Abstract
Background
Tibolone is a synthetic steroid used for the treatment of menopausal symptoms, on the basis of short‐term data suggesting its efficacy. We considered the balance between the benefits and risks of tibolone.
Objectives
To evaluate the effectiveness and safety of tibolone for treatment of postmenopausal and perimenopausal women.
Search methods
In October 2015, we searched the Gynaecology and Fertility Group (CGF) Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase and PsycINFO (from inception), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and clinicaltrials.gov. We checked the reference lists in articles retrieved.
Selection criteria
We included randomised controlled trials (RCTs) comparing tibolone versus placebo, oestrogens and/or combined hormone therapy (HT) in postmenopausal and perimenopausal women.
Data collection and analysis
We used standard methodological procedures of The Cochrane Collaboration. Primary outcomes were vasomotor symptoms, unscheduled vaginal bleeding and long‐term adverse events. We evaluated safety outcomes and bleeding in studies including women either with or without menopausal symptoms.
Main results
We included 46 RCTs (19,976 women). Most RCTs evaluated tibolone for treating menopausal vasomotor symptoms. Some had other objectives, such as assessment of bleeding patterns, endometrial safety, bone health, sexuality and safety in women with a history of breast cancer. Two included women with uterine leiomyoma or lupus erythematosus.
Tibolone versus placebo
Vasomotor symptoms
Tibolone was more effective than placebo (standard mean difference (SMD) ‐0.99, 95% confidence interval (CI) ‐1.10 to ‐0.89; seven RCTs; 1657 women; moderate‐quality evidence), but removing trials at high risk of attrition bias attenuated this effect (SMD ‐0.61, 95% CI ‐0.73 to ‐0.49; odds ratio (OR) 0.33, 85% CI 0.27 to 0.41). This suggests that if 67% of women taking placebo experience vasomotor symptoms, between 35% and 45% of women taking tibolone will do so.
Unscheduled bleeding
Tibolone was associated with greater likelihood of bleeding (OR 2.79, 95% CI 2.10 to 3.70; nine RCTs; 7814 women; I2 = 43%; moderate‐quality evidence). This suggests that if 18% of women taking placebo experience unscheduled bleeding, between 31% and 44% of women taking tibolone will do so.
Long‐term adverse events
Most of the studies reporting these outcomes provided follow‐up of two to three years (range three months to three years).
Breast cancer
We found no evidence of differences between groups among women with no history of breast cancer (OR 0.52, 95% CI 0.21 to 1.25; four RCTs; 5500 women; I2= 17%; very low‐quality evidence). Among women with a history of breast cancer, tibolone was associated with increased risk (OR 1.5, 95% CI 1.21 to 1.85; two RCTs; 3165 women; moderate‐quality evidence).
Cerebrovascular events
We found no conclusive evidence of differences between groups in cerebrovascular events (OR 1.74, 95% CI 0.99 to 3.04; four RCTs; 7930 women; I2 = 0%; very low‐quality evidence). We obtained most data from a single RCT (n = 4506) of osteoporotic women aged 60 to 85 years, which was stopped prematurely for increased risk of stroke.
Other outcomes
Evidence on other outcomes was of low or very low quality, with no clear evidence of any differences between the groups. Effect estimates were as follows:
• Endometrial cancer: OR 2.04, 95% CI 0.79 to 5.24; nine RCTs; 8504 women; I2 = 0%.
• Cardiovascular events: OR 1.38, 95% CI 0.84 to 2.27; four RCTs; 8401 women; I2 = 0%.
• Venous thromboembolic events: OR 0.85, 95% CI 0.37 to 1.97; 9176 women; I2 = 0%.
• Mortality from any cause: OR 1.06, 95% CI 0.79 to 1.41; four RCTs; 8242 women; I2 = 0%.
Tibolone versus combined HT
Vasomotor symptoms
Combined HT was more effective than tibolone (SMD 0.17, 95% CI 0.06 to 0.28; OR 1.36, 95% CI 1.11 to 1.66; nine studies; 1336 women; moderate‐quality evidence). This result was robust to a sensitivity analysis that excluded trials with high risk of attrition bias, suggesting a slightly greater disadvantage of tibolone (SMD 0.25, 95% CI 0.09 to 0.41; OR 1.57, 95% CI 1.18 to 2.10). This suggests that if 7% of women taking combined HT experience vasomotor symptoms, between 8% and 14% of women taking tibolone will do so.
Unscheduled bleeding
Tibolone was associated with a lower rate of bleeding (OR 0.32, 95% CI 0.24 to 0.41; 16 RCTs; 6438 women; I2 = 72%; moderate‐quality evidence). This suggests that if 47% of women taking combined HT experience unscheduled bleeding, between 18% and 27% of women taking tibolone will do so.
Long‐term adverse events
Most studies reporting these outcomes provided follow‐up of two to three years (range three months to three years). Evidence was of very low quality, with no clear evidence of any differences between the groups. Effect estimates were as follows:
• Endometrial cancer: OR 1.47, 95% CI 0.23 to 9.33; five RCTs; 3689 women; I2 = 0%.
• Breast cancer: OR 1.69, 95% CI 0.78 to 3.67; five RCTs; 4835 women; I2 = 0%.
• Venous thromboembolic events: OR 0.44, 95% CI 0.09 to 2.14; four RCTs; 4529 women; I2 = 0%.
• Cardiovascular events: OR 0.63, 95% CI 0.24 to 1.66; two RCTs; 3794 women; I2 = 0%.
• Cerebrovascular events: OR 0.76, 95% CI 0.16 to 3.66; four RCTs; 4562 women; I2 = 0%.
• Mortality from any cause: only one event reported (two RCTs; 970 women).
Authors' conclusions
Moderate‐quality evidence suggests that tibolone is more effective than placebo but less effective than HT in reducing menopausal vasomotor symptoms, and that tibolone is associated with a higher rate of unscheduled bleeding than placebo but with a lower rate than HT.
Compared with placebo, tibolone increases recurrent breast cancer rates in women with a history of breast cancer, and may increase stroke rates in women over 60 years of age. No evidence indicates that tibolone increases the risk of other long‐term adverse events, or that it differs from HT with respect to long‐term safety.
Much of the evidence was of low or very low quality. Limitations included high risk of bias and imprecision. Most studies were financed by drug manufacturers or failed to disclose their funding source.
Plain language summary
Short‐term and long‐term effects of tibolone in postmenopausal women
Review question
Cochrane review authors aimed to evaluate the effectiveness and safety of tibolone for treatment of postmenopausal and perimenopausal women.
Background
Tibolone is an available option for the treatment of menopausal symptoms, and short‐term data suggest its efficacy. However, healthcare providers must consider the balance between benefits and risks of tibolone, as concerns have arisen about breast and endometrial cancer and stroke.
Study characteristics
We included 46 randomised controlled trials (RCTs), which included 19,976 postmenopausal women. Most studies evaluated tibolone for treatment of menopausal vasomotor symptoms. Some studies reported other objectives: Four RCTs aimed to assess endometrial safety, four bleeding patterns, five bone loss or fracture prevention, one sexual outcomes and three safety in women with a history of breast cancer; two studies examined use of tibolone in women with fibroids or lupus erythematosus. The evidence is current to October 2015.
Key results
Moderate‐quality evidence suggests that tibolone is more effective than placebo and less effective than combined hormone therapy (HT) in reducing vasomotor symptoms in postmenopausal women. Data suggest that if 67% of women taking placebo experience vasomotor symptoms, then between 35% and 45% of women taking tibolone will do so; and if 7% of women taking combined HT experience vasomotor symptoms, then between 8% and 14% of women taking tibolone will do so. Moderate‐quality evidence also suggests that tibolone is associated with a higher rate of unscheduled bleeding than placebo, but a lower rate than HT.
Compared with placebo, tibolone increases the risk of recurrent breast cancer in women with a history of breast cancer, and may increase the risk of stroke in women over 60 years of age. No evidence suggests that tibolone increases the risk of other serious adverse events, and no evidence shows differences between tibolone and HT with respect to long‐term adverse events. Nearly all evidence on adverse events was of very low quality, and reported events were scarce.
Quality of the evidence
Much of the evidence obtained was of low or very low quality. Limitations included high risk of bias in the included trials, very low event rates and potential conflicts of interest. Twenty‐six of the studies were financed by drug manufacturers, and another 14 studies failed to disclose their source of funding.
Summary of findings
Summary of findings for the main comparison. Tibolone compared with placebo for treatment of vasomotor symptoms in postmenopausal women.
| Tibolone compared with placebo: vasomotor symptoms | ||||||
| Population: postmenopausal women with vasomotor symptoms Settings: outpatient or community Intervention: tibolone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tibolone | |||||
|
Vasomotor symptoms (all doses) Follow‐up: 12 weeks to 1 year |
670 per 1000 | 400 per 1000 (350 to 450) |
OR 0.33 (0.27 to 0.41) | 842 (5 RCTs) |
⊕⊕⊝⊝ moderatea | Three studies at high risk of attrition bias were excluded from this analysis. Inclusion of these studies was associated with stronger effect of tibolone but with extreme heterogeneity (I2= 97%) |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded one level for serious risk of bias: poor reporting of study methods and potential conflict of interest (pharmaceutical funding) in most studies; standard deviations imputed for some studies. Effect estimate robust to a sensitivity analysis excluding studies at high risk of attrition bias
Summary of findings 2. Tibolone compared with placebo for postmenopausal women: adverse events.
| Tibolone compared with placebo: adverse events | ||||||
| Population: postmenopausal women with or without vasomotor symptoms Settings: outpatient or community Intervention: tibolone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tibolone | |||||
| Endometrial cancer (all doses) Follow‐up: 1 to 3 years (median 1) | See comment | OR 2.04 (0.79 to 5.24) | 8504 (9 studies) | ⊕⊝⊝⊝ very lowa,b,c | Events very rare in both groups. Total of 21 events: 16/4486 in tibolone group, 5/4018 in placebo group | |
| Breast cancer; women without previous breast cancer (all doses) Follow‐up: 12 weeks to 3 years | 4 per 1000 | 1 per 1000 (1 to 5) |
OR 0.52 (0.21 to 1.25) | 5500 (4 studies) | ⊕⊝⊝⊝ very lowa,b | In women with a history of breast cancer, risk increased in the tibolone group at 1 to 2.75 years' follow up: OR 1.50 (1.21 to 1.85, 2 RCTs, 3165 women, moderate‐quality evidence ) |
|
Unscheduled bleeding (all doses) Follow‐up: 1 to 3 years (median 2) |
177 per 1000 | 374 per 1000 (310 to 442) |
OR 2.79 (2.1 to 3.7) | 7814 (9 studies) | ⊕⊕⊝⊝ moderated | |
| Venous thromboembolic events (clinical evaluation) all doses Follow‐up: 1 to 2.75 years (median 1.5) | See comment | OR 0.85 (0.37 to 1.97) | 9176 (5 studies) | ⊕⊝⊝⊝ very lowa,b,c | Events very rare in both groups. Total of 24 events: 12/5054 in tibolone group, 12/4122 in placebo group | |
|
Cardiovascular events (all doses) Follow‐up: 2 to 3 years (median 2.75) |
10 per 1000 | 13 per 1000 (8 to 22) |
1.38 (0.84 to 2.27) |
8401 (4 studies) |
⊕⊝⊝⊝ very lowa,b,c | |
| Cerebrovascular events (all doses) Follow‐up: 14 days to 2.8 years | 5 per 1000 | 8 per 1000 (4 to 14) |
OR 1.74 (0.99 to 3.04) | 7930 (4 studies) | ⊕⊝⊝⊝ very lowa,b | |
| Mortality from any cause (all doses) Follow‐up: 1 to 3 years (median 2.77) | 10 per 1000 | 10 per 1000 (8 to 14) |
OR 1.06 (0.79 to 1.41) | 8242 (4 studies) | ⊕⊕⊝⊝ lowb,e | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded two levels for very serious risk of bias: poor reporting of study methods, high attrition and/or potential conflict of interest in most studies
bDowngraded one level for serious imprecision: low event rate. Findings compatible with meaningful benefit in one or both arms, or with no effect
cDowngraded one level for serious risk of low applicability: Some studies compare doses of tibolone that have not been marketed (although downgrading has no effect on rating, as study already rated very low)
dDowngraded one level for serious risk of bias: poor reporting of study methods and potential conflict of interest in most studies
eDowngraded one level for potential conflict of interest (funding by pharmaceutical companies)
Summary of findings 3. Tibolone compared with combined HT for treatment of vasomotor symptoms in postmenopausal women.
| Tibolone compared with combined HT for postmenopausal women: vasomotor symptoms | ||||||
| Population: postmenopausal women with vasomotor symptoms Settings: outpatient or community Intervention: tibolone Comparison: combined HT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combined HT | Tibolone | |||||
| Vasomotor symptoms (tibolone 2.5 mg/d) Follow‐up: 3 to 12 months | 70 per 1000 | 110 per 1000 (80 to 140) |
OR 1.57 (1.18 to 2.1) | 646 (4 studies) |
⊕⊝⊝⊝ moderatea | From a sensitivity analysis excluding studies with high risk of attrition bias. An inclusive analysis (9 studies, 1336 participants) suggests a similar but slightly reduced disadvantage of tibolone (OR (95% CI) 1.36 (1.11 to 1.66)) |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded one level for serious risk of bias: poor reporting of study methods and potential conflict of interest in all studies. Effect estimate robust to a sensitivity analysis excluding studies at high risk of attrition bias
Summary of findings 4. Tibolone compared with combined HT for postmenopausal women: adverse events.
| Tibolone compared with combined HT for postmenopausal women: adverse events | ||||||
| Population: postmenopausal women with or without vasomotor symptoms Settings: outpatient or community Intervention: tibolone Comparison: combined HT | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Combined HT | Tibolone | |||||
| Unscheduled bleeding (all doses) Follow‐up: 3 to 36 months (median 12) | 474 per 1000 | 224 per 1000 (178 to 270) | OR 0.32 (0.24 to 0.41) | 6438 (16 studies) | ⊕⊕⊝⊝ moderatea | |
| Endometrial cancer (all doses) Follow‐up: 6.8 to 36 months (median 12) | See comments | OR 1.47 (0.23 to 9.33) | 3689 (5 studies) | ⊕⊝⊝⊝ very lowb,c | Events very rare in both groups. Total of 3 events: 2/1826 in tibolone group, 1/1863 in combined HT group | |
| Breast cancer; women without previous breast cancer (all doses) Follow‐up: 6.8 to 36 months (median 24) | 3 per 1000 | 6 per 1000 (3 to 13) |
OR 1.69 (0.78 to 3.67) | 4835 (5 studies) | ⊕⊝⊝⊝ very lowb,c | |
| Venous thromboembolic events (clinical evaluation; all doses) Follow‐up: 6.8 to 24 months (median 12) | 3 per 1000 | 1 per 1000 (0 to 6) |
OR 0.44 (0.09 to 2.14) | 4529 (4 studies) | ⊕⊝⊝⊝ very lowb,c | |
| Cardiovascular events (all doses) Follow‐up: 2 to 3 years | 17 per 1000 | 10 per 1000 (4 to 27) |
OR 0.63 (0.24 to 1.66) | 3794 (2 studies) | ⊕⊝⊝⊝ very lowb,c | |
| Cerebrovascular event (all doses) Follow‐up: 3.4 to 24 (median 9.4) months | 1 per 1000 | 1 per 1000 (0 to 3) |
OR 0.76 (0.16 to 3.66) | 4562 (4 studies) | ⊕⊝⊝⊝ very lowb,c | |
| Mortality from any cause (tibolone 2.5 mg/d) Follow‐up: 3.4 to 24 (median 9.4) months | See comments | OR 3.05 (0.12 to 75.2) | 970 (2 studies) | ⊕⊝⊝⊝ very lowb,c | Only 1 event (in tibolone group): 1/485 vs 0/485 | |
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded one level for serious risk of bias: poor reporting of study methods and potential conflict of interest in some studies
bDowngraded two levels for very serious risk of bias: poor reporting of study methods and potential conflict of interest in some studies
cDowngraded one level for serious imprecision: low event rate. Findings compatible with meaningful benefit in one or both arms, or with no effect
Background
Description of the condition
Hot flushes are among the most characteristic clinical symptoms of menopause (Politi 2008); they are probably caused by lability in the hypothalamic thermoregulatory centre induced by reduction of oestrogen and progesterone levels (Freedman 1995). Hot flushes and sweats of increasing severity can occur during the night, leading to sleep problems (Porter 1996). Hot flushes and sweats are described as vasomotor symptoms.
Many postmenopausal women report a variety of symptoms such as vaginal dryness (Suckling 2006), sexual discomfort, urinary incontinence (Cody 2012) and frequent urinary infection, probably resulting from the natural decline of oestrogen levels (Speroff 2004).
All symptoms tend to fluctuate, and their perceived severity varies greatly among individuals, with some reporting intense discomfort and a substantial reduction in quality of life.
Researchers have successfully used oestrogens and progestogens to ameliorate vasomotor (MacLennan 2004) and vaginal symptoms (Suckling 2006), anxiety and low mood (NCC‐WCH 2015). Urinary tract infections are less clearly influenced by combined hormone therapy (HT) (Soc Obstetr Gynaecol Canada 2014).
Description of the intervention
Tibolone (Livial®, ORG OD 14) is a synthetic steroid widely prescribed to postmenopausal women in Europe.
How the intervention might work
After its commercialisation, tibolone gained some popularity for combining oestrogenic and progestogen actions. Its mechanism of action is not well known, although many studies, most sponsored by the drug manufacturer, indicate that the drug undergoes different tissue‐selective metabolic transformations and may exert weak oestrogen, progestogen and/or androgen activities (Modelska 2002). The oestrogenic effects, exerted mainly in brain, bone and vaginal tissues, are weaker on the endometrium, where the drug is transformed into progestogen metabolites. In breast tissue, limited conversion of oestrone to oestradiol may reduce the oestrogenic effects. In brain and liver, tibolone seems to have androgenic effects. Some randomised controlled clinical trials (RCTs) have suggested that tibolone decreases vasomotor symptoms and ameliorates vaginal dryness and discomfort, but results are not consistent. An RCT published in 2009 (Kenemans 2009) highlighted that tibolone increases recurrence of breast cancer, revealing a contraindication for women with a history of breast cancer. Although the drug is thought to have a possible role in preserving bone mineral density, control of osteoporosis is not a recommended indication.
Why it is important to do this review
The safety profile of tibolone has not been well defined, and trials evaluating its use to treat patients with vasomotor symptoms usually provide follow‐up periods that are too short for assessment of potential long‐term adverse events such as increased risk of endometrial (Beral 2005) and breast (Kenemans 2009; Beral 2003) cancer and of cardiovascular events (Cummings 2008). For this reason, safety has been evaluated in a wider population, and RCTs including women who did not take tibolone for symptomatic relief have been considered.
Objectives
To evaluate the effectiveness and safety of tibolone for treatment of postmenopausal and perimenopausal women.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We did not include quasi‐randomised and cross‐over trials.
Types of participants
Menopausal and perimenopausal women with or without vasomotor and/or genital symptoms, defined as women with surgical menopause or with spontaneous menopause, or women who had menstruated irregularly over the past 12 months.
Types of interventions
Tibolone use versus placebo
Tibolone use versus oestrogens
Tibolone use versus combined HT (referring to two different formulations: sequential combined and continuous combined)
This review did not consider tibolone use versus no treatment.
Types of outcome measures
Primary outcomes
Vasomotor symptoms measured as occurrences or through scales, defined as any otherwise unexplained sensation of flushing/sweating experienced by the participant. We included studies that measured hot flushes (with or without night sweats), provided that they measured hot flushes as an outcome of efficacy in populations including symptomatic women
Unscheduled bleeding (vaginal bleeding and/or spotting)
Long‐term adverse events: endometrial cancer, breast cancer, venous thromboembolic events, cardiovascular events, cerebrovascular events, mortality from any cause
Secondary outcomes
Insomnia (frequency or continuous outcome)
Genital symptoms: vaginal dryness and painful sexual intercourse (measured as frequency or severity), vaginal infection (inflammation of the vagina usually related to one of three infectious conditions: bacterial vaginosis, vulvovaginal candidiasis, trichomoniasis), urinary tract infection
Endometrial hyperplasia
We measured all outcomes other than vasomotor symptoms in women with or without vasomotor symptoms.
We included studies assessing at least one of these specific outcomes, even if they did not report useable data. We excluded studies not assessing such outcomes.
Search methods for identification of studies
Electronic searches
We searched for all relevant published and unpublished RCTs, without language restriction, and in consultation with the Cochrane Gynaecology and Fertility Group (CGF) Information Specialist.
We searched the CGF Specialised Register (formerly known as the Menstrual Disorders and Subfertility Group Specialised Register), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), from inception until 15 October 2015, using the strategies shown in Appendix 1, Appendix 2, Appendix 3, Appendix 4, Appendix 5 and Appendix 6. For the search of clinicaltrials.gov, we used "tibolone" as a keyword. We contacted individual researchers and the current manufacturer of tibolone to ask them to identify unpublished and ongoing trials.
Searching other resources
We contacted individual researchers working in relevant fields (gynaecology, endocrinology) and the current manufacturer of tibolone (Merck Sharp & Dome) to check for additional relevant references and unpublished and ongoing trials. We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Four review authors (GF, EP, SM, SB) independently screened the titles and abstracts of articles found in the search for inclusion. We searched for outcomes of interest in the full texts, even if they had not been reported in the abstracts. We resolved disagreements by discussion and by consultation with two additional review authors (VB, a gynaecologist; and EM, an endocrinologist). We sought further information from study authors who published papers containing insufficient information to permit a decision about eligibility. We recorded reasons for excluding studies after debate and agreement.
Data extraction and management
Five review authors (GF, EP, SM, SB, JW) independently extracted details of study design, participants, interventions, follow‐up, quality components, efficacy outcomes and adverse events.
Three other review authors (VB, a gynaecologist; EM, an endocrinologist; and AMM, a cardiologist) resolved discrepancies regarding extraction of quantitative data or risk of bias assessment of RCTs. When a trial was presented in abstract form, we sought further information by searching the Internet, by contacting study authors and by checking for the next best available resource or publication. We contacted study authors for further insight on study design and results, when we considered this necessary. For studies with more than one publication, we extracted data from all publications, but we considered the final or updated version of each trial to be the primary reference.
We extracted the following information from the studies included in the review (see also Characteristics of included studies table).
Trial characteristics
Randomisation
Allocation concealment
Trial design: multi‐centre or single‐centre
Number of women randomised, excluded and analysed
Duration, timing and location of the trial
Source of funding and conflicts of interest
Baseline characteristics of studied groups
Definition and duration of preexisting menopausal condition
Age of the women
Previously administered treatment(s)
Interventions
Type of intervention and control
Dose regimen
Treatment duration
Outcomes
Outcomes reported
Definitions of outcomes
The way outcomes were measured
Timing of outcome measurement
If data were reported only in figures, we used Microsoft PowerPoint to extract data from the figures. We opened the figure in the software and overlaid a grid. We drew horizontal or vertical lines as needed, and we ‘snapped’ (aligned) them to this grid, to ensure that they were parallel/perpendicular to the plot axes, as required. We could move lines drawn in the software vertically and horizontally, so we could read off the value corresponding to a given data point in a scatterplot or the height of a bar in a bar chart against the appropriate axis. A single review author (JW) extracted data from figures.
Assessment of risk of bias in included studies
We assessed risk of bias of included trials by taking six components into account: generation of the allocation sequence (participant randomisation), allocation concealment, blinding (or masking) of participants and personnel, blinding of outcome assessment, completeness of follow‐up (attrition bias) and selective reporting. We used the following definitions when assessing risk of bias.
Generation of the allocation sequence
Adequate: if the allocation sequence was generated by a computer or by a random number table. We considered drawing of lots, tossing of a coin, shuffling of cards or throwing of die as adequate if a person not otherwise involved in recruitment of participants performed the procedure
Unclear: if the trial was described as randomised, but the method used for generation of the allocation sequence was not described
Inadequate: if a system involving dates, names or admittance numbers was used for allocation of women. We excluded these studies, known as quasi‐randomised, from the present review
We also excluded trials with alternating allocation.
Allocation concealment
Adequate: if allocation of women involved a central, independent unit; an on‐site locked computer; identical appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator; or sealed, opaque envelopes
Unclear: if the trial was described as randomised but the method used to conceal the allocation was not described
Inadequate: if the allocation sequence was known to investigators who assigned participants, envelopes were unsealed or transparent or the study was quasi‐randomised
Blinding (or masking) of participants and personnel
-
Adequate: if the trial was described as double‐blind and the method of blinding involved identical placebo or active drugs, particularly:
double‐blind (method described and use of a placebo(s) or dummy technique meant neither the participant nor the care provider or assessor knew which treatment was given)
single‐blind (participant, care provider or assessor was aware of the treatment given)
Unclear: if the trial was described as double‐blind or single‐blind but the method of blinding was not described
Not performed: if the trial was open‐label (all parties aware of treatment)
Blinding of outcome assessment
Adequate: if in the absence of blinding of outcome assessment, review authors judged that outcome measurement was not likely to be influenced by lack of blinding; or if blinding of outcome assessment was ensured and it was unlikely that blinding could have been broken
Unclear: if information was insufficient to permit judgement of ‘low risk’ or ‘high risk’, or if the study did not address this outcome
Inadequate: if no blinding of outcome assessment occurred and outcome measurement was likely to be influenced by lack of blinding; or if blinding of outcome assessment was present but blinding could have been broken, and if outcome measurement was likely to be influenced by lack of blinding
Completeness of follow‐up (attrition bias)
Adequate: if numbers and reasons for dropouts and withdrawals in all intervention groups were described and 90% or more of randomised participants were included in the analysis; or if it was specified that no dropouts or withdrawals occurred
Unclear: if the report gave the impression that no dropouts or withdrawals occurred but this was not specifically stated
Inadequate: if less than 90% of randomised participants were included in the analysis; or numbers or reasons for dropouts and withdrawals were not provided
We contacted the authors of primary trial reports when necessary to request clarification of data and to obtain missing information.
