Abstract
OBJECTIVES
The study evaluated whether lipoprotein apheresis produces immediate changes in resting perfusion in subjects with severe hypercholesterolemia, and whether there is a difference in the response between peripheral and coronary microcirculations.
BACKGROUND
Lipoprotein apheresis is used in patients with severe hypercholesterolemia to reduce plasma levels of low-density lipoprotein cholesterol.
METHODS
Quantitative contrast-enhanced ultrasound perfusion imaging of the myocardium at rest and skeletal muscle at rest and during calibrated contractile exercise was performed before and immediately after lipoprotein apheresis in 8 subjects with severe hypercholesterolemia, 7 of whom had a diagnosis of familial hypercholesterolemia. Myocardial perfusion imaging was also performed in 14 normal control subjects. Changes in myocardial work and left ventricular function were assessed by echocardiography. Ex vivo ovine coronary and femoral artery ring tension assays were assessed in the presence of pre- and post-apheresis plasma.
RESULTS
Apheresis acutely decreased low-density lipoprotein cholesterol (234.9 ± 103.2 mg/dl vs. 67.1 ± 49.5 mg/dl; p < 0.01) and oxidized phospholipid on apolipoprotein B-100 (60.2 ± 55.2 nmol/l vs. 47.0 ± 24.5 nmol/l; p = 0.01), and acutely increased resting myocardial perfusion (55.1 [95% confidence interval: 77.2 to 73.1] vs. 135 [95% confidence interval: 81.2 to 189.6] IU/s; p = 0.01), without changes in myocardial work. Myocardial longitudinal strain improved in those subjects with reduced pre-apheresis function. Skeletal muscle perfusion at rest and during contractile exercise was unchanged by apheresis. Acetylcholine-mediated dilation of ex vivo ovine coronary but not femoral arteries was impaired in pre-apheresis plasma and was completely reversed in post-apheresis plasma.
CONCLUSIONS
Lipoprotein apheresis produces an immediate improvement in coronary microvascular function, which increases myocardial perfusion and normalizes endothelial-dependent vasodilation. These changes are not observed in the periphery. (Acute Microvascular Changes With LDL Apheresis; NCT02388633).
Keywords: apheresis, contrast ultrasound, familial hypercholesterolemia, myocardial contrast echocardiography
In patients with severe hypercholesterolemia, including those with familial hypercholesterolemia (FH), statins are the first-line approach for lowering low-density lipoprotein cholesterol (LDL-C) and reducing risk for major adverse acute or chronic ischemic complications (1). Myocardial ischemia with hypercholesterolemia may be mediated not only by obstructive atherosclerotic disease, but also by abnormal microvascular function that occurs from vasoconstrictor/vasodilator imbalance or to abnormal microvascular rheology (2–5).
Lipoprotein apheresis is a therapeutic option for patients with severe hypercholesterolemia in whom adequate LDL-C cannot be achieved with maximally tolerated pharmacotherapy (3,6,7). Although apheresis is intended to reduce risk for atherothrombotic events, the partial removal of LDL-C or other lipoprotein particles could potentially improve microvascular function by reducing oxidative and inflammatory mediators, restoring vasomotor balance, and improving rheology (3,6). There are conflicting results regarding whether apheresis in patients with FH improves endothelial-dependent vasodilation in large peripheral arteries (8,9). Improvement in coronary Doppler-derived flow reserve has been more consistent (10,11), although in some studies flow reserve was increased by apheresis not by augmentation of hyperemic flow, but rather by reduction in resting flow, possibly reflecting changes in myocardial oxygen demand (10).
In this study, we used myocardial contrast echocardiography (MCE) and skeletal muscle contrast-enhanced ultrasound (CEU) to test whether resting microvascular perfusion is increased immediately after lipoprotein apheresis in subjects with severe hypercholesterolemia, and whether the peripheral and coronary microcirculations differ in their response. Further mechanistic insight was provided by evaluation of coronary and peripheral arterial endothelial-dependent vasodilation in the presence of pre- and post-apheresis plasma, and examination of acute changes in oxidatively modified phospholipids (OxPL) and blood rheology.
METHODS
PATIENT POPULATION AND PROTOCOL.
The study was approved by the Institutional Review Board at Oregon Health & Science University (NCT02388633). We recruited 8 patients with a diagnosis of severe hypercholesterolemia, 7 of whom had a diagnosis of FH, who were undergoing first or recurrent lipoprotein apheresis in whom diet, lifestyle modification, and maximum drug therapy was ineffective or not tolerated. Exclusion criteria included pregnant or lactating women, large right-to-left shunt, left ventricular ejection fraction <40%, or more than mild valve regurgitation or stenosis. Subjects were studied immediately before, and again within 2 h after completion of extracorporeal lipoprotein apheresis performed with dextran sulfate adsorption (Liposorber LA-15, Kaneka, Osaka, Japan). Comprehensive transthoracic echocardiography was conducted to evaluate left and right ventricular function. Blood was drawn for determination of plasma lipids, plasma viscosity, and erythrocyte deformability. Quantitative ultrasound perfusion imaging was used to assess resting myocardial blood flow as well as forearm skeletal muscle blood flow at rest and during moderate-intensity contractile exercise. Myocardial perfusion imaging data from 14 age-matched control patients without any coronary artery disease on coronary computed tomography angiography were also included for analysis.
LIPID AND OxPL ANALYSIS.
See the Online Appendix.
ECHOCARDIOGRAPHY.
