Abstract
Background
One of the major issues in the field of acute liver failure (ALF) is the lack of reliable biomarkers that predict outcome. Many cases present with very limited treatment options and prognostic indicators are invaluable. We tested whether leukocyte cell derived chemotaxin 2 can be used as a prognostic biomarker to predict patient survival either alone or in combination with other routine clinical parameters.
Methods
Serum samples and associated clinical data from came from two independent sources, the Acute Liver Failure Study Group (ALFSG) registry and the University of Kansas Medical Center. We analyzed a total of 61 cases, each with individual time points collected over a period of 0 to 7 days after hospital admission. Analysis was developed to compare responses in survivors vs. non-survivors.
Results
The data indicate that survivors had significantly lower serum levels of leukocyte cell derived chemotaxin 2 compared to non-survivors (P=0.03). Further, it was able to predict patient survival when taken together with either international normalized ratio (INR) alone (71% concordance) or INR and bilirubin (76% concordance) or INR and serum albumin (77% concordance). Furthermore, when we analyzed data for each day, serum Lect2 and INR taken together were able to predict survival at day three after hospital admission with 86.3% concordance.
Conclusions
These studies have revealed test batteries consisting of easily available serum tests that are concordant with survival status of ALF patients early during the clinical course.
Keywords: Survival rate; liver failure, acute; liver regeneration, predictive value of tests
Introduction
In the field of hepatology, fulminant acute liver failure (ALF) exemplifies the complex decisions that patients, families, and clinicians must face with incomplete information. In many cases, treatment options are limited and predictors of survival are essential. This is highlighted in drug induced liver injury. Since its first clinical use in 1893 and commercial introduction in the 1950’s, acetaminophen (APAP) has become one of the most widely used over the counter pharmaceuticals to treat pain and fever (1-4). At therapeutic doses, it is safe and effective with limited adverse effects. However, overdosing can cause hepatotoxicity and can lead to ALF. APAP overdose is associated with approximately 56,000 emergency department visits and 26,000 hospitalizations every year and almost 50% of all ALF cases in the United States (1-4). The treatments for APAP-induced ALF are very limited. In patients who present within 24 hour after a single time point overdose, intravenous N-acetylcysteine therapy is effective, but less so for late presenting patients (2,5,6). If recovery is not expected, the final treatment option is liver transplantation which is complicated by increasing demand and limited supply (7). Additionally, 65% of APAP overdose patients will spontaneously survive without reliable tests that can predict who will require transplantation (8-12).
Leukocyte cell derived chemokine 2 (Lect2) is a chemokine which has been studied as a biomarker in liver diseases including nonalcoholic fatty liver disease, hepatocellular carcinoma, and ALF prognosis (13-15). Lect2 is directly regulated by β-catenin, which is known to play a significant role in liver pathobiology, including in acetaminophen-induced overdose (16). Similarly, Lect2 is a chemokine involved in the chemotaxis of neutrophils and other inflammatory cells that infiltrate the liver (17). Based on these studies, we hypothesized that Lect2 could be a prognostic marker in ALF. We sought to analyze if Lect2, either alone or with other clinical parameters, could form a ‘survival test battery’ that would be concordant with survival of ALF patients earlier than existing tests.
