Abstract
Aims
Klotho, an essential co-receptor for fibroblast growth factor (FGF)-23, has potentially beneficial inhibitory effects on the renin-angiotensin system. Limited data exist on the prognostic value of Klotho and FGF-23 in combination or their ability to predict benefit from angiotensin-converting enzyme (ACE)-inhibition.
Methods and Results
3,555 patients with stable ischemic heart disease and LVEF >40% enrolled in the PEACE trial of trandolapril vs placebo had Klotho levels drawn at randomization. Patients were characterized by quartiles of Klotho and FGF-23 concentrations. 6-year Kaplan-Meier rates and adjusted risk were calculated in the placebo arm for the composite of CV death or hospitalization for heart failure and its components. Low (quartile [Q]1–3) Klotho concentration was associated with an increased rate of CV death or hospitalization for heart failure as compared with Q4 (8.2 vs 4.2%; p=0.03). After multivariable adjustment for clinical variables and renal and CV biomarkers (eGFR, cystatin-C, UACR, FGF-23, hsTnT, NT-proBNP, and hsCRP), low Klotho concentration remained strongly associated with increased risk of CV death or hospitalization for heart failure (HRadj 2.62 [1.35–5.08]; p<0.01). The combination of low Klotho and high (Q4) FGF-23 concentration identified patients at particularly elevated risk (HRadj 3.99 [1.67–9.56]; p<0.01). This high risk combination additionally predicted benefit from trandolapril (HR 0.39 [0.23–0.68]; pint<0.01).
Conclusions
Low Klotho concentration is associated with an increased risk of CV death or heart failure hospitalization in patients with stable ischemic heart disease. The combination of low Klotho and high FGF-23 further identifies patients at distinctly elevated risk who derive clinical benefit from the ACE-inhibitor trandolapril.
Keywords: Heart failure, FGF-23, Klotho, biomarker, ACE-inhibitor, cardiovascular outcomes
Introduction
The renin-angiotensin system (RAS) connects cardiovascular (CV) and renal function in mammals and provides targets for essential medical therapies across a spectrum of disease states. Chronic kidney disease (CKD) is associated with adverse CV outcomes by numerous mechanisms, including RAS dysregulation.1, 2 α-Klotho, hereafter referred to as Klotho, is a single-pass transmembrane protein located primarily in the renal distal convoluted tubule.3 The extracellular domain may be cleaved, producing a soluble form with distant actions, including in the vasculature.3 Low Klotho levels are associated with findings similar to those seen in CKD, including left ventricular hypertrophy (LVH), myocardial fibrosis, and increased mortality.1, 2
RAS and Klotho function are closely linked, with low Klotho levels associated with RAS upregulation.4 Klotho’s actions are also intimately tied to fibroblast growth factor (FGF)-23, a protein hormone for which Klotho is a co-receptor in the kidney and vasculature.5–8 Increased FGF-23 levels are associated with LVH, vascular calcification, and adverse CV events.9–11 This relationship is complex, however, with FGF-23 correlating in some studies with myocardial fibrosis and LVH in the setting of Klotho deficiency, but not at normal Klotho levels.2 This interaction among FGF-23, Klotho, and CV events has potential implications for prognostication as well as patient selection for clinical trials and therapies.
Previous work by our group showed elevated FGF-23 levels in patients enrolled in the PEACE (Prevention of Events with Angiotensin-Converting Enzyme) Trial12 to be associated with increased risk of CV death and heart failure and also predict benefit from the ACE-inhibitor trandolapril.13 Klotho levels were not available for this prior analysis. We now investigate here the relationships between Klotho, FGF-23, ACE-inhibitor exposure, and CV outcomes in patients with stable ischemic heart disease enrolled in the PEACE trial.
Methods
Study Design and Participants
The study design, baseline patient characteristics, and primary results of the PEACE trial (NCT00000558) have been described previously.12, 14 The PEACE trial was a double-blind, placebo-controlled study which randomized 8,290 patients with stable coronary artery disease and normal or mildly reduced left ventricular ejection fraction (LVEF) to the ACE-inhibitor trandolapril or placebo. Median follow-up was 4.8 years.12 In addition to coronary artery disease, patients were required to have an LVEF >40%, serum creatinine ≤2.0 mg/dL, and age ≥50 years. There was no overall benefit with trandolapril for the primary end point of cardiovascular death, myocardial infarction, or coronary revascularization.
The primary end point for this post hoc, exploratory analysis was the composite of CV death or hospitalization for heart failure. Heart failure hospitalization was defined as a hospitalization for which heart failure was the primary cause as determined by centrally trained local staff and confirmed via review of hospital records by the clinical and statistical coordinating center at George Washington University, Rockville, Maryland.12, 15 All CV deaths were adjudicated by an independent and blinded clinical events committee.12, 14
All participants provided written informed consent. The study protocol was approved by all relevant institutional review boards.
Measurement of Biomarkers
All subjects enrolled in the United States and Canada were invited to participate in biomarker sampling at study enrollment and approximately half agreed. Venous blood samples obtained at randomization were shipped frozen to the TIMI Clinical Trials Laboratory (Boston, MA), where they were stored at −80ºC or colder and subsequently tested by laboratory personnel blinded to treatment allocation and clinical outcome. Klotho was assayed on the third thaw of the samples, occurring in June 2014.
Klotho concentrations were measured using a manual enzyme-linked immunosorbent assay (soluble α-Klotho Assay, Immuno-Biological Laboratories, Inc., Minneapolis, Minnesota) targeting the 130 kD form of soluble Klotho16 and standard washers/readers (Tecan, Männnedorf, Switzerland). The coefficients of variation (CVs) observed in the TIMI Clinical Trials Laboratory were 3.5% at 187 pg/mL and 3.1% at 2969 pg/mL for intra-assay precision and 9.2% at 664 pg/mL and 6.2% at 1439 pg/mL for inter-assay precision. FGF-23 was measured using the Human FGF-23 (C-term) ELISA Kit (Immutopics, Inc., San Clemente, CA). Observed intra-assay CVs were 4.7% at 33.6 RU/mL and 2.4% at 293 RU/mL while inter-assay CVs were 12.8% at 30.3 RU/mL and 4.9% at 279 RU/mL. The reference range for this assay in adults with preserved renal function is 55+50 reference units (RU)/mL.17
Baseline concentrations of high-sensitivity troponin-T (hs-TnT), N-terminal pro-B-type natriuretic peptide (NT-proBNP), high-sensitivity C-reactive protein (hsCRP), cystatin-C, and urine albumin-to-creatinine ratio (UACR) have been reported for the PEACE trial cohort previously.13, 15, 18, 19
Statistical Analyses
All patients with available Klotho levels were included in the current analysis. Baseline patient characteristics by quartile of Klotho concentration were described with mean and standard deviation for continuous variables and number and percent for categorical variables. Comparisons of baseline characteristics were made using a linear trend test.
