetracycline‐enzyme based antibiotic resulted in a significantly shorter fever resolution time compared with intravenous penicillin G (MD ‐5.03, 95% CI ‐5.77 to ‐4.29; women = 100; studies = 1) (Analysis 4.1)(Table 4).
4.1. Analysis.
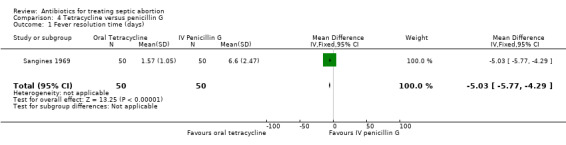
Comparison 4 Tetracycline versus penicillin G, Outcome 1 Fever resolution time (days).
3. Short‐term complications
In Chow 1977, two women in the clindamycin group developed pelvic abscess (tubo‐ovarian abscess) compared to one woman in the penicillin plus chloramphenicol group, while one woman developed salpingitis and another developed a urinary tract infection in the clindamycin group (RR 4.32, 95% CI 0.51 to 36.95; women = 77; studies = 1) (Analysis 5.1). The other two studies reported no short‐term complications. Two women in the clindamycin group experienced treatment failure (RR 5.39, 95% CI 0.27 to 108.80; women = 77; studies = 1) (Analysis 5.2).
5.2. Analysis.
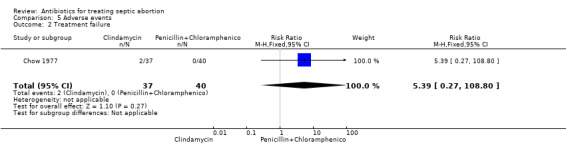
Comparison 5 Adverse events, Outcome 2 Treatment failure.
4. Reduction in levels of markers of inflammation
None of the three included studies reported reductions in levels of any inflammatory marker. However, Sangines 1969 reported the mean number of days until leukocytosis resolved after starting antibiotics as 5.1 ± 1.3 and 9.89 ± 3.76 for tetracycline and penicillin G, respectively (MD ‐4.88, 95% CI ‐5.98 to ‐3.78; women = 100; studies = 1).
5. Quality of life
None of the included studies reported on any measures of quality of life.
6. Adverse events
Only one study Chow 1977, reported on adverse events (Table 5). A woman in the clindamycin group had drug reaction (RR 3.24, 95% CI 0.14 to 77.06; women = 77; studies = 1) (Analysis 5.3), while a woman in the penicillin plus chloramphenicol group developed phlebitis (RR 0.36, 95% CI 0.02 to 8.56; women = 77; studies = 1) (Analysis 5.4). These adverse events were not statistically significant between the two interventions.
5.3. Analysis.
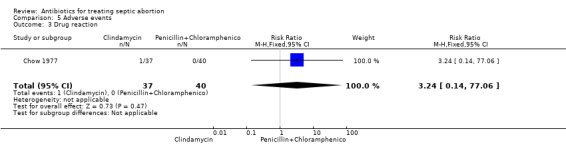
Comparison 5 Adverse events, Outcome 3 Drug reaction.
5.4. Analysis.
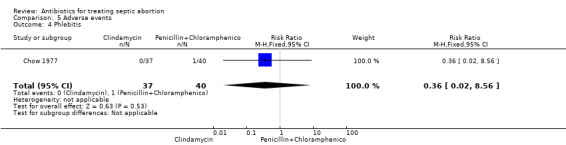
Comparison 5 Adverse events, Outcome 4 Phlebitis.
Discussion
Septic abortion often follows incompletely induced abortion, but may also complicate spontaneous miscarriage. Unnecessary and largely avoidable deaths continue to occur due to sepsis from unsafe abortion. The target should always be the prevention of septic abortion, and where it has already occurred, prevention of mortality. The consequences of septic abortion cannot be predicted, including its short‐term morbidities and long‐term disabilities. When septic abortion occurs, early detection and prompt and effective treatment should reduce morbidity and limit mortality. The restrictive abortion laws in some countries may have made unsafe abortion prevalent, thereby increasing the risks of septic abortion in many women who seek out the procedure.