Selective reporting
Adequate: if the study protocol was available and all of the study’s prespecified (primary and secondary) outcomes of interest in the review were reported in the prespecified way
Unclear: if information was insufficient to permit judgement of ‘low risk’ or ‘high risk’
Inadequate: if not all of the study’s prespecified primary outcomes were reported; if one or more primary outcomes were reported via measurements, analysis methods or subsets of data (e.g. subscales) that were not prespecified; if one or more reported primary outcomes were not prespecified (unless clear justification for their reporting was provided, such as an unexpected adverse effect); if one or more outcomes of interest in the review were reported incompletely and could not be included in a meta‐analysis; or if the study report failed to include results for a key outcome that would have been expected to be reported for such a study
Measures of treatment effect
We evaluated efficacy and safety outcomes by considering the number of women in the control and intervention groups of each study experiencing at least one event (dichotomous outcomes) to calculate Mantel‐Haenszel odds ratios (DerSimonian 1986) with 95% confidence intervals (CIs), or (for continuous outcomes) mean scores, standard deviations and the number of women in each group, using the inverse variance method. The primary outcome ‘vasomotor symptoms’ and the secondary outcomes vaginal dryness and sleep were exceptions; we reported these outcomes as binary or continuous variables ‐ the first two using several scales. Accordingly, we converted all treatment effect estimates from binary or continuous variables to standardised mean differences (SMDs), as this permitted pooling of these variants in a meta‐analysis. Pooled SMDs computed in this manner can be transformed and interpreted as odds ratios, at the cost of information related to symptom severity (Higgins 2011).
Unit of analysis issues
This systematic review considered only RCTs. The unit of analysis in each RCT was the women who were randomised to one of the treatment arms. For vaginal bleeding, we considered endometrial hyperplasia and endometrial cancer only in women with a uterus.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible by including all randomised participants in the groups to which they were allocated. Missing data in included studies compromised realisation of this strategy. Moreover, options to rectify the matter were limited in the absence of individual participant data. Accordingly, we took the approach of penalising trials with notable rates of attrition in the risk of bias assessment and conducting sensitivity analyses that were restricted to trials with low risk of bias in this domain. We incorporated these sensitivity analyses into our conclusions.
Assessment of heterogeneity
We included in the meta‐analysis all outcomes reported by individual studies, noting heterogeneity by using Chi2 and I2 statistics (Higgins 2002). We stated that the Chi2 statistic was statistically significant if P < 0.10. The I2statistic indicated the percent of variability due to between‐study (or interstudy) variability, as opposed to within‐study (or intrastudy) variability. We considered an I2value greater than 50% to be large (Higgins 2002). When statistically significant heterogeneity existed, we conducted a careful clinical review of the data to seek the source of such heterogeneity and to decide whether statistical combining of trials was warranted.
Assessment of reporting biases
We graphically assessed publication bias by using contour‐enhanced funnel plots.
Data synthesis
We used a random‐effects model, except for vasomotor symptoms, vaginal dryness and sleep, for which we combined data from dichotomous and continuous outcomes in a fixed‐effect model by converting all treatment effect estimates to standardised mean differences (SMDs). We deemed this necessary because the key assumption of random‐effects meta‐analysis ‐ that all observed treatment effects represent realisations from a common underlying distribution ‐ did not appear to be warranted, given the diversity of outcome reporting scales used. Poor reporting standards required that we impute standard deviations for several studies reporting on menopausal symptoms to combine their results; we calculated all effect sizes and corresponding standard errors by using the metaphor package (Viechtbauer 2010) in R (R Core Team 2015). If results for this outcome were available at several time points, we used results corresponding to the longest period of use. Table 5 and Table 6 provide details of methods used in analyses of menopausal symptoms and vaginal dryness, as well as reasons for exclusion of several RCTs from these meta‐analyses.
1. Details on RCTs assessing vasomotor symptoms requiring additional data or analysis before data synthesis.
| Study | Comparator | Outcome measure | Information available | Notes | Results for meta‐analysis | SMD |
| Al‐Azzawi 1999 | HT | Presence of vasomotor symptoms, severity measured by Greene menopausal symptoms scale | 6 HRT and 9 tibolone patients were without symptoms at baseline. 67 HRT and 58 tibolone patients were free at month 3 | Contacted study authors, no reply | ||
| Baracat 2002 | HT | Total score: mean number of hot flushes per day multiplied by severity score | Means plotted as bar chart in Figure 1. Baseline, 11 for tibolone (n = 40), 12 for control (n = 45). At 3 months, 1.8 for tibolone and 1.5 for control. At 13 months, 0.2 for both |
Would have to impute SDs – ‘no significant difference’ Unclear how to do this, given the available info Unable to find contact details |
||
| Benedeck‐Jaszmann 1987 | Placebo | 0 to 3 severity score | 12 months From Fig 1: Mean P: 1.6 T: 0.6 SD P: 1 T: 0.9 N P: 19 T: 24 (assuming 30 per arm to start, not explicitly stated) |
Extracted from figure | Mean P: 1.6 T: 0.6 SD P: 1 T: 0.9 N P: 19 T: 24 |
SMD: ‐1.0384784 SE: 0.3268612 |
| Bouchard 2012 | Placebo | Severity score | Calculate 12 week values P: 1.59 T: 1.16 Sample sizes of 150 (P) and 164 (T) Wk 12 |
Use SD from sample size calc, which is in line with other studies | P: Mean 1.59 SD 0.9 N = 150 T: Mean 1.16 SD: 0.9 N = 164 |
SMD: ‐0.4766282 SE: 0.1145686 |
| Egarter 1996 | HT | Severity of hot flushes (modified Kupperman Index) | Baseline mean C: 2.1 T: 2.2 6 months C: 0.4 T: 0.4 ‘N/S’ N = 34 (C) N = 62 (T) |
Impute SD ‐ unclear how to Contacted study authors: no reply |
||
| Hammar 2007 | HT | Number of hot flushes | Week 48, baseline mean of both groups 6, follow‐up mean ≤ 1 Baseline SD C: 4.40 T: 4.37 N = 241 (C) N = 222 (T) |
Use baseline SDs (these appear reasonable, given Landgren 2002) | C: mean 1, SD 4.40; N = 241 T: mean 1, SD 4.37 N = 222 |
SMD: 0.00 SE: 0.09302624 |
| Hudita 2003 | Placebo (3 –arm study) | 5‐point severity scale for hot flushes | Week 24 P: 3 T: 1.25 mg: 0.2 T: 2.5mg: 0.1 N = 34 N = 45 N = 41 P < 0.01 for both compared with placebo |
Split control group size between 2 arms Used P value to calculate SD Get implausible answers. Used known value instead (e.g. Hammar 1998) |
Mean P: 3 T: 1.25 mg: 0.2 T: 2.5 mg: 0.1 N N = 34/2 = 17 N = 45 N = 41 SD P: 0.63 T: 1.25: 0.87 T: 2.5: 0.87 |
1.25 SMD: ‐3.4009511 SE: 0.4175209 2.5 SMD: ‐3.5375963 SE: 0.4371477 |
| Kokcu 2000 | HT | Occurrence of hot flushes | OR: 4.16 (0.75 to 22.9) | 2/19 have symptoms in C 12/19 have symptoms in T |
SMD: 1.6236743 SE: 0.5369759 |
|
| Landgren 2002 | Placebo (5‐arm study) | Frequency of hot flushes | Read means and SEs at 12 weeks from Figure 1 Mean P = 5.2 T 0.625 = 5 T 1.25 = 2.1 T 2.5 = 1.8 T 5.0 = 1.6 Standard error P = 0.37 T 0.625 = 0.37 T 1.25 = 0.40 T 2.5 = 0.43 T 5.0 = 0.37 Ns (calculated as all evaluable – dropouts ‐this assumes dropout occurred after 1st measurement at week 4) P = 113 T 0.625 = 129 T 1.25 = 124 T 2.5 = 139 T 5.0 = 136 |
Read means and SEs from Figure 1 Calculated SDs using SEs and sample sizes Split placebo group size in 4 113/4 = 28.25 |
Mean P = 5.2 T 0.625 = 5 T 1.25 = 2.1 T 2.5 = 1.8 T 5.0 = 1.6 SD P = 3.93 T 0.625 = 4.20 T 1.25 = 4.45 T 2.5 = 5.07 T 5.0 = 4.31 N (calculated as all evaluable – dropouts – this assumes dropout occurred after 1st measurement at week 4) P = 28.25 T 0.625 = 129 T 1.25 = 124 T 2.5 = 139 T 5.0 = 136 |
0.625 SMD: ‐0.04792794 SE: 0.20552850 1.25 SMD: ‐0.7077526 SE: 0.2102005 2.5 SMD: ‐0.6912512 SE: 0.2076033 5.0 SMD: ‐0.8437215 SE: 0.2097448 |
| Mendoza 2002 | HT | Flushes subscore of the Modified Kupperman Index, 0 to 2 score Number (%) reduced |
Have number and percentage that improved in terms of vasomotor symptoms after 1 year Have 2 possible control groups – choose the best performing to give a conservative estimate 25/26 reduced in control group 27/29 reduced in T groups |
Calculate odds ratio for reduced vasomotor symptoms. Turn this into an SMD for combination (27/2)/(25/1) = 0.54 SE log(OR) = Sqrt(1/27+1/2+1/25+1/1) = 1.26 |
OR for improvement: OR = 0.54 SE(log(OR)) = 1.26 (so T worse) |
SMD: 0.3734461 SE: 0.7610917 |
| Nappi 2006a | HT | Vasomotor symptoms (0 to 3 severity score) | At 6 months Means from Figure 4 C: 1.75 T: 1.5 P value for treatment term in ANOVA given as ‘P < 0.4’ N = 20 in both groups |
Assume ANOVA P value is 0.4 and work out SDs as though this was a t‐test Gives SD of 0.657, assuming same in both groups |
SMD: ‐0.3729492 SE: 0.3189649 |
|
| Ross 1999 | HT | Greene Climacteric Scale subscore | Nothing usable. Only present 1 of 6 relevant comparisons because it is almost significant. Do not present 3 month score | |||
| Siseles 1995 | HT | Kupperman Index | No information given for vasomotor subscale | Have contacted study authors, no reply | ||
| Swanson 2006 | Placebo (3‐arm study) | Number of hot flushes per day | Median change from baseline at week 12 ‐5.5 P ‐9.7 T 2.5 ‐8.3 T 1.25 P < 0.001 for T 2.5 vs P P < 0.003 for T 125 vs P N P: 133 T 2.5: 125 T 1.25: 133 Actually, mean changes at week 12 and P values given in abstract T 2.5 vs P ‐10.14 vs ‐5.85, P < 0.001 T 1.25 vs P, week12 ‐8.32 P < 0.003 |
Use reported values and calculate as for t‐tests. Split placebo group in half. Will have to impute SDs and final scores, as changes cannot be pooled with final scores if SMDs are used. For baseline, take median of values from Hammar 2007 and Landgren 2002 6,6,8,8,8,9,9.7 Mean 7.8. Too low – Figure 2 shows large changes. Say, 10 P: 10 ‐ 5.85 = 4.15 T 2.5: 10 ‐ 10 = 0 T 1.25: 10 ‐ 8.32 = 1.68 SDs too large when calculated from t‐test. Use values from Langren: P: 3.93 T 2.5: 5.07 T 1.25: 4.45 |
Mean P: 10 ‐ 5.85 = 4.15 T 2.5: 10 ‐ 10 = 0 T 1.25: 10 ‐ 8.32 = 1.68 SD P: 3.93 T 2.5: 5.07 T 1.25: 4.45 N P: 66 T 2.5: 125 T 1.25: 133 |
1.25 SMD: ‐0.5741771133 SE: 0.1532927 2.5 SMD: ‐0.9661562 SE: 0.1599848 |
| Vieira 2009 | Placebo | Kupperman Index | Only overall Kupperman Index shown | Have contacted study authors, no reply | ||
| Volpe 1986 | Placebo HT |
0 to 9 score, with 0 = absent, 3 = mild, 6 = moderate, 9 = severe Unclear whether intermediate scores are possible |
Can extract means for 24 weeks for tibolone arm, placebo arm and each of several HT arms, which have been partially combined, from Figure 1 in the paper | No real way to calculate SD from info in the paper, and the scale is different from those used in other studies (so not reasonable to use one from another study) | ||
| Wender 2004 | Placebo | Kupperman Index | Only overall Kupperman Index shown | Have contacted study authors, no reply |
2. Details on RCTs assessing vaginal dryness requiring additional data or analysis before data synthesis.
| Study | Comparator | Outcome measure | Information available | Method used | Results for meta‐analysis | SMD |
| Hudita 2003 | Placebo (3‐arm study) | 0 to 4 scale | From figure Week 24 P: 2.6 T 1.25 mg: 1 T 2.5 mg: 0.9 N = 34/2 = 17 N = 45 N = 41 |
Split control group size between 2 arms Use known value from other study for SD Use those from Nappi 2006a SD T: 0.89 HT: 0.89 |
Mean P: 2.6 T 1.25 mg: 1 T 2.5 mg: 0.9 N N = 34/2 = 17 N = 45 N = 41 SD P: 0.89 T 1.25: 0.89 T 2.5: 0.89 |
1.25mg SMD: ‐1.7751711 SE: 0.3262804 2.5mg SMD: ‐1.8843965 SE: 0.3373802 |
| Kenemans 2009 | Placebo | Vaginal dryness as binary | P: 33/1558 T: 19/1575 |
Convert OR to SMD | P: 33/1558 T: 19/1575 |
|
| Swanson 2006 | Placebo (3‐arm study) | 0 to 3 score | Mean change from baseline at week 12 P: ‐0.2 T 2.5: ‐0.26 T 1.25: ‐0.39 N P: 133 T 2.5: 125 T 1.25: 133 |
Split control group size between 2 arms Calculate final means using baseline and change – but no baseline values given Would also need to use SDs from another study |
Cannot use | |
| Huber 2002 | HT | Vaginal dryness as binary | HT: 7/166 T: 6/158 |
Convert OR to SMD | HT: 7/166 T: 6/158 |
SMD: ‐0.06613757 SE: 0.34411866 |
| Kokcu 2000 | HT | Vaginal dryness as binary | HT: 0/21 T: 1/23 |
Convert OR to SMD | HT: 0/21 T: 1/23 |
SMD: 0.6382727 SE: 1.0064298 |
| Ziaei 2010 | HT and placebo | Vaginal dryness as binary Also, lubrication scores 1 to 5, higher is better – can reverse signs of mean differences |
HT: 20/42 T: 33/47 P: 37/48 Mean HT: 4.93 T: 4.58 P: 3.65 SD HT: 1.95 T: 1.26 P: 1.81 |
Use the continuous data Calculate and reverse sign, so that greater = increased vaginal dryness |
HT: 20/42 T: 33/47 P: 37/48 |
Using OR to SMD vs HT SMD: 0.5774306 0.2691251 vs placebo SMD ‐0.5904427 SE: 0.2096301 Using lubrication scores vs HT: SMD after switching sign: 0.2138954 SE: 0.2129393 vs placebo: SMD after switching sign: ‐0.1313959 SE: 0.2185150 |
| Nappi 2006a | HT | Vaginal dryness 0 to 3 score | From Figure 4, mean at 6 months Mean T: 0.7 HC: 0.6 SD: can read SE off Figure 4 and calculate SD N = 20 both groups |
SD: can read SE off Figure 4 and calculate SD T: 0.89 HT: 0.89 |
Mean T: 0.7 HC: 0.6 SD T: 0.89 HT: 0.89 N = 20 |
SMD: 0.1101248 SE: 0.3164674 |
| Uygur 2005 | HT | 7‐point scale with ‐3 as worsened a lot and 3 as improved a lot | 6 months Mean (higher is better) HT: 0 T: 0.56 N HT: 34 T: 38 |
P < 0.05 given. Assume P = 0.05 and calculate SD, assuming equal in 2 groups: Gives SD = 1.7 |
Mean (higher is better) HT: 0 T: 0.56 N HT: 34 T: 38 Sd=1.7 for both |
SMD after sign change: ‐0.3258676 0.2376236 |
We sought the following comparisons.
Tibolone use, stratified by dose, versus placebo.
Tibolone use, stratified by dose, versus oestrogens.
Tibolone use, stratified by dose, versus combined HT.
To avoid multiple‐counting of a control group in RevMan, we split the numbers of events and of exposed participants in studies with multiple arms, depending on the number of comparisons, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011; see paragraph 16.5.4). We did not perform this procedure in cases of rare events (e.g. when one or three cases should have been split) or when estimated odds ratios differed by more than 0.05 from the non‐stratified analysis. In the latter case, we combined intervention groups (e.g. different doses of tibolone) to create a single pair‐wise comparison versus the control group.
Subgroup analysis and investigation of heterogeneity
We stratified results according to tibolone dose. Two of the largest RCTs, which assessed the occurrence of breast cancer and cardio‐cerebrovascular events, selected very specific and heterogeneous populations; therefore, we considered that it would be informative to present results on breast cancer separately for women who had a history of breast cancer and those who had no such history, and results on cardiovascular and cerebrovascular events that distinguished women younger than and over 60 years of age. We did not prespecify these subgroup analyses.
Sensitivity analysis
We conducted sensitivity analyses of the primary outcome to determine whether conclusions were robust to arbitrary decisions regarding eligibility and analysis. In performing these analyses, we considered whether conclusions would have differed if:
eligibility had been restricted to studies without high risk of attrition bias; and
eligibility had been further restricted to studies that used validated scales to measure vasomotor symptoms.
Overall quality of the body of evidence ‐ Summary of findings table
We used GRADEPRO software and methods of The Cochrane Collaboration to prepare a Summary of findings table (Higgins 2011). This table portrayed the overall quality of the body of evidence for main review outcomes (occurrence of vasomotor symptoms, vaginal bleeding, breast cancer, endometrial cancer, venous thromboembolic events, cardiovascular events, cerebrovascular events and mortality from any cause) and main comparisons (tibolone vs placebo, tibolone vs HT) on the basis of GRADE criteria (study limitations (i.e. risk of bias), consistency of effect, imprecision, indirectness and publication bias). We justified, documented and incorporated Judgements about evidence quality (high, moderate, low or very low) into the reporting of results for each outcome.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The original systematic search performed in 2011 through seven databases produced 540 records (after duplicates were removed). After selecting 57 papers of potential interest from their titles and abstracts, we eventually included 33 RCTs. Two of these articles (Ziaei 2010; Ziaei 2010b) appeared to report different outcomes for the same study; we have amalgamated these and counted them as a single study in the 2016 update.
We performed additional searches in 2015: we initially selected 62 additional abstracts and found 14 additional RCTs, plus another publication (Bots 2006) for one of the studies already included (Langer 2006). (See Figure 1 for study flow.) We have included in this update six studies that were excluded in the previous version of the review (see Differences between protocol and review). Therefore this review update includes a total of 46 studies (32 studies from the previous version of the review, six that were excluded from the previous version of the review and eight new studies).
1.
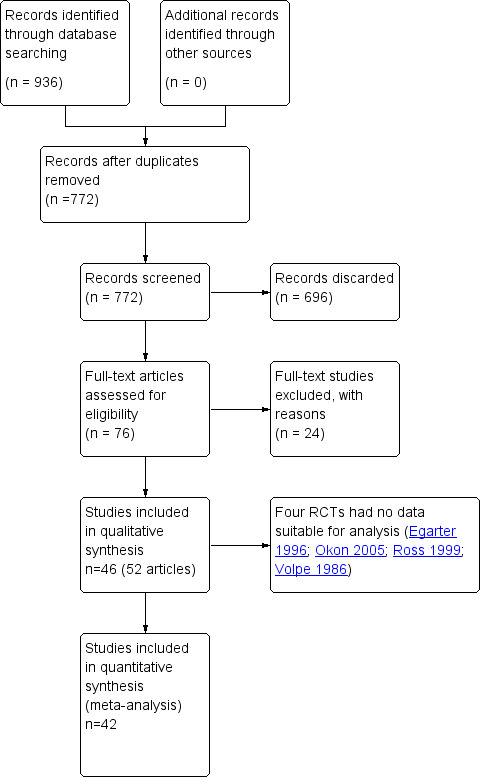
Study flow diagram.
Of these newly included reports, five (Bouchard 2012; Gupta 2013; Jacobsen 2012; Morais‐Socorro 2012; Polisseni 2013) were published since 2012, and three (Baracat 2002;Doren 1999;Wender 2004) were cited among references provided in other studies. We asked drug manufacturers, as well as authors of conference proceedings, about possibly unpublished studies but obtained no information on this.
Included studies
Study design and setting
We included 46 RCTs of parallel design; 18 were multi‐centre studies.
Participants
All selected RCTs included postmenopausal or perimenopausal women (n = 19,976), and in most of these RCTs, all or some participants had menopausal symptoms. A few studies did not clearly specify whether women were symptomatic, or whether investigators had other reasons to test the effectiveness of tibolone. Among these, five RCTs (Archer 2007; Hänggi 1997; Doren 1999; Okon 2005; Wender 2004) were carried out with the main objective to assess endometrial safety associated with the use of tibolone, and four RCTs (Elfituri 2005; Huber 2002; Winkler 2000; Ziaei 2010) had as their main objective assessment of bleeding patterns.
Five of the included RCTs (Cummings 2008; Gallagher 2001; Jacobsen 2012; Langer 2006; Roux 2002) assessed effects of tibolone on bone loss in postmenopausal women, in addition to its safety profile and its effects on menopausal symptoms. One study (Cummings 2008) also evaluated the reduction in fractures among women with osteoporosis.
Three RCTs (Kenemans 2009; Kroiss 2005; Kubista 2007) specifically studied individuals with breast cancer: Kenemans 2009 assessed the recurrence of breast cancer in women with vasomotor symptoms who were previously treated surgically; Kroiss 2005 evaluated the safety profile of tibolone administered to postmenopausal women after breast cancer surgery to prevent, relieve or delay the occurrence of menopausal symptoms; Kubista 2007 assessed the safety of 14‐day tibolone treatment of breast tissue in patients with invasive cancer without metastatic spread, and we included this study because an ischaemic stroke occurred.
Among populations with specific characteristics other than menopausal symptoms, one RCT (de Aloysio 1998) selected patients with uterine leiomyomas to assess the effects of tibolone on bleeding patterns. Another RCT (Vieira 2009) assessed the frequency of flares in patients with lupus erythematosus.
Most of the included RCTs studied women in natural menopause only, although a few studies also included women without a uterus. In these cases, investigators evaluated endometrial outcomes (bleeding, hyperplasia, cancer) only in women with an intact uterus.
The mean age of women in most of the selected studies was between 52 and 55 years. In two trials (Cummings 2008; Jacobsen 2012) that selected women older than 60 years of age, researchers observed much higher means, whereas in one trial (Elfituri 2005) on Lybian women with natural or surgical menopause, the mean age of participants was lower (44 years). Mean time since menopause ranged from 1.5 to 17 years.
All but three of the selected RCTs included fewer than 1000 participants. Each of the three largest RCTs (Archer 2007; Cummings 2008; Kenemans 2009) actually included more than 3000 participants. Follow‐up periods ranged from two weeks to four years.
Interventions
The included studies administered oral tibolone (usually 2.5 mg daily: range 0.625 mg to 5 mg daily) compared with placebo, unopposed oestrogen or combined HT, as detailed below. Unless otherwise stated, doses were daily and progesterone was continuous. Several studies included more than one comparator.
Placebo (17 RCTs): Benedek‐Jaszmann 1987, Berning 2000, Bouchard 2012, Cummings 2008, Gallagher 2001, Hudita 2003, Jacobsen 2012, Kenemans 2009, Kroiss 2005, Kubista 2007, Landgren 2002, Meeuwsen 2002, Morais‐Socorro 2012, Swanson 2006, Vieira 2009, Volpe 1986, Wender 2004
-
Unopposed oestrogen (three RCTs)
Conjugated equine oestrogen (CEE) 0.0625 (Gupta 2013)
Oestriol 2 to 4 mg (Volpe 1986)
17β‐Oestradiol patch 50 μg (Mendoza 2000)
-
Combined HT (28 RCTs)
CEE 0.625 mg plus medroxyprogesterone acetate 2.5 to 5 mg (Archer 2007;Baracat 2002;de Aloysio 1998;Huber 2002;Kökçü 2000;Langer 2006;Uygur 2005;Wu 2001;Ziaei 2010)
Oestradiol valerate 2 mg and norethisterone 0.7 to 2mg (Al‐Azzawi 1999;Okon 2005)
Oestradiol 50 μg + norethisterone acetate (140 microgr) in the form of a transdermal patch (Nijland 2009)
Oestradiol valerate 2 mg plus dienogest 2 mg (Osmanağaoğlu 2006)
Oestradiol 2 mg + oestriol 1 mg/d + norethindrone acetate 1 mg/d (Winkler 2000)
Oestradiol 1 to 2 mg plus norethindrone 0.5 to 1 mg (Polisseni 2013;Roux 2002)
17β‐Oestradiol 1 to 2 mg + norethisterone 0.5 to 1 mg (Doren 1999;Hammar 1998;Hammar 2007;Nappi 2006a;Nathorst‐Böös 1997)
Oestradiol 2 mg + medrogestone 10 mg (Egarter 1996)
CEE 0.625 mg plus sequential 150 μg norgestrel (Ross 1999)
CEE 0.625 mg plus sequential medroxyprogesterone 5 mg (Siseles 1995)
CEE 0.625 mg plus sequential norethisterone 5 mg (Siseles 1995;Volpe 1986)
CEE 0.625 mg + sequential cyproterone acetate 12.5 mg/d (Volpe 1986)
Oestradiol valerate 2 mg plus sequential cyproterone acetate 12.5 mg (Volpe 1986)
Oestradiol valerate 2 mg plus sequential norethisterone 5 mg (Volpe 1986)
17β‐Oestradiol oral 2 mg or patch 50 μg plus sequential oral dydrogesterone 10 mg (Elfituri 2005;Hänggi 1997)
17β‐Oestradiol patch 50 μg plus sequential norethisterone 0.25 mg (Mendoza 2002)
Transdermal β‐oestradiol patch 50 μg plus micronised natural progesterone 200 mg twice a week (Mendoza 2002)
Outcomes
Of 46 RCTs, 23 evaluated the effectiveness of tibolone for treatment of vasomotor symptoms in symptomatic women, measured as occurrence (Kökçü 2000;Meeuwsen 2002), as frequency (Bouchard 2012;Hammar 2007;Landgren 2002;Swanson 2006) or with the use of scales (Benedek‐Jaszmann 1987; Elfituri 2005;Hammar 1998;Huber 2002;Hudita 2003;Morais‐Socorro 2012;Polisseni 2013;Wu 2001;Ziaei 2010). Data from eight other RCTs (Al‐Azzawi 1999;Baracat 2002;Egarter 1996;Ross 1999;Siseles 1995;Vieira 2009;Volpe 1986; Wender 2004) that evaluated vasomotor symptoms were unsuitable for analysis (see Table 5 for detailed explanations).
Twenty‐eight of 46 RCTs evaluated unscheduled bleeding (24 could be considered for meta‐analyses).
Ten of 46 RCTs evaluated breast cancer.
Thirteen of 46 RCTs evaluated endometrial cancer.
Nine of 46 RCTs evaluated venous thromboembolic events.
Five of 46 RCTs evaluated cardiovascular events.
