Abstract
Reef-building corals typically live close to the upper limits of their thermal tolerance and even small increases in summer water temperatures can lead to bleaching and mortality. Projections of coral reef futures based on forecasts of ocean temperatures indicate that by the end of this century, corals will experience their current thermal thresholds annually, which would lead to the widespread devastation of coral reef ecosystems. Here, we use skeletal cores of long-lived Porites corals collected from 14 reefs across the northern Great Barrier Reef, the Coral Sea, and New Caledonia to evaluate changes in their sensitivity to heat stress since 1815. High-density ‘stress bands’—indicative of past bleaching—first appear during a strong pre-industrial El Niño event in 1877 but become significantly more frequent in the late twentieth and early twenty-first centuries in accordance with rising temperatures from anthropogenic global warming. However, the proportion of cores with stress bands declines following successive bleaching events in the twenty-first century despite increasing exposure to heat stress. Our findings demonstrate an increase in the thermal tolerance of reef-building corals and offer a glimmer of hope that at least some coral species can acclimatize fast enough to keep pace with global warming.
Keywords: coral reefs, acclimatization, climate change, ocean warming, coral bleaching
1. Introduction
Since the beginning of the industrial era in the mid-nineteenth century, anthropogenic greenhouse gas emissions have driven an increase in tropical sea surface temperature (SST) of +0.7°C, with an even greater warming of +0.9°C in coral reef regions [1,2]. Global climate models project an additional +1°C warming by the end of this century even if drastic emissions reductions are implemented to avoid the +2 to +4°C warming likely to occur under a business-as-usual scenario [1]. Rising SST is devastating for reef-building corals because warm anomalies of even +1°C can cause them to lose the symbiotic algae that are of paramount importance to their survival. This phenomenon is termed ‘coral bleaching’ as normally colourful corals turn white after expelling their pigmented symbionts [3,4]. Regardless of which actions are taken to tackle climate change, the world is already locked in to a level of warming considered sufficient to trigger annual mass bleaching on more than 90% of coral reefs worldwide by the end of the century [5,6].
The dire projections of coral reef futures are rooted in the assumption that corals bleach when water temperature exceeds a threshold level, and critically, that this threshold remains constant over time [6]. Coral bleaching is expected to occur when degree heating weeks (DHW, °C-weeks), a metric of heat stress that incorporates both the duration and magnitude of warming above the maximum monthly mean (MMM) temperature climatology, exceeds a threshold between 2 and 4°C-weeks [7–9], with mortality occurring between 3 and 8°C-weeks [8,9]. As global warming pushes summertime temperatures beyond the MMM more frequently, coral bleaching is anticipated to become more common until mass bleaching events reach annual frequency later this century [5,6]. Therefore, a future only exists for most coral reef communities if the threshold DHW that triggers bleaching keeps pace with rising SST. Yet, with few exceptions [10–12], little is known about whether coral tolerance to heat stress has changed over time, and whether there is potential for it to increase in the future as the oceans continue to warm.
Here, we use the skeletons of long-lived Porites corals to evaluate their tolerance to rising ocean temperatures from 1815 to 2017 [2]. When Porites corals are bleached or otherwise physiologically perturbed by anomalous temperatures, they record their reaction within their skeletons as discrete high-density ‘stress bands’ that are visible in micro-computed tomography (μCT) scans. These historical stress events can be accurately dated using the annual density bands of the skeleton [13–19]. Together, this information enables (i) detection of stressful events—such as bouts of mass bleaching—that were not directly observed, and (ii) evaluation of the corals' sensitivity to heatwaves observed within recent decades. Our cores, collected in late 2017, span the northern Great Barrier Reef (GBR), northern Coral Sea atolls, and western New Caledonia (Nouméa) (figure 1a). Corals on these reefs were exposed to repeated warm SST anomalies in 2002, 2004, 2010, and 2015–2017 (figure 1; electronic supplementary material, figure S1), allowing us to assess their short-term (annual) responses to the increasing frequency of heat stress in the twenty-first century, in addition to their long-term (centennial) response to global warming since the early nineteenth century.
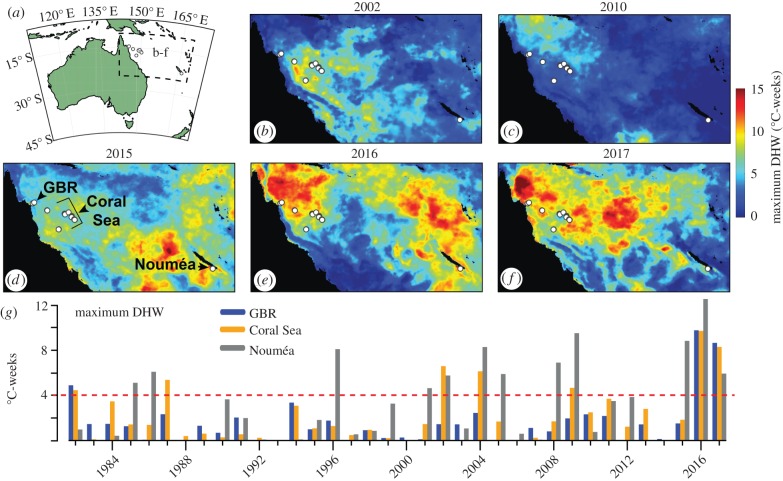
Figure 1.