Echocardiography (iE33, Philips Ultrasound, Andover, Massachusetts) was performed to assess chamber dimensions according to guidelines published by the American Society of Echocardiography (12). EF was calculated using the modified Simpson’s method. Stroke volume was calculated using the product of left ventricular outflow tract area and the time-velocity integral measured by pulsed-wave spectral Doppler. Myocardial work was calculated by the product of stroke volume index, systolic blood pressure, and heart rate. Arterial elastance as a measure of afterload was calculated by: (0.9 × systolic blood pressure)/(stroke volume index). Systemic vascular resistance was calculated by the difference in mean blood pressure and right atrial pressure estimated by inferior vena cava imaging divided by cardiac output (product of stroke volume and heart rate). Global longitudinal strain (GLS) was measured offline (Epsilon Imaging, Ann Arbor, Michigan) from DICOM image sets using speckle-tracking echocardiography of the LV from long-axis views.
CONTRAST-ENHANCED ULTRASOUND.
Perfusion imaging was performed with a multipulse contrast-specific detection algorithm (amplitude modulation imaging) at 1.6 MHz and a mechanical index of 0.12. Lipid-shelled octafluoropropane microbubbles (Definity, Lantheus Medical Imaging, North Billerica, Massachusetts) were diluted 1:15 in normal saline and infused intravenously at a rate of 0.375 ml/min for obtaining blood pool signal from the brachial artery while gating acquisition (1 Hz) to end-diastole. For MCE, the infusion rate was increased to 1 ml/min. The heart was imaged in the apical 4- and 3-chamber views, and frames triggered to end-systole by electrocardiography gating were obtained after a 5-frame high-power (mechanical index >0.9) pulse sequence. For muscle CEU, the infusion rate was increased to 1.5 ml/min. The proximal forearm flexor muscle group was imaged in the transaxial plane and frames were acquired gated to end-diastole after a destructive pulse sequence. Image acquisition was repeated immediately after 1 min of high-intensity exercise performed by handgrip at 0.2 Hz on a calibrated ergometer (Detecto Scales, Inc., Webb City, Missouri) at 75% of their predetermined maximal force. For assessing perfusion, regions of interest were placed over 2 myocardial regions representing 2 separate coronary artery territories for the heart, and over the proximal forearm flexor muscle groups for skeletal muscle. The first post-destructive frame was digitally subtracted from all subsequent frames and background-subtracted time-intensity data were fit to the following function:
where y is signal intensity at time t, A is the plateau intensity reflecting relative microvascular blood volume (MBV), and β is the rate constant reflecting microvascular blood flux rate (13). Microvascular blood flow was quantified by the product of MBV and β (13). For skeletal muscle perfusion imaging, assessment for only the distal microvascular compartment (capillaries and small arterioles and venules) was made by digitally subtracting frames obtained 1 s after the post-destructive pulse sequence, which eliminates large intramuscular vessels (14).
BLOOD RHEOLOGY.
See the Online Appendix.
PERIPHERAL AND CORONARY ARTERIAL ENDOTHELIAL-DERIVED VASODILATION.
An ex vivo arterial ring tension assay was used to assess vascular reactivity to acetylcholine (Ach) in the presence of plasma obtained before or after apheresis. See the Online Appendix for methods.
NO PRODUCTION.
See the Online Appendix.
STATISTICAL METHODS.
Data were analyzed using Prism version 6.0 (GraphPad Software, La Jolla, California). Normal versus non-normal distribution of data was determined by the D’Agostino-Pearson omnibus test. For non-normally distributed data, a Wilcoxon rank sum test was used to compare pre- and post-apheresis data, while a Mann-Whitney test was used to test apheresis patients to control subjects. For normally distributed data, paired Student’s t-tests were used. Linear regression analysis was used to evaluate correlations between lipid and perfusion data. Differences in Ach dose-response were assessed by comparison of individual half maximal inhibitory concentration values. Numerical values are listed as mean ± SD or as median (95% confidence interval), unless stated otherwise; differences were considered significant at p < 0.05 (2-sided).
RESULTS
CLINICAL DATA, LIPID LEVELS, AND BLOOD RHEOLOGY.
The indication for lipoprotein apheresis in this cohort was inability to achieve LDL-C goal with maximally tolerated LDL-C-lowering therapy in all subjects. Three subjects were not taking a statin due to severe intolerance, but were on other lipid-lowering medications. Demographic and clinical data are presented in Table 1. Three of the subjects were studied at the time of their first lipoprotein apheresis treatment while the remaining subjects were studied an average of 27 ± 8 days after their previous recurrent apheresis treatment.
Table 1.
Subject Demographics and Characteristics
| Age, yrs | 56 ± 11 |
| Male/female | 2/6 |
| Body mass index, kg/m2 | 31.8 ± 5.8 |
| First apheresis | 3 (38) |
| Days since last apheresis* | 28 (17–37) |
| Number of preceding apheresis sessions* | 60 (16–129) |
| Diabetes | 1 (13) |
| Hypertension | 5 (63) |
| Smoking | 1 (13) |
| Coronary revascularization | 2 (25) |
| Myocardial infarction | 2 (25) |
| Medications | |
| Statin | 5 (63) |
| Ezetimibe | 3 (38) |
| Bile acid sequestrant | 1 (13) |
| PCSK9 inhibitor | 1 (13) |
| Aspirin | 6 (75) |
| P2Y12 inhibitor | 1 (13) |
| ACE inhibitor or ARB | 3 (38) |
| Beta-blocker | 6 (75) |
| Calcium-channel blocker | 2 (25) |
Values are mean ± SD, n, n (%), or median (95% confidence interval).
Data for non-first-time apheresis patients.
ACE = angiotensin-converting enzyme; ARB = angiotensin receptor blocker; PCSK9 = proprotein convertase subtilisin/kexin type-9.
