Abstract
Background:
Electronic cigarettes (ECs) are nicotine delivery devices that produce aerosol without combustion of tobacco; therefore, they do not produce sidestream smoke. Nevertheless, many users exhale large clouds of aerosol that can result in passive exposure of non-users. Analogous to thirdhand cigarette smoke, the exhaled aerosol also settles on indoor surfaces where it can produce a residue. We refer to this residue as EC exhaled aerosol residue (ECEAR). Our objective was to determine if exhaled EC aerosol transferred from a vape shop in a multiple-tenant retail building, where it was produced, to a nearby business (field site) where it could deposit as ECEAR.
Methods:
We examined the buildup of ECEAR in commonly used materials (cotton towel and paper towels) placed inside the field site across from the vape shop. Materials were subjected to short-term (days) and long-term (months) exposures. Nicotine, other alkaloids, and tobacco-specific nitrosamines (TSNAs) were identified and quantified in unexposed controls and field site samples using analytical chemical techniques.
Results:
Nicotine and other alkaloids were detected after 1 day of exposure in the field site, and these chemicals generally increased as exposure times increased. TSNAs, which have been linked to carcinogenesis, were also detected in short and long-term exposed samples from the field site.
Conclusions:
In a multiple-tenant retail building, chemicals in EC aerosol traveled from a vape shop into an adjacent business where they deposited forming ECEAR. Regulatory agencies and tenants occupying such buildings should be aware of this potential environmental hazard.
INTRODUCTION
Electronic cigarettes (ECs) deliver nicotine in an aerosol that is produced by heating a fluid containing a solvent, e.g., 1,2-propanediol (propylene glycol, PG) and/or glycerol (vegetable glycerin, VG), nicotine, and flavor chemicals [1–4]. EC aerosols also contain volatile organic compounds (VOCs), including carbonyls, and metals [5–10]. EC users may exhale large quantities of aerosol that contains nicotine [11–14] and the exhaled aerosol forms a residue on indoor surfaces [15,16], in much the same way that thirdhand smoke (THS) forms indoors after secondhand smoke has settled [17,18]. We refer to EC exhaled aerosol residue as ECEAR. Both the exhaled aerosol and ECEAR can result in passive exposure of non-users, and these exposures are a growing environmental and health concern.
Several toxicants including formaldehyde, acetaldehyde and acrolein are created by the oxidation and/or dehydration of VG or PG, and have been reported in EC aerosol [5,19,20]. Significant amounts of 1,2-propanediol, glycerin, nicotine and PM2.5 particles were present indoors during 2 hours of vaping [21]. Moreover, indoor air quality study showed that a large room with active EC users contained PM2.5 at concentrations that were higher than in hookah cafes and bars that allow cigarette smoking [22]. Studies done inside the homes of EC users showed airborne nicotine levels of 0.13µg/m3, in contrast to non-smokers’ homes which had 0.02 µg/m3 [23]. In the same study, salivary cotinine concentrations were significantly higher in non-smokers living in a home where ECs were used than in non-smokers living in non-smokers’ homes. B cause EC users as well as bystanders who do not use ECs can be exposed to the chemicals and particles in suspended EC aerosols, the International Union Against Tuberculosis and Lung Disease has recommended that EC should not be used in public places, workplaces, or on public transportation [24]. A surface sampling study of indoor environments where vaping occurred showed that ECEAR contained nicotine, although concentrations were not as high as in the homes of smokers [15]. Passive exposure to nicotine and other chemicals in ECEAR could occur through dermal absorption, ingestion, or the inhalation of re-emitted chemicals.
In vape shops, multiple EC users exhale aerosols that could potentially move through the heating, ventilating, and air conditioning system (HVAC) to adjacent businesses. The purpose of this study was to determine if tobacco specific chemicals in the aerosol from an active vape shop transferred into a nearby business in a multiple-tenant retail building (mall) by examining deposition of nicotine and nicotine derivatives in the nearby business.