Spearman correlation coefficients were calculated among Klotho, FGF-23, estimated glomerular filtration rate (eGFR) calculated by the Modification of Diet in Renal Dysfunction (MDRD) formula, cystatin-C, UACR, NT-proBNP, hsTnT, and hsCRP.
Six-year Kaplan-Meier event rates for the primary end point of CV death or heart failure hospitalization as well as its individual components were calculated by quartile of Klotho concentration (Q1: lowest concentrations; Q4: highest concentrations) in the placebo arm. Event rates in the placebo arm and by randomized treatment were also calculated after simultaneous stratification by FGF-23 and Klotho with resulting assignment to a “low risk” (either FGF-23 Q1–3 or Klotho Q4) or “high risk” (both FGF-23 Q4 and Klotho Q1–3) group. FGF-23 quartiles were used in this exploratory analysis based on their previously reported association with CV outcomes in this patient cohort.13
Unadjusted risk for CV outcomes was calculated by quartile of Klotho concentration in the placebo arm. Adjusted risk was calculated using the following covariates: age, sex, current smoker, hypertension, diabetes mellitus, prior MI, prior revascularization, weight, systolic blood pressure (SBP), LVEF, eGFR, cystatin-C, UACR, FGF-23, NT-proBNP, hsTnT, and hsCRP (sensitivity analyses were performed removing each of cystatin-C and eGFR; Supplemental Table S8). Patients were then simultaneously stratified by FGF-23 Q1–3 vs Q4 and Klotho Q1–3 vs Q4 and unadjusted and adjusted risks were calculated comparing each of the four resulting categories in the placebo arm. The interaction between treatment and risk groups was calculated accounting for a linear trend. Finally, metrics of discrimination (net reclassification index [NRI], integrated discrimination improvement [IDI], and Harrell’s C-statistic) were calculated for the addition of Klotho and FGF-23 to the clinical model including the clinical variables in the multivariate model above.
All analyses were performed by the TIMI Study Group using commercial statistical software (SAS version 9.4, SAS institute, Cary, NC). A two-sided p value of 0.05 was considered significant for all tests.
Results
Baseline Characteristics
3,555 patients had baseline Klotho levels available for analysis. The median Klotho concentration was 564.95 (interquartile range 463.12 to 691.45) pg/mL. Baseline patient characteristics according to Klotho quartile are presented in Table 1. Patients with the lowest Klotho levels were older with lower eGFR and were less likely to have diabetes or a prior MI. Baseline characteristics for the full trial population and the biomarker subgroup are shown in Supplemental Table S1.
Table 1.
Baseline patient characteristics by Klotho level.
| Klotho Quartile | ||||||
|---|---|---|---|---|---|---|
| Characteristic | Total N=3555 | 1st (<463.12) N=889 |
2nd (463.12, 564.95) N=889 |
3rd (564.95, 691.45) N=889 |
4th (>691.45) N=888 |
P-trend |
| Demographics | ||||||
| Age (years) | 64.0 (58.0, 70.0) | 65.0 (58.0, 71.0) | 65.0 (58.0, 71.0) | 64.0 (57.0, 70.0) | 63.0 (57.0, 69.0) | <.001 |
| Male sex, n(%) | 2882 (81.1%) | 690 (77.6%) | 708 (79.6%) | 748 (84.1%) | 736 (82.9%) | 0.001 |
| Past Medical History | ||||||
| Hypertension, n(%) | 1587 (44.7%) | 416 (46.8%) | 406 (45.7%) | 366 (41.2%) | 399 (45.0%) | 0.183 |
| Current smoker, n(%) | 530 (14.9%) | 130 (14.6%) | 153 (17.2%) | 132 (14.9%) | 115 (13.0%) | 0.174 |
| Diabetes mellitus, n(%) | 573 (16.1%) | 108 (12.1%) | 116 (13%) | 137 (15.4%) | 212 (23.9%) | <.001 |
| eGFR<60 (mL/min/1.73m2), n(%) | 556 (15.7%) | 177 (20.0%) | 147 (16.6%) | 127 (14.3%) | 105 (11.8%) | <.001 |
| Prior MI, n(%) | 1992 (56.0%) | 464 (52.2%) | 497 (55.9%) | 509 (57.3%) | 522 (58.9%) | 0.004 |
| Prior PCI or CABG, n(%) | 2570 (72.3%) | 658 (74.1%) | 622 (70%) | 637 (71.7%) | 653 (73.6%) | 0.971 |
| Medications | ||||||
| Aspirin/other antiplatelet, n(%) | 3241 (91.2%) | 822 (92.5%) | 809 (91%) | 814 (91.7%) | 796 (89.7%) | 0.077 |
| Beta-blocker, n(%) | 2195 (61.8%) | 569 (64%) | 537 (60.4%) | 519 (58.4%) | 570 (64.3%) | 0.869 |
| Lipid-lowering agent, n(%) | 2553 (71.9%) | 663 (74.7%) | 637 (71.7%) | 621 (69.9%) | 632 (71.3%) | 0.077 |
| Calcium-channel blocker, n(%) | 1194 (33.6%) | 296 (33.3%) | 317 (35.7%) | 299 (33.7%) | 282 (31.8%) | 0.360 |
| Any diuretic, n(%) | 427 (12%) | 118 (13.3%) | 107 (12%) | 95 (10.7%) | 107 (12.1%) | 0.308 |
| Measurements at Enrollment | ||||||
| Weight (kg) | 82.0 (73.0, 93.0) | 81.5 (72.0, 91.0) | 84.0 (75.0, 94.0) | 82.0 (73.0, 93.0) | 84.0 (75.0, 94.0) | <.001 |
| BMI (kg/m2) | 27.8 (25.3, 30.8) | 27.7 (24.9, 30.4) | 28.1 (25.3, 31.2) | 27.8 (25.3, 30.9) | 28.4 (25.8, 31.5) | <.001 |
| SBP (mmHg) | 132.0 (120.0, 144.0) | 132.0 (122.0, 146.0) | 132.0 (120.0, 145.0) | 130.0 (120.0, 142.0) | 130.0 (120.0, 142.0) | 0.045 |
