Abstract
Genetic variants in APOL1, encoding apolipoprotein L1, are major drivers of glomerular disease in peoples of sub-Saharan African descent. APOL1-associated primary glomerular diseases include focal segmental glomerulosclerosis (FSGS), HIV-associated nephropathies and aterionephrosclerosis. Other conditions where APOL1 variants affect outcomes include membranous nephropathy, lupus nephritis, diabetic nephropathy, pre-eclampsia, and kidney transplant. In FSGS, APOL1-variants are associated with upregulation of RNA encoding chemokine C-X-C motif receptor 3 (CXCR3) ligands and ubiquitin D; the significance of these findings remains unclear but may provide valuable insight into disease mechanisms.
Introduction
Using admixture scans, two groups identified a genetic region on chromosome 22 with an excess of African ancestry among African Americans with kidney disease compared to African Americans without kidney disease1, 2 . In 2010, two research groups identified the main signal was coming from APOL1 genetic variants within this region3, 4 . In the intervening eight years, much has been learned about the spectrum and manifestations of APOL1 nephropathies, including histopathologic findings, clinical course, response to therapy, role in kidney transplant, and molecular and cellular mechanisms of disease. It is estimated that the APOL1 genetic variants account for approximately 70% of the excess risk for kidney disease that characterizes the African American population5 . The field is highly active, and while much has been learned much remains to be understood about the mechanism of APOL1-mediated injury.
APOL1 genetic variants
APOL1 encodes apolipoprotein L1, a minor protein constituent of high density lipoprotein. In 2010, genetic variants in APOl1 were found to be strongly associated with glomerular disease3, 4 . The two kidney risk variants are located in the C terminal domain of the protein, which participates in host defenses against trypanosomal infection. The G1 variant comprises two point mutations, S342G and I384M; these are very closely linked but occasional individuals will have only the first mutation (S342G), which is thought to be pathogenic and the second to be carried forward by linkage. The G2 variant consists of a 6 base pair deletion, resulting in deletion of amino acids 388N-389Y.
It is estimated that these variants arose ~10,000 years ago. There were several out-of-Africa dispersals prior to this time point, which explains why the variants are restricted to populations with (recent) African ancestry; by contrast with the African ancestry inherent in all humans. Current genetic and archaeological evidence suggests that there were migrations of modern humans (as distinct from Neanderthal and other extinct populations) out of Africa and into Eurasia beginning between 75,000 and 45,00 years ago (or perhaps, more conservatively, 40,00 to 100,000 years ago)6, 7 . Among populations with present-day descendants, it appears that one out of Africa expansion occurred ~70,000 years ago, giving rise to south Asia and Australian populations, and another expansion occurred prior to ~55,000 years ago, giving rise to European and north Asian populations.
Focal segmental glomerulosclerosis
In 2011, Kopp and colleagues studied 217 African Americans and 168 European Americans with focal segmental glomerulosclerosis (FSGS,) most of whom presented as adults8 . A histopathologic diagnosis, in particular presence or absence of FSGS (any pattern), was extracted from the pathology reports. Carriers of two APOL1 risk alleles had an odds ratio (OR) for FSGS cases of 17, compared to healthy volunteers. APOL1-status was defined as high risk (HR) if 2 risk alleles were present or low risk (LR) if zero or 1 risk allele was present. Among APOL1 HR FSGS cases, 70% presented between the ages of 15 and 39, compared to a bimodal distribution -- 15–39 (42%) and >40 yr (48%) -- for non-APOL1 LR FSGS. Response rates to 8 weeks of glucocorticoid therapy were similar between individuals with HR genotypes (29%) and LR genotypes (33%). However, HR genotype subjects progressed more rapidly to end-stage kidney disease (ESKD), with a median renal survival of 5 years for the HR genotypes and 13 years for the LR genotype. More rapid progression to ESKD is a finding that characterizes essentially all APOL1-associated renal syndromes.
