Summary
People with repeated rapid meal ingestion have been reported to have increased risk of insulin resistance, impaired glucose tolerance and obesity. To explore whether speed of eating a breakfast influences the postprandial rise of glucose, insulin and the incretin hormones, 24 healthy subjects (12 men and 12 women, mean age 62 years) ingested a standardized solid breakfast consisting of 524 kcal (60% from carbohydrate, 20% from protein, 20% from fat) over 5 or 12 minutes on separate days in random order. Breakfast ingestion increased circulating glucose and insulin with maximal levels seen at 30 minutes after start of meal ingestion with no significant difference in the two tests. Similarly, breakfast increased circulating levels of total (reflecting secretion) glucose‐dependent insulinotropic polypeptide (GIP) and glucagon‐like peptide‐1 (GLP‐1) with, again, no difference between the tests. Furthermore, gastric emptying, as revealed by the indirect paracetamol test, did not differ between the tests. We therefore conclude that the speed of breakfast ingestion does not affect the postprandial rise of glucose, insulin or incretin hormones in healthy subjects.
Keywords: GIP, GLP‐1, insulin, meal ingestion
1. INTRODUCTION
Besides excessive food intake as an important risk factor, disruption of regular eating is associated with obesity and hyperglycaemia. Thus, comparing a large group of individuals who eat breakfast only 3‐5 times/wk or less with a group having breakfast everyday showed that the former group had an increased risk of developing type 2 diabetes over an eight‐year follow‐up period.1 Furthermore, skipping breakfast has been shown to be associated with a higher glucose levels through the day in subjects with type 2 diabetes.2
In addition, fast speed of eating has been shown to be associated with health hazards, such as impaired glucose tolerance, insulin resistance and obesity.3, 4 This could be due to a larger caloric intake in repeated rapid eating together with metabolic and hormonal consequences of rapid ingestion of a meal compared to slow ingestion.
Whether the speed of eating affects postprandial metabolic consequences is, however, not known. It may theoretically be speculated that fast eating results in rapid increases in glucose and insulin, which may have long‐term consequences. One such consequence would be that lipolysis would be inhibited more efficiently, since insulin is known to inhibit lipolysis,9 which may promote fat accumulation. This hypothesis is not, however, supported by results of studies comparing rapid or slow ingestion of a liquid meal ingestion10 or ice cream.11, 12 in healthy subjects11 or in subjects with type 2 diabetes.10, 12 Thus, these studies showed no difference in postprandial levels of glucose or insulin between rapid (5 minutes) or slow (20 or 30 minutes) intake.
Also, the incretin hormone levels may be different after rapid vs slow eating, since their release has been demonstrated to depend on the amount of load presented to the duodenum.13 Differences in the incretin hormones may have relevance for a potential difference in insulin levels, since they stimulate insulin secretion.14 It may also be of importance for regulation of food intake since the incretin hormone glucagon‐like peptide‐1 (GLP‐1) is a powerful satiety factor.15 One study has indeed reported higher postprandial GLP‐1 levels after 60 minutes after the slow compared to fast ingestion in healthy individuals11 but this was not confirmed in subjects with type 2 diabetes.10, 12 Effects of fast versus slow ingestion of a standardized solid meal have, however, not been examined. Therefore, this study examined levels of glucose, insulin and the two incretin hormones glucose‐dependent insulinotropic polypeptide (GIP) and GLP‐1 after a rapid (5 minutes) vs a slow (12 minutes) intake of a standardized solid breakfast in healthy nondiabetic subjects.