Methods
Sample acquisition
The Institutional Review Board at the University of Kansas Medical Center approved all research activities and all clinical investigation has been conducted according to the principles expressed in the 1975 Declaration of Helsinki. Two sets of serum samples of confirmed ALF were used for this study. The first set of 30 samples was obtained from the Acute Liver Failure Study Group (ALFSG) registry where further information can be found at https://repository.niddk.nih.gov/studies/aalf/ (18). One sample case in this set was excluded because the outcome was unknown and another case was excluded because the patient received a liver transplant. All patients had ALF defined as an international normalized ratio (INR) >1.5 and hepatic encephalopathy within the first 26 weeks of liver disease or INR >2.0 without encephalopathy (18). The primary etiology of ALF was determined by the site investigator who enrolled the patient into the database. The second set of 33 samples was provided by Dr. Hartmut Jaeschke and collected under a separate IRB (#MOD00021641) in the states of Kansas and Arizona. These were collected samples and clinical data on patients who had ALF from APAP overdose (19). The definition of ALF was the same for both datasets. In the second data set, diagnosis of APAP overdose was made by reported history of APAP overdose ingestion, detectable serum APAP, and/or AST/ALT level of >1,500 IU/l (19). The primary sample selection criterion for this study was overall survival vs. non-survival of ALF patients. Patients who received liver transplantation were not included in this study. Informed consent was obtained from patients or written assent from next of kin were obtained when patients with ALF were unable to give consent (for example, if the subject was encephalopathic). We analyzed 42 survivors and 19 non-survivors with a total of 418 individual time points. Each case had laboratory and clinical data on day of admission with a follow up period of 7 days (once a day). However, some individual time points either did not have full clinical data (such as full laboratory data) or serum available for analysis and this is reflected and reported in each analysis where appropriate. Patient #15 had outlier Lect2 values on days 5–7 that were excluded from analysis. Clinical data associated with each case was available for further analysis.
Determination of serum Lect2 and bile acids concentrations
Serum Lect2 levels were determined using a commercially available kit (USCN, China) and used according to manufacturer’s guidelines. Serum bile acids (BA) were determined using a commercially available kit (BQ Kits, San Diego, CA, USA). Other data including INR, serum Albumin, bilirubin, MELD and KCC were extracted from the ALFSG PI-submitted clinical data and used for analyses.
Statistical analysis
Simple means, standard errors and frequencies are reported. Two sample t-tests were conducted to compare continuous variables across survival groups. Discrete variables were compared using binomial and chi-square tests for differences in proportions. We used contingency table analyses and related chi-square tests to examine the association of individual variables in relation to outcome. Binary logistic regression analysis was used to develop and compare predictive models for survival outcome. Receiver operating characteristic curves (ROC) was calculated for Lect2 to determine sensitivity and specificity as a biomarker. A P value <0.05 was considered significant throughout. Multiple regression analysis was performed, but did not reveal significant results (data not included).
Results
Lower Lect2 levels during first three days is associated with higher probability of survival
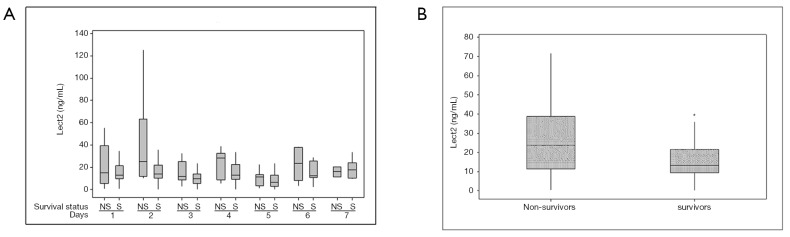
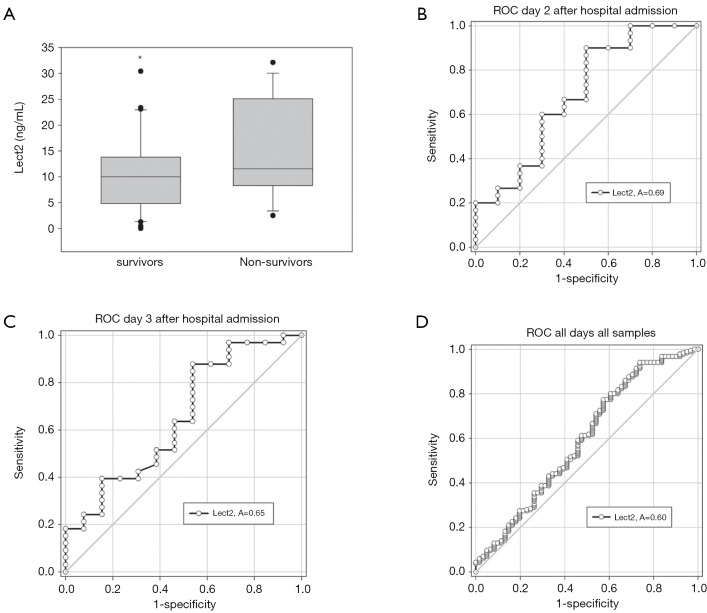
There were 61 total patients included in our study with 19 non-survivors. Etiology of liver disease varied including 3 patients with drug induced liver injury, 5 patients with shock/ischemia, 5 patients with auto-immune hepatitis, and the remaining with APAP overdose (n=50). In addition to relevant clinical data, serum samples of ALF patients collected over the first 7 days after admission to the hospital were used for ELISA based detection of Lect2 levels. Mean ALT trends were similar between both groups and we did not find prognostic information for this biomarker (Figure 1). Overall, mean Lect2 levels were lower in the survivors as compared to non-survivors (P=0.024, Tables 1,2 and Figure 2). The Lect2 levels of survivors were also found to be lower than non-survivors (Figures 2,3). Serum Lect2 levels from the non-survivors exhibited significant variation as compared to survivors.