Women with septic abortion are provided antibiotic treatment either before or after uterine evacuation procedures including medical abortion, use of manual vacuum aspiration, electric vacuum aspiration, or surgical D&C. The polymicrobial nature of organisms involved in septic abortion suggests that a single antibiotic alone may be inadequate for its treatment and that a combination of antibiotics would be an effective treatment strategy. In spite of this, very few vigorous studies have been conducted to determine which antibiotic regimen is effective. All three included studies in this review are over three decades old. Microbial organisms encountered in septic abortion may have varied over time, this worsened further by the emergence of antibiotic‐resistant strains. It seems reasonable to assume that the development and use of very potent antibiotics has in part contributed to a decline in morbidity and mortality from septic abortion.
Summary of main results
There was moderate‐quality evidence that intravenous clindamycin had similar effects on fever index, a quantitative measure of amount of fever, when compared to penicillin plus chloramphenicol in all women with septic abortion. Moderate‐quality evidence showed that both interventions had the same effect on duration of hospital stay for women with septic abortion.
Low‐quality evidence showed that penicillin plus chloramphenicol had a similar effect on duration of fever when compared with cephalothin plus kanamycin in women with septic abortion. Both combinations of antibiotics also had similar effects on duration of fever when D&C was performed during antibiotic therapy.
In addition, moderate‐quality evidence showed that performing D&C before commencing antibiotics resulted in a non‐significant lower fever index when compared to D&C performed 12 to 24 hours after antibiotic therapy. This effect was found irrespective of the antibiotic regimen and whether a single or a combination of antibiotics was used.
Moderate‐quality evidence from one included study found very few adverse events in women treated with clindamycin when compared to penicillin plus chloramphenicol (Chow 1977).
The fever resolution time was significantly less when tetracycline‐enzymes complex was used compared with intravenous penicillin G (Sangines 1969). In addition, the reduction in leukocytosis (a marker of inflammation) took significantly less time with tetracycline‐enzymes complex compared with penicillin G.
None of the included studies reported microbiological cure, death, or other long‐term outcomes such as chronic pelvic pain and infertility.
Overall completeness and applicability of evidence
In general, there was a paucity of studies required to generate evidence regarding the primary objective of this review. The available evidence pertains to antibiotic regimens some of which are not used in current day‐to‐day gynaecological practice. In addition, the included studies were conducted over three decades ago. However, it seems that a single antibiotic regimen led to early reduction of fever, while the different combinations of antibiotic regimens appeared similar in terms of fever lysis, irrespective of whether D&C was performed during antibiotics treatment or antibiotics were administered before D&C.
The current practice of parenteral administration of broad‐spectrum antibiotics, including an antibiotic to which anaerobic bacteria are susceptible, in addition to evacuation of retained products of conception from the uterus should limit risk of bacteraemia in women with septic abortion. However, due to the small number of studies and participants, the risks of bias in the included studies, and the likelihood of latter‐day antibiotic resistance, we are unable to recommend any specific antibiotic regimen at this time.
Quality of the evidence
This review found only 3 trials, involving 233 women, suitable for inclusion. Many of the primary outcome data were from one study. While Chow 1977 had unclear risk of bias in one domain, it was at low risk of bias in all other domains. Ostergard 1970 was at low risk of bias in four domains and unclear in others. In Sangines 1969, the risk of bias was unclear in four domains whereas it was low in three other domains. The small number of women and the few events that occurred in all three included studies preclude any confident conclusions on the effectiveness of single antibiotic versus combination of antibiotics to treat septic abortion.
Potential biases in the review process
We reduced potential biases by using a comprehensive search strategy. As the search strategy found a trial as far back as the late 1960s, it is unlikely we missed any important studies. However, it is possible that the technological evolution of the pharmaceutical industries and the production of many potent antibiotics in recent years have discouraged further trials. The non‐inclusion of a trial from any of the regions in which septic abortion is prevalent may also have introduced some bias.
Agreements and disagreements with other studies or reviews
An RCT involving 56 women with septic abortion reported cure after 10 days of oral doxycycline and metronidazole (Savaris 2011). However, women were only randomised after intravenous antibiotics had been administered. Also, a retrospective cohort study reported similar cure rates in women with septic abortion who used clindamycin one, three, or four times daily (Guigno 2013). While antibiotic treatment is essential for women with septic abortion, it appears a combination of antibiotics may be slightly more effective than a single antibiotic.
Authors' conclusions
Implications for practice.
The available data and quality of the evidence from 3 small trials conducted over 30 years ago using antibiotics some of which are no longer in use in clinical practice is insufficient to advocate for a change in the existing treatment regimen for septic abortion. Furthermore, the emergence of new and antibiotic‐resistant strains of organisms suggests practice needs to be based on these realities.
