Abstract
Purpose.
To compare the observed risk of femoral fracture in primary soft-tissue sarcoma (STS) of the thigh/groin treated with IMRT to expected risk calculated using the Princess Margaret Hospital (PMH) nomogram.
Methods.
Expected femoral fracture risk was calculated using the PMH nomogram. Cumulative risk of fracture was estimated using Kaplan-Meier statistics. Prognostic factors were assessed with univariate and multivariate analysis using Cox’s stepwise regression.
Results.
Between February 2002 and December 2010, 92 consecutive eligible patients were assessed. Median follow-up was 73 months (106 months in surviving patients). IMRT was delivered preoperatively (50 Gy) in 13 (14%) patients, and postoperatively in 79 (86%) patients (median dose of 63 Gy, range 59.4–66.6 Gy). The observed crude risk of fractures was 6.5% compared to 25.6% expected risk from the nomogram; the cumulative risk of fracture using IMRT at 5 years was 6.7% (95% CI 2.8–16.0%). The median time to fracture was 23 months (range 6.9–88.6). Significant predictors of fracture on univariate analysis were age ≥ 60 years (p = 0.03), tumor location in the anterior thigh (p = 0.008), and periosteal stripping to > 20 cm (p < 0.0001). On multivariate analysis, age ≥ 60 years and periosteal stripping > 20 cm retained significance (p = 0.04 and p = 0.009, respectively).
Conclusions.
In this study, the cumulative risk of femur fracture in patients treated with IMRT (6.7%) is less than the expected risk using the PMH nomogram (25.6%). Established predictors of femur fracture such as gender, tumor size, and dose of RT seem to have less impact on fracture risk when using IMRT.
Keywords: Extremity sarcoma, femur, IMRT, fracture
Introduction
The overall rate of radiation-associated bone fracture in soft tissue sarcoma (STS) of the extremity is about 4–6%.1–3 Most of these fractures occur in patients with lower extremity lesions, particularly the thigh.4 Even within the thigh, there is a higher rate of fracture in anterior compartment tumors compared to medial or posterior compartments.3,5 The dose of radiation also influence the rate of femoral fracture, with 9% rate being reported for lower extremity STS treated to > 60Gy compared to 1% for those treated to 50 Gy.4
One potential means of reducing fracture risk in STS is intensity-modulated radiation therapy (IMRT), which can spare at least a portion of the circumference of a long bone from receiving the full dose of radiation.7 In a previous report from Memorial Sloan Kettering Cancer Center (MSKCC), the rate of bone fracture using adjuvant IMRT for primary STS of the extremity was 4.8%.8 Determining the true impact of IMRT on the rate of fracture, however, is challenging. First, several factors, apart from radiation techniques, influence the risk of fracture, such as patient age, gender, tumor size, location, and the extent of periosteal stripping.2,3 Second, these radiation-related fractures tend to manifest long after treatment, with a median time to fracture of close to 40 months.4,8
Investigators from Princess Margaret Hospital (PMH) incorporated the above risk factors for femur fracture into a predictive nomogram.11 Here, we sought to examine the impact of IMRT on fracture risk by comparing the observed risk of femoral fracture in primary STS treated with limb-sparing surgery and IMRT to the expected risk using the PMH nomogram. Using this nomogram rather than simply comparing fracture rates with other reports in the literature accounts for the influence of various factors and thus allows for more accurate estimation of the true impact of IMRT on fracture rate. The IMRT cohort was limited to patients whose median follow-up (surviving patients) exceeded 8 years.
Methods
Patients
The prospective database at our institution was reviewed to identify patients with primary nonmetastatic soft tissue sarcoma (STS) of the thigh/groin who underwent both limb-sparing surgery and adjuvant IMRT at MSKCC between February 2002 and December 2010. Patients receiving prophylactic internal fixation were excluded. This retrospective analysis was approved by our Institutional Review Board.
