Abstract
Background
Pembrolizumab shows robust antitumor activity and favorable safety in metastatic melanoma. KEYNOTE-151 evaluated pembrolizumab in Chinese patients, who have more aggressive melanoma subtypes than other populations.
Methods
Chinese patients aged ≥18 years with advanced melanoma previously treated with one line of therapy received pembrolizumab 2 mg/kg every 3 weeks for 35 cycles or until confirmed disease progression, intolerable toxicity, or study withdrawal. Primary end points were objective response rate (ORR) per RECIST v1.1 by blinded independent central review and safety. Key secondary end points included duration of response (DOR) and progression-free survival (PFS) per RECIST v1.1 and overall survival (OS).
Results
Median age was 52 years (N = 103); 37.9% had acral and 14.6% had mucosal melanoma. Median follow-up was 7.9 months at data cutoff (December 27, 2017). ORR was 16.7% (95% CI, 10.0–25.3%) (1 complete, 16 partial responses). Disease control rate was 38.2%. ORR was 15.8% for acral, 13.3% for mucosal melanoma. Median DOR was 8.4 months; 65.6% of patients had response duration ≥6 months. Median PFS was 2.8 months (95% CI, 2.7–3.5 months); 6-month rate was 20.4%. Median OS was 12.1 months (95% CI, 9.6 months–not reached); 6-month rate, 75.7%; 12-month rate, 50.6%. Treatment-related AEs (TRAEs) occurred in 87 (84.5%) patients; 9 (8.7%) experienced grade 3/4 TRAE and 2 (1.9%) discontinued because of TRAE; none died. Two deaths occurred that were unrelated to treatment.
Conclusions
Pembrolizumab was well tolerated and provided clinically meaningful antitumor activity as second-line therapy in Chinese patients with advanced melanoma.
Introduction
Melanoma is generally managed successfully with surgical resection; however, advanced or metastatic disease is associated with poor prognosis [1]. Although the incidence of melanoma remains low in China, it is growing at an annual rate of 3–5%, and approximately 20,000 new cases are reported each year [1]. For patients with advanced melanoma, chemotherapy is often ineffective, and there is a paucity of second-line treatment options for patients with advanced disease in China. Use of immune checkpoint inhibitors has significantly improved survival outcomes in white patients with diagnoses of advanced or metastatic melanoma [2], [3], [4], [5], [6]. Treatment with checkpoint inhibitors of Chinese patients with more aggressive melanoma subtypes has not been well characterized.
The subtypes of melanoma most common in Asian patients, unlike white patients, are acral and mucosal, and they account for up to 58% of all melanoma tumors in that patient population [7]. Acral and mucosal melanomas are more frequently characterized by DNA structural changes and mutation signatures of unknown etiology. Although cutaneous melanoma is characterized by defects in BRAF, CDKN2A, NRAS, and TP53, acral melanoma is associated with genetic mutations of BRAF, NRAS, KIT, MAP2K2, and NF1, and mucosal melanoma is associated with mutations of SF3B1 [8]. Acral and mucosal melanomas are generally regarded as more aggressive [9]; in addition, because they manifest in hidden locations, these tumors often remain undiagnosed until they reach an advanced stage [10], [11]. In an analysis of 82 Chinese patients with acral (39.0%), nodular (37.8%), lentigo (12.2%), and superficial spreading (11.0%) melanoma, the 3- and 5-year survival rates were 39.0% and 10.9%, respectively [12]. To date, the antitumor activity of immunotherapeutic agents in this patient population has not been well characterized.
Pembrolizumab blocks the interaction between programmed death 1 (PD-1) and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2) [13]. Pembrolizumab demonstrated robust and efficacious antitumor tumor activity in the phase I KEYNOTE-001 study [14], [15], the phase II KEYNOTE-002 study [16], and the phase III KEYNOTE-006 study [17]. Pembrolizumab has been approved in more than 80 countries for the treatment of one or more malignancies, including melanoma. The efficacy of pembrolizumab in Asian populations has been assessed. In the phase Ib trial of pembrolizumab in Japanese patients with advanced melanoma (KEYNOTE-041), the objective response rate (ORR) in 42 patients who received treatment was 24.1% for cutaneous melanoma and 25.0% for mucosal melanoma [18]. In a Korean population of 37 patients who were treated with either pembrolizumab or nivolumab, the ORRs were 9.1% in cutaneous melanoma and 11.5% in acral/mucosal melanoma [19]. In 52 Chinese patients, the ORRs were 0%, 20%, and 25% for patients with advanced/metastatic disease treated with ipilimumab, pembrolizumab plus ipilimumab, and pembrolizumab monotherapy, respectively [9]. Nonetheless, further prospective reports assessing the treatment of Chinese patients with advanced melanoma are needed.
In this analysis, we present the first results of the KEYNOTE-151 trial (NCT02821000), which evaluated the safety and efficacy of pembrolizumab in Chinese patients with advanced melanoma that progressed following first-line chemotherapy or targeted therapy.
Materials and methods
Patients.