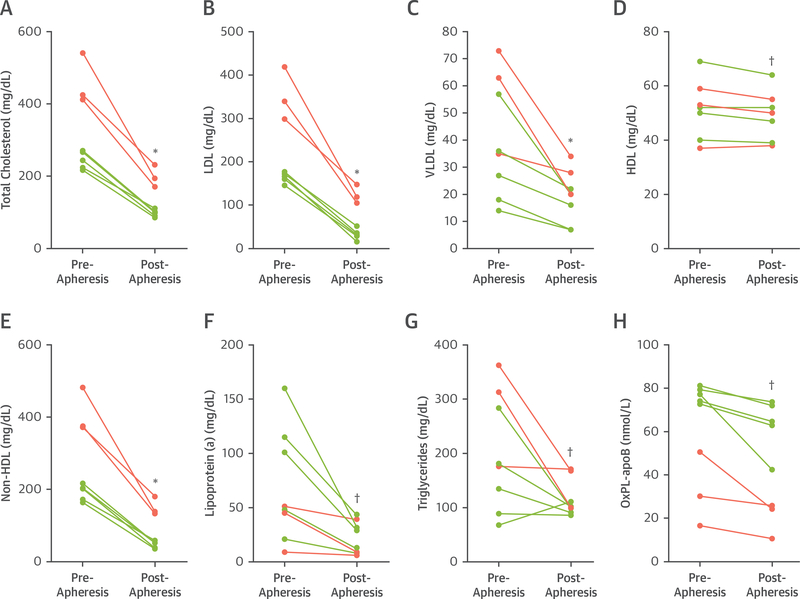
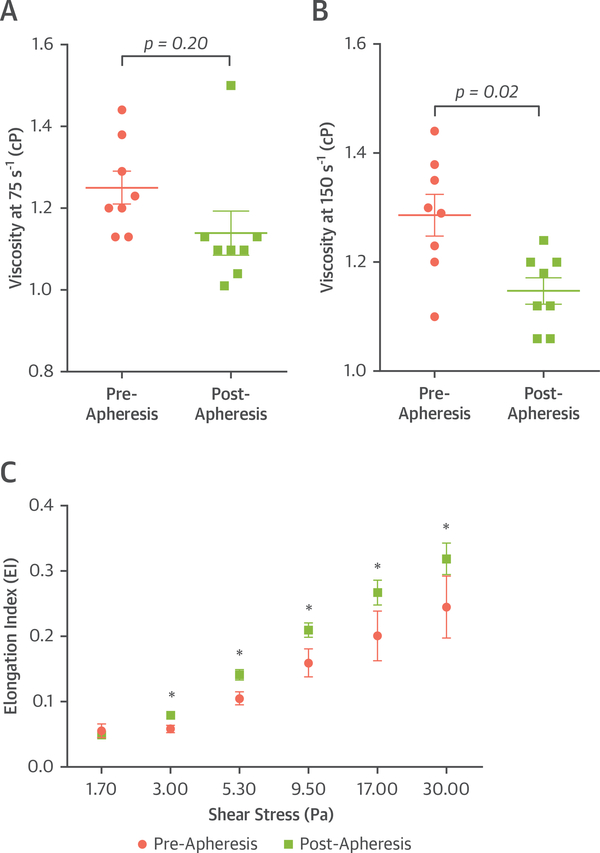
Lipoprotein apheresis resulted in reductions in total cholesterol (325.1 ± 118.8 mg/dl vs. 135.8 ± 55.4 mg/dl; p < 0.01), non–high-density lipoprotein cholesterol (273.5 ± 118.9 mg/dl vs. 86.4 ± 56.2 mg/dl; p < 0.01), LDL-C (234.9 ± 103.2 mg/dl vs. 67.1 ± 49.5 mg/dl; p < 0.01), and Lipoprotein(a) (71.3 ± 55.2 mg/dl vs. 19.9 ± 14.6 mg/dl; p < 0.05) (Figure 1). The concentration of OxPL-apolipoprotein B-100 (apo B-100) lipoproteins was reduced on average by 24 ± 17% (60.2 ± 55.2 nmol/l vs. 47.0 ± 24.5 nmol/l; p = 0.01). Apheresis significantly and uniformly increased erythrocyte deformability and decreased plasma viscosity (Figure 2).
FIGURE 1. Plasma Lipoprotein Lipid Measurements in Subjects Pre- and Post-Apheresis.
Individual values are illustrated for patients on chronic apheresis (green) and those undergoing apheresis for the first time (pink). *p < 0.01 versus pre-apheresis. †p < 0.05 versus pre-apheresis. See text for mean values. HDL = high-density lipoprotein; LDL = low-density lipoprotein; OxPL-apoB = oxidized phospholipid on apolipoprotein B-100; VLDL = very low-density lipoprotein.
FIGURE 2. Blood Characteristics Influencing Microvascular Rheology.
Mean ± SEM values for plasma viscosity are shown for rotational shear of (A) 75 s−1 and (B) 150 s−1. (C) Mean ± SEM values for erythrocyte deformability. *p < 0.05 versus pre-apheresis.
HEMODYNAMIC AND ECHOCARDIOGRAPHIC DATA.
There were no significant differences in heart rate, blood pressure, or double product before and after apheresis (Table 2). Similarly, there were no significant changes in calculated stroke volume, cardiac output, myocardial work, arterial elastance, systemic vascular resistance, or indexes of contractility (LV end-systolic pressure-volume relation) after apheresis. Although LV longitudinal strain improved in all 4 subjects with reduced strain at baseline (defined as GLS >−0.17) (−14.4 ± 2.2 to −18.0 ± 4.1), there was no change in those with normal pre-apheresis strain, resulting in no significant difference for the group as a whole (Table 2).
Table 2.
