Abstract
Schizophrenia is a debilitating and heritable mental disorder associated with lower reproductive success. However, the prevalence of schizophrenia is stable over populations and time, resulting in an evolutionary puzzle: how is schizophrenia maintained in the population, given its apparent fitness costs? One possibility is that increased genetic liability for schizophrenia, in the absence of the disorder itself, may confer some reproductive advantage. We assessed the correlation and causal effect of genetic liability for schizophrenia with number of children, age at first birth and number of sexual partners using data from the Psychiatric Genomics Consortium and UK Biobank. Linkage disequilibrium score regression showed little evidence of genetic correlation between genetic liability for schizophrenia and number of children (rg = 0.002, p = 0.84), age at first birth (rg = −0.007, p = 0.45) or number of sexual partners (rg = 0.007, p = 0.42). Mendelian randomization indicated no robust evidence of a causal effect of genetic liability for schizophrenia on number of children (mean difference: 0.003 increase in number of children per doubling in the natural log odds ratio of schizophrenia risk, 95% confidence interval (CI): −0.003 to 0.009, p = 0.39) or age at first birth (−0.004 years lower age at first birth, 95% CI: −0.043 to 0.034, p = 0.82). We find some evidence of a positive effect of genetic liability for schizophrenia on number of sexual partners (0.165 increase in the number of sexual partners, 95% CI: 0.117–0.212, p = 5.30×10−10). These results suggest that increased genetic liability for schizophrenia does not confer a fitness advantage but does increase mating success.
Keywords: schizophrenia, Mendelian randomization, stabilizing selection, cliff-edge fitness, reproductive success
1. Introduction
Schizophrenia is a severe, debilitating mental disorder that is substantially heritable [1]. The prevalence of schizophrenia remains stable over populations and time, and yet is associated with lower reproductive success for those diagnosed [1–5]. This creates an evolutionary puzzle: how is schizophrenia maintained in the population despite apparent negative selection? Multiple theories have been proposed to explain this paradox [3,6,7]. One is mutation–selection balance, which suggests that selection against detrimental variants is counteracted by the continuous occurrence of new mutations [8,9]. Another is that effects over many common genetic variants are individually too weak to be under negative selection [1,8,10].
Another popular theory is that stabilizing selection operates, where the optimum fitness level for a trait is approximately at the mean of the trait and fitness declines along a normal distribution on either side of this optimum [3,6,11,12]. A related hypothesis is that schizophrenia-related traits may demonstrate ‘cliff-edge’ effects on fitness, so that fitness increases with increased expression of the trait until a threshold, where increased expression then results in a steep decline in fitness for some individuals [1,12]. Some have suggested that this peak occurs at levels of symptoms that could result in a diagnosis of schizophrenia, with a reproductive advantage among healthy individuals with an increased genetic liability for the disorder (in the absence of the disorder itself) compensating for the lower reproductive success of those with the disorder itself [1,4,12–14]. Behaviourally, it is possible that higher genetic liability for schizophrenia may be associated with attractive traits (e.g. creativity) and therefore also with a greater number of children [4,13]. For example, schizotypy, a personality measure of schizophrenia-proneness, has been shown to be associated with creativity, short-term mating interest and mating success [4,13,15], while genetic liability for schizophrenia is associated with the increased risk of unprotected sex [16].
Relatives of people with schizophrenia are assumed to have an intermediate level of genetic liability for the highly heritable disorder [17]. Studies into whether cliff-edge fitness maintains the prevalence of schizophrenia have therefore largely focused on family studies. However, despite extensive research, there is no clear evidence of increased fecundity in relatives of individuals with schizophrenia [2,7,17]. Del Giudice [17] argued that family studies underestimate the reproductive benefits of schizophrenia-proneness in the general population. He highlights that relatives share not only genetic liability for schizophrenia but also their environments, which may hinder fitness and result in apparent negative selection [17]. It is therefore important to investigate a potential reproductive advantage of schizophrenia-proneness in the wider population, rather than relying on family studies alone. Moreover, it is important to investigate causal relationships between schizophrenia risk and reproductive success, rather than relying on observational methods previously used, which do not support strong causal inference due to bias from residual confounding and reverse causation [18]. These family studies also suggest that optimum fitness could occur before the appearance of symptoms that might result in the diagnosis of schizophrenia. The principal measure of fitness is the number of children, used in the present study; however, both earlier age at first birth and increased numbers of sexual partners have previously been used as indicators of reproductive success, particularly in developed populations in which contraception is commonly used to control family size [4,8,19,20]. Earlier age at first birth probably results in a longer reproductive period, whereas the number of sexual partners captures mating success and hence potential reproductive success [4].
Recent developments in genetic epidemiology mean that it is now possible to investigate the effects of genetic liability for schizophrenia in the wider population. A genome-wide association study (GWAS) identified 128 independent genetic variants from 108 loci associated with schizophrenia that explained approximately 3.4% of the observed variation in schizophrenia risk [21]. These variants have been used to show that genetic liability for schizophrenia (using a risk score comprising these individual variants) is positively associated with creativity [22]. Evidence for associations between genetic liability for schizophrenia and age at first birth is mixed. Higher genetic liability for schizophrenia was found for those with a young (e.g. below 20 years) age at first birth compared to those with an intermediate age at first birth [23,24]. Another study found no clear evidence for linear or quadratic associations between a genetic liability for schizophrenia and age at first birth [8]. Two previous studies also used schizophrenia-associated variants to investigate whether genetic liability for schizophrenia is associated with number of children, but results were again inconclusive, perhaps due to limited power [8,25]. The studies showed estimates in the direction of a reproductive advantage, but confidence intervals are typically wide and consistent with no effect [8,25]. Nevertheless, these studies demonstrate how genetic liability for schizophrenia can be measured in the wider population.
We applied a range of methods with roots in genetic epidemiology to test part of the cliff-edge hypothesis. For our main analysis, we examine whether increasing genetic liability for schizophrenia increases reproductive success in a largely post-reproductive population-based sample which is not selected on schizophrenia status, and, therefore, includes very few cases. This linear increase is predicted for part of cliff-edge fitness where a reproductive advantage among healthy individuals with higher genetic liability for the disorder compensates for lower reproductive success of those with the disorder itself. Additionally, given suggestions from family studies that there may be a fitness decline of healthy individuals with high genetic liability for the disorder, we conducted sensitivity analyses to investigate a possible nonlinear relationship where at very high levels of genetic score for schizophrenia liability, there is decreased reproductive success in the absence of schizophrenia itself [2,7,17].
For our main analysis, we calculated genetic correlations using linkage disequilibrium (LD) score regression between genetic liability for schizophrenia and reproductive success, measured as number of children, age at first birth and number of sexual partners. Furthermore, we used genetic variants associated with schizophrenia within a Mendelian randomization (MR) framework to estimate the causal effect of genetic liability for schizophrenia on these measures of reproductive success. MR uses single-nucleotide polymorphisms (SNPs), which are assigned at conception and are mostly independent from other variants or environments. MR therefore overcomes some limitations of observational studies previously used to investigate this evolutionary paradox, by reducing bias from confounding and reverse causation [18]. Finally, we estimated the effect of genetically predicted educational attainment on the number of children and age at first birth. Higher genetically predicted education is known to be associated with fewer children and delayed age at first birth [25–29]. As the present study applied MR in a novel context to this evolutionary paradox, we therefore included educational attainment as an exposure with these two outcomes (using the same outcome datasets used for our schizophrenia analysis) as a positive control.
