Summary
Context and Objective
Bilateral adrenal vein sampling (AVS), the diagnostic standard for identifying surgically remediable aldosteronism (SRA), is commonly performed after cosyntropin stimulation (post‐ACTHstim). The role of AVS without cosyntropin stimulation (pre‐ACTHstim) has not been established. The selectivity index (SI), the adrenal vein (av) serum cortisol concentration divided by that in a peripheral vein, confirms av sampling. The minimally acceptable SI is controversial. The objectives of this study were to determine the role of pre‐ACTHstim AVS and a predetermined SI.
Design
Using biochemical cure as the endpoint, we performed a retrospective head‐to‐head comparison of pre‐ACTHstim AVS to post‐ACTHstim AVS. The specificity of a predetermined minimum SI of 1.5 in pre‐ACTHstim AVS was determined.
Patients
At a regional AVS referral centre, we analysed 32 patients who had undergone simultaneous bilateral AVS both pre‐ and post‐ACTHstim and had returned for postadrenalectomy evaluation.
Measurements
Simultaneous bilateral AVS was performed with measurements of venous concentrations of aldosterone and cortisol. End points were postadrenalectomy plasma renin activity, serum aldosterone concentration, and number of antihypertensive medications.
Results
All 32 patients achieved a biochemical cure following adrenalectomy. The two AVS protocols were complementary. Notably, seven patients (22%; CI = 11‐38) were found to have SRA by a lateralization index (LI) > 4 on the pre‐ACTHstim AVS, but not on the post‐ACTHstim AVS. SI pre‐ACTHstim was divided into tertiles. Specificity was 100% in all.
Conclusions
Simultaneous bilateral AVS performed both pre‐ACTHstim and post‐ACTHstim maximizes SRA identification. A SI of 1.5 pre‐ACTHstim does not reduce specificity.
Keywords: adrenal vein sampling, adrenalectomy, aldosterone, hyperaldosteronism
1. INTRODUCTION
Primary aldosteronism (PA), the most common cause of secondary hypertension, affects 11%‐20% of patients with drug‐resistant hypertension.1, 2 As compared to essential hypertension, PA is associated with approximately a two fold increase in cardiovascular events,3, 4 suggesting that excess aldosterone may cause damage independent of the hypertension.3, 5, 6, 7, 8 About two‐thirds of SRA is caused by either unilateral functioning adrenocortical adenomas or unilateral hyperplasia, whereas bilateral hyperplasia accounts for one‐third of PA.9 Subtype differentiation of PA directs clinical management.
Bilateral adrenal vein sampling (AVS) is considered to be the best diagnostic test for SRA identification.10, 11 However, there is no consensus as to the best AVS protocol.12, 13 Approximately two‐third of centres perform AVS following cosyntropin administration.15 Since cosyntropin increases the blood flow into and out of the adrenal glands, AVS is technically easier and can be performed more rapidly following cosyntropin administration. However, AVS performed only after cosyntropin administration may reduce SRA yield.16 Furthermore, there is no agreement as to the correct selectivity index (SI) (the ratio of serum cortisol concentration in the adrenal vein to the serum cortisol concentration in a peripheral vein). If the SI is too restrictive, a proportion of true positive cases that could benefit from adrenalectomy may be excluded. Conversely, the use of a too permissive threshold may compromise specificity. Correlation of different AVS protocols with biochemical cure will determine the optimum AVS protocol.
In this study, we determine the diagnostic utility of simultaneous bilateral AVS performed both without cosyntropin stimulation (pre‐ACTHstim) and following cosyntropin stimulation (post‐ACTHstim) in the same patients and use of biochemical cure as the endpoint. Furthermore, we determine whether a lower limit pre‐ACTH SI of 1.5 decreases AVS specificity.
2. MATERIALS AND METHODS
This study was approved by the Institutional Review Board (IRB) at the University of Connecticut Health Center. We retrospectively analysed all the PA patients who underwent simultaneous bilateral AVS at our institution between 1996 and May 2018. We correlated AVS results with biochemical cure following adrenalectomy. The primary endpoint indicating successful response to adrenalectomy was biochemical cure defined as serum aldosterone concentration <250 pmol/L (9 ng/dL) and/or plasma renin activity >2 ng/mL/h while the patient was in the sitting position and taking no agents that antagonized aldosterone or its intracellular effects. The secondary endpoints indicating successful response to adrenalectomy were reduction in the number of antihypertensive medications and resolution of hypokalemia. The terms sensitivity and specificity refer to the ability to the indicated AVS result to predict the primary end point in the patients that meet all the study inclusion criteria. The diagnostic accuracy of AVS was evaluated at different SI.
