Abstract
Background
Non‐nutritive sucking (NNS) is used during gavage feeding and in the transition from gavage to breast/bottle feeding in preterm infants to improve the development of sucking behavior and the digestion of enteral feedings.
Objectives
To assess the effects of non‐nutritive sucking on physiologic stability and nutrition in preterm infants.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), MEDLINE via PubMed (1966 to 25 February 2016), Embase (1980 to 25 February 2016), and CINAHL (1982 to 25 February 2016). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials.
Selection criteria
Randomised controlled trials and quasi‐randomised trials that compared non‐nutritive sucking versus no provision of non‐nutritive sucking in preterm infants. We excluded cross‐over trials.
Data collection and analysis
Two review authors assessed trial eligibility and risk of bias and undertook data extraction independently. We analysed the treatment effects in the individual trials and reported mean differences (MD) for continuous data, with 95% confidence intervals (CIs). We used a fixed‐effect model in meta‐analyses. We did not perform subgroup analyses because of the small number of studies related to the relevant outcomes. We used the GRADE approach to assess the quality of evidence.
Main results
We identified 12 eligible trials enrolling a total of 746 preterm infants. Meta‐analysis, though limited by data quality, demonstrated a significant effect of NNS on transition from gavage to full oral feeding (MD −5.51 days, 95% CI −8.20 to −2.82; N = 87), transition from start of oral feeding to full oral feeding (MD −2.15 days, 95% CI −3.12 to −1.17; N = 100), and the length of hospital stay (MD −4.59 days, 95% CI −8.07 to −1.11; N = 501). Meta‐analysis revealed no significant effect of NNS on weight gain. One study found that the NNS group had a significantly shorter intestinal transit time during gavage feeding compared to the control group (MD −10.50 h, 95% CI −13.74 to −7.26; N = 30). Other individual studies demonstrated no clear positive effect of NNS on age of infant at full oral feeds, days from birth to full breastfeeding, rates and proportion of infants fully breastfeeding at discharge, episodes of bradycardia, or episodes of oxygen desaturation. None of the studies reported any negative outcomes. These trials were generally small and contained various methodological weaknesses including lack of blinding of intervention and outcome assessors and variability on outcome measures. The quality of the evidence on outcomes assessed according to GRADE was low to very low.
Authors' conclusions
Meta‐analysis demonstrated a significant effect of NNS on the transition from gavage to full oral feeding, transition from start of oral feeding to full oral feeding, and length of hospital stay. None of the trials reported any adverse effects. Well‐designed, adequately powered studies using reliable methods of randomisation, concealment of treatment allocation and blinding of the intervention and outcome assessors are needed. In order to facilitate meta‐analysis of these data, future research should involve outcome measures consistent with those used in previous studies.
Plain language summary
Non‐nutritive sucking for increasing physiologic stability and nutrition in preterm infants
Review question
Does non‐nutritive sucking increase physiological stability and nutrition in preterm infants?
Background
An infant born prematurely may be fed through a tube into the stomach and often receives a pacifier to suck on to improve nutrition. An infant needs coordinated sucking, swallowing and breathing to feed. The ability to suck and to swallow is present by 28 weeks gestation, but infants are not fully coordinated until 32 to 34 weeks. This means that preterm infants born at less than 32 weeks gestation are usually not able to feed effectively from the breast or a bottle. They are fed by a small tube that is placed up the nose into the stomach (gavage feeding). Sucking on a pacifier (non‐nutritive sucking) during gavage feeding may encourage the development of sucking behavior and improve digestion of the feeding. Non‐nutritive sucking may also have a calming effect on infants, although it does have the potential to interfere with breastfeeding.
Study characteristics
We identified 12 eligible trials enrolling a total of 746 preterm infants in searches updated to February 2016.
Key results
An overall analysis suggests that non‐nutritive sucking reduces the time infants need to transition from tube to full oral feeding, and from start of oral feeding to full oral feeding. It also reduces the length of hospital stay. Non‐nutritive sucking did not demonstrate a positive effect on weight gain.
Quality of evidence
Participants numbers in these studies were small, and we judged the quality of the evidence on outcomes assessed to be low or very low. Large well‐designed randomised controlled trials are necessary for further evaluating the effectiveness and safety of non‐nutritive sucking for increasing physiologic stability and nutrition in preterm infants.
Summary of findings
Summary of findings for the main comparison. Non‐nutritive sucking (NNS) compared with no NNS for physiologic stability and nutrition.
| Non‐nutritive sucking compared with no non‐nutritive sucking for physiologic stability and nutrition | |||||
|
Patient or population: preterm infants Settings: neonatal unit Intervention: NNS Comparison: no NNS | |||||
| Outcomes | Anticipated absolute effects (95% CI) * | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Risk without NNS | Risk with NNS | ||||
| Gavage to full oral feeding (days) | The mean gavage to full oral feeding was 0 days | The mean days from gavage to full oral feeding in the intervention group was 5.51 lower (8.20 lower to 2.82 lower) |
— | 87 (2 trials) |
⊕⊕⊝⊝ Lowa,b,c |
| Start oral feeding to full oral feeding (days) | The mean start oral feeding to full oral feeding was 0 days | The mean days from start of oral feeding to full oral feeding in the intervention group was 2.15 lower (3.12 lower to 1.17 lower) | — | 100 (2 trials) |
⊕⊝⊝⊝ Very lowa,b,c,d |
| Days from birth to full oral breastfeeding | The mean days from birth to full breastfeeding was 0 days | The mean days from birth to full breastfeeding was 1 day lower (6.71 lower to 4.71 higher) | — | 303 (1 trial) |
⊕⊕⊝⊝ Lowb,e,f |
| Full breastfeeding at discharge | 520 per 1000 | 0 per 1000 (0 to 0) | Not estimable | 303 (1 trial) |
⊕⊕⊝⊝ Lowb,e,f |
| Length of hospital stay (days) | The mean length of hospital stay was 0 days | The mean length of hospital stay in the intervention group was 4.59 days lower (8.07 lower to 1.11 lower) | — | 501 (5 trials) |
⊕⊕⊝⊝ Lowa,b,c |
| Weight gain (g/day) | the mean weight gain 0 grams/day | The mean weight gain in the intervention group was 1.57 g lower (3.5 lower to 0.37 higher) | — | 103 (3 trials) |
⊕⊕⊝⊝ Lowa,b,c |
| Postconceptual age at full oral feeds (days) | The mean post menstrual age at full oral feeding was 0 days | The mean postconceptual age at full oral feeding in the intervention group was 1.70 days lower (46.06 lower to 42.66 higher) | — | 28 (1 trial) |
⊕⊝⊝⊝ Very lowa,b,c,e |
| Intestinal transit time (hours) | The mean intestinal transit time was 0 hours | The mean intestinal transit time in the intervention group was 10.50 h lower (13.74 lower to 7.26 lower) | — | 30 (1 trial) |
⊕⊝⊝⊝ Very lowa,b,c,e |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group CI: confidence interval. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate of the effect. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate of the effect. Very low quality: we are very uncertain about the estimate of the effect. | |||||
aHigh risk of selection bias. bHigh risk of performance bias. cHigh risk of detection bias. dModerate heterogeneity. eOne study. fHigh non‐adherence.
Background
Description of the condition
The early components of sucking have been demonstrated to occur in fetal life from about seven to eight weeks postconceptual age. Oral and gag reflexes appear at about 12 to 16 weeks and sucking at 15 to 18 weeks gestation (Breton 2008; Poore 2008). At 28 weeks gestation, some infants may have established a suck‐swallow‐breathe cycle, although they lack the physiological stability to maintain this cycle whilst feeding, which can lead to variable oxygenation, irregular breathing sequence and poor digestion (Jones 2012). The smooth integration of sucking, swallowing and breathing during nutritive feeding allows the infant to feed efficiently and effectively. Full coordination may not occur until 32 to 34 weeks gestation (Amaizu 2008; Goldson 1987; Neiva 2007).This means that preterm infants with less than 32 weeks gestation are usually not able to feed effectively from the breast or a bottle. They are fed by a small tube that is placed up the nose into the stomach (gavage feeding). In the gavage‐fed infant, NNS is extremely important to facilitate a positive association between sucking and swallowing with satiation in order to avoid the development of feed aversion (Barlow 2009; Hawdon 2000). The effect of feeding experience contributes to the development of feeding skills (Amaizu 2008).
The development of both nutritive and non‐nutritive sucking behaviours in preterm infants is thought to reflect neurobehavioral maturation and organization (Lau 2003; Pickler 2009). An organized stable temporal pattern has been identified for both nutritive and non‐nutritive sucking, enabling the features of each to be analysed by quantitative techniques (Wolf 1992). From a clinical perspective, the ability to feed depends on a coordinated sucking, swallowing, and breathing pattern. In preterm infants of less than 32 weeks gestation, this ability is not usually effective enough to sustain full oral feeds (Wolf 1992). In the interim, infants are fed by gavage tube until they are mature enough to take milk directly from the breast or bottle (Greer 2001). The development of oral feeding in infants requires complex anatomical and physiological coordination. The integration of structures involving the lips, cheeks, jaws, tongue, palate, pharynx, and larynx allow for the infant to create the appropriate pressure required for suction and swallowing during oral feeding (Miller 2007). Similarly, the ability to maintain stable physiologic functions such as heart rate, respiratory rate and function, and oxygen saturation are essential to prevent variable oxygenation, bradycardia, and irregular breathing sequence during feeding (Pickler 2004). The coordination of the autonomic, motoric, and behavioral subsystems required to perform these actions may not be fully developed in the preterm infant, resulting in difficulty establishing a stable suck‐swallow‐breathe cycle prior to full‐term gestation (McGrath 2004).The inability of the premature infant to develop a stable suck‐swallow‐breathe cycle can be linked to factors such as poor motor skills and posture, multiple medical complications or an immature autonomic nervous system, which can then be further exacerbated by congenital abnormalities, low birth weight, or developmental complications (Boiron 2007; Dodrill 2008; Miller 2007).
Description of the intervention
Breastfeeding, bottle feeding and cup feeding are considered a form of nutritive sucking (NS) because the purpose of NS is to obtain nutrition in the form of breast milk or formula. Non‐nutritive sucking (NNS) occurs in the absence of nutrient flow and may be used to satisfy an infant's basic sucking urge or as a state regulatory mechanism (Wolf 1992). Pacifiers play a significant role in non‐nutritive sucking, one of which replaces the role of thumb sucking that has been shown to occur in the womb from as early as 12 weeks gestation (Jenik 2009). A gloved finger and empty breast are also described as methods of non‐nutritive sucking (Medeiros 2011).
Most often pacifiers have been used during gavage feeding to facilitate the co‐ordination of sucking and swallowing.The development of the stable suck‐swallow‐breathe cycle present in full term infants is viewed as a sign of neurobehavioral stability and maturation. Hence achieving and maintaining this cycle for preterm infants is often fraught with difficulty, as neural and physiological pathways are often immature and uncoordinated (McGrath 2004). Non‐nutritive sucking has been shown to assist the infant in achieving and maintaining physiological homeostasis and behavioral state (Jenik 2009). During nutritive sucking, if fluid is swallowed incorrectly it can lead to aspiration pneumonia, bradycardia, hypoxia, and fatigue (Crowe 2012; Miller 2007). Non‐nutritive sucking has the ability to create oral feeding experiences without the added stress of fluid. In addition, the act of NNS is one of an infant's first methods of self‐organization and self‐soothing, which is repetitive and rhythmic in nature. Non‐nutritive sucking, particularly with a pacifier, is believed to have a calming effect on infants and is commonly used as an intervention in nurseries and neonatal intensive care units (Kimble 1992b).
How the intervention might work
The rationale for this intervention is that non‐nutritive sucking facilitates the development of sucking behavior and improves digestion of enteral feeds. A number of enzymes/hormones have been implicated in the facilitation of digestion through non‐nutritive sucking: lingual lipase, gastrin, insulin, and motilin. Experts believe that non‐nutritive sucking stimulates the secretion of these enzymes/hormones through vagal innervation in the oral mucosa (Chey 1980; Hamosh 1979; Wiener 1987). NNS use has also been linked to improving the initiation and duration of the first nutritive suck, enhancing weight gain and reducing transition time between gavage and oral feeding (Boiron 2007). Investigators have shown that NNS accelerates the acquisition of mature NNS patterns and improves feeding skills (Barlow 2008).
An improved ability to modulate behavioral state is particularly important for the preterm infant at higher risk of developmental problems. Evidence suggests that providing non‐nutritive sucking opportunities to premature infants during gavage feeding may have beneficial effects on oxygen saturation, gastrointestinal function, growth, and development (Hack 1985). Literature reporting the effectiveness of NNS as a pain relief strategy for infants is also increasing (Tsa 2008).
Why it is important to do this review
Our literature review found a meta‐analysis of five studies of non‐nutritive sucking in preterm infants (Schwartz 1987). The authors concluded that non‐nutritive sucking reduced the time to first bottle feeding and reduced the days of hospitalisation. Outcome data related to weight gain were inconclusive.
A meta‐analysis of the non‐nutritive sucking research in preterm infants by Steer 1992 included eight randomised trials. The major outcome variables studied in these trials included weight gain, gastrointestinal transit, readiness for nipple feedings and length of hospitalisation. Lack of blinding to the intervention or outcome measurement in all studies affected the methodological quality of the findings. The authors concluded that in view of the limitations in the available research, there was insufficient beneficial evidence to support the use of non‐nutritive sucking in the management of tube‐fed preterm infants.
Non‐nutritive sucking within newborn care settings has become common practice. Recent evidence further supports the notion that improving feeding skills through the use of NNS shortens the length of hospital stay (Barlow 2008). As a component of developmentally supportive care, NNS is widely promoted though neonatal intensive care units (NICU) and other newborn care centres. Although commonly considered a benign intervention, further synthesis of the literature is required to support the ongoing use of NNS.
Objectives
To assess the effects of non‐nutritive sucking on physiologic stability and nutrition in preterm infants.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials and quasi‐randomised trials which compared non‐nutritive sucking in preterm infants to no provision of non‐nutritive sucking. We excluded trials evaluating the effect of NNS on pain in preterm infants, and for the 2016 update, we excluded cross‐over trials.
Types of participants
All infants born at less than 37 weeks postconceptual age. We excluded studies involving both preterm and term (greater than or equal to 37 weeks) infants.
Types of interventions
Non‐nutritive sucking involving the use of a pacifier or other method. The intervention can occur before, during or after gavage feeding by a naso/orogastric tube; before or after oral (bottle or breast) feeding; or outside of feeding times.