The Nephrotic Syndrome Study network (NEPTUNE) was initiated in 2010 to broaden our knowledge of primary nephrotic diseases. At entry, as part of a diagnostic kidney biopsy, a core is taken and glomeruli, tubules, and interstium are microdissected, yielding tissue for two transcriptional profiles (glomeruli versus tubulointerstitium). Sampson and colleagues compared transcriptional profiles between APOL1 HR and APOL1 LR African American subjects (a total of 90 samples) and found the former was characterized by increased glomerular expression of CXCL9, encoding C-X-C chemokine ligand 9, CXCL11 and UBD (encoding ubiquitin D, also known as FAT10) and increased tubulointerstitial expression of SNOR14B (small non-coding RNA 14B) and MUC139 . Interestingly, the transcripts were found to be up-regulated in APOL1 transgenic mice, in which the tetracycline-inducible nephrin promoter drives APOL1 transgene expression10 . This striking replication suggests that the transcriptional findings are robust –- but how does it inform our understanding of pathogenesis of APOL1-related nephropathies? We are just beginning to get answers to this question.
APOL1 variant pathways: CXCR3 signaling
Further study of CXCR3 pathway activation in podocytes may help decipher how the APOL1 variants induce glomerular damage. CXCL9, CXCL10, and CXCL11 are closely related cytokines that are induced by interferon gamma (including in renal tubular epithelium) and that are ligands for the chemokine receptor CXCR3 (CD183). By contrast, CXCR3 is expressed by immune cells (T lymphocytes, NK cells, and macrophages). Ligand binding to and activation of CXCR3 is believed to be a key driver of the influx of these cells into the renal interstitium (A comprehensive review of this receptor and its ligands in the setting of renal transplant is available11 ).
Podocytes also manifest CXCR3 signaling. In cultured podocytes, antibodies to CXCL10 decreased the differentiation state, with reduced expression of slit-diaphragm proteins12 . CXCL10 induced expression of the cyclin-dependent kinase inhibitor 1B (p27/Kip1) and reduced expression of cyclins A and E, associated with reduced podocyte proliferation in vivo. As podocytes generally do not proliferate in vivo, the relevance of these latter findings is uncertain, although it might relate also to parietal epithelial cells, a likely proliferative compartment in the adult glomerulus. More recently, Yu and colleagues showed that advanced glycation end-products, applied to mouse podocytes, increased expression of CXCL9 and CXCR3, leading to increased Stat3 phosphorylation, which could be inhibited by JNK inhibitors13 .11 . CXCR3 has roles in non-inflammatory conditions as well. Subjects with nephropathy in the setting of type 2 diabetes have increased urine levels of CXCL914 . These findings raise the question as to whether APOL1 is driving cytokine expression or, perhaps more likely, that APOL1 and cytokine expression is driven by a common interferon-mediated mechanism.
CXCR3 activation has diverse effects on cell signaling. First, it triggers G protein signaling, leading to activation of several signaling pathways. Second, it activates JAK/STAT signaling which promotes cell activation. Third, CXCR3 activates phosphoinositide-3 kinase (PI-3K), which in turn activates protein kinase C (promoting B cell survival) and Rho GTPase activity (actin reorganization, affecting cell polarity adhesion and motility). CXCR3 signaling also activates protein kinase C, which via generation of diacylglycerol activates phospholipase C beta which generates inositol triphosphate, triggering intracellular calcium release and thereby activating the extracellular receptor kinase (ERK), p38 kinase and reinforcing JNK activation.
Animal models of kidney disease have been used to study modulation of CXCR3 activation. These studies suggest that CXCR3 signaling in the kidney has protective roles, at least in some settings, and that down-regulation of this pathway in the setting may have adverse outcomes. Thus blockade of CXCL10/CXCR3 signaling had significant but modest effects to promote TGFβ signaling and progressive renal fibrosis in mouse model; this occurred without affecting macrophage or T cell infiltration, raising the possibility of direct effects on renal cells15 . Paust and colleagues generated mice lacking CXCR3 expression in regulatory T cells (Tregs) (Paust, Riedel et al. 2016). Following experimental induction of glomerulonephritis, fewer Tregs were recruited to the kidneys of the mutant mice and the mice manifested more severe glomerulonephritis, demonstrating a role for CXCR3+ Tregs in tamping down immune activation in this model.