Figure 1.
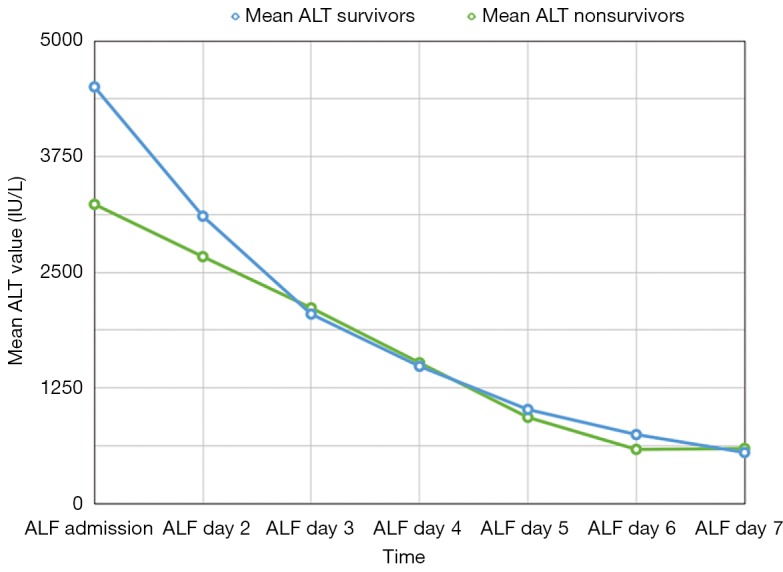
Mean alanine transferase (ALT) levels over time for survivors and non-survivors.
Table 1. Number of patient time points and Lect 2 levels.
| Variable | Survivors | Non-survivors |
|---|---|---|
| N | 195 | 65 |
| Mean Lect2 levels (ng/mL) | 14.8 | 21.6 |
| STDEV | 13.8 | 22.2 |
| SE mean | 0.99 | 2.8 |
Table 2. Statistics showing overall Lect2 changes between survivors and non-survivors.
| Variable | Outcome |
|---|---|
| Estimate for difference | 6.732 |
| Median Lect2 levels (ng/mL) | 12.16 |
| 95% CI for difference | (0.90662, 12.557) |
| P value | 0.024 |
| DF | 80 |
Figure 2.
Modulation of serum Lect2 in ALF patients (A) boxplot showing Lect2 levels every day over a period of seven days after hospital admission (B) boxplot showing overall change between survivors and non-survivors. * indicates significant difference at P<0.05. ALF, acute liver failure.
Figure 3.
Receiver operating characteristic (ROC) analysis for serum Lect2 in ALF patients. (A) Boxplot showing serum Lect2 in ALF patients only on day three after admission. * indicates significant difference at P<0.05. ROC curve analysis of serum Lect2 at (B) day two (P=0.07), (C) day three after admission (P=0.11), and (D) for all days considered together (P=0.02). ALF, acute liver failure.