Radiation therapy
Treatment plans were generated using our in-house treatment-planning system (Top Module, New York, NY, USA). Details regarding treatment planning and delivery have been previously described.7 In brief, when expanding the target gross tumor volume (GTV) for preoperative IMRT or tumor bed for postoperative IMRT to the clinical target volume (CTV), nearby bone is excluded. Typical expansion from CTV to planning target volume (PTV) was 1 cm. Routine portal imaging was performed on a weekly basis. The median dose of preoperative IMRT was 50 Gy, and for postoperative IMRT was 63 Gy. For patients who subsequently developed fractures, the location of the fracture was digitized into the patient’s original treatment plan and dosimetric factors associated with the fracture were assessed.
Calculation of expected fracture risk
Expected risk of fracture was calculated using the PMH nomogram,11 which incorporates risk factors including gender, age at index surgery (in years), compartment of thigh (anterior, posterior, adductor, or “other”), tumor size (in cm), tumor dose (< 60 Gy or ≥ 60 Gy), and extent of periosteal stripping (< 10 cm, 10–20 cm, or > 20 cm). The probability of fracture (Pfrac) was calculated using maximum likelihood estimates for each factor in the nomogram, using the equation
Assessment/follow-up
The status of all patients was checked at least once weekly while on treatment by the treating radiation oncologist. Follow-up evaluation included physical examination and imaging. All patient follow-up was prospectively performed at our institution. Toxicity was graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, with the highest grade of any observed toxicity reported for each patient.
Statistics
All outcomes were measured from time of definitive surgery to time of event. Fracture-free interval was defined as the time from definitive surgery to highest fracture grade. Lower grade fractures were noted but excluded from our analysis as they were outside the treatment field. Patients were censored at the date of last follow-up or death. Outcomes were estimated using the Kaplan-Meier method and cumulative incidence functions; confidence intervals at median follow-up times were calculated using Wilson’s interval with continuity correction. Log-rank test and Cox proportional hazards regression models were used for univariate and multivariate analysis. Receiver-operating characteristic (ROC) analysis was performed to calculate the area under the receiver operator curve (AUC) and test the predictive power of the PHM nomogram in the study population treated with IMRT. Statistical analysis was performed using SPSS v22.0.0 (SPSS Inc.).
Results
Patients
Between February 2002 and December 2010, 92 consecutive eligible patients were treated with limb-sparing surgery and IMRT. Median follow-up was 73 months (106 months in surviving patients). Patient characteristics are provided in Table 1. Of the patients treated, 36 (39%) were female. The average age was 58 years (range, 19–88). Thigh compartment was anterior in 43 (47%) patients, posterior in 28 (30%), medial in 17 (18%), and groin in 4 (4%). The median tumor maximum dimension was 11.1 cm (range 2.5–31 cm), and 56 tumors (61%) were >10 cm in size. Periosteal stripping was performed in 20 (22%) patients; the extent of periosteal stripping was < 10 cm in 4 patients, 10–20 cm in 9 patients, and > 20 cm in 7 patients (one patient had a partial cortical resection). Preoperative IMRT to 50 Gy was delivered in 13 (14%) patients, and postoperative IMRT was delivered in 79 (86%) patients to a median dose of 63 Gy (range 59.4–66.6 Gy). Neoadjuvant or adjuvant chemotherapy was administered in 33 (36%) patients.
Table 1.
Demographic and clinical characteristics of the studied cohort (n = 92).
