Abstract
Bacterial exopolysaccharides (EPS) play a critical role in sequestration of metals from contaminated environment. Considering these, this study was aimed at extracting EPS from metal tolerant Pseudomonas aeruginosa CPSB1 and Azotobacter chroococcum CAZ3 and to ascertain its role in metal removal. P. aeruginosa CPSB1 and A. chroococcum CAZ3 secreted 1306.7 and 1660 µg mL−1 EPS, respectively in the presence of 200 and 100 µg mL−1 Pb, respectively with glucose as C source. The binding of metal ions to bacterial EPS was validated by SEM and EDX. The functional group involved in metal chelation was revealed by FT-IR. The metal ions were adsorbed onto EPS and hence, EPS could play a crucial role in metal detoxification. Due to this novel trait, P. aeruginosa CPSB1 and A. chroococcum CAZ3 could be developed as bioinoculant to cleanup metal contaminated sites.
Keywords: Exopolysaccharides, Heavy metals, Metal sequestration, P. aeruginosa, A. chroococcum
Heavy metals are significant environmental threat, which, when present beyond threshold levels in the environment, disrupt the ecological niches. However, certain metal tolerant bacterial strains have been found potentially magical in detoxifying polluted environment. For this, the metal tolerant bacterial strains have evolved multiple strategies to remediate metal contaminated soils. For example, metal biosorption, extracellular precipitation, conversion of toxic metal ions into less toxic forms and flush out (efflux pumping) of metals to exterior environment are some of the approaches adopted by bacteria to thrive well even under metal stressed conditions [1]. Apart from these, bacterial cells also synthesize extracellular polymeric substances which allow them to survive even in the presence of stressor molecules by masking their toxic impact [2, 3]. Also, EPS plays a significant role in metal chelation, wherein, the ionic forms of metals bind to the complex polymeric structures of EPS. The secretion of EPS by bacterial cells is thus an interesting biological phenomenon to shield themselves from the harsh environment by forming a biofilm matrix on solid substrates. Moreover, the structure and composition of EPS is such that it amply allows easy sequestration of metal ions. Due to these functional properties, microbial polymers have largely been employed in metal removal from polluted environments [4]. Considering the importance of EPS in metal sequestration and detoxification, the present study was aimed at searching metal tolerant bacterial strains capable of synthesizing EPS under both metal stressed and conventional environments. Also, the uptake and localization of metals within EPS was determined by SEM and EDX while functional moieties of EPS were detected by FTIR analysis.
The production of EPS by metal tolerant bacterial strains under conventional and metal stressed conditions was determined by growing P. aeruginosa CPSB1 and A. chroococcum CAZ3 in 50 mL nutrient broth containing 5% glucose and treated with 0, 25, 50, 100 and 200 µg mL−1 each of Cu, Cd, Cr, Ni and Pb. The bacterized flasks were incubated at 28 ± 2 °C for 5 days on a rotary shaker incubator at 120 r min−1. The culture broth was spun at 8000 rpm for 30 min and three volume of chilled acetone was added to one volume of supernatant and EPS was extracted. The extracted EPS was washed three times with distilled water and acetone and later transferred to a filter paper (No. 42) and weighed. The structural composition, metal distribution and sequestration of powdered EPS were later characterized by SEM, EDX and FTIR techniques.
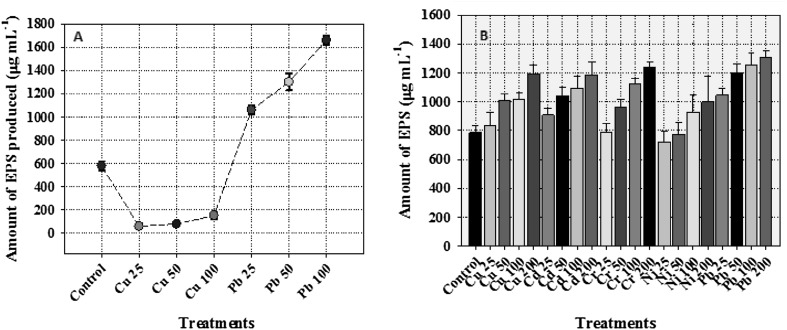
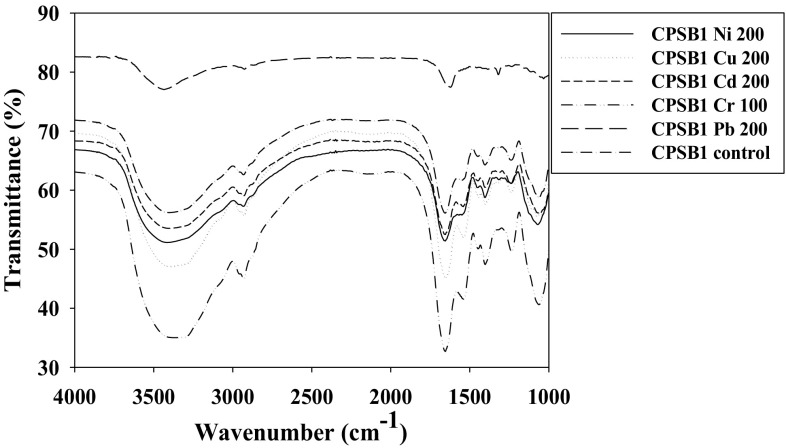