Currently, combination antibiotic regimens such as intravenous gentamicin and clindamycin; ampicillin, gentamicin, and metronidazole; and levofloxacin and metronidazole or single broad‐spectrum agents such as imipenem, piperacillin‐tazobactam, and ticarcillin‐clavulanate have been recommended to target gram‐positive, gram‐negative, and anaerobic organisms. These should be started promptly prior to, during, and continued after evacuation of the uterus. Given this review's paucity of effectiveness data, we have no reason not to recommend this strategy. However, prompt and early diagnosis as well as cultures of appropriate specimen are imperative. The need for other adjunctive measures in the management of sepsis such as resuscitation with fluids, blood transfusion, oxygen therapy, surgical removal of uterus or tubes, and intensive care should be borne in mind. It seems reasonable to stress the importance of focusing on strategies to prevent the occurrence of unsafe abortions. In this regard, advocacy for health policy change, removal of appropriate legal restrictions, and the scaling up of public health measures to lessen the stigma associated with abortion are necessary. In addition, utilising preventative interventions such as testing for and treating sexually transmitted infections, routine use of perioperative antibiotics, training of caregivers in the use of sterile techniques, and improved access to effective contraception should be of great benefit.
Implications for research.
The findings of this review cannot confidently address the question of which policy of antibiotic treatment should be adopted for treating septic abortion. Well‐powered, multisetting, high‐quality RCTs that can provide reliable evidence as to whether or not the more commonly used antibiotics such as metronidazole for anaerobic bacteria, quinolones like ciprofloxacin, third‐generation cephalosporins like ceftriaxone, and other antibiotics like imipenem are effective either alone or in combination for treating septic abortion are needed.
Newer trials on this subject should focus on important endpoints like treatment failure, microbiological cure, fever resolution time, duration of bacteraemia, adverse events of the medications used, as well as long‐term morbidities such as chronic pelvic pain and subfertility. It is also important to consider antibiotic resistance patterns during the design of the studies. The challenge of poor antibiotic stewardship in resource‐poor settings such as sub‐Saharan Africa, Latin America and the Caribbean, and Southeast Asia with high burden of septic abortion and health systems challenges should also be borne in mind.
What's new
| Date | Event | Description |
|---|---|---|
| 19 April 2016 | Amended | New search conducted on 19 April 2016 |
History
Protocol first published: Issue 2, 2015 Review first published: Issue 7, 2016
| Date | Event | Description |
|---|---|---|
| 2 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
AU was supported by a fellowship offered by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy. We wished to acknowledge the contribution of Dr Bela Ganatra who offered her advice as a content expert to the review authors and Carol Manion for doing the study search for this review.
Appendices
Appendix 1. Appendix 1
1950 to 13 December 2014 (strategy 1)
MEDLINE via PubMed (abortion, septic OR ((sepsis OR septic) AND abortion)) AND (anti‐biotic agents OR antibiotic agents OR antibiotic* ) Results: 381 refs
December 2014 to 19 April 2016
Results:487 refs
1950 to 13 December 2014 (strategy 2) (abortion, septic OR ((sepsis OR septic) AND abortion)) AND (anti‐biotic agents OR antibiotic agents OR antibiotic* ) limited to Clinical Trial, Comparative Study, Controlled Clinical Trial, Multicenter Study, Randomized Controlled Trial, Meta‐Analysis, Review, Systematic Reviews. Results: 76 refs
December 2014 to 19 April 2016
Results: 0 Embase 1974 to 13 Dec 2014 (abortion, septic OR septic abortion) OR ((septic OR sepsis OR septicaemia) AND abortion) AND (antibiotics OR antibiotic agents)
Results: 16
Popline Accessed 13 December 2014 ("septic abortion" OR (sepsis AND abortion)) AND antibiotic* Results: 28 refs Lilacs 1982 to 13 Dec 2014 septic abortion* AND (antibiotic agents OR antibiotic*) (Strategy 1) Results: 0
septic abortion* (Strategy 2) Results: 75 septic abortion* (Strategy 3) limited to major focus Results: 54 refs
December 2014 to 19 April 2016
Results:2
AIM (African Index Medicus) 13 Dec 2014 septic AND abortion AND antibiotics Results: 0
Data and analyses
Comparison 1. Clindamycin versus penicillin plus chloramphenicol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever index (mean degree‐hour) ‐ all women | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐12.3 [‐25.12, 0.52] |
| 2 Fever index (mean degree‐hour) ‐ bacteraemic patients | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐11.70 [‐55.44, 32.04] |
| 3 Duration of hospitalisation | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.54, 0.54] |
| 4 Complications | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.57 [0.98, 58.61] |