Combined test batteries including Lect2 can predict survival in humans with ALF with high concordance
We studied the combination of serum Lect2 with other biochemical markers of liver function to improve the prediction of regeneration and survival in ALF. Laboratory data on serum ALT, albumin, phosphate, INR, and bilirubin levels were used in combination with Lect2 to build logistic regression based statistical models to predict clinical outcomes. Models built using serum phosphate and ALT levels were not predictive of survival (data not shown). However, a baseline concordance was found with Lect2 and INR such that lower Lect2 (odds ratio 0.98) and lower INR (odds ratio 0.65) together are associated with survival with 71.3% concordance (Table 3).
Table 3. The Lect2-INR model of survival prediction.
| Predictor | Coefficient | SE Coeff | Z score | P value | Odds ratio | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|---|---|---|
| Lect2 (ng/mL) | −0.016 | 0.085 | −1.96 | 0.05 | 0.98 | 0.97 | 1.00 |
| INR | −0.424 | 0.094 | −4.48 | 0.001 | 0.65 | 0.54 | 0.79 |
Total sample number =249, survivors =188, non-survivors =61, concordance =71.3. INR, international normalized ratio.
The test battery was further refined using the combination of serum Lect2, INR and serum albumin. When taken together, lower serum Lect2 (odds ratio 0.98), lower INR (odds ratio 0.59) and higher serum albumin (odds ratio 1.46) were associated with survival with 76.9% concordance (Table 4). We further developed a test battery using serum Lect2, INR and serum bilirubin levels. This test battery was also associated with survival with 76.9% concordance when serum lect2 was lower (odds ratio 0.98), INR was lower (odds ratio 0.62) and serum bilirubin was lower (odds ratio 0.89, Table 5).
Table 4. The Lect2-INR-Albumin model of survival prediction.
| Predictor | Coefficient | SE Coeff | Z score | P value | Odds ratio | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|---|---|---|
| Lect2 (ng/mL) | −0.017 | 0.0090 | −1.92 | 0.054 | 0.98 | 0.97 | 1.00 |
| INR | −0.524 | 0.104 | −5.04 | 0.001 | 0.59 | 0.48 | 0.73 |
| Albumin | 0.378 | 0.353 | 1.07 | 0.284 | 1.46 | 0.73 | 2.92 |
Total sample number =226, survivors =174, non-survivors =52, concordance =76.9. INR, international normalized ratio.
Table 5. The Lect2-INR-Bilirubin model of survival prediction.
| Predictor | Coefficient | SE Coeff | Z score | P value | Odds ratio | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|---|---|---|
| Lect2 (ng/mL) | −0.02 | 0.0093 | −2.25 | 0.024 | 0.98 | 0.96 | 0.99 |
| INR | −0.475 | 0.1 | −4.73 | 0.001 | 0.62 | 0.51 | 0.76 |
| Bilirubin | −0.121 | 0.335 | −3.60 | 0.001 | 0.89 | 0.83 | 0.95 |
Total sample number =244, survivors =185, non-survivors =59, concordance =76.9. INR, international normalized ratio.
Finally, we tested a model containing serum Lect2, creatinine, bilirubin, bile acid concentration, INR and MELD scores. When controlling for other parameters including creatinine, bilirubin, bile acids and INR; serum Lect2 and MELD scores were associated with survival with lower serum Lect2 (odds ratio, 0.97) and lower MELD score (odds ratio 0.89) giving 80.9% concordance (Table 6). Interestingly, when only serum Lect2 and MELD scores were used to build a model without controlling for other parameters it reduced the concordance to survival to 75%.
Table 6. The Lect2-MELD model predicts survival.
| Predictor | Coefficient | SE Coeff | Z score | P value | Odds ratio | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|---|---|---|
| Lect2 (ng/mL) | −0.028 | 0.01 | −2.84 | 0.005 | 0.97 | 0.95 | 0.99 |
| MELD | −0.113 | 0.043 | −2.61 | 0.009 | 0.89 | 0.82 | 0.97 |
Total sample number =217, survivors =154, non-survivors =63, concordance =80.9, when controlling for serum creatinine, bilirubin, INR and bile acid concentrations. MELD, Model for End Stage Liver Disease.