METHODS
Collection of Fabrics
Cotton towels (Easydry, easydry.com), paper towels (Bounty®, Stater Bros. Riverside, CA, USA), terrycloth towels (Jo-Anne Fabric and Craft Stores, Riverside, CA, USA), and two air filters [3M high performance 20×25×1 Filtrete™ air filter (amazon.com) and Rabbit Air Classic BioGS Replacement HEPA filter (amazon.com)] were placed in the field site for short or long-term exposures. The area of 1 gram of each fabric is: terrycloth towel 5.5 cm × 4.75 cm (26.125 cm2), air filter 10.5 cm × 9.5 cm (99.75 cm2), paper towel 15.5 cm × 13 mm (201.5 cm2), and cotton towel 13.5 cm × 12.5 cm (168.75 cm2). Short-term exposure samples were collected after 1 (24 hrs.), 4 (96 hrs.) and 8 days (192 hrs.), while long-term exposure samples were collected after 1, 2 and 3 months. Mall control fabrics (terrycloth towel) were exposed in a hallway on a separate HVAC system outside the field site for 1 day (24 hrs.), 3 days (72 hrs.), and 1 week. Additional control samples of fabrics (terrycloth towels) were collected from a non-smoker home in the same community after exposure for 1 day (24 hrs.), 4 (96 hrs.) days, and 1 week. Control samples inside the non-smoker home were placed inside the front room of the house and in the garage. Unexposed samples of each type of fabric (cotton towels, paper towels, terrycloth towels, and both air filters) were also used as controls. After exposure, samples were placed in Ziploc® bags and/or envelops and either extracted immediately or stored at −80°C in heat sealed, cut to size, Mylar bags (ULINE, Pleasant Prairie, Wisconsin, USA).
Extraction of Nicotine, Other Alkaloids, and Tobacco-Specific Nitrosamines (TSNAs) from ECEAR Fabrics
ECEAR was extracted from control (unexposed) and exposed samples of cotton towel, paper towel, terrycloth towel, and air filters. Fabrics were cut into small pieces and immersed into cell culture medium consisting of Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO), 5% horse serum (Invitrogen, Carlsbad, CA), 1% sodium pyruvate (Lonza, Walkersville, D) and 1% penicillin–streptomycin (GIBCO, Invitrogen, Carlsbad, California) [25,26] at a concentration of 0.05 g of fabric /ml of medium. This mix was agitated in 50 ml Falcon tubes on rocker for 1 h at room temperature [27]. The contents of the tubes were extruded through a 30 ml plastic syringe (Sigma-Aldrich, St. Louis, MO) to obtain only the medium. The extracts were then filtered using 0.22 µm sterile filters (Pall Corporation, Port Washington, NY) and aliquoted into 1.5 ml vials for storage at −80 °C and later shipment on dry ice to the Clinical Pharmacology Laboratory at the University of California at San Francisco.
Chemical Analysis of ECEAR Fabrics for Nicotine, Other Alkaloids, and TSNAs
The quantification of nicotine, nicotine derivatives, and TSNAs was done by liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) at the University of California San Francisco. Sample prep has been described previously [27–29]. The LC-MS/MS analysis, including the method for determining the LOQ, is described in Whitehead et al. [27–29]. The limits of quantification for each chemical was as follows: nicotine = 2 ng/ml, cotinine = 1ng/ml, n-formylnornicotine = 1, bipyridine = 1 ng/ml, nicotelline = 0.2 ng/ml, myosmine = 1 ng/ml, NAB (N-nitrosoanatabine) = 1 ng/ml, NNK (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone) = 0.1 ng/ml, NNN (N’-nitrosonornicotine) = 0.1 ng/ml, NAT (N-nitrosoanatabine) = 0.2 ng/ml, NNA (1-(N-methyl-N-nitrosamino)-1-(3-pyridinyl)-4-butanal) = 1 ng/ml.
RESULTS
Field Site
The field site is located on the basement floor of a two-story mall in a metropolitan area. The commercial building is over 50 years old. The field site for this study included the three basement suites shown in Figure 1. Suite #1 was actively operated shop, and the site where the cotton fabrics, pap r towels, and filter samples were placed for collection of ECEAR. The Filtrete™ air filter was placed in the return vent towards the back of Suite #1 while the Rabbit air filter was placed in the middle of the suite. Suite #3 is an active vape shop that blends and manufactures refill fluids which they sell in the store and online. The vape shop allows vaping inside the shop and has a bar and lounge where customers can try out ECs or refill fluids. Suite #2 is a shop that was seldom used during the course of our study. Approximate dimensions of Suite #1, Suite #2, and Suite #3 were 398 ft2 (37 m2), 405 ft2 (37 m2), 311 ft2 (28 m2), respectively.
Figure 1: Map of the field site in the mall and airflow between Suites.
