Abstract
Temporary social parasite ant queens initiate new colonies by entering colonies of host species, where they begin laying eggs. As the resident queen can be killed during this process, host colonies may lose their entire future reproductive output. Selection thus favours the evolution of defence mechanisms, before and after parasite intrusion. Most studies on social parasites focus on host worker discrimination of parasite queens and their offspring. However, ant larvae can also influence brood composition by consuming eggs. This raises the question whether host larvae can aid in preventing colony takeover by consuming eggs laid by parasite queens. To test whether larvae could play a role in anti-parasite defence, we compared the rates at which larvae of a common host species, Formica fusca, consumed eggs laid by social parasite, non-parasite, nest-mate, or conspecific non-nest-mate queens. Larvae consumed social parasite eggs more than eggs laid by a heterospecific non-parasite queen, irrespective of the chemical distance between the egg cuticular profiles. Also, larvae consumed eggs laid by conspecific non-nest-mate queens more than those laid by nest-mate queens. Our study suggests that larvae may act as players in colony defence against social parasitism, and that social parasitism is a key factor shaping discrimination behaviour in ants.
Keywords: larvae, ants, recognition, cannibalism, social parasites, colony defence
1. Introduction
Parasitism is the most common lifestyle on Earth [1,2], and parasites can target hosts across the full range of biological complexity, from single cells to individuals, and entire societies. Hosts in turn can fend off parasites via their genetic constitution and/or physiological responses (innate and induced immunity [3–5]), and by adjusting their behaviour to avoid and/or treat infection (behavioural immunity [6]). Over evolutionary time, parasites and their hosts are locked in an arms race, where parasites continually evolve mechanisms to exploit hosts more effectively, while hosts continually evolve better defences [7].
Nests of social insects (ants, bees, wasps, and termites) can be especially attractive to parasites, because they usually contain high densities of genetically similar individuals, and rich resource stores [8]. Within social insects, some species have evolved to parasitize other social insects [9–12]. Social parasites are especially numerous in ants, where they fall roughly into three categories: permanent inquilines, slave-makers, and temporary social parasites [9,13]. Permanent inquilines coexist with the host species, whereas slave-making ants raid other colonies to capture and enslave their brood [13,14]. In temporary social parasite species, parasite queens invade host colonies, kill the host queen(s), initiate egg-laying, and take advantage of the brood care behaviour of host workers. If successful, this can lead to the loss of the entire future reproductive output of the host colony [9].
Given these high stakes, hosts of temporary social parasites are predicted to invest in defence mechanisms both pre- and post-infection [15–17], whereas social parasites should evolve ways to overcome these defences. Social parasite queens need to enter the host colony, be accepted by the host workers, and ensure that the host workers rear their offspring. The host can either prevent social parasite queens from entering the nest and initiating egg-laying (pre-infection defence), or discriminate against parasite offspring (post-infection defence). In ants, discrimination against intruders is typically based on chemical cues, which consist of a mixture of cuticular hydrocarbons [18,19]. In ant populations routinely exposed to temporary social parasites, host workers are better at discriminating against parasites compared to host workers in populations that are parasite-free [20]. Yet, this first line of defence can fail, allowing a parasite queen to succeed in entering a host nest, where she may avoid detection by blending into the host colony using chemical mimicry (i.e. biosynthesis of host cues), or camouflage (actively or passively obtaining host cues) [9,21–23]. Once a parasite queen has managed to enter a host nest undetected, targeting the parasite offspring is the only way a host colony may still manage to alleviate the costs of parasite invasion [17,24–26].
Host species may harness their own larvae to consume eggs laid by social parasites, and so defend their nest if a social parasite queen has successfully entered the colony. Indeed, studies on Formica ants show that larvae can influence the composition of brood within nests by consuming eggs, and that they cannibalize less when surrounded by eggs that are close kin [27,28]. In the context of nest defence against social parasites, host larvae should adjust their behaviour according to egg identity, with higher levels of consumption expected when the host larvae encounter parasite eggs, compared to non-parasite eggs. In addition, as cannibalism directed towards relatives potentially results in inclusive fitness costs, consumption of related eggs should be avoided [28]. Much like in adults, the surface chemistry of eggs is predicted to play a key role in such discriminatory behaviour [27,29–32].
To test whether larvae could play a role in nest defence against social parasites, we compared the rates at which larvae of the ant Formica fusca, a common host of temporary social parasites [33], consumed eggs laid by a queen that was a social parasite, a non-parasite, a nest-mate, or a conspecific non-nest-mate. For each species, we documented hatching success of eggs and egg size, as these traits may also influence a larva's decision to engage in egg consumption [34,35]. We also determined the sex of larvae, because sex has been shown to play a role in larval propensity to cannibalize [27]. Finally, in order to exclude that larvae are merely responding to chemical dissimilarity, rather than parasite status per se, we measured chemical similarity between eggs of all species. The results of this study allow us to assess both the potential ecological role of larvae in host–social parasite systems, and give further insight into the propensity of ant larvae to discriminate among eggs of different origins.
2. Methods
Our focal species, F. fusca, is a common species in Finland, and inhabits forest clearings as well as other types of semi-open habitats [33,36]. It is often the first ant species to colonize a clear-cut forest, dominating the ant fauna for the first several years [37,38]. As the ecological succession of the forest advances, species that found colonies through temporary social parasitism follow, replacing F. fusca [39]. The discrimination abilities of this species are very precise, and adult workers are known to discriminate against both foreign workers and queens, as well as foreign brood [20,25,26,40–43].
To test whether the propensity of F. fusca larvae to consume eggs differs depending on the origin of eggs, we collected entire colonies of the host species F. fusca (n = 28). In addition, we collected colony fragments with queens and workers of three social parasite species (Formica pressilabris, n = 5; Formica exsecta, n = 10; Formica truncorum, n = 7), and two non-parasite species (Formica cinerea, n = 13; Formica lemani, n = 7) on the Hanko peninsula in southwestern Finland in the vicinity of Tvärminne Zoological Station. After collection, the ants were kept in the dark at +4°C in the laboratory, until the onset of the experiments. Nineteen of the 28 field-collected F. fusca colonies were set aside to produce larvae (host nests). These nests contained 3–14 queens and approximately 200 workers, and were housed at room temperature in 30 × 20 × 5 cm glass-roofed nests (Ytongnest, Antstore) with six nest chambers, a watering chamber, and a chamber leading to a feeding arena. The glass tops of the nest-boxes were covered with red plastic sheets to allow easy observation of brood development yet prevent light from disturbing the ants. Water was supplied via the watering chamber, and the ants were fed Bhatkar diet [44] daily.
The remaining nine F. fusca colonies, and all parasite and non-parasite colony fragments, were designated as egg donor nests (donor nests) (table 1). To obtain eggs for the bioassays, queens of the donor nests, kept at+4°C until this time point, were isolated on individual Petri dishes without workers, and allowed to lay eggs. This ensured that the eggs were free of possible nest-derived cues. In each case, the queens originated from several different field colonies (table 1). To obtain nest-mate, and in some cases conspecific non-nest-mate eggs, queens were also isolated from the host nests (table 1). All egg donor queens were kept in the dark at room temperature with strips of wet sponge cloth to ensure adequate humidity, and freshly laid eggs (1–3 days old) were picked from the Petri dishes for the bioassays.