2.1. Inclusion criteria and patient population
The inclusion criteria for our study were successful simultaneous bilateral AVS both pre‐ and post‐ACTHstim, adrenalectomy followed by clinical evaluation with biochemical testing within 6 months following adrenalectomy. All patients undergoing AVS had PA with persistent hypertension (BP > 140/90), spontaneous serum potassium concentration <4.0 mmol/L and, on at least two consecutive measurements, all three of the following: sitting plasma renin activity (PRA) of <1.0 ng/mL/h, sitting serum aldosterone concentration >277 pmol/L (10 ng/dL) and serum aldosterone concentration divided by plasma renin activity ratio (aldosterone to renin ratio) of >550 pmol/L/ng/mL/h (20 ng/dL/ng/mL/h). Adrenal gland imaging results did not influence the decision to perform AVS or adrenalectomy.
2.2. Adrenal vein sampling protocol
Patients were prepared for adrenal vein sampling by replacing potassium so that the serum potassium concentration was >3.5 mmol/L and by decreasing diuretic therapy so that sitting PRA was <0.5 ng/mL/h. Adrenal veins were catheterized according to the method described by Doppman10 except that both catheters were inserted through a single femoral vein access. The location of the adrenal veins was determined fluoroscopically by gentle injection of a small amount of isotonic and nonionic contrast media, both before and after the adrenal venous sampling as well as when necessary between different sampling sequences. In some patients, it was necessary to modify the catheter tip for sampling of the right adrenal vein by creating a single extra hole within the 3‐4 mm of the catheter tip along the superior margin, so that the effluent immediately exiting the adrenal vein could be sampled without the complete occlusion of the small vein. Simultaneous venous blood samplings from the right adrenal vein, left adrenal veins and the right common iliac vein (peripheral blood) were supervised by an experienced endocrinologist (CDM or BT) and collected at approximately 5 and 2 minutes prior to cosyntropin administration (pre‐ACTHstim) and at 5, 15 and 20 minutes after cosyntropin administration (post‐ACTHstim). The sample drawn 5 minutes post‐ACTHstim was for confirmation of catheter placement in the adrenal vein, but not for calculating the lateralization index (LI). If there was concern that a catheter might have been dislodged, then the catheter was replaced and repeat sampling was performed. Cosyntropin was administered as a bolus of 250 mcg followed immediately by a cosyntropin infusion at 5 mcg/minute, as described by Doppman.10
For the pre‐ACTHstim measurements, the AVS was considered successful if SI was >1.5. For the post‐ACTHstim measurements, the AVS was considered successful if the SI was >2.
2.3. Biochemical assays and evaluation of biochemical results
Serum aldosterone was measured at ARUP laboratories by chemiluminescent immunoassay. The current interassay coefficient of variation (CV) at a concentration of 6.9 ng/dL is 10.1 per cent; at 26.4 ng/dL it is 6.0 per cent, and at 59.5 ng/dL it is 6.9 per cent. Plasma renin activity was measured at ARUP laboratories by a quantitative enzyme‐linked immunosorbent assay. The current interassay CV at 1.9 ng/mL/h is 12.4 per cent; at 5.6 ng/mL/h it is 11.2 per cent; and at 17.0 ng/mL/h it is 8.8 per cent. Serum cortisol was measured in a variety of different commercial assays over the years of the study, but all with similar performance and always the same assay for any given patient. The most recent measurements are made using the ARCHTECT chemiluminescent microparticle immunoassay (Abbott Laboratories, Abbott Park, IL 60064).
The SI is the ratio of serum cortisol concentration in the adrenal vein to that of the peripheral vein drawn simultaneously. The prespecified SI to confirm successful adrenal vein cannulation pre‐ACTHstim was >1.5 and post‐ACTHstim was >2.0. At our institution, the current cortisol assay is calibrated using a six‐point standard reference concentration curve. The standard reference concentrations are 0, 83, 149, 295, 695 and 1650 nmol/L (0, 3.0, 5.4, 10.7, 25.2, and 59.8 mcg/dL). Using quality control material, current coefficient of variation (CV) for mean cortisol concentration of 99.3 nmol/L (3.6 mcg/dL) CV is 3.5%, for a mean cortisol concentration of 510 nmol/L (18.5 mcg/dL) CV is 2.4%, and for a mean concentration of 767 nmol/L (27.8 mcg/dL) CV is 2.4%. We calculated that for a CV of 10 per cent that a SI > 1.5 is highly unlikely (P < 0.0025) to be due to a laboratory variability and indicates successful adrenal vein cannulation. Therefore, we predicted that a lower limit LI of 1.5 would be large enough to confirm successful AVS. Based on this calculation and discussions with Dr John Doppman prior to the initiation of this study in 1996, we prospectively selected a SI of ≥1.5 to confirm successful av catheterization pre‐ACTHstim and a SI ≥ 2.0 to confirm successful av catheterization post‐ACTHstim. To determine if these predictions were correct, we evaluated the sensitivity and specificity of the predetermined SI. The SI of the same 32 patients was divided into tertiles, and the sensitivity and specificity of the SI for predicting SRA was determined at each tertile both pre‐ and post‐ACTHstim. Thirty‐two patients had 65 pre‐ACTHstim samples and 76 post‐ACTHstim samples. For each bilateral sampling, we report the lowest SI value of the right or left adrenal veins. Chi‐square test was used to determine the statistical significance of differences in specificity and sensitivity between the tertiles.