Several clinical studies have addressed the correlates of CXCR3 activation in the kidney, which serves to limit inflammatory cell immigration and dysfunction. In patients with anti-neutrophil cytoplasmic antibody (ANCA) vasculitis, kidney infiltrates are enriched in Tregs expressing CXCR316 . In human renal transplant rejection, most studies suggest that CXCR3 is activated and elevation of ligand levels, expressed in kidney or present in urine, is associated with rejection or risk of rejection11 . In human lupus nephritis, increased CXCR3 expression is associated with impaired renal function. In membranous nephropathy, CXCR3 is up-regulated in podocytes17 . Urine levels of CXCL9 are increased in human diabetic nephropathy14 .
The significance of these findings for APOL1 glomerular disease is far from clear. It may be that signaling via CXCR3 contributes to podocyte injury and targeting this pathway might be beneficial. On the other hand, inhibiting the effects of CXCR3 to limit interstitial inflammation could theoretically worsen renal disease. Further studies in relevant transgenic mouse models of APOL1 nephropathy may help to clarify these uncertain areas, but as human immunobiology and mouse immunobiology may differ, clinical trials will ultimately be required for definitive answers in humans
APOL1 variant pathways: ubiquitin and SNOR14B
Ubiquitins function by attaching themselves to other protein, which are then targeted for transport to the 28S proteasome where the multiprotein complex undergoes degradation. UBD may have other functions, including in tumor necrosis factor (TNF)-induced translocation of p65 to the nucleus, and mediation of apoptosis in a caspase dependent manner. This function has been shown to play a role in tubular cell dysfunction in polycystic kidney disease and in HIV-associated nephropathy, the kidney disease most strongly associated with APOL1 variants18 . Somewhat surprisingly, ubiquitins may also function in the extracellular space. CXCR4 binds ubiquitin, as well as binding CXCL12 (SDF1) and HIV envelope protein gp12019 . There have been no studies addressing potential molecular interactions between UBD and CXCR3, the ligand for CXCL9, 10 and 11 discussed above. How ubiquitin might contribute to APOL1-related pathways remains unknown.
Finally, SNOR14B is a small non-coding nucleolar RNA, an RNA class that has multiple functions. SNORs facilitate the processing of other non-coding RNAs, especially ribosomal RNAs. Thus, they guide chemical modification (e.g. methylation) of other RNA species, including ribosomal RNA and transfer RNA. SNOR also contribute to RNA splicing and to miRNA dependent gene silencing. Jorjani and colleagues used data from the ENCODE project to report that the human genome contains at least 750 expressed SNOR sequences20 . The typical SNOR is 70–160 bp long. SNORs originate from intronic sequences. SNOR RNAs may be present in the nucleus and cytoplasm. What the target of SNOR14B might be and how this particular SNOR might affect glomerular pathobiology are unknown.
FSGS classification: where does APOL1 FSGS fit?
FSGS can be divided, according to etiology, into six parts, making it twice as complex as Caesar’s Gaul, which had only three parts. These include three common forms, primary FSGS, adaptive FSGS, and APOL1 FSGS (the latter being common among countries with African-descent populations), as well as three less-common forms, high-penetrance genetic FSGS (currently identified with variants over 50 genes, both nuclear and mitochondrial), infection-associated FSGS (including cases associated with HIV, CMV and EBV infection ), and medication associated FSGS (e.g. lithium, pamidronate, and others). In this classification scheme, APOL1 has its own class of FSGS, defined by genetic testing but also contributes disproportionately to viral FSGS (most notably HIV-associated FSGS and HIV-associated nephropathy). The odds ratio (OR) for HIV collapsing glomerulopathy or HIV FSGS among subjects with HIV carrying two APOL1 risk alleles is 29 in the USA, among African Americans, and 89 in South Africa, among Africans. The OR for APOL1 two risk alleles for other forms for FSGS among African Americans in the USA is 17.
