Abstract
Escherichia coli (E. coli) colonizes human intestinal tract and is usually harmless to the host. However, several strains of E. coli have acquired virulent genes and could cause enteric diseases, urinary tract and even brain infections. Shiga toxin producing Escherichia coli (STEC) is an enterohaemorrhagic E. coli (EHEC) which can result in bloody diarrhoea and could potentially lead to deadly heamolytic uremic syndrome (HUS). STEC is one of the important food borne pathogens that causes food poisoning leading to diarrhoea and number of STEC outbreaks have occurred across the world. The use of standard antibiotics to treat STEC infection is not recommended as it increases the production of shiga toxin which could lead to HUS. Therefore, use of alternative approaches which include use of plant products to treat STEC infections have been gaining attention. The objective of this study was to evaluate the antibacterial and anti-biofilm activity of garlic (Allium sativum) against STEC strains isolated from various patient and food samples using in vitro assays. The microbiological isolation of STEC from various patient and food samples resulted in eight STEC isolates of which seven strains were multidrug resistant. Antibacterial assay results indicated that all the strains exhibited dose dependent sensitivity towards garlic with zone of inhibition diameters ranging from 7 to 24 mm with 15 µl of fresh garlic extract (FGE). Minimum inhibitory concentration (MIC) of FGE for isolates ranged from 30 to 140 µl/ml. Interestingly, the biofilm formation of all isolates in presence of 4% of FGE decreased by 35 to 59%. FTIR analysis indicated that treatment with 1% FGE results in compositional and content changes in the biofilm. In addition, the total carbohydrate content of biofilm was reduced by 40% upon 1% FGE treatment. The results of the present study report for the first time the antibacterial and anti-biofilm activity of garlic against STEC. The findings will enable development of novel garlic organosulfide based drugs for the prevention and treatment of STEC infections.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00784-3) contains supplementary material, which is available to authorized users.
Keywords: Shiga-toxin producing E. coli (STEC), Garlic, Antibacterial activity, Disc diffusion assay, Anti-biofilm
Introduction
Escherichia coli are commensal bacteria that reside in human colon and rarely cause any disease. However, certain clones of E. coli are pathogenic by the virtue of acquiring virulence factors and are referred as E. coli pathotypes. Shiga toxin producing E. coli (STEC) constitute to enterohaemorrhagic E. coli (EHEC) class of intestinal pathotype. STEC infections cause bloody diarrhoea (haemorrhagic colitis), non-bloody diarrhoea and could lead to potentially deadly heamolytic uremic syndrome (HUS) [1]. The intestine of ruminants is the main reservoir of STEC and consumption of contaminated meat and produce is the main cause of infection. Shiga toxin (Stx) is the primary toxin produced by STEC and based on lipopolysaccharide (O) and flagellar (H) antigens they are categorized into various serotypes. O157:H7 strain produces Stx and contains pathogenicity island called locus of enterocyte effacement (LEE) that codes for other virulence factors resulting in human disease [2]. Most of the non-O157:H7 STEC strains are also associated with human illness but go unreported due to lack of laboratory tests and clinical awareness. Stx1 and Stx2 are two subgroups of Stx family of toxin that cause apoptosis of intestinal cells and necrosis leading to enterocolitis, bloody diarrhoea and intestinal perforation. In addition, Stx can enter the blood stream and cause fatal renal failure due to kidney endothelial damage, microvascular occlusion and inflammation [3, 4].
A report estimated that around 2.8 million acute illnesses were caused by STEC infections annually between 1990 and 2012 in 21 countries effecting children of age 0–4 years more severely [5]. In USA alone O157:H7 strains causes 100,000 infections yearly whereas in Europe 6109 confirmed cases of STEC infections were reported in 2014 [6, 7]. The estimation of STEC infections in India is obscure due to paucity of data. However, according to some studies STEC was isolated from 1.4 to 1.6% of bloody diarrhoea samples [8]. A sizeable number of STEC infections also occur in Africa [9]. In addition, 1999 outbreak in China caused due to a new O157:H7 strain resulted in 177 deaths due to HUS out of 195 infected individuals, mainly elderly females [10]. It has been noticed that childern < 5 years are particularly more susceptible to STEC infections [11, 12]. Altogether, STEC is one of the main enteropathogens with prevalence all over the globe that can result in severe disease conditions with potential to cause death [13].