Exopolysaccharides synthesized by microbial cells provide protection against stressful conditions including those against heavy metals and desiccation [5, 6] besides acting as a biosorbent [4]. Apart from these, the EPS synthesized by microbial communities also participates in biofilm formation (quorum sensing) and hence, acts as a barrier against antibacterial drugs. However, the QS inhibitors (QSIs) of diverse origins have been shown to act as potential antipathogens [7]. Considering these, the production of EPS by P. aeruginosa CPSB1 (GenBank Accession number KX821717) and A. chroococcum CAZ3 (GenBank Accession number MG252731) was assessed under metal stress. Interestingly, both Gram negative bacterial genera secreted considerable amounts of EPS even under metal stressed conditions though the level of production differed among bacterial strains and were greatly influenced by metal species. For example, A. chroococcum CAZ3 secreted 153.3 µg mL−1 EPS when grown in the presence of 100 µg mL−1 of Cu which was however, increased by 65% when it was grown in the presence of 100 µg mL−1 of Pb when compared with control (Fig. 1a). Similarly, EPS production by P. aeruginosa CPSB1 was enhanced by 34, 34, 37, 21 and 40% when it was grown at 200 µg mL−1 each of Cu, Cd, Cr, Ni and Pb, respectively relative to untreated control (Fig. 1b). While comparing the secretion of EPS by two strains, A. chroococcum CAZ3 in general demonstrated maximum production under metal stress. Among metals, Pb was found as the strongest inducer of EPS synthesis. The EPS micrographs of metal treated bacterial cells under SEM revealed a definite polymeric structure whereas the uptake and localization of metal by P. aeruginosa CPSB1 (Fig. 2a) and A. chroococcum CAZ3 (Fig. 2b) was confirmed by EDX spectra. The fact that EPS could chelate heavy metal ions was strengthened by comparing the EDX spectra of EPS obtained from metal treated and control cells. The FT-IR data indicating functional groups was recorded in the range of 1000–4000 cm−1. Multiple peaks corresponding to varied functional groups were obtained in the spectra of metal loaded and unloaded EPS. Some deviations and shifting in peaks of functional groups was observed in the presence of metals. The EPS extracted from P. aeruginosa CPSB1 for instance, displayed a broad peak in the range of wave number 2819–1928 cm−1 in the presence of Cu which could be designated to asymmetrical stretching of –CH3 group of fatty acids. Similarly in the presence of Cd and Cr, broad peaks in the range of 2606–1874 and 2522–1866 cm−1 respectively, were recorded which also corresponded to -CH3 stretching in fatty acids. Lead in contrast, resulted in a peak that ranged between 2926 and 1775 which represented asymmetrical stretching of > CH2 group. Several small peaks were also observed at different regions of EPS which were slightly shifted in position compared to control (Fig. 3). Similar variation in FTIR spectra representing modifications in the functional group moieties of metal loaded EPS extracted from A. chroococcum CAZ3 was observed (data not shown). The shifting in peaks relative to control could possibly be due to the disturbances in various functional groups attached to the surface of metal loaded EPS resulting due to the binding of metal ions with the bacterial EPS. The disappearance and irregularity in peaks of EPS extracted from other bacterial genera while growing in the presence of metal stress have also been reported by [8].
Fig. 1.
Quantification of EPS synthesized by A. chroococcum CAZ3 (a) and P. aeruginosa CPSB1 (b) under controlled and metal stressed environments
Fig. 2.
