Abstract
Background:
Children adopted from orphanages or other such institutions tend to display blunted reactivity to stressors - even years after arriving in their generally supportive and highly resourced postadoption homes. Puberty, a proposed sensitive period for environmental influences on stress-mediating systems, may provide an opportunity for post-institutionalized children to recalibrate stress response systems in accordance with their now more supportive living situations.
Methods:
This cross-sectional study examined the HPA-axis reactivity of 280 children ages 7 through 14 years; 122 children were adopted from institutions in 14 countries between the ages of 6 months and 5 years, after spending an average of 95% of their lives in institutional care, and 158 children of similarly high socioeconomic status in their biological families served as the nonadopted comparison group. All of the children were assessed by nurses for Tanner stage and, on a different day, completed the Trier Social Stress Test for Children.
Results:
Using a linear mixed-effects model and 7 measures of salivary cortisol, results indicated that early-pubertal post-institutionalized children showed blunted HPA-axis reactivity compared to nonadopted children, but mid/late-pubertal post-institutionalized children displayed higher reactivity similar to the nonadopted comparison children.
Conclusions:
This is the first evidence of possible pubertal recalibration of HPA axis reactivity to a psychosocial stressor in post-institutionalized children, which provides a promising avenue for future research regarding the protective factors of the postadoption environment and subsequent physiological, behavioral, and psychopathological outcomes.
Keywords: Stress, Puberty, Institutions, Endocrinology
Introduction
The hypothalamic-pituitary-adrenal (HPA) axis is a critical mediator of physical and psychological stressors that has potent impacts on almost all organs and tissues of the body (Ulrich-Lai & Herman, 2009). The brain is a major target organ for glucocorticoids, where they influence numerous neurobehavioral systems (de Kloet, 2003). Animal models have shown that, in numerous species, the HPA axis calibrates to the harshness of the natal environment (Gutman & Nemeroff, 2002; Sanchez, 2006). Similar findings have been noted in maltreated children living with their families and those in foster care (e.g., Bernard et al., 2010; Bruce, Fisher, Pears, & Levine, 2009; Cicchetti & Rogosch, 2001); however, studies of children adopted internationally from institutions (e.g., orphanages) provide a closer human analogue to the animal models, in that for both animal models and post-institutionalized (PI) children, the adverse care is confined to infancy and early development (Julian, 2013). Thus, sequelae beyond that period can be examined while the children live in relatively supportive, resource-rich environment.
Physiological systems calibrate themselves to the demands of the environment during development; thus, for example, individuals born at high altitudes have great lung capacity and different haematological activity than those raised at sea level (Collins, 1999). Similarly, rats raised with dams who provide less and/or less organized maternal care develop a higher set-point of reactivity of the HPA axis via altered levels of glucocorticoid receptors in the hippocampus (Liu et al., 1997); while non-human primates raised without maternal care develop a hypo-responsive HPA axis and low basal levels of cortisol (Capitanio, Mendoza, Mason, & Maninger, 2005). These changes have been viewed as calibration of these systems to environmental demands in order to facilitate survival (e.g., Del Guidice, Ellis, & Shirtcliff, 2011), presumably during sensitive periods of development. Recently, it has been argued that for the HPA axis, there is a second period of calibration, or recalibration, that occurs during the peripubertal period (Eiland & Romeo, 2013). Animal studies have shown that stress during the peripubertal period has larger, less reversible impacts on physiology compared to stress in adulthood. However, there has been less attention paid to the possibility that if the individual starts life under harsh conditions known to alter activity of the HPA axis even after those conditions are relieved, then the pubertal period might be one in which the system could recalibrate to match the harshness or demands of the current context. For individuals reared initially in harsh, non-supportive conditions and then moving into more supportive, benign conditions, pubertal recalibration would mean that activity of the HPA axis would approximate those individuals who had lived all their lives under similar supportive, benign conditions.
Data from children reared in institutions show that deprivation of early parental care results in a hypo-responsive HPA axis (Koss, Mliner, Donzella, & Gunnar, 2016; McLaughlin et al., 2015). This may be achieved through an altered mineralocorticoid/glucocorticoid receptor (MR/GR) ratio (Strüber, Strüber, & Roth, 2014) or increased corticotropin-releasing hormone (CRH) expression in the brain which results in downregulation in peripheral regions (Centeno, Sanchez, Reddy, Cameron, & Bethea, 2007). Though the exact cause of this hypoactivity is still unknown, presumably it is an adaptive mechanism to prevent the physical and mental sequelae of prolonged cortisol elevations (Fries, Hesse, Hellhammer, & Hellhammer, 2005). However, both hyper- and hypoactivity of the HPA axis are associated with adverse health outcomes, as discussed in the allostatic load model of adaptation to chronic stress (Juster, McEwen, & Lupien, 2010). In PI children, hypoactivity of the HPA axis following early institutional care is associated with deficits in attention and behavior regulation, as well as higher levels of externalizing symptoms (Koss et al., 2016; Kroupina et al., 2012; Pitula et al., 2017). A key question for the field, however, is whether hypoactivity of the HPA axis persists throughout development in PI youth.
In typically developing children, pubertal development is generally associated with an increase in cortisol levels (Gunnar, Wewerka, Frenn, Long & Griggs, 2009; Stroud et al., 2009) and possibly in reactivity (Blumenthal et al., 2014; Sumter, Bokhorst, Miers, Van Pelt, & Westenberg, 2010). With pubertal development, self-reports of stress also increase, particularly in social-evaluative situations (Peper & Dahl, 2013), which may be influenced by increased emotional and physiological reactivity to stressors (Dahl & Gunnar, 2009; Sumter et al., 2010). This increased stress reactivity may also be influenced by increased production of gonadal hormones during puberty (Peper & Dahl, 2013). Pubertal hormones have wide-ranging influence on brain and behavioral development (Sisk & Zehr, 2005), which may allow for heightened plasticity of stress and emotion regulatory systems (Fuhrmann, Knoll, & Blakemore, 2015; Galván & McGlennan, 2012; Somerville, Jones, & Casey, 2010). In animal models, exposure to a significant stressor during the peripubertal period causes more enduring effects on the HPA axis compared to exposure to the same stressor in adulthood (Romeo, 2013). However, plasticity cuts both ways and may conversely facilitate the recalibration of systems previously impacted by early life stress following an improvement in the environmental circumstances, as is the case with PI children.