The current treatment strategy for STEC infections is mainly supportive therapy such as rehydration due to lack of effective treatment options [4]. The use of antibiotic is not recommended due to the risk of HUS progression [9, 14, 15]. It has been reported that antibiotic treatment increases Stx production either by releasing intracellular Stx due to lysis or by induction of Stx expression from prophage due to genomic insult caused by antibiotics [16]. Thus, there is a lot of interest in developing novel alternative therapies to treat STEC infection such as antibodies, Stx receptor analogs, novel antibacterial agents, probiotics, phages and plant derived natural products [17–19]. The antibacterial activity of Chinese cinnamon, Spanish oregano and savory essential oils against STEC was found to be mediated by compromising the cell wall and membrane [20]. Green tea and cranberry has also been reported to exhibit antibacterial activity against STEC [21]. The antibacterial activity of garlic (Allium sativum) is well documented against many pathogenic bacteria including antibiotic resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) [22–26]. A very important aspect of STEC infection is survival of the bacteria outside the host or reservoir under rash condition until it infects a new host or reservoir. Biofilm formation facilitates such survival on contaminated food surfaces and plays a major role in foodborne bacterial infections [27]. Biofilm is accumulation of microorganisms on animate and inanimate surfaces with the help of extracellular polymeric substance which is composed of proteins, polysaccharides and nucleic acids which plays an important role in infection and bacterial resistance [28]. Quorum sensing (QS) is chemical communication system through which bacteria interact with each other when present in high densities. Among other phenomenon, formation of biofilm is mediated by QS and can lead to expression of attachment and virulent genes that are critical for colonization and pathogenesis [17]. Inhibition of biofilm formation by inhibiting QS is a clever approach to fight bacterial infections and diseases. Extensive research and recent advancements in technology has enabled development of various assays to monitor QS which can be used to screen and discover QS inhibitors (QSI) as antipathogens [29]. Natural products are of great importance as many of them can be used as therapeutics to treat bacterial infections by their virtue to inhibit biofilm formation by interfering with QS. It is recently reported that phytochemicals such as limonoids, coumarins, harmaline and nonharmane along with antibacterial activity also exhibit anti-biofilm activity against E. coli O157:O7 [30, 31]. The need to keep looking for new antibacterial and anti-biofilm agents is always there due to emergence of drug and QSI resistant bacterial strains [32]. It is suggested that biofilm formation could have an influence on the pathogenesis of STEC, however more studies are required to better understand the relevance of biofilm in the STEC pathogenesis [33].
In the present study, we isolated STEC from patient samples and common food samples such as water, milk and food from local stalls. The sensitivity of these isolates against antibiotics and garlic was investigated. As attachment and formation of biofilm plays an important role in STEC infection and pathogenesis, we investigated the effect of garlic on the biofilm formation of STEC isolates.
Materials and Method
Isolation of STEC
Food and water samples were collected from different sites in and around Sagar district, Madhya Pradesh, India. Stool samples from patients suffering from diarrhoea were collected from Bundelkand Medical College, Sagar, Madhya Pradesh, India and King George’s Medical University, Lucknow, Uttar Pradesh, India. In brief, the samples were transported in sterile container and initially plated on eosin methylene blue (EMB) agar media (selective and differential media) and were incubated at 37 °C overnight to selectively grow Gram-negative bacteria. Lactose fermenting E. coli were differentiated from non-fermenters due to their metallic green colonies following overnight incubation. In order to differentiate between non-pathogenic and enterohaemorrhagic E. coli, these colonies were picked and streaked on MacConkey agar with Sorbitol (SMAC) differential media and were incubated overnight at 37 °C [34]. The following day colorless colonies were picked and were further confirmed by plating on cefixime and tellurite (CT)-SMAC selective media [35]. The colonies which grew on (CT)-SMAC were collected and considered as STEC.
Preparation of Fresh Garlic Extract
The garlic bulbs were washed with double distilled water and wiped with ethanol before homogenizing by crushing in a mortar and pestle. The crushed garlic was placed in a muslin cloth and pressed to obtain fresh garlic extract. The extract was centrifuged at 6000 rpm for 10 min and supernatant was collected as fresh garlic extract (FGE), which was stored at − 80 °C till further use.
Antibacterial Activity Assays
Antibiotic sensitivity and antimicrobial activity of FGE was tested with disc and well diffusion method respectively. In brief, 100 µl of 0.5 McFarland bacterial culture was evenly spread on Luria–Bertani (LB) agar plates. The growth of the isolates was not uniform on Mueller–Hinton Agar (MHA) therefore LB agar plates were used for antibacterial studies. Antibacterial sensitivity was performed using discs of Amoxicillin (Amx-10 µg), Vancomycin (Va-10 µg), Ciprofloxacin (Cip-10 µg), Linezolid (Lz-10 µg), Clindamycin (Cd-10 µg), Lincomycin (L-10 µg), Teicoplanin (Te-30 µg), Nfacillin (Nf-1 µg) and Rifampicin (Rif-15 µg). The antibacterial activity of FGE was tested by puncturing wells in the plates and different volumes (5, 10 and 15 µl) of FGE was added [36, 37]. The plates were incubated at 37 °C for 18 h. The zone of inhibition was measured and recorded around the disc and well after the incubation. Broth serial dilution technique was used to determine MIC in 24 well plates. 750 µl FGE serially diluted with LB media to a final volume of 1500 µl to which 500 µl of 0.5 McFarland inoculum of STEC isolates were added and incubated at 37 °C for 18 h. The concentration were no turbidity was noticed was recorded as the MIC [38].