Eight of 46 RCTs evaluated cerebrovascular events.
Six of 46 RCTs evaluated mortality from any cause.
Nine of 46 RCTs evaluated endometrial hyperplasia (extra one is Volpe 1986).
Sixteen of 46 RCTs evaluated vaginal dryness and painful sexual intercourse (seven could be considered for meta‐analyses) (extra ones are Mendoza 2000 and Uygur 2005).
Four of 46 RCTs evaluated insomnia.
Two of 46 RCTs evaluated vaginal infection.
One of 46 RCTs evaluated urinary tract infection.
Excluded studies
We excluded 24 studies from the review. Following are the most common reasons for exclusion (occurring in more than one RCT).
Three of 24 were not randomised.
Fifteen of 24 did not assess outcomes of interest.
Four of 24 did not include a comparator of interest.
Risk of bias in included studies
See also Characteristics of included studies,Figure 2 and Figure 3.
2.
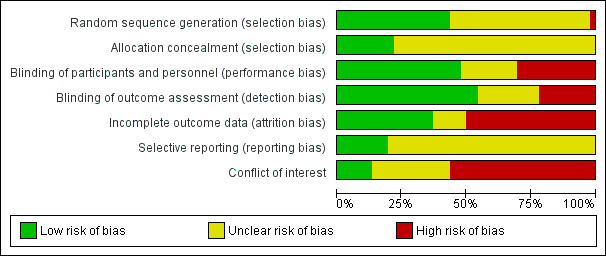
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
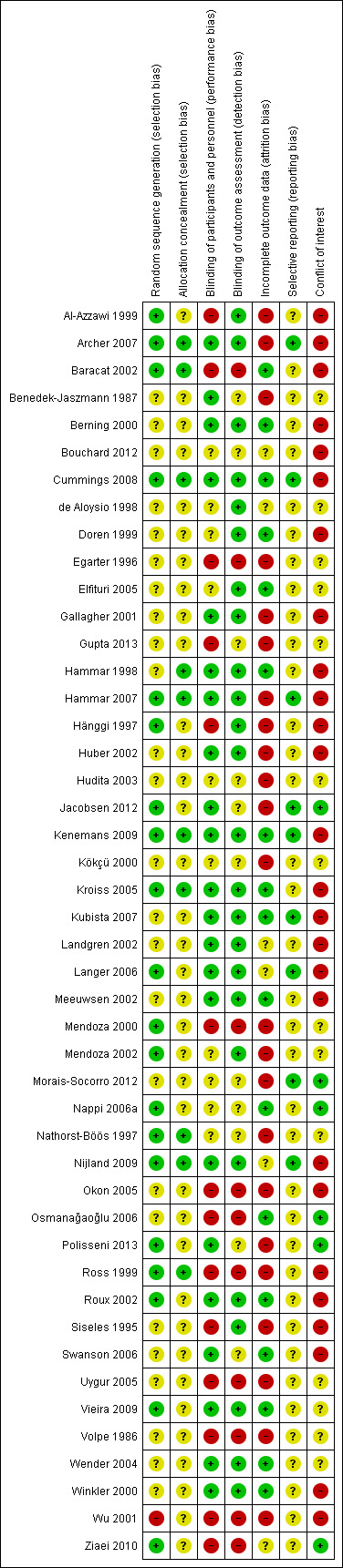
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Twenty RCTs described adequate methods of sequence generation; we rated them as having low risk of bias in this domain. We rated 25 studies as having unclear risk. We rated one study (Wu 2001) as having high risk of bias; investigators stated they allocated to treatment groups randomly selected pairs of two women.
Allocation concealment
Most of the selected RCTs provided no information regarding allocation concealment. Only 10 of 46 RCTs specified that researchers used a system for concealing allocation (low risk of bias): an interactive voice response system in five RCTs, another computerised system (the Almedica Drug Labelling System; Almedica, Parsippany, NJ, USA) in one RCT and opaque envelopes in four RCTs. We rated remaining studies as having unclear risk of bias.
Blinding
Performance bias
In 22 out of 46 RCTs, participants and/or personnel were blinded (low risk of bias). Fourteen RCTs were open trials or blinding appeared unlikely (high risk of bias), and 10 provided insufficient or no information by which this domain could be assessed (unclear risk).
Detection bias
We considered risk of bias as low in 25 of 46 RCTs, whereas 10 RCTs did not provide enough information for assessment, and we rated 13 studies as having high risk of bias in this domain.
Incomplete outcome data
We considered 17 of 46 RCTs to have low risk of attrition bias. Several RCTs reported some reasons for concern (lack of intention‐to‐treat analysis, loss to follow‐up with no reasons specified). In particular, investigators gave no clear reasons for excluding participants from treatment and/or evaluation in six RCTs (rated as having unclear risk), and more than 10% of participants were lost to follow‐up in 23 RCTs (rated as having high risk).
Selective reporting
Only nine of 46 study protocols were available; we judged risk of selective reporting bias as low in all of these studies, as they reported expected outcomes of interest for this review, or they reported data on adverse events that were not indicated in the study protocol but could be expected in the study report. We rated all other studies as having unclear risk.
Other potential sources of bias
The drug producer sponsored most of the RCTs, and its employees often authored the articles. We rated 26 as having high risk of bias and 10 unclear risk. Just six of 46 RCTs appeared truly independent, and we rated them as having low risk of bias in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Tibolone versus placebo
Primary outcomes
Vasomotor symptoms
Eight RCTs reported useable data on this outcome; three other RCTs reported data that could not be used (see Table 5). A substantial effect of tibolone on vasomotor symptoms compared with placebo is suggested (see Analysis 1.1 and Figure 4), with a pooled estimate of the SMD of ‐0.99 (95% CI ‐1.10 to ‐0.89; n = 1657; I2 = 96%; moderate‐quality evidence). Multiplying this by the pooled standard deviation from Hammar 1998 (0.76) suggests that tibolone could improve vasomotor symptoms by around 0.75 (0.7 to 0.8) points on a 5‐point severity scale. A sensitivity analysis (see Analysis 1.15) excluding three RCTs with attrition bias (Benedek‐Jaszmann 1987;Hudita 2003; Morais‐Socorro 2012 ‐ the latter two also have very large estimates) still shows an effect of tibolone, with reduced heterogeneity and effect size (SMD ‐0.61, 95% CI ‐0.73 to ‐0.49; I2 = 54%). The corresponding odds ratio (OR) is 0.33 (95% CI 0.27 to 0.41). These estimates can be translated to meaningful scales; multiplying the SMD by the pooled standard deviation from Hammar 1998 (0.76) suggests that tibolone could improve vasomotor symptoms by around 0.5 (0.4 to 0.6) points on a 5‐point severity scale; this probably would not constitute a clinically meaningful effect.
1.1. Analysis.
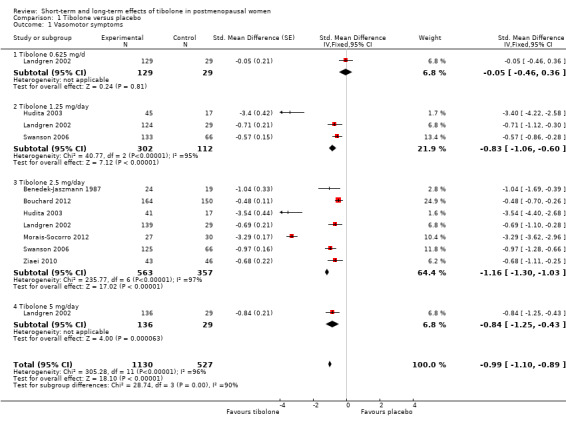
Comparison 1 Tibolone versus placebo, Outcome 1 Vasomotor symptoms.
4.
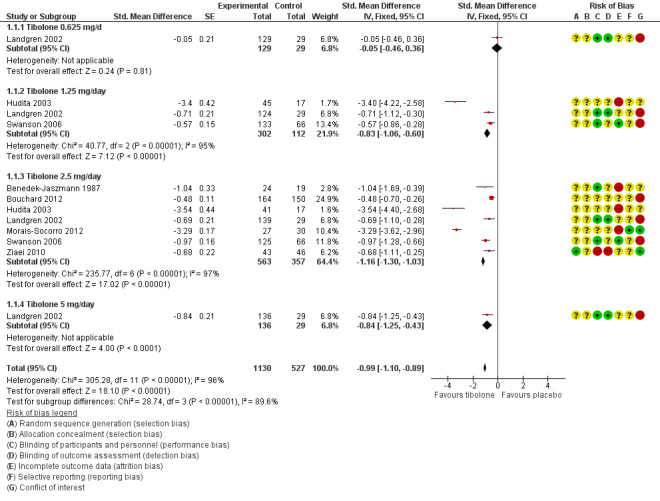
Forest plot of comparison: 1 Tibolone versus placebo, outcome: 1.1 Vasomotor symptoms.
1.15. Analysis.
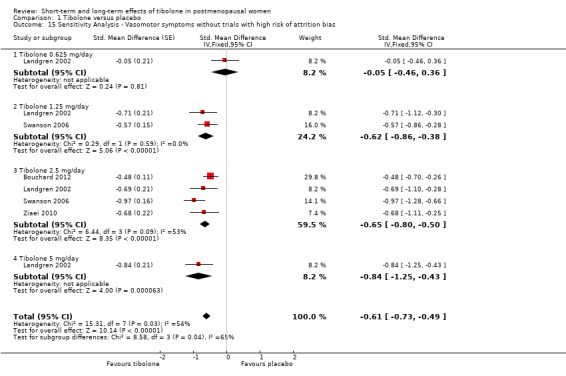
Comparison 1 Tibolone versus placebo, Outcome 15 Sensitivity Analysis ‐ Vasomotor symptoms without trials with high risk of attrition bias.
Subgroup analysis by dose
We found strong evidence (P < 0.00001) of differences between subgroups defined by tibolone dose, although this was diminished when we removed trials with high risk of attrition bias, which were likely to provide overestimates (P = 0.04). Furthermore, once we removed these trials, we noted the suggestion of a dose‐response relationship (Analysis 1.15; Figure 5), although trials were too few to allow formal investigation of this through meta‐regression.
5.
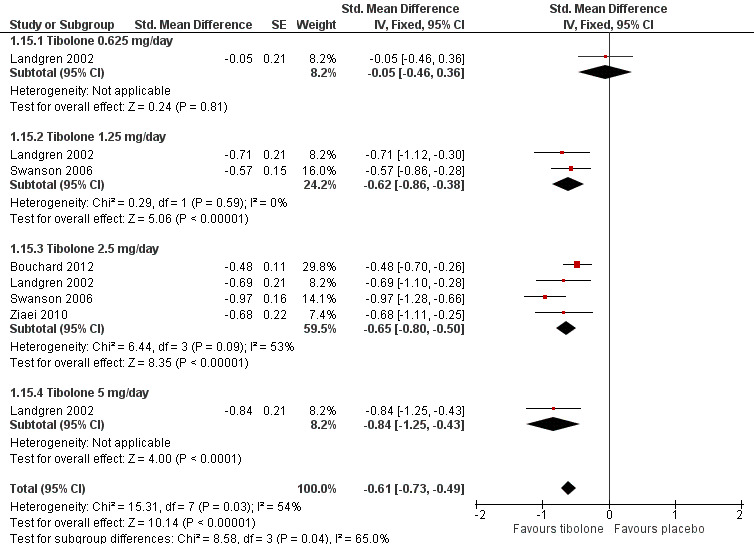
Forest plot of comparison: 1 Tibolone versus placebo, outcome: 1.15 Sensitivity analysis ‐ Vasomotor symptoms without trials with high risk of attrition bias.
Subgroup analysis by duration
We noted some scope, albeit limited, for review authors to consider the impact of treatment duration on the effect; estimates from four of the included studies (Bouchard 2012;Landgren 2002;Morais‐Socorro 2012; Swanson 2006) corresponded to 12 weeks, from one (Hudita 2003) to 24 weeks, from one (Ziaei 2010) to six months and from one (Benedek‐Jaszmann 1987) to 12 months. All seven studies appeared in the stratum corresponding to a dose of 2.5 mg/d. Accordingly, we were able to look at estimates in this stratum to see whether duration modified the treatment effect when dose was held constant. As we recalled the high risk of attrition bias in Hudita 2003 and Morais‐Socorro 2012, we noted that no such relationship was evident; neither the estimate from Benedek‐Jaszmann 1987 (12 months) nor that from Ziaei 2010 (six months) was notably different from the 12 week estimates.
Unscheduled bleeding
Nine RCTs reported this outcome (Analysis 1.2). Unscheduled bleeding was more likely to occur in the tibolone group (OR 2.79, 95% CI 2.10 to 3.70; nine RCTs; n = 7814; I2 = 43%; moderate‐quality evidence). This suggests that if 18% of women taking placebo experience unscheduled bleeding, then between 31% and 44% of women taking tibolone will do so. Statistical significance persisted if we excluded the two largest RCTs (Cummings 2008; Kenemans 2009), which provided 47% of the total weight and about 85% of the population of interest.
1.2. Analysis.
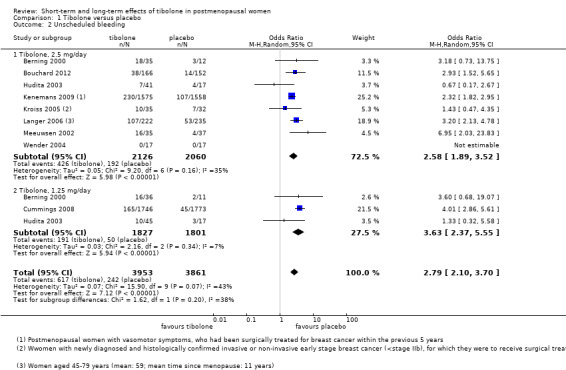
Comparison 1 Tibolone versus placebo, Outcome 2 Unscheduled bleeding.
Subgroup analysis by dose
Results were stratified by dose (2.5 and 1.25 mg daily). Effect estimates were similar in the two groups.
Long‐term adverse events
Endometrial cancer
Eight RCTs reported this outcome (Analysis 1.3). We found no evidence of a difference between groups, although the event rate was low, with 16 cases reported in the tibolone arms and five in the placebo arms (OR 2.04, 95% CI 0.79 to 5.24; eight RCTs; 8504 women; I2 = 0%; very low‐quality evidence).
1.3. Analysis.
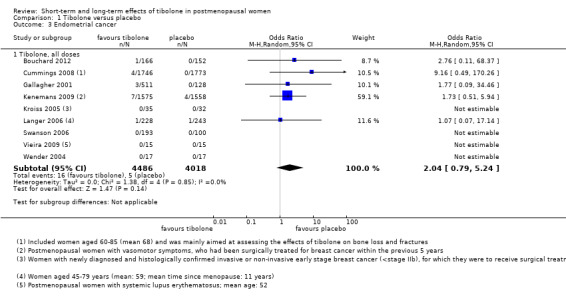
Comparison 1 Tibolone versus placebo, Outcome 3 Endometrial cancer.
Evidence suggests that if one woman in a thousand taking placebo develops endometrial cancer, then between one and six women in a thousand who take tibolone may do so. Seven and four cases, respectively, occurred in Kenemans 2009 (with 2.5 mg/d; n = 3133), and four versus zero cases in Cummings 2008 (with 1.25 mg; n = 3519). Fifteen cases (11 in tibolone arms vs four in placebo arms) occurred in studies recruiting younger postmenopausal women (average age < 55 years).
Breast cancer
Six RCTs assessed this outcome: four in women without a history of breast cancer (Analysis 1.4) and two in women with a history of breast cancer (Analysis 1.5).
1.4. Analysis.
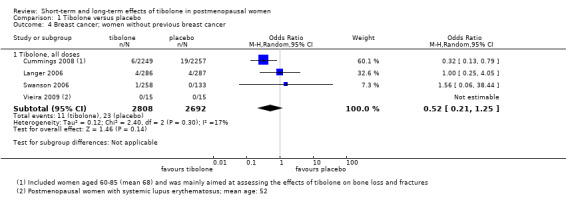
Comparison 1 Tibolone versus placebo, Outcome 4 Breast cancer; women without previous breast cancer.
1.5. Analysis.
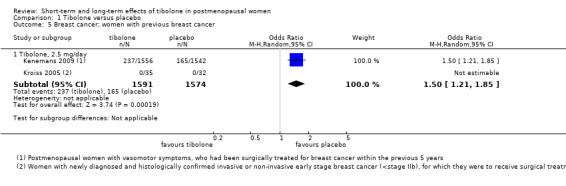
Comparison 1 Tibolone versus placebo, Outcome 5 Breast cancer; women with previous breast cancer.
Among women without a history of breast cancer, we found no evidence of a difference between groups (OR 0.52, 95% CI 0.21 to 1.25; four RCTs; 5500 women; I2 = 17%; very low‐quality evidence).
Among women with a history of breast cancer, we noted increased risk in the tibolone group (OR 1.5, 95% CI 1.21 to 1.85; two RCTs; 3165 women; moderate‐quality evidence). All events occurred in the largest of the studies (Kenemans 2009), which administered 2.5 mg/d of tibolone and was stopped prematurely owing to increased risk in the intervention group.
Venous thromboembolic events
Five RCTs assessed this outcome; three of them (Cummings 2008; Kenemans 2009;Landgren 2002) reported the occurrence of events (Analysis 1.6). We found no evidence of a difference between groups (OR 0.85, 95% CI 0.37 to 1.97; n = 9176; I2 = 0%; very low‐quality evidence).
1.6. Analysis.
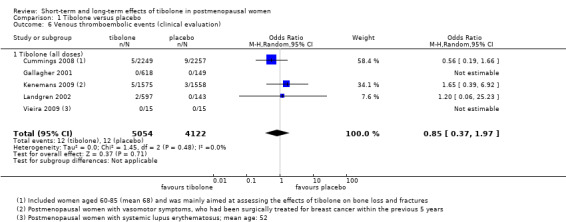
Comparison 1 Tibolone versus placebo, Outcome 6 Venous thromboembolic events (clinical evaluation).
Ten cases (seven in tibolone arms vs three in placebo arms) of a total of 24 occurred in studies recruiting younger postmenopausal women (average age < 55).
Cardiovascular events
We found no evidence of a difference between groups (OR 1.38, 95% CI 0.84 to 2.27; four RCTs; n = 8401; I2 = 0%; very low‐quality evidence; Analysis 1.7).
1.7. Analysis.
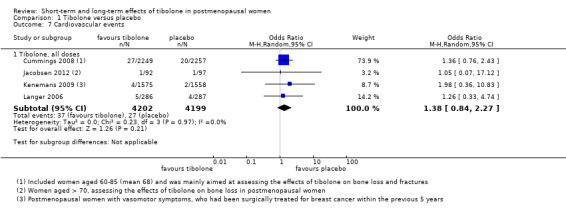
Comparison 1 Tibolone versus placebo, Outcome 7 Cardiovascular events.
The four RCTs assessing this outcome involved women of very different age groups (Cummings 2008, mean age 68; Jacobsen 2012, mean age 74; Kenemans 2009, mean age 53 years; Langer 2006, mean age 59), but we observed no statistical heterogeneity between these studies.
Cerebrovascular events
Four RCTs assessed this outcome (Analysis 1.8) and provided no conclusive evidence of a difference between groups (OR 1.74, 95% CI 0.99 to 3.04; four RCTs; n = 7930; I2 = 0%).
1.8. Analysis.
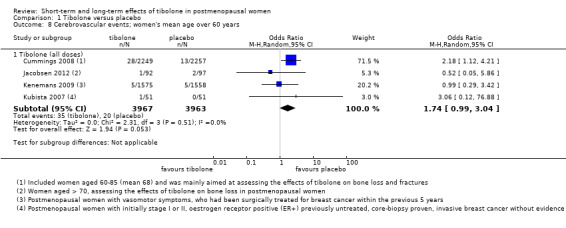
Comparison 1 Tibolone versus placebo, Outcome 8 Cerebrovascular events; women's mean age over 60 years.
One RCT (Cummings 2008; n = 4506), which selected osteoporotic women aged 60 to 85 years, provided most of the data; this trial was stopped prematurely for increased risk of stroke with 1.25 mg/d of tibolone (28 vs 13 cases; OR 2.18, 95% CI 1.12 to 4.21). Among women younger than 60 years old (Kenemans 2009), five cases occurred in each group (OR 0.99, 95% CI 0.29 to 3.42; n = 3133).
Mortality from any cause
Four RCTs assessed this outcome, and three reported events (Analysis 1.9), providing no evidence of a difference between groups (OR 1.06, 95% CI 0.79 to 1.41; five RCTs; n = 8242; I2 = 0%; low‐quality evidence).
1.9. Analysis.
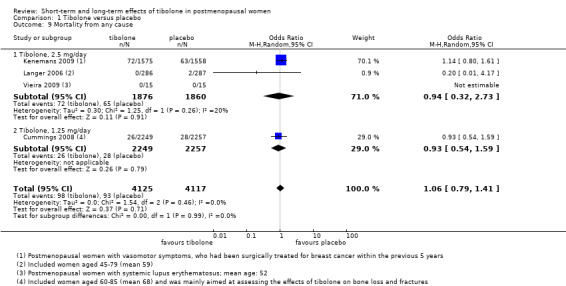
Comparison 1 Tibolone versus placebo, Outcome 9 Mortality from any cause.
Secondary outcomes
Insomnia
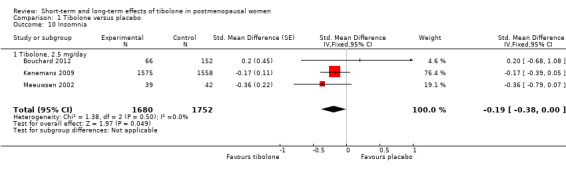
Three RCTs reported insomnia or "sleep" (Analysis 1.10).
1.10. Analysis.
Comparison 1 Tibolone versus placebo, Outcome 10 Insomnia.
Results suggested an advantage of tibolone over placebo related to insomnia or quality of sleep (SMD ‐0.19, 95% CI ‐0.38 to 0.00; three RCTs; n = 3432; I2 = 0%).
Genital symptoms
Vaginal dryness
Three RCTs (Hudita 2003;Kenemans 2009;Ziaei 2010) reported useable data on this outcome (see Analysis 1.11 and Table 6), suggesting an advantage of tibolone over placebo for vaginal dryness, although this would barely be evident if the two arms from Hudita 2003, which had a high dropout rate, were excluded. The SMD (95% CI) including Hudita 2003 was ‐0.66 (‐0.90 to ‐0.43), which corresponds to improvement on a 0 to 3 severity score of 0.6 (0.4 to 0.8) points with a standard deviation (SD) of 0.89. This probably would not amount to a clinically meaningful difference.
1.11. Analysis.
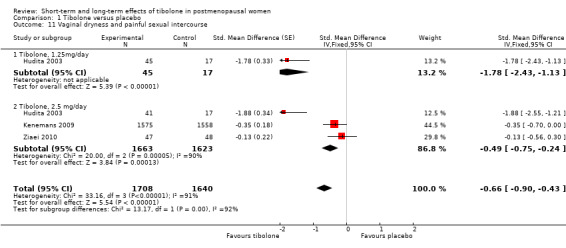
Comparison 1 Tibolone versus placebo, Outcome 11 Vaginal dryness and painful sexual intercourse.
Vaginal infection
Two RCTs reported this outcome (Analysis 1.12). The rate of vaginal infection was higher in the tibolone group (OR 2.50, 95% CI 1.24 to 5.06; two RCTs; n = 7639; I2 = 88%). The direction of effect was consistent, but considerable statistical heterogeneity was probably due to differences in the population studied (osteoporotic women aged 60 to 85 years in Cummings 2008, and younger women who had experienced breast cancer in Kenemans 2009).
1.12. Analysis.
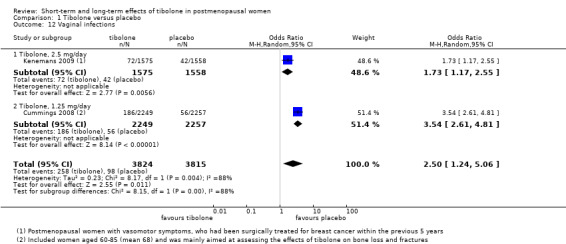
Comparison 1 Tibolone versus placebo, Outcome 12 Vaginal infections.
Urinary tract infection
One RCT (Kenemans 2009) reported this outcome (Analysis 1.13) and revealed no evidence of a difference between groups (OR 0.70, 95% CI 0.46 to 1.06; one RCT; n = 3133).
1.13. Analysis.
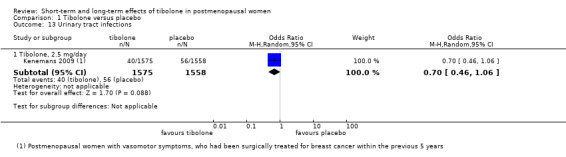
Comparison 1 Tibolone versus placebo, Outcome 13 Urinary tract infections.
Endometrial hyperplasia
Four RCTs assessed this outcome, and two reported events (Analysis 1.14), providing no evidence of a difference between groups, although results revealed only seven events in total (OR 1.20, 95% CI 0.23 to 6.25; n = 4518; I2 = 0%).
1.14. Analysis.
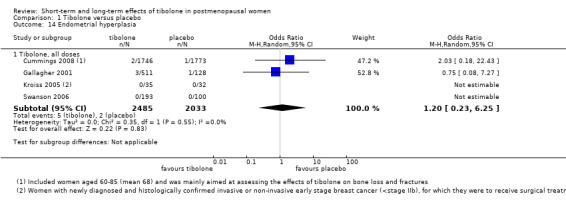
Comparison 1 Tibolone versus placebo, Outcome 14 Endometrial hyperplasia.
Tibolone versus oestrogens
Primary outcomes
Two RCTs (Gupta 2013;Mendoza 2002) compared tibolone versus oestrogens and reported data on three outcomes (vasomotor symptoms, vaginal dryness and painful sexual intercourse, insomnia).
Vasomotor symptoms
We found no evidence of a difference between groups (OR 1.23, 95% CI 0.35 to 4.34; two RCTs; n = 108; I2 = 0%; low‐quality evidence), although the small number of events observed meant that large effects in either direction could not be ruled out. See Analysis 2.1 and Figure 6.
2.1. Analysis.
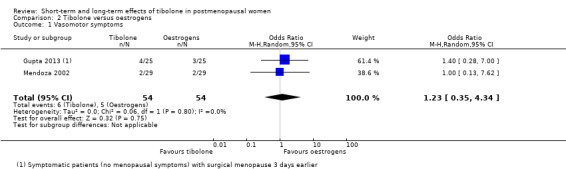
Comparison 2 Tibolone versus oestrogens, Outcome 1 Vasomotor symptoms.
6.