Heat stress on coral reefs in the twenty-first century. (a) Map of the Australia region, with dashed box indicating the GBR, Coral Sea, and Nouméa area. (b–f) Maps of maximum DHW at 5 km resolution per annum (colours) for key years with the highest DHW. (g) Annual maximum DHW for the northern GBR (blue), Coral Sea (orange), and Nouméa (grey). Dashed red line indicates the nominal 4°C-weeks bleaching threshold.
2. Methods
(a). Climate data
We used the National Oceanic and Atmospheric Administration (NOAA) Coral Reef Watch (CRW) 5 km (v. 3.1) and 25 km SST and DHW products [9,20] to assess heat stress at our coral collection sites from 1982 to 2017. The 5 km dataset covered 1985–2017, and the 25 km dataset was used to extend the temporal coverage to include 1982–1984. We obtained the daily SST and DHW data for the single pixel covering each coring location, and for each year, we extracted the maximum DHW value. To make figure 1g, we averaged the maximum DHW per year for our northern GBR sites and our Coral Sea sites, separately. To make figure 1b–f, we downloaded the full daily 5 km CRW dataset for each year, and plotted (in colours) the maximum DHW at each pixel during each year. We validated these satellite SST data by comparing them to in situ temperature logger measurements wherever possible (electronic supplementary material, table S1 and figure S2) [21,22], which showed that the satellite-derived temperatures fall close to 1 : 1 lines with the logger data, with little evidence of local amplification effects [23].
(b). Coral core collection
Coral skeletal cores were extracted from massive (i.e. dome- or hemispherical-shaped) Porites colonies using underwater pneumatic drills operated by divers. Cores from the northern GBR (n = 26) were collected between 1 and 10 m depth in September and October 2017 at Yonge Reef (n = 10; an outer barrier reef), MacGillivray Reef (n = 6; a fringing reef surrounding a sand cay), and Lizard Island (n = 10; sampling conducted on fringing reefs). In Nouméa, eight Porites colonies at 3 m depth were marked with submerged floats in March 2016, four that were bleached (those with names beginning with ‘B’) and four that were normally pigmented (those with names beginning with ‘Z’), and these eight colonies were then drilled in September 2017. Cores from the Coral Sea (n = 30) were sampled from colonies living between 1 and 20 m depth in November and December 2017 at Bougainville Reef (n = 9), Moore Reefs (n = 5), Diane Bank (n = 3), Willis Cays (n = 3), Magdalene Cays (n = 7), and Flinders Reef (n = 3). See electronic supplementary material, tables S2–S4 for additional core details. Northern GBR and Coral Sea cores were drilled with 5 cm diameter bits, and those from Nouméa with 2.5 cm diameter bits. Core holes were filled with cement plugs to prevent infestation by bioeroding organisms and to provide the corals with a hard surface to grow over during recovery. Each core was sonicated in an ultrasonic bath filled with deionized water for 15 min to remove particles, and then dried for at least 24 h in an oven at 50°C.
(c). Micro-computed tomography scanning
All cores were scanned with a Skyscan 1176 (Bruker-microCT, Kontich, Belgium) to visualize stress bands, partial mortality scars, and annual density bands. Scan settings included voltage of 90 kV, current of 272 µA, a 0.11 mm Cu filter, 36 µm isotropic voxels, and step angles of either 0.7° or 1.1° over 180° rotations with no averaging. For Nouméa samples, we scanned the full core diameter with 350 ms exposures. For northern GBR and Coral Sea cores, we scanned the central 3 cm diameter of the cores with exposure times ranging from 500 to 1250 ms depending on density because longer exposures were required to achieve sufficient X-ray transmission for higher density cores. The scanner has a maximum scan length of 23 cm, so longer cores were scanned piece-wise and compiled to develop full chronologies (electronic supplementary material, figure S3).
