Abstract
Human engineering of the outdoors led to the development of the indoor niche, including home construction. However, it is unlikely that domicile construction mechanics are under direct selection for humans. Nonetheless, our preferences within indoor environments are, or once were, consequential to our fitness. The research of human homes does not usually consider human evolution, and, therefore, we are without previous predictions about indoor climate preference. We worked with citizen scientists to collect indoor climate data from homes (n = 37) across the USA. We then compared these data to recent global terrestrial climate data (0.5° grid cells, n = 67 420) using a climate dissimilarity index. We also compared some climate-related physiological parameters (e.g. thermoneutral zone (TNZ)) between humans and a selection of non-human primates. On average, our study homes were most similar in climate to the outdoor conditions of west central Kenya. We found that the indoor climates of our study homes largely matched the TNZ of humans and other primates. Overall, we identified the geographical distribution of the global outdoor climate that is most similar to the interiors of our study homes and summarized study home indoor climate preferences.
Keywords: climate dissimilarity, human niche construction, human associates, thermal comfort, indoor biome
1. Introduction
Climate plays an important role in the life history of most organisms, and the influence of climate on the ecology, evolution and distribution of organisms has been the subject of many thousands of studies. Similarly, outdoor climates themselves have been the subject of a rich body of work, both in terms of current climate, projected future climate and modelled or measured historic climates. Yet, somehow, the relationship between humans and climate, particularly the climate in the ecological realm we spend the most time in, our homes, remains poorly studied, particularly with regard to the ecology and evolution of humans and the many thousands of species that live alongside us [1,2].
Dawkins coined the term extended phenotype to describe the extent to which an organism's genes encode not only its body and behaviour but also the ways in which that organism might manipulate the environment [3]. The termite's nest is part of its extended phenotype [4] and is mediated both by genes associated with behaviour and the rules those genes influence, just as the warren of a mouse is part of its [5]. Recent work has even begun to understand the individual genes associated with deer mice (Peromyscus spp.) and when they build one type of warren relative to another [6]. But what about humans? It would be difficult to convincingly argue that the behaviours leading to the construction of human houses are under direct selection. Many humans (the authors of this paper included) could not build a modern house if their life depended on it, yet we persist. However, the issue may be more subtle than it at first seems; human preferences influence human houses. Our houses are built to reflect both comfortable temperatures and levels of humidity [7,8]. If our house is too hot or cold, we modify it in such a way as to produce more heat and vice versa [9]. However, for thousands of years before air conditioning, we also modified conditions through construction or placement of homes that buffered outdoor climates with passive measures such as sun shading, thermal mass and ceiling architecture, to both to make them liveable and to make them comfortable [10,11].
For ectotherms, a large body of the literature considers how individual organisms alter their climate [12]. Species seek favoured climates or employ body postures that alter the temperature to which they are exposed [13–16]. In social insects, some species even alter the climate around them, and particularly their brood, whether through collective behaviours (e.g. honey bees [17]) or through the constructions the behaviours create (e.g. nests [18]). Similar phenomena are reported for mammals, but often anecdotally, especially for primates including humans [19]. The relationship of humans with climate is complex [20,21]. We thermoregulate [22], acclimate [23], and, over time, we have even adapted in as much as individual human lineages appear to demonstrate physiological and anatomical differences associated with their historic climates [24]. Yet, the defining way in which we have responded to outdoor climate, since the advent clothing, no less than 20 000 years ago, is to modify the climate we are exposed to in order to maximize thermal comfort [25].
A rich literature considers the many proximate factors that influence thermal comfort. Thermal comfort can be influenced by culture [11,26], by wind speed and humidity [27–30] and mean radiant temperature [31,32]. This literature suggests that the many ways in which the climate people prefer for their homes might be modulated and why. But what these do not change is the reality that thermal comfort itself, evolved.
What do we favour about these indoor climatic conditions? Are they similar to the climate of our ancestors? Which (outdoor) climate are we attempting to reconstruct when we turn the heat up or down? These questions seem to have been given little consideration, perhaps for two reasons. First, there is a paucity of reported indoor climate data across seasons for occupied homes, which would allow direct comparison with outdoor climates except where specific house types are being compared (e.g. traditional versus modern homes [26,27]). Second, the people who study indoor environmental quality (e.g. homes and their interior climates) do so in the context of creating interior spaces that promote comfort and productivity rather than in an ecological or evolutionary context [33,34]. Understanding the climates humans construct in light of human ecology and evolution has relevance not only to understanding why we build homes the way we do (and how we might make more reasoned decisions in the future), but also the climate that we create for other organisms indoors. The indoor biome is one of the most rapidly growing biomes on Earth [35], yet its climatic features have not been well characterized with regards to species ecology, nor have they been compared to other, outdoor climates. Such a comparison is necessary in order to understand which climates we have replicated indoors and which species might be most predisposed, in terms of climate, to live with us in the future, whether wanted or unwanted. As many as several hundred thousand species have been found living in homes [1,2], and the question of the climate that these species inhabit is relevant to the basic biology of a broad swathe of life.
Here, we worked with citizen scientists to record the climate within homes across the USA. We first characterize the indoor climates of these homes, then we compare these indoor climates with what is known about the climatic tolerances of non-human primates, and finally, we identify specific geographies from across the globe whose climate is most similar to the observed indoor climates. In considering which (global) outdoor climates these North American homes are most similar to, we argue that there are two consequences of the conditions that we prefer in our homes. First, the climates we prefer have strong effects on global energy usage and how that usage varies geographically. Second, and perhaps less obviously, in constructing our homes and modulating their climate as an extension of our phenotype (and to some extent culture) we might also recreate specific climates for other organisms, favouring the subset of species that prefer the same climates as we do [35].