| DBP (mmHg) | 80.0 (70.0, 84.0) | 80.0 (70.0, 84.0) | 80.0 (70.0, 84.0) | 80.0 (70.0, 84.0) | 80.0 (70.0, 86.0) | 0.043 |
| Apo B (mg/dL) | 135.2 (121.3, 152.1) | 137.4 (121.3, 155.6) | 135.6 (122.8, 151.8) | 135.2 (121.8, 151.7) | 134.1 (119.4, 149.8) | 0.953 |
| Apo A (mg/dL) | 105.0 (91.1, 120.5) | 106.4 (91.5, 121.2) | 104.5 (89.9, 119.0) | 105.1 (91.6, 121.8) | 104.5 (91.3, 121.9) | <.001 |
| Ratio Apo B/Apo A | 0.8 (0.6, 0.9) | 0.8 (0.6, 0.9) | 0.8 (0.6, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.007 |
| LV ejection fraction (%) | 57.0 (50.0, 65.0) | 58.0 (52.0, 65.0) | 59.0 (51.0, 65.0) | 58.0 (50.0, 65.0) | 59.5 (50.0, 65.0) | 0.729 |
| UACR (ug/mg) | 8.6 (5.2, 19.6) | 8.6 (5.1, 20.1) | 8.5 (5.4, 17.6) | 8.2 (5.1, 17.0) | 8.9 (5.3, 23.7) | 0.728 |
| hsCRP (mg/L) | 1.7 (0.8, 3.5) | 2.0 (0.9, 4.0) | 1.8 (0.9, 3.6) | 1.5 (0.8, 3.1) | 1.5 (0.7, 3.2) | <.001 |
| NT-proBNP (pg/mL) | 139.0 (71.0, 272.0) | 165.0 (84.0, 333.5) | 140.0 (74.0, 274.0) | 133.0 (67.0, 247.0) | 121.0 (63.0, 237.5) | <.001 |
| TnT (ng/mL) | 0.0 (0.0, 0.0) | 0.006 (0.004, 0.010) | 0.006 (0.004, 0.009) | 0.006 (0.004, 0.009) | 0.006 (0.004, 0.009) | 0.247 |
| Demographics | ||||||
| Age (years) | 64.0 (58.0, 70.0) | 65.0 (58.0, 71.0) | 65.0 (58.0, 71.0) | 64.0 (57.0, 70.0) | 63.0 (57.0, 69.0) | <.001 |
| Male sex, n(%) | 2882 (81.1%) | 690 (77.6%) | 708 (79.6%) | 748 (84.1%) | 736 (82.9%) | 0.001 |
| Past Medical History | ||||||
| Hypertension, n(%) | 1587 (44.7%) | 416 (46.8%) | 406 (45.7%) | 366 (41.2%) | 399 (45.0%) | 0.183 |
| Current smoker, n(%) | 530 (14.9%) | 130 (14.6%) | 153 (17.2%) | 132 (14.9%) | 115 (13.0%) | 0.174 |
| Diabetes mellitus, n(%) | 573 (16.1%) | 108 (12.1%) | 116 (13%) | 137 (15.4%) | 212 (23.9%) | <.001 |
| eGFR<60 (mL/min/1.73m2), n(%) | 556 (15.7%) | 177 (20.0%) | 147 (16.6%) | 127 (14.3%) | 105 (11.8%) | <.001 |
| Prior MI, n(%) | 1992 (56.0%) | 464 (52.2%) | 497 (55.9%) | 509 (57.3%) | 522 (58.9%) | 0.004 |
| Prior PCI or CABG, n(%) | 2570 (72.3%) | 658 (74.1%) | 622 (70%) | 637 (71.7%) | 653 (73.6%) | 0.971 |
| Medications | ||||||
| Aspirin/other antiplatelet, n(%) | 3241 (91.2%) | 822 (92.5%) | 809 (91%) | 814 (91.7%) | 796 (89.7%) | 0.077 |
| Beta-blocker, n(%) | 2195 (61.8%) | 569 (64%) | 537 (60.4%) | 519 (58.4%) | 570 (64.3%) | 0.869 |
| Lipid-lowering agent, n(%) | 2553 (71.9%) | 663 (74.7%) | 637 (71.7%) | 621 (69.9%) | 632 (71.3%) | 0.077 |
| Calcium-channel blocker, n(%) | 1194 (33.6%) | 296 (33.3%) | 317 (35.7%) | 299 (33.7%) | 282 (31.8%) | 0.360 |
| Any diuretic, n(%) | 427 (12%) | 118 (13.3%) | 107 (12%) | 95 (10.7%) | 107 (12.1%) | 0.308 |
| Measurements at Enrollment | ||||||
| Weight (kg) | 82.0 (73.0, 93.0) | 81.5 (72.0, 91.0) | 84.0 (75.0, 94.0) | 82.0 (73.0, 93.0) | 84.0 (75.0, 94.0) | <.001 |
| BMI (kg/m2) | 27.8 (25.3, 30.8) | 27.7 (24.9, 30.4) | 28.1 (25.3, 31.2) | 27.8 (25.3, 30.9) | 28.4 (25.8, 31.5) | <.001 |
| SBP (mmHg) | 132.0 (120.0, 144.0) | 132.0 (122.0, 146.0) | 132.0 (120.0, 145.0) | 130.0 (120.0, 142.0) | 130.0 (120.0, 142.0) | 0.045 |
| DBP (mmHg) | 80.0 (70.0, 84.0) | 80.0 (70.0, 84.0) | 80.0 (70.0, 84.0) | 80.0 (70.0, 84.0) | 80.0 (70.0, 86.0) | 0.043 |
| Apo B (mg/dL) | 135.2 (121.3, 152.1) | 137.4 (121.3, 155.6) | 135.6 (122.8, 151.8) | 135.2 (121.8, 151.7) | 134.1 (119.4, 149.8) | 0.953 |
| Apo A (mg/dL) | 105.0 (91.1, 120.5) | 106.4 (91.5, 121.2) | 104.5 (89.9, 119.0) | 105.1 (91.6, 121.8) | 104.5 (91.3, 121.9) | <.001 |
| Ratio Apo B/Apo A | 0.8 (0.6, 0.9) | 0.8 (0.6, 0.9) | 0.8 (0.6, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.007 |
| LV ejection fraction (%) | 57.0 (50.0, 65.0) | 58.0 (52.0, 65.0) | 59.0 (51.0, 65.0) | 58.0 (50.0, 65.0) | 59.5 (50.0, 65.0) | 0.729 |
| UACR (ug/mg) | 8.6 (5.2, 19.6) | 8.6 (5.1, 20.1) | 8.5 (5.4, 17.6) | 8.2 (5.1, 17.0) | 8.9 (5.3, 23.7) | 0.728 |
| hsCRP (mg/L) | 1.7 (0.8, 3.5) | 2.0 (0.9, 4.0) | 1.8 (0.9, 3.6) | 1.5 (0.8, 3.1) | 1.5 (0.7, 3.2) | <.001 |
| NT-proBNP (pg/mL) | 139.0 (71.0, 272.0) | 165.0 (84.0, 333.5) | 140.0 (74.0, 274.0) | 133.0 (67.0, 247.0) | 121.0 (63.0, 237.5) | <.001 |
| TnT (ng/mL) | 0.0 (0.0, 0.0) | 0.006 (0.004, 0.010) | 0.006 (0.004, 0.009) | 0.006 (0.004, 0.009) | 0.006 (0.004, 0.009) | 0.247 |
Continuous variable presented as median (interquartile range). BMI – body mass index; CABG – coronary artery bypass graft; eGFR – estimated glomerular filtration rate; hsCRP – high sensitivity C-reactive protein; LV – left ventricle; MI – myocardial infarction; NT-proBNP – N-terminal B-type pro-natriuretic peptide; TnT – troponin-T; UACR – urine albumin/creatinine ratio
Correlation with other Biomarkers
Klotho concentration showed only weak correlations with FGF-23 (r=−0.06, p<0.001) and with eGFR (r=0.12, p<0.001), cystatin-C (r=−0.11, p<0.001), NT-proBNP (r=−0.12, p<0.001), and hsCRP (r=−0.09, p<0.001). There was no correlation with hsTnT (r=−0.005, p=0.78) or UACR (r=0.03, p=0.13) (Supplemental Table S2).