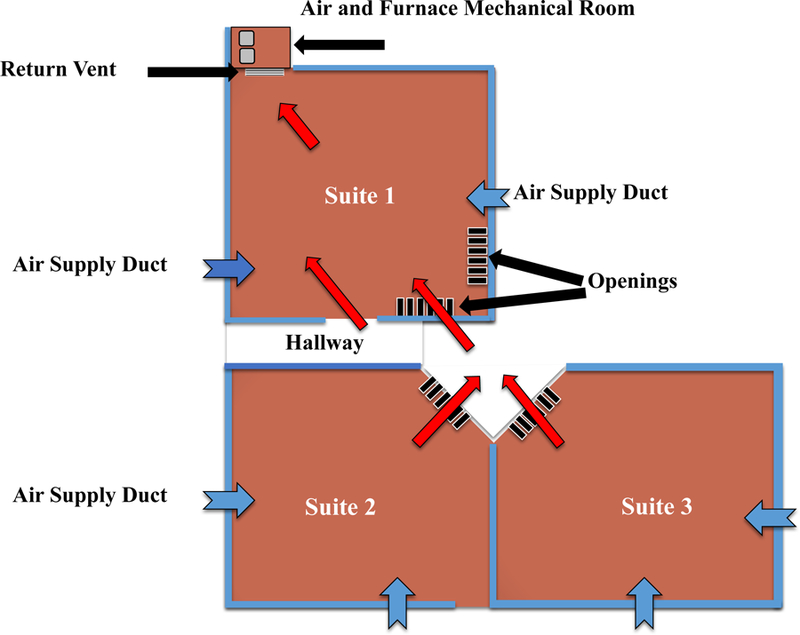
A return vent, located at the back of Suite #1, intakes air (red arrows) from the surrounding spaces creating negative pressure inside Suite #1. Air can enter Suite #1 through the door and also through openings in the upper portion of the front and side walls. Air is redistributed to the three Suites through vents (blue arrows) from the air and furnace mechanical room located in the back of Suite #1.
The air intake to the building is from an adjacent alley. The HVAC system for the suites is a gas forced air furnace located in a mechanical room in back of Suite #1 (Figure 1). All suites have supply ducts in the floor, but only suite #1 has a return vent, which draws air f om all three suites into the mechanical room. Each suite also contains storefront screen partitions that allow the air from suites #2 and 3 to enter the return vent in suite #1. Air pulled through Suite #1 was recirculated to the three Suites through the air supply ducts in the floor. There are no dedicated exhaust systems for any of the suites.
The furnace in the mechanical room was produced by International Comfort Products Corporation (Louisburg, TN.) and the manufacturer’s model number was GNE150J20A. Air flow through the field site was approximately 1401 to 1709 cubic feet per minute. The air filter in the return vent was a 3M Filtrete ™ healthy living air filter (20 inches (L) × 25 inches (W) × 1.0 inches (D)), which was made of polypropylene. The basement also had a locked thermostat in the hall that controlled the furnace in the mechanical room.
Table 1 summarizes the types of samples collected from the field site (Suite #1) and their exposure dates. All short-term and some long-term samples were exposed at the front top openings of Suite #1 in the field site, while some long-term samples were exposed at the back near the r turn vent (Figure 1). The 3M Filtrete™ is labeled as Filter A while the Rabbit air filter is lab l as Filter B.
Table 1:
Samples and exposure dates
| Cotton Towel | Paper Towel | ||||
|---|---|---|---|---|---|
| Sample | Exposure Date | Sample | Exposure Date | ||