KEYNOTE-151 was an open-label, nonrandomized, multicenter, phase Ib trial. Patients were ≥ 18 years of age, were of Chinese descent, and had histologically confirmed diagnoses of locally advanced or metastatic melanoma not amenable to local therapy. Patients were enrolled if their disease progressed on or after first-line chemotherapy (excluding adjuvant or neoadjuvant therapy) or targeted therapy for melanoma, if they had at least a measurable lesion as defined by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1, if they had an Eastern Cooperative Oncology Group performance status of 0 or 1, if they had a life expectancy ≥3 months, if they had provided tissue (archival, newly obtained, or excisional biopsy of a tumor lesion that was not previously irradiated) for evaluation of PD-L1 expression (patients were enrolled irrespective of PD-L1 expression status) and if they had known BRAF mutational status or were willing to provide tumor tissue for BRAF genotyping. Key exclusion criteria were diagnosis of uveal or ocular melanoma; previous therapy with an anti–PD-1, anti–PD-L1, or anti–PD-L2 agent; known active central nervous system tumors/metastases and/or carcinomatous meningitis; autoimmune disease that necessitated systemic treatment in the past 2 years; HIV positivity; history of (noninfectious) pneumonitis that necessitated treatment with steroids or current pneumonitis; and known active hepatitis B or C.
Study Design and Assessments.
Patients received pembrolizumab 2 mg/kg on day 1 of each 3-week cycle for up to 35 cycles (approximately 2 years). Pembrolizumab was administered intravenously over a 30-minute period and continued until disease progression, onset of unacceptable toxicities, investigator decision to discontinue treatment, or withdrawal of patient consent. The primary end points were ORR, defined as complete response (CR) or partial response (PR) based on RECIST v1.1 and assessed by blinded independent central radiology review (BICR), and safety and tolerability, assessed by clinical review of all relevant parameters including adverse event (AEs), laboratory tests, and vital signs. Secondary end points were duration of response (DOR; defined as the time from the first documented response to subsequent disease progression or death from any cause) per RECIST v1.1 by BICR and progression-free survival (PFS; defined as the time from the first day of study treatment to disease progression or death from any cause) per RECIST v1.1 and immune-related RECIST (irRECIST) by BICR, ORR per irRECIST by BICR, overall survival (OS; defined as the time from the first day of study treatment to death from any cause), and pharmacokinetics (PK). PD-L1 expression in tumor samples was assessed centrally using an immunohistochemistry assay, PD-L1 22C3 pharmDx (Agilent Technologies, Carpinteria, CA, USA). PD-L1 positivity was assessed using the Allred Proportion Score (APS) method in which positively stained mononuclear tumor-infiltrating inflammatory cells in immediate contact with tumor cells are counted in conjunction with neoplastic cells. PD-L1 positivity was defined as staining on at least 1% of tumor cells or mononuclear inflammatory cells within or contiguous to nests of tumor cells.
Radiological disease assessment.
A baseline imaging assessment was made within 4 weeks of the first dose of study treatment, and the first on-study imaging assessment was performed 12 weeks after the first dose of pembrolizumab. Subsequent imaging assessments were performed every 6 weeks (±7 days) until 48 weeks and then every 12 weeks (±7 days). Response was confirmed by subsequent imaging 4 weeks later or at the next scheduled scan (6 or 12 weeks later), as clinically indicated. Imaging was continued until disease progression, start of new anticancer therapy, withdrawal of consent, death, or study end (whichever occurred first).
Statistical Assessments.
The full-analysis set consisted of all allocated patients who received ≥1 dose of study treatment and had baseline data (measurable disease per RECIST v1.1 at baseline) for analyses that required baseline data. ORR and PFS were assessed in the full-analysis set; DOR was assessed in all responders; OS and safety were assessed in the all-subjects-as-treated population. ORR was evaluated by providing the point estimate and 95% confidence internal (CI) using an exact method based on binomial distribution. For PFS, DOR, and OS, Kaplan–Meier curves and median estimated from the curves were provided as appropriate. Summary statistics included count, percentage, mean, and standard deviation (SD). No adjustment was made for multiplicity. The planned sample size was 100 patients. The data cutoff was December 27, 2017 (per protocol, 24 weeks after last patient enrolled).
Study Oversight.
The original protocol and all amendments were approved by the relevant institutional review board or independent ethics committee at each trial center. The trial was conducted in accordance with the protocol, Good Clinical Practice guidelines, and the provisions of the Declaration of Helsinki. All patients provided written informed consent.
Role of the Funding Source.
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, sponsored this study. The sponsor collaborated with academic advisers to design the study and gather, analyze, and interpret the results. All authors had full access to all study data and approved the decision to submit the manuscript for publication.
Results
Patient Disposition and Demographics.
KEYNOTE-151 enrolled 103 patients who were included in the all-subjects-as-treated population; 102 patients were included in the full-analysis set. Overall, 42.7% (n = 44) were male, the median age was 52 years (range, 22–77 years), 43.7% (n = 45) had an Eastern Cooperative Oncology Group performance status of 0, 53.4% (n = 55) had stage M1c disease, and 51.5% (n = 53) were PD-L1 positive (Table 1 [20]). Of those enrolled, 37.9% (n = 39) had acral melanoma and 14.6% (n = 15) had mucosal melanoma. Prior therapies included chemotherapy in 68.9% (n = 71), ipilimumab in 16.5% (n = 17), immunotherapy other than ipilimumab in 17.5% (n = 18), BRAF/MEK inhibitor in 2.9% (n = 3), and other in 31.1% (n = 32). As of the data cutoff of December 27, 2017, the median follow-up duration was 7.9 months (range, 5.6–13.1 months); 73.8% (n = 76) had discontinued treatment—60.2% (n = 62) because of progressive disease (PD), 8.7% (n = 9) because of clinical progression, 3.9% (n = 4) because of an AE—and 1.0% (n = 1) of patients withdrew. Treatment was ongoing in 26.2% (n = 27).