Predictors of Klotho Concentration
Univariate clinical predictors of dichotomized Klotho concentration (Q1–3 vs Q4) in the full cohort were age (p<0.001), diabetes mellitus (p<0.0001), eGFR (p<0.001), body mass (p=0.003), and diastolic blood pressure (p=0.020). The independent predictors of Klotho concentration identified using the logistic multivariable model described above were diabetes mellitus (p<0.0001), eGFR (p<0.001), diastolic blood pressure (p=0.02), current smoking (p=0.01), and the biomarkers hsCRP (p=0.04) and UACR (p=0.002). Systolic blood pressure (p=0.08), age (p=0.10), prior MI (p=0.19), left ventricular ejection fraction (p=0.26), and the biomarkers NT-proBNP (p=0.07) and TnT (p=0.12) were not independent predictors of Klotho concentration.
Event Rates and Unadjusted Risk by Klotho Quartile in the Placebo Arm
Six-year Kaplan-Meier event rates for CV death or heart failure hospitalization in the placebo arm were highest among patients with low Klotho concentrations (Figure 1). Based on the apparent event rate threshold between patients in Q3 and Q4 of Klotho concentration, patients were subsequently analyzed as being in Q1–3 vs Q4 (Supplemental Figure S1). Specifically, the rates of CV death or heart failure hospitalization were nearly twice as high in patients in Q1–3 of Klotho concentration (8.2 vs 4.2%; HR 1.78; 95% CI 1.06–2.99; p=0.028; Figure 1), with similar patterns for the individual outcomes of CV death (5.1 vs 1.8%; HR 2.32; 95% CI 1.11–4.87; p=0.026) and heart failure hospitalization (3.9 vs 2.5%; HR 1.62; 95% CI 0.79–3.31; p=0.189). Rates of additional outcomes by dichotomized Klotho concentration are provided in Supplemental Table S3. The interaction with eGFR (<60 vs >=60 mL/min/1.73m2) for CV death or heart failure hospitalization by dichotomized Klotho concentration in the placebo arm did not reach statistical significance, though the increased risk with low Klotho concentration tended to be more pronounced in patients with low eGFR (pint=0.10; Supplemental Table S4).
Figure 1.

6-year Kaplan-Meier event rates and observed risk for CV death or heart failure hospitalization by quartile of Klotho concentration in the placebo arm.
Multivariable Adjustment
After multivariable adjustment for the clinical factors and biomarkers previously described, patients in Q1–3 of Klotho concentration remained at increased risk of CV death or heart failure hospitalization (HRadj 2.62; 95% CI 1.35–5.08; p=0.004) with directional consistency for both CV death (HRadj 5.38; 95% CI 1.60–18.08; p=0.007) and heart failure hospitalization (HRadj 2.00; 95% CI 0.89–4.50; p=0.095) individually in the placebo arm (results not shown). The inverse relationship between Klotho concentration and CV risk persisted when Klotho was modeled as a continuous variable, with a 22% reduction in adjusted risk of CV death or heart failure hospitalization observed for each standard deviation increase in Klotho concentration (p=0.048).
Concurrent Stratification by Klotho and FGF-23
In addition to Klotho, FGF-23 was also a significant independent predictor of CV death or hospitalization for heart failure, with a HRadj of 1.83 (95% CI 1.14–2.93; p=0.012) for Q4 vs Q1–3 FGF-23 concentration. When patients were stratified simultaneously by both FGF-23 quartile and Klotho quartile in the placebo arm, the 6-year rate of CV death or hospitalization for heart failure was nearly five times higher in patients with both a Klotho level in Q1–3 and an FGF-23 level in Q4 (n=339) as compared to patients with high Klotho and low FGF-23 concentrations (n=340; 16.9 vs 3.4%). This risk persisted after multivariable adjustment with HRadj 3.99 (95% CI 1.67–9.56; p=0.002; Figure 2 and Supplemental Table S5A), as well as with stratification by baseline eGFR (pinteraction=0.096; Supplemental Table S5B). Similar findings were observed for the individual outcomes of CV death and hospitalization for heart failure (Supplemental Figure S2). Metrics of discrimination are shown in Supplemental Table S6. Whereas FGF-23 added value to the clinical model, Klotho did not.
Figure 2.