Two studies, both of the cortisol awakening response (CAR), suggest that across pubertal development youth with histories of adverse early experiences may transition from a blunted CAR to either a typical CAR (Quevedo et al., 2012) or one that has overcorrected and become larger than that of youth without histories of adverse early experiences (King et al., 2016). A study of PI Romanian individuals, however, continued to note a blunted CAR into adulthood (van der Vegt, van der Ende, Kirschbaum, Verhulst, & Tiemeier, 2009). To our knowledge with regard to the cortisol stress response, however, there are no studies of possible pubertal recalibration of the stress response that have been conducted with PI or any other children.
The present study investigated whether 1) at earlier stages of puberty, PI children show a smaller cortisol stress response compared to non-adopted children earlier in puberty while 2) at later stages of puberty, there would be no difference between PI and non-adopted children’s cortisol responses, suggesting pubertal recalibration of the stress response. Children completed the Trier Social Stress Test for Children (TSST-C), a common method for evoking a cortisol response in children and adolescents.
Methods
Participants
Participants were internationally adopted youth (PI) and non-adopted youth (NA). Inclusion criteria were: for the PI youth, international adoption after having spent 50% of preadoption life in institutional care; for NA youth, born and raised in his/her family of origin. A total of 8 PI youth were excluded from the analyses due to FAS/FAE (n=6), Autism (n=1), or dropping out before the TSST-C session (n=1), while 6 NA youth were excluded due to Autism (n=2) and dropping out before the TSST-C session (n=4). FedEx lost one shipment of saliva being sent for assay resulting in exclusion for lack of cortisol data of 9 PI and 13 NA youth. The final sample included 122 (81 female) PI and 158 (85 female) NA youth. PI youth spent an average of 95% (SD=9.6%) of their preadoption lives in institutional care. Age at adoption ranged from 5.5 to 59 months (M=19.4, SD=12.7 months). These children were adopted from a number of regions: 60 (49.1%) from Eastern Europe; 16 (13.1%) from India; 16 (13.1%) from Latin America; 26 (21.3%) from Asia; and 4 (3.2%) from Africa. The PI youth had been in their adoptive families an average of 10.6 years (range 4–15 years). Family demographics can be found in Table 1, separated for PI and NA youth. Income and education level for the two groups was comparable. Consent was received from a caregiver for each participant and written and verbal assent were received from all participants. This study was conducted in accordance with university Institutional Review Board approval standards.
Table 1.
Demographics for PI and NA youth.
| Post-institutionalized (PI) n = 122 |
Non-adopted (NA) n = 158 |
|
|---|---|---|
| Child age, years, M (SD) | 11.4 (2.4) | 11.2 (2.2) |
| Child race, n (%) | ||
| African American | 6 (4.9) | 3 (1.9) |
| Asian | 47 (38.5) | 1 (0.6) |
| Caucasian | 49 (40.2) | 141 (89.2) |
| Latin American | 13 (10.7) | — |
| Mixed race or other | 7 (5.7) | 13 (8.2) |
| Child BMI, M (SD) | 18.4 (3.4) | 19.2 (4.0) |
| Granger medication score, % 0 | 73.0 | 85.4 |
| Proportion who are receiving/have ever received mental health treatment (%) | 23.8 | 7.0 |
| Primary caregiver education, n (%) | ||
| Bachelor’s degree or higher | 104 (85.2) | 130 (82.3) |
| Annual family income | ||
| Median | $100,001–150 | $100,001–150 |
| Range | $40,001–200,000+ | $10,001–200,000+ |
Procedure
Youth participated in two laboratory sessions, approximately one week apart. During one session, typically first, participants completed the Trier Social Stress Test (see below for details). During a second session, participants underwent a physical exam that included Tanner staging as a pubertal assessment (see below for details).
Measures
Trier Social Stress Test for Children.
Participants engaged in a modified version of the TSST-C, involving a socially-evaluative public speaking task and verbal math performance, as described previously (Hostinar, Johnson, & Gunnar, 2015). The story stem for the speech involved introducing oneself to a new school class (Yim, Quas, Cahill, & Hayakawa, 2010), while the physical arrangements involved facing a two-way mirror while being filmed, with judges behind the mirror represented by audiotaped voices (Jansen et al., 2000). Though it is a departure from original TSST protocol to not have the judges physically in the room with the participant, Jansen and colleagues (2000) successfully elicited a cortisol response using this method. Participants had a 40-minute acclimation period in order to control for the effects of arrival/unfamiliarity on cortisol production. Then, participants were escorted to a private room and given 5 minutes to prepare a speech in which they were told to introduce themselves to a new class of students. They were then brought to another small room to deliver the speech and math. This room contained only a one-way mirror and an obvious camera. Participants were told that the experimenter and one other adult were behind the mirror to judge their performance. Both judges introduced themselves. One was male and one was female. If participants stopped their speech before five minutes, they were told to “continue” by the experimenter via intercom. Following the speech, participants performed an age-adjusted verbal subtraction task aloud for an additional 5 minutes. Specifically, participants aged 7–8 began with the number 397 and were asked to subtract by 3’s, 9–10 year olds began with 307 and subtracted by 3’s, and 11-year-olds and up began with 758 and subtracted by 7’s. When an error was made, the experimenter interrupted to indicate the answer was incorrect and to restart the sequence. Subtraction difficulty level was titrated based on each child’s response rate and accuracy (i.e., bumped up a level if responding rapidly and accurately for several numbers in a row). Simpler and more complex difficulty levels were available if participants needed to be bumped down from the 7–8 starting point or bumped up from the 11+ starting point, respectively. Participants then returned to the prep room and completed questionnaires and saliva samples with the experimenter in the room before returning to their caregiver (see Figure 1 for session timeline). Experimenters maintained a neutral demeanor while interacting with the participants before and after the TSST-C.