Table 1.
Baseline characteristics.
| Characteristic |
n (%) N = 103 |
|---|---|
| Sex | |
| Male | 44 (42.7) |
| Female | 59 (57.3) |
| Age, years | |
| <65 | 84 (81.6) |
| ≥65 | 19 (18.4) |
| Mean | 50.5 |
| SD | 14.2 |
| Median | 52.0 |
| Range | 22 to 77 |
| ECOG performance status | |
| 0 | 45 (43.7) |
| 1 | 58 (56.3) |
| PD-L1 status | |
| Positive | 53 (51.5) |
| Negative | 45 (43.7) |
| Unknown | 5 (4.9) |
| Histology | |
| Cutaneous, nonacral | 41 (39.8) |
| Cutaneous, acral | 39 (37.9) |
| Mucosal | 15 (14.6) |
| Primary site unknown | 8 (7.8) |
| Metastatic stage | |
| M0 | 4 (3.9) |
| M1 | 8 (7.8) |
| M1A | 13 (12.6) |
| M1B | 23 (22.3) |
| M1C | 55 (53.4) |
| Overall stagea | |
| IIIC | 2 (1.9) |
| III | 1 (1.0) |
| IV | 100 (97.1) |
| BRAF mutation status | |
| Wild type | 82 (79.6) |
| Mutant | 20 (19.4) |
| Unknown | 1 (1.0) |
| Baseline lactate dehydrogenase level | |
| Normal (<1.1× ULN) | 70 (68.0) |
| Elevated (≥1.1× ULN) | 33 (32.0) |
| Brain metastases | |
| Yes | 2 (1.9) |
| No | 101 (98.1) |
| Baseline tumor size (mm)b | |
| Mean | 86.1 |
| SD | 70.6 |
| Median | 60.8 |
| Range | 11.3 to 270.1 |
| Prior adjuvant/neoadjuvant therapy | |
| Yes | 69 (67.0) |
| No | 34 (33.0) |
| Type of first-line therapyc | |
| Chemotherapy | 71 (68.9) |
| Ipilimumab | 17 (16.5) |
| Immunotherapy (ipilimumab excluded) | 18 (17.5) |
| BRAF/MEK inhibitor | 3 (2.9) |
| Other | 32 (31.1) |
| Type of prior adjuvant/neoadjuvant therapy | |
| Chemotherapy | 14 (13.6) |
| Immunotherapy (ipilimumab excluded) | 65 (63.1) |
| Other | 5 (4.9) |
ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed death ligand 1; SD, standard deviation; ULN, upper limit of normal.
Per American Joint Committee on Cancer Staging Manual, version 7 [20].
Data were missing for one patient.
Some patients received more than one therapy in the first-line setting.
Efficacy.
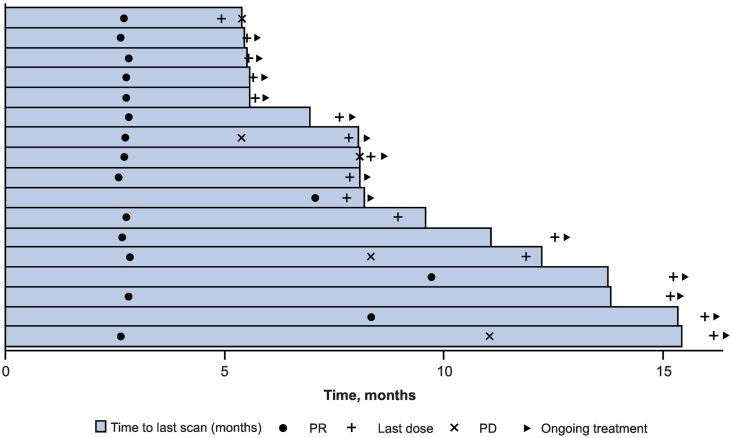
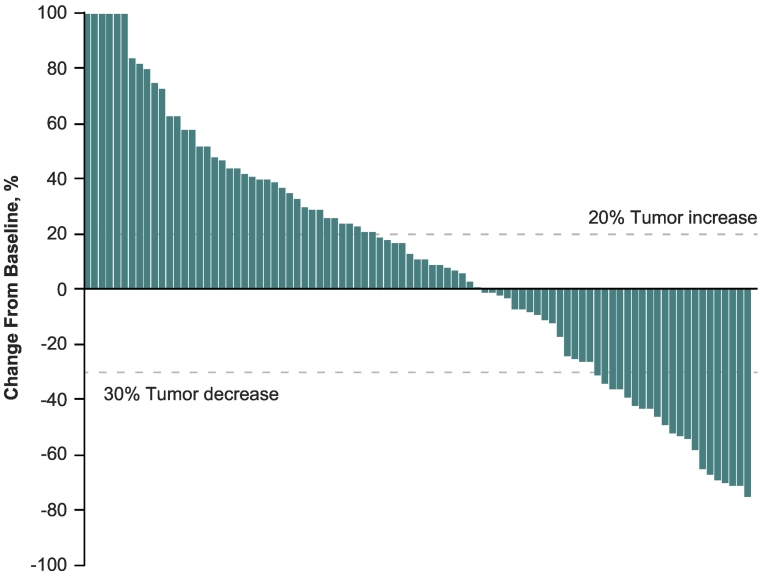
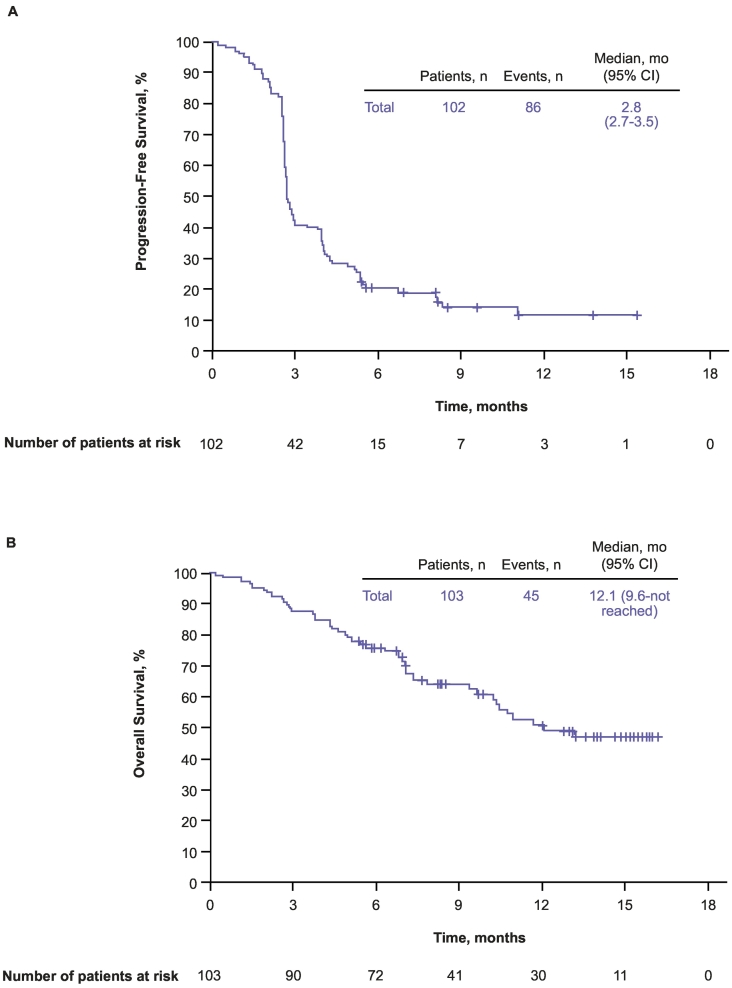
