Abstract
Multi-trophic interactions maintain critical ecosystem functions. Biodiversity is declining globally, while responses of trophic interactions to biodiversity change are largely unclear. Thus, studying responses of multi-trophic interaction robustness to biodiversity change is crucial for understanding ecosystem functioning and persistence. We investigate plant–Hemiptera (antagonism) and Hemiptera–ant (mutualism) interaction networks in response to experimental manipulation of tree diversity. We show increased diversity at both higher trophic levels (Hemiptera and ants) and increased robustness through redundancy of lower level species of multi-trophic interactions when tree diversity increased. Hemiptera and ant diversity increased with tree diversity through non-additive diversity effects. Network analyses identified that tree diversity also increased the number of tree and Hemiptera species used by Hemiptera and ant species, and decreased the specialization on lower trophic level species in both mutualistic and antagonist interactions. Our results demonstrate that bottom-up effects of tree diversity ascend through trophic levels regardless of interaction type. Thus, local tree diversity is a key driver of multi-trophic community diversity and interaction robustness in forests.
Keywords: tri-trophic, insect–plant interactions, bottom-up, stability, redundancy, BEF-China
1. Introduction
Biodiversity and trophic interactions among organisms at multiple trophic levels affect the stability and functioning of ecosystems [1–6]. Forests are the largest global terrestrial biodiversity repositories and provide crucial ecosystem services [7]. Therefore, understanding the mechanisms and interrelationships between biotic interactions and diversity is a core theme in ecology, which is especially challenging in (sub-)tropical forest systems [4,8–12].
Across almost all ecosystems, organisms can be grouped into communities of different trophic levels with energy flowing from the lowest (plants) via medium (herbivores) to the highest level (predators). Functional complementarity of species within the lower (plant) level increases productivity in this trophic level and contributes to maintaining species abundance and richness at higher trophic levels [5,13–15]. Consequently, trophic interactions become more abundant and diverse when plant diversity increases, which, in theory, promotes stability of natural communities [3,4,16–18].
Following the predictions of the ‘insurance hypothesis’, the stability (resistance to perturbations) of communities is expected to increase with increasing functional redundancy [3,19], and robustness against secondary extinctions with network connectivity (number of interaction partners per species) [20]. Communities and interactions of a higher trophic level are expected to be more robust against perturbations when the diversity of the respective lower trophic level is high, because more interactions can be realized, which increases functional redundancy of lower trophic level interaction partners and network connectivity [3,19]. Although different responses to interaction partner diversity were proposed for antagonism and mutualism [21], functional redundancy should increase higher trophic level community robustness regardless of the interaction type (e.g. consumers in antagonism and mutualism). This is supported by studies from a grassland biodiversity experiment, in which plant–insect interaction relationships across multiple trophic groups were analysed (e.g. [14]), and few studies exist from forest ecosystems (e.g. [22,23]) but experimental evidence is still scarce [11].
Especially in complex ecosystems, such as forests, natural food webs are interlinked and surpass bipartite interactions among two trophic levels, but separating direct and indirect effects in multi-trophic interactions with more than two interacting levels is challenging. To test the relationship of abundance, diversity, and interaction partner redundancy of connected trophic interaction networks in response to experimentally manipulated tree species richness, we analysed trophobioses in a controlled Biodiversity-Ecosystem-Functioning (BEF) experiment [24,25]. Trophobioses are the interactions between plants, Hemiptera, and ants, and represent a model for multi-trophic interaction networks including antagonistic (i.e. plant–Hemiptera) and mutualistic (i.e. Hemiptera–ant) interactions [26]. While Hemiptera (e.g. Aphids, Coccids, Membracids) extract plant sap, ants provide protection to Hemiptera in return for their supply of honeydew (see electronic supplementary material, figure S1). We hypothesized that species richness at the lower trophic level (trees, Hemiptera) directly propagates species richness at the higher trophic level (Hemiptera, ants) and that this effect can be seen for both mutualistic and antagonistic interactions. Furthermore, we expected that diversity at the lower trophic level increases robustness at the higher trophic level regardless of interaction type, because species richness at the lower trophic level increases interaction partner redundancy, irrespective if interactions are antagonistic or mutualistic.
2. Methods
(a). Study site
The BEF-China Main Experiment field sites are located in subtropical south-east China near Xingangshan, Jiangxi Province (117°54′ E, 29°07′ N) [24]. The surrounding landscape is a patchwork of small agricultural fields in the valleys and conifer plantations or diverse secondary forests on the slopes. Local forests consist of 50% evergreen and 50% deciduous tree species. For detailed descriptions of the region and forest tree compositions see Bruelheide et al. [27]. The BEF-China Main Experiment is replicated at two field sites, 4 km apart from each other, and comprising a total area of 50 ha (see fig. 4 in Bruelheide et al. [27]). It is currently the largest tree diversity experiment in the world [24]. In total, 566 plots each of 25.8 × 25.8 m in orthogonal projection were planted with 400 tree seedlings in a 20 × 20 grid system with 1.29 m distance between seedlings. At each field site, a tree species richness gradient of 24 tree species was planted (1, 2, 4, 8, 16, and 24 species), obtained from a total species pool of 40 tree species in 2009 and 2010. The species pool on both sites contains eight shared tree species and 16 site-specific tree species. All species are represented in monocultures and all higher diversity mixtures. Plots of different tree species richness are randomly distributed within each field site. A comprehensive description of the BEF-China Main Experiment was provided by Yang et al. [28] and Bruelheide et al. [24].
