Abstract
Anthrax is a potentially life-threatening bacterial disease that can spread between wild and livestock animals and humans. Transmission typically occurs indirectly via environmental exposure, with devastating consequences for human and animal health, as well as pastoralist economies. India has a high annual occurrence of anthrax in some regions, but a country-wide delineation of risk has not yet been undertaken. The current study modelled the geographical suitability of anthrax across India and its associated environmental features using a biogeographic application of machine learning. Both biotic and abiotic features contributed to risk across multiple scales of influence. The elephant–livestock interface was the dominant feature in delineating anthrax suitability. In addition, water–soil balance, soil chemistry and historical forest loss were also influential. These findings suggest that the elephant–livestock interface plays an important role in the cycling of anthrax in India. Livestock prevention efforts targeting this interface, particularly within anthropogenic ecotones, may yield successes in reducing ongoing transmission between animal hosts and subsequent zoonotic transmission to humans.
Keywords: anthrax, landscape epidemiology, infection ecology, wildlife–livestock interface, India
1. Introduction
Anthrax is a disease of wide global distribution that primarily affects pastoralist communities [1]. A worldwide incidence of between 20 000 and 100 000 human cases per year has been estimated [2], with approximately 63.8 million pastoralists and livestock holders and 1.1 billion livestock animals estimated to be at risk [3]. Risk of human infection is greater in those that process or consume contaminated carcasses or animal (livestock/wildlife) products. Humans are accidental dead-end hosts and generally do not transmit the disease, whereas herbivores do because they often present with external hemorrhaging, edema, shock and sudden death, which contributes to environmental contamination and thus transmission to other hosts [4]. Sporadic, epizootic transmission also occurs in wildlife and can have devastating impacts, particularly when introduced to wild herbivore populations from livestock as in the case of Wood Bison in Canada or in wildlife in the small, anthropogenically stressed nature reserves adjacent to Kruger National Park in South Africa [1,5].
The causative agent of anthrax, Bacillus anthracis, can remain inactive, yet stable, in soil for up to several decades and across a spectrum of environmental conditions owing to the generation of spores. The formation of these spores is often assumed to require contact with atmospheric oxygen following the release of vegetative bacilli from the host at the time of death [1]; however, some evidence suggests that sporulation is due to the change in the partial pressure of carbon dioxide that follows release from the carcass [6]. Spores germinate into the vegetative form and replicate in the gut of mammalian hosts following accidental ingestion [1,6,7]. When these animals succumb to infection, they contaminate their environment and thereby continue the infection cycle. Additional dissemination in the environment can be modulated to a varying degree by scavengers and calliphorid or tabanid flies [8], which can promote subsequent infections in distal or proximate herbivores, while shared watering holes can facilitate spore aggregation and seasonal outbreaks [5].
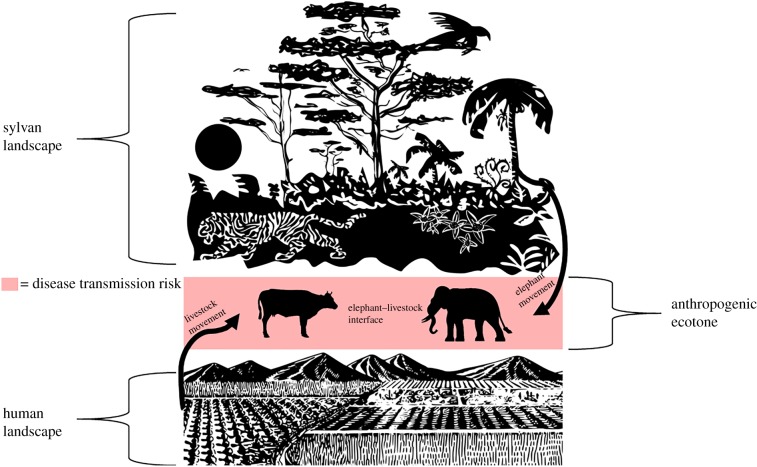
Anthropogenic pressure operates within several spheres of influence in domestic and sylvan landscapes to promote disease emergence [9]. Two such spheres of influence, the wildlife–livestock interface and habitat loss, may be particularly important in propagating the anthrax infection cycle. The wildlife–livestock interface, defined as the extent to which wildlife species and livestock interact (or have the potential to interact) directly or indirectly in a given landscape, has been recognized for some time as driving inter-species anthrax transmission between animals (figure 1), and subsequent potential spillover to humans [5,9–15]. The wildlife–livestock interface has been shown to enhance the ability of anthrax to persist in some areas, and may be a driver of enzootic transmission in tropical settings [10,12,16]. Habitat loss, particularly over long periods of time, can substantially alter the abundance, richness and movement patterns of the wildlife species that occupy the transitional spaces or ecotones [9,16–21]. This in turn can directly or indirectly influence wildlife–livestock interfaces [13,15]. Moreover, in the context of anthrax ecology, once a wildlife–livestock interface has been established in an ecotone, the characteristics of B. anthracis are such that interspecific transmission among mammals can modulate plant–animal interaction and vice versa. For example, it has been shown that soil contaminated by anthrax-infected carcasses can promote grasses favoured by grazing herbivores, which subsequently draw these mammals to the grasses in spore-containing soils [22,23].
Figure 1.
Representation of the elephant–livestock interface in anthropogenic ecotones. (Online version in colour.)
India has a high burden of anthrax, with some locations experiencing annual or near annual outbreaks of disease, with up to a dozen cases reported in a year [24–26]. In comparison, the European Union sees an average of fewer than 10 cases per year across the continent [27]. Most published multiyear annual incidence data for India is old and regionally specific, and formal anthrax surveillance is inconsistent across the country. This is because the Indian government is a federalist system and so investment in human and animal health services and surveillance is highly dependent on the commitments of individual states, which vary widely in resources and agenda over time. There is also regular detection of anthrax in wildlife in India, with a preponderance of reported cases in elephants [11,28–31], although comprehensive formal surveillance of wildlife is almost non-existent and so anthrax occurrence is probably under-reported.