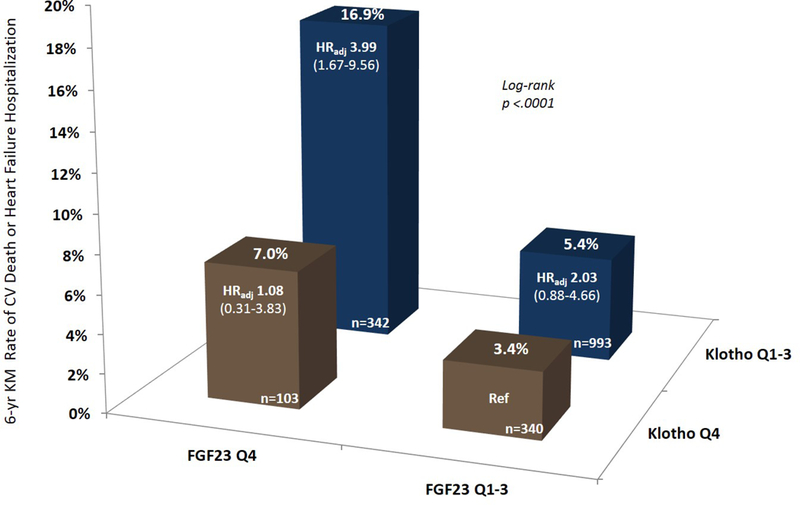
6-year Kaplan-Meier event rates and adjusted risk of CV death or heart failure hospitalization in patients in the placebo arm stratified by Klotho and FGF-23 concentrations. As compared to Klotho Q4/FGF23 Q1–3 as the referent, the adjusted risk for CV death or heart failure hospitalization was HRadj 1.08 (95% CI 0.31–3.83) for Klotho Q4/FGF23 Q4, HRadj 2.03 (95% CI 0.88–4.66) for Klotho Q1–3/FGF23 Q1–3, and HRadj 3.99 (95% CI 1.67–9.56) for Klotho Q1–3/FGF23 Q4. Adjustment covariates: age, sex, current smoker, HTN, T2DM, prior MI, prior revascularization, weight, SBP, LVEF, eGFR, cystatin-C, UACR, NT-proBNP, hsTnT, and hsCRP.
Trandolapril Effect by Klotho and FGF-23 Stratification
Patients with Q1–3 Klotho concentrations derived benefit from trandolapril for the end point of CV death or heart failure hospitalization (HR 0.69; 95% CI 0.50–0.95), whereas patients with Q4 Klotho concentrations had similar risk of CV death or heart failure hospitalization irrespective of randomized treatment assignment (HR 1.37; 95% CI 0.73–2.54; pinteraction =0.054).
Patients were classified as high risk if they were in both Q1–3 Klotho concentration and Q4 FGF-23 concentration; all other combinations were classified as low risk. High risk patients assigned to placebo had a 16.7% rate of CV death or heart failure hospitalization at 6 years as compared to 6.0% for high risk patients assigned to trandolapril (HR 0.39; 95% CI 0.23–0.68; Figure 3). Low risk patients did not benefit from trandolapril, however, with rates of CV death or heart failure hospitalization of 5.1% and 5.5% for placebo-treated and trandolapril-treated patients, respectively (HR 1.08; 95% CI 0.77–1.51; pint=0.002; Figure 3 and Supplemental Table S7A). These results were consistent when patients were stratified by baseline eGFR (Supplemental Table S7B). Among high risk patients, trandolapril also showed benefit for the two components of the primary composite outcome of CV death (HR 0.37; 95% CI 0.18–0.77; p=0.008) and heart failure hospitalization (HR 0.39; 95% CI 0.19–0.81; p=0.012) individually. This benefit was not seen in low risk patients.
Figure 3.
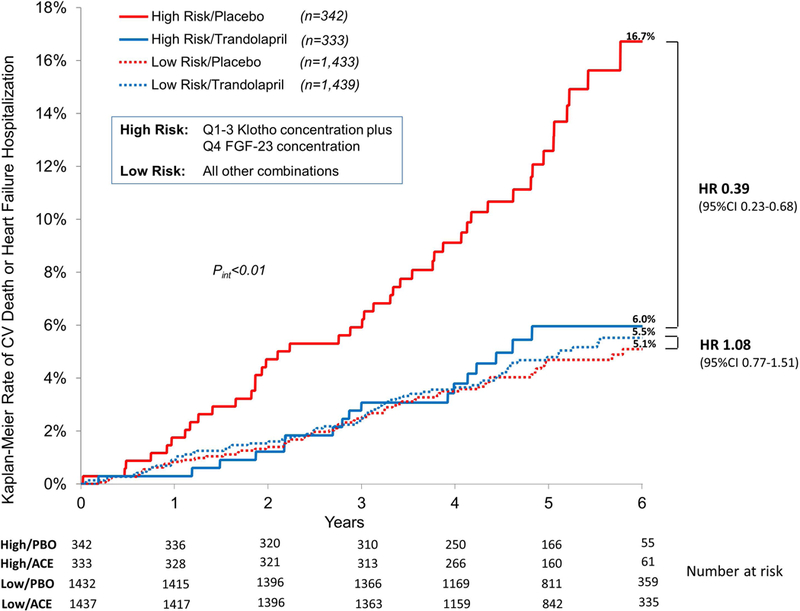
Kaplan-Meier event rates for CV death or heart failure hospitalization by high risk or low risk status and randomized treatment. High risk status was defined as both Klotho concentration in Q1–3 and FGF-23 concentration in Q4. All other combinations were considered low risk.
The additional ability of Klotho to predict benefit from trandolapril beyond FGF-23 alone was assessed in two ways. First, we examined trandolapril efficacy by high (Q4) or low (Q1–3) Klotho concentration in the subset of patients with high (Q4) FGF-23 concentration (n=885). Among these patients, a reduced risk of CV death or heart failure hospitalization was observed among patients with Q1–3 Klotho concentrations assigned to trandolapril (HR 0.39; 95% CI 0.23–0.68), whereas no significant benefit was seen with trandolapril in patients with Q4 Klotho concentrations (HR 0.79; 95% CI 0.27–2.35; pinteraction=0.27). In the former group, the absolute risk reduction was 10.7% and therefore the number needed to treat (NNT) with trandolapril to prevent one CV death or heart failure hospitalization event in the high risk group was 9. Secondly, in a model that already contained an interaction term for trandolapril and FGF-23, we also added a term for the interaction between trandolapril and Klotho, which had a p value of 0.08.
Discussion
Among patients with stable ischemic heart disease and LVEF >40%, low circulating levels of Klotho are associated with an increased risk of CV death or hospitalization for heart failure independent of clinical risk factors, renal biomarkers, and traditional CV biomarkers. Moreover, low Klotho concentration remained an independent predictor of CV death or hospitalization for heart failure after adjusting for circulating levels of its ligand, FGF-23. Indeed, patients with low levels of Klotho and high levels of FGF-23 were at distinctly elevated risk, with an adjusted hazard ratio of nearly four. This high risk group derived particularly large benefit from ACE inhibition with trandolapril.
Klotho and FGF-23 have been shown individually to predict adverse CV outcomes across a variety of disease states and patient populations.1, 2, 9–11, 20–30 Specifically, we previously reported the association between FGF-23 and CV outcomes in the PEACE trial13 along with the ability of FGF-23 to predict response to trandolapril. This current analysis is the first to our knowledge to combine FGF-23 level, Klotho level, and randomized ACE-inhibitor treatment.