Types of outcome measures
Primary outcomes
Time (days) taken to achieve exclusive oral feeding, defined as when the infant ingests all nutrient volumes in a 24 hour period without any gavage (McCain 2001)
Breastfeeding (at discharge)
Length of hospital stay (days)
Weight gain (grams/day) during hospital stay
Secondary outcomes
Time (days) spent in NICU
Age of infant (days) at full oral feeding (postmenstrual age or postconceptual age)
Episodes of bradycardia (during or immediately after feeding) during hospital stay
Episodes of oxygen desaturation (during or immediately after feeding) during hospital stay
Activity or behavior (measured during or immediately after feeding by a validated tool, e.g. Brazelton Neonatal Behavioral Assessment Scale, Anderson's Behavioral State Scale) during hospital stay
Intestinal transit time (hours)
Neurodevelopmental outcomes at 12 months or more of age (corrected for preterm birth) measured using validated assessment tools such as Bayley Scales of Infant Development, and classifications of disability, including auditory and visual disability. Severe neurodevelopmental disability will be defined as any one or combination of the following: non‐ambulant cerebral palsy, developmental delay (developmental quotient less than 70), auditory and visual impairment
Any other clinically relevant outcomes as determined by authors
Search methods for identification of studies
Electronic searches
For the 2016 update, we conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 1) in The Cochrane Library; MEDLINE via PubMed (1966 to 25 February 2016); Embase (1980 to 25 February 2016); and CINAHL (1982 to 25 February 2016) using the following search terms: ((non‐nutritive AND suck*) OR (nonnutritive AND suck*) OR pacifier OR dummy), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. Additionally, we did not apply any date limits.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/, and the ISRCTN Registry).
Searching other resources
We retrieved all potentially relevant titles and abstracts identified during the search. Review authors independently handsearched the bibliographies of each article for additional relevant titles and these were also retrieved. We sent the resulting list of all relevant articles to two major authors in this area and asked them if they knew of any other published or unpublished studies relevant to the area that were not included in the original list.
Data collection and analysis
The systematic review followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions and by the Cochrane Neonatal Review Group (Higgins 2011).
Selection of studies
Two review authors (JF and TP) screened the title and abstract of all identified studies. We re‐assessed the full text of any potentially eligible reports and excluded the studies that did not meet all of the inclusion criteria. We discussed any disagreements until we achieved consensus.
Data extraction and management
We used the data extraction form available within Review Manager software (RevMan) to extract data on the participants, interventions and control(s), and outcomes of each included trial (RevMan 2014).
Two review authors (JF and KP) independently extracted data from each study without blinding to authorship or journal publication.
In case of any disagreement, two review authors resolved them by discussion until reaching a consensus.
Where data were missing, unclear, or incomplete, we made reasonable attempts to contact the trial authors to obtain the required information.
One review author (JF) entered data into RevMan, and a second review author (KP) verified them (RevMan 2014).
We extracted the following data.
Participant characteristics.
Inclusion and exclusion criteria.
Numbers of enrolled participants and attrition rates (wherever possible).
Details of intervention.
Outcomes measured.
Duration of study and frequency of measurements.
Assessment of risk of bias in included studies
Review authors independently assessed risk of bias for the included studies using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements successfully by discussion, so it was unnecessary to involve a review arbiter. We completed the 'Risk of bias' table addressing the following methodological domains.
-
Selection bias.
Random sequence generation (biased allocation to interventions) due to inadequate generation of a randomised sequence.
Allocation concealment: selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment.
Blinding of participants and personnel: performance bias due to knowledge of the allocated interventions by participants and personnel during the study.
Blinding of outcome assessment: detection bias due to knowledge of the allocated interventions by outcome assessors.
Incomplete outcome data: attrition bias due to amount, nature or handling of incomplete outcome data.
Selective reporting: reporting bias due to selective outcome reporting.
Other sources of bias: bias due to problems not covered elsewhere in the table.
Overall bias.
See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using RevMan (RevMan 2014). We calculated risk ratios (RR) and risk differences (RD) for dichotomous data, and we analysed continuous data using mean differences (MDs). We reported the 95% confidence interval (CI) on all estimates.
Dealing with missing data
We include additional data obtained from Collins 2004 for the outcomes 'full breastfeeding at discharge and 'days from birth to full breastfeeding'.
Assessment of heterogeneity
We assessed the heterogeneity between the included trials, using the formal and commonly applied statistic to assess heterogeneity: the I2 statistic. This test describes the percentage of total variation observed across studies due to heterogeneity rather than sampling (random) error (Higgins 2011). We graded the degree of heterogeneity as non‐existent or minimal for an I2 value of less than 25%, low for an I2 value of 25% to 49%, moderate for an I2 value of 50% to 74%, and high for an I2 value of 75% to 100%. Had there been evidence of apparent or statistical heterogeneity, we planned to assess the source of the heterogeneity using sensitivity and subgroup analyses looking for sources of bias or methodological differences between the heterogeneous trials (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses.
Data synthesis
We performed meta‐analysis using RevMan (RevMan 2014). For estimates of typical risk ratio and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analyses because of the small number of studies related to the relevant outcomes.
Summary of findings table
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: gavage to full oral feeding (days), start oral feeding to full oral feeding (days), days from birth to full breastfeeding, proportion of infants fully breastfeeding at discharge, length of hospital stay, weight gain (grams/day), postconceptual age at full oral feeds and intestinal transit time.
Two review authors (JF and KP) independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded it one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro 2008 Guideline Development Tool to create a 'Summary of findings' table to report the main findings and the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
The search yielded 706 unique records (Figure 1). We included five additional studies in this review update (Collins 2004; Harding 2014a; Lau 2012; Moreira 2014; Zhang 2014).
1.
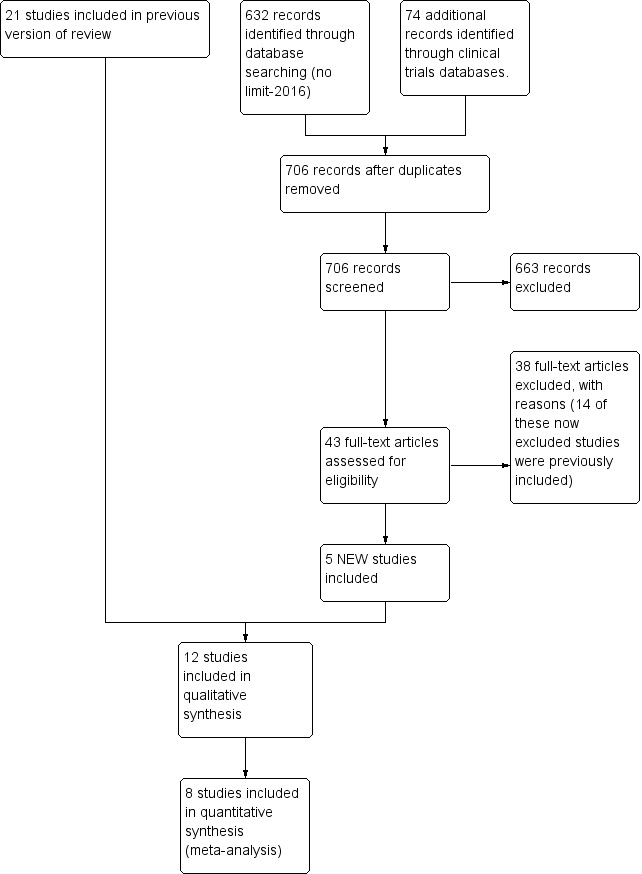
Study flow diagram: review update
We also found three ongoing RCTs (IRCT201106062324N8; IRCT2013120815458N2; IRCT201501205163N2). See Characteristics of ongoing studies.
Included studies
We describe each study below and in the Characteristics of included studies table.
In total, we included 12 studies (N = 746) in this review: Bernbaum 1983 (N = 30); Collins 2004 (N = 319); Ernst 1989 (N = 18); Field 1982 (N = 57); Gill 1988 (N = 24); Gill 1992 (N = 42); Harding 2014a and Harding 2014b, a single study with two intervention arms (N = 59); Kanarek 1992 (N = 21); Lau 2012 (N = 48); Mattes 1996 (N = 28); Moreira 2014 (N = 40); Zhang 2014 (N = 60). The 12 included trials took place in neonatal centres in North America, the UK, Brazil, China, and Australia.
Participants
Bernbaum 1983 was a single‐centre RCT that included 30 preterm infants with a mean gestational age of 31.5 weeks, weight of less than 1500 grams and mean postnatal age of 10 days. Eligible infants had a birth weight of less than 1500 grams; did not need a surgical intervention or further management from the intensive care team at the time nasogastric feeding commenced; and had no seizures, central nervous system (CNS) haemorrhages or cardiac or pulmonary diseases. The trial excluded infants if they were small for gestational age.
Collins 2004 was a multicentre RCT that included 319 preterm infants with mean birth weight of 1325 grams in the cup and no NNS group, 1508 grams in the bottle no NNS group, 1344 grams in the cup and NNS group, and 1382 g in the bottle and NNS group. Authors did not report postnatal age. Inclusion criteria were women with singleton or twin infants of less than 34 weeks' gestation who wanted to breast feed. The trial excluded infants with congenital abnormalities precluding enteral feeding.
Ernst 1989 was a single‐centre RCT that included 18 preterm infants with mean birth weight 1185 grams in the control group and 1256 grams in the NNS group and a mean gestational age of 29 weeks in the control group and 29 weeks in the NNS group. The study began on day of birth, including infants if they were very low birth weight babies between 890 grams and 1400 grams with an appropriate weight, length, and head circumference for their gestational age (27 to 30 weeks). The trial excluded infants if they experienced seizures or CNS haemorrhage, or if they required surgery, fluid restrictions, prolonged mechanical ventilation, significant supplemental oxygen, discontinued feeding, or formula that was different from the routinely used premature feeding formula.
Field 1982 was a a single‐centre RCT that included 57 infants with a mean birth weight of 1300 grams and mean 32 weeks' gestation. Postnatal age was not reported; however, the study began on day 1. Eligible infants had a birth weight of less than 1800 grams and less than 35 weeks gestational age, and they were free from major congenital abnormalities, chromosomal abnormalities, oropharyngeal problems, and conditions known to be incompatible with life.
Gill 1988 was a single‐centre RCT that included 24 preterm infants with a median birth weight of 1270 grams in the NNS group and 1570 grams in the no NNS group; median gestational age was 30.5 weeks in the NNS group and 31.5 weeks in the no NNS group. Median postnatal age was 19 days in the NNS group and 15 days in the no NNS group. Infants were included if they had a birth weight of less than 2000 grams, gestational age of 34 weeks or less, and appropriate weight for gestational age. They had to be receiving gavage feeding and be ready to have a first bottle feeding. No infants had intraventricular haemorrhage of grades III or IV or congenital or neurological anomalies.
Gill 1992 was a single‐centre RCT that included 42 preterm infants with a mean birth weight of 1254 grams in the control group and 1408 grams in the NNS group, a mean gestational age of 29.3 weeks in the control group and 30.2 weeks in the NNS group, and a mean postnatal age of 25.9 days in the control group and 22.8 days in the NNS group. Infants were included if they weighed under 2000 grams, had a gestational age of 34 weeks or less, had an appropriate weight for gestational age, were being fed by gavage, and were ready to have a first bottle feeding. No infants had intraventricular haemorrhage of grades III or IV or congenital or neurological anomalies.
Harding 2014a (and Harding 2014b) included 59 preterm infants in a single‐centre RCT with two intervention arms. For Intervention 1 (NNS before gavage feeding), the 19 included infants had a mean gestational age of 32.53 weeks and birth weight of 1651 grams, and for intervention 2 (NNS during gavage feeding) the 20 included infants had a mean gestational age of 31.60 weeks and a mean birth weight of 1651 grams. The control group had a mean gestational age of 30.95 weeks and mean birth weight of 1676 grams. Authors did not report postnatal age, but the trial included infants when they started to show oral readiness. They were eligible if they were preterm and excluded if they were identified as having congenital disorders, intraventricular haemorrhages III or IV, severe respiratory problems, or necrotizing enterocolitis.
Kanarek 1992 was a single‐centre RCT that included 21 preterm infants with a mean birth weight of 1450 grams in the control group and 1320 grams in the NNS group, a mean gestational age of 31.8 weeks in the control group and 31.0 weeks in the NNS group, and postnatal age of one day. The trial included infants if their weight was appropriate for gestational age and if they were free from major congenital abnormalities, perinatal asphyxia, infection and respiratory distress.
Lau 2012 included infants with a mean birth weight of 1121 grams in the control group and 1076 grams in the NNS group and with a mean gestational age of 28.1 weeks in both groups. Mean postmenstrual age was 38.8 weeks in the control group and 39.0 weeks in the NNS group. The trial is a single‐centre RCT and included infants identified as 'feeders and growers' with a primary diagnosis of prematurity, and it excluded infants if they had gastrointestinal complications, congenital anomalies or chronic medical conditions: intraventricular haemorrhage grade III and IV, periventricular leukomalacia, necrotizing enterocolitis or bronchopulmonary dysplasia.
Mattes 1996 was a multicenter RCT that included 28 preterm infants with a mean birth weight of 1321 grams in the control group and 1377 grams in the NNS group, a mean gestational age of 30.5 weeks in the control group and 31.1 weeks in the NNS group, and a mean postconceptual age of 33.7 weeks in the control group and 33.6 weeks in the NNS group. The trials included infants if their body weight was 1250 grams or more, had a gestational age of less than 34 weeks, had growth parameters appropriate for gestational age, Apgar scores of more than 3 at one minute and more than 5 at five minutes, no clinical evidence of seizure activity or grade III or IV intraventricular haemorrhage, no congenital heart disease other than patent ductus arteriosus or peripheral pulmonic stenosis that was haemodynamically significant, and no respiratory distress syndrome. Excluded from the study were infants with signs of necrotizing enterocolitis, hepatic disorder, congenital infection, metabolic disease, or anomalies affecting the central nervous system or gastrointestinal tract.
Moreira 2014 was a single‐centre RCT that included 40 preterm infants with a birth weight of 1256 grams in the control group and 1306 grams in the NNS group and a mean gestational age of 29.9 weeks in the control group and 30.1 weeks in the NNS group. Authors did not report postnatal age. The study included infants if they had a birth weight of less than 1500 grams, gestational age at birth of 32 weeks or less, five‐minute Apgar score of 6 or more, clinical (respiratory and haemodynamic) stability on enrolment and during the study, initiation of enteral feeding by oral or nasogastric tube associated or not with parenteral nutrition. Exclusion criteria were infants with grades III/IV intraventricular haemorrhage, clinical instability on enrolment or during the study, including necrotizing enterocolitis, sepsis, bronchopulmonary dysplasia and other clinical respiratory or haemodynamic instabilities, five‐minute Apgar score of 5 or less, presence of genetic syndromes, neurological disorders, or head, neck or central nervous system congenital malformations.