Figure 1.
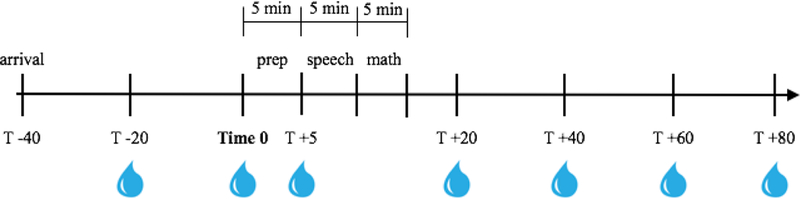
TSST session timeline. Saliva samples are indicated by water droplets beneath the timeline.
Additionally, self-reported stress ratings were collected following the completion of the TSST-C. This was assessed with a 5-item rating scale asking the child how stressed they felt at arrival, during speech prep, during the speech and math portions, and at that moment on a 5-point scale (1=not at all to 5=a whole lot). The average of these ratings was taken as the child’s self-reported stress rating during the session.
Salivary cortisol.
Saliva samples were collected seven times during the session (−20, 0, +5, +20, +40, +60, and +80 minutes, where 0 represents the beginning of 5-minute preparatory period before the TSST-C; see Figure 1 for sample timing). The beginning of speech prep was used as time 0 because previous studies have shown that cortisol production begins to increase in anticipation of the speech rather than due to giving the speech itself (Sumter et al., 2010). Peak salivary cortisol levels are generally reached 20–40 minutes post-stressor onset (Kirschbaum & Hellhammer, 1994), and we have included several samples at 20-minute intervals post-stressor to capture peak levels as well as decline back to baseline. In order to control for time of day effects that may emerge from diurnal variation in cortisol levels, all sessions began between 3:00 PM and 4:30 PM and ran for exactly 2 hours. Whole unstimulated samples were obtained. Participants were asked to refrain from eating or drinking during the session and reported on recent food, caffeine, and medication intake and recent illness. The samples were stored in a laboratory freezer at −20°C until being shipped to the University of Trier, Germany. All seven saliva samples were assayed for cortisol concentration in duplicate using a time-resolved fluorescence immunoassay (DELFIA). The intra-assay coefficients of variation were between 4.0% and 6.7%, and the corresponding inter-assay coefficients of variation were between 7.1% −9.0%. All of the samples from each participant were included in the same assay batch. Biologically implausible cortisol values above 2 μg/dL were removed. With regard to the impact of medication use, a Granger score was calculated for each participant following guidelines of Granger and colleagues (2009), in which each medication taken that day was rated regarding its likelihood of affecting cortisol production (0=no plausible effect to 2=very plausible effect) and summing each of these scores together. Fifty-six participants had a score greater than 0 (M=2.6 excluding zeros) and this variable was investigated as a possible covariate. Complete salivary cortisol data are available for all participants included in this sample. Log10 transformations were performed for all cortisol values to resolve positive skew.
Tanner staging.
Participants underwent a nurse exam for Tanner staging (range 1–5, M=2.55, SD=1.50). Three nurses conducted a physical exam to measure pubertal stage using Marshal and Tanner criteria (Marshal & Tanner 1969; 1970). Study nurses received Tanner stage training from an expert in performing this assessment in clinical trials and a pediatric endocrinologist. Cohen’s κ was run to determine if there was agreement between the three nurses’ Tanner stage assessment on breast and genital measures in 33 boys and 50 girls. There was almost perfect agreement between the nurses’ assessments, κ=.87, p<.001 using Landis and Koch (1977) criteria of >.81 (more details on Tanner staging in Reid et al., 2017). Tanner stage was treated continuously in the models described below but dichotomized at a median split (Tanner=2; grouping 1/2, n=156, vs. 3/4/5, n=124) for graphical purposes. This may also be a meaningful inflection point, as shifts in activity of the HPA axis have been reported to occur around Tanner stage 3 (Netherton, Goodyer, Tamplin & Herbert, 2004).
Pubertal status was also assessed using child self-report and caregiver-report of body changes associated with puberty using the Peterson Pubertal Development Scale (Carskadon & Acebo, 1993; Petersen, Crockett, Richards, & Boxer, 1988). For boys, measures included growth in height, body hair, skin changes, deepening of voice, and facial hair. For girls, measures included growth in height, body hair, skin changes, breast development, and onset of menarche. Responses were 1=not yet started, 2=barely started, 3=definitely started, and 4=seems complete (39). Menstruation was coded as 4 if it begun and 1 if it had not begun, alongside a self-report of age in months of menarche. This measure yields a mean score from 1 (puberty has not begun) to 4 (puberty is complete). Child and caregiver report were highly correlated with each other (r=0.92) and each were highly correlated with Tanner stage (r=.82 and.83, respectively). Sixteen participants (5.7%) refused Tanner staging, and so pubertal status was imputed based on average Peterson score. This was done by calculating a Peterson z-score, which was back-transformed to a Tanner score from 1–5 to account for the difference in scales. As previously reported, there was no evidence of early onset puberty in this sample of PI children (Reid et al., 2017).
Family Environment Scale.
The Family Environment Scale (FES; Moos & Moos, 1981) is a 90-item questionnaire filled out by the parent during the TSST session. Items are statements about processes, routines, and relationships in the home and answers are given as true/false. Two scales resulting from this questionnaire are cohesion (9 items, α=.62, M=7.8, SD=1.5) and conflict (9 items, α=.66, M=2.8, SD=1.9) that assess support/commitment and conflict between family members, respectively.