Table 1.
Experimental set-up in numbers. Larvae of host nests were offered eggs laid by either conspecific or heterospecific queens.
| egg donors |
hosts (Formica fusca) |
|||||
|---|---|---|---|---|---|---|
| colonies | queens | colonies | mean replicates per colony (range) | replicates | ||
| conspecific | ||||||
| nest-mates | F. fusca | 9 | 21 | 9 | 3 (2–14) | 56 |
| non-nest-mates | 13 | 20 | 19 | 8 (3–13) | 154 | |
| heterospecific | ||||||
| non-parasites | F. cinerea | 13 | 17 | 19 | 7 (3–16) | 139 |
| F. lemani | 7 | 20 | 19 | 8 (2–16) | 148 | |
| parasites | F. exsecta | 10 | 14 | 18 | 7 (2–14) | 139 |
| F. pressilabris | 5 | 22 | 19 | 8 (2–16) | 144 | |
| F. truncorum | 7 | 17 | 19 | 7 (1–18) | 141 | |
(a). Bioassays
The F. fusca larvae to be used in bioassays were removed from their respective nests, and sorted visually by size. The number of larval development stages in F. fusca is not known, but related species exhibit three (Formica japonica) or four (F. polyctena) larval instars (reviewed in [45]). Based on our visual inspection of larvae (see electronic supplementary material, figure S1), only young, i.e. first or second instar larvae were included in the experiments. For each set of replicates, we placed seven visually size-matched larvae individually on a Petri dish. We then presented the larvae with an egg produced by: (i) a nest-mate F. fusca queen, (ii) a non-nest-mate F. fusca queen, (iii) a non-parasite F. cinerea queen, (iv) a non-parasite F. lemani queen, (v) a parasite F. exsecta queen, (vi) a parasite F. pressilabris queen, or (vii) a parasite F. truncorum queen (table 1). As larvae are practically immobile, we placed each larva on top of an egg, with its mouthparts touching the egg, and added strips of wet sponge cloth around the larvae to maintain moisture. We counted the number of eggs consumed after 24 and 48 h. For 170/921 replicates (ca 18%), the number of consumed eggs was counted only once after 24 h, due to time constraints. We decided to include these replicates in the final analysis because there was no difference in the number of eggs consumed during the first 24 h in the 24 h replicates compared to the 48 h replicates (generalized linear model (GLM), T921,1 = 0.581, p = 0.562). Larvae consume eggs by piercing the eggshell and feeding on its contents. Hence, we counted an egg as consumed, when either only the eggshell remained or we observed a larva actively feeding on an egg (figure 1, for an additional video of cannibalistic behaviour of larvae, see [46]). Although we always set up one replicate per treatment in parallel, with visually size-matched larvae, larval mortality and/or lack of suitable eggs led to differences in the final number of replicates (table 1).
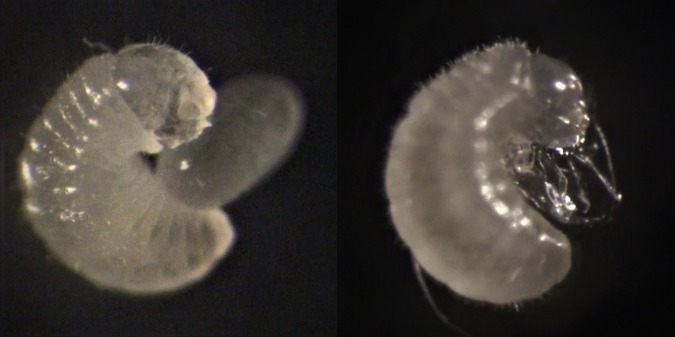
Figure 1.
Formica fusca larva consuming a F. pressilabris egg, with the remains of the eggshell in the picture on the right. (Online version in colour.)
(b). Determination of larval sex
Given that male Formica larvae show a higher propensity to cannibalize [27,28], we determined the sex of F. fusca larvae (diploid females and haploid males) from genotype data [28]. A subset of larvae used in the bioassays (n = 486, 5–68 larvae from each of the 19 host colonies) were collected in individual Eppendorf tubes with 90% ethanol. Larvae were dried on a paper towel, placed in individual wells with 2.5 : 100 µl Proteinase K–Chelex (6%) solution for DNA extraction, left to incubate overnight at 56°C, and inactivated at 100°C for 10 min. Samples were then amplified at eight microsatellite loci previously tested in F. fusca (Fe19, Fe51, Fl12, Fl20, Fl21, Fy13, Fy4, Fy7 [28]), using the QIAGEN Type-it Microsatellite Multiplex Protocol, with 5 µl Type-it multiplex buffer, 3 µl deionized water, 1 µl optimized primer mix, and 1 µl extracted DNA per sample. Reactions were run according to QIAGEN recommendations. PCR products were analysed in a 3730 ABI sequencer (Applied Biosystems), and microsatellite peaks scored individually using Genemapper software (5.0 Applied Biosystems).
We scored individuals as females if they were heterozygous at one or more loci and as males if they were homozygous at all eight loci. For cases where not all eight loci amplified successfully (63/486 genotyped larvae), we calculated the probability of wrongly assigning a larva as a male, as in Schultner et al. [28], and scored larvae with a misclassification probability below 3% as males (six male candidates excluded). For each host nest, the average larval sex ratio was calculated from sexed individuals (from 0, all females, to 1, all males), and this score given to the six larvae with inconclusive sex, as well as the rest of the larvae not sampled (the remaining 435 larvae).
(c). Egg size and hatching success
The size of eggs—relative to the size of larvae—may influence the propensity to consume eggs, because large egg size may act as a barrier to consumption [35]. We therefore compared egg sizes between species by photographing and measuring the length (longest measurable distance) of eggs from one to five colonies per species (n = 40–72 eggs per species, table 2) using the ImageJ software [47]. In ants, queens and workers can produce trophic eggs destined for consumption [48]. These eggs are not viable, and are preferred by larvae over viable eggs [34]. To estimate whether differences in the rates of egg consumption could be caused by differential production of trophic eggs across species, we measured egg hatching success by placing clean, freshly laid eggs (3–5 colonies per species, 4–7 queens each, n = 36–65 eggs per species, table 2) on a Petri dish with small sponge cloth strips for moisture. Petri dishes were kept in the dark at room temperature. We checked the eggs daily, documented hatching success and removed hatched eggs. Fungus-infested or otherwise compromised eggs were removed, and their spot on the Petri dish cleaned with cleaning alcohol to contain infections.
Table 2.