| n (%) | ||
|---|---|---|
| Gender | Female | 36 (39.1%) |
| Male | 56 (60.9%) | |
| Age | < 60 | 51 (55.4%) |
| ≥ 60 | 41 (44.5%) | |
| Compartment | Anterior | 43 (46.7%) |
| Posterior | 28 (30.4%) | |
| Medial/groin | 21 (22.8%) | |
| Tumor size | < 10 cm | 36 (39%) |
| ≥ 10 cm | 56 (61%) | |
| Extent of periosteal stripping | < 10 cm | 76 (82.6%) |
| 10–20 cm | 9 (9.8%) | |
| > 20 cm | 7 (7.6%) | |
| Radiation dose | > 60 Gy | 76 (85.2%) |
| ≤ 60 Gy | 16 (17.4%) | |
| Chemotherapy | Yes | 33 (35.9%) |
| No | 59 (64.1%) |
Fractures
There were 6 (6.5%) Grade 3 femoral fractures. The median time to fracture was 23 months (range 6.9–88.6 months). All 6 patients with Grade 3 fractures were > 50 years of age when treated for primary STS (median age of 61 years) and all had tumors located in the anterior compartment of the thigh. Of the 6 patients experiencing Grade 3 fractures, 5 patients (83.3%) were treated to a dose of >60 Gy and had a tumor >10 cm, 4 patients (66.7%) were female, 2 patients (33.3%) underwent significant bone manipulation (both with >20 cm of periosteal stripping), and 2 patients (33.3%) received adjuvant chemotherapy as part of initial definitive treatment. In 5 patients (83.3%), the fracture was an acute event requiring open reduction and internal fixation. The sixth patient started developing symptoms and signs of avascular necrosis of the femoral head 4 years after IMRT, ultimately leading to planned hip replacement 3 years later (7 years after IMRT).
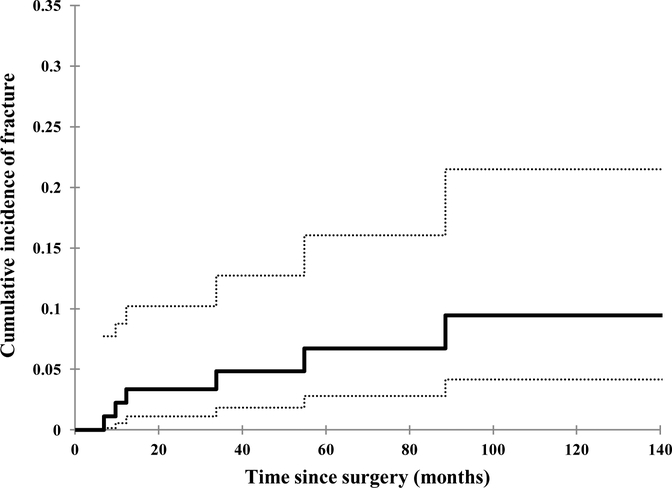
The cumulative risk of fracture at 5 years was 6.7% (95% CI 2.8–16.0%) (Fig. 1) Significant predictors of fracture on univariate analysis were age ≥ 60 (p = 0.03), tumor location in the anterior compartment of the thigh (p = 0.008), and periosteal stripping > 20 cm (p < 0.0001). On multivariate analysis, age ≥ 60 and periosteal stripping > 20 cm retained significance (p = 0.04 and p = 0.009, respectively). The risk of femur fracture among patient groups classified according to the assessed clinical and treatment variables is provided in Table 2.
Figure 1.
Cumulative rate of Grade 3 fracture over time among patients with STS of the thigh treated by limb-sparing surgery and IMRT between February 2002 and December 2010 at MSKCC. Dotted lines indicate 95% CI bounds.
Table 2.
Risk of Grade 3 femur fracture at 5 years among patients classified according to clinical and treatment variables.