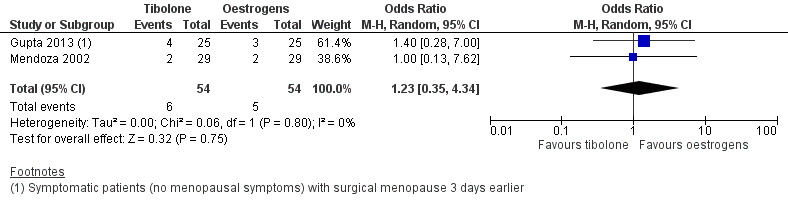
Forest plot of comparison: 2 Tibolone versus oestrogens, outcome: 2.1 Vasomotor symptoms.
Secondary outcomes
Insomnia
No events occurred in either group (Analysis 2.2).
2.2. Analysis.
Comparison 2 Tibolone versus oestrogens, Outcome 2 Insomnia.
Genital symptoms
Vaginal dryness and painful sexual intercourse
We found no evidence of a difference between groups (OR 0.32, 95% CI 0.01 to 8.25; one RCT; n = 50), although the estimate was so imprecise as to be completely uninformative (Analysis 2.3).
2.3. Analysis.
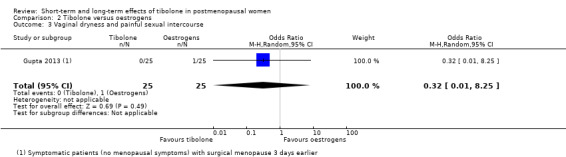
Comparison 2 Tibolone versus oestrogens, Outcome 3 Vaginal dryness and painful sexual intercourse.
Tibolone versus combined HT
Primary outcomes
Vasomotor symptoms
Nine RCTs reported useable data on this outcome, and five other RCTs provided data that could not be used (see Table 5). Results suggested a small disadvantage of tibolone compared with combined HT (see Analysis 3.1 and Figure 7), with a pooled estimate of the SMD of 0.17 (95% CI 0.06 to 0.28; n = 1336; I2 = 67%; moderate‐quality evidence). Multiplying this estimate by the pooled standard deviation from Hammar 1998 (0.76) suggests that combined HT improves vasomotor symptoms by around 0.15 (0.08 to 0.23) compared with tibolone on a 5‐point severity scale. The corresponding OR was 1.36 (95% CI 1.11 to 1.66). A sensitivity analysis (see Analysis 3.11) excluding five RCTs with high attrition bias provided slightly larger but similar estimates (SMD 0.25, 95% CI 0.09 to 0.41; I2 = 0%). A further sensitivity analysis excluding the latter five RCTs plus Hammar 1998 (using a non‐validated scale) revealed no evidence of a difference between treatments because the estimate lacked precision once other studies were excluded (see Analysis 3.12).
3.1. Analysis.
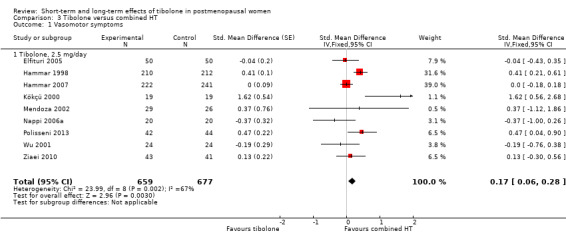
Comparison 3 Tibolone versus combined HT, Outcome 1 Vasomotor symptoms.
7.
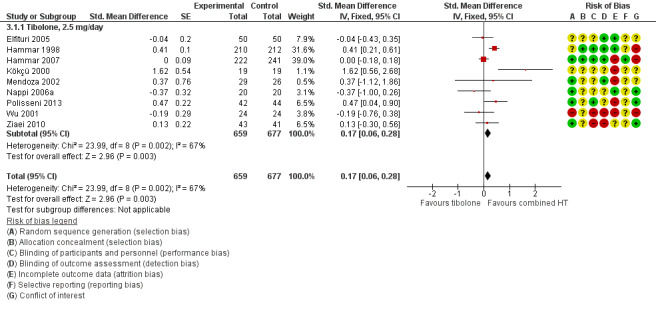
Forest plot of comparison: 3 Tibolone versus combined HT, outcome: 3.1 Vasomotor symptoms.
3.11. Analysis.
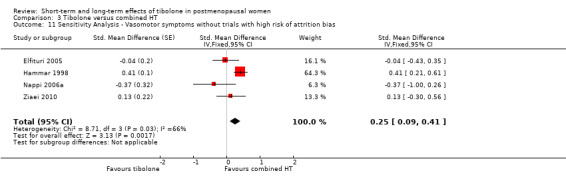
Comparison 3 Tibolone versus combined HT, Outcome 11 Sensitivity Analysis ‐ Vasomotor symptoms without trials with high risk of attrition bias.
3.12. Analysis.
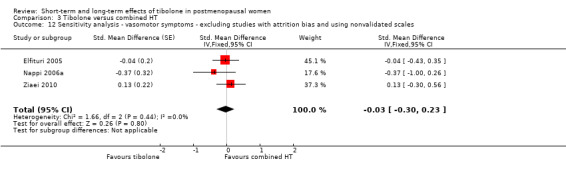
Comparison 3 Tibolone versus combined HT, Outcome 12 Sensitivity analysis ‐ vasomotor symptoms ‐ excluding studies with attrition bias and using nonvalidated scales.
Subgroup analysis by duration
Duration of treatment in this comparison ranged from 12 weeks to 12 months, while dose was the same in all studies (2.5 mg/d); therefore, a tentative investigation of the impact of treatment duration on treatment effect could be undertaken. Although we identified too few studies to permit a formal analysis (e.g. using meta‐regression), we were able to order the studies according to duration so as to inspect whether a trend in the size of the SMDs was suggested (Analysis 3.13). However, we observed no clear trend, and consequently found no evidence that the difference between tibolone and HT varies according to the duration of treatment.
3.13. Analysis.
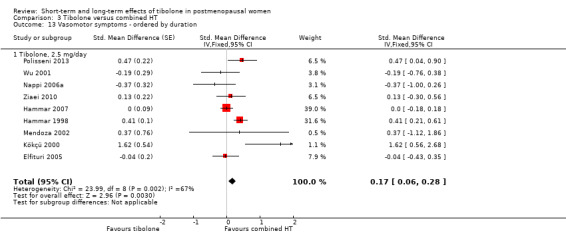
Comparison 3 Tibolone versus combined HT, Outcome 13 Vasomotor symptoms ‐ ordered by duration.
Unscheduled bleeding
Seventeen RCTs reported this outcome: 15 compared tibolone with continuous combined HT, two with continuous sequential HT (Analysis 3.2). The latter studies included cases of bleeding if they had been reported as side effects by study authors.
3.2. Analysis.
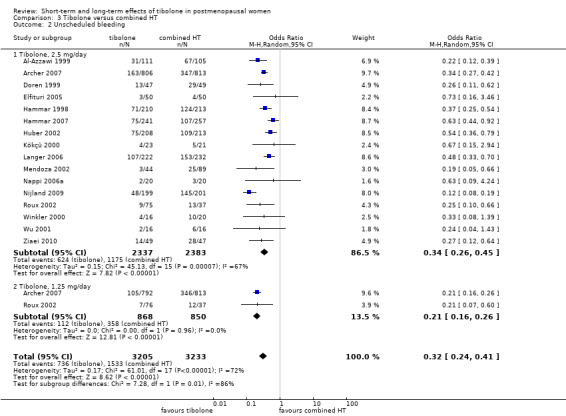
Comparison 3 Tibolone versus combined HT, Outcome 2 Unscheduled bleeding.
Tibolone was associated with fewer breakthrough events than combined HT (OR 0.32, 95% CI 0.24 to 0.41; 16 RCTs; n = 6438; I2 = 72%; low‐quality evidence), suggesting that if 47% of women taking combined HT experience unscheduled bleeding, then between 18% and 27% of those taking tibolone will do so. High heterogeneity was attributable in part to an RCT (Nijland 2009) in which HT was delivered in patch form, and also to a difference between dose subgroups, as noted below.
Statistical significance persisted if we excluded the largest RCT (Archer 2007, which provided about half of the population of interest).
One RCT (Okon 2005) reported this outcome as days of bleeding over one year of follow‐up. Study authors reported no significant differences between groups.
Subgroup analysis by dose
We stratified results by dose, revealing a statistically significant difference between 2.5 mg and 1.25 mg subgroups (test for subgroup differences: Chi² = 7.28; df = 1 (P = 0.007); I² = 86.3%), which suggested that the lower dose of tibolone was associated with a more beneficial effect when compared with HT (OR 0.21, 95% CI 0.16 to 0.26; two RCTs; n = 1718; I2 = 0%).
Long‐term adverse events
Endometrial cancer
Five RCTs reported this outcome (Analysis 3.3). Few events occurred (two cases in tibolone arms vs one in combined HT arms in three trials), and investigators provided no evidence of a difference between groups (OR 1.47, 95% CI 0.23 to 9.33; five RCTs; n = 3689; I2 = 0%; very low‐quality evidence).
3.3. Analysis.
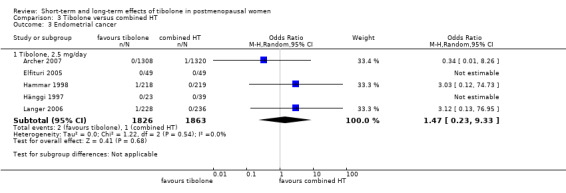
Comparison 3 Tibolone versus combined HT, Outcome 3 Endometrial cancer.
Breast cancer
Five RCTs assessed this outcome (Analysis 3.4). All included women without a history of breast cancer. Few events occurred (17 cases in tibolone arms vs 10 in combined HT arms), and researchers provided no evidence of a difference between groups (OR 1.69, 95% CI 0.78 to 3.67; n = 4835; I2 = 0%; very low‐quality evidence).
3.4. Analysis.
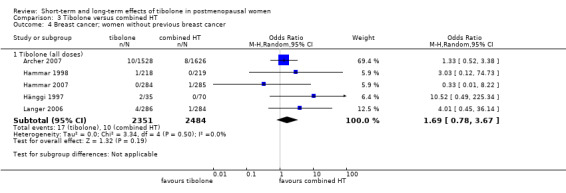
Comparison 3 Tibolone versus combined HT, Outcome 4 Breast cancer; women without previous breast cancer.
Twenty‐two cases (13 in tibolone arms vs nine in placebo arms) occurred in studies recruiting younger postmenopausal women (average age < 55).
Venous thromboembolic events
Four RCTs assessed this outcome (Analysis 3.5). Few events occurred (one case of pulmonary embolism in tibolone arms vs two cases of pulmonary embolism and three of deep venous thrombosis in combined HT arms), and researchers provided no evidence of a difference between groups (OR 0.44, 95% CI 0.09 to 2.14; four RCTs; n = 4529; I2 = 0%; very low‐quality evidence).
3.5. Analysis.
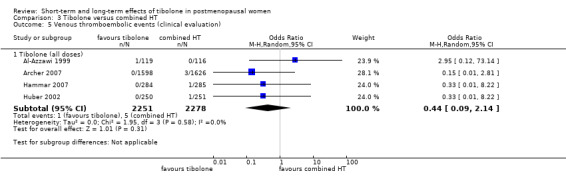
Comparison 3 Tibolone versus combined HT, Outcome 5 Venous thromboembolic events (clinical evaluation).
Cardiovascular events
Two RCTs assessed this outcome (Archer 2007; Langer 2006). Few events occurred (seven in tibolone arms vs 11 in combined HT arms), and results showed no evidence of a difference between groups (OR 0.63, 95% CI 0.24 to 1.66; two RCTs; n = 3794; I2 = 0%; very low‐quality evidence; Analysis 3.6). The mean age of women in these RCTs was less than 60 years.
3.6. Analysis.
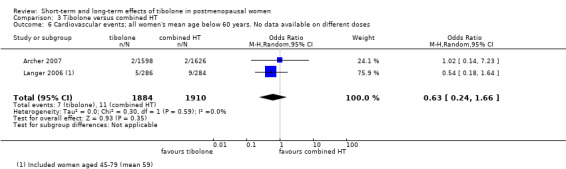
Comparison 3 Tibolone versus combined HT, Outcome 6 Cardiovascular events; all women's mean age below 60 years. No data available on different doses.
Cerebrovascular events
Four RCTs assessed this outcome (Analysis 3.7). Few events occurred (two cases in tibolone arms vs four cases in combined HT arms), and data show no evidence of a difference between groups (pooled OR 0.76, 95% CI 0.16 to 3.66; four RCTs; n = 4562; I2 = 0%; very low‐quality evidence). The mean age of women in these RCTs was less than 60 years.
3.7. Analysis.
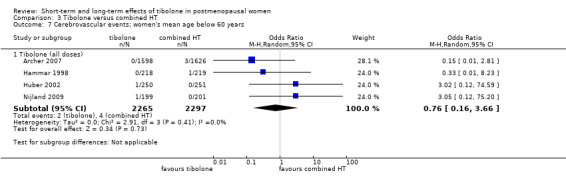
Comparison 3 Tibolone versus combined HT, Outcome 7 Cerebrovascular events; women's mean age below 60 years.
Mortality from any cause
Two RCTs (Langer 2006; Nijland 2009; n = 970) reported this outcome, with only one case noted in the tibolone arm (Analysis 3.8).
3.8. Analysis.
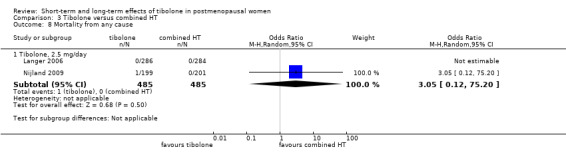
Comparison 3 Tibolone versus combined HT, Outcome 8 Mortality from any cause.
Secondary outcomes
Insomnia
Just one RCT (Egarter 1996) used a validated scale (a domain of the Kupperman Index) to assess this outcome but provided no data suitable for analysis (SD was not reported and could not be calculated sensibly via the information provided). The publication reported no evidence of a difference between tibolone and combined HT.
Genital symptoms
Vaginal dryness and painful sexual intercourse
Evidence at face value suggested little or no difference between tibolone and combined HT in relation to vaginal dryness (SMD 0.02, 95% CI ‐0.12 to 0.17; seven RCTs; n = 1098; moderate‐quality evidence; Analysis 3.10).
3.10. Analysis.
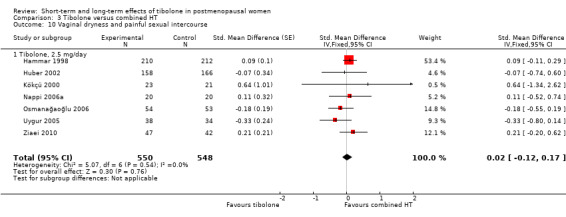
Comparison 3 Tibolone versus combined HT, Outcome 10 Vaginal dryness and painful sexual intercourse.
Mendoza 2000 (n = 76) also measured painful sexual intercourse as an outcome but provided no data suitable for analysis; study authors reported no significant difference between groups.
Similarly, Nathorst‐Böös 1997 evaluated dyspareunia but provided no data suitable for analysis, and study authors reported that they found no evidence of a difference between groups.
Vaginal infection
None of the selected RCTs reported useable data on this outcome
Urinary tract infection
None of the selected RCTs reported useable data on this outcome.
Endometrial hyperplasia
Five RCTs assessed this outcome (Analysis 3.9), reporting few events (zero cases in tibolone arms vs three cases in the combined HT arm) and no evidence of a difference between groups (OR 0.35, 95% CI 0.05 to 2.21; five RCTs; n = 2846; I2 = 0%).
3.9. Analysis.
Comparison 3 Tibolone versus combined HT, Outcome 9 Endometrial hyperplasia.
Sensitivity analyses
Aside from sensitivity analyses performed for evaluation of vasomotor symptoms, as described above (see Results 1.1 and 3.1), review authors performed sensitivity analyses for primary outcomes, considering alternative scenarios in participants lost to follow‐up. We performed three analyses on placebo‐controlled RCTs (specifically on venous thromboembolic events and breast cancer in women who had or had no history of breast cancer) and two on combined HT controlled RCTs (specifically on unscheduled bleeding and vasomotor symptoms). None of these analyses showed differences in terms of direction of effect and statistical significance.
Assessment of review‐wide reporting bias
Funnel plot analyses were not helpful to review authors in assessing the presence of publication bias, given the relative scarcity of studies and data. Vasomotor symptoms and unscheduled bleeding were the only outcomes with sufficient RCTs to permit such an assessment, which revealed no evidence of bias for this outcome. As for the other outcomes, we cannot exclude the occurrence of publication bias because the drug manufacturer, who sponsored almost all of the published RCTs, was asked for possibly unpublished data but provided no written response.
Discussion
Summary of main results
For this review, we retrieved randomised controlled trials (RCTs) comparing tibolone versus placebo and versus combined hormone therapy (HT). We identified only three RCTs comparing tibolone versus oestrogens without progestogens (Gupta 2013; Mendoza 2000; Volpe 1986), and only two of these were suitable for analysis. The addition of progestogens is considered important for lowering the risk of endometrial carcinoma in women with a uterus.
Effectiveness in treatment of menopausal symptoms
Our findings suggest that tibolone reduces vasomotor symptoms compared with placebo and is less effective than combined HT. The clinical relevance of observed differences is disputable ‐ especially for comparison versus combined HT ‐ as their magnitude is limited. It should be noted that the quality of evidence for this outcome was moderate. In particular, attrition bias and use of non‐validated scales were frequently observed, as was statistical heterogeneity, although sensitivity analyses excluding RCTs with high risk of attrition bias confirmed both statistical significance and direction of effects. Available evidence suggests at most a modest effect of tibolone on insomnia and vaginal dryness compared with placebo. No clinically relevant differences are apparent between tibolone and combined HT in relation to vaginal dryness outcomes.
Short‐term safety
This review suggests that tibolone has a better bleeding profile than combined HT and is associated with more numerous breakthrough bleeding events than placebo.
Evidence is scarce and unclear on vaginal and urinary tract infections. Only two RCTs (Cummings 2008; Kenemans 2009) provided data on vaginal infection. Cummings 2008 performed cervical cytological smears annually in women with a cervix, whereas Kenemans 2009 provided no information on diagnostic technique. Both RCTs suggested that tibolone increases vaginal infection and provided no information on specific aetiologic agents. Only one study reported urinary tract infections.
Long‐term safety
For this systematic review, we found few RCTs providing data that could be used to assess the long‐term safety of tibolone. Nearly all of the evidence on adverse events was of very low quality, and events were scarce.
Available evidence indicates that compared with placebo, tibolone increases the risk of recurrent breast cancer in women with a history of breast cancer, and may increase the risk of stroke among women over 60 years of age. No evidence suggests that tibolone increases the risk of other long‐term adverse events, and no evidence reveals a difference between tibolone and HT with respect to long‐term adverse events.
In particular, the LIBERATE study (Kenemans 2009) confirmed that tibolone could significantly increase breast cancer among high‐risk women who were surgically treated within five years for breast cancer (for whom usual oestrogen and combined HT therapies were contraindicated) and who were using adjuvant therapy and/or chemotherapy in about seven cases out of 10. A daily dose of 2.5 mg led to an average of 15 extra recurrences each year for every 1000 women. It is a matter of concern that more than 70% of recurrence events were distant metastases, ultimately leading to death. This study failed to confirm the initial hypothesis of non‐inferiority of tibolone versus placebo for breast cancer risk, and was stopped after 3.1 years.
The latter findings sharply contrast with results from the LIFT study (Cummings 2008), in which 1.25 mg of tibolone, administered to osteoporotic women to reduce the risk of vertebral fracture, slightly but significantly reduced new‐onset breast cancer (about two fewer cases for every 1000 women each year). However, the absolute number of events in this study was low (six for tibolone vs 19 for placebo, for a total population of about 4500 women between 60 and 85 years of age). We should also note that LIFT researchers used half of the recommended dose for menopausal symptoms in women over 60 years of age (mean age 68). The Million Women Study (Beral 2011) suggested that breast cancer risk may be greater in women starting hormonal therapies within five years of menopause.
Populations for the LIBERATE and LIFT studies were too different for results to be combined meaningfully, and populations in both studies are not a typical target for HT addressing menopausal symptoms, so transferability of their results is a matter of concern. Other RCTs have not added useful data for better assessment of the breast cancer hypothesis. We should consider that follow‐up in available RCTs was between 12 weeks and three years, which may be too short a period for a drug therapy to induce cancer, except for the LIBERATE study, in which high‐risk women were treated and the study was powered for assessment of breast cancer recurrence.
We found 13 RCTs reporting on endometrial cancer, which occurred in only seven of these trials. Its incidence was low (most cases occurred in placebo‐controlled trials ‐ 15 cases in tibolone arms vs five cases in placebo arms ‐ most in Kenemans 2009), so that the hypothesis emerging from observational studies of greater risk with tibolone could not be confirmed. In this case, we should also consider that study follow‐up ranged between 12 weeks and three years ‐ an inadequate duration for a drug therapy to induce cancer.
Data on cerebrovascular events provide some suggestion of higher risk of stroke with tibolone versus placebo. This result was driven by the LIFT study (Cummings 2008), which recruited women over 60 years of age and stopped after 33 months for such an unexpected difference of 2.3 more events every 1000 women per year, which was even greater during the first year of treatment. These data are consistent with data from systematic reviews of RCTs testing combined HT therapies versus placebo; among those, a Cochrane review (Sanchez 2005) including 10 RCTs with a total of 24,283 women randomised to hormone therapy (HT) or placebo for an average of five years (risk ratio (RR) for stroke 1.25, 95% confidence interval (CI) 1.07 to 1.45). As for RCTs directly comparing tibolone versus combined HT, our review did not show differences between treatments, but data were scant. Unpublished data from the Million Women Study (available as rapid response; Beral 2007) had suggested higher risk of fatal stroke with tibolone versus other hormonal therapies (RR 1.58, 95% CI 1.06 to 2.37).
Our review provides no evidence of an increase in cardiovascular events with tibolone versus placebo, whereas data on thromboembolic events are very scant and unhelpful. As for combined HT, Sanchez 2005 found no increase in cardiovascular events and total mortality with HT but reported an increase in thromboembolic events. Randomised controlled trials directly comparing tibolone versus combined HT have provided few data and have revealed no statistically significant differences.
Last, two large RCTs (Cummings 2008; Kenemans 2009), which included higher‐risk women than were included in other studies (for previous cancer or more advanced age), provided most of the data on mortality, revealing no statistically significant differences or trends.
Summary of benefits and harms
Moderate‐quality evidence suggests that tibolone is more effective than placebo and less effective overall than combined HT in reducing postmenopausal symptoms, although the magnitude of observed differences is low. Tibolone provides a clear advantage in terms of less vaginal bleeding, but available data from RCTs on its long‐term safety compared with other hormonal therapies are insufficient.
We found no evidence that tibolone increases the risk of serious adverse events for women taking it over a short term to treat vasomotor symptoms, provided they have had no history of breast cancer, but data are scarce and more evidence is required. Evidence indicates that tibolone is associated with increased risk of serious adverse events when used in other contexts. Tibolone leads to increased risk of breast cancer among women with a history of breast cancer and appears to increase the risk of stroke in older women. Data on endometrial cancer are inconclusive.
Overall completeness and applicability of evidence
Moderate‐quality evidence on symptomatic relief may limit its applicability and clinical relevance. Very little evidence is available on the risks of breast and endometrial cancer in women typically treated for menopausal symptoms. In addition to this, we found no unpublished studies and did not obtain such information from the drug manufacturer. It should be highlighted that absence of publication bias is unusual in therapeutic areas with strong commercial interests, especially as almost all of the published RCTs were sponsored by the drug manufacturer (Bekelman 2003; Lexchin 2003).
Most of the included RCTs assessed effects of tibolone 2.5 mg ‐ the most frequently used dose. Therapeutic schemes and doses of active controls (combined HT) also reflect those normally used. Most of the selected RCTs included postmenopausal women with menopausal symptoms. Two of the largest RCTs, which strongly influenced results on several outcomes, included very specific populations (patients with breast cancer and those with osteoporosis, respectively), and findings of these studies are of limited applicability to women taking tibolone for menopausal symptoms.
Quality of the evidence
We rated the quality of the evidence for the primary outcome of our review ‘vasomotor symptoms’ as moderate for comparisons of tibolone versus placebo and combined HT, and very low for the comparison against oestrogens. We consider the quality to be very low for the comparison versus oestrogen because we identified only two small studies, both of which were compromised by attrition bias. Given that dropout in these studies is very likely to be informative (women with poorer responses will be more likely to drop out), attrition could be fatal to the validity of a trial. In relation to comparisons against combined HT and placebo, we have identified weaknesses in many of the individual studies. However, on the basis of our sensitivity analyses, we believe we can be reasonably confident in our conclusions related to vasomotor symptoms, for the following reasons.
First, many of the relevant studies in these comparisons are subject to attrition bias, which, as noted above, could undermine the validity of a trial. However, we have shown that our conclusions are quite robust if we include only studies without high risk of attrition bias. Another concern is the matter of poor reporting in these studies. This is a matter of concern because we had to make some assumptions about variance in some studies, and we had to pool outcomes measured on different scales. However, although this may have had some impact on the exact size (and precision) of the estimate, it is probably unlikely that we arrived at estimates in the wrong direction (i.e. it is unlikely that placebo is actually better than tibolone, or that HT is worse than tibolone, with respect to vasomotor symptoms). Heterogeneity among studies is notable, but for the comparison versus placebo, we appear to explain much of it as the result of dose effects and artificially large estimates due to attrition bias in several studies. Substantial heterogeneity remains for the comparison versus HT, which we cannot explain; we see no evidence of a difference in treatment effectiveness according to treatment duration, and considerable variation remains after studies with high risk of attrition bias were excluded. One study (Hammar 1998) dominates this comparison: It is reasonably sized and appears to be of fair quality (given its use of a non‐validated measurement scale). This study has a conflict of interest, as the manufacturer of tibolone is involved. However, the estimate from this trial actually suggests a disadvantage of tibolone, so the conflict of interest is not really a concern. Many of the other included studies have similar conflicts of interest. However, specific concerns in relation to this would involve selective reporting and publication bias, and we would expect these to manifest as artificial exaggeration of the benefits of tibolone. We have ended up concluding that tibolone is inferior to HT in relation to vasomotor symptoms; it seems unlikely that companies would be hiding studies or analyses that showed tibolone as superior to HT, so it is unlikely that our conclusion would change if we discovered new studies. These biases may have affected our estimate of the effect of tibolone compared with placebo, although we tentatively note that trials with no apparent conflict of interest also demonstrated benefit in relation to vasomotor symptoms (tentatively, because these studies are themselves subject to other sources of bias). In summary, although the individual studies have weaknesses, we believe we can be fairly confident in our conclusions related to vasomotor symptoms, given the collective evidence. Although the exact size and precision of our estimates could change in light of further research, we believe that our clinical conclusions are reasonably unlikely to do so. In our view, this warrants a GRADE assessment of moderate quality.
