Abstract
We examined the utility of coronary artery calcium (CAC) for cardiovascular risk stratification among hypertensive adults, including those fitting eligibility for SPRINT (Systolic Blood Pressure Intervention Trial). Additionally, we used CAC to identify hypertensive adults with CVD mortality rates equivalent to those observed in SPRINT who may therefore benefit from the most intensive blood pressure therapy. Our study population included 16,167 hypertensive patients from the CAC Consortium, among whom 6,375 constituted a “SPRINT-like” population. We compared multivariable-adjusted hazard ratios of coronary heart disease (CHD) and cardiovascular disease (CVD) deaths by CAC category (0, 1–99, 100–399, ≥400). Additionally, we generated a CAC-CVD mortality curve for patients aged >50 years to determine what CAC scores were associated with CVD death rates observed in SPRINT.
Mean age was 58.1±10.6 years. Over a mean follow-up of 11.6±3.6 years, there were 409 CVD deaths and 207 CHD deaths. Increasing CAC scores were associated with increased CHD and CVD mortality [CHD – CAC 100–399: Hazard ratio (95% confidence interval) 1.88 (1.04–3.40), CAC≥400: 4.16 (2.34–7.39); CVD – CAC 100–399: 1.93 (1.31–2.83), CAC≥400: 3.51 (2.40–5.13)]. A similar increased risk was observed across 10-year ASCVD risk categories and in the SPRINT-like population. A CAC score of 220 (confidence range 165–270) was associated with the CVD mortality rate observed in SPRINT.
CAC risk stratifies adults with hypertension, including those who are SPRINT-eligible. A CAC score of 220 can identify hypertensive adults with SPRINT-level CVD mortality risk, and therefore may be reasonable for identifying candidates for aggressive blood pressure therapy.
Keywords: blood pressure, hypertension, coronary artery calcium, cardiovascular disease, cardiovascular risk
BACKGROUND
Elevated blood pressure is a major risk factor for cardiovascular events.1 According to the 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines, hypertension is currently defined as blood pressure (BP) ≥130/80 mmHg.2 Based on the new definition, the crude prevalence of hypertension in US adults is 45.6%, which is a substantial 13.7% absolute increase from the prevalence based on the prior definition of hypertension.3,4
While treating hypertension has been shown to decrease cardiovascular events, there remains considerable debate regarding the optimal BP targets in patients with hypertension. The landmark 2015 Systolic Blood Pressure Intervention Trial (SPRINT) reported a 25% reduction in the primary composite cardiovascular outcome and a 27% reduction in all-cause mortality among hypertensive non-diabetic individuals aged >50 years at high cardiovascular risk who were treated intensively to the systolic BP target ≤120 mmHg.5 However, findings from SPRINT appear to differ from results from the Heart Outcomes Prevention Evaluation–3 (HOPE-3) trial, which showed no statistical benefit of additional BP lowering among intermediate cardiovascular risk adults.6 While various differences may contribute to the disparate findings, a primary difference in the studies was the baseline risk of the population.7–9 For instance, the population enrolled in SPRINT had more than twice the risk of cardiovascular disease (CVD) compared to that enrolled in HOPE-3 (annual CVD event rate: 2.2% vs 0.8%). Based in part on these data, the new guidelines – for the first time ever in the United States – incorporated the use of risk scoring to guide hypertension therapy in stage 1 hypertension (systolic BP: 130–139 mmHg or diastolic BP: 80–89 mmHg), although advanced risk stratification tools were not specifically recommended.2
Coronary artery calcium (CAC) detected by non-contrast cardiac computerized tomography (CT) estimates the burden of coronary atherosclerosis and is a strong independent predictor of future cardiovascular events. CAC=0 is associated with low event rates and all-cause mortality,10,11 whereas an increasing CAC score is associated with a high risk of cardiovascular events.12–14 Additionally, CAC improves risk reclassification for cardiovascular events beyond traditional risk factors.15 There is an increasing interest in using CAC scoring to risk stratify hypertensives for selecting personalized BP goals; for example, a combined CVD risk-CAC approach has been shown to identify individuals who might benefit from more or less aggressive anti-hypertensive therapy.16
Therefore, in this study, we aimed to quantify the ability of CAC to further stratify cardiovascular risk in patients with hypertension. Additionally, we assessed if CAC scoring could be used to identify hypertensive individuals aged >50 years who, by virtue of their CAC scores, have observed event rates similar to those enrolled in the SPRINT trial. We hypothesized that CAC may identify such patients that could potentially benefit from intensive BP therapy, consistent with the guidelines emphasis on a risk-based approach.
METHODS
Data and study materials are available from the corresponding author on reasonable request.