2. Methods
2.1. Exposure data
We used independent SNPs associated with schizophrenia (p < 5 × 10−8) from the Psychiatric Genomics Consortium GWAS (N = 35 123 cases and 109 657 controls) [21]. The 128 SNPs identified explained approximately 3.4% of the observed variance in schizophrenia risk. A total of 101 SNPs remained due to availability in UK Biobank, availability of proxies and meeting exclusion criteria (see electronic supplementary material). Odds ratios (ORs) and standard errors (s.e.) for the 101 SNP and schizophrenia associations were recorded using GWAS data for Europeans only [30]. The final 101 SNPs and effect estimates for schizophrenia genetic variants are listed in electronic supplementary material, table S1.
For educational attainment, we used SNPs associated with educational attainment (p < 5 × 10−8) from a recent GWAS by the Social Science Genetic Association Consortium [31]. As the GWAS conducted a replication in UK Biobank, effect estimates from the pooled sex analysis of the discovery sample were used to avoid sample overlap. Sixty-seven SNPs were available in UK Biobank data and met exclusion criteria. Effect estimates used for educational attainment genetic variants are listed in electronic supplementary material, table S2.
2.2. Outcome data
The exposure-associated SNPs described above were extracted from UK Biobank to derive SNP-outcome associations for our outcome data. Extraction was done using PLINK (v. 2.00) and best guess algorithms for determining alleles (full genotyping information below).
2.2.1. Sample
UK Biobank is a population-based health research resource consisting of approximately 500 000 people, aged between 38 and 73 years, who were recruited between the years 2006 and 2010 from across the UK [32]. Particularly focused on identifying determinants of human diseases in middle-aged and older individuals, participants provided a range of information (such as demographics, health status, lifestyle measures, cognitive testing, personality self-report, and physical and mental health measures) via questionnaires and interviews; anthropometric measures, blood pressure readings and samples of blood, urine and saliva were also taken. A full description of the study design, participants and quality control (QC) methods has been published [33].
2.2.2. Genotyping information in UK Biobank
The full data release contains the cohort of successfully genotyped samples (N = 488 377). A total of 49 979 individuals were genotyped using the UK BiLEVE array and 438 398 using the UK Biobank axiom array. Pre-imputation QC, phasing and imputation are described elsewhere [34]. In brief, prior to phasing, multiallelic SNPs or those with minor allele frequency (MAF) less than or equal to 1% were removed. Phasing of genotype data was performed using a modified version of the SHAPEIT2 algorithm [35]. Genotype imputation to a reference set combining the UK10 K haplotype and Haplotype Reference Consortium (HRC) reference panels [36] was performed using IMPUTE2 algorithms [37]. The analyses presented here were restricted to autosomal variants within the HRC site list using a graded filtering with varying imputation quality for different allele frequency ranges. Therefore, rarer genetic variants are required to have a higher imputation Info score (Info > 0.3 for MAF > 3%; Info > 0.6 for MAF 1–3%; Info > 0.8 for MAF 0.5–1%; Info > 0.9 for MAF 0.1–0.5%) with MAF and Info scores having been recalculated on an in-house-derived ‘European’ subset. Individuals with sex-mismatch (derived by comparing genetic sex and reported sex) or individuals with sex-chromosome aneuploidy were excluded from the analysis (N = 814). We restricted the sample to individuals of white British ancestry who self-report as ‘White British’ and who have very similar ancestral backgrounds according to the principal component analysis (PCA) (N = 409 703), as described by Bycroft et al. [34]. Estimated kinship coefficients using the KING toolset [38] identified 107 162 pairs of individuals [34]. An in-house algorithm was then applied to this list and preferentially removed the individuals related to the greatest number of other individuals until no related pairs remain. These individuals were excluded (N = 79 448). Additionally, two individuals were removed due to them relating to a very large number (greater than 200) of individuals. QC protocol is described elsewhere [39].
2.2.3. Outcome measures
We derived the number of children and age at first birth similarly to previous analyses in UK Biobank [28]. Participants were either asked how many children they had given birth to or how many children they had fathered. We further derived a binary variable to indicate if participants were childless or not (childlessness coded as 1). Age at first birth was only measured in females in UK Biobank, with participants asked: ‘How old were you when you had your first child?’. If participants indicated that they had had sexual intercourse, they were asked ‘About how many sexual partners have you had in your lifetime?’. Participants were given the information that ‘Sexual intercourse includes vaginal, oral or anal intercourse’ if they activated the help button. We coded responses to missing if above the 99th percentile. We then derived a binary measure indicating if participants were in approximately the top 10th percentile for the highest number of sexual partners (equal to or above 12 partners coded as 1). Although no age restrictions were applied in analyses, the nature of UK Biobank data meant that participants were aged towards the end of their reproductive lives.
2.3. Data analysis
We used LD score regression [40,41] to calculate the genome-wide genetic correlation (rg) between schizophrenia liability or predicted educational attainment and number of children, age at first birth and number of sexual partners. Genome-wide associations were conducted for these outcomes using linear regression, implemented in PLINK v. 2.00 through the Medical Research Council Integrative Epidemiology Unit GWAS pipeline [42]. In this, we adjusted for the top 10 principal components. For the number of children and number of sexual partners analysis, age and sex were also included as covariates. We then filtered results on MAF (greater than 0.01) and imputation quality (greater than 0.8) separately.
In MR analyses, data were harmonized to ensure that the effect of the SNP on the exposure and the SNP on the outcome corresponded to the same allele. The increasing allele for schizophrenia liability and educational attainment was used. Associations for exposure SNPs and all outcome measures were then calculated in R, fitting the same covariates as listed above. Effect sizes for number of children, age at first birth and number of sexual partners analysis are listed in electronic supplementary material, tables S1 and S2. SNP-exposure and SNP-outcome data were combined using an inverse variance weighted (IVW) approach which is analogous to a weighted regression of SNP-outcome coefficients on SNP-exposure coefficients with the intercept constrained to zero [43], and further assumes all variants are valid instruments or allows pleiotropy to be balanced across instruments when using the random effect [44] with Cochran's Q providing a measure of any overdispersion (see electronic supplementary material).
The IVW effect estimate will only be consistent if all genetic variants in the analysis are valid. Weighted median, mode-based estimator and MR-Egger regression are complementary approaches that can be used to investigate the impact of invalid instruments on our effect estimates. The weighted median estimates a consistent effect estimate if at least 50% of the instruments are valid [45]. The mode-based estimator provides a consistent effect estimate when the largest number of similar individual-instrument estimates come from valid instruments, even if the majority are invalid [46]. A tuning parameter of 0.5 was set for mode-based estimator analysis. One of the main assumptions underpinning MR is that of no horizontal pleiotropy (i.e. no direct effect of the genetic variant on the outcome that does not act through the exposure) [47]. MR-Egger regression analysis can be used to further investigate this; MR-Egger does not constrain the intercept to zero and the intercept term therefore estimates overall directional pleiotropy [48]. We calculated F-statistics (mean of the squared SNP-exposure association divided by the squared s.e. for SNP-outcome association) to indicate the strength of instrument, and I2GX statistics to assess the suitability of MR-Egger (above 0.9 is desired) [47]. Analysis was repeated after removing the few schizophrenia cases in our sample. All analysis was also conducted with SNP-outcome associations additionally adjusted for genotype array.