Confirmed CR and PR were achieved in 1 (1.0%) and 16 (15.7%) patients, respectively, for an ORR per RECIST v1.1 of 16.7% (95% CI, 10.0–25.3%) (Table 2, Fig. 1). An additional 22 (21.6%) patients achieved stable disease for a disease control rate (defined as CR + PR + SD) of 38.2% (95% CI, 28.8–48.4%) during the overall treatment period. Overall, 36 patients (35.3%) experienced a reduction from baseline in the size of their target lesions (Fig. 2). The median time to response per RECIST v1.1 was 2.8 months (range, 2.6–9.7 months), and the median DOR was 8.4 months (1.1 + −11.0+ months). The DOR was ≥3 months in 87.5% and ≥ 6 months in 65.6% of patients. The median PFS per RECIST v1.1 was 2.8 months (95% CI, 2.7–3.5 months) (Fig. 3A). At 6 and 12 months, PFS rates were 20.4% and 11.9%, respectively. The confirmed ORR, the median DOR, and the median PFS were the same per RECIST v1.1 and irRECIST. At data cutoff, 45 (43.7%) deaths had been recorded. The estimated median OS was 12.1 months (95% CI, 9.6 months–not reached) (Fig. 3B). Six-month and 12-month OS rates were 75.7% and 50.6%, respectively.
Table 2.
Objective responsesa for all patients and for the acral and mucosal subpopulations (full-analysis set).
|
All Patients N = 102 (%) |
Acral n = 38 |
Mucosal n = 15 |
|
|---|---|---|---|
| CR | 1 (1.0) | 0 (0) | 1 (6.7) |
| PR | 16 (15.7) | 6 (15.8) | 1 (6.7) |
| Objective response rate (CR + PR) | 17 (16.7) | 6 (15.8) | 2 (13.3) |
| SD | 22 (21.6) | 10 (26.3) | 1 (6.7) |
| Disease control rate (CR + PR + SD) | 39 (38.2) | 16 (42.1) | 3 (20.0) |
| Progressive disease | 52 (51.0) | 18 (47.4) | 8 (53.3) |
| No assessment | 11 (10.8) | 4 (10.5) | 4 (26.7) |
CR, complete response; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease.
Based on RECIST v1.1 per blinded independent central review.
Fig. 1.
Duration of response based on RECIST v1.1 per BICR. BICR, blinded independent central review; PD, progressive disease; PR, partial response; Q3W, every 3 weeks; RECIST, Response Evaluation Criteria in Solid Tumors.
Fig. 2.
Best percentage change in target lesion size from baseline based on RECIST v1.1 per BICR in the full-analysis set. Values ≥100 were set to 100. Data were not available for 13 patients (12.7%). BICR, blinded independent central review; Q3W, every 3 weeks; RECIST, Response Evaluation Criteria in Solid Tumors.
Fig. 3.