| Crude risk | Cumulative risk over 5 years | 95% CI | Univariate P value | Multivariate P value | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 4/36 (11.1%) | 10.2% | 0.0–21.5% | 0.17 | |
| Male | 2/56 (3.6%) | 4.1% | 0.0–9.6% | ||
| Age | |||||
| Age < 60 | 1/51 (2.0%) | 2.0% | 0.0–6.0% | 0.03 | 0.04 |
| Age ≥ 60 | 5/41 (12.2%) | 13.4% | 0.5–26.3% | ||
| Compartment | |||||
| Anterior | 6/43 (14.0%) | 14.0% | 2.3–25.8% | 0.008 | 0.95 |
| Other | 0/49 (0.0%) | 0.0% | 0.0–0.0% | ||
| Tumor size | |||||
| < 10 cm | l*/36 (2.8%) | 0.0% | 0.0–0.0% | 0.13 | |
| ≥ 10cm | 5/56 (8.9%) | 12.7% | 1.5–23.9% | ||
| Extent of periosteal stripping | |||||
| ≤ 20 cm | 4/85 (4.7%) | 4.7% | 0.0–9.9% | <0.0001 | 0.009 |
| > 20 cm | 2/7 (28.6%) | 31.4% | 0.0–67.9% | ||
| Dose | |||||
| ≤ 60 Gy | 1/16 (6.3%) | 8.3% | 0.0–24.0% | 0.95 | |
| > 60 Gy | 5/76 (6.6%) | 6.3% | 0.1–12.6% | ||
| Chemotherapy | |||||
| No chemotherapy | 4/59 (6.8%) | 6.6% | 0.0–13.9% | 0.99 | |
| Chemotherapy | 2/33 (6.1%) | 6.3% | 0.0–14.7% |
This patient developed fracture past 5 years (88.6 months).
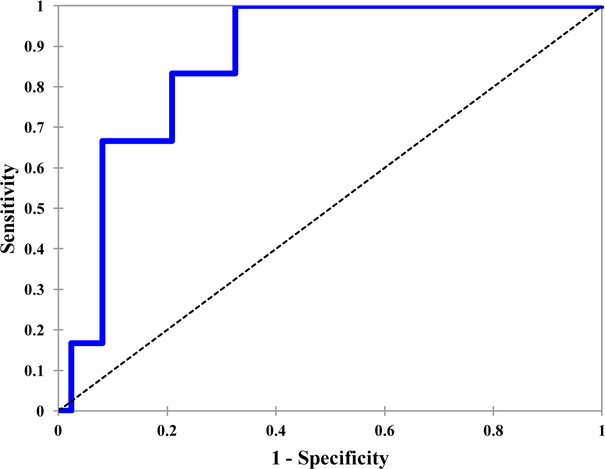
The PMH nomogram was predictive of femoral fracture in the current cohort of patients treated with IMRT, with an ROC AUC of 0.866 (p = 0.003). (Fig. 2). The expected risk calculated using the PMH nomogram was 25.6%, whereas the observed risk of fracture at 5 years was only 6.7%.
Figure 2.
Predictive power of the PMH femur fracture nomogram among patients with STS of the thigh treated by limb-sparing surgery and IMRT between February 2002 and December 2010 at MSKCC (blue line). AUC = 0.866, p = .003.
Dosimetric analysis
The locations of all fractures noted in this population were digitized into the patient’s original treatment plan and dosimetric factors associated with the fracture were assessed. For the site of fracture, the median maximum dose (Dmax) was 65.6 Gy (range, 62.9–74.1 Gy) and the median Dmean was 52.2 Gy (range, 46.3–65.7 Gy). For the irradiated femur, the median Dmax was 65.9 Gy (range, 65.5–76 Gy), the median Dmean was 42.5 Gy (range, 31.2–60.2 Gy), and the median V40 (volume of femur irradiated to ≥ 40 Gy) was 0.63 (range, .38–.916).
Discussion
At a median follow-up of 73 months (106 months in surviving patients), the crude rate of femoral fracture was 6.5% in our cohort of 92 patients with primary thigh/groin STS treated by limb-sparing surgery and IMRT at our institution. The overall crude rate of femoral fracture with conventional RT ranges from as low as 2% in some series to as high as 22% in others.3,4,7–12 This wide range in outcomes reflects the varied influence of several prognostic factors on the risk of femoral fractures such as age, gender, tumor size, compartment within the thigh, extent of periosteal stripping, and radiation dose and technique on the risk of femoral fractures. This makes direct comparisons between the end outcomes in published series uninformative.