Hemodynamic and Echocardiographic Data
| Pre-Apheresis | Post-Apheresis | p Value* | |
|---|---|---|---|
| Heart rate, min−1 | 76 ± 15 | 68 ± 9 | 0.10 |
| Systolic BP, mm Hg | 126 ± 16 | 128 ± 11 | 0.69 |
| Diastolic BP, mm Hg | 68 ± 11 | 71 ± 10 | 0.09 |
| Double product, mm Hg/min | 9,566 ± 2,186 | 8,744 ± 1,580 | 0.36 |
| LVESV, ml | 29.8 ± 19.0 | 26.9 ± 12.7 | 0.45 |
| LVEDV, ml | 88.2 ± 31.8 | 88.2 ± 36.5 | 0.99 |
| LVEF, % | 68.1 ± 11.0 | 66.5 ± 12.4 | 0.66 |
| Stroke volume index, ml/m2 | 36.7 ± 8.9 | 34.7 ± 9.9 | 0.37 |
| Myocardial work, (mm Hg · ml)/(m2·min) | 5,832 ± 1,712 | 6,112 ± 1,678 | 0.59 |
| EA, mm Hg/(ml· m2) | 1.67 ± 0.51 | 1.84 ± 0.64 | 0.13 |
| SVR, dynes ·s/cm5 | 1,226 ± 480 | 1,633 ± 710 | 0.07 |
| Global longitudinal strain | −16.9 ± 3.2 | −18.2 ± 2.8 | 0.25 |
Values are mean ± SEM.
Pre- versus post-apheresis.
BP = blood pressure; Ea = arterial elastance; Ees = end-systolic left ventricular elastance; LVEF = left ventricular ejection fraction; LVEDV = left ventricular end-diastolic volume; LVESV = left ventricular end-systolic volume; SVR = systemic vascular resistance.
SKELETAL MUSCLE AND MYOCARDIAL MICROVASCULAR PERFUSION.
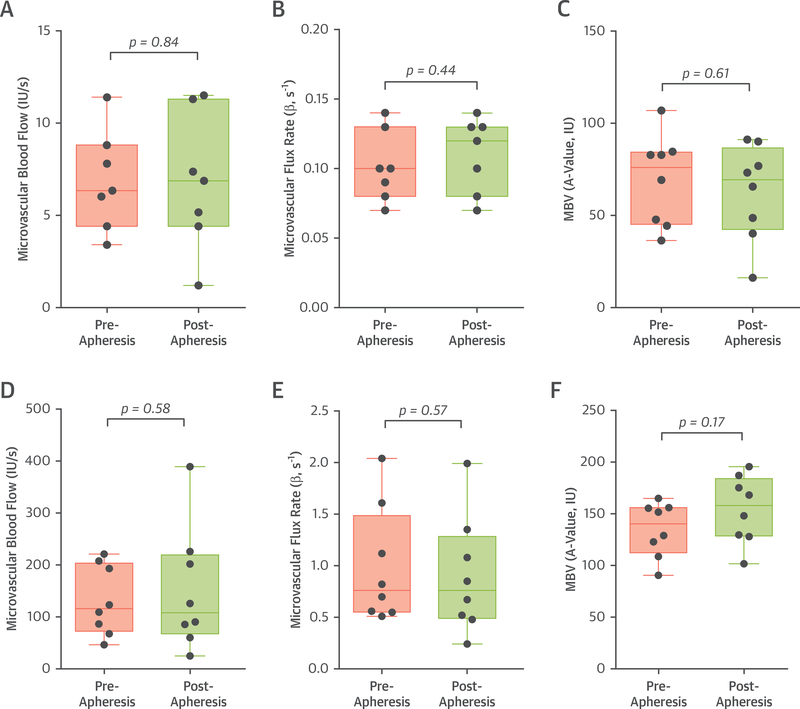
Lipoprotein apheresis did not significantly change skeletal muscle perfusion or any of the parametric components of MBV (A-value) or microvascular flux rate (β–value) when measured either at rest or during exercise (Figure 3). This finding persisted when image analysis was performed to eliminate all but the very distal microcirculation (microvessels with mean blood velocity <4 mm/s) (Online Figure 1). Pre-apheresis resting skeletal muscle CEU perfusion imaging values were found to be similar without significant differences to values from normal control populations studied previously (15).
FIGURE 3. Skeletal Muscle Perfusion Imaging Data.
Box-and-whisker plots for median (bar), 25% to 75% confidence interval (box), and range (whiskers) of values for resting skeletal muscle contrast-enhanced ultrasound data depicting: (A) microvascular blood flow, (B) blood flux rate or β, and (C) microvascular blood volume (MBV). Data from 1 outlying individual with extremely high resting β was removed after performing Grubb’s test for outliers. (D to F) Similar plots are illustrated for skeletal muscle during contractile exercise. p = NS for all pre- versus post-apheresis values. IU = video intensity units.
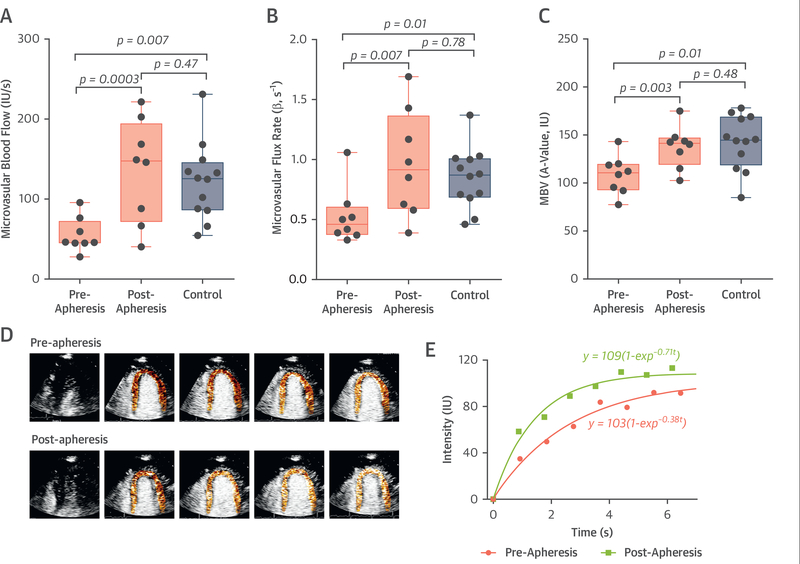
In contrast, apheresis significantly increased resting myocardial blood flow (Figure 4). The greatest increases in flow occurred in those undergoing apheresis for the first time (Online Figure 2). The increase in perfusion was attributable to improvements in both myocardial microvascular flux rate and MBV. On average, the increase in myocardial blood flow tended to be greater in the 3 subjects undergoing apheresis for the first time (342 ± 257%) compared with those undergoing recurrent apheresis (86 ± 101%), but the difference was not statistically significant. Pre-apheresis parameters of myocardial microvascular perfusion were all significantly lower than in age-matched control subjects and skeletal muscle blood flow during exercise approximated that of myocardial blood flow, suggesting that improvements in rheology, which should affect perfusion similarly in the 2 tissues, were not solely responsible for the increases in flow that were unique to the myocardium.