Study Design and Study Population
The CAC Consortium is a multi-center, retrospective cohort of 66,636 participants, aimed at examining the association between clinical CAC scoring and long-term cause-specific mortality. The methods and baseline results of the cohort have been described previously.17
Briefly, 4 high volume centers contributed detailed patient information including demographics, risk factor data, and CAC score results to a centralized coordinating center. All patients in the cohort were free of clinical CVD at baseline and were clinically referred for CAC-based risk stratification. Patients were enrolled between 1991 to 2010 and follow-up was data collected through 2014. Comparison of the CAC Consortium with the contemporary NHANES 2001–2002, Multi-ethnic Study of Atherosclerosis (MESA) and Framingham Offspring/3rd Generation cohorts have been previously published.17 Consent for participation in research was collected at the centers at the time of CAC scanning and IRB approval for coordinating center activities was obtained at the Johns Hopkins Hospital.
The primary population for this study includes CAC Consortium participants with baseline hypertension. Thus, 16,167 participants were included for the primary analysis. A secondary analysis was also conducted using participants who were hypertensive, were >50 years of age, and had Framingham risk score (FRS) >15%. This sub-population, which was similar to the standard-treatment arm of SPRINT, was defined as the ‘SPRINT-like’ population and included 6,375 individuals.
Coronary Artery Calcium Scoring
Non-contrast, cardiac-gated CT scans were performed for CAC scoring at each individual site using a common protocol for each CT scanner technology. The Agatston method was used to quantify CAC for all patients. Approximately 93% of the scans were performed by electron beam tomography (EBT) while 7% of the scans were obtained by multi-detector CT (MDCT). Prior studies revealed no meaningful clinical differences between CAC scores derived from EBT versus MDCT scanners.18 For this analysis, patients were categorized into four CAC score groups – CAC=0, CAC 1–99, CAC 100–399 and CAC ≥ 400.
Outcome Ascertainment - Cause-Specific Mortality
The primary outcomes for the study were coronary heart disease (CHD) and CVD mortality assessed over mean 11.6±3.6 years of follow-up. Mortality was accessed by linking patient records with the Social Security Administration (SSA) Death Master File (DMF) using a previously validated algorithm. Death certificates were obtained from the National Death Index (NDI), and underlying cause of death was categorized into common causes of death using ICD 9 and ICD10 codes as previously described.17 Internal validation studies for the CAC Consortium against known deaths identified via the electronic medical record revealed sensitivity ranging from 72 to 90% for identifying known deaths, with >90% specificity. Detailed comparison of death rates in the CAC Consortium with the U.S. Census and MESA has been previously published.17
Risk Factor Definitions
Definitions of traditional risk factors have been described previously.17 Hypertension was defined as a prior diagnosis of hypertension or use of anti-hypertensive medications. This was based on the old definition of hypertension, prior to the 2017 ACC/AHA guideline (systolic BP >=140 mmHg or diastolic BP >=90 mmHg). Similarly, diabetes was defined as prior diagnosis of diabetes or treatment with oral hypoglycemic drugs or insulin. Dyslipidemia was defined as: prior diagnosis of primary hyperlipidemia (LDL-C >160 mg/dL), prior diagnosis of dyslipidemia (elevated triglycerides >150 mg/dL and/or low HDL-C <40 mg/dL in men and <50 mg/dL in women), or treatment with any lipid-lowering drug. Current smoking was categorized as a binary variable (considered present/absent). Family history of CHD was generally defined as the presence of a first degree relative with a history of CHD, although one site (with 11% of patients) used a definition of premature family history (<55 years old in a male relative and <65 years old in a female relative). The 10-year ASCVD risk score was calculated using the Pooled Cohort Equations.19 Simple rule-based imputation method was used in the event of missing continuous data for blood pressure and lipid measurements for the calculation of the ASCVD risk score.17
Statistical Analysis
Baseline characteristics of the study population were compared across the four CAC score categories. For categorical variables, proportions were calculated and for continuous variables mean ± standard deviation or median ± IQR were calculated based on the normality of the data. For formal comparisons, Chi-square test and ANOVA or Kruskal-Wallis testing were used as appropriate.
Absolute CHD and CVD mortality rates were calculated by dividing the total number of deaths by the total number of patient-years of follow-up, and then reported per 1000 patient-years.
