Abstract
Low birth weight is associated with a greater prevalence of hypertension and an earlier age at menopause in women in later life. Yet, the association between birth weight and blood pressure in women as they age is not well-defined. In a rodent model of low birth weight induced by placental insufficiency, intrauterine growth restriction programs a significant increase in blood pressure by 12 months of age in female growth- restricted offspring that is associated with early reproductive senescence, increased testosterone, and a shift in the hormonal milieu. Thus, this study tested the hypothesis that increased blood pressure in female growth-restricted offspring is abolished by chronic estradiol supplementation. Placebo or 17β-estradiol valerate minipellets (1.5 mg for 60-day release) were administered at 12 months of age for 6 weeks. Blood pressure, measured in conscious catheterized rats, was significantly increased in placebo-treated growth-restricted relative to placebo-treated control. However, blood pressure was not elevated in estradiol-treated growth-restricted relative to placebo-treated growth-restricted. Estradiol mediates its effects on blood pressure via its receptors and the renin angiotensin system. Blood pressure was decreased in growth-restricted offspring treated with a G-protein coupled receptor agonist, G1 (400 mg/kg for 2 weeks). Renal angiotensin type 1a and 1b receptor and renal androgen receptor mRNA expression were elevated in vehicle-treated growth-restricted offspring but not in G1-treated growth-restricted. Therefore, these data suggest that chronic estradiol supplementation prevents the increase in blood pressure that develops in female growth-restricted offspring via actions that may involve its G-protein coupled receptor and the renin angiotensin system.
Keywords: IUGR, women’s health, blood pressure, estrogen, renin angiotensin system
Introduction:
Cardiovascular (CV) disease is the number one cause of death among women, and results in 1 in 3 deaths each year (1). Typically, prior to menopause, men have a higher prevalence of hypertension than women (2). However, after menopause, this relationship is reversed with the prevalence of hypertension greater in postmenopausal women compared to premenopausal women (3). Standard treatment options for postmenopausal hypertension are not clear (4). According to results derived from the Women’s Health Initiative, diuretics and calcium channel blockers were the most common prescribed therapy for postmenopausal hypertension (5). Yet, two-thirds of menopausal women, who are most at risk for stroke and cardiovascular events, do not have their hypertension adequately controlled while taking medications; thus, leading to the conclusion that more aggressive therapies are needed to prevent the progression of hypertension in this subset of the population (4). The etiology of hypertension in women is multifactorial and includes activation of the renin-angiotensin system (6), the endothelin system (7), and the sympathetic nervous system (8). Another suggested contributor to the etiology of postmenopausal hypertension is an imbalance in the hormonal milieu (9); in particular, a decrease in estrogens relative to androgens. Yet, further investigation into the role of sex hormones in the etiology of the increased prevalence of hypertension that develops in women in later life is warranted.
Birth weight is inversely associated with blood pressure (BP) in men and women (10). This relationship is amplified with age (11) with low birth weight (LBW) women twice as likely to become hypertensive by the age of 60 compared to their normal birth weight counterparts (12). Yet, the mechanisms that contribute to the greater prevalence of hypertension in LBW women in later life is unknown. Experimental models of developmental origins report that males are hypertensive during young adulthood, whereas female are protected in early life (13, 14, 15). In addition to hypertension, LBW in women is also associated with an increase in adiposity (10) and an earlier age at menopause (16); both of which are risk factors for hypertension.
Our laboratory uses a model of developmental origins induced by placental insufficiency in the rat to study the inverse relationship between BP and birth weight (13). Female intrauterine growth restricted (IUGR) offspring are normotensive during young adulthood. However, at 12 months of age, BP is elevated in female growth-restricted offspring compared to control indicating that female rats born growth-restricted are no longer protected at 12 months of age (17). In our experimental model, female growth-restricted offspring enter into a state of persistent estrous by 12 months of age, or 6 months prior to female control, indicative of early reproductive senescence (18). Previous studies from our laboratory also indicate that circulating levels of testosterone are elevated in female growth-restricted offspring in association with the increase in BP; estradiol levels do not differ (18) implicating a shift towards a prevalence of testosterone. Blockade of the androgen receptor abolishes the increase in BP in female growth-restricted offspring at 12 months of age (19). Androgen blockade also abolishes the increase in circulating testosterone (19). Thus, the aim of this study was to test the hypothesis that chronic supplementation of 17β -estradiol (E2), resulting in a shift to higher circulating levels of estradiol, would be protective against increased BP in female growth-restricted offspring in later life.
MATERIALS AND METHODS
Data Availability and the Transparency and Openness Promotion Guidelines Statement.
Upon acceptance, we will make all data underlying the findings described in our manuscript fully available without restriction in compliance with Hypertension’s policy.
Animals.
All experimental procedures were conducted in accordance with National Institutes of Health guidelines. All protocols were approved by the Animal Care and Use Committee at the University of Mississippi Medical Center. Rats were housed in a temperature-controlled room (23°C) with a 12:12 hr light-dark cycle with food and water available ad libitum. Timed pregnant Sprague Dawley rats were purchased from Envigo Inc. (Indianapolis, IN). At day 14 of gestation, pregnant rats were randomly selected to undergo either the reduced uterine perfusion or a sham procedure as described in the Online Supplement. All pregnant rats were allowed to deliver at term with birth weights recorded within 12 hours of delivery. Pups of control and reduced uterine perfusion litters were culled to four female and four male pups per dam at 48 hours after birth in order to allow equal nutrition access for all offspring while maintaining a 1 to 1 sex ratio per litter. Only female control offspring from sham-operated dams and female growth-restricted offspring from reduced uterine perfusion dams were used in this study. Final groups included offspring from 18 sham-operated and 16 reduced uterine perfusion pressure (RUPP) pregnant rats. To ensure diversity and that programmed influences were not representative of a litter effect, one - two pups per sex per litter were used per study parameter. All animals undergoing surgical procedures were anesthetized using 2–5% isoflurane by inhalation. Offspring were randomly selected for the different study groups. Systemic and molecular experimental endpoints for the 17β-estradiol supplementation study and the selective G protein coupled estrogen receptor 1 (GPER) agonist, G1, study were performed in separate cohort of animals with measured blood pressure measured at 13.5 and 14.5 months of age, respectively, followed by the harvest of kidney cortex, kidney medulla, visceral fat, uterine weight, serum, and plasma. Vaginal cytology was performed in an additional cohort. A more detailed Methods section is available in the online-only Data Supplement.