FIGURE 4. Myocardial Perfusion Imaging Data.
Box-and-whisker plots are depicted for myocardial contrast echocardiography data on (A) microvascular blood flow, (B) blood flux rate or β, and (C) microvascular blood volume (MBV) in the myocardium at rest. Individual changes are provided in Online Figure 2. (D) Background-subtracted color-coded myocardial contrast echocardiography images obtained at each sequential end-systole (left to right) after a high-power pulse sequence, and (E) time-intensity data illustrating changes in resting myocardial perfusion from single subject who had a modest increase in perfusion after apheresis. IU = video intensity units.
CORRELATIONS BETWEEN LIPID VALUES AND MYOCARDIAL PERFUSION.
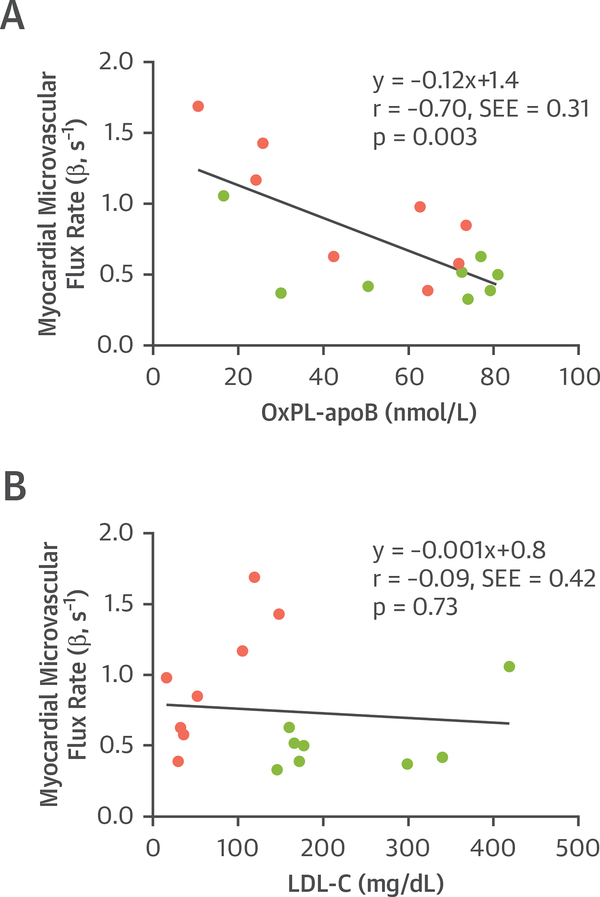
Although the small number of subjects limited statistical power, the apheresis-related change in microvascular flux rate was modestly correlated with certain baseline pre-apheresis lipid values including cholesterol (r = 0.70; p = 0.05), LDL-C (r = 0.70; p = 0.05), and OxPL-apo B-100 (r = 0.75; p = 0.03) (Online Table 1). There were no significant correlations found between change in myocardial perfusion and the degree of change in any of the lipid values or rheologic parameters. When combining all pre- and post-apheresis data, there was a significant but modest inverse correlation between OxPL-apo B-100 and myocardial perfusion (microvascular flux rate), but not for any other lipid variable including LDL-C (Figure 5).
FIGURE 5. Lipid Variables and Myocardial Blood Flow.
Correlations are shown for (A) OxPL on apo B-100 lipoproteins or (B) LDL cholesterol with myocardial perfusion on myocardial contrast echocardiography expressed as the microvascular blood flux rate (β). Data are combined for pre-apheresis (green) and post-apheresis (pink) stages. SEE = standard error of the estimate; other abbreviations as in Figure 1.
VASCULAR REACTIVITY.
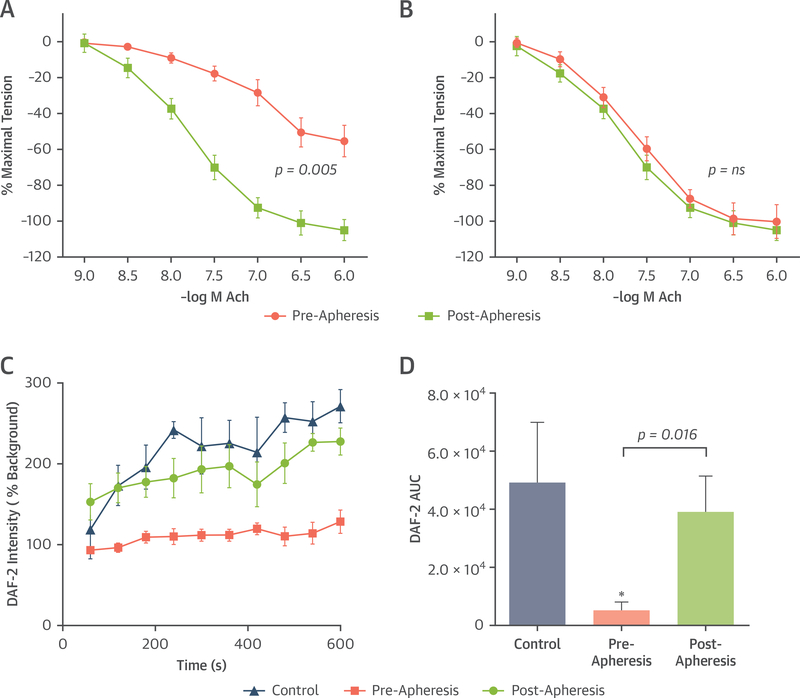
Vascular reactivity to the endothelium-dependent vasodilator Ach was tested in isolated ovine coronary and peripheral artery rings in the presence of plasma obtained pre- and post-apheresis. Reduction in arterial tension in response to Ach was entirely abolished by inhibition of endothelial nitric oxide synthase (eNOS) with L-nitroarginine methyl ester (100 μmol/l) (Online Figure 3). For coronary arteries, Ach-mediated relaxation (but not relaxation with sodium nitroprusside) was reduced in the presence of pre-apheresis plasma and was restored completely in the presence of post-apheresis plasma (Figure 6A, Online Figure 3).