To evaluate the association of CAC score with CHD and CVD mortality, survival analyses were conducted using individual subject time-to CHD or CVD-mortality data. After graphical confirmation of the proportional hazards assumption, hazard ratios (HRs) and 95% confidence intervals were calculated using the Cox proportional hazards regression model with CAC=0 as the reference group, adjusting for age and sex (Model 1) and further adjusting for hyperlipidemia, smoking status, family history of CHD and diabetes (Model 2). The regression analysis was also repeated: (1) after stratifying by 10-year ASCVD risk groups (<10%, ≥10%); (2) within the dedicated SPRINT-like population (age >50 and FRS >15%); (3) after excluding participants with diabetes in the SPRINT-like population; and (4) among the study population not eligible for SPRINT (age <50 or age ≥50 and FRS <15%). The ASCVD 10% cutpoint was based on the new AHA/ACC hypertension guidelines recommendations for risk stratification of the stage 1 hypertension group.
We performed a separate analysis to further compare CHD and CVD-mortality risk across different CAC and ASCVD risk groups using Cox proportional hazards model with CAC=0 and ASCVD <10% as the reference group. Hazard ratio estimates in this analysis was adjusted for age, sex, hyperlipidemia, smoking status, family history of CHD and diabetes (Model 2). While hazards are compared across CAC/ASCVD groups, formal statistical reclassification analysis (i.e. net reclassification improvement [NRI]) was not performed in this study due to the different outcomes studied (ASCVD vs. CHD/CVD mortality in the CAC Consortium).
An additional analysis was conducted among hypertensive participants aged >50 years to determine what CAC scores would translate into CVD death rates equivalent to those observed in the standard-treatment group of the SPRINT trial (0.43%/year). To accomplish this, CVD mortality rates observed in SPRINT were first age-standardized to the population structure of the CAC Consortium (age-standardized annual CVD mortality rate = 0.35%/year). Then, using a plot of CAC score (x-axis) vs. annual CVD mortality rates (y-axis), a y-axis line was placed at the annual CVD mortality rate observed in SPRINT, and its intersection with the CAC-CVD mortality plot was interpreted as the CAC-score equivalent of SPRINT-level risk (i.e. the CAC score that would produce risk equivalent to the standard treatment arm of the SPRINT trial). A confidence band was applied to reflect the possible 15% underestimation of risk within the CAC Consortium. All analyses were performed using Stata 13.0 (StataCorp, College Station, TX), and a two-tailed p <0.05 was considered to be significant.
RESULTS
The baseline characteristics of the 16,167 participants are shown in Table 1. The mean age was 58.1±10.6, with 35.8% women and 85.8% Whites. About 32% participants had CAC=0, and 36.2% participants had CAC ≥100. The mean 10-year ASCVD risk score was 12.1%. Participants with higher CAC scores had an increased burden of ASCVD risk factors. For instance, those with CAC>100 were more likely to be older, have hyperlipidemia and diabetes, and were more likely to be current smokers compared to those with CAC<100.
Table 1:
Baseline characteristics of individuals with hypertension in the CAC Consortium by CAC score categories.
| Variable | Overall (N=16,167) |
CAC=0 (N=5114) |
CAC 1–99 (N = 5202) |
CAC 100–399 (N=2928) |
CAC ≥400 (N=2923) |
P-value* |
|---|---|---|---|---|---|---|
| Age, years | 58.1 ± 10.6 | 52.9 ± 9.6 | 57.2 ± 9.7 | 61.7 ± 9.7 | 65.3 ± 9.6 | <0.001 |
| Women | 5795 (35.8%) | 2597 (50.8%) | 1789 (34.4%) |
846 (28.9%) | 563 (19.3%) | <0.001 |
| Men | 10372 (64.2%) | 2517 (49.2%) | 3413 (65.6%) |
2082 (71.1%) | 2360 (80.7%) | |
| Race | 0.07 | |||||
| White | 10557 (85.8%) | 3325 (85.9%) | 3361 (84.8%) | 1959 (85.9%) | 1912 (87%) | |
| Black | 519 (4.22%) |
169 (4.4%) |
187 (4.7%) |
95 (4.2%) |
68 (3.1%) |
|
| Hispanic | 490 (3.98%) |
161 (4.2%) |
159 (4.0%) |
76 (3.33%) | 94 (4.3%) |
|
| Hyperlipidemia | 10043 (62.1%) | 2821 (55.2%) | 3226 (62.0%) | 1959 (66.9%) | 2037 (69.7%) | <0.001 |
| Current Smoker | 1541 (9.5%) | 435 (8.5%) | 466 (9.0%) |
332 (11.3%) | 308 (10.5%) | <0.001 |
| Family History of CHD | 7711 (47.7%) | 2431 (47.5%) | 2504 (48.1%) | 1371 (46.8%) | 1405 (48.1%) | 0.68 |
| Diabetes | 2047 (12.7%) | 391 (7.7%) | 599 (11.5%) |
427 (14.6%) | 630 (21.6%) | <0.001 |
| 10-y ASCVD risk score | 12.1 ± 11.9 | 6.4 ± 6.8 | 10.7 ± 9.8 | 15.7±12.6 | 21.2 ± 14.8 | <0.001 |
| 10-y Framingham risk score | 15.9 ± 11.4 | 11.3 ± 7.6 | 15.2 ± 9.8 | 18.6 ± 12.2 | 22.7 ± 14.5 | <0.001 |
Continuous variables shown as mean±SD, categorical variables shown as n (%).