MR results were multiplied by 0.693 to represent the causal estimate per doubling in odds of schizophrenia risk [49]. For childlessness and highest number of sexual partners as outcomes, all MR results were multiplied by 0.693 on the log-odds scale, and then exponentiated. The reported estimates therefore indicate the effect of doubling the odds of schizophrenia on the odds of childlessness or being in the highest number of sexual partners category. The effects of education on childlessness were converted to ORs by exponentiating log ORs.
As an illustration of shape of the schizophrenia liability–reproductive success relationship, we created an additive unweighted genetic score for schizophrenia liability in UK Biobank. The score was created in R (v. 3.2.0), with missing SNP data replaced with the mean value for that SNP across individuals. We then divided this score into quintiles and deciles and plotted the mean number of children, age at first birth and number of sexual partners across these categories of the genetic score. We additionally plotted these relationships with reproductive success on the x-axis. As sensitivity analysis to assess if there was any decline in reproductive success within our sample at very high levels of genetic liability, we conducted a series of linear regressions in Stata (v. MP 15.1) between this genetic score for schizophrenia liability and our outcomes, systematically removing cumulative centiles from the maximum. This analysis included adjustment for the top 10 principal components and was repeated after removing the few schizophrenia cases in our sample. Similarly, to further investigate a possible peak in reproductive success at high genetic liability for schizophrenia, we conducted quadratic regression analysis of the genetic score for schizophrenia liability and our outcomes (adjusted for the top 10 principal components and additionally adjusted for sex and age at assessment where appropriate). Lastly, we repeated quadratic analysis separately for each sex.
Analysis scripts are available on GitHub [50].
3. Results
In our sample, from UK Biobank, there were more females than males, a majority had children and a minority had college or university degree qualifications (electronic supplementary material, table S3). The mean age was 56.9 years (s.d.: 8.0) and the mean years of education was 13.3 (s.d.: 4.4). For our outcomes, the mean number of children was 1.8 (s.d.: 1.2), mean number of sexual partners was 5.8 (s.d.: 8.6) and the mean age at first birth was 25.4 years (s.d.: 4.5).
3.1. Genetically predicted educational attainment
We found a modest negative genetic correlation between educational attainment-associated variants and number of children (rg = −0.35, p = 8.57 × 10−41) and a strong positive genetic correlation between educational attainment-associated variants and age at first birth (rg = 0.81, p < 5 × 10−41) (table 1). The number of individuals in this analysis was 283 723 for educational attainment, 333 628 for number of children and 123 310 for age at first birth data. There was a total of 1 117 154 SNPs included in analysis.
Table 1.
Genetic correlations of genetic liability for schizophrenia and genetically predicted educational attainment on number of children, age at first birth and number of sexual partners using LD score regression.
| no. of childrena |
age at first birthb |
no. of sexual partnersc |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| rg | s.e. | p-value | rg | s.e. | p-value | rg | s.e. | p-value | |
| genetic liability for schizophreniad | 0.002 | 0.008 | 0.84 | −0.007 | 0.009 | 0.45 | 0.007 | 0.009 | 0.42 |
| genetically predicted educational attainmente | −0.347 | 0.026 | 8.57 × 10−41 | 0.805 | 0.019 | <5 × 10−41 | — | — | — |
aNumber of children data from UK Biobank (N = 333 628).
bAge at first birth data from UK Biobank (N = 123 310).
cNumber of sexual partners data from UK Biobank (N = 273 970).
dSchizophrenia data from the Psychiatric Genomics Consortium GWAS (N = 35 123 cases and 109 657 controls).
eEducational attainment from the Social Science Genetic Association Consortium GWAS (N = 283 723). There were 1 114 456 SNPs included in schizophrenia analyses and 1 117 154 included in educational attainment analyses.
Educational attainment variants showed a mean F-statistic (strength of instrument) of 33.23, with above 10 indicating acceptable levels of relative bias (less than 10%) [44,51]. We applied multiple MR methods with IVW results reported throughout the text, and other methods only when not consistent. We found that educational attainment had a negative effect on number of children (mean difference: −0.16, 95% confidence interval (CI): −0.21 to −0.12, p = 3.63 × 10−10 per year increase in educational attainment) and a positive effect on age at first birth (mean difference: 2.68, 95% CI: 2.40–2.95, p < 5 × 10−14) per year increase in educational attainment (table 2). We also found an effect of increased education on increased likelihood of being childless (OR: 1.60, 95% CI: 1.47–1.75, p = 1.60 × 10−14 per year increase in educational attainment). Results for all educational attainment analysis with genotype array included as a covariate in our outcome summary statistics are presented in electronic supplementary material, tables S4 and S5.
Table 2.
Estimates of the causal effect of genetic liability for schizophrenia and genetically predicted educational attainment on number of children, age at first birth and number of sexual partners using IVW, mode-based estimator, MR-Egger and weighted median MR approaches.
|
β (95% CI), p-value |
OR (95% CI), p-value |
||||
|---|---|---|---|---|---|
| method | no. of childrenb | age at first birthc | no. of sexual partnersd | childlessnesse | highest number of sexual partnersf |
| genetic liability for schizophrenia: 101 SNPsa | |||||
| inverse variance weighted | 0.003 (−0.003, 0.009), 0.39 | −0.004 (−0.043, 0.034), 0.82 | 0.165 (0.117, 0.212), 5.30 × 10−10 | 0.998 (0.985, 1.012), 0.79 | 1.057 (1.038, 1.077), 4.52 × 10−8 |
| MR-Egger intercept | −0.001 (−0.004, 0.001), 0.29 | −0.016 (−0.031, −0.001), 0.04 | −0.005 (−0.023, 0.014), 0.61 | 0.998 (0.993, 1.004), 0.55 | 0.994 (0.987, 1.001), 0.08 |
| MR-Egger slope | 0.020 (−0.013, 0.053), 0.23 | 0.214 (0.007, 0.420), 0.04 | 0.229 (−0.024, 0.482), 0.08 | 1.019 (0.950, 1.094), 0.59 | 1.151 (1.043, 1.271), 0.01 |
| weighted median | 0.006 (−0.004, 0.015), 0.23 | 0.023 (−0.042, 0.089), 0.49 | 0.172 (0.092, 0.230), 7.50 × 10−2 | 0.995 (0.975, 1.016), 0.65 | 1.034 (1.003, 1.066), 0.03 |
| simple mode-based estimator | 0.020 (−0.014, 0.055), 0.25 | 0.067 (−0.196, 0.331), 0.62 | 0.376 (−0.072, 0.823), 0.10 | 0.988 (0.912, 1.070), 0.76 | 1.121 (0.977, 1.287), 0.11 |
| weighted mode-based estimator | 0.020 (−0.012, 0.052), 0.22 | 0.060 (−0.175, 0.294), 0.62 | 0.389 (−0.032, 0.810), 0.07 | 0.992 (0.924, 1.065), 0.83 | 1.010 (0.884, 1.154), 0.88 |
| genetically predicted educational attainment: 67 SNPsg | |||||
| inverse variance weighted | −0.162 (−0.206, −0.118), 3.63 × 10−10 | 2.677 (2.401, 2.952), <5 × 10−14 | — | 1.589 (1.446, 1.746), 1.60 × 10−14 | — |
| MR-Egger intercept | 0.004 (0.001, 0.008), 2.50 × 10−02 | −0.031 (−0.054, −0.008), 9.52 × 10−03 | — | 0.990 (0.982, 0.997), 0.010 | — |
| MR-Egger slope | −0.391 (−0.595, −0.187), 2.99 × 10−04 | 4.348 (3.069, 5.628), 4.12 × 10−09 | — | 2.812 (1.813, 4.362), 1.38 × 10−05 | — |
| weighted median | −0.206 (−0.276, −0.135), 2.93 × 10−07 | 2.828 (2.387, 3.270), <5 × 10−14 | — | 1.567 (1.343, 1.829), 3.00 × 10−07 | — |
| simple mode-based estimator | −0.253 (−0.5107, 0.005), 0.06 | 3.454 (1.938, 4.969), 3.18 × 10−05 | — | 1.474 (0.884, 2.457), 0.14 | — |
| weighted mode-based estimator | −0.249 (−0.478, −0.020), 0.04 | 1.649 (0.303, 2.995), 1.92 × 10−02 | — | 1.513 (0.952, 2.404), 0.085 | — |
aSchizophrenia genetic data from the Psychiatric Genomics Consortium GWAS (N = 35 123 cases and 109 657 controls).