Species-specific measures of average egg consumption, egg size, and egg hatching success.
| egg consumption | egg size |
egg hatching successa |
||||
|---|---|---|---|---|---|---|
| egg donor class | mean % (2.5 and 97.5% percentiles) | mean (mm) ± s.d. | n | % | n | |
| F. fusca | nest-mate | 0 (0, 0) | 0.87 ± 0.07 | 44 | 63.63 | 44 |
| non-nest-mate | 9.09 (4.55, 13.63) | |||||
| F. cinerea | non-parasite | 6.47 (2.87, 10.79) | 0.87 ± 0.06 | 69 | 5.67 | 53 |
| F. lemani | non-parasite | 4.05 (1.35, 7.43) | 0.98 ± 0.12 | 40 | 57.50 | 40 |
| F. exsecta | parasite | 11.51 (6.47, 17.27) | 0.62 ± 0.04 | 72 | 44.44 | 36 |
| F. pressilabris | parasite | 16.67 (10.42, 22.92) | 0.70 ± 0.10 | 64 | 7.69 | 65 |
| F. truncorum | parasite | 7.09 (3.53, 11.35) | 0.84 ± 0.06 | 40 | 50.00 | 40 |
aEgg hatching success percentage refers to eggs that hatched during the observation period, whereas those that did not hatch succumbed to infection.
(d). Chemical analysis of egg surface hydrocarbons
Data on egg surface hydrocarbons were obtained from Helanterä & d'Ettorre [31] (available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.38204) for five of the six study species. (F. fusca, F. lemani, F. cinerea, F. pressilabris, and F. exsecta (=F. fennica [49])). We obtained chemical profiles of F. truncorum eggs by sampling eggs laid by queens isolated on Petri dishes. As in Helanterä & d'Ettorre [30], we sampled two pools of 10 eggs each, as well as 15 single eggs (three eggs from five colonies), which were placed in 2 ml glass vials (Sigma Aldrich), and frozen at −20°C. For the analysis of surface chemicals with gas chromatography–mass spectrometry (GC–MS), 10 µl of pentane (HPLC grade) was added to the thawed sample vials for 1 min, after which 2 µl of the extract was injected into a Thermo Fisher Scientific Trace 1300 series gas chromatograph, with a Restek RXi-5sil MS column (20 m × 0.18 mm × 0.18 µm), a splitless injector and a Thermo Fisher Scientific ISQ series Mass Spectrometer. Helium was used as the carrier gas, and after an initial hold of 2 min at 70°C, the temperature was raised to 200°C at a rate of 20°C min−1 and then to 250°C in 3°C steps min−1 and finally to 320°C in 5°C steps min−1, where it was held for 3 min. The egg surface hydrocarbons of F. truncorum were identified and integrated as in Helanterä & d'Ettorre [30], using individual samples to estimate the surface area of each hydrocarbon peak, and pooled samples to identify the compounds (see electronic supplementary material, figure S2 shows a typical egg chemical profile of F. truncorum). Only peaks that represented greater than 1% of the total area in at least one F. truncorum sample were retained.
To obtain an estimate of the chemical distance between different types of eggs, we first compiled a dataset containing the raw peak areas of all compounds that represented greater than 1% of the total area in at least one sample within a species. This dataset contained 14–72 individual egg samples from 4 to 9 nests of each species. To compile the chemical data, we had to pool some compounds present on the eggs of F. fusca and F. cinerea (methyls and dimethyls of C25 and C26), because these were not identified more precisely in Helanterä & d'Ettorre [30] (see electronic supplementary material, table S1). This only affects the chemical distance between eggs of these two species, so the impact on the overall results is most probably small. Nonetheless, pooling may have affected the estimates of chemical distance within F. fusca (i.e. between nest-mate eggs, between nest-mate and non-nest-mate eggs), because dimethyls of C25 are expected to play an important role in F. fusca nest-mate recognition [50]. The dataset also contained some coeluting compounds, so to estimate the peak areas of compounds that were assigned to a single peak in Helanterä & d'Ettorre [30], we divided each peak area in half and assigned one half of the total area to each of the two compounds. There were three such peaks representing six compounds in F. fusca profiles, one of which was also present in F. cinerea profiles (see electronic supplementary material, table S1). Again, the possible error introduced by this method is negligible, because any effect on chemical distance is the same for all comparisons. Finally, because the profiles differed in their overall composition across species, and some compounds were absent in one or several species (see electronic supplementary material, table S1), we added a peak area of 1 for all absent compounds. This was necessary because the transformations required in our statistical analysis demand non-zero data. Across all species and compounds, the mean peak area was 85 750 000 with a range of 6127–1.97 × 10+10, thus we are confident that substituting zero values with 1 had no effect on the final results (see electronic supplementary material, table S1).
In order to estimate the chemical distance between host nest-mate and non-nest-mate eggs, as well as between host and heterospecific eggs based on quantitative comparisons, we first transformed the raw peak areas according to Aitchison [51]. We then reduced variation with a principal component analysis (command princomp from library MASS). We retained the first five principal components according to their eigenvalues and the scree plot [52] (see electronic supplementary material, table S2). We calculated the average coordinates of the five principal components for each sample and used these averages to calculate the pairwise Euclidean distance between all samples. Estimates of chemical distance were calculated as the average distance between F. fusca samples, and samples of each of the other five species. Chemical distance between nest-mate and non-nest-mate eggs was estimated as the average distance between F. fusca samples from different nests. Finally, estimates of chemical distance between F. fusca nest-mate eggs were obtained by calculating the average distance between samples from the same nests.
(e). Statistical analyses
To analyse the effect of egg donor class on egg consumption, we used a mixed logistic regression model (generalized linear mixed model, GLMM). We excluded data from the nest-mate treatment from all analyses, as no nest-mate eggs were consumed. The full model contained egg consumption as a binomial response variable, three explanatory variables: egg species nested within egg donor class (three levels: parasites, non-parasites, conspecific non-nest-mates), and larval sex ratio, as well as two random variables: donor nest, and host nest (function glmer, package lme4). Non-significant variables were sequentially removed from the model, and the reduced models compared to the full model using log-likelihood tests (function lrtest, package lmtest), until only significant terms remained. Our final model contained egg consumption as a binomial response variable, egg donor class as a fixed explanatory variable, and donor nest and host nest as random variables.
We then tested whether the hatching success of eggs from different egg donor classes differed, using a logistic regression model (GLM), with egg hatching success as a binomial response variable, and egg donor class as the explanatory variable. We furthermore tested whether the egg sizes of conspecifics, parasites, and non-parasites differed, using a GLM with egg size as the response variable, and egg donor class as the explanatory variable. Finally, we analysed the effect of chemical similarity on egg consumption using a binomial GLM with average egg consumption as the response variable, and the average chemical distance as the explanatory variable. Given that the experimental eggs had been consumed, we could not measure individual eggs used in the assays for egg hatching success, egg size, or chemical distance. Instead, we used representative values for each species (data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q499457 [53]), which consequently were highly collinear with species/donor class. Therefore, we could not include these variables in the model testing the effect of egg donor class on egg consumption, without either seriously inflating sample size or overfitting the model. We thus tested the importance of these variables separately, as described above. All analyses were run in R, v. 3.5.1 [54], and binomial GLM(M) models with logit link.