P-value was calculated for continuous variables using a nonparametric test for trend and for categorical variables using the Chi-square test.
ASCVD indicates atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CHD, coronary heart disease.
A total of 6,375 (39.4%) participants qualified for the SPRINT-like sub-population (age >50 years and FRS >15%). The mean age in this sub-population was 63 years and there were 75.1% males and 86.5% Whites. Additionally, 17.7% participants had CAC=0, and 51.5% participants had CAC ≥100.
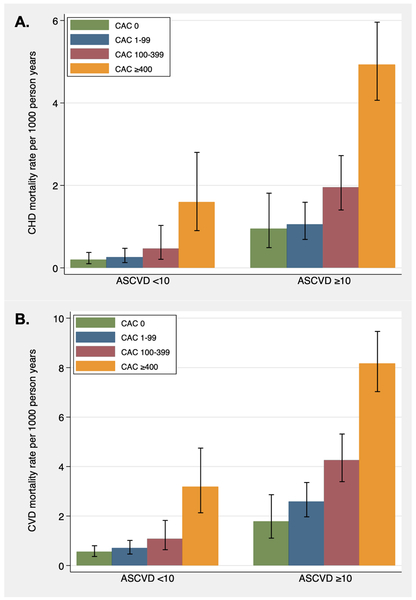
Over the mean follow-up of 11.6±3.6 years, there were 409 CVD deaths and 207 CHD deaths in the total study population. The overall mortality rate in the study population by the CAC categories CAC=0, 1–99, >100 and 100–399 were 0.3, 0.5, 1.3, 4.1 per 1000-person years for CHD, and 0.8, 1.4, 2.9, 6.9 per 1000-person years for CVD, respectively. Figure 1 shows the CHD and CVD mortality rates per 1000-person years by increasing CAC scores stratified by ASCVD risk groups (<10% vs ≥10%). In each risk group, mortality rates increased with increasing CAC scores. For instance, CVD mortality among individuals with CAC ≥400 was twice that of CAC 100–399 among the ASCVD ≥10% group (8.15 vs 4.24 per 1000 person-years). Notably, mortality rates at higher CAC scores (≥400) in ASCVD <10% was greater than mortality rates of lower CAC scores (CAC 1–99) in the ASCVD≥10% group. This pattern was more pronounced for CHD compared to CVD.
Figure 1: Absolute CHD and CVD mortality rates among hypertensives in the CAC Consortium.
Absolute (A) coronary heart disease (CHD) and (B) cardiovascular disease (CVD) mortality rates per 1000 person-years by ASCVD risk groups and CAC score categories.
ASCVD indicates atherosclerotic cardiovascular disease; CAC, coronary artery calcium.
Table 2 shows the multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for CHD and CVD deaths across increasing CAC score categories in the total study population. Increased CAC scores were associated with increased risk of CHD and CVD deaths, most notably among the CAC100–399 and CAC ≥400 categories. In the fully adjusted model, individuals with CAC ≥400 had approximately 4 and 3.5 times increased risk of experiencing a CHD and CVD death respectively compared to those with CAC=0.
Table 2:
Multivariable-adjusted hazard ratios (95% CIs) for CHD and CVD deaths, by CAC score group
| CAC Score Group | N (%) | Unadjusted HR (95% CI) |
Model 1 HR (95% CI)* |
Model 2 HR (95% CI)† |
|---|---|---|---|---|
| CHD death | ||||
| CAC=0 | 18 (8.7) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 31 (15) | 1.67 (0.94, 3.0) | 1.22 (0.67, 2.21) | 1.15 (0.63, 2.08) |
| CAC 100–399 | 41 (19.8) | 4.02 (2.31, 7.01) | 2.11 (1.17, 3.83) | 1.88 (1.04, 3.40) |
| CAC ≥400 | 117 (56.5) | 11.84 (7.21, 19.45) | 4.9 (2.74, 8.76) | 4.16 (2.34, 7.39) |
| CVD death | ||||
| CAC=0 | 42 (10.3) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 79 (19.3) | 1.82 (1.25, 2.65) | 1.39 (0.95, 2.03) | 1.33 (0.91, 1.95) |
| CAC 100–399 | 90 (22) | 3.80 (2.64, 5.48) | 2.09 (1.42, 3.08) | 1.93 (1.31, 2.83) |
| CAC ≥400 | 198 (48.4) | 8.68 (6.22, 12.11) | 3.92 (2.68, 5.73) | 3.51 (2.40, 5.13) |
N (%) represent number of events in each category.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, hyperlipidemia, smoker, family history of CHD and diabetes.