Zhang 2014 was a single‐centre RCT that included 55 preterm infants. Infants in the NNS group had a mean birth weight of 1548 grams and mean gestational age of 30.9 weeks, and infants in the control group had a mean birth weight of 1651 grams and a mean gestational age of 31.1 weeks. The trial is a single‐centre RCT. Postnatal age was not reported, but all included infants were born in other hospitals and transported within 24 hours to 48 hours to the NICU. The trial included infants born at 29 to 34 weeks gestation, who had an appropriate weight for gestational age, Apgar scores of more than 3 at one minute and more than 5 at at five minutes, and who received all feedings by tube. The trial excluded infants with congenital abnormalities (oral, heart etc.) and infants who developed chronic medical complications during NICU admission such as intraventricular haemorrhage grades III and IV, bronchopulmonary dysplasia, or necrotizing enterocolitis.
Interventions
The NNS intervention was via a pacifier in all but one study, which used a gloved finger (Moreira 2014). There was substantial variability as to when and how the NNS was used. Non‐nutritive sucking occurred before gavage feeding (Harding 2014a; Moreira 2014; Zhang 2014), during gavage feeding (Bernbaum 1983; Field 1982; Harding 2014b; Mattes 1996), during and after gavage feeding (Ernst 1989; Kanarek 1992), before bottle feeding (Gill 1988; Gill 1992), or not directly related to feeding (Collins 2004; Lau 2012).
NNS before gavage feeding
Harding 2014a reported on NNS prior to gavage feeding. Parents were encouraged to use a pacifier to elicit three sequential sucks and to encourage sequential sucking for a minimum of five minutes. Infants received the intervention after they started showing signs of oral readiness. The intervention lasted a minimum of three days until they were taking all of their feeds orally. Moreira 2014 reported on the use of NNS with gloved finger for 10 minutes, three times a day for three days a week before gavage feeding. Zhang 2014 reported on the use of NNS, and NNS and oral stimulation 30 minutes prior to gavage feeding. Infants in the NNS group were allowed to suck on pacifiers for five minutes, 7 to 8 times a day (Zhang 2014). The pacifier was placed in the infant's mouth whether or not they made an attempt to suck; however, where necessary the nurse would manipulate the pacifier to encourage sucking.
NNS during gavage feeding
Bernbaum 1983 reported on NNS during gavage feeding, where infants did not have sucking opportunities between feeding periods. The pacifier, which was constructed from an unperforated standard‐sized disposable nipple plugged with the plunger of a 20 mL syringe to prevent swallowing of air, was manipulated to encourage sucking and was placed so that it remained in the infant's mouth during the entire feeding. All infants were gavage fed until they attained a weight of 1700 grams, at which time they began oral feedings that advanced in frequency and amount according to the infant's tolerance. Field 1982 reported on NNS during gavage feeding, in which infants were given the pacifiers whether or not they made an effort to suck. Infants in both groups were allowed to have a pacifier at any other time, but only the treatment group received the pacifier during gavage feeding. Infants began bottle feeding when they weighed 1500 grams and their medical condition was stable. Harding 2014b reported on NNS during gavage feeding, whereby parents were encouraged to use a pacifier to elicit three sequential sucks and to encourage sequential sucking for a minimum of five minutes. Infants received the intervention after they started showing signs of oral readiness. The intervention was provided for a minimum of three days until they were taking all of their feeds orally. The study did not describe the pacifier used for the study. Mattes 1996 reported NNS during gavage feeding whereby infants were provided a standard latex pacifier during all feedings until they were able to tolerate full oral feedings.
NNS during and after gavage feeding
Ernst 1989 reported on NNS during and after gavage feeding, in which infants received a pacifier constructed from a standard blue premature nipple, stuffed with gauze for resistance and attached with tape to a rolled terry cloth for each of manipulation and positioning in the infant's mouths, with each feeding. Pacifiers were positioned in the mouth throughout and after and maintained in the mouth throughout and after the feeding for a total of 30 minutes. Manipulation of the pacifier in the infant's mouth and stroking of the infant's cheek were used to stimulate NNS when infants stopped sucking during each 30 minute treatment. The treatment phase of the study ended when infants weighed approximately 1700 grams, gavage feedings were discontinued, and all infants began nipple feedings. Kanarek 1992 reported on NNS during and after gavage feeding, whereby infants received a commercial pacifier beginning on the first day of life, during and after all feedings and when they were awake. Blood measurements were taken before and 72 hours after the initiation of continuous gavage feedings. In infants who were bolus‐fed, investigators obtained the second specimen 20 minutes after the feed.
NNS before bottle feeding
Gill 1988 reported on the use of NNS five minutes before bottle feeding every three hours for the first 48 hours. The trial used two sizes of 'firm slow‐feed' commercial nipples as pacifiers by inserting the distal end of a plastic disposable 3 mL syringe cover into the nipple as a prop and to block air entry. When necessary, nurses cut the nipple flat on one side to facilitate entry into the infant's mouth. Gill 1992 reported on the use of NNS, using commercial bottle nipples that varied in size and firmness but matched the nipple being used for bottle feedings, five minutes before bottle feeding every three hours and beginning with the first bottle feed, for the first 48 hours. The infant received a pacifier that was kept in the infant's mouth, whether or not sucking occurred.
NNS not directly related to feeding
Lau 2012 reported on a sucking exercise that consisted of NNS with the commercial pacifiers that were routinely used in the NICU. This was achieved by gently moving the pacifier in a rhythmic up/down motion that stimulated the NNS. All infants received the use of the pacifier, but only infants in the NNS group received this exercise. Experienced research feeding therapists provided NNS between, but not within the 30 minutes prior to oral feedings, 15 minutes per day, five days a week.
Collins 2004 reported on the use of bottle/cup feeding and NNS and bottle/cup feeding and no NNS. Infants in the experimental group received a dummy in between feeds. Cup or bottle feeding commenced at the discretion of the attending nurse/midwife or neonatologist and occurred when the mother was unavailable to breast feed or when additional milk, given orally, was required after a breast feed. Investigators used small plastic medicine cups. Infants randomised to NNS groups had dummies available on trial entry; their use was encouraged during tube feeds and when the infant was restless. Infants received NNS between feeds when needed.
Outcomes
The 12 studies used a large number of outcomes, but only a few were common among them. In addition, there was considerable variability or lack of reporting in relation to how and when the outcomes were measured.
Bernbaum 1983 reported on intraoral negative (sucking) pressures, sucking patterns, daily caloric intake, anthropometric measures (weight, length, and head circumference), gastrointestinal transit time, frequency of bowel movements, time taken until first five bottle feeds were achieved, time to reach 2000 grams weight, days for transition from partial to full oral feeds, and length of hospital stay. Collins 2004 reported on the proportion of infants fully breastfeeding (compared with partially and not) at discharge, days from birth to full breastfeeding, and length of hospital stay.
Ernst 1989 reported on anthropometric measures (weight, length, head and arm circumferences, and subscapular and triceps skin folds), gastrointestinal transit time, description and frequency of stools, amount of aspirated gastric residue, serum protein determinations, energy expenditure, energy and fat excretions/energy expenditure, and total caloric value. Field 1982 reported on number of days of tube feedings, number of tube feeds, weight, length of hospital stay, cost of hospital stay, behavioral assessment, and feeding behaviours, including incidence of regurgitation, volume of formula intake and length of feeding time. Gill 1988 and Gill 1992 reported on behavioral state. Harding 2014a and Harding 2014b reported on time taken to achieve full oral feeding, number of days in hospital, type of sucking pattern, and average age of gestation for oral feeding. They also reported number of re‐admissions, difficulties with oral feeding, and receptive and expressive language ratings at follow‐up. Kanarek 1992 reported on gastrin, motilin, insulin, and insulin‐like growth factor‐1 concentrations. Lau 2012 reported on days from start to independent oral feedings, feeding performance, volume taken at five minutes, volume taken during entire feeding, and duration of oral feeding. Mattes 1996 reported on anthropometric measurements (body weight, length, head circumference, midarm circumference, triceps skinfold, subscapular skinfold), sucking measures and age at full oral feeds. Moreira 2014 reported on readiness to commence suck feeds, transition time to oral feeding, exclusive maternal breastfeeding, and occurrence of distress signals. Zhang 2014 reported on number of days transitioning from introduction of oral feeding to autonomous oral feeding, rate of milk transfer, feeding proficiency, volume transfer, weight gain, length of hospital stay, behavioral state, episodes of apnoea, bradycardia, and oxygen desaturation.
Excluded studies
See Characteristics of excluded studies.
We excluded a total of 38 studies from the review (Barlow 2014a; Barlow 2014b; Bingham 2003; Burroughs 1981; Burroughs 1978; Cevasco 2005; Corvaglia 2016; Daniels 1988; De Curtis 1986; DiPietro 1994; Gilliam 2011; Jaafar 2011; Kamhawy 2014; Kimble 1992a; Kronborg 2009; Marchini 1987; McCain 1992; McCain 1995; Measel 1979; Miller 1993; Narayanan 1991; Neeley 1979; Orenstein 1988; Paludetto 1984; Paludetto 1986; Pickler 1993; Pickler 1996; Pickler 2004; Pimenta 2008; Sehgal 1990; Song 2014; Standley 2003; Szabo 1985; Widstrom 1988; Woodson 1985; Woodson 1988; Yildiz 2012; Yu 1999). The reason for exclusion in all cases was because they did not meet one or more of the inclusion criteria.
Risk of bias in included studies
Generally, the trials poorly described the randomisation methods, allocation concealment and blinding of personnel and outcome assessors. We present details of the methodological quality assessments in the Characteristics of included studies table. We completed a 'Risk of bias' table for each eligible study, and our overall assessment of risk of bias using a 'Risk of bias' graph (Figure 2) and 'Risk of bias' summary (Figure 3).
2.
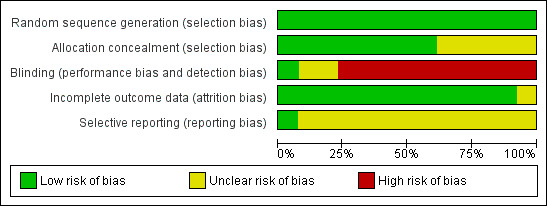
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
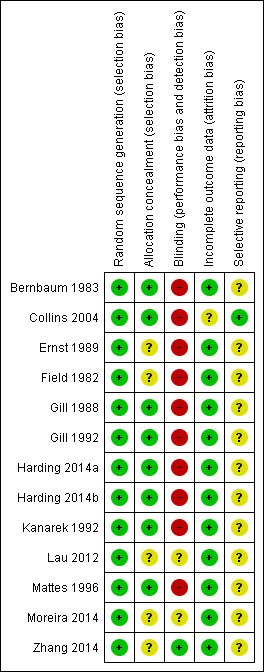
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven of the 12 RCTs were at low risk of bias for allocation concealment (Bernbaum 1983; Collins 2004; Gill 1988; Gill 1992; Harding 2014a; Kanarek 1992; Mattes 1996).
Blinding
Only one study was at low risk of bias for blinding of participants or personnel to the intervention and blinding of outcome assessors (Zhang 2014).
Incomplete outcome data
All 12 included studies were at low risk of bias for incomplete outcome data. Non‐adherence to the intervention was high in Collins 2004. Of the infants randomised to no dummy, 31% (47/152) had a dummy introduced, usually because the baby was unsettled (37%, 14/38) or caregivers wanted to teach the baby to suck (29%, 11/38). We analysed data as intention‐to‐treat.
Selective reporting
Protocols (or trials registration material) were unavailable for the majority of included studies.
Effects of interventions
See: Table 1
Primary outcomes
Time (days) taken to achieve exclusive oral feeding
Three studies reported on transition from gavage to full oral feeding (days) (Bernbaum 1983; Field 1982; Moreira 2014). We were able to include two studies in the meta‐analysis (Bernbaum 1983; Field 1982), and we found a statistically significant reduction in transition from gavage to full oral feeding in the NNS group (MD −5.51 days, 95% CI −8.20 to −2.82; N = 87; I2 = 0%; Analysis 1.1; low‐quality evidence).
1.1. Analysis.
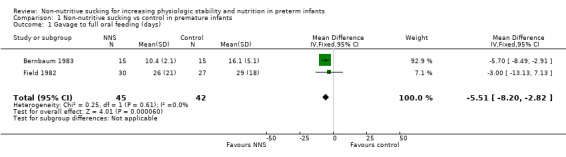
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 1 Gavage to full oral feeding (days).
Moreira 2014 also reported on the transition from gavage feeding to bottle feeding, finding a significant decrease in transition time in the group receiving NNS prior to gavage feeding compared to the control group (3 days versus 5 days, P = 0.001).
Lau 2012 and Zhang 2014 reported on transition from start of oral feeding to full oral feeding and found a statistically significant reduction in NNS group (during, and before gavage feeding) compared to the control group (MD −2.15 days, 95% CI −3.12 to −1.17; N = 100; Analysis 1.2; very low‐quality evidence); however, the I2 value of 59% indicated moderate heterogeneity.
1.2. Analysis.
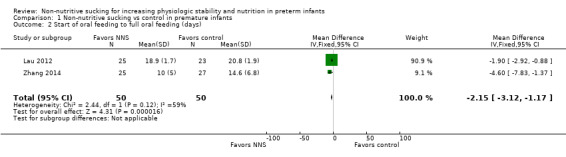
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 2 Start of oral feeding to full oral feeding (days).
Collins 2004 reported on days from birth to full breastfeeding and found no difference between the NNS and no NNS groups (MD −1.00 days, 95% CI −6.71 to 4.71; N = 303; Analysis 1.3; low‐quality evidence). The trial defined breastfeeding as mother's milk given by direct breastfeeding or other feeding device.
1.3. Analysis.
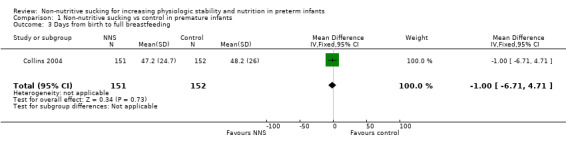
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 3 Days from birth to full breastfeeding.
Full breastfeeding at discharge
Collins 2004 reported on the proportion of infants fully breastfeeding at discharge and found no difference between the NNS and no NNS groups (typical RR 1.08, 95% CI 0.88 to 1.33; typical RD 0.04, 95% CI −0.07 to 0.16, N = 303; Analysis 1.4; low‐quality evidence). The trial defined breastfeeding as mother's milk given by direct breastfeeding or other feeding device.
1.4. Analysis.
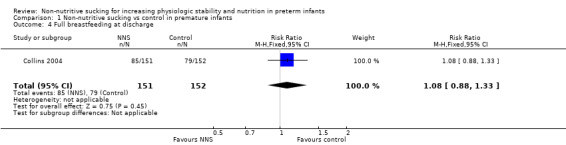
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 4 Full breastfeeding at discharge.
Moreira 2014 also reported no statistical difference between the the NNS and control groups in rates of exclusive maternal breastfeeding on hospital discharge (P = 0.41).