Klotho levels in this cohort from the PEACE trial were intermediate relative to other reports in the literature. Because Klotho concentrations fall with worsened renal dysfunction and overall severity of illness, the distribution of Klotho concentrations in any cohort is determined by the particular population sampled. The median value of 565 pg/mL observed here among patients with stable ischemic heart disease is higher than the mean observed in a study of patients with CKD and type 2 diabetes (430 pg/mL),21 but lower than in the general older adult population (middle tertile 575–763 pg/mL).30
Klotho and FGF-23
The combination of low Klotho and elevated FGF-23 concentrations is a powerful marker of increased CV risk. It is possible that risk is increased with this pairing relative to either abnormality in isolation simply because two high-risk biomarkers portend a more ominous prognosis than one alone. But taking into consideration the close physiologic relationship of these two proteins, the findings reported here support the potential importance of FGF-23 resistance as a distinct, high-risk state amenable to RAS inhibition. FGF-23 signaling in the kidney and vasculature occurs largely through its co-receptor, Klotho, though Klotho-independent actions of FGF-23 do exist.5–8, 31 In the setting of systemic disease, inflammation, and renal dysfunction, however, Klotho levels fall, impairing FGF-23 signaling and potentially leading to FGF-23 resistance.31 FGF-23 levels are observed to rise in these same circumstances. FGF-23 appears to have directly deleterious cardiovascular effects, including promotion of myocardial fibrosis and altered myocardial calcium handling, via Klotho-independent mechanisms.9, 10, 32–35 A framework of Klotho depletion resulting in FGF-23 resistance anticipates low Klotho and high FGF-23 levels, which are observed here among patients at the highest risk for CV events.
Simultaneous stratification by Klotho and FGF-23 provided clear risk discrimination beyond that seen with either biomarker alone. Our group previously found Q4 FGF-23 concentration to be associated with an increased risk of CV death or heart failure hospitalization as compared to Q1 after adjustment for clinical variables and biomarkers (not including Klotho; HRadj 1.73; 95% CI 1.09–2.4).13 In the present analysis, this association was again observed in the presence of low Klotho, but not elevated Klotho. In parallel, the heightened risk seen with low Klotho concentration tended to be greater in those with elevated FGF-23. Finally, the previously observed finding of high FGF-23 as a marker of benefit with trandolapril was seen here only with concurrent low Klotho. These findings support the principal physiological relevance of paired decrease in Klotho and elevation in FGF-23 over either single abnormality.
Klotho/FGF-23 and the Renin-Angiotensin System
Function of the Klotho/FGF-23 axis is closely linked to the RAS. Kidney injury is associated with RAS upregulation and Klotho deficiency.1, 2, 21, 25, 36 Klotho and angiontensin II are mutually inhibitory4, 37and exogenous Klotho suppresses RAS activation, normalizes blood pressure, and improves renal fibrotic lesions in mice.36 In humans, pharmacologic RAS blockade is associated with increased Klotho levels, reduced vascular calcification, normalization of blood pressure, and improvement in LVH and myocardial fibrosis.21, 25
Mice with Klotho deletions develop extensive vascular calcification, increased aldosterone levels, LVH, and myocardial fibrosis.21–25 In humans, Klotho levels fall early in CKD,26 a finding which is associated with prevalent CAD27, 28 and mortality.30 Reverse findings have been seen with FGF-23, in which high FGF-23 is associated with RAS upregulation31, 38 and potential benefit from ACE-inhibition.13, 39
Here we observed a modulation of treatment effect with the ACE-inhibitor trandolapril based on the Klotho/FGF-23 status. Patients with the high-risk combination of Q1–3 Klotho and Q4 FGF-23 concentrations showed significant benefit with trandolapril whereas no effect was seen in patients with low risk Klotho and FGF-23 levels. Indeed, the NNT with trandolapril to prevent one CV death or heart failure hospitalization event in the high risk group as defined by concurrent Klotho and FGF-23 stratification was 9, which is 25% fewer patients than when risk stratifying with FGF-23 alone (NNT=12).13 These observations are consistent with the combination of low Klotho and high FGF-23 concentrations serving as a marker of increased RAS activation. Further, while the interaction between eGFR and Klotho concentration for risk of CV death or heart failure was not statistically significant, there was a trend toward more apparent risk stratification by Klotho concentration among subjects with low eGFR.
Implications
Biomarkers allow for increasingly nuanced definitions of disease states, prognosis, and potential risks or benefits from therapies on individual and population levels. As strategies for primary and secondary prevention evolve, specific delineation of risk status and potential benefit is essential. Although Klotho did not add to FGF-23 in terms of measures of discrimination, stratification with both of these biomarkers at the intersection of cardiovascular and renal function provided important prognostication of recurrent cardiovascular events in patients with stable ischemic heart disease and predicted benefit from one of the most commonly used classes of cardiovascular medicines. This finding is particularly striking in the setting of an overall neutral trial and, if validated in other data sets, would support the use of biomarker concentrations in patient selection for future trials.
Limitations
While this analysis benefits from a large, prospectively collected sample with randomized ACE-inhibitor assignment and adjudicated outcomes, there are important limitations. First, this analysis is post hoc and exploratory and the trial was not designed to achieve sufficient power for these specific sub-analyses, particularly tests for subgroup interactions. Second, only baseline Klotho and FGF-23 values are available. A more complete analysis with response to treatment could be undertaken with serial biomarker recordings, including measurement of renin, angiotensinogen, and aldosterone. Third, biomarker cut-points for the univariate and multivariate analyses were established post hoc, perhaps limiting reproducibility in other cohorts. Fourth, the specificity of commercially available assays for α-Klotho is debated40 and the relevance of measured circulating level to biological activity is not certain; nor is the long term stability of Klotho in frozen samples known. Finally, PEACE was an overall negative trial, which limits the ability to show differential treatment response. It might be fruitful to perform a similar analysis in a contemporary positive trial of an agent acting on the RAS.
Conclusion
In a population of stable patients with ischemic heart disease and preserved LV systolic function, low circulating levels of Klotho are associated with an increased risk of CV death or hospitalization for heart failure independent of clinical risk factors, renal biomarkers, and traditional CV biomarkers. Further, the combination of low Klotho and elevated FGF-23 concentrations identifies patients at elevated risk who derive clinical benefit from the ACE-inhibitor trandolapril.
Supplementary Material
Acknowledgments
Dr. Udell was supported by funding from a Heart and Stroke Foundation of Canada National New Investigator/Ontario Clinician Scientist Award; Ontario Ministry of Research and Innovation Early Researcher Award; Women’s College Research Institute and Department of Medicine, Women’s College Hospital; Peter Munk Cardiac Centre, University Health Network; and the Department of Medicine and Heart and Stroke Richard Lewar Centre of Excellence in Cardiovascular Research, University of Toronto.
