Abstract
Febrile neutropenia is a medical emergency that complicates the clinical course and treatment of haematological malignancies, significantly enhancing the financial burden and worsening the overall outcome. This study was carried out to evaluate the efficacy of institution’s current first-line antibiotic regimen for febrile neutropenia in view of recent spectrum of institution’s local flora and its susceptibility pattern. 163 episodes of microbiologically documented infections in 110 adult patients were studied over a period of 1 year. Of 110 patients, 61 patients were male. The mean age of the patient population, mean absolute neutrophil count and temperature as documented were 30.1 years (SD ± 16.8), 450 cells/ul, and 101.9 °C respectively. Gram-negative and gram-positive organisms accounted for 79% and 21% of the febrile neutropenic infections respectively. E. coli and Staphylococcus aureus were the most common gram positive and gram negative pathogens respectively. A susceptibility pattern of > 60% was documented for all the gram negative pathogen’s associated febrile neutropenic infections for the current first-line antibiotic combination of Piperacillin/Tazobactum and Amikacin. Comparative analysis of results with the institutional data of 2015 study revealed no statistically significant difference in the resistance pattern of the organisms hence, validating the persistent use of Piperacillin/Tazobacum and Amikacin combination as a potent and efficacious therapy for febrile neutropenia patients with haematological malignancies. However, continuous surveillance remains prudent for the emerging changes in the spectrum and resistance pattern of local flora so that timely revision of empirical antibiotic regimens can save the added financial burdens and associated high morbidity and mortality.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00789-y) contains supplementary material, which is available to authorized users.
Keywords: Febrile neutropenia, Microbiological spectrum, Antibiotic sensitivity, Haematological malignancies, Microbiologically documented infections
Introduction
Advent of aggressive chemotherapy has significantly improved the survival of patients suffering from haematological malignancies but at the stake of increased risk of bacterial infections and sepsis which are a major cause of morbidity and mortality [1]. Febrile neutropenia is a medical emergency which complicates the clinical course and treatment of haematological malignancies [2–4]. It is estimated to cause life-threatening events in 48–60% of patients. Empiric use of antibiotics has declined mortality due to febrile neutropenia from 75 to < 10% [5]. An infectious aetiology is documented in 30–60% of the febrile neutropenia episodes in the setting of haematological malignancies; bacteremia being most common accounting for up to 25% of the cases [6, 7].
A substantial shift has been documented over the last 40 years in the spectrum of organisms causing febrile neutropenia [8]. Gram-positivecocci (especially Staphylococcus aureus) were the main culprit of this devastating condition in the 1950s and 1960s. The advent of Beta lactamase resistant anti-staphylococcal penicillins substantially attenuated gram-positive febrile neuropenic infecions, giving way to a higher incidence and predominance of gram negative pathogen’s associated febrile neutropenic infecions [9].
A second major shift from gram-negative pathogens to gram-positive organisms has collectively been demonstrated in various studies conducted since 1980. Various management-related factors have been implicated in this new surge of gram-positive bugs. Notable among them is the use of intensely toxic chemotherapeutic regimens causing significant damage to upper and lower gastrointestinal mucosa along with the use of quinolone prophylaxis in the high risk febrile neutropenia patients. The quinolone prophylaxis potently suppresses the infections associated with colonizer aerobic gram-negative organisms by inhibiting their growth but remains useless against microaerophillicgram-positive organisms. Similarly, the more frequent use of H2 receptor blockers and proton pump inhibitors increase the risk of infection by gram positive organisms. Furthermore, the widespread use of indwelling central venous access catheters has markedly increased the incidence of gram-positive organisms associated infection and sepsis. However, in recent years a trend back towards the higher incidence of gram-negative infection and sepsis has evolved due to the emergence of antibiotic resistance gram-negative bugs. This is in contrast to the observations of pre-2000 era [10].
The reported mortality rate in febrile neutropenia is 3–8% [11]. Mortality rate as high as 11% is documented due to febrile neutropenia in some haematologic malignancies [12]. Apt management to counter this decimating emergency of chemotherapy includes prompt hospitalization, immediate institution of efficacious broad-spectrum empirical antibiotics, and hyper vigilant monitoring.