Length of hospital stay (days)
Five trials examined the effect of NNS on length of hospital stay (in days) and contributed data to the meta‐analysis (Bernbaum 1983; Collins 2004; Field 1982; Harding 2014a (Harding 2014b); Zhang 2014). These trials used NNS before or during gavage feeding. Meta‐analysis showed a statistically significant shorter length of hospital stay for infants in the NNS compared to the control infants (MD −4.59 days, 95% CI −8.07 to −1.11; N = 501; Analysis 1.5; low‐quality evidence).The I2 statistic of 19% indicated minimal heterogeneity.
1.5. Analysis.
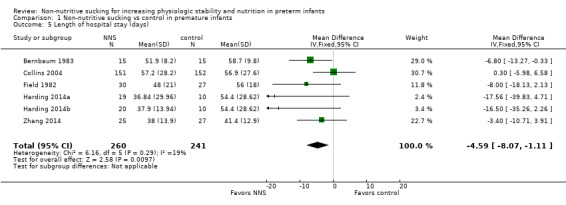
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 5 Length of hospital stay (days).
Weight gain (grams/day)
Five trials reported the effect of NNS on weight gain (Bernbaum 1983; Ernst 1989; Field 1982; Mattes 1996; Zhang 2014). We could include three randomised trials in the meta‐analysis (Ernst 1989; Field 1982; Mattes 1996), which showed no significant difference between the NNS and control groups (MD −1.57 grams/day, 95% CI −3.50 to 0.37; N = 103; Analysis 1.6; low‐quality evidence) and with an I2 statistic of 0% indicating no heterogeneity.
1.6. Analysis.
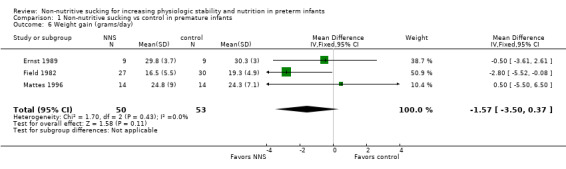
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 6 Weight gain (grams/day).
Bernbaum 1983 found a significant difference in weight gain favouring the NNS (during gavage feeding) group by the second week, and the difference remained significant throughout the six‐week study period. We did not include this study in the meta‐analysis because the standard deviations were unavailable from the authors. Zhang 2014 reported the average weight gain rate (%) and found no difference in weight at independent oral feeding between the NNS, NNS plus oral stimulation, and control groups. Based on the results of the studies, there is no clear benefit of NNS with respect to weight gain.
Secondary outcomes
Time (days) spent in NICU
Time spent in NICU was not reported in any of the included studies.
Age of infant at full oral feeding
Lau 2012 found no significant difference between the NNS (not directly related to feeding) and control groups for postmenstrual age at full oral feeding (MD −0.10 days, 95% CI −0.36 to 0.16; N = 48; Analysis 1.7).
1.7. Analysis.
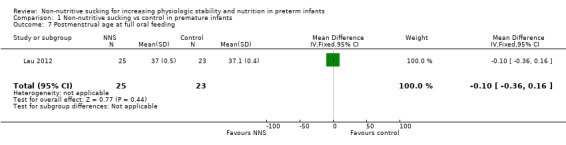
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 7 Postmenstrual age at full oral feeding.
Mattes 1996 similarly found no difference between the NNS (during gavage feeding) and control groups for postconceptual age at full oral feeds (MD −1.70 days, 95% CI −46.06 to 42.66; N = 28; Analysis 1.8; low‐quality evidence).
1.8. Analysis.
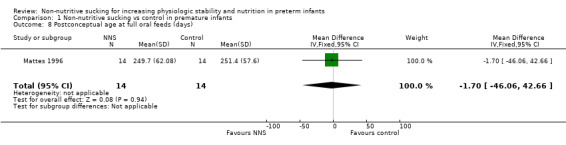
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 8 Postconceptual age at full oral feeds (days).
Episodes of bradycardia
Zhang 2014 reported no significant difference in bradycardia between the NNS and control groups. No data was provided by the authors.
Episodes of oxygen desaturation
Zhang 2014 reported no significant difference in oxygen desaturations between the NNS and control groups. No data was provided by the authors.
Behavioral state
Four studies reported on behavioral state; however, we were unable to combine the results due to the method of reporting and therefore report them in narrative form. Zhang 2014 used the Anderson 12‐level Behavioral State Scale and reported no difference in behavioral state between NNS (given before gavage feedings) and control groups. Field 1982 used the Brazelton Neonatal Behavioral Assessment Scale (NBAS) and found that NNS during gavage feeding had no effect on behavioral state. Gill 1988 used the Anderson 12‐level Behavioral State Scale and reported that the most frequent transition was from quiet sleep to drowsy for NNS (before bottle feeding) and from quiet sleep to restless awake in the control group. Gill 1992 also used the Anderson 12‐level Behavioral State Scale and found sleep states occurred more frequently in the NNS (before bottle feeding) group, and restless states were twice as frequent in the control group.
Intestinal transit time
Bernbaum 1983 reported on intestinal transit time and found that the NNS group (during gavage feeding) had a significantly shorter intestinal transit time compared to the control group (MD −10.50 hours, 95% CI −13.74 to −7.26; N = 30; Analysis 1.9; very low‐quality evidence).
1.9. Analysis.
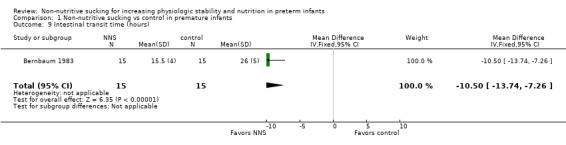
Comparison 1 Non‐nutritive sucking vs control in premature infants, Outcome 9 Intestinal transit time (hours).
Ernst 1989 reported no effect of NNS on gastric emptying (during and after gavage feeding), but no data were available for this trial. Kanarek 1992 studied the effect of NNS in gavage‐fed infants on specific hormones and found NNS (during and after gavage feeding) to have no apparent effect on the blood concentrations of motilin, gastrin, insulin, or insulin‐like growth factor‐1 three days after commencing feeds.
Neurodevelopmental outcomes
No studies reported on neurodevelopmental outcomes.
Discussion
Summary of main results
Meta‐analysis demonstrated a significant effect favouring NNS on transition from gavage to full oral feeding (days), transition from start of oral feeding to full oral feeding (days), the length of hospital stay (days) and intestinal transit time. We found no significant effect for NNS on weight gain. One study found that NNS (during gavage feeding) significantly shortened intestinal transit time. Other individual studies demonstrated no clear positive effect of NNS on age of infant at full oral feeds, days from birth to full breastfeeding, rates and proportion of infants fully breastfeeding at discharge, episodes of bradycardia, and episodes of oxygen desaturation. The evidence for a positive effect on infant behavioral state is inconclusive, with two studies reporting that NNS had no effect on behavioral state and two studies reporting positive effects on sleep states. One study reported that NNS had no apparent effect on motilin, gastrin, insulin, or insulin‐like growth factor‐1. None of the studies reported any negative outcomes.Meta‐analysis demonstrated a significant effect of NNS on transition from gavage to full oral feeding (days), transition from start of oral feeding to full oral feeding (days), and the length of hospital stay (days). Meta‐analysis revealed no significant effect of NNS on weight gain. Individual studies demonstrated no clear positive effect of NNS on age of infant at full oral feeds, days from birth to full breastfeeding, rates and proportion of infants fully breastfeeding at discharge, episodes of bradycardia or episodes of oxygen desaturation. The evidence for positive effect on infant behavioral state is not consistent, with two studies reporting that NNS had no effect on behavioral state and two studies reporting positive effects on sleep states. One study found that NNS decreased intestinal transit time, and one study reported that NNS had no apparent effect on motilin, gastrin, insulin, or insulin‐like growth factor‐1. None of the studies reported any negative outcomes. The studies range in size but are mainly small and often poorly designed, particularly the earlier studies. Readers should interpret study results with caution and consider methodological limitations.
Quality of the evidence
The quality of the evidence ranged from low to very low according to the GRADE approach for all of the major outcomes. Moreover, few trials contributed to meta‐analyses of the primary outcomes, and most of the trials that did not provide numerical data did not demonstrate any effect for NNS.
There were a number of limitations on the presently available evidence.
Design limitations. Because of the nature of the intervention, blinding of the intervention only occurred in 1 of the 12 studies. Likewise, blinding of outcome assessors, although possible, was evident in only one of the studies reviewed.
Outcome variability. Meta‐analysis was limited in this review due to the large variation in outcomes and the limited number of randomised trials that were included in each outcome. Although many of the studies measured similar outcomes, the outcomes were too dissimilar to be included in a meta‐analysis. Alternatively, the authors reported the significance level but did not provide specific data. In addition, the context of the measurement of the outcomes varied greatly among studies. For example, investigators measured outcomes before, during or after gavage feeding; before or after bottle feeding; separately from feeding; with variable timing; or they did not report the timing. Because of the small number of studies in each category that measured comparable outcomes, we combined all studies regardless of context. However, readers should consider these contextual differences when interpreting the results of the review.
Lack of long‐term data. The studies reviewed included no short‐ or long‐term negative outcomes. The outcomes that trialists chose showed either a positive short‐term effect or no effect as a result of NNS. None of included studies reported on long‐term developmental outcomes in the infants.
Despite limiting the included studies to randomised controlled trials and quasi‐randomised trials in this update of the systematic review, the certainty of the conclusions was not strengthened due to the methodological limitations of the included studies. Therefore, readers should interpret the study results with caution.
Agreements and disagreements with other studies or reviews
The findings from this review are consistent with the findings of three earlier systematic reviews (Premji 2000; Schwartz 1987; Steer 1992). Premji 2000 synthesized eight randomized trials and concluded that NNS reduced length of hospitalisation but its effects on sucking response, gastric emptying and weight gain were inconclusive. Schwartz 1987 synthesized five studies of non‐nutritive sucking in preterm infants in a meta‐analysis. The authors concluded that non‐nutritive sucking reduced the time to first bottle feeding and reduced the days of hospitalisation. Outcome data related to weight gain were inconclusive. A meta‐analysis of the non‐nutritive sucking research in preterm infants by Steer, Lucas and Sinclair (Steer 1992) included eight randomised trials. The major outcome variables studied in these trials included weight gain, gastrointestinal transit, readiness for nipple feedings and length of hospitalisation. A lack of blinding to the intervention and/or outcome measurement in all studies affected the methodologic quality of the findings. The authors concluded that in view of the limitations in the available research, there was insufficient beneficial evidence to support the use of non‐nutritive sucking in the management of tube‐fed preterm infants.
Authors' conclusions
Implications for practice.
Meta‐analysis demonstrated a significant effect for NNS on transition from gavage to full oral feeding (days), transition from start of oral feeding to full oral feeding (days), the length of hospital stay (days), and intestinal transit time. We found no significant effect for NNS on weight gain. Single studies demonstrated no clear positive effect for NNS on age of infant at full oral feeds, episodes of bradycardia or episodes of oxygen desaturation. The evidence for positive effect on infant behavioral state was not consistent between trials.
Although a number of outcomes demonstrated no difference with or without NNS, there do not appear to be any short‐term negative effects as a result of this intervention. Based on the available evidence, NNS in preterm infants would appear to have some clinical benefit. Although not specifically studied, NNS does not appear to have any negative short‐term effect. No long‐term data on the effects of NNS are presently available.
Implications for research.
Well‐designed, adequately powered studies using reliable methods of randomisation, concealment of treatment allocation and blinding of the intervention and outcome assessors are needed. In order to facilitate meta‐analysis of these data, future research should involve outcome measures consistent with those used in previous studies. In view of the fact that there are no long‐term data, we recommend further investigation. In addition, published reports should include all relevant data including postnatal age of infants upon enrolment and age of infants when the outcomes are measured.
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2017 | Amended | Author affiliations updated |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 15 August 2016 | New citation required but conclusions have not changed | No change to conclusions. |
| 25 February 2016 | New search has been performed | This review updates the review "Non‐nutritive sucking for promoting physiologic stability in preterm infants" published in the Cochrane Database of Systematic Reviews. Updated search found five new trials. We excluded 14 previously included trials based on study design. |
| 6 April 2010 | New search has been performed | This review updates the review "Non‐nutritive sucking for promoting physiologic stability and nutrition in preterm infants" published in the Cochrane Database of Systematic Reviews (Pinelli 2005). Updated search found no new trials. No changes to conclusions. |
| 28 October 2008 | Amended | Converted to new review format. |
| 14 July 2005 | New search has been performed | This review updates the existing review of "Non‐nutritive sucking for promoting physiologic stability and nutrition in preterm infants" which was published in The Cochrane Library, Issue 3, 2003 (Pinelli 2003). One new trial (Pickler 2004a) was identified and included as a result of the most recent search. |
| 14 July 2005 | New citation required but conclusions have not changed | Substantive amendment |
Acknowledgements
We would like to thank Lisa Jones at Australasian Satellite NRG for her assistance with the 'Summary of findings' table in the second review update.
We would like to thank Colleen Ovelman and Roger Soll at Cochrane Neonatal for their editorial assistance.
We would like to thank Patricia Austin for her assistance in retrieving the references for this review for the first review update.
We would like to thank the review's original authors, Janet Pinelli and Amanda Symington, for their work on the protocol and the previously published versions of the review.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Risk of bias tool
Random sequence generation
Selection bias (biased allocation to interventions) due to inadequate generation of a randomized sequence.
Criteria for a judgement of 'low risk' bias, for example:
referring to a random number table;
using a computer random number generator;
coin tossing;
shuffling cards or envelopes;
throwing dice;
drawing of lots;
minimisation (may be implemented without a random element and this is considered to be equivalent to being random).
Criteria for the judgement of 'high risk'bias:the investigators described a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example:
sequence generated by odd or even date of birth;
sequence generated by some rule based on date (or day) of admission;
sequence generated by some rule based on hospital or clinic record number.
Other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorization of participants, for example:
allocation by judgement of the clinician;
allocation by preference of the participant;
allocation based on the results of a laboratory test or a series of tests;
allocation by availability of the intervention.
Criteria for the judgement of 'unclear risk'of bias: insufficient information about the sequence generation process to permit judgement of 'low risk' or 'high risk'
Allocation concealment
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment.
Criteria for a judgement of 'low risk'of bias. Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
central allocation (including telephone, web‐based and pharmacy‐controlled randomisation);
sequentially numbered drug containers of identical appearance;
sequentially numbered, opaque, sealed envelopes.
Criteria for the judgement of 'high risk'of bias. Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
using an open random allocation schedule (e.g. a list of random numbers);
assignment envelopes were used without appropriate standards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered);
alternation or rotation;
date of birth;
case record number;
any other explicitly unconcealed procedure.
Criteria for the judgement of 'unclear risk'of bias. Insufficient information to permit judgement of 'low risk' or 'high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed.
Blinding
Blinding of participants and personnel
Performance bias due to knowledge of the allocated interventions by participants and personnel during the study.