The lack of robust anthrax surveillance notwithstanding, the over-representation of elephants may be due to their unique biological and ecological requirements in landscapes increasingly shared with humans and their livestock. Common points of interface between elephants and livestock in settings relevant to anthrax transmission include shared waterholes, salt licks and grazing meadows, particularly in the forest fringe areas. These also tend to be the areas where backyard animal husbandry, in the form of small and mixed holdings of grazing animals, is in closest proximity to wildlife. Conversely, higher reporting of anthrax in elephants may simply reflect their role as a sentinel species. Typically in the wild, dying or dead animals are quickly devoured by predators or scavengers. Thus detection of anthrax cases in wildlife is difficult because they are cleared before samples can be obtained. However, elephants remain in the landscape longer because their larger mass is not as readily cleared by scavengers. As such these large herbivores are likely to be epidemiologically important because a larger mass present for a longer time has the potential to contaminate more of the environment with spores. They are also logistically relevant because a larger mass present for a longer time is also more likely to be found and identified as an anthrax case. The current investigation sought to map geographical anthrax suitability, as projected from environmental feature-space, as a representation of epidemiological risk, while inferring ecological relationships between anthrax outbreaks and abiotic and biotic features. Specifically, we hypothesized that an increasing elephant–livestock interface and proximity to human-modified landscapes (specifically, historical forest loss) would delineate greater anthrax suitability in India.
2. Material and methods
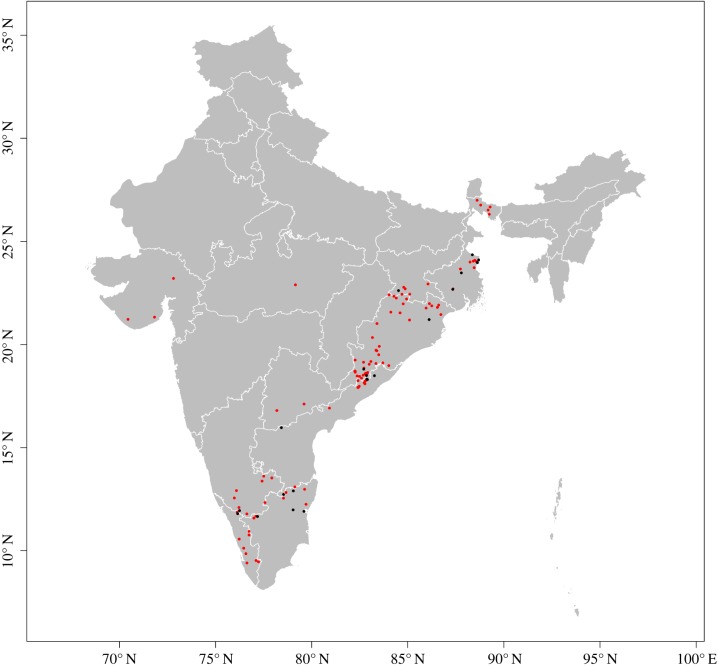
One hundred and three anthrax outbreaks between 1 January 2000 and 1 May 2018 were identified from the ProMED-mail electronic surveillance system. This system is maintained by the International Society of Infectious Diseases and provides archival documentation of formal and informal reports of infectious disease occurrences [25]. ProMED-mail reports are screened by a large team of editors, moderators, correspondents and sometimes country managers, who evaluate reports as well as engage the large group of locally sourced subscribers to offer additional insight in support of or against the alerts [32]. The data thus represent a specific cross-section of disease experience rather than a population-based sample. As such, while we emphasize that the scope of this study does not apply to the full spectrum of anthrax experience in India, we do correct for potential reporting bias inherent in the data (see Statistical Methods below), which minimizes reporting bias in the assessment of anthrax suitability in anthropogenic environments. Since the validity of ProMED-mail data for use in disease risk modelling was a secondary aim of this study, we also evaluated model performance using an independent sample of 22 laboratory-confirmed anthrax outbreaks, with investigations reported in the scientific literature (n = 17) [28,33–43] or by government agencies (n = 5) as captured by the EMPRES Global Animal Disease Information System (EMPRES-i; http://empres-i.fao.org), which is maintained by the Food and Agriculture Organization. Only one of these 22 outbreaks included wildlife (one elephant). This latter evaluation (see §3) has the added benefit of providing the first validation testing of ProMED-mail surveillance data in India to delineate risk of an important zoonotic infection. The distribution of reported ProMED-mail (red), and independent laboratory-confirmed (black), anthrax outbreaks in India from 1 January 2000 to 1 May 2018 is presented in figure 2.
Figure 2.
Distribution of anthrax outbreaks documented by the Pro-MED mail surveillance mechanism between 2000 and 2018 (red) and an independent sample of laboratory-confirmed outbreaks (black) used for model testing and evaluation. All maps are displayed only for the purposes of depicting the distribution of disease occurrence and risk, and do not reflect the authors' assertion of territory or borders of any sovereign country including India. Map direction and scale are represented by the latitude and longitude grid along the box. All maps are created in R (v. 3.3.1). (Online version in colour.)
The Gridded Livestock of the World (GLW) provided livestock densities for cattle, sheep and goats at 30 arcsec resolution (approx. 1 km) [44]. While subject to considerable spatial heterogeneity in error globally, within India livestock estimates were adjusted for (and thus constrained by) reported animal census data by polygon units at the 2nd and 3rd stage administrative levels, which corresponds to the district and taluk level, respectively, and provides an acceptable level of data verification at a sub-national and sub-state scale of moderate resolution [44]. Because the current aim was to assess anthrax suitability associated with livestock presence, a combined livestock raster was created based on the sum of the absolute number of cattle, sheep and goats per unit area, rather than calculating livestock units [45], because evaluating the differential impact of different livestock species on the grazing and browsing capacity of land parcels was beyond the scope and capacity of this study given the lack of sufficiently fine-scaled environmental or outbreak data. The Global Biodiversity Information Facility (GBIF) was used to identify observed wild elephants (Elephas maximus) only across India so their ecological niche could be modelled and used as an anthrax sentinel species (http://www.gbif.org/). These two data products, livestock density and the elephant niche, were then applied to the quantification of the elephant–livestock interface (see below), which delineates a sentinel interface only rather than a broader interface between livestock and wildlife more generally. The elephant niche was chosen because of the species's importance as both an anthrax sentinel [11,28–31] and the common overlap of their range with that of grazing livestock in forest fringe areas [34]. In addition, we tested the validity of the elephant–livestock interface by substituting the modelled niche, which was based on GBIF observations, with the accepted species range according to IUCN data, which was obtained from the Socioeconomic Data and Applications Center (SEDAC) [46] at the same 30 arcmin resolution (see below).