Kaplan–Meier estimates of PFS (A) in the full-analysis set per BICR and OS (B) in all-patients-as-treated population. BICR, blinded independent central review; OS, overall survival; PFS, progression-free survival; Q3W, every 3 weeks.
ORR by Subgroup.
The ORR was 15.8% (95% CI, 6.0–31.3%) for the acral subtype, 19.5% (95% CI, 8.8–34.9%) for the non-acral subtype, 13.3% (95% CI, 1.7–40.5%) for the mucosal subtype, and 12.5% (95% CI, 0.3–52.7%) for primary site unknown subtypes. The ORR was 21.2% (95% CI, 11.1–34.7%) in patients with PD-L1–positive tumors and 13.3% (95% CI, 5.1–26.8%) in patients with PD-L1–negative tumors. The ORR was 15.0% (95% CI, 3.2–37.9%) in patients with BRAF mutant status and 17.3% (95% CI, 9.8–27.3%) in patients with BRAF wild-type status.
Safety.
TRAEs were reported in 84.5% (n = 87) of patients (Table 3); the most common were hypothyroidism in 23.3% (n = 24), hypertriglyceridemia in 20.4% (n = 21), and increased blood lactate dehydrogenase in 15.5% (n = 16). Grade 3/4 TRAEs were reported in 8.7% (n = 9) of patients; of these, 1.9% (n = 2; autoimmune hepatitis, n = 1, and pneumonia, n = 1) of patients discontinued treatment. Two deaths were reported that were unrelated to study treatment; 1 death was a result of shock and 1 death was a result of pulmonary embolism. No patients died because of a TRAE.
Table 3.
Treatment-related AEs occurring in ≥2% of patients.
| Adverse Events, n (%) |
All Treated Patients N = 103 |
|
|---|---|---|
| Any Grade | Grade 3–5 | |
| Blood and lymphatic system disorders | ||
| Anemia | 11 (10.7) | 5 (4.9) |
| Endocrine disorders | ||
| Hypothyroidism | 24 (23.3) | 0 |
| Hyperthyroidism | 6 (5.8) | 0 |
| Autoimmune thyroiditis | 3 (2.9) | 0 |
| Gastrointestinal disorders | ||
| Nausea | 4 (3.9) | 0 |
| Diarrhea | 3 (2.9) | 0 |
| General disorders and administration site conditions | ||
| Fatigue | 15 (14.6) | 1 (1.0) |
| Asthenia | 6 (5.8) | 0 |
| Pyrexia | 4 (3.9) | 0 |
| Peripheral swelling | 3 (2.9) | 0 |
| Hepatobiliary disorders | ||
| Hepatic function abnormal | 4 (3.9) | 1 (1.0) |
| Laboratory investigations | ||
| Blood lactate dehydrogenase increased | 16 (15.5) | 0 |
| Alanine aminotransferase increased | 15 (14.6) | 0 |
| Blood bilirubin increased | 11 (10.7) | 0 |
| Aspartate aminotransferase increased | 10 (9.7) | 0 |
| Bilirubin conjugated increased | 7 (6.8) | 2 (1.9) |
| Blood creatine phosphokinase increased | 7 (6.8) | 0 |
| Blood cholesterol increased | 6 (5.8) | 0 |
| Blood bilirubin unconjugated increased | 5 (4.9) | 0 |
| Blood glucose increased | 5 (4.9) | 0 |
| Blood thyroid stimulating hormone increased | 5 (4.9) | 0 |
| Total bile acids increased | 5 (4.9) | 0 |
| Blood urea increased | 4 (3.9) | 0 |
| Blood uric acid increased | 4 (3.9) | 0 |
| Neutrophil count decreased | 10 (9.7) | 0 |
| Platelet count decreased | 3 (2.9) | 1 (1.0) |
| Transaminases increased | 4 (3.9) | 0 |
| Weight decreased | 3 (2.9) | 1 (1.0) |
| White blood cell count decreased | 12 (11.7) | 3 (2.9) |
| Metabolism and nutrition disorders | ||
| Hypertriglyceridemia | 21 (20.4) | 0 |
| Hyperglycemia | 11 (10.7) | 0 |
| Hyperuricemia | 9 (8.7) | 0 |
| Decreased appetite | 8 (7.8) | 1 (1.0) |
| Hypercholesterolemia | 5 (4.9) | 1 (1.0) |
| Musculoskeletal and connective tissue disorders | ||
| Myalgia | 4 (3.9) | 0 |
| Arthralgia | 3 (2.9) | 0 |
| Backpain | 3 (2.9) | 0 |
| Neoplasms benign, malignant, and unspecifieda | ||
| Tumor pain | 5 (4.9) | 0 |
| Nervous system disorders | 5 (4.9) | 0 |
| Renal and urinary disorders | ||
| Proteinuria | 4 (3.9) | 0 |
| Respiratory, thoracic and mediastinal disorders | 6 (5.8) | 0 |
| Skin and subcutaneous tissue disorders | ||
| Rash | 14 (13.6) | 0 |
| Pruritus | 11 (10.7) | 0 |
| Skin depigmentation | 4 (3.9) | 0 |
| Skin hypopigmentation | 4 (3.9) | 0 |
AE, adverse event.
aIncluding cysts and polyps.