The mean LI was used to predict the response to adrenalectomy. First, the adrenal vein serum aldosterone concentration was corrected for flow and dilution by dividing the serum aldosterone concentration by the serum cortisol concentration in that same sample. This is referred to as the cortisol corrected aldosterone concentration (A/C). The lateralization index (LI) was calculated by dividing the dominant A/C (D) by the nondominant A/C (ND). The mean LI (usually of duplicate samples but sometimes additional samples had been drawn, and these were also used) was the LI used to predict response to adrenalectomy.
Prior to the initiation of this study in 1996, we decided upon the following prespecified criteria. We offered adrenalectomy to all patients with a LI > 4.0, either pre‐ACTHstim or post‐ACTHstim and to those patients with contralateral suppression defined as A/C of the nondominant adrenal vein (ND) < A/C of the peripheral vein (P).
2.4. Statistical analysis
Confidence intervals were used to determine the statistical significance of proportions. Wilcoxon signed‐rank test was used to compare the pre‐ and postadrenalectomy aldosterone to renin ratios as well as the number of blood pressure medications before and following adrenalectomy. Chi‐square test was used to determine statistical significance of differences in specificity and sensitivity between the SI tertiles. P < 0.05 was considered statistically significant.
3. RESULTS
A total of 63 patients were confirmed biochemically to have simultaneous bilateral AVS both pre‐ and post‐ACTHstim based on the SI meeting the predetermined criteria. Of this group, 32 patients were predicted to have SRA and met all three inclusion criteria: successful simultaneous bilateral AVS both pre‐ and post‐ACTHstim, unilateral adrenalectomy, and postadrenalectomy biochemical testing. This group of 32 patients was a subset of 45 patients who underwent successful bilateral AVS both pre‐ and post‐ACTHstim and were predicted to have SRA. Of those 45 patients, seven declined adrenalectomy, and six underwent adrenalectomy, but did not return for postadrenalectomy evaluation. Eighteen of the 63 patients were diagnosed with bilateral hyperplasia based upon a LI < 4 both pre‐ and post‐ACTHstim.
We first evaluated the primary end point of biochemical cure postadrenalectomy to confirm that the biochemical abnormality of the renin‐aldosterone system that had been persistently present prior to adrenalectomy had resolved postadrenalectomy. All 32 patients who met our inclusion criteria experienced a biochemical cure based on postadrenalectomy serum aldosterone concentration <250 pmol/L (9 ng/dL) and/or plasma renin activity (PRA) >2.0 ng/mL/h, indicating an overall specificity of SRA prediction of 100 per cent. This outcome is summarized by the ratios of serum aldosterone concentration to PRA ratios shown in Figure 1. The mean aldosterone (pmol/L) to PRA (ng/mL/h) ratio prior to adrenalectomy was 11 080 (400 ng/dL/ng/mL/h) with a range of 554‐30 441 (20.4‐1099 ng/dL/ng/mL/h). Postadrenalectomy it decreased to 211 pmol/L/ng/mL/h (7.6 ng/dL/ng/mL/h) with a range of 2.2‐1110 pmol/L/ng/mL/h (0.08‐40 ng/dL/ng/mL/h). The mean decrease was 11 050 pmol/L/ng/mL/h (399 ng/dL/ng/mL/h) with a 95% CI = 7820‐14 300 pmol/L/ng/mL/h (282‐516 ng/dL/ng/mL/h) and P < 0.0001. There was one patient with postadrenalectomy aldosterone to renin ratio of 1110 pmol/L/ng/mL/h) (40 ng/dL/ng/mL/h). This individual had a sitting serum aldosterone of 222 pmol/L (8 ng/dL), and therefore met the criterion for cure (serum aldosterone concentration < 250 pmol/L (9 ng/dL) and also may have low renin hypertension in addition to primary aldosteronism. We then determined whether biochemical cure was associated with an improvement in clinical outcome, as measured by the secondary end points. All patients met both secondary endpoints: resolution of hypokalemia and reduction in number of antihypertensive medications. Following adrenalectomy, the mean decrease in number of antihypertensive medications per patient was 2.0 (95% CI: 1.1‐2.3; P < 0.0001; Figure 2). We reviewed the adrenal pathology. There were 13 right cortical adenomas, 13 left cortical adenomas, two right unilateral hyperplasia, three multinodular right adrenal glands and one multinodular left adrenal gland.
Figure 1.