| Short Term | SF 1D | March 5, 2014 | Short Term | SF 1D | March 5, 2014 |
| SF 1D | March 15, 2015 | SF 4D | March 1, 2014 – March 5, 2014 | ||
| SF 4D | March 1, 2014 – March 5, 2014 | SF 4D | March 15, 2014 – March 19, 2014 | ||
| SF 10D | January 27, 2015 – February 6, 2015 | SF 8D | March 7, 2014 – March 15, 2014 | ||
| Long Term | SF 28D | January 27, 2015 – February 24, 2015 | Long Term | SF 18D | February 6, 2015 – February 24, 2015 |
| SF 35D | January 31, 2015 – March 7, 2015 | SF 28D | January 27, 2015 – February 24, 2015 | ||
| SF 31D | March 7, 2015 – April 7, 2015 | SF 30D | March 15, 2014 – April 15, 2014 | ||
| SF 31D | March 7, 2015 – April 7, 2015 | RV 59D | February 2014 – April 2014 | ||
| SF 74D | October 1, 2014 – December 13, 2014 | SF 61D | May 2014 – July 2014 | ||
| Filter A 181D | February 2014 – August 2014 | RV 61D | May 2014 – July 2014 | ||
| Filter B 365D | April 2014 – April 2015 | RV 74D | October 1, 2014 – December 13, 2014 | ||
| SF 75D | August 1, 2014 – October 15, 2014 |
Nicotine, Other Alkaloids, and TSNAs Detected in Suite #1 ECEAR Samples
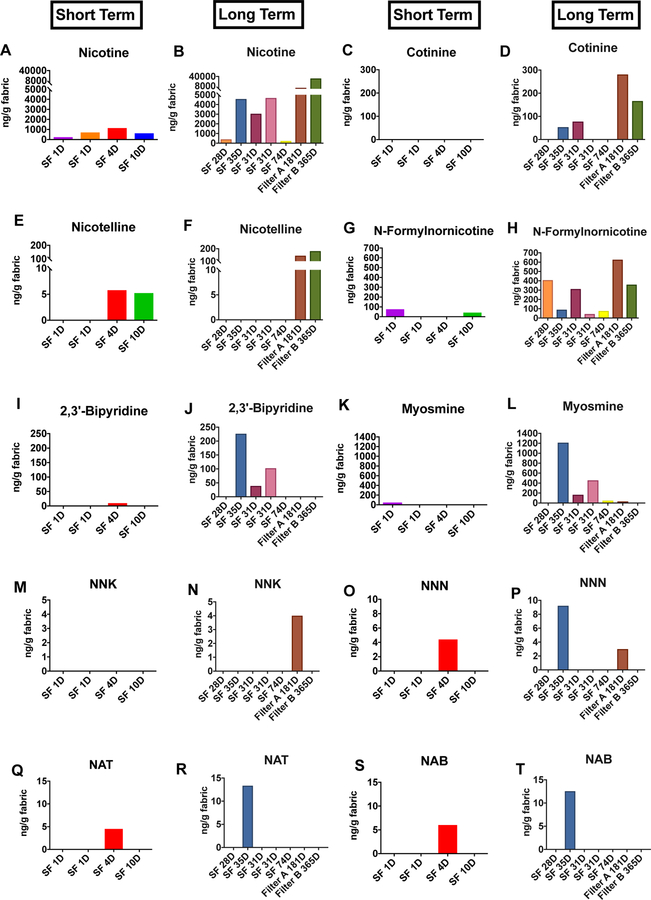
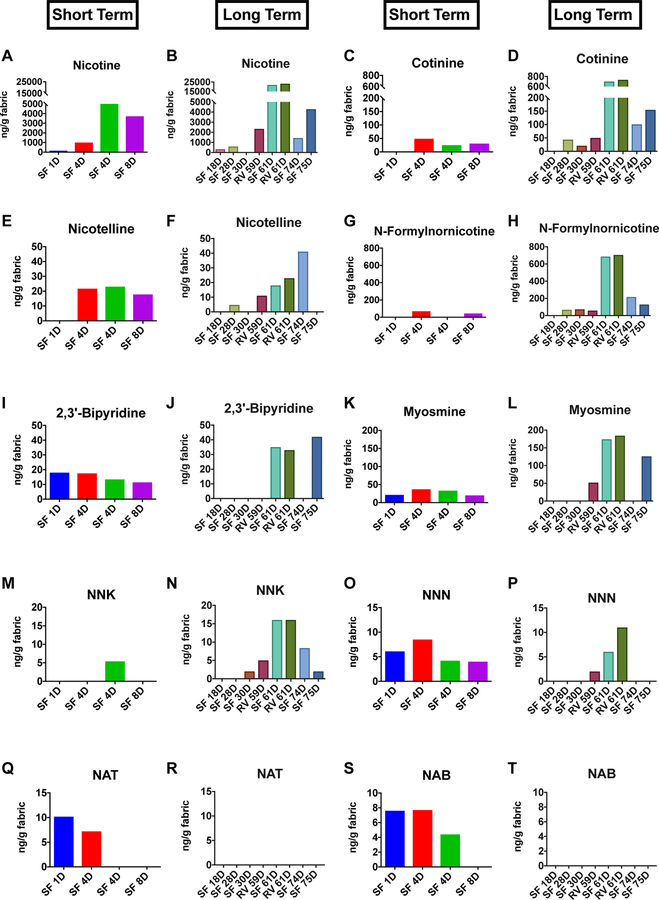
Nicotine, other alkaloids, and TSNAs were detected in ECEAR extracts of cotton towels (Figure 2) and paper towels (Figure 3) from Suite #1. Nicotine was the most abundant marker of EC aerosol contamination in Suite #1 (Figures 2A-B and 3A-B) (highest concentration = 23,260 ng/g of fabric). Nicotine was present in extracts of both short and long-term samples of cotton towel, paper towel, and terrycloth towel, and its concentration generally increased with exposure time (Figure 2A, B and 3A, B). Even samples exposed for only 1 day had detectable amounts of nicotine, e.g., paper towel exposed 1 day had 154 ng of nicotine/g of paper towel. Both air filter A and air filter B had high concentrations of nicotine (Figure 2B). Control sample of paper towels and terrycloth towels exposed both in the home of a non-smoker and in the mall had no detectable nicotine except for a low level(107ng/gand93ng/g)in two samples (Supplementary Table 1).