(b). Sampling design
We conducted field surveys for trophobiotic Hemiptera (hereafter referred to as ‘Hemiptera’) and tending ants (hereafter referred to as ‘ants’) at 300 plots and over 12 000 trees at the BEF-China Main Experiment (see electronic supplementary material, table S1; [24,29]). Depending on tree species identity and sampling year, tree height was between 1 and 6 m. We selected 150 plots at each of the two experimental sites (‘site A’ and ‘site B’) representing each tree species at similar proportions across all tree species richness levels. Each site was surveyed three times for the presence of Hemiptera and interacting ants (trophobiosis), site A once in 2011, site B once in 2013, and both sites twice in 2014 (see electronic supplementary material, table S2). To avoid the effects of neighbouring plots and edge-effects, our surveys used a ‘core area’ approach on the central tree individuals guaranteeing a minimum buffer distance of 5.16 m to the adjacent plot margin. The number of plots available for sampling decreased with increasing tree species richness. To sample the same number of tree individuals for each tree species in each tree species richness level, we increased the number of trees sampled per plot with increasing tree species richness, from 36 trees in monocultures to 144 trees in 24 species mixtures (see electronic supplementary material, table S1). For each individual tree, a total of 20 leaves from three branch sections and their bark were visually inspected for the presence of Hemiptera and interacting ants [29,30]. Ants were only considered as interacting when direct tending behaviour, such as honeydew excretion in response to ant antennation or the presence of ant constructed sheetings, was observed. Each unique trophobiosis between one ant species and one Hemiptera species per leaf or bark section was defined as one interaction, regardless of the number of interaction partners involved [31,32]. All Hemiptera species were recorded and only Hemiptera species that occurred in trophobioses were used for analyses. In other words, Hemiptera species not observed at least once in a trophobioses were not considered trophobiotic Hemiptera and were excluded from the analysis. For ants, only interacting ant species were noted, therefore the occurrence of ants is equal to the number of trophobioses. Common ant and Hemiptera species were identified in the field. For all others, voucher specimens were collected and stored in 99.7% ethanol for later identification. Auchenorrhyncha specimens (cicada) were compared with material deposited in the Insect Collection of the Institute of Zoology, Chinese Academy of Sciences, Beijing, China. All aphids where identified using the online key ‘Aphids on the world's trees’ (adopted from [33]). Ants were identified to species or morphospecies by the senior author. Species that could not be identified were delineated into morphospecies to which we also refer as species in all analyses (see electronic supplementary material, table S3).
(c). Data organization
Data were analysed using the software R 3.4.3 (www.r-project.org). For all analyses we considered the 24 species plots within the 16 species plots (there are only two 24 species plots at each field site). We calculated Hemiptera species richness for each plot to identify the ‘overall effect’ of tree species richness. In this ‘overall effect’ dataset tree identity (selection and sampling effects) and additive effects caused by Hemiptera host plant specificity are captured. In contrast, for all other analyses, including tests for the effect of tree species richness on insect diversity and interaction networks, we created tree, Hemiptera, and ant communities by combining all observed interactions of the same tree species richness level (1, 2, 4, 8, 16). With two field sites and three sampling rounds this resulted in 30 multi-trophic communities. Each community, regardless of tree species richness, therefore included observations of all 24 tree species planted at the respective field site. Accordingly, in our analyses, all tree species are represented in each tree species richness level and interaction networks could, in principle, include 24 tree species regardless of the tree species richness level. Therefore, potential ‘additive’ and ‘selection’ effects were excluded and tree species richness effects can be seen as ‘non-additive’ effects (through competition, niche partitioning, and facilitation), caused by tree species richness [34–36]. Community metrics and network indices (see below) were calculated for each community.
To test for the possible influence of different sampling effort of plots with different tree species richness and small imbalances in the number of trees sampled per plot, we generated a ‘rarefied’ dataset in which tree species-specific individual numbers are equal across tree species richness levels [28]. The aim was to rarefy all three trophic levels at once simulating a perfectly balanced hypothetical field survey. We used this dataset to repeat all analysis and confirmed that sampling effort did not affect the results of the study (for more details on ‘rarefaction’ and the results on rarefied data see electronic supplementary material, methods S1). As community metrics we used species richness and species occurrence of Hemiptera and ants. We use occurrence as metric of abundance instead of actual individual counts to avoid overestimations caused by reproductive and social behaviour of Hemiptera and ants, respectively (see electronic supplementary material, table S2). To describe changes in the interaction networks, we used two mathematically independent quantitative network indices, calculated with the R package ‘bipartite’ [37]. We use generality Gqw (weighted number of interaction partners) to describe functional redundancy (generality on interaction partners) and specialization (weighted complementary specialization on interaction partners) to quantify specialization on interaction partners (complementary specialization on interaction partners) of the antagonistic and mutualistic interaction [37–39]. is standardized against variation in species' abundances and network size and thus comparable across networks of different dimensions and types. Gqw provides realistic estimates for the number of interaction partners and is insensitive to small variation in network size [40]. To include redundancy from a lower trophic level perspective, we use interaction network ‘vulnerability’ (Vqw), representing the weighted number of higher level species per lower level species [37]. For more details on interaction network indices see electronic supplementary material, methods S1.