2. METHODS
2.1. Study population and design
The study was performed at the Clinical Research Center at Skåne University Hospital, Lund, Sweden. Twelve healthy male and 12 healthy female subjects (age 52‐69 years, mean 62 ± 6 years [mean ± SD], BMI 18‐36 kg/m2, mean 25.5 ± 3.8 kg/m2, body weight 74 ± 12 kg) were studied. The subjects were recruited through advertisements at public spaces and in local newspaper. Inclusion criteria were that the subjects should not have diabetes, liver disease, kidney disease or thyroid disorder (diagnosed as normal HbA1c [mean was, 35.8 ± 4.5 (SD) mmol/mol] and liver enzymes, serum creatinine, TSH and thyroid hormone levels within the normal range). Treatment of hypercholesterolaemia and hypertension was accepted provided that lipid levels were within the normal range and blood pressure <135/85 mm Hg. Five subjects were taking statins, and eight subjects were on antihypertensive treatment. All subjects were regular breakfast eaters and able to eat all contents of the study breakfast.
The study had a single‐centre crossover design. Each participant was studied twice, separated by at least 1 week. Therefore, for the two tests, the study populations, including medication, were exactly the same. After an overnight fast at 8 am, subjects were provided with an intravenous cannula and paracetamol (1.5 g; GlaxoSmithKline, Mölndal, Sweden) was given for assessment of gastric emptying. Thirty minutes later, that is at 8.30 am, a breakfast was served. The breakfast consisted of rye and wheat bread (67% carbohydrate; 60 g), 40% margarine (10 g), smoked ham, from pork (3% fat; 15 g), cheese with 17% fat (15 g), juice (285 g), green pepper (40 g), light sour milk with 0.5% fat (200 g), mix‐muesli cereal with fruit (40 g), water and nonsweetened coffee or tea (total 524 kcal; 60% from carbohydrate, 20% from protein, 20% from fat). The breakfast ingestion was closely supervised by the study nurse to be exactly 5 minutes in one test and exactly 12 minutes in the other test. The two tests were performed in a randomized order. These time ranges were chosen to reflect ranges in common habits when ingesting a relatively small amount of food as the breakfast represents. Blood was sampled at specific time points. First sample was taken 5 minutes before start of breakfast ingestion, which was performed at time t = 0.
2.2. Assay
Blood was collected in chilled tubes containing EDTA (7.4 mmol/L; glucose, insulin and C‐peptide) or EDTA and the DPP‐4 inhibitor diprotin A (0.1 mmol/L; Bachem, Bubendorf, Switzerland; GIP and GLP‐1). Samples were immediately centrifuged at 4°C, and plasma was frozen at −20°C. Glucose was measured using the glucose oxidase method, insulin using ELISA (Mercodia, Uppsala, Sweden, detection limit 6.9 pmol/L, interassay coefficient of variation [CV] 2.6%‐3.6% and intraassay CV 2.1%‐2.9% at low and high levels), total GIP using ELISA (Merck, detection limit 0.8 pmol/L, interassay CV 3.0%‐8.8% and intraassay CV 2.1%‐3.9% at low and high levels), and total GLP‐1 using ELISA (Merck, Burlington, MA, USA, detection limit 1.5 pmol/L, interassay CV 2.8%‐3.2% and intra‐assay CV 1.0%‐2.0%). Paracetamol was analysed colorimetrically (Cambridge Life Science, Ely, Cambridgeshire, UK).
2.3. Data analysis
Means ± SEM are shown if not stated otherwise. Both total and incremental areas under the curve (AUC) were calculated using the trapezoid rule. For statistical analyses, data for insulin, GLP‐1 and GIP were logarithmically transformed. Paired t test was used to test the differences between fast and slow ingestion.
2.4. Ethics statement
The study was performed according to the Declaration of Helsinki and approved by the Ethic Committee, Lund, Sweden (no 2013/2). All participants gave written informed consent after full explanation of the purpose and nature of all procedures used. The study was registered at clinicaltrials.gov database (NCT01779622) and conducted using good clinical practice.
3. RESULTS
3.1. Glucose, insulin
Glucose and insulin levels increased after breakfast ingestion; the peaks were observed at 30 minutes (Figure 1). There was no significant difference at any time point in glucose or insulin levels when breakfast was ingested over 5 or 12 minutes. Furthermore, there was no difference in time of peak levels of glucose and insulin or their peak concentrations between the tests.
Figure 1.