1.4. Analysis.
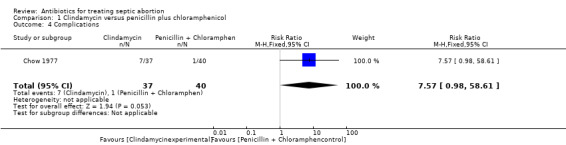
Comparison 1 Clindamycin versus penicillin plus chloramphenicol, Outcome 4 Complications.
Comparison 2. Penicillin plus chloramphenicol versus cephalothin plus kanamycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to fever resolution (mean time) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐17.31, 12.71] |
| 2 Absence of fever during D&C (mean time) | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐13.84, 11.84] |
Comparison 3. Antibiotics after D&C versus D&C during antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Absence of fever (degree‐hour) | 1 | 76 | Mean Difference (IV, Fixed, 95% CI) | ‐4.24 [‐17.08, 8.60] |
| 1.1 Clindamycin after D&C versus D&C during clindamycin | 1 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐6.0 [‐20.74, 8.74] |
| 1.2 Penicillin + chloramphenicol after D&C versus D&C during penicillin + chloramphenicol therapy) | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [‐24.84, 27.44] |
Comparison 4. Tetracycline versus penicillin G.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever resolution time (days) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐5.03 [‐5.77, ‐4.29] |
| 2 Leukocytosis resolution time (days) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐4.88 [‐5.98, ‐3.78] |
4.2. Analysis.
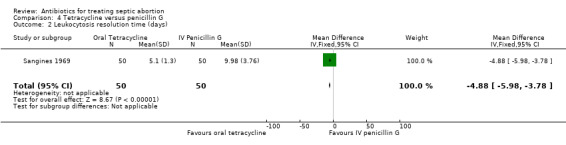
Comparison 4 Tetracycline versus penicillin G, Outcome 2 Leukocytosis resolution time (days).
Comparison 5. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pelvic abscess, salpingitis, urinary tract infection | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 4.32 [0.51, 36.95] |
| 2 Treatment failure | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.39 [0.27, 108.80] |
| 3 Drug reaction | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.14, 77.06] |
| 4 Phlebitis | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.56] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chow 1977.
| Methods | Randomised controlled trial | |
| Participants | 77 febrile women (fever =100.4°F or 38°C) with a clinical diagnosis of septic abortion and no known history of allergy to penicillin | |
| Interventions | Clindamycin alone versus penicillin plus chloramphenicol. Clindamycin (300 mg) was administered intravenously every 6 hours, and a bottle of 0.85% NaCl was also administered intravenously to keep the procedure double blind. Doses of 5 x 106 units of penicillin and 1 g of chloramphenicol were administered by separate iv infusions every 6 hours.Therapy continued until women became afebrile for 48 hr | |
| Outcomes | Woman’s fever index (quantitative measure of the overall amount of fever reported in degree hours), duration of hospitalisation, and incidence of complications | |
| Notes | Place of study: California, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned a therapeutic regimen" |
| Allocation concealment (selection bias) | Low risk | All medications were mixed by the hospital pharmacy in unmarked infusion bottles, and treatment was determined by sequentially drawing a sealed envelope containing the antibiotic protocol |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Doses of 5 x 106 units of penicillin and 1 g of chloramphenicol were administered by separate iv infusions every 6 hr. Clindamycin (300 mg) was administered iv every 6 hrs, and a bottle of 0.85% NaCl was also administered iv to keep the procedure double blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The code was only broken if woman's condition deteriorated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All results of all outcomes were presented adequately including adverse events |
| Other bias | Low risk | None |
Ostergard 1970.