Figure 2: Concentrations of nicotine, nicotine alkaloids, and TSNAs in cotton towels and air filters from the field site.
Short term vs. long term samples are shown for each sample collected from the field site. Nicotine (A-B), cotinine (C-D), nicotelline (E-F), n-formylnornicotine (G-H), 2,3’-bipyridine (I-J), myosmine (K-L), NNK (M-N), NNN (O-P), NAT (Q-R), NAB (S-T). SF = Store Front. RV = Return Vent. Air Filter A =3M Filtrete™ air filter. Air Filter B = Rabbit Air HEPA filter.
Figure 3: Concentrations of nicotine, nicotine alkaloids and TSNAs in paper towels from the field site.
Short term vs. long term samples are shown side by side for each sample collected from the field site. Nicotine (A-B), cotinine (C-D), nicotelline (E-F), n-formylnornicotine (G-H), 2,3’-bipyridine (I-J), myosmine (K-L), NNK (M-N), NNN (O-P), NAT (Q-R), NAB (S-T). SF = Store Front.
Several tobacco alkaloids (cotinine, nicotelline, N-formylnornicotine, 2,3’-bipyridine, and myosmine) were found in ECEAR extracts from Suite #1, and their concentrations generally increased as exposure time increased (Figres 2C-L, 3C–L). The alkaloids were found more frequently in paper towel extracts than in cotton towel, and their concentrations were generally higher in paper towel samples. The air filters appeared to trap nicotine and the alkaloids, except for 2,3’ bipyridine and myosmine, which were not detected in the filter samples.
TSNAs were found in many of the paper towel extracts and in some of the terrycloth towel samples (Figures 2M-T and 3M–T). When TSNAs were found in the cotton towel extracts, concentrations w re g n rally higher after long-term exposure (Figures 2 M-T). In paper towel samples, NNK and NNN tended to increase with long-term exposure, while NAT and NAB were only present in the short-term paper towel samples (Figures 3 Q–T). Both NNK and NNN were detected in air filter A, but not in air filter B (Figure 2N). NNA was analyzed but not found in any of the samples.
The frequency with which chemicals appeared in paper and cotton samples is given in Supplementary Table 2. Almost all samples contained nicotine. Cotinine, nicotelline, NNK, and NNN were detected more frequently in paper than in cotton. N-Formylnornicotine, 2,3’-bipyridine, myosmine, NAT and NAB appeared with about equal frequency in cotton and paper. Both air filters contained nicotine, cotinine, nicotelline, and n-formylnornicotine. However, they did not contain 2,3 bipyridine, NAT, and NAB. Air filter A did contain myosmine, NNK, and NNN, while air filter B did not.
DISCUSSION
Our results demonstrate that EC aerosols generated in vape shop can travel into a nearby business where they deposit on surfaces forming ECEAR. In our field site, this likely occurred because air circulated from Suites #2 and #3 (vape shop) to Suite #1 where samples were collected. The ECEAR in Suite #1 contained nicotine, other alkaloids, and nitrosamines, consistent with it originating in the vape shop. According to the American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc. (ASHRAE), environmental tobacco smoke (ETS) includes emissions produced by electronic smoking devices [30] and air should not recirculate or transfer from an ETS area to an ETS free area (5.17.5). The transfer of exhaled EC aerosol to Suite #1 may have been avoided or reduced if each suite had its own air distribution system. Since many types of HVAC systems are currently in buildings worldwide, the possibility of transfer of EC chemicals to businesses near vape shops should be addressed in building codes and regulated.
Our conclusion regarding the transfer of exhaled EC aerosol to a nearby business is based mainly on finding chemicals known to be present in EC aerosol in Suite #1, but not in the mall away from Suite #1 or in the control home. Nicotine and other alkaloids have been found in EC liquids and aerosols [31] and in exhaled EC aerosol [11]. The chemical markers did t c me from cigarette smoke as smoking had been banned in the mall since 2009 (Public Act 188, www.michigan.gov). TSNAs have been reported in both refill fluids [32] and EC aerosols [5], and those found in the field site may have originated in the aerosol and/or formed after deposition in Suite #1 through interconversion of nicotine, as described previously for THS [33]. Laboratory studies have shown that 93–99% of the inhaled nicotine in EC aerosol is retained in the lungs by EC users [14,34], but the extent of nicotine exhalation depends on the user’s propensity to produce clouds of aerosol. In our real world study, nicotine generated by vape shop occupants reached Suite #1 and contributed to ECEAR.