CAC indicates coronary artery calcium; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Table 3 demonstrates the multi-variable adjusted HRs (95% CIs) for CHD and CVD deaths by CAC score categories stratified by the ASVCD risk groups (<10%, ≥10%). A similar pattern was observed in this analysis – higher CAC score categories were positively associated with risk for both CHD and CVD deaths in both risk groups. Higher CAC score groups, such as CAC 100–399 and ≥400, had higher HRs (vs CAC=0) in the ASCVD <10% group compared to lower CAC score groups, such as 1–99, in the ASCVD ≥10% group. Similarly, when CAC=0 and ASCVD <10% was used as a common reference group, the higher CAC score groups in the ASCVD <10% (for instance, CAC 100–399) group had higher HRs compared to the lower CAC score groups (CAC 1–99) in the ASCVD ≥10% group (Table S1).
Table 3:
Multivariable-adjusted hazard ratios (95% CIs) for CHD and CVD deaths stratified by ASCVD risk, by CAC score group
| CAC Score Group |
N (%) | Unadjusted HR (95% CI) |
Model 1 HR (95% CI)* |
Model 2 HR (95% CI)† |
|---|---|---|---|---|
| CHD death | ||||
| ASCVD risk <10% | ||||
| CAC=0 | 9 (25) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 9 (25) | 1.29 (0.51 – 3.24) | 1.33 (0.52 – 3.38) | 1.30 (0.52 – 3.30) |
| CAC 100–399 | 6 (16.7) | 2.38 (0.85 – 6.67) | 2.51 (0.87 – 7.23) | 2.39 (0.85 – 6.70) |
| CAC ≥400 | 12 (33.3) | 8.02 (3.38 – 19.05) | 8.60 (3.42 – 21.65) | 8.11 (3.24 – 20.29) |
| ASCVD risk ≥10% | ||||
| CAC=0 | 9 (5.3) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 22 (12.9) | 1.10 (0.51 – 2.38) | 1.05 (0.48 – 2.30) | 1.03 (0.47 – 2.24) |
| CAC 100–399 | 35 (20.5) | 2.02 (0.97 – 4.20) | 1.70 (0.80 – 3.61) | 1.60 (0.76 – 3.40) |
| CAC ≥400 | 105 (61.4) | 4.91 (2.49 – 9.68) | 3.74 (1.81 – 7.74) | 3.36 (1.63 – 6.94) |
| CVD death | ||||
| ASCVD risk <10% | ||||
| CAC=0 | 25 (28.4) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 25 (28.4) | 1.28 (0.73,2.22) | 1.36 (0.76,2.44) | 1.32 (0.74,2.36) |
| CAC 100–399 | 14 (15.9) | 1.99 (1.03,3.83) | 2.16 (1.09,4.27) | 2.00 (1.01,3.95) |
| CAC ≥400 | 24 (27.3) | 5.75 (3.27,10.09) | 6.46 (3.39,12.32) | 5.92 (3.11,11.26) |
| ASCVD risk ≥10% | ||||
| CAC=0 | 17 (5.3) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 54 (16.8) | 1.43 (0.83,2.46) | 1.43 (0.83,2.47) | 1.4 (0.81,2.43) |
| CAC 100–399 | 76 (23.7) | 2.33 (1.38,3.94) | 2.02 (1.19,3.45) | 1.93 (1.13,3.30) |
| CAC ≥400 | 174 (54.2) | 4.37 (2.66,7.18) | 3.52 (2.09,5.90) | 3.28 (1.95,5.51) |
N (%) represent number of events in each category.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, hyperlipidemia, smoker, family history of CHD and diabetes.
ASCVD indicates atherosclerotic cardiovascular disease; CAC, coronary artery calcium; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Increasing CAC score was also similarly positively associated with increased risk of both CHD and CVD deaths in the SPRINT-like population (Table 4), after the exclusion of participants with diabetes in the SPRINT-like population (Table S2), and among the study population not eligible for SPRINT (Table S3).