We would similarly assess the quality of the evidence for the outcome unscheduled bleeding. We found no evidence for the comparison against oestrogens, but we would consider the evidence to be of moderate quality when taken collectively for the comparisons against placebo and combined HT, because estimates from studies with conflicts of interest and showing attrition bias appear to be generally similar to those from studies not revealing these weaknesses. We have rated the quality of evidence related to other adverse events as very low, as the result of low or very low event rates, leading to imprecision in our estimates and a corresponding inability to comment on the effects of tibolone on these endpoints.
Potential biases in the review process
As stated above, we asked the drug manufacturer, which sponsored almost all of the published RCTs, to provide possibly unpublished data but received no written response. Funnel plot analyses did not help review authors in assessing the presence of publication bias, given the relative scarcity of studies and data, although we were able to produce such plots for both unscheduled bleeding and vasomotor symptoms, and these suggested no obvious bias.
Agreements and disagreements with other studies or reviews
Use of tibolone for the treatment of menopausal symptoms has never been supported by demonstrated advantages over oestrogens and combined HT therapies, such as lower risks of breast and endometrial cancer. On the contrary, observational data from the Million Women Study (Beral 2003; Beral 2005) suggested greater risk of breast cancer (RR 1.45, 95% CI 1.25 to 1.68) and endometrial cancer (RR 1.79, 95% CI 1.43 to 2.25) versus non‐users of HT, and two more recent RCTs included in this review (Cummings 2008; Kenemans 2009) have raised concerns about the benefit/risk profile of this drug. The latter two trials targeted very specific populations (women over 60 years of age and women who had already had breast cancer), and their results are not easily generalisable, although it may be wise to apply a precautionary principle and not exclude the possibility of safety problems for other groups. It should be noted that the Food and Drug Administration rejected the application for the registration of tibolone in the United States, although the reason for this is unknown.
With regard to the effectiveness of tibolone for treating menopausal symptoms, the effectiveness of combined HT over placebo has been shown more convincingly (MacLennan 2004).
Authors' conclusions
Implications for practice.
Moderate‐quality evidence suggests that tibolone is more effective than placebo and is less effective than combined hormone therapy (HT) in treating vasomotor symptoms. Tibolone is associated with a higher rate of unscheduled bleeding than placebo but a lower rate than combined HT.
Compared with placebo, tibolone increases the risk of recurrent breast cancer in women with a history of breast cancer, and may increase the risk of stroke in women over 60 years of age. No evidence indicates that tibolone increases the risk of other long‐term adverse events, and no evidence has revealed a difference between tibolone and HT with respect to long‐term adverse events.
Many of the included randomised controlled trials (RCTs) were of low or very low quality. Limitations included high risk of bias in the included trials, very low event rates and potential conflicts of interest. Twenty‐four studies were financed by drug manufacturers, and another 10 failed to disclose their source of funding.
Implications for research.
This review may reveal a systematic misunderstanding of RCT methods in this field, with study authors routinely misinterpreting their own trials. In particular, trial authors frequently interpret change from baseline in a study arm as evidence of a treatment effect. Change from baseline within a treatment group, even if statistically significant, can never be interpreted in this way; even in the absence of any effect of treatment at all, the appearance of improvement would be due to the twin spectres of variation in repeated responses of any given individual and so‐called regression to the mean, whereby subsequent measurements will tend to be closer to the population average compared with relatively severe baseline measurements introduced by a study’s inclusion criteria. Patient‐reported outcome measures, such as those commonly used in this field, are particularly susceptible to these phenomena. It may help researchers to consider the fact that, were it possible to make a conclusion of treatment effectiveness based on the evolution of a single group, no comparator group, and therefore no RCT, would be required. Researchers should keep this in mind before making erroneous inferences that may be used as the basis for clinical decision making.
Other areas of statistical weakness in these trials include poor methods for handling missing outcome data due to dropout and for analysing longitudinal outcomes. In relation to the former, we found that it was common to ignore participants who had dropped out or to carry their last observation forward for analysis. These approaches may introduce serious bias if a patient is more or less likely to drop out depending on her symptoms. Researchers should instead employ such appropriate methods as multiple imputation (Sterne 2009). In relation to longitudinal analysis, researchers generally analysed separately mean responses at each of several time points. This is problematic because it both ignores the variation in patterns of response over time and increases the possibility of false‐positive results due to multiple testing. Researchers instead should employ linear mixed models for which statistical expertise is available (Diggle 1994), or should perform analyses based on summary measures of longitudinal responses when it is not (Matthews 1990).
Finally, we would appeal to researchers to adhere to CONSORT guidelines when reporting RCTs. Reporting was poor in the included studies, representing a considerable obstacle to meta‐analysis in this review.
In this specific clinical area, well‐designed comparative RCTs are needed to better assess whether, in women with troublesome menopausal symptoms who use short‐term therapies, tibolone is as effective as combined HT in relieving symptoms. Although no evidence indicates that use of tibolone for up to three years increases the risk of serious adverse events in younger postmenopausal women without a history of breast cancer, observational studies and RCTs in other populations have raised serious doubts on the risks of long‐term use of both tibolone and combined HT. Therefore, RCTs realised to better clarify the comparative safety of these drugs would be unethical. A systematic review of observational studies may be warranted to improve our understanding in this regard.
What's new
| Date | Event | Description |
|---|---|---|
| 23 November 2016 | Review declared as stable | We have made this a stable review as further evidence is unlikely to change its conclusions. |
History
Protocol first published: Issue 6, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 15 September 2016 | New search has been performed | Updated version |
| 15 September 2016 | New citation required but conclusions have not changed | Thirteen new studies (Baracat 2002;Benedek‐Jaszmann 1987;Bouchard 2012;Gupta 2013;Jacobsen 2012;Mendoza 2000;Morais‐Socorro 2012;Okon 2005;Polisseni 2013;Ross 1999;Uygur 2005;Volpe 1986;Wender 2004) added and additional data included for one study (Langer 2006). Two reports of the same study (Ziaei 2010) amalgamated. Total of 46 studies in updated review |
| 20 September 2010 | New search has been performed | Contact details updated. |
| 9 February 2010 | Amended | made corrections according to Editorial Board's requests |
| 23 March 2006 | New citation required and major changes | Substantive amendment |
Acknowledgements
The review authors acknowledge Jane Marjoribanks for her support in reviewing the final version, Marian Showell and Maria Camerlingo for their support in conducting literature searches and retrieval and the three peer reviewers for their useful suggestions.
Appendices
Appendix 1. Gynaecology and Fertility (GF) Specialised Register search strategy
Formerly known as the Menstrual Disorders and Subfertility database (MDSG)
Inception until 14.10.15
Keywords CONTAINS "climacteric "or "menopausal" or "*Menopause" or "postmenopausal" or "postmenopause" or "perimenopausal" or "perimenopause" or "vasomotor" or "hot flashes" or "hot flushes" or "night sweats" or "night time awakenings" or Title CONTAINS "climacteric "or "menopausal" or "*Menopause" or "postmenopausal" or "postmenopause" or "perimenopausal" or "perimenopause" or "vasomotor" or "hot flashes" or "hot flushes" or "night sweats" or "night time awakenings"
AND
Keywords CONTAINS "tibolone" or "Livial" or Title CONTAINS "tibolone" or "Livial" (289 hits)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials (Ovid platform)
From inception until 14.10.15
1 exp climacteric/ or exp menopause/ or exp menopause, premature/ or exp perimenopause/ or exp postmenopause/ (5805) 2 (climacteric or menopaus$).tw. (5145) 3 (postmenopaus$ or perimenopaus$).tw. (10135) 4 exp Hot Flashes/ (514) 5 (hot flush$ or hot flash$).tw. (1247) 6 vasomotor.tw. (1057) 7 or/1‐6 (14853) 8 (tibolone or tibilone).tw. (430) 9 17 hydroxy.tw. (29) 10 17 alpha.tw. (149) 11 (boltin or livial).tw. (44) 12 (liviella or tibofem).tw. (0) 13 xyvion.tw. (0) 14 (org od 14 or org od 4).tw. (26) 15 17 beta hydroxy$.tw. (8) 16 or/8‐15 (625) 17 7 and 16 (399)
Appendix 3. MEDLINE(R) search strategy
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)
From inception to 14.10.15
1 exp climacteric/ or exp menopause/ or exp menopause, premature/ or exp perimenopause/ or exp postmenopause/ (52515) 2 (climacteric or menopaus$).tw. (41594) 3 (postmenopaus$ or perimenopaus$).tw. (47307) 4 exp Hot Flashes/ (2625) 5 (hot flush$ or hot flash$).tw. (3908) 6 vasomotor.tw. (11184) 7 or/1‐6 (100994) 8 (tibolone or tibilone).tw. (912) 9 17 hydroxy.tw. (553) 10 17 alpha.tw. (5521) 11 (boltin or livial).tw. (66) 12 (liviella or tibofem).tw. (0) 13 xyvion.tw. (0) 14 (org od 14 or org od 4).tw. (44) 15 17 beta hydroxy$.tw. (1658) 16 or/8‐15 (8186) 17 randomized controlled trial.pt. (414057) 18 controlled clinical trial.pt. (91918) 19 randomized.ab. (335509) 20 placebo.tw. (173417) 21 clinical trials as topic.sh. (179333) 22 randomly.ab. (242103) 23 trial.ti. (147798) 24 (crossover or cross‐over or cross over).tw. (66140) 25 or/17‐24 (1025496) 26 (animals not (humans and animals)).sh. (4035803) 27 25 not 26 (944593) 28 7 and 16 and 27 (391)
Appendix 4. Embase search strategy
Database: Ovid Embase
From inception until 14.10.15
1 exp "menopause and climacterium"/ or exp climacterium/ or exp early menopause/ or exp menopause/ or exp postmenopause/ (94150) 2 (climacteric or menopaus$).tw. (56667) 3 (postmenopaus$ or perimenopaus$).tw. (61730) 4 vasomotor.tw. (12794) 5 exp hot flush/ (12544) 6 (hot flush$ or hot flash$).tw. (5365) 7 or/1‐6 (145116) 8 exp Tibolone/ (2591) 9 (tibilone or tibolone).tw. (1219) 10 (boltin or livial).tw. (425) 11 17 beta hydroxy$.tw. (488) 12 17 hydroxy.tw. (573) 13 17 alpha.tw. (2144) 14 (liviella or tibofem).tw. (25) 15 xyvion.tw. (7) 16 (org od 14 or org od 4).tw. (105) 17 or/8‐16 (5728) 18 Clinical Trial/ (851552) 19 Randomized Controlled Trial/ (385597) 20 exp randomization/ (68366) 21 Single Blind Procedure/ (21090) 22 Double Blind Procedure/ (124054) 23 Crossover Procedure/ (44662) 24 Placebo/ (264312) 25 Randomi?ed controlled trial$.tw. (124841) 26 Rct.tw. (18429) 27 random allocation.tw. (1456) 28 randomly allocated.tw. (23397) 29 allocated randomly.tw. (2061) 30 (allocated adj2 random).tw. (738) 31 Single blind$.tw. (16431) 32 Double blind$.tw. (155332) 33 ((treble or triple) adj blind$).tw. (491) 34 placebo$.tw. (221733) 35 prospective study/ (309654) 36 or/18‐35 (1510043) 37 case study/ (34071) 38 case report.tw. (292028) 39 abstract report/ or letter/ (940292) 40 or/37‐39 (1259859) 41 36 not 40 (1470095) 42 7 and 17 and 41 (979)
Appendix 5. PsycINFO search strategy
Database: Ovid PsycINFO
From inception until 14.10.15
1 exp menopause/ (3151) 2 (climacteric or menopaus$).tw. (4257) 3 (postmenopaus$ or perimenopaus$).tw. (2524) 4 vasomotor.tw. (1224) 5 or/1‐4 (6700) 6 (tibilone or tibolone).tw. (33) 7 (boltin or livial).tw. (3) 8 (liviella or tibofem).tw. (0) 9 xyvion.tw. (0) 10 (org od 14 or org od 4).tw. (0) 11 17 hydroxy.tw. (34) 12 17 alpha.tw. (27) 13 17 beta hydroxy$.tw. (6) 14 or/6‐13 (99) 15 5 and 14 (30)
Appendix 6. CINAHL search strategy
Database: Ovid CINAHL
From inception until 13.12.07
S28 S9 AND S26 AND S27 (80)
S27 S10 OR S11 OR S12 OR S13 OR S14 (593)
S26 S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 (921,449)
S25 TX allocat* random* (4,096)
S24 (MH "Quantitative Studies") (12,613)
S23 (MH "Placebos") (8,922)
S22 TX placebo* (32,488)
S21 TX random* allocat* (4,096)
S20 (MH "Random Assignment") (38,014)
S19 TX randomi* control* trial* (78,710)
S18 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) (739,304)
S17 TX clinic* n1 trial* (166,477)
S16 PT Clinical trial (76,624)
S15 (MH "Clinical Trials+") (179,629)
S14 TX 17 beta hydroxy (5)
S13 TX (boltin or livial) (16)
S12 TX 17 alpha (314)
S11 TX 17 hydroxy* (251)
S10 TX (tibilone or tibolone) (147)
S9 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 (21,930)
S8 TX climacteri* (1,622)
S7 TX (postmenopaus* or perimenopaus*) (13,847)
S6 TX menopaus* (11,430)
S5 TX hot flash* (1,998)
S4 TX hot flush* (465)
S3 TX vasomotor (1,060)
S2 (MM "Postmenopause") (3,318)
S1 (MM "Climacteric"# OR #MM "Perimenopause"# OR #MM "Perimenopausal Symptoms"# OR #MM "Menopause+"# OR #MM "Hot Flashes"# (8,921)
Database: Ebsco CINAHL
From inception until 14.10.15
| # | Query | Results |
| S28 | S9 AND S26 AND S27 | 82 |
| S27 | S10 OR S11 OR S12 OR S13 OR S14 | 645 |
| S26 | S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 990,143 |
| S25 | TX allocat* random* | 4,464 |
| S24 | (MH "Quantitative Studies") | 13,814 |
| S23 | (MH "Placebos") | 9,427 |
| S22 | TX placebo* | 34,772 |
| S21 | TX random* allocat* | 4,464 |
| S20 | (MH "Random Assignment") | 39,802 |
| S19 | TX randomi* control* trial* | 93,467 |
| S18 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 789,912 |
| S17 | TX clinic* n1 trial* | 175,948 |
| S16 | PT Clinical trial | 78,685 |
| S15 | (MH "Clinical Trials+") | 192,364 |
| S14 | TX 17 beta hydroxy | 5 |
| S13 | TX (boltin or livial) | 18 |
| S12 | TX 17 alpha | 338 |
| S11 | TX 17 hydroxy* | 281 |
| S10 | TX (tibilone or tibolone) | 153 |
| S9 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 | 23,371 |
| S8 | TX climacteri* | 1,799 |
| S7 | TX (postmenopaus* or perimenopaus*) | 14,679 |
| S6 | TX menopaus* | 12,192 |
| S5 | TX hot flash* | 2,163 |
| S4 | TX hot flush* | 484 |
| S3 | TX vasomotor | 1,147 |
| S2 | (MM "Postmenopause") | 3,604 |
| S1 | (MM "Climacteric") OR (MM "Perimenopause") OR (MM "Perimenopausal Symptoms") OR (MM "Menopause+") OR (MM "Hot Flashes") | 9,562 |
Data and analyses
Comparison 1. Tibolone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vasomotor symptoms | 7 | 1657 | Std. Mean Difference (Fixed, 95% CI) | ‐0.99 [‐1.10, ‐0.89] |
| 1.1 Tibolone 0.625 mg/d | 1 | 158 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.46, 0.36] |
| 1.2 Tibolone 1.25 mg/day | 3 | 414 | Std. Mean Difference (Fixed, 95% CI) | ‐0.83 [‐1.06, ‐0.60] |
| 1.3 Tibolone 2.5 mg/day | 7 | 920 | Std. Mean Difference (Fixed, 95% CI) | ‐1.16 [‐1.30, ‐1.03] |
| 1.4 Tibolone 5 mg/day | 1 | 165 | Std. Mean Difference (Fixed, 95% CI) | ‐0.84 [‐1.25, ‐0.43] |
| 2 Unscheduled bleeding | 9 | 7814 | Odds Ratio (M‐H, Random, 95% CI) | 2.79 [2.10, 3.70] |
| 2.1 Tibolone, 2.5 mg/day | 8 | 4186 | Odds Ratio (M‐H, Random, 95% CI) | 2.58 [1.89, 3.52] |
| 2.2 Tibolone, 1.25 mg/day | 3 | 3628 | Odds Ratio (M‐H, Random, 95% CI) | 3.63 [2.37, 5.55] |
| 3 Endometrial cancer | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tibolone, all doses | 9 | 8504 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [0.79, 5.24] |
| 4 Breast cancer; women without previous breast cancer | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Tibolone, all doses | 4 | 5500 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.21, 1.25] |
| 5 Breast cancer; women with previous breast cancer | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Tibolone, 2.5 mg/day | 2 | 3165 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [1.21, 1.85] |
| 6 Venous thromboembolic events (clinical evaluation) | 5 | 9176 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.97] |
| 6.1 Tibolone (all doses) | 5 | 9176 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.97] |
| 7 Cardiovascular events | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Tibolone, all doses | 4 | 8401 | Odds Ratio (M‐H, Random, 95% CI) | 1.38 [0.84, 2.27] |
| 8 Cerebrovascular events; women's mean age over 60 years | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Tibolone (all doses) | 4 | 7930 | Odds Ratio (M‐H, Random, 95% CI) | 1.74 [0.99, 3.04] |
| 9 Mortality from any cause | 4 | 8242 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.79, 1.41] |
| 9.1 Tibolone, 2.5 mg/day | 3 | 3736 | Odds Ratio (M‐H, Random, 95% CI) | 0.94 [0.32, 2.73] |
| 9.2 Tibolone, 1.25 mg/day | 1 | 4506 | Odds Ratio (M‐H, Random, 95% CI) | 0.93 [0.54, 1.59] |
| 10 Insomnia | 3 | 3432 | Std. Mean Difference (Fixed, 95% CI) | ‐0.19 [‐0.38, ‐0.00] |
| 10.1 Tibolone, 2.5 mg/day | 3 | 3432 | Std. Mean Difference (Fixed, 95% CI) | ‐0.19 [‐0.38, ‐0.00] |
| 11 Vaginal dryness and painful sexual intercourse | 3 | 3348 | Std. Mean Difference (Fixed, 95% CI) | ‐0.66 [‐0.90, ‐0.43] |
| 11.1 Tibolone, 1.25mg/day | 1 | 62 | Std. Mean Difference (Fixed, 95% CI) | ‐1.78 [‐2.43, ‐1.13] |
| 11.2 Tibolone, 2.5 mg/day | 3 | 3286 | Std. Mean Difference (Fixed, 95% CI) | ‐0.49 [‐0.75, ‐0.24] |
| 12 Vaginal infections | 2 | 7639 | Odds Ratio (M‐H, Random, 95% CI) | 2.50 [1.24, 5.06] |
| 12.1 Tibolone, 2.5 mg/day | 1 | 3133 | Odds Ratio (M‐H, Random, 95% CI) | 1.73 [1.17, 2.55] |
| 12.2 Tibolone, 1.25 mg/day | 1 | 4506 | Odds Ratio (M‐H, Random, 95% CI) | 3.54 [2.61, 4.81] |
| 13 Urinary tract infections | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Tibolone, 2.5 mg/day | 1 | 3133 | Odds Ratio (M‐H, Random, 95% CI) | 0.70 [0.46, 1.06] |
| 14 Endometrial hyperplasia | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Tibolone, all doses | 4 | 4518 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.23, 6.25] |
| 15 Sensitivity Analysis ‐ Vasomotor symptoms without trials with high risk of attrition bias | 4 | Std. Mean Difference (Fixed, 95% CI) | ‐0.61 [‐0.73, ‐0.49] | |
| 15.1 Tibolone 0.625 mg/day | 1 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.46, 0.36] | |
| 15.2 Tibolone 1.25 mg/day | 2 | Std. Mean Difference (Fixed, 95% CI) | ‐0.62 [‐0.86, ‐0.38] | |
| 15.3 Tibolone 2.5 mg/day | 4 | Std. Mean Difference (Fixed, 95% CI) | ‐0.65 [‐0.80, ‐0.50] | |
| 15.4 Tibolone 5 mg/day | 1 | Std. Mean Difference (Fixed, 95% CI) | ‐0.84 [‐1.25, ‐0.43] |
Comparison 2. Tibolone versus oestrogens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vasomotor symptoms | 2 | 108 | Odds Ratio (M‐H, Random, 95% CI) | 1.23 [0.35, 4.34] |
| 2 Insomnia | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Vaginal dryness and painful sexual intercourse | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 8.25] |
Comparison 3. Tibolone versus combined HT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vasomotor symptoms | 9 | 1336 | Std. Mean Difference (Fixed, 95% CI) | 0.17 [0.06, 0.28] |
| 1.1 Tibolone, 2.5 mg/day | 9 | 1336 | Std. Mean Difference (Fixed, 95% CI) | 0.17 [0.06, 0.28] |
| 2 Unscheduled bleeding | 16 | 6438 | Odds Ratio (M‐H, Random, 95% CI) | 0.32 [0.24, 0.41] |
| 2.1 Tibolone, 2.5 mg/day | 16 | 4720 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.26, 0.45] |
| 2.2 Tibolone, 1.25 mg/day | 2 | 1718 | Odds Ratio (M‐H, Random, 95% CI) | 0.21 [0.16, 0.26] |
| 3 Endometrial cancer | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Tibolone, 2.5 mg/day | 5 | 3689 | Odds Ratio (M‐H, Random, 95% CI) | 1.47 [0.23, 9.33] |
| 4 Breast cancer; women without previous breast cancer | 5 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Tibolone (all doses) | 5 | 4835 | Odds Ratio (M‐H, Random, 95% CI) | 1.69 [0.78, 3.67] |
| 5 Venous thromboembolic events (clinical evaluation) | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Tibolone (all doses) | 4 | 4529 | Odds Ratio (M‐H, Random, 95% CI) | 0.44 [0.09, 2.14] |
| 6 Cardiovascular events; all women's mean age below 60 years. No data available on different doses | 2 | 3794 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.24, 1.66] |
| 7 Cerebrovascular events; women's mean age below 60 years | 4 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Tibolone (all doses) | 4 | 4562 | Odds Ratio (M‐H, Random, 95% CI) | 0.76 [0.16, 3.66] |
| 8 Mortality from any cause | 2 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Tibolone, 2.5 mg/day | 2 | 970 | Odds Ratio (M‐H, Random, 95% CI) | 3.05 [0.12, 75.20] |
| 9 Endometrial hyperplasia | 5 | 2846 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.05, 2.21] |
| 9.1 Tibolone, 2.5 mg/day | 5 | 1549 | Odds Ratio (M‐H, Random, 95% CI) | 0.35 [0.04, 3.36] |
| 9.2 Tibolone, 1.25 mg/day | 1 | 1297 | Odds Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.48] |
| 10 Vaginal dryness and painful sexual intercourse | 7 | 1098 | Std. Mean Difference (Fixed, 95% CI) | 0.02 [‐0.12, 0.17] |
| 10.1 Tibolone, 2.5 mg/day | 7 | 1098 | Std. Mean Difference (Fixed, 95% CI) | 0.02 [‐0.12, 0.17] |
| 11 Sensitivity Analysis ‐ Vasomotor symptoms without trials with high risk of attrition bias | 4 | Std. Mean Difference (Fixed, 95% CI) | 0.25 [0.09, 0.41] | |
| 12 Sensitivity analysis ‐ vasomotor symptoms ‐ excluding studies with attrition bias and using nonvalidated scales | 3 | Std. Mean Difference (Fixed, 95% CI) | ‐0.03 [‐0.30, 0.23] | |
| 13 Vasomotor symptoms ‐ ordered by duration | 9 | Std. Mean Difference (Fixed, 95% CI) | 0.17 [0.06, 0.28] | |
| 13.1 Tibolone, 2.5 mg/day | 9 | Std. Mean Difference (Fixed, 95% CI) | 0.17 [0.06, 0.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Azzawi 1999.
| Methods | Randomised open‐label controlled trial | |
| Participants | 235 healthy women with intact uteri, ≥ 12 months postmenopausal (mean 61 months), with serum FSH exceeding 20 IU/L. None of the women enrolled in the study had received hormone therapy during the 3 months before enrolment. Mean age: 54 years | |
| Interventions |
Administered for 1 year |
|
| Outcomes | Vaginal bleeding (0 to 3 months), menopausal symptoms, pulmonary embolism | |
| Notes | Commented on menopausal symptoms that were assessed according to the Greene menopausal symptoms scale but provided no data on women who completed ≥ 3 months of treatment 12‐Month data on vaginal bleeding not available. Cumulative data available only for the first 3 months Timing: unclear Location: unclear (UK?) Multi‐centre: 15 sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified but, given the nature of the outcomes assessed, evaluation likely to be "objective". Open design may affect evaluation of climacteric symptoms, but these were not taken into consideration (score) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants analysed was variable for different outcomes and throughout the study, depending on the number of completed diaries. Cumulative 12‐month incidence of vaginal bleeding not available |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by the drug manufacturer. Study authors have conflicts of interest |
Archer 2007.
| Methods | Randomised controlled trial | |
| Participants | 3240 postmenopausal healthy women, with an intact uterus and with a screening biopsy classified as atrophic or inactive endometrium and a double‐layer endometrial thickness ≤ 6 mm as assessed by transvaginal ultrasonography (TVUS). Mean time since menopause: 4.5 years. Mean age: 54.4 years | |
| Interventions |
Administered for 2 years |
|
| Outcomes | Unscheduled bleeding, breast cancer, endometrial cancer, endometrial hyperplasia, ovarian cancer, cardiovascular events, cerebrovascular events, thromboembolic events | |
| Notes | Timing: not reported Location: USA, Europe, Chile Multi‐centre:146 centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No details on random generation of the allocation sequence, but use of an interactive voice response system should keep risk of selection bias very low |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified but, given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information on withdrawals/dropouts |
| Selective reporting (reporting bias) | Low risk | No difference between study protocol and assessed outcomes |
| Conflict of interest | High risk | Financed by the drug manufacturer; some study authors are employees of the drug manufacturer |
Baracat 2002.