(d). Statistical analyses
Prior to regression analyses, the explanatory variable tree species richness was log2-transformed. We used generalized linear mixed-effects models (R-package ‘lme4’; [41]). To account for the hierarchical organization of the sampling and the potential variability of the insect community between surveys and field sites, field site (levels = A and B) and survey (levels = 1, 2, and 3) were included as random effects in all models. As the BEF-China Main Experiment manipulates tree species richness (i.e. the lowest trophic level), we expect bottom-up effects to act on higher trophic levels. To explore how Hemiptera respond to tree species richness and if ant species richness is directly linked to the trees or indirectly via the intermediate Hemiptera level, we used a structural equation model (SEM; [42]). For SEM construction we used the generalized linear mixed-effects models described above as component models in the ‘picewiseSEM’ package [42]. Each component model included one response, all downstream responses, and tree species richness as explanatory variables and both random effects. The SEM approach requires approximation of degrees of freedom (d.f.) to calculate p-values. We used Satterthwaite's approximation in the R-package ‘lmerTest’ to obtain d.f.s [43]. The ‘full’ model included all potential bottom-up paths, from species richness of trees (first level) to occurrence and species richness of Hemiptera (second level) and occurrence and species richness of ants (third level), and paths from abundance to species richness. The full model was backward-selected to achieve parsimony using Akaike Information Criterion (AICc). A direct path between tree species richness and ants was included, since Hemiptera enable ants to access plant sap and ants might respond directly to tree species richness [44]. The model included five variables and 30 data points (sample-size/variable ratio = six) and is expected to have sufficient power to estimate relationships among different variables [42]. Based on overall model fit calculated by Fishers' C statistic, we evaluated whether any paths are missing from the model. Here, a minimum AICc and a non-significant Fishers' C indicate the best model balancing parsimony and necessity of parameters [42].
Interaction network indices can vary with the community composition of higher and lower trophic levels. Our analyses included similar tree compositions at all tree species richness levels, therefore we expect similar community compositions for Hemiptera and ant communities at all tree species richness levels. To test this, we calculated ‘Morisita Horn’ dissimilarity indexes for Hemiptera and ant communities of each tree species richness level and compared the community compositions using an analysis of similarities (ANOSIM, number of permutations = 10 000) based on ‘Bray–Curtis’ distance.
(e). Honeydew analysis
To test for complementarity effects as a potential driver of direct or indirect ant responses to tree species richness we analysed the amino acid composition of honeydew from tree species-specific Hemiptera. For five of the planted tree species, honeydew was sampled at three to nine trophobioses from trees randomly distributed across all study plots. For each tree species, one specific Hemiptera interaction partner was sampled by extracting honeydew from interacting ants (see electronic supplementary material, methods S1). Proteinogenic amino acids of the honeydew were determined using high-performance liquid chromatography (see electronic supplementary material, methods S1 and [45]) to compare the similarity of tree species-specific amino acid compositions. The tree species-specific composition of the 20 proteinogenic amino acids was compared using an analysis of similarities (ANOSIM, ‘Bray–Curtis’ distance, number of permutations = 10 000) with the ‘vegan’ package [46].
3. Results
In total, 3500 occurrences of trophobioses were observed across all trees, representing a 10% occupancy rate. We found 67 Hemiptera species attended by 37 ant species on 32 of the 40 tree species (see also electronic supplementary material, results S1 and a species list in table S3). Hemiptera and ant community composition did not vary significantly with tree species richness (ANOSIM, ants R2 = 0.062, p = 0.063; Hemiptera R2 = 0.0068, p > 0.586, electronic supplementary material, figure S2). The structural equation model identified a direct response of Hemiptera to tree species richness and that all changes in ant occurrence and species richness were indirectly mediated by Hemiptera, the intermediate trophic level (figure 1; electronic supplementary material, table S4). Hemiptera occurrence and species richness increased with tree species richness. Ant occurrence was explained by Hemiptera occurrence . Ant species richness was explained by ant occurrence and Hemiptera species richness .
Figure 1.
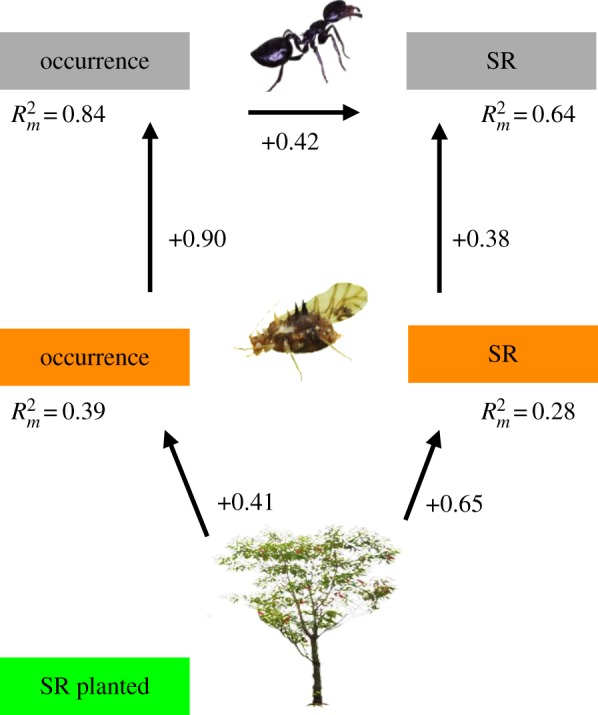
Significant paths of indirect and direct effects of tree species richness on multi-trophic interactions, estimated by the most-parsimonious structural equation model, see electronic supplementary material, table S4 for coefficients. Species richness (SR) in the lowest level (log2 of planted tree species richness) affected primary consumer (Hemiptera) abundance and species richness, at the intermediate level. Hemiptera interacting in mutualistic trophobioses with ants (highest level), affected abundance and species richness of their partners. Arrows represent significant paths with positive correlation coefficients (p < 0.05; standardized path coefficients are given next to arrows); amount of variance explained by the model is given as the marginal coefficient of determination (, based on variance of fixed effects). Absent paths are validated by non-significant difference of Fisher's C compared to a χ2 distribution (n = 30, Fisher's C = 2.33, d.f. = 2, p = 0.312). (Online version in colour.)