Climate data were obtained from the WorldClim Global Climate database [47]. The mean annual temperature was calculated using aggregate spatio-temporal weather station data between 1950 and 2000, and extracted as a 30 arcsec resolution raster [48]. The Priestley–Taylor α coefficient (P-Tα) was used to represent water–soil balance [49,50]. The P-Tα is the ratio of actual evapotranspiration to potential evapotranspiration, and captures water availability in the soil, as well as the local vegetation's water requirements, as contrasted with the solar energy input. Thus, P-Tα is a robust estimate of environmental water stress through soil–water balance. The raster data were acquired at 30 arcsec resolution from the Consultative Group for International Agricultural Research (CGIAR) Consortium for Spatial Information. The ratio is dimensionless and ranges from 0 (extreme water stress) to 1 (no water stress) [51].
Soil pH and organic content data were obtained from the Global Soil Dataset for Earth System Modelling, which is based on an improved protocol of the Harmonized World Soil Database [52]. These two rasters are recorded at 5 arcmin (approx. 10 km).
Historical forest data from 1900 were derived from the History Database of Global Environment (HYDE) anthrome data product at 5 arcmin resolution and compared to forest cover in 2000 [53,54]. The HYDE database is the result of an ongoing development by the Netherlands Environmental Assessment Agency to describe human population growth and land use change from 10 000 BCE to the present day. Estimation of land cover and land use is based on an amalgam of satellite image data, historical sub-national statistical inventories, ecosystem envelopes based on climate and geological (soil) properties, and archeological data [53,54]. The difference in proximity to historical forest cover (i.e. forest cover that was present in 1900) and modern forest cover (i.e. forest cover present in 2000) was thus calculated and evaluated with respect to anthrax suitability.
The sampling of background points was weighted using the human footprint (HFP) to correct for potential reporting bias in anthrax presence points (see modelling description below).The HFP was quantified using data obtained from SEDAC [55], and comprises two stages [56]. First, the human influence index (HII) describes the impact of human influence based on eight domains: (i) population density, (ii) proximity to railroads, (iii) proximity to roads, (iv) proximity to navigable rivers, (v) proximity to coastlines, (vi) intensity of night-time artificial light, (vii) location in or outside urban space, and (viii) land cover. These domains are scored and quantify the level of human impact per item per 1 km2. The eight domains are then combined to form a composite index, which ranges from 0, indicating an absence of human impact, to 64, indicating maximal human impact. The HII is then normalized to the 15 terrestrial biomes defined by the World Wildlife Fund to obtain the HFP. The normalization is the ratio of the range of minimum and maximum HII in each biome to the range of minimum and maximum HII across all biomes, and is expressed as a percentage [56].
3. Statistical analysis
Anthrax suitability in India, as well as the ecological niche of elephants, was modelled using maximum entropy (Maxent) machine learning. Machine learning is now widely applied to the modelling of the geographical suitability of many zoonoses [57–59], and Maxent is an analytically appealing approach because the model is not constrained by a specific functional form. Additionally, the system can be modelled without knowledge of the locations of unknown (and unknowable) anthrax outbreak absences [59,60]. Maxent has become a popular implementation to ecological niche modelling due to its robustness [61].
A metric for the elephant–livestock interface was constructed by weighting the density of all livestock in each 1 km2 space by the ecological niche of E. maximus, which was estimated by a separate Maxent model using observations of E. maximus from the GBIF, as described above. The new weighted metric raster was the product of the livestock density raster and the probability density function raster of the estimated niche. As such, the interface is a representation of the number of livestock present in a given space adjusted by the probability of that space being a suitable habitat for elephants.
Annual temperature, P-Tα, soil chemistry, the difference in forest cover between 1900 and 2000, the elephant–livestock interface, and livestock density were included in the Maxent model of anthrax suitability (the distributions of environmental features are presented in electronic supplementary material; figure S1). All covariates were aggregated to scales of 5 and 30 arcmin, respectively, for the two spatial scales modelled separately in this study (see below). Correlation was low (all values of ρ were less than 0.6) among all the environmental covariates under consideration. A total of 10 000 background points were sampled, weighted by HFP as described above to correct for reporting bias in anthrax surveillance [62]. Five-fold cross-validation was used to train the model, and the area under the receiver operating characteristic curve (AUC) was used to evaluate model performance. To correct for overfitting, the regularization parameter was set at 1.0. Covariates were ranked by permutation importance, which is a random permutation of their values between background and presence points during training [59,63]. With all available covariates under consideration, the best model was selected based on performance and fit using (i) the test AUCs between the full and reduced models, (ii) the Akaike information criterion (AIC) based on a Poisson point process to measure goodness-of-fit [64] and (iii) a jackknife variable selection procedure wherein each covariate's single contribution to the loss function is compared to the loss function when the covariate is withheld from the model. Because the surveillance data were derived from the ProMED system, the model performance was evaluated using the independent testing dataset described above, which comprised a separate independent sample of laboratory-confirmed anthrax outbreaks. Reported AUCs, therefore, reflect the externally validated model performance.
As some recent work identified, the relationship between infectious diseases and abiotic and biotic features may depend on a spatial scale [65]. We therefore include two sensitivity analyses to evaluate whether these features operate differently at different scales. First, the models were evaluated at 5 arcmin and 30 arcmin to determine whether predicted anthrax suitability was robust to scale and whether the abiotic and biotic features in the model demonstrated scale-dependence. Second, two additional reduced models, one abiotic (climate, soil chemistry, and forest loss) and one biotic (livestock density and the wildlife–livestock interface), were compared to the best-fitting model with respect to model performance (AUC), model fit (AIC) and niche equivalency. The latter is an assessment of the degree to which predicted niches are coincident with each other [66]. The final sensitivity analysis included the evaluation of the representation of the elephant–livestock interface, as constructed first using the modelled niche, and second using the IUCN delineated range, for elephant presence. The effect of each interface construct on anthrax suitability was compared with respect to AUC, AIC, and niche equivalency. The Maxent function (dismo package; v. 0.9-3) was used to fit the models [60,67,68]. All analyses were performed using R statistical software v. 3.1.3 [69]. The vector images used to create figure 1 was created by the authors with use of some public domain content obtained from https://publicdomainvectors.org/en/ and distributed without restriction under Creative Commons CC0 1.0 Universal.