3. Results
(a). Egg consumption
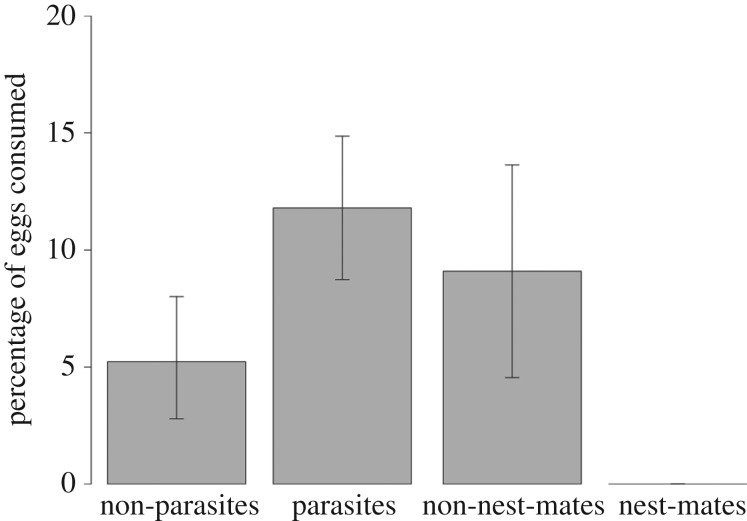
When confronted with heterospecific eggs, F. fusca larvae consumed between 4.1 ± 3.5% CI (F. lemani (non-parasite) eggs) and 16.7 ± 6.4% (F. pressilabris (parasite) eggs) of the eggs offered (table 2). Parasite eggs were consumed at a significantly higher rate than non-parasite eggs (11.8 ± 3.2% and 5.2 ± 2.8%, respectively) (GLMM, Z865,3 = −2.05, p = 0.041, figure 2). We recorded no cannibalism incidents when larvae were offered conspecific nest-mate eggs, but conspecific non-nest-mate eggs were cannibalized in 9.1 ± 4.9% of cases. The rate at which conspecific non-nest-mate eggs were consumed, compared to parasite or non-parasite eggs, did not differ (GLMM, parasite eggs: Z865,3 = 0.95, p = 0.34; non-parasite eggs: Z865,3 = 0.77, p = 0.44, figure 2). The sex ratios of larvae from host nests ranged from 0 (all females) to 0.8 (mostly males), with an on average strongly female-biased sex ratio of 0.06 ± 0.18 (mean ± s.d.). Larval sex ratios did not affect egg consumption levels significantly (GLMM, Z865,3 = 1.51, p = 0.13).
Figure 2.
Average rates of egg consumption by F. fusca larvae, when offered non-parasite eggs (n = 287), parasite eggs (n = 424), non-nest-mate eggs (n = 154), or nest-mate eggs (n = 56), with 2.5 and 97.5% quantiles.
Species-specific egg sizes ranged from 0.62 ± 0.04 mm (mean ± s.d.) in F. exsecta (parasite), to 0.98 ± 0.12 mm in F. lemani (non-parasite) (table 2). The eggs of parasite species were significantly smaller than those of non-parasite species (GLM: T329,1 = −16.76, p < 0.001). The size of F. fusca eggs was intermediate between non-parasites and parasites, being significantly larger than parasite eggs and smaller than non-parasite eggs (GLM: T329,1 = 9.90, p < 0.001, and T329,1 = −2.10, p = 0.040, respectively). Egg hatching success ranged from 6 to 64% (table 2), and we found no significant difference in the hatching success of eggs from parasite versus non-parasite species (GLM: Z278,1 = 0.19, p = 0.853). The hatching success of F. fusca eggs was higher than that of the other species (GLM (F. fusca—parasites): Z278,1 = 3.99, p < 0.001; GLM: (F. fusca—non-parasites) Z278,1 = 3.87, p < 0.001). The low hatching success rates in F. cinerea (non-parasite), and F. pressilabris (parasite), were probably caused by fungal infections. Owing to the limited number of eggs produced by these species, we could not monitor more eggs, but we saw no sign of trophic egg production, as all eggs that did not succumb to fungus infection, hatched successfully.
(b). Egg surface chemistry
The Formica egg profiles encompassed on average 21 compounds (range: 14–37), and included alkanes, alkenes, and mono-, di-, and tri-methylated alkanes ([30]; this study). Most species had the same major alkanes and alkenes, but differed in the number of methylated compounds (see electronic supplementary material, table S1; [30]). The egg profiles of F. truncorum (parasite), analysed in this study, consisted of 13 major alkanes and alkenes (see electronic supplementary material, table S1 and figure S2), as well as several methylated compounds, none of which reached the cut-off at greater than 1% relative abundance. We also identified several prominent heavy compounds (greater than C32, see electronic supplementary material, figure S2), which could not be included in the comparison, as heavier compounds were not identified in the Helanterä & d'Ettorre [30] dataset.
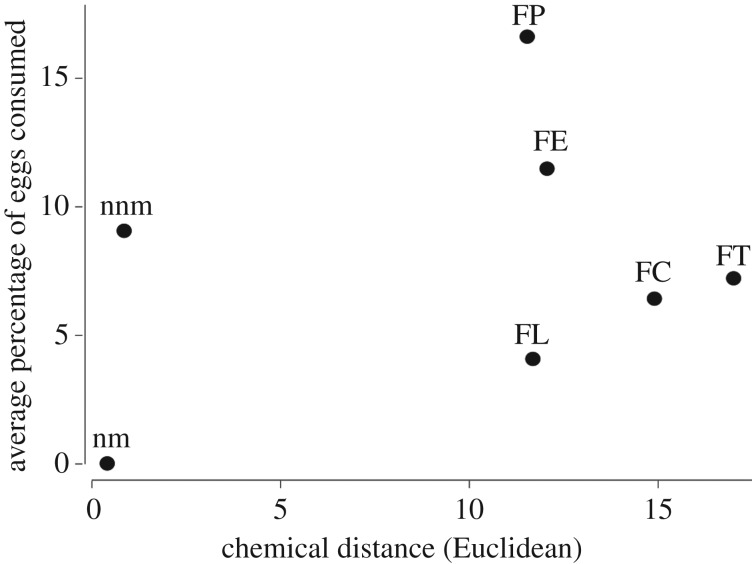
The chemical distances of egg odours between the host (F. fusca), and the other species ranged from 11.53 ± 0.45 (F. pressilabris (parasite)) to 17.05 ± 0.48 (F. truncorum (parasite)) (figure 3), and did not reflect phylogenetic patterns. For example, F. lemani (non-parasite) eggs are chemically strikingly different from F. fusca eggs ([30] figure 3), although these two species are closely related [50,55,56]. The average chemical distance between eggs from different F. fusca nests was 0.80 ± 0.31, whereas the distance between F. fusca nest-mate eggs was shorter, averaging 0.41 ± 0.23. We found no association between the average chemical distance between species, and the proportion of eggs consumed in each category (GLM: Z7,1 = 0.15, p = 0.879, figure 3), but we note that the statistical power of the test is low due to small sample size.
Figure 3.
Average percentage of eggs consumed, plotted against average pairwise chemical distances among F. fusca eggs and between F. fusca eggs and eggs of the other species. FE, F. exsecta (Euclidean distance: 12.03 ± 0.62); FP, F. pressilabris (11.53 ± 0.45); FT, F. truncorum (17.05 ± 0.48); FC, F. cinerea (14.84 ± 0.59); FL, F. lemani (11.66 ± 0.44); nnm, F. fusca non-nest-mate (0.80 ± 0.31); nm, F. fusca nest-mate (0.41 ± 0.23).