Supplementation with 17β-estradiol
17β-estradiol valerate minipellets (1.5 mg for 60-day release, E2-treated) or placebo pellet (placebo-treated) from Innovative Research of America of Sarasota, FL were inserted subcutaneously (SC) in the dorsum of the neck for continuous release of hormone at a dose chosen to mimic the normal E2 levels observed in adult Sprague-Dawley female control animals (25±2 ng/dL) (20).
Administration of the selective G protein-coupled estrogen receptor 1 (GPER) agonist, G1
The GPER agonist, G1 (400 µg/kg/day, Caymen Chemical, Ann Arbor, MI) dissolved in DMSO and saline, or vehicle were administered via osmotic pumps (model 2ML2; Alzet: Cupertino, CA) implanted at the dorsum of the neck for 2 weeks beginning at 14 months of age.
Statistics.
Graphpad PRISM version 4 (Graph Pad Software, San Diego, CA) was used for all statistical analysis. Comparisons among groups were performed by two-way ANOVA. Unpaired student t-test was used for comparison of birth weight, baseline body weight, and litter before cull analysis. The level of significance was set at P<0.05. All results are presented as mean±SEM.
RESULTS:
17β-Estradiol Study
Mean Arterial Pressure.
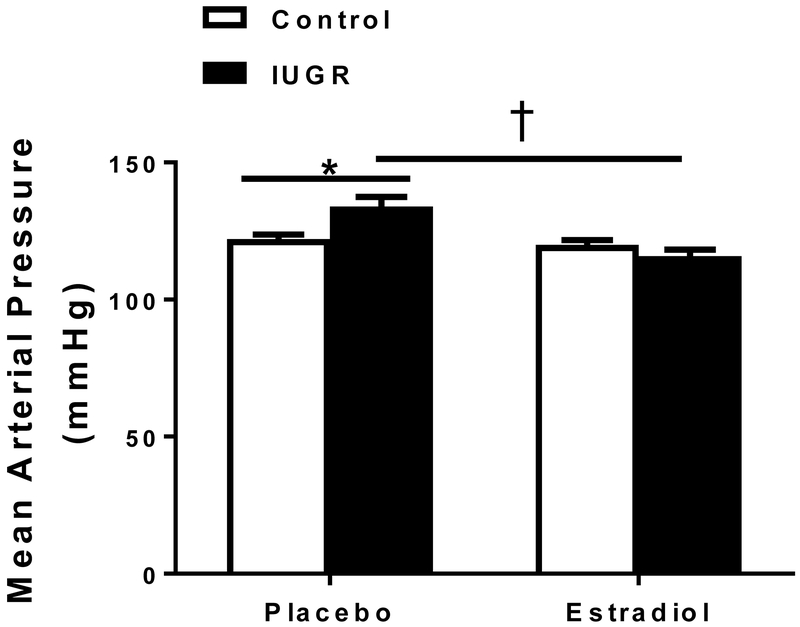
Mean arterial pressure (MAP) was significantly increased in placebo-treated growth-restricted offspring relative to placebo-treated control at 13.5 months of age (Figure 1); a finding previously reported by our lab (17). MAP was significantly reduced in E2-treated growth-restricted relative to placebo-treated growth-restricted (Figure 1). Importantly, MAP in E2-treated growth-restricted offspring was not significantly elevated compared to placebo-treated control. MAP did not differ between placebo-treated and E2-treated control groups.
Figure 1.
Effect of chronic estradiol (E2) treatment on mean arterial pressure (MAP) in female control and intrauterine growth restricted (IUGR) rats treated for 6 weeks with 17β-estradiol pellets (1.5mg; 60-day release) or placebo. MAP was measured at 13.5 months in conscious animals chronically instrumented with a carotid catheter, (n=7-10/group).*P<0.05 vs. placebo-treated control; †P<0.05 vs. placebo-treated IUGR. P<0.05 denotes significance. Data values represent mean±SEM.
Uterine Weight, Serum, and Plasma Estradiol.