(d). Stress band analysis
All μCT scans were reconstructed into two-dimensional slices using Bruker Nrecon software with the following settings: beam hardening correction of 20%, ring artefact reduction of 12, smoothing of 3, and misalignment compensation adjusted each scanning day. Reconstructed images were converted to DICOM files in Hounsfield units (HU) with a range of −1500 to 500 HU. The reconstructed images were post-processed in MATLAB, averaging to 71 µm isotropic voxels to reduce file sizes, and then imported into Osirix software for visual analysis. We created digital slabs in the mean intensity projection with thicknesses between 2.5 and 3.0 mm to visualize both annual density banding and stress bands. The digital slabs were rotated and tilted within the three-dimensional image to locate the primary growth axis where banding patterns were most clearly visible. Annual density bands were counted backwards in time from the collection year of 2017 to establish a chronology for each core, following the well-established interpretation of annual density banding patterns [18,24]. Stress bands were identified following established methods [14,16,23] as either discrete, anomalous high-density bands, or partial mortality scars that may also include high-density stress bands slightly below the scar.
(e). Dissepiment analysis
Dissepiments (thin horizontal skeletal elements accreted every lunar month [18]) were counted in the top 4 cm of cores following previously developed techniques [18]. Slabs were cut with a rock saw as close as possible to the primary growth axis, embedded in epoxy, and polished to a thin section. The sections were then imaged with an Aperio ScanScope XT digital slide scanner at 20× magnification. Dissepiments were traced on the digital images and counted back in time based on the number of full moons elapsed prior to the collection day [18]. Accurate analysis of dissepiment counts was only possible where the section was cut precisely along the growth axis [18], which precluded analysis of many sections and left us with a subset of 13 cores from the northern GBR with quality-controlled dissepiment counts. For two cores from Yonge Reef with dissepiment measurements reaching stress bands, the years of stress band formation interpreted from the μCT scans were confirmed by dissepiment counts. In other cases, the average dissepiment spacing was used to confirm the chronology interpreted from the μCT scans (see ‘Validation of chronologies').
(f). Validation of chronologies
To validate our chronologies interpreted from μCT scanning, we compared our μCT-derived annual extension rates to those determined from dissepiment measurements (described above) and luminescent bands visible under ultraviolet (UV) light. An exceptionally bright luminescent band was visible in all the northern GBR cores between 7 and 10 cm from the top. By comparing the location of this bright luminescent band with μCT analysis of a core with clear density banding, we determined the bright luminescent band corresponds to late 2010 or early 2011 (electronic supplementary material, figure S4). We then divided the down-core depth of the bright luminescent band in all cores by 7 (approximately the number of years between its formation and the sample collection), and compared that to the average annual extension rate inferred from density banding. Additionally, we compared the average of the most recent 1–3 years of annual extension inferred from density bands to the average dissepiment spacing for these same corals multiplied by 12.4 (the number of lunar cycles per year). Both comparisons produced positive correlations close to 1 : 1 lines (electronic supplementary material, figure S5), thus generally confirming our interpretations of chronologies in the μCT scans.
(g). Statistical analysis
We compiled our stress band identifications into a dataset filled with 0 for all years without any sign of a stress band and 1 for any year with a stress band and/or partial mortality scar. To test for temporal changes in the occurrence of stress bands, we fitted our stress band dataset to a generalized linear model with a binomial distribution and a logit link using the glmer function within the lme4 package in R. This test was performed with fixed effects of time and depth, reef location as a fixed factor, and random effects of individual cores.
We tested for temporal changes of sensitivity (proportion of cores with stress bands/maximum DHW per year) across the major stress events of the twenty-first century by fitting sensitivity per region (GBR, Coral Sea, Nouméa) to time with a simple linear model. For this test, we assessed the uncertainty of each sensitivity observation by conducting a Monte Carlo analysis and assuming that 10% of stress band assignments were false positives. The calculations were repeated 103 times, and in each iteration, 10% of stress bands were randomly removed, enabling calculation of a standard deviation of sensitivity for each year.
The relationship between stress band occurrence and DHW during the twenty-first century bleaching events was tested by fitting with glmer the stress band dataset (0 and 1 s) to maximum annual DHW for each core during 2002, 2010, 2015, 2016, and 2017; including reef location and depth as fixed effects, and individual cores as random effects.
Additionally, we used odds ratios to evaluate whether cores with stress bands in a previous year were more likely to have another stress band in a latter year. Specifically, we calculated the odds ratios for the formation of 2010 stress bands compared to 2002 stress bands (i.e. were corals with 2002 stress bands more likely to have 2010 stress bands than corals without 2002 stress bands), and the same for 2010–2015, and 2015–2016. Odds ratios exceeding 1 indicated that prior stress banding had a positive effect on the likelihood of stress band formation in a latter year.
Summaries of statistical tests are provided in electronic supplementary material, table S5.