FIGURE 6. Arterial Tension Assays and Endothelial NO Production.
Tension assay dose-response curves illustrate degree of relaxation as a percentage of maximal tension (mean ± SEM) according to acetylcholine (Ach) concentration for the (A) coronary and (B) femoral branch arteries in a water bath containing pre- and post-apheresis plasma. For coronary analysis, p < 0.05 versus pre-apheresis by half maximal inhibitory concentration analysis. (C) Mean ± SEM values for 4,5-diaminofluorescein diacetate-2 (DAF-2) fluorescence over time, reflecting nitric oxide (NO) production, in cultured SVEC4–10 endothelial cells exposed to plasma from healthy volunteers or familial hypercholesterolemia patients pre- and post-apheresis. (D) Mean ± SEM area under the curve (AUC) for 10-min acquisition. *p < 0.05 versus healthy control subjects.
Vasoreactivity for femoral artery rings was not different in the presence of pre- or post-apheresis plasma (Figure 6B). Production of NO by endothelial cells in vitro measured by 4,5-diaminofluorescein diacetate-2 intensity was substantially lower after cells were exposed to pre-apheresis versus post-apheresis plasma (Figures 6C and 6D).
DISCUSSION
Severe hypercholesterolemia has been implicated as a cause of microvascular dysfunction through pathways that include reduced bioavailability of endothelial-derived vasodilators, increased oxidative signaling, enhanced vasoconstrictor pathway signaling, and increased effective viscosity at the capillary level (2,16–19). Evaluation of the severity of microvascular dysfunction in FH and whether it is improved by drug or apheresis therapy has generally involved the assessment of flow reserve, or of flow-dependent vasodilation in a peripheral artery as an indicator of endothelial NO production. In this study, by using quantitative perfusion imaging we have demonstrated for the first time that lipoprotein apheresis in patients with severe hypercholesterolemia results in an immediate increase in resting myocardial perfusion to a level similar to that in control subjects. These beneficial effects on myocardial perfusion, which could not be attributed to changes in myocardial work, were not observed for the peripheral microcirculation.
In humans and other large mammals, mechanisms that control microvascular tone vary according to vascular hierarchy (20). Shear-dependent arterial responses, which are known to be influenced by LDL-C levels (5), tend to predominate in larger arterioles (>100 μm). Metabolic mediators such as adenosine, adenosine triphosphate, H2O2, and prostanoids that are influenced by inflammatory status, oxidative stress, sympathetic tone, and other factors tend to act in smaller precapillary arteriolar networks. Rheological properties such as erythrocyte deformability and viscosity, which are influenced by plasma lipoprotein concentration, are most impactful at the capillary level (21). In the current study, quantitative CEU perfusion imaging was used to evaluate tissue perfusion at the capillary level and, accordingly, to assess the net vascular effects of apheresis. Parametric analysis helped identify the potential mechanisms by which apheresis affected the myocardial microcirculation. The largest increases in resting myocardial flow were found in those undergoing apheresis for the first time, in whom large increases in microvascular flux rate (β) occurred, implying either a major reduction in tone at the resistance arteriolar level or improvement in rheology (22,23).
CEU also provided the unique opportunity to compare responses within myocardial and skeletal muscle microcirculations nearly simultaneously, and to assess muscle perfusion during physiological hyperemia produced by exercise. Our results indicate that lipoprotein apheresis increased resting myocardial perfusion but, unexpectedly, there was no effect on forearm muscle perfusion even during exercise. During contractile exercise, muscle blood flow and flux rate (β) approximated that seen in the resting myocardium after apheresis. As differences in coronary and skeletal muscle capillary diameter are negligible (24), one can conclude that improvement in rheology (viscosity and erythrocyte deformability) after apheresis was not the primary reason for improving myocardial perfusion because it should have produced a similar benefit in exercising skeletal muscle. Our finding that apheresis did not affect muscle perfusion is different from a prior study using plethysmography to evaluate response to Ach (25). In the prior study, flow augmentation with Ach increased 4- to 5-fold compared with the 20-fold increase during exercise in our study. Because NO-dependent pathways account for only a small component of muscle vascular response to exercise (26), we believe that our findings more accurately reflect a physiological response, whereas the prior study tested 1 pathway. By performing echocardiography immediately before MCE, we were also able to exclude the possibility that apheresis-related increases in myocardial perfusion were a consequence of changes in myocardial work or contractility.
The most plausible explanation for differences in coronary and peripheral microvascular response to apheresis is that they respond differently to severe hypercholesterolemia. Previous studies have suggested that endothelial-dependent vasomotor response and other vasodilator/vasoconstrictor responses are not tightly correlated between the 2 beds (27,28). We investigated endothelial-derived pathways based largely on a preponderance of data indicating impaired NO signaling in hypercholesterolemia (5,11,17,25). Arterial ring tension assays confirmed abnormal endothelial-dependent vasodilation in the presence of pre-apheresis plasma for coronary but not femoral artery rings, with complete normalization of vasodilation in the presence of post-apheresis plasma.