The 3M Filtrete ™ air filter d Rabbit Air HEPA filter, which were in the field site for 6 months and 1 year, respectively, picked up nicotine, cotinine, nicotelline, and n-formylnornicotine, and the 3M Filtrete ™ also trapped NNK and NNN. Concentrations of the Chemical markers in these filters were likely higher than in the paper towel and terrycloth towel samples due to their longer exposure period. These data suggest that filtering air helps reduce exposure to nicotine and it alkaloids, but several chemicals (2,3’ bipyridine and myosmine) were not trap in either filter. Our study did not determine the efficiency with which these filters removed the chemical markers; however, ECEAR clearly collected on our cotton and paper samples before air circulated through the filters.
ECEAR chemicals in Suite #1 could be internalized passively through dermal contact and/or inhalation. Ingestion could also occur if a toddler mouthed fabrics or surfaces in Suite #1. Nicotine, the most abundant chemical we detected in ECEAR, is water and lipid soluble, and it readily permeated an in vitro skin model exposed to refill fluids containing nicotine, even when the period of dermal contact with refill fluids was short (10 minutes) [35,36]. Research into dermal uptake of nicotine from contact with ECEAR in realistic scenarios, such as the ne n this study, is needed. Volatile organic compounds (VOCs), which are present in EC aerosol [8,37] and were found in ECEAR [38], could be passively inhaled. Acrolein and formaldehyde have been reported in EC aerosols [39] and likely contributed to ECEAR, although we did not analyze them in this study. Inhalation of these and related VOCs would be a concern given their known toxicity [40–41].
While EC refill fluids can produce cytotoxic effects in vitro [42–44] and adverse health effects have been reported in EC users [45–47], the effects of exposure to ECEAR chemicals on human health are not yet known. The concentrations of these chemicals are likely much higher in the vape shop, but those chemicals reaching Suite #1 could build up over time. After 35 days in the field site, a cotton towel collected 4.571 g of nicotine. If toddler sucked on 0.3 m2 or about 1 foot2 of cotton fabric from Suite #1, they would be exposed to 81.26 µg of nicotine. Surface wipes measuring indirect exposure to cigarette smoke inside households where smokers only used cigarettes outside the home contained about 3.2 µg of nicotine/ per 0.3 m2 at the average of the mean nicotine level per household [48], indicating greater transfer of nicotine from the vape shop to Suite #1 than to the household with indirect cigarette smoke exposure.
Others have shown that secondhand tobacco smoke also settles on indoor surfaces and forms THS [18,48]. While the health effects of THS are not fully known, it does have toxicity both in vitro and in mice [25,28,49,50]. Further monitoring of ECEAR and its health effects would be important in the future.
The data collected in this study pertains to a specific commercial vape shop and may not be generalizable to multiple-tenant residences or to vape shops in malls with alternative HVAC systems. ECEAR was not measured on all surface in the field site, and it extraction may vary with different surfaces.
Store owners and tenants of malls should be aware that EC aerosols from nearby vape shops can enter their units and deposit as ECEAR. Chemicals in ECEAR include nicotine, minor alkaloids, and TSNAs, the latter of which are potential carcinogens. Passive and unwanted exposure to these chemicals could occur through dermal contact, ingestion, or inhalation. The identification of chemicals, their concentrations, and their secondary products in ECEAR will be a necessary first step towards understanding the health and environmental effects of ECEAR. Building codes will need to be developed and enforced to protect those who do not wish to be exposed to ECEAR. Vape shop air quality is not currently regulated nor has it been thoroughly studied. Regulatory agencies should exercise authority over malls to ensure that employees and tenants do not receive unwanted exposure to EC aerosol and its residue.
Supplementary Material
WHAT THIS PAPER ADDS?
There are currently thousands of vape shops in the United States, some in buildings with other tenants and businesses working close by.
ECEAR containing nicotine, other alkaloids, and TSNAs was detected and quantified in a business adjacent to a vape shop.
ECEAR was detected after only 1 day and generally increased over time.
ECEAR is a potential environmental hazard that should be evaluated for regulation.
Acknowledgments:
We thank Jessica Miranda Bustamante for her assistance in sample extraction and shipment and Christopher Havel and Olivia Yturralde for their help in analyzing the samples.
Funding: This research was supported by Grant #26IR-0018 from the Tobacco-Related Disease Research Program of California (TRDRP) to PT and g ant P30 DA012393 from the National Institute on Drug Abuse, and grant # S10 RR026437 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of TRDRP, NIH, the FDA, or other granting agencies.