2. Material and methods
2.1. Climate datasets
With the assistance of citizen scientists, we collected indoor and outdoor climate data from homes from each state of the USA and Washington, DC using a temperature (°C) and relative humidity (%) data logger (iButton model DS1923-F5, Maxim Integrated Products, Inc., Sunnyvale, CA, USA) that is commonly used in ecological studies [36]. For indoor climate measurements, participants were instructed to place the data logger on a surface with a low risk of physical disturbance, and away from any air vents, windows or direct sunlight (e.g. shelf, bookcase). Participants were asked to place the outdoor climate logger in a location that was disturbance free and shaded. The data collection period was February 2013–April 2014, and temperature and humidity were recorded once per hour. During initial data processing, prior to analysis, we removed homes that did not have records from summer, winter and, at least, spring or autumn; 37 homes were retained (additional information on study homes available as electronic supplementary material, table S1). To align the indoor air moisture variable with that of the global outdoor data, we calculated vapour pressure (hPa) from indoor temperature and relative humidity observations using the August–Roche–Magnus equation [37]. Home climate data were converted to monthly averages prior to analyses. We examined the relationship between indoor and outdoor home temperatures with linear regression, fitting regressions for both vapour pressure and temperature by season. All analyses were performed in R [38] (version 3.3.2; http://www.R-project.org). This research was approved by the NC State University IRB review board under IRB Protocol 2177. We received written consent from all participants.
Global, outdoor climate data were acquired from the University of East Anglia Climatic Research Unit's Time-Series Version 3.21 High Resolution Gridded Data [39] (CRU TS3.21; http://catalogue.ceda.ac.uk). This dataset is constructed from monthly observations from terrestrial meteorological stations from across the globe. Station anomalies are interpolated to 0.5° grid cells (n = 67 420 terrestrial cells excluding Antarctica) and combined with an existing climatology [40] to derive absolute monthly values. We used the 2012 CRU TS3.21 monthly air temperature (°C) and vapour pressure (hPa) data for our analyses.
2.2. Climate dissimilarity
We calculated the dissimilarity between North American indoor and global outdoor climates, using the climatic parameters air temperature (°C) and vapour pressure (hPa), to determine if indoor climates approximated outdoor climates of specific geographies. For our dissimilarity analyses, we used six climate variables: minimum mean air temperature and mean vapour pressure for winter, mean air temperature and mean vapour pressure for spring/autumn, and maximum air temperature and mean vapour pressure for summer. Seasons were defined as follows for the Northern and Southern Hemispheres, respectively: December–February (winter/summer), March–May (spring/autumn), June–August (summer/winter), September–November (autumn/spring). Spring and autumn were analysed as one season, averaging spring and autumn values as needed. Air temperature and air moisture are often-used climatic variables when considering indoor climate and human thermal comfort [29,41]. These parameters have also been used in studies of climate analogues [42].
We used a standardized Euclidian distance to compute a climate dissimilarity index [42,43] between each home and global grid cell (equation (2.1)), using the climate variables described above.
| 2.1 |
where C is the climate dissimilarity index between each indoor i and outdoor j location. Where k is the climate variable (n = 6), h is the mean of the indoor climate variable k at i, g is the mean outdoor climate variable k at j, and is the standard deviation of the indoor climate variable. Climate dissimilarity indices are a common tool used to compare climates separated by space and/or time and to find the climate that is most or least similar to a focal climate [42,44–46]. We also calculated the root mean square errors for temperature and vapour pressure between each home and global grid cell, and the methods and results of these analyses can be found in the electronic supplementary material, appendix A.
3. Results
3.1. Indoor climates
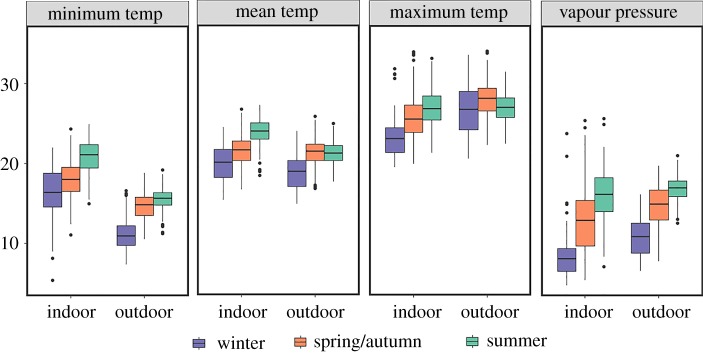
The mean maximum temperature in the summer for the 37 homes ranged from 22.22 to 34.63°C, with a mean of 27.27 ± 0.46°C (standard error of the mean); mean vapour pressure ranged from 10.22 to 25.28 hPa with a mean of 16.15 ± 0.46 hPa (figure 1). The mean minimum temperature in winter ranged from 8.38 to 22°C, with a mean of 16.44 ± 0.52°C, and mean vapour pressure ranged from 4.98 to 22.33, with a mean of 8.75 ± 0.54 hPa. The mean temperature of spring/autumn ranged from 17.52 to 25.37°C, with a mean of 21.51 ± 0.28°C, and mean vapour pressure ranged from 8.26 to 23.59 hPa, with a mean of 12.82 ± 0.46 hPa.
Figure 1.
Boxplots for the climatic variables air temperature (°C) and vapour pressure (hPa) by season (spring and autumn are averaged; winter = purple, spring/autumn = orange, summer = green) and location. Minimum temp is the mean minimum air temperature, mean temp is mean air temperature, maximum temp is the mean maximum air temperature and vapour pressure is the mean vapour pressure. The indoor climate is from our study homes and outdoor climate values are from the 100 grid cells that are the most climatically similar to the mean home indoor climate. The box plots display data range, quartiles and median with dots as outliers. Figure was generated with R (version 3.3.2; http://www.R-project.org) package ggplot2 (version 2.2.0; http://CRAN.R-project.org/package=ggplot2).