4. Results
The best fitting and performing model from all those considered (electronic supplementary material, Model 6, table S2 and figure S3) is presented in figure 3. The elephant–livestock interface was the dominant feature delineating anthrax suitability (permutation importance (PI) = 67.7%). Livestock density (PI = 10.5%), soil–water balance (PI = 7.1%), soil pH (4.7%), and proximity to forest lost between 1900 and 2000 (PI = 4.0%) were also influential to anthrax suitability. When the model was validated against the independent testing data, it performed reasonably well with AUC equal to 89%, and demonstrated the best fit (lowest AIC), compared to the other models (electronic supplementary material, table S2). The jackknife variable selection model largely agreed with the final model above with respect to variable importance (electronic supplementary material, figure S4), although the livestock density appeared to contribute the least information to the model by itself and to have relatively little information present that is not already present in other variables. Therefore, a further reduced model with the livestock density omitted was also considered (electronic supplementary material, Model 7, table S2). The jackknife results notwithstanding, the reduced model with livestock omitted performed only slightly worse, but provided a noticeably poorer fit and so the model with livestock density included was retained as the final model.
Figure 3.
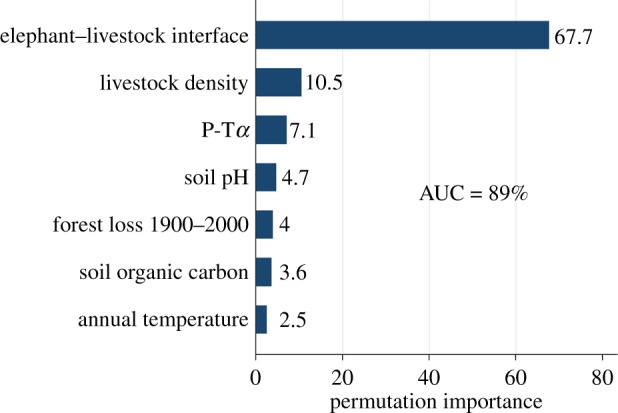
Environmental feature ranking by permutation importance in the Maxent model. The area under the curve (AUC) is reported as a percentage. (Online version in colour.)
Given the strong association with the elephant–livestock interface, which used the elephant ecological niche to construct the interface, there was concern that the model fit and performance may be driven by elephant anthrax outbreaks specifically. As a sensitivity analysis, the 20 elephant outbreaks were removed from the training data and the Maxent model refitted to the remaining 83 outbreaks. This model was similar in performance (AUC = 87%) and fit (AIC = 224) and demonstrated exceptional overlap in suitability (niche equivalency = 0.99; electronic supplementary material, figure S5), suggesting that the inclusion of elephant outbreaks was not responsible for the close association between anthrax suitability and the elephant–livestock interface.
The elephant–livestock interface did not appear to operate differently with respect to anthrax suitability at different scales (electronic supplementary material, table S2, Model 6: 5 arcmin versus 30 arcmin), while soil pH and forest loss did appear to exhibit a moderately increased impact at a smaller scale (30 arcmin) than at a larger scale (5 arcmin). This scale dependence was further confirmed by the abiotic model (electronic supplementary material, table S2, Model 6: 5 arcmin versus 30 arcmin), which evaluated these features independently of the livestock density and the elephant–livestock interface. Despite the moderate scale dependence observed for some of the abiotic features, the predicted suitability equivalency remained high when comparing the full model to the biotic model (niche equivalency at 5 arcmin = 0.97; niche equivalency at 30 arcmin = 0.97) or comparing the full model to the abiotic model (niche equivalency at 5 arcmin = 0.95; niche equivalency at 30 arcmin = 0.95). Finally, the use of the modelled elephant niche based on GBIF observations, compared to the IUCN-delineated elephant range, demonstrated better fit (AIC = 216 versus AIC = 220, respectively), better performance (AUC = 89 versus AUC = 87.5, respectively), and high equivalency (niche equivalency = 0.97). As such, and because the GBIF-modelled niche is based on observations recorded over roughly the same period of time as the period of recorded anthrax occurrences, the modelled niche is retained for the elephant–livestock interface construct.
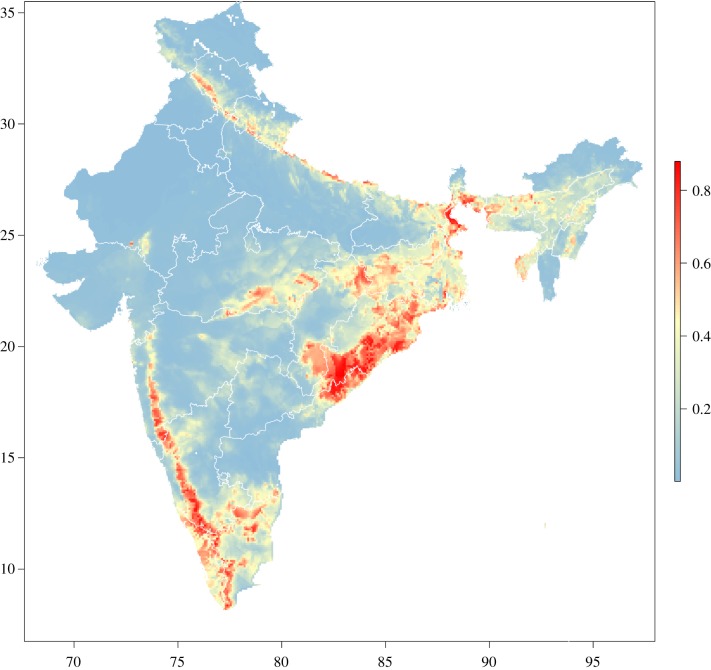
Predicted anthrax suitability across India based on the best-fitting and best-performing model is displayed in figure 4. Two distinct corridors emerged: a wide distribution of high suitability running from northeast Andhra Pradesh to West Bengal in the east, and a narrower distribution of high suitability running the length of the Western Ghats and their ecotonal fringes from north Kerala to southern Maharashtra in the west.
Figure 4.
Predicted geographical anthrax suitability across India based on the best-fitting and best-performing Maxent model (Model 6). Boundaries reflect individual states. The colour spectrum of anthrax suitability runs from blue (low suitability) to red (high suitability). All maps are displayed only for the purposes of depicting the distribution of disease occurrence and risk, and do not reflect the authors’ assertion of territory or borders of any sovereign country including India. Map direction and scale are represented by the latitude and longitude grid along the box. All maps are created in R (v. 3.3.1).