We partitioned overall diversity effects (results based on data of individual plots) and non-additive effects (results based on data pooled by tree species richness level) and showed that both correlate significantly with increasing Hemiptera species richness (overall: 500% species richness increase, linear regression estimate ± standard error 0.45 ± 0.02, z = 19.49, p < 0.001; non-additive: 38%, 0.86 ± 0.21, F = 17.21, p < 0.001; see electronic supplementary material, figure S3 and table S5).
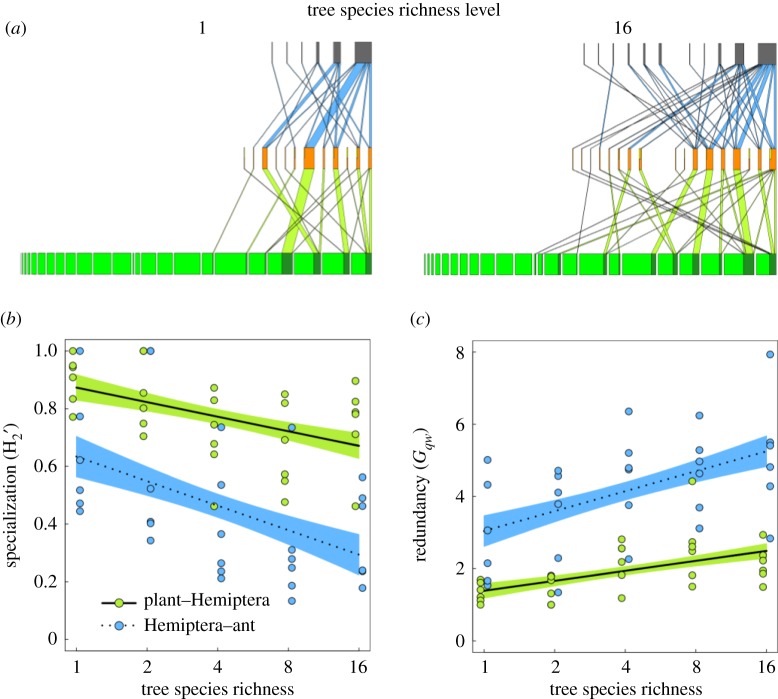
We showed for 30 multi-trophic interaction networks that redundancy significantly increased (by 79% for antagonism and 72% for mutualism) and specialization decreased (30% and 116%) with increasing tree species richness (figure 2a–c; electronic supplementary material, table S6). Antagonism was more specialized and less redundant than mutualism (mean ± standard deviation Gqw: 1.9 ± 0.7 and 4.1 ± 1.5; : 0.77 ± 0.16 and 0.46 ± 0.25, respectively, figure 2b and c). The degree of change in network indices with tree species richness was similar for both network types (figure 2b,c; electronic supplementary material, table S6). The results remain quantitatively similar after ‘rarefaction’ to equal numbers of trees per tree species (Gqw: 55% for antagonism and 49% for mutualism, mean ± standard deviation 1.7 ± 0.6 and 3.5 ± 1.4; : 20% and 43%, 0.82 ± 0.14 and 0.54 ± 0.22, respectively; electronic supplementary material, figure S4a and b, table S7). Representing the Hemipteran's perspective of redundancy in the mutualistic interaction with ants, interaction network vulnerability (number of interacting ant species per Hemiptera species) increased with increasing tree species richness. This indicates increasing robustness of Hemiptera interactions during mutualism (electronic supplementary material, figure S5, table S6).
Figure 2.
Multi-trophic networks in response to experimentally manipulated tree species richness. Example multi-trophic networks (a) of lowest (1) and highest (16) tree species richness. Ants (grey bars), the highest trophic level, interact with Hemiptera (orange bars) in trophobioses (mutualism, blue links). The intermediate trophic level Hemiptera (orange bars) interacts with trees, the lowest trophic level (green bars), as herbivores (antagonism, light green links). Each box represents one species and the box width the relative share of all interacting individuals, light green and yellow indicate the share of the non-interacting tree and Hemiptera individuals. Connecting links represent trophic links between two species and their width the relative share of all interactions of the two connected trophic levels. To illustrate interaction network changes the depicted networks are based on rarefied data (the abundance of each tree species is the same for each tree species richness level, here shown for levels 1 and 16) and the position of each species is identical in both networks. Network indices describing these networks in relation to tree species richness are shown in the lower panels (for rarefied data, see electronic supplementary material, figure S2, and table S7). (b) Network specialization of plant–Hemiptera (green dots, solid line ± standard error show model predictions) and Hemiptera–ant (blue dots, dashed line ± standard error show model predictions) interactions decreased with tree species richness, while (c) the corresponding network interaction partner redundancy (Gqw) increased. The antagonistic interaction is more specialized, as indicated by higher and lower Gqw, than the mutualistic interaction (see electronic supplementary material, table S6). In (b) and (c) tree species richness was log2-transformed. (Online version in colour.)