The PEACE trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI; N01HC65149) with support from Knoll Pharmaceuticals and Abbott Laboratories.
Footnotes
Disclosures
B.A. Bergmark: Research Grant: MedImmune; Consultant/Advisory Board: Janssen, Daiichi Sankyo, Quark Pharmaceuticals, Abbott Vascular.
J.A. Udell: Consulting: Amgen, Boehringer-Ingelheim, Janssen, Merck, Novartis, Sanofi Pasteur; Honoraria: Novartis (registry steering committee), Sanofi Pasteur, Servier; Grant support: Novartis.
D.A. Morrow: Research Grant; Significant; Abbott Laboratories, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GlaxoSmithKline, Merck, Novartis, Roche Diagnostics. Consultant/Advisory Board; Modest; Abbott Laboratories, AstraZeneca, Bayer, GlaxoSmithKline, Merck, Peloton, Roche Diagnostics, and Verseon.
P. Jarolim: Research Grant; Significant; Abbott Laboratories, AstraZeneca, DaiichiSankyo, GlaxoSmithKline, Janssen Scientific Affairs, Merck & Co., Roche Diagnostics Corp, Takeda Global Research and Development Corp, Waters Technologies Corporation.
J.F. Kuder: None.
S.D. Solomon: Research Grant; Significant: Roche Diagnostics.
M.A. Pfeffer: Research Grant; Significant; Amgen, Celladon, Novartis, Sanofi. Consultant/Advisory Board; Modest; Bayer, Genzyme, GlaxoSmithKline, Janssen, Lilly, Medicines Company, Merck, Novartis, Novo Nordisk, Relypsa, Salix, Sanofi, Thrasos and Vericel. Consultant/Advisory Board; Significant; Boehringer Ingelheim, DalCor, Teva. Other; Modest; The Brigham and Women’s Hospital has patents for the use of inhibitors of the reninangiotensin system in survivors of MI with Novartis, with licensing agreement is irrevocably transferred to charity.
E. Braunwald: Research Grant; Significant; AstraZeneca, Duke University, Novartis, Merck, and DaiichiSankyo. Consultant/Advisory Board; Novartis, Medscape, Merck, The Medicines Company, Theravance.
M.S. Sabatine: Research Grant; Significant; Abbott Laboratories, Amgen, AstraZeneca, Critical Diagnostics, Daiichi Sankyo, Eisai, Genzyme, Gilead, GlaxoSmithKline, Intarcia, the Medicines Company, Merck, Roche Diagnostics, Takeda, Novartis, Pfizer, Poxel, Janssen Research Dev., MedImmune, Consultant/Advisory Board; Modest; Alnylam, AstraZeneca, CVS Caremark, MedImmune, Merck, MyoKardia, Bristol-Myers Squibb, Esperion, the Medicines Company, and Ionis.
Contributor Information
Brian A. Bergmark, Thrombolysis in Myocardial Infarction (TIMI) Study Group, Women’s College Hospital and the University Health Network, Toronto, Canada.
Jacob A. Udell, Brigham and Women’s Hospital and Harvard Medical School, Boston; Department of Medicine, Women’s College Hospital and the University Health Network, Toronto, Canada.
David A. Morrow, Thrombolysis in Myocardial Infarction (TIMI) Study Group, Women’s College Hospital and the University Health Network, Toronto, Canada.
Petr Jarolim, Department of Medicine, and Department of Pathology, Women’s College Hospital and the University Health Network, Toronto, Canada.
Julia F. Kuder, Thrombolysis in Myocardial Infarction (TIMI) Study Group, Women’s College Hospital and the University Health Network, Toronto, Canada.
Scott D. Solomon, Cardiovascular Division, Women’s College Hospital and the University Health Network, Toronto, Canada.
Marc A. Pfeffer, Cardiovascular Division, Women’s College Hospital and the University Health Network, Toronto, Canada.
Eugene Braunwald, Thrombolysis in Myocardial Infarction (TIMI) Study Group, Women’s College Hospital and the University Health Network, Toronto, Canada.
Marc S. Sabatine, Thrombolysis in Myocardial Infarction (TIMI) Study Group, Women’s College Hospital and the University Health Network, Toronto, Canada.
References
- 1.Bernheim J and Benchetrit S. The potential roles of FGF23 and Klotho in the prognosis of renal and cardiovascular diseases. Nephrology Dialysis Transplantation. 2011;26:2433–2438. [DOI] [PubMed] [Google Scholar]
- 2.Hu MC, Shi M, Cho HJ, Adams-Huet B, Paek J, Hill K, Shelton J, Amaral AP, Faul C and Taniguchi M. Klotho and phosphate are modulators of pathologic uremic cardiac remodeling. Journal of the American Society of Nephrology. 2014;26:1290–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim K, Lu T-S, Molostvov G, Lee C, Lam F, Zehnder D and Hsiao L-L. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. [DOI] [PubMed] [Google Scholar]
- 4.Mitani H, Ishizaka N, Aizawa T, Ohno M, Usui S-i, Suzuki T, Amaki T, Mori I, Nakamura Y and Sato M. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension (Dallas, Tex : 1979) 2002;39:838–843. [DOI] [PubMed] [Google Scholar]
- 5.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ and Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study). Journal of the American College of Cardiology. 2012;60:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S and Quarles LD. How fibroblast growth factor 23 works. Journal of the American Society of Nephrology. 2007;18:1637–1647. [DOI] [PubMed] [Google Scholar]
- 7.Donate-Correa J, Mora-Fernández C, Martínez-Sanz R, Muros-de-Fuentes M, Pérez H, Meneses-Pérez B, Cazaña-Pérez V and Navarro-González JF. Expression of FGF23/KLOTHO system in human vascular tissue. International journal of cardiology. 2013;165:179–183. [DOI] [PubMed] [Google Scholar]
- 8.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S and Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. [DOI] [PubMed] [Google Scholar]
- 9.Negishi K, Kobayashi M, Ochiai I, Yamazaki Y, Hasegawa H, Yamashita T, Shimizu T, Kasama S and Kurabayashi M. Association Between Fibroblast Growth Factor 23 and Left Ventricular Hypertrophy in Maintenance Hemodialysis Patients-Comparison With B-Type Natriuretic Peptide and Cardiac Troponin T. Circulation Journal. 2010;74:2734–2740. [DOI] [PubMed] [Google Scholar]
- 10.Hsu HJ and Wu M-S. Fibroblast growth factor 23: a possible cause of left ventricular hypertrophy in hemodialysis patients. The American Journal of the Medical Sciences. 2009;337:116–122. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R and Jüppner H. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. New England Journal of Medicine. 2008;359:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Investigators TPT. Angiotensin-converting–enzyme inhibition in stable coronary artery disease. New England Journal of Medicine. 2004;2004:2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udell JA, Morrow DA, Jarolim P, Sloan S, Hoffman EB, O’Donnell TF, Vora AN, Omland T, Solomon SD, Pfeffer MA, Braunwald E and Sabatine MS. Fibroblast growth factor-23, cardiovascular prognosis, and benefit of angiotensin-converting enzyme inhibition in stable ischemic heart disease. Journal of the American College of Cardiology. 2014;63:2421–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfeffer MA, Domanski M, Rosenberg Y, Verter J, Geller N, Albert P, Hsia J and Braunwald E. Prevention of events with angiotensin-converting enzyme inhibition (the PEACE study design). The American journal of cardiology. 1998;82:25–30. [DOI] [PubMed] [Google Scholar]