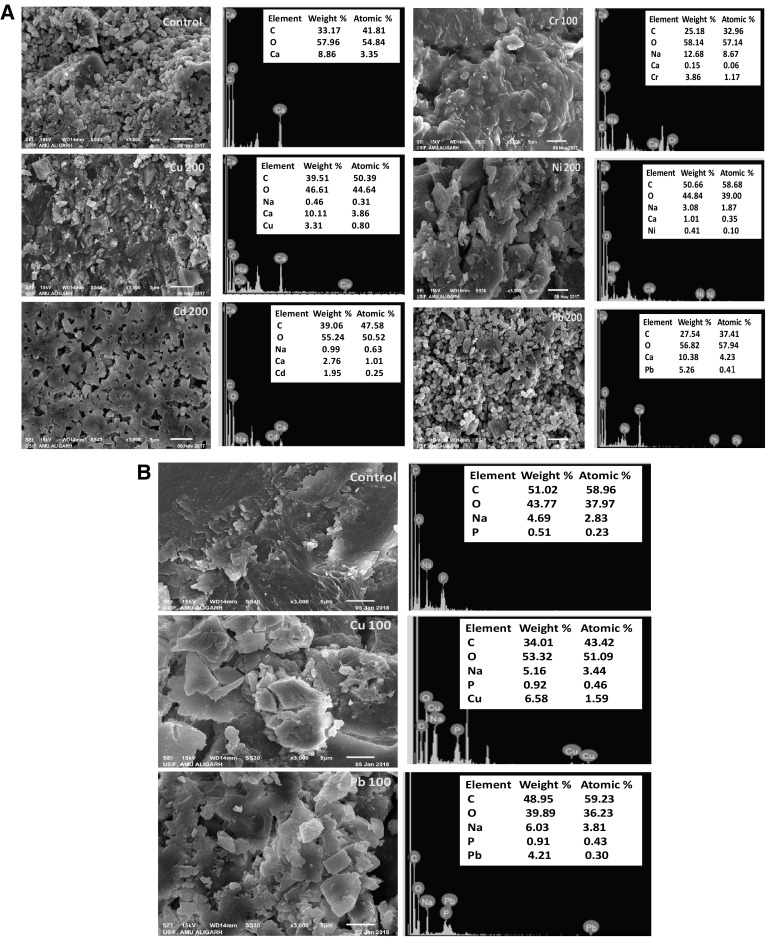
a SEM micrographs and EDX spectra of EPS secreted by P. aeruginosa CPSB1 while growing under metal stressed and free conditions. The EDX shows elemental composition of EPS. b SEM micrographs and EDX spectra of EPS secreted by A. chroococcum CAZ3 under metal stressed and free conditions. The EDX spectra represent the elemental composition of EPS
Fig. 3.
FT-IR spectra of EPS extracted from metal treated and untreated cells of P. aeruginosa CPSB1 showing characteristic peaks corresponding to various functional groups involved in metal ion binding to EPS
The secretion of EPS by metal tolerant Gram negative P. aeruginosa and A. chroococcum under metal stress and its further characterization by SEM, EDX and FTIR techniques explicitly revealed that EPS could serve as a potential and reliable biomolecule in safeguarding the rhizosphere microflora from the destruction/damage caused by heavy metals while allowing them to maintain their metabolic activities even under stressful conditions. Summarily, the synthesis of EPS by bacterial cells is likely to serve as an important emerging eco-friendly approach for heavy metal detoxification and hence, could practically be applied as a bioremediation strategy in metal contaminated environments.
Acknowledgements
The funding for this work was provided by Department of Science and Technology, New Delhi, India as INSPIRE fellowship (DST/INSPIRE Fellowship/2014/IF140773). One of the authors AR is highly thankful to University Sophisticated Instruments Facility (USIF), Aligarh Muslim University, Aligarh and Macrogen Inc., Seoul, South Korea for the analyses.
Compliance with Ethical Standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan MS, Zaidi A, Wani PA, Oves M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett. 2009;7:1–9. doi: 10.1007/s10311-008-0155-0. [DOI] [Google Scholar]
- 2.Batool R, Marghoob U, Kalsoom A (2017) Estimation of exopolysaccharides (EPS) producing ability of Cr(VI) resistant bacterial strains from tannery effluent. J Basic Appl Sci 13: 589–596. ISSN: 1927-5129
- 3.Mohite BV, Koli SH, Patil SV. Heavy metal stress and its consequences on exopolysaccharide (EPS)-producing Pantoea agglomerans. Appl Biochem Biotechnol. 2018;19:1–8. doi: 10.1007/s12010-018-2727-1. [DOI] [PubMed] [Google Scholar]
- 4.Muthu M, Wu HF, Gopal J, Sivanesan I, Chun S. Exploiting microbial polysaccharides for biosorption of trace elements in aqueous environments-scope for expansion via nanomaterial intervention. Polymers. 2017;9:721. doi: 10.3390/polym9120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta P, Diwan B. Bacterial exopolysaccharide mediated heavy metal removal: a review on biosynthesis, mechanism and remediation strategies. Biotechnol Rep. 2017;13:58–71. doi: 10.1016/j.btre.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa OY, Raaijmakers JM, Kuramae EE. Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalia VC, Patel SKSP, Kang YC, Lee JK. Quorum sensing inhibitors as antipathogens: biotechnological applications. Biotechnol Adv. 2019;37:68–90. doi: 10.1016/j.biotechadv.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Seneviratne M, Gunaratne S, Bandara T, Weerasundara L, Rajakaruna N, Seneviratne G, Vithanage M. Plant growth promotion by Bradyrhizobium japonicum under heavy metal stress. S Afr J Bot. 2016;105:19–24. doi: 10.1016/j.sajb.2016.02.206. [DOI] [Google Scholar]