3. Results and discussion
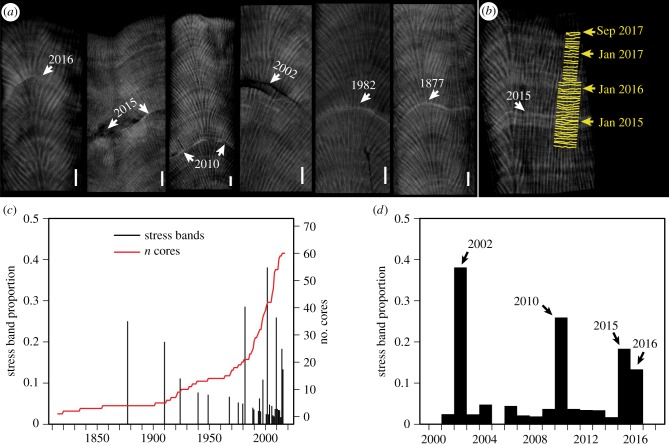
The oldest stress band in our record corresponds to 1877 (figure 2), a year with a strong El Niño but prior to substantial industrial warming [2]. Following this pre-industrial heat stress event, the first widespread occurrence of stress bands was more than a century later in 1982 (figure 2c), also a strong El Niño year, but superimposed onto an anthropogenic warming trend. The next major event, in 2002, marked the onset of frequent heat stress in the twenty-first century, with multiple stress bands also present in 2010, 2015, and 2016 (figure 2). Accordingly, there was a significant increase in stress band occurrence over time since 1815 (p < 0.0001, mixed effects model), with some influence of reef location (significantly more stress bands in Yonge Reef and Nouméa compared to the Coral Sea) but no significant effect of depth (p = 0.34). Continuous direct observations since the 1970s around Lizard Island in the northern GBR generally confirm the history recorded in our cores, with the timing of stress bands matching coral bleaching reports in 1982, 2002, and 2016 [7,25]. Our cores also reveal evidence of stress responses in 2010 and 2015 (figure 2), even though DHW values were moderate (less than 4°C-weeks). However, the proportion of cores with stress bands decreases between 2015 and 2016, with no stress bands observed in 2017, even though 2016 and 2017 were the years with highest DHW (figure 1).
Figure 2.
Coral skeletal core records of heat stress. (a) μCT scans (dark/light shading = low/high density) reveal discrete high-density stress bands and partial mortality scars preserved within the skeletons of long-lived Porites corals. Characteristic signatures of heat stress are shown for the years with the most stress bands recorded (2016, 2015, 2010, 2002, and 1982) and the oldest stress band in our dataset (1877). Scale bars are 5 mm. (b) Verification of the timing of a stress band as January–March 2015 is confirmed by monthly dissepiment counts (yellow) superimposed onto a μCT image of the same coral. (c) Stress band proportion (black bars) in all cores from 1815 to 2017 and (d) 2000–2017. The red line in (c) indicates the number of cores in the dataset per year.
Although early studies of stress bands in Orbicella (formerly Montastrea) corals from the western Atlantic Ocean suggested that stress bands may form under exposure to either anomalously warm or cold water [13,14], more recent studies with Indo-Pacific Porites have consistently linked stress bands to high-temperature events [16–19,23]. Indeed, our stress band analysis successfully captured known bleaching events in the northern GBR and Coral Sea during 1982 and 2002 [25,26]. Yet, widespread bleaching of corals, including Porites, was also reported across the northern GBR and the Coral Sea in 2016 and 2017 [7,8,27], and half of the cored colonies in Nouméa bleached in 2016. The presence of only a few stress bands in 2016 and none in 2017 is thus the first identified decoupling between coral bleaching and the occurrence of stress bands in Porites skeletal cores. When corals lose the symbionts that provide most of their energy, they begin to consume their own tissues (i.e. catabolysis), eventually dying if bleaching persists for long enough that their tissue reserves are depleted [28]. Stress bands form as a result of the cessation of upward growth and depletion of the tissue layer during catabolysis [18]. Therefore, the absence of stress bands and continued skeletal accretion during summer heatwaves in 2016–2017 suggests that the corals may have nourished themselves with heterotrophic feeding [29], stored energy in expanded tissue reserves prior to bleaching, or diverted resources from other physiological processes such as reproduction [28]. The increase in tolerance to repeated heat stress is also consistent with recent suggestions that coral bleaching may be viewed as an immune system response with ‘memory’ of previous exposures [30]. Whichever mechanism was in place, our results indicate that the observed tolerance to high DHW in 2016–2017 was established only after 2015 because higher proportions of stress bands were found in previous years (figure 2).