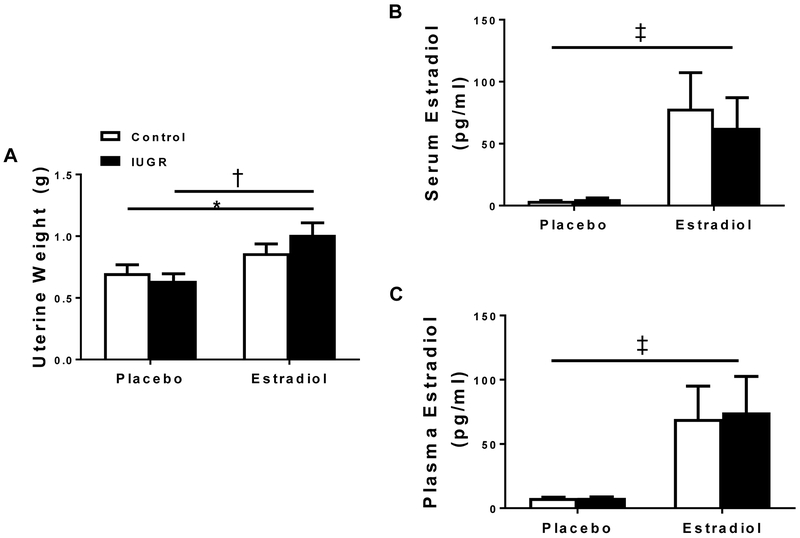
Uterine weight, a crude marker of circulating E2, did not differ in control offspring regardless of treatment group; yet, uterine weight was significantly increased in E2-treated growth-restricted offspring relative to placebo-treated control and placebo-treated growth-restricted (Figure 2A). Circulating E2 levels did not differ between control and growth-restricted offspring regardless of treatment group (Figures 2B and 2C). However, circulating E2 was significantly increased in E2-treated groups compared to placebo groups (Figures 2B and 2C).
Figure 2.
Uterine weight (=7-10/group) and serum estradiol (E2) (n=5-8/group) measured by ELISA or plasma E2 measured by RIA in placebo-treated and E2-treated control and intrauterine growth restricted (IUGR) groups. *P<0.05 vs. placebo-treated control; †P<0.05 vs. placebo-treated IUGR, ‡P<0.05 vs. placebo-treated groups. P<0.05 denotes significance. Data values represent mean±SEM.
Renal mRNA Expression
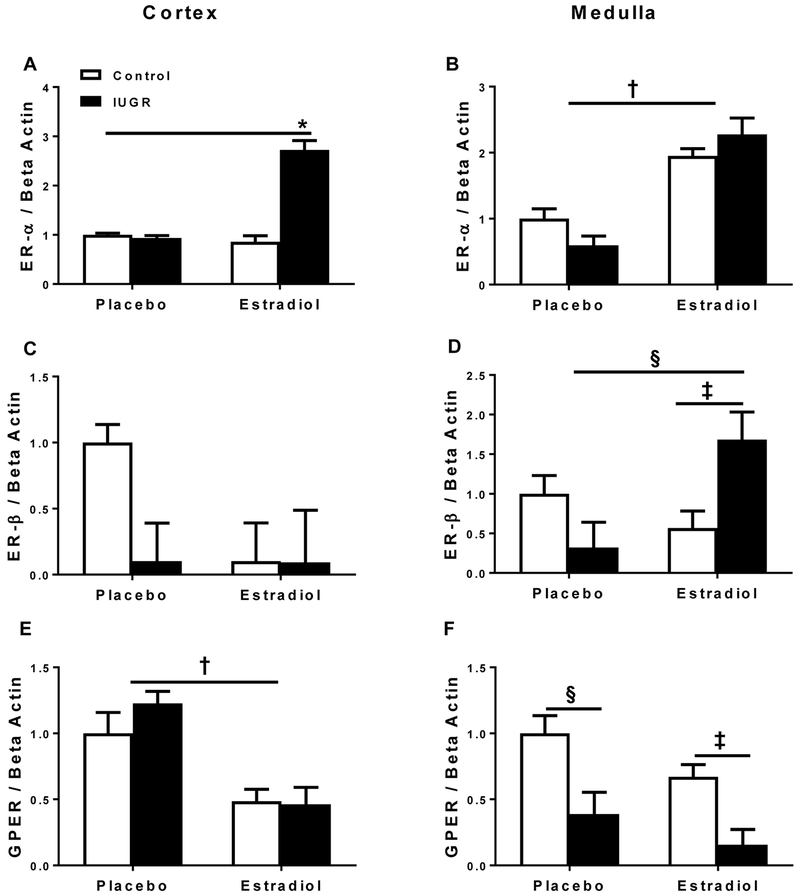
Cortical estrogen receptor alpha (ER-α) mRNA expression did not differ in placebo-treated growth-restricted versus placebo-treated control offspring. However, cortical ER-α mRNA expression was significantly increased in E2-treated growth-restricted offspring relative to all other groups (Figure 3A). Medullary ER-α mRNA expression was significantly increased in E2-treated control and growth-restricted offspring compared to placebo-treated groups (Figure 3B). There was no significant difference in cortical estrogen receptor beta (ER-β) mRNA expression among groups regardless of birth weight or treatment (Figure 3C). Medullary ER-β mRNA expression was significantly increased in E2-treated growth-restricted relative to placebo-treated growth-restricted and E2-treated control (Figure 3D). Cortical mRNA expression of GPER was decreased in both control and growth-restricted E2-treated groups relative to placebo-treated groups (Figure 3E), whereas medullary GPER mRNA expression was decreased in growth-restricted offspring relative to control counterpart (Figure 3F).
Figure 3.
Estrogen receptor alpha (ER-α), estrogen receptor beta (ER-β), and G-protein coupled estrogen receptor 1 (GPER) mRNA expression in the kidney cortex and medulla from placebo-treated and E2-treated control and intrauterine growth restricted (IUGR) rats at 13.5 months of age. Cortical ER-α mRNA expression (A), medullary ER-α mRNA expression (B), cortical ER-β mRNA expression (C), medullary ER-β mRNA expression (D), cortical GPER mRNA expression (E), and medullary GPER mRNA expression (F) in placebo-treated and E2-treated control and IUGR, (n=5-6/group). Data are expressed as fold changes relative to the mean expression level of the placebo-treated control, which is arbitrarily defined as 1. *P<0.05 vs. all groups; †P<0.05 vs. placebo-treated groups; ‡P<0.05 vs. E2-treated control; §P<0.05 vs. placebo-treated IUGR. P<0.05 denotes significance. Data values represent mean±SEM.