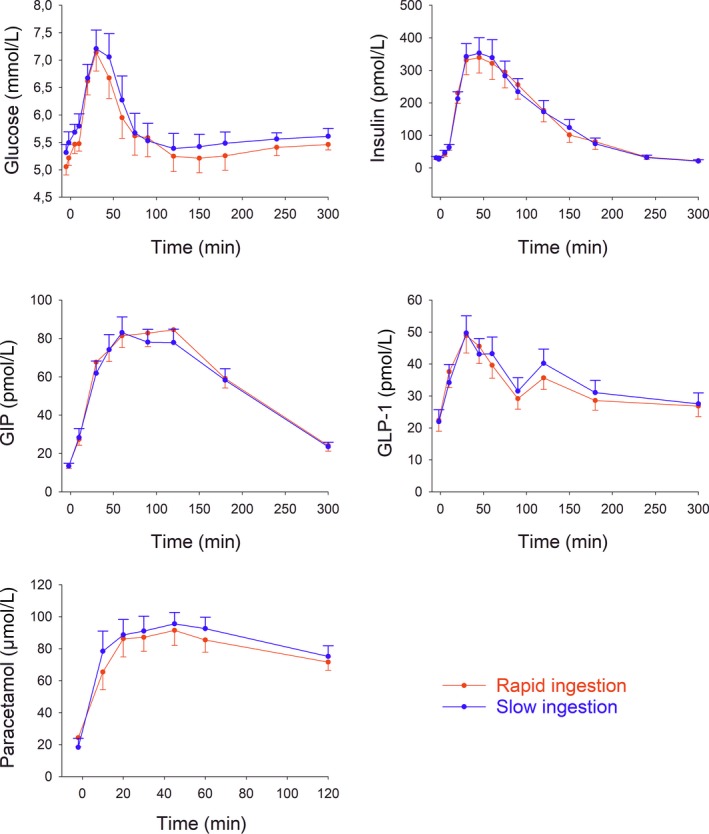
Plasma levels of glucose, insulin, GIP, GLP‐1 and paracetamol after rapid or slow ingestion of a standard breakfast (524 kcal) in 24 healthy volunteers (12 men and 12 women). Paracetamol (1.5 g) was given 30 min before start of breakfast ingestion, which was at time t = 0. Means ± SEM are shown
3.2. GLP‐1, GIP
Levels of GLP‐1 and GIP increased after breakfast ingestion with, again, no significant difference between ingestion over 5 vs 12 minutes at any time point (Figure 1). Furthermore, there was no difference in time of peak levels of GLP‐1 and GIP or their peak concentrations between the tests.
3.3. Paracetamol
Plasma paracetamol concentrations throughout the test and the 120 minutes AUCparacetamol (3.4 ± 0.7 and 3.6 ± 0.8 mmol/L minutes after rapid and slow breakfast ingestion, respectively) did not different significantly between the two tests (Figure 1).
4. DISCUSSION
The main finding in this study is that rapid and slow ingestion of a standardized solid breakfast within the time limit of 5‐12 minutes has no differential impact on postprandial glucose, insulin, GIP or GLP‐1 in healthy subjects. The rationale of the study was that it is known that fast eating is associated with insulin resistance, impaired glucose tolerance and obesity4, 5 and it would therefore be of importance to know whether postprandial glucose, insulin and incretin hormones are affected by the speed of intake of a standardized solid breakfast. Previous studies have shown similarly no difference in postprandial glucose and insulin after slow vs rapid intake of liquid meal or ice cream in healthy subjects.10, 11 Besides that these studies examined liquid meals and our study standardized solid meal, a difference between the studies was that the first blood sample after meal ingestion was taken at 30 minutes in these previous studies,10, 11 and therefore, important early time points were not analysed.