As a center majorly dealing with haematological malignancies, this study was carried out to establish the current spectrum of local flora and its susceptibility to the standard first line antibiotic regimen hence, evaluating the efficacy of institution’s current first line antibiotic regimen for febrile neutropenia. A regular review of local flora and the susceptibility pattern is conducted every few years, as per institution’s protocol, to devise better antibiotic policies and to dock the emergence of resistant organisms. Spectrum of microflora and susceptibility patterns are compared with the similar institutional study conducted in 2015 by Mehwish et al. [13] to document any substantial differences in the local flora and any statistically significant differences in their susceptibility patterns.
Materials and Methods
Data was collected from all the patients receiving chemotherapeutic treatment for haematological malignancies at the National Institute of Blood disease and Bone Marrow Transplant hospital from Aug’2017 to Aug’2018, and were reported to have microbiologically documented infections (MDI). Febrile neutropenia was defined as a single reading of oral temperature of 38.5 °C or 38 °C on two or more occasions in 12 h in the presence of absolute neutrophil count of less than 1000cells/mm3. The MDIs were defined as positive blood cultures in the absence of any localizing signs/symptoms or documentation of localized microbiologically documented infections with or without positive blood cultures. Each new admission in a previously afebrile patient with documented MDI was counted as a new episode over the defined one-year course.
After thorough history and examination of each patient, pan cultures inclusive of blood cultures from both central line (if in place) and peripheral blood along with urine cultures and throat swab were sent. Cultures from other sites e.g. pus swab, sputum culture and stool cultures were also sent if localizing signs and symptoms were present. Patients were then started on first line antibiotic regimen that comprised of intravenous piperacillin/tazobactum in dose of90 mg per kilogram of body weight (maximum 4.5 gm)given eight hourly along with intravenous amikacin 15 mg per kilogram body weight given in two divided doses. If fever spikes persisted beyond 48 h after institution of first line empirical antibiotics, repeat cultures were sent and patients were shifted to carbapanem group along with amikacin.
Identification of organisms followed standard bacteriological procedures. Disc Diffusion method was employed to establish the organism’s susceptibility pattern to first line antibiotics i.e. piperacillin/tazobactum and amikacin, the inhibitory zones being 22-mm and 17-mm respectively.
Data was recorded and analyzed by using MS Excel 2010 and SPSS version 23. Frequencies and percentages were calculated for categorical variables, and mean and standard deviation were computed for quantitative variables. Chi square test was applied to observe the association. P value ≤ 0.05 was considered significant.
Results and Discussion
One hundred sixty-three episodes of MDI in 110 patients with febrile neutropenia, suffering from haematological malignancies, were studied over a period of 1 year. Of 110 patients, 61 patients were male. The mean age of the patient population, mean absolute neutrophil count and temperature as documented were 30.1 years (SD ± 16.8), 450cells/ul, and 101.9°Crespectively.Disease distribution revealed acute lymphoid leukemia (44%) as the most common malignancy followed by acute myeloid leukemia (39%). Frequencies of other haematological malignancies are given in Fig. 1.
Fig. 1.
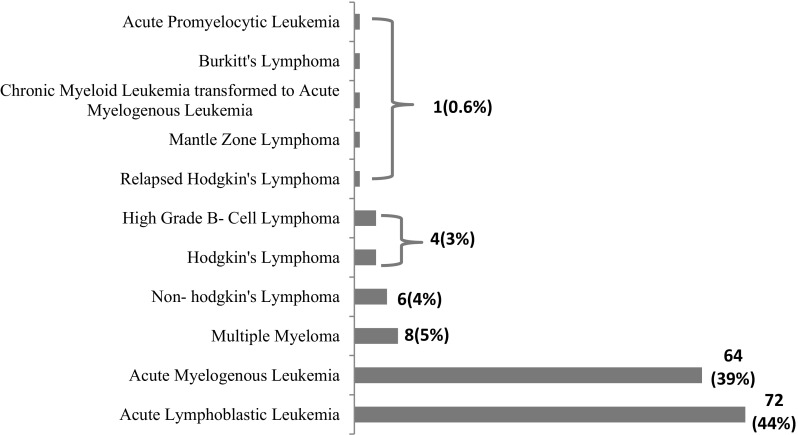
Disease distribution
Isolation Sites, Microbiological Profile, and Sensitivity Patterns
The highest topographical infection site was genitourinary tract followed by blood stream infections (combined central line and peripheral line)Table-S1.Of 163 microbiologically proven infectious episodes, gram-negative organisms accounted for 79% of infections whereas; gram positive organisms comprised 21% of all the infections Table-S2. E. coli and Staphylococcus aureus were the most common gram negative and gram positive isolates respectively. The distribution and frequencies of various pathogens is delineated in Table-S2. Susceptibility patterns of isolated pathogens to piperacillin/tazobactum and Amikacin are elaborated in Table-S3. Comparative analysis, for the statistically significant differences, with the previously published 2015 data is shown in Table-S4.