Outdoor air temperature was a significant predictor of indoor home temperature by season, but the strength of these associations was modest (table 1). The relationship between outdoor and indoor temperature was especially weak in winter (t = 2.88, adjusted R2 = 0.04, p < 0.001). Associations of outdoor and indoor home vapour pressure were generally stronger than the same comparisons for temperature (table 1). The weakest relationship between outdoor and indoor home vapour pressure was found in summer (t = 7.69, adjusted R2 = 0.30, p < 0.001).
Table 1.
Results of linear models evaluating indoor home climate (temperature, vapour pressure) by outdoor home climate.
| season | d.f. | variable | estimate (s.e.) | t value | adj. R2 |
|---|---|---|---|---|---|
| winter | 159 | temperature (°C) | 0.063 (0.02) | 2.88** | 0.044 |
| vapour pressure (hPa) | 0.757 (0.03) | 23.41*** | 0.774 | ||
| spring/autumn | 218 | temperature (°C) | 0.246 (0.02) | 14.13*** | 0.476 |
| vapour pressure (hPa) | 5.862 (0.33) | 23.44*** | 0.715 | ||
| summer | 136 | temperature (°C) | 0.245 (0.03) | 8.23*** | 0.328 |
| vapour pressure (hPa) | 0.351 (0.05) | 7.69*** | 0.298 |
Significance levels **p < 0.01, ***p < 0.001.
3.2. Most similar indoor and outdoor climates
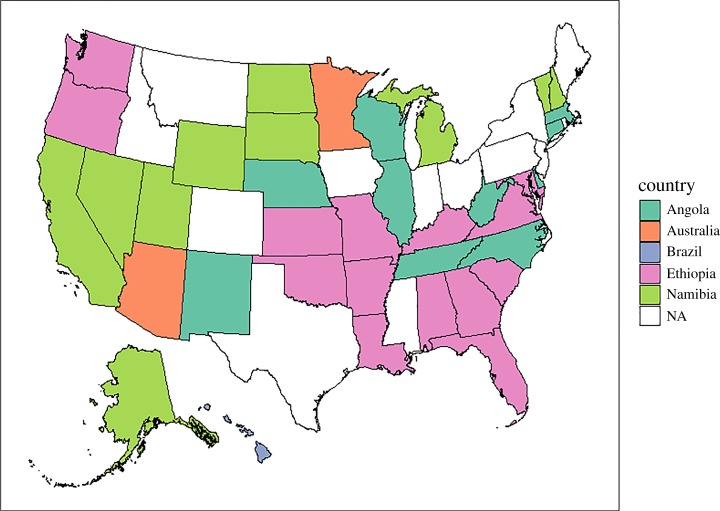
We identified the outdoor location(s) with the most similar climate for each of our study homes (table 2). The indoor climate from the Oregon home, for example, had the smallest observed C and was a close match (C = 0.3812) with a grid cell in Kenya (0.25° N, 35.25° E). By contrast, the indoor climate for the Missouri home had the greatest minimum C (3.765) for its most similar outdoor climate (1.75° N, 35.25° E) which was also located within Kenya. To generalize the climate similarities, we also considered the 100 most similar outdoor climates for each home. The Hawaii home had the lowest mean C (0.900 ± 0.014) and these global grid cell centres that were most often located in Brazil (figure 2) and the Missouri home also had the greatest minimum mean C (4.120 ± 0.017), and the locations of these global grids most often occurred in Ethiopia.
Table 2.
Results of climate dissimilarity analysis between the indoor climate of a North American home (n = 37) and 67 420 global terrestrial grid cells. Cnearest is the minimum value of the climate dissimilarity index (C) for that state. The country where the centre (latitude and longitude) of the grid cell is located is listed as the nearest country. CTop 100 is the mean minimum value of C (standard error) for the 100 most climatically similar global grid cells for that state, the corresponding country represents the most frequently observed country from the 100 most climatically grid cells.
| state | Cnearest | country (nearest) | latitude | longitude | CTop 100 | country (top 100) |
|---|---|---|---|---|---|---|
| Alabama | 2.557 | Kenya | 1.75 | 35.25 | 3.181 (0.022) | Ethiopia |
| Alaska | 1.273 | Namibia | 13.75 | −19.75 | 1.879 (0.023) | Namibia |
| Arizona | 2.146 | Namibia | −22.75 | 15.75 | 2.547 (0.012) | Australia |
| Arkansas | 1.221 | Ethiopia | 7.75 | 35.25 | 1.79 (0.019) | Ethiopia |
| California | 1.263 | Namibia | −21.25 | 14.75 | 1.868 (0.019) | Namibia |
| Connecticut | 2.197 | Namibia | −22.25 | 15.25 | 2.625 (0.018) | Angola |
| Delaware | 0.770 | Angola | −13.75 | 16.25 | 1.272 (0.025) | Angola |
| Florida | 1.705 | Ethiopia | 3.75 | 38.75 | 2.339 (0.028) | Ethiopia |
| Georgia | 1.755 | Ethiopia | 9.75 | 35.25 | 2.317 (0.017) | Ethiopia |
| Hawaii | 0.561 | Brazil | −11.25 | −38.25 | 0.9 (0.014) | Brazil |
| Illinois | 2.648 | Namibia | −21.75 | 15.25 | 3.249 (0.017) | Angola |
| Kansas | 2.087 | Kenya | 0.75 | 35.75 | 2.64 (0.016) | Ethiopia |
| Kentucky | 2.163 | Kenya | 1.75 | 35.25 | 2.62 (0.017) | Ethiopia |
| Louisiana | 1.258 | Kenya | −1.25 | 38.25 | 1.658 (0.015) | Ethiopia |
| Maryland | 3.243 | Ethiopia | 9.75 | 35.25 | 3.759 (0.019) | Ethiopia |
| Massachusetts | 0.736 | Angola | −15.75 | 14.75 | 1.329 (0.023) | Angola |
| Michigan | 2.188 | Namibia | −22.75 | 15.25 | 2.742 (0.014) | Namibia |
| Minnesota | 3.079 | Bermuda | 32.25 | −64.75 | 3.562 (0.011) | Australia |
| Missouri | 3.580 | Ethiopia | 12.75 | 37.25 | 4.12 (0.017) | Ethiopia |
| Nebraska | 1.745 | Angola | −10.75 | 22.25 | 2.347 (0.019) | Angola |
| Nevada | 3.210 | Namibia | −21.75 | 15.75 | 3.945 (0.027) | Namibia |
| New Hampshire | 1.898 | Namibia | −21.25 | 14.75 | 2.457 (0.018) | Namibia |