4. Discussion
In agreement with our predictions, larvae of the ant F. fusca, a common host to temporary social parasites, discriminated against eggs depending on their origins. Eggs produced by social parasite queens were consumed at a higher rate than eggs produced by non-parasite queens. Conspecific non-nest-mate eggs were consumed at a rate similar to those produced by social parasite queens, whereas nest-mate eggs were left untouched. Egg consumption levels did not depend on chemical distance between the eggs.
Larvae consumed social parasite eggs at a significantly higher rate than those of non-parasites. This suggests that larvae participate in post-infection defence by selectively removing parasite eggs, thus acting as a second line of defence, and potentially improving colony survival [17,24–26,57]. Earlier studies have shown, that while workers of social insect hosts destroy social parasite eggs [24–26,29,58,59], workers of many ant species forgo discrimination between nest-mate and non-nest-mate eggs [60–63]. However, F. fusca workers in southern Finland can, and do, discriminate against both social parasite and non-nest-mate eggs [25,41,42,64]. As colonies of F. fusca are under high parasite pressure in this area, they even exhibit better overall discrimination abilities compared to populations without parasites [20]. Our study suggests that parasite pressure may also shape the behaviour of immature stages, and echoes earlier results that Formica ant larvae may have precise recognition abilities [27,28].
How can investing in a second line of defence pay off after the demise of the host queen? There are several avenues to benefits. Hosts can rebel by killing the parasite brood, or by reproducing in the presence of the parasite queen [17]. Host workers may also gain direct fitness by producing unfertilized male-destined eggs [25,26,65,66], or in the case of primitively eusocial species, also fertilized eggs [67]. Consumption of social parasite eggs can furthermore benefit host larvae directly, for instance, by increasing their survival [27]. Any female brood of the host queen present in the colony at the time of parasite takeover may also benefit from larval defence, as female F. fusca larvae are more likely to develop into new queens—with direct fitness returns in its wake—in the absence of the queen [65]. Larvae as a secondary line of defence may, however, not work for species that (unlike F. fusca) maintain their eggs and larvae separately [34,68], or which have sterile workers incapable of producing male offspring, and thus unable to gain direct fitness in the absence of a queen [69]. In addition, some parasite species may inhibit their host from reproducing (complete or partial parasitic castration) [67,70,71].
Larvae also cannibalized conspecific non-nest-mate eggs, leaving their nest-mate eggs untouched. This result is in agreement with inclusive fitness models of cannibalism, which predict that individuals should avoid consuming relatives [28,35]. Earlier studies also found that Formica ant larvae cannibalized eggs at a lower rate when relatedness between larvae and eggs was high [27,28]. Moreover, these studies found that female larvae are less likely to engage in cannibalism of kin than their male counterparts [27,28]. In the present study, the proportion of female larvae was extremely high, which may explain why we did not observe any acts of nest-mate cannibalism.
Ultimately, the precise recognition abilities of both adults [20,25,26,32,40–43,64] and larvae of F. fusca may have emanated from the threat of intrusion by social parasite queens, conspecific queens, or both. For example, discrimination against alien conspecific eggs occurs in the social wasp Polistes biglumis, and it has been suggested that brood discrimination abilities evolved as counter-adaptations against intra- and interspecific social parasitism [59]. Similarly, in ants of the genera Myrmica and Solenopsis, conspecific queens can act as intraspecific parasites [72,73]. In F. fusca, relatedness among nest-mate queens is highly variable [40,74], and several mitochondrial lineages may be present within a colony (H Johansson 2017, personal communication). This suggests the presence of intruder queens, which may pose an inclusive fitness cost to the resident workers and brood, and thus select for precise recognition abilities.
The production of more trophic (i.e. non-viable) eggs by parasite, compared to non-parasite queens, could have generated a pattern with higher consumption rates of parasite eggs [34]. However, we found no evidence for consistent differences in hatching success between non-parasite and parasite eggs. Furthermore, the production of trophic eggs was probably negligible, because egg mortality was in most cases due to infection, including in the two species with very low hatching success. Finally, the hatching success of F. fusca eggs was significantly higher than that of the other species, yet F. fusca eggs were consumed either at relatively high rates (non-nest-mate eggs) or not at all (nest-mate eggs). This suggests that egg viability as such does not influence larval egg consumption behaviour.
The relative size of intraspecific prey (in this case the egg) to predator (in this case the larva) may also influence egg consumption behaviour, with relatively smaller prey more likely to be consumed [75]. In agreement with this, our species-level measurements show that parasite eggs are, on average, smaller than non-parasite eggs. Nonetheless, two lines of evidence suggest that egg size is not the sole determinant of larval behaviour. First, larvae readily consumed non-nest-mate F. fusca eggs, but did not consume nest-mate eggs (see electronic supplementary material, figure S3), yet the eggs show little intraspecific variation in size (this study [76]). Second, if egg size is the sole determinant of egg consumption, the smallest eggs (F. exsecta (parasite)) should be eaten at the highest rate, yet, F. pressilabris (parasite) eggs, which are larger, were eaten at a 1.5-fold rate compared to F. exsecta (see electronic supplementary material, figure S3). These results suggest that egg size is neither a strict cue inducing egg consumption (i.e. ‘always eat smallest eggs'), nor a hindrance preventing egg consumption (i.e. ‘unable to eat large eggs’).
The chemical distance between F. fusca eggs and eggs of the five species did not influence the rate of egg consumption, which agrees with earlier observations that chemical profiles of parasite eggs are not more similar to each other than those of non-parasite eggs [30]. However, this does not mean that larvae do not use chemical information when making their behavioural decision. In fact, eggs carried both species- and nest-specific odours ([30], this study), indicating that sufficient chemical information is available to larvae to discriminate against both con- and heterospecific intruders. When it comes to estimates of chemical distance within F. fusca (i.e. between nest-mate eggs, and between nest-mate and non-nest-mate eggs), pooling potentially important compounds for nest-mate recognition [50] has likely underestimated the chemical distances. This prevents us from detecting a possible effect of chemical distance on the consumption of conspecific non-nest-mate eggs.
As the sensory modalities of ant larvae are virtually unexplored, we can speculate that larval egg recognition is likely a complex process, contingent on a combination of different cues. One possible scenario is that larvae use both egg size and egg odour as cues. Thus, larvae may preferentially eat small (i.e. parasite) eggs irrespective of their odour, and use odour as a cue when encountering eggs of similar size to their own (i.e. intermediate size in F. fusca). Alternatively, larvae may use chemical surface cues not detected with our methods, such as long-chained hydrocarbons or lipids [77]. Future studies will help identify the proximate mechanisms behind larval egg discrimination behaviour.