Honeydew amino acid composition sampled from tree-specific Hemiptera species was significantly different for the five tree species analysed (ANOSIM, p < 0.001, R2 = 0.32, see electronic supplementary material, figure S6).
4. Discussion
Our study showed that tree species richness increased Hemiptera species richness through non-additive effects; subsequently, Hemiptera species richness increased ant species richness. Our network analysis revealed for both connected interaction networks (antagonistic and mutualist interaction), that the robustness, indicated by interaction partner redundancy, increased with species richness at the lower trophic level. This increase in robustness may serve as a mechanistic explanation for the positive relationship between the diversities of the connecting trophic levels, and indicates a stabilizing effect of biodiversity. With this we highlight bottom-up effects of plant diversity on multiple trophic levels, and that responses of separate trophic levels are tightly interwoven in complex ecosystems such as subtropical forests.
(a). Overall and non-additive biodiversity effects
Overall biodiversity effects, including additive, selection, and non-additive effects, may be observed through comparing monocultures to more diverse species mixtures. For example, many Hemiptera species are specialized on certain plant families or genera. Therefore, Hemiptera diversity should increase with the addition of new tree species [33]. The analysis of overall biodiversity effects showed that Hemiptera species richness increased on average from 0.45 to 2.75 species when tree species richness increased from 1 to 16 species. The extrapolation of Hemiptera species based on the observations of tree monocultures would lead to 7.2 Hemiptera species at 16 tree species mixtures (16 tree species × 0.45 Hemiptera species). This difference of observed and expected species richness could indicate that ‘dilution’ effects mitigate the overall species richness increase [47].
Through the inclusion of all tree species at equal abundance at all diversity levels, non-additive effects can be distinguished from overall diversity effects [34–36]. Non-additive effects increased Hemiptera species richness from 8.96 to 12.4 Hemiptera species. Because non-additive effects can only be identified after data pooling we cannot determine the relative contribution of non-additive to the overall diversity effect. Nevertheless, the number of additional species found at high tree species richness was similar (overall 2.3 species, non-additive 3.44 species, respectively). While the overall effect might be best explained by host complementarity (i.e. an additive effect), the non-additive effect might be a result of facilitation. This means, the combination of trees and their complementary expression of traits might open new niches for additional species to co-occur (e.g. increased spatial heterogeneity, dietary mixing, or predator avoidance) [48]. The structural equation model showed that changes in abundance and species richness of the highest trophic level were only related to changes in the next lower trophic level. Although Hemiptera enable ants to access plant sap [44], ants did not directly respond to changes in local tree species richness. These coupled, yet not directly linked responses, highlight the importance of including intermediate trophic levels for the assessment of interactions, as intermediate trophic levels can modify resources and alter their provision to species in other levels [49]. Such responses are likely not restricted to the multi-trophic interactions investigated by us and may equally apply to trophic interactions involving predators, according to the predictions of the ‘enemies hypothesis’ [50].
(b). Interaction networks
Exceeding the evidence obtained from correlating species diversity of co-occurring trophic levels (e.g. in [14,51]), quantitative interaction networks revealed a more detailed picture of the multi-trophic community responses to changing plant diversity. For both antagonistic and mutualistic networks, redundancy increased and specialization decreased with increasing tree species richness. However, the species community compositions of Hemiptera and ants were similar for all tree species richness levels. Taken together, this indicates that higher level species interacted with more partners of the same species pool, with increasing tree species richness. At high tree species richness, Hemiptera might colonize ‘sink’ tree species (e.g. low attractiveness for Hemiptera) more often because ‘source’ trees are in proximity. Similarly, ants might exploit less productive Hemiptera species (e.g. low honeydew quality or quantity) more readily if productive Hemiptera species are in proximity. This might be due to increased encounter probability with ephemeral resources like mobile Cicada species, or shifted cost–benefit ratios (e.g. decreased distance between resources).
In theory, via complementary responses of functionally redundant species (response diversity), the diversity of interaction partners at the lower trophic level may enhance the resistance of the higher trophic level towards perturbations such as species loss or environmental change [3,19]. The changes in network structure therefore identify increased community robustness of ants and Hemiptera, each mediated bottom-up.
(c). Benefits for higher trophic levels in antagonism and mutualism
In the antagonistic tree–Hemiptera interaction, the Hemiptera community should benefit from diverse tree communities. In mutualism, both interacting levels should benefit from the diversity of the other level. Hence, ants should benefit from redundant Hemiptera partners and Hemiptera from increased functional redundancy (e.g. protection, sheeting, cleaning) provided by ants. For antagonism, functional complementarity of tree species at high tree species richness might increase plant resource abundance and structural complexity [15,52,53]. These changes in niche dimensions, for example via reduced pressure from natural enemies, favourable microclimate conditions, or higher food availability, might explain the non-additive increase in the number of Hemiptera species [54]. The thereby likely increased functional redundancy of plants results in increased Hemiptera community robustness.