| Methods | Randomised controlled trial | |
| Participants | 56 women with diagnosis of septic abortion | |
| Interventions | Penicillin and chloramphenicol group (10 million units and 1.0 g intravenously at 4 hr, 8 hr, and 12 hr) versus cephalothin (2.0 g intravenously every 6 hr) and kanamycin (15 mg/kg body weight intramuscularly every 24 hr in divided doses for 5 days or until woman was afebrile. Women in both groups also had D&C within 12 to 24 hr after starting antibiotic therapy | |
| Outcomes |
|
|
| Notes | Place of study: California, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Before the study was started, 2 protocols were prepared, each differing only in the antibiotic therapy employed. An equal number of each of the protocols was placed in plain envelopes, sealed, and randomly distributed |
| Allocation concealment (selection bias) | Unclear risk | When a woman was admitted with the diagnosis of septic abortion, a sealed envelope was opened and the antibiotic therapy outlined within it was assigned to the woman. It is unclear whether the envelope was transparent or opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not stated that participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated that investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of women lost to follow‐up was not stated. However, all women assigned into each group were included in the final analysis |
| Selective reporting (reporting bias) | Low risk | All specified outcomes were reported |
| Other bias | Low risk | None |
Sangines 1969.
| Methods | Randomised controlled trial | |
| Participants | Women living in Mexico City hospitalised with a diagnosis of septic abortion defined as abortion or miscarriage syndrome accompanied by 1 or more of the following criteria: history of abortion manoeuvres, fever, leukocytosis over 10,000/mm3, purulent vaginal discharge Exclusion: women who had received any antibiotic treatment before entering the study 100 women were randomised, 50 in each group |
|
| Interventions | Group 1: Tetracycline with streptokinase and streptodornase (Ledermycin, Varidase) capsules taken orally every 8 hours starting from the entry date to6 days after the discharge. The daily dose of tetracycline was 600 mg per day. No specification of the doses of streptokinase and streptodornase Group 2: Intravenous 10,000,000 units of penicillin G sodium |
|
| Outcomes |
|
|
| Notes | All the women received oxytocic drugs and analgesic. Most had D&C, 39 in tetracycline group and 47 in penicillin G group. A reference to the dosage and numbers of days penicillin G group will be treated was cited, but the translator of Sangines 1969 could not access it | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. It was only mentioned that study was randomised |
| Allocation concealment (selection bias) | Unclear risk | How allocation was concealed was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not described if the participants or the personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not described if the assessor of the cultures of the vaginal discharge and the curettage were blinded to the clinical details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the women initially randomised were followed up. However, for 1 (days to fever resolution) data were incomplete |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported in the published report |
| Other bias | Low risk | None |
D&C: dilation and curettage iv: intravenous
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abudu 1986 | No control arm |
| Dahm 1973 | 18 women in the control arm were treated with penicillin alone or in combination with streptomycin, or chloramphenicol and ampicillin in the case of 1 woman. Effect of each treatment was not separated. There is no way to know which treatment contributed to improvement or harm |
| Horta 1971 | Study did not define what treatment was administered to the control arm. Historical controls used |
| Savaris 2011 | Women were randomised into intervention groups after they had received antibiotics and D&C and been discharged from hospital. Day 1 of the study was 48 hours after hospital discharge |
| Sivasamboo 1968 | Study included cases of threatened abortion with clinical features similar to septic abortion. Data could not be separated from that for septic abortion |
Characteristics of ongoing studies [ordered by study ID]
NCT02309346.
| Trial name or title | Clindamycin Once a Day in Septic Abortion (CLINDA‐PRO) |
| Methods | Randomised clinical trial |
| Participants | Women with clinical diagnosis of septic abortion |
| Interventions | Clindamycin once and thrice a day |
| Outcomes | Cure (clinical improvement defined as reduction of pain and bleeding and no fever for 48 hr) |
| Starting date | November 2014 |
| Contact information | Daniel M da Silva +555133597542; danielmsilva@hcpa.ufrgs.br; Ernesto P Guedes Neto, MD, PhD: +555132211455; epgneto@gmail.com |
| Notes |
Differences between protocol and review
We included fever resolution time as an additional secondary outcome.
Contributions of authors
AU wrote the Background and Methods sections of this review under the guidance of EEE. OO extracted data directly into RevMan, and BOO cross‐checked entry for accuracy. OO and EEE wrote the results, while BOO and EEE discussed the results. .
Sources of support
Internal sources
University of Calabar and University of Calabar Teaching Hospital, Nigeria.
Cochrane Nigeria, Nigeria.
External sources
No sources of support supplied
Declarations of interest
Atim Udoh: None known. Emmanuel E Effa: None known. Olabisi Oduwole: None known. Babasola O Okusanya: None known. .