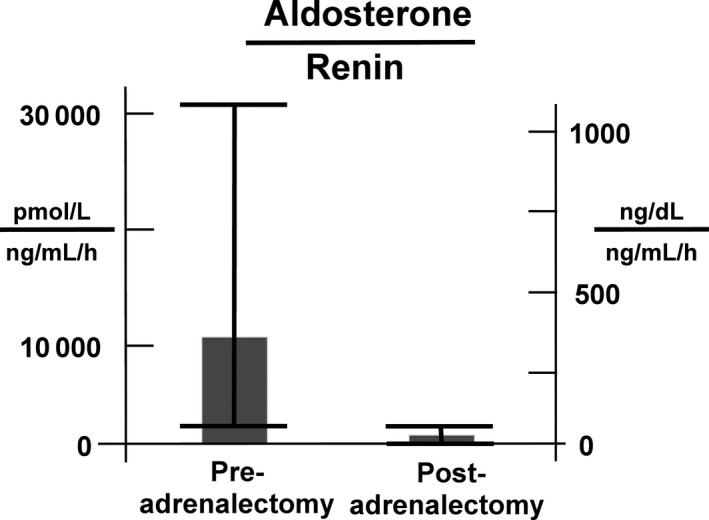
Biochemical cure is summarized by the mean (and range) ratio of serum aldosterone concentration to plasma renin activity (PRA) before and after adrenalectomy. The mean ratio of serum aldosterone concentration (pmol/L) to PRA (ng/mL/h) prior to adrenalectomy was 11 080 (range = 554‐30 440). Postadrenalectomy the ratio decreased to 211 pmol/L/ng/mL/h (range = 2.2‐1110 pmol/L/ng/mL/h). The mean decrease was 11 050 pmol/L/ng/mL/h (95% CI = 7820‐14 300 pmol/L/ng/mL/h; P < 0.0001). In common units, the mean ratio of serum aldosterone concentration (ng/dL) to PRA (ng/mL/h) prior to adrenalectomy was 400 (range = 20.4‐1099). Postadrenalectomy the ratio decreased to 7.6 (range = 0.08‐40). The mean decrease was 399 (95% CI: 282‐516; P < 0.0001)
Figure 2.
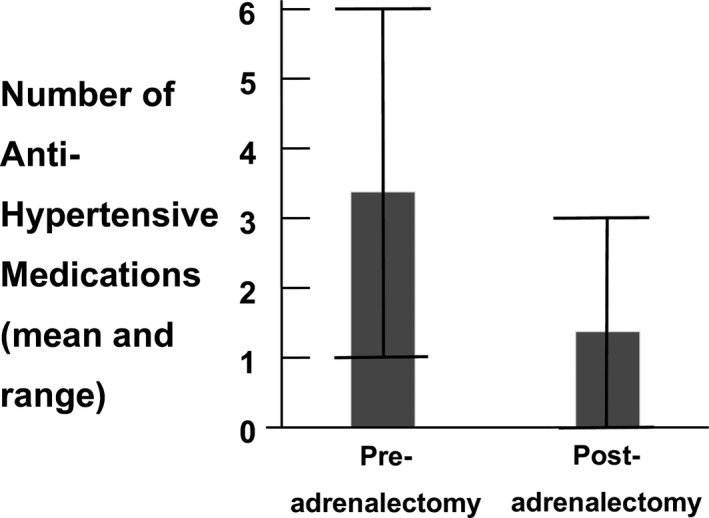
Number of antihypertensive medications per patient pre‐ and postadrenalectomy. Preadrenalectomy the average number of medications per patient was 3.3 (range 1‐6), and postadrenalectomy this decreased to 1.3 (range 0‐3). The mean decrease in number of antihypertensive medications per patient was 2.0 (95% CI: 1.1‐2.3; P < 0.0001)
To further verify that the resected adrenal gland was the source of aldosterone production, we reviewed the ratio of the aldosterone concentration in the vein of the dominant adrenal gland to that in the peripheral vein in all patients included in this study. The peripheral vein aldosterone concentration approximates the arterial aldosterone concentration; therefore, the adrenal vein aldosterone concentration divided by the peripheral vein aldosterone concentration approximates the venous/arterial gradient. For those patients for whom the diagnosis of SRA is made using LI > 4 pre‐ACTHstim, the minimum gradient is 3.4 (range 3.4‐473). For those patients for whom the diagnosis of SRA is made using LI > 4 post‐ACTHstim, the minimum gradient is 4.7 (range 4.7‐850). These gradients, a classic criterion for hormone production by a gland, are further confirmation of excess aldosterone production by the resected adrenal gland.
We evaluated the SRA yield of the different AVS protocols. The SRA yields of the two different AVS protocols were complementary as summarized in Figure 3. Notably, seven out of the 32 patients (22%; CI = 11‐38; P < 0.01) had an LI > 4 pre‐ACTHstim, but not post‐ACTHstim. Therefore, 22% of the patients with biochemically confirmed SRA, were diagnosed with SRA only by simultaneous bilateral AVS performed pre‐ACTHstim. Six patients had an isolated LI > 4 only post‐ACTHstim, and 18 patients had a LI > 4 both pre‐ and post‐ACTHstim. Figure 4A shows the LI pre‐ and post‐ACTHstim in those seven patients who were predicted to have SRA based upon pre‐ACTHstim only. Figure 4B shows the LI of the six patients with isolated LI > 4 post‐ACTHstim only. There was one patient who did not have a LI > 4, but underwent adrenalectomy because of contralateral suppression (ND < P). This patient was also cured. In summary, the two AVS protocols were complementary.