Vegetation-foraging ants receive much of their nitrogen from honeydew excreted by trophobiotic Hemiptera and diverse Hemiptera access plant sap of more tree species [44,49,55]. Thus, for ants, functional complementarity in resource quantity, quality, and temporal provision is likely increased through redundant interactions with more Hemiptera species. A potential behavioural modulation in honeydew provisioning by different Hemiptera species is supported by our findings on amino acid composition in honeydew. Amino acids varied with host tree species identity. Plant sap and honeydew show the same amino acid composition [45], but ants did not directly respond to tree species richness. Hence, although we cannot fully distinguish between Hemiptera and plant identity effects, the complementarity of Hemiptera instead of plants might be of greater importance for explaining the observed responses of ants to Hemiptera species richness.
From a Hemipteran's perspective, increased mutualistic partner diversity may increase robustness of the protective function delivered by ants. This function is likely complementary in space and time, for instance when different ants interact at different times of the day or weather conditions [56]. Therefore, the functional relationship of both mutualistic partners may be stabilized under high tree diversity, and, in the future, experimental manipulations of higher trophic levels will help to validate causality. Besides protecting mutualistic Hemiptera, ants could reduce chewing herbivore occurrence and indirectly benefit plant primary production and growth in more diverse environments [57,58].
5. Conclusion
Our study shows that tree diversity has multiple positive effects on trophic interactions. With increasing lower trophic level diversity (e.g. plants), higher trophic level diversity and abundance and interaction partner redundancy increases regardless if interactions are antagonistic or mutualistic. Thus, the size, diversity, and robustness of interaction networks might generally be dependent on lower trophic level species richness. These stabilizing bottom-up effects likely transcend multi-trophic interaction networks regardless of interaction type and potentially affect communities within the entire ecosystem (sensu [59]). Therefore, biodiversity loss at the level of primary producers and primary consumers likely jeopardizes the many ecosystem processes maintained by multiple trophic levels.
Supplementary Material
Acknowledgements
We thank H. Bruelheide, K. Ma, B. Schmid, and all members of the BEF-consortium for initiation and maintenance of the experiment, and Merle Noack, Simon Willy, Marina Roth, and Sarah Radford for assistance at the field surveys recording trophobioses. Laila Berning helped with the amino acid analyses and Yang Bo and Chen Lin provided crucial infrastructure at the field sites.
Ethics
This research complies with the laws, guidelines, and ethical standards of China and the European Union, where the research was performed.
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.847km00 [60].
Authors' contributions
All authors conceived the study; F.F. and M.S. carried out data collection and species identification. F.F. carried out the data analysis and wrote the paper, each with conceptual input from M.S., A.-M.K., and N.B.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the German Research Foundation (DFG FOR 891/2, FOR 891/3, KL 1849/6-1, KL 1849/6-2).
References
- 1.MacArthur R. 1955. Fluctuations of animal populations and a measure of community stability. Ecology 36, 533–536. ( 10.2307/1929601) [DOI] [Google Scholar]
- 2.Yodzis P. 1981. The stability of real ecosystems. Nature 289, 674–676. ( 10.1038/289674a0) [DOI] [Google Scholar]
- 3.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCann KS. 2000. The diversity–stability debate. Nature 405, 228–233. ( 10.1038/35012234) [DOI] [PubMed] [Google Scholar]
- 5.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 6.Soliveres S, et al. 2016. Biodiversity at multiple trophic levels is needed for ecosystem multifunctionality. Nature 536, 456–459. ( 10.1038/nature19092) [DOI] [PubMed] [Google Scholar]
- 7.FAO. 2016. State of the World's Forests 2016 (SOFO). Rome, Italy: FAO; See http://www.fao.org/documents/card/en/c/ffed061b-82e0-4c74-af43-1a999a443fbf/. [Google Scholar]
- 8.Tilman D, Reich PB, Knops JMH. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441, 629–632. ( 10.1038/nature04742) [DOI] [PubMed] [Google Scholar]
- 9.Hector A, et al. 2010. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91, 2213–2220. ( 10.1890/09-1162.1) [DOI] [PubMed] [Google Scholar]
- 10.Gamfeldt L, et al. 2013. Higher levels of multiple ecosystem services are found in forests with more tree species. Nat. Commun. 4, 1340 ( 10.1038/ncomms2328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mori AS, Lertzman KP, Gustafsson L. 2017. Biodiversity and ecosystem services in forest ecosystems: a research agenda for applied forest ecology. J. Appl. Ecol. 54, 12–27. ( 10.1111/1365-2664.12669) [DOI] [Google Scholar]
- 12.Clarke DA, York PH, Rasheed MA, Northfield TD. 2017. Does biodiversity–ecosystem function literature neglect tropical ecosystems? Trends Ecol. Evol. 32, 320–323. ( 10.1016/j.tree.2017.02.012) [DOI] [PubMed] [Google Scholar]
- 13.Ives AR, Cardinale BJ, Snyder WE. 2005. A synthesis of subdisciplines: predator–prey interactions, and biodiversity and ecosystem functioning. Ecol. Lett. 8, 102–116. ( 10.1111/j.1461-0248.2004.00698.x) [DOI] [Google Scholar]
- 14.Scherber C, et al. 2010. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468, 553–556. ( 10.1038/nature09492) [DOI] [PubMed] [Google Scholar]