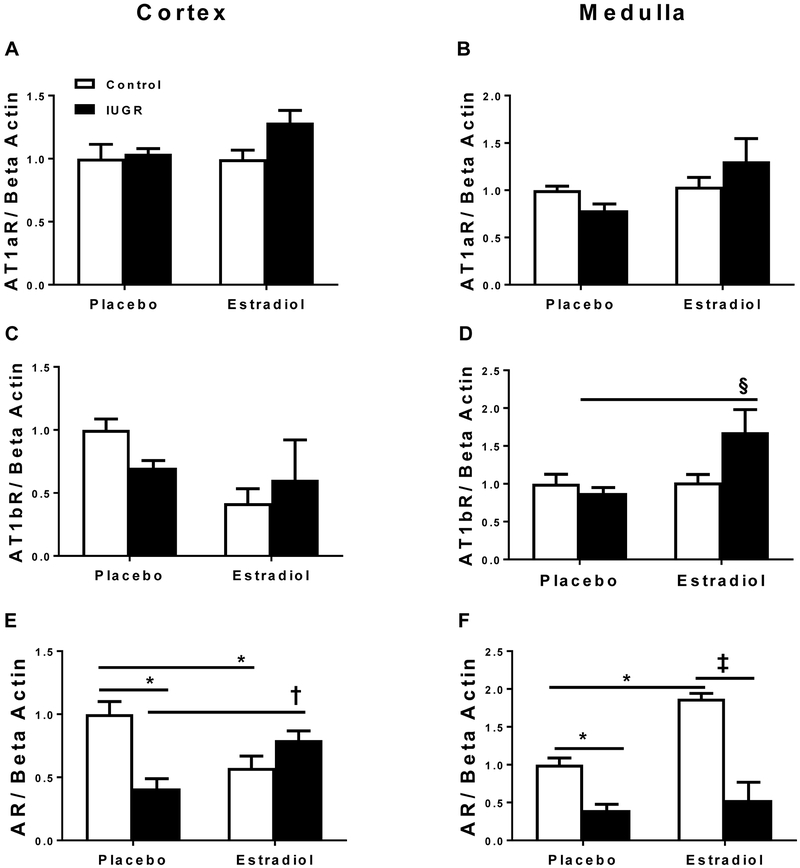
Cortical and medullary Angiotensin Type 1a receptor (AT1aR) mRNA expression did not differ between placebo-treated and E2-treated offspring (Figures 4A and 4B). Cortical Angiotensin Type 1b (AT1bR) mRNA expression also did not differ between groups (Figure 4C). However, medullary AT1bR (Figure 4D) mRNA expression was significantly increased in E2-treated growth-restricted relative to all other groups. Cortical and medullary androgen receptor (AR) mRNA expression was significantly elevated in placebo-treated control in comparison to placebo-treated growth-restricted offspring (Figure 4E and 4F). After E2 supplementation, cortical AR mRNA expression was decreased in E2-treated controls but increased in E2-treated growth-restricted rats relative to their placebo-treated counterparts (Figure 4E). However, in the medulla, AR mRNA expression was increased in E2-treated controls relative to their placebo-treated counterpart (Figure 4F).
Figure 4.
Angiotensin type 1a receptor (AT1aR), angiotensin type 1b receptor (AT1bR), and androgen receptor (AR) mRNA expression in the kidney cortex and medulla from placebo-treated and E2-treated control and intrauterine growth restricted (IUGR) rats at 13.5 months of age. Cortical AT1aR mRNA expression (A), medullary AT1aR mRNA expression (B), cortical AT1bR mRNA expression (C), medullary AT1bR mRNA expression (D), cortical AR mRNA expression (E), and medullary AR mRNA expression (F), in placebo-treated and E2-treated control and IUGR, (n= 5-6/group). Data are expressed as fold changes relative to the mean expression level of the placebo-treated control, which is arbitrarily defined as 1. *P<0.05 vs. placebo-treated control; †P<0.05 vs. placebo-treated IUGR; ‡ P<0.05 vs. E2-treated control; § P<0.05 vs. all groups. P<0.05 denotes significance. Data values represent mean±SEM.
G1 Agonist Study
Mean Arterial Pressure.
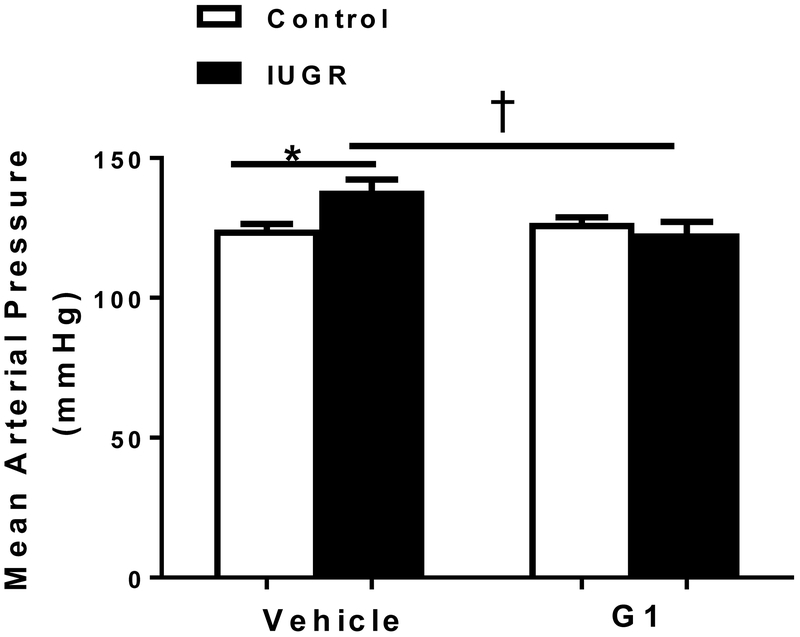
MAP was significantly elevated in vehicle-treated growth-restricted relative to vehicle-treated control (Figure 5). However, MAP was significantly decreased in G1-treated growth-restricted relative to vehicle-treated growth-restricted offspring and did not differ relative to placebo-treated female control (Figure 5).