There are at least two alternative explanations for the complete absence of 2017 stress bands. The first is that our sampling occurred too early after the 2017 bleaching event for the corals to accrete clearly visible stress bands. However, our cores were collected six to nine months after peak temperatures in 2017, and a previous study found that this is sufficient time for the corals to continue growing above any newly formed stress bands such that they are visible in CT scans [17]. Thus, the lack of 2017 stress bands is unlikely to be an artefact of the time of sampling. Alternatively, it is possible that other environmental factors, such as localized currents or cloud cover, dampened heat stress for these corals in 2017. Indeed, the occurrence of stress bands in non-heatwave years, albeit in small proportions of our cores, supports the notion that local environmental factors can influence stress, and thus potentially stress band presence or absence. Nevertheless, that we found high proportions of stress bands in previous bleaching years, combined with the success of Porites stress bands in recording bleaching events in several other studies [16–19,23], suggest that the absence of 2017 stress bands is unlikely a result of local environmental variability across our broad study region, but rather it is more likely due to prior exposure to heat stress events in 2015 and 2016.
Throughout the satellite SST era (1982–present), the relationship between stress bands and DHW over time follows neither a line of constant sensitivity (grey lines in figure 3a) nor a threshold response above a certain DHW value, as is assumed in projections of coral reef futures [5,6]. Instead, the data cluster around high-stress band proportion under low-DHW in 2002, 2010, and 2015; and low-stress band proportion despite high DHW in 2016–2017 (figure 3a). Even though there is a positive correlation between DHW and stress band proportion spatially across sites in 2016–2017, the relationship between DHW and stress bands (i.e. sensitivity) changed drastically among years, with a significant decline in sensitivity over time when data from all reefs are pooled (p = 0.013, simple linear model) or treated separately for the Coral Sea (p = 0.049) and GBR (p = 0.017). Sensitivity declined most significantly on the GBR, mainly due to the higher proportions of stress bands there in 2002 and 2010. Sensitivity either remained stable or slightly decreased between 2002 and 2015, followed by a sharp decline in 2016 and 2017 (figure 3b). Similarly, there is a significant negative correlation (p = 0.007, mixed effects model) between DHW and stress band occurrence when considering only the anomalously warm years of 2002, 2010, and 2015–2017. Evidence for acclimatization may also be found in the timeline of stress bands within individual cores. Corals with 2002 stress bands were more likely to have 2010 stress bands (odds ratio 1.95), and those with 2010 stress bands were more likely to have 2015 stress bands (odds ratio 1.71). By contrast, corals with a stress band in 2015 were less likely to have one in 2016 (odds ratio 0.64), and no corals had stress bands in 2017. Thus, our data suggest that sensitivity to DHW remains stable when the return time of heat stress exceeds several years (e.g. 2002, 2010, and 2015), but corals can develop tolerance (i.e. decreased sensitivity) to heat stress in response to more frequent heatwaves (e.g. 2015, 2016, and 2017). Whereas a previous study inferred that acclimatization mechanisms will be quickly overridden by global warming [31], our findings indicate that the temperature tolerance of Porites has increased as the return time of heat stress events approaches 1 or 2 years.
Figure 3.
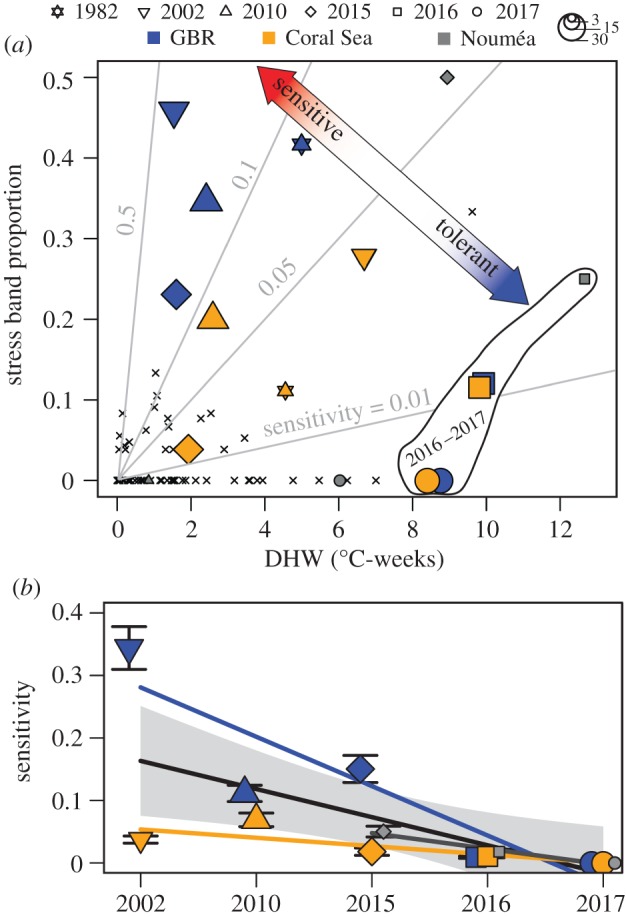
Declining sensitivity to heat stress. (a) Proportion of cores with stress bands in all years of the satellite era (1982–2017, crosses) and the years with the most evidence of heat stress (symbols indicate year, colours indicate location, and symbol size indicates number of replicates). Light grey lines show constant sensitivities to heat stress, defined as the ratio of stress band proportion to maximum DHW. (b) Sensitivity to heat stress in the twenty-first century (colours are the same as a). Regression lines are shown for each region (blue, grey, and orange) and for the pooled data (black), with the grey error bound indicating the 95% confidence interval of the pooled fit. Error bars indicate uncertainty (1 σ) based on a 10% rate of false positives in stress band assignments.