Febrile neutropenia remains the major cause of morbidity and mortality in patients suffering from and undergoing treatment for haematological malignancies. As an institute dealing majorly with haematological malignancies, knowledge of the local flora of our own institute, their susceptibility patterns, and response to the first line empirical antibiotics are of paramount importance [14].
Blood stream infections are the most common site of microbiologically documented infections in most of the studies [15]. In our study, urinary tract infections (37%) were commonest followed by blood stream infections (35%). The previous institutional study conducted in 2015 demonstrated a higher topographical representation of blood stream infections 43.75%. The topographical microbiological difference between the two studies can be explained by the under-representation of female patients in the 2015 study and consequently an overall reduced incidence of urinary tract infections.
The study showed that the gram-negative organisms are still the major cause of concern accounting for 79% of the neutropenic infections, a finding concurring with multiple previous institutional and regional studies. A recent study conducted in Iran demonstrated gram negative and gram-positive organisms accounting for 67%, and 29.8% of the infections respectively [16]. Similarly, a 2014 study from Saudi Arabia attributed 59.68% infections to gram-negative organisms and 40.32% infections to gram-positive organisms in patients presenting with febrile neutropenia in haematological malignancies [17]. 63.4% gram negative isolates were reported in a 2013 febrile neutropenia study conducted in South India in patients with haematological malignancies, reporting E. coli as the most common bug isolated [18]. Addressing the similar concern regarding the paucity of information on the most prevalent pathogens, a study from Eastern India published their results in 2015 reporting 61.5% and 34.6% gram negative and gram positive isolates respectively, obtained from febrile neutropenia patients suffering from haematological malignancies. The most prevalent gram negative and gram positive organisms were Pseudomonas aeruginosa, and methicillin resistant Staphylococcus aureus respectively [19]. A study published from Turkey in the year 2015 demonstrated gram positive pathogens accounting for 52% of the infections, with Methicillin-resistant coagulase-negative Staphylococci being the most frequent isolate, a finding not concurring with our results [20].
Of the gram negative organisms, E. coli was the predominant cause of infection and again, this finding is not only concordant with the previous findings of the same institute but also with the various international studies conducted in India, Turkey, and Brazil [21, 22].
Other prominent gram-negative pathogens in the decreasing order of frequency included Klebsiella pneumoniae, Pseudomonas aeruginosa, and Pseudomonas spp Table-S2. Although the susceptibility pattern of all these pathogens to institutional first line antibiotics remained more than 60%, an apparent decline in the susceptibility pattern was observed as compared to the results of 2015 data. The comparative analysis though remained statistically insignificant except for the higher sensitivity of Klebsiella pneumoniae to the first line antibiotics. (p value = 0.02) Table-S4.
Multiple case series studies have been conducted at NIBD with the intent to improvise the first line antibiotic regimen and from the first series in 2003 [23] followed by a study in 2006 [24] and then in 2015, there has been a significant decline in the proportion of gram positive infections. However, in this study, the percentage of infections caused by gram-positive infections is marginally increased (21 vs. 15%) as compared to 2015 data. This recent rise can be attributed to a more frequent institutional utilization of central lines as a measure to circumvent patient discomfort associated with multiple pricks and associated phlebitis. Staphylococcus aureus remained the most common gram-positive pathogen. All the Methicillin resistant strains of Staphylococcus aureus were sensitive to Vancomycin. Two cases of Vancomycin resistant Enterococci were documented which were effectively treated with Linezolid.