Biofilm Formation
Biofilm assay was performed as per the protocol described by Merritt et al. [39]. In brief, different STEC isolated were grown in LB media in the presence of 0, 2, 3 and 4% of FGE in 96 well plates for 18 h at 37 °C. After incubation, the wells were washed twice with phosphate buffered saline and once with double distilled water. The biofilm was stained with 100 µl of 0.1% crystal violet for 10 min and rinsed once with double distilled water. Finally, crystal violet was solubilized using 33% acetic acid and OD at 570 nm was recorded (Multiskan GO,Thermo Fisher Scientific) to determine the biofilm formation.
Protein and Carbohydrate Estimation
Protein and carbohydrate content of the biofilm were estimated using protocol mentioned by Kim et al. [40] with some modifications. LN12 isolate (0.5 McFarland) was incubated in 6 wellplate with 0, 0.5 and 1% FGE for 72 h at 37 °C and resultant biofilm was pelleted by centrifugation at 6000 rpm for 10 min and washed once with PBS. Collected samples were dried for 24 h and sonicated in PBS followed by centrifugation at 6000 rpm for 5 min and supernatant was used for protein and carbohydrate estimation. Protein content was estimated by adding Bradford reagent to the supernatant and after 30 min incubation absorbance was recorded at 595 nm (Multiskan GO Thermo Fisher Scientific). To estimate total carbohydrate content, the supernatant was incubated with H2SO4 and 5% phenol for 10 min and absorbance was recorded at 490 nm (Multiskan GO ThermoFisher Scientific).
FTIR Analysis
The powder sample of LN12 biofilm was prepared in the presence of 0 and 1% FGE as mentioned earlier. The powder samples were characterized using Bruker Vertex 70 V FT-IR spectrometer (Bruker Optics), equipped with KBr beam splitters and IR spectra were recorded in transmission mode from 4500 to 1000 cm−1 at a spectral resolution of 4 cm−1. OPUS software version 7.8 was used to perform data analysis.
Statistical Analysis
Experiments were repeated in triplicates three times. The data were subjected to analysis of variance, and mean values in each treatment were compared using Duncan’s multiple range test (DMRT) using SPSS (version 18) at 5% probability (p ¼ 0.05) [41].
Results
Antibiotic Sensitivity of STEC Isolates
The treatment of STEC infections with antibiotics is generally not recommended due to risk of progression to HUS [9, 42]. Although the use of antibiotics for the treatment of STEC infections is discouraged and controversial, to understand the general antibiotic resistance of the isolates antibiotic sensitivity of all eight STEC isolates was performed with eight different antibiotics. According to the results shown in Supplementary Table 1, isolate CKN1 is sensitive to two antibiotics i.e. ciprofloxacin and rifampicin with multiple antibiotic resistances (MAR) index of 0.77 and isolates CKN2, LN12, LN2, LN9.1, LN10.1 and ECD were sensitive to only one antibiotic i.e. rifampicin with MAR index of 0.88 out of nine antibiotics that were tested. Isolate LN6.2 was sensitive to eight antibiotics out of nine that were tested with MAR index of 0.11. The antibiotic sensitivity data indicates that except LN6.2 all isolates had MAR index more than 0.6 making them highly drug resistant STEC isolates.
Antibacterial Activity of Garlic Against STEC Isolates
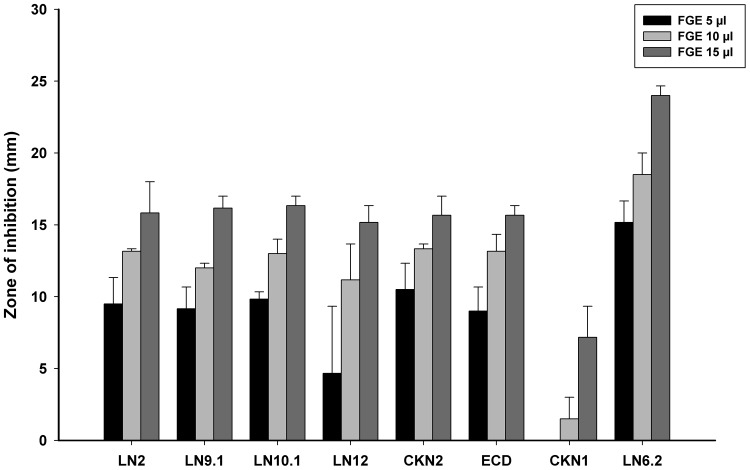
The antibacterial activity of FGE was tested against all of the eight STEC isolates. The MIC value of FGE ranging from 30 to 140 µl/ml against each isolates (Supplementary Table 2) indicates that garlic exhibits antibacterial activity against all STEC isolates. In addition, we also tested the antibacterial activity of FGE using well diffusion assay and Fig. 1 shows the zones of inhibition with 5, 10 and 15 µl of FGE against all the isolates. All the STEC isolates exhibit susceptibility to FGE extract in a dose dependent manner. Both MIC and well diffusion antibacterial assays indicate that garlic exhibits antibacterial activity against STEC isolates including highly drug resistant isolates.
Fig. 1.