| New Mexico | 2.487 | Ethiopia | 12.75 | 37.25 | 3.1 (0.019) | Angola |
| North Carolina | 1.519 | Ethiopia | 10.75 | 35.75 | 1.792 (0.009) | Angola |
| North Dakota | 2.970 | Namibia | −21.75 | 15.25 | 3.613 (0.018) | Namibia |
| Oklahoma | 2.820 | Kenya | 1.75 | 35.25 | 3.387 (0.019) | Ethiopia |
| Oregon | 0.387 | Kenya | 0.25 | 35.25 | 1.109 (0.023) | Ethiopia |
| South Carolina | 1.967 | Ethiopia | 4.25 | 39.25 | 2.511 (0.021) | Ethiopia |
| South Dakota | 2.721 | Namibia | −21.25 | 14.75 | 3.267 (0.018) | Namibia |
| Tennessee | 1.272 | Namibia | −22.25 | 15.25 | 2.08 (0.021) | Angola |
| Utah | 1.982 | Namibia | −21.25 | 14.75 | 2.565 (0.018) | Namibia |
| Vermont | 1.719 | Mexico | 25.25 | −106.75 | 2.078 (0.012) | Namibia |
| Virginia | 1.658 | Ethiopia | 9.75 | 35.25 | 2.328 (0.018) | Ethiopia |
| Washington | 1.870 | Kenya | 1.25 | 35.75 | 2.498 (0.02) | Ethiopia |
| West Virginia | 1.561 | Ethiopia | 10.75 | 35.75 | 1.941 (0.012) | Angola |
| Wisconsin | 1.997 | Namibia | −21.75 | 14.75 | 2.538 (0.016) | Angola |
| Wyoming | 3.433 | Namibia | −22.75 | 15.75 | 4.119 (0.023) | Namibia |
Figure 2.
Map of the USA (not to scale). Each state represents one study home (n = 37). State fill colour represents the country (Angola, aquamarine; Australia, salmon; Brazil, light purple; Ethiopia, magenta; Namibia, green) that was identified as most frequent from a subset of the 100 global grid cells with most similar climate to each study home. States not included in the analysis are shown in white. Map was generated with R (version 3.3.2; http://www.R-project.org) packages ggplot2 (http://CRAN.R-project.org/package=ggplot2), mapproj (version 1.2-4; https://cran.r-project.org/package=mapproj), rgdal (version 1.2-7; https://cran.r-project.org/package=rgdal) and sp (version 1.2-4; https://cran.r-project.org/package=sp). State boundaries (5 m resolution) were obtained from the US Census Bureau (https://www.census.gov/geo/maps-data/data/cbf/cbf_state.html).
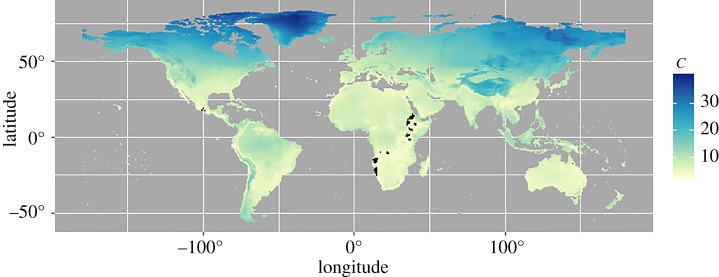
Considering all global cells (n = 67 420), the location with the least similar climate to the mean North American indoor climate was located within northern Greenland (79.75° N, 39.25° W; C = 39.874). In other words, to achieve the indoor conditions found in North America, someone in Greenland would have to alter indoor conditions relative to outdoor conditions more than anywhere else on Earth. Conversely, the location with the most similar climate was located in west central Kenya (1.25° N, 35.75° E; C = 2.938). In west central Kenya, outdoor conditions are essentially the same as the mean conditions created inside homes in North America.
We were interested in identifying potential global, outdoor locations from which the species associated with North American homes might be most expected to have come. To this end, we used the overall mean climatic dissimilarity metric (C) from our study homes to identify the 100 (of 67 420) most similar global grid cells (figure 3). The value distributions of the climate variables used in the climate dissimilarity index can be viewed in figure 1.
Figure 3.
Map depicting the climate dissimilarity index (C) between the mean indoor climate of the North American homes (n = 37; 2013–2014) and the outdoor climate of terrestrial 0.5° global grid cells (n = 67 420; 2012). Dissimilarity increases as C increases (yellow to blue). Cells depicted in black are those grid cells with the climatic conditions most similar to the average North American home in terms of temperature and humidity (n = 100). Map was generated with R (version 3.3.2; http://www.R-project.org) packages ggplot2 (version 2.2.0; http://CRAN.R-project.org/package=ggplot2), rgdal (version 1.2-7; https://cran.r-project.org/package=rgdal), sp (version 1.2-4; https://cran.r-project.org/package=sp), and rworldmap (version 1.3-4; https://cran.r-project.org/package=rworldmap), which uses Natural Earth data (version 1.4.0; http://www.naturalearthdata.com) for country borders.
4. Discussion
Here, we present data on observed indoor climate from homes across the North America (figure 1). Indoor environments are important for humans; the average person in the USA spends, for example, less than 10% of their time outdoors [47]. In spite of numerous reports of human thermal preferences inside buildings and codified climatic prescriptions (e.g. ASHRAE Standard 55) for construction of interior spaces, data on the climates actually achieved in houses, throughout the year, have not been widely reported.