New
References
References to studies included in this review
Chow 1977 {published data only}
- Chow AW, Marshall JR, Guze LB. A double‐blind comparison of clindamycin with penicillin plus chloramphenicol in treatment of septic abortion. The Journal of Infectious Diseases 1977;135:535‐9. [DOI] [PubMed] [Google Scholar]
Ostergard 1970 {published data only}
- Ostergard DR. Comparison of two antibiotic regimens in the treatment of septic abortion. Obstetrics and Gynecology 1970;36(3):473‐4. [PubMed] [Google Scholar]
Sangines 1969 {published data only}
- Sangines A, Salum M, Gonzãlez R, Ahued JR. Tetracycline‐enzymes in septic abortion. Ginecología y Obstetricia de México 1969;26(157):605–11. [PubMed] [Google Scholar]
References to studies excluded from this review
Abudu 1986 {published data only}
- Abudu O, Rotimi V, Uguru V, Aboola A. Cefoxitin: single agent treatment of septic abortion. African Journal of Medicine and Medical Sciences 1986;15:35‐40. [PubMed] [Google Scholar]
Dahm 1973 {published data only}
- Dahm CH, Ostapowicz F, Cavanagh D. Use of cephalothin in septic abortion. Obstetrics & Gynecology 1973;41(5):693‐6. [PubMed] [Google Scholar]
Horta 1971 {published data only}
- Horta S, Franck C, Ramirez J. Treatment of septic abortion with rifampicin [Tratamiento del abortion septico con rifampicina]. Revista Chilena de Obstetricia y Ginecologia 1971;36(3):119‐21. [PubMed] [Google Scholar]
Savaris 2011 {published data only}
- Savaris RF, Moraes GS, Cristovam RA, Braun RD. Are antibiotics necessary after 48 hours of improvement in infected/septic abortions? A randomized controlled trial followed by a cohort study. American Journal of Obstetrics and Gynecology 2011;204(4):301.e1‐5. [DOI: ] [DOI] [PubMed] [Google Scholar]
Sivasamboo 1968 {published data only}
- Sivasamboo R, Lean TH, Goon SM. Ampicillin in septic abortion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1968;75:264‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT02309346 {published data only}
- NCT02309346. Clindamycin Once a Day in Septic Abortion (CLINDA‐PRO). clinicaltrials.gov/show/NCT02309346 (accessed 23 August, 2015).
Additional references
Achilles 2012
- Achilles SL, Reeves MF. Prevention of infection after induced abortion. Contraception 2011;83(4):295‐309. [DOI] [PubMed] [Google Scholar]
Adler 2012
- Adler AJ, Filippi V, Thomas SL, Ronsmans C. Quantifying the global burden of morbidity due to unsafe abortion: Magnitude in hospital‐based studies and methodological issues. International Journal of Gynecology & Obstetrics 2012;118(Suppl 2):S65–S77. [DOI] [PubMed] [Google Scholar]
Ahman 2011
- Ahman E, Shah I. New estimates and trends regarding unsafe abortion mortality. International Journal of Gynecology & Obstetrics 2011;115:121–6. [DOI] [PubMed] [Google Scholar]
Aldape 2006
- Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clinical Infectious Disease 2006;43:1436. [DOI] [PubMed] [Google Scholar]
ASRM 2013
- Practice Committee of the American Society for Reproductive Medicine in collaboration with the Society of Reproductive Surgeons. Pathogenesis, consequences, and control of peritoneal adhesions in gynecologic surgery: a committee opinion. Fertility and Sterility 2013;99(6):1550‐5. [DOI] [PubMed] [Google Scholar]
Barbacci 1986
- Barbacci MB, Spence MR, Kappus EW, Burkman RC, Rao L, Quinn TC. Postabortal endometritis and isolation of Chlamydia trachomatis. Obstetrics & Gynecology 1986;68(5):686‐90. [PubMed] [Google Scholar]
Bhattacharya 2010
- Bhattacharya S, Mukherjee G, Mistri P, Pati S. Safe abortion – still a neglected scenario: A study of septic abortions in a tertiary hospital of rural India. Online Journal of Health and Allied Sciences 2010;9(2):7. [Google Scholar]
Blackwell 1993
- Blackwell AL, Thomas PD, Wareham K, Emery SJ. Health gains from screening for infection of the lower genital tract in women attending for termination of pregnancy. The Lancet 1993;342(8865):206‐10. [DOI] [PubMed] [Google Scholar]
Butt 2012
- Butt S, Saydain G. Hypotension after medical termination of pregnancy: think outside of the uterus. Journal of Emergency Medicine 2012;43(1):50‐3. [DOI] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. Morbidity and Mortality Weekly Report 2015;64(3):81. [PMC free article] [PubMed] [Google Scholar]