Potential reasons for abnormal NO-dependent vasomotion in association with dyslipidemia include reduced L-arginine transport; reduced eNOS phosphorylation; increased production of eNOS inhibitors such as asymmetric dimethylarginine; and the end results of increased oxidative stress including uncoupling of eNOS, reduced activity of tetrahydrobiopterin, and oxidative metabolism of NO (4,16). Our results with 4,5-diaminofluorescein diacetate-2 suggested a role of reduced production of NO after exposure to pre-apheresis plasma. Inactivation of NO through oxidative modification could have also contributed to reduced coronary vasomotion, a notion supported by the reduction in OxPL-apo B-100 by apheresis. Prior studies have demonstrated that reduction in oxidatively modified LDL improves arterial response to Ach (29) and that apheresis for elevated Lipoprotein(a), which contains the highest proportion of OxPL among lipoproteins, reduces angina and improves myocardial blood flow during vasodilator stress (30). In a recent study examining proangiogenic growth factor gene therapy in patients with refractory angina, improvement in resting myocardial blood flow was inversely correlated with OxPL-apo B-100, suggesting that patients with low OxPL have the greatest potential for improvement (31). Our own findings that baseline pre-apheresis OxPL-apo B-100 levels correlated inversely with improvement in myocardial perfusion and that OxPL-apo B-100 from all stages correlated with myocardial perfusion suggest a potential role of oxidative stress in governing myocardial perfusion.
STUDY LIMITATIONS.
The number of patients was small despite studying all patients undergoing apheresis in our institution over a 2-year period willing to participate. However, the magnitude of the treatment effects was greater than that anticipated by initial power calculations and obviated the need for a larger study. We did not restudy patients sequentially after apheresis to determine the rate at which improvement in myocardial perfusion is lost. Although our strain echocardiography data suggests apheresis can improve LV function in those with subtle pre-apheresis dysfunction, we cannot verify this finding based on the small number of subjects studied and inconsistent changes in GLS in the group as a whole. It should be noted that ex vivo tension studies were performed in large arteries rather than for microvascular preparations. However, our primary outcome data from CEU primarily reflect changes that occur at the microvascular level. In our study, apheresis was performed with the charge-based adsorption method, so it is uncertain if other apheresis approaches would produce the same results.
In summary, we have demonstrated that lipoprotein apheresis produces an immediate coronary-specific improvement in resting myocardial perfusion which is likely secondary to changes in the regulation of microvascular tone, which does not occur for skeletal muscle even during contractile exercise. It will important to determine whether improvements in resting myocardial perfusion are related to improvement in symptoms or to the reduction in circulating inflammatory markers.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Apheresis can result in an immediate improvement in myocardial perfusion in patients with FH in whom maximal medical therapy has not achieved targeted lipid lowering.
TRANSLATIONAL OUTLOOK:
Treatments that acutely correct hyperlipidemia can increase resting myocardial blood flow independent of changes in myocardial work.
Acknowledgments
Dr. Lindner is supported by grants R01-HL078610 and R01-HL120046 from the National Institutes of Health; and grant 14NSBRI1-0025 from the National Space Biomedical Research Institute (National Aeronautics and Space Administration). Dr. Wu is supported by grants T32-HL094294 and K08-HL133493 from the National Institutes of Health. Dr. Moccetti is funded by grant P2BSP3-158853 from the Swiss National Science Foundation. Dr. Fazio is supported by grant R01-132985 from the National Institutes of Health. Dr. Tsimikas is supported by the Fondation Leducq and National Institutes of Health grants R01-HL119828, R01-HL078610, R01-HL106579, R01 HL128550, R01 HL136098, P01 HL136275, and R35 HL135737. Dr. Duell has served as a consultant for Retrophin, Esperion, RegenxBio, Kastle, Daiichi-Sankyo, and Akcea; and has received institutional grant support from Regeneron, Retrophin, and Esperion. Dr. Fazio has served as a consultant for Kowa, Amgen, Amarin, and Aegerion. Dr. Tsimikas is a co-inventor and receives royalties from patents owned by the University of California, San Diego, on oxidation-specific antibodies and of oxidative biomarkers; and has a dual appointment at the University of California, San Diego, and as an employee of Ionis Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- Ach
acetylcholine
- apo B
apolipoprotein B-100
- CEU
contrast-enhanced ultrasound
- Enos
endothelial nitric oxide synthase
- FH
familial hypercholesterolemia
- GLS
global longitudinal strain
- LDL-C
low-density lipoprotein cholesterol
- MBV
microvascular blood volume
- MCE
myocardial contrast echocardiography
- OxPL
oxidatively modified phospholipids
Footnotes
APPENDIX For an expanded Methods section and supplemental table and figures, please see the online version of this paper.