FSGS can be categorized by histologic appearance, as per the Columbia classification: collapsing variant, tip-lesion, variant, perihilar variant, cellular variant and not otherwise specified FSGS. Among these, collapsing FSGS is particularly associated with APOL1 variants, but it appears that APOL1 nephropathy can manifest as most if not all of the other forms.
FSGS can be categorized by steroid sensitivity. APOL1 FSGS can manifest as both steroid sensitive and steroid -resistant FSGS8 . While it might seem odd that a genetic form of FSGS can be steroid sensitive, it may be that steroids suppresses renal inflammation and inflammation contributed to stimulation of interferon expression, one could imagine that inflammation would be associated with enhanced APOL1 expression.
APOL1 and Hypertension
Persistent hypertension is associated with CKD in many populations, although it is not always clear whether hypertension is primary or whether intrinsic glomerular or tubular disease that promotes hypertension. Hypertension-attributed kidney disease is generally characterized by reduced glomerular filtration rate, modest glomerular proteinuria (urine protein/creatinine <1 g/g and often < 0.5 g/g), and a history of sustained hypertension, usually poorly controlled. Hypertension-attributed chronic kidney disease (CKD) is common in most populations around the world. Control of blood pressure often halts or slows progressive loss of glomerular filtration rate (GFR)
African Americans have higher rates of hypertension, but he data assessing the relationship between APOL1 variants and hypertension per se are conflicting. In the longitudinal CARDIA study, involving 1330 blacks followed from young adulthood through middle age, no association of hypertension with APOL1 variants was shown21 . By contrast, in a much larger longitudinal study (5201 subjects in the discovery cohort and 3999 subjects in the replication cohorts), APOL1 risk alleles were associated with higher systolic blood pressure (1 mm Hg) and diastolic blood pressures, starting in the third decade, followed by a decline in estimated GFR in the fourth decade22 . The differences between these two studies may well result from the greater statistical power of the larger cohort and future replication efforts will help to clarify these relationships.
Arteriosclerosis and arteriolosclerosis
Chronic hypertension is associated with CKD in all racial and ethnic populations studied, and the causal relationships are likely bidirectional. Physiologically, hypertension drives renal injury via activation of the renal-angiotensin-aldosterone system (RAAS), glomerular hypertension, oxidative stress, and endothelial dysfunction23 , while CKD promotes hypertension via similar pathways, as well as by promoting sodium retention. Johnson and colleagues have proposed that subtle renal injury, such as arteriolosclerosis, may impair the ability to excrete sodium, leading to salt-sensitive hypertension24 . Salt sensitivity is more common among individuals of sub-Saharan African ancestry, and a genetic predisposition may play a role. However, to date, there is no evidence that APOL1 variants are involved in the pathogenesis of essential hypertension25 .
Absent an association between APOL1 genetic variants and hypertension risk, it is plausible consider a relationship between APOL1 variants and subtle renal microvascular injury (predisposing to overt glomerular injury) in arteriosclerosis. However, the data do not yet support this. In a study of autopsy kidneys from Mississippi, Hughson et al. found that APOL1 high risk status was associated with more severe arteriosclerosis in the interlobular arteries, vessels generally associated with hypertension, but were unable to find an association between arteriolar hyalinization and APOL1 high risk status (Hughson 2106). However, it remains unclear whether hypertension or interlobular arteriosclerosis is the initiator of the dysregulation of renal microvascular function. Another alternative that remains to be tested is whether there may be physiologic changes in arteriolar function in these individuals that is not apparent on histopathologic assessment.26 .