Data analytic plan
Group differences (PI vs. NA) in family environment were tested via a t-test to support our claim that the PI children in this study are reared in similarly low-risk adoptive families compared to the children raised in their biological family. Chi-squared tests of difference indicated that the groups did not differ in BMI (χ2=97.14, p=.45), but PI children had higher Granger scores (χ2=10.08, p=.02) and more mental health treatment (χ2=15.93, p<.001) compared to NA children. The association between self-reported stress ratings and Tanner stage was assessed via Pearson correlation. A linear mixed-effects model was fit to analyze cortisol levels and reactivity. Linear, quadratic, and cubic effects of time were tested given that values are expected to decrease with adaptation to the lab, increase in response to the TSST-C, and then decrease back to baseline. The highest-order effect of time was tested to assess whether there was a significant response to the TSST-C for the entire sample. Given our hypotheses regarding pubertal recalibration, this analysis was conducted on the full sample (n=280) as well as the subgroup that were determined to be at or past Tanner stage 3 (n=124). Direct effects of predictors in the model were used to assess associations with overall level of cortisol production, while moderation effects with time variables represent that predictor’s association with cortisol reactivity. Random intercepts and slopes (up to the highest-order effect of time that was retained in the first step) were also tested. Group (PI vs. NA), pubertal stage, and the interaction between group and pubertal stage were considered as focal time-invariant predictors of overall cortisol production as well as reactivity.
Age was highly correlated with Tanner stage (r=0.83) and so was not considered as an additional predictor given that pubertal stage was a focal predictor. If age was included as a covariate, we would then be assessing the effect of pubertal timing rather than the effect of puberty itself. Age at adoption, sex, family income, Granger score, and self-reported stress ratings were all considered as potential time-invariant covariates but did not change results so they were removed from the final model. Similarly, the pattern of results remained the same when only examining the subset of participants that had a Granger score of 0 (n=224). While PI children also experience dramatic improvements in physical growth/nutritional status following adoption (van IJzendoorn, Bakermans-Kranenburg, & Juffer, 2007), we did not find a significant group x puberty moderation in predicting child body mass index (BMI; t=1.54, p=.13). Thus, nutritional status was not investigated further as a potential covariate. No moderating effects of sex were found; however, these analyses should be interpreted with caution due to the small cell sizes of these interactions.
Results
Descriptive statistics
Descriptive statistics for focal variables can be found in Table 2. Of note, later Tanner stage was significantly associated with higher cortisol levels at time +40 and +80, reflecting peak reactivity and recovery cortisol, respectively. Groups significantly differed in sex and pubertal stage (χ2=4.02, p=.05; χ2=17.73, p=.001, respectively) such that PI children were less likely to be male, less likely to be in Tanner stage 2, and more likely to be in stage 3 than NA children. Pubertal stage also varied by sex (χ2=24.82, p<.001) such that males were more likely to be in stage 5 and less likely to be in stage 4 than females, which was slightly unexpected because females tend to be farther along in puberty than males. However, when controlling for sex and age, there was no longer a group difference in Tanner stage (Ordinal logistic regression coefficient=−0.07, t=−0.26). There were no group differences in post-acclimation baseline cortisol (time 0; t=−0.85, p=.39).
Table 2.
Descriptive statistics and correlations between focal variables.
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. Child age at adoption (in months) | — | ||||
| 2. Tanner stage | 0.03 | — | |||
| 3. Time 0 cortisol (post-acclimation baseline) | −0.14* | 0.03 | — | ||
| 4. Time +40 cortisol (peak reactivity) | −0.14* | 0.19*** | 0.66*** | — | |
| 5. Time +80 cortisol (recovery) | −0.16** | 0.13* | 0.62*** | 0.78*** | — |
| Mean (SD) | 8.47 (12.76) | 2.55 (1.50) | −1.13 (0.31) | −1.12 (0.34) | −1.28 (0.33) |
Note. p <.05,
p <.001.
All correlations are Pearson correlations except those with Tanner stage, which are Spearman’s rank correlations. Cortisol descriptive statistics and correlations are using log10-transformed values.
There were no differences found in parent-reported family environment across groups (cohesion: t=0.02, p=.99; conflict: t=0.08, p=.93). Similarly, there was no difference in self-reported stress ratings across groups (t=−1.23, p=.22) and there was not a significant association between stress ratings and Tanner stage (r=0.06, p=.32); however, there was a difference in association across groups. As displayed in Figure 2, for PI children, stress ratings were not associated with Tanner stage (r=−0.04, p=.66), but for NA children stress ratings were significantly positively associated with Tanner stage (r=0.15, p=.05).
Figure 2.
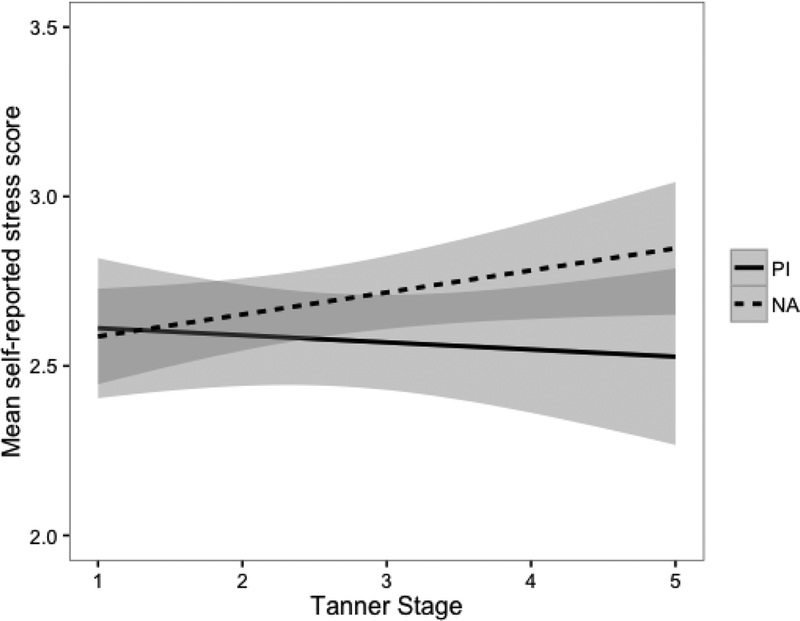
Mean self-reported stress scores by group and Tanner stage. The shaded areas represent the 95% confidence envelope. * p <.05
Cortisol reactivity to TSST-C
Chi-squared tests of difference determined that the shape of the curve for cortisol is indeed cubic (Cubic vs. quartic: χ2=0.69, p=.41). This was also consistent using only the subsample of participants that were at or past Tanner stage 3. Random intercept, as well as random slopes for the linear and quadratic effects of time were retained. Cubic time significantly predicted cortisol levels (t=10.74, p<.001), suggesting that the hypothesized pattern of adaptation, reactivity, and recovery is present in the overall sample. Age at adoption, sex, and family income did not significantly predict cortisol levels (age at adoption: t=−1.94; sex: t=1.74; family income: t=−0.52) and so were not retained in future models.