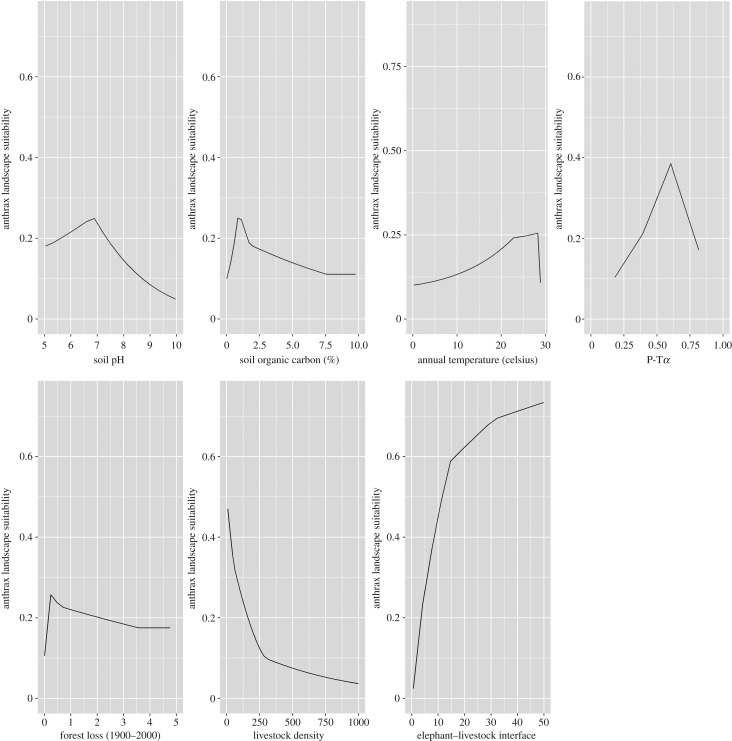
Figure 5 presents the response curves depicting the functional relationship between anthrax suitability and each environmental feature conditional on all others. Anthrax suitability was highest with a soil pH in the range of 6–8 and soil organic carbon content in the range of 1%–2%. Suitability increased with increasing soil–water balance until peaking at P-Tα = 0.6, which signifies low water stress. Anthrax suitability increased sharply with an increasing elephant–livestock interface. Finally, increasing proximity to lost forest was associated with increasing anthrax suitability, with peak suitability within a window of 10 km of forest loss between 1900 and 2000.
Figure 5.
Response curves showing the functional relationships between each feature and anthrax suitability. (Online version in colour.)
5. Discussion
This study presents the first description of anthrax suitability across India. The elephant–livestock interface was the most influential feature to anthrax suitability at relatively large and small scales, and even when anthrax outbreaks did not directly involve elephants. Moreover, this finding was consistent with the highest anthrax suitability manifesting in relatively small livestock herds, which would be more likely to occupy forest fringe than large commercial herds.
The relationship between anthrax suitability and the elephant–livestock interface is unsurprising because it has been shown that intense competition for resources exists between livestock and wild grazing and browsing species, especially elephants, in precisely the same areas as those identified as high risk in the current study [34]. Moreover, relatively small free-ranging livestock herds will present the greatest opportunity for interface with sylvan species in anthropogenic ecotones. This was evident in the current study wherein greater anthrax suitability was associated with smaller herds. Both the Eastern and Western Ghats have stretches of forest covering several states with free ranging wild elephants through known corridors. These forests are often occupied by human habitations, particularly traditional forest-living people, for whom this elephant–livestock interface is a constant reality. Moreover, the strong association between anthrax outbreaks and the elephant–livestock interface was not simply driven by the inclusion of elephant outbreaks in the training data, as this association remained strong even after the elephant outbreaks were removed (electronic supplementary material, figure S5). This lends further credence to this interface representing an important feature of anthrax transmission in the landscape, whether the transmission involves elephants or livestock. Similar wildlife–livestock interface foci have been identified in anthrax hotspots in other tropical settings as well [10,12], although investigation of the interface between livestock and other wildlife species was beyond the scope or capacity of the current study.
Soil pH in this study was generally reflective of the preferred pedological profile of B. anthracis with suitability peaking in the 6–8 range, which is typical for these bacteria in many parts of the world [6,7,70,71]. In contrast to temperate climate regimes [72], anthrax suitability peaked at the lowest levels of the organic carbon content, but this was probably indicative of the relative homogeneity of generally low soil organic carbon across much of India. Similar homogeneity was observed for annual temperature but not soil–water balance. The relationship with soil–water balance was interesting because, while the current study identified decreased water stress to be associated with greater anthrax suitability in India, increased water stress was associated with greater suitability across the temperate zones of the Northern Hemisphere [72].
The association between anthrax suitability and forest loss, albeit considerably weaker than the elephant–livestock interface, is also intuitive and likely to be an important modulator of the interface. This novel finding may suggest a ‘revenant’ forest presence that reflects historical cycling of anthrax between elephants, or wildlife more generally, and livestock, or it may simply reflect an increased proclivity to modern cycling in transformed sylvan landscapes. Large mammalian herbivores require extensive forest range in which to graze or browse. Elephant caloric requirements, for example, represent an extreme in plant intake and consequent home range. Adults will consume an average of 150 kg of vegetation per day, and can forage a variety of plants comprising 75 species across a range of 25 km2 (monsoon season) to 64 km2 (dry season) in southern India [73]. The loss of forested habitat across India has reduced the available sylvan range for elephants, forcing them increasingly into anthropogenic ecotones wherein the potential for interspecific contact is substantial [74,75]. It is therefore expected that these transitional zones will reflect a trajectory of forest loss and, given the ability of spores to persist for decades in the environment, potential for long-term cycling of anthrax between wildlife and livestock. However, the current data are too limited in temporal granularity to make this claim definitely, as anthrax cycling could also reflect a more recent introduction into ecotonal areas by relatively new livestock herds. Nevertheless, the association with the elephant–livestock interface combined with close proximity to historical forest loss suggests the protection of wildlife populations and forest management, with concerted effort to maintain separation between wildlife and livestock, may be a fruitful approach to anthrax prevention in those areas of highest risk in India.