Figure 5.
Effect of a G protein coupled estrogen receptor 1 (GPER) agonist (G1) on mean arterial pressure (MAP) at 14.5 months of age. Female control and intrauterine growth restricted (IUGR) rats were treated for 2 weeks with a G1 agonist (400 μg/kg/day) or vehicle; (n=5/ group). MAP was measured at 14.5 months of age in conscious animals chronically instrumented animals with a carotid catheter. * P<0.05 vs. vehicle-treated control; †P<0.05 vs. vehicle-treated IUGR. P<0.05 denotes significance. Data values represent mean±SEM.
Renal mRNA Expression
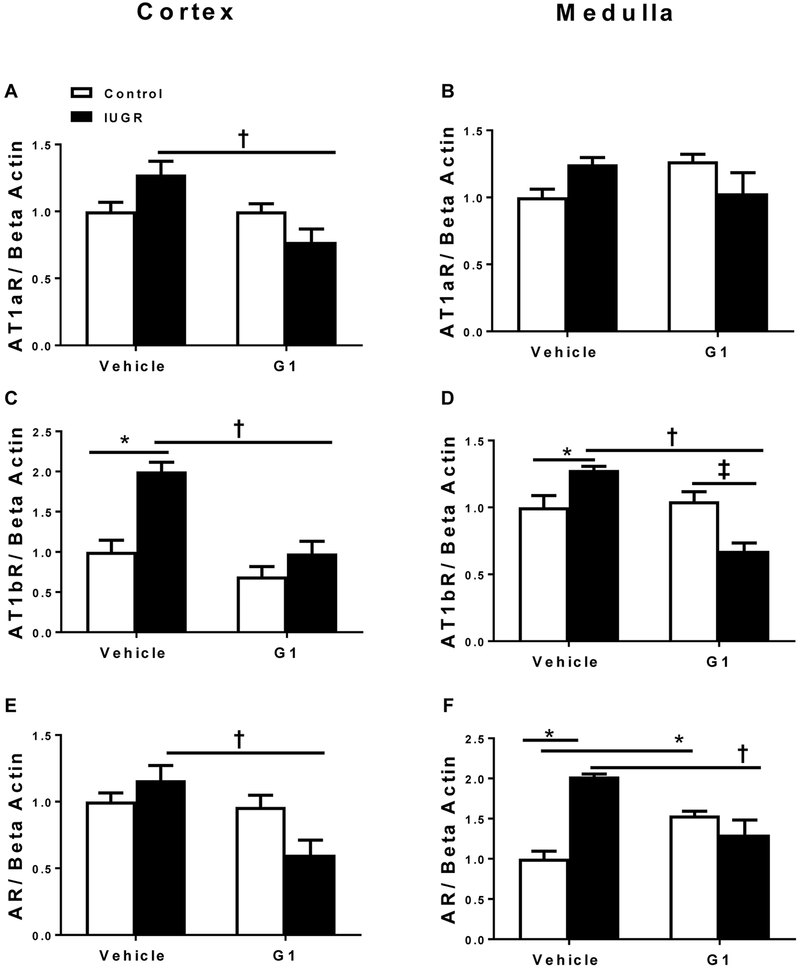
AT1aR cortical mRNA expression was significantly decreased in G1-treated growth-restricted compared to vehicle-treated growth-restricted (Figure 6A). Medullary AT1aR mRNA expression did not differ regardless of birth weight or treatment (Figure 6B). Cortical and medullary mRNA expression of AT1bR was significantly elevated in vehicle-treated growth-restricted relative to vehicle-treated control (Figure 6C and 6D). Cortical and medullary AT1bR mRNA expression was decreased in G1-treated growth-restricted relative to vehicle-treated growth restricted (Figure 6C and 6D). Cortical AR mRNA expression was decreased in G1-treated growth-restricted relative to vehicle-treated growth restricted (Figure 6E). In addition, medullary AR mRNA expression was significantly increased in vehicle-treated growth-restricted compared to vehicle-treated control (Figure 6F). However, medullary AR mRNA expression was decreased in G1-treated growth-restricted relative to vehicle-treated growth restricted. However, AR mRNA expression was increased in G1-treated controls compared to their vehicle-treated counterparts (Figure 6F).
Figure 6.
Effect of a G protein coupled estrogen receptor 1 (GPER) agonist (G1) administration on angiotensin type 1a receptor (AT1aR), angiotensin type 1b receptor (AT1bR), and androgen receptor (AR) mRNA expression in the kidney cortex and medulla from vehicle-treated and G1-treated control and intrauterine growth restricted (IUGR) rats at 14.5 months of age. Cortical AT1aR mRNA expression (A), medullary AT1aR mRNA expression (B), cortical AT1bR mRNA expression (C), medullary AT1bR mRNA expression (D), cortical AR mRNA expression (E), and medullary AR mRNA expression (F), in female vehicle-treated and G1-treated control and IUGR (n=5-6/group). * P<0.05 vs. vehicle-treated control; †P<0.05 vs. vehicle-treated IUGR; ‡P<0.05 vs. G1-treated control. P<0.05 denotes significance. Data values represent mean±SEM.