Differences by Tanner stage and adoption status
Adoption status significantly predicted cortisol levels such that PI children exhibited lower cortisol levels than NA children (t=2.56, p=.01). Furthermore, pubertal status significantly predicted cortisol levels such that children at later stages of puberty had higher overall cortisol levels compared to those at earlier pubertal stages (t=1.98, p<.05). Subsequently, there was a significant group by puberty interaction and a significant interaction between puberty and cubic time. Final model results can be found in Table 3. Raw/log-transformed and model-predicted least-squares means for each cortisol time point across groups can be found in Table S1. In order to probe these interaction effects, cortisol reactivity broken down by group and pubertal status is displayed graphically in Figure 3. Post-hoc least-squares comparisons suggest that early-pubertal PI children show the lowest cortisol production and reactivity compared to all mid/late-pubertal children and early-pubertal NA children (early-pubertal PI 95%CI [−1.35,−1.20]; late-pubertal PI [−1.16,−1.03]; early-pubertal NA [−1.18,−1.06]; late-pubertal NA [−1.17,−1.04]). These associations also held when controlling for self-reported stress ratings and medication use (data available upon request).
Table 3.
Final mixed-effects model results for cortisol reactivity.
| Fixed effects | Estimate (SE) | t-value |
|---|---|---|
| Intercept | −1.105 (.074) | −15.01*** |
| Time | −0.138 (.052) | −2.64** |
| Time2 | 0.029 (.014) | 2.03* |
| Time3 | −0.002 (.001) | −2.06* |
| Group (PI vs. NA) | 0.211 (.079) | 2.67** |
| Pubertal status | 0.059 (.023) | 2.63** |
| Time x Group | −0.015 (.045) | −0.33 |
| Time x Pubertal status | −0.027 (.015) | −1.82 |
| Group x Pubertal status | −0.052 (.021) | −2.47* |
| Time2 x Group | 0.007 (.012) | 0.53 |
| Time2 x Pubertal status | 0.011 (.004) | 2.73** |
| Time3 x Group | −0.001 (.001) | −0.61 |
| Time3 x Pubertal status | −0.001 (.000) | −3.12** |
| Random effects | Variance | SD |
| Intercept | 0.094 | 0.306 |
| Time | 0.010 | 0.098 |
| Time2 | 0.000 | 0.010 |
| Residual | 0.015 | 0.124 |
Note. p <.05,
p <.01,
p <.001.
PI is the reference group for “Group”.
Figure 3.
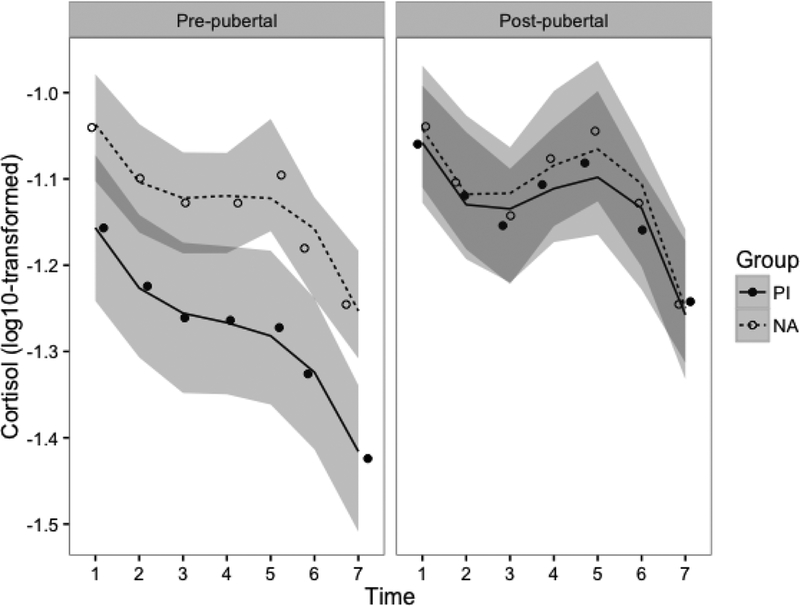
Estimated cortisol reactivity by group (PI vs. NA) and pubertal stage (median split: Tanner < 3 vs. Tanner ≥ 3). Model results include pubertal stage as a continuous predictor; the median split was used purely for visualization purposes. Lines indicate model-predicted values, and points indicate raw group means at each time point. Lines are separated by group (PI vs. NA) and graphs are faceted by pubertal status. The shaded areas represent the 95% confidence envelope. Cortisol values are log10-transformed to account for positive skew.