- 15.Huang Y, et al. 2018. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 362, 80–83. ( 10.1126/science.aat6405) [DOI] [PubMed] [Google Scholar]
- 16.Hector A, et al. 1999. Plant diversity and productivity experiments in European grasslands. Science 286, 1123–1127. ( 10.1126/science.286.5442.1123) [DOI] [PubMed] [Google Scholar]
- 17.Novotny V, Drozd P, Miller SE, Kulfan M, Janda M, Basset Y, Weiblen GD. 2006. Why are there so many species of herbivorous insects in tropical rainforests? Science 313, 1115–1118. ( 10.1126/science.1129237) [DOI] [PubMed] [Google Scholar]
- 18.Tylianakis JM, Klein A-M, Lozada T, Tscharntke T. 2006. Spatial scale of observation affects α, β and γ diversity of cavity-nesting bees and wasps across a tropical land-use gradient. J. Biogeogr. 33, 1295–1304. ( 10.1111/j.1365-2699.2006.01493.x) [DOI] [Google Scholar]
- 19.Blüthgen N, Klein A-M. 2011. Functional complementarity and specialisation: the role of biodiversity in plant–pollinator interactions. Basic Appl. Ecol. 12, 282–291. ( 10.1016/j.baae.2010.11.001) [DOI] [Google Scholar]
- 20.Dunne JA, Williams RJ, Martinez ND. 2002. Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol. Lett. 5, 558–567. ( 10.1046/j.1461-0248.2002.00354.x) [DOI] [Google Scholar]
- 21.Thébault E, Fontaine C. 2010. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329, 853–856. ( 10.1126/science.1188321) [DOI] [PubMed] [Google Scholar]
- 22.Basset Y, et al. 2012. Arthropod diversity in a tropical forest. Science 338, 1481–1484. ( 10.1126/science.1226727) [DOI] [PubMed] [Google Scholar]
- 23.Zhang K, et al. 2016. Plant diversity accurately predicts insect diversity in two tropical landscapes. Mol. Ecol. 25, 4407–4419. ( 10.1111/mec.13770) [DOI] [PubMed] [Google Scholar]
- 24.Bruelheide H, et al. 2014. Designing forest biodiversity experiments: general considerations illustrated by a new large experiment in subtropical China. Methods Ecol. Evol. 5, 74–89. ( 10.1111/2041-210X.12126) [DOI] [Google Scholar]
- 25.Grossman JJ, et al. 2018. Synthesis and future research directions linking tree diversity to growth, survival, and damage in a global network of tree diversity experiments. Environ. Exp. Bot. 152, 68–89. ( 10.1016/j.envexpbot.2017.12.015) [DOI] [Google Scholar]
- 26.Ivens ABF, von Beeren C, Blüthgen N, Kronauer DJC. 2016. Studying the complex communities of ants and their symbionts using ecological network analysis. Annu. Rev. Entomol. 61, 353–371. ( 10.1146/annurev-ento-010715-023719) [DOI] [PubMed] [Google Scholar]
- 27.Bruelheide H, et al. 2011. Community assembly during secondary forest succession in a Chinese subtropical forest. Ecol. Monogr. 81, 25–41. ( 10.1890/09-2172.1) [DOI] [Google Scholar]
- 28.Yang X, et al. 2013. Establishment success in a forest biodiversity and ecosystem functioning experiment in subtropical China (BEF-China). Eur. J. For. Res. 132, 593–606. ( 10.1007/s10342-013-0696-z) [DOI] [Google Scholar]
- 29.Staab M, Blüthgen N, Klein A-M. 2015. Tree diversity alters the structure of a tri-trophic network in a biodiversity experiment. Oikos 124, 827–834. ( 10.1111/oik.01723) [DOI] [Google Scholar]
- 30.Zhang S, Zhang Y, Ma K. 2015. Mutualism with aphids affects the trophic position, abundance of ants and herbivory along an elevational gradient. Ecosphere 6, 1–11. ( 10.1890/ES15-00229.1) [DOI] [Google Scholar]
- 31.Blüthgen N, Mezger D, Linsenmair KE. 2006. Ant-hemipteran trophobioses in a Bornean rainforest—diversity, specificity and monopolisation. Insectes Soc. 53, 194–203. ( 10.1007/s00040-005-0858-1) [DOI] [Google Scholar]
- 32.Blüthgen N, Menzel F, Hovestadt T, Fiala B, Blüthgen N. 2007. Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346. ( 10.1016/j.cub.2006.12.039) [DOI] [PubMed] [Google Scholar]
- 33.Blackman RL, Eastop VF. 1994. Aphids on the world's trees: an identification and information guide. Wallingford, UK: CABI International; See https://www.cabdirect.org/cabdirect/abstract/19941105799. [Google Scholar]
- 34.Aarssen LW. 1997. High productivity in grassland ecosystems: effected by species diversity or productive species? Oikos 80, 183–184. ( 10.2307/3546531) [DOI] [Google Scholar]
- 35.Huston MA. 1997. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110, 449–460. ( 10.1007/s004420050180) [DOI] [PubMed] [Google Scholar]
- 36.Loreau M, Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. ( 10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 37.Dormann CF, Fründ J, Blüthgen N, Gruber B. 2009. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24. ( 10.2174/1874213000902010007) [DOI] [Google Scholar]
- 38.Bersier L-F, Banašek-Richter C, Cattin M-F. 2002. Quantitative descriptors of food-web matrices. Ecology 83, 2394–2407. ( 10.1890/0012-9658(2002)083[2394:QDOFWM]2.0.CO;2) [DOI] [Google Scholar]
- 39.Blüthgen N, Menzel F, Blüthgen N. 2006. Measuring specialization in species interaction networks. BMC Ecol. 6, 9 ( 10.1186/1472-6785-6-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fründ J, McCann KS, Williams NM. 2016. Sampling bias is a challenge for quantifying specialization and network structure: lessons from a quantitative niche model. Oikos 125, 502–513. ( 10.1111/oik.02256) [DOI] [Google Scholar]
- 41.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 42.Lefcheck JS. 2016. piecewiseSEM: piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. ( 10.1111/2041-210X.12512) [DOI] [Google Scholar]
- 43.Kuznetsova A, Brockhoff PB, Christensen RHB. 2016. lmerTest: tests in linear mixed effects models. See https://cran.r-project.org/web/packages/lmerTest/index.html.