Criteria for a judgement of 'low risk'of bias. For example:
no blinding or incomplete blinding but the review authors judge that the outcome is not likely to be influence by lack of blinding;
blinding of participants and key study personnel ensured, an unlikely that the blinding could have been broken.
Criteria for the judgement of 'high risk'of bias. Any one of the following:
no blinding or incomplete blinding, and the outcome is likely to be influence by the lack of blinding;
blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding.
Criteria for the judgement of 'unclear risk'of bias. Anyone of the following:
insufficient information to permit judgement of 'low risk' or 'high risk';
the study did not address this outcome.
Blinding of outcome assessment
Detection bias due to knowledge of the allocated interventions by outcome assessors.
Criteria for a judgement of 'low risk 'of bias. For example:
no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding;
blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Criteria for the judgement of 'high risk'of bias. Any one of the following:
no blinding of outcome assessment, and the outcome measurement is likely to be influenced by the lack of blinding;
blinding of outcome assessment but likely that the blinding could not have been broken, and the outcome measurement is likely to influence by lack of blinding.
Criteria for the judgement of 'unclear risk'of bias. Any one of the following:
insufficient information to permit judgement of 'low risk' or 'high risk';
the study did not address this outcome.
Incomplete outcome data
Attrition bias due to amount, nature or handling of incomplete data.
Criteria for a judgement 'low risk'of bias. Any one of the following:
no missing outcome data;
reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias);
missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups;
for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standard difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size;
missing data have been imputed using appropriate methods.
Criteria for the judgement of 'high risk'of bias. Any one of the following:
reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups;
for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk enough to induce clinically relevant bias in intervention effect estimate;
for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size;
'as treated' analysis done with substantial departure of the intervention received from that assigned at randomisation;
potentially inappropriate application of simple imputation.
Criteria for judgement of 'unclear risk'of bias. Any one of the following:
insufficient reporting of attrition/ exclusions to permit judgement of 'low risk' or 'high risk' (e.g. number randomised not stated, no reasons for missing data provided);
the study did not address this outcome.
Selective reporting
Reporting bias due to selective outcome reporting.
Criteria for a judgement of 'low risk'of bias. Any one of the following:
the study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way;
the study protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
Criteria for the judgement of 'high risk'of bias. Any one of the following:
not all of the study's pre‐specified primary outcomes have been reported;
one of more primary outcomes is reported using measurements, analysis methods or subjects of the data (e.g. subscales) that were not pre‐specified;
one of more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as un unexpected adverse effect);
one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis;
the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Criteria for the judgement of 'unclear risk'of bias: insufficient information to permit judgement of 'low risk' or 'high risk'. It is likely that the majority of studies will fall into this category.
Other bias
Bias due to problems not covered elsewhere in the table (only included if authors find other sources of bias to report).
Criteria for a judgement of 'low risk'of bias: the study appears to be free of other sources of bias.
Criteria for a judgement of 'high risk'bias: there is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used;
has been claimed to have been fraudulent;
has some other problem.
Criteria for the judgement of 'unclear risk'of bias: there may be a risk of bias, but there is:
insufficient information to assess whether an important risk of bias exists;
insufficient rationale or evidence that an identified problem will introduce bias.
Data and analyses
Comparison 1. Non‐nutritive sucking vs control in premature infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gavage to full oral feeding (days) | 2 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐5.51 [‐8.20, ‐2.82] |
| 2 Start of oral feeding to full oral feeding (days) | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐2.15 [‐3.12, ‐1.17] |
| 3 Days from birth to full breastfeeding | 1 | 303 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐6.71, 4.71] |
| 4 Full breastfeeding at discharge | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.33] |
| 5 Length of hospital stay (days) | 6 | 501 | Mean Difference (IV, Fixed, 95% CI) | ‐4.59 [‐8.07, ‐1.11] |
| 6 Weight gain (grams/day) | 3 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐1.57 [‐3.50, 0.37] |
| 7 Postmenstrual age at full oral feeding | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.36, 0.16] |
| 8 Postconceptual age at full oral feeds (days) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐46.06, 42.66] |
| 9 Intestinal transit time (hours) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐10.5 [‐13.74, ‐7.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bernbaum 1983.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 30 preterm infants Birth weight < 1500 grams (inclusion criteria) Mean gestational age: 31.5 weeks Mean postnatal age: 10 days Setting: the Children's Hospital of Philadelphia, USA. Inclusion criteria: infants with a birth weight < 1500 grams, with no requirement for surgical intervention, no seizures or CNS haemorrhages, no cardiac or pulmonary diseases and no requirement for further management from the intensive care team at the time nasogastric feeding commenced Exclusion criteria: premature infants that were small for gestational age |
|
| Interventions |
Experimental group: pacifier during gavage feeding. Sucking opportunities were not allowed between feeding periods. The pacifier which was constructed from an unperforated standard‐sized disposable nipple plugged with the plunger of a 20 mL syringe to prevent swallowing of air. Caregivers manipulated it to encourage sucking and placed it so that it remained in the infant's mouth during the entire feeding. All infants were gavage fed until they attained a weight of 1700 grams, at which time they began oral feedings that increased in frequency and amount according to the infant's tolerance. Control: no NNS Sucking opportunities were not allowed between feeding periods in either group. |
|
| Outcomes | Intraoral negative (sucking) pressures measured via a specially designed nipple that was attached to pressure transducer. Sucking patterns: classified into two categories:
Daily caloric intake Anthropometric measures (weight, length and head circumference) Gastrointestinal transit time: determined by the time interval between the nasogastric feed with 125 mg of Carmine red and its appearance in the stools Frequency of bowel movements Time taken until first 5 bottle feeds are achieved Time to reach 2 kg weight Days for transition from partial to full oral feeds Length of hospital stay |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random envelope assignment (information supplied by author) |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation ‐ yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ noBlinding of outcome assessors ‐ not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | We were unable to obtain the study protocol. |
Collins 2004.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: preterm infants Birth weight: 1325 grams ‐ 1508 grams Mean gestational age: 23‐33 weeks Mean postnatal age: not reported Setting: 2 large tertiary hospitals, 54 peripheral hospitals, Australlia Inclusion criteria: women with singleton or twin infants < 34 weeks' gestation who wanted to breast feed Exclusion criteria: infants with congenital abnormalities precluding enteral feeding |
|
| Interventions |
Experimental group: bottle or cup and pacifier ‐ cup or bottle feeding commenced at the discretion of the attending nurse/midwife or neonatologist and occurred when the mother was unavailable to breast feed or when additional milk, given orally, was required after a breast feed. Small plastic medicine cups were used. Infants randomised to NNS groups had dummies available on trial entry; their use was encouraged during tube feeds and when the infant was restless. Control: bottle or cup and no pacifier ‐ for infants who did not receive NNS, alternate soothing methods were promoted (for example, facilitation of hand‐to‐mouth action promoting self‐quieting behavior). |
|
| Outcomes | Proportion of infants fully breastfeeding (compared with partially and not). Full breastfeeding meant that no other types of milk or solids were given except vitamins or minerals. Proportion of infants receiving any breastfeeding (compared with none) on discharge home. Length of hospital stay Prevalence of breastfeeding at 3 and 6 months after discharge Note: breastfeeding defined as mother's milk given by direct breastfeeding or other feeding device. |
|
| Notes | Non‐adherence: of the infants randomised to cup feeding, 56% (85/151) had a bottle introduced, and of the infants randomised to no dummy, 31% (47/152) had a dummy introduced. Reasons dummies were introduced were: baby was unsettled (37%, 14/38) and to teach the baby to suck (29%, 11/38). Data analysed as intention‐to‐treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent researcher developed a separate randomisation schedule for each recruiting hospital by using by using a random number table to select balanced blocks of varying size with stratification for gestation. |
| Allocation concealment (selection bias) | Low risk | Assignments were sealed in sequentially numbered, opaque envelopes. Researchers determined allocation by telephoning an independent ward, available 24 hours a day, within the recruiting hospitals. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 16 infants excluded ‐ cup or bottle and NNS (n = 6), cup or bottle and no NNS (n = 10). Reasons given for attrition. |
| Selective reporting (reporting bias) | Low risk | Reporting of outcomes same as protocol |
Ernst 1989.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 18 preterm infants Mean birth weight: 1185 grams in the control group and 1256 grams in the NNS group Mean gestational age: 29 weeks in the control group and 29 weeks in the NNS group Mean postnatal age: study began on day 1 Setting: Indiana University Medical Center, Indianapolis, IN, USA Inclusion criteria: infants were included if they were very low birth weight babies between 890‐1400 grams with an appropriate weight, length, and head circumference for their gestational age (27 to 30 weeks) Exclusion criteria: infants with seizures, CNS haemorrhage, requirement of surgery, fluid restrictions, prolonged mechanical ventilation, significant supplemental oxygen, discontinued feeding or formula that was different from the routinely used premature feeding formula |
|
| Interventions |
Experimental group: NNS during and after gavage feeding. Pacifier given at the commencement of nasogastric tube feeding and remained in mouth post‐feeding. Total time of pacifier in mouth was 30 min. Movement of the pacifier or stroking of the infants' cheek was used to restimulate non‐nutritive sucking during the 30‐minute period. Control: gavage feeding with no pacifier. Infants in the control group were not allowed to suck on pacifiers between feeding times. Treatment phase of the study concluded when infants weighed approximately 1700 grams. At this point gavage feeding was discontinued and replaced by nipple feeding. Both groups: no pacifier between feedings. Intake was held constant in both groups. |
|
| Outcomes | Anthropometric measures: weight, length, head and arm circumferences, skin folds (subscapular and triceps) obtained weekly. Gastrointestinal transit time: determined using carmine markers that were mixed with initial feeding on day 1 of the study and at weekly intervals. Transit time was determined upon the first appearance when the marker appeared in the stools for the first time. Other measures: description and frequency of stools and amount of aspirated gastric residue Blood samples were obtained individually from each infant on day 1 of the study by venepuncture, and by heel stick weekly for serum protein determinations. Energy expenditure: estimated where cumulative heart rate measurements were correlated with energy expenditure in premature infants. Infants were monitored for 4‐6 consecutive hours during the 72 hour fecal collections. Energy and fat excretions/energy expenditure: determined in a subgroup of 8 baby boys (4 control, 4 NNS). Measured at baseline, and 1 and 2 weeks post‐treatment from 72 hour fecal collections. A second carmine marker was administered to infant boys 72 hours after the first marker so that stool collections exactly corresponded to 72 hours of formula intake. The total caloric value was determined by bomb calorimetry. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were "randomly assigned" to the intervention or treatment group based on sex and birth weight. No further information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome assessors ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained. |
Field 1982.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 57 preterm infants Mean birth weight: 1300 grams Mean gestational age: 32 weeks Mean postnatal age: study began day 1 Setting: neonatal unit, USA Inclusion criteria: birth weight < 1800 grams and < 35 weeks gestational age. Exclusion criteria: major congenital abnormalities, chromosomal abnormalities, oropharyngeal problems, and conditions known to be incompatible with life |
|
| Interventions |
Experimental group: NNS during gavage feeding. Infants were given the pacifiers whether or not they made an effort to suck. Bottle feeding began when infants weighed 1500 grams, and their medical condition was stable Control: no pacifier during gavage feeding Both groups: infants were allowed a pacifier at any other time, but only the treatment babies received the pacifier during gavage feedings. |
|
| Outcomes | Days of tube feedings
Number of tube feeds
Weight gain
Length of hospital stay
Cost of hospital stay Brazelton Neonatal Behavioral Assessment undertaken after each infant was placed in their crib. This was divided into 4 dimensions:
Bottle feeding interactions: were observed when the infant was placed in a crib in a minimal care nursery. Behaviors considered included: looking at the infant, talking to the infant, repositioning the infant and "jiggling" the infant's bottle. To assess feeding performance, trialists coded the incidence of regurgitation and the volume of formula intake and recorded the length of feeding time. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified random sampling technique |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome assessors ‐ unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Gill 1988.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 24 preterm infants Median birth weight: 1270 grams in the NNS group and 1570 grams in the no NNS group Median gestational age: 30.5 weeks in the NNS group and 31.5 weeks in the no NNS group Mean postnatal age: 19 days in the NNS group and 15 days in the no NNS group Setting: neonatal unit in a teaching hospital, USA Inclusion criteria: birth weight < 2000 grams, ≤ 34 weeks gestational age, appropriate weight for gestational age, being fed by gavage, and ready to have a first bottle feeding Exclusion criteria: no infants had intraventricular haemorrhage grades III or IV or congenital or neurological anomalies |
|
| Interventions |
Experimental group: NNS before bottle feeding. NNS was given 5 minutes before bottle feeding every 3 hours for the first 48 hours. Two sizes of 'firm slow‐feed' commercial nipples were used as pacifiers by inserting the distal end of a plastic disposable 3 mL syringe cover into the nipple as a prop and to block air entry. When necessary, the nipple was cut flat on one side to facilitate entry into the infant's mouth. Control: no NNS |
|
| Outcomes | Behavioral state: Anderson Behavioral State Scale: used to assess behavioral state. The assessment began before disturbing the infant for bottle feeding. Each assessment lasted 30 seconds and the highest behavioral state was recorded. After 5‐minute rest a second behavioral state assessment performed. During these 5 minutes infants in the NNS group received NNS. After this period ended both groups were fed based on the individual infant's weight and age. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned by precoded envelope" |
| Allocation concealment (selection bias) | Low risk | "Randomly assigned by precoded envelope" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome assessors ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Protocol not obtained |
Gill 1992.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 42 preterm infants Mean birth weight: 1254 grams in the control group and 1408 grams in the NNS group Mean gestational age: mean gestational age 29.3 weeks in the control group and 30.2 weeks in the NNS group Mean postnatal age: mean postnatal age 25.9 days in the control group and 22.8 days in the NNS group Setting: neonatal unit in a teaching hospital, USA Inclusion criteria: birth weight < 2000 grams, ≤ 34 weeks gestational age, appropriate weight for gestational age, being fed by gavage, and ready to have a first bottle feeding. Exclusion criteria: no infants had intraventricular haemorrhage grades III or IV or congenital or neurological anomalies |
|
| Interventions |
Experimental group: NNS before bottle feeding. The researcher held the commercially made premature‐sized pacifier in the infant's mouth for 10 minutes prior to a scheduled bottle feed over a 2‐3 day period between 6 am and 10 pm Control: no NNS |
|
| Outcomes | Behavioral state: Anderson Behavioral State Scale: used to assess behavioral state. The assessment began before disturbing the infant for bottle feeding. Each assessment lasted 30 seconds and the highest behavioral state was recorded. After 5‐minute rest a second behavioral state assessment performed. During these 5 minutes infants in the NNS group received NNS. After this period ended both groups were fed based on the individual infant's weight and age. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised: "The sample consisted of 42 preterm infants who were randomly assigned by pre‐coded envelope." No further information provided |