| Methods | Randomised controlled trial; open label, multi‐centre | |
| Participants | 85 generally healthy postmenopausal women, with an intact uterus, in menopause for ≥ 4 years, absence of endometrial hyperplasia, mean age 52 years | |
| Interventions |
For 13 treatment cycles, each of 28 days |
|
| Outcomes | Hot flushes, unscheduled bleeding, vaginal dryness, painful intercourse, endometrial hyperplasia | |
| Notes | Timing: not available Location: Brasil Multi‐centre: number of sites not specified Hot flushes not measured with a validated score (frequency and intensity of hot flushes for each participant in each cycle were calculated as the sum of the mean # of hot flushes per day multiplied by the respective score (1 = mild, 2 = moderate, 3 = severe) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Describe: “the randomization was performed in balanced blocks of ten subjects using the table of aleatory numbers; each study center received 20 envelopes with the number of the subject and respective code (treatment group)” (p 62) |
| Allocation concealment (selection bias) | Low risk | Describe: “the randomization was performed in balanced blocks of ten subjects using the table of aleatory numbers; each study center received 20 envelopes with the number of the subject and respective code (treatment group)” (p 62) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Describe: open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Describe: participants unblinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe: similar rates of discontinuation, reasons given |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Describe: sponsored by manufacturer of CEE/MPA |
Benedek‐Jaszmann 1987.
| Methods | Parallel‐group RCT | |
| Participants | 60 healthy postmenopausal women 44 to 61 years old, with hot flushes, who had undergone natural or surgical menopause and were experiencing hot flushes and associated symptoms | |
| Interventions |
Administered for 1 year |
|
| Outcomes | Hot flushes, insomnia Following scoring system used for clinical parameters: absent = 0, mild = 1, moderate = 2, strong = 3 |
|
| Notes | Menopausal symptoms measured on a non‐validated scale Timing: unclear Trial location: Netherlands Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study authors state that the trial is double‐blind and that identical‐looking placebo tablets have been used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes evaluated through a questionnaire with insufficient information to judge whether outcome measurement could have been influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17/60 participants dropped out. Unclear how many were randomised to each group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No information provided |
Berning 2000.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 94 healthy non‐smoking women, 1 to 3 years following spontaneous menopause (mean 22 months), with body mass index < 27 kg/m2, free of diseases or medication known to influence calcium metabolism or to contraindicate the trial medication. Mean age: 53 years | |
| Interventions |
Administered for 2 years |
|
| Outcomes | Vaginal bleeding | |
| Notes | Timing: unclear Location: Netherlands Multi‐centre: number of sites not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear what "random medication number" means |
| Allocation concealment (selection bias) | Unclear risk | Method not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although study authors do not state whether trial is double‐blind or single‐blind, they used identical looking interventions and a placebo control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of the outcome assessed, evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Bleeding: all randomised participants assessed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by the drug manufacturer. Study authors have conflicts of interest |
Bouchard 2012.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | 485 postmenopausal women 40 to 65 years of age, seeking treatment for hot flushes, who had completed their last natural menstrual period 12 months before screening (or had a follicle‐stimulating hormone (FSH) level 40 mIU/mL). Women had intact uterus, BMI ≤ 34 and minimum of 7 moderate and severe hot flushes per day, or 50 moderate and severe hot flushes per week, recorded for 7 consecutive days during screening. Mean age: 53.6 years | |
| Interventions | Tibolone 2.5 mg/d, placebo, desvenlafaxine 100 mg/d (not considered in meta‐analyses) | |
| Outcomes | Hot flushes (frequency), hot flushes (severity, through the Greene climacteric scale), uterine bleeding, endometrial cancer | |
| Notes | Multi‐centre trial (35 sites in Europe, 2 sites in South Africa, 1 site in Mexico) Timing: unclear Follow‐up: 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study authors declare that this is a double‐blind trial but do not provide information on blinding methods |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in each of tibolone and placebo groups not assessed for taking study medications for less than 5 days |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Study sponsored by Wyeth; 4 study authors are former Wyeth or current Pfizer employees |
Cummings 2008.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 4538 women between 60 and 85 years of age (mean 68) who had bone mineral density T score ≤ −2.5 at the hip or lumbar spine or T score ≤ −2.0 with radiological evidence of vertebral fracture | |
| Interventions |
Administered for 34 months (median) |
|
| Outcomes | Vaginal bleeding, vaginal infection, endometrial cancer and endometrial hyperplasia, breast cancer, stroke, coronary heart disease, venous thromboembolism, mortality from any cause | |
| Notes | Timing: July 2001 to Feb 2006, when trial was stopped because increased risk of stroke was identified Location: Europe, the Americas Multi‐centre: 80 sites in 22 countries All participants received 2 to 4 tablets of calcium + vit D daily |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No details on random generation of the allocation sequence, but use of an interactive voice response system should keep risk of selection bias very low |
| Allocation concealment (selection bias) | Low risk | Centralised interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled and identical looking interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 of 4538 participants not evaluated for not receiving any dose of the interventions under study |
| Selective reporting (reporting bias) | Low risk | Study reported data on outcomes as indicated in the protocol. Additional data on vaginal bleeding, vaginal infection, endometrial cancer and endometrial hyperplasia, breast cancer, stroke, coronary heart disease, venous thromboembolism and mortality from any cause were available in the study publication and were included in this review |
| Conflict of interest | High risk | Financed by the drug manufacturer. Study authors have conflicts of interest |
de Aloysio 1998.
| Methods | Randomised controlled trial | |
| Participants | 50 women, 13 to 30 months since menopause (mean 20 months); 1 to 4 submucous or intramural asymptomatic uterine leiomyomas (with longest diameter ranging from 3 to 8 cm); body mass index (BMI) < 28; without blood coagulation disease; without endometrial pathology. Mean age: 51 years | |
| Interventions |
Administered for twelve 28‐day cycles |
|
| Outcomes | Irregular bleeding, endometrial hyperplasia | |
| Notes | Bleeding measured as incidence of bleeding cycles/number of cycles Timing and trial location unclear Multi‐centre: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of the outcome assessed (endometrial hyperplasia), its evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants excluded from analysis for non‐compliance (reasons not related to the study but not better specified) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Not financed by drug manufacturer; other conflicts of interest not stated |
Doren 1999.
| Methods | Randomised double‐blind placebo‐controlled study | |
| Participants | 98 healthy postmenopausal women, with intact uterus (mean age 56 years), mean BMI 25 kg/m2, mean time since menopause 6 years | |
| Interventions |
For 12 months |
|
| Outcomes | Unscheduled bleeding | |
| Notes | Timing: unclear Location: Netherlands; single centre Hot flashes and sleeplessness reported as adverse events, each by 1 participant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Describe: no details on randomisation |
| Allocation concealment (selection bias) | Unclear risk | Describe: no details given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Describe: participants blinded but no details on personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Describe: participants recorded bleeding episodes in a diary |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe: reasons for withdrawal explained |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Describe: study sponsored by manufacturer of tibolone; employer among study authors |
Egarter 1996.
| Methods | Randomised controlled trial | |
| Participants | 129 women with physiological menopause (for ≥ 12 months), mean age 53 years | |
| Interventions |
For 6 months |
|
| Outcomes | Unscheduled bleeding, severity of menopausal symptoms (hot flashes, insomnia, vaginal dryness) | |
| Notes | Data on unscheduled bleeding reported in a graph but number of events unclear Timing: not reported Location: Austria Multi‐centre: 5 sites To register severity of climacteric symptoms, a modified Kupperman Index was used |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Participants lost to follow‐up: 19.4% in tibolone group, 34.6% in combined HT group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Not reported |
Elfituri 2005.
| Methods | Randomised controlled trial | |
| Participants | 100 healthy Lybian women with a uterus, with natural or surgical menopause, with menopausal symptoms. All had received no previous oestrogen and/or progestogen in preceding 12 months. 1 to 9 years since menopause (mean 2 years). Mean age 44.3 years | |
| Interventions |
For 1 year |
|
| Outcomes | Unscheduled bleeding, endometrial cancer, vasomotor symptoms quantified as none (0), mild (1), moderate (2) and severe (3) | |
| Notes | Timing: not reported
Location: Lybia Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for withdrawals/dropouts (2 women) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Not reported |
Gallagher 2001.
| Methods | Pooled data from 1 randomised placebo‐controlled trials | |
| Participants | 770 healthy postmenopausal Caucasian or Asian women, mean duration of menopause 2.5 years, without osteoporosis (BMD of lumbar vertebrae within 2 standard deviations of age‐matched mean). Mean age: 52.4 years | |
| Interventions |
For 2 years. All groups also received 500 mg/d of calcium |
|
| Outcomes | Hot flashes, endometrial hyperplasia, endometrial cancer, thromboembolic events | |
| Notes | Timing: not reported Location: USA Multi‐centre: more than 20 centres per study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Defined by study authors as randomised but no details given on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical appearing tibolone and placebo tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 85% of randomised participants analysed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by drug manufacturer, no declaration of conflicts of interest |
Gupta 2013.
| Methods | Randomised controlled trial | |
| Participants | 100 asymptomatic patients (no menopausal symptoms) with surgical menopause 3 days earlier (total abdominal hysterectomy with bilateral salpingo‐oophorectomy) | |
| Interventions | Tibolone 2.5 mg/d; CEE 0.625 mg; DHEA 25 mg/d (all administered orally); no treatment. Latter 2 arms not considered in the meta‐analysis | |
| Outcomes | Vasomotor symptoms (occurrence of hot flushes and night sweats), insomnia (occurrence), vaginal dryness | |
| Notes | Trial location: India (single centre) Follow‐up: 12 months Timing: 2005 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This RCT is presumably an open trial ‐ includes a "no treatment" arm |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors acknowledged losses to follow‐up, but total number of lost participants is unclear |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No information provided |
Hammar 1998.
| Methods | Randomised double‐blind controlled trial | |
| Participants | 437 women with menopausal symptoms, in good physical and mental health, ≥ 1 year since last menstrual bleeding, menopausal symptoms, intact uterus, body mass index (BMI) < 30 kg/m2. Mean age 55 years | |
| Interventions |
Administered for 48 weeks |
|
| Outcomes | Vaginal bleeding (more than 1 sanitary napkin per day)/spotting (just 1 sanitary napkin per day), hot flushes (1 = none, 2 = light, 3 = moderate, 4 = severe, 5 = very severe), sweating, vaginal dryness, endometrial cancer, breast cancer, cerebrovascular events | |
| Notes | Timing: June 1992 to Feb 1995 Location: Denmark, Norway, Sweden Multi‐centre: 44 sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes eventually assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14/437 participants not assessed for lack of post‐baseline assessment |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by drug manufacturer. Study authors have conflicts of interest |
Hammar 2007.
| Methods | Randomised controlled trial | |
| Participants | 572 postmenopausal healthy women with an intact uterus, with or without vasomotor symptoms. Mean age 55 years. Time since menopause 5 years. Mean number of hot flashes at baseline 5.8 | |
| Interventions |
Administered for 48 weeks |
|
| Outcomes | Unscheduled vaginal bleeding or spotting, hot flashes, thromboembolic events, breast cancer | |
| Notes | Hot flashes measured as median number per treatment period and reported as graph Timing: from November 2002 to March 2005 Location: 7 Northern European countries Multi‐centre: 32 centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Restricted block‐wise randomisation (1:1 ratio within each specific site). No details on random generation of the allocation sequence, but use of an interactive voice response system should keep risk of selection bias very low |
| Allocation concealment (selection bias) | Low risk | Automatic interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, study site personnel and participants remained blinded until after database was locked |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 87% of randomised participants analysed but reasons for withdrawals/dropouts not given |
| Selective reporting (reporting bias) | Low risk | Outcomes assessed in the study and of specific interest for the review had been indicated in the protocol |
| Conflict of interest | High risk | Financed by the drug producer. One study author was an employee of the drug producer |
Huber 2002.
| Methods | Randomised controlled trial | |
| Participants | 502 postmenopausal women, with last menstrual period ≥ 12 months previously, younger than 65 years of age (mean age 55). If the date of natural menopause could not be established because of hormonal treatment, participants had to be ≥ 53 years of age and must have been receiving hormonal therapy for ≥ 2 years; if applicable, hormone therapy had to end with a progestogen phase. All participants were required to have an intact uterus and a body mass index (BMI) of 18 to 29 kg/m2 | |
| Interventions |
Administered for 12 months |
|
| Outcomes | Vaginal bleeding/spotting (defined as requiring sanitary protection with more than 1 sanitary pad per day vs just 1 or none), dyspareunia, severity of VM symptoms, stroke, pulmonary embolism | |
| Notes | Severity of VM symptoms quantified as none = 0, light = 1, moderate = 2, severe = 3, very severe = 4 Timing: Feb 1996 to June 1998 Location: Austria, Denmark, Spain, Sweden, Switzerland, UK Multi‐centre: 37 sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed and/or self‐evaluation by blind patients, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Several participants (about 80, depending on different outcomes) were excluded from final analyses for adverse events and insufficient compliance/efficacy |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by the drug producer. One study author was the employee of a drug producer |
Hudita 2003.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 162 healthy, non‐obese, postmenopausal women (with evidence of ≥ 12 months of amenorrhoea with levels of FSH > 30 mlU/mL and of 17β‐oestradiol < 50 pg/mL), between 40 and 65 years of age (mean age 55), with an intact uterus | |
| Interventions |
Administered for 24 weeks |
|
| Outcomes | Vaginal bleeding and spotting, hot flushes, sweating, vaginal dryness | |
| Notes | Used a non‐validated scale to assess menopausal symptoms; they were reported also as frequency reduction from baseline Timing: unclear Location: Romania Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Defined as "double‐blind" but no other specific information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided about assessment of vaginal bleeding; unclear if trial is truly "double‐blind" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 42/162 participants not analysed because of adverse events, loss to follow‐up, lack of efficacy, etc |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Not reported |
Hänggi 1997.
| Methods | Randomised controlled trial | |
| Participants | 140 healthy early postmenopausal women between 45 and 55 years of age (mean age 52) with an amenorrhoeic interval >12 months or serum FSH > 30 IU/L. In addition, women > 55 years of age were included if they had a menopausal age < 5 years | |
| Interventions |
Administered for 24 months |
|
| Outcomes | Endometrial hyperplasia, endometrial cancer, breast cancer | |
| Notes | No‐treatment arm with 35 women not considered (as stated in our protocol; moreover they were not randomised) Timing: unclear Location: Switzerland Multi‐centre: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial because women in 1 study arm were treated with an oestrogen patch |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 55/105 (after 12 months) and 46/105 (after 24 months) participants were evaluated through endometrial biopsy. Reasons why remaining women were not assessed were not specified |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Sponsored by the drug manufacturer. Study authors' conflicts of interest not reported |
Jacobsen 2012.
| Methods | Randomised double‐blind double‐dummy placebo‐controlled trial | |
| Participants | 318 community‐living women > 70 years of age | |
| Interventions | Tibolone 1.25 mg/d, placebo, raloxifene 60 mg/d (not considered in the meta‐analysis) for 24 months | |
| Outcomes | Cardiovascular events (TIA; cerebrovascular events; myocardial infarction) | |
| Notes | Trial location: Netherlands (single centre) Timing: July 2003 to Jan 2008 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation with computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial; study authors declared that use of double dummy blinded participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial losses to follow‐up (already > 20% at 3 months) |
| Selective reporting (reporting bias) | Low risk | Study reported data on outcomes as indicated in the protocol. Additional data on cardiovascular and cerebrovascular events available in the study publication and included in this review |
| Conflict of interest | Low risk | Sponsored by the Dutch Organization for Health Research and Development. Study authors declare that they have no conflicts of interest |
Kenemans 2009.
| Methods | Randomised placebo‐controlled non‐inferiority trial | |
| Participants | 3148 postmenopausal women with vasomotor symptoms, in menopause for ≥ 12 months, who were surgically treated for breast cancer (T1‐3, N0‐2, M0) within the previous 5 years; excluded women with endometrial abnormalities at transvaginal ultrasonography. Mean time since menopause 6.2 years. Mean age 52.7 years. At study entry, 67% of participants were using tamoxifen | |
| Interventions |
Administered for 2.75 years |
|
| Outcomes | Unscheduled bleeding, vulvovaginal dryness, vaginal infection, urinary tract infection, insomnia, recurrence of breast cancer, endometrial cancer, venous thromboembolic events, cardiovascular and cerebrovascular events, mortality | |
| Notes | Women who did not have adequate relief of their vasomotor symptoms were allowed to use concomitant non‐hormonal medication, such as soy products, clonidine and antidepressants Timing: from June 2002 to July 2007 (study prematurely interrupted for safety reasons) Location: USA, Europe, Asia, Australia Multi‐centre: 245 centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by use of a centralised interactive voice response system, stratified by centre, with a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Centralised interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98% of randomised participants were analysed; reasons given for withdrawals/dropouts |
| Selective reporting (reporting bias) | Low risk | Data on all outcomes indicated in the protocol were eventually available in the study publication |
| Conflict of interest | High risk | Financed by the drug producer. Some study authors with conflicts of interest |
Kroiss 2005.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 70 postmenopausal women (hospital outpatients; < 75 years old; body mass index 18 to 30 kg/m2) with newly diagnosed and histologically confirmed invasive or non‐invasive early‐stage breast cancer (< stage IIb), for which they were to receive surgical treatment (conservation therapy or modified radical mastectomy) followed by tamoxifen (20 mg/d). The women were required to have had their last natural menstrual period > 1 year before diagnosis of breast cancer (mean time since menopause 107 months) and to have a serum oestradiol concentration < 30 pg/mL. Mean age 58 years | |
| Interventions |
Administered for 12 months |
|
| Outcomes | Vaginal bleeding/spotting, endometrial hyperplasia, endometrial cancer, recurrence of breast cancer, hot flushes, sweating, vaginal dryness | |
| Notes | Menopausal symptoms were evaluated as frequency reduction from baseline (for participants who could be evaluated) and as mean change in number and severity from baseline. No data available on vaginal dryness Timing: July 1996 to July 2000 Location: unclear Multi‐centre: described as multi‐centre trial but unclear number and locations of sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated random assignment using ADLS system |
| Allocation concealment (selection bias) | Low risk | Automated random assignment using ADLS system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, double‐blind (identical medication) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/35 participants in the placebo group did not receive study treatment |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Two study authors were employees of the drug manufacturer |
Kubista 2007.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 102 postmenopausal women with initially stage I or II, oestrogen receptor–positive (ER+), previously untreated, core‐biopsy proven, invasive breast cancer without evidence of metastatic spread; any endocrine or enzyme modulator therapy was stopped ≥ 3 months before randomisation. Mean age 65 years. Mean time since menopause 17 years | |
| Interventions |
Administered for 14 days |
|
| Outcomes | Ischaemic stroke, breast tumoural markers | |
| Notes | Tumoural markers (surrogate outcome) measured as median/mean Timing: March 2003 to April 2005 Location: unclear Multi‐centre: 14 sites in 5 countries (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled and defined as "double‐blind" (1 pill administered per day) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of the outcome assessed (stroke), its evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stroke evaluated referring to the "all subject treated group" |
| Selective reporting (reporting bias) | Low risk | Some of the outcomes indicated in the protocol were assessed and reported in the study publication. Those not reported were of no interest for the review. Additional data on ischaemic stroke were available in the study publication and were included in this review |
| Conflict of interest | High risk | Financed by the drug manufacturer. Two study authors were employees of the drug manufacturer |
Kökçü 2000.
| Methods | Randomised controlled trial | |
| Participants | 50 women in spontaneous menopause ≥ 1 year (mean 25 months), still sexually active with a partner with no sexual problems, did not have any gynaecological surgery and had no absolute contraindication for HRT. Mean age 52 years | |
| Interventions |
Administered for 1 year |
|
| Outcomes | Vaginal dryness/dyspareunia, vasomotor symptoms, irregular spotting/bleeding | |
| Notes | Timing: unclear Location: Turkey Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study authors did not specify whether study drugs were identical looking. They stated that (1) the trial was single‐blind; and (2) the women did not have any previous knowledge and did not receive any information on the possible effects on sexual function of the study drugs. It is then unclear whether they were intended as "blind" just because they were not provided any information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified (and not clear whether the women were blind) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/50 women were not evaluated for not attending visits |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No information about funding or study authors' conflicts of interest |
Landgren 2002.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 775 women with a uterus between 40 and 60 years (mean 52 years), with absence of spontaneous vaginal bleeding for ≥ 10 months and presence of menopausal symptoms (≥ 1 moderate to severe hot flush per day). Body weight had to be between 80% and 130% of ideal body weight. Mean time since menopause 35 months | |
| Interventions |
Administered for 12 weeks |
|
| Outcomes | Hot flashes, sweats, vaginal bleeding, thromboembolic events | |
| Notes | Menopausal symptoms were evaluated as intensity and as frequency for participants with a decrease from baseline of 3 or more hot flushes and sweats per day; vaginal bleeding reported only on a graph Timing: March 1994 to July 1995 Location: Sweden, Netherlands, Finland, Norway Multi‐centre: 28 sites (9 in Sweden; 8 in Netherlands; 7 in Finland; 4 in Norway) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not explained how the randomisation list was generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified whether assignment of the corresponding number on the randomisation list was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled, double‐blind (use of identical tablets) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of the outcome assessed (thromboembolism), its evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 30 of 770 participants who started treatment were not evaluable (reasons not specified) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | 3 out of 4 study authors were employees of the drug producer |
Langer 2006.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 866 healthy postmenopausal women (45 to 79 years of age with a body mass index > 19 and < 32 kg/m2) who had been amenorrhoeic for ≥ 1 year (mean time since menopause 11 years), with or without intact uterus. If the date of final menstruation was unclear, the woman was to have used hormone therapy (HT) for > 2 years and had to be > 53 years old or fulfil the US Food and Drug Administration (FDA) criteria for menopause (serum oestradiol ≤ 20pg/mL [or 73 pmol/L] and follicle‐stimulating hormone ≥ 40 mIU/mL). Mean age 59 years | |
| Interventions |
Administered for 3 years (39 cycles of 28 days) CF336 study numbers |
|
| Outcomes | Vaginal bleeding (requiring more than 1 sanitary napkin or tampon per day), vaginal spotting (requiring just 1 sanitary napkin or tampon per day), breast cancer, cardiovascular events, mortality from any cause, endometrial cancer
|
|
| Notes | Data on endometrial cancer considered in separate publication Timing: unclear Location: United States and Europe Multi‐centre: 11 sites (6 in the United States, 5 in Europe) All participants also received oral calcium (500 mg/d) 707/857 women taking ≥ 1 dose of study medication had intact uterus |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | No information provided in the published article. In a private communication, the main study author assured that study treatments were allocated through random codes generated by a central co‐ordinating group |
| Allocation concealment (selection bias) | Unclear risk | In another published article describing the study methods (Bots ML; Cont Clin Trials 2003;24:752‐75), it is stated: "code numbers were assigned to subjects in the order of their randomisation in the trial, that is, the first subject received the first number (the lowest), the second subject received the next number in sequence, and so on". This specification made the allocation concealment issue unclear, but in a private communication, the main study author assured that such process was concealed to investigators but provided no further details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled with double‐dummy technique |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of women not completing the trial and with no assessment of outcomes of interest is unclear |
| Selective reporting (reporting bias) | Low risk | Study reported data on outcomes as indicated in the protocol. Additional data on breast and endometrial cancer, cardiovascular events and mortality from any cause available in the study publication and included in this review |
| Conflict of interest | High risk | Financed by the drug manufacturer. One study author was an employee of the drug manufacturer |
Meeuwsen 2002.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 85 healthy postmenopausal women, who were ≥ 1 year and at maximum 15 years after natural menopause. Mean age 54.2 years | |
| Interventions |
Administered for 1 year |
|
| Outcomes | Vasomotor symptoms, unscheduled bleeding and sleep | |
| Notes | Timing: not reported Location: Netherlands Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not explained how the randomisation list was generated |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tablets of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of the outcome assessed, its evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for withdrawals (4 women) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Drug manufacturer was involved in the trial (random sequence generation was performed by the drug manufacturer) |
Mendoza 2000.
| Methods | Parallel‐group RCT | |
| Participants | 76 hysterectomised women < 50 years old. Excluded if had had any previous malignant gynaecological process, oestrogen‐producing tumour, endocrinological or metabolic problems, cardiovascular disease, uncontrolled hypertension, active hepatic disease, serious skin illness, intestinal sickness or chronic obstructive respiratory disease. Patients with psychiatric problems or receiving anxiolytic or antidepressive drugs were also excluded Unclear whether all women were symptomatic |
|
| Interventions |
Administered for 1 year |
|
| Outcomes | Climacteric symptoms through a modified version of the Kupperman Index Vasomotor symptoms measured as frequently (2), occasionally (1) or never (0) Reports binary measure of "reduction in vasomotor symptoms" Dyspareunia reported as part of a composite outcome of sexual symptoms (“behavioural changes”), which included libido |
|
| Notes | Timing: Feb 1, 1995, to January 31, 1996 Trial location: Nicaragua Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers with simple blind randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of participants and personnel not stated and therefore unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14/76 participants interrupted or changed therapy, or were lost to follow‐up; 6/76 did not start therapy |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No information provided |
Mendoza 2002.
| Methods | Randomised controlled trial | |
| Participants | 165 women with intact uterus younger than 60 years (mean 50 years), who had been amenorrhoeic for 1 to 5 years (mean 22.3 months). Women who had had a hysterectomy or had received hormone treatment in the 3 months before the trial were excluded, as were those with a history of a malignant gynaecological process, oestrogen‐producing tumour or obesity (body mass index > 32) | |
| Interventions |
For 1 year |
|
| Outcomes | Irregular bleeding, vasomotor symptoms frequency 0 = never, 1 = occasionally, 2 = frequently | |
| Notes | Data on vasomotor symptoms expressed as number of women with reduced symptoms Timing: September 1996 to April 1998 Location: Spain Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done following a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Defined as "simple‐blind", but no details given |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32/165 women did not start HRT, no reasons given |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Not reported |
Morais‐Socorro 2012.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | 65 women between 40 and 55 years of age, with menstrual irregularity during the previous 6 months but fewer than 12 months of amenorrhoea, presence of a uterus without anomalies in an initial vaginal ultrasonography evaluation and an endometrial thickness measurement ≤ 10 mm; Kupperman Menopausal Index (KMI) score ≥ 14 points. Mean age 48.5 years | |
| Interventions | Tibolone 2.5 mg/d, placebo for 12 weeks | |
| Outcomes | Greene scale (vasomotor symptoms), Kupperman Index, vaginal bleeding‐spotting (based on number of days of uninterrupted bleeding and number of pads or tampons/d required) | |
| Notes | Trial location: Brazil (unclear if multi‐centre) Timing: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study authors declare that this is a double‐blind trial but do not provide information on blinding methods |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10% and 14% dropout in tibolone and placebo arms, respectively |
| Selective reporting (reporting bias) | Low risk | Some of the outcomes indicated in the protocol (Kupperman Index, Greene scale) were assessed and reported in the study publication. Those not reported were of no interest for this review. Additional information on vaginal bleeding‐spotting was available in the study publication and was considered for this review |
| Conflict of interest | Low risk | Supported by grant from the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico ‐ "National Counsel of Technological and Scientific Development") |
Nappi 2006a.