LECT2 was significant in predicting overall outcome with similar results to MELD score and best prediction on day 3
The test batteries mentioned above considered data from all seven days after hospital admission. We examined if Lect2 alone or in combination with any other clinical parameter was predictive of the outcome on a day-by-day basis, with major focus on the first three days after hospital admission. The binary logistic regression found that LECT2 alone had an overall ability to predict survival with an odds ratio of 0.97 (P=0.005) controlling for creatinine, bilirubin, INR value, and BA concentration. On a day-by-day analysis, this was most apparent on day 3 where the odds ratio was 0.92 (P=0.032). KCC was not found to be predictive of outcome with a likelihood ratio chi-square of 0.793 (P=0.373), though missing KCC data limited interpretation.
Further, a model containing serum Lect2, creatinine, bilirubin bile acids, and INR was built using only day 3 values. In this model lower Lect2 (odds ratio 0.92) and lower INR (odds ratio 0.35) were associated with survival with 86.3% concordance when controlled for serum creatinine, bilirubin and bile acid concentrations (Table 7).
Table 7. The Lect2-INR day 3 only model predicts survival.
| Predictor | Coefficient | SE Coeff | Z score | P value | Odds ratio | 95% CI lower limit | 95% CI upper limit |
|---|---|---|---|---|---|---|---|
| Lect2 (ng/mL) | −0.0845 | 0.039 | −2.14 | 0.032 | 0.92 | 0.85 | 0.99 |
| INR | −1.057 | 0.399 | −2.64 | 0.008 | 0.35 | 0.16 | 0.76 |
Total sample number =59, survivors =43, non-survivors =16, concordance =86.3, when controlling for serum creatinine, bilirubin and bile acid concentrations. INR, international normalized ratio.
Finally, we generated ROC curves to determine sensitivity and specificity of Lect2 association with survival in the first three days of presentation and overall (Figure 3). Using this analysis, Lect2 did not have significant specificity and sensitivity on each individual day. However, it was able to predict overall survival using all data points with a sensitivity of 41.5% and specificity of 78.4% if using a cut off value of 20.04 ng/mL.
Full patient clinical information is available for review from the authors by request.
Discussion
The field of hepatology lacks reliable individual tests or scoring systems that can easily predict patient outcome after the onset of ALF. Current scoring methods for adult patients include the King’s College Criteria (KCC), Model for End Stage Liver Disease (MELD) scores, and the ALFSG index (20,21). Other scoring algorithms have been described but are not widely utilized at this time (22-24). Each system has strengths, but also opportunities for refinement. Despite widespread use, the MELD score becomes more reliable during later stages of pathogenesis either after patient is spontaneously recovering or rapidly deteriorating (20). The ALFSG Index has proved more accurate than KCC and MELD Scores with a sensitivity of 85.6% and specificity of 64.7% to identify patients who require liver transplantation or will die (25). However, until these prognostic indicators reach 100% specificity and sensitivity there is still room for refinement and discovery of new biomarkers to improve predictive potential.
Recently, the high mobility group box 1 (HMGB1) protein and cytokeratin-18 (full-length and caspase cleaved forms) were shown to provide information on the mode of cell death in APAP overdose patients (26). Markers of mitochondrial damage including mtDNA, glutamate dehydrogenase (GDH), glycodeoxycholate, and carbamoyl phosphate synthetase-1 (CPS1) have also been evaluated (25-28). Many of these show predictive potential for negative outcome, (i.e., death or need for a transplant); however, they were better able to define the severity of liver injury as opposed to being true predictors of outcome (19,25,27,28). These studies were able to predict poor outcome in a large group of patients, but the wide variability between individual patients made the parameters less useful in predicting the outcome of individual patients (19,25,27,28). Other groups have assessed whether serum levels of alpha-feto protein (AFP), serum phosphate levels, and alpha-NH(2)-butyric acid may be used as markers of liver regeneration (8,10,11,29,30). However, they are not reliable throughout the entire hospital admission and are obscured by other liver diseases. For example, serum phosphate can be elevated in the setting of renal failure, which often occurs with ALF. Further study to determination a test or a test battery that is geared towards predicting patient survival would be extremely beneficial to make informed therapy decisions.