| Allocation concealment (selection bias) | Low risk | "42 preterm infants who were randomly assigned by pre‐coded envelope." No further information provided |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome assessors ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Harding 2014a.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 59 preterm infants Mean birth weight: 1651 grams Mean gestational age: 32.53 weeks Mean postnatal age: not reported, but infants were included in the study when they started to show oral readiness Setting: neonatal unit, UK Inclusion criteria: preterm Exclusion criteria: congenital disorders, intraventricular haemorrhages grade III or IV, severe respiratory problems or necrotizing enterocolitis |
|
| Interventions | Experimental group 1: NNS before gavage feeding (n = 19). Parents were encouraged to use a pacifier to elicit 3 sequential sucks and to encourage sequential sucking for a minimum of 5 minutes. Infants received the intervention after they started showing signs of oral readiness. The intervention was provided for a minimum of 3 days until they were taking all of their feeds orally. Control: no NNS (n = 10). Infants received the intervention after they started showing signs of oral readiness. The intervention was provided for a minimum of 3 days until they were taking all of their feeds orally |
|
| Outcomes | Time taken to achieve full oral feeding Number of days in hospital Type of sucking pattern using the Neonatal Oral Motor Schedule (NOMAS) Average age of gestation for oral feeding |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | "A computer generated randomisation assigned infants to one of three groups" |
| Blinding (performance bias and detection bias) All outcomes | High risk | "[R]andomzed non‐blinded controlled study" Blinding of intervention ‐ no Blinding of outcome assessors ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A final total of 60 infants were recruited, and 59 infants completed the offered intervention. At 6 months follow‐up, data were gathered on 56 infants. 7% dropout rate |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Harding 2014b.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 59 preterm infants (intervention groups I and II and control) Mean birth weight: 1651 grams Mean gestational age: 31.60 weeks Mean postnatal age: postnatal age not reported, but infants were included in the study when they started to show oral readiness Setting: neonatal unit, UK Inclusion criteria: preterm Exclusion criteria: congenital disorders, intraventricular haemorrhages grade III or IV, severe respiratory problems or necrotizing enterocolitis |
|
| Interventions |
Experimental group 2: NNS during gavage feeding (n = 20). Parents were encouraged to use a pacifier to elicit 3 sequential sucks and to encourage sequential sucking for a minimum of 5 minutes. Infants received the intervention after they started showing signs of oral readiness. The intervention was provided for a minimum of 3 days until they were taking all of their feeds orally. Description of the pacifier used for the study not provided Control: no NNS (n = 10) |
|
| Outcomes | Time taken to achieve full oral feeding Number of days in hospital Type of sucking pattern using the Neonatal Oral Motor Schedule (NOMAS) Average age of gestation for oral feeding |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | "A computer generated randomisation assigned infants to one of three groups" |
| Blinding (performance bias and detection bias) All outcomes | High risk | "[R]andomized non‐blinded controlled study" Blinding of intervention ‐ no Blinding of outcome assessors ‐ no |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A final total of 60 infants were recruited, and 59 infants completed the offered intervention. At 6 months follow‐up, data were gathered on 56 infants. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Kanarek 1992.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 21 preterm infants Mean birth weight: 1450 grams in the control group and 1320 grams in the NNS group Mean gestational age: 31.8 weeks in the control group and 31.0 weeks in the NNS group Mean postnatal age: day 1 Setting: neonatal unit NICU, Tampa General Hospital, USA Inclusion criteria: appropriate weight for gestational age, free from major congenital abnormalities, perinatal asphyxia, infection and respiratory distress Exclusion criteria: no other criteria reported |
|
| Interventions |
Experimental group: NNS during and after gavage feeding Infants were given a commercial pacifier beginning on the first day of life, during and after all feedings and when they were awake. Blood measurements were taken before and 72 hours after the initiation continuous gavage feedings. In infants who were bolus‐fed, the second specimen was obtained 20 minutes after the feed. Control: no NNS but infants were stroked when restless |
|
| Outcomes | Blood specimens:
Specimens were obtained by venepuncture at 08:00‐09:00 just before feeding was initiated. Venepuncture was performed by skilled research nurses, and efforts were made to calm the infants during the procedure to minimize the release of catecholamines. Feedings were then commenced either via bolus (every 3 hours) or continuous feeding via nasogastric tube. For infants who were bolus fed, a second blood specimen was taken 20 minutes after the feed. All other infant had their blood specimens collected 72 hours later. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[W]e conducted a controlled, randomized study in healthy premature infants receiving enteral feedings with and without NNS." No further information provided |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation ‐ yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ no Blinding of outcome assessors ‐ unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Lau 2012.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 88 preterm infants Mean birth weight: 1121 grams in the control group and 1076 grams in the NNS group Mean gestational age: 28.1 weeks in the control group and 28.1 weeks in the NNS group Mean postnatal age: mean postmenstrual age 38.8 weeks in the control group and 39.0 weeks in the NNS group Setting: neonatal unit, Texas Children's Hospital, USA Inclusion criteria: only infants identified as 'feeders and growers' with a primary diagnosis of prematurity Exclusion criteria: gastrointestinal complications, congenital anomalies or chronic medical conditions, for example, intraventricular haemorrhage III and IV (4), periventricular leukomalacia, necrotizing enterocolitis and bronchopulmonary dysplasia. |
|
| Interventions |
Experimental groups: NNS not directly related to feeding The experimental groups included infants who, in addition to the NICU standard care, received a defined non‐nutritive sucking or a swallowing exercise program. The sucking and swallowing exercises were provided by experienced research feeding therapists between, but not within the 30 min prior to oral feedings, 15 minutes per day, 5 days a week and only if infants were clinically stable as per the medical staff's recommendation Experimental group 1: the sucking exercise consisted of active non‐nutritive sucking on the pacifiers routinely used in NICU Experimental group 2: the swallowing exercise consisted of placing a bolus of 0.05–0.2 mL of the type of milk they were receiving at the time, that is, mother's milk or formula, via a 1 mL syringe directly on the medial–posterior part of the tongue approximately at the level of the hard and soft palate junction, close to the site where the bolus rests prior to entering the pharynx. The infants started with 0.05 mL, and the volume was increased in increments of 0.05 mL to a maximum of 0.2 mL until the swallowing reflex was observed or as tolerated. This exercise was provided every 30 seconds over the 15‐minute program or as tolerated. Control: no NNS Data for experimental group 1 and control group reported (n = 48) |
|
| Outcomes | Days from start to independent oral feedings. Feeding performance monitored at 1‐2, 3‐5 and 6‐8 oral feedings per day. Collected measures: Total volume prescribed Volume taken at 5 minutes Volume taken during entire feeding Duration of oral feeding |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Infants were randomized into 3 groups with balanced gestational age and gender distribution." No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention ‐ yes: "A screen around the isolette was placed to blind parents and caregivers to group assignments." Blinding of outcome assessors ‐ unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Eighty‐eight very low birthweight infants were recruited. Eighteen infants did not complete the study owing to medical outliers, death, transfer or discharge on tube feeding. Seventy infants completed the study." 20.5% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Mattes 1996.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 42 preterm infants Mean birth weight: 1321 grams in the control group and 1377 grams in the NNS group Mean gestational age: 30.5 weeks in the control group and 31.1 weeks in the NNS group Mean postnatal age: postconceptual age 33.7 weeks in the control group and 33.6 weeks in the NNS group Setting: NICU from across 4 general hospitals, USA Inclusion criteria: body weight greater than or equal to 1250 grams, gestational age < 34 weeks, growth parameters appropriate for gestational age, Apgar scores greater than 3 at 1 minute and greater than 5 at 5 minutes, no clinical evidence of seizure activity, grade 3 or 4 intraventricular haemorrhages, congential heart disease other than patent ductus arteriosus or peripheral pulmonic stenosis that was haemodynamically significant, respiratory distress syndrome Exclusion criteria: presence or signs of necrotizing enterocolitis, hepatic disorder, congenital infection, metabolic disease or anomalies affecting the central nervous system or gastrointestinal tract; infants with parents or guardians who were concerned that exposure could adversely influence future feeding or pacifier preference |
|
| Interventions |
Experimental group 1: infants were provided with a sweetened edible pacifier for a 4 minute trial followed by a 1 minute rest and another 4 minute trial with a latex pacifier Experimental group 2: infants in the control group received their pacifiers in the reverse order. Infants were initially provided with a latex pacifier for 4 minutes, followed by a 1 minute rest, and a 4 minute trial with a sucrose flavoured pacifier. Sucking was stimulated only at the beginning of the trial by moving the pacifier in and out of the mouth 3 times. Control: no NNS. Maternal heart beat played during tube feeds Data reported for experimental group 2 and control (n = 28) |
|
| Outcomes | Anthropometric measurements
Sucking measures
Age at full oral feeds Sucking measures were evaluated weekly before a midday feeding. Sucking related pressures on the pacifier were translated through a pressure transducer into audio signals via a modified stereo cassette tape deck and recorded. Frequency and strength were used to assess sucking performance |
|
| Notes | Latex pacifier group used as experimental group for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised: "All infants meeting stipulated criteria were recruited at four participating hospitals." No further information provided |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation ‐ yes |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention ‐ noBlinding of outcome assessors ‐ unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Moreira 2014.
| Methods | Randomised controlled trial | |
| Participants |
Participants: 40 preterm infants Mean birth weight: 1256 grams in control group and 1306 grams in NNS stimulation group Mean gestational age: 29.9 weeks in control group and 30.1 weeks in NNS stimulation group Setting: neonatal unit, Hospital de Clínicas da Universidade Federal do Paraná, Brazil Inclusion criteria: birth weight < 1500 grams, gestational age at birth ≤ 32 weeks, 5‐minute Apgar score ≥ 6, clinical (respiratory and haemodynamic) stability on enrolment and during the study, initiation of enteral feeding by oral or nasogastric tube associated or not with parenteral nutrition, and free and informed consent form signed by the parents. Exclusion criteria: grades III/IV intraventricular haemorrhage, clinical instability on enrolment or during the study, including necrotizing enterocolitis, sepsis, bronchopulmonary dysplasia and other clinical respiratory or haemodynamic instabilities, 5‐minute Apgar ≤ 5, presence of genetic syndromes, neurological disorders, as well as head, neck or central nervous system congenital malformations |
|
| Interventions |
Experimental group: infants received a 10‐minute NNS stimulation with a gloved finger before feeding, 3 times a day, 3 times a week, with the newborn on a supine, semiflexed position receiving perioral and oral stimulation Control: no NNS |
|
| Outcomes | Transition time to oral feeds | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Newborns randomly and equally distributed into 2 groups" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention ‐ unclear Blinding of outcome assessors ‐ unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up ‐ yes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
Zhang 2014.
| Methods | Parallel‐group randomised controlled trial | |
| Participants |
Participants: 112 preterm infants (NNS and control) Mean birth weight: 1548 grams Mean gestational age: 30.9 weeks Mean postnatal age: postnatal age was not reported but all included infants were born in other hospitals and transported within 24‐48 hours to the NICU Setting: neonatal unit, Children's Hospital of Fudan University, China Inclusion criteria: born at 29 to 34 weeks gestation, weight appropriate for gestational age, Apgar scores of > 3 at 1 minute and > 5 at 5 minutes, received all feedings by tube Exclusion criteria: congenital abnormalities (oral, heart etc.) and infants who developed chronic medical complications during NICU admission such as intraventricular haemorrhage grades III and IV, bronchopulmonary dysplasia, or necrotizing enterocolitis |
|
| Interventions | Infants were randomised to 4 groups (N = 112) Experimental group 1: NNS before gavage feeding Infants were allowed to suck on pacifiers for 5 minutes, 7‐8 times a day. The pacifier was placed in the infant's mouth whether or not they made an attempt to suck; however, where necessary the pacifier would be manipulated by the nurse to encourage sucking. NNS and oral stimulation group: the combined group was administered by the oral motor program including oral stimulation for 12 minutes and NNS for 3 minutes, once a day Experimental group 2: oral stimulation group ‐ consisted of stroking the cheeks, lips, gums, and tongues for 12 minutes All interventions took place 30 minutes prior to feeding. All interventions started 48 hours after discontinuation of nasal continuous positive airway pressure and were continued until the newborn began exclusively oral diet Experimental group 3: NNS and oral stimulation ‐ The combined group was administered by the oral motor program including oral stimulation for 12min and NNS for 3min, once a day. All infants in the three groups received the interventions 30 min before the beginning of scheduled feeding Control: no NNS Data only reported for the NNS group (experimental 1) and control group (n = 55) |
|
| Outcomes |
Primary outcomes: Transition time: defined as the number of days needed from introduction of oral feeding to autonomous oral feeding Age and weight: the infants postmenstrual age and weight were recorded at the commencement of oral feeds and when autonomous oral feeding was established Secondary outcomes: Rate of transfer Feeding proficiency Volume transfer Length of stay Behavioral state: Anderson Behavioral State Scale taken at the start of each feeding session Episodes of apnoea, bradycardia, and oxygen desaturation during the final feeding session were recorded. |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "infants were randomized into one of four groups using a stratified block randomisation." No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Blinding of randomisation ‐ yes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention ‐ yes Blinding of assessors ‐ yes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One infant did not complete the study in the control group and 2 infants did not complete the study in the NNS group. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not obtained |
CNS: central nervous system;NICU: neonatal intensive care unit; NNS: non‐nutritive sucking.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Barlow 2014a | Study related to development, not effect of non‐nutritive sucking |
| Barlow 2014b | Study related to development, not effect of non‐nutritive sucking |
| Bingham 2003 | Non‐nutritive sucking is not the intervention |
| Burroughs 1978 | Non‐RCT: before‐and‐after design |
| Burroughs 1981 | Not experimental or quasi‐experimental |
| Cevasco 2005 | No outcomes of clinical relevance Pacifier activated lullaby |
| Corvaglia 2016 | Outcome of interest was beyond the scope of this review (GORD) |
| Daniels 1988 | Not experimental or quasi‐experimental |
| De Curtis 1986 | Cross‐over trial |
| DiPietro 1994 | Cross‐over trial |
| Gilliam 2011 | Term infants |
| Jaafar 2011 | Term infants |
| Kamhawy 2014 | Non‐RCT, alternate assignment |
| Kimble 1992a | Term infants No clinical outcomes Not experimental or quasi‐experimental |
| Kronborg 2009 | Term infants |
| Marchini 1987 | Term infants |
| McCain 1992 | Cross‐over trial |
| McCain 1995 | Cross‐over trial |
| Measel 1979 | Sequential allocation of infants into groups |
| Miller 1993 | Term infants |
| Narayanan 1991 | No intervention |
| Neeley 1979 | Term infants |
| Orenstein 1988 | Term infants |
| Paludetto 1984 | Not experimental or quasi‐experimental |
| Paludetto 1986 | Not experimental or quasi‐experimental |
| Pickler 1993 | Cross‐over trial |
| Pickler 1996 | Cross‐over trial |
| Pickler 2004 | Cross‐over trial |
| Pimenta 2008 | Intervention NNS and oral stimulation |
| Sehgal 1990 | Method of allocation of infants into groups uncertain |
| Song 2014 | Study related to development, not effect of non‐nutritive sucking |
| Standley 2003 | Non‐nutritive sucking not the primary intervention |
| Szabo 1985 | Cross‐over trial |
| Widstrom 1988 | Cross‐over trial |
| Woodson 1985 | Term infants Not experimental or quasi‐experimental |
| Woodson 1988 | Non‐RCT cross‐over |
| Yildiz 2012 | Non‐RCT |
| Yu 1999 | Cross‐over trial |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
IRCT201106062324N8.