We also identified outdoor climates from around the world that are most climatically similar (e.g. in terms of temperature and humidity, by season) to the indoor climate of the homes we studied. North American homes were most similar in climate to the outdoor conditions of west central Kenya (C = 2.938). The mean maximum temperature (average of all seasons) in the North American homes was 25.35°C compared with 25.06°C for the conditions outdoors in west central Kenya. The mean vapour pressure was 12.58 hPa for North American homes and was similar to the outdoor conditions in west central Kenya (12.96 hPa).
When humans adjust the climates within their homes, it is unlikely that most are consciously attempting to emulate the climatic conditions of some outdoor location in another country or continent. Instead, they are almost certainly attempting to achieve climatic conditions that result in thermal comfort. They do so to such an extent that indoor climate is no longer well correlated to outdoor climate (table 1). Based purely on its indoor temperature and humidity, you would be unlikely to discern whether a house from our dataset was in Wyoming or Mississippi. Of the two climatic variables, we considered, indoor humidity was more strongly correlated with outdoor conditions than was the case for temperature, but this correlation was weak. The extent to which humans have decoupled indoor and outdoor climate is likely to be the most extreme in nature. Even honeybee nests, for example, which are actively buffered from outdoor conditions, still vary in response to outdoor conditions.
In general, mammals, including humans, have evolved the ability to regulate their body temperatures via behaviour and autonomic responses. Human autonomic control has the capacity to maintain brain and core temperature over a range of environmental conditions [48]. Moreover, humans acclimate relatively quickly to new climatic conditions [49] and the evolution of hypothalamic controlled body temperatures, along with behavioural and cultural advances, may have allowed humans to expand the range of climatic conditions of their niche. So why do humans expend such extraordinary expense to maintain constant indoor climates [50] when such climates are not necessary for survival, especially given the plasticity of human temperature acclimation (e.g. ama divers to endurance athletes)? Probably, it is because these climates are comfortable.
In mammals, the perception of whether a climate is comfortable or not is an important driver of climate seeking behaviour [51], as a comfortable climate produces conditions that allow an individual to remain within their thermoneutral zone (TNZ). The TNZ is the range of environmental conditions where, for a given animal, heat loss equals gain and core body temperature is maintained [52]. When an individual is outside of this range of conditions, the individual may adjust climatic conditions behaviourally, physiologically or psychologically to adapt to the climatic conditions and ultimately perceive thermal comfort [51,53]. These TNZs are mutable and may change with an individual's climatic history or habituation of an indoor space (i.e. the Adaptive Comfort Model), but the methods to achieve thermal comfort remain the same [53,54]. Interestingly, the range of the mean indoor temperature recorded by citizen scientists in their homes and the 100 most climatically similar global grid cells (figure 3) largely fall within the TNZs (24–30°C) for primates including humans [23]. A comparison of climate-related physiological parameters between humans and a selection of non-human primates is included in table 3. We hypothesize that indoor climates largely correspond with our TNZ because our ancestors evolved thermal preferences that led them to favour (and ultimately build) these climates.
Table 3.
Climate-related values for select primate species. Variables include animal husbandry recommendations for temperature (THusbandry) and relative humidity (RHHusbandry), natural habitat temperature (THabitat), normal adult body temperature (TBody) and thermoneutral zone (TNZ). THusbandry and RHHusbandry values, [55]. THabitat values, Primate Info Network, Wisconsin National Primate Research Center, University of Wisconsin – Madison, accessed 10 April 2017; http://pin.primate.wisc.edu). TBody and TNZ values, [23,56–60].
| species | THusbandry(°C) | RHHusbandry (%) | THabitat (°C) | TBody (°C) | TNZ (°C) |
|---|---|---|---|---|---|
| Gorilla beringei | 18.3–29.4 | 30–70 | 3.9–14.5 | unknown | unknown |
| Gorilla gorilla | 18.3–29.4 | 30–70 | 23 | 35.5 | unknown |
| Homo sapiens | n.a. | n.a. | n.a. | 37 | 25–30 |
| Pan paniscus | 18–22 | 50–60 | 20–30 | unknown | unknown |
| Pan troglodytes | 15.6–29.4 | 30–70 | 18.5–30 | 37.25 | 17–29 |
| Pongo abelii | 18–28 | 30–70 | 17–34.2 | unknown | unknown |
| Pongo pygmaeus | 18–28 | 30–70 | 18–37.5 | 37 | unknown |
Perhaps not surprising, in light of the TNZ hypothesis, the temperature people prefer overlaps with much of the geographical area in which key events in hominid evolution and, for that matter, early civilization occurred [48]. We hypothesize that natural selection favoured human preferences and thermal traits that allowed human ancestors to live in those climates. However, as humans moved out of those environments they faced new climates. Strong evidence suggests that the selective pressures imparted by climate has altered human genomes [24,61,62]. In addition, new climates led to cultural responses such as the use of fire for heat [63], clothing [26] and shelter [64], all of which modified the climate to which individuals were exposed. We argue that modern temperatures in homes are a continuation of this same effort, but the technological ability of humans to modify climate has led to the extreme scenario, where fossil fuels are cheap, and (North American) indoor climates closely align with TNZs. Moreover, air-conditioned buildings with closed ventilation combined with changing indoor climatic expectations have also led to narrower ranges of human thermal comfort [30,53]. However, many questions remain. For example, do wealthy homeowners (or striving homeowners) keep their homes colder than is preferred in hot places to display wealth (and vice versa)? Do genetic backgrounds of homeowners influence preferred climates? How do these climates affect our health and well-being? For example, indoor climates are less variable [65] than outdoor climates and this reduced variability may lead to health issues such as obesity or diabetes [66,67].