Initially isolated in the 1990’s, Lect2 has shown involvement in multiple diseases and organ systems including amyloidosis, rheumatoid arthritis, sepsis, non-alcoholic fatty liver disease, insulin resistance, among others (21-36). Studies have shown that Lect2 may be a good biomarker of survival in various liver disease states as well (13). Sato et al. were able to show that serum Lect2 was inversely proportionate to serum AST and ALT levels in cases of ALF (13). Lect2 then increased in the serum several days after presentation as liver function recovered. The same group also studied serum Lect2 in donors and recipients of adult living related liver transplants (14). These patients had similar results where initial serum Lect2 levels were low and associated with an elevated AST and ALT that then inversely changed 1–3 days after the initial insult (14).
However, both studies by Sato et al. were conducted on few samples of mixed etiologies and their application to clinical practice is still limited. In our study, we sought to expand on these initial findings and determine whether serum Lect2 could be used as a biomarker for outcome prediction in adult ALF and help improve current existing prediction models. Our studies are the first to demonstrate that lower serum Lect2 is associated with better prognosis (survival) in adult ALF patients. This result is significant because Lect2 is a target gene of the Wnt/β-catenin pathway. It is secreted into the serum by the liver and plays a critical role in stimulation of liver regeneration (16,37). However, Lect2 is also chemotactic factor for neutrophils as well as macrophages (38). The fact that neutrophils and monocyte-derived macrophages are activated and recruited into the liver mainly during the recovery phase patients suggests that lower serum Lect2 levels may indicate overall lower tissue necrosis and/or faster recovery and resolution of the inflammatory response (39,40). Furthermore, the intrahepatic role of Lect2 may be different than the systemic role of Lect2, which needs to be further investigated.
We investigated whether Lect2 in combination with other laboratory data routinely obtained on patients can be a better predictor of survival. Logistic regression models were built using Lect2 and several other parameters including ALT, serum phosphate, INR, MELD, serum creatinine, bile acids and KCC. Our studies show that the association of lower serum Lect2 with survival increases when taken together with lower serum bilirubin, lower INR, lower MELD, and higher serum albumin. Interestingly, these parameters were not associated with survival by themselves without Lect2. Serum bilirubin, INR, albumin are routinely assessed in ALF patients and MELD calculations are also routinely performed. Adding serum Lect2 assessments, which can be performed rapidly using a commercially available ELISA kits on patient serum samples, can help form new survival test batteries or further develop existing prognostic indicators such as the ALFSG index. These refined test batteries and indicators will significantly improve the clinical decision-making by physicians to list patients in ALF for transplantation and improve organ allotment in ALF patients.
Acknowledgements
The authors of the study would like to acknowledge and thank Michael Brimacombe for his assistance with the statistical analysis of this data.
Funding: This study was supported by following grants: R01 DK098414, R01 DK070195 and a pilot grant from the KUMC Liver Center; P20 GM103549, P30 GM118247, U01 58369-17. The Acute Liver Failure Study Group is supported by U-01 DK 58369.