Footnotes
Competing financial interests: All authors declare that they have no actual or potential competing financial interest.
Patient consent: The project did not involve humans.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All of the relevant data are included in the manuscript.
REFERENCES
- 1.Trtchounian A, Williams M, Talbot P. Conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res 2010;12:905–12. [DOI] [PubMed] [Google Scholar]
- 2.Trtchounian A, Talbot P. Electronic nicotine delivery systems: is there a need for regulation? Tob Control 2010;20:47–52. [DOI] [PubMed] [Google Scholar]
- 3.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014;129:1972–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Services UDOHAH, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, et al. E-Cigarette use among youth and young adults. A report of the Surgeon General 2016;:1–298
- 5.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutzler C, Paschke M, Kruschinski S, et al. Chemical hazards present in liquids and vapors of electronic cigarettes. Arch Toxicol 2014;88:1295–308. [DOI] [PubMed] [Google Scholar]
- 7.Kosmider L, Sobczak A, Fik M, et al. Carbonyl compounds in electronic cigarette vapors: effects of nicotine solvent and battery output voltage. Nicotine Tob Res 2014;16:1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sleiman M, Logue JM, Montesinos VN, et al. Emissions from Electronic Cigarettes: Key Parameters Affecting the Release of Harmful Chemicals. Environ Sci Technol 2016;50:9644–51. [DOI] [PubMed] [Google Scholar]
- 9.Williams M, Villarreal A, Bozhilov K, et al. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS ONE 2013;8:e57987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams M, Bozhilov K, Ghai S, et al. Elements including metals in the atomizer and aerosol of disposable electronic cigarettes and electronic hookahs. PLoS ONE 2017;12:e0175430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czogala J, Goniewicz ML, Fidelus B, et al. Secondhand exposure to vapors from electronic cigarettes. Nicotine Tob Res 2014;16:655–62. 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell G, Colard S, Cahours X, et al. An Assessment of Indoor Air Quality before, during and after Unrestricted Use of E-Cigarettes in a Small Room. Int J Environ Res Publ Health 2015;12:4889–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schripp T, Markewitz D, Uhde E, et al. Does e-cigarette consumption cause passive vaping? Indoor Air 2013;23:25–31. [DOI] [PubMed] [Google Scholar]
- 14.Helen G, Havel C, Dempsey DA, et al. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction 2016;111:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bush D, Goniewicz ML. A pilot study on nicotine residues in houses of electronic cigarette users, tobacco smokers, and non-users of nicotine-containing products. Int J Drug Policy 2015;26:609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Liang Q, Oldham MJ, et al. Determination of Selected Chemical Levels in Room Air and on Surfaces after the Use of Cartridge- and Tank-Based E-Vapor Products or Conventional Cigarettes. Int J Environ Res Publ Health 2017;14:969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matt GE, Quintana PJE, Destaillats H, et al. Thirdhand Tobacco Smoke: Emerging Evidence and Arguments for a Multidisciplinary Research Agenda. Environ Health Perspect 2011;119:1218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob P III, Benowitz NL, Destaillats H, et al. Thirdhand Smoke: New Evidence, Challenges, and Future Directions. Chem Res Toxicol 2017;30:270–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flora JW, Meruva N, Huang CB, et al. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul Toxicol Pharmacol 2016;74:1–11. [DOI] [PubMed] [Google Scholar]
- 20.Jensen RP, Luo W. Hidden Formaldehyde in E-Cigarette Aerosols. N Engl J M d 2015;372:389–92. [DOI] [PubMed] [Google Scholar]
- 21.Schober W, Szendrei K, Matzen W, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. Int J Hyg Environ Health 2014;217:628–37. [DOI] [PubMed] [Google Scholar]
- 22.Soule EK, Maloney SF, Spindle TR, et al. Electronic cigarette use and indoor air quality in a natural setting. Tob Control 2017;26:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballbè M, Martínez-Sánchez JM, Sureda X, et al. Cigarettes vs. e-cigarettes: Passive exposure at home measured by means of airborne marker and biomarkers. Environ Res 2014;135:76–80. [DOI] [PubMed] [Google Scholar]
- 24.Bam TS, Bellew W, Berezhnova I, et al. Position statement on electronic cigarettes or electronic nicotine delivery systems. Int. J. Tuberc. Lung Dis 2014;18:5–7. [DOI] [PubMed] [Google Scholar]