DISCUSSION
The main findings from this study demonstrated that chronic E2 supplementation abolished the increase in blood pressure that naturally develops around one year of age in intact, female growth-restricted offspring. Administration of the GPER agonist, G1, also abolished the age-related increase in blood pressure in female growth-restricted offspring. Additionally, renal AT1aR and AT1bR mRNA expression were not elevated in G1-treated growth-restricted offspring relative to placebo-treated counterparts. Collectively, these data suggest that administration of E2 was protective against increased blood pressure in female growth-restricted offspring via a mechanism that may involve activation of GPER and its actions on the renin angiotensin system (RAS).
We previously reported that blood pressure is not elevated in female growth-restricted offspring in young adulthood (12), but is elevated in female growth-restricted offspring at 12 months of age in association with an increase in circulating testosterone, but no change in circulating estradiol (18, 19). This shift in the hormonal milieu in female growth-restricted offspring at 12 months of age is associated with cessation of estrous cyclicity 6 months prior to female control, indicative of accelerated reproductive senescence (18). Early onset reproductive senescence (21, 22, 23) and the development of increased blood pressure (24, 25) in female offspring is also reported in other experimental models of developmental origins. Collectively, our studies, in addition to others, suggest that adverse fetal exposures may influence the age at reproductive senescence and program an increased susceptibility to hypertension in female offspring in later life. Therefore, the goal of this project was to determine the importance of the hormonal imbalance in the pathogenesis of elevated BP that develops in intact female growth-restricted offspring by one year of age.
Hypertension in women after menopause is associated with a decrease in estrogens and a shift in the testosterone to estradiol ratio (9). Numerous experimental models of hypertension indicate a protective effect by E2 on BP in ovarian deficient rats in young adulthood (26, 27, 28) or middle age (12 months or older) (29, 30). In the current study, chronic E2 supplementation, initiated at 12 months of age in intact female growth-restricted offspring, resulted in a decrease in BP associated with an increase in uterine weight, a crude marker of circulating estradiol. These data suggest that a shift in the hormonal milieu to a sustained increase in circulating estradiol contributed to the decrease in BP. However, the benefit of hormone replacement in post-menopausal women is highly controversial. The ‘timing’ hypothesis, as discussed in the review by Miller and Harman (31), suggests that administration of hormone replacement within a time span of 10 years or less from the onset of menopause may be beneficial against cardiovascular risk. Thus, in accordance with the timing hypothesis, E2 supplementation in our study may have been administered within a timeframe that was beneficial.
Experimental studies indicate that the beneficial effect of ovarian hormones may involve a role for the RAS, which may differ with age (32). We previously reported that an increase in BP and circulating testosterone in female growth-restricted offspring in later life is associated with an increase in medullary AT1aR and AT1bR mRNA expression (19). This study by Dasinger et al. also demonstrated that BP and medullary mRNA expression of AT1aR and AT1bR are decreased in female growth-restricted offspring chronically treated with flutamide, an androgen receptor antagonist, relative to vehicle-treated counterparts. (19). Moreover, medullary ACE2 mRNA expression was increased in flutamide-treated female growth-restricted offspring (19). Collectively, these data suggested that the protective effect of chronic androgen receptor blockade in female growth-restricted offspring involved a shift in the RAS from the classic vasoconstrictive to the counter-regulatory arm. We also previously reported that BP in female growth-restricted offspring in later life is abolished by the ACE inhibitor, enalapril, indicating a key role for the RAS in the pathogenesis of increased BP that develops by 12 months of age in female growth-restricted offspring (19). In the current study, chronic supplementation with E2 was not associated with a decrease in renal mRNA expression of the AT1 receptors; yet, medullary mRNA expression of ACE2 was increased in E2-treated growth-restricted relative to placebo-treated counterparts.
The biological actions of E2 involve its receptors ER-α, ER-β, GPER (33). E2’s protective role on the cardiovascular system includes increased angiogenesis, increased vasodilation of the vasculature, reduced oxidative stress, and reduced fibrosis (34). E2 may also contribute to blood pressure regulation via its actions on the RAS (35). However, the exact mechanisms by which E2 mediates these effects are not well understood. Recent studies report a vascular (36) and centrally mediated role (37) for ER-β in protection from hypertension. Yet, ER-α and GPER mRNA expression are the predominant receptors found in the kidney of female Sprague Dawley rats (38). Lindsey and colleagues report that activation of GPER, via G1, lowers blood pressure in female mRen2.Lewis rats that develop ovariectomy-induced hypertension (39). Aortic AT1 receptor mRNA expression is also reduced in female ovariectomized, G1-treated mRen2.Lewis rats, (39) implicating a role for GPER in E2-mediated effects on the RAS. Whereas our initial hypothesis was to determine the beneficial effect of chronic E2 supplementation on BP, subsequently we wanted to test the hypothesis that the membrane-bound receptor GPER contributes to the regulation of blood pressure in female growth-restricted offspring through a mechanism that may also involve the renal RAS. Although BP was decreased in G1-treated female growth-restricted offspring, uterine weight did not differ between vehicle and G1-treated offspring indicating that chronic treatment with G1 did not have an effect on ER-α or ER-β activation (40, 41). However, renal ATR1a and ATR1b mRNA expression were not elevated in G1-treated growth-restricted offspring relative to vehicle-treated counterparts suggesting that GPER’s actions on BP may be mediated by suppression of the renal RAS. Renal androgen receptor mRNA expression was also decreased in G1-treated growth-restricted offspring. Vancher et al report that activation of GPER, via G1, results in a decrease in testosterone production in Leydig cells of adult rat and human testis (42). Collectively, these findings suggest a role for GPER activation on BP regulation in female growth-restricted offspring that is mediated via suppression of the angiotensin receptor-dependent vasoconstrictor arm of the renal RAS that may also involve suppression of androgen-dependent mechanisms.