Figure 3.
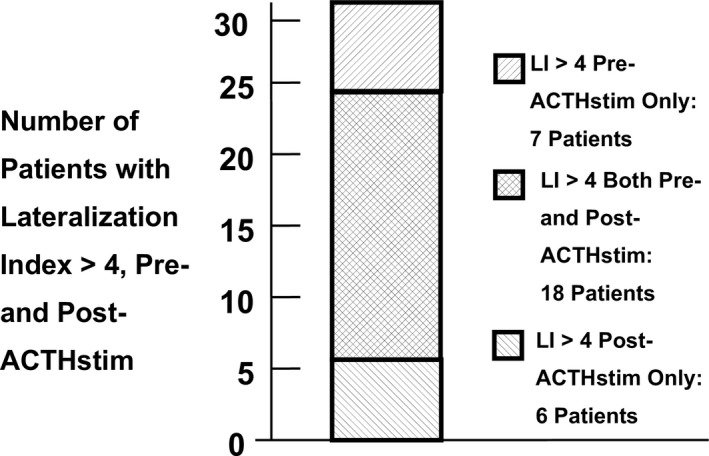
Yield of SRA based on AVS performed pre‐ACTHstim and post‐ACTHstim. The total number of patients with a lateralization index (LI) > 4, (n = 31) is shown in the bar. Not shown is a single patient that was cured based upon a suppressed contralateral side. Of the patients predicted to have SRA by a LI > 4, seven patients (22%; CI = 11‐38) only met this threshold when simultaneous bilateral AVS was performed pre‐ACTH stim; 18 patients met this threshold when bilateral simultaneous AVS was performed pre‐ and post‐ACTHstim; six patients only met this threshold when simultaneous bilateral AVS was performed post‐ACTH stimulation
Figure 4.
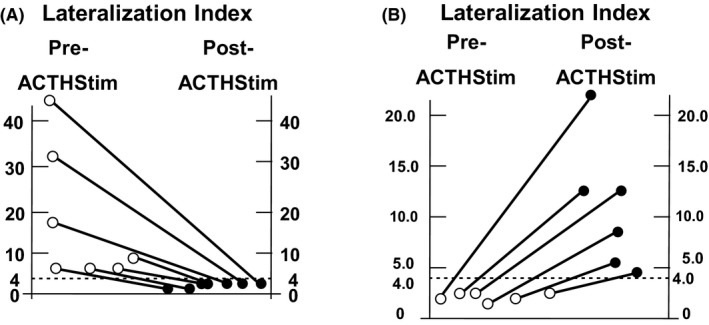
The lateralization index (LI) of the seven patients identified with SRA with LI > 4 only found pre‐ACTHstim and the six patients identified with SRA only found post‐ACTHstim. A, the LI pre‐ACTHstim (open circles) and post‐ACTHstim (closed circles) for each of the seven patients who were predicted to have SRA based upon an isolated pre‐ACTHstim LI > 4. If AVS had been performed only post‐ACTH stimulation, these patients would not have been predicted to have SRA, and would not have been cured. B, the LI pre‐ACTHstim (open circles) and post‐ACTHstim (closed circles) for each of the six patients who were predicted to have surgically SRA based upon an isolated post‐ACTHstim LI > 4. If AVS had been performed only pre‐ACTHstim, these patients would not have been predicted to have SRA, and would not have been cured. The dotted line indicates the predetermined LI of 4.0 used to predict SRA
We evaluated the sensitivity and specificity of the predetermined SI. The SI of the 32 patients was divided into tertiles, and the sensitivity and specificity of the SI for predicting SRA was determined at each tertile both pre‐ and post‐ACTHstim. The results are summarized in Table 1. For each bilateral sampling, we report the lowest SI value of the right or left adrenal veins. The clinical utility of a relatively low prespecified SI was determined by examining the yield of SRA in the lowest tertile both pre‐ and post‐ACTHstim. The pre‐ACTHstim SI tertiles were 1.5‐2.1, 2.3‐3.5 and 3.7‐42. Specificity was 100% in all tertiles. Sensitivity was 50% at the lowest SI tertile, 82% at the middle SI tertile and 81% at the greatest SI tertile (P = 0.03; chi‐square). Despite the relatively low overall sensitivity of the lowest SI tertile, there were four patients in the lowest SI tertile pre‐ACTHstim that did not meet SRA criteria post‐ACTHstim. This is a statistically significant proportion of the total group (4 out of 32; P < 0.05), and without accepting a relatively low SI (1.5‐2.1) these patients would not have been predicted to have an aldosteronoma and would not have been cured by adrenalectomy. The post‐ACTHstim LI tertiles were 3.2‐26, 27‐41 and 42‐130. Specificity was 100% in all tertiles. Sensitivity was 72% at the lowest SI tertile, 92% at the middle tertile and 73% at the greatest tertile (P = ns; chi‐square). In summary, the lowest SI tertile (1.5‐2.1) pre‐ACTHstim does not reduce the AVS specificity and may increase the SRA yield by about 14%. Finally, the tertile with the lowest SI post‐ACTHstim (3.2‐26) is greater than the prespecified minimum SI of 2 and does not decrease AVS specificity.