In conclusion, we show that ant larvae are capable of fine-scale discriminatory behaviour, and that they possibly harness this ability in their best inclusive fitness interests through a novel role in colony defence against social parasites. In most host–parasite systems, this interaction is reversed, with parasite offspring attacking host offspring, as occurs, for instance, in socially parasitic bees [78] and cuckoos [7]. Active participation in defence by larvae has, however, been demonstrated in other insect species. For instance, larvae of social sawflies and moth caterpillars take part in cooperative chemical defence against predators by regurgitating a resinous fluid in response to harassment, often accompanied by vigorous whipping and arching of their bodies [79–81]. Lepidopteran collective defences, such as head-flicking and biting, are also used towards parasitoids in Baltimore checkerspot and Forest tent caterpillars [82,83]. Concordantly, selection on hosts to evolve defences against temporary social parasites may have favoured accurate egg recognition by all colony members—including larvae—in F. fusca, a common host to social parasites. Our study thus adds to the evidence that immature social insects are not merely passive members of the colony, but actively participate in colony life [84].
Supplementary Material
Acknowledgements
We thank Jelle van Zweden for his help with GC–MS analyses.
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q499457 [53].
Competing interests
We have no competing interests.
Funding
The work was supported by the LUOVA Doctoral Programme (U.P.), the Academy of Finland (grant numbers 252411, 284666 to the Centre of Excellence in Biological Interactions to L.S.), the Kone Foundation (H.H.), the University of Helsinki, and the Bayrisches Staatsministerium für Bildung und Kultus, Wissenschaft und Kunst (E.S.).
References
- 1.Thompson J. 1994. The coevolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 2.Poulin R. 2000. EBSCO host: the diversity of parasites. Q. Rev. Biol. 75, 277–293. ( 10.1086/393500) [DOI] [PubMed] [Google Scholar]
- 3.Müller U, Vogel P, Alber G, Schaub GA. 2008. The innate immune system of mammals and insects. In Trends in innate immunity (eds Egesten A, Schmidt A, Herwald H), pp. 21–44. Basel, Switzerland: Karger. [DOI] [PubMed] [Google Scholar]
- 4.Rolff J, Reynolds SE. 2009. Insect infection and immunity: evolution, ecology, and mechanisms. Oxford, NY: Oxford University Press. [Google Scholar]
- 5.Riera RM, Pérez-Martínez D, Castillo Ferrer C. 2016. Innate immunity in vertebrates: an overview. Immunology 148, 125–139. ( 10.1111/imm.12597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Roode JC, Lefèvre T. 2012. Behavioral immunity in insects. Insects 3, 789–820. ( 10.3390/insects3030789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies NB, Bourke AF, Brooke M. 1989. Cuckoos and parasitic ants: interspecific brood parasitism as an evolutionary arms race. Trends Ecol. Evol. 4, 274–278. ( 10.1016/0169-5347(89)90202-4) [DOI] [PubMed] [Google Scholar]
- 8.Schmid-Hempel P. 1995. Parasites and social insects. Apidologie 26, 255–271. ( 10.1051/apido:19950307) [DOI] [Google Scholar]
- 9.Buschinger A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol. News 12, 219–235. [Google Scholar]
- 10.Martin SJ, Carruthers JM, Williams PH, Drijfhout FP. 2010. Host specific social parasites (Psithyrus) indicate chemical recognition system in bumblebees. J. Chem. Ecol. 36, 855–863. ( 10.1007/s10886-010-9805-3) [DOI] [PubMed] [Google Scholar]
- 11.Kilner RM, Langmore NE. 2011. Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol. Rev. Camb. Philos. Soc. 86, 836–852. ( 10.1111/j.1469-185X.2010.00173.x) [DOI] [PubMed] [Google Scholar]
- 12.Lopez-Osorio F, Perrard A, Pickett KM, Carpenter JM, Agnarsson I. 2015. Phylogenetic tests reject Emery's rule in the evolution of social parasitism in yellowjackets and hornets (Hymenoptera: Vespidae, Vespinae). R. Soc. Open Sci. 2, 150159 ( 10.1098/rsos.150159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 14.d'Ettorre P, Heinze J. 2001. Sociobiology of slave-making ants. Acta Ethol. 3, 67–82. ( 10.1007/s102110100038) [DOI] [Google Scholar]
- 15.Alloway TM. 1990. Slave-species ant colonies recognize slavemakers as enemies. Anim. Behav. 39, 1218–1220. ( 10.1016/S0003-3472(05)80797-3) [DOI] [Google Scholar]
- 16.Foitzik S, Herbers JM. 2001. Colony structure of a slavemaking ant. II. Frequency of slave raids and impact on the host population. Evolution 55, 316–323. [DOI] [PubMed] [Google Scholar]
- 17.Grüter C, Jongepier E, Foitzik S. 2017. Insect societies fight back: the evolution of defensive traits against social parasites. Phil. Trans. R. Soc. B 373, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenoir A, Fresneau D, Errard C, Hefetz A. 1999. Individuality and colonial identity in ants: the emergence of the social representation concept. In Information processing in social insects (eds Detrain C, Deneubourg JL, Pasteels JM), pp. 219–237. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 19.van Zweden JS, d'Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In Insect hydrocarbons: biology, biochemistry and chemical ecology (eds Blomquist GJ, Bagneres A), pp. 222–243. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 20.Martin SJ, Helanterä H, Drijfhout FP. 2011. Is parasite pressure a driver of chemical cue diversity in ants? Proc. R. Soc. B 278, 496–503. ( 10.1098/rspb.2010.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topoff H, Zimmerli E. 1993. Colony takeover by a socially parasitic ant, Polyergus breviceps: the role of chemicals obtained during host-queen killing. Anim. Behav. 46, 479–486. ( 10.1006/anbe.1993.1216) [DOI] [Google Scholar]
- 22.Lenoir A, d'Ettorre P, Errard C, Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46, 573–599. ( 10.1146/annurev.ento.46.1.573) [DOI] [PubMed] [Google Scholar]
- 23.Guillem RM, Drijfhout F, Martin SJ. 2014. Chemical deception among ant social parasites. Curr. Zool. 60, 62–75. ( 10.1093/czoolo/60.1.62) [DOI] [Google Scholar]
- 24.Achenbach A, Foitzik S. 2009. First evidence for slave rebellion: enslaved ant workers systematically kill the brood of their social parasite Protomognathus americanus. Evolution 63, 1068–1075. ( 10.1111/j.1558-5646.2009.00591.x) [DOI] [PubMed] [Google Scholar]