bNumber of children data from UK Biobank (N = 318 921–335 758 for genetic liability of schizophrenia analysis and 268 658–335 758 for educational attainment analysis).
cAge at first birth data from UK Biobank (N = 117 844–124 093 for genetic liability of schizophrenia analysis and 99 317–124 093 for educational attainment analysis).
dNumber of sexual partners data from UK Biobank (N = 261 931–275 700).
eChildlessness data from UK Biobank (N = 318 921–335 758 for genetic liability of schizophrenia analysis and 268 658–335 758 for educational attainment analysis). Childlessness was coded as 1.
fHighest number of sexual partners data from UK Biobank (N = 261 931–275 700). Highest tenth percentile was coded as 1. Schizophrenia results were multiplied by 0.693 to represent the estimate per doubling in odds of the binary exposure. Results were converted to ORs for schizophrenia by multiplying log ORs by 0.693 and then exponentiating to represent the OR per doubling in odds of the binary exposure. Results were converted to ORs for educational attainment by exponentiating log ORs.
gEducational attainment from the Social Science Genetic Association Consortium GWAS (N = 283 723). It should be noted that the I2GX statistic for an unweighted MR-Egger regression was 0.33 for educational attainment and 0.20 for genetic liability of schizophrenia, which is deemed too low to conduct a SIMEX adjustment, and MR-Egger results should be treated with caution [47].
3.2. Genetic liability for schizophrenia
Using LD score regression, we found little evidence of genetic correlations (rg) between schizophrenia liability and number of children (rg = 0.002, p = 0.84), age at first birth (rg = −0.007, p = 0.45) and number of sexual partners (rg = 0.007, p = 0.42) (table 1). The number of individuals included was 35 123 cases and 109 657 controls for schizophrenia liability, 333 628 for number of children, 123 310 for age at first birth and 273 970 for number of sexual partners data. The analysis included 1 114 456 SNPs.
The mean F-statistic for schizophrenia genetic liability was 35.15. There was little evidence that higher genetic liability for schizophrenia increased number of children (mean difference: 0.003 increase in the number of children per doubling in the natural log OR of schizophrenia liability, 95% CI: −0.003 to 0.009, p = 0.39) or decreased age at first birth (−0.004 years lower age at first birth, 95% CI: −0.043 to 0.034, p = 0.82) (table 2). We further tested childlessness as an outcome and found no strong evidence of an effect of genetic liability for schizophrenia on childlessness (table 2). We found that higher genetic liability for schizophrenia had a positive effect on number of sexual partners (0.165 increase in number of sexual partners, 95% CI: 0.117–0.212, p = 5.30 × 10−10) (table 2). A positive effect was also seen in the analysis of our binary measure for the highest number of sexual partners (table 2). We repeated the MR analysis after removing the few schizophrenia cases in our sample (maximum N = 207) with no clear change in results. Results for these analyses with genotype array included as a covariate in our outcome summary statistics are presented in electronic supplementary material, tables S4 and S5.
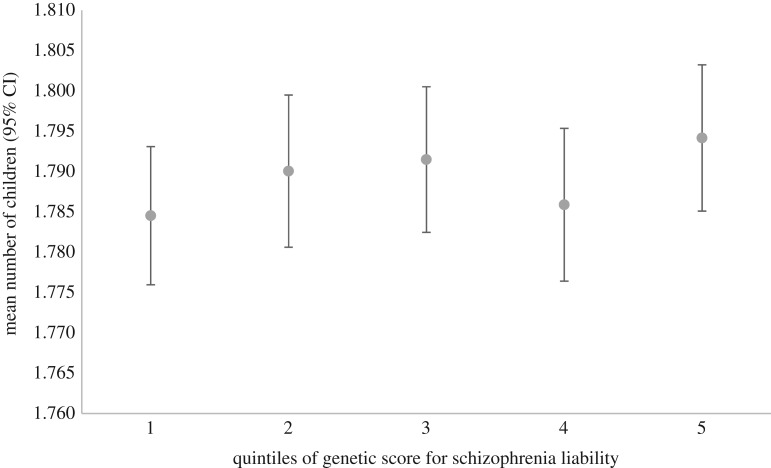
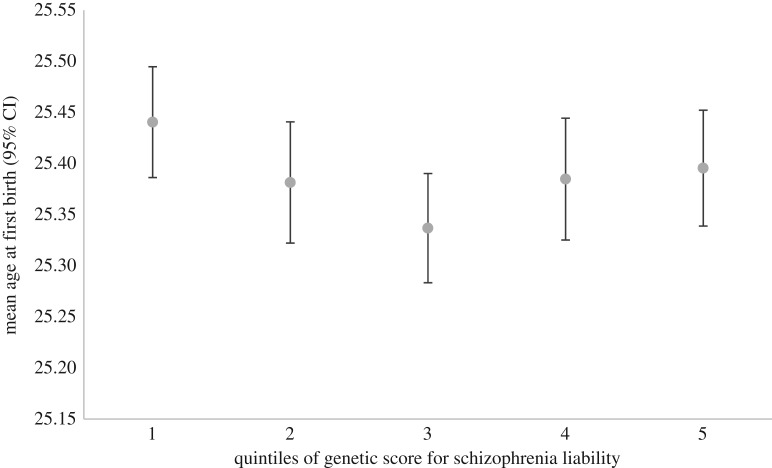
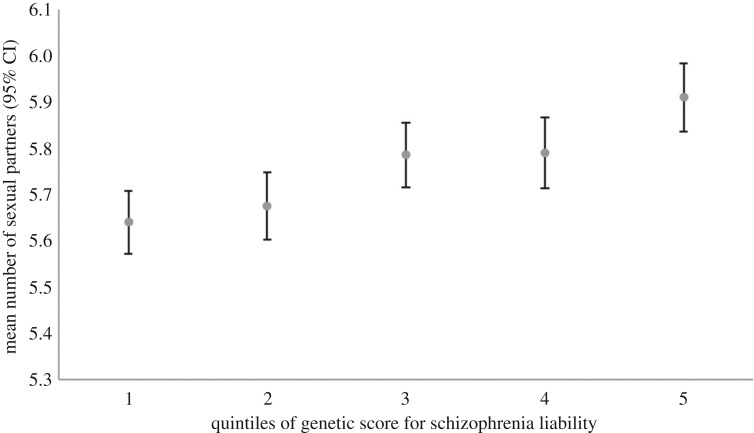
Our sensitivity analysis investigating a possible nonlinear relationship is presented in figures 1–3, showing the mean number of children, sexual partners and age at first birth for quintiles of an unweighted additive genetic score for schizophrenia liability. Although these figures are somewhat suggestive of a nonlinear relationship between the genetic score for schizophrenia liability and mean age at first birth, there is little evidence of heterogeneity across values of the schizophrenia score. The relationship between the genetic score for schizophrenia liability and number of sexual partners appears more linear. Similar patterns are seen across deciles of the genetic score for schizophrenia liability and when plotting these measures of reproductive success on the x-axis (electronic supplementary material, figures S1–S6). A series of regressions between the genetic score and age at first birth, systematically removing cumulative centiles from the maximum, suggests that the relationship is strongest at intermediate levels. It appeared that estimates became slightly stronger in the analysis with number of children, although there was little statistical support (table 3). This analysis was repeated after removing the few schizophrenia cases in UK Biobank (maximum N = 207), which did not alter these results (electronic supplementary material, table S6). Regression of the genetic score for schizophrenia liability and number of children showed no clear evidence, also when including a quadratic term for genetic liability for schizophrenia and when stratified by sex (table 4). This quadratic relationship suggested a slight peak in fitness at intermediate levels of the genetic liability, particularly for females, but again with little statistical support (electronic supplementary material, figures S7–S9). Regression of the genetic score for schizophrenia liability on age at first birth showed no clear evidence; however, there was a weak association when including a quadratic term for the genetic score, suggesting lowest age at first birth was seen at intermediate levels of genetic liability (table 4; electronic supplementary material, figure S10). For the number of sexual partners, the relationship appeared to be linear, with no clear evidence when including a quadratic term (table 4; electronic supplementary material, figures S11–S13)
Figure 1.