PERSPECTIVES
Previous studies from our laboratory suggest that a shift in the hormonal milieu towards androgen excess precipitates the development of hypertension in female growth-restricted offspring in later life. This study demonstrated that chronic E2 supplementation, resulting in a sustained increase in circulating E2, abolished the increase in BP that spontaneously develops in female growth-restricted offspring by 12 months of age. Whether the greater prevalence of hypertension in LBW women in later life (12) is positively associated with testosterone, and whether hormone replacement in LBW women would be beneficial is unknown. Yet, this study provides insight into one potential mechanism that may contribute to the greater prevalence of increased cardiovascular risk that develops in LBW women as they age, the importance of a shift in the hormonal milieu, and also highlights that timing of hormone supplementation may be most beneficial if administered in close proximity to reproductive senescence.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
Chronic E2 supplementation abolishes increased blood pressure in female growth-restricted offspring that develops in later life via the GPER and its actions on the RAS in a manner that may also involve suppression of androgen-dependent mechanisms.
What Is Relevant?
Timing of hormone supplementation may be most beneficial in low birth weight women if administered in close proximity to reproductive senescence.
Summary
LBW predisposes an individual to increased cardiovascular risk. In LBW women the greater prevalence of hypertension in later life may be a contributory factor. Although the role of hormone replacement therapy in women after menopause is controversial, the current study suggests that correcting an imbalance in the hormonal milieu may be cardio-protective in LBW women.
ACKNOWLEDGEMENTS
Special thanks to Daniel R. Bamrick for technical assistance.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health Grants (NIH) HL074927 and R56HL143459 and the American Heart Grant (AHA) GRNT19900004 (Alexander). Additional funding was provided by HL51971 with support for work performed through the Analytical and Assay Core and the UMMC Molecular and Genomics Facility (MGF) supported in part by funds from P20GM104357and P20GM121334 with additional funds for the MGF provided by P20GM103476. Ms. Davis and Ms. Newsome received funding from NIH T32HL105324, and AHA PRE34060010 (Davis) and NIH F30DK112718 (Newsome). The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
None.
REFERENCES:
- 1.Benjamin EJ, Virani SS, Callaway CW et al. Heart disease and stroke statistics- 2018 update: a repost from the American Heart Association. Circulation. 2018; 137:e67–492. [DOI] [PubMed] [Google Scholar]
- 2.Muiesan ML, Salvetti M, Rosei CA, Paini A. Gender difference in antihypeternsive treatment: myths or legends? High Blood Press Cardiovasc Prev. 2016; 23:105–13. [DOI] [PubMed] [Google Scholar]
- 3.Barton M, Meyer MR. Postmenopausal hypertension: mechanism and therapy. Hypertension. 2009; 54:11–8. [DOI] [PubMed] [Google Scholar]
- 4.Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. A Single Pill to Treat Postmenopausal Hypertension? Not Yet. Current Topics in Medicinal Chemistry. 2011; 11(13):1736–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and Its Treatment in Postmenopausal Women. Hypertension. 2000;36:780–789 [DOI] [PubMed] [Google Scholar]
- 6.Yanes LL, Romero DG, Iliescu R, Zhang H, Davis D, Reckelhoff JF. Postmenopausal hypertension: role of the Renin-Angiotensin system. Hypertension. 2010; 56(3):359–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanes LL, Romero DG, Cucchiarelli VE, Fortepiani LA, Gomez-Sanchez CE, Santacruz F, Reckelhoff JF. Role of endothelin in mediating postmenopausal hypertension in a rat model. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2005; 288(1):R229–R233. [DOI] [PubMed] [Google Scholar]
- 8.Maranon RO, Lima R, Mathbout M, do Carmo JM, Hall JE, Roman RJ, Reckelhoff JF. Postmenopausal hypertension: role of the sympathetic nervous system in an animal model. Am J Physiol Regul Integr Comp Physiol. 2014; 306(4):R248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yanes LL, Reckelhoff JF. Postmenopausal Hypertension. American Journal of Hypertension. 2011; 24(7):740–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lackland DT, Egan BM, Ferguson PL. Low Birth Weight as a Risk Factor for Hypertension. The Journal of Clinical Hypertension. 2003; 5:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson SW, Lapidus L, Niklasson A, Hallberg L, Bengtsson C, Hulthen L. Blood pressure and hypertension in middle-aged women in relation to weight and length at birth: a follow-up study. J Hypertens. 2000; 18:1753–61. [DOI] [PubMed] [Google Scholar]
- 12.Alexander BT. Placental insufficiency leads to development of hypertension in growth restricted offspring. Hypertension. 2003; 41(3): 457–62. [DOI] [PubMed] [Google Scholar]
- 13.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005; 289: R1131–R1136. [DOI] [PubMed] [Google Scholar]
- 14.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003; 41(2): 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002; 105:1088–1092. [DOI] [PubMed] [Google Scholar]