- 25.Bahl V, Shim HJ, Jacob P, et al. Thirdhand smoke: Chemical dynamics, cytotoxicity, and genotoxicity in outdoor and indoor environments. Toxicol In Vitro 2016;32:220–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarker AH, Chatterjee A, Williams M, et al. NEIL2 protects against oxidative DNA damage induced by sidestream smoke in human cells. PLoS ONE 2014;9:e90261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahl V, Jacob P, Havel C, et al. Thirdhand Cigarette Smoke: Factors Affecting Exposure and Remediation. PLoS ONE 2014;9:e108258–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hang B, Sarker AH, Havel C, et al. Thirdhand smoke causes DNA damage in human cells. Mutagenesis 2013;28:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitehead TP, Havel C, Metayer C, et al. Tobacco alkaloids and tobacco-specific nitrosamines in dust from homes of smokeless tobacco users, active smokers, and nontobacco users. Chem Res Toxicol 2015;28:1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Society of Heating RAA-CE. Ventilation for acceptable indoor air quality 2016. www.ashrae.org
- 31.Lisko JG, Tran H, Stanfill SB, et al. Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions. Nicotine Tob Res 2015;17:1270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H-J, Shin H-S. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J Chromatogr A 2013;1291:48–55. [DOI] [PubMed] [Google Scholar]
- 33.Sleiman M, Gundel LA, Pankow JF, et al. Formation of carcinogens indoors by surface-mediated reactions of nicotine with nitrous acid, leading to potential thirdhand smoke hazards. Proc Natl Acad Sci USA 2010;107:6576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connell G, Colard S, Breiev K, et al. An experimental method to determine the concentration of nicotine in exhaled breath and its retention rate following use of an electronic cigarette. J Environ Anal Chem 2015;2:100161. [Google Scholar]
- 35.Maina G, Castagnoli C, Passini V, et al. Transdermal nicotine absorption handling e-cigarette refill liquids. Regul Toxicol Pharmacol 2016;74:31–3. [DOI] [PubMed] [Google Scholar]
- 36.Maina G, Castagnoli C, Ghione G, et al. Skin contamination as pathway for nicotine intoxication in vapers. Toxicology in Vitro 2017;41:102–5. [DOI] [PubMed] [Google Scholar]
- 37.Logue JM, Sleiman M, Montesinos VN, et al. Emi ions from Electronic Cigarettes: Assessing Vapers’ Intake of Toxic Compounds, Secondhand Exposures, and the Associated Health Impacts. Environ Sci Technol 2017;51:9271–9. [DOI] [PubMed] [Google Scholar]
- 38.McAuley TR, Hopke PK, Zhao J, et al. Supplementary: Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol 2012;24:850–7. [DOI] [PubMed] [Google Scholar]
- 39.Gillman IG, Kistler KA, Stewart EW, et al. Effect of variable power levels on the yield of total aerosol mass and formation of aldehydes in e-cigarette aerosols. Regul Toxicol Pharmacol 2016;75:58–65. [DOI] [PubMed] [Google Scholar]
- 40.Acrolein, Agency for Toxic Substances & Disease Registry, 3 Mar. 2011, www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=102.
- 41.Formaldehyde, Agency for Toxic Substances & Disease Registry, 21 Oct. 2014, www.asdr.cdc.gov/mmg/mmg.asp?id=216&tid=39
- 42.Bahl V, Lin S, Xu N, et al. Comparison of electronic cigarette refill fluid cytotoxicity using mbryonic and adult models. Reprod Toxicol 2012;34:529–37. [DOI] [PubMed] [Google Scholar]
- 43.Bahar RZ, Luo W, Lin SC, et al. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob Control 2016;25:ii94–ii102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2017;:tobaccocontrol-2016-053472. [DOI] [PMC free article] [PubMed]
- 45.Hua M, Alfi M, Talbot P. Health-Related Effects Reported by Electronic Cigarette Users in Online Forums. J Med Internet Res 2013;15:e59–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pisinger C Why public health people are more worried than excited over e-cigarettes. BMC Med 2014;12:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hua M, Talbot P. Potential health effects of electronic cigarettes: A systematic review f case reports. Prev Med Rep 2016;4:169–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matt GE, Quintana PJE, Hovell MF, et al. Households contaminated by environmental tobacco smoke: sources of infant exposures. Tob Control 2004;13:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bahl V, Weng NJH, Schick SF, et al. Cytotoxicity of Thirdhand Smoke and Identification of Acrolein as a Volatile Thirdhand Smoke Chemical That Inhibits Cell Proliferation. Toxicol Sci 2016;150:234–46. [DOI] [PubMed] [Google Scholar]
- 50.Martins-Green M, Adhami N, Frankos M, et al. Cigarette smoke toxins deposited on surfaces: implications for human health. PLoS ONE 2014;9:e86391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.