While our results demonstrate that massive Porites corals increased their thermal tolerance during 2016–2017 in response to modest heat stress in 2015, it is possible that similar pre-conditioning responses have been expressed before, and potentially by other coral species. In the broader Coral Sea and northern GBR region, heat stress in 2004 exceeded that of 2002 in most areas, and even rivalled that of 2016/2017 at some reefs (figure 1; electronic supplementary material, figure S1). While both mass coral bleaching [26] and stress band formation (figure 2) occurred in 2002, there are neither reports of bleaching [26] nor a high proportion of stress bands (only 2/24) during 2004 in the northern GBR. Thus, it is conceivable that increased thermal tolerance after the modest 2002 warming event mitigated corals from bleaching in 2004, a notion that is consistent with reports of both species- and community-level acclimatization in other field and laboratory studies [4,10–12,31–33]. Conversely, record-breaking temperatures in 2016 and 2017 together killed approximately half of the corals on the GBR [8], even though they were exposed to modest warming in 2015. This can be interpreted in several ways. First, the concurrence of a strong El Niño with peak summer in 2016 may have created such an extreme heatwave [34] that many corals succumbed irrespective of their ability to augment their thermal tolerance. Alternatively, exposure to modest warming in 2015 may have diminished the effects of heat stress on the survivors during the following 2 years, leaving populations that are better able to withstand recurring heatwaves [8,35]. Finally, because many massive-morphology corals, including Porites, exhibit characteristics of a stress-tolerant life-history strategy [36], they may have greater ability to survive heat stress than corals with weedy or competitive life-history strategies [8,36].
As the rate of severe heat stress events increases [26,37], adaptation and acclimatization in reef-building corals is essential to mitigate against rapid environmental change [8,35] and preserve ecosystem services [26,38]. The capacity of the hundreds of coral species that inhabit reefs to increase their thermal tolerance in the face of global warming will determine whether coral reefs' rich biodiversity [39] will persist into the next century, or whether future generations will inherit reefs covered by depauperate coral communities that would be unrecognizable to us today as functioning coral reef ecosystems. Our findings indicate that natural mechanisms to tolerate the increased frequency of marine heatwaves have been triggered and provide a glimmer of hope that at least some corals can acclimatize to repeated heat stress, enabling them to persist in a warmer world.
Supplementary Material
Acknowledgements
Sanna Persson (James Cook University), Anton Kuret (University of Western Australia), and Bertrand Bourgeois (Institut de Recheche pour le Développement) assisted with fieldwork. The authors wish to thank the staff at Parks Australia, particularly Andy Warmbrunn, in addition to Captain Peter Sayre and the crew of the MV Phoenix. The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Centre for Microscopy, Characterisation and Analysis, The University of Western Australia.
Data accessibility
Datasets supporting this article have been uploaded as electronic supplementary material, and the raw data and code can be found online at https://codeocean.com/capsule/9809895/tree/v1 (https://doi.org/10.24433/CO.7136833.v1).
Authors' contributions
T.M.D. designed the study. T.M.D., H.B.H., L.G., J.D., R.R.-M., and M.T.M. designed and conducted fieldwork. T.M.D. conducted the μCT scanning with assistance from D.P. D.A. conducted the dissepiment and luminescent analyses. All authors contributed to interpreting the results. T.M.D. led the writing of the manuscript with input from all authors.
Competing interests
The authors declare that they have no competing interests.
Funding
Funding for GBR sampling was provided by The John and Laurine Proud Postdoctoral Fellowship (awarded to T.M.D.) and supported by the Australian Museum's Lizard Island Research Station under Great Barrier Reef Marine Park Authority Permit No. G39589.1. Sampling in the Coral Sea Marine Park was funded by an ARC Discovery Early Career Research Award to H.B.H. (DE160101141) and the ARC Centre of Excellence for Coral Reef Studies under Permit No. AU-COM-2017-386. Fieldwork in Nouméa was granted by the project BLANCO (IRD—Ifrecor—Ministère de l'Outre mer). T.M.D., J.D., and M.T.M. were also supported by ARC grants CE140100020 and FL120100049.