This study has some limitations that warrant further discussion. First, the anthrax outbreaks used to train the models in this study are based on ProMED-mail surveillance, which we recognize does not identify all outbreaks. In particular, the responsiveness potential of the surveillance system varies by state according to the quality of veterinary services and reporting infrastructure. This may lead to reporting bias in the identified anthrax locations. We attempted to correct for such reporting bias by selecting background points weighted by the presence of the human footprint as a proxy for reporting infrastructure. In addition, we tested the fitted model against an independent laboratory-confirmed sample of anthrax outbreaks to provide a less biased, externally validated, assessment of performance. Nevertheless, we concede that the data may not be representative of the complete anthrax experience in India over the last two decades and that there may be some residual bias toward larger outbreaks. Second, even at the larger of the two scales considered here, the scale of the study is coarse by virtue of the scale of ProMED-mail reporting and the available environmental data. While this is unlikely to be of substantial influence to abiotic environmental features, which are expected to dominate at small (i.e. coarse) spatial scale, it may be influential to biotic features, which are expected to dominate at large (i.e. fine) scale [65]. Third, climate features (PT-α and mean annual temperature) were constructs of decadal averages from 1950 to 2000, and therefore the models presented here assume temporal homogeneity of these aggregates both during the period in which they were recorded as well as during the period of observed anthrax outbreaks under current investigation. Fourth, we acknowledge that the elephant–livestock interface was not based on our own systematic observations and counts of animals in the field, but rather relied on estimates of elephant and livestock presence based on GBIF and GLW data, respectively, which may be subject to spatial heterogeneity that could not be completely accounted for in this study.
In conclusion, this study has provided the first country-wide predictions of anthrax suitability for India and has found that this suitability is strongly associated with the elephant–livestock interface as represented by the presence of livestock across the spectrum of the elephant niche. While this study cannot claim whether this association is due to the specific ecology of elephants in ecotones, or whether it is simply due to their capacity to function as important anthrax sentinels, we can claim that interventions directed at preventing transmission at this specific interface may be an important step toward averting outbreaks of anthrax. Moreover, while not as impactful as the elephant–livestock interface, the concurrent influence of historical forest loss lends further support to the potential importance of anthropogenic ecotones in the ongoing transmission of anthrax in India. The high suitability corridors running from Andhra Pradesh to West Bengal in the east, and the forest fringes of the Western Ghats from Kerala to southern Maharashtra in the west would be appropriate targets for improved biosurveillance, as well as deeper field investigations of anthrax disease ecology. More targeted biosurveillance in these areas, coupled with an informed distribution of vaccines, could help in the allocation of limited resources at the state level to improve both human and animal health. Finally, these findings highlight the potential benefits of a One Health approach to anthrax prevention and control, incorporating the expertise and spheres of influence of state veterinary, forest management, and human health services. Collaborative surveillance of human and animal disease events, coupled with policy advocacy for prevention strategies such as targeted vaccine deployment and environmental conservation, have demonstrated the successful One Health paradigm for anthrax control in the European Union [27]. Given the findings from the current study, similar successes could be expected from equitable transdisciplinary approaches to anthrax control in India.
Supplementary Material
Data accessibility
Outbreak data are available at https://figshare.com/s/2ca888024bc1d873842e.
Authors' contributions
M.G.W. carried out methodological conceptualization, modelling, and writing the manuscript and editing drafts; S.M.M. carried out methodological conceptualization, writing the manuscript and editing drafts; S.H. carried out experiential and methodological conceptualization, modelling, and writing the manuscript and editing drafts.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.WHO. 2008. Anthrax in humans and animals. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- 2.Friedlander AM. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20, 335–349. [PubMed] [Google Scholar]
- 3.Carlson CJ, et al. 2018. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock, and wildlife. bioRxiv . ( ) [DOI]
- 4.Bauerfeind R, et al. 2016. Zoonoses: infectious diseases transmissible between animals and humans, 4th edn., pp. 175–179. Washington, DC: American Society for Microbiology Press. [Google Scholar]
- 5.Hugh-Jones ME, de Vos V.. 2002. Anthrax and wildlife. Rev. Sci. Tech. 21, 359–383. ( 10.20506/rst.21.2.1336) [DOI] [PubMed] [Google Scholar]
- 6.Hugh-Jones M, Blackburn J. 2009. The ecology of Bacillus anthracis. Mol. Aspects Med. 30, 356–367. ( 10.1016/j.mam.2009.08.003) [DOI] [PubMed] [Google Scholar]
- 7.Dragon DC, Rennie RP. 1995. The ecology of anthrax spores: tough but not invincible. Can. Vet. J. 36, 295–301. [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos V. 1990. The ecology of anthrax in the Kruger National Park, South Africa. Salisbury Med. Bull. 68, 19–23. [Google Scholar]
- 9.Hassell JM, Begon M, Ward MJ, Fèvre EM. 2017. Urbanization and disease emergence: dynamics at the wildlife–livestock–human interface. Trends Ecol. Evol. 32, 55–67. ( 10.1016/j.tree.2016.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwakapeje ER, Høgset S, Fyumagwa R, Nonga HE, Mdegela RH, Skjerve E. 2018. Anthrax outbreaks in the humans–livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006–2016. BMC Public Health 18, 106 ( 10.1186/s12889-017-5007-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh BB, Gajadhar AA. 2014. Role of India's wildlife in the emergence and re-emergence of zoonotic pathogens, risk factors and public health implications. Acta Trop. 138, 67–77. ( 10.1016/j.actatropica.2014.06.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turnbull PC, Doganay M, Lindeque PM, Aygen B, McLaughlin J. 1992. Serology and anthrax in humans, livestock and Etosha National Park wildlife. Epidemiol. Infect. 108, 299–313. ( 10.1017/S0950268800049773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones BA, et al. 2013. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci USA 110, 8399–8404. ( 10.1073/pnas.1208059110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiethoelter AK, Beltrán-Alcrudo D, Kock R, Mor SM. 2015. Global trends in infectious diseases at the wildlife-livestock interface. Proc. Natl. Acad. Sci. USA 112, 9662–9667. ( 10.1073/pnas.1422741112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones B, et al. 2011. Zoonoses (Project 1): Wildlife/domestic livestock interactions. London, UK: Royal Veterinary College.