Our results also offer a hypothesis about the likely origin of human home-associated species, as indoor climates probably favour certain lineages, those pre-adapted to indoor climates. We hypothesize that the assemblage of species that colonize our homes are likely to be those with thermal preferences/tolerances similar to us, which is to say species from relatively dry, relatively warm climates, including north and eastern Africa, but also much of the Middle East. Moreover, predictions can be made about the communities of home associates through time and space, as climate, home technologies and fortunes change. We know that climate preferences in homes differ among regions [25,29], and the USA is probably an extreme case, where indoor climates most reflect resource availability and culture, rather than economic and environmental costs.
Our characterization of the indoor climate of North American homes and the identification of the outdoor climates most similar to these homes opens a new line of inquiry. Why do we prefer these climatic conditions? Do the climates of modern houses reflect our ancestral climates? When and where did we evolve these modern climate preferences, and what are the contributions of genetic and cultural evolution to these preferences? Interestingly, the majority of 100 most climatically similar outdoor locations were located in the hot and seasonably dry northeastern Africa, a region rich in hominid fossils and evolution [68].
As a first step, we presented a simple comparison between the indoor climate of these North American homes and the climatic conditions experienced by some non-human primates (i.e. great apes). We found that climatic conditions generally overlapped. However, no a priori predictions seem to exist for which global climate we might favour in our homes, and future work should test the simplest one, namely that we tend to attempt to recreate the conditions from which we evolved, before we had the ability to make homes, the ones to which our physiologies are adapted.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the citizen scientists who collected the climatic data from their homes, and Lea Shell and Meghan Thoemmes for their help coordinating citizen science participation.
Ethics
This research was approved by the NC State University IRB review board under IRB Protocol 2177. We received written consent from all participants.
Data accessibility
The datasets supporting this article have been uploaded as part of the supplementary material.
Authors' contributions
M.G.J., L.M.N. and R.R.D. conceived and designed the research and revised the manuscript; M.G.J. analysed the data and prepared the draft manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by a National Science Foundation CAREER Award (no. 953390) to R.R.D.
References
- 1.Bertone MA, Leong M, Bayless KM, Malow TLF, Dunn RR, Trautwein MD. 2016. Arthropods of the great indoors: characterizing diversity inside urban and suburban homes. PeerJ 4, e1582 ( 10.7717/peerj.1582) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberán A, et al. 2015. The ecology of microscopic life in household dust. Proc. R. Soc. B 282, 20151139 ( 10.1098/rspb.2015.1139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawkins R. 1982. The extended phenotype: the gene as the unit of selection. Oxford, UK: Oxford University Press. [Google Scholar]
- 4.Jouquet P, Mery T, Rouland C, Lepage M. 2003. Modulated effect of the termite Ancistrotermes cavithorax (Isoptera, Macrotermitinae) on soil properties according to the internal mound structures. Sociobiology 42, 1–10. [Google Scholar]
- 5.Weber JN, Hoekstra HE. 2009. The evolution of burrowing behaviour in deer mice (genus Peromyscus). Anim. Behav. 77, 603–609. ( 10.1016/j.anbehav.2008.10.031) [DOI] [Google Scholar]
- 6.Hu CK, Hoekstra HE. 2017. Peromyscus burrowing: a model system for behavioral evolution. Semin. Cell Dev. Biol. 61, 107–114. ( 10.1016/j.semcdb.2016.08.001) [DOI] [PubMed] [Google Scholar]
- 7.Karjalainen S. 2007. Gender differences in thermal comfort and use of thermostats in everyday thermal environments. Build. Environ. 42, 1594–1603. ( 10.1016/j.buildenv.2006.01.009) [DOI] [Google Scholar]
- 8.Kempton W. 1986. Two theories of home heat control. Cogn. Sci. 90, 75–90. ( 10.1207/s15516709cog1001_3) [DOI] [Google Scholar]
- 9.Peffer T, Pritoni M, Meier A, Aragon C, Perry D. 2011. How people use thermostats in homes: a review. Build. Environ. 46, 2529–2541. ( 10.1016/j.buildenv.2011.06.002) [DOI] [Google Scholar]
- 10.Cook J. 1996. Architecture indigenous to extreme climates. Energy Build. 23, 277–291. ( 10.1016/0378-7788(95)00953-1) [DOI] [Google Scholar]
- 11.Zhai Z, Previtali JM. 2010. Ancient vernacular architecture: characteristics categorization and energy performance evaluation. Energy Build. 42, 357–365. ( 10.1016/j.enbuild.2009.10.002) [DOI] [Google Scholar]
- 12.Stevenson R. 1985. The relative importance of behavioral and physiological adjustments controlling body temperature in terrestrial ectotherms. Am. Nat. 126, 362–386. (http://www.jstor.org/stable/2461361) [Google Scholar]
- 13.Kearney M, Predavec M. 2000. Do nocturnal ectotherms thermoregulate? A study of the temperate gecko Christinus marmoratus. Ecology 81, 2984–2996. [Google Scholar]