Discussion
There were three findings of note. First, as in other studies that have examined early-pubertal PI children, we found evidence of blunted stress responding. Second, among PI children who were at Tanner stage 3 and beyond, we observed no evidence of blunted cortisol response to the TSST-C. Third, cortisol levels increased with pubertal stage, although it was not clear that reactivity increased for the non-adopted children. These findings are consistent with the pubertal stress recalibration hypothesis and the first to show this for cortisol reactivity in children. However, because this is a cross-sectional study, and age and puberty were so highly correlated, these findings should be viewed as suggestive, not conclusive.
There is a good deal of evidence that early life adversity, particularly a lack of supportive caregiving is associated with a down-regulation of adrenal production of cortisol. This has been shown in the flattening of the diurnal rhythm due to lower early morning levels (Gunnar & Vazquez, 2001), a smaller cortisol awakening response (Mangold, Wand, Javors, & Mintz, 2010; Kumsta, et al., 2017), and lower reactivity to psychosocial challenge (Koss et al., 2016; McLaughlin et al., 2015). This is typically shown in early-pubertal children, which corroborates our finding, shown in Figure 3, that lower pubertal stages were associated with a less marked cortisol response to the TSST. Even though early-pubertal PI children reported that they were similarly stressed by the TSST-C as their non-adopted counterparts, they showed little to no cortisol response to this stressor. Indeed, their levels were lower at arrival and declined on average across the session.
Several cross-sectional studies have found evidence that the cortisol awakening response increases with pubertal stage in children who experienced early adverse care, beginning to resemble or perhaps exceeding the magnitude of non-adopted children from low risk backgrounds (Quevedo et al., 2012, King et al., 2016). In the present study, we also found evidence that cortisol reactivity to the TSST-C was larger and resembled that of non-adopted children with increasing pubertal stage. Indeed, as shown in Figure 3, not only were cortisol levels higher for the PI youth who were further along in pubertal development than those at earlier stages, but they also showed an increase in cortisol in response to the TSST-C rather than the decrease seen in children at earlier stages of puberty. This pattern of findings does not seem to be explained by PI children having a differentially supportive family environment compared to NA children because we found no differences in family conflict or cohesion between groups. Both groups had a fairly high median income, and caregivers in both groups tended to be very well educated (approximately 85% of caregivers had a bachelor’s degree or higher). However, future studies should examine whether the degree of supportiveness and/or stressfulness of the current environment is associated with pubertal recalibration processes in PI children.
Because of the considerations outlined above, our findings suggest recalibration of the HPA axis with puberty; however, more conclusive evidence will come later once we have longitudinal data and can examine within-individual change in reactivity across pubertal development. This will be especially important in differentiating age from puberty as the factor most associated with the shift in HPA axis functioning for youth with early life stress histories. Additionally, longitudinal data will allow us to examine whether these PI children initially resemble their NA counterparts, but then eventually overshoot them in terms of cortisol reactivity to stressors. The nonsignificant difference found between PI and NA children at later stages of puberty is consistent with Quevedo et al. (2012) which examined the cortisol awakening response, but this may be influenced by the fact that the majority of the children in this study were still fairly young (oldest=14 years) and only 18% of the sample had reached Tanner stage 5, while 36% were at stage 1. Once more of these children progress to the final stage of puberty, PI children’s cortisol reactivity may continue rising, mirroring the findings seen in King et al. (2016) with the cortisol awakening response. Longitudinal data of stress reactivity poses a challenge, however, because the HPA axis is particularly responsive to novel stressors, which complicates our ability to assess within-individual change in HPA reactivity to the same stressor over time. Thus, cross-sectional analyses still provide a useful tool to assess pubertal recalibration of HPA axis reactivity.
We also did not observe any sex differences or interactions between sex and pubertal stage or group. Adolescence tends to be the period in development when consistent sex differences in HPA activity emerge (McCormick & Matthews, 2007), potentially mediated by changing levels of sex hormones across this period. Particularly, post-pubertal and adult females tend to have a cortisol stress response that initiates more quickly and is larger than that of males, which may mediate the increased risk of depression seen in adolescent females compared to adolescent males (Natsuaki et al., 2009). However, due to limitations in study design (including the fact that many participants were still in the very early stages of puberty) and the small cell sizes that result when parsing the sample by group, pubertal status, and sex, we cannot interpret our null results with any amount of certainty. While we did not find any evidence for sex-specific recalibration in this study, future studies should assess whether pubertal recalibration of HPA reactivity mediates risk for later psychopathology in PI children.
Interestingly, it does not seem that the children’s perceived stress during the TSST-C can explain our findings. If children’s perceived stress paralleled our findings with cortisol, then extra-hypothalamic changes might explain recalibration of stress reactivity for PI youth. Indeed, for NA children, ratings of perceived stressfulness of the TSST-C correlated positively with pubertal stage, which is expected given the heightened sensitivity of adolescents to social evaluation (Dahl & Gunnar, 2009; Peper & Dahl, 2013) and reactivity to social-evaluative stressors such that higher pubertal stages are associated with greater anticipatory cortisol responses to a stressor that involves evaluation by peers (Sumter et al., 2010). Surprisingly, we did not observe this pattern for the PI children. Self-reports of stress rarely correlate with measures of HPA reactivity during stressors; nonetheless, it was notable that PI children did not exhibit heightened sensitivity to social evaluation with pubertal development. Because this was unexpected, it will be important to replicate this finding.
It should also be noted that the PI youth had early histories of nutritional deprivation that could also be contributing to physiological changes seen post-adoption. However, analyses of current BMI did not explain the variation in HPA axis functioning associated with pubertal stages. It is also important to note that from a neurophysiological perspective, we have little idea of the alterations that produce a down-regulation of the HPA axis with chronic early life adversity, nor the mechanisms that might allow this to be reversed if conditions are more benign during puberty. It is important to recognize, too, that we are only measuring the adrenal level of the axis. It is entirely possible that at higher levels of the axis, including regions of the brain upstream of the hypothalamus that help to regulate HPA activity, different patterns emerge after early adversity and/or puberty that may shed light on these processes. Animal models are needed to help explicate the neurophysiological underpinnings both the hypo-functioning of the HPA axis following early adversity and subsequent recalibration with pubertal development.