Antibacterial activity of garlic against STEC isolates. The graph represents average zone of inhibition (mm) with increasing volume of FGE against STEC isolates as labelled in the figure. The graph shows that there is dose dependent increase in the antibacterial activity of FGE against STEC isolates. The graph represents the data of three sets of triplicate data
Anti-biofilm Activity of Garlic Against STEC Isolates
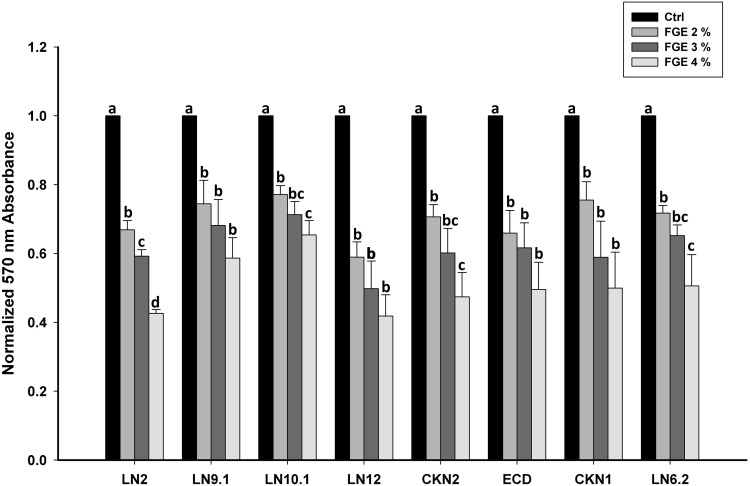
The ability of garlic to inhibit biofilm formation of STEC isolates was also investigated by treating STEC with sub-MIC concentration of FGE. The effect of 2, 3 and 4% of FGE on the biofilm formation ability of STEC isolates is shown in Fig. 2. The data indicates that the FGE reduced biofilm formation of all STEC isolates in a dose dependent manner and the data shown is the average of three independent experiments. The ability of biofilm formation by all STEC isolates was reduced significantly in the presence of 4% of FGE. The data clearly indicates that FGE extract significantly reduces the biofilm formation of all the STEC isolates.
Fig. 2.
Anti-biofilm activity of garlic against STEC isolates. The graph shows the average 590 nm reading of crystal violet staining of biofilm which is normalized to untreated control sample. The effect of increasing percentage of FGE against STEC isolates shows decrease in biofilm formation as labelled. Different letters indicate mean values (n = 3) that are significantly different at p < 0.05, according to DMRT for each different parameter
Garlic Effects the Composition and Content of STEC Biofilm
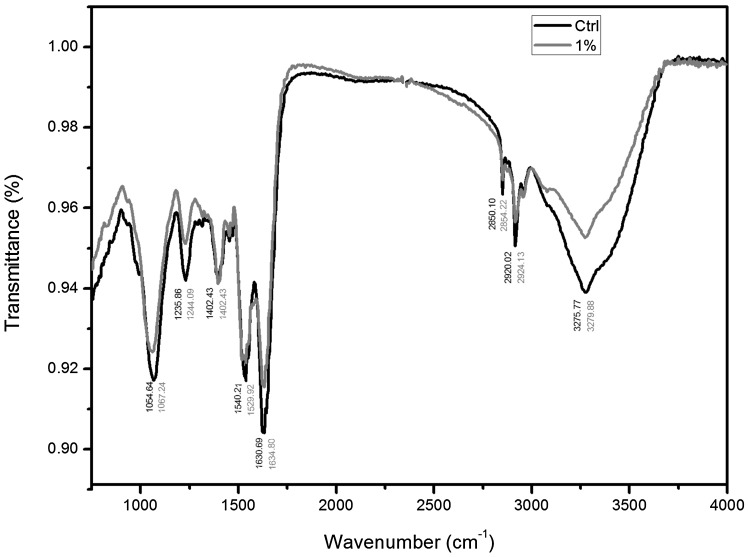
In order to get more insights into the mode of action through which garlic inhibits biofilm formation ability of STEC isolates FTIR analysis was performed. The FTIR analysis of biofilm formed by LN 12 in absence and presence of 1% FGE was analyzed using FTIR. The biofilm sample was obtained with 1% FGE since large amount of sample is required for FTIR analysis which is difficult to obtain considering that in the presence of higher concentration of FGE formation of biofilm is reduced by almost 50%. As expected the FTIR data (Fig. 3) shows peaks in four distinct regions: I-3000 to 2800 cm−1, II-1700 to 1500 cm−1, III-1500 to 1200 cm−1 and IV-1200 to 900 cm−1 that correspond to membrane fatty acids; amides of protein and peptides; proteins, lipids, carbohydrates and phosphate containing molecules (nucleic acids and phospholipids); and polysaccharides and carbohydrates respectively [43]. Although FGE treatment resulted in shifts in all peaks, prominent shifts occurred in peaks 1540, 1235 and 1054 cm−1 that correspond to amide-II of peptides and proteins, nucleic acids and polysaccharides respectively indicating changes in chemical composition of the biofilm. In addition, notable decrease in the intensity of peaks 3275, 1630, 1235 and 1054 cm−1 indicate that amounts of proteins and polysaccharides, proteins, nucleic acids and polysaccharides decreased respectively. Although the amide-I peak intensity decreased there was no change in overall protein content of the biofilm (data not shown). The total carbohydrate content of the biofilm was estimated to understand the effect of FGE on the polysaccharide content of LN12 biofilm. In agreement with the FTIR data the total carbohydrate of biofilm in the presence of 0.5 and 1% of FGE decreased approximately by 20 and 40% respectively compared to untreated control (Supplementary Fig. 1). The data indicates that garlic inhibits biofilm formation ability of STEC by changing the composition of the biofilm and also by reducing the amount of polysaccharide content of biofilm.