Genetic score for schizophrenia liability (in quintiles) and mean number of children in UK Biobank data showing little evidence of heterogeneity across values of the score.
Figure 2.
Genetic score for schizophrenia liability (in quintiles) and mean age at first birth in women from UK Biobank data also showing little evidence of heterogeneity across values of the score.
Figure 3.
Genetic score for schizophrenia liability (in quintiles) and mean number of sexual partners in UK Biobank data suggesting a linear relationship.
Table 3.
Associations of the genetic score for schizophrenia liability and reproductive outcomes removing cumulative deciles of the score. Adjusted for the top 10 principal components.
| number of children | age at first birth | number of sexual partners | |
|---|---|---|---|
| schizophrenia genetic score | β (95% CI), p-value | ||
| highest 10% removed | 0.0005 (−0.0003, 0.0013), 0.19 N = 302 190 |
−0.004 (−0.009, 0.001), 0.15 N = 111 632 |
0.011 (0.005, 0.017), 5.57 × 10−4 N = 248 251 |
| highest 20% removed | 0.0002 (−0.0007, 0.0011), 0.70 N = 268 604 |
−0.005 (−0.010, 0.001), 0.11 N = 99 134 |
0.012 (0.005, 0.020), 9.10 × 10−4 N = 220 847 |
| highest 30% removed | 0.0005 (−0.0006, 0.0016), 0.35 N = 235 030 |
−0.008 (−0.014, −0.001), 0.03 N = 86 620 |
0.017 (0.008, 0.025), 1.13 × 10−4 N = 193 353 |
| highest 40% removed | 0.0006 (−0.0006, 0.0019), 0.30 N = 208 433 |
−0.009 (−0.016, −0.001), 0.02 N = 76 833 |
0.015 (0.006, 0.025), 1.98 × 10−3 N = 171 567 |
| highest 50% removed | 0.0008 (−0.0006, 0.0023), 0.27 N = 167 860 |
−0.008 (−0.018, 0.001), 0.08 N = 61 822 |
0.012 (0.001, 0.024), 0.04 N = 138 280 |
Table 4.
Quadratic regression of the genetic score for schizophrenia liability with number of children, age at first birth and number of sexual partners in UK Biobank data.
| number of childrena | age at first birthb,c | number of sexual partnersa | |
|---|---|---|---|
| β (95% CI), p-value | |||
| genetic score | |||
| combined sexes | 0.0002 (−0.0004, 0.0008), 0.53 N = 335 758 |
— | 0.016 (0.011, 0.021), 7.6 × 10−11 N = 275 700 |
| females | 0.0006 (−0.0002, 0.0014), 0.16 N = 181 255 |
−0.001 (−0.005, 0.0027), 0.54 N = 124 093 |
0.005 (0.002, 0.009), 0.004 N = 148 630 |
| males | −0.0003 (−0.0012, 0.0007), 0.60 N = 154 503 |
— | 0.029 (0.019, 0.038), 4.9 × 10−9 N = 127, 070 |
| including quadratic term for genetic score | |||
| combined sexes | 0.0025 (−0.0108, 0.0157), 0.72 N = 335 758 |
— | −0.046 (−0.148, 0.056), 0.38 N = 275 700 |
| females | −0.0001 (−0.0174, 0.0172), 0.99 N = 181 255 |
−0.088 (−0.171, −0.004), 0.04 N = 124 093 |
−0.017 (−0.092, 0.057), 0.65 N = 148 630 |
| males | 0.0055 (−0.0149, 0.0259), 0.60 N = 154 503 |
— | −0.085 (−0.289, 0.118), 0.41 N = 127 070 |
aAdjusted for the top 10 principal components, age at assessment and sex (in combined sex analysis).
bAdjusted for the top 10 principal components.
cAge at first birth measured in females only in UK Biobank data.
4. Discussion
Our results do not indicate a genetic correlation between genetic liability for schizophrenia and reproductive success using LD score regression, or a linear causal effect on number of children and age at first birth using MR techniques. This is inconsistent with cliff-edge fitness maintaining schizophrenia in the population, which would predict an increase in fitness with increased genetic liability in the general population. These results support previous research suggesting no strong evidence of a relationship between genetic liability for schizophrenia and number of offspring [8,25]. We find an effect of increasing genetic liability for schizophrenia on increasing number of sexual partners, suggesting liability for the disorder increases mating success in the wider population and could reflect potential reproductive success [4]. Results of our positive control analyses were as expected and in line with previous genetic research, suggesting that educational attainment is under negative selection [25–29], which suggests that the overall approach we adopted here is valid.
Also consistent with previous research, we found no clear evidence for a linear association between genetic liability of schizophrenia and age at first birth [8] and weak evidence of a nonlinear association [24]. In these sensitivity analyses, we found some suggestion of a possible peak in fitness at intermediate to high levels of genetic liability, but there was no statistical evidence for this, suggesting that if this nonlinear association exists, it is very weak, and not reliably detectable even in a large study such as UK Biobank. A previous study also showed little evidence of quadratic associations between genetic liability for schizophrenia and number of children [8]. These sensitivity analyses further suggest that the relationship between genetic liability for schizophrenia and number of sexual partners is linear in this healthy population.