Table 4:
Multivariable-adjusted hazard ratios (95% CIs) for CHD and CVD deaths in the SPRINT-like population (age >50 and FRS>15%), by CAC score group
| CAC Score Group | N (%) | Unadjusted HR (95% CI) |
Model 1 HR (95% CI)* |
Model 2 HR (95% CI)† |
|---|---|---|---|---|
| CHD deaths | ||||
| CAC=0 | 6 (5) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 11 (9.2) | 1.05 (0.39, 2.85) | 0.76 (0.28, 2.06) | 0.73 (0.27, 1.99) |
| CAC 100–399 | 25 (20.8) | 3.28 (1.34, 8.0) | 1.71 (0.66, 4.41) | 1.57 (0.62, 4.0) |
| CAC ≥400 | 78 (65) | 8.54 (3.71, 19.6) | 3.24 (1.30, 8.07) | 2.86 (1.17, 7.0) |
| CVD deaths | ||||
| CAC=0 | 10 (4.5) | 1.0 | 1.0 | 1.0 |
| CAC 1–99 | 34 (15.3) | 1.96 (0.97, 3.97) | 1.64 (0.81, 3.4) | 1.57 (0.78, 3.2) |
| CAC 100–399 | 49 (22.1) | 3.87 (1.96, 7.65) | 2.55 (1.25, 5.22) | 2.36 (1.16, 4.8) |
| CAC ≥400 | 129 (58.1) | 8.61 (4.51, 16.4) | 4.33 (2.16, 8.65) | 3.95 (1.98, 7.9) |
N (%) represent number of events in each category.
Model 1 adjusted for age and sex.
Model 2 adjusted for age, sex, hyperlipidemia, smoker, family history of CHD and diabetes.
CAC indicates coronary artery calcium; CHD, coronary heart disease; CI, confidence interval; CVD, cardiovascular disease; FRS, Framingham risk score; HR, hazard ratio; SPRINT, Systolic Blood Pressure Intervention Trial.
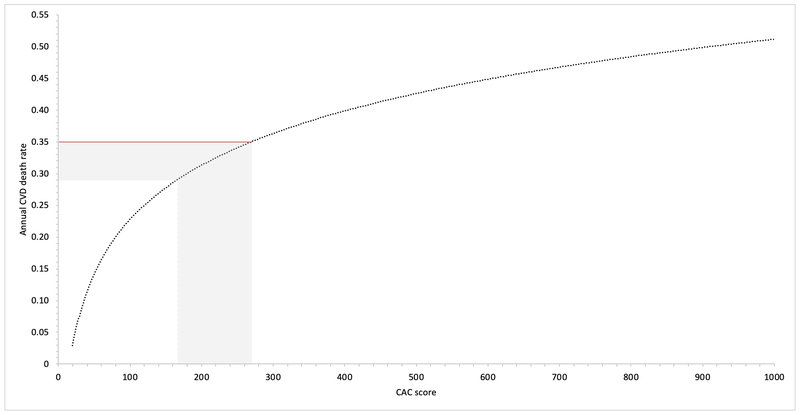
Figure 2 shows the graphical calculation of the CAC score threshold associated with SPRINT-level risk. A CAC score in the range of 165–270 was associated with the age-adjusted annual CVD mortality rate observed in the SPRINT trial (0.35% per year). A score in this range is thus associated with CVD mortality rates equivalent to the observed mortality rate in the standard therapy arm of SPRINT.
Figure 2: CAC score equivalent of SPRINT-level risk among participants age >50 years.
Graph shows the annual cardiovascular disease (CVD) mortality rate as a function of coronary artery calcium (CAC) scores among hypertensive patients age >50 years.
Horizontal red line represents the age-adjusted CVD death rate observed in the SPRINT trial (0.35%/year).
These lines intersect at CAC=270, with lower limit of confidence (accounting for possible 15% underestimation of risk in the CAC Consortium) at CAC=165.
DISCUSSION
In our study of a large, clinical cohort of asymptomatic patients with hypertension free of baseline CVD, increasing CAC scores were strongly associated with CHD and CVD death rates after adjustment for traditional cardiovascular risk factors. Additionally, CAC stratified risk of CVD and CHD deaths across guideline-based ASCVD risk categories, resulting in re-categorization of risk across the 10% ASCVD risk threshold. CAC stratified risk similarly well in a subgroup of patients who would be eligible for the SPRINT trial. Additionally, our modeling suggests that hypertensive persons aged >50 years with a CAC score near 220 (estimated range 165–270) have equivalent risk to the SPRINT trial population, and therefore may be candidates for the most aggressive blood pressure goals.
There are a limited number of studies on the predictive ability of CAC among those with hypertension. Our results add to the growing evidence of the predictive value of CAC scoring on CHD and CVD outcomes among hypertensives. For example, our findings were similar to that reported by McEvoy et. al, who showed, among 3,733 hypertensive participants of mean age 65 years from the Multi-Ethnic Study of Atherosclerosis (MESA), a similarly increased hazard for incident all-cause CVD events and heart failure with higher CAC scores (CAC 1–99 and CAC >100) compared to CAC=0, specifically among those with systolic BP in the range of 120–159 mmHg.16 Similarly, Erbel et al, in 2012, evaluated the predictive ability of CAC in different stages of hypertension among 4,181 participants from the Heinz Nixdorf Recall study.20 Consistent with our findings, the authors showed increasing CAC scores were associated with increased primary and secondary endpoints in all stages of hypertension. However, point estimates reported were larger compared to our estimates, which is likely explained by the authors’ choice of a reference group of normotensives with CAC=0, compared to our reference group of hypertensives with CAC=0.