Fig. 3.
FTIR analysis of LN12 biofilm. The graph shows that FTIR spectra of LN12 biofilm in the absence of FGE (control-black line) and in the presence of 1% FGE (blue/gray line) (color figure online)
Discussion
The first STEC infection occurred in 1983 due to consumption of undercooked hamburgers in United States [44]. Since then the occurrence and severity of STEC infections has increased and incidence has spread all over the world. Haemorrhagic colitis and diarrhoea caused by STEC is one of the important emerging infectious diseases of recent past. Although, STEC is not considered as one of the main reasons of diarrhoea in India but several articles have been published describing isolation of STEC from patient samples and various food sources [45]. In this study we isolated eight STEC strains from patient and food samples indicating the prevalence of STEC in central and western India. It was noticed that seven of these eight isolates were highly drug resistant. Rifampicin was the only antibiotic to which all the isolates were sensitive and CKN1 was sensitive to both ciprofloxacin and rifampicin. Whereas, isolate LN6.2 was sensitive to all the tested antibiotics except for Nfacillin. The antibiotics resistance was much higher in our isolates compared to a pervious study by Khan et al. [46].
The current treatment options of STEC infection is supportive care in the form of rehydration and administration of antibiotics is discouraged due to exacerbation of Stx production and/or release increasing the chances of HUS progression [7]. Thus, alternative treatment such as antibodies against Stx [47], use of probiotics [48] and divalent metals are being evaluated [49]. In addition, phytochemical such as essential oils and other phytochemicals are also being evaluated for anti-STEC activity [21, 44, 45]. Novel alternative treatment options for STEC infections using natural products have been investigated with encouraging results [19]. Recently, derivatization of cationic polysaccharides has been shown to exhibit activity against E. coli [50]. Similarly, we also wanted to test the anti-STEC activity of garlic which has been reported to have excellent antibacterial activity against many pathogenic bacteria. It has also been reported that garlic exhibits excellent antibacterial activity even against multi drug resistant pathogenic bacteria. As seven of the eight STEC isolates were highly drug resistant. Therefore, we also wanted to test, if garlic exhibits similar antibacterial activity against highly drug resistant STEC isolates. As expect garlic was highly effective against all the STEC isolates including the highly drug resistance isolates. The amount of FGE required for anti-STEC activity was higher for CKN1 isolate compared to other isolates. The MIC for CKN1 was 140 µl/ml whereas zone of inhibition started from 10 µl of FGE. The antibacterial activity of garlic against STEC has been studied earlier [4, 12, 51]. However, these tests were done using standard strains and not with isolates. In addition, in these studies the anti-STEC activity was noticed when garlic was used in combination with other plant products. In the present study, we report anti-STEC activity of FGE against STEC isolates that were highly drug resistance.
Further, we have reported the anti-biofilm activity of garlic against STEC for the first time. On an average the biofilm formation of STEC isolates was decreased by approximately 48 percent at 4% FGE. The anti-biofilm activity of garlic against STEC is of great importance as formation of biofilm plays a very important role in colonization of STEC in reservoir animal and also in persistence at other sites (green leafy vegetables). It was found that garlic alters the biofilm composition and in particular reduces the carbohydrate content of the biofilm while it had no effect on the protein content indicating that garlic reduces biofilm formation by reducing the amount of carbohydrate in the biofilm. This observation is very important because cellulose along with curli protein has synergistic effect on attachment and biofilm development of E. coli [52] and expression of polysaccharide by STEC play a major role in attachment [53]. In addition, expression of cellulose was noticed in E. coli isolated human gastrointestinal tract suggest the role of cellulose in attachment and colonization [54]. Development of novel garlic-organosulfide based agents with anti-biofilm activity against STEC could help in evolution of strategies to tackle the problem of STEC infection and spread. Overall, the present study comprehensively indicates that garlic exhibits antibacterial and anti-biofilm activity against STEC isolates. Thus more research should be directed towards understanding the mechanism of action through with garlic is exhibiting antibacterial and anti-biofilm activities.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was financially supported by UGC-BSR research startup Grant [F, 30-12/2014 (BSR)], Govt. of India, sanctioned to RA. We acknowledge the Department of Chemistry, Dr. Harisingh Gour Central University, Sagar (MP)-India for providing FTIR instrument. We also would like to thank Dr. Kalpataru Das, Department of Chemistry, Dr. Harisingh Gour Central University, Sagar (MP)-India for providing invaluable help with FTIR data. We extend our thanks to Dr. Ashwani Kumar, Department of Botany, Dr. Harisingh Gour Central University, Sagar (MP)-India for his invaluable help with statistical analysis. SBB thanks UGC-RGNF.