Cliff-edge fitness suggests that schizophrenia prevalence is sustained because the negative reproductive effects in those with an underlying genetic liability and the disorder are offset by a reproductive advantage to those who have an underlying genetic liability but do not develop the disorder [8]. We therefore examined only part of the cliff-edge hypothesis by studying only those without the disorder, testing whether there is a linear effect on reproductive success with increasing genetic liability. Although it is hard to estimate the size of effect on fecundity necessary to sustain the prevalence of schizophrenia (or indeed whether this effect size may fall within the confidence intervals of our estimate), our results provide no support for a cliff-edge fitness effect maintaining schizophrenia prevalence. However, in the modern environment (with available contraception), there are limits to the conclusions we can make about historical evolutionary forces on schizophrenia-associated alleles from these present-day fitness associations. We do find evidence of increased mating success with increasing genetic liability which may proxy reproductive success in past environments and partly explain how the disorder has been maintained in the population. We see stronger evidence for a positive association between genetic liability for schizophrenia and number of sexual partners in males than females, in line with sex differences in reproductive strategies [4]. As variance in reproductive success is higher in males than in females, it has been argued that males obtain higher reproductive benefits from additional matings than females [52]. It has been suggested that creative displays are a form of sexual competition that reflect these evolutionary pressures [4,53]. It is possible that the associations seen in females here are a by-product of male reproductive behaviour, although there are, of course, also benefits to females of attracting additional mates, such as higher mate quality [4]. We assume that, on average, increasing numbers of partners is a reasonable proxy for fitness; however, number of sexual partners has probably also undergone changes since the introduction of contraception, which has allowed for decoupling of sexual and reproductive partners. We therefore cannot conclude that cliff-edge fitness has sustained the prevalence of schizophrenia within the population and provide no evidence for a cliff-edge effect on current fitness.
This leaves us with two further theories for how schizophrenia prevalence is maintained. One is that as schizophrenia is a highly heterogeneous disorder and exhibits a highly polygenic architecture, and effects of genetic variants are individually too weak to be under negative selection [1,8,10]. Our results are consistent with this possibility and suggest that identified schizophrenia risk variants are not under strong selection in the general population. Another explanation is that mutation–selection balance maintains the prevalence of schizophrenia; rare recurrent DNA copy number variants, which are also risk factors for schizophrenia, are filtered out of the population by selection and replenished by de novo mutations [9]. Rare copy number variants conferring risk to psychiatric illness are under strong negative selection [8,9], with most persisting in the population for only two generations [9]. We used results from GWAS, which mainly detect common alleles and therefore cannot determine whether mutation–selection balance sustains the prevalence of schizophrenia through rare variants, although rare variants have been shown to associate with the number of children [1,8]. Other explanations could include an increased likelihood of symptom diagnosis, changes in the environment [54,55] and/or selection bias. UK Biobank data are unrepresentative of the population, given a response rate of approximately 5%, which may introduce selection bias [32,56]. This can generate spurious results in genotypic associations when selection is based on phenotypes associated with the genetic variants and could attenuate associations towards the null if schizophrenia-proneness and increased number of children reduced participation [57–59]. Previous studies have found that higher genetic liability for schizophrenia is associated with lower participation in cohort studies which could bias estimates between genetic liability and traits that lead to non-participation in genetic associations and MR [58,60].
A key strength of this study is the use of MR, which can provide stronger evidence of causality than observational studies [18,61]. We showed agreement between various MR methods that rely on differing assumptions and agreement between methods provides greater confidence in the robustness of the results [62]. We further conducted a positive control analysis to confirm that our approach was valid. Additionally, our MR offers large sample sizes which are necessary for investigating small effect sizes common in such genetic analysis [43]. However, there are also some limitations that should be considered with the current evidence. Firstly, MR relies on genetic variants naturally randomizing an exposure, and therefore inferring causality from genetic liability for schizophrenia as the exposure requires careful interpretation. Our outcome sample was not selected on schizophrenia status, so it contained only few cases of diagnosed schizophrenia. Therefore, we assume that schizophrenia SNPs are associated with subdiagnostic schizophrenia traits that could cause a reproductive advantage within the wider population [4,13,15]. Although debated [63,64], schizophrenia symptoms have been suggested to exist on a continuum, and this assumption could therefore be met [63,65–67]. Within this, we assume that the instrumental variable assumptions are satisfied for this continuous liability to provide a valid test of causality using the binary exposure [49]. Secondly, variants are non-specific, and it is difficult to fully remove population structure, which can induce spurious associations through confounding, even within a sample of European ancestry and adjusting for principal components as we have done [30,68]. Thirdly, age at first birth was measured only in females in UK Biobank, and therefore consists of a different population to the exposure data (which includes data from both females and males). However, the correlation between male and female estimates for age at first birth in a recent GWAS was high [28]. Similarly, the schizophrenia-associated genetic variants used in our sex-stratified analyses were identified from a mixed sex population, although we used an unweighted genetic score to minimize any bias. Lastly, the exposure and outcome samples were each quality controlled for relatedness; however, it is not possible to determine whether participants had relatives across the samples due to the use of summary-level GWAS data for our exposures.
The present study highlights the continued importance of investigating differential fertility and contributes to understanding the maintenance of schizophrenia, and educational attainment, in the population [3,20,69]. Educational attainment has previously been shown to predict human longevity [70] and highlights how even traits with a positive effect on longevity can be maladaptive, although other influences on educational attainment in the population are also identified [29]. This work additionally demonstrates how epidemiological methods can be repurposed to study evolutionary theories. Future research should investigate causal methods for estimating nonlinear relationships as well as other explanations for this evolutionary paradox, such as mutation–selection balance.
Supplementary Material
Acknowledgements
The authors thank Dr Suzi Gage, Dr Jack Bowden, Dr Hannah Jones and Dr Dan Lawson for their technical support and comments. The authors also thank Dr Ruth Mitchell, Dr Gibran Hemani, Mr Tom Dudding and Dr Lavinia Paternoster for conducting the quality control filtering of UK Biobank data. The authors are grateful to the participants of UK Biobank and those who contributed to the PGC and SSGAC GWAS, as well as research staff who worked on the data collection.
Ethics
UK Biobank received ethics approval from the Research Ethics Committee (REC reference for UK Biobank is 11/NW/0382).
Data accessibility
Genome-wide summary data for schizophrenia and educational attainment can be downloaded from the Psychiatric Genomics Consortium and Social Science Genetic Association Consortium websites (http://pgc.unc.edu; https://www.thessgac.org/). UK Biobank data are available upon application (www.ukbiobank.ac.uk). Analysis scripts are available on GitHub (https://github.com/MRCIEU/Schizophrenia_Fertility_Paper.git).
Authors' contributions
A.F., M.R.M. and I.S.P.-V. conceived the study. R.B.L. conducted the analysis and drafted the initial manuscript. H.M.S., R.E.W., G.H., G.D.S., N.M.D. and A.E.T. assisted with analysis and interpretation. All authors assisted with interpretation, commented on drafts of the manuscript and approved the final version.
Competing interests
The authors declare no competing interests.
Funding
R.B.L., H.M.S., A.E.T., R.E.W., G.D.S., N.M.D., G.H., A.F. and M.R.M. are members of the Medical Research Council Integrative Epidemiology Unit at the University of Bristol, which is supported by the Medical Research Council and the University of Bristol and funds RBL's PhD studentship (grant number: MC_UU_00011/7). A.F. is supported by a personal fellowship from the UK Medical Research Council (MR/M009351/1). A.E.T. and M.R.M. are members of the UK Centre for Tobacco and Alcohol Studies, a UKCRC Public Health Research: Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. G.H. is funded by the Wellcome Trust (208806/Z/17/Z). The Economics and Social Research Council (ESRC) supports N.M.D. via a Future Research Leaders grant (ES/N000757/1). R.E.W., I.S.P.-V. and M.R.M. are supported by the NIHR Biomedical Research Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. This research has been conducted using the UK Biobank Resource under Application number 6326.