Therefore, we utilized the PMH nomogram, which incorporates several of these prognostic factors. In that study, in which all patients were treated with conventional radiation therapy (and none with IMRT), the crude rate of femoral fracture was 21.7% (22/101) with an estimated 5-year rate of 15%. The PMH nomogram was predictive of femoral fracture in the current cohort of patients treated with IMRT, with an ROC AUC of 0.866 (p = .003). More importantly, the calculated expected risk at 5 years was 25.6%, whereas the observed risk was only 6.7%, indicating that the use of IMRT was associated with a reduced risk of femoral fracture.
The association between a lower rate of femoral fracture and the use of IMRT is likely due to reduction in the radiation dose to the whole circumference of the femur. Bishop et al. reported that the rate of femoral fracture was 0% when none or part of the bone circumference was treated, compared with 7% when the whole circumference was treated to doses of ≥ 50 Gy (p < 0.001).9 When treating the whole circumference of femur to doses of ≥ 50 Gy, combined with adjuvant chemotherapy and periosteal stripping/bone exposure, the femoral fracture rate increased to 37% at 10 years.
Among patients who developed fractures in our cohort, the median radiation dose (Dmean) to the femur was 42.5 Gy and the median Dmax was 65.9 Gy, which are both greater than the current widely used dose constraints of Dmean < 37 Gy and Dmax < 59 Gy. This discordance with guidelines results from the fact that the study cohort was treated prior to the publication of the two studies on which recommendations are based, comparing RT dose/volume parameters between patients who developed fractures and those who did not. In the first study, the Dmean to the femur among patients who experienced fractures was 44 Gy vs. 38 Gy among those who did not, and the Dmax was 65 Gy vs. 59 Gy;15 in the second study, the Dmean was 57.6 Gy vs. 22.9 Gy.16 Our findings thus lend further credence and support to the importance of dose constraints for reducing the risk of femoral fracture. Because doses are lower when IMRT is delivered preoperatively, this approach is particularly appropriate for patients at high risk for fracture, such as those with anterior thigh tumors that require significant periosteal stripping.
Another important finding in this cohort treated exclusively with IMRT was that there were no observed instances of nonunion or complications secondary to fixation. This is in contrast to reports in the literature, where rates of non-union ranging from 27 to 45% have been observed in patients who developed fractures after receiving conventional RT.2,3,14
This study is not without its limitations, due to its retrospective nature and the inherent biases present in such research. To limit these biases, we restricted our analysis to patients with primary STS of the thigh/groin who received all surgical and radiation treatment at our institution. Limiting reported fractures to those classified as Grade 3 makes this outcome clear and unequivocal, and comprehensive dosimetric information was available for all patients in the study cohort. We excluded patients treated after 2010 to allow more than adequate time to capture any potential fracture several years beyond the completion date of IMRT. After 2010 we implemented the above-mentioned dose constraints for weight-bearing bone to further limit the radiation dose to the femur. Whether such dose constraints will influence the rate of femoral fractures will be the subject of future investigation.
In conclusion, the use of IMRT was associated with a low crude rate (6.5%) of femoral fracture, despite the high dose of radiation delivered to the target volume. More importantly, the observed cumulative risk of femoral fracture in this cohort of patients treated with IMRT was less than the expected risk calculated using the PMH nomogram.
Synopsis.
Radiation therapy to treat soft-tissue sarcoma of the thigh is associated with increased risk of femoral fracture. As IMRT can reduce the dose delivered to bone, we compared the observed fracture risk in patients treated with IMRT with the nomogram-predicted risk.
Acknowledgments
This research was supported by the NIH/NCI grant P50 CA140146 (SPORE in Soft Tissue Sarcoma) and the NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosures: This work was supported by the National Cancer Institute via the SPORE in Soft Tissue Sarcoma (P50 CA140146 to SS) and the Cancer Center Support Grant P30 CA008748. Michael R. Folkert receives travel funding from Varian, Inc. and research support from Augmenix, Inc. None of the other authors have conflicts to disclose. None of the authors have a personal or institutional financial interest in the materials or devices described in this submission.