In conclusion, the persistent higher institutional incidence (79%) of gram negative pathogens associated febrile neutropenia and their substantial sensitivity to piperacillin/tazobactum and amikacin combination validate its use as a potent and efficacious first line empirical therapy. A definitive edge has also been observed associated to this combination in the literature [25]. However, it should be considered highly prudent that continuous surveillance of the local microbiological flora of a institute is performed time and again so that antibiotics regimens can be revised according to the microbiological spectrum and the susceptibility patterns. hence, extracting maximum financial and morality benefit for the patients.
Limitation of this Study
A major limitation of this study is the fact that antibiotic susceptibility to Carbapenem group, which is the cornerstone of our second line empirical combination along with amikacin in the setting of febrile neutropenia, is not analysed, the knowledge of which could have profoundly enhanced our knowledge on the susceptibility patterns of our local flora.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Authors’ Contribution
AJ conceptualized and investigated the study and wrote the manuscript. NF and BK performed the statistical analysis. QR, UZ and MB played their part in searching literature and supervision of project. TS and SS supervised the study and critically reviewed the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This study was approved by the Institutional Review Board and informed consent was taken from the participants prior to the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Babady NE. Laboratory diagnosis of infections in cancer patients: challenges and opportunities. J Clin Microbiol. 2016;54:2635–2646. doi: 10.1128/JCM.00604-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi F, Ashrafi F, Moghaddas A, Derakhshandeh A. Management of febrile neutropenia: a description of clinical and microbiological findings by focusing on risk factors and pitfalls. J Res Pharm Pract. 2018;7:147–156. doi: 10.4103/jrpp.JRPP_18_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13:e552–e561. doi: 10.1200/JOP.2016.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor R, Simalti AK, Roy S, Agarwal P. Clinicohematological profile of febrile neutropenia in childhood acute leukemia and utility of serum procalcitonin levels in neutropenic patients. Indian J Crit Care Med. 2018;22:336–339. doi: 10.4103/ijccm.IJCCM_516_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattaneo C, Quaresmini G, Casari S, Capucci MA, Micheletti M, Borlenghi E, Signorini L, Re A, Carosi G, Rossi G. Recent changes in bacterial epidemiology and the emergence of fluoroquinolone-resistant Escherichia coli among patients with haematological malignancies: results of a prospective study on 823 patients at a single institution. J Antimicrob Chemother. 2008;61:721–728. doi: 10.1093/jac/dkm514. [DOI] [PubMed] [Google Scholar]
- 6.Villafuerte-Gutierrez P, Villalon L, Losa JE, Henriquez-Camacho C. Treatment of febrile neutropenia and prophylaxis in hematologic malignancies: a critical review and update. Adv Hematol. 2014;27:1–9. doi: 10.1155/2014/986938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gustinetti G, Mikulska M. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence. 2016;7:280–297. doi: 10.1080/21505594.2016.1156821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babu KG, Lokanatha D, Lakshmaiah KC, Babu MS, Jacob LA, Bhat GR, Vardhana H, Sinha M, Vijaykumar BR, Sumati BG, Jayshree RS. Bloodstream infections in febrile neutropenic patients at a tertiary cancer institute in South India: a timeline of clinical and microbial trends through the years. Ind J Med Paediatr Oncol. 2016;37:174–182. doi: 10.4103/0971-5851.190352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Lokeshwar N. Febrile neutropenia in haematological malignancies. J Postgrad Med. 2005;51:S42–S48. [PubMed] [Google Scholar]