Ethical Statement: The study was approved by The Institutional Review Board at the University of Kansas Medical Center.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lee WM. Etiologies of acute liver failure. Semin Liver Dis 2008;28:142-52. 10.1055/s-2008-1073114 [DOI] [PubMed] [Google Scholar]
- 2.Lee WM, Squires RH, Jr, Nyberg SL, Acute liver failure: Summary of a workshop. Hepatology 2008;47:1401-15. 10.1002/hep.22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prescott LF. Paracetamol: past, present, and future. Am J Ther 2000;7:143-7. 10.1097/00045391-200007020-00011 [DOI] [PubMed] [Google Scholar]
- 4.Nourjah P, Ahmad SR, Karwoski C, Estimates of acetaminophen (Paracetomal)-associated overdoses in the United States. Pharmacoepidemiol Drug Saf 2006;15:398-405. 10.1002/pds.1191 [DOI] [PubMed] [Google Scholar]
- 5.Smilkstein MJ, Knapp GL, Kulig KW, Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985). N Engl J Med 1988;319:1557-62. 10.1056/NEJM198812153192401 [DOI] [PubMed] [Google Scholar]
- 6.Fontana RJ. Acute liver failure including acetaminophen overdose. Med Clin North Am 2008;92:761-94. 10.1016/j.mcna.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalimar, Acharya SK. Management in Acute Liver Failure. J Clin Exp Hepatol 2015;5:S104-15. 10.1016/j.jceh.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee WM. Liver: Determining prognosis in acute liver failure. Nat Rev Gastroenterol Hepatol 2012;9:192-4. 10.1038/nrgastro.2012.34 [DOI] [PubMed] [Google Scholar]
- 9.Dalhoff K, Laursen H, Bangert K, et al. Autoprotection in acetaminophen intoxication in rats: the role of liver regeneration. Pharmacol Toxicol 2001;88:135-41. 10.1034/j.1600-0773.2001.d01-94.x [DOI] [PubMed] [Google Scholar]
- 10.Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology 2002;36:659-65. 10.1053/jhep.2002.35069 [DOI] [PubMed] [Google Scholar]
- 11.Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology 2005;41:26-31. 10.1002/hep.20511 [DOI] [PubMed] [Google Scholar]
- 12.Bhushan B, Walesky C, Manley M, et al. Pro-regenerative signaling after acetaminophen-induced acute liver injury in mice identified using a novel incremental dose model. Am J Pathol 2014;184:3013-25. 10.1016/j.ajpath.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato Y, Watanabe H, Kameyama H, et al. Serum LECT2 level as a prognostic indicator in acute liver failure. Transplant Proc 2004;36:2359-61. 10.1016/j.transproceed.2004.07.007 [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, Watanabe H, Kameyama H, et al. Changes in serum LECT 2 levels during the early period of liver regeneration after adult living related donor liver transplantation. Transplant Proc 2004;36:2357-8. 10.1016/j.transproceed.2004.07.006 [DOI] [PubMed] [Google Scholar]
- 15.Okabe H, Delgado E, Lee JM, et al. Role of leukocyte cell-derived chemotaxin 2 as a biomarker in hepatocellular carcinoma. PLoS One 2014;9:e98817. 10.1371/journal.pone.0098817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ovejero C, Cavard C, Perianin A, et al. Identification of the leukocyte cell-derived chemotaxin 2 as a direct target gene of beta-catenin in the liver. Hepatology 2004;40:167-76. 10.1002/hep.20286 [DOI] [PubMed] [Google Scholar]
- 17.Woolbright BL, Jaeschke H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J Hepatol 2017;66:836-48. 10.1016/j.jhep.2016.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karvellas CJ, Speiser JL, Tremblay M, Elevated FABP1 serum levels are associated with poorer survival in acetaminophen-induced acute liver failure. Hepatology 2017;65:938-49. 10.1002/hep.28945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGill MR, Sharpe MR, Williams CD, The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest 2012;122:1574-83. 10.1172/JCI59755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katoonizadeh A, Decaestecker J, Wilmer A, et al. MELD score to predict outcome in adult patients with non-acetaminophen-induced acute liver failure. Liver Int 2007;27:329-34. 10.1111/j.1478-3231.2006.01429.x [DOI] [PubMed] [Google Scholar]