It has been demonstrated before that there is a caloric‐dependent regulation of insulin and incretin hormone responses after meal ingestion, since a larger meal elicits a higher postprandial insulin and incretin hormone response than a smaller meal.16, 17 In fact, the release of GLP‐1 has been shown to correlate to the rate of calories delivered to the gut.18 This would theoretically suggest that rapid eating would elicit a faster and higher incretin hormone response. However, our results do not support this hypothesis, since within the time frame of the eating period (5‐12 minutes), there was no difference in the responses. Also, glucose and insulin levels were similar after the two meal ingestion rates, which support previous results after liquid and soft meal ingestions.10, 11
A major determinant for release of incretin hormones is gastric emptying.13 If eating rate impacts gastric emptying, a difference in incretin hormone levels between the rapid and slow meal ingestion would be anticipated. However, the regulation of gastric emptying is strictly controlled by neurohormonal and adaptive mechanisms.19, 20 Therefore, an initial larger load to the duodenum after a rapid ingestion may be followed by adaptive responses to inhibit gastric emptying. This could result in no difference in gastric emptying between rapid and slow meal ingestion. To study gastric emptying in our present study, we determined paracetamol concentrations after 1.5 g paracetamol was taken 30 minutes before breakfast. This test of gastric emptying is easy to perform and non‐invasive, but it is important to acknowledge that this technique is not the best test for estimating gastric emptying.21 Nonetheless, it has previously been validated as an indirect determination of gastric emptying.22, 23 We found that there was no significant difference in gastric emptying between the two tests, which illustrates the finely tuned regulation of gastric emptying and also corroborates the data on incretin hormones.
A strength of the study was that we examined a larger number of subjects (24 subjects), to avoid conclusions drawn on an insufficient number of subjects. We also studied healthy nondiabetic subjects to assure a normal regulation of islet function. Another strength was that we studied both men and women. A strength is also that we sampled blood repeatedly over the study period, and therefore, it is unlikely that we have missed a difference at some specific postprandial time point. A limitation of our study was that we tested a single ingestion of rapid and fast eating, and therefore, it remains to be studied whether long‐term difference in the speed of food ingestion impacts glucose, insulin and incretin hormones. Another limitation may be that we studied middle‐aged subjects with a mean age of 62 years that are known to have a slightly reduced gastric emptying.24
Based on the data achieved from this study, we conclude that the speed of a standardized solid breakfast ingestion within 5‐12 minutes does not affect the postprandial rise of glucose, insulin or incretin hormones in healthy subjects.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
The study was designed by BA and conducted by WA and BA who also collected and analysed data and wrote the manuscript. BA is the guarantors of the study.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge research nurse Bertil Nilsson and biomedical technician Kristina Andersson for expert assistance.
Alsalim W, Ahrén B. Insulin and incretin hormone responses to rapid versus slow ingestion of a standardized solid breakfast in healthy subjects. Endocrinol Diab Metab. 2019;2:e00056 10.1002/edm2.56
Funding information
The study was supported by the Swedish Medical Research Council, Region Skåne, and the Faculty of Medicine.
DATA ACCESSIBILITY
The data that support the findings of this study are available from the corresponding author (BA), upon reasonable request.