References
- 1.Van Dongen J, Boomsma DI. 2013. The evolutionary paradox and the missing heritability of schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 162, 122–136. ( 10.1002/ajmg.b.32135) [DOI] [PubMed] [Google Scholar]
- 2.Bundy H, Stahl D, MacCabe JH. 2011. A systematic review and meta-analysis of the fertility of patients with schizophrenia and their unaffected relatives. Acta Psychiatr. Scand. 123, 98–106. ( 10.1111/j.1600-0447.2010.01623.x) [DOI] [PubMed] [Google Scholar]
- 3.Essen-Möller E. 1959. Mating and fertility patterns in families with schizophrenia. Eugen. Q. 6, 142–147. ( 10.1080/19485565.1959.9987406) [DOI] [Google Scholar]
- 4.Nettle D, Clegg H. 2006. Schizotypy, creativity and mating success in humans. Proc. R. Soc. B 273, 611–615. ( 10.1098/rspb.2005.3349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jablensky A, et al. 1992. Schizophrenia: manifestations, incidence and course in different cultures: a World Health Organization ten country study. Psychol. Med. 20, 1–97. ( 10.1017/S0264180100000904) [DOI] [PubMed] [Google Scholar]
- 6.Huxley J, Mayr E, Osmond H, Hoffer A. 1964. Schizophrenia as a genetic morphism. Nature 204, 220–221. ( 10.1038/204220a0) [DOI] [PubMed] [Google Scholar]
- 7.Power R, et al. 2013. Fecundity of patients with schizophrenia, autism, bipolar disorder, depression, anorexia nervosa, or substance abuse vs their unaffected siblings. JAMA Psychiatry 70, 22–30. ( 10.1001/jamapsychiatry.2013.268) [DOI] [PubMed] [Google Scholar]
- 8.Mullins N, et al. 2017. Reproductive fitness and genetic risk of psychiatric disorders in the general population. Nat. Commun. 8, 15833 ( 10.1038/ncomms15833) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rees E, Moskvina V, Owen MJ, O'Donovan MC, Kirov G. 2011. De novo rates and selection of schizophrenia-associated copy number variants. Biol. Psychiatry. 70, 1109–1114. ( 10.1016/j.biopsych.2011.07.011) [DOI] [PubMed] [Google Scholar]
- 10.Loh P-R, et al. 2015. Contrasting genetic architectures of schizophrenia and other complex diseases using fast variance-components analysis. Nat. Genet. 47, 1385–1392. ( 10.1038/ng.3431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis A. 1958. Fertility and mental illness. Eugen. Rev. 50, 91–106. [PMC free article] [PubMed] [Google Scholar]
- 12.Nesse RM. 2004. Cliff-edged fitness functions and the persistence of schizophrenia. Behav. Brain Sci. 27, 862–863. ( 10.1017/S0140525X04300191) [DOI] [Google Scholar]
- 13.Del Giudice M, Angeleri R, Brizio A, Elena MR. 2010. The evolution of autistic-like and schizotypal traits: a sexual selection hypothesis. Front Psychol. 1, 1–18. ( 10.3389/fpsyg.2010.00041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller MC, Miller G. 2006. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? Behav. Brain Sci. 29, 385–404. ( 10.1017/S0140525X06009095) [DOI] [PubMed] [Google Scholar]
- 15.Crow TJ. 2008. The ‘big bang’ theory of the origin of psychosis and the faculty of language. Schizophr. Res. 102, 31–52. ( 10.1016/j.schres.2008.03.010) [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Polimanti R, Kranzler HR, Farrer LA, Zhao H, Gelernter J. 2016. Genetic factor common to schizophrenia and HIV infection is associated with risky sexual behavior: antagonistic vs. synergistic pleiotropic SNPs enriched for distinctly different biological functions. Hum. Genet. 136, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Giudice M. 2010. Reduced fertility in patients' families is consistent with the sexual selection model of schizophrenia and schizotypy. PLoS ONE 5, e16040 ( 10.1371/journal.pone.0016040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davey SG, Ebrahim S. 2003. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int. J. Epidemiol. 32, 1–22. ( 10.1093/ije/dyg070) [DOI] [PubMed] [Google Scholar]
- 19.Westendorp RGJ, Kirkwood TBL. 1998. Human longevity at the cost of reproductive success. Nature 396, 743–746. ( 10.1038/25519) [DOI] [PubMed] [Google Scholar]
- 20.Tropf FC, Stulp G, Barban N, Visscher PM, Yang J, Snieder H, Mills MC, Perry JRB. 2015. Human fertility, molecular genetics, and natural selection in modern societies. PLoS ONE 10, e0126821 ( 10.1371/journal.pone.0126821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schizophrenia Working Group of the Psychiatric Genomics Consortium. 2014. Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. ( 10.1038/nature13595) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Power RA, et al. 2015. Polygenic risk scores for schizophrenia and bipolar disorder predict creativity. Nat. Neurosci. 18, 953–955. ( 10.1038/nn.4040) [DOI] [PubMed] [Google Scholar]
- 23.Mehta D, et al. 2016. Evidence for genetic overlap between schizophrenia and age at first birth in women. JAMA Psychiatry 73, 497–505. ( 10.1001/jamapsychiatry.2016.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ni G, et al. 2018. Age at first birth in women is genetically associated with increased risk of schizophrenia. Sci. Rep. 8, 10168 ( 10.1038/s41598-018-28160-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beauchamp JP. 2016. Genetic evidence for natural selection in humans in the contemporary United States. Proc. Natl Acad. Sci. USA 113, 7774–7779. ( 10.1073/pnas.1600398113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Courtiol A, Tropf FC, Mills MC. 2016. When genes and environment disagree: making sense of trends in recent human evolution. Proc. Natl Acad. Sci. USA 113, 7693–7695. ( 10.1073/pnas.1608532113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong A, et al. 2017. Selection against variants in the genome associated with educational attainment. Proc. Natl Acad. Sci. USA 114, E727–E732. ( 10.1073/pnas.1612113114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barban N, et al. 2016. Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nat. Genet. 48, 1462 ( 10.1038/ng.3698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanjak JS, Sidorenko J, Robinson MR, Thornton KR, Visscher PM. 2017. Evidence of directional and stabilizing selection in contemporary humans. Proc. Natl Acad. Sci. USA 115, 151–156. ( 10.1073/pnas.1707227114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis D. 2018. Polygenic risk score for schizophrenia is more strongly associated with ancestry than with schizophrenia. Psychiatr. Genet. 28, 85–89. ( 10.1097/YPG.0000000000000206) [DOI] [PubMed] [Google Scholar]
- 31.Okbay A, et al. 2016. Genome-wide association study identifies 74 loci associated with educational attainment. Nature 533, 539–542. ( 10.1038/nature17671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen NE, Sudlow C, Peakman T, Collins R. 2014. UK Biobank data: come and get it. Sci. Transl. Med. 6, 224ed4 ( 10.1126/scitranslmed.3008601) [DOI] [PubMed] [Google Scholar]
- 33.Collins R. 2012. What makes UK Biobank special? Lancet 379, 1173–1174. ( 10.1016/S0140-6736(12)60404-8) [DOI] [PubMed] [Google Scholar]
- 34.Bycroft C, et al. 2018. The UK Biobank resource with deep phenotyping and genomic data. Nature 562, 203–209. ( 10.1038/s41586-018-0579-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connell J, et al. 2016. Haplotype estimation for biobank-scale data sets. Nat. Genet. 48, 817–820. ( 10.1038/ng.3583) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, et al. 2015. Improved imputation of low-frequency and rare variants using the UK10 K haplotype reference panel. Nat. Commun. 6, 1–9. ( 10.1038/ncomms9111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howie B, Marchini J, Stephens M. 2011. Genotype imputation with thousands of genomes. G3 Genes, Genomes, Genet. 1, 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen WM. 2010. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873. ( 10.1093/bioinformatics/btq559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell R, Hemani G, Dudding T, Paternoster L. 2017. UK Biobank genetic data: MRC-IEU quality control, Version 1.