- 44.Davidson DW, Cook SC, Snelling RR, Chua TH. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300, 969–972. ( 10.1126/science.1082074) [DOI] [PubMed] [Google Scholar]
- 45.Staab M, Fornoff F, Klein A-M, Blüthgen N. 2017. Ants at plant wounds: a little-known trophic interaction with evolutionary implications for ant–plant interactions. Am. Nat. 190, 442–450. ( 10.1086/692735) [DOI] [PubMed] [Google Scholar]
- 46.Oksanen J, et al. 2017. Vegan: community ecology package. See https://cran.r-project.org/web/packages/vegan/index.html.
- 47.Otway SJ, Hector A, Lawton JH. 2005. Resource dilution effects on specialist insect herbivores in a grassland biodiversity experiment. J. Anim. Ecol. 74, 234–240. ( 10.1111/j.1365-2656.2005.00913.x) [DOI] [Google Scholar]
- 48.Wright AJ, Wardle DA, Callaway R, Gaxiola A. 2017. The overlooked role of facilitation in biodiversity experiments. Trends Ecol. Evol. 32, 383–390. ( 10.1016/j.tree.2017.02.011) [DOI] [PubMed] [Google Scholar]
- 49.Douglas AE. 2006. Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57, 747–754. ( 10.1093/jxb/erj067) [DOI] [PubMed] [Google Scholar]
- 50.Root RB. 1973. Organization of a plant-arthropod association in simple and diverse habitats: the fauna of Collards (Brassica oleracea). Ecol. Monogr. 43, 95–124. ( 10.2307/1942161) [DOI] [Google Scholar]
- 51.Schuldt A, et al. 2015. Multitrophic diversity in a biodiverse forest is highly nonlinear across spatial scales. Nat. Commun. 6, 10169 ( 10.1038/ncomms10169) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dixon AFG, Kindlmann P, Leps J, Holman J. 1987. Why there are so few species of aphids, especially in the tropics. Am. Nat. 129, 580–592. ( 10.1086/284659) [DOI] [Google Scholar]
- 53.Aquilino KM, Cardinale BJ, Ives AR. 2005. Reciprocal effects of host plant and natural enemy diversity on herbivore suppression: an empirical study of a model tritrophic system. Oikos 108, 275–282. ( 10.1111/j.0030-1299.2005.13418.x) [DOI] [Google Scholar]
- 54.Singer MS, Stireman JO. 2005. The tri-trophic niche concept and adaptive radiation of phytophagous insects. Ecol. Lett. 8, 1247–1255. ( 10.1111/j.1461-0248.2005.00835.x) [DOI] [Google Scholar]
- 55.Pringle EG, Novo A, Ableson I, Barbehenn RV, Vannette RL. 2014. Plant-derived differences in the composition of aphid honeydew and their effects on colonies of aphid-tending ants. Ecol. Evol. 4, 4065–4079. ( 10.1002/ece3.1277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houadria M, Blüthgen N, Salas-Lopez A, Schmitt M-I, Arndt J, Schneider E, Orivel J, Menzel F. 2016. The relation between circadian asynchrony, functional redundancy, and trophic performance in tropical ant communities. Ecology 97, 225–235. ( 10.1890/14-2466.1) [DOI] [PubMed] [Google Scholar]
- 57.Moreira X, Mooney KA, Zas R, Sampedro L. 2012. Bottom-up effects of host-plant species diversity and top-down effects of ants interactively increase plant performance. Proc. R. Soc. B 279, 4464–4472. ( 10.1098/rspb.2012.0893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuldt A, Fornoff F, Bruelheide H, Klein A-M, Staab M. 2017. Tree species richness attenuates the positive relationship between mutualistic ant–hemipteran interactions and leaf chewer herbivory. Proc. R. Soc. B 284, 20171489 ( 10.1098/rspb.2017.1489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hutchinson MC, Mora BB, Pilosof S, Barner AK, Kéfi S, Thébault E, Jordano P, Stouffer DB. 2019. Seeing the forest for the trees: putting multilayer networks to work for community ecology. Funct. Ecol. 33, 206–217. ( 10.1111/1365-2435.13237) [DOI] [Google Scholar]
- 60.Fornoff F, Klein A-M, Blüthgen N, Staab M. 2019. Data from: Tree diversity increases robustness of multi-trophic interactions Dryad Digital Repository. ( 10.5061/dryad.847km00) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Fornoff F, Klein A-M, Blüthgen N, Staab M. 2019. Data from: Tree diversity increases robustness of multi-trophic interactions Dryad Digital Repository. ( 10.5061/dryad.847km00) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.847km00 [60].