| Trial name or title | The impact of non‐nutritive sucking with pacifier and finger on weight gaining, initiation of breastfeeding and discharge of preterm newborns |
| Methods | Parallel‐group, randomised controlled trial |
| Participants | Target number of required participants: 90 healthy preterm newborns Age: between 30‐34 weeks Weight: appropriate for gestational age Maximum age: 1 year Sex: boys and girls Setting: neonatal intensive care unit of one University hospital in Shiraz City Inclusion criteria: gestational age between 30‐34 weeks; birth weight appropriate for gestational age (AGA); Apgar score at 1 and 5 min after birth ≤ 7; Normal neonate (no evidence of congenital anomaly such as respiratory and heart problems, seizure, CNS haemorrhage …); non‐addicted mothers; no need for phototherapy; no need for complete parental feeding. Exclusion criteria: unwillingness of the parents for complete the study; need for transfer to other wards or other hospitals; received drugs that affect central nerves system for example: phenobarbital and phenytoin; need for surgical and medical interventions during study; need for phototherapy during study; received other supportive intervention such as touch therapy, kangaroo care, music therapy and other intervention during study; abnormal physiologic response such as heart rate above 200 beat per min or under 100 beat per min, deceleration of oxygen saturation level under 80% during intervention; missing of intervention at least twice during study |
| Interventions | Experimental group: non‐nutritive sucking with pacifier and finger Control: no intervention will be done in control group |
| Outcomes | Primary outcome measures: weight (measured daily) Secondary outcome measures: initiation of breastfeeding (the first time the neonate will be able to suck and breast feed); time of discharge |
| Starting date | 23 October 2011 |
| Contact information | Maryam Kehavarz Tehran University of Medical Sciences Islamic Republic of Iran Keshavarz_m@tums.ac.ir; m_keshir@yahoo.com |
| Notes | — |
IRCT2013120815458N2.
| Trial name or title | The effect of sucking in preterm infants without give milk that admitted to hospital |
| Methods | Parallel‐group randomised controlled trial |
| Participants | Target number of required participants: 69 Maximum age: 1 year Sex: boys and girls Setting: Shariati Hospital and Children's Hospital, Iran Inclusion criteria: informed consent of the mother, gestational age between 32‐26 weeks and birth weight less than 1500 grams. Exclusion criteria: neonates with severe asphyxia, malformations, cranial haemorrhage grade III or IV infections at the onset or during the study and very ill newborns |
| Interventions | Experimental group: neonates will be stimulated 15 min twice a day for 10 days and the breastfeeding will be done free from milk or a pacifier until the start of oral feeding in preterm infants. Oral intake will begin when neonates are clinically and haemodynamically stable. Depending on the physical conditions, oral feeding for the newborn will be done 20 mL per kg per day. Control: neonates will not be stimulated with breastfeeding or pacifier. Oral intake will begin when neonates are clinically and haemodynamically stable. Depending on the physical conditions, oral feeding for the newborn will be done 20 mL per kg per day. |
| Outcomes | Primary outcome measures: weight: taken after feeding (daily) and at discharge; head circumference (weekly) and at discharge; RASA microbalance Secondary outcome measure: duration of hospital stay |
| Starting date | 9 June 2012 |
| Contact information | Dr Rakhshaneh Goodarzi Hormozgan University of Medical sciences Islamic Republic of Iran Rgoodarzi@oldhums.ac.ir |
| Notes | — |
IRCT201501205163N2.
| Trial name or title | Effectiveness of non‐nutritive sucking performed by the mother on the physiological indexes and full oral feeding in premature infants in the neonatal intensive care unit |
| Methods | Parallel‐group, randomised controlled trial |
| Participants | Target number of required participants: 80 Sex: boys and girls Setting: Tehran University Medical sciences Inclusion criteria: neonate: gestational age 26‐34 weeks; weight 1000‐2500 grams; score of Apgar at 1 and 5 min above 7; normal without congenital, respiratory or heart disease; did not have respiratory assistant; did not use any drug that can effect respiratory and nervous system; did not use other kind of complementary care; have normal physiologic signs. Mother: does not use drugs or alcohol; can do 2 interventions a day; does not have scare on finger. Exclusion criteria: mother does not want to do intervention; neonate cannot tolerate intervention or will change situation |
| Interventions | Experimental group: non‐nutritive sucking by sucking of the mother's finger Control: routine nursing intervention |
| Outcomes | Primary outcome measures: physiologic signs: before, during and after intervention; oxygen saturation: via pulse oximetry before, during and after intervention; vital signs: before during and after trial Secondary outcome measures: complete feeding by mouth, oral feeding: total milk (mls) consumed. |
| Starting date | 22 January 2015 |
| Contact information | Akram Sadat Sadat Hoseini Tehran University Medical Sciences Islamic Republic of Iran ashoseini@tums.ac.ir |
| Notes | — |
Differences between protocol and review
We reported several outcomes that were not predefined, including some new physiological outcomes.
We include only randomised and quasi‐randomised controlled trials in this update. We have now excluded non‐experimental designs and cross‐over trials to increase the validity of the review. Most of the cross‐over studies we found did not report whether there was a washout period, and as an intervention can have a lasting carry‐over effect that compromises entry to subsequent periods of the trial, we excluded them from the review.
We added the methodology and plan for the 'Summary of findings' tables and GRADE recommendations, which were not included in the original protocol or the previous versions of the review.
Contributions of authors
Janet Pinelli (JP) and Amanda J Symington (AS) wrote the original review and updated it in 2001, 2003, and 2005.
The Cochrane Neonatal Review Group staff (Yolanda Montagne, Diane Haughton, and Roger Soll) conducted the April 2010 update centrally. JP reviewed and approved that update.
Jann Foster, Kim Psaila and Tiffany Patterson carried out the present (2016) update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
Jann Foster: none known.
Kim Psaila: none known.
Tiffany Patterson: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Bernbaum 1983 {published data only}
- Bernbaum JC, Pereira GR, Watkins JB, Peckham GJ. Nonnutritive sucking during gavage feeding enhances growth and maturation in premature infants. Pediatrics 1983;71:41‐5. [PubMed] [Google Scholar]
Collins 2004 {published data only}
- Collins CT, Ryan P, Crowther C, McPhee AJ, Paterson S, Hiller JE. Effect of bottles, cups, and dummies on breast feeding in preterm infants: a randomised controlled trial. BMJ Online 2004;329:193. [DOI: 10.1136/bmj.38131.675914.55] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 1989 {published data only}
- Ernst JA, Rickard KA, Neal PR, Yu PL, Oei, TO, Lemons, JA. Lack of improved growth outcome related to nonnutritive sucking in very low birth weight premature infants fed a controlled nutrient intake: A randomized prospective study. Pediatrics 1989;83:706‐16. [PubMed] [Google Scholar]
Field 1982 {published data only}
- Field T, Ignatoff E, Stringer S, Brennan J, Greenberg R, Widmayer S, Anderson GC. Nonnutritive sucking during tube feedings: Effects on preterm neonates in an intensive care unit. Pediatrics 1982;70:381‐4. [PubMed] [Google Scholar]
Gill 1988 {published data only}
- Gill NE, Behnke M, Conlon M, McNeely JB, Anderson GC. Effect of nonnutritive sucking on behavioral state in preterm infants before feeding. Nursing Research 1988;37:347‐50. [PubMed] [Google Scholar]
Gill 1992 {published data only}
- Gill NE, Behnke M, Conlon M, Anderson GC. Nonnutritive sucking modulates behavioral state for preterm infants before feeding. Scandinavian Journal of Caring Sciences 1992;6:3‐7. [DOI] [PubMed] [Google Scholar]
Harding 2014a {published data only}
- Harding C, Frank L, Someren V, Hilari K, Botting N. How does non‐nutritive sucking support infant feeding?. Infant Behavior & Development 2014;37(4):457‐64. [PUBMED: 24974134] [DOI] [PubMed] [Google Scholar]
Harding 2014b {published data only}
- Harding C, Frank L, Someren V, Hilari K, Botting N. How does non nutritive sucking support infant feeding. Infant Behaviour and Development 2014;37(4):457‐64. [DOI] [PubMed] [Google Scholar]
Kanarek 1992 {published data only}
- Kanarek KS, Shulman D. Non‐nutritive sucking does not increase blood levels of gastrin, motilin, insulin and insulin‐like growth factor 1 in premature infants receiving enteral feedings. Acta Paediatrica 1992;81:974‐7. [DOI] [PubMed] [Google Scholar]
Lau 2012 {published data only}
- Lau C, Smith EO. Interventions to improve the oral feeding performance of preterm infants. Acta Paediatrica 2012;101(7):e269‐74. [DOI] [PubMed] [Google Scholar]
Mattes 1996 {published data only}
- Mattes RD, Wager‐Page S, Beauchamp G, Bernbaum J, Stallings V, Pereira G, et al. Effects of sweet taste stimulation on growth and sucking in preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1996;25:407‐14. [DOI] [PubMed] [Google Scholar]
Moreira 2014 {published data only}
- Moreira CMD, Cavalcante‐Silva RPGV, Miyaki M, Ide Fujinaga C. Effects of nonnutritive sucking stimulation with gloved finger on feeding transition in very low birth weight premature infants. Revista CEFAC 2014;16(4):1187‐92. [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y, Lyu T, Hu X, Shi P, Cao Y, Latour JM. Effect of nonnutritive sucking and oral stimulation on feeding performance in preterm infants: a randomized controlled trial. Pediatric Critical Care Medicine 2014;15(7):608‐14. [PUBMED: 24977689] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Barlow 2014a {published data only}
- Barlow S, Lee J, Wang J, Oder A, Oh H, Hall S, et al. Effects of oral stimulus frequency spectra on the development of non‐nutritive suck in preterm infants with respiratory distress syndrome or chronic lung disease, and preterm infants of diabetic mothers. Journal of Neonatal Nursing 2014;20(4):178‐88. [PUBMED: 25018662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 2014b {published data only}
- Barlow SM, Jegatheesan P, Weiss S, Govindaswami B, Wang J, Lee J, et al. Amplitude‐integrated EEG and range‐EEG modulation associated with pneumatic orocutaneous stimulation in preterm infants. Journal of Perinatology 2014;34(3):213‐9. [PUBMED: 24310443] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bingham 2003 {published data only}
- Bingham PM, Abassi S, Sivirei E. A pilot study of milk odor effect on non‐nutritive sucking by premature newborns. Archives of Pediatric and Academic Medicine 2003;157:72‐5. [PubMed] [Google Scholar]
Burroughs 1978 {published data only}
- Burroughs AK, Asonye UO, Anderson‐Shanklin GC, Vidyasagar D. The effect of nonnutritive sucking on transcutaneous oxygen tension in noncrying, preterm neonates. Research in Nursing & Health 1978;1:69‐75. [DOI] [PubMed] [Google Scholar]
Burroughs 1981 {published data only}
- Burroughs AK, Anderson GC, Patel MK, Vidyasagar D. Relation of nonnutritive sucking pressures to tcPO2 and gestational age in preterm infants. Perinatology‐Neonatology 1981;2:54‐62. [Google Scholar]
Cevasco 2005 {published data only}
- Cevasco AM. Effects of the pacifier activated lullaby on weight gain of premature infants. Journal of Music Therapy 2005;42(2):123‐39. [DOI] [PubMed] [Google Scholar]
Corvaglia 2016 {published data only}
- Corvaglia L, Martini S, Corrado MF, Mariani E, Legnani E, Bosi I, et al. Does the use of pacifier affect gastro‐esophageal reflux in preterm infants?. Journal of Pediatrics 2016;172:205‐8. [DOI: 10.1016/j.jpeds.2016.01.022; PUBMED: S0022‐3476(16)00024‐X] [DOI] [PubMed] [Google Scholar]
Daniels 1988 {published data only}
- Daniels H, Devlieger H, Casaer P, Callens M, Eggermont E. Nutritive and non‐nutritive sucking in preterm infants. Journal of Developmental Psychology 1986;8:117‐21. [PubMed] [Google Scholar]
De Curtis 1986 {published data only}
- Curtis M, McIntosh N, Ventura V, Brooke O. Effect of nonnutritive sucking on nutrient retention in preterm infants. Journal of Pediatrics 1986;109:888‐90. [DOI] [PubMed] [Google Scholar]
DiPietro 1994 {published data only}
- DiPietro JA, Cusson RM, Caughy MO, Fox NA. Behavioral and physiologic effects of nonnutritive sucking during gavage feeding in preterm infants. Pediatric Research 1994;36:207‐14. [DOI] [PubMed] [Google Scholar]
Gilliam 2011 {published data only}
- Gilliam ML. Pacifier use compared with no pacifier use in breastfeeding term infants for increasing duration of breastfeeding. Obstetrics and Gynecology 2011;118(1):164‐5. [DOI] [PubMed] [Google Scholar]
Jaafar 2011 {published data only}
- Jaafar SH, Jahanfar S, Angolkar M, Ho JJ. Pacifier use versus no pacifier use in breastfeeding term infants for increasing duration of breastfeeding. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD007202.pub2] [DOI] [PubMed] [Google Scholar]
Kamhawy 2014 {published data only}
- Kamhawy H, Holditch‐Davis D, Alsharkawy S, AlrafayS, Corazzini K. Non‐nutritive sucking for preterm infants in Egypt. Journal of Obstetric, Gynecologic, and Neonatal Nursing 2014;43(3):330‐40. [DOI] [PubMed] [Google Scholar]
Kimble 1992a {published data only}
- Kimble C. Nonnutritive sucking: Adaptation and health for the neonate. Neonatal Network 1992;11:29‐33. [PubMed] [Google Scholar]
Kronborg 2009 {published data only}
- Kronborg H, Vaeth M. How are effective breastfeeding technique and pacifier use related to breastfeeding problems and breastfeeding duration?. Birth 2009;36(1):34‐42. [DOI] [PubMed] [Google Scholar]
Marchini 1987 {published data only}