- 16.Bengis RG, Kock RA, Fischer J. 2002. Infectious animal diseases: the wildlife/livestock interface. Rev. Sci. Tech. 21, 53–65. ( 10.20506/rst.21.1.1322) [DOI] [PubMed] [Google Scholar]
- 17.Turner IM. 1996. Species loss in fragments of tropical rain forest: a review of the evidence. J. Appl. Ecol. 33, 200 ( 10.2307/2404743) [DOI] [Google Scholar]
- 18.Gascon C, et al. 1999. Matrix habitat and species richness in tropical forest remnants. Biol. Conserv. 91, 223–229. ( 10.1016/S0006-3207(99)00080-4) [DOI] [Google Scholar]
- 19.Cleaveland S, Laurenson MK, Taylor LH. 2001. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Phil. Trans. R. Soc. Lond. B 356, 991–999. ( 10.1098/rstb.2001.0889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurance WF. 1997. Responses of mammals to rainforest fragmentation in tropical Queensland: a review and synthesis. Wildl. Res. 24, 603 ( 10.1071/WR96039) [DOI] [Google Scholar]
- 21.Steinfeld H, de Haan C, Blackburn H. 1998. Livestock and the environment: issues and options. In Agriculture and the environment: perspectives on sustainable rural development, p. 383 Washington, DC: World Bank. [Google Scholar]
- 22.Turner WC, et al. 2014. Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proc. R. Soc. B 281, 20141785 ( 10.1098/rspb.2014.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganz HH, et al. 2014. Interactions between Bacillus anthracis and plants may promote anthrax transmission. PLoS Negl. Trop. Dis. 8, e2903 ( 10.1371/journal.pntd.0002903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thappa DM, Karthikeyan K. 2001. Anthrax: an overview within the Indian subcontinent. Int. J. Dermatol. 40, 216–222. ( 10.1046/j.1365-4362.2001.01174.x) [DOI] [PubMed] [Google Scholar]
- 25.Lalitha MK, Kumar A. 1996. Anthrax in southern India. Lancet 348, 553–554. ( 10.1016/S0140-6736(05)64718-6) [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Kanungo R, Bhattacharya S, Badrinath S, Dutta TK, Swaminathan RP. 2000. Human anthrax in India: urgent need for effective prevention. J. Commun. Dis. 32, 240–246. [PubMed] [Google Scholar]
- 27.Cross AR, Baldwin VM, Roy S, Essex-Lopresti AE, Prior JL, Harmer NJ. 2018. Zoonoses under our noses. Microbes Infect. 21, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Priya PM, Mini M, Rameshkumar P, Jayesh V, Jayesh V. 2009. A case of anthrax in wild elephant from the Western Ghats region of Kerala, India, J. Threat Taxa. 1, 192–193. ( 10.11609/JoTT.o1756.192-3) [DOI] [Google Scholar]
- 29.Krishnamurthy V, Wemmer C. 1995. Veterinary care of Asian timber elephants in India: historical accounts and current observations. Zoo Biol. 14, 123–133. ( 10.1002/zoo.1430140206) [DOI] [Google Scholar]
- 30.Mustafa AH. 1984. Isolation of Anthrax bacillus from an elephant in Bangladesh. Vet. Rec. 114, 590 ( 10.1136/vr.114.24.590) [DOI] [PubMed] [Google Scholar]
- 31.Choudhury A. 1999. Status and conservation of the Asian Elephant Elephas maximus in north-eastern India. Mamm. Rev. 29, 141–174. ( 10.1046/j.1365-2907.1999.00045.x) [DOI] [Google Scholar]
- 32.International Society for Infectious Diseases. ProMED-mail. See http://www.promedmail.org/.
- 33.Rao T, Venkatachalam K, Ahmed K, Padmaja I, Bharthi M, Rao P. 2009. A mini outbreak of cutaneous anthrax in Vizianagaram district, Andhra Pradesh, India. Indian J. Dermatol. Venereol. Leprol. 75, 416 ( 10.4103/0378-6323.53158) [DOI] [PubMed] [Google Scholar]
- 34.Madhusudan MD. 2004. Recovery of wild large herbivores following livestock decline in a tropical Indian wildlife reserve. J. Appl. Ecol. 41, 858–869. ( 10.1111/j.0021-8901.2004.00950.x) [DOI] [Google Scholar]
- 35.Rao GRR, Padmaja J, Lalitha MK, Rao PVK, Kumar HK, Gopal KVT, Mohanraj P. 2007. Cutaneous anthrax in a remote tribal area? Araku Valley, Visakhapatnam district, Andhra Pradesh, southern India. Int. J. Dermatol. 46, 55–58. ( 10.1111/j.1365-4632.2006.03043.x) [DOI] [PubMed] [Google Scholar]
- 36.Vijaikumar M, Thappa DM, Karthikeyan K. 2002. Cutaneous anthrax: an endemic outbreak in south India. J. Trop. Pediatr. 48, 225–226. ( 10.1093/tropej/48.4.225) [DOI] [PubMed] [Google Scholar]
- 37.Rao GRR, et al. 2018. An outbreak of cutaneous anthrax in a non-endemic district—Visakhapatnam in Andhra Pradesh. Indian J. Dermatol. Venereol. Leprol. 71, 102–105. ( 10.4103/0378-6323.13994) [DOI] [PubMed] [Google Scholar]
- 38.Ray TK, Hutin YJ, Murhekar MV. 2009. Cutaneous Anthrax, West Bengal, India, 2007. Emerg. Infect. Dis. 15, 497–499. ( 10.3201/eid1503.080972) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharya G, Bhattacharyya I, Kundu PK, Hoque MS. 2013. Cutaneous anthrax: A case report. J. Evol. Med. Dent. Sci. 2, 9576–9580. ( 10.14260/jemds/1660) [DOI] [Google Scholar]
- 40.Chakraborty PP, et al. 2012. Outbreak of cutaneous anthrax in a tribal village: a clinico-epidemiological study. J. Assoc. Physicians India. 60, 89–93. [PubMed] [Google Scholar]
- 41.Reddy R, Parasadini G, Rao P, Uthappa CK, Murhekar MV. 2012. Outbreak of cutaneous anthrax in Musalimadugu village, Chittoor district, Andhra Pradesh, India, July–August 2011. J. Infect. Dev. Countries. 6, 695–699. ( 10.3855/jidc.2635) [DOI] [PubMed] [Google Scholar]
- 42.Sekhar PC, Singh RS, Sridhar MS, Bhaskar CJ, Rao YS. 1990. Outbreak of human anthrax in Ramabhadrapuram village of Chittoor district in Andhra Pradesh. Indian J. Med. Res. 91, 448–452. [PubMed] [Google Scholar]
- 43.Mondal T, Ghosh S, Dasgupta S, Sarkar A. 2015. Suspected anthrax outbreak: investigation in a rural block of west Bengal and public health response. Indian J. Public Health. 59, 302 ( 10.4103/0019-557X.169662) [DOI] [PubMed] [Google Scholar]
- 44.Robinson TP, et al. 2014. Mapping the global distribution of livestock. PLoS ONE 9, e96084 ( 10.1371/journal.pone.0096084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Upton M. 2011. Guidelines for the preparation of livestock sector review. In FAO animal production and health guidelines. Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 46.Socioeconomic Data and Applications Center (SEDAC). 2015. Global Mammal Richness Grids. See http://sedac.ciesin.columbia.edu/data/set/species-global-mammal-richness-2015.