References
- 1.Alektiar KM, Zelefsky MJ, Brennan MF. Morbidity of adjuvant brachytherapy in soft tissue sarcoma of the extremity and superficial trunk. Int J Radiat Oncol Biol Phys. 2000;47(5):1273–1279. [DOI] [PubMed] [Google Scholar]
- 2.Blaes AH, Lindgren B, Mulrooney DA, Willson L, Cho LC. Pathologic femur fractures after limb-sparing treatment of soft-tissue sarcomas. J Cancer Surviv. 2010;4(4):399–404. [DOI] [PubMed] [Google Scholar]
- 3.Helmstedter CS, Goebel M, Zlotecki R, Scarborough MT. Pathologic fractures after surgery and radiation for soft tissue tumors. Clin Orthop Relat Res. 2001(389):165–172. [DOI] [PubMed] [Google Scholar]
- 4.Holt GE, Griffin AM, Pintilie M, et al. Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas. A comparison of high-dose and low-dose radiotherapy. J Bone Joint Surg Am. 2005;87(2):315–319. [DOI] [PubMed] [Google Scholar]
- 5.Lin PP, Boland PJ, Healey JH. Treatment of femoral fractures after irradiation. Clin Orthop Relat Res. 1998(352):168–178. [PubMed] [Google Scholar]
- 6.Kim HJ, Healey JH, Morris CD, Boland PJ. Site-dependent replacement or internal fixation for postradiation femur fractures after soft tissue sarcoma resection. Clin Orthop Relat Res. 2010;468(11):3035–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong L, Alektiar KM, Hunt M, Venkatraman E, Leibel SA. Intensity-modulated radiotherapy for soft tissue sarcoma of the thigh. Int J Radiat Oncol Biol Phys. 2004;59(3):752–759. [DOI] [PubMed] [Google Scholar]
- 8.Folkert MR, Singer S, Brennan MF, et al. Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol. 2014;32(29):3236–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bishop AJ, Zagars GK, Allen PK, et al. Treatment-related fractures after combined modality therapy for soft tissue sarcomas of the proximal lower extremity: Can the risk be mitigated? Pract Radiat Oncol. 2016;6(3):194–200. [DOI] [PubMed] [Google Scholar]
- 10.Rimner A, Brennan MF, Zhang Z, Singer S, Alektiar KM. Influence of compartmental involvement on the patterns of morbidity in soft tissue sarcoma of the thigh. Cancer. 2009;115(1):149–157. [DOI] [PubMed] [Google Scholar]
- 11.Gortzak Y, Lockwood GA, Mahendra A, et al. Prediction of pathologic fracture risk of the femur after combined modality treatment of soft tissue sarcoma of the thigh. Cancer. 2010;116(6):1553–1559. [DOI] [PubMed] [Google Scholar]
- 12.Cannon CP, Ballo MT, Zagars GK, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer. 2006;107(10):2455–2461. [DOI] [PubMed] [Google Scholar]
- 13.Livi L, Santoni R, Paiar F, et al. Late treatment-related complications in 214 patients with extremity soft-tissue sarcoma treated by surgery and postoperative radiation therapy. Am J Surg. 2006;191(2):230–234. [DOI] [PubMed] [Google Scholar]
- 14.McGee L, Indelicato DJ, Dagan R, et al. Long-term results following postoperative radiotherapy for soft tissue sarcomas of the extremity. Int J Radiat Oncol Biol Phys. 2012;84(4):1003–1009. [DOI] [PubMed] [Google Scholar]
- 15.Dickie CI, Parent AL, Griffin AM, et al. Bone fractures following external beam radiotherapy and limb-preservation surgery for lower extremity soft tissue sarcoma: relationship to irradiated bone length, volume, tumor location and dose. Int J Radiat Oncol Biol Phys. 2009;75(4):1119–1124. [DOI] [PubMed] [Google Scholar]
- 16.Pak D, Vineberg KA, Griffith KA, et al. Dose--effect relationships for femoral fractures after multimodality limb-sparing therapy of soft-tissue sarcomas of the proximal lower extremity. Int J Radiat Oncol Biol Phys. 2012;83(4):1257–1263. [DOI] [PubMed] [Google Scholar]