- 16.Tom SE, Cooper R, Kuh D, Guralnik JM, Hardy R, Power C. Fetal environment and early age at natural menopause in a British birth cohort study. Human Reproduction. 2010; 25(3):791–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Intapad S, Tull L, Brown A, Dasinger J, Ojeda N, Fahling J, Alexander B. Renal Denervation Abolishes the Age-Dependent Increase in Blood Pressure in Female Intrauterine Growth-Restricted Rats at 12 Months of Age. Hypertension. 2013; 61(4):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Intapad S, Dasinger J, Brown A, Fahling J, Esters J, Alexander B. Glucose intolerance develops prior to increased adiposity and accelerated cessation of estrous cyclicity in female growth-restricted rats. Pediatric Research. 2016; 79(6):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasinger JH, Intapad S, Rudsenske BR, Davis GK, Newsome AD, Alexander BT. Chronic Blockade of the Androgen Receptor Abolishes Age-Dependent Increases in Blood Pressure in Female Growth-Restricted Rats. Hypertension. 2016; 67(6):1281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojeda N, Grigore D, Robertson E, Alexander B. Estrogen Protects Against Increased Blood Pressure in Postpubertal Female Growth Restricted Offspring. Hypertension. 2007;50(4):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernal AB, Vickers MH, Hampton MB, Poynton RA, Sloboda DM. Maternal undernutrition significantly impacts ovarian follicle number and increases ovarian oxidative stress in adult rat offspring. PLoS One. 2010; 5(12):e15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossa F, Carter F, Walsh SW, Kenny DA, Smith GW, Ireland JL, Hildebrandt TB, Lonergan P, Ireland JJ, Evans AC. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol Reprod. 2013; 88(4):92. [DOI] [PubMed] [Google Scholar]
- 23.Khorram O, Keen-Rinehart E, Chuang TD, Ross MG, Desai M. Maternal undernutrition induces premature reproductive senescence in adult female rat offspring. Fertil Steril. 2015; 103(1):291–8.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pijacka W, Clifford B, Tilburgs C, Joles JA, Langley-Evans S, McMullen S. Protective role of female gender in programmed accelerated renal aging in the rat. Physiol Rep. 2015; 3(4).pii: e12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran M, Young ME, Jefferies AJ, Hryciw DH, Ward MM, Fletcher EL, Wlodek ME, Wadley GD. Uteroplacental insufficiency leads to hypertension, but not glucose intolerance or impaired skeletal muscle mitochondrial biogenesis, in 12-month-old rats. Physiol Rep. 2015; 3(9). pii: e12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue B, Beltz TG, Guo F, Johnson AK. Sex differences in maternal gestational hypertension-induced sensitization of angiotensin II hypertension in rat offspring: the protective effect of estrogen. Am J Physiol Regul Integr Comp Physiol. 2018; 314(2):R274–R281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chappell MC, Gallagher PE, Averill DB, Ferrario CM, Brosnihan KB Estrogen or the AT1 antagonist olmesartan reverses the development of profound hypertension in the congenic mRen2. Lewis rat. Hypertension. 2003;42(4):781–6 [DOI] [PubMed] [Google Scholar]
- 28.Xiao D, Huang X, Yang S, Zhang L. Estrogen normalizes perinatal nicotine-induced hypertensive responses in adult female rat offspring. Hypertension. 2013; 61(6):1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004; 44(4):405–9. [DOI] [PubMed] [Google Scholar]
- 30.Peng N, Clark JT, Wei CC, Wyss JM. Estrogen depletion increases blood pressure and hypothalamic norepinephrine in middle-aged spontaneously hypertensive rats. Hypertension. 2003; 41(5):1164–7. [DOI] [PubMed] [Google Scholar]
- 31.Miller VM, Harman SM. An update on hormone therapy in postmenopausal women: mini-review for the basic scientist. American Journal of Physiology. Heart and Circulatory Physiology. 2017; 313(5):H1013–H1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2.Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med. 2008; 5 Suppl A:S65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhushankar R, Krueger C, Manrique C. Membrane estrogen receptors: their role in blood pressure regulation and cardiovascular disease. Curr Hypertens Rep. 2014; 16(1):408. [DOI] [PubMed] [Google Scholar]
- 34.Iorga A, Cunningham CM, Moazeni S, Ruffenach G, Umar S, Eghbali M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biology of Sex Differences. 2017; 8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Donnell E, Floras JS, Harvey PJ. Estrogen status and the renin angiotensin aldosterone system. Am J Physiol Regul Integr Comp Physiol. 2014; 307(5): R498–500. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Dhaul PW, Thorén P, Smithies O, Gustafsson J, Mendelsohn ME. Abnormal Vascular Function and Hypertension in Mice Deficient in Estrogen Receptor β. Science. 2002; 505–508. [DOI] [PubMed] [Google Scholar]
- 37.Xue B, Zhang Z, Beltz TG, et al. Estrogen receptor-β in the paraventricular nucleus and rostroventrolateral medulla plays an essential protective role in aldosterone/salt-induced hypertension in female rats. Hypertension. 2013; 61(6):1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutson DD, Gurrala R, Ogola BO, et al. Estrogen receptor profiles across tissues from male and female Rattus norvegicus. Biol Sex Differ. 2019; 10(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsey SH, Cohen JA, Brosnihan KB, Gallagher PE, Chappell MC. Chronic treatment with the G protein-coupled receptor 30 agonist G-1 decreases blood pressure in ovariectomized mRen2.Lewis rats. Endocrinology. 2009; 150(8):3753–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci U S A. 1993; 90(23):11162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada-Hiraike O, Hiraike H, Okinaga H, et al. Role of estrogen receptor beta in uterine stroma and epithelium: Insights from estrogen receptor beta−/− mice. Proc Natl Acad Sci U S A. 2006; 103(48):18350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaucher L, Funaro MG, Mehta A, et al. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS One. 2014; 9(4):e92425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon acceptance, we will make all data underlying the findings described in our manuscript fully available without restriction in compliance with Hypertension’s policy.