- 14.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB.. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goller M, Goller F, French SS. 2014. A heterogeneous thermal environment enables remarkable behavioral thermoregulation in Uta stansburiana. Ecol. Evol. 4, 3319–3329. ( 10.1002/ece3.1141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barton M, Porter W, Kearney M. 2014. Behavioural thermoregulation and the relative roles of convection and radiation in a basking butterfly. J. Therm. Biol. 41, 65–71. ( 10.1016/j.jtherbio.2014.02.004) [DOI] [PubMed] [Google Scholar]
- 17.Kronenberg F, Heller HC. 1982. Colonial thermoregulation in honey bees (Apis mellifera). J. Comp. Physiol. B 148, 65–76. ( 10.1007/BF00688889) [DOI] [Google Scholar]
- 18.Jones J, Oldroyd B. 2006. Nest thermoregulation in social insects. Adv. Insect Physiol. 33, 153–191. ( 10.1016/S0065-2806(06)33003-2) [DOI] [Google Scholar]
- 19.Terrien J, Perret M, Aujard F. 2011. Behavioral thermoregulation in mammals: a review. Front. Biosci. 16, 1428–1444. ( 10.2741/3797) [DOI] [PubMed] [Google Scholar]
- 20.Schlader ZJ, Simmons SE, Stannard SR, Mündel T. 2011. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol. Behav. 103, 217–224. ( 10.1016/j.physbeh.2011.02.002) [DOI] [PubMed] [Google Scholar]
- 21.Parsons K. 2014. Human thermal environments, 3rd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 22.Werner J. 1981. Control aspects of human temperature regulation. Automatica 17, 351–362. ( 10.1016/0005-1098(81)90052-2) [DOI] [Google Scholar]
- 23.Hanna JM, Brown DE. 1983. Human heat tolerance: an anthropological perspective. Annu. Rev. Anthropol. 12, 259–284. ( 10.1146/annurev.an.12.100183.001355) [DOI] [Google Scholar]
- 24.Hancock AM, et al. 2011. Adaptations to climate-mediated selective pressures in humans. PLoS Genet. 7, e1001375 ( 10.1371/journal.pgen.1001375) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Djongyang N, Tchinda R, Njomo D. 2010. Thermal comfort: a review paper. Renew. Sustain. Energy Rev. 14, 2626–2640. ( 10.1016/j.rser.2010.07.040) [DOI] [Google Scholar]
- 26.Gilligan I. 2010. The prehistoric development of clothing: Archaeological implications of a thermal model. J. Archaeol. Method Theory 17, 15–80. ( 10.1007/s10816-009-9076-x) [DOI] [Google Scholar]
- 27.Pappenberger F, Jendritzky G, Staiger H, Dutra E, Di Giuseppe F, Richardson DS, Cloke HL.. 2015. Global forecasting of thermal health hazards: the skill of probabilistic predictions of the Universal Thermal Climate Index (UTCI). Int. J. Biometeorol. 59, 311–323. ( 10.1007/s00484-014-0843-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staiger H, Laschewski G, Grätz A. 2012. The perceived temperature: a versatile index for the assessment of the human thermal environment. Part A: scientific basics. Int. J. Biometeorol. 56, 165–176. ( 10.1007/s00484-011-0409-6) [DOI] [PubMed] [Google Scholar]
- 29.Mayer H, Höppe P. 1987. Thermal comfort of man in different urban environments. Theor. Appl. Climatol. 49, 43–49. (http://link.springer.com/article/10.1007/BF00866252) [Google Scholar]
- 30.Ubbelohde M, Loisos G, McBride R.. 2003. Advanced comfort criteria & annotated bibliography on adapted comfort (No. P500-04-009-A4). Sacramento, CA: California Energy Commission.
- 31.Lin Z, Deng S. 2008. A study on the thermal comfort in sleeping environments in the subtropics—developing a thermal comfort model for sleeping environments. Build. Environ. 43, 70–81. ( 10.1016/j.buildenv.2006.11.026) [DOI] [Google Scholar]
- 32.Malama A, Sharples S. 1997. Thermal performance of traditional and contemporary housing in the cool season of Zambia. Build. Environ. 32, 69–78. ( 10.1016/S0360-1323(96)00036-4) [DOI] [Google Scholar]
- 33.Karjalainen S. 2009. Thermal comfort and use of thermostats in Finnish homes and offices. Build. Environ. 44, 1237–1245. ( 10.1016/j.buildenv.2008.09.002) [DOI] [Google Scholar]
- 34.Indraganti M, Rao KD. 2010. Effect of age, gender, economic group and tenure on thermal comfort: a field study in residential buildings in hot and dry climate with seasonal variations. Energy Build. 42, 273–281. ( 10.1016/j.enbuild.2009.09.003) [DOI] [Google Scholar]
- 35.Martin LJ, et al. 2015. Evolution of the indoor biome. Trends Ecol. Evol. 30, 223–232. ( 10.1016/j.tree.2015.02.001) [DOI] [PubMed] [Google Scholar]
- 36.Terando AJ, Youngsteadt E, Meineke EK, Prado SG. 2017. Ad hoc instrumentation methods in ecological studies produce highly biased temperature measurements. Ecol. Evol. 7, 9890–9904. ( 10.1002/ece3.3499) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawrence MG. 2005. The relationship between relative humidity and the dewpoint temperature in moist air: a simple conversion and applications. Bull. Am. Meteorol. Soc. 86, 225–233. ( 10.1175/BAMS-86-2-225) [DOI] [Google Scholar]
- 38.R Core Team. 2016. R (3.3.2): A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/) [Google Scholar]
- 39.Harris I, Jones PD, Osborn TJ, Lister DH. 2014. Updated high-resolution grids of monthly climatic observations: the CRU TS3.10 dataset. Int. J. Climatol. 34, 623–642. ( 10.1002/joc.3711) [DOI] [Google Scholar]
- 40.New M, Hulme M, Jones P. 1999. Representing twentieth-century space–time climate variability. Part I: development of a 1961–90 mean monthly terrestrial climatology. J. Clim. 12, 829–856. [Google Scholar]
- 41.Fountain M, Brager G, de Dear R. 1996. Expectations of indoor climate control. Energy Build. 24, 179–182. ( 10.1016/S0378-7788(96)00988-7) [DOI] [Google Scholar]