- 40.Bulik-Sullivan BK, et al. 2015. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295. ( 10.1038/ng.3211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bulik-Sullivan B, et al. 2015. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 47, 1236–1241. ( 10.1038/ng.3406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elsworth B, Mitchell R, Raistrick C, Paternoster L, Hemani G, Gaunt T. 2017. MRC IEU UK Biobank GWAS pipeline, version 1.
- 43.Gage SH, et al. 2016. Assessing causality in associations between cannabis use and schizophrenia risk: a two-sample Mendelian randomization study. Psychol. Med. 47, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burgess S, Butterworth A, Thompson SG. 2013. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. ( 10.1002/gepi.21758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bowden J, Davey Smith G, Haycock PC, Burgess S. 2016. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. ( 10.1002/gepi.21965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hartwig FP, Davey Smith G, Bowden J. 2017. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46, 1985–1998. ( 10.1093/ije/dyx102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowden J, Fabiola Del Greco M, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. 2016. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int. J. Epidemiol. 45, 1961–1974. ( 10.1093/ije/dyw252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. 2017. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802. ( 10.1002/sim.7221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burgess S, Labrecque JA. 2018. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur. J. Epidemiol. 33, 947–952. ( 10.1007/s10654-018-0424-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn RB, et al. 2018. Analysis scripts for: Schizophrenia risk and reproductive success: a Mendelian randomization study. [Internet]. GitHub. See https://github.com/MRCIEU/Schizophrenia_Fertility_Paper.git .
- 51.Pierce BL, Ahsan H, Vanderweele TJ. 2011. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int. J. Epidemiol. 40, 740–752. ( 10.1093/ije/dyq151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trivers R. 1972. Parental investment and sexual selection. In Sexual Selection and the Descent of Man, 1871–1971 (ed. Campbell B.), pp. 136–207. Chicago, IL: Aldine. [Google Scholar]
- 53.Miller GF. 1999. Sexual selection for cultural displays. In The evolution of culture (eds Dunbar R, Knight C, Power C), pp. 71–91. Edinburgh, Scotland: Edinburgh University Press. [Google Scholar]
- 54.Weinstein Y, Levav I, Gelkopf M, Roe D, Yoffe R, Pugachova I. 2018. Association of maternal exposure to terror attacks during pregnancy and the risk of schizophrenia in the offspring: a population-based study. Schizophr. Res. 199, 163–167. ( 10.1016/j.schres.2018.04.024) [DOI] [PubMed] [Google Scholar]
- 55.Gage SH, Jones HJ, Taylor AE, Burgess S, Zammit S, Munafò MR. 2017. Investigating causality in associations between smoking initiation and schizophrenia using Mendelian randomization. Sci. Rep. 7, 40653 ( 10.1038/srep40653) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. 2017. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with the general population. Am. J. Epidemiol. 186, 1026–1034. ( 10.1093/aje/kwx246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conde LC, et al. 2017. Higher genetic risk for schizophrenia is associated with living in urban and populated areas. Eur. Neuropsychopharmacol. 27, S488 ( 10.1016/j.euroneuro.2016.09.579) [DOI] [Google Scholar]
- 58.Taylor AE, et al. 2018. Exploring the association of genetic factors with participation in the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 47, 1207–1216. ( 10.1093/ije/dyy060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G. 2018. Collider scope: when selection bias can substantially influence observed associations. Int. J. Epidemiol. 47, 226–235. ( 10.1093/ije/dyx206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin J, Tilling K, Hubbard L, Stergiakouli E, Thapar A, Davey Smith G, O'Donovan MC, Zammit S. 2016. Association of genetic risk for schizophrenia with nonparticipation over time in a population-based cohort study. Am. J. Epidemiol. 183, 1149–1158. ( 10.1093/aje/kww009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gage SH, Munafò MR, Davey Smith G. 2016. Causal inference in developmental origins of health and disease (DOHaD) research. Annu. Rev. Psychol. 67, 567–585. ( 10.1146/annurev-psych-122414-033352) [DOI] [PubMed] [Google Scholar]
- 62.Lawlor DA, Tilling K, Davey Smith G. 2016. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 45, 1866–1886. ( 10.1093/ije/dyw127) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L.. 2009. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol. Med. 39, 179–195. ( 10.1017/S0033291708003814) [DOI] [PubMed] [Google Scholar]
- 64.Zammit S, et al. 2013. Psychotic experiences and psychotic disorders at age 18 in relation to psychotic experiences at age 12 in a longitudinal population-based cohort study. Am. J. Psychiatry 170, 742–750. ( 10.1176/appi.ajp.2013.12060768) [DOI] [PubMed] [Google Scholar]
- 65.Taylor AE, et al. 2016. Investigating causality in the association between 25(OH)D and schizophrenia. Sci. Rep. 6, 1–9. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. 2000. Children's self-reported psychotic symptoms and adult schizophreniform disorder. Arch. Gen. Psychiatry 57, 1053 ( 10.1001/archpsyc.57.11.1053) [DOI] [PubMed] [Google Scholar]
- 67.Kendler KS, et al. 1993. Examining the validity of DSM-III-R schizoaffective disorder and its putative subtypes in the Roscommon Family Study. Am. J. Psychiatry 152, 755–764. [DOI] [PubMed] [Google Scholar]
- 68.Haworth S, et al. 2018. Common genetic variants and health outcomes appear geographically structured in the UK Biobank sample: old concerns returning and their implications. bioRxiv. 294876.
- 69.Lewontin RC. 2016. Senior lecture, annual short course on medical and experimental mammalian genetics, Bar Harbor, Maine, 1968. Int. J. Epidemiol. 45, 654–664. ( 10.1093/ije/dyw189) [DOI] [PubMed] [Google Scholar]
- 70.Marioni RE, et al. 2016. Genetic variants linked to education predict longevity. Proc. Natl Acad. Sci. USA 113, 13 366–13 371. ( 10.1073/pnas.1605334113) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genome-wide summary data for schizophrenia and educational attainment can be downloaded from the Psychiatric Genomics Consortium and Social Science Genetic Association Consortium websites (http://pgc.unc.edu; https://www.thessgac.org/). UK Biobank data are available upon application (www.ukbiobank.ac.uk). Analysis scripts are available on GitHub (https://github.com/MRCIEU/Schizophrenia_Fertility_Paper.git).