- 21.Rutherford A, King LY, Hynan LS, et al. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology 2012:143:1237-43. 10.1053/j.gastro.2012.07.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch DG, Tillman H, Durkalski V, Development of a Model to Predict Transplant-free Survival of Patients With Acute Liver Failure. Clin Gastroenterol Hepatol 2016;14:1199-1206.e2. 10.1016/j.cgh.2016.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Speiser JL, Lee WM, Karvellas CJ, Predicting outcome on admission and post-admission for acetaminophen-induced acute liver failure using classification and regression tree models. PLoS One 2015;10:e0122929. 10.1371/journal.pone.0122929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gregory B, Larson AM, Reisch J, Acetaminophen dose does not predict outcome in acetaminophen-induced acute liver failure. J Investig Med 2010;58:707-10. 10.2310/JIM.0b013e3181db8764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woolbright BL, McGill MR, Staggs VS, et al. Glycodeoxycholic acid levels as prognostic biomarker in acetaminophen-induced acute liver failure patients. Toxicol Sci 2014;142:436-44. 10.1093/toxsci/kfu195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antoine DJ, Jenkins RE, Dear JW, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol 2012;56:1070-9. 10.1016/j.jhep.2011.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Weerasinghe SV, Jang YJ, Fontana RJ, Carbamoyl phosphate synthetase-1 is a rapid turnover biomarker in mouse and human acute liver injury. Am J Physiol Gastrointest Liver Physiol 2014;307:G355-64. 10.1152/ajpgi.00303.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGill MR, Staggs VS, Sharpe MR, Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology 2014;60:1336-45. 10.1002/hep.27265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azhar N, Ziraldo C, Barclay D, et al. Analysis of serum inflammatory mediators identifies unique dynamic networks associated with death and spontaneous survival in pediatric acute liver failure. PLoS One 2013;8:e78202. 10.1371/journal.pone.0078202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudnick DA, Dietzen DJ, Turmelle YP, et al. Serum alpha-NH-butyric acid may predict spontaneous survival in pediatric acute liver failure. Pediatr Transplant 2009;13:223-30. 10.1111/j.1399-3046.2008.00998.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graessler J, Verloren M, Grassier A, et al. Association of chondromodulin-II Val58Ile polymorphism with radiographic joint destruction in rheumatoid arthritis. J Rheumatol 2005;32:1654-61. [PubMed] [Google Scholar]
- 32.Yamagoe S, Yamakawa Y, Matsuo Y, Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett 1996;52:9-13. 10.1016/0165-2478(96)02572-2 [DOI] [PubMed] [Google Scholar]
- 33.Kowalski A, Cabrera J, Nasr S, Renal LECT2 amyloidosis: a newly described disorder gaining greater recognition. Clin Nephrol 2015;84:236-40. 10.5414/CN108549 [DOI] [PubMed] [Google Scholar]
- 34.Ando K, Kato H, Kotani T, Plasma leukocyte cell-derived chemotaxin 2 is associated with the severity of systemic inflammation in patients with sepsis. Microbiol Immunol 2012;56:708-18. 10.1111/j.1348-0421.2012.00488.x [DOI] [PubMed] [Google Scholar]
- 35.Okumura A, Enoki-Kubota H, Matsushita Y, et al. Increased serum leukocyte cell-derived chemotaxis 2 (Lect2) levels in obesity and fatty liver. Biosci Trends 2013;7:276-83. [PubMed] [Google Scholar]
- 36.Lan F, Misu H, Chikamoto K, et al. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance: Diabetes 2014;63:1649-64. 10.2337/db13-0728 [DOI] [PubMed] [Google Scholar]
- 37.Apte U, Singh S, Zeng G, et al. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am J Pathol 2009;175:1056-65. 10.2353/ajpath.2009.080976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu XJ, Chen J, Yu CH, et al. LECT2 protects mice against bacterial sepsis by activating macrophages via the CD209a receptor. J Exp Med 2013;210:5-13. 10.1084/jem.20121466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antoniades CG, Quaglia A, Taams LS, et al. Source and characterization of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology 2012;56:735-46. 10.1002/hep.25657 [DOI] [PubMed] [Google Scholar]
- 40.Williams CD, Bajt ML, Sharpe MR, Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol Appl Pharmacol 2014;275:122-33. 10.1016/j.taap.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]