Other topics: characteristic APOL1 renal pathologies, glomerular diseases, glomerular numbers
The histopathologic pattern most closely associated with APOL1 variants is collapsing glomerulopathy. In addition to this pattern being predominantly observed in HIV associated nephropathy, collapsing glomerulopathy has now been associated with APOL1 status in the setting of systemic lupus27 , membranous nephropathy28 and jn association with exogenous interferons29 . Larsen and colleagues ascribed the solidified/disappearing pattern of global glomerulosclerosis and the thyroidization and microcystic dilatation pattern of tubular atrophy, in the absence of HIV, to the ApoL1 high risk allele status and arterionephrosclerosis {30 }. By contrast, zero APOL1 risk alleles had more severe arteriosclerosis. By contrast, less apparent from a histopathologic perspective, is the epidemiologic association in a case control study between APOL1 high risk status and more rapid progression to ESKD type 2 diabetic subjects5, 31 . It remains unclear whether characteristic features of APOL1 nephropathy such as FSGS and more severe renal arteriosclerosis are also present and future cohorts exploring the histology of these individuals will be needed5 .
These observations, taken together, are consistent with a view that African Americans with zero APOL1 risk alleles have a form of nephrosclerosis where hypertension, smoking, obesity, hyperlipidemia, and chronic inflammation may contribute to renal microvascular injury. By contrast, those factors may contribute in subjects with two APOL1 risk alleles but the most important driver of histopathologic aberration may be the APOL1 risk variants.
One more confounder of African American kidney disease risk is the reduced nephron number, as demonstrated by painstaking studies of autopsy kidneys in Black population from Mississippi32 . These autopsies were generally carried out for sudden death cases, and kidneys showing glomerular or tubular disease were excluded. Over the first 38 years of life, regression analysis suggested that nephrons were lost at a rate of approximately 9000 nephrons per kidney per year, out of an average total glomerular number at baseline of about 900,000. Over that time span, the data suggested that APOL1 high risk subjects (combing one and two risk alleles) lost 350,000 nephrons per single kidney, while APOL1 low risk subjects lost 300,000 nephrons, although this did not reach statistical significance. Interestingly, the effect was similar among those with one and with two APOL1 risk alleles, although the sample size was limited. Age-related nephron losses correlated with increases in glomerular size in APOL1 high risk subjects. This suggests the possibility that compensatory increases in glomerular size, likely associated with increased pressures and flows in the glomerular capillaries, may contribute to glomerular injury and ultimately to glomerular senescence.
Conclusions
Since the identification of the APOL1 renal risk variants in 2010, much has been learned but more remains to be done. In cell culture models, the risk variants alter the function of diverse cellular pathways. For prospective interventions to ameliorate disease progression, we need to know which pathways are relevant in vivo. The intriguing data from human subjects relating to altered expression of chemokines and ubiquitin D may be useful to better define potential therapeutic targets. Various transgenic mouse models have been developed and can be exploited to answer some of these questions and to test novel therapeutic approaches.
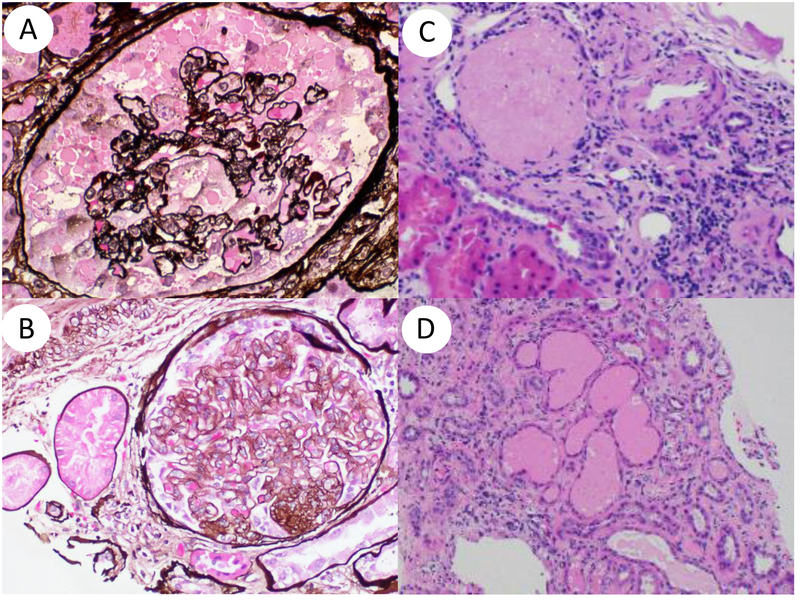
Figure 1. APOL1nephropathy histopathologic patterns.