Table 1.
Sensitivity and specificity of the LI in predicting SRA at each tertile of SI both pre‐ and post‐ACTHstim
| SI range | Number of AVS measurements | Specificity (percent) | Sensitivity (percent) |
|---|---|---|---|
| SI Pre‐ACTHstim | |||
| 1.5‐2.1 | 22 | 100 | 50 |
| 2.3‐3.5 | 22 | 100 | 82 |
| 3.7‐42 | 21 | 100 | 81 |
| SI Post‐ACTHstim | |||
| 3.2‐26 | 25 | 100 | 72 |
| 27‐41 | 25 | 100 | 92 |
| 42‐130 | 26 | 100 | 73 |
4. DISCUSSION
Adrenal vein sampling is considered the diagnostic standard for identifying SRA.10, 11, 13, 17 However, the optimal protocols for performing AVS have not been determined. A majority of centres may employ cosyntropin stimulation.15 Cosyntropin stimulation has two advantages. First, it increases blood flow into and out the adrenal glands making AVS technically less challenging. Second, in theory, it ensures constant adrenal aldosterone and cortisol production, so that catheterization can be performed sequentially making the AVS technically less challenging and quicker. Simultaneous bilateral AVS is preferred over sequential sampling based on theoretical considerations of variable hormone secretion rates that are likely to occur when sampling is done without cosyntropin stimulation. There is no agreement as which protocol will optimize patient outcome. Doppman preferred simultaneous bilateral AVS both pre‐ and post‐ACTHstim,10 whereas Young has published extensively on results using sequential AVS performed only post‐ACTHstim.18, 19 Others recommend against the use of cosyntropin stimulation.20 Since we had performed simultaneous bilateral AVS both pre‐ACTHstim and post‐ACTHstim in all PA patients undergoing adrenalectomy, we can compare the performance of these two protocols in the same patients.
We demonstrate that simultaneous bilateral AVS performed pre‐ACTHstim increases the SRA yield by 28 per cent over performing AVS post‐ACTHstim alone. Neither protocol alone identified all patients with SRA. Both protocols had 100 per cent specificity, an important criterion when recommending an adrenalectomy for a patient. Another study with a head‐to‐head comparison and using biochemical cure as an endpoint demonstrated similar findings.16 The reproducibility of these findings at different institutions in different countries suggests that it may be appropriate to incorporate this protocol AVS guidelines.
The mechanisms to explain the dissociation of pre‐ACTHstim and post‐ACTHstim AVS results are unknown. We propose three potential mechanisms for the failure of post‐ACTH stimulation to identify seven patients with SRA. First, these unresponsive aldosteronomas may lack the ACTH receptor or subsequent downstream signalling molecules necessary for an ACTH response. Second, aldosterone production by an adenoma is maximal and cannot be increased further. Third, ACTH paradoxically inhibits the aldosterone production by these aldosteronomas. Recent studies suggest that some aldosteronomas produce enough cortisol to suppress the cortisol production by contralateral adrenal gland.21 This would lower the LI, possibly masking an aldosteronoma. These may be uncovered by cosyntropin stimulation and might explain why six patients had an isolated LI > 4, post‐ACTHstim. Regardless of the mechanisms that cause the dissociation of pre‐ACTHstim testing and post‐ACTHstim AVS results, our findings support that use of pre‐ACTHstim sampling to maximize the yield of AVS. Regardless of the mechanisms that cause the dissociation of pre‐ACTHstim and post‐ACTHstim AVS results, our findings support that use of pre‐ACTHstim sampling to maximize the yield of AVS.
In comparison to many prior studies,12 we used a lower SI limit (1.5 pre‐ACTHstim and 2.0 post‐ACTHstim) to confirm adequate sampling of the adrenal veins. Since we employed a predetermined SI in all patients, then we are in a position to evaluate the performance of this relatively low SI. Based on the CV of the cortisol assay, we calculated that a SI > 1.5 is highly unlikely to be due to a laboratory artefact or variability, and likely should indicate successful cannulation and sampling of the adrenal vein. Although our laboratory used different cortisol assays over the period of the study, the CVs were similar. The specificity of AVS in the lowest SI tertile was 100 per cent, indicating that our prediction was correct. Furthermore, the yield of SRA may be increased by using the lowest tertile of SI pre‐ACTHstim, but more patients will be required to determine whether this is reproducible. In summary, the lowest SI tertile (1.5‐2.1) pre‐ACTH stimulation does not reduce the AVS specificity and may increase the SRA yield. Although the predetermined SI post‐ACTHstim was 2.0, the lowest SI encountered was 3.2 consistent with the well‐known observation that ACTHstim increases the SI.12, 22, 23 Other studies have examined the sensitivity and specificity of different SI, but do not use surgical cure, not biochemical cure, as the outcome.22, 23 We argue that the gold standard outcome is biochemical cure.