Cherpes 2002
- Cherpes TL, Kusne S, Hillier SL. Haemophilus influenza septic abortion. Infectious Diseases in Obstetrics and Gynecology 2002;10:161‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dellinger 2008
- Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock. Critical Care Medicine 2008;36(1):296‐327. [DOI] [PubMed] [Google Scholar]
Dragoman 2014
- Dragoman M, Sheldon WR, Qureshi Z, Blum J, Winikoff B, Ganatra B, on behalf of the WHO Multicountry Survey on Maternal Newborn Health Research Network. Overview of abortion cases with severe maternal outcomes in the WHO Multicountry Survey on Maternal and Newborn Health: a descriptive analysis. British Journal of Obstetrics and Gynaecology 2014;121(Suppl 1):25–31. [DOI] [PubMed] [Google Scholar]
Eschenbach 2015
- Eschenbach DA. Treating spontaneous and induced septic abortions. Obstetrics & Gynecology 2015;125:1042–8. [DOI] [PubMed] [Google Scholar]
Fischer 2005
- Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Meter SH, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. The New England Journal of Medicine 2005;353:2352‐60. [DOI] [PubMed] [Google Scholar]
Gerdts 2013
- Gerdts C, Vohra D, Ahern J. Measuring unsafe abortion‐related mortality: a systematic review of the existing methods. PLoS One 2013;8(1):e53346. [DOI] [PMC free article] [PubMed] [Google Scholar]
GradeproGDT 2015
- GRADE Working Group. GRADE's software for Summary of Findings tables, Health Technology Assessment and Guidelines. www.gradepro.org (accessed 15 August 2016).
Gravett 2012
- Gravett CA, Gravett MG, Martin ET, Bernson JD, Khan S, Boyle DS, et al. Serious and life‐threatening pregnancy‐related infections: Opportunities to reduce the global burden. PLoS Medicine 2012;9:e1001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2006
- Grimes DA, Benson J, Singh S, Romero M, Ganatra B, Okonofua FE, et al. Unsafe abortion: the preventable pandemic. The Lancet 2006;368(9550):1908‐19. [DOI] [PubMed] [Google Scholar]
Guigno 2013
- Guigno CS, Silva AL, Fuhrich DG, Rabaioli PS, Goncalves KG, Sartor NC, et al. Daily dose of clindamycin versus standard divided doses in obstetrical and gynaecological infections: a retrospective cohort study. International Journal of STD & AIDS 2013;24(11):893‐8. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
Hazra 2013
- Hazra SK, Sarkar PK, Chaudhuri A, Mitra G, Banerjee D, Guha S. Septic abortion managed in a tertiary hospital in West Bengal. Journal of Basic and Clinical Reproductive Sciences 2013;2:38‐41. [Google Scholar]
Henderson 2013
- Henderson JT, Puri M, Blum M, Harper CC, Rana A, Gurung G. Effects of abortion legalization in Nepal, 2001–2010. PLoS ONE 2013;8(5):e64775. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Sytematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kassebaum 2014
- Kassebaum N, Bertozzi‐Villa A, Coggeshall M, Shackelford K, Steiner C, Heuton K, et al. Global, regional, and national levels and causes of maternal mortality during 1990–2013: a systematic analysis for the global burden of disease study 2013. The Lancet 2014;384:980–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kohanski 2010
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nature Review Microbiology 2010;8(6):423‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ledger 1975
- Ledger WJ, Kriewall TJ, Gee C. The fever index: a technique for evaluating the clinical response to bacteremia. Obstetrics & Gynecology 1975;45(6):603‐8. [DOI] [PubMed] [Google Scholar]
Low 2012
- Low N, Mueller M, Vliet HAAM, Kapp N. Perioperative antibiotics to prevent infection after first‐trimester abortion. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD005217.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
May 2007
- May W, Gülmezoglu AM, Ba‐Thike K. Antibiotics for incomplete abortion. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001779.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meites 2010
- Meites E, Zane S, Gould C. Fatal Clostridium sordellii infections after medical abortions. The New England Journal of Medicine 2010;363(14):1382‐3. [DOI] [PubMed] [Google Scholar]
NGCH 2012
- National Guideline Clearinghouse. Bacterial sepsis in pregnancy. https://www.guideline.gov/content.aspx?id=36901 (accessed 23 March 2014).