There has been an increasing interest in the use of cardiovascular risk assessment to help define individual BP treatment goals. The new AHA/ACC guidelines have focused on the ASCVD 10% cut-point to guide anti-hypertensive treatment.2 In our analysis, we demonstrate that CAC can re-categorize risk around the 10% threshold; CAC >100 identified individuals in the low risk group (ASCVD <10%) having higher risk of CVD and CHD death compared to those with lower CAC scores in the higher risk group (ASCVD ≥10%). In line with our findings, McEvoy et al16 have previously demonstrated, among individuals with hypertension, a higher HR for higher CAC scores (CAC >100 vs CAC=0) in ASCVD <15% group compared to the lower HR for lower CAC scores in the ASCVD >15% group.
Besides recommending a risk-based approach to hypertension therapy, the 2017 ACC/AHA guidelines also propose more intensive BP targets compared to previous guidelines. A major influence for this was the SPRINT trial. In our study, we demonstrated that CAC can identify people with risk equivalent to the SPRINT study. This has clinical implications for identifying candidates with advanced subclinical atherosclerosis who may be more likely to benefit from the most from aggressive BP lowering. Hypertensive individuals with a CAC score of approximately ≥220 (range 165–270) and aged >50 years would have event rates similar to that of SPRINT and may therefore benefit from intensive BP therapy. Our results thus strengthen the evidence for a combined CAC/ASCVD-risk approach that can help identify individuals at the greatest risk who could benefit from an aggressive BP therapy.
Strengths of our study include using one of the largest study populations with hypertension, a long follow-up and ascertainment of cause-specific mortality (CHD and CVD deaths). To our knowledge, this is also the first CAC-based analysis using the ASCVD 10% cut-point of the 2017 AHA/ACC guidelines. Additionally, we have also modeled CAC for the first time in the context of the SPRINT trial results, using an innovative approach for identifying a threshold of CAC score that would identify SPRINT-level risk.
Our study also has some limitations. Hypertension and other risk factors were predominantly obtained by self-report or prior treatment which could potentially introduce recall bias. However, self-reporting of hypertension and other risk factors has been validated to assess risk factor data of participants.21 Additionally, study participants - who were free of baseline CVD - were clinically referred to the centers for CAC scoring for risk stratification which could potentially limit generalizability to the overall healthy population. We also did not possess other measurements that were used by the SPRINT trial to determine trial eligibility such as the presence of chronic kidney disease.
In conclusion, we demonstrated that: (1) CAC risk stratifies hypertensives, including those who are SPRINT eligible; (2) CAC re-categorizes risk around the ASCVD 10% threshold; (3) A CAC score of around 220 can identify hypertensives with risk equivalent to the SPRINT study. This score threshold can therefore help identify candidates for the most aggressive BP lowering
Supplementary Material
PERSPECTIVES.
This study greatly strengthens the evidence of CAC scoring as an advanced risk-stratification tool among adults with hypertension and demonstrates the utility of CAC to inform making more personalized BP goals. The findings support stronger endorsement of the use of CAC scoring in future guidelines.
NOVELTY AND SIGNIFICANCE.
What is New?
This study examined the utility of coronary artery calcium (CAC) for cardiovascular disease mortality risk stratification among hypertensive adults, including those who would be eligible for SPRINT (Systolic Blood Pressure Intervention Trial).
Using an innovative approach, a threshold of CAC score was identified that would identify individuals with cardiovascular mortality rates similar to those observed in the standard-treatment arm of SPRINT.
What is relevant?
CAC is a robust test for cardiovascular risk stratification among adults with hypertension, including those who are eligible for SPRINT.
A CAC score of 220 can identify hypertensive adults with SPRINT-level risk, and therefore may be reasonable for identifying candidates for aggressive blood pressure therapy.
Summary:
CAC risk-stratifies adults with hypertension, and can identify individuals who could benefit from intensive blood pressure therapy.
SOURCE OF FUNDING
Dr. Blaha has received support from NIH award L30 HL110027 for this project.
Footnotes
DISCLOSURES
The authors have no disclosures pertinent to this work.