| Methods | Randomised controlled trial | |
| Participants | 40 women with menopausal symptoms and primary headache (migraine without aura [MwA] and ETTH) of premenopausal onset (history ≥ 10 years), spontaneous menopausal status ≥ 12 months (mean 18 months) with follicle‐stimulating hormone levels > 30 IU/L, age between 51 and 55 years (mean age 53 years), body mass index > 19 and < 30 kg/m2 | |
| Interventions |
Administered for 6 months |
|
| Outcomes | Vaginal bleeding/spotting, vasomotor symptoms, vaginal dryness | |
| Notes | Women had been using symptomatic medications and headache drug prophylaxis ≥ 3 months before entering the study Results on vasomotor symptoms and vaginal dryness (evaluated using Greene scale) available only as a graph Timing: unclear Location: Italy Multi‐centre: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is stated that outcome measures were evaluated by a blind study author, although it is not clear whether this referred to the database level or to the clinical assessment of outcomes, which was not likely to be conducted in a blind fashion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study authors state that all women completed the study following appropriate evaluation |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Low risk | Supported by a grant from the Italian Ministry of Health. No conflicts of interest reported |
Nathorst‐Böös 1997.
| Methods | Randomised controlled trial | |
| Participants | 437 healthy women, ≥ 1 year postmenopausal or had been using hormone replacement therapy (HRT) > 2 years. Women were older than 53 years at entry and had been without HRT for longer than 1 month. All had had hot flushes and sweating, had a body mass index < 30 and had an intact uterus | |
| Interventions |
Administered for 12 months |
|
| Outcomes | Vaginal dryness and pain during sexual intercourse as score at baseline and as differences between pretreatment and post‐treatment | |
| Notes | Timing: unclear Location : Denmark, Norway, Sweden Unclear number of sites, but locations in 3 Scandinavian countries |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list and codes |
| Allocation concealment (selection bias) | Low risk | Sealed envelope containing the code |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind, double‐dummy not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective outcomes (McCoy’s Sex Scale Questionnaire) that may be subject to bias in the absence of double‐blind (double‐dummy not specified). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 264/437 (60.4%) completed all assessments (baseline, at 24 and 48 weeks) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Unclear risk: not specified |
Nijland 2009.
| Methods | Randomised controlled trial | |
| Participants | 403 healthy women who had undergone natural menopause, with an intact uterus and with female sexual dysfunction associated with sexuality‐related personal distress. Mean age 55.8 years | |
| Interventions |
Administered for 24 weeks |
|
| Outcomes | Unscheduled bleeding, cerebrovascular events, mortality from any cause | |
| Notes | Timing: June 2004 to November 2005 Location: Europa, USA, Australia Multi‐centre: 29 centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised automatic interactive voice response system was used |
| Allocation concealment (selection bias) | Low risk | Computerised automatic interactive voice response system was used, and treatment assignment was stored electronically |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6% to 10% were not analysed for unspecified protocol violations |
| Selective reporting (reporting bias) | Low risk | Some outcomes indicated in the protocol (vaginal bleeding and spotting rate) were assessed and reported in the study publication. Those not reported were of no interest for this review. Additional information on cerebrovascular events and mortality from any cause was available in the study publication and was considered for this review |
| Conflict of interest | High risk | Study sponsored by the drug manufacturer, and some study authors were employees of the drug firm |
Okon 2005.
| Methods | Parallel RCT | |
| Participants | 30 postmenopausal women with an intact uterus, requesting HT, who had had ≥ 12 months of amenorrhoea with plasma follicle‐stimulating hormone (FSH) > 20 IU/L; < 65 years old | |
| Interventions | Tibolone 2.5 mg daily 2 mg micronised oestradiol valerate and norethisterone acetate 0.7 mg daily for 12 months |
|
| Outcomes | Irregular bleeding ‐ reported as days of bleeding over 1 year | |
| Notes | Timing: unclear Single centre UK |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of sequence allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 19/30 women included in analysis (5 in tibolone group and 6 in HT group withdrew; 1 was excluded from analysis) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Funded by pharmaceutical company |
Osmanağaoğlu 2006.
| Methods | Randomised controlled trial | |
| Participants | 165 naturally postmenopausal women; absence of menstruation > 1 year; FSH ≥ 30 IU/L; not undergone any gynaecological operation; no absolute contraindication for HT. Mean age 50 years | |
| Interventions |
Administered for 6 months |
|
| Outcomes | Lubrication and pain during sexual intercourse as score at baseline and at post treatment | |
| Notes | Only 107 women were considered in the analyses (excluding women assigned to "no treatment") Even if not specified in the protocol, lubrication has been evaluated as a measure of vaginal dryness Timing: unclear Location: Turkey? Multi‐centre: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors declare that the study is single‐blind (participant), but in some cases, women were given doctor samples from drug companies |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were evaluated through a self‐administered questionnaire, but it is unclear whether participating women were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/165 participants without follow‐up data |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Low risk | Study authors declare that they did not receive external funding and that they do not have conflicts of interest |
Polisseni 2013.
| Methods | Randomised double‐blind controlled trial | |
| Participants | 174 postmenopausal women between 45 and 60 years of age with moderate or pronounced vasomotor symptoms and a Blatt–Kupperman menopausal index (BKMI) ≥ 20 points, with no treatment for menopausal symptoms in the past 6 months | |
| Interventions | Tibolone 2.5 mg/d; 1 mg oestradiol + 0.5 mg norethindrone acetate; 50 mg calcium carbonate and 200 UI vitamin D3 (not considered in the meta‐analysis) | |
| Outcomes | Vasomotor symptoms, insomnia (measured through the Women’s Health Questionnaire) | |
| Notes | Trial location: Brazil (single centre) Follow‐up: 12 weeks Timing: June 2009 to June 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial; study authors declared that all capsules appeared identical |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 47 participants lost to follow‐up (with differential attrition among groups); only treated women appear to have been assessed |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Low risk | Study authors declare that they have no conflicts of interest |
Ross 1999.
| Methods | Parallel‐group RCT | |
| Participants | 36 perimenopausal women (amenorrhoea ≥ 3 months), > 45 years old, with no past psychotic history nor current use of antidepressants or psychotherapeutic agents. All participants "suffering from menopausal symptoms and requesting HRT" | |
| Interventions | • Tibolone 2.5 mg/d • 0.625 mg conjugated oestrogens daily for 28 days, plus 150 μg norgestrel daily on days 17 to 28 Administered for 12 weeks |
|
| Outcomes | Women’s Health Questionnaire (subscales on vasomotor symptoms, sleep ) Greene’s Climacteric Scale (subscale on vasomotor symptoms) |
|
| Notes | Timing: unclear Trial location: Scotland Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by pre‐generated, sequential randomisation lists |
| Allocation concealment (selection bias) | Low risk | Used a block size of 10, and each packet was given a code number. Copies of the code were kept in opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study authors state that some of the women knew which drug they were on. Therefore, it is likely that clinicians/researchers had been unblinded too |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Incomplete blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22% of participants withdrew (2 in tibolone group and 6 in HT group) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Study funded by Organon |
Roux 2002.
| Methods | Randomised controlled trial | |
| Participants | 225 healthy women with physiological menopause (time since menopause 3.9 years, mean age 53.3 years) | |
| Interventions |
Administered for 24 months |
|
| Outcomes | Menopausal vaginal bleeding | |
| Notes | Each participant also received 1 tablet of 500 mg calcium supplement daily Timing: not specified Trial location: France Multi‐centre: 66 participating centres |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size of 6) |
| Allocation concealment (selection bias) | Unclear risk | Not clear if centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Bleeding was evaluated for all randomised women |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Study sponsored by drug manufacturer |
Siseles 1995.
| Methods | Randomised open‐label controlled trial | |
| Participants | 30 postmenopausal women ≥ 1 year postmenopausal and reporting hot flushes and other menopausal symptoms (but otherwise healthy). Age range 48 to 62 years | |
| Interventions |
Administered for six 28‐day cycles |
|
| Outcomes | Hot flushes, sweating, sleeplessness, irregular bleeding, endometrial hyperplasia | |
| Notes | Menopausal symptoms measured through Kupperman Index but results available only as a graph Bleeding not evaluable because insufficient information provided Timing: June to Dec 1990 Trial location: Argentina Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of the outcome of interest (endometrial hyperplasia), its evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/30 patients excluded from final analyses |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by drug manufacturer. Study authors' conflicts of interest not stated |
Swanson 2006.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 396 healthy postmenopausal women (≥ 40 years of age; mean age 52 years) who had been amenorrhoeic ≥ 6 months (women with a uterus only) and who were experiencing a minimum of 7 moderate to severe hot flashes per day (or 60 per week). In addition, women had to be within 70% to 140% of their ideal body weight, smoke fewer than 15 cigarettes daily and have tested negative for pregnancy. Mean time since menopause 84 months | |
| Interventions |
Administered for 12 weeks |
|
| Outcomes | Hot flashes, vaginal dryness, dyspareunia, endometrial hyperplasia, endometrial cancer, breast cancer | |
| Notes | Menopausal symptoms evaluated as mean change from baseline using a non‐validated scale: 1 = mild sensation of heat without perspiration; 2 = moderate sensation of heat with perspiration, able to continue activity; 3 = severe sensation of heat with sweating, causing the woman to stop activity Timing: unclear Location: United States Multi‐centre: 31 sites |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled. Defined as "double‐blind"; 3 daily interventions were compared |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Evaluation of endometrial hyperplasia and cancers should not suffer from detection bias. Methods for (and blinding when) diagnosing heart failure not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/396 excluded for not receiving any study treatment |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by the drug manufacturer. Two study authors were employees of drug manufacturer |
Uygur 2005.
| Methods | Parallel‐group RCT | |
| Participants | 80 postmenopausal women (56 years old), married, with spontaneous menopausal status ≥ 1 year with follicle‐stimulating hormone level > 30 mIU/L and no contraindication to use of HRT, without chronic disease. Participants were not selected on the basis of sexual function or dysfunction | |
| Interventions |
Administered for 6 months (n = 40) |
|
| Outcomes | Vaginal dryness, pain during sexual intercourse as score at baseline and at post treatment | |
| Notes | Sexual function measured on a non‐validated questionnaire Timing: unclear Trial location: Turkey Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and providers. States "not double blind" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, with outcomes evaluated through a questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/80 dropped out (2 from tibolone group because of bleeding, 6 from CEE/MPA group ‐ 1 for mastalgia, 1 for menorrhagia, 2 for weight gain, 2 for loss to follow‐up) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No information provided |
Vieira 2009.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 30 postmenopausal women with systemic lupus erythematosus, between 30 and 65 years of age (mean age 51.7 years), who had not menstruated for over a year (mean 7.1 years); had follicle‐stimulating hormone (FSH) levels > 20 mIU/mL in 2 (chemiluminescence) tests performed 30 days apart; had not used any HRT for ≥ 6 months; and had presented with symptoms of hypoestrogenism (night sweats, hot flashes or symptoms of urogenital atrophy) at inclusion. Other than oral corticosteroids, use of other medications for treatment of SLE was allowed if doses remained stable for ≥ 3 months before study outset | |
| Interventions |
For 1 year |
|
| Outcomes | Menopausal symptoms, breast cancer, endometrial cancer, venous thromboembolic events, mortality from any cause | |
| Notes | Data on menopausal symptoms were assessed through Kupperman Index; it is not possible to derive results on those specific symptoms provided in the protocol Timing: enrolment between March 2002 and December 2004 Location: Brazil Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | GraphPad StatMate® (Graphpad Software, San Diego, CA) software programme was used to randomise participants into 2 groups |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given the nature of outcomes assessed, their evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/30 excluded owing to SLE reactivation |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | Not reported |
Volpe 1986.
| Methods | Parallel‐group RCT | |
| Participants | 113 postmenopausal women with menopausal symptoms: 81 were naturally menopausal (mean age 51 years); 32 were post hysterectomy and oophorectomy (mean age 41 years) Last menstrual period 1 to 5 years previously Excluded women who had received hormone preparations during preceding 8 weeks or in whom oestrogen therapy was contraindicated Dropouts: 11/15 in placebo group dropped out by 6 months |
|
| Interventions | Tibolone 2.5 mg daily (n = 27) vs
All for 6 cycles |
|
| Outcomes | Hot flushes, scored as follows: 0 = absent, 3 = mild, 6 = moderate, 9 = severe No comparative data on AEs were reported. Endometrial hyperplasia was reported, but no histology was done in the placebo group |
|
| Notes | Menopausal symptoms measured on a non‐validated questionnaire Timing: unclear Trial location: Italy Multi‐centre: no; single site |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States “randomly allocated". Baseline characteristics of groups not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information about blinding provided. Blindness unlikely at least for providers/researchers (it is a placebo‐controlled trial) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Histology assessment blinded, but symptoms evaluated through a questionnaire; unlikely that providers/researchers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition in placebo group (11/15), numbers assessed for hot flushes in active groups not reported |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No information provided about conflicts of interest. Non‐validated measure used for VM symptoms |
Wender 2004.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | 40 healthy postmenopausal women, mean age 55 years, mean time since natural menopause 5 to 7.7 years, mean BMI 26 kg/m2 | |
| Interventions |
For 1 year |
|
| Outcomes | Endometrial thickness, endometrial cancer, uterine bleeding | |
| Notes | Timing: not specified Location: Brasil Single centre |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | page 424: “the tibolone and the placebo tablets and bottles looked identical; the bottles were identified with numbers from 1 to 40. The correspondence between the numbers and the group to which the participant belonged was not disclosed until the end of the study” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | page 424: “all ultrasonographic exams were performed at the Hospital’s Gynecology and Obstetrics Service by the same operator, who was blinded to information concerning participant groups. […] “ The material was analysed twice by 2 pathologists who were also blinded to participant information” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal given: 3 participants/group withdrew from the study
|
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Unclear risk | No details given |
Winkler 2000.
| Methods | Randomised controlled trial | |
| Participants | 62 healthy postmenopausal women, between 45 and 70 years of age (mean age 54 years), spontaneous menopause with last menstrual period ≥ 36 months before enrolment or artificial menopause (hysterectomy and/or oophorectomy) with FSH level > 30 IU/L (mean time since menopause 8.5 years) | |
| Interventions |
Administered for 24 weeks |
|
| Outcomes | Vaginal bleeding/spotting (defined as requiring > 1/just 1 tampon/d), hot flushes, sweating | |
| Notes | Menopausal symptoms measured as frequency but number of participants evaluated is unclear Timing: Feb 1995 to 1996 Location: Germany Multi‐centre: no; participants were selected from private practices of 2 specialists in Germany |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. Concern because participants were selected from private practices of 2 specialists |
| Allocation concealment (selection bias) | Unclear risk | No information provided. Concern because participants were selected from private practices of 2 specialists |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not specified, but given self‐assessment of the outcome of interest (vaginal bleeding/spotting), its evaluation is likely to be "objective" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women with a uterus were evaluated for vaginal bleeding/spotting |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Financed by the drug producer. One study author was an employee of the drug producer |
Wu 2001.
| Methods | Randomised controlled trial | |
| Participants | 48 healthy postmenopausal women (52 years old), postmenopausal for 12 to 36 months (confirmation by FSH > 40 mIU/mL and oestradiol < 20 pg/mL), with ≥ 1 climacteric symptom according to the Greene Climateric Scale | |
| Interventions |
Administered for 3 months |
|
| Outcomes | Menopausal symptoms (assessed using Greene's Climateric Scale), attitudes of sexuality (assessed using McCoy Sex Scale), unscheduled bleeding | |
| Notes | Timing: not clear Not clear if multi‐centre or not |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomly selected pairs of 2 women were allocated to treatment groups |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/48 dropped out, but reasons given |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | High risk | Study sponsored by the manufacturer. Study authors declare that they have no conflicts of interest |
Ziaei 2010.
| Methods | Randomised controlled trial | |
| Participants | 150 healthy postmenopausal women (mean age at menopause: 49 years), 45 to 60 years of age (mean age 52 years), whose last menstrual period was more than a year ago with plasma 17β‐oestradiol < 35 pg/mL | |
| Interventions |
Administered for 6 months |
|
| Outcomes | Vaginal bleeding (requiring > 1 sanitary napkin per day), vaginal spotting (requiring just 1 sanitary napkin per day), vaginal dryness, vasomotor symptoms, lubrication and pain during sexual intercourse, as scored at baseline and at post treatment | |
| Notes | An arm with 50 women who received only 1 Cal + D tablet (500 mg + 200 IU) was not considered Timing: unclear Location: Iran Multi‐centre: only 2 sites (in Tehran) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not specified whether blind/double‐blind trial. All women received Ca + vit D but 1 control group did not receive active treatments; no dummy placebo mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Only blood samples stated to have been assessed in blinded fashion (corresponding outcome is not of interest for this review) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/150 lost to follow‐up for bleeding outcomes; 20/150 (13%) for vasomotor symptoms |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Conflict of interest | Low risk | Publicly financed; study authors state no competing interests |
ADLS: Almedica Drug Labeling System
AE: adverse event.
BKMI: Blatt‐Kupperman menopausal index.
BMD: bone mineral density.
BMI: body mass index.
CE: conjugated oestrogen.
CEE: conjugated equine oestrogen.
ETTH: Episodic tension‐type headache
EV: oestradiol valerate.
FDA: Food and Drug Administration.
FSH: follicle‐stimulating hormone.
HRT: hormone replacement therapy.
HT: hormone therapy.
MPA: medroxyprogesterone acetate.
RCT: randomized controlled trial.
SLE: systemic lupus erythematosus.
TIA: transient ischaemic attack.
TVUS: transvaginal ultrasonography.
VM: vasomotor symptoms.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Argyroudis 1997 | Not clear whether randomised or not; impossible to contact study author to ask for details on methods |
| Baksu 2005 | Inclusion not apparently limited to women who were experiencing vasomotor symptoms at baseline. No other outcomes of interest measured |
| Beardsworth 1999 | Study vs no treatment |
| Berlanga 2003 | Inclusion not apparently limited to women who were experiencing vasomotor symptoms at baseline. No other outcomes of interest measured |
| Bhattacharya 2008 | Results on somatovegetative and urogenital symptoms assessed through score but specific outcomes of interest to this review not measured |
| Bhattacharya 2010 | Results on somatovegetative and urogenital symptoms assessed through score but specific outcomes of interest to this review not measured |
| Bukulmez 2001 | Measured no outcomes of interest |
| Cagnacci 2004 | Measured no outcomes of interest |
| Cayan 2008 | No available data explicitly comparing tibolone vs combined hormone therapy |
| De Censi 2013 | Participants not randomised to tibolone |
| Fedele 2000 | No outcomes of interest measured |
| Gambacciani 2004 | Study vs no treatment |
| Genazzani 2011 | Wrote to study authors to ask for data but received no response |
| Inan 2005 | No outcomes of interest measured |
| Lundstrom 2011 | Ineligible outcomes (breast density) |
| Nappi 2006b | Sexual dysfunction as vaginal health index (not provided for in the protocol) |
| Onalan 2005 | No outcomes of interest measured |
| Palacios 1995 | Compared tibolone vs calcium tablets |
| Silva 2015 | Conference proceeding with no data on outcomes of interest |
| Simsek 2002 | Measured no outcomes of interest |
| Stefanos 2010 | Included participants with regular menstruation |
| Stevenson 2011 | Not an RCT; review with unretrievable full text |
| Tasic 2011 | Measured no outcomes of interest |
| Yuk 2012 | Ineligible outcomes (changes in body composition and body size), unretrievable full text |
Differences between protocol and review
Title: We changed the protocol title ("Tibolone for menopausal symptoms") to "Short‐term and long‐term effects of tibolone in postmenopausal women", because the review is focused mostly on the long‐term safety of tibolone (in particular for the incidence of breast and endometrial cancer and of cardiovascular events, which were included among the primary outcomes ‐ see next paragraph), in addition to its efficacy for symptoms. Protocol criteria allowed the inclusion of RCTs testing tibolone also in women without menopausal symptoms, as far as safety data were reported; in fact, the largest trial in the review tested the effects of tibolone in osteoporotic women. Therefore, the title "Tibolone for menopausal symptoms" would have been misleading. The new title is consistent with Cochrane editorial policies, using the [Intervention] in OR for [participant group/location] structure, as proposed in the Cochrane Handbook for Systematic Reviews of Interventions (Table 4.2.a: Structure for Cochrane review titles). Moreover, "short‐term and long‐term effects" helps to suggest that the goal is to review short‐term and especially long‐term safety, in addition to symptom improvement in the short term. Outcomes: Given the importance of safety in the objectives of the review, we followed reviewers' suggestions to include major adverse events (breast cancer, endometrial cancer, venous thromboembolic events, cardiovascular events, cerebrovascular events and mortality from any cause) as primary outcomes, along with reduction in symptoms and shifting genital symptoms (excluding vaginal bleeding because it may also be a drug‐related adverse event) as secondary outcomes. We evaluated cardiovascular and cerebrovascular events separately and added endometrial hyperplasia as a secondary outcome. We no longer considered irregular menstrual periods.
In previous versions of the review, we applied the criterion that To be eligible for inclusion in the review, studies had to report useable data on one of more of the outcomes listed below (in our list of included outcomes), although we did not explicitly state this. In line with current Cochrane methods, we now include all studies that measured our outcomes of interest, even if they were not reported in a useable format.
Statistical methods: We did not fully anticipate at the protocol stage the variation in reporting of the primary outcome, vasomotor symptoms, and so, some of the methods for combining these data in meta‐analysis (explained in the Methods section) are necessarily post hoc and data driven in nature. Although we believe we have reached the most appropriate conclusion given the available information, another review team may have made different decisions in relation to the analysis and could plausibly have arrived at a different conclusion. We have attempted to make our methods transparent, so that the competent reader may determine their suitability for herself.
Aside from data on vasomotor symptoms, vaginal dryness and sleep (as explained in the Methods section), we did not combine outcomes by using the fixed‐effect model (as stated in the protocol); we used the random‐effects model instead, because it takes population heterogeneity into better account. We considered that two of the major RCTs (Cummings 2008; Kenemans 2009) studied very heterogeneous populations (women who had had breast cancer and osteoporotic women, respectively), whose characteristics differ widely from women taking hormonal therapies for postmenopausal symptoms. A recent textbook (Borenstein 2009) highlights (page 86): “The selection of a model must be based solely on the question of which model fits the distribution of effect sizes, and takes account of the relevant source(s) of error. When studies are gathered from the published literature, the random‐effects model is generally a more plausible match”.
Subgroup analyses: As two of the largest RCTs selected very specific populations, it was considered informative to present, together with a full analysis set, results on breast cancer distinguishing patients who had already had breast cancer from those who had not, and distinguishing results on cardiovascular and cerebrovascular events for patients under and over 60 years of age. As stated in the protocol, we also considered subgroup analyses based on methodological risks of bias components and duration of treatment. We eventually did not perform these, given the lack of studies in most of the strata.
We took the "multi‐centre" item out of the risk of bias tables because participation of more centres in an RCT should mainly increase its external rather than internal validity. However, we kept this information in the "Notes" items under Characteristics of included studies.
Contributions of authors
Giulio Formoso: co‐ordinated the review; contributed to study screening, quality appraisal, data extraction and interpretation; wrote the final texts.
Enrica Perrone: contributed to study screening, quality appraisal, data extraction and interpretation; provided relevant feedback on the final texts.
Susanna Maltoni: performed previous work that was the foundation of the current study; participated in conceiving the review and co‐ordinated protocol development; contributed to study screening, quality appraisal, data extraction and interpretation.
Sara Balduzzi: contributed to study screening, quality appraisal, data extraction and interpretation; provided statistical support.
Jack Wilkinson: extracted, analysed and contributed to interpretation of data from RCTs assessing vasomotor symptoms and vaginal dryness; contributed to related portions of the text.
Vittorio Basevi: performed previous work that was the foundation of the current study; participated in conceiving the review and in protocol development; provided constant support in interpreting the clinical relevance of data; provided relevant feedback on the final texts.
Anna Maria Marata: performed previous work that was the foundation of the current study; participated in conceiving the review and in protocol development; provided support in interpreting the clinical relevance of data.
Nicola Magrini: provided relevant feedback in interpreting the clinical relevance of data and in reviewing the final texts.
Roberto D’Amico: participated in conceiving the statistical methods of the review and in protocol development; participated in study screening; provided statistical support.
Chiara Bassi: designed search strategies at the protocol stage.
Emilio Maestri: performed previous work that was the foundation of the current study; participated extensively in conceiving the review and in protocol development; provided support in interpreting the clinical relevance of data; provided relevant feedback on the final texts.
Sources of support
Internal sources
-
Emilia‐Romagna Health and Social Policies Directorate, Italy.
Emilia‐Romagna Health and Social Policies Directorate, Italy, provided the salary for reviewers
External sources
-
Cochrane Gynaecology and Fertility Group, New Zealand.