REFERENCES
- 1.Besseling J, Hovingh GK, Huijgen R, Kastelein JJ, Hutten BA. Statins in familial hypercholesterolemia: consequences for coronary artery disease and all-cause mortality. J Am Coll Cardiol 2016;68:252–60. [DOI] [PubMed] [Google Scholar]
- 2.Bender SB, de Beer VJ, Tharp DL, et al. Severe familial hypercholesterolemia impairs the regulation of coronary blood flow and oxygen supply during exercise. Basic Res Cardiol 2016;111:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellwig KP, Pulawski E, Horstkotte D, van Buuren F. Lipid apheresis: oxidative stress, rheology, and vasodilatation. Clin Res Cardiol Suppl 2012;7:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landmesser U, Hornig B, Drexler H. Endothelial dysfunction in hypercholesterolemia: mechanisms, pathophysiological importance, and therapeutic interventions. Semin Thromb Hemost 2000;26: 529–37. [DOI] [PubMed] [Google Scholar]
- 5.Creager MA, Cooke JP, Mendelsohn ME, et al. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J Clin Invest 1990;86:228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossenbach J, Mueller GA, Lange K, et al. Lipid-apheresis improves microcirculation of the upper limbs. J Clin Apher 2011;26:167–73. [DOI] [PubMed] [Google Scholar]
- 7.Gordon BR, Kelsey SF, Dau PC, et al. Long-term effects of low-density lipoprotein apheresis using an automated dextran sulfate cellulose adsorption system. Liposorber Study Group. Am J Cardiol 1998;81:407–11. [DOI] [PubMed] [Google Scholar]
- 8.Lu Q, Eriksson M, Jogestrand T, Henriksson P, Freyschuss A. Micro- and macrocirculatory effects of apheresis in patients with familial hyperlipidemia. Ther Apher Dial 2003;7:115–8. [DOI] [PubMed] [Google Scholar]
- 9.Ellins EA, New KJ, Datta DB, et al. Validation of a new method for non-invasive assessment of vasomotor function. Eur J Prev Cardiol 2016;23: 577–83. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Yamashita K, Tasaki H, et al. Evaluation of improved coronary flow velocity reserve using transthoracic Doppler echocardiography after single LDL apheresis. Ther Apher Dial 2004;8:383–9. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi K, Tsuji M, Nishimura M, Horimoto M. Improvement of endothelium-dependent coronary vasodilation after a single LDL apheresis in patients with hypercholesterolemia. J Clin Apher 2004;19:11–6. [DOI] [PubMed] [Google Scholar]
- 12.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 13.Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998;97:473–83. [DOI] [PubMed] [Google Scholar]
- 14.Pascotto M, Leong-Poi H, Kaufmann B, et al. Assessment of ischemia-induced microvascular remodeling using contrast-enhanced ultrasound vascular anatomic mapping. J Am Soc Echocardiogr 2007;20:1100–8. [DOI] [PubMed] [Google Scholar]
- 15.Womack L, Peters D, Barrett EJ, Kaul S, Price W, Lindner JR. Abnormal skeletal muscle capillary recruitment during exercise in patients with type 2 diabetes mellitus and microvascular complications. J Am Coll Cardiol 2009;53: 2175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stapleton PA, Goodwill AG, James ME, Brock RW, Frisbee JC. Hypercholesterolemia and microvascular dysfunction: interventional strategies. J Inflamm (Lond) 2010;7:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feron O, Dessy C, Moniotte S, Desager JP, Balligand JL. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J Clin Invest 1999;103:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardillo C, Kilcoyne CM, Cannon RO 3rd, Panza JA. Increased activity of endogenous endothelin in patients with hypercholesterolemia. J Am Coll Cardiol 2000;36:1483–8. [DOI] [PubMed] [Google Scholar]
- 19.Jay RH, Rampling MW, Betteridge DJ. Abnormalities of blood rheology in familial hypercholesterolaemia: effects of treatment. Atherosclerosis 1990;85:249–56. [DOI] [PubMed] [Google Scholar]
- 20.Duncker DJ, Koller A, Merkus D, Canty JM Jr. Regulation of coronary blood flow in health and ischemic heart disease. Prog Cardiovasc Dis 2015; 57:409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pries AR, Secomb TW, Gaehtgens P. Biophysical aspects of blood flow in the microvasculature. Cardiovasc Res 1996;32:654–67. [PubMed] [Google Scholar]
- 22.Rim SJ, Leong-Poi H, Lindner JR, Wei K, Fisher NG, Kaul S. Decrease in coronary blood flow reserve during hyperlipidemia is secondary to an increase in blood viscosity. Circulation 2001;104: 2704–9. [DOI] [PubMed] [Google Scholar]
- 23.Wei K, Le E, Bin JP, Coggins M, Jayawera AR, Kaul S. Mechanism of reversible (99m)Tc-sestamibi perfusion defects during pharmacologically induced vasodilatation. Am J Physiol Heart Circ Physiol 2001;280:H1896–904. [DOI] [PubMed] [Google Scholar]
- 24.Potter RF, Groom AC. Capillary diameter and geometry in cardiac and skeletal muscle studied by means of corrosion casts. Microvasc Res 1983; 25:68–84. [DOI] [PubMed] [Google Scholar]
- 25.Tamai O, Matsuoka H, Itabe H, Wada Y, Kohno K, Imaizumi T. Single LDL apheresis improves endothelium-dependent vasodilatation in hypercholesterolemic humans. Circulation 1997; 95:76–82. [DOI] [PubMed] [Google Scholar]
- 26.Sarelius I, Pohl U. Control of muscle blood flow during exercise: local factors and integrative mechanisms. Acta Physiol (Oxf) 2010;199:349–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teragawa H, Ueda K, Matsuda K, et al. Relationship between endothelial function in the coronary and brachial arteries. Clin Cardiol 2005;28: 460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrer M, Encabo A, Conde MV, Marin J, Balfagon G. Heterogeneity of endothelium-dependent mechanisms in different rabbit arteries. J Vasc Res 1995;32:339–46. [DOI] [PubMed] [Google Scholar]
- 29.Penny WF, Ben-Yehuda O, Kuroe K, et al. Improvement of coronary artery endothelial dysfunction with lipid-lowering therapy: heterogeneity of segmental response and correlation with plasma-oxidized low density lipoprotein. J Am Coll Cardiol 2001;37:766–74. [DOI] [PubMed] [Google Scholar]
- 30.Khan TZ, Hsu LY, Arai AE, et al. Apheresis as novel treatment for refractory angina with raised lipoprotein(a): a randomized controlled cross-over trial. Eur Heart J 2017;38:1561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartikainen J, Hassinen I, Hedman A, et al. Adenoviral intramyocardial VEFG-DdNdC gene transfer increases myocardial perfusion reserve in refractory angina patients: a phase I/IIa study with 1 year follow-up. Eur Heart J 2017;38: 2547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.