REFERENCES
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: A report from the american heart association. Circulation. 2018;137(12):e492. doi: 10.1161/CIR.0000000000000558 [doi]. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2017. doi: S0735-1097(17)41519-1 [pii]. [DOI] [PubMed] [Google Scholar]
- 3.Muntner P, Carey RM, Gidding S, et al. Potential U.S. population impact of the 2017 American College of Cardiology/American Heart Association High Blood Pressure Guideline. J Am Coll Cardiol. 2017. doi: S0735-1097(17)41474-4 [pii]. [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560 [doi]. [DOI] [PubMed] [Google Scholar]
- 5.Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. SPRINT Research Group. N Engl J Med. 2015;373(22):2103–2116. 10.1056/NEJMoa1511939. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonn EM, Bosch J, Lopez-Jaramillo P, et al. Blood-pressure lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374(21):2009–2020. doi: 10.1056/NEJMoa1600175 [doi]. [DOI] [PubMed] [Google Scholar]
- 7.Whelton PK, Reboussin DM, Fine LJ. Comparing the SPRINT and the HOPE-3 blood pressure trial. JAMA Cardiol. 2016;1(8):855–856. doi: 10.1001/jamacardio.2016.2051 [doi]. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf S, Lonn E. The SPRINT and the HOPE-3 trial in the context of other blood pressure-lowering trials. JAMA Cardiol. 2016;1(8):857–858. doi: 10.1001/jamacardio.2016.2169 [doi]. [DOI] [PubMed] [Google Scholar]
- 9.Oparil S, Lewis CE. Should patients with cardiovascular risk factors receive intensive treatment of hypertension to <120/80 mm hg target? A protagonist view from the SPRINT trial (systolic blood pressure intervention trial). Circulation. 2016;134(18):1308–1310. doi: CIRCULATIONAHA.116.023263 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joshi PH, Blaha MJ, Budoff MJ, et al. The 10-year prognostic value of zero and minimal CAC. JACC: Cardiovascular Imaging. 2017;10(8):957–958. http://www.sciencedirect.com/science/article/pii/S1936878X17305107. doi: 10.1016/j.jcmg.2017.04.016 “. [DOI] [PubMed] [Google Scholar]
- 11.Blaha M, Budoff MJ, Shaw LJ, et al. Absence of coronary artery calcification and all-cause mortality. JACC: Cardiovascular Imaging. 2009;2(6):692–700. http://www.sciencedirect.com/science/article/pii/S1936878X09001739. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228(3):826–833. doi: 10.1148/radiol.2283021006 [doi]. [DOI] [PubMed] [Google Scholar]
- 13.Keelan PC, Bielak LF, Ashai K, et al. Long-term prognostic value of coronary calcification detected by electron-beam computed tomography in patients undergoing coronary angiography. Circulation. 2001;104(4):412–417. [DOI] [PubMed] [Google Scholar]
- 14.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358(13):1336–1345. doi: 10.1056/NEJMoa072100 [doi]. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann U, Massaro JM, D’Agostino RB S, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the framingham heart study. J Am Heart Assoc. 2016;5(2):10.1161/JAHA.115.003144. doi: 10.1161/JAHA.115.003144 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEvoy JW, Martin SS, Dardari ZA, et al. Coronary artery calcium to guide a personalized risk-based approach to initiation and intensification of antihypertensive therapy. Circulation. 2017;135(2):153–165. doi: 10.1161/CIRCULATIONAHA.116.025471 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaha MJ, Whelton SP, Al Rifai M, et al. Rationale and design of the coronary artery calcium consortium: A multicenter cohort study. J Cardiovasc Comput Tomogr. 2017;11(1):54–61. doi: S1934-5925(16)30286-6 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao SS, Pal RS, McKay CR, et al. Comparison of coronary artery calcium scores between electron beam computed tomography and 64-multidetector computed tomographic scanner. J Comput Assist Tomogr. 2009;33(2):175–178. doi: 10.1097/RCT.0b013e31817579ee [doi]. [DOI] [PubMed] [Google Scholar]
- 19.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the american college of cardiology/american heart association task force on practice guidelines. Circulation. 2014;129(25 Suppl 2):49. doi: 10.1161/01.cir.0000437741.48606.98 [doi]. [DOI] [PubMed] [Google Scholar]
- 20.Erbel R, Lehmann N, Mohlenkamp S, et al. Subclinical coronary atherosclerosis predicts cardiovascular risk in different stages of hypertension: Result of the heinz nixdorf recall study. Hypertension. 2012;59(1):44–53. doi: 10.1161/HYPERTENSIONAHA.111.180489 [doi]. [DOI] [PubMed] [Google Scholar]
- 21.Blair SN, Goodyear NN, Gibbons LW, Cooper KH. Physical fitness and incidence of hypertension in healthy normotensive men and women. JAMA. 1984;252(4):487–490. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.