Conclusion
The present study is the first assessment of pubertal recalibration of HPA stress reactivity in post-institutionalized children. This population provides a good model for investigating the impact of circumscribed early life adversity, followed by recalibration of the body’s stress system during pubertal development – a second period of plasticity when the environment is comparatively more supportive than the institutions in infancy. While these data are cross-sectional and thus preliminary, cortisol was sampled frequently before and after the stressor, and time of day was strictly controlled, so as to allow for nuanced and dynamic changes in cortisol trajectories across the session. There was a 40-minute adaptation period, which enhances our confidence that the reactivity seen above is in response to the stressor and not an anticipatory increase upon arrival to the session.
In sum, early-pubertal post-institutionalized children show blunted HPA axis reactivity compared to nonadopted peers. At later pubertal stages, this pattern seems to normalize, providing support for the pubertal recalibration hypothesis following early institutionalization. These results are hopeful and underscore the possibility of recovery following severe early adversity even several years later in development if the environment can be improved. Future studies should attempt to replicate these findings with longitudinal data, investigate whether the degree of supportiveness of the pubertal environment predicts the degree of recalibration, and assess whether recalibration reduces the risk for later psychopathology in post-institutionalized children.
Supplementary Material
Key points:
– Replicating extant research, early-pubertal post-institutionalized children show blunted cortisol reactivity to a psychosocial stressor
– However, children at later stages of puberty resemble nonadopted peers in stress reactivity
– There were no differences by sex, and results could not be explained by differences in age at adoption, the family environment, self-reported perceived stress, medication use, or time of day
– These results provide preliminary cross-sectional support for pubertal recalibration of the HPA axis following severe early adversity, which may set the stage for resilient development in post-institutionalized children
Acknowledgements
The authors would like to thank the families, who devoted many hours to the longitudinal study from which the current data was taken. To Bao Moua, Lea Neumann, Heather Taylor, Chris Desjardins, and all nurses, staff, and students who helped recruit participants and collect and process the data. This research was supported by a grant from the National Institute of Child Health and Human Development through the National Institutes of Health [5R01 HD075349] to the final author. The authors have declared that they have no competing or potential conflicts of interest.
Abbreviations:
- HPA
hypothalamic-pituitary-adrenocortical
- PI
post-institutionalized
- NA
nonadopted
- TSST-C
Trier Social Stress Test for Children
Footnotes
Conflict of interest statement:
No conflicts declared.
References
- Bernard K, Butzin-Dozier Z, Rittenhouse J, & Dozier M (2010). Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement of Child Protective Services. Archives of Pediatrics & Adolescent Medicine, 164(5), 438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Badour CL, Trainor CD, & Babson KA (2014). Pubertal maturation and cortisol level in response to a novel social environment among female adolescents. Journal of Adolescence, 37(6), 893–900. doi: 10.1016/j.adolescence.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Bruce J, Fisher PA, Pears KC, & Levine S (2009). Morning cortisol levels in preschool‐aged foster children: Differential effects of maltreatment type. Developmental Psychobiology, 51(1), 14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, & Maninger N (2005). Rearing environment and hypothalamic‐pituitary‐adrenal regulation in young rhesus monkeys (Macaca mulatta). Developmental Psychobiology, 46(4), 318–330. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, & Acebo C (1993). A self-administered rating scale for pubertal development. Journal of Adolescent Health, 14, 190–195. [DOI] [PubMed] [Google Scholar]
- Centeno ML, Sanchez RL, Reddy AP, Cameron JL, & Bethea CL (2007). Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology, 86(4), 277–288. doi: 10.1159/000109877 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (2001). Diverse patterns of neuroendocrine activity in maltreated children. Development and Psychopathology, 13(3), 677–693. [DOI] [PubMed] [Google Scholar]
- Collins KJ (1999). Physiological variation and adaptability in human populations. Annals of Human Biology, 26(1), 19–38. [DOI] [PubMed] [Google Scholar]
- Dahl RE, & Gunnar MR (2009). Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Development and Psychopathology, 21(1), 1–6. [DOI] [PubMed] [Google Scholar]
- de Kloet ER (2003). Hormones, brain and stress. Endocrine Regulations, 37(2), 51. [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, & Shirtcliff EA (2011). The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews, 35(7), 1562–1592. doi: 10.1016/j.neubiorev.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, & Romeo RD (2013). Stress and the developing adolescent brain. Neuroscience, 249, 162–171. doi: 10.1016/j.neuroscience.2012.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, & Hellhammer D (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30(10), 1010–1016. doi: 10.1016/j.psyneuen.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, & Blakemore SJ (2015). Adolescence as a sensitive period of brain development. Trends in Cognitive Sciences, 19(10), 558–566. doi: 10.1016/j.tics.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Galván A, & McGlennen KM (2013). Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. Journal of Cognitive Neuroscience, 25(2), 284–296. doi: 10.1162/jocn_a_00326 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, & Vazquez D (2001). Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development & Psychopathology, 13(3), 515–538. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, & Griggs C (2009). Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Development and Psychopathology, 21(1), 69–85. doi: 10.1017/S0954579409000054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, & Nemeroff CB (2002). Neurobiology of early life stress: Rodent studies. Seminars in Clinical Neuropsychiatry 7(2), 89–95. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, & Gunnar MR (2015). Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Science, 18(2), 281–297. doi: 10.1111/desc.12195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LMC, Gispen-de Wied CC, Van der Gaag RJ, Ten Hove F, Willemsen-Swinkels SWM, Harteveld E, & Van Engeland H (2000). Unresponsiveness to psychosocial stress in a subgroup of autistic-like children, multiple complex developmental disorder. Psychoneuroendocrinology, 25(8), 753–764. doi: 10.1016/S0306-4530(00)00020-2 [DOI] [PubMed] [Google Scholar]