Abbreviation
- STEC
Shiga toxin producing E. coli
- MIC
Minimum inhibitory concentration
- FGE
Fresh garlic extract
- EMB
Eosin methylene blue agar
- SMAC
Sorbitol MacConkey agar
- CT-SMAC
Cefixime tellurite sorbital MacConkey agar
- QS
Quorum sensing
Conflict of interest
The authors declare that they have no conflict of interest.
Human and Animal Rights
No human or animal were used in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sushma Bagde Bhatwalkar, Email: sushmabagde123@gmail.com.
Surendra Singh Gound, Email: goundsurendrasingh@gmail.com.
Rajesh Mondal, Email: drrajeshmondal@gmail.com.
Rupesh K. Srivastava, Email: rupesh_srivastava13@yahoo.co.in
Rajaneesh Anupam, Email: rajaneeshanupam@gmail.com.
References
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Clements A, Young JC, Constantinou N, Frankel G. Infection strategies of enteric pathogenic Escherichia coli. Gut Microbes. 2012;3:71–87. doi: 10.4161/gmic.19182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacheco AR, Sperandio V. Shiga toxin in enterohemorrhagic E. coli: regulation and novel anti-virulence strategies. Front Cell Infect Microbiol. 2012;2:81. doi: 10.3389/fcimb.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thorpe CM. Shiga toxin-producing Escherichia coli infection. Clin Infect Dis. 2004;38:1298–1303. doi: 10.1086/383473. [DOI] [PubMed] [Google Scholar]
- 5.Majowicz SE, Scallan E, Jones-Bitton A, Sargeant JM, Stapleton J, Angulo FJ, Yeung DH, Kirk MD. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathog Dis. 2014;11:447–455. doi: 10.1089/fpd.2013.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eppinger M, Cebula TA. Future perspectives, applications and challenges of genomic epidemiology studies for food-borne pathogens: a case study of Enterohemorrhagic Escherichia coli (EHEC) of the O157:H7 serotype. Gut Microbes. 2015;6:194–201. doi: 10.4161/19490976.2014.969979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Control ECfDPa (2015) Annual Epidemiological Report 2016—Shigatoxin/verocytotoxin-producing Escherichia coli infection. Stockholm: ECDC; 2016
- 8.Kumar A, Taneja N, Bharti B, Sharma M (2014) Characterization of Shiga-toxigenic Escherichia coli isolated from cases of diarrhoea & haemolytic uremic syndrome in north India. Indian J Med Res 140:778–784. http://www.ijmr.org.in/text.asp?2014/140/6/778/152463 [PMC free article] [PubMed]
- 9.Freedman SB, Xie J, Neufeld MS, Hamilton WL, Hartling L, Tarr PI, Alberta Provincial Pediatric Enteric Infection T, Nettel-Aguirre A, Chuck A, Lee B, Johnson D, Currie G, Talbot J, Jiang J, Dickinson J, Kellner J, MacDonald J, Svenson L, Chui L, Louie M, Lavoie M, Eltorki M, Vanderkooi O, Tellier R, Ali S, Drews S, Graham T, Pang XL (2016) Shiga toxin-producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin Infect Dis 62:1251–1258. 10.1093/cid/ciw099 [DOI] [PMC free article] [PubMed]
- 10.Xiong Y, Wang P, Lan R, Ye C, Wang H, Ren J, Jing H, Wang Y, Zhou Z, Bai X, Cui Z, Luo X, Zhao A, Wang Y, Zhang S, Sun H, Wang L, Xu J. A novel Escherichia coli O157:H7 clone causing a major hemolytic uremic syndrome outbreak in China. PLoS ONE. 2012;7:e36144. doi: 10.1371/journal.pone.0036144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, Griffin PM. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin Infect Dis. 2009;49:1480–1485. doi: 10.1086/644621. [DOI] [PubMed] [Google Scholar]
- 12.Byrne L, Jenkins C, Launders N, Elson R, Adak GK. The epidemiology, microbiology and clinical impact of Shiga toxin-producing Escherichia coli in England, 2009–2012. Epidemiol Infect. 2015;143:3475–3487. doi: 10.1017/S0950268815000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro VS, Carvalho RCT, Conte-Junior CA, Figuiredo EES. Shiga-toxin producing Escherichia coli: pathogenicity, supershedding, diagnostic methods, occurrence, and foodborne outbreaks. Compr Rev Food Sci Food Saf. 2017;16:1269–1280. doi: 10.1111/1541-4337.12302. [DOI] [PubMed] [Google Scholar]
- 14.Safdar N, Said A, Gangnon RE, Maki DG. Risk of hemolytic uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 enteritis: a meta-analysis. JAMA. 2002;288:996–1001. doi: 10.1001/jama.288.8.996. [DOI] [PubMed] [Google Scholar]