In contrast to current Endocrine Society Guidelines, we no longer routinely perform confirmatory testing for PA prior to AVS. We reserve confirmatory testing for cases considered clinically less obvious and elect against salt loading in patients in whom it is contraindicated. We do agree with the Endocrine Society Guidelines’ conclusions that there is no gold standard confirmatory test and that the results can be ambiguous.13 If confirmatory testing is necessary, then there are two possible consequences of not routinely performing confirmatory testing: First, unsuccessful adrenalectomy and, second, a low SRA yield. We examined both these possibilities. We have already discussed the first consequence; adrenalectomy effected a resolution of the renin‐aldosterone abnormalities in all patients (100 per cent specificity), and all SRA patients had an apparent venous/arterial aldosterone gradient greater than 4. Therefore, they met classic criteria for hormone production by a tumour. In brief, bypassing confirmatory testing did not result in unnecessary adrenalectomies. Second, we did not have a low SRA yield. We reported 63 patients with successful simultaneous bilateral AVS, and adrenalectomy was recommended for 45 (71 per cent). This is similar to the yield of SRA in a study that performed AVS both pre‐ and post‐ACTHstim only following confirmatory testing with a salt loading protocol.16 Our study calls into question the utility of routine confirmatory testing.
There are a number of strengths to this study. Foremost, this is a head‐to‐head comparison of two different AVS protocols in the same patients that is superior to a randomized study that has been proposed by others.12 We were able to identify clinically important differences in the performance of the two protocols with a relatively modest number of patients. A second strength is the use of strict adherence to predetermined criteria for establishing successful AVS and for predicting SRA. The third major strength of this study is the primary endpoint of biochemical cure. PA is defined by biochemical abnormalities of the renin‐angiotensin‐aldosterone system, and we argue that gold standard for cure should be defined by normalization of these biochemical abnormalities. In contrast, one large study defined successful adrenalectomy as the identification of an adrenal cortical adenoma without regards to biochemical cure.19 Since the hormone production of an adrenal cortical adenoma cannot be determined by conventional pathology, this endpoint may be misleading. In summary, there are three strengths of this study not found in most other studies evaluating the efficacy of adrenalectomy for PA.
A potential weakness of the study is that not all patients predicted to have SRA underwent adrenalectomy and had a postadrenalectomy evaluation. Our study analysed 32 patients. This group of 32 patients came from a larger group of 45 patients who were diagnosed with SRA based upon AVS results. Seven of these patients were excluded from our analysis because they elected against adrenalectomy, and another six were excluded because they did not return for follow‐up biochemical testing following adrenalectomy. However, of the 13 patients excluded, 4 (31 per cent) were predicted to have SRA based upon an isolated pre‐ACTHstim LI > 4; a fraction similar to that of our study group.
We acknowledge that a potential weakness of our study is the modest number of patients. Therefore, the precise percentage of subjects identified to have SRA by pre‐ACTHstim should be considered as an estimate. One patient with a LI < 4 both pre‐ and post‐ACTHstim underwent adrenalectomy based upon the finding of the ND < P ratios of A/C. However, too few patients had this isolated criterion to determine its sensitivity and specificity.
In summary, performing simultaneous bilateral AVS both pre‐ACTHstim and post‐ACTHstim increases the yield of SRA as compared to preforming AVS post‐ACTHstim alone. A lower SI of 1.5 to confirm adequate AVS pre‐ACTHstim does not reduce specificity, and may increase the SRA yield. Excluding routine confirmatory testing for primary aldosteronism from the clinical protocol does not decrease the yield of SRA following AVS, nor does it result in unnecessary adrenalectomies.
We conclude that simultaneous bilateral AVS performed both pre‐ACTHstim and post‐ACTHstim maximizes SRA identification without sacrificing specificity. A minimum SI of 1.5 pre‐ACTHstim does not reduce specificity.
CONFLICTS OF INTEREST
The authors have no disclosures or conflicts of interest.
AUTHORS’ CONTRIBUTIONS
The major hypotheses and hypotheses testing methodologies were developed by a collaboration between ECV, MA, CS and CDM. Data collection and analysis was performed by EG, SJS, CMC, JG, BRT and CDM. Data interpretation was performed by EGV, MA, CKS, CSG, BRT, SJS and CDM. Manuscript was drafted and revised by EGV, CMC and CDM. All authors reviewed and approved of the final manuscript.
ETHICS STATEMENT
This study was approved by the institutional review board of the UConn Health Center. Signed informed consent was not required since this was a retrospective study extending back over 10 years and the results were presented in the aggregate.
DATA ACCESSIBILITY STATEMENT
The primary de‐identified data is available by contacting CDM. The data that support the findings of this study are available from the corresponding author upon reasonable request.
ACKNOWLEDGEMENTS
We would like to thank Dr. John Doppman for his advice during the initiation of this study.