- 25.Chernenko A, Helanterä H, Sundström L. 2011. Egg recognition and social parasitism in Formica ants. Ethology 117, 1081–1092. ( 10.1111/j.1439-0310.2011.01972.x) [DOI] [Google Scholar]
- 26.Chernenko A, Vidal-Garcia M, Helanterä H, Sundström L. 2013. Colony take-over and brood survival in temporary social parasite of the ant genus Formica. Behav. Ecol. Sociobiol. 67, 727–735. ( 10.1007/s00265-013-1496-7) [DOI] [Google Scholar]
- 27.Schultner E, d'Ettorre P, Helanterä H. 2013. Social conflict in ant larvae: egg cannibalism occurs mainly in males and larvae prefer alien eggs. Behav. Ecol. 24, 1306–1311. ( 10.1093/beheco/art067) [DOI] [Google Scholar]
- 28.Schultner E, Gardner A, Karhunen M, Helanterä H. 2014. Ant larvae as players in social conflict: relatedness and individual identity mediate cannibalism intensity. Am. Nat. 184, E161–E174. ( 10.1086/678459) [DOI] [PubMed] [Google Scholar]
- 29.Johnson CA, Topoff H, Vander Meer RK, Lavine B. 2005. Do these eggs smell funny to you?: an experimental study of egg discrimination by hosts of the social parasite Polyergus breviceps (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 57, 245–255. ( 10.1007/s00265-004-0851-0) [DOI] [Google Scholar]
- 30.Helanterä H, d'Ettorre P. 2014. A comparative study of egg recognition signature mixtures in Formica ants. Evolution 69, 520–529. ( 10.1111/evo.12590) [DOI] [PubMed] [Google Scholar]
- 31.Helanterä H, d'Ettorre P. 2014. Data from: A comparative study of egg recognition signature mixtures in Formica ants Dryad Digital Repository. ( 10.5061/dryad.38204) [DOI] [PubMed]
- 32.Helanterä H, Martin SJ, Ratnieks F. 2014. Recognition of nestmate eggs in the ant Formica fusca is based on queen derived cues. Cur. Zool. 60, 131–136. ( 10.1093/czoolo/60.1.131) [DOI] [Google Scholar]
- 33.Collingwood CA. 1979. The Formicidae (Hymenoptera) of Fennoscandia and Denmark. Klampenborg, Denmark: Scandinavian Science Press Ltd. [Google Scholar]
- 34.Baroni UC. 1991. Indiscriminate oophagy by ant larvae: an explanation for brood serial organization? Insectes Soc. 38, 229–239. ( 10.1007/BF01314909) [DOI] [Google Scholar]
- 35.Crespi B. 1992. Cannibalism and trophic eggs in subsocial and eusocial insects. In Cannibalism: ecology and evolution among diverse taxa (eds Elgar M, Crespi B), pp. 176–213. Oxford, NY: Oxford University Press. [Google Scholar]
- 36.Seifert B. 2007. Die Ameisen Mittel- und Nordeuropas. Görlitz, Tauer, Germany: Lutra.Verlags-und Vertriebsgesesellschaft. [Google Scholar]
- 37.Punttila P, Haila Y, Pajunen T, Tukia H. 1991. Colonisation of clearcut forests by ants in the southern Finnish taiga: a quantitative survey. Oikos 61, 250–262. ( 10.2307/3545343) [DOI] [Google Scholar]
- 38.Punttila P, Haila Y. 1996. Colonisation of a burned forest by ants in the Southern Finnish boreal forest. Silva Fenn. 30, 421–435. ( 10.14214/sf.a8502) [DOI] [Google Scholar]
- 39.Punttila P. 1996. Succession, forest fragmentation and the distribution of wood ants. Oikos 75, 291–298. ( 10.2307/3546252) [DOI] [Google Scholar]
- 40.Hannonen M, Sundström L. 2003. Sociobiology: worker nepotism among polygynous ants. Nature 421, 910 ( 10.1038/421910a) [DOI] [PubMed] [Google Scholar]
- 41.Helanterä H, Sundström L. 2007. Worker policing and nest mate recognition in the ant Formica fusca. Behav. Ecol. Sociobiol. 61, 1143–1149. ( 10.1007/s00265-006-0327-5) [DOI] [Google Scholar]
- 42.Helanterä H, Ratnieks FLW. 2009. Two independent mechanisms of egg recognition in worker Formica fusca ants. Behav. Ecol. Sociobiol. 63, 573–580. ( 10.1007/s00265-008-0692-3) [DOI] [Google Scholar]
- 43.Helanterä H, Lee YR, Drijfhout FP, Martin SJ. 2011. Genetic diversity, colony chemical phenotype, and nest mate recognition in the ant Formica fusca. Behav. Ecol. 22, 710–716. ( 10.1093/beheco/arr037) [DOI] [Google Scholar]
- 44.Bhatkar A, Whitcomb WH. 1970. Artificial diet for rearing various species of ants. Florida Entomol. 53, 229–232. ( 10.2307/3493193) [DOI] [Google Scholar]
- 45.Solis DR, Fox EGP, Rossi ML, Bueno OC. 2010. Description of the immatures of Linepithema humile Mayr (Hymenoptera: Formicidae). Biol. Res. 43, 19–30. ( 10.4067/S0716-97602010000100004) [DOI] [PubMed] [Google Scholar]
- 46.Outgroup—Science Visually. 2017. Cannibal larvae. [online video]. See https://youtu.be/UM4KhpmqpqY (accessed: 5 February 2019).
- 47.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gösswald K. 1989. Die Waldameise: Biologie, Oekologie und Forstliche Nutzung. Wiesbaden, Germany: Aula-Verlag. [Google Scholar]
- 49.Hakala SM, Seppä P, Heikkilä M, Punttila P, Sorvari J, Helanterä H. 2018. Genetic analysis reveals Finnish Formica fennica populations do not form a separate genetic entity from F. exsecta. PeerJ 6, e6013 ( 10.7717/peerj.6013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin SJ, Helanterä H, Drijfhout FP. 2008. Evolution of species-specific cuticular hydrocarbon patterns in Formica ants. Biol. J. Linn. Soc. 95, 131–140. ( 10.1111/j.1095-8312.2008.01038.x) [DOI] [Google Scholar]
- 51.Aitchison J. 1986. The statistical analysis of compositional data. London, UK: Chapman & Hall. [Google Scholar]
- 52.Cattell RB. 1966. The scree test for the number of factors. Multivariate Behav. Res. 1, 245–276. ( 10.1207/s15327906mbr0102_10) [DOI] [PubMed] [Google Scholar]
- 53.Pulliainen U, Helanterä H, Sundström L, Schultner E. 2019. Data from: The possible role of ant larvae in the defence against social parasites Dryad Digital Repository. ( 10.5061/dryad.q499457) [DOI] [PMC free article] [PubMed]
- 54.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 55.Sameshima S, Hasegawa E, Kitade O, Minaka N, Matsumoto T. 1999. Phylogenetic comparison of endosymbionts with their host ants based on molecular evidence. Zool. Sci. 16, 993–1000. ( 10.2108/zsj.16.993) [DOI] [Google Scholar]
- 56.Seppä P, Helanterä H, Trontti K, Punttila P, Chernenko A, Martin SJ, Sundström L. 2010. The many ways to delimit species: hairs, genes and surface chemistry. Myrmecol. News 15, 31–41. [Google Scholar]
- 57.Stevens M. 2013. Bird brood parasitism. Curr. Biol. 23, R909–R913. ( 10.1016/j.cub.2013.08.025) [DOI] [PubMed] [Google Scholar]