Shown are collapsing glomerulopathy (A), focal segmental glomerulosclerosis (B), focal global glomerulosclerosis (disappearing obsolescent glomerulus) (C) and microcystic dilatation and tubular atrophy (D).
Table 1.
Associations of APOL1 with kidney diseases – would this be useful?
| Disease | Associations of APOL1 high risk status |
|---|---|
| HIV-associated nephropathy | OR 29 in USA, OR 89 in South African |
| Focal segmental glomerulosclerosis | OR 17 |
| Lupus nephritis | Progression to ESKD, OR 2.7 Collapsing glomerulopathy, OR 5.4 |
| Hypertension-attributed nephropathy | Progression to serum creatinine >3, OR 4.6 |
| Diabetes and kidney disease | Progression rate, 1.32 ml/min/yr in APOL1 HR group, 1.07 ml/min/yr in APOL1 LR group |
Shown are the OR for carriage of two APOL1 risk alleles for the various conditions shown.
Table 2.
Proposed APOL1 variant-driven injury mechanisms
| Mechanisms | Downstream effects |
|---|---|
| CXCR3 stimulation via CXCL9, CXCL10, CXCL11 | Activation of stress kinases (p44/42 MAPK), phosphoinositide 3-kinase |
| Opening cation channels | Activation of stress kinases (p44/42 MAPK) |
| Activation of integrin alpha V beta 3 | Altered cell adhesion |
| Mitochondrial dysfunction | Potential energy deficit., altered metabolic function |
| Impaired endolysosmal dysfunction | Impaired cell function, possibly premature senescence |
| NLRP3 inflammasome activation | Generation of IL-1beta, inflammation |
| PKR activation | Impairment of protein synthesis |
Most injury pathways have been studied in cell culture models and a few have been studied in mouse models; hence the relevance of particular mechanisms to human disease states and in particular whether the pathway is of central relevance to human disease pathogenesis remains to be established.
Acknowledgement.
This work was supported in part by the Intramural Research Program, NIDDK, NIH via project ZO1 DK043308 (JBK) and the National Kidney Foundation of the National Capital Area, Joseph M. Krainin, MD, Memorial Young Investigator Award (NKF). we appreciated the manuscript review by Dr. Ben Afzali.
Footnotes
Disclosure. The authors declare no conflict of interest.
Contributor Information
Jeffrey B. Kopp, Kidney Diseases Branch, NIDDK, NIH, Bethesda, MD.
Avi Z. Rosenberg, Department of Pathology, Johns Hopkins Medical Institutions, Baltimore, MD.
References
- [1].Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet 2008;40: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet 2008;40: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010;329: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010;128: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. The New England journal of medicine 2013;369: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nielsen R, Akey JM, Jakobsson M, et al. Tracing the peopling of the world through genomics. Nature 2017;541: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Groucutt HS, Petraglia MD, Bailey G, et al. Rethinking the dispersal of Homo sapiens out of Africa. Evol Anthropol 2015;24: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kopp JB, Nelson GW, Sampath K, et al. APOL1 Genetic Variants in Focal Segmental Glomerulosclerosis and HIV-Associated Nephropathy. J Ame Soc Nephrol 2011;22: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sampson MG, Robertson CC, Martini S, et al. Integrative Genomics Identifies Novel Associations with APOL1 Risk Genotypes in Black NEPTUNE Subjects. J Am Soc Nephrol 2016;27: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beckerman P, Bi-Karchin J, Park AS, et al. Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dabiri S, Kariminik A, Kennedy D. The role of CXCR3 and its ligands in renal transplant outcome. Eur Cytokine Netw 2016;27: [DOI] [PubMed] [Google Scholar]
- [12].Han GD, Suzuki K, Koike H, et al. IFN-inducible protein-10 plays a pivotal role in maintaining slit-diaphragm function by regulating podocyte cell-cycle balance. J Am Soc Nephrol 2006;17: [DOI] [PubMed] [Google Scholar]