Violari EG, Arici M, Singh CK, et al. Adrenal vein sampling with and without cosyntropin stimulation for detection of surgically remediable aldosteronism. Endocrinol Diab Metab. 2019;2:e00066 10.1002/edm2.66
REFERENCES
- 1. Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371:1921‐1926. [DOI] [PubMed] [Google Scholar]
- 2. Eide IK, Torjesen PA, Drolsum A, Babovic A, Lilledahl NP. Low‐renin status in therapy‐resistant hypertension: a clue to efficient treatment. J Hypertens. 2004;22:2217‐2226. [DOI] [PubMed] [Google Scholar]
- 3. Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6:41‐50. [DOI] [PubMed] [Google Scholar]
- 4. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6:51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanabe A, Naruse M, Naruse K, et al. Left ventricular hypertrophy is more prominent in patients with primary aldosteronism than in patients with other types of secondary hypertension. Hypertens Res. 1997;20:85‐90. [DOI] [PubMed] [Google Scholar]
- 6. Strauch B, Petrak O, Wichterle D, Zelinka T, Holaj R, Widimsky J Jr. Increased arterial wall stiffness in primary aldosteronism in comparison with essential hypertension. Am J Hypertens. 2006;19:909‐914. [DOI] [PubMed] [Google Scholar]
- 7. Rossi GP, Bernini G, Desideri G, et al. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232‐238. [DOI] [PubMed] [Google Scholar]
- 8. Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross‐sectional study. Hypertension. 2013;62:331‐336. [DOI] [PubMed] [Google Scholar]
- 9. Young WF Jr. Adrenal causes of hypertension: pheochromocytoma and primary aldosteronism. Rev Endocr Metab Disord. 2007;8:309‐320. [DOI] [PubMed] [Google Scholar]
- 10. Doppman JL, Gill J. Hyperaldosteronism: sampling the adrenal veins. Radiology. 1996;198:309‐312. [DOI] [PubMed] [Google Scholar]
- 11. Rossi GP, Sacchetto A, Chiesura‐Corona M, et al. Identification of the etiology of primary aldosteronism with adrenal vein sampling in patients with equivocal computed tomography and magnetic resonance findings: results in 104 consecutive cases. J Clin Endocrinol Metab. 2001;86:1083‐1090. [DOI] [PubMed] [Google Scholar]
- 12. Buffolo F, Monticone S, Williams TA, et al. Subtype diagnosis of primary aldosteronism: is adrenal vein sampling always necessary? Int J Mol Sci. 2017;18:E848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889‐1916. [DOI] [PubMed] [Google Scholar]
- 14. Rossi GP, Auchus RJ, Brown M, et al. An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151‐160. [DOI] [PubMed] [Google Scholar]
- 15. Rossi GP, Barisa M, Allolio B, et al. The Adrenal Vein Sampling International Study (AVIS) for identifying the major subtypes of primary aldosteronism. J Clin Endocrinol Metab. 2012;97:1606‐1614. [DOI] [PubMed] [Google Scholar]
- 16. El Ghorayeb N, Mazzuco TL, Bourdeau I, et al. Basal and Post‐ACTH aldosterone and its ratios are useful during adrenal vein sampling in primary aldosteronism. J Clin Endocrinol Metab. 2016;101:1826‐1835. [DOI] [PubMed] [Google Scholar]
- 17. Doppman J, Gill JJ, Miller D, et al. Distinction between hyperaldosteronism due to bilateral hyperplasia and unilateral aldosteronoma: reliability of CT. Radiology. 1992;184:677‐682. [DOI] [PubMed] [Google Scholar]
- 18. Lim V, Guo Q, Grant CS, et al. Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism. J Clin Endocrinol Metab. 2014;99:2712‐2719. [DOI] [PubMed] [Google Scholar]
- 19. Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227‐1235. [DOI] [PubMed] [Google Scholar]
- 20. Seccia TM, Miotto D, De Toni R, et al. Adrenocorticotropic hormone stimulation during adrenal vein sampling for identifying surgically curable subtypes of primary aldosteronism: comparison of 3 different protocols. Hypertension. 2009;53:761‐766. [DOI] [PubMed] [Google Scholar]
- 21. Arlt W, Lang K, Sitch AJ, et al. Steroid metabolome analysis reveals prevalent glucocorticoid excess in primary aldosteronism. JCI Insight. 2017;2:93136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mailhot JP, Traistaru M, Soulez G, et al. Adrenal vein sampling in primary aldosteronism: sensitivity and specificity of basal adrenal vein to peripheral vein cortisol and aldosterone ratios to confirm catheterization of the adrenal vein. Radiology. 2015;277:887‐894. [DOI] [PubMed] [Google Scholar]
- 23. Mulatero P, Bertello C, Sukor N, et al. Impact of different diagnostic criteria during adrenal vein sampling on reproducibility of subtype diagnosis in patients with primary aldosteronism. Hypertension. 2010;55:667‐673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary de‐identified data is available by contacting CDM. The data that support the findings of this study are available from the corresponding author upon reasonable request.