Immune-mediated AEs were reported in 30 patients (29.1%) (Table 4). The most frequently reported immune-mediated AEs were hypothyroidism (23.3%), hyperthyroidism (5.8%), and autoimmune thyroiditis (2.9%). Most cases of hypothyroidism were subclinical in nature, as diagnosed with thyroid function tests. Two patients (1.9%) experienced grade 3 or 4 immune-mediated AEs (rhabdomyolysis, grade 3; autoimmune hepatitis, grade 4). One patient discontinued study treatment because of autoimmune hepatitis. There were no infusion reactions.
Table 4.
Immune-mediated AEs occurring in >1% of the population.
| Characteristic |
n (%) N = 103 |
|---|---|
| All grade, >1% of patients | |
| Patients with ≥1 immune-mediated AEs | 30 (29.1) |
| Hypothyroidism | 24 (23.3) |
| Hyperthyroidism | 6 (5.8) |
| Autoimmune thyroiditis | 3 (2.9) |
| Autoimmune hepatitis | 1 (1.0) |
| Hypersensitivity | 1 (1.0) |
| Rhabdomyolysis | 1 (1.0) |
| Interstitial lung disease | 1 (1.0) |
AE, adverse event.
Discussion
Chinese patients with locally advanced or metastatic melanoma who were treated with pembrolizumab had an ORR of 16.7% (95% CI, 10.0–25.3%). The ORRs in patients with acral and mucosal subtypes were similar, with rates of 15.8% (95% CI, 6.0–31.3%) and 13.3% (95% CI, 1.7–40.5%), respectively. Of the patients who responded in the total patient population, 65.6% (Kaplan–Meier estimate) had a response lasting ≥6 months. The estimated 6- and 12-month OS rates were 75.7% and 50.6%, respectively, and the plateau in the Kaplan–Meier curve suggests that pembrolizumab may provide Chinese patients with long-term survival benefit. These analyses of KEYNOTE-151 support the use of pembrolizumab for the treatment of Chinese patients with melanoma that progresses following first-line therapy.
The historical standard-of-care, first-line treatment for Chinese patients with advanced or metastatic melanoma has been dacarbazine, and its use has been associated with poor outcomes. Results of a phase II study in Chinese patients with advanced melanoma (N = 110; 42.6% and 13.0% had acral and mucosal melanoma, respectively) treated with first-line dacarbazine plus placebo experienced a median PFS of 1.5 months and a median OS of 8.0 months; ORR was 3.7% [21]. Patients in that trial who received the recombinant human endostatin (“endostar” plus dacarbazine) experienced a median PFS of 4.5 months and a median OS of 120 months [21]. The 12-month OS rate was 49.7% in the endostar/dacarbazine arm and 22.5% in the placebo/dacarbazine arm. Furthermore, no CRs were reported, and only 5 (8.9%) and 2 (3.7%) patients achieved PR in each arm. More recent trials have aimed to assess dacarbazine combination therapy with a variety of agents, including ipilimumab and BRAF inhibitors. However, in a pooled analysis of nine randomized controlled trials, combination therapy was associated with a higher incidence of AEs and only moderately improved outcomes compared with dacarbazine monotherapy [22]. Results of a phase Ib study of pembrolizumab in Japanese patients with advanced melanoma (N = 42) indicate that they experienced ORRs of 24.1% for cutaneous melanoma and 25.0% for mucosal melanoma [18]. Median OS was not reached; 12-month OS rates were 82.7% and 51.4% for patients with cutaneous and mucosal melanoma, respectively. In the analysis of KEYNOTE-151 presented herein, pembrolizumab was associated with a median OS of 12.1 months, with a 12-month survival rate of 50.6%; the ORR was 16.7%. Taken together, results of these studies indicate that Asian patients with advanced melanoma who are treated with pembrolizumab have better clinical outcomes in the second-line setting than patients treated with dacarbazine in the first-line setting.
Recently, vemurafenib was assessed in a phase I study that enrolled 46 treatment-naive or previously treated Chinese patients with BRAFV600 mutation–positive unresectable or metastatic melanoma [23]. Confirmed best ORR was 52.2%, median PFS was 8.3 months, and median OS was 13.5 months. Nine grade 3/4 AEs were reported in 7 patients (15.2%). Although BRAF inhibitors have high initial response rates in BRAF-mutant, metastatic melanoma, almost all patients who are treated with them ultimately become resistant. Consistent with this observation, approximately 50% of patients experience disease progression by 6 months [24]. The incidence of BRAF mutations in Chinese patients with melanoma (25.5%) [25] was much lower than that in the white patients (approximately 50%). For patients without BRAF mutations, the standard first-line therapy in China is still limited to dacarbazine. No effective second-line therapy is available.
In KEYNOTE-151, pembrolizumab had an acceptable safety profile for the treatment of Chinese patients with unresectable and metastatic melanoma. The safety of pembrolizumab in the current study was consistent with reports in white patients [15], [16], [17]. Most treatment-related AEs were low grade. Grade 3/4 treatment-related AEs were experienced in just 8.7% (n = 9) of patients, with less than 2% resulting in patient discontinuation and none resulting in death. In KEYNOTE-151, 29.1% of patients experienced immune-mediated AEs, which was similar to incidences reported in the global melanoma pivotal studies and the Japanese registration study [14], [16], [17], [18]. In the current study, the most common immune-mediated AEs were hypothyroidism (23.3%), hyperthyroidism (5.8%), and autoimmune thyroiditis (2.9%). Most cases of hypothyroidism were subclinical, based on results of thyroid function tests, and none of the patients required study treatment interruption or discontinuation. Some patients with hypothyroidism received hormone replacement therapy to manage their hypothyroidism (n = 6, 5.8%). None of the immune-mediated AEs led to death. No new safety signals or immune-mediated AEs associated with pembrolizumab were identified.