REFERENCES
- 1. Uemura M, Yatsuya H, Hilawe EH, et al. Breakfast skipping is positively associated with incidence of type 2 diabetes mellitus: evidence from the Aichi workers cohort study. J Epidemiol. 2015;25:351‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jakubowicz D, Wainstein J, Ahrén B, et al. Fasting until noon triggers increased postprandial hyperglycemia and impaired insulin response after lunch and dinner in individuals with type 2 diabetes: a randomized clinical trial. Diabetes Care. 2015;38:1820‐1826. [DOI] [PubMed] [Google Scholar]
- 3. Hurst Y, Fukuda H. Effects of changes in eating speed on obesity in patients with diabetes: a secondary analysis of longitudinal check‐up data. BMJ Open. 2018;8:e019589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Otsuka R, Tamakoshi K, Yatsuva H, et al. Eating fast leads to insulin resistance: findings in middle‐aged Japanese men and women. Prev Med. 2008;46:154‐159. [DOI] [PubMed] [Google Scholar]
- 5. Totsuka K, Maeno T, Saito K, et al. Self‐reported fast eating is a potent predictor of development of impaired glucose tolerance in Japanese men and women: Tsukuba Medical Center Study. Diabetes Res Clin Pract. 2011;94:e72‐74. [DOI] [PubMed] [Google Scholar]
- 6. Otsuka R, Tamakoshi K, Yatsuya H, et al. Eating fast leads to obesity: findings based on self‐administered questionnaires among middle‐aged Japanese men and women. J Epidemiol. 2006;16:117‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maruyama K, Sato S, Ohira T, et al. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: cross sectional survey. BMJ. 2008;337:a2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohkuma T, Hirakawa Y, Nakamura U, et al. Association between eating rate and obesity: a systematic review and meta‐analysis. Int J Obes. 2015;39:1589‐1596. [DOI] [PubMed] [Google Scholar]
- 9. Ferrannini E, Galvan AQ, Gastaldelli A, et al. Insulin: new roles for an ancient hormone. Eur J Clin Invest. 1999;29:842‐852. [DOI] [PubMed] [Google Scholar]
- 10. Kamiko K, Aoki K, Kamiyama H, et al. Comparison of plasma glucose and gut hormone levels between drinking enteral formula over a period of 5 and 20 minutes in Japanese patients with type 2 diabetes: a pilot study. J Clin Med Res. 2016;20:749‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kokkinos A, le Roux CW, Alexiadou K, et al. Easting slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon‐like peptide‐1. J Clin Endocrinol Metab. 2010;95:333‐337. [DOI] [PubMed] [Google Scholar]
- 12. Angelopoulos T, Kokkinos A, Liaskos C, et al. The effect of slow spaced eating on hunger and satiety in overweight and obese patients with type 2 diabetes mellitus.BMJ. Open Diabetes Res Care. 2014;2:e000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holst JJ, Gribble F, Horowitz M, Rayner CK. Role of the gut in glucose homeostasis. Diabetes Care. 2016;39:884‐892. [DOI] [PubMed] [Google Scholar]
- 14. Deacon CF, Ahrén B. Physiology of incretins in health and disease. Rev Diabet Stud. 2011;8:293‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andersen A, Lund A, Knop FK, Vilsbøll T. Glucagon‐like peptide 1 in health and disease. Nat Rev Endocrinol. 2018;14:390‐403. [DOI] [PubMed] [Google Scholar]
- 16. Vilsbøll T, Krarup T, Sonne J, et al. Incretin secretion in relation to meal size and body weight in healthy subjects and people with type 1 and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:2706‐2713. [DOI] [PubMed] [Google Scholar]
- 17. Alsalim W, Omar B, Pacini G, et al. Incretin and islet hormone adaptation to meals of increasing size in healthy subjects. J Clin Endocrinol Metab. 2015;100:561‐568. [DOI] [PubMed] [Google Scholar]
- 18. Schirra J, Katschinski M, Weidmann C, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97:92‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mussa EM, Sood S, Verberne AJ. Implication of neurohormonal‐coupled mechanisms of gastric emptying and pancreatic secretory function in diabetic gastroparesis. World J Gastroenterol. 2018;24:3821‐3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vella A, Camilleri M. The gastrointestinal tract as an integrator of mechanical and hormonal response to nutrient ingestion. Diabetes. 2017;66:2729‐2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Phillips LK, Rayner CK, Jones KL, Horowitz M. Measurement of gastric emptying in diabetes. J Diabetes Complications. 2014;28:894‐903. [DOI] [PubMed] [Google Scholar]
- 22. Tarling MM, Toner CC, Withington PS, et al. A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med. 1997;23:256‐260. [DOI] [PubMed] [Google Scholar]
- 23. Medhus AW, Lofthus CM, Bredesen J, Husebye E. Gastric emptying: the validity of the paracetamol absorption test adjusted for individual pharmacokinetics. Neurogastroenterol Motil. 2001;13:179‐185. [DOI] [PubMed] [Google Scholar]
- 24. Kuo P, Rayner CK, Horowitz M. Gastric emptying, diabetes, and aging. Clin Geriatr Med. 2007;23(785):808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (BA), upon reasonable request.