- 58.Fisher RM. 1987. Queen-worker conflict and social parasitism in bumble bees (Hymenoptera: Apidae). Anim. Behav. 35, 1026–1036. ( 10.1016/S0003-3472(87)80159-8) [DOI] [Google Scholar]
- 59.Lorenzi MC, Filippone F. 2000. Opportunistic discrimination of alien eggs by social wasps (Polistes biglumis, Hymenoptera Vespidae): a defense against social parasitism? Behav. Ecol. Sociobiol. 48, 402–406. ( 10.1007/s002650000251) [DOI] [Google Scholar]
- 60.Haskins CP, Haskins EF. 1950. Notes on the biology and social behavior of the archaic Ponerine ants of the Genera Myrmecia and Promyrmecia. Ann. Entomol. Soc. Am. 43, 461–491. ( 10.1093/aesa/43.4.461) [DOI] [Google Scholar]
- 61.Foster KR, Ratnieks FLW. 2001. Convergent evolution of worker policing by egg eating in the honeybee and common wasp. Proc. R. Soc. Lond. B 268, 169–174. ( 10.1098/rspb.2000.1346) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.d'Ettorre P, Heinze J, Ratnieks FLW. 2004. Worker policing by egg eating in the ponerine ant Pachycondyla inversa. Proc. R. Soc. Lond. B 271, 1427–1434. ( 10.1098/rspb.2004.2742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Endler A, Liebig J, Schmitt T, Parker JE, Jones GR, Schreier P, Hölldobler B. 2004. Surface hydrocarbons of queen eggs regulate worker reproduction in a social insect. Proc. Natl Acad. Sci. USA 101, 2945–2950. ( 10.1073/pnas.0308447101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Helanterä H, Martin SJ, Ratnieks FLW. 2007. Prior experience with eggs laid by non-nestmate queens induces egg acceptance errors in ant workers. Behav. Ecol. Sociobiol. 62, 223–228. ( 10.1007/s00265-007-0456-5) [DOI] [Google Scholar]
- 65.Helanterä H, Sundström L. 2005. Worker reproduction in the ant Formica fusca. J. Evol. Biol. 18, 162–171. ( 10.1111/j.1420-9101.2004.00777.x) [DOI] [PubMed] [Google Scholar]
- 66.Helanterä H, Sundström L. 2007. Worker reproduction in Formica ants. Am. Nat. 170, E14–E25. ( 10.1086/518185) [DOI] [PubMed] [Google Scholar]
- 67.Cini A, Nieri R, Dapporto L, Monnin T, Cervo R. 2014. Almost royal: incomplete suppression of host worker ovarian development by a social parasite wasp. Behav. Ecol. Sociobiol. 68, 467–475. ( 10.1007/s00265-013-1661-z) [DOI] [Google Scholar]
- 68.Franks NR, Sendova-Franks AB. 1992. Brood sorting by ants: distributing the workload over the work-surface. Behav. Ecol. Sociobiol. 30, 109–123. ( 10.1007/BF00173947) [DOI] [Google Scholar]
- 69.Ronai I, Vergoz V, Oldroyd BP. 2016. The mechanistic, genetic, and evolutionary basis of worker sterility in the social hymenoptera. Adv. Study Behav. 48, 251–317. ( 10.1016/bs.asb.2016.03.002) [DOI] [Google Scholar]
- 70.Baudoin M. 1975. Host castration as a parasitic strategy. Evolution 29, 335–352. ( 10.1111/j.1558-5646.1975.tb00213.x) [DOI] [PubMed] [Google Scholar]
- 71.Hurd H. 2001. Host fecundity reduction: a strategy for damage limitation? Trends Parasitol. 17, 363–368. ( 10.1016/S1471-4922(01)01927-4) [DOI] [PubMed] [Google Scholar]
- 72.Steiner FM, Schlick-Steiner BC, Konrad H, Moder K, Christian E, Seifert B, Crozier RH, Stauffer C, Buschinger A. 2006. No sympatric speciation here: multiple data sources show that the ant Myrmica microrubra is not a separate species but an alternate reproductive morph of Myrmica rubra. J. Evol. Biol. 19, 777–787. ( 10.1111/j.1420-9101.2005.01053.x) [DOI] [PubMed] [Google Scholar]
- 73.Helms JA, Godfrey A. 2016. Dispersal polymorphisms in invasive fire ants. PLoS ONE 11, e0153955 ( 10.1371/journal.pone.0153955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hannonen M, Helanterä H, Sundström L. 2004. Habitat age, breeding system and kinship in the ant Formica fusca. Mol. Ecol. 13, 1579–1588. ( 10.1111/j.1365-294X.2004.02136.x) [DOI] [PubMed] [Google Scholar]
- 75.Polis GA. 1981. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 12, 225–251. ( 10.1146/annurev.es.12.110181.001301) [DOI] [Google Scholar]
- 76.Ozan M. 2016. Reproductive partitioning in the polygynous black ant Formica fusca. Doctoral dissertation, University of Helsinki. [Google Scholar]
- 77.Akino T. 2006. Cuticular hydrocarbons of Formica truncorum (Hymenoptera: Formicidae): description of new very long chained hydrocarbon components. Appl. Entomol. Zool. 41, 667–677. ( 10.1303/aez.2006.667) [DOI] [Google Scholar]
- 78.Michener CD. 1974. The social behavior of the bees; a comparative study. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 79.Codella JRSG, Raffa KF. 1996. Individual and social components of wood ant response to conifer sawfly defence (Hymenoptera: Formicidae, Diprionidae). Anim. Behav. 52, 801–811. ( 10.1006/anbe.1996.0225) [DOI] [Google Scholar]
- 80.Costa JT. 2006. The other insect societies. Cambridge, MA: Belknap Press of Harvard University Press. [Google Scholar]
- 81.Lindstedt C, Huttunen H, Kakko M, Mappes J. 2011. Disengtangling the evolution of weak warning signals: high detection risk and low production costs of chemical defences in gregarious pine sawfly larvae. Evol. Ecol. 25, 1029–1046. ( 10.1007/s10682-010-9456-4) [DOI] [Google Scholar]
- 82.Stamp NE. 1982. Behavioral interactions of parasitoids and Baltimore checkerspot caterpillars (Euphydryas phaeton). Environ. Entomol. 11, 100–104. ( 10.1093/ee/11.1.100) [DOI] [Google Scholar]
- 83.McClure M, Despland E. 2011. Defensive responses by a social caterpillar are tailored to different predators and change with larval instar and group size. Naturwissenschaften 98, 425–434. ( 10.1007/s00114-011-0788-x) [DOI] [PubMed] [Google Scholar]
- 84.Schultner E, Oettler J, Helanterä H. 2017. The role of brood in eusocial hymenoptera. Q. Rev. Biol. 92, 39–78. ( 10.1086/690840) [DOI] [PubMed] [Google Scholar]
- 85.Morandin C, Pulliainen U, Bos N, Schultner E. 2018. De novo transcriptome assembly and its annotation for the black ant Formica fusca at the larval stage. Sci. Data 5, 180282 ( 10.1038/sdata.2018.282) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Helanterä H, d'Ettorre P. 2014. Data from: A comparative study of egg recognition signature mixtures in Formica ants Dryad Digital Repository. ( 10.5061/dryad.38204) [DOI] [PubMed]
- Pulliainen U, Helanterä H, Sundström L, Schultner E. 2019. Data from: The possible role of ant larvae in the defence against social parasites Dryad Digital Repository. ( 10.5061/dryad.q499457) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.q499457 [53].