Disclaimer
The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect the views of Parks Australia, the Director of National Parks, the Australian Government, NOAA or the U.S. Department of Commerce.
References
- 1.Hoegh-Guldberg O, Cai R, Poloczanska E, Brewer P, Sundby S, Helmi K, Fabry V, Jung S. 2014. The ocean. In Climate change 2014: impacts, adaptation, and vulnerability. Contribution of working group 2 to the fifth assessment report of the intergovernmental panel on climate change (eds Barros V, et al.), Cambridge, UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
- 2.Lough JM, Anderson KD, Hughes TP. 2018. Increasing thermal stress for tropical coral reefs: 1871–2017. Sci. Rep. 8, 6079 ( 10.1038/s41598-018-24530-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glynn PW. 1993. Coral reef bleaching: ecological perspectives. Coral Reefs 12, 1–17. ( 10.1007/BF00303779) [DOI] [Google Scholar]
- 4.Berkelmans R, Willis BL. 1999. Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18, 219–228. ( 10.1007/s003380050186) [DOI] [Google Scholar]
- 5.Frieler K, Meinshausen M, Golly A, Mengel M, Lebek K, Donner SD, Hoegh-Guldberg O. 2012. Limiting global warming to 2°C is unlikely to save most coral reefs. Nat. Clim. Chang. 3, 165–170. ( 10.1038/nclimate1674) [DOI] [Google Scholar]
- 6.van Hooidonk R, Maynard JA, Planes S.. 2013. Temporary refugia for coral reefs in a warming world. Nat. Clim. Chang. 3, 508–511. ( 10.1038/nclimate1829) [DOI] [Google Scholar]
- 7.Hughes TP, et al. 2017. Global warming and recurrent mass bleaching of corals. Nature 543, 373–377. ( 10.1038/nature21707) [DOI] [PubMed] [Google Scholar]
- 8.Hughes TP, et al. 2018. Global warming transforms coral reef assemblages. Nature 556, 492–496. ( 10.1038/s41586-018-0041-2) [DOI] [PubMed] [Google Scholar]
- 9.Heron S, et al. 2016. Validation of reef-scale thermal stress satellite products for coral bleaching monitoring. Remote Sens. 8, 59 ( 10.3390/rs8010059) [DOI] [Google Scholar]
- 10.Pratchett MS, McCowan D, Maynard JA, Heron SF. 2013. Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS ONE 8, e70443 ( 10.1371/journal.pone.0070443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guest JR, Baird AH, Maynard JA, Muttaqin E, Edwards AJ, Campbell SJ, Yewdall K, Affendi YA, Chou LM. 2012. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 7, e33353 ( 10.1371/journal.pone.0033353) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gintert BE, Manzello DP, Enochs IC, Kolodziej G, Carlton R, Gleason ACR, Gracias N. 2018. Marked annual coral bleaching resilience of an inshore patch reef in the Florida Keys: a nugget of hope, aberrance, or last man standing? Coral Reefs 37, 533–547. ( 10.1007/s00338-018-1678-x) [DOI] [Google Scholar]
- 13.Hudson JH, Shinn EA, Halley RB, Lidz B. 1976. Sclerochronology: a tool for interpreting past environments. Geology 4, 361–364. ( 10.1130/0091-7613(1976)4) [DOI] [Google Scholar]
- 14.Carilli JE, Norris RD, Black BA, Walsh SM, McField M. 2009. Local stressors reduce coral resilience to bleaching. PLoS ONE 4, e6324 ( 10.1371/journal.pone.0006324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lough JM, Cooper TF. 2011. New insights from coral growth band studies in an era of rapid environmental change. Earth-Sci. Rev. 108, 170–184. ( 10.1016/j.earscirev.2011.07.001) [DOI] [Google Scholar]
- 16.Cantin NE, Lough JM. 2014. Surviving coral bleaching events: Porites growth anomalies on the Great Barrier Reef. PLoS ONE 9, e88720 ( 10.1371/journal.pone.0088720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkley HC, Cohen AL. 2016. Skeletal records of community-level bleaching in Porites corals from Palau. Coral Reefs 35, 1407–1417. ( 10.1007/s00338-016-1483-3) [DOI] [Google Scholar]
- 18.DeCarlo TM, Cohen AL. 2017. Dissepiments, density bands and signatures of thermal stress in Porites skeletons. Coral Reefs 36, 749–761. ( 10.1007/s00338-017-1566-9) [DOI] [Google Scholar]