Given the lower incidence in white populations, limited data exist for acral and mucosal melanoma in Western populations. In an analysis of 1567 patients enrolled in KEYNOTE-001, KEYNOTE-002, and KEYNOTE-006, 84 (5%) had mucosal melanoma. Of these, the ORR was 19%, median PFS was 2.8 months, and median OS was 11.3 months [26]. In an additional multi-institutional retrospective analysis conducted in the United States in 60 patients with either acral (n = 25) or mucosal (n = 35) melanoma, 67% (n = 40) of patients received pembrolizumab and 33% (n = 20) received nivolumab for the treatment of advanced disease [27]. The ORR was 32% in the acral and 23% in the mucosal subgroups. After a median follow-up of 20 months (acral) and 10.6 months (mucosal), the median PFS was 4.1 months and 3.9 months, respectively. Median OS was 31.7 months in acral melanoma and not reported in mucosal melanoma because follow-up was not mature enough. Nonetheless, though these rates were comparable with those observed in cutaneous melanoma, the trial was limited by its retrospective nature. Furthermore, the data should be interpreted with caution because they were derived from a pooled analysis of varying doses and schedules. Results of a pooled analysis of data from 889 patients with mucosal (n = 86) or cutaneous (n = 665) melanoma treated with nivolumab monotherapy showed median PFS of 3.0 months and 6.2 months, respectively, and ORRs of 23.3% and 40.9%, respectively [28]. Data were also pooled for patients with mucosal (n = 35) or cutaneous (n = 326) melanoma treated with nivolumab combined with ipilimumab [28]. The combination showed greater activity than nivolumab monotherapy overall, but activity in mucosal melanoma was still lower than activity in cutaneous melanoma; median PFS was 5.9 months and 11.7 months respectively, and ORR was 37.1% and 60.4%, respectively. In KEYNOTE-151, we report an ORR of 15.8% in acral melanoma and 13.3% in mucosal melanoma. The ORRs by subtype were similar to those observed in the overall patient population (16.7%), supporting the use of PD-1 inhibition as second-line therapy, regardless of melanoma subtype.
In conclusion, the treatment of Chinese patients with advanced melanoma, particularly those with acral or mucosal subtypes, represents a strong unmet medical need. The standard of care for first-line treatment of Chinese patients with advanced melanoma is dacarbazine; there is no standard of care for second-line treatment. Poor survival outcomes have led to the recent exploration of immunomodulators in this population. In this first analysis of KEYNOTE-151, pembrolizumab was well tolerated, and ORRs in patients with acral or mucosal melanoma were similar to those in the overall patient population. Moreover, responses were durable, with a median DOR of 8.4 months and a median OS of 12.1 months. These data support the use of pembrolizumab for second-line treatment in Chinese patients with advanced or metastatic melanoma.
Declarations.
Competing Interests
L. S., X. Z., Y. S., H. P., D. W., J. Liu, F. L., L. M., X. Wa., X. We., Y. G., L. Z., S. L., X. C., and J. Gu. have nothing to disclose. S. D. reports employment at Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and holds stocks in the company. Y. Z. and J. Ge report employment at MSD, Shanghai, China. J. Li and H. W. report employment at MSD, Beijing, China.
Funding
This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, sponsored this study, and collaborated with academic advisers to design the study and to gather, analyze, and interpret the results.
Acknowledgments
Medical writing and/or editorial assistance was provided by Cathy Winter, PhD, of the ApotheCom pembrolizumab team (Yardley, PA). This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Footnotes
Trial registration:ClinicalTrials.gov, NCT02821000.
Registration date: July 1, 2016.
Funding: This work was supported by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2019.02.007.
Contributor Information
Lu Si, Email: silu15_silu@126.com.
Xiaoshi Zhang, Email: zhangxsh@sysucc.org.cn.
Yongqian Shu, Email: shuyongqian@csco.org.cn.
Hongming Pan, Email: panhongming@zju.edu.cn.
Di Wu, Email: wudi888991@163.com.
Jiwei Liu, Email: Jiweiliudl@126.com.
Fang Lou, Email: loufang101@126.com.
Lili Mao, Email: yunzhongmanbu7848@163.com.
Xuan Wang, Email: w_xuan_md@126.com.
Xizhi Wen, Email: wenxzh@sysucc.org.cn.
Yanhong Gu, Email: guyanhong@njmu.edu.cn.
Lingjun Zhu, Email: zhulingjun@njmu.edu.cn.
Shijie Lan, Email: lanjie3@sina.com.
Xin Cai, Email: Babydocone@126.com.
Scott J. Diede, Email: scott.diede@merck.com.
Yu Zhou, Email: yu.zhou1@merck.com.
Jun Ge, Email: jun.ge@merck.com.
Jianfeng Li, Email: Jianfeng.li@merck.com.
Haiyan Wu, Email: hai.yan.wu1@merck.com.
Jun Guo, Email: guoj307@126.com.