- Marchini G, Lagercrantz H, Feuerberg Y, Winberg J, Uvnas‐Moberg K. The effect of non‐nutritive sucking on plasma insulin, gastrin and somatostatin levels in infants. Acta Paediatrica Scandinavica 1987;76:573‐8. [DOI] [PubMed] [Google Scholar]
McCain 1992 {published data only}
- McCain GC. Facilitating inactive awake states in preterm infants: A study of three interventions. Nursing Research 1992;41:157‐60. [PubMed] [Google Scholar]
McCain 1995 {published data only}
- McCain GC. Promotion of preterm infant nipple feeding with nonnutritive sucking. Journal of Pediatric Nursing 1995;10:3‐8. [DOI] [PubMed] [Google Scholar]
Measel 1979 {published data only}
- Measel CP, Anderson GC. Nonnutritive sucking during tube feedings: Effects on clinical course in premature infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1979;8:265‐72. [DOI] [PubMed] [Google Scholar]
Miller 1993 {published data only}
- Miller HD, Anderson GC. Nonnutritive sucking: Effects on crying and heart rate in intubated infants requiring assisted mechanical ventilation. Nursing Research 1993;42:305‐7. [PubMed] [Google Scholar]
Narayanan 1991 {published data only}
- Narayanan I, Mehta R, Choudhury DK, Jain BK. Sucking on the 'emptied' breast: non‐nutritive sucking with a difference. Archives of Disease in Childhood 1991;66:241‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Neeley 1979 {published data only}
- Neeley CA. Effects of non‐nutritive sucking upon the behavioral arousal of the newborn. In: Anderson GC, Raff B editor(s). Newborn Behavioral Organization: Nursing Research and Implications. Birth Defects Original Article Series. Vol. 15, Ann Arbor, MI: University of Michigan, 1979:173‐200. [PubMed] [Google Scholar]
Orenstein 1988 {published data only}
- Orenstein SR. Effect of nonnutritive sucking on infant gastroesophageal reflux. Pediatric Research 1988;24:38‐40. [DOI] [PubMed] [Google Scholar]
Paludetto 1984 {published data only}
- Paludetto, R, Robertson SS, Hack M, Shivpuri CR, Martin RJ. Transcutaneous oxygen tension during nonnutritive sucking in preterm infants. Pediatrics 1984;74:539‐42. [PubMed] [Google Scholar]
Paludetto 1986 {published data only}
- Paludetto R, Robertson SS, Martin RJ. Interaction between nonnutritive sucking and respiration in preterm infants. Biology of the Neonate 1986;49:198‐203. [DOI] [PubMed] [Google Scholar]
Pickler 1993 {published data only}
- Pickler RH, Higgins KE, Crummette BD. The effect of nonnutritive sucking on bottle‐feeding stress in preterm infants. Journal of Obstetric, Gynecologic, and Neonatal Nursing 1993;22:230‐4. [DOI] [PubMed] [Google Scholar]
Pickler 1996 {published data only}
- Pickler RH, Frankel HB, Walsh KM, Thompson NM. Effects of nonnutritive sucking on behavioral organization and feeding performance in preterm infants. Nursing Research 1996;45:132‐5. [DOI] [PubMed] [Google Scholar]
Pickler 2004 {published data only}
- Pickler RH, Reyna BA. Effects of non‐nutritive sucking on nutritive sucking, breathing and behavior during bottle feedings of preterm infants. Advances in Neonatal Care 2004;4:226‐34. [DOI] [PubMed] [Google Scholar]
Pimenta 2008 {published data only}
- Pimenta HP, Moreira MEL, Duarte Rocha A, Gomes SC, Pinto LW, Lucena SL. Effects of non‐nutritive sucking and oral stimulation on breastfeeding rates for preterm, low birth weight infants:a randomized clinical trial. Journal of Pediatrics 2008;84(5):423‐27. [DOI] [PubMed] [Google Scholar]
Sehgal 1990 {published data only}
- Sehgal SK, Prakash O, Gupta A, Mohan M, Anand NK. Evaluation of beneficial effects of nonnutritive sucking in preterm infants. Indian Pediatrics 1990;27:263‐6. [PubMed] [Google Scholar]
Song 2014 {published data only}
- Song D, Jegatheesan P, Weiss S, Govindaswami B, Wang J, Lee J, et al. Modulation of EEG spectral edge frequency during patterned pneumatic oral stimulation in preterm infants. Pediatric Research 2014;75(1‐1):85‐92. [PUBMED: 24129553] [DOI] [PMC free article] [PubMed] [Google Scholar]
Standley 2003 {published data only}
- Standley JM. The effect of music‐reinforced non‐nutritive sucking on feeding rate of premature infants. Journal of Pediatric Nursing 2003;18(3):169‐73. [DOI] [PubMed] [Google Scholar]
Szabo 1985 {published data only}
- Szabo JS, Hillemeier AC, Oh W. Effect of non‐nutritive and nutritive suck on gastric emptying in premature infants. Journal of Pediatric Gastroenterology and Nutrition 1985;4:348‐51. [DOI] [PubMed] [Google Scholar]
Widstrom 1988 {published data only}
- Widstrom AM, Marchini G, Matthiesen AS, Werner S, Winberg J, Uvnan‐Moberg K. Nonnutritive sucking in tube‐fed preterm infants: effects on gastric motility and gastric contents of somatostatin. Journal of Pediatric Gastroenterology and Nutrition 1988;7:517‐23. [DOI] [PubMed] [Google Scholar]
Woodson 1985 {published data only}
- Woodson R, Drinkwin J, Hamilton C. Effects of nonnutritive sucking on state and activity: term‐preterm comparisons. Infant Behaviour and Development 1985;8:435‐41. [Google Scholar]
Woodson 1988 {published data only}
- Woodson R, Hamilton C. The effect of nonnutritive sucking on heart rate in preterm infants. Developmental Psychobiology 1988;21:207‐13. [DOI] [PubMed] [Google Scholar]
Yildiz 2012 {published data only}
- Yildiz A, Arikan D. The effects of giving pacifiers to premature infants and making them listen to lullabies on their transition period for total oral feeding and sucking success. Journal of Clinical Nursing 2012;21(5‐6):644‐56. [DOI] [PubMed] [Google Scholar]
Yu 1999 {published data only}
- Yu M, Chen Y. The effects of nonnutritive sucking on behavioral state and feeding in premature infants before feeding. Nursing Research (China) 1999;7:468‐78. [Google Scholar]
References to ongoing studies
IRCT201106062324N8 {unpublished data only}
- IRCT201106062324N8. The impact of non‐nutritive sucking with pacifier and finger on weight gaining, initiation of breastfeeding and discharge of preterm newborns [Non‐nutritive sucking with pacifier and finger on weight gaining, initiation of breastfeeding and discharge of preterm newborns, in neonatal intensive care unit, Hazrat Zynab hospital, Shiraz]. apps.who.int/trialsearch/Trial2.aspx?trialid=IRCT201106062324N8 First received 17 March 2012.
IRCT2013120815458N2 {unpublished data only}
- IRCT2013120815458N2. The effect of sucking in preterm infants without give milk that admitted to hospital [Effects of non‐nutritive sucking and feeding in preterm infants growth that admitted to Children's Hospital and Shariati in Bandar Abbas in 2012]. apps.who.int/trialsearch/Trial2.aspx?trialid=IRCT2013120815458N2 First received 1 March 2014.
IRCT201501205163N2 {unpublished data only}
- IRCT201501205163N2. Effectiveness of non‐nutritive sucking performed by the mother on the physiological indexes and full oral feeding in premature infants in the neonatal intensive care unit. www.irct.ir/searchresult.php?keyword=&id=5163&number=2&prt=8300&total=10&m=1 First received 10 June 2015.
Additional references
Amaizu 2008
- Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatrica 2008;97(1):61‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 2008
- Barlow SM, Finan DS, Lee J, Chu S. Synthetic orocutaneous stimulation entrains pre‐term infants with feeding difficulties to suck. Journal of Perinatology 2008;28(8):541‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 2009
- Barlow SM. Oral and respiratory control for preterm feeding. Current Opinion in Otolaryngology & Head and Neck Surgery 209;17(3):179‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boiron 2007
- Boiron M, Nobrega L, Roux S, Henrot A, Saliba E. Effects of oral stimulation and oral support on non‐nutritive sucking and feeding performance in preterm infants. Developmental Medicine and Child Neurology 2007;49(6):439‐44. [DOI] [PubMed] [Google Scholar]
Breton 2008
- Breton S, Steinwender S. Timing introduction and transition to oral feeding in preterm infants: current trends and practice. Newborn and Infant Nursing Reviews: NAINR 2008;8(3):153‐9. [Google Scholar]
Chey 1980
- Chey WY, Lee KY. Motilin. Clinics in Gastroenterology 1980;9:645‐56. [PubMed] [Google Scholar]
Crowe 2012
- Crowe L, Chang A, Wallace K. Instruments for assessing readiness to commence suck feeds in preterm infants: effects on time to establish full oral feeding and duration of hospitalisation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005586.pub3] [DOI] [PubMed] [Google Scholar]
Dodrill 2008
- Dodrill P, McMahon S, Donovan T, Cleghorn G. Current management of transitional feeding issues in preterm neonates born in Queensland, Australia. Early Human Development 2008;84(10):637‐43. [DOI: 10.1016/j.earlhumdev.2008.04.004] [DOI] [PubMed] [Google Scholar]
Goldson 1987
- Goldson E. Non‐nutritive sucking in the sick infant. Journal of Perinatology 1987;7:30‐4. [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. The GRADE Working Group, 2008.
Greer 2001
- Greer FR. Feeding the premature infant in the 20th century. The Journal of Nutrition 2001;131(2):426S‐30S. [DOI] [PubMed] [Google Scholar]
Hack 1985
- Hack M, Estabrook MM, Robertson SS. Development of sucking rhythm in preterm infants. Early Human Development 1985;11(2):133‐40. [DOI] [PubMed] [Google Scholar]
Hamosh 1979
- Hamosh M. A review. Fat digestion in the newborn: Role of lingual lipase and preduodenal digestion. Pediatric Research 1979;13:615‐22. [DOI] [PubMed] [Google Scholar]
Hawdon 2000
- Hawdon JM, Beauregard N, Slattery J, Kennedy G. Identification of neonates at risk of developing feeding problems in infancy. Developmental Medicine Child Neurology 2000;42(4):235‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jenik 2009
- Jenik AG, Vain N. The pacifier debate. Early Human Development 2009;85(10 Suppl):S89‐91. [DOI: 10.1016/j.earlhumdev.2009.08.025] [DOI] [PubMed] [Google Scholar]
Jones 2012
- Jones L. Oral feeding readiness in the neonatal intensive care unit. Neonatal Network 2012;31(3):148‐55. [DOI: 10.1891/0730-0832.31.3.148] [DOI] [PubMed] [Google Scholar]
Kimble 1992b
- Kimble C. Nonnutritive sucking: adaptation and health for the neonate. Neonatal Network 1992;11:29‐33. [PubMed] [Google Scholar]
Lau 2003
- Lau C, Smith EO, Schanler RJ. Coordination of suck‐swallow and swallow respiration in preterm infants. Acta Paediatrica 2003;92(6):721‐7. [PubMed] [Google Scholar]
McCain 2001
- MCain GC. A feeding protocol for healthy preterm infants that shortens time to oral feeding. Journal of Pediatrics 2001;139(3):374‐9. [DOI] [PubMed] [Google Scholar]
McGrath 2004
- McGrath JM, Bodea Braescu AV. Feeding readiness in the preterm infant. Journal of Perinatal & Neonatal Nursing 2004;18(4):353‐68. [DOI] [PubMed] [Google Scholar]
Medeiros 2011
- Medeiros AM, Oliveira AR, Fernandes AM, Guardachoni GA, Aquino JP, Rubinick ML, et al. Characterization of the transition technique from enteral tube feeding to breastfeeding in preterm newborns. Jornal da Sociedade Brasileira de Fonoaudiologia 2011;23(1):57‐65. [DOI] [PubMed] [Google Scholar]
Miller 2007
- Miller JL, Kang SM. Preliminary ultrasound observation of lingual movement patterns during nutritive verus non‐nutritive sucking in a premature infant. Dysphagia 2007;22(2):150‐60. [DOI: 10.1007/s00455-006-9058-z] [DOI] [PubMed] [Google Scholar]
Neiva 2007
- Neiva FC, Leone CR. Development of sucking rhythm and the influence of stimulation in premature infants. Pro Fono 2007;19(39):241–8. [DOI] [PubMed] [Google Scholar]
Pickler 2009
- Pickler RH, Best A, Crosson D. The effect of feeding experience on clinical outcomes in preterm infants. Journal of Perinatology 2009;29(2):124‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poore 2008
- Poore M, Zimmerman E, Barlow SM, Wang J, Gu F. Patterned orocutaneous therapy improves sucking and oral feeding in preterm infants. Acta Paediatrica 2008;97(7):920‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Premji 2000
- Premji SS, Paes B. Gastrointestinal function and growth in premature infants: is non‐nutritive sucking vital?. Journal of Perinatology 2000;20(1):46‐53. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schwartz 1987
- Schwartz R, Moody L, Yarandi H, Anderson G. A meta‐analysis of critical outcome variables in non‐nutritive sucking in preterm infants. Nursing Research 1987;36:292‐5. [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors), Grade Working Group. GRADE handbook for grading quality of evidence and strength of recommendations (updated October 2013). Available from www.guidelinedevelopment.org/handbook.
Steer 1992
- Steer PA, Lucas A, Sinclair JC. Feeding the low birthweight infant. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:128‐130. [Google Scholar]
Tsa 2008
- Tsao JC, Evans S, Meldrum M, Altman T, Zeltzer LK. A review of CAM for procedural pain in infancy: part I. Sucrose and non‐nutritive sucking. Evidence‐based Complementary and Alternative Medicine : ECAM 2008;5(4):371‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiener 1987
- Wiener I, Khalil T, Thompson JC, Rayford PL. Gastrin. In: Thompson JC editor(s). Gastrointestinal Endocrinology. New York: McGraw Hill, 1987:194‐212. [Google Scholar]
Wolf 1992
- Wolf LS, Glass RP. Functional anatomy and physiology of the suck/swallow/ breathe triad. In: Wolf LS, Glass RP editor(s). Feeding and Swallowing Disorders in Infancy: Assessment and Management. Austin, Texas: Hammill Institute on Disabilities, 1992:3‐71. [Google Scholar]
References to other published versions of this review
Pinelli 1998
- Pinelli J, Symington A. Non‐nutritive sucking in premature infants. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001071] [DOI] [Google Scholar]
Pinelli 2001
- Pinelli J, Symington A. Non‐nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD001071] [DOI] [PubMed] [Google Scholar]
Pinelli 2003
- Pinelli J, Symington. Non‐nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database of Systematic Reviews 3, Issue 2003. [DOI: 10.1002/14651858.CD001071.pub2] [DOI] [Google Scholar]
Pinelli 2005
- Pinelli J, Symington AJ. Non‐nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001071.pub2] [DOI] [PubMed] [Google Scholar]