- 19.Barkley H, et al. 2018. Repeat bleaching of a central Pacific coral reef over the past six decades (1960–2016). Commun. Biol. 1, 177 ( 10.1038/s42003-018-0183-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu G, et al. 2014. Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sens. 6, 11 579–11 606. ( 10.3390/rs61111579) [DOI] [Google Scholar]
- 21.Varillon D, et al. 2018. ReefTEMPS: the observation network of the coastal sea waters of the South, West and South-West Pacific. ( 10.17882/55128) [DOI]
- 22.Australian Institute of Marine Science. 2018. Sea temperatures. See https://www.aims.gov.au/docs/research/climate-change/climate-monitoring/sst.html (accessed: 15 June 2018)
- 23.DeCarlo TM, Cohen AL, Wong GTF, Davis KA, Lohmann P, Soong K. 2017. Mass coral mortality under local amplification of 2°C ocean warming. Sci. Rep. 7, 44586 ( 10.1038/srep44586) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutson DW, Buddemeier RW, Smith SV. 1972. Coral chronometers: seasonal growth bands in reef corals. Science 177, 270–272. ( 10.1126/science.177.4045.270) [DOI] [PubMed] [Google Scholar]
- 25.Harriott VJ. 1985. Mortality rates of scleractinian corals before and during a mass bleaching event. Mar. Ecol. Prog. Ser. 21, 81–88. ( 10.2307/24816918) [DOI] [Google Scholar]
- 26.Hughes TP, et al. 2018. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83. ( 10.1126/science.aan8048) [DOI] [PubMed] [Google Scholar]
- 27.Harrison HB, Álvarez-Noriega M, Baird AH, Heron SF, MacDonald C, Hughes TP. 2018. Back-to-back coral bleaching events on isolated atolls in the Coral Sea. Coral Reefs 1–7. ( 10.1007/s00338-018-01749-6) [DOI] [Google Scholar]
- 28.Mendes JM, Woodley JD. 2002. Effect of the 1995–1996 bleaching event on polyp tissue depth, growth, reproduction and skeletal band formation in Montastraea annularis. Mar. Ecol. Prog. Ser. 235, 93–102. ( 10.3354/meps235093) [DOI] [Google Scholar]
- 29.Houlbreque F, Ferrier-Pagès C. 2009. Heterotrophy in tropical scleractinian corals. Biol. Rev. 84, 1–17. ( 10.1111/j.1469-185X.2008.00058.x) [DOI] [PubMed] [Google Scholar]
- 30.Palmer CV. 2018. Immunity and the coral crisis. Commun. Biol. 1, 91 ( 10.1038/s42003-018-0097-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ainsworth TD, Heron SF, Ortiz JC, Mumby PJ, Grech A, Ogawa D, Eakin CM, Leggat W. 2016. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 352, 338–342. ( 10.1126/science.aac7125) [DOI] [PubMed] [Google Scholar]
- 32.Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA. 2014. Mechanisms of reef coral resistance to future climate change. Science 344, 895–898. ( 10.1126/science.1251336) [DOI] [PubMed] [Google Scholar]
- 33.Putnam HM, Barott KL, Ainsworth TD, Gates RD. 2017. The vulnerability and resilience of reef-building corals. Curr. Biol. 27, R528–R540. ( 10.1016/J.CUB.2017.04.047) [DOI] [PubMed] [Google Scholar]
- 34.Benthuysen JA, Oliver ECJ, Feng M, Marshall AG. 2018. Extreme marine warming across tropical Australia during Austral Summer 2015–2016. J. Geophys. Res. Ocean 123, 1301–1326. ( 10.1002/2017JC013326) [DOI] [Google Scholar]
- 35.Torda G, et al. 2017. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 7, 627–636. ( 10.1038/nclimate3374) [DOI] [Google Scholar]
- 36.Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM. 2012. Evaluating life-history strategies of reef corals from species traits. Ecol. Lett. 15, 1378–1386. ( 10.1111/j.1461-0248.2012.01861.x) [DOI] [PubMed] [Google Scholar]
- 37.Hobday A, et al. 2018. Categorizing and Naming Marine Heatwaves. Oceanography 31, 162–173. ( 10.5670/oceanog.2018.205) [DOI] [Google Scholar]
- 38.Perry CT, et al. 2018. Loss of coral reef growth capacity to track future increases in sea level. Nature 558, 396–400. ( 10.1038/s41586-018-0194-z) [DOI] [PubMed] [Google Scholar]
- 39.Knowlton N, Brainard RE, Fisher R, Moews M, Plaisance L, Caley M. 2010. Coral reef biodiversity. In Life in the world's oceans: diversity, distribution, and abundance. Oxford, UK: Wiley-Blackwell. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets supporting this article have been uploaded as electronic supplementary material, and the raw data and code can be found online at https://codeocean.com/capsule/9809895/tree/v1 (https://doi.org/10.24433/CO.7136833.v1).