Appendix A. Supplementary data
Supplementary material
References
- 1.Guo J, Qin S, Liang J, Lin T, Si L, Chen X, et al. Chinese guidelines on the diagnosis and treatment of melanoma (2015 ed). Ann Transl Med 2015;3:322. [DOI] [PMC free article] [PubMed]
- 2.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Thomas L, Bondarenko I, O'Day S, JW MD, Garbe C. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Dummer R, Gutzmer R, Lorigan P, Kim KB, Nyakas M. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol. 2013;14:733–740. doi: 10.1016/S1470-2045(13)70237-7. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Joshua AM, Weber JS, Ribas A, Hodi FS, Kefford RF. Pembrolizumab (pembro; MK-3475) for advanced melanoma (MEL): randomized comparison of two dosing schedules. Ann Oncol. 2014;25(suppl):1–41. [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang JW, Yeh KY, Wang CH, Yang TS, Chiang HF, Wei FC. Malignant melanoma in Taiwan: a prognostic study of 181 cases. Melanoma Res. 2004;14:537–541. doi: 10.1097/00008390-200412000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Hayward NK, Wilmott JS, Waddell N, Johansson PA, Field MA, Nones K. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 9.Wen X, Ding Y, Li J, Zhao J, Peng R, Li D. The experience of immune checkpoint inhibitors in Chinese patients with metastatic melanoma: a retrospective case series. Cancer Immunol Immunother. 2017;66:1153–1162. doi: 10.1007/s00262-017-1989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagore E, Oliver V, Moreno-Picot S, Fortea JM. Primary cutaneous melanoma in hidden sites is associated with thicker tumours—a study of 829 patients. Eur J Cancer. 2001;37:79–82. doi: 10.1016/s0959-8049(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 11.Slingluff CL, Jr., Vollmer R, Seigler HF. Acral melanoma: a review of 185 patients with identification of prognostic variables. J Surg Oncol. 1990;45:91–98. doi: 10.1002/jso.2930450207. [DOI] [PubMed] [Google Scholar]
- 12.Yu J, Luo X, Huang H, Zhai Z, Shen Z, Lin H. Clinical characteristics of malignant melanoma in southwest China: a single-center series of 82 consecutive cases and a meta-analysis of 958 reported cases. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merck Sharp & Dohme Corp., KEYTRUDA® (pembrolizumab) injection, for intravenous use. Whitehouse Station, NJ USA; 08/2018.
- 14.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 15.Ribas A, Hamid O, Daud A, Hodi FS, Wolchok JD, Kefford R. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. JAMA. 2016;315:1600–1609. doi: 10.1001/jama.2016.4059. [DOI] [PubMed] [Google Scholar]
- 16.Hamid O, Puzanov I, Dummer R, Schachter A, Daud A, Schadendorf D. Final analysis of a randomized trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37–45. doi: 10.1016/j.ejca.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki N, Takenouchi T, Fujimoto M, Ihn H, Uchi H, Inozume T. Phase 1b study of pembrolizumab (MK-3475; anti-PD-1 monoclonal antibody) in Japanese patients with advanced melanoma (KEYNOTE-041) Cancer Chemother Pharmacol. 2017;79:651–660. doi: 10.1007/s00280-016-3237-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J, Ahn S, Yoo KH, Kim JH, Choi SH, Jang KT. Treatment outcome of PD-1 immune checkpoint inhibitor in Asian metastatic melanoma patients: correlative analysis with PD-L1 immunohistochemistry. Invest New Drugs. 2016;34:677–684. doi: 10.1007/s10637-016-0373-4. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Cui C, Mao L, Chi Z, Si L, Sheng X, Kong Y. A phase II, randomized, double-blind, placebo-controlled multicenter trial of Endostar in patients with metastatic melanoma. Mol Ther. 2013;21:1456–1463. doi: 10.1038/mt.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang G, Li RH, Sun C, Liu YQ, Zheng JN. Dacarbazine combined targeted therapy versus dacarbazine alone in patients with malignant melanoma: a meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0111920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Si L, Zhang X, Xu Z, Jiang Q, Bu L, Wang X. Vemurafenib in Chinese patients with BRAFV600 mutation–positive unresectable or metastatic melanoma: an open-label, multicenter phase I study. BMC Cancer. 2018;18:520. doi: 10.1186/s12885-018-4336-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–1977. doi: 10.1158/1078-0432.CCR-13-3122. [DOI] [PubMed] [Google Scholar]
- 25.Si L, Kong Y, Xu X, Flaherty KT, Sheng X, Cui C. Prevalence of BRAF V600E mutation in Chinese melanoma patients: large scale analysis of BRAF and NRAS mutations in a 432-case cohort. Eur J Cancer. 2012;48:94–100. doi: 10.1016/j.ejca.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 26.Butler M, Hamid O, Ribas A, Hodi FS, Walpole E, Dauad A. Efficacy of pembrolizumab in patients with advanced mucosal melanoma enrolled in the KEYNOTE-001, 002, and 006 studies. Eur J Cancer. 2017;72(suppl):S123. [Google Scholar]
- 27.Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK. The efficacy of anti-PD-1 agents in acral and mucosal melanoma. Cancer. 2016;122:3354–3362. doi: 10.1002/cncr.30259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Angelo SP, Larkin J, Sosman JA, Lebbe C, Brady B, Neyns B. Efficacy and safety of nivolumab alone or in combination with ipilimumab in patients with mucosal melanoma: a pooled analysis. J Clin Oncol. 2017;35:226–235. doi: 10.1200/JCO.2016.67.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material