- 15.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA and Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. New England Journal of Medicine. 2009;361:2538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K and Saito Y. Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochemical and biophysical research communications. 2010;398:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H and Ljunggren Ö. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. New England Journal of Medicine. 2003;348:1656–1663. [DOI] [PubMed] [Google Scholar]
- 18.Omland T, Sabatine MS, Jablonski KA, Rice MM, Hsia J, Wergeland R, Landaas S, Rouleau JL, Domanski MJ and Hall C. Prognostic value of B-type natriuretic peptides in patients with stable coronary artery disease: the PEACE Trial. Journal of the American College of Cardiology. 2007;50:205–214. [DOI] [PubMed] [Google Scholar]
- 19.Sabatine MS, Morrow DA, de Lemos JA, Omland T, Sloan S, Jarolim P, Solomon S, Pfeffer MA and Braunwald E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation. 2012;125:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizobuchi M, Towler D and Slatopolsky E. Vascular calcification: the killer of patients with chronic kidney disease. Journal of the American Society of Nephrology. 2009;20:1453–1464. [DOI] [PubMed] [Google Scholar]
- 21.Karalliedde J, Maltese G, Hill B, Viberti G and Gnudi L. Effect of renin-angiotensin system blockade on soluble Klotho in patients with type 2 diabetes, systolic hypertension, and albuminuria. Clinical Journal of the American Society of Nephrology. 2013;8:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuro-o M The FGF23 and Klotho system beyond mineral metabolism. Clinical and Experimental Nephrology. 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 23.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T and Kume E. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. nature. 1997;390:45–51. [DOI] [PubMed] [Google Scholar]
- 24.Maltese G, Viberti G, Gnudi L and Karalliedde J. Effect of renin angiotensin system blockade on soluble Klotho, arterial stiffness and albuminuria in patients with Type 2 Diabetes and Systolic Hypertension. Artery Research. 2013;7:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon HE, Ghee JY, Piao S, Song J-H, Han DH, Kim S, Ohashi N, Kobori H, Kuro-o M and Yang CW. Angiotensin II blockade upregulates the expression of Klotho, the anti-ageing gene, in an experimental model of chronic cyclosporine nephropathy. Nephrology Dialysis Transplantation. 2011;26:800–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimamura Y, Hamada K, Inoue K, Ogata K, Ishihara M, Kagawa T, Inoue M, Fujimoto S, Ikebe M and Yuasa K. Serum levels of soluble secreted α-Klotho are decreased in the early stages of chronic kidney disease, making it a probable novel biomarker for early diagnosis. Clinical and experimental nephrology. 2012;16:722–729. [DOI] [PubMed] [Google Scholar]
- 27.Navarro-González JF, Donate-Correa J, de Fuentes MM, Pérez-Hernández H, Martínez-Sanz R and Mora-Fernández C. Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart. 2014;100:34–40. [DOI] [PubMed] [Google Scholar]
- 28.Arking DE, Becker DM, Yanek LR, Fallin D, Judge DP, Moy TF, Becker LC and Dietz HC. KLOTHO allele status and the risk of early-onset occult coronary artery disease. The American Journal of Human Genetics. 2003;72:1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maltese G and Karalliedde J. The putative role of the antiageing protein klotho in cardiovascular and renal disease. International Journal of Hypertension. 2012;2012:757–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, Guralnik JM and Ferrucci L. Plasma klotho and mortality risk in older community-dwelling adults. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Borst MH, Vervloet MG, ter Wee PM and Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. Journal of the American Society of Nephrology. 2011;22:1603–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faul C, Amaral AP, Oskouei B, Hu M-C, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J and Hare JM. FGF23 induces left ventricular hypertrophy. The Journal of Clinical Investigation. 2011;121:4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E and Christenson R. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirza MA, Larsson A, Melhus H, Lind L and Larsson TE. Serum intact FGF23 associate with left ventricular mass, hypertrophy and geometry in an elderly population. Atherosclerosis. 2009;207:546–551. [DOI] [PubMed] [Google Scholar]
- 35.Unsal A, Kose Budak S, Koc Y, Basturk T, Sakaci T, Ahbap E and Sinangil A. Relationship of fibroblast growth factor 23 with left ventricle mass index and coronary calcificaton in chronic renal disease. Kidney and Blood Pressure Research. 2012;36:55–64. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou FF and Liu Y. Klotho ameliorates kidney injury and fibrosis and normalizes blood pressure by targeting the renin-angiotensin system. The American journal of pathology. 2015;185:3211–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jimbo R, Kawakami-Mori F, Mu S, Hirohama D, Majtan B, Shimizu Y, Yatomi Y, Fukumoto S, Fujita T and Shimosawa T. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of Klotho deficiency. Kidney international. 2014;85:1103–1111. [DOI] [PubMed] [Google Scholar]
- 38.Boehm M and Nabel EG. Angiotensin-converting enzyme 2—a new cardiac regulator. New England Journal of Medicine. 2002;347:1795–1797. [DOI] [PubMed] [Google Scholar]
- 39.Wohlfahrt P, Melenovsky V, Kotrc M, Benes J, Jabor A, Franekova J, Lemaire S, Kautzner J and Jarolim P. Association of fibroblast growth factor-23 levels and angiotensin-converting enzyme inhibition in chronic systolic heart failure. JACC: Heart Failure. 2015;3:829–839. [DOI] [PubMed] [Google Scholar]
- 40.Barker SL, Pastor J, Carranza D, Quinones H, Griffith C, Goetz R, Mohammadi M, Ye J, Zhang J, Hu MC, Kuro-o M, Moe OW and Sidhu SS. The demonstration of alphaKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