- 10.Perez F, Adachi J, Bonomo RA. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin Infect Dis. 2014;59:S335–S339. doi: 10.1093/cid/ciu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demirel A, Tabak F, Ar MC, Mete B, Öngören Ş, Yemişen M, Özaras R, Eşkazan E, Başlar Z, Mert A, Soysal T. Secondary infections in febrile neutropenia in hematological malignancies: more than another febrile neutropenic episode. Turk J Haematol. 2015;32:243–250. doi: 10.4274/tjh.2013.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Naurois J, Novitzky-Basso I, Gill MJ, Marti FM, Cullen MH, Roila F. ESMO Guidelines Working Group. Management of febrile neutropenia: ESMO clinical practice guidelines. Ann Oncol. 2010;21:v252–v2566. doi: 10.1093/annonc/mdq196. [DOI] [PubMed] [Google Scholar]
- 13.Taj ME, Farzana T, Shah T, Maqsood S, Ahmed SS, Shamsi TS. Clinical and microbiological profile of pathogens in febrile neutropenia in hematological malignancies: a single center prospective analysis. J Oncol. 2015;2015:1–5. doi: 10.1155/2015/596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005;40:S240–S245. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 15.Karanwal AB, Parikh BJ, Goswami P, Panchal HP, Parekh BB, Patel KB. Review of clinical profile and bacterial spectrum and sensitivity patterns of pathogens in febrile neutropenic patients in hematological malignancies: a retrospective analysis from a single center. Ind J Med Paediatr Oncol. 2013;34:85–88. doi: 10.4103/0971-5851.116184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadegarynia D, Fatemi A, Mahdizadeh M, Movahhed RK, Alizadeh MA. Current spectrum of bacterial infections with nosocomial fever and neutropenia. Caspian J Inter Med. 2013;4:698. [PMC free article] [PubMed] [Google Scholar]
- 17.Sirkhazi M, Sarriff A, Aziz NA, Almana F, Arafat O, Shorman M. Bacterial spectrum, isolation sites and susceptibility patterns of pathogens in adult febrile neutropenic cancer patients at a specialist Hospital in Saudi Arabia. World J Oncol. 2014;5:196–203. doi: 10.14740/wjon850w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakshmaiah KC, Malabagi AS, Govindbabu RS, Sinha M, Jayashree RS. Febrile neutropenia in hematological malignancies: clinical and microbiological profile and outcome in high risk patients. J Lab Physicians. 2015;7:116–120. doi: 10.4103/0974-2727.163126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandal PK, Maji SK, Dolai TK, De R, Dutta S, Saha S, Bhattacharyya M. Micro-organisms associated with febrile neutropenia in patients with haematological malignancies in a tertiary care hospital in Eastern India. Ind J Hematol Blood Transfus. 2015;31:46–50. doi: 10.1007/s12288-014-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Görük M, Dal MS, Dal T, Karakus A, Tekin R, Özcan N, Ayyildiz O. Evaluation of febrile neutropenic patients hospitalized in a hematology clinic. Asian Pac J Trop Biomed. 2015;5:1051–1054. doi: 10.1016/j.apjtb.2015.09.014. [DOI] [Google Scholar]
- 21.Sacar S, Hacioglu SK, Keskin A, Turgut H. Evaluation of febrile neutropenic attacks in a tertiary care medical center in Turkey. J Inf Dev Ctries. 2008;2:359–363. doi: 10.3855/jidc.197. [DOI] [PubMed] [Google Scholar]
- 22.Lima SS, Franca MS, LA MartinhoGH Jesus, Romanelli RMC, Clemente WT. Neutropenic patients and their infectious complications at a University Hospital. Rev Bras Hematol Hemoter. 2013;35:18–22. doi: 10.5581/1516-8484.20130009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamsi TS, Farzana T, Ansari SH, Ahmed A, Ishaque A. Febrile neutropenia in haematological disorders: a single centre review of antibiotic policy and the outcome. J Pak Med Assoc. 2003;53:190–193. [Google Scholar]
- 24.Ansari SH, Nasim S, Ahmed A, Irfan M, Ishaque A, Farzana T, Panjwani VK, Taj M, Shamsi TS. Febrile neutropenia in paediatric peripheral blood Stem cell transplantation, in -vitro sensitivity data And clinical response to empirical antibiotic Therapy. J Coll Phys Surg Pak. 2006;16:704–708. [PubMed] [Google Scholar]
- 25.Ghalaut PS, Chaudhary U, Ghalaut VS, Dhingra A, Dixit G, Aggarwal S. Piperacillin-tazobactum plus Amikacin versus ceftazidime plus Amikacin as empirical therapy for fever in neutropenic patients with hematological malignancies. Ind J Hematol Blood Transfus. 2011;27:131–135. doi: 10.1007/s12288-011-0076-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