- 47.WorldClim—Global Climate. 2014. Data for current conditions (∼1950–2000). See http://www.worldclim.org/current.
- 48.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 49.Priestley CHB, Taylor RJ. 1972. On the assessment of surface heat flux and evaporation using large-scale parameters. Mon. Weather Rev. 100, 81–92. () [DOI] [Google Scholar]
- 50.Khaldi A, Khaldi A, Hamimed A. 2014. Using the Priestley-Taylor expression for estimating actual evapotranspiration from satellite Landsat ETM+data. Proc. Int. Assoc. Hydrol. Sci. 364, 398–403. [Google Scholar]
- 51.Trabucco A, Zomer RJ. 2010. Global soil water balance geospatial database. Montpellier, France: CGIAR Consortium for Spatial Information. [Google Scholar]
- 52.Shangguan W, Dai Y, Duan Q, Liu B, Yuan H. 2014. A global soil data set for earth system modeling. J. Adv. Model. Earth Syst. 6, 249–263. ( 10.1002/2013MS000293) [DOI] [Google Scholar]
- 53.Klein GK, Beusen A, Van Drecht G, De Vos M.. 2011. The HYDE 3.1 spatially explicit database of human-induced global land-use change over the past 12,000 years. Glob. Ecol. Biogeogr. 20, 73–86. ( 10.1111/j.1466-8238.2010.00587.x) [DOI] [Google Scholar]
- 54.Klein GK, Beusen A, Doelman J, Stehfest E. 2017. Anthropogenic land use estimates for the Holocene: HYDE 3.2. Earth Syst. Sci. Data 9, 927–953. ( 10.5194/essd-9-927-2017) [DOI] [Google Scholar]
- 55.Socioeconomic Data and Applications Center 2014 Last of the Wild, v2. see http://sedac.ciesin.columbia.edu/data/collection/wildareas-v2/methods. [cited cited 23 December 2014].
- 56.Sanderson EW, Jaiteh M, Levy MA, Redford KH, Wannebo AV, Woolmer G. 2002. the human footprint and the last of the wild. Bioscience 52, 891 ( 10.1641/0006-3568(2002)052[0891:THFATL]2.0.CO;2) [DOI] [Google Scholar]
- 57.Hay SI, et al. 2013. Global mapping of infectious disease. Phil. Trans. R. Soc. B 368, 20120250 ( 10.1098/rstb.2012.0250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stevens KB, Pfeiffer DU. 2011. Spatial modelling of disease using data- and knowledge-driven approaches. Spat. Spatiotemporal Epidemiol. 2, 125–133. ( 10.1016/j.sste.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 59.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. ( 10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 60.Franklin J. 2010. Mapping species distributions: spatial inference and prediction. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 61.Duan R-Y, Kong X-Q, Huang M-Y, Fan W-Y, Wang Z-G. 2014. The predictive performance and stability of six species distribution models. PLoS ONE 9, e112764 ( 10.1371/journal.pone.0112764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phillips SJ, et al. 2009. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. esa 19, 181–197. ( 10.1890/07-2153.1) [DOI] [PubMed] [Google Scholar]
- 63.Phillips SJ, Dudík M. 2008. Modeling of species distribution with Maxent: new extensions and a comprehensive evalutation. Ecograpy 31, 161–175. ( 10.1111/j.0906-7590.2008.5203.x) [DOI] [Google Scholar]
- 64.Renner IW, Warton DI. 2013. Equivalence of MAXENT and Poisson point process models for species distribution modeling in ecology. Biometrics 69, 274–281. ( 10.1111/j.1541-0420.2012.01824.x) [DOI] [PubMed] [Google Scholar]
- 65.Cohen JM, Civitello DJ, Brace AJ, Feichtinger EM, Ortega CN, Richardson JC, Sauer EL, Liu X, Rohr JR. 2016. Spatial scale modulates the strength of ecological processes driving disease distributions. Proc. Natl. Acad. Sci. USA 113, E3359–E3364. ( 10.1073/pnas.1521657113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warren DL, Glor RE, Turelli M. 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868–2883. ( 10.1111/j.1558-5646.2008.00482.x) [DOI] [PubMed] [Google Scholar]
- 67.Hijmans RJ, Phillips S, Leathwick JR, Elith J. 2014. Package ‘dismo.’ see https://cran.r-project.org/web/packages/dismo/dismo.pdf. [Google Scholar]
- 68.Fielding AH, Bell JF. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49. ( 10.1017/S0376892997000088) [DOI] [Google Scholar]
- 69.R Core Team. 2016. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 70.Foerster HF, Foster JW. 1966. Endotrophic calcium, strontium, and barium spores of Bacillus megaterium and Bacillus cereus. J. Bacteriol. 91, 1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stastná J, Vinter V. 1970. Spores of microorganisms. 23. Interdependence of intra- and extra-cellular levels of calcium: its effect on the germination of bacterial spores in different media. Folia Microbiol (Praha). 15, 103–110. ( 10.1007/BF02880091) [DOI] [PubMed] [Google Scholar]
- 72.Walsh MG, de Smalen AW, Mor SM. 2018. Climatic influence on anthrax suitability in warming northern latitudes. Sci. Rep. 8, 9269 ( 10.1038/s41598-018-27604-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shoshani J, Eisenberg JF. 1982. Elephas maximus Mamm. Species. 182, 1–8. ( 10.2307/3504045) [DOI] [Google Scholar]
- 74.Choudhury A. 2004. Human–elephant conflicts in Northeast India. Hum. Dimens. Wildl. 9, 261–270. ( 10.1080/10871200490505693) [DOI] [Google Scholar]
- 75.Kumar MA, Mudappa D, Raman TRS. 2010. Asian elephant Elephas maximus habitat use and ranging in fragmented rainforest and plantations in the Anamalai Hills, India. Trop. Conserv. Sci. 3, 143–158. ( 10.1177/194008291000300203) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Outbreak data are available at https://figshare.com/s/2ca888024bc1d873842e.