- [13].Yu J, Wu H, Liu ZY, et al. Advanced glycation end products induce the apoptosis of and inflammation in mouse podocytes through CXCL9-mediated JAK2/STAT3 pathway activation. Int J Mol Med 2017;40: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Higurashi M, Ohya Y, Joh K, et al. Increased urinary levels of CXCL5, CXCL8 and CXCL9 in patients with Type 2 diabetic nephropathy. J Diabetes Complications 2009;23: [DOI] [PubMed] [Google Scholar]
- [15].Nakaya I, Wada T, Furuichi K, et al. Blockade of IP-10/CXCR3 promotes progressive renal fibrosis. Nephron Exp Nephrol 2007;107: [DOI] [PubMed] [Google Scholar]
- [16].Paust HJ, Riedel JH, Krebs CF, et al. CXCR3+ Regulatory T Cells Control TH1 Responses in Crescentic GN. J Am Soc Nephrol 2016;27: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huber TB, Reinhardt HC, Exner M, et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. J Immunol 2002;168: [DOI] [PubMed] [Google Scholar]
- [18].Kasembeli AN, Duarte R, Ramsay M, et al. APOL1 Risk Variants Are Strongly Associated with HIV-Associated Nephropathy in Black South Africans. J Am Soc Nephrol 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Peng D, Cao B, Zhou YJ, et al. The chemical diversity and structure-based evolution of non-peptide CXCR4 antagonists with diverse therapeutic potential. Eur J Med Chem 2018;149: [DOI] [PubMed] [Google Scholar]
- [20].Jorjani H, Kehr S, Jedlinski DJ, et al. An updated human snoRNAome. Nucleic Acids Res 2016;44: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen TK, Estrella MM, Vittinghoff E, et al. APOL1 genetic variants are not associated with longitudinal blood pressure in young black adults. Kidney Int 2017;92: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nadkarni GN, Galarneau G, Ellis SB, et al. Apolipoprotein L1 Variants and Blood Pressure Traits in African Americans. J Am Coll Cardiol 2017;69: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mennuni S, Rubattu S, Pierelli G, et al. Hypertension and kidneys: unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens 2014;28: [DOI] [PubMed] [Google Scholar]
- [24].Johnson RJ, Herrera-Acosta J, Schreiner GF, et al. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. The New England journal of medicine 2002;346: [DOI] [PubMed] [Google Scholar]
- [25].Richardson SI, Freedman BI, Ellison DH, et al. Salt sensitivity: a review with a focus on non-Hispanic blacks and Hispanics. J Am Soc Hypertens 2013;7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hughson MD, Hoy WE, Mott SA, et al. APOL1 Risk Alleles are Associated with More Severe Arteriosclerosis in Renal Resistance Vessels with Aging and Hypertension. Kidney Int Rep 2016;1: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Larsen CP, Beggs ML, Saeed M, et al. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 2013;24: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Larsen CP, Beggs ML, Walker PD, et al. Histopathologic effect of APOL1 risk alleles in PLA2R-associated membranous glomerulopathy. Am J Kidney Dis 2014;64: [DOI] [PubMed] [Google Scholar]
- [29].Nichols B, Jog P, Lee JH, et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Larsen CP, Beggs ML, Saeed M, et al. Histopathologic findings associated with APOL1 risk variants in chronic kidney disease. Mod Pathol 2015;28: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bailey JNC, Palmer ND, Ng MCY, et al. Analysis of coding variants identified from exome sequencing resources for association with diabetic and non-diabetic nephropathy in African Americans. Hum Genet 2014;133: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hoy WE, Hughson MD, Kopp JB, et al. APOL1 Risk Alleles Are Associated with Exaggerated Age-Related Changes in Glomerular Number and Volume in African-American Adults: An Autopsy Study. J Am Soc Nephrol 2015;26: [DOI] [PMC free article] [PubMed] [Google Scholar]