- 42.Veloz S, Williams JW, Lorenz D, Notaro M, Vavrus S, Vimont DJ. 2012. Identifying climatic analogs for Wisconsin under 21st-century climate-change scenarios. Clim. Change 112, 1037–1058. ( 10.1007/s10584-011-0261-z) [DOI] [Google Scholar]
- 43.Williams JW, Jackson ST, Kutzbach JE. 2007. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742. ( 10.1073/pnas.0606292104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grenier P, Parent AC, Huard D, Anctil F, Chaumont D. 2013. An assessment of six dissimilarity metrics for climate analogs. J. Appl. Meteorol. Climatol. 52, 733–752. ( 10.1175/JAMC-D-12-0170.1) [DOI] [Google Scholar]
- 45.Hallegatte S, Hourcade JC, Ambrosi P. 2007. Using climate analogues for assessing climate change economic impacts in urban areas. Clim. Change 82, 47–60. ( 10.1007/s10584-006-9161-z) [DOI] [Google Scholar]
- 46.Kopf S, Ha-Duong M, Hallegatte S. 2008. Using maps of city analogues to display and interpret climate change scenarios and their uncertainty. Nat. Hazards Earth Syst. Sci. 8, 905–918. ( 10.5194/nhess-8-905-2008) [DOI] [Google Scholar]
- 47.Klepeis N, Nelson W, Ott W, Robinson J, Tsang A, Switzer P, Behar JV, Hern SC, Engelmann WH.. 2001. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Expo. Anal. Environ. Epidemiol. 11, 231–252. ( 10.1038/sj.jea.7500165) [DOI] [PubMed] [Google Scholar]
- 48.Gisolfi C, Mora F. 2000. What's so important about a body temperature of 37°C? The hot brain, pp. 95–119. Cambridge, MA: MIT Press. [Google Scholar]
- 49.Taylor NAS. 2014. Human heat adaptation. Compr. Physiol. 4, 325–365. ( 10.1002/cphy.c130022) [DOI] [PubMed] [Google Scholar]
- 50.IEA. 2004. Oil crises & climate changes: 30 years of energy use in IEA countries. Paris, France: International Energy Association. [Google Scholar]
- 51.Frank SM, Raja SN, Bulcao CF, Goldstein DS. Relative contribution of core and cutaneous temperatures to thermal comfort and autonomic responses in humans. J. Appl. Physiol. 86, 1588–1593. (http://jap.physiology.org/content/86/5/1588.abstract) [DOI] [PubMed] [Google Scholar]
- 52.Kingma BR, Frijns AJH, Schellen L, van Marken Lichtenbelt WD. 2014. Beyond the classic thermoneutral zone: including thermal comfort. Temperature 1, 142–149. ( 10.4161/temp.29702) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Dear RJ, Brager GS, Reardon J, Nicol F.. 1998. Developing an adaptive model of thermal comfort and preference Am Soc Heating, Refrig Air Cond Eng Inc, Macquarie Res Ltd., 4106. [Google Scholar]
- 54.Filingeri D, Zhang H, Arens EA. 2017. Characteristics of the local cutaneous sensory thermoneutral zone. J. Neurophysiol. 117, 1797–1806. ( 10.1152/jn.00845.2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy HW. 2015. Great apes. In Fowler's zoo and wild animal medicine, 8th edn (eds Miller E, Fowler M), pp. 336–354. St. Louis, MO: Elsevier Saunders. [Google Scholar]
- 56.Brown CS, Finnegan JM. 2007. Resting heart rate and tympanic temperature in operant conditioned western lowland gorillas (Gorilla gorilla gorilla). J. Zoo Wildl. Med. 38, 345–347. ( 10.1638/1042-7260(2007)038[0345:RHRATT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 57.Morrison P. 1962. An analysis of body temperature in the chimpanzee. J. Mammal. 43, 166–171. [Google Scholar]
- 58.Fahlman A, Bosi EJ, Nyman G. 2006. Reversible anesthesia of Southeast Asian primates with medetomidine, zolazepam, and tiletamine. J. Zoo Wildl. Med. 37, 558–561. ( 10.1638/05-044.1) [DOI] [PubMed] [Google Scholar]
- 59.Benedict FG, Bruhn JM. 1936. Chimpanzee metabolism. Proc. Natl Acad. Sci. USA 22, 394–397. (http://www.jstor.org/stable/86571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duncan LM, Pillay N.. Shade as a thermoregulatory resource for captive chimpanzees. J. Therm. Biol. 2013;38: 169–177. ( 10.1016/j.jtherbio.2013.02.009) [DOI] [Google Scholar]
- 61.Fumagalli M, et al. 2015. Greenlandic Inuit show genetic signatures of diet and climate adaptation. Science 349, 1343–1347. ( 10.1126/science.aab2319) [DOI] [PubMed] [Google Scholar]
- 62.Hancock AM, Witonsky DB, Gordon AS, Eshel G, Pritchard JK, Coop G, Di Rienzo A.. 2008. Adaptations to climate in candidate genes for common metabolic disorders. PLoS Genet. 4, e32 ( 10.1371/journal.pgen.0040032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roebroeks W, Villa P. 2011. On the earliest evidence for habitual use of fire in Europe. Proc. Natl Acad. Sci. USA 108, 5209–5214. ( 10.1073/pnas.1018116108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu W. 2009. A functional approach to Paleolithic open-air habitation structures. World Archaeol. 41, 348–362. ( 10.1080/00438240903112179) [DOI] [Google Scholar]
- 65.Healy S. 2008. Air-conditioning and the ‘homogenization’ of people and built environments. Build. Res. Inf. 36, 312–322. ( 10.1080/09613210802076351) [DOI] [Google Scholar]
- 66.Johnson F, Mavrogianni A, Ucci M, Vidal-Puig A, Wardle J. 2011. Could increased time spent in a thermal comfort zone contribute to population increases in obesity? Obes. Rev. 12, 543–551. ( 10.1111/j.1467-789X.2010.00851.x) [DOI] [PubMed] [Google Scholar]
- 67.Chechi K, Carpentier AC, Richard D. 2013. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 24, 408–420. ( 10.1016/j.tem.2013.04.002) [DOI] [PubMed] [Google Scholar]
- 68.Maslin MA, Brierley CM, Milner AM, Shultz S, Trauth MH, Wilson KE. 2014. East African climate pulses and early human evolution. Quat. Sci. Rev. 101, 1–17. ( 10.1016/j.quascirev.2014.06.012) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the supplementary material.