- 15.Wong CS, Jelacic S, Habeeb RL, Watkins SL, Tarr PI. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimmitt PT, Harwood CR, Barer MR. Toxin gene expression by shiga toxin-producing Escherichia coli: the role of antibiotics and the bacterial SOS response. Emerg Infect Dis. 2000;6:458–465. doi: 10.3201/eid0605.000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahal EA, Fadlallah SM, Nassar FJ, Kazzi N, Matar GM. Approaches to treatment of emerging Shiga toxin-producing Escherichia coli infections highlighting the O104:H4 serotype. Front Cell Infect Microbiol. 2015;5:24. doi: 10.3389/fcimb.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luz D, Amaral MM, Sacerdoti F, Bernal AM, Quintilio W, Moro AM, Palermo MS, Ibarra C, Piazza RMF. Human recombinant Fab fragment neutralizes shiga toxin type 2 cytotoxic effects in vitro and in vivo. Toxins. 2018;10:508. doi: 10.3390/toxins10120508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howard-Varona C, Vik DR, Solonenko NE, Li YF, Gazitua MC, Chittick L, Samiec JK, Jensen AE, Anderson P, Howard-Varona A, Kinkhabwala AA, Abedon ST, Sullivan MB. Fighting fire with fire: phage potential for the treatment of E. coli O157 infection. Antibiotics. 2018;7:101. doi: 10.3390/antibiotics7040101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oussalah M, Caillet S, Lacroix M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157:H7 and Listeria monocytogenes. J Food Prot. 2006;69:1046–1055. doi: 10.4315/0362-028X-69.5.1046. [DOI] [PubMed] [Google Scholar]
- 21.Lacombe A, Wu VC, Tyler S, Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. Int J Food Microbiol. 2010;139:102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Borlinghaus J, Albrecht F, Gruhlke MC, Nwachukwu ID, Slusarenko AJ. Allicin: chemistry and biological properties. Molecules. 2014;19:12591–12618. doi: 10.3390/molecules190812591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rattanachaikunsopon P, Phumkhachorn P. Diallyl sulfide content and antimicrobial activity against food-borne pathogenic bacteria of chives (Allium schoenoprasum) Biosci Biotechnol Biochem. 2008;72:2987–2991. doi: 10.1271/bbb.80482. [DOI] [PubMed] [Google Scholar]
- 24.Jain I, Jain P, Bisht D, Sharma A, Srivastava B, Gupta N. Comparative evaluation of antibacterial efficacy of six indian plant extracts against streptococcus mutans. J Clin Diagn Res. 2015;9:ZC50–ZC53. doi: 10.7860/JCDR/2015/11526.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G, Ma X, Deng L, Zhao X, Wei Y, Gao Z, Jia J, Xu J, Sun C. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J Microbiol. 2015;8:e14814. doi: 10.5812/jjm.14814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Santos RR, Fink-Gremmels J. Analyzing the antibacterial effects of food ingredients: model experiments with allicin and garlic extracts on biofilm formation and viability of Staphylococcus epidermidis. Food Sci Nutr. 2015;3:158–168. doi: 10.1002/fsn3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar CG, Anand SK. Significance of microbial biofilms in food industry: a review. Int J Food Microbiol. 1998;42:9–27. doi: 10.1016/S0168-1605(98)00060-9. [DOI] [PubMed] [Google Scholar]
- 28.Saxena P, Joshi Y, Rawat K, Bisht R. Biofilms: architecture, resistance, quorum sensing and control mechanisms. Indian J Microbiol. 2018 doi: 10.1007/s12088-018-0757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalia VC. Quorum sensing inhibitors: an overview. Biotechnol Adv. 2013;31:224–245. doi: 10.1016/j.biotechadv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Kim YG, Cho HS, Ryu SY, Cho MH, Lee J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine. 2014;21:1037–1042. doi: 10.1016/j.phymed.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Kim YG, Shim SH, Lee J. Antibiofilm activities of norharmane and its derivatives against Escherichia coli O157:H7 and other bacteria. Phytomedicine. 2017;36:254–261. doi: 10.1016/j.phymed.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Koul S, Prakash J, Mishra A, Kalia VC. Potential emergence of multi-quorum sensing inhibitor resistant (MQSIR) bacteria. Indian J Microbiol. 2016;56:1–18. doi: 10.1007/s12088-015-0558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biscola FT, Abe CM, Guth BE. Determination of adhesin gene sequences in, and biofilm formation by, O157 and non-O157 Shiga toxin-producing Escherichia coli strains isolated from different sources. Appl Environ Microbiol. 2011;77:2201–2208. doi: 10.1128/AEM.01920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.March SB, Ratnam S. Sorbitol-MacConkey medium for detection of Escherichia coli O157:H7 associated with hemorrhagic colitis. J Clin Microbiol. 1986;23:869–872. doi: 10.1128/jcm.23.5.869-872.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zadik PM, Chapman PA, Siddons CA. Use of tellurite for the selection of verocytotoxigenic Escherichia coli O157. J Med Microbiol. 1993;39:155–158. doi: 10.1099/00222615-39-2-155. [DOI] [PubMed] [Google Scholar]