- Julian M (2013). Age at adoption from institutional care as a window into the lasting effects of early experiences. Clinical Child and Family Psychology Review, 16(2), 101–145. doi: 10.1007/s10567-013-0130-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster RPP, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. doi: 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- King LS, Colich NL, LeMoult J, Humphreys KL, Ordaz SJ, Price AN, & Gotlib IH (2016). The impact of the severity of early life stress on diurnal cortisol: The role of puberty. Psychoneuroendocrinology, 77, 68–74. doi: 10.1016/j.psyneuen.2016.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, & Hellhammer DH (1994). Salivary cortisol in psychoneuroendocrine research: Recent developments and applications. Psychoneuroendocrinology, 19(4), 313–333. [DOI] [PubMed] [Google Scholar]
- Koss KJ, Mliner SB, Donzella B, & Gunnar MR (2016). Early adversity, hypocortisolism, and behavior problems at school entry: A study of internationally adopted children. Psychoneuroendocrinology, 66, 31–38. doi: 10.1016/j.psyneuen.2015.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroupina MG, Fuglestad AJ, Iverson SL, Himes JH, Mason PW, Gunnar MR, … & Johnson DE (2012). Adoption as an intervention for institutionally reared children: HPA functioning and developmental status. Infant Behavior and Development, 35(4), 829–837. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Schlotz W, Golm D, Moser D, Kennedy M, Knights N, … & Sonuga-Barke E (2017). HPA axis dysregulation in adult adoptees twenty years after severe institutional deprivation in childhood. Psychoneuroendocrinology, 86, 196–202. doi: 10.1016/j.psyneuen.2017.09.021 [DOI] [PubMed] [Google Scholar]
- Landis JR, & Koch GG (1977). The measurement of observer agreement for categorical data. Biometrics, 33(1), 159–174. [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, … & Meaney MJ (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277(5332), 1659–1662. [DOI] [PubMed] [Google Scholar]
- Mangold D, Wand G, Javors M, & Mintz J (2010). Acculturation, childhood trauma and the cortisol awakening response in Mexican–American adults. Hormones and Behavior, 58(4), 637–646. doi: 10.1016/j.yhbeh.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1969). Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood, 44, 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, & Tanner JM (1970). Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood, 45, 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, & Nelson CA (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences of the United States of America, 112(18), 5637–42. doi: 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH & Moos BS (1981). Family Environment Scale manual. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Natsuaki MN, Klimes-Dougan B, Ge X, Shirtcliff EA, Hastings PD, & Zahn-Waxler C (2009). Early pubertal maturation and internalizing problems in adolescence: Sex differences in the role of cortisol reactivity to interpersonal stress. Journal of Clinical Child & Adolescent Psychology, 38(4), 513–524. doi: 10.1080/15374410902976320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, & Herbert J (2004). Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology, 29(2), 125–140. doi: 10.1016/S0306-4530(02)00150-6 [DOI] [PubMed] [Google Scholar]
- Peper JS, & Dahl RE (2013). The teenage brain: Surging hormones—Brain-behavior interactions during puberty. Current Directions in Psychological Science, 22(2), 134–139. doi: 10.1177/0963721412473755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. [DOI] [PubMed] [Google Scholar]
- Pitula CE, DePasquale CE, Mliner SB, & Gunnar MR (2017). Peer problems among postinstitutionalized, internationally adopted children: Relations to hypocortisolism, parenting quality, and ADHD symptoms. Child Development. doi: 10.1111/cdev.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson A, Loman M, Lafavor T, & Gunnar M (2012). The confluence of adverse early experience and puberty on the cortisol awakening response. International Journal of Behavioral Development, 36(1), 19–28. doi: 10.1177/0165025411406860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Miller B, Dorn L, Desjardins C, Donzella B, & Gunnar MR (2017). Early-growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatric Research, 82, 278–284. doi: 10.1038/pr.2017.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD (2013). The teenage brain: The stress response and the adolescent brain. Current Directions in Psychological Science, 22(2), 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM (2006). The impact of early adverse care on HPA axis development: Nonhuman primate models. Hormones and Behavior, 50(4), 623–631. [DOI] [PubMed] [Google Scholar]
- Sisk CL, & Zehr JL (2005). Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology, 26(3–4), 163–174. doi: 10.1016/j.yfrne.2005.10.003 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, & Casey BJ (2010). A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–133. doi: 10.1016/j.bandc.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, & Niaura R (2009). Stress response and the adolescent transition: Performance versus peer rejection stressors. Development and Psychopathology, 21(1), 47–68. doi: 10.1007/s10964-008-9292-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strüber N, Strüber D, & Roth G (2014). Impact of early adversity on glucocorticoid regulation and later mental disorders. Neuroscience & Biobehavioral Reviews, 38, 17–37. doi: 10.1016/j.neubiorev.2013.10.015 [DOI] [PubMed] [Google Scholar]
- Sumter SR, Bokhorst CL, Miers AC, Van Pelt J, & Westenberg PM (2010). Age and puberty differences in stress responses during a public speaking task: Do adolescents grow more sensitive to social evaluation? Psychoneuroendocrinology, 35(10), 1510–1516. doi: 10.1016/j.psyneuen.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, & Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience, 10(6), 397–409. doi: 10.1038/nrn2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vegt EJ, van der Ende J, Kirschbaum C, Verhulst FC, & Tiemeier H (2009). Early neglect and abuse predict diurnal cortisol patterns in adults: A study of international adoptees. Psychoneuroendocrinology, 34(5), 660–669. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ, & Juffer F (2007). Plasticity of growth in height, weight, and head circumference: Meta-analytic evidence of massive catch-up after international adoption. Journal of Developmental & Behavioral Pediatrics, 28(4), 334–343. doi: 10.1097/DBP.0b013e31811320aa [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, & Hayakawa CM (2010). Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrinology, 35(2), 241–248. doi: 10.1016/j.psyneuen.2009.06.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