Provided feedback and support during the whole review process; provided bibliographic support
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Al‐Azzawi 1999 {published data only}
- Al‐Azzawi F, Wahab M, Habiba M, Akkad A, Mason T. Continuous combined hormone replacement therapy compared with tibolone. Obstetrics and Gynecology 1999;93(2):258‐64. [DOI] [PubMed] [Google Scholar]
Archer 2007 {published data only}
- Archer DF, Hendrix S, Gallagher JC, Rymer J, Skouby S, Ferenczy A, et al. Endometrial effects of tibolone. Journal of Clinical Endocrinology and Metabolism 2007;99(3):911‐8. [DOI] [PubMed] [Google Scholar]
Baracat 2002 {published data only}
- Baracat EC, Barbosa IC, Giordano MG, Haidar MA, Marinho RM, Menegocci JC, et al. A randomized, open‐label study of conjugated equine estrogens plus medroxyprogesterone acetate versus tibolone: effects on symptom control, bleeding pattern, lipid profile and tolerability. Climacteric 2002;5:60‐9. [PubMed] [Google Scholar]
Benedek‐Jaszmann 1987 {published data only}
- Benedek‐Jaszmann LJ. Long‐term placebo‐controlled efficacy and safety study of ORG OD 14 in climacteric women. Maturitas 1987;Suppl 1:25‐33. [DOI] [PubMed] [Google Scholar]
Berning 2000 {published data only}
- Berning B, Kuijk C, Coelingh Bennink HJT, Fauser BCJ. Absent correlation between vaginal bleeding and oestradiol levels or endometrial morphology during tibolone use in early postmenopausal women. Maturitas 2000;35:81‐8. [DOI] [PubMed] [Google Scholar]
Bouchard 2012 {published data only}
- Bouchard P, Panay N, Villiers TJ, Vincendon P, Bao W, Cheng RJ, et al. Randomized placebo‐ and active‐controlled study of desvenlafaxine for menopausal vasomotor symptoms. Climacteric 2012;15:12‐20. [DOI] [PubMed] [Google Scholar]
Cummings 2008 {published data only}
- Cummings SR, Ettinger B, Delmas PD, Kenemans P, Stathopoulos V, Verweij P, et al. LIFT Trial Investigators. The effects of tibolone in older postmenopausal women. New England Journal of Medicine 2008;359(7):697‐708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger B, Kenemans P, Johnson SR, Mol‐Arts M, Os S, Seifert W, et al. Endometrial effects of tibolone in elderly, osteoporotic women. Obstetrics and Gynaecology 2008;112:653‐9. [DOI] [PubMed] [Google Scholar]
de Aloysio 1998 {published data only}
- Aloysio D, Altieri P, Penacchioni P, Salgarello M, Ventura V. Bleeding patterns in recent postmenopausal outpatients with uterine myomas: comparison between two regimens of HRT. Maturitas 1998;29:261‐4. [DOI] [PubMed] [Google Scholar]
Doren 1999 {published data only}
- Dören M, Rübig A, Coelingh Bennink HJ, Holzgreve W. Impact on uterine bleeding and endometrial thickness: tibolone compared with continuous combined estradiol and norethisterone acetate replacement therapy. Menopause 1999;6:299‐306. [DOI] [PubMed] [Google Scholar]
Egarter 1996 {published data only}
- Egarter C, Huber J, Leikermoser R, Haidbauer R, Pusch H, Fischl F, et al. Tibolone versus conjugated estrogens and sequential progestogen in the treatment of climacteric complaints. Maturitas 1996;23(1):55‐62. [DOI] [PubMed] [Google Scholar]
Elfituri 2005 {published data only}
- Elfituri A, Sherif F, Elmahaishi M, Chrystyn H. Two hormone replacement therapy (HRT) regimens for middle‐eastern postmenopausal women. Maturitas 2005;52:52‐9. [DOI] [PubMed] [Google Scholar]
Gallagher 2001 {published data only}
- Gallagher JC, Baylink DJ, Freeman R, McClung M. Prevention of bone loss with tibolone in postmenopausal women: results of two randomized, double‐blind, placebo‐controlled, dose‐finding studies. Journal of Clinical Endocrinology and Metabolism 2001;86(10):4717‐26. [DOI] [PubMed] [Google Scholar]
Gupta 2013 {published data only}
- Gupta B, Mittal P, Khuteta R, Bhargava A. A comparative study of CEE, tibolone, and DHEA as hormone replacement therapy for surgical menopause. Journal of Obstetrics and Gynaecology India 2013;63:194‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammar 1998 {published data only}
- Hammar M, Christau S, Nathorst‐Böös J, Rud T, Garre K. A double‐blind, randomised trial comparing the effects of tibolone and continuous combined hormone replacement therapy in postmenopausal women with menopausal symptoms. British Journal of Obstetrics and Gynaecology 1998;105(8):904‐11. [DOI] [PubMed] [Google Scholar]
Hammar 2007 {published data only}
- Hammar ML, Weijer P, Franke HR, Pornel B, Mauw EM, Nijland EA, TOTAL Study Investigators Group. Tibolone and low‐dose continuous combined hormone treatment: vaginal bleeding pattern, efficacy and tolerability. BJOG 2007;114(12):1522‐9. [DOI] [PubMed] [Google Scholar]
Hänggi 1997 {published data only}
- Hänggi W, Bersinger N, Altermatt HJ, Birkhäuser MH. Comparison of transvaginal ultrasonography and endometrial biopsy in endometrial surveillance in postmenopausal HRTusers. Maturitas 1997;27(2):133‐43. [DOI] [PubMed] [Google Scholar]
Huber 2002 {published data only}
- Huber J, Palacios S, Berglund L, Hänggi W, Sathanandan SM, Christau S, et al. Effects of tibolone and continuous combined hormone replacement therapy on bleeding rates, quality of life and tolerability in postmenopausal women. BJOG 2002;109(8):886‐93. [DOI] [PubMed] [Google Scholar]
Hudita 2003 {published data only}
- Hudita D, Posea C, Ceausu I, Rusu M. Efficacy and safety of oral tibolone 1.25 or 2.5 mg/day vs. placebo in postmenopausal women. European Review for Medical and Pharmacological Sciences 2003;7(5):117‐25. [PubMed] [Google Scholar]
Jacobsen 2012 {published data only}
- Jacobsen DE, Melis RJ, Verhaar HJ, Olde Rikkert MG. Raloxifene and tibolone in elderly women: a randomized, double‐blind, double‐dummy, placebo‐controlled trial. Journal of the American Medical Directors Association 2012;13:189.e1‐7. [DOI] [PubMed] [Google Scholar]
Kenemans 2009 {published data only}
- Bundred NJ, Kenemans P, Yip CH, Beckmann MW, Foidart JM, Sismondi P, et al. Tibolone increases bone mineral density but also relapse in breast cancer survivors: LIBERATE trial bone substudy. Breast Cancer Research 2012;14:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenemans P, Bundred NJ, Foidart JM, Kubista E, Schoultz B, Sismondi P, et al. LIBERATE Study Group. Safety and efficacy of tibolone in breast‐cancer patients with vasomotor symptoms: a double‐blind, randomised, non‐inferiority trial. Lancet Oncology 2009;10(2):135‐46. [DOI] [PubMed] [Google Scholar]
- Sismondi P, Kimmig R, Kubista E, et al. Effects of tibolone on climacteric symptoms and quality of life in breast cancer patients ‐ data from LIBERATE trial. Maturitas 2011;70(4):365‐72. [DOI] [PubMed] [Google Scholar]
Kökçü 2000 {published data only}
- Kökçü A, Çetinkaya MB, Yanik F, Alper T, Malatyalioğlu E. The comparison of effects of tibolone and conjugated estrogen‐medroxyprogesterone acetate therapy on sexual performance in postmenopausal women. Maturitas 2000;36:75‐80. [DOI] [PubMed] [Google Scholar]
Kroiss 2005 {published data only}
- Kroiss R, Fentiman IS, Helmond FA, Rymer J, Foidart JM, Bundred N, et al. The effect of tibolone in postmenopausal women receiving tamoxifen after surgery for breast cancer: a randomised, double‐blind, placebo‐controlled trial. BJOG 2005;112(2):228‐33. [DOI] [PubMed] [Google Scholar]
Kubista 2007 {published data only}
- Kubista E, Planellas Gomez JVM, Dowsett M, Foidart JM, Pohlodek K, Serreyn R, et al. Effect of tibolone on breast cancer cell proliferation in postmenopausal ER+ patients: results from STEM trial. Clinical Cancer Research 2007;13(14):4185‐90. [DOI] [PubMed] [Google Scholar]
Landgren 2002 {published data only}
- Landgren MB, Bennink HJ, Helmond FA, Engelen S. Dose‐response analysis of effects of tibolone on climacteric symptoms. BJOG 2002;109(10):1109‐14. [DOI] [PubMed] [Google Scholar]
- Landgren MB, Helmond FA, Engelen S. Tibolone relieves climacteric symptoms in highly symptomatic women with at least seven hot flushes and sweats per day. Maturitas 2005;14:222‐30. [DOI] [PubMed] [Google Scholar]
Langer 2006 {published data only}
- Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, et al. OPAL Investigators. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima‐media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. European Heart Journal 2006;27:746‐55. [DOI] [PubMed] [Google Scholar]
- Langer RD, Landgren BM, Rymer J, Helmond FA, OPAL Investigators. Effects of tibolone and continuous combined conjugated equine estrogen/medroxyprogesterone acetate on the endometrium and vaginal bleeding: results of the OPAL study. American Journal of Obstetrics and Gynecology 2006;195(5):1320‐7. [DOI] [PubMed] [Google Scholar]
Meeuwsen 2002 {published data only}
- Meeuwsen IB, Samson MM, Duursma SA, Verhaar HJ. The influence of tibolone on quality of life in postmenopausal women. Maturitas 2002;41(1):35‐43. [DOI] [PubMed] [Google Scholar]
Mendoza 2000 {published data only}
- Mendoza N, Suárez AM, Álamo F, Bartual E, Vergara F, Herruzo A. Lipid effects, effectiveness and acceptability of tibolone versus transdermic 17β‐estradiol for hormonal replacement therapy in women with surgical menopause. Maturitas 2000;37(1):37‐43. [DOI] [PubMed] [Google Scholar]
Mendoza 2002 {published data only}
- Mendoza N, Pisón JA, Fernández M, Sánchez MC, Malde J, Miranda JA. Prospective, randomised study with three HRT regimens in postmenopausal women with an intact uterus. Maturitas 2002;41:289‐98. [DOI] [PubMed] [Google Scholar]
Morais‐Socorro 2012 {published data only}
- Morais‐Socorro M, Cavalcanti MA, Martins R, Neto Francisco P, Rezende A, Azevedo G, et al. Safety and efficacy of tibolone and menopausal transition: a randomized, double‐blind placebo‐controlled trial. Gynecologic Endocrinology 2012;28:483‐7. [DOI] [PubMed] [Google Scholar]
Nappi 2006a {published data only}
- Nappi RE, Sances G, Sommacal A, Detaddei S, Facchinetti F, Cristina S, et al. Different effects of tibolone and low‐dose EPT in the management of postmenopausal women with primary headaches. Menopause 2006;13(5):818‐25. [DOI] [PubMed] [Google Scholar]
Nathorst‐Böös 1997 {published data only}
- Nathorst‐Böös J, Hammar M. Effect on sexual life ‐ a comparison between tibolone and a continuous estradiol‐norethisterone acetate regimen. Maturitas 1997;26(1):15‐20. [DOI] [PubMed] [Google Scholar]
Nijland 2009 {published data only}
- Nijland EA, Nathorst‐Boos J, Palacios S, Weijer PW, Davis S, Stathopoulos VM, et al. Improved bleeding profile and tolerability of tibolone versus transdermal E2/NETA treatment in postmenopausal women with female sexual dysfunction. Climateric 2009;12:114‐21. [DOI] [PubMed] [Google Scholar]
Okon 2005 {published data only}
- Okon MA, Lee S, Laird SM, Li TC. A prospective randomized controlled study comparing the morphological and biochemical responses of the endometrium to two different forms of 'period‐free' hormone replacement therapy. Human Reproduction 1998;13(8):2261‐5. [DOI] [PubMed] [Google Scholar]
Osmanağaoğlu 2006 {published data only}
- Osmanağaoğlu MA, Atasaral T, Baltaci D, Bozkaya H. Effect of different preparations of hormone therapy on sexual dysfunction in naturally postmenopausal women. Climacteric 2006;9:464‐72. [DOI] [PubMed] [Google Scholar]
Polisseni 2013 {published data only}
- Polisseni AF, Andrade AT, Ribeiro LC, Castro IQ, Brandão M, Polisseni F, et al. Effects of a continuous‐combined regimen of low‐dose hormone therapy (oestradiol and norethindrone acetate) and tibolone on the quality of life in symptomatic postmenopausal women: a double‐blind, randomised study. Maturitas 2013;74:172‐8. [DOI] [PubMed] [Google Scholar]
Ross 1999 {published data only}
- Ross LA, Alder EM, Cawood EHH, Brown J, Gebbie AE. Psychological effects of hormone replacement therapy: a comparison of tibolone and a sequential estrogen therapy. Journal of Psychosomatic Obstetrics and Gynecology 1999;20:88‐96. [DOI] [PubMed] [Google Scholar]
Roux 2002 {published data only}
- Roux C, Pelissier C, Fechtenbaum J, Loiseau‐Peres S, Benhamou CL. Randomized, double‐masked, 2‐year comparison of tibolone with 17beta‐estradiol and norethindrone acetate in preventing postmenopausal bone loss. Osteoporosis International 2002;13:241‐8. [DOI] [PubMed] [Google Scholar]
Siseles 1995 {published data only}
- Siseles NO, Halperin H, Benencia HJ, Berg G, Pilnik S, Mesch V, et al. A comparative study of two hormone replacement therapy regimens on safety and efficacy variables. Maturitas 1995;21(3):201‐10. [DOI] [PubMed] [Google Scholar]
Swanson 2006 {published data only}
- Swanson SG, Drosman S, Helmond FA, Stathopoulos VM. Tibolone for the treatment of moderate to severe vasomotor symptoms and genital atrophy in postmenopausal women: a multicenter, randomized, double‐blind, placebo‐controlled study. Menopause 2006;13(6):917‐25. [DOI] [PubMed] [Google Scholar]
Uygur 2005 {published data only}
- Uygur D, Yeşildaglar N, Erkaya S. Effect on sexual life ‐ a comparison between tibolone and continuous combined conjugated equine estrogens and medroxyprogesterone acetate. Gynecologic Endocrinology 2005;20(4):209‐12. [DOI] [PubMed] [Google Scholar]
Vieira 2009 {published data only}
- Vieira CS, Pereira FV, Sá MFS, Paulo LJ, Martins WP, Ferriani RA. Tibolone in postmenopausal women with systemic lupus erythematosus: a pilot study. Maturitas 2009;62:311‐6. [DOI] [PubMed] [Google Scholar]
Volpe 1986 {published data only}
- Volpe A, Facchinetti F, Grasso A, Petraglia F, Campanini D, Genazzani AR. Benefits and risks of different hormonal replacement therapies in post‐menopausal women. Maturitas 1986;8:327‐34. [DOI] [PubMed] [Google Scholar]
Wender 2004 {published data only}
- Wender MC, Edelweiss MI, Campos LS, de Castro JA, Spritzer PM. Endometrial assessment in women using tibolone or placebo: 1‐year randomized trial and 2‐year observational study. Menopause 2004;11:423‐9. [DOI] [PubMed] [Google Scholar]
Winkler 2000 {published data only}
- Winkler UH, Altkemper R, Kwee B, Helmond FA, Coelingh Bennink HJT. Effects of tibolone and continuous combined hormone replacement therapy on parameters in the clotting cascade: a multicenter, double‐blind, randomized study. Fertility and Sterility 2000;74(1):10‐9. [DOI] [PubMed] [Google Scholar]
Wu 2001 {published data only}
- Wu MH, Pan HA, Wang ST, Hsu CC, Chang FM, Huang KE. Quality of life and sexuality changes in postmenopausal women receiving tibolone therapy. Climacteric 2001;4(4):314‐9. [PubMed] [Google Scholar]
Ziaei 2010 {published data only}
- Ziaei S, Masoumi R, Faghihzadeh S. Comparing the effects of continuous hormone replacement therapy and tibolone on the genital tract of menopausal women. A randomized controlled trial. Journal of Reproduction and Infertility 2010;11(3):183‐7. [PMC free article] [PubMed] [Google Scholar]
- Ziaei S, Moghasemi M, Faghihzadeh S. Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women. Climacteric 2010;13:147‐56. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Argyroudis 1997 {published data only}
- Argyroudis EM, Iatrakis G, Kourkoubas A, Georgoulias N, Kourounis G, Tsionis C, et al. Tibolone in the treatment of psychosomatic symptoms in menopause. Clinical and Experimental Obstetrics and Gynecology 1997;24(3):167‐8. [PubMed] [Google Scholar]
Baksu 2005 {published data only}
- Baksu A, Ayas B, Citak S, Kalan A, Baksu B, Goker N. Efficacy of tibolone and transdermal estrogen therapy on psychological symptoms in women following surgical menopause. International Journal of Gynaecology and Obstetrics 2005;91(1):58‐62. [DOI] [PubMed] [Google Scholar]
Beardsworth 1999 {published data only}
- Beardsworth SA, Kearney CE, Purdie DW. Prevention of postmenopausal bone loss at lumbar spine and upper femur with tibolone: a two‐year randomised controlled trial. British Journal of Obstetrics and Gynaecology 1999;106(7):678‐83. [DOI] [PubMed] [Google Scholar]
Berlanga 2003 {published data only}
- Berlang C, Mendieta D, Alva G, Carmen LM. Failure of tibolone to potentiate the pharmacological effect of fluoxetine in postmenopausal major depression. Journal of Women's Health 2003;12(1):33‐9. [DOI] [PubMed] [Google Scholar]
Bhattacharya 2008 {published data only}
- Bhattacharya SM. Tibolone versus conjugated oestrogen ‐ what is the short‐term treatment effect on health‐related quality of life (HRQOL) in surgical menopausal women?. Journal of the Turkish‐German Gynecology Association 2008;9(4):195‐201. [Google Scholar]
Bhattacharya 2010 {published data only}
- Bhattacharya SM, Jha A. Effects of transdermal estradiol gel and oral tibolone on health‐related quality of life after surgical menopause. International Journal of Gynaecology and Obstetrics 2010;110(3):213‐6. [DOI] [PubMed] [Google Scholar]
Bukulmez 2001 {published data only}
- Bukulmez O, Al A, Gurdal H, Yarali H, Ulug B, Gurgan T. Short‐term effects of three continuous hormone replacement therapy regimens on platelet tritiated imipramine binding and mood scores: a prospective randomized trial. Fertility and Sterility 2001;75(4):737‐43. [DOI] [PubMed] [Google Scholar]
Cagnacci 2004 {published data only}
- Cagnacci A, Baldassari F, Arangino S, Alessandrini C, Volpe A. Administration of tibolone decreases 24 h heart rate but not blood pressure of post‐menopausal women. Maturitas 2004;48(2):155‐60. [DOI] [PubMed] [Google Scholar]
Cayan 2008 {published data only}
- Cayan F, Dilek U, Pata O, Dilek S. Comparison of the effects of hormone therapy regimens, oral and vaginal estradiol, estradiol + drospirenone and tibolone, on sexual function in healthy postmenopausal women. Journal of Sexual Medicine 2008;5(1):132‐8. [DOI] [PubMed] [Google Scholar]
De Censi 2013 {published data only}
- DeCensi A, Bonanni B, Maisonneuve P, Serrano D, Omodei U, Varricchio C, et al. A phase‐III prevention trial of low dose tamoxifen in postmenopausal hormone replacement therapy users: the HOT study. Annals Oncology 2013;24:2753‐60. [DOI] [PubMed] [Google Scholar]
Fedele 2000 {published data only}
- Fedele L, Bianchi S, Raffaelli R, Zanconato G. A randomized study of the effects of tibolone and transdermal estrogen replacement therapy in postmenopausal women with uterine myomas. European Journal of Obstetric and Gynecological Reproductive Biology 2000;88(1):91‐4. [DOI] [PubMed] [Google Scholar]
Gambacciani 2004 {published data only}
- Gambacciani M, Ciaponi M, Cappagli B, Monteleone P, Benussi C, Bevilacqua G, et al. A longitudinal evaluation of the effect of two doses of tibolone on bone density and metabolism in early postmenopausal women. Gynecologic Endocrinology 2004;18(1):9‐16. [DOI] [PubMed] [Google Scholar]
Genazzani 2011 {published data only}
- Genazzani AR, Stomati M, Valentino V, et al. Effect of 1‐year, low‐dose DHEA therapy on climacteric symptoms and female sexuality. Climacteric 2011;14(6):661‐8. [DOI] [PubMed] [Google Scholar]
Inan 2005 {published data only}
- Inan I, Kelecki S, Yilmaz B. Psychological effects of tibolone and sequential estrogen‐progestogen therapy in postmenopausal women. Gynecologic Endocrinology 2005;20(2):64‐7. [DOI] [PubMed] [Google Scholar]
Lundstrom 2011 {published data only}
- Lundstrom E, Hirschberg AL, Soderqvist G. Digitized assessment of mammographic breast density ‐ effects of continuous combined hormone therapy, tibolone and black cohosh compared to placebo. Maturitas 2011;70(4):361‐4. [DOI] [PubMed] [Google Scholar]
Nappi 2006b {published data only}
- Nappi RE, Ferdeghini F, Sampaolo P, Vaccaro P, Leonardis C, Albani F, et al. Clitoral circulation in postmenopausal women with sexual dysfunction: a pilot randomized study with hormone therapy. Maturitas 2006;55(3):288‐95. [DOI] [PubMed] [Google Scholar]
Onalan 2005 {published data only}
- Onalan G, Onalan R, Selam B, Akar M, Gunenc Z, Topcuoglu A. Mood scores in relation to hormone replacement therapies during menopause: a prospective randomized trial. Tohoku Journal of Experimental Medicine 2005;207(3):223‐31. [DOI] [PubMed] [Google Scholar]
Palacios 1995 {published data only}
- Palacios S, Menendez C, Jurado AR, Castaño R, Vargas JC. Changes in sex behaviour after menopause: effects of tibolone. Maturitas 1995;22(2):155‐61. [DOI] [PubMed] [Google Scholar]
Silva 2015 {published data only}
- Silva G, Lima S, Guazzelle R, Yamada S, Martins C. Hypoactive sexual desire disorder: tibolone and Tribulus terrestris are really effective?. Journal of Sexual Medicine 2015;12:30‐1. [Google Scholar]
Simsek 2002 {published data only}
- Simsek T, Karakus C, Trak B. Impact of different hormone replacement therapy regimens on the size of myoma uteri in postmenopausal period. Tibolone versus transdermal hormonal replacement system. Maturitas 2002;42:243‐6. [DOI] [PubMed] [Google Scholar]
Stefanos 2010 {published data only}
- Stefanos Z, Georgios I, Panagiotis P, Iordanis N, Panagiotis T, Georgios G, et al. Sequential tibolone versus progestin for premenopausal bleeding. Gineco (Ro) 2010;6:37‐40. [Google Scholar]
Stevenson 2011 {published data only}
- Stevenson JC. Prevention of osteoporosis: one step forward, two steps back. Menopause International 2011;17(4):137‐41. [DOI] [PubMed] [Google Scholar]
Tasic 2011 {published data only}
- Tasic L, Dunjic R, Vasiljevic M, Dikic S, Jurisic A, Tasic D. Comparison of two different types of hormone therapy effects on haemostatic parameters in early postmenopausal women. Srpski Arhiv za Celokupno Lekarstvo 2011;139(1‐2):52‐7. [DOI] [PubMed] [Google Scholar]
Yuk 2012 {published data only}
- Yuk JS, Yi KW, Kim T, Hur JY, Kim SH, Shin JH. Preliminary study: the body compositional change according to drospirenone (DRSP) containing lowdose hormone therapy and tibolone. Maturitas 2012;71:S46. [Google Scholar]
Additional references
Bekelman 2003
- Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA 2003;289:454‐65. [DOI] [PubMed] [Google Scholar]
Beral 2003
- Beral V, Million Women Study Collaborators. Breast cancer and hormone‐replacement therapy in the Million Women Study. Lancet 2003;362:419‐27. [DOI] [PubMed] [Google Scholar]
Beral 2005
- Beral V, Bull D, Reeves G, Million Women Study Collaborators. Endometrial cancer and hormone‐replacement therapy in the Million Women Study. Lancet 2005;365:1543‐51. [DOI] [PubMed] [Google Scholar]
Beral 2007
- Beral V. LIFT study is discontinued. BMJ (rapid response) 2006;332:667. http://www.bmj.com/content/332/7542/667.1/reply. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beral 2011
- Beral V, Reeves G, Bull D, Green J. Breast cancer risk in relation to the interval between menopause and starting hormone therapy. Journal of the National Cancer Institute 2011;103:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis. New York: John Wiley & Sons, 2009. [Google Scholar]
Bots 2006
- Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, OPAL Investigators. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima‐media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. European Heart Journal 2006;27:746‐55. [DOI] [PubMed] [Google Scholar]
Cody 2012
- Cody JD, Jacobs ML, Richardson K, Moehrer B, Hextall A. Oestrogen therapy for urinary incontinence in post‐menopausal women. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001405.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Diggle 1994
- Diggle P, Liang KY, Zeger SL. Longitudinal Data Analysis. New York: Oxford University Press, 1994. [Google Scholar]
Freedman 1995
- Freedman RR, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. Journal of Clinical Endocrinology and Metabolism 1995;80(8):2354‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011], The Cochrane Collaboration. www.cochrane‐handbook.org. 2011.
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLennan 2004
- MacLennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD002978.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 1990
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Modelska 2002
- Modelska K, Cummings S. Tibolone for postmenopausal women: systematic review of randomized trials. Journal of Clinical Endocrinology and Metabolism 2002;87(1):16‐23. [DOI] [PubMed] [Google Scholar]
NCC‐WCH 2015
- NCC‐WCH National Collaborating Centre for Women’s and Children’s Health. Menopause. Commissioned by the National Institute for Health and Care Excellence. London: NCC‐WCH, 2015.
Politi 2008
- Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta‐analysis. Journal of General Internal Medicine 2008;23:1507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 1996
- Porter M, Penney GC, Russell D, Russell E, Templeton A. A population based survey of women's experience of the menopause. British Journal of Obstetrics and Gynaecology 1996;103(10):1025‐8. [DOI] [PubMed] [Google Scholar]
R Core Team 2015
- R Core Team. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2015. [https://www.R‐project.org/]
Sanchez 2005
- Gabriel Sanchez R, Sanchez Gomez LM, Carmona L, Roqué i Figuls M, Bonfill Cosp X. Hormone replacement therapy for preventing cardiovascular disease in post‐menopausal women. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002229.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soc Obstetr Gynaecol Canada 2014
- Society of Obstetricians and Gynaecologist Canada. Managing menopause. Journal of Obstetrics and Gynaecology in Canada 2014;36 (9 e Suppl A):S1–S80. [DOI] [PubMed] [Google Scholar]
Speroff 2004
- Speroff L, Fritz MA. The Clinical Gynecologic Endocrinology and Infertility: The Cervical Spine Research Society Editorial Committee (Clinical Gynecologic Endocrinology and Infertility). Baltimore, MD: Lippincott Williams & Wilkins, 2004. [Google Scholar]
Sterne 2009
- Sterne JAC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suckling 2006
- Suckling J, Kennedy R, Lethaby A, Roberts H. Local oestrogen therapy for vaginal atrophy in postmenopausal women. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD001500.pub2] [DOI] [PubMed] [Google Scholar]
Viechtbauer 2010
- Viechtbauer W. Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software 2010;36:1‐48. [Google Scholar]
Ziaei 2010b
- Ziaei S, Moghasemi M, Faghihzadeh S. Comparative effects of conventional hormone replacement therapy and tibolone on climacteric symptoms and sexual dysfunction in postmenopausal women. Climacteric 2010;13:147‐56. [DOI] [PubMed] [Google Scholar]