Nielsen 1995
- Nielsen S, Hahlin M. Expectant management of first‐trimester spontaneous abortion. The Lancet 1995;345(8942):84‐5. [DOI] [PubMed] [Google Scholar]
Nwagbara 2014 [pers comm]
- Nwagbara AB. (Melbourne School of Population and Global Health, University of Melbourne, Carlton Victoria). [personal communication]. Conversation with: Olabisi Oduwole (University of Calabar Teaching Hospital, Calabar) February 2014.
Nwogu‐Ikojo 2007
- Nwogu‐Ikojo EE, Ezegwui HU. Abortion related mortality in a tertiary medical center in Enugu, Nigeria. Journal of Obstetrics and Gynaecology 2007;27:835–7. [DOI] [PubMed] [Google Scholar]
Porter 2008
- Porter TF, Branch D, Scott J. Early pregnancy loss. In: Gibbs RS editor(s). Danforth's Obstetrics and Gynecology. 10th Edition. Philadelphia: Lippincott Williams and Wilkins, 2008:62‐70. [Google Scholar]
Rasch 2011
- Rasch V. Unsafe abortion and postabortion care ‐ an overview. Acta Obstetricia et Gynecologica Scandinavica 2011;90(7):692‐700. [DOI] [PubMed] [Google Scholar]
Raymond 2012
- Raymond EG, Grimes DA. The comparative safety of legal induced abortion and childbirth in the United States. Obstetrics & Gynecology 2012;119(2 Part 1):215‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Singh 2006
- Singh S. Hospital admissions resulting from unsafe abortion: estimates from 13 developing countries. The Lancet 2006;368:1887–92. [DOI] [PubMed] [Google Scholar]
Soper 2010
- Soper DE. Pelvic inflammatory disease. Obstetrics & Gynecology 2010;116(2 Part 1):419‐28. [DOI] [PubMed] [Google Scholar]
Stubblefield 1994
- Stubblefield PG, Grimes DA. Septic abortion. The New England Journal of Medicine 1994;331(5):310‐4. [DOI] [PubMed] [Google Scholar]
ten Broek 2013
- Broek RP, Issa Y, Santbrink EJ, Bouvy ND, Kruitwagen RF, Jeekel J, et al. Burden of adhesions in abdominal and pelvic surgery: systematic review and meta‐analysis. BMJ 2013;347(f5):588. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toure 1992
- Toure B, Thonneau P, Cantrelle P, Barry TM, Ngo‐Khac T, Papiernik E. Level and causes of maternal morality in Guinea (West Africa). International Journal of Gynecology & Obstetrics 1992;37:89‐95. [DOI] [PubMed] [Google Scholar]
Trinder 2006
- Trinder J, Brocklehurst P, Porter R, Read M, VyasS, Smith L. Management of miscarriage: expectant, medical, or surgical? Results of randomised controlled trial (miscarriage treatment (MIST) trial). BMJ 2006;332(7552):1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warriner 2006
- Warriner IK, Shah IH (editors). Preventing Unsafe Abortion and Its Consequences: Priorities for Research and Action. New York: Guttmacher Institute, 2006. [Google Scholar]
WHO 1994
- World Health Organization, Department of Reproductive Health and Research. Clinical management of abortion complications: a practical guide. http://www.who.int/reproductivehealth/publications/unsafe_abortion/MSM_94_1/en/ (accessed 17 September 2014).
WHO 2007
- World Health Organization. Unsafe Abortion: Global and Regional Estimates of the Incidence of Unsafe Abortion and Associated Mortality in 2003. 5th Edition. Geneva: WHO, 2007. [Google Scholar]
WHO 2011
- World Health Organization Department of Reproductive Health and Research. Unsafe Abortion: Global and Regional Estimates of Incidence of Unsafe Abortion and Associated Mortality in 2008. 6th Edition. Geneva: WHO, 2011. [Google Scholar]
WHO 2012
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd Edition. Geneva: WHO, 2012. [PubMed] [Google Scholar]
Zane 2011
- Zane S, Guarner J. Gynecologic Clostridial toxic shock in women of reproductive age. Current Infectious Disease Reports 2011;13:561‐70. [DOI] [PubMed] [Google Scholar]