- 36.Jain S, Mehata MS. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci Rep. 2017;7:15867. doi: 10.1038/s41598-017-15724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal. 2016;6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakri IM, Douglas CW. Inhibitory effect of garlic extract on oral bacteria. Arch Oral Biol. 2005;50:645–651. doi: 10.1016/j.archoralbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Merritt JH, Kadouri DE, O’Toole GA. Growing and analyzing static biofilms. Curr Protoc Microbiol. 2005 doi: 10.1002/9780471729259.mc01b01s00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HS, Park HD. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS ONE. 2013;8:e76106. doi: 10.1371/journal.pone.0076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A, Sharma S, Mishra A. Evaluating effect of arbuscular mycorrhizal fungal consortia and Azotobacter chroococcum in improving biomass yield of Jatropha curcas. Plant Biosyst. 2015;150:1056–1064. doi: 10.1080/11263504.2014.1001001. [DOI] [Google Scholar]
- 42.Mor M, Ashkenazi S. The dilemma of antimicrobial treatment of Shiga toxin-producing Escherichia coli. Pediatr Infect Dis J. 2014;33:979–981. doi: 10.1097/INF.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 43.Lu X, Rasco BA, Jabal JM, Aston DE, Lin M, Konkel ME. Investigating antibacterial effects of garlic (Allium sativum) concentrate and garlic-derived organosulfur compounds on Campylobacter jejuni by using Fourier transform infrared spectroscopy, Raman spectroscopy, and electron microscopy. Appl Environ Microbiol. 2011;77:5257–5269. doi: 10.1128/AEM.02845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, Blake PA, Cohen ML. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 45.Wani SA, Pandit F, Samanta I, Bhat MA, Buch AS (2004) Molecular epidemiology of Shiga toxinproducing Escherichia coli in India. Curr Sci 87:1345–1353. http://www.jstor.org/stable/24109474
- 46.Khan A, Das SC, Ramamurthy T, Sikdar A, Khanam J, Yamasaki S, Takeda Y, Nair GB. Antibiotic resistance, virulence gene, and molecular profiles of Shiga toxin-producing Escherichia coli isolates from diverse sources in Calcutta, India. J Clin Microbiol. 2002;40:2009–2015. doi: 10.1128/JCM.40.6.2009-2015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez EL, Contrini MM, Glatstein E, Gonzalez Ayala S, Santoro R, Allende D, Ezcurra G, Teplitz E, Koyama T, Matsumoto Y, Sato H, Sakai K, Hoshide S, Komoriya K, Morita T, Harning R, Brookman S. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with Shiga-like toxin-producing Escherichia coli. Antimicrob Agents Chemother. 2010;54:239–243. doi: 10.1128/AAC.00343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tahamtan Y, Kargar M, Namdar N, Rahimian A, Hayati M, Namavari MM. Probiotic inhibits the cytopathic effect induced by Escherichia coli O157:H7 in vero cell line model. Lett Appl Microbiol. 2011;52:527–531. doi: 10.1111/j.1472-765X.2011.03037.x. [DOI] [PubMed] [Google Scholar]
- 49.Mukhopadhyay S, Linstedt AD. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science. 2012;335:332–335. doi: 10.1126/science.1215930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel SKS, Kim J, Kalia VC, Lee J. Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol. 2019;59:96–99. doi: 10.1007/s12088-018-0764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta S, Ravishankar S. A comparison of the antimicrobial activity of garlic, ginger, carrot, and turmeric pastes against Escherichia coli O157:H7 in laboratory buffer and ground beef. Foodborne Pathog Dis. 2005;2:330–340. doi: 10.1089/fpd.2005.2.330. [DOI] [PubMed] [Google Scholar]
- 52.Saldana Z, Xicohtencatl-Cortes J, Avelino F, Phillips AD, Kaper JB, Puente JL, Giron JA. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ Microbiol. 2009;11:992–1006. doi: 10.1111/j.1462-2920.2008.01824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matthysse AG, Deora R, Mishra M, Torres AG. Polysaccharides cellulose, poly-beta-1,6-n-acetyl-d-glucosamine, and colanic acid are required for optimal binding of Escherichia coli O157:H7 strains to alfalfa sprouts and K-12 strains to plastic but not for binding to epithelial cells. Appl Environ Microbiol. 2008;74:2384–2390. doi: 10.1128/AEM.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bokranz W, Wang X, Tschape H, Romling U. Expression of cellulose and curli fimbriae by Escherichia coli isolated from the gastrointestinal tract. J Med Microbiol. 2005;54:1171–1182. doi: 10.1099/jmm.0.46064-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.